User login
Journal of Hospital Medicine – Nov. 2017
BACKGROUND AND OBJECTIVES: Adherence to American Academy of Pediatrics (AAP) bronchiolitis clinical practice guideline recommendations improved significantly through the AAP’s multi-institutional collaborative the Bronchiolitis Quality Improvement Project (BQIP). We assessed sustainability of improvements at participating institutions for 1 year following completion of the collaborative.
METHODS: Twenty-one multidisciplinary hospital-based teams provided monthly data for key inpatient bronchiolitis measures during baseline and intervention bronchiolitis seasons. Nine sites provided data in the season following completion of the collaborative. Encounters included children younger than 24 months who were hospitalized for bronchiolitis without comorbid chronic illness, prematurity, or intensive care. Changes between baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with site-specific random effects. Differences between hospital characteristics, baseline performance, and initial improvement among sites that did and did not participate in the sustainability season were compared.
RESULTS: A total of 2,275 discharges were reviewed, comprising 995 baseline, 877 intervention, and 403 sustainability-season encounters. Improvements in all key bronchiolitis quality measures achieved during the intervention season were maintained during the sustainability season, and orders for intermittent pulse oximetry increased from 40.6% (95% confidence interval, 22.8-61.1) to 79.2% (95% CI, 58.0-91.3). Sites that did and did not participate in the sustainability season had similar characteristics.
DISCUSSION: BQIP participating sites maintained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. This approach, which provided an evidence-based best-practice toolkit while building the quality-improvement capacity of local interdisciplinary teams, may support performance gains that persist beyond the active phase of the collaborative.
Also in JHM this month
The effect of an inpatient smoking cessation treatment program on hospital readmissions and length of stayAUTHORS: Eline M. van den Broek-Altenburg, MS, MA, Adam J. Atherly, PhD
Treatment trends and outcomes in healthcare-associated pneumoniaAUTHORS: Sarah Haessler, MD; Tara Lagu, MD, MPH; Peter K. Lindenauer, MD, MSc; Daniel J. Skiest, MD; Aruna Priya, MA, MSc; Penelope S. Pekow, PhD; Marya D. Zilberberg, MD, MPH; Thomas L. Higgins, MD, MBA; Michael B. Rothberg, MD, MPH
What’s the purpose of rounds? A qualitative study examining the perceptions of faculty and studentsAUTHORS: Oliver Hulland; Jeanne Farnan, MD, MHPE; Raphael Rabinowitz; Lisa Kearns, MD, MS; Michele Long, MD; Bradley Monash, MD; Priti Bhansali, MD; H. Barrett Fromme, MD, MHPE
Association between anemia and fatigue in hospitalized patients: does the measure of anemia matter?AUTHORS: Micah T. Prochaska, MD, MS; Richard Newcomb, BA; Graham Block, BA; Brian Park, BA; David O. Meltzer MD, PhD
Helping seniors plan for posthospital discharge needs before a hospitalization occurs: Results from the randomized control trial of planyourlifespan.orgAUTHORS: Lee A. Lindquist, MD, MPH, MBA; Vanessa Ramirez-Zohfeld, MPH; Priya D. Sunkara, MA; Chris Forcucci, RN, BSN; Dianne S. Campbell, BS; Phyllis Mitzen, MA; Jody D. Ciolino, PhD; Gayle Kricke, MSW; Anne Seltzer, LSW; Ana V. Ramirez, BA; Kenzie A. Cameron, PhD, MPH
BACKGROUND AND OBJECTIVES: Adherence to American Academy of Pediatrics (AAP) bronchiolitis clinical practice guideline recommendations improved significantly through the AAP’s multi-institutional collaborative the Bronchiolitis Quality Improvement Project (BQIP). We assessed sustainability of improvements at participating institutions for 1 year following completion of the collaborative.
METHODS: Twenty-one multidisciplinary hospital-based teams provided monthly data for key inpatient bronchiolitis measures during baseline and intervention bronchiolitis seasons. Nine sites provided data in the season following completion of the collaborative. Encounters included children younger than 24 months who were hospitalized for bronchiolitis without comorbid chronic illness, prematurity, or intensive care. Changes between baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with site-specific random effects. Differences between hospital characteristics, baseline performance, and initial improvement among sites that did and did not participate in the sustainability season were compared.
RESULTS: A total of 2,275 discharges were reviewed, comprising 995 baseline, 877 intervention, and 403 sustainability-season encounters. Improvements in all key bronchiolitis quality measures achieved during the intervention season were maintained during the sustainability season, and orders for intermittent pulse oximetry increased from 40.6% (95% confidence interval, 22.8-61.1) to 79.2% (95% CI, 58.0-91.3). Sites that did and did not participate in the sustainability season had similar characteristics.
DISCUSSION: BQIP participating sites maintained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. This approach, which provided an evidence-based best-practice toolkit while building the quality-improvement capacity of local interdisciplinary teams, may support performance gains that persist beyond the active phase of the collaborative.
Also in JHM this month
The effect of an inpatient smoking cessation treatment program on hospital readmissions and length of stayAUTHORS: Eline M. van den Broek-Altenburg, MS, MA, Adam J. Atherly, PhD
Treatment trends and outcomes in healthcare-associated pneumoniaAUTHORS: Sarah Haessler, MD; Tara Lagu, MD, MPH; Peter K. Lindenauer, MD, MSc; Daniel J. Skiest, MD; Aruna Priya, MA, MSc; Penelope S. Pekow, PhD; Marya D. Zilberberg, MD, MPH; Thomas L. Higgins, MD, MBA; Michael B. Rothberg, MD, MPH
What’s the purpose of rounds? A qualitative study examining the perceptions of faculty and studentsAUTHORS: Oliver Hulland; Jeanne Farnan, MD, MHPE; Raphael Rabinowitz; Lisa Kearns, MD, MS; Michele Long, MD; Bradley Monash, MD; Priti Bhansali, MD; H. Barrett Fromme, MD, MHPE
Association between anemia and fatigue in hospitalized patients: does the measure of anemia matter?AUTHORS: Micah T. Prochaska, MD, MS; Richard Newcomb, BA; Graham Block, BA; Brian Park, BA; David O. Meltzer MD, PhD
Helping seniors plan for posthospital discharge needs before a hospitalization occurs: Results from the randomized control trial of planyourlifespan.orgAUTHORS: Lee A. Lindquist, MD, MPH, MBA; Vanessa Ramirez-Zohfeld, MPH; Priya D. Sunkara, MA; Chris Forcucci, RN, BSN; Dianne S. Campbell, BS; Phyllis Mitzen, MA; Jody D. Ciolino, PhD; Gayle Kricke, MSW; Anne Seltzer, LSW; Ana V. Ramirez, BA; Kenzie A. Cameron, PhD, MPH
BACKGROUND AND OBJECTIVES: Adherence to American Academy of Pediatrics (AAP) bronchiolitis clinical practice guideline recommendations improved significantly through the AAP’s multi-institutional collaborative the Bronchiolitis Quality Improvement Project (BQIP). We assessed sustainability of improvements at participating institutions for 1 year following completion of the collaborative.
METHODS: Twenty-one multidisciplinary hospital-based teams provided monthly data for key inpatient bronchiolitis measures during baseline and intervention bronchiolitis seasons. Nine sites provided data in the season following completion of the collaborative. Encounters included children younger than 24 months who were hospitalized for bronchiolitis without comorbid chronic illness, prematurity, or intensive care. Changes between baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with site-specific random effects. Differences between hospital characteristics, baseline performance, and initial improvement among sites that did and did not participate in the sustainability season were compared.
RESULTS: A total of 2,275 discharges were reviewed, comprising 995 baseline, 877 intervention, and 403 sustainability-season encounters. Improvements in all key bronchiolitis quality measures achieved during the intervention season were maintained during the sustainability season, and orders for intermittent pulse oximetry increased from 40.6% (95% confidence interval, 22.8-61.1) to 79.2% (95% CI, 58.0-91.3). Sites that did and did not participate in the sustainability season had similar characteristics.
DISCUSSION: BQIP participating sites maintained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. This approach, which provided an evidence-based best-practice toolkit while building the quality-improvement capacity of local interdisciplinary teams, may support performance gains that persist beyond the active phase of the collaborative.
Also in JHM this month
The effect of an inpatient smoking cessation treatment program on hospital readmissions and length of stayAUTHORS: Eline M. van den Broek-Altenburg, MS, MA, Adam J. Atherly, PhD
Treatment trends and outcomes in healthcare-associated pneumoniaAUTHORS: Sarah Haessler, MD; Tara Lagu, MD, MPH; Peter K. Lindenauer, MD, MSc; Daniel J. Skiest, MD; Aruna Priya, MA, MSc; Penelope S. Pekow, PhD; Marya D. Zilberberg, MD, MPH; Thomas L. Higgins, MD, MBA; Michael B. Rothberg, MD, MPH
What’s the purpose of rounds? A qualitative study examining the perceptions of faculty and studentsAUTHORS: Oliver Hulland; Jeanne Farnan, MD, MHPE; Raphael Rabinowitz; Lisa Kearns, MD, MS; Michele Long, MD; Bradley Monash, MD; Priti Bhansali, MD; H. Barrett Fromme, MD, MHPE
Association between anemia and fatigue in hospitalized patients: does the measure of anemia matter?AUTHORS: Micah T. Prochaska, MD, MS; Richard Newcomb, BA; Graham Block, BA; Brian Park, BA; David O. Meltzer MD, PhD
Helping seniors plan for posthospital discharge needs before a hospitalization occurs: Results from the randomized control trial of planyourlifespan.orgAUTHORS: Lee A. Lindquist, MD, MPH, MBA; Vanessa Ramirez-Zohfeld, MPH; Priya D. Sunkara, MA; Chris Forcucci, RN, BSN; Dianne S. Campbell, BS; Phyllis Mitzen, MA; Jody D. Ciolino, PhD; Gayle Kricke, MSW; Anne Seltzer, LSW; Ana V. Ramirez, BA; Kenzie A. Cameron, PhD, MPH
Sustainability in the AAP Bronchiolitis Quality Improvement Project
Acute viral bronchiolitis is the most common cause of hospitalization for children less than 1 year of age.1 Overuse of ineffective therapies has persisted despite the existence of the evidence-based American Academy of Pediatrics (AAP) clinical practice guideline (CPG), which recommends primarily supportive care.2-8 Adherence to the AAP CPG recommendations for management of bronchiolitis improved significantly through the AAP’s Bronchiolitis Quality Improvement Project (BQIP), a 12-month, multiinstitutional collaborative of community and free-standing children’s hospitals.9 This subsequent study investigates if these improvements were sustained after completion of the formal 12-month project.
Published multiinstitutional bronchiolitis quality improvement (QI) work is limited to 1 study5 that describes the results of a single intervention season at academic medical centers. Multiyear bronchiolitis QI projects are limited to single-center studies, and results have been mixed.5,6,8,10-13 One study11 observed continued improvement in bronchodilator use in subsequent seasons, whereas a second study10 observed a return to baseline bronchodilator use in the following season. Mittal6 observed inconsistent improvements in key bronchiolitis measures during postintervention seasons.
Our specific aim was to assess the sustainability of improvements in bronchiolitis management at participating institutions 1 year following completion of the AAP BQIP collaborative.9 Because no studies demonstrate the most effective way to support long-term improvement through a QI collaborative, we hypothesized that the initial collaborative activities, which were designed to build the capacity of local interdisciplinary teams while providing standardized evidence-based care pathways, would lead to performance in the subsequent season at levels similar to or better than those observed during the active phase of the collaborative, without additional project interventions.
METHODS
Study Design and Setting
This was a follow-up study of the AAP Quality Improvement Innovation Networks project entitled “A Quality Collaborative for Improving Hospital Compliance with the AAP Bronchiolitis Guideline” (BQIP).9 The AAP Institutional Review Board approved this project.
Twenty-one multidisciplinary, hospital-based teams participated in the BQIP collaborative and provided monthly data during the January through March bronchiolitis season. Teams submitted 2013 baseline data and 2014 intervention data. Nine sites provided 2015 sustainability data following the completion of the collaborative.
Participants
Hospital encounters with a primary diagnosis of acute viral bronchiolitis were eligible for inclusion among patients from 1 month to 2 years of age. Encounters were excluded for prematurity (<35 weeks gestational age), congenital heart disease, bronchopulmonary dysplasia, genetic, congenital or neuromuscular abnormalities, and pediatric intensive-care admission.
Data Collection
Hospital characteristics were collected, including hospital type (academic, community), bed size, location (urban, rural), hospital distributions of race/ethnicity and public payer, cases of bronchiolitis per year, presence of an electronic medical record and a pediatric respiratory therapist, and self-rated QI knowledge of the multidisciplinary team (very knowledgeable, knowledgeable, and somewhat knowledgeable). A trained member at each site collected data through structured chart review in baseline, intervention, and sustainability bronchiolitis seasons for January, February, and March. Site members reviewed the first 20 charts per month that met the inclusion criteria or all charts if there were fewer than 20 eligible encounters. Sites input data about key quality measures into the AAP’s Quality Improvement Data Aggregator, a web-based data repository.
Intervention
The BQIP project was designed as a virtual collaborative consisting of monthly education webinars about QI methods and bronchiolitis management, opportunities for collaboration via teleconference and e-mail listserv, and individual site-coaching by e-mail or telephone.9 A change package was shared with sites that included examples of evidence-based pathways, ordersets, a respiratory scoring tool, communication tools for parents and referring physicians, and slide sets for individual site education efforts. Following completion of the collaborative, written resources remained available to participants, although virtual collaboration ceased and no additional project interventions to promote sustainability were introduced.
Bronchiolitis Process and Outcome Measures
Process measures following admission included the following: severity assessment using a respiratory score, respiratory score use to assess response to bronchodilators, bronchodilator use, bronchodilator doses, steroid doses per patient encounter, chest radiographs per encounter, and presence of an order to transition to intermittent pulse oximetry monitoring. Outcome measures included length of stay and readmissions within 72 hours.
Analysis
Changes among baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with random effect for study sites. Negative binomial models were used for count variables to allow for overdispersion. Length of stay was log-transformed to achieve a normal distribution. We also analyzed each site individually to assess whether sustained improvements were the result of broad sustainability across all sites or whether they represented an aggregation of some sites that continued to improve while other sites actually worsened.
To address any bias introduced by the voluntary and incomplete participation of sites in the sustainability season, we planned a priori to conduct 3 additional analyses. First, we compared the characteristics of sites that did participate in the sustainability season with those that did not participate by using Chi-squared tests for differences in proportions and t tests for differences in means. Second, we determined whether the baseline-season process and outcome measures were different between sites that did and did not participate using descriptive statistics. Third, we assessed whether improvements between the baseline and intervention seasons were different between sites that did and did not participate using a linear mixed-effects model for normally distributed outcomes and generalized linear mixed-effects model with site-specific random effects for nonnormally distributed outcomes. All study outcomes were summarized in terms of model-adjusted means along with the corresponding 95% confidence intervals. All P values are 2-sided, and P < 0.05 was used to define statistical significance. Data analyses were conducted using SAS software (SAS Institute Inc., Cary, North Carolina) version 9.4.
RESULTS
Differences in baseline bronchiolitis quality measures between sites that did and did not participate in the sustainability season are displayed in Table 3. Sustainability sites had significantly lower baseline use of a respiratory score, both to assess severity of illness at any point after hospitalization as well as to assess responsiveness following bronchodilator treatments (P < 0.001). At baseline they also had fewer orders for intermittent pulse oximetry use (P = 0.01) and fewer doses of bronchodilators per encounter (P = 0.04). Sites were not significantly different in their baseline use of bronchodilators, oral steroid doses, or chest radiographs. Sites that participated in the sustainability season demonstated larger magnitude improvement between baseline and intervention seasons for respiratory score use (P < 0.001 for any use and P = 0.02 to assess bronchodilator responsiveness; Appendix 1b).
DISCUSSION
To our knowledge, this is the first report of sustained improvements in care achieved through a multiinstitutional QI collaborative of community and academic hospitals focused on bronchiolitis care. We found that overall sites participating in a national bronchiolitis QI project sustained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. For the aggregate group no measures worsened, and one measure, orders for intermittent pulse oximetry monitoring, continued to increase during the sustainability season. Furthermore, the sustained improvements were primarily the result of consistent sustained performance of each individual site, as opposed to averages wherein some sites worsened while others improved (Appendix 1a). These findings suggest that designing a collaborative approach, which provides an evidence-based best-practice toolkit while building the QI capacity of local interdisciplinary teams, can support performance gains that persist beyond the project’s active phase.
There are a number of possible reasons why improvements were sustained following the collaborative. The BQIP requirement for institutional leadership buy-in may have motivated accountability to local leaders in subsequent bronchiolitis seasons at each site. We suspect that culture change such as flattened hierarchies through multidisciplinary teams,14 which empowered nurse and respiratory therapy staff, may have facilitated consistent use of tools created locally. The synergy of interdisciplinary teams composed of physician, nurse, and respiratory therapy champions may have created accountability to perpetuate the previous year’s efforts.15 In addition, the sites adopted elements of the evidence-based toolkit, such as pathways,16,17 forcing function tools13,18 and order sets that limited management decision options and bronchodilator use contingent on respiratory scores,9,19 which may have driven desired behaviors.
Moreover, the 2014 AAP CPG for the management of bronchiolitis,20 released prior to the sustainability bronchiolitis season, may have underscored the key concepts of the collaborative. Similarly, national exposure of best practices for bronchiolitis management, including the 3 widespread Choosing Wisely recommendations related to bronchiolitis,21 might have been a compelling reason for sites to maintain their improvement efforts and contribute to secular trends toward decreasing interventions in bronchiolitis management nationally.3 Lastly, the mechanisms developed for local data collection may have created opportunities at each site to conduct ongoing evaluation of performance on key bronchiolitis quality measures through data-driven feedback systems.22 Our study highlights the need for additional research in order to understand why improvements are or are not sustained.
Even with substantial, sustained improvements in this initiative, further reduction in unnecessary care may be possible. Findings from previous studies suggest that even multifaceted QI interventions, including provider education, guidelines and use of respiratory scores, may only modestly reduce bronchodilators, steroids, and chest radiograph use.8,13 To achieve continued improvements in bronchiolitis care, additional active efforts may be needed to develop new interventions that target root causes for areas of overuse at individual sites.
Future multiinstitutional collaboratives might benefit their participants if they include a focus on helping sites develop skills to ensure that local improvement activities continue after the collaborative phases are completed. Proactively scheduling intermittent check-ins with collaborative members to discuss experiences with both sustainability and ongoing improvement may be valuable and likely needs to be incorporated into the initial collaborative planning.
As these sustainability data represent a subset of 9 of the original 21 BQIP sites, there is concern for potential selection bias related to factors that could have motivated sites to participate in the sustainability season’s data collection and simultaneously influenced their performance. These concerns were mitigated to some extent through 3 specific analyses: finding limited differences in hospital characteristics, baseline performance in key bronchiolitis measures, and performance change from baseline to intervention seasons between sites that did and did not participate in the sustainability season.
Notably, sites that participated in the sustainability phase actually had lower baseline respiratory score use and fewer orders for intermittent pulse oximetry at baseline. Theoretically, if participation in the collaborative highlighted this disparity for these sites, it could have been a motivating factor for their continued participation and sustained performance across these measures. Similarly, sites that recognized their higher baseline performance through participation in the collaborative might have felt less motivation to participate in ongoing data collection during the sustainability season. Whether they might have also sustained, declined, or continued improving is not known. Additionally, the magnitude of improvement in the collaborative period might have also motivated ongoing participation during the sustainability phase. For example, although all sites improved in score use during the collaborative, sites participating in the sustainability season demonstrated significantly more improvement in these measures. Sites with a higher magnitude of improvement in collaborative measures might have more enthusiasm about the project, more commitment to the project activities, or feel a sense of obligation to respond to requests for additional data collection.
This work has several limitations. Selection bias may limit generalizability of the results, as sites that did not participate in the sustainability season may have had different results than those that did participate. It is unknown whether sites that regressed toward their baseline were deterred from participating in the sustainability season. The analyses that we were able to preform, however, suggest that the 2 groups were similar in their characteristics as well as in their baseline and improvement performance.
We have limited knowledge of the local improvement work that sites conducted between the completion of the collaborative and the sustainability season. Site-specific factors may have influenced improvement sustainability. For example, qualitative research with the original group found that team engagement had a quantitative association with better performance, but only for the bronchodilator use measure.23 Sites were responsible for their own data collection, and despite attempts to centralize and standardize the process, data collection inconsistencies may have occurred. For instance, it is unknown how closely that orders for intermittent pulse oximetry correlate with intermittent use at the bedside. Lastly, the absence of a control group limits examination of the causal relationships of interventions and the influence of secular trends.
CONCLUSIONS
Improvements gained during the BQIP collaborative were sustained at 1 year following completion of the collaborative. These findings are encouraging, as national QI collaborative efforts are increasingly common. Our findings suggest that opportunities exist to even further reduce unnecessary care in the management of bronchiolitis. Such opportunities highlight the importance of integrating strategies to both measure sustainability and plan for ongoing independent local activities after completion of the collaborative. Future efforts should focus on supporting local sites to continue individual practice-improvement as they transition from collaborative to independent quality initiatives.
Acknowledgments
The authors thank the 21 hospitals that participated in the BQIP collaborative, and in particular the 9 hospital teams that contributed sustainability data for their ongoing dedication. There was no external funding for this manuscript.
Disclosure
The authors report no financial conflicts of interest.
1. Healthcare Cost and Utilization Project (HCUP) KID Trends Supplemental File. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=2C331B13FB40957D&Form=DispTab&JS=Y&Action=Accept. 2012. Accessed July 21, 2016.
2. Ralston S, Parikh K, Goodman D. Benchmarking overuse of medical interventions for bronchiolitis. JAMA Pediatr. 2015;169:805-806. PubMed
3. Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133:e1-e7. PubMed
4. Johnson LW, Robles J, Hudgins A, Osburn S, Martin D, Thompson A. Management of bronchiolitis in the emergency department: impact of evidence-based guidelines? Pediatrics. 2013;131 Suppl 1:S103-S109. PubMed
5. Kotagal UR, Robbins JM, Kini NM, Schoettker PJ, Atherton HD, Kirschbaum MS. Impact of a bronchiolitis guideline: a multisite demonstration project. Chest. 2002;121:1789-1797. PubMed
6. Mittal V, Darnell C, Walsh B, et al. Inpatient bronchiolitis guideline implementation and resource utilization. Pediatrics. 2014;133:e730-e737. PubMed
7. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165:570.e3-576.e3. PubMed
8. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25-30. PubMed
9. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137. PubMed
10. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154:1001-1007. PubMed
11. Walker C, Danby S, Turner S. Impact of a bronchiolitis clinical care pathway on treatment and hospital stay. Eur J Pediatr. 2012;171:827-832. PubMed
12. Cheney J, Barber S, Altamirano L, et al. A clinical pathway for bronchiolitis is effective in reducing readmission rates. J Pediatr. 2005;147:622-626. PubMed
13. Ralston S, Comick A, Nichols E, Parker D, Lanter P. Effectiveness of quality improvement in hospitalization for bronchiolitis: a systematic review. Pediatrics. 2014;134:571-581. PubMed
14. Schwartz RW, Tumblin TF. The power of servant leadership to transform health care organizations for the 21st-century economy. Arch Surg. 2002;137:1419-1427; discussion 27. PubMed
15. Schalock RL, Verdugo M, Lee T. A systematic approach to an organization’s sustainability. Eval Program Plann. 2016;56:56-63. PubMed
16. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17:195-199. PubMed
17. Muething S, Schoettker PJ, Gerhardt WE, Atherton HD, Britto MT, Kotagal UR. Decreasing overuse of therapies in the treatment of bronchiolitis by incorporating evidence at the point of care. J Pediatr. 2004;144:703-710. PubMed
18. Streiff MB, Carolan HT, Hobson DB, et al. Lessons from the Johns Hopkins multi-disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935. PubMed
19. Todd J, Bertoch D, Dolan S. Use of a large national database for comparative evaluation of the effect of a bronchiolitis/viral pneumonia clinical care guideline on patient outcome and resource utilization. Arch Pediatr Adolesc Med. 2002;156:1086-1090. PubMed
20. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502. PubMed
21. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8:479-485. PubMed
22. Stone S, Lee HC, Sharek PJ. Perceived factors associated with sustained improvement following participation in a multicenter quality improvement collaborative. Jt Comm J Qual Patient Saf. 2016;42:309-315. PubMed
23. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. PubMed
Acute viral bronchiolitis is the most common cause of hospitalization for children less than 1 year of age.1 Overuse of ineffective therapies has persisted despite the existence of the evidence-based American Academy of Pediatrics (AAP) clinical practice guideline (CPG), which recommends primarily supportive care.2-8 Adherence to the AAP CPG recommendations for management of bronchiolitis improved significantly through the AAP’s Bronchiolitis Quality Improvement Project (BQIP), a 12-month, multiinstitutional collaborative of community and free-standing children’s hospitals.9 This subsequent study investigates if these improvements were sustained after completion of the formal 12-month project.
Published multiinstitutional bronchiolitis quality improvement (QI) work is limited to 1 study5 that describes the results of a single intervention season at academic medical centers. Multiyear bronchiolitis QI projects are limited to single-center studies, and results have been mixed.5,6,8,10-13 One study11 observed continued improvement in bronchodilator use in subsequent seasons, whereas a second study10 observed a return to baseline bronchodilator use in the following season. Mittal6 observed inconsistent improvements in key bronchiolitis measures during postintervention seasons.
Our specific aim was to assess the sustainability of improvements in bronchiolitis management at participating institutions 1 year following completion of the AAP BQIP collaborative.9 Because no studies demonstrate the most effective way to support long-term improvement through a QI collaborative, we hypothesized that the initial collaborative activities, which were designed to build the capacity of local interdisciplinary teams while providing standardized evidence-based care pathways, would lead to performance in the subsequent season at levels similar to or better than those observed during the active phase of the collaborative, without additional project interventions.
METHODS
Study Design and Setting
This was a follow-up study of the AAP Quality Improvement Innovation Networks project entitled “A Quality Collaborative for Improving Hospital Compliance with the AAP Bronchiolitis Guideline” (BQIP).9 The AAP Institutional Review Board approved this project.
Twenty-one multidisciplinary, hospital-based teams participated in the BQIP collaborative and provided monthly data during the January through March bronchiolitis season. Teams submitted 2013 baseline data and 2014 intervention data. Nine sites provided 2015 sustainability data following the completion of the collaborative.
Participants
Hospital encounters with a primary diagnosis of acute viral bronchiolitis were eligible for inclusion among patients from 1 month to 2 years of age. Encounters were excluded for prematurity (<35 weeks gestational age), congenital heart disease, bronchopulmonary dysplasia, genetic, congenital or neuromuscular abnormalities, and pediatric intensive-care admission.
Data Collection
Hospital characteristics were collected, including hospital type (academic, community), bed size, location (urban, rural), hospital distributions of race/ethnicity and public payer, cases of bronchiolitis per year, presence of an electronic medical record and a pediatric respiratory therapist, and self-rated QI knowledge of the multidisciplinary team (very knowledgeable, knowledgeable, and somewhat knowledgeable). A trained member at each site collected data through structured chart review in baseline, intervention, and sustainability bronchiolitis seasons for January, February, and March. Site members reviewed the first 20 charts per month that met the inclusion criteria or all charts if there were fewer than 20 eligible encounters. Sites input data about key quality measures into the AAP’s Quality Improvement Data Aggregator, a web-based data repository.
Intervention
The BQIP project was designed as a virtual collaborative consisting of monthly education webinars about QI methods and bronchiolitis management, opportunities for collaboration via teleconference and e-mail listserv, and individual site-coaching by e-mail or telephone.9 A change package was shared with sites that included examples of evidence-based pathways, ordersets, a respiratory scoring tool, communication tools for parents and referring physicians, and slide sets for individual site education efforts. Following completion of the collaborative, written resources remained available to participants, although virtual collaboration ceased and no additional project interventions to promote sustainability were introduced.
Bronchiolitis Process and Outcome Measures
Process measures following admission included the following: severity assessment using a respiratory score, respiratory score use to assess response to bronchodilators, bronchodilator use, bronchodilator doses, steroid doses per patient encounter, chest radiographs per encounter, and presence of an order to transition to intermittent pulse oximetry monitoring. Outcome measures included length of stay and readmissions within 72 hours.
Analysis
Changes among baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with random effect for study sites. Negative binomial models were used for count variables to allow for overdispersion. Length of stay was log-transformed to achieve a normal distribution. We also analyzed each site individually to assess whether sustained improvements were the result of broad sustainability across all sites or whether they represented an aggregation of some sites that continued to improve while other sites actually worsened.
To address any bias introduced by the voluntary and incomplete participation of sites in the sustainability season, we planned a priori to conduct 3 additional analyses. First, we compared the characteristics of sites that did participate in the sustainability season with those that did not participate by using Chi-squared tests for differences in proportions and t tests for differences in means. Second, we determined whether the baseline-season process and outcome measures were different between sites that did and did not participate using descriptive statistics. Third, we assessed whether improvements between the baseline and intervention seasons were different between sites that did and did not participate using a linear mixed-effects model for normally distributed outcomes and generalized linear mixed-effects model with site-specific random effects for nonnormally distributed outcomes. All study outcomes were summarized in terms of model-adjusted means along with the corresponding 95% confidence intervals. All P values are 2-sided, and P < 0.05 was used to define statistical significance. Data analyses were conducted using SAS software (SAS Institute Inc., Cary, North Carolina) version 9.4.
RESULTS
Differences in baseline bronchiolitis quality measures between sites that did and did not participate in the sustainability season are displayed in Table 3. Sustainability sites had significantly lower baseline use of a respiratory score, both to assess severity of illness at any point after hospitalization as well as to assess responsiveness following bronchodilator treatments (P < 0.001). At baseline they also had fewer orders for intermittent pulse oximetry use (P = 0.01) and fewer doses of bronchodilators per encounter (P = 0.04). Sites were not significantly different in their baseline use of bronchodilators, oral steroid doses, or chest radiographs. Sites that participated in the sustainability season demonstated larger magnitude improvement between baseline and intervention seasons for respiratory score use (P < 0.001 for any use and P = 0.02 to assess bronchodilator responsiveness; Appendix 1b).
DISCUSSION
To our knowledge, this is the first report of sustained improvements in care achieved through a multiinstitutional QI collaborative of community and academic hospitals focused on bronchiolitis care. We found that overall sites participating in a national bronchiolitis QI project sustained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. For the aggregate group no measures worsened, and one measure, orders for intermittent pulse oximetry monitoring, continued to increase during the sustainability season. Furthermore, the sustained improvements were primarily the result of consistent sustained performance of each individual site, as opposed to averages wherein some sites worsened while others improved (Appendix 1a). These findings suggest that designing a collaborative approach, which provides an evidence-based best-practice toolkit while building the QI capacity of local interdisciplinary teams, can support performance gains that persist beyond the project’s active phase.
There are a number of possible reasons why improvements were sustained following the collaborative. The BQIP requirement for institutional leadership buy-in may have motivated accountability to local leaders in subsequent bronchiolitis seasons at each site. We suspect that culture change such as flattened hierarchies through multidisciplinary teams,14 which empowered nurse and respiratory therapy staff, may have facilitated consistent use of tools created locally. The synergy of interdisciplinary teams composed of physician, nurse, and respiratory therapy champions may have created accountability to perpetuate the previous year’s efforts.15 In addition, the sites adopted elements of the evidence-based toolkit, such as pathways,16,17 forcing function tools13,18 and order sets that limited management decision options and bronchodilator use contingent on respiratory scores,9,19 which may have driven desired behaviors.
Moreover, the 2014 AAP CPG for the management of bronchiolitis,20 released prior to the sustainability bronchiolitis season, may have underscored the key concepts of the collaborative. Similarly, national exposure of best practices for bronchiolitis management, including the 3 widespread Choosing Wisely recommendations related to bronchiolitis,21 might have been a compelling reason for sites to maintain their improvement efforts and contribute to secular trends toward decreasing interventions in bronchiolitis management nationally.3 Lastly, the mechanisms developed for local data collection may have created opportunities at each site to conduct ongoing evaluation of performance on key bronchiolitis quality measures through data-driven feedback systems.22 Our study highlights the need for additional research in order to understand why improvements are or are not sustained.
Even with substantial, sustained improvements in this initiative, further reduction in unnecessary care may be possible. Findings from previous studies suggest that even multifaceted QI interventions, including provider education, guidelines and use of respiratory scores, may only modestly reduce bronchodilators, steroids, and chest radiograph use.8,13 To achieve continued improvements in bronchiolitis care, additional active efforts may be needed to develop new interventions that target root causes for areas of overuse at individual sites.
Future multiinstitutional collaboratives might benefit their participants if they include a focus on helping sites develop skills to ensure that local improvement activities continue after the collaborative phases are completed. Proactively scheduling intermittent check-ins with collaborative members to discuss experiences with both sustainability and ongoing improvement may be valuable and likely needs to be incorporated into the initial collaborative planning.
As these sustainability data represent a subset of 9 of the original 21 BQIP sites, there is concern for potential selection bias related to factors that could have motivated sites to participate in the sustainability season’s data collection and simultaneously influenced their performance. These concerns were mitigated to some extent through 3 specific analyses: finding limited differences in hospital characteristics, baseline performance in key bronchiolitis measures, and performance change from baseline to intervention seasons between sites that did and did not participate in the sustainability season.
Notably, sites that participated in the sustainability phase actually had lower baseline respiratory score use and fewer orders for intermittent pulse oximetry at baseline. Theoretically, if participation in the collaborative highlighted this disparity for these sites, it could have been a motivating factor for their continued participation and sustained performance across these measures. Similarly, sites that recognized their higher baseline performance through participation in the collaborative might have felt less motivation to participate in ongoing data collection during the sustainability season. Whether they might have also sustained, declined, or continued improving is not known. Additionally, the magnitude of improvement in the collaborative period might have also motivated ongoing participation during the sustainability phase. For example, although all sites improved in score use during the collaborative, sites participating in the sustainability season demonstrated significantly more improvement in these measures. Sites with a higher magnitude of improvement in collaborative measures might have more enthusiasm about the project, more commitment to the project activities, or feel a sense of obligation to respond to requests for additional data collection.
This work has several limitations. Selection bias may limit generalizability of the results, as sites that did not participate in the sustainability season may have had different results than those that did participate. It is unknown whether sites that regressed toward their baseline were deterred from participating in the sustainability season. The analyses that we were able to preform, however, suggest that the 2 groups were similar in their characteristics as well as in their baseline and improvement performance.
We have limited knowledge of the local improvement work that sites conducted between the completion of the collaborative and the sustainability season. Site-specific factors may have influenced improvement sustainability. For example, qualitative research with the original group found that team engagement had a quantitative association with better performance, but only for the bronchodilator use measure.23 Sites were responsible for their own data collection, and despite attempts to centralize and standardize the process, data collection inconsistencies may have occurred. For instance, it is unknown how closely that orders for intermittent pulse oximetry correlate with intermittent use at the bedside. Lastly, the absence of a control group limits examination of the causal relationships of interventions and the influence of secular trends.
CONCLUSIONS
Improvements gained during the BQIP collaborative were sustained at 1 year following completion of the collaborative. These findings are encouraging, as national QI collaborative efforts are increasingly common. Our findings suggest that opportunities exist to even further reduce unnecessary care in the management of bronchiolitis. Such opportunities highlight the importance of integrating strategies to both measure sustainability and plan for ongoing independent local activities after completion of the collaborative. Future efforts should focus on supporting local sites to continue individual practice-improvement as they transition from collaborative to independent quality initiatives.
Acknowledgments
The authors thank the 21 hospitals that participated in the BQIP collaborative, and in particular the 9 hospital teams that contributed sustainability data for their ongoing dedication. There was no external funding for this manuscript.
Disclosure
The authors report no financial conflicts of interest.
Acute viral bronchiolitis is the most common cause of hospitalization for children less than 1 year of age.1 Overuse of ineffective therapies has persisted despite the existence of the evidence-based American Academy of Pediatrics (AAP) clinical practice guideline (CPG), which recommends primarily supportive care.2-8 Adherence to the AAP CPG recommendations for management of bronchiolitis improved significantly through the AAP’s Bronchiolitis Quality Improvement Project (BQIP), a 12-month, multiinstitutional collaborative of community and free-standing children’s hospitals.9 This subsequent study investigates if these improvements were sustained after completion of the formal 12-month project.
Published multiinstitutional bronchiolitis quality improvement (QI) work is limited to 1 study5 that describes the results of a single intervention season at academic medical centers. Multiyear bronchiolitis QI projects are limited to single-center studies, and results have been mixed.5,6,8,10-13 One study11 observed continued improvement in bronchodilator use in subsequent seasons, whereas a second study10 observed a return to baseline bronchodilator use in the following season. Mittal6 observed inconsistent improvements in key bronchiolitis measures during postintervention seasons.
Our specific aim was to assess the sustainability of improvements in bronchiolitis management at participating institutions 1 year following completion of the AAP BQIP collaborative.9 Because no studies demonstrate the most effective way to support long-term improvement through a QI collaborative, we hypothesized that the initial collaborative activities, which were designed to build the capacity of local interdisciplinary teams while providing standardized evidence-based care pathways, would lead to performance in the subsequent season at levels similar to or better than those observed during the active phase of the collaborative, without additional project interventions.
METHODS
Study Design and Setting
This was a follow-up study of the AAP Quality Improvement Innovation Networks project entitled “A Quality Collaborative for Improving Hospital Compliance with the AAP Bronchiolitis Guideline” (BQIP).9 The AAP Institutional Review Board approved this project.
Twenty-one multidisciplinary, hospital-based teams participated in the BQIP collaborative and provided monthly data during the January through March bronchiolitis season. Teams submitted 2013 baseline data and 2014 intervention data. Nine sites provided 2015 sustainability data following the completion of the collaborative.
Participants
Hospital encounters with a primary diagnosis of acute viral bronchiolitis were eligible for inclusion among patients from 1 month to 2 years of age. Encounters were excluded for prematurity (<35 weeks gestational age), congenital heart disease, bronchopulmonary dysplasia, genetic, congenital or neuromuscular abnormalities, and pediatric intensive-care admission.
Data Collection
Hospital characteristics were collected, including hospital type (academic, community), bed size, location (urban, rural), hospital distributions of race/ethnicity and public payer, cases of bronchiolitis per year, presence of an electronic medical record and a pediatric respiratory therapist, and self-rated QI knowledge of the multidisciplinary team (very knowledgeable, knowledgeable, and somewhat knowledgeable). A trained member at each site collected data through structured chart review in baseline, intervention, and sustainability bronchiolitis seasons for January, February, and March. Site members reviewed the first 20 charts per month that met the inclusion criteria or all charts if there were fewer than 20 eligible encounters. Sites input data about key quality measures into the AAP’s Quality Improvement Data Aggregator, a web-based data repository.
Intervention
The BQIP project was designed as a virtual collaborative consisting of monthly education webinars about QI methods and bronchiolitis management, opportunities for collaboration via teleconference and e-mail listserv, and individual site-coaching by e-mail or telephone.9 A change package was shared with sites that included examples of evidence-based pathways, ordersets, a respiratory scoring tool, communication tools for parents and referring physicians, and slide sets for individual site education efforts. Following completion of the collaborative, written resources remained available to participants, although virtual collaboration ceased and no additional project interventions to promote sustainability were introduced.
Bronchiolitis Process and Outcome Measures
Process measures following admission included the following: severity assessment using a respiratory score, respiratory score use to assess response to bronchodilators, bronchodilator use, bronchodilator doses, steroid doses per patient encounter, chest radiographs per encounter, and presence of an order to transition to intermittent pulse oximetry monitoring. Outcome measures included length of stay and readmissions within 72 hours.
Analysis
Changes among baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with random effect for study sites. Negative binomial models were used for count variables to allow for overdispersion. Length of stay was log-transformed to achieve a normal distribution. We also analyzed each site individually to assess whether sustained improvements were the result of broad sustainability across all sites or whether they represented an aggregation of some sites that continued to improve while other sites actually worsened.
To address any bias introduced by the voluntary and incomplete participation of sites in the sustainability season, we planned a priori to conduct 3 additional analyses. First, we compared the characteristics of sites that did participate in the sustainability season with those that did not participate by using Chi-squared tests for differences in proportions and t tests for differences in means. Second, we determined whether the baseline-season process and outcome measures were different between sites that did and did not participate using descriptive statistics. Third, we assessed whether improvements between the baseline and intervention seasons were different between sites that did and did not participate using a linear mixed-effects model for normally distributed outcomes and generalized linear mixed-effects model with site-specific random effects for nonnormally distributed outcomes. All study outcomes were summarized in terms of model-adjusted means along with the corresponding 95% confidence intervals. All P values are 2-sided, and P < 0.05 was used to define statistical significance. Data analyses were conducted using SAS software (SAS Institute Inc., Cary, North Carolina) version 9.4.
RESULTS
Differences in baseline bronchiolitis quality measures between sites that did and did not participate in the sustainability season are displayed in Table 3. Sustainability sites had significantly lower baseline use of a respiratory score, both to assess severity of illness at any point after hospitalization as well as to assess responsiveness following bronchodilator treatments (P < 0.001). At baseline they also had fewer orders for intermittent pulse oximetry use (P = 0.01) and fewer doses of bronchodilators per encounter (P = 0.04). Sites were not significantly different in their baseline use of bronchodilators, oral steroid doses, or chest radiographs. Sites that participated in the sustainability season demonstated larger magnitude improvement between baseline and intervention seasons for respiratory score use (P < 0.001 for any use and P = 0.02 to assess bronchodilator responsiveness; Appendix 1b).
DISCUSSION
To our knowledge, this is the first report of sustained improvements in care achieved through a multiinstitutional QI collaborative of community and academic hospitals focused on bronchiolitis care. We found that overall sites participating in a national bronchiolitis QI project sustained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. For the aggregate group no measures worsened, and one measure, orders for intermittent pulse oximetry monitoring, continued to increase during the sustainability season. Furthermore, the sustained improvements were primarily the result of consistent sustained performance of each individual site, as opposed to averages wherein some sites worsened while others improved (Appendix 1a). These findings suggest that designing a collaborative approach, which provides an evidence-based best-practice toolkit while building the QI capacity of local interdisciplinary teams, can support performance gains that persist beyond the project’s active phase.
There are a number of possible reasons why improvements were sustained following the collaborative. The BQIP requirement for institutional leadership buy-in may have motivated accountability to local leaders in subsequent bronchiolitis seasons at each site. We suspect that culture change such as flattened hierarchies through multidisciplinary teams,14 which empowered nurse and respiratory therapy staff, may have facilitated consistent use of tools created locally. The synergy of interdisciplinary teams composed of physician, nurse, and respiratory therapy champions may have created accountability to perpetuate the previous year’s efforts.15 In addition, the sites adopted elements of the evidence-based toolkit, such as pathways,16,17 forcing function tools13,18 and order sets that limited management decision options and bronchodilator use contingent on respiratory scores,9,19 which may have driven desired behaviors.
Moreover, the 2014 AAP CPG for the management of bronchiolitis,20 released prior to the sustainability bronchiolitis season, may have underscored the key concepts of the collaborative. Similarly, national exposure of best practices for bronchiolitis management, including the 3 widespread Choosing Wisely recommendations related to bronchiolitis,21 might have been a compelling reason for sites to maintain their improvement efforts and contribute to secular trends toward decreasing interventions in bronchiolitis management nationally.3 Lastly, the mechanisms developed for local data collection may have created opportunities at each site to conduct ongoing evaluation of performance on key bronchiolitis quality measures through data-driven feedback systems.22 Our study highlights the need for additional research in order to understand why improvements are or are not sustained.
Even with substantial, sustained improvements in this initiative, further reduction in unnecessary care may be possible. Findings from previous studies suggest that even multifaceted QI interventions, including provider education, guidelines and use of respiratory scores, may only modestly reduce bronchodilators, steroids, and chest radiograph use.8,13 To achieve continued improvements in bronchiolitis care, additional active efforts may be needed to develop new interventions that target root causes for areas of overuse at individual sites.
Future multiinstitutional collaboratives might benefit their participants if they include a focus on helping sites develop skills to ensure that local improvement activities continue after the collaborative phases are completed. Proactively scheduling intermittent check-ins with collaborative members to discuss experiences with both sustainability and ongoing improvement may be valuable and likely needs to be incorporated into the initial collaborative planning.
As these sustainability data represent a subset of 9 of the original 21 BQIP sites, there is concern for potential selection bias related to factors that could have motivated sites to participate in the sustainability season’s data collection and simultaneously influenced their performance. These concerns were mitigated to some extent through 3 specific analyses: finding limited differences in hospital characteristics, baseline performance in key bronchiolitis measures, and performance change from baseline to intervention seasons between sites that did and did not participate in the sustainability season.
Notably, sites that participated in the sustainability phase actually had lower baseline respiratory score use and fewer orders for intermittent pulse oximetry at baseline. Theoretically, if participation in the collaborative highlighted this disparity for these sites, it could have been a motivating factor for their continued participation and sustained performance across these measures. Similarly, sites that recognized their higher baseline performance through participation in the collaborative might have felt less motivation to participate in ongoing data collection during the sustainability season. Whether they might have also sustained, declined, or continued improving is not known. Additionally, the magnitude of improvement in the collaborative period might have also motivated ongoing participation during the sustainability phase. For example, although all sites improved in score use during the collaborative, sites participating in the sustainability season demonstrated significantly more improvement in these measures. Sites with a higher magnitude of improvement in collaborative measures might have more enthusiasm about the project, more commitment to the project activities, or feel a sense of obligation to respond to requests for additional data collection.
This work has several limitations. Selection bias may limit generalizability of the results, as sites that did not participate in the sustainability season may have had different results than those that did participate. It is unknown whether sites that regressed toward their baseline were deterred from participating in the sustainability season. The analyses that we were able to preform, however, suggest that the 2 groups were similar in their characteristics as well as in their baseline and improvement performance.
We have limited knowledge of the local improvement work that sites conducted between the completion of the collaborative and the sustainability season. Site-specific factors may have influenced improvement sustainability. For example, qualitative research with the original group found that team engagement had a quantitative association with better performance, but only for the bronchodilator use measure.23 Sites were responsible for their own data collection, and despite attempts to centralize and standardize the process, data collection inconsistencies may have occurred. For instance, it is unknown how closely that orders for intermittent pulse oximetry correlate with intermittent use at the bedside. Lastly, the absence of a control group limits examination of the causal relationships of interventions and the influence of secular trends.
CONCLUSIONS
Improvements gained during the BQIP collaborative were sustained at 1 year following completion of the collaborative. These findings are encouraging, as national QI collaborative efforts are increasingly common. Our findings suggest that opportunities exist to even further reduce unnecessary care in the management of bronchiolitis. Such opportunities highlight the importance of integrating strategies to both measure sustainability and plan for ongoing independent local activities after completion of the collaborative. Future efforts should focus on supporting local sites to continue individual practice-improvement as they transition from collaborative to independent quality initiatives.
Acknowledgments
The authors thank the 21 hospitals that participated in the BQIP collaborative, and in particular the 9 hospital teams that contributed sustainability data for their ongoing dedication. There was no external funding for this manuscript.
Disclosure
The authors report no financial conflicts of interest.
1. Healthcare Cost and Utilization Project (HCUP) KID Trends Supplemental File. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=2C331B13FB40957D&Form=DispTab&JS=Y&Action=Accept. 2012. Accessed July 21, 2016.
2. Ralston S, Parikh K, Goodman D. Benchmarking overuse of medical interventions for bronchiolitis. JAMA Pediatr. 2015;169:805-806. PubMed
3. Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133:e1-e7. PubMed
4. Johnson LW, Robles J, Hudgins A, Osburn S, Martin D, Thompson A. Management of bronchiolitis in the emergency department: impact of evidence-based guidelines? Pediatrics. 2013;131 Suppl 1:S103-S109. PubMed
5. Kotagal UR, Robbins JM, Kini NM, Schoettker PJ, Atherton HD, Kirschbaum MS. Impact of a bronchiolitis guideline: a multisite demonstration project. Chest. 2002;121:1789-1797. PubMed
6. Mittal V, Darnell C, Walsh B, et al. Inpatient bronchiolitis guideline implementation and resource utilization. Pediatrics. 2014;133:e730-e737. PubMed
7. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165:570.e3-576.e3. PubMed
8. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25-30. PubMed
9. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137. PubMed
10. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154:1001-1007. PubMed
11. Walker C, Danby S, Turner S. Impact of a bronchiolitis clinical care pathway on treatment and hospital stay. Eur J Pediatr. 2012;171:827-832. PubMed
12. Cheney J, Barber S, Altamirano L, et al. A clinical pathway for bronchiolitis is effective in reducing readmission rates. J Pediatr. 2005;147:622-626. PubMed
13. Ralston S, Comick A, Nichols E, Parker D, Lanter P. Effectiveness of quality improvement in hospitalization for bronchiolitis: a systematic review. Pediatrics. 2014;134:571-581. PubMed
14. Schwartz RW, Tumblin TF. The power of servant leadership to transform health care organizations for the 21st-century economy. Arch Surg. 2002;137:1419-1427; discussion 27. PubMed
15. Schalock RL, Verdugo M, Lee T. A systematic approach to an organization’s sustainability. Eval Program Plann. 2016;56:56-63. PubMed
16. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17:195-199. PubMed
17. Muething S, Schoettker PJ, Gerhardt WE, Atherton HD, Britto MT, Kotagal UR. Decreasing overuse of therapies in the treatment of bronchiolitis by incorporating evidence at the point of care. J Pediatr. 2004;144:703-710. PubMed
18. Streiff MB, Carolan HT, Hobson DB, et al. Lessons from the Johns Hopkins multi-disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935. PubMed
19. Todd J, Bertoch D, Dolan S. Use of a large national database for comparative evaluation of the effect of a bronchiolitis/viral pneumonia clinical care guideline on patient outcome and resource utilization. Arch Pediatr Adolesc Med. 2002;156:1086-1090. PubMed
20. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502. PubMed
21. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8:479-485. PubMed
22. Stone S, Lee HC, Sharek PJ. Perceived factors associated with sustained improvement following participation in a multicenter quality improvement collaborative. Jt Comm J Qual Patient Saf. 2016;42:309-315. PubMed
23. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. PubMed
1. Healthcare Cost and Utilization Project (HCUP) KID Trends Supplemental File. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=2C331B13FB40957D&Form=DispTab&JS=Y&Action=Accept. 2012. Accessed July 21, 2016.
2. Ralston S, Parikh K, Goodman D. Benchmarking overuse of medical interventions for bronchiolitis. JAMA Pediatr. 2015;169:805-806. PubMed
3. Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133:e1-e7. PubMed
4. Johnson LW, Robles J, Hudgins A, Osburn S, Martin D, Thompson A. Management of bronchiolitis in the emergency department: impact of evidence-based guidelines? Pediatrics. 2013;131 Suppl 1:S103-S109. PubMed
5. Kotagal UR, Robbins JM, Kini NM, Schoettker PJ, Atherton HD, Kirschbaum MS. Impact of a bronchiolitis guideline: a multisite demonstration project. Chest. 2002;121:1789-1797. PubMed
6. Mittal V, Darnell C, Walsh B, et al. Inpatient bronchiolitis guideline implementation and resource utilization. Pediatrics. 2014;133:e730-e737. PubMed
7. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165:570.e3-576.e3. PubMed
8. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25-30. PubMed
9. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137. PubMed
10. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154:1001-1007. PubMed
11. Walker C, Danby S, Turner S. Impact of a bronchiolitis clinical care pathway on treatment and hospital stay. Eur J Pediatr. 2012;171:827-832. PubMed
12. Cheney J, Barber S, Altamirano L, et al. A clinical pathway for bronchiolitis is effective in reducing readmission rates. J Pediatr. 2005;147:622-626. PubMed
13. Ralston S, Comick A, Nichols E, Parker D, Lanter P. Effectiveness of quality improvement in hospitalization for bronchiolitis: a systematic review. Pediatrics. 2014;134:571-581. PubMed
14. Schwartz RW, Tumblin TF. The power of servant leadership to transform health care organizations for the 21st-century economy. Arch Surg. 2002;137:1419-1427; discussion 27. PubMed
15. Schalock RL, Verdugo M, Lee T. A systematic approach to an organization’s sustainability. Eval Program Plann. 2016;56:56-63. PubMed
16. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17:195-199. PubMed
17. Muething S, Schoettker PJ, Gerhardt WE, Atherton HD, Britto MT, Kotagal UR. Decreasing overuse of therapies in the treatment of bronchiolitis by incorporating evidence at the point of care. J Pediatr. 2004;144:703-710. PubMed
18. Streiff MB, Carolan HT, Hobson DB, et al. Lessons from the Johns Hopkins multi-disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935. PubMed
19. Todd J, Bertoch D, Dolan S. Use of a large national database for comparative evaluation of the effect of a bronchiolitis/viral pneumonia clinical care guideline on patient outcome and resource utilization. Arch Pediatr Adolesc Med. 2002;156:1086-1090. PubMed
20. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502. PubMed
21. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8:479-485. PubMed
22. Stone S, Lee HC, Sharek PJ. Perceived factors associated with sustained improvement following participation in a multicenter quality improvement collaborative. Jt Comm J Qual Patient Saf. 2016;42:309-315. PubMed
23. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. PubMed
© 2017 Society of Hospital Medicine
Choosing Wisely in Pediatric Medicine
Overuse in medicine is a significant and under‐recognized problem. Don Berwick estimated that waste accounts for at least 20% of healthcare expenditures in the United States, with overtreatment as one of the largest categories.[1] A commentary by Schroeder et al. challenged pediatricians to incorporate this knowledge into our own patient safety and quality movement.[2] Recently published data suggest that we are far from achieving the patient safety goals set forth in the Institute of Medicine's landmark To Err is Human[3] report, despite more than a decade of national, local, and regional efforts.[4] One way to reduce waste and improve patient safety is to eliminate practices of unproven benefit. Therapies or tests that may initially seem promising are often proven to be not only unhelpful but actually harmful. The recommendation of the US Preventive Services Task Force against routine screening for prostate specific antigen is an example of how a common test initially thought of as lifesaving actually increases harm.[5]
The American Board of Internal Medicine Foundation (ABIM‐F) recently announced the Choosing Wisely campaign. Through this campaign the Foundation encourages physicians, patients and other healthcare stakeholders to think and talk about medical tests and procedures that may be unnecessary.[6] The primary output of this challenge is the development of a list of 5 tests and or therapies that physicians and patients should question. The ABIM‐F approached different medical societies to develop these lists within their own specialties. The Society of Hospital Medicine (SHM) joined the Choosing Wisely campaign in April 2012, and agreed to develop a list of 5 therapies and tests for adult hospital medicine and pediatric hospital medicine. Here we present the contribution of the pediatric workgroup detailing the methodology and process for developing the list, as well as summarizing the evidence supporting each recommendation.
METHODS
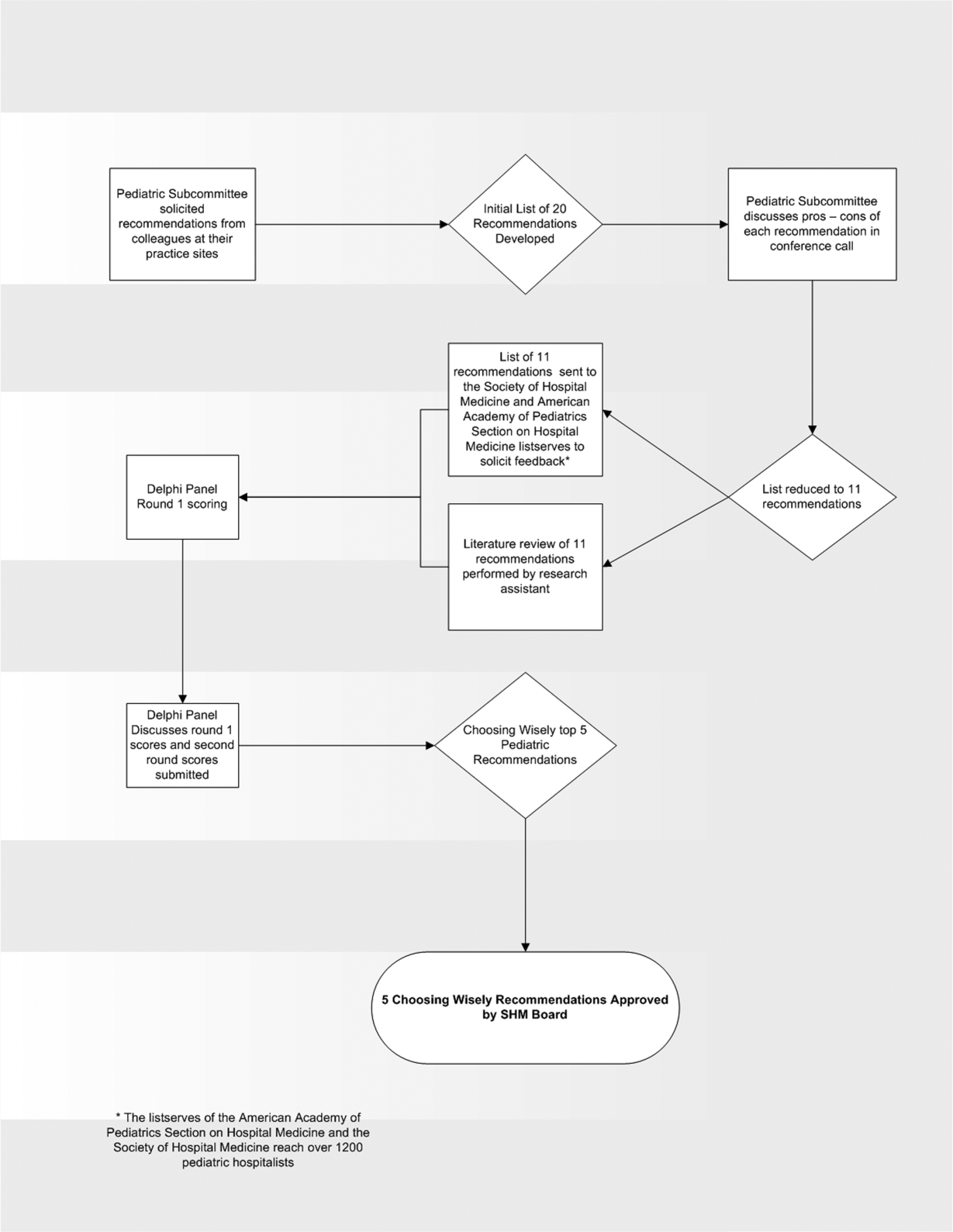
In the spring of 2012, the pediatric committee of the SHM convened a workgroup of pediatric hospitalists to develop a top 5 list for the field. This workgroup was composed of experienced pediatric hospitalists representing diverse geographic locations of the United States and a mix of academic and nonacademic practice settings. The group, consisting of 4 women and 9 men, began by proposing candidate recommendations after discussion with colleagues at their different practice sites. The group was charged to maintain a focus on overuse practices that had a strong basis in evidence, were frequently encountered at their practice sites, and achieved significant consensus among their colleagues. Figure 1 shows the process map describing the method for the development of the pediatric recommendations. All workgroup participants were queried as to conflict of interest relevant to this work and none were identified.
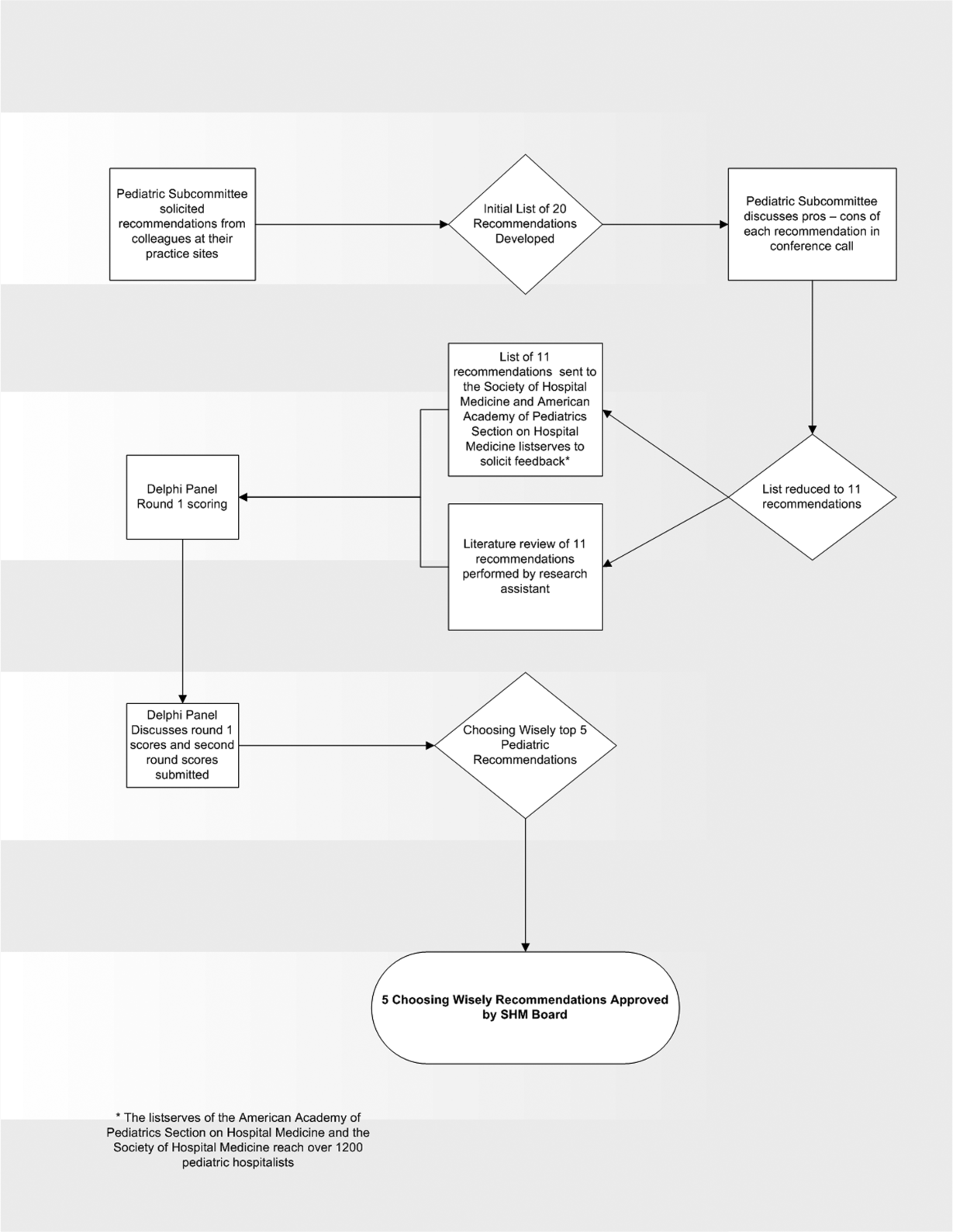
Literature Review
After the generation of the initial top 20 list, 2 reviewers conducted independent literature searches in PubMed, MEDLINE, and the Cochrane Library on the proposed topics. The reviewers also conducted generic Internet searches. Key search terms included pediatric asthma, bronchiolitis, chest radiograph, systemic corticosteroids, gastroesophageal reflux disease (GERD), infant, child, acid suppression therapy, continuous pulse oximetry, pneumonia, gastroenteritis, viral testing, blood culture, and soft tissue infections. To ensure that the reviewers included all studies relevant to the searches, they utilized broad terms. The search included all literature published through 2012, and nonEnglish language publications were included in the search. Studies selected and included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, included a pediatric population in the guidelines or study, reviewed the harm associated with the administration of a particular test or treatment, and explored the cost associated with the test or treatment.
The Delphi Panel
Members of the workgroup formed a Delphi panel except for 1 member (R.Q.) who served as the nonvoting moderator. The members of the Delphi panel considered the results of the literature search for each recommendation along with the collated feedback from hospitalist listserves as described in Figure 1. Each panel member received a voting instrument with the candidate tests and treatments for the first round of Delphi voting. The panel utilized a modified Delphi method or the RAND Corporation (RAND)/University of California at Los Angeles (UCLA) appropriateness method as described in previous publications of quality indicator development in pediatrics.[7] Each panelist scored the candidate tests and treatments and forwarded the scores to the moderator. Subsequently, all the members of the Delphi panel met through a conference call to carry out the second round of voting. The deidentified collated results of the first round of Delphi voting were made available and discussed during the call. The moderator collated the final results, and the final 5 recommendations were those that had the highest score after the second round of Delphi voting.
Volume and Costs
During deliberations, the committee took into account the prevalence and cost rankings of our most common pediatric inpatient diagnoses. This was done using the Agency for Healthcare Research and Quality's (AHRQ) Healthcare Utilization Project (HCUP), specifically, the Kids' Inpatient Database (KID). HCUP includes the largest collection of longitudinal hospital care data in the United States, encompassing all‐payer discharge‐level information. We excluded normal newborn hospitalizations, and looked at the top 10 acute inpatient diagnoses in terms of both volume and aggregate costs.
RESULTS
The initial list of 20 candidate tests and treatments as well as the refined list of 11 recommendations can be found as electronic supplements to this publication (see Supporting Table 1 and Supporting Table 2 in the online version of this article). The format and language of the list of 11 recommendations were chosen to mesh with that typically used in the ABIM‐F Choosing Wisely campaign. During the Delphi panel, there was strong group consensus about combining items 1 and 2 (chest radiographs in asthma and bronchiolitis) into a single recommendation.
| Do not order chest radiographs in children with asthma or bronchiolitis. |
| Do not use bronchodilators in children with bronchiolitis. |
| Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection. |
| Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
The top 5 recommendations based on the result of the second round of Delphi scoring are shown in Table 1 and described below along with a detailed evidence summary.
Do not order chest radiographs in children with asthma or bronchiolitis.
The National Heart and Lung Institute's guidelines for the management of asthma, published in 1987, recommend against routinely obtaining chest radiographs in patients with asthma or asthma exacerbations.[8] Supporting this recommendation are several studies that show a low overall yield when obtaining chest radiographs for wheezing patients.[9, 10, 11] Most relevant, studies that evaluated the clinical utility of radiographs in patients with asthma have demonstrated that they influence clinical management in less than 2% of cases.[12] A quality improvement project aimed at decreasing the rate of chest radiographs obtained in patients with asthma demonstrated that close to 60% of patients admitted to the hospital had chest radiographs performed, and that significant overall reductions can be achieved (45.3%28.9%, P=0.0005) without impacting clinical outcomes negatively.[13]
Similarly, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely obtaining radiographs during the evaluation for bronchiolitis.[14] Studies assessing the utility of chest x‐rays in these children demonstrate an even lower incidence of abnormalities (0.75%) and indicate that, despite this low incidence, physicians are more likely to treat with antibiotics when radiographs are obtained.[15] There is also evidence that chest radiographs in patients with bronchiolitis are not useful in predicting severity of illness.[16] Furthermore, cost‐effective analyses have demonstrated that omitting chest radiographs in bronchiolitis is actually cost‐effective, without compromising diagnostic accuracy.[17] In a recently published national benchmarking inpatient collaborative, Ralston et al. demonstrated that the majority of patients admitted to the hospital with bronchiolitis have chest radiographs performed at a rate of 64% (interquartile range [IQR], 54%81%).[18]
In both bronchiolitis and asthma, the elimination of unnecessary radiographs has the potential to decrease costs, reduce radiation exposure, and minimize the overuse of antibiotics that often occurs secondary to false positive results.
Do not use bronchodilators in children with bronchiolitis.
Ralston showed that 70% (IQR, 59%83%) of admitted bronchiolitis patients received bronchodilators with an average of 7.9 doses per patient (IQR, 4.69.8). National guidelines for bronchiolitis suggest a very limited role of bronchodilators in patients with bronchiolitis.[14] The first meta‐analyses of studies related to the question of ‐agonist efficacy in bronchiolitis were published in the late 1990s, revealing minimal or no treatment effects.[19, 20] Since then, further research has solidified these findings, and fairly definitive statements can be made based on a recent comprehensive meta‐analysis.[21] The pooled data do not show any effect on hospitalization rates, hospital length of stay, or other inpatient outcomes in bronchiolitis. They do show a small change in clinical scores documented in the outpatient setting, though these scores have not correlated with any detectable difference in outcomes. Routine use of ‐agonists in the inpatient setting has no proven benefit, and given the large amount of consistent data, there is no compelling reason for further study of this therapy in the inpatient setting.
Epinephrine, a combined ‐ and ‐agonist, has been extensively evaluated in bronchiolitis as well. Like albuterol, epinephrine has been reported to have no effect on hospital length of stay in bronchiolitis.[22] The issue of admission rates after epinephrine is complicated by 1 very large study that combined epinephrine with dexamethasone and reported a decreased admission rate, though only at 7 days after therapy; however, this effect was nullified after adjustment for multiple comparisons.[23] When the end point is improvement of respiratory scores, epinephrine may perform better than albuterol in studies where they are directly compared; however, there is no evidence that repeated usage of epinephrine has any impact on any clinical outcome for inpatients.[24, 25]
Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection
In their summary of evidence, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely using systemic corticosteroids for infants with bronchiolitis.[14] The previously reference bronchiolitis benchmarking study demonstrated that admitted patients received steroids at a rate of 21% (IQR, 14%26%). The poor efficacy of corticosteroids in children with bronchiolitis under 2 years of age is well demonstrated in the literature. A large, blinded, randomized, controlled study compared systemic oral corticosteroids to placebo in hospitalized children 10 months to 6 years of age with viral wheezing.[26] This study showed no benefit of corticosteroids over placebo in length of stay or parental report of symptoms 1 week later. In the study, a subanalysis of children with eczema and family history of asthma also demonstrated no benefit of systemic corticosteroids. Large systematic reviews further argue that there is no effect of corticosteroids on the likelihood of admission or length of stay in infants with bronchiolitis.[27, 28] One 4‐armed prospective study of children 6 weeks to 12 months of age found no efficacy of dexamethasone over placebo.[23] There was modest benefit of dexamethasone in conjunction with racemic epinephrine; however, this benefit disappeared after adjustment for multiple comparisons. Three smaller studies showing benefit of systemic corticosteroids, however, were highly problematic. They have included older children, were retrospective, or demonstrated inconsistent results.[29, 30] A smaller study showed benefit for children over 2 years of age, but none for children under 2 years of age.[31] Premature infants are at increased risk of asthma, which typically responds well to corticosteroids as these children get older. However, a retrospective study of premature infants under 2 years of age with bronchiolitis demonstrated no association between corticosteroid use and length of stay, even in the subset of premature infants responding to albuterol.[32]
Systemic corticosteroid use in children is not harmless. Children under 2 years of age are especially vulnerable to the decreased growth velocity seen as a side effect of systemic corticosteroids.[33] Corticosteroids may also negatively impact the course of infectious illness. For instance, in children hospitalized with pneumonia but not receiving ‐agonists (ie, patients who are unlikely to have asthma), length of stay is prolonged and readmission is higher in those who receive corticosteroids.[34]
Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
From 2000 to 2005, the incidence of infants diagnosed with gastroeshopaheal reflux (GER) tripled (3.4%12.3%), and the use of proton pump inhibitors (PPIs) doubled (31.5%62.6%).[35] Patients diagnosed with GER and treated with antireflux medication incurred 1.8 times higher healthcare costs in 1 study compared to healthy controls.[36] Though common, the use of acid suppressive medications in infants lacks evidence for efficacy in the majority of the clinical scenarios in which they are prescribed.[37, 38] PPIs have failed to outperform placebo for typical infant reflux, which is generally developmental and not pathologic.[39, 40] Furthermore, prompted by findings in adults, multiple pediatric investigators have now catalogued the potential risks associated with acid blockade in children in multiple clinical settings. Specifically, increased risk of pneumonia has been documented in inpatients and outpatients, and increased risk of necrotizing enterocolitis and other serious infections have been documented in intensive care unit settings.[41] In the absence of data supporting efficacy and given the emerging data on risk, empiric acid suppression in infants with reflux is wasteful and potentially harmful.
Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
Pulse oximetry use has become widespread in the management of infants with bronchiolitis and likely accounts for the dramatic increase in bronchiolitis hospitalization rates in recent years.[14, 42, 43, 44, 45, 46, 47] Despite this increase in hospitalization rate, there was no change in mortality from bronchiolitis between 1979 and 1997.[48] The continuous monitoring of oxygen saturations in hospitalized infants with bronchiolitis may lead to overdiagnosis of hypoxemia and subsequent oxygen use that is of no apparent benefit to the child. Schroeder et al. demonstrated that 26% of a sample of infants hospitalized with bronchiolitis had a prolonged length of stay because of a perceived need for oxygen based on pulse oximetry readings.[43] Unger and Cunningham showed that the need for oxygen was the final determinant of length of stay in 58% of cases, and Cunningham and Murray suggested that using an oxygen saturation cutoff of 94% instead of 90% might increase the length of stay by 22 hours.[44, 49]
It has been previously shown that hypoxia is normative in infants. Healthy infants experience multiple episodes of SpO2 90% while sleeping.[50] This finding strengthens the notion that detection of low saturations in infants convalescing from bronchiolitis may simply reflect overdiagnosis. Among children with chronic severe asthma, who presumably have experienced episodes of hypoxia throughout childhood, there is no difference in school performance compared to healthy controls.[51]
The practice parameter on bronchiolitis from the American Academy of Pediatrics states: as the child's clinical course improves, continuous measurement of SpO2 is not routinely needed, which is a recommendation based on expert consensus.[14] There is at least one ongoing randomized trial comparing the use of continuous versus intermittent pulse oximetry in hospitalized infants with bronchiolitis who are weaned off oxygen (
DISCUSSION
Berwick and Hackbarth define overtreatment as: waste that comes from subjecting patients to care that, according to sound science and the patients' own preferences, cannot possibly help themcare rooted in outmoded habits, supply‐driven behaviors, and ignoring science.[1] With this project, we tried to capture common clinical sources of waste in the inpatient pediatric setting. This is an inherently difficult project because of the absence of solid evidence to inform every decision point in medicine. Although there is always room for improvement in our evidence base, our group intentionally gravitated to areas where the evidence was robust.
The primary strength of this work is the use of the RAND/UCLA appropriateness method or modified Delphi method. Several publications have validated this methodology as a sound strategy to assess quality indicators and issues related to overuse.[7, 53] To our knowledge, we are the first group to report the use of this methodology to develop a list such as the list reported here.
There were some challenges inherent to this project that can be considered limitations of the work. One perceived limitation of our list is the heavy concentration on respiratory diagnoses, especially bronchiolitis and asthma. We do not feel this is a genuine limitation, as the recommendations were partly driven by volume and costs as assessed by the KID database. Among the top 10 acute inpatient diagnoses in pediatrics, respiratory diagnoses are the most common, including bronchiolitis, pneumonia, and asthma. Pneumonia or bronchiolitis has been the most common medical diagnosis in inpatient pediatrics for the past decade, and both are always in the top 10 for costs as well.[54] Thus, the impact of decreasing overuse for these conditions will be highly significant from a simple volume standpoint.
The primary limitation of this work is the lack of implementation strategies. Although the Choosing Wisely campaign has plans for dissemination of the lists, compliance with the recommendations may be suboptimal. Although the development process followed an accepted methodology, shortcomings include the lack of wide, local, multidisciplinary (including parents or caretakers) consultation. Other barriers to compliance with these recommendations exist. Despite evidence that bronchiolitis is a benign self‐limited disease that does not respond to bronchodilators and steroids, the drive to identify and correct all abnormalities, such as wheezing or low oxygen saturation in a nontoxic infant with bronchiolitis, seems to trump the obligation to do no harm in daily practice.[55] This behavior may result from pressure by patients, families, nurses, or peers and is deeply embedded in our medical culture, where action is preferred to inaction without full knowledge or consideration of risks. Doctors and nurses have become attached to the pulse oximeter, believing somehow that the number displayed is less subjective and holds more predictive value than careful evaluation of the patient's respiratory status. Other pressures, such as direct to consumer marketing have made acid reflux a household term that is easily treated with over‐the‐counter medications. Considerations of the care continuum will also serve as barriers. Chest x‐rays, for example, are frequently obtained prior to admission to the hospital before the hospitalist is involved.
To overcome these limitations, the study of individual and organizational adoption of innovation might be relevant. Though it is complex and often more descriptive than proscriptive, a few salient features have emerged. Champions and opinion leaders make a difference, local culture is dominant, social networking is important, simple innovations that can be trialed on a small scale are adaptable by the user and have observable benefits, are more likely to be adopted.[56] Fortunately, the top 5 list meets many of these criteria, but also faces the daunting challenges of inertia, lack of financial incentive, inability to break with old habits, and fear of lawsuits and perceived patient/parent dissatisfaction. Ongoing evaluation, feedback, and audit will be necessary to detect and sustain change.
CONCLUSION
We have identified 5 tests or therapies overused in inpatient general pediatrics. One goal of the Choosing Wisely campaign is to begin to change social norms related to physician behavior. We hope by asking clinicians to consider doing less for common conditions in inpatient pediatrics, that they will increasingly consider the known and unanticipated risks of any medical interventions they choose to use. Finally, we would like to encourage all pediatricians to embrace the idea of good stewardship and join us in prioritizing and addressing waste and overuse as important patient safety issues as well as threats to the sustainability of our healthcare system.
Acknowledgments
The authors thank Drs. Doug Carlson, James O'Callaghan, and Karen Smith from the Society of Hospital Medicine's Pediatric and Quality and Safety Committees for their support of this effort.
Disclosure: Nothing to report.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- , , . Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128:e1596–e1597.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363:2124–2134.
- . Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- , , , et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357:1515–1523.
- National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138.
- , . The chest x‐ray and childhood acute asthma. Aust Clin Rev. 1993;13:153–156.
- , , , . Clinical factors associated with focal infiltrates in wheezing infants and toddlers. Clin Pediatr (Phila). 2000;39:387–393.
- , , , . Chest radiographs in the pediatric emergency department for children < or = 18 months of age with wheezing. Clin Pediatr (Phila). 1999;38:395–399.
- , , , , , . Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124:e29–e36.
- , . Reduce the rads: a quality assurance project on reducing unnecessary chest X‐rays in children with asthma. J Paediatr Child Health. 2005;41:107–111.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- , , , et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150:429–433.
- , , , et al. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100:e17–e23.
- , , , et al. A cost effectiveness analysis of omitting radiography in diagnosis of acute bronchiolitis. Pediatr Pulmonol. 2009;44:122–127.
- , , , et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25–30.
- , , , . Efficacy of bronchodilator therapy in bronchiolitis. A meta‐analysis. Arch Pediatr Adolesc Med. 1996;150:1166–1172.
- , . Efficacy of beta2‐agonists in bronchiolitis: a reappraisal and meta‐analysis. Pediatrics. 1997;100:233–239.
- , . Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2010;(12):CD001266.
- , , , et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011;(6):CD003123.
- , , , et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079–2089.
- , , , et al. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27–35.
- , , , . A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–824.
- , , , et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. N Engl J Med. 2009;360:329–338.
- , , , et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2010;(10):CD004878.
- , , , , . Systemic corticosteroids in infant bronchiolitis: a meta‐analysis. Pediatrics. 2000;105:E44.
- , , , . Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518.
- , , . Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356.
- , , , , . Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet. 1987;1:879–882.
- , , , , . The clinical management of preterm infants with bronchiolitis. Hosp Pediatr. 2013;3:244–250.
- , . Glucocorticoids and growth in asthmatic children. Pediatr Allergy Immunol. 1995;6:145–154.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127:e255–e263.
- , , , , , . Pediatric gastroesophageal reflux disease and acid‐related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12:348–355.
- , , , , , . Healthcare costs of GERD and acid‐related conditions in pediatric patients, with comparison between histamine‐2 receptor antagonists and proton pump inhibitors. Curr Med Res Opin. 2009;25:2703–2709.
- , , , . Are we overprescribing antireflux medications for infants with regurgitation? Pediatrics. 2007;120:946–949.
- , , , , . Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45:421–427.
- , , , , , . Efficacy of proton‐pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127:925–935.
- . Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease. Ann Pharmacother. 2010;44:572–576.
- . Are there risks associated with empric acid suppression treatment of infants and children suspected of having gastroesophageal reflux disease? Hosp Pediatr. 2013;3:16–23.
- , , , . Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics. 2003;111:e45–e51.
- , , , . Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158:527–530.
- , . Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121:470–475.
- . Oxygen therapy for bronchiolitis. Pediatrics. 2007;120:686–687; author reply 687–688.
- , , , , , . Bronchiolitis‐associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446.
- , . Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–349.
- , , , , . Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16–22.
- , . Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97:361–363.
- , , , et al. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatr. 2011;159:377–383.e1.
- , . The impact of severe asthma on schoolchildren. J Asthma. 1999;36:409–417.
- , . Multi‐center, randomized trial of pulse oximetry monitoring strategies for children hospitalized for bronchiolitis. Abstract presented at: ID Week 2012; October 2012; San Diego, CA.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- Agency for Healthcare Research and Quality. HCUPnet. Kids inpatient database 2009. Available at: http://hcupnet.ahrq.gov. Accessed November 6, 2012.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- . How to implement change in clinical practice. Paediatr Respir Rev. 2003;4:340–346.
Overuse in medicine is a significant and under‐recognized problem. Don Berwick estimated that waste accounts for at least 20% of healthcare expenditures in the United States, with overtreatment as one of the largest categories.[1] A commentary by Schroeder et al. challenged pediatricians to incorporate this knowledge into our own patient safety and quality movement.[2] Recently published data suggest that we are far from achieving the patient safety goals set forth in the Institute of Medicine's landmark To Err is Human[3] report, despite more than a decade of national, local, and regional efforts.[4] One way to reduce waste and improve patient safety is to eliminate practices of unproven benefit. Therapies or tests that may initially seem promising are often proven to be not only unhelpful but actually harmful. The recommendation of the US Preventive Services Task Force against routine screening for prostate specific antigen is an example of how a common test initially thought of as lifesaving actually increases harm.[5]
The American Board of Internal Medicine Foundation (ABIM‐F) recently announced the Choosing Wisely campaign. Through this campaign the Foundation encourages physicians, patients and other healthcare stakeholders to think and talk about medical tests and procedures that may be unnecessary.[6] The primary output of this challenge is the development of a list of 5 tests and or therapies that physicians and patients should question. The ABIM‐F approached different medical societies to develop these lists within their own specialties. The Society of Hospital Medicine (SHM) joined the Choosing Wisely campaign in April 2012, and agreed to develop a list of 5 therapies and tests for adult hospital medicine and pediatric hospital medicine. Here we present the contribution of the pediatric workgroup detailing the methodology and process for developing the list, as well as summarizing the evidence supporting each recommendation.
METHODS
In the spring of 2012, the pediatric committee of the SHM convened a workgroup of pediatric hospitalists to develop a top 5 list for the field. This workgroup was composed of experienced pediatric hospitalists representing diverse geographic locations of the United States and a mix of academic and nonacademic practice settings. The group, consisting of 4 women and 9 men, began by proposing candidate recommendations after discussion with colleagues at their different practice sites. The group was charged to maintain a focus on overuse practices that had a strong basis in evidence, were frequently encountered at their practice sites, and achieved significant consensus among their colleagues. Figure 1 shows the process map describing the method for the development of the pediatric recommendations. All workgroup participants were queried as to conflict of interest relevant to this work and none were identified.

Literature Review
After the generation of the initial top 20 list, 2 reviewers conducted independent literature searches in PubMed, MEDLINE, and the Cochrane Library on the proposed topics. The reviewers also conducted generic Internet searches. Key search terms included pediatric asthma, bronchiolitis, chest radiograph, systemic corticosteroids, gastroesophageal reflux disease (GERD), infant, child, acid suppression therapy, continuous pulse oximetry, pneumonia, gastroenteritis, viral testing, blood culture, and soft tissue infections. To ensure that the reviewers included all studies relevant to the searches, they utilized broad terms. The search included all literature published through 2012, and nonEnglish language publications were included in the search. Studies selected and included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, included a pediatric population in the guidelines or study, reviewed the harm associated with the administration of a particular test or treatment, and explored the cost associated with the test or treatment.
The Delphi Panel
Members of the workgroup formed a Delphi panel except for 1 member (R.Q.) who served as the nonvoting moderator. The members of the Delphi panel considered the results of the literature search for each recommendation along with the collated feedback from hospitalist listserves as described in Figure 1. Each panel member received a voting instrument with the candidate tests and treatments for the first round of Delphi voting. The panel utilized a modified Delphi method or the RAND Corporation (RAND)/University of California at Los Angeles (UCLA) appropriateness method as described in previous publications of quality indicator development in pediatrics.[7] Each panelist scored the candidate tests and treatments and forwarded the scores to the moderator. Subsequently, all the members of the Delphi panel met through a conference call to carry out the second round of voting. The deidentified collated results of the first round of Delphi voting were made available and discussed during the call. The moderator collated the final results, and the final 5 recommendations were those that had the highest score after the second round of Delphi voting.
Volume and Costs
During deliberations, the committee took into account the prevalence and cost rankings of our most common pediatric inpatient diagnoses. This was done using the Agency for Healthcare Research and Quality's (AHRQ) Healthcare Utilization Project (HCUP), specifically, the Kids' Inpatient Database (KID). HCUP includes the largest collection of longitudinal hospital care data in the United States, encompassing all‐payer discharge‐level information. We excluded normal newborn hospitalizations, and looked at the top 10 acute inpatient diagnoses in terms of both volume and aggregate costs.
RESULTS
The initial list of 20 candidate tests and treatments as well as the refined list of 11 recommendations can be found as electronic supplements to this publication (see Supporting Table 1 and Supporting Table 2 in the online version of this article). The format and language of the list of 11 recommendations were chosen to mesh with that typically used in the ABIM‐F Choosing Wisely campaign. During the Delphi panel, there was strong group consensus about combining items 1 and 2 (chest radiographs in asthma and bronchiolitis) into a single recommendation.
| Do not order chest radiographs in children with asthma or bronchiolitis. |
| Do not use bronchodilators in children with bronchiolitis. |
| Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection. |
| Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
The top 5 recommendations based on the result of the second round of Delphi scoring are shown in Table 1 and described below along with a detailed evidence summary.
Do not order chest radiographs in children with asthma or bronchiolitis.
The National Heart and Lung Institute's guidelines for the management of asthma, published in 1987, recommend against routinely obtaining chest radiographs in patients with asthma or asthma exacerbations.[8] Supporting this recommendation are several studies that show a low overall yield when obtaining chest radiographs for wheezing patients.[9, 10, 11] Most relevant, studies that evaluated the clinical utility of radiographs in patients with asthma have demonstrated that they influence clinical management in less than 2% of cases.[12] A quality improvement project aimed at decreasing the rate of chest radiographs obtained in patients with asthma demonstrated that close to 60% of patients admitted to the hospital had chest radiographs performed, and that significant overall reductions can be achieved (45.3%28.9%, P=0.0005) without impacting clinical outcomes negatively.[13]
Similarly, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely obtaining radiographs during the evaluation for bronchiolitis.[14] Studies assessing the utility of chest x‐rays in these children demonstrate an even lower incidence of abnormalities (0.75%) and indicate that, despite this low incidence, physicians are more likely to treat with antibiotics when radiographs are obtained.[15] There is also evidence that chest radiographs in patients with bronchiolitis are not useful in predicting severity of illness.[16] Furthermore, cost‐effective analyses have demonstrated that omitting chest radiographs in bronchiolitis is actually cost‐effective, without compromising diagnostic accuracy.[17] In a recently published national benchmarking inpatient collaborative, Ralston et al. demonstrated that the majority of patients admitted to the hospital with bronchiolitis have chest radiographs performed at a rate of 64% (interquartile range [IQR], 54%81%).[18]
In both bronchiolitis and asthma, the elimination of unnecessary radiographs has the potential to decrease costs, reduce radiation exposure, and minimize the overuse of antibiotics that often occurs secondary to false positive results.
Do not use bronchodilators in children with bronchiolitis.
Ralston showed that 70% (IQR, 59%83%) of admitted bronchiolitis patients received bronchodilators with an average of 7.9 doses per patient (IQR, 4.69.8). National guidelines for bronchiolitis suggest a very limited role of bronchodilators in patients with bronchiolitis.[14] The first meta‐analyses of studies related to the question of ‐agonist efficacy in bronchiolitis were published in the late 1990s, revealing minimal or no treatment effects.[19, 20] Since then, further research has solidified these findings, and fairly definitive statements can be made based on a recent comprehensive meta‐analysis.[21] The pooled data do not show any effect on hospitalization rates, hospital length of stay, or other inpatient outcomes in bronchiolitis. They do show a small change in clinical scores documented in the outpatient setting, though these scores have not correlated with any detectable difference in outcomes. Routine use of ‐agonists in the inpatient setting has no proven benefit, and given the large amount of consistent data, there is no compelling reason for further study of this therapy in the inpatient setting.
Epinephrine, a combined ‐ and ‐agonist, has been extensively evaluated in bronchiolitis as well. Like albuterol, epinephrine has been reported to have no effect on hospital length of stay in bronchiolitis.[22] The issue of admission rates after epinephrine is complicated by 1 very large study that combined epinephrine with dexamethasone and reported a decreased admission rate, though only at 7 days after therapy; however, this effect was nullified after adjustment for multiple comparisons.[23] When the end point is improvement of respiratory scores, epinephrine may perform better than albuterol in studies where they are directly compared; however, there is no evidence that repeated usage of epinephrine has any impact on any clinical outcome for inpatients.[24, 25]
Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection
In their summary of evidence, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely using systemic corticosteroids for infants with bronchiolitis.[14] The previously reference bronchiolitis benchmarking study demonstrated that admitted patients received steroids at a rate of 21% (IQR, 14%26%). The poor efficacy of corticosteroids in children with bronchiolitis under 2 years of age is well demonstrated in the literature. A large, blinded, randomized, controlled study compared systemic oral corticosteroids to placebo in hospitalized children 10 months to 6 years of age with viral wheezing.[26] This study showed no benefit of corticosteroids over placebo in length of stay or parental report of symptoms 1 week later. In the study, a subanalysis of children with eczema and family history of asthma also demonstrated no benefit of systemic corticosteroids. Large systematic reviews further argue that there is no effect of corticosteroids on the likelihood of admission or length of stay in infants with bronchiolitis.[27, 28] One 4‐armed prospective study of children 6 weeks to 12 months of age found no efficacy of dexamethasone over placebo.[23] There was modest benefit of dexamethasone in conjunction with racemic epinephrine; however, this benefit disappeared after adjustment for multiple comparisons. Three smaller studies showing benefit of systemic corticosteroids, however, were highly problematic. They have included older children, were retrospective, or demonstrated inconsistent results.[29, 30] A smaller study showed benefit for children over 2 years of age, but none for children under 2 years of age.[31] Premature infants are at increased risk of asthma, which typically responds well to corticosteroids as these children get older. However, a retrospective study of premature infants under 2 years of age with bronchiolitis demonstrated no association between corticosteroid use and length of stay, even in the subset of premature infants responding to albuterol.[32]
Systemic corticosteroid use in children is not harmless. Children under 2 years of age are especially vulnerable to the decreased growth velocity seen as a side effect of systemic corticosteroids.[33] Corticosteroids may also negatively impact the course of infectious illness. For instance, in children hospitalized with pneumonia but not receiving ‐agonists (ie, patients who are unlikely to have asthma), length of stay is prolonged and readmission is higher in those who receive corticosteroids.[34]
Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
From 2000 to 2005, the incidence of infants diagnosed with gastroeshopaheal reflux (GER) tripled (3.4%12.3%), and the use of proton pump inhibitors (PPIs) doubled (31.5%62.6%).[35] Patients diagnosed with GER and treated with antireflux medication incurred 1.8 times higher healthcare costs in 1 study compared to healthy controls.[36] Though common, the use of acid suppressive medications in infants lacks evidence for efficacy in the majority of the clinical scenarios in which they are prescribed.[37, 38] PPIs have failed to outperform placebo for typical infant reflux, which is generally developmental and not pathologic.[39, 40] Furthermore, prompted by findings in adults, multiple pediatric investigators have now catalogued the potential risks associated with acid blockade in children in multiple clinical settings. Specifically, increased risk of pneumonia has been documented in inpatients and outpatients, and increased risk of necrotizing enterocolitis and other serious infections have been documented in intensive care unit settings.[41] In the absence of data supporting efficacy and given the emerging data on risk, empiric acid suppression in infants with reflux is wasteful and potentially harmful.
Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
Pulse oximetry use has become widespread in the management of infants with bronchiolitis and likely accounts for the dramatic increase in bronchiolitis hospitalization rates in recent years.[14, 42, 43, 44, 45, 46, 47] Despite this increase in hospitalization rate, there was no change in mortality from bronchiolitis between 1979 and 1997.[48] The continuous monitoring of oxygen saturations in hospitalized infants with bronchiolitis may lead to overdiagnosis of hypoxemia and subsequent oxygen use that is of no apparent benefit to the child. Schroeder et al. demonstrated that 26% of a sample of infants hospitalized with bronchiolitis had a prolonged length of stay because of a perceived need for oxygen based on pulse oximetry readings.[43] Unger and Cunningham showed that the need for oxygen was the final determinant of length of stay in 58% of cases, and Cunningham and Murray suggested that using an oxygen saturation cutoff of 94% instead of 90% might increase the length of stay by 22 hours.[44, 49]
It has been previously shown that hypoxia is normative in infants. Healthy infants experience multiple episodes of SpO2 90% while sleeping.[50] This finding strengthens the notion that detection of low saturations in infants convalescing from bronchiolitis may simply reflect overdiagnosis. Among children with chronic severe asthma, who presumably have experienced episodes of hypoxia throughout childhood, there is no difference in school performance compared to healthy controls.[51]
The practice parameter on bronchiolitis from the American Academy of Pediatrics states: as the child's clinical course improves, continuous measurement of SpO2 is not routinely needed, which is a recommendation based on expert consensus.[14] There is at least one ongoing randomized trial comparing the use of continuous versus intermittent pulse oximetry in hospitalized infants with bronchiolitis who are weaned off oxygen (
DISCUSSION
Berwick and Hackbarth define overtreatment as: waste that comes from subjecting patients to care that, according to sound science and the patients' own preferences, cannot possibly help themcare rooted in outmoded habits, supply‐driven behaviors, and ignoring science.[1] With this project, we tried to capture common clinical sources of waste in the inpatient pediatric setting. This is an inherently difficult project because of the absence of solid evidence to inform every decision point in medicine. Although there is always room for improvement in our evidence base, our group intentionally gravitated to areas where the evidence was robust.
The primary strength of this work is the use of the RAND/UCLA appropriateness method or modified Delphi method. Several publications have validated this methodology as a sound strategy to assess quality indicators and issues related to overuse.[7, 53] To our knowledge, we are the first group to report the use of this methodology to develop a list such as the list reported here.
There were some challenges inherent to this project that can be considered limitations of the work. One perceived limitation of our list is the heavy concentration on respiratory diagnoses, especially bronchiolitis and asthma. We do not feel this is a genuine limitation, as the recommendations were partly driven by volume and costs as assessed by the KID database. Among the top 10 acute inpatient diagnoses in pediatrics, respiratory diagnoses are the most common, including bronchiolitis, pneumonia, and asthma. Pneumonia or bronchiolitis has been the most common medical diagnosis in inpatient pediatrics for the past decade, and both are always in the top 10 for costs as well.[54] Thus, the impact of decreasing overuse for these conditions will be highly significant from a simple volume standpoint.
The primary limitation of this work is the lack of implementation strategies. Although the Choosing Wisely campaign has plans for dissemination of the lists, compliance with the recommendations may be suboptimal. Although the development process followed an accepted methodology, shortcomings include the lack of wide, local, multidisciplinary (including parents or caretakers) consultation. Other barriers to compliance with these recommendations exist. Despite evidence that bronchiolitis is a benign self‐limited disease that does not respond to bronchodilators and steroids, the drive to identify and correct all abnormalities, such as wheezing or low oxygen saturation in a nontoxic infant with bronchiolitis, seems to trump the obligation to do no harm in daily practice.[55] This behavior may result from pressure by patients, families, nurses, or peers and is deeply embedded in our medical culture, where action is preferred to inaction without full knowledge or consideration of risks. Doctors and nurses have become attached to the pulse oximeter, believing somehow that the number displayed is less subjective and holds more predictive value than careful evaluation of the patient's respiratory status. Other pressures, such as direct to consumer marketing have made acid reflux a household term that is easily treated with over‐the‐counter medications. Considerations of the care continuum will also serve as barriers. Chest x‐rays, for example, are frequently obtained prior to admission to the hospital before the hospitalist is involved.
To overcome these limitations, the study of individual and organizational adoption of innovation might be relevant. Though it is complex and often more descriptive than proscriptive, a few salient features have emerged. Champions and opinion leaders make a difference, local culture is dominant, social networking is important, simple innovations that can be trialed on a small scale are adaptable by the user and have observable benefits, are more likely to be adopted.[56] Fortunately, the top 5 list meets many of these criteria, but also faces the daunting challenges of inertia, lack of financial incentive, inability to break with old habits, and fear of lawsuits and perceived patient/parent dissatisfaction. Ongoing evaluation, feedback, and audit will be necessary to detect and sustain change.
CONCLUSION
We have identified 5 tests or therapies overused in inpatient general pediatrics. One goal of the Choosing Wisely campaign is to begin to change social norms related to physician behavior. We hope by asking clinicians to consider doing less for common conditions in inpatient pediatrics, that they will increasingly consider the known and unanticipated risks of any medical interventions they choose to use. Finally, we would like to encourage all pediatricians to embrace the idea of good stewardship and join us in prioritizing and addressing waste and overuse as important patient safety issues as well as threats to the sustainability of our healthcare system.
Acknowledgments
The authors thank Drs. Doug Carlson, James O'Callaghan, and Karen Smith from the Society of Hospital Medicine's Pediatric and Quality and Safety Committees for their support of this effort.
Disclosure: Nothing to report.
Overuse in medicine is a significant and under‐recognized problem. Don Berwick estimated that waste accounts for at least 20% of healthcare expenditures in the United States, with overtreatment as one of the largest categories.[1] A commentary by Schroeder et al. challenged pediatricians to incorporate this knowledge into our own patient safety and quality movement.[2] Recently published data suggest that we are far from achieving the patient safety goals set forth in the Institute of Medicine's landmark To Err is Human[3] report, despite more than a decade of national, local, and regional efforts.[4] One way to reduce waste and improve patient safety is to eliminate practices of unproven benefit. Therapies or tests that may initially seem promising are often proven to be not only unhelpful but actually harmful. The recommendation of the US Preventive Services Task Force against routine screening for prostate specific antigen is an example of how a common test initially thought of as lifesaving actually increases harm.[5]
The American Board of Internal Medicine Foundation (ABIM‐F) recently announced the Choosing Wisely campaign. Through this campaign the Foundation encourages physicians, patients and other healthcare stakeholders to think and talk about medical tests and procedures that may be unnecessary.[6] The primary output of this challenge is the development of a list of 5 tests and or therapies that physicians and patients should question. The ABIM‐F approached different medical societies to develop these lists within their own specialties. The Society of Hospital Medicine (SHM) joined the Choosing Wisely campaign in April 2012, and agreed to develop a list of 5 therapies and tests for adult hospital medicine and pediatric hospital medicine. Here we present the contribution of the pediatric workgroup detailing the methodology and process for developing the list, as well as summarizing the evidence supporting each recommendation.
METHODS
In the spring of 2012, the pediatric committee of the SHM convened a workgroup of pediatric hospitalists to develop a top 5 list for the field. This workgroup was composed of experienced pediatric hospitalists representing diverse geographic locations of the United States and a mix of academic and nonacademic practice settings. The group, consisting of 4 women and 9 men, began by proposing candidate recommendations after discussion with colleagues at their different practice sites. The group was charged to maintain a focus on overuse practices that had a strong basis in evidence, were frequently encountered at their practice sites, and achieved significant consensus among their colleagues. Figure 1 shows the process map describing the method for the development of the pediatric recommendations. All workgroup participants were queried as to conflict of interest relevant to this work and none were identified.

Literature Review
After the generation of the initial top 20 list, 2 reviewers conducted independent literature searches in PubMed, MEDLINE, and the Cochrane Library on the proposed topics. The reviewers also conducted generic Internet searches. Key search terms included pediatric asthma, bronchiolitis, chest radiograph, systemic corticosteroids, gastroesophageal reflux disease (GERD), infant, child, acid suppression therapy, continuous pulse oximetry, pneumonia, gastroenteritis, viral testing, blood culture, and soft tissue infections. To ensure that the reviewers included all studies relevant to the searches, they utilized broad terms. The search included all literature published through 2012, and nonEnglish language publications were included in the search. Studies selected and included in the review were based upon common criteria including whether the article discussed an evaluation of efficacy and/or utility of treatment, included a pediatric population in the guidelines or study, reviewed the harm associated with the administration of a particular test or treatment, and explored the cost associated with the test or treatment.
The Delphi Panel
Members of the workgroup formed a Delphi panel except for 1 member (R.Q.) who served as the nonvoting moderator. The members of the Delphi panel considered the results of the literature search for each recommendation along with the collated feedback from hospitalist listserves as described in Figure 1. Each panel member received a voting instrument with the candidate tests and treatments for the first round of Delphi voting. The panel utilized a modified Delphi method or the RAND Corporation (RAND)/University of California at Los Angeles (UCLA) appropriateness method as described in previous publications of quality indicator development in pediatrics.[7] Each panelist scored the candidate tests and treatments and forwarded the scores to the moderator. Subsequently, all the members of the Delphi panel met through a conference call to carry out the second round of voting. The deidentified collated results of the first round of Delphi voting were made available and discussed during the call. The moderator collated the final results, and the final 5 recommendations were those that had the highest score after the second round of Delphi voting.
Volume and Costs
During deliberations, the committee took into account the prevalence and cost rankings of our most common pediatric inpatient diagnoses. This was done using the Agency for Healthcare Research and Quality's (AHRQ) Healthcare Utilization Project (HCUP), specifically, the Kids' Inpatient Database (KID). HCUP includes the largest collection of longitudinal hospital care data in the United States, encompassing all‐payer discharge‐level information. We excluded normal newborn hospitalizations, and looked at the top 10 acute inpatient diagnoses in terms of both volume and aggregate costs.
RESULTS
The initial list of 20 candidate tests and treatments as well as the refined list of 11 recommendations can be found as electronic supplements to this publication (see Supporting Table 1 and Supporting Table 2 in the online version of this article). The format and language of the list of 11 recommendations were chosen to mesh with that typically used in the ABIM‐F Choosing Wisely campaign. During the Delphi panel, there was strong group consensus about combining items 1 and 2 (chest radiographs in asthma and bronchiolitis) into a single recommendation.
| Do not order chest radiographs in children with asthma or bronchiolitis. |
| Do not use bronchodilators in children with bronchiolitis. |
| Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection. |
| Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
The top 5 recommendations based on the result of the second round of Delphi scoring are shown in Table 1 and described below along with a detailed evidence summary.
Do not order chest radiographs in children with asthma or bronchiolitis.
The National Heart and Lung Institute's guidelines for the management of asthma, published in 1987, recommend against routinely obtaining chest radiographs in patients with asthma or asthma exacerbations.[8] Supporting this recommendation are several studies that show a low overall yield when obtaining chest radiographs for wheezing patients.[9, 10, 11] Most relevant, studies that evaluated the clinical utility of radiographs in patients with asthma have demonstrated that they influence clinical management in less than 2% of cases.[12] A quality improvement project aimed at decreasing the rate of chest radiographs obtained in patients with asthma demonstrated that close to 60% of patients admitted to the hospital had chest radiographs performed, and that significant overall reductions can be achieved (45.3%28.9%, P=0.0005) without impacting clinical outcomes negatively.[13]
Similarly, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely obtaining radiographs during the evaluation for bronchiolitis.[14] Studies assessing the utility of chest x‐rays in these children demonstrate an even lower incidence of abnormalities (0.75%) and indicate that, despite this low incidence, physicians are more likely to treat with antibiotics when radiographs are obtained.[15] There is also evidence that chest radiographs in patients with bronchiolitis are not useful in predicting severity of illness.[16] Furthermore, cost‐effective analyses have demonstrated that omitting chest radiographs in bronchiolitis is actually cost‐effective, without compromising diagnostic accuracy.[17] In a recently published national benchmarking inpatient collaborative, Ralston et al. demonstrated that the majority of patients admitted to the hospital with bronchiolitis have chest radiographs performed at a rate of 64% (interquartile range [IQR], 54%81%).[18]
In both bronchiolitis and asthma, the elimination of unnecessary radiographs has the potential to decrease costs, reduce radiation exposure, and minimize the overuse of antibiotics that often occurs secondary to false positive results.
Do not use bronchodilators in children with bronchiolitis.
Ralston showed that 70% (IQR, 59%83%) of admitted bronchiolitis patients received bronchodilators with an average of 7.9 doses per patient (IQR, 4.69.8). National guidelines for bronchiolitis suggest a very limited role of bronchodilators in patients with bronchiolitis.[14] The first meta‐analyses of studies related to the question of ‐agonist efficacy in bronchiolitis were published in the late 1990s, revealing minimal or no treatment effects.[19, 20] Since then, further research has solidified these findings, and fairly definitive statements can be made based on a recent comprehensive meta‐analysis.[21] The pooled data do not show any effect on hospitalization rates, hospital length of stay, or other inpatient outcomes in bronchiolitis. They do show a small change in clinical scores documented in the outpatient setting, though these scores have not correlated with any detectable difference in outcomes. Routine use of ‐agonists in the inpatient setting has no proven benefit, and given the large amount of consistent data, there is no compelling reason for further study of this therapy in the inpatient setting.
Epinephrine, a combined ‐ and ‐agonist, has been extensively evaluated in bronchiolitis as well. Like albuterol, epinephrine has been reported to have no effect on hospital length of stay in bronchiolitis.[22] The issue of admission rates after epinephrine is complicated by 1 very large study that combined epinephrine with dexamethasone and reported a decreased admission rate, though only at 7 days after therapy; however, this effect was nullified after adjustment for multiple comparisons.[23] When the end point is improvement of respiratory scores, epinephrine may perform better than albuterol in studies where they are directly compared; however, there is no evidence that repeated usage of epinephrine has any impact on any clinical outcome for inpatients.[24, 25]
Do not use systemic corticosteroids in children under 2 years of age with a lower respiratory tract infection
In their summary of evidence, the Subcommittee on Diagnosis and Management of Bronchiolitis of the American Academy of Pediatrics recommends against routinely using systemic corticosteroids for infants with bronchiolitis.[14] The previously reference bronchiolitis benchmarking study demonstrated that admitted patients received steroids at a rate of 21% (IQR, 14%26%). The poor efficacy of corticosteroids in children with bronchiolitis under 2 years of age is well demonstrated in the literature. A large, blinded, randomized, controlled study compared systemic oral corticosteroids to placebo in hospitalized children 10 months to 6 years of age with viral wheezing.[26] This study showed no benefit of corticosteroids over placebo in length of stay or parental report of symptoms 1 week later. In the study, a subanalysis of children with eczema and family history of asthma also demonstrated no benefit of systemic corticosteroids. Large systematic reviews further argue that there is no effect of corticosteroids on the likelihood of admission or length of stay in infants with bronchiolitis.[27, 28] One 4‐armed prospective study of children 6 weeks to 12 months of age found no efficacy of dexamethasone over placebo.[23] There was modest benefit of dexamethasone in conjunction with racemic epinephrine; however, this benefit disappeared after adjustment for multiple comparisons. Three smaller studies showing benefit of systemic corticosteroids, however, were highly problematic. They have included older children, were retrospective, or demonstrated inconsistent results.[29, 30] A smaller study showed benefit for children over 2 years of age, but none for children under 2 years of age.[31] Premature infants are at increased risk of asthma, which typically responds well to corticosteroids as these children get older. However, a retrospective study of premature infants under 2 years of age with bronchiolitis demonstrated no association between corticosteroid use and length of stay, even in the subset of premature infants responding to albuterol.[32]
Systemic corticosteroid use in children is not harmless. Children under 2 years of age are especially vulnerable to the decreased growth velocity seen as a side effect of systemic corticosteroids.[33] Corticosteroids may also negatively impact the course of infectious illness. For instance, in children hospitalized with pneumonia but not receiving ‐agonists (ie, patients who are unlikely to have asthma), length of stay is prolonged and readmission is higher in those who receive corticosteroids.[34]
Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
From 2000 to 2005, the incidence of infants diagnosed with gastroeshopaheal reflux (GER) tripled (3.4%12.3%), and the use of proton pump inhibitors (PPIs) doubled (31.5%62.6%).[35] Patients diagnosed with GER and treated with antireflux medication incurred 1.8 times higher healthcare costs in 1 study compared to healthy controls.[36] Though common, the use of acid suppressive medications in infants lacks evidence for efficacy in the majority of the clinical scenarios in which they are prescribed.[37, 38] PPIs have failed to outperform placebo for typical infant reflux, which is generally developmental and not pathologic.[39, 40] Furthermore, prompted by findings in adults, multiple pediatric investigators have now catalogued the potential risks associated with acid blockade in children in multiple clinical settings. Specifically, increased risk of pneumonia has been documented in inpatients and outpatients, and increased risk of necrotizing enterocolitis and other serious infections have been documented in intensive care unit settings.[41] In the absence of data supporting efficacy and given the emerging data on risk, empiric acid suppression in infants with reflux is wasteful and potentially harmful.
Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
Pulse oximetry use has become widespread in the management of infants with bronchiolitis and likely accounts for the dramatic increase in bronchiolitis hospitalization rates in recent years.[14, 42, 43, 44, 45, 46, 47] Despite this increase in hospitalization rate, there was no change in mortality from bronchiolitis between 1979 and 1997.[48] The continuous monitoring of oxygen saturations in hospitalized infants with bronchiolitis may lead to overdiagnosis of hypoxemia and subsequent oxygen use that is of no apparent benefit to the child. Schroeder et al. demonstrated that 26% of a sample of infants hospitalized with bronchiolitis had a prolonged length of stay because of a perceived need for oxygen based on pulse oximetry readings.[43] Unger and Cunningham showed that the need for oxygen was the final determinant of length of stay in 58% of cases, and Cunningham and Murray suggested that using an oxygen saturation cutoff of 94% instead of 90% might increase the length of stay by 22 hours.[44, 49]
It has been previously shown that hypoxia is normative in infants. Healthy infants experience multiple episodes of SpO2 90% while sleeping.[50] This finding strengthens the notion that detection of low saturations in infants convalescing from bronchiolitis may simply reflect overdiagnosis. Among children with chronic severe asthma, who presumably have experienced episodes of hypoxia throughout childhood, there is no difference in school performance compared to healthy controls.[51]
The practice parameter on bronchiolitis from the American Academy of Pediatrics states: as the child's clinical course improves, continuous measurement of SpO2 is not routinely needed, which is a recommendation based on expert consensus.[14] There is at least one ongoing randomized trial comparing the use of continuous versus intermittent pulse oximetry in hospitalized infants with bronchiolitis who are weaned off oxygen (
DISCUSSION
Berwick and Hackbarth define overtreatment as: waste that comes from subjecting patients to care that, according to sound science and the patients' own preferences, cannot possibly help themcare rooted in outmoded habits, supply‐driven behaviors, and ignoring science.[1] With this project, we tried to capture common clinical sources of waste in the inpatient pediatric setting. This is an inherently difficult project because of the absence of solid evidence to inform every decision point in medicine. Although there is always room for improvement in our evidence base, our group intentionally gravitated to areas where the evidence was robust.
The primary strength of this work is the use of the RAND/UCLA appropriateness method or modified Delphi method. Several publications have validated this methodology as a sound strategy to assess quality indicators and issues related to overuse.[7, 53] To our knowledge, we are the first group to report the use of this methodology to develop a list such as the list reported here.
There were some challenges inherent to this project that can be considered limitations of the work. One perceived limitation of our list is the heavy concentration on respiratory diagnoses, especially bronchiolitis and asthma. We do not feel this is a genuine limitation, as the recommendations were partly driven by volume and costs as assessed by the KID database. Among the top 10 acute inpatient diagnoses in pediatrics, respiratory diagnoses are the most common, including bronchiolitis, pneumonia, and asthma. Pneumonia or bronchiolitis has been the most common medical diagnosis in inpatient pediatrics for the past decade, and both are always in the top 10 for costs as well.[54] Thus, the impact of decreasing overuse for these conditions will be highly significant from a simple volume standpoint.
The primary limitation of this work is the lack of implementation strategies. Although the Choosing Wisely campaign has plans for dissemination of the lists, compliance with the recommendations may be suboptimal. Although the development process followed an accepted methodology, shortcomings include the lack of wide, local, multidisciplinary (including parents or caretakers) consultation. Other barriers to compliance with these recommendations exist. Despite evidence that bronchiolitis is a benign self‐limited disease that does not respond to bronchodilators and steroids, the drive to identify and correct all abnormalities, such as wheezing or low oxygen saturation in a nontoxic infant with bronchiolitis, seems to trump the obligation to do no harm in daily practice.[55] This behavior may result from pressure by patients, families, nurses, or peers and is deeply embedded in our medical culture, where action is preferred to inaction without full knowledge or consideration of risks. Doctors and nurses have become attached to the pulse oximeter, believing somehow that the number displayed is less subjective and holds more predictive value than careful evaluation of the patient's respiratory status. Other pressures, such as direct to consumer marketing have made acid reflux a household term that is easily treated with over‐the‐counter medications. Considerations of the care continuum will also serve as barriers. Chest x‐rays, for example, are frequently obtained prior to admission to the hospital before the hospitalist is involved.
To overcome these limitations, the study of individual and organizational adoption of innovation might be relevant. Though it is complex and often more descriptive than proscriptive, a few salient features have emerged. Champions and opinion leaders make a difference, local culture is dominant, social networking is important, simple innovations that can be trialed on a small scale are adaptable by the user and have observable benefits, are more likely to be adopted.[56] Fortunately, the top 5 list meets many of these criteria, but also faces the daunting challenges of inertia, lack of financial incentive, inability to break with old habits, and fear of lawsuits and perceived patient/parent dissatisfaction. Ongoing evaluation, feedback, and audit will be necessary to detect and sustain change.
CONCLUSION
We have identified 5 tests or therapies overused in inpatient general pediatrics. One goal of the Choosing Wisely campaign is to begin to change social norms related to physician behavior. We hope by asking clinicians to consider doing less for common conditions in inpatient pediatrics, that they will increasingly consider the known and unanticipated risks of any medical interventions they choose to use. Finally, we would like to encourage all pediatricians to embrace the idea of good stewardship and join us in prioritizing and addressing waste and overuse as important patient safety issues as well as threats to the sustainability of our healthcare system.
Acknowledgments
The authors thank Drs. Doug Carlson, James O'Callaghan, and Karen Smith from the Society of Hospital Medicine's Pediatric and Quality and Safety Committees for their support of this effort.
Disclosure: Nothing to report.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- , , . Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128:e1596–e1597.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363:2124–2134.
- . Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- , , , et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357:1515–1523.
- National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138.
- , . The chest x‐ray and childhood acute asthma. Aust Clin Rev. 1993;13:153–156.
- , , , . Clinical factors associated with focal infiltrates in wheezing infants and toddlers. Clin Pediatr (Phila). 2000;39:387–393.
- , , , . Chest radiographs in the pediatric emergency department for children < or = 18 months of age with wheezing. Clin Pediatr (Phila). 1999;38:395–399.
- , , , , , . Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124:e29–e36.
- , . Reduce the rads: a quality assurance project on reducing unnecessary chest X‐rays in children with asthma. J Paediatr Child Health. 2005;41:107–111.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- , , , et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150:429–433.
- , , , et al. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100:e17–e23.
- , , , et al. A cost effectiveness analysis of omitting radiography in diagnosis of acute bronchiolitis. Pediatr Pulmonol. 2009;44:122–127.
- , , , et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25–30.
- , , , . Efficacy of bronchodilator therapy in bronchiolitis. A meta‐analysis. Arch Pediatr Adolesc Med. 1996;150:1166–1172.
- , . Efficacy of beta2‐agonists in bronchiolitis: a reappraisal and meta‐analysis. Pediatrics. 1997;100:233–239.
- , . Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2010;(12):CD001266.
- , , , et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011;(6):CD003123.
- , , , et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079–2089.
- , , , et al. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27–35.
- , , , . A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–824.
- , , , et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. N Engl J Med. 2009;360:329–338.
- , , , et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2010;(10):CD004878.
- , , , , . Systemic corticosteroids in infant bronchiolitis: a meta‐analysis. Pediatrics. 2000;105:E44.
- , , , . Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518.
- , , . Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356.
- , , , , . Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet. 1987;1:879–882.
- , , , , . The clinical management of preterm infants with bronchiolitis. Hosp Pediatr. 2013;3:244–250.
- , . Glucocorticoids and growth in asthmatic children. Pediatr Allergy Immunol. 1995;6:145–154.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127:e255–e263.
- , , , , , . Pediatric gastroesophageal reflux disease and acid‐related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12:348–355.
- , , , , , . Healthcare costs of GERD and acid‐related conditions in pediatric patients, with comparison between histamine‐2 receptor antagonists and proton pump inhibitors. Curr Med Res Opin. 2009;25:2703–2709.
- , , , . Are we overprescribing antireflux medications for infants with regurgitation? Pediatrics. 2007;120:946–949.
- , , , , . Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45:421–427.
- , , , , , . Efficacy of proton‐pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127:925–935.
- . Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease. Ann Pharmacother. 2010;44:572–576.
- . Are there risks associated with empric acid suppression treatment of infants and children suspected of having gastroesophageal reflux disease? Hosp Pediatr. 2013;3:16–23.
- , , , . Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics. 2003;111:e45–e51.
- , , , . Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158:527–530.
- , . Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121:470–475.
- . Oxygen therapy for bronchiolitis. Pediatrics. 2007;120:686–687; author reply 687–688.
- , , , , , . Bronchiolitis‐associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446.
- , . Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–349.
- , , , , . Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16–22.
- , . Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97:361–363.
- , , , et al. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatr. 2011;159:377–383.e1.
- , . The impact of severe asthma on schoolchildren. J Asthma. 1999;36:409–417.
- , . Multi‐center, randomized trial of pulse oximetry monitoring strategies for children hospitalized for bronchiolitis. Abstract presented at: ID Week 2012; October 2012; San Diego, CA.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- Agency for Healthcare Research and Quality. HCUPnet. Kids inpatient database 2009. Available at: http://hcupnet.ahrq.gov. Accessed November 6, 2012.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- . How to implement change in clinical practice. Paediatr Respir Rev. 2003;4:340–346.
- , . Eliminating waste in US health care. JAMA. 2012;307:1513–1516.
- , , . Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128:e1596–e1597.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , , . Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363:2124–2134.
- . Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134.
- , . Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802.
- , , , et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357:1515–1523.
- National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138.
- , . The chest x‐ray and childhood acute asthma. Aust Clin Rev. 1993;13:153–156.
- , , , . Clinical factors associated with focal infiltrates in wheezing infants and toddlers. Clin Pediatr (Phila). 2000;39:387–393.
- , , , . Chest radiographs in the pediatric emergency department for children < or = 18 months of age with wheezing. Clin Pediatr (Phila). 1999;38:395–399.
- , , , , , . Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124:e29–e36.
- , . Reduce the rads: a quality assurance project on reducing unnecessary chest X‐rays in children with asthma. J Paediatr Child Health. 2005;41:107–111.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- , , , et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150:429–433.
- , , , et al. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100:e17–e23.
- , , , et al. A cost effectiveness analysis of omitting radiography in diagnosis of acute bronchiolitis. Pediatr Pulmonol. 2009;44:122–127.
- , , , et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25–30.
- , , , . Efficacy of bronchodilator therapy in bronchiolitis. A meta‐analysis. Arch Pediatr Adolesc Med. 1996;150:1166–1172.
- , . Efficacy of beta2‐agonists in bronchiolitis: a reappraisal and meta‐analysis. Pediatrics. 1997;100:233–239.
- , . Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2010;(12):CD001266.
- , , , et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011;(6):CD003123.
- , , , et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079–2089.
- , , , et al. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27–35.
- , , , . A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–824.
- , , , et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. N Engl J Med. 2009;360:329–338.
- , , , et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2010;(10):CD004878.
- , , , , . Systemic corticosteroids in infant bronchiolitis: a meta‐analysis. Pediatrics. 2000;105:E44.
- , , , . Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518.
- , , . Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356.
- , , , , . Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet. 1987;1:879–882.
- , , , , . The clinical management of preterm infants with bronchiolitis. Hosp Pediatr. 2013;3:244–250.
- , . Glucocorticoids and growth in asthmatic children. Pediatr Allergy Immunol. 1995;6:145–154.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127:e255–e263.
- , , , , , . Pediatric gastroesophageal reflux disease and acid‐related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12:348–355.
- , , , , , . Healthcare costs of GERD and acid‐related conditions in pediatric patients, with comparison between histamine‐2 receptor antagonists and proton pump inhibitors. Curr Med Res Opin. 2009;25:2703–2709.
- , , , . Are we overprescribing antireflux medications for infants with regurgitation? Pediatrics. 2007;120:946–949.
- , , , , . Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45:421–427.
- , , , , , . Efficacy of proton‐pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127:925–935.
- . Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease. Ann Pharmacother. 2010;44:572–576.
- . Are there risks associated with empric acid suppression treatment of infants and children suspected of having gastroesophageal reflux disease? Hosp Pediatr. 2013;3:16–23.
- , , , . Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics. 2003;111:e45–e51.
- , , , . Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158:527–530.
- , . Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics. 2008;121:470–475.
- . Oxygen therapy for bronchiolitis. Pediatrics. 2007;120:686–687; author reply 687–688.
- , , , , , . Bronchiolitis‐associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446.
- , . Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–349.
- , , , , . Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16–22.
- , . Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97:361–363.
- , , , et al. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatr. 2011;159:377–383.e1.
- , . The impact of severe asthma on schoolchildren. J Asthma. 1999;36:409–417.
- , . Multi‐center, randomized trial of pulse oximetry monitoring strategies for children hospitalized for bronchiolitis. Abstract presented at: ID Week 2012; October 2012; San Diego, CA.
- , , , . The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol. 2012;65:1133–1143.
- Agency for Healthcare Research and Quality. HCUPnet. Kids inpatient database 2009. Available at: http://hcupnet.ahrq.gov. Accessed November 6, 2012.
- , , . Too little? Too much? Primary care physicians' views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585.
- . How to implement change in clinical practice. Paediatr Respir Rev. 2003;4:340–346.
Copyright © 2013 Society of Hospital Medicine