User login
FDA allows marketing of morcellation containment system
The Food and Drug Administration has cleared for marketing a novel tissue containment system for use with certain laparoscopic power morcellators.
The PneumoLiner system is intended to be used to contain morcellated uterine tissue in a limited population of patients, including women without uterine fibroids undergoing hysterectomy and some premenopausal women with fibroids who want to maintain their fertility, according to the FDA. The agency is requiring the manufacturer to warn patients and physicians that the device has not been proven to reduce the risk of spreading cancer during surgery.
This approval – through the FDA’s de novo classification process for novel, low- and moderate-risk medical devices – comes about 2 years after the FDA first warned physicians and patients about the risk of spreading unsuspected uterine sarcomas during laparoscopic power morcellation in hysterectomy or myomectomy.
“This new device does not change our position on the risks associated with power morcellation,” Dr. William Maisel, deputy director for science and chief scientist at the FDA’s Center for Devices and Radiological Health, said in an April 7 statement. “We are continuing to warn against the use of power morcellators for the vast majority of women undergoing removal of the uterus or uterine fibroids.”
The device, which is manufactured by Advanced Surgical Concepts of Bray, Ireland, consists of a containment bag and a tube-like plunger to deliver the device into the abdominal cavity. The tissue being removed is placed in the bag and the bag is sealed and inflated. The inflation is intended to create working space around the tissue and better visualization during morcellation to prevent the morcellator tip or other surgical instruments from puncturing the bag. During laboratory testing, the containment bag was found to be impermeable to substances similar in molecular size to tissues, cells, and body fluids, according to the FDA.
Risks associated with the device include dissemination of morcellated tissue, injury to surrounding tissues or organs, infections, and a potentially longer surgical time. The FDA is requiring that the device’s label state that use of the PneumoLiner system is limited to physicians who have successfully completed the manufacturer’s validated training program.
On Twitter @maryellenny
The Food and Drug Administration has cleared for marketing a novel tissue containment system for use with certain laparoscopic power morcellators.
The PneumoLiner system is intended to be used to contain morcellated uterine tissue in a limited population of patients, including women without uterine fibroids undergoing hysterectomy and some premenopausal women with fibroids who want to maintain their fertility, according to the FDA. The agency is requiring the manufacturer to warn patients and physicians that the device has not been proven to reduce the risk of spreading cancer during surgery.
This approval – through the FDA’s de novo classification process for novel, low- and moderate-risk medical devices – comes about 2 years after the FDA first warned physicians and patients about the risk of spreading unsuspected uterine sarcomas during laparoscopic power morcellation in hysterectomy or myomectomy.
“This new device does not change our position on the risks associated with power morcellation,” Dr. William Maisel, deputy director for science and chief scientist at the FDA’s Center for Devices and Radiological Health, said in an April 7 statement. “We are continuing to warn against the use of power morcellators for the vast majority of women undergoing removal of the uterus or uterine fibroids.”
The device, which is manufactured by Advanced Surgical Concepts of Bray, Ireland, consists of a containment bag and a tube-like plunger to deliver the device into the abdominal cavity. The tissue being removed is placed in the bag and the bag is sealed and inflated. The inflation is intended to create working space around the tissue and better visualization during morcellation to prevent the morcellator tip or other surgical instruments from puncturing the bag. During laboratory testing, the containment bag was found to be impermeable to substances similar in molecular size to tissues, cells, and body fluids, according to the FDA.
Risks associated with the device include dissemination of morcellated tissue, injury to surrounding tissues or organs, infections, and a potentially longer surgical time. The FDA is requiring that the device’s label state that use of the PneumoLiner system is limited to physicians who have successfully completed the manufacturer’s validated training program.
On Twitter @maryellenny
The Food and Drug Administration has cleared for marketing a novel tissue containment system for use with certain laparoscopic power morcellators.
The PneumoLiner system is intended to be used to contain morcellated uterine tissue in a limited population of patients, including women without uterine fibroids undergoing hysterectomy and some premenopausal women with fibroids who want to maintain their fertility, according to the FDA. The agency is requiring the manufacturer to warn patients and physicians that the device has not been proven to reduce the risk of spreading cancer during surgery.
This approval – through the FDA’s de novo classification process for novel, low- and moderate-risk medical devices – comes about 2 years after the FDA first warned physicians and patients about the risk of spreading unsuspected uterine sarcomas during laparoscopic power morcellation in hysterectomy or myomectomy.
“This new device does not change our position on the risks associated with power morcellation,” Dr. William Maisel, deputy director for science and chief scientist at the FDA’s Center for Devices and Radiological Health, said in an April 7 statement. “We are continuing to warn against the use of power morcellators for the vast majority of women undergoing removal of the uterus or uterine fibroids.”
The device, which is manufactured by Advanced Surgical Concepts of Bray, Ireland, consists of a containment bag and a tube-like plunger to deliver the device into the abdominal cavity. The tissue being removed is placed in the bag and the bag is sealed and inflated. The inflation is intended to create working space around the tissue and better visualization during morcellation to prevent the morcellator tip or other surgical instruments from puncturing the bag. During laboratory testing, the containment bag was found to be impermeable to substances similar in molecular size to tissues, cells, and body fluids, according to the FDA.
Risks associated with the device include dissemination of morcellated tissue, injury to surrounding tissues or organs, infections, and a potentially longer surgical time. The FDA is requiring that the device’s label state that use of the PneumoLiner system is limited to physicians who have successfully completed the manufacturer’s validated training program.
On Twitter @maryellenny
FDA investigates Boston Scientific’s surgical mesh
The Food and Drug Administration is investigating allegations that Boston Scientific is using counterfeit raw materials in its urogynecologic surgical mesh.
For women who already have the Boston Scientific mesh implanted, the FDA is not recommending removal. The available data do not suggest any decreased benefit with the device and the risk of mesh removal outweighs any potential risk from mesh manufactured from alleged counterfeit raw materials, according to the FDA.
The investigation, announced by the FDA on April 1, 2016, comes after the agency received a citizen’s petition alleging that Boston Scientific was using a resin manufactured in China rather than the authentic Marlex HGX-030-01 resin.
Teresa Stevens, who is also involved in a federal class action lawsuit against Boston Scientific, submitted the citizen’s petition asking the FDA to begin an immediate Class I recall of all Boston Scientific mesh products made with the alleged counterfeit resin.
Boston Scientific is denying the allegations made in the petition.
“Boston Scientific does not use ‘counterfeit’ or ‘adulterated’ materials in our medical devices,” company officials wrote in an April 1 statement. “We have the highest confidence in the safety of our mesh devices. We have shared our test data with the Food and Drug Administration and are fully cooperating with the agency’s requests for information as part of our ongoing discussions. Additionally, we have offered to conduct further biocompatibility and chemical characterization testing to complement the results from existing tests.”
Company officials said they located a new supplier of Marlex resin in 2011 and put samples of the materials through a battery of tests to demonstrate equivalency.
The FDA acknowledged that it is not uncommon for companies to change the source of their raw materials after a device has been cleared for marketing and that the change often does not require premarket review by the agency. But due to the allegations in the citizen’s petition, the FDA will evaluate the results of new testing conducted by the company, including chemical characterization and toxicologic risk assessment of the raw material alleged to be counterfeit, as well as chemical characterization and biocompatibility of the final finished urogynecologic surgical mesh.
“The additional testing should be sufficient for the FDA to determine whether or not the urogynecologic surgical mesh manufactured from the alleged counterfeit raw material are equivalent to the urogynecologic surgical mesh manufactured from the original raw material supplier,” FDA officials wrote. “We expect that this testing will take several months to complete.”
On Twitter @maryellenny
The Food and Drug Administration is investigating allegations that Boston Scientific is using counterfeit raw materials in its urogynecologic surgical mesh.
For women who already have the Boston Scientific mesh implanted, the FDA is not recommending removal. The available data do not suggest any decreased benefit with the device and the risk of mesh removal outweighs any potential risk from mesh manufactured from alleged counterfeit raw materials, according to the FDA.
The investigation, announced by the FDA on April 1, 2016, comes after the agency received a citizen’s petition alleging that Boston Scientific was using a resin manufactured in China rather than the authentic Marlex HGX-030-01 resin.
Teresa Stevens, who is also involved in a federal class action lawsuit against Boston Scientific, submitted the citizen’s petition asking the FDA to begin an immediate Class I recall of all Boston Scientific mesh products made with the alleged counterfeit resin.
Boston Scientific is denying the allegations made in the petition.
“Boston Scientific does not use ‘counterfeit’ or ‘adulterated’ materials in our medical devices,” company officials wrote in an April 1 statement. “We have the highest confidence in the safety of our mesh devices. We have shared our test data with the Food and Drug Administration and are fully cooperating with the agency’s requests for information as part of our ongoing discussions. Additionally, we have offered to conduct further biocompatibility and chemical characterization testing to complement the results from existing tests.”
Company officials said they located a new supplier of Marlex resin in 2011 and put samples of the materials through a battery of tests to demonstrate equivalency.
The FDA acknowledged that it is not uncommon for companies to change the source of their raw materials after a device has been cleared for marketing and that the change often does not require premarket review by the agency. But due to the allegations in the citizen’s petition, the FDA will evaluate the results of new testing conducted by the company, including chemical characterization and toxicologic risk assessment of the raw material alleged to be counterfeit, as well as chemical characterization and biocompatibility of the final finished urogynecologic surgical mesh.
“The additional testing should be sufficient for the FDA to determine whether or not the urogynecologic surgical mesh manufactured from the alleged counterfeit raw material are equivalent to the urogynecologic surgical mesh manufactured from the original raw material supplier,” FDA officials wrote. “We expect that this testing will take several months to complete.”
On Twitter @maryellenny
The Food and Drug Administration is investigating allegations that Boston Scientific is using counterfeit raw materials in its urogynecologic surgical mesh.
For women who already have the Boston Scientific mesh implanted, the FDA is not recommending removal. The available data do not suggest any decreased benefit with the device and the risk of mesh removal outweighs any potential risk from mesh manufactured from alleged counterfeit raw materials, according to the FDA.
The investigation, announced by the FDA on April 1, 2016, comes after the agency received a citizen’s petition alleging that Boston Scientific was using a resin manufactured in China rather than the authentic Marlex HGX-030-01 resin.
Teresa Stevens, who is also involved in a federal class action lawsuit against Boston Scientific, submitted the citizen’s petition asking the FDA to begin an immediate Class I recall of all Boston Scientific mesh products made with the alleged counterfeit resin.
Boston Scientific is denying the allegations made in the petition.
“Boston Scientific does not use ‘counterfeit’ or ‘adulterated’ materials in our medical devices,” company officials wrote in an April 1 statement. “We have the highest confidence in the safety of our mesh devices. We have shared our test data with the Food and Drug Administration and are fully cooperating with the agency’s requests for information as part of our ongoing discussions. Additionally, we have offered to conduct further biocompatibility and chemical characterization testing to complement the results from existing tests.”
Company officials said they located a new supplier of Marlex resin in 2011 and put samples of the materials through a battery of tests to demonstrate equivalency.
The FDA acknowledged that it is not uncommon for companies to change the source of their raw materials after a device has been cleared for marketing and that the change often does not require premarket review by the agency. But due to the allegations in the citizen’s petition, the FDA will evaluate the results of new testing conducted by the company, including chemical characterization and toxicologic risk assessment of the raw material alleged to be counterfeit, as well as chemical characterization and biocompatibility of the final finished urogynecologic surgical mesh.
“The additional testing should be sufficient for the FDA to determine whether or not the urogynecologic surgical mesh manufactured from the alleged counterfeit raw material are equivalent to the urogynecologic surgical mesh manufactured from the original raw material supplier,” FDA officials wrote. “We expect that this testing will take several months to complete.”
On Twitter @maryellenny
FDA updates labeling for mifepristone for early abortions
The Food and Drug Administration has approved new labeling for the early abortion drug mifepristone, which women’s health advocates say now puts it in line with current medical standards.
Under the revised labeling, the FDA has approved the use of mifepristone (Mifeprex) in combination with misoprostol, to end a pregnancy through 70 days’ gestation. On the first day of the new regimen, 200 mg of mifepristone is taken orally. Then, 24-48 hours later, women should take 800 mcg of misoprostol buccally (in the cheek pouch). About 7-14 days after taking mifepristone, the FDA recommends that women follow up with their health care provider.
This is the first labeling change since the agency first approved mifepristone in 2000.
Previously, the FDA labeling information had stated that mifepristone was indicated for medication abortion through 49 days of gestation. On day 1, they recommended a 600-mg oral dose of mifepristone, followed by 400 mcg of misoprostol orally 2 days later. The FDA label also previously called for a follow-up office visit about 14 days after the administration of mifepristone.
The FDA is continuing to require a Risk Evaluation and Mitigation Strategy (REMS) for mifepristone, but they are expanding the types of providers who can prescribe the drug beyond just physicians.
The American Congress of Obstetricians and Gynecologists praised the move, saying that the updated labeling reflects the current available scientific evidence and best practices, including many of ACOG’s own recommendations.
“The evidence-based regimen for medication abortion has been shown to improve efficacy, reduce adverse effects, and even lower the cost of medication abortion,” Dr. Mark S. DeFrancesco, ACOG president, said in a statement. “And by allowing more qualified health care providers to prescribe and administer mifepristone, and removing the in-person follow-up appointment, the updated label will also make medication abortion accessible to more women, including previously underserved and rural women.”
But he said that more work is needed. For example, ACOG wants the FDA to eliminate the REMS requirements, noting that they are no longer necessary given the history of safe use of the drug.
Dr. Carolyn Westhoff, senior medical adviser for the Planned Parenthood Federation of America, said the FDA’s revised labeling won’t affect the majority of women seeking medication abortion, since most abortion providers around the country – including Planned Parenthood affiliates – have been using a different medication abortion regimen for about a decade.
But the change will make a difference in Texas, Ohio, and North Dakota, where state legislators have enacted laws requiring abortion providers to follow the FDA’s 2000 labeling, despite objections by physician groups that the regimen in the original labeling was outdated and potentially less effective. Similar restrictions had been enacted in Arizona, Arkansas, and Oklahoma but have been blocked by the courts.
While those laws will stay on the books, physicians will now be free to follow the most recent labeling from the FDA.
On Twitter @maryellenny
The Food and Drug Administration has approved new labeling for the early abortion drug mifepristone, which women’s health advocates say now puts it in line with current medical standards.
Under the revised labeling, the FDA has approved the use of mifepristone (Mifeprex) in combination with misoprostol, to end a pregnancy through 70 days’ gestation. On the first day of the new regimen, 200 mg of mifepristone is taken orally. Then, 24-48 hours later, women should take 800 mcg of misoprostol buccally (in the cheek pouch). About 7-14 days after taking mifepristone, the FDA recommends that women follow up with their health care provider.
This is the first labeling change since the agency first approved mifepristone in 2000.
Previously, the FDA labeling information had stated that mifepristone was indicated for medication abortion through 49 days of gestation. On day 1, they recommended a 600-mg oral dose of mifepristone, followed by 400 mcg of misoprostol orally 2 days later. The FDA label also previously called for a follow-up office visit about 14 days after the administration of mifepristone.
The FDA is continuing to require a Risk Evaluation and Mitigation Strategy (REMS) for mifepristone, but they are expanding the types of providers who can prescribe the drug beyond just physicians.
The American Congress of Obstetricians and Gynecologists praised the move, saying that the updated labeling reflects the current available scientific evidence and best practices, including many of ACOG’s own recommendations.
“The evidence-based regimen for medication abortion has been shown to improve efficacy, reduce adverse effects, and even lower the cost of medication abortion,” Dr. Mark S. DeFrancesco, ACOG president, said in a statement. “And by allowing more qualified health care providers to prescribe and administer mifepristone, and removing the in-person follow-up appointment, the updated label will also make medication abortion accessible to more women, including previously underserved and rural women.”
But he said that more work is needed. For example, ACOG wants the FDA to eliminate the REMS requirements, noting that they are no longer necessary given the history of safe use of the drug.
Dr. Carolyn Westhoff, senior medical adviser for the Planned Parenthood Federation of America, said the FDA’s revised labeling won’t affect the majority of women seeking medication abortion, since most abortion providers around the country – including Planned Parenthood affiliates – have been using a different medication abortion regimen for about a decade.
But the change will make a difference in Texas, Ohio, and North Dakota, where state legislators have enacted laws requiring abortion providers to follow the FDA’s 2000 labeling, despite objections by physician groups that the regimen in the original labeling was outdated and potentially less effective. Similar restrictions had been enacted in Arizona, Arkansas, and Oklahoma but have been blocked by the courts.
While those laws will stay on the books, physicians will now be free to follow the most recent labeling from the FDA.
On Twitter @maryellenny
The Food and Drug Administration has approved new labeling for the early abortion drug mifepristone, which women’s health advocates say now puts it in line with current medical standards.
Under the revised labeling, the FDA has approved the use of mifepristone (Mifeprex) in combination with misoprostol, to end a pregnancy through 70 days’ gestation. On the first day of the new regimen, 200 mg of mifepristone is taken orally. Then, 24-48 hours later, women should take 800 mcg of misoprostol buccally (in the cheek pouch). About 7-14 days after taking mifepristone, the FDA recommends that women follow up with their health care provider.
This is the first labeling change since the agency first approved mifepristone in 2000.
Previously, the FDA labeling information had stated that mifepristone was indicated for medication abortion through 49 days of gestation. On day 1, they recommended a 600-mg oral dose of mifepristone, followed by 400 mcg of misoprostol orally 2 days later. The FDA label also previously called for a follow-up office visit about 14 days after the administration of mifepristone.
The FDA is continuing to require a Risk Evaluation and Mitigation Strategy (REMS) for mifepristone, but they are expanding the types of providers who can prescribe the drug beyond just physicians.
The American Congress of Obstetricians and Gynecologists praised the move, saying that the updated labeling reflects the current available scientific evidence and best practices, including many of ACOG’s own recommendations.
“The evidence-based regimen for medication abortion has been shown to improve efficacy, reduce adverse effects, and even lower the cost of medication abortion,” Dr. Mark S. DeFrancesco, ACOG president, said in a statement. “And by allowing more qualified health care providers to prescribe and administer mifepristone, and removing the in-person follow-up appointment, the updated label will also make medication abortion accessible to more women, including previously underserved and rural women.”
But he said that more work is needed. For example, ACOG wants the FDA to eliminate the REMS requirements, noting that they are no longer necessary given the history of safe use of the drug.
Dr. Carolyn Westhoff, senior medical adviser for the Planned Parenthood Federation of America, said the FDA’s revised labeling won’t affect the majority of women seeking medication abortion, since most abortion providers around the country – including Planned Parenthood affiliates – have been using a different medication abortion regimen for about a decade.
But the change will make a difference in Texas, Ohio, and North Dakota, where state legislators have enacted laws requiring abortion providers to follow the FDA’s 2000 labeling, despite objections by physician groups that the regimen in the original labeling was outdated and potentially less effective. Similar restrictions had been enacted in Arizona, Arkansas, and Oklahoma but have been blocked by the courts.
While those laws will stay on the books, physicians will now be free to follow the most recent labeling from the FDA.
On Twitter @maryellenny
Q&A: Identifying gaps in the treatment of endometriosis
Despite the high prevalence of endometriosis – a condition that is estimated to affect about 10% of women of reproductive age – available treatments are inadequate for some women with chronic pain, and diagnosis is often delayed.
Stacey A. Missmer, Sc.D., the scientific director of the Boston Center for Endometriosis, is looking to advance understanding of the condition through the Women’s Health Study: From Adolescence to Adulthood. In an interview, Dr. Missmer, who is also the director of epidemiologic research in reproductive medicine at Brigham and Women’s Hospital in Boston, shared the progress of the study and her assessment of the treatment landscape.
Question: How would you rate the currently available treatments – both in terms of medications and surgery – in relieving the symptoms of endometriosis?
Dr. Missmer: The currently available treatments work well for some women and girls with pelvic pain, but it gets more difficult once that pain becomes chronic. If women have geographic and financial access to assisted reproductive treatment, then this works well for most women with endometriosis who also have subfertility. The great dilemma is that for pelvic pain, new nonhormonal/nonsurgical treatments have been slow to come for those who do not find relief with current treatments. These girls and young women are impacted just when they most need to be afforded a high quality of life that allows them to pursue all of their goals and dreams unimpeded by the symptoms associated with endometriosis.
Question: How does the Women’s Health Study: From Adolescence to Adulthood help advance treatment options?
Dr. Missmer: I am very proud of the Women’s Health Study: From Adolescence to Adulthood (A2A). Most studies of endometriosis have focused on women in their 30s and 40s, particularly those who present with infertility as their main health concern. However, we know that for women with endometriosis, the majority experience pain as their presenting symptom, and most report that endometriotic pain began during their teens and 20s. Uniquely, the A2A is a cohort of girl as young as 7 and young women, as well as those up to age 50. It is also uniquely a longitudinal cohort. By enrolling these girls and women now and following them into the future, we will be able to identify what characteristics in adolescence and young adulthood predicted who responds well to current treatments both in the short term and long into their reproductive and productive years.
We’ll also be able to discover the characteristics of those who did not respond as well, and we expect that this will drive us toward identifying unique groups of patients – and consequent biologic markers and patterns that are the basis for developing new treatments and prognostic tools for endometriosis.
Since November 2012, we have enrolled nearly 1,000 participants. Our initial enrollees are now completing their third year of lifestyle and symptom questionnaires, clinical details, and providing biologic samples. Their contributions are invaluable and are designed to be consistent with the harmonized data collection tools that we developed in collaboration with our national and international colleagues through the World Endometriosis Research Foundation (WERF). These Endometriosis Phenome and Biobanking Harmonisation Project (EPHect) tools are publicly available and will allow us to combine data from our cohort with participants at other sites to begin to detect geographic and patient-specific treatment response differences across the globe.
Question: What other studies are you closely watching?
Dr. Missmer: I am thrilled about the opportunities that the WERF EPHect tools offer us and our respected colleagues across the globe. I’m quite excited to see what new collaborations are formed and what creative hypotheses are tested. We’re in the midst of a renaissance in endometriosis understanding. Despite its prevalence and potentially debilitating impact on girls and women, endometriosis research is dramatically underfunded. This impacts discovery itself, but it also diminishes the ability for young enthusiastic brilliant scientists to be drawn to and remain dedicated to our field.
Question: How far are we from a noninvasive test that physicians could use to diagnose endometriosis and monitor its progression?
Dr. Missmer: How far in terms of a time line is difficult to determine. Again, I stress that I believe wholeheartedly that we are in the midst of a renaissance for endometriosis. We now have the tools to advance multidisciplinary scientific discovery with the ability to conduct studies with large numbers of diverse girls and women and to rigorously detect changes in their symptoms across the life course. I have no doubt that this will lead to advancement of precision medicine that allows endometriosis-focused scientists to develop noninvasive diagnostics and novel treatments. The key is that everyone in the field of endometriosis work together with this goal in mind.
On Twitter @maryellenny
Despite the high prevalence of endometriosis – a condition that is estimated to affect about 10% of women of reproductive age – available treatments are inadequate for some women with chronic pain, and diagnosis is often delayed.
Stacey A. Missmer, Sc.D., the scientific director of the Boston Center for Endometriosis, is looking to advance understanding of the condition through the Women’s Health Study: From Adolescence to Adulthood. In an interview, Dr. Missmer, who is also the director of epidemiologic research in reproductive medicine at Brigham and Women’s Hospital in Boston, shared the progress of the study and her assessment of the treatment landscape.
Question: How would you rate the currently available treatments – both in terms of medications and surgery – in relieving the symptoms of endometriosis?
Dr. Missmer: The currently available treatments work well for some women and girls with pelvic pain, but it gets more difficult once that pain becomes chronic. If women have geographic and financial access to assisted reproductive treatment, then this works well for most women with endometriosis who also have subfertility. The great dilemma is that for pelvic pain, new nonhormonal/nonsurgical treatments have been slow to come for those who do not find relief with current treatments. These girls and young women are impacted just when they most need to be afforded a high quality of life that allows them to pursue all of their goals and dreams unimpeded by the symptoms associated with endometriosis.
Question: How does the Women’s Health Study: From Adolescence to Adulthood help advance treatment options?
Dr. Missmer: I am very proud of the Women’s Health Study: From Adolescence to Adulthood (A2A). Most studies of endometriosis have focused on women in their 30s and 40s, particularly those who present with infertility as their main health concern. However, we know that for women with endometriosis, the majority experience pain as their presenting symptom, and most report that endometriotic pain began during their teens and 20s. Uniquely, the A2A is a cohort of girl as young as 7 and young women, as well as those up to age 50. It is also uniquely a longitudinal cohort. By enrolling these girls and women now and following them into the future, we will be able to identify what characteristics in adolescence and young adulthood predicted who responds well to current treatments both in the short term and long into their reproductive and productive years.
We’ll also be able to discover the characteristics of those who did not respond as well, and we expect that this will drive us toward identifying unique groups of patients – and consequent biologic markers and patterns that are the basis for developing new treatments and prognostic tools for endometriosis.
Since November 2012, we have enrolled nearly 1,000 participants. Our initial enrollees are now completing their third year of lifestyle and symptom questionnaires, clinical details, and providing biologic samples. Their contributions are invaluable and are designed to be consistent with the harmonized data collection tools that we developed in collaboration with our national and international colleagues through the World Endometriosis Research Foundation (WERF). These Endometriosis Phenome and Biobanking Harmonisation Project (EPHect) tools are publicly available and will allow us to combine data from our cohort with participants at other sites to begin to detect geographic and patient-specific treatment response differences across the globe.
Question: What other studies are you closely watching?
Dr. Missmer: I am thrilled about the opportunities that the WERF EPHect tools offer us and our respected colleagues across the globe. I’m quite excited to see what new collaborations are formed and what creative hypotheses are tested. We’re in the midst of a renaissance in endometriosis understanding. Despite its prevalence and potentially debilitating impact on girls and women, endometriosis research is dramatically underfunded. This impacts discovery itself, but it also diminishes the ability for young enthusiastic brilliant scientists to be drawn to and remain dedicated to our field.
Question: How far are we from a noninvasive test that physicians could use to diagnose endometriosis and monitor its progression?
Dr. Missmer: How far in terms of a time line is difficult to determine. Again, I stress that I believe wholeheartedly that we are in the midst of a renaissance for endometriosis. We now have the tools to advance multidisciplinary scientific discovery with the ability to conduct studies with large numbers of diverse girls and women and to rigorously detect changes in their symptoms across the life course. I have no doubt that this will lead to advancement of precision medicine that allows endometriosis-focused scientists to develop noninvasive diagnostics and novel treatments. The key is that everyone in the field of endometriosis work together with this goal in mind.
On Twitter @maryellenny
Despite the high prevalence of endometriosis – a condition that is estimated to affect about 10% of women of reproductive age – available treatments are inadequate for some women with chronic pain, and diagnosis is often delayed.
Stacey A. Missmer, Sc.D., the scientific director of the Boston Center for Endometriosis, is looking to advance understanding of the condition through the Women’s Health Study: From Adolescence to Adulthood. In an interview, Dr. Missmer, who is also the director of epidemiologic research in reproductive medicine at Brigham and Women’s Hospital in Boston, shared the progress of the study and her assessment of the treatment landscape.
Question: How would you rate the currently available treatments – both in terms of medications and surgery – in relieving the symptoms of endometriosis?
Dr. Missmer: The currently available treatments work well for some women and girls with pelvic pain, but it gets more difficult once that pain becomes chronic. If women have geographic and financial access to assisted reproductive treatment, then this works well for most women with endometriosis who also have subfertility. The great dilemma is that for pelvic pain, new nonhormonal/nonsurgical treatments have been slow to come for those who do not find relief with current treatments. These girls and young women are impacted just when they most need to be afforded a high quality of life that allows them to pursue all of their goals and dreams unimpeded by the symptoms associated with endometriosis.
Question: How does the Women’s Health Study: From Adolescence to Adulthood help advance treatment options?
Dr. Missmer: I am very proud of the Women’s Health Study: From Adolescence to Adulthood (A2A). Most studies of endometriosis have focused on women in their 30s and 40s, particularly those who present with infertility as their main health concern. However, we know that for women with endometriosis, the majority experience pain as their presenting symptom, and most report that endometriotic pain began during their teens and 20s. Uniquely, the A2A is a cohort of girl as young as 7 and young women, as well as those up to age 50. It is also uniquely a longitudinal cohort. By enrolling these girls and women now and following them into the future, we will be able to identify what characteristics in adolescence and young adulthood predicted who responds well to current treatments both in the short term and long into their reproductive and productive years.
We’ll also be able to discover the characteristics of those who did not respond as well, and we expect that this will drive us toward identifying unique groups of patients – and consequent biologic markers and patterns that are the basis for developing new treatments and prognostic tools for endometriosis.
Since November 2012, we have enrolled nearly 1,000 participants. Our initial enrollees are now completing their third year of lifestyle and symptom questionnaires, clinical details, and providing biologic samples. Their contributions are invaluable and are designed to be consistent with the harmonized data collection tools that we developed in collaboration with our national and international colleagues through the World Endometriosis Research Foundation (WERF). These Endometriosis Phenome and Biobanking Harmonisation Project (EPHect) tools are publicly available and will allow us to combine data from our cohort with participants at other sites to begin to detect geographic and patient-specific treatment response differences across the globe.
Question: What other studies are you closely watching?
Dr. Missmer: I am thrilled about the opportunities that the WERF EPHect tools offer us and our respected colleagues across the globe. I’m quite excited to see what new collaborations are formed and what creative hypotheses are tested. We’re in the midst of a renaissance in endometriosis understanding. Despite its prevalence and potentially debilitating impact on girls and women, endometriosis research is dramatically underfunded. This impacts discovery itself, but it also diminishes the ability for young enthusiastic brilliant scientists to be drawn to and remain dedicated to our field.
Question: How far are we from a noninvasive test that physicians could use to diagnose endometriosis and monitor its progression?
Dr. Missmer: How far in terms of a time line is difficult to determine. Again, I stress that I believe wholeheartedly that we are in the midst of a renaissance for endometriosis. We now have the tools to advance multidisciplinary scientific discovery with the ability to conduct studies with large numbers of diverse girls and women and to rigorously detect changes in their symptoms across the life course. I have no doubt that this will lead to advancement of precision medicine that allows endometriosis-focused scientists to develop noninvasive diagnostics and novel treatments. The key is that everyone in the field of endometriosis work together with this goal in mind.
On Twitter @maryellenny
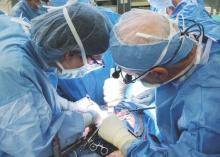
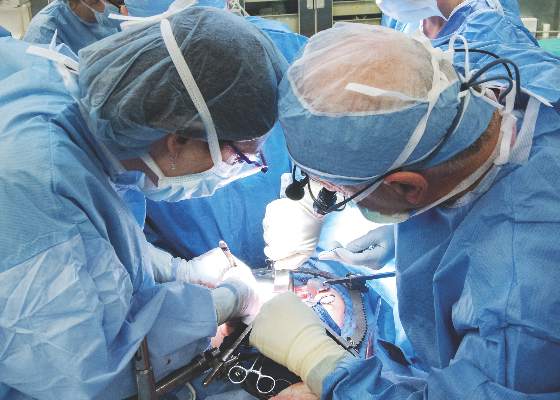
Historic Cleveland Clinic uterus transplant fails
Surgeons at the Cleveland Clinic had to remove the transplanted uterus of a 26-year-old woman who was the first patient in a groundbreaking U.S.-based study aimed at achieving pregnancy in women with uterine factor infertility.
Removal of the uterus, which was announced March 9, came after the patient experienced a sudden complication. The study was originally slated to include 10 women and is still ongoing, according to a statement from the Cleveland Clinic.
“At this time, the circumstance of the complication is under review and more information will be shared as it becomes available,” according to a statement. “There is a known risk in solid organ transplantation that the transplanted organ may have to be removed should a complication arise. The medical team took all necessary precautions and measures to ensure the safety of our patient.”
On Feb. 24, a transplant team at the Cleveland Clinic made history by performing the first uterus transplant in the United States. Prior to that, there had been nine successful uterus transplants performed at the University of Gothenburg in Sweden, with the first posttransplant baby born in 2014.
The Cleveland Clinic transplant recipient, known as “Lindsey” to protect her privacy, was selected from more than 250 applicants to undergo the 9-hour procedure involving transplantation of a uterus from a deceased organ donor of reproductive age.
If the transplant had been a successful, Lindsey was set to undergo a year of antirejection treatment followed by in vitro fertilization. Under the study protocol, the uterus transplant would be temporary and the uterus would be removed after a patient had delivered one or two babies.
Officials at the Cleveland Clinic said Lindsey was doing well and recovering after the removal of the transplanted uterus.
“I just wanted to take a moment to express my gratitude toward all of my doctors,” Lindsey and her husband said in a statement. “They acted very quickly to ensure my health and safety. Unfortunately, I did lose the uterus to complications. However, I am doing okay and appreciate all of your prayers and good thoughts.”
On Twitter @maryellenny
Surgeons at the Cleveland Clinic had to remove the transplanted uterus of a 26-year-old woman who was the first patient in a groundbreaking U.S.-based study aimed at achieving pregnancy in women with uterine factor infertility.
Removal of the uterus, which was announced March 9, came after the patient experienced a sudden complication. The study was originally slated to include 10 women and is still ongoing, according to a statement from the Cleveland Clinic.
“At this time, the circumstance of the complication is under review and more information will be shared as it becomes available,” according to a statement. “There is a known risk in solid organ transplantation that the transplanted organ may have to be removed should a complication arise. The medical team took all necessary precautions and measures to ensure the safety of our patient.”
On Feb. 24, a transplant team at the Cleveland Clinic made history by performing the first uterus transplant in the United States. Prior to that, there had been nine successful uterus transplants performed at the University of Gothenburg in Sweden, with the first posttransplant baby born in 2014.
The Cleveland Clinic transplant recipient, known as “Lindsey” to protect her privacy, was selected from more than 250 applicants to undergo the 9-hour procedure involving transplantation of a uterus from a deceased organ donor of reproductive age.
If the transplant had been a successful, Lindsey was set to undergo a year of antirejection treatment followed by in vitro fertilization. Under the study protocol, the uterus transplant would be temporary and the uterus would be removed after a patient had delivered one or two babies.
Officials at the Cleveland Clinic said Lindsey was doing well and recovering after the removal of the transplanted uterus.
“I just wanted to take a moment to express my gratitude toward all of my doctors,” Lindsey and her husband said in a statement. “They acted very quickly to ensure my health and safety. Unfortunately, I did lose the uterus to complications. However, I am doing okay and appreciate all of your prayers and good thoughts.”
On Twitter @maryellenny
Surgeons at the Cleveland Clinic had to remove the transplanted uterus of a 26-year-old woman who was the first patient in a groundbreaking U.S.-based study aimed at achieving pregnancy in women with uterine factor infertility.
Removal of the uterus, which was announced March 9, came after the patient experienced a sudden complication. The study was originally slated to include 10 women and is still ongoing, according to a statement from the Cleveland Clinic.
“At this time, the circumstance of the complication is under review and more information will be shared as it becomes available,” according to a statement. “There is a known risk in solid organ transplantation that the transplanted organ may have to be removed should a complication arise. The medical team took all necessary precautions and measures to ensure the safety of our patient.”
On Feb. 24, a transplant team at the Cleveland Clinic made history by performing the first uterus transplant in the United States. Prior to that, there had been nine successful uterus transplants performed at the University of Gothenburg in Sweden, with the first posttransplant baby born in 2014.
The Cleveland Clinic transplant recipient, known as “Lindsey” to protect her privacy, was selected from more than 250 applicants to undergo the 9-hour procedure involving transplantation of a uterus from a deceased organ donor of reproductive age.
If the transplant had been a successful, Lindsey was set to undergo a year of antirejection treatment followed by in vitro fertilization. Under the study protocol, the uterus transplant would be temporary and the uterus would be removed after a patient had delivered one or two babies.
Officials at the Cleveland Clinic said Lindsey was doing well and recovering after the removal of the transplanted uterus.
“I just wanted to take a moment to express my gratitude toward all of my doctors,” Lindsey and her husband said in a statement. “They acted very quickly to ensure my health and safety. Unfortunately, I did lose the uterus to complications. However, I am doing okay and appreciate all of your prayers and good thoughts.”
On Twitter @maryellenny
Core outcomes needed for endometriosis research
The clinical usefulness of endometriosis research is being hindered by a lack of a uniform core outcomes set in published trials, according to the findings of a systematic review.
Martin Hirsch of Barts and The London School of Medicine and Dentistry and his colleagues reviewed 54 randomized controlled trials with 5,427 participants evaluating a surgical intervention – with or without medical adjuvant therapy – for the treatment of endometriosis symptoms. They included all randomized, controlled trials in the Cochrane Central Register of Controlled Trials, Embase, and MEDLINE from inception to November 2014 and found a wide variation in the outcomes reported.
Across all trials, there were 164 outcomes and 113 outcomes measures reported. The three most commonly reported primary outcomes were dysmenorrhea (23 trials), dyspareunia (21 trials), and pregnancy (26 trials).
This level of variation among trials makes it difficult to make comparisons and synthesize data, according to the researchers. “This limits the usefulness of research to inform clinical practice, enhance patient care, and improve patient outcomes.”
Mr. Hirsch and his colleagues called for an international consensus on a core outcome set for endometriosis trials. Until then, they suggested the use of the three most common pain-related outcomes – dysmenorrhea, dyspareunia, and pelvic pain – as well as subfertility outcomes, measured by pregnancy, miscarriage, and live birth.
Read the full study in the American Journal of Obstetrics & Gynecology (doi: 10.1016/j.ajog.2015.12.039).
On Twitter @maryellenny
The clinical usefulness of endometriosis research is being hindered by a lack of a uniform core outcomes set in published trials, according to the findings of a systematic review.
Martin Hirsch of Barts and The London School of Medicine and Dentistry and his colleagues reviewed 54 randomized controlled trials with 5,427 participants evaluating a surgical intervention – with or without medical adjuvant therapy – for the treatment of endometriosis symptoms. They included all randomized, controlled trials in the Cochrane Central Register of Controlled Trials, Embase, and MEDLINE from inception to November 2014 and found a wide variation in the outcomes reported.
Across all trials, there were 164 outcomes and 113 outcomes measures reported. The three most commonly reported primary outcomes were dysmenorrhea (23 trials), dyspareunia (21 trials), and pregnancy (26 trials).
This level of variation among trials makes it difficult to make comparisons and synthesize data, according to the researchers. “This limits the usefulness of research to inform clinical practice, enhance patient care, and improve patient outcomes.”
Mr. Hirsch and his colleagues called for an international consensus on a core outcome set for endometriosis trials. Until then, they suggested the use of the three most common pain-related outcomes – dysmenorrhea, dyspareunia, and pelvic pain – as well as subfertility outcomes, measured by pregnancy, miscarriage, and live birth.
Read the full study in the American Journal of Obstetrics & Gynecology (doi: 10.1016/j.ajog.2015.12.039).
On Twitter @maryellenny
The clinical usefulness of endometriosis research is being hindered by a lack of a uniform core outcomes set in published trials, according to the findings of a systematic review.
Martin Hirsch of Barts and The London School of Medicine and Dentistry and his colleagues reviewed 54 randomized controlled trials with 5,427 participants evaluating a surgical intervention – with or without medical adjuvant therapy – for the treatment of endometriosis symptoms. They included all randomized, controlled trials in the Cochrane Central Register of Controlled Trials, Embase, and MEDLINE from inception to November 2014 and found a wide variation in the outcomes reported.
Across all trials, there were 164 outcomes and 113 outcomes measures reported. The three most commonly reported primary outcomes were dysmenorrhea (23 trials), dyspareunia (21 trials), and pregnancy (26 trials).
This level of variation among trials makes it difficult to make comparisons and synthesize data, according to the researchers. “This limits the usefulness of research to inform clinical practice, enhance patient care, and improve patient outcomes.”
Mr. Hirsch and his colleagues called for an international consensus on a core outcome set for endometriosis trials. Until then, they suggested the use of the three most common pain-related outcomes – dysmenorrhea, dyspareunia, and pelvic pain – as well as subfertility outcomes, measured by pregnancy, miscarriage, and live birth.
Read the full study in the American Journal of Obstetrics & Gynecology (doi: 10.1016/j.ajog.2015.12.039).
On Twitter @maryellenny
Brazilian study identifies fetal abnormalities linked to Zika virus
Fetal abnormalities were detected among more than a quarter of pregnant women who underwent ultrasound examinations after testing positive for Zika virus infection.
The small study, which included 88 pregnant women enrolled from September 2015 through February 2016 in Rio de Janeiro, was published online March 4 in the New England Journal of Medicine (doi: 10.1056/NEJMoa1602412).
“Our findings provide further support for a link between maternal ZIKV infection and fetal and placental abnormalities that is not unlike that of other viruses that are known to cause congenital infections characterized by intrauterine growth restriction and placental insufficiency,” investigators from Brazil and California reported.
The women in the study had developed a rash within the previous 5 days and were tested for Zika virus infection using reverse transcriptase polymerase chain reaction (RT-PCR) assays. Of the 88 women tested, 72 (82%) tested positive for Zika virus in blood, urine, or both.
Acute Zika infection was found throughout the course of pregnancy, though more than half of the women presented with acute infection during the second trimester. Along with a macular or maculopapular rash with pruritus, other distinctive clinical features of Zika virus infection included conjunctival injection, lymphadenopathy, and an absence of respiratory symptoms.
Two women who were positive for Zika virus had miscarriages during the first trimester. The investigators performed ultrasound for 42 of the remaining 70 women who had tested positive for Zika virus, as well as all women who tested negative for the virus. The other women who tested positive for Zika virus declined the imaging studies.
Fetal abnormalities were detected in 12 (29%) of the 42 women who were Zika virus positive and none of the women who had tested negative.
Among the 12 fetuses with abnormalities, there were two fetal deaths noted on ultrasound after 30 weeks of gestation. There were five fetuses with in utero growth restriction with or without microcephaly on ultrasound. Four fetuses had cerebral calcifications, and other central nervous system alterations were noted in two fetuses. Ultrasound detected abnormal arterial flow in the cerebral or umbilical arteries in four fetuses. Also, oligohydramnios and anhydramnios were seen in two fetuses.
At the time of this report, there had been six live births and two stillbirths among the study cohort and the ultrasound findings had been confirmed. The study was not supported by any research funds. The investigators reported having no financial disclosures.
On Twitter @maryellenny
Fetal abnormalities were detected among more than a quarter of pregnant women who underwent ultrasound examinations after testing positive for Zika virus infection.
The small study, which included 88 pregnant women enrolled from September 2015 through February 2016 in Rio de Janeiro, was published online March 4 in the New England Journal of Medicine (doi: 10.1056/NEJMoa1602412).
“Our findings provide further support for a link between maternal ZIKV infection and fetal and placental abnormalities that is not unlike that of other viruses that are known to cause congenital infections characterized by intrauterine growth restriction and placental insufficiency,” investigators from Brazil and California reported.
The women in the study had developed a rash within the previous 5 days and were tested for Zika virus infection using reverse transcriptase polymerase chain reaction (RT-PCR) assays. Of the 88 women tested, 72 (82%) tested positive for Zika virus in blood, urine, or both.
Acute Zika infection was found throughout the course of pregnancy, though more than half of the women presented with acute infection during the second trimester. Along with a macular or maculopapular rash with pruritus, other distinctive clinical features of Zika virus infection included conjunctival injection, lymphadenopathy, and an absence of respiratory symptoms.
Two women who were positive for Zika virus had miscarriages during the first trimester. The investigators performed ultrasound for 42 of the remaining 70 women who had tested positive for Zika virus, as well as all women who tested negative for the virus. The other women who tested positive for Zika virus declined the imaging studies.
Fetal abnormalities were detected in 12 (29%) of the 42 women who were Zika virus positive and none of the women who had tested negative.
Among the 12 fetuses with abnormalities, there were two fetal deaths noted on ultrasound after 30 weeks of gestation. There were five fetuses with in utero growth restriction with or without microcephaly on ultrasound. Four fetuses had cerebral calcifications, and other central nervous system alterations were noted in two fetuses. Ultrasound detected abnormal arterial flow in the cerebral or umbilical arteries in four fetuses. Also, oligohydramnios and anhydramnios were seen in two fetuses.
At the time of this report, there had been six live births and two stillbirths among the study cohort and the ultrasound findings had been confirmed. The study was not supported by any research funds. The investigators reported having no financial disclosures.
On Twitter @maryellenny
Fetal abnormalities were detected among more than a quarter of pregnant women who underwent ultrasound examinations after testing positive for Zika virus infection.
The small study, which included 88 pregnant women enrolled from September 2015 through February 2016 in Rio de Janeiro, was published online March 4 in the New England Journal of Medicine (doi: 10.1056/NEJMoa1602412).
“Our findings provide further support for a link between maternal ZIKV infection and fetal and placental abnormalities that is not unlike that of other viruses that are known to cause congenital infections characterized by intrauterine growth restriction and placental insufficiency,” investigators from Brazil and California reported.
The women in the study had developed a rash within the previous 5 days and were tested for Zika virus infection using reverse transcriptase polymerase chain reaction (RT-PCR) assays. Of the 88 women tested, 72 (82%) tested positive for Zika virus in blood, urine, or both.
Acute Zika infection was found throughout the course of pregnancy, though more than half of the women presented with acute infection during the second trimester. Along with a macular or maculopapular rash with pruritus, other distinctive clinical features of Zika virus infection included conjunctival injection, lymphadenopathy, and an absence of respiratory symptoms.
Two women who were positive for Zika virus had miscarriages during the first trimester. The investigators performed ultrasound for 42 of the remaining 70 women who had tested positive for Zika virus, as well as all women who tested negative for the virus. The other women who tested positive for Zika virus declined the imaging studies.
Fetal abnormalities were detected in 12 (29%) of the 42 women who were Zika virus positive and none of the women who had tested negative.
Among the 12 fetuses with abnormalities, there were two fetal deaths noted on ultrasound after 30 weeks of gestation. There were five fetuses with in utero growth restriction with or without microcephaly on ultrasound. Four fetuses had cerebral calcifications, and other central nervous system alterations were noted in two fetuses. Ultrasound detected abnormal arterial flow in the cerebral or umbilical arteries in four fetuses. Also, oligohydramnios and anhydramnios were seen in two fetuses.
At the time of this report, there had been six live births and two stillbirths among the study cohort and the ultrasound findings had been confirmed. The study was not supported by any research funds. The investigators reported having no financial disclosures.
On Twitter @maryellenny
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Zika virus infection in pregnancy appears to be associated with in utero growth restriction, central nervous system lesions, and fetal death.
Major finding: Fetal abnormalities were detected by ultrasound in 12 of 42 pregnant women who tested positive for Zika virus infection.
Data source: A prospective study of 88 pregnant women with a rash from September 2015 through February 2016 in Rio de Janeiro.
Disclosures: The study was not supported by any research funds. The investigators reported having no financial disclosures.
New evidence strengthens link between Zika and microcephaly
While scientists can’t say with certainty that congenital Zika virus is causing the massive spike in cases of microcephaly seen in Brazil, evidence of a strong association continues to mount.
Two reports, published Feb. 10 in the Morbidity and Mortality Weekly Report and in the New England Journal of Medicine, confirm through laboratory testing that fetuses and infants with microcephaly also were positive for Zika virus infection.
In the MMWR report, researchers from the United States and Brazil present evidence of a link between Zika virus infection and microcephaly and fetal demise through detection of viral RNA and antigens in brain tissues with infants with microcephaly, as well as placental tissues from early miscarriages.
The findings are based on laboratory testing of tissue samples from two newborns with microcephaly who died within 20 hours of birth and two miscarriages (at 11 and 13 weeks’ gestation). The samples were submitted to the Centers for Disease Control and Prevention from the state of Rio Grande do Norte, Brazil, in December 2015. All four mothers had clinical signs of Zika virus infection during the first trimester but did not have signs of active infection at the time of delivery or miscarriage.
Specimens from all four cases were positive by reverse transcription-polymerase chain reaction (RT-PCR) testing, and sequence analysis provided additional evidence of Zika virus infection (Morb Mortal Wkly Rep. 2016 Feb;65:1-2. doi: 10.15585/mmwr.mm6506e1er).
“To better understand the pathogenesis of Zika virus infection and associated congenital anomalies and fetal death, it is necessary to evaluate autopsy and placental tissues from additional cases, and to determine the effect of gestational age during maternal illness on fetal outcomes,” the researchers wrote.
In the New England Journal of Medicine report, Dr. Jernej Mlakar of the University of Ljubljana, Slovenia, and colleagues, presented the case of a previously healthy 25-year-old pregnant woman who had become ill while living in Brazil. During the 13th week of gestation, she had a high fever, followed by severe musculoskeletal and retro-ocular pain, as well as an itchy generalized maculopapular rash. Zika virus was suspected at the time but virologic diagnostic testing was not performed.
Ultrasound at 14 weeks and 20 weeks showed normal fetal growth and anatomy, but ultrasound at 29 weeks showed signs of fetal abnormalities. At 32 weeks, physicians confirmed intrauterine growth retardation and microcephaly with calcifications in the fetal brain and placenta.
The woman requested termination of the pregnancy and an autopsy was performed on the fetus. Positive results for Zika virus were obtained on RT-PCR assay in the fetal brain sample. All autopsy samples were tested on PCR assay and found to be negative for other flaviviruses, including dengue, yellow fever, West Nile, and tick-borne encephalitis (N Engl J Med. 2016 Feb 10. doi: 10.1056/NEJMoa1600651).
In an editorial accompanying the report, physicians from the Harvard School of Public Health and Massachusetts General Hospital, both in Boston, wrote that there are still many unanswered questions about Zika virus in pregnancy. Assuming the association between Zika virus and microcephaly exists, researchers do not know whether the timing of the infection during pregnancy has an effect on the risk of fetal abnormalities. Additionally, it’s unknown whether asymptomatic or minimally symptomatic disease poses a risk to the fetus (N Engl J Med. 2016 Feb 10. doi: 10.1056/NEJMe1601862).
The researchers for both case reports had no financial disclosures.
On Twitter @maryellenny
While scientists can’t say with certainty that congenital Zika virus is causing the massive spike in cases of microcephaly seen in Brazil, evidence of a strong association continues to mount.
Two reports, published Feb. 10 in the Morbidity and Mortality Weekly Report and in the New England Journal of Medicine, confirm through laboratory testing that fetuses and infants with microcephaly also were positive for Zika virus infection.
In the MMWR report, researchers from the United States and Brazil present evidence of a link between Zika virus infection and microcephaly and fetal demise through detection of viral RNA and antigens in brain tissues with infants with microcephaly, as well as placental tissues from early miscarriages.
The findings are based on laboratory testing of tissue samples from two newborns with microcephaly who died within 20 hours of birth and two miscarriages (at 11 and 13 weeks’ gestation). The samples were submitted to the Centers for Disease Control and Prevention from the state of Rio Grande do Norte, Brazil, in December 2015. All four mothers had clinical signs of Zika virus infection during the first trimester but did not have signs of active infection at the time of delivery or miscarriage.
Specimens from all four cases were positive by reverse transcription-polymerase chain reaction (RT-PCR) testing, and sequence analysis provided additional evidence of Zika virus infection (Morb Mortal Wkly Rep. 2016 Feb;65:1-2. doi: 10.15585/mmwr.mm6506e1er).
“To better understand the pathogenesis of Zika virus infection and associated congenital anomalies and fetal death, it is necessary to evaluate autopsy and placental tissues from additional cases, and to determine the effect of gestational age during maternal illness on fetal outcomes,” the researchers wrote.
In the New England Journal of Medicine report, Dr. Jernej Mlakar of the University of Ljubljana, Slovenia, and colleagues, presented the case of a previously healthy 25-year-old pregnant woman who had become ill while living in Brazil. During the 13th week of gestation, she had a high fever, followed by severe musculoskeletal and retro-ocular pain, as well as an itchy generalized maculopapular rash. Zika virus was suspected at the time but virologic diagnostic testing was not performed.
Ultrasound at 14 weeks and 20 weeks showed normal fetal growth and anatomy, but ultrasound at 29 weeks showed signs of fetal abnormalities. At 32 weeks, physicians confirmed intrauterine growth retardation and microcephaly with calcifications in the fetal brain and placenta.
The woman requested termination of the pregnancy and an autopsy was performed on the fetus. Positive results for Zika virus were obtained on RT-PCR assay in the fetal brain sample. All autopsy samples were tested on PCR assay and found to be negative for other flaviviruses, including dengue, yellow fever, West Nile, and tick-borne encephalitis (N Engl J Med. 2016 Feb 10. doi: 10.1056/NEJMoa1600651).
In an editorial accompanying the report, physicians from the Harvard School of Public Health and Massachusetts General Hospital, both in Boston, wrote that there are still many unanswered questions about Zika virus in pregnancy. Assuming the association between Zika virus and microcephaly exists, researchers do not know whether the timing of the infection during pregnancy has an effect on the risk of fetal abnormalities. Additionally, it’s unknown whether asymptomatic or minimally symptomatic disease poses a risk to the fetus (N Engl J Med. 2016 Feb 10. doi: 10.1056/NEJMe1601862).
The researchers for both case reports had no financial disclosures.
On Twitter @maryellenny
While scientists can’t say with certainty that congenital Zika virus is causing the massive spike in cases of microcephaly seen in Brazil, evidence of a strong association continues to mount.
Two reports, published Feb. 10 in the Morbidity and Mortality Weekly Report and in the New England Journal of Medicine, confirm through laboratory testing that fetuses and infants with microcephaly also were positive for Zika virus infection.
In the MMWR report, researchers from the United States and Brazil present evidence of a link between Zika virus infection and microcephaly and fetal demise through detection of viral RNA and antigens in brain tissues with infants with microcephaly, as well as placental tissues from early miscarriages.
The findings are based on laboratory testing of tissue samples from two newborns with microcephaly who died within 20 hours of birth and two miscarriages (at 11 and 13 weeks’ gestation). The samples were submitted to the Centers for Disease Control and Prevention from the state of Rio Grande do Norte, Brazil, in December 2015. All four mothers had clinical signs of Zika virus infection during the first trimester but did not have signs of active infection at the time of delivery or miscarriage.
Specimens from all four cases were positive by reverse transcription-polymerase chain reaction (RT-PCR) testing, and sequence analysis provided additional evidence of Zika virus infection (Morb Mortal Wkly Rep. 2016 Feb;65:1-2. doi: 10.15585/mmwr.mm6506e1er).
“To better understand the pathogenesis of Zika virus infection and associated congenital anomalies and fetal death, it is necessary to evaluate autopsy and placental tissues from additional cases, and to determine the effect of gestational age during maternal illness on fetal outcomes,” the researchers wrote.
In the New England Journal of Medicine report, Dr. Jernej Mlakar of the University of Ljubljana, Slovenia, and colleagues, presented the case of a previously healthy 25-year-old pregnant woman who had become ill while living in Brazil. During the 13th week of gestation, she had a high fever, followed by severe musculoskeletal and retro-ocular pain, as well as an itchy generalized maculopapular rash. Zika virus was suspected at the time but virologic diagnostic testing was not performed.
Ultrasound at 14 weeks and 20 weeks showed normal fetal growth and anatomy, but ultrasound at 29 weeks showed signs of fetal abnormalities. At 32 weeks, physicians confirmed intrauterine growth retardation and microcephaly with calcifications in the fetal brain and placenta.
The woman requested termination of the pregnancy and an autopsy was performed on the fetus. Positive results for Zika virus were obtained on RT-PCR assay in the fetal brain sample. All autopsy samples were tested on PCR assay and found to be negative for other flaviviruses, including dengue, yellow fever, West Nile, and tick-borne encephalitis (N Engl J Med. 2016 Feb 10. doi: 10.1056/NEJMoa1600651).
In an editorial accompanying the report, physicians from the Harvard School of Public Health and Massachusetts General Hospital, both in Boston, wrote that there are still many unanswered questions about Zika virus in pregnancy. Assuming the association between Zika virus and microcephaly exists, researchers do not know whether the timing of the infection during pregnancy has an effect on the risk of fetal abnormalities. Additionally, it’s unknown whether asymptomatic or minimally symptomatic disease poses a risk to the fetus (N Engl J Med. 2016 Feb 10. doi: 10.1056/NEJMe1601862).
The researchers for both case reports had no financial disclosures.
On Twitter @maryellenny
CDC’s emergency operations center moves to Level 1 for Zika
Officials at the Centers for Disease Control and Prevention are ramping up their response to the Zika virus – moving their Emergency Operations Center to Level 1 activation.
The agency’s Emergency Operations Center (EOC) was initially activated for Zika response on Jan. 22 to better coordinate the response to the Zika outbreak and bring together CDC scientists in arboviruses, reproductive health, and birth and developmental defects. On Feb. 8, the CDC accelerated its efforts “in anticipation of local Zika virus transmission by mosquitoes in the continental U.S.”
The EOC is currently at work on developing diagnostic tests for Zika virus, investigating links between the virus and microcephaly and Guillain-Barré syndrome, conducting surveillance in the United States, and providing on-the-ground support in Puerto Rico, Brazil, and Colombia.
The CDC recently updated its guidance on Zika virus, advising pregnant women to use condoms or abstain from sex with men who have traveled to Zika-infected areas. The agency also advised offering testing to pregnant women without symptoms of Zika virus 2-12 weeks after returning from areas with ongoing Zika virus transmission.
The CDC’s current Zika virus travel alert includes American Samoa, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Costa Rica, Curacao, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Martinique, Mexico, Nicaragua, Panama, Paraguay, Puerto Rico, Saint Martin, Samoa, Suriname, Tonga, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
An up-to-date list of affected countries and regions is available at www.cdc.gov/zika/geo/index.html.
On Twitter @maryelleny
Officials at the Centers for Disease Control and Prevention are ramping up their response to the Zika virus – moving their Emergency Operations Center to Level 1 activation.
The agency’s Emergency Operations Center (EOC) was initially activated for Zika response on Jan. 22 to better coordinate the response to the Zika outbreak and bring together CDC scientists in arboviruses, reproductive health, and birth and developmental defects. On Feb. 8, the CDC accelerated its efforts “in anticipation of local Zika virus transmission by mosquitoes in the continental U.S.”
The EOC is currently at work on developing diagnostic tests for Zika virus, investigating links between the virus and microcephaly and Guillain-Barré syndrome, conducting surveillance in the United States, and providing on-the-ground support in Puerto Rico, Brazil, and Colombia.
The CDC recently updated its guidance on Zika virus, advising pregnant women to use condoms or abstain from sex with men who have traveled to Zika-infected areas. The agency also advised offering testing to pregnant women without symptoms of Zika virus 2-12 weeks after returning from areas with ongoing Zika virus transmission.
The CDC’s current Zika virus travel alert includes American Samoa, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Costa Rica, Curacao, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Martinique, Mexico, Nicaragua, Panama, Paraguay, Puerto Rico, Saint Martin, Samoa, Suriname, Tonga, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
An up-to-date list of affected countries and regions is available at www.cdc.gov/zika/geo/index.html.
On Twitter @maryelleny
Officials at the Centers for Disease Control and Prevention are ramping up their response to the Zika virus – moving their Emergency Operations Center to Level 1 activation.
The agency’s Emergency Operations Center (EOC) was initially activated for Zika response on Jan. 22 to better coordinate the response to the Zika outbreak and bring together CDC scientists in arboviruses, reproductive health, and birth and developmental defects. On Feb. 8, the CDC accelerated its efforts “in anticipation of local Zika virus transmission by mosquitoes in the continental U.S.”
The EOC is currently at work on developing diagnostic tests for Zika virus, investigating links between the virus and microcephaly and Guillain-Barré syndrome, conducting surveillance in the United States, and providing on-the-ground support in Puerto Rico, Brazil, and Colombia.
The CDC recently updated its guidance on Zika virus, advising pregnant women to use condoms or abstain from sex with men who have traveled to Zika-infected areas. The agency also advised offering testing to pregnant women without symptoms of Zika virus 2-12 weeks after returning from areas with ongoing Zika virus transmission.
The CDC’s current Zika virus travel alert includes American Samoa, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Costa Rica, Curacao, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Martinique, Mexico, Nicaragua, Panama, Paraguay, Puerto Rico, Saint Martin, Samoa, Suriname, Tonga, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
An up-to-date list of affected countries and regions is available at www.cdc.gov/zika/geo/index.html.
On Twitter @maryelleny
CDC expands Zika virus travel warnings again
Officials at the Centers for Disease Control and Prevention have added four more destinations to their Zika virus travel alert – American Samoa, Costa Rica, Curacao, and Nicaragua.
The Level 2 travel alert means that individuals are urged to take enhanced precautions against mosquito bites while in these regions to minimize their chances of contracting the Zika virus. Pregnant women are being advised to consider postponing travel to areas where Zika virus transmission in ongoing. Pregnant women and those trying to become pregnant who must travel to these areas are advised to consult with their physician before traveling and take steps to prevent mosquito bites.
The CDC has already issued a Level 2 travel alert for these areas where Zika virus transmission is ongoing: Puerto Rico, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
An up-to-date list of affected countries and regions is available at www.cdc.gov/zika/geo/index.html.
On Twitter @maryelleny
Officials at the Centers for Disease Control and Prevention have added four more destinations to their Zika virus travel alert – American Samoa, Costa Rica, Curacao, and Nicaragua.
The Level 2 travel alert means that individuals are urged to take enhanced precautions against mosquito bites while in these regions to minimize their chances of contracting the Zika virus. Pregnant women are being advised to consider postponing travel to areas where Zika virus transmission in ongoing. Pregnant women and those trying to become pregnant who must travel to these areas are advised to consult with their physician before traveling and take steps to prevent mosquito bites.
The CDC has already issued a Level 2 travel alert for these areas where Zika virus transmission is ongoing: Puerto Rico, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
An up-to-date list of affected countries and regions is available at www.cdc.gov/zika/geo/index.html.
On Twitter @maryelleny
Officials at the Centers for Disease Control and Prevention have added four more destinations to their Zika virus travel alert – American Samoa, Costa Rica, Curacao, and Nicaragua.
The Level 2 travel alert means that individuals are urged to take enhanced precautions against mosquito bites while in these regions to minimize their chances of contracting the Zika virus. Pregnant women are being advised to consider postponing travel to areas where Zika virus transmission in ongoing. Pregnant women and those trying to become pregnant who must travel to these areas are advised to consult with their physician before traveling and take steps to prevent mosquito bites.
The CDC has already issued a Level 2 travel alert for these areas where Zika virus transmission is ongoing: Puerto Rico, Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, Venezuela, the U.S. Virgin Islands, and the Dominican Republic.
An up-to-date list of affected countries and regions is available at www.cdc.gov/zika/geo/index.html.
On Twitter @maryelleny