User login
FDA places CAR-T cell trial on hold following patient deaths
The Food and Drug Administration placed Juno Therapeutics’ phase II ROCKET trial, involving CAR-T cell therapy, on clinical hold following two treatment-related patient deaths caused by excess fluid accumulation in the brain.
The ROCKET trial is a single-arm, multicenter phase II study treating adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia with an infusion of the patient’s own T cells that have been genetically modified to express a chimeric antigen receptor (CAR) that will bind to CD19-expressing leukemia cells. This treatment is referred to as JCAR015, and the ROCKET trial is only one of three current clinical trials testing its safety and efficacy.
Just before the ROCKET trial commenced, researchers added the chemotherapy drug fludarabine, which was successful in improving the performance of other immunotherapies, to the JCAR015 infusion. Researchers involved in the trial reported that the addition of this drug was likely the cause of the patient deaths.
Juno Therapeuticswill submit a revised trial protocol and patient consent form to the FDA before the hold is lifted, Juno reported in a written statement. The other trials led by Juno Therapeutics involving CAR-T cell product candidates are not affected.
On Twitter @jessnicolecraig
The Food and Drug Administration placed Juno Therapeutics’ phase II ROCKET trial, involving CAR-T cell therapy, on clinical hold following two treatment-related patient deaths caused by excess fluid accumulation in the brain.
The ROCKET trial is a single-arm, multicenter phase II study treating adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia with an infusion of the patient’s own T cells that have been genetically modified to express a chimeric antigen receptor (CAR) that will bind to CD19-expressing leukemia cells. This treatment is referred to as JCAR015, and the ROCKET trial is only one of three current clinical trials testing its safety and efficacy.
Just before the ROCKET trial commenced, researchers added the chemotherapy drug fludarabine, which was successful in improving the performance of other immunotherapies, to the JCAR015 infusion. Researchers involved in the trial reported that the addition of this drug was likely the cause of the patient deaths.
Juno Therapeuticswill submit a revised trial protocol and patient consent form to the FDA before the hold is lifted, Juno reported in a written statement. The other trials led by Juno Therapeutics involving CAR-T cell product candidates are not affected.
On Twitter @jessnicolecraig
The Food and Drug Administration placed Juno Therapeutics’ phase II ROCKET trial, involving CAR-T cell therapy, on clinical hold following two treatment-related patient deaths caused by excess fluid accumulation in the brain.
The ROCKET trial is a single-arm, multicenter phase II study treating adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia with an infusion of the patient’s own T cells that have been genetically modified to express a chimeric antigen receptor (CAR) that will bind to CD19-expressing leukemia cells. This treatment is referred to as JCAR015, and the ROCKET trial is only one of three current clinical trials testing its safety and efficacy.
Just before the ROCKET trial commenced, researchers added the chemotherapy drug fludarabine, which was successful in improving the performance of other immunotherapies, to the JCAR015 infusion. Researchers involved in the trial reported that the addition of this drug was likely the cause of the patient deaths.
Juno Therapeuticswill submit a revised trial protocol and patient consent form to the FDA before the hold is lifted, Juno reported in a written statement. The other trials led by Juno Therapeutics involving CAR-T cell product candidates are not affected.
On Twitter @jessnicolecraig
Pediatric Cancer Survivors at Increased Risk for Endocrine Abnormalities
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Pediatric cancer survivors at increased risk for endocrine abnormalities
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
On Twitter @jessnicolecraig
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
On Twitter @jessnicolecraig
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Survivors of pediatric-onset cancer are at increased risk for developing endocrine abnormalities.
Major finding: Overall, 44% of childhood cancer survivors had at least one endocrinopathy. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Data source: A multi-institutional retrospective study of 14,290 men and women who survived pediatric cancer.
Disclosures: The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
Cisplatin-based chemo may be linked to hearing loss
In male patients with adult-onset germ cell tumors, cisplatin-based chemotherapy may be associated with hearing loss, according to the results of the large, multicenter Platinum Study.
For every 100-mg/m2 increase in cumulative cisplatin dose, a 3.2-dB decline in overall hearing threshold occurred, Robert Frisina, PhD, of the University of South Florida, Tampa, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8822).
A total of 488 men with adult-onset germ cell tumors who were treated with cisplatin-based chemotherapy were consented into this study, and completed questionnaires concerning neurotoxic symptoms, lifestyle habits, and medication use. Each patient underwent bone-conduction and speech-conducting threshold testing. Pure-tone air conduction thresholds were obtained bilaterally at speech frequency range (0.25 to 12 kHz). Classification of hearing loss and assessment of severity followed standardized criteria as defined by the American Speech-Language-Hearing Association. Median age at cancer diagnosis was 31 years; the median interval between chemotherapy and audiometric testing was 4.25 years. Median cumulative cisplatin dose was 400 mg/m2. Increasing cumulative cisplatin dose was associated with increasing (worse) hearing thresholds at 4 kHz (P = .021), 6 kHz (P = .0017), 8 kHz (P less than .001), 10 kHz (P less than .001), and 12 kHz (P = .0013) after correcting for age.
Cumulative cisplatin doses above 300 mg/m2 were associated with more severe hearing loss, compared with doses less than 300 mg/m2 (odds ratio, 1.59; 95% confidence interval, 1.14-2.21; P = .0066).
Conductive hearing loss in the middle ear was not associated with drug exposure dosage levels. Hypertension was identified as a risk factor for hearing loss as impaired overall hearing threshold was significantly associated with hypertension when correcting for age and cisplatin dose (n = 60, P = .0066).
“Because alterations in the highly successful [germ cell tumor] regimens are unlikely, our results point to the importance of ongoing research aimed at the identification of genetic variants associated with cisplatin-related ototoxicity,” investigators wrote. They also suggested that cancer patients treated with cisplatin should be careful to avoid noise exposure, ototoxic drugs, and other factors that could further increase damage.
This study was funded by the National Cancer Institute. Dr. Frisina reported holding patents related to hearing loss products. Six other investigators reported serving in advisory roles, receiving financial compensation or honoraria from multiple pharmaceutical and biomedical companies.
On Twitter @jessnicolecraig
In male patients with adult-onset germ cell tumors, cisplatin-based chemotherapy may be associated with hearing loss, according to the results of the large, multicenter Platinum Study.
For every 100-mg/m2 increase in cumulative cisplatin dose, a 3.2-dB decline in overall hearing threshold occurred, Robert Frisina, PhD, of the University of South Florida, Tampa, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8822).
A total of 488 men with adult-onset germ cell tumors who were treated with cisplatin-based chemotherapy were consented into this study, and completed questionnaires concerning neurotoxic symptoms, lifestyle habits, and medication use. Each patient underwent bone-conduction and speech-conducting threshold testing. Pure-tone air conduction thresholds were obtained bilaterally at speech frequency range (0.25 to 12 kHz). Classification of hearing loss and assessment of severity followed standardized criteria as defined by the American Speech-Language-Hearing Association. Median age at cancer diagnosis was 31 years; the median interval between chemotherapy and audiometric testing was 4.25 years. Median cumulative cisplatin dose was 400 mg/m2. Increasing cumulative cisplatin dose was associated with increasing (worse) hearing thresholds at 4 kHz (P = .021), 6 kHz (P = .0017), 8 kHz (P less than .001), 10 kHz (P less than .001), and 12 kHz (P = .0013) after correcting for age.
Cumulative cisplatin doses above 300 mg/m2 were associated with more severe hearing loss, compared with doses less than 300 mg/m2 (odds ratio, 1.59; 95% confidence interval, 1.14-2.21; P = .0066).
Conductive hearing loss in the middle ear was not associated with drug exposure dosage levels. Hypertension was identified as a risk factor for hearing loss as impaired overall hearing threshold was significantly associated with hypertension when correcting for age and cisplatin dose (n = 60, P = .0066).
“Because alterations in the highly successful [germ cell tumor] regimens are unlikely, our results point to the importance of ongoing research aimed at the identification of genetic variants associated with cisplatin-related ototoxicity,” investigators wrote. They also suggested that cancer patients treated with cisplatin should be careful to avoid noise exposure, ototoxic drugs, and other factors that could further increase damage.
This study was funded by the National Cancer Institute. Dr. Frisina reported holding patents related to hearing loss products. Six other investigators reported serving in advisory roles, receiving financial compensation or honoraria from multiple pharmaceutical and biomedical companies.
On Twitter @jessnicolecraig
In male patients with adult-onset germ cell tumors, cisplatin-based chemotherapy may be associated with hearing loss, according to the results of the large, multicenter Platinum Study.
For every 100-mg/m2 increase in cumulative cisplatin dose, a 3.2-dB decline in overall hearing threshold occurred, Robert Frisina, PhD, of the University of South Florida, Tampa, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8822).
A total of 488 men with adult-onset germ cell tumors who were treated with cisplatin-based chemotherapy were consented into this study, and completed questionnaires concerning neurotoxic symptoms, lifestyle habits, and medication use. Each patient underwent bone-conduction and speech-conducting threshold testing. Pure-tone air conduction thresholds were obtained bilaterally at speech frequency range (0.25 to 12 kHz). Classification of hearing loss and assessment of severity followed standardized criteria as defined by the American Speech-Language-Hearing Association. Median age at cancer diagnosis was 31 years; the median interval between chemotherapy and audiometric testing was 4.25 years. Median cumulative cisplatin dose was 400 mg/m2. Increasing cumulative cisplatin dose was associated with increasing (worse) hearing thresholds at 4 kHz (P = .021), 6 kHz (P = .0017), 8 kHz (P less than .001), 10 kHz (P less than .001), and 12 kHz (P = .0013) after correcting for age.
Cumulative cisplatin doses above 300 mg/m2 were associated with more severe hearing loss, compared with doses less than 300 mg/m2 (odds ratio, 1.59; 95% confidence interval, 1.14-2.21; P = .0066).
Conductive hearing loss in the middle ear was not associated with drug exposure dosage levels. Hypertension was identified as a risk factor for hearing loss as impaired overall hearing threshold was significantly associated with hypertension when correcting for age and cisplatin dose (n = 60, P = .0066).
“Because alterations in the highly successful [germ cell tumor] regimens are unlikely, our results point to the importance of ongoing research aimed at the identification of genetic variants associated with cisplatin-related ototoxicity,” investigators wrote. They also suggested that cancer patients treated with cisplatin should be careful to avoid noise exposure, ototoxic drugs, and other factors that could further increase damage.
This study was funded by the National Cancer Institute. Dr. Frisina reported holding patents related to hearing loss products. Six other investigators reported serving in advisory roles, receiving financial compensation or honoraria from multiple pharmaceutical and biomedical companies.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: In male patients with adult-onset germ cell tumors, cisplatin-based chemotherapy may be associated with hearing loss.
Major finding: For every 100-mg/m2 increase in cumulative cisplatin dose, a 3.2-dB decline in overall hearing threshold occurred.
Data source: A multicenter study of 488 men with adult-onset germ cell tumors.
Disclosures: This study was funded by the National Cancer Institute. Dr. Frisina reported holding patents related to hearing loss. Six other investigators reported serving in advisory roles, receiving financial compensation or honoraria from multiple pharmaceutical and biomedical companies.
FDA partners with analytics firm to study real world evidence on NSCLC
The Food and Drug Administration is partnering with a health analytics company to gather and analyze real world evidence on immune checkpoint inhibitors for the treatment of advanced non–small-cell lung cancer.
The partnership, which will eventually extend to the study of other cancer types, is designed “to determine how real world evidence derived from deidentified, HIPAA-compliant patient data captured outside of clinical trials can provide new insights into the safety and effectiveness of emerging anticancer therapies,” the company, Flatiron Health, said in a written statement.
“Flatiron will be responsible for collecting all of the electronic health records and processing them using [their] proprietary technology abstraction method. The FDA will be responsible for analysis, although this is intended to be a collaborative process with FDA and Flatiron working together on the analysis,” Amy Abernethy, MD, chief medical officer and senior vice president, oncology, Flatiron Health, said in an interview.
A unique advantage of this study is that it will span multiple payers, geographies, and demographics unlike previous studies and trials that consist largely of white patients living in urban settings who rely on Medicare or Medicaid to cover health care costs, she said.
“The goal is to generate a data set that represents the broad story of real people, from across the country, who are being treated with immuno-oncology agents for lung cancer,” said Dr. Abernethy.
Immune checkpoint inhibitors are the first focus because “things are changing so fast that clinical trials cannot always fill in the details,” Dr. Abernethy said. “It is not currently clear how providers are treating advanced non–small-cell lung cancer patients with immune therapies and how immune therapies are used in relation to other newly approved targeted therapies or traditional chemotherapy.”
This project and others will help define how real world evidence is best used, and whether it will be of adequate quality to meet a regulatory standard.
“Maybe the FDA finds that real world evidence is only applicable in certain settings or to answer certain questions. This might restrict the potential for Flatiron products, but ultimately helps to define where real world evidence is best used,” Dr. Abernethy said.
The Food and Drug Administration is partnering with a health analytics company to gather and analyze real world evidence on immune checkpoint inhibitors for the treatment of advanced non–small-cell lung cancer.
The partnership, which will eventually extend to the study of other cancer types, is designed “to determine how real world evidence derived from deidentified, HIPAA-compliant patient data captured outside of clinical trials can provide new insights into the safety and effectiveness of emerging anticancer therapies,” the company, Flatiron Health, said in a written statement.
“Flatiron will be responsible for collecting all of the electronic health records and processing them using [their] proprietary technology abstraction method. The FDA will be responsible for analysis, although this is intended to be a collaborative process with FDA and Flatiron working together on the analysis,” Amy Abernethy, MD, chief medical officer and senior vice president, oncology, Flatiron Health, said in an interview.
A unique advantage of this study is that it will span multiple payers, geographies, and demographics unlike previous studies and trials that consist largely of white patients living in urban settings who rely on Medicare or Medicaid to cover health care costs, she said.
“The goal is to generate a data set that represents the broad story of real people, from across the country, who are being treated with immuno-oncology agents for lung cancer,” said Dr. Abernethy.
Immune checkpoint inhibitors are the first focus because “things are changing so fast that clinical trials cannot always fill in the details,” Dr. Abernethy said. “It is not currently clear how providers are treating advanced non–small-cell lung cancer patients with immune therapies and how immune therapies are used in relation to other newly approved targeted therapies or traditional chemotherapy.”
This project and others will help define how real world evidence is best used, and whether it will be of adequate quality to meet a regulatory standard.
“Maybe the FDA finds that real world evidence is only applicable in certain settings or to answer certain questions. This might restrict the potential for Flatiron products, but ultimately helps to define where real world evidence is best used,” Dr. Abernethy said.
The Food and Drug Administration is partnering with a health analytics company to gather and analyze real world evidence on immune checkpoint inhibitors for the treatment of advanced non–small-cell lung cancer.
The partnership, which will eventually extend to the study of other cancer types, is designed “to determine how real world evidence derived from deidentified, HIPAA-compliant patient data captured outside of clinical trials can provide new insights into the safety and effectiveness of emerging anticancer therapies,” the company, Flatiron Health, said in a written statement.
“Flatiron will be responsible for collecting all of the electronic health records and processing them using [their] proprietary technology abstraction method. The FDA will be responsible for analysis, although this is intended to be a collaborative process with FDA and Flatiron working together on the analysis,” Amy Abernethy, MD, chief medical officer and senior vice president, oncology, Flatiron Health, said in an interview.
A unique advantage of this study is that it will span multiple payers, geographies, and demographics unlike previous studies and trials that consist largely of white patients living in urban settings who rely on Medicare or Medicaid to cover health care costs, she said.
“The goal is to generate a data set that represents the broad story of real people, from across the country, who are being treated with immuno-oncology agents for lung cancer,” said Dr. Abernethy.
Immune checkpoint inhibitors are the first focus because “things are changing so fast that clinical trials cannot always fill in the details,” Dr. Abernethy said. “It is not currently clear how providers are treating advanced non–small-cell lung cancer patients with immune therapies and how immune therapies are used in relation to other newly approved targeted therapies or traditional chemotherapy.”
This project and others will help define how real world evidence is best used, and whether it will be of adequate quality to meet a regulatory standard.
“Maybe the FDA finds that real world evidence is only applicable in certain settings or to answer certain questions. This might restrict the potential for Flatiron products, but ultimately helps to define where real world evidence is best used,” Dr. Abernethy said.
Dr. Richard Pazdur named acting director of FDA’s Oncology Center of Excellence
Food and Drug Administration Commissioner Robert M. Califf, MD, has named Richard Pazdur, MD, to serve as the acting director of the FDA’s Oncology Center of Excellence (OCE), an outgrowth of Vice President Biden’s National Cancer Moonshot Initiative.
The center will expedite the development of novel combination products and support an integrated approach to the disease, according to a written statement from the FDA.
Dr. Pazdur joined the FDA in 1999 and most recently served as the director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. In his new role as acting director of the OCE, Dr. Pazdur will be responsible for leading the effort to develop and execute an integrated regulatory approach for clinical product review with coordination between centers and collaboration with stakeholders.
“Dr. Pazdur is the person the FDA needs to get the OCE up and running, because of his in-depth understanding of the inner workings of the FDA, his deep expertise in treating this complex disease, and his ability to move the agency forward in this complicated task,” Dr. Califf said.
“I am honored to be selected by Commissioner Califf today as the acting director of FDA’s new Oncology Center of Excellence in support of the Vice President’s National Cancer Moonshot Initiative,” Dr. Pazdur wrote in a separate written statement. “I look forward to guiding the agency through this initial phase, building our cross-disciplinary review staff, providing external outreach to diverse stakeholders, and streamlining administrative processes to ensure rapid review of important cancer products to the American public,” he added.
Specific details about how the center will function to accomplish its goals have not yet been released.
“Establishing a Center of Excellence in a disease as complex as cancer requires a thoughtful approach, and we recognize that the framework of the OCE will evolve. At the same time, we remain committed to the sense of urgency that is central to the Cancer Moonshot. That is why we determined the best way to accelerate the formation of the OCE without disrupting the ongoing work within the FDA’s centers would be to appoint an internal leader in an acting capacity who can focus on bringing together oncologists across the FDA,” Dr. Califf said.
Dr. Pazdur will work with directors from the Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, and the Center for Devices and Radiological Health to formalize a seamless cross-center regulatory approach. He will also be responsible for streamlining engagement and collaboration with oncology stakeholders, including patient-focused advocacy groups, professional associations, industry, academia, and sister agencies such as the National Institutes of Health.
On Twitter @jessnicolecraig
Food and Drug Administration Commissioner Robert M. Califf, MD, has named Richard Pazdur, MD, to serve as the acting director of the FDA’s Oncology Center of Excellence (OCE), an outgrowth of Vice President Biden’s National Cancer Moonshot Initiative.
The center will expedite the development of novel combination products and support an integrated approach to the disease, according to a written statement from the FDA.
Dr. Pazdur joined the FDA in 1999 and most recently served as the director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. In his new role as acting director of the OCE, Dr. Pazdur will be responsible for leading the effort to develop and execute an integrated regulatory approach for clinical product review with coordination between centers and collaboration with stakeholders.
“Dr. Pazdur is the person the FDA needs to get the OCE up and running, because of his in-depth understanding of the inner workings of the FDA, his deep expertise in treating this complex disease, and his ability to move the agency forward in this complicated task,” Dr. Califf said.
“I am honored to be selected by Commissioner Califf today as the acting director of FDA’s new Oncology Center of Excellence in support of the Vice President’s National Cancer Moonshot Initiative,” Dr. Pazdur wrote in a separate written statement. “I look forward to guiding the agency through this initial phase, building our cross-disciplinary review staff, providing external outreach to diverse stakeholders, and streamlining administrative processes to ensure rapid review of important cancer products to the American public,” he added.
Specific details about how the center will function to accomplish its goals have not yet been released.
“Establishing a Center of Excellence in a disease as complex as cancer requires a thoughtful approach, and we recognize that the framework of the OCE will evolve. At the same time, we remain committed to the sense of urgency that is central to the Cancer Moonshot. That is why we determined the best way to accelerate the formation of the OCE without disrupting the ongoing work within the FDA’s centers would be to appoint an internal leader in an acting capacity who can focus on bringing together oncologists across the FDA,” Dr. Califf said.
Dr. Pazdur will work with directors from the Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, and the Center for Devices and Radiological Health to formalize a seamless cross-center regulatory approach. He will also be responsible for streamlining engagement and collaboration with oncology stakeholders, including patient-focused advocacy groups, professional associations, industry, academia, and sister agencies such as the National Institutes of Health.
On Twitter @jessnicolecraig
Food and Drug Administration Commissioner Robert M. Califf, MD, has named Richard Pazdur, MD, to serve as the acting director of the FDA’s Oncology Center of Excellence (OCE), an outgrowth of Vice President Biden’s National Cancer Moonshot Initiative.
The center will expedite the development of novel combination products and support an integrated approach to the disease, according to a written statement from the FDA.
Dr. Pazdur joined the FDA in 1999 and most recently served as the director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. In his new role as acting director of the OCE, Dr. Pazdur will be responsible for leading the effort to develop and execute an integrated regulatory approach for clinical product review with coordination between centers and collaboration with stakeholders.
“Dr. Pazdur is the person the FDA needs to get the OCE up and running, because of his in-depth understanding of the inner workings of the FDA, his deep expertise in treating this complex disease, and his ability to move the agency forward in this complicated task,” Dr. Califf said.
“I am honored to be selected by Commissioner Califf today as the acting director of FDA’s new Oncology Center of Excellence in support of the Vice President’s National Cancer Moonshot Initiative,” Dr. Pazdur wrote in a separate written statement. “I look forward to guiding the agency through this initial phase, building our cross-disciplinary review staff, providing external outreach to diverse stakeholders, and streamlining administrative processes to ensure rapid review of important cancer products to the American public,” he added.
Specific details about how the center will function to accomplish its goals have not yet been released.
“Establishing a Center of Excellence in a disease as complex as cancer requires a thoughtful approach, and we recognize that the framework of the OCE will evolve. At the same time, we remain committed to the sense of urgency that is central to the Cancer Moonshot. That is why we determined the best way to accelerate the formation of the OCE without disrupting the ongoing work within the FDA’s centers would be to appoint an internal leader in an acting capacity who can focus on bringing together oncologists across the FDA,” Dr. Califf said.
Dr. Pazdur will work with directors from the Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, and the Center for Devices and Radiological Health to formalize a seamless cross-center regulatory approach. He will also be responsible for streamlining engagement and collaboration with oncology stakeholders, including patient-focused advocacy groups, professional associations, industry, academia, and sister agencies such as the National Institutes of Health.
On Twitter @jessnicolecraig
Nearly 200 practices expected to participate in Oncology Care Model
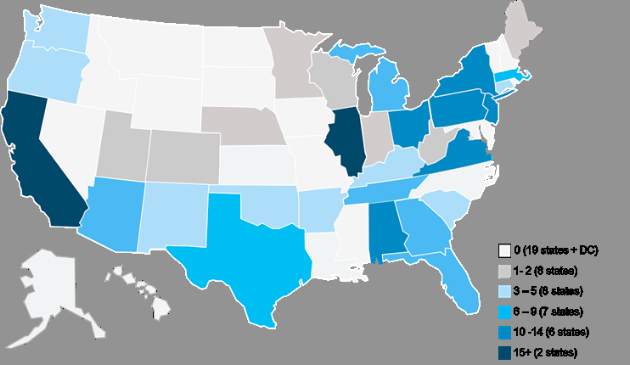
The Department of Health and Human Services has announced that nearly 200 physician group practices – more than double the number expected – and 17 health insurance companies will be participating in the Oncology Care Model, a voluntary payment and care delivery model developed by the CMS Innovation Center and advanced by the Affordable Care Act. The 5-year program, designed to improve cancer care by providing financial incentives to physician practices that provide effective treatment, is set to begin July 1, 2016.
The Medicare arm of the Oncology Care Model (OCM) will include more than 3,200 oncologists and will cover approximately 155,000 Medicare beneficiaries nationwide, according to a written statement from the HHS.
“CMS is thrilled with how many physician groups chose to be a part of the Oncology Care Model,” Patrick Conway, MD, CMS principal deputy administrator and chief medical officer, said in the statement.
Physician practices from 31 states will be participating, with the highest levels of provider participation in Alabama, California, Illinois, New Jersey, New York, Ohio, Pennsylvania, and Virginia, according to an analysis conducted by Avalere Health, a Washington, DC–based health care consulting firm.
The CMS first announced the OCM project in February 2015 and originally aimed to have 100 physician practices participating in the first-ever oncology-specific payment reform model.
“Based on feedback from the medical, consumer, and business communities, we are launching this new model of care to support clinicians’ work with their patients,” HHS Secretary Sylvia M. Burwell said in a written statement in February 2015.
“We aim to provide Medicare beneficiaries struggling with cancer with high-quality care around the clock and to reward doctors for the value, not volume, of care they provide. Improving the way we pay providers and deliver care to patients will result in healthier people,” she said.
The OCM encourages practices to improve care and lower costs through episodic and performance-based payments that reward high-quality patient care. It is a multipayer model that includes Medicare’s fee-for-service (OCM-FFS) and commercial payers.
OCM participants will receive regular OCM-FFS payments during the model. To create incentives to improve the quality of care, reimbursement will include a monthly payment of $160 per beneficiary for delivery of OCM enhanced services, and a performance-based payment for OCM episodes, according to a CMS fact sheet.
An OCM-FFS episode begins on the date of initial Part B or D chemotherapy claim and includes all Medicare Part A and B (and some Part D) services received during the episode period which lasts 6 months. Beneficiaries who receive chemotherapy after the end of an episode will begin a new episode.
Enhanced services include patient navigation, a care plan based on the Institute of Medicine care management report, patient access 24 hours a day, 7 days a week, and treatment with therapies that are consistent with nationally recognized clinical guidelines.
View the complete list of participating practices at https://innovation.cms.gov/initiatives/Oncology-Care.
On Twitter @jessnicolecraig
The Department of Health and Human Services has announced that nearly 200 physician group practices – more than double the number expected – and 17 health insurance companies will be participating in the Oncology Care Model, a voluntary payment and care delivery model developed by the CMS Innovation Center and advanced by the Affordable Care Act. The 5-year program, designed to improve cancer care by providing financial incentives to physician practices that provide effective treatment, is set to begin July 1, 2016.
The Medicare arm of the Oncology Care Model (OCM) will include more than 3,200 oncologists and will cover approximately 155,000 Medicare beneficiaries nationwide, according to a written statement from the HHS.
“CMS is thrilled with how many physician groups chose to be a part of the Oncology Care Model,” Patrick Conway, MD, CMS principal deputy administrator and chief medical officer, said in the statement.
Physician practices from 31 states will be participating, with the highest levels of provider participation in Alabama, California, Illinois, New Jersey, New York, Ohio, Pennsylvania, and Virginia, according to an analysis conducted by Avalere Health, a Washington, DC–based health care consulting firm.
The CMS first announced the OCM project in February 2015 and originally aimed to have 100 physician practices participating in the first-ever oncology-specific payment reform model.
“Based on feedback from the medical, consumer, and business communities, we are launching this new model of care to support clinicians’ work with their patients,” HHS Secretary Sylvia M. Burwell said in a written statement in February 2015.
“We aim to provide Medicare beneficiaries struggling with cancer with high-quality care around the clock and to reward doctors for the value, not volume, of care they provide. Improving the way we pay providers and deliver care to patients will result in healthier people,” she said.
The OCM encourages practices to improve care and lower costs through episodic and performance-based payments that reward high-quality patient care. It is a multipayer model that includes Medicare’s fee-for-service (OCM-FFS) and commercial payers.
OCM participants will receive regular OCM-FFS payments during the model. To create incentives to improve the quality of care, reimbursement will include a monthly payment of $160 per beneficiary for delivery of OCM enhanced services, and a performance-based payment for OCM episodes, according to a CMS fact sheet.
An OCM-FFS episode begins on the date of initial Part B or D chemotherapy claim and includes all Medicare Part A and B (and some Part D) services received during the episode period which lasts 6 months. Beneficiaries who receive chemotherapy after the end of an episode will begin a new episode.
Enhanced services include patient navigation, a care plan based on the Institute of Medicine care management report, patient access 24 hours a day, 7 days a week, and treatment with therapies that are consistent with nationally recognized clinical guidelines.
View the complete list of participating practices at https://innovation.cms.gov/initiatives/Oncology-Care.
On Twitter @jessnicolecraig
The Department of Health and Human Services has announced that nearly 200 physician group practices – more than double the number expected – and 17 health insurance companies will be participating in the Oncology Care Model, a voluntary payment and care delivery model developed by the CMS Innovation Center and advanced by the Affordable Care Act. The 5-year program, designed to improve cancer care by providing financial incentives to physician practices that provide effective treatment, is set to begin July 1, 2016.
The Medicare arm of the Oncology Care Model (OCM) will include more than 3,200 oncologists and will cover approximately 155,000 Medicare beneficiaries nationwide, according to a written statement from the HHS.
“CMS is thrilled with how many physician groups chose to be a part of the Oncology Care Model,” Patrick Conway, MD, CMS principal deputy administrator and chief medical officer, said in the statement.
Physician practices from 31 states will be participating, with the highest levels of provider participation in Alabama, California, Illinois, New Jersey, New York, Ohio, Pennsylvania, and Virginia, according to an analysis conducted by Avalere Health, a Washington, DC–based health care consulting firm.
The CMS first announced the OCM project in February 2015 and originally aimed to have 100 physician practices participating in the first-ever oncology-specific payment reform model.
“Based on feedback from the medical, consumer, and business communities, we are launching this new model of care to support clinicians’ work with their patients,” HHS Secretary Sylvia M. Burwell said in a written statement in February 2015.
“We aim to provide Medicare beneficiaries struggling with cancer with high-quality care around the clock and to reward doctors for the value, not volume, of care they provide. Improving the way we pay providers and deliver care to patients will result in healthier people,” she said.
The OCM encourages practices to improve care and lower costs through episodic and performance-based payments that reward high-quality patient care. It is a multipayer model that includes Medicare’s fee-for-service (OCM-FFS) and commercial payers.
OCM participants will receive regular OCM-FFS payments during the model. To create incentives to improve the quality of care, reimbursement will include a monthly payment of $160 per beneficiary for delivery of OCM enhanced services, and a performance-based payment for OCM episodes, according to a CMS fact sheet.
An OCM-FFS episode begins on the date of initial Part B or D chemotherapy claim and includes all Medicare Part A and B (and some Part D) services received during the episode period which lasts 6 months. Beneficiaries who receive chemotherapy after the end of an episode will begin a new episode.
Enhanced services include patient navigation, a care plan based on the Institute of Medicine care management report, patient access 24 hours a day, 7 days a week, and treatment with therapies that are consistent with nationally recognized clinical guidelines.
View the complete list of participating practices at https://innovation.cms.gov/initiatives/Oncology-Care.
On Twitter @jessnicolecraig
Early results positive for nivolumab as first-line therapy in advanced NSCLC
The PD-1 immune checkpoint inhibitor nivolumab may be safe and effective as a first-line therapy in adult patients with non–small cell lung cancer (NSCLC), according to the results of the phase I CheckMate 012 trial.
Of 52 adult patients with advanced NSCLC who received nivolumab, 19% experienced grade three or four adverse events, and the overall response rate was 23% with four ongoing complete responses, reported Scott Gettinger, MD, of the Yale Cancer Center, New Haven, Conn., and his associates (J Clin Oncol. 2016 June. doi: 10.1200/JCO.2016.66.9929).
In the study cohort, 94% had stage IV NSCLC, 79% were former or current smokers, and 65% had received either radiotherapy, adjuvant or neoadjuvant chemotherapy.
Treatment-related adverse events were reported in 71% of patients, the most common being fatigue (29%), rash (19%), and nausea (14%). Grade 3 or 4 adverse events including rash, cardiac failure, and lung infection occurred in 10 patients (19%). There were no treatment-related deaths, but adverse events led to the discontinuation of the drug treatment in six patients.
Responses to nivolumab (overall response rate, 23%) were durable with duration of responses ranging from 4.2 to 25.8 months. In addition, the median overall survival was 19.4 months, median progression-free survival was 3.5 months, and the 24-week progression-free survival rate was 31% (95% confidence interval, 28%-60%).
Forty-six patients had tumor specimens evaluable for PD-L1 expression. Clinical activity was observed regardless of PD-L1 expression, the investigators reported. However, the overall response rate was higher in patients with tumors that expressed PD-L1, compared with non-PD-L1-expressing tumors.
All investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock in multiple companies including Bristol-Myers Squibb, which funded the study.
On Twitter @jessnicolecraig
The PD-1 immune checkpoint inhibitor nivolumab may be safe and effective as a first-line therapy in adult patients with non–small cell lung cancer (NSCLC), according to the results of the phase I CheckMate 012 trial.
Of 52 adult patients with advanced NSCLC who received nivolumab, 19% experienced grade three or four adverse events, and the overall response rate was 23% with four ongoing complete responses, reported Scott Gettinger, MD, of the Yale Cancer Center, New Haven, Conn., and his associates (J Clin Oncol. 2016 June. doi: 10.1200/JCO.2016.66.9929).
In the study cohort, 94% had stage IV NSCLC, 79% were former or current smokers, and 65% had received either radiotherapy, adjuvant or neoadjuvant chemotherapy.
Treatment-related adverse events were reported in 71% of patients, the most common being fatigue (29%), rash (19%), and nausea (14%). Grade 3 or 4 adverse events including rash, cardiac failure, and lung infection occurred in 10 patients (19%). There were no treatment-related deaths, but adverse events led to the discontinuation of the drug treatment in six patients.
Responses to nivolumab (overall response rate, 23%) were durable with duration of responses ranging from 4.2 to 25.8 months. In addition, the median overall survival was 19.4 months, median progression-free survival was 3.5 months, and the 24-week progression-free survival rate was 31% (95% confidence interval, 28%-60%).
Forty-six patients had tumor specimens evaluable for PD-L1 expression. Clinical activity was observed regardless of PD-L1 expression, the investigators reported. However, the overall response rate was higher in patients with tumors that expressed PD-L1, compared with non-PD-L1-expressing tumors.
All investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock in multiple companies including Bristol-Myers Squibb, which funded the study.
On Twitter @jessnicolecraig
The PD-1 immune checkpoint inhibitor nivolumab may be safe and effective as a first-line therapy in adult patients with non–small cell lung cancer (NSCLC), according to the results of the phase I CheckMate 012 trial.
Of 52 adult patients with advanced NSCLC who received nivolumab, 19% experienced grade three or four adverse events, and the overall response rate was 23% with four ongoing complete responses, reported Scott Gettinger, MD, of the Yale Cancer Center, New Haven, Conn., and his associates (J Clin Oncol. 2016 June. doi: 10.1200/JCO.2016.66.9929).
In the study cohort, 94% had stage IV NSCLC, 79% were former or current smokers, and 65% had received either radiotherapy, adjuvant or neoadjuvant chemotherapy.
Treatment-related adverse events were reported in 71% of patients, the most common being fatigue (29%), rash (19%), and nausea (14%). Grade 3 or 4 adverse events including rash, cardiac failure, and lung infection occurred in 10 patients (19%). There were no treatment-related deaths, but adverse events led to the discontinuation of the drug treatment in six patients.
Responses to nivolumab (overall response rate, 23%) were durable with duration of responses ranging from 4.2 to 25.8 months. In addition, the median overall survival was 19.4 months, median progression-free survival was 3.5 months, and the 24-week progression-free survival rate was 31% (95% confidence interval, 28%-60%).
Forty-six patients had tumor specimens evaluable for PD-L1 expression. Clinical activity was observed regardless of PD-L1 expression, the investigators reported. However, the overall response rate was higher in patients with tumors that expressed PD-L1, compared with non-PD-L1-expressing tumors.
All investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock in multiple companies including Bristol-Myers Squibb, which funded the study.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: A phase I trial indicates that nivolumab is safe and shows durable activity as a first-line therapy in treating patients with advanced NSCLC.
Major finding: Grade 3 or 4 adverse events were reported in 19% of patients. The overall response rate was 23% with four ongoing complete responses.
Data source: A phase I trial of 52 patients with stage 3 or 4 non–small-cell lung cancer.
Disclosures: All investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock interest in multiple companies including Bristol-Myers Squibb, which funded the study.
Nivolumab plus chemo shows increased response, decreased safety in NSCLC
In a phase I study, the PD-1 checkpoint inhibitor nivolumab administered in combination with platinum-based doublet chemotherapy (PT-DC) showed improved response rates but higher-than-expected occurrence of adverse events, investigators reported.
In the CheckMate 012 phase I, multicohort trial of nivolumab for first-line treatment of advanced non–small-cell lung cancer, treatment-related adverse events occurred in 95% of patients, and the overall response rates ranged from 33% to 47%.
“It is unclear at this time whether nivolumab plus PT-DC offers improved long-term [overall survival] benefit compared with nivolumab monotherapy,” wrote Naiyer Rizvi, MD, of Memorial Sloan-Kettering Cancer Center, New York, and his associates (J Clin Oncol. 2016 June. doi: 10.1200/JCO.2016.66.9861).
Fifty-six patients with newly-diagnosed advanced NSCLC were assigned by histology to receive one of four drug regimens: nivolumab at 10 mg/kg plus gemcitabine-cisplatin (squamous, n = 12), nivolumab at 10 mg/kg plus pemetrexed-cisplatin (nonsquamous, n = 15), nivolumab at 10mg/kg plus paclitaxel-carboplatin (any histology, n = 15), or nivolumab at 5 mg/kg plus paclitaxel-carboplatin (any histology, n = 14).
For all patients, nivolumab and PT-DC were administered intravenously on day 1 of each 21-day cycle for four cycles followed by nivolumab monotherapy every 3 weeks. Median follow-up time for safety and efficacy was 19.0 months.
No dose-limiting toxicities occurred in the first 6 weeks of treatment. In the overall population, 95% of patients experienced treatment-related adverse events, the most common being fatigue, nausea, and alopecia. Grade three or four adverse events occurred in 45% of patients. Specifically, among those treated with nivolumab at 10 mg/kg, 93% experienced any grade and 50% experienced grade 3 or 4 treatment-related adverse events. Adverse events led to the discontinuation of all study therapy in 21% of patients. No treatment-related deaths were reported.
Overall response was highest among patients receiving nivolumab plus pemetrexed-cisplatin or paclitaxel-carboplatin, both with rates of 47% (95% confidence interval, 21-73 for both treatment arms).
In addition, median progression-free survival ranged from 4.8 to 7.1 months across treatment arms and was longest among patients receiving nivolumab at 5 mg/kg in combination with paclitaxel-carboplatin.
Finally, there was no clear association between PD-L1 expression and outcome.
All 17 investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock interest in multiple companies including Bristol-Myers Squibb, which funded the CheckMate 012 trial.
On Twitter @jessnicolecraig
In a phase I study, the PD-1 checkpoint inhibitor nivolumab administered in combination with platinum-based doublet chemotherapy (PT-DC) showed improved response rates but higher-than-expected occurrence of adverse events, investigators reported.
In the CheckMate 012 phase I, multicohort trial of nivolumab for first-line treatment of advanced non–small-cell lung cancer, treatment-related adverse events occurred in 95% of patients, and the overall response rates ranged from 33% to 47%.
“It is unclear at this time whether nivolumab plus PT-DC offers improved long-term [overall survival] benefit compared with nivolumab monotherapy,” wrote Naiyer Rizvi, MD, of Memorial Sloan-Kettering Cancer Center, New York, and his associates (J Clin Oncol. 2016 June. doi: 10.1200/JCO.2016.66.9861).
Fifty-six patients with newly-diagnosed advanced NSCLC were assigned by histology to receive one of four drug regimens: nivolumab at 10 mg/kg plus gemcitabine-cisplatin (squamous, n = 12), nivolumab at 10 mg/kg plus pemetrexed-cisplatin (nonsquamous, n = 15), nivolumab at 10mg/kg plus paclitaxel-carboplatin (any histology, n = 15), or nivolumab at 5 mg/kg plus paclitaxel-carboplatin (any histology, n = 14).
For all patients, nivolumab and PT-DC were administered intravenously on day 1 of each 21-day cycle for four cycles followed by nivolumab monotherapy every 3 weeks. Median follow-up time for safety and efficacy was 19.0 months.
No dose-limiting toxicities occurred in the first 6 weeks of treatment. In the overall population, 95% of patients experienced treatment-related adverse events, the most common being fatigue, nausea, and alopecia. Grade three or four adverse events occurred in 45% of patients. Specifically, among those treated with nivolumab at 10 mg/kg, 93% experienced any grade and 50% experienced grade 3 or 4 treatment-related adverse events. Adverse events led to the discontinuation of all study therapy in 21% of patients. No treatment-related deaths were reported.
Overall response was highest among patients receiving nivolumab plus pemetrexed-cisplatin or paclitaxel-carboplatin, both with rates of 47% (95% confidence interval, 21-73 for both treatment arms).
In addition, median progression-free survival ranged from 4.8 to 7.1 months across treatment arms and was longest among patients receiving nivolumab at 5 mg/kg in combination with paclitaxel-carboplatin.
Finally, there was no clear association between PD-L1 expression and outcome.
All 17 investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock interest in multiple companies including Bristol-Myers Squibb, which funded the CheckMate 012 trial.
On Twitter @jessnicolecraig
In a phase I study, the PD-1 checkpoint inhibitor nivolumab administered in combination with platinum-based doublet chemotherapy (PT-DC) showed improved response rates but higher-than-expected occurrence of adverse events, investigators reported.
In the CheckMate 012 phase I, multicohort trial of nivolumab for first-line treatment of advanced non–small-cell lung cancer, treatment-related adverse events occurred in 95% of patients, and the overall response rates ranged from 33% to 47%.
“It is unclear at this time whether nivolumab plus PT-DC offers improved long-term [overall survival] benefit compared with nivolumab monotherapy,” wrote Naiyer Rizvi, MD, of Memorial Sloan-Kettering Cancer Center, New York, and his associates (J Clin Oncol. 2016 June. doi: 10.1200/JCO.2016.66.9861).
Fifty-six patients with newly-diagnosed advanced NSCLC were assigned by histology to receive one of four drug regimens: nivolumab at 10 mg/kg plus gemcitabine-cisplatin (squamous, n = 12), nivolumab at 10 mg/kg plus pemetrexed-cisplatin (nonsquamous, n = 15), nivolumab at 10mg/kg plus paclitaxel-carboplatin (any histology, n = 15), or nivolumab at 5 mg/kg plus paclitaxel-carboplatin (any histology, n = 14).
For all patients, nivolumab and PT-DC were administered intravenously on day 1 of each 21-day cycle for four cycles followed by nivolumab monotherapy every 3 weeks. Median follow-up time for safety and efficacy was 19.0 months.
No dose-limiting toxicities occurred in the first 6 weeks of treatment. In the overall population, 95% of patients experienced treatment-related adverse events, the most common being fatigue, nausea, and alopecia. Grade three or four adverse events occurred in 45% of patients. Specifically, among those treated with nivolumab at 10 mg/kg, 93% experienced any grade and 50% experienced grade 3 or 4 treatment-related adverse events. Adverse events led to the discontinuation of all study therapy in 21% of patients. No treatment-related deaths were reported.
Overall response was highest among patients receiving nivolumab plus pemetrexed-cisplatin or paclitaxel-carboplatin, both with rates of 47% (95% confidence interval, 21-73 for both treatment arms).
In addition, median progression-free survival ranged from 4.8 to 7.1 months across treatment arms and was longest among patients receiving nivolumab at 5 mg/kg in combination with paclitaxel-carboplatin.
Finally, there was no clear association between PD-L1 expression and outcome.
All 17 investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock interest in multiple companies including Bristol-Myers Squibb, which funded the CheckMate 012 trial.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Nivolumab administered in combination with platinum-based doublet chemotherapy may improve response rates. However, the combination therapy may also increase the occurrence of adverse events.
Major finding: Treatment-related adverse events occurred in 95% of patients. The overall response rates ranged from 33% to 47% across treatment arms.
Data source: The CheckMate 012 phase I, multicohort trial.
Disclosures: All 17 investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or having ownership or stock interest in multiple companies including Bristol-Myers Squibb, which funded the study.
Racial bias linked with shorter, less-supportive appointments with black patients
Higher implicit racial bias among non-black oncologists was associated with aspects of their interactions and care of non-black patients, investigators report.
In a study of 18 non-black oncologists and 112 black patients, oncologists who were higher in implicit racial bias had shorter interactions with the black patients, and those interactions were rated less supportive by observers and patients. Higher implicit bias was also associated with more patient difficulty remembering contents of the interaction, Louis A. Penner, Ph.D., of Karmanos Cancer Institute, Detroit, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.66.3658).
“We acknowledge it is unlikely racial bias alone [that] is the major source of the well-documented, widespread racial disparities in cancer treatment. Factors such as patient socioeconomic status, limited access to high-quality health care, and patients’ health-related attitudes also contribute to racial disparities in cancer treatment. However, our data suggest that oncologist implicit racial bias may uniquely contribute to these disparities and should be further explored,” wrote Dr. Penner and his associates.
For the study, oncologists completed the Implicit Association Test that measured implicit racial bias prior to professional interaction. Patients also completed a baseline questionnaire prior to their appointment with an oncologist. Patients and physicians then had an appointment to discuss initial treatment for a current cancer. Patient questionnaires measuring perception of oncologist, the interaction, and recommended treatments, and physician questionnaires measuring patient participation were completed following the appointment. In addition, 96 of 112 appointments were videotaped and reviewed by four – two black and two white – researchers to assess various aspects of the physician-patient interaction.
Bivariate multilevel models revealed that higher implicit racial bias among oncologists was significantly associated to shorter appointment times (P = .02) and decreased use of supportive communication (P less than .01 when controlling for physician age). Implicit racial bias was not significantly correlated to talk-time ratio (P = .27) nor to the extent to which oncologists involved their patients in treatment decisions (P = .22).
Higher implicit racial bias was associated with patients experiencing greater difficulty remembering conversation contents (P less than .01) and patients perceiving the conversation as being less patient-centered (P = .01). Higher implicit racial bias was not significantly correlated to patient’s perception of treatment plans discussed (P = .19), post-visit distress (P = .57), or trust in their oncologist (P = .08).
This study was funded by the National Cancer Institute and the research advisory committee of the Southeast Michigan Partners Against Cancer. Eleven investigators had no relevant disclosures to report. The three other investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or participating in the speakers bureau of multiple companies.
On Twitter @jessnicolecraig
Higher implicit racial bias among non-black oncologists was associated with aspects of their interactions and care of non-black patients, investigators report.
In a study of 18 non-black oncologists and 112 black patients, oncologists who were higher in implicit racial bias had shorter interactions with the black patients, and those interactions were rated less supportive by observers and patients. Higher implicit bias was also associated with more patient difficulty remembering contents of the interaction, Louis A. Penner, Ph.D., of Karmanos Cancer Institute, Detroit, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.66.3658).
“We acknowledge it is unlikely racial bias alone [that] is the major source of the well-documented, widespread racial disparities in cancer treatment. Factors such as patient socioeconomic status, limited access to high-quality health care, and patients’ health-related attitudes also contribute to racial disparities in cancer treatment. However, our data suggest that oncologist implicit racial bias may uniquely contribute to these disparities and should be further explored,” wrote Dr. Penner and his associates.
For the study, oncologists completed the Implicit Association Test that measured implicit racial bias prior to professional interaction. Patients also completed a baseline questionnaire prior to their appointment with an oncologist. Patients and physicians then had an appointment to discuss initial treatment for a current cancer. Patient questionnaires measuring perception of oncologist, the interaction, and recommended treatments, and physician questionnaires measuring patient participation were completed following the appointment. In addition, 96 of 112 appointments were videotaped and reviewed by four – two black and two white – researchers to assess various aspects of the physician-patient interaction.
Bivariate multilevel models revealed that higher implicit racial bias among oncologists was significantly associated to shorter appointment times (P = .02) and decreased use of supportive communication (P less than .01 when controlling for physician age). Implicit racial bias was not significantly correlated to talk-time ratio (P = .27) nor to the extent to which oncologists involved their patients in treatment decisions (P = .22).
Higher implicit racial bias was associated with patients experiencing greater difficulty remembering conversation contents (P less than .01) and patients perceiving the conversation as being less patient-centered (P = .01). Higher implicit racial bias was not significantly correlated to patient’s perception of treatment plans discussed (P = .19), post-visit distress (P = .57), or trust in their oncologist (P = .08).
This study was funded by the National Cancer Institute and the research advisory committee of the Southeast Michigan Partners Against Cancer. Eleven investigators had no relevant disclosures to report. The three other investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or participating in the speakers bureau of multiple companies.
On Twitter @jessnicolecraig
Higher implicit racial bias among non-black oncologists was associated with aspects of their interactions and care of non-black patients, investigators report.
In a study of 18 non-black oncologists and 112 black patients, oncologists who were higher in implicit racial bias had shorter interactions with the black patients, and those interactions were rated less supportive by observers and patients. Higher implicit bias was also associated with more patient difficulty remembering contents of the interaction, Louis A. Penner, Ph.D., of Karmanos Cancer Institute, Detroit, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.66.3658).
“We acknowledge it is unlikely racial bias alone [that] is the major source of the well-documented, widespread racial disparities in cancer treatment. Factors such as patient socioeconomic status, limited access to high-quality health care, and patients’ health-related attitudes also contribute to racial disparities in cancer treatment. However, our data suggest that oncologist implicit racial bias may uniquely contribute to these disparities and should be further explored,” wrote Dr. Penner and his associates.
For the study, oncologists completed the Implicit Association Test that measured implicit racial bias prior to professional interaction. Patients also completed a baseline questionnaire prior to their appointment with an oncologist. Patients and physicians then had an appointment to discuss initial treatment for a current cancer. Patient questionnaires measuring perception of oncologist, the interaction, and recommended treatments, and physician questionnaires measuring patient participation were completed following the appointment. In addition, 96 of 112 appointments were videotaped and reviewed by four – two black and two white – researchers to assess various aspects of the physician-patient interaction.
Bivariate multilevel models revealed that higher implicit racial bias among oncologists was significantly associated to shorter appointment times (P = .02) and decreased use of supportive communication (P less than .01 when controlling for physician age). Implicit racial bias was not significantly correlated to talk-time ratio (P = .27) nor to the extent to which oncologists involved their patients in treatment decisions (P = .22).
Higher implicit racial bias was associated with patients experiencing greater difficulty remembering conversation contents (P less than .01) and patients perceiving the conversation as being less patient-centered (P = .01). Higher implicit racial bias was not significantly correlated to patient’s perception of treatment plans discussed (P = .19), post-visit distress (P = .57), or trust in their oncologist (P = .08).
This study was funded by the National Cancer Institute and the research advisory committee of the Southeast Michigan Partners Against Cancer. Eleven investigators had no relevant disclosures to report. The three other investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or participating in the speakers bureau of multiple companies.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Higher implicit racial bias among oncologists was linked with less-supportive care for black patients.
Major finding: Higher oncologist racial bias was significantly associated to shorter appointment times (P = .02) and less-supportive communication (P less than .01).
Data source: A randomized study involving 18 non-black oncologists and 112 black patients.
Disclosures: This study was funded by the National Cancer Institute and the research advisory committee of the Southeast Michigan Partners Against Cancer. Eleven investigators had no relevant disclosures to report. The three other investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or participating in the speakers bureau of Eli Lilly, Albrecht Pharmaceutical Consulting, GE Healthcare, and Karyopharm Therapeutics.