User login
Ureteral reimplantation for injuries not easily managed with stenting
The risk of lower urinary tract injury (bladder or ureters) at the time of benign gynecologic surgery is estimated to be between 0.3% and 4%. The majority are bladder injuries, with ureteral injuries occurring in 0.3%-1.8% of hysterectomies. While urologic procedures account for the majority of iatrogenic ureteral injuries, gynecologic surgery is the second leading cause, followed by general surgery and colorectal surgery.
With respect to hysterectomy in particular, the risk of ureteral injury is less than 1%. In a large prospective cohort of women undergoing hysterectomy for benign indications in 53 hospitals in Finland, rates of ureteral injury varied based on the route of hysterectomy, with laparoscopic and abdominal routes having an injury rate of 0.3% and the vaginal route having an injury rate of 0.04% (Human Reprod. 2011;26[7]:1741-51). The risk generally is higher with procedures for endometriosis, large fibroids, cancer, or pelvic organ prolapse.
During hysterectomy and with gynecologic surgery overall, ureteral injuries occur most commonly at three locations: at the level of the infundibulopelvic ligament and ovarian vessels, at the level of the uterine artery, and near the vaginal cuff. Identification and knowledge of the course of the ureter at these three locations is essential in preventing ureteral injury during pelvic surgery.

SOURCE: DR. MUELLER AND DR. KENTON
Perioperative ureteral stenting has been proposed as a method of preventing iatrogenic injury by allowing surgeons to more easily identify the ureters during surgery. Available reports suggest, however, that the actual risk of injury is not decreased and may even be increased by placing prophylactic ureteral stents, and most surgeons have moved away from this practice. The use of lighted ureteral stents during complex laparoscopic endometriosis resections may be helpful.
Many health care systems recommend intraoperative cystoscopy with bladder and ureteral survey to evaluate the integrity of the lower urinary tract at the time of all hysterectomies. A recent study of nearly 3,000 women undergoing benign hysterectomies at the University of Michigan, Ann Arbor, showed a significant decrease in the rate of delayed diagnosis of urinary tract injuries with implementation of a universal cystoscopy policy. While the rate of lower urinary tract injury was fairly consistent before and after implementation of the policy (2.6% and 1.8%, respectively), the rate of delayed detection of a lower urinary tract injury decreased from 0.7% before the policy to 0.1% after implementation (Obstet Gynecol. 2016;127[2]:369-75). The study also showed that hospital costs nearly doubled with a delayed detection of a lower urinary tract injury.
Unfortunately, even a normal postoperative cystoscopy does not ensure there is no lower urinary tract injury, especially considering that thermal injuries resulting from the use of energy devices typically do not present until 7-14 days after surgery. Overall, however, ureteral injury detection rates with universal cystoscopy approach 97% (Obstet Gynecol. 2009;113:6-10).
Identifying injuries
Intraoperative recognition and repair always is preferred, and when ureteral injuries are discovered or suspected in the operating room, cystoscopy and retrograde pyelography is the most helpful imaging tool. Contrast dye is injected during cystoscopy directly into the renal collecting system through the ureteral orifices; with fluoroscopy, the surgeon can visualize the integrity of the ureter from the bladder to the renal pelvis to diagnosis a ureteral injury, including ureteral transection, kinking, or ligation caused by a suture or sealing device.
If retrograde pyelography shows a transection or injury from a crushing clamp or sealing device, we recommend ureteroureteral anastomosis or urethral neocystostomy depending on the extent and location of the injury. If the ureter just appears kinked, sometime simply releasing the cuff sutures or uterosacral ligament sutures will resolve the obstruction. If there is extravasation of contrast suggesting a partial tear, placing a double-J ureteral stent for 6-8 weeks is frequently sufficient.
Patients with delayed iatrogenic ureteral injuries present with symptoms that often are nonspecific and that include abdominal or flank pain, fever, nausea, vomiting, back pain, and leukocytosis.
We recommend that patients with a history of surgery and symptoms suggestive of a ureteral injury be initially evaluated with CT urography that images the renal collecting system both as contrast dye is instilled and again several minutes later as it has progressed through the entire urinary tract. Alternatively, if CT urography is unavailable, a retrograde pyelogram can be performed as an emergency procedure to determine the location of renal injury.
Surgical management
Delayed ureteral injuries resulting in partial ureteral obstruction or extravasation of urine into the pelvis can sometimes be managed conservatively though placement of an internalized double J stent. The stent can be placed in a retrograde fashion via cystoscopy by a
We typically manage delayed ureteral injuries that are not amenable to, or do not heal with, ureteral stenting with ureteroneocystotomy, or ureteral reimplantation, into the bladder. This technique is effective for distal ureteral injuries that result in obstruction or fistula and are in close proximity to the bladder (most iatrogenic gynecologic injuries). We perform ureteroneocystotomy via an open, robotic, or laparoscopic route of access depending on the circumstance.
Our preferred route of access is minimally invasive with the da Vinci robot. A camera port is placed at the umbilicus, two robotic ports are placed on the patient’s left side at the level of the umbilicus, and one is placed on the patient’s right side at the level of the umbilicus with an additional assistant port on the right side. It is helpful if each port is at least 8 cm apart. If obstruction or transection is suspected to be more proximal, the ports may have been shifted above the umbilicus to optimally mobilize the ureter.
First, the ureter is identified and dissected. Regardless of the site of injury, which is usually identifiable with inflammation and scar tissue, it is always easiest to identify the ureter at the bifurcation of the common iliac vessels. The isolated ureter is inspected proximally and above the area of injury, and we find it helpful to place a vessel loop around the ureter for easy manipulation and counter traction. Care must be taken not to disturb the adventitia and blood supply. We do not transect the ureter until we’re ready to reimplant it in the bladder.
To mobilize the bladder and prepare for a tension-free anastomosis, an adequate retropubic dissection is performed, starting with an incision in the anterior abdominal wall peritoneum and taking it down to the level of the pubic bone and into the retropubic space. It is important to be mindful of the location of the obturator neurovascular bundle when performing this dissection.
Achieving a tension-free and water-tight anastomosis of the ureter to the bladder is critical. The bladder should be mobilized such that it reaches to above the injured portion of the ureter. The bladder is retrograde filled with approximately 300 mL and a reimplantation site of the posterior bladder is identified. When there is concern about tension, a psoas hitch suture can be placed to keep the bladder in a superior position with reduced tension. Because of high rates of the congenital absence of the psoas tendon minor, we advocate direct visualization of the genitofemoral nerve by incising the peritoneum; this will avoid nerve entrapment.
Once the bladder is mobilized and the ureter isolated, we perform an intentional cystotomy in the posterior lateral aspect of the bladder. The ureter, which is on the vessel loop, must be transected proximal to the site of injury. To facilitate this, we spatulate the ureter, making a vertical incision of often about 5 mm in length to increase our surface area for anastomosis. Placement of a suture at the apex of the spatulated ureter helps us maintain orientation.
Anastomosis of the ureter and bladder is achieved in a mucosa-to-mucosa fashion using a series of interrupted monofilament fine absorbable sutures; we use a 3-0 monocryl suture. The most posterior anastomotic sutures are placed first to allow for optimal visualization, and prior to completing the anastomosis, a guide wire is placed through the open ureter and a double-J stent is introduced into the renal pelvis. The wire is then removed and the distal end of the stent coiled in the bladder. This stent will protect the ureter for about 6 weeks while it heals. The anastomosis is then completed on the anterior aspect, with a watertight closure ensured.
Postoperatively, we routinely perform an x-ray to ensure proper placement of the stent in the reimplanted ureter. To determine correct stent placement, the last rib is identified at T12 vertebrae. The renal pelvis is located at the level of the L2-L3 with the left being slightly higher than the right. A Foley catheter is maintained in the bladder for approximately 2 weeks, and the stent is maintained for approximately 6 weeks. Both the catheter and the stent can be removed in the office with cystoscopic guidance.
Imaging at 4-6 weeks after removal of the stent is performed to rule out development of an obstruction or a stricture. In patients who did not have a dilated ureter and renal collecting system prior to reimplantation, a renal ultrasound is sufficient to identify hydroureter/hydronephrosis or a urinoma. Many patients with a markedly dilated renal-collecting system prior to ureteral reimplantation will have persistent hydroureter/hydronephrosis (similar to a latex balloon that does not return to its original size after it is blown up) after reimplantation. A Lasix renal scan is a better imaging modality in these patients because it can differentiate a ureter that is dilated from one that is dilated and obstructed.
It is important to note that prompt ureteroneocystotomy is feasible only when the delayed ureteral injury presents within approximately 7 days of surgery. If the patient presents more than a week after surgery, inflammation is so significant that conservative management is necessary with reevaluation for reimplantation in another 6 weeks. Decompression of the system prior to reimplantation can be achieved through either stent placement or placement of a percutaneous nephrostomy tube. We prefer the latter because it reduces inflammation around the ureter that may make subsequent dissection and surgery more difficult.
Dr. Kenton is chief of urogynecology, Northwestern University, Chicago, and Dr. Mueller also is in the division of female pelvic medicine and reconstructive surgery–urogynecology at Northwestern. Dr. Kenton discloses grant funding from Boston Scientific.
The risk of lower urinary tract injury (bladder or ureters) at the time of benign gynecologic surgery is estimated to be between 0.3% and 4%. The majority are bladder injuries, with ureteral injuries occurring in 0.3%-1.8% of hysterectomies. While urologic procedures account for the majority of iatrogenic ureteral injuries, gynecologic surgery is the second leading cause, followed by general surgery and colorectal surgery.
With respect to hysterectomy in particular, the risk of ureteral injury is less than 1%. In a large prospective cohort of women undergoing hysterectomy for benign indications in 53 hospitals in Finland, rates of ureteral injury varied based on the route of hysterectomy, with laparoscopic and abdominal routes having an injury rate of 0.3% and the vaginal route having an injury rate of 0.04% (Human Reprod. 2011;26[7]:1741-51). The risk generally is higher with procedures for endometriosis, large fibroids, cancer, or pelvic organ prolapse.
During hysterectomy and with gynecologic surgery overall, ureteral injuries occur most commonly at three locations: at the level of the infundibulopelvic ligament and ovarian vessels, at the level of the uterine artery, and near the vaginal cuff. Identification and knowledge of the course of the ureter at these three locations is essential in preventing ureteral injury during pelvic surgery.

SOURCE: DR. MUELLER AND DR. KENTON
Perioperative ureteral stenting has been proposed as a method of preventing iatrogenic injury by allowing surgeons to more easily identify the ureters during surgery. Available reports suggest, however, that the actual risk of injury is not decreased and may even be increased by placing prophylactic ureteral stents, and most surgeons have moved away from this practice. The use of lighted ureteral stents during complex laparoscopic endometriosis resections may be helpful.
Many health care systems recommend intraoperative cystoscopy with bladder and ureteral survey to evaluate the integrity of the lower urinary tract at the time of all hysterectomies. A recent study of nearly 3,000 women undergoing benign hysterectomies at the University of Michigan, Ann Arbor, showed a significant decrease in the rate of delayed diagnosis of urinary tract injuries with implementation of a universal cystoscopy policy. While the rate of lower urinary tract injury was fairly consistent before and after implementation of the policy (2.6% and 1.8%, respectively), the rate of delayed detection of a lower urinary tract injury decreased from 0.7% before the policy to 0.1% after implementation (Obstet Gynecol. 2016;127[2]:369-75). The study also showed that hospital costs nearly doubled with a delayed detection of a lower urinary tract injury.
Unfortunately, even a normal postoperative cystoscopy does not ensure there is no lower urinary tract injury, especially considering that thermal injuries resulting from the use of energy devices typically do not present until 7-14 days after surgery. Overall, however, ureteral injury detection rates with universal cystoscopy approach 97% (Obstet Gynecol. 2009;113:6-10).
Identifying injuries
Intraoperative recognition and repair always is preferred, and when ureteral injuries are discovered or suspected in the operating room, cystoscopy and retrograde pyelography is the most helpful imaging tool. Contrast dye is injected during cystoscopy directly into the renal collecting system through the ureteral orifices; with fluoroscopy, the surgeon can visualize the integrity of the ureter from the bladder to the renal pelvis to diagnosis a ureteral injury, including ureteral transection, kinking, or ligation caused by a suture or sealing device.
If retrograde pyelography shows a transection or injury from a crushing clamp or sealing device, we recommend ureteroureteral anastomosis or urethral neocystostomy depending on the extent and location of the injury. If the ureter just appears kinked, sometime simply releasing the cuff sutures or uterosacral ligament sutures will resolve the obstruction. If there is extravasation of contrast suggesting a partial tear, placing a double-J ureteral stent for 6-8 weeks is frequently sufficient.
Patients with delayed iatrogenic ureteral injuries present with symptoms that often are nonspecific and that include abdominal or flank pain, fever, nausea, vomiting, back pain, and leukocytosis.
We recommend that patients with a history of surgery and symptoms suggestive of a ureteral injury be initially evaluated with CT urography that images the renal collecting system both as contrast dye is instilled and again several minutes later as it has progressed through the entire urinary tract. Alternatively, if CT urography is unavailable, a retrograde pyelogram can be performed as an emergency procedure to determine the location of renal injury.
Surgical management
Delayed ureteral injuries resulting in partial ureteral obstruction or extravasation of urine into the pelvis can sometimes be managed conservatively though placement of an internalized double J stent. The stent can be placed in a retrograde fashion via cystoscopy by a
We typically manage delayed ureteral injuries that are not amenable to, or do not heal with, ureteral stenting with ureteroneocystotomy, or ureteral reimplantation, into the bladder. This technique is effective for distal ureteral injuries that result in obstruction or fistula and are in close proximity to the bladder (most iatrogenic gynecologic injuries). We perform ureteroneocystotomy via an open, robotic, or laparoscopic route of access depending on the circumstance.
Our preferred route of access is minimally invasive with the da Vinci robot. A camera port is placed at the umbilicus, two robotic ports are placed on the patient’s left side at the level of the umbilicus, and one is placed on the patient’s right side at the level of the umbilicus with an additional assistant port on the right side. It is helpful if each port is at least 8 cm apart. If obstruction or transection is suspected to be more proximal, the ports may have been shifted above the umbilicus to optimally mobilize the ureter.
First, the ureter is identified and dissected. Regardless of the site of injury, which is usually identifiable with inflammation and scar tissue, it is always easiest to identify the ureter at the bifurcation of the common iliac vessels. The isolated ureter is inspected proximally and above the area of injury, and we find it helpful to place a vessel loop around the ureter for easy manipulation and counter traction. Care must be taken not to disturb the adventitia and blood supply. We do not transect the ureter until we’re ready to reimplant it in the bladder.
To mobilize the bladder and prepare for a tension-free anastomosis, an adequate retropubic dissection is performed, starting with an incision in the anterior abdominal wall peritoneum and taking it down to the level of the pubic bone and into the retropubic space. It is important to be mindful of the location of the obturator neurovascular bundle when performing this dissection.
Achieving a tension-free and water-tight anastomosis of the ureter to the bladder is critical. The bladder should be mobilized such that it reaches to above the injured portion of the ureter. The bladder is retrograde filled with approximately 300 mL and a reimplantation site of the posterior bladder is identified. When there is concern about tension, a psoas hitch suture can be placed to keep the bladder in a superior position with reduced tension. Because of high rates of the congenital absence of the psoas tendon minor, we advocate direct visualization of the genitofemoral nerve by incising the peritoneum; this will avoid nerve entrapment.
Once the bladder is mobilized and the ureter isolated, we perform an intentional cystotomy in the posterior lateral aspect of the bladder. The ureter, which is on the vessel loop, must be transected proximal to the site of injury. To facilitate this, we spatulate the ureter, making a vertical incision of often about 5 mm in length to increase our surface area for anastomosis. Placement of a suture at the apex of the spatulated ureter helps us maintain orientation.
Anastomosis of the ureter and bladder is achieved in a mucosa-to-mucosa fashion using a series of interrupted monofilament fine absorbable sutures; we use a 3-0 monocryl suture. The most posterior anastomotic sutures are placed first to allow for optimal visualization, and prior to completing the anastomosis, a guide wire is placed through the open ureter and a double-J stent is introduced into the renal pelvis. The wire is then removed and the distal end of the stent coiled in the bladder. This stent will protect the ureter for about 6 weeks while it heals. The anastomosis is then completed on the anterior aspect, with a watertight closure ensured.
Postoperatively, we routinely perform an x-ray to ensure proper placement of the stent in the reimplanted ureter. To determine correct stent placement, the last rib is identified at T12 vertebrae. The renal pelvis is located at the level of the L2-L3 with the left being slightly higher than the right. A Foley catheter is maintained in the bladder for approximately 2 weeks, and the stent is maintained for approximately 6 weeks. Both the catheter and the stent can be removed in the office with cystoscopic guidance.
Imaging at 4-6 weeks after removal of the stent is performed to rule out development of an obstruction or a stricture. In patients who did not have a dilated ureter and renal collecting system prior to reimplantation, a renal ultrasound is sufficient to identify hydroureter/hydronephrosis or a urinoma. Many patients with a markedly dilated renal-collecting system prior to ureteral reimplantation will have persistent hydroureter/hydronephrosis (similar to a latex balloon that does not return to its original size after it is blown up) after reimplantation. A Lasix renal scan is a better imaging modality in these patients because it can differentiate a ureter that is dilated from one that is dilated and obstructed.
It is important to note that prompt ureteroneocystotomy is feasible only when the delayed ureteral injury presents within approximately 7 days of surgery. If the patient presents more than a week after surgery, inflammation is so significant that conservative management is necessary with reevaluation for reimplantation in another 6 weeks. Decompression of the system prior to reimplantation can be achieved through either stent placement or placement of a percutaneous nephrostomy tube. We prefer the latter because it reduces inflammation around the ureter that may make subsequent dissection and surgery more difficult.
Dr. Kenton is chief of urogynecology, Northwestern University, Chicago, and Dr. Mueller also is in the division of female pelvic medicine and reconstructive surgery–urogynecology at Northwestern. Dr. Kenton discloses grant funding from Boston Scientific.
The risk of lower urinary tract injury (bladder or ureters) at the time of benign gynecologic surgery is estimated to be between 0.3% and 4%. The majority are bladder injuries, with ureteral injuries occurring in 0.3%-1.8% of hysterectomies. While urologic procedures account for the majority of iatrogenic ureteral injuries, gynecologic surgery is the second leading cause, followed by general surgery and colorectal surgery.
With respect to hysterectomy in particular, the risk of ureteral injury is less than 1%. In a large prospective cohort of women undergoing hysterectomy for benign indications in 53 hospitals in Finland, rates of ureteral injury varied based on the route of hysterectomy, with laparoscopic and abdominal routes having an injury rate of 0.3% and the vaginal route having an injury rate of 0.04% (Human Reprod. 2011;26[7]:1741-51). The risk generally is higher with procedures for endometriosis, large fibroids, cancer, or pelvic organ prolapse.
During hysterectomy and with gynecologic surgery overall, ureteral injuries occur most commonly at three locations: at the level of the infundibulopelvic ligament and ovarian vessels, at the level of the uterine artery, and near the vaginal cuff. Identification and knowledge of the course of the ureter at these three locations is essential in preventing ureteral injury during pelvic surgery.

SOURCE: DR. MUELLER AND DR. KENTON
Perioperative ureteral stenting has been proposed as a method of preventing iatrogenic injury by allowing surgeons to more easily identify the ureters during surgery. Available reports suggest, however, that the actual risk of injury is not decreased and may even be increased by placing prophylactic ureteral stents, and most surgeons have moved away from this practice. The use of lighted ureteral stents during complex laparoscopic endometriosis resections may be helpful.
Many health care systems recommend intraoperative cystoscopy with bladder and ureteral survey to evaluate the integrity of the lower urinary tract at the time of all hysterectomies. A recent study of nearly 3,000 women undergoing benign hysterectomies at the University of Michigan, Ann Arbor, showed a significant decrease in the rate of delayed diagnosis of urinary tract injuries with implementation of a universal cystoscopy policy. While the rate of lower urinary tract injury was fairly consistent before and after implementation of the policy (2.6% and 1.8%, respectively), the rate of delayed detection of a lower urinary tract injury decreased from 0.7% before the policy to 0.1% after implementation (Obstet Gynecol. 2016;127[2]:369-75). The study also showed that hospital costs nearly doubled with a delayed detection of a lower urinary tract injury.
Unfortunately, even a normal postoperative cystoscopy does not ensure there is no lower urinary tract injury, especially considering that thermal injuries resulting from the use of energy devices typically do not present until 7-14 days after surgery. Overall, however, ureteral injury detection rates with universal cystoscopy approach 97% (Obstet Gynecol. 2009;113:6-10).
Identifying injuries
Intraoperative recognition and repair always is preferred, and when ureteral injuries are discovered or suspected in the operating room, cystoscopy and retrograde pyelography is the most helpful imaging tool. Contrast dye is injected during cystoscopy directly into the renal collecting system through the ureteral orifices; with fluoroscopy, the surgeon can visualize the integrity of the ureter from the bladder to the renal pelvis to diagnosis a ureteral injury, including ureteral transection, kinking, or ligation caused by a suture or sealing device.
If retrograde pyelography shows a transection or injury from a crushing clamp or sealing device, we recommend ureteroureteral anastomosis or urethral neocystostomy depending on the extent and location of the injury. If the ureter just appears kinked, sometime simply releasing the cuff sutures or uterosacral ligament sutures will resolve the obstruction. If there is extravasation of contrast suggesting a partial tear, placing a double-J ureteral stent for 6-8 weeks is frequently sufficient.
Patients with delayed iatrogenic ureteral injuries present with symptoms that often are nonspecific and that include abdominal or flank pain, fever, nausea, vomiting, back pain, and leukocytosis.
We recommend that patients with a history of surgery and symptoms suggestive of a ureteral injury be initially evaluated with CT urography that images the renal collecting system both as contrast dye is instilled and again several minutes later as it has progressed through the entire urinary tract. Alternatively, if CT urography is unavailable, a retrograde pyelogram can be performed as an emergency procedure to determine the location of renal injury.
Surgical management
Delayed ureteral injuries resulting in partial ureteral obstruction or extravasation of urine into the pelvis can sometimes be managed conservatively though placement of an internalized double J stent. The stent can be placed in a retrograde fashion via cystoscopy by a
We typically manage delayed ureteral injuries that are not amenable to, or do not heal with, ureteral stenting with ureteroneocystotomy, or ureteral reimplantation, into the bladder. This technique is effective for distal ureteral injuries that result in obstruction or fistula and are in close proximity to the bladder (most iatrogenic gynecologic injuries). We perform ureteroneocystotomy via an open, robotic, or laparoscopic route of access depending on the circumstance.
Our preferred route of access is minimally invasive with the da Vinci robot. A camera port is placed at the umbilicus, two robotic ports are placed on the patient’s left side at the level of the umbilicus, and one is placed on the patient’s right side at the level of the umbilicus with an additional assistant port on the right side. It is helpful if each port is at least 8 cm apart. If obstruction or transection is suspected to be more proximal, the ports may have been shifted above the umbilicus to optimally mobilize the ureter.
First, the ureter is identified and dissected. Regardless of the site of injury, which is usually identifiable with inflammation and scar tissue, it is always easiest to identify the ureter at the bifurcation of the common iliac vessels. The isolated ureter is inspected proximally and above the area of injury, and we find it helpful to place a vessel loop around the ureter for easy manipulation and counter traction. Care must be taken not to disturb the adventitia and blood supply. We do not transect the ureter until we’re ready to reimplant it in the bladder.
To mobilize the bladder and prepare for a tension-free anastomosis, an adequate retropubic dissection is performed, starting with an incision in the anterior abdominal wall peritoneum and taking it down to the level of the pubic bone and into the retropubic space. It is important to be mindful of the location of the obturator neurovascular bundle when performing this dissection.
Achieving a tension-free and water-tight anastomosis of the ureter to the bladder is critical. The bladder should be mobilized such that it reaches to above the injured portion of the ureter. The bladder is retrograde filled with approximately 300 mL and a reimplantation site of the posterior bladder is identified. When there is concern about tension, a psoas hitch suture can be placed to keep the bladder in a superior position with reduced tension. Because of high rates of the congenital absence of the psoas tendon minor, we advocate direct visualization of the genitofemoral nerve by incising the peritoneum; this will avoid nerve entrapment.
Once the bladder is mobilized and the ureter isolated, we perform an intentional cystotomy in the posterior lateral aspect of the bladder. The ureter, which is on the vessel loop, must be transected proximal to the site of injury. To facilitate this, we spatulate the ureter, making a vertical incision of often about 5 mm in length to increase our surface area for anastomosis. Placement of a suture at the apex of the spatulated ureter helps us maintain orientation.
Anastomosis of the ureter and bladder is achieved in a mucosa-to-mucosa fashion using a series of interrupted monofilament fine absorbable sutures; we use a 3-0 monocryl suture. The most posterior anastomotic sutures are placed first to allow for optimal visualization, and prior to completing the anastomosis, a guide wire is placed through the open ureter and a double-J stent is introduced into the renal pelvis. The wire is then removed and the distal end of the stent coiled in the bladder. This stent will protect the ureter for about 6 weeks while it heals. The anastomosis is then completed on the anterior aspect, with a watertight closure ensured.
Postoperatively, we routinely perform an x-ray to ensure proper placement of the stent in the reimplanted ureter. To determine correct stent placement, the last rib is identified at T12 vertebrae. The renal pelvis is located at the level of the L2-L3 with the left being slightly higher than the right. A Foley catheter is maintained in the bladder for approximately 2 weeks, and the stent is maintained for approximately 6 weeks. Both the catheter and the stent can be removed in the office with cystoscopic guidance.
Imaging at 4-6 weeks after removal of the stent is performed to rule out development of an obstruction or a stricture. In patients who did not have a dilated ureter and renal collecting system prior to reimplantation, a renal ultrasound is sufficient to identify hydroureter/hydronephrosis or a urinoma. Many patients with a markedly dilated renal-collecting system prior to ureteral reimplantation will have persistent hydroureter/hydronephrosis (similar to a latex balloon that does not return to its original size after it is blown up) after reimplantation. A Lasix renal scan is a better imaging modality in these patients because it can differentiate a ureter that is dilated from one that is dilated and obstructed.
It is important to note that prompt ureteroneocystotomy is feasible only when the delayed ureteral injury presents within approximately 7 days of surgery. If the patient presents more than a week after surgery, inflammation is so significant that conservative management is necessary with reevaluation for reimplantation in another 6 weeks. Decompression of the system prior to reimplantation can be achieved through either stent placement or placement of a percutaneous nephrostomy tube. We prefer the latter because it reduces inflammation around the ureter that may make subsequent dissection and surgery more difficult.
Dr. Kenton is chief of urogynecology, Northwestern University, Chicago, and Dr. Mueller also is in the division of female pelvic medicine and reconstructive surgery–urogynecology at Northwestern. Dr. Kenton discloses grant funding from Boston Scientific.
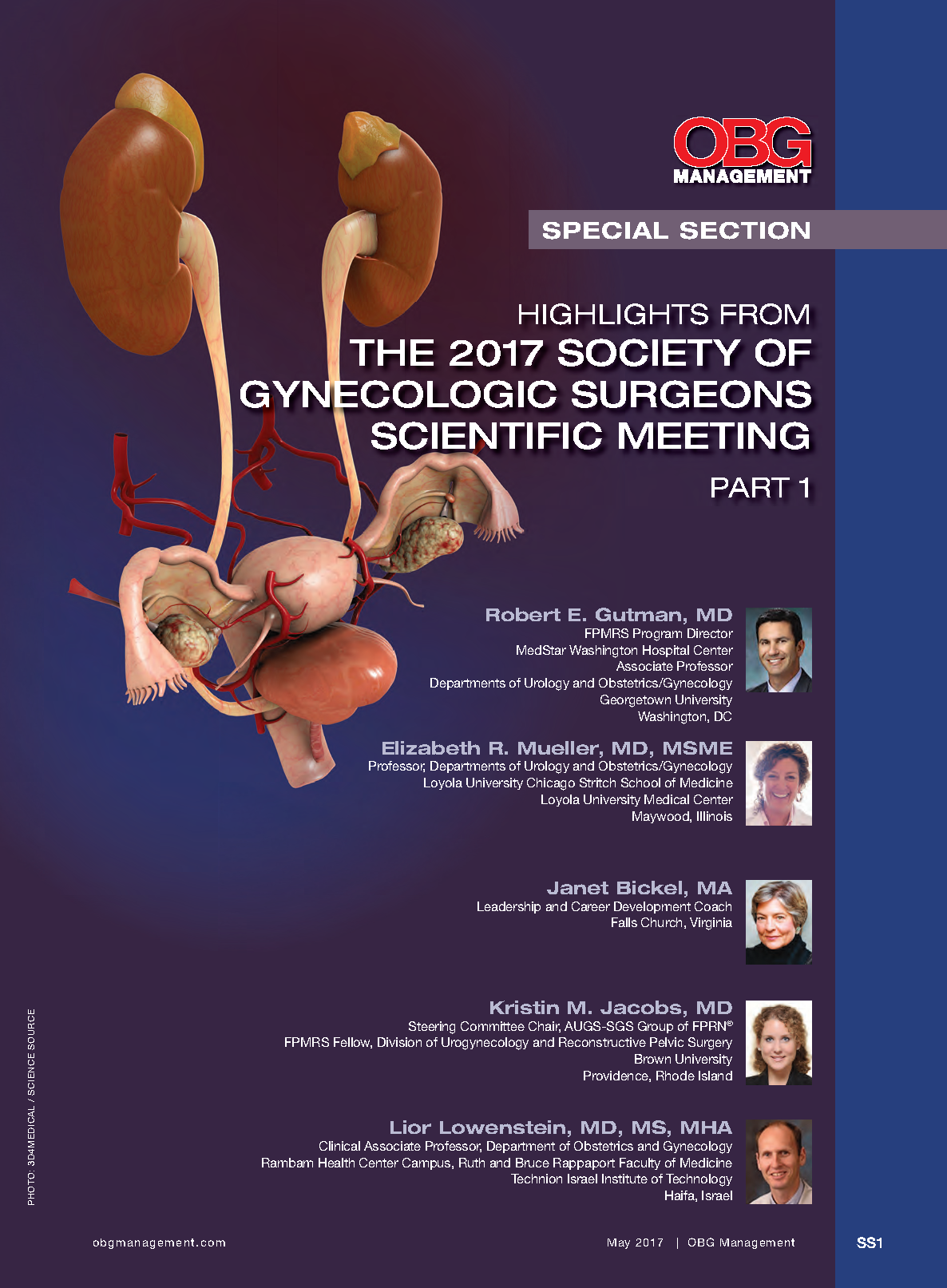
Highlights from The 2017 Society of Gynecologic Surgeons Scientific Meeting
PART 1

Robert E. Gutman, MD
FPMRS Program Director
MedStar Washington Hospital Center
Associate Professor
Departments of Urology and Obstetrics/Gynecology
Georgetown University
Washington, DC

Elizabeth R. Mueller, MD, MSME
Professor, Departments of Urology and Obstetrics/Gynecology
Loyola University Chicago Stritch School of Medicine
Loyola University Medical Center
Maywood, Illinois

Janet Bickel, MA
Leadership and Career Development Coach
Falls Church, Virginia

Kristin M. Jacobs, MD
Steering Committee Chair, AUGS-SGS Group of FPRN®
FPMRS Fellow, Division of Urogynecology and Reconstructive Pelvic Surgery
Brown University
Providence, Rhode Island

Lior Lowenstein, MD, MS, MHA
Clinical Associate Professor, Department of Obstetrics and Gynecology
Rambam Health Center Campus, Ruth and Bruce Rappaport Faculty of Medicine
Technion Israel Institute of Technology
Haifa, Israel
Drs. Gutman, Jacobs, and Lowenstein and Ms. Bickel report no financial relationships relevant to their articles. Dr. Mueller reports that she is an investigator for and is on the advisory board of Astellas Medical and Scientific Affairs.
PART 2

Geoffrey W. Cundiff, MD
Dr. Victor Gomel Professor and Head
Department of Obstetrics and Gynaecology
University of Bristish Columbia
Vancouver, British Columbia

Kimberly Kenton, MD, MS
Professor, Obstetrics and Gynaecology and Urology
Divison Chief and Fellowship Program Director
Female Pelvis Medicine and Reconstructive Surgery
Medicial Director, Women's Integrated Pelvic Health Program
Northwestern Medicine/Northwestern University Feinberg School of Medicince
Chicago, Illinois

Denise M. Elser, MD
Urogynecologist
Women's Health Institute of Illinois
Oak Lawn, Illinois
Drs. Cundiff and Elser report no financial relationships relevant to their articles. Dr. Kenton reports that she receives grant or research support from Boston Scientific and the National Institutes of Health, and that she serves as an expert witness for the Butler Snow Law Firm/Ethicon.

PART 1

Robert E. Gutman, MD
FPMRS Program Director
MedStar Washington Hospital Center
Associate Professor
Departments of Urology and Obstetrics/Gynecology
Georgetown University
Washington, DC

Elizabeth R. Mueller, MD, MSME
Professor, Departments of Urology and Obstetrics/Gynecology
Loyola University Chicago Stritch School of Medicine
Loyola University Medical Center
Maywood, Illinois

Janet Bickel, MA
Leadership and Career Development Coach
Falls Church, Virginia

Kristin M. Jacobs, MD
Steering Committee Chair, AUGS-SGS Group of FPRN®
FPMRS Fellow, Division of Urogynecology and Reconstructive Pelvic Surgery
Brown University
Providence, Rhode Island

Lior Lowenstein, MD, MS, MHA
Clinical Associate Professor, Department of Obstetrics and Gynecology
Rambam Health Center Campus, Ruth and Bruce Rappaport Faculty of Medicine
Technion Israel Institute of Technology
Haifa, Israel
Drs. Gutman, Jacobs, and Lowenstein and Ms. Bickel report no financial relationships relevant to their articles. Dr. Mueller reports that she is an investigator for and is on the advisory board of Astellas Medical and Scientific Affairs.

PART 2

Geoffrey W. Cundiff, MD
Dr. Victor Gomel Professor and Head
Department of Obstetrics and Gynaecology
University of Bristish Columbia
Vancouver, British Columbia

Kimberly Kenton, MD, MS
Professor, Obstetrics and Gynaecology and Urology
Divison Chief and Fellowship Program Director
Female Pelvis Medicine and Reconstructive Surgery
Medicial Director, Women's Integrated Pelvic Health Program
Northwestern Medicine/Northwestern University Feinberg School of Medicince
Chicago, Illinois

Denise M. Elser, MD
Urogynecologist
Women's Health Institute of Illinois
Oak Lawn, Illinois
Drs. Cundiff and Elser report no financial relationships relevant to their articles. Dr. Kenton reports that she receives grant or research support from Boston Scientific and the National Institutes of Health, and that she serves as an expert witness for the Butler Snow Law Firm/Ethicon.

PART 1

Robert E. Gutman, MD
FPMRS Program Director
MedStar Washington Hospital Center
Associate Professor
Departments of Urology and Obstetrics/Gynecology
Georgetown University
Washington, DC

Elizabeth R. Mueller, MD, MSME
Professor, Departments of Urology and Obstetrics/Gynecology
Loyola University Chicago Stritch School of Medicine
Loyola University Medical Center
Maywood, Illinois

Janet Bickel, MA
Leadership and Career Development Coach
Falls Church, Virginia

Kristin M. Jacobs, MD
Steering Committee Chair, AUGS-SGS Group of FPRN®
FPMRS Fellow, Division of Urogynecology and Reconstructive Pelvic Surgery
Brown University
Providence, Rhode Island

Lior Lowenstein, MD, MS, MHA
Clinical Associate Professor, Department of Obstetrics and Gynecology
Rambam Health Center Campus, Ruth and Bruce Rappaport Faculty of Medicine
Technion Israel Institute of Technology
Haifa, Israel
Drs. Gutman, Jacobs, and Lowenstein and Ms. Bickel report no financial relationships relevant to their articles. Dr. Mueller reports that she is an investigator for and is on the advisory board of Astellas Medical and Scientific Affairs.

PART 2

Geoffrey W. Cundiff, MD
Dr. Victor Gomel Professor and Head
Department of Obstetrics and Gynaecology
University of Bristish Columbia
Vancouver, British Columbia

Kimberly Kenton, MD, MS
Professor, Obstetrics and Gynaecology and Urology
Divison Chief and Fellowship Program Director
Female Pelvis Medicine and Reconstructive Surgery
Medicial Director, Women's Integrated Pelvic Health Program
Northwestern Medicine/Northwestern University Feinberg School of Medicince
Chicago, Illinois

Denise M. Elser, MD
Urogynecologist
Women's Health Institute of Illinois
Oak Lawn, Illinois
Drs. Cundiff and Elser report no financial relationships relevant to their articles. Dr. Kenton reports that she receives grant or research support from Boston Scientific and the National Institutes of Health, and that she serves as an expert witness for the Butler Snow Law Firm/Ethicon.