User login
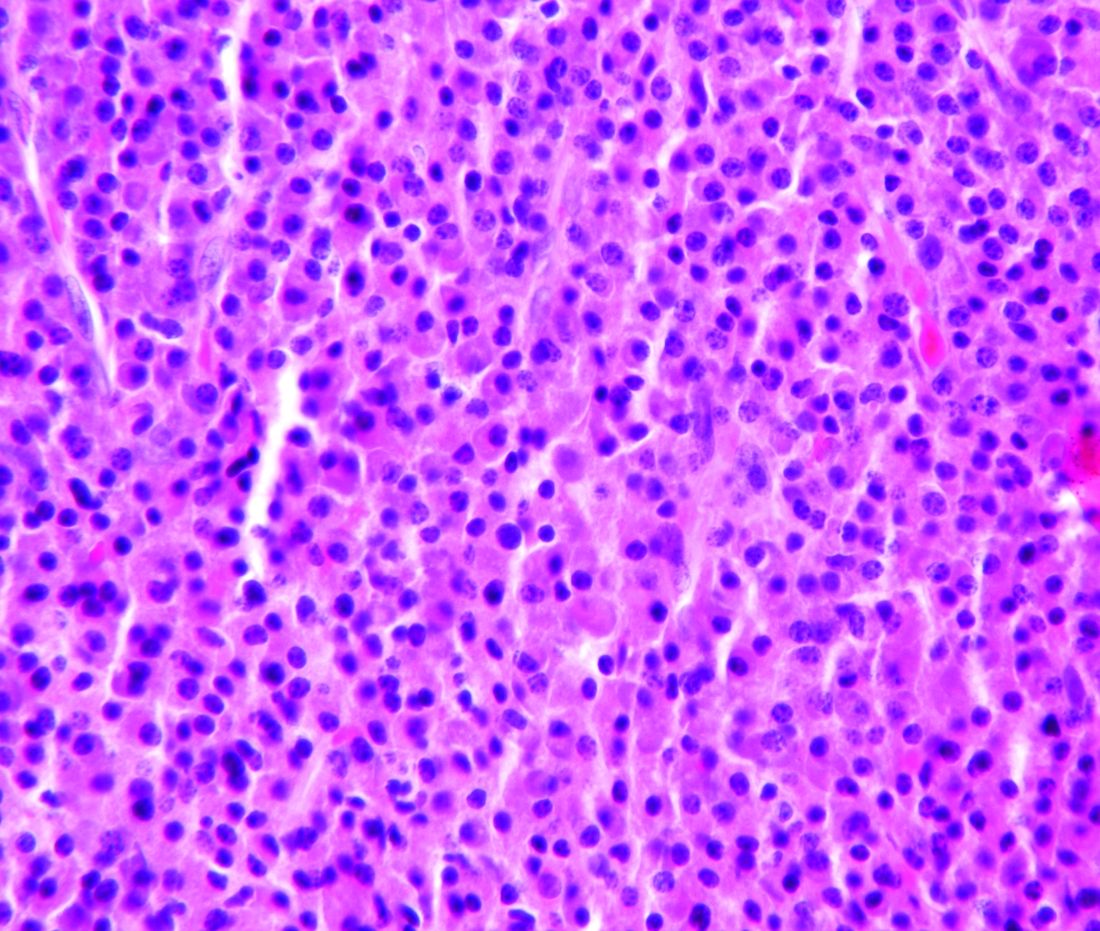
Combo therapy with melflufen promising in small r/r multiple myeloma study
Small phase 1 and phase 2 multi-center, open label studies of melflufen toxicity and efficacy in combination with dexamethasone showed significant benefits in overall response rate in patients with relapsed/refractory multiple myeloma. A report on these studies was published in Lancet Hematology.
The studies enrolled patients aged 18 years and older with relapsed/refractory multiple myeloma (MM), who had received two or more previous lines of therapy and were refractory to their last line of therapy. All patients had an Eastern Cooperative Oncology Group performance status of 2 or less.
Phase 1 was a dose-tolerance study of 23 patients, which found that the maximum tolerated dose tested of intravenous infusion of melflufen was 40 mg for 30 min on day 1 in 21-day cycles plus oral dexamethasone at 40 mg weekly.
In phase 2, 45 patients were tested at the maximum tolerated dose (40 mg) identified in phase 1 in combination with dexamethasone and 13 patients were treated with melflufen as a single agent (ClinicalTrials.gov, NCT01897714). The single-agent arm of the study was discontinued for ethical reasons once evidence became available of the benefit of combination therapy in comparison.
In phase 2, patients treated with combination therapy achieved an overall response rate of 31% (14/45 patients) and a clinical benefit rate of 49% (22/45 patients). In the phase 2 single-agent cohort, the overall response rate was 8% (1/13 patients) and the clinical benefit rate was 23% (3/13 patients) before this aspect of the study was discontinued.
In terms of adverse events, among the 45 phase 2 combination–treatment patients, the most common grade 3-4 adverse events were clinically manageable thrombocytopenia (62% of patients) and neutropenia (58%) In addition, 24 serious adverse events were reported in 38% patients, most commonly pneumonia (11%).
Because of the benefits seen and the need for relapsed/refractory MM treatments, further studies on melflufen in combination therapies are ongoing, according to the researchers “with encouraging early results to date”: NCT03151811, NCT02963493, and NCT03481556.
“Melflufen represents a novel treatment concept with a unique mechanism of action that might find future use in combinations with key partners, such as proteasome inhibitors and monoclonal antibodies,” the researchers concluded.
The study was sponsored by Oncopeptides AB. The authors received funding from a variety of pharmaceutical companies.
SOURCE: Richardson PG et al. Lancet Hematol. 2020; doi.org/10.1016/S2352-3026(20)30044-2.
Small phase 1 and phase 2 multi-center, open label studies of melflufen toxicity and efficacy in combination with dexamethasone showed significant benefits in overall response rate in patients with relapsed/refractory multiple myeloma. A report on these studies was published in Lancet Hematology.
The studies enrolled patients aged 18 years and older with relapsed/refractory multiple myeloma (MM), who had received two or more previous lines of therapy and were refractory to their last line of therapy. All patients had an Eastern Cooperative Oncology Group performance status of 2 or less.
Phase 1 was a dose-tolerance study of 23 patients, which found that the maximum tolerated dose tested of intravenous infusion of melflufen was 40 mg for 30 min on day 1 in 21-day cycles plus oral dexamethasone at 40 mg weekly.
In phase 2, 45 patients were tested at the maximum tolerated dose (40 mg) identified in phase 1 in combination with dexamethasone and 13 patients were treated with melflufen as a single agent (ClinicalTrials.gov, NCT01897714). The single-agent arm of the study was discontinued for ethical reasons once evidence became available of the benefit of combination therapy in comparison.
In phase 2, patients treated with combination therapy achieved an overall response rate of 31% (14/45 patients) and a clinical benefit rate of 49% (22/45 patients). In the phase 2 single-agent cohort, the overall response rate was 8% (1/13 patients) and the clinical benefit rate was 23% (3/13 patients) before this aspect of the study was discontinued.
In terms of adverse events, among the 45 phase 2 combination–treatment patients, the most common grade 3-4 adverse events were clinically manageable thrombocytopenia (62% of patients) and neutropenia (58%) In addition, 24 serious adverse events were reported in 38% patients, most commonly pneumonia (11%).
Because of the benefits seen and the need for relapsed/refractory MM treatments, further studies on melflufen in combination therapies are ongoing, according to the researchers “with encouraging early results to date”: NCT03151811, NCT02963493, and NCT03481556.
“Melflufen represents a novel treatment concept with a unique mechanism of action that might find future use in combinations with key partners, such as proteasome inhibitors and monoclonal antibodies,” the researchers concluded.
The study was sponsored by Oncopeptides AB. The authors received funding from a variety of pharmaceutical companies.
SOURCE: Richardson PG et al. Lancet Hematol. 2020; doi.org/10.1016/S2352-3026(20)30044-2.
Small phase 1 and phase 2 multi-center, open label studies of melflufen toxicity and efficacy in combination with dexamethasone showed significant benefits in overall response rate in patients with relapsed/refractory multiple myeloma. A report on these studies was published in Lancet Hematology.
The studies enrolled patients aged 18 years and older with relapsed/refractory multiple myeloma (MM), who had received two or more previous lines of therapy and were refractory to their last line of therapy. All patients had an Eastern Cooperative Oncology Group performance status of 2 or less.
Phase 1 was a dose-tolerance study of 23 patients, which found that the maximum tolerated dose tested of intravenous infusion of melflufen was 40 mg for 30 min on day 1 in 21-day cycles plus oral dexamethasone at 40 mg weekly.
In phase 2, 45 patients were tested at the maximum tolerated dose (40 mg) identified in phase 1 in combination with dexamethasone and 13 patients were treated with melflufen as a single agent (ClinicalTrials.gov, NCT01897714). The single-agent arm of the study was discontinued for ethical reasons once evidence became available of the benefit of combination therapy in comparison.
In phase 2, patients treated with combination therapy achieved an overall response rate of 31% (14/45 patients) and a clinical benefit rate of 49% (22/45 patients). In the phase 2 single-agent cohort, the overall response rate was 8% (1/13 patients) and the clinical benefit rate was 23% (3/13 patients) before this aspect of the study was discontinued.
In terms of adverse events, among the 45 phase 2 combination–treatment patients, the most common grade 3-4 adverse events were clinically manageable thrombocytopenia (62% of patients) and neutropenia (58%) In addition, 24 serious adverse events were reported in 38% patients, most commonly pneumonia (11%).
Because of the benefits seen and the need for relapsed/refractory MM treatments, further studies on melflufen in combination therapies are ongoing, according to the researchers “with encouraging early results to date”: NCT03151811, NCT02963493, and NCT03481556.
“Melflufen represents a novel treatment concept with a unique mechanism of action that might find future use in combinations with key partners, such as proteasome inhibitors and monoclonal antibodies,” the researchers concluded.
The study was sponsored by Oncopeptides AB. The authors received funding from a variety of pharmaceutical companies.
SOURCE: Richardson PG et al. Lancet Hematol. 2020; doi.org/10.1016/S2352-3026(20)30044-2.
FROM THE LANCET HEMATOLOGY
REACH2: Ruxolitinib outperformed control treatment for refractory acute GVHD
Ruxolitinib produced significantly better efficacy outcomes in patients with glucocorticoid-refractory acute graft-versus-host disease (GVHD), compared with investigator’s choice of control therapy, in the phase 3 REACH2 trial.
However, there was a higher incidence of thrombocytopenia with ruxolitinib than with control treatment, according to a report by Robert Zeiser, MD, of University of Freiburg (Germany) and colleagues on behalf of the REACH2 research group. The report was published in the New England Journal of Medicine.
The REACH2 trial (NCT02913261) is a randomized, open-label, phase 3 trial comparing the efficacy and safety of oral ruxolitinib (10 mg twice daily) with investigator’s choice of therapy for control treatment using a list of nine commonly used options.
Patients were 12 years of age or older with glucocorticoid-refractory acute GVHD after allogeneic stem cell transplant. A total of 154 patients were assigned to the ruxolitinib group, and 155 patients were in the control group.
Most patients – 152 in the ruxolitinib group and 150 in the control group – received at least one dose of trial treatment.
Treatment discontinuation occurred in 72% (111/154) of patients in the ruxolitinib group and in 85% (132/155) of those in the control group. The most common reason for discontinuation was lack of efficacy (in 21% and 44%, respectively).
Outcomes
The overall response at day 28 (the primary endpoint) was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; odds ratio, 2.64; P < .001). The durable overall response at day 56 was also significantly higher in the ruxolitinib group than in the control group (40% vs. 22%; OR, 2.38; P < .001).
The estimated cumulative incidence of loss of response at 6 months was 10% in the ruxolitinib group compared with 39% in the control group.
The median failure-free survival was considerably longer with ruxolitinib than with control treatment (5.0 months vs. 1.0 month; hazard ratio for relapse or progression of hematologic disease, non–relapse-related death, or the use of new systemic therapy for acute GVHD, 0.46).
The median overall survival was 11.1 months in the ruxolitinib group and 6.5 months in the control group (HR, 0.83).
Overall, 72 patients (47%) in the ruxolitinib group and 77 (51%) in the control group died by the data cutoff date. Most deaths were attributed to acute GVHD (22% in the ruxolitinib group and 25% in the control group).
The most common adverse events at day 28 (in the ruxolitinib and control arms, respectively) were thrombocytopenia (33% and 18%), anemia (30% and 28%), and cytomegalovirus infection (26% and 21%).
Praise for ‘successful’ randomized trial in GVHD
“The authors are to be congratulated for completing this successful randomized trial, which showed convincingly that ruxolitinib was more effective than the investigator’s choice of therapy ... in patients in whom glucocorticoid therapy had failed,” wrote Nelson Chao, MD, of Duke University in Durham, N.C., in his invited editorial.
He went on to speculate on the possible mechanism for ruxolitinib in these patients, discussing the possible role of the STAT3 and STAT1 signaling pathways.
Dr. Chao also found it “interesting that the incidence of infectious complications or relapse was apparently not greater with ruxolitinib than with control therapy,” but he noted that the total follow-up time was short.
“As with all good research, these observations raise important questions and set the stage for further work in this area,” he concluded.
The REACH2 trial was funded by Novartis. The study authors disclosed relationships with a variety of pharmaceutical companies, including Novartis. Dr. Chao reported having no relevant disclosures.
SOURCE: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
Ruxolitinib produced significantly better efficacy outcomes in patients with glucocorticoid-refractory acute graft-versus-host disease (GVHD), compared with investigator’s choice of control therapy, in the phase 3 REACH2 trial.
However, there was a higher incidence of thrombocytopenia with ruxolitinib than with control treatment, according to a report by Robert Zeiser, MD, of University of Freiburg (Germany) and colleagues on behalf of the REACH2 research group. The report was published in the New England Journal of Medicine.
The REACH2 trial (NCT02913261) is a randomized, open-label, phase 3 trial comparing the efficacy and safety of oral ruxolitinib (10 mg twice daily) with investigator’s choice of therapy for control treatment using a list of nine commonly used options.
Patients were 12 years of age or older with glucocorticoid-refractory acute GVHD after allogeneic stem cell transplant. A total of 154 patients were assigned to the ruxolitinib group, and 155 patients were in the control group.
Most patients – 152 in the ruxolitinib group and 150 in the control group – received at least one dose of trial treatment.
Treatment discontinuation occurred in 72% (111/154) of patients in the ruxolitinib group and in 85% (132/155) of those in the control group. The most common reason for discontinuation was lack of efficacy (in 21% and 44%, respectively).
Outcomes
The overall response at day 28 (the primary endpoint) was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; odds ratio, 2.64; P < .001). The durable overall response at day 56 was also significantly higher in the ruxolitinib group than in the control group (40% vs. 22%; OR, 2.38; P < .001).
The estimated cumulative incidence of loss of response at 6 months was 10% in the ruxolitinib group compared with 39% in the control group.
The median failure-free survival was considerably longer with ruxolitinib than with control treatment (5.0 months vs. 1.0 month; hazard ratio for relapse or progression of hematologic disease, non–relapse-related death, or the use of new systemic therapy for acute GVHD, 0.46).
The median overall survival was 11.1 months in the ruxolitinib group and 6.5 months in the control group (HR, 0.83).
Overall, 72 patients (47%) in the ruxolitinib group and 77 (51%) in the control group died by the data cutoff date. Most deaths were attributed to acute GVHD (22% in the ruxolitinib group and 25% in the control group).
The most common adverse events at day 28 (in the ruxolitinib and control arms, respectively) were thrombocytopenia (33% and 18%), anemia (30% and 28%), and cytomegalovirus infection (26% and 21%).
Praise for ‘successful’ randomized trial in GVHD
“The authors are to be congratulated for completing this successful randomized trial, which showed convincingly that ruxolitinib was more effective than the investigator’s choice of therapy ... in patients in whom glucocorticoid therapy had failed,” wrote Nelson Chao, MD, of Duke University in Durham, N.C., in his invited editorial.
He went on to speculate on the possible mechanism for ruxolitinib in these patients, discussing the possible role of the STAT3 and STAT1 signaling pathways.
Dr. Chao also found it “interesting that the incidence of infectious complications or relapse was apparently not greater with ruxolitinib than with control therapy,” but he noted that the total follow-up time was short.
“As with all good research, these observations raise important questions and set the stage for further work in this area,” he concluded.
The REACH2 trial was funded by Novartis. The study authors disclosed relationships with a variety of pharmaceutical companies, including Novartis. Dr. Chao reported having no relevant disclosures.
SOURCE: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
Ruxolitinib produced significantly better efficacy outcomes in patients with glucocorticoid-refractory acute graft-versus-host disease (GVHD), compared with investigator’s choice of control therapy, in the phase 3 REACH2 trial.
However, there was a higher incidence of thrombocytopenia with ruxolitinib than with control treatment, according to a report by Robert Zeiser, MD, of University of Freiburg (Germany) and colleagues on behalf of the REACH2 research group. The report was published in the New England Journal of Medicine.
The REACH2 trial (NCT02913261) is a randomized, open-label, phase 3 trial comparing the efficacy and safety of oral ruxolitinib (10 mg twice daily) with investigator’s choice of therapy for control treatment using a list of nine commonly used options.
Patients were 12 years of age or older with glucocorticoid-refractory acute GVHD after allogeneic stem cell transplant. A total of 154 patients were assigned to the ruxolitinib group, and 155 patients were in the control group.
Most patients – 152 in the ruxolitinib group and 150 in the control group – received at least one dose of trial treatment.
Treatment discontinuation occurred in 72% (111/154) of patients in the ruxolitinib group and in 85% (132/155) of those in the control group. The most common reason for discontinuation was lack of efficacy (in 21% and 44%, respectively).
Outcomes
The overall response at day 28 (the primary endpoint) was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; odds ratio, 2.64; P < .001). The durable overall response at day 56 was also significantly higher in the ruxolitinib group than in the control group (40% vs. 22%; OR, 2.38; P < .001).
The estimated cumulative incidence of loss of response at 6 months was 10% in the ruxolitinib group compared with 39% in the control group.
The median failure-free survival was considerably longer with ruxolitinib than with control treatment (5.0 months vs. 1.0 month; hazard ratio for relapse or progression of hematologic disease, non–relapse-related death, or the use of new systemic therapy for acute GVHD, 0.46).
The median overall survival was 11.1 months in the ruxolitinib group and 6.5 months in the control group (HR, 0.83).
Overall, 72 patients (47%) in the ruxolitinib group and 77 (51%) in the control group died by the data cutoff date. Most deaths were attributed to acute GVHD (22% in the ruxolitinib group and 25% in the control group).
The most common adverse events at day 28 (in the ruxolitinib and control arms, respectively) were thrombocytopenia (33% and 18%), anemia (30% and 28%), and cytomegalovirus infection (26% and 21%).
Praise for ‘successful’ randomized trial in GVHD
“The authors are to be congratulated for completing this successful randomized trial, which showed convincingly that ruxolitinib was more effective than the investigator’s choice of therapy ... in patients in whom glucocorticoid therapy had failed,” wrote Nelson Chao, MD, of Duke University in Durham, N.C., in his invited editorial.
He went on to speculate on the possible mechanism for ruxolitinib in these patients, discussing the possible role of the STAT3 and STAT1 signaling pathways.
Dr. Chao also found it “interesting that the incidence of infectious complications or relapse was apparently not greater with ruxolitinib than with control therapy,” but he noted that the total follow-up time was short.
“As with all good research, these observations raise important questions and set the stage for further work in this area,” he concluded.
The REACH2 trial was funded by Novartis. The study authors disclosed relationships with a variety of pharmaceutical companies, including Novartis. Dr. Chao reported having no relevant disclosures.
SOURCE: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Ruxolitinib was significantly more effective against acute graft-versus-host disease than was control treatment.
Major finding: The overall response at day 28 was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; P < .001).
Study details: Phase 3 trial of 154 patients randomized to ruxolitinib and 155 patients randomized to investigator’s choice of control therapy.
Disclosures: The trial was funded by Novartis. Authors disclosed relationships with a variety of pharmaceutical companies, including Novartis.
Source: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
Five prognostic indexes come up short for planning early CLL treatment
Prognostic indexes have been developed recently to assess time to first treatment in early-stage chronic lymphocytic leukemia (CLL) patients. However, none of five indexes evaluated in a study showed more than a moderate prognostic value or were precise enough to permit clinical decisions to be made, according to a report by Spanish researchers.
Their study, published in Clinical Lymphoma, Myeloma and Leukemia, examined the comparative prognostic value of five prognostic indexes – the CLL-IPI, the Barcelona-Brno, the IPS-A, the CLL-01, and the Tailored approach – on evaluating 428 Binet A CLL patients from a multicenter Spanish database which contained the relevant necessary clinical and biological information. The predictive value of the scores was assessed with Harrell´s C index and receiver operating characteristic curve (area under the curve, AUC).
The researchers found a significant association between time to first treatment and risk subgroups for all the indexes used. The most accurate index was the IPS-A (Harrell´s C, 0.72; AUC, 0.76), followed by the CLL-01 (Harrell´s C, 0.69; AUC, 0.70), the CLL-IPI (Harrell´s C, .69; AUC, 0.69), the Barcelona-Brno (Harrell´s C: 0.67, AUC, 0.69) and the Tailored approach (Harrell´s C, 0.61 and 0.58, AUCs, 0.58 and 0.54).
However, the concordance between four of the five indexes (the Tailored approach was not included for technical reasons) compared was low (44%): 146 cases were classified as low risk with all four indexes tested, 36 as intermediate risk, and 4 as high risk. In the remaining 242 patients (56%) at least one discrepancy was detected in the allocation among prognostic subgroups between the indexes. However, only 12 patients (3%) were allocated as low and high risk at the same time with different indexes, showing the extremes of the discordance.
These data suggest that, although all of these indexes “significantly improve clinical staging and help physicians in routine clinical practice, it is necessary to harmonize larger cohorts of patients in order to define the best index for treatment decision making in the real world,” the authors stated.
“All the models had a moderate prognostic value to predict time to first therapy. ... None of them was precise enough to allow clinical decisions based exclusively on it,” the authors concluded.
The study was supported by grants from the Spanish government and a variety of nonprofit institutions. The authors reported no commercial disclosures.
SOURCE: Gascon y Marín IG et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 13. doi: 10.1016/j.clml.2020.03.003.
Prognostic indexes have been developed recently to assess time to first treatment in early-stage chronic lymphocytic leukemia (CLL) patients. However, none of five indexes evaluated in a study showed more than a moderate prognostic value or were precise enough to permit clinical decisions to be made, according to a report by Spanish researchers.
Their study, published in Clinical Lymphoma, Myeloma and Leukemia, examined the comparative prognostic value of five prognostic indexes – the CLL-IPI, the Barcelona-Brno, the IPS-A, the CLL-01, and the Tailored approach – on evaluating 428 Binet A CLL patients from a multicenter Spanish database which contained the relevant necessary clinical and biological information. The predictive value of the scores was assessed with Harrell´s C index and receiver operating characteristic curve (area under the curve, AUC).
The researchers found a significant association between time to first treatment and risk subgroups for all the indexes used. The most accurate index was the IPS-A (Harrell´s C, 0.72; AUC, 0.76), followed by the CLL-01 (Harrell´s C, 0.69; AUC, 0.70), the CLL-IPI (Harrell´s C, .69; AUC, 0.69), the Barcelona-Brno (Harrell´s C: 0.67, AUC, 0.69) and the Tailored approach (Harrell´s C, 0.61 and 0.58, AUCs, 0.58 and 0.54).
However, the concordance between four of the five indexes (the Tailored approach was not included for technical reasons) compared was low (44%): 146 cases were classified as low risk with all four indexes tested, 36 as intermediate risk, and 4 as high risk. In the remaining 242 patients (56%) at least one discrepancy was detected in the allocation among prognostic subgroups between the indexes. However, only 12 patients (3%) were allocated as low and high risk at the same time with different indexes, showing the extremes of the discordance.
These data suggest that, although all of these indexes “significantly improve clinical staging and help physicians in routine clinical practice, it is necessary to harmonize larger cohorts of patients in order to define the best index for treatment decision making in the real world,” the authors stated.
“All the models had a moderate prognostic value to predict time to first therapy. ... None of them was precise enough to allow clinical decisions based exclusively on it,” the authors concluded.
The study was supported by grants from the Spanish government and a variety of nonprofit institutions. The authors reported no commercial disclosures.
SOURCE: Gascon y Marín IG et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 13. doi: 10.1016/j.clml.2020.03.003.
Prognostic indexes have been developed recently to assess time to first treatment in early-stage chronic lymphocytic leukemia (CLL) patients. However, none of five indexes evaluated in a study showed more than a moderate prognostic value or were precise enough to permit clinical decisions to be made, according to a report by Spanish researchers.
Their study, published in Clinical Lymphoma, Myeloma and Leukemia, examined the comparative prognostic value of five prognostic indexes – the CLL-IPI, the Barcelona-Brno, the IPS-A, the CLL-01, and the Tailored approach – on evaluating 428 Binet A CLL patients from a multicenter Spanish database which contained the relevant necessary clinical and biological information. The predictive value of the scores was assessed with Harrell´s C index and receiver operating characteristic curve (area under the curve, AUC).
The researchers found a significant association between time to first treatment and risk subgroups for all the indexes used. The most accurate index was the IPS-A (Harrell´s C, 0.72; AUC, 0.76), followed by the CLL-01 (Harrell´s C, 0.69; AUC, 0.70), the CLL-IPI (Harrell´s C, .69; AUC, 0.69), the Barcelona-Brno (Harrell´s C: 0.67, AUC, 0.69) and the Tailored approach (Harrell´s C, 0.61 and 0.58, AUCs, 0.58 and 0.54).
However, the concordance between four of the five indexes (the Tailored approach was not included for technical reasons) compared was low (44%): 146 cases were classified as low risk with all four indexes tested, 36 as intermediate risk, and 4 as high risk. In the remaining 242 patients (56%) at least one discrepancy was detected in the allocation among prognostic subgroups between the indexes. However, only 12 patients (3%) were allocated as low and high risk at the same time with different indexes, showing the extremes of the discordance.
These data suggest that, although all of these indexes “significantly improve clinical staging and help physicians in routine clinical practice, it is necessary to harmonize larger cohorts of patients in order to define the best index for treatment decision making in the real world,” the authors stated.
“All the models had a moderate prognostic value to predict time to first therapy. ... None of them was precise enough to allow clinical decisions based exclusively on it,” the authors concluded.
The study was supported by grants from the Spanish government and a variety of nonprofit institutions. The authors reported no commercial disclosures.
SOURCE: Gascon y Marín IG et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 13. doi: 10.1016/j.clml.2020.03.003.
FROM CLINICAL LYMPHOMA, MYELOMA AND LEUKEMIA
EHA and TIF explore how COVID-19 is affecting thalassemia and SCD patients
In a webinar designed to guide physicians in the care of hematology patients during the COVID-19 pandemic, three world experts on thalassemia and sickle cell disease (SCD) provided on-the-ground information from physicians who were dealing with the height of the crisis in their countries.
The webinar was organized by the European Hematology Association (EHA) and the Thalassemia International Federation (TIF).
Moderator Francesco Cerisoli, MD, head of research and mentoring at EHA, led the discussion with three guest speakers: Maria-Domenica Cappellini, MD, PhD, professor of hematology at the University of Milan; Androulla Eleftheriou, MD, executive director of TIF in Cyprus; and Raffaella Colombatti , MD, of the University of Padova in Italy, coordinator of the Red Cell Reserve Working Group of the Italian Association of Pediatric Hematology and Oncology.
Italian experience with thalassemia and COVID-19
Dr. Cappellini discussed the Italian experience with 11 thalassemia patients followed by a network survey who developed COVID-19 in the northern part of Italy, where the pandemic has been most widespread.
There are no published data focusing specifically on SARS-CoV-2 infection in patients with thalassemic syndromes, but patients with preexisting comorbidities are likely to be more severely affected by SARS-CoV-2, according to Dr. Cappellini.
Of particular concern is the fact that patients with thalassemia, especially older ones, are frequently splenectomized, which renders them more vulnerable to bacterial infections and can trigger life-threatening sepsis. However, splenectomy is not known to increase the risk of viral infection or severe viral illness. Of additional concern is the fact that many thalassemia patients need routine and frequent transfusions.
Overall, the 11 thalassemia patients who developed COVID-19 experienced only mild to moderate symptoms. This is despite the fact that 72% of the patients were splenectomized, which did not appear to affect the clinical course, and all of the patients had thalassemia-related comorbidities.
Around half of the patients were hospitalized, but none of them required transfer to the ICU. One patient who was treated with chemotherapy for diffuse large B-cell lymphoma in 2019 but is now in remission required more intense ventilation support with the use of continuous positive airway pressure.
Only three patients received specific treatment for COVID-19: one with hydroxychloroquine (HCQ) alone, one with HCQ plus anakinra, and one with HCQ plus ritonavir/darunavir.
Overall, “the number of infected thalassemia patients was lower than expected, likely due to earlier and more vigilant self-isolation compared to the general population,” Dr. Cappellini said. She pointed out that the first early response in February by thalassemia physicians was to warn their patients via email and phone calls about the need for self-isolation and precautions against the pandemic.
Physicians “rapidly reorganized activities, postponing nonessential ones” and managed to provide patients “a safe track at the hospital to receive their life-saving treatment in COVID-19–free areas with health care personnel wearing protective equipment” and assessment of all entering patients for COVID-19 infection, Dr. Cappellini said.
Results in additional thalassemia patients and SCD patients
Dr. Eleftheriou described 51 cases of thalassemia patients with SARS-CoV-2 infection reported to TIF as of April 16. Patients were from Cyprus, Italy, the United Kingdom, France, Turkey, Iran, Pakistan, and Indonesia.
Of the 51 patients, 46 presented with mild to moderate symptoms. Five patients had severe respiratory symptoms and required hospitalization, two were hospitalized and discharged, and three died between day 5 and day 15 post hospitalization.
Dr. Colombatti followed with a brief presentation of the intersection of COVID-19 with SCD patients. She presented anecdotal data involving 32 SCD patients who exhibited COVID-19 symptoms. Dr. Colombatti obtained the data via personal communication with Pablo Bartolucci, of Hôpitaux Universitaires Henri Mondor in Créteil, France.
All 32 SCD patients were screened and treated for COVID-19, and 17 of them continued treatment for 10 days. In all, 22 patients were hospitalized, 11 were transferred to the ICU, and 1 died.
Ensuring adequate blood supply
Dr. Eleftheriou also discussed the TIF response to the COVID-19 pandemic, which focused on the adequacy of blood supplies for these patients who so often need transfusions.
Dr. Eleftheriou stated that a shortage of blood was reported in 75% of the 62 member countries of the TIF, with 58% reporting severe shortages and 35% reporting moderate to severe shortages.
The shortages resulted in many countries returning to older family/friends donation practices, rare use of whole blood transfusions, and the use of older blood transfusions (older than 28 days).
In addition, physicians have modified their transfusion strategy. They have reduced the amount of blood given to thalassemia patients from two units to one unit during any transfusion, while making arrangements for more frequent transfusions; for example, one transfusion per week but with precautions made to “limit the time spent in the clinic and to control blood supplies while safeguarding that all [thalassemia] patients will be able to get their transfusion,” Dr. Eleftheriou said.
The information in the webinar was provided with the caveat that “no general evidence-based guidance can be derived from this discussion.” There were no other disclosures given.
In a webinar designed to guide physicians in the care of hematology patients during the COVID-19 pandemic, three world experts on thalassemia and sickle cell disease (SCD) provided on-the-ground information from physicians who were dealing with the height of the crisis in their countries.
The webinar was organized by the European Hematology Association (EHA) and the Thalassemia International Federation (TIF).
Moderator Francesco Cerisoli, MD, head of research and mentoring at EHA, led the discussion with three guest speakers: Maria-Domenica Cappellini, MD, PhD, professor of hematology at the University of Milan; Androulla Eleftheriou, MD, executive director of TIF in Cyprus; and Raffaella Colombatti , MD, of the University of Padova in Italy, coordinator of the Red Cell Reserve Working Group of the Italian Association of Pediatric Hematology and Oncology.
Italian experience with thalassemia and COVID-19
Dr. Cappellini discussed the Italian experience with 11 thalassemia patients followed by a network survey who developed COVID-19 in the northern part of Italy, where the pandemic has been most widespread.
There are no published data focusing specifically on SARS-CoV-2 infection in patients with thalassemic syndromes, but patients with preexisting comorbidities are likely to be more severely affected by SARS-CoV-2, according to Dr. Cappellini.
Of particular concern is the fact that patients with thalassemia, especially older ones, are frequently splenectomized, which renders them more vulnerable to bacterial infections and can trigger life-threatening sepsis. However, splenectomy is not known to increase the risk of viral infection or severe viral illness. Of additional concern is the fact that many thalassemia patients need routine and frequent transfusions.
Overall, the 11 thalassemia patients who developed COVID-19 experienced only mild to moderate symptoms. This is despite the fact that 72% of the patients were splenectomized, which did not appear to affect the clinical course, and all of the patients had thalassemia-related comorbidities.
Around half of the patients were hospitalized, but none of them required transfer to the ICU. One patient who was treated with chemotherapy for diffuse large B-cell lymphoma in 2019 but is now in remission required more intense ventilation support with the use of continuous positive airway pressure.
Only three patients received specific treatment for COVID-19: one with hydroxychloroquine (HCQ) alone, one with HCQ plus anakinra, and one with HCQ plus ritonavir/darunavir.
Overall, “the number of infected thalassemia patients was lower than expected, likely due to earlier and more vigilant self-isolation compared to the general population,” Dr. Cappellini said. She pointed out that the first early response in February by thalassemia physicians was to warn their patients via email and phone calls about the need for self-isolation and precautions against the pandemic.
Physicians “rapidly reorganized activities, postponing nonessential ones” and managed to provide patients “a safe track at the hospital to receive their life-saving treatment in COVID-19–free areas with health care personnel wearing protective equipment” and assessment of all entering patients for COVID-19 infection, Dr. Cappellini said.
Results in additional thalassemia patients and SCD patients
Dr. Eleftheriou described 51 cases of thalassemia patients with SARS-CoV-2 infection reported to TIF as of April 16. Patients were from Cyprus, Italy, the United Kingdom, France, Turkey, Iran, Pakistan, and Indonesia.
Of the 51 patients, 46 presented with mild to moderate symptoms. Five patients had severe respiratory symptoms and required hospitalization, two were hospitalized and discharged, and three died between day 5 and day 15 post hospitalization.
Dr. Colombatti followed with a brief presentation of the intersection of COVID-19 with SCD patients. She presented anecdotal data involving 32 SCD patients who exhibited COVID-19 symptoms. Dr. Colombatti obtained the data via personal communication with Pablo Bartolucci, of Hôpitaux Universitaires Henri Mondor in Créteil, France.
All 32 SCD patients were screened and treated for COVID-19, and 17 of them continued treatment for 10 days. In all, 22 patients were hospitalized, 11 were transferred to the ICU, and 1 died.
Ensuring adequate blood supply
Dr. Eleftheriou also discussed the TIF response to the COVID-19 pandemic, which focused on the adequacy of blood supplies for these patients who so often need transfusions.
Dr. Eleftheriou stated that a shortage of blood was reported in 75% of the 62 member countries of the TIF, with 58% reporting severe shortages and 35% reporting moderate to severe shortages.
The shortages resulted in many countries returning to older family/friends donation practices, rare use of whole blood transfusions, and the use of older blood transfusions (older than 28 days).
In addition, physicians have modified their transfusion strategy. They have reduced the amount of blood given to thalassemia patients from two units to one unit during any transfusion, while making arrangements for more frequent transfusions; for example, one transfusion per week but with precautions made to “limit the time spent in the clinic and to control blood supplies while safeguarding that all [thalassemia] patients will be able to get their transfusion,” Dr. Eleftheriou said.
The information in the webinar was provided with the caveat that “no general evidence-based guidance can be derived from this discussion.” There were no other disclosures given.
In a webinar designed to guide physicians in the care of hematology patients during the COVID-19 pandemic, three world experts on thalassemia and sickle cell disease (SCD) provided on-the-ground information from physicians who were dealing with the height of the crisis in their countries.
The webinar was organized by the European Hematology Association (EHA) and the Thalassemia International Federation (TIF).
Moderator Francesco Cerisoli, MD, head of research and mentoring at EHA, led the discussion with three guest speakers: Maria-Domenica Cappellini, MD, PhD, professor of hematology at the University of Milan; Androulla Eleftheriou, MD, executive director of TIF in Cyprus; and Raffaella Colombatti , MD, of the University of Padova in Italy, coordinator of the Red Cell Reserve Working Group of the Italian Association of Pediatric Hematology and Oncology.
Italian experience with thalassemia and COVID-19
Dr. Cappellini discussed the Italian experience with 11 thalassemia patients followed by a network survey who developed COVID-19 in the northern part of Italy, where the pandemic has been most widespread.
There are no published data focusing specifically on SARS-CoV-2 infection in patients with thalassemic syndromes, but patients with preexisting comorbidities are likely to be more severely affected by SARS-CoV-2, according to Dr. Cappellini.
Of particular concern is the fact that patients with thalassemia, especially older ones, are frequently splenectomized, which renders them more vulnerable to bacterial infections and can trigger life-threatening sepsis. However, splenectomy is not known to increase the risk of viral infection or severe viral illness. Of additional concern is the fact that many thalassemia patients need routine and frequent transfusions.
Overall, the 11 thalassemia patients who developed COVID-19 experienced only mild to moderate symptoms. This is despite the fact that 72% of the patients were splenectomized, which did not appear to affect the clinical course, and all of the patients had thalassemia-related comorbidities.
Around half of the patients were hospitalized, but none of them required transfer to the ICU. One patient who was treated with chemotherapy for diffuse large B-cell lymphoma in 2019 but is now in remission required more intense ventilation support with the use of continuous positive airway pressure.
Only three patients received specific treatment for COVID-19: one with hydroxychloroquine (HCQ) alone, one with HCQ plus anakinra, and one with HCQ plus ritonavir/darunavir.
Overall, “the number of infected thalassemia patients was lower than expected, likely due to earlier and more vigilant self-isolation compared to the general population,” Dr. Cappellini said. She pointed out that the first early response in February by thalassemia physicians was to warn their patients via email and phone calls about the need for self-isolation and precautions against the pandemic.
Physicians “rapidly reorganized activities, postponing nonessential ones” and managed to provide patients “a safe track at the hospital to receive their life-saving treatment in COVID-19–free areas with health care personnel wearing protective equipment” and assessment of all entering patients for COVID-19 infection, Dr. Cappellini said.
Results in additional thalassemia patients and SCD patients
Dr. Eleftheriou described 51 cases of thalassemia patients with SARS-CoV-2 infection reported to TIF as of April 16. Patients were from Cyprus, Italy, the United Kingdom, France, Turkey, Iran, Pakistan, and Indonesia.
Of the 51 patients, 46 presented with mild to moderate symptoms. Five patients had severe respiratory symptoms and required hospitalization, two were hospitalized and discharged, and three died between day 5 and day 15 post hospitalization.
Dr. Colombatti followed with a brief presentation of the intersection of COVID-19 with SCD patients. She presented anecdotal data involving 32 SCD patients who exhibited COVID-19 symptoms. Dr. Colombatti obtained the data via personal communication with Pablo Bartolucci, of Hôpitaux Universitaires Henri Mondor in Créteil, France.
All 32 SCD patients were screened and treated for COVID-19, and 17 of them continued treatment for 10 days. In all, 22 patients were hospitalized, 11 were transferred to the ICU, and 1 died.
Ensuring adequate blood supply
Dr. Eleftheriou also discussed the TIF response to the COVID-19 pandemic, which focused on the adequacy of blood supplies for these patients who so often need transfusions.
Dr. Eleftheriou stated that a shortage of blood was reported in 75% of the 62 member countries of the TIF, with 58% reporting severe shortages and 35% reporting moderate to severe shortages.
The shortages resulted in many countries returning to older family/friends donation practices, rare use of whole blood transfusions, and the use of older blood transfusions (older than 28 days).
In addition, physicians have modified their transfusion strategy. They have reduced the amount of blood given to thalassemia patients from two units to one unit during any transfusion, while making arrangements for more frequent transfusions; for example, one transfusion per week but with precautions made to “limit the time spent in the clinic and to control blood supplies while safeguarding that all [thalassemia] patients will be able to get their transfusion,” Dr. Eleftheriou said.
The information in the webinar was provided with the caveat that “no general evidence-based guidance can be derived from this discussion.” There were no other disclosures given.
CLAM trial regimen shown safe, effective for r/r AML
A clofarabine-based treatment was found to be safe and effective in refractory/relapsed acute myeloid leukemia (AML) in the phase 2 CLAM trial.
The CLAM protocol treatment was clofarabine, cytarabine, and mitoxantrone (intravenous infusion, days 1‐5), cytarabine (intravenous infusion starting 4 hours after clofarabine, days 1‐5), and mitoxantrone (intravenous infusion, days 3‐5).
Bone marrow aspiration and trephine biopsy were performed on day 28. A total of 52 patients (16 women), with an age range of 22-65 years and refractory/relapsed AML were treated.
The overall response rate after the first cycle of CLAM was 90.4% (complete remission, 69.2%; CR with incomplete hematologic recovery, 21.2%). In addition, the efficacy of CLAM was not apparently affected by high‐risk karyotypes and genetic mutations among the patients.
Patients with a response (marrow < 5% blasts) received a maximum of two cycles of CLAM consolidation, each at 50% dose reduction, given 6‐8 weeks apart. Responding patients with an HLA‐matched sibling or volunteer‐unrelated donor were offered allogeneic hematopoietic stem cell transplantation (HSCT). Toxicity of CLAM was manageable and did not compromise subsequent allogeneic HSCT, the researchers added.
“In this era of molecular targeting, CLAM might still have a role to play,” according to the researchers. “It offers the advantage of a highly effective regimen that is readily available. It provides a median DOR of 5 months, which is meaningful for organization of HSCT. Delays associated with recruitment into clinical trials or sourcing of targeted drugs are obviated. Precious time is saved, so that patients can quickly be bridged to a potentially curative allogeneic HSCT.”
No disclosures or conflicts of interest were reported.
SOURCE: Gill H et al. Cancer Med. 2020 Mar 20. doi:10.1002/cam4.2865.
A clofarabine-based treatment was found to be safe and effective in refractory/relapsed acute myeloid leukemia (AML) in the phase 2 CLAM trial.
The CLAM protocol treatment was clofarabine, cytarabine, and mitoxantrone (intravenous infusion, days 1‐5), cytarabine (intravenous infusion starting 4 hours after clofarabine, days 1‐5), and mitoxantrone (intravenous infusion, days 3‐5).
Bone marrow aspiration and trephine biopsy were performed on day 28. A total of 52 patients (16 women), with an age range of 22-65 years and refractory/relapsed AML were treated.
The overall response rate after the first cycle of CLAM was 90.4% (complete remission, 69.2%; CR with incomplete hematologic recovery, 21.2%). In addition, the efficacy of CLAM was not apparently affected by high‐risk karyotypes and genetic mutations among the patients.
Patients with a response (marrow < 5% blasts) received a maximum of two cycles of CLAM consolidation, each at 50% dose reduction, given 6‐8 weeks apart. Responding patients with an HLA‐matched sibling or volunteer‐unrelated donor were offered allogeneic hematopoietic stem cell transplantation (HSCT). Toxicity of CLAM was manageable and did not compromise subsequent allogeneic HSCT, the researchers added.
“In this era of molecular targeting, CLAM might still have a role to play,” according to the researchers. “It offers the advantage of a highly effective regimen that is readily available. It provides a median DOR of 5 months, which is meaningful for organization of HSCT. Delays associated with recruitment into clinical trials or sourcing of targeted drugs are obviated. Precious time is saved, so that patients can quickly be bridged to a potentially curative allogeneic HSCT.”
No disclosures or conflicts of interest were reported.
SOURCE: Gill H et al. Cancer Med. 2020 Mar 20. doi:10.1002/cam4.2865.
A clofarabine-based treatment was found to be safe and effective in refractory/relapsed acute myeloid leukemia (AML) in the phase 2 CLAM trial.
The CLAM protocol treatment was clofarabine, cytarabine, and mitoxantrone (intravenous infusion, days 1‐5), cytarabine (intravenous infusion starting 4 hours after clofarabine, days 1‐5), and mitoxantrone (intravenous infusion, days 3‐5).
Bone marrow aspiration and trephine biopsy were performed on day 28. A total of 52 patients (16 women), with an age range of 22-65 years and refractory/relapsed AML were treated.
The overall response rate after the first cycle of CLAM was 90.4% (complete remission, 69.2%; CR with incomplete hematologic recovery, 21.2%). In addition, the efficacy of CLAM was not apparently affected by high‐risk karyotypes and genetic mutations among the patients.
Patients with a response (marrow < 5% blasts) received a maximum of two cycles of CLAM consolidation, each at 50% dose reduction, given 6‐8 weeks apart. Responding patients with an HLA‐matched sibling or volunteer‐unrelated donor were offered allogeneic hematopoietic stem cell transplantation (HSCT). Toxicity of CLAM was manageable and did not compromise subsequent allogeneic HSCT, the researchers added.
“In this era of molecular targeting, CLAM might still have a role to play,” according to the researchers. “It offers the advantage of a highly effective regimen that is readily available. It provides a median DOR of 5 months, which is meaningful for organization of HSCT. Delays associated with recruitment into clinical trials or sourcing of targeted drugs are obviated. Precious time is saved, so that patients can quickly be bridged to a potentially curative allogeneic HSCT.”
No disclosures or conflicts of interest were reported.
SOURCE: Gill H et al. Cancer Med. 2020 Mar 20. doi:10.1002/cam4.2865.
FROM CANCER MEDICINE
Should ART for HIV be initiated prior to tuberculosis testing results?
Tuberculosis symptoms as defined by the World Health Organization were effective in identifying patients with TB for the purposes of same-day antiretroviral therapy (ART) initiation in patients diagnosed with HIV, according to a pooled study of patients in two clinical trials. Guidelines suggest that patients with one or more TB symptoms be investigated for active TB before initiation of ART.
However, more than 80% of patients with TB symptoms did not have the disease and faced a delay of ART initiation, despite the many benefits of same-day ART initiation, according to the study presented online at the Conference on Retroviruses & Opportunistic Infections. This year CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In her presentation, Alana T. Brennan, PhD, of the Boston University School of Public Health discussed the pooled results of 834 patients in the SLATE (Simple Algorithm for Treatment Eligibility) I and SLATE II trials. These two trials, conducted in South Africa and Kenya, respectively, assessed two variations of a simplified algorithm for eligibility for same-day ART initiation.
A total of 834 patients at baseline reported any self-described symptoms of TB using the WHO four-symptom TB screen (cough, fever, weight loss, night sweats). Those patients with any TB symptoms were assessed by sputum samples. The outcomes were prevalence of TB symptoms, TB diagnosis, and treatment.
Among the 834 patients, 493 (60%) reported no symptoms; 215 (26%) reported one to two symptoms, and 120 (14%) reported three to four symptoms. Only 66% of the patients with one to two symptoms were tested for TB; 78% of the patients with three to four symptoms were tested. Of these, only 1% of the patients with one to two symptoms tested positive for TB, and only 2% of the patients with three to four symptoms tested positive, according to Dr. Brennan.
“More than 80% of patients with TB symptoms did not have TB, but faced delay in ART initiation. No same-day [ART] initiators reported adverse events, so we hope that there would be some reconsideration of the requirement of TB testing prior to ART initiation due to any symptom of TB. … A potential consideration of the severity of the symptoms a patient has is necessary,” Dr. Brennan concluded.
Dr. Brennan reported that there were no disclosures.
SOURCE: Brennan AT et al. CROI 2020, Abstract 720.
Tuberculosis symptoms as defined by the World Health Organization were effective in identifying patients with TB for the purposes of same-day antiretroviral therapy (ART) initiation in patients diagnosed with HIV, according to a pooled study of patients in two clinical trials. Guidelines suggest that patients with one or more TB symptoms be investigated for active TB before initiation of ART.
However, more than 80% of patients with TB symptoms did not have the disease and faced a delay of ART initiation, despite the many benefits of same-day ART initiation, according to the study presented online at the Conference on Retroviruses & Opportunistic Infections. This year CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In her presentation, Alana T. Brennan, PhD, of the Boston University School of Public Health discussed the pooled results of 834 patients in the SLATE (Simple Algorithm for Treatment Eligibility) I and SLATE II trials. These two trials, conducted in South Africa and Kenya, respectively, assessed two variations of a simplified algorithm for eligibility for same-day ART initiation.
A total of 834 patients at baseline reported any self-described symptoms of TB using the WHO four-symptom TB screen (cough, fever, weight loss, night sweats). Those patients with any TB symptoms were assessed by sputum samples. The outcomes were prevalence of TB symptoms, TB diagnosis, and treatment.
Among the 834 patients, 493 (60%) reported no symptoms; 215 (26%) reported one to two symptoms, and 120 (14%) reported three to four symptoms. Only 66% of the patients with one to two symptoms were tested for TB; 78% of the patients with three to four symptoms were tested. Of these, only 1% of the patients with one to two symptoms tested positive for TB, and only 2% of the patients with three to four symptoms tested positive, according to Dr. Brennan.
“More than 80% of patients with TB symptoms did not have TB, but faced delay in ART initiation. No same-day [ART] initiators reported adverse events, so we hope that there would be some reconsideration of the requirement of TB testing prior to ART initiation due to any symptom of TB. … A potential consideration of the severity of the symptoms a patient has is necessary,” Dr. Brennan concluded.
Dr. Brennan reported that there were no disclosures.
SOURCE: Brennan AT et al. CROI 2020, Abstract 720.
Tuberculosis symptoms as defined by the World Health Organization were effective in identifying patients with TB for the purposes of same-day antiretroviral therapy (ART) initiation in patients diagnosed with HIV, according to a pooled study of patients in two clinical trials. Guidelines suggest that patients with one or more TB symptoms be investigated for active TB before initiation of ART.
However, more than 80% of patients with TB symptoms did not have the disease and faced a delay of ART initiation, despite the many benefits of same-day ART initiation, according to the study presented online at the Conference on Retroviruses & Opportunistic Infections. This year CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In her presentation, Alana T. Brennan, PhD, of the Boston University School of Public Health discussed the pooled results of 834 patients in the SLATE (Simple Algorithm for Treatment Eligibility) I and SLATE II trials. These two trials, conducted in South Africa and Kenya, respectively, assessed two variations of a simplified algorithm for eligibility for same-day ART initiation.
A total of 834 patients at baseline reported any self-described symptoms of TB using the WHO four-symptom TB screen (cough, fever, weight loss, night sweats). Those patients with any TB symptoms were assessed by sputum samples. The outcomes were prevalence of TB symptoms, TB diagnosis, and treatment.
Among the 834 patients, 493 (60%) reported no symptoms; 215 (26%) reported one to two symptoms, and 120 (14%) reported three to four symptoms. Only 66% of the patients with one to two symptoms were tested for TB; 78% of the patients with three to four symptoms were tested. Of these, only 1% of the patients with one to two symptoms tested positive for TB, and only 2% of the patients with three to four symptoms tested positive, according to Dr. Brennan.
“More than 80% of patients with TB symptoms did not have TB, but faced delay in ART initiation. No same-day [ART] initiators reported adverse events, so we hope that there would be some reconsideration of the requirement of TB testing prior to ART initiation due to any symptom of TB. … A potential consideration of the severity of the symptoms a patient has is necessary,” Dr. Brennan concluded.
Dr. Brennan reported that there were no disclosures.
SOURCE: Brennan AT et al. CROI 2020, Abstract 720.
FROM CROI 2020
Severe COVID-19 may lower hemoglobin levels
A meta-analysis of four applicable studies found that the hemoglobin value was significantly lower in COVID-19 patients with severe disease, compared with those with milder forms, according to a letter to the editor of Hematology Transfusion and Cell Therapy by Giuseppe Lippi, MD, of the University of Verona (Italy) and colleague.
The four studies comprised 1,210 COVID-19 patients (224 with severe disease; 18.5%). The primary endpoint was defined as a composite of admission to the ICU, need of mechanical ventilation or death. The heterogeneity among the studies was high.
Overall, the hemoglobin value was found to be significantly lower in COVID-19 patients with severe disease than in those with milder forms, yielding a weighted mean difference of −7.1 g/L, with a 95% confidence interval of −8.3 g/L to −5.9 g/L.
“Initial assessment and longitudinal monitoring of hemoglobin values seems advisable in patients with the SARS-CoV-2 infection, whereby a progressive decrease in the hemoglobin concentration may reflect a worse clinical progression,” the authors stated. They also suggested that studies should be “urgently planned to assess whether transfusion support (e.g., with administration of blood or packed red blood cells) may be helpful in this clinical setting to prevent evolution into severe disease and death.”
The authors declared the had no conflicts of interest.
SOURCE: Lippi G et al. Hematol Transfus Cell Ther. 2020 Apr 11; doi:10.1016/j.htct.2020.03.001.
A meta-analysis of four applicable studies found that the hemoglobin value was significantly lower in COVID-19 patients with severe disease, compared with those with milder forms, according to a letter to the editor of Hematology Transfusion and Cell Therapy by Giuseppe Lippi, MD, of the University of Verona (Italy) and colleague.
The four studies comprised 1,210 COVID-19 patients (224 with severe disease; 18.5%). The primary endpoint was defined as a composite of admission to the ICU, need of mechanical ventilation or death. The heterogeneity among the studies was high.
Overall, the hemoglobin value was found to be significantly lower in COVID-19 patients with severe disease than in those with milder forms, yielding a weighted mean difference of −7.1 g/L, with a 95% confidence interval of −8.3 g/L to −5.9 g/L.
“Initial assessment and longitudinal monitoring of hemoglobin values seems advisable in patients with the SARS-CoV-2 infection, whereby a progressive decrease in the hemoglobin concentration may reflect a worse clinical progression,” the authors stated. They also suggested that studies should be “urgently planned to assess whether transfusion support (e.g., with administration of blood or packed red blood cells) may be helpful in this clinical setting to prevent evolution into severe disease and death.”
The authors declared the had no conflicts of interest.
SOURCE: Lippi G et al. Hematol Transfus Cell Ther. 2020 Apr 11; doi:10.1016/j.htct.2020.03.001.
A meta-analysis of four applicable studies found that the hemoglobin value was significantly lower in COVID-19 patients with severe disease, compared with those with milder forms, according to a letter to the editor of Hematology Transfusion and Cell Therapy by Giuseppe Lippi, MD, of the University of Verona (Italy) and colleague.
The four studies comprised 1,210 COVID-19 patients (224 with severe disease; 18.5%). The primary endpoint was defined as a composite of admission to the ICU, need of mechanical ventilation or death. The heterogeneity among the studies was high.
Overall, the hemoglobin value was found to be significantly lower in COVID-19 patients with severe disease than in those with milder forms, yielding a weighted mean difference of −7.1 g/L, with a 95% confidence interval of −8.3 g/L to −5.9 g/L.
“Initial assessment and longitudinal monitoring of hemoglobin values seems advisable in patients with the SARS-CoV-2 infection, whereby a progressive decrease in the hemoglobin concentration may reflect a worse clinical progression,” the authors stated. They also suggested that studies should be “urgently planned to assess whether transfusion support (e.g., with administration of blood or packed red blood cells) may be helpful in this clinical setting to prevent evolution into severe disease and death.”
The authors declared the had no conflicts of interest.
SOURCE: Lippi G et al. Hematol Transfus Cell Ther. 2020 Apr 11; doi:10.1016/j.htct.2020.03.001.
FROM HEMATOLOGY, TRANSFUSION AND CELL THERAPY
ASH tackles COVID-19 with hematology-related FAQ, promotes new registries
The American Society of Hematology has committed a portion of its website to providing continually updated information addressing specific hematologic disorders in relation to COVID-19.
“As the world grapples with the novel coronavirus, ASH believes that we can help each other be as knowledgeable and prepared as possible,” wrote the society’s president, Stephanie J. Lee, MD, MPH.
On its website, ASH provides relevant COVID-19 information in a series of FAQ divided into malignant and nonmalignant hematologic diseases and disorders. In the malignant category, the various lymphomas and leukemias are individually addressed, as well as other conditions such as myelodysplastic syndromes, myeloproliferative neoplasms, and multiple myeloma. In the nonmalignant category, ASH has provided FAQ on aplastic anemia, thalassemia, sickle cell disease, pulmonary embolism, venous thromboembolism/anticoagulation, coagulopathy, and immune as well as thrombotic thrombocytopenic purpura.
In addition to the continually updated series of relevant FAQ, as part of its response to the pandemic ASH is promoting two unique COVID-19 registries for physicians: the ASH Research Collaborative’s (ASH RC) Data Hub COVID-19 Registry and the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion Sickle Cell Disease (SECURE-SCD) Registry.
“The ASH Research Collaborative’s (ASH RC) Data Hub launched the COVID-19 Registry and is currently capturing data on people who test positive for COVID-19 and have been or are currently being treated for hematologic malignancy,” according to the website. The intention is to provide “near real-time observational data summaries,” which will hopefully provide useful information to clinicians treating hematologic malignancies in patients in the midst of the COVID-19 pandemic.
The registry allows clinicians to enter their own cases in a specified format to allow data analysis on clinical practice and patient outcomes that will be aggregated to provide rapid insights for clinicians to help them care for their patients, according to ASH.
The second registry specifically deals with COVID-19 cases in patients with sickle cell disease. It also allows clinicians to add cases with a similar intention of aggregating data to provide near real-time insights into patient care. “We are asking providers caring for these patients to report all of their cases of COVID-19 to this registry,” according to the registry website. The registry is for reporting COVID-19 cases in sickle cell disease patients “after sufficient time has passed to observe the disease course through resolution of acute illness and/or death.”
ASH also provides more generalized information for hematology practitioners dealing with COVID-19 on the topics of conducting their practice and using telemedicine, among others.
Correction, April 15, 2020: This story originally said incorrectly that ASH developed the 2 new registries. The registries are merely being promoted on the ASH website.
The American Society of Hematology has committed a portion of its website to providing continually updated information addressing specific hematologic disorders in relation to COVID-19.
“As the world grapples with the novel coronavirus, ASH believes that we can help each other be as knowledgeable and prepared as possible,” wrote the society’s president, Stephanie J. Lee, MD, MPH.
On its website, ASH provides relevant COVID-19 information in a series of FAQ divided into malignant and nonmalignant hematologic diseases and disorders. In the malignant category, the various lymphomas and leukemias are individually addressed, as well as other conditions such as myelodysplastic syndromes, myeloproliferative neoplasms, and multiple myeloma. In the nonmalignant category, ASH has provided FAQ on aplastic anemia, thalassemia, sickle cell disease, pulmonary embolism, venous thromboembolism/anticoagulation, coagulopathy, and immune as well as thrombotic thrombocytopenic purpura.
In addition to the continually updated series of relevant FAQ, as part of its response to the pandemic ASH is promoting two unique COVID-19 registries for physicians: the ASH Research Collaborative’s (ASH RC) Data Hub COVID-19 Registry and the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion Sickle Cell Disease (SECURE-SCD) Registry.
“The ASH Research Collaborative’s (ASH RC) Data Hub launched the COVID-19 Registry and is currently capturing data on people who test positive for COVID-19 and have been or are currently being treated for hematologic malignancy,” according to the website. The intention is to provide “near real-time observational data summaries,” which will hopefully provide useful information to clinicians treating hematologic malignancies in patients in the midst of the COVID-19 pandemic.
The registry allows clinicians to enter their own cases in a specified format to allow data analysis on clinical practice and patient outcomes that will be aggregated to provide rapid insights for clinicians to help them care for their patients, according to ASH.
The second registry specifically deals with COVID-19 cases in patients with sickle cell disease. It also allows clinicians to add cases with a similar intention of aggregating data to provide near real-time insights into patient care. “We are asking providers caring for these patients to report all of their cases of COVID-19 to this registry,” according to the registry website. The registry is for reporting COVID-19 cases in sickle cell disease patients “after sufficient time has passed to observe the disease course through resolution of acute illness and/or death.”
ASH also provides more generalized information for hematology practitioners dealing with COVID-19 on the topics of conducting their practice and using telemedicine, among others.
Correction, April 15, 2020: This story originally said incorrectly that ASH developed the 2 new registries. The registries are merely being promoted on the ASH website.
The American Society of Hematology has committed a portion of its website to providing continually updated information addressing specific hematologic disorders in relation to COVID-19.
“As the world grapples with the novel coronavirus, ASH believes that we can help each other be as knowledgeable and prepared as possible,” wrote the society’s president, Stephanie J. Lee, MD, MPH.
On its website, ASH provides relevant COVID-19 information in a series of FAQ divided into malignant and nonmalignant hematologic diseases and disorders. In the malignant category, the various lymphomas and leukemias are individually addressed, as well as other conditions such as myelodysplastic syndromes, myeloproliferative neoplasms, and multiple myeloma. In the nonmalignant category, ASH has provided FAQ on aplastic anemia, thalassemia, sickle cell disease, pulmonary embolism, venous thromboembolism/anticoagulation, coagulopathy, and immune as well as thrombotic thrombocytopenic purpura.
In addition to the continually updated series of relevant FAQ, as part of its response to the pandemic ASH is promoting two unique COVID-19 registries for physicians: the ASH Research Collaborative’s (ASH RC) Data Hub COVID-19 Registry and the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion Sickle Cell Disease (SECURE-SCD) Registry.
“The ASH Research Collaborative’s (ASH RC) Data Hub launched the COVID-19 Registry and is currently capturing data on people who test positive for COVID-19 and have been or are currently being treated for hematologic malignancy,” according to the website. The intention is to provide “near real-time observational data summaries,” which will hopefully provide useful information to clinicians treating hematologic malignancies in patients in the midst of the COVID-19 pandemic.
The registry allows clinicians to enter their own cases in a specified format to allow data analysis on clinical practice and patient outcomes that will be aggregated to provide rapid insights for clinicians to help them care for their patients, according to ASH.
The second registry specifically deals with COVID-19 cases in patients with sickle cell disease. It also allows clinicians to add cases with a similar intention of aggregating data to provide near real-time insights into patient care. “We are asking providers caring for these patients to report all of their cases of COVID-19 to this registry,” according to the registry website. The registry is for reporting COVID-19 cases in sickle cell disease patients “after sufficient time has passed to observe the disease course through resolution of acute illness and/or death.”
ASH also provides more generalized information for hematology practitioners dealing with COVID-19 on the topics of conducting their practice and using telemedicine, among others.
Correction, April 15, 2020: This story originally said incorrectly that ASH developed the 2 new registries. The registries are merely being promoted on the ASH website.
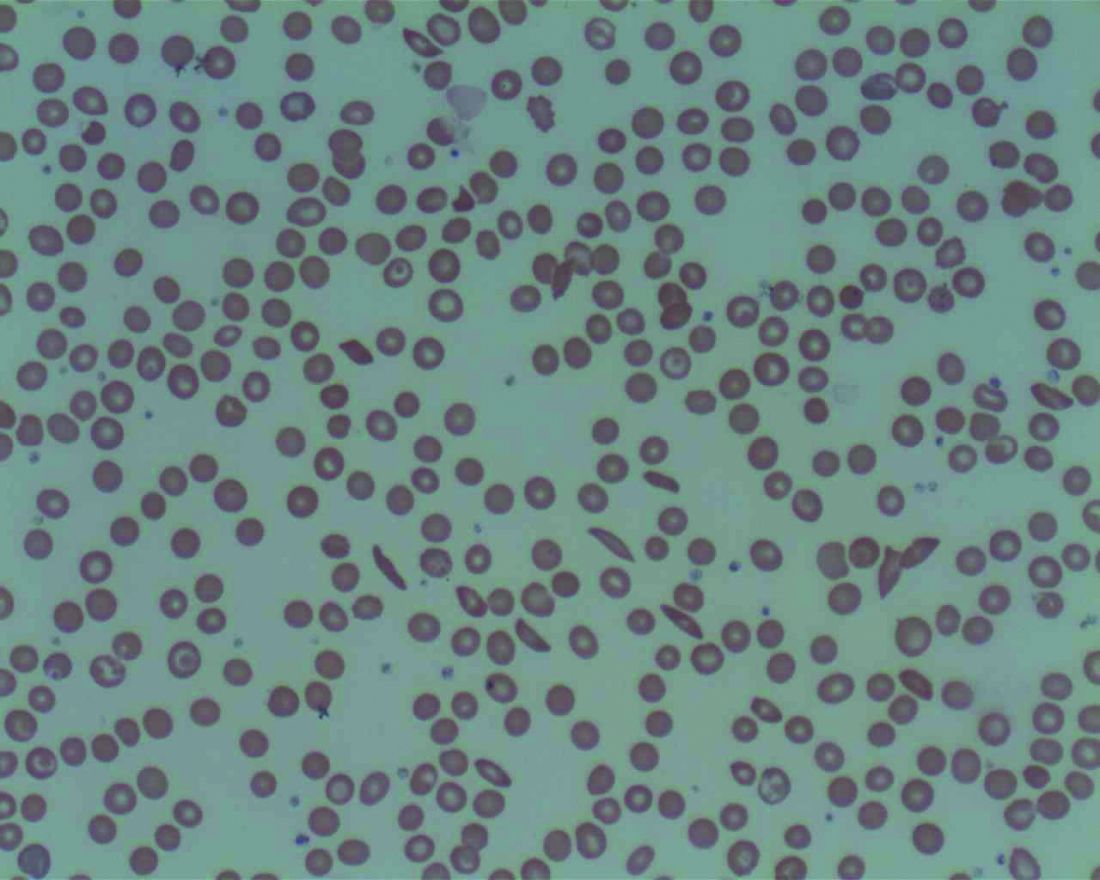
Low plasma sodium levels can predict complications in acute painful episodes of SCD
Although a low blood sodium level has been shown to be a prognostic factor for a number of disorders, it has not been reported on for sickle cell disease, according to French researchers Jean-Simon Rech, MD, and colleagues.
They found that hyponatremia at hospital admission was predictive of complications in initially uncomplicated episodes of painful episodes of sickle cell disease (SCD), according to their study published online in the American Journal of Medicine. Dr. Rech is with the department of internal medicine, Sickle Cell Disease Reference Center, Tenon Hospital, Assistance Publique-Hôpitaux de Paris.
The study assessed 1,218 stays (406 patients) admitted to a single center and the analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count.
The researchers found that hyponatremia (defined as plasma sodium ≤ 135 mmol/L) was significantly associated with the primary endpoint of a composite criterion including acute chest syndrome, intensive care unit transfer, red blood cell transfusion, or inpatient death (P = .001).
With regard to the components of the primary endpoint, hyponatremia was significantly associated with acute chest syndrome (P = .008), as well as with receiving a red blood cell transfusion (P < .001) However, hyponatremia at admission was not significantly associated with intensive care unit transfer (P = .074) and there were no patient deaths.
In addition, hyponatremia was significantly associated with longer stays: 1.1 days adjusted mean length of stay (P < .001).
“Hyponatremia may lead to direct clinical consequences in the management of sickle cell disease patients. Indeed, a plasma sodium concentration ≤ 135mmol/L at admission or a decreasing natremia over the first days of an acute painful episode could be regarded as early signs of incipient acute chest syndrome, prompting clinicians to closely monitor the clinical status of their patients,” the researchers concluded.
The authors declared that they had no conflicts and that there was no funding source.
SOURCE: Rech JS et al. Am J Med. 2020 Mar 18. doi.org/10.1016/j.amjmed.2020.02.017.
Although a low blood sodium level has been shown to be a prognostic factor for a number of disorders, it has not been reported on for sickle cell disease, according to French researchers Jean-Simon Rech, MD, and colleagues.
They found that hyponatremia at hospital admission was predictive of complications in initially uncomplicated episodes of painful episodes of sickle cell disease (SCD), according to their study published online in the American Journal of Medicine. Dr. Rech is with the department of internal medicine, Sickle Cell Disease Reference Center, Tenon Hospital, Assistance Publique-Hôpitaux de Paris.
The study assessed 1,218 stays (406 patients) admitted to a single center and the analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count.
The researchers found that hyponatremia (defined as plasma sodium ≤ 135 mmol/L) was significantly associated with the primary endpoint of a composite criterion including acute chest syndrome, intensive care unit transfer, red blood cell transfusion, or inpatient death (P = .001).
With regard to the components of the primary endpoint, hyponatremia was significantly associated with acute chest syndrome (P = .008), as well as with receiving a red blood cell transfusion (P < .001) However, hyponatremia at admission was not significantly associated with intensive care unit transfer (P = .074) and there were no patient deaths.
In addition, hyponatremia was significantly associated with longer stays: 1.1 days adjusted mean length of stay (P < .001).
“Hyponatremia may lead to direct clinical consequences in the management of sickle cell disease patients. Indeed, a plasma sodium concentration ≤ 135mmol/L at admission or a decreasing natremia over the first days of an acute painful episode could be regarded as early signs of incipient acute chest syndrome, prompting clinicians to closely monitor the clinical status of their patients,” the researchers concluded.
The authors declared that they had no conflicts and that there was no funding source.
SOURCE: Rech JS et al. Am J Med. 2020 Mar 18. doi.org/10.1016/j.amjmed.2020.02.017.
Although a low blood sodium level has been shown to be a prognostic factor for a number of disorders, it has not been reported on for sickle cell disease, according to French researchers Jean-Simon Rech, MD, and colleagues.
They found that hyponatremia at hospital admission was predictive of complications in initially uncomplicated episodes of painful episodes of sickle cell disease (SCD), according to their study published online in the American Journal of Medicine. Dr. Rech is with the department of internal medicine, Sickle Cell Disease Reference Center, Tenon Hospital, Assistance Publique-Hôpitaux de Paris.
The study assessed 1,218 stays (406 patients) admitted to a single center and the analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count.
The researchers found that hyponatremia (defined as plasma sodium ≤ 135 mmol/L) was significantly associated with the primary endpoint of a composite criterion including acute chest syndrome, intensive care unit transfer, red blood cell transfusion, or inpatient death (P = .001).
With regard to the components of the primary endpoint, hyponatremia was significantly associated with acute chest syndrome (P = .008), as well as with receiving a red blood cell transfusion (P < .001) However, hyponatremia at admission was not significantly associated with intensive care unit transfer (P = .074) and there were no patient deaths.
In addition, hyponatremia was significantly associated with longer stays: 1.1 days adjusted mean length of stay (P < .001).
“Hyponatremia may lead to direct clinical consequences in the management of sickle cell disease patients. Indeed, a plasma sodium concentration ≤ 135mmol/L at admission or a decreasing natremia over the first days of an acute painful episode could be regarded as early signs of incipient acute chest syndrome, prompting clinicians to closely monitor the clinical status of their patients,” the researchers concluded.
The authors declared that they had no conflicts and that there was no funding source.
SOURCE: Rech JS et al. Am J Med. 2020 Mar 18. doi.org/10.1016/j.amjmed.2020.02.017.
FROM THE AMERICAN JOURNAL OF MEDICINE
CAR T-cell therapy effective for r/r B-cell lymphoma of the GI tract
The use of anti-CD22/CD19 CAR-T sequential infusion was shown to have promising efficacy and safety for relapsed/refractory aggressive B-cell lymphoma with GI involvement, according to the results of a small study reported in Cytotherapy.
The open-label, single-center study enrolled 14 patients with relapsed/refractory aggressive B-cell lymphoma involving the GI tract between November 2017 and January 2019. The researchers examined treatment with sequential infusion of anti-CD22 and anti-CD19 CAR T cells in terms of safety and effectiveness.
An objective response was seen in 10 patients, with 7 of these having a complete response. However, 6 of the patients with partial response or stable disease went on to develop progressive disease. In terms of safety, cytokine-release syndrome and GI adverse events were generally mild and manageable, according to the authors. The most serious events were infections: Two of the patients developed bacterial infections in the GI tract, and one of these died of sepsis early after CAR T-cell infusion.
“The [CD22/CD19 CAR T sequential infusion] regimen was generally safe; however, special attention should be paid to the risk of infection in patients with lymphoma involving the GI tract,” the researchers concluded.
The study was funded by the National Science Foundation of China. The authors reported they had no conflicts of interest.
SOURCE: Zheng C et al. Cytotherapy. 2020;22:166-71.
The use of anti-CD22/CD19 CAR-T sequential infusion was shown to have promising efficacy and safety for relapsed/refractory aggressive B-cell lymphoma with GI involvement, according to the results of a small study reported in Cytotherapy.
The open-label, single-center study enrolled 14 patients with relapsed/refractory aggressive B-cell lymphoma involving the GI tract between November 2017 and January 2019. The researchers examined treatment with sequential infusion of anti-CD22 and anti-CD19 CAR T cells in terms of safety and effectiveness.
An objective response was seen in 10 patients, with 7 of these having a complete response. However, 6 of the patients with partial response or stable disease went on to develop progressive disease. In terms of safety, cytokine-release syndrome and GI adverse events were generally mild and manageable, according to the authors. The most serious events were infections: Two of the patients developed bacterial infections in the GI tract, and one of these died of sepsis early after CAR T-cell infusion.
“The [CD22/CD19 CAR T sequential infusion] regimen was generally safe; however, special attention should be paid to the risk of infection in patients with lymphoma involving the GI tract,” the researchers concluded.
The study was funded by the National Science Foundation of China. The authors reported they had no conflicts of interest.
SOURCE: Zheng C et al. Cytotherapy. 2020;22:166-71.
The use of anti-CD22/CD19 CAR-T sequential infusion was shown to have promising efficacy and safety for relapsed/refractory aggressive B-cell lymphoma with GI involvement, according to the results of a small study reported in Cytotherapy.
The open-label, single-center study enrolled 14 patients with relapsed/refractory aggressive B-cell lymphoma involving the GI tract between November 2017 and January 2019. The researchers examined treatment with sequential infusion of anti-CD22 and anti-CD19 CAR T cells in terms of safety and effectiveness.
An objective response was seen in 10 patients, with 7 of these having a complete response. However, 6 of the patients with partial response or stable disease went on to develop progressive disease. In terms of safety, cytokine-release syndrome and GI adverse events were generally mild and manageable, according to the authors. The most serious events were infections: Two of the patients developed bacterial infections in the GI tract, and one of these died of sepsis early after CAR T-cell infusion.
“The [CD22/CD19 CAR T sequential infusion] regimen was generally safe; however, special attention should be paid to the risk of infection in patients with lymphoma involving the GI tract,” the researchers concluded.
The study was funded by the National Science Foundation of China. The authors reported they had no conflicts of interest.
SOURCE: Zheng C et al. Cytotherapy. 2020;22:166-71.
FROM CYTOTHERAPY