User login
Diet and Atopic Dermatitis
Atopic dermatitis (AD) is the leading diagnosis among pediatric dermatologists,1 and this condition is commonly seen worldwide by dermatologists and allergists.2 There is a widespread misconception held by many patients and their guardians who believe that AD is caused by a food allergy.3 Although AD is related to and part of the atopic complex of disorders associated with food allergies, the role of diet in AD is not well defined. Previously it was recommended to delay early exposure to foods, but now it is recommended to do the opposite in certain situations. In fact, delaying exposure to certain types of foods can increase the likelihood of food allergies (eg, early exposure to peanut butter lowers the statistical chance of developing peanut allergies). This article reviews recent data on the role of diet in AD regarding disease activity as well as new and emerging data on dietary modifications for prevention and intervention. Emerging data on the relationship between AD and food allergies also are presented.
Pathogenesis of AD
The skin barrier plays a vital role in the prevention of pathogens, allergen exposure, and sensitization. There is no solitary root cause of AD, rather it is a combination of inflammation and barrier dysfunction associated with allergic diathesis (eg, atopy). Many patients with AD, especially those with persistent disease, have an intrinsic barrier dysfunction as part of the root cause of their illness, which may be caused by genetically mediated filaggrin defects or alternative barrier dysfunction such as decreased ceramide content that predisposes to percutaneous and mucosal sensitization.4,5 Another source of percutaneous exposure to allergens is macroscopic breaks in the skin caused by scratching, which allows dendritic termini of Langerhans cells to be exposed to percutaneous antigens4,6 through binding to high-affinity IgE receptors.
Langerhans cells exposed to allergens can trigger either an immediate or delayed-type (type I or type II) reaction (sensitization phase) in the lymph node causing inflammatory activation (elicitation). Inflammatory activity in AD is broad and complex and includes the release of IL-4, elevated IgE levels, and eosinophilia, which trigger the helper T cell TH2 and TH17 cascade of cytokines, including IL-2, IL-4, IL-5, IL-8, IL-10, IL-13, IL-17α, tumor necrosis factor α, and IFN-γ,7-9 with the latter worsening barrier defect via downregulation of intercellular substances (eg, filaggrin) and intercellular adhesion expression (eg, claudin 1).6,7,10
Atopic dermatitis does not exist in isolation. The barrier dysfunction associated with AD allows for sensitization to allergens, including those found in food and/or the environment. The atopic march, which occurs via barrier abnormalities facilitating sensitization, can result in further atopy, such as food allergies, environmental allergies, asthma, and eosinophilic esophagitis.11
|
AD and Food Allergies
Many patients and guardians believe AD is caused by a food allergy and that diet restrictions will resolve the disease. Although the latter is not true, in reality many patients with AD do have food allergies. Approximately 40% of infants and young children with moderate to severe AD and 8% of the general population of children will manifest a specific IgE-based food allergy. Food-specific IgE can be triggered or exacerbated by AD through the induction of hives, cutaneous activation of mast cells, increased “spontaneous” basophil histamine release, and food-related lymphocyte-proliferative responses measurable by food patch testing.12 Allergists generally recommend avoidance of or use of heavily denatured food (in the case of a milk/egg allergy) in the setting of documented IgE-mediated allergens.13 Food allergies in AD can manifest with flares, hives, pruritus, and/or other cutaneous symptoms in the absence of flaring AD disease.
Guidelines from the American Academy of Dermatology (AAD)(Table) for the management of AD have recently recommended testing for food allergies in children younger than 5 years who have intractable AD or known food-induced reactions.14 This technique will largely identify children at risk for anaphylaxis but may not yield information contributing to AD improvement. Furthermore, withdrawal of allergens with known IgE-mediated response was classified by the AAD as having consistent good-quality patient-oriented evidence, and asking about allergic reactions as well as acting on a reported allergic history had inconsistent or limited-quality patient-oriented evidence. It is believed that atopy can progress, or march, into a food and/or environmental allergy at any point in life; therefore, testing for a food allergy should be considered in all patients with recent onset of severe and/or persistent AD and/or food-aggravated AD due to a lifetime risk of sensitization.14,15 A food introduction plan may require collaboration with an allergist, especially in high-risk patients (eg, those with known food reactions, family history of food allergies, severe atopy).
Prevention of AD Through Dietary Modification
The National Institute of Allergy and Infectious Diseases consensus group published guidelines on food allergies that affect AD management, including avoidance of proven allergens but not random elimination of food allergens in AD; the group identifies AD and family history of AD as risk factors for food allergies.16 The best data in support of avoidance of documented food allergens to reduce AD severity has been found for egg white allergy and avoidance. Active egg allergy also is linked to staphylococcal superantigen IgE sensitizations,17 but the reason for the link is not yet clear. For the pediatric population, exclusive breastfeeding until 4 to 6 months of age and introduction of solids within the first 4 to 6 months as well as avoidance of maternal dietary restriction during pregnancy and lactation was further endorsed, with use of hydrolyzed formulas as an alternative to exclusive breastfeeding in infants who are not exclusively breastfed (cost permitting).16,18
A Cochrane review of maternal dietary restrictions during pregnancy found no benefit of maternal prenatal dietary restriction on AD prevalence in the first 18 months of life but did note an association with lower mean gestational weight.19
There is currently an effort to produce foods, such as soybeans and corn, that are genetically modified to reduce exposure to the allergenic component, but it is possible that when large-scale challenges occur, these foods also will be allergenic.20,21 In the case of a modified apple, some promising reduction in allergy symptoms has been reported.22 Although genetically modified foods may benefit children with food allergies in the future, they are a source of some controversy.
Complementary and Alternative Medicine
The AAD guidelines do not recommend complementary and alternative medicine (CAM) to treat AD,14 but it remains a commonly used therapy in the United States. A 2014 analysis of data from the 2007 US-based national health interview survey of 9417 children (age range, 0–17 years) demonstrated that 46.9% of children used 1 or more CAM, of which 0.99% used CAM specifically for AD. In this study, herbal therapy, vitamins, homeopathy, diet, and movement techniques were associated with increased prevalence of AD.23 Although some herbals have been shown to be beneficial in AD,24 hepatotoxicity has been reported with some herbal therapies.25 Complementary techniques with evidence-based support include massage therapy,26 relocation to an alternative climate, acupuncture that rivals cetirizine in efficacy, and supportive nutritional advice.24,27
Factors Affecting the Incidence of AD
Atopic dermatitis is of greater prevalence in children in developed wealthy nations such as the United States, supporting the role of enhanced hygiene and overall good health through vaccination as a possible contributor to the rise in AD prevalence in the last 4 decades.28,29 Alternatively, viruses such as respiratory syncytial virus may trigger AD, suggesting vaccination against the virus may reduce the risk for AD.30 Overall, vaccination improves life expectancy and should be conducted on schedule without reservation. Other aspects of hygiene that could conceptually affect prevalence of AD are raw food ingestion and the effects of foodborne microbes on the intestinal microbiome in relationship to AD development. Probiotics have been tested for this purpose.
Probiotics and prebiotics have been theorized to work through a reduction in inflammation; these agents have some evidence in their favor, but they were not endorsed in the AAD guidelines14 despite showing promise in meta-analysis. In particular prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus shows promise.31-33 Food elimination diets and supplements including vitamin D, selenium, fish oil, borage oil, and zinc were not found to be beneficial and were not recommended in the AAD guidelines.14,34
Percutaneous exposure to peanuts, possibly in household dust, may be the mechanism of peanut sensitization in AD27 via an inherent adjuvant effect of peanut protein.28 The recent LEAP (Learning Early About Peanut Allergy) trial randomized 530 infants aged 4 to 11 months to peanut-avoidant versus peanut-exposed diets for 60 months. The results showed statistically reduced (approximately one-twelfth of the risk) peanut allergy even in infants known to be sensitized (approximately one-third of the risk).35 It is now recommended in countries with a high prevalence of peanut allergies to introduce peanuts to an infant’s diet between 4 and 11 months of age (evidence level 1 [highest level of evidence]), with referral to an allergist for introduction in known sensitization cases and severe AD.36 In the setting of known or documented peanut allergy and for evaluation of potential food allergies, an allergist should be consulted.
Other interventions have been described as promising in mouse models. Those supplements include Lithospermum erythrorhizon,37Platycodon grandiflorus,38Hypsizygus marmoreous,39 fortified ginseng extract,40 polyunsaturated fatty acids,41 and galactooligosaccharide.42 Prebiotic oligosaccharides also are promising for early prevention of AD symptoms in infants, but otherwise these agents have remained largely untested in AD.43 None of these therapies have been endorsed by the AAD, and the long-term safety and efficacy in humans remains to be proven.
Risks of Dietary Restriction
Dietary restrictions in treating AD can have negative consequences, including reduced birth weight when initiated in pregnancy,19 osteomalacia from vitamin D deficiency,44 and nutritional deficiencies (eg, calcium, phosphorus, iron, vitamin K, vitamin D, zinc, vitamin A, B1, B2, B6, niacin, cholesterol, and/or vitamin C deficiencies).45 Excess dietary intake of vegetables in individuals with extensive food allergies can result in carotenemia.46 Protein-restricted diets from use of rice milk or dietary protein restriction can result in kwashiorkorlike protein malnutrition and marasmus.47-49 Nutritional counseling and/or supplementation is recommended for patients with food-restricted diets.
Avoiding Fragrance in Food
Food intolerance often is reported by AD patients. In allergies, food intolerance refers to side effects such as gastrointestinal symptoms; in dermatology, food intolerance can include itching, systemic flares of allergic contact dermatitis (eg, fragrance allergy), or true IgE-mediated allergies such as oral allergy syndrome. Oral allergy syndrome (pollen-food allergy syndrome) is an epitope-spread phenomenon related to an allergy to tree pollen, causing broad allergy to specific groups of fruits and nuts.50 Food triggers in AD include kiwi, milk, apple, tomato, citrus fruits, tree nuts, and peanuts. Oral allergy syndrome is common in food-sensitive AD patients (51.2%) followed by gastrointestinal symptoms (23.5%) and worsening AD (11.4%).51 Sensitization to fragrance can cross-react with foods (eg, balsam of Peru and tomatoes).52 A tomato allergy can be detected either by a skin-prick test or a food patch test in this setting.53 An allergist should be consulted if oral allergy syndrome is suspected.
Conclusion
Food allergies are more common in AD patients and patients should be referred to an allergist for evaluation and management. Strict dietary practice is not recommended, while avoiding proven food allergens in AD could be beneficial. Dermatologists should be aware that patients with dietary restrictions may lack key nutrients, manifesting with nutritional deficiencies in the skin; therefore, nutrition counseling may be needed in the most severe AD/allergy patients. This field is evolving; therefore, ongoing study and evaluation of interventions as they relate to AD will be needed to assess best practices for diet in AD over time.
1. Schachner L, Ling NS, Press S. A statistical analysis of a pediatric dermatology clinic. Pediatr Dermatol. 1983;1:157-164.
2. Kiprono SK, Muchunu JW, Masenga JE. Skin diseases in pediatric patients attending a tertiary dermatology hospital in Northern Tanzania: a cross-sectional study. BMC Dermatol. 2015;15:16.
3. Wensink M, Timmer C, Brand PL. Atopic dermatitis in infants not caused by food allergy [in Dutch]. Ned Tijdschr Geneeskd. 2008;152:4-9.
4. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(3, pt 2):949-963.
5. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
6. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
7. Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
8. Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
9. Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
10. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
11. Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review [published online July 22, 2015]. Clin Rev Allergy Immunol. doi:10.1111/all.12846.
12. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis; pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999;104(3, pt 2):S114-S122.
13. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.
14. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
15. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the epidermal differentiation complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
16. Burks AW, Jones SM, Boyce JA, et al. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics. 2011;128:955-965.
17. Ong PY. Association between egg and staphylococcal superantigen IgE sensitizations in atopic dermatitis. Allergy Asthma Proc. 2014;35:346-348.
18. Botteman M, Detzel P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in Southeast Asia. Ann Nutr Metab. 2015;66(suppl 1):26-32.
19. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.
20. Yum HY, Lee SY, Lee KE, et al. Genetically modified and wild soybeans: an immunologic comparison. Allergy Asthma Proc. 2005;26:210-216.
21. Mathur C, Kathuria PC, Dahiya P, et al. Lack of detectable allergenicity in genetically modified maize containing “Cry” proteins as compared to native maize based on in silico & in vitro analysis. PLoS One. 2015;10:e0117340.
22. Dubois AE, Pagliarani G, Brouwer RM, et al. First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar [published online July 24, 2015]. Allergy. 2015;70:1406-1412.
23. Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
24. Pfab F, Schalock PC, Napadow V, et al. Complementary integrative approach for treating pruritus. Dermatol Ther. 2013;26:149-156.
25. Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
26. Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
27. Pfab F, Schalock PC, Napadow V, et al. Acupuncture for allergic disease therapy–the current state of evidence. Expert Rev Clin Immunol. 2014;10:831-841.
28. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
29. Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
30. Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
31. Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
32. Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
33. Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
34. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I: atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
35. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
36. Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants [published online October 2015]. Allergy. 2015;70:1193-1195.
37. Kim J, Cho Y. Gromwell (Lithospermum erythrorhizon) supplementation enhances epidermal levels of cera-mides, glucosylceramides, β-glucocerebrosidase, and acidicsphingomyelinase in NC/Nga mice. J Med Food. 2013;16:927-933.
38. Choi JH, Jin SW, Han EH, et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21:1053-1061.
39. Kim T, Park K, Jung HS, et al. Evaluation of anti-atopic dermatitis activity of Hypsizigus marmoreus extract. Phytother Res. 2014;28:1539-1546.
40. Kim JR, Choi J, Kim J, et al. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365-371.
41. Weise C, Ernst D, van Tol EA, et al. Dietary polyunsaturated fatty acids and non-digestible oligosaccharides reduce dermatitis in mice. Pediatr Allergy Immunol. 2013;24:361-367.
42. Tanabe S, Hochi S. Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Int J Mol Med. 2010;25:331-336.
43. Arslanoglu S, Moro GE, Boehm G, et al. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J Biol Regul Homeost Agents. 2012;26(3 suppl):49-59.
44. Shikino K, Ikusaka M, Yamashita T. Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis [published online July 4, 2014] . BMJ Case Rep.
45. Kim J, Kwon J, Noh G, et al. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7:488-494.
46. Silverberg NB, Lee-Wong M. Generalized yellow discoloration of the skin. Cutis. 2014;93:E11-E12.
47. Hon KL, Nip SY, Cheung KL. A tragic case of atopic eczema: malnutrition and infections despite multivitamins and supplements. Iran J Allergy Asthma Immunol. 2012;11:267-270.
48. Diamanti A, Pedicelli S, D’Argenio P, et al. Iatrogenic kwashiorkor in three infants on a diet of rice beverages. Pediatr Allergy Immunol. 2011;22:878-879.
49. Pillai K, Acharya S. Iatrogenic kwashiorkar. Indian Pediatr. 2010;47:540-541.
50. Price A, Ramachandran S, Smith GP, et al. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015;26:78-88.
51. Mattila L, Kilpeläinen M, Terho EO, et al. Food hypersensitivity among Finnish university students: association with atopic diseases. Clin Exp Allergy. 2003;33:600-606.
52. Paulsen E, Christensen LP, Andersen KE. Tomato contact dermatitis. Contact Dermatitis. 2012;67:321-327.
53. Di Leo E, Nettis E, Cardinale F, et al. Tomato atopy patch test in adult atopic dermatitis: diagnostic value and comparison among different methods. Allergy. 2009;64:659-663.
Atopic dermatitis (AD) is the leading diagnosis among pediatric dermatologists,1 and this condition is commonly seen worldwide by dermatologists and allergists.2 There is a widespread misconception held by many patients and their guardians who believe that AD is caused by a food allergy.3 Although AD is related to and part of the atopic complex of disorders associated with food allergies, the role of diet in AD is not well defined. Previously it was recommended to delay early exposure to foods, but now it is recommended to do the opposite in certain situations. In fact, delaying exposure to certain types of foods can increase the likelihood of food allergies (eg, early exposure to peanut butter lowers the statistical chance of developing peanut allergies). This article reviews recent data on the role of diet in AD regarding disease activity as well as new and emerging data on dietary modifications for prevention and intervention. Emerging data on the relationship between AD and food allergies also are presented.
Pathogenesis of AD
The skin barrier plays a vital role in the prevention of pathogens, allergen exposure, and sensitization. There is no solitary root cause of AD, rather it is a combination of inflammation and barrier dysfunction associated with allergic diathesis (eg, atopy). Many patients with AD, especially those with persistent disease, have an intrinsic barrier dysfunction as part of the root cause of their illness, which may be caused by genetically mediated filaggrin defects or alternative barrier dysfunction such as decreased ceramide content that predisposes to percutaneous and mucosal sensitization.4,5 Another source of percutaneous exposure to allergens is macroscopic breaks in the skin caused by scratching, which allows dendritic termini of Langerhans cells to be exposed to percutaneous antigens4,6 through binding to high-affinity IgE receptors.
Langerhans cells exposed to allergens can trigger either an immediate or delayed-type (type I or type II) reaction (sensitization phase) in the lymph node causing inflammatory activation (elicitation). Inflammatory activity in AD is broad and complex and includes the release of IL-4, elevated IgE levels, and eosinophilia, which trigger the helper T cell TH2 and TH17 cascade of cytokines, including IL-2, IL-4, IL-5, IL-8, IL-10, IL-13, IL-17α, tumor necrosis factor α, and IFN-γ,7-9 with the latter worsening barrier defect via downregulation of intercellular substances (eg, filaggrin) and intercellular adhesion expression (eg, claudin 1).6,7,10
Atopic dermatitis does not exist in isolation. The barrier dysfunction associated with AD allows for sensitization to allergens, including those found in food and/or the environment. The atopic march, which occurs via barrier abnormalities facilitating sensitization, can result in further atopy, such as food allergies, environmental allergies, asthma, and eosinophilic esophagitis.11
|
AD and Food Allergies
Many patients and guardians believe AD is caused by a food allergy and that diet restrictions will resolve the disease. Although the latter is not true, in reality many patients with AD do have food allergies. Approximately 40% of infants and young children with moderate to severe AD and 8% of the general population of children will manifest a specific IgE-based food allergy. Food-specific IgE can be triggered or exacerbated by AD through the induction of hives, cutaneous activation of mast cells, increased “spontaneous” basophil histamine release, and food-related lymphocyte-proliferative responses measurable by food patch testing.12 Allergists generally recommend avoidance of or use of heavily denatured food (in the case of a milk/egg allergy) in the setting of documented IgE-mediated allergens.13 Food allergies in AD can manifest with flares, hives, pruritus, and/or other cutaneous symptoms in the absence of flaring AD disease.
Guidelines from the American Academy of Dermatology (AAD)(Table) for the management of AD have recently recommended testing for food allergies in children younger than 5 years who have intractable AD or known food-induced reactions.14 This technique will largely identify children at risk for anaphylaxis but may not yield information contributing to AD improvement. Furthermore, withdrawal of allergens with known IgE-mediated response was classified by the AAD as having consistent good-quality patient-oriented evidence, and asking about allergic reactions as well as acting on a reported allergic history had inconsistent or limited-quality patient-oriented evidence. It is believed that atopy can progress, or march, into a food and/or environmental allergy at any point in life; therefore, testing for a food allergy should be considered in all patients with recent onset of severe and/or persistent AD and/or food-aggravated AD due to a lifetime risk of sensitization.14,15 A food introduction plan may require collaboration with an allergist, especially in high-risk patients (eg, those with known food reactions, family history of food allergies, severe atopy).
Prevention of AD Through Dietary Modification
The National Institute of Allergy and Infectious Diseases consensus group published guidelines on food allergies that affect AD management, including avoidance of proven allergens but not random elimination of food allergens in AD; the group identifies AD and family history of AD as risk factors for food allergies.16 The best data in support of avoidance of documented food allergens to reduce AD severity has been found for egg white allergy and avoidance. Active egg allergy also is linked to staphylococcal superantigen IgE sensitizations,17 but the reason for the link is not yet clear. For the pediatric population, exclusive breastfeeding until 4 to 6 months of age and introduction of solids within the first 4 to 6 months as well as avoidance of maternal dietary restriction during pregnancy and lactation was further endorsed, with use of hydrolyzed formulas as an alternative to exclusive breastfeeding in infants who are not exclusively breastfed (cost permitting).16,18
A Cochrane review of maternal dietary restrictions during pregnancy found no benefit of maternal prenatal dietary restriction on AD prevalence in the first 18 months of life but did note an association with lower mean gestational weight.19
There is currently an effort to produce foods, such as soybeans and corn, that are genetically modified to reduce exposure to the allergenic component, but it is possible that when large-scale challenges occur, these foods also will be allergenic.20,21 In the case of a modified apple, some promising reduction in allergy symptoms has been reported.22 Although genetically modified foods may benefit children with food allergies in the future, they are a source of some controversy.
Complementary and Alternative Medicine
The AAD guidelines do not recommend complementary and alternative medicine (CAM) to treat AD,14 but it remains a commonly used therapy in the United States. A 2014 analysis of data from the 2007 US-based national health interview survey of 9417 children (age range, 0–17 years) demonstrated that 46.9% of children used 1 or more CAM, of which 0.99% used CAM specifically for AD. In this study, herbal therapy, vitamins, homeopathy, diet, and movement techniques were associated with increased prevalence of AD.23 Although some herbals have been shown to be beneficial in AD,24 hepatotoxicity has been reported with some herbal therapies.25 Complementary techniques with evidence-based support include massage therapy,26 relocation to an alternative climate, acupuncture that rivals cetirizine in efficacy, and supportive nutritional advice.24,27
Factors Affecting the Incidence of AD
Atopic dermatitis is of greater prevalence in children in developed wealthy nations such as the United States, supporting the role of enhanced hygiene and overall good health through vaccination as a possible contributor to the rise in AD prevalence in the last 4 decades.28,29 Alternatively, viruses such as respiratory syncytial virus may trigger AD, suggesting vaccination against the virus may reduce the risk for AD.30 Overall, vaccination improves life expectancy and should be conducted on schedule without reservation. Other aspects of hygiene that could conceptually affect prevalence of AD are raw food ingestion and the effects of foodborne microbes on the intestinal microbiome in relationship to AD development. Probiotics have been tested for this purpose.
Probiotics and prebiotics have been theorized to work through a reduction in inflammation; these agents have some evidence in their favor, but they were not endorsed in the AAD guidelines14 despite showing promise in meta-analysis. In particular prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus shows promise.31-33 Food elimination diets and supplements including vitamin D, selenium, fish oil, borage oil, and zinc were not found to be beneficial and were not recommended in the AAD guidelines.14,34
Percutaneous exposure to peanuts, possibly in household dust, may be the mechanism of peanut sensitization in AD27 via an inherent adjuvant effect of peanut protein.28 The recent LEAP (Learning Early About Peanut Allergy) trial randomized 530 infants aged 4 to 11 months to peanut-avoidant versus peanut-exposed diets for 60 months. The results showed statistically reduced (approximately one-twelfth of the risk) peanut allergy even in infants known to be sensitized (approximately one-third of the risk).35 It is now recommended in countries with a high prevalence of peanut allergies to introduce peanuts to an infant’s diet between 4 and 11 months of age (evidence level 1 [highest level of evidence]), with referral to an allergist for introduction in known sensitization cases and severe AD.36 In the setting of known or documented peanut allergy and for evaluation of potential food allergies, an allergist should be consulted.
Other interventions have been described as promising in mouse models. Those supplements include Lithospermum erythrorhizon,37Platycodon grandiflorus,38Hypsizygus marmoreous,39 fortified ginseng extract,40 polyunsaturated fatty acids,41 and galactooligosaccharide.42 Prebiotic oligosaccharides also are promising for early prevention of AD symptoms in infants, but otherwise these agents have remained largely untested in AD.43 None of these therapies have been endorsed by the AAD, and the long-term safety and efficacy in humans remains to be proven.
Risks of Dietary Restriction
Dietary restrictions in treating AD can have negative consequences, including reduced birth weight when initiated in pregnancy,19 osteomalacia from vitamin D deficiency,44 and nutritional deficiencies (eg, calcium, phosphorus, iron, vitamin K, vitamin D, zinc, vitamin A, B1, B2, B6, niacin, cholesterol, and/or vitamin C deficiencies).45 Excess dietary intake of vegetables in individuals with extensive food allergies can result in carotenemia.46 Protein-restricted diets from use of rice milk or dietary protein restriction can result in kwashiorkorlike protein malnutrition and marasmus.47-49 Nutritional counseling and/or supplementation is recommended for patients with food-restricted diets.
Avoiding Fragrance in Food
Food intolerance often is reported by AD patients. In allergies, food intolerance refers to side effects such as gastrointestinal symptoms; in dermatology, food intolerance can include itching, systemic flares of allergic contact dermatitis (eg, fragrance allergy), or true IgE-mediated allergies such as oral allergy syndrome. Oral allergy syndrome (pollen-food allergy syndrome) is an epitope-spread phenomenon related to an allergy to tree pollen, causing broad allergy to specific groups of fruits and nuts.50 Food triggers in AD include kiwi, milk, apple, tomato, citrus fruits, tree nuts, and peanuts. Oral allergy syndrome is common in food-sensitive AD patients (51.2%) followed by gastrointestinal symptoms (23.5%) and worsening AD (11.4%).51 Sensitization to fragrance can cross-react with foods (eg, balsam of Peru and tomatoes).52 A tomato allergy can be detected either by a skin-prick test or a food patch test in this setting.53 An allergist should be consulted if oral allergy syndrome is suspected.
Conclusion
Food allergies are more common in AD patients and patients should be referred to an allergist for evaluation and management. Strict dietary practice is not recommended, while avoiding proven food allergens in AD could be beneficial. Dermatologists should be aware that patients with dietary restrictions may lack key nutrients, manifesting with nutritional deficiencies in the skin; therefore, nutrition counseling may be needed in the most severe AD/allergy patients. This field is evolving; therefore, ongoing study and evaluation of interventions as they relate to AD will be needed to assess best practices for diet in AD over time.
Atopic dermatitis (AD) is the leading diagnosis among pediatric dermatologists,1 and this condition is commonly seen worldwide by dermatologists and allergists.2 There is a widespread misconception held by many patients and their guardians who believe that AD is caused by a food allergy.3 Although AD is related to and part of the atopic complex of disorders associated with food allergies, the role of diet in AD is not well defined. Previously it was recommended to delay early exposure to foods, but now it is recommended to do the opposite in certain situations. In fact, delaying exposure to certain types of foods can increase the likelihood of food allergies (eg, early exposure to peanut butter lowers the statistical chance of developing peanut allergies). This article reviews recent data on the role of diet in AD regarding disease activity as well as new and emerging data on dietary modifications for prevention and intervention. Emerging data on the relationship between AD and food allergies also are presented.
Pathogenesis of AD
The skin barrier plays a vital role in the prevention of pathogens, allergen exposure, and sensitization. There is no solitary root cause of AD, rather it is a combination of inflammation and barrier dysfunction associated with allergic diathesis (eg, atopy). Many patients with AD, especially those with persistent disease, have an intrinsic barrier dysfunction as part of the root cause of their illness, which may be caused by genetically mediated filaggrin defects or alternative barrier dysfunction such as decreased ceramide content that predisposes to percutaneous and mucosal sensitization.4,5 Another source of percutaneous exposure to allergens is macroscopic breaks in the skin caused by scratching, which allows dendritic termini of Langerhans cells to be exposed to percutaneous antigens4,6 through binding to high-affinity IgE receptors.
Langerhans cells exposed to allergens can trigger either an immediate or delayed-type (type I or type II) reaction (sensitization phase) in the lymph node causing inflammatory activation (elicitation). Inflammatory activity in AD is broad and complex and includes the release of IL-4, elevated IgE levels, and eosinophilia, which trigger the helper T cell TH2 and TH17 cascade of cytokines, including IL-2, IL-4, IL-5, IL-8, IL-10, IL-13, IL-17α, tumor necrosis factor α, and IFN-γ,7-9 with the latter worsening barrier defect via downregulation of intercellular substances (eg, filaggrin) and intercellular adhesion expression (eg, claudin 1).6,7,10
Atopic dermatitis does not exist in isolation. The barrier dysfunction associated with AD allows for sensitization to allergens, including those found in food and/or the environment. The atopic march, which occurs via barrier abnormalities facilitating sensitization, can result in further atopy, such as food allergies, environmental allergies, asthma, and eosinophilic esophagitis.11
|
AD and Food Allergies
Many patients and guardians believe AD is caused by a food allergy and that diet restrictions will resolve the disease. Although the latter is not true, in reality many patients with AD do have food allergies. Approximately 40% of infants and young children with moderate to severe AD and 8% of the general population of children will manifest a specific IgE-based food allergy. Food-specific IgE can be triggered or exacerbated by AD through the induction of hives, cutaneous activation of mast cells, increased “spontaneous” basophil histamine release, and food-related lymphocyte-proliferative responses measurable by food patch testing.12 Allergists generally recommend avoidance of or use of heavily denatured food (in the case of a milk/egg allergy) in the setting of documented IgE-mediated allergens.13 Food allergies in AD can manifest with flares, hives, pruritus, and/or other cutaneous symptoms in the absence of flaring AD disease.
Guidelines from the American Academy of Dermatology (AAD)(Table) for the management of AD have recently recommended testing for food allergies in children younger than 5 years who have intractable AD or known food-induced reactions.14 This technique will largely identify children at risk for anaphylaxis but may not yield information contributing to AD improvement. Furthermore, withdrawal of allergens with known IgE-mediated response was classified by the AAD as having consistent good-quality patient-oriented evidence, and asking about allergic reactions as well as acting on a reported allergic history had inconsistent or limited-quality patient-oriented evidence. It is believed that atopy can progress, or march, into a food and/or environmental allergy at any point in life; therefore, testing for a food allergy should be considered in all patients with recent onset of severe and/or persistent AD and/or food-aggravated AD due to a lifetime risk of sensitization.14,15 A food introduction plan may require collaboration with an allergist, especially in high-risk patients (eg, those with known food reactions, family history of food allergies, severe atopy).
Prevention of AD Through Dietary Modification
The National Institute of Allergy and Infectious Diseases consensus group published guidelines on food allergies that affect AD management, including avoidance of proven allergens but not random elimination of food allergens in AD; the group identifies AD and family history of AD as risk factors for food allergies.16 The best data in support of avoidance of documented food allergens to reduce AD severity has been found for egg white allergy and avoidance. Active egg allergy also is linked to staphylococcal superantigen IgE sensitizations,17 but the reason for the link is not yet clear. For the pediatric population, exclusive breastfeeding until 4 to 6 months of age and introduction of solids within the first 4 to 6 months as well as avoidance of maternal dietary restriction during pregnancy and lactation was further endorsed, with use of hydrolyzed formulas as an alternative to exclusive breastfeeding in infants who are not exclusively breastfed (cost permitting).16,18
A Cochrane review of maternal dietary restrictions during pregnancy found no benefit of maternal prenatal dietary restriction on AD prevalence in the first 18 months of life but did note an association with lower mean gestational weight.19
There is currently an effort to produce foods, such as soybeans and corn, that are genetically modified to reduce exposure to the allergenic component, but it is possible that when large-scale challenges occur, these foods also will be allergenic.20,21 In the case of a modified apple, some promising reduction in allergy symptoms has been reported.22 Although genetically modified foods may benefit children with food allergies in the future, they are a source of some controversy.
Complementary and Alternative Medicine
The AAD guidelines do not recommend complementary and alternative medicine (CAM) to treat AD,14 but it remains a commonly used therapy in the United States. A 2014 analysis of data from the 2007 US-based national health interview survey of 9417 children (age range, 0–17 years) demonstrated that 46.9% of children used 1 or more CAM, of which 0.99% used CAM specifically for AD. In this study, herbal therapy, vitamins, homeopathy, diet, and movement techniques were associated with increased prevalence of AD.23 Although some herbals have been shown to be beneficial in AD,24 hepatotoxicity has been reported with some herbal therapies.25 Complementary techniques with evidence-based support include massage therapy,26 relocation to an alternative climate, acupuncture that rivals cetirizine in efficacy, and supportive nutritional advice.24,27
Factors Affecting the Incidence of AD
Atopic dermatitis is of greater prevalence in children in developed wealthy nations such as the United States, supporting the role of enhanced hygiene and overall good health through vaccination as a possible contributor to the rise in AD prevalence in the last 4 decades.28,29 Alternatively, viruses such as respiratory syncytial virus may trigger AD, suggesting vaccination against the virus may reduce the risk for AD.30 Overall, vaccination improves life expectancy and should be conducted on schedule without reservation. Other aspects of hygiene that could conceptually affect prevalence of AD are raw food ingestion and the effects of foodborne microbes on the intestinal microbiome in relationship to AD development. Probiotics have been tested for this purpose.
Probiotics and prebiotics have been theorized to work through a reduction in inflammation; these agents have some evidence in their favor, but they were not endorsed in the AAD guidelines14 despite showing promise in meta-analysis. In particular prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus shows promise.31-33 Food elimination diets and supplements including vitamin D, selenium, fish oil, borage oil, and zinc were not found to be beneficial and were not recommended in the AAD guidelines.14,34
Percutaneous exposure to peanuts, possibly in household dust, may be the mechanism of peanut sensitization in AD27 via an inherent adjuvant effect of peanut protein.28 The recent LEAP (Learning Early About Peanut Allergy) trial randomized 530 infants aged 4 to 11 months to peanut-avoidant versus peanut-exposed diets for 60 months. The results showed statistically reduced (approximately one-twelfth of the risk) peanut allergy even in infants known to be sensitized (approximately one-third of the risk).35 It is now recommended in countries with a high prevalence of peanut allergies to introduce peanuts to an infant’s diet between 4 and 11 months of age (evidence level 1 [highest level of evidence]), with referral to an allergist for introduction in known sensitization cases and severe AD.36 In the setting of known or documented peanut allergy and for evaluation of potential food allergies, an allergist should be consulted.
Other interventions have been described as promising in mouse models. Those supplements include Lithospermum erythrorhizon,37Platycodon grandiflorus,38Hypsizygus marmoreous,39 fortified ginseng extract,40 polyunsaturated fatty acids,41 and galactooligosaccharide.42 Prebiotic oligosaccharides also are promising for early prevention of AD symptoms in infants, but otherwise these agents have remained largely untested in AD.43 None of these therapies have been endorsed by the AAD, and the long-term safety and efficacy in humans remains to be proven.
Risks of Dietary Restriction
Dietary restrictions in treating AD can have negative consequences, including reduced birth weight when initiated in pregnancy,19 osteomalacia from vitamin D deficiency,44 and nutritional deficiencies (eg, calcium, phosphorus, iron, vitamin K, vitamin D, zinc, vitamin A, B1, B2, B6, niacin, cholesterol, and/or vitamin C deficiencies).45 Excess dietary intake of vegetables in individuals with extensive food allergies can result in carotenemia.46 Protein-restricted diets from use of rice milk or dietary protein restriction can result in kwashiorkorlike protein malnutrition and marasmus.47-49 Nutritional counseling and/or supplementation is recommended for patients with food-restricted diets.
Avoiding Fragrance in Food
Food intolerance often is reported by AD patients. In allergies, food intolerance refers to side effects such as gastrointestinal symptoms; in dermatology, food intolerance can include itching, systemic flares of allergic contact dermatitis (eg, fragrance allergy), or true IgE-mediated allergies such as oral allergy syndrome. Oral allergy syndrome (pollen-food allergy syndrome) is an epitope-spread phenomenon related to an allergy to tree pollen, causing broad allergy to specific groups of fruits and nuts.50 Food triggers in AD include kiwi, milk, apple, tomato, citrus fruits, tree nuts, and peanuts. Oral allergy syndrome is common in food-sensitive AD patients (51.2%) followed by gastrointestinal symptoms (23.5%) and worsening AD (11.4%).51 Sensitization to fragrance can cross-react with foods (eg, balsam of Peru and tomatoes).52 A tomato allergy can be detected either by a skin-prick test or a food patch test in this setting.53 An allergist should be consulted if oral allergy syndrome is suspected.
Conclusion
Food allergies are more common in AD patients and patients should be referred to an allergist for evaluation and management. Strict dietary practice is not recommended, while avoiding proven food allergens in AD could be beneficial. Dermatologists should be aware that patients with dietary restrictions may lack key nutrients, manifesting with nutritional deficiencies in the skin; therefore, nutrition counseling may be needed in the most severe AD/allergy patients. This field is evolving; therefore, ongoing study and evaluation of interventions as they relate to AD will be needed to assess best practices for diet in AD over time.
1. Schachner L, Ling NS, Press S. A statistical analysis of a pediatric dermatology clinic. Pediatr Dermatol. 1983;1:157-164.
2. Kiprono SK, Muchunu JW, Masenga JE. Skin diseases in pediatric patients attending a tertiary dermatology hospital in Northern Tanzania: a cross-sectional study. BMC Dermatol. 2015;15:16.
3. Wensink M, Timmer C, Brand PL. Atopic dermatitis in infants not caused by food allergy [in Dutch]. Ned Tijdschr Geneeskd. 2008;152:4-9.
4. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(3, pt 2):949-963.
5. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
6. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
7. Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
8. Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
9. Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
10. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
11. Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review [published online July 22, 2015]. Clin Rev Allergy Immunol. doi:10.1111/all.12846.
12. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis; pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999;104(3, pt 2):S114-S122.
13. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.
14. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
15. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the epidermal differentiation complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
16. Burks AW, Jones SM, Boyce JA, et al. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics. 2011;128:955-965.
17. Ong PY. Association between egg and staphylococcal superantigen IgE sensitizations in atopic dermatitis. Allergy Asthma Proc. 2014;35:346-348.
18. Botteman M, Detzel P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in Southeast Asia. Ann Nutr Metab. 2015;66(suppl 1):26-32.
19. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.
20. Yum HY, Lee SY, Lee KE, et al. Genetically modified and wild soybeans: an immunologic comparison. Allergy Asthma Proc. 2005;26:210-216.
21. Mathur C, Kathuria PC, Dahiya P, et al. Lack of detectable allergenicity in genetically modified maize containing “Cry” proteins as compared to native maize based on in silico & in vitro analysis. PLoS One. 2015;10:e0117340.
22. Dubois AE, Pagliarani G, Brouwer RM, et al. First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar [published online July 24, 2015]. Allergy. 2015;70:1406-1412.
23. Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
24. Pfab F, Schalock PC, Napadow V, et al. Complementary integrative approach for treating pruritus. Dermatol Ther. 2013;26:149-156.
25. Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
26. Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
27. Pfab F, Schalock PC, Napadow V, et al. Acupuncture for allergic disease therapy–the current state of evidence. Expert Rev Clin Immunol. 2014;10:831-841.
28. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
29. Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
30. Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
31. Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
32. Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
33. Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
34. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I: atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
35. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
36. Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants [published online October 2015]. Allergy. 2015;70:1193-1195.
37. Kim J, Cho Y. Gromwell (Lithospermum erythrorhizon) supplementation enhances epidermal levels of cera-mides, glucosylceramides, β-glucocerebrosidase, and acidicsphingomyelinase in NC/Nga mice. J Med Food. 2013;16:927-933.
38. Choi JH, Jin SW, Han EH, et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21:1053-1061.
39. Kim T, Park K, Jung HS, et al. Evaluation of anti-atopic dermatitis activity of Hypsizigus marmoreus extract. Phytother Res. 2014;28:1539-1546.
40. Kim JR, Choi J, Kim J, et al. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365-371.
41. Weise C, Ernst D, van Tol EA, et al. Dietary polyunsaturated fatty acids and non-digestible oligosaccharides reduce dermatitis in mice. Pediatr Allergy Immunol. 2013;24:361-367.
42. Tanabe S, Hochi S. Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Int J Mol Med. 2010;25:331-336.
43. Arslanoglu S, Moro GE, Boehm G, et al. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J Biol Regul Homeost Agents. 2012;26(3 suppl):49-59.
44. Shikino K, Ikusaka M, Yamashita T. Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis [published online July 4, 2014] . BMJ Case Rep.
45. Kim J, Kwon J, Noh G, et al. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7:488-494.
46. Silverberg NB, Lee-Wong M. Generalized yellow discoloration of the skin. Cutis. 2014;93:E11-E12.
47. Hon KL, Nip SY, Cheung KL. A tragic case of atopic eczema: malnutrition and infections despite multivitamins and supplements. Iran J Allergy Asthma Immunol. 2012;11:267-270.
48. Diamanti A, Pedicelli S, D’Argenio P, et al. Iatrogenic kwashiorkor in three infants on a diet of rice beverages. Pediatr Allergy Immunol. 2011;22:878-879.
49. Pillai K, Acharya S. Iatrogenic kwashiorkar. Indian Pediatr. 2010;47:540-541.
50. Price A, Ramachandran S, Smith GP, et al. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015;26:78-88.
51. Mattila L, Kilpeläinen M, Terho EO, et al. Food hypersensitivity among Finnish university students: association with atopic diseases. Clin Exp Allergy. 2003;33:600-606.
52. Paulsen E, Christensen LP, Andersen KE. Tomato contact dermatitis. Contact Dermatitis. 2012;67:321-327.
53. Di Leo E, Nettis E, Cardinale F, et al. Tomato atopy patch test in adult atopic dermatitis: diagnostic value and comparison among different methods. Allergy. 2009;64:659-663.
1. Schachner L, Ling NS, Press S. A statistical analysis of a pediatric dermatology clinic. Pediatr Dermatol. 1983;1:157-164.
2. Kiprono SK, Muchunu JW, Masenga JE. Skin diseases in pediatric patients attending a tertiary dermatology hospital in Northern Tanzania: a cross-sectional study. BMC Dermatol. 2015;15:16.
3. Wensink M, Timmer C, Brand PL. Atopic dermatitis in infants not caused by food allergy [in Dutch]. Ned Tijdschr Geneeskd. 2008;152:4-9.
4. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(3, pt 2):949-963.
5. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
6. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
7. Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
8. Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
9. Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
10. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
11. Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review [published online July 22, 2015]. Clin Rev Allergy Immunol. doi:10.1111/all.12846.
12. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis; pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999;104(3, pt 2):S114-S122.
13. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.
14. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
15. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the epidermal differentiation complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
16. Burks AW, Jones SM, Boyce JA, et al. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics. 2011;128:955-965.
17. Ong PY. Association between egg and staphylococcal superantigen IgE sensitizations in atopic dermatitis. Allergy Asthma Proc. 2014;35:346-348.
18. Botteman M, Detzel P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in Southeast Asia. Ann Nutr Metab. 2015;66(suppl 1):26-32.
19. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.
20. Yum HY, Lee SY, Lee KE, et al. Genetically modified and wild soybeans: an immunologic comparison. Allergy Asthma Proc. 2005;26:210-216.
21. Mathur C, Kathuria PC, Dahiya P, et al. Lack of detectable allergenicity in genetically modified maize containing “Cry” proteins as compared to native maize based on in silico & in vitro analysis. PLoS One. 2015;10:e0117340.
22. Dubois AE, Pagliarani G, Brouwer RM, et al. First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar [published online July 24, 2015]. Allergy. 2015;70:1406-1412.
23. Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
24. Pfab F, Schalock PC, Napadow V, et al. Complementary integrative approach for treating pruritus. Dermatol Ther. 2013;26:149-156.
25. Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
26. Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
27. Pfab F, Schalock PC, Napadow V, et al. Acupuncture for allergic disease therapy–the current state of evidence. Expert Rev Clin Immunol. 2014;10:831-841.
28. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
29. Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
30. Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
31. Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
32. Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
33. Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
34. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I: atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
35. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
36. Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants [published online October 2015]. Allergy. 2015;70:1193-1195.
37. Kim J, Cho Y. Gromwell (Lithospermum erythrorhizon) supplementation enhances epidermal levels of cera-mides, glucosylceramides, β-glucocerebrosidase, and acidicsphingomyelinase in NC/Nga mice. J Med Food. 2013;16:927-933.
38. Choi JH, Jin SW, Han EH, et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21:1053-1061.
39. Kim T, Park K, Jung HS, et al. Evaluation of anti-atopic dermatitis activity of Hypsizigus marmoreus extract. Phytother Res. 2014;28:1539-1546.
40. Kim JR, Choi J, Kim J, et al. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365-371.
41. Weise C, Ernst D, van Tol EA, et al. Dietary polyunsaturated fatty acids and non-digestible oligosaccharides reduce dermatitis in mice. Pediatr Allergy Immunol. 2013;24:361-367.
42. Tanabe S, Hochi S. Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Int J Mol Med. 2010;25:331-336.
43. Arslanoglu S, Moro GE, Boehm G, et al. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J Biol Regul Homeost Agents. 2012;26(3 suppl):49-59.
44. Shikino K, Ikusaka M, Yamashita T. Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis [published online July 4, 2014] . BMJ Case Rep.
45. Kim J, Kwon J, Noh G, et al. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7:488-494.
46. Silverberg NB, Lee-Wong M. Generalized yellow discoloration of the skin. Cutis. 2014;93:E11-E12.
47. Hon KL, Nip SY, Cheung KL. A tragic case of atopic eczema: malnutrition and infections despite multivitamins and supplements. Iran J Allergy Asthma Immunol. 2012;11:267-270.
48. Diamanti A, Pedicelli S, D’Argenio P, et al. Iatrogenic kwashiorkor in three infants on a diet of rice beverages. Pediatr Allergy Immunol. 2011;22:878-879.
49. Pillai K, Acharya S. Iatrogenic kwashiorkar. Indian Pediatr. 2010;47:540-541.
50. Price A, Ramachandran S, Smith GP, et al. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015;26:78-88.
51. Mattila L, Kilpeläinen M, Terho EO, et al. Food hypersensitivity among Finnish university students: association with atopic diseases. Clin Exp Allergy. 2003;33:600-606.
52. Paulsen E, Christensen LP, Andersen KE. Tomato contact dermatitis. Contact Dermatitis. 2012;67:321-327.
53. Di Leo E, Nettis E, Cardinale F, et al. Tomato atopy patch test in adult atopic dermatitis: diagnostic value and comparison among different methods. Allergy. 2009;64:659-663.
Practice Points
- Test children younger than 5 years with moderate to severe atopic dermatitis (AD) for food allergies if they have persistently severe AD or known food-induced reactions.
- Food elimination diets are not recommended for management of AD.
- There is not enough evidence supporting the use of complementary and alternative medicine, probiotics/prebiotics, or supplements for the treatment of AD.
Generalized Yellow Discoloration of the Skin
The Diagnosis: Carotenemia
Laboratory parameters including thyroid function testing as well as total protein and bilirubin levels were within reference range. Testing revealed multiple food allergies to almonds, oranges, cashews, garlic, peanuts, and cantaloupe. The patient was treated with a dietary expansion based on his allergy testing.
ß-Carotene converts to vitamin A in the intestine and acts as a lipochrome. Lack of conversion can be noted as an inborn error of metabolism.1 Many green, yellow, and orange fruits and vegetables contain ß-carotene, including carrots, sweet potatoes, squash, green beans, papayas, and pumpkins.1-3 ß-Carotene also is used as a vitamin supplement4 or therapeutic agent in photosensitive disorders such as genetic porphyrias.5
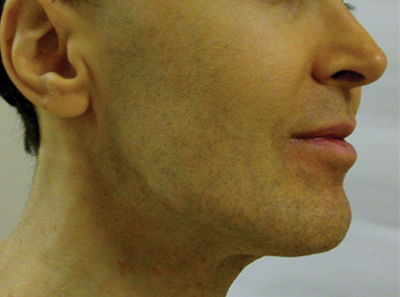
ß-Carotene can accumulate in the stratum corneum and impart a yellow color to the skin when the circulating levels are high; this coloration is termed carotenemia.1,4 Carotenemia is common in infants and young children who have diets rich in green and orange vegetable purees.6 Carotenemia limited to thick areas of the skin, such as the palms and soles, can be seen in adults who eat large amounts of carrots; generalized carotenemia is rare.1,4
Carotenemia is a benign condition of excess cutaneous buildup of ß-carotene through excessive intake of carotene-rich foods1-4 or nutritional supplements7 or through association with anorexia, liver disease, renal disease, hypothyroidism, or diabetes mellitus.1,4,8,9 Carotene deposits usually are most notable in areas with thick stratum corneum, such as the nasolabial folds, palms, and soles, as opposed to areas such as the conjunctivae and mucosa.1,4
Carotenemia may mimic jaundice and should be differentiated through scleral examination for icterus and bilirubin levels. Carotene levels can be tested but generally are unnecessary. Carotenemia can be seen in liver or renal disease and can exacerbate the yellow coloration seen in jaundiced individuals.1,4,9
Because it is a benign condition, the pathology usually is limited to skin discoloration, as seen in our patient. Although this condition can be reversed with a modified diet, our patient had multiple food allergies that further restricted his vegetarian diet, thereby limiting the modifications that he was willing to make to his diet.
1. Schwartz RA. Carotenemia. Emedicine. http://emedicine.medscape.com/article/1104368-overview. Updated April 8, 2014. Accessed April 30, 2014.
2. Sale TA, Stratman E. Carotenemia associated with green bean ingestion. Pediatr Dermatol. 2004;21:657-659.
3. Costanza DJ. Carotenemia associated with papaya ingestion. Calif Med. 1968;109:319-320.
4. Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
5. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
6. Karthik SV, Campbell-Davidson D, Isherwood D. Carotenemia in infancy and its association with prevalent feeding practices. Pediatr Dermatol. 2006;23:571-573.
7. Takita Y, Ichimiya M, Hamamoto Y, et al. A case of carotenemia associated with ingestion of nutrient supplements. J Dermatol. 2006;2:132-134.
8. Thibault L, Roberge AG. The nutritional status of subjects with nervosa. Int J Vitam Nutr Res. 1987;57:447-452.
9. Matthews-Roth M, Gulbrandsen CL. Transport of beta-carotene in serum of individuals with carotenemia. Clin Chem. 1974;20:1578-1579.
The Diagnosis: Carotenemia
Laboratory parameters including thyroid function testing as well as total protein and bilirubin levels were within reference range. Testing revealed multiple food allergies to almonds, oranges, cashews, garlic, peanuts, and cantaloupe. The patient was treated with a dietary expansion based on his allergy testing.
ß-Carotene converts to vitamin A in the intestine and acts as a lipochrome. Lack of conversion can be noted as an inborn error of metabolism.1 Many green, yellow, and orange fruits and vegetables contain ß-carotene, including carrots, sweet potatoes, squash, green beans, papayas, and pumpkins.1-3 ß-Carotene also is used as a vitamin supplement4 or therapeutic agent in photosensitive disorders such as genetic porphyrias.5
ß-Carotene can accumulate in the stratum corneum and impart a yellow color to the skin when the circulating levels are high; this coloration is termed carotenemia.1,4 Carotenemia is common in infants and young children who have diets rich in green and orange vegetable purees.6 Carotenemia limited to thick areas of the skin, such as the palms and soles, can be seen in adults who eat large amounts of carrots; generalized carotenemia is rare.1,4
Carotenemia is a benign condition of excess cutaneous buildup of ß-carotene through excessive intake of carotene-rich foods1-4 or nutritional supplements7 or through association with anorexia, liver disease, renal disease, hypothyroidism, or diabetes mellitus.1,4,8,9 Carotene deposits usually are most notable in areas with thick stratum corneum, such as the nasolabial folds, palms, and soles, as opposed to areas such as the conjunctivae and mucosa.1,4
Carotenemia may mimic jaundice and should be differentiated through scleral examination for icterus and bilirubin levels. Carotene levels can be tested but generally are unnecessary. Carotenemia can be seen in liver or renal disease and can exacerbate the yellow coloration seen in jaundiced individuals.1,4,9
Because it is a benign condition, the pathology usually is limited to skin discoloration, as seen in our patient. Although this condition can be reversed with a modified diet, our patient had multiple food allergies that further restricted his vegetarian diet, thereby limiting the modifications that he was willing to make to his diet.
The Diagnosis: Carotenemia
Laboratory parameters including thyroid function testing as well as total protein and bilirubin levels were within reference range. Testing revealed multiple food allergies to almonds, oranges, cashews, garlic, peanuts, and cantaloupe. The patient was treated with a dietary expansion based on his allergy testing.
ß-Carotene converts to vitamin A in the intestine and acts as a lipochrome. Lack of conversion can be noted as an inborn error of metabolism.1 Many green, yellow, and orange fruits and vegetables contain ß-carotene, including carrots, sweet potatoes, squash, green beans, papayas, and pumpkins.1-3 ß-Carotene also is used as a vitamin supplement4 or therapeutic agent in photosensitive disorders such as genetic porphyrias.5
ß-Carotene can accumulate in the stratum corneum and impart a yellow color to the skin when the circulating levels are high; this coloration is termed carotenemia.1,4 Carotenemia is common in infants and young children who have diets rich in green and orange vegetable purees.6 Carotenemia limited to thick areas of the skin, such as the palms and soles, can be seen in adults who eat large amounts of carrots; generalized carotenemia is rare.1,4
Carotenemia is a benign condition of excess cutaneous buildup of ß-carotene through excessive intake of carotene-rich foods1-4 or nutritional supplements7 or through association with anorexia, liver disease, renal disease, hypothyroidism, or diabetes mellitus.1,4,8,9 Carotene deposits usually are most notable in areas with thick stratum corneum, such as the nasolabial folds, palms, and soles, as opposed to areas such as the conjunctivae and mucosa.1,4
Carotenemia may mimic jaundice and should be differentiated through scleral examination for icterus and bilirubin levels. Carotene levels can be tested but generally are unnecessary. Carotenemia can be seen in liver or renal disease and can exacerbate the yellow coloration seen in jaundiced individuals.1,4,9
Because it is a benign condition, the pathology usually is limited to skin discoloration, as seen in our patient. Although this condition can be reversed with a modified diet, our patient had multiple food allergies that further restricted his vegetarian diet, thereby limiting the modifications that he was willing to make to his diet.
1. Schwartz RA. Carotenemia. Emedicine. http://emedicine.medscape.com/article/1104368-overview. Updated April 8, 2014. Accessed April 30, 2014.
2. Sale TA, Stratman E. Carotenemia associated with green bean ingestion. Pediatr Dermatol. 2004;21:657-659.
3. Costanza DJ. Carotenemia associated with papaya ingestion. Calif Med. 1968;109:319-320.
4. Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
5. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
6. Karthik SV, Campbell-Davidson D, Isherwood D. Carotenemia in infancy and its association with prevalent feeding practices. Pediatr Dermatol. 2006;23:571-573.
7. Takita Y, Ichimiya M, Hamamoto Y, et al. A case of carotenemia associated with ingestion of nutrient supplements. J Dermatol. 2006;2:132-134.
8. Thibault L, Roberge AG. The nutritional status of subjects with nervosa. Int J Vitam Nutr Res. 1987;57:447-452.
9. Matthews-Roth M, Gulbrandsen CL. Transport of beta-carotene in serum of individuals with carotenemia. Clin Chem. 1974;20:1578-1579.
1. Schwartz RA. Carotenemia. Emedicine. http://emedicine.medscape.com/article/1104368-overview. Updated April 8, 2014. Accessed April 30, 2014.
2. Sale TA, Stratman E. Carotenemia associated with green bean ingestion. Pediatr Dermatol. 2004;21:657-659.
3. Costanza DJ. Carotenemia associated with papaya ingestion. Calif Med. 1968;109:319-320.
4. Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
5. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
6. Karthik SV, Campbell-Davidson D, Isherwood D. Carotenemia in infancy and its association with prevalent feeding practices. Pediatr Dermatol. 2006;23:571-573.
7. Takita Y, Ichimiya M, Hamamoto Y, et al. A case of carotenemia associated with ingestion of nutrient supplements. J Dermatol. 2006;2:132-134.
8. Thibault L, Roberge AG. The nutritional status of subjects with nervosa. Int J Vitam Nutr Res. 1987;57:447-452.
9. Matthews-Roth M, Gulbrandsen CL. Transport of beta-carotene in serum of individuals with carotenemia. Clin Chem. 1974;20:1578-1579.
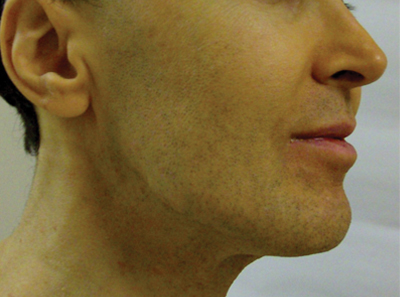
A 50-year-old man presented with yellow, pruritic, xerotic skin and lethargy. The patient also reported nasal congestion and sneezing, especially when eating peanuts. He was fearful of allergic reactions and restricted his diet to “safe foods” such as squash, green beans, and sweet potatoes. On examination the patient had marked generalized yellow discoloration of the skin with pale mucous membranes, nonicteric sclerae, infraocular violaceous and hyperpigmented skin (allergic shiners), and Dennie-Morgan folds.