User login
Freezing the biological clock: A 2023 update on preserving fertility
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
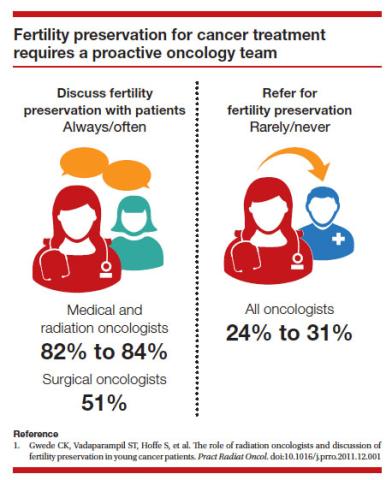
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.
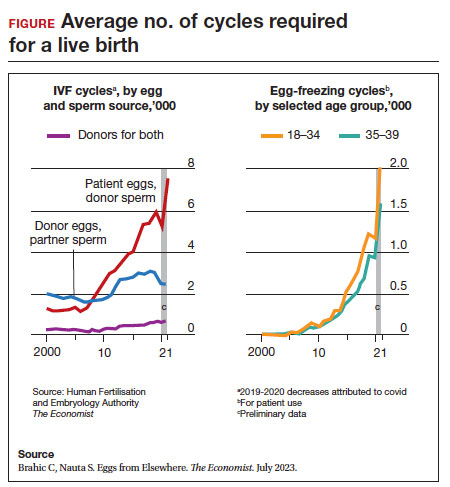
Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
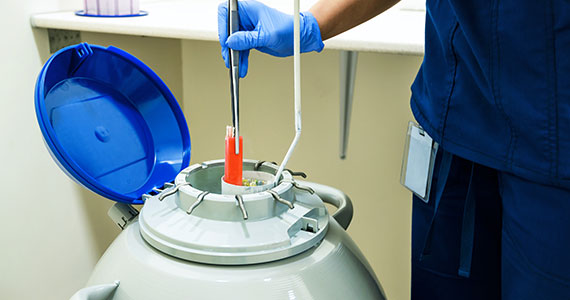
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.

Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.

Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003
Recurrent pregnancy loss and inherited thrombophilias: Does low molecular weight heparin improve the live birth rate?
Quenby S, Booth K, Hiller L, et al; ALIFE2 Block Writing Committee and ALIFE2 Investigators. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): an international open-label, randomised controlled trial. Lancet. 2023;402:54-61. doi:10.1016/S0140-6736(23)00693-1.
EXPERT COMMENTARY
“Follow the evidence to where it leads, even if the conclusion is uncomfortable.”
—Steven James, author
Women with RPL have endured overzealous evaluations and management despite a lack of proven efficacy. From alloimmune testing that results in paternal leukocyte immunization1 and the long-entrusted metroplasty for a septate uterus recently put under fire2 to the “hammer and nail” approach of preimplantation genetic testing for embryo aneuploid screening,3 patients have been subjected to unsubstantiated treatments.
While the evaluation of RPL has evolved, guidelines from the American Society for Reproductive Medicine (ASRM), American College of Obstetricians and Gynecologists (ACOG), and Royal College of Obstetricians and Gynaecologists (RCOG) do not recommend testing for inherited thrombophilias outside of a history for venous thromboembolism.4-6 These 3 societies support treating acquired thrombophilias that represent the antiphospholipid antibody syndrome.
Citing insufficient evidence for reducing adverse pregnancy outcomes, ACOG recommends the use of prophylactic- or intermediate-dose LMWH or unfractionated heparin (UFH) for patients with “high-risk” thrombophilias only to prevent venous thromboembolism during pregnancy and continuing postpartum.4 (High-risk thrombophilias are defined as factor V Leiden homozygosity, prothrombin gene G20210A mutation homozygosity, heterozygosity for both factor V Leiden homozygosity and prothrombin gene G20210A mutation, or an antithrombin deficiency.4)
To determine the impact of LMWH treatment versus no treatment on live birth rate, Quenby and colleagues conducted a prospective randomized controlled trial of women with RPL and inherited thrombophilias (the ALIFE2 trial). This was a follow-up to their 2010 randomized controlled trial that demonstrated no effect of LMWH with low-dose aspirin versus low-dose aspirin alone compared with placebo in women with unexplained RPL.7
 PHOTO: BETAVERSO/SHUTTERSTOCK
PHOTO: BETAVERSO/SHUTTERSTOCK

Continue to: Details of the study...
Details of the study
The ALIFE2 study took place over 8 years and involved 5 countries, including the United States, with the 2 main centers in the Netherlands and the United Kingdom. Women eligible for the study were aged 18 to 42 years, had an inherited thrombophilia (confirmed by 2 tests), experienced recurrent miscarriages (2 or more consecutive miscarriages, nonconsecutive miscarriages, or intrauterine fetal deaths, irrespective of gestational age), and were less than 7 weeks’ estimated gestational age. Study patients were randomly allocated with a positive pregnancy test to either surveillance or LMWH treatment, which was continued throughout pregnancy.
The primary outcome was live birth rate, and secondary outcomes were a history of miscarriage, ectopic pregnancy, and obstetric complications. A total of 164 women were allocated to LMWH plus standard care, and 162 women to standard care alone. LMWH was shown to be safe without major/minor bleeding or maternal heparin-induced thrombocytopenia.
The statistical calculation was by “intention to treat,” which considers all enrolled participants, including those who dropped out of the study, as opposed to a “per protocol” analysis in which only patients who completed the study were analyzed.
Results. Primary outcome data were available for 320 participants. Of the 162 women in the LMWH-treated group, 116 (72%) had live birth rates, as did 112 (71%) of 158 in the standard care group. There was no significant difference between groups (OR, 1.04; 95% CI, 0.64–1.68).
Study strengths and limitations
The outcome of the ALIFE2 study is consistent with that of a Cochrane review that found insufficient evidence for improved live birth rate in patients with RPL and inherited thrombophilias treated with LMWH versus low-dose aspirin. Of their review of the studies at low risk of bias, only 1 was placebo controlled.8
This study by Quenby and colleagues was well designed and ensured a sufficient number of enrolled participants to comply with their power analysis. However, by beginning LMWH at 7 weeks’ gestation, patients may not have received a therapeutic benefit as opposed to initiation of treatment with a positive pregnancy test. The authors did not describe when testing for thrombophilias occurred or explain the protocol and reason for repeat testing.
Study limitations included a deviation from protocol in the standard care group, which was the initiation of LMWH after 7 weeks’ gestation. In the standard care group, 30 participants received LMWH, 18 of whom started heparin treatment before 12 weeks of gestation. The other 12 participants received LMWH after 12 weeks’ gestation, and 6 of those 12 started after 28 weeks’ gestation, since they were determined to need LMWH for thromboprophylaxis according to RCOG guidelines. While this had the potential to influence outcomes, only 18 of 162 (11%) patients were involved.
The authors did not define RPL based on a clinical versus a biochemical pregnancy loss as the latter is more common and is without agreed upon criteria for testing. Additionally, a lack of patient masking to medication could play an undetermined role in affecting the outcome. ●
This elegant, and vital, randomized controlled trial provides double take-home messages: There is no value in testing for inherited thrombophilias in RPL, as they occur in a similar prevalence in the general population, and there is no significant difference in live birth rate from LMWH treatment in women with RPL and inherited thrombophilias compared with surveillance. Consequently, the increased cost of medication and testing can be averted.
MARK P. TROLICE, MD, MBA
- Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2014; CD000112. doi:10.1002/14651858.CD000112
- Trolice MP. The septate uterus and metroplasty—another dogma under siege. Fertil Steril. 2021;116:693-694. doi:10.1016/j.fertnstert.2021.06.063
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. doi:10.1093 /humrep/deab194
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi:10.1097 /AOG.0000000000002703
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103-1111. doi:10.1016/j.fertnstert.2012.06.048
- Regan L, Rai R, Saravelos S, et al; Royal College of Obstetricians and Gynaecologists. Recurrent Miscarriage Green‐top Guideline No. 17. BJOG. June 19, 2023. doi:10.1111/1471 -0528.17515
- Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362:1586-1596. doi:10.1056 /NEJMoa1000641
- de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;CD004734. doi:10.1002/14651858.CD004734 .pub4
Quenby S, Booth K, Hiller L, et al; ALIFE2 Block Writing Committee and ALIFE2 Investigators. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): an international open-label, randomised controlled trial. Lancet. 2023;402:54-61. doi:10.1016/S0140-6736(23)00693-1.
EXPERT COMMENTARY
“Follow the evidence to where it leads, even if the conclusion is uncomfortable.”
—Steven James, author
Women with RPL have endured overzealous evaluations and management despite a lack of proven efficacy. From alloimmune testing that results in paternal leukocyte immunization1 and the long-entrusted metroplasty for a septate uterus recently put under fire2 to the “hammer and nail” approach of preimplantation genetic testing for embryo aneuploid screening,3 patients have been subjected to unsubstantiated treatments.
While the evaluation of RPL has evolved, guidelines from the American Society for Reproductive Medicine (ASRM), American College of Obstetricians and Gynecologists (ACOG), and Royal College of Obstetricians and Gynaecologists (RCOG) do not recommend testing for inherited thrombophilias outside of a history for venous thromboembolism.4-6 These 3 societies support treating acquired thrombophilias that represent the antiphospholipid antibody syndrome.
Citing insufficient evidence for reducing adverse pregnancy outcomes, ACOG recommends the use of prophylactic- or intermediate-dose LMWH or unfractionated heparin (UFH) for patients with “high-risk” thrombophilias only to prevent venous thromboembolism during pregnancy and continuing postpartum.4 (High-risk thrombophilias are defined as factor V Leiden homozygosity, prothrombin gene G20210A mutation homozygosity, heterozygosity for both factor V Leiden homozygosity and prothrombin gene G20210A mutation, or an antithrombin deficiency.4)
To determine the impact of LMWH treatment versus no treatment on live birth rate, Quenby and colleagues conducted a prospective randomized controlled trial of women with RPL and inherited thrombophilias (the ALIFE2 trial). This was a follow-up to their 2010 randomized controlled trial that demonstrated no effect of LMWH with low-dose aspirin versus low-dose aspirin alone compared with placebo in women with unexplained RPL.7
 PHOTO: BETAVERSO/SHUTTERSTOCK
PHOTO: BETAVERSO/SHUTTERSTOCK

Continue to: Details of the study...
Details of the study
The ALIFE2 study took place over 8 years and involved 5 countries, including the United States, with the 2 main centers in the Netherlands and the United Kingdom. Women eligible for the study were aged 18 to 42 years, had an inherited thrombophilia (confirmed by 2 tests), experienced recurrent miscarriages (2 or more consecutive miscarriages, nonconsecutive miscarriages, or intrauterine fetal deaths, irrespective of gestational age), and were less than 7 weeks’ estimated gestational age. Study patients were randomly allocated with a positive pregnancy test to either surveillance or LMWH treatment, which was continued throughout pregnancy.
The primary outcome was live birth rate, and secondary outcomes were a history of miscarriage, ectopic pregnancy, and obstetric complications. A total of 164 women were allocated to LMWH plus standard care, and 162 women to standard care alone. LMWH was shown to be safe without major/minor bleeding or maternal heparin-induced thrombocytopenia.
The statistical calculation was by “intention to treat,” which considers all enrolled participants, including those who dropped out of the study, as opposed to a “per protocol” analysis in which only patients who completed the study were analyzed.
Results. Primary outcome data were available for 320 participants. Of the 162 women in the LMWH-treated group, 116 (72%) had live birth rates, as did 112 (71%) of 158 in the standard care group. There was no significant difference between groups (OR, 1.04; 95% CI, 0.64–1.68).
Study strengths and limitations
The outcome of the ALIFE2 study is consistent with that of a Cochrane review that found insufficient evidence for improved live birth rate in patients with RPL and inherited thrombophilias treated with LMWH versus low-dose aspirin. Of their review of the studies at low risk of bias, only 1 was placebo controlled.8
This study by Quenby and colleagues was well designed and ensured a sufficient number of enrolled participants to comply with their power analysis. However, by beginning LMWH at 7 weeks’ gestation, patients may not have received a therapeutic benefit as opposed to initiation of treatment with a positive pregnancy test. The authors did not describe when testing for thrombophilias occurred or explain the protocol and reason for repeat testing.
Study limitations included a deviation from protocol in the standard care group, which was the initiation of LMWH after 7 weeks’ gestation. In the standard care group, 30 participants received LMWH, 18 of whom started heparin treatment before 12 weeks of gestation. The other 12 participants received LMWH after 12 weeks’ gestation, and 6 of those 12 started after 28 weeks’ gestation, since they were determined to need LMWH for thromboprophylaxis according to RCOG guidelines. While this had the potential to influence outcomes, only 18 of 162 (11%) patients were involved.
The authors did not define RPL based on a clinical versus a biochemical pregnancy loss as the latter is more common and is without agreed upon criteria for testing. Additionally, a lack of patient masking to medication could play an undetermined role in affecting the outcome. ●
This elegant, and vital, randomized controlled trial provides double take-home messages: There is no value in testing for inherited thrombophilias in RPL, as they occur in a similar prevalence in the general population, and there is no significant difference in live birth rate from LMWH treatment in women with RPL and inherited thrombophilias compared with surveillance. Consequently, the increased cost of medication and testing can be averted.
MARK P. TROLICE, MD, MBA
Quenby S, Booth K, Hiller L, et al; ALIFE2 Block Writing Committee and ALIFE2 Investigators. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): an international open-label, randomised controlled trial. Lancet. 2023;402:54-61. doi:10.1016/S0140-6736(23)00693-1.
EXPERT COMMENTARY
“Follow the evidence to where it leads, even if the conclusion is uncomfortable.”
—Steven James, author
Women with RPL have endured overzealous evaluations and management despite a lack of proven efficacy. From alloimmune testing that results in paternal leukocyte immunization1 and the long-entrusted metroplasty for a septate uterus recently put under fire2 to the “hammer and nail” approach of preimplantation genetic testing for embryo aneuploid screening,3 patients have been subjected to unsubstantiated treatments.
While the evaluation of RPL has evolved, guidelines from the American Society for Reproductive Medicine (ASRM), American College of Obstetricians and Gynecologists (ACOG), and Royal College of Obstetricians and Gynaecologists (RCOG) do not recommend testing for inherited thrombophilias outside of a history for venous thromboembolism.4-6 These 3 societies support treating acquired thrombophilias that represent the antiphospholipid antibody syndrome.
Citing insufficient evidence for reducing adverse pregnancy outcomes, ACOG recommends the use of prophylactic- or intermediate-dose LMWH or unfractionated heparin (UFH) for patients with “high-risk” thrombophilias only to prevent venous thromboembolism during pregnancy and continuing postpartum.4 (High-risk thrombophilias are defined as factor V Leiden homozygosity, prothrombin gene G20210A mutation homozygosity, heterozygosity for both factor V Leiden homozygosity and prothrombin gene G20210A mutation, or an antithrombin deficiency.4)
To determine the impact of LMWH treatment versus no treatment on live birth rate, Quenby and colleagues conducted a prospective randomized controlled trial of women with RPL and inherited thrombophilias (the ALIFE2 trial). This was a follow-up to their 2010 randomized controlled trial that demonstrated no effect of LMWH with low-dose aspirin versus low-dose aspirin alone compared with placebo in women with unexplained RPL.7
 PHOTO: BETAVERSO/SHUTTERSTOCK
PHOTO: BETAVERSO/SHUTTERSTOCK

Continue to: Details of the study...
Details of the study
The ALIFE2 study took place over 8 years and involved 5 countries, including the United States, with the 2 main centers in the Netherlands and the United Kingdom. Women eligible for the study were aged 18 to 42 years, had an inherited thrombophilia (confirmed by 2 tests), experienced recurrent miscarriages (2 or more consecutive miscarriages, nonconsecutive miscarriages, or intrauterine fetal deaths, irrespective of gestational age), and were less than 7 weeks’ estimated gestational age. Study patients were randomly allocated with a positive pregnancy test to either surveillance or LMWH treatment, which was continued throughout pregnancy.
The primary outcome was live birth rate, and secondary outcomes were a history of miscarriage, ectopic pregnancy, and obstetric complications. A total of 164 women were allocated to LMWH plus standard care, and 162 women to standard care alone. LMWH was shown to be safe without major/minor bleeding or maternal heparin-induced thrombocytopenia.
The statistical calculation was by “intention to treat,” which considers all enrolled participants, including those who dropped out of the study, as opposed to a “per protocol” analysis in which only patients who completed the study were analyzed.
Results. Primary outcome data were available for 320 participants. Of the 162 women in the LMWH-treated group, 116 (72%) had live birth rates, as did 112 (71%) of 158 in the standard care group. There was no significant difference between groups (OR, 1.04; 95% CI, 0.64–1.68).
Study strengths and limitations
The outcome of the ALIFE2 study is consistent with that of a Cochrane review that found insufficient evidence for improved live birth rate in patients with RPL and inherited thrombophilias treated with LMWH versus low-dose aspirin. Of their review of the studies at low risk of bias, only 1 was placebo controlled.8
This study by Quenby and colleagues was well designed and ensured a sufficient number of enrolled participants to comply with their power analysis. However, by beginning LMWH at 7 weeks’ gestation, patients may not have received a therapeutic benefit as opposed to initiation of treatment with a positive pregnancy test. The authors did not describe when testing for thrombophilias occurred or explain the protocol and reason for repeat testing.
Study limitations included a deviation from protocol in the standard care group, which was the initiation of LMWH after 7 weeks’ gestation. In the standard care group, 30 participants received LMWH, 18 of whom started heparin treatment before 12 weeks of gestation. The other 12 participants received LMWH after 12 weeks’ gestation, and 6 of those 12 started after 28 weeks’ gestation, since they were determined to need LMWH for thromboprophylaxis according to RCOG guidelines. While this had the potential to influence outcomes, only 18 of 162 (11%) patients were involved.
The authors did not define RPL based on a clinical versus a biochemical pregnancy loss as the latter is more common and is without agreed upon criteria for testing. Additionally, a lack of patient masking to medication could play an undetermined role in affecting the outcome. ●
This elegant, and vital, randomized controlled trial provides double take-home messages: There is no value in testing for inherited thrombophilias in RPL, as they occur in a similar prevalence in the general population, and there is no significant difference in live birth rate from LMWH treatment in women with RPL and inherited thrombophilias compared with surveillance. Consequently, the increased cost of medication and testing can be averted.
MARK P. TROLICE, MD, MBA
- Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2014; CD000112. doi:10.1002/14651858.CD000112
- Trolice MP. The septate uterus and metroplasty—another dogma under siege. Fertil Steril. 2021;116:693-694. doi:10.1016/j.fertnstert.2021.06.063
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. doi:10.1093 /humrep/deab194
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi:10.1097 /AOG.0000000000002703
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103-1111. doi:10.1016/j.fertnstert.2012.06.048
- Regan L, Rai R, Saravelos S, et al; Royal College of Obstetricians and Gynaecologists. Recurrent Miscarriage Green‐top Guideline No. 17. BJOG. June 19, 2023. doi:10.1111/1471 -0528.17515
- Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362:1586-1596. doi:10.1056 /NEJMoa1000641
- de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;CD004734. doi:10.1002/14651858.CD004734 .pub4
- Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2014; CD000112. doi:10.1002/14651858.CD000112
- Trolice MP. The septate uterus and metroplasty—another dogma under siege. Fertil Steril. 2021;116:693-694. doi:10.1016/j.fertnstert.2021.06.063
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. doi:10.1093 /humrep/deab194
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi:10.1097 /AOG.0000000000002703
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103-1111. doi:10.1016/j.fertnstert.2012.06.048
- Regan L, Rai R, Saravelos S, et al; Royal College of Obstetricians and Gynaecologists. Recurrent Miscarriage Green‐top Guideline No. 17. BJOG. June 19, 2023. doi:10.1111/1471 -0528.17515
- Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362:1586-1596. doi:10.1056 /NEJMoa1000641
- de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;CD004734. doi:10.1002/14651858.CD004734 .pub4
Can cffDNA technology be used to determine the underlying cause of pregnancy loss to better inform future pregnancy planning?
Hartwig TJ, Ambye L, Gruhn JR, et al. Cell-free fetal DNA for genetic evaluation in Copenhagen Pregnancy Loss Study (COPL): a prospective cohort study. Lancet. 2023;401:762-771. https://doi.org/10.1016/S0140-6736(22)02610-1.
Expert Commentary
A devastating outcome for women, pregnancy loss is directly proportional to maternal age, estimated to occur in approximately 15% of clinically recognized pregnancies and 30% of preclinical pregnancies.1 Approximately 80% of pregnancy losses occur in the first trimester.2 The frequency of clinically recognized early pregnancy loss for women aged 20–30 years is 9% to 17%, and these rates increase sharply, from 20% at age 35 years to 40% at age 40 years, and 80% at age 45 years. Recurrent pregnancy loss (RPL), defined as the spontaneous loss of 2 or more clinically recognized pregnancies, affects less than 5% of women.3 Genetic testing using chromosomal microarray analysis (CMA) has identified aneuploidy in about 55% of cases of miscarriage.4
Following ASRM guidelines for the evaluation of RPL, which consists of analyzing parental chromosomal abnormalities, congenital and acquired uterine anomalies, endocrine imbalances, and autoimmune factors (including antiphospholipid syndrome), no explainable cause is determined in 50% of cases.3 Recently, it has been shown that more than 90% of patients with RPL will have a probable or definitive cause identified when CMA testing on miscarriage tissue with the ASRM evaluation guidelines.5
Details of the study
In this prospective cohort study from Denmark, the authors analyzed maternal serum for cell-free fetal DNA (cffDNA) to determine the ploidy status of the pregnancy loss. One thousand women older than age 18 were included (those who demonstrated an ultrasound-confirmed intrauterine pregnancy loss prior to 22 weeks’ gestation). Maternal blood was obtained while pregnancy tissue was in situ or within 24 hours of passage of products of conception (POC), then analyzed by genome-wide sequencing of cffDNA.
For the first 333 recruited women (validation phase), direct sequencing of the POC was performed for sensitivity and specificity. Following the elimination of inconclusive samples, 302 of the 333 cases demonstrated a sensitivity of 85% and specificity of 93%. In the subsequent evaluation of 667 women, researchers analyzed maternal serum from the gestational age of fetuses ranging from 35 days to 149 days.
Results. In total, nearly 90% of cases yielded conclusive results, with 50% euploid, 46% aneuploid, and 4% multiple aneuploidies. Earlier gestational ages (less than 7 weeks) had a no-call rate (ie, inconclusive) of approximately 50% (only based on 16 patients), with results typically obtained in maternal serum following passage of POC; in pregnancies at gestational ages past 7 weeks, the no-call rate was about 10%. In general, the longer the time after the pregnancy tissue passed, the higher likelihood of a no-call result.
Applying the technology of single-nucleotide polymorphism (SNP)-based CMA can improve identification of fetal and/or maternal sources as causes of pregnancy loss with accuracy, but it does require collection of POC. Of note, samples were deficient in this study, the authors cite, in one-third of the cases. Given this limitation of collection, the authors argue for use of the noninvasive method of cffDNA, obtained from maternal serum.
Study strengths and weaknesses
Several weaknesses of this study are highlighted. Of the validation cohort, one-third of pregnancy tissue could not be analyzed due to insufficient collection. Only 73% of cases allowed for DNA isolation from fetal tissue or chorionic villi; in 27% of cases samples were labeled “unknown tissue.” In those cases classified as unknown, 70% were further determined to be maternal. When all female and monosomy cases were excluded in an effort to assuredly reduce the risk of contamination with maternal DNA, sensitivity of the cffDNA testing process declined to 78%. Another limitation was the required short window for maternal blood sampling (within 24 hours) and its impact on the no-call rate.
The authors note an association with later-life morbidity in patients with a history of pregnancy loss and RPL (including cardiovascular disease, type 2 diabetes, and mental health disorders), thereby arguing for cffDNA-based testing versus no causal testing; however, no treatment has been proven to be effective at reducing pregnancy loss. ●
The best management course for unexplained RPL is uncertain. Despite its use for a euploid miscarriage or parental chromosomal structural rearrangement, in vitro fertilization with preimplantation genetic testing remains an unproven modality.6,7 Given that approximately 70% of human conceptions never achieve viability, and 50% fail spontaneously before being detected,8 the authors’ findings demonstrate peripheral maternal blood can provide a reasonably high sensitivity and specificity for fetal ploidy status when compared with direct sequencing of pregnancy tissue. As fetal aneuploidy offers a higher percentage of subsequent successful pregnancy outcomes, cffDNA may offer reassurance, or direct further testing, following a pregnancy loss. As an application of their results, evaluation may be deferred for an aneuploid miscarriage.
—MARK P. TROLICE, MD, MBA
- Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391-400. doi: 10.1055/s-0028-1087105.
- Wang X, Chen C , Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577-584.
- Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;98: 1103-1111.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/tacg.s320778.
- Popescu F, Jaslow FC, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579-587. https://doi.org/10.1093/humrep/dey021.
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. https://doi.org/10.1093/humrep/deab194.
- Iews M, Tan J, Taskin O, et al. Does preimplantation genetic diagnosis improve reproductive outcome in couples with recurrent pregnancy loss owing to structural chromosomal rearrangement? A systematic review. Reproductive Bio Medicine Online. 2018;36:677-685. https://doi.org/10.1016 /j.rbmo.2018.03.005.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/TACG.S320778.
Hartwig TJ, Ambye L, Gruhn JR, et al. Cell-free fetal DNA for genetic evaluation in Copenhagen Pregnancy Loss Study (COPL): a prospective cohort study. Lancet. 2023;401:762-771. https://doi.org/10.1016/S0140-6736(22)02610-1.
Expert Commentary
A devastating outcome for women, pregnancy loss is directly proportional to maternal age, estimated to occur in approximately 15% of clinically recognized pregnancies and 30% of preclinical pregnancies.1 Approximately 80% of pregnancy losses occur in the first trimester.2 The frequency of clinically recognized early pregnancy loss for women aged 20–30 years is 9% to 17%, and these rates increase sharply, from 20% at age 35 years to 40% at age 40 years, and 80% at age 45 years. Recurrent pregnancy loss (RPL), defined as the spontaneous loss of 2 or more clinically recognized pregnancies, affects less than 5% of women.3 Genetic testing using chromosomal microarray analysis (CMA) has identified aneuploidy in about 55% of cases of miscarriage.4
Following ASRM guidelines for the evaluation of RPL, which consists of analyzing parental chromosomal abnormalities, congenital and acquired uterine anomalies, endocrine imbalances, and autoimmune factors (including antiphospholipid syndrome), no explainable cause is determined in 50% of cases.3 Recently, it has been shown that more than 90% of patients with RPL will have a probable or definitive cause identified when CMA testing on miscarriage tissue with the ASRM evaluation guidelines.5
Details of the study
In this prospective cohort study from Denmark, the authors analyzed maternal serum for cell-free fetal DNA (cffDNA) to determine the ploidy status of the pregnancy loss. One thousand women older than age 18 were included (those who demonstrated an ultrasound-confirmed intrauterine pregnancy loss prior to 22 weeks’ gestation). Maternal blood was obtained while pregnancy tissue was in situ or within 24 hours of passage of products of conception (POC), then analyzed by genome-wide sequencing of cffDNA.
For the first 333 recruited women (validation phase), direct sequencing of the POC was performed for sensitivity and specificity. Following the elimination of inconclusive samples, 302 of the 333 cases demonstrated a sensitivity of 85% and specificity of 93%. In the subsequent evaluation of 667 women, researchers analyzed maternal serum from the gestational age of fetuses ranging from 35 days to 149 days.
Results. In total, nearly 90% of cases yielded conclusive results, with 50% euploid, 46% aneuploid, and 4% multiple aneuploidies. Earlier gestational ages (less than 7 weeks) had a no-call rate (ie, inconclusive) of approximately 50% (only based on 16 patients), with results typically obtained in maternal serum following passage of POC; in pregnancies at gestational ages past 7 weeks, the no-call rate was about 10%. In general, the longer the time after the pregnancy tissue passed, the higher likelihood of a no-call result.
Applying the technology of single-nucleotide polymorphism (SNP)-based CMA can improve identification of fetal and/or maternal sources as causes of pregnancy loss with accuracy, but it does require collection of POC. Of note, samples were deficient in this study, the authors cite, in one-third of the cases. Given this limitation of collection, the authors argue for use of the noninvasive method of cffDNA, obtained from maternal serum.
Study strengths and weaknesses
Several weaknesses of this study are highlighted. Of the validation cohort, one-third of pregnancy tissue could not be analyzed due to insufficient collection. Only 73% of cases allowed for DNA isolation from fetal tissue or chorionic villi; in 27% of cases samples were labeled “unknown tissue.” In those cases classified as unknown, 70% were further determined to be maternal. When all female and monosomy cases were excluded in an effort to assuredly reduce the risk of contamination with maternal DNA, sensitivity of the cffDNA testing process declined to 78%. Another limitation was the required short window for maternal blood sampling (within 24 hours) and its impact on the no-call rate.
The authors note an association with later-life morbidity in patients with a history of pregnancy loss and RPL (including cardiovascular disease, type 2 diabetes, and mental health disorders), thereby arguing for cffDNA-based testing versus no causal testing; however, no treatment has been proven to be effective at reducing pregnancy loss. ●
The best management course for unexplained RPL is uncertain. Despite its use for a euploid miscarriage or parental chromosomal structural rearrangement, in vitro fertilization with preimplantation genetic testing remains an unproven modality.6,7 Given that approximately 70% of human conceptions never achieve viability, and 50% fail spontaneously before being detected,8 the authors’ findings demonstrate peripheral maternal blood can provide a reasonably high sensitivity and specificity for fetal ploidy status when compared with direct sequencing of pregnancy tissue. As fetal aneuploidy offers a higher percentage of subsequent successful pregnancy outcomes, cffDNA may offer reassurance, or direct further testing, following a pregnancy loss. As an application of their results, evaluation may be deferred for an aneuploid miscarriage.
—MARK P. TROLICE, MD, MBA
Hartwig TJ, Ambye L, Gruhn JR, et al. Cell-free fetal DNA for genetic evaluation in Copenhagen Pregnancy Loss Study (COPL): a prospective cohort study. Lancet. 2023;401:762-771. https://doi.org/10.1016/S0140-6736(22)02610-1.
Expert Commentary
A devastating outcome for women, pregnancy loss is directly proportional to maternal age, estimated to occur in approximately 15% of clinically recognized pregnancies and 30% of preclinical pregnancies.1 Approximately 80% of pregnancy losses occur in the first trimester.2 The frequency of clinically recognized early pregnancy loss for women aged 20–30 years is 9% to 17%, and these rates increase sharply, from 20% at age 35 years to 40% at age 40 years, and 80% at age 45 years. Recurrent pregnancy loss (RPL), defined as the spontaneous loss of 2 or more clinically recognized pregnancies, affects less than 5% of women.3 Genetic testing using chromosomal microarray analysis (CMA) has identified aneuploidy in about 55% of cases of miscarriage.4
Following ASRM guidelines for the evaluation of RPL, which consists of analyzing parental chromosomal abnormalities, congenital and acquired uterine anomalies, endocrine imbalances, and autoimmune factors (including antiphospholipid syndrome), no explainable cause is determined in 50% of cases.3 Recently, it has been shown that more than 90% of patients with RPL will have a probable or definitive cause identified when CMA testing on miscarriage tissue with the ASRM evaluation guidelines.5
Details of the study
In this prospective cohort study from Denmark, the authors analyzed maternal serum for cell-free fetal DNA (cffDNA) to determine the ploidy status of the pregnancy loss. One thousand women older than age 18 were included (those who demonstrated an ultrasound-confirmed intrauterine pregnancy loss prior to 22 weeks’ gestation). Maternal blood was obtained while pregnancy tissue was in situ or within 24 hours of passage of products of conception (POC), then analyzed by genome-wide sequencing of cffDNA.
For the first 333 recruited women (validation phase), direct sequencing of the POC was performed for sensitivity and specificity. Following the elimination of inconclusive samples, 302 of the 333 cases demonstrated a sensitivity of 85% and specificity of 93%. In the subsequent evaluation of 667 women, researchers analyzed maternal serum from the gestational age of fetuses ranging from 35 days to 149 days.
Results. In total, nearly 90% of cases yielded conclusive results, with 50% euploid, 46% aneuploid, and 4% multiple aneuploidies. Earlier gestational ages (less than 7 weeks) had a no-call rate (ie, inconclusive) of approximately 50% (only based on 16 patients), with results typically obtained in maternal serum following passage of POC; in pregnancies at gestational ages past 7 weeks, the no-call rate was about 10%. In general, the longer the time after the pregnancy tissue passed, the higher likelihood of a no-call result.
Applying the technology of single-nucleotide polymorphism (SNP)-based CMA can improve identification of fetal and/or maternal sources as causes of pregnancy loss with accuracy, but it does require collection of POC. Of note, samples were deficient in this study, the authors cite, in one-third of the cases. Given this limitation of collection, the authors argue for use of the noninvasive method of cffDNA, obtained from maternal serum.
Study strengths and weaknesses
Several weaknesses of this study are highlighted. Of the validation cohort, one-third of pregnancy tissue could not be analyzed due to insufficient collection. Only 73% of cases allowed for DNA isolation from fetal tissue or chorionic villi; in 27% of cases samples were labeled “unknown tissue.” In those cases classified as unknown, 70% were further determined to be maternal. When all female and monosomy cases were excluded in an effort to assuredly reduce the risk of contamination with maternal DNA, sensitivity of the cffDNA testing process declined to 78%. Another limitation was the required short window for maternal blood sampling (within 24 hours) and its impact on the no-call rate.
The authors note an association with later-life morbidity in patients with a history of pregnancy loss and RPL (including cardiovascular disease, type 2 diabetes, and mental health disorders), thereby arguing for cffDNA-based testing versus no causal testing; however, no treatment has been proven to be effective at reducing pregnancy loss. ●
The best management course for unexplained RPL is uncertain. Despite its use for a euploid miscarriage or parental chromosomal structural rearrangement, in vitro fertilization with preimplantation genetic testing remains an unproven modality.6,7 Given that approximately 70% of human conceptions never achieve viability, and 50% fail spontaneously before being detected,8 the authors’ findings demonstrate peripheral maternal blood can provide a reasonably high sensitivity and specificity for fetal ploidy status when compared with direct sequencing of pregnancy tissue. As fetal aneuploidy offers a higher percentage of subsequent successful pregnancy outcomes, cffDNA may offer reassurance, or direct further testing, following a pregnancy loss. As an application of their results, evaluation may be deferred for an aneuploid miscarriage.
—MARK P. TROLICE, MD, MBA
- Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391-400. doi: 10.1055/s-0028-1087105.
- Wang X, Chen C , Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577-584.
- Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;98: 1103-1111.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/tacg.s320778.
- Popescu F, Jaslow FC, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579-587. https://doi.org/10.1093/humrep/dey021.
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. https://doi.org/10.1093/humrep/deab194.
- Iews M, Tan J, Taskin O, et al. Does preimplantation genetic diagnosis improve reproductive outcome in couples with recurrent pregnancy loss owing to structural chromosomal rearrangement? A systematic review. Reproductive Bio Medicine Online. 2018;36:677-685. https://doi.org/10.1016 /j.rbmo.2018.03.005.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/TACG.S320778.
- Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391-400. doi: 10.1055/s-0028-1087105.
- Wang X, Chen C , Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577-584.
- Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;98: 1103-1111.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/tacg.s320778.
- Popescu F, Jaslow FC, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579-587. https://doi.org/10.1093/humrep/dey021.
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. https://doi.org/10.1093/humrep/deab194.
- Iews M, Tan J, Taskin O, et al. Does preimplantation genetic diagnosis improve reproductive outcome in couples with recurrent pregnancy loss owing to structural chromosomal rearrangement? A systematic review. Reproductive Bio Medicine Online. 2018;36:677-685. https://doi.org/10.1016 /j.rbmo.2018.03.005.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/TACG.S320778.
Is there still a role for tubal surgery in the modern world of IVF?
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
Advances in fertility preservation: Q & A
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.