User login
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
Psoriasis treatments have come a long way in the past 20 years. We now have more than a dozen systemic targeted treatments for psoriatic disease, with more on the way; however, with each successive class of medications introduced, the gap has narrowed in terms of increasing efficacy. In an era of medications reporting complete clearance rates in the 70% range, the average improvement in Psoriasis Area and Severity Index (PASI) for most biologics has remained at 90% to 95% in the past half-decade. While this is a far cry from the mean PASI improvements of 70% seen with the first biologics,1 it is becoming more challenging to base our treatment decisions solely on PASI outcome measures.
How, then, do we approach rational selection of a systemic psoriasis treatment? We could try to delineate based on mechanism of action, but it may be disingenuous to dissect minor differences in pathways (eg, IL-17 vs IL-23) that are fundamentally related and on the same continuum in psoriasis pathophysiology. Therefore, the most meaningful way to select an appropriate therapeutic may be to adopt a patient-centered approach that accounts for both individual preferences and specific medical needs by evaluating for other comorbidities2 to exclude or select certain medicines or types of treatments. We have long known to avoid tumor necrosis factor (TNF) α inhibitors in patients with congestive heart failure or a history of demyelinating disorders while regularly considering the presence of psoriatic arthritis and family planning when making treatment decisions. Now, we can be more nuanced in our approaches to psoriasis biologics. Specifically, the most important comorbidities to consider broadly encompass cardiometabolic disorders, gastrointestinal conditions, and psychiatric conditions.
Cardiometabolic Disorders
Possibly the hottest topic in psoriasis for some years now, the relationship between cardiometabolic disorders and psoriasis is of great interest to clinicians, scientists, and patients alike. There is a clear link between development of atherosclerosis and Th17-related immune mechanisms that also are implicated in the pathogenesis of psoriasis.3 Furthermore, the incidence of cardiovascular disease is markedly increased in patients with psoriasis, which is an independent risk factor for myocardial infarction, particularly among younger patients.4,5 Although several retrospective studies6-8 have shown that TNF-α inhibitors are associated with a reduction in cardiovascular outcomes, it is yet to be seen whether biologic treatment actually has a direct impact on cardiovascular outcomes, multiple studies investigating the effect of biologics on arterial inflammation markers notwithstanding.9
There are some direct factors to keep in mind when considering cardiometabolic comorbidities in patients with psoriasis. Obesity is common in the psoriasis population and can have a direct negative effect on cardiovascular health.10 However, the data on obesity and psoriasis are somewhat mixed with regard to treatment outcomes. In general, with increased volumes of distribution for biologics in patients with obesity, it has been shown that treatment success is more difficult to achieve in those with a body mass index greater than 30.11 Rather surprisingly, a separate nationwide study in South Korea found that patients on biologics for psoriasis were more likely to experience weight gain, even after controlling for factors such as exercise, smoking, and drinking,12 but it is unclear whether this is driven mostly by a known connection between weight gain and TNF-α inhibitors.13 These contrasting results point to the need for further studies in this area, as our intuitive approach would involve promoting weight loss while starting on a systemic treatment for psoriasis—but perhaps it is important not to assume that one will come with the other in tow, reinforcing the need to discuss a healthy diet with our patients with psoriasis regardless of treatment decisions.
The data that we have do not directly answer the big questions about biologic treatment and cardiovascular health, but we are starting to see interesting signals. For example, in a report of tildrakizumab treatment in patients with and without metabolic syndrome, the rates of major adverse cardiovascular events as well as cardiac disorders were essentially the same in both groups after receiving treatment for up to 244 weeks.14 This is interesting, more because of the lack of an increase in cardiovascular adverse events in the metabolic syndrome group, who entered the trial on average 25 kg to 30 kg heavier than those without metabolic syndrome. There is an increased risk for adverse cardiovascular events among patients with metabolic syndrome, a roughly 2-fold relative risk in as few as 5 to 6 years of follow-up.15 While the cohorts in the tildrakizumab study14 were too small to draw firm conclusions, the data are interesting and a step in the right direction; we need much larger data sets for analysis. Among other agents, similar efficacy and safety have been reported for guselkumab in a long-term psoriasis study; as a class, IL-23 inhibitors also tend to perform well from an efficacy standpoint in patients with obesity.16
Overall, when assessing the evidence for cardiometabolic disorders, it is reasonable to consider starting a biologic from the IL-17 or IL-23 inhibitor classes— thus avoiding both the potential downside of weight gain and contraindication in patients with congestive heart failure associated with TNF-α inhibitors. It is important to counsel patients about weight loss in conjunction with these treatments, both to improve efficacy and reduce cardiovascular risk factors. There may be a preference for IL-23 inhibitors in patients with obesity, as this class of medications maintains efficacy particularly well in these patients. Patients with psoriasis should be counseled to follow up with a primary care physician given their higher risk for metabolic syndrome and adverse cardiovascular outcomes.
Gastrointestinal Conditions
Psoriasis and inflammatory bowel disease (IBD) have a bidirectional association, and patients with psoriasis are about 1.7 times more likely to have either Crohn disease or ulcerative colitis.17,18 This association may be related to a shared pathogenesis with regard to immune dysregulation and overactivated inflammatory pathways, but there are some important differences to consider from a therapeutic standpoint. Given the increased expression of IL-17 in patients with IBD,19 a phase II trial of secukinumab yielded surprising results—not only was secukinumab ineffective in treating Crohn disease, but there also were higher rates of adverse events20 (as noted on the product label for all IL-17 inhibitors). We have come to understand that there are regulatory subsets of IL-17 cells that are important in mucosal homeostasis and also regulate IL-10, which generally is considered an anti-inflammatory cytokine.21 Thus, while IL-17 inhibition can reduce some component of inflammatory signaling, it also can increase inflammatory signaling through indirect pathways while increasing intestinal permeability to microbes. Importantly, this process seems to occur via IL-23–independent pathways; as such, while direct inhibition of IL-17 can be deleterious, IL-23 inhibitors have become important therapeutics for IBD.22
IL-17 family, IL-17A clearly is the culprit for worsening colitis as evidenced by both human and animal models. On the contrary, IL-17F blockade has been shown to ameliorate colitis in a murine model, whereas IL-17A inhibition worsens it.23 Furthermore, dual blockade of IL-17A and IL-17F has a protective effect against colitis, suggesting that the IL-17F inhibition is dominant. This interesting finding has some mechanistic backing, since blockade of IL-17F induces Treg cells that serve to maintain gut epithelium homeostasis and integrity.24
Overall, IL-17A inhibitors should be avoided in patients with a history of IBD—namely, secukinumab and ixekizumab. While there may be theoretical reasons that brodalumab or bimekizumab may confer a somewhat different risk for IBD exacerbation, there may be better choices that would be expected to effectively treat both the psoriasis and IBD manifestations. Given the US Food and Drug Administration approval of IL-23 inhibitors for IBD and their high levels of efficacy in treating psoriasis, these likely will be the best choice for most patients. Another mainstay of IBD treatment is TNF-α inhibitors, but they come with other risks such as increased immunosuppression and increased risk for nonmelanoma skin cancer.
An important question remains: What about patients who do not have known IBD? Do we proactively change our treatment choice due to fear of IBD development given the higher incidence of both Crohn disease and ulcerative colitis in patients with psoriasis? What about patients with a family history of IBD? First-degree relatives of patients with Crohn disease and ulcerative colitis have an 8- and 4-fold higher risk for those same conditions, respectively.25 Postmarketing surveillance and database findings of low rates of IBD development with IL-17 inhibitors gives only modest reassurance, as dermatologists generally know to avoid these medications for patients with even questionable IBD symptoms. It is important to emphasize to our patients that in no case do we believe that a psoriasis medication actually will cause IBD—rather, someone with subclinical IBD could experience a flare and a first manifestation of colitis. The drug is not the culprit in inducing IBD but rather may serve to unmask existing disease.
One study suggested that for patients who move on to the IL-17 inhibitor secukinumab after being treated with TNF-α inhibitors for psoriasis, the rates of IBD development are higher (4.8%) than in those who start IL-17A inhibition without prior treatment (1%)(OR, 8.38; P=.018).26 This begs the question of whether subclinical IBD in many patients with psoriasis who are treated with TNF-α inhibitors can be unmasked later when they are transitioned to a treatment that either does not treat the IBD or could worsen it. There may be a mechanistic drive behind this sequencing of treatments that predisposes patients to colitis, which would suggest selecting an IL-23 inhibitor after failing/trying a TNF-α inhibitor. However, the data are very preliminary, and in real practice, other concerns such as severe psoriatic arthritis may outweigh these considerations, as the IL-17 inhibitor class still is considered to be more effective than IL-23 inhibition at treating psoriatic arthritis overall. For most patients with no personal history of IBD and no strong family history of IBD (ie, first-degree relatives), the choice of biologic should not be affected by concern over gastrointestinal issues.
Psychiatric Conditions
It has been well established that psoriasis is linked to higher rates of depression, anxiety, and suicidality.27 How do we take this into account when treating patients with psoriasis, especially when we have biologics with a warning label for suicidality and a Risk Evaluation and Mitigation Strategies program (brodalumab) and language around suicidal ideation in the label (bimekizumab)? While it is challenging to discuss mental health, it is not a conversation that we as dermatologists should shy away from. Appropriate treatment of psoriasis is an important tool to get our patients on the path to better mental health. A recent database study of more than 4000 patients showed that patients with psoriasis treated with biologics had a 17% lower risk for depression than those treated with conventional disease-modifying drugs such as methotrexate.28 The comparator of the conventional disease-modifying drug class is important as it serves as a control for disease severity. Too often, a higher rate of depression, anxiety, or suicidality can be attributed to a medication when we in fact may just be capturing the background of higher incidence of all 3 in patients with severe psoriasis.
Indeed, even with the medication that many worry about on this front (brodalumab), multiple studies have confirmed that the effect on mental health generally is a positive one, with decreases in depressive symptoms.29 In a cohort switched from TNF-α inhibitors to brodalumab, symptoms of depression actually improved,30 so attributing a direct treatment effect to negative mental health outcomes does not seem to be justified, especially in light of the low number of suicide events in global postmarketing surveillance for brodalumab, comparable to or lower than other biologics for psoriasis.31 Similarly, bimekizumab has language in the label about discussing suicidality with patients, although the rates of suicidal ideation and behavior are no different from other biologics and rates of depression improved with its use.32
Heightened awareness of our patients’ mental health is something that we as providers should embrace, even when it seems that we do not have much time to see each patient. The priority when a patient comes in with mental health symptoms should be to treat what is within our scope (ie, psoriasis) as quickly and effectively as possible— with a newer-generation biologic such as an IL-17 or IL-23 inhibitor—while encouraging the patient to seek care from a mental health professional. In these cases, one might even argue that the rapidity of action of IL-17 inhibitors may be of additional benefit.
Final Thoughts
We as dermatologists generally are tasked with seeing high volumes of patients, and an initial psoriasis consultation can be a lengthy visit; however, it is rewarding to establish this relationship with patients and a reminder of why we practice medicine to begin with. Psoriasis can be satisfying to treat, and we have so many highly effective medicines that can completely transform our patients’ lives. Applying an understanding of the interplay between psoriasis, its related comorbidities, and treatment choices can be a fulfilling exercise that captures the essence of shared decision-making, which can lead to better outcomes and satisfaction for both providers and patients.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022. doi:10.1056/NEJMoa030409
- Thatiparthi A, Martin A, Liu J, et al. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22:425-442. doi:10.1007/s40257-021-00603-w
- Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5-22. doi:10.1007 /s00281-009-0153-8
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. doi:10.1001/jama.296.14.1735
- Miller IM, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014-1024. doi:10.1016/j.jaad.2013.06.053
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90. doi:10.1016/j.jaad.2016.07.042
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250. doi:10.1001 /archdermatol.2012.2502
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68. doi:10.1016/j.jaad.2018.02.050
- Cai J, Cui L, Wang Y, et al. Cardiometabolic comorbidities in patients with psoriasis: focusing on risk, biological therapy, and pathogenesis. Front Pharmacol. 2021;12:774808. doi:10.3389/fphar.2021.774808
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:E984-E1010. doi:10.1161/CIR.0000000000000973
- Pirro F, Caldarola G, Chiricozzi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig. 2021;41:917-925. doi:10.1007 /s40261-021-01080-z
- Kim H, Hong JY, Cheong S, et al. Impact of biologic agents on body weight and obesity-related disorders in patients with psoriasis: a nationwide population-based cohort study. Obes Res Clin Pract. 2023;17:210-217. doi:10.1016/j.orcp.2023.05.004
- Saraceno R, Schipani C, Mazzotta A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57:290-295. doi:10.1016/j.phrs.2008.02.006
- Fernandez AP, Dauden E, Gerdes S, et al. Tildrakizumab efficacy and safety in patients with psoriasis and concomitant metabolic syndrome: post hoc analysis of 5-year data from reSURFACE 1 and reSURFACE 2. J Eur Acad Dermatol Venereol. 2022;36:1774-1783. doi:10.1111/jdv.18167
- Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. doi:10.1016/j.jacc.2010.05.034
- Ricceri F, Chiricozzi A, Peris K, et al. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: a multicentric retrospective study. Dermatol Ther. 2022;35:E15793. doi:10.1111/dth.15793
- Alinaghi F, Tekin HG, Burisch J, et al. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease— a systematic review and meta-analysis. J Crohns Colitis. 2020;14:351-360. doi:10.1093/ecco-jcc/jjz152
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. doi:10.1136/gut.52.1.65
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebocontrolled trial. Gut. 2012;61:1693-1700. doi:10.1136 /gutjnl-2011-301668
- Brockmann L, Tran A, Huang Y, et al. Intestinal microbiotaspecific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. 2023;56:2719-2735 e7. doi:10.1016/j.immuni.2023.11.003
- Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727-738. doi:10.1016/j.immuni.2015.09.003
- Wedebye Schmidt EG, Larsen HL, Kristensen NN, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19:1567-1576. doi:10.1097 /MIB.0b013e318286fa1c
- Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing T(reg) cells through modification of the intestinal microbiota. Nat Immunol. 2018;19:755-765. doi:10.1038/s41590-018-0134-y
- El Hadad J, Schreiner P, Vavricka SR, Greuter T. The genetics of inflammatory bowel disease. Mol Diagn Ther. 2024;28:27-35. doi:10.1007 /s40291-023-00678-7
- Albayrak F, Gür M, Karatas¸ A, et al. Is the use of secukinumab after anti-TNF therapy greater than expected for the risk of developing inflammatory bowel disease? Reumatol Clin (Engl Ed). 2024;20:123-127. doi:10.1016/j.reumae.2023.11.002
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Strober B, Soliman AM, Truong B, et al. Association between biologic exposure and the risk of depression in patients with psoriasis: a retrospective analysis of large US administrative claims data. Am J Clin Dermatol. 2024;25:853-856. doi:10.1007/s40257 -024-00877-w
- Koo J, Ho RS, Thibodeaux Q. Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review. Cutis. 2019;104:361-365.
- Andersch-Bjorkman Y, Micu E, Seifert O, et al. Effects of brodalumab on psoriasis and depressive symptoms in patients with insufficient response to TNF-alpha inhibitors. J Dermatol. 2023;50:1401-1414. doi:10.1111/1346-8138.16917
- Yeroushalmi S, Chung M, Bartholomew E, et al. Examining worldwide postmarketing suicides from biologics used for psoriasis with a focus on brodalumab: a cross-sectional analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS). JAAD Int. 2022;9:119-121. doi:10.1016/j.jdin.2022.08.010
- Blauvelt A, Armstrong A, Merola JF, et al. Mental health outcomes in patients with moderate to severe psoriasis treated with bimekizumab: analysis of phase 2/3 randomized trials. J Am Acad Dermatol. 2024;91:72-81. doi:10.1016/j.jaad.2024.02.039
Psoriasis treatments have come a long way in the past 20 years. We now have more than a dozen systemic targeted treatments for psoriatic disease, with more on the way; however, with each successive class of medications introduced, the gap has narrowed in terms of increasing efficacy. In an era of medications reporting complete clearance rates in the 70% range, the average improvement in Psoriasis Area and Severity Index (PASI) for most biologics has remained at 90% to 95% in the past half-decade. While this is a far cry from the mean PASI improvements of 70% seen with the first biologics,1 it is becoming more challenging to base our treatment decisions solely on PASI outcome measures.
How, then, do we approach rational selection of a systemic psoriasis treatment? We could try to delineate based on mechanism of action, but it may be disingenuous to dissect minor differences in pathways (eg, IL-17 vs IL-23) that are fundamentally related and on the same continuum in psoriasis pathophysiology. Therefore, the most meaningful way to select an appropriate therapeutic may be to adopt a patient-centered approach that accounts for both individual preferences and specific medical needs by evaluating for other comorbidities2 to exclude or select certain medicines or types of treatments. We have long known to avoid tumor necrosis factor (TNF) α inhibitors in patients with congestive heart failure or a history of demyelinating disorders while regularly considering the presence of psoriatic arthritis and family planning when making treatment decisions. Now, we can be more nuanced in our approaches to psoriasis biologics. Specifically, the most important comorbidities to consider broadly encompass cardiometabolic disorders, gastrointestinal conditions, and psychiatric conditions.
Cardiometabolic Disorders
Possibly the hottest topic in psoriasis for some years now, the relationship between cardiometabolic disorders and psoriasis is of great interest to clinicians, scientists, and patients alike. There is a clear link between development of atherosclerosis and Th17-related immune mechanisms that also are implicated in the pathogenesis of psoriasis.3 Furthermore, the incidence of cardiovascular disease is markedly increased in patients with psoriasis, which is an independent risk factor for myocardial infarction, particularly among younger patients.4,5 Although several retrospective studies6-8 have shown that TNF-α inhibitors are associated with a reduction in cardiovascular outcomes, it is yet to be seen whether biologic treatment actually has a direct impact on cardiovascular outcomes, multiple studies investigating the effect of biologics on arterial inflammation markers notwithstanding.9
There are some direct factors to keep in mind when considering cardiometabolic comorbidities in patients with psoriasis. Obesity is common in the psoriasis population and can have a direct negative effect on cardiovascular health.10 However, the data on obesity and psoriasis are somewhat mixed with regard to treatment outcomes. In general, with increased volumes of distribution for biologics in patients with obesity, it has been shown that treatment success is more difficult to achieve in those with a body mass index greater than 30.11 Rather surprisingly, a separate nationwide study in South Korea found that patients on biologics for psoriasis were more likely to experience weight gain, even after controlling for factors such as exercise, smoking, and drinking,12 but it is unclear whether this is driven mostly by a known connection between weight gain and TNF-α inhibitors.13 These contrasting results point to the need for further studies in this area, as our intuitive approach would involve promoting weight loss while starting on a systemic treatment for psoriasis—but perhaps it is important not to assume that one will come with the other in tow, reinforcing the need to discuss a healthy diet with our patients with psoriasis regardless of treatment decisions.
The data that we have do not directly answer the big questions about biologic treatment and cardiovascular health, but we are starting to see interesting signals. For example, in a report of tildrakizumab treatment in patients with and without metabolic syndrome, the rates of major adverse cardiovascular events as well as cardiac disorders were essentially the same in both groups after receiving treatment for up to 244 weeks.14 This is interesting, more because of the lack of an increase in cardiovascular adverse events in the metabolic syndrome group, who entered the trial on average 25 kg to 30 kg heavier than those without metabolic syndrome. There is an increased risk for adverse cardiovascular events among patients with metabolic syndrome, a roughly 2-fold relative risk in as few as 5 to 6 years of follow-up.15 While the cohorts in the tildrakizumab study14 were too small to draw firm conclusions, the data are interesting and a step in the right direction; we need much larger data sets for analysis. Among other agents, similar efficacy and safety have been reported for guselkumab in a long-term psoriasis study; as a class, IL-23 inhibitors also tend to perform well from an efficacy standpoint in patients with obesity.16
Overall, when assessing the evidence for cardiometabolic disorders, it is reasonable to consider starting a biologic from the IL-17 or IL-23 inhibitor classes— thus avoiding both the potential downside of weight gain and contraindication in patients with congestive heart failure associated with TNF-α inhibitors. It is important to counsel patients about weight loss in conjunction with these treatments, both to improve efficacy and reduce cardiovascular risk factors. There may be a preference for IL-23 inhibitors in patients with obesity, as this class of medications maintains efficacy particularly well in these patients. Patients with psoriasis should be counseled to follow up with a primary care physician given their higher risk for metabolic syndrome and adverse cardiovascular outcomes.
Gastrointestinal Conditions
Psoriasis and inflammatory bowel disease (IBD) have a bidirectional association, and patients with psoriasis are about 1.7 times more likely to have either Crohn disease or ulcerative colitis.17,18 This association may be related to a shared pathogenesis with regard to immune dysregulation and overactivated inflammatory pathways, but there are some important differences to consider from a therapeutic standpoint. Given the increased expression of IL-17 in patients with IBD,19 a phase II trial of secukinumab yielded surprising results—not only was secukinumab ineffective in treating Crohn disease, but there also were higher rates of adverse events20 (as noted on the product label for all IL-17 inhibitors). We have come to understand that there are regulatory subsets of IL-17 cells that are important in mucosal homeostasis and also regulate IL-10, which generally is considered an anti-inflammatory cytokine.21 Thus, while IL-17 inhibition can reduce some component of inflammatory signaling, it also can increase inflammatory signaling through indirect pathways while increasing intestinal permeability to microbes. Importantly, this process seems to occur via IL-23–independent pathways; as such, while direct inhibition of IL-17 can be deleterious, IL-23 inhibitors have become important therapeutics for IBD.22
IL-17 family, IL-17A clearly is the culprit for worsening colitis as evidenced by both human and animal models. On the contrary, IL-17F blockade has been shown to ameliorate colitis in a murine model, whereas IL-17A inhibition worsens it.23 Furthermore, dual blockade of IL-17A and IL-17F has a protective effect against colitis, suggesting that the IL-17F inhibition is dominant. This interesting finding has some mechanistic backing, since blockade of IL-17F induces Treg cells that serve to maintain gut epithelium homeostasis and integrity.24
Overall, IL-17A inhibitors should be avoided in patients with a history of IBD—namely, secukinumab and ixekizumab. While there may be theoretical reasons that brodalumab or bimekizumab may confer a somewhat different risk for IBD exacerbation, there may be better choices that would be expected to effectively treat both the psoriasis and IBD manifestations. Given the US Food and Drug Administration approval of IL-23 inhibitors for IBD and their high levels of efficacy in treating psoriasis, these likely will be the best choice for most patients. Another mainstay of IBD treatment is TNF-α inhibitors, but they come with other risks such as increased immunosuppression and increased risk for nonmelanoma skin cancer.
An important question remains: What about patients who do not have known IBD? Do we proactively change our treatment choice due to fear of IBD development given the higher incidence of both Crohn disease and ulcerative colitis in patients with psoriasis? What about patients with a family history of IBD? First-degree relatives of patients with Crohn disease and ulcerative colitis have an 8- and 4-fold higher risk for those same conditions, respectively.25 Postmarketing surveillance and database findings of low rates of IBD development with IL-17 inhibitors gives only modest reassurance, as dermatologists generally know to avoid these medications for patients with even questionable IBD symptoms. It is important to emphasize to our patients that in no case do we believe that a psoriasis medication actually will cause IBD—rather, someone with subclinical IBD could experience a flare and a first manifestation of colitis. The drug is not the culprit in inducing IBD but rather may serve to unmask existing disease.
One study suggested that for patients who move on to the IL-17 inhibitor secukinumab after being treated with TNF-α inhibitors for psoriasis, the rates of IBD development are higher (4.8%) than in those who start IL-17A inhibition without prior treatment (1%)(OR, 8.38; P=.018).26 This begs the question of whether subclinical IBD in many patients with psoriasis who are treated with TNF-α inhibitors can be unmasked later when they are transitioned to a treatment that either does not treat the IBD or could worsen it. There may be a mechanistic drive behind this sequencing of treatments that predisposes patients to colitis, which would suggest selecting an IL-23 inhibitor after failing/trying a TNF-α inhibitor. However, the data are very preliminary, and in real practice, other concerns such as severe psoriatic arthritis may outweigh these considerations, as the IL-17 inhibitor class still is considered to be more effective than IL-23 inhibition at treating psoriatic arthritis overall. For most patients with no personal history of IBD and no strong family history of IBD (ie, first-degree relatives), the choice of biologic should not be affected by concern over gastrointestinal issues.
Psychiatric Conditions
It has been well established that psoriasis is linked to higher rates of depression, anxiety, and suicidality.27 How do we take this into account when treating patients with psoriasis, especially when we have biologics with a warning label for suicidality and a Risk Evaluation and Mitigation Strategies program (brodalumab) and language around suicidal ideation in the label (bimekizumab)? While it is challenging to discuss mental health, it is not a conversation that we as dermatologists should shy away from. Appropriate treatment of psoriasis is an important tool to get our patients on the path to better mental health. A recent database study of more than 4000 patients showed that patients with psoriasis treated with biologics had a 17% lower risk for depression than those treated with conventional disease-modifying drugs such as methotrexate.28 The comparator of the conventional disease-modifying drug class is important as it serves as a control for disease severity. Too often, a higher rate of depression, anxiety, or suicidality can be attributed to a medication when we in fact may just be capturing the background of higher incidence of all 3 in patients with severe psoriasis.
Indeed, even with the medication that many worry about on this front (brodalumab), multiple studies have confirmed that the effect on mental health generally is a positive one, with decreases in depressive symptoms.29 In a cohort switched from TNF-α inhibitors to brodalumab, symptoms of depression actually improved,30 so attributing a direct treatment effect to negative mental health outcomes does not seem to be justified, especially in light of the low number of suicide events in global postmarketing surveillance for brodalumab, comparable to or lower than other biologics for psoriasis.31 Similarly, bimekizumab has language in the label about discussing suicidality with patients, although the rates of suicidal ideation and behavior are no different from other biologics and rates of depression improved with its use.32
Heightened awareness of our patients’ mental health is something that we as providers should embrace, even when it seems that we do not have much time to see each patient. The priority when a patient comes in with mental health symptoms should be to treat what is within our scope (ie, psoriasis) as quickly and effectively as possible— with a newer-generation biologic such as an IL-17 or IL-23 inhibitor—while encouraging the patient to seek care from a mental health professional. In these cases, one might even argue that the rapidity of action of IL-17 inhibitors may be of additional benefit.
Final Thoughts
We as dermatologists generally are tasked with seeing high volumes of patients, and an initial psoriasis consultation can be a lengthy visit; however, it is rewarding to establish this relationship with patients and a reminder of why we practice medicine to begin with. Psoriasis can be satisfying to treat, and we have so many highly effective medicines that can completely transform our patients’ lives. Applying an understanding of the interplay between psoriasis, its related comorbidities, and treatment choices can be a fulfilling exercise that captures the essence of shared decision-making, which can lead to better outcomes and satisfaction for both providers and patients.
Psoriasis treatments have come a long way in the past 20 years. We now have more than a dozen systemic targeted treatments for psoriatic disease, with more on the way; however, with each successive class of medications introduced, the gap has narrowed in terms of increasing efficacy. In an era of medications reporting complete clearance rates in the 70% range, the average improvement in Psoriasis Area and Severity Index (PASI) for most biologics has remained at 90% to 95% in the past half-decade. While this is a far cry from the mean PASI improvements of 70% seen with the first biologics,1 it is becoming more challenging to base our treatment decisions solely on PASI outcome measures.
How, then, do we approach rational selection of a systemic psoriasis treatment? We could try to delineate based on mechanism of action, but it may be disingenuous to dissect minor differences in pathways (eg, IL-17 vs IL-23) that are fundamentally related and on the same continuum in psoriasis pathophysiology. Therefore, the most meaningful way to select an appropriate therapeutic may be to adopt a patient-centered approach that accounts for both individual preferences and specific medical needs by evaluating for other comorbidities2 to exclude or select certain medicines or types of treatments. We have long known to avoid tumor necrosis factor (TNF) α inhibitors in patients with congestive heart failure or a history of demyelinating disorders while regularly considering the presence of psoriatic arthritis and family planning when making treatment decisions. Now, we can be more nuanced in our approaches to psoriasis biologics. Specifically, the most important comorbidities to consider broadly encompass cardiometabolic disorders, gastrointestinal conditions, and psychiatric conditions.
Cardiometabolic Disorders
Possibly the hottest topic in psoriasis for some years now, the relationship between cardiometabolic disorders and psoriasis is of great interest to clinicians, scientists, and patients alike. There is a clear link between development of atherosclerosis and Th17-related immune mechanisms that also are implicated in the pathogenesis of psoriasis.3 Furthermore, the incidence of cardiovascular disease is markedly increased in patients with psoriasis, which is an independent risk factor for myocardial infarction, particularly among younger patients.4,5 Although several retrospective studies6-8 have shown that TNF-α inhibitors are associated with a reduction in cardiovascular outcomes, it is yet to be seen whether biologic treatment actually has a direct impact on cardiovascular outcomes, multiple studies investigating the effect of biologics on arterial inflammation markers notwithstanding.9
There are some direct factors to keep in mind when considering cardiometabolic comorbidities in patients with psoriasis. Obesity is common in the psoriasis population and can have a direct negative effect on cardiovascular health.10 However, the data on obesity and psoriasis are somewhat mixed with regard to treatment outcomes. In general, with increased volumes of distribution for biologics in patients with obesity, it has been shown that treatment success is more difficult to achieve in those with a body mass index greater than 30.11 Rather surprisingly, a separate nationwide study in South Korea found that patients on biologics for psoriasis were more likely to experience weight gain, even after controlling for factors such as exercise, smoking, and drinking,12 but it is unclear whether this is driven mostly by a known connection between weight gain and TNF-α inhibitors.13 These contrasting results point to the need for further studies in this area, as our intuitive approach would involve promoting weight loss while starting on a systemic treatment for psoriasis—but perhaps it is important not to assume that one will come with the other in tow, reinforcing the need to discuss a healthy diet with our patients with psoriasis regardless of treatment decisions.
The data that we have do not directly answer the big questions about biologic treatment and cardiovascular health, but we are starting to see interesting signals. For example, in a report of tildrakizumab treatment in patients with and without metabolic syndrome, the rates of major adverse cardiovascular events as well as cardiac disorders were essentially the same in both groups after receiving treatment for up to 244 weeks.14 This is interesting, more because of the lack of an increase in cardiovascular adverse events in the metabolic syndrome group, who entered the trial on average 25 kg to 30 kg heavier than those without metabolic syndrome. There is an increased risk for adverse cardiovascular events among patients with metabolic syndrome, a roughly 2-fold relative risk in as few as 5 to 6 years of follow-up.15 While the cohorts in the tildrakizumab study14 were too small to draw firm conclusions, the data are interesting and a step in the right direction; we need much larger data sets for analysis. Among other agents, similar efficacy and safety have been reported for guselkumab in a long-term psoriasis study; as a class, IL-23 inhibitors also tend to perform well from an efficacy standpoint in patients with obesity.16
Overall, when assessing the evidence for cardiometabolic disorders, it is reasonable to consider starting a biologic from the IL-17 or IL-23 inhibitor classes— thus avoiding both the potential downside of weight gain and contraindication in patients with congestive heart failure associated with TNF-α inhibitors. It is important to counsel patients about weight loss in conjunction with these treatments, both to improve efficacy and reduce cardiovascular risk factors. There may be a preference for IL-23 inhibitors in patients with obesity, as this class of medications maintains efficacy particularly well in these patients. Patients with psoriasis should be counseled to follow up with a primary care physician given their higher risk for metabolic syndrome and adverse cardiovascular outcomes.
Gastrointestinal Conditions
Psoriasis and inflammatory bowel disease (IBD) have a bidirectional association, and patients with psoriasis are about 1.7 times more likely to have either Crohn disease or ulcerative colitis.17,18 This association may be related to a shared pathogenesis with regard to immune dysregulation and overactivated inflammatory pathways, but there are some important differences to consider from a therapeutic standpoint. Given the increased expression of IL-17 in patients with IBD,19 a phase II trial of secukinumab yielded surprising results—not only was secukinumab ineffective in treating Crohn disease, but there also were higher rates of adverse events20 (as noted on the product label for all IL-17 inhibitors). We have come to understand that there are regulatory subsets of IL-17 cells that are important in mucosal homeostasis and also regulate IL-10, which generally is considered an anti-inflammatory cytokine.21 Thus, while IL-17 inhibition can reduce some component of inflammatory signaling, it also can increase inflammatory signaling through indirect pathways while increasing intestinal permeability to microbes. Importantly, this process seems to occur via IL-23–independent pathways; as such, while direct inhibition of IL-17 can be deleterious, IL-23 inhibitors have become important therapeutics for IBD.22
IL-17 family, IL-17A clearly is the culprit for worsening colitis as evidenced by both human and animal models. On the contrary, IL-17F blockade has been shown to ameliorate colitis in a murine model, whereas IL-17A inhibition worsens it.23 Furthermore, dual blockade of IL-17A and IL-17F has a protective effect against colitis, suggesting that the IL-17F inhibition is dominant. This interesting finding has some mechanistic backing, since blockade of IL-17F induces Treg cells that serve to maintain gut epithelium homeostasis and integrity.24
Overall, IL-17A inhibitors should be avoided in patients with a history of IBD—namely, secukinumab and ixekizumab. While there may be theoretical reasons that brodalumab or bimekizumab may confer a somewhat different risk for IBD exacerbation, there may be better choices that would be expected to effectively treat both the psoriasis and IBD manifestations. Given the US Food and Drug Administration approval of IL-23 inhibitors for IBD and their high levels of efficacy in treating psoriasis, these likely will be the best choice for most patients. Another mainstay of IBD treatment is TNF-α inhibitors, but they come with other risks such as increased immunosuppression and increased risk for nonmelanoma skin cancer.
An important question remains: What about patients who do not have known IBD? Do we proactively change our treatment choice due to fear of IBD development given the higher incidence of both Crohn disease and ulcerative colitis in patients with psoriasis? What about patients with a family history of IBD? First-degree relatives of patients with Crohn disease and ulcerative colitis have an 8- and 4-fold higher risk for those same conditions, respectively.25 Postmarketing surveillance and database findings of low rates of IBD development with IL-17 inhibitors gives only modest reassurance, as dermatologists generally know to avoid these medications for patients with even questionable IBD symptoms. It is important to emphasize to our patients that in no case do we believe that a psoriasis medication actually will cause IBD—rather, someone with subclinical IBD could experience a flare and a first manifestation of colitis. The drug is not the culprit in inducing IBD but rather may serve to unmask existing disease.
One study suggested that for patients who move on to the IL-17 inhibitor secukinumab after being treated with TNF-α inhibitors for psoriasis, the rates of IBD development are higher (4.8%) than in those who start IL-17A inhibition without prior treatment (1%)(OR, 8.38; P=.018).26 This begs the question of whether subclinical IBD in many patients with psoriasis who are treated with TNF-α inhibitors can be unmasked later when they are transitioned to a treatment that either does not treat the IBD or could worsen it. There may be a mechanistic drive behind this sequencing of treatments that predisposes patients to colitis, which would suggest selecting an IL-23 inhibitor after failing/trying a TNF-α inhibitor. However, the data are very preliminary, and in real practice, other concerns such as severe psoriatic arthritis may outweigh these considerations, as the IL-17 inhibitor class still is considered to be more effective than IL-23 inhibition at treating psoriatic arthritis overall. For most patients with no personal history of IBD and no strong family history of IBD (ie, first-degree relatives), the choice of biologic should not be affected by concern over gastrointestinal issues.
Psychiatric Conditions
It has been well established that psoriasis is linked to higher rates of depression, anxiety, and suicidality.27 How do we take this into account when treating patients with psoriasis, especially when we have biologics with a warning label for suicidality and a Risk Evaluation and Mitigation Strategies program (brodalumab) and language around suicidal ideation in the label (bimekizumab)? While it is challenging to discuss mental health, it is not a conversation that we as dermatologists should shy away from. Appropriate treatment of psoriasis is an important tool to get our patients on the path to better mental health. A recent database study of more than 4000 patients showed that patients with psoriasis treated with biologics had a 17% lower risk for depression than those treated with conventional disease-modifying drugs such as methotrexate.28 The comparator of the conventional disease-modifying drug class is important as it serves as a control for disease severity. Too often, a higher rate of depression, anxiety, or suicidality can be attributed to a medication when we in fact may just be capturing the background of higher incidence of all 3 in patients with severe psoriasis.
Indeed, even with the medication that many worry about on this front (brodalumab), multiple studies have confirmed that the effect on mental health generally is a positive one, with decreases in depressive symptoms.29 In a cohort switched from TNF-α inhibitors to brodalumab, symptoms of depression actually improved,30 so attributing a direct treatment effect to negative mental health outcomes does not seem to be justified, especially in light of the low number of suicide events in global postmarketing surveillance for brodalumab, comparable to or lower than other biologics for psoriasis.31 Similarly, bimekizumab has language in the label about discussing suicidality with patients, although the rates of suicidal ideation and behavior are no different from other biologics and rates of depression improved with its use.32
Heightened awareness of our patients’ mental health is something that we as providers should embrace, even when it seems that we do not have much time to see each patient. The priority when a patient comes in with mental health symptoms should be to treat what is within our scope (ie, psoriasis) as quickly and effectively as possible— with a newer-generation biologic such as an IL-17 or IL-23 inhibitor—while encouraging the patient to seek care from a mental health professional. In these cases, one might even argue that the rapidity of action of IL-17 inhibitors may be of additional benefit.
Final Thoughts
We as dermatologists generally are tasked with seeing high volumes of patients, and an initial psoriasis consultation can be a lengthy visit; however, it is rewarding to establish this relationship with patients and a reminder of why we practice medicine to begin with. Psoriasis can be satisfying to treat, and we have so many highly effective medicines that can completely transform our patients’ lives. Applying an understanding of the interplay between psoriasis, its related comorbidities, and treatment choices can be a fulfilling exercise that captures the essence of shared decision-making, which can lead to better outcomes and satisfaction for both providers and patients.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022. doi:10.1056/NEJMoa030409
- Thatiparthi A, Martin A, Liu J, et al. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22:425-442. doi:10.1007/s40257-021-00603-w
- Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5-22. doi:10.1007 /s00281-009-0153-8
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. doi:10.1001/jama.296.14.1735
- Miller IM, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014-1024. doi:10.1016/j.jaad.2013.06.053
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90. doi:10.1016/j.jaad.2016.07.042
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250. doi:10.1001 /archdermatol.2012.2502
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68. doi:10.1016/j.jaad.2018.02.050
- Cai J, Cui L, Wang Y, et al. Cardiometabolic comorbidities in patients with psoriasis: focusing on risk, biological therapy, and pathogenesis. Front Pharmacol. 2021;12:774808. doi:10.3389/fphar.2021.774808
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:E984-E1010. doi:10.1161/CIR.0000000000000973
- Pirro F, Caldarola G, Chiricozzi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig. 2021;41:917-925. doi:10.1007 /s40261-021-01080-z
- Kim H, Hong JY, Cheong S, et al. Impact of biologic agents on body weight and obesity-related disorders in patients with psoriasis: a nationwide population-based cohort study. Obes Res Clin Pract. 2023;17:210-217. doi:10.1016/j.orcp.2023.05.004
- Saraceno R, Schipani C, Mazzotta A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57:290-295. doi:10.1016/j.phrs.2008.02.006
- Fernandez AP, Dauden E, Gerdes S, et al. Tildrakizumab efficacy and safety in patients with psoriasis and concomitant metabolic syndrome: post hoc analysis of 5-year data from reSURFACE 1 and reSURFACE 2. J Eur Acad Dermatol Venereol. 2022;36:1774-1783. doi:10.1111/jdv.18167
- Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. doi:10.1016/j.jacc.2010.05.034
- Ricceri F, Chiricozzi A, Peris K, et al. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: a multicentric retrospective study. Dermatol Ther. 2022;35:E15793. doi:10.1111/dth.15793
- Alinaghi F, Tekin HG, Burisch J, et al. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease— a systematic review and meta-analysis. J Crohns Colitis. 2020;14:351-360. doi:10.1093/ecco-jcc/jjz152
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. doi:10.1136/gut.52.1.65
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebocontrolled trial. Gut. 2012;61:1693-1700. doi:10.1136 /gutjnl-2011-301668
- Brockmann L, Tran A, Huang Y, et al. Intestinal microbiotaspecific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. 2023;56:2719-2735 e7. doi:10.1016/j.immuni.2023.11.003
- Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727-738. doi:10.1016/j.immuni.2015.09.003
- Wedebye Schmidt EG, Larsen HL, Kristensen NN, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19:1567-1576. doi:10.1097 /MIB.0b013e318286fa1c
- Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing T(reg) cells through modification of the intestinal microbiota. Nat Immunol. 2018;19:755-765. doi:10.1038/s41590-018-0134-y
- El Hadad J, Schreiner P, Vavricka SR, Greuter T. The genetics of inflammatory bowel disease. Mol Diagn Ther. 2024;28:27-35. doi:10.1007 /s40291-023-00678-7
- Albayrak F, Gür M, Karatas¸ A, et al. Is the use of secukinumab after anti-TNF therapy greater than expected for the risk of developing inflammatory bowel disease? Reumatol Clin (Engl Ed). 2024;20:123-127. doi:10.1016/j.reumae.2023.11.002
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Strober B, Soliman AM, Truong B, et al. Association between biologic exposure and the risk of depression in patients with psoriasis: a retrospective analysis of large US administrative claims data. Am J Clin Dermatol. 2024;25:853-856. doi:10.1007/s40257 -024-00877-w
- Koo J, Ho RS, Thibodeaux Q. Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review. Cutis. 2019;104:361-365.
- Andersch-Bjorkman Y, Micu E, Seifert O, et al. Effects of brodalumab on psoriasis and depressive symptoms in patients with insufficient response to TNF-alpha inhibitors. J Dermatol. 2023;50:1401-1414. doi:10.1111/1346-8138.16917
- Yeroushalmi S, Chung M, Bartholomew E, et al. Examining worldwide postmarketing suicides from biologics used for psoriasis with a focus on brodalumab: a cross-sectional analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS). JAAD Int. 2022;9:119-121. doi:10.1016/j.jdin.2022.08.010
- Blauvelt A, Armstrong A, Merola JF, et al. Mental health outcomes in patients with moderate to severe psoriasis treated with bimekizumab: analysis of phase 2/3 randomized trials. J Am Acad Dermatol. 2024;91:72-81. doi:10.1016/j.jaad.2024.02.039
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022. doi:10.1056/NEJMoa030409
- Thatiparthi A, Martin A, Liu J, et al. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22:425-442. doi:10.1007/s40257-021-00603-w
- Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5-22. doi:10.1007 /s00281-009-0153-8
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. doi:10.1001/jama.296.14.1735
- Miller IM, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014-1024. doi:10.1016/j.jaad.2013.06.053
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90. doi:10.1016/j.jaad.2016.07.042
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250. doi:10.1001 /archdermatol.2012.2502
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68. doi:10.1016/j.jaad.2018.02.050
- Cai J, Cui L, Wang Y, et al. Cardiometabolic comorbidities in patients with psoriasis: focusing on risk, biological therapy, and pathogenesis. Front Pharmacol. 2021;12:774808. doi:10.3389/fphar.2021.774808
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:E984-E1010. doi:10.1161/CIR.0000000000000973
- Pirro F, Caldarola G, Chiricozzi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig. 2021;41:917-925. doi:10.1007 /s40261-021-01080-z
- Kim H, Hong JY, Cheong S, et al. Impact of biologic agents on body weight and obesity-related disorders in patients with psoriasis: a nationwide population-based cohort study. Obes Res Clin Pract. 2023;17:210-217. doi:10.1016/j.orcp.2023.05.004
- Saraceno R, Schipani C, Mazzotta A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57:290-295. doi:10.1016/j.phrs.2008.02.006
- Fernandez AP, Dauden E, Gerdes S, et al. Tildrakizumab efficacy and safety in patients with psoriasis and concomitant metabolic syndrome: post hoc analysis of 5-year data from reSURFACE 1 and reSURFACE 2. J Eur Acad Dermatol Venereol. 2022;36:1774-1783. doi:10.1111/jdv.18167
- Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. doi:10.1016/j.jacc.2010.05.034
- Ricceri F, Chiricozzi A, Peris K, et al. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: a multicentric retrospective study. Dermatol Ther. 2022;35:E15793. doi:10.1111/dth.15793
- Alinaghi F, Tekin HG, Burisch J, et al. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease— a systematic review and meta-analysis. J Crohns Colitis. 2020;14:351-360. doi:10.1093/ecco-jcc/jjz152
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. doi:10.1136/gut.52.1.65
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebocontrolled trial. Gut. 2012;61:1693-1700. doi:10.1136 /gutjnl-2011-301668
- Brockmann L, Tran A, Huang Y, et al. Intestinal microbiotaspecific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. 2023;56:2719-2735 e7. doi:10.1016/j.immuni.2023.11.003
- Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727-738. doi:10.1016/j.immuni.2015.09.003
- Wedebye Schmidt EG, Larsen HL, Kristensen NN, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19:1567-1576. doi:10.1097 /MIB.0b013e318286fa1c
- Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing T(reg) cells through modification of the intestinal microbiota. Nat Immunol. 2018;19:755-765. doi:10.1038/s41590-018-0134-y
- El Hadad J, Schreiner P, Vavricka SR, Greuter T. The genetics of inflammatory bowel disease. Mol Diagn Ther. 2024;28:27-35. doi:10.1007 /s40291-023-00678-7
- Albayrak F, Gür M, Karatas¸ A, et al. Is the use of secukinumab after anti-TNF therapy greater than expected for the risk of developing inflammatory bowel disease? Reumatol Clin (Engl Ed). 2024;20:123-127. doi:10.1016/j.reumae.2023.11.002
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Strober B, Soliman AM, Truong B, et al. Association between biologic exposure and the risk of depression in patients with psoriasis: a retrospective analysis of large US administrative claims data. Am J Clin Dermatol. 2024;25:853-856. doi:10.1007/s40257 -024-00877-w
- Koo J, Ho RS, Thibodeaux Q. Depression and suicidality in psoriasis and clinical studies of brodalumab: a narrative review. Cutis. 2019;104:361-365.
- Andersch-Bjorkman Y, Micu E, Seifert O, et al. Effects of brodalumab on psoriasis and depressive symptoms in patients with insufficient response to TNF-alpha inhibitors. J Dermatol. 2023;50:1401-1414. doi:10.1111/1346-8138.16917
- Yeroushalmi S, Chung M, Bartholomew E, et al. Examining worldwide postmarketing suicides from biologics used for psoriasis with a focus on brodalumab: a cross-sectional analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS). JAAD Int. 2022;9:119-121. doi:10.1016/j.jdin.2022.08.010
- Blauvelt A, Armstrong A, Merola JF, et al. Mental health outcomes in patients with moderate to severe psoriasis treated with bimekizumab: analysis of phase 2/3 randomized trials. J Am Acad Dermatol. 2024;91:72-81. doi:10.1016/j.jaad.2024.02.039
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
The Post-PASI Era: Considering Comorbidities to Select Appropriate Systemic Psoriasis Treatments
Expanding the Psoriasis Framework: Immunopathogenesis and Treatment Updates
Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.
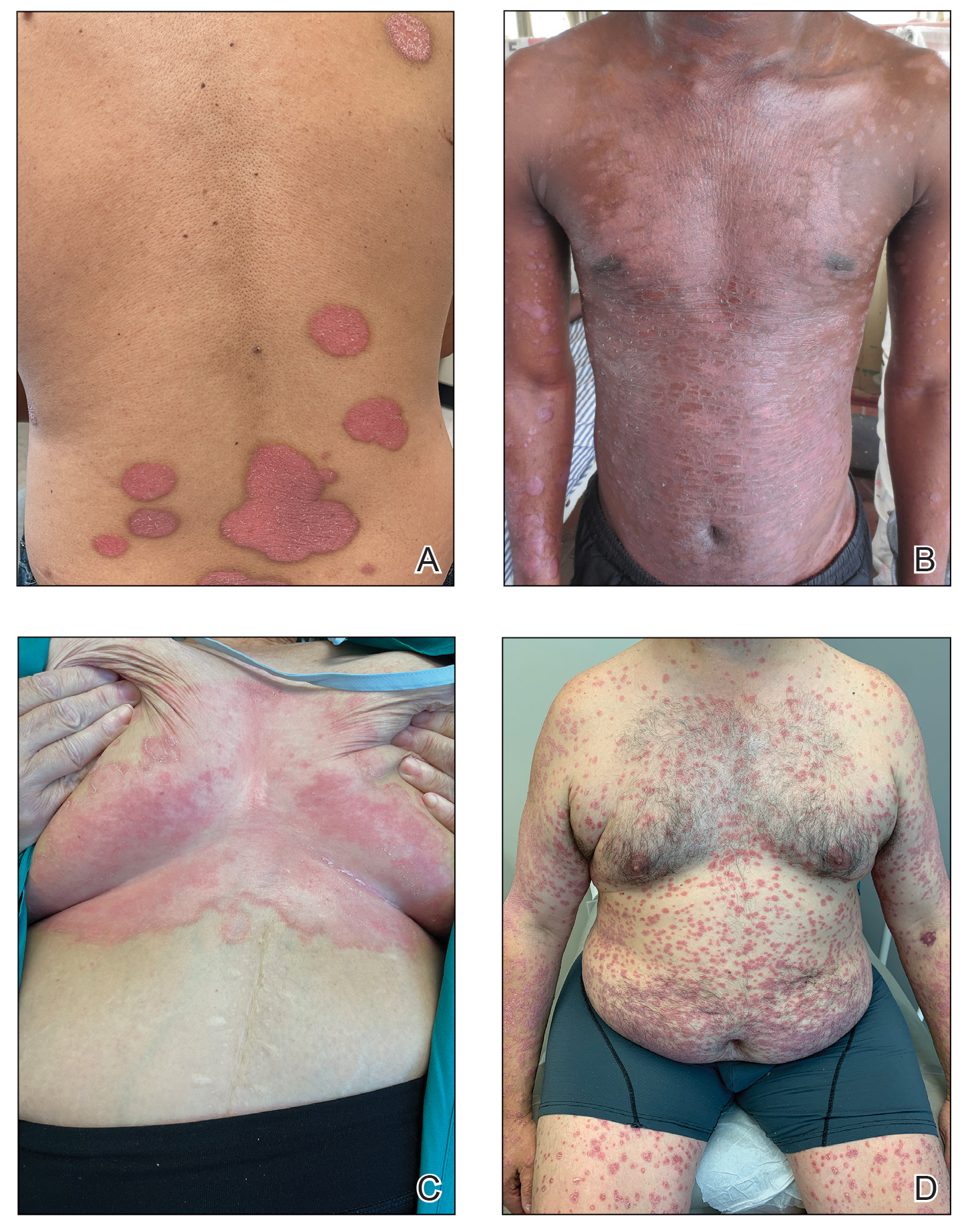
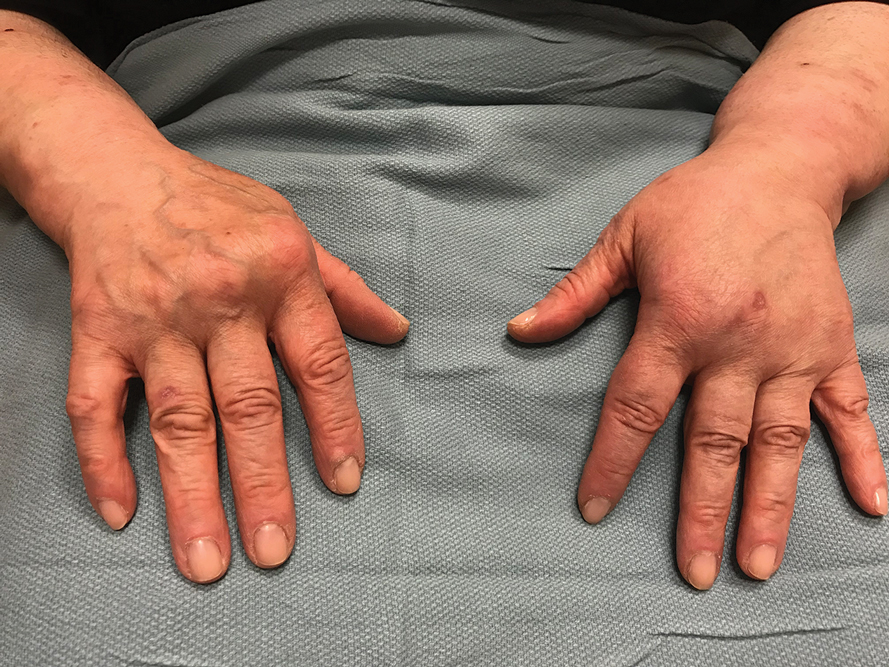
A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.
The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
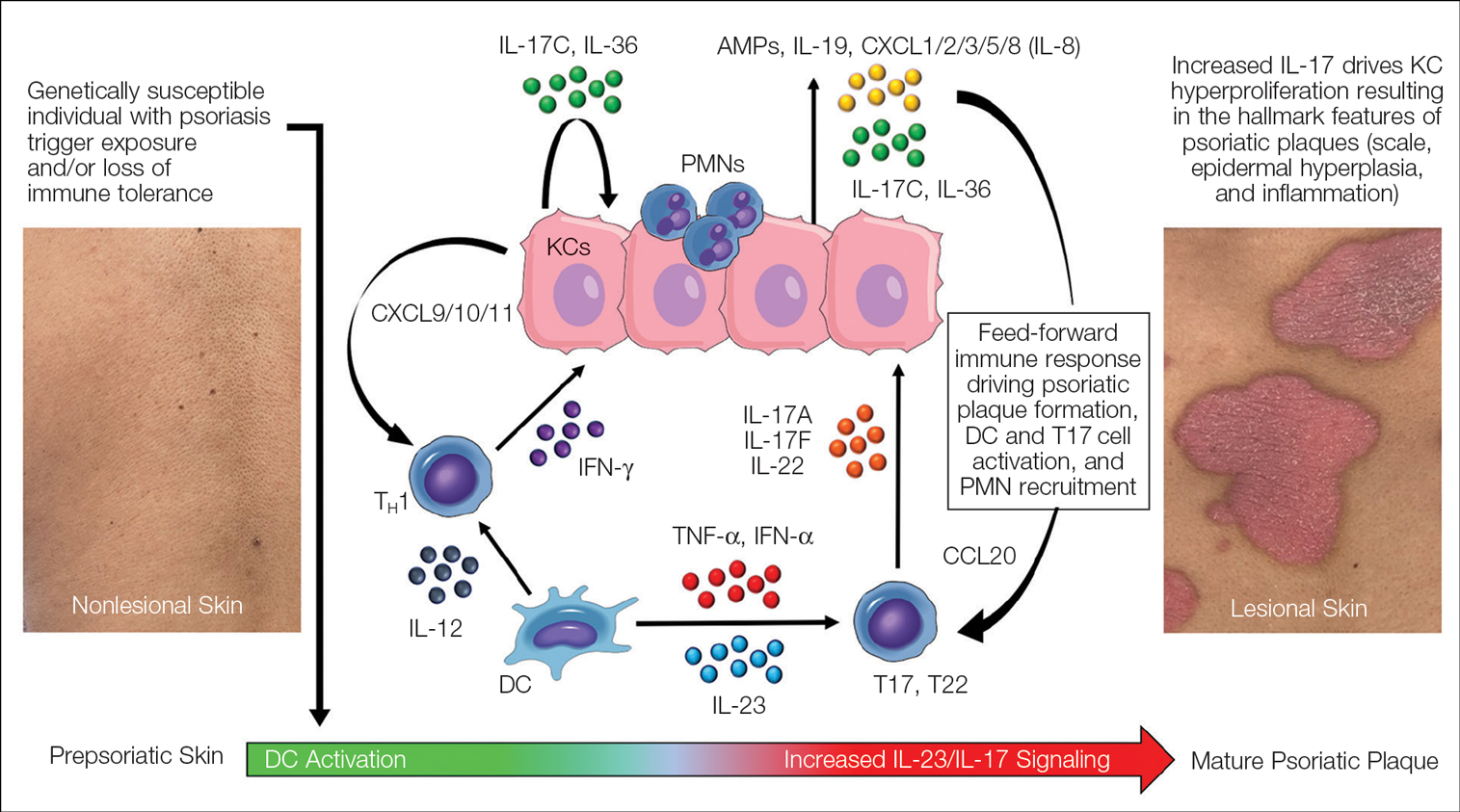
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).
Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.
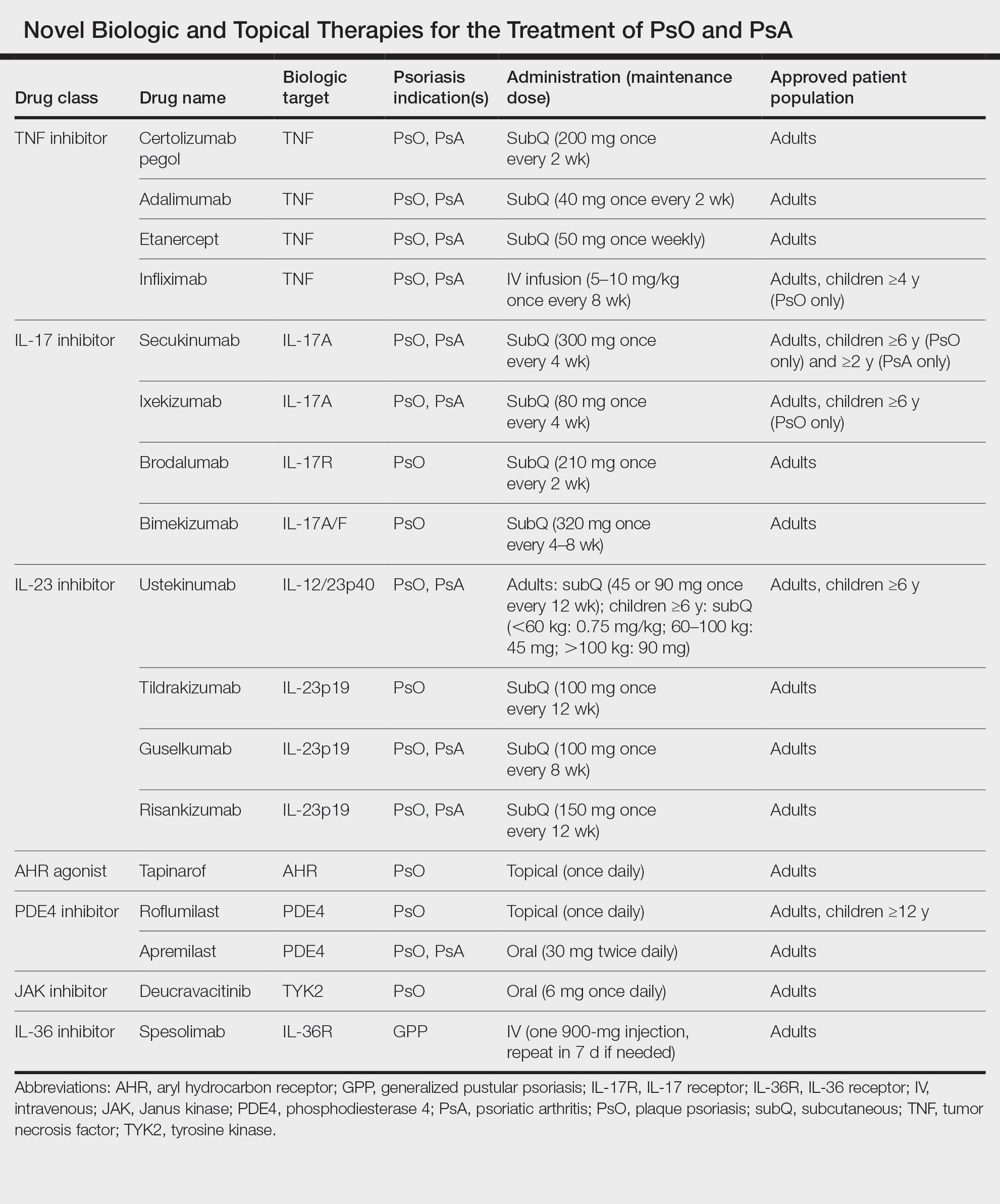
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.

A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.

The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).

Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.

Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.

A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.

The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).

Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.

- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
Practice Points
- Psoriasis is a chronic inflammatory condition characterized by systemic inflammation and dysregulated IL-23/IL-17 signaling.
- Modern discoveries highlight the role of additional immune signals in psoriatic disease such as IL-17C, IL-17F, IL-36, and tyrosine kinase 2, which also contribute to disease development.
- Novel systemic, oral, and topical therapies have become available and add to the rapidly growing armamentarium of safe and effective treatments for psoriatic disease.
Teledermatology: A Postpandemic Update
The rapid expansion of teledermatology in the United States due to the COVID-19 pandemic has been well documented, 1 but where do we stand now that health care and society as a whole are back to a new version of normal? It is important to consider why telemedicine was able to grow so quickly during that period—the Centers for Medicare & Medicaid Services (CMS) unilaterally changed policies related to provision of services and reimbursement thereof due to the public health emergency (PHE), which was declared by the Department of Health and Human Services in January 2020 to provide increased access to care for patients. Under the PHE, reimbursement rates for virtual visits improved, providers could care for patients from their homes and across state lines, and the use of video platforms that were not Health Insurance Portability and Accountability Act compliant was allowed. 2,3
The trajectory of teledermatology after the pandemic, however, remains unclear. In a survey assessing dermatologists’ perceptions of telemedicine (N=4356), 97% used telemedicine during the pandemic but only 58% reported that they intended to continue using teledermatology postpandemic,1 which is driven, at least in part, by the potential concern that dermatologists will again experience the same regulatory and logistical barriers that limited teledermatology utilization prepandemic.
What has changed in reimbursement for teledermatology since the PHE ended?
The PHE ended on May 11, 2023, and already video platforms that were used during the pandemic to provide telemedicine visits but are not Health Insurance Portability and Accountability Act compliant are now forbidden,2 Medicare virtual check-in appointments can only be conducted with established patients,4 and medical licensing requirements have been reinstated in most states such that patients must be located in the state where the provider is licensed to practice medicine at the time of a virtual visit.3 Although the CMS was granted wide freedoms to waive and suspend certain rules, this was only in the context of the PHE, and any lasting changes must be established by Congress.
Reassuringly, recent legislation via the Consolidated Appropriations Act, 2023, authorized an extension of many of the CMS telehealth flexibilities that were in place during the PHE through December 31, 2024 (Table),2 such as allowing access to telehealth services in any geographic area in the United States rather than only rural areas, allowing patients to stay in their homes for telehealth visits rather than traveling to an approved health care facility, and allowing the delivery of telemedicine via audio-only technology if a patient is unable to use both audio and video. As of now, the place of service (POS) designation for telehealth visits will not revert back to the former code (POS 02) but will remain at POS 11 with the telehealth modifier -95 so physicians will be reimbursed at the full level of a non-facility physician’s office rate.4 The CMS has indicated that there will be no change in the reimbursement policy until after December 31, 20234; however, the sense of uncertainty around what happens after this date has made it hard for organizations and practices to fully commit to teledermatology services without knowing what the long-term financial impact may be. Some organizations have already noted that they plan to continue supporting telemedicine after the CMS flexibilities expire. Accountable Care Organizations have the ability to offer services that allow participating practitioners to continue the use of telemedicine visits to expand access to care. Medicaid and Children’s Health Insurance Program policies vary by state and private health insurance policies vary by individual plans, but it should be noted that commercial coverage for telemedicine visits was already strong prior to the pandemic.2
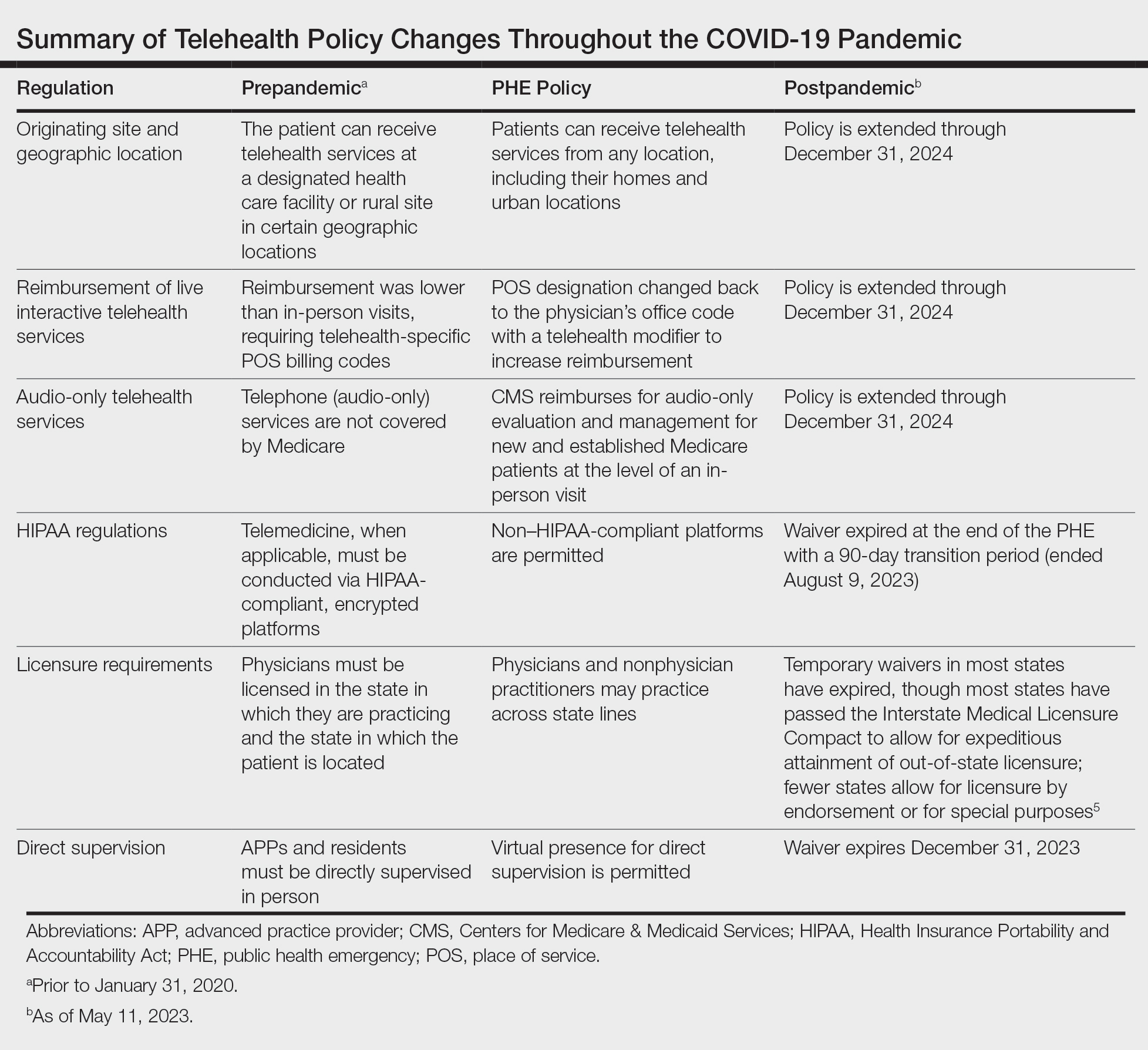
What medical licensing requirements are in place now for telehealth?
During the PHE, medical licensing requirements also were relaxed, enabling providers to deliver telemedicine service in states where they were not licensed.3 As the PHE orders ended, some states including New York discontinued cross-state licensing waivers altogether,6 whereas others have enacted legislation to make them permanent or extend them for brief periods of time.3,6 One potential solution is the Interstate Medical Licensure Compact (https://www.imlcc.org/), which includes 39 states as of October 2023. This program expedites the process for physicians already licensed in participating states to obtain their medical license in another participating state, though licensing fees are required for each state in which a physician wants to practice. Furthermore, some states such as North Dakota, Hawaii, and Virginia have licensure by endorsement policies, which enable licensed physicians with specific qualifications to provide telehealth services in the endorsing state. Other states such as Florida, New Jersey, Louisiana, Minnesota, Nevada, and New Mexico have special telehealth registries that allow physicians in good standing who are licensed in other states to deliver telehealth services to in-state residents barring they do not provide in-person, in-state services.6 Lastly, some states have temporary practice laws to allow existing patients who need medical attention while traveling out of state to see their home providers virtually or in person under certain circumstances for a limited period of time.3,5 In Hawaii and New Hampshire, physicians with out-of-state licenses can provide consultative services in some circumstances.5
What changes have been made to make it easier for patients to use telehealth?
As the legislation around telemedicine is shifting postpandemic, it is important to address additional logistical barriers to teledermatology on a larger scale if the discipline is to stay in practice. On November 15, 2021, the Infrastructure Investment and Jobs Act provided $65 billion in funding for broadband to expand access to high-speed internet. Some of this money was allocated to the Affordable Connectivity Program, which provides eligible households with a discount on broadband service and internet-connected devices. Eligible patrons can qualify for a discount of up to $75 per month for internet service and a one-time discount up to $100 on a laptop, desktop computer, or tablet purchased through a participating provider.6 Although a step in the right direction, the effects of this program on telemedicine encounters remains to be proven. Additionally, these programs do not address educational barriers to understanding how to utilize telemedicine platforms or provide incentives for practitioners to offer telemedicine services.
Final Thoughts
The pandemic taught our specialty a great deal about how to utilize telemedicine. For many dermatologists a return to in-person business as usual could not come fast enough; however, many practices have continued to offer at least some teledermatology services. Although the PHE waivers have ended, the extension of numerous CMS flexibilities through the end of 2024 allows us more time to develop sustainable policies to support the long-term health of telemedicine as a whole, both to sustain practices and to expand access to care in dermatology. The favorable attitudes of both patients and physicians about teledermatology have been clearly documented,1,7 and we should continue to safely expand the use of this technology.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- US Department of Health and Human Services. HHS fact sheet: telehealth flexibilities and resources and the COVID-19 public health emergency. Published May 10, 2023. Accessed October 18, 2023. https://www.hhs.gov/aboutnews/2023/05/10/hhs-fact-sheet-telehealth-flexibilities-resources-covid-19-public-health-emergency.html
- US Department of Health and Human Services. Licensing across state lines. Updated May 11, 2023. Accessed October 25, 2023. https://telehealth.hhs.gov/licensure/licensing-across-state-lines
- American Academy of Dermatology. Teledermatology and the COVID-19 pandemic. Accessed October 12, 2023. https://www.aad.org/member/practice/telederm/covid-19
- American Medical Association. Licensure & Telehealth. Accessed October 12, 2023. https://www.ama-assn.org/system/files/issue-brief-licensure-telehealth.pdf
- Federal Communications Commission. Affordable Connectivity Program. Updated June 29, 2023. Accessed October 12, 2023. https://www.fcc.gov/affordable-connectivity-program
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
The rapid expansion of teledermatology in the United States due to the COVID-19 pandemic has been well documented, 1 but where do we stand now that health care and society as a whole are back to a new version of normal? It is important to consider why telemedicine was able to grow so quickly during that period—the Centers for Medicare & Medicaid Services (CMS) unilaterally changed policies related to provision of services and reimbursement thereof due to the public health emergency (PHE), which was declared by the Department of Health and Human Services in January 2020 to provide increased access to care for patients. Under the PHE, reimbursement rates for virtual visits improved, providers could care for patients from their homes and across state lines, and the use of video platforms that were not Health Insurance Portability and Accountability Act compliant was allowed. 2,3
The trajectory of teledermatology after the pandemic, however, remains unclear. In a survey assessing dermatologists’ perceptions of telemedicine (N=4356), 97% used telemedicine during the pandemic but only 58% reported that they intended to continue using teledermatology postpandemic,1 which is driven, at least in part, by the potential concern that dermatologists will again experience the same regulatory and logistical barriers that limited teledermatology utilization prepandemic.
What has changed in reimbursement for teledermatology since the PHE ended?
The PHE ended on May 11, 2023, and already video platforms that were used during the pandemic to provide telemedicine visits but are not Health Insurance Portability and Accountability Act compliant are now forbidden,2 Medicare virtual check-in appointments can only be conducted with established patients,4 and medical licensing requirements have been reinstated in most states such that patients must be located in the state where the provider is licensed to practice medicine at the time of a virtual visit.3 Although the CMS was granted wide freedoms to waive and suspend certain rules, this was only in the context of the PHE, and any lasting changes must be established by Congress.
Reassuringly, recent legislation via the Consolidated Appropriations Act, 2023, authorized an extension of many of the CMS telehealth flexibilities that were in place during the PHE through December 31, 2024 (Table),2 such as allowing access to telehealth services in any geographic area in the United States rather than only rural areas, allowing patients to stay in their homes for telehealth visits rather than traveling to an approved health care facility, and allowing the delivery of telemedicine via audio-only technology if a patient is unable to use both audio and video. As of now, the place of service (POS) designation for telehealth visits will not revert back to the former code (POS 02) but will remain at POS 11 with the telehealth modifier -95 so physicians will be reimbursed at the full level of a non-facility physician’s office rate.4 The CMS has indicated that there will be no change in the reimbursement policy until after December 31, 20234; however, the sense of uncertainty around what happens after this date has made it hard for organizations and practices to fully commit to teledermatology services without knowing what the long-term financial impact may be. Some organizations have already noted that they plan to continue supporting telemedicine after the CMS flexibilities expire. Accountable Care Organizations have the ability to offer services that allow participating practitioners to continue the use of telemedicine visits to expand access to care. Medicaid and Children’s Health Insurance Program policies vary by state and private health insurance policies vary by individual plans, but it should be noted that commercial coverage for telemedicine visits was already strong prior to the pandemic.2

What medical licensing requirements are in place now for telehealth?
During the PHE, medical licensing requirements also were relaxed, enabling providers to deliver telemedicine service in states where they were not licensed.3 As the PHE orders ended, some states including New York discontinued cross-state licensing waivers altogether,6 whereas others have enacted legislation to make them permanent or extend them for brief periods of time.3,6 One potential solution is the Interstate Medical Licensure Compact (https://www.imlcc.org/), which includes 39 states as of October 2023. This program expedites the process for physicians already licensed in participating states to obtain their medical license in another participating state, though licensing fees are required for each state in which a physician wants to practice. Furthermore, some states such as North Dakota, Hawaii, and Virginia have licensure by endorsement policies, which enable licensed physicians with specific qualifications to provide telehealth services in the endorsing state. Other states such as Florida, New Jersey, Louisiana, Minnesota, Nevada, and New Mexico have special telehealth registries that allow physicians in good standing who are licensed in other states to deliver telehealth services to in-state residents barring they do not provide in-person, in-state services.6 Lastly, some states have temporary practice laws to allow existing patients who need medical attention while traveling out of state to see their home providers virtually or in person under certain circumstances for a limited period of time.3,5 In Hawaii and New Hampshire, physicians with out-of-state licenses can provide consultative services in some circumstances.5
What changes have been made to make it easier for patients to use telehealth?
As the legislation around telemedicine is shifting postpandemic, it is important to address additional logistical barriers to teledermatology on a larger scale if the discipline is to stay in practice. On November 15, 2021, the Infrastructure Investment and Jobs Act provided $65 billion in funding for broadband to expand access to high-speed internet. Some of this money was allocated to the Affordable Connectivity Program, which provides eligible households with a discount on broadband service and internet-connected devices. Eligible patrons can qualify for a discount of up to $75 per month for internet service and a one-time discount up to $100 on a laptop, desktop computer, or tablet purchased through a participating provider.6 Although a step in the right direction, the effects of this program on telemedicine encounters remains to be proven. Additionally, these programs do not address educational barriers to understanding how to utilize telemedicine platforms or provide incentives for practitioners to offer telemedicine services.
Final Thoughts
The pandemic taught our specialty a great deal about how to utilize telemedicine. For many dermatologists a return to in-person business as usual could not come fast enough; however, many practices have continued to offer at least some teledermatology services. Although the PHE waivers have ended, the extension of numerous CMS flexibilities through the end of 2024 allows us more time to develop sustainable policies to support the long-term health of telemedicine as a whole, both to sustain practices and to expand access to care in dermatology. The favorable attitudes of both patients and physicians about teledermatology have been clearly documented,1,7 and we should continue to safely expand the use of this technology.
The rapid expansion of teledermatology in the United States due to the COVID-19 pandemic has been well documented, 1 but where do we stand now that health care and society as a whole are back to a new version of normal? It is important to consider why telemedicine was able to grow so quickly during that period—the Centers for Medicare & Medicaid Services (CMS) unilaterally changed policies related to provision of services and reimbursement thereof due to the public health emergency (PHE), which was declared by the Department of Health and Human Services in January 2020 to provide increased access to care for patients. Under the PHE, reimbursement rates for virtual visits improved, providers could care for patients from their homes and across state lines, and the use of video platforms that were not Health Insurance Portability and Accountability Act compliant was allowed. 2,3
The trajectory of teledermatology after the pandemic, however, remains unclear. In a survey assessing dermatologists’ perceptions of telemedicine (N=4356), 97% used telemedicine during the pandemic but only 58% reported that they intended to continue using teledermatology postpandemic,1 which is driven, at least in part, by the potential concern that dermatologists will again experience the same regulatory and logistical barriers that limited teledermatology utilization prepandemic.
What has changed in reimbursement for teledermatology since the PHE ended?
The PHE ended on May 11, 2023, and already video platforms that were used during the pandemic to provide telemedicine visits but are not Health Insurance Portability and Accountability Act compliant are now forbidden,2 Medicare virtual check-in appointments can only be conducted with established patients,4 and medical licensing requirements have been reinstated in most states such that patients must be located in the state where the provider is licensed to practice medicine at the time of a virtual visit.3 Although the CMS was granted wide freedoms to waive and suspend certain rules, this was only in the context of the PHE, and any lasting changes must be established by Congress.
Reassuringly, recent legislation via the Consolidated Appropriations Act, 2023, authorized an extension of many of the CMS telehealth flexibilities that were in place during the PHE through December 31, 2024 (Table),2 such as allowing access to telehealth services in any geographic area in the United States rather than only rural areas, allowing patients to stay in their homes for telehealth visits rather than traveling to an approved health care facility, and allowing the delivery of telemedicine via audio-only technology if a patient is unable to use both audio and video. As of now, the place of service (POS) designation for telehealth visits will not revert back to the former code (POS 02) but will remain at POS 11 with the telehealth modifier -95 so physicians will be reimbursed at the full level of a non-facility physician’s office rate.4 The CMS has indicated that there will be no change in the reimbursement policy until after December 31, 20234; however, the sense of uncertainty around what happens after this date has made it hard for organizations and practices to fully commit to teledermatology services without knowing what the long-term financial impact may be. Some organizations have already noted that they plan to continue supporting telemedicine after the CMS flexibilities expire. Accountable Care Organizations have the ability to offer services that allow participating practitioners to continue the use of telemedicine visits to expand access to care. Medicaid and Children’s Health Insurance Program policies vary by state and private health insurance policies vary by individual plans, but it should be noted that commercial coverage for telemedicine visits was already strong prior to the pandemic.2

What medical licensing requirements are in place now for telehealth?
During the PHE, medical licensing requirements also were relaxed, enabling providers to deliver telemedicine service in states where they were not licensed.3 As the PHE orders ended, some states including New York discontinued cross-state licensing waivers altogether,6 whereas others have enacted legislation to make them permanent or extend them for brief periods of time.3,6 One potential solution is the Interstate Medical Licensure Compact (https://www.imlcc.org/), which includes 39 states as of October 2023. This program expedites the process for physicians already licensed in participating states to obtain their medical license in another participating state, though licensing fees are required for each state in which a physician wants to practice. Furthermore, some states such as North Dakota, Hawaii, and Virginia have licensure by endorsement policies, which enable licensed physicians with specific qualifications to provide telehealth services in the endorsing state. Other states such as Florida, New Jersey, Louisiana, Minnesota, Nevada, and New Mexico have special telehealth registries that allow physicians in good standing who are licensed in other states to deliver telehealth services to in-state residents barring they do not provide in-person, in-state services.6 Lastly, some states have temporary practice laws to allow existing patients who need medical attention while traveling out of state to see their home providers virtually or in person under certain circumstances for a limited period of time.3,5 In Hawaii and New Hampshire, physicians with out-of-state licenses can provide consultative services in some circumstances.5
What changes have been made to make it easier for patients to use telehealth?
As the legislation around telemedicine is shifting postpandemic, it is important to address additional logistical barriers to teledermatology on a larger scale if the discipline is to stay in practice. On November 15, 2021, the Infrastructure Investment and Jobs Act provided $65 billion in funding for broadband to expand access to high-speed internet. Some of this money was allocated to the Affordable Connectivity Program, which provides eligible households with a discount on broadband service and internet-connected devices. Eligible patrons can qualify for a discount of up to $75 per month for internet service and a one-time discount up to $100 on a laptop, desktop computer, or tablet purchased through a participating provider.6 Although a step in the right direction, the effects of this program on telemedicine encounters remains to be proven. Additionally, these programs do not address educational barriers to understanding how to utilize telemedicine platforms or provide incentives for practitioners to offer telemedicine services.
Final Thoughts
The pandemic taught our specialty a great deal about how to utilize telemedicine. For many dermatologists a return to in-person business as usual could not come fast enough; however, many practices have continued to offer at least some teledermatology services. Although the PHE waivers have ended, the extension of numerous CMS flexibilities through the end of 2024 allows us more time to develop sustainable policies to support the long-term health of telemedicine as a whole, both to sustain practices and to expand access to care in dermatology. The favorable attitudes of both patients and physicians about teledermatology have been clearly documented,1,7 and we should continue to safely expand the use of this technology.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- US Department of Health and Human Services. HHS fact sheet: telehealth flexibilities and resources and the COVID-19 public health emergency. Published May 10, 2023. Accessed October 18, 2023. https://www.hhs.gov/aboutnews/2023/05/10/hhs-fact-sheet-telehealth-flexibilities-resources-covid-19-public-health-emergency.html
- US Department of Health and Human Services. Licensing across state lines. Updated May 11, 2023. Accessed October 25, 2023. https://telehealth.hhs.gov/licensure/licensing-across-state-lines
- American Academy of Dermatology. Teledermatology and the COVID-19 pandemic. Accessed October 12, 2023. https://www.aad.org/member/practice/telederm/covid-19
- American Medical Association. Licensure & Telehealth. Accessed October 12, 2023. https://www.ama-assn.org/system/files/issue-brief-licensure-telehealth.pdf
- Federal Communications Commission. Affordable Connectivity Program. Updated June 29, 2023. Accessed October 12, 2023. https://www.fcc.gov/affordable-connectivity-program
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- US Department of Health and Human Services. HHS fact sheet: telehealth flexibilities and resources and the COVID-19 public health emergency. Published May 10, 2023. Accessed October 18, 2023. https://www.hhs.gov/aboutnews/2023/05/10/hhs-fact-sheet-telehealth-flexibilities-resources-covid-19-public-health-emergency.html
- US Department of Health and Human Services. Licensing across state lines. Updated May 11, 2023. Accessed October 25, 2023. https://telehealth.hhs.gov/licensure/licensing-across-state-lines
- American Academy of Dermatology. Teledermatology and the COVID-19 pandemic. Accessed October 12, 2023. https://www.aad.org/member/practice/telederm/covid-19
- American Medical Association. Licensure & Telehealth. Accessed October 12, 2023. https://www.ama-assn.org/system/files/issue-brief-licensure-telehealth.pdf
- Federal Communications Commission. Affordable Connectivity Program. Updated June 29, 2023. Accessed October 12, 2023. https://www.fcc.gov/affordable-connectivity-program
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
New Treatments for Psoriasis: An Update on a Therapeutic Frontier
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
PRACTICE POINTS
- Roflumilast, a phosphodiesterase 4 inhibitor, and tapinarof, an aryl hydrocarbon receptor–modulating agent, are 2 novel nonsteroidal topical treatments safe for regular long-term use on all affected areas of the skin in adult patients with plaque psoriasis.
- Deucravacitinib is an oral selective tyrosine kinase 2 allosteric inhibitor that has demonstrated a favorable safety profile and greater levels of efficacy than other available oral medications for plaque psoriasis.
- The dual inhibition of IL-17A and IL-17F with bimekizumab provides faster responses and greater clinical benefits for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone, achieving higher levels of efficacy than has been reported with any other biologic therapy.
- Spesolimab, an IL-36 receptor inhibitor, is an effective, US Food and Drug Administration–approved treatment for patients with generalized pustular psoriasis.
Management of Psoriasis With Topicals: Applying the 2020 AAD-NPF Guidelines of Care to Clinical Practice
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.
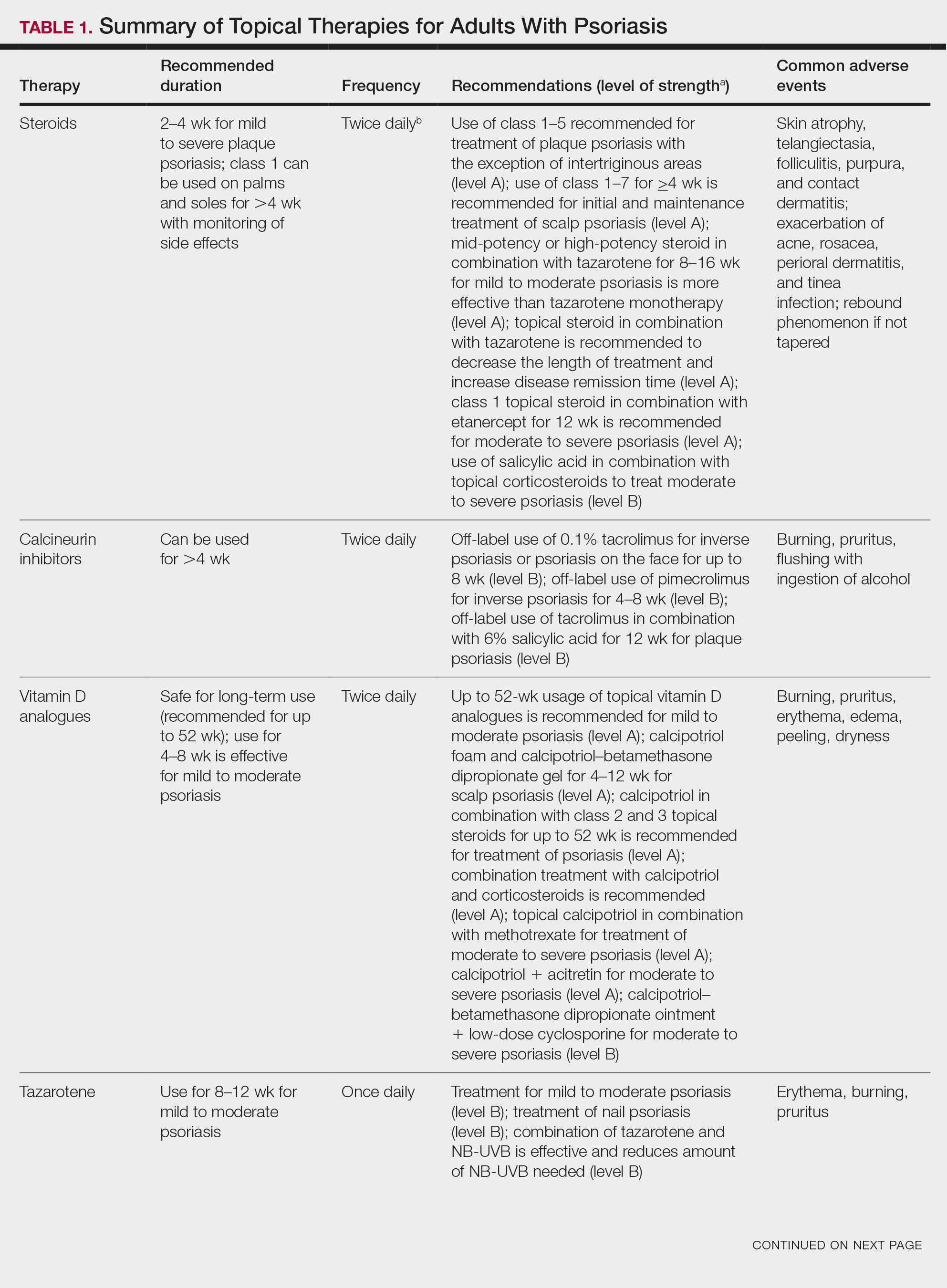
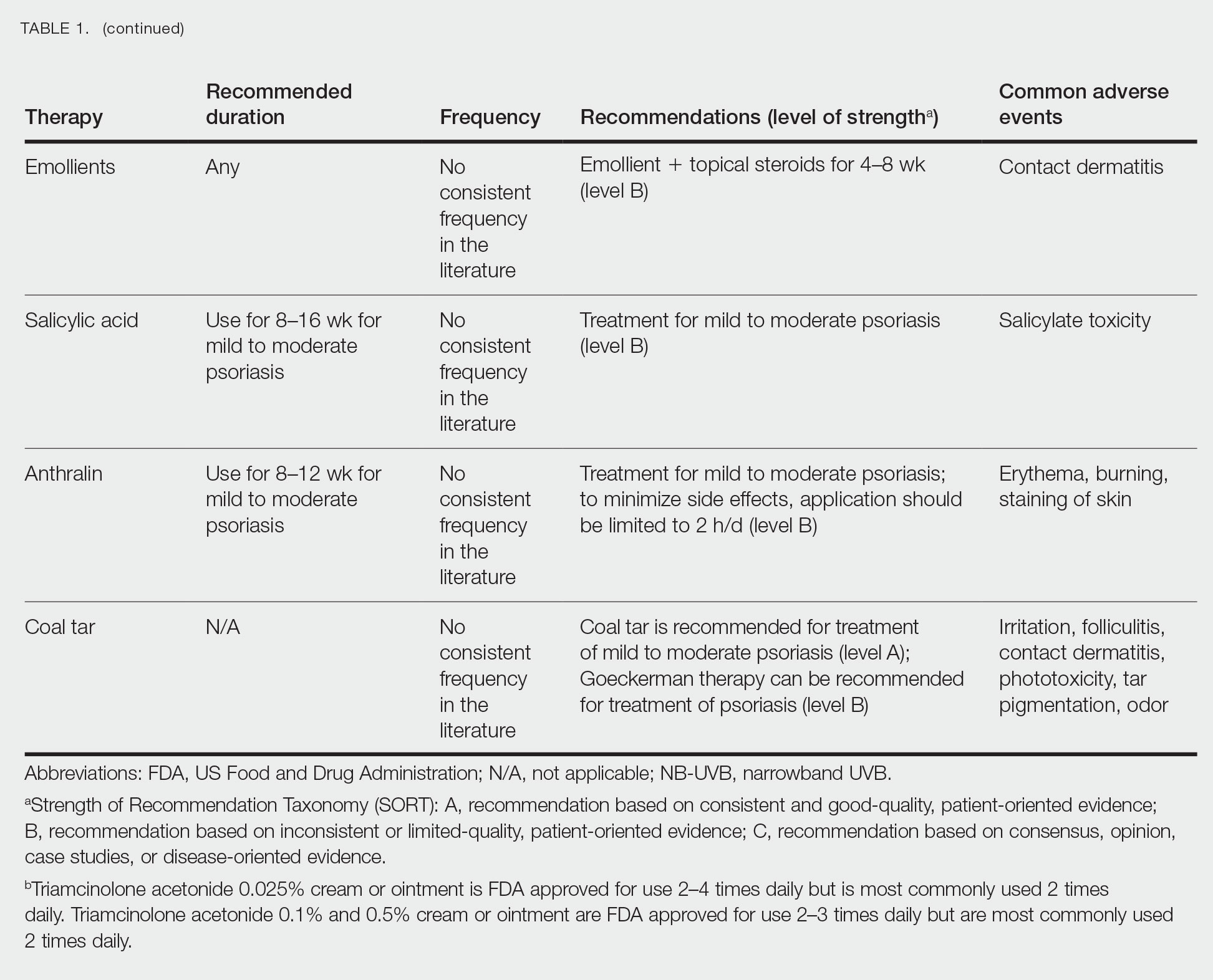
Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.
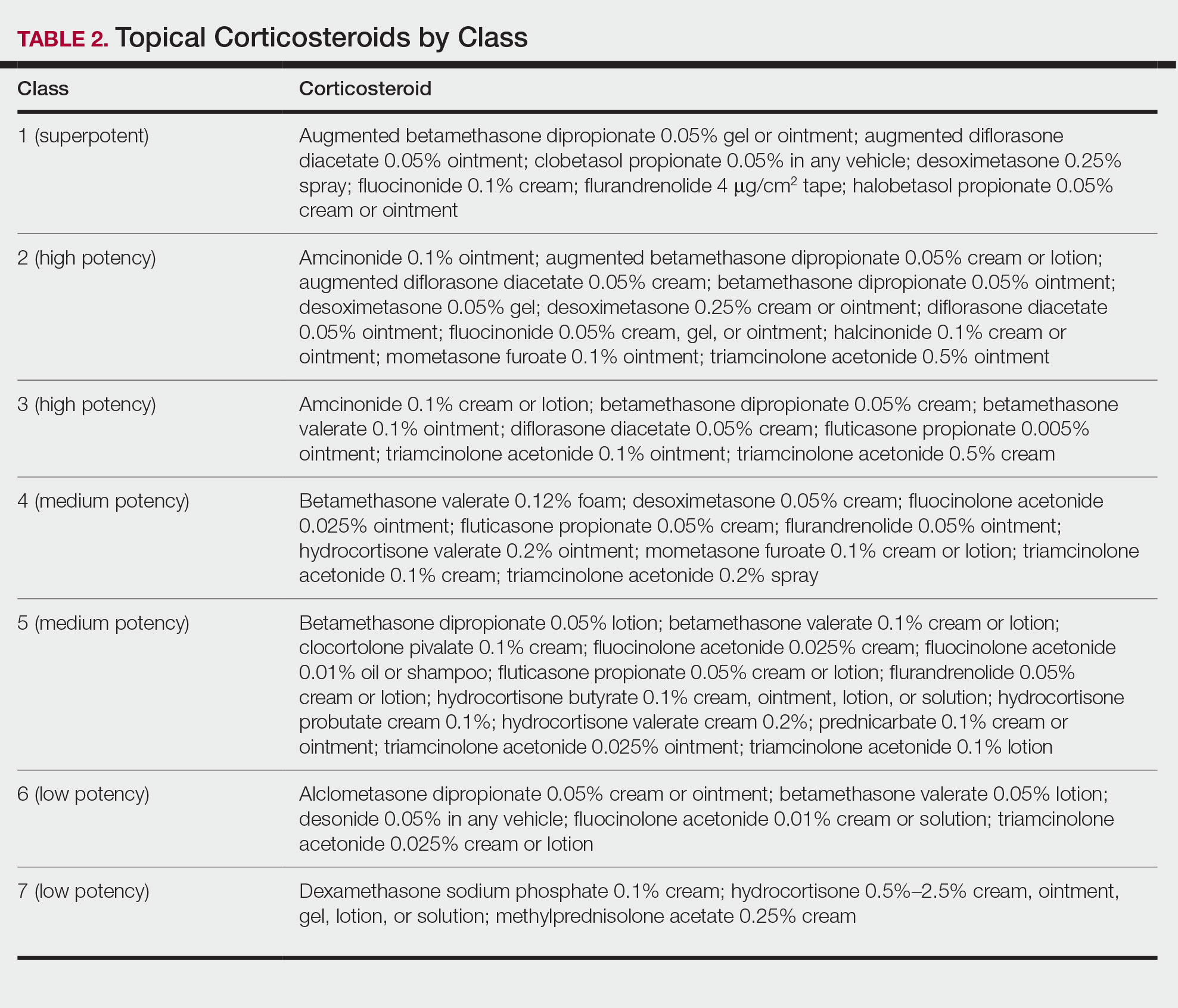
For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Practice Points
- Topical medications collectively represent the most common form of psoriasis treatment. Depending on disease severity and distribution, topical agents can be used as monotherapy or adjunct therapy, offering the benefit of localized treatment without systemic side effects.
- Dermatologists should base the selection of an appropriate topical medication on factors including adverse effects, potency, vehicle, and anatomic localization of disease.
Teledermatology During the COVID-19 Pandemic: Lessons Learned and Future Directions
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
Translating the 2019 AAD-NPF Guidelines of Care for Psoriasis With Attention to Comorbidities
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.

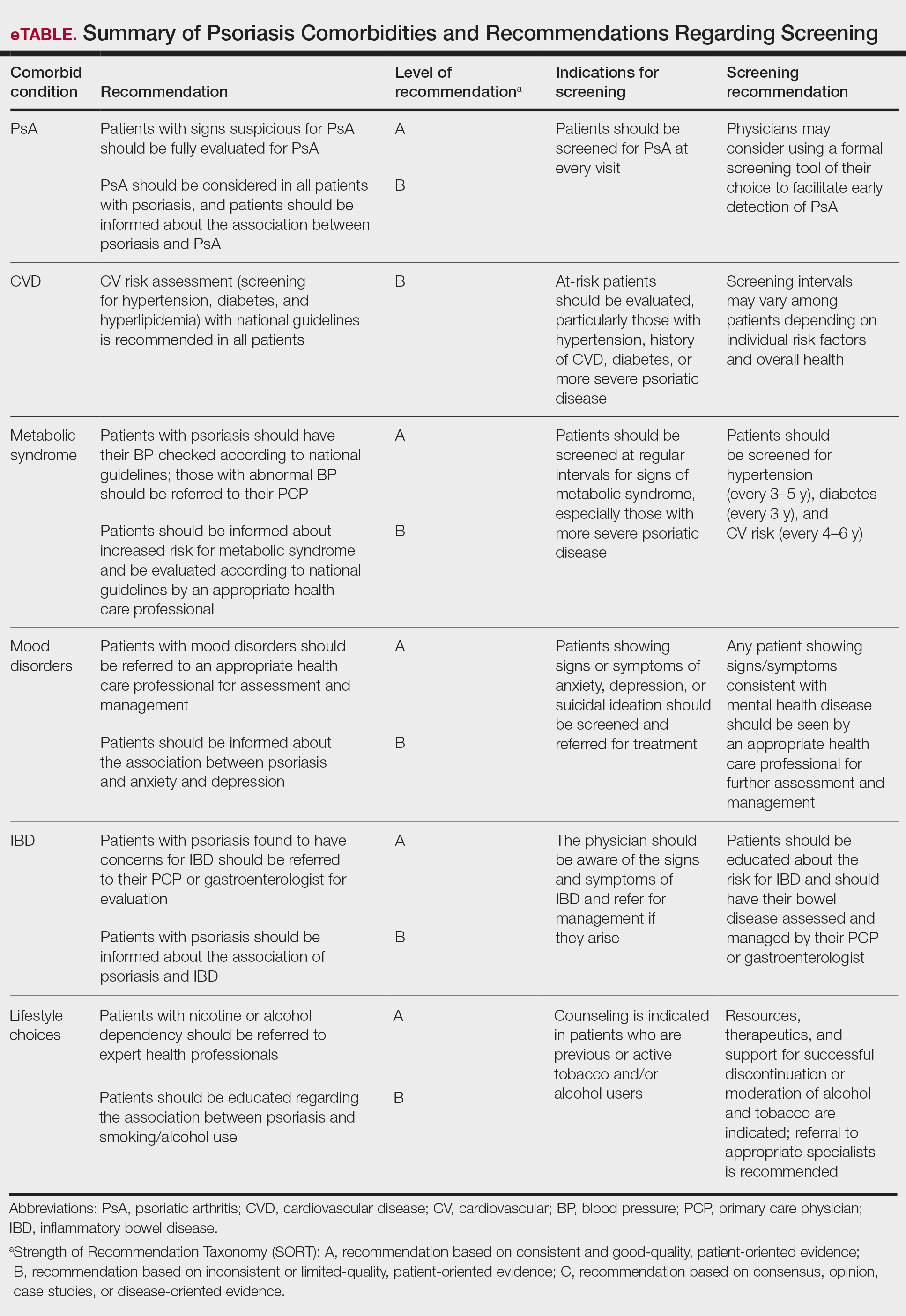
Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Practice Points
- Educating patients about psoriasis and its extracutaneous manifestations, available treatment options, and the impact of lifestyle choices is advised to maximize their patient’s disease awareness and to promote a collaborative physician-patient partnership.
- Physicians are strongly recommended to screen patients with psoriasis for the presence of disease comorbidities to ensure comprehensive management of their disease.
- Managing psoriasis as a multisystem inflammatory disorder requires the combined effort of dermatologists and other specialists to prevent and treat disease comorbidities and enhance patients’ quality of life.
Psoriatic Arthritis Diagnosis and Management in the Era of Telehealth
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
Translating the 2020 AAD-NPF Guidelines of Care for the Management of Psoriasis With Systemic Nonbiologics to Clinical Practice
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2
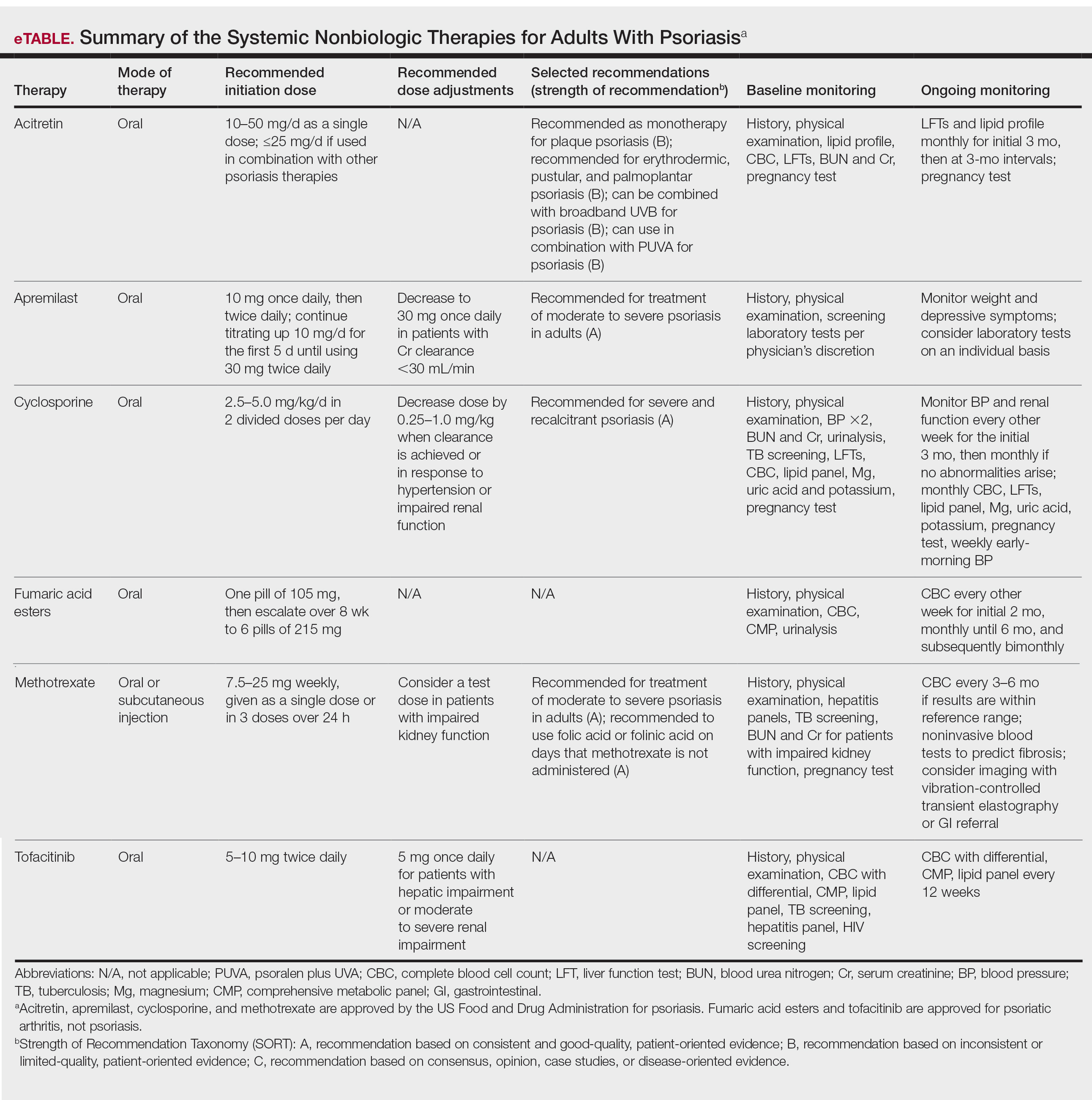
Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2

Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2

Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Practice Points
- Systemic nonbiologic therapies are effective treatments for adults with psoriasis. The benefits of these treatments include ease of administration and the ability to control widespread disease.
- When selecting a therapy, a thorough evaluation of patient characteristics and commitment to lifestyle adjustments is necessary, including careful consideration in women of childbearing potential and those with plans of starting a family.
- Regular drug monitoring and patient follow-up is crucial to ensure safe dosing adjustments and to mitigate potential adverse effects.
Reimbursement for Teledermatology During the COVID-19 Public Health Emergency: Change Has Come, But Will It Stay?
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.

