User login
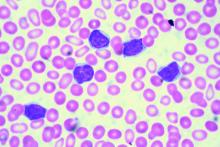
Age of blood did not affect mortality in transfused patients
In-hospital mortality did not vary for patients who received transfusions of blood that had been stored for 2 weeks and for patients who got blood that had been stored for 4 weeks, based on results from 20,858 hospitalized patients in the randomized, controlled INFORM (Informing Fresh versus Old Red Cell Management) trial conducted at six hospitals in four countries.
While previous trials have concluded that the storage time of blood did not affect patient mortality, those studies largely included high-risk patients and were not statistically powered to detect small mortality differences, Nancy M. Heddle, professor of medicine and director of the McMaster (University) transfusion research program, Hamilton, Ont., and colleagues reported in an article published online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1609014). Standard practice is to transfuse with the oldest available blood, which can be stored up to 42 days.
Their study included general hospitalized patients who required a red cell transfusion. From April 2012 through October 2015, patients were randomly assigned in a 1:2 ratio patients to receive blood that had been stored for the shortest duration (mean duration 13 days, 6,936 patients) or the longest duration (mean duration 23.6 days, 13,922 patients).
Only patients with type A or O blood were included in the study’s primary analysis, because of the difficulty of achieving a difference of at least 10 days in the mean duration of blood storage with other blood types.
There were 634 deaths (9.1% mortality) among patients in the short-term blood storage group and 1,213 deaths (8.7% mortality) in the long-term blood storage group. The difference was not statistically significant. Similar results were seen when the analysis was expanded to include all 24,736 patients with any blood type; the mortality rates were 9.1% and 8.8%, respectively.
An additional analysis found similar results in three prespecified high-risk subgroups – patients undergoing cardiovascular surgery, those admitted to intensive care, and those with cancer.
INFORM, Current Controlled Trials number ISRCTN08118744, was funded by the Canadian Institutes of Health Research, Canadian Blood Services, and Health Canada. Ms. Heddle had no relevant financial disclosures.
[email protected]
On Twitter @maryjodales
The results of the INFORM trial should end the debate regarding whether short-term or long-term storage of blood is advantageous. However, questions remain about whether red cells transfused during the last allowed week of storage (35-42 days) pose more risk. Observational studies continue to raise concerns about the use of the oldest blood.
The INFORM trial, with its large numbers of patients, should permit researchers to analyze enough data to address this remaining issue. The transfusion medicine community needs to know whether the storage period should be reduced to less than 35 and whether new preservative solutions should be sought.
Aaron A.R. Tobian, MD, PhD, and Paul M. Ness, MD, are with the division of transfusion medicine, department of pathology, Johns Hopkins University, Baltimore. They had no relevant financial conflicts of interest and made their remarks in an editorial (10.1056/NEJMe1612444) that accompanied the published study.
The results of the INFORM trial should end the debate regarding whether short-term or long-term storage of blood is advantageous. However, questions remain about whether red cells transfused during the last allowed week of storage (35-42 days) pose more risk. Observational studies continue to raise concerns about the use of the oldest blood.
The INFORM trial, with its large numbers of patients, should permit researchers to analyze enough data to address this remaining issue. The transfusion medicine community needs to know whether the storage period should be reduced to less than 35 and whether new preservative solutions should be sought.
Aaron A.R. Tobian, MD, PhD, and Paul M. Ness, MD, are with the division of transfusion medicine, department of pathology, Johns Hopkins University, Baltimore. They had no relevant financial conflicts of interest and made their remarks in an editorial (10.1056/NEJMe1612444) that accompanied the published study.
The results of the INFORM trial should end the debate regarding whether short-term or long-term storage of blood is advantageous. However, questions remain about whether red cells transfused during the last allowed week of storage (35-42 days) pose more risk. Observational studies continue to raise concerns about the use of the oldest blood.
The INFORM trial, with its large numbers of patients, should permit researchers to analyze enough data to address this remaining issue. The transfusion medicine community needs to know whether the storage period should be reduced to less than 35 and whether new preservative solutions should be sought.
Aaron A.R. Tobian, MD, PhD, and Paul M. Ness, MD, are with the division of transfusion medicine, department of pathology, Johns Hopkins University, Baltimore. They had no relevant financial conflicts of interest and made their remarks in an editorial (10.1056/NEJMe1612444) that accompanied the published study.
In-hospital mortality did not vary for patients who received transfusions of blood that had been stored for 2 weeks and for patients who got blood that had been stored for 4 weeks, based on results from 20,858 hospitalized patients in the randomized, controlled INFORM (Informing Fresh versus Old Red Cell Management) trial conducted at six hospitals in four countries.
While previous trials have concluded that the storage time of blood did not affect patient mortality, those studies largely included high-risk patients and were not statistically powered to detect small mortality differences, Nancy M. Heddle, professor of medicine and director of the McMaster (University) transfusion research program, Hamilton, Ont., and colleagues reported in an article published online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1609014). Standard practice is to transfuse with the oldest available blood, which can be stored up to 42 days.
Their study included general hospitalized patients who required a red cell transfusion. From April 2012 through October 2015, patients were randomly assigned in a 1:2 ratio patients to receive blood that had been stored for the shortest duration (mean duration 13 days, 6,936 patients) or the longest duration (mean duration 23.6 days, 13,922 patients).
Only patients with type A or O blood were included in the study’s primary analysis, because of the difficulty of achieving a difference of at least 10 days in the mean duration of blood storage with other blood types.
There were 634 deaths (9.1% mortality) among patients in the short-term blood storage group and 1,213 deaths (8.7% mortality) in the long-term blood storage group. The difference was not statistically significant. Similar results were seen when the analysis was expanded to include all 24,736 patients with any blood type; the mortality rates were 9.1% and 8.8%, respectively.
An additional analysis found similar results in three prespecified high-risk subgroups – patients undergoing cardiovascular surgery, those admitted to intensive care, and those with cancer.
INFORM, Current Controlled Trials number ISRCTN08118744, was funded by the Canadian Institutes of Health Research, Canadian Blood Services, and Health Canada. Ms. Heddle had no relevant financial disclosures.
[email protected]
On Twitter @maryjodales
In-hospital mortality did not vary for patients who received transfusions of blood that had been stored for 2 weeks and for patients who got blood that had been stored for 4 weeks, based on results from 20,858 hospitalized patients in the randomized, controlled INFORM (Informing Fresh versus Old Red Cell Management) trial conducted at six hospitals in four countries.
While previous trials have concluded that the storage time of blood did not affect patient mortality, those studies largely included high-risk patients and were not statistically powered to detect small mortality differences, Nancy M. Heddle, professor of medicine and director of the McMaster (University) transfusion research program, Hamilton, Ont., and colleagues reported in an article published online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1609014). Standard practice is to transfuse with the oldest available blood, which can be stored up to 42 days.
Their study included general hospitalized patients who required a red cell transfusion. From April 2012 through October 2015, patients were randomly assigned in a 1:2 ratio patients to receive blood that had been stored for the shortest duration (mean duration 13 days, 6,936 patients) or the longest duration (mean duration 23.6 days, 13,922 patients).
Only patients with type A or O blood were included in the study’s primary analysis, because of the difficulty of achieving a difference of at least 10 days in the mean duration of blood storage with other blood types.
There were 634 deaths (9.1% mortality) among patients in the short-term blood storage group and 1,213 deaths (8.7% mortality) in the long-term blood storage group. The difference was not statistically significant. Similar results were seen when the analysis was expanded to include all 24,736 patients with any blood type; the mortality rates were 9.1% and 8.8%, respectively.
An additional analysis found similar results in three prespecified high-risk subgroups – patients undergoing cardiovascular surgery, those admitted to intensive care, and those with cancer.
INFORM, Current Controlled Trials number ISRCTN08118744, was funded by the Canadian Institutes of Health Research, Canadian Blood Services, and Health Canada. Ms. Heddle had no relevant financial disclosures.
[email protected]
On Twitter @maryjodales
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: There were 634 deaths (9.1% mortality) among patients in the short-term blood storage group and 1,213 deaths (8.7% mortality) in the long-term blood storage group.
Data source: The randomized, controlled INFORM (Informing Fresh versus Old Red Cell Management) trial.
Disclosures: INFORM, Current Controlled Trials number ISRCTN08118744, was funded by the Canadian Institutes of Health Research, Canadian Blood Services, and Health Canada. Ms. Heddle had no relevant financial disclosures.
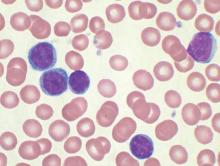
Minimal residual disease status predicts 10-year survival in CLL
Patients who have chronic lymphocytic leukemia and achieve minimal residual disease negativity have a high probability of long-term progression-free and overall survival, irrespective of the type of therapy they receive, reported Marwan Kwok, MD, of Queen Elizabeth Hospital Birmingham (England) and colleagues.
Minimal residual disease (MRD) negativity, defined as less than 1 chronic lymphocytic leukemic (CLL) cell detectable per 10,000 leukocytes, has been shown to independently predict clinical outcome in the front line setting, but the long-term prognostic value of MRD status in other therapeutic settings remains unclear. “Our results demonstrate the long-term benefit of achieving MRD negativity regardless of the therapeutic setting and treatment modality, and support its use as a prognostic marker for long-term PFS (progression-free survival) and as a potential therapeutic goal in CLL,” the authors wrote (Blood. 2016 Oct 3. doi: 10.1182/blood-2016-05-714162).
The researchers retrospectively analyzed, with up to 18 years of follow-up, all 133 CLL patients at St. James’s University Hospital in Leeds, England, who achieved at least a partial response with various therapies between 1996 and 2007 and who received a bone marrow MRD assessment at the end of treatment, according to the international harmonized approach.
MRD negativity correlated with progression-free and overall survival, and the association was independent of the type and line of treatment, as well as adverse cytogenetic findings, the investigators said.
For those who achieved MRD negativity in front-line treatment, the 10-year progression-free survival was 65%; survival in patients who did not achieve MRD negativity was 10%. Overall survival at 10 years was 70% for those who achieved MRD negativity and 30% for MRD-positive patients.
The authors had no relevant financial disclosures.
Patients who have chronic lymphocytic leukemia and achieve minimal residual disease negativity have a high probability of long-term progression-free and overall survival, irrespective of the type of therapy they receive, reported Marwan Kwok, MD, of Queen Elizabeth Hospital Birmingham (England) and colleagues.
Minimal residual disease (MRD) negativity, defined as less than 1 chronic lymphocytic leukemic (CLL) cell detectable per 10,000 leukocytes, has been shown to independently predict clinical outcome in the front line setting, but the long-term prognostic value of MRD status in other therapeutic settings remains unclear. “Our results demonstrate the long-term benefit of achieving MRD negativity regardless of the therapeutic setting and treatment modality, and support its use as a prognostic marker for long-term PFS (progression-free survival) and as a potential therapeutic goal in CLL,” the authors wrote (Blood. 2016 Oct 3. doi: 10.1182/blood-2016-05-714162).
The researchers retrospectively analyzed, with up to 18 years of follow-up, all 133 CLL patients at St. James’s University Hospital in Leeds, England, who achieved at least a partial response with various therapies between 1996 and 2007 and who received a bone marrow MRD assessment at the end of treatment, according to the international harmonized approach.
MRD negativity correlated with progression-free and overall survival, and the association was independent of the type and line of treatment, as well as adverse cytogenetic findings, the investigators said.
For those who achieved MRD negativity in front-line treatment, the 10-year progression-free survival was 65%; survival in patients who did not achieve MRD negativity was 10%. Overall survival at 10 years was 70% for those who achieved MRD negativity and 30% for MRD-positive patients.
The authors had no relevant financial disclosures.
Patients who have chronic lymphocytic leukemia and achieve minimal residual disease negativity have a high probability of long-term progression-free and overall survival, irrespective of the type of therapy they receive, reported Marwan Kwok, MD, of Queen Elizabeth Hospital Birmingham (England) and colleagues.
Minimal residual disease (MRD) negativity, defined as less than 1 chronic lymphocytic leukemic (CLL) cell detectable per 10,000 leukocytes, has been shown to independently predict clinical outcome in the front line setting, but the long-term prognostic value of MRD status in other therapeutic settings remains unclear. “Our results demonstrate the long-term benefit of achieving MRD negativity regardless of the therapeutic setting and treatment modality, and support its use as a prognostic marker for long-term PFS (progression-free survival) and as a potential therapeutic goal in CLL,” the authors wrote (Blood. 2016 Oct 3. doi: 10.1182/blood-2016-05-714162).
The researchers retrospectively analyzed, with up to 18 years of follow-up, all 133 CLL patients at St. James’s University Hospital in Leeds, England, who achieved at least a partial response with various therapies between 1996 and 2007 and who received a bone marrow MRD assessment at the end of treatment, according to the international harmonized approach.
MRD negativity correlated with progression-free and overall survival, and the association was independent of the type and line of treatment, as well as adverse cytogenetic findings, the investigators said.
For those who achieved MRD negativity in front-line treatment, the 10-year progression-free survival was 65%; survival in patients who did not achieve MRD negativity was 10%. Overall survival at 10 years was 70% for those who achieved MRD negativity and 30% for MRD-positive patients.
The authors had no relevant financial disclosures.
FROM BLOOD
Key clinical point:
Major finding: Overall survival at 10 years was 70% for those who achieved MRD negativity and 30% for those who did not.
Data source: Retrospective, single-center study of 133 patients with chronic lymphocytic leukemia.
Disclosures: The authors had no relevant financial disclosures.
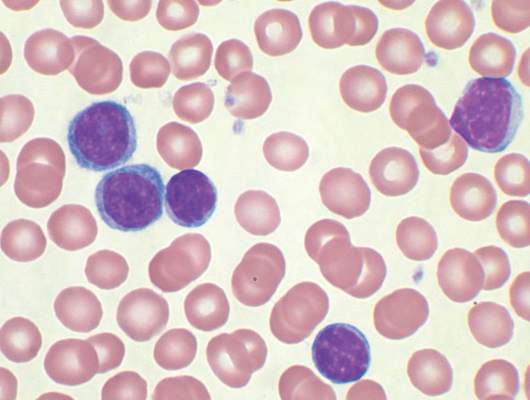
CD49d trumps novel recurrent mutations for predicting overall survival in CLL
CD49d was a strong predictor of overall survival in a cohort of 778 unselected patients with chronic lymphocytic leukemia, reported Michele Dal Bo, MD, of Centro di Riferimento Oncologico, in Aviano, Italy, and colleagues.
High CD49d expression was an independent predictor of poor overall survival in a multivariate Cox analysis (hazard ratio = 1.88, P less than .0001) and in each category of a risk stratification model. Among other biological prognosticators, CD49d was among the top predictors of overall survival (variable importance = 0.0410) along with immunoglobulin heavy chain variable (IGHV) gene mutational status and TP53 abnormalities. “In this context, TP53 disruption and NOTCH1 mutations retained prognostic relevance, in keeping with their roles in CLL cell immuno-chemoresistance,” the authors wrote.
CD49d was a strong predictor of overall survival in a cohort of 778 unselected patients with chronic lymphocytic leukemia, reported Michele Dal Bo, MD, of Centro di Riferimento Oncologico, in Aviano, Italy, and colleagues.
High CD49d expression was an independent predictor of poor overall survival in a multivariate Cox analysis (hazard ratio = 1.88, P less than .0001) and in each category of a risk stratification model. Among other biological prognosticators, CD49d was among the top predictors of overall survival (variable importance = 0.0410) along with immunoglobulin heavy chain variable (IGHV) gene mutational status and TP53 abnormalities. “In this context, TP53 disruption and NOTCH1 mutations retained prognostic relevance, in keeping with their roles in CLL cell immuno-chemoresistance,” the authors wrote.
CD49d was a strong predictor of overall survival in a cohort of 778 unselected patients with chronic lymphocytic leukemia, reported Michele Dal Bo, MD, of Centro di Riferimento Oncologico, in Aviano, Italy, and colleagues.
High CD49d expression was an independent predictor of poor overall survival in a multivariate Cox analysis (hazard ratio = 1.88, P less than .0001) and in each category of a risk stratification model. Among other biological prognosticators, CD49d was among the top predictors of overall survival (variable importance = 0.0410) along with immunoglobulin heavy chain variable (IGHV) gene mutational status and TP53 abnormalities. “In this context, TP53 disruption and NOTCH1 mutations retained prognostic relevance, in keeping with their roles in CLL cell immuno-chemoresistance,” the authors wrote.
VTE risk appears to vary over time in patients with multiple myeloma
Venous thromboembolism may occur later in the disease process of multiple myeloma than has historically been reported, based on data reported by Brea Lipe, MD, of the University of Kansas Medical Center, Kansas City, and her colleagues.
The risk for VTE appears to change over time. Patients with multiple myeloma should be assessed for VTE risk and thromboprophylaxis on an ongoing basis throughout the disease course, the researchers recommended at the annual meeting of the American Society of Clinical Oncology.
In a study originally designed to examine the adoption and utility of the International Myeloma Working Group thromboprophylaxis guidelines, the researchers used the Healthcare Enterprise Repository for Ontological Narration (HERON) database to identify case patients with multiple myeloma and a venous thromboembolism. Patients who had multiple myeloma and had not experienced a VTE were matched to the cases based on gender, age, and time of diagnosis. Patient charts were manually extracted to identify treatment history, disease history, risk factors for VTE, and guideline adherence regarding use of prophylactic anticoagulation for the matched patients at the time of diagnosis and at the time of the VTE.
There were 86 cases and 211 controls in the final cohort. The median time from diagnosis to VTE was 952 days. In accordance with the guidelines, patients with a higher risk of VTE were more likely to be on low-molecular-weight heparin (LMWH) or warfarin versus patients with a lower risk of VTE on aspirin or no prophylaxis (P less than .001). Risk category or prophylactic medication were not associated with the rate of VTE when considering baseline risk factors. Over time, however, the risk category of case patients changed to a higher risk group and this was associated with a higher risk of VTE (P = .06).
“While we were unable to validate the IMWG recommendations for thromboprophylaxis at diagnosis, our data suggest that VTE in multiple myeloma may occur later in the disease process than has historically been reported,” the researchers concluded.
Dr. Lipe has been an adviser to Takeda.
On Twitter @maryjodales
Venous thromboembolism may occur later in the disease process of multiple myeloma than has historically been reported, based on data reported by Brea Lipe, MD, of the University of Kansas Medical Center, Kansas City, and her colleagues.
The risk for VTE appears to change over time. Patients with multiple myeloma should be assessed for VTE risk and thromboprophylaxis on an ongoing basis throughout the disease course, the researchers recommended at the annual meeting of the American Society of Clinical Oncology.
In a study originally designed to examine the adoption and utility of the International Myeloma Working Group thromboprophylaxis guidelines, the researchers used the Healthcare Enterprise Repository for Ontological Narration (HERON) database to identify case patients with multiple myeloma and a venous thromboembolism. Patients who had multiple myeloma and had not experienced a VTE were matched to the cases based on gender, age, and time of diagnosis. Patient charts were manually extracted to identify treatment history, disease history, risk factors for VTE, and guideline adherence regarding use of prophylactic anticoagulation for the matched patients at the time of diagnosis and at the time of the VTE.
There were 86 cases and 211 controls in the final cohort. The median time from diagnosis to VTE was 952 days. In accordance with the guidelines, patients with a higher risk of VTE were more likely to be on low-molecular-weight heparin (LMWH) or warfarin versus patients with a lower risk of VTE on aspirin or no prophylaxis (P less than .001). Risk category or prophylactic medication were not associated with the rate of VTE when considering baseline risk factors. Over time, however, the risk category of case patients changed to a higher risk group and this was associated with a higher risk of VTE (P = .06).
“While we were unable to validate the IMWG recommendations for thromboprophylaxis at diagnosis, our data suggest that VTE in multiple myeloma may occur later in the disease process than has historically been reported,” the researchers concluded.
Dr. Lipe has been an adviser to Takeda.
On Twitter @maryjodales
Venous thromboembolism may occur later in the disease process of multiple myeloma than has historically been reported, based on data reported by Brea Lipe, MD, of the University of Kansas Medical Center, Kansas City, and her colleagues.
The risk for VTE appears to change over time. Patients with multiple myeloma should be assessed for VTE risk and thromboprophylaxis on an ongoing basis throughout the disease course, the researchers recommended at the annual meeting of the American Society of Clinical Oncology.
In a study originally designed to examine the adoption and utility of the International Myeloma Working Group thromboprophylaxis guidelines, the researchers used the Healthcare Enterprise Repository for Ontological Narration (HERON) database to identify case patients with multiple myeloma and a venous thromboembolism. Patients who had multiple myeloma and had not experienced a VTE were matched to the cases based on gender, age, and time of diagnosis. Patient charts were manually extracted to identify treatment history, disease history, risk factors for VTE, and guideline adherence regarding use of prophylactic anticoagulation for the matched patients at the time of diagnosis and at the time of the VTE.
There were 86 cases and 211 controls in the final cohort. The median time from diagnosis to VTE was 952 days. In accordance with the guidelines, patients with a higher risk of VTE were more likely to be on low-molecular-weight heparin (LMWH) or warfarin versus patients with a lower risk of VTE on aspirin or no prophylaxis (P less than .001). Risk category or prophylactic medication were not associated with the rate of VTE when considering baseline risk factors. Over time, however, the risk category of case patients changed to a higher risk group and this was associated with a higher risk of VTE (P = .06).
“While we were unable to validate the IMWG recommendations for thromboprophylaxis at diagnosis, our data suggest that VTE in multiple myeloma may occur later in the disease process than has historically been reported,” the researchers concluded.
Dr. Lipe has been an adviser to Takeda.
On Twitter @maryjodales
FROM ASCO 16
Key clinical point: Patients with multiple myeloma should be assessed for VTE risk and thromboprophylaxis on an ongoing basis throughout the disease course.
Major finding: Over time, the risk category of case patients changed to a higher risk group and this was associated with a higher risk of VTE (P = .06).
Data source: The Healthcare Enterprise Repository for Ontological Narration (HERON) database.
Disclosures: Dr. Lipe has been an adviser to Takeda.
Quality of life measures comparable for panobinostat or placebo in combination with bortezomib and dexamethasone
Quality of life measures were comparable for multiple myeloma patients whether they received panobinostat or placebo in combination with bortezomib and dexamethasone, Paul G. Richardson, MD, of Dana-Farber Cancer Institute, Boston, and his associates in the PANORAMA-1 trial reported at the annual meeting of the American Society of Clinical Oncology.
Symptoms, function, and health-related quality of life were measured using the EORTC-QLQ-30, QLQ-MY20, and FACT/GOG-Ntx scales at screening, the first day of the first therapy cycle, and every 6 weeks thereafter in multiple myeloma patients randomly assigned to receive 21-day cycles of either the triple regimen or a placebo plus bortezomib and dexamethasone.
Panobinostat, in combination with bortezomib and dexamethasone, is approved for multiple myeloma patients who have received at least two prior regimens, including bortezomib and an immunomodulatory drug
In the U.S.-label population of the PANORAMA-1 study, patient-reported outcomes data were available at baseline for 94 and 92 patients based on the EORTC QLQ-C30, Global Health Status/QoL scale and for 91 and 95 patients based on the QLQMY20, Disease Symptoms (DS) subscale and FACT/GOG-Ntx Neurotoxicity subscale.
After 24 weeks of therapy, FACT-GOG-Ntx scores were similar (32.03 vs. 33.92, respectively), suggesting no difference in neurotoxicity symptoms. In the QLQ-MY20 DS subscale, scores initially improved and stabilized, with no difference between the two groups of patients (20.65 vs. 15.85). After initially declining, EORTC QLQ-C30 Global Health Status/QoL scores were also comparable (54.84 vs. 58.55).
The study was sponsored by Novartis Pharmaceuticals, the maker of panobinostat. Dr. Richardson is an adviser to Novartis as well as several other drug companies.
On Twitter @maryjodales
Quality of life measures were comparable for multiple myeloma patients whether they received panobinostat or placebo in combination with bortezomib and dexamethasone, Paul G. Richardson, MD, of Dana-Farber Cancer Institute, Boston, and his associates in the PANORAMA-1 trial reported at the annual meeting of the American Society of Clinical Oncology.
Symptoms, function, and health-related quality of life were measured using the EORTC-QLQ-30, QLQ-MY20, and FACT/GOG-Ntx scales at screening, the first day of the first therapy cycle, and every 6 weeks thereafter in multiple myeloma patients randomly assigned to receive 21-day cycles of either the triple regimen or a placebo plus bortezomib and dexamethasone.
Panobinostat, in combination with bortezomib and dexamethasone, is approved for multiple myeloma patients who have received at least two prior regimens, including bortezomib and an immunomodulatory drug
In the U.S.-label population of the PANORAMA-1 study, patient-reported outcomes data were available at baseline for 94 and 92 patients based on the EORTC QLQ-C30, Global Health Status/QoL scale and for 91 and 95 patients based on the QLQMY20, Disease Symptoms (DS) subscale and FACT/GOG-Ntx Neurotoxicity subscale.
After 24 weeks of therapy, FACT-GOG-Ntx scores were similar (32.03 vs. 33.92, respectively), suggesting no difference in neurotoxicity symptoms. In the QLQ-MY20 DS subscale, scores initially improved and stabilized, with no difference between the two groups of patients (20.65 vs. 15.85). After initially declining, EORTC QLQ-C30 Global Health Status/QoL scores were also comparable (54.84 vs. 58.55).
The study was sponsored by Novartis Pharmaceuticals, the maker of panobinostat. Dr. Richardson is an adviser to Novartis as well as several other drug companies.
On Twitter @maryjodales
Quality of life measures were comparable for multiple myeloma patients whether they received panobinostat or placebo in combination with bortezomib and dexamethasone, Paul G. Richardson, MD, of Dana-Farber Cancer Institute, Boston, and his associates in the PANORAMA-1 trial reported at the annual meeting of the American Society of Clinical Oncology.
Symptoms, function, and health-related quality of life were measured using the EORTC-QLQ-30, QLQ-MY20, and FACT/GOG-Ntx scales at screening, the first day of the first therapy cycle, and every 6 weeks thereafter in multiple myeloma patients randomly assigned to receive 21-day cycles of either the triple regimen or a placebo plus bortezomib and dexamethasone.
Panobinostat, in combination with bortezomib and dexamethasone, is approved for multiple myeloma patients who have received at least two prior regimens, including bortezomib and an immunomodulatory drug
In the U.S.-label population of the PANORAMA-1 study, patient-reported outcomes data were available at baseline for 94 and 92 patients based on the EORTC QLQ-C30, Global Health Status/QoL scale and for 91 and 95 patients based on the QLQMY20, Disease Symptoms (DS) subscale and FACT/GOG-Ntx Neurotoxicity subscale.
After 24 weeks of therapy, FACT-GOG-Ntx scores were similar (32.03 vs. 33.92, respectively), suggesting no difference in neurotoxicity symptoms. In the QLQ-MY20 DS subscale, scores initially improved and stabilized, with no difference between the two groups of patients (20.65 vs. 15.85). After initially declining, EORTC QLQ-C30 Global Health Status/QoL scores were also comparable (54.84 vs. 58.55).
The study was sponsored by Novartis Pharmaceuticals, the maker of panobinostat. Dr. Richardson is an adviser to Novartis as well as several other drug companies.
On Twitter @maryjodales
FROM 2016 ASCO ANNUAL MEETING
Key clinical point: Quality of-life measures were comparable for multiple myeloma patients whether they received panobinostat or placebo in combination with bortezomib and dexamethasone.
Major finding: After 24 weeks of therapy, FACT-GOG-Ntx scores were similar (32.03 vs. 33.92, respectively), suggesting no difference in neurotoxicity symptoms.
Data source: U.S. participants in the PANORAMA-1 trial.
Disclosures: The study was sponsored by Novartis Pharmaceuticals, the maker of panobinostat. Dr. Richardson is an adviser to Novartis as well as several other drug companies.
CAR T-cell therapy eyed for CLL patients with residual disease
Four of eight patients with residual chronic lymphocytic leukemia (CLL) following initial chemotherapy had complete or partial responses to an outpatient therapy that used autologous T cells genetically targeted to the B cell–specific antigen CD19, Mark Blaine Geyer, MD, of Memorial Sloan Kettering Cancer Center, New York, reported at the annual meeting of the American Society of Clinical Oncology.
The therapy employing T cells genetically modified to express CD19-targeted 19-28z chimeric antigen receptors (CARs) was well tolerated but had limited observed efficacy, especially in patients with enlarged lymph nodes. The study goal was to find a safe dose of modified T cells for patients who have disease remaining after initial chemotherapy.
For the phase I dose escalation study (NCT01416974), Dr. Geyer and his associates enrolled eight CLL patients who had residual disease after upfront therapy consisting of six cycles of pentostatin, cyclophosphamide, and rituximab.
Five patients had clearly enlarged lymph nodes prior to T cell infusion.
Patients received cyclophosphamide 600 mg/m2 followed 2 days later by escalating doses of 19-28z T cells. Four of the five patients who received at least a 1 × 107 dose of 19-28z T cells/kg were admitted with fevers and mild cytokine release syndrome.
Maximal levels of CAR T cell persistence were detected at 8 weeks. With a median patient follow-up of 32 months, clinical complete response has been seen in two patients, partial response in two patients, and stable disease in one patient. Disease has progressed in three patients: one had a rising absolute lymphocyte count by the time of infusion and two had marrow response with progressive disease in lymph nodes. The median time to disease progression was 13.6 months, Dr. Geyer said.
Five of seven evaluable patients have received further CLL-directed therapy.
The researchers speculated that low-dose cyclophosphamide monotherapy, used before the CAR T-cell therapy, may be insufficient for lymphodepletion. Additionally, CAR T cell expansion and antitumor efficacy may be limited by a hostile CLL microenvironment. Strategies to enhance CAR T cell expansion and efficacy in patients with CLL are in preparation, Dr. Geyer reported.
Dr. Geyer had no financial disclosures. His colleagues reported various financial relationships with Juno Therapeutics, a developer of CAR technology.
On Twitter @maryjodales
Four of eight patients with residual chronic lymphocytic leukemia (CLL) following initial chemotherapy had complete or partial responses to an outpatient therapy that used autologous T cells genetically targeted to the B cell–specific antigen CD19, Mark Blaine Geyer, MD, of Memorial Sloan Kettering Cancer Center, New York, reported at the annual meeting of the American Society of Clinical Oncology.
The therapy employing T cells genetically modified to express CD19-targeted 19-28z chimeric antigen receptors (CARs) was well tolerated but had limited observed efficacy, especially in patients with enlarged lymph nodes. The study goal was to find a safe dose of modified T cells for patients who have disease remaining after initial chemotherapy.
For the phase I dose escalation study (NCT01416974), Dr. Geyer and his associates enrolled eight CLL patients who had residual disease after upfront therapy consisting of six cycles of pentostatin, cyclophosphamide, and rituximab.
Five patients had clearly enlarged lymph nodes prior to T cell infusion.
Patients received cyclophosphamide 600 mg/m2 followed 2 days later by escalating doses of 19-28z T cells. Four of the five patients who received at least a 1 × 107 dose of 19-28z T cells/kg were admitted with fevers and mild cytokine release syndrome.
Maximal levels of CAR T cell persistence were detected at 8 weeks. With a median patient follow-up of 32 months, clinical complete response has been seen in two patients, partial response in two patients, and stable disease in one patient. Disease has progressed in three patients: one had a rising absolute lymphocyte count by the time of infusion and two had marrow response with progressive disease in lymph nodes. The median time to disease progression was 13.6 months, Dr. Geyer said.
Five of seven evaluable patients have received further CLL-directed therapy.
The researchers speculated that low-dose cyclophosphamide monotherapy, used before the CAR T-cell therapy, may be insufficient for lymphodepletion. Additionally, CAR T cell expansion and antitumor efficacy may be limited by a hostile CLL microenvironment. Strategies to enhance CAR T cell expansion and efficacy in patients with CLL are in preparation, Dr. Geyer reported.
Dr. Geyer had no financial disclosures. His colleagues reported various financial relationships with Juno Therapeutics, a developer of CAR technology.
On Twitter @maryjodales
Four of eight patients with residual chronic lymphocytic leukemia (CLL) following initial chemotherapy had complete or partial responses to an outpatient therapy that used autologous T cells genetically targeted to the B cell–specific antigen CD19, Mark Blaine Geyer, MD, of Memorial Sloan Kettering Cancer Center, New York, reported at the annual meeting of the American Society of Clinical Oncology.
The therapy employing T cells genetically modified to express CD19-targeted 19-28z chimeric antigen receptors (CARs) was well tolerated but had limited observed efficacy, especially in patients with enlarged lymph nodes. The study goal was to find a safe dose of modified T cells for patients who have disease remaining after initial chemotherapy.
For the phase I dose escalation study (NCT01416974), Dr. Geyer and his associates enrolled eight CLL patients who had residual disease after upfront therapy consisting of six cycles of pentostatin, cyclophosphamide, and rituximab.
Five patients had clearly enlarged lymph nodes prior to T cell infusion.
Patients received cyclophosphamide 600 mg/m2 followed 2 days later by escalating doses of 19-28z T cells. Four of the five patients who received at least a 1 × 107 dose of 19-28z T cells/kg were admitted with fevers and mild cytokine release syndrome.
Maximal levels of CAR T cell persistence were detected at 8 weeks. With a median patient follow-up of 32 months, clinical complete response has been seen in two patients, partial response in two patients, and stable disease in one patient. Disease has progressed in three patients: one had a rising absolute lymphocyte count by the time of infusion and two had marrow response with progressive disease in lymph nodes. The median time to disease progression was 13.6 months, Dr. Geyer said.
Five of seven evaluable patients have received further CLL-directed therapy.
The researchers speculated that low-dose cyclophosphamide monotherapy, used before the CAR T-cell therapy, may be insufficient for lymphodepletion. Additionally, CAR T cell expansion and antitumor efficacy may be limited by a hostile CLL microenvironment. Strategies to enhance CAR T cell expansion and efficacy in patients with CLL are in preparation, Dr. Geyer reported.
Dr. Geyer had no financial disclosures. His colleagues reported various financial relationships with Juno Therapeutics, a developer of CAR technology.
On Twitter @maryjodales
FROM THE 2016 ASCO ANNUAL MEETING
Key clinical point: CAR T-cell therapy may be an option for chronic lymphocytic leukemia patients with residual disease after upfront therapy.
Major finding: Four of eight patients with residual CLL following initial chemotherapy had complete or partial responses to an outpatient therapy that used autologous T cells genetically targeted to the B cell–specific antigen CD19.
Data source: A phase I dose-finding and efficacy study in 8 patients with CLL.
Disclosures: Dr. Geyer had no financial disclosures. His colleagues reported various financial relationships with Juno Therapeutics, a developer of CAR technology.
The liver drives RBC elimination, iron recycling
“The liver, not the spleen, is the major on-demand site of red blood cell elimination and iron recycling,” according to Filip Swirski, PhD, of the Massachusetts General Hospital Center for Systems Biology, and his colleagues.
The liver relies on a buffer system consisting of bone marrow–derived monocytes that consume damaged red blood cells (RBCs) in the blood and settle in the liver, where they become the transient macrophages capable of iron recycling, the researchers concluded in a study published in Nature Medicine.
The study was designed to examine how the body clears old and damaged RBCs without releasing toxic levels of free iron. Damaged RBCs can release unbound forms of iron-carrying hemoglobin, which can cause kidney injury and can lead to anemia.
The researchers used several different models of RBC damage to examine RBC clearance and iron recycling in a mouse model. Damaged RBCs in the bloodstream prompted an increase in monocytes that took up the damaged cells and traveled to both the liver and the spleen. Within hours, almost all of the damaged RBCs were located within specialized macrophages seen only in the liver. Chemokines drew the monocytes to the liver, resulting in the accumulation of the iron-recycling macrophages.
Monocytes that express high levels of lymphocyte antigen 6 complex, locus C1 ingest stressed and senescent erythrocytes, accumulate in the liver via coordinated chemotactic cues, and differentiate into ferroportin 1–expressing macrophages that can deliver iron to hepatocytes. Monocyte-derived FPN1+Tim-4neg macrophages are transient, reside alongside embryonically derived T-cell immunoglobulin and mucin domain containing 4high Kupffer cells, and depend on the growth factor Csf1 and the transcription factor Nrf2, the researchers wrote.
Blocking that process resulted in impaired RBC clearance, toxic levels of free iron and hemoglobin, and signs of liver and kidney damage.
“If overactive, (the mechanism) could remove too many RBCs, but if (the mechanism is) sluggish or otherwise impaired, it could lead to iron toxicity. Further study could provide us with details of how this mechanism occurs in the first place and help us understand how to harness or suppress it in various conditions,” Dr. Swirski said in a press release.
The researchers had no financial conflicts of interest. The study was funded by the National Institutes of Health.
On Twitter @maryjodales
“The liver, not the spleen, is the major on-demand site of red blood cell elimination and iron recycling,” according to Filip Swirski, PhD, of the Massachusetts General Hospital Center for Systems Biology, and his colleagues.
The liver relies on a buffer system consisting of bone marrow–derived monocytes that consume damaged red blood cells (RBCs) in the blood and settle in the liver, where they become the transient macrophages capable of iron recycling, the researchers concluded in a study published in Nature Medicine.
The study was designed to examine how the body clears old and damaged RBCs without releasing toxic levels of free iron. Damaged RBCs can release unbound forms of iron-carrying hemoglobin, which can cause kidney injury and can lead to anemia.
The researchers used several different models of RBC damage to examine RBC clearance and iron recycling in a mouse model. Damaged RBCs in the bloodstream prompted an increase in monocytes that took up the damaged cells and traveled to both the liver and the spleen. Within hours, almost all of the damaged RBCs were located within specialized macrophages seen only in the liver. Chemokines drew the monocytes to the liver, resulting in the accumulation of the iron-recycling macrophages.
Monocytes that express high levels of lymphocyte antigen 6 complex, locus C1 ingest stressed and senescent erythrocytes, accumulate in the liver via coordinated chemotactic cues, and differentiate into ferroportin 1–expressing macrophages that can deliver iron to hepatocytes. Monocyte-derived FPN1+Tim-4neg macrophages are transient, reside alongside embryonically derived T-cell immunoglobulin and mucin domain containing 4high Kupffer cells, and depend on the growth factor Csf1 and the transcription factor Nrf2, the researchers wrote.
Blocking that process resulted in impaired RBC clearance, toxic levels of free iron and hemoglobin, and signs of liver and kidney damage.
“If overactive, (the mechanism) could remove too many RBCs, but if (the mechanism is) sluggish or otherwise impaired, it could lead to iron toxicity. Further study could provide us with details of how this mechanism occurs in the first place and help us understand how to harness or suppress it in various conditions,” Dr. Swirski said in a press release.
The researchers had no financial conflicts of interest. The study was funded by the National Institutes of Health.
On Twitter @maryjodales
“The liver, not the spleen, is the major on-demand site of red blood cell elimination and iron recycling,” according to Filip Swirski, PhD, of the Massachusetts General Hospital Center for Systems Biology, and his colleagues.
The liver relies on a buffer system consisting of bone marrow–derived monocytes that consume damaged red blood cells (RBCs) in the blood and settle in the liver, where they become the transient macrophages capable of iron recycling, the researchers concluded in a study published in Nature Medicine.
The study was designed to examine how the body clears old and damaged RBCs without releasing toxic levels of free iron. Damaged RBCs can release unbound forms of iron-carrying hemoglobin, which can cause kidney injury and can lead to anemia.
The researchers used several different models of RBC damage to examine RBC clearance and iron recycling in a mouse model. Damaged RBCs in the bloodstream prompted an increase in monocytes that took up the damaged cells and traveled to both the liver and the spleen. Within hours, almost all of the damaged RBCs were located within specialized macrophages seen only in the liver. Chemokines drew the monocytes to the liver, resulting in the accumulation of the iron-recycling macrophages.
Monocytes that express high levels of lymphocyte antigen 6 complex, locus C1 ingest stressed and senescent erythrocytes, accumulate in the liver via coordinated chemotactic cues, and differentiate into ferroportin 1–expressing macrophages that can deliver iron to hepatocytes. Monocyte-derived FPN1+Tim-4neg macrophages are transient, reside alongside embryonically derived T-cell immunoglobulin and mucin domain containing 4high Kupffer cells, and depend on the growth factor Csf1 and the transcription factor Nrf2, the researchers wrote.
Blocking that process resulted in impaired RBC clearance, toxic levels of free iron and hemoglobin, and signs of liver and kidney damage.
“If overactive, (the mechanism) could remove too many RBCs, but if (the mechanism is) sluggish or otherwise impaired, it could lead to iron toxicity. Further study could provide us with details of how this mechanism occurs in the first place and help us understand how to harness or suppress it in various conditions,” Dr. Swirski said in a press release.
The researchers had no financial conflicts of interest. The study was funded by the National Institutes of Health.
On Twitter @maryjodales
FROM NATURE MEDICINE
Three-drug regimen boosts progression-free survival in patients with relapsed CLL and adverse prognostic features
Progression-free survival was improved for poor prognosis patients with relapsed chronic lymphocytic leukemia when idelalisib was added to bendamustine and rituximab, based on the results of a randomized, double-blind, placebo-controlled phase III study.
The findings support the three-drug approach as an important treatment option for patients with relapsed CLL and adverse prognostic features, but the regimen also was associated with more grade 3 or greater adverse events, primarily neutropenia and opportunistic infections, that led to more study drug discontinuation, Jacqueline Claudia Barrientos, M.D., and her colleagues reported at the annual meeting of the American Society of Clinical Oncology.
The overall response rates were 68% in the three-drug group and 45% in the two-drug plus placebo group.
For the study, 416 patients were stratified based on whether they had 17p deletions or TP53 mutations, their IGHV mutation status, and whether they had refractory or relapsed disease. The 207 patients who received idelalisib, bendamustine, and rituximab had consistently better progression-free survival than the 209 patients who received placebo, bendamustine, and rituximab. The median progression-free survival was 23 months in the group that received idelalisib and 11 months in the group that received placebo, with an overall hazard ratio of 0.33 at a median follow-up of 12 months.
For those with 17p deletions or TP53 mutations, the median progression-free survival was 11 months with the three-drug regimen and 8 months for the two-drug plus placebo regimen. For those with neither of these abnormalities, progression-free survival was more than 24 months for patients given the three active drugs and 11 months for patients given two drugs plus placebo.
For those with the 11q deletion, median progression-free survival was 23 months with idelalisib, bendamustine, and rituximab and 9 months with placebo, bendamustine, and rituximab, said Dr. Barrientos of Hofstra University, Hempstead, N.Y.
For those with IGHV mutations, the median progression-free survival has not been reached in the idelalisib, bendamustine, and rituximab group and was 11 months in the placebo, bendamustine, and rituximab group.
Among patients with tumor burdens exceeding 5 cm, median progression-free survival was 23 months with idelalisib, bendamustine, and rituximab and about 10 months with placebo, bendamustine, and rituximab.
Grade 3 or greater adverse events affected 97% of patients given the three active drugs and 76% of patients given bendamustine and rituximab plus placebo. Adverse events resulted in drug dose reductions in 11% of those given the three active drugs and in 6% of those given bendamustine and rituximab plus placebo. The study drug was discontinued in 26% of those in the idelalisib, bendamustine, and rituximab group and in 13% of the placebo, bendamustine, and rituximab group. Death occurred in 10% of the study patients given idelalisib, bendamustine, and rituximab and in 7% of the patients given placebo, bendamustine, and rituximab.
To be eligible for the study (NCT01569295), patients needed to have previously treated recurrent CLL, have measurable lymphadenopathy, require therapy for CLL, and have experienced CLL progression for less than 36 months since the completion of their last prior therapy.
The treatment regimen consisted of 6 cycles every 28 days of bendamustine (70 mg/m2 on day 1 and day 2 of each cycle), rituximab (375 mg/m2 in cycle 1 and 500 mg/m2 in cycles 2-6), and idelalisib (150 mg twice daily) or placebo. Idelalisib or placebo was continued until an independent review committee confirmed disease progression, death, intolerable toxicity, or withdrawal of consent.
The study was sponsored by Gilead Sciences, the maker of idelalisib (Zydelig). Dr. Barrientos receives research funding from Gilead Sciences and Abbvie, and serves as a consultant or adviser to Celgene, Genentech, and Pharmacyclics.
On Twitter @maryjodales
Progression-free survival was improved for poor prognosis patients with relapsed chronic lymphocytic leukemia when idelalisib was added to bendamustine and rituximab, based on the results of a randomized, double-blind, placebo-controlled phase III study.
The findings support the three-drug approach as an important treatment option for patients with relapsed CLL and adverse prognostic features, but the regimen also was associated with more grade 3 or greater adverse events, primarily neutropenia and opportunistic infections, that led to more study drug discontinuation, Jacqueline Claudia Barrientos, M.D., and her colleagues reported at the annual meeting of the American Society of Clinical Oncology.
The overall response rates were 68% in the three-drug group and 45% in the two-drug plus placebo group.
For the study, 416 patients were stratified based on whether they had 17p deletions or TP53 mutations, their IGHV mutation status, and whether they had refractory or relapsed disease. The 207 patients who received idelalisib, bendamustine, and rituximab had consistently better progression-free survival than the 209 patients who received placebo, bendamustine, and rituximab. The median progression-free survival was 23 months in the group that received idelalisib and 11 months in the group that received placebo, with an overall hazard ratio of 0.33 at a median follow-up of 12 months.
For those with 17p deletions or TP53 mutations, the median progression-free survival was 11 months with the three-drug regimen and 8 months for the two-drug plus placebo regimen. For those with neither of these abnormalities, progression-free survival was more than 24 months for patients given the three active drugs and 11 months for patients given two drugs plus placebo.
For those with the 11q deletion, median progression-free survival was 23 months with idelalisib, bendamustine, and rituximab and 9 months with placebo, bendamustine, and rituximab, said Dr. Barrientos of Hofstra University, Hempstead, N.Y.
For those with IGHV mutations, the median progression-free survival has not been reached in the idelalisib, bendamustine, and rituximab group and was 11 months in the placebo, bendamustine, and rituximab group.
Among patients with tumor burdens exceeding 5 cm, median progression-free survival was 23 months with idelalisib, bendamustine, and rituximab and about 10 months with placebo, bendamustine, and rituximab.
Grade 3 or greater adverse events affected 97% of patients given the three active drugs and 76% of patients given bendamustine and rituximab plus placebo. Adverse events resulted in drug dose reductions in 11% of those given the three active drugs and in 6% of those given bendamustine and rituximab plus placebo. The study drug was discontinued in 26% of those in the idelalisib, bendamustine, and rituximab group and in 13% of the placebo, bendamustine, and rituximab group. Death occurred in 10% of the study patients given idelalisib, bendamustine, and rituximab and in 7% of the patients given placebo, bendamustine, and rituximab.
To be eligible for the study (NCT01569295), patients needed to have previously treated recurrent CLL, have measurable lymphadenopathy, require therapy for CLL, and have experienced CLL progression for less than 36 months since the completion of their last prior therapy.
The treatment regimen consisted of 6 cycles every 28 days of bendamustine (70 mg/m2 on day 1 and day 2 of each cycle), rituximab (375 mg/m2 in cycle 1 and 500 mg/m2 in cycles 2-6), and idelalisib (150 mg twice daily) or placebo. Idelalisib or placebo was continued until an independent review committee confirmed disease progression, death, intolerable toxicity, or withdrawal of consent.
The study was sponsored by Gilead Sciences, the maker of idelalisib (Zydelig). Dr. Barrientos receives research funding from Gilead Sciences and Abbvie, and serves as a consultant or adviser to Celgene, Genentech, and Pharmacyclics.
On Twitter @maryjodales
Progression-free survival was improved for poor prognosis patients with relapsed chronic lymphocytic leukemia when idelalisib was added to bendamustine and rituximab, based on the results of a randomized, double-blind, placebo-controlled phase III study.
The findings support the three-drug approach as an important treatment option for patients with relapsed CLL and adverse prognostic features, but the regimen also was associated with more grade 3 or greater adverse events, primarily neutropenia and opportunistic infections, that led to more study drug discontinuation, Jacqueline Claudia Barrientos, M.D., and her colleagues reported at the annual meeting of the American Society of Clinical Oncology.
The overall response rates were 68% in the three-drug group and 45% in the two-drug plus placebo group.
For the study, 416 patients were stratified based on whether they had 17p deletions or TP53 mutations, their IGHV mutation status, and whether they had refractory or relapsed disease. The 207 patients who received idelalisib, bendamustine, and rituximab had consistently better progression-free survival than the 209 patients who received placebo, bendamustine, and rituximab. The median progression-free survival was 23 months in the group that received idelalisib and 11 months in the group that received placebo, with an overall hazard ratio of 0.33 at a median follow-up of 12 months.
For those with 17p deletions or TP53 mutations, the median progression-free survival was 11 months with the three-drug regimen and 8 months for the two-drug plus placebo regimen. For those with neither of these abnormalities, progression-free survival was more than 24 months for patients given the three active drugs and 11 months for patients given two drugs plus placebo.
For those with the 11q deletion, median progression-free survival was 23 months with idelalisib, bendamustine, and rituximab and 9 months with placebo, bendamustine, and rituximab, said Dr. Barrientos of Hofstra University, Hempstead, N.Y.
For those with IGHV mutations, the median progression-free survival has not been reached in the idelalisib, bendamustine, and rituximab group and was 11 months in the placebo, bendamustine, and rituximab group.
Among patients with tumor burdens exceeding 5 cm, median progression-free survival was 23 months with idelalisib, bendamustine, and rituximab and about 10 months with placebo, bendamustine, and rituximab.
Grade 3 or greater adverse events affected 97% of patients given the three active drugs and 76% of patients given bendamustine and rituximab plus placebo. Adverse events resulted in drug dose reductions in 11% of those given the three active drugs and in 6% of those given bendamustine and rituximab plus placebo. The study drug was discontinued in 26% of those in the idelalisib, bendamustine, and rituximab group and in 13% of the placebo, bendamustine, and rituximab group. Death occurred in 10% of the study patients given idelalisib, bendamustine, and rituximab and in 7% of the patients given placebo, bendamustine, and rituximab.
To be eligible for the study (NCT01569295), patients needed to have previously treated recurrent CLL, have measurable lymphadenopathy, require therapy for CLL, and have experienced CLL progression for less than 36 months since the completion of their last prior therapy.
The treatment regimen consisted of 6 cycles every 28 days of bendamustine (70 mg/m2 on day 1 and day 2 of each cycle), rituximab (375 mg/m2 in cycle 1 and 500 mg/m2 in cycles 2-6), and idelalisib (150 mg twice daily) or placebo. Idelalisib or placebo was continued until an independent review committee confirmed disease progression, death, intolerable toxicity, or withdrawal of consent.
The study was sponsored by Gilead Sciences, the maker of idelalisib (Zydelig). Dr. Barrientos receives research funding from Gilead Sciences and Abbvie, and serves as a consultant or adviser to Celgene, Genentech, and Pharmacyclics.
On Twitter @maryjodales
FROM 2016 ASCO ANNUAL MEETING
Key clinical point: Progression-free survival was improved for poor prognosis patients with relapsed chronic lymphocytic leukemia when idelalisib was added to bendamustine, and rituximab.
Major finding: The median progression-free survival was 23 months in the group that received idelalisib and 11 months in the group that received placebo, with an overall hazard ratio of 0.33 at a median follow-up of 12 months.
Data source: A randomized, double-blind, placebo-controlled phase III study of 416 patients stratified on the basis of 17p deletions or TP53 mutations, IGHV mutation status, and whether they had refractory or relapsed disease.
Disclosures: The study was sponsored by Gilead Sciences, the maker of idelalisib (Zydelig). Dr. Barrientos receives research funding from Gilead Sciences and Abbvie, and serves as a consultant or adviser to Celgene, Genentech, and Pharmacyclics.
Treatment-free remissions achieved in patients with chronic myeloid leukemia
Treatment-free remission attempts are safe and are achievable in most patients with chronic myeloid leukemia (CML) in chronic phase, Timothy P. Hughes, MD, and his colleagues in the international ENESTop trial reported at the annual meeting of the American Society of Clinical Oncology.
The conclusion is based on follow-up data on 126 patients who achieved a sustained deep molecular response (MR4.5) after switching from imatinib (Gleevec) to nilotinib (Tasigna) and discontinued nilotinib. So far, these are the largest prospective treatment-free remission data set in a population of patients who achieved a sustained deep molecular response after switching from imatinib to nilotinib, Dr. Hughes, head of hematology at the University of Adelaide and his colleagues wrote in a poster presentation.
The ENESTop study is a single-arm, phase II study. Patients eligible for the study started treatment with imatinib when they were first diagnosed with CML, then switched to nilotinib for at least 2 years with the combined time on the drugs of at least 3 years and small amounts of leukemia cells remaining after the nilotinib treatment.
For the consolidation phase of the study, patients continued their nilotinib therapy for 1 year. Patients without confirmed loss of MR4.5 after 1 year were eligible to stop nilotinib. RQ-PCR (reverse transcriptase–polymerase chain reaction) was monitored every 12 weeks in the consolidation phase of the study and every 4 weeks during first 48 weeks of treatment-free remission. Nilotinib was restarted if patients had confirmed loss of deep molecular response (MR4 [consecutive BCR-ABL1IS greater than 0.01%]) or loss of major molecular response ([MMR] BCR-ABL1IS greater than 0.1%).
Of the 163 patients in the consolidation phase of the study, 126 entered treatment-free remission. Their median duration of tyrosine kinase inhibitor use prior to treatment-free remission was nearly 88 months, with a 53-month median duration of nilotinib therapy. At data cut-off, with median follow up of 50 weeks, 58% of the 126 patients were still in treatment-free remission at 48 weeks.
During treatment-free remission, 18 patients had confirmed loss of MR4 and 34 lost MMR. One patient had atypical transcript and came off the study. All but one of the 52 patients reinitiated nilotinib; 50 (98%) regained at least MMR by data cut-off, 48 (94%) regained MR4, and 47 (92%) regained MR4.5. One patient switched to another tyrosine kinase inhibitor at 22 weeks after restarting therapy.
Of those who restarted therapy, the median time was 12 weeks to regain MR4 and was 13 weeks to regain MR4.5. No new safety findings were observed on treatment.
The study is sponsored by Novartis, the maker of nilotinib (Tasigna). Dr. Hughes receives research support and honoraria from, and is a consultant or advisor to Novartis as well as Ariad and Bristol-Myers Squibb.
On Twitter @maryjodales
Treatment-free remission attempts are safe and are achievable in most patients with chronic myeloid leukemia (CML) in chronic phase, Timothy P. Hughes, MD, and his colleagues in the international ENESTop trial reported at the annual meeting of the American Society of Clinical Oncology.
The conclusion is based on follow-up data on 126 patients who achieved a sustained deep molecular response (MR4.5) after switching from imatinib (Gleevec) to nilotinib (Tasigna) and discontinued nilotinib. So far, these are the largest prospective treatment-free remission data set in a population of patients who achieved a sustained deep molecular response after switching from imatinib to nilotinib, Dr. Hughes, head of hematology at the University of Adelaide and his colleagues wrote in a poster presentation.
The ENESTop study is a single-arm, phase II study. Patients eligible for the study started treatment with imatinib when they were first diagnosed with CML, then switched to nilotinib for at least 2 years with the combined time on the drugs of at least 3 years and small amounts of leukemia cells remaining after the nilotinib treatment.
For the consolidation phase of the study, patients continued their nilotinib therapy for 1 year. Patients without confirmed loss of MR4.5 after 1 year were eligible to stop nilotinib. RQ-PCR (reverse transcriptase–polymerase chain reaction) was monitored every 12 weeks in the consolidation phase of the study and every 4 weeks during first 48 weeks of treatment-free remission. Nilotinib was restarted if patients had confirmed loss of deep molecular response (MR4 [consecutive BCR-ABL1IS greater than 0.01%]) or loss of major molecular response ([MMR] BCR-ABL1IS greater than 0.1%).
Of the 163 patients in the consolidation phase of the study, 126 entered treatment-free remission. Their median duration of tyrosine kinase inhibitor use prior to treatment-free remission was nearly 88 months, with a 53-month median duration of nilotinib therapy. At data cut-off, with median follow up of 50 weeks, 58% of the 126 patients were still in treatment-free remission at 48 weeks.
During treatment-free remission, 18 patients had confirmed loss of MR4 and 34 lost MMR. One patient had atypical transcript and came off the study. All but one of the 52 patients reinitiated nilotinib; 50 (98%) regained at least MMR by data cut-off, 48 (94%) regained MR4, and 47 (92%) regained MR4.5. One patient switched to another tyrosine kinase inhibitor at 22 weeks after restarting therapy.
Of those who restarted therapy, the median time was 12 weeks to regain MR4 and was 13 weeks to regain MR4.5. No new safety findings were observed on treatment.
The study is sponsored by Novartis, the maker of nilotinib (Tasigna). Dr. Hughes receives research support and honoraria from, and is a consultant or advisor to Novartis as well as Ariad and Bristol-Myers Squibb.
On Twitter @maryjodales
Treatment-free remission attempts are safe and are achievable in most patients with chronic myeloid leukemia (CML) in chronic phase, Timothy P. Hughes, MD, and his colleagues in the international ENESTop trial reported at the annual meeting of the American Society of Clinical Oncology.
The conclusion is based on follow-up data on 126 patients who achieved a sustained deep molecular response (MR4.5) after switching from imatinib (Gleevec) to nilotinib (Tasigna) and discontinued nilotinib. So far, these are the largest prospective treatment-free remission data set in a population of patients who achieved a sustained deep molecular response after switching from imatinib to nilotinib, Dr. Hughes, head of hematology at the University of Adelaide and his colleagues wrote in a poster presentation.
The ENESTop study is a single-arm, phase II study. Patients eligible for the study started treatment with imatinib when they were first diagnosed with CML, then switched to nilotinib for at least 2 years with the combined time on the drugs of at least 3 years and small amounts of leukemia cells remaining after the nilotinib treatment.
For the consolidation phase of the study, patients continued their nilotinib therapy for 1 year. Patients without confirmed loss of MR4.5 after 1 year were eligible to stop nilotinib. RQ-PCR (reverse transcriptase–polymerase chain reaction) was monitored every 12 weeks in the consolidation phase of the study and every 4 weeks during first 48 weeks of treatment-free remission. Nilotinib was restarted if patients had confirmed loss of deep molecular response (MR4 [consecutive BCR-ABL1IS greater than 0.01%]) or loss of major molecular response ([MMR] BCR-ABL1IS greater than 0.1%).
Of the 163 patients in the consolidation phase of the study, 126 entered treatment-free remission. Their median duration of tyrosine kinase inhibitor use prior to treatment-free remission was nearly 88 months, with a 53-month median duration of nilotinib therapy. At data cut-off, with median follow up of 50 weeks, 58% of the 126 patients were still in treatment-free remission at 48 weeks.
During treatment-free remission, 18 patients had confirmed loss of MR4 and 34 lost MMR. One patient had atypical transcript and came off the study. All but one of the 52 patients reinitiated nilotinib; 50 (98%) regained at least MMR by data cut-off, 48 (94%) regained MR4, and 47 (92%) regained MR4.5. One patient switched to another tyrosine kinase inhibitor at 22 weeks after restarting therapy.
Of those who restarted therapy, the median time was 12 weeks to regain MR4 and was 13 weeks to regain MR4.5. No new safety findings were observed on treatment.
The study is sponsored by Novartis, the maker of nilotinib (Tasigna). Dr. Hughes receives research support and honoraria from, and is a consultant or advisor to Novartis as well as Ariad and Bristol-Myers Squibb.
On Twitter @maryjodales
FROM 2016 ASCO ANNUAL MEETING
Key clinical point: Treatment-free remission attempts are safe and are achievable in most patients with chronic myeloid leukemia in chronic phase.
Major finding: At data cut-off, with median follow-up of 50 weeks, 58% of the 126 patients who entered the treatment-free stage of the study were still in treatment-free remission at 48 weeks.
Data source: The ENESTop study is a single-arm, phase II study that included 163 patients.
Disclosures: The study is sponsored by Novartis, the maker of nilotinib (Tasigna). Dr. Hughes receives research support and honoraria from, and is a consultant or advisor to Novartis as well as Ariad and Bristol-Myers Squibb.
Smoldering multiple myeloma affects 1 in 7 patients
About 1 in 7 cases of multiple myeloma diagnosed in the United States are cases of smoldering disease, according to an analysis of data from the National Cancer Data Base, which represents 70% of cancer cases.
The prevalence of smoldering multiple myeloma varied among various socio- and geodemographic subgroups, but overall survival did not, Aishwarya Ravindran, MBBS, of Mayo Clinic, Rochester, Minn., and colleagues reported at the annual meeting of the American Society of Clinical Oncology. “Our results can be used in the future to study the health care impact of SMM,” the researchers wrote in a poster presentation.
Epidemiologic studies of smoldering multiple myeloma have been limited by the lack of International Classification of Diseases codes specific for smoldering status, the researchers said.
They analyzed 86,327 cases of multiple myeloma, considering socio- and geodemographic subgroups and type of treatment facility. Overall survival was compared for smoldering and active multiple myeloma. The researchers included patients enrolled in the database during 2003-2011; records were examined from the time to initial treatment and they considered reasons for patients not receiving treatment.
Patients who did not require treatment within the first 120 days after diagnosis were considered to have smoldering disease. This group comprised almost 14% of the cases.
The proportion of cases that were smoldering disease did not change significantly during the study period (P = .23 and .34, respectively). Smoldering disease was more likely to be diagnosed among women, black patients, older patients (median age at diagnosis was 67 years), and less educated patients. Smoldering disease was more common in patients with fewer medical comorbidities, those living closer to a treatment facility, and those evaluated for their disease in the Northeast United States. The proportions of cases diagnosed at academic and nonacademic facilities were similar.
The median overall survival for smoldering disease was 63 months; for active disease, 33 months. Overall survival in those with smoldering disease did not differ among the racial groups (P = .27).
The researchers had no financial conflicts.
On Twitter @maryjodales
About 1 in 7 cases of multiple myeloma diagnosed in the United States are cases of smoldering disease, according to an analysis of data from the National Cancer Data Base, which represents 70% of cancer cases.
The prevalence of smoldering multiple myeloma varied among various socio- and geodemographic subgroups, but overall survival did not, Aishwarya Ravindran, MBBS, of Mayo Clinic, Rochester, Minn., and colleagues reported at the annual meeting of the American Society of Clinical Oncology. “Our results can be used in the future to study the health care impact of SMM,” the researchers wrote in a poster presentation.
Epidemiologic studies of smoldering multiple myeloma have been limited by the lack of International Classification of Diseases codes specific for smoldering status, the researchers said.
They analyzed 86,327 cases of multiple myeloma, considering socio- and geodemographic subgroups and type of treatment facility. Overall survival was compared for smoldering and active multiple myeloma. The researchers included patients enrolled in the database during 2003-2011; records were examined from the time to initial treatment and they considered reasons for patients not receiving treatment.
Patients who did not require treatment within the first 120 days after diagnosis were considered to have smoldering disease. This group comprised almost 14% of the cases.
The proportion of cases that were smoldering disease did not change significantly during the study period (P = .23 and .34, respectively). Smoldering disease was more likely to be diagnosed among women, black patients, older patients (median age at diagnosis was 67 years), and less educated patients. Smoldering disease was more common in patients with fewer medical comorbidities, those living closer to a treatment facility, and those evaluated for their disease in the Northeast United States. The proportions of cases diagnosed at academic and nonacademic facilities were similar.
The median overall survival for smoldering disease was 63 months; for active disease, 33 months. Overall survival in those with smoldering disease did not differ among the racial groups (P = .27).
The researchers had no financial conflicts.
On Twitter @maryjodales
About 1 in 7 cases of multiple myeloma diagnosed in the United States are cases of smoldering disease, according to an analysis of data from the National Cancer Data Base, which represents 70% of cancer cases.
The prevalence of smoldering multiple myeloma varied among various socio- and geodemographic subgroups, but overall survival did not, Aishwarya Ravindran, MBBS, of Mayo Clinic, Rochester, Minn., and colleagues reported at the annual meeting of the American Society of Clinical Oncology. “Our results can be used in the future to study the health care impact of SMM,” the researchers wrote in a poster presentation.
Epidemiologic studies of smoldering multiple myeloma have been limited by the lack of International Classification of Diseases codes specific for smoldering status, the researchers said.
They analyzed 86,327 cases of multiple myeloma, considering socio- and geodemographic subgroups and type of treatment facility. Overall survival was compared for smoldering and active multiple myeloma. The researchers included patients enrolled in the database during 2003-2011; records were examined from the time to initial treatment and they considered reasons for patients not receiving treatment.
Patients who did not require treatment within the first 120 days after diagnosis were considered to have smoldering disease. This group comprised almost 14% of the cases.
The proportion of cases that were smoldering disease did not change significantly during the study period (P = .23 and .34, respectively). Smoldering disease was more likely to be diagnosed among women, black patients, older patients (median age at diagnosis was 67 years), and less educated patients. Smoldering disease was more common in patients with fewer medical comorbidities, those living closer to a treatment facility, and those evaluated for their disease in the Northeast United States. The proportions of cases diagnosed at academic and nonacademic facilities were similar.
The median overall survival for smoldering disease was 63 months; for active disease, 33 months. Overall survival in those with smoldering disease did not differ among the racial groups (P = .27).
The researchers had no financial conflicts.
On Twitter @maryjodales
FROM ASCO 2016
Key clinical point: The prevalence of smoldering multiple myeloma varied among various socio- and geodemographic subgroups, but overall survival did not.
Major finding: About 1 in 7 cases of multiple myeloma diagnosed in the United States are cases of smoldering disease.
Data source: At total of 86,327 cases of multiple myeloma from the National Cancer Data Base, which represents 70% of cancer cases.
Disclosures: The researchers had no financial conflicts.