User login
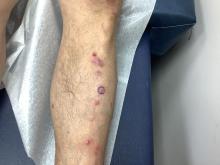
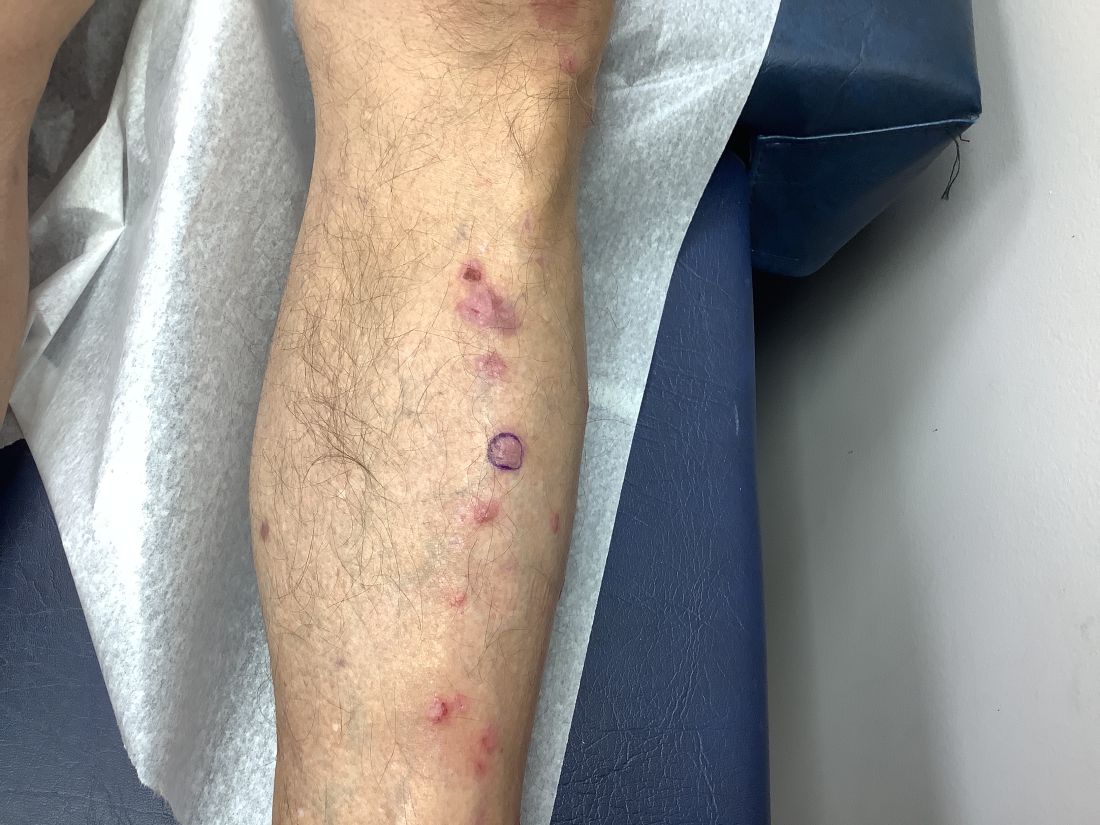
Pruritic, violaceous papules in a patient with renal cell carcinoma
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.