User login
Abortion Rate Holds Steady in 2008; Early Medication Abortions Increase
While the U.S. abortion rate leveled out between 2005 and 2008, the number of early medication abortions increased over the same period, according to a report released online Jan. 11 by the Guttmacher Institute.
In 2008 there were 19.6 abortions per 1,000 women aged 15-44 years, compared with 19.4 abortions per 1,000 women in 2005 – a 1% change. The total number of abortions in 2008 was virtually identical to the number in 2005 – 1.21 million, according to the report (Perspect. Sex. Reprod. Health 2011 [doi:10.1363/4304111]).
The new data mark the end of a long-term decline in abortion rates, dating back to the 1981 peak of 29.3 abortions for every 1,000 women, lead author Rachel K. Jones said during the teleconference.
"The long-term decline in abortion has stalled. We’re not going to say that that’s unilaterally a bad thing. If it’s an indicator that more women are having access to abortion, this would be good," she said.
However, the abortion ratio has not changed from 2005 to 2008 (22 abortions per 100 pregnancies). "So what this actually suggests is that we’re not making progress in helping women avoid unintended pregnancies," Ms. Jones said.
Interestingly, the number of medication abortions has gone up. The use of mifepristone (Mifeprix) in combination with misoprostol to terminate early pregnancies increased from 161,000 in 2005 to 199,000 in 2008.
The proportion of all nonhospital abortions performed as early medication procedures rose from 14% in 2005 to 17% in 2008. Mifepristone is indicated for the medical termination of pregnancy in the first 7 weeks.
"Early medical abortion has become an integral part of abortion care," said Ms. Jones. "This provides women with options."
The researchers also estimated that a quarter of early abortions (within the first 9 weeks) were medication abortions. In addition, more than half of all abortion facilities (59%) offered medication abortions in 2008.
"Our survey also suggests that mifepristone may be increasing access to abortion services," said Ms. Jones, who is a senior research associate at the Guttmacher Institute, New York.
The number of providers who offer only medication abortions increased from 119 in 2005 to 164 in 2008. If medication-only providers are in areas were there are no surgical abortion providers, this could increase access for women, she noted.
Medical abortions also may account for increasing rates of very early abortions. The percentage of early abortions (first trimester) has not changed much in the last 2 decades – hovering around 88%. However, "we’re seeing more and more very early abortions – abortions at 6 weeks or earlier," said Ms. Jones. "Our expectation is that mifepristone is contributing to that trend."
The number of providers offering abortion is virtually unchanged since 2005 (1,787 vs. 1,793 in 2008), according to the report, "Abortion Incidence and Access to Services in the United States, 2008."
A majority of U.S. counties (87%) have no abortion providers; 35% of women of reproductive age live in those counties.
Ms. Jones and her associate, Kathryn Kooistra of the State University of New York Downstate, Brooklyn, N.Y., said they had no relevant financial disclosures.
While the U.S. abortion rate leveled out between 2005 and 2008, the number of early medication abortions increased over the same period, according to a report released online Jan. 11 by the Guttmacher Institute.
In 2008 there were 19.6 abortions per 1,000 women aged 15-44 years, compared with 19.4 abortions per 1,000 women in 2005 – a 1% change. The total number of abortions in 2008 was virtually identical to the number in 2005 – 1.21 million, according to the report (Perspect. Sex. Reprod. Health 2011 [doi:10.1363/4304111]).
The new data mark the end of a long-term decline in abortion rates, dating back to the 1981 peak of 29.3 abortions for every 1,000 women, lead author Rachel K. Jones said during the teleconference.
"The long-term decline in abortion has stalled. We’re not going to say that that’s unilaterally a bad thing. If it’s an indicator that more women are having access to abortion, this would be good," she said.
However, the abortion ratio has not changed from 2005 to 2008 (22 abortions per 100 pregnancies). "So what this actually suggests is that we’re not making progress in helping women avoid unintended pregnancies," Ms. Jones said.
Interestingly, the number of medication abortions has gone up. The use of mifepristone (Mifeprix) in combination with misoprostol to terminate early pregnancies increased from 161,000 in 2005 to 199,000 in 2008.
The proportion of all nonhospital abortions performed as early medication procedures rose from 14% in 2005 to 17% in 2008. Mifepristone is indicated for the medical termination of pregnancy in the first 7 weeks.
"Early medical abortion has become an integral part of abortion care," said Ms. Jones. "This provides women with options."
The researchers also estimated that a quarter of early abortions (within the first 9 weeks) were medication abortions. In addition, more than half of all abortion facilities (59%) offered medication abortions in 2008.
"Our survey also suggests that mifepristone may be increasing access to abortion services," said Ms. Jones, who is a senior research associate at the Guttmacher Institute, New York.
The number of providers who offer only medication abortions increased from 119 in 2005 to 164 in 2008. If medication-only providers are in areas were there are no surgical abortion providers, this could increase access for women, she noted.
Medical abortions also may account for increasing rates of very early abortions. The percentage of early abortions (first trimester) has not changed much in the last 2 decades – hovering around 88%. However, "we’re seeing more and more very early abortions – abortions at 6 weeks or earlier," said Ms. Jones. "Our expectation is that mifepristone is contributing to that trend."
The number of providers offering abortion is virtually unchanged since 2005 (1,787 vs. 1,793 in 2008), according to the report, "Abortion Incidence and Access to Services in the United States, 2008."
A majority of U.S. counties (87%) have no abortion providers; 35% of women of reproductive age live in those counties.
Ms. Jones and her associate, Kathryn Kooistra of the State University of New York Downstate, Brooklyn, N.Y., said they had no relevant financial disclosures.
While the U.S. abortion rate leveled out between 2005 and 2008, the number of early medication abortions increased over the same period, according to a report released online Jan. 11 by the Guttmacher Institute.
In 2008 there were 19.6 abortions per 1,000 women aged 15-44 years, compared with 19.4 abortions per 1,000 women in 2005 – a 1% change. The total number of abortions in 2008 was virtually identical to the number in 2005 – 1.21 million, according to the report (Perspect. Sex. Reprod. Health 2011 [doi:10.1363/4304111]).
The new data mark the end of a long-term decline in abortion rates, dating back to the 1981 peak of 29.3 abortions for every 1,000 women, lead author Rachel K. Jones said during the teleconference.
"The long-term decline in abortion has stalled. We’re not going to say that that’s unilaterally a bad thing. If it’s an indicator that more women are having access to abortion, this would be good," she said.
However, the abortion ratio has not changed from 2005 to 2008 (22 abortions per 100 pregnancies). "So what this actually suggests is that we’re not making progress in helping women avoid unintended pregnancies," Ms. Jones said.
Interestingly, the number of medication abortions has gone up. The use of mifepristone (Mifeprix) in combination with misoprostol to terminate early pregnancies increased from 161,000 in 2005 to 199,000 in 2008.
The proportion of all nonhospital abortions performed as early medication procedures rose from 14% in 2005 to 17% in 2008. Mifepristone is indicated for the medical termination of pregnancy in the first 7 weeks.
"Early medical abortion has become an integral part of abortion care," said Ms. Jones. "This provides women with options."
The researchers also estimated that a quarter of early abortions (within the first 9 weeks) were medication abortions. In addition, more than half of all abortion facilities (59%) offered medication abortions in 2008.
"Our survey also suggests that mifepristone may be increasing access to abortion services," said Ms. Jones, who is a senior research associate at the Guttmacher Institute, New York.
The number of providers who offer only medication abortions increased from 119 in 2005 to 164 in 2008. If medication-only providers are in areas were there are no surgical abortion providers, this could increase access for women, she noted.
Medical abortions also may account for increasing rates of very early abortions. The percentage of early abortions (first trimester) has not changed much in the last 2 decades – hovering around 88%. However, "we’re seeing more and more very early abortions – abortions at 6 weeks or earlier," said Ms. Jones. "Our expectation is that mifepristone is contributing to that trend."
The number of providers offering abortion is virtually unchanged since 2005 (1,787 vs. 1,793 in 2008), according to the report, "Abortion Incidence and Access to Services in the United States, 2008."
A majority of U.S. counties (87%) have no abortion providers; 35% of women of reproductive age live in those counties.
Ms. Jones and her associate, Kathryn Kooistra of the State University of New York Downstate, Brooklyn, N.Y., said they had no relevant financial disclosures.
TELECONFERENCE HELD BY THE GUTTMACHER INSTITUTE
Fentanyl Transmucosal Tablets Approved for Breakthrough Cancer Pain
The Food and Drug Administration’s approval of immediate-release Abstral (fentanyl) transmucosal tablets provides a new option for managing breakthrough pain for adults with cancer.
Approved on Jan. 7, the drug is indicated for the management of breakthrough pain in patients aged 18 years and older with cancer, who already use around-the-clock opioid pain medication and who need and are able to safely use high doses of an additional opioid medicine.
Breakthrough pain is defined as pain that comes on suddenly for short periods of time and is not alleviated by a patient’s usual pain management plan. These patients should be considered opioid tolerant because of their current opioid medication use, according to a news release from the agency.
Immediate-release transmucosal medications, such as Abstral, are administered on the soft surfaces of the mouth, the nasal passages or throat where they dissolve and are absorbed.
The approval comes with the condition that the drug only be made available through through a Risk Evaluation and Mitigation Strategy (REMS) program, in order to minimize the risk of misuse, abuse, addiction, and overdose. Fentanyl is a schedule II opioid under the Controlled Substances Act.
The program requires pharmacies, distributors, and health care professionals who prescribe to outpatients to enroll in the program in order to prescribe, dispense, and distribute this product.
Common adverse reactions include nausea, constipation, drowsiness, and headache. Serious adverse events, including deaths, have been reported in patients with other immediate-release transmucosal fentanyl products, according to the FDA. The deaths occurred as a result of improper patient selection and/or improper dosing.
Health care professionals are encouraged to report adverse side effects or medication errors from the use of this drug to the FDA’s MedWatch Adverse Event Reporting program or by calling 800-332-1088.
Abstral is manufacturedby ProStrakan Inc.
The Food and Drug Administration’s approval of immediate-release Abstral (fentanyl) transmucosal tablets provides a new option for managing breakthrough pain for adults with cancer.
Approved on Jan. 7, the drug is indicated for the management of breakthrough pain in patients aged 18 years and older with cancer, who already use around-the-clock opioid pain medication and who need and are able to safely use high doses of an additional opioid medicine.
Breakthrough pain is defined as pain that comes on suddenly for short periods of time and is not alleviated by a patient’s usual pain management plan. These patients should be considered opioid tolerant because of their current opioid medication use, according to a news release from the agency.
Immediate-release transmucosal medications, such as Abstral, are administered on the soft surfaces of the mouth, the nasal passages or throat where they dissolve and are absorbed.
The approval comes with the condition that the drug only be made available through through a Risk Evaluation and Mitigation Strategy (REMS) program, in order to minimize the risk of misuse, abuse, addiction, and overdose. Fentanyl is a schedule II opioid under the Controlled Substances Act.
The program requires pharmacies, distributors, and health care professionals who prescribe to outpatients to enroll in the program in order to prescribe, dispense, and distribute this product.
Common adverse reactions include nausea, constipation, drowsiness, and headache. Serious adverse events, including deaths, have been reported in patients with other immediate-release transmucosal fentanyl products, according to the FDA. The deaths occurred as a result of improper patient selection and/or improper dosing.
Health care professionals are encouraged to report adverse side effects or medication errors from the use of this drug to the FDA’s MedWatch Adverse Event Reporting program or by calling 800-332-1088.
Abstral is manufacturedby ProStrakan Inc.
The Food and Drug Administration’s approval of immediate-release Abstral (fentanyl) transmucosal tablets provides a new option for managing breakthrough pain for adults with cancer.
Approved on Jan. 7, the drug is indicated for the management of breakthrough pain in patients aged 18 years and older with cancer, who already use around-the-clock opioid pain medication and who need and are able to safely use high doses of an additional opioid medicine.
Breakthrough pain is defined as pain that comes on suddenly for short periods of time and is not alleviated by a patient’s usual pain management plan. These patients should be considered opioid tolerant because of their current opioid medication use, according to a news release from the agency.
Immediate-release transmucosal medications, such as Abstral, are administered on the soft surfaces of the mouth, the nasal passages or throat where they dissolve and are absorbed.
The approval comes with the condition that the drug only be made available through through a Risk Evaluation and Mitigation Strategy (REMS) program, in order to minimize the risk of misuse, abuse, addiction, and overdose. Fentanyl is a schedule II opioid under the Controlled Substances Act.
The program requires pharmacies, distributors, and health care professionals who prescribe to outpatients to enroll in the program in order to prescribe, dispense, and distribute this product.
Common adverse reactions include nausea, constipation, drowsiness, and headache. Serious adverse events, including deaths, have been reported in patients with other immediate-release transmucosal fentanyl products, according to the FDA. The deaths occurred as a result of improper patient selection and/or improper dosing.
Health care professionals are encouraged to report adverse side effects or medication errors from the use of this drug to the FDA’s MedWatch Adverse Event Reporting program or by calling 800-332-1088.
Abstral is manufacturedby ProStrakan Inc.
From the Food and Drug Administration
Fentanyl Transmucosal Tablets Approved for Breakthrough Cancer Pain
The Food and Drug Administration’s approval of immediate-release Abstral (fentanyl) transmucosal tablets provides a new option for managing breakthrough pain for adults with cancer.
Approved on Jan. 7, the drug is indicated for the management of breakthrough pain in patients aged 18 years and older with cancer, who already use around-the-clock opioid pain medication and who need and are able to safely use high doses of an additional opioid medicine.
Breakthrough pain is defined as pain that comes on suddenly for short periods of time and is not alleviated by a patient’s usual pain management plan. These patients should be considered opioid tolerant because of their current opioid medication use, according to a news release from the agency.
Immediate-release transmucosal medications, such as Abstral, are administered on the soft surfaces of the mouth, the nasal passages or throat where they dissolve and are absorbed.
The approval comes with the condition that the drug only be made available through through a Risk Evaluation and Mitigation Strategy (REMS) program, in order to minimize the risk of misuse, abuse, addiction, and overdose. Fentanyl is a schedule II opioid under the Controlled Substances Act.
The program requires pharmacies, distributors, and health care professionals who prescribe to outpatients to enroll in the program in order to prescribe, dispense, and distribute this product.
Common adverse reactions include nausea, constipation, drowsiness, and headache. Serious adverse events, including deaths, have been reported in patients with other immediate-release transmucosal fentanyl products, according to the FDA. The deaths occurred as a result of improper patient selection and/or improper dosing.
Health care professionals are encouraged to report adverse side effects or medication errors from the use of this drug to the FDA’s MedWatch Adverse Event Reporting program or by calling 800-332-1088.
Abstral is manufacturedby ProStrakan Inc.
The Food and Drug Administration’s approval of immediate-release Abstral (fentanyl) transmucosal tablets provides a new option for managing breakthrough pain for adults with cancer.
Approved on Jan. 7, the drug is indicated for the management of breakthrough pain in patients aged 18 years and older with cancer, who already use around-the-clock opioid pain medication and who need and are able to safely use high doses of an additional opioid medicine.
Breakthrough pain is defined as pain that comes on suddenly for short periods of time and is not alleviated by a patient’s usual pain management plan. These patients should be considered opioid tolerant because of their current opioid medication use, according to a news release from the agency.
Immediate-release transmucosal medications, such as Abstral, are administered on the soft surfaces of the mouth, the nasal passages or throat where they dissolve and are absorbed.
The approval comes with the condition that the drug only be made available through through a Risk Evaluation and Mitigation Strategy (REMS) program, in order to minimize the risk of misuse, abuse, addiction, and overdose. Fentanyl is a schedule II opioid under the Controlled Substances Act.
The program requires pharmacies, distributors, and health care professionals who prescribe to outpatients to enroll in the program in order to prescribe, dispense, and distribute this product.
Common adverse reactions include nausea, constipation, drowsiness, and headache. Serious adverse events, including deaths, have been reported in patients with other immediate-release transmucosal fentanyl products, according to the FDA. The deaths occurred as a result of improper patient selection and/or improper dosing.
Health care professionals are encouraged to report adverse side effects or medication errors from the use of this drug to the FDA’s MedWatch Adverse Event Reporting program or by calling 800-332-1088.
Abstral is manufacturedby ProStrakan Inc.
The Food and Drug Administration’s approval of immediate-release Abstral (fentanyl) transmucosal tablets provides a new option for managing breakthrough pain for adults with cancer.
Approved on Jan. 7, the drug is indicated for the management of breakthrough pain in patients aged 18 years and older with cancer, who already use around-the-clock opioid pain medication and who need and are able to safely use high doses of an additional opioid medicine.
Breakthrough pain is defined as pain that comes on suddenly for short periods of time and is not alleviated by a patient’s usual pain management plan. These patients should be considered opioid tolerant because of their current opioid medication use, according to a news release from the agency.
Immediate-release transmucosal medications, such as Abstral, are administered on the soft surfaces of the mouth, the nasal passages or throat where they dissolve and are absorbed.
The approval comes with the condition that the drug only be made available through through a Risk Evaluation and Mitigation Strategy (REMS) program, in order to minimize the risk of misuse, abuse, addiction, and overdose. Fentanyl is a schedule II opioid under the Controlled Substances Act.
The program requires pharmacies, distributors, and health care professionals who prescribe to outpatients to enroll in the program in order to prescribe, dispense, and distribute this product.
Common adverse reactions include nausea, constipation, drowsiness, and headache. Serious adverse events, including deaths, have been reported in patients with other immediate-release transmucosal fentanyl products, according to the FDA. The deaths occurred as a result of improper patient selection and/or improper dosing.
Health care professionals are encouraged to report adverse side effects or medication errors from the use of this drug to the FDA’s MedWatch Adverse Event Reporting program or by calling 800-332-1088.
Abstral is manufacturedby ProStrakan Inc.
From the Food and Drug Administration
Arthrodesis Seems to Be Effective in Midfoot Arthritis
Major Finding: Union occurred in 92% of 104 feet after the primary operation, and fusion after revision occurred in 99%. Most of the patients (90%) were satisfied with the results.
Data Source: Retrospective study of 95 patients with midfoot arthritis.
Disclosures: Dr. Nemec and his coinvestigators reported that they have no relevant financial relationships.
NATIONAL HARBOR, MD. ' Arthrodesis is a safe procedure for midfoot arthritis, with an excellent union rate and high patient satisfaction, according to findings in a small, retrospective study.
Union was achieved in 92% of 104 feet after the primary operation, and fusion after revision was achieved in 99%. In addition, most of the patients (90%) were satisfied with the results of their procedures, reported Dr. Scott A. Nemec.
Patients included in the study had primary midfoot arthritis with or without radiologic or clinical arch collapse. The researchers identified 95 patients (104 feet) who had undergone arthrodesis. Most were women and their mean age was 61 years. The primary indication for surgery was disabling foot pain that was not relieved by other measures. On radiograph, pre-and postoperative measurements were made of the anteroposterior talus and first metatarsal angle, the lateral talus and first metatarsal angle, the medial cuneiform height, and the talonavicular uncoverage.
Other outcomes included complications and reoperations, the AOFAS midfoot score, the visual analog scale pain score (0-10), and patient satisfaction.
In all, 297 joints were fused – roughly 3 per patient. Gastrocnemius recession was performed in 80% of the feet, and 56% had a forefoot procedure. In terms of bone grafts used, autograft was used in 91% of the feet, allograft in 4%, and no grafts were used for 5%. The most commonly fused joints were the first, second, and third tarsometatarsals and the naviculocuneiform. No further surgery was required for one asymptomatic third TMT nonunion. One delayed union was consolidated with immobilization. Radiographically, improvement was seen in all parameters.
Major complications included three deep infections and one instance of chronic pain. The infections were treated with debridement. The patient with chronic pain was referred to a pain clinic.
There were 11 reoperations—7 for refusion, 3 for debridement, and 1 gastrocnemius recession. Four of the refusion patients and one debridement patient were not satisfied with their results. Hardware removal was required for a quarter of the feet at an average of 20 months, said Dr. Nemec, who is an orthopedic specialist in private practice in Petoskey, Mich.
Patient-reported outcome data were available for 68 patients (74 feet), with a mean follow-up of 56 months. The visual analog scale pain score dropped by a significant average of 4.6 points after surgery. The AOFAS score increased by a significant 47 points (maximum 100).
Major Finding: Union occurred in 92% of 104 feet after the primary operation, and fusion after revision occurred in 99%. Most of the patients (90%) were satisfied with the results.
Data Source: Retrospective study of 95 patients with midfoot arthritis.
Disclosures: Dr. Nemec and his coinvestigators reported that they have no relevant financial relationships.
NATIONAL HARBOR, MD. ' Arthrodesis is a safe procedure for midfoot arthritis, with an excellent union rate and high patient satisfaction, according to findings in a small, retrospective study.
Union was achieved in 92% of 104 feet after the primary operation, and fusion after revision was achieved in 99%. In addition, most of the patients (90%) were satisfied with the results of their procedures, reported Dr. Scott A. Nemec.
Patients included in the study had primary midfoot arthritis with or without radiologic or clinical arch collapse. The researchers identified 95 patients (104 feet) who had undergone arthrodesis. Most were women and their mean age was 61 years. The primary indication for surgery was disabling foot pain that was not relieved by other measures. On radiograph, pre-and postoperative measurements were made of the anteroposterior talus and first metatarsal angle, the lateral talus and first metatarsal angle, the medial cuneiform height, and the talonavicular uncoverage.
Other outcomes included complications and reoperations, the AOFAS midfoot score, the visual analog scale pain score (0-10), and patient satisfaction.
In all, 297 joints were fused – roughly 3 per patient. Gastrocnemius recession was performed in 80% of the feet, and 56% had a forefoot procedure. In terms of bone grafts used, autograft was used in 91% of the feet, allograft in 4%, and no grafts were used for 5%. The most commonly fused joints were the first, second, and third tarsometatarsals and the naviculocuneiform. No further surgery was required for one asymptomatic third TMT nonunion. One delayed union was consolidated with immobilization. Radiographically, improvement was seen in all parameters.
Major complications included three deep infections and one instance of chronic pain. The infections were treated with debridement. The patient with chronic pain was referred to a pain clinic.
There were 11 reoperations—7 for refusion, 3 for debridement, and 1 gastrocnemius recession. Four of the refusion patients and one debridement patient were not satisfied with their results. Hardware removal was required for a quarter of the feet at an average of 20 months, said Dr. Nemec, who is an orthopedic specialist in private practice in Petoskey, Mich.
Patient-reported outcome data were available for 68 patients (74 feet), with a mean follow-up of 56 months. The visual analog scale pain score dropped by a significant average of 4.6 points after surgery. The AOFAS score increased by a significant 47 points (maximum 100).
Major Finding: Union occurred in 92% of 104 feet after the primary operation, and fusion after revision occurred in 99%. Most of the patients (90%) were satisfied with the results.
Data Source: Retrospective study of 95 patients with midfoot arthritis.
Disclosures: Dr. Nemec and his coinvestigators reported that they have no relevant financial relationships.
NATIONAL HARBOR, MD. ' Arthrodesis is a safe procedure for midfoot arthritis, with an excellent union rate and high patient satisfaction, according to findings in a small, retrospective study.
Union was achieved in 92% of 104 feet after the primary operation, and fusion after revision was achieved in 99%. In addition, most of the patients (90%) were satisfied with the results of their procedures, reported Dr. Scott A. Nemec.
Patients included in the study had primary midfoot arthritis with or without radiologic or clinical arch collapse. The researchers identified 95 patients (104 feet) who had undergone arthrodesis. Most were women and their mean age was 61 years. The primary indication for surgery was disabling foot pain that was not relieved by other measures. On radiograph, pre-and postoperative measurements were made of the anteroposterior talus and first metatarsal angle, the lateral talus and first metatarsal angle, the medial cuneiform height, and the talonavicular uncoverage.
Other outcomes included complications and reoperations, the AOFAS midfoot score, the visual analog scale pain score (0-10), and patient satisfaction.
In all, 297 joints were fused – roughly 3 per patient. Gastrocnemius recession was performed in 80% of the feet, and 56% had a forefoot procedure. In terms of bone grafts used, autograft was used in 91% of the feet, allograft in 4%, and no grafts were used for 5%. The most commonly fused joints were the first, second, and third tarsometatarsals and the naviculocuneiform. No further surgery was required for one asymptomatic third TMT nonunion. One delayed union was consolidated with immobilization. Radiographically, improvement was seen in all parameters.
Major complications included three deep infections and one instance of chronic pain. The infections were treated with debridement. The patient with chronic pain was referred to a pain clinic.
There were 11 reoperations—7 for refusion, 3 for debridement, and 1 gastrocnemius recession. Four of the refusion patients and one debridement patient were not satisfied with their results. Hardware removal was required for a quarter of the feet at an average of 20 months, said Dr. Nemec, who is an orthopedic specialist in private practice in Petoskey, Mich.
Patient-reported outcome data were available for 68 patients (74 feet), with a mean follow-up of 56 months. The visual analog scale pain score dropped by a significant average of 4.6 points after surgery. The AOFAS score increased by a significant 47 points (maximum 100).
Geriatric Oncology Comes of Age
The increasing number and efficacy of chemotherapy drugs for a host of cancers – coupled with growth of the older population – not only means that elderly patients have more options for treatment, but also that more of these patients, even among the older old, can be considered candidates for chemotherapy.
This marks a big shift in the way that oncologists approach cancer treatment for patients of advanced age. Until recently, palliative care was the only treatment offered, according to interviews with leaders in geriatric oncology.
"Starting in the mid-1980s, people began to talk about these issues and the need for data and information. Just talking about it began to raise the consciousness that age was not a contraindication per se," said Dr. James S. Goodwin, director of the Sealy Center on Aging at the University of Texas at Galveston.
The numbers are clear: The baby boom is about to turn into a geriatric blast for oncologists. In 2050, the number of Americans aged 65 and older is projected to be 88.5 million, more than double the 40.2 million projected today, according to the U.S. Census Bureau. In addition, total cancer incidence is projected to increase by approximately 45%, from 1.6 million this year to 2.3 million in 2030. A 67% increase in cancer incidence is anticipated for older adults (J. Clin. Oncol. 2009;27:2758-65).
With more people living longer, some say the expansion has already begun. "Oncologists are seeing these patients in everyday practice," observed Dr. Arti Hurria, director of the Cancer and Aging Research Program at City of Hope in Duarte, Calif.
But defining these patients is turning out to be a challenge for geriatric oncologists, and determining the best treatment can be even more difficult.
How Old Is Old?
It’s not clear what "geriatric" means in this context. Intuitively, most people have a mental picture but putting it into words – or finding an agreed-upon age cutoff – is a bit elusive.
"Chronologic age doesn’t equal functional age," said Dr. Hurria An individual can be quite healthy at 80 years with little comorbidity; alternatively a 50-year-old with several health conditions can be quite sick. "In our research studies, we’ve really been forced to have to think about some sort of chronologic cutoff."
Age 65 has frequently been used because historically it’s a time when people retire and become eligible for benefits in the United States, she explained. But many facets of aging appear after age 70, and using data from a study of 500 cancer patients at least 65 years of age, Dr. Hurria and her colleagues found age 73 to be a good cutoff.
"In some ways, that was very reassuring because it really added evidence to our feeling that the seventh decade of life is a time when people become more vulnerable," she said. "It also happens to be a group – age 73 or 75 and older – that has been very underrepresented in clinical trials."
That’s not to say that everyone in their mid-70s or older is feeble and in poor health. Instead, it signals the need for further evaluation of an individual’s physiologic status. "From an epidemiologic point of view, what happens in the 70s is that a lot of people start developing health problems," said Dr. Martine Extermann, who has developed an instrument for evaluation of these patients and is professor of oncology at the University of South Florida and attending physician at Moffitt Cancer Center, in Tampa.
For Dr. Goodwin, the cutoff can be even higher. "People live 10 years longer today on average than when I graduated from medical school," said Dr. Goodwin. "So whatever idea we have about what an old person is, needs to be shifted up by 10 years."
In conversations about geriatric oncology with oncologists, he tends to start at age 80. "If you say 80 and older, you can let age be the factor," he explained. "Age is a very strong predictor of health and mortality. You can’t make that go away by factoring in all of the measurements that you might make about how well they function or how fast they walk."
Dr. Hurria said she likens geriatric oncology to pediatric oncology, a specialty that is also defined by age. The term geriatric oncology "highlights that there is a segment of the population that is potentially vulnerable because of things other than chronologic age. ... We also need to develop an evidence base."
"The definition of geriatric oncology is that we have a population, in which we need to do additional screening and evaluation in order to give the proper treatment," agreed Dr. Extermann, professor of oncology and internal medicine at the H. Lee Moffitt Cancer Center and Research Institute in Tampa.
Who Needs an In-Depth Assessment?
If not all older patients are especially vulnerable, the challenge becomes determining which patients need extra attention. The following two-tiered approach to the evaluation and management of geriatric oncology patients would divide the elderly into two groups (Crit. Rev. Oncol. Hematol. 2005;55:241-52):
• The first step would be to screen all older patients for vulnerability to stressors – such as chemotherapy. This should include the assessment of nutritional status, performance status, psychological state (depression), cognition, daily activity, and comorbidities. Those who are considered fit would be managed like younger patients.
• Next, frailer patients would undergo a more thorough evaluation – a comprehensive geriatric assessment – so that an optimal treatment plan could be created. The in-depth evaluation would look at an elderly person’s functional ability, physical health, cognitive and mental health, and socioenvironmental situation.
With the two-tier evaluation, "the idea is that we need a more integrated approach that is going to include a good geriatric evaluation," said Dr. Extermann, a member of the task force that proposed the approach. The ultimate goal is to match the treatment to the patient and the cancer.
In a large academic setting, a multidisciplinary team can do a more in-depth screening if necessary, she added. For private practice oncologists, the initial evaluation may indicate which patients should be referred for special care.
Where Are the Clinical Trial Data?
Another hurdle in geriatric oncology is the need for more clinical trial data that include this patient population. It’s estimated by the National Cancer Institute that more than 60% of new cancer cases occur among the elderly, but they account for about a quarter of the patients enrolled in randomized clinical trials.
"As the population is aging and most of our patients are older adults, what we’re realizing is that the inclusion criteria for those trials [in the past] did not necessarily reflect the patient who’s sitting in front of us in daily practice," Dr. Hurria said.
Although there are exceptions (notably, recent trials assessing a carboplatin-paclitaxel regimen for non–small-cell lung cancer and the VISTA [Velcade as Initial Standard Therapy for Multiple Myeloma] regimen), the lack of data on the efficacy and safety of chemotherapy regimens in the elderly has left most oncologists to rely on their own judgment.
"There is a need to develop trials for patients who might not be fit enough to participate in standard clinical trials. We still realize that there is an evidence base that we need for those patients," she said.
Research in geriatric oncology has become a priority for several organizations, among them the Geriatric Oncology Consortium and the International Society of Geriatric Oncology.
Chemotoxicity Risk
A prominent area of geriatric oncology research is the assessment of chemotoxicity in an older patient population. Dr. Extermann and Dr. Hurria each presented data at this year’s annual meeting of the American Society of Clinical Oncology on the development of risk models/scores to assess an older patient’s risk of developing chemotoxicity.
Despite her experience in geriatric oncology, Dr. Extermann was surprised to find that sometimes her opinion of a patient could be widely divergent from that of the scoring tool she developed. In other cases, "when I was sitting on the fence about whether or not to treat a patient, the score helped me decide," she said.
"It’s also very important to realize that having one such toxicity does not necessarily make the patient sick," she noted. Usually toxicities lead to some modification of the treatment. So while greater risk of chemotoxicities may look foreboding, patients are often able to tolerate the regimen with modification and can function with some help.
It’s not just a question of tolerating classic chemotoxicities, however. "If they’re already having trouble getting around their apartment and they fell once a year ago ... any kind of assault on the body may lead to a decompensation," said Dr. Goodwin.
The CARG Chemotoxicity Risk Score
Dr. Hurria and her coinvestigators followed 500 cancer patients 65 years and older throughout chemotherapy to find factors predictive of chemotoxicity, and used their findings to develop the Cancer and Aging Research Group (CARG) Chemotoxicity Risk Score.
Age, cancer type, chemotherapy dose, number of chemotherapy drugs, anemia, and low creatinine clearance were among 11 key factors identified by the study. The rest were based on the answers to five key questions assessing various geriatric measures: self-rated hearing, number of falls in the last 6 months, need for help in taking medications, ability to walk one block, and level of social activities.
Dr. Hurria reported a significant association between the patients’ baseline risk scores and their subsequent risk of grade 3-5 chemotherapy toxicity. Those with a score greater than 12 had an 83% overall risk of grade 3-5 toxicity,compared with 27% for those with a score of 0-5. The investigators divided the study population into low-risk, intermediate-risk, and high-risk categories based on the scores.
Cancer patients 65 years and older were eligible for the study, if they were going to start a new chemotherapy regimen. Geriatric assessment began prior to the start of treatment.
Mean patient age was 73 years, 85% were white, and 56% were women. Nearly two-thirds had stage IV disease, and 70% were on polychemotherapy. In all, 53% had grade 3-5 toxicities due to chemotherapy – of these, 26% had hematologic and 43% had nonhematologic toxicities. The majority of toxicities were grade 3
The study captured laboratory values, documented the chemotherapy the patient underwent – the number of drugs, normal or modified dosing, first-line therapy or other – and asked a host of questions in a geriatric assessment that included:
• Functional status (Activities of Daily Living, Instrumental Activities of Daily Living [IADL], Karnofsky Performance Rating Scale, timed up-and-go test [time needed to rise from a chair and start walking], the number of falls in the last 6 months).
• Comorbidity.
• Cognition (Blessed Orientation-Memory-Concentration Test).
• Psychological state (Hospital Anxiety and Depression Scale).
• Social functioning (Medical Outcomes Study Social Activity Limitations Measure).
• Social support (Medical Outcomes Study Social Support Survey, Seeman and Berkman Social Ties).
• Nutritional status (body mass index, percentage unintentional weight loss in the last 6 months).
"We really looked at what are the factors – other than chronologic age – that can tell us about this individual," said Dr. Hurria.
Two physicians graded chemotherapy toxicities using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (v3.0) to determine whether the event was due to disease or chemotherapy. Patients were followed until the end of the regimen.
"We felt that in order for a measure to be utilized in clinical practice, we would need to increase both the ease of administration and the ease of the scoring by having health care providers be able to ask single questions to the patient in order to assess their risk," she said.
Therefore, the researchers developed the scoring algorithm, assigning points to each risk factor, and then used the whole cohort to determine how total score relates to chemotoxicity risk.
The CRASH Score
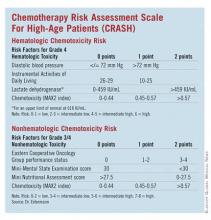
Developed by Dr. Extermann and her coinvestigators, the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score also evaluates an elderly patient’s risk for grade 4 hematologic and grade 3/4 nonhematologic toxicities during chemotherapy.
"This score is helping define – a priori – the level of risk that a patient has for side effects. This way, we know how closely we need to watch the patient during chemotherapy, what strength of chemotherapy we should give, or if we should give chemotherapy at all," said Dr. Extermann.
Investigators enrolled patients from the Moffitt Cancer Center and six community centers. The process included a baseline assessment within 2 weeks of starting chemotherapy and weekly complete blood cell counts. Any published chemotherapy regimen could be used, and clinicians were free to manage care without restriction.
Toxicity was prospectively evaluated using the MAX2 index, previously developed by Dr. Extermann and her coinvestigators. Patients with dementia or planned concomitant radiotherapy were excluded.
A total of 518 patients were included in the analysis. The median age was 76 years. In all, 23 tumor types, and 111 chemotherapy regimens were included. Almost a third of patients (32%) had grade 4 hematologic toxicities, 56% had grade 3/4 nonhematologic toxicity, and 68% had a combination. The median time to first toxicity was 22 days. Development of the model was based on two-thirds of the patient population, and validation on the remaining third.
The study followed patients through the course of chemotherapy up to 1 month after the last cycle. If chemotherapy continued beyond 6 months, assessment was ended at 6 months. The primary end point was the first occurrence of a grade 4 hematologic toxicity and/or a grade 3/4 nonhematologic toxicity.
The researchers assessed several independent variables, including age, sex, body mass index, diastolic blood pressure, comorbidities (Cumulative Illness Rating Scale for Geriatrics [CIRS-G]), CBC count, liver test results, creatinine clearance, albumin level, self-rated health, Eastern Cooperative Oncology Group (ECOG) Performance Status, Instrumental Activities of Daily Living, Geriatric Depression Scale (GDS) score, Mini-Mental State Examination (MMSE) results, Mini Nutritional Assessment (MNA) results, cancer stage, marrow invasion, pretreatment with chemotherapy, tumor response, and chemotoxicity (MAX2 index).
Dr. Extermann noted that other research has suggested that grade 4 hematologic toxicities and grade 3/4 nonhematologic toxicities have different predictors. And her group found:
• On univariate analysis for predictors of hematologic toxicities, diastolic blood pressure, albumin, lactate dehydrogenase, and IADL were significant (after adjustment for chemotoxicity).
• ECOG Performance Status, hemoglobin, creatinine clearance, albumin, MMSE, self-rated health, MNA, and the Cumulative Illness Rating Scale for Geriatrics severity index were significant predictors of nonhematologic toxicities.
The researchers then developed a scoring system for predicting hematologic and nonhematologic chemotoxicity. Scores from both subscales are totaled, and patients with a score of 0-3 are considered low risk while those with a score of 4-6 are grouped as intermediate low risk and those with 7-9 are intermediate high risk. Patients with a score greater than 9 are considered high risk.
A Young Field Looks Ahead
Dr. Extermann is planning to extend her scoring tool to include chemoradiation therapy. The score will also need to be validated for specific tumor types, and she would like to assess what happens once a patient has a severe toxicity. What predicts that they are going to have another one? Another question is whether a score can help identify a starting chemotherapy dose.
Dr. Hurria plans to validate her group’s model in a larger group of patients. It also needs to be validated for patients with specific cancers, such as breast or prostate. This could provide an evidence base to identify specific interventions to reduce or prevent severe chemotoxicities. Work also needs to be done on the pharmacology of chemotherapy drugs and how the pharmacology changes with the aging process.
No less important will be gaining insight into the decision making process as more choices become available to the elderly, she added. How can an oncologist understand what’s meaningful to the patient choosing among options? What’s the best way to communicate with this growing patient group?
Dr. Extermann reported that she has received honoraria from Amgen Inc. Dr. Hurria reported that she has significant financial relationships with Amgen, Genentech Inc., Abraxis BioScience Inc., and Pfizer Inc.
The increasing number and efficacy of chemotherapy drugs for a host of cancers – coupled with growth of the older population – not only means that elderly patients have more options for treatment, but also that more of these patients, even among the older old, can be considered candidates for chemotherapy.
This marks a big shift in the way that oncologists approach cancer treatment for patients of advanced age. Until recently, palliative care was the only treatment offered, according to interviews with leaders in geriatric oncology.
"Starting in the mid-1980s, people began to talk about these issues and the need for data and information. Just talking about it began to raise the consciousness that age was not a contraindication per se," said Dr. James S. Goodwin, director of the Sealy Center on Aging at the University of Texas at Galveston.
The numbers are clear: The baby boom is about to turn into a geriatric blast for oncologists. In 2050, the number of Americans aged 65 and older is projected to be 88.5 million, more than double the 40.2 million projected today, according to the U.S. Census Bureau. In addition, total cancer incidence is projected to increase by approximately 45%, from 1.6 million this year to 2.3 million in 2030. A 67% increase in cancer incidence is anticipated for older adults (J. Clin. Oncol. 2009;27:2758-65).
With more people living longer, some say the expansion has already begun. "Oncologists are seeing these patients in everyday practice," observed Dr. Arti Hurria, director of the Cancer and Aging Research Program at City of Hope in Duarte, Calif.
But defining these patients is turning out to be a challenge for geriatric oncologists, and determining the best treatment can be even more difficult.
How Old Is Old?
It’s not clear what "geriatric" means in this context. Intuitively, most people have a mental picture but putting it into words – or finding an agreed-upon age cutoff – is a bit elusive.
"Chronologic age doesn’t equal functional age," said Dr. Hurria An individual can be quite healthy at 80 years with little comorbidity; alternatively a 50-year-old with several health conditions can be quite sick. "In our research studies, we’ve really been forced to have to think about some sort of chronologic cutoff."
Age 65 has frequently been used because historically it’s a time when people retire and become eligible for benefits in the United States, she explained. But many facets of aging appear after age 70, and using data from a study of 500 cancer patients at least 65 years of age, Dr. Hurria and her colleagues found age 73 to be a good cutoff.
"In some ways, that was very reassuring because it really added evidence to our feeling that the seventh decade of life is a time when people become more vulnerable," she said. "It also happens to be a group – age 73 or 75 and older – that has been very underrepresented in clinical trials."
That’s not to say that everyone in their mid-70s or older is feeble and in poor health. Instead, it signals the need for further evaluation of an individual’s physiologic status. "From an epidemiologic point of view, what happens in the 70s is that a lot of people start developing health problems," said Dr. Martine Extermann, who has developed an instrument for evaluation of these patients and is professor of oncology at the University of South Florida and attending physician at Moffitt Cancer Center, in Tampa.
For Dr. Goodwin, the cutoff can be even higher. "People live 10 years longer today on average than when I graduated from medical school," said Dr. Goodwin. "So whatever idea we have about what an old person is, needs to be shifted up by 10 years."
In conversations about geriatric oncology with oncologists, he tends to start at age 80. "If you say 80 and older, you can let age be the factor," he explained. "Age is a very strong predictor of health and mortality. You can’t make that go away by factoring in all of the measurements that you might make about how well they function or how fast they walk."
Dr. Hurria said she likens geriatric oncology to pediatric oncology, a specialty that is also defined by age. The term geriatric oncology "highlights that there is a segment of the population that is potentially vulnerable because of things other than chronologic age. ... We also need to develop an evidence base."
"The definition of geriatric oncology is that we have a population, in which we need to do additional screening and evaluation in order to give the proper treatment," agreed Dr. Extermann, professor of oncology and internal medicine at the H. Lee Moffitt Cancer Center and Research Institute in Tampa.
Who Needs an In-Depth Assessment?
If not all older patients are especially vulnerable, the challenge becomes determining which patients need extra attention. The following two-tiered approach to the evaluation and management of geriatric oncology patients would divide the elderly into two groups (Crit. Rev. Oncol. Hematol. 2005;55:241-52):
• The first step would be to screen all older patients for vulnerability to stressors – such as chemotherapy. This should include the assessment of nutritional status, performance status, psychological state (depression), cognition, daily activity, and comorbidities. Those who are considered fit would be managed like younger patients.
• Next, frailer patients would undergo a more thorough evaluation – a comprehensive geriatric assessment – so that an optimal treatment plan could be created. The in-depth evaluation would look at an elderly person’s functional ability, physical health, cognitive and mental health, and socioenvironmental situation.
With the two-tier evaluation, "the idea is that we need a more integrated approach that is going to include a good geriatric evaluation," said Dr. Extermann, a member of the task force that proposed the approach. The ultimate goal is to match the treatment to the patient and the cancer.
In a large academic setting, a multidisciplinary team can do a more in-depth screening if necessary, she added. For private practice oncologists, the initial evaluation may indicate which patients should be referred for special care.
Where Are the Clinical Trial Data?
Another hurdle in geriatric oncology is the need for more clinical trial data that include this patient population. It’s estimated by the National Cancer Institute that more than 60% of new cancer cases occur among the elderly, but they account for about a quarter of the patients enrolled in randomized clinical trials.
"As the population is aging and most of our patients are older adults, what we’re realizing is that the inclusion criteria for those trials [in the past] did not necessarily reflect the patient who’s sitting in front of us in daily practice," Dr. Hurria said.
Although there are exceptions (notably, recent trials assessing a carboplatin-paclitaxel regimen for non–small-cell lung cancer and the VISTA [Velcade as Initial Standard Therapy for Multiple Myeloma] regimen), the lack of data on the efficacy and safety of chemotherapy regimens in the elderly has left most oncologists to rely on their own judgment.
"There is a need to develop trials for patients who might not be fit enough to participate in standard clinical trials. We still realize that there is an evidence base that we need for those patients," she said.
Research in geriatric oncology has become a priority for several organizations, among them the Geriatric Oncology Consortium and the International Society of Geriatric Oncology.
Chemotoxicity Risk
A prominent area of geriatric oncology research is the assessment of chemotoxicity in an older patient population. Dr. Extermann and Dr. Hurria each presented data at this year’s annual meeting of the American Society of Clinical Oncology on the development of risk models/scores to assess an older patient’s risk of developing chemotoxicity.
Despite her experience in geriatric oncology, Dr. Extermann was surprised to find that sometimes her opinion of a patient could be widely divergent from that of the scoring tool she developed. In other cases, "when I was sitting on the fence about whether or not to treat a patient, the score helped me decide," she said.
"It’s also very important to realize that having one such toxicity does not necessarily make the patient sick," she noted. Usually toxicities lead to some modification of the treatment. So while greater risk of chemotoxicities may look foreboding, patients are often able to tolerate the regimen with modification and can function with some help.
It’s not just a question of tolerating classic chemotoxicities, however. "If they’re already having trouble getting around their apartment and they fell once a year ago ... any kind of assault on the body may lead to a decompensation," said Dr. Goodwin.
The CARG Chemotoxicity Risk Score
Dr. Hurria and her coinvestigators followed 500 cancer patients 65 years and older throughout chemotherapy to find factors predictive of chemotoxicity, and used their findings to develop the Cancer and Aging Research Group (CARG) Chemotoxicity Risk Score.
Age, cancer type, chemotherapy dose, number of chemotherapy drugs, anemia, and low creatinine clearance were among 11 key factors identified by the study. The rest were based on the answers to five key questions assessing various geriatric measures: self-rated hearing, number of falls in the last 6 months, need for help in taking medications, ability to walk one block, and level of social activities.
Dr. Hurria reported a significant association between the patients’ baseline risk scores and their subsequent risk of grade 3-5 chemotherapy toxicity. Those with a score greater than 12 had an 83% overall risk of grade 3-5 toxicity,compared with 27% for those with a score of 0-5. The investigators divided the study population into low-risk, intermediate-risk, and high-risk categories based on the scores.
Cancer patients 65 years and older were eligible for the study, if they were going to start a new chemotherapy regimen. Geriatric assessment began prior to the start of treatment.
Mean patient age was 73 years, 85% were white, and 56% were women. Nearly two-thirds had stage IV disease, and 70% were on polychemotherapy. In all, 53% had grade 3-5 toxicities due to chemotherapy – of these, 26% had hematologic and 43% had nonhematologic toxicities. The majority of toxicities were grade 3
The study captured laboratory values, documented the chemotherapy the patient underwent – the number of drugs, normal or modified dosing, first-line therapy or other – and asked a host of questions in a geriatric assessment that included:
• Functional status (Activities of Daily Living, Instrumental Activities of Daily Living [IADL], Karnofsky Performance Rating Scale, timed up-and-go test [time needed to rise from a chair and start walking], the number of falls in the last 6 months).
• Comorbidity.
• Cognition (Blessed Orientation-Memory-Concentration Test).
• Psychological state (Hospital Anxiety and Depression Scale).
• Social functioning (Medical Outcomes Study Social Activity Limitations Measure).
• Social support (Medical Outcomes Study Social Support Survey, Seeman and Berkman Social Ties).
• Nutritional status (body mass index, percentage unintentional weight loss in the last 6 months).
"We really looked at what are the factors – other than chronologic age – that can tell us about this individual," said Dr. Hurria.
Two physicians graded chemotherapy toxicities using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (v3.0) to determine whether the event was due to disease or chemotherapy. Patients were followed until the end of the regimen.
"We felt that in order for a measure to be utilized in clinical practice, we would need to increase both the ease of administration and the ease of the scoring by having health care providers be able to ask single questions to the patient in order to assess their risk," she said.
Therefore, the researchers developed the scoring algorithm, assigning points to each risk factor, and then used the whole cohort to determine how total score relates to chemotoxicity risk.
The CRASH Score
Developed by Dr. Extermann and her coinvestigators, the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score also evaluates an elderly patient’s risk for grade 4 hematologic and grade 3/4 nonhematologic toxicities during chemotherapy.
"This score is helping define – a priori – the level of risk that a patient has for side effects. This way, we know how closely we need to watch the patient during chemotherapy, what strength of chemotherapy we should give, or if we should give chemotherapy at all," said Dr. Extermann.
Investigators enrolled patients from the Moffitt Cancer Center and six community centers. The process included a baseline assessment within 2 weeks of starting chemotherapy and weekly complete blood cell counts. Any published chemotherapy regimen could be used, and clinicians were free to manage care without restriction.
Toxicity was prospectively evaluated using the MAX2 index, previously developed by Dr. Extermann and her coinvestigators. Patients with dementia or planned concomitant radiotherapy were excluded.
A total of 518 patients were included in the analysis. The median age was 76 years. In all, 23 tumor types, and 111 chemotherapy regimens were included. Almost a third of patients (32%) had grade 4 hematologic toxicities, 56% had grade 3/4 nonhematologic toxicity, and 68% had a combination. The median time to first toxicity was 22 days. Development of the model was based on two-thirds of the patient population, and validation on the remaining third.
The study followed patients through the course of chemotherapy up to 1 month after the last cycle. If chemotherapy continued beyond 6 months, assessment was ended at 6 months. The primary end point was the first occurrence of a grade 4 hematologic toxicity and/or a grade 3/4 nonhematologic toxicity.
The researchers assessed several independent variables, including age, sex, body mass index, diastolic blood pressure, comorbidities (Cumulative Illness Rating Scale for Geriatrics [CIRS-G]), CBC count, liver test results, creatinine clearance, albumin level, self-rated health, Eastern Cooperative Oncology Group (ECOG) Performance Status, Instrumental Activities of Daily Living, Geriatric Depression Scale (GDS) score, Mini-Mental State Examination (MMSE) results, Mini Nutritional Assessment (MNA) results, cancer stage, marrow invasion, pretreatment with chemotherapy, tumor response, and chemotoxicity (MAX2 index).
Dr. Extermann noted that other research has suggested that grade 4 hematologic toxicities and grade 3/4 nonhematologic toxicities have different predictors. And her group found:
• On univariate analysis for predictors of hematologic toxicities, diastolic blood pressure, albumin, lactate dehydrogenase, and IADL were significant (after adjustment for chemotoxicity).
• ECOG Performance Status, hemoglobin, creatinine clearance, albumin, MMSE, self-rated health, MNA, and the Cumulative Illness Rating Scale for Geriatrics severity index were significant predictors of nonhematologic toxicities.
The researchers then developed a scoring system for predicting hematologic and nonhematologic chemotoxicity. Scores from both subscales are totaled, and patients with a score of 0-3 are considered low risk while those with a score of 4-6 are grouped as intermediate low risk and those with 7-9 are intermediate high risk. Patients with a score greater than 9 are considered high risk.
A Young Field Looks Ahead
Dr. Extermann is planning to extend her scoring tool to include chemoradiation therapy. The score will also need to be validated for specific tumor types, and she would like to assess what happens once a patient has a severe toxicity. What predicts that they are going to have another one? Another question is whether a score can help identify a starting chemotherapy dose.
Dr. Hurria plans to validate her group’s model in a larger group of patients. It also needs to be validated for patients with specific cancers, such as breast or prostate. This could provide an evidence base to identify specific interventions to reduce or prevent severe chemotoxicities. Work also needs to be done on the pharmacology of chemotherapy drugs and how the pharmacology changes with the aging process.
No less important will be gaining insight into the decision making process as more choices become available to the elderly, she added. How can an oncologist understand what’s meaningful to the patient choosing among options? What’s the best way to communicate with this growing patient group?
Dr. Extermann reported that she has received honoraria from Amgen Inc. Dr. Hurria reported that she has significant financial relationships with Amgen, Genentech Inc., Abraxis BioScience Inc., and Pfizer Inc.
The increasing number and efficacy of chemotherapy drugs for a host of cancers – coupled with growth of the older population – not only means that elderly patients have more options for treatment, but also that more of these patients, even among the older old, can be considered candidates for chemotherapy.
This marks a big shift in the way that oncologists approach cancer treatment for patients of advanced age. Until recently, palliative care was the only treatment offered, according to interviews with leaders in geriatric oncology.
"Starting in the mid-1980s, people began to talk about these issues and the need for data and information. Just talking about it began to raise the consciousness that age was not a contraindication per se," said Dr. James S. Goodwin, director of the Sealy Center on Aging at the University of Texas at Galveston.
The numbers are clear: The baby boom is about to turn into a geriatric blast for oncologists. In 2050, the number of Americans aged 65 and older is projected to be 88.5 million, more than double the 40.2 million projected today, according to the U.S. Census Bureau. In addition, total cancer incidence is projected to increase by approximately 45%, from 1.6 million this year to 2.3 million in 2030. A 67% increase in cancer incidence is anticipated for older adults (J. Clin. Oncol. 2009;27:2758-65).
With more people living longer, some say the expansion has already begun. "Oncologists are seeing these patients in everyday practice," observed Dr. Arti Hurria, director of the Cancer and Aging Research Program at City of Hope in Duarte, Calif.
But defining these patients is turning out to be a challenge for geriatric oncologists, and determining the best treatment can be even more difficult.
How Old Is Old?
It’s not clear what "geriatric" means in this context. Intuitively, most people have a mental picture but putting it into words – or finding an agreed-upon age cutoff – is a bit elusive.
"Chronologic age doesn’t equal functional age," said Dr. Hurria An individual can be quite healthy at 80 years with little comorbidity; alternatively a 50-year-old with several health conditions can be quite sick. "In our research studies, we’ve really been forced to have to think about some sort of chronologic cutoff."
Age 65 has frequently been used because historically it’s a time when people retire and become eligible for benefits in the United States, she explained. But many facets of aging appear after age 70, and using data from a study of 500 cancer patients at least 65 years of age, Dr. Hurria and her colleagues found age 73 to be a good cutoff.
"In some ways, that was very reassuring because it really added evidence to our feeling that the seventh decade of life is a time when people become more vulnerable," she said. "It also happens to be a group – age 73 or 75 and older – that has been very underrepresented in clinical trials."
That’s not to say that everyone in their mid-70s or older is feeble and in poor health. Instead, it signals the need for further evaluation of an individual’s physiologic status. "From an epidemiologic point of view, what happens in the 70s is that a lot of people start developing health problems," said Dr. Martine Extermann, who has developed an instrument for evaluation of these patients and is professor of oncology at the University of South Florida and attending physician at Moffitt Cancer Center, in Tampa.
For Dr. Goodwin, the cutoff can be even higher. "People live 10 years longer today on average than when I graduated from medical school," said Dr. Goodwin. "So whatever idea we have about what an old person is, needs to be shifted up by 10 years."
In conversations about geriatric oncology with oncologists, he tends to start at age 80. "If you say 80 and older, you can let age be the factor," he explained. "Age is a very strong predictor of health and mortality. You can’t make that go away by factoring in all of the measurements that you might make about how well they function or how fast they walk."
Dr. Hurria said she likens geriatric oncology to pediatric oncology, a specialty that is also defined by age. The term geriatric oncology "highlights that there is a segment of the population that is potentially vulnerable because of things other than chronologic age. ... We also need to develop an evidence base."
"The definition of geriatric oncology is that we have a population, in which we need to do additional screening and evaluation in order to give the proper treatment," agreed Dr. Extermann, professor of oncology and internal medicine at the H. Lee Moffitt Cancer Center and Research Institute in Tampa.
Who Needs an In-Depth Assessment?
If not all older patients are especially vulnerable, the challenge becomes determining which patients need extra attention. The following two-tiered approach to the evaluation and management of geriatric oncology patients would divide the elderly into two groups (Crit. Rev. Oncol. Hematol. 2005;55:241-52):
• The first step would be to screen all older patients for vulnerability to stressors – such as chemotherapy. This should include the assessment of nutritional status, performance status, psychological state (depression), cognition, daily activity, and comorbidities. Those who are considered fit would be managed like younger patients.
• Next, frailer patients would undergo a more thorough evaluation – a comprehensive geriatric assessment – so that an optimal treatment plan could be created. The in-depth evaluation would look at an elderly person’s functional ability, physical health, cognitive and mental health, and socioenvironmental situation.
With the two-tier evaluation, "the idea is that we need a more integrated approach that is going to include a good geriatric evaluation," said Dr. Extermann, a member of the task force that proposed the approach. The ultimate goal is to match the treatment to the patient and the cancer.
In a large academic setting, a multidisciplinary team can do a more in-depth screening if necessary, she added. For private practice oncologists, the initial evaluation may indicate which patients should be referred for special care.
Where Are the Clinical Trial Data?
Another hurdle in geriatric oncology is the need for more clinical trial data that include this patient population. It’s estimated by the National Cancer Institute that more than 60% of new cancer cases occur among the elderly, but they account for about a quarter of the patients enrolled in randomized clinical trials.
"As the population is aging and most of our patients are older adults, what we’re realizing is that the inclusion criteria for those trials [in the past] did not necessarily reflect the patient who’s sitting in front of us in daily practice," Dr. Hurria said.
Although there are exceptions (notably, recent trials assessing a carboplatin-paclitaxel regimen for non–small-cell lung cancer and the VISTA [Velcade as Initial Standard Therapy for Multiple Myeloma] regimen), the lack of data on the efficacy and safety of chemotherapy regimens in the elderly has left most oncologists to rely on their own judgment.
"There is a need to develop trials for patients who might not be fit enough to participate in standard clinical trials. We still realize that there is an evidence base that we need for those patients," she said.
Research in geriatric oncology has become a priority for several organizations, among them the Geriatric Oncology Consortium and the International Society of Geriatric Oncology.
Chemotoxicity Risk
A prominent area of geriatric oncology research is the assessment of chemotoxicity in an older patient population. Dr. Extermann and Dr. Hurria each presented data at this year’s annual meeting of the American Society of Clinical Oncology on the development of risk models/scores to assess an older patient’s risk of developing chemotoxicity.
Despite her experience in geriatric oncology, Dr. Extermann was surprised to find that sometimes her opinion of a patient could be widely divergent from that of the scoring tool she developed. In other cases, "when I was sitting on the fence about whether or not to treat a patient, the score helped me decide," she said.
"It’s also very important to realize that having one such toxicity does not necessarily make the patient sick," she noted. Usually toxicities lead to some modification of the treatment. So while greater risk of chemotoxicities may look foreboding, patients are often able to tolerate the regimen with modification and can function with some help.
It’s not just a question of tolerating classic chemotoxicities, however. "If they’re already having trouble getting around their apartment and they fell once a year ago ... any kind of assault on the body may lead to a decompensation," said Dr. Goodwin.
The CARG Chemotoxicity Risk Score
Dr. Hurria and her coinvestigators followed 500 cancer patients 65 years and older throughout chemotherapy to find factors predictive of chemotoxicity, and used their findings to develop the Cancer and Aging Research Group (CARG) Chemotoxicity Risk Score.
Age, cancer type, chemotherapy dose, number of chemotherapy drugs, anemia, and low creatinine clearance were among 11 key factors identified by the study. The rest were based on the answers to five key questions assessing various geriatric measures: self-rated hearing, number of falls in the last 6 months, need for help in taking medications, ability to walk one block, and level of social activities.
Dr. Hurria reported a significant association between the patients’ baseline risk scores and their subsequent risk of grade 3-5 chemotherapy toxicity. Those with a score greater than 12 had an 83% overall risk of grade 3-5 toxicity,compared with 27% for those with a score of 0-5. The investigators divided the study population into low-risk, intermediate-risk, and high-risk categories based on the scores.
Cancer patients 65 years and older were eligible for the study, if they were going to start a new chemotherapy regimen. Geriatric assessment began prior to the start of treatment.
Mean patient age was 73 years, 85% were white, and 56% were women. Nearly two-thirds had stage IV disease, and 70% were on polychemotherapy. In all, 53% had grade 3-5 toxicities due to chemotherapy – of these, 26% had hematologic and 43% had nonhematologic toxicities. The majority of toxicities were grade 3
The study captured laboratory values, documented the chemotherapy the patient underwent – the number of drugs, normal or modified dosing, first-line therapy or other – and asked a host of questions in a geriatric assessment that included:
• Functional status (Activities of Daily Living, Instrumental Activities of Daily Living [IADL], Karnofsky Performance Rating Scale, timed up-and-go test [time needed to rise from a chair and start walking], the number of falls in the last 6 months).
• Comorbidity.
• Cognition (Blessed Orientation-Memory-Concentration Test).
• Psychological state (Hospital Anxiety and Depression Scale).
• Social functioning (Medical Outcomes Study Social Activity Limitations Measure).
• Social support (Medical Outcomes Study Social Support Survey, Seeman and Berkman Social Ties).
• Nutritional status (body mass index, percentage unintentional weight loss in the last 6 months).
"We really looked at what are the factors – other than chronologic age – that can tell us about this individual," said Dr. Hurria.
Two physicians graded chemotherapy toxicities using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (v3.0) to determine whether the event was due to disease or chemotherapy. Patients were followed until the end of the regimen.
"We felt that in order for a measure to be utilized in clinical practice, we would need to increase both the ease of administration and the ease of the scoring by having health care providers be able to ask single questions to the patient in order to assess their risk," she said.
Therefore, the researchers developed the scoring algorithm, assigning points to each risk factor, and then used the whole cohort to determine how total score relates to chemotoxicity risk.
The CRASH Score
Developed by Dr. Extermann and her coinvestigators, the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score also evaluates an elderly patient’s risk for grade 4 hematologic and grade 3/4 nonhematologic toxicities during chemotherapy.
"This score is helping define – a priori – the level of risk that a patient has for side effects. This way, we know how closely we need to watch the patient during chemotherapy, what strength of chemotherapy we should give, or if we should give chemotherapy at all," said Dr. Extermann.
Investigators enrolled patients from the Moffitt Cancer Center and six community centers. The process included a baseline assessment within 2 weeks of starting chemotherapy and weekly complete blood cell counts. Any published chemotherapy regimen could be used, and clinicians were free to manage care without restriction.
Toxicity was prospectively evaluated using the MAX2 index, previously developed by Dr. Extermann and her coinvestigators. Patients with dementia or planned concomitant radiotherapy were excluded.
A total of 518 patients were included in the analysis. The median age was 76 years. In all, 23 tumor types, and 111 chemotherapy regimens were included. Almost a third of patients (32%) had grade 4 hematologic toxicities, 56% had grade 3/4 nonhematologic toxicity, and 68% had a combination. The median time to first toxicity was 22 days. Development of the model was based on two-thirds of the patient population, and validation on the remaining third.
The study followed patients through the course of chemotherapy up to 1 month after the last cycle. If chemotherapy continued beyond 6 months, assessment was ended at 6 months. The primary end point was the first occurrence of a grade 4 hematologic toxicity and/or a grade 3/4 nonhematologic toxicity.
The researchers assessed several independent variables, including age, sex, body mass index, diastolic blood pressure, comorbidities (Cumulative Illness Rating Scale for Geriatrics [CIRS-G]), CBC count, liver test results, creatinine clearance, albumin level, self-rated health, Eastern Cooperative Oncology Group (ECOG) Performance Status, Instrumental Activities of Daily Living, Geriatric Depression Scale (GDS) score, Mini-Mental State Examination (MMSE) results, Mini Nutritional Assessment (MNA) results, cancer stage, marrow invasion, pretreatment with chemotherapy, tumor response, and chemotoxicity (MAX2 index).
Dr. Extermann noted that other research has suggested that grade 4 hematologic toxicities and grade 3/4 nonhematologic toxicities have different predictors. And her group found:
• On univariate analysis for predictors of hematologic toxicities, diastolic blood pressure, albumin, lactate dehydrogenase, and IADL were significant (after adjustment for chemotoxicity).
• ECOG Performance Status, hemoglobin, creatinine clearance, albumin, MMSE, self-rated health, MNA, and the Cumulative Illness Rating Scale for Geriatrics severity index were significant predictors of nonhematologic toxicities.
The researchers then developed a scoring system for predicting hematologic and nonhematologic chemotoxicity. Scores from both subscales are totaled, and patients with a score of 0-3 are considered low risk while those with a score of 4-6 are grouped as intermediate low risk and those with 7-9 are intermediate high risk. Patients with a score greater than 9 are considered high risk.
A Young Field Looks Ahead
Dr. Extermann is planning to extend her scoring tool to include chemoradiation therapy. The score will also need to be validated for specific tumor types, and she would like to assess what happens once a patient has a severe toxicity. What predicts that they are going to have another one? Another question is whether a score can help identify a starting chemotherapy dose.
Dr. Hurria plans to validate her group’s model in a larger group of patients. It also needs to be validated for patients with specific cancers, such as breast or prostate. This could provide an evidence base to identify specific interventions to reduce or prevent severe chemotoxicities. Work also needs to be done on the pharmacology of chemotherapy drugs and how the pharmacology changes with the aging process.
No less important will be gaining insight into the decision making process as more choices become available to the elderly, she added. How can an oncologist understand what’s meaningful to the patient choosing among options? What’s the best way to communicate with this growing patient group?
Dr. Extermann reported that she has received honoraria from Amgen Inc. Dr. Hurria reported that she has significant financial relationships with Amgen, Genentech Inc., Abraxis BioScience Inc., and Pfizer Inc.
AMG 479 Fails to Alter Resistance to Endocrine Therapy for Breast Cancer
SAN ANTONIO – The investigational agent AMG 479 failed to alter resistance to hormonal therapy in patients with endocrine therapy–resistant, hormone receptor–positive metastatic breast cancer in the adjuvant setting. Indeed, the drug showed a trend toward worse progression-free survival and objective response in a phase II trial.
When AMG 479 was paired with exemestane or fulvestrant, patients in the experimental arm had a median progression-free survival of 3.9 months, compared with 5.7 months for patients on exemestane or fulvestrant alone (hazard ratio, 1.17; P = .435).
"AMG 479 in combination with either fulvestrant or exemestane does not appear to delay or reverse resistance to hormonal therapy in this population of patients with prior endocrine therapy–resistant hormone receptor-positive metastatic breast cancer," Dr. Peter A. Kaufman said at the San Antonio Breast Cancer Symposium.
[Check out our comprehensive coverage of the San Antonio Breast Cancer Symposium.]
AMG 479 is an investigational fully human monoclonal antibody antagonist of the type I insulinlike growth factor (IGF-I) receptor, blocking the binding of both IGF-I and IGF-II to the receptor.
Resistance to hormonal therapy is thought to possibly occur through increased IGF-I receptor (IGF-IR) signaling, and the researchers hypothesized that inhibition of the IGF-IR signaling pathway may enhance the activity of a second line of hormonal therapy in breast cancer patients, according to Dr. Kaufman of Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
In this study, patients were randomized to receive either treatment with AMG 479 along with exemestane or fulvestrant, or placebo plus exemestane or fulvestrant. (The choice was left to the investigator.) Stratification factors included which hormonal therapy the patient received, as well as the extent of disease
AMG 479 (12 mg/kg) and placebo were given intravenously every 2 weeks. Exemestane (25 mg) was given orally every day; oral fulvestrant was given in a dose of 500 mg on day 1 and then at a dose of 250 mg on days 15 and 29 and every 4 weeks thereafter. The primary end point was progression-free survival, as measured by modified RECIST (Response Evaluation Criteria in Solid Tumors) v1.0.
In all, 106 women were included in the AMG 479 group and 50 in the placebo group. In the treatment group, 98% of women were estrogen receptor positive, 70% were progesterone receptor positive, and 7% were HER2 positive. In the placebo group, 94% of women were ER positive, 70% were PR positive, and 2% were HER2 positive.
Median profession-free survival among women who received exemestane was 4.8 months with AMG 479 and 7.3 months with placebo (HR, 1.31). Among women who received fulvestrant, it was 3.7 months and 5.4, respectively (HR, 1.11).
"There really is no difference in the overall response rate," said Dr. Kaufman. The objective response rate for patients in the AMG 479 group was 8% vs. 13% for those in the control group. The overall clinical benefit rate was 35% for women on AMG 479 and 31% of women on placebo, which was not significant.
Serious adverse events occurred in 25% of patients who were given AMG 479 and 18% of the placebo group. "We saw no meaningful difference in any of those symptoms," said Dr. Kaufman. He did note elevated but nonsignificant rates of hyperglycemia, neutropenia, and thrombocytopenia for those on AMG, however.
Dr. Kaufman disclosed that he receives grant support from Amgen. He has also disclosed that he is a shareholder of Amgen. A coauthor reported that he receives grant support from Amgen. Three of the study authors are employees of Amgen. The study was sponsored by Amgen.
SAN ANTONIO – The investigational agent AMG 479 failed to alter resistance to hormonal therapy in patients with endocrine therapy–resistant, hormone receptor–positive metastatic breast cancer in the adjuvant setting. Indeed, the drug showed a trend toward worse progression-free survival and objective response in a phase II trial.
When AMG 479 was paired with exemestane or fulvestrant, patients in the experimental arm had a median progression-free survival of 3.9 months, compared with 5.7 months for patients on exemestane or fulvestrant alone (hazard ratio, 1.17; P = .435).
"AMG 479 in combination with either fulvestrant or exemestane does not appear to delay or reverse resistance to hormonal therapy in this population of patients with prior endocrine therapy–resistant hormone receptor-positive metastatic breast cancer," Dr. Peter A. Kaufman said at the San Antonio Breast Cancer Symposium.
[Check out our comprehensive coverage of the San Antonio Breast Cancer Symposium.]
AMG 479 is an investigational fully human monoclonal antibody antagonist of the type I insulinlike growth factor (IGF-I) receptor, blocking the binding of both IGF-I and IGF-II to the receptor.
Resistance to hormonal therapy is thought to possibly occur through increased IGF-I receptor (IGF-IR) signaling, and the researchers hypothesized that inhibition of the IGF-IR signaling pathway may enhance the activity of a second line of hormonal therapy in breast cancer patients, according to Dr. Kaufman of Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
In this study, patients were randomized to receive either treatment with AMG 479 along with exemestane or fulvestrant, or placebo plus exemestane or fulvestrant. (The choice was left to the investigator.) Stratification factors included which hormonal therapy the patient received, as well as the extent of disease
AMG 479 (12 mg/kg) and placebo were given intravenously every 2 weeks. Exemestane (25 mg) was given orally every day; oral fulvestrant was given in a dose of 500 mg on day 1 and then at a dose of 250 mg on days 15 and 29 and every 4 weeks thereafter. The primary end point was progression-free survival, as measured by modified RECIST (Response Evaluation Criteria in Solid Tumors) v1.0.
In all, 106 women were included in the AMG 479 group and 50 in the placebo group. In the treatment group, 98% of women were estrogen receptor positive, 70% were progesterone receptor positive, and 7% were HER2 positive. In the placebo group, 94% of women were ER positive, 70% were PR positive, and 2% were HER2 positive.
Median profession-free survival among women who received exemestane was 4.8 months with AMG 479 and 7.3 months with placebo (HR, 1.31). Among women who received fulvestrant, it was 3.7 months and 5.4, respectively (HR, 1.11).
"There really is no difference in the overall response rate," said Dr. Kaufman. The objective response rate for patients in the AMG 479 group was 8% vs. 13% for those in the control group. The overall clinical benefit rate was 35% for women on AMG 479 and 31% of women on placebo, which was not significant.
Serious adverse events occurred in 25% of patients who were given AMG 479 and 18% of the placebo group. "We saw no meaningful difference in any of those symptoms," said Dr. Kaufman. He did note elevated but nonsignificant rates of hyperglycemia, neutropenia, and thrombocytopenia for those on AMG, however.
Dr. Kaufman disclosed that he receives grant support from Amgen. He has also disclosed that he is a shareholder of Amgen. A coauthor reported that he receives grant support from Amgen. Three of the study authors are employees of Amgen. The study was sponsored by Amgen.
SAN ANTONIO – The investigational agent AMG 479 failed to alter resistance to hormonal therapy in patients with endocrine therapy–resistant, hormone receptor–positive metastatic breast cancer in the adjuvant setting. Indeed, the drug showed a trend toward worse progression-free survival and objective response in a phase II trial.
When AMG 479 was paired with exemestane or fulvestrant, patients in the experimental arm had a median progression-free survival of 3.9 months, compared with 5.7 months for patients on exemestane or fulvestrant alone (hazard ratio, 1.17; P = .435).
"AMG 479 in combination with either fulvestrant or exemestane does not appear to delay or reverse resistance to hormonal therapy in this population of patients with prior endocrine therapy–resistant hormone receptor-positive metastatic breast cancer," Dr. Peter A. Kaufman said at the San Antonio Breast Cancer Symposium.
[Check out our comprehensive coverage of the San Antonio Breast Cancer Symposium.]
AMG 479 is an investigational fully human monoclonal antibody antagonist of the type I insulinlike growth factor (IGF-I) receptor, blocking the binding of both IGF-I and IGF-II to the receptor.
Resistance to hormonal therapy is thought to possibly occur through increased IGF-I receptor (IGF-IR) signaling, and the researchers hypothesized that inhibition of the IGF-IR signaling pathway may enhance the activity of a second line of hormonal therapy in breast cancer patients, according to Dr. Kaufman of Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
In this study, patients were randomized to receive either treatment with AMG 479 along with exemestane or fulvestrant, or placebo plus exemestane or fulvestrant. (The choice was left to the investigator.) Stratification factors included which hormonal therapy the patient received, as well as the extent of disease
AMG 479 (12 mg/kg) and placebo were given intravenously every 2 weeks. Exemestane (25 mg) was given orally every day; oral fulvestrant was given in a dose of 500 mg on day 1 and then at a dose of 250 mg on days 15 and 29 and every 4 weeks thereafter. The primary end point was progression-free survival, as measured by modified RECIST (Response Evaluation Criteria in Solid Tumors) v1.0.
In all, 106 women were included in the AMG 479 group and 50 in the placebo group. In the treatment group, 98% of women were estrogen receptor positive, 70% were progesterone receptor positive, and 7% were HER2 positive. In the placebo group, 94% of women were ER positive, 70% were PR positive, and 2% were HER2 positive.
Median profession-free survival among women who received exemestane was 4.8 months with AMG 479 and 7.3 months with placebo (HR, 1.31). Among women who received fulvestrant, it was 3.7 months and 5.4, respectively (HR, 1.11).
"There really is no difference in the overall response rate," said Dr. Kaufman. The objective response rate for patients in the AMG 479 group was 8% vs. 13% for those in the control group. The overall clinical benefit rate was 35% for women on AMG 479 and 31% of women on placebo, which was not significant.
Serious adverse events occurred in 25% of patients who were given AMG 479 and 18% of the placebo group. "We saw no meaningful difference in any of those symptoms," said Dr. Kaufman. He did note elevated but nonsignificant rates of hyperglycemia, neutropenia, and thrombocytopenia for those on AMG, however.
Dr. Kaufman disclosed that he receives grant support from Amgen. He has also disclosed that he is a shareholder of Amgen. A coauthor reported that he receives grant support from Amgen. Three of the study authors are employees of Amgen. The study was sponsored by Amgen.
Major Finding: When paired with exemestane or fulvestrant, AMG 479 produced a median progression-free survival of 3.9 months, compared with 5.7 months for patients on exemestane or fulvestrant alone (HR, 1.17; P = .435).
Data Source: A phase II study in 156 women with hormone receptor–positive metastatic or locally advanced breast cancer.
Disclosures: Dr. Kaufman received grant support from trial sponsor Amgen Inc. He has also disclosed that he is a shareholder of Amgen. Three of the study authors are employees of Amgen.
ABCSG-12 Shows Benefit for Zoledronic Acid in Early Breast Cancer
SAN ANTONIO – Despite the negative results overall of the AZURE trial, it may not be time to put the final nail in coffin of adjuvant zoledronic acid for the treatment of hormone-positive breast cancer.
[Zoledronic Acid Sinks as Breast Cancer Therapy in AZURE Trial]
Updated results for the ABCSG (Austrian Breast and Colorectal Cancer Study Group) Trial 12 continue to show disease-free and overall survival benefits 2 years after stopping adjuvant endocrine treatment with zoledronic acid (Zometa), according to an update presented at the annual San Antonio Breast Cancer Symposium.
The positive Austrian trial randomized 899 premenopausal women on goserelin (Zoladex) to 3 years of zoledronic acid with either tamoxifen or anastrozole (Arimidex) and 904 comparators to endocrine therapy alone (N. Engl. J. Med. 2009;360:679-91).
At a median follow-up of 62 months (2 years after treatment completion), zoledronic acid reduced the risk of disease-free survival events by 32%, compared with endocrine therapy alone (hazard ratio, 0.68; P = .008). Better disease-free survival was seen whether the women were on tamoxifen (HR, 0.67) or anastrozole (HR, 0.68). A trend toward reduced risk of death with zoledronic acid also endured (HR, 0.67; P = .09).
"We observed persistence of benefit with adjuvant zoledronic acid in the ABCSG-12 trial," said Dr. Michael Gnant, president of the ABCSG, at the press briefing where Dr. Robert Coleman reported no disease-free survival benefit overall (adjusted HR, 0.98; P = .79) from adjuvant zoledronic acid in the AZURE (Adjuvant Treatment With Zoledronic Acid in Stage II/III Breast Cancer) trial.
"Why are the ABCSG-12 results so different from the premenopausal [women] in AZURE?" asked Dr. Gnant, professor of surgery at the Medical University of Vienna. "I believe that when you look closely, there is minimal overlap in patient selection and treatment," he said.
"All of them [in the ABCSG-12 trial] were put into artificial menopause for 3 years with the use of goserelin. None of these patients had adjuvant chemotherapy. I think that’s an important difference from the AZURE trial," said Dr. Gnant.
In the AZURE trial, 96% of patients had adjuvant chemotherapy, and none got ovarian function-suppression treatment. Also, in the ABCSG-12 trial, about 70% of patients had low-risk, stage I breast cancer. Patients in the AZURE trial had stage II or III breast cancer.
Patients in the ABCSG-12 trial were randomized to one of four treatment groups: 20 mg/day tamoxifen; 20 mg/day tamoxifen plus 4 mg zoledronic acid every 6 months; 1 mg/day anastrozole; or 1 mg/day anastrozole plus 4 mg zoledronic acid every 6 months. Treatment lasted for 3 years. All patients received 3.6-mg subcutaneous goserelin every 28 days.
In all, 1,803 patients were enrolled between 1999 and 2006. As of May 18, 2010, 186 disease-free survival events and 73 deaths were recorded: 45 locoregional relapse events, 100 distant relapse events (including 53 bone metastasis events), and 14 contralateral breast cancer events. Adding zoledronic acid to endocrine therapy was associated with a continued reduction in recurrences inside and outside bone.
[FDA: Bevacizumab Should No Longer Be Indicated for Breast Cancer]
Adverse events associated with zoledronic acid treatment (arthralgia, bone pain, and pyrexia) were generally mild and consistent with the established safety profile. Zoledronic acid did not increase the occurrence of serious adverse events, compared with endocrine therapy alone. Importantly, there was no significant renal toxicity and no confirmed cases of osteonecrosis of the jaw.
"I find it quite intriguing and actually reassuring that with this therapeutic intervention that lasted 3 years – just an infusion every 6 months – you can change something in the long-term outcome of these patients," said Dr. Gnant. "When we break it down according to additional preplanned subgroups, actually we see that ... adjuvant bisphosphonate treatment works in every subgroup, at least if you look at the tumor-derived parameters."
The addition of adjuvant zoledronic acid may not make much difference in patients younger than 40 years, he suggested: "It’s exactly that group of patients where we cannot assume that the goserelin treatment will lead to full suppression of ovarian function and to very low estrogen levels," he said.
Among patients aged 40 years or older in ABCSG-12, however, there was a roughly 40% difference in disease-free survival, which translated into a 40% reduction in the risk of dying from breast cancer, although this did not reach statistical significance.
[Dual Anti-HER2 Blockade Called the Future of Breast Cancer Therapy]
The AZURE researchers also did extensive planned subgroup analyses. "One clearly stands out from all the others. That is the treatment effect on disease-free survival according to menopausal status," observed Dr. Coleman, a professor of medical oncology at the University of Sheffield (England). There was a clear overall survival benefit for women at least 5 years out from menopause on zoledronic acid. These women had a 29% reduction in the risk of death (P = .017).
"While obviously it’s fair to say that the overall result of AZURE is a little bit disappointing, scientifically it makes perfect sense," said Dr. Gnant. "Perfect suppression – or a natural lack of estrogen – in combination with adjuvant zoledronic acid leads to significant disease-free and overall survival benefit."
To put the results in practical terms, Dr. Gnant noted that for the past 2 years, he would have put his 42-year-old wife on zoledronic acid if she developed ER-positive breast cancer, but not his 83-year-old mother. "This has changed this afternoon. Now, my 83-year-old mother would also receive zoledronic acid," he said.
The ABCSG-12 trial was sponsored by Austrian Breast & Colorectal Cancer Study Group, with support from AstraZenaca (which makes Arimedex) and Novartis Pharmaceutical (which makes Zometa). Dr. Gnant disclosed that he has significant financial relationships with several pharmaceutical companies, including Novartis.
[Women's Health Initiative: New Findings on Big-Three Cancer Rates]
The AZURE trial was sponsored by the University of Sheffield. Dr. Coleman disclosed that he receives grant support from Novartis and is a speaker for Novartis and Amgen.
SAN ANTONIO – Despite the negative results overall of the AZURE trial, it may not be time to put the final nail in coffin of adjuvant zoledronic acid for the treatment of hormone-positive breast cancer.
[Zoledronic Acid Sinks as Breast Cancer Therapy in AZURE Trial]
Updated results for the ABCSG (Austrian Breast and Colorectal Cancer Study Group) Trial 12 continue to show disease-free and overall survival benefits 2 years after stopping adjuvant endocrine treatment with zoledronic acid (Zometa), according to an update presented at the annual San Antonio Breast Cancer Symposium.
The positive Austrian trial randomized 899 premenopausal women on goserelin (Zoladex) to 3 years of zoledronic acid with either tamoxifen or anastrozole (Arimidex) and 904 comparators to endocrine therapy alone (N. Engl. J. Med. 2009;360:679-91).
At a median follow-up of 62 months (2 years after treatment completion), zoledronic acid reduced the risk of disease-free survival events by 32%, compared with endocrine therapy alone (hazard ratio, 0.68; P = .008). Better disease-free survival was seen whether the women were on tamoxifen (HR, 0.67) or anastrozole (HR, 0.68). A trend toward reduced risk of death with zoledronic acid also endured (HR, 0.67; P = .09).
"We observed persistence of benefit with adjuvant zoledronic acid in the ABCSG-12 trial," said Dr. Michael Gnant, president of the ABCSG, at the press briefing where Dr. Robert Coleman reported no disease-free survival benefit overall (adjusted HR, 0.98; P = .79) from adjuvant zoledronic acid in the AZURE (Adjuvant Treatment With Zoledronic Acid in Stage II/III Breast Cancer) trial.
"Why are the ABCSG-12 results so different from the premenopausal [women] in AZURE?" asked Dr. Gnant, professor of surgery at the Medical University of Vienna. "I believe that when you look closely, there is minimal overlap in patient selection and treatment," he said.
"All of them [in the ABCSG-12 trial] were put into artificial menopause for 3 years with the use of goserelin. None of these patients had adjuvant chemotherapy. I think that’s an important difference from the AZURE trial," said Dr. Gnant.
In the AZURE trial, 96% of patients had adjuvant chemotherapy, and none got ovarian function-suppression treatment. Also, in the ABCSG-12 trial, about 70% of patients had low-risk, stage I breast cancer. Patients in the AZURE trial had stage II or III breast cancer.
Patients in the ABCSG-12 trial were randomized to one of four treatment groups: 20 mg/day tamoxifen; 20 mg/day tamoxifen plus 4 mg zoledronic acid every 6 months; 1 mg/day anastrozole; or 1 mg/day anastrozole plus 4 mg zoledronic acid every 6 months. Treatment lasted for 3 years. All patients received 3.6-mg subcutaneous goserelin every 28 days.
In all, 1,803 patients were enrolled between 1999 and 2006. As of May 18, 2010, 186 disease-free survival events and 73 deaths were recorded: 45 locoregional relapse events, 100 distant relapse events (including 53 bone metastasis events), and 14 contralateral breast cancer events. Adding zoledronic acid to endocrine therapy was associated with a continued reduction in recurrences inside and outside bone.
[FDA: Bevacizumab Should No Longer Be Indicated for Breast Cancer]
Adverse events associated with zoledronic acid treatment (arthralgia, bone pain, and pyrexia) were generally mild and consistent with the established safety profile. Zoledronic acid did not increase the occurrence of serious adverse events, compared with endocrine therapy alone. Importantly, there was no significant renal toxicity and no confirmed cases of osteonecrosis of the jaw.
"I find it quite intriguing and actually reassuring that with this therapeutic intervention that lasted 3 years – just an infusion every 6 months – you can change something in the long-term outcome of these patients," said Dr. Gnant. "When we break it down according to additional preplanned subgroups, actually we see that ... adjuvant bisphosphonate treatment works in every subgroup, at least if you look at the tumor-derived parameters."
The addition of adjuvant zoledronic acid may not make much difference in patients younger than 40 years, he suggested: "It’s exactly that group of patients where we cannot assume that the goserelin treatment will lead to full suppression of ovarian function and to very low estrogen levels," he said.
Among patients aged 40 years or older in ABCSG-12, however, there was a roughly 40% difference in disease-free survival, which translated into a 40% reduction in the risk of dying from breast cancer, although this did not reach statistical significance.
[Dual Anti-HER2 Blockade Called the Future of Breast Cancer Therapy]
The AZURE researchers also did extensive planned subgroup analyses. "One clearly stands out from all the others. That is the treatment effect on disease-free survival according to menopausal status," observed Dr. Coleman, a professor of medical oncology at the University of Sheffield (England). There was a clear overall survival benefit for women at least 5 years out from menopause on zoledronic acid. These women had a 29% reduction in the risk of death (P = .017).
"While obviously it’s fair to say that the overall result of AZURE is a little bit disappointing, scientifically it makes perfect sense," said Dr. Gnant. "Perfect suppression – or a natural lack of estrogen – in combination with adjuvant zoledronic acid leads to significant disease-free and overall survival benefit."
To put the results in practical terms, Dr. Gnant noted that for the past 2 years, he would have put his 42-year-old wife on zoledronic acid if she developed ER-positive breast cancer, but not his 83-year-old mother. "This has changed this afternoon. Now, my 83-year-old mother would also receive zoledronic acid," he said.
The ABCSG-12 trial was sponsored by Austrian Breast & Colorectal Cancer Study Group, with support from AstraZenaca (which makes Arimedex) and Novartis Pharmaceutical (which makes Zometa). Dr. Gnant disclosed that he has significant financial relationships with several pharmaceutical companies, including Novartis.
[Women's Health Initiative: New Findings on Big-Three Cancer Rates]
The AZURE trial was sponsored by the University of Sheffield. Dr. Coleman disclosed that he receives grant support from Novartis and is a speaker for Novartis and Amgen.
SAN ANTONIO – Despite the negative results overall of the AZURE trial, it may not be time to put the final nail in coffin of adjuvant zoledronic acid for the treatment of hormone-positive breast cancer.
[Zoledronic Acid Sinks as Breast Cancer Therapy in AZURE Trial]
Updated results for the ABCSG (Austrian Breast and Colorectal Cancer Study Group) Trial 12 continue to show disease-free and overall survival benefits 2 years after stopping adjuvant endocrine treatment with zoledronic acid (Zometa), according to an update presented at the annual San Antonio Breast Cancer Symposium.
The positive Austrian trial randomized 899 premenopausal women on goserelin (Zoladex) to 3 years of zoledronic acid with either tamoxifen or anastrozole (Arimidex) and 904 comparators to endocrine therapy alone (N. Engl. J. Med. 2009;360:679-91).
At a median follow-up of 62 months (2 years after treatment completion), zoledronic acid reduced the risk of disease-free survival events by 32%, compared with endocrine therapy alone (hazard ratio, 0.68; P = .008). Better disease-free survival was seen whether the women were on tamoxifen (HR, 0.67) or anastrozole (HR, 0.68). A trend toward reduced risk of death with zoledronic acid also endured (HR, 0.67; P = .09).
"We observed persistence of benefit with adjuvant zoledronic acid in the ABCSG-12 trial," said Dr. Michael Gnant, president of the ABCSG, at the press briefing where Dr. Robert Coleman reported no disease-free survival benefit overall (adjusted HR, 0.98; P = .79) from adjuvant zoledronic acid in the AZURE (Adjuvant Treatment With Zoledronic Acid in Stage II/III Breast Cancer) trial.
"Why are the ABCSG-12 results so different from the premenopausal [women] in AZURE?" asked Dr. Gnant, professor of surgery at the Medical University of Vienna. "I believe that when you look closely, there is minimal overlap in patient selection and treatment," he said.
"All of them [in the ABCSG-12 trial] were put into artificial menopause for 3 years with the use of goserelin. None of these patients had adjuvant chemotherapy. I think that’s an important difference from the AZURE trial," said Dr. Gnant.
In the AZURE trial, 96% of patients had adjuvant chemotherapy, and none got ovarian function-suppression treatment. Also, in the ABCSG-12 trial, about 70% of patients had low-risk, stage I breast cancer. Patients in the AZURE trial had stage II or III breast cancer.
Patients in the ABCSG-12 trial were randomized to one of four treatment groups: 20 mg/day tamoxifen; 20 mg/day tamoxifen plus 4 mg zoledronic acid every 6 months; 1 mg/day anastrozole; or 1 mg/day anastrozole plus 4 mg zoledronic acid every 6 months. Treatment lasted for 3 years. All patients received 3.6-mg subcutaneous goserelin every 28 days.
In all, 1,803 patients were enrolled between 1999 and 2006. As of May 18, 2010, 186 disease-free survival events and 73 deaths were recorded: 45 locoregional relapse events, 100 distant relapse events (including 53 bone metastasis events), and 14 contralateral breast cancer events. Adding zoledronic acid to endocrine therapy was associated with a continued reduction in recurrences inside and outside bone.
[FDA: Bevacizumab Should No Longer Be Indicated for Breast Cancer]
Adverse events associated with zoledronic acid treatment (arthralgia, bone pain, and pyrexia) were generally mild and consistent with the established safety profile. Zoledronic acid did not increase the occurrence of serious adverse events, compared with endocrine therapy alone. Importantly, there was no significant renal toxicity and no confirmed cases of osteonecrosis of the jaw.
"I find it quite intriguing and actually reassuring that with this therapeutic intervention that lasted 3 years – just an infusion every 6 months – you can change something in the long-term outcome of these patients," said Dr. Gnant. "When we break it down according to additional preplanned subgroups, actually we see that ... adjuvant bisphosphonate treatment works in every subgroup, at least if you look at the tumor-derived parameters."
The addition of adjuvant zoledronic acid may not make much difference in patients younger than 40 years, he suggested: "It’s exactly that group of patients where we cannot assume that the goserelin treatment will lead to full suppression of ovarian function and to very low estrogen levels," he said.
Among patients aged 40 years or older in ABCSG-12, however, there was a roughly 40% difference in disease-free survival, which translated into a 40% reduction in the risk of dying from breast cancer, although this did not reach statistical significance.
[Dual Anti-HER2 Blockade Called the Future of Breast Cancer Therapy]
The AZURE researchers also did extensive planned subgroup analyses. "One clearly stands out from all the others. That is the treatment effect on disease-free survival according to menopausal status," observed Dr. Coleman, a professor of medical oncology at the University of Sheffield (England). There was a clear overall survival benefit for women at least 5 years out from menopause on zoledronic acid. These women had a 29% reduction in the risk of death (P = .017).
"While obviously it’s fair to say that the overall result of AZURE is a little bit disappointing, scientifically it makes perfect sense," said Dr. Gnant. "Perfect suppression – or a natural lack of estrogen – in combination with adjuvant zoledronic acid leads to significant disease-free and overall survival benefit."
To put the results in practical terms, Dr. Gnant noted that for the past 2 years, he would have put his 42-year-old wife on zoledronic acid if she developed ER-positive breast cancer, but not his 83-year-old mother. "This has changed this afternoon. Now, my 83-year-old mother would also receive zoledronic acid," he said.
The ABCSG-12 trial was sponsored by Austrian Breast & Colorectal Cancer Study Group, with support from AstraZenaca (which makes Arimedex) and Novartis Pharmaceutical (which makes Zometa). Dr. Gnant disclosed that he has significant financial relationships with several pharmaceutical companies, including Novartis.
[Women's Health Initiative: New Findings on Big-Three Cancer Rates]
The AZURE trial was sponsored by the University of Sheffield. Dr. Coleman disclosed that he receives grant support from Novartis and is a speaker for Novartis and Amgen.
Major Finding: At a median follow-up of 62 months, adjuvant zoledronic acid continued to improve disease-free survival by 32%, compared with endocrine therapy alone (HR, 0.68; P = .008).
Data Source: A phase III trial including more than 1,800 premenopausal women with early breast cancer, who where randomized to receive endocrine therapy with or without zoledronic acid for 3 years. All patients received goserelin to suppress ovarian function.
Disclosures: The ABCSG-12 trial was sponsored by Austrian Breast & Colorectal Cancer Study Group, with support from AstraZenaca (which makes Arimedex) and Novartis Pharmaceutical (which makes Zometa). Dr. Gnant disclosed that he has significant financial relationships with several pharmaceutical companies, including Novartis. The AZURE trial was sponsored by the University of Sheffield. Dr. Coleman disclosed that he receives grant support from Novartis, and is a speaker for Novartis and Amgen.
Surgical Excision Can Spread Tumor Cells to Sentinel Node
SAN ANTONIO – Surgical excision of breast cancer prior to sentinel lymph node dissection can displace isolated tumor cells to the sentinel lymph node, but these tumor cells appear to have little clinical significance, according to an analysis of more than 17,000 patients in a large database.
"Earlier surgical excision leads to a nearly fourfold increase in the risk of having isolated tumor cells in the sentinel node indicating iatrogenic displacement," Dr. Tove F. Tvedskov said at the annual San Antonio Breast Cancer Symposium. She advised, however, that these cells are probably without clinical significance, and that the omission of axillary lymph node dissection should be considered.
[Women's Health Initiative: New Findings on Big-Three Cancer Rates]
The study was based on data from the Danish Breast Cancer Cooperative Group database, which includes more than 80,000 women with breast cancer. Approximately 3,000 new sentinel lymph node dissections (SLNDs) are included in the database each year, with clinical and histopathologic data prospectively collected for these cases. Data from this database were combined with data from the Danish National Health Registry, which includes all surgical procedures performed in Danish hospitals.
The researchers identified 414 breast cancer patients who underwent surgical excision up to 2 months before SLND and compared them with 16,960 breast cancer patients who underwent SLND without prior surgical tumor excision.
"The proportion of patients with isolated tumor cells was almost three times higher in the group with earlier surgical excision, compared to the group without earlier surgical excision," said Dr. Tvedskov of Copenhagen University Hospital. "One obvious explanation for these differences between groups is, of course, that the group with earlier surgical excision is a highly selected group of patients, where the cancer diagnosis was not obvious from the beginning."
Therefore, the researchers used a multivariate model to take into account these differences by adjusting for tumor size, histologic type and malignancy grade.
"We found that the risk of having isolated tumor cells in the sentinel node was nearly fourfold increased when surgical excision was performed, compared to patients without any earlier surgical excision," Dr. Tvedskov said. "Surgical excision leads to some degree of iatrogenic displacement."
[Trastuzumab/Pertuzumab Doublet Eradicates Some Early Breast Cancer]
In all, 64% of patients who underwent earlier surgical excision and 59% of those without earlier surgical excision were negative for sentinel lymph node metastases. Among the prior-excision group, 9% had isolated tumor cells in the sentinel lymph node, compared with 3% of the control group (odds ratio, 3.90; P less than .001).
Likewise, micrometastases in the sentinel lymph node were more common (15%) among the prior-excision group, compared with 12% in the control group (OR, 1.48; P = .006). However, macrometastases in the sentinel lymph node were more common in the control group (26% vs. 12%).
The researchers next looked at whether the histologic type of tumor mattered. After adjustment, "we found that the risk of having isolated tumor cells was three times higher in patients with lobular carcinomas, compared to patients with ductal carcinomas," said Dr. Tvedskov. The odds ratio was 3.02 (P less than .0001), after adjusting for tumor size, malignancy grade, and earlier surgical excision.
"Finding these results, we expected that the extra isolated tumor cells after earlier surgical excision would especially come from lobular carcinomas," she said. However, in a subanalysis, "we found that in the group of patients with ductal carcinomas, the risk of isolated tumor cells after surgical excision was nearly fivefold increased, compared to patients without surgical excision, whereas, in the group of patients with lobular carcinomas, there was no significant increased odds ratio."
Among patients with ductal carcinoma, isolated tumor cells were significantly more likely to be present in patients who underwent prior surgical excision compared with control patients (OR, 4.91; P less than .0001), after adjusting for tumor size and malignancy grade. Among lobular carcinoma patients though, isolated tumor cells were not significantly more likely to be present in those who underwent prior surgical excision (OR, 1.27; P = .69), after adjusting for tumor size and malignancy grade.
"So, despite the fact that the lobular carcinomas are, in general, more likely to present with isolated tumor cells in the sentinel node, the extra isolated tumor cells caused by the earlier surgical excision seems to come from ductal carcinomas," said Dr. Tvedskov.
The investigators also considered the question of clinical significance: Were the isolated tumor cells related to nonsentinel node metastases, or could an axillary lymph node dissection be omitted in these patients?
Looking at the distribution of nonsentinel node metastases in patients with either isolated tumor cells or micrometastases in the sentinel node, they found that isolated tumor cells were not present in nonsentinel lymph nodes among patients who underwent prior surgical excision. These cells, however, were found in nonsentinel lymph nodes in 12% of control patients.
Dr. Tvedskov pointed out that this difference did not achieve significance because of the small number of patients. There was no significant difference in the likelihood of micrometastases in nonsentinel lymph nodes between the two groups.
"This indicates that isolated tumor cells in patients with earlier surgical excision are not related to nonsentinel node metastases, and are therefore without clinical significance," she said.
The investigators reported that they have no relevant financial relationships.
[Bevacizumab Short of Mark in First Neoadjuvant Breast Cancer Trial]
SAN ANTONIO – Surgical excision of breast cancer prior to sentinel lymph node dissection can displace isolated tumor cells to the sentinel lymph node, but these tumor cells appear to have little clinical significance, according to an analysis of more than 17,000 patients in a large database.
"Earlier surgical excision leads to a nearly fourfold increase in the risk of having isolated tumor cells in the sentinel node indicating iatrogenic displacement," Dr. Tove F. Tvedskov said at the annual San Antonio Breast Cancer Symposium. She advised, however, that these cells are probably without clinical significance, and that the omission of axillary lymph node dissection should be considered.
[Women's Health Initiative: New Findings on Big-Three Cancer Rates]
The study was based on data from the Danish Breast Cancer Cooperative Group database, which includes more than 80,000 women with breast cancer. Approximately 3,000 new sentinel lymph node dissections (SLNDs) are included in the database each year, with clinical and histopathologic data prospectively collected for these cases. Data from this database were combined with data from the Danish National Health Registry, which includes all surgical procedures performed in Danish hospitals.
The researchers identified 414 breast cancer patients who underwent surgical excision up to 2 months before SLND and compared them with 16,960 breast cancer patients who underwent SLND without prior surgical tumor excision.
"The proportion of patients with isolated tumor cells was almost three times higher in the group with earlier surgical excision, compared to the group without earlier surgical excision," said Dr. Tvedskov of Copenhagen University Hospital. "One obvious explanation for these differences between groups is, of course, that the group with earlier surgical excision is a highly selected group of patients, where the cancer diagnosis was not obvious from the beginning."
Therefore, the researchers used a multivariate model to take into account these differences by adjusting for tumor size, histologic type and malignancy grade.
"We found that the risk of having isolated tumor cells in the sentinel node was nearly fourfold increased when surgical excision was performed, compared to patients without any earlier surgical excision," Dr. Tvedskov said. "Surgical excision leads to some degree of iatrogenic displacement."
[Trastuzumab/Pertuzumab Doublet Eradicates Some Early Breast Cancer]
In all, 64% of patients who underwent earlier surgical excision and 59% of those without earlier surgical excision were negative for sentinel lymph node metastases. Among the prior-excision group, 9% had isolated tumor cells in the sentinel lymph node, compared with 3% of the control group (odds ratio, 3.90; P less than .001).
Likewise, micrometastases in the sentinel lymph node were more common (15%) among the prior-excision group, compared with 12% in the control group (OR, 1.48; P = .006). However, macrometastases in the sentinel lymph node were more common in the control group (26% vs. 12%).
The researchers next looked at whether the histologic type of tumor mattered. After adjustment, "we found that the risk of having isolated tumor cells was three times higher in patients with lobular carcinomas, compared to patients with ductal carcinomas," said Dr. Tvedskov. The odds ratio was 3.02 (P less than .0001), after adjusting for tumor size, malignancy grade, and earlier surgical excision.
"Finding these results, we expected that the extra isolated tumor cells after earlier surgical excision would especially come from lobular carcinomas," she said. However, in a subanalysis, "we found that in the group of patients with ductal carcinomas, the risk of isolated tumor cells after surgical excision was nearly fivefold increased, compared to patients without surgical excision, whereas, in the group of patients with lobular carcinomas, there was no significant increased odds ratio."
Among patients with ductal carcinoma, isolated tumor cells were significantly more likely to be present in patients who underwent prior surgical excision compared with control patients (OR, 4.91; P less than .0001), after adjusting for tumor size and malignancy grade. Among lobular carcinoma patients though, isolated tumor cells were not significantly more likely to be present in those who underwent prior surgical excision (OR, 1.27; P = .69), after adjusting for tumor size and malignancy grade.
"So, despite the fact that the lobular carcinomas are, in general, more likely to present with isolated tumor cells in the sentinel node, the extra isolated tumor cells caused by the earlier surgical excision seems to come from ductal carcinomas," said Dr. Tvedskov.
The investigators also considered the question of clinical significance: Were the isolated tumor cells related to nonsentinel node metastases, or could an axillary lymph node dissection be omitted in these patients?
Looking at the distribution of nonsentinel node metastases in patients with either isolated tumor cells or micrometastases in the sentinel node, they found that isolated tumor cells were not present in nonsentinel lymph nodes among patients who underwent prior surgical excision. These cells, however, were found in nonsentinel lymph nodes in 12% of control patients.
Dr. Tvedskov pointed out that this difference did not achieve significance because of the small number of patients. There was no significant difference in the likelihood of micrometastases in nonsentinel lymph nodes between the two groups.
"This indicates that isolated tumor cells in patients with earlier surgical excision are not related to nonsentinel node metastases, and are therefore without clinical significance," she said.
The investigators reported that they have no relevant financial relationships.
[Bevacizumab Short of Mark in First Neoadjuvant Breast Cancer Trial]
SAN ANTONIO – Surgical excision of breast cancer prior to sentinel lymph node dissection can displace isolated tumor cells to the sentinel lymph node, but these tumor cells appear to have little clinical significance, according to an analysis of more than 17,000 patients in a large database.
"Earlier surgical excision leads to a nearly fourfold increase in the risk of having isolated tumor cells in the sentinel node indicating iatrogenic displacement," Dr. Tove F. Tvedskov said at the annual San Antonio Breast Cancer Symposium. She advised, however, that these cells are probably without clinical significance, and that the omission of axillary lymph node dissection should be considered.
[Women's Health Initiative: New Findings on Big-Three Cancer Rates]
The study was based on data from the Danish Breast Cancer Cooperative Group database, which includes more than 80,000 women with breast cancer. Approximately 3,000 new sentinel lymph node dissections (SLNDs) are included in the database each year, with clinical and histopathologic data prospectively collected for these cases. Data from this database were combined with data from the Danish National Health Registry, which includes all surgical procedures performed in Danish hospitals.
The researchers identified 414 breast cancer patients who underwent surgical excision up to 2 months before SLND and compared them with 16,960 breast cancer patients who underwent SLND without prior surgical tumor excision.
"The proportion of patients with isolated tumor cells was almost three times higher in the group with earlier surgical excision, compared to the group without earlier surgical excision," said Dr. Tvedskov of Copenhagen University Hospital. "One obvious explanation for these differences between groups is, of course, that the group with earlier surgical excision is a highly selected group of patients, where the cancer diagnosis was not obvious from the beginning."
Therefore, the researchers used a multivariate model to take into account these differences by adjusting for tumor size, histologic type and malignancy grade.
"We found that the risk of having isolated tumor cells in the sentinel node was nearly fourfold increased when surgical excision was performed, compared to patients without any earlier surgical excision," Dr. Tvedskov said. "Surgical excision leads to some degree of iatrogenic displacement."
[Trastuzumab/Pertuzumab Doublet Eradicates Some Early Breast Cancer]
In all, 64% of patients who underwent earlier surgical excision and 59% of those without earlier surgical excision were negative for sentinel lymph node metastases. Among the prior-excision group, 9% had isolated tumor cells in the sentinel lymph node, compared with 3% of the control group (odds ratio, 3.90; P less than .001).
Likewise, micrometastases in the sentinel lymph node were more common (15%) among the prior-excision group, compared with 12% in the control group (OR, 1.48; P = .006). However, macrometastases in the sentinel lymph node were more common in the control group (26% vs. 12%).
The researchers next looked at whether the histologic type of tumor mattered. After adjustment, "we found that the risk of having isolated tumor cells was three times higher in patients with lobular carcinomas, compared to patients with ductal carcinomas," said Dr. Tvedskov. The odds ratio was 3.02 (P less than .0001), after adjusting for tumor size, malignancy grade, and earlier surgical excision.
"Finding these results, we expected that the extra isolated tumor cells after earlier surgical excision would especially come from lobular carcinomas," she said. However, in a subanalysis, "we found that in the group of patients with ductal carcinomas, the risk of isolated tumor cells after surgical excision was nearly fivefold increased, compared to patients without surgical excision, whereas, in the group of patients with lobular carcinomas, there was no significant increased odds ratio."
Among patients with ductal carcinoma, isolated tumor cells were significantly more likely to be present in patients who underwent prior surgical excision compared with control patients (OR, 4.91; P less than .0001), after adjusting for tumor size and malignancy grade. Among lobular carcinoma patients though, isolated tumor cells were not significantly more likely to be present in those who underwent prior surgical excision (OR, 1.27; P = .69), after adjusting for tumor size and malignancy grade.
"So, despite the fact that the lobular carcinomas are, in general, more likely to present with isolated tumor cells in the sentinel node, the extra isolated tumor cells caused by the earlier surgical excision seems to come from ductal carcinomas," said Dr. Tvedskov.
The investigators also considered the question of clinical significance: Were the isolated tumor cells related to nonsentinel node metastases, or could an axillary lymph node dissection be omitted in these patients?
Looking at the distribution of nonsentinel node metastases in patients with either isolated tumor cells or micrometastases in the sentinel node, they found that isolated tumor cells were not present in nonsentinel lymph nodes among patients who underwent prior surgical excision. These cells, however, were found in nonsentinel lymph nodes in 12% of control patients.
Dr. Tvedskov pointed out that this difference did not achieve significance because of the small number of patients. There was no significant difference in the likelihood of micrometastases in nonsentinel lymph nodes between the two groups.
"This indicates that isolated tumor cells in patients with earlier surgical excision are not related to nonsentinel node metastases, and are therefore without clinical significance," she said.
The investigators reported that they have no relevant financial relationships.
[Bevacizumab Short of Mark in First Neoadjuvant Breast Cancer Trial]
Surgical Excision Can Spread Tumor Cells to Sentinel Node
SAN ANTONIO – Surgical excision of breast cancer prior to sentinel lymph node dissection can displace isolated tumor cells to the sentinel lymph node, but these tumor cells appear to have little clinical significance, according to an analysis of more than 17,000 patients in a large database.
"Earlier surgical excision leads to a nearly fourfold increase in the risk of having isolated tumor cells in the sentinel node indicating iatrogenic displacement," Dr. Tove F. Tvedskov said at the annual San Antonio Breast Cancer Symposium. She advised, however, that these cells are probably without clinical significance, and that the omission of axillary lymph node dissection should be considered.
[Women's Health Initiative: New Findings on Big-Three Cancer Rates]
The study was based on data from the Danish Breast Cancer Cooperative Group database, which includes more than 80,000 women with breast cancer. Approximately 3,000 new sentinel lymph node dissections (SLNDs) are included in the database each year, with clinical and histopathologic data prospectively collected for these cases. Data from this database were combined with data from the Danish National Health Registry, which includes all surgical procedures performed in Danish hospitals.
The researchers identified 414 breast cancer patients who underwent surgical excision up to 2 months before SLND and compared them with 16,960 breast cancer patients who underwent SLND without prior surgical tumor excision.
"The proportion of patients with isolated tumor cells was almost three times higher in the group with earlier surgical excision, compared to the group without earlier surgical excision," said Dr. Tvedskov of Copenhagen University Hospital. "One obvious explanation for these differences between groups is, of course, that the group with earlier surgical excision is a highly selected group of patients, where the cancer diagnosis was not obvious from the beginning."
Therefore, the researchers used a multivariate model to take into account these differences by adjusting for tumor size, histologic type and malignancy grade.
"We found that the risk of having isolated tumor cells in the sentinel node was nearly fourfold increased when surgical excision was performed, compared to patients without any earlier surgical excision," Dr. Tvedskov said. "Surgical excision leads to some degree of iatrogenic displacement."
[Trastuzumab/Pertuzumab Doublet Eradicates Some Early Breast Cancer]
In all, 64% of patients who underwent earlier surgical excision and 59% of those without earlier surgical excision were negative for sentinel lymph node metastases. Among the prior-excision group, 9% had isolated tumor cells in the sentinel lymph node, compared with 3% of the control group (odds ratio, 3.90; P less than .001).
Likewise, micrometastases in the sentinel lymph node were more common (15%) among the prior-excision group, compared with 12% in the control group (OR, 1.48; P = .006). However, macrometastases in the sentinel lymph node were more common in the control group (26% vs. 12%).
The researchers next looked at whether the histologic type of tumor mattered. After adjustment, "we found that the risk of having isolated tumor cells was three times higher in patients with lobular carcinomas, compared to patients with ductal carcinomas," said Dr. Tvedskov. The odds ratio was 3.02 (P less than .0001), after adjusting for tumor size, malignancy grade, and earlier surgical excision.
"Finding these results, we expected that the extra isolated tumor cells after earlier surgical excision would especially come from lobular carcinomas," she said. However, in a subanalysis, "we found that in the group of patients with ductal carcinomas, the risk of isolated tumor cells after surgical excision was nearly fivefold increased, compared to patients without surgical excision, whereas, in the group of patients with lobular carcinomas, there was no significant increased odds ratio."
Among patients with ductal carcinoma, isolated tumor cells were significantly more likely to be present in patients who underwent prior surgical excision compared with control patients (OR, 4.91; P less than .0001), after adjusting for tumor size and malignancy grade. Among lobular carcinoma patients though, isolated tumor cells were not significantly more likely to be present in those who underwent prior surgical excision (OR, 1.27; P = .69), after adjusting for tumor size and malignancy grade.
"So, despite the fact that the lobular carcinomas are, in general, more likely to present with isolated tumor cells in the sentinel node, the extra isolated tumor cells caused by the earlier surgical excision seems to come from ductal carcinomas," said Dr. Tvedskov.
The investigators also considered the question of clinical significance: Were the isolated tumor cells related to nonsentinel node metastases, or could an axillary lymph node dissection be omitted in these patients?
Looking at the distribution of nonsentinel node metastases in patients with either isolated tumor cells or micrometastases in the sentinel node, they found that isolated tumor cells were not present in nonsentinel lymph nodes among patients who underwent prior surgical excision. These cells, however, were found in nonsentinel lymph nodes in 12% of control patients.
Dr. Tvedskov pointed out that this difference did not achieve significance because of the small number of patients. There was no significant difference in the likelihood of micrometastases in nonsentinel lymph nodes between the two groups.
"This indicates that isolated tumor cells in patients with earlier surgical excision are not related to nonsentinel node metastases, and are therefore without clinical significance," she said.
The investigators reported that they have no relevant financial relationships.
[Bevacizumab Short of Mark in First Neoadjuvant Breast Cancer Trial]
SAN ANTONIO – Surgical excision of breast cancer prior to sentinel lymph node dissection can displace isolated tumor cells to the sentinel lymph node, but these tumor cells appear to have little clinical significance, according to an analysis of more than 17,000 patients in a large database.
"Earlier surgical excision leads to a nearly fourfold increase in the risk of having isolated tumor cells in the sentinel node indicating iatrogenic displacement," Dr. Tove F. Tvedskov said at the annual San Antonio Breast Cancer Symposium. She advised, however, that these cells are probably without clinical significance, and that the omission of axillary lymph node dissection should be considered.
[Women's Health Initiative: New Findings on Big-Three Cancer Rates]
The study was based on data from the Danish Breast Cancer Cooperative Group database, which includes more than 80,000 women with breast cancer. Approximately 3,000 new sentinel lymph node dissections (SLNDs) are included in the database each year, with clinical and histopathologic data prospectively collected for these cases. Data from this database were combined with data from the Danish National Health Registry, which includes all surgical procedures performed in Danish hospitals.
The researchers identified 414 breast cancer patients who underwent surgical excision up to 2 months before SLND and compared them with 16,960 breast cancer patients who underwent SLND without prior surgical tumor excision.
"The proportion of patients with isolated tumor cells was almost three times higher in the group with earlier surgical excision, compared to the group without earlier surgical excision," said Dr. Tvedskov of Copenhagen University Hospital. "One obvious explanation for these differences between groups is, of course, that the group with earlier surgical excision is a highly selected group of patients, where the cancer diagnosis was not obvious from the beginning."
Therefore, the researchers used a multivariate model to take into account these differences by adjusting for tumor size, histologic type and malignancy grade.
"We found that the risk of having isolated tumor cells in the sentinel node was nearly fourfold increased when surgical excision was performed, compared to patients without any earlier surgical excision," Dr. Tvedskov said. "Surgical excision leads to some degree of iatrogenic displacement."
[Trastuzumab/Pertuzumab Doublet Eradicates Some Early Breast Cancer]
In all, 64% of patients who underwent earlier surgical excision and 59% of those without earlier surgical excision were negative for sentinel lymph node metastases. Among the prior-excision group, 9% had isolated tumor cells in the sentinel lymph node, compared with 3% of the control group (odds ratio, 3.90; P less than .001).
Likewise, micrometastases in the sentinel lymph node were more common (15%) among the prior-excision group, compared with 12% in the control group (OR, 1.48; P = .006). However, macrometastases in the sentinel lymph node were more common in the control group (26% vs. 12%).
The researchers next looked at whether the histologic type of tumor mattered. After adjustment, "we found that the risk of having isolated tumor cells was three times higher in patients with lobular carcinomas, compared to patients with ductal carcinomas," said Dr. Tvedskov. The odds ratio was 3.02 (P less than .0001), after adjusting for tumor size, malignancy grade, and earlier surgical excision.
"Finding these results, we expected that the extra isolated tumor cells after earlier surgical excision would especially come from lobular carcinomas," she said. However, in a subanalysis, "we found that in the group of patients with ductal carcinomas, the risk of isolated tumor cells after surgical excision was nearly fivefold increased, compared to patients without surgical excision, whereas, in the group of patients with lobular carcinomas, there was no significant increased odds ratio."
Among patients with ductal carcinoma, isolated tumor cells were significantly more likely to be present in patients who underwent prior surgical excision compared with control patients (OR, 4.91; P less than .0001), after adjusting for tumor size and malignancy grade. Among lobular carcinoma patients though, isolated tumor cells were not significantly more likely to be present in those who underwent prior surgical excision (OR, 1.27; P = .69), after adjusting for tumor size and malignancy grade.
"So, despite the fact that the lobular carcinomas are, in general, more likely to present with isolated tumor cells in the sentinel node, the extra isolated tumor cells caused by the earlier surgical excision seems to come from ductal carcinomas," said Dr. Tvedskov.
The investigators also considered the question of clinical significance: Were the isolated tumor cells related to nonsentinel node metastases, or could an axillary lymph node dissection be omitted in these patients?
Looking at the distribution of nonsentinel node metastases in patients with either isolated tumor cells or micrometastases in the sentinel node, they found that isolated tumor cells were not present in nonsentinel lymph nodes among patients who underwent prior surgical excision. These cells, however, were found in nonsentinel lymph nodes in 12% of control patients.
Dr. Tvedskov pointed out that this difference did not achieve significance because of the small number of patients. There was no significant difference in the likelihood of micrometastases in nonsentinel lymph nodes between the two groups.
"This indicates that isolated tumor cells in patients with earlier surgical excision are not related to nonsentinel node metastases, and are therefore without clinical significance," she said.
The investigators reported that they have no relevant financial relationships.
[Bevacizumab Short of Mark in First Neoadjuvant Breast Cancer Trial]
SAN ANTONIO – Surgical excision of breast cancer prior to sentinel lymph node dissection can displace isolated tumor cells to the sentinel lymph node, but these tumor cells appear to have little clinical significance, according to an analysis of more than 17,000 patients in a large database.
"Earlier surgical excision leads to a nearly fourfold increase in the risk of having isolated tumor cells in the sentinel node indicating iatrogenic displacement," Dr. Tove F. Tvedskov said at the annual San Antonio Breast Cancer Symposium. She advised, however, that these cells are probably without clinical significance, and that the omission of axillary lymph node dissection should be considered.
[Women's Health Initiative: New Findings on Big-Three Cancer Rates]
The study was based on data from the Danish Breast Cancer Cooperative Group database, which includes more than 80,000 women with breast cancer. Approximately 3,000 new sentinel lymph node dissections (SLNDs) are included in the database each year, with clinical and histopathologic data prospectively collected for these cases. Data from this database were combined with data from the Danish National Health Registry, which includes all surgical procedures performed in Danish hospitals.
The researchers identified 414 breast cancer patients who underwent surgical excision up to 2 months before SLND and compared them with 16,960 breast cancer patients who underwent SLND without prior surgical tumor excision.
"The proportion of patients with isolated tumor cells was almost three times higher in the group with earlier surgical excision, compared to the group without earlier surgical excision," said Dr. Tvedskov of Copenhagen University Hospital. "One obvious explanation for these differences between groups is, of course, that the group with earlier surgical excision is a highly selected group of patients, where the cancer diagnosis was not obvious from the beginning."
Therefore, the researchers used a multivariate model to take into account these differences by adjusting for tumor size, histologic type and malignancy grade.
"We found that the risk of having isolated tumor cells in the sentinel node was nearly fourfold increased when surgical excision was performed, compared to patients without any earlier surgical excision," Dr. Tvedskov said. "Surgical excision leads to some degree of iatrogenic displacement."
[Trastuzumab/Pertuzumab Doublet Eradicates Some Early Breast Cancer]
In all, 64% of patients who underwent earlier surgical excision and 59% of those without earlier surgical excision were negative for sentinel lymph node metastases. Among the prior-excision group, 9% had isolated tumor cells in the sentinel lymph node, compared with 3% of the control group (odds ratio, 3.90; P less than .001).
Likewise, micrometastases in the sentinel lymph node were more common (15%) among the prior-excision group, compared with 12% in the control group (OR, 1.48; P = .006). However, macrometastases in the sentinel lymph node were more common in the control group (26% vs. 12%).
The researchers next looked at whether the histologic type of tumor mattered. After adjustment, "we found that the risk of having isolated tumor cells was three times higher in patients with lobular carcinomas, compared to patients with ductal carcinomas," said Dr. Tvedskov. The odds ratio was 3.02 (P less than .0001), after adjusting for tumor size, malignancy grade, and earlier surgical excision.
"Finding these results, we expected that the extra isolated tumor cells after earlier surgical excision would especially come from lobular carcinomas," she said. However, in a subanalysis, "we found that in the group of patients with ductal carcinomas, the risk of isolated tumor cells after surgical excision was nearly fivefold increased, compared to patients without surgical excision, whereas, in the group of patients with lobular carcinomas, there was no significant increased odds ratio."
Among patients with ductal carcinoma, isolated tumor cells were significantly more likely to be present in patients who underwent prior surgical excision compared with control patients (OR, 4.91; P less than .0001), after adjusting for tumor size and malignancy grade. Among lobular carcinoma patients though, isolated tumor cells were not significantly more likely to be present in those who underwent prior surgical excision (OR, 1.27; P = .69), after adjusting for tumor size and malignancy grade.
"So, despite the fact that the lobular carcinomas are, in general, more likely to present with isolated tumor cells in the sentinel node, the extra isolated tumor cells caused by the earlier surgical excision seems to come from ductal carcinomas," said Dr. Tvedskov.
The investigators also considered the question of clinical significance: Were the isolated tumor cells related to nonsentinel node metastases, or could an axillary lymph node dissection be omitted in these patients?
Looking at the distribution of nonsentinel node metastases in patients with either isolated tumor cells or micrometastases in the sentinel node, they found that isolated tumor cells were not present in nonsentinel lymph nodes among patients who underwent prior surgical excision. These cells, however, were found in nonsentinel lymph nodes in 12% of control patients.
Dr. Tvedskov pointed out that this difference did not achieve significance because of the small number of patients. There was no significant difference in the likelihood of micrometastases in nonsentinel lymph nodes between the two groups.
"This indicates that isolated tumor cells in patients with earlier surgical excision are not related to nonsentinel node metastases, and are therefore without clinical significance," she said.
The investigators reported that they have no relevant financial relationships.
[Bevacizumab Short of Mark in First Neoadjuvant Breast Cancer Trial]
FROM THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: Surgical excision prior to sentinel lymph node dissection leads to a nearly fourfold increase in the risk of having isolated tumor cells in the sentinel node (OR = 3.90, P less than .001).
Data Source: Analysis of 414 breast cancer patients who underwent surgical excision up to 2 months before SLND and 16,960 breast cancer patients who underwent SLND without prior surgical tumor excision. The women were included in Danish Breast Cancer Cooperative Group database.
Disclosures: The investigators reported that they have no relevant financial relationships.
Bevacizumab Short of Mark in First Neoadjuvant Breast Cancer Trial
SAN ANTONIO – Adding bevacizumab to neoadjuvant chemotherapy with epirubicin, cyclophosphamide, and docetaxel did not improve pathological complete response in a large, European trial among women with primary HER2-negative breast cancer.
Only patients with triple-negative disease appeared to benefit from the addition of bevacizumab (Avastin) in a planned subgroup analysis of the first phase III trial to report efficacy outcomes for the monoclonal antibody in this setting.
"Hormone receptor–positive patients did have almost identical pCR rates with or without bevacizumab," said Dr. Gunter von Minckwitz, managing director of the German Breast Group in Neu-Isenburg, Germany, at the annual San Antonio Breast Cancer Symposium. "However, the triple-negative patients ... had an increase of around 40% when bevacizumab was added, and this was just statistically significant."
Pathological complete response (pCR) overall – 15% for chemotherapy alone and 17.5% for chemotherapy plus bevacizumab – did not differ significantly between the two arms in the study, which was part of the German Breast Group’s GEPARquinto trial. The primary end point was the pCR rate (defined as no invasive or noninvasive residual viable tumor cells in any resected specimens of the breast and axillary nodes).
This GEPARquinto trial is the first randomized, phase III study to investigate the effect of bevacizumab plus chemotherapy in early or locally advanced breast cancer. An antiangiogenesis agent targeting the vascular endothelial growth factor (VEGF), bevacizumab is approved for first-line treatment of metastatic breast cancer in the United States. The Food and Drug Administration is considering a near-unanimous advisory panel recommendation that this approval be rescinded, however, because follow-up studies failed to confirm the progression-free survival data on which the approval was based.
The GEPARquinto trial randomized women with early or locally advanced HER2-negative breast cancer to receive either standard epirubicin (Ellence)/cyclophosphamide (EC) therapy followed by standard docetaxel (Taxotere) therapy with or without bevacizumab.
Women with untreated primary breast carcinoma were included if they were HER2 negative based on local pathology (immunohistochemical score of 0-1, or negative status by fluorescence in situ hybridization). Participants had to have breast lesions of at least 2 cm as determined by palpation or at least 1 cm as determined by ultrasound. The following tumor stages were included: cT4 or cT3; cT2, if the tumor was hormone receptor negative or clinically node positive; or cT1 if the tumor was hormone receptor negative or non–sentinel lymph node positive. The women also had to have normal organ function, including a left ventricular ejection fraction of at least 55%.
In the first part of the study (four 3-week cycles), epirubicin was administered at a dose of 90 mg/m2, cyclophosphamide at a dose of 600 mg/m2, and bevacizumab at a dose of 15 mg/kg .
"We had an assessment of response after four cycles [EC with or without bevacizumab], and those patients who did not achieve a complete or partial remission were taken out of the initial treatment," said Dr. von Minckwitz.
Patients with a clinical response, as determined by sonographic evaluation, switched to chemotherapy with 100 mg/m2 docetaxel with or without bevacizumab for four 3-week cycles. Surgery was delayed by 1 week for patients in the bevacizumab arm (at least 28 days after the last infusion of bevacizumab).
A total of 968 women started standard treatment and 959 started the same treatment plus bevacizumab. In the standard therapy arm, 36% of patients discontinued treatment (mostly because of lack of response); 30% of patients discontinued all treatment in the bevacizumab arm, and 4% discontinued bevacizumab only.
The median patient age was 48 years in the standard treatment arm and 49 years in the bevacizumab arm. The median palpable tumor size was 4 cm for both groups. In addition, 35% of women in each group were hormone receptor negative.
The researchers also looked at other definitions of pCR. When pCR was defined as no invasive residual tumor cells in the breast and nodes, they still found no significant difference: 18.5% for standard chemotherapy and 20.3% for chemotherapy plus bevacizumab. When pCR was defined as no invasive residual tumor cells in the breast, the difference also was not significant: 21.3% for standard chemotherapy and 23.9% for chemotherapy plus bevacizumab.
The breast conservation rate also did not differ significantly, at two-thirds of women in each arm.
As of Dec. 1, 2010, serious adverse events have occurred in 12% of women on standard EC therapy, 16% for women on EC plus bevacizumab, 13% of those on docetaxel alone, and 23% of those on docetaxel and bevacizumab.
Febrile neutropenia occurred in 13 women on standard EC therapy, 27 women on EC plus bevacizumab, 13 women on docetaxel alone, and 23 women on docetaxel and bevacizumab.
Dr. von Minckwitz reported that he receives grant support from Sanofi-Aventis, Roche, GlaxoSmithKline, and Novartis. Several of his coinvestigators also reported relevant financial relationships with multiple pharmaceutical companies.
SAN ANTONIO – Adding bevacizumab to neoadjuvant chemotherapy with epirubicin, cyclophosphamide, and docetaxel did not improve pathological complete response in a large, European trial among women with primary HER2-negative breast cancer.
Only patients with triple-negative disease appeared to benefit from the addition of bevacizumab (Avastin) in a planned subgroup analysis of the first phase III trial to report efficacy outcomes for the monoclonal antibody in this setting.
"Hormone receptor–positive patients did have almost identical pCR rates with or without bevacizumab," said Dr. Gunter von Minckwitz, managing director of the German Breast Group in Neu-Isenburg, Germany, at the annual San Antonio Breast Cancer Symposium. "However, the triple-negative patients ... had an increase of around 40% when bevacizumab was added, and this was just statistically significant."
Pathological complete response (pCR) overall – 15% for chemotherapy alone and 17.5% for chemotherapy plus bevacizumab – did not differ significantly between the two arms in the study, which was part of the German Breast Group’s GEPARquinto trial. The primary end point was the pCR rate (defined as no invasive or noninvasive residual viable tumor cells in any resected specimens of the breast and axillary nodes).
This GEPARquinto trial is the first randomized, phase III study to investigate the effect of bevacizumab plus chemotherapy in early or locally advanced breast cancer. An antiangiogenesis agent targeting the vascular endothelial growth factor (VEGF), bevacizumab is approved for first-line treatment of metastatic breast cancer in the United States. The Food and Drug Administration is considering a near-unanimous advisory panel recommendation that this approval be rescinded, however, because follow-up studies failed to confirm the progression-free survival data on which the approval was based.
The GEPARquinto trial randomized women with early or locally advanced HER2-negative breast cancer to receive either standard epirubicin (Ellence)/cyclophosphamide (EC) therapy followed by standard docetaxel (Taxotere) therapy with or without bevacizumab.
Women with untreated primary breast carcinoma were included if they were HER2 negative based on local pathology (immunohistochemical score of 0-1, or negative status by fluorescence in situ hybridization). Participants had to have breast lesions of at least 2 cm as determined by palpation or at least 1 cm as determined by ultrasound. The following tumor stages were included: cT4 or cT3; cT2, if the tumor was hormone receptor negative or clinically node positive; or cT1 if the tumor was hormone receptor negative or non–sentinel lymph node positive. The women also had to have normal organ function, including a left ventricular ejection fraction of at least 55%.
In the first part of the study (four 3-week cycles), epirubicin was administered at a dose of 90 mg/m2, cyclophosphamide at a dose of 600 mg/m2, and bevacizumab at a dose of 15 mg/kg .
"We had an assessment of response after four cycles [EC with or without bevacizumab], and those patients who did not achieve a complete or partial remission were taken out of the initial treatment," said Dr. von Minckwitz.
Patients with a clinical response, as determined by sonographic evaluation, switched to chemotherapy with 100 mg/m2 docetaxel with or without bevacizumab for four 3-week cycles. Surgery was delayed by 1 week for patients in the bevacizumab arm (at least 28 days after the last infusion of bevacizumab).
A total of 968 women started standard treatment and 959 started the same treatment plus bevacizumab. In the standard therapy arm, 36% of patients discontinued treatment (mostly because of lack of response); 30% of patients discontinued all treatment in the bevacizumab arm, and 4% discontinued bevacizumab only.
The median patient age was 48 years in the standard treatment arm and 49 years in the bevacizumab arm. The median palpable tumor size was 4 cm for both groups. In addition, 35% of women in each group were hormone receptor negative.
The researchers also looked at other definitions of pCR. When pCR was defined as no invasive residual tumor cells in the breast and nodes, they still found no significant difference: 18.5% for standard chemotherapy and 20.3% for chemotherapy plus bevacizumab. When pCR was defined as no invasive residual tumor cells in the breast, the difference also was not significant: 21.3% for standard chemotherapy and 23.9% for chemotherapy plus bevacizumab.
The breast conservation rate also did not differ significantly, at two-thirds of women in each arm.
As of Dec. 1, 2010, serious adverse events have occurred in 12% of women on standard EC therapy, 16% for women on EC plus bevacizumab, 13% of those on docetaxel alone, and 23% of those on docetaxel and bevacizumab.
Febrile neutropenia occurred in 13 women on standard EC therapy, 27 women on EC plus bevacizumab, 13 women on docetaxel alone, and 23 women on docetaxel and bevacizumab.
Dr. von Minckwitz reported that he receives grant support from Sanofi-Aventis, Roche, GlaxoSmithKline, and Novartis. Several of his coinvestigators also reported relevant financial relationships with multiple pharmaceutical companies.
SAN ANTONIO – Adding bevacizumab to neoadjuvant chemotherapy with epirubicin, cyclophosphamide, and docetaxel did not improve pathological complete response in a large, European trial among women with primary HER2-negative breast cancer.
Only patients with triple-negative disease appeared to benefit from the addition of bevacizumab (Avastin) in a planned subgroup analysis of the first phase III trial to report efficacy outcomes for the monoclonal antibody in this setting.
"Hormone receptor–positive patients did have almost identical pCR rates with or without bevacizumab," said Dr. Gunter von Minckwitz, managing director of the German Breast Group in Neu-Isenburg, Germany, at the annual San Antonio Breast Cancer Symposium. "However, the triple-negative patients ... had an increase of around 40% when bevacizumab was added, and this was just statistically significant."
Pathological complete response (pCR) overall – 15% for chemotherapy alone and 17.5% for chemotherapy plus bevacizumab – did not differ significantly between the two arms in the study, which was part of the German Breast Group’s GEPARquinto trial. The primary end point was the pCR rate (defined as no invasive or noninvasive residual viable tumor cells in any resected specimens of the breast and axillary nodes).
This GEPARquinto trial is the first randomized, phase III study to investigate the effect of bevacizumab plus chemotherapy in early or locally advanced breast cancer. An antiangiogenesis agent targeting the vascular endothelial growth factor (VEGF), bevacizumab is approved for first-line treatment of metastatic breast cancer in the United States. The Food and Drug Administration is considering a near-unanimous advisory panel recommendation that this approval be rescinded, however, because follow-up studies failed to confirm the progression-free survival data on which the approval was based.
The GEPARquinto trial randomized women with early or locally advanced HER2-negative breast cancer to receive either standard epirubicin (Ellence)/cyclophosphamide (EC) therapy followed by standard docetaxel (Taxotere) therapy with or without bevacizumab.
Women with untreated primary breast carcinoma were included if they were HER2 negative based on local pathology (immunohistochemical score of 0-1, or negative status by fluorescence in situ hybridization). Participants had to have breast lesions of at least 2 cm as determined by palpation or at least 1 cm as determined by ultrasound. The following tumor stages were included: cT4 or cT3; cT2, if the tumor was hormone receptor negative or clinically node positive; or cT1 if the tumor was hormone receptor negative or non–sentinel lymph node positive. The women also had to have normal organ function, including a left ventricular ejection fraction of at least 55%.
In the first part of the study (four 3-week cycles), epirubicin was administered at a dose of 90 mg/m2, cyclophosphamide at a dose of 600 mg/m2, and bevacizumab at a dose of 15 mg/kg .
"We had an assessment of response after four cycles [EC with or without bevacizumab], and those patients who did not achieve a complete or partial remission were taken out of the initial treatment," said Dr. von Minckwitz.
Patients with a clinical response, as determined by sonographic evaluation, switched to chemotherapy with 100 mg/m2 docetaxel with or without bevacizumab for four 3-week cycles. Surgery was delayed by 1 week for patients in the bevacizumab arm (at least 28 days after the last infusion of bevacizumab).
A total of 968 women started standard treatment and 959 started the same treatment plus bevacizumab. In the standard therapy arm, 36% of patients discontinued treatment (mostly because of lack of response); 30% of patients discontinued all treatment in the bevacizumab arm, and 4% discontinued bevacizumab only.
The median patient age was 48 years in the standard treatment arm and 49 years in the bevacizumab arm. The median palpable tumor size was 4 cm for both groups. In addition, 35% of women in each group were hormone receptor negative.
The researchers also looked at other definitions of pCR. When pCR was defined as no invasive residual tumor cells in the breast and nodes, they still found no significant difference: 18.5% for standard chemotherapy and 20.3% for chemotherapy plus bevacizumab. When pCR was defined as no invasive residual tumor cells in the breast, the difference also was not significant: 21.3% for standard chemotherapy and 23.9% for chemotherapy plus bevacizumab.
The breast conservation rate also did not differ significantly, at two-thirds of women in each arm.
As of Dec. 1, 2010, serious adverse events have occurred in 12% of women on standard EC therapy, 16% for women on EC plus bevacizumab, 13% of those on docetaxel alone, and 23% of those on docetaxel and bevacizumab.
Febrile neutropenia occurred in 13 women on standard EC therapy, 27 women on EC plus bevacizumab, 13 women on docetaxel alone, and 23 women on docetaxel and bevacizumab.
Dr. von Minckwitz reported that he receives grant support from Sanofi-Aventis, Roche, GlaxoSmithKline, and Novartis. Several of his coinvestigators also reported relevant financial relationships with multiple pharmaceutical companies.
FROM THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: Pathological complete response overall did not differ significantly at 15% in woman treated with neoadjuvant chemotherapy alone and 17.5% among those given chemotherapy plus bevacizumab.
Data Source: A total of 1,927 women with HER2-negative, early-stage breast cancer in the German Breast Group’s GEPARquinto trial program.
Disclosures: Dr. von Minckwitz reported that he receives grant support from Sanofi-Aventis, Roche, GlaxoSmithKline, and Novartis. Several of his coinvestigators also reported relevant financial relationships with multiple pharmaceutical companies.