User login
Where Dysphagia Begins: Polypharmacy and Xerostomia
Xerostomia, the subjective sensation of dry mouth, is a common problem developed by geriatric patients. In practice, xerostomia can impair swallowing, speech, and oral hygiene, and if left unchecked, symptoms such as dysphagia and dysarthria can diminish patients’ quality of life (QOL). Salivary gland hypofunction (SGH) is the objective measure of decreased saliva production, determined by sialometry. Although xerostomia and SGH can coexist, the 2 conditions are not necessarily related.1-4 For this discussion, the term xerostomia will denote dry mouth with or without a concomitant diagnosis of SGH.
Xerostomia is seen in a wide variety of patients with varied comorbidities. It is commonly associated with Sjögren syndrome and head and neck irradiation. The diagnosis and treatment of xerostomia often involves rheumatologists, dentists, otolaryngologists, and oncologists. Additionally, most of the scientific literature about this topic exists in dental journals, such as the Journal of the American Dental Association and the British Dental Journal. Rarer still are studies in the veteran population.5
Faced with increasing time pressure to treat the many chronic diseases affecting aging veterans, health care providers (HCPs) tend to deprioritize diagnosing dry mouth. To that point, saliva is often not considered in the same category as other bodily fluids. According to Mandel, “It lacks the drama of blood, the sincerity of sweat…[and] the emotional appeal of tears.”6 In reality, saliva plays a critical role in the oral-digestive tract and in swallowing. It contains the first digestive enzymes in the gastrointestinal tract and is key for maintaining homeostasis in the oral cavity.7 Decreased saliva production results in difficulties with speech and mastication as well as problems of dysphagia, esophageal dysfunction, dysgeusia, nutritional compromises, new and recurrent dental caries, candidiasis, glossitis, impaired use of dentures, halitosis, and susceptibility to mucosal injury.7,8 Problems with the production of saliva may lead to loss of QOL, such as enjoying a meal or conversing with others.4
Although xerostomia is often associated with advanced age, it is more often explained by the diseases that afflict geriatric patients and the arsenal of medications used to treat them.2,9-16 Polypharmacy, the simultaneous use of multiple drugs by a single patient for ≥ 1 conditions, is an independent risk factor for xerostomia regardless of the types of medication taken.16 From 2005 to 2011, older adults in the US significantly increased their prescription medication use and dietary supplements. More than one-third of older adults used ≥ 5 prescription medications concurrently, and two-thirds of older adults used combinations of prescribed medications, over-the-counter medications, and dietary supplements.17 Several drug classes have the capacity to induce xerostomia, such as antihypertensives, antiulcer agents, anticholinergics, and antidepressants.2,5,12 Prevalence of dry mouth also can range from 10% to 46%, and women typically are more medicated and symptomatic.2,3,9,13,14,16 Xerostomia can also lead to depression and even reduce patients’ will to live.18 Despite xerostomia’s prevalence and impact on QOL, few patients report it as their chief symptom, and few physicians attempt to treat it.19
In order to target polypharmacy as a cause of dry mouth, the objectives for this study were to evaluate (1) the prevalence of xerostomia; (2) the relationship between xerostomia and other oral conditions; and (3) the impact of polypharmacy on dry mouth in a veteran population.
Methods
This is a retrospective cross-sectional study of all outpatient visits in fiscal year (FY) 2015 (October 1, 2014 to September 30, 2015) at the VA Palo Alto Health Care System (VAPAHCS), a tertiary care US Department of Veterans Affairs (VA) hospital. This study was approved by the Stanford University Institutional Review Board. All patients diagnosed with xerostomia in the 1-year study period were identified using ICD-9 diagnosis codes for dry mouth or disturbance of salivary gland secretion (527.7, 527.8, R68.2) and Systemized Nomenclature of Medicine Clinical Terms (SNOMED CT) codes covering dry mouth, xerostomia, aptyalism, absent salivary secretion, and disturbance of salivary secretion (87715008, 78948009). Data analysts in the VA Office of Business Analytics assisted in gathering data from the Veterans Information Systems and Technology Architecture (VistA) electronic health record.
The statistical analysis of that data was performed using Microsoft Excel. Age and gender distributions were determined for the patients. The relationship between xerostomia and the number and types of medications taken by patients also was examined. A previous Swedish study examining the link between dry mouth and quantities of medications used a scale ranging from 0 to ≥ 7 medications.16 The scale for this study was made wider to include the following groups: 0-2, 3-5, 6-8, 9-11, and ≥ 12 medications. Items that do not have xerogenic risks, such as medical supplies (eg, gloves, syringes, etc) and topical medications, were excluded from the analysis. Finally, the number of subjects with comorbid problems with speech, dentition, or swallowing (SDS) was recorded. Non-VA medications were included to capture any self- or externally prescribed xerogenic medications.
Results
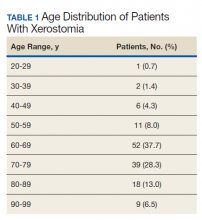
Of the patients seen at VAPAHCS during FY 2015, 138 had a diagnostic code for xerostomia, including 129 men (93.5%) and 9 women (6.5%). The average (SD) age of this xerostomia cohort was 69.3 (12.6) years, and the 3 most common age groups were 60 to 69 years (37.7%), 70 to 79 years (28.3%), and 80 to 89 years (13.0%) (Table 1). Of those 138 patients with a xerostomia diagnosis, a majority (84; 60.9%) had at least 1 documented SDS problem (Table 2). Conversely, during FY 2015, although 4,971 patients seen at VAPAHCS had documented SDS problems, only 77 (1.5%) had a recorded diagnosis of xerostomia
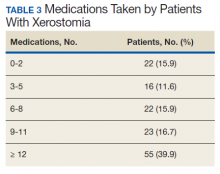
Of the 138 patients with xerostomia, 55 (39.9%) were taking ≥ 12 medications, more than twice as many patients as in any of the other groups studied (0-2, 3-5, 6-8, and 9-11 medications taken) (Table 3). On average, each patient with xerostomia filled prescriptions for 10.4 (SD, 7.2) different drugs. In this cohort of 138 patients diagnosed with xerostomia, antihypertensive medications or analgesics were taken by > 50% of patients, while statins, psychiatric medications, antibiotics, proton pump inhibitors, or drugs known to have anticholinergic activity were taken by > 40%. Antihistamines, anticonvulsants, diuretics, or inhaled respiratory agents were used by > 20% of the patients in this cohort (Table 4).
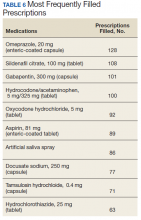
Data on each individual medication were split into 2 categories: the percentage of patients that filled ≥ 1 prescription for that drug, and the total number of prescriptions filled and/or refilled for that drug (ie, including all fills and refills made by individual patients). The 5 most widely used medications in this cohort were omeprazole (39.1%), docusate sodium (29.7%), gabapentin (29.7%), aspirin (27.5%), and hydrocodone/acetaminophen (26.1%) (Table 5). The 5 prescriptions that were cumulatively most filled and/or refilled were omeprazole (128), sildenafil citrate (108), gabapentin (101), hydrocodone/acetaminophen (100), and oxycodone (92) (Table 6). Though sildenafil citrate and oxycodone were among the most-filled prescriptions, these were not included in Table 5 as neither was taken by > 15% of the patients studied. These prescriptions were filled multiple times by a small subset of patients.
Regarding treatment for dry mouth, artificial saliva spray was one of the most widely used (23.2%) and the seventh most-filled prescription within this cohort (86). The only other medication taken by > 15% of patients in a formulation other than a tablet or capsule was chlorhexidine, a germicidal mouthwash used to improve oral care.
Also, 30 (21.7%) patients with a documented xerostomia diagnosis had a history of substance misuse involving use of ≥ 1 of tobacco, alcohol, marijuana, or other illicit drugs.
Discussion
Saliva is an essential component for the maintenance of normal oral health.20,21 Decreased saliva production causes problems, including difficulties with speech, mastication, dysphagia, changes in taste, dental caries, impaired use of prostheses, recurrent infections, halitosis, deterioration of soft tissues, and compromised QOL.22,23 Among patients with a diagnosed SDS abnormality who were seen at this facility during FY 2015, the prevalence of xerostomia was only 1.5%. However, the true prevalence and incidence of xerostomia among veterans is not known. Given the role of xerostomia as a common risk factor for SDS problems and the polypharmacy exhibited by those presented here with SDS problems, it is probable that xerostomia was underreported in this veteran cohort.
Additionally, although salivary acinar cells are known to atrophy with age, as is consistent with this xerostomia cohort’s average age (SD) of 69.3 (12.6) years, the development of dry mouth is a multifactorial process. The current scientific literature asserts that most salivary loss is due to local and systemic diseases, immunologic disorders, external radiation, and as was highlighted by this study, multiple prescription and nonprescription medications.24-26
It has also been demonstrated previously that dry mouth complaints and low salivary flow rates are directly proportional to the number of medications taken by patients.2,27-30 Polypharmacy is therefore an area of great interest, and ≥ 40 categories of xerogenic medications have been identified by investigators such as Sreebny and Schwartz.31 Among those, some of the most xerogenic medication classes include antihypertensives, antiulcer agents, anticholinergics, and antidepressants, are all very commonly consumed in this cohort of patients with dry mouth (58.7%, 42.0%, 47.1%, and 38.4%, respectively). The medication regimens within this cohort of veterans with xerostomia were prime examples of polypharmacy as each patient took an average (SD) of 10.4 (7.2) medications, 39.9% took ≥ 12, and 72.5% of patients with xerostomia were taking ≥ 6 prescription drugs during a 1-year period.
Given the dangers of polypharmacy, a more conservative approach to prescribing medications could feasibly help with preventing xerostomia and SGH. In practice, while clinicians try to avoid prescribing anticholinergics, antimuscarinics, and antihistaminergic drugs for geriatric patients, they are tasked with the complex management of medication adverse effects (AEs) when dealing with multiple health conditions. The clinicians’ primary responsibilities are to follow the standard of care and not to introduce unnecessary harm when managing patients, but they also must push for, stay abreast of, and conduct more basic research and clinical trials to inform, adjust, and improve our current standard.
Research into polypharmacy and its role in inducing dry mouth is ongoing. Twenty years ago, Thomson and colleagues identified reduced salivary flow in patients who used antianginals, thyroxine, diuretics, antidepressants, and medications for asthma, while only 5 years earlier Loesche and colleagues reported the role of antiulcer medications, such as proton pump inhibitors, in the development of xerostomia.2,32 Within the past 5 years, Viljakainen and colleagues and Ohara and colleagues have echoed some of those findings by identifying associations between xerostomia and agents that impact digestive organs.33,34 A strong association recently was identified between the use of antipsychotic drugs and xerostomia.35 Additionally, when attributing xerostomia to polypharmacy, the interaction between medications is often overlooked in favor of considering the raw number of prescriptions taken. Whereas 1 medication alone may not have drying properties, combinations of medications might be more likely to induce xerostomia. Thomson and colleagues suggested such a situation regarding the interaction between thyroxine and diuretics.36 Future studies should focus on identifying viable substitutes for existing medications that reduce risk for xerostomia without compromising the management of other serious conditions.
Treatment
Another pressing question for clinicians concerns artificial saliva. Although 23.2% of patients with dry mouth in this xerostomia cohort used artificial saliva, the efficacy of this treatment is still unproven. Saliva substitutes are often used by patients who cannot produce sufficient amounts of natural saliva. In practice, artificial saliva produces, at best, modest temporary improvement in dry mouth symptoms in up to 40% of patients. At worst, as put forth by the Cochrane Review, artificial saliva may be no better than placebo in treating dry mouth.37,38 The volumes needed for symptom relief are large, ranging between 40 mL and 150 mL per day depending on the substitute’s composition. Saliva substitutes also must be reapplied throughout each day. This is particularly bothersome when patients must wake up repeatedly to reapply the treatment at night.37 In short, these substances do not seem wholly effective in managing dry mouth, and other options must be made available to patients with refractory xerostomia when artificial saliva and lifestyle modifications fail.
For now, few alternatives exist. Chewing gums and lozenges help to stimulate salivary flow, as do muscarinic agonists like pilocarpine. Unfortunately, muscarinic agonists are seldom used due to cholinergic AEs. Humidifiers are effective in increasing nighttime moisture but are contraindicated in patients with dust mite allergies.39 Reservoir-based devices with automated pumps funnel water and/or salivary substitutes from a fanny pack into patients’ mouths for lubrication.37 Other more esoteric pharmacologic treatments include D-limonene, yohimbine, and amifostine, which purportedly protect salivary progenitor cells, increase peripheral cholinergic activity, and protect salivary glands from free-radical damage during radiation treatment, respectively. Although these agents have shown some promise, D-limonene is difficult to administer given the high dosage required for treatment, yohimbine hasn’t been seriously investigated for improving salivary secretion since 1997, and amifostine isn’t used widely due to its AE profile despite its US Food and Drug Administration approval for prevention of xerostomia.39
Substance Abuse
The impact of smoking on xerostomia remains controversial. Some studies report an association between active smoking and xerostomia; others suggest that the local irritant effect of tobacco smoke may increase salivary gland output.40,41 The same may be true for chronic alcohol use as there are no epidemiologic studies showing a causal relationship between alcohol use and xerostomia. Studies with rats that are chronically exposed to ethanol have found increased salivary flow rates.42 In the xerostomia cohort presented here, 30 patients (21.7%) had a documented history of substance misuse. That percentage is likely underestimated, as substance misuse is often underreported, and frequent use may not always constitute misuse. Therefore, nicotine exposure, alcohol exposure, illicit drug use, and vaping all should be considered during the workup of a patient with xerostomia.
Limitations
It is common for medications to remain in a patient’s health record long after that patient stops taking them. Developing methods to track when patients discontinue their prescriptions will be essential for clearing up uncertainty in our data and in other similar studies. This study also did not account for patients’ medication adherence and the duration of exposure to medications and illicit substances. Furthermore, the results of this veteran study are not easily generalizable as this cohort is disproportionately male, of advanced age, and especially prone to exhibiting both substance use and psychiatric diagnoses relative to the general population. As described by Viljakainen and colleagues, risk factors for xerostomia include advanced age, female gender, low body mass index (BMI), malnutrition, and depressive symptoms, but because the demographic scope of this veteran population was narrow, it was not possible to discern the impact of, for example, gender.33 Data on variables like BMI, malnutrition, and depressive symptoms were not available. For this study, xerostomia could only be considered as an all-or-nothing phenomenon because the dataset did not describe different levels of dry mouth severity (eg, mild, moderate, severe).
Additionally, past polypharmacy studies have acknowledged an inability to tell whether xerostomia is mainly due to medications or to underlying medical conditions. For example, for emphysema, ß-adrenergic stimulation from bronchodilators could cause dry mouth by thickening saliva and decreasing salivary volume, but the pathophysiology and/or cardinal symptoms of emphysema, including chronic obstructive pulmonary disease-associated tachypnea, might contribute independently to dryness.
Though we can make inferences based on the medications taken by this cohort (eg, those taking antihypertensives have high blood pressure), this dataset did not explicitly detail comorbid conditions and ICD codes for chronic diseases that commonly arise with xerostomia. Those conditions, however, are of great clinical importance. Diabetes mellitus, HIV/AIDS, and, classically, Sjögren syndrome, all are known to cause dry mouth.43 Identifying new conditions that co-occur with xerostomia would allow clinicians to describe the root causes of and risk factors for dry mouth and SDS conditions in greater detail. Patients with dry mouth without SDS problems in this dataset are of particular interest as closer examination of their medications and comorbid conditions could help us understand why some individuals and not others develop SDS problems. The subjects of how comorbidities contribute to dry mouth and how their influences can be judged independently from the effects of medications are of great interest to us and will be investigated rigorously in our future studies.
Conclusions
In this cohort, few patients with SDS problems had documentation of a concomitant xerostomia diagnosis. This could represent a true infrequency of dry mouth or more likely, an underrecognition by clinicians. Heightened physician awareness regarding the signs and symptoms of and risk factors for xerostomia is needed to improve providers’ ability to diagnose this condition.
In particular, polypharmacy should be a major consideration when evaluating patients for xerostomia. This continues to be an important area of research, and some of the latest data on polypharmacy among older patients were compiled in a recent meta-analysis by Tan and colleagues. The authors of that systematic review reiterated the significant association between salivary gland hypofunction and the number of medications taken by patients. They also advocated for the creation of a risk score for medication-induced dry mouth to aid in medication management.44 Per their recommendations, it is now as crucial as ever to consider the numbers and types of medications taken by patients, to discontinue unnecessary prescriptions when possible, and to continue developing new strategies for preventing and treating xerostomia.
1. Thomson WM, Chalmers JM, Spencer AJ, Ketabi M. The occurrence of xerostomia and salivary gland hypofunction in a population-based sample of older South Australians. Spec Care Dentist. 1999;19(1):20-23.
2. Thomson WM, Chalmers JM, Spencer AJ, Slade GD. Medication and dry mouth: findings from a cohort study of older people. J Public Health Dent. 2000;60(1):12-20.
3. Sasportas LS, Hosford DN, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
4. Bivona PL. Xerostomia. A common problem among the elderly. N Y State Dent J. 1998;64(6):46-52.
5. Ness J, Hoth A, Barnett MJ, Shorr RI, Kaboli PJ. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4(1):42-51.
6. Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19(3):119-125.
7. Friedman PK, Isfeld D. Xerostomia: the “invisible” oral health condition. J Mass Dent Soc. 2008;57(3):42-44.
8. Ship JA, McCutcheon JA, Spivakovsky S, Kerr AR. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. J Oral Rehabil. 2007;34(10):724-732.
9. Field EA, Fear S, Higham SM, et al. Age and medication are significant risk factors for xerostomia in an English population, attending general dental practice. Gerodontology. 2001;18(1):21-24.
10. Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag. 2015;11:45-51.
11. Geuiros LA, Soares MS, Leao JC. Impact of ageing and drug consumption on oral health. Gerodontology. 2009;26(4):297-301.
12. Singh ML, Papas A. Oral implications of polypharmacy in the elderly. Dent Clin North Am. 2014;58(4):783-796.
13. Shinkai RS, Hatch JP, Schmidt CB, Sartori EA. Exposure to the oral side effects of medication in a community-based sample. Spec Care Dentist. 2006;26(3):116-120.
14. Hopcraft MS, Tan C. Xerostomia: an update for clinicians. Aust Dent J. 2010;55(3):238-244; quiz 353.
15. Ettinger RL. Review: xerostomia: a symptom which acts like a disease. Age Ageing. 1996;25(5):409-412.
16. Nederfors T, Isaksson R, Mornstad H, Dahlof C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population—relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997;25(3):211-216.
17. Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
18. Dirix P, Nuyts S, Vander Poorten V, Delaere P, Van den Boaert W. The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer. Support Care Cancer. 2008;16(2):171-179.
19. Sreebny LM, Valdini A. Xerostomia. A neglected symptom. Arch Intern Med. 1987;147(7):1333-1337.
20. Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50(3):140-161.
21. Amerongen AV, Veeran EC. Saliva—the defender of the oral cavity. Oral Dis. 2002;8(1):12-22.
22. Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134(1):61-69; quiz 118-119.
23. Atkinson JC, Baum BJ. Salivary enhancement: current status and future therapies. J Dent Educ. 2001;65(10):1096-1101.
24. Narhi TO, Meurman JH, Ainamo A. Xerostomia and hyposalivation: causes, consequences and treatment in the elderly. Drugs Aging. 1999;15(2):103-116.
25. Ship JA, Baum BJ. Is reduced salivary flow normal in old people? Lancet. 1990;336(8729):1507.
26. Ghezzi EM, Wagner-Lange LA, Schork MA, et al. Longitudinal influence of age, menopause, hormone replacement therapy, and other medications on parotid flow rates in healthy women. J Gerontol A Biol Sci Med Sci. 2000;55(1):M34-M42.
27. Fox PC. Acquired salivary dysfunction. Drugs and radiation. Ann N Y Acad Sci. 1998;842:132-137.
28. Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res. 2000;79(9):1652-1658.
29. Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50(3):535-543.
30. Sreebny LM, Valdini A, Yu A. Xerostomia. Part II: Relationship to nonoral symptoms, drugs, and diseases. Oral Surg Oral Med Oral Pathol. 1989;68(4):419-427.
31. Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth—2nd edition. Gerodontology. 1997;14(1):33-47.
32. Loesche WJ, Bromberg J, Terpenning MS, et al. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J Am Geriatr Soc. 1995;43(4):401-407.

33. Viljakainen S, Nykanen I, Ahonen R, et al. Xerostomia among older home care clients. Community Dent Oral Epidemiol. 2016;44(3):232-238.
34. Ohara Y, Hirano H, Yoshida H, et al. Prevalence and factors associated with xerostomia and hyposalivation among community-dwelling older people in Japan. Gerodontology. 2016;33(1):20-27.
35. Okamoto A, Miyachi H, Tanaka K, Chikazu D, Miyaoka H. Relationship between xerostomia and psychotropic drugs in patients with schizophrenia: evaluation using an oral moisture meter. J Clin Pharm Ther. 2016;41(6):684-688.
36. Thomson WM. Dry mouth and older people. Aust Dent J. 2015;60(suppl 1):54-63.
37. Sasportas LS, Hosford AT, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
38. Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev. 2011;(12):CD008924.
39. Roblegg E, Coughran A, Sirjani D. Saliva: an all-rounder of our body. Eur J Pharm Biopharm. 2019;142:133-141.
40. Billings RJ, Proskin HM, Moss ME. Xerostomia and associated factors in a community-dwelling adult population. Community Dent Oral Epidemiol. 1996;24(5):312-316.
41. Norlen P, Ostberg H, Bjorn AL. Relationship between general health, social factors and oral health in women at the age of retirement. Community Dent Oral Epidemiol. 1991;19(5):296-301.
42. Berry MR, Scott J. Functional and structural adaptation of the parotid gland to medium-term chronic ethanol exposure in the rat. Alcohol Alcoholism. 1990;25(5):523-531.
43. von Bultzingslowen I, Sollecito TP, Fox PC, et al. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2007;103:S57.e1-e15.
44. Tan ECK, Lexomboon D, Sandborgh-Englund G, et al. Medications that cause dry mouth as an adverse effect in older people: a systematic review and metaanalysis. J Am Geriatr Soc. 2018;66(1):76-84.
Xerostomia, the subjective sensation of dry mouth, is a common problem developed by geriatric patients. In practice, xerostomia can impair swallowing, speech, and oral hygiene, and if left unchecked, symptoms such as dysphagia and dysarthria can diminish patients’ quality of life (QOL). Salivary gland hypofunction (SGH) is the objective measure of decreased saliva production, determined by sialometry. Although xerostomia and SGH can coexist, the 2 conditions are not necessarily related.1-4 For this discussion, the term xerostomia will denote dry mouth with or without a concomitant diagnosis of SGH.
Xerostomia is seen in a wide variety of patients with varied comorbidities. It is commonly associated with Sjögren syndrome and head and neck irradiation. The diagnosis and treatment of xerostomia often involves rheumatologists, dentists, otolaryngologists, and oncologists. Additionally, most of the scientific literature about this topic exists in dental journals, such as the Journal of the American Dental Association and the British Dental Journal. Rarer still are studies in the veteran population.5
Faced with increasing time pressure to treat the many chronic diseases affecting aging veterans, health care providers (HCPs) tend to deprioritize diagnosing dry mouth. To that point, saliva is often not considered in the same category as other bodily fluids. According to Mandel, “It lacks the drama of blood, the sincerity of sweat…[and] the emotional appeal of tears.”6 In reality, saliva plays a critical role in the oral-digestive tract and in swallowing. It contains the first digestive enzymes in the gastrointestinal tract and is key for maintaining homeostasis in the oral cavity.7 Decreased saliva production results in difficulties with speech and mastication as well as problems of dysphagia, esophageal dysfunction, dysgeusia, nutritional compromises, new and recurrent dental caries, candidiasis, glossitis, impaired use of dentures, halitosis, and susceptibility to mucosal injury.7,8 Problems with the production of saliva may lead to loss of QOL, such as enjoying a meal or conversing with others.4
Although xerostomia is often associated with advanced age, it is more often explained by the diseases that afflict geriatric patients and the arsenal of medications used to treat them.2,9-16 Polypharmacy, the simultaneous use of multiple drugs by a single patient for ≥ 1 conditions, is an independent risk factor for xerostomia regardless of the types of medication taken.16 From 2005 to 2011, older adults in the US significantly increased their prescription medication use and dietary supplements. More than one-third of older adults used ≥ 5 prescription medications concurrently, and two-thirds of older adults used combinations of prescribed medications, over-the-counter medications, and dietary supplements.17 Several drug classes have the capacity to induce xerostomia, such as antihypertensives, antiulcer agents, anticholinergics, and antidepressants.2,5,12 Prevalence of dry mouth also can range from 10% to 46%, and women typically are more medicated and symptomatic.2,3,9,13,14,16 Xerostomia can also lead to depression and even reduce patients’ will to live.18 Despite xerostomia’s prevalence and impact on QOL, few patients report it as their chief symptom, and few physicians attempt to treat it.19
In order to target polypharmacy as a cause of dry mouth, the objectives for this study were to evaluate (1) the prevalence of xerostomia; (2) the relationship between xerostomia and other oral conditions; and (3) the impact of polypharmacy on dry mouth in a veteran population.
Methods
This is a retrospective cross-sectional study of all outpatient visits in fiscal year (FY) 2015 (October 1, 2014 to September 30, 2015) at the VA Palo Alto Health Care System (VAPAHCS), a tertiary care US Department of Veterans Affairs (VA) hospital. This study was approved by the Stanford University Institutional Review Board. All patients diagnosed with xerostomia in the 1-year study period were identified using ICD-9 diagnosis codes for dry mouth or disturbance of salivary gland secretion (527.7, 527.8, R68.2) and Systemized Nomenclature of Medicine Clinical Terms (SNOMED CT) codes covering dry mouth, xerostomia, aptyalism, absent salivary secretion, and disturbance of salivary secretion (87715008, 78948009). Data analysts in the VA Office of Business Analytics assisted in gathering data from the Veterans Information Systems and Technology Architecture (VistA) electronic health record.
The statistical analysis of that data was performed using Microsoft Excel. Age and gender distributions were determined for the patients. The relationship between xerostomia and the number and types of medications taken by patients also was examined. A previous Swedish study examining the link between dry mouth and quantities of medications used a scale ranging from 0 to ≥ 7 medications.16 The scale for this study was made wider to include the following groups: 0-2, 3-5, 6-8, 9-11, and ≥ 12 medications. Items that do not have xerogenic risks, such as medical supplies (eg, gloves, syringes, etc) and topical medications, were excluded from the analysis. Finally, the number of subjects with comorbid problems with speech, dentition, or swallowing (SDS) was recorded. Non-VA medications were included to capture any self- or externally prescribed xerogenic medications.
Results
Of the patients seen at VAPAHCS during FY 2015, 138 had a diagnostic code for xerostomia, including 129 men (93.5%) and 9 women (6.5%). The average (SD) age of this xerostomia cohort was 69.3 (12.6) years, and the 3 most common age groups were 60 to 69 years (37.7%), 70 to 79 years (28.3%), and 80 to 89 years (13.0%) (Table 1). Of those 138 patients with a xerostomia diagnosis, a majority (84; 60.9%) had at least 1 documented SDS problem (Table 2). Conversely, during FY 2015, although 4,971 patients seen at VAPAHCS had documented SDS problems, only 77 (1.5%) had a recorded diagnosis of xerostomia
Of the 138 patients with xerostomia, 55 (39.9%) were taking ≥ 12 medications, more than twice as many patients as in any of the other groups studied (0-2, 3-5, 6-8, and 9-11 medications taken) (Table 3). On average, each patient with xerostomia filled prescriptions for 10.4 (SD, 7.2) different drugs. In this cohort of 138 patients diagnosed with xerostomia, antihypertensive medications or analgesics were taken by > 50% of patients, while statins, psychiatric medications, antibiotics, proton pump inhibitors, or drugs known to have anticholinergic activity were taken by > 40%. Antihistamines, anticonvulsants, diuretics, or inhaled respiratory agents were used by > 20% of the patients in this cohort (Table 4).
Data on each individual medication were split into 2 categories: the percentage of patients that filled ≥ 1 prescription for that drug, and the total number of prescriptions filled and/or refilled for that drug (ie, including all fills and refills made by individual patients). The 5 most widely used medications in this cohort were omeprazole (39.1%), docusate sodium (29.7%), gabapentin (29.7%), aspirin (27.5%), and hydrocodone/acetaminophen (26.1%) (Table 5). The 5 prescriptions that were cumulatively most filled and/or refilled were omeprazole (128), sildenafil citrate (108), gabapentin (101), hydrocodone/acetaminophen (100), and oxycodone (92) (Table 6). Though sildenafil citrate and oxycodone were among the most-filled prescriptions, these were not included in Table 5 as neither was taken by > 15% of the patients studied. These prescriptions were filled multiple times by a small subset of patients.
Regarding treatment for dry mouth, artificial saliva spray was one of the most widely used (23.2%) and the seventh most-filled prescription within this cohort (86). The only other medication taken by > 15% of patients in a formulation other than a tablet or capsule was chlorhexidine, a germicidal mouthwash used to improve oral care.
Also, 30 (21.7%) patients with a documented xerostomia diagnosis had a history of substance misuse involving use of ≥ 1 of tobacco, alcohol, marijuana, or other illicit drugs.
Discussion
Saliva is an essential component for the maintenance of normal oral health.20,21 Decreased saliva production causes problems, including difficulties with speech, mastication, dysphagia, changes in taste, dental caries, impaired use of prostheses, recurrent infections, halitosis, deterioration of soft tissues, and compromised QOL.22,23 Among patients with a diagnosed SDS abnormality who were seen at this facility during FY 2015, the prevalence of xerostomia was only 1.5%. However, the true prevalence and incidence of xerostomia among veterans is not known. Given the role of xerostomia as a common risk factor for SDS problems and the polypharmacy exhibited by those presented here with SDS problems, it is probable that xerostomia was underreported in this veteran cohort.
Additionally, although salivary acinar cells are known to atrophy with age, as is consistent with this xerostomia cohort’s average age (SD) of 69.3 (12.6) years, the development of dry mouth is a multifactorial process. The current scientific literature asserts that most salivary loss is due to local and systemic diseases, immunologic disorders, external radiation, and as was highlighted by this study, multiple prescription and nonprescription medications.24-26
It has also been demonstrated previously that dry mouth complaints and low salivary flow rates are directly proportional to the number of medications taken by patients.2,27-30 Polypharmacy is therefore an area of great interest, and ≥ 40 categories of xerogenic medications have been identified by investigators such as Sreebny and Schwartz.31 Among those, some of the most xerogenic medication classes include antihypertensives, antiulcer agents, anticholinergics, and antidepressants, are all very commonly consumed in this cohort of patients with dry mouth (58.7%, 42.0%, 47.1%, and 38.4%, respectively). The medication regimens within this cohort of veterans with xerostomia were prime examples of polypharmacy as each patient took an average (SD) of 10.4 (7.2) medications, 39.9% took ≥ 12, and 72.5% of patients with xerostomia were taking ≥ 6 prescription drugs during a 1-year period.
Given the dangers of polypharmacy, a more conservative approach to prescribing medications could feasibly help with preventing xerostomia and SGH. In practice, while clinicians try to avoid prescribing anticholinergics, antimuscarinics, and antihistaminergic drugs for geriatric patients, they are tasked with the complex management of medication adverse effects (AEs) when dealing with multiple health conditions. The clinicians’ primary responsibilities are to follow the standard of care and not to introduce unnecessary harm when managing patients, but they also must push for, stay abreast of, and conduct more basic research and clinical trials to inform, adjust, and improve our current standard.
Research into polypharmacy and its role in inducing dry mouth is ongoing. Twenty years ago, Thomson and colleagues identified reduced salivary flow in patients who used antianginals, thyroxine, diuretics, antidepressants, and medications for asthma, while only 5 years earlier Loesche and colleagues reported the role of antiulcer medications, such as proton pump inhibitors, in the development of xerostomia.2,32 Within the past 5 years, Viljakainen and colleagues and Ohara and colleagues have echoed some of those findings by identifying associations between xerostomia and agents that impact digestive organs.33,34 A strong association recently was identified between the use of antipsychotic drugs and xerostomia.35 Additionally, when attributing xerostomia to polypharmacy, the interaction between medications is often overlooked in favor of considering the raw number of prescriptions taken. Whereas 1 medication alone may not have drying properties, combinations of medications might be more likely to induce xerostomia. Thomson and colleagues suggested such a situation regarding the interaction between thyroxine and diuretics.36 Future studies should focus on identifying viable substitutes for existing medications that reduce risk for xerostomia without compromising the management of other serious conditions.
Treatment
Another pressing question for clinicians concerns artificial saliva. Although 23.2% of patients with dry mouth in this xerostomia cohort used artificial saliva, the efficacy of this treatment is still unproven. Saliva substitutes are often used by patients who cannot produce sufficient amounts of natural saliva. In practice, artificial saliva produces, at best, modest temporary improvement in dry mouth symptoms in up to 40% of patients. At worst, as put forth by the Cochrane Review, artificial saliva may be no better than placebo in treating dry mouth.37,38 The volumes needed for symptom relief are large, ranging between 40 mL and 150 mL per day depending on the substitute’s composition. Saliva substitutes also must be reapplied throughout each day. This is particularly bothersome when patients must wake up repeatedly to reapply the treatment at night.37 In short, these substances do not seem wholly effective in managing dry mouth, and other options must be made available to patients with refractory xerostomia when artificial saliva and lifestyle modifications fail.
For now, few alternatives exist. Chewing gums and lozenges help to stimulate salivary flow, as do muscarinic agonists like pilocarpine. Unfortunately, muscarinic agonists are seldom used due to cholinergic AEs. Humidifiers are effective in increasing nighttime moisture but are contraindicated in patients with dust mite allergies.39 Reservoir-based devices with automated pumps funnel water and/or salivary substitutes from a fanny pack into patients’ mouths for lubrication.37 Other more esoteric pharmacologic treatments include D-limonene, yohimbine, and amifostine, which purportedly protect salivary progenitor cells, increase peripheral cholinergic activity, and protect salivary glands from free-radical damage during radiation treatment, respectively. Although these agents have shown some promise, D-limonene is difficult to administer given the high dosage required for treatment, yohimbine hasn’t been seriously investigated for improving salivary secretion since 1997, and amifostine isn’t used widely due to its AE profile despite its US Food and Drug Administration approval for prevention of xerostomia.39
Substance Abuse
The impact of smoking on xerostomia remains controversial. Some studies report an association between active smoking and xerostomia; others suggest that the local irritant effect of tobacco smoke may increase salivary gland output.40,41 The same may be true for chronic alcohol use as there are no epidemiologic studies showing a causal relationship between alcohol use and xerostomia. Studies with rats that are chronically exposed to ethanol have found increased salivary flow rates.42 In the xerostomia cohort presented here, 30 patients (21.7%) had a documented history of substance misuse. That percentage is likely underestimated, as substance misuse is often underreported, and frequent use may not always constitute misuse. Therefore, nicotine exposure, alcohol exposure, illicit drug use, and vaping all should be considered during the workup of a patient with xerostomia.
Limitations
It is common for medications to remain in a patient’s health record long after that patient stops taking them. Developing methods to track when patients discontinue their prescriptions will be essential for clearing up uncertainty in our data and in other similar studies. This study also did not account for patients’ medication adherence and the duration of exposure to medications and illicit substances. Furthermore, the results of this veteran study are not easily generalizable as this cohort is disproportionately male, of advanced age, and especially prone to exhibiting both substance use and psychiatric diagnoses relative to the general population. As described by Viljakainen and colleagues, risk factors for xerostomia include advanced age, female gender, low body mass index (BMI), malnutrition, and depressive symptoms, but because the demographic scope of this veteran population was narrow, it was not possible to discern the impact of, for example, gender.33 Data on variables like BMI, malnutrition, and depressive symptoms were not available. For this study, xerostomia could only be considered as an all-or-nothing phenomenon because the dataset did not describe different levels of dry mouth severity (eg, mild, moderate, severe).
Additionally, past polypharmacy studies have acknowledged an inability to tell whether xerostomia is mainly due to medications or to underlying medical conditions. For example, for emphysema, ß-adrenergic stimulation from bronchodilators could cause dry mouth by thickening saliva and decreasing salivary volume, but the pathophysiology and/or cardinal symptoms of emphysema, including chronic obstructive pulmonary disease-associated tachypnea, might contribute independently to dryness.
Though we can make inferences based on the medications taken by this cohort (eg, those taking antihypertensives have high blood pressure), this dataset did not explicitly detail comorbid conditions and ICD codes for chronic diseases that commonly arise with xerostomia. Those conditions, however, are of great clinical importance. Diabetes mellitus, HIV/AIDS, and, classically, Sjögren syndrome, all are known to cause dry mouth.43 Identifying new conditions that co-occur with xerostomia would allow clinicians to describe the root causes of and risk factors for dry mouth and SDS conditions in greater detail. Patients with dry mouth without SDS problems in this dataset are of particular interest as closer examination of their medications and comorbid conditions could help us understand why some individuals and not others develop SDS problems. The subjects of how comorbidities contribute to dry mouth and how their influences can be judged independently from the effects of medications are of great interest to us and will be investigated rigorously in our future studies.
Conclusions
In this cohort, few patients with SDS problems had documentation of a concomitant xerostomia diagnosis. This could represent a true infrequency of dry mouth or more likely, an underrecognition by clinicians. Heightened physician awareness regarding the signs and symptoms of and risk factors for xerostomia is needed to improve providers’ ability to diagnose this condition.
In particular, polypharmacy should be a major consideration when evaluating patients for xerostomia. This continues to be an important area of research, and some of the latest data on polypharmacy among older patients were compiled in a recent meta-analysis by Tan and colleagues. The authors of that systematic review reiterated the significant association between salivary gland hypofunction and the number of medications taken by patients. They also advocated for the creation of a risk score for medication-induced dry mouth to aid in medication management.44 Per their recommendations, it is now as crucial as ever to consider the numbers and types of medications taken by patients, to discontinue unnecessary prescriptions when possible, and to continue developing new strategies for preventing and treating xerostomia.
Xerostomia, the subjective sensation of dry mouth, is a common problem developed by geriatric patients. In practice, xerostomia can impair swallowing, speech, and oral hygiene, and if left unchecked, symptoms such as dysphagia and dysarthria can diminish patients’ quality of life (QOL). Salivary gland hypofunction (SGH) is the objective measure of decreased saliva production, determined by sialometry. Although xerostomia and SGH can coexist, the 2 conditions are not necessarily related.1-4 For this discussion, the term xerostomia will denote dry mouth with or without a concomitant diagnosis of SGH.
Xerostomia is seen in a wide variety of patients with varied comorbidities. It is commonly associated with Sjögren syndrome and head and neck irradiation. The diagnosis and treatment of xerostomia often involves rheumatologists, dentists, otolaryngologists, and oncologists. Additionally, most of the scientific literature about this topic exists in dental journals, such as the Journal of the American Dental Association and the British Dental Journal. Rarer still are studies in the veteran population.5
Faced with increasing time pressure to treat the many chronic diseases affecting aging veterans, health care providers (HCPs) tend to deprioritize diagnosing dry mouth. To that point, saliva is often not considered in the same category as other bodily fluids. According to Mandel, “It lacks the drama of blood, the sincerity of sweat…[and] the emotional appeal of tears.”6 In reality, saliva plays a critical role in the oral-digestive tract and in swallowing. It contains the first digestive enzymes in the gastrointestinal tract and is key for maintaining homeostasis in the oral cavity.7 Decreased saliva production results in difficulties with speech and mastication as well as problems of dysphagia, esophageal dysfunction, dysgeusia, nutritional compromises, new and recurrent dental caries, candidiasis, glossitis, impaired use of dentures, halitosis, and susceptibility to mucosal injury.7,8 Problems with the production of saliva may lead to loss of QOL, such as enjoying a meal or conversing with others.4
Although xerostomia is often associated with advanced age, it is more often explained by the diseases that afflict geriatric patients and the arsenal of medications used to treat them.2,9-16 Polypharmacy, the simultaneous use of multiple drugs by a single patient for ≥ 1 conditions, is an independent risk factor for xerostomia regardless of the types of medication taken.16 From 2005 to 2011, older adults in the US significantly increased their prescription medication use and dietary supplements. More than one-third of older adults used ≥ 5 prescription medications concurrently, and two-thirds of older adults used combinations of prescribed medications, over-the-counter medications, and dietary supplements.17 Several drug classes have the capacity to induce xerostomia, such as antihypertensives, antiulcer agents, anticholinergics, and antidepressants.2,5,12 Prevalence of dry mouth also can range from 10% to 46%, and women typically are more medicated and symptomatic.2,3,9,13,14,16 Xerostomia can also lead to depression and even reduce patients’ will to live.18 Despite xerostomia’s prevalence and impact on QOL, few patients report it as their chief symptom, and few physicians attempt to treat it.19
In order to target polypharmacy as a cause of dry mouth, the objectives for this study were to evaluate (1) the prevalence of xerostomia; (2) the relationship between xerostomia and other oral conditions; and (3) the impact of polypharmacy on dry mouth in a veteran population.
Methods
This is a retrospective cross-sectional study of all outpatient visits in fiscal year (FY) 2015 (October 1, 2014 to September 30, 2015) at the VA Palo Alto Health Care System (VAPAHCS), a tertiary care US Department of Veterans Affairs (VA) hospital. This study was approved by the Stanford University Institutional Review Board. All patients diagnosed with xerostomia in the 1-year study period were identified using ICD-9 diagnosis codes for dry mouth or disturbance of salivary gland secretion (527.7, 527.8, R68.2) and Systemized Nomenclature of Medicine Clinical Terms (SNOMED CT) codes covering dry mouth, xerostomia, aptyalism, absent salivary secretion, and disturbance of salivary secretion (87715008, 78948009). Data analysts in the VA Office of Business Analytics assisted in gathering data from the Veterans Information Systems and Technology Architecture (VistA) electronic health record.
The statistical analysis of that data was performed using Microsoft Excel. Age and gender distributions were determined for the patients. The relationship between xerostomia and the number and types of medications taken by patients also was examined. A previous Swedish study examining the link between dry mouth and quantities of medications used a scale ranging from 0 to ≥ 7 medications.16 The scale for this study was made wider to include the following groups: 0-2, 3-5, 6-8, 9-11, and ≥ 12 medications. Items that do not have xerogenic risks, such as medical supplies (eg, gloves, syringes, etc) and topical medications, were excluded from the analysis. Finally, the number of subjects with comorbid problems with speech, dentition, or swallowing (SDS) was recorded. Non-VA medications were included to capture any self- or externally prescribed xerogenic medications.
Results
Of the patients seen at VAPAHCS during FY 2015, 138 had a diagnostic code for xerostomia, including 129 men (93.5%) and 9 women (6.5%). The average (SD) age of this xerostomia cohort was 69.3 (12.6) years, and the 3 most common age groups were 60 to 69 years (37.7%), 70 to 79 years (28.3%), and 80 to 89 years (13.0%) (Table 1). Of those 138 patients with a xerostomia diagnosis, a majority (84; 60.9%) had at least 1 documented SDS problem (Table 2). Conversely, during FY 2015, although 4,971 patients seen at VAPAHCS had documented SDS problems, only 77 (1.5%) had a recorded diagnosis of xerostomia
Of the 138 patients with xerostomia, 55 (39.9%) were taking ≥ 12 medications, more than twice as many patients as in any of the other groups studied (0-2, 3-5, 6-8, and 9-11 medications taken) (Table 3). On average, each patient with xerostomia filled prescriptions for 10.4 (SD, 7.2) different drugs. In this cohort of 138 patients diagnosed with xerostomia, antihypertensive medications or analgesics were taken by > 50% of patients, while statins, psychiatric medications, antibiotics, proton pump inhibitors, or drugs known to have anticholinergic activity were taken by > 40%. Antihistamines, anticonvulsants, diuretics, or inhaled respiratory agents were used by > 20% of the patients in this cohort (Table 4).
Data on each individual medication were split into 2 categories: the percentage of patients that filled ≥ 1 prescription for that drug, and the total number of prescriptions filled and/or refilled for that drug (ie, including all fills and refills made by individual patients). The 5 most widely used medications in this cohort were omeprazole (39.1%), docusate sodium (29.7%), gabapentin (29.7%), aspirin (27.5%), and hydrocodone/acetaminophen (26.1%) (Table 5). The 5 prescriptions that were cumulatively most filled and/or refilled were omeprazole (128), sildenafil citrate (108), gabapentin (101), hydrocodone/acetaminophen (100), and oxycodone (92) (Table 6). Though sildenafil citrate and oxycodone were among the most-filled prescriptions, these were not included in Table 5 as neither was taken by > 15% of the patients studied. These prescriptions were filled multiple times by a small subset of patients.
Regarding treatment for dry mouth, artificial saliva spray was one of the most widely used (23.2%) and the seventh most-filled prescription within this cohort (86). The only other medication taken by > 15% of patients in a formulation other than a tablet or capsule was chlorhexidine, a germicidal mouthwash used to improve oral care.
Also, 30 (21.7%) patients with a documented xerostomia diagnosis had a history of substance misuse involving use of ≥ 1 of tobacco, alcohol, marijuana, or other illicit drugs.
Discussion
Saliva is an essential component for the maintenance of normal oral health.20,21 Decreased saliva production causes problems, including difficulties with speech, mastication, dysphagia, changes in taste, dental caries, impaired use of prostheses, recurrent infections, halitosis, deterioration of soft tissues, and compromised QOL.22,23 Among patients with a diagnosed SDS abnormality who were seen at this facility during FY 2015, the prevalence of xerostomia was only 1.5%. However, the true prevalence and incidence of xerostomia among veterans is not known. Given the role of xerostomia as a common risk factor for SDS problems and the polypharmacy exhibited by those presented here with SDS problems, it is probable that xerostomia was underreported in this veteran cohort.
Additionally, although salivary acinar cells are known to atrophy with age, as is consistent with this xerostomia cohort’s average age (SD) of 69.3 (12.6) years, the development of dry mouth is a multifactorial process. The current scientific literature asserts that most salivary loss is due to local and systemic diseases, immunologic disorders, external radiation, and as was highlighted by this study, multiple prescription and nonprescription medications.24-26
It has also been demonstrated previously that dry mouth complaints and low salivary flow rates are directly proportional to the number of medications taken by patients.2,27-30 Polypharmacy is therefore an area of great interest, and ≥ 40 categories of xerogenic medications have been identified by investigators such as Sreebny and Schwartz.31 Among those, some of the most xerogenic medication classes include antihypertensives, antiulcer agents, anticholinergics, and antidepressants, are all very commonly consumed in this cohort of patients with dry mouth (58.7%, 42.0%, 47.1%, and 38.4%, respectively). The medication regimens within this cohort of veterans with xerostomia were prime examples of polypharmacy as each patient took an average (SD) of 10.4 (7.2) medications, 39.9% took ≥ 12, and 72.5% of patients with xerostomia were taking ≥ 6 prescription drugs during a 1-year period.
Given the dangers of polypharmacy, a more conservative approach to prescribing medications could feasibly help with preventing xerostomia and SGH. In practice, while clinicians try to avoid prescribing anticholinergics, antimuscarinics, and antihistaminergic drugs for geriatric patients, they are tasked with the complex management of medication adverse effects (AEs) when dealing with multiple health conditions. The clinicians’ primary responsibilities are to follow the standard of care and not to introduce unnecessary harm when managing patients, but they also must push for, stay abreast of, and conduct more basic research and clinical trials to inform, adjust, and improve our current standard.
Research into polypharmacy and its role in inducing dry mouth is ongoing. Twenty years ago, Thomson and colleagues identified reduced salivary flow in patients who used antianginals, thyroxine, diuretics, antidepressants, and medications for asthma, while only 5 years earlier Loesche and colleagues reported the role of antiulcer medications, such as proton pump inhibitors, in the development of xerostomia.2,32 Within the past 5 years, Viljakainen and colleagues and Ohara and colleagues have echoed some of those findings by identifying associations between xerostomia and agents that impact digestive organs.33,34 A strong association recently was identified between the use of antipsychotic drugs and xerostomia.35 Additionally, when attributing xerostomia to polypharmacy, the interaction between medications is often overlooked in favor of considering the raw number of prescriptions taken. Whereas 1 medication alone may not have drying properties, combinations of medications might be more likely to induce xerostomia. Thomson and colleagues suggested such a situation regarding the interaction between thyroxine and diuretics.36 Future studies should focus on identifying viable substitutes for existing medications that reduce risk for xerostomia without compromising the management of other serious conditions.
Treatment
Another pressing question for clinicians concerns artificial saliva. Although 23.2% of patients with dry mouth in this xerostomia cohort used artificial saliva, the efficacy of this treatment is still unproven. Saliva substitutes are often used by patients who cannot produce sufficient amounts of natural saliva. In practice, artificial saliva produces, at best, modest temporary improvement in dry mouth symptoms in up to 40% of patients. At worst, as put forth by the Cochrane Review, artificial saliva may be no better than placebo in treating dry mouth.37,38 The volumes needed for symptom relief are large, ranging between 40 mL and 150 mL per day depending on the substitute’s composition. Saliva substitutes also must be reapplied throughout each day. This is particularly bothersome when patients must wake up repeatedly to reapply the treatment at night.37 In short, these substances do not seem wholly effective in managing dry mouth, and other options must be made available to patients with refractory xerostomia when artificial saliva and lifestyle modifications fail.
For now, few alternatives exist. Chewing gums and lozenges help to stimulate salivary flow, as do muscarinic agonists like pilocarpine. Unfortunately, muscarinic agonists are seldom used due to cholinergic AEs. Humidifiers are effective in increasing nighttime moisture but are contraindicated in patients with dust mite allergies.39 Reservoir-based devices with automated pumps funnel water and/or salivary substitutes from a fanny pack into patients’ mouths for lubrication.37 Other more esoteric pharmacologic treatments include D-limonene, yohimbine, and amifostine, which purportedly protect salivary progenitor cells, increase peripheral cholinergic activity, and protect salivary glands from free-radical damage during radiation treatment, respectively. Although these agents have shown some promise, D-limonene is difficult to administer given the high dosage required for treatment, yohimbine hasn’t been seriously investigated for improving salivary secretion since 1997, and amifostine isn’t used widely due to its AE profile despite its US Food and Drug Administration approval for prevention of xerostomia.39
Substance Abuse
The impact of smoking on xerostomia remains controversial. Some studies report an association between active smoking and xerostomia; others suggest that the local irritant effect of tobacco smoke may increase salivary gland output.40,41 The same may be true for chronic alcohol use as there are no epidemiologic studies showing a causal relationship between alcohol use and xerostomia. Studies with rats that are chronically exposed to ethanol have found increased salivary flow rates.42 In the xerostomia cohort presented here, 30 patients (21.7%) had a documented history of substance misuse. That percentage is likely underestimated, as substance misuse is often underreported, and frequent use may not always constitute misuse. Therefore, nicotine exposure, alcohol exposure, illicit drug use, and vaping all should be considered during the workup of a patient with xerostomia.
Limitations
It is common for medications to remain in a patient’s health record long after that patient stops taking them. Developing methods to track when patients discontinue their prescriptions will be essential for clearing up uncertainty in our data and in other similar studies. This study also did not account for patients’ medication adherence and the duration of exposure to medications and illicit substances. Furthermore, the results of this veteran study are not easily generalizable as this cohort is disproportionately male, of advanced age, and especially prone to exhibiting both substance use and psychiatric diagnoses relative to the general population. As described by Viljakainen and colleagues, risk factors for xerostomia include advanced age, female gender, low body mass index (BMI), malnutrition, and depressive symptoms, but because the demographic scope of this veteran population was narrow, it was not possible to discern the impact of, for example, gender.33 Data on variables like BMI, malnutrition, and depressive symptoms were not available. For this study, xerostomia could only be considered as an all-or-nothing phenomenon because the dataset did not describe different levels of dry mouth severity (eg, mild, moderate, severe).
Additionally, past polypharmacy studies have acknowledged an inability to tell whether xerostomia is mainly due to medications or to underlying medical conditions. For example, for emphysema, ß-adrenergic stimulation from bronchodilators could cause dry mouth by thickening saliva and decreasing salivary volume, but the pathophysiology and/or cardinal symptoms of emphysema, including chronic obstructive pulmonary disease-associated tachypnea, might contribute independently to dryness.
Though we can make inferences based on the medications taken by this cohort (eg, those taking antihypertensives have high blood pressure), this dataset did not explicitly detail comorbid conditions and ICD codes for chronic diseases that commonly arise with xerostomia. Those conditions, however, are of great clinical importance. Diabetes mellitus, HIV/AIDS, and, classically, Sjögren syndrome, all are known to cause dry mouth.43 Identifying new conditions that co-occur with xerostomia would allow clinicians to describe the root causes of and risk factors for dry mouth and SDS conditions in greater detail. Patients with dry mouth without SDS problems in this dataset are of particular interest as closer examination of their medications and comorbid conditions could help us understand why some individuals and not others develop SDS problems. The subjects of how comorbidities contribute to dry mouth and how their influences can be judged independently from the effects of medications are of great interest to us and will be investigated rigorously in our future studies.
Conclusions
In this cohort, few patients with SDS problems had documentation of a concomitant xerostomia diagnosis. This could represent a true infrequency of dry mouth or more likely, an underrecognition by clinicians. Heightened physician awareness regarding the signs and symptoms of and risk factors for xerostomia is needed to improve providers’ ability to diagnose this condition.
In particular, polypharmacy should be a major consideration when evaluating patients for xerostomia. This continues to be an important area of research, and some of the latest data on polypharmacy among older patients were compiled in a recent meta-analysis by Tan and colleagues. The authors of that systematic review reiterated the significant association between salivary gland hypofunction and the number of medications taken by patients. They also advocated for the creation of a risk score for medication-induced dry mouth to aid in medication management.44 Per their recommendations, it is now as crucial as ever to consider the numbers and types of medications taken by patients, to discontinue unnecessary prescriptions when possible, and to continue developing new strategies for preventing and treating xerostomia.
1. Thomson WM, Chalmers JM, Spencer AJ, Ketabi M. The occurrence of xerostomia and salivary gland hypofunction in a population-based sample of older South Australians. Spec Care Dentist. 1999;19(1):20-23.
2. Thomson WM, Chalmers JM, Spencer AJ, Slade GD. Medication and dry mouth: findings from a cohort study of older people. J Public Health Dent. 2000;60(1):12-20.
3. Sasportas LS, Hosford DN, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
4. Bivona PL. Xerostomia. A common problem among the elderly. N Y State Dent J. 1998;64(6):46-52.
5. Ness J, Hoth A, Barnett MJ, Shorr RI, Kaboli PJ. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4(1):42-51.
6. Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19(3):119-125.
7. Friedman PK, Isfeld D. Xerostomia: the “invisible” oral health condition. J Mass Dent Soc. 2008;57(3):42-44.
8. Ship JA, McCutcheon JA, Spivakovsky S, Kerr AR. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. J Oral Rehabil. 2007;34(10):724-732.
9. Field EA, Fear S, Higham SM, et al. Age and medication are significant risk factors for xerostomia in an English population, attending general dental practice. Gerodontology. 2001;18(1):21-24.
10. Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag. 2015;11:45-51.
11. Geuiros LA, Soares MS, Leao JC. Impact of ageing and drug consumption on oral health. Gerodontology. 2009;26(4):297-301.
12. Singh ML, Papas A. Oral implications of polypharmacy in the elderly. Dent Clin North Am. 2014;58(4):783-796.
13. Shinkai RS, Hatch JP, Schmidt CB, Sartori EA. Exposure to the oral side effects of medication in a community-based sample. Spec Care Dentist. 2006;26(3):116-120.
14. Hopcraft MS, Tan C. Xerostomia: an update for clinicians. Aust Dent J. 2010;55(3):238-244; quiz 353.
15. Ettinger RL. Review: xerostomia: a symptom which acts like a disease. Age Ageing. 1996;25(5):409-412.
16. Nederfors T, Isaksson R, Mornstad H, Dahlof C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population—relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997;25(3):211-216.
17. Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
18. Dirix P, Nuyts S, Vander Poorten V, Delaere P, Van den Boaert W. The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer. Support Care Cancer. 2008;16(2):171-179.
19. Sreebny LM, Valdini A. Xerostomia. A neglected symptom. Arch Intern Med. 1987;147(7):1333-1337.
20. Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50(3):140-161.
21. Amerongen AV, Veeran EC. Saliva—the defender of the oral cavity. Oral Dis. 2002;8(1):12-22.
22. Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134(1):61-69; quiz 118-119.
23. Atkinson JC, Baum BJ. Salivary enhancement: current status and future therapies. J Dent Educ. 2001;65(10):1096-1101.
24. Narhi TO, Meurman JH, Ainamo A. Xerostomia and hyposalivation: causes, consequences and treatment in the elderly. Drugs Aging. 1999;15(2):103-116.
25. Ship JA, Baum BJ. Is reduced salivary flow normal in old people? Lancet. 1990;336(8729):1507.
26. Ghezzi EM, Wagner-Lange LA, Schork MA, et al. Longitudinal influence of age, menopause, hormone replacement therapy, and other medications on parotid flow rates in healthy women. J Gerontol A Biol Sci Med Sci. 2000;55(1):M34-M42.
27. Fox PC. Acquired salivary dysfunction. Drugs and radiation. Ann N Y Acad Sci. 1998;842:132-137.
28. Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res. 2000;79(9):1652-1658.
29. Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50(3):535-543.
30. Sreebny LM, Valdini A, Yu A. Xerostomia. Part II: Relationship to nonoral symptoms, drugs, and diseases. Oral Surg Oral Med Oral Pathol. 1989;68(4):419-427.
31. Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth—2nd edition. Gerodontology. 1997;14(1):33-47.
32. Loesche WJ, Bromberg J, Terpenning MS, et al. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J Am Geriatr Soc. 1995;43(4):401-407.

33. Viljakainen S, Nykanen I, Ahonen R, et al. Xerostomia among older home care clients. Community Dent Oral Epidemiol. 2016;44(3):232-238.
34. Ohara Y, Hirano H, Yoshida H, et al. Prevalence and factors associated with xerostomia and hyposalivation among community-dwelling older people in Japan. Gerodontology. 2016;33(1):20-27.
35. Okamoto A, Miyachi H, Tanaka K, Chikazu D, Miyaoka H. Relationship between xerostomia and psychotropic drugs in patients with schizophrenia: evaluation using an oral moisture meter. J Clin Pharm Ther. 2016;41(6):684-688.
36. Thomson WM. Dry mouth and older people. Aust Dent J. 2015;60(suppl 1):54-63.
37. Sasportas LS, Hosford AT, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
38. Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev. 2011;(12):CD008924.
39. Roblegg E, Coughran A, Sirjani D. Saliva: an all-rounder of our body. Eur J Pharm Biopharm. 2019;142:133-141.
40. Billings RJ, Proskin HM, Moss ME. Xerostomia and associated factors in a community-dwelling adult population. Community Dent Oral Epidemiol. 1996;24(5):312-316.
41. Norlen P, Ostberg H, Bjorn AL. Relationship between general health, social factors and oral health in women at the age of retirement. Community Dent Oral Epidemiol. 1991;19(5):296-301.
42. Berry MR, Scott J. Functional and structural adaptation of the parotid gland to medium-term chronic ethanol exposure in the rat. Alcohol Alcoholism. 1990;25(5):523-531.
43. von Bultzingslowen I, Sollecito TP, Fox PC, et al. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2007;103:S57.e1-e15.
44. Tan ECK, Lexomboon D, Sandborgh-Englund G, et al. Medications that cause dry mouth as an adverse effect in older people: a systematic review and metaanalysis. J Am Geriatr Soc. 2018;66(1):76-84.
1. Thomson WM, Chalmers JM, Spencer AJ, Ketabi M. The occurrence of xerostomia and salivary gland hypofunction in a population-based sample of older South Australians. Spec Care Dentist. 1999;19(1):20-23.
2. Thomson WM, Chalmers JM, Spencer AJ, Slade GD. Medication and dry mouth: findings from a cohort study of older people. J Public Health Dent. 2000;60(1):12-20.
3. Sasportas LS, Hosford DN, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
4. Bivona PL. Xerostomia. A common problem among the elderly. N Y State Dent J. 1998;64(6):46-52.
5. Ness J, Hoth A, Barnett MJ, Shorr RI, Kaboli PJ. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4(1):42-51.
6. Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19(3):119-125.
7. Friedman PK, Isfeld D. Xerostomia: the “invisible” oral health condition. J Mass Dent Soc. 2008;57(3):42-44.
8. Ship JA, McCutcheon JA, Spivakovsky S, Kerr AR. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. J Oral Rehabil. 2007;34(10):724-732.
9. Field EA, Fear S, Higham SM, et al. Age and medication are significant risk factors for xerostomia in an English population, attending general dental practice. Gerodontology. 2001;18(1):21-24.
10. Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag. 2015;11:45-51.
11. Geuiros LA, Soares MS, Leao JC. Impact of ageing and drug consumption on oral health. Gerodontology. 2009;26(4):297-301.
12. Singh ML, Papas A. Oral implications of polypharmacy in the elderly. Dent Clin North Am. 2014;58(4):783-796.
13. Shinkai RS, Hatch JP, Schmidt CB, Sartori EA. Exposure to the oral side effects of medication in a community-based sample. Spec Care Dentist. 2006;26(3):116-120.
14. Hopcraft MS, Tan C. Xerostomia: an update for clinicians. Aust Dent J. 2010;55(3):238-244; quiz 353.
15. Ettinger RL. Review: xerostomia: a symptom which acts like a disease. Age Ageing. 1996;25(5):409-412.
16. Nederfors T, Isaksson R, Mornstad H, Dahlof C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population—relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997;25(3):211-216.
17. Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
18. Dirix P, Nuyts S, Vander Poorten V, Delaere P, Van den Boaert W. The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer. Support Care Cancer. 2008;16(2):171-179.
19. Sreebny LM, Valdini A. Xerostomia. A neglected symptom. Arch Intern Med. 1987;147(7):1333-1337.
20. Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50(3):140-161.
21. Amerongen AV, Veeran EC. Saliva—the defender of the oral cavity. Oral Dis. 2002;8(1):12-22.
22. Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134(1):61-69; quiz 118-119.
23. Atkinson JC, Baum BJ. Salivary enhancement: current status and future therapies. J Dent Educ. 2001;65(10):1096-1101.
24. Narhi TO, Meurman JH, Ainamo A. Xerostomia and hyposalivation: causes, consequences and treatment in the elderly. Drugs Aging. 1999;15(2):103-116.
25. Ship JA, Baum BJ. Is reduced salivary flow normal in old people? Lancet. 1990;336(8729):1507.
26. Ghezzi EM, Wagner-Lange LA, Schork MA, et al. Longitudinal influence of age, menopause, hormone replacement therapy, and other medications on parotid flow rates in healthy women. J Gerontol A Biol Sci Med Sci. 2000;55(1):M34-M42.
27. Fox PC. Acquired salivary dysfunction. Drugs and radiation. Ann N Y Acad Sci. 1998;842:132-137.
28. Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res. 2000;79(9):1652-1658.
29. Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50(3):535-543.
30. Sreebny LM, Valdini A, Yu A. Xerostomia. Part II: Relationship to nonoral symptoms, drugs, and diseases. Oral Surg Oral Med Oral Pathol. 1989;68(4):419-427.
31. Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth—2nd edition. Gerodontology. 1997;14(1):33-47.
32. Loesche WJ, Bromberg J, Terpenning MS, et al. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J Am Geriatr Soc. 1995;43(4):401-407.

33. Viljakainen S, Nykanen I, Ahonen R, et al. Xerostomia among older home care clients. Community Dent Oral Epidemiol. 2016;44(3):232-238.
34. Ohara Y, Hirano H, Yoshida H, et al. Prevalence and factors associated with xerostomia and hyposalivation among community-dwelling older people in Japan. Gerodontology. 2016;33(1):20-27.
35. Okamoto A, Miyachi H, Tanaka K, Chikazu D, Miyaoka H. Relationship between xerostomia and psychotropic drugs in patients with schizophrenia: evaluation using an oral moisture meter. J Clin Pharm Ther. 2016;41(6):684-688.
36. Thomson WM. Dry mouth and older people. Aust Dent J. 2015;60(suppl 1):54-63.
37. Sasportas LS, Hosford AT, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
38. Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev. 2011;(12):CD008924.
39. Roblegg E, Coughran A, Sirjani D. Saliva: an all-rounder of our body. Eur J Pharm Biopharm. 2019;142:133-141.
40. Billings RJ, Proskin HM, Moss ME. Xerostomia and associated factors in a community-dwelling adult population. Community Dent Oral Epidemiol. 1996;24(5):312-316.
41. Norlen P, Ostberg H, Bjorn AL. Relationship between general health, social factors and oral health in women at the age of retirement. Community Dent Oral Epidemiol. 1991;19(5):296-301.
42. Berry MR, Scott J. Functional and structural adaptation of the parotid gland to medium-term chronic ethanol exposure in the rat. Alcohol Alcoholism. 1990;25(5):523-531.
43. von Bultzingslowen I, Sollecito TP, Fox PC, et al. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2007;103:S57.e1-e15.
44. Tan ECK, Lexomboon D, Sandborgh-Englund G, et al. Medications that cause dry mouth as an adverse effect in older people: a systematic review and metaanalysis. J Am Geriatr Soc. 2018;66(1):76-84.
Faster Triage of Veterans With Head and Neck Cancer
High-risk patients with a growing mass require proper assessment, including a thorough history, physical examination, and fine-needle aspiration for diagnosis.
A 65-year-old Vietnam veteran with a history of posttraumatic stress disorder (PTSD), transient ischemic attack, alcohol dependence, and a smoking history of 50 pack-years presents with a neck mass that has been growing for 2 months and unintentional weight loss of 25 pounds over 6 months. What is the differential diagnosis? How does a general practitioner evaluate, manage, and efficiently triage this patient?
Such cases, in which a head and neck cancer (HNC) diagnosis is suspected, can be unnerving for both physician and patient. However, knowledgeable general practitioners (GPs) can play a pivotal role in recognizing high-risk patients, initiating workups, and referring to appropriate specialists, resulting in earlier detection and potentially better outcomes.
This article outlines the authors’ recommended best practices for GPs treating patients with presumed HNC. Although the focus here is on the veteran population, in which HNC rates are significantly higher, many of the suggestions presented are applicable to the general population.
Background
Head and neck cancers represent a diverse family of malignancies of the nasopharynx, oropharynx, hypopharynx, larynx, oral cavity, paranasal sinuses, and salivary glands. This article does not cover thyroid, ophthalmologic, neurologic, or skin malignancies. The yearly worldwide incidence of all HNC cases is more than 550,000.1 In the U.S., 55,000 new HNC cases, representing 3% of all new malignancies, are reported annually. The 5-year survival rate is 60%, but 12,000 Americans die each year of head and neck cancer.2 Most HNCs occur in males aged ≥ 50 years, and the incidence increases with age. Almost $3.6 billion is spent treating HNC in the U.S. annually.3
Risk Factors
Alcohol and tobacco consumption strongly predisposes patients to squamous cell carcinoma (SCC), which accounts for 90% of HNCs. Together, alcohol and tobacco act multiplicatively.4,5 For instance, heavy drinkers (≥ 10 drinks daily) are at 5-fold increased risk for oral and pharyngeal cancers, heavy smokers (≥ 1 pack daily) are at 20-fold increased risk, and people who both drink and smoke heavily are at 50-fold increased risk.6 Head and neck cancer rates are significantly elevated even for moderate/light drinkers and smokers.
Veterans have disproportionately high rates of alcohol drinking and tobacco smoking,7,8 in part because these habits are ingrained in military culture. During World Wars I and II, tobacco companies supplied soldiers with daily rations of cigarettes,9 and advertisers targeted military personnel by linking smoking with patriotism, strength, and toughness.10 The VHA and the DoD reported that 33% of veterans and active-duty service personnel smoke—compared with 23% of civilians.11 Vietnam veterans, 47% of whom smoke, are at particular risk for HNC.12
Cessation of alcohol drinking and tobacco smoking is essential for overall prognosis, especially after HNC has been diagnosed. Continued smoking after HNC treatment increases the recurrence rate 4-fold.13 There also is mounting evidence that cessation of drinking and smoking can reverse the risk for HNC over time. According to a meta-analysis, quitting smoking for just 1 year begins to lower the risk for HNC. Quitting smoking for 20 years reduces the risk to the level of never smokers, and abstaining from alcohol for 20 years decreases the risk by nearly 40%.14
Viral infections are risk factors for development of oral cavity, oropharyngeal, laryngeal, and nasopharyngeal carcinoma (NPC). Sixty percent of oropharyngeal cancers are positive for human papillomavirus (HPV) infection,15 and most NPCs are associated with prior Epstein-Barr virus (EBV) exposure, particularly in populations from southern China, Southeast Asia, North Africa, and the Middle East.16
Evaluation
Workup of a possible HNC starts with taking a thorough history. Early HNC symptoms that may prompt a patient to seek medical care include neck mass, nonhealing oral ulcer, voice change, sore throat for more than 2 weeks, ear pain, nasal obstruction, serous otitis media, dysphagia, and odynophagia. Patients with advanced HNC may present with unintentional weight loss, decreased appetite, and cranial nerve deficits. For alcohol or tobacco users who present with any of the symptoms, SCC should lead the differential diagnosis, prompting examination of the head and neck. The authors present a general outline for performing this examination and detail the most common types of HNC encountered in the GP setting.
Physical Examination
The GP should perform a bimanual examination of the oral cavity, ears, nose, thyroid, and cranial nerve function with the help of a headlight. The physician should use 2 tongue blades to explore the oral cavity and palpate for suspicious oral lesions. It is often possible to feel a lesion before visualizing it on the base of the tongue. If there is a presenting mass, the physician should document the mass site, size, shape, consistency, tenderness, mobility, and accompanying deficits or symptoms.
Also recommended is a thorough examination of the facial, submandibular, and other cervical lymph nodes. The drainage patterns of these nodes can help the GP track potential routes of malignant infiltration. The submental and submandibular lymph nodes (level 1) drain the lower lip, floor of mouth, anterior tongue, and side of nose. The nodes along the mid and internal jugular vein (levels 2-4) and between the sternocleidomastoid and trapezius muscles (level 5) drain the oropharynx, mid tongue, larynx, hypopharynx, parotid gland, and skin of the face and ear. Nontender hard nodes are more likely to be malignant, as are nodes of the posterior triangle (level 5).17
Malignancy by Site
Oral cavity. The oral cavity includes the lips, buccal mucosa, teeth, gums, anterior two-thirds of tongue, floor of mouth, alveolar ridge, retromolar trigone, and hard palate. The oral cavity is the most common site for HNCs.18 The most common symptoms of malignancy of the oral cavity include dysphonia, nonhealing oral ulcers, loose teeth, bleeding, change in denture fit, and chin numbness, which could indicate mandibular invasion with inferior alveolar nerve involvement.19
For thorough assessment of the oral cavity, the patient should remove all temporary dental appliances. Then, with a tongue blade in each hand, the physician should thoroughly examine the oral mucosa, moving the tongue laterally to evaluate the floor of mouth, and palpate the mucosal surfaces to identify submucosal cancers in the posterior tongue and floor of mouth. Minor salivary glands are ubiquitous in the oral cavity and may be involved by cancer. Ulcerated painful lesions that last longer than 2 weeks are less likely to be common viral or aphthous ulcers. For either an oral cavity mass or a nonhealing ulcer that persists more than 4 weeks, malignancy should be suspected, and the patient should be referred for imaging and biopsy.
Leukoplakias are white patches in the oral cavity that develop from squamous epithelial hyperplasia and cannot be scraped away with a tonghpvue blade. The lesions are usually benign, but, if there is an element of redness (erythroplakia), the risk for harboring dysplasia is much higher, though the differential diagnosis includes trauma from adjacent teeth or lichen planus. If leukoplakia is seen, the physician should accurately note the size, location, and site and should monitor every 3 to 4 months. If erythroplakia, enlargement of leukoplakia, or any evidence of mucosal invasion is noted, the physician should refer to otolaryngologyhead and neck surgery (Oto-HNS). The authors advise against lasering leukoplakia; it is unnecessary, can make subsequent evaluation more difficult, and can mask recurrent malignancy.
Oropharynx. The oropharynx includes the posterior third of tongue, soft palate, palatine and lingual tonsils, and the posterior and lateral pharyngeal walls superior to tip of epiglottis. Cancers can arise in any of these locations and may present with dysphagia, odynophagia, referred
otalgia, hoarseness, and enlarged lymph nodes. In advanced cases, there may be bleeding, airway obstruction, and aspiration. Nonsmokers with oropharyngeal SCC are likely to be HPV positive and may be younger than the typical patient with alcohol- or tobacco-related HNC. Human papillomavirus positive oropharyngeal carcinoma has a much better prognosis than its tobaccorelated counterpart does. Physical examination should include assessment of tonsillar size and symmetry, palpation of neck lymph nodes, and palpation of base of tongue. Treatment may involve surgery, radiation, or chemoradiation, depending on factors such as extent of disease and comorbidities.
Nasopharynx. The nasopharynx extends from the nasal cavity (posterior to nasal septum) to the oropharynx. The most common NPC symptoms are middle-ear effusion and enlarged neck nodes. Nasal obstruction, epistaxis, or cranial nerve deficits also may occur. The nasopharynx
is best assessed with a fiberoptic scope. Most NPCs are associated with EBV infection, and viral levels can be used to monitor response to treatment.20 Early biopsy is indicated if a nasopharyngeal mass is found.
Larynx. As with the nasopharynx, the larynx is best seen with a fiberoptic scope. Malignancy generally presents with hoarseness, voice changes, cough, sore throat, or, if more advanced, airway compromise such as stridor and neck adenopathy. As larynx HNCs may be associated
with aspiration, the authors recommend asking “Does food go down the wrong pipe?” or “Do you cough when you eat?” and having the patient drink and document any difficulty. A smoker with hoarseness lasting more than 2 weeks should be referred to Oto-HNS for endoscopic assessment. Among veterans, other causes of hoarseness include polyps,Candida infection associated with inhalation of steroids for chronic obstructive pulmonary disease, and recurrent nerve paralysis from thyroid or lung cancer.
Neck. Patients with HNCs commonly present with a neck mass. Fifty percent to 80% of adults with a nontender neck mass are harboring a malignancy.21,22 In a patient without HIV, a neck mass larger than 2 cm should be evaluated for cancer, especially if the mass is hard and nontender.23 Computed tomography (CT) is recommended for initial evaluation, which, if there is FNA confirmed carcinoma, should be followed by positron emission tomography (PET). If there is concern for parotidor skull base tumors, magnetic resonance imaging (MRI) is preferable for demonstrating soft-tissue definition and disease extent.
If the patient is aged < 40 years and lymph nodes have been present for less than 2 to 4 weeks, are tender, or are associated with fever or poor dental hygiene, then an infection may instead be the cause. Dentistry referral and/or an antibiotic trial should be considered. Lymphomas, also common in the neck, may be accompanied by “B symptoms” (fever, night sweats, unintentional weight loss of > 10%).24 If lymphoma is suspected, fine-needle aspiration (FNA) for cytology and flow cytometry should be performed. If lymphoma is confirmed, the GP should refer the patient to an appropriate medical oncologist for further evaluation, which may include referral to Oto-HNS for core or open biopsy. Contraindications to FNA of a neck mass include paragangliomas, such as a carotid body tumor.
Other cancers of the upper aerodigestive tract also often spread to the neck nodes and may initially present as a neck mass. A thorough examination can usually point to the primary cancer, and FNA will provide the diagnosis with high specificity and sensitivity.25 Midline cystic neck masses in close proximity to the hyoid bone are likely thyroglossal duct cysts. If these cysts grow, they likely require removal.
Salivary glands. The submandibular, sublingual, and parotid are the major salivary glands. There also are hundreds of small salivary glands scattered through the oral and pharyngeal mucosa. Tumors arising from the salivary glands represent about 6% of all head and neck masses; these tumors are nearly 3 times more common in men than in women.26 About 80% of salivary gland tumors originate in the parotid gland; patients with such tumors typically present with a painless parotid mass.26 In advanced cases, patients may present with skin infiltration and facial paralysis secondary to involvement of
the facial nerve that courses through the parotid gland after it exits the temporal bone near the mastoid tip.
Salivary gland tumors are most commonly benign, and pleomorphic adenomas are the most common benign parotid neoplasm.27 The incidence of malignancy is highest in submandibular, sublingual, and minor salivary glands. There are numerous primary salivary gland malignancies, such as mucoepidermoid carcinoma, adenocarcinoma, and adenoid cystic carcinoma. Facial skin SCC may metastasize to periparotid nodes. There are also multiple nonneoplastic causes of salivary gland inflammation. Recurrent diffuse, painful gland enlargement may be suggestive of recurrent sialadenitis and may be
secondary to a stone or xerostomia associated with dehydration or use of diuretics, antidepressants, or lithium. Multiple lymphoepithelial cysts may be associated with HIV and do not require resection.28
Management
After taking a thorough history and performing a physical examination, the physician evaluating a patient for HNC should proceed with diagnostic testing followed by referral to a specialist.
Diagnostic Testing
Laboratory values. Although laboratory values are unlikely to help in evaluation of a malignancy, elevated white blood cell count, erythrocyte sedimentation
rate, and C-reactive protein level are markers of a general inflammatory process that may support a clinically suspected diagnosis of infection. Values that decrease over time may represent progress toward disease resolution.29
Imaging. If malignancy is suspected, imaging should be obtained. Imaging has an important role in corroborating examination findings of a mass. Imaging
also provides an accurate baseline assessment of tumor size and extent. Recommended imaging modalities include:
- Ultrasonography (US). This quick and inexpensive modality can be used to visualize suspicious neck lesions. It is helpful in performing real-time assessments and differentiating cysts from solid masses and abscesses from reactive lymph nodes or infiltrative tumors. Challenges with US include its inability to penetrate bone and practitioners’ variable interpretation of images. A different modality invariably is needed to document location and spread of suspected HNC.
- MRI and CT. These are necessary for HNC evaluation and staging. Generally, they are equivalent in node assessment, but MRI is preferable in tongue and pharynx evaluation, and CT is preferable in the larynx. An ideal image should extend from the skull base to the clavicles, demonstrating the extent of the primary tumor and potential metastases to the neck nodes. As MRI is best protocoled by an experienced head and neck radiologist, it is preferable to refer the patient to such a specialist and allow Oto-HNS to arrange the imaging. Contraindications to MRI include pacemakers and shrapnel (common among veteran patients) and claustrophobia (common among patients with PTSD).
- PET-CT. This modality helps in staging, detecting distant metastases, assessing treatment response after chemoradiation, and locating the primary cancer when a proven neck metastasis has no obvious source. Whether PET-CT should be performed before initial referral should be discussed with the specialist. A case with a proven distant metastasis likely is not operable and would be better served with a referral to medical oncology.
Biopsy. For almost all HNCs, the initial biopsy modality should be FNA. Although intraoral lesions may benefit from incisional biopsy, this procedure should not delay triage and may be outside the scope of practice for many GPs. A GP can arrange for FNA to be performed before the referral appointment. This modality has excellent diagnostic sensitivity and specificity.30,31 In the setting of equivocal or negative results despite a high index of suspicion, having a more experienced cytopathologist repeat the FNA is often warranted. Excisional biopsy may be warranted if FNA is nondiagnostic or lymphoma is diagnosed.
Other Interventions
In some cases, the GP has additional important roles— in preparing the patient for the possibility of surgery, treating related conditions, helping the patient cope with this new medical challenge, improving nutrition, and promoting cessation of alcohol drinking and tobacco smoking.
Surgery. For patients with biopsy-proven HNC, preoperative assessment by the GP helps provide clearance for surgery, reduces time to treatment, and lessens the likelihood of postoperative complications. A recent study found that VA patients aged ≥ 70 years had a 30-day postoperative mortality rate of 6% and at least a ≥ 20% risk for a major complication during their hospital stay.32 Given these risks and the overall higher rate of chronic diseases among veterans, the authors recommend preoperative evaluation of comorbidities with particular emphasis on cardiac, renal, and pulmonary status. In addition, specific examinations (eg, electrocardiogram, chest radiograph, basic laboratory tests, liver profile test) are recommended for patients with a history of alcohol abuse.
Malnutrition. At initial diagnosis, many patients with HNC have significant weight loss.33 Unfortunately, the required complex treatment modalities increase malnutrition rates and decrease quality of life.34 Preventive strategies are, therefore, key in improving patients’ overall health. The authors recommend that GPs consider early nutritional consultations and as-needed speech therapy evaluations to provide preventive strategies and exercises to maintain proper swallowing function.35 Patients who are unable to eat because of aspiration caused by a large tumor should be admitted for preoperative gastric tube placement to improve nutrition and, ultimately, surgical outcome. A large percentage of veterans with HNC also have depression, which may lead to decreased appetite.36 Mental health consultations can help in these circumstances, as can use of mirtazapine, which increases appetite and treats depression-related symptoms.
Pain. Nonsteroidal anti-inflammatory drugs (NSAIDs) can be recommended for relief of uncontrolled mild to moderate pain, but they must be discontinued 1 week before surgery to reduce the risk for bleeding complications. The NSAIDs should be avoided entirely in patients with untreated friable tumors of the aerodigestive tract. In patients with biopsy-proven cancer, pain control can involve opiates per World Health Organization guidelines.37 Patients with head and neck SCC often have neuropathic pain, which is more effectively treated with gabapentin.
Alcohol drinking and tobacco smoking. Promoting cessation of these habits is essential for all patients, including those already diagnosed with cancer. Encouraging cessation as well as overall healthy lifestyle choices can reduce cancer risk and improve overall health—and may be the single most efficacious intervention a physician can offer.
Referral
Most patients with suspected HNC should be referred to Oto-HNS. In cases in which lymphoma is most highly suspected, medical oncology is the most appropriate initial referral. Early dental consultation is also necessary if an obturator will be needed (eg, as with a hard palate malignancy) or if irradiation is planned (radiation-induced xerostomia significantly increases the risk for dental caries).38 For all new cancer diagnoses, the GP can contact the Oto-HNS specialist for help in tailoring the patient evaluation to the practices and resources at the GP’s home institution and reduce time to treatment.
Conclusion
General practitioners are essential in identifying and triaging veterans with HNC. High-risk patients with a growing mass require proper assessment, including a thorough history and physical examination, FNA for diagnosis, and appropriate specialist referral. Although this article provides a helpful framework for thinking about patients with HNC, the authors encourage GPs to check the National Comprehensive Cancer Network guidelines for additional information on the topics covered here. With this knowledge, GPs can improve outcomes for veterans with HNC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
3. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2): 117-128.
4. Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135(3):451-457.
5. Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541-550.
6. Rodriguez T, Altieri A, Chatenoud L, et al. Risk factors for oral and pharyngeal cancer in young adults. Oral Oncol. 2004;40(2):207-213.
7. National Survey on Drug Use and Health. Alcohol Use and Alcohol-Related Risk Behaviors Among Veterans. NIH publication 99-4323. Rockville, MD: National Institutes of Health, U.S. Dept of Health and Human Services; 2005.
8. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-217.
9. Smith EA, Malone RE. “Everywhere the soldier will be”: wartime tobacco promotion in the US military. Am J Public Health. 2009;99(9):1595-1602.
10. James R, Olstad S. Cigarette advertising. Time. June 15, 2009.
11. Miller DR, Kalman D, Ren XS, Lee AF, Niu Z, Kazis LE. Health Behaviors of Veterans in the VHA: Tobacco Use: 1999 Large Health Survey of VHA Enrollees. Washington, DC: Office of Quality and Performance, Veterans Health Administration, U.S. Dept of Veterans Affairs; 2001.
12. Bray RM, Hourani LL, Rae Olmstead KL, et al. 2005 Department of Defense Survey of Health Related Behaviors Among Active Duty Military Personnel: A Component of the Defense Lifestyle Assessment Program (DLAP). Report prepared for U.S. Dept of Defense (Cooperative Agreement No. DAMD17-00-2-0057). Research Triangle Park, NC: RTI International; 2006.
13. Stevens MH, Gardner JW, Parkin JL, Johnson LP. Head and neck cancer survival and life-style change. Arch Otolaryngol. 1983;109(11):746-749.
14. Marron M, Boffetta P, Zhang ZF, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol. 2010;39(1):182-196.
15. Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31(6):744-754.
16. Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333(11):693-698.
17. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66(11):2103-2110.
18. American Cancer Society. Oral cavity and oropharyngeal cancer. American Cancer Society website. http://www.cancer.org/acs/groups/cid/documents/webcontent/003128-pdf.pdf. Updated January 27, 2016. Accessed July 6, 2016.
19. Pandey M, Rao LP, Das SR, Mathews A, Chacko EM, Naik BR. Patterns of mandibular invasion in oral squamous cell carcinoma of the mandibular region. World J Surg Oncol. 2007;5:12.
20. Leung SF, Zee B, Ma BB, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006;24(34):5414-5418.
21. Dickson PV, Davidoff AM. Malignant neoplasms of the head and neck. Semin Pediatr Surg. 2006;15(2):92-98.
22. Lefebvre JL, Coche-Dequeant B, Van JT, Buisset E, Adenis A. Cervical lymph nodes from an unknown primary tumor in 190 patients. Am J Surg. 1990;160(4):443-446.
23. Bhattacharyya N. Predictive factors for neoplasia and malignancy in a neck mass. Arch Otolaryngol Head Neck Surg. 1999;125(3):303-307.
24. Dailey SH, Sataloff RT. Lymphoma: an update on evolving trends in staging and management. Ear Nose Throat J. 2001;80(3):164-170.
25. Schwarz R, Chan NH, MacFarlane JK. Fine needle aspiration cytology in the evaluation of head and neck masses. Am J Surg. 1990;159(5):482-485.
26. Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806-816.
27. Jones AV, Craig GT, Speight PM, Franklin CD. The range and demographics of salivary gland tumours diagnosed in a UK population. Oral Oncol. 2008;44(4):407-417.
28. Shebl FM, Bhatia K, Engels EA. Salivary gland and nasopharyngeal cancers in individuals with acquired immunodeficiency syndrome in United States. Int J Cancer. 2010;126(10):2503-2508.
29. Bien´ E, Balcerska A. Clinical significance of erythrocyte sedimentation rate, C-reactive protein and serum lactate dehydrogenase levels in the diagnosis, prognosis and treatment monitoring of children suffering from cancer [in Polish]. Med Wieku Rozwoj. 2004;8(4, pt 2):1081-1089.
30. Wu M, Burstein DE, Yuan S, et al. A comparative study of 200 fine needle aspiration biopsies performed by clinicians and cytopathologists. Laryngoscope. 2006;116(7):1212-1215.
31. Liu ES, Bernstein JM, Sculerati N, Wu HC. Fine needle aspiration biopsy of pediatric head and neck masses. Int J Pediatr Otorhinolaryngol. 2001;60(2):135-140.
32. Story DA. Postoperative complications in elderly patients and their significance for long-term prognosis. Curr Opin Anaesthesiol. 2008;21(3):375-379.
33. Head BA, Heitz L, Keeney C, et al. The relationship between weight loss and health-related quality of life in persons treated for head and neck cancer. Support Care Cancer. 2011;19(10):1511-1518.
34. van den Berg MG, Rasmussen-Conrad EL, van Nispen L, van Binsbergen JJ,
Merkx MA. A prospective study on malnutrition and quality of life in patients
with head and neck cancer. Oral Oncol. 2008;44(9):830-837.
35. Murphy BA, Deng J. Advances in supportive care for late effects of head and neck cancer. J Clin Oncol. 2015;33(29):3314-3321.
36. Pandey M, Devi N, Thomas BC, Kumar SV, Krishnan R, Ramdas K. Distress overlaps with anxiety and depression in patients with head and neck cancer. Psychooncology. 2007;16(6):582-586.
37. World Health Organization. Cancer Pain Relief: With a Guide to Opioid Availability.
2nd ed. Geneva, Switzerland: World Health Organization; 1996.
38. Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and cancer
therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62(6):400-422.
High-risk patients with a growing mass require proper assessment, including a thorough history, physical examination, and fine-needle aspiration for diagnosis.
A 65-year-old Vietnam veteran with a history of posttraumatic stress disorder (PTSD), transient ischemic attack, alcohol dependence, and a smoking history of 50 pack-years presents with a neck mass that has been growing for 2 months and unintentional weight loss of 25 pounds over 6 months. What is the differential diagnosis? How does a general practitioner evaluate, manage, and efficiently triage this patient?
Such cases, in which a head and neck cancer (HNC) diagnosis is suspected, can be unnerving for both physician and patient. However, knowledgeable general practitioners (GPs) can play a pivotal role in recognizing high-risk patients, initiating workups, and referring to appropriate specialists, resulting in earlier detection and potentially better outcomes.
This article outlines the authors’ recommended best practices for GPs treating patients with presumed HNC. Although the focus here is on the veteran population, in which HNC rates are significantly higher, many of the suggestions presented are applicable to the general population.
Background
Head and neck cancers represent a diverse family of malignancies of the nasopharynx, oropharynx, hypopharynx, larynx, oral cavity, paranasal sinuses, and salivary glands. This article does not cover thyroid, ophthalmologic, neurologic, or skin malignancies. The yearly worldwide incidence of all HNC cases is more than 550,000.1 In the U.S., 55,000 new HNC cases, representing 3% of all new malignancies, are reported annually. The 5-year survival rate is 60%, but 12,000 Americans die each year of head and neck cancer.2 Most HNCs occur in males aged ≥ 50 years, and the incidence increases with age. Almost $3.6 billion is spent treating HNC in the U.S. annually.3
Risk Factors
Alcohol and tobacco consumption strongly predisposes patients to squamous cell carcinoma (SCC), which accounts for 90% of HNCs. Together, alcohol and tobacco act multiplicatively.4,5 For instance, heavy drinkers (≥ 10 drinks daily) are at 5-fold increased risk for oral and pharyngeal cancers, heavy smokers (≥ 1 pack daily) are at 20-fold increased risk, and people who both drink and smoke heavily are at 50-fold increased risk.6 Head and neck cancer rates are significantly elevated even for moderate/light drinkers and smokers.
Veterans have disproportionately high rates of alcohol drinking and tobacco smoking,7,8 in part because these habits are ingrained in military culture. During World Wars I and II, tobacco companies supplied soldiers with daily rations of cigarettes,9 and advertisers targeted military personnel by linking smoking with patriotism, strength, and toughness.10 The VHA and the DoD reported that 33% of veterans and active-duty service personnel smoke—compared with 23% of civilians.11 Vietnam veterans, 47% of whom smoke, are at particular risk for HNC.12
Cessation of alcohol drinking and tobacco smoking is essential for overall prognosis, especially after HNC has been diagnosed. Continued smoking after HNC treatment increases the recurrence rate 4-fold.13 There also is mounting evidence that cessation of drinking and smoking can reverse the risk for HNC over time. According to a meta-analysis, quitting smoking for just 1 year begins to lower the risk for HNC. Quitting smoking for 20 years reduces the risk to the level of never smokers, and abstaining from alcohol for 20 years decreases the risk by nearly 40%.14
Viral infections are risk factors for development of oral cavity, oropharyngeal, laryngeal, and nasopharyngeal carcinoma (NPC). Sixty percent of oropharyngeal cancers are positive for human papillomavirus (HPV) infection,15 and most NPCs are associated with prior Epstein-Barr virus (EBV) exposure, particularly in populations from southern China, Southeast Asia, North Africa, and the Middle East.16
Evaluation
Workup of a possible HNC starts with taking a thorough history. Early HNC symptoms that may prompt a patient to seek medical care include neck mass, nonhealing oral ulcer, voice change, sore throat for more than 2 weeks, ear pain, nasal obstruction, serous otitis media, dysphagia, and odynophagia. Patients with advanced HNC may present with unintentional weight loss, decreased appetite, and cranial nerve deficits. For alcohol or tobacco users who present with any of the symptoms, SCC should lead the differential diagnosis, prompting examination of the head and neck. The authors present a general outline for performing this examination and detail the most common types of HNC encountered in the GP setting.
Physical Examination
The GP should perform a bimanual examination of the oral cavity, ears, nose, thyroid, and cranial nerve function with the help of a headlight. The physician should use 2 tongue blades to explore the oral cavity and palpate for suspicious oral lesions. It is often possible to feel a lesion before visualizing it on the base of the tongue. If there is a presenting mass, the physician should document the mass site, size, shape, consistency, tenderness, mobility, and accompanying deficits or symptoms.
Also recommended is a thorough examination of the facial, submandibular, and other cervical lymph nodes. The drainage patterns of these nodes can help the GP track potential routes of malignant infiltration. The submental and submandibular lymph nodes (level 1) drain the lower lip, floor of mouth, anterior tongue, and side of nose. The nodes along the mid and internal jugular vein (levels 2-4) and between the sternocleidomastoid and trapezius muscles (level 5) drain the oropharynx, mid tongue, larynx, hypopharynx, parotid gland, and skin of the face and ear. Nontender hard nodes are more likely to be malignant, as are nodes of the posterior triangle (level 5).17
Malignancy by Site
Oral cavity. The oral cavity includes the lips, buccal mucosa, teeth, gums, anterior two-thirds of tongue, floor of mouth, alveolar ridge, retromolar trigone, and hard palate. The oral cavity is the most common site for HNCs.18 The most common symptoms of malignancy of the oral cavity include dysphonia, nonhealing oral ulcers, loose teeth, bleeding, change in denture fit, and chin numbness, which could indicate mandibular invasion with inferior alveolar nerve involvement.19
For thorough assessment of the oral cavity, the patient should remove all temporary dental appliances. Then, with a tongue blade in each hand, the physician should thoroughly examine the oral mucosa, moving the tongue laterally to evaluate the floor of mouth, and palpate the mucosal surfaces to identify submucosal cancers in the posterior tongue and floor of mouth. Minor salivary glands are ubiquitous in the oral cavity and may be involved by cancer. Ulcerated painful lesions that last longer than 2 weeks are less likely to be common viral or aphthous ulcers. For either an oral cavity mass or a nonhealing ulcer that persists more than 4 weeks, malignancy should be suspected, and the patient should be referred for imaging and biopsy.
Leukoplakias are white patches in the oral cavity that develop from squamous epithelial hyperplasia and cannot be scraped away with a tonghpvue blade. The lesions are usually benign, but, if there is an element of redness (erythroplakia), the risk for harboring dysplasia is much higher, though the differential diagnosis includes trauma from adjacent teeth or lichen planus. If leukoplakia is seen, the physician should accurately note the size, location, and site and should monitor every 3 to 4 months. If erythroplakia, enlargement of leukoplakia, or any evidence of mucosal invasion is noted, the physician should refer to otolaryngologyhead and neck surgery (Oto-HNS). The authors advise against lasering leukoplakia; it is unnecessary, can make subsequent evaluation more difficult, and can mask recurrent malignancy.
Oropharynx. The oropharynx includes the posterior third of tongue, soft palate, palatine and lingual tonsils, and the posterior and lateral pharyngeal walls superior to tip of epiglottis. Cancers can arise in any of these locations and may present with dysphagia, odynophagia, referred
otalgia, hoarseness, and enlarged lymph nodes. In advanced cases, there may be bleeding, airway obstruction, and aspiration. Nonsmokers with oropharyngeal SCC are likely to be HPV positive and may be younger than the typical patient with alcohol- or tobacco-related HNC. Human papillomavirus positive oropharyngeal carcinoma has a much better prognosis than its tobaccorelated counterpart does. Physical examination should include assessment of tonsillar size and symmetry, palpation of neck lymph nodes, and palpation of base of tongue. Treatment may involve surgery, radiation, or chemoradiation, depending on factors such as extent of disease and comorbidities.
Nasopharynx. The nasopharynx extends from the nasal cavity (posterior to nasal septum) to the oropharynx. The most common NPC symptoms are middle-ear effusion and enlarged neck nodes. Nasal obstruction, epistaxis, or cranial nerve deficits also may occur. The nasopharynx
is best assessed with a fiberoptic scope. Most NPCs are associated with EBV infection, and viral levels can be used to monitor response to treatment.20 Early biopsy is indicated if a nasopharyngeal mass is found.
Larynx. As with the nasopharynx, the larynx is best seen with a fiberoptic scope. Malignancy generally presents with hoarseness, voice changes, cough, sore throat, or, if more advanced, airway compromise such as stridor and neck adenopathy. As larynx HNCs may be associated
with aspiration, the authors recommend asking “Does food go down the wrong pipe?” or “Do you cough when you eat?” and having the patient drink and document any difficulty. A smoker with hoarseness lasting more than 2 weeks should be referred to Oto-HNS for endoscopic assessment. Among veterans, other causes of hoarseness include polyps,Candida infection associated with inhalation of steroids for chronic obstructive pulmonary disease, and recurrent nerve paralysis from thyroid or lung cancer.
Neck. Patients with HNCs commonly present with a neck mass. Fifty percent to 80% of adults with a nontender neck mass are harboring a malignancy.21,22 In a patient without HIV, a neck mass larger than 2 cm should be evaluated for cancer, especially if the mass is hard and nontender.23 Computed tomography (CT) is recommended for initial evaluation, which, if there is FNA confirmed carcinoma, should be followed by positron emission tomography (PET). If there is concern for parotidor skull base tumors, magnetic resonance imaging (MRI) is preferable for demonstrating soft-tissue definition and disease extent.
If the patient is aged < 40 years and lymph nodes have been present for less than 2 to 4 weeks, are tender, or are associated with fever or poor dental hygiene, then an infection may instead be the cause. Dentistry referral and/or an antibiotic trial should be considered. Lymphomas, also common in the neck, may be accompanied by “B symptoms” (fever, night sweats, unintentional weight loss of > 10%).24 If lymphoma is suspected, fine-needle aspiration (FNA) for cytology and flow cytometry should be performed. If lymphoma is confirmed, the GP should refer the patient to an appropriate medical oncologist for further evaluation, which may include referral to Oto-HNS for core or open biopsy. Contraindications to FNA of a neck mass include paragangliomas, such as a carotid body tumor.
Other cancers of the upper aerodigestive tract also often spread to the neck nodes and may initially present as a neck mass. A thorough examination can usually point to the primary cancer, and FNA will provide the diagnosis with high specificity and sensitivity.25 Midline cystic neck masses in close proximity to the hyoid bone are likely thyroglossal duct cysts. If these cysts grow, they likely require removal.
Salivary glands. The submandibular, sublingual, and parotid are the major salivary glands. There also are hundreds of small salivary glands scattered through the oral and pharyngeal mucosa. Tumors arising from the salivary glands represent about 6% of all head and neck masses; these tumors are nearly 3 times more common in men than in women.26 About 80% of salivary gland tumors originate in the parotid gland; patients with such tumors typically present with a painless parotid mass.26 In advanced cases, patients may present with skin infiltration and facial paralysis secondary to involvement of
the facial nerve that courses through the parotid gland after it exits the temporal bone near the mastoid tip.
Salivary gland tumors are most commonly benign, and pleomorphic adenomas are the most common benign parotid neoplasm.27 The incidence of malignancy is highest in submandibular, sublingual, and minor salivary glands. There are numerous primary salivary gland malignancies, such as mucoepidermoid carcinoma, adenocarcinoma, and adenoid cystic carcinoma. Facial skin SCC may metastasize to periparotid nodes. There are also multiple nonneoplastic causes of salivary gland inflammation. Recurrent diffuse, painful gland enlargement may be suggestive of recurrent sialadenitis and may be
secondary to a stone or xerostomia associated with dehydration or use of diuretics, antidepressants, or lithium. Multiple lymphoepithelial cysts may be associated with HIV and do not require resection.28
Management
After taking a thorough history and performing a physical examination, the physician evaluating a patient for HNC should proceed with diagnostic testing followed by referral to a specialist.
Diagnostic Testing
Laboratory values. Although laboratory values are unlikely to help in evaluation of a malignancy, elevated white blood cell count, erythrocyte sedimentation
rate, and C-reactive protein level are markers of a general inflammatory process that may support a clinically suspected diagnosis of infection. Values that decrease over time may represent progress toward disease resolution.29
Imaging. If malignancy is suspected, imaging should be obtained. Imaging has an important role in corroborating examination findings of a mass. Imaging
also provides an accurate baseline assessment of tumor size and extent. Recommended imaging modalities include:
- Ultrasonography (US). This quick and inexpensive modality can be used to visualize suspicious neck lesions. It is helpful in performing real-time assessments and differentiating cysts from solid masses and abscesses from reactive lymph nodes or infiltrative tumors. Challenges with US include its inability to penetrate bone and practitioners’ variable interpretation of images. A different modality invariably is needed to document location and spread of suspected HNC.
- MRI and CT. These are necessary for HNC evaluation and staging. Generally, they are equivalent in node assessment, but MRI is preferable in tongue and pharynx evaluation, and CT is preferable in the larynx. An ideal image should extend from the skull base to the clavicles, demonstrating the extent of the primary tumor and potential metastases to the neck nodes. As MRI is best protocoled by an experienced head and neck radiologist, it is preferable to refer the patient to such a specialist and allow Oto-HNS to arrange the imaging. Contraindications to MRI include pacemakers and shrapnel (common among veteran patients) and claustrophobia (common among patients with PTSD).
- PET-CT. This modality helps in staging, detecting distant metastases, assessing treatment response after chemoradiation, and locating the primary cancer when a proven neck metastasis has no obvious source. Whether PET-CT should be performed before initial referral should be discussed with the specialist. A case with a proven distant metastasis likely is not operable and would be better served with a referral to medical oncology.
Biopsy. For almost all HNCs, the initial biopsy modality should be FNA. Although intraoral lesions may benefit from incisional biopsy, this procedure should not delay triage and may be outside the scope of practice for many GPs. A GP can arrange for FNA to be performed before the referral appointment. This modality has excellent diagnostic sensitivity and specificity.30,31 In the setting of equivocal or negative results despite a high index of suspicion, having a more experienced cytopathologist repeat the FNA is often warranted. Excisional biopsy may be warranted if FNA is nondiagnostic or lymphoma is diagnosed.
Other Interventions
In some cases, the GP has additional important roles— in preparing the patient for the possibility of surgery, treating related conditions, helping the patient cope with this new medical challenge, improving nutrition, and promoting cessation of alcohol drinking and tobacco smoking.
Surgery. For patients with biopsy-proven HNC, preoperative assessment by the GP helps provide clearance for surgery, reduces time to treatment, and lessens the likelihood of postoperative complications. A recent study found that VA patients aged ≥ 70 years had a 30-day postoperative mortality rate of 6% and at least a ≥ 20% risk for a major complication during their hospital stay.32 Given these risks and the overall higher rate of chronic diseases among veterans, the authors recommend preoperative evaluation of comorbidities with particular emphasis on cardiac, renal, and pulmonary status. In addition, specific examinations (eg, electrocardiogram, chest radiograph, basic laboratory tests, liver profile test) are recommended for patients with a history of alcohol abuse.
Malnutrition. At initial diagnosis, many patients with HNC have significant weight loss.33 Unfortunately, the required complex treatment modalities increase malnutrition rates and decrease quality of life.34 Preventive strategies are, therefore, key in improving patients’ overall health. The authors recommend that GPs consider early nutritional consultations and as-needed speech therapy evaluations to provide preventive strategies and exercises to maintain proper swallowing function.35 Patients who are unable to eat because of aspiration caused by a large tumor should be admitted for preoperative gastric tube placement to improve nutrition and, ultimately, surgical outcome. A large percentage of veterans with HNC also have depression, which may lead to decreased appetite.36 Mental health consultations can help in these circumstances, as can use of mirtazapine, which increases appetite and treats depression-related symptoms.
Pain. Nonsteroidal anti-inflammatory drugs (NSAIDs) can be recommended for relief of uncontrolled mild to moderate pain, but they must be discontinued 1 week before surgery to reduce the risk for bleeding complications. The NSAIDs should be avoided entirely in patients with untreated friable tumors of the aerodigestive tract. In patients with biopsy-proven cancer, pain control can involve opiates per World Health Organization guidelines.37 Patients with head and neck SCC often have neuropathic pain, which is more effectively treated with gabapentin.
Alcohol drinking and tobacco smoking. Promoting cessation of these habits is essential for all patients, including those already diagnosed with cancer. Encouraging cessation as well as overall healthy lifestyle choices can reduce cancer risk and improve overall health—and may be the single most efficacious intervention a physician can offer.
Referral
Most patients with suspected HNC should be referred to Oto-HNS. In cases in which lymphoma is most highly suspected, medical oncology is the most appropriate initial referral. Early dental consultation is also necessary if an obturator will be needed (eg, as with a hard palate malignancy) or if irradiation is planned (radiation-induced xerostomia significantly increases the risk for dental caries).38 For all new cancer diagnoses, the GP can contact the Oto-HNS specialist for help in tailoring the patient evaluation to the practices and resources at the GP’s home institution and reduce time to treatment.
Conclusion
General practitioners are essential in identifying and triaging veterans with HNC. High-risk patients with a growing mass require proper assessment, including a thorough history and physical examination, FNA for diagnosis, and appropriate specialist referral. Although this article provides a helpful framework for thinking about patients with HNC, the authors encourage GPs to check the National Comprehensive Cancer Network guidelines for additional information on the topics covered here. With this knowledge, GPs can improve outcomes for veterans with HNC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
High-risk patients with a growing mass require proper assessment, including a thorough history, physical examination, and fine-needle aspiration for diagnosis.
A 65-year-old Vietnam veteran with a history of posttraumatic stress disorder (PTSD), transient ischemic attack, alcohol dependence, and a smoking history of 50 pack-years presents with a neck mass that has been growing for 2 months and unintentional weight loss of 25 pounds over 6 months. What is the differential diagnosis? How does a general practitioner evaluate, manage, and efficiently triage this patient?
Such cases, in which a head and neck cancer (HNC) diagnosis is suspected, can be unnerving for both physician and patient. However, knowledgeable general practitioners (GPs) can play a pivotal role in recognizing high-risk patients, initiating workups, and referring to appropriate specialists, resulting in earlier detection and potentially better outcomes.
This article outlines the authors’ recommended best practices for GPs treating patients with presumed HNC. Although the focus here is on the veteran population, in which HNC rates are significantly higher, many of the suggestions presented are applicable to the general population.
Background
Head and neck cancers represent a diverse family of malignancies of the nasopharynx, oropharynx, hypopharynx, larynx, oral cavity, paranasal sinuses, and salivary glands. This article does not cover thyroid, ophthalmologic, neurologic, or skin malignancies. The yearly worldwide incidence of all HNC cases is more than 550,000.1 In the U.S., 55,000 new HNC cases, representing 3% of all new malignancies, are reported annually. The 5-year survival rate is 60%, but 12,000 Americans die each year of head and neck cancer.2 Most HNCs occur in males aged ≥ 50 years, and the incidence increases with age. Almost $3.6 billion is spent treating HNC in the U.S. annually.3
Risk Factors
Alcohol and tobacco consumption strongly predisposes patients to squamous cell carcinoma (SCC), which accounts for 90% of HNCs. Together, alcohol and tobacco act multiplicatively.4,5 For instance, heavy drinkers (≥ 10 drinks daily) are at 5-fold increased risk for oral and pharyngeal cancers, heavy smokers (≥ 1 pack daily) are at 20-fold increased risk, and people who both drink and smoke heavily are at 50-fold increased risk.6 Head and neck cancer rates are significantly elevated even for moderate/light drinkers and smokers.
Veterans have disproportionately high rates of alcohol drinking and tobacco smoking,7,8 in part because these habits are ingrained in military culture. During World Wars I and II, tobacco companies supplied soldiers with daily rations of cigarettes,9 and advertisers targeted military personnel by linking smoking with patriotism, strength, and toughness.10 The VHA and the DoD reported that 33% of veterans and active-duty service personnel smoke—compared with 23% of civilians.11 Vietnam veterans, 47% of whom smoke, are at particular risk for HNC.12
Cessation of alcohol drinking and tobacco smoking is essential for overall prognosis, especially after HNC has been diagnosed. Continued smoking after HNC treatment increases the recurrence rate 4-fold.13 There also is mounting evidence that cessation of drinking and smoking can reverse the risk for HNC over time. According to a meta-analysis, quitting smoking for just 1 year begins to lower the risk for HNC. Quitting smoking for 20 years reduces the risk to the level of never smokers, and abstaining from alcohol for 20 years decreases the risk by nearly 40%.14
Viral infections are risk factors for development of oral cavity, oropharyngeal, laryngeal, and nasopharyngeal carcinoma (NPC). Sixty percent of oropharyngeal cancers are positive for human papillomavirus (HPV) infection,15 and most NPCs are associated with prior Epstein-Barr virus (EBV) exposure, particularly in populations from southern China, Southeast Asia, North Africa, and the Middle East.16
Evaluation
Workup of a possible HNC starts with taking a thorough history. Early HNC symptoms that may prompt a patient to seek medical care include neck mass, nonhealing oral ulcer, voice change, sore throat for more than 2 weeks, ear pain, nasal obstruction, serous otitis media, dysphagia, and odynophagia. Patients with advanced HNC may present with unintentional weight loss, decreased appetite, and cranial nerve deficits. For alcohol or tobacco users who present with any of the symptoms, SCC should lead the differential diagnosis, prompting examination of the head and neck. The authors present a general outline for performing this examination and detail the most common types of HNC encountered in the GP setting.
Physical Examination
The GP should perform a bimanual examination of the oral cavity, ears, nose, thyroid, and cranial nerve function with the help of a headlight. The physician should use 2 tongue blades to explore the oral cavity and palpate for suspicious oral lesions. It is often possible to feel a lesion before visualizing it on the base of the tongue. If there is a presenting mass, the physician should document the mass site, size, shape, consistency, tenderness, mobility, and accompanying deficits or symptoms.
Also recommended is a thorough examination of the facial, submandibular, and other cervical lymph nodes. The drainage patterns of these nodes can help the GP track potential routes of malignant infiltration. The submental and submandibular lymph nodes (level 1) drain the lower lip, floor of mouth, anterior tongue, and side of nose. The nodes along the mid and internal jugular vein (levels 2-4) and between the sternocleidomastoid and trapezius muscles (level 5) drain the oropharynx, mid tongue, larynx, hypopharynx, parotid gland, and skin of the face and ear. Nontender hard nodes are more likely to be malignant, as are nodes of the posterior triangle (level 5).17
Malignancy by Site
Oral cavity. The oral cavity includes the lips, buccal mucosa, teeth, gums, anterior two-thirds of tongue, floor of mouth, alveolar ridge, retromolar trigone, and hard palate. The oral cavity is the most common site for HNCs.18 The most common symptoms of malignancy of the oral cavity include dysphonia, nonhealing oral ulcers, loose teeth, bleeding, change in denture fit, and chin numbness, which could indicate mandibular invasion with inferior alveolar nerve involvement.19
For thorough assessment of the oral cavity, the patient should remove all temporary dental appliances. Then, with a tongue blade in each hand, the physician should thoroughly examine the oral mucosa, moving the tongue laterally to evaluate the floor of mouth, and palpate the mucosal surfaces to identify submucosal cancers in the posterior tongue and floor of mouth. Minor salivary glands are ubiquitous in the oral cavity and may be involved by cancer. Ulcerated painful lesions that last longer than 2 weeks are less likely to be common viral or aphthous ulcers. For either an oral cavity mass or a nonhealing ulcer that persists more than 4 weeks, malignancy should be suspected, and the patient should be referred for imaging and biopsy.
Leukoplakias are white patches in the oral cavity that develop from squamous epithelial hyperplasia and cannot be scraped away with a tonghpvue blade. The lesions are usually benign, but, if there is an element of redness (erythroplakia), the risk for harboring dysplasia is much higher, though the differential diagnosis includes trauma from adjacent teeth or lichen planus. If leukoplakia is seen, the physician should accurately note the size, location, and site and should monitor every 3 to 4 months. If erythroplakia, enlargement of leukoplakia, or any evidence of mucosal invasion is noted, the physician should refer to otolaryngologyhead and neck surgery (Oto-HNS). The authors advise against lasering leukoplakia; it is unnecessary, can make subsequent evaluation more difficult, and can mask recurrent malignancy.
Oropharynx. The oropharynx includes the posterior third of tongue, soft palate, palatine and lingual tonsils, and the posterior and lateral pharyngeal walls superior to tip of epiglottis. Cancers can arise in any of these locations and may present with dysphagia, odynophagia, referred
otalgia, hoarseness, and enlarged lymph nodes. In advanced cases, there may be bleeding, airway obstruction, and aspiration. Nonsmokers with oropharyngeal SCC are likely to be HPV positive and may be younger than the typical patient with alcohol- or tobacco-related HNC. Human papillomavirus positive oropharyngeal carcinoma has a much better prognosis than its tobaccorelated counterpart does. Physical examination should include assessment of tonsillar size and symmetry, palpation of neck lymph nodes, and palpation of base of tongue. Treatment may involve surgery, radiation, or chemoradiation, depending on factors such as extent of disease and comorbidities.
Nasopharynx. The nasopharynx extends from the nasal cavity (posterior to nasal septum) to the oropharynx. The most common NPC symptoms are middle-ear effusion and enlarged neck nodes. Nasal obstruction, epistaxis, or cranial nerve deficits also may occur. The nasopharynx
is best assessed with a fiberoptic scope. Most NPCs are associated with EBV infection, and viral levels can be used to monitor response to treatment.20 Early biopsy is indicated if a nasopharyngeal mass is found.
Larynx. As with the nasopharynx, the larynx is best seen with a fiberoptic scope. Malignancy generally presents with hoarseness, voice changes, cough, sore throat, or, if more advanced, airway compromise such as stridor and neck adenopathy. As larynx HNCs may be associated
with aspiration, the authors recommend asking “Does food go down the wrong pipe?” or “Do you cough when you eat?” and having the patient drink and document any difficulty. A smoker with hoarseness lasting more than 2 weeks should be referred to Oto-HNS for endoscopic assessment. Among veterans, other causes of hoarseness include polyps,Candida infection associated with inhalation of steroids for chronic obstructive pulmonary disease, and recurrent nerve paralysis from thyroid or lung cancer.
Neck. Patients with HNCs commonly present with a neck mass. Fifty percent to 80% of adults with a nontender neck mass are harboring a malignancy.21,22 In a patient without HIV, a neck mass larger than 2 cm should be evaluated for cancer, especially if the mass is hard and nontender.23 Computed tomography (CT) is recommended for initial evaluation, which, if there is FNA confirmed carcinoma, should be followed by positron emission tomography (PET). If there is concern for parotidor skull base tumors, magnetic resonance imaging (MRI) is preferable for demonstrating soft-tissue definition and disease extent.
If the patient is aged < 40 years and lymph nodes have been present for less than 2 to 4 weeks, are tender, or are associated with fever or poor dental hygiene, then an infection may instead be the cause. Dentistry referral and/or an antibiotic trial should be considered. Lymphomas, also common in the neck, may be accompanied by “B symptoms” (fever, night sweats, unintentional weight loss of > 10%).24 If lymphoma is suspected, fine-needle aspiration (FNA) for cytology and flow cytometry should be performed. If lymphoma is confirmed, the GP should refer the patient to an appropriate medical oncologist for further evaluation, which may include referral to Oto-HNS for core or open biopsy. Contraindications to FNA of a neck mass include paragangliomas, such as a carotid body tumor.
Other cancers of the upper aerodigestive tract also often spread to the neck nodes and may initially present as a neck mass. A thorough examination can usually point to the primary cancer, and FNA will provide the diagnosis with high specificity and sensitivity.25 Midline cystic neck masses in close proximity to the hyoid bone are likely thyroglossal duct cysts. If these cysts grow, they likely require removal.
Salivary glands. The submandibular, sublingual, and parotid are the major salivary glands. There also are hundreds of small salivary glands scattered through the oral and pharyngeal mucosa. Tumors arising from the salivary glands represent about 6% of all head and neck masses; these tumors are nearly 3 times more common in men than in women.26 About 80% of salivary gland tumors originate in the parotid gland; patients with such tumors typically present with a painless parotid mass.26 In advanced cases, patients may present with skin infiltration and facial paralysis secondary to involvement of
the facial nerve that courses through the parotid gland after it exits the temporal bone near the mastoid tip.
Salivary gland tumors are most commonly benign, and pleomorphic adenomas are the most common benign parotid neoplasm.27 The incidence of malignancy is highest in submandibular, sublingual, and minor salivary glands. There are numerous primary salivary gland malignancies, such as mucoepidermoid carcinoma, adenocarcinoma, and adenoid cystic carcinoma. Facial skin SCC may metastasize to periparotid nodes. There are also multiple nonneoplastic causes of salivary gland inflammation. Recurrent diffuse, painful gland enlargement may be suggestive of recurrent sialadenitis and may be
secondary to a stone or xerostomia associated with dehydration or use of diuretics, antidepressants, or lithium. Multiple lymphoepithelial cysts may be associated with HIV and do not require resection.28
Management
After taking a thorough history and performing a physical examination, the physician evaluating a patient for HNC should proceed with diagnostic testing followed by referral to a specialist.
Diagnostic Testing
Laboratory values. Although laboratory values are unlikely to help in evaluation of a malignancy, elevated white blood cell count, erythrocyte sedimentation
rate, and C-reactive protein level are markers of a general inflammatory process that may support a clinically suspected diagnosis of infection. Values that decrease over time may represent progress toward disease resolution.29
Imaging. If malignancy is suspected, imaging should be obtained. Imaging has an important role in corroborating examination findings of a mass. Imaging
also provides an accurate baseline assessment of tumor size and extent. Recommended imaging modalities include:
- Ultrasonography (US). This quick and inexpensive modality can be used to visualize suspicious neck lesions. It is helpful in performing real-time assessments and differentiating cysts from solid masses and abscesses from reactive lymph nodes or infiltrative tumors. Challenges with US include its inability to penetrate bone and practitioners’ variable interpretation of images. A different modality invariably is needed to document location and spread of suspected HNC.
- MRI and CT. These are necessary for HNC evaluation and staging. Generally, they are equivalent in node assessment, but MRI is preferable in tongue and pharynx evaluation, and CT is preferable in the larynx. An ideal image should extend from the skull base to the clavicles, demonstrating the extent of the primary tumor and potential metastases to the neck nodes. As MRI is best protocoled by an experienced head and neck radiologist, it is preferable to refer the patient to such a specialist and allow Oto-HNS to arrange the imaging. Contraindications to MRI include pacemakers and shrapnel (common among veteran patients) and claustrophobia (common among patients with PTSD).
- PET-CT. This modality helps in staging, detecting distant metastases, assessing treatment response after chemoradiation, and locating the primary cancer when a proven neck metastasis has no obvious source. Whether PET-CT should be performed before initial referral should be discussed with the specialist. A case with a proven distant metastasis likely is not operable and would be better served with a referral to medical oncology.
Biopsy. For almost all HNCs, the initial biopsy modality should be FNA. Although intraoral lesions may benefit from incisional biopsy, this procedure should not delay triage and may be outside the scope of practice for many GPs. A GP can arrange for FNA to be performed before the referral appointment. This modality has excellent diagnostic sensitivity and specificity.30,31 In the setting of equivocal or negative results despite a high index of suspicion, having a more experienced cytopathologist repeat the FNA is often warranted. Excisional biopsy may be warranted if FNA is nondiagnostic or lymphoma is diagnosed.
Other Interventions
In some cases, the GP has additional important roles— in preparing the patient for the possibility of surgery, treating related conditions, helping the patient cope with this new medical challenge, improving nutrition, and promoting cessation of alcohol drinking and tobacco smoking.
Surgery. For patients with biopsy-proven HNC, preoperative assessment by the GP helps provide clearance for surgery, reduces time to treatment, and lessens the likelihood of postoperative complications. A recent study found that VA patients aged ≥ 70 years had a 30-day postoperative mortality rate of 6% and at least a ≥ 20% risk for a major complication during their hospital stay.32 Given these risks and the overall higher rate of chronic diseases among veterans, the authors recommend preoperative evaluation of comorbidities with particular emphasis on cardiac, renal, and pulmonary status. In addition, specific examinations (eg, electrocardiogram, chest radiograph, basic laboratory tests, liver profile test) are recommended for patients with a history of alcohol abuse.
Malnutrition. At initial diagnosis, many patients with HNC have significant weight loss.33 Unfortunately, the required complex treatment modalities increase malnutrition rates and decrease quality of life.34 Preventive strategies are, therefore, key in improving patients’ overall health. The authors recommend that GPs consider early nutritional consultations and as-needed speech therapy evaluations to provide preventive strategies and exercises to maintain proper swallowing function.35 Patients who are unable to eat because of aspiration caused by a large tumor should be admitted for preoperative gastric tube placement to improve nutrition and, ultimately, surgical outcome. A large percentage of veterans with HNC also have depression, which may lead to decreased appetite.36 Mental health consultations can help in these circumstances, as can use of mirtazapine, which increases appetite and treats depression-related symptoms.
Pain. Nonsteroidal anti-inflammatory drugs (NSAIDs) can be recommended for relief of uncontrolled mild to moderate pain, but they must be discontinued 1 week before surgery to reduce the risk for bleeding complications. The NSAIDs should be avoided entirely in patients with untreated friable tumors of the aerodigestive tract. In patients with biopsy-proven cancer, pain control can involve opiates per World Health Organization guidelines.37 Patients with head and neck SCC often have neuropathic pain, which is more effectively treated with gabapentin.
Alcohol drinking and tobacco smoking. Promoting cessation of these habits is essential for all patients, including those already diagnosed with cancer. Encouraging cessation as well as overall healthy lifestyle choices can reduce cancer risk and improve overall health—and may be the single most efficacious intervention a physician can offer.
Referral
Most patients with suspected HNC should be referred to Oto-HNS. In cases in which lymphoma is most highly suspected, medical oncology is the most appropriate initial referral. Early dental consultation is also necessary if an obturator will be needed (eg, as with a hard palate malignancy) or if irradiation is planned (radiation-induced xerostomia significantly increases the risk for dental caries).38 For all new cancer diagnoses, the GP can contact the Oto-HNS specialist for help in tailoring the patient evaluation to the practices and resources at the GP’s home institution and reduce time to treatment.
Conclusion
General practitioners are essential in identifying and triaging veterans with HNC. High-risk patients with a growing mass require proper assessment, including a thorough history and physical examination, FNA for diagnosis, and appropriate specialist referral. Although this article provides a helpful framework for thinking about patients with HNC, the authors encourage GPs to check the National Comprehensive Cancer Network guidelines for additional information on the topics covered here. With this knowledge, GPs can improve outcomes for veterans with HNC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
3. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2): 117-128.
4. Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135(3):451-457.
5. Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541-550.
6. Rodriguez T, Altieri A, Chatenoud L, et al. Risk factors for oral and pharyngeal cancer in young adults. Oral Oncol. 2004;40(2):207-213.
7. National Survey on Drug Use and Health. Alcohol Use and Alcohol-Related Risk Behaviors Among Veterans. NIH publication 99-4323. Rockville, MD: National Institutes of Health, U.S. Dept of Health and Human Services; 2005.
8. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-217.
9. Smith EA, Malone RE. “Everywhere the soldier will be”: wartime tobacco promotion in the US military. Am J Public Health. 2009;99(9):1595-1602.
10. James R, Olstad S. Cigarette advertising. Time. June 15, 2009.
11. Miller DR, Kalman D, Ren XS, Lee AF, Niu Z, Kazis LE. Health Behaviors of Veterans in the VHA: Tobacco Use: 1999 Large Health Survey of VHA Enrollees. Washington, DC: Office of Quality and Performance, Veterans Health Administration, U.S. Dept of Veterans Affairs; 2001.
12. Bray RM, Hourani LL, Rae Olmstead KL, et al. 2005 Department of Defense Survey of Health Related Behaviors Among Active Duty Military Personnel: A Component of the Defense Lifestyle Assessment Program (DLAP). Report prepared for U.S. Dept of Defense (Cooperative Agreement No. DAMD17-00-2-0057). Research Triangle Park, NC: RTI International; 2006.
13. Stevens MH, Gardner JW, Parkin JL, Johnson LP. Head and neck cancer survival and life-style change. Arch Otolaryngol. 1983;109(11):746-749.
14. Marron M, Boffetta P, Zhang ZF, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol. 2010;39(1):182-196.
15. Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31(6):744-754.
16. Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333(11):693-698.
17. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66(11):2103-2110.
18. American Cancer Society. Oral cavity and oropharyngeal cancer. American Cancer Society website. http://www.cancer.org/acs/groups/cid/documents/webcontent/003128-pdf.pdf. Updated January 27, 2016. Accessed July 6, 2016.
19. Pandey M, Rao LP, Das SR, Mathews A, Chacko EM, Naik BR. Patterns of mandibular invasion in oral squamous cell carcinoma of the mandibular region. World J Surg Oncol. 2007;5:12.
20. Leung SF, Zee B, Ma BB, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006;24(34):5414-5418.
21. Dickson PV, Davidoff AM. Malignant neoplasms of the head and neck. Semin Pediatr Surg. 2006;15(2):92-98.
22. Lefebvre JL, Coche-Dequeant B, Van JT, Buisset E, Adenis A. Cervical lymph nodes from an unknown primary tumor in 190 patients. Am J Surg. 1990;160(4):443-446.
23. Bhattacharyya N. Predictive factors for neoplasia and malignancy in a neck mass. Arch Otolaryngol Head Neck Surg. 1999;125(3):303-307.
24. Dailey SH, Sataloff RT. Lymphoma: an update on evolving trends in staging and management. Ear Nose Throat J. 2001;80(3):164-170.
25. Schwarz R, Chan NH, MacFarlane JK. Fine needle aspiration cytology in the evaluation of head and neck masses. Am J Surg. 1990;159(5):482-485.
26. Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806-816.
27. Jones AV, Craig GT, Speight PM, Franklin CD. The range and demographics of salivary gland tumours diagnosed in a UK population. Oral Oncol. 2008;44(4):407-417.
28. Shebl FM, Bhatia K, Engels EA. Salivary gland and nasopharyngeal cancers in individuals with acquired immunodeficiency syndrome in United States. Int J Cancer. 2010;126(10):2503-2508.
29. Bien´ E, Balcerska A. Clinical significance of erythrocyte sedimentation rate, C-reactive protein and serum lactate dehydrogenase levels in the diagnosis, prognosis and treatment monitoring of children suffering from cancer [in Polish]. Med Wieku Rozwoj. 2004;8(4, pt 2):1081-1089.
30. Wu M, Burstein DE, Yuan S, et al. A comparative study of 200 fine needle aspiration biopsies performed by clinicians and cytopathologists. Laryngoscope. 2006;116(7):1212-1215.
31. Liu ES, Bernstein JM, Sculerati N, Wu HC. Fine needle aspiration biopsy of pediatric head and neck masses. Int J Pediatr Otorhinolaryngol. 2001;60(2):135-140.
32. Story DA. Postoperative complications in elderly patients and their significance for long-term prognosis. Curr Opin Anaesthesiol. 2008;21(3):375-379.
33. Head BA, Heitz L, Keeney C, et al. The relationship between weight loss and health-related quality of life in persons treated for head and neck cancer. Support Care Cancer. 2011;19(10):1511-1518.
34. van den Berg MG, Rasmussen-Conrad EL, van Nispen L, van Binsbergen JJ,
Merkx MA. A prospective study on malnutrition and quality of life in patients
with head and neck cancer. Oral Oncol. 2008;44(9):830-837.
35. Murphy BA, Deng J. Advances in supportive care for late effects of head and neck cancer. J Clin Oncol. 2015;33(29):3314-3321.
36. Pandey M, Devi N, Thomas BC, Kumar SV, Krishnan R, Ramdas K. Distress overlaps with anxiety and depression in patients with head and neck cancer. Psychooncology. 2007;16(6):582-586.
37. World Health Organization. Cancer Pain Relief: With a Guide to Opioid Availability.
2nd ed. Geneva, Switzerland: World Health Organization; 1996.
38. Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and cancer
therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62(6):400-422.
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
3. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2): 117-128.
4. Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135(3):451-457.
5. Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541-550.
6. Rodriguez T, Altieri A, Chatenoud L, et al. Risk factors for oral and pharyngeal cancer in young adults. Oral Oncol. 2004;40(2):207-213.
7. National Survey on Drug Use and Health. Alcohol Use and Alcohol-Related Risk Behaviors Among Veterans. NIH publication 99-4323. Rockville, MD: National Institutes of Health, U.S. Dept of Health and Human Services; 2005.
8. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-217.
9. Smith EA, Malone RE. “Everywhere the soldier will be”: wartime tobacco promotion in the US military. Am J Public Health. 2009;99(9):1595-1602.
10. James R, Olstad S. Cigarette advertising. Time. June 15, 2009.
11. Miller DR, Kalman D, Ren XS, Lee AF, Niu Z, Kazis LE. Health Behaviors of Veterans in the VHA: Tobacco Use: 1999 Large Health Survey of VHA Enrollees. Washington, DC: Office of Quality and Performance, Veterans Health Administration, U.S. Dept of Veterans Affairs; 2001.
12. Bray RM, Hourani LL, Rae Olmstead KL, et al. 2005 Department of Defense Survey of Health Related Behaviors Among Active Duty Military Personnel: A Component of the Defense Lifestyle Assessment Program (DLAP). Report prepared for U.S. Dept of Defense (Cooperative Agreement No. DAMD17-00-2-0057). Research Triangle Park, NC: RTI International; 2006.
13. Stevens MH, Gardner JW, Parkin JL, Johnson LP. Head and neck cancer survival and life-style change. Arch Otolaryngol. 1983;109(11):746-749.
14. Marron M, Boffetta P, Zhang ZF, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol. 2010;39(1):182-196.
15. Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31(6):744-754.
16. Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333(11):693-698.
17. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66(11):2103-2110.
18. American Cancer Society. Oral cavity and oropharyngeal cancer. American Cancer Society website. http://www.cancer.org/acs/groups/cid/documents/webcontent/003128-pdf.pdf. Updated January 27, 2016. Accessed July 6, 2016.
19. Pandey M, Rao LP, Das SR, Mathews A, Chacko EM, Naik BR. Patterns of mandibular invasion in oral squamous cell carcinoma of the mandibular region. World J Surg Oncol. 2007;5:12.
20. Leung SF, Zee B, Ma BB, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006;24(34):5414-5418.
21. Dickson PV, Davidoff AM. Malignant neoplasms of the head and neck. Semin Pediatr Surg. 2006;15(2):92-98.
22. Lefebvre JL, Coche-Dequeant B, Van JT, Buisset E, Adenis A. Cervical lymph nodes from an unknown primary tumor in 190 patients. Am J Surg. 1990;160(4):443-446.
23. Bhattacharyya N. Predictive factors for neoplasia and malignancy in a neck mass. Arch Otolaryngol Head Neck Surg. 1999;125(3):303-307.
24. Dailey SH, Sataloff RT. Lymphoma: an update on evolving trends in staging and management. Ear Nose Throat J. 2001;80(3):164-170.
25. Schwarz R, Chan NH, MacFarlane JK. Fine needle aspiration cytology in the evaluation of head and neck masses. Am J Surg. 1990;159(5):482-485.
26. Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806-816.
27. Jones AV, Craig GT, Speight PM, Franklin CD. The range and demographics of salivary gland tumours diagnosed in a UK population. Oral Oncol. 2008;44(4):407-417.
28. Shebl FM, Bhatia K, Engels EA. Salivary gland and nasopharyngeal cancers in individuals with acquired immunodeficiency syndrome in United States. Int J Cancer. 2010;126(10):2503-2508.
29. Bien´ E, Balcerska A. Clinical significance of erythrocyte sedimentation rate, C-reactive protein and serum lactate dehydrogenase levels in the diagnosis, prognosis and treatment monitoring of children suffering from cancer [in Polish]. Med Wieku Rozwoj. 2004;8(4, pt 2):1081-1089.
30. Wu M, Burstein DE, Yuan S, et al. A comparative study of 200 fine needle aspiration biopsies performed by clinicians and cytopathologists. Laryngoscope. 2006;116(7):1212-1215.
31. Liu ES, Bernstein JM, Sculerati N, Wu HC. Fine needle aspiration biopsy of pediatric head and neck masses. Int J Pediatr Otorhinolaryngol. 2001;60(2):135-140.
32. Story DA. Postoperative complications in elderly patients and their significance for long-term prognosis. Curr Opin Anaesthesiol. 2008;21(3):375-379.
33. Head BA, Heitz L, Keeney C, et al. The relationship between weight loss and health-related quality of life in persons treated for head and neck cancer. Support Care Cancer. 2011;19(10):1511-1518.
34. van den Berg MG, Rasmussen-Conrad EL, van Nispen L, van Binsbergen JJ,
Merkx MA. A prospective study on malnutrition and quality of life in patients
with head and neck cancer. Oral Oncol. 2008;44(9):830-837.
35. Murphy BA, Deng J. Advances in supportive care for late effects of head and neck cancer. J Clin Oncol. 2015;33(29):3314-3321.
36. Pandey M, Devi N, Thomas BC, Kumar SV, Krishnan R, Ramdas K. Distress overlaps with anxiety and depression in patients with head and neck cancer. Psychooncology. 2007;16(6):582-586.
37. World Health Organization. Cancer Pain Relief: With a Guide to Opioid Availability.
2nd ed. Geneva, Switzerland: World Health Organization; 1996.
38. Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and cancer
therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62(6):400-422.