User login
Where to find guidance on using pharmacogenomics in psychiatric practice
Pharmacogenomics—the study of how genetic variability influences drug response—is increasingly being used to personalize pharmacotherapy. Used in the context of other clinical variables, genetic-based drug selection and dosing could help clinicians choose the right therapy for a patient, thus minimizing the incidence of treatment failure and intolerable side effects. Pharmacogenomics could be particularly useful in psychiatric pharmacotherapy, where response rates are low and the risk of adverse effects and nonadherence is high.
Despite the potential benefits of pharmacogenetic testing, many barriers prevent its routine use in practice, including a lack of knowledge about how to (1) order gene tests, (2) interpret results for an individual patient, and (3) apply those results to care. To help bridge this knowledge gap, we list practical, freely available pharmacogenomics resources that a psychiatric practitioner can use.
CPIC guidelines
The Clinical Pharmacogenetics Implementation Consortium (CPIC) is an international collaboration of pharmacogenomics experts that publishes clinical practice guidelines on using pharmacogenetic test results to optimize drug therapy.1 Note: These guidelines do not address when tests should be ordered, but rather how results should be used to guide prescribing.
- how to convert genotype to phenotype
- how to modify drug selection or dosing based on these results
- the level of evidence for each recommendation.
CPIC guidelines and supplementary information are available on the CPIC Web site (https://www.cpicpgx.org) and are updated regularly. Table 1 provides current CPIC guidelines for neuropsychiatric drugs.
PharmGKB
Providing searchable annotations of pharmacogenetic variants, PharmGKB summarizes the clinical implications of important pharmacogenes, and includes FDA drug labels containing pharmacogenomics information (https://www.pharmgkb.org).2 The Web site also provides users with evidence-based figures illustrating the pharmacokinetic and pharmacodynamic pathways of drugs that have pharmacogenetic implications.
PharmGKB is an excellent resource to consult for a summary of available evidence when a CPIC guideline does not exist for a given gene or drug.
Other resources
Table 23-8 lists other online resources for practitioners to aid in advancing pharmacogenomics knowledge as it relates to practice.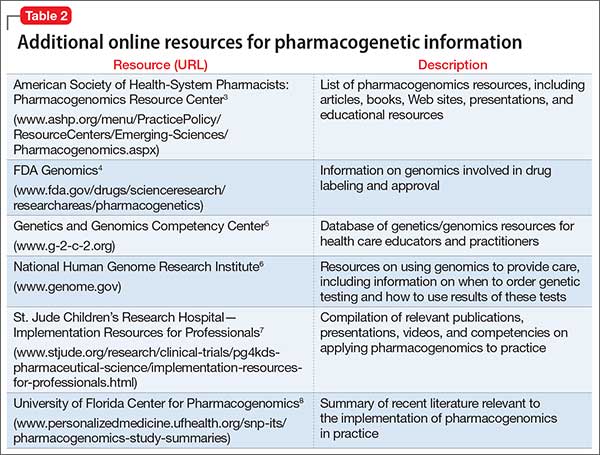
Putting guidance to best use
Familiarity with resources such as CPIC guidelines and PharmGKB can help ensure that patients with pharmacogenetic test results receive genetically tailored therapy that is more likely to be effective and less likely to cause adverse effects.9,10
1. Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209-217.
2. Thorn CF, Klein TE, Altman RB. PharmGKB: the Pharmacogenomics Knowledge Base. Methods Mol Biol. 2013;1015:311-320.
3. American Society of Health-System Pharmacists. Pharmacogenomics resource center. http://www.ashp.org/menu/PracticePolicy/ResourceCenters/Emerging-Sciences/Pharmacogenomics.aspx. Accessed July 21, 2016.
4. Genomics. Food and Drug Administration. http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics. Updated May 5, 2016. Accessed July 27, 2016.
5. National Human Genome Research Institute. Genetics/genomics competency center. http://g-2-c-2.org. Accessed July 21, 2016.
6. National Human Genome Research Institute. https://www.genome.gov. Accessed July 21, 2016.
7. Implementation resources for professionals. St. Jude Children’s Research Hospital. https://www.stjude.org/research/clinical-trials/pg4kds-pharmaceutical-science/implementation-resources-for-professionals.html. Accessed July 21, 2016.
8. SNPits study summaries. University of Florida Health Personalized Medicine Program. http://personalizedmedicine.ufhealth.org/snp-its/pharmacogenomics-study-summaries. Updated June 1, 2016. Accessed July 21, 2016.
9. Zhang G, Zhang Y, Ling Y. Web resources for pharmacogenomics. Genomics Proteomics Bioinformatics. 2015;13(1):51-54.
10. Johnson G. Leading clinical pharmacogenomics implementation: advancing pharmacy practice. Am J Health Syst Pharm. 2015;72(15):1324-1328.
Pharmacogenomics—the study of how genetic variability influences drug response—is increasingly being used to personalize pharmacotherapy. Used in the context of other clinical variables, genetic-based drug selection and dosing could help clinicians choose the right therapy for a patient, thus minimizing the incidence of treatment failure and intolerable side effects. Pharmacogenomics could be particularly useful in psychiatric pharmacotherapy, where response rates are low and the risk of adverse effects and nonadherence is high.
Despite the potential benefits of pharmacogenetic testing, many barriers prevent its routine use in practice, including a lack of knowledge about how to (1) order gene tests, (2) interpret results for an individual patient, and (3) apply those results to care. To help bridge this knowledge gap, we list practical, freely available pharmacogenomics resources that a psychiatric practitioner can use.
CPIC guidelines
The Clinical Pharmacogenetics Implementation Consortium (CPIC) is an international collaboration of pharmacogenomics experts that publishes clinical practice guidelines on using pharmacogenetic test results to optimize drug therapy.1 Note: These guidelines do not address when tests should be ordered, but rather how results should be used to guide prescribing.
- how to convert genotype to phenotype
- how to modify drug selection or dosing based on these results
- the level of evidence for each recommendation.
CPIC guidelines and supplementary information are available on the CPIC Web site (https://www.cpicpgx.org) and are updated regularly. Table 1 provides current CPIC guidelines for neuropsychiatric drugs.
PharmGKB
Providing searchable annotations of pharmacogenetic variants, PharmGKB summarizes the clinical implications of important pharmacogenes, and includes FDA drug labels containing pharmacogenomics information (https://www.pharmgkb.org).2 The Web site also provides users with evidence-based figures illustrating the pharmacokinetic and pharmacodynamic pathways of drugs that have pharmacogenetic implications.
PharmGKB is an excellent resource to consult for a summary of available evidence when a CPIC guideline does not exist for a given gene or drug.
Other resources
Table 23-8 lists other online resources for practitioners to aid in advancing pharmacogenomics knowledge as it relates to practice.
Putting guidance to best use
Familiarity with resources such as CPIC guidelines and PharmGKB can help ensure that patients with pharmacogenetic test results receive genetically tailored therapy that is more likely to be effective and less likely to cause adverse effects.9,10
Pharmacogenomics—the study of how genetic variability influences drug response—is increasingly being used to personalize pharmacotherapy. Used in the context of other clinical variables, genetic-based drug selection and dosing could help clinicians choose the right therapy for a patient, thus minimizing the incidence of treatment failure and intolerable side effects. Pharmacogenomics could be particularly useful in psychiatric pharmacotherapy, where response rates are low and the risk of adverse effects and nonadherence is high.
Despite the potential benefits of pharmacogenetic testing, many barriers prevent its routine use in practice, including a lack of knowledge about how to (1) order gene tests, (2) interpret results for an individual patient, and (3) apply those results to care. To help bridge this knowledge gap, we list practical, freely available pharmacogenomics resources that a psychiatric practitioner can use.
CPIC guidelines
The Clinical Pharmacogenetics Implementation Consortium (CPIC) is an international collaboration of pharmacogenomics experts that publishes clinical practice guidelines on using pharmacogenetic test results to optimize drug therapy.1 Note: These guidelines do not address when tests should be ordered, but rather how results should be used to guide prescribing.
- how to convert genotype to phenotype
- how to modify drug selection or dosing based on these results
- the level of evidence for each recommendation.
CPIC guidelines and supplementary information are available on the CPIC Web site (https://www.cpicpgx.org) and are updated regularly. Table 1 provides current CPIC guidelines for neuropsychiatric drugs.
PharmGKB
Providing searchable annotations of pharmacogenetic variants, PharmGKB summarizes the clinical implications of important pharmacogenes, and includes FDA drug labels containing pharmacogenomics information (https://www.pharmgkb.org).2 The Web site also provides users with evidence-based figures illustrating the pharmacokinetic and pharmacodynamic pathways of drugs that have pharmacogenetic implications.
PharmGKB is an excellent resource to consult for a summary of available evidence when a CPIC guideline does not exist for a given gene or drug.
Other resources
Table 23-8 lists other online resources for practitioners to aid in advancing pharmacogenomics knowledge as it relates to practice.
Putting guidance to best use
Familiarity with resources such as CPIC guidelines and PharmGKB can help ensure that patients with pharmacogenetic test results receive genetically tailored therapy that is more likely to be effective and less likely to cause adverse effects.9,10
1. Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209-217.
2. Thorn CF, Klein TE, Altman RB. PharmGKB: the Pharmacogenomics Knowledge Base. Methods Mol Biol. 2013;1015:311-320.
3. American Society of Health-System Pharmacists. Pharmacogenomics resource center. http://www.ashp.org/menu/PracticePolicy/ResourceCenters/Emerging-Sciences/Pharmacogenomics.aspx. Accessed July 21, 2016.
4. Genomics. Food and Drug Administration. http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics. Updated May 5, 2016. Accessed July 27, 2016.
5. National Human Genome Research Institute. Genetics/genomics competency center. http://g-2-c-2.org. Accessed July 21, 2016.
6. National Human Genome Research Institute. https://www.genome.gov. Accessed July 21, 2016.
7. Implementation resources for professionals. St. Jude Children’s Research Hospital. https://www.stjude.org/research/clinical-trials/pg4kds-pharmaceutical-science/implementation-resources-for-professionals.html. Accessed July 21, 2016.
8. SNPits study summaries. University of Florida Health Personalized Medicine Program. http://personalizedmedicine.ufhealth.org/snp-its/pharmacogenomics-study-summaries. Updated June 1, 2016. Accessed July 21, 2016.
9. Zhang G, Zhang Y, Ling Y. Web resources for pharmacogenomics. Genomics Proteomics Bioinformatics. 2015;13(1):51-54.
10. Johnson G. Leading clinical pharmacogenomics implementation: advancing pharmacy practice. Am J Health Syst Pharm. 2015;72(15):1324-1328.
1. Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209-217.
2. Thorn CF, Klein TE, Altman RB. PharmGKB: the Pharmacogenomics Knowledge Base. Methods Mol Biol. 2013;1015:311-320.
3. American Society of Health-System Pharmacists. Pharmacogenomics resource center. http://www.ashp.org/menu/PracticePolicy/ResourceCenters/Emerging-Sciences/Pharmacogenomics.aspx. Accessed July 21, 2016.
4. Genomics. Food and Drug Administration. http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics. Updated May 5, 2016. Accessed July 27, 2016.
5. National Human Genome Research Institute. Genetics/genomics competency center. http://g-2-c-2.org. Accessed July 21, 2016.
6. National Human Genome Research Institute. https://www.genome.gov. Accessed July 21, 2016.
7. Implementation resources for professionals. St. Jude Children’s Research Hospital. https://www.stjude.org/research/clinical-trials/pg4kds-pharmaceutical-science/implementation-resources-for-professionals.html. Accessed July 21, 2016.
8. SNPits study summaries. University of Florida Health Personalized Medicine Program. http://personalizedmedicine.ufhealth.org/snp-its/pharmacogenomics-study-summaries. Updated June 1, 2016. Accessed July 21, 2016.
9. Zhang G, Zhang Y, Ling Y. Web resources for pharmacogenomics. Genomics Proteomics Bioinformatics. 2015;13(1):51-54.
10. Johnson G. Leading clinical pharmacogenomics implementation: advancing pharmacy practice. Am J Health Syst Pharm. 2015;72(15):1324-1328.
Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia
First-episode psychosis (FEP) in schizophrenia is characterized by high response rates to antipsychotic therapy, followed by frequent antipsychotic discontinuation and elevated relapse rates soon after maintenance treatment begins.1,2 With subsequent episodes, time to response progressively increases and likelihood of response decreases.3,4
To address these issues, this article—the second of 2 parts5—describes the rationale and evidence for using nonstandard first-line antipsychotic therapies to manage FEP. Specifically, we discuss when clinicians might consider monotherapy exceeding FDA-approved maximum dosages, combination therapy, long-acting injectable antipsychotics (LAIA), or clozapine.
Monotherapy beyond FDA-approved dosages
Treatment guidelines for FEP recommend oral antipsychotic dosages in the lower half of the treatment range and lower than those that are required for multi-episode schizophrenia.6-16 Ultimately, clinicians prescribe individualized dosages for their patients based on symptom improvement and tolerability. The optimal dosage at which to achieve a favorable D2 receptor occupancy likely will vary from patient to patient.17
To control symptoms, higher dosages may be needed than those used in FEP clinical trials, recommended by guidelines for FEP or multi-episode patients, or approved by the FDA. Patients seen in everyday practice may be more complicated (eg, have a comorbid condition or history of nonresponse) than study populations. Higher dosages also may be reasonable to overcome drug−drug interactions (eg, cigarette smoking-mediated cytochrome P450 1A2 induction, resulting in increased olanzapine metabolism),18 or to establish antipsychotic failure if adequate trials at lower dosages have resulted in a suboptimal response and the patient is not experiencing tolerability or safety concerns.
In a study of low-, full-, and high-dosage antipsychotic therapy in FEP, an additional 15% of patients responded to higher dosages of olanzapine and risperidone after failing to respond to a standard dosage.19 A study of data from the Recovery After an Initial Schizophrenia Episode Project’s Early Treatment Program (RAISE-ETP) found that, of participants identified who may benefit from therapy modification, 8.8% were prescribed an antipsychotic (often, olanzapine, risperidone, and haloperidol) at a higher-than-recommended dosage.20 Of note, only olanzapine was prescribed at higher than FDA-approved dosages.
Antipsychotic combination therapy
Prescribing combinations of antipsychotics—antipsychotic polypharmacy (APP)— has a negative connotation because of limited efficacy and safety data,21 and limited endorsement in schizophrenia treatment guidelines.9,13 Caution with APP is warranted; a complex medication regimen may increase the potential for adverse effects, poorer adherence, and adverse drug-drug interactions.9 APP has been shown to independently predict both shorter treatment duration and discontinuation before 1 year.22
Nonetheless, the clinician and patient may share the decision to implement APP and observe whether benefits outweigh risks in situations such as:
• to optimize neuroreceptor occupancy and targets (eg, attempting to achieve adequate D2 receptor blockade while minimizing side effects secondary to binding other receptors)
• to manage co-existing symptom domains (eg, mood changes, aggression, negative symptoms, disorganization, and cognitive deficits)
• to mitigate antipsychotic-induced side effects (eg, initiating aripiprazole to treat hyperprolactinemia induced by another antipsychotic to which the patient has achieved a favorable response).23
Clinicians report using APP to treat as many as 50% of patients with a history of multiple psychotic episodes.23 For FEP patients, 23% of participants in the RAISE-ETP trial who were identified as possibly benefiting from therapy modification were prescribed APP.20 Regrettably, researchers have not found evidence to support a reported rationale for using APP—that lower dosages of individual antipsychotics when used in combination may avoid high-dosage prescriptions.24
Before implementing APP, thoroughly explore and manage reasons for a patient’s suboptimal response to monotherapy.25 An adequate trial with any antipsychotic should be at the highest tolerated dosage for 12 to 16 weeks. Be mindful that response to an APP trial may be the result of additional time on the original antipsychotic.
Long-acting injectable antipsychotics in FEP
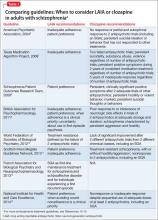
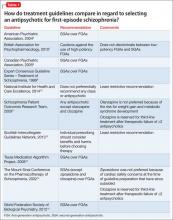
Guideline recommendations. Most older guidelines for schizophrenia treatment suggest LAIA after multiple relapses related to medication nonadherence or when a patient prefers injected medication (Table 1).6-13 Expert consensus guidelines also recommend considering LAIA in patients who lack insight into their illness. The Texas Medication Algorithm Project (TMAP) guidelines7 state LAIA can be considered for inadequate adherence at any stage, whereas the 2010 British Association for Psychopharmacology (BAP) guidelines9 express uncertainty about their use in FEP, because of limited evidence. Both the BAP and National Institute for Health and Care Excellence guidelines13 urge clinicians to consider LAIA when avoiding nonadherence is a treatment priority.
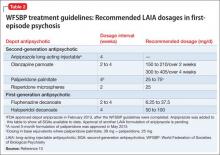
Recently, the French Association for Biological Psychiatry and Neuro-psychopharmacology (AFPBN) created expert consensus guidelines12 on using LAIA in practice. They recommend long-acting injectable second-generation antipsychotics (SGAs) as first-line maintenance treatment for schizophrenia and schizoaffective disorder and for individuals experiencing a first recurrent episode. The World Federation of Societies of Biological Psychiatry guidelines contain LAIA dosage recommendations for FEP (Table 2).10
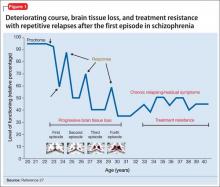
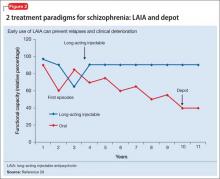
Advances have been made in understanding the serious neurobiological adverse effects of psychotic relapses, including neuroinflammation and oxidative stress, that may explain the atrophic changes observed with psychotic episodes starting with the FEP. Protecting the patient from a second episode has become a vital therapeutic management goal26 (Figure 127).
Concerns. Compared with oral antipsychotics, LAIA offers clinical advantages:
• improved pharmacokinetic profile (lower “peaks” and higher “valleys”)
• more consistent plasma concentrations (no variability related to administration timing or food effects)
• no first-pass metabolism, which can ease the process of finding the lowest effective and safe dosage
• reduced administration burden and objective tracking of adherence with typical dosing every 2 to 4 weeks
• less stigmatizing than oral medication for FEP patients, such as college students living in a dormitory.28,29
Barriers to LAIA use include:
• slow dosage titration and increased time to reach steady state drug level
• oral supplementation for some (eg, risperidone microspheres and aripiprazole long-acting injectable)
• logistical challenges for some (eg, 3-hour post-injection monitoring for delirium sedation syndrome with olanzapine pamoate)
• additional planning to coordinate care for scheduled injections
• higher expenses up front
• local injection site reactions
• dosage adjustment difficulties if adverse effects occur.28,29
Adoption rates of LAIA are low, especially for FEP.30 Most surveys indicate that (1) physicians believe LAIA treatment is ineffective for FEP31 and (2) patients do not prefer injectable to oral antipsychotics,32 despite evidence to the contrary.33,34 A survey of 198 psychiatrists identified 3 factors that influenced their decisions against using LAIA patients with FEP:
• limited availability of SGA depot formulations (4, to date, in the United States)
• frequent rejection by the patient when LAIA is offered without adequate explanation or encouragement
• skepticism of FEP patients (and their family) who lack experience with relapse.35
In reality, when SGA depots were introduced in the United Kingdom, prescribing rates of LAIA did not increase. As for patient rejection being a major reason for not prescribing LAIA, few patients (5% to 36%) are offered depot injections, particularly in FEP.29 Most patients using LAIA are chronic, multi-episode, violent people who are receiving medications involuntarily.29 Interestingly, this survey did not find 2 factors to be influential in psychiatrists’ decision not to use LAIA in FEP:
• guidelines do not explicitly recommend depot treatment in FEP
• treatment in FEP may be limited to 1 year, therefore depot administration is not worthwhile.35
Preliminary evidence. At least a dozen studies have explored LAIA treatment for FEP, with the use of fluphenazine decanoate,36 perphenazine enanthate37 (discontinued), and risperidone microspheres.37-48 The research demonstrates the efficacy and safety of LAIA in FEP as measured by these endpoints:
• improved symptom control38,40-43,46,48
• adherence43,44,48
• reduced relapse rates37,43 and rehospitalizations37,47
• lesser reductions in white matter brain volume45
• no differences in extrapyramidal side effects or prolactin-associated adverse effects.48
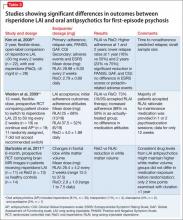
A few small studies demonstrate significant differences in outcomes between risperidone LAIA and oral comparator groups (Table 3).43-45 Ongoing studies of LAIA use in FEP are comparing paliperidone palmitate with risperidone microspheres and other oral antipsychotics.49-51 No studies are examining olanzapine pamoate in FEP, likely because several guidelines do not recommended its use. No studies have been published regarding aripiprazole long-acting injectable in FEP. This LAIA formulation was approved in February 2013, and robust studies of the oral formulation in FEP are limited.52
Discussion and recommendations. Psychiatrists relying on subjective measures of antipsychotic adherence may inaccurately assess whether patients meet this criterion for LAIA use.53 LAIA could combat the high relapse rate in FEP, yet depot antipsychotics are prescribed infrequently for FEP patients (eg, for only 9.5% of participants in the RAISE-ETP study).20 Most schizophrenia treatment guidelines do not discuss LAIA use specifically in FEP, although the AFPBN expert consensus guidelines published in 2013 do recommend SGA depot formulations in FEP.12 SGA LAIA may be preferable, given its neuroprotective effects, in contrast to the neurotoxicity concerns of FGA LAIA.54,55
Relapses begin within a few months of illness stabilization after FEP, and >50% of patients relapse within 1 or 2 years2—the recommended minimum treatment duration for FEP.8,9,13 The use of LAIA is advisable in any patient with schizophrenia for whom long-term antipsychotic therapy is indicated.56 LAIA administration requirements objectively track medication adherence, which allows clinicians to be proactive in relapse prevention. Not using an intervention in FEP that improves adherence and decreases relapse rates contradicts our goal of instituting early, effective treatment to improve long-term functional outcomes (Figure 2).29
Considering clozapine in FEP
Guideline recommendations. Schizo-phrenia treatment guidelines and FDA labeling57 reserve clozapine for third-line treatment of refractory schizophrenia after 2 adequate antipsychotic trials have failed despite optimal dosing (Table 1).6-13 Some guidelines specify 1 of the 2 failed antipsychotic trials must include an SGA.6,7,10,11,13-16 Most say clozapine may be considered in patients with chronic aggression or hostility,7-9,14,16 or suicidal thoughts and behaviors.6-8,14,16 TMAP guidelines recommend a clozapine trial with concomitant substance abuse, persistent positive symptoms during 2 years of consistent medication treatment, and after 5 years of inadequate response (“treatment resistance”), regardless of the number of antipsychotic trials.7
Rationale and concerns. Clozapine is a superior choice for treatment-refractory delusions or hallucinations of schizophrenia, because it markedly enhances the response rate to antipsychotic therapy.58 Researchers therefore have investigated whether clozapine, compared with other antipsychotics, would yield more favorable initial and long-term outcomes when used first-line in FEP.
Preliminary evidence. Five studies have explored the use of clozapine as first-line therapy in FEP (Table 4).59-63 Interpreting the results is difficult because clozapine trials may be brief (mostly, 12 to 52 weeks); lack a comparator arm; suffer from a high attrition rate; enroll few patients; and lack potentially important outcome measures such as negative symptoms, suicidality, and functional assessment.
Overall, these studies demonstrate clozapine is as efficacious in this patient population as chlorpromazine (no difference in remission at 1-year, although clozapine-treated patients remitted faster and stayed in remission longer)60,61 or risperidone (no difference in Positive and Negative Syndrome Scale scores).62
At present, clozapine has not been shown superior to other antipsychotics as a first-line treatment for FEP. Research does underscore the importance of a clozapine trial as third-line treatment for FEP patients who have not responded well to 2 SGA trials.63 Many of these nonresponders (77%) have demonstrated a favorable response when promptly switched to clozapine.64
Discussion and recommendations. The limited evidence argues against using clozapine earlier than as third-line treatment in FEP. Perhaps the high treatment response that characterizes FEP creates a ceiling effect that obscures differences in antipsychotic efficacy at this stage.65 Clozapine use as first-line treatment should be re-evaluated with more robust methodology. One approach could be to assess its benefit in FEP by the duration of untreated psychosis.
The odds of achieving remission have been shown to decrease by 15% for each year that psychosis has not been treated.59 Studies exploring the use of clozapine as a second-line agent for FEP also are warranted, as antipsychotic response during subsequent trials is substantially reduced. In fact, the Scottish Intercollegiate Guidelines Network guidelines recommend this as an area for future research.11
For now, clozapine should continue to be reserved as second- or third-line treatment in a patient with FEP. The risks of clozapine’s potentially serious adverse effects (eg, agranulocytosis, seizures, obesity, diabetes, dyslipidemia, myocarditis, pancreatitis, hypotension, sialorrhea, severe sedation, ileus) can be justified only in the treatment of severe and persistent psychotic symptoms.57
Bottom Line
Nonstandard use of antipsychotic monotherapy dosages beyond the approved FDA limit and combination antipsychotic therapy may be reasonable for select first-episode psychosis (FEP) patients. Strongly consider long-acting injectable antipsychotics in FEP to proactively combat the high relapse rate and more easily identify antipsychotic failure. Continue to use clozapine as second- or third-line therapy in FEP: Studies have not found that it is more efficacious than other antipsychotics for first-line use.
Related Resource
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
Drug Brand Names
Aripiprazole • Abilify, Abilify Maintena
Chlorpromazine • Thorazine
Clozapine • Clozaril
Fluphenazine decanoate • Prolixin-D
Haloperidol • Haldol
Haloperidol decanoate • Haldol-D
Olanzapine • Zyprexa
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone microspheres • Risperdal Consta
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products. Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Lieberman JA, Koreen AR, Chakos M, et al. Factors influencing treatment response and outcome of first-episode schizophrenia: implications for understanding the pathophysiology of schizophrenia. J Clin Psychiatry. 1996;57(suppl 9):5-9.
4. Agid O, Arenovich T, Sajeev G, et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72(11):1439-1444.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis. An early stage of schizophrenia with distinct treatment needs. Current Psychiatry. 2015;14(5):32-34,36-40,42.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Texas Department of State Health Services. Texas Medication Algorithm Project (TMAP) Procedural Manual. Schizophrenia Treatment Algorithms. http://www.jpshealthnet.org/sites/default/files/ tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed June 11, 2015.
8. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
9. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
10. Hasan A, Falkai P, Wobrok T, et al; WFSBP Task force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
11. Scottish Intercollegiate Guidelines Network. SIGN 131: Management of schizophrenia. http://www.sign.ac.uk/ pdf/sign131.pdf. Published March 2013. Accessed June 11, 2015.
12. Llorca PM, Abbar M, Courtet P, et al. Guidelines for the use and management of long-acting injectable antipsychotics in serous mental illness. BMC Psychiatry. 2013;13:340.
13. National Institute for Health and Care Excellence. NICE clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. https://www.nice.org. uk/guidance/cg178/resources/guidance-psychosis-and-schizophrenia-in-adults-treatment-and-management-pdf. Updated March 2014. Accessed June 16, 2015.
14. Canadian Psychiatric Association. Clinical practice guidelines. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
15. McEvoy JP, Scheifler PL, Frances A. The expert consensus guideline series: treatment of schizophrenia. J Clin Psychiatry. 1999;60(suppl 11):3-80.
16. Marder SR, Essock SM, Miller AL, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
17. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
18. Fankhauser MP. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
19. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
20. Robinson DG, Schooler NR, John M, et al. Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry. 2015;172(3):237-248.
21. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
22. Fisher MD, Reilly K, Isenberg K, et al. Antipsychotic patterns of use in patients with schizophrenia: polypharmacy versus monotherapy. BMC Psychiatry. 2014;14(1):341.
23. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
24. John AP, Dragovic M. Antipsychotic polypharmacy is not associated with reduced dose of individual antipsychotics in schizophrenia. J Clin Psychopharmacol. 2015;35(2):193-195.
25. Nasrallah HA. Treatment-resistant schizophrenia. Current Psychiatry. http://www.currentpsychiatry.com/specialty-focus/schizophrenia-other-psychotic-disorders/article/ treatment-resistant-schizophrenia/9be7bba3713d4a4cd68aa 8c92b79e5b1.html. Accessed June 16, 2015.
26. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
27. Nasrallah HA, Smeltzer DJ. Contemporary diagnosis and management of the patient with schizophrenia. 2nd ed. Newton, PA: Handbooks in Health Care Co; 2011.
28. McEvoy JP. Risks versus benefits of different types of long-acting injectable antipsychotics. J Clin Psychiatry. 2006;67(suppl 5):15-18.
29. Agid O, Foussias G, Remington G. Long-acting injectable antipsychotics in the treatment of schizophrenia: their role in relapse prevention. Expert Opin Pharmacother. 2010;11(14):2301-2317.
30. Kirschner M, Theodoridou A, Fusar-Poli P, et al. Patients’ and clinicians’ attitude towards long-acting depot antipsychotics in subjects with a first episode psychosis. Ther Adv Psychophamacol. 2013;3(2):89-99.
31. Heres S, Hamann J, Mendel R, et al. Identifying the profile of optimal candidates for antipsychotic depot therapy: A cluster analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1987-1993.
32. Heres S, Lambert M, Vauth R. Treatment of early episode in patents with schizophrenia: the role of long acting antipsychotics. Eur Psychiatry. 2014;29(suppl 2):1409-1413.
33. Heres S, Schmitz FS, Leucht S, et al. The attitude of patients towards antipsychotic depot treatment. Int Clin Psychopharmacol. 2007;22(5):275-282.
34. Weiden PJ, Schooler NR, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
35. Heres S, Reichhart T, Hamann J, et al. Psychiatrists’ attitude to antipsychotic depot treatment in patients with first-episode schizophrenia. Eur Psychiatry. 2011;26(5):297-301.
36. Kane JM, Rifkin A, Quitkin F, et al. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch Gen Psychiatry. 1982;39(1):70-73.
37. Tiihonen J, Wahlbeck K, Lönnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in a community care after first hospitalization due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333(7561):224.
38. Parellada E, Andrezina R, Milanova V, et al. Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol. 2005;19(suppl 5):5-14.
39. Malla A, Binder C, Chue P. Comparison of long-acting injectable risperidone and oral novel antipsychotic drugs for treatment in early phase of schizophrenia spectrum psychosis. Proceedings of the 61st Annual Convention Society of Biological Psychiatry; Toronto, Canada; 2006.
40. Lasser RA, Bossie CA, Zhu Y, et al. Long-acting risperidone in young adults with early schizophrenia or schizoaffective illness. Ann Clin Psychiatry. 2007;19(2):65-71.
41. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
42. Emsley R, Oosthuizen P, Koen L, et al. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
43. Kim B, Lee SH, Choi TK, et al. Effectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1231-1235.
44. Weiden PJ, Schooler NJ, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
45. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011;132(1):35-41.
46. Napryeyenko O, Burba B, Martinez G, et al. Risperidone long-acting injectable in recent-onset schizophrenia examined with clinician and patient self-report measures. J Clin Psychopharmacol. 2010;30(2):200-202.
47. Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609.
48. Dubois V, Megens J, Mertens C, et al. Long-acting risperidone in early-episode schizophrenia. Acta Psychiatrica Belgica. 2011;111(1):9-21.
49. ClinicalTrials.gov. Oral risperidone versus injectable paliperidone palmitate for treating first-episode schizophrenia. https://clinicaltrials.gov/ct2/show/ NCT01451736. Accessed June 16, 2015.
50. ClinicalTrials.gov. Brain myelination effects of paliperidone palmitate versus oral risperidone in first episode schizophrenia. https://clinicaltrials.gov/ct2/ show/NCT01458379. Accessed June 16, 2015.
51. ClinicalTrials.gov. Effects of paliperidone palmitate versus oral antipsychotics on clinical outcomes and MRI measures. https://clinicaltrials.gov/ct2/show/NCT01359293. Accessed June 16, 2016.
52. U.S. Food and Drug Administration. Drugs@FDA. http:// www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed January 11, 2015.
53. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
54. Nandra KS, Agius M. The difference between typical and atypical antipsychotics: the effects on neurogenesis. Psychiatr Danub. 2012;24(suppl 1):S95-S99.
55. Nasrallah HA. Haloperidol is clearly neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
56. Kane JM, Garcia-Ribora C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry. 2009;52:S63-S67.
57. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
58. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
59. Woerner MG, Robinson DG, Alvir JMJ, et al. Clozapine as a first treatment for schizophrenia. Am J Psychiatry. 2003;160(8):1514-1516.
60. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
61. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
62. Sanz-Fuentenebro J, Taboada D, Palomo T, et al. Randomized trial of clozapine vs. risperidone in treatment-naïve first-episode schizophrenia: results after one year. Schizophr Res. 2013;149(1-3):156-161.
63. Yang PD, Ji Z. The efficacy and related factors of clozapine on first-episode schizophrenia. Chin J Nerv Ment Dis. 1997;23:155-158.
64. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
65. Remington G, Agid O, Foussias G, et al. Clozapine’s role in the treatment of first-episode schizophrenia. Am J Psychiatry. 2013;170(2):146-151.
First-episode psychosis (FEP) in schizophrenia is characterized by high response rates to antipsychotic therapy, followed by frequent antipsychotic discontinuation and elevated relapse rates soon after maintenance treatment begins.1,2 With subsequent episodes, time to response progressively increases and likelihood of response decreases.3,4
To address these issues, this article—the second of 2 parts5—describes the rationale and evidence for using nonstandard first-line antipsychotic therapies to manage FEP. Specifically, we discuss when clinicians might consider monotherapy exceeding FDA-approved maximum dosages, combination therapy, long-acting injectable antipsychotics (LAIA), or clozapine.
Monotherapy beyond FDA-approved dosages
Treatment guidelines for FEP recommend oral antipsychotic dosages in the lower half of the treatment range and lower than those that are required for multi-episode schizophrenia.6-16 Ultimately, clinicians prescribe individualized dosages for their patients based on symptom improvement and tolerability. The optimal dosage at which to achieve a favorable D2 receptor occupancy likely will vary from patient to patient.17
To control symptoms, higher dosages may be needed than those used in FEP clinical trials, recommended by guidelines for FEP or multi-episode patients, or approved by the FDA. Patients seen in everyday practice may be more complicated (eg, have a comorbid condition or history of nonresponse) than study populations. Higher dosages also may be reasonable to overcome drug−drug interactions (eg, cigarette smoking-mediated cytochrome P450 1A2 induction, resulting in increased olanzapine metabolism),18 or to establish antipsychotic failure if adequate trials at lower dosages have resulted in a suboptimal response and the patient is not experiencing tolerability or safety concerns.
In a study of low-, full-, and high-dosage antipsychotic therapy in FEP, an additional 15% of patients responded to higher dosages of olanzapine and risperidone after failing to respond to a standard dosage.19 A study of data from the Recovery After an Initial Schizophrenia Episode Project’s Early Treatment Program (RAISE-ETP) found that, of participants identified who may benefit from therapy modification, 8.8% were prescribed an antipsychotic (often, olanzapine, risperidone, and haloperidol) at a higher-than-recommended dosage.20 Of note, only olanzapine was prescribed at higher than FDA-approved dosages.
Antipsychotic combination therapy
Prescribing combinations of antipsychotics—antipsychotic polypharmacy (APP)— has a negative connotation because of limited efficacy and safety data,21 and limited endorsement in schizophrenia treatment guidelines.9,13 Caution with APP is warranted; a complex medication regimen may increase the potential for adverse effects, poorer adherence, and adverse drug-drug interactions.9 APP has been shown to independently predict both shorter treatment duration and discontinuation before 1 year.22
Nonetheless, the clinician and patient may share the decision to implement APP and observe whether benefits outweigh risks in situations such as:
• to optimize neuroreceptor occupancy and targets (eg, attempting to achieve adequate D2 receptor blockade while minimizing side effects secondary to binding other receptors)
• to manage co-existing symptom domains (eg, mood changes, aggression, negative symptoms, disorganization, and cognitive deficits)
• to mitigate antipsychotic-induced side effects (eg, initiating aripiprazole to treat hyperprolactinemia induced by another antipsychotic to which the patient has achieved a favorable response).23
Clinicians report using APP to treat as many as 50% of patients with a history of multiple psychotic episodes.23 For FEP patients, 23% of participants in the RAISE-ETP trial who were identified as possibly benefiting from therapy modification were prescribed APP.20 Regrettably, researchers have not found evidence to support a reported rationale for using APP—that lower dosages of individual antipsychotics when used in combination may avoid high-dosage prescriptions.24
Before implementing APP, thoroughly explore and manage reasons for a patient’s suboptimal response to monotherapy.25 An adequate trial with any antipsychotic should be at the highest tolerated dosage for 12 to 16 weeks. Be mindful that response to an APP trial may be the result of additional time on the original antipsychotic.
Long-acting injectable antipsychotics in FEP
Guideline recommendations. Most older guidelines for schizophrenia treatment suggest LAIA after multiple relapses related to medication nonadherence or when a patient prefers injected medication (Table 1).6-13 Expert consensus guidelines also recommend considering LAIA in patients who lack insight into their illness. The Texas Medication Algorithm Project (TMAP) guidelines7 state LAIA can be considered for inadequate adherence at any stage, whereas the 2010 British Association for Psychopharmacology (BAP) guidelines9 express uncertainty about their use in FEP, because of limited evidence. Both the BAP and National Institute for Health and Care Excellence guidelines13 urge clinicians to consider LAIA when avoiding nonadherence is a treatment priority.
Recently, the French Association for Biological Psychiatry and Neuro-psychopharmacology (AFPBN) created expert consensus guidelines12 on using LAIA in practice. They recommend long-acting injectable second-generation antipsychotics (SGAs) as first-line maintenance treatment for schizophrenia and schizoaffective disorder and for individuals experiencing a first recurrent episode. The World Federation of Societies of Biological Psychiatry guidelines contain LAIA dosage recommendations for FEP (Table 2).10
Advances have been made in understanding the serious neurobiological adverse effects of psychotic relapses, including neuroinflammation and oxidative stress, that may explain the atrophic changes observed with psychotic episodes starting with the FEP. Protecting the patient from a second episode has become a vital therapeutic management goal26 (Figure 127).
Concerns. Compared with oral antipsychotics, LAIA offers clinical advantages:
• improved pharmacokinetic profile (lower “peaks” and higher “valleys”)
• more consistent plasma concentrations (no variability related to administration timing or food effects)
• no first-pass metabolism, which can ease the process of finding the lowest effective and safe dosage
• reduced administration burden and objective tracking of adherence with typical dosing every 2 to 4 weeks
• less stigmatizing than oral medication for FEP patients, such as college students living in a dormitory.28,29
Barriers to LAIA use include:
• slow dosage titration and increased time to reach steady state drug level
• oral supplementation for some (eg, risperidone microspheres and aripiprazole long-acting injectable)
• logistical challenges for some (eg, 3-hour post-injection monitoring for delirium sedation syndrome with olanzapine pamoate)
• additional planning to coordinate care for scheduled injections
• higher expenses up front
• local injection site reactions
• dosage adjustment difficulties if adverse effects occur.28,29
Adoption rates of LAIA are low, especially for FEP.30 Most surveys indicate that (1) physicians believe LAIA treatment is ineffective for FEP31 and (2) patients do not prefer injectable to oral antipsychotics,32 despite evidence to the contrary.33,34 A survey of 198 psychiatrists identified 3 factors that influenced their decisions against using LAIA patients with FEP:
• limited availability of SGA depot formulations (4, to date, in the United States)
• frequent rejection by the patient when LAIA is offered without adequate explanation or encouragement
• skepticism of FEP patients (and their family) who lack experience with relapse.35
In reality, when SGA depots were introduced in the United Kingdom, prescribing rates of LAIA did not increase. As for patient rejection being a major reason for not prescribing LAIA, few patients (5% to 36%) are offered depot injections, particularly in FEP.29 Most patients using LAIA are chronic, multi-episode, violent people who are receiving medications involuntarily.29 Interestingly, this survey did not find 2 factors to be influential in psychiatrists’ decision not to use LAIA in FEP:
• guidelines do not explicitly recommend depot treatment in FEP
• treatment in FEP may be limited to 1 year, therefore depot administration is not worthwhile.35
Preliminary evidence. At least a dozen studies have explored LAIA treatment for FEP, with the use of fluphenazine decanoate,36 perphenazine enanthate37 (discontinued), and risperidone microspheres.37-48 The research demonstrates the efficacy and safety of LAIA in FEP as measured by these endpoints:
• improved symptom control38,40-43,46,48
• adherence43,44,48
• reduced relapse rates37,43 and rehospitalizations37,47
• lesser reductions in white matter brain volume45
• no differences in extrapyramidal side effects or prolactin-associated adverse effects.48
A few small studies demonstrate significant differences in outcomes between risperidone LAIA and oral comparator groups (Table 3).43-45 Ongoing studies of LAIA use in FEP are comparing paliperidone palmitate with risperidone microspheres and other oral antipsychotics.49-51 No studies are examining olanzapine pamoate in FEP, likely because several guidelines do not recommended its use. No studies have been published regarding aripiprazole long-acting injectable in FEP. This LAIA formulation was approved in February 2013, and robust studies of the oral formulation in FEP are limited.52
Discussion and recommendations. Psychiatrists relying on subjective measures of antipsychotic adherence may inaccurately assess whether patients meet this criterion for LAIA use.53 LAIA could combat the high relapse rate in FEP, yet depot antipsychotics are prescribed infrequently for FEP patients (eg, for only 9.5% of participants in the RAISE-ETP study).20 Most schizophrenia treatment guidelines do not discuss LAIA use specifically in FEP, although the AFPBN expert consensus guidelines published in 2013 do recommend SGA depot formulations in FEP.12 SGA LAIA may be preferable, given its neuroprotective effects, in contrast to the neurotoxicity concerns of FGA LAIA.54,55
Relapses begin within a few months of illness stabilization after FEP, and >50% of patients relapse within 1 or 2 years2—the recommended minimum treatment duration for FEP.8,9,13 The use of LAIA is advisable in any patient with schizophrenia for whom long-term antipsychotic therapy is indicated.56 LAIA administration requirements objectively track medication adherence, which allows clinicians to be proactive in relapse prevention. Not using an intervention in FEP that improves adherence and decreases relapse rates contradicts our goal of instituting early, effective treatment to improve long-term functional outcomes (Figure 2).29
Considering clozapine in FEP
Guideline recommendations. Schizo-phrenia treatment guidelines and FDA labeling57 reserve clozapine for third-line treatment of refractory schizophrenia after 2 adequate antipsychotic trials have failed despite optimal dosing (Table 1).6-13 Some guidelines specify 1 of the 2 failed antipsychotic trials must include an SGA.6,7,10,11,13-16 Most say clozapine may be considered in patients with chronic aggression or hostility,7-9,14,16 or suicidal thoughts and behaviors.6-8,14,16 TMAP guidelines recommend a clozapine trial with concomitant substance abuse, persistent positive symptoms during 2 years of consistent medication treatment, and after 5 years of inadequate response (“treatment resistance”), regardless of the number of antipsychotic trials.7
Rationale and concerns. Clozapine is a superior choice for treatment-refractory delusions or hallucinations of schizophrenia, because it markedly enhances the response rate to antipsychotic therapy.58 Researchers therefore have investigated whether clozapine, compared with other antipsychotics, would yield more favorable initial and long-term outcomes when used first-line in FEP.
Preliminary evidence. Five studies have explored the use of clozapine as first-line therapy in FEP (Table 4).59-63 Interpreting the results is difficult because clozapine trials may be brief (mostly, 12 to 52 weeks); lack a comparator arm; suffer from a high attrition rate; enroll few patients; and lack potentially important outcome measures such as negative symptoms, suicidality, and functional assessment.
Overall, these studies demonstrate clozapine is as efficacious in this patient population as chlorpromazine (no difference in remission at 1-year, although clozapine-treated patients remitted faster and stayed in remission longer)60,61 or risperidone (no difference in Positive and Negative Syndrome Scale scores).62
At present, clozapine has not been shown superior to other antipsychotics as a first-line treatment for FEP. Research does underscore the importance of a clozapine trial as third-line treatment for FEP patients who have not responded well to 2 SGA trials.63 Many of these nonresponders (77%) have demonstrated a favorable response when promptly switched to clozapine.64
Discussion and recommendations. The limited evidence argues against using clozapine earlier than as third-line treatment in FEP. Perhaps the high treatment response that characterizes FEP creates a ceiling effect that obscures differences in antipsychotic efficacy at this stage.65 Clozapine use as first-line treatment should be re-evaluated with more robust methodology. One approach could be to assess its benefit in FEP by the duration of untreated psychosis.
The odds of achieving remission have been shown to decrease by 15% for each year that psychosis has not been treated.59 Studies exploring the use of clozapine as a second-line agent for FEP also are warranted, as antipsychotic response during subsequent trials is substantially reduced. In fact, the Scottish Intercollegiate Guidelines Network guidelines recommend this as an area for future research.11
For now, clozapine should continue to be reserved as second- or third-line treatment in a patient with FEP. The risks of clozapine’s potentially serious adverse effects (eg, agranulocytosis, seizures, obesity, diabetes, dyslipidemia, myocarditis, pancreatitis, hypotension, sialorrhea, severe sedation, ileus) can be justified only in the treatment of severe and persistent psychotic symptoms.57
Bottom Line
Nonstandard use of antipsychotic monotherapy dosages beyond the approved FDA limit and combination antipsychotic therapy may be reasonable for select first-episode psychosis (FEP) patients. Strongly consider long-acting injectable antipsychotics in FEP to proactively combat the high relapse rate and more easily identify antipsychotic failure. Continue to use clozapine as second- or third-line therapy in FEP: Studies have not found that it is more efficacious than other antipsychotics for first-line use.
Related Resource
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
Drug Brand Names
Aripiprazole • Abilify, Abilify Maintena
Chlorpromazine • Thorazine
Clozapine • Clozaril
Fluphenazine decanoate • Prolixin-D
Haloperidol • Haldol
Haloperidol decanoate • Haldol-D
Olanzapine • Zyprexa
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone microspheres • Risperdal Consta
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products. Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
First-episode psychosis (FEP) in schizophrenia is characterized by high response rates to antipsychotic therapy, followed by frequent antipsychotic discontinuation and elevated relapse rates soon after maintenance treatment begins.1,2 With subsequent episodes, time to response progressively increases and likelihood of response decreases.3,4
To address these issues, this article—the second of 2 parts5—describes the rationale and evidence for using nonstandard first-line antipsychotic therapies to manage FEP. Specifically, we discuss when clinicians might consider monotherapy exceeding FDA-approved maximum dosages, combination therapy, long-acting injectable antipsychotics (LAIA), or clozapine.
Monotherapy beyond FDA-approved dosages
Treatment guidelines for FEP recommend oral antipsychotic dosages in the lower half of the treatment range and lower than those that are required for multi-episode schizophrenia.6-16 Ultimately, clinicians prescribe individualized dosages for their patients based on symptom improvement and tolerability. The optimal dosage at which to achieve a favorable D2 receptor occupancy likely will vary from patient to patient.17
To control symptoms, higher dosages may be needed than those used in FEP clinical trials, recommended by guidelines for FEP or multi-episode patients, or approved by the FDA. Patients seen in everyday practice may be more complicated (eg, have a comorbid condition or history of nonresponse) than study populations. Higher dosages also may be reasonable to overcome drug−drug interactions (eg, cigarette smoking-mediated cytochrome P450 1A2 induction, resulting in increased olanzapine metabolism),18 or to establish antipsychotic failure if adequate trials at lower dosages have resulted in a suboptimal response and the patient is not experiencing tolerability or safety concerns.
In a study of low-, full-, and high-dosage antipsychotic therapy in FEP, an additional 15% of patients responded to higher dosages of olanzapine and risperidone after failing to respond to a standard dosage.19 A study of data from the Recovery After an Initial Schizophrenia Episode Project’s Early Treatment Program (RAISE-ETP) found that, of participants identified who may benefit from therapy modification, 8.8% were prescribed an antipsychotic (often, olanzapine, risperidone, and haloperidol) at a higher-than-recommended dosage.20 Of note, only olanzapine was prescribed at higher than FDA-approved dosages.
Antipsychotic combination therapy
Prescribing combinations of antipsychotics—antipsychotic polypharmacy (APP)— has a negative connotation because of limited efficacy and safety data,21 and limited endorsement in schizophrenia treatment guidelines.9,13 Caution with APP is warranted; a complex medication regimen may increase the potential for adverse effects, poorer adherence, and adverse drug-drug interactions.9 APP has been shown to independently predict both shorter treatment duration and discontinuation before 1 year.22
Nonetheless, the clinician and patient may share the decision to implement APP and observe whether benefits outweigh risks in situations such as:
• to optimize neuroreceptor occupancy and targets (eg, attempting to achieve adequate D2 receptor blockade while minimizing side effects secondary to binding other receptors)
• to manage co-existing symptom domains (eg, mood changes, aggression, negative symptoms, disorganization, and cognitive deficits)
• to mitigate antipsychotic-induced side effects (eg, initiating aripiprazole to treat hyperprolactinemia induced by another antipsychotic to which the patient has achieved a favorable response).23
Clinicians report using APP to treat as many as 50% of patients with a history of multiple psychotic episodes.23 For FEP patients, 23% of participants in the RAISE-ETP trial who were identified as possibly benefiting from therapy modification were prescribed APP.20 Regrettably, researchers have not found evidence to support a reported rationale for using APP—that lower dosages of individual antipsychotics when used in combination may avoid high-dosage prescriptions.24
Before implementing APP, thoroughly explore and manage reasons for a patient’s suboptimal response to monotherapy.25 An adequate trial with any antipsychotic should be at the highest tolerated dosage for 12 to 16 weeks. Be mindful that response to an APP trial may be the result of additional time on the original antipsychotic.
Long-acting injectable antipsychotics in FEP
Guideline recommendations. Most older guidelines for schizophrenia treatment suggest LAIA after multiple relapses related to medication nonadherence or when a patient prefers injected medication (Table 1).6-13 Expert consensus guidelines also recommend considering LAIA in patients who lack insight into their illness. The Texas Medication Algorithm Project (TMAP) guidelines7 state LAIA can be considered for inadequate adherence at any stage, whereas the 2010 British Association for Psychopharmacology (BAP) guidelines9 express uncertainty about their use in FEP, because of limited evidence. Both the BAP and National Institute for Health and Care Excellence guidelines13 urge clinicians to consider LAIA when avoiding nonadherence is a treatment priority.
Recently, the French Association for Biological Psychiatry and Neuro-psychopharmacology (AFPBN) created expert consensus guidelines12 on using LAIA in practice. They recommend long-acting injectable second-generation antipsychotics (SGAs) as first-line maintenance treatment for schizophrenia and schizoaffective disorder and for individuals experiencing a first recurrent episode. The World Federation of Societies of Biological Psychiatry guidelines contain LAIA dosage recommendations for FEP (Table 2).10
Advances have been made in understanding the serious neurobiological adverse effects of psychotic relapses, including neuroinflammation and oxidative stress, that may explain the atrophic changes observed with psychotic episodes starting with the FEP. Protecting the patient from a second episode has become a vital therapeutic management goal26 (Figure 127).
Concerns. Compared with oral antipsychotics, LAIA offers clinical advantages:
• improved pharmacokinetic profile (lower “peaks” and higher “valleys”)
• more consistent plasma concentrations (no variability related to administration timing or food effects)
• no first-pass metabolism, which can ease the process of finding the lowest effective and safe dosage
• reduced administration burden and objective tracking of adherence with typical dosing every 2 to 4 weeks
• less stigmatizing than oral medication for FEP patients, such as college students living in a dormitory.28,29
Barriers to LAIA use include:
• slow dosage titration and increased time to reach steady state drug level
• oral supplementation for some (eg, risperidone microspheres and aripiprazole long-acting injectable)
• logistical challenges for some (eg, 3-hour post-injection monitoring for delirium sedation syndrome with olanzapine pamoate)
• additional planning to coordinate care for scheduled injections
• higher expenses up front
• local injection site reactions
• dosage adjustment difficulties if adverse effects occur.28,29
Adoption rates of LAIA are low, especially for FEP.30 Most surveys indicate that (1) physicians believe LAIA treatment is ineffective for FEP31 and (2) patients do not prefer injectable to oral antipsychotics,32 despite evidence to the contrary.33,34 A survey of 198 psychiatrists identified 3 factors that influenced their decisions against using LAIA patients with FEP:
• limited availability of SGA depot formulations (4, to date, in the United States)
• frequent rejection by the patient when LAIA is offered without adequate explanation or encouragement
• skepticism of FEP patients (and their family) who lack experience with relapse.35
In reality, when SGA depots were introduced in the United Kingdom, prescribing rates of LAIA did not increase. As for patient rejection being a major reason for not prescribing LAIA, few patients (5% to 36%) are offered depot injections, particularly in FEP.29 Most patients using LAIA are chronic, multi-episode, violent people who are receiving medications involuntarily.29 Interestingly, this survey did not find 2 factors to be influential in psychiatrists’ decision not to use LAIA in FEP:
• guidelines do not explicitly recommend depot treatment in FEP
• treatment in FEP may be limited to 1 year, therefore depot administration is not worthwhile.35
Preliminary evidence. At least a dozen studies have explored LAIA treatment for FEP, with the use of fluphenazine decanoate,36 perphenazine enanthate37 (discontinued), and risperidone microspheres.37-48 The research demonstrates the efficacy and safety of LAIA in FEP as measured by these endpoints:
• improved symptom control38,40-43,46,48
• adherence43,44,48
• reduced relapse rates37,43 and rehospitalizations37,47
• lesser reductions in white matter brain volume45
• no differences in extrapyramidal side effects or prolactin-associated adverse effects.48
A few small studies demonstrate significant differences in outcomes between risperidone LAIA and oral comparator groups (Table 3).43-45 Ongoing studies of LAIA use in FEP are comparing paliperidone palmitate with risperidone microspheres and other oral antipsychotics.49-51 No studies are examining olanzapine pamoate in FEP, likely because several guidelines do not recommended its use. No studies have been published regarding aripiprazole long-acting injectable in FEP. This LAIA formulation was approved in February 2013, and robust studies of the oral formulation in FEP are limited.52
Discussion and recommendations. Psychiatrists relying on subjective measures of antipsychotic adherence may inaccurately assess whether patients meet this criterion for LAIA use.53 LAIA could combat the high relapse rate in FEP, yet depot antipsychotics are prescribed infrequently for FEP patients (eg, for only 9.5% of participants in the RAISE-ETP study).20 Most schizophrenia treatment guidelines do not discuss LAIA use specifically in FEP, although the AFPBN expert consensus guidelines published in 2013 do recommend SGA depot formulations in FEP.12 SGA LAIA may be preferable, given its neuroprotective effects, in contrast to the neurotoxicity concerns of FGA LAIA.54,55
Relapses begin within a few months of illness stabilization after FEP, and >50% of patients relapse within 1 or 2 years2—the recommended minimum treatment duration for FEP.8,9,13 The use of LAIA is advisable in any patient with schizophrenia for whom long-term antipsychotic therapy is indicated.56 LAIA administration requirements objectively track medication adherence, which allows clinicians to be proactive in relapse prevention. Not using an intervention in FEP that improves adherence and decreases relapse rates contradicts our goal of instituting early, effective treatment to improve long-term functional outcomes (Figure 2).29
Considering clozapine in FEP
Guideline recommendations. Schizo-phrenia treatment guidelines and FDA labeling57 reserve clozapine for third-line treatment of refractory schizophrenia after 2 adequate antipsychotic trials have failed despite optimal dosing (Table 1).6-13 Some guidelines specify 1 of the 2 failed antipsychotic trials must include an SGA.6,7,10,11,13-16 Most say clozapine may be considered in patients with chronic aggression or hostility,7-9,14,16 or suicidal thoughts and behaviors.6-8,14,16 TMAP guidelines recommend a clozapine trial with concomitant substance abuse, persistent positive symptoms during 2 years of consistent medication treatment, and after 5 years of inadequate response (“treatment resistance”), regardless of the number of antipsychotic trials.7
Rationale and concerns. Clozapine is a superior choice for treatment-refractory delusions or hallucinations of schizophrenia, because it markedly enhances the response rate to antipsychotic therapy.58 Researchers therefore have investigated whether clozapine, compared with other antipsychotics, would yield more favorable initial and long-term outcomes when used first-line in FEP.
Preliminary evidence. Five studies have explored the use of clozapine as first-line therapy in FEP (Table 4).59-63 Interpreting the results is difficult because clozapine trials may be brief (mostly, 12 to 52 weeks); lack a comparator arm; suffer from a high attrition rate; enroll few patients; and lack potentially important outcome measures such as negative symptoms, suicidality, and functional assessment.
Overall, these studies demonstrate clozapine is as efficacious in this patient population as chlorpromazine (no difference in remission at 1-year, although clozapine-treated patients remitted faster and stayed in remission longer)60,61 or risperidone (no difference in Positive and Negative Syndrome Scale scores).62
At present, clozapine has not been shown superior to other antipsychotics as a first-line treatment for FEP. Research does underscore the importance of a clozapine trial as third-line treatment for FEP patients who have not responded well to 2 SGA trials.63 Many of these nonresponders (77%) have demonstrated a favorable response when promptly switched to clozapine.64
Discussion and recommendations. The limited evidence argues against using clozapine earlier than as third-line treatment in FEP. Perhaps the high treatment response that characterizes FEP creates a ceiling effect that obscures differences in antipsychotic efficacy at this stage.65 Clozapine use as first-line treatment should be re-evaluated with more robust methodology. One approach could be to assess its benefit in FEP by the duration of untreated psychosis.
The odds of achieving remission have been shown to decrease by 15% for each year that psychosis has not been treated.59 Studies exploring the use of clozapine as a second-line agent for FEP also are warranted, as antipsychotic response during subsequent trials is substantially reduced. In fact, the Scottish Intercollegiate Guidelines Network guidelines recommend this as an area for future research.11
For now, clozapine should continue to be reserved as second- or third-line treatment in a patient with FEP. The risks of clozapine’s potentially serious adverse effects (eg, agranulocytosis, seizures, obesity, diabetes, dyslipidemia, myocarditis, pancreatitis, hypotension, sialorrhea, severe sedation, ileus) can be justified only in the treatment of severe and persistent psychotic symptoms.57
Bottom Line
Nonstandard use of antipsychotic monotherapy dosages beyond the approved FDA limit and combination antipsychotic therapy may be reasonable for select first-episode psychosis (FEP) patients. Strongly consider long-acting injectable antipsychotics in FEP to proactively combat the high relapse rate and more easily identify antipsychotic failure. Continue to use clozapine as second- or third-line therapy in FEP: Studies have not found that it is more efficacious than other antipsychotics for first-line use.
Related Resource
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
Drug Brand Names
Aripiprazole • Abilify, Abilify Maintena
Chlorpromazine • Thorazine
Clozapine • Clozaril
Fluphenazine decanoate • Prolixin-D
Haloperidol • Haldol
Haloperidol decanoate • Haldol-D
Olanzapine • Zyprexa
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone microspheres • Risperdal Consta
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products. Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Lieberman JA, Koreen AR, Chakos M, et al. Factors influencing treatment response and outcome of first-episode schizophrenia: implications for understanding the pathophysiology of schizophrenia. J Clin Psychiatry. 1996;57(suppl 9):5-9.
4. Agid O, Arenovich T, Sajeev G, et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72(11):1439-1444.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis. An early stage of schizophrenia with distinct treatment needs. Current Psychiatry. 2015;14(5):32-34,36-40,42.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Texas Department of State Health Services. Texas Medication Algorithm Project (TMAP) Procedural Manual. Schizophrenia Treatment Algorithms. http://www.jpshealthnet.org/sites/default/files/ tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed June 11, 2015.
8. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
9. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
10. Hasan A, Falkai P, Wobrok T, et al; WFSBP Task force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
11. Scottish Intercollegiate Guidelines Network. SIGN 131: Management of schizophrenia. http://www.sign.ac.uk/ pdf/sign131.pdf. Published March 2013. Accessed June 11, 2015.
12. Llorca PM, Abbar M, Courtet P, et al. Guidelines for the use and management of long-acting injectable antipsychotics in serous mental illness. BMC Psychiatry. 2013;13:340.
13. National Institute for Health and Care Excellence. NICE clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. https://www.nice.org. uk/guidance/cg178/resources/guidance-psychosis-and-schizophrenia-in-adults-treatment-and-management-pdf. Updated March 2014. Accessed June 16, 2015.
14. Canadian Psychiatric Association. Clinical practice guidelines. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
15. McEvoy JP, Scheifler PL, Frances A. The expert consensus guideline series: treatment of schizophrenia. J Clin Psychiatry. 1999;60(suppl 11):3-80.
16. Marder SR, Essock SM, Miller AL, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
17. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
18. Fankhauser MP. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
19. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
20. Robinson DG, Schooler NR, John M, et al. Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry. 2015;172(3):237-248.
21. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
22. Fisher MD, Reilly K, Isenberg K, et al. Antipsychotic patterns of use in patients with schizophrenia: polypharmacy versus monotherapy. BMC Psychiatry. 2014;14(1):341.
23. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
24. John AP, Dragovic M. Antipsychotic polypharmacy is not associated with reduced dose of individual antipsychotics in schizophrenia. J Clin Psychopharmacol. 2015;35(2):193-195.
25. Nasrallah HA. Treatment-resistant schizophrenia. Current Psychiatry. http://www.currentpsychiatry.com/specialty-focus/schizophrenia-other-psychotic-disorders/article/ treatment-resistant-schizophrenia/9be7bba3713d4a4cd68aa 8c92b79e5b1.html. Accessed June 16, 2015.
26. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
27. Nasrallah HA, Smeltzer DJ. Contemporary diagnosis and management of the patient with schizophrenia. 2nd ed. Newton, PA: Handbooks in Health Care Co; 2011.
28. McEvoy JP. Risks versus benefits of different types of long-acting injectable antipsychotics. J Clin Psychiatry. 2006;67(suppl 5):15-18.
29. Agid O, Foussias G, Remington G. Long-acting injectable antipsychotics in the treatment of schizophrenia: their role in relapse prevention. Expert Opin Pharmacother. 2010;11(14):2301-2317.
30. Kirschner M, Theodoridou A, Fusar-Poli P, et al. Patients’ and clinicians’ attitude towards long-acting depot antipsychotics in subjects with a first episode psychosis. Ther Adv Psychophamacol. 2013;3(2):89-99.
31. Heres S, Hamann J, Mendel R, et al. Identifying the profile of optimal candidates for antipsychotic depot therapy: A cluster analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1987-1993.
32. Heres S, Lambert M, Vauth R. Treatment of early episode in patents with schizophrenia: the role of long acting antipsychotics. Eur Psychiatry. 2014;29(suppl 2):1409-1413.
33. Heres S, Schmitz FS, Leucht S, et al. The attitude of patients towards antipsychotic depot treatment. Int Clin Psychopharmacol. 2007;22(5):275-282.
34. Weiden PJ, Schooler NR, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
35. Heres S, Reichhart T, Hamann J, et al. Psychiatrists’ attitude to antipsychotic depot treatment in patients with first-episode schizophrenia. Eur Psychiatry. 2011;26(5):297-301.
36. Kane JM, Rifkin A, Quitkin F, et al. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch Gen Psychiatry. 1982;39(1):70-73.
37. Tiihonen J, Wahlbeck K, Lönnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in a community care after first hospitalization due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333(7561):224.
38. Parellada E, Andrezina R, Milanova V, et al. Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol. 2005;19(suppl 5):5-14.
39. Malla A, Binder C, Chue P. Comparison of long-acting injectable risperidone and oral novel antipsychotic drugs for treatment in early phase of schizophrenia spectrum psychosis. Proceedings of the 61st Annual Convention Society of Biological Psychiatry; Toronto, Canada; 2006.
40. Lasser RA, Bossie CA, Zhu Y, et al. Long-acting risperidone in young adults with early schizophrenia or schizoaffective illness. Ann Clin Psychiatry. 2007;19(2):65-71.
41. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
42. Emsley R, Oosthuizen P, Koen L, et al. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
43. Kim B, Lee SH, Choi TK, et al. Effectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1231-1235.
44. Weiden PJ, Schooler NJ, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
45. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011;132(1):35-41.
46. Napryeyenko O, Burba B, Martinez G, et al. Risperidone long-acting injectable in recent-onset schizophrenia examined with clinician and patient self-report measures. J Clin Psychopharmacol. 2010;30(2):200-202.
47. Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609.
48. Dubois V, Megens J, Mertens C, et al. Long-acting risperidone in early-episode schizophrenia. Acta Psychiatrica Belgica. 2011;111(1):9-21.
49. ClinicalTrials.gov. Oral risperidone versus injectable paliperidone palmitate for treating first-episode schizophrenia. https://clinicaltrials.gov/ct2/show/ NCT01451736. Accessed June 16, 2015.
50. ClinicalTrials.gov. Brain myelination effects of paliperidone palmitate versus oral risperidone in first episode schizophrenia. https://clinicaltrials.gov/ct2/ show/NCT01458379. Accessed June 16, 2015.
51. ClinicalTrials.gov. Effects of paliperidone palmitate versus oral antipsychotics on clinical outcomes and MRI measures. https://clinicaltrials.gov/ct2/show/NCT01359293. Accessed June 16, 2016.
52. U.S. Food and Drug Administration. Drugs@FDA. http:// www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed January 11, 2015.
53. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
54. Nandra KS, Agius M. The difference between typical and atypical antipsychotics: the effects on neurogenesis. Psychiatr Danub. 2012;24(suppl 1):S95-S99.
55. Nasrallah HA. Haloperidol is clearly neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
56. Kane JM, Garcia-Ribora C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry. 2009;52:S63-S67.
57. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
58. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
59. Woerner MG, Robinson DG, Alvir JMJ, et al. Clozapine as a first treatment for schizophrenia. Am J Psychiatry. 2003;160(8):1514-1516.
60. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
61. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
62. Sanz-Fuentenebro J, Taboada D, Palomo T, et al. Randomized trial of clozapine vs. risperidone in treatment-naïve first-episode schizophrenia: results after one year. Schizophr Res. 2013;149(1-3):156-161.
63. Yang PD, Ji Z. The efficacy and related factors of clozapine on first-episode schizophrenia. Chin J Nerv Ment Dis. 1997;23:155-158.
64. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
65. Remington G, Agid O, Foussias G, et al. Clozapine’s role in the treatment of first-episode schizophrenia. Am J Psychiatry. 2013;170(2):146-151.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Lieberman JA, Koreen AR, Chakos M, et al. Factors influencing treatment response and outcome of first-episode schizophrenia: implications for understanding the pathophysiology of schizophrenia. J Clin Psychiatry. 1996;57(suppl 9):5-9.
4. Agid O, Arenovich T, Sajeev G, et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72(11):1439-1444.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis. An early stage of schizophrenia with distinct treatment needs. Current Psychiatry. 2015;14(5):32-34,36-40,42.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Texas Department of State Health Services. Texas Medication Algorithm Project (TMAP) Procedural Manual. Schizophrenia Treatment Algorithms. http://www.jpshealthnet.org/sites/default/files/ tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed June 11, 2015.
8. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
9. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
10. Hasan A, Falkai P, Wobrok T, et al; WFSBP Task force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
11. Scottish Intercollegiate Guidelines Network. SIGN 131: Management of schizophrenia. http://www.sign.ac.uk/ pdf/sign131.pdf. Published March 2013. Accessed June 11, 2015.
12. Llorca PM, Abbar M, Courtet P, et al. Guidelines for the use and management of long-acting injectable antipsychotics in serous mental illness. BMC Psychiatry. 2013;13:340.
13. National Institute for Health and Care Excellence. NICE clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. https://www.nice.org. uk/guidance/cg178/resources/guidance-psychosis-and-schizophrenia-in-adults-treatment-and-management-pdf. Updated March 2014. Accessed June 16, 2015.
14. Canadian Psychiatric Association. Clinical practice guidelines. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
15. McEvoy JP, Scheifler PL, Frances A. The expert consensus guideline series: treatment of schizophrenia. J Clin Psychiatry. 1999;60(suppl 11):3-80.
16. Marder SR, Essock SM, Miller AL, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
17. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
18. Fankhauser MP. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
19. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
20. Robinson DG, Schooler NR, John M, et al. Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry. 2015;172(3):237-248.
21. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
22. Fisher MD, Reilly K, Isenberg K, et al. Antipsychotic patterns of use in patients with schizophrenia: polypharmacy versus monotherapy. BMC Psychiatry. 2014;14(1):341.
23. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
24. John AP, Dragovic M. Antipsychotic polypharmacy is not associated with reduced dose of individual antipsychotics in schizophrenia. J Clin Psychopharmacol. 2015;35(2):193-195.
25. Nasrallah HA. Treatment-resistant schizophrenia. Current Psychiatry. http://www.currentpsychiatry.com/specialty-focus/schizophrenia-other-psychotic-disorders/article/ treatment-resistant-schizophrenia/9be7bba3713d4a4cd68aa 8c92b79e5b1.html. Accessed June 16, 2015.
26. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
27. Nasrallah HA, Smeltzer DJ. Contemporary diagnosis and management of the patient with schizophrenia. 2nd ed. Newton, PA: Handbooks in Health Care Co; 2011.
28. McEvoy JP. Risks versus benefits of different types of long-acting injectable antipsychotics. J Clin Psychiatry. 2006;67(suppl 5):15-18.
29. Agid O, Foussias G, Remington G. Long-acting injectable antipsychotics in the treatment of schizophrenia: their role in relapse prevention. Expert Opin Pharmacother. 2010;11(14):2301-2317.
30. Kirschner M, Theodoridou A, Fusar-Poli P, et al. Patients’ and clinicians’ attitude towards long-acting depot antipsychotics in subjects with a first episode psychosis. Ther Adv Psychophamacol. 2013;3(2):89-99.
31. Heres S, Hamann J, Mendel R, et al. Identifying the profile of optimal candidates for antipsychotic depot therapy: A cluster analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1987-1993.
32. Heres S, Lambert M, Vauth R. Treatment of early episode in patents with schizophrenia: the role of long acting antipsychotics. Eur Psychiatry. 2014;29(suppl 2):1409-1413.
33. Heres S, Schmitz FS, Leucht S, et al. The attitude of patients towards antipsychotic depot treatment. Int Clin Psychopharmacol. 2007;22(5):275-282.
34. Weiden PJ, Schooler NR, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
35. Heres S, Reichhart T, Hamann J, et al. Psychiatrists’ attitude to antipsychotic depot treatment in patients with first-episode schizophrenia. Eur Psychiatry. 2011;26(5):297-301.
36. Kane JM, Rifkin A, Quitkin F, et al. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch Gen Psychiatry. 1982;39(1):70-73.
37. Tiihonen J, Wahlbeck K, Lönnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in a community care after first hospitalization due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333(7561):224.
38. Parellada E, Andrezina R, Milanova V, et al. Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol. 2005;19(suppl 5):5-14.
39. Malla A, Binder C, Chue P. Comparison of long-acting injectable risperidone and oral novel antipsychotic drugs for treatment in early phase of schizophrenia spectrum psychosis. Proceedings of the 61st Annual Convention Society of Biological Psychiatry; Toronto, Canada; 2006.
40. Lasser RA, Bossie CA, Zhu Y, et al. Long-acting risperidone in young adults with early schizophrenia or schizoaffective illness. Ann Clin Psychiatry. 2007;19(2):65-71.
41. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
42. Emsley R, Oosthuizen P, Koen L, et al. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
43. Kim B, Lee SH, Choi TK, et al. Effectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1231-1235.
44. Weiden PJ, Schooler NJ, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
45. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011;132(1):35-41.
46. Napryeyenko O, Burba B, Martinez G, et al. Risperidone long-acting injectable in recent-onset schizophrenia examined with clinician and patient self-report measures. J Clin Psychopharmacol. 2010;30(2):200-202.
47. Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609.
48. Dubois V, Megens J, Mertens C, et al. Long-acting risperidone in early-episode schizophrenia. Acta Psychiatrica Belgica. 2011;111(1):9-21.
49. ClinicalTrials.gov. Oral risperidone versus injectable paliperidone palmitate for treating first-episode schizophrenia. https://clinicaltrials.gov/ct2/show/ NCT01451736. Accessed June 16, 2015.
50. ClinicalTrials.gov. Brain myelination effects of paliperidone palmitate versus oral risperidone in first episode schizophrenia. https://clinicaltrials.gov/ct2/ show/NCT01458379. Accessed June 16, 2015.
51. ClinicalTrials.gov. Effects of paliperidone palmitate versus oral antipsychotics on clinical outcomes and MRI measures. https://clinicaltrials.gov/ct2/show/NCT01359293. Accessed June 16, 2016.
52. U.S. Food and Drug Administration. Drugs@FDA. http:// www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed January 11, 2015.
53. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
54. Nandra KS, Agius M. The difference between typical and atypical antipsychotics: the effects on neurogenesis. Psychiatr Danub. 2012;24(suppl 1):S95-S99.
55. Nasrallah HA. Haloperidol is clearly neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
56. Kane JM, Garcia-Ribora C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry. 2009;52:S63-S67.
57. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
58. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
59. Woerner MG, Robinson DG, Alvir JMJ, et al. Clozapine as a first treatment for schizophrenia. Am J Psychiatry. 2003;160(8):1514-1516.
60. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
61. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
62. Sanz-Fuentenebro J, Taboada D, Palomo T, et al. Randomized trial of clozapine vs. risperidone in treatment-naïve first-episode schizophrenia: results after one year. Schizophr Res. 2013;149(1-3):156-161.
63. Yang PD, Ji Z. The efficacy and related factors of clozapine on first-episode schizophrenia. Chin J Nerv Ment Dis. 1997;23:155-158.
64. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
65. Remington G, Agid O, Foussias G, et al. Clozapine’s role in the treatment of first-episode schizophrenia. Am J Psychiatry. 2013;170(2):146-151.
Managing first-episode psychosis: An early stage of schizophrenia with distinct treatment needs
The less time that passes between the onset of psychosis and initiation of appropriate treatment, the greater the patient’s odds of recovery.1 However, relapse prevention is a major clinical challenge because >80% of patients will relapse within 5 years, and, on average, 40% to 50% of patients with a first-episode schizophrenia will relapse within 2 years depending on the definition used and patient characteristics.2 Although there are several explanations and contributing factors to relapses, nonadherence—partial or complete discontinuation of antipsychotics—is a primary risk factor, contributing to a 5-fold increase in relapse risk.3
As such, optimal antipsychotic selection, dosing, and monitoring play an important role in managing this illness. Patients with first-episode psychosis (FEP) are unusual in some ways, compared with patients with multiple episodes of psychosis and represent a different stage of schizophrenia.
In this 2-part series, we will discuss pharmacotherapy for FEP. This article focuses on antipsychotic selection, dosage, and duration of treatment among these patients. The second article, in the July 2015 issue, reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Defining FEP
FEP refers to a patient who has presented, been evaluated, and received treatment for the first psychotic episode associated with a schizophrenia spectrum diagnosis.4 FEP is part of a trajectory marked by tran sitional periods. The patient transitions from being “healthy” to a prodromal state characterized by: (1) nonpsychotic behavioral disturbances such as depression or obsessive-compulsive disorder, (2) attenuated psychotic symptoms not requiring treatment, then converting to (3) psychotic symptoms prompting initial presentation for antipsychotic pharmacotherapy, leading to (4) a formal diagnosis of schizophreniform disorder and, subsequently, schizophrenia, requiring treatment to stabilize symptoms.
There are 2 critical periods along this continuum: prodromal stage and the duration of untreated psychosis (DUP). The prodromal period is a retrospectively identified time where the patient shows initial nonpsychotic disturbances (eg, cognitive and behavioral symptoms) before exhibiting clinical diagnostic criteria for a schizophrenia spectrum disorder. Approximately one-third of patients exhibiting these symptoms convert to psychosis within 1 year, and early treatment engagement at this stage has been shown to improve outcomes.5 The DUP is the time from when a patient has noticeable psychotic symptoms to initiation of drug treatment. The DUP is a consistent predictor of clinical outcome in schizophrenia, including negative symptoms, quality of life, and functional capacity.1
Antipsychotic selection
Treatment goals for FEP patients include:
• minimizing the DUP
• rapidly stabilizing psychosis
• achieving full symptomatic remission
• preventing relapse.
Several treatment guidelines for managing schizophrenia offer variable recommendations for initial antipsychotic treatment in patients with first-episode schizophrenia (Table 1).6-15 Most recommend second-generation antipsychotics (SGAs) over first-generation antipsychotics (FGAs)6,8,9,13,15 with specific recommendations on minimizing neurologic and metabolic adverse effects—to which FEP patients are susceptible—by avoiding high-potency and neurotoxic FGAs (eg, haloperidol and fluphenazine),7 clozapine,11,14 olanzapine,11 or ziprasidone.14 Two guidelines—the National Institute for Health and Care Excellence and the Scottish Intercollegiate Guidelines Network—do not state a preference for antipsychotic selection.10,12
The rationale for these recommendations is based on efficacy data, tolerability differences, FDA-approved indications, and recent FDA approvals with sparse post-marketing data. Of note, there are a lack of robust data for newer antipsychotics (eg, aripiprazole, paliperidone, iloperidone, asenapine, and lurasidone) in effectively and safely treating FEP; however, given the results of other antipsychotics studies, it is likely the efficacy and tolerability of these drugs can be extrapolated from experience with multi-episode patients.
Study design and demographics. Research studies of FEP share some similarities in study design; however, there is enough variability to make it difficult to compare studies and generalize findings (Table 2).16 The variability of DUP is a limitation when comparing studies because it is a significant predictor of clinical outcome. Patients who abuse substances—and often are more challenging to treat17—typically are excluded from these trials, which could explain the high response rate documented in studies of first-episode schizophrenia.
In addition, some FEP patients included in clinical trials might not be truly antipsychotic naïve; an estimated 25% to 75% of patients in these studies are antipsychotic naïve. This is an important consideration when comparing data on adverse effects that occur early in treatment. Additionally, acknowledging the advantages and disadvantages of how to handle missing data is critical because of the high dropout rate observed in these studies.18
Efficacy. There is a high response rate to antipsychotic therapy—ranging from 46% to 96%, depending on the study—in patients with first-episode schizophrenia.3 The response mainly is seen in reduction of positive symptoms because typically negative and cognitive symptoms do not respond to antipsychotics. One study reported only 29% of patients achieved both positive and negative symptom remission.19 It is likely that secondary negative symptoms caused by social withdrawal, reduced speech, and avoidance improve when positive symptoms subside, but primary negative symptoms endure.In general, there is a lack of evidence suggesting that 1 antipsychotic class or agent is more effective than another. Studies mainly assess effectiveness using the primary outcome measure of all-cause discontinuation, such as the Clinical Antipsychotic Trials of Intervention Effectiveness study.20 This outcome measure is a mixture of patient preference, tolerability, and efficacy that provides a more generalizable gauge on how well the treatment works in the clinic rather than tightly regulated settings such as clinical trials. A recent meta-analysis supports no differences in efficacy among antipsychotics in early-episode psychosis.21
Tolerability. Because there are no significant differences among antipsychotic classes or agents in terms of efficacy in first-episode schizophrenia, drug selection is guided mainly by (1) the adverse effect profile and (2) what should be avoided depending on patient-specific variables. Evidence suggests first-episode patients are more sensitive to adverse effects of antipsychotics, particularly neurologic side effects (see this article at CurrentPsychiatry.com for a table comparing adverse effects of antipsychotics in first-episode psychosis).18,22-29 Overall adverse effect profiles remain similar across FEP or multi-episode patients, but tend to be more exaggerated in drug-naïve patients with FEP.
Regarding FGA side effects, McEvoy et al18 demonstrated the neuroleptic threshold occurs at 50% lower haloperidol dosages in patients with first-episode schizophrenia (2.1 mg/d) compared with multi-episode schizophrenia (4.3 mg/d). Other trials suggest SGAs are associated with a lower risk of extrapyramidal side effects (EPS) or use of adjunctive therapies such as anticholinergic drugs or benzodiazepines.23-27 An exception to this statement is that higher risperidone dosages (≥4 to 6 mg/d) have been found to have higher rates of EPS and use of adjunctive medications to treat these symptoms in FEP.26 This is important because studies report higher discontinuation rates with more severe adverse effects of antipsychotics.
Cardiometabolic effects are of particular concern in first-episode patients because most weight gain happens in the first 3 to 4 months of treatment and remains throughout the first year.18,24,29,30 Studies have shown that olanzapine, quetiapine, and risperidone are associated with more clinically significant weight gain compared with haloperidol and ziprasidone.23-25 Olanzapine-associated weight gain has been reported to be twice that of quetiapine and risperidone.18 Regardless, the EUFEST trial did not find a difference in clinically significant weight gain after 12 months among the antipsychotics studied, including haloperidol and ziprasidone.25
Weight gain associated with these antipsychotics is accompanied by changes in fasting triglycerides, glucose, total cholesterol,23 and high-density lipoprotein cholesterol as well as an increase in body mass index (BMI) categorization29 (eg, shift from normal to overweight).18,25 Patients with lower baseline BMI and in racial minority groups might experience more rapid weight gain regardless of antipsychotic selection.29,30
Hyperprolactinemia could be under-recognized and could contribute to early treatment discontinuation.31 Evidence in patients with first-episode schizophrenia suggests similar outcomes as those seen in multi-episode patients, in whom risperidone is associated with higher prolactin elevations and clinically significant hyperprolactinemia (eg, galactorrhea and gynecomastia) compared with olanzapine, quetiapine, and low-dose haloperidol.18,23,24 However, there is a lack of studies that assess whether long-term therapy with strong D2 receptor antagonists increases the risk of bone demineralization or pathological fractures when started before patients’ bones reach maximum density in their mid-20s.31
Antipsychotic dosing
Given the high rate of treatment response in FEP and patients’ higher sensitivity to antipsychotic adverse effects, particularly EPS, guidelines recommend antipsychotic dosages lower than those used for multi-episode schizophrenia,11 especially FGAs. Based on trial data, commonly used dosages include:
• haloperidol, ≤5 mg/d23-25,29
• olanzapine, 10 mg/d18,23,25,29
• risperidone, ≤4 to 6 mg/d.18,24,29,32
In general, haloperidol and risperidone, 2 to 3 mg/d, were well tolerated and effective in trials. Higher quetiapine dosages of 500 to 600 mg/d could be required.11,18,25
According to a survey on prescribing practices of antipsychotic selection and dosing in first-episode schizophrenia,4 clinical prescribing practices tend to use unnecessarily high initial antipsychotic dosing compared with trial data. There also is variability in the usual target antipsychotic dosage ranging from 50% lower dosages to normal dosages in chronic schizophrenia to above FDA-approved maximum dosages for olanzapine (which may be necessary to counteract tobacco-induced cytochrome P450 1A2 enzyme induction).
In addition, these clinicians reported prescribing aripiprazole, an antipsychotic with weaker evidence (eg, case reports, case series, open-label studies) supporting its efficacy and tolerability in FEP. These prescribing practices could reflect attempts to reduce the DUP and achieve symptom remission, so long as tolerability is not a concern.
Essentially, prescribed dosages should be based on symptom improvement and tolerability. This ideal dosage will vary as illustrated by Kapur et al,33 who reported that FEP patients (N = 20) given haloperidol, 1 mg or 2.5 mg/d, had D2 receptor occupancy rates of 38% to 87%, which was significantly dose-related (1 mg/d mean = 59%, 2.5 mg/d mean = 75%). Clinical response and EPS significantly increased as D2 receptor occupancy exceeded 65% and 78%, respectively.
Antipsychotic response
When should you expect to see symptom improvement in patients with first-episode schizophrenia?
Emsley et al34 reported a 77.6% response rate among first-episode patients (N = 522) treated with low dosages of risperidone (mean modal dosage [MMD] = 3.3 mg/d) and haloperidol (MMD = 2.9 mg/d). They found variable response times that were evenly dispersed over a 10-week period. Nearly one-quarter (22.5%) did not respond until after week 4 and 11.2% did not respond until after week 8. In a study of FEP patients (N = 112) treated with olanzapine (MMD = 11.8 mg/d) or risperidone (MMD = 3.9 mg/d), Gallego et al35 reported a cumulative response of 39.6% at week 8 and 65.1% at week 16.
Although there is evidence that, among multi-episode patients, early nonresponse to antipsychotic therapy could predict subsequent nonresponse,36 the evidence is mixed for first-episode schizophrenia. Studies by Emsley et al34 and Gallego et al35 did not find that early nonresponse at weeks 1 or 2 predicted subsequent nonresponse at week 4 or later. However, other studies support the idea that early nonresponse predicts subsequent nonresponse and early antipsychotic response predicts future response in first-episode patients, with good specificity and sensitivity.37,38
Overall, treatment response in first-episode schizophrenia is variable. An adequate antipsychotic trial may be longer, 8 to 16 weeks, compared with 4 to 8 weeks in multi-episode patients. Because research suggests that failure to respond to treatment may lead to medication nonadherence,39 it is reasonable to consider switching antipsychotics when a patient experiences minimal or no response to antipsychotic therapy at week 2; however, this should be a patient-specific decision.
How long should you continue therapy after symptom remission?
There is a lack of consensus on the duration of therapy for a patient treated for first-episode schizophrenia because a small percentage (10% to 20%) do not relapse after the first psychotic episode.3 In general, treatment guidelines and expert consensus statements recommend at least 1 to 2 years of treatment before considering a discontinuation trial.7,10-11 Discuss the benefits and risks of maintenance treatment with your patient and obtain informed consent. With patients with minimal insight, obtaining proper consent is not possible and the physician must exercise judgment unilaterally, if necessary, after educating the family.
After at least 12 months of treatment, antipsychotic therapy could continue indefinitely, depending on patient-specific factors. There are no predictors for identifying patients who do not require maintenance therapy beyond the first psychotic episode. The absence of negative and cognitive deficits could provide clues that a patient might be a candidate for antipsychotic tapering.
Predicting the treatment course
Research investigating clinical predictors or biomarkers that forecast whether a patient will respond to treatment is preliminary. Many characteristics have been identified (Table 31,3,4,23,25,40) and include shorter DUP,1 poorer premorbid function,3 antipsychotic discontinuation,3 a trusting patient-doctor relationship,41 and antipsychotic-related adverse effects,23,25 which are predictive of response, nonresponse, relapse, adherence, and nonadherence, respectively.
Bottom Line
The goals of pharmacological treatment of first-episode schizophrenia are to minimize the duration of untreated psychosis and target full remission of positive symptoms using the lowest possible antipsychotic dosages. Pharmacotherapy should continued for 1 to 2 years, with longer duration considered if it is discussed with the patient and with vigilant monitoring for adverse effects and suboptimal medication nonadherence to prevent relapse.
Editor’s note: The second article in this series in the July 2015 issue reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Related Resources
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
• Martens L, Baker S. Promoting recovery from first episode psychosis: a guide for families. Centre for Addiction and Mental Health. http://www.camh.ca/en/hospital/ Documents/www.camh.net/AboutCAMH/Guideto CAMH/MentalHealthPrograms/SchizophreniaProgram/ 3936PromotingRecoveryFirstEpisodePsychosisfinal.pdf.
Drug Brand Names
Aripiprazole • Abilify Lurasidone • Latuda
Asenapine • Saphris Olanzapine • Zyprexa
Clozapine • Clozaril Paliperidone • Invega
Fluphenazine • Prolixin Quetiapine • Seroquel
Iloperidone • Fanapt Risperidone • Risperdal
Haloperidol • Haldol Ziprasidone • Geodon
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following a response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
4. Weiden PJ, Buckley PF, Grody M. Understanding and treating “first-episode” schizophrenia. Psychiatr Clin North Am. 2007;30(3):481-510.
5. Madaan V, Bestha DP, Kolli V. Schizophrenia prodrome: an optimal approach. Current Psychiatry. 2014;13(3):16-20, 29-30.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
8. Canadian Psychiatric Association. Clinical practice guideline. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
9. McEvoy JP, Scheifler PL, Frances A. Treatment of schizophrenia 1999. Expert consensus guideline series. J Clin Psychiatry. 1999;60(suppl 11):4-80.
10. National Institute for Health and Care Excellence (NICE). Clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. London, United Kingdom: National Institute for Health and Care Excellence (NICE); 2014.
11. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
12. Scottish Intercollegiate Guidelines Network (SIGN). Management of schizophrenia. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network; 2013. SIGN publication no. 131.
13. Argo TR, Crismon ML, Miller AL, et al. Texas Medication Algorithm Project procedural manual. Schizophrenia treatment algorithms. Austin, Texas: Texas Department of State Health Services; 2008.
14. Marder SR, Essock SM, Miller Al, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
15. Bandelow B, Zohar J, Hollander E, et al; WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J Biol Psychiatry. 2008;9(4):248-312.
16. Robinson DG, Woerner MG, Alvir JMJ, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psych. 1999;56(3):241-247.
17. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
18. McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164(7): 1050-1060.
19. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71(6):716-728.
20. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New Engl J Med. 2005; 353(12):1209-1223.
21. Crossley NA, Constante M, McGuire P, et al. Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta-analysis. Br J Psychiatry. 2010;196(6):434-439.
22. McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psych. 1991;48(8):739-745.
23. Lieberman JA, Tollefson G, Tohen M, et al; HGDH Study Group. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396-1404.
24. Schooler N, Rabinowitz J, Davidson M, et al; Early Psychosis Global Working Group. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry. 2005;162(5):947-953.
25. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
26. Emsley RA; Risperidone Working Group. Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Schizophr Bull. 1999;25(4):721-729.
27. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
28. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
29. Robinson DG, Woerner MG, Napolitano B, et al. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006;163(12):2096-2102.
30. Zipursky RB, Gu H, Green AI, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537-543.
31. Taylor M, Waight A, Leonard B. Advances in the understanding and challenges facing the management of first-episode schizophrenia. J Psychopharmacol. 2012; 26(suppl 5):3-5.
32. Merlo MC, Hofer H, Gekle W, et al. Risperidone, 2mg/day vs. 4mg/day, in first-episode, acutely psychotic patients: treatment efficacy and effects on fine motor functioning. J Clin Psychiatry. 2002;63(10):885-891.
33. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
34. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163(4):743-745.
35. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72(12):1691-1696.
36. Gardner KN, Bostwick JR. Antipsychotic treatment response in schizophrenia. Am J Health Sys Pharm. 2012;69(21):1872-1879.
37. Stauffer VL, Case M, Kinon BJ, et al. Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res. 2011;187(1-2):42-48.
38. Schennach-Wolff R, Seemüller FH, Mayr A, et al. An early improvement threshold to predict response and remission in first-episode schizophrenia. Br J Psychiatry. 2010;196(6):460-466.
39. Perkins DO, Gu H, Weiden PJ, et al; Comparison of Atypicals in First Episode study group. Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter study. J Clin Psychiatry. 2008;69(1):106-113.
40. Garner B, Berger GE, Nicolo JP, et al. Pituitary volume and early treatment response in drug-naïve first-episode psychosis patients. Schizophr Res. 2009;113(1):65-71.
41. Sapra M, Weiden PJ, Schooler NR, et al. Reasons for adherence and nonadherence: a pilot study comparing first-and multi-episode schizophrenia patients. Clin Schizophr Relat Psychoses. 2014;7(4):199-206.
The less time that passes between the onset of psychosis and initiation of appropriate treatment, the greater the patient’s odds of recovery.1 However, relapse prevention is a major clinical challenge because >80% of patients will relapse within 5 years, and, on average, 40% to 50% of patients with a first-episode schizophrenia will relapse within 2 years depending on the definition used and patient characteristics.2 Although there are several explanations and contributing factors to relapses, nonadherence—partial or complete discontinuation of antipsychotics—is a primary risk factor, contributing to a 5-fold increase in relapse risk.3
As such, optimal antipsychotic selection, dosing, and monitoring play an important role in managing this illness. Patients with first-episode psychosis (FEP) are unusual in some ways, compared with patients with multiple episodes of psychosis and represent a different stage of schizophrenia.
In this 2-part series, we will discuss pharmacotherapy for FEP. This article focuses on antipsychotic selection, dosage, and duration of treatment among these patients. The second article, in the July 2015 issue, reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Defining FEP
FEP refers to a patient who has presented, been evaluated, and received treatment for the first psychotic episode associated with a schizophrenia spectrum diagnosis.4 FEP is part of a trajectory marked by tran sitional periods. The patient transitions from being “healthy” to a prodromal state characterized by: (1) nonpsychotic behavioral disturbances such as depression or obsessive-compulsive disorder, (2) attenuated psychotic symptoms not requiring treatment, then converting to (3) psychotic symptoms prompting initial presentation for antipsychotic pharmacotherapy, leading to (4) a formal diagnosis of schizophreniform disorder and, subsequently, schizophrenia, requiring treatment to stabilize symptoms.
There are 2 critical periods along this continuum: prodromal stage and the duration of untreated psychosis (DUP). The prodromal period is a retrospectively identified time where the patient shows initial nonpsychotic disturbances (eg, cognitive and behavioral symptoms) before exhibiting clinical diagnostic criteria for a schizophrenia spectrum disorder. Approximately one-third of patients exhibiting these symptoms convert to psychosis within 1 year, and early treatment engagement at this stage has been shown to improve outcomes.5 The DUP is the time from when a patient has noticeable psychotic symptoms to initiation of drug treatment. The DUP is a consistent predictor of clinical outcome in schizophrenia, including negative symptoms, quality of life, and functional capacity.1
Antipsychotic selection
Treatment goals for FEP patients include:
• minimizing the DUP
• rapidly stabilizing psychosis
• achieving full symptomatic remission
• preventing relapse.
Several treatment guidelines for managing schizophrenia offer variable recommendations for initial antipsychotic treatment in patients with first-episode schizophrenia (Table 1).6-15 Most recommend second-generation antipsychotics (SGAs) over first-generation antipsychotics (FGAs)6,8,9,13,15 with specific recommendations on minimizing neurologic and metabolic adverse effects—to which FEP patients are susceptible—by avoiding high-potency and neurotoxic FGAs (eg, haloperidol and fluphenazine),7 clozapine,11,14 olanzapine,11 or ziprasidone.14 Two guidelines—the National Institute for Health and Care Excellence and the Scottish Intercollegiate Guidelines Network—do not state a preference for antipsychotic selection.10,12
The rationale for these recommendations is based on efficacy data, tolerability differences, FDA-approved indications, and recent FDA approvals with sparse post-marketing data. Of note, there are a lack of robust data for newer antipsychotics (eg, aripiprazole, paliperidone, iloperidone, asenapine, and lurasidone) in effectively and safely treating FEP; however, given the results of other antipsychotics studies, it is likely the efficacy and tolerability of these drugs can be extrapolated from experience with multi-episode patients.
Study design and demographics. Research studies of FEP share some similarities in study design; however, there is enough variability to make it difficult to compare studies and generalize findings (Table 2).16 The variability of DUP is a limitation when comparing studies because it is a significant predictor of clinical outcome. Patients who abuse substances—and often are more challenging to treat17—typically are excluded from these trials, which could explain the high response rate documented in studies of first-episode schizophrenia.
In addition, some FEP patients included in clinical trials might not be truly antipsychotic naïve; an estimated 25% to 75% of patients in these studies are antipsychotic naïve. This is an important consideration when comparing data on adverse effects that occur early in treatment. Additionally, acknowledging the advantages and disadvantages of how to handle missing data is critical because of the high dropout rate observed in these studies.18
Efficacy. There is a high response rate to antipsychotic therapy—ranging from 46% to 96%, depending on the study—in patients with first-episode schizophrenia.3 The response mainly is seen in reduction of positive symptoms because typically negative and cognitive symptoms do not respond to antipsychotics. One study reported only 29% of patients achieved both positive and negative symptom remission.19 It is likely that secondary negative symptoms caused by social withdrawal, reduced speech, and avoidance improve when positive symptoms subside, but primary negative symptoms endure.In general, there is a lack of evidence suggesting that 1 antipsychotic class or agent is more effective than another. Studies mainly assess effectiveness using the primary outcome measure of all-cause discontinuation, such as the Clinical Antipsychotic Trials of Intervention Effectiveness study.20 This outcome measure is a mixture of patient preference, tolerability, and efficacy that provides a more generalizable gauge on how well the treatment works in the clinic rather than tightly regulated settings such as clinical trials. A recent meta-analysis supports no differences in efficacy among antipsychotics in early-episode psychosis.21
Tolerability. Because there are no significant differences among antipsychotic classes or agents in terms of efficacy in first-episode schizophrenia, drug selection is guided mainly by (1) the adverse effect profile and (2) what should be avoided depending on patient-specific variables. Evidence suggests first-episode patients are more sensitive to adverse effects of antipsychotics, particularly neurologic side effects (see this article at CurrentPsychiatry.com for a table comparing adverse effects of antipsychotics in first-episode psychosis).18,22-29 Overall adverse effect profiles remain similar across FEP or multi-episode patients, but tend to be more exaggerated in drug-naïve patients with FEP.
Regarding FGA side effects, McEvoy et al18 demonstrated the neuroleptic threshold occurs at 50% lower haloperidol dosages in patients with first-episode schizophrenia (2.1 mg/d) compared with multi-episode schizophrenia (4.3 mg/d). Other trials suggest SGAs are associated with a lower risk of extrapyramidal side effects (EPS) or use of adjunctive therapies such as anticholinergic drugs or benzodiazepines.23-27 An exception to this statement is that higher risperidone dosages (≥4 to 6 mg/d) have been found to have higher rates of EPS and use of adjunctive medications to treat these symptoms in FEP.26 This is important because studies report higher discontinuation rates with more severe adverse effects of antipsychotics.
Cardiometabolic effects are of particular concern in first-episode patients because most weight gain happens in the first 3 to 4 months of treatment and remains throughout the first year.18,24,29,30 Studies have shown that olanzapine, quetiapine, and risperidone are associated with more clinically significant weight gain compared with haloperidol and ziprasidone.23-25 Olanzapine-associated weight gain has been reported to be twice that of quetiapine and risperidone.18 Regardless, the EUFEST trial did not find a difference in clinically significant weight gain after 12 months among the antipsychotics studied, including haloperidol and ziprasidone.25
Weight gain associated with these antipsychotics is accompanied by changes in fasting triglycerides, glucose, total cholesterol,23 and high-density lipoprotein cholesterol as well as an increase in body mass index (BMI) categorization29 (eg, shift from normal to overweight).18,25 Patients with lower baseline BMI and in racial minority groups might experience more rapid weight gain regardless of antipsychotic selection.29,30
Hyperprolactinemia could be under-recognized and could contribute to early treatment discontinuation.31 Evidence in patients with first-episode schizophrenia suggests similar outcomes as those seen in multi-episode patients, in whom risperidone is associated with higher prolactin elevations and clinically significant hyperprolactinemia (eg, galactorrhea and gynecomastia) compared with olanzapine, quetiapine, and low-dose haloperidol.18,23,24 However, there is a lack of studies that assess whether long-term therapy with strong D2 receptor antagonists increases the risk of bone demineralization or pathological fractures when started before patients’ bones reach maximum density in their mid-20s.31
Antipsychotic dosing
Given the high rate of treatment response in FEP and patients’ higher sensitivity to antipsychotic adverse effects, particularly EPS, guidelines recommend antipsychotic dosages lower than those used for multi-episode schizophrenia,11 especially FGAs. Based on trial data, commonly used dosages include:
• haloperidol, ≤5 mg/d23-25,29
• olanzapine, 10 mg/d18,23,25,29
• risperidone, ≤4 to 6 mg/d.18,24,29,32
In general, haloperidol and risperidone, 2 to 3 mg/d, were well tolerated and effective in trials. Higher quetiapine dosages of 500 to 600 mg/d could be required.11,18,25
According to a survey on prescribing practices of antipsychotic selection and dosing in first-episode schizophrenia,4 clinical prescribing practices tend to use unnecessarily high initial antipsychotic dosing compared with trial data. There also is variability in the usual target antipsychotic dosage ranging from 50% lower dosages to normal dosages in chronic schizophrenia to above FDA-approved maximum dosages for olanzapine (which may be necessary to counteract tobacco-induced cytochrome P450 1A2 enzyme induction).
In addition, these clinicians reported prescribing aripiprazole, an antipsychotic with weaker evidence (eg, case reports, case series, open-label studies) supporting its efficacy and tolerability in FEP. These prescribing practices could reflect attempts to reduce the DUP and achieve symptom remission, so long as tolerability is not a concern.
Essentially, prescribed dosages should be based on symptom improvement and tolerability. This ideal dosage will vary as illustrated by Kapur et al,33 who reported that FEP patients (N = 20) given haloperidol, 1 mg or 2.5 mg/d, had D2 receptor occupancy rates of 38% to 87%, which was significantly dose-related (1 mg/d mean = 59%, 2.5 mg/d mean = 75%). Clinical response and EPS significantly increased as D2 receptor occupancy exceeded 65% and 78%, respectively.
Antipsychotic response
When should you expect to see symptom improvement in patients with first-episode schizophrenia?
Emsley et al34 reported a 77.6% response rate among first-episode patients (N = 522) treated with low dosages of risperidone (mean modal dosage [MMD] = 3.3 mg/d) and haloperidol (MMD = 2.9 mg/d). They found variable response times that were evenly dispersed over a 10-week period. Nearly one-quarter (22.5%) did not respond until after week 4 and 11.2% did not respond until after week 8. In a study of FEP patients (N = 112) treated with olanzapine (MMD = 11.8 mg/d) or risperidone (MMD = 3.9 mg/d), Gallego et al35 reported a cumulative response of 39.6% at week 8 and 65.1% at week 16.
Although there is evidence that, among multi-episode patients, early nonresponse to antipsychotic therapy could predict subsequent nonresponse,36 the evidence is mixed for first-episode schizophrenia. Studies by Emsley et al34 and Gallego et al35 did not find that early nonresponse at weeks 1 or 2 predicted subsequent nonresponse at week 4 or later. However, other studies support the idea that early nonresponse predicts subsequent nonresponse and early antipsychotic response predicts future response in first-episode patients, with good specificity and sensitivity.37,38
Overall, treatment response in first-episode schizophrenia is variable. An adequate antipsychotic trial may be longer, 8 to 16 weeks, compared with 4 to 8 weeks in multi-episode patients. Because research suggests that failure to respond to treatment may lead to medication nonadherence,39 it is reasonable to consider switching antipsychotics when a patient experiences minimal or no response to antipsychotic therapy at week 2; however, this should be a patient-specific decision.
How long should you continue therapy after symptom remission?
There is a lack of consensus on the duration of therapy for a patient treated for first-episode schizophrenia because a small percentage (10% to 20%) do not relapse after the first psychotic episode.3 In general, treatment guidelines and expert consensus statements recommend at least 1 to 2 years of treatment before considering a discontinuation trial.7,10-11 Discuss the benefits and risks of maintenance treatment with your patient and obtain informed consent. With patients with minimal insight, obtaining proper consent is not possible and the physician must exercise judgment unilaterally, if necessary, after educating the family.
After at least 12 months of treatment, antipsychotic therapy could continue indefinitely, depending on patient-specific factors. There are no predictors for identifying patients who do not require maintenance therapy beyond the first psychotic episode. The absence of negative and cognitive deficits could provide clues that a patient might be a candidate for antipsychotic tapering.
Predicting the treatment course
Research investigating clinical predictors or biomarkers that forecast whether a patient will respond to treatment is preliminary. Many characteristics have been identified (Table 31,3,4,23,25,40) and include shorter DUP,1 poorer premorbid function,3 antipsychotic discontinuation,3 a trusting patient-doctor relationship,41 and antipsychotic-related adverse effects,23,25 which are predictive of response, nonresponse, relapse, adherence, and nonadherence, respectively.
Bottom Line
The goals of pharmacological treatment of first-episode schizophrenia are to minimize the duration of untreated psychosis and target full remission of positive symptoms using the lowest possible antipsychotic dosages. Pharmacotherapy should continued for 1 to 2 years, with longer duration considered if it is discussed with the patient and with vigilant monitoring for adverse effects and suboptimal medication nonadherence to prevent relapse.
Editor’s note: The second article in this series in the July 2015 issue reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Related Resources
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
• Martens L, Baker S. Promoting recovery from first episode psychosis: a guide for families. Centre for Addiction and Mental Health. http://www.camh.ca/en/hospital/ Documents/www.camh.net/AboutCAMH/Guideto CAMH/MentalHealthPrograms/SchizophreniaProgram/ 3936PromotingRecoveryFirstEpisodePsychosisfinal.pdf.
Drug Brand Names
Aripiprazole • Abilify Lurasidone • Latuda
Asenapine • Saphris Olanzapine • Zyprexa
Clozapine • Clozaril Paliperidone • Invega
Fluphenazine • Prolixin Quetiapine • Seroquel
Iloperidone • Fanapt Risperidone • Risperdal
Haloperidol • Haldol Ziprasidone • Geodon
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
The less time that passes between the onset of psychosis and initiation of appropriate treatment, the greater the patient’s odds of recovery.1 However, relapse prevention is a major clinical challenge because >80% of patients will relapse within 5 years, and, on average, 40% to 50% of patients with a first-episode schizophrenia will relapse within 2 years depending on the definition used and patient characteristics.2 Although there are several explanations and contributing factors to relapses, nonadherence—partial or complete discontinuation of antipsychotics—is a primary risk factor, contributing to a 5-fold increase in relapse risk.3
As such, optimal antipsychotic selection, dosing, and monitoring play an important role in managing this illness. Patients with first-episode psychosis (FEP) are unusual in some ways, compared with patients with multiple episodes of psychosis and represent a different stage of schizophrenia.
In this 2-part series, we will discuss pharmacotherapy for FEP. This article focuses on antipsychotic selection, dosage, and duration of treatment among these patients. The second article, in the July 2015 issue, reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Defining FEP
FEP refers to a patient who has presented, been evaluated, and received treatment for the first psychotic episode associated with a schizophrenia spectrum diagnosis.4 FEP is part of a trajectory marked by tran sitional periods. The patient transitions from being “healthy” to a prodromal state characterized by: (1) nonpsychotic behavioral disturbances such as depression or obsessive-compulsive disorder, (2) attenuated psychotic symptoms not requiring treatment, then converting to (3) psychotic symptoms prompting initial presentation for antipsychotic pharmacotherapy, leading to (4) a formal diagnosis of schizophreniform disorder and, subsequently, schizophrenia, requiring treatment to stabilize symptoms.
There are 2 critical periods along this continuum: prodromal stage and the duration of untreated psychosis (DUP). The prodromal period is a retrospectively identified time where the patient shows initial nonpsychotic disturbances (eg, cognitive and behavioral symptoms) before exhibiting clinical diagnostic criteria for a schizophrenia spectrum disorder. Approximately one-third of patients exhibiting these symptoms convert to psychosis within 1 year, and early treatment engagement at this stage has been shown to improve outcomes.5 The DUP is the time from when a patient has noticeable psychotic symptoms to initiation of drug treatment. The DUP is a consistent predictor of clinical outcome in schizophrenia, including negative symptoms, quality of life, and functional capacity.1
Antipsychotic selection
Treatment goals for FEP patients include:
• minimizing the DUP
• rapidly stabilizing psychosis
• achieving full symptomatic remission
• preventing relapse.
Several treatment guidelines for managing schizophrenia offer variable recommendations for initial antipsychotic treatment in patients with first-episode schizophrenia (Table 1).6-15 Most recommend second-generation antipsychotics (SGAs) over first-generation antipsychotics (FGAs)6,8,9,13,15 with specific recommendations on minimizing neurologic and metabolic adverse effects—to which FEP patients are susceptible—by avoiding high-potency and neurotoxic FGAs (eg, haloperidol and fluphenazine),7 clozapine,11,14 olanzapine,11 or ziprasidone.14 Two guidelines—the National Institute for Health and Care Excellence and the Scottish Intercollegiate Guidelines Network—do not state a preference for antipsychotic selection.10,12
The rationale for these recommendations is based on efficacy data, tolerability differences, FDA-approved indications, and recent FDA approvals with sparse post-marketing data. Of note, there are a lack of robust data for newer antipsychotics (eg, aripiprazole, paliperidone, iloperidone, asenapine, and lurasidone) in effectively and safely treating FEP; however, given the results of other antipsychotics studies, it is likely the efficacy and tolerability of these drugs can be extrapolated from experience with multi-episode patients.
Study design and demographics. Research studies of FEP share some similarities in study design; however, there is enough variability to make it difficult to compare studies and generalize findings (Table 2).16 The variability of DUP is a limitation when comparing studies because it is a significant predictor of clinical outcome. Patients who abuse substances—and often are more challenging to treat17—typically are excluded from these trials, which could explain the high response rate documented in studies of first-episode schizophrenia.
In addition, some FEP patients included in clinical trials might not be truly antipsychotic naïve; an estimated 25% to 75% of patients in these studies are antipsychotic naïve. This is an important consideration when comparing data on adverse effects that occur early in treatment. Additionally, acknowledging the advantages and disadvantages of how to handle missing data is critical because of the high dropout rate observed in these studies.18
Efficacy. There is a high response rate to antipsychotic therapy—ranging from 46% to 96%, depending on the study—in patients with first-episode schizophrenia.3 The response mainly is seen in reduction of positive symptoms because typically negative and cognitive symptoms do not respond to antipsychotics. One study reported only 29% of patients achieved both positive and negative symptom remission.19 It is likely that secondary negative symptoms caused by social withdrawal, reduced speech, and avoidance improve when positive symptoms subside, but primary negative symptoms endure.In general, there is a lack of evidence suggesting that 1 antipsychotic class or agent is more effective than another. Studies mainly assess effectiveness using the primary outcome measure of all-cause discontinuation, such as the Clinical Antipsychotic Trials of Intervention Effectiveness study.20 This outcome measure is a mixture of patient preference, tolerability, and efficacy that provides a more generalizable gauge on how well the treatment works in the clinic rather than tightly regulated settings such as clinical trials. A recent meta-analysis supports no differences in efficacy among antipsychotics in early-episode psychosis.21
Tolerability. Because there are no significant differences among antipsychotic classes or agents in terms of efficacy in first-episode schizophrenia, drug selection is guided mainly by (1) the adverse effect profile and (2) what should be avoided depending on patient-specific variables. Evidence suggests first-episode patients are more sensitive to adverse effects of antipsychotics, particularly neurologic side effects (see this article at CurrentPsychiatry.com for a table comparing adverse effects of antipsychotics in first-episode psychosis).18,22-29 Overall adverse effect profiles remain similar across FEP or multi-episode patients, but tend to be more exaggerated in drug-naïve patients with FEP.
Regarding FGA side effects, McEvoy et al18 demonstrated the neuroleptic threshold occurs at 50% lower haloperidol dosages in patients with first-episode schizophrenia (2.1 mg/d) compared with multi-episode schizophrenia (4.3 mg/d). Other trials suggest SGAs are associated with a lower risk of extrapyramidal side effects (EPS) or use of adjunctive therapies such as anticholinergic drugs or benzodiazepines.23-27 An exception to this statement is that higher risperidone dosages (≥4 to 6 mg/d) have been found to have higher rates of EPS and use of adjunctive medications to treat these symptoms in FEP.26 This is important because studies report higher discontinuation rates with more severe adverse effects of antipsychotics.
Cardiometabolic effects are of particular concern in first-episode patients because most weight gain happens in the first 3 to 4 months of treatment and remains throughout the first year.18,24,29,30 Studies have shown that olanzapine, quetiapine, and risperidone are associated with more clinically significant weight gain compared with haloperidol and ziprasidone.23-25 Olanzapine-associated weight gain has been reported to be twice that of quetiapine and risperidone.18 Regardless, the EUFEST trial did not find a difference in clinically significant weight gain after 12 months among the antipsychotics studied, including haloperidol and ziprasidone.25
Weight gain associated with these antipsychotics is accompanied by changes in fasting triglycerides, glucose, total cholesterol,23 and high-density lipoprotein cholesterol as well as an increase in body mass index (BMI) categorization29 (eg, shift from normal to overweight).18,25 Patients with lower baseline BMI and in racial minority groups might experience more rapid weight gain regardless of antipsychotic selection.29,30
Hyperprolactinemia could be under-recognized and could contribute to early treatment discontinuation.31 Evidence in patients with first-episode schizophrenia suggests similar outcomes as those seen in multi-episode patients, in whom risperidone is associated with higher prolactin elevations and clinically significant hyperprolactinemia (eg, galactorrhea and gynecomastia) compared with olanzapine, quetiapine, and low-dose haloperidol.18,23,24 However, there is a lack of studies that assess whether long-term therapy with strong D2 receptor antagonists increases the risk of bone demineralization or pathological fractures when started before patients’ bones reach maximum density in their mid-20s.31
Antipsychotic dosing
Given the high rate of treatment response in FEP and patients’ higher sensitivity to antipsychotic adverse effects, particularly EPS, guidelines recommend antipsychotic dosages lower than those used for multi-episode schizophrenia,11 especially FGAs. Based on trial data, commonly used dosages include:
• haloperidol, ≤5 mg/d23-25,29
• olanzapine, 10 mg/d18,23,25,29
• risperidone, ≤4 to 6 mg/d.18,24,29,32
In general, haloperidol and risperidone, 2 to 3 mg/d, were well tolerated and effective in trials. Higher quetiapine dosages of 500 to 600 mg/d could be required.11,18,25
According to a survey on prescribing practices of antipsychotic selection and dosing in first-episode schizophrenia,4 clinical prescribing practices tend to use unnecessarily high initial antipsychotic dosing compared with trial data. There also is variability in the usual target antipsychotic dosage ranging from 50% lower dosages to normal dosages in chronic schizophrenia to above FDA-approved maximum dosages for olanzapine (which may be necessary to counteract tobacco-induced cytochrome P450 1A2 enzyme induction).
In addition, these clinicians reported prescribing aripiprazole, an antipsychotic with weaker evidence (eg, case reports, case series, open-label studies) supporting its efficacy and tolerability in FEP. These prescribing practices could reflect attempts to reduce the DUP and achieve symptom remission, so long as tolerability is not a concern.
Essentially, prescribed dosages should be based on symptom improvement and tolerability. This ideal dosage will vary as illustrated by Kapur et al,33 who reported that FEP patients (N = 20) given haloperidol, 1 mg or 2.5 mg/d, had D2 receptor occupancy rates of 38% to 87%, which was significantly dose-related (1 mg/d mean = 59%, 2.5 mg/d mean = 75%). Clinical response and EPS significantly increased as D2 receptor occupancy exceeded 65% and 78%, respectively.
Antipsychotic response
When should you expect to see symptom improvement in patients with first-episode schizophrenia?
Emsley et al34 reported a 77.6% response rate among first-episode patients (N = 522) treated with low dosages of risperidone (mean modal dosage [MMD] = 3.3 mg/d) and haloperidol (MMD = 2.9 mg/d). They found variable response times that were evenly dispersed over a 10-week period. Nearly one-quarter (22.5%) did not respond until after week 4 and 11.2% did not respond until after week 8. In a study of FEP patients (N = 112) treated with olanzapine (MMD = 11.8 mg/d) or risperidone (MMD = 3.9 mg/d), Gallego et al35 reported a cumulative response of 39.6% at week 8 and 65.1% at week 16.
Although there is evidence that, among multi-episode patients, early nonresponse to antipsychotic therapy could predict subsequent nonresponse,36 the evidence is mixed for first-episode schizophrenia. Studies by Emsley et al34 and Gallego et al35 did not find that early nonresponse at weeks 1 or 2 predicted subsequent nonresponse at week 4 or later. However, other studies support the idea that early nonresponse predicts subsequent nonresponse and early antipsychotic response predicts future response in first-episode patients, with good specificity and sensitivity.37,38
Overall, treatment response in first-episode schizophrenia is variable. An adequate antipsychotic trial may be longer, 8 to 16 weeks, compared with 4 to 8 weeks in multi-episode patients. Because research suggests that failure to respond to treatment may lead to medication nonadherence,39 it is reasonable to consider switching antipsychotics when a patient experiences minimal or no response to antipsychotic therapy at week 2; however, this should be a patient-specific decision.
How long should you continue therapy after symptom remission?
There is a lack of consensus on the duration of therapy for a patient treated for first-episode schizophrenia because a small percentage (10% to 20%) do not relapse after the first psychotic episode.3 In general, treatment guidelines and expert consensus statements recommend at least 1 to 2 years of treatment before considering a discontinuation trial.7,10-11 Discuss the benefits and risks of maintenance treatment with your patient and obtain informed consent. With patients with minimal insight, obtaining proper consent is not possible and the physician must exercise judgment unilaterally, if necessary, after educating the family.
After at least 12 months of treatment, antipsychotic therapy could continue indefinitely, depending on patient-specific factors. There are no predictors for identifying patients who do not require maintenance therapy beyond the first psychotic episode. The absence of negative and cognitive deficits could provide clues that a patient might be a candidate for antipsychotic tapering.
Predicting the treatment course
Research investigating clinical predictors or biomarkers that forecast whether a patient will respond to treatment is preliminary. Many characteristics have been identified (Table 31,3,4,23,25,40) and include shorter DUP,1 poorer premorbid function,3 antipsychotic discontinuation,3 a trusting patient-doctor relationship,41 and antipsychotic-related adverse effects,23,25 which are predictive of response, nonresponse, relapse, adherence, and nonadherence, respectively.
Bottom Line
The goals of pharmacological treatment of first-episode schizophrenia are to minimize the duration of untreated psychosis and target full remission of positive symptoms using the lowest possible antipsychotic dosages. Pharmacotherapy should continued for 1 to 2 years, with longer duration considered if it is discussed with the patient and with vigilant monitoring for adverse effects and suboptimal medication nonadherence to prevent relapse.
Editor’s note: The second article in this series in the July 2015 issue reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Related Resources
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
• Martens L, Baker S. Promoting recovery from first episode psychosis: a guide for families. Centre for Addiction and Mental Health. http://www.camh.ca/en/hospital/ Documents/www.camh.net/AboutCAMH/Guideto CAMH/MentalHealthPrograms/SchizophreniaProgram/ 3936PromotingRecoveryFirstEpisodePsychosisfinal.pdf.
Drug Brand Names
Aripiprazole • Abilify Lurasidone • Latuda
Asenapine • Saphris Olanzapine • Zyprexa
Clozapine • Clozaril Paliperidone • Invega
Fluphenazine • Prolixin Quetiapine • Seroquel
Iloperidone • Fanapt Risperidone • Risperdal
Haloperidol • Haldol Ziprasidone • Geodon
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following a response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
4. Weiden PJ, Buckley PF, Grody M. Understanding and treating “first-episode” schizophrenia. Psychiatr Clin North Am. 2007;30(3):481-510.
5. Madaan V, Bestha DP, Kolli V. Schizophrenia prodrome: an optimal approach. Current Psychiatry. 2014;13(3):16-20, 29-30.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
8. Canadian Psychiatric Association. Clinical practice guideline. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
9. McEvoy JP, Scheifler PL, Frances A. Treatment of schizophrenia 1999. Expert consensus guideline series. J Clin Psychiatry. 1999;60(suppl 11):4-80.
10. National Institute for Health and Care Excellence (NICE). Clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. London, United Kingdom: National Institute for Health and Care Excellence (NICE); 2014.
11. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
12. Scottish Intercollegiate Guidelines Network (SIGN). Management of schizophrenia. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network; 2013. SIGN publication no. 131.
13. Argo TR, Crismon ML, Miller AL, et al. Texas Medication Algorithm Project procedural manual. Schizophrenia treatment algorithms. Austin, Texas: Texas Department of State Health Services; 2008.
14. Marder SR, Essock SM, Miller Al, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
15. Bandelow B, Zohar J, Hollander E, et al; WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J Biol Psychiatry. 2008;9(4):248-312.
16. Robinson DG, Woerner MG, Alvir JMJ, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psych. 1999;56(3):241-247.
17. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
18. McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164(7): 1050-1060.
19. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71(6):716-728.
20. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New Engl J Med. 2005; 353(12):1209-1223.
21. Crossley NA, Constante M, McGuire P, et al. Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta-analysis. Br J Psychiatry. 2010;196(6):434-439.
22. McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psych. 1991;48(8):739-745.
23. Lieberman JA, Tollefson G, Tohen M, et al; HGDH Study Group. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396-1404.
24. Schooler N, Rabinowitz J, Davidson M, et al; Early Psychosis Global Working Group. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry. 2005;162(5):947-953.
25. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
26. Emsley RA; Risperidone Working Group. Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Schizophr Bull. 1999;25(4):721-729.
27. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
28. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
29. Robinson DG, Woerner MG, Napolitano B, et al. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006;163(12):2096-2102.
30. Zipursky RB, Gu H, Green AI, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537-543.
31. Taylor M, Waight A, Leonard B. Advances in the understanding and challenges facing the management of first-episode schizophrenia. J Psychopharmacol. 2012; 26(suppl 5):3-5.
32. Merlo MC, Hofer H, Gekle W, et al. Risperidone, 2mg/day vs. 4mg/day, in first-episode, acutely psychotic patients: treatment efficacy and effects on fine motor functioning. J Clin Psychiatry. 2002;63(10):885-891.
33. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
34. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163(4):743-745.
35. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72(12):1691-1696.
36. Gardner KN, Bostwick JR. Antipsychotic treatment response in schizophrenia. Am J Health Sys Pharm. 2012;69(21):1872-1879.
37. Stauffer VL, Case M, Kinon BJ, et al. Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res. 2011;187(1-2):42-48.
38. Schennach-Wolff R, Seemüller FH, Mayr A, et al. An early improvement threshold to predict response and remission in first-episode schizophrenia. Br J Psychiatry. 2010;196(6):460-466.
39. Perkins DO, Gu H, Weiden PJ, et al; Comparison of Atypicals in First Episode study group. Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter study. J Clin Psychiatry. 2008;69(1):106-113.
40. Garner B, Berger GE, Nicolo JP, et al. Pituitary volume and early treatment response in drug-naïve first-episode psychosis patients. Schizophr Res. 2009;113(1):65-71.
41. Sapra M, Weiden PJ, Schooler NR, et al. Reasons for adherence and nonadherence: a pilot study comparing first-and multi-episode schizophrenia patients. Clin Schizophr Relat Psychoses. 2014;7(4):199-206.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following a response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
4. Weiden PJ, Buckley PF, Grody M. Understanding and treating “first-episode” schizophrenia. Psychiatr Clin North Am. 2007;30(3):481-510.
5. Madaan V, Bestha DP, Kolli V. Schizophrenia prodrome: an optimal approach. Current Psychiatry. 2014;13(3):16-20, 29-30.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
8. Canadian Psychiatric Association. Clinical practice guideline. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
9. McEvoy JP, Scheifler PL, Frances A. Treatment of schizophrenia 1999. Expert consensus guideline series. J Clin Psychiatry. 1999;60(suppl 11):4-80.
10. National Institute for Health and Care Excellence (NICE). Clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. London, United Kingdom: National Institute for Health and Care Excellence (NICE); 2014.
11. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
12. Scottish Intercollegiate Guidelines Network (SIGN). Management of schizophrenia. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network; 2013. SIGN publication no. 131.
13. Argo TR, Crismon ML, Miller AL, et al. Texas Medication Algorithm Project procedural manual. Schizophrenia treatment algorithms. Austin, Texas: Texas Department of State Health Services; 2008.
14. Marder SR, Essock SM, Miller Al, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
15. Bandelow B, Zohar J, Hollander E, et al; WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J Biol Psychiatry. 2008;9(4):248-312.
16. Robinson DG, Woerner MG, Alvir JMJ, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psych. 1999;56(3):241-247.
17. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
18. McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164(7): 1050-1060.
19. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71(6):716-728.
20. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New Engl J Med. 2005; 353(12):1209-1223.
21. Crossley NA, Constante M, McGuire P, et al. Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta-analysis. Br J Psychiatry. 2010;196(6):434-439.
22. McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psych. 1991;48(8):739-745.
23. Lieberman JA, Tollefson G, Tohen M, et al; HGDH Study Group. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396-1404.
24. Schooler N, Rabinowitz J, Davidson M, et al; Early Psychosis Global Working Group. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry. 2005;162(5):947-953.
25. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
26. Emsley RA; Risperidone Working Group. Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Schizophr Bull. 1999;25(4):721-729.
27. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
28. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
29. Robinson DG, Woerner MG, Napolitano B, et al. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006;163(12):2096-2102.
30. Zipursky RB, Gu H, Green AI, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537-543.
31. Taylor M, Waight A, Leonard B. Advances in the understanding and challenges facing the management of first-episode schizophrenia. J Psychopharmacol. 2012; 26(suppl 5):3-5.
32. Merlo MC, Hofer H, Gekle W, et al. Risperidone, 2mg/day vs. 4mg/day, in first-episode, acutely psychotic patients: treatment efficacy and effects on fine motor functioning. J Clin Psychiatry. 2002;63(10):885-891.
33. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
34. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163(4):743-745.
35. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72(12):1691-1696.
36. Gardner KN, Bostwick JR. Antipsychotic treatment response in schizophrenia. Am J Health Sys Pharm. 2012;69(21):1872-1879.
37. Stauffer VL, Case M, Kinon BJ, et al. Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res. 2011;187(1-2):42-48.
38. Schennach-Wolff R, Seemüller FH, Mayr A, et al. An early improvement threshold to predict response and remission in first-episode schizophrenia. Br J Psychiatry. 2010;196(6):460-466.
39. Perkins DO, Gu H, Weiden PJ, et al; Comparison of Atypicals in First Episode study group. Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter study. J Clin Psychiatry. 2008;69(1):106-113.
40. Garner B, Berger GE, Nicolo JP, et al. Pituitary volume and early treatment response in drug-naïve first-episode psychosis patients. Schizophr Res. 2009;113(1):65-71.
41. Sapra M, Weiden PJ, Schooler NR, et al. Reasons for adherence and nonadherence: a pilot study comparing first-and multi-episode schizophrenia patients. Clin Schizophr Relat Psychoses. 2014;7(4):199-206.