User login
Anastrozole-Induced Subacute Cutaneous Lupus Erythematosus
Drug-induced subacute cutaneous lupus erythematosus (DI-SCLE) was first described in 1985 in 5 patients who had been taking hydrochlorothiazide.1 The skin lesions in these patients were identical to those seen in idiopathic subacute cutaneous lupus erythematosus (SCLE) and were accompanied by the same autoantibodies (anti-Ro/Sjögren syndrome antigen A [SS-A] and anti-La/Sjögren syndrome antigen B [SS-B]) and HLA type (HLA-DR2/DR3) that are known to be associated with idiopathic SCLE. The skin lesions of SCLE in these 5 patients resolved spontaneously after discontinuing hydrochlorothiazide; however, anti-Ro/SS-A antibodies persisted in all except 1 patient.1 Over the last decade, an increasing number of drugs from different classes have been implicated to be associated with DI-SCLE. Since the concept of DI-SCLE was introduced, it has been reported to look identical to idiopathic SCLE, both clinically and histopathologically; however, one report suggested that the 2 entities can be distinguished based on clinical variations.2 In general, patients with DI-SCLE develop the same anti-Ro antibodies as seen in idiopathic SCLE. In addition, although the rash in DI-SCLE typically resolves with withdrawal of the offending drug, the antibodies tend to persist. Herein, we report a case of a patient being treated with an aromatase inhibitor who presented with clinical, serologic, and histopathologic evidence of DI-SCLE.
Case Report
A 69-year-old woman diagnosed with breast cancer 4 years prior to her presentation to dermatology initially underwent a lumpectomy and radiation treatment. She was subsequently started on anastrozole 2 years later. After 16 months of treatment with anastrozole, she developed an erythematous scaly rash on sun-exposed areas of the skin. The patient was seen by an outside dermatologist who treated her for a patient-perceived drug rash based on biopsy results that simply demonstrated interface dermatitis. She was treated with both topical and oral steroids with little improvement and therefore presented to our office approximately 6 months after starting treatment seeking a second opinion.
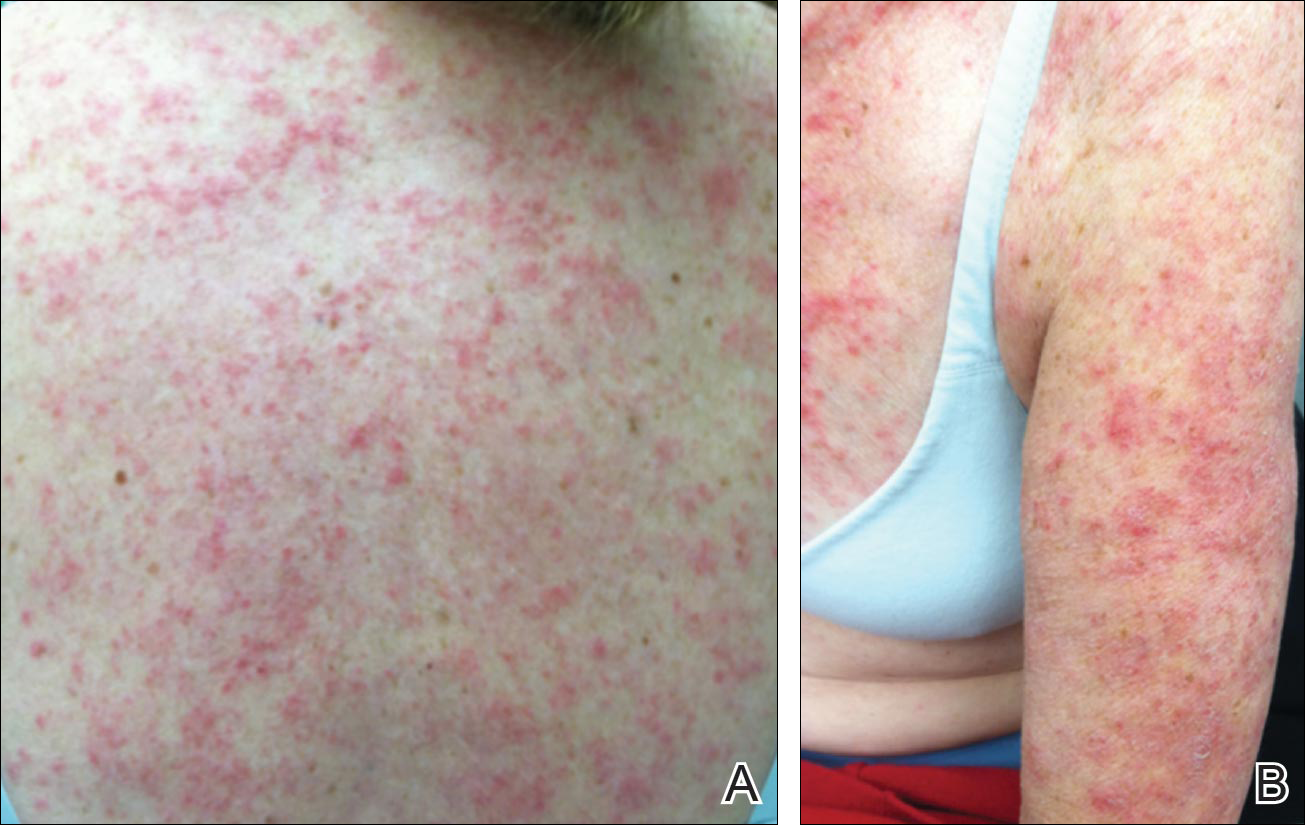
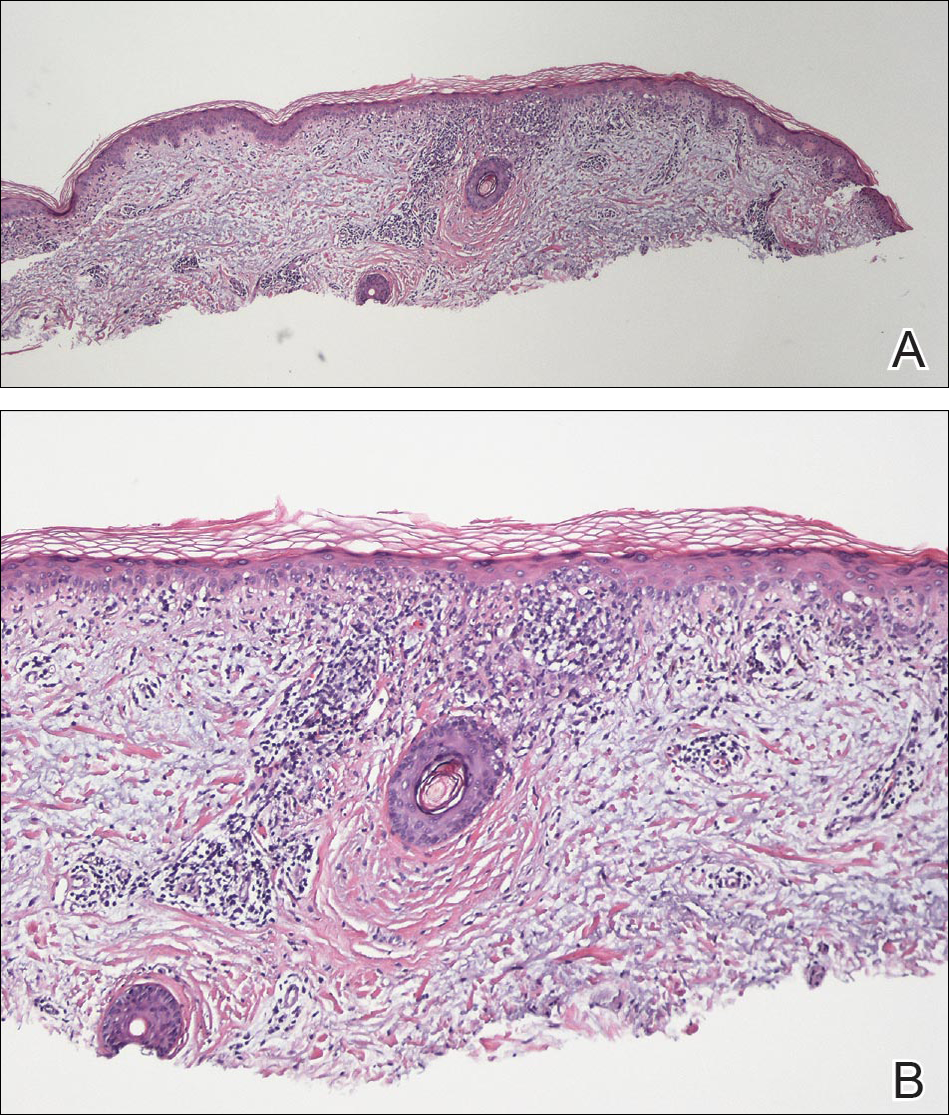
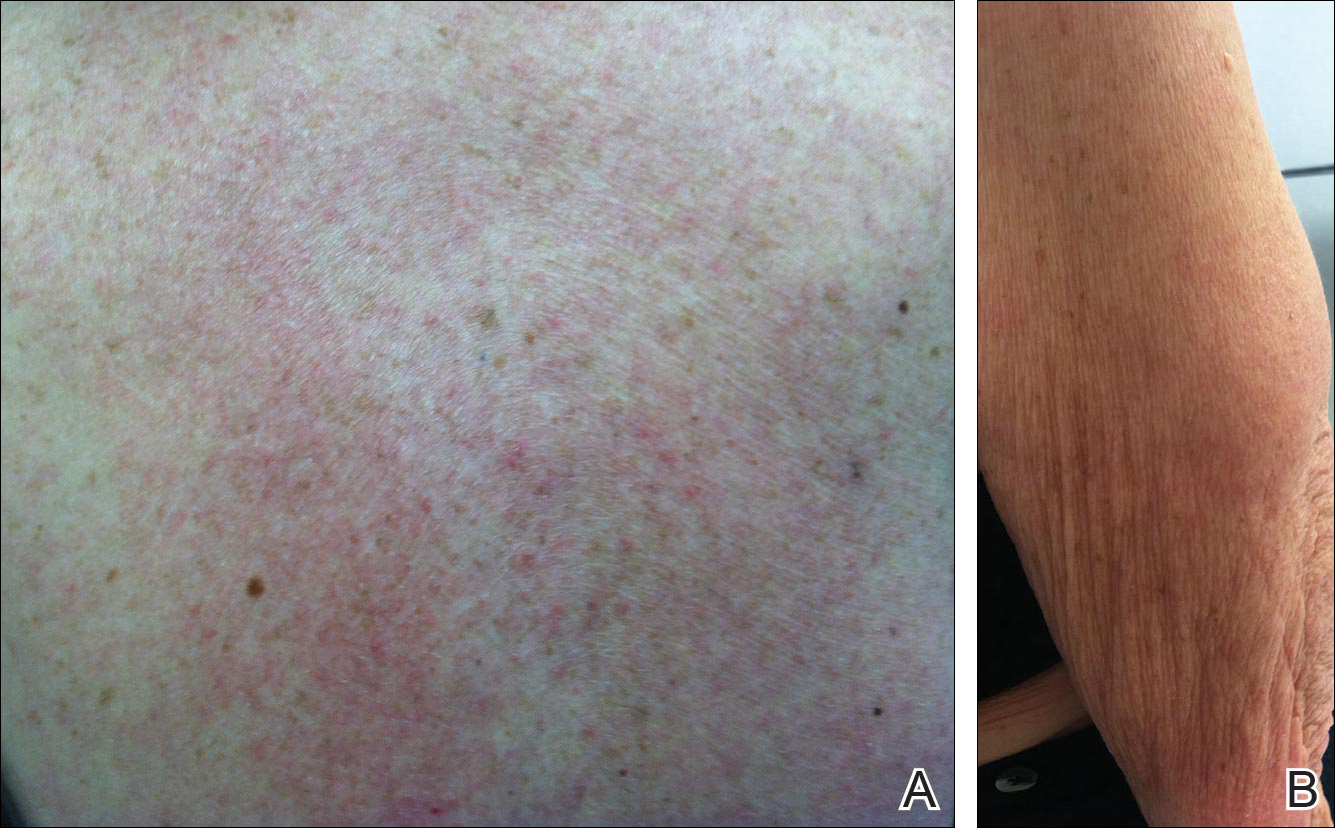
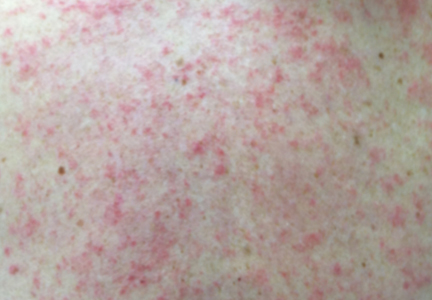
Physical examination revealed numerous erythematous scaly papules and plaques in a photodistributed pattern on the chest, back, legs, and arms (Figure 1). On further questioning, the patient noted that the rash became worse when she was at the beach or playing tennis outside as well as under indoor lights. A repeat biopsy was performed, revealing interface and perivascular dermatitis with an infiltrate composed of lymphocytes, histiocytes, and scattered pigment-laden macrophages (Figure 2). Given the appearance and distribution of the rash as well as the clinical scenario, drug-induced lupus was suspected. Anastrozole was the only medication being taken. Laboratory evaluation was performed and was negative for antinuclear antibodies, antihistone antibodies, and anti-La/SS-B antibodies but was positive for anti-Ro/SS-A antibodies (>8.0 U [reference range, <1.0 U]). Based on these findings, anastrozole-induced SCLE was the most likely explanation for this presentation. The patient was started on a sun-protective regimen (ie, wide-brimmed hat, daily sunscreen) and anastrozole was discontinued by her oncologist; the combination led to moderate improvement in symptoms. One week later, oral hydroxychloroquine 200 mg twice daily was started, which led to notable improvement (Figure 3). The patient was seen for 2 additional follow-up visits, each time with sustained resolution of the rash. The hydroxychloroquine was then stopped at her last visit 3 months after diagnosis. The patient was subsequently lost to follow-up.
Comment
Presentation of SCLE
Subacute cutaneous lupus erythematosus is a form of lupus erythematosus characterized by nonscarring, annular, scaly, erythematous plaques that occur on sun-exposed skin. The lesions are classically distributed on the upper back, chest, dorsal arms, and lateral neck but also can be found in other locations.3,4 Subacute cutaneous lupus erythematosus may be idiopathic; may occur in patients with systemic lupus erythematosus, Sjögren syndrome, or deficiency of the second component of complement (C2d); or may be drug induced.5 On histology SCLE presents as a lichenoid tissue reaction with focal vacuolization of the epidermal basal layer and perivascular lymphocytic infiltrate. On direct immunofluorescence, both idiopathic and drug-induced SCLE present with granular deposition of IgM, IgG, and C3 in a bandlike array at the dermoepidermal junction and circulating anti-Ro/SS-A antibodies. Therefore, histopathologically and immunologically, DI-SCLE is indistinguishable from idiopathic cases.6
Differential Diagnosis
It was previously thought that the clinical presentation of DI-SCLE and idiopathic SCLE were indistinguishable; however, Marzano et al2 described remarkable differences in the cutaneous manifestations of the 2 diseases. Drug-induced SCLE lesions are more widespread, occur more frequently on the legs, and may be bullous or erythema multiforme–like versus the idiopathic lesions, which tend to be more concentrated on the upper body and classically present as scaly erythematous plaques. Additionally, malar rash and vasculitic lesions, such as purpura and necrotic-ulcerative lesions, are seen more often in DI-SCLE.
Drug-induced systemic lupus erythematosus (DI-SLE) is a lupuslike syndrome that can be differentiated from DI-SCLE by virtue of its clinical and serological presentation. It differs from DI-SCLE in that DI-SLE typically does not present with skin symptoms; rather, systemic symptoms such as fever, weight loss, arthralgia, polyarthritis, pericarditis, and pleuritis are more commonly seen. Additionally, it has been associated with antihistone antibodies.4 More than 80 drugs have been reported to cause DI-SLE, including procainamide, hydralazine, and quinidine.7
To be classified as either DI-SCLE or DI-SLE, symptoms need to present after administration of the triggering drug and must resolve after the drug is discontinued.7 The drugs most commonly associated with DI-SCLE are thiazides, calcium channel blockers, tumor necrosis factor α inhibitors, angiotensin-converting enzyme inhibitors, and terbinafine, with few cases citing anastrozole as the inciting agent.4,6,8,9 The incubation period for DI-SCLE varies substantially. Thiazide diuretics and calcium channel blockers typically have the longest incubation period, ranging from 6 months to 5 years for thiazides,1,6,10,11 while calcium channel blockers have an average incubation period of 3 years.12 Drug-induced SCLE associated with antifungals, however, usually is much more rapid in onset; the incubation period on average is 5 weeks for terbinafine and 2 weeks for griseofulvin.13-15
Antiestrogen Drugs and SCLE
Anastrozole, the inciting agent in our case, is a third-generation, selective, nonsteroidal, aromatase inhibitor with no progestogenic, androgenic, or estrogenic activity. Anastrozole, when taken at its recommended dosage of 1 mg daily, will suppress estradiol. It is used as an adjuvant treatment of estrogen-sensitive breast cancer in postmenopausal women. In contrast to a prior case of DI-SCLE secondary to anastrozole in which the incubation period was approximately 1 month,8 our patient had an incubation period of approximately 16 months. Tamoxifen, another antiestrogen drug, also has been associated with DI-SCLE.9 In cases of tamoxifen-induced SCLE, the incubation period was several years, which is more similar to our patient. Although these drugs do not have the same mechanism of action, they both have antiestrogen properties.9 A systemic review of DI-SCLE reported that incubation periods between drug exposure and appearance of DI-SCLE varied greatly and were drug class dependent. It is possible that reactions associated with antiestrogen medications have a delayed presentation; however, given there are limited cases of anastrozole-induced DI-SCLE, we cannot make a clear statement on incubation periods.6
Reports of DI-SCLE caused by antiestrogen drugs are particularly interesting because sex hormones in relation to lupus disease activity have been the subject of debate for decades. Women are considerably more likely to develop autoimmune diseases than men, suggesting that steroid hormones, especially estrogen and progesterone, influence the immune system.16 Estrogen actions are proinflammatory, while the actions of progesterone, androgens, and glucocorticoids are anti-inflammatory.17 Studies in women with lupus revealed an increased rate of mild- to moderate-intensity disease flares associated with estrogen-containing hormone replace-ment therapy.18-20
Over the years, several antiestrogen therapies have been used in murine models, which showed remarkable clinical improvement in the course of SLE. The precise mechanisms involved in disease immunomodulation by these therapies have not been elucidated.21-23 It is thought that estrogen plays a role in the synthesis and expression of Ro antigens on the surface of keratinocytes, increasing the fixation of anti-Ro antibodies in keratinocytes and provoking the appearance of a cutaneous eruption in patients with a susceptible HLA profile.6
Conclusion
We report a rare case of SCLE induced by anastrozole use. Cases such as ours and others that implicate antiestrogen drugs in association with DI-SCLE are particularly noteworthy, considering many studies are looking at the potential usefulness of antiestrogen therapy in the treatment of SLE. Further research on this relationship is warranted.
- Reed B, Huff J, Jones S, et al. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med. 1985;103:49-51.
- Marzano A, Lazzari R, Polloni I, et al. Drug-induced subacute cutaneous lupus erythematosus: evidence for differences from its idiopathic counterpart. Br J Dermatol. 2011;165:335-341.
- Bonsmann G, Schiller M, Luger T, et al. Terbinafine-induced subacute cutaneous lupus erythematosus. J Am Acad Dermatol. 2001;44:925-931.
- Callen J. Review: drug induced subacute cutaneous lupus erythematosus. Lupus. 2010;19:1107-1111.
- Lin J, Callen JP. Subacute cutaneous lupus erythematosus (SCLE). Medscape website. http://emedicine.medscape.com/article/1065657-overview. Updated March 7, 2016. Accessed April 29, 2016.
- Lowe GC, Henderson CL, Grau RH, et al. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164:465-472.
- Vedove C, Giglio M, Schena D, et al. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99-105.
- Trancart M, Cavailhes A, Balme B, et al. Anastrozole-induced subacute cutaneous lupus erythematosus [published online December 6, 2007]. Br J Dermatol. 2008;158:628-629.
- Fumal I, Danchin A, Cosserat F, et al. Subacute cutaneous lupus erythematosus associated with tamoxifen therapy: two cases. Dermatology. 2005;210:251-252.
- Brown C, Deng J. Thiazide diuretics induce cutaneous lupus-like adverse reaction. J Toxicol Clin Toxicol. 1995;33:729-733.
- Sontheimer R. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263.
- Crowson A, Magro C. Subacute cutaneous lupus erythematosus arising in the setting of calcium channel blocker therapy. Hum Pathol. 1997;28:67-73.
- Lorentz K, Booken N, Goerdt S, et al. Subacute cutaneous lupus erythematosus induced by terbinafine: case report and review of literature. J Dtsch Dermatol Ges. 2008;6:823-837.
- Kasperkiewicz M, Anemüller W, Angelova-Fischer I, et al. Subacute cutaneous lupus erythematosus associated with terbinafine. Clin Exp Dermatol. 2009;34:403-404.
- Miyagawa S, Okuchi T, Shiomi Y, et al. Subacute cutaneous lupus erythematosus lesions precipitated by griseofulvin. J Am Acad Dermatol. 1989;21:343-346.
- Inman RD. Immunologic sex differences and the female predominance in systemic lupus erythematosus. Arthritis Rheum. 1978;21:849-854.
- Cutolo M, Wilder RL. Different roles of androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum Dis Clin North Am. 2000;26:825-839.
- Petri M. Sex hormones and systemic lupus erythematosus. Lupus. 2008;17:412-415.
- Lateef A, Petri M. Hormone replacement and contraceptive therapy in autoimmune diseases [published online January 18, 2012]. J Autoimmun. 2012;38:J170-J176.
- Buyon JP, Petri M, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:954-962.
- Wu W, Suen J, Lin B, et al. Tamoxifen alleviates disease severity and decreases double negative T cells in autoimmune MRL-lpr/lpr mice. Immunology. 2000;100:110-118.
- Dayan M, Zinger H, Kalush F, et al. The beneficial effects of treatment with tamoxifen and anti-oestradiol antibody on experimental systemic lupus erythematosus are associated with cytokine modulations. Immunology. 1997;90:101-108.
- Sthoeger Z, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBxNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheum Dis. 2003;62:341-346.
Drug-induced subacute cutaneous lupus erythematosus (DI-SCLE) was first described in 1985 in 5 patients who had been taking hydrochlorothiazide.1 The skin lesions in these patients were identical to those seen in idiopathic subacute cutaneous lupus erythematosus (SCLE) and were accompanied by the same autoantibodies (anti-Ro/Sjögren syndrome antigen A [SS-A] and anti-La/Sjögren syndrome antigen B [SS-B]) and HLA type (HLA-DR2/DR3) that are known to be associated with idiopathic SCLE. The skin lesions of SCLE in these 5 patients resolved spontaneously after discontinuing hydrochlorothiazide; however, anti-Ro/SS-A antibodies persisted in all except 1 patient.1 Over the last decade, an increasing number of drugs from different classes have been implicated to be associated with DI-SCLE. Since the concept of DI-SCLE was introduced, it has been reported to look identical to idiopathic SCLE, both clinically and histopathologically; however, one report suggested that the 2 entities can be distinguished based on clinical variations.2 In general, patients with DI-SCLE develop the same anti-Ro antibodies as seen in idiopathic SCLE. In addition, although the rash in DI-SCLE typically resolves with withdrawal of the offending drug, the antibodies tend to persist. Herein, we report a case of a patient being treated with an aromatase inhibitor who presented with clinical, serologic, and histopathologic evidence of DI-SCLE.
Case Report
A 69-year-old woman diagnosed with breast cancer 4 years prior to her presentation to dermatology initially underwent a lumpectomy and radiation treatment. She was subsequently started on anastrozole 2 years later. After 16 months of treatment with anastrozole, she developed an erythematous scaly rash on sun-exposed areas of the skin. The patient was seen by an outside dermatologist who treated her for a patient-perceived drug rash based on biopsy results that simply demonstrated interface dermatitis. She was treated with both topical and oral steroids with little improvement and therefore presented to our office approximately 6 months after starting treatment seeking a second opinion.



Physical examination revealed numerous erythematous scaly papules and plaques in a photodistributed pattern on the chest, back, legs, and arms (Figure 1). On further questioning, the patient noted that the rash became worse when she was at the beach or playing tennis outside as well as under indoor lights. A repeat biopsy was performed, revealing interface and perivascular dermatitis with an infiltrate composed of lymphocytes, histiocytes, and scattered pigment-laden macrophages (Figure 2). Given the appearance and distribution of the rash as well as the clinical scenario, drug-induced lupus was suspected. Anastrozole was the only medication being taken. Laboratory evaluation was performed and was negative for antinuclear antibodies, antihistone antibodies, and anti-La/SS-B antibodies but was positive for anti-Ro/SS-A antibodies (>8.0 U [reference range, <1.0 U]). Based on these findings, anastrozole-induced SCLE was the most likely explanation for this presentation. The patient was started on a sun-protective regimen (ie, wide-brimmed hat, daily sunscreen) and anastrozole was discontinued by her oncologist; the combination led to moderate improvement in symptoms. One week later, oral hydroxychloroquine 200 mg twice daily was started, which led to notable improvement (Figure 3). The patient was seen for 2 additional follow-up visits, each time with sustained resolution of the rash. The hydroxychloroquine was then stopped at her last visit 3 months after diagnosis. The patient was subsequently lost to follow-up.
Comment
Presentation of SCLE
Subacute cutaneous lupus erythematosus is a form of lupus erythematosus characterized by nonscarring, annular, scaly, erythematous plaques that occur on sun-exposed skin. The lesions are classically distributed on the upper back, chest, dorsal arms, and lateral neck but also can be found in other locations.3,4 Subacute cutaneous lupus erythematosus may be idiopathic; may occur in patients with systemic lupus erythematosus, Sjögren syndrome, or deficiency of the second component of complement (C2d); or may be drug induced.5 On histology SCLE presents as a lichenoid tissue reaction with focal vacuolization of the epidermal basal layer and perivascular lymphocytic infiltrate. On direct immunofluorescence, both idiopathic and drug-induced SCLE present with granular deposition of IgM, IgG, and C3 in a bandlike array at the dermoepidermal junction and circulating anti-Ro/SS-A antibodies. Therefore, histopathologically and immunologically, DI-SCLE is indistinguishable from idiopathic cases.6
Differential Diagnosis
It was previously thought that the clinical presentation of DI-SCLE and idiopathic SCLE were indistinguishable; however, Marzano et al2 described remarkable differences in the cutaneous manifestations of the 2 diseases. Drug-induced SCLE lesions are more widespread, occur more frequently on the legs, and may be bullous or erythema multiforme–like versus the idiopathic lesions, which tend to be more concentrated on the upper body and classically present as scaly erythematous plaques. Additionally, malar rash and vasculitic lesions, such as purpura and necrotic-ulcerative lesions, are seen more often in DI-SCLE.
Drug-induced systemic lupus erythematosus (DI-SLE) is a lupuslike syndrome that can be differentiated from DI-SCLE by virtue of its clinical and serological presentation. It differs from DI-SCLE in that DI-SLE typically does not present with skin symptoms; rather, systemic symptoms such as fever, weight loss, arthralgia, polyarthritis, pericarditis, and pleuritis are more commonly seen. Additionally, it has been associated with antihistone antibodies.4 More than 80 drugs have been reported to cause DI-SLE, including procainamide, hydralazine, and quinidine.7
To be classified as either DI-SCLE or DI-SLE, symptoms need to present after administration of the triggering drug and must resolve after the drug is discontinued.7 The drugs most commonly associated with DI-SCLE are thiazides, calcium channel blockers, tumor necrosis factor α inhibitors, angiotensin-converting enzyme inhibitors, and terbinafine, with few cases citing anastrozole as the inciting agent.4,6,8,9 The incubation period for DI-SCLE varies substantially. Thiazide diuretics and calcium channel blockers typically have the longest incubation period, ranging from 6 months to 5 years for thiazides,1,6,10,11 while calcium channel blockers have an average incubation period of 3 years.12 Drug-induced SCLE associated with antifungals, however, usually is much more rapid in onset; the incubation period on average is 5 weeks for terbinafine and 2 weeks for griseofulvin.13-15
Antiestrogen Drugs and SCLE
Anastrozole, the inciting agent in our case, is a third-generation, selective, nonsteroidal, aromatase inhibitor with no progestogenic, androgenic, or estrogenic activity. Anastrozole, when taken at its recommended dosage of 1 mg daily, will suppress estradiol. It is used as an adjuvant treatment of estrogen-sensitive breast cancer in postmenopausal women. In contrast to a prior case of DI-SCLE secondary to anastrozole in which the incubation period was approximately 1 month,8 our patient had an incubation period of approximately 16 months. Tamoxifen, another antiestrogen drug, also has been associated with DI-SCLE.9 In cases of tamoxifen-induced SCLE, the incubation period was several years, which is more similar to our patient. Although these drugs do not have the same mechanism of action, they both have antiestrogen properties.9 A systemic review of DI-SCLE reported that incubation periods between drug exposure and appearance of DI-SCLE varied greatly and were drug class dependent. It is possible that reactions associated with antiestrogen medications have a delayed presentation; however, given there are limited cases of anastrozole-induced DI-SCLE, we cannot make a clear statement on incubation periods.6
Reports of DI-SCLE caused by antiestrogen drugs are particularly interesting because sex hormones in relation to lupus disease activity have been the subject of debate for decades. Women are considerably more likely to develop autoimmune diseases than men, suggesting that steroid hormones, especially estrogen and progesterone, influence the immune system.16 Estrogen actions are proinflammatory, while the actions of progesterone, androgens, and glucocorticoids are anti-inflammatory.17 Studies in women with lupus revealed an increased rate of mild- to moderate-intensity disease flares associated with estrogen-containing hormone replace-ment therapy.18-20
Over the years, several antiestrogen therapies have been used in murine models, which showed remarkable clinical improvement in the course of SLE. The precise mechanisms involved in disease immunomodulation by these therapies have not been elucidated.21-23 It is thought that estrogen plays a role in the synthesis and expression of Ro antigens on the surface of keratinocytes, increasing the fixation of anti-Ro antibodies in keratinocytes and provoking the appearance of a cutaneous eruption in patients with a susceptible HLA profile.6
Conclusion
We report a rare case of SCLE induced by anastrozole use. Cases such as ours and others that implicate antiestrogen drugs in association with DI-SCLE are particularly noteworthy, considering many studies are looking at the potential usefulness of antiestrogen therapy in the treatment of SLE. Further research on this relationship is warranted.
Drug-induced subacute cutaneous lupus erythematosus (DI-SCLE) was first described in 1985 in 5 patients who had been taking hydrochlorothiazide.1 The skin lesions in these patients were identical to those seen in idiopathic subacute cutaneous lupus erythematosus (SCLE) and were accompanied by the same autoantibodies (anti-Ro/Sjögren syndrome antigen A [SS-A] and anti-La/Sjögren syndrome antigen B [SS-B]) and HLA type (HLA-DR2/DR3) that are known to be associated with idiopathic SCLE. The skin lesions of SCLE in these 5 patients resolved spontaneously after discontinuing hydrochlorothiazide; however, anti-Ro/SS-A antibodies persisted in all except 1 patient.1 Over the last decade, an increasing number of drugs from different classes have been implicated to be associated with DI-SCLE. Since the concept of DI-SCLE was introduced, it has been reported to look identical to idiopathic SCLE, both clinically and histopathologically; however, one report suggested that the 2 entities can be distinguished based on clinical variations.2 In general, patients with DI-SCLE develop the same anti-Ro antibodies as seen in idiopathic SCLE. In addition, although the rash in DI-SCLE typically resolves with withdrawal of the offending drug, the antibodies tend to persist. Herein, we report a case of a patient being treated with an aromatase inhibitor who presented with clinical, serologic, and histopathologic evidence of DI-SCLE.
Case Report
A 69-year-old woman diagnosed with breast cancer 4 years prior to her presentation to dermatology initially underwent a lumpectomy and radiation treatment. She was subsequently started on anastrozole 2 years later. After 16 months of treatment with anastrozole, she developed an erythematous scaly rash on sun-exposed areas of the skin. The patient was seen by an outside dermatologist who treated her for a patient-perceived drug rash based on biopsy results that simply demonstrated interface dermatitis. She was treated with both topical and oral steroids with little improvement and therefore presented to our office approximately 6 months after starting treatment seeking a second opinion.



Physical examination revealed numerous erythematous scaly papules and plaques in a photodistributed pattern on the chest, back, legs, and arms (Figure 1). On further questioning, the patient noted that the rash became worse when she was at the beach or playing tennis outside as well as under indoor lights. A repeat biopsy was performed, revealing interface and perivascular dermatitis with an infiltrate composed of lymphocytes, histiocytes, and scattered pigment-laden macrophages (Figure 2). Given the appearance and distribution of the rash as well as the clinical scenario, drug-induced lupus was suspected. Anastrozole was the only medication being taken. Laboratory evaluation was performed and was negative for antinuclear antibodies, antihistone antibodies, and anti-La/SS-B antibodies but was positive for anti-Ro/SS-A antibodies (>8.0 U [reference range, <1.0 U]). Based on these findings, anastrozole-induced SCLE was the most likely explanation for this presentation. The patient was started on a sun-protective regimen (ie, wide-brimmed hat, daily sunscreen) and anastrozole was discontinued by her oncologist; the combination led to moderate improvement in symptoms. One week later, oral hydroxychloroquine 200 mg twice daily was started, which led to notable improvement (Figure 3). The patient was seen for 2 additional follow-up visits, each time with sustained resolution of the rash. The hydroxychloroquine was then stopped at her last visit 3 months after diagnosis. The patient was subsequently lost to follow-up.
Comment
Presentation of SCLE
Subacute cutaneous lupus erythematosus is a form of lupus erythematosus characterized by nonscarring, annular, scaly, erythematous plaques that occur on sun-exposed skin. The lesions are classically distributed on the upper back, chest, dorsal arms, and lateral neck but also can be found in other locations.3,4 Subacute cutaneous lupus erythematosus may be idiopathic; may occur in patients with systemic lupus erythematosus, Sjögren syndrome, or deficiency of the second component of complement (C2d); or may be drug induced.5 On histology SCLE presents as a lichenoid tissue reaction with focal vacuolization of the epidermal basal layer and perivascular lymphocytic infiltrate. On direct immunofluorescence, both idiopathic and drug-induced SCLE present with granular deposition of IgM, IgG, and C3 in a bandlike array at the dermoepidermal junction and circulating anti-Ro/SS-A antibodies. Therefore, histopathologically and immunologically, DI-SCLE is indistinguishable from idiopathic cases.6
Differential Diagnosis
It was previously thought that the clinical presentation of DI-SCLE and idiopathic SCLE were indistinguishable; however, Marzano et al2 described remarkable differences in the cutaneous manifestations of the 2 diseases. Drug-induced SCLE lesions are more widespread, occur more frequently on the legs, and may be bullous or erythema multiforme–like versus the idiopathic lesions, which tend to be more concentrated on the upper body and classically present as scaly erythematous plaques. Additionally, malar rash and vasculitic lesions, such as purpura and necrotic-ulcerative lesions, are seen more often in DI-SCLE.
Drug-induced systemic lupus erythematosus (DI-SLE) is a lupuslike syndrome that can be differentiated from DI-SCLE by virtue of its clinical and serological presentation. It differs from DI-SCLE in that DI-SLE typically does not present with skin symptoms; rather, systemic symptoms such as fever, weight loss, arthralgia, polyarthritis, pericarditis, and pleuritis are more commonly seen. Additionally, it has been associated with antihistone antibodies.4 More than 80 drugs have been reported to cause DI-SLE, including procainamide, hydralazine, and quinidine.7
To be classified as either DI-SCLE or DI-SLE, symptoms need to present after administration of the triggering drug and must resolve after the drug is discontinued.7 The drugs most commonly associated with DI-SCLE are thiazides, calcium channel blockers, tumor necrosis factor α inhibitors, angiotensin-converting enzyme inhibitors, and terbinafine, with few cases citing anastrozole as the inciting agent.4,6,8,9 The incubation period for DI-SCLE varies substantially. Thiazide diuretics and calcium channel blockers typically have the longest incubation period, ranging from 6 months to 5 years for thiazides,1,6,10,11 while calcium channel blockers have an average incubation period of 3 years.12 Drug-induced SCLE associated with antifungals, however, usually is much more rapid in onset; the incubation period on average is 5 weeks for terbinafine and 2 weeks for griseofulvin.13-15
Antiestrogen Drugs and SCLE
Anastrozole, the inciting agent in our case, is a third-generation, selective, nonsteroidal, aromatase inhibitor with no progestogenic, androgenic, or estrogenic activity. Anastrozole, when taken at its recommended dosage of 1 mg daily, will suppress estradiol. It is used as an adjuvant treatment of estrogen-sensitive breast cancer in postmenopausal women. In contrast to a prior case of DI-SCLE secondary to anastrozole in which the incubation period was approximately 1 month,8 our patient had an incubation period of approximately 16 months. Tamoxifen, another antiestrogen drug, also has been associated with DI-SCLE.9 In cases of tamoxifen-induced SCLE, the incubation period was several years, which is more similar to our patient. Although these drugs do not have the same mechanism of action, they both have antiestrogen properties.9 A systemic review of DI-SCLE reported that incubation periods between drug exposure and appearance of DI-SCLE varied greatly and were drug class dependent. It is possible that reactions associated with antiestrogen medications have a delayed presentation; however, given there are limited cases of anastrozole-induced DI-SCLE, we cannot make a clear statement on incubation periods.6
Reports of DI-SCLE caused by antiestrogen drugs are particularly interesting because sex hormones in relation to lupus disease activity have been the subject of debate for decades. Women are considerably more likely to develop autoimmune diseases than men, suggesting that steroid hormones, especially estrogen and progesterone, influence the immune system.16 Estrogen actions are proinflammatory, while the actions of progesterone, androgens, and glucocorticoids are anti-inflammatory.17 Studies in women with lupus revealed an increased rate of mild- to moderate-intensity disease flares associated with estrogen-containing hormone replace-ment therapy.18-20
Over the years, several antiestrogen therapies have been used in murine models, which showed remarkable clinical improvement in the course of SLE. The precise mechanisms involved in disease immunomodulation by these therapies have not been elucidated.21-23 It is thought that estrogen plays a role in the synthesis and expression of Ro antigens on the surface of keratinocytes, increasing the fixation of anti-Ro antibodies in keratinocytes and provoking the appearance of a cutaneous eruption in patients with a susceptible HLA profile.6
Conclusion
We report a rare case of SCLE induced by anastrozole use. Cases such as ours and others that implicate antiestrogen drugs in association with DI-SCLE are particularly noteworthy, considering many studies are looking at the potential usefulness of antiestrogen therapy in the treatment of SLE. Further research on this relationship is warranted.
- Reed B, Huff J, Jones S, et al. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med. 1985;103:49-51.
- Marzano A, Lazzari R, Polloni I, et al. Drug-induced subacute cutaneous lupus erythematosus: evidence for differences from its idiopathic counterpart. Br J Dermatol. 2011;165:335-341.
- Bonsmann G, Schiller M, Luger T, et al. Terbinafine-induced subacute cutaneous lupus erythematosus. J Am Acad Dermatol. 2001;44:925-931.
- Callen J. Review: drug induced subacute cutaneous lupus erythematosus. Lupus. 2010;19:1107-1111.
- Lin J, Callen JP. Subacute cutaneous lupus erythematosus (SCLE). Medscape website. http://emedicine.medscape.com/article/1065657-overview. Updated March 7, 2016. Accessed April 29, 2016.
- Lowe GC, Henderson CL, Grau RH, et al. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164:465-472.
- Vedove C, Giglio M, Schena D, et al. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99-105.
- Trancart M, Cavailhes A, Balme B, et al. Anastrozole-induced subacute cutaneous lupus erythematosus [published online December 6, 2007]. Br J Dermatol. 2008;158:628-629.
- Fumal I, Danchin A, Cosserat F, et al. Subacute cutaneous lupus erythematosus associated with tamoxifen therapy: two cases. Dermatology. 2005;210:251-252.
- Brown C, Deng J. Thiazide diuretics induce cutaneous lupus-like adverse reaction. J Toxicol Clin Toxicol. 1995;33:729-733.
- Sontheimer R. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263.
- Crowson A, Magro C. Subacute cutaneous lupus erythematosus arising in the setting of calcium channel blocker therapy. Hum Pathol. 1997;28:67-73.
- Lorentz K, Booken N, Goerdt S, et al. Subacute cutaneous lupus erythematosus induced by terbinafine: case report and review of literature. J Dtsch Dermatol Ges. 2008;6:823-837.
- Kasperkiewicz M, Anemüller W, Angelova-Fischer I, et al. Subacute cutaneous lupus erythematosus associated with terbinafine. Clin Exp Dermatol. 2009;34:403-404.
- Miyagawa S, Okuchi T, Shiomi Y, et al. Subacute cutaneous lupus erythematosus lesions precipitated by griseofulvin. J Am Acad Dermatol. 1989;21:343-346.
- Inman RD. Immunologic sex differences and the female predominance in systemic lupus erythematosus. Arthritis Rheum. 1978;21:849-854.
- Cutolo M, Wilder RL. Different roles of androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum Dis Clin North Am. 2000;26:825-839.
- Petri M. Sex hormones and systemic lupus erythematosus. Lupus. 2008;17:412-415.
- Lateef A, Petri M. Hormone replacement and contraceptive therapy in autoimmune diseases [published online January 18, 2012]. J Autoimmun. 2012;38:J170-J176.
- Buyon JP, Petri M, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:954-962.
- Wu W, Suen J, Lin B, et al. Tamoxifen alleviates disease severity and decreases double negative T cells in autoimmune MRL-lpr/lpr mice. Immunology. 2000;100:110-118.
- Dayan M, Zinger H, Kalush F, et al. The beneficial effects of treatment with tamoxifen and anti-oestradiol antibody on experimental systemic lupus erythematosus are associated with cytokine modulations. Immunology. 1997;90:101-108.
- Sthoeger Z, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBxNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheum Dis. 2003;62:341-346.
- Reed B, Huff J, Jones S, et al. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med. 1985;103:49-51.
- Marzano A, Lazzari R, Polloni I, et al. Drug-induced subacute cutaneous lupus erythematosus: evidence for differences from its idiopathic counterpart. Br J Dermatol. 2011;165:335-341.
- Bonsmann G, Schiller M, Luger T, et al. Terbinafine-induced subacute cutaneous lupus erythematosus. J Am Acad Dermatol. 2001;44:925-931.
- Callen J. Review: drug induced subacute cutaneous lupus erythematosus. Lupus. 2010;19:1107-1111.
- Lin J, Callen JP. Subacute cutaneous lupus erythematosus (SCLE). Medscape website. http://emedicine.medscape.com/article/1065657-overview. Updated March 7, 2016. Accessed April 29, 2016.
- Lowe GC, Henderson CL, Grau RH, et al. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164:465-472.
- Vedove C, Giglio M, Schena D, et al. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99-105.
- Trancart M, Cavailhes A, Balme B, et al. Anastrozole-induced subacute cutaneous lupus erythematosus [published online December 6, 2007]. Br J Dermatol. 2008;158:628-629.
- Fumal I, Danchin A, Cosserat F, et al. Subacute cutaneous lupus erythematosus associated with tamoxifen therapy: two cases. Dermatology. 2005;210:251-252.
- Brown C, Deng J. Thiazide diuretics induce cutaneous lupus-like adverse reaction. J Toxicol Clin Toxicol. 1995;33:729-733.
- Sontheimer R. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263.
- Crowson A, Magro C. Subacute cutaneous lupus erythematosus arising in the setting of calcium channel blocker therapy. Hum Pathol. 1997;28:67-73.
- Lorentz K, Booken N, Goerdt S, et al. Subacute cutaneous lupus erythematosus induced by terbinafine: case report and review of literature. J Dtsch Dermatol Ges. 2008;6:823-837.
- Kasperkiewicz M, Anemüller W, Angelova-Fischer I, et al. Subacute cutaneous lupus erythematosus associated with terbinafine. Clin Exp Dermatol. 2009;34:403-404.
- Miyagawa S, Okuchi T, Shiomi Y, et al. Subacute cutaneous lupus erythematosus lesions precipitated by griseofulvin. J Am Acad Dermatol. 1989;21:343-346.
- Inman RD. Immunologic sex differences and the female predominance in systemic lupus erythematosus. Arthritis Rheum. 1978;21:849-854.
- Cutolo M, Wilder RL. Different roles of androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum Dis Clin North Am. 2000;26:825-839.
- Petri M. Sex hormones and systemic lupus erythematosus. Lupus. 2008;17:412-415.
- Lateef A, Petri M. Hormone replacement and contraceptive therapy in autoimmune diseases [published online January 18, 2012]. J Autoimmun. 2012;38:J170-J176.
- Buyon JP, Petri M, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:954-962.
- Wu W, Suen J, Lin B, et al. Tamoxifen alleviates disease severity and decreases double negative T cells in autoimmune MRL-lpr/lpr mice. Immunology. 2000;100:110-118.
- Dayan M, Zinger H, Kalush F, et al. The beneficial effects of treatment with tamoxifen and anti-oestradiol antibody on experimental systemic lupus erythematosus are associated with cytokine modulations. Immunology. 1997;90:101-108.
- Sthoeger Z, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBxNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheum Dis. 2003;62:341-346.
Practice Points
- There are numerous cases of drug-induced subacute cutaneous lupus erythematosus (DI-SCLE) published in the literature; however, there are limited reports with anastrozole implicated as the causative agent.
- Cases of DI-SCLE are clinically and histologically indistinguishable from idiopathic cases. It is important to recognize and withdraw the offending agent.