User login
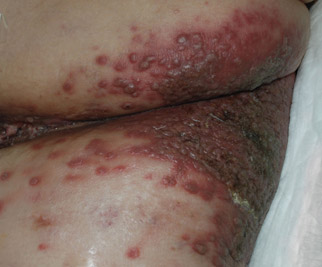
Round Purple Erythematous Tumors
The Diagnosis: Skin Metastases of Vulvar Carcinoma
A Tzanck cytodiagnosis was performed and a punch biopsy specimen was obtained for histologic examination. The patient’s Tzanck cytodiagnosis was initially interpreted as a viral cytopathic effect due to the presence of multinucleated atypical cells; hence, she was treated with intravenous acyclovir. The biopsy specimen showed a preserved epidermis and a dense infiltrate in the mid and deep dermis (Figure 1) formed by atypical squamous cells with enlarged nuclei and anisokaryosis, evident nucleoli, atypical mitoses, and multinucleated cells (Figure 2). The histopathologic diagnosis was skin metastases of moderately differentiated squamous cell carcinoma. The subsequent clinical course was unfavorableand the patient died during hospitalization from septic shock.
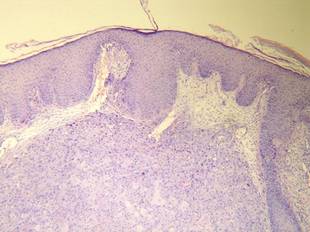
Figure 1. Histology revealed a preserved epidermis with a dense infiltrate in the mid and deep dermis (H&E, original magnification ×40). | 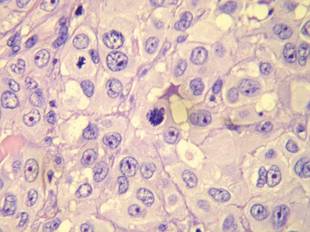
Figure 2. Atypical squamous cells with enlarged nuclei and anisokaryosis, evident nucleoli, atypical mitoses, and multinucleated cells were evident (H&E, original magnification ×400). |
Cutaneous metastases occur in 0.7% to 9% of solid tumors in advanced stages of disease progression or occasionally as an initial manifestation; they are a predictor of poor prognosis and breast cancer is the most frequent cause in women.1 Vulvar carcinoma comprises 5% of all malignant neoplasms of the female genital tract and 95% are squamous cell carcinoma; metastases appear most frequently in the inguinal and pelvic lymph nodes, followed by the lungs, liver, and bones.2 Skin metastases are extremely rare, with few cases documented.2,3
Clinically, skin metastases present most frequently as nodules, either solitary or multiple, that are sometimes ulcerated. However, a wide spectrum of metastases has been described, including erysipeloid, sclerodermiform (en cuirasse), telangiectatic papulovesicles, purpuric plaques mimicking vasculitis, alopecia areata–like scalp lesions, and others. The zosteriform pattern has been described in few cases, including vesiculobullous herpetiform lesions or nodular metastases with metameric distribution.4 In more than half of cases, the lesions were initially interpreted as herpes zoster and hence treated with acyclovir.5
Regarding cutaneous metastases of vulvar carcinoma, a case of metastases mimicking primary varicella-zoster virus infection has been reported,3 but our case represents a zosteriform pattern, which is unique.
Multiple theories have been proposed to explain the pathogenic mechanism of zosteriform spread, though none have been adequately proven.4-7 Some of the patients described in the literature had a history of viral infection by serology or polymerase chain reaction for herpes simplex virus types 1 and 2 and varicella-zoster virus in the same dermatome where metastases were observed. It has been suggested that neural damage caused by the herpesvirus results in an immune function impairment of the overlying skin, which consequently may be more receptive to the inflow of metastatic cells. In such cases, the zosteriform pattern could be due to a Wolf or Köbner phenomenon in an area of vulnerable skin.4-9
Other possible explanations for the zosteriform spread are the invasion of perineural lymphatic vessels or of the dorsal root ganglion, direct invasion from deeper structures, or the surgical implantation of neoplastic cells,4-7 though the latter 2 mechanisms do not correspond to true metastases.
1. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2, pt 1):161-182.
2. Wang AR, O’Brien M, Ross R, et al. Epidermotropic metastasis from vulvar squamous cell carcinoma: a rare cutaneous manifestation. J Am Acad Dermatol. 2010;63:1088-1091.
3. Mailhot J, O’Donnell P, Han R, et al. Metastatic squamous cell carcinoma of the vulva mimicking primary varicella-zoster virus infection. J Am Acad Dermatol. 2011;65:e63-e64.
4. Savoia P, Fava P, Deboli T, et al. Zosteriform cutaneous metastases: a literature meta-analysis and a clinical report of three melanoma cases. Dermatol Surg. 2009;35:1355-1363.
5. Niiyama S, Satoh K, Kaneko S, et al. Zosteriform skin involvement of nodal T-cell lymphoma: a review of the published work of cutaneous malignancies mimicking herpes zoster. J Dermatol. 2007;34:68-73.
6. Zalaudek I, Leinweber B, Richtig E, et al. Cutaneous zosteriform melanoma metastases arising after herpes zoster infection: a case report and review of the literature. Melanoma Res. 2003;13:635-639.
7. LeSueur BW, Abraham LJ, DiCaudo DJ, et al. Zosteriform skin metastases. Int J Dermatol. 2004;43:126-128.
8. Ruocco V, Ruocco E, Brunetti G, et al. Opportunistic localization of skin lesions on vulnerable areas. Clin Dermatol. 2011;29:483-488.
9. Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
The Diagnosis: Skin Metastases of Vulvar Carcinoma
A Tzanck cytodiagnosis was performed and a punch biopsy specimen was obtained for histologic examination. The patient’s Tzanck cytodiagnosis was initially interpreted as a viral cytopathic effect due to the presence of multinucleated atypical cells; hence, she was treated with intravenous acyclovir. The biopsy specimen showed a preserved epidermis and a dense infiltrate in the mid and deep dermis (Figure 1) formed by atypical squamous cells with enlarged nuclei and anisokaryosis, evident nucleoli, atypical mitoses, and multinucleated cells (Figure 2). The histopathologic diagnosis was skin metastases of moderately differentiated squamous cell carcinoma. The subsequent clinical course was unfavorableand the patient died during hospitalization from septic shock.

Figure 1. Histology revealed a preserved epidermis with a dense infiltrate in the mid and deep dermis (H&E, original magnification ×40). | 
Figure 2. Atypical squamous cells with enlarged nuclei and anisokaryosis, evident nucleoli, atypical mitoses, and multinucleated cells were evident (H&E, original magnification ×400). |
Cutaneous metastases occur in 0.7% to 9% of solid tumors in advanced stages of disease progression or occasionally as an initial manifestation; they are a predictor of poor prognosis and breast cancer is the most frequent cause in women.1 Vulvar carcinoma comprises 5% of all malignant neoplasms of the female genital tract and 95% are squamous cell carcinoma; metastases appear most frequently in the inguinal and pelvic lymph nodes, followed by the lungs, liver, and bones.2 Skin metastases are extremely rare, with few cases documented.2,3
Clinically, skin metastases present most frequently as nodules, either solitary or multiple, that are sometimes ulcerated. However, a wide spectrum of metastases has been described, including erysipeloid, sclerodermiform (en cuirasse), telangiectatic papulovesicles, purpuric plaques mimicking vasculitis, alopecia areata–like scalp lesions, and others. The zosteriform pattern has been described in few cases, including vesiculobullous herpetiform lesions or nodular metastases with metameric distribution.4 In more than half of cases, the lesions were initially interpreted as herpes zoster and hence treated with acyclovir.5
Regarding cutaneous metastases of vulvar carcinoma, a case of metastases mimicking primary varicella-zoster virus infection has been reported,3 but our case represents a zosteriform pattern, which is unique.
Multiple theories have been proposed to explain the pathogenic mechanism of zosteriform spread, though none have been adequately proven.4-7 Some of the patients described in the literature had a history of viral infection by serology or polymerase chain reaction for herpes simplex virus types 1 and 2 and varicella-zoster virus in the same dermatome where metastases were observed. It has been suggested that neural damage caused by the herpesvirus results in an immune function impairment of the overlying skin, which consequently may be more receptive to the inflow of metastatic cells. In such cases, the zosteriform pattern could be due to a Wolf or Köbner phenomenon in an area of vulnerable skin.4-9
Other possible explanations for the zosteriform spread are the invasion of perineural lymphatic vessels or of the dorsal root ganglion, direct invasion from deeper structures, or the surgical implantation of neoplastic cells,4-7 though the latter 2 mechanisms do not correspond to true metastases.
The Diagnosis: Skin Metastases of Vulvar Carcinoma
A Tzanck cytodiagnosis was performed and a punch biopsy specimen was obtained for histologic examination. The patient’s Tzanck cytodiagnosis was initially interpreted as a viral cytopathic effect due to the presence of multinucleated atypical cells; hence, she was treated with intravenous acyclovir. The biopsy specimen showed a preserved epidermis and a dense infiltrate in the mid and deep dermis (Figure 1) formed by atypical squamous cells with enlarged nuclei and anisokaryosis, evident nucleoli, atypical mitoses, and multinucleated cells (Figure 2). The histopathologic diagnosis was skin metastases of moderately differentiated squamous cell carcinoma. The subsequent clinical course was unfavorableand the patient died during hospitalization from septic shock.

Figure 1. Histology revealed a preserved epidermis with a dense infiltrate in the mid and deep dermis (H&E, original magnification ×40). | 
Figure 2. Atypical squamous cells with enlarged nuclei and anisokaryosis, evident nucleoli, atypical mitoses, and multinucleated cells were evident (H&E, original magnification ×400). |
Cutaneous metastases occur in 0.7% to 9% of solid tumors in advanced stages of disease progression or occasionally as an initial manifestation; they are a predictor of poor prognosis and breast cancer is the most frequent cause in women.1 Vulvar carcinoma comprises 5% of all malignant neoplasms of the female genital tract and 95% are squamous cell carcinoma; metastases appear most frequently in the inguinal and pelvic lymph nodes, followed by the lungs, liver, and bones.2 Skin metastases are extremely rare, with few cases documented.2,3
Clinically, skin metastases present most frequently as nodules, either solitary or multiple, that are sometimes ulcerated. However, a wide spectrum of metastases has been described, including erysipeloid, sclerodermiform (en cuirasse), telangiectatic papulovesicles, purpuric plaques mimicking vasculitis, alopecia areata–like scalp lesions, and others. The zosteriform pattern has been described in few cases, including vesiculobullous herpetiform lesions or nodular metastases with metameric distribution.4 In more than half of cases, the lesions were initially interpreted as herpes zoster and hence treated with acyclovir.5
Regarding cutaneous metastases of vulvar carcinoma, a case of metastases mimicking primary varicella-zoster virus infection has been reported,3 but our case represents a zosteriform pattern, which is unique.
Multiple theories have been proposed to explain the pathogenic mechanism of zosteriform spread, though none have been adequately proven.4-7 Some of the patients described in the literature had a history of viral infection by serology or polymerase chain reaction for herpes simplex virus types 1 and 2 and varicella-zoster virus in the same dermatome where metastases were observed. It has been suggested that neural damage caused by the herpesvirus results in an immune function impairment of the overlying skin, which consequently may be more receptive to the inflow of metastatic cells. In such cases, the zosteriform pattern could be due to a Wolf or Köbner phenomenon in an area of vulnerable skin.4-9
Other possible explanations for the zosteriform spread are the invasion of perineural lymphatic vessels or of the dorsal root ganglion, direct invasion from deeper structures, or the surgical implantation of neoplastic cells,4-7 though the latter 2 mechanisms do not correspond to true metastases.
1. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2, pt 1):161-182.
2. Wang AR, O’Brien M, Ross R, et al. Epidermotropic metastasis from vulvar squamous cell carcinoma: a rare cutaneous manifestation. J Am Acad Dermatol. 2010;63:1088-1091.
3. Mailhot J, O’Donnell P, Han R, et al. Metastatic squamous cell carcinoma of the vulva mimicking primary varicella-zoster virus infection. J Am Acad Dermatol. 2011;65:e63-e64.
4. Savoia P, Fava P, Deboli T, et al. Zosteriform cutaneous metastases: a literature meta-analysis and a clinical report of three melanoma cases. Dermatol Surg. 2009;35:1355-1363.
5. Niiyama S, Satoh K, Kaneko S, et al. Zosteriform skin involvement of nodal T-cell lymphoma: a review of the published work of cutaneous malignancies mimicking herpes zoster. J Dermatol. 2007;34:68-73.
6. Zalaudek I, Leinweber B, Richtig E, et al. Cutaneous zosteriform melanoma metastases arising after herpes zoster infection: a case report and review of the literature. Melanoma Res. 2003;13:635-639.
7. LeSueur BW, Abraham LJ, DiCaudo DJ, et al. Zosteriform skin metastases. Int J Dermatol. 2004;43:126-128.
8. Ruocco V, Ruocco E, Brunetti G, et al. Opportunistic localization of skin lesions on vulnerable areas. Clin Dermatol. 2011;29:483-488.
9. Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
1. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2, pt 1):161-182.
2. Wang AR, O’Brien M, Ross R, et al. Epidermotropic metastasis from vulvar squamous cell carcinoma: a rare cutaneous manifestation. J Am Acad Dermatol. 2010;63:1088-1091.
3. Mailhot J, O’Donnell P, Han R, et al. Metastatic squamous cell carcinoma of the vulva mimicking primary varicella-zoster virus infection. J Am Acad Dermatol. 2011;65:e63-e64.
4. Savoia P, Fava P, Deboli T, et al. Zosteriform cutaneous metastases: a literature meta-analysis and a clinical report of three melanoma cases. Dermatol Surg. 2009;35:1355-1363.
5. Niiyama S, Satoh K, Kaneko S, et al. Zosteriform skin involvement of nodal T-cell lymphoma: a review of the published work of cutaneous malignancies mimicking herpes zoster. J Dermatol. 2007;34:68-73.
6. Zalaudek I, Leinweber B, Richtig E, et al. Cutaneous zosteriform melanoma metastases arising after herpes zoster infection: a case report and review of the literature. Melanoma Res. 2003;13:635-639.
7. LeSueur BW, Abraham LJ, DiCaudo DJ, et al. Zosteriform skin metastases. Int J Dermatol. 2004;43:126-128.
8. Ruocco V, Ruocco E, Brunetti G, et al. Opportunistic localization of skin lesions on vulnerable areas. Clin Dermatol. 2011;29:483-488.
9. Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
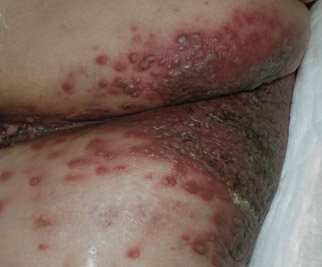
A 48-year-old woman presented with a dermatosis on the left thigh. She had a history of hypertension, obesity, and a stage IIIC vulvar carcinoma treated with radical vulvectomy and bilateral inguinal lymphadenectomy. Two months after surgery she was hospitalized because of a left popliteal deep vein thrombosis and an abscess involving the site of the lymphadenectomy. One month later she presented with a painful dermatosis on the left thigh. Physical examination revealed multiple round-shaped, purple, erythematous tumors with a smooth surface on the upper third of the left thigh extending to the lower abdomen. The tumors measured 0.5 cm in diameter and were grouped to form an indurated plaque on an erythematous base; some tumors were arranged as satellite lesions in the periphery. The dermatosis had a distinct zosteriform appearance.