User login
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
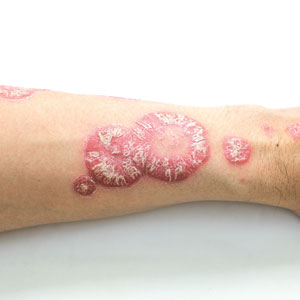
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

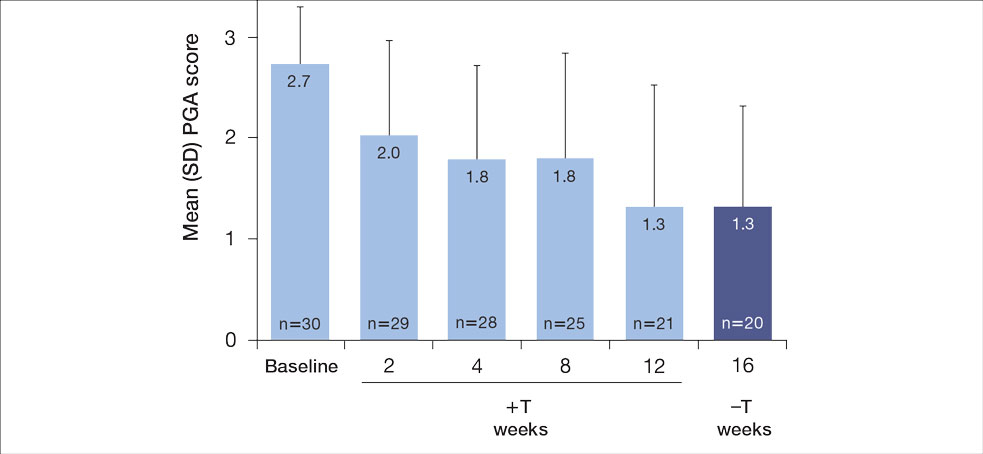
For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.
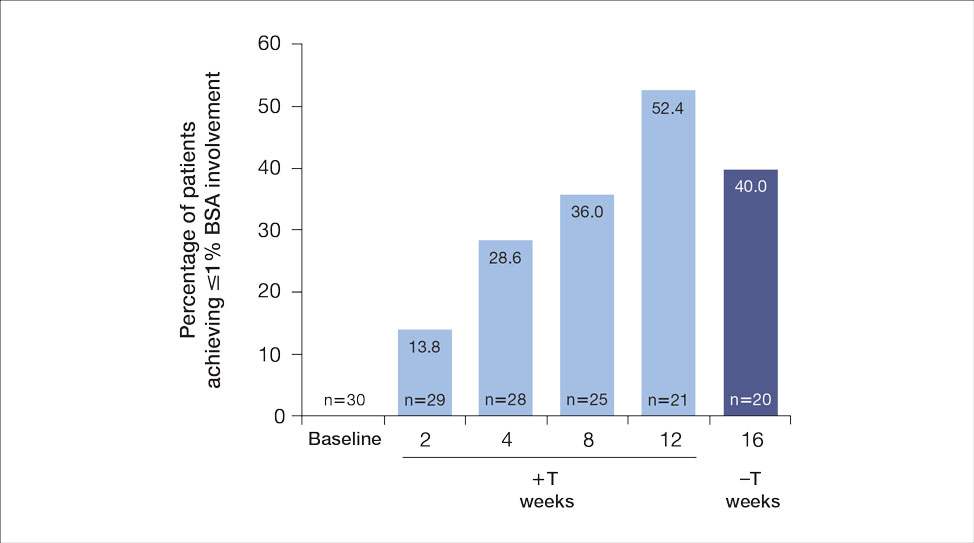
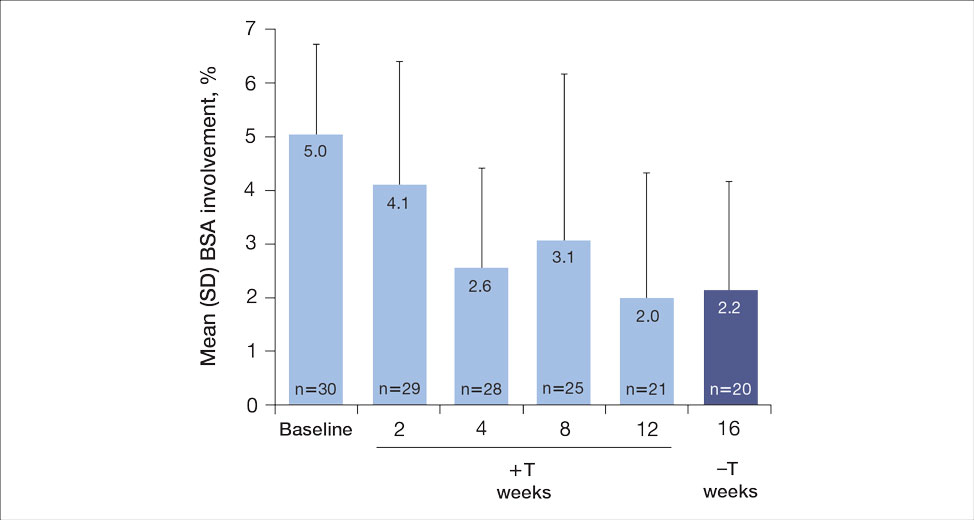
After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
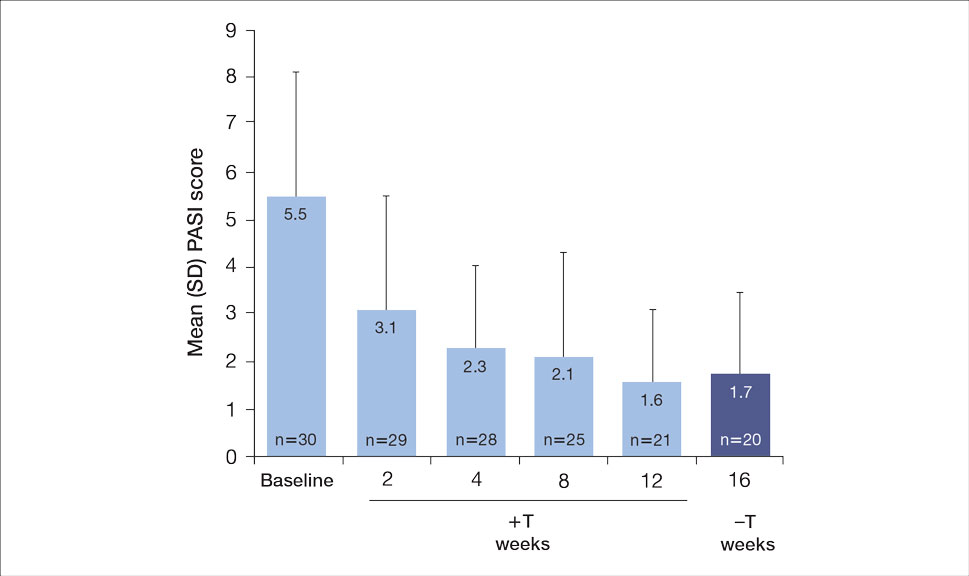
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.
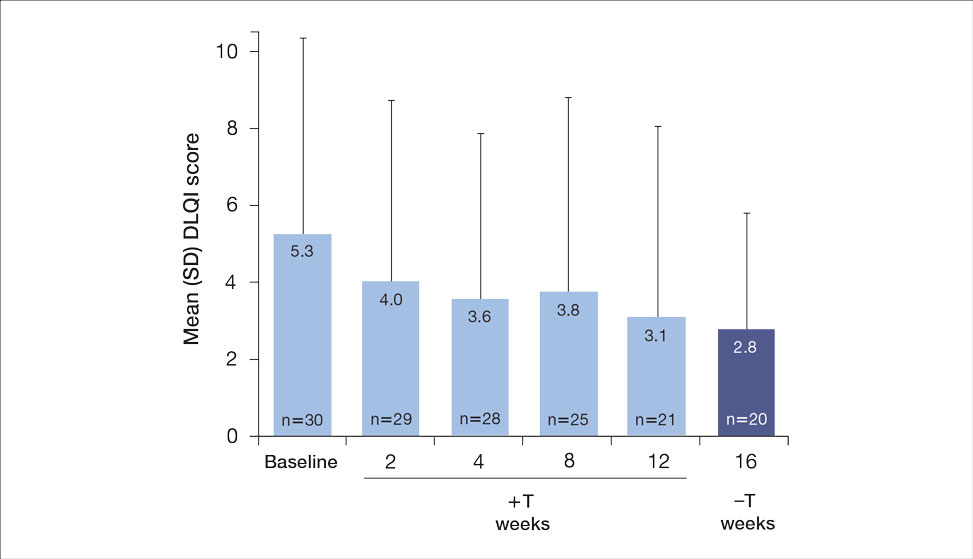
Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.

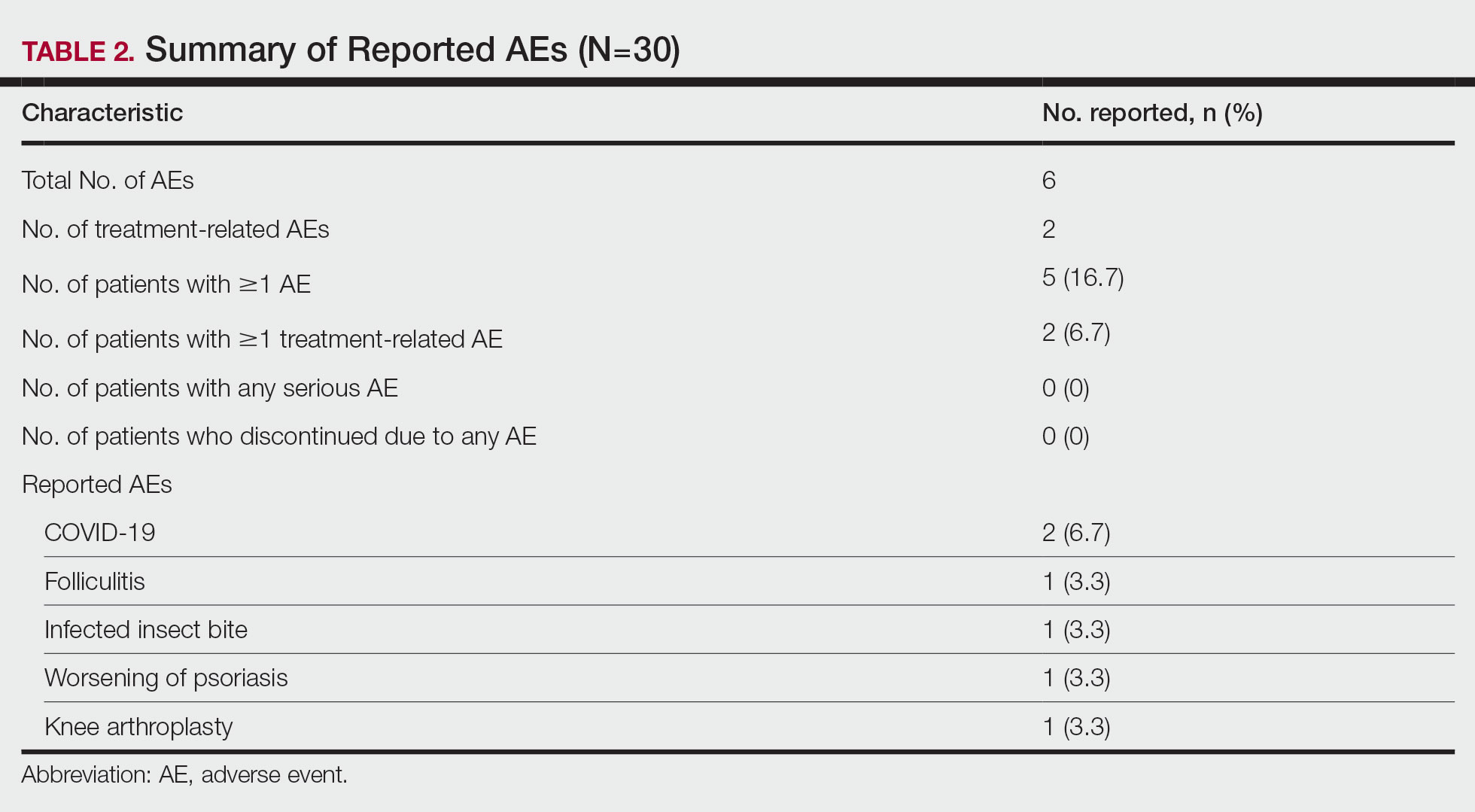
A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.
Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.



After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.

Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.


A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.

Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.



After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.

Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.


A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.

Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Practice Points
- Patients with moderate to severe psoriasis do not always reach treatment goals with biologic therapy alone.
- Adjunctive use of nonsteroidal tapinarof cream 1% may enhance the effects of ongoing biologic therapy in patients with moderate to severe plaque psoriasis, possibly avoiding the need to switch to another biologic.
- Patients with moderate to severe plaque psoriasis who are not adequately responding to biologics may benefit from adding tapinarof cream 1% to their current regimen.
Halobetasol Propionate for the Management of Psoriasis
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).
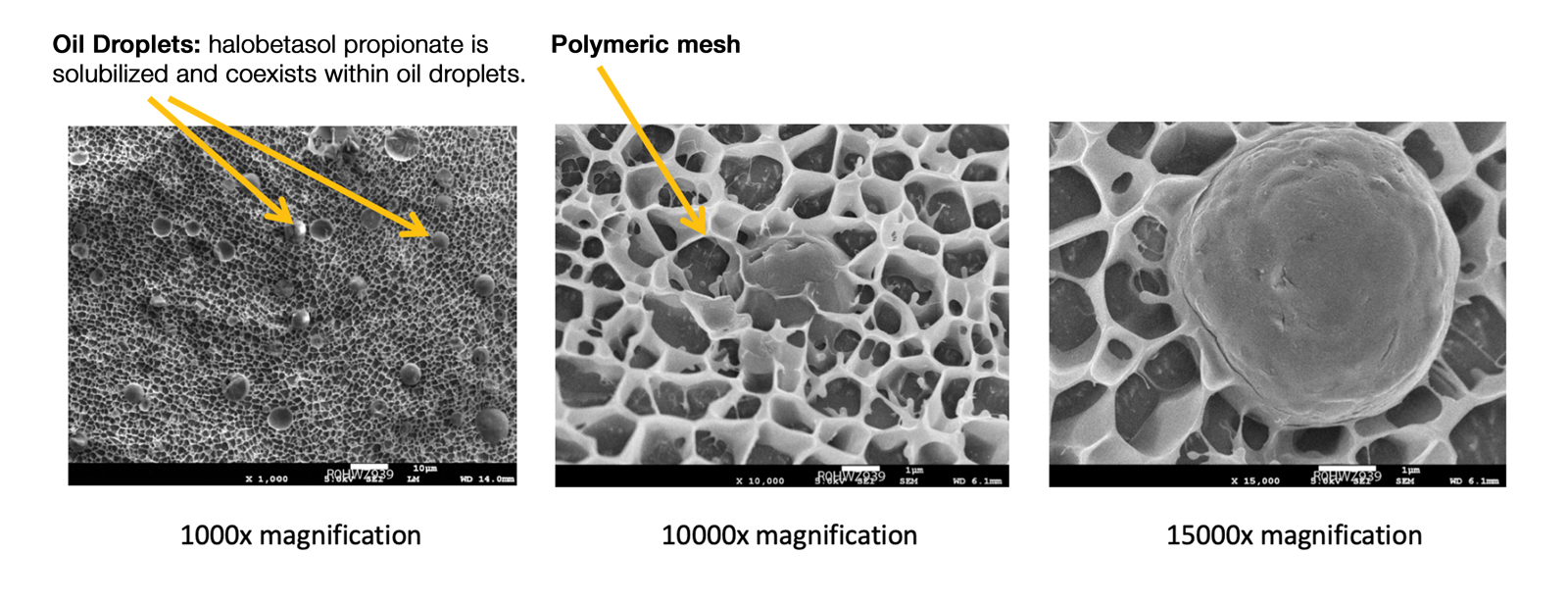
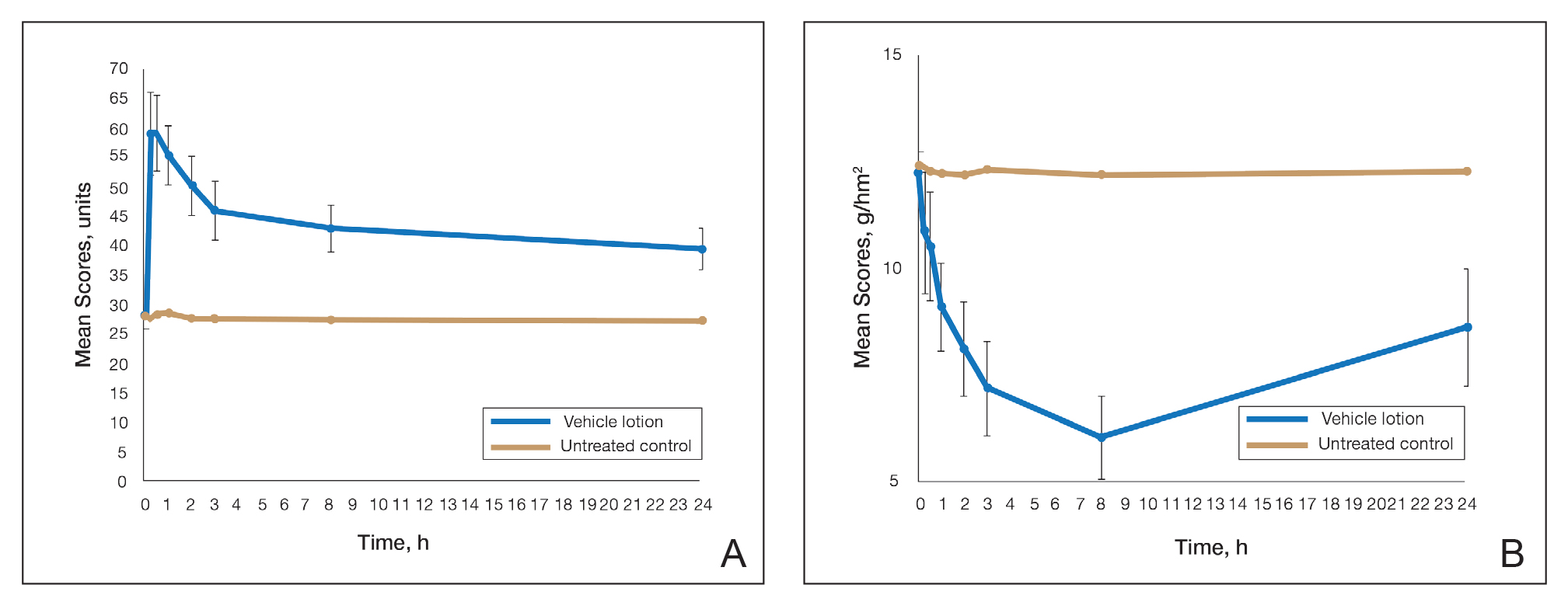
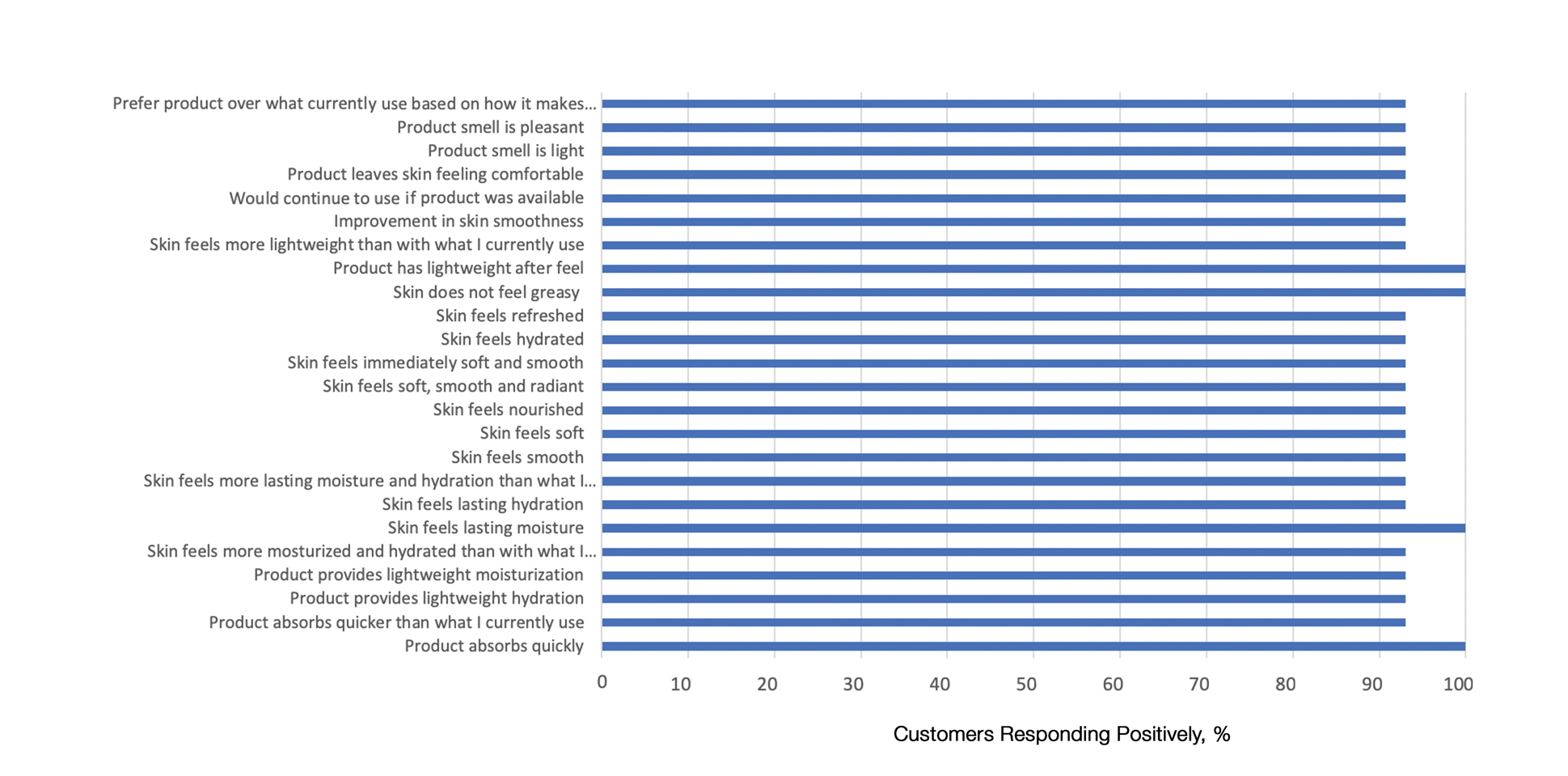
This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).
Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
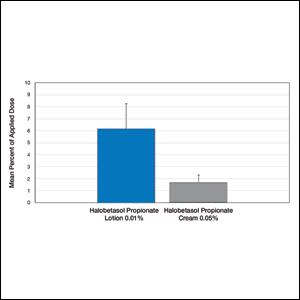
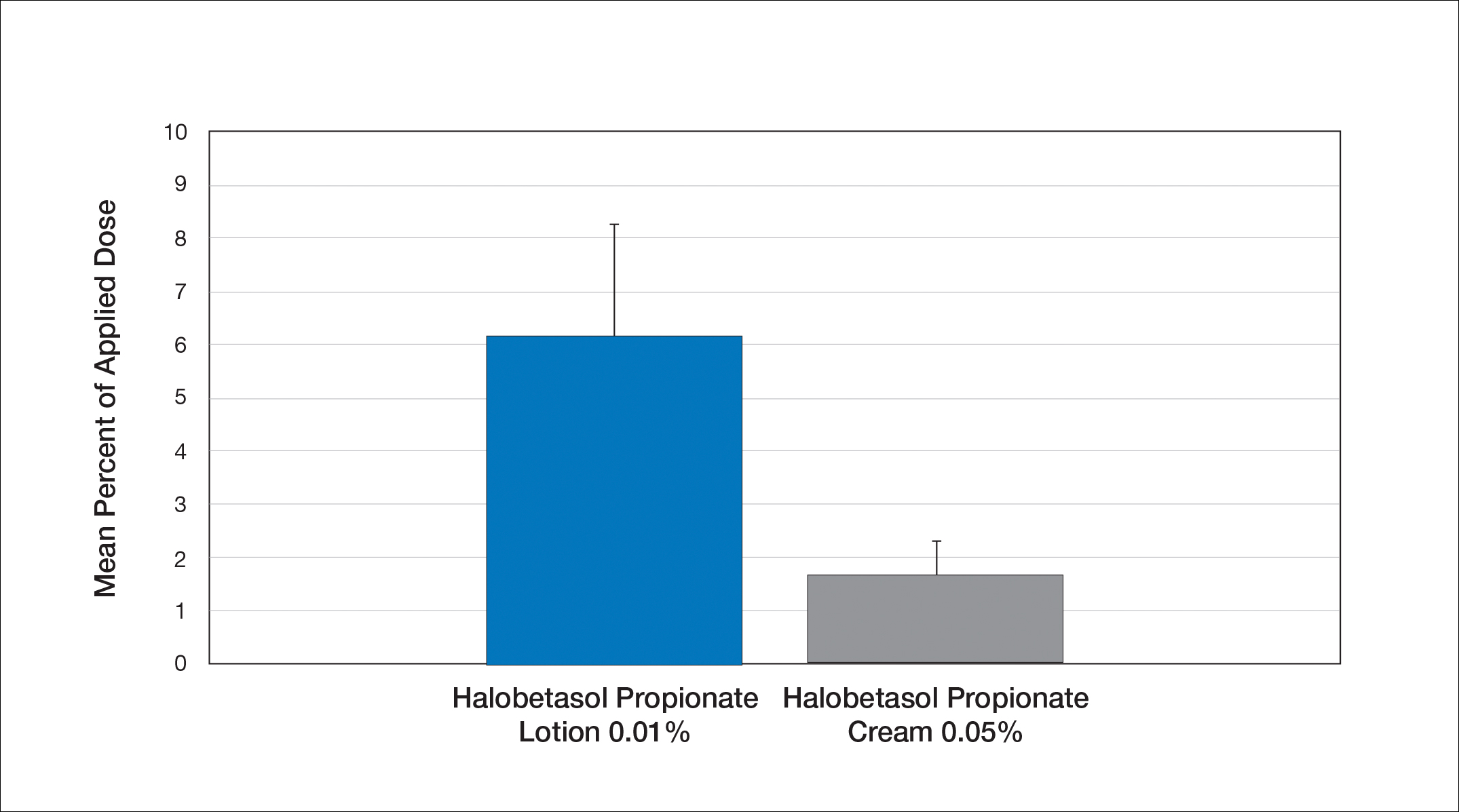
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).
Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
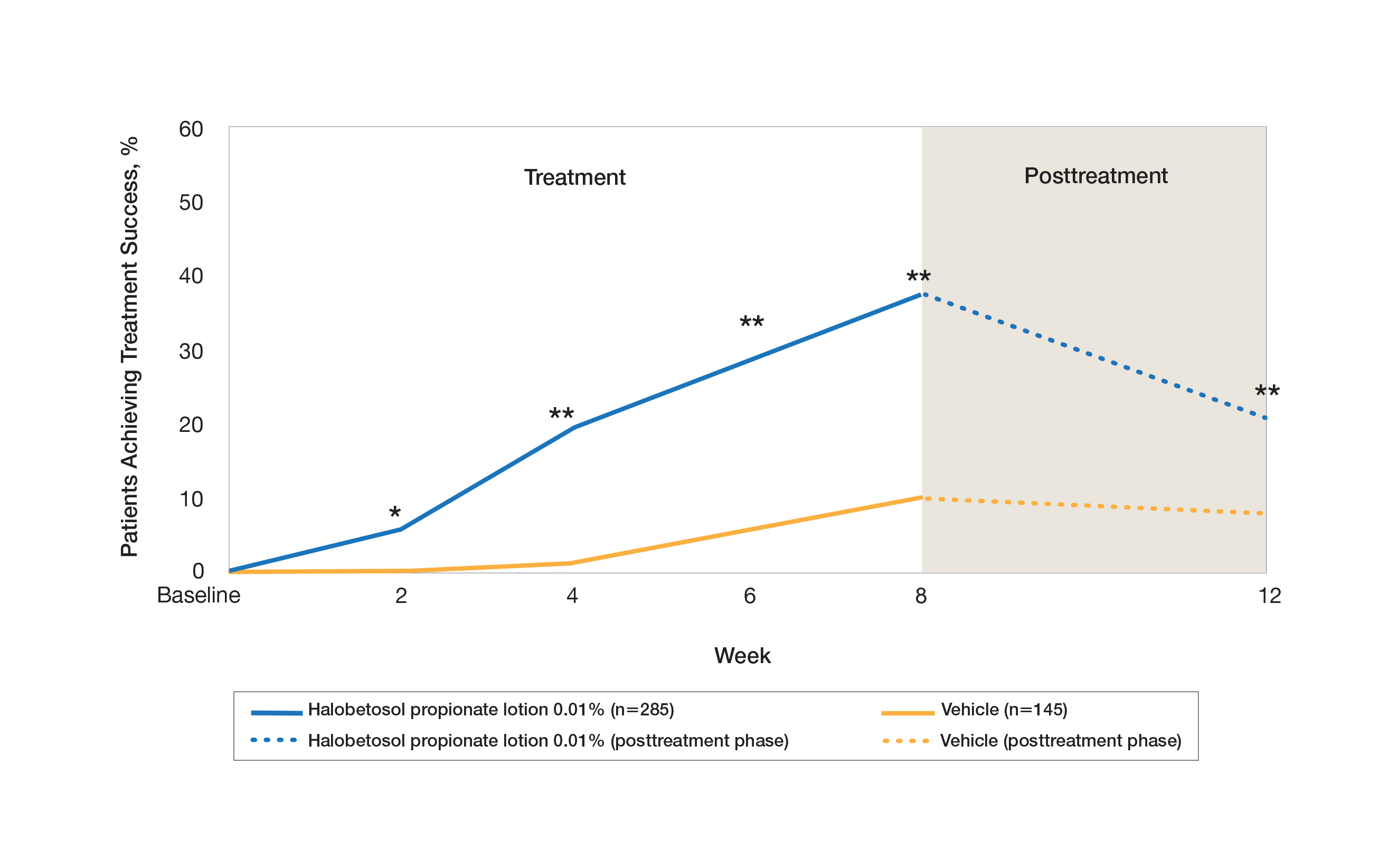
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20
HP Lotion 0.01% Compared to HP Cream 0.05%
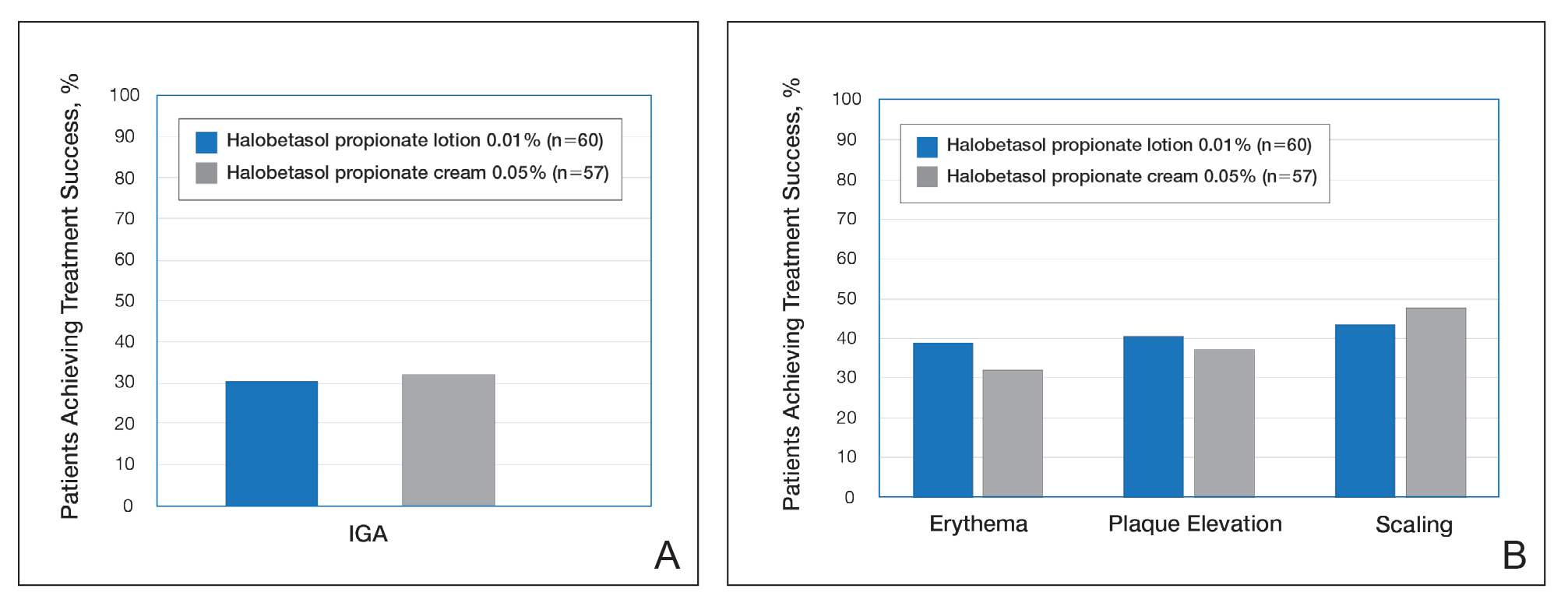
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21
Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).

This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).


Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).

Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20

HP Lotion 0.01% Compared to HP Cream 0.05%
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21

Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).

This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).


Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).

Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20

HP Lotion 0.01% Compared to HP Cream 0.05%
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21

Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
Practice Points
- The widespread use of superpotent topical corticosteroids in treating psoriasis is limited by labelling that restricts short-term use, concerns about side effects, and a paucity of clinical data with longer-term use.
- Long-term management and treatment options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life.
- A novel formulation of halobetasol propionate lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets.
Betamethasone Dipropionate Spray 0.05% Alleviates Troublesome Symptoms of Plaque Psoriasis
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.
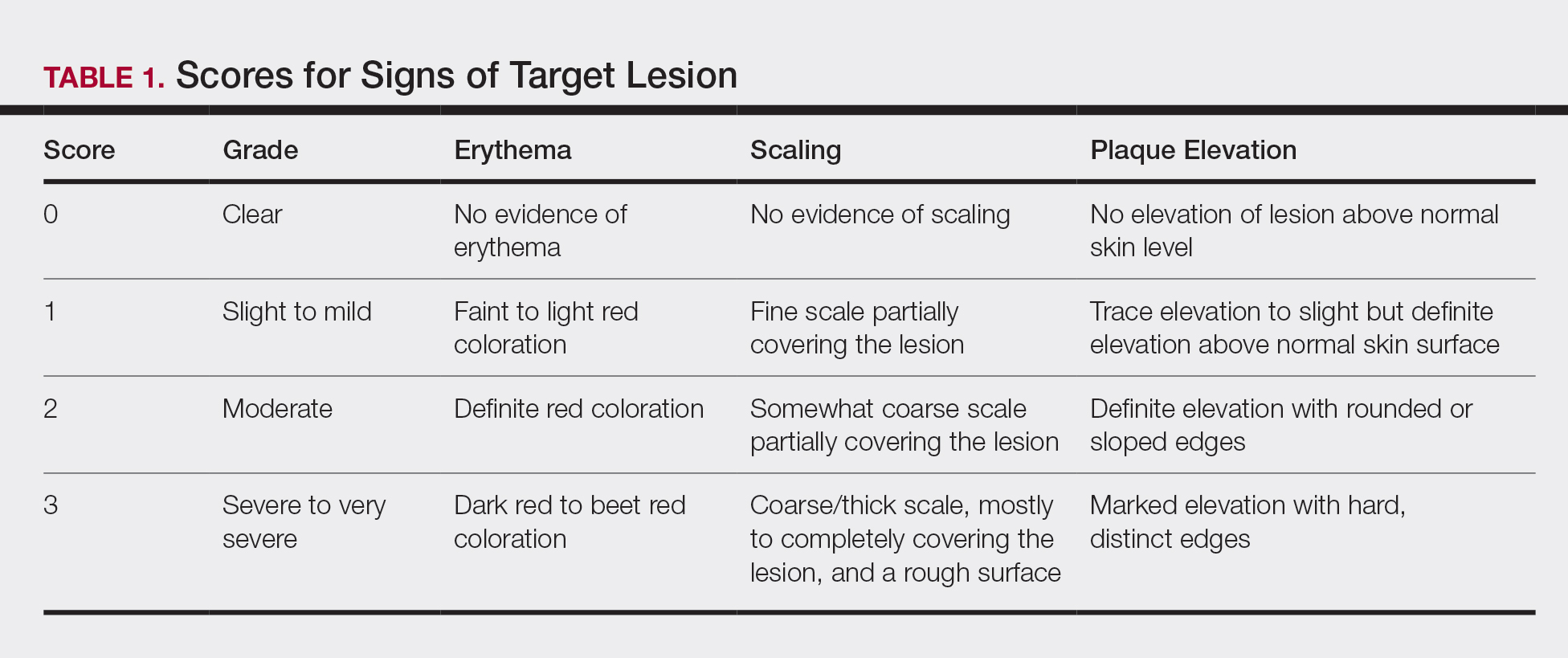
Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.
A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.
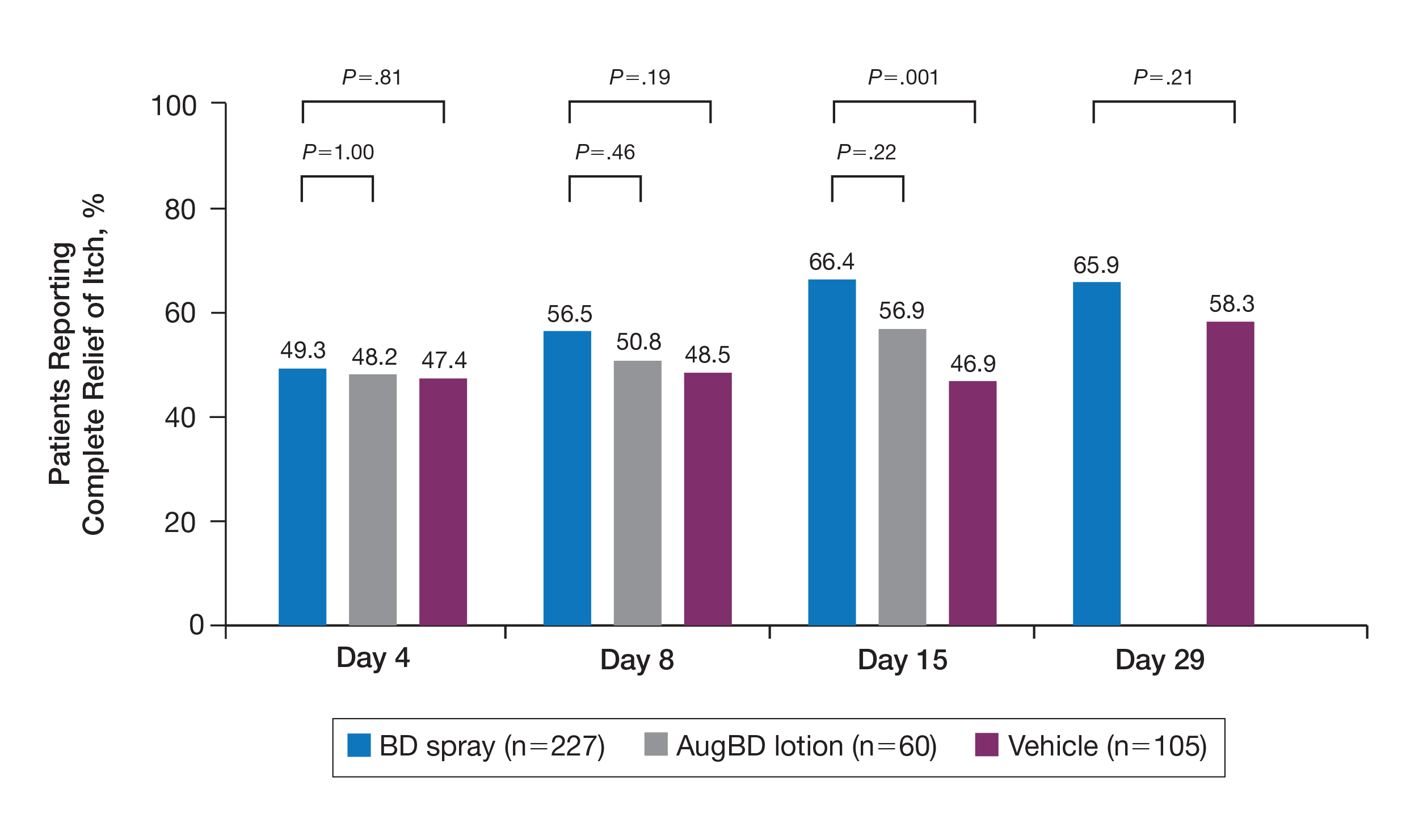
Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).
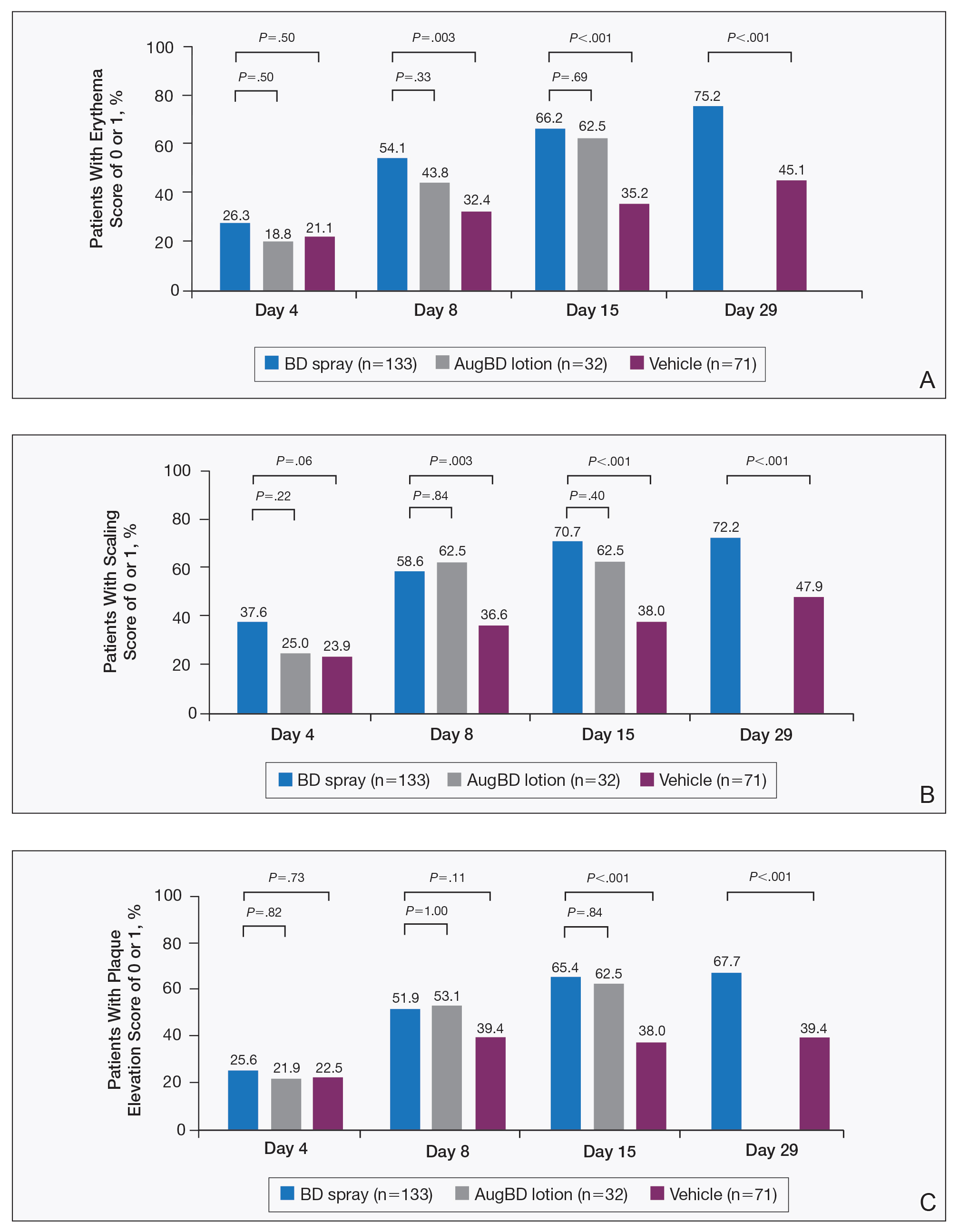
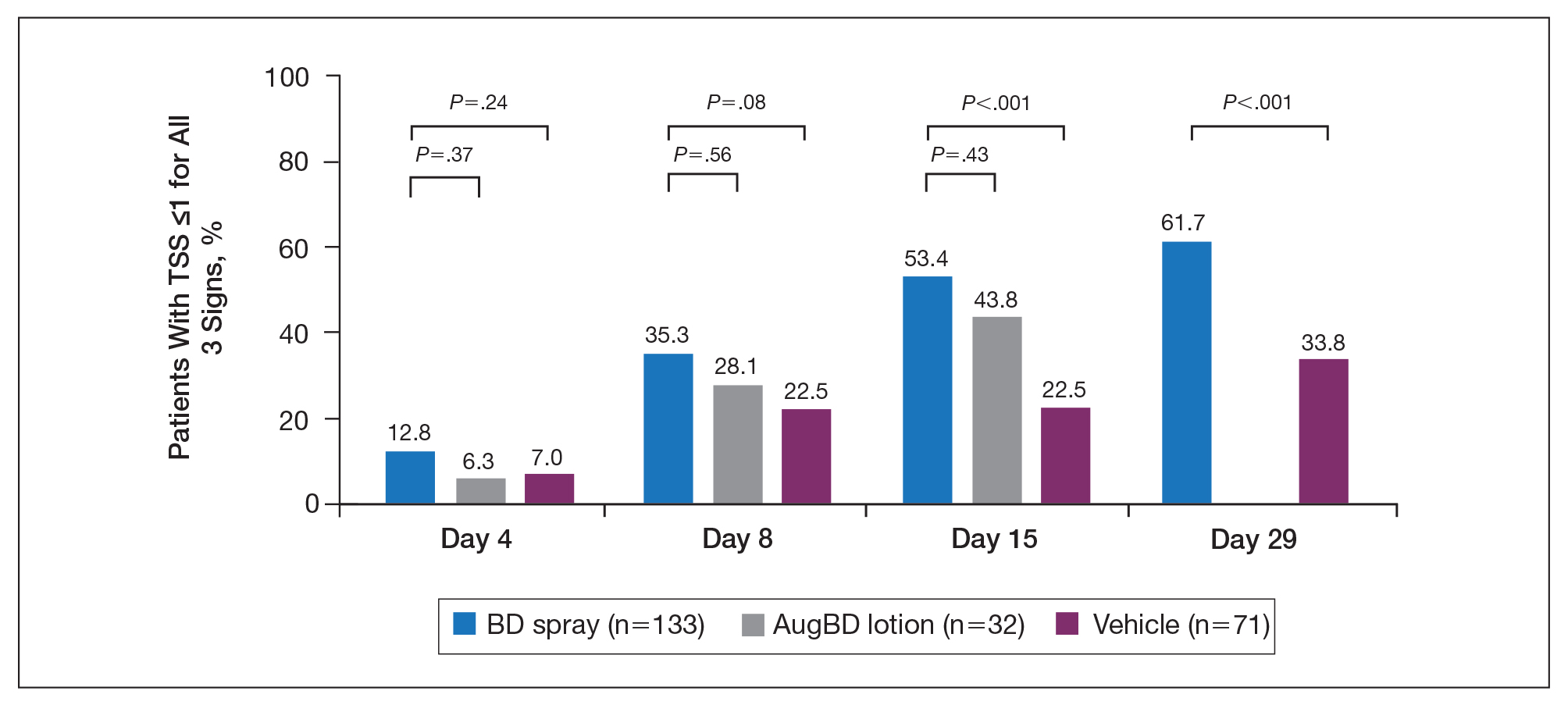
The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4
The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.

Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.

A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.

Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).


The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4

The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.

Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.

A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.

Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).


The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4

The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
Practice Points
- Pruritus is one of the most bothersome symptoms of psoriasis; plaques located on the knees and elbows remain hard to treat.
- Topical corticosteroids are the initial form of treatment of localized plaque psoriasis.
- The choice of vehicle can change the penetration of the medication, alter the efficacy, and minimize side effects of the drug.
- Betamethasone dipropionate spray 0.05% is a mid-potent corticosteroid that provides fast symptom relief and early efficacy in clearing plaques, similar to a high-potency topical corticosteroid but with less potential for systemic absorption and adverse events.