User login
Part D Reduced Nondrug Medical Spending
Major Finding: Medicare Part D reduced nondrug medical spending for beneficiaries who had limited drug coverage prior to enrolling in the federal prescription drug plan by 10.6%.
Data Source: Data from the Health and Retirement Study survey linked with Medicare claims from 2004 to 2007.
Disclosures: The authors had no conflicts of interest to disclose. The study was supported by grants from several charitable foundations.
Medicare Part D coverage significantly reduced nondrug medical spending for beneficiaries who had limited drug coverage prior to the start of the federal prescription drug plan, Harvard Medical School researchers reported.
The 10.6% savings was mostly due to a decrease in spending on acute and postacute care under Medicare Part A (JAMA 2011;306:402-409).
“These reductions in nondrug medical spending suggest that Part D has not cost as much as what we initially thought,” Dr. J. Michael McWilliams, the study's lead author, said in an interview.
The findings could also lend support to the Affordable Care Act's goal of closing the “doughnut hole,” the gap in drug coverage under Part D, he added. “The cost of closing the doughnut hole could be less than what we might expect because of these partially offsetting reductions in spending on nondrug medical care.”
The results also highlight a need for better coordination between all parts of Medicare, the investigators wrote. “Even though Part D plans function completely separately from Part A and Part B of the Medicare program, and even though they have no financial incentive to lower copayments, particularly for beneficial medications, clearly providing this benefit to seniors through stand-alone Part D plans has been quite effective,” Dr. McWilliams said.
The authors used data from the Health and Retirement Study and linked it to Medicare claims data from 2004 to 2007 on 6,001 beneficiaries, then categorized the beneficiaries as having had generous (2,538) and limited (3,463) drug coverage prior to implementation of Part D. Nontraditional Medicare beneficiaries, such as those who qualified for Medicare before age 65, were excluded.
For the control cohort, they selected a similar group of 5,988 beneficiaries who had generous (2,537) and limited (3,451) drug coverage in 2002. They studied the group up to 2005.
They found total nondrug medical spending before Part D implementation was not significantly higher for beneficiaries with limited drug coverage compared with those who had generous drug coverage (7.6% relative difference). However, after implementation of Part D, nondrug medical spending for beneficiaries who previously had limited drug coverage was 3.9% lower than those with generous drug coverage, leading to a significant differential reduction of 10.6%.
In dollars, Medicare spent nearly $306 per quarter less than expected on beneficiaries who previously had limited drug coverage.
“The economic and clinical benefits suggested by these reductions may be enhanced by further expansions in prescription drug coverage for seniors, improvements in benefit designs for drug-sensitive conditions, and policies that integrate Medicare payment and delivery systems across drug and nondrug services,” the authors wrote.
Previous studies have shown that the implementation of Part D has been associated with reduced out-of-pocket costs and better medication adherence (N. Engl. J. Med. 2009;361:52-61).
'The cost of closing the doughnut hole could be' partially offset by these reductions in nondrug spending.
Source DR. MCWILLIAMS
Major Finding: Medicare Part D reduced nondrug medical spending for beneficiaries who had limited drug coverage prior to enrolling in the federal prescription drug plan by 10.6%.
Data Source: Data from the Health and Retirement Study survey linked with Medicare claims from 2004 to 2007.
Disclosures: The authors had no conflicts of interest to disclose. The study was supported by grants from several charitable foundations.
Medicare Part D coverage significantly reduced nondrug medical spending for beneficiaries who had limited drug coverage prior to the start of the federal prescription drug plan, Harvard Medical School researchers reported.
The 10.6% savings was mostly due to a decrease in spending on acute and postacute care under Medicare Part A (JAMA 2011;306:402-409).
“These reductions in nondrug medical spending suggest that Part D has not cost as much as what we initially thought,” Dr. J. Michael McWilliams, the study's lead author, said in an interview.
The findings could also lend support to the Affordable Care Act's goal of closing the “doughnut hole,” the gap in drug coverage under Part D, he added. “The cost of closing the doughnut hole could be less than what we might expect because of these partially offsetting reductions in spending on nondrug medical care.”
The results also highlight a need for better coordination between all parts of Medicare, the investigators wrote. “Even though Part D plans function completely separately from Part A and Part B of the Medicare program, and even though they have no financial incentive to lower copayments, particularly for beneficial medications, clearly providing this benefit to seniors through stand-alone Part D plans has been quite effective,” Dr. McWilliams said.
The authors used data from the Health and Retirement Study and linked it to Medicare claims data from 2004 to 2007 on 6,001 beneficiaries, then categorized the beneficiaries as having had generous (2,538) and limited (3,463) drug coverage prior to implementation of Part D. Nontraditional Medicare beneficiaries, such as those who qualified for Medicare before age 65, were excluded.
For the control cohort, they selected a similar group of 5,988 beneficiaries who had generous (2,537) and limited (3,451) drug coverage in 2002. They studied the group up to 2005.
They found total nondrug medical spending before Part D implementation was not significantly higher for beneficiaries with limited drug coverage compared with those who had generous drug coverage (7.6% relative difference). However, after implementation of Part D, nondrug medical spending for beneficiaries who previously had limited drug coverage was 3.9% lower than those with generous drug coverage, leading to a significant differential reduction of 10.6%.
In dollars, Medicare spent nearly $306 per quarter less than expected on beneficiaries who previously had limited drug coverage.
“The economic and clinical benefits suggested by these reductions may be enhanced by further expansions in prescription drug coverage for seniors, improvements in benefit designs for drug-sensitive conditions, and policies that integrate Medicare payment and delivery systems across drug and nondrug services,” the authors wrote.
Previous studies have shown that the implementation of Part D has been associated with reduced out-of-pocket costs and better medication adherence (N. Engl. J. Med. 2009;361:52-61).
'The cost of closing the doughnut hole could be' partially offset by these reductions in nondrug spending.
Source DR. MCWILLIAMS
Major Finding: Medicare Part D reduced nondrug medical spending for beneficiaries who had limited drug coverage prior to enrolling in the federal prescription drug plan by 10.6%.
Data Source: Data from the Health and Retirement Study survey linked with Medicare claims from 2004 to 2007.
Disclosures: The authors had no conflicts of interest to disclose. The study was supported by grants from several charitable foundations.
Medicare Part D coverage significantly reduced nondrug medical spending for beneficiaries who had limited drug coverage prior to the start of the federal prescription drug plan, Harvard Medical School researchers reported.
The 10.6% savings was mostly due to a decrease in spending on acute and postacute care under Medicare Part A (JAMA 2011;306:402-409).
“These reductions in nondrug medical spending suggest that Part D has not cost as much as what we initially thought,” Dr. J. Michael McWilliams, the study's lead author, said in an interview.
The findings could also lend support to the Affordable Care Act's goal of closing the “doughnut hole,” the gap in drug coverage under Part D, he added. “The cost of closing the doughnut hole could be less than what we might expect because of these partially offsetting reductions in spending on nondrug medical care.”
The results also highlight a need for better coordination between all parts of Medicare, the investigators wrote. “Even though Part D plans function completely separately from Part A and Part B of the Medicare program, and even though they have no financial incentive to lower copayments, particularly for beneficial medications, clearly providing this benefit to seniors through stand-alone Part D plans has been quite effective,” Dr. McWilliams said.
The authors used data from the Health and Retirement Study and linked it to Medicare claims data from 2004 to 2007 on 6,001 beneficiaries, then categorized the beneficiaries as having had generous (2,538) and limited (3,463) drug coverage prior to implementation of Part D. Nontraditional Medicare beneficiaries, such as those who qualified for Medicare before age 65, were excluded.
For the control cohort, they selected a similar group of 5,988 beneficiaries who had generous (2,537) and limited (3,451) drug coverage in 2002. They studied the group up to 2005.
They found total nondrug medical spending before Part D implementation was not significantly higher for beneficiaries with limited drug coverage compared with those who had generous drug coverage (7.6% relative difference). However, after implementation of Part D, nondrug medical spending for beneficiaries who previously had limited drug coverage was 3.9% lower than those with generous drug coverage, leading to a significant differential reduction of 10.6%.
In dollars, Medicare spent nearly $306 per quarter less than expected on beneficiaries who previously had limited drug coverage.
“The economic and clinical benefits suggested by these reductions may be enhanced by further expansions in prescription drug coverage for seniors, improvements in benefit designs for drug-sensitive conditions, and policies that integrate Medicare payment and delivery systems across drug and nondrug services,” the authors wrote.
Previous studies have shown that the implementation of Part D has been associated with reduced out-of-pocket costs and better medication adherence (N. Engl. J. Med. 2009;361:52-61).
'The cost of closing the doughnut hole could be' partially offset by these reductions in nondrug spending.
Source DR. MCWILLIAMS
Early Podiatry Referral Eased Pain, Preserved Function
If patients in early stages of rheumatoid arthritis have foot problems, it is “crucial” to refer them to podiatrists, according to recent findings.
Roughly 90% of people with RA eventually develop foot or ankle symptoms, according to investigator Marike van der Leeden, Ph.D., a senior researcher and project leader at Reade, rehabilitation and rheumatology in Amsterdam.
Prescription foot orthoses is one of the ways to manage the patients' foot problems.
However, “indications for foot orthoses are not clear, and the effectiveness of the intervention is highly variable among patients,” according to the study.
To determine the clinical and demographic factors that predict the outcome of customized foot orthoses on related pain and disability, researchers conducted a prospective cohort study, which included 135 RA patients who were given customized foot orthoses made by a podiatrist.
Pain and disability were measured before and after the intervention period using a Numeric Rating Scale (NRS) for foot pain, the Foot Function Index (FFI), the Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC), and a 10-meter walking time test, according to the study.
The intervention period included one or more podiatrist appointments during which the foot problem was diagnosed and managed.
The results showed that the duration of RA was negatively associated with score changes in NRS foot pain (P = .018), WOMAC pain (P = .001), FFI disability (P = .003), and WOMAC physical function (P = .002).
Age was negatively associated with the change score in 10-meter walking time (P = .008).
Statistically significant improvements after the intervention with foot orthoses were found on all outcome measures (P less than .001), according to the study.
“Shorter disease duration predicted greater improvements in self-reported foot pain and disability after intervention with foot orthoses,” the authors concluded.
“Younger age predicted greater improvements in walking time. Referral for conservative management with foot orthoses in the early stage of RA seems important when aiming to achieve reduction in pain and improvement in daily activities.”
“To date, no evidence is available for which types of foot orthoses are most effective for RA. Further research is needed for evidence-based prescription protocols for foot problems in RA.” Dr. van der Leeden said.
The authors reported no financial disclosures.
To date there are no data on the most effective type of foot orthoses for patients with RA-induced foot pain.
Source DR. VAN DER LEEDEN
If patients in early stages of rheumatoid arthritis have foot problems, it is “crucial” to refer them to podiatrists, according to recent findings.
Roughly 90% of people with RA eventually develop foot or ankle symptoms, according to investigator Marike van der Leeden, Ph.D., a senior researcher and project leader at Reade, rehabilitation and rheumatology in Amsterdam.
Prescription foot orthoses is one of the ways to manage the patients' foot problems.
However, “indications for foot orthoses are not clear, and the effectiveness of the intervention is highly variable among patients,” according to the study.
To determine the clinical and demographic factors that predict the outcome of customized foot orthoses on related pain and disability, researchers conducted a prospective cohort study, which included 135 RA patients who were given customized foot orthoses made by a podiatrist.
Pain and disability were measured before and after the intervention period using a Numeric Rating Scale (NRS) for foot pain, the Foot Function Index (FFI), the Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC), and a 10-meter walking time test, according to the study.
The intervention period included one or more podiatrist appointments during which the foot problem was diagnosed and managed.
The results showed that the duration of RA was negatively associated with score changes in NRS foot pain (P = .018), WOMAC pain (P = .001), FFI disability (P = .003), and WOMAC physical function (P = .002).
Age was negatively associated with the change score in 10-meter walking time (P = .008).
Statistically significant improvements after the intervention with foot orthoses were found on all outcome measures (P less than .001), according to the study.
“Shorter disease duration predicted greater improvements in self-reported foot pain and disability after intervention with foot orthoses,” the authors concluded.
“Younger age predicted greater improvements in walking time. Referral for conservative management with foot orthoses in the early stage of RA seems important when aiming to achieve reduction in pain and improvement in daily activities.”
“To date, no evidence is available for which types of foot orthoses are most effective for RA. Further research is needed for evidence-based prescription protocols for foot problems in RA.” Dr. van der Leeden said.
The authors reported no financial disclosures.
To date there are no data on the most effective type of foot orthoses for patients with RA-induced foot pain.
Source DR. VAN DER LEEDEN
If patients in early stages of rheumatoid arthritis have foot problems, it is “crucial” to refer them to podiatrists, according to recent findings.
Roughly 90% of people with RA eventually develop foot or ankle symptoms, according to investigator Marike van der Leeden, Ph.D., a senior researcher and project leader at Reade, rehabilitation and rheumatology in Amsterdam.
Prescription foot orthoses is one of the ways to manage the patients' foot problems.
However, “indications for foot orthoses are not clear, and the effectiveness of the intervention is highly variable among patients,” according to the study.
To determine the clinical and demographic factors that predict the outcome of customized foot orthoses on related pain and disability, researchers conducted a prospective cohort study, which included 135 RA patients who were given customized foot orthoses made by a podiatrist.
Pain and disability were measured before and after the intervention period using a Numeric Rating Scale (NRS) for foot pain, the Foot Function Index (FFI), the Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC), and a 10-meter walking time test, according to the study.
The intervention period included one or more podiatrist appointments during which the foot problem was diagnosed and managed.
The results showed that the duration of RA was negatively associated with score changes in NRS foot pain (P = .018), WOMAC pain (P = .001), FFI disability (P = .003), and WOMAC physical function (P = .002).
Age was negatively associated with the change score in 10-meter walking time (P = .008).
Statistically significant improvements after the intervention with foot orthoses were found on all outcome measures (P less than .001), according to the study.
“Shorter disease duration predicted greater improvements in self-reported foot pain and disability after intervention with foot orthoses,” the authors concluded.
“Younger age predicted greater improvements in walking time. Referral for conservative management with foot orthoses in the early stage of RA seems important when aiming to achieve reduction in pain and improvement in daily activities.”
“To date, no evidence is available for which types of foot orthoses are most effective for RA. Further research is needed for evidence-based prescription protocols for foot problems in RA.” Dr. van der Leeden said.
The authors reported no financial disclosures.
To date there are no data on the most effective type of foot orthoses for patients with RA-induced foot pain.
Source DR. VAN DER LEEDEN
Medicare Part D Reduced Spending on Medical Care
Medicare Part D coverage significantly reduced nondrug medical spending for beneficiaries who had limited drug coverage prior to the start of the federal prescription drug plan, Harvard Medical School researchers reported July 26 in JAMA.
The 10.6% savings was mostly due to a decrease in spending on acute and postacute care under Medicare Part A (JAMA 2011;306:402-9).
"These reductions in nondrug medical spending suggest that Part D has not cost as much as what we initially thought," Dr. J. Michael McWilliams, the study’s lead author, said in an interview.
The findings could also lend support to the Affordable Care Act’s goal of closing the "doughnut hole," the gap in drug coverage under Part D, he added. "The cost of closing the doughnut hole could be less than what we might expect because of these partially offsetting reductions in spending on nondrug medical care."
The results also highlight a need for better coordination between all parts of Medicare, the investigators wrote.
"Even though Part D plans function completely separately from Part A and Part B of the Medicare program, and even though they have no financial incentive to lower copayments, particularly for beneficial medications, clearly providing this benefit to seniors through stand-alone Part D plans has been quite effective," Dr. McWilliams said.
The authors used data from the Health and Retirement Study and linked it to Medicare claims data from 2004 to 2007 on 6,001 beneficiaries, then categorized the beneficiaries as having had generous (2,538) and limited (3,463) drug coverage prior to implementation of Part D. Nontraditional Medicare beneficiaries, such as those who qualified for Medicare before age 65 or those with veterans’ health benefits, were excluded.
For the control cohort, they selected a similar group of 5,988 beneficiaries who had generous (2,537) and limited (3,451) drug coverage in 2002. They studied the group up to 2005.
They found that total nondrug medical spending before Part D implementation was not significantly higher for beneficiaries with limited drug coverage compared with those who had generous drug coverage (7.6% relative difference).
However, after implementation of Part D, nondrug medical spending for beneficiaries who previously had limited drug coverage was 3.9% lower than those who previously had generous drug coverage, leading to a significant differential reduction of 10.6%.
In dollars, Medicare spent nearly $306 per quarter less than expected on beneficiaries who previously had a limited drug coverage.
"The economic and clinical benefits suggested by these reductions may be enhanced by further expansions in prescription drug coverage for seniors, improvements in benefit designs for drug-sensitive conditions, and policies that integrate Medicare payment and delivery systems across drug and nondrug services," the authors wrote.
Previous studies have shown that the implementation of Part D has been associated with reduced out-of-pocket costs, increased medication use, and better medication adherence (N. Engl. J. Med. 2009;361:52-61) as well as decreased hospitalization for ambulatory care-sensitive conditions (Health Serv. Res. 2011;46:1104-23).
The authors had no conflicts of interest to disclose. The study was supported by grants from several charitable foundations.
Medicare Part D coverage significantly reduced nondrug medical spending for beneficiaries who had limited drug coverage prior to the start of the federal prescription drug plan, Harvard Medical School researchers reported July 26 in JAMA.
The 10.6% savings was mostly due to a decrease in spending on acute and postacute care under Medicare Part A (JAMA 2011;306:402-9).
"These reductions in nondrug medical spending suggest that Part D has not cost as much as what we initially thought," Dr. J. Michael McWilliams, the study’s lead author, said in an interview.
The findings could also lend support to the Affordable Care Act’s goal of closing the "doughnut hole," the gap in drug coverage under Part D, he added. "The cost of closing the doughnut hole could be less than what we might expect because of these partially offsetting reductions in spending on nondrug medical care."
The results also highlight a need for better coordination between all parts of Medicare, the investigators wrote.
"Even though Part D plans function completely separately from Part A and Part B of the Medicare program, and even though they have no financial incentive to lower copayments, particularly for beneficial medications, clearly providing this benefit to seniors through stand-alone Part D plans has been quite effective," Dr. McWilliams said.
The authors used data from the Health and Retirement Study and linked it to Medicare claims data from 2004 to 2007 on 6,001 beneficiaries, then categorized the beneficiaries as having had generous (2,538) and limited (3,463) drug coverage prior to implementation of Part D. Nontraditional Medicare beneficiaries, such as those who qualified for Medicare before age 65 or those with veterans’ health benefits, were excluded.
For the control cohort, they selected a similar group of 5,988 beneficiaries who had generous (2,537) and limited (3,451) drug coverage in 2002. They studied the group up to 2005.
They found that total nondrug medical spending before Part D implementation was not significantly higher for beneficiaries with limited drug coverage compared with those who had generous drug coverage (7.6% relative difference).
However, after implementation of Part D, nondrug medical spending for beneficiaries who previously had limited drug coverage was 3.9% lower than those who previously had generous drug coverage, leading to a significant differential reduction of 10.6%.
In dollars, Medicare spent nearly $306 per quarter less than expected on beneficiaries who previously had a limited drug coverage.
"The economic and clinical benefits suggested by these reductions may be enhanced by further expansions in prescription drug coverage for seniors, improvements in benefit designs for drug-sensitive conditions, and policies that integrate Medicare payment and delivery systems across drug and nondrug services," the authors wrote.
Previous studies have shown that the implementation of Part D has been associated with reduced out-of-pocket costs, increased medication use, and better medication adherence (N. Engl. J. Med. 2009;361:52-61) as well as decreased hospitalization for ambulatory care-sensitive conditions (Health Serv. Res. 2011;46:1104-23).
The authors had no conflicts of interest to disclose. The study was supported by grants from several charitable foundations.
Medicare Part D coverage significantly reduced nondrug medical spending for beneficiaries who had limited drug coverage prior to the start of the federal prescription drug plan, Harvard Medical School researchers reported July 26 in JAMA.
The 10.6% savings was mostly due to a decrease in spending on acute and postacute care under Medicare Part A (JAMA 2011;306:402-9).
"These reductions in nondrug medical spending suggest that Part D has not cost as much as what we initially thought," Dr. J. Michael McWilliams, the study’s lead author, said in an interview.
The findings could also lend support to the Affordable Care Act’s goal of closing the "doughnut hole," the gap in drug coverage under Part D, he added. "The cost of closing the doughnut hole could be less than what we might expect because of these partially offsetting reductions in spending on nondrug medical care."
The results also highlight a need for better coordination between all parts of Medicare, the investigators wrote.
"Even though Part D plans function completely separately from Part A and Part B of the Medicare program, and even though they have no financial incentive to lower copayments, particularly for beneficial medications, clearly providing this benefit to seniors through stand-alone Part D plans has been quite effective," Dr. McWilliams said.
The authors used data from the Health and Retirement Study and linked it to Medicare claims data from 2004 to 2007 on 6,001 beneficiaries, then categorized the beneficiaries as having had generous (2,538) and limited (3,463) drug coverage prior to implementation of Part D. Nontraditional Medicare beneficiaries, such as those who qualified for Medicare before age 65 or those with veterans’ health benefits, were excluded.
For the control cohort, they selected a similar group of 5,988 beneficiaries who had generous (2,537) and limited (3,451) drug coverage in 2002. They studied the group up to 2005.
They found that total nondrug medical spending before Part D implementation was not significantly higher for beneficiaries with limited drug coverage compared with those who had generous drug coverage (7.6% relative difference).
However, after implementation of Part D, nondrug medical spending for beneficiaries who previously had limited drug coverage was 3.9% lower than those who previously had generous drug coverage, leading to a significant differential reduction of 10.6%.
In dollars, Medicare spent nearly $306 per quarter less than expected on beneficiaries who previously had a limited drug coverage.
"The economic and clinical benefits suggested by these reductions may be enhanced by further expansions in prescription drug coverage for seniors, improvements in benefit designs for drug-sensitive conditions, and policies that integrate Medicare payment and delivery systems across drug and nondrug services," the authors wrote.
Previous studies have shown that the implementation of Part D has been associated with reduced out-of-pocket costs, increased medication use, and better medication adherence (N. Engl. J. Med. 2009;361:52-61) as well as decreased hospitalization for ambulatory care-sensitive conditions (Health Serv. Res. 2011;46:1104-23).
The authors had no conflicts of interest to disclose. The study was supported by grants from several charitable foundations.
FROM JAMA
Major Finding: Medicare Part D reduced nondrug medical spending for beneficiaries who had limited drug coverage prior to enrolling in the federal prescription drug plan by 10.6%.
Data Source: Data from the Health and Retirement Study survey linked with Medicare claims from 2004 to 2007.
Disclosures: The authors had no conflicts of interest to disclose. The study was supported by grants from several charitable foundations.
Yellow Fever Vaccination Raises Multiple Sclerosis Relapse Rate
Patients with relapsing-remitting multiple sclerosis had a more than 10-fold increase in their relapse rate after they received vaccination against yellow fever, according to a small prospective study.
Although relapsing-remitting MS and yellow fever (YF) vaccination do not overlap often, "depending on specific patient travel plans, potential local epidemics, and length of stay, the final decision on whether to administer the vaccine should result from a careful balance between the risk of MS exacerbation and the likelihood of exposure to the YF virus, which can lead to death," Dr. Jorge Correale, one of the authors of the study, wrote in an e-mail (Arch. Neurol. 2011 June 13 [doi: 10.1001/archneurol.2011.131]).
Dr. Correale added that most other vaccines do not increase the risk of new exacerbations in MS patients (J. Neurol. 2011;258:1197-206).
"However, in contrast to other vaccines, YF vaccine is prepared with live attenuated virus, and probably for other vaccines prepared in the same way we need to consider this possibility," wrote Dr. Correale and his coauthor, Dr. Mauricio F. Farez. Both work in the department of neurology at the Dr. Raúl Carrea Institute for Neurological Research, Buenos Aires.
YF is a potentially deadly disease found in tropical regions of Africa and in parts of South America. It affects approximately 200,000 people each year and leads to 30,000 deaths annually. Although there are no treatments for the disease, the YF 17D-204 vaccine has been proven a safe and effective preventive measure.
Observations of two patients who presented with worsened MS after YF immunization prompted the authors to conduct the study. There is very little data available on the overlap of YF vaccination and MS.
The authors recruited seven patients – five women and two men with an average age of 45 years – with relapsing-remitting MS who received a single dose of YF 17D-204 before traveling. After vaccination, the patients underwent clinical, radiological, and immunological evaluations every 3 months for 2 years. The investigators asked the patients to return for examination within 72 hours if they had a relapse.
To serve as control subjects, the investigators recruited seven unvaccinated patients with MS of the same age and sex; seven patients with MS of the same age and sex who had received influenza vaccinations; and seven healthy individuals of the same age and sex.
They defined an MS exacerbation as the development of a new symptom or worsening of preexisting symptoms lasting at least 48 hours and preceded by stability or improvement lasting at least 30 days. They considered patients to be at risk between 1 to 5 weeks after immunization.
During the at-risk period, five of the seven patients who received YF 17D-204 had exacerbations. Four of those five patients had a significant and persistent increase in their Expanded Disability Status Scale score 1 year after the exacerbation, according to the study. Exacerbations were transient in the other 3 patients.
The annual exacerbation rate in the at-risk period was significantly higher than during the non-risk period (8.57 vs. 0.67, respectively). During 2 years of follow-up, the overall annual exacerbation rate was 0.99. In contrast, immunization against influenza did not affect the annual exacerbation rate.
MRI showed a significantly higher mean number of new or enlarging T2 lesions at 3 months after YF vaccination, compared with the remaining follow-up period (2.6 vs. 0.1), as well as significantly more gadolinium-enhancing lesions (2.14 vs. 0).
The number of myelin basic protein- and myelin oligodendrocyte glycoprotein-peptide-specific cells that secreted the proinflammatory cytokines and chemokines IL-1alpha, IL-1beta, IFN-gamma, TNF, and IP-10 were significantly higher in samples collected from YF vaccinated patients with MS, compared with unvaccinated patients with MS, influenza-vaccinated patients with MS, or controls. The numbers of these cells reached maximum levels between 2 and 5 weeks after vaccination.
The study’s limitations were the small number of patients and its unblinded design for clinical and radiological assessments.
The authors added that it is not clear how YF immunization can trigger autoimmune reactions. They proposed molecular mimicry, epitope spreading, bystander activation, and polyclonal activation as possible mechanisms.
"Whether the more than 10-fold increase in the relapse rate observed in these seven patients can be confirmed by larger studies remains to be seen," the authors wrote.
Dr. Correale is a board member of Merck Serono Argentina, Biogen Idec LATAM, and Merck Serono LATAM. He has received reimbursement to develop educational presentations for Merck Serono Argentina, Merck Serono LATAM, Biogen Idec Argentina, and Teva-Tuteur Argentina.
The study was supported by a research grant from his institution.
Patients with relapsing-remitting multiple sclerosis had a more than 10-fold increase in their relapse rate after they received vaccination against yellow fever, according to a small prospective study.
Although relapsing-remitting MS and yellow fever (YF) vaccination do not overlap often, "depending on specific patient travel plans, potential local epidemics, and length of stay, the final decision on whether to administer the vaccine should result from a careful balance between the risk of MS exacerbation and the likelihood of exposure to the YF virus, which can lead to death," Dr. Jorge Correale, one of the authors of the study, wrote in an e-mail (Arch. Neurol. 2011 June 13 [doi: 10.1001/archneurol.2011.131]).
Dr. Correale added that most other vaccines do not increase the risk of new exacerbations in MS patients (J. Neurol. 2011;258:1197-206).
"However, in contrast to other vaccines, YF vaccine is prepared with live attenuated virus, and probably for other vaccines prepared in the same way we need to consider this possibility," wrote Dr. Correale and his coauthor, Dr. Mauricio F. Farez. Both work in the department of neurology at the Dr. Raúl Carrea Institute for Neurological Research, Buenos Aires.
YF is a potentially deadly disease found in tropical regions of Africa and in parts of South America. It affects approximately 200,000 people each year and leads to 30,000 deaths annually. Although there are no treatments for the disease, the YF 17D-204 vaccine has been proven a safe and effective preventive measure.
Observations of two patients who presented with worsened MS after YF immunization prompted the authors to conduct the study. There is very little data available on the overlap of YF vaccination and MS.
The authors recruited seven patients – five women and two men with an average age of 45 years – with relapsing-remitting MS who received a single dose of YF 17D-204 before traveling. After vaccination, the patients underwent clinical, radiological, and immunological evaluations every 3 months for 2 years. The investigators asked the patients to return for examination within 72 hours if they had a relapse.
To serve as control subjects, the investigators recruited seven unvaccinated patients with MS of the same age and sex; seven patients with MS of the same age and sex who had received influenza vaccinations; and seven healthy individuals of the same age and sex.
They defined an MS exacerbation as the development of a new symptom or worsening of preexisting symptoms lasting at least 48 hours and preceded by stability or improvement lasting at least 30 days. They considered patients to be at risk between 1 to 5 weeks after immunization.
During the at-risk period, five of the seven patients who received YF 17D-204 had exacerbations. Four of those five patients had a significant and persistent increase in their Expanded Disability Status Scale score 1 year after the exacerbation, according to the study. Exacerbations were transient in the other 3 patients.
The annual exacerbation rate in the at-risk period was significantly higher than during the non-risk period (8.57 vs. 0.67, respectively). During 2 years of follow-up, the overall annual exacerbation rate was 0.99. In contrast, immunization against influenza did not affect the annual exacerbation rate.
MRI showed a significantly higher mean number of new or enlarging T2 lesions at 3 months after YF vaccination, compared with the remaining follow-up period (2.6 vs. 0.1), as well as significantly more gadolinium-enhancing lesions (2.14 vs. 0).
The number of myelin basic protein- and myelin oligodendrocyte glycoprotein-peptide-specific cells that secreted the proinflammatory cytokines and chemokines IL-1alpha, IL-1beta, IFN-gamma, TNF, and IP-10 were significantly higher in samples collected from YF vaccinated patients with MS, compared with unvaccinated patients with MS, influenza-vaccinated patients with MS, or controls. The numbers of these cells reached maximum levels between 2 and 5 weeks after vaccination.
The study’s limitations were the small number of patients and its unblinded design for clinical and radiological assessments.
The authors added that it is not clear how YF immunization can trigger autoimmune reactions. They proposed molecular mimicry, epitope spreading, bystander activation, and polyclonal activation as possible mechanisms.
"Whether the more than 10-fold increase in the relapse rate observed in these seven patients can be confirmed by larger studies remains to be seen," the authors wrote.
Dr. Correale is a board member of Merck Serono Argentina, Biogen Idec LATAM, and Merck Serono LATAM. He has received reimbursement to develop educational presentations for Merck Serono Argentina, Merck Serono LATAM, Biogen Idec Argentina, and Teva-Tuteur Argentina.
The study was supported by a research grant from his institution.
Patients with relapsing-remitting multiple sclerosis had a more than 10-fold increase in their relapse rate after they received vaccination against yellow fever, according to a small prospective study.
Although relapsing-remitting MS and yellow fever (YF) vaccination do not overlap often, "depending on specific patient travel plans, potential local epidemics, and length of stay, the final decision on whether to administer the vaccine should result from a careful balance between the risk of MS exacerbation and the likelihood of exposure to the YF virus, which can lead to death," Dr. Jorge Correale, one of the authors of the study, wrote in an e-mail (Arch. Neurol. 2011 June 13 [doi: 10.1001/archneurol.2011.131]).
Dr. Correale added that most other vaccines do not increase the risk of new exacerbations in MS patients (J. Neurol. 2011;258:1197-206).
"However, in contrast to other vaccines, YF vaccine is prepared with live attenuated virus, and probably for other vaccines prepared in the same way we need to consider this possibility," wrote Dr. Correale and his coauthor, Dr. Mauricio F. Farez. Both work in the department of neurology at the Dr. Raúl Carrea Institute for Neurological Research, Buenos Aires.
YF is a potentially deadly disease found in tropical regions of Africa and in parts of South America. It affects approximately 200,000 people each year and leads to 30,000 deaths annually. Although there are no treatments for the disease, the YF 17D-204 vaccine has been proven a safe and effective preventive measure.
Observations of two patients who presented with worsened MS after YF immunization prompted the authors to conduct the study. There is very little data available on the overlap of YF vaccination and MS.
The authors recruited seven patients – five women and two men with an average age of 45 years – with relapsing-remitting MS who received a single dose of YF 17D-204 before traveling. After vaccination, the patients underwent clinical, radiological, and immunological evaluations every 3 months for 2 years. The investigators asked the patients to return for examination within 72 hours if they had a relapse.
To serve as control subjects, the investigators recruited seven unvaccinated patients with MS of the same age and sex; seven patients with MS of the same age and sex who had received influenza vaccinations; and seven healthy individuals of the same age and sex.
They defined an MS exacerbation as the development of a new symptom or worsening of preexisting symptoms lasting at least 48 hours and preceded by stability or improvement lasting at least 30 days. They considered patients to be at risk between 1 to 5 weeks after immunization.
During the at-risk period, five of the seven patients who received YF 17D-204 had exacerbations. Four of those five patients had a significant and persistent increase in their Expanded Disability Status Scale score 1 year after the exacerbation, according to the study. Exacerbations were transient in the other 3 patients.
The annual exacerbation rate in the at-risk period was significantly higher than during the non-risk period (8.57 vs. 0.67, respectively). During 2 years of follow-up, the overall annual exacerbation rate was 0.99. In contrast, immunization against influenza did not affect the annual exacerbation rate.
MRI showed a significantly higher mean number of new or enlarging T2 lesions at 3 months after YF vaccination, compared with the remaining follow-up period (2.6 vs. 0.1), as well as significantly more gadolinium-enhancing lesions (2.14 vs. 0).
The number of myelin basic protein- and myelin oligodendrocyte glycoprotein-peptide-specific cells that secreted the proinflammatory cytokines and chemokines IL-1alpha, IL-1beta, IFN-gamma, TNF, and IP-10 were significantly higher in samples collected from YF vaccinated patients with MS, compared with unvaccinated patients with MS, influenza-vaccinated patients with MS, or controls. The numbers of these cells reached maximum levels between 2 and 5 weeks after vaccination.
The study’s limitations were the small number of patients and its unblinded design for clinical and radiological assessments.
The authors added that it is not clear how YF immunization can trigger autoimmune reactions. They proposed molecular mimicry, epitope spreading, bystander activation, and polyclonal activation as possible mechanisms.
"Whether the more than 10-fold increase in the relapse rate observed in these seven patients can be confirmed by larger studies remains to be seen," the authors wrote.
Dr. Correale is a board member of Merck Serono Argentina, Biogen Idec LATAM, and Merck Serono LATAM. He has received reimbursement to develop educational presentations for Merck Serono Argentina, Merck Serono LATAM, Biogen Idec Argentina, and Teva-Tuteur Argentina.
The study was supported by a research grant from his institution.
FROM ARCHIVES OF NEUROLOGY
Major Finding: The annual exacerbation rate between 1 to 5 weeks after yellow fever vaccination was significantly higher than during the rest of a 2-year follow-up period (8.57 vs. 0.67, respectively).
Data Source: A self-controlled case series study of seven MS patients.
Disclosures: Dr. Correale is a board member of Merck Serono Argentina, Biogen Idec LATAM, and Merck Serono LATAM. He has received reimbursement to develop educational presentations for Merck Serono Argentina, Merck Serono LATAM, Biogen Idec Argentina, and Teva-Tuteur Argentina. The study was supported by a research grant from his institution.
NIAMS Celebrates 25 Years of Advances in Medical Research
BETHESDA, MD. – It’s been 25 years since the establishment of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and great strides have been made in diagnosis, treatment, and management of numerous conditions, "but you ain’t seen nothing yet," said Dr. Francis Collins, director of the National Institutes of Health.
Opportunities for medical research have never been as great as they are today, said Dr. Collins, who gave the welcome address for NIAMS’ 25th anniversary at the NIH campus in Bethesda, Md.
Although prominent researchers in the field agreed that research has come a long way in the past 25 years, they stressed that there is still a long way to go. Currently, the molecular basis for 4,000 diseases is known, said Dr. Collins. "But we have effective treatment for only 200."
In broad strokes, the day-long event touched upon the past, present, and future of major diseases of bones, joints, muscles, and skin – including muscular dystrophies, osteoporosis, rheumatoid arthritis, and lupus – through panels and discussion involving prominent researchers, physicians, and patient advocates.
"These diseases are chronic, crippling, and common," said Dr. Stephen Katz, director of NIAMS, in his opening address. "They affect every family in the United States."
Among the attendees were many researchers and clinicians who said they felt loyalty and appreciation for receiving funding from NIAMS at some point in their career. For some, the progress in the past 2 decades was quite tangible.
"Public investment in osteoporosis research has really changed how we take care of the patients," said Dr. Sundeep Khosla, president of the American Society for Bone and Mineral Research. Dr. Khosla, professor at the Mayo Medical School, Rochester, Minn., recalled a time more than 2 decades ago when calcium, vitamin D, and estrogen were the only options he could offer to patients with osteoporosis.
A few years later, bisphosphonates became available. Then came anabolic drugs, and now more drugs are in the pipeline. Patient diagnosis also has advanced, he said. Although he agreed that the field still has a long way to go, he was optimistic about more progress. "Who knows what will happen in the next 25 years," he said.
There was talk of individualized therapy, balancing research and treatment, and a closer collaboration among scientists, all in the spirit of bringing better diagnosis and treatment to patients.
"We’re in a different world from when all we had was aspirin," said Dr. Daniel Kastner, a scientific director at the National Human Genome Research Institute. "But what we really want is a cure. And we’re not there yet."
BETHESDA, MD. – It’s been 25 years since the establishment of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and great strides have been made in diagnosis, treatment, and management of numerous conditions, "but you ain’t seen nothing yet," said Dr. Francis Collins, director of the National Institutes of Health.
Opportunities for medical research have never been as great as they are today, said Dr. Collins, who gave the welcome address for NIAMS’ 25th anniversary at the NIH campus in Bethesda, Md.
Although prominent researchers in the field agreed that research has come a long way in the past 25 years, they stressed that there is still a long way to go. Currently, the molecular basis for 4,000 diseases is known, said Dr. Collins. "But we have effective treatment for only 200."
In broad strokes, the day-long event touched upon the past, present, and future of major diseases of bones, joints, muscles, and skin – including muscular dystrophies, osteoporosis, rheumatoid arthritis, and lupus – through panels and discussion involving prominent researchers, physicians, and patient advocates.
"These diseases are chronic, crippling, and common," said Dr. Stephen Katz, director of NIAMS, in his opening address. "They affect every family in the United States."
Among the attendees were many researchers and clinicians who said they felt loyalty and appreciation for receiving funding from NIAMS at some point in their career. For some, the progress in the past 2 decades was quite tangible.
"Public investment in osteoporosis research has really changed how we take care of the patients," said Dr. Sundeep Khosla, president of the American Society for Bone and Mineral Research. Dr. Khosla, professor at the Mayo Medical School, Rochester, Minn., recalled a time more than 2 decades ago when calcium, vitamin D, and estrogen were the only options he could offer to patients with osteoporosis.
A few years later, bisphosphonates became available. Then came anabolic drugs, and now more drugs are in the pipeline. Patient diagnosis also has advanced, he said. Although he agreed that the field still has a long way to go, he was optimistic about more progress. "Who knows what will happen in the next 25 years," he said.
There was talk of individualized therapy, balancing research and treatment, and a closer collaboration among scientists, all in the spirit of bringing better diagnosis and treatment to patients.
"We’re in a different world from when all we had was aspirin," said Dr. Daniel Kastner, a scientific director at the National Human Genome Research Institute. "But what we really want is a cure. And we’re not there yet."
BETHESDA, MD. – It’s been 25 years since the establishment of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and great strides have been made in diagnosis, treatment, and management of numerous conditions, "but you ain’t seen nothing yet," said Dr. Francis Collins, director of the National Institutes of Health.
Opportunities for medical research have never been as great as they are today, said Dr. Collins, who gave the welcome address for NIAMS’ 25th anniversary at the NIH campus in Bethesda, Md.
Although prominent researchers in the field agreed that research has come a long way in the past 25 years, they stressed that there is still a long way to go. Currently, the molecular basis for 4,000 diseases is known, said Dr. Collins. "But we have effective treatment for only 200."
In broad strokes, the day-long event touched upon the past, present, and future of major diseases of bones, joints, muscles, and skin – including muscular dystrophies, osteoporosis, rheumatoid arthritis, and lupus – through panels and discussion involving prominent researchers, physicians, and patient advocates.
"These diseases are chronic, crippling, and common," said Dr. Stephen Katz, director of NIAMS, in his opening address. "They affect every family in the United States."
Among the attendees were many researchers and clinicians who said they felt loyalty and appreciation for receiving funding from NIAMS at some point in their career. For some, the progress in the past 2 decades was quite tangible.
"Public investment in osteoporosis research has really changed how we take care of the patients," said Dr. Sundeep Khosla, president of the American Society for Bone and Mineral Research. Dr. Khosla, professor at the Mayo Medical School, Rochester, Minn., recalled a time more than 2 decades ago when calcium, vitamin D, and estrogen were the only options he could offer to patients with osteoporosis.
A few years later, bisphosphonates became available. Then came anabolic drugs, and now more drugs are in the pipeline. Patient diagnosis also has advanced, he said. Although he agreed that the field still has a long way to go, he was optimistic about more progress. "Who knows what will happen in the next 25 years," he said.
There was talk of individualized therapy, balancing research and treatment, and a closer collaboration among scientists, all in the spirit of bringing better diagnosis and treatment to patients.
"We’re in a different world from when all we had was aspirin," said Dr. Daniel Kastner, a scientific director at the National Human Genome Research Institute. "But what we really want is a cure. And we’re not there yet."
FROM A SCIENTIFIC SYMPOSIUM SPONSORED BY NIAMS
Combined Hormonal Contraceptives Should Not Be Used for First 21 Days Post Partum
Regardless of their breastfeeding status, women who are less than 21 days post partum should not use combined hormonal contraception because of the high risk for venous thromboembolism, according to an update to the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria for Contraceptive Use 2010.
Previously, the guidelines recommended that women less than 21 days post partum "generally should not use combined hormonal contraceptives." After 21 days, combined hormonal contraceptives could be used with no restrictions.
The revised guidelines now recommend that nonbreastfeeding women who are 21-42 days post partum and have other risk factors for venous thromboembolism (VTE) generally not use combined hormonal contraceptives. These risk factors include age of 35 years or more, previous VTE, thrombophilia, immobility, transfusion at delivery, body mass index of 30 kg/m2 or more, postpartum hemorrhage, postcesarean delivery, preeclampsia, or smoking.
There are no restrictions for combined hormonal contraceptive use in women who are more than 42 days post partum (MMWR 2011;60:878-83).
Recommendations for breastfeeding women have not been changed. Recommendations for use of other contraceptives, including progestin-only hormonal contraceptive and IUDs, remain unchanged, according to the report.
"We do really want providers to know that progestin-only methods are safe [immediately post partum], and that it’s important for them to talk to their patients about postpartum contraception," said Dr. Naomi Tepper, an ob.gyn. at the CDC’s Division of Reproductive Health, and the lead author of the report.
Although it is important for physicians to use the recommendations, the updates "won’t be a huge change in practice in terms of what’s already being done," Dr. Eve Espey, professor of obstetrics and gynecology at the University of New Mexico, Albuquerque, commented in an interview. Dr. Espey was not involved in the MMWR report.
In a survey of roughly 400 New Mexico physicians and midwives, she and her colleagues showed that "the majority (70%) of providers prescribe progestin-only contraceptive methods to breastfeeding women within the first 6 weeks [post partum]" (Contraception 2006;74:389-93).
Dr. Espey, who chairs the Long-Acting Reversible Contraception (LARC) Working Group at the American College of Obstetricians and Gynecologists, added that the updated recommendations are consistent with the college’s guidelines. Those guidelines recommend no estrogen-containing hormonal contraception during the first 4 weeks post partum, and that breastfeeding women wait until their breastfeeding is well established before they use combined hormonal contraceptives ("ACOG Practice Bulletin No. 73," in Obstet. Gynecol. 2006;107:1453-73).
Current research suggests that women wait 18-24 months between pregnancies, said Dr. Tepper. Meanwhile, studies have shown that short birth intervals can lead to negative health outcomes for mother and baby, highlighting the importance of using contraception during the postpartum period. However, the safety of various forms of contraceptives also needs to be considered, because of an increased risk of VTE among postpartum women.
The CDC published the U.S. Medical Eligibility Criteria for Contraceptive Use (U.S. MEC) for the first time in 2010, adapting some of the World Health Organization’s Medical Eligibility Criteria for Contraceptive Use guidelines.
The current updates follow WHO’s 2010 updated guidance, which recommended that use of combined hormonal contraceptives among nonbreastfeeding postpartum women be more restrictive during the first 42 days, especially in women who are at a high risk for VTE.
To make the revisions, the CDC recruited 13 experts who reviewed WHO’s revised recommendations. "A key issue identified was that immediate postpartum use of combined hormonal contraceptives would impose a high risk for VTE without any substantial benefit in pregnancy prevention, because most nonlactating women will not have a fertile ovulation until at least 42 days post partum," the authors wrote in MMWR.
As in the 2010 U.S. MEC, the updated recommendations use categories 1 to 4, with 1 representing a safe contraceptive method with no restrictions and 4 representing an unacceptable health risk. The use of combined hormonal contraceptives during the first 21 days post partum was upgraded from category 3 to category 4.
"Our goal with these entire sets of guidelines is to keep them up to date and based on the latest evidence," said Dr. Tepper.
Dr. Tepper and Dr. Espey reported no relevant financial disclosures.
Regardless of their breastfeeding status, women who are less than 21 days post partum should not use combined hormonal contraception because of the high risk for venous thromboembolism, according to an update to the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria for Contraceptive Use 2010.
Previously, the guidelines recommended that women less than 21 days post partum "generally should not use combined hormonal contraceptives." After 21 days, combined hormonal contraceptives could be used with no restrictions.
The revised guidelines now recommend that nonbreastfeeding women who are 21-42 days post partum and have other risk factors for venous thromboembolism (VTE) generally not use combined hormonal contraceptives. These risk factors include age of 35 years or more, previous VTE, thrombophilia, immobility, transfusion at delivery, body mass index of 30 kg/m2 or more, postpartum hemorrhage, postcesarean delivery, preeclampsia, or smoking.
There are no restrictions for combined hormonal contraceptive use in women who are more than 42 days post partum (MMWR 2011;60:878-83).
Recommendations for breastfeeding women have not been changed. Recommendations for use of other contraceptives, including progestin-only hormonal contraceptive and IUDs, remain unchanged, according to the report.
"We do really want providers to know that progestin-only methods are safe [immediately post partum], and that it’s important for them to talk to their patients about postpartum contraception," said Dr. Naomi Tepper, an ob.gyn. at the CDC’s Division of Reproductive Health, and the lead author of the report.
Although it is important for physicians to use the recommendations, the updates "won’t be a huge change in practice in terms of what’s already being done," Dr. Eve Espey, professor of obstetrics and gynecology at the University of New Mexico, Albuquerque, commented in an interview. Dr. Espey was not involved in the MMWR report.
In a survey of roughly 400 New Mexico physicians and midwives, she and her colleagues showed that "the majority (70%) of providers prescribe progestin-only contraceptive methods to breastfeeding women within the first 6 weeks [post partum]" (Contraception 2006;74:389-93).
Dr. Espey, who chairs the Long-Acting Reversible Contraception (LARC) Working Group at the American College of Obstetricians and Gynecologists, added that the updated recommendations are consistent with the college’s guidelines. Those guidelines recommend no estrogen-containing hormonal contraception during the first 4 weeks post partum, and that breastfeeding women wait until their breastfeeding is well established before they use combined hormonal contraceptives ("ACOG Practice Bulletin No. 73," in Obstet. Gynecol. 2006;107:1453-73).
Current research suggests that women wait 18-24 months between pregnancies, said Dr. Tepper. Meanwhile, studies have shown that short birth intervals can lead to negative health outcomes for mother and baby, highlighting the importance of using contraception during the postpartum period. However, the safety of various forms of contraceptives also needs to be considered, because of an increased risk of VTE among postpartum women.
The CDC published the U.S. Medical Eligibility Criteria for Contraceptive Use (U.S. MEC) for the first time in 2010, adapting some of the World Health Organization’s Medical Eligibility Criteria for Contraceptive Use guidelines.
The current updates follow WHO’s 2010 updated guidance, which recommended that use of combined hormonal contraceptives among nonbreastfeeding postpartum women be more restrictive during the first 42 days, especially in women who are at a high risk for VTE.
To make the revisions, the CDC recruited 13 experts who reviewed WHO’s revised recommendations. "A key issue identified was that immediate postpartum use of combined hormonal contraceptives would impose a high risk for VTE without any substantial benefit in pregnancy prevention, because most nonlactating women will not have a fertile ovulation until at least 42 days post partum," the authors wrote in MMWR.
As in the 2010 U.S. MEC, the updated recommendations use categories 1 to 4, with 1 representing a safe contraceptive method with no restrictions and 4 representing an unacceptable health risk. The use of combined hormonal contraceptives during the first 21 days post partum was upgraded from category 3 to category 4.
"Our goal with these entire sets of guidelines is to keep them up to date and based on the latest evidence," said Dr. Tepper.
Dr. Tepper and Dr. Espey reported no relevant financial disclosures.
Regardless of their breastfeeding status, women who are less than 21 days post partum should not use combined hormonal contraception because of the high risk for venous thromboembolism, according to an update to the Centers for Disease Control and Prevention’s U.S. Medical Eligibility Criteria for Contraceptive Use 2010.
Previously, the guidelines recommended that women less than 21 days post partum "generally should not use combined hormonal contraceptives." After 21 days, combined hormonal contraceptives could be used with no restrictions.
The revised guidelines now recommend that nonbreastfeeding women who are 21-42 days post partum and have other risk factors for venous thromboembolism (VTE) generally not use combined hormonal contraceptives. These risk factors include age of 35 years or more, previous VTE, thrombophilia, immobility, transfusion at delivery, body mass index of 30 kg/m2 or more, postpartum hemorrhage, postcesarean delivery, preeclampsia, or smoking.
There are no restrictions for combined hormonal contraceptive use in women who are more than 42 days post partum (MMWR 2011;60:878-83).
Recommendations for breastfeeding women have not been changed. Recommendations for use of other contraceptives, including progestin-only hormonal contraceptive and IUDs, remain unchanged, according to the report.
"We do really want providers to know that progestin-only methods are safe [immediately post partum], and that it’s important for them to talk to their patients about postpartum contraception," said Dr. Naomi Tepper, an ob.gyn. at the CDC’s Division of Reproductive Health, and the lead author of the report.
Although it is important for physicians to use the recommendations, the updates "won’t be a huge change in practice in terms of what’s already being done," Dr. Eve Espey, professor of obstetrics and gynecology at the University of New Mexico, Albuquerque, commented in an interview. Dr. Espey was not involved in the MMWR report.
In a survey of roughly 400 New Mexico physicians and midwives, she and her colleagues showed that "the majority (70%) of providers prescribe progestin-only contraceptive methods to breastfeeding women within the first 6 weeks [post partum]" (Contraception 2006;74:389-93).
Dr. Espey, who chairs the Long-Acting Reversible Contraception (LARC) Working Group at the American College of Obstetricians and Gynecologists, added that the updated recommendations are consistent with the college’s guidelines. Those guidelines recommend no estrogen-containing hormonal contraception during the first 4 weeks post partum, and that breastfeeding women wait until their breastfeeding is well established before they use combined hormonal contraceptives ("ACOG Practice Bulletin No. 73," in Obstet. Gynecol. 2006;107:1453-73).
Current research suggests that women wait 18-24 months between pregnancies, said Dr. Tepper. Meanwhile, studies have shown that short birth intervals can lead to negative health outcomes for mother and baby, highlighting the importance of using contraception during the postpartum period. However, the safety of various forms of contraceptives also needs to be considered, because of an increased risk of VTE among postpartum women.
The CDC published the U.S. Medical Eligibility Criteria for Contraceptive Use (U.S. MEC) for the first time in 2010, adapting some of the World Health Organization’s Medical Eligibility Criteria for Contraceptive Use guidelines.
The current updates follow WHO’s 2010 updated guidance, which recommended that use of combined hormonal contraceptives among nonbreastfeeding postpartum women be more restrictive during the first 42 days, especially in women who are at a high risk for VTE.
To make the revisions, the CDC recruited 13 experts who reviewed WHO’s revised recommendations. "A key issue identified was that immediate postpartum use of combined hormonal contraceptives would impose a high risk for VTE without any substantial benefit in pregnancy prevention, because most nonlactating women will not have a fertile ovulation until at least 42 days post partum," the authors wrote in MMWR.
As in the 2010 U.S. MEC, the updated recommendations use categories 1 to 4, with 1 representing a safe contraceptive method with no restrictions and 4 representing an unacceptable health risk. The use of combined hormonal contraceptives during the first 21 days post partum was upgraded from category 3 to category 4.
"Our goal with these entire sets of guidelines is to keep them up to date and based on the latest evidence," said Dr. Tepper.
Dr. Tepper and Dr. Espey reported no relevant financial disclosures.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Thyrotoxicosis Guidelines Stress Patient-Centered Graves Approach
A patient-centered approach to treating Graves’ disease is among new recommendations for diagnosing and treating thyrotoxicosis.
The guidelines, commissioned by the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE), include 100 recommendations and took a 13-member task force 2 years to produce (Thyroid 2011;21:593-646 [doi:10.1089/thy.2010.0417] and Endocr. Pract. 2011;17:e1-e65).
The guidelines update the 1995 ATA recommendations and the 2002 AACE recommendations on treating thyrotoxicosis and, for the first time, the recommendations are given evidence-based ratings, Dr. Rebecca S. Bahn, chair of the task force, said in an interview.
"Physicians who don’t treat hyperthyroidism often may not be aware of new developments in the field and recent changes in practice," said Dr. Bahn.
The guidelines address in specific detail initial evaluation and management of thyrotoxicosis; management of Graves’ hyperthyroidism using radioactive iodine, antithyroid drugs, or surgery; management of toxic multinodular goiter or toxic adenoma using radioactive iodine or surgery; Graves’ disease in children, adolescents, or pregnant patients; subclinical hyperthyroidism; hyperthyroidism in patients with Graves’ ophthalmopathy; and management of other miscellaneous causes of thyrotoxicosis.
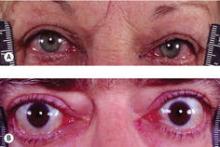
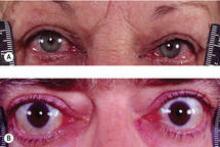
Among the recommendations that could lead to a change in practice is the concept that antithyroid drugs could be used more often in place of radioactive iodine in Graves’ disease, and a recommendation that patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with methimazole or surgery, said Dr. Bahn.
Patients with Graves’ disease have traditionally been prescribed radioactive iodine, but the new guidelines acknowledge the three treatment options: surgery, antithyroid medications, and radioactive iodine.
"It’s important to talk to patients about the pros and cons of each treatment and make appropriate choices. It’s about making patient-centric choices," said Dr. Bahn, past president of the American Thyroid Association and professor of medicine at the Mayo Clinic in Rochester, Minn.
Also recommended is a more cautious approach to the use of propylthiouracil (PTU). PTU can cause hepatic necrosis, "so our guideline is that methimazole should be the first-line treatment," she said.
PTU is still recommended as the first-line medication for pregnant women in their first trimester. "So, [pregnant women] should start with PTU, but then switch to methimazole during the second trimester should they need it."
Another recommendation advises that if surgery is the main therapy for the Graves’ disease, the patient should be sent to a surgeon who does a high volume of thyroid procedures.
"This means that if a high-volume surgeon is not available, then surgery should not be the choice," said Dr. Bahn.
In an editorial, Dr. Elizabeth N. Pearce of Boston University and her colleagues wrote that the guidelines "are much needed." (Thyroid 2011;21:573-6 [doi:10.1089/thy.2011.0104]).
But an important shortcoming of the new guidelines "is that they are based on relatively limited available evidence," she added.
"This indicates that clinical management of hyperthyroidism is still largely rooted in expert opinion and personal experience. Given this, it is perhaps remarkable that 98 of the recommendations were unanimous, whereas two of the recommendations had a single dissenting member," wrote Dr. Pearce and colleagues, who disclosed that they had no conflicts of interest.
The fact that there are not a lot of high-quality randomized trials "means that there does need to be more research in this area. We would hope that the NIH [National Institutes of Health] agrees with that" assessment, said Dr. Bahn.
Dr. Hossein Gharib, who was not involved in the task force, said that the new recommendations are useful. But he added that guidelines are only recommendations and do not necessarily apply to every practice and every patient.
"Doctors should read the guidelines and then apply their own experience, expertise, and resources," said Dr. Gharib, a clinical endocrinologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Bahn, Dr. Pearce, and Dr. Gharib reported no relevant financial disclosures.
Selected recommendations:
Recommendation 13: Methimazole should be used in virtually every patient who chooses antithyroid drug therapy for GD [Graves’ disease], except during the first trimester of pregnancy when propylthiouracil is preferred, in the treatment of thyroid storm, and in patients with minor reactions to methimazole who refuse radioactive iodine therapy or surgery.
Recommendation 25: If surgery is chosen as the primary therapy for GD, the patient should be referred to a high-volume thyroid surgeon.
Recommendation 31: We suggest that patients with overtly TMNG [toxic multinodar goiter] or TA [thyroid adenoma] be treated with either 131I therapy or thyroidectomy. On occasion, long-term, low-dose treatment with methimazole may be appropriate.
Recommendation 50: Children with GD should be treated with methimazole, 131I therapy, or thyroidectomy. 131I therapy should be avoided in very young children [younger than 5 years]. 131I therapy in patients between 5 and 10 years of age is acceptable if the calculated 131I administered activity is [less than] 10 mCi. 131I therapy in patients older than 10 years of age is acceptable if the activity is [greater than] 150 mcCi/g of thyroid tissue. Thyroidectomy should be chosen when definitive therapy is required, the child is too young for 131I, and surgery can be performed by a high-volume thyroid surgeon.
Recommendation 71: We suggest that patients taking methimazole who decide to become pregnant obtain pregnancy testing at the earliest suggestion of pregnancy and be switched to propylthiouracil as soon as possible in the first trimester and changed back to methimazole at the beginning of the second trimester. Similarly, we suggest that patients started on propylthiouracil during the first trimester be switched to methimazole at the beginning of the second trimester
Recommendation 86: Patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with either methimazole or surgery.
A patient-centered approach to treating Graves’ disease is among new recommendations for diagnosing and treating thyrotoxicosis.
The guidelines, commissioned by the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE), include 100 recommendations and took a 13-member task force 2 years to produce (Thyroid 2011;21:593-646 [doi:10.1089/thy.2010.0417] and Endocr. Pract. 2011;17:e1-e65).
The guidelines update the 1995 ATA recommendations and the 2002 AACE recommendations on treating thyrotoxicosis and, for the first time, the recommendations are given evidence-based ratings, Dr. Rebecca S. Bahn, chair of the task force, said in an interview.
"Physicians who don’t treat hyperthyroidism often may not be aware of new developments in the field and recent changes in practice," said Dr. Bahn.
The guidelines address in specific detail initial evaluation and management of thyrotoxicosis; management of Graves’ hyperthyroidism using radioactive iodine, antithyroid drugs, or surgery; management of toxic multinodular goiter or toxic adenoma using radioactive iodine or surgery; Graves’ disease in children, adolescents, or pregnant patients; subclinical hyperthyroidism; hyperthyroidism in patients with Graves’ ophthalmopathy; and management of other miscellaneous causes of thyrotoxicosis.
Among the recommendations that could lead to a change in practice is the concept that antithyroid drugs could be used more often in place of radioactive iodine in Graves’ disease, and a recommendation that patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with methimazole or surgery, said Dr. Bahn.
Patients with Graves’ disease have traditionally been prescribed radioactive iodine, but the new guidelines acknowledge the three treatment options: surgery, antithyroid medications, and radioactive iodine.
"It’s important to talk to patients about the pros and cons of each treatment and make appropriate choices. It’s about making patient-centric choices," said Dr. Bahn, past president of the American Thyroid Association and professor of medicine at the Mayo Clinic in Rochester, Minn.
Also recommended is a more cautious approach to the use of propylthiouracil (PTU). PTU can cause hepatic necrosis, "so our guideline is that methimazole should be the first-line treatment," she said.
PTU is still recommended as the first-line medication for pregnant women in their first trimester. "So, [pregnant women] should start with PTU, but then switch to methimazole during the second trimester should they need it."
Another recommendation advises that if surgery is the main therapy for the Graves’ disease, the patient should be sent to a surgeon who does a high volume of thyroid procedures.
"This means that if a high-volume surgeon is not available, then surgery should not be the choice," said Dr. Bahn.
In an editorial, Dr. Elizabeth N. Pearce of Boston University and her colleagues wrote that the guidelines "are much needed." (Thyroid 2011;21:573-6 [doi:10.1089/thy.2011.0104]).
But an important shortcoming of the new guidelines "is that they are based on relatively limited available evidence," she added.
"This indicates that clinical management of hyperthyroidism is still largely rooted in expert opinion and personal experience. Given this, it is perhaps remarkable that 98 of the recommendations were unanimous, whereas two of the recommendations had a single dissenting member," wrote Dr. Pearce and colleagues, who disclosed that they had no conflicts of interest.
The fact that there are not a lot of high-quality randomized trials "means that there does need to be more research in this area. We would hope that the NIH [National Institutes of Health] agrees with that" assessment, said Dr. Bahn.
Dr. Hossein Gharib, who was not involved in the task force, said that the new recommendations are useful. But he added that guidelines are only recommendations and do not necessarily apply to every practice and every patient.
"Doctors should read the guidelines and then apply their own experience, expertise, and resources," said Dr. Gharib, a clinical endocrinologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Bahn, Dr. Pearce, and Dr. Gharib reported no relevant financial disclosures.
Selected recommendations:
Recommendation 13: Methimazole should be used in virtually every patient who chooses antithyroid drug therapy for GD [Graves’ disease], except during the first trimester of pregnancy when propylthiouracil is preferred, in the treatment of thyroid storm, and in patients with minor reactions to methimazole who refuse radioactive iodine therapy or surgery.
Recommendation 25: If surgery is chosen as the primary therapy for GD, the patient should be referred to a high-volume thyroid surgeon.
Recommendation 31: We suggest that patients with overtly TMNG [toxic multinodar goiter] or TA [thyroid adenoma] be treated with either 131I therapy or thyroidectomy. On occasion, long-term, low-dose treatment with methimazole may be appropriate.
Recommendation 50: Children with GD should be treated with methimazole, 131I therapy, or thyroidectomy. 131I therapy should be avoided in very young children [younger than 5 years]. 131I therapy in patients between 5 and 10 years of age is acceptable if the calculated 131I administered activity is [less than] 10 mCi. 131I therapy in patients older than 10 years of age is acceptable if the activity is [greater than] 150 mcCi/g of thyroid tissue. Thyroidectomy should be chosen when definitive therapy is required, the child is too young for 131I, and surgery can be performed by a high-volume thyroid surgeon.
Recommendation 71: We suggest that patients taking methimazole who decide to become pregnant obtain pregnancy testing at the earliest suggestion of pregnancy and be switched to propylthiouracil as soon as possible in the first trimester and changed back to methimazole at the beginning of the second trimester. Similarly, we suggest that patients started on propylthiouracil during the first trimester be switched to methimazole at the beginning of the second trimester
Recommendation 86: Patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with either methimazole or surgery.
A patient-centered approach to treating Graves’ disease is among new recommendations for diagnosing and treating thyrotoxicosis.
The guidelines, commissioned by the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE), include 100 recommendations and took a 13-member task force 2 years to produce (Thyroid 2011;21:593-646 [doi:10.1089/thy.2010.0417] and Endocr. Pract. 2011;17:e1-e65).
The guidelines update the 1995 ATA recommendations and the 2002 AACE recommendations on treating thyrotoxicosis and, for the first time, the recommendations are given evidence-based ratings, Dr. Rebecca S. Bahn, chair of the task force, said in an interview.
"Physicians who don’t treat hyperthyroidism often may not be aware of new developments in the field and recent changes in practice," said Dr. Bahn.
The guidelines address in specific detail initial evaluation and management of thyrotoxicosis; management of Graves’ hyperthyroidism using radioactive iodine, antithyroid drugs, or surgery; management of toxic multinodular goiter or toxic adenoma using radioactive iodine or surgery; Graves’ disease in children, adolescents, or pregnant patients; subclinical hyperthyroidism; hyperthyroidism in patients with Graves’ ophthalmopathy; and management of other miscellaneous causes of thyrotoxicosis.
Among the recommendations that could lead to a change in practice is the concept that antithyroid drugs could be used more often in place of radioactive iodine in Graves’ disease, and a recommendation that patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with methimazole or surgery, said Dr. Bahn.
Patients with Graves’ disease have traditionally been prescribed radioactive iodine, but the new guidelines acknowledge the three treatment options: surgery, antithyroid medications, and radioactive iodine.
"It’s important to talk to patients about the pros and cons of each treatment and make appropriate choices. It’s about making patient-centric choices," said Dr. Bahn, past president of the American Thyroid Association and professor of medicine at the Mayo Clinic in Rochester, Minn.
Also recommended is a more cautious approach to the use of propylthiouracil (PTU). PTU can cause hepatic necrosis, "so our guideline is that methimazole should be the first-line treatment," she said.
PTU is still recommended as the first-line medication for pregnant women in their first trimester. "So, [pregnant women] should start with PTU, but then switch to methimazole during the second trimester should they need it."
Another recommendation advises that if surgery is the main therapy for the Graves’ disease, the patient should be sent to a surgeon who does a high volume of thyroid procedures.
"This means that if a high-volume surgeon is not available, then surgery should not be the choice," said Dr. Bahn.
In an editorial, Dr. Elizabeth N. Pearce of Boston University and her colleagues wrote that the guidelines "are much needed." (Thyroid 2011;21:573-6 [doi:10.1089/thy.2011.0104]).
But an important shortcoming of the new guidelines "is that they are based on relatively limited available evidence," she added.
"This indicates that clinical management of hyperthyroidism is still largely rooted in expert opinion and personal experience. Given this, it is perhaps remarkable that 98 of the recommendations were unanimous, whereas two of the recommendations had a single dissenting member," wrote Dr. Pearce and colleagues, who disclosed that they had no conflicts of interest.
The fact that there are not a lot of high-quality randomized trials "means that there does need to be more research in this area. We would hope that the NIH [National Institutes of Health] agrees with that" assessment, said Dr. Bahn.
Dr. Hossein Gharib, who was not involved in the task force, said that the new recommendations are useful. But he added that guidelines are only recommendations and do not necessarily apply to every practice and every patient.
"Doctors should read the guidelines and then apply their own experience, expertise, and resources," said Dr. Gharib, a clinical endocrinologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Bahn, Dr. Pearce, and Dr. Gharib reported no relevant financial disclosures.
Selected recommendations:
Recommendation 13: Methimazole should be used in virtually every patient who chooses antithyroid drug therapy for GD [Graves’ disease], except during the first trimester of pregnancy when propylthiouracil is preferred, in the treatment of thyroid storm, and in patients with minor reactions to methimazole who refuse radioactive iodine therapy or surgery.
Recommendation 25: If surgery is chosen as the primary therapy for GD, the patient should be referred to a high-volume thyroid surgeon.
Recommendation 31: We suggest that patients with overtly TMNG [toxic multinodar goiter] or TA [thyroid adenoma] be treated with either 131I therapy or thyroidectomy. On occasion, long-term, low-dose treatment with methimazole may be appropriate.
Recommendation 50: Children with GD should be treated with methimazole, 131I therapy, or thyroidectomy. 131I therapy should be avoided in very young children [younger than 5 years]. 131I therapy in patients between 5 and 10 years of age is acceptable if the calculated 131I administered activity is [less than] 10 mCi. 131I therapy in patients older than 10 years of age is acceptable if the activity is [greater than] 150 mcCi/g of thyroid tissue. Thyroidectomy should be chosen when definitive therapy is required, the child is too young for 131I, and surgery can be performed by a high-volume thyroid surgeon.
Recommendation 71: We suggest that patients taking methimazole who decide to become pregnant obtain pregnancy testing at the earliest suggestion of pregnancy and be switched to propylthiouracil as soon as possible in the first trimester and changed back to methimazole at the beginning of the second trimester. Similarly, we suggest that patients started on propylthiouracil during the first trimester be switched to methimazole at the beginning of the second trimester
Recommendation 86: Patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with either methimazole or surgery.
FROM ENDOCRINE PRACTICE
Thyrotoxicosis Guidelines Stress Patient-Centered Graves Approach
A patient-centered approach to treating Graves’ disease is among new recommendations for diagnosing and treating thyrotoxicosis.
The guidelines, commissioned by the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE), include 100 recommendations and took a 13-member task force 2 years to produce (Thyroid 2011;21:593-646 [doi:10.1089/thy.2010.0417] and Endocr. Pract. 2011;17:e1-e65).
The guidelines update the 1995 ATA recommendations and the 2002 AACE recommendations on treating thyrotoxicosis and, for the first time, the recommendations are given evidence-based ratings, Dr. Rebecca S. Bahn, chair of the task force, said in an interview.
"Physicians who don’t treat hyperthyroidism often may not be aware of new developments in the field and recent changes in practice," said Dr. Bahn.
The guidelines address in specific detail initial evaluation and management of thyrotoxicosis; management of Graves’ hyperthyroidism using radioactive iodine, antithyroid drugs, or surgery; management of toxic multinodular goiter or toxic adenoma using radioactive iodine or surgery; Graves’ disease in children, adolescents, or pregnant patients; subclinical hyperthyroidism; hyperthyroidism in patients with Graves’ ophthalmopathy; and management of other miscellaneous causes of thyrotoxicosis.
Among the recommendations that could lead to a change in practice is the concept that antithyroid drugs could be used more often in place of radioactive iodine in Graves’ disease, and a recommendation that patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with methimazole or surgery, said Dr. Bahn.
Patients with Graves’ disease have traditionally been prescribed radioactive iodine, but the new guidelines acknowledge the three treatment options: surgery, antithyroid medications, and radioactive iodine.
"It’s important to talk to patients about the pros and cons of each treatment and make appropriate choices. It’s about making patient-centric choices," said Dr. Bahn, past president of the American Thyroid Association and professor of medicine at the Mayo Clinic in Rochester, Minn.
Also recommended is a more cautious approach to the use of propylthiouracil (PTU). PTU can cause hepatic necrosis, "so our guideline is that methimazole should be the first-line treatment," she said.
PTU is still recommended as the first-line medication for pregnant women in their first trimester. "So, [pregnant women] should start with PTU, but then switch to methimazole during the second trimester should they need it."
Another recommendation advises that if surgery is the main therapy for the Graves’ disease, the patient should be sent to a surgeon who does a high volume of thyroid procedures.
"This means that if a high-volume surgeon is not available, then surgery should not be the choice," said Dr. Bahn.
In an editorial, Dr. Elizabeth N. Pearce of Boston University and her colleagues wrote that the guidelines "are much needed." (Thyroid 2011;21:573-6 [doi:10.1089/thy.2011.0104]).
But an important shortcoming of the new guidelines "is that they are based on relatively limited available evidence," she added.
"This indicates that clinical management of hyperthyroidism is still largely rooted in expert opinion and personal experience. Given this, it is perhaps remarkable that 98 of the recommendations were unanimous, whereas two of the recommendations had a single dissenting member," wrote Dr. Pearce and colleagues, who disclosed that they had no conflicts of interest.
The fact that there are not a lot of high-quality randomized trials "means that there does need to be more research in this area. We would hope that the NIH [National Institutes of Health] agrees with that" assessment, said Dr. Bahn.
Dr. Hossein Gharib, who was not involved in the task force, said that the new recommendations are useful. But he added that guidelines are only recommendations and do not necessarily apply to every practice and every patient.
"Doctors should read the guidelines and then apply their own experience, expertise, and resources," said Dr. Gharib, a clinical endocrinologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Bahn, Dr. Pearce, and Dr. Gharib reported no relevant financial disclosures.
Selected recommendations:
Recommendation 13: Methimazole should be used in virtually every patient who chooses antithyroid drug therapy for GD [Graves’ disease], except during the first trimester of pregnancy when propylthiouracil is preferred, in the treatment of thyroid storm, and in patients with minor reactions to methimazole who refuse radioactive iodine therapy or surgery.
Recommendation 25: If surgery is chosen as the primary therapy for GD, the patient should be referred to a high-volume thyroid surgeon.
Recommendation 31: We suggest that patients with overtly TMNG [toxic multinodar goiter] or TA [thyroid adenoma] be treated with either 131I therapy or thyroidectomy. On occasion, long-term, low-dose treatment with methimazole may be appropriate.
Recommendation 50: Children with GD should be treated with methimazole, 131I therapy, or thyroidectomy. 131I therapy should be avoided in very young children [younger than 5 years]. 131I therapy in patients between 5 and 10 years of age is acceptable if the calculated 131I administered activity is [less than] 10 mCi. 131I therapy in patients older than 10 years of age is acceptable if the activity is [greater than] 150 mcCi/g of thyroid tissue. Thyroidectomy should be chosen when definitive therapy is required, the child is too young for 131I, and surgery can be performed by a high-volume thyroid surgeon.
Recommendation 71: We suggest that patients taking methimazole who decide to become pregnant obtain pregnancy testing at the earliest suggestion of pregnancy and be switched to propylthiouracil as soon as possible in the first trimester and changed back to methimazole at the beginning of the second trimester. Similarly, we suggest that patients started on propylthiouracil during the first trimester be switched to methimazole at the beginning of the second trimester
Recommendation 86: Patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with either methimazole or surgery.
A patient-centered approach to treating Graves’ disease is among new recommendations for diagnosing and treating thyrotoxicosis.
The guidelines, commissioned by the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE), include 100 recommendations and took a 13-member task force 2 years to produce (Thyroid 2011;21:593-646 [doi:10.1089/thy.2010.0417] and Endocr. Pract. 2011;17:e1-e65).
The guidelines update the 1995 ATA recommendations and the 2002 AACE recommendations on treating thyrotoxicosis and, for the first time, the recommendations are given evidence-based ratings, Dr. Rebecca S. Bahn, chair of the task force, said in an interview.
"Physicians who don’t treat hyperthyroidism often may not be aware of new developments in the field and recent changes in practice," said Dr. Bahn.
The guidelines address in specific detail initial evaluation and management of thyrotoxicosis; management of Graves’ hyperthyroidism using radioactive iodine, antithyroid drugs, or surgery; management of toxic multinodular goiter or toxic adenoma using radioactive iodine or surgery; Graves’ disease in children, adolescents, or pregnant patients; subclinical hyperthyroidism; hyperthyroidism in patients with Graves’ ophthalmopathy; and management of other miscellaneous causes of thyrotoxicosis.
Among the recommendations that could lead to a change in practice is the concept that antithyroid drugs could be used more often in place of radioactive iodine in Graves’ disease, and a recommendation that patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with methimazole or surgery, said Dr. Bahn.
Patients with Graves’ disease have traditionally been prescribed radioactive iodine, but the new guidelines acknowledge the three treatment options: surgery, antithyroid medications, and radioactive iodine.
"It’s important to talk to patients about the pros and cons of each treatment and make appropriate choices. It’s about making patient-centric choices," said Dr. Bahn, past president of the American Thyroid Association and professor of medicine at the Mayo Clinic in Rochester, Minn.
Also recommended is a more cautious approach to the use of propylthiouracil (PTU). PTU can cause hepatic necrosis, "so our guideline is that methimazole should be the first-line treatment," she said.
PTU is still recommended as the first-line medication for pregnant women in their first trimester. "So, [pregnant women] should start with PTU, but then switch to methimazole during the second trimester should they need it."
Another recommendation advises that if surgery is the main therapy for the Graves’ disease, the patient should be sent to a surgeon who does a high volume of thyroid procedures.
"This means that if a high-volume surgeon is not available, then surgery should not be the choice," said Dr. Bahn.
In an editorial, Dr. Elizabeth N. Pearce of Boston University and her colleagues wrote that the guidelines "are much needed." (Thyroid 2011;21:573-6 [doi:10.1089/thy.2011.0104]).
But an important shortcoming of the new guidelines "is that they are based on relatively limited available evidence," she added.
"This indicates that clinical management of hyperthyroidism is still largely rooted in expert opinion and personal experience. Given this, it is perhaps remarkable that 98 of the recommendations were unanimous, whereas two of the recommendations had a single dissenting member," wrote Dr. Pearce and colleagues, who disclosed that they had no conflicts of interest.
The fact that there are not a lot of high-quality randomized trials "means that there does need to be more research in this area. We would hope that the NIH [National Institutes of Health] agrees with that" assessment, said Dr. Bahn.
Dr. Hossein Gharib, who was not involved in the task force, said that the new recommendations are useful. But he added that guidelines are only recommendations and do not necessarily apply to every practice and every patient.
"Doctors should read the guidelines and then apply their own experience, expertise, and resources," said Dr. Gharib, a clinical endocrinologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Bahn, Dr. Pearce, and Dr. Gharib reported no relevant financial disclosures.
Selected recommendations:
Recommendation 13: Methimazole should be used in virtually every patient who chooses antithyroid drug therapy for GD [Graves’ disease], except during the first trimester of pregnancy when propylthiouracil is preferred, in the treatment of thyroid storm, and in patients with minor reactions to methimazole who refuse radioactive iodine therapy or surgery.
Recommendation 25: If surgery is chosen as the primary therapy for GD, the patient should be referred to a high-volume thyroid surgeon.
Recommendation 31: We suggest that patients with overtly TMNG [toxic multinodar goiter] or TA [thyroid adenoma] be treated with either 131I therapy or thyroidectomy. On occasion, long-term, low-dose treatment with methimazole may be appropriate.
Recommendation 50: Children with GD should be treated with methimazole, 131I therapy, or thyroidectomy. 131I therapy should be avoided in very young children [younger than 5 years]. 131I therapy in patients between 5 and 10 years of age is acceptable if the calculated 131I administered activity is [less than] 10 mCi. 131I therapy in patients older than 10 years of age is acceptable if the activity is [greater than] 150 mcCi/g of thyroid tissue. Thyroidectomy should be chosen when definitive therapy is required, the child is too young for 131I, and surgery can be performed by a high-volume thyroid surgeon.
Recommendation 71: We suggest that patients taking methimazole who decide to become pregnant obtain pregnancy testing at the earliest suggestion of pregnancy and be switched to propylthiouracil as soon as possible in the first trimester and changed back to methimazole at the beginning of the second trimester. Similarly, we suggest that patients started on propylthiouracil during the first trimester be switched to methimazole at the beginning of the second trimester
Recommendation 86: Patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with either methimazole or surgery.
A patient-centered approach to treating Graves’ disease is among new recommendations for diagnosing and treating thyrotoxicosis.
The guidelines, commissioned by the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE), include 100 recommendations and took a 13-member task force 2 years to produce (Thyroid 2011;21:593-646 [doi:10.1089/thy.2010.0417] and Endocr. Pract. 2011;17:e1-e65).
The guidelines update the 1995 ATA recommendations and the 2002 AACE recommendations on treating thyrotoxicosis and, for the first time, the recommendations are given evidence-based ratings, Dr. Rebecca S. Bahn, chair of the task force, said in an interview.
"Physicians who don’t treat hyperthyroidism often may not be aware of new developments in the field and recent changes in practice," said Dr. Bahn.
The guidelines address in specific detail initial evaluation and management of thyrotoxicosis; management of Graves’ hyperthyroidism using radioactive iodine, antithyroid drugs, or surgery; management of toxic multinodular goiter or toxic adenoma using radioactive iodine or surgery; Graves’ disease in children, adolescents, or pregnant patients; subclinical hyperthyroidism; hyperthyroidism in patients with Graves’ ophthalmopathy; and management of other miscellaneous causes of thyrotoxicosis.
Among the recommendations that could lead to a change in practice is the concept that antithyroid drugs could be used more often in place of radioactive iodine in Graves’ disease, and a recommendation that patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with methimazole or surgery, said Dr. Bahn.
Patients with Graves’ disease have traditionally been prescribed radioactive iodine, but the new guidelines acknowledge the three treatment options: surgery, antithyroid medications, and radioactive iodine.
"It’s important to talk to patients about the pros and cons of each treatment and make appropriate choices. It’s about making patient-centric choices," said Dr. Bahn, past president of the American Thyroid Association and professor of medicine at the Mayo Clinic in Rochester, Minn.
Also recommended is a more cautious approach to the use of propylthiouracil (PTU). PTU can cause hepatic necrosis, "so our guideline is that methimazole should be the first-line treatment," she said.
PTU is still recommended as the first-line medication for pregnant women in their first trimester. "So, [pregnant women] should start with PTU, but then switch to methimazole during the second trimester should they need it."
Another recommendation advises that if surgery is the main therapy for the Graves’ disease, the patient should be sent to a surgeon who does a high volume of thyroid procedures.
"This means that if a high-volume surgeon is not available, then surgery should not be the choice," said Dr. Bahn.
In an editorial, Dr. Elizabeth N. Pearce of Boston University and her colleagues wrote that the guidelines "are much needed." (Thyroid 2011;21:573-6 [doi:10.1089/thy.2011.0104]).
But an important shortcoming of the new guidelines "is that they are based on relatively limited available evidence," she added.
"This indicates that clinical management of hyperthyroidism is still largely rooted in expert opinion and personal experience. Given this, it is perhaps remarkable that 98 of the recommendations were unanimous, whereas two of the recommendations had a single dissenting member," wrote Dr. Pearce and colleagues, who disclosed that they had no conflicts of interest.
The fact that there are not a lot of high-quality randomized trials "means that there does need to be more research in this area. We would hope that the NIH [National Institutes of Health] agrees with that" assessment, said Dr. Bahn.
Dr. Hossein Gharib, who was not involved in the task force, said that the new recommendations are useful. But he added that guidelines are only recommendations and do not necessarily apply to every practice and every patient.
"Doctors should read the guidelines and then apply their own experience, expertise, and resources," said Dr. Gharib, a clinical endocrinologist and professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Bahn, Dr. Pearce, and Dr. Gharib reported no relevant financial disclosures.
Selected recommendations:
Recommendation 13: Methimazole should be used in virtually every patient who chooses antithyroid drug therapy for GD [Graves’ disease], except during the first trimester of pregnancy when propylthiouracil is preferred, in the treatment of thyroid storm, and in patients with minor reactions to methimazole who refuse radioactive iodine therapy or surgery.
Recommendation 25: If surgery is chosen as the primary therapy for GD, the patient should be referred to a high-volume thyroid surgeon.
Recommendation 31: We suggest that patients with overtly TMNG [toxic multinodar goiter] or TA [thyroid adenoma] be treated with either 131I therapy or thyroidectomy. On occasion, long-term, low-dose treatment with methimazole may be appropriate.
Recommendation 50: Children with GD should be treated with methimazole, 131I therapy, or thyroidectomy. 131I therapy should be avoided in very young children [younger than 5 years]. 131I therapy in patients between 5 and 10 years of age is acceptable if the calculated 131I administered activity is [less than] 10 mCi. 131I therapy in patients older than 10 years of age is acceptable if the activity is [greater than] 150 mcCi/g of thyroid tissue. Thyroidectomy should be chosen when definitive therapy is required, the child is too young for 131I, and surgery can be performed by a high-volume thyroid surgeon.
Recommendation 71: We suggest that patients taking methimazole who decide to become pregnant obtain pregnancy testing at the earliest suggestion of pregnancy and be switched to propylthiouracil as soon as possible in the first trimester and changed back to methimazole at the beginning of the second trimester. Similarly, we suggest that patients started on propylthiouracil during the first trimester be switched to methimazole at the beginning of the second trimester
Recommendation 86: Patients with Graves’ hyperthyroidism and active moderate to severe or sight-threatening ophthalmopathy should be treated with either methimazole or surgery.
FROM ENDOCRINE PRACTICE
Policy & Practice : Want more health reform news? Subscribe to our podcast – search 'Policy & Practice' in the iTunes store
Technology Boosts Diabetes Care
Diabetes care and outcomes were superior in facilities using electronic health records, compared with care at facilities with paper-based records, according a report by Better Health Greater Cleveland for the Robert Wood Johnson Foundation. Researchers compiled data on care such as eye exams and outcomes such as blood sugar control from 32 health centers in the Cleveland area using EHRs and from 5 using paper. More than half of the patients at EHR-based clinics received all necessary care; only 7% of patients who went to paper-based clinics did. Similarly, 44% of those at EHR-based clinics achieved positive outcomes, compared with 16% at paper-based clinics. The report noted that the federal government has committed $27 billion to EHRs and other health information technology over the next 10 years. It added that the Cleveland data show that using EHRs results in “dramatically better patient care.”
New Diabetes-Help Videos
A new series of 3- to 5-minute videos, part of the redesigned National Diabetes Education Program online library, addressin topics such as living with diabetes, finding support, preventing diabetes, and setting goals to improve health. “For more than 14 years, the NDEP has been in the forefront of raising awareness about diabetes, but more needs to be done to provide resources and tools to support health care providers and their patients when it comes to achieving and sustaining health goals,” Dr. Griffin P. Rodgers, director of the National Institute of Diabetes and Digestive and Kidney Diseases, one of the sponsors of NDEP, said in a statement. The videos are available at
www.YourDiabetesInfo.org/HealthSense
Another Try for Diabetes Bill
Two congressmen have reintroduced the Gestational Diabetes Act, which passed the House last year but stalled in the Senate. Rep. Eliot Engel (D-N.Y.) and Rep. Michael C. Burgess (R-Tex.), a physician, said that the fight against gestational diabetes is a bipartisan effort and needs continued attention. “The statistics speak for themselves – mothers diagnosed with gestational diabetes are more likely to develop type 2 diabetes, and their children are at an increased risk not only for diabetes but other prenatal complications as well,” Rep. Burgess said in a statement. The bill (H.R.2194) aims to enhance surveillance and public health research on gestational diabetes, provide grants for reducing incidence of the condition, and expand research and treatments.
Smoking Is Risk in Obese Girls
Adolescent girls who are obese are twice as likely as others to become heavy smokers, according to the National Longitudinal Study of Adolescent Health survey. The authors controlled for demographic differences and factors such as smoking by parents and friends in analyzing the more than 4,000 survey responses. Family smoking turned out to be the strongest predictor of nicotine addiction, and a high grade point average mediated the girls' risk of becoming heavy smokers. “Obese, adolescent females may require targeted interventions to address their risk of subsequent high-level nicotine addiction, especially if risk factors such as parental smoking and poor school performance are present,” the authors concluded in the Journal of Adolescent Health.
IOM Addresses Childhood Obesity
Health care providers should monitor weight and height as part of every well-child visit and should help parents learn ways to increase physical activity and decrease sedentary behavior to curb early childhood obesity, according to a report from the Institute of Medicine. The report urges pediatricians and others who work with infants to encourage breastfeeding and to provide guidance on healthy eating strategies. In addition, health care providers should counsel parents on limiting television and other media use, and should urge parents and other caregivers not to permit televisions or other media devices in young children's bedrooms or sleeping areas. The IOM report details steps policy makers and federal programs can take to help curb early childhood obesity.
Privacy Notification Planned
A proposed change in health insurance-privacy rules would give patients the right to see reports on who has accessed their protected health information electronically. The change was mandated by the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act. Although health care providers are required to track this information, they don't have to share it with patients. “This proposed rule represents an important step in our continued efforts to promote accountability across the health care system, ensuring that providers properly safeguard private health information,” said Office of Civil Rights Director Georgina Verdugo. “We need to protect people's rights so that they know how their health information has been used or disclosed.”
Technology Boosts Diabetes Care
Diabetes care and outcomes were superior in facilities using electronic health records, compared with care at facilities with paper-based records, according a report by Better Health Greater Cleveland for the Robert Wood Johnson Foundation. Researchers compiled data on care such as eye exams and outcomes such as blood sugar control from 32 health centers in the Cleveland area using EHRs and from 5 using paper. More than half of the patients at EHR-based clinics received all necessary care; only 7% of patients who went to paper-based clinics did. Similarly, 44% of those at EHR-based clinics achieved positive outcomes, compared with 16% at paper-based clinics. The report noted that the federal government has committed $27 billion to EHRs and other health information technology over the next 10 years. It added that the Cleveland data show that using EHRs results in “dramatically better patient care.”
New Diabetes-Help Videos
A new series of 3- to 5-minute videos, part of the redesigned National Diabetes Education Program online library, addressin topics such as living with diabetes, finding support, preventing diabetes, and setting goals to improve health. “For more than 14 years, the NDEP has been in the forefront of raising awareness about diabetes, but more needs to be done to provide resources and tools to support health care providers and their patients when it comes to achieving and sustaining health goals,” Dr. Griffin P. Rodgers, director of the National Institute of Diabetes and Digestive and Kidney Diseases, one of the sponsors of NDEP, said in a statement. The videos are available at
www.YourDiabetesInfo.org/HealthSense
Another Try for Diabetes Bill
Two congressmen have reintroduced the Gestational Diabetes Act, which passed the House last year but stalled in the Senate. Rep. Eliot Engel (D-N.Y.) and Rep. Michael C. Burgess (R-Tex.), a physician, said that the fight against gestational diabetes is a bipartisan effort and needs continued attention. “The statistics speak for themselves – mothers diagnosed with gestational diabetes are more likely to develop type 2 diabetes, and their children are at an increased risk not only for diabetes but other prenatal complications as well,” Rep. Burgess said in a statement. The bill (H.R.2194) aims to enhance surveillance and public health research on gestational diabetes, provide grants for reducing incidence of the condition, and expand research and treatments.
Smoking Is Risk in Obese Girls
Adolescent girls who are obese are twice as likely as others to become heavy smokers, according to the National Longitudinal Study of Adolescent Health survey. The authors controlled for demographic differences and factors such as smoking by parents and friends in analyzing the more than 4,000 survey responses. Family smoking turned out to be the strongest predictor of nicotine addiction, and a high grade point average mediated the girls' risk of becoming heavy smokers. “Obese, adolescent females may require targeted interventions to address their risk of subsequent high-level nicotine addiction, especially if risk factors such as parental smoking and poor school performance are present,” the authors concluded in the Journal of Adolescent Health.
IOM Addresses Childhood Obesity
Health care providers should monitor weight and height as part of every well-child visit and should help parents learn ways to increase physical activity and decrease sedentary behavior to curb early childhood obesity, according to a report from the Institute of Medicine. The report urges pediatricians and others who work with infants to encourage breastfeeding and to provide guidance on healthy eating strategies. In addition, health care providers should counsel parents on limiting television and other media use, and should urge parents and other caregivers not to permit televisions or other media devices in young children's bedrooms or sleeping areas. The IOM report details steps policy makers and federal programs can take to help curb early childhood obesity.
Privacy Notification Planned
A proposed change in health insurance-privacy rules would give patients the right to see reports on who has accessed their protected health information electronically. The change was mandated by the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act. Although health care providers are required to track this information, they don't have to share it with patients. “This proposed rule represents an important step in our continued efforts to promote accountability across the health care system, ensuring that providers properly safeguard private health information,” said Office of Civil Rights Director Georgina Verdugo. “We need to protect people's rights so that they know how their health information has been used or disclosed.”
Technology Boosts Diabetes Care
Diabetes care and outcomes were superior in facilities using electronic health records, compared with care at facilities with paper-based records, according a report by Better Health Greater Cleveland for the Robert Wood Johnson Foundation. Researchers compiled data on care such as eye exams and outcomes such as blood sugar control from 32 health centers in the Cleveland area using EHRs and from 5 using paper. More than half of the patients at EHR-based clinics received all necessary care; only 7% of patients who went to paper-based clinics did. Similarly, 44% of those at EHR-based clinics achieved positive outcomes, compared with 16% at paper-based clinics. The report noted that the federal government has committed $27 billion to EHRs and other health information technology over the next 10 years. It added that the Cleveland data show that using EHRs results in “dramatically better patient care.”
New Diabetes-Help Videos
A new series of 3- to 5-minute videos, part of the redesigned National Diabetes Education Program online library, addressin topics such as living with diabetes, finding support, preventing diabetes, and setting goals to improve health. “For more than 14 years, the NDEP has been in the forefront of raising awareness about diabetes, but more needs to be done to provide resources and tools to support health care providers and their patients when it comes to achieving and sustaining health goals,” Dr. Griffin P. Rodgers, director of the National Institute of Diabetes and Digestive and Kidney Diseases, one of the sponsors of NDEP, said in a statement. The videos are available at
www.YourDiabetesInfo.org/HealthSense
Another Try for Diabetes Bill
Two congressmen have reintroduced the Gestational Diabetes Act, which passed the House last year but stalled in the Senate. Rep. Eliot Engel (D-N.Y.) and Rep. Michael C. Burgess (R-Tex.), a physician, said that the fight against gestational diabetes is a bipartisan effort and needs continued attention. “The statistics speak for themselves – mothers diagnosed with gestational diabetes are more likely to develop type 2 diabetes, and their children are at an increased risk not only for diabetes but other prenatal complications as well,” Rep. Burgess said in a statement. The bill (H.R.2194) aims to enhance surveillance and public health research on gestational diabetes, provide grants for reducing incidence of the condition, and expand research and treatments.
Smoking Is Risk in Obese Girls
Adolescent girls who are obese are twice as likely as others to become heavy smokers, according to the National Longitudinal Study of Adolescent Health survey. The authors controlled for demographic differences and factors such as smoking by parents and friends in analyzing the more than 4,000 survey responses. Family smoking turned out to be the strongest predictor of nicotine addiction, and a high grade point average mediated the girls' risk of becoming heavy smokers. “Obese, adolescent females may require targeted interventions to address their risk of subsequent high-level nicotine addiction, especially if risk factors such as parental smoking and poor school performance are present,” the authors concluded in the Journal of Adolescent Health.
IOM Addresses Childhood Obesity
Health care providers should monitor weight and height as part of every well-child visit and should help parents learn ways to increase physical activity and decrease sedentary behavior to curb early childhood obesity, according to a report from the Institute of Medicine. The report urges pediatricians and others who work with infants to encourage breastfeeding and to provide guidance on healthy eating strategies. In addition, health care providers should counsel parents on limiting television and other media use, and should urge parents and other caregivers not to permit televisions or other media devices in young children's bedrooms or sleeping areas. The IOM report details steps policy makers and federal programs can take to help curb early childhood obesity.
Privacy Notification Planned
A proposed change in health insurance-privacy rules would give patients the right to see reports on who has accessed their protected health information electronically. The change was mandated by the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act. Although health care providers are required to track this information, they don't have to share it with patients. “This proposed rule represents an important step in our continued efforts to promote accountability across the health care system, ensuring that providers properly safeguard private health information,” said Office of Civil Rights Director Georgina Verdugo. “We need to protect people's rights so that they know how their health information has been used or disclosed.”
Drop in Oophorectomies at the Time of Benign Hysterectomy
Major Finding: There was an 8% absolute decrease in the performance of benign oophorectomy at the time of benign hysterectomy, between 2001 and 2006.
Data Source: A cross-sectional analysis of the New York State Department of Health database of 146,494 women aged 18 years or older who had undergone benign hysterectomy.
Disclosures: Dr. Novetsky said he had no relevant financial disclosures.
WASHINGTON – The rate of bilateral oophorectomies in women under age 55 significantly decreased from 2001 to 2005, according to a study based on a cross-sectional analysis of the New York State Department of Health database.
The study results presented at the meetingoalso showed that age, route of hysterectomy, and associated gynecological diagnoses influenced the rate of oophorectomy.
“Recent studies on complications of HRT [hormone replacement therapy] and prophylactic bilateral oophorectomy may have influenced patients' and physicians' decision-making, leading to lower rates,” according to the researchers (ACOG 2011, A.P. Novetsky et al., abstract).
Dr. Akiva P. Novetsky, lead researcher and chief resident at New York University Langone Medical Center, said that although hysterectomy is among the most common operations performed in U.S. women, prophylactic oophorectomy has remained controversial.
Among the studies that have argued for the preservation of ovaries is the landmark 2005 study by Dr. W.H. Parker, which concluded that “ovarian conservation until at least age 65 benefits long-term survival for women at average risk of ovarian cancer when undergoing hysterectomy for benign disease” (Obstet. Gynecol. 2005;106:–26).
Meanwhile, the results of a 2011 study based on data from the Women's Health Initiative showed that bilateral salpingo-oophorectomy “may not have an adverse effect on cardiovascular health, hip fracture, cancer, or total mortality compared with hysterectomy and ovarian conservation” (Arch. Intern. Med. 2011;171:760–8).
Roughly 90% of women who get ovarian cancer are older than 40 years of age, and the greatest number includes women aged 55 years or older, according to statistics from the Centers for Disease Control and Prevention.
Ovarian cancer is the second most common and deadliest gynecologic cancer.
What prompted the study was the team's observation of a trend in ovarian retention in the past decade, Dr. Novetsky said in an interview.
The analysis included 146,494 women aged 18 years or older who had undergone benign hysterectomy. The results showed an 8% absolute decrease in the performance of benign oophorectomy at the time of benign hysterectomy, between 2001 and 2006.
The findings, “didn't come as a huge shock to us,” said Dr. Novetsky. “What was interesting to us was the temporal relationship to the decline in HRT use, although we can't make an argument for a cause and effect relationship.”
Nearly half (47%) of hysterectomies in the database included oophorectomy. Race and insurance status were associated with performance of oophorectomy.
The results also showed that women who underwent oophorectomy were older; more likely to have a family history of breast/ovarian cancer; and more likely to have a personal history of breast cancer, ovarian cancer, or endometriosis.
Women were less likely to have an oophorectomy if they underwent vaginal or laparoscopic hysterectomy, or had uterine prolapse.
“We didn't see a decline [in oophorectomy] in women over age 55,” said Dr. Novetsky.
Although the study was based on a New York State database, he predicted that the trend toward fewer oophorectomies at the time of benign hysterectomy is prevalent nationwide, and “will continue to increase among premenopausal women.”
The study had some limitations, including the accuracy of the database and the fact that risk factors for the performance of oophorectomy may not have been adequately recorded.
Dr. Novetsky said while oophorectomy at the time of benign hysterectomy might not have been as much in question a decade ago, “a lot more patient counseling and research go into it now. … It's become a lot more personalized.”
Major Finding: There was an 8% absolute decrease in the performance of benign oophorectomy at the time of benign hysterectomy, between 2001 and 2006.
Data Source: A cross-sectional analysis of the New York State Department of Health database of 146,494 women aged 18 years or older who had undergone benign hysterectomy.
Disclosures: Dr. Novetsky said he had no relevant financial disclosures.
WASHINGTON – The rate of bilateral oophorectomies in women under age 55 significantly decreased from 2001 to 2005, according to a study based on a cross-sectional analysis of the New York State Department of Health database.
The study results presented at the meetingoalso showed that age, route of hysterectomy, and associated gynecological diagnoses influenced the rate of oophorectomy.
“Recent studies on complications of HRT [hormone replacement therapy] and prophylactic bilateral oophorectomy may have influenced patients' and physicians' decision-making, leading to lower rates,” according to the researchers (ACOG 2011, A.P. Novetsky et al., abstract).
Dr. Akiva P. Novetsky, lead researcher and chief resident at New York University Langone Medical Center, said that although hysterectomy is among the most common operations performed in U.S. women, prophylactic oophorectomy has remained controversial.
Among the studies that have argued for the preservation of ovaries is the landmark 2005 study by Dr. W.H. Parker, which concluded that “ovarian conservation until at least age 65 benefits long-term survival for women at average risk of ovarian cancer when undergoing hysterectomy for benign disease” (Obstet. Gynecol. 2005;106:–26).
Meanwhile, the results of a 2011 study based on data from the Women's Health Initiative showed that bilateral salpingo-oophorectomy “may not have an adverse effect on cardiovascular health, hip fracture, cancer, or total mortality compared with hysterectomy and ovarian conservation” (Arch. Intern. Med. 2011;171:760–8).
Roughly 90% of women who get ovarian cancer are older than 40 years of age, and the greatest number includes women aged 55 years or older, according to statistics from the Centers for Disease Control and Prevention.
Ovarian cancer is the second most common and deadliest gynecologic cancer.
What prompted the study was the team's observation of a trend in ovarian retention in the past decade, Dr. Novetsky said in an interview.
The analysis included 146,494 women aged 18 years or older who had undergone benign hysterectomy. The results showed an 8% absolute decrease in the performance of benign oophorectomy at the time of benign hysterectomy, between 2001 and 2006.
The findings, “didn't come as a huge shock to us,” said Dr. Novetsky. “What was interesting to us was the temporal relationship to the decline in HRT use, although we can't make an argument for a cause and effect relationship.”
Nearly half (47%) of hysterectomies in the database included oophorectomy. Race and insurance status were associated with performance of oophorectomy.
The results also showed that women who underwent oophorectomy were older; more likely to have a family history of breast/ovarian cancer; and more likely to have a personal history of breast cancer, ovarian cancer, or endometriosis.
Women were less likely to have an oophorectomy if they underwent vaginal or laparoscopic hysterectomy, or had uterine prolapse.
“We didn't see a decline [in oophorectomy] in women over age 55,” said Dr. Novetsky.
Although the study was based on a New York State database, he predicted that the trend toward fewer oophorectomies at the time of benign hysterectomy is prevalent nationwide, and “will continue to increase among premenopausal women.”
The study had some limitations, including the accuracy of the database and the fact that risk factors for the performance of oophorectomy may not have been adequately recorded.
Dr. Novetsky said while oophorectomy at the time of benign hysterectomy might not have been as much in question a decade ago, “a lot more patient counseling and research go into it now. … It's become a lot more personalized.”
Major Finding: There was an 8% absolute decrease in the performance of benign oophorectomy at the time of benign hysterectomy, between 2001 and 2006.
Data Source: A cross-sectional analysis of the New York State Department of Health database of 146,494 women aged 18 years or older who had undergone benign hysterectomy.
Disclosures: Dr. Novetsky said he had no relevant financial disclosures.
WASHINGTON – The rate of bilateral oophorectomies in women under age 55 significantly decreased from 2001 to 2005, according to a study based on a cross-sectional analysis of the New York State Department of Health database.
The study results presented at the meetingoalso showed that age, route of hysterectomy, and associated gynecological diagnoses influenced the rate of oophorectomy.
“Recent studies on complications of HRT [hormone replacement therapy] and prophylactic bilateral oophorectomy may have influenced patients' and physicians' decision-making, leading to lower rates,” according to the researchers (ACOG 2011, A.P. Novetsky et al., abstract).
Dr. Akiva P. Novetsky, lead researcher and chief resident at New York University Langone Medical Center, said that although hysterectomy is among the most common operations performed in U.S. women, prophylactic oophorectomy has remained controversial.
Among the studies that have argued for the preservation of ovaries is the landmark 2005 study by Dr. W.H. Parker, which concluded that “ovarian conservation until at least age 65 benefits long-term survival for women at average risk of ovarian cancer when undergoing hysterectomy for benign disease” (Obstet. Gynecol. 2005;106:–26).
Meanwhile, the results of a 2011 study based on data from the Women's Health Initiative showed that bilateral salpingo-oophorectomy “may not have an adverse effect on cardiovascular health, hip fracture, cancer, or total mortality compared with hysterectomy and ovarian conservation” (Arch. Intern. Med. 2011;171:760–8).
Roughly 90% of women who get ovarian cancer are older than 40 years of age, and the greatest number includes women aged 55 years or older, according to statistics from the Centers for Disease Control and Prevention.
Ovarian cancer is the second most common and deadliest gynecologic cancer.
What prompted the study was the team's observation of a trend in ovarian retention in the past decade, Dr. Novetsky said in an interview.
The analysis included 146,494 women aged 18 years or older who had undergone benign hysterectomy. The results showed an 8% absolute decrease in the performance of benign oophorectomy at the time of benign hysterectomy, between 2001 and 2006.
The findings, “didn't come as a huge shock to us,” said Dr. Novetsky. “What was interesting to us was the temporal relationship to the decline in HRT use, although we can't make an argument for a cause and effect relationship.”
Nearly half (47%) of hysterectomies in the database included oophorectomy. Race and insurance status were associated with performance of oophorectomy.
The results also showed that women who underwent oophorectomy were older; more likely to have a family history of breast/ovarian cancer; and more likely to have a personal history of breast cancer, ovarian cancer, or endometriosis.
Women were less likely to have an oophorectomy if they underwent vaginal or laparoscopic hysterectomy, or had uterine prolapse.
“We didn't see a decline [in oophorectomy] in women over age 55,” said Dr. Novetsky.
Although the study was based on a New York State database, he predicted that the trend toward fewer oophorectomies at the time of benign hysterectomy is prevalent nationwide, and “will continue to increase among premenopausal women.”
The study had some limitations, including the accuracy of the database and the fact that risk factors for the performance of oophorectomy may not have been adequately recorded.
Dr. Novetsky said while oophorectomy at the time of benign hysterectomy might not have been as much in question a decade ago, “a lot more patient counseling and research go into it now. … It's become a lot more personalized.”
From the Annual Meeting of the American College of Obstetricians and Gynecologists