User login
Jennifer Smith is the editor of Oncology Practice, part of MDedge Hematology/Oncology. She was previously the editor of Hematology Times, an editor at Principal Investigators Association, and a reporter at The Oneida Daily Dispatch. She has a BS in journalism.
Hypoxia-related discoveries net Nobel Prize
Three researchers have won the 2019 Nobel Prize in Physiology or Medicine “for their discoveries of how cells sense and adapt to oxygen availability.”
William G. Kaelin Jr., MD; Sir Peter J. Ratcliffe, MB ChB, MD; and Gregg L. Semenza, MD, PhD, described the molecular machinery that regulates gene activity in response to oxygen levels.
Their work “established the basis for our understanding of how oxygen levels affect cellular metabolism and physiological function” and “paved the way for promising new strategies to fight anemia, cancer, and many other diseases,” according to a statement by The Nobel Assembly at Karolinska Institutet.
Dr. Semenza, of Johns Hopkins Medicine in Baltimore, studied how the erythropoietin (EPO) gene is regulated by oxygen levels. Via experiments in mice, he identified DNA segments next to the EPO gene that mediate the response to hypoxia.
Dr. Ratcliffe, of the University of Oxford (England) and the Francis Crick Institute in London, also studied oxygen-dependent regulation of the EPO gene. Both his and Dr. Semenza’s groups found the oxygen-sensing mechanism was present in nearly all tissues.
Dr. Semenza also discovered a protein complex, hypoxia-inducible factor (HIF), that binds to the identified DNA segments in an oxygen-dependent manner. Additional investigation revealed that HIF consists of two transcription factors, HIF-1a and ARNT.
Several research groups found that HIF-1a is protected from degradation in hypoxia. In low-oxygen conditions, the amount of HIF-1a increases so it can bind to and regulate EPO and other genes with HIF-binding DNA segments. However, at normal oxygen levels, ubiquitin is added to HIF-1a, tagging it for degradation in the proteasome. It wasn’t clear how ubiquitin binds to HIF-1a in an oxygen-dependent manner, but Dr. Kaelin’s work provided some insight.
Dr. Kaelin, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, was researching von Hippel-Lindau’s (VHL) syndrome, an inherited disorder in which mutations can lead to tumors in multiple organs. He found the VHL gene encodes a protein that prevents cancer onset, and cancer cells without a functional VHL gene express high levels of hypoxia-regulated genes.
Research by other groups showed that VHL is part of a complex that labels proteins with ubiquitin, tagging them for degradation. Dr. Ratcliffe and his group found that VHL is required for the degradation of HIF-1a at normal oxygen levels.
Dr. Kaelin’s and Dr. Ratcliffe’s groups also showed that, under normal oxygen conditions, hydroxyl groups are added at two locations in HIF-1a. This modification – prolyl hydroxylation – allows VHL to bind to HIF-1a. So the researchers found that normal oxygen levels control HIF-1a degradation with the help of prolyl hydroxylases.
Additional research by Dr. Ratcliffe’s group and others revealed the specific prolyl hydroxylases involved in HIF-1a degradation. The researchers also found that HIF-1a’s gene-activating function was regulated by oxygen-dependent hydroxylation.
This work has improved the understanding of how different oxygen levels regulate physiological processes. In particular, oxygen sensing is essential for erythropoiesis, so these findings have implications for the treatment of anemia.
“There are several drugs that are now in clinical trials that serve to increase HIF activity and, as a result, will increase the production of erythropoietin and stimulate red blood cell production,” Dr. Semenza said in an interview after the announcement of his Nobel win. “These are all small molecules that can be given by mouth, and that may be a great convenience for patients who, at the present time, may require injections of recombinant human erythropoietin.”
Three researchers have won the 2019 Nobel Prize in Physiology or Medicine “for their discoveries of how cells sense and adapt to oxygen availability.”
William G. Kaelin Jr., MD; Sir Peter J. Ratcliffe, MB ChB, MD; and Gregg L. Semenza, MD, PhD, described the molecular machinery that regulates gene activity in response to oxygen levels.
Their work “established the basis for our understanding of how oxygen levels affect cellular metabolism and physiological function” and “paved the way for promising new strategies to fight anemia, cancer, and many other diseases,” according to a statement by The Nobel Assembly at Karolinska Institutet.
Dr. Semenza, of Johns Hopkins Medicine in Baltimore, studied how the erythropoietin (EPO) gene is regulated by oxygen levels. Via experiments in mice, he identified DNA segments next to the EPO gene that mediate the response to hypoxia.
Dr. Ratcliffe, of the University of Oxford (England) and the Francis Crick Institute in London, also studied oxygen-dependent regulation of the EPO gene. Both his and Dr. Semenza’s groups found the oxygen-sensing mechanism was present in nearly all tissues.
Dr. Semenza also discovered a protein complex, hypoxia-inducible factor (HIF), that binds to the identified DNA segments in an oxygen-dependent manner. Additional investigation revealed that HIF consists of two transcription factors, HIF-1a and ARNT.
Several research groups found that HIF-1a is protected from degradation in hypoxia. In low-oxygen conditions, the amount of HIF-1a increases so it can bind to and regulate EPO and other genes with HIF-binding DNA segments. However, at normal oxygen levels, ubiquitin is added to HIF-1a, tagging it for degradation in the proteasome. It wasn’t clear how ubiquitin binds to HIF-1a in an oxygen-dependent manner, but Dr. Kaelin’s work provided some insight.
Dr. Kaelin, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, was researching von Hippel-Lindau’s (VHL) syndrome, an inherited disorder in which mutations can lead to tumors in multiple organs. He found the VHL gene encodes a protein that prevents cancer onset, and cancer cells without a functional VHL gene express high levels of hypoxia-regulated genes.
Research by other groups showed that VHL is part of a complex that labels proteins with ubiquitin, tagging them for degradation. Dr. Ratcliffe and his group found that VHL is required for the degradation of HIF-1a at normal oxygen levels.
Dr. Kaelin’s and Dr. Ratcliffe’s groups also showed that, under normal oxygen conditions, hydroxyl groups are added at two locations in HIF-1a. This modification – prolyl hydroxylation – allows VHL to bind to HIF-1a. So the researchers found that normal oxygen levels control HIF-1a degradation with the help of prolyl hydroxylases.
Additional research by Dr. Ratcliffe’s group and others revealed the specific prolyl hydroxylases involved in HIF-1a degradation. The researchers also found that HIF-1a’s gene-activating function was regulated by oxygen-dependent hydroxylation.
This work has improved the understanding of how different oxygen levels regulate physiological processes. In particular, oxygen sensing is essential for erythropoiesis, so these findings have implications for the treatment of anemia.
“There are several drugs that are now in clinical trials that serve to increase HIF activity and, as a result, will increase the production of erythropoietin and stimulate red blood cell production,” Dr. Semenza said in an interview after the announcement of his Nobel win. “These are all small molecules that can be given by mouth, and that may be a great convenience for patients who, at the present time, may require injections of recombinant human erythropoietin.”
Three researchers have won the 2019 Nobel Prize in Physiology or Medicine “for their discoveries of how cells sense and adapt to oxygen availability.”
William G. Kaelin Jr., MD; Sir Peter J. Ratcliffe, MB ChB, MD; and Gregg L. Semenza, MD, PhD, described the molecular machinery that regulates gene activity in response to oxygen levels.
Their work “established the basis for our understanding of how oxygen levels affect cellular metabolism and physiological function” and “paved the way for promising new strategies to fight anemia, cancer, and many other diseases,” according to a statement by The Nobel Assembly at Karolinska Institutet.
Dr. Semenza, of Johns Hopkins Medicine in Baltimore, studied how the erythropoietin (EPO) gene is regulated by oxygen levels. Via experiments in mice, he identified DNA segments next to the EPO gene that mediate the response to hypoxia.
Dr. Ratcliffe, of the University of Oxford (England) and the Francis Crick Institute in London, also studied oxygen-dependent regulation of the EPO gene. Both his and Dr. Semenza’s groups found the oxygen-sensing mechanism was present in nearly all tissues.
Dr. Semenza also discovered a protein complex, hypoxia-inducible factor (HIF), that binds to the identified DNA segments in an oxygen-dependent manner. Additional investigation revealed that HIF consists of two transcription factors, HIF-1a and ARNT.
Several research groups found that HIF-1a is protected from degradation in hypoxia. In low-oxygen conditions, the amount of HIF-1a increases so it can bind to and regulate EPO and other genes with HIF-binding DNA segments. However, at normal oxygen levels, ubiquitin is added to HIF-1a, tagging it for degradation in the proteasome. It wasn’t clear how ubiquitin binds to HIF-1a in an oxygen-dependent manner, but Dr. Kaelin’s work provided some insight.
Dr. Kaelin, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, was researching von Hippel-Lindau’s (VHL) syndrome, an inherited disorder in which mutations can lead to tumors in multiple organs. He found the VHL gene encodes a protein that prevents cancer onset, and cancer cells without a functional VHL gene express high levels of hypoxia-regulated genes.
Research by other groups showed that VHL is part of a complex that labels proteins with ubiquitin, tagging them for degradation. Dr. Ratcliffe and his group found that VHL is required for the degradation of HIF-1a at normal oxygen levels.
Dr. Kaelin’s and Dr. Ratcliffe’s groups also showed that, under normal oxygen conditions, hydroxyl groups are added at two locations in HIF-1a. This modification – prolyl hydroxylation – allows VHL to bind to HIF-1a. So the researchers found that normal oxygen levels control HIF-1a degradation with the help of prolyl hydroxylases.
Additional research by Dr. Ratcliffe’s group and others revealed the specific prolyl hydroxylases involved in HIF-1a degradation. The researchers also found that HIF-1a’s gene-activating function was regulated by oxygen-dependent hydroxylation.
This work has improved the understanding of how different oxygen levels regulate physiological processes. In particular, oxygen sensing is essential for erythropoiesis, so these findings have implications for the treatment of anemia.
“There are several drugs that are now in clinical trials that serve to increase HIF activity and, as a result, will increase the production of erythropoietin and stimulate red blood cell production,” Dr. Semenza said in an interview after the announcement of his Nobel win. “These are all small molecules that can be given by mouth, and that may be a great convenience for patients who, at the present time, may require injections of recombinant human erythropoietin.”
Cleveland Clinic taps Abraham as chair
Jame Abraham, MD, has been appointed chair of the hematology/medical oncology department at Cleveland Clinic in Ohio. In this new role, Dr. Abraham will “recruit and develop staff and guide the department’s focus on patient access and a multidisciplinary approach to care,” according to a statement.
Dr. Abraham is also director of the breast oncology program at Taussig Cancer Institute, codirector of the Cleveland Clinic comprehensive breast cancer program, and a professor of medicine at Cleveland Clinic Lerner College of Medicine. He takes the helm from hematologist Matt Kalaycio, MD. Dr. Kalaycio also serves as editor-in-chief of Hematology News.
In other news, Zhe Ying, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, has received a 5-year Pathway to Independence Award from the National Institute of Dental and Craniofacial Research.
With this award funding, Dr. Ying will investigate oncogene-induced differentiation in PI3K-mutant head and neck squamous cell carcinoma. Specifically, he aims to determine if genetic mutations and niche factors can overcome oncogene-induced differentiation to promote tumorigenesis.
Another grant winner is Gina Mantia-Smaldone, MD, of Fox Chase Cancer Center in Philadelphia. She will receive 3 years of funding from the Gynecologic Oncology Group Foundation and NRG Oncology to study gynecologic malignancies.
This award will also provide Dr. Mantia-Smaldone with research mentorship and opportunities to collaborate with other researchers. Her research is focused on developing targeted therapies for ovarian and endometrial cancers that will, ideally, improve patients’ quality of life.
Lastly, Edna (Eti) Cukierman, PhD, of Fox Chase Cancer Center, received a grant to conduct research with Ashani Weeraratna, PhD, of Johns Hopkins University in Baltimore, and Vivek Shenoy, PhD, and Arjun Raj, PhD, both of the University of Pennsylvania in Philadelphia.
The grant, from the National Cancer Institute, will be used to investigate the link between cell aging and melanoma. Dr. Cukierman, Dr. Weeraratna, Dr. Shenoy, and Dr. Raj will focus their research "on better understanding the deterioration of collagen integrity via cellular aging and its role in melanoma metastasis,” according to a statement.
Jame Abraham, MD, has been appointed chair of the hematology/medical oncology department at Cleveland Clinic in Ohio. In this new role, Dr. Abraham will “recruit and develop staff and guide the department’s focus on patient access and a multidisciplinary approach to care,” according to a statement.
Dr. Abraham is also director of the breast oncology program at Taussig Cancer Institute, codirector of the Cleveland Clinic comprehensive breast cancer program, and a professor of medicine at Cleveland Clinic Lerner College of Medicine. He takes the helm from hematologist Matt Kalaycio, MD. Dr. Kalaycio also serves as editor-in-chief of Hematology News.
In other news, Zhe Ying, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, has received a 5-year Pathway to Independence Award from the National Institute of Dental and Craniofacial Research.
With this award funding, Dr. Ying will investigate oncogene-induced differentiation in PI3K-mutant head and neck squamous cell carcinoma. Specifically, he aims to determine if genetic mutations and niche factors can overcome oncogene-induced differentiation to promote tumorigenesis.
Another grant winner is Gina Mantia-Smaldone, MD, of Fox Chase Cancer Center in Philadelphia. She will receive 3 years of funding from the Gynecologic Oncology Group Foundation and NRG Oncology to study gynecologic malignancies.
This award will also provide Dr. Mantia-Smaldone with research mentorship and opportunities to collaborate with other researchers. Her research is focused on developing targeted therapies for ovarian and endometrial cancers that will, ideally, improve patients’ quality of life.
Lastly, Edna (Eti) Cukierman, PhD, of Fox Chase Cancer Center, received a grant to conduct research with Ashani Weeraratna, PhD, of Johns Hopkins University in Baltimore, and Vivek Shenoy, PhD, and Arjun Raj, PhD, both of the University of Pennsylvania in Philadelphia.
The grant, from the National Cancer Institute, will be used to investigate the link between cell aging and melanoma. Dr. Cukierman, Dr. Weeraratna, Dr. Shenoy, and Dr. Raj will focus their research "on better understanding the deterioration of collagen integrity via cellular aging and its role in melanoma metastasis,” according to a statement.
Jame Abraham, MD, has been appointed chair of the hematology/medical oncology department at Cleveland Clinic in Ohio. In this new role, Dr. Abraham will “recruit and develop staff and guide the department’s focus on patient access and a multidisciplinary approach to care,” according to a statement.
Dr. Abraham is also director of the breast oncology program at Taussig Cancer Institute, codirector of the Cleveland Clinic comprehensive breast cancer program, and a professor of medicine at Cleveland Clinic Lerner College of Medicine. He takes the helm from hematologist Matt Kalaycio, MD. Dr. Kalaycio also serves as editor-in-chief of Hematology News.
In other news, Zhe Ying, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, has received a 5-year Pathway to Independence Award from the National Institute of Dental and Craniofacial Research.
With this award funding, Dr. Ying will investigate oncogene-induced differentiation in PI3K-mutant head and neck squamous cell carcinoma. Specifically, he aims to determine if genetic mutations and niche factors can overcome oncogene-induced differentiation to promote tumorigenesis.
Another grant winner is Gina Mantia-Smaldone, MD, of Fox Chase Cancer Center in Philadelphia. She will receive 3 years of funding from the Gynecologic Oncology Group Foundation and NRG Oncology to study gynecologic malignancies.
This award will also provide Dr. Mantia-Smaldone with research mentorship and opportunities to collaborate with other researchers. Her research is focused on developing targeted therapies for ovarian and endometrial cancers that will, ideally, improve patients’ quality of life.
Lastly, Edna (Eti) Cukierman, PhD, of Fox Chase Cancer Center, received a grant to conduct research with Ashani Weeraratna, PhD, of Johns Hopkins University in Baltimore, and Vivek Shenoy, PhD, and Arjun Raj, PhD, both of the University of Pennsylvania in Philadelphia.
The grant, from the National Cancer Institute, will be used to investigate the link between cell aging and melanoma. Dr. Cukierman, Dr. Weeraratna, Dr. Shenoy, and Dr. Raj will focus their research "on better understanding the deterioration of collagen integrity via cellular aging and its role in melanoma metastasis,” according to a statement.
Vaping-associated lung injury cases exceed 1,000
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email [email protected]. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email [email protected]. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email [email protected]. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email [email protected]. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email [email protected]. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email [email protected]. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
Targeted agents vs. chemoimmunotherapy as first-line treatment of CLL
SAN FRANCISCO – Should targeted agents replace chemoimmunotherapy (CIT) as first-line treatment for chronic lymphocytic leukemia (CLL)? A recent debate suggests there’s no consensus.
William G. Wierda, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, debated the topic at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Wierda argued that CLL patients should receive a BTK inhibitor or BCL2 inhibitor, with or without obinutuzumab, as first-line therapy because these targeted agents have been shown to provide better progression-free survival (PFS) than CIT, and the targeted therapies may prolong overall survival (OS) as well.
Dr. Brown countered that targeted agents don’t improve PFS for all CLL patients, improved PFS doesn’t always translate to improved OS, and targeted agents cost more than CIT.
No role for CIT as first-line treatment
“We have two approaches right now, with nonchemoimmunotherapy-based treatment,” Dr. Wierda said. “One approach, with small-molecule inhibitors, is to have a sustained and durable period of disease control, particularly with BTK inhibitors. The other strategy that has emerged is deep remissions with fixed-duration treatment with BCL2 small-molecule inhibitor-based therapy, which, I would argue, is better than being exposed to genotoxic chemoimmunotherapy.”
Dr. Wierda went on to explain that the BTK inhibitor ibrutinib has been shown to improve PFS, compared with CIT, in phase 3 trials.
In the iLLUMINATE trial, researchers compared ibrutinib plus obinutuzumab to chlorambucil plus obinutuzumab as first-line treatment in CLL. At a median follow-up of 31.3 months, the median PFS was not reached in the ibrutinib arm and was 19 months in the chlorambucil arm (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:43-56).
In the A041202 study, researchers compared ibrutinib alone (Ib) or in combination with rituximab (Ib-R) to bendamustine plus rituximab (BR) in untreated, older patients with CLL. The 2-year PFS estimates were 74% in the BR arm, 87% in the Ib arm, and 88% in the Ib-R arm (P less than .001 for BR vs. Ib or Ib-R; N Engl J Med. 2018; 379:2517-28).
In the E1912 trial, researchers compared Ib-R to fludarabine, cyclophosphamide, and rituximab (FCR) in younger, untreated CLL patients. The 3-year PFS was 89.4% with Ib-R and 72.9% with FCR (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43).
Dr. Wierda noted that the E1912 trial also showed superior OS with Ib-R. The 3-year OS rate was 98.8% with Ib-R and 91.5% with FCR (P less than .001). However, there was no significant difference in OS between the treatment arms in the A041202 trial or the iLLUMINATE trial.
“But I would argue that is, in part, because of short follow-up,” Dr. Wierda said. “The trials were all designed to look at progression-free survival, not overall survival. With longer follow-up, we may see differences in overall survival emerging.”
Dr. Wierda went on to say that fixed‐duration treatment with the BCL2 inhibitor venetoclax can improve PFS over CIT.
In the phase 3 CLL14 trial, researchers compared fixed-duration treatment with venetoclax plus obinutuzumab to chlorambucil plus obinutuzumab in previously untreated CLL patients with comorbidities. The estimated PFS at 2 years was 88.2% in the venetoclax group and 64.1% in the chlorambucil group (P less than .001; N Engl J Med. 2019; 380:2225-36).
“[There was] no difference in overall survival,” Dr. Wierda noted. “But, again, I would argue ... that follow-up is relatively limited. We may ultimately see a difference in overall survival.”
Based on these findings, Dr. Wierda made the following treatment recommendations:
- Any CLL patient with del(17p) or TP53 mutation, and older, unfit patients with unmutated IGHV should receive a BTK inhibitor, with or without obinutuzumab.
- All young, fit patients, and older, unfit patients with mutated IGHV should receive a BCL2 inhibitor plus obinutuzumab.
Dr. Wierda also noted that ibrutinib and venetoclax in combination have shown early promise for patients with previously untreated CLL (N Engl J Med. 2019; 380:2095-2103).
CIT still has a role as first-line treatment
Dr. Brown suggested that a PFS benefit may not be enough to recommend targeted agents over CIT. For one thing, the PFS benefit doesn’t apply to all patients, as the IGHV-mutated subgroup does equally well with CIT and targeted agents.
In the IGHV-mutated group from the E1912 trial, the 3-year PFS was 88% for patients who received Ib-R and those who received FCR (N Engl J Med. 2019 Aug 1;381:432-43). In the A041202 study, the 2-year PFS among IGHV-mutated patients was 87% in the BR arm, 86% in the Ib arm, and 88% in the Ib-R arm (N Engl J Med. 2018; 379:2517-28).
In the CLL14 trial, PFS rates were similar among IGHV-mutated patients who received chlorambucil plus obinutuzumab and IGHV-mutated or unmutated patients who received venetoclax and obinutuzumab (N Engl J Med. 2019; 380:2225-36).
Dr. Brown also noted that the overall improvement in PFS observed with ibrutinib and venetoclax doesn’t always translate to improved OS.
In the A041202 study, there was no significant difference in OS between the Ib, Ib-R, and BR arms (N Engl J Med. 2018; 379:2517-28). There was no significant difference in OS between the ibrutinib and chlorambucil arms in the iLLUMINATE trial (Lancet Oncol. 2019 Jan;20[1]:43-56). And there was no significant difference in OS between the venetoclax and chlorambucil arms in the CLL14 trial (N Engl J Med. 2019; 380:2225-36).
However, in the RESONATE-2 trial, ibrutinib provided an OS benefit over chlorambucil. The 2-year OS was 95% and 84%, respectively (P = .0145; Haematologica. Sept 2018;103:1502-10). Dr. Brown said the OS advantage in this study was due to the “very poor comparator of chlorambucil and very limited crossover.”
As Dr. Wierda mentioned, the OS rate was higher with Ib-R than with FCR in the E1912 trial. The 3-year OS rate was 98.8% and 91.5%, respectively (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43). Dr. Brown noted, however, that there were few deaths in this study, and many of them “were not clearly related to the disease or its treatment.”
Dr. Brown also pointed out that FCR has been shown to have curative potential in IGHV-mutated CLL in both the FCR300 trial (Blood. 2016 127:303-9) and the CLL8 trial (Blood. 2016 127:208-15).
Another factor to consider is the greater cost of targeted agents. One analysis suggested the per-patient lifetime cost of CLL treatment in the United States will increase from $147,000 to $604,000 as targeted therapies overtake CIT as first-line treatment (J Clin Oncol. 2017 Jan 10;35[2]:166-174).
“Given all of the above, chemoimmunotherapy is going to remain part of the treatment repertoire for CLL,” Dr. Brown said. “It’s our only known potential cure for the fit, mutated patients ... and can also result in prolonged treatment-free intervals for patients who are older. As we manage CLL as a chronic disease over a lifetime, we need to continue to have this in our armamentarium.”
Specifically, Dr. Brown said CIT is appropriate for patients who don’t have del(17p) or mutated TP53. FCR should be given to young, fit patients with IGHV-mutated CLL, and FCR or BR should be given to older patients and young, fit patients with IGHV-unmutated CLL.
Dr. Brown and Dr. Wierda reported financial ties to multiple pharmaceutical companies, including makers of CLL treatments.
SAN FRANCISCO – Should targeted agents replace chemoimmunotherapy (CIT) as first-line treatment for chronic lymphocytic leukemia (CLL)? A recent debate suggests there’s no consensus.
William G. Wierda, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, debated the topic at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Wierda argued that CLL patients should receive a BTK inhibitor or BCL2 inhibitor, with or without obinutuzumab, as first-line therapy because these targeted agents have been shown to provide better progression-free survival (PFS) than CIT, and the targeted therapies may prolong overall survival (OS) as well.
Dr. Brown countered that targeted agents don’t improve PFS for all CLL patients, improved PFS doesn’t always translate to improved OS, and targeted agents cost more than CIT.
No role for CIT as first-line treatment
“We have two approaches right now, with nonchemoimmunotherapy-based treatment,” Dr. Wierda said. “One approach, with small-molecule inhibitors, is to have a sustained and durable period of disease control, particularly with BTK inhibitors. The other strategy that has emerged is deep remissions with fixed-duration treatment with BCL2 small-molecule inhibitor-based therapy, which, I would argue, is better than being exposed to genotoxic chemoimmunotherapy.”
Dr. Wierda went on to explain that the BTK inhibitor ibrutinib has been shown to improve PFS, compared with CIT, in phase 3 trials.
In the iLLUMINATE trial, researchers compared ibrutinib plus obinutuzumab to chlorambucil plus obinutuzumab as first-line treatment in CLL. At a median follow-up of 31.3 months, the median PFS was not reached in the ibrutinib arm and was 19 months in the chlorambucil arm (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:43-56).
In the A041202 study, researchers compared ibrutinib alone (Ib) or in combination with rituximab (Ib-R) to bendamustine plus rituximab (BR) in untreated, older patients with CLL. The 2-year PFS estimates were 74% in the BR arm, 87% in the Ib arm, and 88% in the Ib-R arm (P less than .001 for BR vs. Ib or Ib-R; N Engl J Med. 2018; 379:2517-28).
In the E1912 trial, researchers compared Ib-R to fludarabine, cyclophosphamide, and rituximab (FCR) in younger, untreated CLL patients. The 3-year PFS was 89.4% with Ib-R and 72.9% with FCR (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43).
Dr. Wierda noted that the E1912 trial also showed superior OS with Ib-R. The 3-year OS rate was 98.8% with Ib-R and 91.5% with FCR (P less than .001). However, there was no significant difference in OS between the treatment arms in the A041202 trial or the iLLUMINATE trial.
“But I would argue that is, in part, because of short follow-up,” Dr. Wierda said. “The trials were all designed to look at progression-free survival, not overall survival. With longer follow-up, we may see differences in overall survival emerging.”
Dr. Wierda went on to say that fixed‐duration treatment with the BCL2 inhibitor venetoclax can improve PFS over CIT.
In the phase 3 CLL14 trial, researchers compared fixed-duration treatment with venetoclax plus obinutuzumab to chlorambucil plus obinutuzumab in previously untreated CLL patients with comorbidities. The estimated PFS at 2 years was 88.2% in the venetoclax group and 64.1% in the chlorambucil group (P less than .001; N Engl J Med. 2019; 380:2225-36).
“[There was] no difference in overall survival,” Dr. Wierda noted. “But, again, I would argue ... that follow-up is relatively limited. We may ultimately see a difference in overall survival.”
Based on these findings, Dr. Wierda made the following treatment recommendations:
- Any CLL patient with del(17p) or TP53 mutation, and older, unfit patients with unmutated IGHV should receive a BTK inhibitor, with or without obinutuzumab.
- All young, fit patients, and older, unfit patients with mutated IGHV should receive a BCL2 inhibitor plus obinutuzumab.
Dr. Wierda also noted that ibrutinib and venetoclax in combination have shown early promise for patients with previously untreated CLL (N Engl J Med. 2019; 380:2095-2103).
CIT still has a role as first-line treatment
Dr. Brown suggested that a PFS benefit may not be enough to recommend targeted agents over CIT. For one thing, the PFS benefit doesn’t apply to all patients, as the IGHV-mutated subgroup does equally well with CIT and targeted agents.
In the IGHV-mutated group from the E1912 trial, the 3-year PFS was 88% for patients who received Ib-R and those who received FCR (N Engl J Med. 2019 Aug 1;381:432-43). In the A041202 study, the 2-year PFS among IGHV-mutated patients was 87% in the BR arm, 86% in the Ib arm, and 88% in the Ib-R arm (N Engl J Med. 2018; 379:2517-28).
In the CLL14 trial, PFS rates were similar among IGHV-mutated patients who received chlorambucil plus obinutuzumab and IGHV-mutated or unmutated patients who received venetoclax and obinutuzumab (N Engl J Med. 2019; 380:2225-36).
Dr. Brown also noted that the overall improvement in PFS observed with ibrutinib and venetoclax doesn’t always translate to improved OS.
In the A041202 study, there was no significant difference in OS between the Ib, Ib-R, and BR arms (N Engl J Med. 2018; 379:2517-28). There was no significant difference in OS between the ibrutinib and chlorambucil arms in the iLLUMINATE trial (Lancet Oncol. 2019 Jan;20[1]:43-56). And there was no significant difference in OS between the venetoclax and chlorambucil arms in the CLL14 trial (N Engl J Med. 2019; 380:2225-36).
However, in the RESONATE-2 trial, ibrutinib provided an OS benefit over chlorambucil. The 2-year OS was 95% and 84%, respectively (P = .0145; Haematologica. Sept 2018;103:1502-10). Dr. Brown said the OS advantage in this study was due to the “very poor comparator of chlorambucil and very limited crossover.”
As Dr. Wierda mentioned, the OS rate was higher with Ib-R than with FCR in the E1912 trial. The 3-year OS rate was 98.8% and 91.5%, respectively (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43). Dr. Brown noted, however, that there were few deaths in this study, and many of them “were not clearly related to the disease or its treatment.”
Dr. Brown also pointed out that FCR has been shown to have curative potential in IGHV-mutated CLL in both the FCR300 trial (Blood. 2016 127:303-9) and the CLL8 trial (Blood. 2016 127:208-15).
Another factor to consider is the greater cost of targeted agents. One analysis suggested the per-patient lifetime cost of CLL treatment in the United States will increase from $147,000 to $604,000 as targeted therapies overtake CIT as first-line treatment (J Clin Oncol. 2017 Jan 10;35[2]:166-174).
“Given all of the above, chemoimmunotherapy is going to remain part of the treatment repertoire for CLL,” Dr. Brown said. “It’s our only known potential cure for the fit, mutated patients ... and can also result in prolonged treatment-free intervals for patients who are older. As we manage CLL as a chronic disease over a lifetime, we need to continue to have this in our armamentarium.”
Specifically, Dr. Brown said CIT is appropriate for patients who don’t have del(17p) or mutated TP53. FCR should be given to young, fit patients with IGHV-mutated CLL, and FCR or BR should be given to older patients and young, fit patients with IGHV-unmutated CLL.
Dr. Brown and Dr. Wierda reported financial ties to multiple pharmaceutical companies, including makers of CLL treatments.
SAN FRANCISCO – Should targeted agents replace chemoimmunotherapy (CIT) as first-line treatment for chronic lymphocytic leukemia (CLL)? A recent debate suggests there’s no consensus.
William G. Wierda, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, debated the topic at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Wierda argued that CLL patients should receive a BTK inhibitor or BCL2 inhibitor, with or without obinutuzumab, as first-line therapy because these targeted agents have been shown to provide better progression-free survival (PFS) than CIT, and the targeted therapies may prolong overall survival (OS) as well.
Dr. Brown countered that targeted agents don’t improve PFS for all CLL patients, improved PFS doesn’t always translate to improved OS, and targeted agents cost more than CIT.
No role for CIT as first-line treatment
“We have two approaches right now, with nonchemoimmunotherapy-based treatment,” Dr. Wierda said. “One approach, with small-molecule inhibitors, is to have a sustained and durable period of disease control, particularly with BTK inhibitors. The other strategy that has emerged is deep remissions with fixed-duration treatment with BCL2 small-molecule inhibitor-based therapy, which, I would argue, is better than being exposed to genotoxic chemoimmunotherapy.”
Dr. Wierda went on to explain that the BTK inhibitor ibrutinib has been shown to improve PFS, compared with CIT, in phase 3 trials.
In the iLLUMINATE trial, researchers compared ibrutinib plus obinutuzumab to chlorambucil plus obinutuzumab as first-line treatment in CLL. At a median follow-up of 31.3 months, the median PFS was not reached in the ibrutinib arm and was 19 months in the chlorambucil arm (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:43-56).
In the A041202 study, researchers compared ibrutinib alone (Ib) or in combination with rituximab (Ib-R) to bendamustine plus rituximab (BR) in untreated, older patients with CLL. The 2-year PFS estimates were 74% in the BR arm, 87% in the Ib arm, and 88% in the Ib-R arm (P less than .001 for BR vs. Ib or Ib-R; N Engl J Med. 2018; 379:2517-28).
In the E1912 trial, researchers compared Ib-R to fludarabine, cyclophosphamide, and rituximab (FCR) in younger, untreated CLL patients. The 3-year PFS was 89.4% with Ib-R and 72.9% with FCR (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43).
Dr. Wierda noted that the E1912 trial also showed superior OS with Ib-R. The 3-year OS rate was 98.8% with Ib-R and 91.5% with FCR (P less than .001). However, there was no significant difference in OS between the treatment arms in the A041202 trial or the iLLUMINATE trial.
“But I would argue that is, in part, because of short follow-up,” Dr. Wierda said. “The trials were all designed to look at progression-free survival, not overall survival. With longer follow-up, we may see differences in overall survival emerging.”
Dr. Wierda went on to say that fixed‐duration treatment with the BCL2 inhibitor venetoclax can improve PFS over CIT.
In the phase 3 CLL14 trial, researchers compared fixed-duration treatment with venetoclax plus obinutuzumab to chlorambucil plus obinutuzumab in previously untreated CLL patients with comorbidities. The estimated PFS at 2 years was 88.2% in the venetoclax group and 64.1% in the chlorambucil group (P less than .001; N Engl J Med. 2019; 380:2225-36).
“[There was] no difference in overall survival,” Dr. Wierda noted. “But, again, I would argue ... that follow-up is relatively limited. We may ultimately see a difference in overall survival.”
Based on these findings, Dr. Wierda made the following treatment recommendations:
- Any CLL patient with del(17p) or TP53 mutation, and older, unfit patients with unmutated IGHV should receive a BTK inhibitor, with or without obinutuzumab.
- All young, fit patients, and older, unfit patients with mutated IGHV should receive a BCL2 inhibitor plus obinutuzumab.
Dr. Wierda also noted that ibrutinib and venetoclax in combination have shown early promise for patients with previously untreated CLL (N Engl J Med. 2019; 380:2095-2103).
CIT still has a role as first-line treatment
Dr. Brown suggested that a PFS benefit may not be enough to recommend targeted agents over CIT. For one thing, the PFS benefit doesn’t apply to all patients, as the IGHV-mutated subgroup does equally well with CIT and targeted agents.
In the IGHV-mutated group from the E1912 trial, the 3-year PFS was 88% for patients who received Ib-R and those who received FCR (N Engl J Med. 2019 Aug 1;381:432-43). In the A041202 study, the 2-year PFS among IGHV-mutated patients was 87% in the BR arm, 86% in the Ib arm, and 88% in the Ib-R arm (N Engl J Med. 2018; 379:2517-28).
In the CLL14 trial, PFS rates were similar among IGHV-mutated patients who received chlorambucil plus obinutuzumab and IGHV-mutated or unmutated patients who received venetoclax and obinutuzumab (N Engl J Med. 2019; 380:2225-36).
Dr. Brown also noted that the overall improvement in PFS observed with ibrutinib and venetoclax doesn’t always translate to improved OS.
In the A041202 study, there was no significant difference in OS between the Ib, Ib-R, and BR arms (N Engl J Med. 2018; 379:2517-28). There was no significant difference in OS between the ibrutinib and chlorambucil arms in the iLLUMINATE trial (Lancet Oncol. 2019 Jan;20[1]:43-56). And there was no significant difference in OS between the venetoclax and chlorambucil arms in the CLL14 trial (N Engl J Med. 2019; 380:2225-36).
However, in the RESONATE-2 trial, ibrutinib provided an OS benefit over chlorambucil. The 2-year OS was 95% and 84%, respectively (P = .0145; Haematologica. Sept 2018;103:1502-10). Dr. Brown said the OS advantage in this study was due to the “very poor comparator of chlorambucil and very limited crossover.”
As Dr. Wierda mentioned, the OS rate was higher with Ib-R than with FCR in the E1912 trial. The 3-year OS rate was 98.8% and 91.5%, respectively (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43). Dr. Brown noted, however, that there were few deaths in this study, and many of them “were not clearly related to the disease or its treatment.”
Dr. Brown also pointed out that FCR has been shown to have curative potential in IGHV-mutated CLL in both the FCR300 trial (Blood. 2016 127:303-9) and the CLL8 trial (Blood. 2016 127:208-15).
Another factor to consider is the greater cost of targeted agents. One analysis suggested the per-patient lifetime cost of CLL treatment in the United States will increase from $147,000 to $604,000 as targeted therapies overtake CIT as first-line treatment (J Clin Oncol. 2017 Jan 10;35[2]:166-174).
“Given all of the above, chemoimmunotherapy is going to remain part of the treatment repertoire for CLL,” Dr. Brown said. “It’s our only known potential cure for the fit, mutated patients ... and can also result in prolonged treatment-free intervals for patients who are older. As we manage CLL as a chronic disease over a lifetime, we need to continue to have this in our armamentarium.”
Specifically, Dr. Brown said CIT is appropriate for patients who don’t have del(17p) or mutated TP53. FCR should be given to young, fit patients with IGHV-mutated CLL, and FCR or BR should be given to older patients and young, fit patients with IGHV-unmutated CLL.
Dr. Brown and Dr. Wierda reported financial ties to multiple pharmaceutical companies, including makers of CLL treatments.
REPORTING FROM NCCN HEMATOLOGIC MALIGNANCIES
Decoding biosimilar approvals
SAN FRANCISCO – Several factors must be considered when extrapolating biosimilar results, according to a speaker at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
In this context, “extrapolation” means expanding the use of an approved biosimilar from one indication to another, based on efficacy and safety data from the first indication, Andrew D. Zelenetz, MD, PhD, of Memorial Sloan Kettering Cancer Center, New York, explained at the meeting.
To determine if extrapolation is appropriate, regulatory agencies consider the biosimilar’s mechanism of action in each indication; pharmacokinetics, pharmacodynamics, and immunogenicity in the different patient populations; differences in expected toxicities for each condition and population; and any other factor that may affect safety or efficacy.
To illustrate the process, Dr. Zelenetz explained how results with a rituximab biosimilar in rheumatoid arthritis (RA) cannot be extrapolated to B‐cell non‐Hodgkin lymphoma (NHL), but results with that same biosimilar in follicular lymphoma can be extrapolated to other types of B-cell NHL.
The biosimilar is rituximab-abbs (CT‐P10, Truxima). In a phase 1 trial of patients with RA, rituximab-abbs demonstrated biosimilarity to the reference product (Ann Rheum Dis. 2017;76[3]:566‐70).
The RA results cannot be extrapolated to B-cell NHL for a few reasons, according to Dr. Zelenetz. He noted that rituximab’s mechanism of action is antibody-dependent cell‐mediated cytotoxicity in both RA and NHL. However, the target in RA is the normal B cell, and the target in NHL is the malignant B cell.
In addition, the pharmacokinetics of rituximab are “drastically different” in RA and NHL, Dr. Zelenetz said. Differences in pharmacokinetics support different dosing approaches in the two diseases.
Another big difference is immunogenicity. Anti‐CD20 antibodies develop in 15%-17% of RA patients, Dr. Zelenetz said, but the risk of antibody development is less than 1% in lymphoma.
Though extrapolation from RA to B‐cell NHL was not possible, it was possible to extrapolate results with rituximab-abbs in follicular lymphoma to other B-cell NHLs.
The study used was a phase 3 trial comparing rituximab-abbs to rituximab – both in combination with cyclophosphamide, vincristine, and prednisone – in patients with newly diagnosed, advanced stage follicular lymphoma.
This study showed no difference in pharmacokinetics or pharmacodynamics between rituximab-abbs and rituximab. The two agents also had comparable safety profiles and produced similar response rates (Lancet Haematol. 2017 Jul 13;4:e362‐73).
Rituximab‐abbs was approved in the United States based on these data, and results from this trial were extrapolated to other types of B-cell NHL. The results were extrapolated because the mechanism of action, pharmacokinetics, pharmacodynamics, and immunogenicity of rituximab are the same across B-cell NHLs, Dr. Zelenetz noted.
“Extrapolation is a critical part of biosimilarity development,” he said. “As long as scientific justification for extrapolation exists, I believe that extrapolation makes good sense.”
Dr. Zelenetz reported relationships with AbbVie, Adaptive Biotechnologies, Amgen, AstraZeneca, BeiGene, Celgene, Genentech, Gilead Sciences, Janssen, MEI Pharma, MorphoSys AG, Novartis, Pharmacyclics, and Roche.
SAN FRANCISCO – Several factors must be considered when extrapolating biosimilar results, according to a speaker at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
In this context, “extrapolation” means expanding the use of an approved biosimilar from one indication to another, based on efficacy and safety data from the first indication, Andrew D. Zelenetz, MD, PhD, of Memorial Sloan Kettering Cancer Center, New York, explained at the meeting.
To determine if extrapolation is appropriate, regulatory agencies consider the biosimilar’s mechanism of action in each indication; pharmacokinetics, pharmacodynamics, and immunogenicity in the different patient populations; differences in expected toxicities for each condition and population; and any other factor that may affect safety or efficacy.
To illustrate the process, Dr. Zelenetz explained how results with a rituximab biosimilar in rheumatoid arthritis (RA) cannot be extrapolated to B‐cell non‐Hodgkin lymphoma (NHL), but results with that same biosimilar in follicular lymphoma can be extrapolated to other types of B-cell NHL.
The biosimilar is rituximab-abbs (CT‐P10, Truxima). In a phase 1 trial of patients with RA, rituximab-abbs demonstrated biosimilarity to the reference product (Ann Rheum Dis. 2017;76[3]:566‐70).
The RA results cannot be extrapolated to B-cell NHL for a few reasons, according to Dr. Zelenetz. He noted that rituximab’s mechanism of action is antibody-dependent cell‐mediated cytotoxicity in both RA and NHL. However, the target in RA is the normal B cell, and the target in NHL is the malignant B cell.
In addition, the pharmacokinetics of rituximab are “drastically different” in RA and NHL, Dr. Zelenetz said. Differences in pharmacokinetics support different dosing approaches in the two diseases.
Another big difference is immunogenicity. Anti‐CD20 antibodies develop in 15%-17% of RA patients, Dr. Zelenetz said, but the risk of antibody development is less than 1% in lymphoma.
Though extrapolation from RA to B‐cell NHL was not possible, it was possible to extrapolate results with rituximab-abbs in follicular lymphoma to other B-cell NHLs.
The study used was a phase 3 trial comparing rituximab-abbs to rituximab – both in combination with cyclophosphamide, vincristine, and prednisone – in patients with newly diagnosed, advanced stage follicular lymphoma.
This study showed no difference in pharmacokinetics or pharmacodynamics between rituximab-abbs and rituximab. The two agents also had comparable safety profiles and produced similar response rates (Lancet Haematol. 2017 Jul 13;4:e362‐73).
Rituximab‐abbs was approved in the United States based on these data, and results from this trial were extrapolated to other types of B-cell NHL. The results were extrapolated because the mechanism of action, pharmacokinetics, pharmacodynamics, and immunogenicity of rituximab are the same across B-cell NHLs, Dr. Zelenetz noted.
“Extrapolation is a critical part of biosimilarity development,” he said. “As long as scientific justification for extrapolation exists, I believe that extrapolation makes good sense.”
Dr. Zelenetz reported relationships with AbbVie, Adaptive Biotechnologies, Amgen, AstraZeneca, BeiGene, Celgene, Genentech, Gilead Sciences, Janssen, MEI Pharma, MorphoSys AG, Novartis, Pharmacyclics, and Roche.
SAN FRANCISCO – Several factors must be considered when extrapolating biosimilar results, according to a speaker at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
In this context, “extrapolation” means expanding the use of an approved biosimilar from one indication to another, based on efficacy and safety data from the first indication, Andrew D. Zelenetz, MD, PhD, of Memorial Sloan Kettering Cancer Center, New York, explained at the meeting.
To determine if extrapolation is appropriate, regulatory agencies consider the biosimilar’s mechanism of action in each indication; pharmacokinetics, pharmacodynamics, and immunogenicity in the different patient populations; differences in expected toxicities for each condition and population; and any other factor that may affect safety or efficacy.
To illustrate the process, Dr. Zelenetz explained how results with a rituximab biosimilar in rheumatoid arthritis (RA) cannot be extrapolated to B‐cell non‐Hodgkin lymphoma (NHL), but results with that same biosimilar in follicular lymphoma can be extrapolated to other types of B-cell NHL.
The biosimilar is rituximab-abbs (CT‐P10, Truxima). In a phase 1 trial of patients with RA, rituximab-abbs demonstrated biosimilarity to the reference product (Ann Rheum Dis. 2017;76[3]:566‐70).
The RA results cannot be extrapolated to B-cell NHL for a few reasons, according to Dr. Zelenetz. He noted that rituximab’s mechanism of action is antibody-dependent cell‐mediated cytotoxicity in both RA and NHL. However, the target in RA is the normal B cell, and the target in NHL is the malignant B cell.
In addition, the pharmacokinetics of rituximab are “drastically different” in RA and NHL, Dr. Zelenetz said. Differences in pharmacokinetics support different dosing approaches in the two diseases.
Another big difference is immunogenicity. Anti‐CD20 antibodies develop in 15%-17% of RA patients, Dr. Zelenetz said, but the risk of antibody development is less than 1% in lymphoma.
Though extrapolation from RA to B‐cell NHL was not possible, it was possible to extrapolate results with rituximab-abbs in follicular lymphoma to other B-cell NHLs.
The study used was a phase 3 trial comparing rituximab-abbs to rituximab – both in combination with cyclophosphamide, vincristine, and prednisone – in patients with newly diagnosed, advanced stage follicular lymphoma.
This study showed no difference in pharmacokinetics or pharmacodynamics between rituximab-abbs and rituximab. The two agents also had comparable safety profiles and produced similar response rates (Lancet Haematol. 2017 Jul 13;4:e362‐73).
Rituximab‐abbs was approved in the United States based on these data, and results from this trial were extrapolated to other types of B-cell NHL. The results were extrapolated because the mechanism of action, pharmacokinetics, pharmacodynamics, and immunogenicity of rituximab are the same across B-cell NHLs, Dr. Zelenetz noted.
“Extrapolation is a critical part of biosimilarity development,” he said. “As long as scientific justification for extrapolation exists, I believe that extrapolation makes good sense.”
Dr. Zelenetz reported relationships with AbbVie, Adaptive Biotechnologies, Amgen, AstraZeneca, BeiGene, Celgene, Genentech, Gilead Sciences, Janssen, MEI Pharma, MorphoSys AG, Novartis, Pharmacyclics, and Roche.
REPORTING FROM NCCN HEMATOLOGIC MALIGNANCIES
Study finds no standard for treatment discontinuation in myeloma
BOSTON — There is “no standard of care and no clear pattern” for discontinuing treatment in multiple myeloma, according to a speaker at the International Myeloma Workshop.
Data from a large, observational study revealed that a wide range of treatment regimens are used for first-, second-, and third-line therapy in multiple myeloma. The duration of therapy and time to next treatment were shorter in this real-world study than in prior clinical trials, and reasons for treatment discontinuation varied by regimen and line of therapy.
Katja Weisel, MD, of University Medical Center Hamburg-Eppendorf in Germany, presented these findings at the workshop, held by the International Myeloma Society.
The study, INSIGHT MM, is the largest global, prospective, observational study of multiple myeloma to date, according to Dr. Weisel. The study, which began July 1, 2016, has enrolled patients in the United States (n = 1,004), Europe (n = 1,612), Latin America (n = 367), and Asia (n = 218).
Dr. Weisel and her colleagues evaluated duration of therapy, reasons for treatment discontinuation, and subsequent therapies in a subset of patients on INSIGHT MM. The researchers’ analysis revealed “broad heterogeneity” across lines of therapy, Dr. Weisel said, adding that patients are receiving multiple regimens in addition to the most commonly prescribed regimens in myeloma.
First-line therapy
“In first-line treatment, we see predominantly bortezomib-based triplets ... regardless of transplant-eligible or transplant-ineligible patients,” Dr. Weisel said. “This is followed by doublets and other more rarely [applied] regimens.”
First-line therapies in 1,175 patients included:
- Bortezomib, cyclophosphamide, and dexamethasone (VCd) – 323 patients.
- Bortezomib, lenalidomide, and dexamethasone (VRd) – 321 patients.
- Bortezomib, thalidomide, and dexamethasone (VTd) – 200 patients.
- Bortezomib and dexamethasone (Vd) – 102 patients.
- Lenalidomide and dexamethasone (Rd) – 90 patients.
- Bortezomib, melphalan, and prednisone (VMP) – 53 patients.
- Carfilzomib, lenalidomide, and dexamethasone (KRd) – 47 patients.
- Daratumumab-based regimens (Dara) – 32 patients.
- Carfilzomib and dexamethasone (Kd) – 5 patients.
- Ixazomib, lenalidomide, and dexamethasone (IRd) – 2 patients.
Of the 1,175 newly diagnosed patients, 894 did not proceed to transplant after first-line therapy, but 281 did. Most of the patients who went on to transplant had received VRd (n = 82), VTd (n = 76), or VCd (n = 75).
Second- and third-line therapies
“In second-line treatment, we have still a dominance of the len-dex regimen all over the world,” Dr. Weisel said. “There is an emerging use of daratumumab in various combinations, and then you see the whole spectrum of approved triplet and doublet regimens.”
In the third line, the most commonly used regimens are daratumumab-based combinations and Rd.
There were 548 patients who received second-line treatment and 332 who received third-line therapy. The regimens used were:
- Rd – 130 patients second line, 71 third line.
- Dara – 121 patients second line, 105 third line.
- KRd – 61 patients second line, 17 third line.
- VCd – 57 patients second line, 19 third line.
- Vd – 48 patients second line, 29 third line.
- VRd – 36 patients second line, 8 third line.
- Kd – 33 patients for both second and third line.
- IRd – 29 patients second line, 43 third line.
- VTd – 25 patients second line, 4 third line.
- VMP – 8 patients second line, 3 third line.
Duration of therapy
Most transplant-eligible patients received any first-line therapy (VRd, VTd, or VCd) for longer than 12 months. Among transplant-ineligible patients, Rd was the first-line therapy most likely to be given for 12 months or more.
None of the second-line regimens lasted longer than 12 months in a majority of patients, but daratumumab-based regimens and IRd were the therapies most likely to exceed 12 months’ duration in both second- and third-line treatment.
Time to next treatment
“The vast majority of [transplant-eligible] patients, close to 90% ... do not need a second-line treatment during the first year of treatment,” Dr. Weisel said. “However, for transplant-ineligible patients, this accounts only for the most effective regimens, VMP and Rd.”
For second- and third-line therapies, a 12-month or longer time to next treatment was most likely among patients who received IRd or daratumumab-based regimens.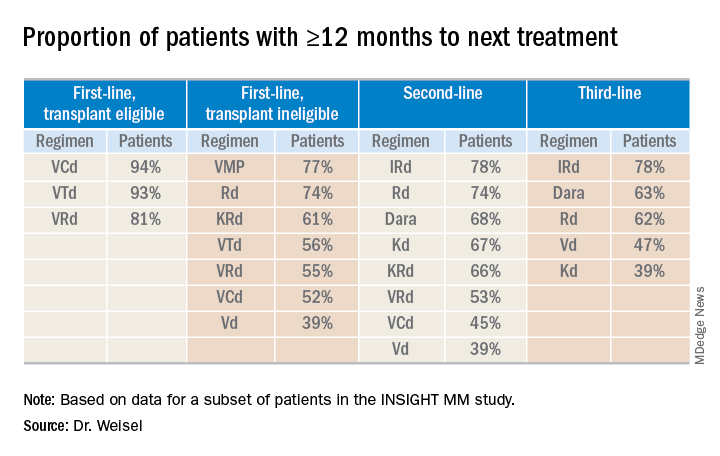
Reasons for discontinuation
“Planned end of therapy only accounts for a small proportion of treatment discontinuations, especially in the relapsed setting,” Dr. Weisel said. “Patients are discontinuing treatment due to reasons other than relapse, ultimately receiving fixed-duration therapy.”
The most common reasons for discontinuation of first-line therapy were:
- Relapse for VCd.
- Planned end of therapy for VRd.
- Adverse events (AEs) for VD and VTd.
- AEs and “other reasons” for Rd.
The most common reasons for discontinuation of second-line therapy were:
- Planned end of therapy for VCd.
- AEs, relapse, and other reasons for VRd.
- Relapse for VD, KRd, and Dara.
- AEs for Rd and IRd.
- AEs and other reasons for Kd.
The most common reasons for discontinuation of third-line therapy were:
- AEs for VCd, Vd, and KRd.
- Relapse for Kd, IRd, and Dara.
- Relapse and other reasons for VRd.
- AEs and other reasons for Rd.
The most common AE leading to discontinuation, across all treatment regimens, was peripheral neuropathy. This suggests peripheral neuropathy is still the “biggest impediment for continuous treatment,” Dr. Weisel said.
INSIGHT MM is sponsored by Takeda. Dr. Weisel reported relationships with Takeda and several other companies.
SOURCE: Weisel K et al. IMW 2019, Abstract OAB-005.
BOSTON — There is “no standard of care and no clear pattern” for discontinuing treatment in multiple myeloma, according to a speaker at the International Myeloma Workshop.
Data from a large, observational study revealed that a wide range of treatment regimens are used for first-, second-, and third-line therapy in multiple myeloma. The duration of therapy and time to next treatment were shorter in this real-world study than in prior clinical trials, and reasons for treatment discontinuation varied by regimen and line of therapy.
Katja Weisel, MD, of University Medical Center Hamburg-Eppendorf in Germany, presented these findings at the workshop, held by the International Myeloma Society.
The study, INSIGHT MM, is the largest global, prospective, observational study of multiple myeloma to date, according to Dr. Weisel. The study, which began July 1, 2016, has enrolled patients in the United States (n = 1,004), Europe (n = 1,612), Latin America (n = 367), and Asia (n = 218).
Dr. Weisel and her colleagues evaluated duration of therapy, reasons for treatment discontinuation, and subsequent therapies in a subset of patients on INSIGHT MM. The researchers’ analysis revealed “broad heterogeneity” across lines of therapy, Dr. Weisel said, adding that patients are receiving multiple regimens in addition to the most commonly prescribed regimens in myeloma.
First-line therapy
“In first-line treatment, we see predominantly bortezomib-based triplets ... regardless of transplant-eligible or transplant-ineligible patients,” Dr. Weisel said. “This is followed by doublets and other more rarely [applied] regimens.”
First-line therapies in 1,175 patients included:
- Bortezomib, cyclophosphamide, and dexamethasone (VCd) – 323 patients.
- Bortezomib, lenalidomide, and dexamethasone (VRd) – 321 patients.
- Bortezomib, thalidomide, and dexamethasone (VTd) – 200 patients.
- Bortezomib and dexamethasone (Vd) – 102 patients.
- Lenalidomide and dexamethasone (Rd) – 90 patients.
- Bortezomib, melphalan, and prednisone (VMP) – 53 patients.
- Carfilzomib, lenalidomide, and dexamethasone (KRd) – 47 patients.
- Daratumumab-based regimens (Dara) – 32 patients.
- Carfilzomib and dexamethasone (Kd) – 5 patients.
- Ixazomib, lenalidomide, and dexamethasone (IRd) – 2 patients.
Of the 1,175 newly diagnosed patients, 894 did not proceed to transplant after first-line therapy, but 281 did. Most of the patients who went on to transplant had received VRd (n = 82), VTd (n = 76), or VCd (n = 75).
Second- and third-line therapies
“In second-line treatment, we have still a dominance of the len-dex regimen all over the world,” Dr. Weisel said. “There is an emerging use of daratumumab in various combinations, and then you see the whole spectrum of approved triplet and doublet regimens.”
In the third line, the most commonly used regimens are daratumumab-based combinations and Rd.
There were 548 patients who received second-line treatment and 332 who received third-line therapy. The regimens used were:
- Rd – 130 patients second line, 71 third line.
- Dara – 121 patients second line, 105 third line.
- KRd – 61 patients second line, 17 third line.
- VCd – 57 patients second line, 19 third line.
- Vd – 48 patients second line, 29 third line.
- VRd – 36 patients second line, 8 third line.
- Kd – 33 patients for both second and third line.
- IRd – 29 patients second line, 43 third line.
- VTd – 25 patients second line, 4 third line.
- VMP – 8 patients second line, 3 third line.
Duration of therapy
Most transplant-eligible patients received any first-line therapy (VRd, VTd, or VCd) for longer than 12 months. Among transplant-ineligible patients, Rd was the first-line therapy most likely to be given for 12 months or more.
None of the second-line regimens lasted longer than 12 months in a majority of patients, but daratumumab-based regimens and IRd were the therapies most likely to exceed 12 months’ duration in both second- and third-line treatment.
Time to next treatment
“The vast majority of [transplant-eligible] patients, close to 90% ... do not need a second-line treatment during the first year of treatment,” Dr. Weisel said. “However, for transplant-ineligible patients, this accounts only for the most effective regimens, VMP and Rd.”
For second- and third-line therapies, a 12-month or longer time to next treatment was most likely among patients who received IRd or daratumumab-based regimens.
Reasons for discontinuation
“Planned end of therapy only accounts for a small proportion of treatment discontinuations, especially in the relapsed setting,” Dr. Weisel said. “Patients are discontinuing treatment due to reasons other than relapse, ultimately receiving fixed-duration therapy.”
The most common reasons for discontinuation of first-line therapy were:
- Relapse for VCd.
- Planned end of therapy for VRd.
- Adverse events (AEs) for VD and VTd.
- AEs and “other reasons” for Rd.
The most common reasons for discontinuation of second-line therapy were:
- Planned end of therapy for VCd.
- AEs, relapse, and other reasons for VRd.
- Relapse for VD, KRd, and Dara.
- AEs for Rd and IRd.
- AEs and other reasons for Kd.
The most common reasons for discontinuation of third-line therapy were:
- AEs for VCd, Vd, and KRd.
- Relapse for Kd, IRd, and Dara.
- Relapse and other reasons for VRd.
- AEs and other reasons for Rd.
The most common AE leading to discontinuation, across all treatment regimens, was peripheral neuropathy. This suggests peripheral neuropathy is still the “biggest impediment for continuous treatment,” Dr. Weisel said.
INSIGHT MM is sponsored by Takeda. Dr. Weisel reported relationships with Takeda and several other companies.
SOURCE: Weisel K et al. IMW 2019, Abstract OAB-005.
BOSTON — There is “no standard of care and no clear pattern” for discontinuing treatment in multiple myeloma, according to a speaker at the International Myeloma Workshop.
Data from a large, observational study revealed that a wide range of treatment regimens are used for first-, second-, and third-line therapy in multiple myeloma. The duration of therapy and time to next treatment were shorter in this real-world study than in prior clinical trials, and reasons for treatment discontinuation varied by regimen and line of therapy.
Katja Weisel, MD, of University Medical Center Hamburg-Eppendorf in Germany, presented these findings at the workshop, held by the International Myeloma Society.
The study, INSIGHT MM, is the largest global, prospective, observational study of multiple myeloma to date, according to Dr. Weisel. The study, which began July 1, 2016, has enrolled patients in the United States (n = 1,004), Europe (n = 1,612), Latin America (n = 367), and Asia (n = 218).
Dr. Weisel and her colleagues evaluated duration of therapy, reasons for treatment discontinuation, and subsequent therapies in a subset of patients on INSIGHT MM. The researchers’ analysis revealed “broad heterogeneity” across lines of therapy, Dr. Weisel said, adding that patients are receiving multiple regimens in addition to the most commonly prescribed regimens in myeloma.
First-line therapy
“In first-line treatment, we see predominantly bortezomib-based triplets ... regardless of transplant-eligible or transplant-ineligible patients,” Dr. Weisel said. “This is followed by doublets and other more rarely [applied] regimens.”
First-line therapies in 1,175 patients included:
- Bortezomib, cyclophosphamide, and dexamethasone (VCd) – 323 patients.
- Bortezomib, lenalidomide, and dexamethasone (VRd) – 321 patients.
- Bortezomib, thalidomide, and dexamethasone (VTd) – 200 patients.
- Bortezomib and dexamethasone (Vd) – 102 patients.
- Lenalidomide and dexamethasone (Rd) – 90 patients.
- Bortezomib, melphalan, and prednisone (VMP) – 53 patients.
- Carfilzomib, lenalidomide, and dexamethasone (KRd) – 47 patients.
- Daratumumab-based regimens (Dara) – 32 patients.
- Carfilzomib and dexamethasone (Kd) – 5 patients.
- Ixazomib, lenalidomide, and dexamethasone (IRd) – 2 patients.
Of the 1,175 newly diagnosed patients, 894 did not proceed to transplant after first-line therapy, but 281 did. Most of the patients who went on to transplant had received VRd (n = 82), VTd (n = 76), or VCd (n = 75).
Second- and third-line therapies
“In second-line treatment, we have still a dominance of the len-dex regimen all over the world,” Dr. Weisel said. “There is an emerging use of daratumumab in various combinations, and then you see the whole spectrum of approved triplet and doublet regimens.”
In the third line, the most commonly used regimens are daratumumab-based combinations and Rd.
There were 548 patients who received second-line treatment and 332 who received third-line therapy. The regimens used were:
- Rd – 130 patients second line, 71 third line.
- Dara – 121 patients second line, 105 third line.
- KRd – 61 patients second line, 17 third line.
- VCd – 57 patients second line, 19 third line.
- Vd – 48 patients second line, 29 third line.
- VRd – 36 patients second line, 8 third line.
- Kd – 33 patients for both second and third line.
- IRd – 29 patients second line, 43 third line.
- VTd – 25 patients second line, 4 third line.
- VMP – 8 patients second line, 3 third line.
Duration of therapy
Most transplant-eligible patients received any first-line therapy (VRd, VTd, or VCd) for longer than 12 months. Among transplant-ineligible patients, Rd was the first-line therapy most likely to be given for 12 months or more.
None of the second-line regimens lasted longer than 12 months in a majority of patients, but daratumumab-based regimens and IRd were the therapies most likely to exceed 12 months’ duration in both second- and third-line treatment.
Time to next treatment
“The vast majority of [transplant-eligible] patients, close to 90% ... do not need a second-line treatment during the first year of treatment,” Dr. Weisel said. “However, for transplant-ineligible patients, this accounts only for the most effective regimens, VMP and Rd.”
For second- and third-line therapies, a 12-month or longer time to next treatment was most likely among patients who received IRd or daratumumab-based regimens.
Reasons for discontinuation
“Planned end of therapy only accounts for a small proportion of treatment discontinuations, especially in the relapsed setting,” Dr. Weisel said. “Patients are discontinuing treatment due to reasons other than relapse, ultimately receiving fixed-duration therapy.”
The most common reasons for discontinuation of first-line therapy were:
- Relapse for VCd.
- Planned end of therapy for VRd.
- Adverse events (AEs) for VD and VTd.
- AEs and “other reasons” for Rd.
The most common reasons for discontinuation of second-line therapy were:
- Planned end of therapy for VCd.
- AEs, relapse, and other reasons for VRd.
- Relapse for VD, KRd, and Dara.
- AEs for Rd and IRd.
- AEs and other reasons for Kd.
The most common reasons for discontinuation of third-line therapy were:
- AEs for VCd, Vd, and KRd.
- Relapse for Kd, IRd, and Dara.
- Relapse and other reasons for VRd.
- AEs and other reasons for Rd.
The most common AE leading to discontinuation, across all treatment regimens, was peripheral neuropathy. This suggests peripheral neuropathy is still the “biggest impediment for continuous treatment,” Dr. Weisel said.
INSIGHT MM is sponsored by Takeda. Dr. Weisel reported relationships with Takeda and several other companies.
SOURCE: Weisel K et al. IMW 2019, Abstract OAB-005.
REPORTING FROM IMW 2019
Immunotherapies under investigation in newly diagnosed B-ALL
SAN FRANCISCO – Positive results with blinatumomab and inotuzumab ozogamicin in the relapsed/refractory setting have prompted trials of these immunotherapies in newly diagnosed B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab and inotuzumab have been shown to improve overall survival, compared with chemotherapy, in patients with relapsed/refractory B-ALL. However, most adults with relapsed/refractory B-ALL still die, so the initial therapy patients receive is “critical,” according to Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York.
“Ideally, we do not want to deal with the relapse,” Dr. Park said. “It’s better to cure the disease the first time ... which is the reason clinical trials are incorporating these agents earlier.”
Dr. Park discussed these points at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Blinatumomab
Dr. Park cited the phase 3 TOWER trial, which showed that blinatumomab produced better response rates and overall survival compared with standard chemotherapy. The trial enrolled 405 patients with Ph-negative relapsed/refractory B-ALL who were randomized to blinatumomab (n = 271) or chemotherapy (n = 134).
The rate of complete response (CR) with full, partial, or incomplete hematologic recovery was 44% with blinatumomab and 25% with chemotherapy (P less than .001). The median overall survival was 7.7 months and 4.0 months, respectively (P = .01; N Engl J Med 2017; 376:836-47).
Based on these data, researchers decided to test blinatumomab in newly diagnosed, elderly patients (65 years and older) with Ph-negative B-ALL in the phase 2 SWOG 1318 study. The study enrolled 31 patients, and 29 were eligible. Their median age at baseline was 75 years (range 66‐84 years).
The patients received blinatumomab for two to five cycles, followed by 18 months of maintenance with prednisone, vincristine, 6-mercaptopurine, and methotrexate. One patient went on to transplant.
In all, 66% of patients achieved a CR or CR with incomplete count recovery. The estimated overall survival was 79% at 6 months and 65% at 1 year. These results were presented at the 2018 annual meeting of the American Society of Hematology (Blood. 2018;132:33).
Another study of blinatumomab as frontline treatment is the ECOG-E1910 trial. In this phase 3 study, researchers are testing chemotherapy, with or without blinatumomab, in adults (aged 30-70 years) with newly diagnosed, BCR-ABL-negative B-ALL. Results from this study are not yet available.
Inotuzumab ozogamicin
Dr. Park also discussed the INOVATE trial, in which inotuzumab ozogamicin bested standard chemotherapy. The trial enrolled patients with Ph-positive or negative, relapsed/refractory B-ALL.
The patients were randomized to inotuzumab (n = 141) or investigator’s choice of chemotherapy (n = 138). Some patients, 41% in the inotuzumab arm and 11% in the chemotherapy arm, went on to transplant.
The CR rate was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P less than .001). The median progression-free survival was 5 months and 1.8 months, respectively (P less than .001). The median overall survival was 7.7 months and 6.7 months, respectively (P = .04; N Engl J Med 2016; 375:740-53).
Based on these results, researchers are testing inotuzumab as frontline therapy in young adults (aged 18-39 years) with CD22-positive, Ph-negative B-ALL. In the phase 3 A041501 trial, patients are receiving inotuzumab after the first and second courses of treatment with the CALGB 10403 chemotherapy regimen. Results from this trial are not yet available.
Dr. Park reported relationships with Allogene Therapeutics, Amgen, AstraZeneca, Incyte, Kite Pharma, Novartis, and Takeda.
SAN FRANCISCO – Positive results with blinatumomab and inotuzumab ozogamicin in the relapsed/refractory setting have prompted trials of these immunotherapies in newly diagnosed B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab and inotuzumab have been shown to improve overall survival, compared with chemotherapy, in patients with relapsed/refractory B-ALL. However, most adults with relapsed/refractory B-ALL still die, so the initial therapy patients receive is “critical,” according to Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York.
“Ideally, we do not want to deal with the relapse,” Dr. Park said. “It’s better to cure the disease the first time ... which is the reason clinical trials are incorporating these agents earlier.”
Dr. Park discussed these points at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Blinatumomab
Dr. Park cited the phase 3 TOWER trial, which showed that blinatumomab produced better response rates and overall survival compared with standard chemotherapy. The trial enrolled 405 patients with Ph-negative relapsed/refractory B-ALL who were randomized to blinatumomab (n = 271) or chemotherapy (n = 134).
The rate of complete response (CR) with full, partial, or incomplete hematologic recovery was 44% with blinatumomab and 25% with chemotherapy (P less than .001). The median overall survival was 7.7 months and 4.0 months, respectively (P = .01; N Engl J Med 2017; 376:836-47).
Based on these data, researchers decided to test blinatumomab in newly diagnosed, elderly patients (65 years and older) with Ph-negative B-ALL in the phase 2 SWOG 1318 study. The study enrolled 31 patients, and 29 were eligible. Their median age at baseline was 75 years (range 66‐84 years).
The patients received blinatumomab for two to five cycles, followed by 18 months of maintenance with prednisone, vincristine, 6-mercaptopurine, and methotrexate. One patient went on to transplant.
In all, 66% of patients achieved a CR or CR with incomplete count recovery. The estimated overall survival was 79% at 6 months and 65% at 1 year. These results were presented at the 2018 annual meeting of the American Society of Hematology (Blood. 2018;132:33).
Another study of blinatumomab as frontline treatment is the ECOG-E1910 trial. In this phase 3 study, researchers are testing chemotherapy, with or without blinatumomab, in adults (aged 30-70 years) with newly diagnosed, BCR-ABL-negative B-ALL. Results from this study are not yet available.
Inotuzumab ozogamicin
Dr. Park also discussed the INOVATE trial, in which inotuzumab ozogamicin bested standard chemotherapy. The trial enrolled patients with Ph-positive or negative, relapsed/refractory B-ALL.
The patients were randomized to inotuzumab (n = 141) or investigator’s choice of chemotherapy (n = 138). Some patients, 41% in the inotuzumab arm and 11% in the chemotherapy arm, went on to transplant.
The CR rate was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P less than .001). The median progression-free survival was 5 months and 1.8 months, respectively (P less than .001). The median overall survival was 7.7 months and 6.7 months, respectively (P = .04; N Engl J Med 2016; 375:740-53).
Based on these results, researchers are testing inotuzumab as frontline therapy in young adults (aged 18-39 years) with CD22-positive, Ph-negative B-ALL. In the phase 3 A041501 trial, patients are receiving inotuzumab after the first and second courses of treatment with the CALGB 10403 chemotherapy regimen. Results from this trial are not yet available.
Dr. Park reported relationships with Allogene Therapeutics, Amgen, AstraZeneca, Incyte, Kite Pharma, Novartis, and Takeda.
SAN FRANCISCO – Positive results with blinatumomab and inotuzumab ozogamicin in the relapsed/refractory setting have prompted trials of these immunotherapies in newly diagnosed B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab and inotuzumab have been shown to improve overall survival, compared with chemotherapy, in patients with relapsed/refractory B-ALL. However, most adults with relapsed/refractory B-ALL still die, so the initial therapy patients receive is “critical,” according to Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York.
“Ideally, we do not want to deal with the relapse,” Dr. Park said. “It’s better to cure the disease the first time ... which is the reason clinical trials are incorporating these agents earlier.”
Dr. Park discussed these points at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Blinatumomab
Dr. Park cited the phase 3 TOWER trial, which showed that blinatumomab produced better response rates and overall survival compared with standard chemotherapy. The trial enrolled 405 patients with Ph-negative relapsed/refractory B-ALL who were randomized to blinatumomab (n = 271) or chemotherapy (n = 134).
The rate of complete response (CR) with full, partial, or incomplete hematologic recovery was 44% with blinatumomab and 25% with chemotherapy (P less than .001). The median overall survival was 7.7 months and 4.0 months, respectively (P = .01; N Engl J Med 2017; 376:836-47).
Based on these data, researchers decided to test blinatumomab in newly diagnosed, elderly patients (65 years and older) with Ph-negative B-ALL in the phase 2 SWOG 1318 study. The study enrolled 31 patients, and 29 were eligible. Their median age at baseline was 75 years (range 66‐84 years).
The patients received blinatumomab for two to five cycles, followed by 18 months of maintenance with prednisone, vincristine, 6-mercaptopurine, and methotrexate. One patient went on to transplant.
In all, 66% of patients achieved a CR or CR with incomplete count recovery. The estimated overall survival was 79% at 6 months and 65% at 1 year. These results were presented at the 2018 annual meeting of the American Society of Hematology (Blood. 2018;132:33).
Another study of blinatumomab as frontline treatment is the ECOG-E1910 trial. In this phase 3 study, researchers are testing chemotherapy, with or without blinatumomab, in adults (aged 30-70 years) with newly diagnosed, BCR-ABL-negative B-ALL. Results from this study are not yet available.
Inotuzumab ozogamicin
Dr. Park also discussed the INOVATE trial, in which inotuzumab ozogamicin bested standard chemotherapy. The trial enrolled patients with Ph-positive or negative, relapsed/refractory B-ALL.
The patients were randomized to inotuzumab (n = 141) or investigator’s choice of chemotherapy (n = 138). Some patients, 41% in the inotuzumab arm and 11% in the chemotherapy arm, went on to transplant.
The CR rate was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P less than .001). The median progression-free survival was 5 months and 1.8 months, respectively (P less than .001). The median overall survival was 7.7 months and 6.7 months, respectively (P = .04; N Engl J Med 2016; 375:740-53).
Based on these results, researchers are testing inotuzumab as frontline therapy in young adults (aged 18-39 years) with CD22-positive, Ph-negative B-ALL. In the phase 3 A041501 trial, patients are receiving inotuzumab after the first and second courses of treatment with the CALGB 10403 chemotherapy regimen. Results from this trial are not yet available.
Dr. Park reported relationships with Allogene Therapeutics, Amgen, AstraZeneca, Incyte, Kite Pharma, Novartis, and Takeda.
EXPERT ANALYSIS FROM NCCN HEMATOLOGIC MALIGNANCIES
Second-generation anti-BCMA CAR T-cell therapy shows promise in myeloma trial
BOSTON – CT053, a chimeric antigen receptor (CAR) T-cell therapy, has demonstrated efficacy and tolerability in a phase 1 trial of patients with relapsed/refractory multiple myeloma.
CT053 produced an objective response rate of 87.5% and a complete response rate of 79.2%. All patients experienced grade 3 or higher adverse events (AEs), but none developed grade 3 or higher cytokine-release syndrome (CRS).
Siguo Hao, MD, of Xinhua Hospital, Shanghai (China) Jiaotong University, presented these results at the International Myeloma Workshop held by the International Myeloma Society.
Dr. Hao explained that CT053 consists of autologous T cells modified with a second-generation CAR that incorporates a fully human anti–B-cell maturation antigen single-chain fragment variant, a 4-1BB costimulatory domain, and a CD3-zeta–signaling domain.
In preclinical studies, CT053 induced dose-dependent cytotoxic effects on multiple myeloma cell lines and completely eradicated myeloma in mice.
Dr. Hao and his colleagues conducted the phase 1 study of CT053 at three sites (NCT03716856, NCT03302403, and NCT03380039). The study enrolled 30 patients with relapsed/refractory multiple myeloma, and 24 ultimately received CT053.
In the 24 patients, the median age was 60.2 years (range, 38.5-70.0 years), and the median time since diagnosis was 3.5 years (range, 0.3-10.8 years). Nine patients had progressive disease at baseline.
The patients had received a median of 5 prior therapies (range, 2-12). All patients had received a proteasome inhibitor, 22 had received an immunomodulatory agent, 10 had undergone a transplant, and 5 had received an anti-CD38 monoclonal antibody.
For this study, patients received conditioning with fludarabine and cyclophosphamide, followed by a single infusion of CT053. Most patients (n = 21) received 1.5 x 108 cells, but three received 0.5 x 108, 1 x 108, and 1.8 x 108 cells, respectively.
The median follow-up was 333 days. CAR T cells were detectable 1-7 days after infusion and peaked at 7-21 days. The cells persisted for a median of 172 days (range, 21-341 days).
A total of 21 patients responded to treatment (87.5%). There were 19 patients with a complete response or stringent complete response, 1 patient with a very good partial response, and 1 with a partial response.
Dr. Hao noted that CT053 was effective even at the lowest dose. The patient who received 0.5 x 108 cells initially achieved a very good partial response that deepened to a stringent complete response on day 502 after infusion.
Ten patients still had a stringent complete response at last follow-up, and two had a complete response. Nine patients progressed, and one patient relapsed after achieving a complete response. Three patients died, two from disease progression and one from a serious AE (neutropenic infection).
All 24 patients experienced a treatment-related AE, and all had grade 3 or higher hematologic AEs. Six patients had grade 3 or higher fever, six had grade 3 or higher infections and infestations, and one had grade 3 or higher neurotoxicity.
Three patients had grade 1 CRS, and 12 had grade 2 CRS. None of the patients had grade 3 or higher CRS.
This trial was sponsored by Xinhua Hospital/Shanghai Jiao Tong University School of Medicine, First Affiliated Hospital of Zhejiang University, and First Affiliated Hospital of Wenzhou Medical University in collaboration with Carsgen Therapeutics. Dr. Hao did not disclose any conflicts of interest.
SOURCE: Hao S et al. IMW 2019, Abstract OAB-082.
BOSTON – CT053, a chimeric antigen receptor (CAR) T-cell therapy, has demonstrated efficacy and tolerability in a phase 1 trial of patients with relapsed/refractory multiple myeloma.
CT053 produced an objective response rate of 87.5% and a complete response rate of 79.2%. All patients experienced grade 3 or higher adverse events (AEs), but none developed grade 3 or higher cytokine-release syndrome (CRS).
Siguo Hao, MD, of Xinhua Hospital, Shanghai (China) Jiaotong University, presented these results at the International Myeloma Workshop held by the International Myeloma Society.
Dr. Hao explained that CT053 consists of autologous T cells modified with a second-generation CAR that incorporates a fully human anti–B-cell maturation antigen single-chain fragment variant, a 4-1BB costimulatory domain, and a CD3-zeta–signaling domain.
In preclinical studies, CT053 induced dose-dependent cytotoxic effects on multiple myeloma cell lines and completely eradicated myeloma in mice.
Dr. Hao and his colleagues conducted the phase 1 study of CT053 at three sites (NCT03716856, NCT03302403, and NCT03380039). The study enrolled 30 patients with relapsed/refractory multiple myeloma, and 24 ultimately received CT053.
In the 24 patients, the median age was 60.2 years (range, 38.5-70.0 years), and the median time since diagnosis was 3.5 years (range, 0.3-10.8 years). Nine patients had progressive disease at baseline.
The patients had received a median of 5 prior therapies (range, 2-12). All patients had received a proteasome inhibitor, 22 had received an immunomodulatory agent, 10 had undergone a transplant, and 5 had received an anti-CD38 monoclonal antibody.
For this study, patients received conditioning with fludarabine and cyclophosphamide, followed by a single infusion of CT053. Most patients (n = 21) received 1.5 x 108 cells, but three received 0.5 x 108, 1 x 108, and 1.8 x 108 cells, respectively.
The median follow-up was 333 days. CAR T cells were detectable 1-7 days after infusion and peaked at 7-21 days. The cells persisted for a median of 172 days (range, 21-341 days).
A total of 21 patients responded to treatment (87.5%). There were 19 patients with a complete response or stringent complete response, 1 patient with a very good partial response, and 1 with a partial response.
Dr. Hao noted that CT053 was effective even at the lowest dose. The patient who received 0.5 x 108 cells initially achieved a very good partial response that deepened to a stringent complete response on day 502 after infusion.
Ten patients still had a stringent complete response at last follow-up, and two had a complete response. Nine patients progressed, and one patient relapsed after achieving a complete response. Three patients died, two from disease progression and one from a serious AE (neutropenic infection).
All 24 patients experienced a treatment-related AE, and all had grade 3 or higher hematologic AEs. Six patients had grade 3 or higher fever, six had grade 3 or higher infections and infestations, and one had grade 3 or higher neurotoxicity.
Three patients had grade 1 CRS, and 12 had grade 2 CRS. None of the patients had grade 3 or higher CRS.
This trial was sponsored by Xinhua Hospital/Shanghai Jiao Tong University School of Medicine, First Affiliated Hospital of Zhejiang University, and First Affiliated Hospital of Wenzhou Medical University in collaboration with Carsgen Therapeutics. Dr. Hao did not disclose any conflicts of interest.
SOURCE: Hao S et al. IMW 2019, Abstract OAB-082.
BOSTON – CT053, a chimeric antigen receptor (CAR) T-cell therapy, has demonstrated efficacy and tolerability in a phase 1 trial of patients with relapsed/refractory multiple myeloma.
CT053 produced an objective response rate of 87.5% and a complete response rate of 79.2%. All patients experienced grade 3 or higher adverse events (AEs), but none developed grade 3 or higher cytokine-release syndrome (CRS).
Siguo Hao, MD, of Xinhua Hospital, Shanghai (China) Jiaotong University, presented these results at the International Myeloma Workshop held by the International Myeloma Society.
Dr. Hao explained that CT053 consists of autologous T cells modified with a second-generation CAR that incorporates a fully human anti–B-cell maturation antigen single-chain fragment variant, a 4-1BB costimulatory domain, and a CD3-zeta–signaling domain.
In preclinical studies, CT053 induced dose-dependent cytotoxic effects on multiple myeloma cell lines and completely eradicated myeloma in mice.
Dr. Hao and his colleagues conducted the phase 1 study of CT053 at three sites (NCT03716856, NCT03302403, and NCT03380039). The study enrolled 30 patients with relapsed/refractory multiple myeloma, and 24 ultimately received CT053.
In the 24 patients, the median age was 60.2 years (range, 38.5-70.0 years), and the median time since diagnosis was 3.5 years (range, 0.3-10.8 years). Nine patients had progressive disease at baseline.
The patients had received a median of 5 prior therapies (range, 2-12). All patients had received a proteasome inhibitor, 22 had received an immunomodulatory agent, 10 had undergone a transplant, and 5 had received an anti-CD38 monoclonal antibody.
For this study, patients received conditioning with fludarabine and cyclophosphamide, followed by a single infusion of CT053. Most patients (n = 21) received 1.5 x 108 cells, but three received 0.5 x 108, 1 x 108, and 1.8 x 108 cells, respectively.
The median follow-up was 333 days. CAR T cells were detectable 1-7 days after infusion and peaked at 7-21 days. The cells persisted for a median of 172 days (range, 21-341 days).
A total of 21 patients responded to treatment (87.5%). There were 19 patients with a complete response or stringent complete response, 1 patient with a very good partial response, and 1 with a partial response.
Dr. Hao noted that CT053 was effective even at the lowest dose. The patient who received 0.5 x 108 cells initially achieved a very good partial response that deepened to a stringent complete response on day 502 after infusion.
Ten patients still had a stringent complete response at last follow-up, and two had a complete response. Nine patients progressed, and one patient relapsed after achieving a complete response. Three patients died, two from disease progression and one from a serious AE (neutropenic infection).
All 24 patients experienced a treatment-related AE, and all had grade 3 or higher hematologic AEs. Six patients had grade 3 or higher fever, six had grade 3 or higher infections and infestations, and one had grade 3 or higher neurotoxicity.
Three patients had grade 1 CRS, and 12 had grade 2 CRS. None of the patients had grade 3 or higher CRS.
This trial was sponsored by Xinhua Hospital/Shanghai Jiao Tong University School of Medicine, First Affiliated Hospital of Zhejiang University, and First Affiliated Hospital of Wenzhou Medical University in collaboration with Carsgen Therapeutics. Dr. Hao did not disclose any conflicts of interest.
SOURCE: Hao S et al. IMW 2019, Abstract OAB-082.
REPORTING FROM IMW 2019
Subcutaneous and IV daratumumab combos appear comparable in myeloma
BOSTON – Subcutaneous daratumumab in combination with standard care is comparable to intravenous daratumumab plus standard care in patients with newly diagnosed or relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop.
Overall response rates (ORRs) observed with subcutaneous daratumumab–based combinations in the phase 2 PLEIADES trial were similar to ORRs observed with intravenous daratumumab–based combinations in three other trials – GRIFFIN, ALCYONE, and POLLUX.
Ajai Chari, MD, of the Icahn School of Medicine at Mount Sinai, New York, presented these findings at the workshop, which is held by the International Myeloma Society.
In the PLEIADES trial, researchers tested subcutaneous daratumumab (D) in combination with:
- Bortezomib, lenalidomide, and dexamethasone (VRd) in transplant-eligible patients with newly diagnosed multiple myeloma
- Bortezomib, melphalan, and prednisone (VMP) in transplant-ineligible patients with newly diagnosed multiple myeloma
- Lenalidomide and dexamethasone (Rd) in patients with relapsed/refractory multiple myeloma who had received at least one prior line of therapy.
There were 67 patients in the D-VRd arm, and they had a median age of 59 years (range, 33-76 years). There were 67 patients in the D-VMP arm, and they had a median age of 75 years (range, 66-86 years). There were 65 patients in the D-Rd arm, they had a median age of 69 years (range, 33-82 years), and they had received a median of one (range, one to five) prior therapies.
Dr. Chari noted that baseline characteristics in this study were “pretty comparable” to characteristics in the studies of intravenous daratumumab. He also pointed out that the median administration time for subcutaneous daratumumab was 5 minutes in this study, which is “substantially” shorter than the typical administration time for intravenous daratumumab.
The median number of treatment cycles was 4 (range, 1-4) in the D-VRd arm, 8 (range, 1-10) in the D-VMP arm, and 12 (range, 1-15) in the D-Rd arm. The median duration of treatment was 2.6 months, 10.6 months, and 11.1 months, respectively.
The proportion of patients who discontinued treatment was 3% in the D-VRd arm, 10.4% in the D-VMP arm, and 20% in the D-Rd arm.
Response
Dr. Chari said response rates in the three arms of PLEAIDES were similar to response rates in corresponding groups from the studies of intravenous daratumumab–based combinations.
After four induction cycles, subcutaneous D-VRd produced an ORR of 97% in PLEAIDES, and intravenous D-VRd produced an ORR of 98% in the GRIFFIN trial (IMW 2019. Abstract OAB-087).
Subcutaneous D-VMP produced an ORR of 89.6% at a median follow-up of 11 months. In the ALCYONE trial, intravenous D-VMP produced an ORR of 90.9% at a median follow-up of 16.5 months (N Engl J Med. 2018; 378:518-28).
Subcutaneous D-Rd produced an ORR of 93.8% at a median follow-up of 11.2 months. In the POLLUX trial, intravenous D-Rd produced an ORR of 92.9% at a median follow-up of 13.5 months (N Engl J Med. 2016; 375:1319-31).
Safety
All patients in PLEIADES had treatment-related adverse events (TEAEs). The rate of serious TEAEs was 28.4% in the D-VRd arm, 38.8% in the D-VMP arm, and 47.7% in the D-Rd arm. The rate of grade 3/4 TEAEs was 56.7%, 68.7%, and 83.1%, respectively. There was one fatal TEAE in the D-VRd arm, two fatal TEAEs in the D-VMP arm, and two in the D-Rd arm.
Infusion-related reactions occurred in 7.5% of all patients (15/199). Most infusion-related reactions were grade 1/2. One patient had a grade 3 reaction, and there were no grade 4 reactions. The median time to onset was 3.3 hours.
“Daratumumab in combination with standard of care, when given subcutaneously, demonstrated comparable clinical activity and safety and corresponded to daratumumab intravenous–containing regimens,” Dr. Chari said. “These results support the use of flat-dose 1,800 mg [subcutaneous daratumumab] in combination with standard treatment regimens.”
The PLEIADES trial was sponsored by Janssen Research & Development. Dr. Chari reported relationships with Janssen and several other companies.
SOURCE: Chari A et al. IMW 2019, Abstract OAB-022.
BOSTON – Subcutaneous daratumumab in combination with standard care is comparable to intravenous daratumumab plus standard care in patients with newly diagnosed or relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop.
Overall response rates (ORRs) observed with subcutaneous daratumumab–based combinations in the phase 2 PLEIADES trial were similar to ORRs observed with intravenous daratumumab–based combinations in three other trials – GRIFFIN, ALCYONE, and POLLUX.
Ajai Chari, MD, of the Icahn School of Medicine at Mount Sinai, New York, presented these findings at the workshop, which is held by the International Myeloma Society.
In the PLEIADES trial, researchers tested subcutaneous daratumumab (D) in combination with:
- Bortezomib, lenalidomide, and dexamethasone (VRd) in transplant-eligible patients with newly diagnosed multiple myeloma
- Bortezomib, melphalan, and prednisone (VMP) in transplant-ineligible patients with newly diagnosed multiple myeloma
- Lenalidomide and dexamethasone (Rd) in patients with relapsed/refractory multiple myeloma who had received at least one prior line of therapy.
There were 67 patients in the D-VRd arm, and they had a median age of 59 years (range, 33-76 years). There were 67 patients in the D-VMP arm, and they had a median age of 75 years (range, 66-86 years). There were 65 patients in the D-Rd arm, they had a median age of 69 years (range, 33-82 years), and they had received a median of one (range, one to five) prior therapies.
Dr. Chari noted that baseline characteristics in this study were “pretty comparable” to characteristics in the studies of intravenous daratumumab. He also pointed out that the median administration time for subcutaneous daratumumab was 5 minutes in this study, which is “substantially” shorter than the typical administration time for intravenous daratumumab.
The median number of treatment cycles was 4 (range, 1-4) in the D-VRd arm, 8 (range, 1-10) in the D-VMP arm, and 12 (range, 1-15) in the D-Rd arm. The median duration of treatment was 2.6 months, 10.6 months, and 11.1 months, respectively.
The proportion of patients who discontinued treatment was 3% in the D-VRd arm, 10.4% in the D-VMP arm, and 20% in the D-Rd arm.
Response
Dr. Chari said response rates in the three arms of PLEAIDES were similar to response rates in corresponding groups from the studies of intravenous daratumumab–based combinations.
After four induction cycles, subcutaneous D-VRd produced an ORR of 97% in PLEAIDES, and intravenous D-VRd produced an ORR of 98% in the GRIFFIN trial (IMW 2019. Abstract OAB-087).
Subcutaneous D-VMP produced an ORR of 89.6% at a median follow-up of 11 months. In the ALCYONE trial, intravenous D-VMP produced an ORR of 90.9% at a median follow-up of 16.5 months (N Engl J Med. 2018; 378:518-28).
Subcutaneous D-Rd produced an ORR of 93.8% at a median follow-up of 11.2 months. In the POLLUX trial, intravenous D-Rd produced an ORR of 92.9% at a median follow-up of 13.5 months (N Engl J Med. 2016; 375:1319-31).
Safety
All patients in PLEIADES had treatment-related adverse events (TEAEs). The rate of serious TEAEs was 28.4% in the D-VRd arm, 38.8% in the D-VMP arm, and 47.7% in the D-Rd arm. The rate of grade 3/4 TEAEs was 56.7%, 68.7%, and 83.1%, respectively. There was one fatal TEAE in the D-VRd arm, two fatal TEAEs in the D-VMP arm, and two in the D-Rd arm.
Infusion-related reactions occurred in 7.5% of all patients (15/199). Most infusion-related reactions were grade 1/2. One patient had a grade 3 reaction, and there were no grade 4 reactions. The median time to onset was 3.3 hours.
“Daratumumab in combination with standard of care, when given subcutaneously, demonstrated comparable clinical activity and safety and corresponded to daratumumab intravenous–containing regimens,” Dr. Chari said. “These results support the use of flat-dose 1,800 mg [subcutaneous daratumumab] in combination with standard treatment regimens.”
The PLEIADES trial was sponsored by Janssen Research & Development. Dr. Chari reported relationships with Janssen and several other companies.
SOURCE: Chari A et al. IMW 2019, Abstract OAB-022.
BOSTON – Subcutaneous daratumumab in combination with standard care is comparable to intravenous daratumumab plus standard care in patients with newly diagnosed or relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop.
Overall response rates (ORRs) observed with subcutaneous daratumumab–based combinations in the phase 2 PLEIADES trial were similar to ORRs observed with intravenous daratumumab–based combinations in three other trials – GRIFFIN, ALCYONE, and POLLUX.
Ajai Chari, MD, of the Icahn School of Medicine at Mount Sinai, New York, presented these findings at the workshop, which is held by the International Myeloma Society.
In the PLEIADES trial, researchers tested subcutaneous daratumumab (D) in combination with:
- Bortezomib, lenalidomide, and dexamethasone (VRd) in transplant-eligible patients with newly diagnosed multiple myeloma
- Bortezomib, melphalan, and prednisone (VMP) in transplant-ineligible patients with newly diagnosed multiple myeloma
- Lenalidomide and dexamethasone (Rd) in patients with relapsed/refractory multiple myeloma who had received at least one prior line of therapy.
There were 67 patients in the D-VRd arm, and they had a median age of 59 years (range, 33-76 years). There were 67 patients in the D-VMP arm, and they had a median age of 75 years (range, 66-86 years). There were 65 patients in the D-Rd arm, they had a median age of 69 years (range, 33-82 years), and they had received a median of one (range, one to five) prior therapies.
Dr. Chari noted that baseline characteristics in this study were “pretty comparable” to characteristics in the studies of intravenous daratumumab. He also pointed out that the median administration time for subcutaneous daratumumab was 5 minutes in this study, which is “substantially” shorter than the typical administration time for intravenous daratumumab.
The median number of treatment cycles was 4 (range, 1-4) in the D-VRd arm, 8 (range, 1-10) in the D-VMP arm, and 12 (range, 1-15) in the D-Rd arm. The median duration of treatment was 2.6 months, 10.6 months, and 11.1 months, respectively.
The proportion of patients who discontinued treatment was 3% in the D-VRd arm, 10.4% in the D-VMP arm, and 20% in the D-Rd arm.
Response
Dr. Chari said response rates in the three arms of PLEAIDES were similar to response rates in corresponding groups from the studies of intravenous daratumumab–based combinations.
After four induction cycles, subcutaneous D-VRd produced an ORR of 97% in PLEAIDES, and intravenous D-VRd produced an ORR of 98% in the GRIFFIN trial (IMW 2019. Abstract OAB-087).
Subcutaneous D-VMP produced an ORR of 89.6% at a median follow-up of 11 months. In the ALCYONE trial, intravenous D-VMP produced an ORR of 90.9% at a median follow-up of 16.5 months (N Engl J Med. 2018; 378:518-28).
Subcutaneous D-Rd produced an ORR of 93.8% at a median follow-up of 11.2 months. In the POLLUX trial, intravenous D-Rd produced an ORR of 92.9% at a median follow-up of 13.5 months (N Engl J Med. 2016; 375:1319-31).
Safety
All patients in PLEIADES had treatment-related adverse events (TEAEs). The rate of serious TEAEs was 28.4% in the D-VRd arm, 38.8% in the D-VMP arm, and 47.7% in the D-Rd arm. The rate of grade 3/4 TEAEs was 56.7%, 68.7%, and 83.1%, respectively. There was one fatal TEAE in the D-VRd arm, two fatal TEAEs in the D-VMP arm, and two in the D-Rd arm.
Infusion-related reactions occurred in 7.5% of all patients (15/199). Most infusion-related reactions were grade 1/2. One patient had a grade 3 reaction, and there were no grade 4 reactions. The median time to onset was 3.3 hours.
“Daratumumab in combination with standard of care, when given subcutaneously, demonstrated comparable clinical activity and safety and corresponded to daratumumab intravenous–containing regimens,” Dr. Chari said. “These results support the use of flat-dose 1,800 mg [subcutaneous daratumumab] in combination with standard treatment regimens.”
The PLEIADES trial was sponsored by Janssen Research & Development. Dr. Chari reported relationships with Janssen and several other companies.
SOURCE: Chari A et al. IMW 2019, Abstract OAB-022.
REPORTING FROM IMW 2019
Quadruplet prolongs progression-free survival in newly diagnosed myeloma
BOSTON – A carfilzomib-based quadruplet can improve outcomes in transplant-eligible patients with newly diagnosed multiple myeloma, a phase 3 trial suggests.
In the Myeloma XI trial, carfilzomib plus cyclophosphamide, lenalidomide, and dexamethasone (KCRD) significantly prolonged progression-free survival (PFS), compared with cyclophosphamide-lenalidomide-dexamethasone (CRD) or cyclophosphamide-thalidomide-dexamethasone (CTD).
“KCRD was associated with a very high response rate and a high MRD [minimal residual disease]-negative rate at the end of induction, and it significantly improved progression-free survival compared to the triplet combinations,” said Charlotte Pawlyn, PhD, of The Institute of Cancer Research in London.
Dr. Pawlyn reported these findings at the International Myeloma Workshop held by the International Myeloma Society.
The phase 3 Myeloma XI trial enrolled 1,056 patients with newly diagnosed myeloma who were eligible for transplant. The patients were randomized to receive KCRD (n = 526), CRD (n = 265), or CTD (n = 265) as induction.
Baseline characteristics were well balanced between the treatment arms. The median age was 61 years in the KCRD and CTD arms and 62 years in the CRD arm (overall range, 33-75 years). Roughly 60% of patients in each arm were men.
About 50%-60% of patients in each arm had standard-risk cytogenetics, which was defined as the absence of any cytogenetic lesions. About 30%-40% of patients in each arm had high-risk cytogenetics, meaning they had one of the following lesions: t(4;14), t(14;16), t(14;20), del (17p), or gain(1q). About 10% of patients in each arm had ultra-high-risk cytogenetics, which was defined as having more than one lesion.
Treatment
For induction, patients were randomized to KCRD, CRD, or CTD. All patients in the KCRD arm and patients in the CRD/CTD arms who achieved a partial response or better went straight to autologous transplant after induction. Nonresponders in the CTD and CRD arms received intensification with cyclophosphamide, bortezomib, and dexamethasone before transplant.
After transplant, all eligible patients were randomized to lenalidomide maintenance or observation. Patients were eligible for this randomization if they didn’t respond to induction, had progressive disease, or had previous or concurrent active malignancies.
The median follow-up was 34.5 months. The median number of induction cycles completed was 4 (range, 1-12) in the KCRD arm, 5 (range, 1-15) in the CRD arm, and 6 (range, 1-13) in the CTD arm.
Response
At the end of induction, the rate of very good partial response or better was 82.3% in the KCRD arm, 64.9% in the CRD arm, 52.8% in the CTD arm, and 58.9% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 4.35 (P less than .0001).
At 100 days after transplant, the rate of very good partial response or better was 91.9% in the KCRD arm, 82.1% in the CRD arm, 76.1% in the CTD arm, and 79.3% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 3.01 (P less than .0001).
KCRD produced a higher proportion of MRD-negative responses both before and after transplant. After induction, the rate of MRD-negative response was 11% in the CTD arm, 21% in the CRD arm, and 55% in the KCRD arm. After transplant, the rates were 51%, 49%, and 77%, respectively.
Survival
KCRD improved PFS. The 3-year PFS rate was 64.5% in the KCRD arm and 50.3% in the CTD-CRD arms combined. The hazard ratio (HR) was 0.63 (P less than .0001).
The PFS benefit with KCRD was present in all patient subgroups. For example, KCRD improved PFS, compared with CTD-CRD, in patients with standard-risk (HR = 0.62), high-risk (HR = 0.68), and ultra-high-risk (HR = 0.50) cytogenetics.
Patients who achieved an MRD-negative response had better PFS, and early achievement of MRD negativity was associated with improved PFS, Dr. Pawlyn noted.
“But what’s also notable ... is that those patients who received KCRD and achieved MRD negativity ... had better outcomes than patients who achieved MRD negativity whilst receiving a triplet combination,” Dr. Pawlyn said. “So this suggests that the induction regimen delivered is important, not just the achievement of MRD negativity at a defined cutoff.”
Dr. Pawlyn added that overall survival data from this study are not yet mature, but the researchers did assess PFS2. PFS2 was defined as the time from randomization to second disease progression. The 3-year PFS2 was 81.8% in the KCRD arm and 75.1% in the CTD-CRD arms combined. The HR was 0.75 (P = .0451).
Myeloma XI is sponsored by University of Leeds in collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn reported relationships with Amgen, Celgene, and other companies.
SOURCE: Pawlyn C et al. IMW 2019, Abstract OAB-002.
BOSTON – A carfilzomib-based quadruplet can improve outcomes in transplant-eligible patients with newly diagnosed multiple myeloma, a phase 3 trial suggests.
In the Myeloma XI trial, carfilzomib plus cyclophosphamide, lenalidomide, and dexamethasone (KCRD) significantly prolonged progression-free survival (PFS), compared with cyclophosphamide-lenalidomide-dexamethasone (CRD) or cyclophosphamide-thalidomide-dexamethasone (CTD).
“KCRD was associated with a very high response rate and a high MRD [minimal residual disease]-negative rate at the end of induction, and it significantly improved progression-free survival compared to the triplet combinations,” said Charlotte Pawlyn, PhD, of The Institute of Cancer Research in London.
Dr. Pawlyn reported these findings at the International Myeloma Workshop held by the International Myeloma Society.
The phase 3 Myeloma XI trial enrolled 1,056 patients with newly diagnosed myeloma who were eligible for transplant. The patients were randomized to receive KCRD (n = 526), CRD (n = 265), or CTD (n = 265) as induction.
Baseline characteristics were well balanced between the treatment arms. The median age was 61 years in the KCRD and CTD arms and 62 years in the CRD arm (overall range, 33-75 years). Roughly 60% of patients in each arm were men.
About 50%-60% of patients in each arm had standard-risk cytogenetics, which was defined as the absence of any cytogenetic lesions. About 30%-40% of patients in each arm had high-risk cytogenetics, meaning they had one of the following lesions: t(4;14), t(14;16), t(14;20), del (17p), or gain(1q). About 10% of patients in each arm had ultra-high-risk cytogenetics, which was defined as having more than one lesion.
Treatment
For induction, patients were randomized to KCRD, CRD, or CTD. All patients in the KCRD arm and patients in the CRD/CTD arms who achieved a partial response or better went straight to autologous transplant after induction. Nonresponders in the CTD and CRD arms received intensification with cyclophosphamide, bortezomib, and dexamethasone before transplant.
After transplant, all eligible patients were randomized to lenalidomide maintenance or observation. Patients were eligible for this randomization if they didn’t respond to induction, had progressive disease, or had previous or concurrent active malignancies.
The median follow-up was 34.5 months. The median number of induction cycles completed was 4 (range, 1-12) in the KCRD arm, 5 (range, 1-15) in the CRD arm, and 6 (range, 1-13) in the CTD arm.
Response
At the end of induction, the rate of very good partial response or better was 82.3% in the KCRD arm, 64.9% in the CRD arm, 52.8% in the CTD arm, and 58.9% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 4.35 (P less than .0001).
At 100 days after transplant, the rate of very good partial response or better was 91.9% in the KCRD arm, 82.1% in the CRD arm, 76.1% in the CTD arm, and 79.3% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 3.01 (P less than .0001).
KCRD produced a higher proportion of MRD-negative responses both before and after transplant. After induction, the rate of MRD-negative response was 11% in the CTD arm, 21% in the CRD arm, and 55% in the KCRD arm. After transplant, the rates were 51%, 49%, and 77%, respectively.
Survival
KCRD improved PFS. The 3-year PFS rate was 64.5% in the KCRD arm and 50.3% in the CTD-CRD arms combined. The hazard ratio (HR) was 0.63 (P less than .0001).
The PFS benefit with KCRD was present in all patient subgroups. For example, KCRD improved PFS, compared with CTD-CRD, in patients with standard-risk (HR = 0.62), high-risk (HR = 0.68), and ultra-high-risk (HR = 0.50) cytogenetics.
Patients who achieved an MRD-negative response had better PFS, and early achievement of MRD negativity was associated with improved PFS, Dr. Pawlyn noted.
“But what’s also notable ... is that those patients who received KCRD and achieved MRD negativity ... had better outcomes than patients who achieved MRD negativity whilst receiving a triplet combination,” Dr. Pawlyn said. “So this suggests that the induction regimen delivered is important, not just the achievement of MRD negativity at a defined cutoff.”
Dr. Pawlyn added that overall survival data from this study are not yet mature, but the researchers did assess PFS2. PFS2 was defined as the time from randomization to second disease progression. The 3-year PFS2 was 81.8% in the KCRD arm and 75.1% in the CTD-CRD arms combined. The HR was 0.75 (P = .0451).
Myeloma XI is sponsored by University of Leeds in collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn reported relationships with Amgen, Celgene, and other companies.
SOURCE: Pawlyn C et al. IMW 2019, Abstract OAB-002.
BOSTON – A carfilzomib-based quadruplet can improve outcomes in transplant-eligible patients with newly diagnosed multiple myeloma, a phase 3 trial suggests.
In the Myeloma XI trial, carfilzomib plus cyclophosphamide, lenalidomide, and dexamethasone (KCRD) significantly prolonged progression-free survival (PFS), compared with cyclophosphamide-lenalidomide-dexamethasone (CRD) or cyclophosphamide-thalidomide-dexamethasone (CTD).
“KCRD was associated with a very high response rate and a high MRD [minimal residual disease]-negative rate at the end of induction, and it significantly improved progression-free survival compared to the triplet combinations,” said Charlotte Pawlyn, PhD, of The Institute of Cancer Research in London.
Dr. Pawlyn reported these findings at the International Myeloma Workshop held by the International Myeloma Society.
The phase 3 Myeloma XI trial enrolled 1,056 patients with newly diagnosed myeloma who were eligible for transplant. The patients were randomized to receive KCRD (n = 526), CRD (n = 265), or CTD (n = 265) as induction.
Baseline characteristics were well balanced between the treatment arms. The median age was 61 years in the KCRD and CTD arms and 62 years in the CRD arm (overall range, 33-75 years). Roughly 60% of patients in each arm were men.
About 50%-60% of patients in each arm had standard-risk cytogenetics, which was defined as the absence of any cytogenetic lesions. About 30%-40% of patients in each arm had high-risk cytogenetics, meaning they had one of the following lesions: t(4;14), t(14;16), t(14;20), del (17p), or gain(1q). About 10% of patients in each arm had ultra-high-risk cytogenetics, which was defined as having more than one lesion.
Treatment
For induction, patients were randomized to KCRD, CRD, or CTD. All patients in the KCRD arm and patients in the CRD/CTD arms who achieved a partial response or better went straight to autologous transplant after induction. Nonresponders in the CTD and CRD arms received intensification with cyclophosphamide, bortezomib, and dexamethasone before transplant.
After transplant, all eligible patients were randomized to lenalidomide maintenance or observation. Patients were eligible for this randomization if they didn’t respond to induction, had progressive disease, or had previous or concurrent active malignancies.
The median follow-up was 34.5 months. The median number of induction cycles completed was 4 (range, 1-12) in the KCRD arm, 5 (range, 1-15) in the CRD arm, and 6 (range, 1-13) in the CTD arm.
Response
At the end of induction, the rate of very good partial response or better was 82.3% in the KCRD arm, 64.9% in the CRD arm, 52.8% in the CTD arm, and 58.9% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 4.35 (P less than .0001).
At 100 days after transplant, the rate of very good partial response or better was 91.9% in the KCRD arm, 82.1% in the CRD arm, 76.1% in the CTD arm, and 79.3% in the CTD-CRD arms combined. The odds ratio for the KCRD group compared to the triplets combined was 3.01 (P less than .0001).
KCRD produced a higher proportion of MRD-negative responses both before and after transplant. After induction, the rate of MRD-negative response was 11% in the CTD arm, 21% in the CRD arm, and 55% in the KCRD arm. After transplant, the rates were 51%, 49%, and 77%, respectively.
Survival
KCRD improved PFS. The 3-year PFS rate was 64.5% in the KCRD arm and 50.3% in the CTD-CRD arms combined. The hazard ratio (HR) was 0.63 (P less than .0001).
The PFS benefit with KCRD was present in all patient subgroups. For example, KCRD improved PFS, compared with CTD-CRD, in patients with standard-risk (HR = 0.62), high-risk (HR = 0.68), and ultra-high-risk (HR = 0.50) cytogenetics.
Patients who achieved an MRD-negative response had better PFS, and early achievement of MRD negativity was associated with improved PFS, Dr. Pawlyn noted.
“But what’s also notable ... is that those patients who received KCRD and achieved MRD negativity ... had better outcomes than patients who achieved MRD negativity whilst receiving a triplet combination,” Dr. Pawlyn said. “So this suggests that the induction regimen delivered is important, not just the achievement of MRD negativity at a defined cutoff.”
Dr. Pawlyn added that overall survival data from this study are not yet mature, but the researchers did assess PFS2. PFS2 was defined as the time from randomization to second disease progression. The 3-year PFS2 was 81.8% in the KCRD arm and 75.1% in the CTD-CRD arms combined. The HR was 0.75 (P = .0451).
Myeloma XI is sponsored by University of Leeds in collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn reported relationships with Amgen, Celgene, and other companies.
SOURCE: Pawlyn C et al. IMW 2019, Abstract OAB-002.
REPORTING FROM IMW 2019
























