User login
Prevention of Periprosthetic Joint Infections of the Hip and Knee
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
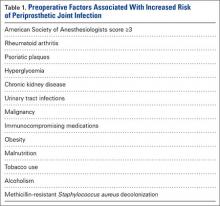
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
A Retrospective Analysis of Hemostatic Techniques in Primary Total Knee Arthroplasty: Traditional Electrocautery, Bipolar Sealer, and Argon Beam Coagulation
Total knee arthroplasty (TKA) is a reliable and successful treatment for end-stage degenerative joint disease of the knee. Given the reproducibility of its generally excellent outcomes, TKA is increasingly being performed.1 However, the potential complications of this procedure can be devastating.2-4 The arthroplasty literature has shed light on the detrimental effects of postoperative blood loss and anemia.5,6 In addition, the increase in transfusion burden among patients is not without risk.7 Given these concerns, surgeons have been tasked with determining the ideal methods for minimizing blood transfusions and postoperative hematomas and anemia. Several strategies have been described.8-11 Hemostasis can be achieved with use of intravenous medications, intra-articular agents, or electrocautery devices. Electrocautery technologies include traditional electrocautery (TE), saline-coupled bipolar sealer (BS), and argon beam coagulation (ABC). There is controversy as to whether outcomes are better with one hemostasis method over another and whether these methods are worth the additional cost.
In traditional (Bovie) electrocautery, a unipolar device delivers an electrical current to tissues through a pencil-like instrument. Intraoperative tissue temperatures can exceed 400°C.12 In BS, radiofrequency energy is delivered through a saline medium, which increases the contact area, acts as an electrode, and maintains a cooler environment during electrocautery. Proposed advantages are reduced tissue destruction and absence of smoke.12 There is evidence both for10,12-16 and against17-20 use of BS in total joint arthroplasty. ABC, a novel hemostasis method, has been studied in the context of orthopedics21,22 but not TKA specifically. ABC establishes a monopolar electric circuit between a handheld device and the target tissues by channeling electrons through ionized argon gas. Hemostasis is achieved through thermal coagulation. Tissue penetration can be adjusted by changing power, probe-to-target distance, and duration of use.23 We conducted a study to assess the efficacy of all 3 electrocautery methods during TKA. We hypothesized the 3 methods would be clinically equivalent with respect to estimated blood loss (EBL), 48-hour wound drainage, operative time, and change from preoperative hemoglobin (Hb) level.
Methods
We conducted a retrospective cohort study of consecutive primary TKAs performed by Dr. Levine between October 2010 and November 2011. Patients were identified by querying an internal database. Exclusion criteria were prior ipsilateral open knee procedure, prior fracture, nonuse of our standard hemostatic protocol, and either tourniquet time under 40 minutes or intraoperative documentation of tourniquet failure. As only 9 patients were initially identified for the TE cohort, the same database was used to add 32 patients treated between April 2009 and October 2009 (before our institution began using BS and ABC).
Clinical charts were reviewed, and baseline demographics (age, body mass index [BMI], preoperative Hb level) were abstracted, as were outcome metrics (EBL, 48-hour wound drainage, operative time, postoperative transfusions, adverse events (AEs) before discharge, and change in Hb level from before surgery to after surgery, in recovery room and on discharge). Statistical analyses were performed with JMP Version 10.0.0 (SAS Institute). Given the hypothesis that the 3 hemostasis methods would be clinically equivalent, 2 one-sided tests (TOSTs) of equivalence were performed with an α of 0.05. With TOST, the traditional null and alternative hypotheses are reversed; thus, P < .05 identifies statistical equivalence. The advantage of this study design is that equivalence can be identified, whereas traditional study designs can identify only a lack of statistical difference.24 We used our consensus opinions to set clinical insignificance thresholds for EBL (150 mL), wound drainage (150 mL), decrease from postoperative Hb level (1 g/dL), and operative time (10 minutes). Patients who received a blood transfusion were subsequently excluded from analysis in order to avoid skewing Hb-level depreciation calculations. Analysis of variance (ANOVA) and χ2 tests were used to compare preoperative variables, transfusion requirements, hospital length of stay, and AE rates by hemostasis type.
Cautery Technique
In all cases, TE was used for surgical dissection, which followed a standard midvastus approach. Then, for meniscal excision, the capsule and meniscal attachment sites were treated with TE, BS, or ABC. During cement hardening, an available supplemental cautery option was used to achieve hemostasis of the suprapatellar fat pad and visible meniscal attachment sites. All other aspects of the procedure and the postoperative protocols—including the anticoagulation and rapid rehabilitation (early ambulation and therapy) protocols—were similar for all patients. The standard anticoagulation protocol was to use low-molecular-weight heparin, unless contraindicated. Tranexamic acid was not used at our institution during the study period.
Results
For the study period, 280 cases (41 TE, 203 BS, 36 ABC) met the inclusion criteria. Of the 280 TKAs, 261 (93.21%) were performed for degenerative arthritis. There was no statistically significant difference among cohorts in indication (χ2 = 1.841, P = .398) or sex (χ2 = 1.176, P= .555).
Table 1 lists the cohorts’ baseline demographics (mean age, BMI, preoperative Hb level) and comparative ANOVA results. TOSTs of equivalence were performed to compare operative time, EBL, 48-hour wound drainage, and postoperative Hb-level depreciation among hemostasis types. Changes in Hb level were calculated for the immediate postoperative period and time of discharge (Table 2). ANOVA of hospital length of stay demonstrated no significant difference in means among groups (P = .09).
The cohorts were compared with respect to use of postoperative transfusions and incidence of postoperative AEs (Table 3). The TE cohort did not have any AEs. Of the 203 BS patients, 14 (7%) had 1 or more AEs, which included acute kidney injury (3 cases), electrolyte disturbance (3), urinary tract infection (2), oxygen desaturation (2), altered mental status (1), pneumonia (1), arrhythmia (1), congestive heart failure exacerbation (1), dehiscence (1), pulmonary embolism (2), and hypotension (1). Of the 36 ABC patients, 1 (3%) had arrhythmia, pneumonia, sepsis, and altered mental status.
Discussion
With the population aging, the demand for TKA is greater than ever.1 As surgical volume increases, the ability to minimize the rates of intraoperative bleeding, postoperative anemia, and transfusion is becoming increasingly important to patients and the healthcare system. There is no consensus as to which cautery method is ideal. Other investigators have identified differences in clinical outcomes between cautery systems, but reported results are largely conflicting.10,12-20 In addition, no one has studied the utility of ABC in TKA. In the present retrospective cohort analysis, we hypothesized that TE, BS, and ABC would be clinically equivalent in primary TKA with respect to EBL, 48-hour wound drainage, operative time, and change from preoperative Hb level.
The data on hemostatic technology in primary TKA are inconclusive. In an age- and sex-matched study comparing TE and BS in primary TKA, BS used with shed blood autotransfusion reduced homologous blood transfusions by a factor of 5.16 In addition, BS patients lost significantly less total visible blood (intraoperative EBL, postoperative drain output), and their magnitude of postoperative Hb-level depreciations at time of discharge was significantly lower. In a multicenter, prospective randomized trial comparing TE with BS, adjusted blood loss and need for autologous blood transfusions were lower in BS patients,10 though there was no significant difference in Knee Society Scale scores between the 2 treatment arms. However, analysis was potentially biased in that multiple authors had financial ties to Salient Surgical Technologies, the manufacturer of the BS device used in the study. Other prospective randomized trials of patients who had primary TKA with either TE or BS did not find any significant difference in postoperative Hb level, postoperative drainage, or transfusion requirements.19 ABC has been studied in the context of orthopedics but not joint arthroplasty specifically. This technology was anecdotally identified as a means of attaining hemostasis in foot and ankle surgery after failure of TE and other conventional means.22 ABC has also been identified as a successful adjuvant to curettage in the treatment of aneurysmal bone cysts.21 However, ABC has not been compared with TE or BS in the orthopedic literature.
In the present study, analysis of preoperative variables revealed a statistically but not clinically significant difference in BMI among cohorts. Mean (SD) BMI was 35.6 (6.5) for TE patients, 35.8 (9.7) for BS patients, and 40.9 (11.3) for ABC patients. (Previously, BMI did not correlate with intraoperative blood loss in TKA.25) Analysis also revealed a statistically significant but clinically insignificant and inconsequential difference in Hb level among cohorts. Mean (SD) preoperative Hb level was 13.5 (1.6) g/dL for TE patients, 12.8 (1.4) g/dL for BS patients, and 13.0 (1.6) g/dL for ABC patients. As decreases from preoperative baseline Hb levels were the intended focus of analysis—not absolute Hb levels—this finding does not refute postoperative analyses.
Our results suggest that, though TE may have relatively longer operative times in primary TKA, it is clinically equivalent to BS and ABC with respect to EBL and postoperative change in Hb levels. In addition, postoperative drainage was lower in TE than in BS and ABC, which were equivalent. No significant differences were found among hemostasis types with respect to postoperative transfusion requirements.
The prevalence distribution of predischarge AEs trended toward significance (χ2 = 5.957, P = .051), despite not meeting the predetermined α level. Rates of predischarge AEs were 0% (0/41) for TE patients, 7% (14/203) for BS patients, and 3% (1/36) for ABC patients. AEs included acute kidney injuries, electrolyte disturbances, urinary tract infections, oxygen desaturation, altered mental status, sepsis/infections, arrhythmias, congestive heart failure exacerbation, dehiscence, pulmonary embolism, and hypotension. Clearly, many of these AEs are not attributable to the hemostasis system used.
Limitations of this study include its retrospective design, documentation inadequate to account for drainage amount reinfused, and limited data on which clinical insignificance thresholds were based. In addition, reliance on historical data may have introduced bias into the analysis. The historical data used to increase the size of the TE cohort may reflect a period of relative inexperience and may have contributed to the longer operative times relative to those of the ABC cohort (Dr. Levine used ABC later in his career).
Traditional electrocautery remains a viable option in primary TKA. With its low cost and hemostasis equivalent to that of BS and ABC, TE deserves consideration equal to that given to these more modern hemostasis technologies. Cost per case is about $10 for TE versus $500 for BS and $110 for ABC.17 Soaring healthcare expenditures may warrant returning to TE or combining cautery techniques and other agents in primary TKA in order to reduce the number of transfusions and associated surgical costs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
2. Leijtens B, Kremers van de Hei K, Jansen J, Koëter S. High complication rate after total knee and hip replacement due to perioperative bridging of anticoagulant therapy based on the 2012 ACCP guideline. Arch Orthop Trauma Surg. 2014;134(9):1335-1341.
3. Park CH, Lee SH, Kang DG, Cho KY, Lee SH, Kim KI. Compartment syndrome following total knee arthroplasty: clinical results of late fasciotomy. Knee Surg Relat Res. 2014;26(3):177-181.
4. Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96-B(4):479-485.
5. Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1(8588):727-729.
6. Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348(9034):1055-1060.
7. Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14(6):671-676.
8. Kang DG, Khurana S, Baek JH, Park YS, Lee SH, Kim KI. Efficacy and safety using autotransfusion system with postoperative shed blood following total knee arthroplasty in haemophilia. Haemophilia. 2014;20(1):129-132.
9. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
10. Marulanda GA, Krebs VE, Bierbaum BE, et al. Hemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
11. Katkhouda N, Friedlander M, Darehzereshki A, et al. Argon beam coagulation versus fibrin sealant for hemostasis following liver resection: a randomized study in a porcine model. Hepatogastroenterology. 2013;60(125):1110-1116.
12. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131.
13. Morris MJ, Berend KR, Lombardi AV Jr. Hemostasis in anterior supine intermuscular total hip arthroplasty: pilot study comparing standard electrocautery and a bipolar sealer. Surg Technol Int. 2010;20:352-356.
14. Clement RC, Kamath AF, Derman PB, Garino JP, Lee GC. Bipolar sealing in revision total hip arthroplasty for infection: efficacy and cost analysis. J Arthroplasty. 2012;27(7):1376-1381.
15. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplasty. 2007;22(4 suppl 1):82-85.
16. Pfeiffer M, Bräutigam H, Draws D, Sigg A. A new bipolar blood sealing system embedded in perioperative strategies vs. a conventional regimen for total knee arthroplasty: results of a matched-pair study. Ger Med Sci. 2005;3:Doc10.
17. Morris MJ, Barrett M, Lombardi AV Jr, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
18. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
19. Plymale MF, Capogna BM, Lovy AJ, Adler ML, Hirsh DM, Kim SJ. Unipolar vs bipolar hemostasis in total knee arthroplasty: a prospective randomized trial. J Arthroplasty. 2012;27(6):1133-1137.e1.
20. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
21. Cummings JE, Smith RA, Heck RK Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231-237.
22. Adams ML, Steinberg JS. Argon beam coagulation in foot and ankle surgery. J Foot Ankle Surg. 2011;50(6):780-782.
23. Neumayer L, Vargo D. Principles of preoperative and operative surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:211-239.
24. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-196.
25. Hrnack SA, Skeen N, Xu T, Rosenstein AD. Correlation of body mass index and blood loss during total knee and total hip arthroplasty. Am J Orthop. 2012;41(10):467-471.
Total knee arthroplasty (TKA) is a reliable and successful treatment for end-stage degenerative joint disease of the knee. Given the reproducibility of its generally excellent outcomes, TKA is increasingly being performed.1 However, the potential complications of this procedure can be devastating.2-4 The arthroplasty literature has shed light on the detrimental effects of postoperative blood loss and anemia.5,6 In addition, the increase in transfusion burden among patients is not without risk.7 Given these concerns, surgeons have been tasked with determining the ideal methods for minimizing blood transfusions and postoperative hematomas and anemia. Several strategies have been described.8-11 Hemostasis can be achieved with use of intravenous medications, intra-articular agents, or electrocautery devices. Electrocautery technologies include traditional electrocautery (TE), saline-coupled bipolar sealer (BS), and argon beam coagulation (ABC). There is controversy as to whether outcomes are better with one hemostasis method over another and whether these methods are worth the additional cost.
In traditional (Bovie) electrocautery, a unipolar device delivers an electrical current to tissues through a pencil-like instrument. Intraoperative tissue temperatures can exceed 400°C.12 In BS, radiofrequency energy is delivered through a saline medium, which increases the contact area, acts as an electrode, and maintains a cooler environment during electrocautery. Proposed advantages are reduced tissue destruction and absence of smoke.12 There is evidence both for10,12-16 and against17-20 use of BS in total joint arthroplasty. ABC, a novel hemostasis method, has been studied in the context of orthopedics21,22 but not TKA specifically. ABC establishes a monopolar electric circuit between a handheld device and the target tissues by channeling electrons through ionized argon gas. Hemostasis is achieved through thermal coagulation. Tissue penetration can be adjusted by changing power, probe-to-target distance, and duration of use.23 We conducted a study to assess the efficacy of all 3 electrocautery methods during TKA. We hypothesized the 3 methods would be clinically equivalent with respect to estimated blood loss (EBL), 48-hour wound drainage, operative time, and change from preoperative hemoglobin (Hb) level.
Methods
We conducted a retrospective cohort study of consecutive primary TKAs performed by Dr. Levine between October 2010 and November 2011. Patients were identified by querying an internal database. Exclusion criteria were prior ipsilateral open knee procedure, prior fracture, nonuse of our standard hemostatic protocol, and either tourniquet time under 40 minutes or intraoperative documentation of tourniquet failure. As only 9 patients were initially identified for the TE cohort, the same database was used to add 32 patients treated between April 2009 and October 2009 (before our institution began using BS and ABC).
Clinical charts were reviewed, and baseline demographics (age, body mass index [BMI], preoperative Hb level) were abstracted, as were outcome metrics (EBL, 48-hour wound drainage, operative time, postoperative transfusions, adverse events (AEs) before discharge, and change in Hb level from before surgery to after surgery, in recovery room and on discharge). Statistical analyses were performed with JMP Version 10.0.0 (SAS Institute). Given the hypothesis that the 3 hemostasis methods would be clinically equivalent, 2 one-sided tests (TOSTs) of equivalence were performed with an α of 0.05. With TOST, the traditional null and alternative hypotheses are reversed; thus, P < .05 identifies statistical equivalence. The advantage of this study design is that equivalence can be identified, whereas traditional study designs can identify only a lack of statistical difference.24 We used our consensus opinions to set clinical insignificance thresholds for EBL (150 mL), wound drainage (150 mL), decrease from postoperative Hb level (1 g/dL), and operative time (10 minutes). Patients who received a blood transfusion were subsequently excluded from analysis in order to avoid skewing Hb-level depreciation calculations. Analysis of variance (ANOVA) and χ2 tests were used to compare preoperative variables, transfusion requirements, hospital length of stay, and AE rates by hemostasis type.
Cautery Technique
In all cases, TE was used for surgical dissection, which followed a standard midvastus approach. Then, for meniscal excision, the capsule and meniscal attachment sites were treated with TE, BS, or ABC. During cement hardening, an available supplemental cautery option was used to achieve hemostasis of the suprapatellar fat pad and visible meniscal attachment sites. All other aspects of the procedure and the postoperative protocols—including the anticoagulation and rapid rehabilitation (early ambulation and therapy) protocols—were similar for all patients. The standard anticoagulation protocol was to use low-molecular-weight heparin, unless contraindicated. Tranexamic acid was not used at our institution during the study period.
Results
For the study period, 280 cases (41 TE, 203 BS, 36 ABC) met the inclusion criteria. Of the 280 TKAs, 261 (93.21%) were performed for degenerative arthritis. There was no statistically significant difference among cohorts in indication (χ2 = 1.841, P = .398) or sex (χ2 = 1.176, P= .555).
Table 1 lists the cohorts’ baseline demographics (mean age, BMI, preoperative Hb level) and comparative ANOVA results. TOSTs of equivalence were performed to compare operative time, EBL, 48-hour wound drainage, and postoperative Hb-level depreciation among hemostasis types. Changes in Hb level were calculated for the immediate postoperative period and time of discharge (Table 2). ANOVA of hospital length of stay demonstrated no significant difference in means among groups (P = .09).
The cohorts were compared with respect to use of postoperative transfusions and incidence of postoperative AEs (Table 3). The TE cohort did not have any AEs. Of the 203 BS patients, 14 (7%) had 1 or more AEs, which included acute kidney injury (3 cases), electrolyte disturbance (3), urinary tract infection (2), oxygen desaturation (2), altered mental status (1), pneumonia (1), arrhythmia (1), congestive heart failure exacerbation (1), dehiscence (1), pulmonary embolism (2), and hypotension (1). Of the 36 ABC patients, 1 (3%) had arrhythmia, pneumonia, sepsis, and altered mental status.
Discussion
With the population aging, the demand for TKA is greater than ever.1 As surgical volume increases, the ability to minimize the rates of intraoperative bleeding, postoperative anemia, and transfusion is becoming increasingly important to patients and the healthcare system. There is no consensus as to which cautery method is ideal. Other investigators have identified differences in clinical outcomes between cautery systems, but reported results are largely conflicting.10,12-20 In addition, no one has studied the utility of ABC in TKA. In the present retrospective cohort analysis, we hypothesized that TE, BS, and ABC would be clinically equivalent in primary TKA with respect to EBL, 48-hour wound drainage, operative time, and change from preoperative Hb level.
The data on hemostatic technology in primary TKA are inconclusive. In an age- and sex-matched study comparing TE and BS in primary TKA, BS used with shed blood autotransfusion reduced homologous blood transfusions by a factor of 5.16 In addition, BS patients lost significantly less total visible blood (intraoperative EBL, postoperative drain output), and their magnitude of postoperative Hb-level depreciations at time of discharge was significantly lower. In a multicenter, prospective randomized trial comparing TE with BS, adjusted blood loss and need for autologous blood transfusions were lower in BS patients,10 though there was no significant difference in Knee Society Scale scores between the 2 treatment arms. However, analysis was potentially biased in that multiple authors had financial ties to Salient Surgical Technologies, the manufacturer of the BS device used in the study. Other prospective randomized trials of patients who had primary TKA with either TE or BS did not find any significant difference in postoperative Hb level, postoperative drainage, or transfusion requirements.19 ABC has been studied in the context of orthopedics but not joint arthroplasty specifically. This technology was anecdotally identified as a means of attaining hemostasis in foot and ankle surgery after failure of TE and other conventional means.22 ABC has also been identified as a successful adjuvant to curettage in the treatment of aneurysmal bone cysts.21 However, ABC has not been compared with TE or BS in the orthopedic literature.
In the present study, analysis of preoperative variables revealed a statistically but not clinically significant difference in BMI among cohorts. Mean (SD) BMI was 35.6 (6.5) for TE patients, 35.8 (9.7) for BS patients, and 40.9 (11.3) for ABC patients. (Previously, BMI did not correlate with intraoperative blood loss in TKA.25) Analysis also revealed a statistically significant but clinically insignificant and inconsequential difference in Hb level among cohorts. Mean (SD) preoperative Hb level was 13.5 (1.6) g/dL for TE patients, 12.8 (1.4) g/dL for BS patients, and 13.0 (1.6) g/dL for ABC patients. As decreases from preoperative baseline Hb levels were the intended focus of analysis—not absolute Hb levels—this finding does not refute postoperative analyses.
Our results suggest that, though TE may have relatively longer operative times in primary TKA, it is clinically equivalent to BS and ABC with respect to EBL and postoperative change in Hb levels. In addition, postoperative drainage was lower in TE than in BS and ABC, which were equivalent. No significant differences were found among hemostasis types with respect to postoperative transfusion requirements.
The prevalence distribution of predischarge AEs trended toward significance (χ2 = 5.957, P = .051), despite not meeting the predetermined α level. Rates of predischarge AEs were 0% (0/41) for TE patients, 7% (14/203) for BS patients, and 3% (1/36) for ABC patients. AEs included acute kidney injuries, electrolyte disturbances, urinary tract infections, oxygen desaturation, altered mental status, sepsis/infections, arrhythmias, congestive heart failure exacerbation, dehiscence, pulmonary embolism, and hypotension. Clearly, many of these AEs are not attributable to the hemostasis system used.
Limitations of this study include its retrospective design, documentation inadequate to account for drainage amount reinfused, and limited data on which clinical insignificance thresholds were based. In addition, reliance on historical data may have introduced bias into the analysis. The historical data used to increase the size of the TE cohort may reflect a period of relative inexperience and may have contributed to the longer operative times relative to those of the ABC cohort (Dr. Levine used ABC later in his career).
Traditional electrocautery remains a viable option in primary TKA. With its low cost and hemostasis equivalent to that of BS and ABC, TE deserves consideration equal to that given to these more modern hemostasis technologies. Cost per case is about $10 for TE versus $500 for BS and $110 for ABC.17 Soaring healthcare expenditures may warrant returning to TE or combining cautery techniques and other agents in primary TKA in order to reduce the number of transfusions and associated surgical costs.
Total knee arthroplasty (TKA) is a reliable and successful treatment for end-stage degenerative joint disease of the knee. Given the reproducibility of its generally excellent outcomes, TKA is increasingly being performed.1 However, the potential complications of this procedure can be devastating.2-4 The arthroplasty literature has shed light on the detrimental effects of postoperative blood loss and anemia.5,6 In addition, the increase in transfusion burden among patients is not without risk.7 Given these concerns, surgeons have been tasked with determining the ideal methods for minimizing blood transfusions and postoperative hematomas and anemia. Several strategies have been described.8-11 Hemostasis can be achieved with use of intravenous medications, intra-articular agents, or electrocautery devices. Electrocautery technologies include traditional electrocautery (TE), saline-coupled bipolar sealer (BS), and argon beam coagulation (ABC). There is controversy as to whether outcomes are better with one hemostasis method over another and whether these methods are worth the additional cost.
In traditional (Bovie) electrocautery, a unipolar device delivers an electrical current to tissues through a pencil-like instrument. Intraoperative tissue temperatures can exceed 400°C.12 In BS, radiofrequency energy is delivered through a saline medium, which increases the contact area, acts as an electrode, and maintains a cooler environment during electrocautery. Proposed advantages are reduced tissue destruction and absence of smoke.12 There is evidence both for10,12-16 and against17-20 use of BS in total joint arthroplasty. ABC, a novel hemostasis method, has been studied in the context of orthopedics21,22 but not TKA specifically. ABC establishes a monopolar electric circuit between a handheld device and the target tissues by channeling electrons through ionized argon gas. Hemostasis is achieved through thermal coagulation. Tissue penetration can be adjusted by changing power, probe-to-target distance, and duration of use.23 We conducted a study to assess the efficacy of all 3 electrocautery methods during TKA. We hypothesized the 3 methods would be clinically equivalent with respect to estimated blood loss (EBL), 48-hour wound drainage, operative time, and change from preoperative hemoglobin (Hb) level.
Methods
We conducted a retrospective cohort study of consecutive primary TKAs performed by Dr. Levine between October 2010 and November 2011. Patients were identified by querying an internal database. Exclusion criteria were prior ipsilateral open knee procedure, prior fracture, nonuse of our standard hemostatic protocol, and either tourniquet time under 40 minutes or intraoperative documentation of tourniquet failure. As only 9 patients were initially identified for the TE cohort, the same database was used to add 32 patients treated between April 2009 and October 2009 (before our institution began using BS and ABC).
Clinical charts were reviewed, and baseline demographics (age, body mass index [BMI], preoperative Hb level) were abstracted, as were outcome metrics (EBL, 48-hour wound drainage, operative time, postoperative transfusions, adverse events (AEs) before discharge, and change in Hb level from before surgery to after surgery, in recovery room and on discharge). Statistical analyses were performed with JMP Version 10.0.0 (SAS Institute). Given the hypothesis that the 3 hemostasis methods would be clinically equivalent, 2 one-sided tests (TOSTs) of equivalence were performed with an α of 0.05. With TOST, the traditional null and alternative hypotheses are reversed; thus, P < .05 identifies statistical equivalence. The advantage of this study design is that equivalence can be identified, whereas traditional study designs can identify only a lack of statistical difference.24 We used our consensus opinions to set clinical insignificance thresholds for EBL (150 mL), wound drainage (150 mL), decrease from postoperative Hb level (1 g/dL), and operative time (10 minutes). Patients who received a blood transfusion were subsequently excluded from analysis in order to avoid skewing Hb-level depreciation calculations. Analysis of variance (ANOVA) and χ2 tests were used to compare preoperative variables, transfusion requirements, hospital length of stay, and AE rates by hemostasis type.
Cautery Technique
In all cases, TE was used for surgical dissection, which followed a standard midvastus approach. Then, for meniscal excision, the capsule and meniscal attachment sites were treated with TE, BS, or ABC. During cement hardening, an available supplemental cautery option was used to achieve hemostasis of the suprapatellar fat pad and visible meniscal attachment sites. All other aspects of the procedure and the postoperative protocols—including the anticoagulation and rapid rehabilitation (early ambulation and therapy) protocols—were similar for all patients. The standard anticoagulation protocol was to use low-molecular-weight heparin, unless contraindicated. Tranexamic acid was not used at our institution during the study period.
Results
For the study period, 280 cases (41 TE, 203 BS, 36 ABC) met the inclusion criteria. Of the 280 TKAs, 261 (93.21%) were performed for degenerative arthritis. There was no statistically significant difference among cohorts in indication (χ2 = 1.841, P = .398) or sex (χ2 = 1.176, P= .555).
Table 1 lists the cohorts’ baseline demographics (mean age, BMI, preoperative Hb level) and comparative ANOVA results. TOSTs of equivalence were performed to compare operative time, EBL, 48-hour wound drainage, and postoperative Hb-level depreciation among hemostasis types. Changes in Hb level were calculated for the immediate postoperative period and time of discharge (Table 2). ANOVA of hospital length of stay demonstrated no significant difference in means among groups (P = .09).
The cohorts were compared with respect to use of postoperative transfusions and incidence of postoperative AEs (Table 3). The TE cohort did not have any AEs. Of the 203 BS patients, 14 (7%) had 1 or more AEs, which included acute kidney injury (3 cases), electrolyte disturbance (3), urinary tract infection (2), oxygen desaturation (2), altered mental status (1), pneumonia (1), arrhythmia (1), congestive heart failure exacerbation (1), dehiscence (1), pulmonary embolism (2), and hypotension (1). Of the 36 ABC patients, 1 (3%) had arrhythmia, pneumonia, sepsis, and altered mental status.
Discussion
With the population aging, the demand for TKA is greater than ever.1 As surgical volume increases, the ability to minimize the rates of intraoperative bleeding, postoperative anemia, and transfusion is becoming increasingly important to patients and the healthcare system. There is no consensus as to which cautery method is ideal. Other investigators have identified differences in clinical outcomes between cautery systems, but reported results are largely conflicting.10,12-20 In addition, no one has studied the utility of ABC in TKA. In the present retrospective cohort analysis, we hypothesized that TE, BS, and ABC would be clinically equivalent in primary TKA with respect to EBL, 48-hour wound drainage, operative time, and change from preoperative Hb level.
The data on hemostatic technology in primary TKA are inconclusive. In an age- and sex-matched study comparing TE and BS in primary TKA, BS used with shed blood autotransfusion reduced homologous blood transfusions by a factor of 5.16 In addition, BS patients lost significantly less total visible blood (intraoperative EBL, postoperative drain output), and their magnitude of postoperative Hb-level depreciations at time of discharge was significantly lower. In a multicenter, prospective randomized trial comparing TE with BS, adjusted blood loss and need for autologous blood transfusions were lower in BS patients,10 though there was no significant difference in Knee Society Scale scores between the 2 treatment arms. However, analysis was potentially biased in that multiple authors had financial ties to Salient Surgical Technologies, the manufacturer of the BS device used in the study. Other prospective randomized trials of patients who had primary TKA with either TE or BS did not find any significant difference in postoperative Hb level, postoperative drainage, or transfusion requirements.19 ABC has been studied in the context of orthopedics but not joint arthroplasty specifically. This technology was anecdotally identified as a means of attaining hemostasis in foot and ankle surgery after failure of TE and other conventional means.22 ABC has also been identified as a successful adjuvant to curettage in the treatment of aneurysmal bone cysts.21 However, ABC has not been compared with TE or BS in the orthopedic literature.
In the present study, analysis of preoperative variables revealed a statistically but not clinically significant difference in BMI among cohorts. Mean (SD) BMI was 35.6 (6.5) for TE patients, 35.8 (9.7) for BS patients, and 40.9 (11.3) for ABC patients. (Previously, BMI did not correlate with intraoperative blood loss in TKA.25) Analysis also revealed a statistically significant but clinically insignificant and inconsequential difference in Hb level among cohorts. Mean (SD) preoperative Hb level was 13.5 (1.6) g/dL for TE patients, 12.8 (1.4) g/dL for BS patients, and 13.0 (1.6) g/dL for ABC patients. As decreases from preoperative baseline Hb levels were the intended focus of analysis—not absolute Hb levels—this finding does not refute postoperative analyses.
Our results suggest that, though TE may have relatively longer operative times in primary TKA, it is clinically equivalent to BS and ABC with respect to EBL and postoperative change in Hb levels. In addition, postoperative drainage was lower in TE than in BS and ABC, which were equivalent. No significant differences were found among hemostasis types with respect to postoperative transfusion requirements.
The prevalence distribution of predischarge AEs trended toward significance (χ2 = 5.957, P = .051), despite not meeting the predetermined α level. Rates of predischarge AEs were 0% (0/41) for TE patients, 7% (14/203) for BS patients, and 3% (1/36) for ABC patients. AEs included acute kidney injuries, electrolyte disturbances, urinary tract infections, oxygen desaturation, altered mental status, sepsis/infections, arrhythmias, congestive heart failure exacerbation, dehiscence, pulmonary embolism, and hypotension. Clearly, many of these AEs are not attributable to the hemostasis system used.
Limitations of this study include its retrospective design, documentation inadequate to account for drainage amount reinfused, and limited data on which clinical insignificance thresholds were based. In addition, reliance on historical data may have introduced bias into the analysis. The historical data used to increase the size of the TE cohort may reflect a period of relative inexperience and may have contributed to the longer operative times relative to those of the ABC cohort (Dr. Levine used ABC later in his career).
Traditional electrocautery remains a viable option in primary TKA. With its low cost and hemostasis equivalent to that of BS and ABC, TE deserves consideration equal to that given to these more modern hemostasis technologies. Cost per case is about $10 for TE versus $500 for BS and $110 for ABC.17 Soaring healthcare expenditures may warrant returning to TE or combining cautery techniques and other agents in primary TKA in order to reduce the number of transfusions and associated surgical costs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
2. Leijtens B, Kremers van de Hei K, Jansen J, Koëter S. High complication rate after total knee and hip replacement due to perioperative bridging of anticoagulant therapy based on the 2012 ACCP guideline. Arch Orthop Trauma Surg. 2014;134(9):1335-1341.
3. Park CH, Lee SH, Kang DG, Cho KY, Lee SH, Kim KI. Compartment syndrome following total knee arthroplasty: clinical results of late fasciotomy. Knee Surg Relat Res. 2014;26(3):177-181.
4. Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96-B(4):479-485.
5. Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1(8588):727-729.
6. Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348(9034):1055-1060.
7. Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14(6):671-676.
8. Kang DG, Khurana S, Baek JH, Park YS, Lee SH, Kim KI. Efficacy and safety using autotransfusion system with postoperative shed blood following total knee arthroplasty in haemophilia. Haemophilia. 2014;20(1):129-132.
9. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
10. Marulanda GA, Krebs VE, Bierbaum BE, et al. Hemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
11. Katkhouda N, Friedlander M, Darehzereshki A, et al. Argon beam coagulation versus fibrin sealant for hemostasis following liver resection: a randomized study in a porcine model. Hepatogastroenterology. 2013;60(125):1110-1116.
12. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131.
13. Morris MJ, Berend KR, Lombardi AV Jr. Hemostasis in anterior supine intermuscular total hip arthroplasty: pilot study comparing standard electrocautery and a bipolar sealer. Surg Technol Int. 2010;20:352-356.
14. Clement RC, Kamath AF, Derman PB, Garino JP, Lee GC. Bipolar sealing in revision total hip arthroplasty for infection: efficacy and cost analysis. J Arthroplasty. 2012;27(7):1376-1381.
15. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplasty. 2007;22(4 suppl 1):82-85.
16. Pfeiffer M, Bräutigam H, Draws D, Sigg A. A new bipolar blood sealing system embedded in perioperative strategies vs. a conventional regimen for total knee arthroplasty: results of a matched-pair study. Ger Med Sci. 2005;3:Doc10.
17. Morris MJ, Barrett M, Lombardi AV Jr, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
18. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
19. Plymale MF, Capogna BM, Lovy AJ, Adler ML, Hirsh DM, Kim SJ. Unipolar vs bipolar hemostasis in total knee arthroplasty: a prospective randomized trial. J Arthroplasty. 2012;27(6):1133-1137.e1.
20. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
21. Cummings JE, Smith RA, Heck RK Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231-237.
22. Adams ML, Steinberg JS. Argon beam coagulation in foot and ankle surgery. J Foot Ankle Surg. 2011;50(6):780-782.
23. Neumayer L, Vargo D. Principles of preoperative and operative surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:211-239.
24. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-196.
25. Hrnack SA, Skeen N, Xu T, Rosenstein AD. Correlation of body mass index and blood loss during total knee and total hip arthroplasty. Am J Orthop. 2012;41(10):467-471.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
2. Leijtens B, Kremers van de Hei K, Jansen J, Koëter S. High complication rate after total knee and hip replacement due to perioperative bridging of anticoagulant therapy based on the 2012 ACCP guideline. Arch Orthop Trauma Surg. 2014;134(9):1335-1341.
3. Park CH, Lee SH, Kang DG, Cho KY, Lee SH, Kim KI. Compartment syndrome following total knee arthroplasty: clinical results of late fasciotomy. Knee Surg Relat Res. 2014;26(3):177-181.
4. Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96-B(4):479-485.
5. Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1(8588):727-729.
6. Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348(9034):1055-1060.
7. Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14(6):671-676.
8. Kang DG, Khurana S, Baek JH, Park YS, Lee SH, Kim KI. Efficacy and safety using autotransfusion system with postoperative shed blood following total knee arthroplasty in haemophilia. Haemophilia. 2014;20(1):129-132.
9. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
10. Marulanda GA, Krebs VE, Bierbaum BE, et al. Hemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
11. Katkhouda N, Friedlander M, Darehzereshki A, et al. Argon beam coagulation versus fibrin sealant for hemostasis following liver resection: a randomized study in a porcine model. Hepatogastroenterology. 2013;60(125):1110-1116.
12. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131.
13. Morris MJ, Berend KR, Lombardi AV Jr. Hemostasis in anterior supine intermuscular total hip arthroplasty: pilot study comparing standard electrocautery and a bipolar sealer. Surg Technol Int. 2010;20:352-356.
14. Clement RC, Kamath AF, Derman PB, Garino JP, Lee GC. Bipolar sealing in revision total hip arthroplasty for infection: efficacy and cost analysis. J Arthroplasty. 2012;27(7):1376-1381.
15. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplasty. 2007;22(4 suppl 1):82-85.
16. Pfeiffer M, Bräutigam H, Draws D, Sigg A. A new bipolar blood sealing system embedded in perioperative strategies vs. a conventional regimen for total knee arthroplasty: results of a matched-pair study. Ger Med Sci. 2005;3:Doc10.
17. Morris MJ, Barrett M, Lombardi AV Jr, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
18. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
19. Plymale MF, Capogna BM, Lovy AJ, Adler ML, Hirsh DM, Kim SJ. Unipolar vs bipolar hemostasis in total knee arthroplasty: a prospective randomized trial. J Arthroplasty. 2012;27(6):1133-1137.e1.
20. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
21. Cummings JE, Smith RA, Heck RK Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231-237.
22. Adams ML, Steinberg JS. Argon beam coagulation in foot and ankle surgery. J Foot Ankle Surg. 2011;50(6):780-782.
23. Neumayer L, Vargo D. Principles of preoperative and operative surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:211-239.
24. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-196.
25. Hrnack SA, Skeen N, Xu T, Rosenstein AD. Correlation of body mass index and blood loss during total knee and total hip arthroplasty. Am J Orthop. 2012;41(10):467-471.