User login
Baby Don’t Cry: Evaluation of Prolonged, Unexplained Crying in Infants
Case
A previously healthy 4-month-old infant was brought to the ED late on a Sunday afternoon by his father, who reported that his son had been crying hysterically since the previous evening. The father stated that the infant was well until he awoke crying in his crib around midnight. Since then, the baby had not slept for more than 20 minutes uninterrupted and had not breastfed as usual due to the persistent crying. According to the father, the patient had been a “good baby” since birth, eating well and even sleeping through the night for the past month; specifically, his son’s continual crying was not typical of his usual behavior.
There was no known trauma, fever, congestion, cough, vomiting, or diarrhea at home, and the father confirmed the patient had been making wet diapers throughout the day. The baby had received his 4-month vaccines and, according to his pediatrician, had been following a normal growth pattern.
As the patient’s father related his son’s history, he appeared exhausted and extremely anxious, pacing the room as he spoke and trying unsuccessfully to quiet the baby. He stated that the patient’s mother, who was at home in bed with mastitis, was afraid their son had become ill because she had breastfed him while she had a fever. The father confessed that he was concerned that their older son, a rambunctious 2-year-old who shares the same room as the patient, may have accidentally done something to harm the baby unbeknownst to him or his wife.
In relating the history, the infant’s father was almost tearful as he admitted that he and his wife felt completely overwhelmed and helpless that they were not able to soothe or comfort the patient.
Overview
While often the healthiest of patients seen in the ED, infants with unexplained, prolonged crying are challenging to evaluate and discharge—especially in the care of distraught parents. The following review is intended to provide a basis for understanding the scope of normal crying in infants, how and if to diagnose infantile colic in the ED, and how to avoid missing common pathology requiring acute management in the infant with new, unexplained crying.
Normal Crying: How Much Is Too Much?
During the first 3 to 4 months of life, infants cry more than at any other time. Developmental pediatrician Harvey Karp, MD, dubs the first 3 months of an infant’s life as the “fourth trimester,” during which period the baby yearns for the calming acoustic and tactile sensations of the womb. He notes that crying in this fourth trimester is merely a response to the abruptly distinct stimulation of the outside world.1 Just as each baby tolerates these new sensations differently after birth, and is soothed in her or his own measure, each parent too has a different perception and tolerance of an infant’s crying—making for a clinical parameter that is difficult to clearly assess.
What, then has been established as the upper limit of normal for crying in infancy? In the 1960s, Brazelton’s studies defined normal crying as 1 hour and 45 minutes per day at age 2 weeks; 2 hours and 45 minutes at age 6 weeks; and less than 1 hour per day by age 12 weeks.2 A more recent meta-analysis reinforces Brazelton’s criteria, indicating that the mean duration of crying is approximately 2 hours per day during the first 6 weeks of life and decreasing to a daily mean of 72 minutes by age 10 to 12 weeks.3
Infantile Colic
As the caregiver’s report of the duration of crying is often subjective, more emphasis has been placed on evaluating patterns of newborn crying by defining what is excessive. Infantile colic, or excessive crying in an otherwise healthy baby, is classically defined as fussing or crying lasting more than 3 hours per day and occurring on more than 3 days per week in a baby who is gaining weight and is otherwise well.4 Severe colic is further described as the persistence of the crying pattern for more than 3 weeks. When using this “rule of 3s,” excessive crying is estimated to be present in 1.5% to 11.9% of the infant population. There are, however, many other definitions of what constitutes excessive crying, including the more inclusive and subjective definition of colic as “intermittent, unexplained crying during the first 3 months of life that reaches a point where the parents complain about it.”5
Depending upon the definition utilized, as many as 43% of infants experience excessive crying.6 What is more uniformly accepted in the extensive literature on infantile colic is the observation that crying because of colic is concentrated during the hours of 3:00 pm to 11:00 pm and is associated with infant behaviors such as clenched fists, back-arching, passing gas, grimacing, and flexing legs, as well as with maternal anxiety.1,2,7,8
Diagnostic Certainty and Setting
Infantile colic is a diagnosis that is made retrospectively in the setting of an otherwise healthy infant who is growing and developing appropriately, and whose excessive crying ultimately resolves without medical intervention. Since colic is difficult to diagnose from any single medical encounter in the ED, it must be a diagnosis reached after excluding other possible causes.
Soothing the Colicky Infant
Extensive parental reassurance is required prior to discharging a colicky infant home—with the understanding that by the time of presentation in the ED, most parents have already exhausted their parental soothing abilities and personal coping mechanisms. Moreover, the typical physician’s promise that “this too, shall pass” is just not a sufficient addition to the parental armamentarium to manage their baby’s colic. Parents instead must be given effective techniques to calm colicky infants. Karp enumerates the following alliterative “5 Ss” guideline for soothing and calming fussy infants:
(1) Swaddling;
(2) Side/Stomach position (not while sleeping);
(3)“Shhhhhing” to provide a soothing sound that recalls the womb;
(4) Swinging the baby rhythmically in parents’ arms; and
(5) Sucking, either a pacifier or the mother’s breast.1
Equally important guidance prior to discharge from the ED is to inform parents that if they become overwhelmed by the baby’s fussing, it is always better to place an infant in the crib and let him or her cry alone for some time rather than allowing frustration to build with the baby in one's arms and increasing the potential of unintentionally harming the infant.
The “Don’t Miss” Differential Diagnoses
Whereas, as much as 43% of the infant population may experience excessive crying, only approximately 5% of infants with colic have underlying organic disease.8 The emergency physician (EP) is responsible for identifying this 5% when these infants present to the ED. A useful way to focus the initial evaluation of excessive crying is to determine the chronicity of the infant’s symptoms. To begin with, it is important to identify those babies who are in the ED merely because they have finally overwhelmed their parents with recurrent, intermittent bouts of prolonged crying despite being otherwise healthy and maintaining eating and sleeping patterns largely unaffected by crying. These are the infants who, after a thorough physical examination revealing none of the causes described below, should be swaddled, “shhhhhh’ed,” swung, suckled, and discharged with parental reassurance.
The EP, however, must be able to differentiate the classically colicky infant described above from the baby who has acutely developed unexplained crying and is at higher risk of serious disease or condition. In a study of afebrile infants experiencing an acute episode of excessive, prolonged crying, approximately 60% had an underlying disease process requiring management.9 Fortunately, many of these diagnoses can be made by an astute physical examination. In addition to evaluating infants for the most typical causes of new, prolonged crying, such as otitis media and anal fissures, the following common diagnoses should be clinically excluded in all infants presenting to the ED with acute, unexplained crying.
Corneal Abrasion
Performing a comprehensive eye examination on an inconsolable infant is not an easy task. However, corneal abrasions and foreign bodies in the eye are notorious causes of acute, excessive crying in infants—ones that are not always accompanied by conspicuous signs such as lacrimation or conjunctival injection.10
Fluorescein staining of both corneas should be performed to evaluate for a corneal abrasion. The infant’s eyelids should also be everted to look for retained foreign bodies, especially when vertical corneal abrasions have been visualized with fluorescein staining. Administering a topical ophthalmic anesthetic prior to fluorescein staining is advisable; this can be both therapeutic and diagnostic since resolution of crying after numbing the infant’s affected eye supports the diagnosis of corneal abrasion or foreign body.
Infants with corneal abrasions can be managed with a topical antibiotic ointment and 24-hour follow-up. Of note, recent studies indicate that asymptomatic corneal abrasions are extremely common in the infant period, suggesting that physicians should be careful to consider and exclude other potential causes of acute, excessive crying before attributing the symptoms to a corneal abrasion identified on examination.11
Hair Tourniquet Syndrome
Also referred to as hair thread tourniquet syndrome, the circumferential constriction of an infant’s appendage with hair, thread, or another fine material may present with the chief complaint of crying. Hair tourniquets most often involve the toes, followed by the fingers and external genitalia, and, if unrecognized or untreated, will lead to ischemia and necrosis of the distal tissue. Sleepwear that encloses the feet is a strong
risk factor for toe tourniquets, and the use of mittens is similarly linked to finger tourniquets.7,12
The crying or irritable infant merits a thorough examination of all digits and external genitalia in search of constricting bands and resultant tissue swelling. If the tourniquet has gone unnoticed over time, severe swelling, embedding of the thread, or re-epithelialization of the skin over lacerated tissue may obscure the band and make simple removal impossible. In cases when the constricting band cannot be directly unwound, treatment options for a hair tourniquet include application of a depilatory agent (eg, Nair), which has been shown to effectively dissolve the hair within 8 minutes.13 If a severe laceration is present and the thread cannot be removed by unwinding, the depilatory agent should not be applied to open tissue; instead, the clinician should perform a dorsal incision of the toe or finger to remove the constricting material in its entirety.
Testicular Torsion
Testicular torsion is a common urologic emergency in the male pediatric population, with up to 12% of all cases occurring within the first year of life. It should be considered in any infant who develops acute, prolonged, unexplained crying. Eighty-five percent of infants with torsion between 1 and 12 months old will present with irritability, and 92% will present with a tender scrotal or inguinal mass14 that may go unnoticed by the parents. This possibility alone is a compelling reason to remove the diaper of an infant with prolonged crying and to perform a thorough physical examination.
While testicular deformity or a high-riding testicle can be difficult to assess on a crying baby, any scrotal enlargement, tenderness, or color change merits an emergent urology consult—and likely ultrasound imaging with Doppler sonography. Efficient and rapid management of infants with testicular torsion is essential as the testicular salvage rate is historically very poor in this population.15
Inguinal Hernia
The reported incidence of pediatric inguinal hernia is between 0.8% and 4.4%, with most patients presenting with a chief complaint of inguinal swelling. Inguinal hernias alone are symptomatic in up to 25% of infants and may present as prolonged crying.16 In these cases, inguinal hernia surgery is usually scheduled as an elective outpatient procedure. Serious issues arise when the bowel becomes edematous and incarcerated in the hernial sac, which can lead to ischemic necrosis and intestinal perforation, intestinal obstruction, and gonadal necrosis.
More than half of all cases of pediatric incarcerated hernias occur within the first 6 months of life. Infants younger than age 12 months—especially neonates—have a more severe and a higher incidence of complications related to incarcerated inguinal hernias than the rest of the pediatric population. In a recent study, one third of newborn patients were diagnosed with incarceration as their initial presentation of an inguinal hernia.17 This is another reason to remove the patient’s diaper and perform a complete physical examination on any infant presenting with acute, unexplained crying. An incarcerated inguinal hernia will likely be identified as a tender, firm, inguinal or scrotal mass with overlying swelling or discoloration and is a surgical emergency if it cannot be reduced manually.
Nonaccidental Trauma
Emergency physicians must maintain a high index of suspicion for nonaccidental trauma in pediatric patients. Studies demonstrate that 95% of serious intracranial injuries and 64% of all head injuries in infants younger than age 12 months are the result of physical abuse. Equally discouraging is the finding that as many as 75% of child abuse cases presenting to EDs are unrecognized.18,19 Infants presenting with excessive crying have elevated risk for nonaccidental trauma both because their crying may be a result of injuries sustained from physical abuse and also because their prolonged crying itself may have been a precipitating factor in nonaccidental trauma.
Stress predisposes a caregiver to child abuse and is particularly important to identify when evaluating an infant with prolonged crying and also when providing guidance to the parents of a child with symptoms of infantile colic. Additional signs of possible nonaccidental trauma include an inconsistent history, a delay in seeking medical care, inappropriate affect of the caregiver, and an injury that is not well explained by the caregiver’s history or is incompatible with the developmental stage of the child.
Other Causes
While there are no specific laboratory tests required for the diagnosis of the abovementioned common neonatal causes of acute, prolonged crying, the EP must always consider life-threatening conditions in the inconsolable or irritable infant, including dehydration, early shock, meningitis, and a surgical abdomen. The pursuit of an appropriate workup for these diseases, accompanied by an awareness that infants may present atypically (eg, hypothermic instead of febrile) will often lead to comprehensive laboratory studies.
The EP additionally cannot undervalue the importance of trending vital signs and should never assume that tachycardia and tachypnea are due to crying alone. The onus is on the EP to consider and exclude serious illness, and nonemergent causes of prolonged crying should not be diagnosed in the setting of lethargy or poor feeding without a thorough workup. Any infant who is crying too much and eating too little per parental history is an infant who should be screened for possible underlying serious disease.
Case Conclusion
The patient in this case was found to be afebrile, tachycardic, tachypneic, and with a normal oxygen saturation while crying inconsolably on initial examination. Upon removal of the infant’s closed-footed pajamas, the distal second toe of his right foot was noted to be markedly swollen and erythematous. The EP recognized the presence of a hair tourniquet but was unable to unwind the constricting hair due to the small size of the infant’s toe and extent of tissue swelling. Topical anesthetic cream was first applied to the toe and, as there was no visible laceration of the skin, depilatory cream was subsequently applied to the tourniquet. After 10 minutes, the toe was rubbed and rinsed with water and the hair tourniquet dissolved. There was significant improvement in tissue swelling and color within 1 hour of removal of the constricting band. The affected toe demonstrated adequate capillary refill and no sign of persistent vascular compromise. The patient’s crying subsided and his vital signs and remainder of his physical examination were normal.
Since there was evidence of superficial skin breakdown at the site of the hair tourniquet, the patient’s father was instructed to apply topical antibiotic ointment to the site and to follow-up with the patient’s pediatrician in 24 hours. Of note, the father stated that the infant’s mother had been experiencing significant postpartum hair loss recently and that they would now be careful to remove loose hair from the baby’s clothing and bedding—especially when dressing him in closed-footed pajamas or mittens.
Dr Leader is a fellow, department of pediatric emergency medicine, Eastern Virginia Medical School, Norfolk, Virginia. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Karp H. The “fourth trimester”: a framework and strategy for understanding and resolving colic. Contemp Pediatrics. 2004;21:94.
- Brazelton TB. Crying in infancy. Pediatrics. 1962;29:579-588.
- Wolke D, Samara M, Alvarez Wolke M. Meta-analysis of fuss/cry durations and colic prevalence across countries. In: Proceedings of the 11th International Infant Cry Research Workshop. 8-10 June 2011. Zeist, The Netherlands. 2011.
- Wessel MA, Cobb JC, Jackson EB, Harris GS Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics. 1954;14(5):421-435.
- Schmitt BD. Colic: excessive crying in newborns. Clin Perinatol. 1985;12(2):441-451.
- Reijneveld SA, Brugman E, Hirasing RA. Excessive infant crying: the impact of varying definitions. Pediatrics. 2001;108(4):893-897.
- Hicks M. An evidence-based, systematic approach to acute, unexplained, excessive crying in infants. Pediatr Emerg Med Pract. 2005;2(2-3):1-50.
- Barr RG. Colic and crying syndromes in infants. Pediatrics. 1998;102(5 Suppl E):1282-1286.
- Poole SR. The infant with acute, unexplained, excessive crying. Pediatrics 1991;88(3):450-455.
- Harkness MJ. Corneal abrasion in infancy as a cause of inconsolable crying. Pediatr Emerg Care. 1989;5(4):242-244.
- Shope TR, Rieg TS, Kathiria NN. Corneal abrasions in young infants. Pediatrics. 2012;125(3):e585-e589.
- Barton DJ, Sloan GM, Nichter LS, Reinisch JF. Hair-thread tourniquet syndrome. Pediatrics. 1988;82(6):925-928.
- Plesa JA, Shoup K, Manole MD, Hickey RW. Effect of depilatory agent on cotton, polyester, and rayon versus human hair in a laboratory setting. Ann Emerg Med. 2015;65(3):256-259.
- Mano R, Livne PM, Nevo A, Sivan B, Ben-Meir D. Testicular torsion in the first year of life—characteristics and treatment outcome. Urology. 2013;82(5):1132-1137.
- Saxena AK, Castellani C, Ruttenstock EM, Höllwarth ME. Testicular torsion: a 15-year single-centre clinical and histological analysis. Acta Paediatr. 2012;101(7):e282-e286.
- Ein SH, Njere I, Ein A. Six thousand three hundred sixty-one pediatric inguinal hernias: a 35-year review. J Ped Surg. 2006;41(5):980-986.
- Erdoğan D, Karaman I, Aslan MK, Karaman A, Cavuşoğlu YH. Analysis of 3,776 pediatric inguinal hernia and hydrocele cases in a tertiary center. J Pediatr Surg. 2013;48(8):1767-1772.
- Sirotnak AP, Grigsby T, Krugman RD. Physical abuse of children. Pediatr Rev. 2004;25(8):264-277
- Kunen S, Hume P, Perret JN, Mandry CV, Patterson TR. Underdiagnosis of child abuse in emergency departments. Acad Emerg Med. 2003;10(5):546-a.
Case
A previously healthy 4-month-old infant was brought to the ED late on a Sunday afternoon by his father, who reported that his son had been crying hysterically since the previous evening. The father stated that the infant was well until he awoke crying in his crib around midnight. Since then, the baby had not slept for more than 20 minutes uninterrupted and had not breastfed as usual due to the persistent crying. According to the father, the patient had been a “good baby” since birth, eating well and even sleeping through the night for the past month; specifically, his son’s continual crying was not typical of his usual behavior.
There was no known trauma, fever, congestion, cough, vomiting, or diarrhea at home, and the father confirmed the patient had been making wet diapers throughout the day. The baby had received his 4-month vaccines and, according to his pediatrician, had been following a normal growth pattern.
As the patient’s father related his son’s history, he appeared exhausted and extremely anxious, pacing the room as he spoke and trying unsuccessfully to quiet the baby. He stated that the patient’s mother, who was at home in bed with mastitis, was afraid their son had become ill because she had breastfed him while she had a fever. The father confessed that he was concerned that their older son, a rambunctious 2-year-old who shares the same room as the patient, may have accidentally done something to harm the baby unbeknownst to him or his wife.
In relating the history, the infant’s father was almost tearful as he admitted that he and his wife felt completely overwhelmed and helpless that they were not able to soothe or comfort the patient.
Overview
While often the healthiest of patients seen in the ED, infants with unexplained, prolonged crying are challenging to evaluate and discharge—especially in the care of distraught parents. The following review is intended to provide a basis for understanding the scope of normal crying in infants, how and if to diagnose infantile colic in the ED, and how to avoid missing common pathology requiring acute management in the infant with new, unexplained crying.
Normal Crying: How Much Is Too Much?
During the first 3 to 4 months of life, infants cry more than at any other time. Developmental pediatrician Harvey Karp, MD, dubs the first 3 months of an infant’s life as the “fourth trimester,” during which period the baby yearns for the calming acoustic and tactile sensations of the womb. He notes that crying in this fourth trimester is merely a response to the abruptly distinct stimulation of the outside world.1 Just as each baby tolerates these new sensations differently after birth, and is soothed in her or his own measure, each parent too has a different perception and tolerance of an infant’s crying—making for a clinical parameter that is difficult to clearly assess.
What, then has been established as the upper limit of normal for crying in infancy? In the 1960s, Brazelton’s studies defined normal crying as 1 hour and 45 minutes per day at age 2 weeks; 2 hours and 45 minutes at age 6 weeks; and less than 1 hour per day by age 12 weeks.2 A more recent meta-analysis reinforces Brazelton’s criteria, indicating that the mean duration of crying is approximately 2 hours per day during the first 6 weeks of life and decreasing to a daily mean of 72 minutes by age 10 to 12 weeks.3
Infantile Colic
As the caregiver’s report of the duration of crying is often subjective, more emphasis has been placed on evaluating patterns of newborn crying by defining what is excessive. Infantile colic, or excessive crying in an otherwise healthy baby, is classically defined as fussing or crying lasting more than 3 hours per day and occurring on more than 3 days per week in a baby who is gaining weight and is otherwise well.4 Severe colic is further described as the persistence of the crying pattern for more than 3 weeks. When using this “rule of 3s,” excessive crying is estimated to be present in 1.5% to 11.9% of the infant population. There are, however, many other definitions of what constitutes excessive crying, including the more inclusive and subjective definition of colic as “intermittent, unexplained crying during the first 3 months of life that reaches a point where the parents complain about it.”5
Depending upon the definition utilized, as many as 43% of infants experience excessive crying.6 What is more uniformly accepted in the extensive literature on infantile colic is the observation that crying because of colic is concentrated during the hours of 3:00 pm to 11:00 pm and is associated with infant behaviors such as clenched fists, back-arching, passing gas, grimacing, and flexing legs, as well as with maternal anxiety.1,2,7,8
Diagnostic Certainty and Setting
Infantile colic is a diagnosis that is made retrospectively in the setting of an otherwise healthy infant who is growing and developing appropriately, and whose excessive crying ultimately resolves without medical intervention. Since colic is difficult to diagnose from any single medical encounter in the ED, it must be a diagnosis reached after excluding other possible causes.
Soothing the Colicky Infant
Extensive parental reassurance is required prior to discharging a colicky infant home—with the understanding that by the time of presentation in the ED, most parents have already exhausted their parental soothing abilities and personal coping mechanisms. Moreover, the typical physician’s promise that “this too, shall pass” is just not a sufficient addition to the parental armamentarium to manage their baby’s colic. Parents instead must be given effective techniques to calm colicky infants. Karp enumerates the following alliterative “5 Ss” guideline for soothing and calming fussy infants:
(1) Swaddling;
(2) Side/Stomach position (not while sleeping);
(3)“Shhhhhing” to provide a soothing sound that recalls the womb;
(4) Swinging the baby rhythmically in parents’ arms; and
(5) Sucking, either a pacifier or the mother’s breast.1
Equally important guidance prior to discharge from the ED is to inform parents that if they become overwhelmed by the baby’s fussing, it is always better to place an infant in the crib and let him or her cry alone for some time rather than allowing frustration to build with the baby in one's arms and increasing the potential of unintentionally harming the infant.
The “Don’t Miss” Differential Diagnoses
Whereas, as much as 43% of the infant population may experience excessive crying, only approximately 5% of infants with colic have underlying organic disease.8 The emergency physician (EP) is responsible for identifying this 5% when these infants present to the ED. A useful way to focus the initial evaluation of excessive crying is to determine the chronicity of the infant’s symptoms. To begin with, it is important to identify those babies who are in the ED merely because they have finally overwhelmed their parents with recurrent, intermittent bouts of prolonged crying despite being otherwise healthy and maintaining eating and sleeping patterns largely unaffected by crying. These are the infants who, after a thorough physical examination revealing none of the causes described below, should be swaddled, “shhhhhh’ed,” swung, suckled, and discharged with parental reassurance.
The EP, however, must be able to differentiate the classically colicky infant described above from the baby who has acutely developed unexplained crying and is at higher risk of serious disease or condition. In a study of afebrile infants experiencing an acute episode of excessive, prolonged crying, approximately 60% had an underlying disease process requiring management.9 Fortunately, many of these diagnoses can be made by an astute physical examination. In addition to evaluating infants for the most typical causes of new, prolonged crying, such as otitis media and anal fissures, the following common diagnoses should be clinically excluded in all infants presenting to the ED with acute, unexplained crying.
Corneal Abrasion
Performing a comprehensive eye examination on an inconsolable infant is not an easy task. However, corneal abrasions and foreign bodies in the eye are notorious causes of acute, excessive crying in infants—ones that are not always accompanied by conspicuous signs such as lacrimation or conjunctival injection.10
Fluorescein staining of both corneas should be performed to evaluate for a corneal abrasion. The infant’s eyelids should also be everted to look for retained foreign bodies, especially when vertical corneal abrasions have been visualized with fluorescein staining. Administering a topical ophthalmic anesthetic prior to fluorescein staining is advisable; this can be both therapeutic and diagnostic since resolution of crying after numbing the infant’s affected eye supports the diagnosis of corneal abrasion or foreign body.
Infants with corneal abrasions can be managed with a topical antibiotic ointment and 24-hour follow-up. Of note, recent studies indicate that asymptomatic corneal abrasions are extremely common in the infant period, suggesting that physicians should be careful to consider and exclude other potential causes of acute, excessive crying before attributing the symptoms to a corneal abrasion identified on examination.11
Hair Tourniquet Syndrome
Also referred to as hair thread tourniquet syndrome, the circumferential constriction of an infant’s appendage with hair, thread, or another fine material may present with the chief complaint of crying. Hair tourniquets most often involve the toes, followed by the fingers and external genitalia, and, if unrecognized or untreated, will lead to ischemia and necrosis of the distal tissue. Sleepwear that encloses the feet is a strong
risk factor for toe tourniquets, and the use of mittens is similarly linked to finger tourniquets.7,12
The crying or irritable infant merits a thorough examination of all digits and external genitalia in search of constricting bands and resultant tissue swelling. If the tourniquet has gone unnoticed over time, severe swelling, embedding of the thread, or re-epithelialization of the skin over lacerated tissue may obscure the band and make simple removal impossible. In cases when the constricting band cannot be directly unwound, treatment options for a hair tourniquet include application of a depilatory agent (eg, Nair), which has been shown to effectively dissolve the hair within 8 minutes.13 If a severe laceration is present and the thread cannot be removed by unwinding, the depilatory agent should not be applied to open tissue; instead, the clinician should perform a dorsal incision of the toe or finger to remove the constricting material in its entirety.
Testicular Torsion
Testicular torsion is a common urologic emergency in the male pediatric population, with up to 12% of all cases occurring within the first year of life. It should be considered in any infant who develops acute, prolonged, unexplained crying. Eighty-five percent of infants with torsion between 1 and 12 months old will present with irritability, and 92% will present with a tender scrotal or inguinal mass14 that may go unnoticed by the parents. This possibility alone is a compelling reason to remove the diaper of an infant with prolonged crying and to perform a thorough physical examination.
While testicular deformity or a high-riding testicle can be difficult to assess on a crying baby, any scrotal enlargement, tenderness, or color change merits an emergent urology consult—and likely ultrasound imaging with Doppler sonography. Efficient and rapid management of infants with testicular torsion is essential as the testicular salvage rate is historically very poor in this population.15
Inguinal Hernia
The reported incidence of pediatric inguinal hernia is between 0.8% and 4.4%, with most patients presenting with a chief complaint of inguinal swelling. Inguinal hernias alone are symptomatic in up to 25% of infants and may present as prolonged crying.16 In these cases, inguinal hernia surgery is usually scheduled as an elective outpatient procedure. Serious issues arise when the bowel becomes edematous and incarcerated in the hernial sac, which can lead to ischemic necrosis and intestinal perforation, intestinal obstruction, and gonadal necrosis.
More than half of all cases of pediatric incarcerated hernias occur within the first 6 months of life. Infants younger than age 12 months—especially neonates—have a more severe and a higher incidence of complications related to incarcerated inguinal hernias than the rest of the pediatric population. In a recent study, one third of newborn patients were diagnosed with incarceration as their initial presentation of an inguinal hernia.17 This is another reason to remove the patient’s diaper and perform a complete physical examination on any infant presenting with acute, unexplained crying. An incarcerated inguinal hernia will likely be identified as a tender, firm, inguinal or scrotal mass with overlying swelling or discoloration and is a surgical emergency if it cannot be reduced manually.
Nonaccidental Trauma
Emergency physicians must maintain a high index of suspicion for nonaccidental trauma in pediatric patients. Studies demonstrate that 95% of serious intracranial injuries and 64% of all head injuries in infants younger than age 12 months are the result of physical abuse. Equally discouraging is the finding that as many as 75% of child abuse cases presenting to EDs are unrecognized.18,19 Infants presenting with excessive crying have elevated risk for nonaccidental trauma both because their crying may be a result of injuries sustained from physical abuse and also because their prolonged crying itself may have been a precipitating factor in nonaccidental trauma.
Stress predisposes a caregiver to child abuse and is particularly important to identify when evaluating an infant with prolonged crying and also when providing guidance to the parents of a child with symptoms of infantile colic. Additional signs of possible nonaccidental trauma include an inconsistent history, a delay in seeking medical care, inappropriate affect of the caregiver, and an injury that is not well explained by the caregiver’s history or is incompatible with the developmental stage of the child.
Other Causes
While there are no specific laboratory tests required for the diagnosis of the abovementioned common neonatal causes of acute, prolonged crying, the EP must always consider life-threatening conditions in the inconsolable or irritable infant, including dehydration, early shock, meningitis, and a surgical abdomen. The pursuit of an appropriate workup for these diseases, accompanied by an awareness that infants may present atypically (eg, hypothermic instead of febrile) will often lead to comprehensive laboratory studies.
The EP additionally cannot undervalue the importance of trending vital signs and should never assume that tachycardia and tachypnea are due to crying alone. The onus is on the EP to consider and exclude serious illness, and nonemergent causes of prolonged crying should not be diagnosed in the setting of lethargy or poor feeding without a thorough workup. Any infant who is crying too much and eating too little per parental history is an infant who should be screened for possible underlying serious disease.
Case Conclusion
The patient in this case was found to be afebrile, tachycardic, tachypneic, and with a normal oxygen saturation while crying inconsolably on initial examination. Upon removal of the infant’s closed-footed pajamas, the distal second toe of his right foot was noted to be markedly swollen and erythematous. The EP recognized the presence of a hair tourniquet but was unable to unwind the constricting hair due to the small size of the infant’s toe and extent of tissue swelling. Topical anesthetic cream was first applied to the toe and, as there was no visible laceration of the skin, depilatory cream was subsequently applied to the tourniquet. After 10 minutes, the toe was rubbed and rinsed with water and the hair tourniquet dissolved. There was significant improvement in tissue swelling and color within 1 hour of removal of the constricting band. The affected toe demonstrated adequate capillary refill and no sign of persistent vascular compromise. The patient’s crying subsided and his vital signs and remainder of his physical examination were normal.
Since there was evidence of superficial skin breakdown at the site of the hair tourniquet, the patient’s father was instructed to apply topical antibiotic ointment to the site and to follow-up with the patient’s pediatrician in 24 hours. Of note, the father stated that the infant’s mother had been experiencing significant postpartum hair loss recently and that they would now be careful to remove loose hair from the baby’s clothing and bedding—especially when dressing him in closed-footed pajamas or mittens.
Dr Leader is a fellow, department of pediatric emergency medicine, Eastern Virginia Medical School, Norfolk, Virginia. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
Case
A previously healthy 4-month-old infant was brought to the ED late on a Sunday afternoon by his father, who reported that his son had been crying hysterically since the previous evening. The father stated that the infant was well until he awoke crying in his crib around midnight. Since then, the baby had not slept for more than 20 minutes uninterrupted and had not breastfed as usual due to the persistent crying. According to the father, the patient had been a “good baby” since birth, eating well and even sleeping through the night for the past month; specifically, his son’s continual crying was not typical of his usual behavior.
There was no known trauma, fever, congestion, cough, vomiting, or diarrhea at home, and the father confirmed the patient had been making wet diapers throughout the day. The baby had received his 4-month vaccines and, according to his pediatrician, had been following a normal growth pattern.
As the patient’s father related his son’s history, he appeared exhausted and extremely anxious, pacing the room as he spoke and trying unsuccessfully to quiet the baby. He stated that the patient’s mother, who was at home in bed with mastitis, was afraid their son had become ill because she had breastfed him while she had a fever. The father confessed that he was concerned that their older son, a rambunctious 2-year-old who shares the same room as the patient, may have accidentally done something to harm the baby unbeknownst to him or his wife.
In relating the history, the infant’s father was almost tearful as he admitted that he and his wife felt completely overwhelmed and helpless that they were not able to soothe or comfort the patient.
Overview
While often the healthiest of patients seen in the ED, infants with unexplained, prolonged crying are challenging to evaluate and discharge—especially in the care of distraught parents. The following review is intended to provide a basis for understanding the scope of normal crying in infants, how and if to diagnose infantile colic in the ED, and how to avoid missing common pathology requiring acute management in the infant with new, unexplained crying.
Normal Crying: How Much Is Too Much?
During the first 3 to 4 months of life, infants cry more than at any other time. Developmental pediatrician Harvey Karp, MD, dubs the first 3 months of an infant’s life as the “fourth trimester,” during which period the baby yearns for the calming acoustic and tactile sensations of the womb. He notes that crying in this fourth trimester is merely a response to the abruptly distinct stimulation of the outside world.1 Just as each baby tolerates these new sensations differently after birth, and is soothed in her or his own measure, each parent too has a different perception and tolerance of an infant’s crying—making for a clinical parameter that is difficult to clearly assess.
What, then has been established as the upper limit of normal for crying in infancy? In the 1960s, Brazelton’s studies defined normal crying as 1 hour and 45 minutes per day at age 2 weeks; 2 hours and 45 minutes at age 6 weeks; and less than 1 hour per day by age 12 weeks.2 A more recent meta-analysis reinforces Brazelton’s criteria, indicating that the mean duration of crying is approximately 2 hours per day during the first 6 weeks of life and decreasing to a daily mean of 72 minutes by age 10 to 12 weeks.3
Infantile Colic
As the caregiver’s report of the duration of crying is often subjective, more emphasis has been placed on evaluating patterns of newborn crying by defining what is excessive. Infantile colic, or excessive crying in an otherwise healthy baby, is classically defined as fussing or crying lasting more than 3 hours per day and occurring on more than 3 days per week in a baby who is gaining weight and is otherwise well.4 Severe colic is further described as the persistence of the crying pattern for more than 3 weeks. When using this “rule of 3s,” excessive crying is estimated to be present in 1.5% to 11.9% of the infant population. There are, however, many other definitions of what constitutes excessive crying, including the more inclusive and subjective definition of colic as “intermittent, unexplained crying during the first 3 months of life that reaches a point where the parents complain about it.”5
Depending upon the definition utilized, as many as 43% of infants experience excessive crying.6 What is more uniformly accepted in the extensive literature on infantile colic is the observation that crying because of colic is concentrated during the hours of 3:00 pm to 11:00 pm and is associated with infant behaviors such as clenched fists, back-arching, passing gas, grimacing, and flexing legs, as well as with maternal anxiety.1,2,7,8
Diagnostic Certainty and Setting
Infantile colic is a diagnosis that is made retrospectively in the setting of an otherwise healthy infant who is growing and developing appropriately, and whose excessive crying ultimately resolves without medical intervention. Since colic is difficult to diagnose from any single medical encounter in the ED, it must be a diagnosis reached after excluding other possible causes.
Soothing the Colicky Infant
Extensive parental reassurance is required prior to discharging a colicky infant home—with the understanding that by the time of presentation in the ED, most parents have already exhausted their parental soothing abilities and personal coping mechanisms. Moreover, the typical physician’s promise that “this too, shall pass” is just not a sufficient addition to the parental armamentarium to manage their baby’s colic. Parents instead must be given effective techniques to calm colicky infants. Karp enumerates the following alliterative “5 Ss” guideline for soothing and calming fussy infants:
(1) Swaddling;
(2) Side/Stomach position (not while sleeping);
(3)“Shhhhhing” to provide a soothing sound that recalls the womb;
(4) Swinging the baby rhythmically in parents’ arms; and
(5) Sucking, either a pacifier or the mother’s breast.1
Equally important guidance prior to discharge from the ED is to inform parents that if they become overwhelmed by the baby’s fussing, it is always better to place an infant in the crib and let him or her cry alone for some time rather than allowing frustration to build with the baby in one's arms and increasing the potential of unintentionally harming the infant.
The “Don’t Miss” Differential Diagnoses
Whereas, as much as 43% of the infant population may experience excessive crying, only approximately 5% of infants with colic have underlying organic disease.8 The emergency physician (EP) is responsible for identifying this 5% when these infants present to the ED. A useful way to focus the initial evaluation of excessive crying is to determine the chronicity of the infant’s symptoms. To begin with, it is important to identify those babies who are in the ED merely because they have finally overwhelmed their parents with recurrent, intermittent bouts of prolonged crying despite being otherwise healthy and maintaining eating and sleeping patterns largely unaffected by crying. These are the infants who, after a thorough physical examination revealing none of the causes described below, should be swaddled, “shhhhhh’ed,” swung, suckled, and discharged with parental reassurance.
The EP, however, must be able to differentiate the classically colicky infant described above from the baby who has acutely developed unexplained crying and is at higher risk of serious disease or condition. In a study of afebrile infants experiencing an acute episode of excessive, prolonged crying, approximately 60% had an underlying disease process requiring management.9 Fortunately, many of these diagnoses can be made by an astute physical examination. In addition to evaluating infants for the most typical causes of new, prolonged crying, such as otitis media and anal fissures, the following common diagnoses should be clinically excluded in all infants presenting to the ED with acute, unexplained crying.
Corneal Abrasion
Performing a comprehensive eye examination on an inconsolable infant is not an easy task. However, corneal abrasions and foreign bodies in the eye are notorious causes of acute, excessive crying in infants—ones that are not always accompanied by conspicuous signs such as lacrimation or conjunctival injection.10
Fluorescein staining of both corneas should be performed to evaluate for a corneal abrasion. The infant’s eyelids should also be everted to look for retained foreign bodies, especially when vertical corneal abrasions have been visualized with fluorescein staining. Administering a topical ophthalmic anesthetic prior to fluorescein staining is advisable; this can be both therapeutic and diagnostic since resolution of crying after numbing the infant’s affected eye supports the diagnosis of corneal abrasion or foreign body.
Infants with corneal abrasions can be managed with a topical antibiotic ointment and 24-hour follow-up. Of note, recent studies indicate that asymptomatic corneal abrasions are extremely common in the infant period, suggesting that physicians should be careful to consider and exclude other potential causes of acute, excessive crying before attributing the symptoms to a corneal abrasion identified on examination.11
Hair Tourniquet Syndrome
Also referred to as hair thread tourniquet syndrome, the circumferential constriction of an infant’s appendage with hair, thread, or another fine material may present with the chief complaint of crying. Hair tourniquets most often involve the toes, followed by the fingers and external genitalia, and, if unrecognized or untreated, will lead to ischemia and necrosis of the distal tissue. Sleepwear that encloses the feet is a strong
risk factor for toe tourniquets, and the use of mittens is similarly linked to finger tourniquets.7,12
The crying or irritable infant merits a thorough examination of all digits and external genitalia in search of constricting bands and resultant tissue swelling. If the tourniquet has gone unnoticed over time, severe swelling, embedding of the thread, or re-epithelialization of the skin over lacerated tissue may obscure the band and make simple removal impossible. In cases when the constricting band cannot be directly unwound, treatment options for a hair tourniquet include application of a depilatory agent (eg, Nair), which has been shown to effectively dissolve the hair within 8 minutes.13 If a severe laceration is present and the thread cannot be removed by unwinding, the depilatory agent should not be applied to open tissue; instead, the clinician should perform a dorsal incision of the toe or finger to remove the constricting material in its entirety.
Testicular Torsion
Testicular torsion is a common urologic emergency in the male pediatric population, with up to 12% of all cases occurring within the first year of life. It should be considered in any infant who develops acute, prolonged, unexplained crying. Eighty-five percent of infants with torsion between 1 and 12 months old will present with irritability, and 92% will present with a tender scrotal or inguinal mass14 that may go unnoticed by the parents. This possibility alone is a compelling reason to remove the diaper of an infant with prolonged crying and to perform a thorough physical examination.
While testicular deformity or a high-riding testicle can be difficult to assess on a crying baby, any scrotal enlargement, tenderness, or color change merits an emergent urology consult—and likely ultrasound imaging with Doppler sonography. Efficient and rapid management of infants with testicular torsion is essential as the testicular salvage rate is historically very poor in this population.15
Inguinal Hernia
The reported incidence of pediatric inguinal hernia is between 0.8% and 4.4%, with most patients presenting with a chief complaint of inguinal swelling. Inguinal hernias alone are symptomatic in up to 25% of infants and may present as prolonged crying.16 In these cases, inguinal hernia surgery is usually scheduled as an elective outpatient procedure. Serious issues arise when the bowel becomes edematous and incarcerated in the hernial sac, which can lead to ischemic necrosis and intestinal perforation, intestinal obstruction, and gonadal necrosis.
More than half of all cases of pediatric incarcerated hernias occur within the first 6 months of life. Infants younger than age 12 months—especially neonates—have a more severe and a higher incidence of complications related to incarcerated inguinal hernias than the rest of the pediatric population. In a recent study, one third of newborn patients were diagnosed with incarceration as their initial presentation of an inguinal hernia.17 This is another reason to remove the patient’s diaper and perform a complete physical examination on any infant presenting with acute, unexplained crying. An incarcerated inguinal hernia will likely be identified as a tender, firm, inguinal or scrotal mass with overlying swelling or discoloration and is a surgical emergency if it cannot be reduced manually.
Nonaccidental Trauma
Emergency physicians must maintain a high index of suspicion for nonaccidental trauma in pediatric patients. Studies demonstrate that 95% of serious intracranial injuries and 64% of all head injuries in infants younger than age 12 months are the result of physical abuse. Equally discouraging is the finding that as many as 75% of child abuse cases presenting to EDs are unrecognized.18,19 Infants presenting with excessive crying have elevated risk for nonaccidental trauma both because their crying may be a result of injuries sustained from physical abuse and also because their prolonged crying itself may have been a precipitating factor in nonaccidental trauma.
Stress predisposes a caregiver to child abuse and is particularly important to identify when evaluating an infant with prolonged crying and also when providing guidance to the parents of a child with symptoms of infantile colic. Additional signs of possible nonaccidental trauma include an inconsistent history, a delay in seeking medical care, inappropriate affect of the caregiver, and an injury that is not well explained by the caregiver’s history or is incompatible with the developmental stage of the child.
Other Causes
While there are no specific laboratory tests required for the diagnosis of the abovementioned common neonatal causes of acute, prolonged crying, the EP must always consider life-threatening conditions in the inconsolable or irritable infant, including dehydration, early shock, meningitis, and a surgical abdomen. The pursuit of an appropriate workup for these diseases, accompanied by an awareness that infants may present atypically (eg, hypothermic instead of febrile) will often lead to comprehensive laboratory studies.
The EP additionally cannot undervalue the importance of trending vital signs and should never assume that tachycardia and tachypnea are due to crying alone. The onus is on the EP to consider and exclude serious illness, and nonemergent causes of prolonged crying should not be diagnosed in the setting of lethargy or poor feeding without a thorough workup. Any infant who is crying too much and eating too little per parental history is an infant who should be screened for possible underlying serious disease.
Case Conclusion
The patient in this case was found to be afebrile, tachycardic, tachypneic, and with a normal oxygen saturation while crying inconsolably on initial examination. Upon removal of the infant’s closed-footed pajamas, the distal second toe of his right foot was noted to be markedly swollen and erythematous. The EP recognized the presence of a hair tourniquet but was unable to unwind the constricting hair due to the small size of the infant’s toe and extent of tissue swelling. Topical anesthetic cream was first applied to the toe and, as there was no visible laceration of the skin, depilatory cream was subsequently applied to the tourniquet. After 10 minutes, the toe was rubbed and rinsed with water and the hair tourniquet dissolved. There was significant improvement in tissue swelling and color within 1 hour of removal of the constricting band. The affected toe demonstrated adequate capillary refill and no sign of persistent vascular compromise. The patient’s crying subsided and his vital signs and remainder of his physical examination were normal.
Since there was evidence of superficial skin breakdown at the site of the hair tourniquet, the patient’s father was instructed to apply topical antibiotic ointment to the site and to follow-up with the patient’s pediatrician in 24 hours. Of note, the father stated that the infant’s mother had been experiencing significant postpartum hair loss recently and that they would now be careful to remove loose hair from the baby’s clothing and bedding—especially when dressing him in closed-footed pajamas or mittens.
Dr Leader is a fellow, department of pediatric emergency medicine, Eastern Virginia Medical School, Norfolk, Virginia. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Karp H. The “fourth trimester”: a framework and strategy for understanding and resolving colic. Contemp Pediatrics. 2004;21:94.
- Brazelton TB. Crying in infancy. Pediatrics. 1962;29:579-588.
- Wolke D, Samara M, Alvarez Wolke M. Meta-analysis of fuss/cry durations and colic prevalence across countries. In: Proceedings of the 11th International Infant Cry Research Workshop. 8-10 June 2011. Zeist, The Netherlands. 2011.
- Wessel MA, Cobb JC, Jackson EB, Harris GS Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics. 1954;14(5):421-435.
- Schmitt BD. Colic: excessive crying in newborns. Clin Perinatol. 1985;12(2):441-451.
- Reijneveld SA, Brugman E, Hirasing RA. Excessive infant crying: the impact of varying definitions. Pediatrics. 2001;108(4):893-897.
- Hicks M. An evidence-based, systematic approach to acute, unexplained, excessive crying in infants. Pediatr Emerg Med Pract. 2005;2(2-3):1-50.
- Barr RG. Colic and crying syndromes in infants. Pediatrics. 1998;102(5 Suppl E):1282-1286.
- Poole SR. The infant with acute, unexplained, excessive crying. Pediatrics 1991;88(3):450-455.
- Harkness MJ. Corneal abrasion in infancy as a cause of inconsolable crying. Pediatr Emerg Care. 1989;5(4):242-244.
- Shope TR, Rieg TS, Kathiria NN. Corneal abrasions in young infants. Pediatrics. 2012;125(3):e585-e589.
- Barton DJ, Sloan GM, Nichter LS, Reinisch JF. Hair-thread tourniquet syndrome. Pediatrics. 1988;82(6):925-928.
- Plesa JA, Shoup K, Manole MD, Hickey RW. Effect of depilatory agent on cotton, polyester, and rayon versus human hair in a laboratory setting. Ann Emerg Med. 2015;65(3):256-259.
- Mano R, Livne PM, Nevo A, Sivan B, Ben-Meir D. Testicular torsion in the first year of life—characteristics and treatment outcome. Urology. 2013;82(5):1132-1137.
- Saxena AK, Castellani C, Ruttenstock EM, Höllwarth ME. Testicular torsion: a 15-year single-centre clinical and histological analysis. Acta Paediatr. 2012;101(7):e282-e286.
- Ein SH, Njere I, Ein A. Six thousand three hundred sixty-one pediatric inguinal hernias: a 35-year review. J Ped Surg. 2006;41(5):980-986.
- Erdoğan D, Karaman I, Aslan MK, Karaman A, Cavuşoğlu YH. Analysis of 3,776 pediatric inguinal hernia and hydrocele cases in a tertiary center. J Pediatr Surg. 2013;48(8):1767-1772.
- Sirotnak AP, Grigsby T, Krugman RD. Physical abuse of children. Pediatr Rev. 2004;25(8):264-277
- Kunen S, Hume P, Perret JN, Mandry CV, Patterson TR. Underdiagnosis of child abuse in emergency departments. Acad Emerg Med. 2003;10(5):546-a.
- Karp H. The “fourth trimester”: a framework and strategy for understanding and resolving colic. Contemp Pediatrics. 2004;21:94.
- Brazelton TB. Crying in infancy. Pediatrics. 1962;29:579-588.
- Wolke D, Samara M, Alvarez Wolke M. Meta-analysis of fuss/cry durations and colic prevalence across countries. In: Proceedings of the 11th International Infant Cry Research Workshop. 8-10 June 2011. Zeist, The Netherlands. 2011.
- Wessel MA, Cobb JC, Jackson EB, Harris GS Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics. 1954;14(5):421-435.
- Schmitt BD. Colic: excessive crying in newborns. Clin Perinatol. 1985;12(2):441-451.
- Reijneveld SA, Brugman E, Hirasing RA. Excessive infant crying: the impact of varying definitions. Pediatrics. 2001;108(4):893-897.
- Hicks M. An evidence-based, systematic approach to acute, unexplained, excessive crying in infants. Pediatr Emerg Med Pract. 2005;2(2-3):1-50.
- Barr RG. Colic and crying syndromes in infants. Pediatrics. 1998;102(5 Suppl E):1282-1286.
- Poole SR. The infant with acute, unexplained, excessive crying. Pediatrics 1991;88(3):450-455.
- Harkness MJ. Corneal abrasion in infancy as a cause of inconsolable crying. Pediatr Emerg Care. 1989;5(4):242-244.
- Shope TR, Rieg TS, Kathiria NN. Corneal abrasions in young infants. Pediatrics. 2012;125(3):e585-e589.
- Barton DJ, Sloan GM, Nichter LS, Reinisch JF. Hair-thread tourniquet syndrome. Pediatrics. 1988;82(6):925-928.
- Plesa JA, Shoup K, Manole MD, Hickey RW. Effect of depilatory agent on cotton, polyester, and rayon versus human hair in a laboratory setting. Ann Emerg Med. 2015;65(3):256-259.
- Mano R, Livne PM, Nevo A, Sivan B, Ben-Meir D. Testicular torsion in the first year of life—characteristics and treatment outcome. Urology. 2013;82(5):1132-1137.
- Saxena AK, Castellani C, Ruttenstock EM, Höllwarth ME. Testicular torsion: a 15-year single-centre clinical and histological analysis. Acta Paediatr. 2012;101(7):e282-e286.
- Ein SH, Njere I, Ein A. Six thousand three hundred sixty-one pediatric inguinal hernias: a 35-year review. J Ped Surg. 2006;41(5):980-986.
- Erdoğan D, Karaman I, Aslan MK, Karaman A, Cavuşoğlu YH. Analysis of 3,776 pediatric inguinal hernia and hydrocele cases in a tertiary center. J Pediatr Surg. 2013;48(8):1767-1772.
- Sirotnak AP, Grigsby T, Krugman RD. Physical abuse of children. Pediatr Rev. 2004;25(8):264-277
- Kunen S, Hume P, Perret JN, Mandry CV, Patterson TR. Underdiagnosis of child abuse in emergency departments. Acad Emerg Med. 2003;10(5):546-a.
Pediatric Orthopedic Basics
Case 1
A mother presented to the ED with her 8-year-old daughter after she witnessed the child fall off her bicycle onto the sidewalk. When she fell, the girl landed onto her outstretched arms and sustained minor abrasions to her palms and knees, but did not hit her head or lose consciousness. Upon falling, the child immediately cried that her left arm hurt and kept holding it guarded near her body.
She was seated on her mother’s lap in the examination room, appearing anxious but in no acute distress. The treating EP observed the superficial abrasions from across the room and obtained a detailed history.
The patient was afebrile, and her vital signs were stable with the exception of mild tachycardia. After a couple of minutes, the EP slowly approached the child and was able to perform a basic examination. There was no obvious deformity to her left upper extremity, and only mild swelling over the wrist. She was able to move her fingers well and had excellent capillary refill distally. The child remained calm during manual palpation of the anatomic snuff box. However, she immediately pulled away and began to cry upon palpation more proximally over the distal forearm. The EP discussed his concerns with the child’s mother and explained that further evaluation was necessary.
Case 2
A mother and father presented to the ED carrying their 6-year-old boy, stating that the child had been limping since they picked him up from a neighbor’s house an hour earlier and was now refusing to walk. The father noted that a group of children had been jumping on a trampoline unsupervised, but he did not witness any injury to his son. Both parents said that the boy had been well up until that day.
At presentation, the child was afebrile and his vital signs were stable. The EP asked the parents to coax the child to walk across the room. During the walk, the boy was reluctant to bear weight on his right foot. Careful inspection of his lower limb revealed no external signs of trauma, and it appeared neurovascularly intact. Careful palpation elicited tenderness directly over the physis at the distal fibula and near the lateral malleolus. While considering the broad differential for a limping child, the physician was primarily concerned with point tenderness on examination and informed the parents that radiographic imaging was warranted.
Overview
Pediatric musculoskeletal (MSK) injury and orthopedic trauma now comprise more than 10% of visits to the ED.1 Fractures in particular are becoming more commonplace with the increasing number of children actively involved in athletic sports and high-risk activities.
The general approach to acute-care management of these children has evolved, trending more toward splinting the fractured extremity and away from traditional casting. There are many benefits to splinting. The most important is arguably the reduced risk of developing compartment syndrome due to a splint’s ability to expand with accompanied swelling.2 This article reviews the unique characteristics of pediatric bone development and initial management of pediatric fractures, as well as basic splinting techniques and unique indications that require further orthopedic consultation.
Physiological Differences in Children
The MSK system of a child differs greatly from that of an adult. The bones themselves are much more porous and malleable during childhood, making them more susceptible to traumatic injury. The growing periosteum and the developing physes are particularly vulnerable, accounting for up to 20% of pediatric fractures (see the Figure illustrating the Salter-Harris classification in the next section).3 This is particularly true at a young age, when ligamentous adherence out-performs the bony integrity itself, making fractures more common than sprains and tears. The opposite is true in adults, who are much more likely to experience sprains before succumbing to fracture. Furthermore, since the periosteum is still very active in children, the fractured bone is much more likely to remodel, lending to less deformity and overall better outcomes in most cases.3 Nonunion is extremely rare in children.
Initial Management
Approaching the Pediatric Patient
Special consideration should be given when initially approaching an injured child, so as not to cause additional undue fear or anxiety to the patient. It is helpful to take an extra moment upon entering the room to simply observe the child’s positioning, posture, or reluctance to move a particular limb. Obtaining a careful, detailed history from a distance is recommended before too quickly approaching the patient. In addition, asking the caregiver to serve as proxy during the initial physical examination may also prove helpful in localizing the pain. In the obscure case, such as the child refusing to bear weight, it is good to keep a broad differential and inspect for non-MSK injury (eg, painful hernia, testicular torsion, foreign body lodged in the bottom of the foot). Utilizing a “log-rolling” technique with the palms of one’s hands on the patient’s thigh may reveal hip pathology. Simply observing the preoccupied child walk around the unit while watching from behind may also aid in the evaluation.
However, when the injury such as an open fracture or severe displacement is obvious, immediate stabilization is critical so as not to permit any additional harm. An arm board is typically used to accomplish this. In addition, pain control should never be overlooked, either with intravenous opioids or more appropriate oral or intranasal analgesia.4,5 In cases of significant trauma, always remember ABC assessment (airway, breathing/oxygenation, and circulation), despite the eagerness to give attention to what may be an obvious fracture.
Workup
Although the use of ultrasound and other modalities is becoming more popular in some settings, it is still commonplace to begin the evaluation of a potential fracture or dislocation with plain film X-rays. Before sending a patient to radiology, always stabilize any unstable fracture to avoid further injury or potentiate neurovascular compromise. In most cases, three views, including anteroposterior, true lateral, and oblique, are obtained. If a fracture is unclear, it may be helpful to image the opposite extremity for comparison. The location of a fracture and its characteristics greatly influence acute-care management, as well as patient disposition, the need for consultation with orthopedics, and follow-up expectations and instructions.
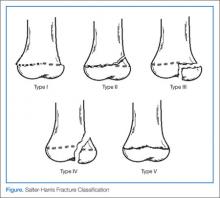
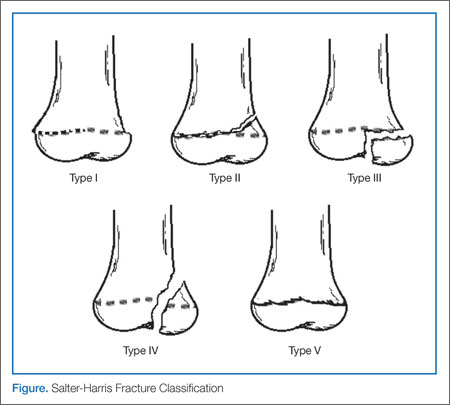
Salter-Harris Fracture Classification
The physes of bones in growing children are particularly vulnerable sites of fracture since they have not yet fused. The five generally accepted types of fracture according to risk of growth disturbance are illustrated in the above Figure and are differentiated as follows:
Type I. This type of fracture is exclusive involvement of the physis itself, separating the metaphysis from the epiphysis. Since plain films may not reveal any visible fracture, the clinician should have a high index of suspicion if the physical examination elicits point tenderness over the growth plate. When in doubt if a fracture is present, always splint. Type I fractures of the physis tend to heal well, without significant consequence.
Type II. As with type I fractures, type II involve the physis, but also have a fragment of displaced metaphysis—the most common of all physeal fractures. Without significant displacement, these fractures also tend to have good outcomes.
Type III. Rather than involving the physis and metaphysis, type III fractures involve the epiphysis and therefore the joint itself. It is because of the epiphyseal displacement that these fractures tend to have a worse prognosis with joint disability and growth arrest. Thus, establishing alignment is imperative. The distal tibial Tillaux fracture is an example and requires internal fixation for optimal healing.
Type IV. Similar to a type III fracture, with the fracture extending proximally through a segment of the metaphysis, type IV fractures are treated similar to type III fractures. Due to joint involvement, an orthopedic consultation is warranted.
Type V. This type represents compression fractures of the physis. As the visibility of these fractures is poor on plain films, diagnosis can be challenging. However, history of axial compression injury may help lead the clinician to an accurate diagnosis. Since there is a high incidence of growth disturbance in type V fractures, compression affecting other areas such as the spine should also be considered.
Certainly not all pediatric fractures will involve a physis. A detailed description and management of other unique types of pediatric fractures is discussed in other articles in this feature.
Splinting Basics
Once the decision is made to apply a splint to a fracture, certain basic precautions should first be taken. Initially, any significant lacerations or abrasions should be thoroughly irrigated, cleansed, and dressed appropriately. Next, the physician should reevaluate and document both neurological status and perfusion of the area, particularly distal to the fracture site.
One commonly overlooked step in management of any fracture is pain control. It is advisable to consider administering medication prior to splinting on a case-by-case basis and for all fractures requiring reduction.
Materials and Methods
Prepackaged fiberglass splints have become a popular, efficient, and less-messy material of choice in pediatric splinting. Alternatively, plaster of Paris—although a bit more cumbersome—has some advantages, including low cost and a tendency to mold more easily to the extremity being splinted.7 When using plaster, strips should be cut a little longer than the anticipated length needed since they may shrink during curing. The unaffected limb should be used to gauge the measurement needed.
Regardless of the material chosen, all splinting should begin with the application of a stockinette tube dressing over the skin, leaving a distal opening over fingers or toes. This should be followed by a padding material (eg, Webril), beginning distally and rolling proximally, being sure to have approximately 50% overlap of each roll. Extra padding should be rolled over any bony prominence (eg, ulnar stylus) to avoid discomfort or pressure sores once the splint is applied.2
Between 8 and 10 layers of plaster (additional layers for lower extremity splints) should be wetted with room-temperature water. Hot water should never be used as this will intensify the exothermic reaction that occurs when curing and could cause burns.2 The limb should be kept in the anatomic position while the plaster is being molded to the shape of the extremity, allowing 15 to 20 minutes to dry.1 Once dry, an elastic bandage such as an Ace wrap may be placed over the entire cast to hold it secure in place. If fiberglass is used, it is helpful to squeeze out extra water before molding to the extremity. Again, an additional padding roll should be employed to avoid any discomfort or pressure beneath the splint.
In both fiberglass and plaster splinting, the edges of either type of material should not be abrasive to the skin; this can be avoided by rolling over excess padding and stockinette to create a round soft edge on either end.7 Finally, the patient should be fitted with a shoulder sling or crutches (if age appropriate) to further immobilize the injured extremity and avoid any movement or weight bearing.
Types of Splints
The type of splint depends of the location and characteristics of the fracture being immobilized. The following are a few examples of the more popular splinting techniques indicated for common pediatric fractures.
Long-Arm Posterior Splint. This splint is useful for most forearm and elbow fractures. The splint length should extend from midlength of the humerus to the palmar crease, and the width should be semicircular. In addition, an anatomic position of 90˚ flexion of the elbow should be maintained, with the hand in a neutral position and slight dorsiflexion. It is generally accepted to slightly pronate the forearm when splinting a supracondylar fracture. Orthopedics should always be consulted if the fracture involves the elbow.
Ulnar “Gutter” Splint. Useful for nondisplaced, minimally-angulated metacarpal “boxer’s fracture” or fourth and fifth phalangeal fractures, the length of the ulnar splint should extend from the distal phalanx to proximal forearm. Splint width should enclose both the volar and dorsum surfaces of the fourth and fifth metacarpals. In addition, padding should be placed between the digits for comfort. The metacarpophalangeal joints should be positioned at 70˚, and the proximal phalangeal angle at approximately 20˚ flexion2; this will help minimize the risk of contractures.
Forearm “Sugar-Tong” Splint. These splints are indicated for immobilization of a distal radius fracture or wrist injury. Distal radial fractures are by far the most common fractures encountered in the pediatric population,8 and splinting for angulation less than 15˚ is preferred.9,10 For proper stabilization, a long U-shaped splint should originate at the palmar crease, wrap around the elbow, and end at the metacarpophalangeal joint dorsally. Again, the hand should be dorsiflexed, and a soft rolled edge should be kept on the palmar crease to allow full finger flexion to near 90˚.
Thumb Spica Splint. A thumb spica splint is useful to immobilize uncomplicated fracture of the first metacarpal or proximal phalanx or when scaphoid (navicular) bone fracture is suspected. A semicircumferential molding of the radial forearm should be formed, extending to the thumbnail bed, and wrapping around the thumb. The proper hand positioning is slightly dorsiflexed, with thumb abducted slightly, as if holding a glass of water.2 If there is any doubt of a navicular fracture (rare in prepubescent children), the clinician should never hesitate to splint!
Long-Leg Posterior Splint. This type of splint is appropriate for immobilization of midshaft tibia/fibula fractures or most knee injuries. Full length of the splint should start beneath the inferior gluteal fold and extend to the ball of the foot, leaving the toes free. The ankle should be at 90˚ flexion and the knee should remain just slightly flexed, never locked straight. Orthopedics should always be consulted in cases of proximal tibia/fibula fractures or knee joint involvement.
Posterior Ankle Splint. Essentially a shorter version of a long-leg splint extending proximally to just below the knee, the posterior ankle splint is useful to immobilize ankle fractures, foot fractures, and severe ankle sprains. The distal fibula and occasional tibia physes are another common site of pediatric fractures, particularly in obese or more active children.11,12 When using either a long- or short-leg posterior ankle splint, it is helpful to hold the foot at 90˚ flexion until the material hardens or the proper angle may be lost. A recall that displaced or Salter-Harris type III or IV physeal fractures justify orthopedics consult. Nonweight-bearing, use of crutches, ice, and elevation are all important points for recovery in 3 to 6 weeks.
Lower Extremity Stirrup “Sugar-Tong” Splint. This splint is indicated for additional ankle stabilization. It runs in a U-shape (not unlike a forearm sugar-tong splint) from just below the knee around the calcaneus, and it must be wide enough to encase the ankle but not so wide that the two sides overlap when molded. It is very important to add extra padding around both malleoli and beneath the calcaneus to reduce the likelihood of pressure sores. Crutches are essential to avoid weight-bearing in patients old enough to use them. Some pediatric orthopedists advise avoiding this type of splint in the smaller, noncompliant, active child.
Complications
Although splinting has many advantages over casting in the acute-care setting, several potential complications may develop. Although rare, thermal burns to the underlying skin may occur if excessively warm or hot water is used on plaster or fiberglass due to the exothermic reaction during the hardening process. Therefore, the use of room-temperature water is always recommended. Despite the noncircumferential nature of a splint, it is still possible to develop significant swelling following splint application, which can lead to neurovascular compromise, compartment syndrome, infection, or pressure ulcers.7 The patient and caregiver should be advised to return to the ED immediately for evaluation if serious signs and symptoms such as pain, numbness, tingling, dusky color of skin, or poor capillary refill develop.
Case 1 Conclusion
The EP in this case elected to obtain plain X-rays of the patient’s left forearm, including the wrist and elbow. The results demonstrated a disruption of the cortical integrity of the distal radius, consistent with a buckle fracture. The angulation was estimated at merely 10˚. The bones of the wrist and elbow appeared normal. The EP concluded that a consult with orthopedics was not required urgently, and immobilized the patient’s arm using a fiberglass sugar-tong splint, keeping her elbow at 90˚, the forearm in a neutral position, and hand slightly dorsiflexed. A nurse assisted in keeping the child still to ensure the splint was shaped around the arm and hardened in this position. The child was provided with a sling, and supportive-care measures, including analgesia with nonsteroidal anti-inflammatory drugs as needed, ice, rest, and the importance of keeping the splint dry, were reviewed with her parents. The EP also stressed the importance of surveying for any loss of sensation or perfusion to the patient’s hand and fingers, and recommended follow up with orthopedics 1 week from discharge.
Case 2 Conclusion
Multiple views of the patient’s ankle were obtained on X-ray and showed no apparent fracture or dislocation. Additional films of the opposite ankle were obtained for comparison, but both appeared quite similar except for mild soft-tissue swelling of the affected side. Since point tenderness was reproducible over the distal fibular physis, the EP elected to place a short-leg posterior splint, maintaining good anatomic position with extra padding around the malleoli. The parents were instructed on proper elevation, ice to reduce inflammation, and the use of pain medication if needed.
One week after discharge, the treating EP received a letter from the child’s orthopedist, informing him that at the follow-up appointment, a repeat ankle film revealed periosteal changes and a type I Salter-Harris distal fibula fracture. Immobilization for an additional 3 weeks and supportive care was indicated.
Dr Del Re is an instructor of pediatrics and an intermediate care pediatrician, Rady Children’s Hospital, San Diego, California. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Bachman D, Santora S. Musculoskeletal trauma. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:1335-1375.
- Klig JE. Splinting procedures. In: King C, Henretig FM, eds. Texbook of Pediatric Emergency Procedures. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:919-931.
- Wilkins KE. The incidence of fractures in children. In: Rockwood CA, Wilkins KE, Beaty JH, eds. Fractures in Children. 4th ed. Philadelphia, PA: Lippincott-Raven; 1996:3-17.
- Mahar PJ, Rana JA, Kennedy CS, Christopher NC. A randomized clinical trial of oral transmucosal fentanyl citrate versus intravenous morphine sulfate for initial control of pain in children with extremity injuries. Pediatr Emerg Care. 2007;23(8):544-548.
- Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med. 2010;17(11):1155-1161.
- Salter RB, Harris WR. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587-622.
- Boyd AS, Benjamin HJ, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-22.
- Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33(6):503-505.
- Boutis K, Willan A, Babyn P, Goeree R, Howard A. Cast versus splint in children with minimally angulated fractures of the distal radius: a randomized controlled trial. CMAJ. 2010;182(14):1507-1512.
- Firmin F, Crouch R. Splinting versus casting of “torus” fractures to the distal radius in the paediatric patient presenting at the emergency department (ED): a literature review. Int Emerg Nurs. 2009;17(3):173-178.
- Peterson HA, Madhok R, Benson JT, Ilstrup DM, Melton LJ 3rd. Physeal fractures: Part 1. Epidemiology in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop. 1994;14(4):423-430.
- Blackburn EW, Aronsson DD, Rubright JH, Lisle JW. Ankle fractures in children. J Bone Joint Surg Am. 2012; 94(13):1234-1244.
Case 1
A mother presented to the ED with her 8-year-old daughter after she witnessed the child fall off her bicycle onto the sidewalk. When she fell, the girl landed onto her outstretched arms and sustained minor abrasions to her palms and knees, but did not hit her head or lose consciousness. Upon falling, the child immediately cried that her left arm hurt and kept holding it guarded near her body.
She was seated on her mother’s lap in the examination room, appearing anxious but in no acute distress. The treating EP observed the superficial abrasions from across the room and obtained a detailed history.
The patient was afebrile, and her vital signs were stable with the exception of mild tachycardia. After a couple of minutes, the EP slowly approached the child and was able to perform a basic examination. There was no obvious deformity to her left upper extremity, and only mild swelling over the wrist. She was able to move her fingers well and had excellent capillary refill distally. The child remained calm during manual palpation of the anatomic snuff box. However, she immediately pulled away and began to cry upon palpation more proximally over the distal forearm. The EP discussed his concerns with the child’s mother and explained that further evaluation was necessary.
Case 2
A mother and father presented to the ED carrying their 6-year-old boy, stating that the child had been limping since they picked him up from a neighbor’s house an hour earlier and was now refusing to walk. The father noted that a group of children had been jumping on a trampoline unsupervised, but he did not witness any injury to his son. Both parents said that the boy had been well up until that day.
At presentation, the child was afebrile and his vital signs were stable. The EP asked the parents to coax the child to walk across the room. During the walk, the boy was reluctant to bear weight on his right foot. Careful inspection of his lower limb revealed no external signs of trauma, and it appeared neurovascularly intact. Careful palpation elicited tenderness directly over the physis at the distal fibula and near the lateral malleolus. While considering the broad differential for a limping child, the physician was primarily concerned with point tenderness on examination and informed the parents that radiographic imaging was warranted.
Overview
Pediatric musculoskeletal (MSK) injury and orthopedic trauma now comprise more than 10% of visits to the ED.1 Fractures in particular are becoming more commonplace with the increasing number of children actively involved in athletic sports and high-risk activities.
The general approach to acute-care management of these children has evolved, trending more toward splinting the fractured extremity and away from traditional casting. There are many benefits to splinting. The most important is arguably the reduced risk of developing compartment syndrome due to a splint’s ability to expand with accompanied swelling.2 This article reviews the unique characteristics of pediatric bone development and initial management of pediatric fractures, as well as basic splinting techniques and unique indications that require further orthopedic consultation.
Physiological Differences in Children
The MSK system of a child differs greatly from that of an adult. The bones themselves are much more porous and malleable during childhood, making them more susceptible to traumatic injury. The growing periosteum and the developing physes are particularly vulnerable, accounting for up to 20% of pediatric fractures (see the Figure illustrating the Salter-Harris classification in the next section).3 This is particularly true at a young age, when ligamentous adherence out-performs the bony integrity itself, making fractures more common than sprains and tears. The opposite is true in adults, who are much more likely to experience sprains before succumbing to fracture. Furthermore, since the periosteum is still very active in children, the fractured bone is much more likely to remodel, lending to less deformity and overall better outcomes in most cases.3 Nonunion is extremely rare in children.
Initial Management
Approaching the Pediatric Patient
Special consideration should be given when initially approaching an injured child, so as not to cause additional undue fear or anxiety to the patient. It is helpful to take an extra moment upon entering the room to simply observe the child’s positioning, posture, or reluctance to move a particular limb. Obtaining a careful, detailed history from a distance is recommended before too quickly approaching the patient. In addition, asking the caregiver to serve as proxy during the initial physical examination may also prove helpful in localizing the pain. In the obscure case, such as the child refusing to bear weight, it is good to keep a broad differential and inspect for non-MSK injury (eg, painful hernia, testicular torsion, foreign body lodged in the bottom of the foot). Utilizing a “log-rolling” technique with the palms of one’s hands on the patient’s thigh may reveal hip pathology. Simply observing the preoccupied child walk around the unit while watching from behind may also aid in the evaluation.
However, when the injury such as an open fracture or severe displacement is obvious, immediate stabilization is critical so as not to permit any additional harm. An arm board is typically used to accomplish this. In addition, pain control should never be overlooked, either with intravenous opioids or more appropriate oral or intranasal analgesia.4,5 In cases of significant trauma, always remember ABC assessment (airway, breathing/oxygenation, and circulation), despite the eagerness to give attention to what may be an obvious fracture.
Workup
Although the use of ultrasound and other modalities is becoming more popular in some settings, it is still commonplace to begin the evaluation of a potential fracture or dislocation with plain film X-rays. Before sending a patient to radiology, always stabilize any unstable fracture to avoid further injury or potentiate neurovascular compromise. In most cases, three views, including anteroposterior, true lateral, and oblique, are obtained. If a fracture is unclear, it may be helpful to image the opposite extremity for comparison. The location of a fracture and its characteristics greatly influence acute-care management, as well as patient disposition, the need for consultation with orthopedics, and follow-up expectations and instructions.
Salter-Harris Fracture Classification
The physes of bones in growing children are particularly vulnerable sites of fracture since they have not yet fused. The five generally accepted types of fracture according to risk of growth disturbance are illustrated in the above Figure and are differentiated as follows:
Type I. This type of fracture is exclusive involvement of the physis itself, separating the metaphysis from the epiphysis. Since plain films may not reveal any visible fracture, the clinician should have a high index of suspicion if the physical examination elicits point tenderness over the growth plate. When in doubt if a fracture is present, always splint. Type I fractures of the physis tend to heal well, without significant consequence.
Type II. As with type I fractures, type II involve the physis, but also have a fragment of displaced metaphysis—the most common of all physeal fractures. Without significant displacement, these fractures also tend to have good outcomes.
Type III. Rather than involving the physis and metaphysis, type III fractures involve the epiphysis and therefore the joint itself. It is because of the epiphyseal displacement that these fractures tend to have a worse prognosis with joint disability and growth arrest. Thus, establishing alignment is imperative. The distal tibial Tillaux fracture is an example and requires internal fixation for optimal healing.
Type IV. Similar to a type III fracture, with the fracture extending proximally through a segment of the metaphysis, type IV fractures are treated similar to type III fractures. Due to joint involvement, an orthopedic consultation is warranted.
Type V. This type represents compression fractures of the physis. As the visibility of these fractures is poor on plain films, diagnosis can be challenging. However, history of axial compression injury may help lead the clinician to an accurate diagnosis. Since there is a high incidence of growth disturbance in type V fractures, compression affecting other areas such as the spine should also be considered.
Certainly not all pediatric fractures will involve a physis. A detailed description and management of other unique types of pediatric fractures is discussed in other articles in this feature.
Splinting Basics
Once the decision is made to apply a splint to a fracture, certain basic precautions should first be taken. Initially, any significant lacerations or abrasions should be thoroughly irrigated, cleansed, and dressed appropriately. Next, the physician should reevaluate and document both neurological status and perfusion of the area, particularly distal to the fracture site.
One commonly overlooked step in management of any fracture is pain control. It is advisable to consider administering medication prior to splinting on a case-by-case basis and for all fractures requiring reduction.
Materials and Methods
Prepackaged fiberglass splints have become a popular, efficient, and less-messy material of choice in pediatric splinting. Alternatively, plaster of Paris—although a bit more cumbersome—has some advantages, including low cost and a tendency to mold more easily to the extremity being splinted.7 When using plaster, strips should be cut a little longer than the anticipated length needed since they may shrink during curing. The unaffected limb should be used to gauge the measurement needed.
Regardless of the material chosen, all splinting should begin with the application of a stockinette tube dressing over the skin, leaving a distal opening over fingers or toes. This should be followed by a padding material (eg, Webril), beginning distally and rolling proximally, being sure to have approximately 50% overlap of each roll. Extra padding should be rolled over any bony prominence (eg, ulnar stylus) to avoid discomfort or pressure sores once the splint is applied.2
Between 8 and 10 layers of plaster (additional layers for lower extremity splints) should be wetted with room-temperature water. Hot water should never be used as this will intensify the exothermic reaction that occurs when curing and could cause burns.2 The limb should be kept in the anatomic position while the plaster is being molded to the shape of the extremity, allowing 15 to 20 minutes to dry.1 Once dry, an elastic bandage such as an Ace wrap may be placed over the entire cast to hold it secure in place. If fiberglass is used, it is helpful to squeeze out extra water before molding to the extremity. Again, an additional padding roll should be employed to avoid any discomfort or pressure beneath the splint.
In both fiberglass and plaster splinting, the edges of either type of material should not be abrasive to the skin; this can be avoided by rolling over excess padding and stockinette to create a round soft edge on either end.7 Finally, the patient should be fitted with a shoulder sling or crutches (if age appropriate) to further immobilize the injured extremity and avoid any movement or weight bearing.
Types of Splints
The type of splint depends of the location and characteristics of the fracture being immobilized. The following are a few examples of the more popular splinting techniques indicated for common pediatric fractures.
Long-Arm Posterior Splint. This splint is useful for most forearm and elbow fractures. The splint length should extend from midlength of the humerus to the palmar crease, and the width should be semicircular. In addition, an anatomic position of 90˚ flexion of the elbow should be maintained, with the hand in a neutral position and slight dorsiflexion. It is generally accepted to slightly pronate the forearm when splinting a supracondylar fracture. Orthopedics should always be consulted if the fracture involves the elbow.
Ulnar “Gutter” Splint. Useful for nondisplaced, minimally-angulated metacarpal “boxer’s fracture” or fourth and fifth phalangeal fractures, the length of the ulnar splint should extend from the distal phalanx to proximal forearm. Splint width should enclose both the volar and dorsum surfaces of the fourth and fifth metacarpals. In addition, padding should be placed between the digits for comfort. The metacarpophalangeal joints should be positioned at 70˚, and the proximal phalangeal angle at approximately 20˚ flexion2; this will help minimize the risk of contractures.
Forearm “Sugar-Tong” Splint. These splints are indicated for immobilization of a distal radius fracture or wrist injury. Distal radial fractures are by far the most common fractures encountered in the pediatric population,8 and splinting for angulation less than 15˚ is preferred.9,10 For proper stabilization, a long U-shaped splint should originate at the palmar crease, wrap around the elbow, and end at the metacarpophalangeal joint dorsally. Again, the hand should be dorsiflexed, and a soft rolled edge should be kept on the palmar crease to allow full finger flexion to near 90˚.
Thumb Spica Splint. A thumb spica splint is useful to immobilize uncomplicated fracture of the first metacarpal or proximal phalanx or when scaphoid (navicular) bone fracture is suspected. A semicircumferential molding of the radial forearm should be formed, extending to the thumbnail bed, and wrapping around the thumb. The proper hand positioning is slightly dorsiflexed, with thumb abducted slightly, as if holding a glass of water.2 If there is any doubt of a navicular fracture (rare in prepubescent children), the clinician should never hesitate to splint!
Long-Leg Posterior Splint. This type of splint is appropriate for immobilization of midshaft tibia/fibula fractures or most knee injuries. Full length of the splint should start beneath the inferior gluteal fold and extend to the ball of the foot, leaving the toes free. The ankle should be at 90˚ flexion and the knee should remain just slightly flexed, never locked straight. Orthopedics should always be consulted in cases of proximal tibia/fibula fractures or knee joint involvement.
Posterior Ankle Splint. Essentially a shorter version of a long-leg splint extending proximally to just below the knee, the posterior ankle splint is useful to immobilize ankle fractures, foot fractures, and severe ankle sprains. The distal fibula and occasional tibia physes are another common site of pediatric fractures, particularly in obese or more active children.11,12 When using either a long- or short-leg posterior ankle splint, it is helpful to hold the foot at 90˚ flexion until the material hardens or the proper angle may be lost. A recall that displaced or Salter-Harris type III or IV physeal fractures justify orthopedics consult. Nonweight-bearing, use of crutches, ice, and elevation are all important points for recovery in 3 to 6 weeks.
Lower Extremity Stirrup “Sugar-Tong” Splint. This splint is indicated for additional ankle stabilization. It runs in a U-shape (not unlike a forearm sugar-tong splint) from just below the knee around the calcaneus, and it must be wide enough to encase the ankle but not so wide that the two sides overlap when molded. It is very important to add extra padding around both malleoli and beneath the calcaneus to reduce the likelihood of pressure sores. Crutches are essential to avoid weight-bearing in patients old enough to use them. Some pediatric orthopedists advise avoiding this type of splint in the smaller, noncompliant, active child.
Complications
Although splinting has many advantages over casting in the acute-care setting, several potential complications may develop. Although rare, thermal burns to the underlying skin may occur if excessively warm or hot water is used on plaster or fiberglass due to the exothermic reaction during the hardening process. Therefore, the use of room-temperature water is always recommended. Despite the noncircumferential nature of a splint, it is still possible to develop significant swelling following splint application, which can lead to neurovascular compromise, compartment syndrome, infection, or pressure ulcers.7 The patient and caregiver should be advised to return to the ED immediately for evaluation if serious signs and symptoms such as pain, numbness, tingling, dusky color of skin, or poor capillary refill develop.
Case 1 Conclusion
The EP in this case elected to obtain plain X-rays of the patient’s left forearm, including the wrist and elbow. The results demonstrated a disruption of the cortical integrity of the distal radius, consistent with a buckle fracture. The angulation was estimated at merely 10˚. The bones of the wrist and elbow appeared normal. The EP concluded that a consult with orthopedics was not required urgently, and immobilized the patient’s arm using a fiberglass sugar-tong splint, keeping her elbow at 90˚, the forearm in a neutral position, and hand slightly dorsiflexed. A nurse assisted in keeping the child still to ensure the splint was shaped around the arm and hardened in this position. The child was provided with a sling, and supportive-care measures, including analgesia with nonsteroidal anti-inflammatory drugs as needed, ice, rest, and the importance of keeping the splint dry, were reviewed with her parents. The EP also stressed the importance of surveying for any loss of sensation or perfusion to the patient’s hand and fingers, and recommended follow up with orthopedics 1 week from discharge.
Case 2 Conclusion
Multiple views of the patient’s ankle were obtained on X-ray and showed no apparent fracture or dislocation. Additional films of the opposite ankle were obtained for comparison, but both appeared quite similar except for mild soft-tissue swelling of the affected side. Since point tenderness was reproducible over the distal fibular physis, the EP elected to place a short-leg posterior splint, maintaining good anatomic position with extra padding around the malleoli. The parents were instructed on proper elevation, ice to reduce inflammation, and the use of pain medication if needed.
One week after discharge, the treating EP received a letter from the child’s orthopedist, informing him that at the follow-up appointment, a repeat ankle film revealed periosteal changes and a type I Salter-Harris distal fibula fracture. Immobilization for an additional 3 weeks and supportive care was indicated.
Dr Del Re is an instructor of pediatrics and an intermediate care pediatrician, Rady Children’s Hospital, San Diego, California. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
Case 1
A mother presented to the ED with her 8-year-old daughter after she witnessed the child fall off her bicycle onto the sidewalk. When she fell, the girl landed onto her outstretched arms and sustained minor abrasions to her palms and knees, but did not hit her head or lose consciousness. Upon falling, the child immediately cried that her left arm hurt and kept holding it guarded near her body.
She was seated on her mother’s lap in the examination room, appearing anxious but in no acute distress. The treating EP observed the superficial abrasions from across the room and obtained a detailed history.
The patient was afebrile, and her vital signs were stable with the exception of mild tachycardia. After a couple of minutes, the EP slowly approached the child and was able to perform a basic examination. There was no obvious deformity to her left upper extremity, and only mild swelling over the wrist. She was able to move her fingers well and had excellent capillary refill distally. The child remained calm during manual palpation of the anatomic snuff box. However, she immediately pulled away and began to cry upon palpation more proximally over the distal forearm. The EP discussed his concerns with the child’s mother and explained that further evaluation was necessary.
Case 2
A mother and father presented to the ED carrying their 6-year-old boy, stating that the child had been limping since they picked him up from a neighbor’s house an hour earlier and was now refusing to walk. The father noted that a group of children had been jumping on a trampoline unsupervised, but he did not witness any injury to his son. Both parents said that the boy had been well up until that day.
At presentation, the child was afebrile and his vital signs were stable. The EP asked the parents to coax the child to walk across the room. During the walk, the boy was reluctant to bear weight on his right foot. Careful inspection of his lower limb revealed no external signs of trauma, and it appeared neurovascularly intact. Careful palpation elicited tenderness directly over the physis at the distal fibula and near the lateral malleolus. While considering the broad differential for a limping child, the physician was primarily concerned with point tenderness on examination and informed the parents that radiographic imaging was warranted.
Overview
Pediatric musculoskeletal (MSK) injury and orthopedic trauma now comprise more than 10% of visits to the ED.1 Fractures in particular are becoming more commonplace with the increasing number of children actively involved in athletic sports and high-risk activities.
The general approach to acute-care management of these children has evolved, trending more toward splinting the fractured extremity and away from traditional casting. There are many benefits to splinting. The most important is arguably the reduced risk of developing compartment syndrome due to a splint’s ability to expand with accompanied swelling.2 This article reviews the unique characteristics of pediatric bone development and initial management of pediatric fractures, as well as basic splinting techniques and unique indications that require further orthopedic consultation.
Physiological Differences in Children
The MSK system of a child differs greatly from that of an adult. The bones themselves are much more porous and malleable during childhood, making them more susceptible to traumatic injury. The growing periosteum and the developing physes are particularly vulnerable, accounting for up to 20% of pediatric fractures (see the Figure illustrating the Salter-Harris classification in the next section).3 This is particularly true at a young age, when ligamentous adherence out-performs the bony integrity itself, making fractures more common than sprains and tears. The opposite is true in adults, who are much more likely to experience sprains before succumbing to fracture. Furthermore, since the periosteum is still very active in children, the fractured bone is much more likely to remodel, lending to less deformity and overall better outcomes in most cases.3 Nonunion is extremely rare in children.
Initial Management
Approaching the Pediatric Patient
Special consideration should be given when initially approaching an injured child, so as not to cause additional undue fear or anxiety to the patient. It is helpful to take an extra moment upon entering the room to simply observe the child’s positioning, posture, or reluctance to move a particular limb. Obtaining a careful, detailed history from a distance is recommended before too quickly approaching the patient. In addition, asking the caregiver to serve as proxy during the initial physical examination may also prove helpful in localizing the pain. In the obscure case, such as the child refusing to bear weight, it is good to keep a broad differential and inspect for non-MSK injury (eg, painful hernia, testicular torsion, foreign body lodged in the bottom of the foot). Utilizing a “log-rolling” technique with the palms of one’s hands on the patient’s thigh may reveal hip pathology. Simply observing the preoccupied child walk around the unit while watching from behind may also aid in the evaluation.
However, when the injury such as an open fracture or severe displacement is obvious, immediate stabilization is critical so as not to permit any additional harm. An arm board is typically used to accomplish this. In addition, pain control should never be overlooked, either with intravenous opioids or more appropriate oral or intranasal analgesia.4,5 In cases of significant trauma, always remember ABC assessment (airway, breathing/oxygenation, and circulation), despite the eagerness to give attention to what may be an obvious fracture.
Workup
Although the use of ultrasound and other modalities is becoming more popular in some settings, it is still commonplace to begin the evaluation of a potential fracture or dislocation with plain film X-rays. Before sending a patient to radiology, always stabilize any unstable fracture to avoid further injury or potentiate neurovascular compromise. In most cases, three views, including anteroposterior, true lateral, and oblique, are obtained. If a fracture is unclear, it may be helpful to image the opposite extremity for comparison. The location of a fracture and its characteristics greatly influence acute-care management, as well as patient disposition, the need for consultation with orthopedics, and follow-up expectations and instructions.
Salter-Harris Fracture Classification
The physes of bones in growing children are particularly vulnerable sites of fracture since they have not yet fused. The five generally accepted types of fracture according to risk of growth disturbance are illustrated in the above Figure and are differentiated as follows:
Type I. This type of fracture is exclusive involvement of the physis itself, separating the metaphysis from the epiphysis. Since plain films may not reveal any visible fracture, the clinician should have a high index of suspicion if the physical examination elicits point tenderness over the growth plate. When in doubt if a fracture is present, always splint. Type I fractures of the physis tend to heal well, without significant consequence.
Type II. As with type I fractures, type II involve the physis, but also have a fragment of displaced metaphysis—the most common of all physeal fractures. Without significant displacement, these fractures also tend to have good outcomes.
Type III. Rather than involving the physis and metaphysis, type III fractures involve the epiphysis and therefore the joint itself. It is because of the epiphyseal displacement that these fractures tend to have a worse prognosis with joint disability and growth arrest. Thus, establishing alignment is imperative. The distal tibial Tillaux fracture is an example and requires internal fixation for optimal healing.
Type IV. Similar to a type III fracture, with the fracture extending proximally through a segment of the metaphysis, type IV fractures are treated similar to type III fractures. Due to joint involvement, an orthopedic consultation is warranted.
Type V. This type represents compression fractures of the physis. As the visibility of these fractures is poor on plain films, diagnosis can be challenging. However, history of axial compression injury may help lead the clinician to an accurate diagnosis. Since there is a high incidence of growth disturbance in type V fractures, compression affecting other areas such as the spine should also be considered.
Certainly not all pediatric fractures will involve a physis. A detailed description and management of other unique types of pediatric fractures is discussed in other articles in this feature.
Splinting Basics
Once the decision is made to apply a splint to a fracture, certain basic precautions should first be taken. Initially, any significant lacerations or abrasions should be thoroughly irrigated, cleansed, and dressed appropriately. Next, the physician should reevaluate and document both neurological status and perfusion of the area, particularly distal to the fracture site.
One commonly overlooked step in management of any fracture is pain control. It is advisable to consider administering medication prior to splinting on a case-by-case basis and for all fractures requiring reduction.
Materials and Methods
Prepackaged fiberglass splints have become a popular, efficient, and less-messy material of choice in pediatric splinting. Alternatively, plaster of Paris—although a bit more cumbersome—has some advantages, including low cost and a tendency to mold more easily to the extremity being splinted.7 When using plaster, strips should be cut a little longer than the anticipated length needed since they may shrink during curing. The unaffected limb should be used to gauge the measurement needed.
Regardless of the material chosen, all splinting should begin with the application of a stockinette tube dressing over the skin, leaving a distal opening over fingers or toes. This should be followed by a padding material (eg, Webril), beginning distally and rolling proximally, being sure to have approximately 50% overlap of each roll. Extra padding should be rolled over any bony prominence (eg, ulnar stylus) to avoid discomfort or pressure sores once the splint is applied.2
Between 8 and 10 layers of plaster (additional layers for lower extremity splints) should be wetted with room-temperature water. Hot water should never be used as this will intensify the exothermic reaction that occurs when curing and could cause burns.2 The limb should be kept in the anatomic position while the plaster is being molded to the shape of the extremity, allowing 15 to 20 minutes to dry.1 Once dry, an elastic bandage such as an Ace wrap may be placed over the entire cast to hold it secure in place. If fiberglass is used, it is helpful to squeeze out extra water before molding to the extremity. Again, an additional padding roll should be employed to avoid any discomfort or pressure beneath the splint.
In both fiberglass and plaster splinting, the edges of either type of material should not be abrasive to the skin; this can be avoided by rolling over excess padding and stockinette to create a round soft edge on either end.7 Finally, the patient should be fitted with a shoulder sling or crutches (if age appropriate) to further immobilize the injured extremity and avoid any movement or weight bearing.
Types of Splints
The type of splint depends of the location and characteristics of the fracture being immobilized. The following are a few examples of the more popular splinting techniques indicated for common pediatric fractures.
Long-Arm Posterior Splint. This splint is useful for most forearm and elbow fractures. The splint length should extend from midlength of the humerus to the palmar crease, and the width should be semicircular. In addition, an anatomic position of 90˚ flexion of the elbow should be maintained, with the hand in a neutral position and slight dorsiflexion. It is generally accepted to slightly pronate the forearm when splinting a supracondylar fracture. Orthopedics should always be consulted if the fracture involves the elbow.
Ulnar “Gutter” Splint. Useful for nondisplaced, minimally-angulated metacarpal “boxer’s fracture” or fourth and fifth phalangeal fractures, the length of the ulnar splint should extend from the distal phalanx to proximal forearm. Splint width should enclose both the volar and dorsum surfaces of the fourth and fifth metacarpals. In addition, padding should be placed between the digits for comfort. The metacarpophalangeal joints should be positioned at 70˚, and the proximal phalangeal angle at approximately 20˚ flexion2; this will help minimize the risk of contractures.
Forearm “Sugar-Tong” Splint. These splints are indicated for immobilization of a distal radius fracture or wrist injury. Distal radial fractures are by far the most common fractures encountered in the pediatric population,8 and splinting for angulation less than 15˚ is preferred.9,10 For proper stabilization, a long U-shaped splint should originate at the palmar crease, wrap around the elbow, and end at the metacarpophalangeal joint dorsally. Again, the hand should be dorsiflexed, and a soft rolled edge should be kept on the palmar crease to allow full finger flexion to near 90˚.
Thumb Spica Splint. A thumb spica splint is useful to immobilize uncomplicated fracture of the first metacarpal or proximal phalanx or when scaphoid (navicular) bone fracture is suspected. A semicircumferential molding of the radial forearm should be formed, extending to the thumbnail bed, and wrapping around the thumb. The proper hand positioning is slightly dorsiflexed, with thumb abducted slightly, as if holding a glass of water.2 If there is any doubt of a navicular fracture (rare in prepubescent children), the clinician should never hesitate to splint!
Long-Leg Posterior Splint. This type of splint is appropriate for immobilization of midshaft tibia/fibula fractures or most knee injuries. Full length of the splint should start beneath the inferior gluteal fold and extend to the ball of the foot, leaving the toes free. The ankle should be at 90˚ flexion and the knee should remain just slightly flexed, never locked straight. Orthopedics should always be consulted in cases of proximal tibia/fibula fractures or knee joint involvement.
Posterior Ankle Splint. Essentially a shorter version of a long-leg splint extending proximally to just below the knee, the posterior ankle splint is useful to immobilize ankle fractures, foot fractures, and severe ankle sprains. The distal fibula and occasional tibia physes are another common site of pediatric fractures, particularly in obese or more active children.11,12 When using either a long- or short-leg posterior ankle splint, it is helpful to hold the foot at 90˚ flexion until the material hardens or the proper angle may be lost. A recall that displaced or Salter-Harris type III or IV physeal fractures justify orthopedics consult. Nonweight-bearing, use of crutches, ice, and elevation are all important points for recovery in 3 to 6 weeks.
Lower Extremity Stirrup “Sugar-Tong” Splint. This splint is indicated for additional ankle stabilization. It runs in a U-shape (not unlike a forearm sugar-tong splint) from just below the knee around the calcaneus, and it must be wide enough to encase the ankle but not so wide that the two sides overlap when molded. It is very important to add extra padding around both malleoli and beneath the calcaneus to reduce the likelihood of pressure sores. Crutches are essential to avoid weight-bearing in patients old enough to use them. Some pediatric orthopedists advise avoiding this type of splint in the smaller, noncompliant, active child.
Complications
Although splinting has many advantages over casting in the acute-care setting, several potential complications may develop. Although rare, thermal burns to the underlying skin may occur if excessively warm or hot water is used on plaster or fiberglass due to the exothermic reaction during the hardening process. Therefore, the use of room-temperature water is always recommended. Despite the noncircumferential nature of a splint, it is still possible to develop significant swelling following splint application, which can lead to neurovascular compromise, compartment syndrome, infection, or pressure ulcers.7 The patient and caregiver should be advised to return to the ED immediately for evaluation if serious signs and symptoms such as pain, numbness, tingling, dusky color of skin, or poor capillary refill develop.
Case 1 Conclusion
The EP in this case elected to obtain plain X-rays of the patient’s left forearm, including the wrist and elbow. The results demonstrated a disruption of the cortical integrity of the distal radius, consistent with a buckle fracture. The angulation was estimated at merely 10˚. The bones of the wrist and elbow appeared normal. The EP concluded that a consult with orthopedics was not required urgently, and immobilized the patient’s arm using a fiberglass sugar-tong splint, keeping her elbow at 90˚, the forearm in a neutral position, and hand slightly dorsiflexed. A nurse assisted in keeping the child still to ensure the splint was shaped around the arm and hardened in this position. The child was provided with a sling, and supportive-care measures, including analgesia with nonsteroidal anti-inflammatory drugs as needed, ice, rest, and the importance of keeping the splint dry, were reviewed with her parents. The EP also stressed the importance of surveying for any loss of sensation or perfusion to the patient’s hand and fingers, and recommended follow up with orthopedics 1 week from discharge.
Case 2 Conclusion
Multiple views of the patient’s ankle were obtained on X-ray and showed no apparent fracture or dislocation. Additional films of the opposite ankle were obtained for comparison, but both appeared quite similar except for mild soft-tissue swelling of the affected side. Since point tenderness was reproducible over the distal fibular physis, the EP elected to place a short-leg posterior splint, maintaining good anatomic position with extra padding around the malleoli. The parents were instructed on proper elevation, ice to reduce inflammation, and the use of pain medication if needed.
One week after discharge, the treating EP received a letter from the child’s orthopedist, informing him that at the follow-up appointment, a repeat ankle film revealed periosteal changes and a type I Salter-Harris distal fibula fracture. Immobilization for an additional 3 weeks and supportive care was indicated.
Dr Del Re is an instructor of pediatrics and an intermediate care pediatrician, Rady Children’s Hospital, San Diego, California. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Bachman D, Santora S. Musculoskeletal trauma. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:1335-1375.
- Klig JE. Splinting procedures. In: King C, Henretig FM, eds. Texbook of Pediatric Emergency Procedures. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:919-931.
- Wilkins KE. The incidence of fractures in children. In: Rockwood CA, Wilkins KE, Beaty JH, eds. Fractures in Children. 4th ed. Philadelphia, PA: Lippincott-Raven; 1996:3-17.
- Mahar PJ, Rana JA, Kennedy CS, Christopher NC. A randomized clinical trial of oral transmucosal fentanyl citrate versus intravenous morphine sulfate for initial control of pain in children with extremity injuries. Pediatr Emerg Care. 2007;23(8):544-548.
- Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med. 2010;17(11):1155-1161.
- Salter RB, Harris WR. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587-622.
- Boyd AS, Benjamin HJ, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-22.
- Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33(6):503-505.
- Boutis K, Willan A, Babyn P, Goeree R, Howard A. Cast versus splint in children with minimally angulated fractures of the distal radius: a randomized controlled trial. CMAJ. 2010;182(14):1507-1512.
- Firmin F, Crouch R. Splinting versus casting of “torus” fractures to the distal radius in the paediatric patient presenting at the emergency department (ED): a literature review. Int Emerg Nurs. 2009;17(3):173-178.
- Peterson HA, Madhok R, Benson JT, Ilstrup DM, Melton LJ 3rd. Physeal fractures: Part 1. Epidemiology in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop. 1994;14(4):423-430.
- Blackburn EW, Aronsson DD, Rubright JH, Lisle JW. Ankle fractures in children. J Bone Joint Surg Am. 2012; 94(13):1234-1244.
- Bachman D, Santora S. Musculoskeletal trauma. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:1335-1375.
- Klig JE. Splinting procedures. In: King C, Henretig FM, eds. Texbook of Pediatric Emergency Procedures. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:919-931.
- Wilkins KE. The incidence of fractures in children. In: Rockwood CA, Wilkins KE, Beaty JH, eds. Fractures in Children. 4th ed. Philadelphia, PA: Lippincott-Raven; 1996:3-17.
- Mahar PJ, Rana JA, Kennedy CS, Christopher NC. A randomized clinical trial of oral transmucosal fentanyl citrate versus intravenous morphine sulfate for initial control of pain in children with extremity injuries. Pediatr Emerg Care. 2007;23(8):544-548.
- Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med. 2010;17(11):1155-1161.
- Salter RB, Harris WR. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587-622.
- Boyd AS, Benjamin HJ, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-22.
- Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33(6):503-505.
- Boutis K, Willan A, Babyn P, Goeree R, Howard A. Cast versus splint in children with minimally angulated fractures of the distal radius: a randomized controlled trial. CMAJ. 2010;182(14):1507-1512.
- Firmin F, Crouch R. Splinting versus casting of “torus” fractures to the distal radius in the paediatric patient presenting at the emergency department (ED): a literature review. Int Emerg Nurs. 2009;17(3):173-178.
- Peterson HA, Madhok R, Benson JT, Ilstrup DM, Melton LJ 3rd. Physeal fractures: Part 1. Epidemiology in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop. 1994;14(4):423-430.
- Blackburn EW, Aronsson DD, Rubright JH, Lisle JW. Ankle fractures in children. J Bone Joint Surg Am. 2012; 94(13):1234-1244.
Pediatric Orthopedic Injuries
| Pediatric orthopedic injuries are a common presentation to the ED, representing 12% of pediatric visits.1 In this special feature, our authors focus on the challenge of evaluating and managing the pediatric orthopedic patient and spotlight conditions where the emergency physician (EP) might not have significant clinical experience. |
While many pediatric orthopedic injuries are the simple “bruises and bumps” of active childhood and need little more than pain control and parental education, there are some age-specific injuries that require truly emergent care in order to salvage an extremity or prevent loss of function. Differentiation between the urgent and emergent patient may not be obvious in the preverbal child or in a child whose radiographs show open growth plates and ossification centers obscuring interpretation.
This educational series begins by covering the physiological differences in pediatric musculoskeletal injuries and reviews both the general approach to examination and some pediatric splinting basics. With an enhanced awareness of the structural differences between growing and mature bones (along with their tendons and ligaments), subtle age-related injuries are less likely to be missed.
With the basics firmly in hand, we turn your attention to several common orthopedic injuries unique to children and review acute-care management. The third article deals with the frustrating diagnostic dilemma of “my child won’t walk” and explores some nontraumatic pediatric orthopedic presentations. Our series concludes with a review of some “high risk/can’t miss” pediatric injury patterns and presenting symptoms and also reminds us of injuries that might be suggestive of nonaccidental trauma as the underlying etiology.
While pediatric bones are indeed not simply little adult bones, EPs need not be intimidated in caring for these patients. A basic understanding of pediatric musculoskeletal physiology and an enhanced clinical awareness as to common injury patterns will equip most EPs with the knowledge necessary to ensure the best possible outcome for these children.
Reference
1. Chamberlain JM, Patel KM, Pollack MM, et al. Recalibration of the pediatric risk of admission (PRISA) score using a multi-institutional sample. Ann Emerg Med. 2004;43(4):461-486.
| Pediatric orthopedic injuries are a common presentation to the ED, representing 12% of pediatric visits.1 In this special feature, our authors focus on the challenge of evaluating and managing the pediatric orthopedic patient and spotlight conditions where the emergency physician (EP) might not have significant clinical experience. |
While many pediatric orthopedic injuries are the simple “bruises and bumps” of active childhood and need little more than pain control and parental education, there are some age-specific injuries that require truly emergent care in order to salvage an extremity or prevent loss of function. Differentiation between the urgent and emergent patient may not be obvious in the preverbal child or in a child whose radiographs show open growth plates and ossification centers obscuring interpretation.
This educational series begins by covering the physiological differences in pediatric musculoskeletal injuries and reviews both the general approach to examination and some pediatric splinting basics. With an enhanced awareness of the structural differences between growing and mature bones (along with their tendons and ligaments), subtle age-related injuries are less likely to be missed.
With the basics firmly in hand, we turn your attention to several common orthopedic injuries unique to children and review acute-care management. The third article deals with the frustrating diagnostic dilemma of “my child won’t walk” and explores some nontraumatic pediatric orthopedic presentations. Our series concludes with a review of some “high risk/can’t miss” pediatric injury patterns and presenting symptoms and also reminds us of injuries that might be suggestive of nonaccidental trauma as the underlying etiology.
While pediatric bones are indeed not simply little adult bones, EPs need not be intimidated in caring for these patients. A basic understanding of pediatric musculoskeletal physiology and an enhanced clinical awareness as to common injury patterns will equip most EPs with the knowledge necessary to ensure the best possible outcome for these children.
| Pediatric orthopedic injuries are a common presentation to the ED, representing 12% of pediatric visits.1 In this special feature, our authors focus on the challenge of evaluating and managing the pediatric orthopedic patient and spotlight conditions where the emergency physician (EP) might not have significant clinical experience. |
While many pediatric orthopedic injuries are the simple “bruises and bumps” of active childhood and need little more than pain control and parental education, there are some age-specific injuries that require truly emergent care in order to salvage an extremity or prevent loss of function. Differentiation between the urgent and emergent patient may not be obvious in the preverbal child or in a child whose radiographs show open growth plates and ossification centers obscuring interpretation.
This educational series begins by covering the physiological differences in pediatric musculoskeletal injuries and reviews both the general approach to examination and some pediatric splinting basics. With an enhanced awareness of the structural differences between growing and mature bones (along with their tendons and ligaments), subtle age-related injuries are less likely to be missed.
With the basics firmly in hand, we turn your attention to several common orthopedic injuries unique to children and review acute-care management. The third article deals with the frustrating diagnostic dilemma of “my child won’t walk” and explores some nontraumatic pediatric orthopedic presentations. Our series concludes with a review of some “high risk/can’t miss” pediatric injury patterns and presenting symptoms and also reminds us of injuries that might be suggestive of nonaccidental trauma as the underlying etiology.
While pediatric bones are indeed not simply little adult bones, EPs need not be intimidated in caring for these patients. A basic understanding of pediatric musculoskeletal physiology and an enhanced clinical awareness as to common injury patterns will equip most EPs with the knowledge necessary to ensure the best possible outcome for these children.
Reference
1. Chamberlain JM, Patel KM, Pollack MM, et al. Recalibration of the pediatric risk of admission (PRISA) score using a multi-institutional sample. Ann Emerg Med. 2004;43(4):461-486.
Reference
1. Chamberlain JM, Patel KM, Pollack MM, et al. Recalibration of the pediatric risk of admission (PRISA) score using a multi-institutional sample. Ann Emerg Med. 2004;43(4):461-486.
The Seizing Child - An Age-Based Approach to Pediatric Seizure Management
Immediately following this episode, the parents took their son to the ED for evaluation. He was afebrile with normal vital signs. The physical examination, which included a neurological examination, was normal, and both parents noted that the child appeared to be completely back to his normal behavior. The patient’s past medical and surgical histories were unremarkable. His parents stated that he had not been on any medication and denied a family history of seizures.
Overview
Seizures, the most common pediatric neurological disorder, are a frequent presentation in the ED. It is estimated that between 4% and 10% of children will have at least one seizure before age 16 years.1,2 The highest incidence of occurrence is seen in children younger than age 3 years; this frequency decreases in older children.2
Seizures are the resulting clinical manifestations of abnormal excessive synchronized neuronal activity within the cerebral cortex. Most seizure activity is stereotypical and self-limited3 and can be divided into two main types: generalized and partial. Generalized seizures involve both cerebral hemispheres and impairment of consciousness, while partial (or focal) seizures involve a single area of one hemisphere. Generalized seizures are usually classified as convulsive or nonconvulsive and include the following subtypes: tonic-clonic, atonic, absence, and myoclonic. Partial seizures can be subdivided into simple or complex, depending on whether consciousness is impaired.
Initial Management
As with critically ill patients, the first and most vital step in managing a seizing patient is to assess the airway, breathing, and circulation. Although the airway is the most frequently compromised component, simple interventions such as jaw thrust, suctioning, or the insertion of an oral or nasopharyngeal airway are usually sufficient to maintain adequate airway. If intubation is required, use of a short-acting paralytic agent should be considered so as not to mask ongoing seizure activity.1 Patients actively seizing at presentation should be assumed to be in status epilepticus, which is defined as seizure activity lasting longer than 5 minutes or as repetitive seizure episodes without return of consciousness between episodes.
Treatment
The benzodiazepines are the first-line treatment for status epilepticus in pediatric patients. Although intravenous (IV) lorazepam has long been the standard for seizure termination, intramuscular midazolam has been shown to be as safe and effective in patients weighing more than 13 kg in whom IV access is either not available or is difficult to obtain.4
Many other forms of benzodiazepines exist for seizure cessation, such as diazepam gel, which is administered rectally. Intranasal and buccal administration of midazolam also has proved effective for pediatric seizure termination.5,6 Second-line therapies include IV fosphenytoin, levetiracetam, phenobarbital, and valproic acid.1,7,8 In infants, phenobarbital is usually the primary second-line therapy after benzodiazepines.
History and Behavioral Observation
Given both the significant number of causes of pediatric seizures and events that can mimic childhood seizure activity, obtaining a thorough history and detailed description of the seizure episode and any preceding events are essential. It is also important to note nonseizure-related activities unique to the pediatric population (eg, Moro reflex in young infants, back-arching with gastroesophageal reflux [Sandifer syndrome], breath-holding spells, daydreaming).
Differentiating normal movements from seizures can be particularly difficult with infants. For example, repetitive bicycling movements of the legs are a common sign of seizure activity in an infant but may be mistaken as normal by parents or inexperienced observers.
West Syndrome.
West syndrome (infantile spasms) is a rare severe seizure syndrome consisting of spasmodic flexural movements of the extremities and trunk that usually presents between ages 4 to 18 months. Subtle episodes can be misinterpreted as Moro reflex, the normal sudden extension of an infant’s extremities and arching of the back occurring from birth to approximately ages 3 to 5 months.9
Breath-holding.
An episode of loss of consciousness with associated cyanosis, pallor, rigidity, limpness, or even twitching that was immediately preceded by vigorous crying in a child ages 6 months to 5 years should prompt the physician to strongly consider a breath-holding spell.
Attention Deficit Disorder Versus Seizure Activity.
Children, especially those with attention deficit disorder, have a propensity for daydreaming. These children will often stare and not respond to voice at times; however, if these episodes are associated with facial movements or a sudden pause in activity (also known as behavior arrest), the possibility of absence or complex partial seizures should be suspected.2
Physical Examination
Along with the patient’s history, the physical examination should focus primarily on determining a possible seizure etiology. The entire body should be thoroughly evaluated for evidence of trauma. The head should be carefully evaluated for signs of deformity or swelling; the fullness of the anterior fontanel should be carefully assessed; and the pupils should be examined for signs of increased intracranial pressure (ICP). In the older pediatric patient with closed fontanelles in whom a cervical spine injury has been ruled out, assessment of the neck for evidence of meningeal irritation should be performed. Stigmata of underlying medical disease, along with the presence of dysmorphic features associated with particular genetic syndromes, also should be evaluated. In addition, signs of common toxidromes should be sought.
Common Etiologies and Diagnostic Evaluation by Age
Since seizure etiologies and diagnostic evaluation vary greatly along the pediatric age spectrum, it is helpful to divide this population into three main groups: neonates and infants (ages 0-12 months), toddlers and young children (ages 12 months-10 years), and preadolescents and teenagers (ages 11-18 years).
In patients with known seizure disorders, the majority of cases are due to subtherapeutic antiepileptic medications either from a patient “outgrowing” his or her weight-based dose or from medication noncompliance. For the purposes of this article, we have limited our discussion to patients with first-time seizures.
Neonates and Infants.
In infants, hypoxic ischemic encephalopathy (HIE) due to perinatal asphyxia is the most common cause of seizures, the vast majority of which present within the first 24 to 48 hours of life.2 Although EPs may encounter children with seizures caused by HIE, it is rare that these represent an initial seizure. Far more common etiologies of first-time seizures in infants are infection (eg, meningitis, encephalitis, sepsis) and nonaccidental trauma.
Electrolyte Imbalance. Although electrolytes are frequently checked in all age groups with seizures, infants are by far the most likely to experience seizures secondary to electrolyte abnormalities. Therefore, this is the only group in which routine evaluation of electrolytes is recommended. Common conditions associated with electrolyte imbalance include hyponatremia, hypocalcemia and hypomagnesemia, as well as errors of metabolism.
Hyponatremia in infants can be secondary to congenital adrenal hyperplasia or formula overdilution. Hypocalcemia and hypomagnesemia, associated with hypoparathyroidism may be the initial presentation in infants with DiGeorge syndrome. In addition, inborn errors of metabolism frequently lead to hypoglycemic seizures. Thus, in all patients with ongoing seizures refractory to medication, electrolyte abnormalities should be strongly considered.
Computed Tomography. A computed tomography (CT) scan of the head should be highly considered in this patient population as it is often difficult to determine if the patient has returned to neurological baseline. Since the fontanelles are open in infants, they can tolerate larger increases in intracranial volume (whether from blood or mass lesion) before evidence of increased ICP becomes clinically evident.
Lumbar Puncture. A lumbar puncture (LP) to analyze the cerebrospinal fluid (CSF) for infection should be considered in all afebrile infants with seizures, though some recent evidence shows a relatively low yield in such testing.10,11
Toddlers and Young Children.
Within this age group, febrile seizures tend to predominate. A febrile seizure is defined as a seizure occurring between ages 6 months and 5 years that is associated with fever (temperature >38˚C [100.4˚F]) but without evidence of intracranial infection, neurological disease, or another defined cause.1 These seizures can be simple or complex. Simple febrile seizures last less than 15 minutes, have generalized clinical features, and occur only once in a 24-hour period. In contrast, complex febrile seizures last longer than 15 minutes, have focal manifestations, or recur multiple times in 24 hours.3
Simple Febrile Seizures. The vast majority of patients presenting with simple febrile seizures require very limited diagnostic evaluation. If the patient has no evidence of intracranial infection, a normal neurological examination, and is back to baseline mental status, then no further evaluation is necessary. Serum electrolytes should only be checked if the patient does not quickly return to neurological baseline, at which point checking a serum glucose level would be prudent. Any further laboratory testing should be for the sole purpose of determining the source of the patient’s fever.
Most patients do not require CSF testing. In the consensus statement on the neurodiagnostic evaluation of patients with simple febrile seizures, the American Academy of Pediatrics listed only being younger than 6 months of age as a strong indicator to perform an LP. An LP should, however, be considered in patients aged 6 to 12 months who are deficient in their immunizations (especially Haemophilus influenza type B and Streptococcus pneumoniae) and in all patients pretreated with antibiotics as this could mask the signs of meningitis and encephalitis.12
Complex Febrile Seizures. Firm recommendations regarding the management of complex febrile seizures are currently lacking. These seizures carry a higher risk of intracranial infections and therefore warrant a low-threshold for both neuroimaging and CSF evaluation, especially in those patients younger than age 18 months.
Afebrile Seizures. Types of afebrile seizure disorders presenting in this age group include juvenile myoclonic epilepsy (JME) and benign rolandic epilepsy (BRE), both of which tend to present between ages 5 and 15 years. The majority of patients with first-time afebrile seizures do not require emergent neuroimaging; however, they should be referred to a pediatric neurologist for outpatient magnetic resonance imaging (MRI) of the brain and an electroencephalogram (EEG). Patients requiring emergent neuroimaging with either CT or MRI include those with signs or symptoms of elevated ICP, a focal seizure or focal findings on neurological examination, failure to return to neurological baseline, and a seizure in the setting of head trauma.1
Juvenile Myoclonic Epilepsy. Also known as Janz syndrome, JME is one of most common types of idiopathic generalized epilepsy in childhood. It presents most commonly in otherwise healthy teenagers with one or more of the following seizure types: myoclonic jerks, generalized tonic-clonic seizures (GTCS), or absence seizures. Myoclonic jerks are unique in that they occur during the morning hours, usually the first hour after awakening. They consist of rapid muscle contractions which are most often symmetric and bilateral. The GTCS occur in about 90% of patients with JME, typically just after awakening or during sleep. Both myoclonic jerks and GTCS are exacerbated by sleep deprivation.
Absence seizures are the least common type of seizure in JME. Intelligence in these patients is normal and there is often a family history of similar seizures. Most patients respond well to treatment with antiepileptic drugs, which are usually required for life.3,13
Benign Rolandic Epilepsy. This is the most common form of partial epilepsy in childhood. The name is derived from the central sulcus of the cerebral cortex (the rolandic fissure) around which these seizures originate. Onset of BRE typically occurs between ages 5 and 15 years, with a peak incidence of initial seizures occurring between ages 8 and 9 years. Males are more commonly affected than females (approximate distribution of 1.5:1).2
Simple partial seizures are the hallmark of this type of epilepsy, with the majority of these seizures occurring during sleep. Cardinal features include unilateral facial sensory-motor symptoms, oropharyngeal symptoms, speech arrest, and hypersalivation. Although all of these manifestations are often present, seizures may be marked by only a single symptom. Although it is uncommon, partial seizures may progress to generalized tonic-clonic activity. The hallmark finding on EEG is centrotemporal spikes. Most children do not require treatment, and the vast majority (98%) outgrow the seizures by age 18 years.3 Children with BRE have normal development and intelligence.
Early Adolescents and Teenagers.
Among this cohort, toxic ingestion and overdose tend to be the most common etiologies of first-time seizures presenting to the ED. Oral hypoglycemics (especially sulfonylureas), tricyclic antidepressants, and isoniazid are the most common prescription medications leading to seizures. Others drugs include salicylates, lithium, anticholinergic medications, and bupropion.1 With respect to nonprescription drugs, alcohol can cause seizures via hypoglycemia; cocaine and amphetamines also have a propensity to induce seizures.14 It is paramount to evaluate serum glucose levels and consider toxicologic etiology early in the management of seizures in this age group.
Case Conclusion
Given the focal nature of this patient’s probable seizures, a CT scan of the brain was ordered without contrast to rule out an intracranial mass lesion. Based on negative findings, no further testing was ordered. The patient remained at neurological baseline throughout the course of his stay in the ED, and was discharged home with a prescription for rectal diazepam and instructions on its use for seizures lasting longer than 5 minutes. He was referred to a pediatric neurologist for further evaluation, which included an EEG study that confirmed a diagnosis of BRE.
Dr. Schneider is a pediatric emergency medicine fellow, Eastern Virginia Medical School, Children’s Hospital of the King’s Daughters, Norfolk.
Dr. Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Chiang VW. Seizures. In: Fleisher, GR, Ludwig, S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:564-570.
- Friedman MJ, Sharieff, GQ. Seizures in children. Pediatr Clin N Am. 2006;53(2):257-277.
- Sidhu R, Velayudam, K, Barnes, G. Pediatric seizures. Pediatr Rev. 2013;34(8):333-342.
- Silbergleit R, Durkalski V, Lowenstein D, et al. NETT Investigators. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366(7):591-600.
- Holsti M, Dudley N, Schunk J, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Arch Pediatr Adolesc Med. 2010;164(8):747-753.
- McIntyre J, Robertson S, Norris E, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366(948):205-210.
- Misra UK, Kalita J, Maurya PK. Levetiracetam versus lorazepam in status epilepticus: a randomized, open labeled pilot study. J Neurol. 2012;2594):645-648.
- McTague A, Kneen R, Kumar R, Spinty S, Appleton R. Intravenous levetiracetam in acute repetitive seizures and status epilepticus in children: experience from a children’s hospital. Seizure. 2012;21(7):529-534.
- Shields WD. Infantile spasms: little seizures, BIG consequences. Epilepsy Curr. 2006;6(3):63-69.
- Sharma S, Riviello JJ, Harper MB, Baskin, MN. The role of emergent neuroimaging in children with new-onset afebrile seizures. Pediatrics. 2003;111(1):1-5.
- Lateef TM, Tsuchida TN, Chang T, Johnson J, Gaillard WD, Nelson KB. Diagnostic value of lumbar puncture in afebrile infants with suspected new-onset seizures. J Pediatr. 2008;153(1):140-142.
- Subcommittee on Febrile Seizures; American Academy of Pediatrics. Neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. 2011;127(2):389-394.
- Genton P, Thomas P, Kasteleijn-Nolst Trenité DG, Medina MT, Salas-Puig J. Clinical aspects of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(suppl 1):S8-S14.
- Thundiyil JG, Kearney TE, Olsen KR. Evolving epidemiology of drug-induced seizures reported to a poison control center system. J Med Toxicol. 2007;3(1):15-19.
Immediately following this episode, the parents took their son to the ED for evaluation. He was afebrile with normal vital signs. The physical examination, which included a neurological examination, was normal, and both parents noted that the child appeared to be completely back to his normal behavior. The patient’s past medical and surgical histories were unremarkable. His parents stated that he had not been on any medication and denied a family history of seizures.
Overview
Seizures, the most common pediatric neurological disorder, are a frequent presentation in the ED. It is estimated that between 4% and 10% of children will have at least one seizure before age 16 years.1,2 The highest incidence of occurrence is seen in children younger than age 3 years; this frequency decreases in older children.2
Seizures are the resulting clinical manifestations of abnormal excessive synchronized neuronal activity within the cerebral cortex. Most seizure activity is stereotypical and self-limited3 and can be divided into two main types: generalized and partial. Generalized seizures involve both cerebral hemispheres and impairment of consciousness, while partial (or focal) seizures involve a single area of one hemisphere. Generalized seizures are usually classified as convulsive or nonconvulsive and include the following subtypes: tonic-clonic, atonic, absence, and myoclonic. Partial seizures can be subdivided into simple or complex, depending on whether consciousness is impaired.
Initial Management
As with critically ill patients, the first and most vital step in managing a seizing patient is to assess the airway, breathing, and circulation. Although the airway is the most frequently compromised component, simple interventions such as jaw thrust, suctioning, or the insertion of an oral or nasopharyngeal airway are usually sufficient to maintain adequate airway. If intubation is required, use of a short-acting paralytic agent should be considered so as not to mask ongoing seizure activity.1 Patients actively seizing at presentation should be assumed to be in status epilepticus, which is defined as seizure activity lasting longer than 5 minutes or as repetitive seizure episodes without return of consciousness between episodes.
Treatment
The benzodiazepines are the first-line treatment for status epilepticus in pediatric patients. Although intravenous (IV) lorazepam has long been the standard for seizure termination, intramuscular midazolam has been shown to be as safe and effective in patients weighing more than 13 kg in whom IV access is either not available or is difficult to obtain.4
Many other forms of benzodiazepines exist for seizure cessation, such as diazepam gel, which is administered rectally. Intranasal and buccal administration of midazolam also has proved effective for pediatric seizure termination.5,6 Second-line therapies include IV fosphenytoin, levetiracetam, phenobarbital, and valproic acid.1,7,8 In infants, phenobarbital is usually the primary second-line therapy after benzodiazepines.
History and Behavioral Observation
Given both the significant number of causes of pediatric seizures and events that can mimic childhood seizure activity, obtaining a thorough history and detailed description of the seizure episode and any preceding events are essential. It is also important to note nonseizure-related activities unique to the pediatric population (eg, Moro reflex in young infants, back-arching with gastroesophageal reflux [Sandifer syndrome], breath-holding spells, daydreaming).
Differentiating normal movements from seizures can be particularly difficult with infants. For example, repetitive bicycling movements of the legs are a common sign of seizure activity in an infant but may be mistaken as normal by parents or inexperienced observers.
West Syndrome.
West syndrome (infantile spasms) is a rare severe seizure syndrome consisting of spasmodic flexural movements of the extremities and trunk that usually presents between ages 4 to 18 months. Subtle episodes can be misinterpreted as Moro reflex, the normal sudden extension of an infant’s extremities and arching of the back occurring from birth to approximately ages 3 to 5 months.9
Breath-holding.
An episode of loss of consciousness with associated cyanosis, pallor, rigidity, limpness, or even twitching that was immediately preceded by vigorous crying in a child ages 6 months to 5 years should prompt the physician to strongly consider a breath-holding spell.
Attention Deficit Disorder Versus Seizure Activity.
Children, especially those with attention deficit disorder, have a propensity for daydreaming. These children will often stare and not respond to voice at times; however, if these episodes are associated with facial movements or a sudden pause in activity (also known as behavior arrest), the possibility of absence or complex partial seizures should be suspected.2
Physical Examination
Along with the patient’s history, the physical examination should focus primarily on determining a possible seizure etiology. The entire body should be thoroughly evaluated for evidence of trauma. The head should be carefully evaluated for signs of deformity or swelling; the fullness of the anterior fontanel should be carefully assessed; and the pupils should be examined for signs of increased intracranial pressure (ICP). In the older pediatric patient with closed fontanelles in whom a cervical spine injury has been ruled out, assessment of the neck for evidence of meningeal irritation should be performed. Stigmata of underlying medical disease, along with the presence of dysmorphic features associated with particular genetic syndromes, also should be evaluated. In addition, signs of common toxidromes should be sought.
Common Etiologies and Diagnostic Evaluation by Age
Since seizure etiologies and diagnostic evaluation vary greatly along the pediatric age spectrum, it is helpful to divide this population into three main groups: neonates and infants (ages 0-12 months), toddlers and young children (ages 12 months-10 years), and preadolescents and teenagers (ages 11-18 years).
In patients with known seizure disorders, the majority of cases are due to subtherapeutic antiepileptic medications either from a patient “outgrowing” his or her weight-based dose or from medication noncompliance. For the purposes of this article, we have limited our discussion to patients with first-time seizures.
Neonates and Infants.
In infants, hypoxic ischemic encephalopathy (HIE) due to perinatal asphyxia is the most common cause of seizures, the vast majority of which present within the first 24 to 48 hours of life.2 Although EPs may encounter children with seizures caused by HIE, it is rare that these represent an initial seizure. Far more common etiologies of first-time seizures in infants are infection (eg, meningitis, encephalitis, sepsis) and nonaccidental trauma.
Electrolyte Imbalance. Although electrolytes are frequently checked in all age groups with seizures, infants are by far the most likely to experience seizures secondary to electrolyte abnormalities. Therefore, this is the only group in which routine evaluation of electrolytes is recommended. Common conditions associated with electrolyte imbalance include hyponatremia, hypocalcemia and hypomagnesemia, as well as errors of metabolism.
Hyponatremia in infants can be secondary to congenital adrenal hyperplasia or formula overdilution. Hypocalcemia and hypomagnesemia, associated with hypoparathyroidism may be the initial presentation in infants with DiGeorge syndrome. In addition, inborn errors of metabolism frequently lead to hypoglycemic seizures. Thus, in all patients with ongoing seizures refractory to medication, electrolyte abnormalities should be strongly considered.
Computed Tomography. A computed tomography (CT) scan of the head should be highly considered in this patient population as it is often difficult to determine if the patient has returned to neurological baseline. Since the fontanelles are open in infants, they can tolerate larger increases in intracranial volume (whether from blood or mass lesion) before evidence of increased ICP becomes clinically evident.
Lumbar Puncture. A lumbar puncture (LP) to analyze the cerebrospinal fluid (CSF) for infection should be considered in all afebrile infants with seizures, though some recent evidence shows a relatively low yield in such testing.10,11
Toddlers and Young Children.
Within this age group, febrile seizures tend to predominate. A febrile seizure is defined as a seizure occurring between ages 6 months and 5 years that is associated with fever (temperature >38˚C [100.4˚F]) but without evidence of intracranial infection, neurological disease, or another defined cause.1 These seizures can be simple or complex. Simple febrile seizures last less than 15 minutes, have generalized clinical features, and occur only once in a 24-hour period. In contrast, complex febrile seizures last longer than 15 minutes, have focal manifestations, or recur multiple times in 24 hours.3
Simple Febrile Seizures. The vast majority of patients presenting with simple febrile seizures require very limited diagnostic evaluation. If the patient has no evidence of intracranial infection, a normal neurological examination, and is back to baseline mental status, then no further evaluation is necessary. Serum electrolytes should only be checked if the patient does not quickly return to neurological baseline, at which point checking a serum glucose level would be prudent. Any further laboratory testing should be for the sole purpose of determining the source of the patient’s fever.
Most patients do not require CSF testing. In the consensus statement on the neurodiagnostic evaluation of patients with simple febrile seizures, the American Academy of Pediatrics listed only being younger than 6 months of age as a strong indicator to perform an LP. An LP should, however, be considered in patients aged 6 to 12 months who are deficient in their immunizations (especially Haemophilus influenza type B and Streptococcus pneumoniae) and in all patients pretreated with antibiotics as this could mask the signs of meningitis and encephalitis.12
Complex Febrile Seizures. Firm recommendations regarding the management of complex febrile seizures are currently lacking. These seizures carry a higher risk of intracranial infections and therefore warrant a low-threshold for both neuroimaging and CSF evaluation, especially in those patients younger than age 18 months.
Afebrile Seizures. Types of afebrile seizure disorders presenting in this age group include juvenile myoclonic epilepsy (JME) and benign rolandic epilepsy (BRE), both of which tend to present between ages 5 and 15 years. The majority of patients with first-time afebrile seizures do not require emergent neuroimaging; however, they should be referred to a pediatric neurologist for outpatient magnetic resonance imaging (MRI) of the brain and an electroencephalogram (EEG). Patients requiring emergent neuroimaging with either CT or MRI include those with signs or symptoms of elevated ICP, a focal seizure or focal findings on neurological examination, failure to return to neurological baseline, and a seizure in the setting of head trauma.1
Juvenile Myoclonic Epilepsy. Also known as Janz syndrome, JME is one of most common types of idiopathic generalized epilepsy in childhood. It presents most commonly in otherwise healthy teenagers with one or more of the following seizure types: myoclonic jerks, generalized tonic-clonic seizures (GTCS), or absence seizures. Myoclonic jerks are unique in that they occur during the morning hours, usually the first hour after awakening. They consist of rapid muscle contractions which are most often symmetric and bilateral. The GTCS occur in about 90% of patients with JME, typically just after awakening or during sleep. Both myoclonic jerks and GTCS are exacerbated by sleep deprivation.
Absence seizures are the least common type of seizure in JME. Intelligence in these patients is normal and there is often a family history of similar seizures. Most patients respond well to treatment with antiepileptic drugs, which are usually required for life.3,13
Benign Rolandic Epilepsy. This is the most common form of partial epilepsy in childhood. The name is derived from the central sulcus of the cerebral cortex (the rolandic fissure) around which these seizures originate. Onset of BRE typically occurs between ages 5 and 15 years, with a peak incidence of initial seizures occurring between ages 8 and 9 years. Males are more commonly affected than females (approximate distribution of 1.5:1).2
Simple partial seizures are the hallmark of this type of epilepsy, with the majority of these seizures occurring during sleep. Cardinal features include unilateral facial sensory-motor symptoms, oropharyngeal symptoms, speech arrest, and hypersalivation. Although all of these manifestations are often present, seizures may be marked by only a single symptom. Although it is uncommon, partial seizures may progress to generalized tonic-clonic activity. The hallmark finding on EEG is centrotemporal spikes. Most children do not require treatment, and the vast majority (98%) outgrow the seizures by age 18 years.3 Children with BRE have normal development and intelligence.
Early Adolescents and Teenagers.
Among this cohort, toxic ingestion and overdose tend to be the most common etiologies of first-time seizures presenting to the ED. Oral hypoglycemics (especially sulfonylureas), tricyclic antidepressants, and isoniazid are the most common prescription medications leading to seizures. Others drugs include salicylates, lithium, anticholinergic medications, and bupropion.1 With respect to nonprescription drugs, alcohol can cause seizures via hypoglycemia; cocaine and amphetamines also have a propensity to induce seizures.14 It is paramount to evaluate serum glucose levels and consider toxicologic etiology early in the management of seizures in this age group.
Case Conclusion
Given the focal nature of this patient’s probable seizures, a CT scan of the brain was ordered without contrast to rule out an intracranial mass lesion. Based on negative findings, no further testing was ordered. The patient remained at neurological baseline throughout the course of his stay in the ED, and was discharged home with a prescription for rectal diazepam and instructions on its use for seizures lasting longer than 5 minutes. He was referred to a pediatric neurologist for further evaluation, which included an EEG study that confirmed a diagnosis of BRE.
Dr. Schneider is a pediatric emergency medicine fellow, Eastern Virginia Medical School, Children’s Hospital of the King’s Daughters, Norfolk.
Dr. Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
Immediately following this episode, the parents took their son to the ED for evaluation. He was afebrile with normal vital signs. The physical examination, which included a neurological examination, was normal, and both parents noted that the child appeared to be completely back to his normal behavior. The patient’s past medical and surgical histories were unremarkable. His parents stated that he had not been on any medication and denied a family history of seizures.
Overview
Seizures, the most common pediatric neurological disorder, are a frequent presentation in the ED. It is estimated that between 4% and 10% of children will have at least one seizure before age 16 years.1,2 The highest incidence of occurrence is seen in children younger than age 3 years; this frequency decreases in older children.2
Seizures are the resulting clinical manifestations of abnormal excessive synchronized neuronal activity within the cerebral cortex. Most seizure activity is stereotypical and self-limited3 and can be divided into two main types: generalized and partial. Generalized seizures involve both cerebral hemispheres and impairment of consciousness, while partial (or focal) seizures involve a single area of one hemisphere. Generalized seizures are usually classified as convulsive or nonconvulsive and include the following subtypes: tonic-clonic, atonic, absence, and myoclonic. Partial seizures can be subdivided into simple or complex, depending on whether consciousness is impaired.
Initial Management
As with critically ill patients, the first and most vital step in managing a seizing patient is to assess the airway, breathing, and circulation. Although the airway is the most frequently compromised component, simple interventions such as jaw thrust, suctioning, or the insertion of an oral or nasopharyngeal airway are usually sufficient to maintain adequate airway. If intubation is required, use of a short-acting paralytic agent should be considered so as not to mask ongoing seizure activity.1 Patients actively seizing at presentation should be assumed to be in status epilepticus, which is defined as seizure activity lasting longer than 5 minutes or as repetitive seizure episodes without return of consciousness between episodes.
Treatment
The benzodiazepines are the first-line treatment for status epilepticus in pediatric patients. Although intravenous (IV) lorazepam has long been the standard for seizure termination, intramuscular midazolam has been shown to be as safe and effective in patients weighing more than 13 kg in whom IV access is either not available or is difficult to obtain.4
Many other forms of benzodiazepines exist for seizure cessation, such as diazepam gel, which is administered rectally. Intranasal and buccal administration of midazolam also has proved effective for pediatric seizure termination.5,6 Second-line therapies include IV fosphenytoin, levetiracetam, phenobarbital, and valproic acid.1,7,8 In infants, phenobarbital is usually the primary second-line therapy after benzodiazepines.
History and Behavioral Observation
Given both the significant number of causes of pediatric seizures and events that can mimic childhood seizure activity, obtaining a thorough history and detailed description of the seizure episode and any preceding events are essential. It is also important to note nonseizure-related activities unique to the pediatric population (eg, Moro reflex in young infants, back-arching with gastroesophageal reflux [Sandifer syndrome], breath-holding spells, daydreaming).
Differentiating normal movements from seizures can be particularly difficult with infants. For example, repetitive bicycling movements of the legs are a common sign of seizure activity in an infant but may be mistaken as normal by parents or inexperienced observers.
West Syndrome.
West syndrome (infantile spasms) is a rare severe seizure syndrome consisting of spasmodic flexural movements of the extremities and trunk that usually presents between ages 4 to 18 months. Subtle episodes can be misinterpreted as Moro reflex, the normal sudden extension of an infant’s extremities and arching of the back occurring from birth to approximately ages 3 to 5 months.9
Breath-holding.
An episode of loss of consciousness with associated cyanosis, pallor, rigidity, limpness, or even twitching that was immediately preceded by vigorous crying in a child ages 6 months to 5 years should prompt the physician to strongly consider a breath-holding spell.
Attention Deficit Disorder Versus Seizure Activity.
Children, especially those with attention deficit disorder, have a propensity for daydreaming. These children will often stare and not respond to voice at times; however, if these episodes are associated with facial movements or a sudden pause in activity (also known as behavior arrest), the possibility of absence or complex partial seizures should be suspected.2
Physical Examination
Along with the patient’s history, the physical examination should focus primarily on determining a possible seizure etiology. The entire body should be thoroughly evaluated for evidence of trauma. The head should be carefully evaluated for signs of deformity or swelling; the fullness of the anterior fontanel should be carefully assessed; and the pupils should be examined for signs of increased intracranial pressure (ICP). In the older pediatric patient with closed fontanelles in whom a cervical spine injury has been ruled out, assessment of the neck for evidence of meningeal irritation should be performed. Stigmata of underlying medical disease, along with the presence of dysmorphic features associated with particular genetic syndromes, also should be evaluated. In addition, signs of common toxidromes should be sought.
Common Etiologies and Diagnostic Evaluation by Age
Since seizure etiologies and diagnostic evaluation vary greatly along the pediatric age spectrum, it is helpful to divide this population into three main groups: neonates and infants (ages 0-12 months), toddlers and young children (ages 12 months-10 years), and preadolescents and teenagers (ages 11-18 years).
In patients with known seizure disorders, the majority of cases are due to subtherapeutic antiepileptic medications either from a patient “outgrowing” his or her weight-based dose or from medication noncompliance. For the purposes of this article, we have limited our discussion to patients with first-time seizures.
Neonates and Infants.
In infants, hypoxic ischemic encephalopathy (HIE) due to perinatal asphyxia is the most common cause of seizures, the vast majority of which present within the first 24 to 48 hours of life.2 Although EPs may encounter children with seizures caused by HIE, it is rare that these represent an initial seizure. Far more common etiologies of first-time seizures in infants are infection (eg, meningitis, encephalitis, sepsis) and nonaccidental trauma.
Electrolyte Imbalance. Although electrolytes are frequently checked in all age groups with seizures, infants are by far the most likely to experience seizures secondary to electrolyte abnormalities. Therefore, this is the only group in which routine evaluation of electrolytes is recommended. Common conditions associated with electrolyte imbalance include hyponatremia, hypocalcemia and hypomagnesemia, as well as errors of metabolism.
Hyponatremia in infants can be secondary to congenital adrenal hyperplasia or formula overdilution. Hypocalcemia and hypomagnesemia, associated with hypoparathyroidism may be the initial presentation in infants with DiGeorge syndrome. In addition, inborn errors of metabolism frequently lead to hypoglycemic seizures. Thus, in all patients with ongoing seizures refractory to medication, electrolyte abnormalities should be strongly considered.
Computed Tomography. A computed tomography (CT) scan of the head should be highly considered in this patient population as it is often difficult to determine if the patient has returned to neurological baseline. Since the fontanelles are open in infants, they can tolerate larger increases in intracranial volume (whether from blood or mass lesion) before evidence of increased ICP becomes clinically evident.
Lumbar Puncture. A lumbar puncture (LP) to analyze the cerebrospinal fluid (CSF) for infection should be considered in all afebrile infants with seizures, though some recent evidence shows a relatively low yield in such testing.10,11
Toddlers and Young Children.
Within this age group, febrile seizures tend to predominate. A febrile seizure is defined as a seizure occurring between ages 6 months and 5 years that is associated with fever (temperature >38˚C [100.4˚F]) but without evidence of intracranial infection, neurological disease, or another defined cause.1 These seizures can be simple or complex. Simple febrile seizures last less than 15 minutes, have generalized clinical features, and occur only once in a 24-hour period. In contrast, complex febrile seizures last longer than 15 minutes, have focal manifestations, or recur multiple times in 24 hours.3
Simple Febrile Seizures. The vast majority of patients presenting with simple febrile seizures require very limited diagnostic evaluation. If the patient has no evidence of intracranial infection, a normal neurological examination, and is back to baseline mental status, then no further evaluation is necessary. Serum electrolytes should only be checked if the patient does not quickly return to neurological baseline, at which point checking a serum glucose level would be prudent. Any further laboratory testing should be for the sole purpose of determining the source of the patient’s fever.
Most patients do not require CSF testing. In the consensus statement on the neurodiagnostic evaluation of patients with simple febrile seizures, the American Academy of Pediatrics listed only being younger than 6 months of age as a strong indicator to perform an LP. An LP should, however, be considered in patients aged 6 to 12 months who are deficient in their immunizations (especially Haemophilus influenza type B and Streptococcus pneumoniae) and in all patients pretreated with antibiotics as this could mask the signs of meningitis and encephalitis.12
Complex Febrile Seizures. Firm recommendations regarding the management of complex febrile seizures are currently lacking. These seizures carry a higher risk of intracranial infections and therefore warrant a low-threshold for both neuroimaging and CSF evaluation, especially in those patients younger than age 18 months.
Afebrile Seizures. Types of afebrile seizure disorders presenting in this age group include juvenile myoclonic epilepsy (JME) and benign rolandic epilepsy (BRE), both of which tend to present between ages 5 and 15 years. The majority of patients with first-time afebrile seizures do not require emergent neuroimaging; however, they should be referred to a pediatric neurologist for outpatient magnetic resonance imaging (MRI) of the brain and an electroencephalogram (EEG). Patients requiring emergent neuroimaging with either CT or MRI include those with signs or symptoms of elevated ICP, a focal seizure or focal findings on neurological examination, failure to return to neurological baseline, and a seizure in the setting of head trauma.1
Juvenile Myoclonic Epilepsy. Also known as Janz syndrome, JME is one of most common types of idiopathic generalized epilepsy in childhood. It presents most commonly in otherwise healthy teenagers with one or more of the following seizure types: myoclonic jerks, generalized tonic-clonic seizures (GTCS), or absence seizures. Myoclonic jerks are unique in that they occur during the morning hours, usually the first hour after awakening. They consist of rapid muscle contractions which are most often symmetric and bilateral. The GTCS occur in about 90% of patients with JME, typically just after awakening or during sleep. Both myoclonic jerks and GTCS are exacerbated by sleep deprivation.
Absence seizures are the least common type of seizure in JME. Intelligence in these patients is normal and there is often a family history of similar seizures. Most patients respond well to treatment with antiepileptic drugs, which are usually required for life.3,13
Benign Rolandic Epilepsy. This is the most common form of partial epilepsy in childhood. The name is derived from the central sulcus of the cerebral cortex (the rolandic fissure) around which these seizures originate. Onset of BRE typically occurs between ages 5 and 15 years, with a peak incidence of initial seizures occurring between ages 8 and 9 years. Males are more commonly affected than females (approximate distribution of 1.5:1).2
Simple partial seizures are the hallmark of this type of epilepsy, with the majority of these seizures occurring during sleep. Cardinal features include unilateral facial sensory-motor symptoms, oropharyngeal symptoms, speech arrest, and hypersalivation. Although all of these manifestations are often present, seizures may be marked by only a single symptom. Although it is uncommon, partial seizures may progress to generalized tonic-clonic activity. The hallmark finding on EEG is centrotemporal spikes. Most children do not require treatment, and the vast majority (98%) outgrow the seizures by age 18 years.3 Children with BRE have normal development and intelligence.
Early Adolescents and Teenagers.
Among this cohort, toxic ingestion and overdose tend to be the most common etiologies of first-time seizures presenting to the ED. Oral hypoglycemics (especially sulfonylureas), tricyclic antidepressants, and isoniazid are the most common prescription medications leading to seizures. Others drugs include salicylates, lithium, anticholinergic medications, and bupropion.1 With respect to nonprescription drugs, alcohol can cause seizures via hypoglycemia; cocaine and amphetamines also have a propensity to induce seizures.14 It is paramount to evaluate serum glucose levels and consider toxicologic etiology early in the management of seizures in this age group.
Case Conclusion
Given the focal nature of this patient’s probable seizures, a CT scan of the brain was ordered without contrast to rule out an intracranial mass lesion. Based on negative findings, no further testing was ordered. The patient remained at neurological baseline throughout the course of his stay in the ED, and was discharged home with a prescription for rectal diazepam and instructions on its use for seizures lasting longer than 5 minutes. He was referred to a pediatric neurologist for further evaluation, which included an EEG study that confirmed a diagnosis of BRE.
Dr. Schneider is a pediatric emergency medicine fellow, Eastern Virginia Medical School, Children’s Hospital of the King’s Daughters, Norfolk.
Dr. Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Chiang VW. Seizures. In: Fleisher, GR, Ludwig, S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:564-570.
- Friedman MJ, Sharieff, GQ. Seizures in children. Pediatr Clin N Am. 2006;53(2):257-277.
- Sidhu R, Velayudam, K, Barnes, G. Pediatric seizures. Pediatr Rev. 2013;34(8):333-342.
- Silbergleit R, Durkalski V, Lowenstein D, et al. NETT Investigators. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366(7):591-600.
- Holsti M, Dudley N, Schunk J, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Arch Pediatr Adolesc Med. 2010;164(8):747-753.
- McIntyre J, Robertson S, Norris E, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366(948):205-210.
- Misra UK, Kalita J, Maurya PK. Levetiracetam versus lorazepam in status epilepticus: a randomized, open labeled pilot study. J Neurol. 2012;2594):645-648.
- McTague A, Kneen R, Kumar R, Spinty S, Appleton R. Intravenous levetiracetam in acute repetitive seizures and status epilepticus in children: experience from a children’s hospital. Seizure. 2012;21(7):529-534.
- Shields WD. Infantile spasms: little seizures, BIG consequences. Epilepsy Curr. 2006;6(3):63-69.
- Sharma S, Riviello JJ, Harper MB, Baskin, MN. The role of emergent neuroimaging in children with new-onset afebrile seizures. Pediatrics. 2003;111(1):1-5.
- Lateef TM, Tsuchida TN, Chang T, Johnson J, Gaillard WD, Nelson KB. Diagnostic value of lumbar puncture in afebrile infants with suspected new-onset seizures. J Pediatr. 2008;153(1):140-142.
- Subcommittee on Febrile Seizures; American Academy of Pediatrics. Neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. 2011;127(2):389-394.
- Genton P, Thomas P, Kasteleijn-Nolst Trenité DG, Medina MT, Salas-Puig J. Clinical aspects of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(suppl 1):S8-S14.
- Thundiyil JG, Kearney TE, Olsen KR. Evolving epidemiology of drug-induced seizures reported to a poison control center system. J Med Toxicol. 2007;3(1):15-19.
- Chiang VW. Seizures. In: Fleisher, GR, Ludwig, S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:564-570.
- Friedman MJ, Sharieff, GQ. Seizures in children. Pediatr Clin N Am. 2006;53(2):257-277.
- Sidhu R, Velayudam, K, Barnes, G. Pediatric seizures. Pediatr Rev. 2013;34(8):333-342.
- Silbergleit R, Durkalski V, Lowenstein D, et al. NETT Investigators. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366(7):591-600.
- Holsti M, Dudley N, Schunk J, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Arch Pediatr Adolesc Med. 2010;164(8):747-753.
- McIntyre J, Robertson S, Norris E, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366(948):205-210.
- Misra UK, Kalita J, Maurya PK. Levetiracetam versus lorazepam in status epilepticus: a randomized, open labeled pilot study. J Neurol. 2012;2594):645-648.
- McTague A, Kneen R, Kumar R, Spinty S, Appleton R. Intravenous levetiracetam in acute repetitive seizures and status epilepticus in children: experience from a children’s hospital. Seizure. 2012;21(7):529-534.
- Shields WD. Infantile spasms: little seizures, BIG consequences. Epilepsy Curr. 2006;6(3):63-69.
- Sharma S, Riviello JJ, Harper MB, Baskin, MN. The role of emergent neuroimaging in children with new-onset afebrile seizures. Pediatrics. 2003;111(1):1-5.
- Lateef TM, Tsuchida TN, Chang T, Johnson J, Gaillard WD, Nelson KB. Diagnostic value of lumbar puncture in afebrile infants with suspected new-onset seizures. J Pediatr. 2008;153(1):140-142.
- Subcommittee on Febrile Seizures; American Academy of Pediatrics. Neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. 2011;127(2):389-394.
- Genton P, Thomas P, Kasteleijn-Nolst Trenité DG, Medina MT, Salas-Puig J. Clinical aspects of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(suppl 1):S8-S14.
- Thundiyil JG, Kearney TE, Olsen KR. Evolving epidemiology of drug-induced seizures reported to a poison control center system. J Med Toxicol. 2007;3(1):15-19.