User login
Ultra-Late Cutaneous Melanoma Recurrence Following 49 Years of Quiescence
To the Editor:
Ultra-late melanoma recurrence represents a minority of cases in which the quiescent period lasts longer than 15 years, and epidemiologic studies have reported recurrence rates of 6% to 10% during the ultra-late period.1 Even more uncommon are cases that span many decades (eg, >30 years), but all are useful in understanding the cellular behavior leading to the reactivation of fully excised melanomas. Few cases have been reported in which recurrence occurs more than 35 years after the original diagnosis of melanoma. Unfortunately, mechanisms underlying this long stable quiescence and subsequent reactivation are poorly understood, which is why it is important to identify and document cases. We present a case of local recurrence of cutaneous melanoma on the patient’s lower back after a 49-year disease-free period.
A 78-year-old White woman presented to a private dermatology office for a full-body skin examination. She had a medical history of a cutaneous melanoma that had been removed on the lower back 49 years prior; Parkinson disease of 10 years’ duration; and an enlarged thyroid nodule with decreased thyrotropin and hyperthyroidism, atrial fibrillation, mitral valve prolapse, osteoarthritis in the knees, and actinic keratoses, all of which were chronic conditions lasting years to decades. She was taking several medications for these medical conditions. Her surgical history included a hysterectomy, hip replacement, hernia repair, cardioversion, and tonsillectomy in childhood. Her family medical history included breast cancer in her paternal grandmother and aunt; hypertension in her father; and sarcoma in her mother at 78 years of age, which initially was identified in the sacrum and metastasized to the lungs causing death. No family history of melanoma or other skin cancers was reported. Prior to the original diagnosis of melanoma at 29 years of age, she had no history of skin cancer or any other medical condition other than acne. The patient did report spending a great deal of time in the sun during high school.
The patient reported developing the original cutaneous melanoma during her second pregnancy at 29 years of age and recalled that it was excised with wide margins. There had been a mole on her back that was present for years but changed in size during pregnancy, prompting the original visit to the primary care physician for evaluation. Remarkably, the original pathology report was obtained from the patient and revealed a specimen consisting of a 3.7×1.7-cm skin
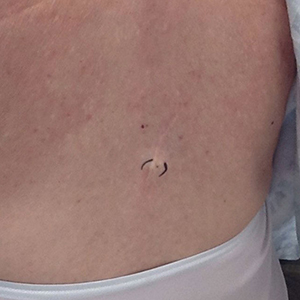
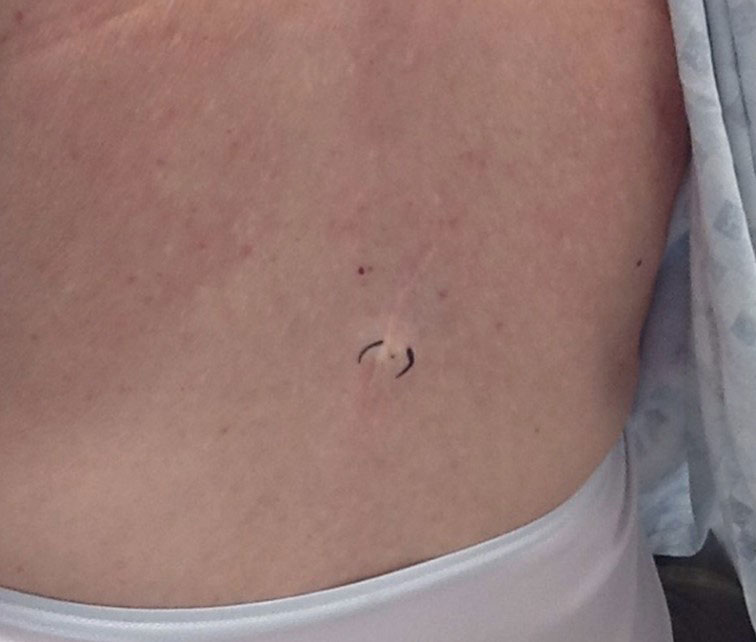
Physical examination at the current presentation 49 years later revealed an even-bordered 2-mm black macule that was located approximately 1 cm from the original melanoma excision scar line (Figure). A biopsy was performed and sent to a dermatopathologist. Microscopic evaluation revealed nests, islands, and sheets of atypical epithelioid melanocytes extending through the dermis between collagen bundles. The melanocytes varied in size and shape with moderate nuclear pleomorphism present. Scattered mitotic figures and necrotic melanocytes were present, which most likely represented cutaneous satellite metastases of melanoma. Subsequent chest radiography, full-body positron emission tomography, and standard laboratory blood tests were unremarkable except for an enlarged right thyroid gland and moderate cardiomegaly. The patient was sent to a surgical oncologist for excision with wide surgical margins, and she elected not to have a sentinel lymph node biopsy. At follow-up 3, 6, 12, and 24 months later, there were no signs of recurrence based on direct clinical examination. The patient subsequently was lost to follow-up.
Recurrence rates of melanoma vary by stage and age at diagnosis, but prior studies have reported a recurrence rate of approximately 6% after 10 or more years following the initial diagnosis.2 Ultra-late recurrences of approximately 4 decades or more are extremely rare. A PubMed search of articles indexed for MEDLINE using the terms melanoma and ultra-late recurrence revealed 4 reported cases with a quiescent period of 38 or more years.3-6 All cases were metastatic melanomas in women; spanned 38, 40, 41, and 45 years from the initial melanoma diagnosis to recurrence; and all of the recurrences except one were regional or distal metastatic lesions (eg, lymph node, brain). In one case, both the original and recurrent lesions occurred on the left elbow.6 The original lesions occurred on the legs, elbow, and back of the neck, and there were no notable concomitant medical conditions. The patients were aged 72, 73, 73, and 84 years at recurrence.3-6 However, generalizations from these cases are limited given the potential for selection bias (eg, men may be less likely to visit a clinic for follow-up and nevi examination) and the likelihood that many cases of ultra-late melanoma recurrence are unrecognized or unreported.
More recently, genomic analyses on melanoma lesions occurring 30 years apart confirmed that the second lesion was indeed a recurrence, although with numerous additional mutations.7 The specific mechanisms underlying the dormancy and subsequent reemergence of metastatic lesions are unclear, but
It also is worth highlighting the concomitant diagnosis of Parkinson disease in our patient. In recent years, Parkinson disease has been linked to melanoma in both epidemiologic and genetic studies. For example, one large-scale study found a 50% increased risk for developing Parkinson disease in patients with melanoma (and vice versa), and this finding has been replicated in other studies.10 Moreover, patients with Parkinson disease have a 2-fold increase in their risk for developing melanoma, demonstrating that it is a bidirectional pathway. Not surprisingly, associations between melanin and neuromelanin pathways have been identified as a potential link between these diseases, and scientists are in the process of understanding the genetic components of both.10 It is unknown if specific genetic mutations contributed to both diseases in our case, but follow-up genetic testing on the recurrent melanoma specimen currently is being pursued.
The 49-year quiescent period in our case of recurrent cutaneous malignant melanoma potentially represents the longest ultra-late recurrence of melanoma in the literature to date based on a review of indexed publications. Moreover, it is relatively unique compared to other similar cases in that the recurrence was within a centimeter of the original excisional scar. Most metastases occur in locoregional lymph nodes or the lungs3; therefore, it is unusual to find one so close to the original lesion, especially one that occurred decades later. Factors associated with ultra-late recurrences are unknown, primarily because of the rarity of these cases as well as the biases and other factors that limit existing studies. However, genetic sequencing may provide information regarding these factors and related processes. Genetic sequencing specifically points to a small cell group remaining after excision of the primary tumor, which mutates while proliferating. Low antigenicity and tolerance to immunity during the quiescent period may explain the long duration of dormancy.6 More recently, there have been efforts to identify immunohistochemical signatures that may predict late recurrences, though the data are preliminary in nature.11
Given the latency period and location of the recurrence, our case demonstrates that even fully excised melanomas may recur locally many decades later, hence patients should be aware of the importance of a lifetime of vigilance after being diagnosed with melanoma.
- Tsao H, Cosimi AB, Sober AJ. Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer. 1997;79:2361-2370.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Mansour D, Kejariwal D. It is never too late: ultra-late recurrence of melanoma with distant metastases [published online March 8, 2012]. BMJ Case Rep. 2012:bcr0120125474. doi:10.1136/bcr.01.2012.5474
- Saleh D, Peach AHS. Ultra-late recurrence of malignant melanoma after 40 years of quiescent disease. J Surg Oncol. 2011;103:290-291.
- Goodenough J, Cozon CL, Liew SH. An incidental finding of a nodal recurrence of cutaneous malignant melanoma after a 45-year disease-free period [published online June 4, 2014]. BMJ Case Rep. 2014:bcr2014204289. doi:10.1136/bcr-2014-204289
- Nakamura M, Obayashi M, Yoshimitsu M, et al. Comparative whole-exome sequencing of an ultra-late recurrent malignant melanoma. Br J Dermatol. 2021;184:762-763.
- Miller JJ, Lofgren KA, Hughes SR, et al. Genomic analysis of melanoma evolution following a 30-year disease-free interval. J Cutan Pathol. 2017;44:805-808.
- North JP, Kageshita T, Pinkel D, et al. Distribution and significance of occult intraepidermal tumor cells surrounding primary melanoma. J Invest Dermatol. 2008;128:2024-2030.
- Massi G, LeBoit PE. Recurrent and persistent melanoma. In: Massi G, LeBoit PE, eds. Histological Diagnosis of Nevi and Melanoma. 2nd ed. Springer-Verlag; 2014:689-698.
- Bose A, Petsko GA, Eliezer D. Parkinson’s disease and melanoma: co-occurrence and mechanisms. J Parkinsons Dis. 2018;8:385-398.
- Reschke R, Dumann K, Ziemer M. Risk stratification and clinical characteristics of patients with late recurrence of melanoma (>10 years).J Clin Med. 2022;11:2026.
To the Editor:
Ultra-late melanoma recurrence represents a minority of cases in which the quiescent period lasts longer than 15 years, and epidemiologic studies have reported recurrence rates of 6% to 10% during the ultra-late period.1 Even more uncommon are cases that span many decades (eg, >30 years), but all are useful in understanding the cellular behavior leading to the reactivation of fully excised melanomas. Few cases have been reported in which recurrence occurs more than 35 years after the original diagnosis of melanoma. Unfortunately, mechanisms underlying this long stable quiescence and subsequent reactivation are poorly understood, which is why it is important to identify and document cases. We present a case of local recurrence of cutaneous melanoma on the patient’s lower back after a 49-year disease-free period.
A 78-year-old White woman presented to a private dermatology office for a full-body skin examination. She had a medical history of a cutaneous melanoma that had been removed on the lower back 49 years prior; Parkinson disease of 10 years’ duration; and an enlarged thyroid nodule with decreased thyrotropin and hyperthyroidism, atrial fibrillation, mitral valve prolapse, osteoarthritis in the knees, and actinic keratoses, all of which were chronic conditions lasting years to decades. She was taking several medications for these medical conditions. Her surgical history included a hysterectomy, hip replacement, hernia repair, cardioversion, and tonsillectomy in childhood. Her family medical history included breast cancer in her paternal grandmother and aunt; hypertension in her father; and sarcoma in her mother at 78 years of age, which initially was identified in the sacrum and metastasized to the lungs causing death. No family history of melanoma or other skin cancers was reported. Prior to the original diagnosis of melanoma at 29 years of age, she had no history of skin cancer or any other medical condition other than acne. The patient did report spending a great deal of time in the sun during high school.
The patient reported developing the original cutaneous melanoma during her second pregnancy at 29 years of age and recalled that it was excised with wide margins. There had been a mole on her back that was present for years but changed in size during pregnancy, prompting the original visit to the primary care physician for evaluation. Remarkably, the original pathology report was obtained from the patient and revealed a specimen consisting of a 3.7×1.7-cm skin
Physical examination at the current presentation 49 years later revealed an even-bordered 2-mm black macule that was located approximately 1 cm from the original melanoma excision scar line (Figure). A biopsy was performed and sent to a dermatopathologist. Microscopic evaluation revealed nests, islands, and sheets of atypical epithelioid melanocytes extending through the dermis between collagen bundles. The melanocytes varied in size and shape with moderate nuclear pleomorphism present. Scattered mitotic figures and necrotic melanocytes were present, which most likely represented cutaneous satellite metastases of melanoma. Subsequent chest radiography, full-body positron emission tomography, and standard laboratory blood tests were unremarkable except for an enlarged right thyroid gland and moderate cardiomegaly. The patient was sent to a surgical oncologist for excision with wide surgical margins, and she elected not to have a sentinel lymph node biopsy. At follow-up 3, 6, 12, and 24 months later, there were no signs of recurrence based on direct clinical examination. The patient subsequently was lost to follow-up.

Recurrence rates of melanoma vary by stage and age at diagnosis, but prior studies have reported a recurrence rate of approximately 6% after 10 or more years following the initial diagnosis.2 Ultra-late recurrences of approximately 4 decades or more are extremely rare. A PubMed search of articles indexed for MEDLINE using the terms melanoma and ultra-late recurrence revealed 4 reported cases with a quiescent period of 38 or more years.3-6 All cases were metastatic melanomas in women; spanned 38, 40, 41, and 45 years from the initial melanoma diagnosis to recurrence; and all of the recurrences except one were regional or distal metastatic lesions (eg, lymph node, brain). In one case, both the original and recurrent lesions occurred on the left elbow.6 The original lesions occurred on the legs, elbow, and back of the neck, and there were no notable concomitant medical conditions. The patients were aged 72, 73, 73, and 84 years at recurrence.3-6 However, generalizations from these cases are limited given the potential for selection bias (eg, men may be less likely to visit a clinic for follow-up and nevi examination) and the likelihood that many cases of ultra-late melanoma recurrence are unrecognized or unreported.
More recently, genomic analyses on melanoma lesions occurring 30 years apart confirmed that the second lesion was indeed a recurrence, although with numerous additional mutations.7 The specific mechanisms underlying the dormancy and subsequent reemergence of metastatic lesions are unclear, but
It also is worth highlighting the concomitant diagnosis of Parkinson disease in our patient. In recent years, Parkinson disease has been linked to melanoma in both epidemiologic and genetic studies. For example, one large-scale study found a 50% increased risk for developing Parkinson disease in patients with melanoma (and vice versa), and this finding has been replicated in other studies.10 Moreover, patients with Parkinson disease have a 2-fold increase in their risk for developing melanoma, demonstrating that it is a bidirectional pathway. Not surprisingly, associations between melanin and neuromelanin pathways have been identified as a potential link between these diseases, and scientists are in the process of understanding the genetic components of both.10 It is unknown if specific genetic mutations contributed to both diseases in our case, but follow-up genetic testing on the recurrent melanoma specimen currently is being pursued.
The 49-year quiescent period in our case of recurrent cutaneous malignant melanoma potentially represents the longest ultra-late recurrence of melanoma in the literature to date based on a review of indexed publications. Moreover, it is relatively unique compared to other similar cases in that the recurrence was within a centimeter of the original excisional scar. Most metastases occur in locoregional lymph nodes or the lungs3; therefore, it is unusual to find one so close to the original lesion, especially one that occurred decades later. Factors associated with ultra-late recurrences are unknown, primarily because of the rarity of these cases as well as the biases and other factors that limit existing studies. However, genetic sequencing may provide information regarding these factors and related processes. Genetic sequencing specifically points to a small cell group remaining after excision of the primary tumor, which mutates while proliferating. Low antigenicity and tolerance to immunity during the quiescent period may explain the long duration of dormancy.6 More recently, there have been efforts to identify immunohistochemical signatures that may predict late recurrences, though the data are preliminary in nature.11
Given the latency period and location of the recurrence, our case demonstrates that even fully excised melanomas may recur locally many decades later, hence patients should be aware of the importance of a lifetime of vigilance after being diagnosed with melanoma.
To the Editor:
Ultra-late melanoma recurrence represents a minority of cases in which the quiescent period lasts longer than 15 years, and epidemiologic studies have reported recurrence rates of 6% to 10% during the ultra-late period.1 Even more uncommon are cases that span many decades (eg, >30 years), but all are useful in understanding the cellular behavior leading to the reactivation of fully excised melanomas. Few cases have been reported in which recurrence occurs more than 35 years after the original diagnosis of melanoma. Unfortunately, mechanisms underlying this long stable quiescence and subsequent reactivation are poorly understood, which is why it is important to identify and document cases. We present a case of local recurrence of cutaneous melanoma on the patient’s lower back after a 49-year disease-free period.
A 78-year-old White woman presented to a private dermatology office for a full-body skin examination. She had a medical history of a cutaneous melanoma that had been removed on the lower back 49 years prior; Parkinson disease of 10 years’ duration; and an enlarged thyroid nodule with decreased thyrotropin and hyperthyroidism, atrial fibrillation, mitral valve prolapse, osteoarthritis in the knees, and actinic keratoses, all of which were chronic conditions lasting years to decades. She was taking several medications for these medical conditions. Her surgical history included a hysterectomy, hip replacement, hernia repair, cardioversion, and tonsillectomy in childhood. Her family medical history included breast cancer in her paternal grandmother and aunt; hypertension in her father; and sarcoma in her mother at 78 years of age, which initially was identified in the sacrum and metastasized to the lungs causing death. No family history of melanoma or other skin cancers was reported. Prior to the original diagnosis of melanoma at 29 years of age, she had no history of skin cancer or any other medical condition other than acne. The patient did report spending a great deal of time in the sun during high school.
The patient reported developing the original cutaneous melanoma during her second pregnancy at 29 years of age and recalled that it was excised with wide margins. There had been a mole on her back that was present for years but changed in size during pregnancy, prompting the original visit to the primary care physician for evaluation. Remarkably, the original pathology report was obtained from the patient and revealed a specimen consisting of a 3.7×1.7-cm skin
Physical examination at the current presentation 49 years later revealed an even-bordered 2-mm black macule that was located approximately 1 cm from the original melanoma excision scar line (Figure). A biopsy was performed and sent to a dermatopathologist. Microscopic evaluation revealed nests, islands, and sheets of atypical epithelioid melanocytes extending through the dermis between collagen bundles. The melanocytes varied in size and shape with moderate nuclear pleomorphism present. Scattered mitotic figures and necrotic melanocytes were present, which most likely represented cutaneous satellite metastases of melanoma. Subsequent chest radiography, full-body positron emission tomography, and standard laboratory blood tests were unremarkable except for an enlarged right thyroid gland and moderate cardiomegaly. The patient was sent to a surgical oncologist for excision with wide surgical margins, and she elected not to have a sentinel lymph node biopsy. At follow-up 3, 6, 12, and 24 months later, there were no signs of recurrence based on direct clinical examination. The patient subsequently was lost to follow-up.

Recurrence rates of melanoma vary by stage and age at diagnosis, but prior studies have reported a recurrence rate of approximately 6% after 10 or more years following the initial diagnosis.2 Ultra-late recurrences of approximately 4 decades or more are extremely rare. A PubMed search of articles indexed for MEDLINE using the terms melanoma and ultra-late recurrence revealed 4 reported cases with a quiescent period of 38 or more years.3-6 All cases were metastatic melanomas in women; spanned 38, 40, 41, and 45 years from the initial melanoma diagnosis to recurrence; and all of the recurrences except one were regional or distal metastatic lesions (eg, lymph node, brain). In one case, both the original and recurrent lesions occurred on the left elbow.6 The original lesions occurred on the legs, elbow, and back of the neck, and there were no notable concomitant medical conditions. The patients were aged 72, 73, 73, and 84 years at recurrence.3-6 However, generalizations from these cases are limited given the potential for selection bias (eg, men may be less likely to visit a clinic for follow-up and nevi examination) and the likelihood that many cases of ultra-late melanoma recurrence are unrecognized or unreported.
More recently, genomic analyses on melanoma lesions occurring 30 years apart confirmed that the second lesion was indeed a recurrence, although with numerous additional mutations.7 The specific mechanisms underlying the dormancy and subsequent reemergence of metastatic lesions are unclear, but
It also is worth highlighting the concomitant diagnosis of Parkinson disease in our patient. In recent years, Parkinson disease has been linked to melanoma in both epidemiologic and genetic studies. For example, one large-scale study found a 50% increased risk for developing Parkinson disease in patients with melanoma (and vice versa), and this finding has been replicated in other studies.10 Moreover, patients with Parkinson disease have a 2-fold increase in their risk for developing melanoma, demonstrating that it is a bidirectional pathway. Not surprisingly, associations between melanin and neuromelanin pathways have been identified as a potential link between these diseases, and scientists are in the process of understanding the genetic components of both.10 It is unknown if specific genetic mutations contributed to both diseases in our case, but follow-up genetic testing on the recurrent melanoma specimen currently is being pursued.
The 49-year quiescent period in our case of recurrent cutaneous malignant melanoma potentially represents the longest ultra-late recurrence of melanoma in the literature to date based on a review of indexed publications. Moreover, it is relatively unique compared to other similar cases in that the recurrence was within a centimeter of the original excisional scar. Most metastases occur in locoregional lymph nodes or the lungs3; therefore, it is unusual to find one so close to the original lesion, especially one that occurred decades later. Factors associated with ultra-late recurrences are unknown, primarily because of the rarity of these cases as well as the biases and other factors that limit existing studies. However, genetic sequencing may provide information regarding these factors and related processes. Genetic sequencing specifically points to a small cell group remaining after excision of the primary tumor, which mutates while proliferating. Low antigenicity and tolerance to immunity during the quiescent period may explain the long duration of dormancy.6 More recently, there have been efforts to identify immunohistochemical signatures that may predict late recurrences, though the data are preliminary in nature.11
Given the latency period and location of the recurrence, our case demonstrates that even fully excised melanomas may recur locally many decades later, hence patients should be aware of the importance of a lifetime of vigilance after being diagnosed with melanoma.
- Tsao H, Cosimi AB, Sober AJ. Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer. 1997;79:2361-2370.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Mansour D, Kejariwal D. It is never too late: ultra-late recurrence of melanoma with distant metastases [published online March 8, 2012]. BMJ Case Rep. 2012:bcr0120125474. doi:10.1136/bcr.01.2012.5474
- Saleh D, Peach AHS. Ultra-late recurrence of malignant melanoma after 40 years of quiescent disease. J Surg Oncol. 2011;103:290-291.
- Goodenough J, Cozon CL, Liew SH. An incidental finding of a nodal recurrence of cutaneous malignant melanoma after a 45-year disease-free period [published online June 4, 2014]. BMJ Case Rep. 2014:bcr2014204289. doi:10.1136/bcr-2014-204289
- Nakamura M, Obayashi M, Yoshimitsu M, et al. Comparative whole-exome sequencing of an ultra-late recurrent malignant melanoma. Br J Dermatol. 2021;184:762-763.
- Miller JJ, Lofgren KA, Hughes SR, et al. Genomic analysis of melanoma evolution following a 30-year disease-free interval. J Cutan Pathol. 2017;44:805-808.
- North JP, Kageshita T, Pinkel D, et al. Distribution and significance of occult intraepidermal tumor cells surrounding primary melanoma. J Invest Dermatol. 2008;128:2024-2030.
- Massi G, LeBoit PE. Recurrent and persistent melanoma. In: Massi G, LeBoit PE, eds. Histological Diagnosis of Nevi and Melanoma. 2nd ed. Springer-Verlag; 2014:689-698.
- Bose A, Petsko GA, Eliezer D. Parkinson’s disease and melanoma: co-occurrence and mechanisms. J Parkinsons Dis. 2018;8:385-398.
- Reschke R, Dumann K, Ziemer M. Risk stratification and clinical characteristics of patients with late recurrence of melanoma (>10 years).J Clin Med. 2022;11:2026.
- Tsao H, Cosimi AB, Sober AJ. Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer. 1997;79:2361-2370.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Mansour D, Kejariwal D. It is never too late: ultra-late recurrence of melanoma with distant metastases [published online March 8, 2012]. BMJ Case Rep. 2012:bcr0120125474. doi:10.1136/bcr.01.2012.5474
- Saleh D, Peach AHS. Ultra-late recurrence of malignant melanoma after 40 years of quiescent disease. J Surg Oncol. 2011;103:290-291.
- Goodenough J, Cozon CL, Liew SH. An incidental finding of a nodal recurrence of cutaneous malignant melanoma after a 45-year disease-free period [published online June 4, 2014]. BMJ Case Rep. 2014:bcr2014204289. doi:10.1136/bcr-2014-204289
- Nakamura M, Obayashi M, Yoshimitsu M, et al. Comparative whole-exome sequencing of an ultra-late recurrent malignant melanoma. Br J Dermatol. 2021;184:762-763.
- Miller JJ, Lofgren KA, Hughes SR, et al. Genomic analysis of melanoma evolution following a 30-year disease-free interval. J Cutan Pathol. 2017;44:805-808.
- North JP, Kageshita T, Pinkel D, et al. Distribution and significance of occult intraepidermal tumor cells surrounding primary melanoma. J Invest Dermatol. 2008;128:2024-2030.
- Massi G, LeBoit PE. Recurrent and persistent melanoma. In: Massi G, LeBoit PE, eds. Histological Diagnosis of Nevi and Melanoma. 2nd ed. Springer-Verlag; 2014:689-698.
- Bose A, Petsko GA, Eliezer D. Parkinson’s disease and melanoma: co-occurrence and mechanisms. J Parkinsons Dis. 2018;8:385-398.
- Reschke R, Dumann K, Ziemer M. Risk stratification and clinical characteristics of patients with late recurrence of melanoma (>10 years).J Clin Med. 2022;11:2026.
Practice Points
- In some cases of ultra-late malignant melanoma recurrence, the quiescent period can last more than 30 years.
- There does not appear to be specificity with location since ultra-late melanoma recurrences can occur locally, regionally, and distally, and original lesions appear to be randomly distributed in these cases.
- Mechanisms for ultra-late melanoma recurrence are poorly understood; histologically, unrecognizable aberrations in the skin beyond the histopathologic margins may represent an early phase of disease that lies dormant for many years before reemerging in response to external or immunologic changes.
- Patients with malignant melanoma are at a higher risk for developing Parkinson disease (and vice versa) given the link between melanin and neuromelanin pathways.