User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
DMDD diagnosis will house only some children with rages
The DSM-5’s new diagnosis of disruptive mood dysregulation disorder was made to address the overdiagnosis of bipolar disorder by creating a diagnostic home for children with explosive rages, but only some of those children will qualify for the diagnosis, according to Dr. Gabrielle A. Carlson.
It "will relieve [only] some of the stress" on bipolar disorder, but "it won’t solve all the problems of false diagnosis," she said at a psychopharmacology update, sponsored by the American Academy of Child and Adolescent Psychiatry.
When the DSM-5 is published in May, disruptive mood dysregulation disorder (DMDD) probably will require at least 12 months of very severe outbursts from trivial triggers that happen more than three times per week and are developmentally inappropriate; persistently irritable mood between outbursts most of the day every day; outbursts or negative mood in at least two separate settings; and onset before the age of 10, among other criteria.
"And there are a number of really important exclusionary criteria. You don’t see [DMDD] if there’s any hint of mania, if there’s major depressive disorder," or if there is dysthymia, psychosis, posttraumatic stress disorder (PTSD), pervasive developmental disorders, or separation anxiety, said Dr. Carlson, director of child and adolescent psychiatry at the State University of New York at Stony Brook.
"Here’s the problem: Many children with explosive outbursts have depression and dysthymia, or PTSD," or some other exclusionary problem, or don’t meet all of the DMDD requirements. "If we follow the rules," only about 25% of inpatient explosive children and a fifth of children on the outpatient side will have DMDD, she said, based on analyses of her own inpatient and outpatient services.
Having a diagnostic home and treatment focus for explosive children is probably a positive development, Dr. Carlson said, because rages are probably the "single most vexing problem we have in child psychiatry. On the other hand, [DMDD] is just as likely to be abused as bipolar disorder, because it doesn’t capture the vast majority of kids with explosive outbursts. In fact, it may well mask conditions we already know about and can treat. If you go rushing right to ‘whoa, explosive outbursts – DMDD,’ you’re not going to look for depression, you are not going to look for ADHD, you’re not going to look for stuff you can treat," Dr. Carlson said.
Remember that "kids [with explosive outbursts] are doing the best they can," she said. "They aren’t getting up in the morning saying, ‘I’m really going to try to anger my parents and teachers.’ "
It’s important to first "maximize treatment of the base condition, whether it’s ADHD, depression, or some other problem." If outbursts remain a symptom, consider adding an atypical or conventional antipsychotic or a mood stabilizer, she said. It might take a while to see benefit, so "keep a record of the frequency, duration, and intensity of outbursts" to show parents that progress is being made.
"Medication is for executive functioning and mood regulation. For language and social" problems, it’s about psychoeducation – trying to understand the rage triggers and how not to feed them. Parent training can help. In addition, "if a parent has a psychiatric disorder, get it treated." Parents will not be able to help their child – and follow through on treatment recommendations – if they don’t have their own issues under control.
Dr. Carlson disclosed research funding from Pfizer, GlaxoSmithKline, Schering-Plough, Bristol-Myers Squibb, and Otsuka.
The DSM-5’s new diagnosis of disruptive mood dysregulation disorder was made to address the overdiagnosis of bipolar disorder by creating a diagnostic home for children with explosive rages, but only some of those children will qualify for the diagnosis, according to Dr. Gabrielle A. Carlson.
It "will relieve [only] some of the stress" on bipolar disorder, but "it won’t solve all the problems of false diagnosis," she said at a psychopharmacology update, sponsored by the American Academy of Child and Adolescent Psychiatry.
When the DSM-5 is published in May, disruptive mood dysregulation disorder (DMDD) probably will require at least 12 months of very severe outbursts from trivial triggers that happen more than three times per week and are developmentally inappropriate; persistently irritable mood between outbursts most of the day every day; outbursts or negative mood in at least two separate settings; and onset before the age of 10, among other criteria.
"And there are a number of really important exclusionary criteria. You don’t see [DMDD] if there’s any hint of mania, if there’s major depressive disorder," or if there is dysthymia, psychosis, posttraumatic stress disorder (PTSD), pervasive developmental disorders, or separation anxiety, said Dr. Carlson, director of child and adolescent psychiatry at the State University of New York at Stony Brook.
"Here’s the problem: Many children with explosive outbursts have depression and dysthymia, or PTSD," or some other exclusionary problem, or don’t meet all of the DMDD requirements. "If we follow the rules," only about 25% of inpatient explosive children and a fifth of children on the outpatient side will have DMDD, she said, based on analyses of her own inpatient and outpatient services.
Having a diagnostic home and treatment focus for explosive children is probably a positive development, Dr. Carlson said, because rages are probably the "single most vexing problem we have in child psychiatry. On the other hand, [DMDD] is just as likely to be abused as bipolar disorder, because it doesn’t capture the vast majority of kids with explosive outbursts. In fact, it may well mask conditions we already know about and can treat. If you go rushing right to ‘whoa, explosive outbursts – DMDD,’ you’re not going to look for depression, you are not going to look for ADHD, you’re not going to look for stuff you can treat," Dr. Carlson said.
Remember that "kids [with explosive outbursts] are doing the best they can," she said. "They aren’t getting up in the morning saying, ‘I’m really going to try to anger my parents and teachers.’ "
It’s important to first "maximize treatment of the base condition, whether it’s ADHD, depression, or some other problem." If outbursts remain a symptom, consider adding an atypical or conventional antipsychotic or a mood stabilizer, she said. It might take a while to see benefit, so "keep a record of the frequency, duration, and intensity of outbursts" to show parents that progress is being made.
"Medication is for executive functioning and mood regulation. For language and social" problems, it’s about psychoeducation – trying to understand the rage triggers and how not to feed them. Parent training can help. In addition, "if a parent has a psychiatric disorder, get it treated." Parents will not be able to help their child – and follow through on treatment recommendations – if they don’t have their own issues under control.
Dr. Carlson disclosed research funding from Pfizer, GlaxoSmithKline, Schering-Plough, Bristol-Myers Squibb, and Otsuka.
The DSM-5’s new diagnosis of disruptive mood dysregulation disorder was made to address the overdiagnosis of bipolar disorder by creating a diagnostic home for children with explosive rages, but only some of those children will qualify for the diagnosis, according to Dr. Gabrielle A. Carlson.
It "will relieve [only] some of the stress" on bipolar disorder, but "it won’t solve all the problems of false diagnosis," she said at a psychopharmacology update, sponsored by the American Academy of Child and Adolescent Psychiatry.
When the DSM-5 is published in May, disruptive mood dysregulation disorder (DMDD) probably will require at least 12 months of very severe outbursts from trivial triggers that happen more than three times per week and are developmentally inappropriate; persistently irritable mood between outbursts most of the day every day; outbursts or negative mood in at least two separate settings; and onset before the age of 10, among other criteria.
"And there are a number of really important exclusionary criteria. You don’t see [DMDD] if there’s any hint of mania, if there’s major depressive disorder," or if there is dysthymia, psychosis, posttraumatic stress disorder (PTSD), pervasive developmental disorders, or separation anxiety, said Dr. Carlson, director of child and adolescent psychiatry at the State University of New York at Stony Brook.
"Here’s the problem: Many children with explosive outbursts have depression and dysthymia, or PTSD," or some other exclusionary problem, or don’t meet all of the DMDD requirements. "If we follow the rules," only about 25% of inpatient explosive children and a fifth of children on the outpatient side will have DMDD, she said, based on analyses of her own inpatient and outpatient services.
Having a diagnostic home and treatment focus for explosive children is probably a positive development, Dr. Carlson said, because rages are probably the "single most vexing problem we have in child psychiatry. On the other hand, [DMDD] is just as likely to be abused as bipolar disorder, because it doesn’t capture the vast majority of kids with explosive outbursts. In fact, it may well mask conditions we already know about and can treat. If you go rushing right to ‘whoa, explosive outbursts – DMDD,’ you’re not going to look for depression, you are not going to look for ADHD, you’re not going to look for stuff you can treat," Dr. Carlson said.
Remember that "kids [with explosive outbursts] are doing the best they can," she said. "They aren’t getting up in the morning saying, ‘I’m really going to try to anger my parents and teachers.’ "
It’s important to first "maximize treatment of the base condition, whether it’s ADHD, depression, or some other problem." If outbursts remain a symptom, consider adding an atypical or conventional antipsychotic or a mood stabilizer, she said. It might take a while to see benefit, so "keep a record of the frequency, duration, and intensity of outbursts" to show parents that progress is being made.
"Medication is for executive functioning and mood regulation. For language and social" problems, it’s about psychoeducation – trying to understand the rage triggers and how not to feed them. Parent training can help. In addition, "if a parent has a psychiatric disorder, get it treated." Parents will not be able to help their child – and follow through on treatment recommendations – if they don’t have their own issues under control.
Dr. Carlson disclosed research funding from Pfizer, GlaxoSmithKline, Schering-Plough, Bristol-Myers Squibb, and Otsuka.
EXPERT ANALYSIS FROM A PSYCHOPHARMACOLOGY UPDATE, SPONSORED BY THE AMERICAN ACADEMY OF CHILD AND ADOLESCENT PSYCHIATRY
Psoriasis response at 2 months guides methotrexate decision
LAS VEGAS – If psoriasis patients don’t improve after 2 months of methotrexate therapy, the drug’s probably not going to work, according to Dr. Bruce Strober, of the University of Connecticut in Farmington.
But "don’t give up" if there’s even a slight improvement at that point. Up to 45% of patients achieve psoriasis area and severity index (PASI) scores of 75 on methotrexate, but "you really have to allow the drug 24 weeks to see that. If you see any hint of efficacy, keep going," he said at the SDEF Las Vegas Dermatology Seminar.
Dr. Strober shared insights about using methotrexate culled from more than a decade of experience. He starts most patients on 15 mg/week, but smaller people at perhaps 10-12.5 mg. He’ll dose down the elderly, as well, if he suspects renal insufficiency.
"I give it all in one dose, except if it’s at 17.5 mg or more per week, then I will divide the dose, either half in the morning, half at night," or a few days apart. "I never do the q [every] 12 hours x 3-day dosing. It’s based on no science, and you don’t have to make your patient’s life so hard," he said.
Not infrequently, Dr. Strober has patients self-inject a subcutaneous formulation in lieu of oral therapy. "It likely has better bioavailability and efficacy, [and] studies suggest avoiding first pass metabolism" is safer for the liver. Liver toxicity will be a problem in up to a quarter of patients, with the obese perhaps facing a higher risk. As with CBC and renal function, monthly liver testing is a must with methotrexate, at least for the first year.
"I don’t do liver biopsies anymore. I think it’s an extremely poor test laden with sampling error, and it has its own risks," he said. Instead, "I use liver function tests pretty exclusively" to look for marked, persistent LFT [liver function test] elevations above baseline, even if patients stay in the normal range. "Not uncommonly, a patient’s LFT could be 15 over 16 AST [aspartate aminotransferase] over ALT [alanine aminotransferase], and then 3 months into methotrexate, 45 over 42. It might be in the normal range, but I think that should give you pause. You caused a threefold increase in the liver tests," he said.
"Liver-induced changes are reversible if you react early enough by reducing the dose or just stopping the drug," Dr. Strober said.
Folate supplementation helps to protect the liver and reduce GI side effects. Dr. Strober often adds folinic acid, three 5 mg-doses per week, if GI side effects persist with folic acid alone. It’s as effective as folic acid, but "folic acid is cheaper. That’s why I start with it," he said.
The risk of pancytopenia is increased with poor renal function or use of sulfonamide-based antibiotics. "Monitor the CBC closely in the first few visits. You need folinic acid for rescue if it becomes a problem," he said.
Dr. Strober is on the advisory board of or a consultant to several pharmaceutical companies, including Janssen, Abbott, Pfizer, and Amgen. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – If psoriasis patients don’t improve after 2 months of methotrexate therapy, the drug’s probably not going to work, according to Dr. Bruce Strober, of the University of Connecticut in Farmington.
But "don’t give up" if there’s even a slight improvement at that point. Up to 45% of patients achieve psoriasis area and severity index (PASI) scores of 75 on methotrexate, but "you really have to allow the drug 24 weeks to see that. If you see any hint of efficacy, keep going," he said at the SDEF Las Vegas Dermatology Seminar.
Dr. Strober shared insights about using methotrexate culled from more than a decade of experience. He starts most patients on 15 mg/week, but smaller people at perhaps 10-12.5 mg. He’ll dose down the elderly, as well, if he suspects renal insufficiency.
"I give it all in one dose, except if it’s at 17.5 mg or more per week, then I will divide the dose, either half in the morning, half at night," or a few days apart. "I never do the q [every] 12 hours x 3-day dosing. It’s based on no science, and you don’t have to make your patient’s life so hard," he said.
Not infrequently, Dr. Strober has patients self-inject a subcutaneous formulation in lieu of oral therapy. "It likely has better bioavailability and efficacy, [and] studies suggest avoiding first pass metabolism" is safer for the liver. Liver toxicity will be a problem in up to a quarter of patients, with the obese perhaps facing a higher risk. As with CBC and renal function, monthly liver testing is a must with methotrexate, at least for the first year.
"I don’t do liver biopsies anymore. I think it’s an extremely poor test laden with sampling error, and it has its own risks," he said. Instead, "I use liver function tests pretty exclusively" to look for marked, persistent LFT [liver function test] elevations above baseline, even if patients stay in the normal range. "Not uncommonly, a patient’s LFT could be 15 over 16 AST [aspartate aminotransferase] over ALT [alanine aminotransferase], and then 3 months into methotrexate, 45 over 42. It might be in the normal range, but I think that should give you pause. You caused a threefold increase in the liver tests," he said.
"Liver-induced changes are reversible if you react early enough by reducing the dose or just stopping the drug," Dr. Strober said.
Folate supplementation helps to protect the liver and reduce GI side effects. Dr. Strober often adds folinic acid, three 5 mg-doses per week, if GI side effects persist with folic acid alone. It’s as effective as folic acid, but "folic acid is cheaper. That’s why I start with it," he said.
The risk of pancytopenia is increased with poor renal function or use of sulfonamide-based antibiotics. "Monitor the CBC closely in the first few visits. You need folinic acid for rescue if it becomes a problem," he said.
Dr. Strober is on the advisory board of or a consultant to several pharmaceutical companies, including Janssen, Abbott, Pfizer, and Amgen. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – If psoriasis patients don’t improve after 2 months of methotrexate therapy, the drug’s probably not going to work, according to Dr. Bruce Strober, of the University of Connecticut in Farmington.
But "don’t give up" if there’s even a slight improvement at that point. Up to 45% of patients achieve psoriasis area and severity index (PASI) scores of 75 on methotrexate, but "you really have to allow the drug 24 weeks to see that. If you see any hint of efficacy, keep going," he said at the SDEF Las Vegas Dermatology Seminar.
Dr. Strober shared insights about using methotrexate culled from more than a decade of experience. He starts most patients on 15 mg/week, but smaller people at perhaps 10-12.5 mg. He’ll dose down the elderly, as well, if he suspects renal insufficiency.
"I give it all in one dose, except if it’s at 17.5 mg or more per week, then I will divide the dose, either half in the morning, half at night," or a few days apart. "I never do the q [every] 12 hours x 3-day dosing. It’s based on no science, and you don’t have to make your patient’s life so hard," he said.
Not infrequently, Dr. Strober has patients self-inject a subcutaneous formulation in lieu of oral therapy. "It likely has better bioavailability and efficacy, [and] studies suggest avoiding first pass metabolism" is safer for the liver. Liver toxicity will be a problem in up to a quarter of patients, with the obese perhaps facing a higher risk. As with CBC and renal function, monthly liver testing is a must with methotrexate, at least for the first year.
"I don’t do liver biopsies anymore. I think it’s an extremely poor test laden with sampling error, and it has its own risks," he said. Instead, "I use liver function tests pretty exclusively" to look for marked, persistent LFT [liver function test] elevations above baseline, even if patients stay in the normal range. "Not uncommonly, a patient’s LFT could be 15 over 16 AST [aspartate aminotransferase] over ALT [alanine aminotransferase], and then 3 months into methotrexate, 45 over 42. It might be in the normal range, but I think that should give you pause. You caused a threefold increase in the liver tests," he said.
"Liver-induced changes are reversible if you react early enough by reducing the dose or just stopping the drug," Dr. Strober said.
Folate supplementation helps to protect the liver and reduce GI side effects. Dr. Strober often adds folinic acid, three 5 mg-doses per week, if GI side effects persist with folic acid alone. It’s as effective as folic acid, but "folic acid is cheaper. That’s why I start with it," he said.
The risk of pancytopenia is increased with poor renal function or use of sulfonamide-based antibiotics. "Monitor the CBC closely in the first few visits. You need folinic acid for rescue if it becomes a problem," he said.
Dr. Strober is on the advisory board of or a consultant to several pharmaceutical companies, including Janssen, Abbott, Pfizer, and Amgen. SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
ACOG calls for routine sexual and reproductive coercion screening
Obstetricians and gynecologists should routinely screen teenagers and women during annual, new-patient, and obstetric visits for sexual and reproductive coercion, according to a new committee opinion from the American College of Obstetricians and Gynecologists.
Sexual and reproductive coercion – a pattern of physical violence or psychologically coercive behaviors intended to control a woman’s sexual decision-making, contraceptive use, or pregnancy – is an "under-recognized" problem, according to Dr. Eve Espey, associate professor of obstetrics and gynecology at the University of New Mexico, Albuquerque, and one of the opinion’s authors (Obstet. Gynecol. 2013;121:411-5).
The coercion can play out as contraceptive sabotage, pressure to become pregnant unwillingly, or forcing a woman to continue or end a pregnancy against her will. "Some male partners go so far as to forcefully remove intrauterine devices and vaginal rings, poke holes in condoms, or destroy birth control pills. Repeated pressure to have sex, forcing sex without a condom, and intentionally exposing a partner to an STI are examples of sexual coercion," according to an ACOG written statement. Unintended pregnancies, sexually transmitted infections (STI), and HIV can be red flags.
Ob.gyns. "are in a unique position to address" the problem, the ACOG opinion states.
If women answer yes to screening questions such as, "Has your partner ever forced you to do something sexually that you did not want to do or refused your request to use condoms?" ob.gyns. should do the following:
• Offer hotline numbers and referrals to local domestic violence shelters and agencies, letting women use the office phone to make the calls.
• Offer long-acting methods of contraception less detectable to partners, such as IUDs, contraceptive implants, or injections.
• Trim IUD strings inside the cervical canal so they are undetectable and are harder for partners to remove, and send emergency contraceptive pills home in plain envelopes.
• Counsel these patients on harm-reduction strategies and safety planning.
The opinion by the ACOG Committee on Health Care for Underserved Women also suggests that ob.gyns. get more education about reproductive and sexual coercion and "include reproductive and sexual coercion and [intimate partner violence] as part of the differential diagnosis when patients are seen for STI testing, emergency contraception, or unintended pregnancies."
Other screening questions can be included: "Has your partner ever tried to get you pregnant when you did not want to be pregnant?" "Are you worried your partner will hurt you if you do not do what he wants with the pregnancy?" and "Does your partner support your decision about when or if you want to become pregnant?" the opinion noted. Additional questions can be found in "Addressing Intimate Partner Violence, Reproductive and Sexual Coercion."
"If a patient responds affirmatively ... the health care provider should validate her experience and commend her for discussing and evaluating her health and relationships. She should be reassured that the situation is not her fault, and further assessment of her safety should be elicited and discreet contraceptive options reviewed," according to the opinion.
"Anybody can be in this situation, but it disproportionally affects disempowered, underserved women," Dr. Espey said in an interview.
Sexual and reproductive coercion tends to travel with physical or sexual violence. In one study, 66% of battered adolescent mothers on public assistance reported birth control sabotage by their dating partner (School Nurse News 2006;23:38-40).
The opinion "is an expansion of ACOG’s long-standing work on intimate partner violence in general," said Dr. Espey, noting that the organization offers educational sessions on sexual and reproductive coercion at its annual meetings.
There is no specific ICD-9/ICD-10 code for screening for sexual and reproductive coercion. Because the physician initially will be performing a screening service, the contraceptive counseling codes would not be the most appropriate codes to report, according to ACOG’s coding department. Code V82.89 (Special screening for other conditions; Other specified conditions) would be a better bet for routine screening.
If coercion seems likely, counseling may be reported with code V65.49 (Other specified counseling) or code V62.89 (Other psychological or physical stress, not elsewhere classified; Other). Payer reimbursement policies will vary, according to ACOG.
Women wouldn’t have a copay for screening and counseling because sexual and reproductive coercion is a subset of intimate partner violence, which is a no-copay preventive care service under the Affordable Care Act.
The National Domestic Violence Hotline is 1-800-799-SAFE (7233); the Rape Abuse & Incest National Network Hotline is at 1-800-656-HOPE (4673). Several websites offer help and guidance as well, including Futures Without Violence, the National Coalition Against Domestic Violence, and the U.S. Department of Justice Office on Violence Against Women.
Dr. Espey said that she has no relevant disclosures.
Obstetricians and gynecologists should routinely screen teenagers and women during annual, new-patient, and obstetric visits for sexual and reproductive coercion, according to a new committee opinion from the American College of Obstetricians and Gynecologists.
Sexual and reproductive coercion – a pattern of physical violence or psychologically coercive behaviors intended to control a woman’s sexual decision-making, contraceptive use, or pregnancy – is an "under-recognized" problem, according to Dr. Eve Espey, associate professor of obstetrics and gynecology at the University of New Mexico, Albuquerque, and one of the opinion’s authors (Obstet. Gynecol. 2013;121:411-5).
The coercion can play out as contraceptive sabotage, pressure to become pregnant unwillingly, or forcing a woman to continue or end a pregnancy against her will. "Some male partners go so far as to forcefully remove intrauterine devices and vaginal rings, poke holes in condoms, or destroy birth control pills. Repeated pressure to have sex, forcing sex without a condom, and intentionally exposing a partner to an STI are examples of sexual coercion," according to an ACOG written statement. Unintended pregnancies, sexually transmitted infections (STI), and HIV can be red flags.
Ob.gyns. "are in a unique position to address" the problem, the ACOG opinion states.
If women answer yes to screening questions such as, "Has your partner ever forced you to do something sexually that you did not want to do or refused your request to use condoms?" ob.gyns. should do the following:
• Offer hotline numbers and referrals to local domestic violence shelters and agencies, letting women use the office phone to make the calls.
• Offer long-acting methods of contraception less detectable to partners, such as IUDs, contraceptive implants, or injections.
• Trim IUD strings inside the cervical canal so they are undetectable and are harder for partners to remove, and send emergency contraceptive pills home in plain envelopes.
• Counsel these patients on harm-reduction strategies and safety planning.
The opinion by the ACOG Committee on Health Care for Underserved Women also suggests that ob.gyns. get more education about reproductive and sexual coercion and "include reproductive and sexual coercion and [intimate partner violence] as part of the differential diagnosis when patients are seen for STI testing, emergency contraception, or unintended pregnancies."
Other screening questions can be included: "Has your partner ever tried to get you pregnant when you did not want to be pregnant?" "Are you worried your partner will hurt you if you do not do what he wants with the pregnancy?" and "Does your partner support your decision about when or if you want to become pregnant?" the opinion noted. Additional questions can be found in "Addressing Intimate Partner Violence, Reproductive and Sexual Coercion."
"If a patient responds affirmatively ... the health care provider should validate her experience and commend her for discussing and evaluating her health and relationships. She should be reassured that the situation is not her fault, and further assessment of her safety should be elicited and discreet contraceptive options reviewed," according to the opinion.
"Anybody can be in this situation, but it disproportionally affects disempowered, underserved women," Dr. Espey said in an interview.
Sexual and reproductive coercion tends to travel with physical or sexual violence. In one study, 66% of battered adolescent mothers on public assistance reported birth control sabotage by their dating partner (School Nurse News 2006;23:38-40).
The opinion "is an expansion of ACOG’s long-standing work on intimate partner violence in general," said Dr. Espey, noting that the organization offers educational sessions on sexual and reproductive coercion at its annual meetings.
There is no specific ICD-9/ICD-10 code for screening for sexual and reproductive coercion. Because the physician initially will be performing a screening service, the contraceptive counseling codes would not be the most appropriate codes to report, according to ACOG’s coding department. Code V82.89 (Special screening for other conditions; Other specified conditions) would be a better bet for routine screening.
If coercion seems likely, counseling may be reported with code V65.49 (Other specified counseling) or code V62.89 (Other psychological or physical stress, not elsewhere classified; Other). Payer reimbursement policies will vary, according to ACOG.
Women wouldn’t have a copay for screening and counseling because sexual and reproductive coercion is a subset of intimate partner violence, which is a no-copay preventive care service under the Affordable Care Act.
The National Domestic Violence Hotline is 1-800-799-SAFE (7233); the Rape Abuse & Incest National Network Hotline is at 1-800-656-HOPE (4673). Several websites offer help and guidance as well, including Futures Without Violence, the National Coalition Against Domestic Violence, and the U.S. Department of Justice Office on Violence Against Women.
Dr. Espey said that she has no relevant disclosures.
Obstetricians and gynecologists should routinely screen teenagers and women during annual, new-patient, and obstetric visits for sexual and reproductive coercion, according to a new committee opinion from the American College of Obstetricians and Gynecologists.
Sexual and reproductive coercion – a pattern of physical violence or psychologically coercive behaviors intended to control a woman’s sexual decision-making, contraceptive use, or pregnancy – is an "under-recognized" problem, according to Dr. Eve Espey, associate professor of obstetrics and gynecology at the University of New Mexico, Albuquerque, and one of the opinion’s authors (Obstet. Gynecol. 2013;121:411-5).
The coercion can play out as contraceptive sabotage, pressure to become pregnant unwillingly, or forcing a woman to continue or end a pregnancy against her will. "Some male partners go so far as to forcefully remove intrauterine devices and vaginal rings, poke holes in condoms, or destroy birth control pills. Repeated pressure to have sex, forcing sex without a condom, and intentionally exposing a partner to an STI are examples of sexual coercion," according to an ACOG written statement. Unintended pregnancies, sexually transmitted infections (STI), and HIV can be red flags.
Ob.gyns. "are in a unique position to address" the problem, the ACOG opinion states.
If women answer yes to screening questions such as, "Has your partner ever forced you to do something sexually that you did not want to do or refused your request to use condoms?" ob.gyns. should do the following:
• Offer hotline numbers and referrals to local domestic violence shelters and agencies, letting women use the office phone to make the calls.
• Offer long-acting methods of contraception less detectable to partners, such as IUDs, contraceptive implants, or injections.
• Trim IUD strings inside the cervical canal so they are undetectable and are harder for partners to remove, and send emergency contraceptive pills home in plain envelopes.
• Counsel these patients on harm-reduction strategies and safety planning.
The opinion by the ACOG Committee on Health Care for Underserved Women also suggests that ob.gyns. get more education about reproductive and sexual coercion and "include reproductive and sexual coercion and [intimate partner violence] as part of the differential diagnosis when patients are seen for STI testing, emergency contraception, or unintended pregnancies."
Other screening questions can be included: "Has your partner ever tried to get you pregnant when you did not want to be pregnant?" "Are you worried your partner will hurt you if you do not do what he wants with the pregnancy?" and "Does your partner support your decision about when or if you want to become pregnant?" the opinion noted. Additional questions can be found in "Addressing Intimate Partner Violence, Reproductive and Sexual Coercion."
"If a patient responds affirmatively ... the health care provider should validate her experience and commend her for discussing and evaluating her health and relationships. She should be reassured that the situation is not her fault, and further assessment of her safety should be elicited and discreet contraceptive options reviewed," according to the opinion.
"Anybody can be in this situation, but it disproportionally affects disempowered, underserved women," Dr. Espey said in an interview.
Sexual and reproductive coercion tends to travel with physical or sexual violence. In one study, 66% of battered adolescent mothers on public assistance reported birth control sabotage by their dating partner (School Nurse News 2006;23:38-40).
The opinion "is an expansion of ACOG’s long-standing work on intimate partner violence in general," said Dr. Espey, noting that the organization offers educational sessions on sexual and reproductive coercion at its annual meetings.
There is no specific ICD-9/ICD-10 code for screening for sexual and reproductive coercion. Because the physician initially will be performing a screening service, the contraceptive counseling codes would not be the most appropriate codes to report, according to ACOG’s coding department. Code V82.89 (Special screening for other conditions; Other specified conditions) would be a better bet for routine screening.
If coercion seems likely, counseling may be reported with code V65.49 (Other specified counseling) or code V62.89 (Other psychological or physical stress, not elsewhere classified; Other). Payer reimbursement policies will vary, according to ACOG.
Women wouldn’t have a copay for screening and counseling because sexual and reproductive coercion is a subset of intimate partner violence, which is a no-copay preventive care service under the Affordable Care Act.
The National Domestic Violence Hotline is 1-800-799-SAFE (7233); the Rape Abuse & Incest National Network Hotline is at 1-800-656-HOPE (4673). Several websites offer help and guidance as well, including Futures Without Violence, the National Coalition Against Domestic Violence, and the U.S. Department of Justice Office on Violence Against Women.
Dr. Espey said that she has no relevant disclosures.
FROM OBSTERICS AND GYNECOLOGY
Medicare hospitalizations, readmissions cut in CMS quality improvement project
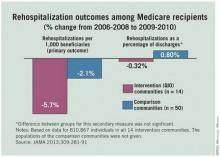
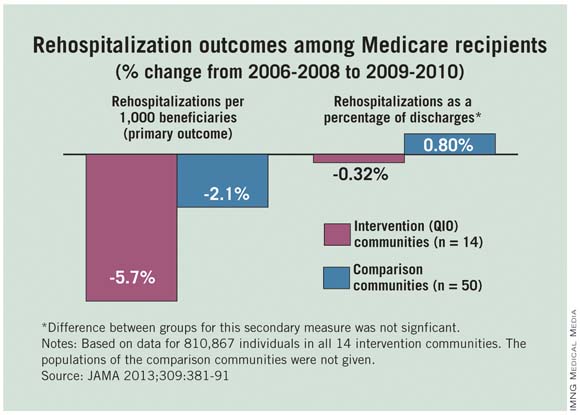
Hospitalizations and 30-day readmissions fell for Medicare patients in 14 communities when providers there worked with local quality improvement organizations to smooth and coordinate transitions between care settings, researchers from the Colorado Foundation for Medical Care and the Centers for Medicare and Medicaid Services reported Jan. 23 in JAMA.
Atlanta, Miami, Pittsburgh, and the other communities that participated in the 2-year CMS project saw mean reductions of 5.74% in hospitalizations and 5.70% in 30-day readmissions per 1,000 Medicare fee-for-service patients during 2009-2010. Fifty control communities saw smaller reductions: 3.17% in hospitalizations and 2.05% in readmissions (JAMA 2013;309:381-91).
"Our results provide evidence of a ... significant association between care transitions improvement interventions initiated by 14 [quality improvement organizations] and reductions in rehospitalizations and hospitalizations" as well as "evidence of a background national decline in hospitalizations and rehospitalizations for Medicare beneficiaries since 2008," the researchers said.
Each state typically has one nonprofit quality improvement organization (QIO) that contracts with CMS to improve beneficiary care.
The goal of the project was to "implement evidence-based improvements in care transitions by community organizing, technical assistance, and monitoring of participation, implementation, effectiveness, and adverse effects," according to the investigators.
But CMS left it up to each community to decide how best to do that. QIOs guided the efforts, working with hospitals, nursing facilities, home care agencies, hospices, social service agencies, clinicians, and others to improve a range of areas, including discharge standardization, medication reconciliation, chronic disease care planning, palliative care counseling, elder care services, and patient self-management.
Each community used three or more evidence-based approaches such as Interventions to Reduce Acute Care Transfers, Re-Engineered Discharges, Transitional Care Nursing, the Care Transitions Program, and the Best Practices Intervention Packages on transitional care.
With such a variety of approaches, the project "can only begin to guide predictions about effective combinations of context and interventions, optimal replication strategies, and effective plans for sustainability. Local community context was clearly relevant," the investigators said.
QIOs bid to be part of the efforts, and were selected by CMS based on application quality and their community’s contribution to geographic, market, and utilization diversity. The agency spent on average about $12 million annually to support their efforts. Medicare fee-for-service populations were quite varied among the 14 communities, ranging from about 22,000 to 90,000.
The investigators disclosed lecture fees, honoraria, or travel expenses from several sources, including the Alliance for Home Health Quality and Innovation, Lilly, and Insignia Health.
"Attempts to optimize care transitions will need to recognize that there is no single solution to address all the issues contributing to patient rehospitalization," wrote Dr. Mark V. Williams in an editorial (JAMA 2013;309:394-6).
"Efforts moving forward should involve implementation of broad patient-centered approaches that engage all members of a care team, especially front-line clinicians, and use proven quality improvement methods ... to identify helpful interventions. As achieved by the quality improvement organization initiative, engaging the community in caring for a patient can facilitate enhanced connections with better care coordination, and may help deliver higher-quality care more cost efficiently – optimizing value," he said.
Dr. Williams is chief of the division of medicine–hospital medicine at the Northwestern University, Chicago.He reported no relevant conflicts of interest.
"Attempts to optimize care transitions will need to recognize that there is no single solution to address all the issues contributing to patient rehospitalization," wrote Dr. Mark V. Williams in an editorial (JAMA 2013;309:394-6).
"Efforts moving forward should involve implementation of broad patient-centered approaches that engage all members of a care team, especially front-line clinicians, and use proven quality improvement methods ... to identify helpful interventions. As achieved by the quality improvement organization initiative, engaging the community in caring for a patient can facilitate enhanced connections with better care coordination, and may help deliver higher-quality care more cost efficiently – optimizing value," he said.
Dr. Williams is chief of the division of medicine–hospital medicine at the Northwestern University, Chicago.He reported no relevant conflicts of interest.
"Attempts to optimize care transitions will need to recognize that there is no single solution to address all the issues contributing to patient rehospitalization," wrote Dr. Mark V. Williams in an editorial (JAMA 2013;309:394-6).
"Efforts moving forward should involve implementation of broad patient-centered approaches that engage all members of a care team, especially front-line clinicians, and use proven quality improvement methods ... to identify helpful interventions. As achieved by the quality improvement organization initiative, engaging the community in caring for a patient can facilitate enhanced connections with better care coordination, and may help deliver higher-quality care more cost efficiently – optimizing value," he said.
Dr. Williams is chief of the division of medicine–hospital medicine at the Northwestern University, Chicago.He reported no relevant conflicts of interest.
Hospitalizations and 30-day readmissions fell for Medicare patients in 14 communities when providers there worked with local quality improvement organizations to smooth and coordinate transitions between care settings, researchers from the Colorado Foundation for Medical Care and the Centers for Medicare and Medicaid Services reported Jan. 23 in JAMA.
Atlanta, Miami, Pittsburgh, and the other communities that participated in the 2-year CMS project saw mean reductions of 5.74% in hospitalizations and 5.70% in 30-day readmissions per 1,000 Medicare fee-for-service patients during 2009-2010. Fifty control communities saw smaller reductions: 3.17% in hospitalizations and 2.05% in readmissions (JAMA 2013;309:381-91).
"Our results provide evidence of a ... significant association between care transitions improvement interventions initiated by 14 [quality improvement organizations] and reductions in rehospitalizations and hospitalizations" as well as "evidence of a background national decline in hospitalizations and rehospitalizations for Medicare beneficiaries since 2008," the researchers said.
Each state typically has one nonprofit quality improvement organization (QIO) that contracts with CMS to improve beneficiary care.
The goal of the project was to "implement evidence-based improvements in care transitions by community organizing, technical assistance, and monitoring of participation, implementation, effectiveness, and adverse effects," according to the investigators.
But CMS left it up to each community to decide how best to do that. QIOs guided the efforts, working with hospitals, nursing facilities, home care agencies, hospices, social service agencies, clinicians, and others to improve a range of areas, including discharge standardization, medication reconciliation, chronic disease care planning, palliative care counseling, elder care services, and patient self-management.
Each community used three or more evidence-based approaches such as Interventions to Reduce Acute Care Transfers, Re-Engineered Discharges, Transitional Care Nursing, the Care Transitions Program, and the Best Practices Intervention Packages on transitional care.
With such a variety of approaches, the project "can only begin to guide predictions about effective combinations of context and interventions, optimal replication strategies, and effective plans for sustainability. Local community context was clearly relevant," the investigators said.
QIOs bid to be part of the efforts, and were selected by CMS based on application quality and their community’s contribution to geographic, market, and utilization diversity. The agency spent on average about $12 million annually to support their efforts. Medicare fee-for-service populations were quite varied among the 14 communities, ranging from about 22,000 to 90,000.
The investigators disclosed lecture fees, honoraria, or travel expenses from several sources, including the Alliance for Home Health Quality and Innovation, Lilly, and Insignia Health.
Hospitalizations and 30-day readmissions fell for Medicare patients in 14 communities when providers there worked with local quality improvement organizations to smooth and coordinate transitions between care settings, researchers from the Colorado Foundation for Medical Care and the Centers for Medicare and Medicaid Services reported Jan. 23 in JAMA.
Atlanta, Miami, Pittsburgh, and the other communities that participated in the 2-year CMS project saw mean reductions of 5.74% in hospitalizations and 5.70% in 30-day readmissions per 1,000 Medicare fee-for-service patients during 2009-2010. Fifty control communities saw smaller reductions: 3.17% in hospitalizations and 2.05% in readmissions (JAMA 2013;309:381-91).
"Our results provide evidence of a ... significant association between care transitions improvement interventions initiated by 14 [quality improvement organizations] and reductions in rehospitalizations and hospitalizations" as well as "evidence of a background national decline in hospitalizations and rehospitalizations for Medicare beneficiaries since 2008," the researchers said.
Each state typically has one nonprofit quality improvement organization (QIO) that contracts with CMS to improve beneficiary care.
The goal of the project was to "implement evidence-based improvements in care transitions by community organizing, technical assistance, and monitoring of participation, implementation, effectiveness, and adverse effects," according to the investigators.
But CMS left it up to each community to decide how best to do that. QIOs guided the efforts, working with hospitals, nursing facilities, home care agencies, hospices, social service agencies, clinicians, and others to improve a range of areas, including discharge standardization, medication reconciliation, chronic disease care planning, palliative care counseling, elder care services, and patient self-management.
Each community used three or more evidence-based approaches such as Interventions to Reduce Acute Care Transfers, Re-Engineered Discharges, Transitional Care Nursing, the Care Transitions Program, and the Best Practices Intervention Packages on transitional care.
With such a variety of approaches, the project "can only begin to guide predictions about effective combinations of context and interventions, optimal replication strategies, and effective plans for sustainability. Local community context was clearly relevant," the investigators said.
QIOs bid to be part of the efforts, and were selected by CMS based on application quality and their community’s contribution to geographic, market, and utilization diversity. The agency spent on average about $12 million annually to support their efforts. Medicare fee-for-service populations were quite varied among the 14 communities, ranging from about 22,000 to 90,000.
The investigators disclosed lecture fees, honoraria, or travel expenses from several sources, including the Alliance for Home Health Quality and Innovation, Lilly, and Insignia Health.
FROM JAMA
Major finding: Communities that worked with local QIOs to improve care transitions saw mean reductions of 5.74% in hospitalizations and 5.70% in 30-day readmissions per 1,000 Medicare fee-for-service patients during 2009-2010.
Data source: Two-year CMS quality improvement project in 14 communities
Disclosures: CMS funded the project. The investigators disclosed lecture fees, honoraria, or travel expenses from several sources, including the Alliance for Home Health Quality and Innovation, Lilly, and Insignia Health.
Laser pill has potential benefits over upper GI endoscopy
A small, swallowed, laser imaging capsule provides full-thickness imaging of the upper gastrointestinal tract without biopsy, and is quicker and less invasive than traditional endoscopy, according to the Harvard University researchers who are developing it.
About the size of a large multivitamin pill, the transparent capsule generates a near-infrared beam that spins rapidly about its circumference during transit. Changes in the reflected light allow cross-sectional imaging of the esophagus in a few minutes. Sequential cross-sections can be compiled into three-dimensional models of the entire lumen (Nat. Med. 2013 Jan. 13 [doi: 10.1038/nm.3052]).
"This system gives us a convenient way to screen for Barrett’s [esophagus] that doesn’t require patient sedation, a specialized setting and equipment, or a physician who has been trained in endoscopy. By showing the three-dimensional, microscopic structure of the esophageal lining, it reveals much more detail than can be seen with even high-resolution endoscopy. The images produced have been some of the best we have seen of the esophagus," investigator Dr. Guillermo Tearney, a Harvard Medical School pathology professor and the associate director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said in a statement.
The capsule is on a tether, which carries its fiber optic line and laser driveshaft and helps with positioning. The capsule is pulled up and out after use, and disinfected for the next patient.
In early testing, 15 cm of esophagus in seven healthy and six Barrett’s esophagus patients was imaged in a mean of 58 seconds; it took about 6 minutes to make two down- and two up-transits. The technique, dubbed tethered capsule endomicroscopy, clearly distinguished the cellular abnormalities of Barrett’s. Standard upper GI endoscopy takes about 90 minutes.
"We originally were concerned that we might miss a lot of data because of the small size of the capsule, but we were surprised to find that, once the pill has been swallowed, it is firmly grasped by the esophagus, allowing complete microscopic imaging of the entire wall," Dr. Tearney said.
There were no complications, and 12 of the 13 subjects said they preferred the capsule to previous endoscopies.
"Because the tethered endomicroscopy pill traverses the gastrointestinal tract without visual guidance, the training required to conduct the procedure is minimal. This fact, combined with the brevity and ease with which the procedure is performed, will enable internal microscopic imaging in almost any health care setting, including in the office of the primary care physician," Dr. Tearney and his colleagues wrote in their paper.
The research was supported by grants from the National Institutes of Health. The researchers said they had no disclosures.
A small, swallowed, laser imaging capsule provides full-thickness imaging of the upper gastrointestinal tract without biopsy, and is quicker and less invasive than traditional endoscopy, according to the Harvard University researchers who are developing it.
About the size of a large multivitamin pill, the transparent capsule generates a near-infrared beam that spins rapidly about its circumference during transit. Changes in the reflected light allow cross-sectional imaging of the esophagus in a few minutes. Sequential cross-sections can be compiled into three-dimensional models of the entire lumen (Nat. Med. 2013 Jan. 13 [doi: 10.1038/nm.3052]).
"This system gives us a convenient way to screen for Barrett’s [esophagus] that doesn’t require patient sedation, a specialized setting and equipment, or a physician who has been trained in endoscopy. By showing the three-dimensional, microscopic structure of the esophageal lining, it reveals much more detail than can be seen with even high-resolution endoscopy. The images produced have been some of the best we have seen of the esophagus," investigator Dr. Guillermo Tearney, a Harvard Medical School pathology professor and the associate director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said in a statement.
The capsule is on a tether, which carries its fiber optic line and laser driveshaft and helps with positioning. The capsule is pulled up and out after use, and disinfected for the next patient.
In early testing, 15 cm of esophagus in seven healthy and six Barrett’s esophagus patients was imaged in a mean of 58 seconds; it took about 6 minutes to make two down- and two up-transits. The technique, dubbed tethered capsule endomicroscopy, clearly distinguished the cellular abnormalities of Barrett’s. Standard upper GI endoscopy takes about 90 minutes.
"We originally were concerned that we might miss a lot of data because of the small size of the capsule, but we were surprised to find that, once the pill has been swallowed, it is firmly grasped by the esophagus, allowing complete microscopic imaging of the entire wall," Dr. Tearney said.
There were no complications, and 12 of the 13 subjects said they preferred the capsule to previous endoscopies.
"Because the tethered endomicroscopy pill traverses the gastrointestinal tract without visual guidance, the training required to conduct the procedure is minimal. This fact, combined with the brevity and ease with which the procedure is performed, will enable internal microscopic imaging in almost any health care setting, including in the office of the primary care physician," Dr. Tearney and his colleagues wrote in their paper.
The research was supported by grants from the National Institutes of Health. The researchers said they had no disclosures.
A small, swallowed, laser imaging capsule provides full-thickness imaging of the upper gastrointestinal tract without biopsy, and is quicker and less invasive than traditional endoscopy, according to the Harvard University researchers who are developing it.
About the size of a large multivitamin pill, the transparent capsule generates a near-infrared beam that spins rapidly about its circumference during transit. Changes in the reflected light allow cross-sectional imaging of the esophagus in a few minutes. Sequential cross-sections can be compiled into three-dimensional models of the entire lumen (Nat. Med. 2013 Jan. 13 [doi: 10.1038/nm.3052]).
"This system gives us a convenient way to screen for Barrett’s [esophagus] that doesn’t require patient sedation, a specialized setting and equipment, or a physician who has been trained in endoscopy. By showing the three-dimensional, microscopic structure of the esophageal lining, it reveals much more detail than can be seen with even high-resolution endoscopy. The images produced have been some of the best we have seen of the esophagus," investigator Dr. Guillermo Tearney, a Harvard Medical School pathology professor and the associate director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said in a statement.
The capsule is on a tether, which carries its fiber optic line and laser driveshaft and helps with positioning. The capsule is pulled up and out after use, and disinfected for the next patient.
In early testing, 15 cm of esophagus in seven healthy and six Barrett’s esophagus patients was imaged in a mean of 58 seconds; it took about 6 minutes to make two down- and two up-transits. The technique, dubbed tethered capsule endomicroscopy, clearly distinguished the cellular abnormalities of Barrett’s. Standard upper GI endoscopy takes about 90 minutes.
"We originally were concerned that we might miss a lot of data because of the small size of the capsule, but we were surprised to find that, once the pill has been swallowed, it is firmly grasped by the esophagus, allowing complete microscopic imaging of the entire wall," Dr. Tearney said.
There were no complications, and 12 of the 13 subjects said they preferred the capsule to previous endoscopies.
"Because the tethered endomicroscopy pill traverses the gastrointestinal tract without visual guidance, the training required to conduct the procedure is minimal. This fact, combined with the brevity and ease with which the procedure is performed, will enable internal microscopic imaging in almost any health care setting, including in the office of the primary care physician," Dr. Tearney and his colleagues wrote in their paper.
The research was supported by grants from the National Institutes of Health. The researchers said they had no disclosures.
FROM NATURE MEDICINE
Major Finding: In early testing, 15 cm of esophagus was imaged in a mean of 58 seconds and clearly distinguished the cellular abnormalities of Barrett’s esophagus; it took about 6 minutes to make two down- and two up-transits.
Data Source: A pilot study to image the esophagus in seven healthy patients and six with Barrett’s esophagus.
Disclosures: The research was supported by grants from the National Institutes of Health. The investigators said they had no disclosures.
FDA approves first flu shot made without eggs
A new trivalent influenza vaccine approved by the Food and Drug Administration Jan. 16 is the first to be made without eggs.
Instead, Flublok’s manufacturer, Protein Sciences Corp. of Meriden, Conn., uses the expression system of baculovirus (an insect virus) and recombinant DNA.
"The new technology offers the potential for faster start-up of the vaccine manufacturing process in the event of a pandemic, because it is not dependent on an egg supply or on availability of the influenza virus," Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement.
The vaccine is indicated for the prevention of seasonal influenza in people aged 18-49 years. It proved 44.6% effective against all circulating influenza strains, not just the three it contains, when tested in about 2,300 people against placebo shots in about the same number.
Side effects were similar to those for egg-based flu vaccines and included injection-site pain, headache, fatigue, and muscle aches. Flublok has a shelf life of 16 weeks, the agency noted, and its manufacturing technique is already in use for other approved vaccines.
Flublok contains full-length, recombinant hemagglutinin proteins against H1N1 and H3N2 influenza A strains and one influenza B strain.
A new trivalent influenza vaccine approved by the Food and Drug Administration Jan. 16 is the first to be made without eggs.
Instead, Flublok’s manufacturer, Protein Sciences Corp. of Meriden, Conn., uses the expression system of baculovirus (an insect virus) and recombinant DNA.
"The new technology offers the potential for faster start-up of the vaccine manufacturing process in the event of a pandemic, because it is not dependent on an egg supply or on availability of the influenza virus," Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement.
The vaccine is indicated for the prevention of seasonal influenza in people aged 18-49 years. It proved 44.6% effective against all circulating influenza strains, not just the three it contains, when tested in about 2,300 people against placebo shots in about the same number.
Side effects were similar to those for egg-based flu vaccines and included injection-site pain, headache, fatigue, and muscle aches. Flublok has a shelf life of 16 weeks, the agency noted, and its manufacturing technique is already in use for other approved vaccines.
Flublok contains full-length, recombinant hemagglutinin proteins against H1N1 and H3N2 influenza A strains and one influenza B strain.
A new trivalent influenza vaccine approved by the Food and Drug Administration Jan. 16 is the first to be made without eggs.
Instead, Flublok’s manufacturer, Protein Sciences Corp. of Meriden, Conn., uses the expression system of baculovirus (an insect virus) and recombinant DNA.
"The new technology offers the potential for faster start-up of the vaccine manufacturing process in the event of a pandemic, because it is not dependent on an egg supply or on availability of the influenza virus," Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement.
The vaccine is indicated for the prevention of seasonal influenza in people aged 18-49 years. It proved 44.6% effective against all circulating influenza strains, not just the three it contains, when tested in about 2,300 people against placebo shots in about the same number.
Side effects were similar to those for egg-based flu vaccines and included injection-site pain, headache, fatigue, and muscle aches. Flublok has a shelf life of 16 weeks, the agency noted, and its manufacturing technique is already in use for other approved vaccines.
Flublok contains full-length, recombinant hemagglutinin proteins against H1N1 and H3N2 influenza A strains and one influenza B strain.
Primary care docs unsure when to refer for pediatric epilepsy surgery
SAN DIEGO – A majority of pediatricians and family practitioners in a central Texas survey were unsure when pediatric epilepsy should be considered refractory and children should be referred for possible surgery.
Researchers from the epilepsy program at the Dell Children’s Medical Center in Austin surveyed 84 pediatricians, 44 family practitioners, and 18 neurologists or neurosurgeons to gauge their understanding of epilepsy management. Respondents treated a median of 2.5 pediatric epilepsy patients per month (range, 0-200), and about half had been in practice for less than 15 years.
The neurologists and neurosurgeons scored well, but among primary care providers, "there was a clear lack of understanding of when you would consider someone refractory and when you would want to refer someone to a specialist or work them up for epilepsy surgery," said investigator Collin Hovinga, Pharm.D., the epilepsy program’s director of neuropharmacology.
Only 39% (57) of respondents correctly agreed that children should be considered to have refractory epilepsy after failing two or three antiseizure medications; the rest were unsure or disagreed with the statement. More than a third (52) were unsure if failing six medications constituted refractory epilepsy.
About 70% (104) did not think or were unsure whether surgery is an effective option for partial epilepsy; 86% (126) were unsure or doubtful if it would help in generalized epilepsy. Only about half of respondents agreed that surgery should be considered for children who have failed 3 years of antiseizure medications.
Surgery for partial or generalized epilepsy "probably has the biggest impact on whether you can cure somebody or hugely decrease their seizure load. If two-thirds of respondents are unsure of that, it tells me we have completely failed in communicating what we do to professionals who refer to us," said coinvestigator Dr. Freedom F. Perkins Jr., a pediatric epileptologist at Dell.
Education is the answer. "It is incumbent on us to reach out to our referral sources" – pediatricians and family practitioners – "to make sure they understand these things." Webinars could help, but might only draw physicians already interested in and knowledgeable about epilepsy. "The old-fashioned shoe leather" approach might be better; "you get in your car, you meet people, and you talk," Dr. Perkins said at the annual meeting of the American Epilepsy Society.
The researchers plan to do just that with primary care physicians around Austin, and then repeat the survey in perhaps a year to see if the efforts improved understanding and referral patterns.
Dr. Hovinga and Dr. Perkins said they had no relevant financial disclosures.
SAN DIEGO – A majority of pediatricians and family practitioners in a central Texas survey were unsure when pediatric epilepsy should be considered refractory and children should be referred for possible surgery.
Researchers from the epilepsy program at the Dell Children’s Medical Center in Austin surveyed 84 pediatricians, 44 family practitioners, and 18 neurologists or neurosurgeons to gauge their understanding of epilepsy management. Respondents treated a median of 2.5 pediatric epilepsy patients per month (range, 0-200), and about half had been in practice for less than 15 years.
The neurologists and neurosurgeons scored well, but among primary care providers, "there was a clear lack of understanding of when you would consider someone refractory and when you would want to refer someone to a specialist or work them up for epilepsy surgery," said investigator Collin Hovinga, Pharm.D., the epilepsy program’s director of neuropharmacology.
Only 39% (57) of respondents correctly agreed that children should be considered to have refractory epilepsy after failing two or three antiseizure medications; the rest were unsure or disagreed with the statement. More than a third (52) were unsure if failing six medications constituted refractory epilepsy.
About 70% (104) did not think or were unsure whether surgery is an effective option for partial epilepsy; 86% (126) were unsure or doubtful if it would help in generalized epilepsy. Only about half of respondents agreed that surgery should be considered for children who have failed 3 years of antiseizure medications.
Surgery for partial or generalized epilepsy "probably has the biggest impact on whether you can cure somebody or hugely decrease their seizure load. If two-thirds of respondents are unsure of that, it tells me we have completely failed in communicating what we do to professionals who refer to us," said coinvestigator Dr. Freedom F. Perkins Jr., a pediatric epileptologist at Dell.
Education is the answer. "It is incumbent on us to reach out to our referral sources" – pediatricians and family practitioners – "to make sure they understand these things." Webinars could help, but might only draw physicians already interested in and knowledgeable about epilepsy. "The old-fashioned shoe leather" approach might be better; "you get in your car, you meet people, and you talk," Dr. Perkins said at the annual meeting of the American Epilepsy Society.
The researchers plan to do just that with primary care physicians around Austin, and then repeat the survey in perhaps a year to see if the efforts improved understanding and referral patterns.
Dr. Hovinga and Dr. Perkins said they had no relevant financial disclosures.
SAN DIEGO – A majority of pediatricians and family practitioners in a central Texas survey were unsure when pediatric epilepsy should be considered refractory and children should be referred for possible surgery.
Researchers from the epilepsy program at the Dell Children’s Medical Center in Austin surveyed 84 pediatricians, 44 family practitioners, and 18 neurologists or neurosurgeons to gauge their understanding of epilepsy management. Respondents treated a median of 2.5 pediatric epilepsy patients per month (range, 0-200), and about half had been in practice for less than 15 years.
The neurologists and neurosurgeons scored well, but among primary care providers, "there was a clear lack of understanding of when you would consider someone refractory and when you would want to refer someone to a specialist or work them up for epilepsy surgery," said investigator Collin Hovinga, Pharm.D., the epilepsy program’s director of neuropharmacology.
Only 39% (57) of respondents correctly agreed that children should be considered to have refractory epilepsy after failing two or three antiseizure medications; the rest were unsure or disagreed with the statement. More than a third (52) were unsure if failing six medications constituted refractory epilepsy.
About 70% (104) did not think or were unsure whether surgery is an effective option for partial epilepsy; 86% (126) were unsure or doubtful if it would help in generalized epilepsy. Only about half of respondents agreed that surgery should be considered for children who have failed 3 years of antiseizure medications.
Surgery for partial or generalized epilepsy "probably has the biggest impact on whether you can cure somebody or hugely decrease their seizure load. If two-thirds of respondents are unsure of that, it tells me we have completely failed in communicating what we do to professionals who refer to us," said coinvestigator Dr. Freedom F. Perkins Jr., a pediatric epileptologist at Dell.
Education is the answer. "It is incumbent on us to reach out to our referral sources" – pediatricians and family practitioners – "to make sure they understand these things." Webinars could help, but might only draw physicians already interested in and knowledgeable about epilepsy. "The old-fashioned shoe leather" approach might be better; "you get in your car, you meet people, and you talk," Dr. Perkins said at the annual meeting of the American Epilepsy Society.
The researchers plan to do just that with primary care physicians around Austin, and then repeat the survey in perhaps a year to see if the efforts improved understanding and referral patterns.
Dr. Hovinga and Dr. Perkins said they had no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN EPILEPSY SOCIETY
Major Finding: When asked the threshold of refractory epilepsy, 61% of physicians surveyed did not know, and most of these were family physicians or pediatricians.
Data Source: Survey of 146 physicians in central Texas.
Disclosures: The investigators said they had no relevant financial disclosures.
Laser ablation rivaled open surgery for epileptogenic lesions
Craniotomies and open resections may soon no longer be necessary to remove focal, well-circumscribed refractory epileptogenic lesions from the brain, two small studies have shown.
Two research teams reported positive, early results for laser ablation, an alternative approach in which a narrow probe is inserted through a small hole in the skull to kill epileptogenic neurons with heat under real-time MRI and EEG guidance.
At the Sutter Neuroscience Institute in Sacramento, "we are having about a 70% [seizure-free] success rate" in the six children and two young adults who have undergone the procedure and been followed for up to a year," said neurologist Michael Chez, director of Sutter’s pediatric and adult epilepsy program. He and his team have treated lesions of the frontal, nonmesial temporal, occipital, and parietal lobes.
"We’ve had some very nice results with a very minimally invasive technique. The outcomes are very promising. We have another six surgeries planned," he said at the annual meeting of the American Epilepsy Society.
Meanwhile, Miami Children’s Hospital reported about a 50% seizure-free success rate following laser ablation in 10 pediatric patients followed for up to 18 months. "The outcomes look comparable" to those achieved with open surgery, and should improve with additional experience. "I think [the technique] has incredible promise," said Dr. Ian Miller, director of neuroinformatics at the hospital.
A handful of epilepsy centers in the United States are developing the technique following the Food and Drug Administration’s approval for soft tissue ablation several years ago.
Hospital stays are much shorter with laser ablation, about 24 hours instead of 3 or more days following open surgery. Laser ablation is also probably less damaging to nearby healthy brain tissue, better able to reach deep-seated foci, more acceptable to patients and parents weary of open techniques, and better tolerated by patients who could not tolerate open procedures and the work-up that precedes them, said Dr. Chez, who noted that developmental disabilities ruled out open procedures in six of his eight patients.
To date, laser ablation has been used in perhaps 85 adult and pediatric epilepsy patients across the United States. Investigators will need to pool their outcomes to compare them to open surgery, and determine the best-case scenario where laser ablation would be helpful, said Miami Children’s neurosurgeon Sanjiv Bhatia, who is also developing the technique there.
After the probe is placed in the operating room – which takes about 30 minutes – patients are rolled into the MRI suite for monitoring during ablation. EEGs with plastic electrodes are monitored, as well. "We can see exactly where the probe is and the spike activity going away during ablation," Dr. Chez said.
Instead of using direct electrophysiologic recording, the investigators are using advanced functional imaging techniques including magnetic EEGs, three-dimensional computer modeling, and stereotactic probe guidance. "The question is if that is ultimately going to be enough to replace direct recordings," Dr. Bhatia said.
For now, the greatest challenge is accurately defining the epileptogenic lesion through a closed skull, without the benefit of the multiple-depth electrodes and grid mapping used during open surgery. "The key to these procedures is having very solid localization," Dr. Miller noted.
The researchers said they had no relevant financial disclosures.
Craniotomies and open resections may soon no longer be necessary to remove focal, well-circumscribed refractory epileptogenic lesions from the brain, two small studies have shown.
Two research teams reported positive, early results for laser ablation, an alternative approach in which a narrow probe is inserted through a small hole in the skull to kill epileptogenic neurons with heat under real-time MRI and EEG guidance.
At the Sutter Neuroscience Institute in Sacramento, "we are having about a 70% [seizure-free] success rate" in the six children and two young adults who have undergone the procedure and been followed for up to a year," said neurologist Michael Chez, director of Sutter’s pediatric and adult epilepsy program. He and his team have treated lesions of the frontal, nonmesial temporal, occipital, and parietal lobes.
"We’ve had some very nice results with a very minimally invasive technique. The outcomes are very promising. We have another six surgeries planned," he said at the annual meeting of the American Epilepsy Society.
Meanwhile, Miami Children’s Hospital reported about a 50% seizure-free success rate following laser ablation in 10 pediatric patients followed for up to 18 months. "The outcomes look comparable" to those achieved with open surgery, and should improve with additional experience. "I think [the technique] has incredible promise," said Dr. Ian Miller, director of neuroinformatics at the hospital.
A handful of epilepsy centers in the United States are developing the technique following the Food and Drug Administration’s approval for soft tissue ablation several years ago.
Hospital stays are much shorter with laser ablation, about 24 hours instead of 3 or more days following open surgery. Laser ablation is also probably less damaging to nearby healthy brain tissue, better able to reach deep-seated foci, more acceptable to patients and parents weary of open techniques, and better tolerated by patients who could not tolerate open procedures and the work-up that precedes them, said Dr. Chez, who noted that developmental disabilities ruled out open procedures in six of his eight patients.
To date, laser ablation has been used in perhaps 85 adult and pediatric epilepsy patients across the United States. Investigators will need to pool their outcomes to compare them to open surgery, and determine the best-case scenario where laser ablation would be helpful, said Miami Children’s neurosurgeon Sanjiv Bhatia, who is also developing the technique there.
After the probe is placed in the operating room – which takes about 30 minutes – patients are rolled into the MRI suite for monitoring during ablation. EEGs with plastic electrodes are monitored, as well. "We can see exactly where the probe is and the spike activity going away during ablation," Dr. Chez said.
Instead of using direct electrophysiologic recording, the investigators are using advanced functional imaging techniques including magnetic EEGs, three-dimensional computer modeling, and stereotactic probe guidance. "The question is if that is ultimately going to be enough to replace direct recordings," Dr. Bhatia said.
For now, the greatest challenge is accurately defining the epileptogenic lesion through a closed skull, without the benefit of the multiple-depth electrodes and grid mapping used during open surgery. "The key to these procedures is having very solid localization," Dr. Miller noted.
The researchers said they had no relevant financial disclosures.
Craniotomies and open resections may soon no longer be necessary to remove focal, well-circumscribed refractory epileptogenic lesions from the brain, two small studies have shown.
Two research teams reported positive, early results for laser ablation, an alternative approach in which a narrow probe is inserted through a small hole in the skull to kill epileptogenic neurons with heat under real-time MRI and EEG guidance.
At the Sutter Neuroscience Institute in Sacramento, "we are having about a 70% [seizure-free] success rate" in the six children and two young adults who have undergone the procedure and been followed for up to a year," said neurologist Michael Chez, director of Sutter’s pediatric and adult epilepsy program. He and his team have treated lesions of the frontal, nonmesial temporal, occipital, and parietal lobes.
"We’ve had some very nice results with a very minimally invasive technique. The outcomes are very promising. We have another six surgeries planned," he said at the annual meeting of the American Epilepsy Society.
Meanwhile, Miami Children’s Hospital reported about a 50% seizure-free success rate following laser ablation in 10 pediatric patients followed for up to 18 months. "The outcomes look comparable" to those achieved with open surgery, and should improve with additional experience. "I think [the technique] has incredible promise," said Dr. Ian Miller, director of neuroinformatics at the hospital.
A handful of epilepsy centers in the United States are developing the technique following the Food and Drug Administration’s approval for soft tissue ablation several years ago.
Hospital stays are much shorter with laser ablation, about 24 hours instead of 3 or more days following open surgery. Laser ablation is also probably less damaging to nearby healthy brain tissue, better able to reach deep-seated foci, more acceptable to patients and parents weary of open techniques, and better tolerated by patients who could not tolerate open procedures and the work-up that precedes them, said Dr. Chez, who noted that developmental disabilities ruled out open procedures in six of his eight patients.
To date, laser ablation has been used in perhaps 85 adult and pediatric epilepsy patients across the United States. Investigators will need to pool their outcomes to compare them to open surgery, and determine the best-case scenario where laser ablation would be helpful, said Miami Children’s neurosurgeon Sanjiv Bhatia, who is also developing the technique there.
After the probe is placed in the operating room – which takes about 30 minutes – patients are rolled into the MRI suite for monitoring during ablation. EEGs with plastic electrodes are monitored, as well. "We can see exactly where the probe is and the spike activity going away during ablation," Dr. Chez said.
Instead of using direct electrophysiologic recording, the investigators are using advanced functional imaging techniques including magnetic EEGs, three-dimensional computer modeling, and stereotactic probe guidance. "The question is if that is ultimately going to be enough to replace direct recordings," Dr. Bhatia said.
For now, the greatest challenge is accurately defining the epileptogenic lesion through a closed skull, without the benefit of the multiple-depth electrodes and grid mapping used during open surgery. "The key to these procedures is having very solid localization," Dr. Miller noted.
The researchers said they had no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN EPILEPSY SOCIETY
Major Finding: Minimally invasive laser ablation of focal epileptogenic lesions produced about a 70% seizure-free success rate in a study of six children and two adults, and a 50% seizure-free success rate in a study of 10 children.
Data Source: Epilepsy patients with a maximum follow-up of 18 months.
Disclosures: The researchers said they had no relevant financial disclosures.
Neurologic impairment before epilepsy affects long-term outcomes
SAN DIEGO – Prior neural impairment is the only independent predictor of long-term epilepsy and major cognitive and motor impairments following convulsive status epilepticus in children, researchers reported at the annual meeting of the American Epilepsy Society.
Investigators with the North London Status Epilepticus in Childhood Surveillance Study assessed 126 children a median of 8.5 years following convulsive status epilepticus (CSE) at a median age of 3.6 years.
Among children with prior neurologic impairment, the odds ratio for active epilepsy at follow-up was 7.1 (95% confidence interval, 1.8-27.7), for cognitive impairment, 16.3 (95% CI, 4.3-61.1), and for motor impairment, 9.8 (95% CI, 3.0-31.2).
Outcomes were not predicted by the duration of the original attack or by whether it was focal or generalized or continuous or intermittent.
Overall, "if you have good neurologic development before status, your outcome is going to be very good," said senior investigator Dr. Richard Chin, director of the epilepsy center at the University of Edinburgh.
That’s often the case with pediatric febrile seizures, the most common cause of pediatric CSE. True to the point, none of the 30 children who entered the study following febrile seizures had active epilepsy or major motor impairments at follow-up; one child with a subsequent autism diagnosis had major cognitive impairment. MRIs were normal in 28 of the children; one child had evidence of neurofibromatosis and another had a unilateral hippocampal volume reduction without signal change.
Although prolonged febrile seizures have long been thought to be a significant risk factor for subsequent mesial temporal sclerosis (MTS), "we didn’t find evidence of that. None of our children with prolonged febrile seizures developed MTS. The relationship between febrile seizures and subsequent mesial temporal sclerosis is not as strong as once believed," Dr. Chin said.
The 51 children who entered the study with remote symptomatic CSE had previous diagnoses of cerebral palsy, hypoxic brain injury, and other problems; 48 (94%) had active epilepsy, 32 (63%) had major motor deficits, and 37 (73%) had major cognitive impairments at follow-up.
Seventeen children had suffered acute symptomatic CSE attacks; only one had active epilepsy and major cognitive impairment at follow-up. MRIs in a child whose attack was brought on by pneumococcal meningitis suggested left MTS.
Most of the rest of the children in the study were classified as having had an idiopathic or cryptogenic CSE attack – the majority had active epilepsy at follow-up and major motor or cognitive impairments.
Dr. Chin said that he had no disclosures.
SAN DIEGO – Prior neural impairment is the only independent predictor of long-term epilepsy and major cognitive and motor impairments following convulsive status epilepticus in children, researchers reported at the annual meeting of the American Epilepsy Society.
Investigators with the North London Status Epilepticus in Childhood Surveillance Study assessed 126 children a median of 8.5 years following convulsive status epilepticus (CSE) at a median age of 3.6 years.
Among children with prior neurologic impairment, the odds ratio for active epilepsy at follow-up was 7.1 (95% confidence interval, 1.8-27.7), for cognitive impairment, 16.3 (95% CI, 4.3-61.1), and for motor impairment, 9.8 (95% CI, 3.0-31.2).
Outcomes were not predicted by the duration of the original attack or by whether it was focal or generalized or continuous or intermittent.
Overall, "if you have good neurologic development before status, your outcome is going to be very good," said senior investigator Dr. Richard Chin, director of the epilepsy center at the University of Edinburgh.
That’s often the case with pediatric febrile seizures, the most common cause of pediatric CSE. True to the point, none of the 30 children who entered the study following febrile seizures had active epilepsy or major motor impairments at follow-up; one child with a subsequent autism diagnosis had major cognitive impairment. MRIs were normal in 28 of the children; one child had evidence of neurofibromatosis and another had a unilateral hippocampal volume reduction without signal change.
Although prolonged febrile seizures have long been thought to be a significant risk factor for subsequent mesial temporal sclerosis (MTS), "we didn’t find evidence of that. None of our children with prolonged febrile seizures developed MTS. The relationship between febrile seizures and subsequent mesial temporal sclerosis is not as strong as once believed," Dr. Chin said.
The 51 children who entered the study with remote symptomatic CSE had previous diagnoses of cerebral palsy, hypoxic brain injury, and other problems; 48 (94%) had active epilepsy, 32 (63%) had major motor deficits, and 37 (73%) had major cognitive impairments at follow-up.
Seventeen children had suffered acute symptomatic CSE attacks; only one had active epilepsy and major cognitive impairment at follow-up. MRIs in a child whose attack was brought on by pneumococcal meningitis suggested left MTS.
Most of the rest of the children in the study were classified as having had an idiopathic or cryptogenic CSE attack – the majority had active epilepsy at follow-up and major motor or cognitive impairments.
Dr. Chin said that he had no disclosures.
SAN DIEGO – Prior neural impairment is the only independent predictor of long-term epilepsy and major cognitive and motor impairments following convulsive status epilepticus in children, researchers reported at the annual meeting of the American Epilepsy Society.
Investigators with the North London Status Epilepticus in Childhood Surveillance Study assessed 126 children a median of 8.5 years following convulsive status epilepticus (CSE) at a median age of 3.6 years.
Among children with prior neurologic impairment, the odds ratio for active epilepsy at follow-up was 7.1 (95% confidence interval, 1.8-27.7), for cognitive impairment, 16.3 (95% CI, 4.3-61.1), and for motor impairment, 9.8 (95% CI, 3.0-31.2).
Outcomes were not predicted by the duration of the original attack or by whether it was focal or generalized or continuous or intermittent.
Overall, "if you have good neurologic development before status, your outcome is going to be very good," said senior investigator Dr. Richard Chin, director of the epilepsy center at the University of Edinburgh.
That’s often the case with pediatric febrile seizures, the most common cause of pediatric CSE. True to the point, none of the 30 children who entered the study following febrile seizures had active epilepsy or major motor impairments at follow-up; one child with a subsequent autism diagnosis had major cognitive impairment. MRIs were normal in 28 of the children; one child had evidence of neurofibromatosis and another had a unilateral hippocampal volume reduction without signal change.
Although prolonged febrile seizures have long been thought to be a significant risk factor for subsequent mesial temporal sclerosis (MTS), "we didn’t find evidence of that. None of our children with prolonged febrile seizures developed MTS. The relationship between febrile seizures and subsequent mesial temporal sclerosis is not as strong as once believed," Dr. Chin said.
The 51 children who entered the study with remote symptomatic CSE had previous diagnoses of cerebral palsy, hypoxic brain injury, and other problems; 48 (94%) had active epilepsy, 32 (63%) had major motor deficits, and 37 (73%) had major cognitive impairments at follow-up.
Seventeen children had suffered acute symptomatic CSE attacks; only one had active epilepsy and major cognitive impairment at follow-up. MRIs in a child whose attack was brought on by pneumococcal meningitis suggested left MTS.
Most of the rest of the children in the study were classified as having had an idiopathic or cryptogenic CSE attack – the majority had active epilepsy at follow-up and major motor or cognitive impairments.
Dr. Chin said that he had no disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN EPILEPSY SOCIETY
Major Finding: Among children with convulsive status epilepticus and prior neurologic impairment, the odds ratio for active epilepsy at long-term follow-up is 7.1 (95% CI, 1.8-27.7), for cognitive impairment, 16.3 (95% CI, 4.3-61.1), and for motor impairment, 9.8 (95% CI, 3.0-31.2).
Data Source: Assessment of 126 children a median of 8.5 years following convulsive status epilepticus.
Disclosures: Dr. Chin said that he had no disclosures.
Use methotrexate at baseline to preserve biologics' effectiveness
LAS VEGAS – To prevent immunogenicity to biologics, it’s best to have psoriasis patients on methotrexate at baseline, according to Dr. Bruce Strober of the University of Connecticut, Farmington.
Once patients develop antibodies to a biologic, “the horse is already out of the barn. It may be too late to recover efficacy by adding methotrexate,” Dr. Strober said. “Add the biologic to the methotrexate, not visa versa” (Br. J. Dermatol. 2012;167:649-57).
Anti–tumor necrosis factors and other biologics often lose their effects as patients build antibodies to the foreign proteins they contain. “Drug levels fall off a cliff when you have a lot of immunogenicity. The major challenge [is] making sure patients 1-3 years out see the response they got in the first 6 months,” Dr. Strober said at the SDEF Las Vegas Dermatology Seminar.
Methotrexate is thought to diminish the antibody response, blocking immunogenicity. “Biologics invariably show greater and more durable efficacy” with methotrexate “even when methotrexate is ineffective on its own,” he said. When oral therapy is indicated, Dr. Strober said he starts most of his psoriasis patients on about 15 mg/wk and waits 8-12 weeks to see whether this works. If there is no response, he will add a biologic and continue the methotrexate.
The dose of methotrexate needed to tame the antibody response remains unclear. “The consensus in the rheumatology world is somewhere around 15 mg weekly, but I don’t always use that dose. I will go down to 7.5 mg to 12.5 mg in many patients,” said Dr. Strober. “It appears anecdotally that there’s good protection of the [biologic] response with those doses. It’s something you might vary based on the size of the patient,” he said. “Episodic dosing gives you the greatest percent of patients getting antibodies, [so] dose biologics without interruption if you can,” he added (J. Am. Acad. Dermatol. 2007;56:31.e1-15).
Immunogenicity to one biologic does not necessarily translate to immunogenicity to another. Also, increasing infusion frequency can help recapture a biologic’s effect, he noted.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Strober is on the advisory board of or a consultant to several pharmaceutical companies, including Janssen, Abbott, Pfizer, and Amgen.
LAS VEGAS – To prevent immunogenicity to biologics, it’s best to have psoriasis patients on methotrexate at baseline, according to Dr. Bruce Strober of the University of Connecticut, Farmington.
Once patients develop antibodies to a biologic, “the horse is already out of the barn. It may be too late to recover efficacy by adding methotrexate,” Dr. Strober said. “Add the biologic to the methotrexate, not visa versa” (Br. J. Dermatol. 2012;167:649-57).
Anti–tumor necrosis factors and other biologics often lose their effects as patients build antibodies to the foreign proteins they contain. “Drug levels fall off a cliff when you have a lot of immunogenicity. The major challenge [is] making sure patients 1-3 years out see the response they got in the first 6 months,” Dr. Strober said at the SDEF Las Vegas Dermatology Seminar.
Methotrexate is thought to diminish the antibody response, blocking immunogenicity. “Biologics invariably show greater and more durable efficacy” with methotrexate “even when methotrexate is ineffective on its own,” he said. When oral therapy is indicated, Dr. Strober said he starts most of his psoriasis patients on about 15 mg/wk and waits 8-12 weeks to see whether this works. If there is no response, he will add a biologic and continue the methotrexate.
The dose of methotrexate needed to tame the antibody response remains unclear. “The consensus in the rheumatology world is somewhere around 15 mg weekly, but I don’t always use that dose. I will go down to 7.5 mg to 12.5 mg in many patients,” said Dr. Strober. “It appears anecdotally that there’s good protection of the [biologic] response with those doses. It’s something you might vary based on the size of the patient,” he said. “Episodic dosing gives you the greatest percent of patients getting antibodies, [so] dose biologics without interruption if you can,” he added (J. Am. Acad. Dermatol. 2007;56:31.e1-15).
Immunogenicity to one biologic does not necessarily translate to immunogenicity to another. Also, increasing infusion frequency can help recapture a biologic’s effect, he noted.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Strober is on the advisory board of or a consultant to several pharmaceutical companies, including Janssen, Abbott, Pfizer, and Amgen.
LAS VEGAS – To prevent immunogenicity to biologics, it’s best to have psoriasis patients on methotrexate at baseline, according to Dr. Bruce Strober of the University of Connecticut, Farmington.
Once patients develop antibodies to a biologic, “the horse is already out of the barn. It may be too late to recover efficacy by adding methotrexate,” Dr. Strober said. “Add the biologic to the methotrexate, not visa versa” (Br. J. Dermatol. 2012;167:649-57).
Anti–tumor necrosis factors and other biologics often lose their effects as patients build antibodies to the foreign proteins they contain. “Drug levels fall off a cliff when you have a lot of immunogenicity. The major challenge [is] making sure patients 1-3 years out see the response they got in the first 6 months,” Dr. Strober said at the SDEF Las Vegas Dermatology Seminar.
Methotrexate is thought to diminish the antibody response, blocking immunogenicity. “Biologics invariably show greater and more durable efficacy” with methotrexate “even when methotrexate is ineffective on its own,” he said. When oral therapy is indicated, Dr. Strober said he starts most of his psoriasis patients on about 15 mg/wk and waits 8-12 weeks to see whether this works. If there is no response, he will add a biologic and continue the methotrexate.
The dose of methotrexate needed to tame the antibody response remains unclear. “The consensus in the rheumatology world is somewhere around 15 mg weekly, but I don’t always use that dose. I will go down to 7.5 mg to 12.5 mg in many patients,” said Dr. Strober. “It appears anecdotally that there’s good protection of the [biologic] response with those doses. It’s something you might vary based on the size of the patient,” he said. “Episodic dosing gives you the greatest percent of patients getting antibodies, [so] dose biologics without interruption if you can,” he added (J. Am. Acad. Dermatol. 2007;56:31.e1-15).
Immunogenicity to one biologic does not necessarily translate to immunogenicity to another. Also, increasing infusion frequency can help recapture a biologic’s effect, he noted.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Strober is on the advisory board of or a consultant to several pharmaceutical companies, including Janssen, Abbott, Pfizer, and Amgen.
EXPERT ANALYSIS FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR