User login
Improving Nephropathy Screening in Appalachian Patients With Diabetes Using Practice-Wide Outreach
From West Virginia University, Morgantown, WV.
Abstract
Objective: To describe the strategies a family medicine clinic in Appalachia utilized to increase nephropathy screening rates as well as to explore the factors predictive of nephropathy screening in patients with diabetes.
Design: This quality improvement project targeted the points in the care process when patients are lost to follow-up for nephropathy screening.
Setting and participants: Patients with diabetes cared for by a primary care provider (PCP) at an academic family medicine practice in Appalachia from January 2018 to November 2018.
Interventions: Bulk orders for albumin-to-creatinine (ACR) testing and urine collection during clinic visit, enhanced patient communication through bulk communication reminders and individual patient outreach, and education of clinic providers.
Measurements: Demographic data and monthly nephropathy screening rates.
Results: The nephropathy screening rate increased by 6.2% during the project. Older patients living closer to the clinic who visited their PCP 3 or more times per year were the most likely to be screened.
Conclusion: Combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy treatment and screening in rural patients with diabetes at a family medicine clinic.
Keywords: rural; kidney disease; albumin-to-creatinine ratio; electronic health record.
According to the Centers for Disease Control and Prevention (CDC), an estimated 30.3 million people in the United States—about 9.4% of the population—have been diagnosed with diabetes.1 Diabetes is the seventh leading cause of death in the United States, and it contributes to other leading causes of death: heart disease and stroke.1 Diabetes also is related to high morbidity risk and is a leading cause of chronic kidney disease.1 The total cost of diagnosed diabetes was estimated at $327 billion in direct medical costs and reduced productivity.2
Residents of Appalachia bear a disproportionate burden of diabetes and other related negative health outcomes; these outcomes are influenced by a number of factors, including socioeconomic status, poverty, rurality, and health care access. Rates of chronic disease, such as diabetes, are most pronounced in Appalachia’s most economically distressed counties.3-5 In 2011, the CDC labeled a 644-county area the “diabetes belt,” which included most of Appalachia.6 As a result of this elevated prevalence of diabetes in Appalachia as compared to the rest of the country, complications directly associated with diabetes are more commonly observed in Appalachian residents. One of the most damaging complications is diabetic nephropathy.
Diabetic nephropathy results from damage to the microvasculature of the kidney due to inadequately controlled blood glucose. This, in turn, leads to decreased renal function, eventually leading to clinically significant renal disease. The long-term complications associated with nephropathy can include many comorbid conditions, the most serious of which are progression to end-stage renal disease, dialysis requirement, and early mortality. Diabetic nephropathy affects approximately 40% of patients with type 1 and type 2 diabetes.7,8
One way to prevent complications of diabetic nephropathy, in addition to good glycemic control in patients with diabetes, is early and regular screening. Currently, the American Diabetes Association (ADA) recommends yearly screening for diabetic nephropathy in the form of a urine albumin-to-creatinine ratio (ACR) for patients 18 to 75 years of age.2 This screening to detect diabetic nephropathy is recognized as a marker of quality care by many public and private insurance agencies and medical specialty associations, such as the Centers for Medicare and Medicaid Services.
Many patients with diabetes are cared for by primary care providers (PCP), and these PCP appointments provide an opportune time to screen and appropriately treat nephropathy. Screening opportunities are often missed, however, due to time constraints and competing health priorities. There are also a number of other factors specific to the Appalachian region that reduce the likelihood of screening for diabetic nephropathy, such as a lack of health insurance, the need to travel long distances to see a PCP, work and household responsibilities, low levels of education and health literacy, and a mistrust of outsiders regarding personal matters, including health.9-11 While nephropathy can have a detrimental impact on patients across populations, it is of particular concern for a state located in the heart of Appalachia, such as West Virginia.
Given the disproportionate burden of diabetes in this region and the potentially severe consequences of undetected nephropathy, clinicians from an academic family medicine clinic in West Virginia undertook a quality improvement project to increase the rate of nephropathy screening and treatment among patients with diabetes. This article describes the intervention strategies the team utilized to increase nephropathy screening and treatment in patients 18 to 75 years of age who met quality measures for nephropathy screening or treatment in the previous 12 months and explores the factors most predictive of nephropathy screening in Appalachian patients in this age group. It also reports the challenges and opportunities encountered and offers suggestions for other providers and clinics attempting to increase their nephropathy screening rates.
Methods
Setting and Study Population
The study population included patients ages 18 to 75 years under the care of providers in an academic family medicine practice in West Virginia who had been diagnosed with diabetes mellitus. The study focused on those patients overdue for diabetic nephropathy screening (ie, had not been screened in previous 12 months). The project began in January 2018 with a screening rate of 83.8%. The goal of this project was to increase this compliance metric by at least 5%. The project protocol was submitted to the West Virginia University Institutional Review Board, and, because it is a quality improvement project, permission was given to proceed without a board review.
Interventions
The team identified and implemented several interventions intended to reduce screening barriers and increase the screening rate.
Bulk orders for ACR and urine collection during clinic visits. Prior to initiation of this project, it was left to individual clinic providers to order nephropathy screening for patients with diabetes during a clinic visit; after receiving the order for “random urine microalbumin/creatinine ratio,” patients then had to travel to a lab to provide a urine sample. For this project and moving forward, the team changed to the procedure of initiating bulk ACR orders and collecting urine samples during clinic visits from all patients ages 18 to 75 years who have diabetes.
Bulk communication reminders. Since many patients with diabetes may not have realized they were overdue for nephropathy screening, the team began sending out bulk communication reminders through either the institution’s electronic health record (EHR; MyChart) or postal service–delivered physical letters (according to patient communication preferences) to remind patients that they were due for screening and to encourage them to schedule an appointment or keep a previously scheduled appointment with their PCP.
Individual patient outreach. A team of pharmacy students led by a licensed pharmacist in the family medicine clinic contacted patients overdue for screening even after bulk communication reminders went out. The students telephoned patients 2 to 3 months following the bulk communication. The students obtained an updated list of patients with diabetes ages 18 to 75 years from an EHR quality report. They began by prescreening the patients on the overdue list for potential candidacy for an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB). Screening for candidacy included evaluation of recent blood pressure readings, electrolytes (ie, basic metabolic panel), and ACR. If the students determined a patient was a candidate, they presented the patient to the preceptor for verification and then reached out to the provider with a recommendation. If the provider agreed, the student contacted the patient by telephone for medication counseling and education. The remaining patients determined not to be candidates for ACE inhibitors or ARBs were contacted by the pharmacy students by telephone to remind them that laboratory work was pending. Up to 3 phone call attempts were made before patients were determined to be unreachable. Students left voice mails with generic reminders if a patient could not be reached. If a patient answered, the student provided a reminder but also reviewed indications for lab work, the reason why the provider wished for follow-up, and updated lab hours. Students also followed up with the results of the work-up, as appropriate. During this outreach process, the student team encountered a number of patients who had moved or changed to a PCP outside of the family medicine clinic. In these cases, the EHR was updated and those patients were removed from the list of patients altogether.
Education of clinic providers. Clinic providers were educated during faculty and resident meetings and didactic learning sessions on identifying patients within the EHR who are due for nephropathy screening. They also received instruction on how to update the EHR to reflect completed screenings.
Data Analysis
All analyses in this study were conducted using SAS (version 9.4, 2013, SAS Institute Inc., Cary, NC). Descriptive analyses were conducted to summarize basic patient demographic information. To compare patients screened within the previous 12 months to those patients overdue for screening, 2-sample t-tests were used to examine differences in patients’ age, HbA1c, ACR, and creatinine level and the distance (in miles) between the patient’s home and the clinic. Chi-square analyses were used to examine the relationship between whether a patient was recently screened for nephropathy and the patient’s insurance, number of patient visits in the previous 12 months, and provider level. Logistic regression analyses were conducted to control for covariates and to explore which factors were most predictive of nephropathy screening. All tests were 2-tailed, and P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
There were 1676 family medicine clinic patients with diabetes between 18 and 75 years of age (Table 1 and Table 2). Of the total sample, 1489 (88.8%) had completed screening for nephropathy in the 12 months prior to evaluation, and 67.5%, 23.7%, and 8.8% of patients had private insurance, Medicare, and Medicaid, respectively. 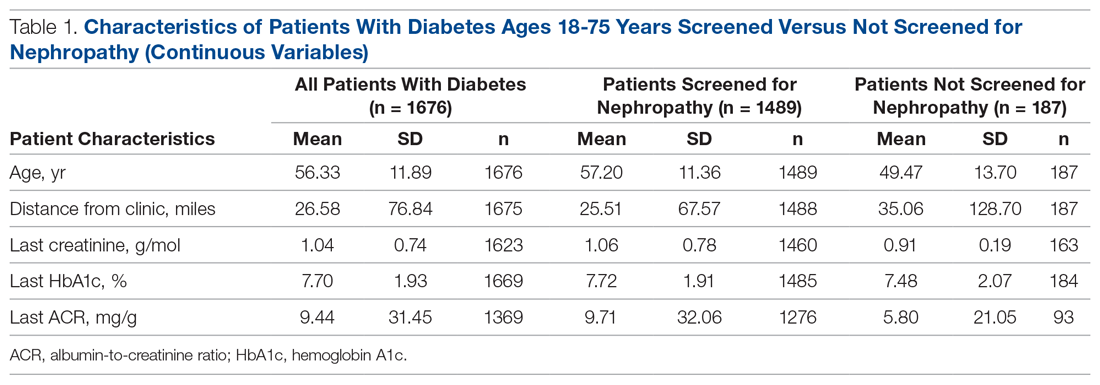
The mean (SD) age of the patients was 56.3 (11.9) years. The mean distance between the patient’s home and the clinic was 26.6 (76.8) miles. The mean number of visits was 3.6 (2.9) per year, and 43.0% of the patientvisited the clinic more than 3 times in a year. The mean values for HbA1c (%), creatinine (g/mol), and ACR (mg/g) were 7.7 (1.9), 1.0 (0.7), and 9.4 (31.4), respectively.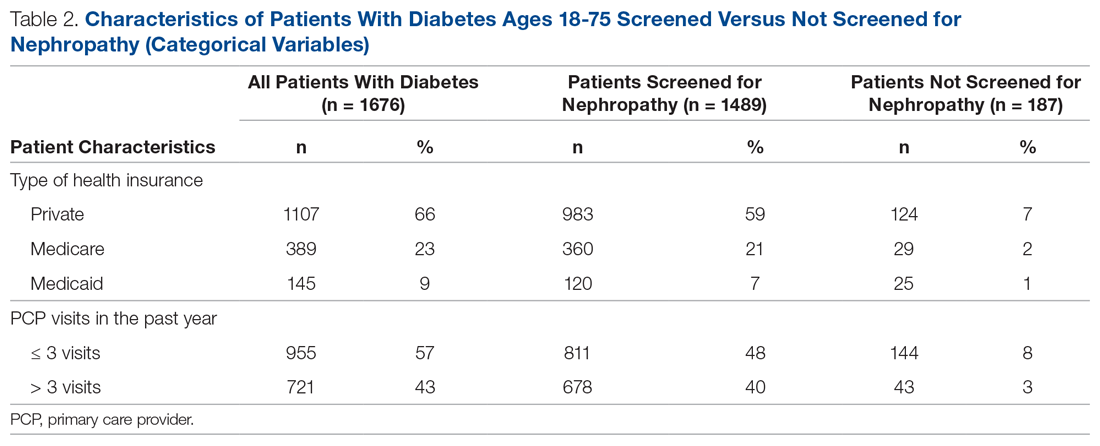
Screening of Patients for Nephropathy
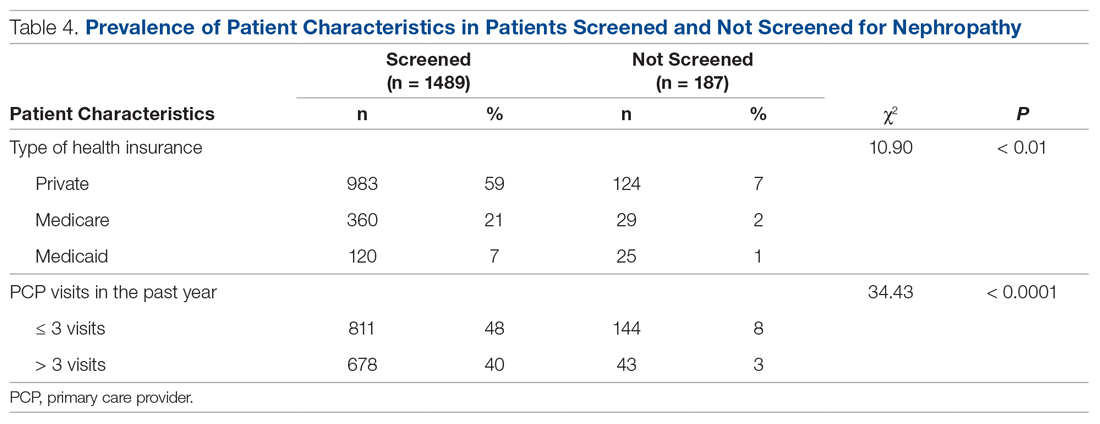
Patients with Medicare and private insurance were more likely to have completed the nephropathy screening than those with Medicaid (92.5% versus 88.8% versus 82.8%, P = 0.004; Table 3 and Table 4). 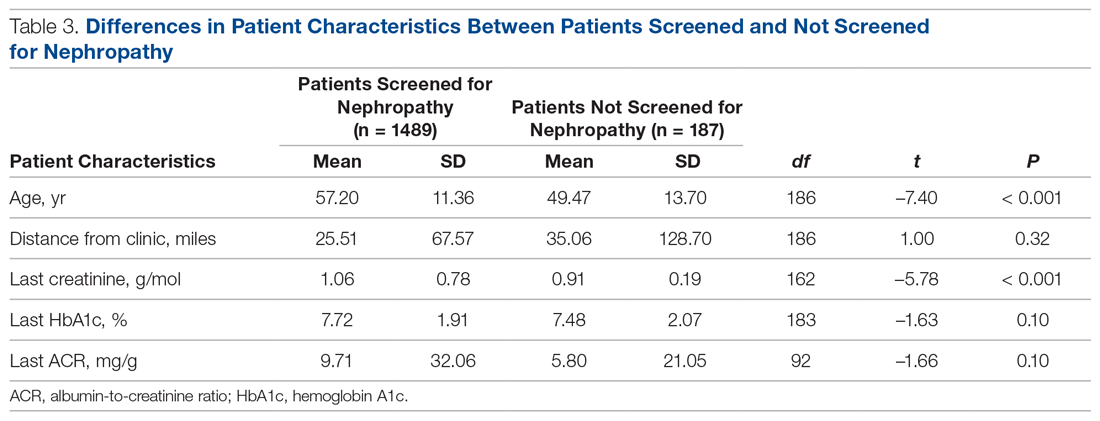
Changes in Screening Rate
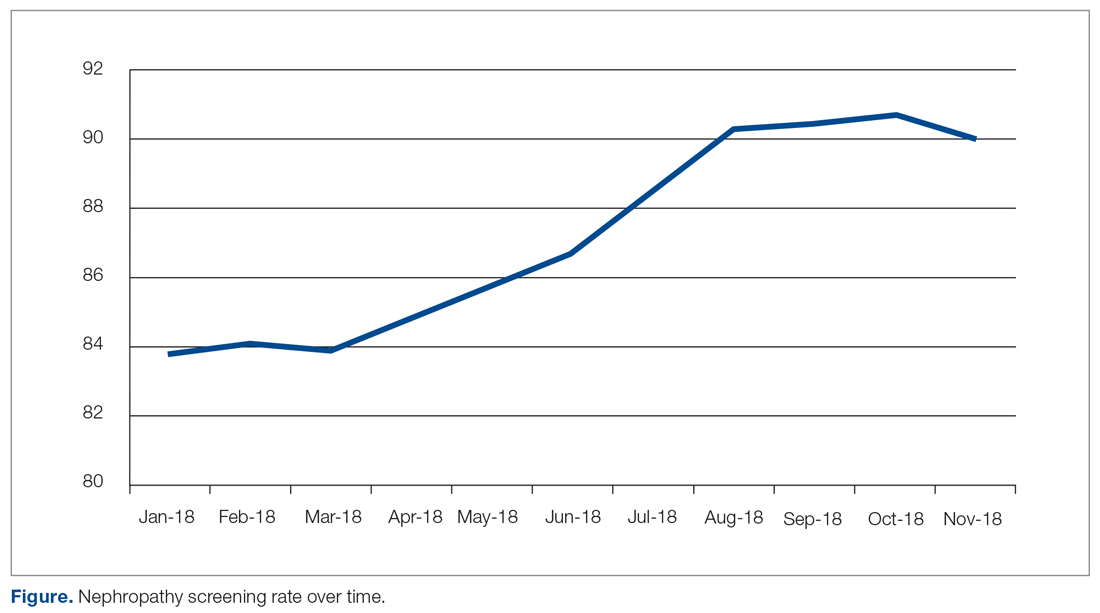
The practice-wide screening rate was 83.8% at the start of this project in January 2018. The screening rate steadily increased throughout 2018, reaching 90.3% in August 2018, and then leveled off around 90% when the project was concluded at the end of November 2018 (Figure). As an added benefit of the increased screening rates, a number of patients were initiated on an ACE inhibitor or ARB based on the team’s screening efforts.
Predictors of Nephropathy Screening
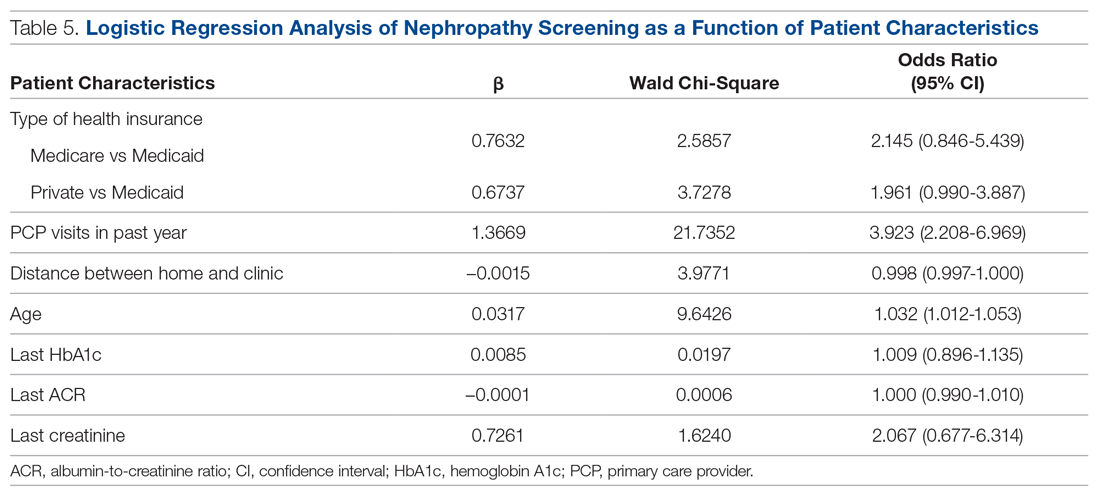
A logistic regression analysis was conducted with nephropathy screening (screened or not screened) as the outcome and 7 patient characteristics as predictors: type of insurance (private, Medicare, or Medicaid), PCP visits in the past 12 months (≤ 3 or > 3), distance in miles of the patient’s residence from the clinic, age, last HbA1c value, last ACR value, and last creatinine value. A test of the full model with all 7 predictors was statistically significant (χ2 (8) = 57.77, P < 0.001). Table 5 shows regression coefficients, Wald statistics, and 95% confidence intervals for odds ratios for each of the 7 predictors. According to the Wald criterion, 3 patient characteristics were significant predictors of nephropathy screening: age, distance between the patient’s home and clinic, and number of PCP visits in the past 12 months. After adjusting for the covariates, there were still significant associations between the nephropathy screening status and age ( χ2(1) = 9.64, P < 0.01); distance between the patient’s home and the clinic (χ2(1) = 3.98, P < 0.05); and the number of PCP visits in the previous year (χ2(1) = 21.74, P < 0.001). With each 1-year increment in age, the odds of completing the nephropathy screening increased by 3.2%. With each 1-mile increase in the distance between the patient’s home and clinic, the odds of completing the nephropathy screening decreased by 0.2%. Patients who visited the clinic more than 3 times in a year were 3.9 times (95% confidence interval, 2.2-7.0) more likely to complete the nephropathy screening than their counterparts who visited fewer than 3 times per year.
In summary, older patients living within about 164 miles of the clinic (ie, within 1 standard deviation from the average miles between patient’s homes and the clinic) who visited their PCP 3 or more times per year were the most likely to be screened.
Discussion
Diabetic nephropathy is a critical issue facing family medicine providers and patients. The morbidity and mortality costs are significant, as diabetic nephropathy is the leading cause of end-stage renal disease. While the ADA recommends annual ACR screening in patients with diabetes and prescription of ACE inhibitors or ARBs in patients who qualify, many patients do not receive these interventions, despite following up with a provider.12-15 There is no current literature that indicates the compliance rates in the rural setting. Due to health disparities in the rural setting noted in the literature, it could be hypothesized that these individuals are at high risk of not meeting these screening and treatment recommendations.16,17 Limited access to care and resources, gaps in insurance coverage, and lower health literacy are a few barriers identified in the rural population that may influence whether these measures are met.17
Considering the disease burden of diabetes and its related complications, including nephropathy, consistent screening is necessary to reduce diabetes-related burdens and cost, while also increasing the quality of life for patients with diabetes. All parties must be involved to ensure appropriate compliance and treatment. Our institution’s implementation of quality improvement strategies has key implications for nephropathy screening and treatment efforts in rural settings.
An additional step of having a health care provider (other than the PCP) screen all patients who are not meeting the standard allows for identification of gaps in care. In our quality improvement workflow, the clinical pharmacist screened all patients for candidacy for ACE inhibitor/ARB therapy. While only a small percentage of patients qualified, many of these patients had previously been on therapy and were discontinued for an unknown reason or were stopped due to an acute condition (eg, acute kidney injury) and never restarted after recovery. Other patients required additional education that therapy would be utilized for nephroprotection versus blood pressure management (secondary to an elevated ACR). This highlights the importance of transitions of care and ongoing, intensive education, not only during initial diagnosis but also throughout the disease-state progression.
Utilization of EHRs and telephone outreach are additional aspects of care that can be provided. Our improved rates of compliance with these care interventions parallel findings from previous studies.15,18 Optimization of an institution’s EHR can aid in standardization of care, workflow management, and communication with patients, as well as alert nursing or support staff of screening needs. Techniques such as best practice reminders, patient chart messages, and nursing-entered physician alerts on daily schedules have been shown to increase rates of compliance with nephropathy standards. These findings underscore an additional opportunity for nursing and support staff to be better integrated into care.
Despite the success of this quality improvement initiative, there remain some limitations. The processes we used in this project may not be applicable to every institution and may have limited external validity. Primarily, while these processes may be implemented at some sites, without additional support staff (ie, extra nursing staff, pharmacists) and students to aid in patient outreach, success may be limited due to provider time constraints. Additionally, our workflow process demonstrates significant incorporation of an EHR system for patient outreach. Institutions and/or clinics that heavily rely on paper charts and paper outreach may face barriers with bulk orders (eg, ACR) and messages, interventions that streamlined our population health management. Finally, this project focuses on only 1 aspect of population health management for patients with diabetes. While nephropathy is a critical aspect of caring for individuals with diabetes, this patient outreach does not address retinopathy screening, HbA1c control, or vaccination rates, which are other components of care.
Conclusion
Although this evaluation does not provide insight into why patients were not treated or screened, it demonstrates processes to improve compliance in patients with diabetic nephropathy. Rural health care facilities require an ongoing program of change and evaluation, with the aim to improve the provision of services, increase screening, and encourage team member involvement in health promotion. This study demonstrates that combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy screening and treatment in rural patients with diabetes at a family medicine clinic.
Corresponding author: Amie M. Ashcraft, West Virginia University, Department of Family Medicine, 1 Medical Center Drive, Box 9152, Morgantown, WV 26506; [email protected].
Financial disclosures: None.
Acknowledgment: The authors thank the faculty, residents, nurses, and clinic staff for their hard work and dedication to this effort: Umama Sadia, Michelle Prestoza, Richard Dattola, Greg Doyle, Dana King, Mike Maroon, Kendra Under, Judy Siebert, Christine Snyder, Rachel Burge, Meagan Gribble, Lisa Metts, Kelsey Samek, Sarah Deavers, Amber Kitzmiller, Angela Lamp, Tina Waldeck, and Andrea Sukeruksa.
1. Centers for Disease Control and Prevention (CDC). National diabetes statistics report. Estimates of diabetes and its burden in the United States. Atlanta, GA: CDC; 2017www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 20, 2020.
2. American Diabetes Association (ADA). Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928.
3. Wood L. Trends in national and regional economic distress, 1960-2000. Washington, DC: Appalachian Regional Commission; 2005.
4. Barker L, Crespo R, Gerzoff RB, et al. Residence in a distressed county in Appalachia as a risk factor for diabetes, Behavioral Risk Factor Surveillance System, 2006-2007. Prev Chronic Dis. 2010;7:A104.
5. Barker L, Kirtland KA, Gregg E, et al. Geographic distribution of diagnosed diabetes in the United States: A diabetes belt. Am J Prev Med. 2011;40:434-439.
6. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176.
7. United States Renal Data System (USRDS). Annual data report. Ann Arbor, MI: USRDS; 2018. www.usrds.org/2018/view/Default.aspx. Accessed December 20, 2020.
8. Halverson JA, Bichak G. Underlying socioeconomic factors influencing health disparities in the Appalachian region. Washington, DC: Appalachian Regional Commission; 2008.
9. Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20:368-373.
10. Hatcher J, Dignan MB, Schoenberg N. How do rural health care providers and patients view barriers to colorectal cancer screening? Insights from Appalachian Kentucky. Nurs Clin North Am. 2011;46:181-192.
11. Scott S, McSpirit S. The suspicious, untrusting hillbilly in political-economic contexts: Stereotypes and social trust in the Appalachian coalfields. Pract Anthropol. 2014;36:42-46.
12. Kirkman MS, Williams SR, Caffrey HH, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care. 2002;25:1946-1951.
13. Byun SH, Ma SH, Jun JK, et al. Screening for diabetic retinopathy and nephropathy in patients with diabetes: A nationwide survey in Korea. PLoS One. 2013;8:e62991.
14. Flood D, Garcia P, Douglas K, et al. Screening for chronic kidney disease in a community-based diabetes cohort in rural Guatemala: A cross-sectional study. BMJ Open. 2018;8:e019778.
15. Anabtawi A, Mathew LM. Improving compliance with screening of diabetic patients for microalbuminuria in primary care practice. ISRN Endocrinology. 2013:893913.
16. Tonks SA, Makwana S, Salanitro AH, et al. Quality of diabetes mellitus care by rural primary care physicians. J Rural Health. 2012;28:364-371.
17. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129:611-620.
18. Weber V, Bloom F, Pierdon S, Wood C. Employing the electronic health record to improve diabetes care: a multifaceted intervention in an integrated delivery system. J Gen Intern Med. 2008;23:379-382.
From West Virginia University, Morgantown, WV.
Abstract
Objective: To describe the strategies a family medicine clinic in Appalachia utilized to increase nephropathy screening rates as well as to explore the factors predictive of nephropathy screening in patients with diabetes.
Design: This quality improvement project targeted the points in the care process when patients are lost to follow-up for nephropathy screening.
Setting and participants: Patients with diabetes cared for by a primary care provider (PCP) at an academic family medicine practice in Appalachia from January 2018 to November 2018.
Interventions: Bulk orders for albumin-to-creatinine (ACR) testing and urine collection during clinic visit, enhanced patient communication through bulk communication reminders and individual patient outreach, and education of clinic providers.
Measurements: Demographic data and monthly nephropathy screening rates.
Results: The nephropathy screening rate increased by 6.2% during the project. Older patients living closer to the clinic who visited their PCP 3 or more times per year were the most likely to be screened.
Conclusion: Combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy treatment and screening in rural patients with diabetes at a family medicine clinic.
Keywords: rural; kidney disease; albumin-to-creatinine ratio; electronic health record.
According to the Centers for Disease Control and Prevention (CDC), an estimated 30.3 million people in the United States—about 9.4% of the population—have been diagnosed with diabetes.1 Diabetes is the seventh leading cause of death in the United States, and it contributes to other leading causes of death: heart disease and stroke.1 Diabetes also is related to high morbidity risk and is a leading cause of chronic kidney disease.1 The total cost of diagnosed diabetes was estimated at $327 billion in direct medical costs and reduced productivity.2
Residents of Appalachia bear a disproportionate burden of diabetes and other related negative health outcomes; these outcomes are influenced by a number of factors, including socioeconomic status, poverty, rurality, and health care access. Rates of chronic disease, such as diabetes, are most pronounced in Appalachia’s most economically distressed counties.3-5 In 2011, the CDC labeled a 644-county area the “diabetes belt,” which included most of Appalachia.6 As a result of this elevated prevalence of diabetes in Appalachia as compared to the rest of the country, complications directly associated with diabetes are more commonly observed in Appalachian residents. One of the most damaging complications is diabetic nephropathy.
Diabetic nephropathy results from damage to the microvasculature of the kidney due to inadequately controlled blood glucose. This, in turn, leads to decreased renal function, eventually leading to clinically significant renal disease. The long-term complications associated with nephropathy can include many comorbid conditions, the most serious of which are progression to end-stage renal disease, dialysis requirement, and early mortality. Diabetic nephropathy affects approximately 40% of patients with type 1 and type 2 diabetes.7,8
One way to prevent complications of diabetic nephropathy, in addition to good glycemic control in patients with diabetes, is early and regular screening. Currently, the American Diabetes Association (ADA) recommends yearly screening for diabetic nephropathy in the form of a urine albumin-to-creatinine ratio (ACR) for patients 18 to 75 years of age.2 This screening to detect diabetic nephropathy is recognized as a marker of quality care by many public and private insurance agencies and medical specialty associations, such as the Centers for Medicare and Medicaid Services.
Many patients with diabetes are cared for by primary care providers (PCP), and these PCP appointments provide an opportune time to screen and appropriately treat nephropathy. Screening opportunities are often missed, however, due to time constraints and competing health priorities. There are also a number of other factors specific to the Appalachian region that reduce the likelihood of screening for diabetic nephropathy, such as a lack of health insurance, the need to travel long distances to see a PCP, work and household responsibilities, low levels of education and health literacy, and a mistrust of outsiders regarding personal matters, including health.9-11 While nephropathy can have a detrimental impact on patients across populations, it is of particular concern for a state located in the heart of Appalachia, such as West Virginia.
Given the disproportionate burden of diabetes in this region and the potentially severe consequences of undetected nephropathy, clinicians from an academic family medicine clinic in West Virginia undertook a quality improvement project to increase the rate of nephropathy screening and treatment among patients with diabetes. This article describes the intervention strategies the team utilized to increase nephropathy screening and treatment in patients 18 to 75 years of age who met quality measures for nephropathy screening or treatment in the previous 12 months and explores the factors most predictive of nephropathy screening in Appalachian patients in this age group. It also reports the challenges and opportunities encountered and offers suggestions for other providers and clinics attempting to increase their nephropathy screening rates.
Methods
Setting and Study Population
The study population included patients ages 18 to 75 years under the care of providers in an academic family medicine practice in West Virginia who had been diagnosed with diabetes mellitus. The study focused on those patients overdue for diabetic nephropathy screening (ie, had not been screened in previous 12 months). The project began in January 2018 with a screening rate of 83.8%. The goal of this project was to increase this compliance metric by at least 5%. The project protocol was submitted to the West Virginia University Institutional Review Board, and, because it is a quality improvement project, permission was given to proceed without a board review.
Interventions
The team identified and implemented several interventions intended to reduce screening barriers and increase the screening rate.
Bulk orders for ACR and urine collection during clinic visits. Prior to initiation of this project, it was left to individual clinic providers to order nephropathy screening for patients with diabetes during a clinic visit; after receiving the order for “random urine microalbumin/creatinine ratio,” patients then had to travel to a lab to provide a urine sample. For this project and moving forward, the team changed to the procedure of initiating bulk ACR orders and collecting urine samples during clinic visits from all patients ages 18 to 75 years who have diabetes.
Bulk communication reminders. Since many patients with diabetes may not have realized they were overdue for nephropathy screening, the team began sending out bulk communication reminders through either the institution’s electronic health record (EHR; MyChart) or postal service–delivered physical letters (according to patient communication preferences) to remind patients that they were due for screening and to encourage them to schedule an appointment or keep a previously scheduled appointment with their PCP.
Individual patient outreach. A team of pharmacy students led by a licensed pharmacist in the family medicine clinic contacted patients overdue for screening even after bulk communication reminders went out. The students telephoned patients 2 to 3 months following the bulk communication. The students obtained an updated list of patients with diabetes ages 18 to 75 years from an EHR quality report. They began by prescreening the patients on the overdue list for potential candidacy for an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB). Screening for candidacy included evaluation of recent blood pressure readings, electrolytes (ie, basic metabolic panel), and ACR. If the students determined a patient was a candidate, they presented the patient to the preceptor for verification and then reached out to the provider with a recommendation. If the provider agreed, the student contacted the patient by telephone for medication counseling and education. The remaining patients determined not to be candidates for ACE inhibitors or ARBs were contacted by the pharmacy students by telephone to remind them that laboratory work was pending. Up to 3 phone call attempts were made before patients were determined to be unreachable. Students left voice mails with generic reminders if a patient could not be reached. If a patient answered, the student provided a reminder but also reviewed indications for lab work, the reason why the provider wished for follow-up, and updated lab hours. Students also followed up with the results of the work-up, as appropriate. During this outreach process, the student team encountered a number of patients who had moved or changed to a PCP outside of the family medicine clinic. In these cases, the EHR was updated and those patients were removed from the list of patients altogether.
Education of clinic providers. Clinic providers were educated during faculty and resident meetings and didactic learning sessions on identifying patients within the EHR who are due for nephropathy screening. They also received instruction on how to update the EHR to reflect completed screenings.
Data Analysis
All analyses in this study were conducted using SAS (version 9.4, 2013, SAS Institute Inc., Cary, NC). Descriptive analyses were conducted to summarize basic patient demographic information. To compare patients screened within the previous 12 months to those patients overdue for screening, 2-sample t-tests were used to examine differences in patients’ age, HbA1c, ACR, and creatinine level and the distance (in miles) between the patient’s home and the clinic. Chi-square analyses were used to examine the relationship between whether a patient was recently screened for nephropathy and the patient’s insurance, number of patient visits in the previous 12 months, and provider level. Logistic regression analyses were conducted to control for covariates and to explore which factors were most predictive of nephropathy screening. All tests were 2-tailed, and P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
There were 1676 family medicine clinic patients with diabetes between 18 and 75 years of age (Table 1 and Table 2). Of the total sample, 1489 (88.8%) had completed screening for nephropathy in the 12 months prior to evaluation, and 67.5%, 23.7%, and 8.8% of patients had private insurance, Medicare, and Medicaid, respectively. 
The mean (SD) age of the patients was 56.3 (11.9) years. The mean distance between the patient’s home and the clinic was 26.6 (76.8) miles. The mean number of visits was 3.6 (2.9) per year, and 43.0% of the patientvisited the clinic more than 3 times in a year. The mean values for HbA1c (%), creatinine (g/mol), and ACR (mg/g) were 7.7 (1.9), 1.0 (0.7), and 9.4 (31.4), respectively.
Screening of Patients for Nephropathy
Patients with Medicare and private insurance were more likely to have completed the nephropathy screening than those with Medicaid (92.5% versus 88.8% versus 82.8%, P = 0.004; Table 3 and Table 4). 

Changes in Screening Rate
The practice-wide screening rate was 83.8% at the start of this project in January 2018. The screening rate steadily increased throughout 2018, reaching 90.3% in August 2018, and then leveled off around 90% when the project was concluded at the end of November 2018 (Figure). As an added benefit of the increased screening rates, a number of patients were initiated on an ACE inhibitor or ARB based on the team’s screening efforts.

Predictors of Nephropathy Screening
A logistic regression analysis was conducted with nephropathy screening (screened or not screened) as the outcome and 7 patient characteristics as predictors: type of insurance (private, Medicare, or Medicaid), PCP visits in the past 12 months (≤ 3 or > 3), distance in miles of the patient’s residence from the clinic, age, last HbA1c value, last ACR value, and last creatinine value. A test of the full model with all 7 predictors was statistically significant (χ2 (8) = 57.77, P < 0.001). Table 5 shows regression coefficients, Wald statistics, and 95% confidence intervals for odds ratios for each of the 7 predictors. According to the Wald criterion, 3 patient characteristics were significant predictors of nephropathy screening: age, distance between the patient’s home and clinic, and number of PCP visits in the past 12 months. After adjusting for the covariates, there were still significant associations between the nephropathy screening status and age ( χ2(1) = 9.64, P < 0.01); distance between the patient’s home and the clinic (χ2(1) = 3.98, P < 0.05); and the number of PCP visits in the previous year (χ2(1) = 21.74, P < 0.001). With each 1-year increment in age, the odds of completing the nephropathy screening increased by 3.2%. With each 1-mile increase in the distance between the patient’s home and clinic, the odds of completing the nephropathy screening decreased by 0.2%. Patients who visited the clinic more than 3 times in a year were 3.9 times (95% confidence interval, 2.2-7.0) more likely to complete the nephropathy screening than their counterparts who visited fewer than 3 times per year.
In summary, older patients living within about 164 miles of the clinic (ie, within 1 standard deviation from the average miles between patient’s homes and the clinic) who visited their PCP 3 or more times per year were the most likely to be screened.

Discussion
Diabetic nephropathy is a critical issue facing family medicine providers and patients. The morbidity and mortality costs are significant, as diabetic nephropathy is the leading cause of end-stage renal disease. While the ADA recommends annual ACR screening in patients with diabetes and prescription of ACE inhibitors or ARBs in patients who qualify, many patients do not receive these interventions, despite following up with a provider.12-15 There is no current literature that indicates the compliance rates in the rural setting. Due to health disparities in the rural setting noted in the literature, it could be hypothesized that these individuals are at high risk of not meeting these screening and treatment recommendations.16,17 Limited access to care and resources, gaps in insurance coverage, and lower health literacy are a few barriers identified in the rural population that may influence whether these measures are met.17
Considering the disease burden of diabetes and its related complications, including nephropathy, consistent screening is necessary to reduce diabetes-related burdens and cost, while also increasing the quality of life for patients with diabetes. All parties must be involved to ensure appropriate compliance and treatment. Our institution’s implementation of quality improvement strategies has key implications for nephropathy screening and treatment efforts in rural settings.
An additional step of having a health care provider (other than the PCP) screen all patients who are not meeting the standard allows for identification of gaps in care. In our quality improvement workflow, the clinical pharmacist screened all patients for candidacy for ACE inhibitor/ARB therapy. While only a small percentage of patients qualified, many of these patients had previously been on therapy and were discontinued for an unknown reason or were stopped due to an acute condition (eg, acute kidney injury) and never restarted after recovery. Other patients required additional education that therapy would be utilized for nephroprotection versus blood pressure management (secondary to an elevated ACR). This highlights the importance of transitions of care and ongoing, intensive education, not only during initial diagnosis but also throughout the disease-state progression.
Utilization of EHRs and telephone outreach are additional aspects of care that can be provided. Our improved rates of compliance with these care interventions parallel findings from previous studies.15,18 Optimization of an institution’s EHR can aid in standardization of care, workflow management, and communication with patients, as well as alert nursing or support staff of screening needs. Techniques such as best practice reminders, patient chart messages, and nursing-entered physician alerts on daily schedules have been shown to increase rates of compliance with nephropathy standards. These findings underscore an additional opportunity for nursing and support staff to be better integrated into care.
Despite the success of this quality improvement initiative, there remain some limitations. The processes we used in this project may not be applicable to every institution and may have limited external validity. Primarily, while these processes may be implemented at some sites, without additional support staff (ie, extra nursing staff, pharmacists) and students to aid in patient outreach, success may be limited due to provider time constraints. Additionally, our workflow process demonstrates significant incorporation of an EHR system for patient outreach. Institutions and/or clinics that heavily rely on paper charts and paper outreach may face barriers with bulk orders (eg, ACR) and messages, interventions that streamlined our population health management. Finally, this project focuses on only 1 aspect of population health management for patients with diabetes. While nephropathy is a critical aspect of caring for individuals with diabetes, this patient outreach does not address retinopathy screening, HbA1c control, or vaccination rates, which are other components of care.
Conclusion
Although this evaluation does not provide insight into why patients were not treated or screened, it demonstrates processes to improve compliance in patients with diabetic nephropathy. Rural health care facilities require an ongoing program of change and evaluation, with the aim to improve the provision of services, increase screening, and encourage team member involvement in health promotion. This study demonstrates that combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy screening and treatment in rural patients with diabetes at a family medicine clinic.
Corresponding author: Amie M. Ashcraft, West Virginia University, Department of Family Medicine, 1 Medical Center Drive, Box 9152, Morgantown, WV 26506; [email protected].
Financial disclosures: None.
Acknowledgment: The authors thank the faculty, residents, nurses, and clinic staff for their hard work and dedication to this effort: Umama Sadia, Michelle Prestoza, Richard Dattola, Greg Doyle, Dana King, Mike Maroon, Kendra Under, Judy Siebert, Christine Snyder, Rachel Burge, Meagan Gribble, Lisa Metts, Kelsey Samek, Sarah Deavers, Amber Kitzmiller, Angela Lamp, Tina Waldeck, and Andrea Sukeruksa.
From West Virginia University, Morgantown, WV.
Abstract
Objective: To describe the strategies a family medicine clinic in Appalachia utilized to increase nephropathy screening rates as well as to explore the factors predictive of nephropathy screening in patients with diabetes.
Design: This quality improvement project targeted the points in the care process when patients are lost to follow-up for nephropathy screening.
Setting and participants: Patients with diabetes cared for by a primary care provider (PCP) at an academic family medicine practice in Appalachia from January 2018 to November 2018.
Interventions: Bulk orders for albumin-to-creatinine (ACR) testing and urine collection during clinic visit, enhanced patient communication through bulk communication reminders and individual patient outreach, and education of clinic providers.
Measurements: Demographic data and monthly nephropathy screening rates.
Results: The nephropathy screening rate increased by 6.2% during the project. Older patients living closer to the clinic who visited their PCP 3 or more times per year were the most likely to be screened.
Conclusion: Combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy treatment and screening in rural patients with diabetes at a family medicine clinic.
Keywords: rural; kidney disease; albumin-to-creatinine ratio; electronic health record.
According to the Centers for Disease Control and Prevention (CDC), an estimated 30.3 million people in the United States—about 9.4% of the population—have been diagnosed with diabetes.1 Diabetes is the seventh leading cause of death in the United States, and it contributes to other leading causes of death: heart disease and stroke.1 Diabetes also is related to high morbidity risk and is a leading cause of chronic kidney disease.1 The total cost of diagnosed diabetes was estimated at $327 billion in direct medical costs and reduced productivity.2
Residents of Appalachia bear a disproportionate burden of diabetes and other related negative health outcomes; these outcomes are influenced by a number of factors, including socioeconomic status, poverty, rurality, and health care access. Rates of chronic disease, such as diabetes, are most pronounced in Appalachia’s most economically distressed counties.3-5 In 2011, the CDC labeled a 644-county area the “diabetes belt,” which included most of Appalachia.6 As a result of this elevated prevalence of diabetes in Appalachia as compared to the rest of the country, complications directly associated with diabetes are more commonly observed in Appalachian residents. One of the most damaging complications is diabetic nephropathy.
Diabetic nephropathy results from damage to the microvasculature of the kidney due to inadequately controlled blood glucose. This, in turn, leads to decreased renal function, eventually leading to clinically significant renal disease. The long-term complications associated with nephropathy can include many comorbid conditions, the most serious of which are progression to end-stage renal disease, dialysis requirement, and early mortality. Diabetic nephropathy affects approximately 40% of patients with type 1 and type 2 diabetes.7,8
One way to prevent complications of diabetic nephropathy, in addition to good glycemic control in patients with diabetes, is early and regular screening. Currently, the American Diabetes Association (ADA) recommends yearly screening for diabetic nephropathy in the form of a urine albumin-to-creatinine ratio (ACR) for patients 18 to 75 years of age.2 This screening to detect diabetic nephropathy is recognized as a marker of quality care by many public and private insurance agencies and medical specialty associations, such as the Centers for Medicare and Medicaid Services.
Many patients with diabetes are cared for by primary care providers (PCP), and these PCP appointments provide an opportune time to screen and appropriately treat nephropathy. Screening opportunities are often missed, however, due to time constraints and competing health priorities. There are also a number of other factors specific to the Appalachian region that reduce the likelihood of screening for diabetic nephropathy, such as a lack of health insurance, the need to travel long distances to see a PCP, work and household responsibilities, low levels of education and health literacy, and a mistrust of outsiders regarding personal matters, including health.9-11 While nephropathy can have a detrimental impact on patients across populations, it is of particular concern for a state located in the heart of Appalachia, such as West Virginia.
Given the disproportionate burden of diabetes in this region and the potentially severe consequences of undetected nephropathy, clinicians from an academic family medicine clinic in West Virginia undertook a quality improvement project to increase the rate of nephropathy screening and treatment among patients with diabetes. This article describes the intervention strategies the team utilized to increase nephropathy screening and treatment in patients 18 to 75 years of age who met quality measures for nephropathy screening or treatment in the previous 12 months and explores the factors most predictive of nephropathy screening in Appalachian patients in this age group. It also reports the challenges and opportunities encountered and offers suggestions for other providers and clinics attempting to increase their nephropathy screening rates.
Methods
Setting and Study Population
The study population included patients ages 18 to 75 years under the care of providers in an academic family medicine practice in West Virginia who had been diagnosed with diabetes mellitus. The study focused on those patients overdue for diabetic nephropathy screening (ie, had not been screened in previous 12 months). The project began in January 2018 with a screening rate of 83.8%. The goal of this project was to increase this compliance metric by at least 5%. The project protocol was submitted to the West Virginia University Institutional Review Board, and, because it is a quality improvement project, permission was given to proceed without a board review.
Interventions
The team identified and implemented several interventions intended to reduce screening barriers and increase the screening rate.
Bulk orders for ACR and urine collection during clinic visits. Prior to initiation of this project, it was left to individual clinic providers to order nephropathy screening for patients with diabetes during a clinic visit; after receiving the order for “random urine microalbumin/creatinine ratio,” patients then had to travel to a lab to provide a urine sample. For this project and moving forward, the team changed to the procedure of initiating bulk ACR orders and collecting urine samples during clinic visits from all patients ages 18 to 75 years who have diabetes.
Bulk communication reminders. Since many patients with diabetes may not have realized they were overdue for nephropathy screening, the team began sending out bulk communication reminders through either the institution’s electronic health record (EHR; MyChart) or postal service–delivered physical letters (according to patient communication preferences) to remind patients that they were due for screening and to encourage them to schedule an appointment or keep a previously scheduled appointment with their PCP.
Individual patient outreach. A team of pharmacy students led by a licensed pharmacist in the family medicine clinic contacted patients overdue for screening even after bulk communication reminders went out. The students telephoned patients 2 to 3 months following the bulk communication. The students obtained an updated list of patients with diabetes ages 18 to 75 years from an EHR quality report. They began by prescreening the patients on the overdue list for potential candidacy for an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB). Screening for candidacy included evaluation of recent blood pressure readings, electrolytes (ie, basic metabolic panel), and ACR. If the students determined a patient was a candidate, they presented the patient to the preceptor for verification and then reached out to the provider with a recommendation. If the provider agreed, the student contacted the patient by telephone for medication counseling and education. The remaining patients determined not to be candidates for ACE inhibitors or ARBs were contacted by the pharmacy students by telephone to remind them that laboratory work was pending. Up to 3 phone call attempts were made before patients were determined to be unreachable. Students left voice mails with generic reminders if a patient could not be reached. If a patient answered, the student provided a reminder but also reviewed indications for lab work, the reason why the provider wished for follow-up, and updated lab hours. Students also followed up with the results of the work-up, as appropriate. During this outreach process, the student team encountered a number of patients who had moved or changed to a PCP outside of the family medicine clinic. In these cases, the EHR was updated and those patients were removed from the list of patients altogether.
Education of clinic providers. Clinic providers were educated during faculty and resident meetings and didactic learning sessions on identifying patients within the EHR who are due for nephropathy screening. They also received instruction on how to update the EHR to reflect completed screenings.
Data Analysis
All analyses in this study were conducted using SAS (version 9.4, 2013, SAS Institute Inc., Cary, NC). Descriptive analyses were conducted to summarize basic patient demographic information. To compare patients screened within the previous 12 months to those patients overdue for screening, 2-sample t-tests were used to examine differences in patients’ age, HbA1c, ACR, and creatinine level and the distance (in miles) between the patient’s home and the clinic. Chi-square analyses were used to examine the relationship between whether a patient was recently screened for nephropathy and the patient’s insurance, number of patient visits in the previous 12 months, and provider level. Logistic regression analyses were conducted to control for covariates and to explore which factors were most predictive of nephropathy screening. All tests were 2-tailed, and P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
There were 1676 family medicine clinic patients with diabetes between 18 and 75 years of age (Table 1 and Table 2). Of the total sample, 1489 (88.8%) had completed screening for nephropathy in the 12 months prior to evaluation, and 67.5%, 23.7%, and 8.8% of patients had private insurance, Medicare, and Medicaid, respectively. 
The mean (SD) age of the patients was 56.3 (11.9) years. The mean distance between the patient’s home and the clinic was 26.6 (76.8) miles. The mean number of visits was 3.6 (2.9) per year, and 43.0% of the patientvisited the clinic more than 3 times in a year. The mean values for HbA1c (%), creatinine (g/mol), and ACR (mg/g) were 7.7 (1.9), 1.0 (0.7), and 9.4 (31.4), respectively.
Screening of Patients for Nephropathy
Patients with Medicare and private insurance were more likely to have completed the nephropathy screening than those with Medicaid (92.5% versus 88.8% versus 82.8%, P = 0.004; Table 3 and Table 4). 

Changes in Screening Rate
The practice-wide screening rate was 83.8% at the start of this project in January 2018. The screening rate steadily increased throughout 2018, reaching 90.3% in August 2018, and then leveled off around 90% when the project was concluded at the end of November 2018 (Figure). As an added benefit of the increased screening rates, a number of patients were initiated on an ACE inhibitor or ARB based on the team’s screening efforts.

Predictors of Nephropathy Screening
A logistic regression analysis was conducted with nephropathy screening (screened or not screened) as the outcome and 7 patient characteristics as predictors: type of insurance (private, Medicare, or Medicaid), PCP visits in the past 12 months (≤ 3 or > 3), distance in miles of the patient’s residence from the clinic, age, last HbA1c value, last ACR value, and last creatinine value. A test of the full model with all 7 predictors was statistically significant (χ2 (8) = 57.77, P < 0.001). Table 5 shows regression coefficients, Wald statistics, and 95% confidence intervals for odds ratios for each of the 7 predictors. According to the Wald criterion, 3 patient characteristics were significant predictors of nephropathy screening: age, distance between the patient’s home and clinic, and number of PCP visits in the past 12 months. After adjusting for the covariates, there were still significant associations between the nephropathy screening status and age ( χ2(1) = 9.64, P < 0.01); distance between the patient’s home and the clinic (χ2(1) = 3.98, P < 0.05); and the number of PCP visits in the previous year (χ2(1) = 21.74, P < 0.001). With each 1-year increment in age, the odds of completing the nephropathy screening increased by 3.2%. With each 1-mile increase in the distance between the patient’s home and clinic, the odds of completing the nephropathy screening decreased by 0.2%. Patients who visited the clinic more than 3 times in a year were 3.9 times (95% confidence interval, 2.2-7.0) more likely to complete the nephropathy screening than their counterparts who visited fewer than 3 times per year.
In summary, older patients living within about 164 miles of the clinic (ie, within 1 standard deviation from the average miles between patient’s homes and the clinic) who visited their PCP 3 or more times per year were the most likely to be screened.

Discussion
Diabetic nephropathy is a critical issue facing family medicine providers and patients. The morbidity and mortality costs are significant, as diabetic nephropathy is the leading cause of end-stage renal disease. While the ADA recommends annual ACR screening in patients with diabetes and prescription of ACE inhibitors or ARBs in patients who qualify, many patients do not receive these interventions, despite following up with a provider.12-15 There is no current literature that indicates the compliance rates in the rural setting. Due to health disparities in the rural setting noted in the literature, it could be hypothesized that these individuals are at high risk of not meeting these screening and treatment recommendations.16,17 Limited access to care and resources, gaps in insurance coverage, and lower health literacy are a few barriers identified in the rural population that may influence whether these measures are met.17
Considering the disease burden of diabetes and its related complications, including nephropathy, consistent screening is necessary to reduce diabetes-related burdens and cost, while also increasing the quality of life for patients with diabetes. All parties must be involved to ensure appropriate compliance and treatment. Our institution’s implementation of quality improvement strategies has key implications for nephropathy screening and treatment efforts in rural settings.
An additional step of having a health care provider (other than the PCP) screen all patients who are not meeting the standard allows for identification of gaps in care. In our quality improvement workflow, the clinical pharmacist screened all patients for candidacy for ACE inhibitor/ARB therapy. While only a small percentage of patients qualified, many of these patients had previously been on therapy and were discontinued for an unknown reason or were stopped due to an acute condition (eg, acute kidney injury) and never restarted after recovery. Other patients required additional education that therapy would be utilized for nephroprotection versus blood pressure management (secondary to an elevated ACR). This highlights the importance of transitions of care and ongoing, intensive education, not only during initial diagnosis but also throughout the disease-state progression.
Utilization of EHRs and telephone outreach are additional aspects of care that can be provided. Our improved rates of compliance with these care interventions parallel findings from previous studies.15,18 Optimization of an institution’s EHR can aid in standardization of care, workflow management, and communication with patients, as well as alert nursing or support staff of screening needs. Techniques such as best practice reminders, patient chart messages, and nursing-entered physician alerts on daily schedules have been shown to increase rates of compliance with nephropathy standards. These findings underscore an additional opportunity for nursing and support staff to be better integrated into care.
Despite the success of this quality improvement initiative, there remain some limitations. The processes we used in this project may not be applicable to every institution and may have limited external validity. Primarily, while these processes may be implemented at some sites, without additional support staff (ie, extra nursing staff, pharmacists) and students to aid in patient outreach, success may be limited due to provider time constraints. Additionally, our workflow process demonstrates significant incorporation of an EHR system for patient outreach. Institutions and/or clinics that heavily rely on paper charts and paper outreach may face barriers with bulk orders (eg, ACR) and messages, interventions that streamlined our population health management. Finally, this project focuses on only 1 aspect of population health management for patients with diabetes. While nephropathy is a critical aspect of caring for individuals with diabetes, this patient outreach does not address retinopathy screening, HbA1c control, or vaccination rates, which are other components of care.
Conclusion
Although this evaluation does not provide insight into why patients were not treated or screened, it demonstrates processes to improve compliance in patients with diabetic nephropathy. Rural health care facilities require an ongoing program of change and evaluation, with the aim to improve the provision of services, increase screening, and encourage team member involvement in health promotion. This study demonstrates that combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy screening and treatment in rural patients with diabetes at a family medicine clinic.
Corresponding author: Amie M. Ashcraft, West Virginia University, Department of Family Medicine, 1 Medical Center Drive, Box 9152, Morgantown, WV 26506; [email protected].
Financial disclosures: None.
Acknowledgment: The authors thank the faculty, residents, nurses, and clinic staff for their hard work and dedication to this effort: Umama Sadia, Michelle Prestoza, Richard Dattola, Greg Doyle, Dana King, Mike Maroon, Kendra Under, Judy Siebert, Christine Snyder, Rachel Burge, Meagan Gribble, Lisa Metts, Kelsey Samek, Sarah Deavers, Amber Kitzmiller, Angela Lamp, Tina Waldeck, and Andrea Sukeruksa.
1. Centers for Disease Control and Prevention (CDC). National diabetes statistics report. Estimates of diabetes and its burden in the United States. Atlanta, GA: CDC; 2017www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 20, 2020.
2. American Diabetes Association (ADA). Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928.
3. Wood L. Trends in national and regional economic distress, 1960-2000. Washington, DC: Appalachian Regional Commission; 2005.
4. Barker L, Crespo R, Gerzoff RB, et al. Residence in a distressed county in Appalachia as a risk factor for diabetes, Behavioral Risk Factor Surveillance System, 2006-2007. Prev Chronic Dis. 2010;7:A104.
5. Barker L, Kirtland KA, Gregg E, et al. Geographic distribution of diagnosed diabetes in the United States: A diabetes belt. Am J Prev Med. 2011;40:434-439.
6. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176.
7. United States Renal Data System (USRDS). Annual data report. Ann Arbor, MI: USRDS; 2018. www.usrds.org/2018/view/Default.aspx. Accessed December 20, 2020.
8. Halverson JA, Bichak G. Underlying socioeconomic factors influencing health disparities in the Appalachian region. Washington, DC: Appalachian Regional Commission; 2008.
9. Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20:368-373.
10. Hatcher J, Dignan MB, Schoenberg N. How do rural health care providers and patients view barriers to colorectal cancer screening? Insights from Appalachian Kentucky. Nurs Clin North Am. 2011;46:181-192.
11. Scott S, McSpirit S. The suspicious, untrusting hillbilly in political-economic contexts: Stereotypes and social trust in the Appalachian coalfields. Pract Anthropol. 2014;36:42-46.
12. Kirkman MS, Williams SR, Caffrey HH, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care. 2002;25:1946-1951.
13. Byun SH, Ma SH, Jun JK, et al. Screening for diabetic retinopathy and nephropathy in patients with diabetes: A nationwide survey in Korea. PLoS One. 2013;8:e62991.
14. Flood D, Garcia P, Douglas K, et al. Screening for chronic kidney disease in a community-based diabetes cohort in rural Guatemala: A cross-sectional study. BMJ Open. 2018;8:e019778.
15. Anabtawi A, Mathew LM. Improving compliance with screening of diabetic patients for microalbuminuria in primary care practice. ISRN Endocrinology. 2013:893913.
16. Tonks SA, Makwana S, Salanitro AH, et al. Quality of diabetes mellitus care by rural primary care physicians. J Rural Health. 2012;28:364-371.
17. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129:611-620.
18. Weber V, Bloom F, Pierdon S, Wood C. Employing the electronic health record to improve diabetes care: a multifaceted intervention in an integrated delivery system. J Gen Intern Med. 2008;23:379-382.
1. Centers for Disease Control and Prevention (CDC). National diabetes statistics report. Estimates of diabetes and its burden in the United States. Atlanta, GA: CDC; 2017www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 20, 2020.
2. American Diabetes Association (ADA). Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928.
3. Wood L. Trends in national and regional economic distress, 1960-2000. Washington, DC: Appalachian Regional Commission; 2005.
4. Barker L, Crespo R, Gerzoff RB, et al. Residence in a distressed county in Appalachia as a risk factor for diabetes, Behavioral Risk Factor Surveillance System, 2006-2007. Prev Chronic Dis. 2010;7:A104.
5. Barker L, Kirtland KA, Gregg E, et al. Geographic distribution of diagnosed diabetes in the United States: A diabetes belt. Am J Prev Med. 2011;40:434-439.
6. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176.
7. United States Renal Data System (USRDS). Annual data report. Ann Arbor, MI: USRDS; 2018. www.usrds.org/2018/view/Default.aspx. Accessed December 20, 2020.
8. Halverson JA, Bichak G. Underlying socioeconomic factors influencing health disparities in the Appalachian region. Washington, DC: Appalachian Regional Commission; 2008.
9. Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20:368-373.
10. Hatcher J, Dignan MB, Schoenberg N. How do rural health care providers and patients view barriers to colorectal cancer screening? Insights from Appalachian Kentucky. Nurs Clin North Am. 2011;46:181-192.
11. Scott S, McSpirit S. The suspicious, untrusting hillbilly in political-economic contexts: Stereotypes and social trust in the Appalachian coalfields. Pract Anthropol. 2014;36:42-46.
12. Kirkman MS, Williams SR, Caffrey HH, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care. 2002;25:1946-1951.
13. Byun SH, Ma SH, Jun JK, et al. Screening for diabetic retinopathy and nephropathy in patients with diabetes: A nationwide survey in Korea. PLoS One. 2013;8:e62991.
14. Flood D, Garcia P, Douglas K, et al. Screening for chronic kidney disease in a community-based diabetes cohort in rural Guatemala: A cross-sectional study. BMJ Open. 2018;8:e019778.
15. Anabtawi A, Mathew LM. Improving compliance with screening of diabetic patients for microalbuminuria in primary care practice. ISRN Endocrinology. 2013:893913.
16. Tonks SA, Makwana S, Salanitro AH, et al. Quality of diabetes mellitus care by rural primary care physicians. J Rural Health. 2012;28:364-371.
17. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129:611-620.
18. Weber V, Bloom F, Pierdon S, Wood C. Employing the electronic health record to improve diabetes care: a multifaceted intervention in an integrated delivery system. J Gen Intern Med. 2008;23:379-382.