User login
Tai Chi Shown to Improve Quality of Life in Heart Failure Patients
A program of regular tai chi exercises improved measures of quality of life in patients with chronic heart failure but failed to improve more conventional measures of exercise efficacy, compared with patients who only received education.
"Tai chi exercise, a multicomponent mind-body training modality that is safe and has good rates of adherence, may provide value in improving daily exercise, quality of life, self-efficacy, and mood in frail, deconditioned patients with systolic HF. A more restricted focus on traditional measured exercise capacity may underestimate the potential benefits of integrated interventions such as tai chi," wrote Dr. Gloria Y. Yeh and her coinvestigators in a study released April 25 in the Archives of Internal Medicine.
Tai chi is a gentle, meditative exercise of flowing circular movements, balance and weight shifting, breathing techniques, visualization, and focused internal awareness. "Tai chi may represent an additional exercise option for patients with HF because it integrates multiple relevant processes, including mild to moderate aerobic activity, upper and lower extremity training, and core strengthening," they said.
The researchers recruited 100 heart failure patients with a left ventricular ejection fraction of 40% or lower in the past 2 years, a stable medical regimen, and New York Heart Association class I-III from Boston medical practices.
They were randomly assigned to receive a 12-week tai chi exercise program or a heart health education program (attention control). All participants continued to receive usual care, noted Dr. Yeh, assistant professor of medicine at Beth Israel Deaconess and in the division for research and education in complementary and integrative medical therapies at Harvard Medical School, both in Boston, and her associates.
All assessments were performed at baseline and at 12 weeks. Patients performed a symptom-limited exercise test using a bicycle ramp protocol to determine peak oxygen uptake, a 6-minute walk test, and a Timed Up and Go functional assessment.
The researchers used the Minnesota Living with Heart Failure Questionnaire (MLHFQ) to assess disease-specific quality of life, the Profile of Mood States to assess emotional states expected to respond to clinical intervention, and the Cardiac Exercise Self-Efficacy Instrument to assess patient confidence in performing exercise-related activities.
The tai chi intervention consisted of 1-hour group classes held twice weekly for 12 weeks by certified instructors. Patients were encouraged to practice at home at least three times a week. Patients in the control group attended nurse practitioner–led education sessions twice weekly and were asked not to start tai chi during the study period.
The mean age of study participants was 67 years, the mean baseline LVEF was 29%, and the median NYHA class was II. Most patients were receiving beta-blockers (86%) and ACE inhibitors (85%).
At 12 weeks, significant improvements were seen in scores on the MLHFQ, Profile of Mood States (total mood disturbance, depression, and vigor subscales), and Cardiac Exercise Self-Efficacy Instrument in the tai chi group, compared with the heart health education group. There were no significant changes in serum biomarkers, which included B-type natriuretic peptide, catecholamines, and C-reactive protein, Dr. Yeh and her associates reported (Arch. Intern. Med. 2011;171:750-7).
Post hoc exploratory analyses pointed to subsets of patients who derived significant benefits from tai chi, compared with education only. Tai chi patients without implanted cardioverter defibrillators devices, those with NYHA class II and III symptoms, and those with a nonischemic etiology of HF improved significantly on the MLHFQ, compared with education-only patients. The researchers also found that participants with a higher baseline resting heart rate had greater improvements in the MLHFQ score in the tai chi group; there was no association in the control group.
"One of the purported mechanisms of mind-body exercises, such as tai chi, is favorable modulation of the autonomic nervous system. In our post hoc analyses, we found that, in participants with higher resting heart rates (and presumably more sympathetic nervous system ‘overdrive’), there was a greater benefit with tai chi," the investigators noted.
At the 6-month follow-up telephone contact, 34 patients in the tai chi group (68.0%) reported continued practice (including daily, weekly, and monthly). There were no adverse events related to the protocol.
Dr. Yeh and her associates reported no significant financial relationships.
Despite borderline improvements in MLHFQ responses seen in the current trial, there was no improvement in 6-minute walk test, peak oxygen uptake, or exercise duration, and there was a trend toward worsening norepinephrine levels in the tai chi exercise group compared with the control group, Dr. John R. Teerlink noted in an accompanying commentary (Arch. Intern. Med. 2011;171:758-9).
The emergence of nonpharmacologic approaches that attempt to directly improve quality of life, such as mind-body medicine, poses new challenges in the evaluation of efficacy in HF trials, he noted. As therapies move from a primary goal of increasing survival beyond physiological or functional surrogates to improving quality of life, a variety of other end points have emerged. "Should the clinical trialist, and eventually the clinician, select primary study end points that emphasize objective increases in functional activity? Or should improvements in the patient’s subjective sense of well-being be the focus?" he asked.
"In many ways, the choice between functional and health-related quality-of-life end points is a false one. The criticism that these measures do not correlate with rehospitalization rates or mortality is not relevant. As long as an intervention is safe, improvements in functional capacity or health-related quality of life are independently important, although underappreciated, goals reflecting different facets of the patient’s response to therapy," Dr. Teerlink wrote.
Despite the improvements in quality of life in the current trial, a much larger trial to assess these outcomes is necessary. Importantly, "further investigation of the mechanism of tai chi’s putative benefit will be of interest. Mind-body medicine holds tremendous potential to improve both functional capacity and health-related quality of life in patients with HF; it is time to give these therapies the studies they deserve," said Dr. Teerlink.
Dr. John R. Teerlink is a cardiologist at the San Francisco Veterans Affairs Medical Center. No significant financial relationships were reported.
Despite borderline improvements in MLHFQ responses seen in the current trial, there was no improvement in 6-minute walk test, peak oxygen uptake, or exercise duration, and there was a trend toward worsening norepinephrine levels in the tai chi exercise group compared with the control group, Dr. John R. Teerlink noted in an accompanying commentary (Arch. Intern. Med. 2011;171:758-9).
The emergence of nonpharmacologic approaches that attempt to directly improve quality of life, such as mind-body medicine, poses new challenges in the evaluation of efficacy in HF trials, he noted. As therapies move from a primary goal of increasing survival beyond physiological or functional surrogates to improving quality of life, a variety of other end points have emerged. "Should the clinical trialist, and eventually the clinician, select primary study end points that emphasize objective increases in functional activity? Or should improvements in the patient’s subjective sense of well-being be the focus?" he asked.
"In many ways, the choice between functional and health-related quality-of-life end points is a false one. The criticism that these measures do not correlate with rehospitalization rates or mortality is not relevant. As long as an intervention is safe, improvements in functional capacity or health-related quality of life are independently important, although underappreciated, goals reflecting different facets of the patient’s response to therapy," Dr. Teerlink wrote.
Despite the improvements in quality of life in the current trial, a much larger trial to assess these outcomes is necessary. Importantly, "further investigation of the mechanism of tai chi’s putative benefit will be of interest. Mind-body medicine holds tremendous potential to improve both functional capacity and health-related quality of life in patients with HF; it is time to give these therapies the studies they deserve," said Dr. Teerlink.
Dr. John R. Teerlink is a cardiologist at the San Francisco Veterans Affairs Medical Center. No significant financial relationships were reported.
Despite borderline improvements in MLHFQ responses seen in the current trial, there was no improvement in 6-minute walk test, peak oxygen uptake, or exercise duration, and there was a trend toward worsening norepinephrine levels in the tai chi exercise group compared with the control group, Dr. John R. Teerlink noted in an accompanying commentary (Arch. Intern. Med. 2011;171:758-9).
The emergence of nonpharmacologic approaches that attempt to directly improve quality of life, such as mind-body medicine, poses new challenges in the evaluation of efficacy in HF trials, he noted. As therapies move from a primary goal of increasing survival beyond physiological or functional surrogates to improving quality of life, a variety of other end points have emerged. "Should the clinical trialist, and eventually the clinician, select primary study end points that emphasize objective increases in functional activity? Or should improvements in the patient’s subjective sense of well-being be the focus?" he asked.
"In many ways, the choice between functional and health-related quality-of-life end points is a false one. The criticism that these measures do not correlate with rehospitalization rates or mortality is not relevant. As long as an intervention is safe, improvements in functional capacity or health-related quality of life are independently important, although underappreciated, goals reflecting different facets of the patient’s response to therapy," Dr. Teerlink wrote.
Despite the improvements in quality of life in the current trial, a much larger trial to assess these outcomes is necessary. Importantly, "further investigation of the mechanism of tai chi’s putative benefit will be of interest. Mind-body medicine holds tremendous potential to improve both functional capacity and health-related quality of life in patients with HF; it is time to give these therapies the studies they deserve," said Dr. Teerlink.
Dr. John R. Teerlink is a cardiologist at the San Francisco Veterans Affairs Medical Center. No significant financial relationships were reported.
A program of regular tai chi exercises improved measures of quality of life in patients with chronic heart failure but failed to improve more conventional measures of exercise efficacy, compared with patients who only received education.
"Tai chi exercise, a multicomponent mind-body training modality that is safe and has good rates of adherence, may provide value in improving daily exercise, quality of life, self-efficacy, and mood in frail, deconditioned patients with systolic HF. A more restricted focus on traditional measured exercise capacity may underestimate the potential benefits of integrated interventions such as tai chi," wrote Dr. Gloria Y. Yeh and her coinvestigators in a study released April 25 in the Archives of Internal Medicine.
Tai chi is a gentle, meditative exercise of flowing circular movements, balance and weight shifting, breathing techniques, visualization, and focused internal awareness. "Tai chi may represent an additional exercise option for patients with HF because it integrates multiple relevant processes, including mild to moderate aerobic activity, upper and lower extremity training, and core strengthening," they said.
The researchers recruited 100 heart failure patients with a left ventricular ejection fraction of 40% or lower in the past 2 years, a stable medical regimen, and New York Heart Association class I-III from Boston medical practices.
They were randomly assigned to receive a 12-week tai chi exercise program or a heart health education program (attention control). All participants continued to receive usual care, noted Dr. Yeh, assistant professor of medicine at Beth Israel Deaconess and in the division for research and education in complementary and integrative medical therapies at Harvard Medical School, both in Boston, and her associates.
All assessments were performed at baseline and at 12 weeks. Patients performed a symptom-limited exercise test using a bicycle ramp protocol to determine peak oxygen uptake, a 6-minute walk test, and a Timed Up and Go functional assessment.
The researchers used the Minnesota Living with Heart Failure Questionnaire (MLHFQ) to assess disease-specific quality of life, the Profile of Mood States to assess emotional states expected to respond to clinical intervention, and the Cardiac Exercise Self-Efficacy Instrument to assess patient confidence in performing exercise-related activities.
The tai chi intervention consisted of 1-hour group classes held twice weekly for 12 weeks by certified instructors. Patients were encouraged to practice at home at least three times a week. Patients in the control group attended nurse practitioner–led education sessions twice weekly and were asked not to start tai chi during the study period.
The mean age of study participants was 67 years, the mean baseline LVEF was 29%, and the median NYHA class was II. Most patients were receiving beta-blockers (86%) and ACE inhibitors (85%).
At 12 weeks, significant improvements were seen in scores on the MLHFQ, Profile of Mood States (total mood disturbance, depression, and vigor subscales), and Cardiac Exercise Self-Efficacy Instrument in the tai chi group, compared with the heart health education group. There were no significant changes in serum biomarkers, which included B-type natriuretic peptide, catecholamines, and C-reactive protein, Dr. Yeh and her associates reported (Arch. Intern. Med. 2011;171:750-7).
Post hoc exploratory analyses pointed to subsets of patients who derived significant benefits from tai chi, compared with education only. Tai chi patients without implanted cardioverter defibrillators devices, those with NYHA class II and III symptoms, and those with a nonischemic etiology of HF improved significantly on the MLHFQ, compared with education-only patients. The researchers also found that participants with a higher baseline resting heart rate had greater improvements in the MLHFQ score in the tai chi group; there was no association in the control group.
"One of the purported mechanisms of mind-body exercises, such as tai chi, is favorable modulation of the autonomic nervous system. In our post hoc analyses, we found that, in participants with higher resting heart rates (and presumably more sympathetic nervous system ‘overdrive’), there was a greater benefit with tai chi," the investigators noted.
At the 6-month follow-up telephone contact, 34 patients in the tai chi group (68.0%) reported continued practice (including daily, weekly, and monthly). There were no adverse events related to the protocol.
Dr. Yeh and her associates reported no significant financial relationships.
A program of regular tai chi exercises improved measures of quality of life in patients with chronic heart failure but failed to improve more conventional measures of exercise efficacy, compared with patients who only received education.
"Tai chi exercise, a multicomponent mind-body training modality that is safe and has good rates of adherence, may provide value in improving daily exercise, quality of life, self-efficacy, and mood in frail, deconditioned patients with systolic HF. A more restricted focus on traditional measured exercise capacity may underestimate the potential benefits of integrated interventions such as tai chi," wrote Dr. Gloria Y. Yeh and her coinvestigators in a study released April 25 in the Archives of Internal Medicine.
Tai chi is a gentle, meditative exercise of flowing circular movements, balance and weight shifting, breathing techniques, visualization, and focused internal awareness. "Tai chi may represent an additional exercise option for patients with HF because it integrates multiple relevant processes, including mild to moderate aerobic activity, upper and lower extremity training, and core strengthening," they said.
The researchers recruited 100 heart failure patients with a left ventricular ejection fraction of 40% or lower in the past 2 years, a stable medical regimen, and New York Heart Association class I-III from Boston medical practices.
They were randomly assigned to receive a 12-week tai chi exercise program or a heart health education program (attention control). All participants continued to receive usual care, noted Dr. Yeh, assistant professor of medicine at Beth Israel Deaconess and in the division for research and education in complementary and integrative medical therapies at Harvard Medical School, both in Boston, and her associates.
All assessments were performed at baseline and at 12 weeks. Patients performed a symptom-limited exercise test using a bicycle ramp protocol to determine peak oxygen uptake, a 6-minute walk test, and a Timed Up and Go functional assessment.
The researchers used the Minnesota Living with Heart Failure Questionnaire (MLHFQ) to assess disease-specific quality of life, the Profile of Mood States to assess emotional states expected to respond to clinical intervention, and the Cardiac Exercise Self-Efficacy Instrument to assess patient confidence in performing exercise-related activities.
The tai chi intervention consisted of 1-hour group classes held twice weekly for 12 weeks by certified instructors. Patients were encouraged to practice at home at least three times a week. Patients in the control group attended nurse practitioner–led education sessions twice weekly and were asked not to start tai chi during the study period.
The mean age of study participants was 67 years, the mean baseline LVEF was 29%, and the median NYHA class was II. Most patients were receiving beta-blockers (86%) and ACE inhibitors (85%).
At 12 weeks, significant improvements were seen in scores on the MLHFQ, Profile of Mood States (total mood disturbance, depression, and vigor subscales), and Cardiac Exercise Self-Efficacy Instrument in the tai chi group, compared with the heart health education group. There were no significant changes in serum biomarkers, which included B-type natriuretic peptide, catecholamines, and C-reactive protein, Dr. Yeh and her associates reported (Arch. Intern. Med. 2011;171:750-7).
Post hoc exploratory analyses pointed to subsets of patients who derived significant benefits from tai chi, compared with education only. Tai chi patients without implanted cardioverter defibrillators devices, those with NYHA class II and III symptoms, and those with a nonischemic etiology of HF improved significantly on the MLHFQ, compared with education-only patients. The researchers also found that participants with a higher baseline resting heart rate had greater improvements in the MLHFQ score in the tai chi group; there was no association in the control group.
"One of the purported mechanisms of mind-body exercises, such as tai chi, is favorable modulation of the autonomic nervous system. In our post hoc analyses, we found that, in participants with higher resting heart rates (and presumably more sympathetic nervous system ‘overdrive’), there was a greater benefit with tai chi," the investigators noted.
At the 6-month follow-up telephone contact, 34 patients in the tai chi group (68.0%) reported continued practice (including daily, weekly, and monthly). There were no adverse events related to the protocol.
Dr. Yeh and her associates reported no significant financial relationships.
FROM ARCHIVES OF INTERNAL MEDICINE
Major Finding: Significant improvements were seen in scores on the Minnesota Living with Heart Failure Questionnaire, Profile of Mood, and Cardiac Exercise Self-Efficacy Instrument among heart failure patients randomized to a 12-week tai chi program, compared with patients who received heart health education for 12 weeks.
Data Source: A prospective study of 100 patients with heart failure.
Disclosures: No disclosures were reported.
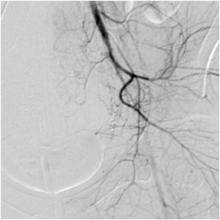
Uterine Artery Embolization Improves Urinary Symptoms
CHICAGO – Uterine artery embolization for the treatment of fibroids also appears to improve lower urinary tract symptoms, a small study has shown.
Women who underwent uterine artery embolization (UAE) for fibroids had an average improvement of 23 points on the Urinary Distress Inventory (UDI-6).
"We had a significant improvement in all of the measures, except for the number of accidents," said Dr. James B. Spies, professor and chairman of radiology at Georgetown University, Washington.
The researchers recruited 46 women (average age, 44 years), who were undergoing UAE at the George Washington University Medical Center between 2008 and 2010. Each patient completed a set of validated questionnaires assessing urinary symptoms and quality of life impact, and fibroid symptoms and quality of life impact, as well as a 48-hour urinary diary before and after UAE. The women also completed these questionnaires and a urinary diary at 3 months post procedure.
Lower urinary tract symptoms included an increased frequency of urination, a sudden urgent need to urinate, difficulty urinating (retention), and incontinence.
The researchers used several validated tools to assess improvement in urinary symptoms, including the UDI-6, the Incontinence Impact Questionnaire (IIQ-7), the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), the Uterine Fibroid Symptom and Quality of Life Questionnaire (UFS-QoL), and a standardized 48-hour bladder diary.
The Patient Global Impression of Improvement (PGI-I) also was used to assess patient satisfaction with the procedure. The primary outcome was the measure of subjective improvement in lower urinary tract symptoms at 3 months, as measured by the UDI-6.
In addition to improvement on the UDI-6, there was a mean reduction of 15 points on the IIQ-7. There were also significant decreases in the mean scores for the PISQ-12 and UFS-QoL, Dr. Spies said at the annual meeting of the Society of Interventional Radiology.
Using regression modeling, the researchers found that uterine volume, dominant fibroid volume, location of the fibroid, and bladder compression did not affect the scores, but obesity did. For each unit increase in body mass index, the improvement in the UDI-6 scores was decreased by 1.8.
Dr. Spies and his associates reported they had no relevant financial disclosures.
CHICAGO – Uterine artery embolization for the treatment of fibroids also appears to improve lower urinary tract symptoms, a small study has shown.
Women who underwent uterine artery embolization (UAE) for fibroids had an average improvement of 23 points on the Urinary Distress Inventory (UDI-6).
"We had a significant improvement in all of the measures, except for the number of accidents," said Dr. James B. Spies, professor and chairman of radiology at Georgetown University, Washington.
The researchers recruited 46 women (average age, 44 years), who were undergoing UAE at the George Washington University Medical Center between 2008 and 2010. Each patient completed a set of validated questionnaires assessing urinary symptoms and quality of life impact, and fibroid symptoms and quality of life impact, as well as a 48-hour urinary diary before and after UAE. The women also completed these questionnaires and a urinary diary at 3 months post procedure.
Lower urinary tract symptoms included an increased frequency of urination, a sudden urgent need to urinate, difficulty urinating (retention), and incontinence.
The researchers used several validated tools to assess improvement in urinary symptoms, including the UDI-6, the Incontinence Impact Questionnaire (IIQ-7), the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), the Uterine Fibroid Symptom and Quality of Life Questionnaire (UFS-QoL), and a standardized 48-hour bladder diary.
The Patient Global Impression of Improvement (PGI-I) also was used to assess patient satisfaction with the procedure. The primary outcome was the measure of subjective improvement in lower urinary tract symptoms at 3 months, as measured by the UDI-6.
In addition to improvement on the UDI-6, there was a mean reduction of 15 points on the IIQ-7. There were also significant decreases in the mean scores for the PISQ-12 and UFS-QoL, Dr. Spies said at the annual meeting of the Society of Interventional Radiology.
Using regression modeling, the researchers found that uterine volume, dominant fibroid volume, location of the fibroid, and bladder compression did not affect the scores, but obesity did. For each unit increase in body mass index, the improvement in the UDI-6 scores was decreased by 1.8.
Dr. Spies and his associates reported they had no relevant financial disclosures.
CHICAGO – Uterine artery embolization for the treatment of fibroids also appears to improve lower urinary tract symptoms, a small study has shown.
Women who underwent uterine artery embolization (UAE) for fibroids had an average improvement of 23 points on the Urinary Distress Inventory (UDI-6).
"We had a significant improvement in all of the measures, except for the number of accidents," said Dr. James B. Spies, professor and chairman of radiology at Georgetown University, Washington.
The researchers recruited 46 women (average age, 44 years), who were undergoing UAE at the George Washington University Medical Center between 2008 and 2010. Each patient completed a set of validated questionnaires assessing urinary symptoms and quality of life impact, and fibroid symptoms and quality of life impact, as well as a 48-hour urinary diary before and after UAE. The women also completed these questionnaires and a urinary diary at 3 months post procedure.
Lower urinary tract symptoms included an increased frequency of urination, a sudden urgent need to urinate, difficulty urinating (retention), and incontinence.
The researchers used several validated tools to assess improvement in urinary symptoms, including the UDI-6, the Incontinence Impact Questionnaire (IIQ-7), the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), the Uterine Fibroid Symptom and Quality of Life Questionnaire (UFS-QoL), and a standardized 48-hour bladder diary.
The Patient Global Impression of Improvement (PGI-I) also was used to assess patient satisfaction with the procedure. The primary outcome was the measure of subjective improvement in lower urinary tract symptoms at 3 months, as measured by the UDI-6.
In addition to improvement on the UDI-6, there was a mean reduction of 15 points on the IIQ-7. There were also significant decreases in the mean scores for the PISQ-12 and UFS-QoL, Dr. Spies said at the annual meeting of the Society of Interventional Radiology.
Using regression modeling, the researchers found that uterine volume, dominant fibroid volume, location of the fibroid, and bladder compression did not affect the scores, but obesity did. For each unit increase in body mass index, the improvement in the UDI-6 scores was decreased by 1.8.
Dr. Spies and his associates reported they had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY OF INTERVENTIONAL RADIOLOGY
Major Finding: Women who underwent UAE for fibroids had an average improvement of 23 points on the UDI-6, which was significant.
Data Source: Prospective study of 46 women treated with UAE for fibroids.
Disclosures: Dr. Spies and his associates reported they had no relevant financial disclosures.
Uterine Artery Embolization Improves Urinary Symptoms
CHICAGO – Uterine artery embolization for the treatment of fibroids also appears to improve lower urinary tract symptoms, a small study has shown.
Women who underwent uterine artery embolization (UAE) for fibroids had an average improvement of 23 points on the Urinary Distress Inventory (UDI-6).
"We had a significant improvement in all of the measures, except for the number of accidents," said Dr. James B. Spies, professor and chairman of radiology at Georgetown University, Washington.
The researchers recruited 46 women (average age, 44 years), who were undergoing UAE at the George Washington University Medical Center between 2008 and 2010. Each patient completed a set of validated questionnaires assessing urinary symptoms and quality of life impact, and fibroid symptoms and quality of life impact, as well as a 48-hour urinary diary before and after UAE. The women also completed these questionnaires and a urinary diary at 3 months post procedure.
Lower urinary tract symptoms included an increased frequency of urination, a sudden urgent need to urinate, difficulty urinating (retention), and incontinence.
The researchers used several validated tools to assess improvement in urinary symptoms, including the UDI-6, the Incontinence Impact Questionnaire (IIQ-7), the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), the Uterine Fibroid Symptom and Quality of Life Questionnaire (UFS-QoL), and a standardized 48-hour bladder diary.
The Patient Global Impression of Improvement (PGI-I) also was used to assess patient satisfaction with the procedure. The primary outcome was the measure of subjective improvement in lower urinary tract symptoms at 3 months, as measured by the UDI-6.
In addition to improvement on the UDI-6, there was a mean reduction of 15 points on the IIQ-7. There were also significant decreases in the mean scores for the PISQ-12 and UFS-QoL, Dr. Spies said at the annual meeting of the Society of Interventional Radiology.
Using regression modeling, the researchers found that uterine volume, dominant fibroid volume, location of the fibroid, and bladder compression did not affect the scores, but obesity did. For each unit increase in body mass index, the improvement in the UDI-6 scores was decreased by 1.8.
Dr. Spies and his associates reported they had no relevant financial disclosures.
CHICAGO – Uterine artery embolization for the treatment of fibroids also appears to improve lower urinary tract symptoms, a small study has shown.
Women who underwent uterine artery embolization (UAE) for fibroids had an average improvement of 23 points on the Urinary Distress Inventory (UDI-6).
"We had a significant improvement in all of the measures, except for the number of accidents," said Dr. James B. Spies, professor and chairman of radiology at Georgetown University, Washington.
The researchers recruited 46 women (average age, 44 years), who were undergoing UAE at the George Washington University Medical Center between 2008 and 2010. Each patient completed a set of validated questionnaires assessing urinary symptoms and quality of life impact, and fibroid symptoms and quality of life impact, as well as a 48-hour urinary diary before and after UAE. The women also completed these questionnaires and a urinary diary at 3 months post procedure.
Lower urinary tract symptoms included an increased frequency of urination, a sudden urgent need to urinate, difficulty urinating (retention), and incontinence.
The researchers used several validated tools to assess improvement in urinary symptoms, including the UDI-6, the Incontinence Impact Questionnaire (IIQ-7), the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), the Uterine Fibroid Symptom and Quality of Life Questionnaire (UFS-QoL), and a standardized 48-hour bladder diary.
The Patient Global Impression of Improvement (PGI-I) also was used to assess patient satisfaction with the procedure. The primary outcome was the measure of subjective improvement in lower urinary tract symptoms at 3 months, as measured by the UDI-6.
In addition to improvement on the UDI-6, there was a mean reduction of 15 points on the IIQ-7. There were also significant decreases in the mean scores for the PISQ-12 and UFS-QoL, Dr. Spies said at the annual meeting of the Society of Interventional Radiology.
Using regression modeling, the researchers found that uterine volume, dominant fibroid volume, location of the fibroid, and bladder compression did not affect the scores, but obesity did. For each unit increase in body mass index, the improvement in the UDI-6 scores was decreased by 1.8.
Dr. Spies and his associates reported they had no relevant financial disclosures.
CHICAGO – Uterine artery embolization for the treatment of fibroids also appears to improve lower urinary tract symptoms, a small study has shown.
Women who underwent uterine artery embolization (UAE) for fibroids had an average improvement of 23 points on the Urinary Distress Inventory (UDI-6).
"We had a significant improvement in all of the measures, except for the number of accidents," said Dr. James B. Spies, professor and chairman of radiology at Georgetown University, Washington.
The researchers recruited 46 women (average age, 44 years), who were undergoing UAE at the George Washington University Medical Center between 2008 and 2010. Each patient completed a set of validated questionnaires assessing urinary symptoms and quality of life impact, and fibroid symptoms and quality of life impact, as well as a 48-hour urinary diary before and after UAE. The women also completed these questionnaires and a urinary diary at 3 months post procedure.
Lower urinary tract symptoms included an increased frequency of urination, a sudden urgent need to urinate, difficulty urinating (retention), and incontinence.
The researchers used several validated tools to assess improvement in urinary symptoms, including the UDI-6, the Incontinence Impact Questionnaire (IIQ-7), the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), the Uterine Fibroid Symptom and Quality of Life Questionnaire (UFS-QoL), and a standardized 48-hour bladder diary.
The Patient Global Impression of Improvement (PGI-I) also was used to assess patient satisfaction with the procedure. The primary outcome was the measure of subjective improvement in lower urinary tract symptoms at 3 months, as measured by the UDI-6.
In addition to improvement on the UDI-6, there was a mean reduction of 15 points on the IIQ-7. There were also significant decreases in the mean scores for the PISQ-12 and UFS-QoL, Dr. Spies said at the annual meeting of the Society of Interventional Radiology.
Using regression modeling, the researchers found that uterine volume, dominant fibroid volume, location of the fibroid, and bladder compression did not affect the scores, but obesity did. For each unit increase in body mass index, the improvement in the UDI-6 scores was decreased by 1.8.
Dr. Spies and his associates reported they had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY OF INTERVENTIONAL RADIOLOGY
Major Finding: Women who underwent UAE for fibroids had an average improvement of 23 points on the UDI-6, which was significant.
Data Source: Prospective study of 46 women treated with UAE for fibroids.
Disclosures: Dr. Spies and his associates reported they had no relevant financial disclosures.
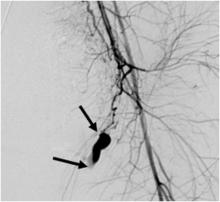
Pelvic Artery Embolization Stops Postpartum Hemorrhage
CHICAGO – Pelvic artery embolization is a highly effective technique for managing postpartum hemorrhage with the added advantage that it preserves the uterus and fertility, according to Dr. Ji Hoon Shin.
In a retrospective study of 225 women who underwent pelvic artery embolization (PAE) to stop postpartum hemorrhage (PPH), a single procedure stopped bleeding in 86% of women. A second procedure stopped bleeding in 89%.
"The major advantage of this procedure is that you can save the uterus and fertility," Dr. Shin said at the annual meeting of the Society for Interventional Radiology.
Standard therapies include fluid resuscitation/blood transfusion, management of the underlying cause, or surgery – uterine artery ligation, suturing, and hysterectomy.
PAE involves inserting a catheter into the femoral artery via a small incision in the groin. The interventional radiologist can then inject small particles or coils into the arteries to stem hemorrhage. Not only is the technique minimally invasive, it can save the uterus and preserve fertility. While the use of PAE was first reported in 1979, obstetrician awareness of this option remains limited.
In this study, researchers identified 225 patients who underwent pulmonary artery embolization for PPH within 24 hours of delivery between 2000 and 2010. Technical success was defined as cessation of bleeding on postembolization angiogram and cessation of vaginal bleeding on physical inspection. Clinical success was defined as the cessation of bleeding following PAE without the need for additional surgery during the hospital stay.
Uterine atony was the most common cause of PPH in this group (81%). Roughly a third (36%) of patients had positive angiographic findings. Contrast extravasation (a sign of acute bleeding) was seen in most patients (86%) and pseudoaneurysm occurred in 14% of patients, said Dr. Shin of the department of radiology at the University of Ulsan in Seoul, South Korea.
Technical success was 89%. Clinical success with only one PAE procedure was 86% and was 89% when women who had an additional PAE procedure were included. Overall bleeding control – including patients who had repeat PAEs and/or surgeries – was 98%.
In terms of safety, major complications occurred in 2% of patients and included puncture site hematoma and uterine artery dissection. Transient numbness – a minor complication – occurred in 1% of women.
In terms of fertility preservation, 97% of 113 women who were available for follow-up resumed regular menstruation. Irregular menses occurred in two women (2%), and early menopause occurred in one patient (1%). Notably, 11 patients (10%) were able to become pregnant.
On statistical analysis, the presence of disseminated intravascular coagulopathy was the most important negative prognostic factor.
Dr. Shin reported that he had no relevant financial disclosures.
CHICAGO – Pelvic artery embolization is a highly effective technique for managing postpartum hemorrhage with the added advantage that it preserves the uterus and fertility, according to Dr. Ji Hoon Shin.
In a retrospective study of 225 women who underwent pelvic artery embolization (PAE) to stop postpartum hemorrhage (PPH), a single procedure stopped bleeding in 86% of women. A second procedure stopped bleeding in 89%.
"The major advantage of this procedure is that you can save the uterus and fertility," Dr. Shin said at the annual meeting of the Society for Interventional Radiology.
Standard therapies include fluid resuscitation/blood transfusion, management of the underlying cause, or surgery – uterine artery ligation, suturing, and hysterectomy.
PAE involves inserting a catheter into the femoral artery via a small incision in the groin. The interventional radiologist can then inject small particles or coils into the arteries to stem hemorrhage. Not only is the technique minimally invasive, it can save the uterus and preserve fertility. While the use of PAE was first reported in 1979, obstetrician awareness of this option remains limited.
In this study, researchers identified 225 patients who underwent pulmonary artery embolization for PPH within 24 hours of delivery between 2000 and 2010. Technical success was defined as cessation of bleeding on postembolization angiogram and cessation of vaginal bleeding on physical inspection. Clinical success was defined as the cessation of bleeding following PAE without the need for additional surgery during the hospital stay.
Uterine atony was the most common cause of PPH in this group (81%). Roughly a third (36%) of patients had positive angiographic findings. Contrast extravasation (a sign of acute bleeding) was seen in most patients (86%) and pseudoaneurysm occurred in 14% of patients, said Dr. Shin of the department of radiology at the University of Ulsan in Seoul, South Korea.
Technical success was 89%. Clinical success with only one PAE procedure was 86% and was 89% when women who had an additional PAE procedure were included. Overall bleeding control – including patients who had repeat PAEs and/or surgeries – was 98%.
In terms of safety, major complications occurred in 2% of patients and included puncture site hematoma and uterine artery dissection. Transient numbness – a minor complication – occurred in 1% of women.
In terms of fertility preservation, 97% of 113 women who were available for follow-up resumed regular menstruation. Irregular menses occurred in two women (2%), and early menopause occurred in one patient (1%). Notably, 11 patients (10%) were able to become pregnant.
On statistical analysis, the presence of disseminated intravascular coagulopathy was the most important negative prognostic factor.
Dr. Shin reported that he had no relevant financial disclosures.
CHICAGO – Pelvic artery embolization is a highly effective technique for managing postpartum hemorrhage with the added advantage that it preserves the uterus and fertility, according to Dr. Ji Hoon Shin.
In a retrospective study of 225 women who underwent pelvic artery embolization (PAE) to stop postpartum hemorrhage (PPH), a single procedure stopped bleeding in 86% of women. A second procedure stopped bleeding in 89%.
"The major advantage of this procedure is that you can save the uterus and fertility," Dr. Shin said at the annual meeting of the Society for Interventional Radiology.
Standard therapies include fluid resuscitation/blood transfusion, management of the underlying cause, or surgery – uterine artery ligation, suturing, and hysterectomy.
PAE involves inserting a catheter into the femoral artery via a small incision in the groin. The interventional radiologist can then inject small particles or coils into the arteries to stem hemorrhage. Not only is the technique minimally invasive, it can save the uterus and preserve fertility. While the use of PAE was first reported in 1979, obstetrician awareness of this option remains limited.
In this study, researchers identified 225 patients who underwent pulmonary artery embolization for PPH within 24 hours of delivery between 2000 and 2010. Technical success was defined as cessation of bleeding on postembolization angiogram and cessation of vaginal bleeding on physical inspection. Clinical success was defined as the cessation of bleeding following PAE without the need for additional surgery during the hospital stay.
Uterine atony was the most common cause of PPH in this group (81%). Roughly a third (36%) of patients had positive angiographic findings. Contrast extravasation (a sign of acute bleeding) was seen in most patients (86%) and pseudoaneurysm occurred in 14% of patients, said Dr. Shin of the department of radiology at the University of Ulsan in Seoul, South Korea.
Technical success was 89%. Clinical success with only one PAE procedure was 86% and was 89% when women who had an additional PAE procedure were included. Overall bleeding control – including patients who had repeat PAEs and/or surgeries – was 98%.
In terms of safety, major complications occurred in 2% of patients and included puncture site hematoma and uterine artery dissection. Transient numbness – a minor complication – occurred in 1% of women.
In terms of fertility preservation, 97% of 113 women who were available for follow-up resumed regular menstruation. Irregular menses occurred in two women (2%), and early menopause occurred in one patient (1%). Notably, 11 patients (10%) were able to become pregnant.
On statistical analysis, the presence of disseminated intravascular coagulopathy was the most important negative prognostic factor.
Dr. Shin reported that he had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INTERVENTIONAL RADIOLOGY
Major Finding: A single pelvic artery embolization procedure stopped postpartum hemorrhage in 86% of women. A second procedure stopped bleeding in 89%.
Data Source: A retrospective study of 225 women who underwent PAE to stop postpartum hemorrhage.
Disclosures: Dr. Shin reported that he had no relevant financial disclosures.
Pelvic Artery Embolization Stops Postpartum Hemorrhage
CHICAGO – Pelvic artery embolization is a highly effective technique for managing postpartum hemorrhage with the added advantage that it preserves the uterus and fertility, according to Dr. Ji Hoon Shin.
In a retrospective study of 225 women who underwent pelvic artery embolization (PAE) to stop postpartum hemorrhage (PPH), a single procedure stopped bleeding in 86% of women. A second procedure stopped bleeding in 89%.
"The major advantage of this procedure is that you can save the uterus and fertility," Dr. Shin said at the annual meeting of the Society for Interventional Radiology.
Standard therapies include fluid resuscitation/blood transfusion, management of the underlying cause, or surgery – uterine artery ligation, suturing, and hysterectomy.
PAE involves inserting a catheter into the femoral artery via a small incision in the groin. The interventional radiologist can then inject small particles or coils into the arteries to stem hemorrhage. Not only is the technique minimally invasive, it can save the uterus and preserve fertility. While the use of PAE was first reported in 1979, obstetrician awareness of this option remains limited.
In this study, researchers identified 225 patients who underwent pulmonary artery embolization for PPH within 24 hours of delivery between 2000 and 2010. Technical success was defined as cessation of bleeding on postembolization angiogram and cessation of vaginal bleeding on physical inspection. Clinical success was defined as the cessation of bleeding following PAE without the need for additional surgery during the hospital stay.
Uterine atony was the most common cause of PPH in this group (81%). Roughly a third (36%) of patients had positive angiographic findings. Contrast extravasation (a sign of acute bleeding) was seen in most patients (86%) and pseudoaneurysm occurred in 14% of patients, said Dr. Shin of the department of radiology at the University of Ulsan in Seoul, South Korea.
Technical success was 89%. Clinical success with only one PAE procedure was 86% and was 89% when women who had an additional PAE procedure were included. Overall bleeding control – including patients who had repeat PAEs and/or surgeries – was 98%.
In terms of safety, major complications occurred in 2% of patients and included puncture site hematoma and uterine artery dissection. Transient numbness – a minor complication – occurred in 1% of women.
In terms of fertility preservation, 97% of 113 women who were available for follow-up resumed regular menstruation. Irregular menses occurred in two women (2%), and early menopause occurred in one patient (1%). Notably, 11 patients (10%) were able to become pregnant.
On statistical analysis, the presence of disseminated intravascular coagulopathy was the most important negative prognostic factor.
Dr. Shin reported that he had no relevant financial disclosures.
CHICAGO – Pelvic artery embolization is a highly effective technique for managing postpartum hemorrhage with the added advantage that it preserves the uterus and fertility, according to Dr. Ji Hoon Shin.
In a retrospective study of 225 women who underwent pelvic artery embolization (PAE) to stop postpartum hemorrhage (PPH), a single procedure stopped bleeding in 86% of women. A second procedure stopped bleeding in 89%.
"The major advantage of this procedure is that you can save the uterus and fertility," Dr. Shin said at the annual meeting of the Society for Interventional Radiology.
Standard therapies include fluid resuscitation/blood transfusion, management of the underlying cause, or surgery – uterine artery ligation, suturing, and hysterectomy.
PAE involves inserting a catheter into the femoral artery via a small incision in the groin. The interventional radiologist can then inject small particles or coils into the arteries to stem hemorrhage. Not only is the technique minimally invasive, it can save the uterus and preserve fertility. While the use of PAE was first reported in 1979, obstetrician awareness of this option remains limited.
In this study, researchers identified 225 patients who underwent pulmonary artery embolization for PPH within 24 hours of delivery between 2000 and 2010. Technical success was defined as cessation of bleeding on postembolization angiogram and cessation of vaginal bleeding on physical inspection. Clinical success was defined as the cessation of bleeding following PAE without the need for additional surgery during the hospital stay.
Uterine atony was the most common cause of PPH in this group (81%). Roughly a third (36%) of patients had positive angiographic findings. Contrast extravasation (a sign of acute bleeding) was seen in most patients (86%) and pseudoaneurysm occurred in 14% of patients, said Dr. Shin of the department of radiology at the University of Ulsan in Seoul, South Korea.
Technical success was 89%. Clinical success with only one PAE procedure was 86% and was 89% when women who had an additional PAE procedure were included. Overall bleeding control – including patients who had repeat PAEs and/or surgeries – was 98%.
In terms of safety, major complications occurred in 2% of patients and included puncture site hematoma and uterine artery dissection. Transient numbness – a minor complication – occurred in 1% of women.
In terms of fertility preservation, 97% of 113 women who were available for follow-up resumed regular menstruation. Irregular menses occurred in two women (2%), and early menopause occurred in one patient (1%). Notably, 11 patients (10%) were able to become pregnant.
On statistical analysis, the presence of disseminated intravascular coagulopathy was the most important negative prognostic factor.
Dr. Shin reported that he had no relevant financial disclosures.
CHICAGO – Pelvic artery embolization is a highly effective technique for managing postpartum hemorrhage with the added advantage that it preserves the uterus and fertility, according to Dr. Ji Hoon Shin.
In a retrospective study of 225 women who underwent pelvic artery embolization (PAE) to stop postpartum hemorrhage (PPH), a single procedure stopped bleeding in 86% of women. A second procedure stopped bleeding in 89%.
"The major advantage of this procedure is that you can save the uterus and fertility," Dr. Shin said at the annual meeting of the Society for Interventional Radiology.
Standard therapies include fluid resuscitation/blood transfusion, management of the underlying cause, or surgery – uterine artery ligation, suturing, and hysterectomy.
PAE involves inserting a catheter into the femoral artery via a small incision in the groin. The interventional radiologist can then inject small particles or coils into the arteries to stem hemorrhage. Not only is the technique minimally invasive, it can save the uterus and preserve fertility. While the use of PAE was first reported in 1979, obstetrician awareness of this option remains limited.
In this study, researchers identified 225 patients who underwent pulmonary artery embolization for PPH within 24 hours of delivery between 2000 and 2010. Technical success was defined as cessation of bleeding on postembolization angiogram and cessation of vaginal bleeding on physical inspection. Clinical success was defined as the cessation of bleeding following PAE without the need for additional surgery during the hospital stay.
Uterine atony was the most common cause of PPH in this group (81%). Roughly a third (36%) of patients had positive angiographic findings. Contrast extravasation (a sign of acute bleeding) was seen in most patients (86%) and pseudoaneurysm occurred in 14% of patients, said Dr. Shin of the department of radiology at the University of Ulsan in Seoul, South Korea.
Technical success was 89%. Clinical success with only one PAE procedure was 86% and was 89% when women who had an additional PAE procedure were included. Overall bleeding control – including patients who had repeat PAEs and/or surgeries – was 98%.
In terms of safety, major complications occurred in 2% of patients and included puncture site hematoma and uterine artery dissection. Transient numbness – a minor complication – occurred in 1% of women.
In terms of fertility preservation, 97% of 113 women who were available for follow-up resumed regular menstruation. Irregular menses occurred in two women (2%), and early menopause occurred in one patient (1%). Notably, 11 patients (10%) were able to become pregnant.
On statistical analysis, the presence of disseminated intravascular coagulopathy was the most important negative prognostic factor.
Dr. Shin reported that he had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INTERVENTIONAL RADIOLOGY
Major Finding: A single pelvic artery embolization procedure stopped postpartum hemorrhage in 86% of women. A second procedure stopped bleeding in 89%.
Data Source: A retrospective study of 225 women who underwent PAE to stop postpartum hemorrhage.
Disclosures: Dr. Shin reported that he had no relevant financial disclosures.
Opioid Abuse Is Rising Concern in Cancer Patients and Survivors
NATIONAL HARBOR, MD. – Opioid misuse, and substance abuse in general, is a growing problem among cancer patients and survivors, according to a panel of experts at the annual meeting of the American Academy of Pain Medicine.
With the number of cancer survivors – people who can live for years and decades after diagnosis – on the rise, clinicians are seeing a corresponding growth in risk for pain medication addiction, they said. In addition, earlier cancer diagnoses can mean more patients may commence cancer therapy with a history of substance abuse.
"We’re starting to see a lot more in the way of substance abuse of every stripe. ... There are a lot of folks making it to tertiary cancer care centers with a history of addiction," said Steven D. Passik, Ph.D., a professor of clinical psychiatry at Vanderbilt University in Nashville, Tenn.
He gave the example of a female patient who had a preexisting polysubstance abuse problem and lived with metastatic breast cancer for 11 years. "When I first started in this field, this woman’s life expectancy would have been measured in months, and now it’s measured in years to decades," he said, introducing the challenges of tailoring long-term pain management programs for patients who are at risk of substance abuse.
Oncologists had not dealt much with substance abuse, because cancer is primarily a disease of older people, and addiction problems tend to manifest by age 35, Dr. Passik said. Oncologists and pain management physicians "will now have to worry about a small but very labor-intensive subpopulation of the cancer population. But you have to identify them," he continued, adding that "screening for substance abuse in the cancer setting ... has still not taken root."
Screening Tool Introduced
Dr. Dhanalakshmi Koyyalagunta, a pain management specialist at the University of Texas M.D. Anderson Cancer Center in Houston, said that clinicians there started using the short form of the SOAPP (Screener and Opioid Assessment for Patients With Pain) tool a little more than 2 years ago. This is a five-question assessment. Dr. Koyyalagunta said that clinicians at M.D. Anderson use a score of 4 or more as an indication that an individual is at high risk for substance abuse.
A review of 524 charts with completed SOAPP data found that more than a quarter of cancer patients (29%) were at high risk, she said, noting that the high-risk group included 43% of patients aged 18-35 years, 27% of patients older than 35 years, 72% of smokers, 40% of those who were unemployed, 60% of those with a history of drug use, and 32% of those with a history of alcohol abuse.
Although the true prevalence of substance abuse in the overall population of long-term cancer survivors is unknown, clinicians do know that there is a high incidence of comorbid anxiety and depression, added Dr. Koyyalagunta. It is very important to address any depression, anxiety, or other comorbid psychiatric conditions along with the pain, she said: "There’s a lot of chemical coping associated with these comorbidities."
The best treatment approach is to put together a multidisciplinary team that can develop an integrated treatment plan, said Diane M. Novy, Ph.D., a professor of pain medicine at M.D. Anderson. For example, a psychologist or social worker can address coexisting conditions such as affective disorders; acute stress related to cancer and cancer treatment; family problems; and work-related issues.
No Self-Titration for Pain Management
Pain management in cancer patients, particularly in older patients, tends to rely on self-titration: Take as much as you need, observed Dr. Passik. "It doesn’t hurt low-risk people to have a self-titration model, but you can’t apply that to high-risk people. That’s the recipe for a public health disaster," he warned.
Add in the need for long-term treatment, and problems associated with opioid exposure "now have ample time to play themselves out in terrible fashion," he said. Any opioid treatment strategy that he might use in high-risk patients with an expected 3-6 months of life would be inappropriate for a high-risk patient who was likely to survive for many years, he observed.
"All of us do have trouble with how we handle these patients long term," agreed Dr. Koyyalagunta. "We see a fair number of patients, and it may not be as easy as in the chronic pain setting, where you may have an exit strategy or have an alternative plan – especially in patients with active cancer."
Nonetheless, strategies and tools from the noncancer chronic pain world can be used in the cancer setting, said Dr. Koyyalagunta. These include assessment and differential diagnosis, screening, informed consent, ongoing psychiatric care, frequent outpatient visits, and documentation. "It’s pretty much the same set of principles, but adapting it to a cancer setting," she said.
Dr. Novy concurred. "For people who are at risk, we see them more frequently. We involve their families in the treatment. We encourage the use of a pill box and pill count. We also monitor with random urine screens," she said, adding that M.D. Anderson is also starting to use an opioid compliance checklist similar to the COMM (Current Opioid Misuse Measure) that was developed by Robert N. Jamison, Ph.D., at Brigham and Women’s Hospital in Boston (Pain 2007;130:144-56).
Dr. Passik reported that he has received honoraria from Cephalon Inc., King Pharmaceuticals Inc. (now part of Pfizer Inc.), Pricara (a division of Ortho-McNeil-Janssen Pharmaceuticals Inc.), and Purdue Pharma LP. Dr. Koyyalagunta and Dr. Novy both reported that they have no relevant financial relationships.
NATIONAL HARBOR, MD. – Opioid misuse, and substance abuse in general, is a growing problem among cancer patients and survivors, according to a panel of experts at the annual meeting of the American Academy of Pain Medicine.
With the number of cancer survivors – people who can live for years and decades after diagnosis – on the rise, clinicians are seeing a corresponding growth in risk for pain medication addiction, they said. In addition, earlier cancer diagnoses can mean more patients may commence cancer therapy with a history of substance abuse.
"We’re starting to see a lot more in the way of substance abuse of every stripe. ... There are a lot of folks making it to tertiary cancer care centers with a history of addiction," said Steven D. Passik, Ph.D., a professor of clinical psychiatry at Vanderbilt University in Nashville, Tenn.
He gave the example of a female patient who had a preexisting polysubstance abuse problem and lived with metastatic breast cancer for 11 years. "When I first started in this field, this woman’s life expectancy would have been measured in months, and now it’s measured in years to decades," he said, introducing the challenges of tailoring long-term pain management programs for patients who are at risk of substance abuse.
Oncologists had not dealt much with substance abuse, because cancer is primarily a disease of older people, and addiction problems tend to manifest by age 35, Dr. Passik said. Oncologists and pain management physicians "will now have to worry about a small but very labor-intensive subpopulation of the cancer population. But you have to identify them," he continued, adding that "screening for substance abuse in the cancer setting ... has still not taken root."
Screening Tool Introduced
Dr. Dhanalakshmi Koyyalagunta, a pain management specialist at the University of Texas M.D. Anderson Cancer Center in Houston, said that clinicians there started using the short form of the SOAPP (Screener and Opioid Assessment for Patients With Pain) tool a little more than 2 years ago. This is a five-question assessment. Dr. Koyyalagunta said that clinicians at M.D. Anderson use a score of 4 or more as an indication that an individual is at high risk for substance abuse.
A review of 524 charts with completed SOAPP data found that more than a quarter of cancer patients (29%) were at high risk, she said, noting that the high-risk group included 43% of patients aged 18-35 years, 27% of patients older than 35 years, 72% of smokers, 40% of those who were unemployed, 60% of those with a history of drug use, and 32% of those with a history of alcohol abuse.
Although the true prevalence of substance abuse in the overall population of long-term cancer survivors is unknown, clinicians do know that there is a high incidence of comorbid anxiety and depression, added Dr. Koyyalagunta. It is very important to address any depression, anxiety, or other comorbid psychiatric conditions along with the pain, she said: "There’s a lot of chemical coping associated with these comorbidities."
The best treatment approach is to put together a multidisciplinary team that can develop an integrated treatment plan, said Diane M. Novy, Ph.D., a professor of pain medicine at M.D. Anderson. For example, a psychologist or social worker can address coexisting conditions such as affective disorders; acute stress related to cancer and cancer treatment; family problems; and work-related issues.
No Self-Titration for Pain Management
Pain management in cancer patients, particularly in older patients, tends to rely on self-titration: Take as much as you need, observed Dr. Passik. "It doesn’t hurt low-risk people to have a self-titration model, but you can’t apply that to high-risk people. That’s the recipe for a public health disaster," he warned.
Add in the need for long-term treatment, and problems associated with opioid exposure "now have ample time to play themselves out in terrible fashion," he said. Any opioid treatment strategy that he might use in high-risk patients with an expected 3-6 months of life would be inappropriate for a high-risk patient who was likely to survive for many years, he observed.
"All of us do have trouble with how we handle these patients long term," agreed Dr. Koyyalagunta. "We see a fair number of patients, and it may not be as easy as in the chronic pain setting, where you may have an exit strategy or have an alternative plan – especially in patients with active cancer."
Nonetheless, strategies and tools from the noncancer chronic pain world can be used in the cancer setting, said Dr. Koyyalagunta. These include assessment and differential diagnosis, screening, informed consent, ongoing psychiatric care, frequent outpatient visits, and documentation. "It’s pretty much the same set of principles, but adapting it to a cancer setting," she said.
Dr. Novy concurred. "For people who are at risk, we see them more frequently. We involve their families in the treatment. We encourage the use of a pill box and pill count. We also monitor with random urine screens," she said, adding that M.D. Anderson is also starting to use an opioid compliance checklist similar to the COMM (Current Opioid Misuse Measure) that was developed by Robert N. Jamison, Ph.D., at Brigham and Women’s Hospital in Boston (Pain 2007;130:144-56).
Dr. Passik reported that he has received honoraria from Cephalon Inc., King Pharmaceuticals Inc. (now part of Pfizer Inc.), Pricara (a division of Ortho-McNeil-Janssen Pharmaceuticals Inc.), and Purdue Pharma LP. Dr. Koyyalagunta and Dr. Novy both reported that they have no relevant financial relationships.
NATIONAL HARBOR, MD. – Opioid misuse, and substance abuse in general, is a growing problem among cancer patients and survivors, according to a panel of experts at the annual meeting of the American Academy of Pain Medicine.
With the number of cancer survivors – people who can live for years and decades after diagnosis – on the rise, clinicians are seeing a corresponding growth in risk for pain medication addiction, they said. In addition, earlier cancer diagnoses can mean more patients may commence cancer therapy with a history of substance abuse.
"We’re starting to see a lot more in the way of substance abuse of every stripe. ... There are a lot of folks making it to tertiary cancer care centers with a history of addiction," said Steven D. Passik, Ph.D., a professor of clinical psychiatry at Vanderbilt University in Nashville, Tenn.
He gave the example of a female patient who had a preexisting polysubstance abuse problem and lived with metastatic breast cancer for 11 years. "When I first started in this field, this woman’s life expectancy would have been measured in months, and now it’s measured in years to decades," he said, introducing the challenges of tailoring long-term pain management programs for patients who are at risk of substance abuse.
Oncologists had not dealt much with substance abuse, because cancer is primarily a disease of older people, and addiction problems tend to manifest by age 35, Dr. Passik said. Oncologists and pain management physicians "will now have to worry about a small but very labor-intensive subpopulation of the cancer population. But you have to identify them," he continued, adding that "screening for substance abuse in the cancer setting ... has still not taken root."
Screening Tool Introduced
Dr. Dhanalakshmi Koyyalagunta, a pain management specialist at the University of Texas M.D. Anderson Cancer Center in Houston, said that clinicians there started using the short form of the SOAPP (Screener and Opioid Assessment for Patients With Pain) tool a little more than 2 years ago. This is a five-question assessment. Dr. Koyyalagunta said that clinicians at M.D. Anderson use a score of 4 or more as an indication that an individual is at high risk for substance abuse.
A review of 524 charts with completed SOAPP data found that more than a quarter of cancer patients (29%) were at high risk, she said, noting that the high-risk group included 43% of patients aged 18-35 years, 27% of patients older than 35 years, 72% of smokers, 40% of those who were unemployed, 60% of those with a history of drug use, and 32% of those with a history of alcohol abuse.
Although the true prevalence of substance abuse in the overall population of long-term cancer survivors is unknown, clinicians do know that there is a high incidence of comorbid anxiety and depression, added Dr. Koyyalagunta. It is very important to address any depression, anxiety, or other comorbid psychiatric conditions along with the pain, she said: "There’s a lot of chemical coping associated with these comorbidities."
The best treatment approach is to put together a multidisciplinary team that can develop an integrated treatment plan, said Diane M. Novy, Ph.D., a professor of pain medicine at M.D. Anderson. For example, a psychologist or social worker can address coexisting conditions such as affective disorders; acute stress related to cancer and cancer treatment; family problems; and work-related issues.
No Self-Titration for Pain Management
Pain management in cancer patients, particularly in older patients, tends to rely on self-titration: Take as much as you need, observed Dr. Passik. "It doesn’t hurt low-risk people to have a self-titration model, but you can’t apply that to high-risk people. That’s the recipe for a public health disaster," he warned.
Add in the need for long-term treatment, and problems associated with opioid exposure "now have ample time to play themselves out in terrible fashion," he said. Any opioid treatment strategy that he might use in high-risk patients with an expected 3-6 months of life would be inappropriate for a high-risk patient who was likely to survive for many years, he observed.
"All of us do have trouble with how we handle these patients long term," agreed Dr. Koyyalagunta. "We see a fair number of patients, and it may not be as easy as in the chronic pain setting, where you may have an exit strategy or have an alternative plan – especially in patients with active cancer."
Nonetheless, strategies and tools from the noncancer chronic pain world can be used in the cancer setting, said Dr. Koyyalagunta. These include assessment and differential diagnosis, screening, informed consent, ongoing psychiatric care, frequent outpatient visits, and documentation. "It’s pretty much the same set of principles, but adapting it to a cancer setting," she said.
Dr. Novy concurred. "For people who are at risk, we see them more frequently. We involve their families in the treatment. We encourage the use of a pill box and pill count. We also monitor with random urine screens," she said, adding that M.D. Anderson is also starting to use an opioid compliance checklist similar to the COMM (Current Opioid Misuse Measure) that was developed by Robert N. Jamison, Ph.D., at Brigham and Women’s Hospital in Boston (Pain 2007;130:144-56).
Dr. Passik reported that he has received honoraria from Cephalon Inc., King Pharmaceuticals Inc. (now part of Pfizer Inc.), Pricara (a division of Ortho-McNeil-Janssen Pharmaceuticals Inc.), and Purdue Pharma LP. Dr. Koyyalagunta and Dr. Novy both reported that they have no relevant financial relationships.
Opioid Abuse Is Rising Concern in Cancer Patients and Survivors
NATIONAL HARBOR, MD. – Opioid misuse, and substance abuse in general, is a growing problem among cancer patients and survivors, according to a panel of experts at the annual meeting of the American Academy of Pain Medicine.
With the number of cancer survivors – people who can live for years and decades after diagnosis – on the rise, clinicians are seeing a corresponding growth in risk for pain medication addiction, they said. In addition, earlier cancer diagnoses can mean more patients may commence cancer therapy with a history of substance abuse.
"We’re starting to see a lot more in the way of substance abuse of every stripe. ... There are a lot of folks making it to tertiary cancer care centers with a history of addiction," said Steven D. Passik, Ph.D., a professor of clinical psychiatry at Vanderbilt University in Nashville, Tenn.
He gave the example of a female patient who had a preexisting polysubstance abuse problem and lived with metastatic breast cancer for 11 years. "When I first started in this field, this woman’s life expectancy would have been measured in months, and now it’s measured in years to decades," he said, introducing the challenges of tailoring long-term pain management programs for patients who are at risk of substance abuse.
Oncologists had not dealt much with substance abuse, because cancer is primarily a disease of older people, and addiction problems tend to manifest by age 35, Dr. Passik said. Oncologists and pain management physicians "will now have to worry about a small but very labor-intensive subpopulation of the cancer population. But you have to identify them," he continued, adding that "screening for substance abuse in the cancer setting ... has still not taken root."
Screening Tool Introduced
Dr. Dhanalakshmi Koyyalagunta, a pain management specialist at the University of Texas M.D. Anderson Cancer Center in Houston, said that clinicians there started using the short form of the SOAPP (Screener and Opioid Assessment for Patients With Pain) tool a little more than 2 years ago. This is a five-question assessment. Dr. Koyyalagunta said that clinicians at M.D. Anderson use a score of 4 or more as an indication that an individual is at high risk for substance abuse.
A review of 524 charts with completed SOAPP data found that more than a quarter of cancer patients (29%) were at high risk, she said, noting that the high-risk group included 43% of patients aged 18-35 years, 27% of patients older than 35 years, 72% of smokers, 40% of those who were unemployed, 60% of those with a history of drug use, and 32% of those with a history of alcohol abuse.
Although the true prevalence of substance abuse in the overall population of long-term cancer survivors is unknown, clinicians do know that there is a high incidence of comorbid anxiety and depression, added Dr. Koyyalagunta. It is very important to address any depression, anxiety, or other comorbid psychiatric conditions along with the pain, she said: "There’s a lot of chemical coping associated with these comorbidities."
The best treatment approach is to put together a multidisciplinary team that can develop an integrated treatment plan, said Diane M. Novy, Ph.D., a professor of pain medicine at M.D. Anderson. For example, a psychologist or social worker can address coexisting conditions such as affective disorders; acute stress related to cancer and cancer treatment; family problems; and work-related issues.
No Self-Titration for Pain Management
Pain management in cancer patients, particularly in older patients, tends to rely on self-titration: Take as much as you need, observed Dr. Passik. "It doesn’t hurt low-risk people to have a self-titration model, but you can’t apply that to high-risk people. That’s the recipe for a public health disaster," he warned.
Add in the need for long-term treatment, and problems associated with opioid exposure "now have ample time to play themselves out in terrible fashion," he said. Any opioid treatment strategy that he might use in high-risk patients with an expected 3-6 months of life would be inappropriate for a high-risk patient who was likely to survive for many years, he observed.
"All of us do have trouble with how we handle these patients long term," agreed Dr. Koyyalagunta. "We see a fair number of patients, and it may not be as easy as in the chronic pain setting, where you may have an exit strategy or have an alternative plan – especially in patients with active cancer."
Nonetheless, strategies and tools from the noncancer chronic pain world can be used in the cancer setting, said Dr. Koyyalagunta. These include assessment and differential diagnosis, screening, informed consent, ongoing psychiatric care, frequent outpatient visits, and documentation. "It’s pretty much the same set of principles, but adapting it to a cancer setting," she said.
Dr. Novy concurred. "For people who are at risk, we see them more frequently. We involve their families in the treatment. We encourage the use of a pill box and pill count. We also monitor with random urine screens," she said, adding that M.D. Anderson is also starting to use an opioid compliance checklist similar to the COMM (Current Opioid Misuse Measure) that was developed by Robert N. Jamison, Ph.D., at Brigham and Women’s Hospital in Boston (Pain 2007;130:144-56).
Dr. Passik reported that he has received honoraria from Cephalon Inc., King Pharmaceuticals Inc. (now part of Pfizer Inc.), Pricara (a division of Ortho-McNeil-Janssen Pharmaceuticals Inc.), and Purdue Pharma LP. Dr. Koyyalagunta and Dr. Novy both reported that they have no relevant financial relationships.
NATIONAL HARBOR, MD. – Opioid misuse, and substance abuse in general, is a growing problem among cancer patients and survivors, according to a panel of experts at the annual meeting of the American Academy of Pain Medicine.
With the number of cancer survivors – people who can live for years and decades after diagnosis – on the rise, clinicians are seeing a corresponding growth in risk for pain medication addiction, they said. In addition, earlier cancer diagnoses can mean more patients may commence cancer therapy with a history of substance abuse.
"We’re starting to see a lot more in the way of substance abuse of every stripe. ... There are a lot of folks making it to tertiary cancer care centers with a history of addiction," said Steven D. Passik, Ph.D., a professor of clinical psychiatry at Vanderbilt University in Nashville, Tenn.
He gave the example of a female patient who had a preexisting polysubstance abuse problem and lived with metastatic breast cancer for 11 years. "When I first started in this field, this woman’s life expectancy would have been measured in months, and now it’s measured in years to decades," he said, introducing the challenges of tailoring long-term pain management programs for patients who are at risk of substance abuse.
Oncologists had not dealt much with substance abuse, because cancer is primarily a disease of older people, and addiction problems tend to manifest by age 35, Dr. Passik said. Oncologists and pain management physicians "will now have to worry about a small but very labor-intensive subpopulation of the cancer population. But you have to identify them," he continued, adding that "screening for substance abuse in the cancer setting ... has still not taken root."
Screening Tool Introduced
Dr. Dhanalakshmi Koyyalagunta, a pain management specialist at the University of Texas M.D. Anderson Cancer Center in Houston, said that clinicians there started using the short form of the SOAPP (Screener and Opioid Assessment for Patients With Pain) tool a little more than 2 years ago. This is a five-question assessment. Dr. Koyyalagunta said that clinicians at M.D. Anderson use a score of 4 or more as an indication that an individual is at high risk for substance abuse.
A review of 524 charts with completed SOAPP data found that more than a quarter of cancer patients (29%) were at high risk, she said, noting that the high-risk group included 43% of patients aged 18-35 years, 27% of patients older than 35 years, 72% of smokers, 40% of those who were unemployed, 60% of those with a history of drug use, and 32% of those with a history of alcohol abuse.
Although the true prevalence of substance abuse in the overall population of long-term cancer survivors is unknown, clinicians do know that there is a high incidence of comorbid anxiety and depression, added Dr. Koyyalagunta. It is very important to address any depression, anxiety, or other comorbid psychiatric conditions along with the pain, she said: "There’s a lot of chemical coping associated with these comorbidities."
The best treatment approach is to put together a multidisciplinary team that can develop an integrated treatment plan, said Diane M. Novy, Ph.D., a professor of pain medicine at M.D. Anderson. For example, a psychologist or social worker can address coexisting conditions such as affective disorders; acute stress related to cancer and cancer treatment; family problems; and work-related issues.
No Self-Titration for Pain Management
Pain management in cancer patients, particularly in older patients, tends to rely on self-titration: Take as much as you need, observed Dr. Passik. "It doesn’t hurt low-risk people to have a self-titration model, but you can’t apply that to high-risk people. That’s the recipe for a public health disaster," he warned.
Add in the need for long-term treatment, and problems associated with opioid exposure "now have ample time to play themselves out in terrible fashion," he said. Any opioid treatment strategy that he might use in high-risk patients with an expected 3-6 months of life would be inappropriate for a high-risk patient who was likely to survive for many years, he observed.
"All of us do have trouble with how we handle these patients long term," agreed Dr. Koyyalagunta. "We see a fair number of patients, and it may not be as easy as in the chronic pain setting, where you may have an exit strategy or have an alternative plan – especially in patients with active cancer."
Nonetheless, strategies and tools from the noncancer chronic pain world can be used in the cancer setting, said Dr. Koyyalagunta. These include assessment and differential diagnosis, screening, informed consent, ongoing psychiatric care, frequent outpatient visits, and documentation. "It’s pretty much the same set of principles, but adapting it to a cancer setting," she said.
Dr. Novy concurred. "For people who are at risk, we see them more frequently. We involve their families in the treatment. We encourage the use of a pill box and pill count. We also monitor with random urine screens," she said, adding that M.D. Anderson is also starting to use an opioid compliance checklist similar to the COMM (Current Opioid Misuse Measure) that was developed by Robert N. Jamison, Ph.D., at Brigham and Women’s Hospital in Boston (Pain 2007;130:144-56).
Dr. Passik reported that he has received honoraria from Cephalon Inc., King Pharmaceuticals Inc. (now part of Pfizer Inc.), Pricara (a division of Ortho-McNeil-Janssen Pharmaceuticals Inc.), and Purdue Pharma LP. Dr. Koyyalagunta and Dr. Novy both reported that they have no relevant financial relationships.
NATIONAL HARBOR, MD. – Opioid misuse, and substance abuse in general, is a growing problem among cancer patients and survivors, according to a panel of experts at the annual meeting of the American Academy of Pain Medicine.
With the number of cancer survivors – people who can live for years and decades after diagnosis – on the rise, clinicians are seeing a corresponding growth in risk for pain medication addiction, they said. In addition, earlier cancer diagnoses can mean more patients may commence cancer therapy with a history of substance abuse.
"We’re starting to see a lot more in the way of substance abuse of every stripe. ... There are a lot of folks making it to tertiary cancer care centers with a history of addiction," said Steven D. Passik, Ph.D., a professor of clinical psychiatry at Vanderbilt University in Nashville, Tenn.
He gave the example of a female patient who had a preexisting polysubstance abuse problem and lived with metastatic breast cancer for 11 years. "When I first started in this field, this woman’s life expectancy would have been measured in months, and now it’s measured in years to decades," he said, introducing the challenges of tailoring long-term pain management programs for patients who are at risk of substance abuse.
Oncologists had not dealt much with substance abuse, because cancer is primarily a disease of older people, and addiction problems tend to manifest by age 35, Dr. Passik said. Oncologists and pain management physicians "will now have to worry about a small but very labor-intensive subpopulation of the cancer population. But you have to identify them," he continued, adding that "screening for substance abuse in the cancer setting ... has still not taken root."
Screening Tool Introduced
Dr. Dhanalakshmi Koyyalagunta, a pain management specialist at the University of Texas M.D. Anderson Cancer Center in Houston, said that clinicians there started using the short form of the SOAPP (Screener and Opioid Assessment for Patients With Pain) tool a little more than 2 years ago. This is a five-question assessment. Dr. Koyyalagunta said that clinicians at M.D. Anderson use a score of 4 or more as an indication that an individual is at high risk for substance abuse.
A review of 524 charts with completed SOAPP data found that more than a quarter of cancer patients (29%) were at high risk, she said, noting that the high-risk group included 43% of patients aged 18-35 years, 27% of patients older than 35 years, 72% of smokers, 40% of those who were unemployed, 60% of those with a history of drug use, and 32% of those with a history of alcohol abuse.
Although the true prevalence of substance abuse in the overall population of long-term cancer survivors is unknown, clinicians do know that there is a high incidence of comorbid anxiety and depression, added Dr. Koyyalagunta. It is very important to address any depression, anxiety, or other comorbid psychiatric conditions along with the pain, she said: "There’s a lot of chemical coping associated with these comorbidities."
The best treatment approach is to put together a multidisciplinary team that can develop an integrated treatment plan, said Diane M. Novy, Ph.D., a professor of pain medicine at M.D. Anderson. For example, a psychologist or social worker can address coexisting conditions such as affective disorders; acute stress related to cancer and cancer treatment; family problems; and work-related issues.
No Self-Titration for Pain Management
Pain management in cancer patients, particularly in older patients, tends to rely on self-titration: Take as much as you need, observed Dr. Passik. "It doesn’t hurt low-risk people to have a self-titration model, but you can’t apply that to high-risk people. That’s the recipe for a public health disaster," he warned.
Add in the need for long-term treatment, and problems associated with opioid exposure "now have ample time to play themselves out in terrible fashion," he said. Any opioid treatment strategy that he might use in high-risk patients with an expected 3-6 months of life would be inappropriate for a high-risk patient who was likely to survive for many years, he observed.
"All of us do have trouble with how we handle these patients long term," agreed Dr. Koyyalagunta. "We see a fair number of patients, and it may not be as easy as in the chronic pain setting, where you may have an exit strategy or have an alternative plan – especially in patients with active cancer."
Nonetheless, strategies and tools from the noncancer chronic pain world can be used in the cancer setting, said Dr. Koyyalagunta. These include assessment and differential diagnosis, screening, informed consent, ongoing psychiatric care, frequent outpatient visits, and documentation. "It’s pretty much the same set of principles, but adapting it to a cancer setting," she said.
Dr. Novy concurred. "For people who are at risk, we see them more frequently. We involve their families in the treatment. We encourage the use of a pill box and pill count. We also monitor with random urine screens," she said, adding that M.D. Anderson is also starting to use an opioid compliance checklist similar to the COMM (Current Opioid Misuse Measure) that was developed by Robert N. Jamison, Ph.D., at Brigham and Women’s Hospital in Boston (Pain 2007;130:144-56).
Dr. Passik reported that he has received honoraria from Cephalon Inc., King Pharmaceuticals Inc. (now part of Pfizer Inc.), Pricara (a division of Ortho-McNeil-Janssen Pharmaceuticals Inc.), and Purdue Pharma LP. Dr. Koyyalagunta and Dr. Novy both reported that they have no relevant financial relationships.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF PAIN MEDICINE
Opioid Abuse Is Rising Concern in Cancer Patients and Survivors
NATIONAL HARBOR, MD. – Opioid misuse, and substance abuse in general, is a growing problem among cancer patients and survivors, according to a panel of experts at the annual meeting of the American Academy of Pain Medicine.
With the number of cancer survivors – people who can live for years and decades after diagnosis – on the rise, clinicians are seeing a corresponding growth in risk for pain medication addiction, they said. In addition, earlier cancer diagnoses can mean more patients may commence cancer therapy with a history of substance abuse.
"We’re starting to see a lot more in the way of substance abuse of every stripe. ... There are a lot of folks making it to tertiary cancer care centers with a history of addiction," said Steven D. Passik, Ph.D., a professor of clinical psychiatry at Vanderbilt University in Nashville, Tenn.
He gave the example of a female patient who had a preexisting polysubstance abuse problem and lived with metastatic breast cancer for 11 years. "When I first started in this field, this woman’s life expectancy would have been measured in months, and now it’s measured in years to decades," he said, introducing the challenges of tailoring long-term pain management programs for patients who are at risk of substance abuse.
Oncologists had not dealt much with substance abuse, because cancer is primarily a disease of older people, and addiction problems tend to manifest by age 35, Dr. Passik said. Oncologists and pain management physicians "will now have to worry about a small but very labor-intensive subpopulation of the cancer population. But you have to identify them," he continued, adding that "screening for substance abuse in the cancer setting ... has still not taken root."
Screening Tool Introduced
Dr. Dhanalakshmi Koyyalagunta, a pain management specialist at the University of Texas M.D. Anderson Cancer Center in Houston, said that clinicians there started using the short form of the SOAPP (Screener and Opioid Assessment for Patients With Pain) tool a little more than 2 years ago. This is a five-question assessment. Dr. Koyyalagunta said that clinicians at M.D. Anderson use a score of 4 or more as an indication that an individual is at high risk for substance abuse.
A review of 524 charts with completed SOAPP data found that more than a quarter of cancer patients (29%) were at high risk, she said, noting that the high-risk group included 43% of patients aged 18-35 years, 27% of patients older than 35 years, 72% of smokers, 40% of those who were unemployed, 60% of those with a history of drug use, and 32% of those with a history of alcohol abuse.
Although the true prevalence of substance abuse in the overall population of long-term cancer survivors is unknown, clinicians do know that there is a high incidence of comorbid anxiety and depression, added Dr. Koyyalagunta. It is very important to address any depression, anxiety, or other comorbid psychiatric conditions along with the pain, she said: "There’s a lot of chemical coping associated with these comorbidities."
The best treatment approach is to put together a multidisciplinary team that can develop an integrated treatment plan, said Diane M. Novy, Ph.D., a professor of pain medicine at M.D. Anderson. For example, a psychologist or social worker can address coexisting conditions such as affective disorders; acute stress related to cancer and cancer treatment; family problems; and work-related issues.
No Self-Titration for Pain Management
Pain management in cancer patients, particularly in older patients, tends to rely on self-titration: Take as much as you need, observed Dr. Passik. "It doesn’t hurt low-risk people to have a self-titration model, but you can’t apply that to high-risk people. That’s the recipe for a public health disaster," he warned.
Add in the need for long-term treatment, and problems associated with opioid exposure "now have ample time to play themselves out in terrible fashion," he said. Any opioid treatment strategy that he might use in high-risk patients with an expected 3-6 months of life would be inappropriate for a high-risk patient who was likely to survive for many years, he observed.
"All of us do have trouble with how we handle these patients long term," agreed Dr. Koyyalagunta. "We see a fair number of patients, and it may not be as easy as in the chronic pain setting, where you may have an exit strategy or have an alternative plan – especially in patients with active cancer."
Nonetheless, strategies and tools from the noncancer chronic pain world can be used in the cancer setting, said Dr. Koyyalagunta. These include assessment and differential diagnosis, screening, informed consent, ongoing psychiatric care, frequent outpatient visits, and documentation. "It’s pretty much the same set of principles, but adapting it to a cancer setting," she said.
Dr. Novy concurred. "For people who are at risk, we see them more frequently. We involve their families in the treatment. We encourage the use of a pill box and pill count. We also monitor with random urine screens," she said, adding that M.D. Anderson is also starting to use an opioid compliance checklist similar to the COMM (Current Opioid Misuse Measure) that was developed by Robert N. Jamison, Ph.D., at Brigham and Women’s Hospital in Boston (Pain 2007;130:144-56).
Dr. Passik reported that he has received honoraria from Cephalon Inc., King Pharmaceuticals Inc. (now part of Pfizer Inc.), Pricara (a division of Ortho-McNeil-Janssen Pharmaceuticals Inc.), and Purdue Pharma LP. Dr. Koyyalagunta and Dr. Novy both reported that they have no relevant financial relationships.
NATIONAL HARBOR, MD. – Opioid misuse, and substance abuse in general, is a growing problem among cancer patients and survivors, according to a panel of experts at the annual meeting of the American Academy of Pain Medicine.
With the number of cancer survivors – people who can live for years and decades after diagnosis – on the rise, clinicians are seeing a corresponding growth in risk for pain medication addiction, they said. In addition, earlier cancer diagnoses can mean more patients may commence cancer therapy with a history of substance abuse.
"We’re starting to see a lot more in the way of substance abuse of every stripe. ... There are a lot of folks making it to tertiary cancer care centers with a history of addiction," said Steven D. Passik, Ph.D., a professor of clinical psychiatry at Vanderbilt University in Nashville, Tenn.
He gave the example of a female patient who had a preexisting polysubstance abuse problem and lived with metastatic breast cancer for 11 years. "When I first started in this field, this woman’s life expectancy would have been measured in months, and now it’s measured in years to decades," he said, introducing the challenges of tailoring long-term pain management programs for patients who are at risk of substance abuse.
Oncologists had not dealt much with substance abuse, because cancer is primarily a disease of older people, and addiction problems tend to manifest by age 35, Dr. Passik said. Oncologists and pain management physicians "will now have to worry about a small but very labor-intensive subpopulation of the cancer population. But you have to identify them," he continued, adding that "screening for substance abuse in the cancer setting ... has still not taken root."
Screening Tool Introduced
Dr. Dhanalakshmi Koyyalagunta, a pain management specialist at the University of Texas M.D. Anderson Cancer Center in Houston, said that clinicians there started using the short form of the SOAPP (Screener and Opioid Assessment for Patients With Pain) tool a little more than 2 years ago. This is a five-question assessment. Dr. Koyyalagunta said that clinicians at M.D. Anderson use a score of 4 or more as an indication that an individual is at high risk for substance abuse.
A review of 524 charts with completed SOAPP data found that more than a quarter of cancer patients (29%) were at high risk, she said, noting that the high-risk group included 43% of patients aged 18-35 years, 27% of patients older than 35 years, 72% of smokers, 40% of those who were unemployed, 60% of those with a history of drug use, and 32% of those with a history of alcohol abuse.
Although the true prevalence of substance abuse in the overall population of long-term cancer survivors is unknown, clinicians do know that there is a high incidence of comorbid anxiety and depression, added Dr. Koyyalagunta. It is very important to address any depression, anxiety, or other comorbid psychiatric conditions along with the pain, she said: "There’s a lot of chemical coping associated with these comorbidities."
The best treatment approach is to put together a multidisciplinary team that can develop an integrated treatment plan, said Diane M. Novy, Ph.D., a professor of pain medicine at M.D. Anderson. For example, a psychologist or social worker can address coexisting conditions such as affective disorders; acute stress related to cancer and cancer treatment; family problems; and work-related issues.
No Self-Titration for Pain Management
Pain management in cancer patients, particularly in older patients, tends to rely on self-titration: Take as much as you need, observed Dr. Passik. "It doesn’t hurt low-risk people to have a self-titration model, but you can’t apply that to high-risk people. That’s the recipe for a public health disaster," he warned.
Add in the need for long-term treatment, and problems associated with opioid exposure "now have ample time to play themselves out in terrible fashion," he said. Any opioid treatment strategy that he might use in high-risk patients with an expected 3-6 months of life would be inappropriate for a high-risk patient who was likely to survive for many years, he observed.
"All of us do have trouble with how we handle these patients long term," agreed Dr. Koyyalagunta. "We see a fair number of patients, and it may not be as easy as in the chronic pain setting, where you may have an exit strategy or have an alternative plan – especially in patients with active cancer."
Nonetheless, strategies and tools from the noncancer chronic pain world can be used in the cancer setting, said Dr. Koyyalagunta. These include assessment and differential diagnosis, screening, informed consent, ongoing psychiatric care, frequent outpatient visits, and documentation. "It’s pretty much the same set of principles, but adapting it to a cancer setting," she said.
Dr. Novy concurred. "For people who are at risk, we see them more frequently. We involve their families in the treatment. We encourage the use of a pill box and pill count. We also monitor with random urine screens," she said, adding that M.D. Anderson is also starting to use an opioid compliance checklist similar to the COMM (Current Opioid Misuse Measure) that was developed by Robert N. Jamison, Ph.D., at Brigham and Women’s Hospital in Boston (Pain 2007;130:144-56).
Dr. Passik reported that he has received honoraria from Cephalon Inc., King Pharmaceuticals Inc. (now part of Pfizer Inc.), Pricara (a division of Ortho-McNeil-Janssen Pharmaceuticals Inc.), and Purdue Pharma LP. Dr. Koyyalagunta and Dr. Novy both reported that they have no relevant financial relationships.
NATIONAL HARBOR, MD. – Opioid misuse, and substance abuse in general, is a growing problem among cancer patients and survivors, according to a panel of experts at the annual meeting of the American Academy of Pain Medicine.
With the number of cancer survivors – people who can live for years and decades after diagnosis – on the rise, clinicians are seeing a corresponding growth in risk for pain medication addiction, they said. In addition, earlier cancer diagnoses can mean more patients may commence cancer therapy with a history of substance abuse.
"We’re starting to see a lot more in the way of substance abuse of every stripe. ... There are a lot of folks making it to tertiary cancer care centers with a history of addiction," said Steven D. Passik, Ph.D., a professor of clinical psychiatry at Vanderbilt University in Nashville, Tenn.
He gave the example of a female patient who had a preexisting polysubstance abuse problem and lived with metastatic breast cancer for 11 years. "When I first started in this field, this woman’s life expectancy would have been measured in months, and now it’s measured in years to decades," he said, introducing the challenges of tailoring long-term pain management programs for patients who are at risk of substance abuse.
Oncologists had not dealt much with substance abuse, because cancer is primarily a disease of older people, and addiction problems tend to manifest by age 35, Dr. Passik said. Oncologists and pain management physicians "will now have to worry about a small but very labor-intensive subpopulation of the cancer population. But you have to identify them," he continued, adding that "screening for substance abuse in the cancer setting ... has still not taken root."
Screening Tool Introduced
Dr. Dhanalakshmi Koyyalagunta, a pain management specialist at the University of Texas M.D. Anderson Cancer Center in Houston, said that clinicians there started using the short form of the SOAPP (Screener and Opioid Assessment for Patients With Pain) tool a little more than 2 years ago. This is a five-question assessment. Dr. Koyyalagunta said that clinicians at M.D. Anderson use a score of 4 or more as an indication that an individual is at high risk for substance abuse.
A review of 524 charts with completed SOAPP data found that more than a quarter of cancer patients (29%) were at high risk, she said, noting that the high-risk group included 43% of patients aged 18-35 years, 27% of patients older than 35 years, 72% of smokers, 40% of those who were unemployed, 60% of those with a history of drug use, and 32% of those with a history of alcohol abuse.
Although the true prevalence of substance abuse in the overall population of long-term cancer survivors is unknown, clinicians do know that there is a high incidence of comorbid anxiety and depression, added Dr. Koyyalagunta. It is very important to address any depression, anxiety, or other comorbid psychiatric conditions along with the pain, she said: "There’s a lot of chemical coping associated with these comorbidities."
The best treatment approach is to put together a multidisciplinary team that can develop an integrated treatment plan, said Diane M. Novy, Ph.D., a professor of pain medicine at M.D. Anderson. For example, a psychologist or social worker can address coexisting conditions such as affective disorders; acute stress related to cancer and cancer treatment; family problems; and work-related issues.
No Self-Titration for Pain Management
Pain management in cancer patients, particularly in older patients, tends to rely on self-titration: Take as much as you need, observed Dr. Passik. "It doesn’t hurt low-risk people to have a self-titration model, but you can’t apply that to high-risk people. That’s the recipe for a public health disaster," he warned.
Add in the need for long-term treatment, and problems associated with opioid exposure "now have ample time to play themselves out in terrible fashion," he said. Any opioid treatment strategy that he might use in high-risk patients with an expected 3-6 months of life would be inappropriate for a high-risk patient who was likely to survive for many years, he observed.
"All of us do have trouble with how we handle these patients long term," agreed Dr. Koyyalagunta. "We see a fair number of patients, and it may not be as easy as in the chronic pain setting, where you may have an exit strategy or have an alternative plan – especially in patients with active cancer."
Nonetheless, strategies and tools from the noncancer chronic pain world can be used in the cancer setting, said Dr. Koyyalagunta. These include assessment and differential diagnosis, screening, informed consent, ongoing psychiatric care, frequent outpatient visits, and documentation. "It’s pretty much the same set of principles, but adapting it to a cancer setting," she said.
Dr. Novy concurred. "For people who are at risk, we see them more frequently. We involve their families in the treatment. We encourage the use of a pill box and pill count. We also monitor with random urine screens," she said, adding that M.D. Anderson is also starting to use an opioid compliance checklist similar to the COMM (Current Opioid Misuse Measure) that was developed by Robert N. Jamison, Ph.D., at Brigham and Women’s Hospital in Boston (Pain 2007;130:144-56).
Dr. Passik reported that he has received honoraria from Cephalon Inc., King Pharmaceuticals Inc. (now part of Pfizer Inc.), Pricara (a division of Ortho-McNeil-Janssen Pharmaceuticals Inc.), and Purdue Pharma LP. Dr. Koyyalagunta and Dr. Novy both reported that they have no relevant financial relationships.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF PAIN MEDICINE
FDA Keeping an Eye on New Malignancy Concerns With Lenalidomide
The Food and Drug Administration has alerted the public that the agency is currently reviewing all available information on the potential for increased risk of new malignancies associated with lenalidomide in patients treated for multiple myeloma or myelodysplatic syndromes.
The agency plans to communicate any new recommendations once it has completed its review of existing data, according to a safety announcement released on April 8, 2011. "At this time, [the] FDA recommends that patients continue their Revlimid [lenalidomide] treatment as prescribed by their health care provider," it said.
The concerns appear to be based in part on results from the phase III Cancer and Leukemia Group B (CALGB) 100104 trial of 460 patients with stage I-III multiple myeloma. In the trial, the estimated time to progression reached 42.3 months with lenalidomide maintenance following transplant vs. 21.8 months with placebo. (The results were reported at the 2010 annual meeting of the American Society of Hematology.)
As of late 2010, though, 25 patients had new malignancies: 15 patients in the lenalidomide group, 6 on placebo, and 4 who developed these before randomization. The second cancers included five cases of acute myeloid leukemia or myelodysplastic syndrome, three of which occurred in patients on lenalidomide maintenance.
Lenalidomide, a less-toxic thalidomide analogue, is one of the more important new therapies in multiple myeloma. In addition to the CALGB trial, results from the Intergroupe Francophone du Myélome (IFM) 2005-02 trial also support maintenance lenalidomide.
Lenalidomide is indicated for the treatment of multiple myeloma, in combination with dexamethasone, in patients who have received at least one prior therapy. It is also indicated for patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"At this time, there is no recommendation to delay, modify, or restrict the use of Revlimid for patients being treated according to the FDA-approved indications," the agency noted. "[The] FDA believes the benefits of Revlimid continue to outweigh the potential risks."
The FDA is also currently reviewing all available information on this potential risk for thalidomide.
Physicians are encouraged to report adverse events involving lenalidomide to the FDA MedWatch program.
Cancer and Leukemia Group B, CALGB,
The Food and Drug Administration has alerted the public that the agency is currently reviewing all available information on the potential for increased risk of new malignancies associated with lenalidomide in patients treated for multiple myeloma or myelodysplatic syndromes.
The agency plans to communicate any new recommendations once it has completed its review of existing data, according to a safety announcement released on April 8, 2011. "At this time, [the] FDA recommends that patients continue their Revlimid [lenalidomide] treatment as prescribed by their health care provider," it said.
The concerns appear to be based in part on results from the phase III Cancer and Leukemia Group B (CALGB) 100104 trial of 460 patients with stage I-III multiple myeloma. In the trial, the estimated time to progression reached 42.3 months with lenalidomide maintenance following transplant vs. 21.8 months with placebo. (The results were reported at the 2010 annual meeting of the American Society of Hematology.)
As of late 2010, though, 25 patients had new malignancies: 15 patients in the lenalidomide group, 6 on placebo, and 4 who developed these before randomization. The second cancers included five cases of acute myeloid leukemia or myelodysplastic syndrome, three of which occurred in patients on lenalidomide maintenance.
Lenalidomide, a less-toxic thalidomide analogue, is one of the more important new therapies in multiple myeloma. In addition to the CALGB trial, results from the Intergroupe Francophone du Myélome (IFM) 2005-02 trial also support maintenance lenalidomide.
Lenalidomide is indicated for the treatment of multiple myeloma, in combination with dexamethasone, in patients who have received at least one prior therapy. It is also indicated for patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"At this time, there is no recommendation to delay, modify, or restrict the use of Revlimid for patients being treated according to the FDA-approved indications," the agency noted. "[The] FDA believes the benefits of Revlimid continue to outweigh the potential risks."
The FDA is also currently reviewing all available information on this potential risk for thalidomide.
Physicians are encouraged to report adverse events involving lenalidomide to the FDA MedWatch program.
The Food and Drug Administration has alerted the public that the agency is currently reviewing all available information on the potential for increased risk of new malignancies associated with lenalidomide in patients treated for multiple myeloma or myelodysplatic syndromes.
The agency plans to communicate any new recommendations once it has completed its review of existing data, according to a safety announcement released on April 8, 2011. "At this time, [the] FDA recommends that patients continue their Revlimid [lenalidomide] treatment as prescribed by their health care provider," it said.
The concerns appear to be based in part on results from the phase III Cancer and Leukemia Group B (CALGB) 100104 trial of 460 patients with stage I-III multiple myeloma. In the trial, the estimated time to progression reached 42.3 months with lenalidomide maintenance following transplant vs. 21.8 months with placebo. (The results were reported at the 2010 annual meeting of the American Society of Hematology.)
As of late 2010, though, 25 patients had new malignancies: 15 patients in the lenalidomide group, 6 on placebo, and 4 who developed these before randomization. The second cancers included five cases of acute myeloid leukemia or myelodysplastic syndrome, three of which occurred in patients on lenalidomide maintenance.
Lenalidomide, a less-toxic thalidomide analogue, is one of the more important new therapies in multiple myeloma. In addition to the CALGB trial, results from the Intergroupe Francophone du Myélome (IFM) 2005-02 trial also support maintenance lenalidomide.
Lenalidomide is indicated for the treatment of multiple myeloma, in combination with dexamethasone, in patients who have received at least one prior therapy. It is also indicated for patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"At this time, there is no recommendation to delay, modify, or restrict the use of Revlimid for patients being treated according to the FDA-approved indications," the agency noted. "[The] FDA believes the benefits of Revlimid continue to outweigh the potential risks."
The FDA is also currently reviewing all available information on this potential risk for thalidomide.
Physicians are encouraged to report adverse events involving lenalidomide to the FDA MedWatch program.
Cancer and Leukemia Group B, CALGB,
Cancer and Leukemia Group B, CALGB,
FDA Keeping an Eye on New Malignancy Concerns With Lenalidomide
The Food and Drug Administration has alerted the public that the agency is currently reviewing all available information on the potential for increased risk of new malignancies associated with lenalidomide in patients treated for multiple myeloma or myelodysplatic syndromes.
The agency plans to communicate any new recommendations once it has completed its review of existing data, according to a safety announcement released on April 8, 2011. "At this time, [the] FDA recommends that patients continue their Revlimid [lenalidomide] treatment as prescribed by their health care provider," it said.
The concerns appear to be based in part on results from the phase III Cancer and Leukemia Group B (CALGB) 100104 trial of 460 patients with stage I-III multiple myeloma. In the trial, the estimated time to progression reached 42.3 months with lenalidomide maintenance following transplant vs. 21.8 months with placebo. (The results were reported at the 2010 annual meeting of the American Society of Hematology.)
As of late 2010, though, 25 patients had new malignancies: 15 patients in the lenalidomide group, 6 on placebo, and 4 who developed these before randomization. The second cancers included five cases of acute myeloid leukemia or myelodysplastic syndrome, three of which occurred in patients on lenalidomide maintenance.
Lenalidomide, a less-toxic thalidomide analogue, is one of the more important new therapies in multiple myeloma. In addition to the CALGB trial, results from the Intergroupe Francophone du Myélome (IFM) 2005-02 trial also support maintenance lenalidomide.
Lenalidomide is indicated for the treatment of multiple myeloma, in combination with dexamethasone, in patients who have received at least one prior therapy. It is also indicated for patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"At this time, there is no recommendation to delay, modify, or restrict the use of Revlimid for patients being treated according to the FDA-approved indications," the agency noted. "[The] FDA believes the benefits of Revlimid continue to outweigh the potential risks."
The FDA is also currently reviewing all available information on this potential risk for thalidomide.
Physicians are encouraged to report adverse events involving lenalidomide to the FDA MedWatch program.
Cancer and Leukemia Group B, CALGB,
The Food and Drug Administration has alerted the public that the agency is currently reviewing all available information on the potential for increased risk of new malignancies associated with lenalidomide in patients treated for multiple myeloma or myelodysplatic syndromes.
The agency plans to communicate any new recommendations once it has completed its review of existing data, according to a safety announcement released on April 8, 2011. "At this time, [the] FDA recommends that patients continue their Revlimid [lenalidomide] treatment as prescribed by their health care provider," it said.
The concerns appear to be based in part on results from the phase III Cancer and Leukemia Group B (CALGB) 100104 trial of 460 patients with stage I-III multiple myeloma. In the trial, the estimated time to progression reached 42.3 months with lenalidomide maintenance following transplant vs. 21.8 months with placebo. (The results were reported at the 2010 annual meeting of the American Society of Hematology.)
As of late 2010, though, 25 patients had new malignancies: 15 patients in the lenalidomide group, 6 on placebo, and 4 who developed these before randomization. The second cancers included five cases of acute myeloid leukemia or myelodysplastic syndrome, three of which occurred in patients on lenalidomide maintenance.
Lenalidomide, a less-toxic thalidomide analogue, is one of the more important new therapies in multiple myeloma. In addition to the CALGB trial, results from the Intergroupe Francophone du Myélome (IFM) 2005-02 trial also support maintenance lenalidomide.
Lenalidomide is indicated for the treatment of multiple myeloma, in combination with dexamethasone, in patients who have received at least one prior therapy. It is also indicated for patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"At this time, there is no recommendation to delay, modify, or restrict the use of Revlimid for patients being treated according to the FDA-approved indications," the agency noted. "[The] FDA believes the benefits of Revlimid continue to outweigh the potential risks."
The FDA is also currently reviewing all available information on this potential risk for thalidomide.
Physicians are encouraged to report adverse events involving lenalidomide to the FDA MedWatch program.
The Food and Drug Administration has alerted the public that the agency is currently reviewing all available information on the potential for increased risk of new malignancies associated with lenalidomide in patients treated for multiple myeloma or myelodysplatic syndromes.
The agency plans to communicate any new recommendations once it has completed its review of existing data, according to a safety announcement released on April 8, 2011. "At this time, [the] FDA recommends that patients continue their Revlimid [lenalidomide] treatment as prescribed by their health care provider," it said.
The concerns appear to be based in part on results from the phase III Cancer and Leukemia Group B (CALGB) 100104 trial of 460 patients with stage I-III multiple myeloma. In the trial, the estimated time to progression reached 42.3 months with lenalidomide maintenance following transplant vs. 21.8 months with placebo. (The results were reported at the 2010 annual meeting of the American Society of Hematology.)
As of late 2010, though, 25 patients had new malignancies: 15 patients in the lenalidomide group, 6 on placebo, and 4 who developed these before randomization. The second cancers included five cases of acute myeloid leukemia or myelodysplastic syndrome, three of which occurred in patients on lenalidomide maintenance.
Lenalidomide, a less-toxic thalidomide analogue, is one of the more important new therapies in multiple myeloma. In addition to the CALGB trial, results from the Intergroupe Francophone du Myélome (IFM) 2005-02 trial also support maintenance lenalidomide.
Lenalidomide is indicated for the treatment of multiple myeloma, in combination with dexamethasone, in patients who have received at least one prior therapy. It is also indicated for patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
"At this time, there is no recommendation to delay, modify, or restrict the use of Revlimid for patients being treated according to the FDA-approved indications," the agency noted. "[The] FDA believes the benefits of Revlimid continue to outweigh the potential risks."
The FDA is also currently reviewing all available information on this potential risk for thalidomide.
Physicians are encouraged to report adverse events involving lenalidomide to the FDA MedWatch program.
Cancer and Leukemia Group B, CALGB,
Cancer and Leukemia Group B, CALGB,