User login
Depression As a Potential Contributing Factor in Hidradenitis Suppurativa and Associated Racial Gaps
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28
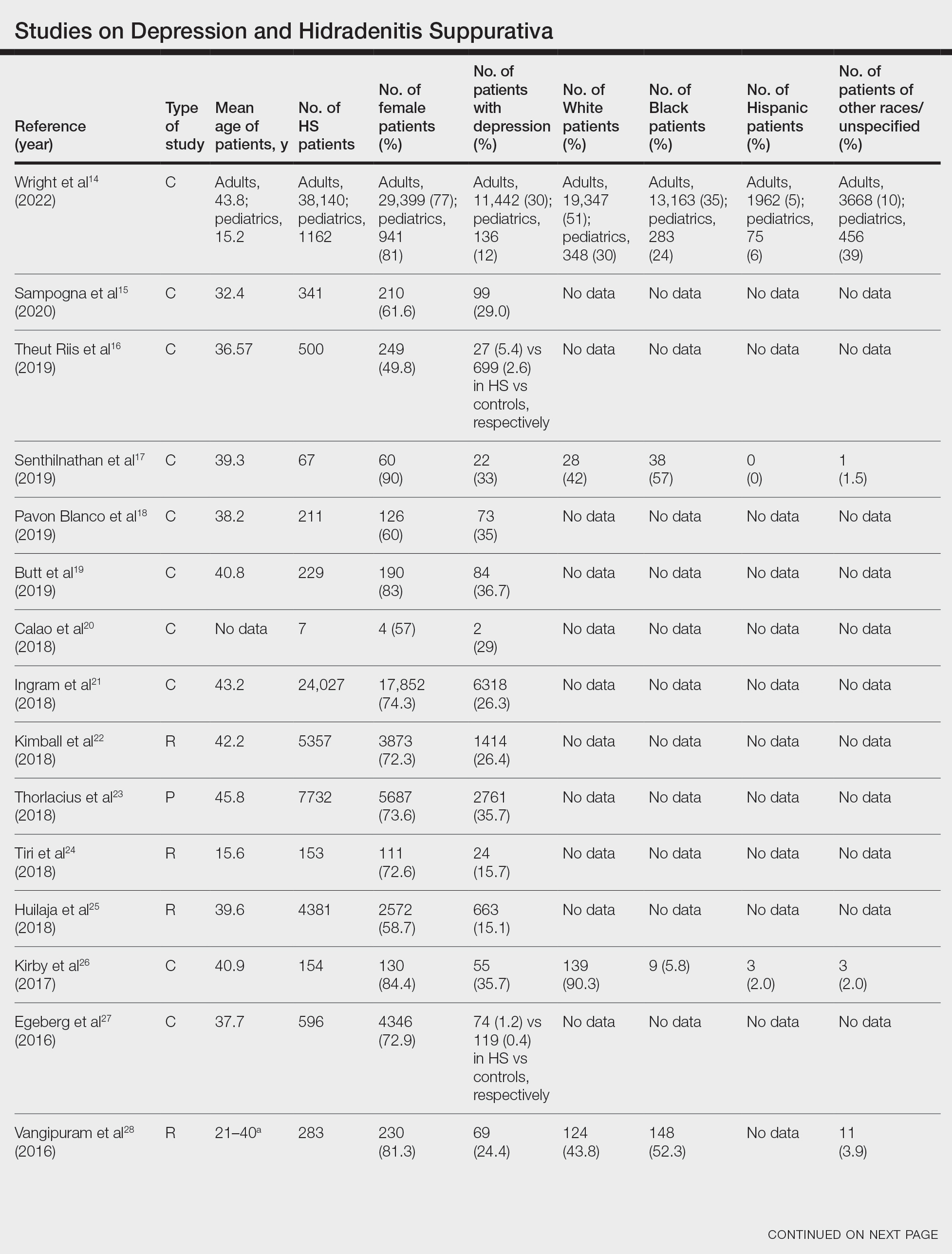
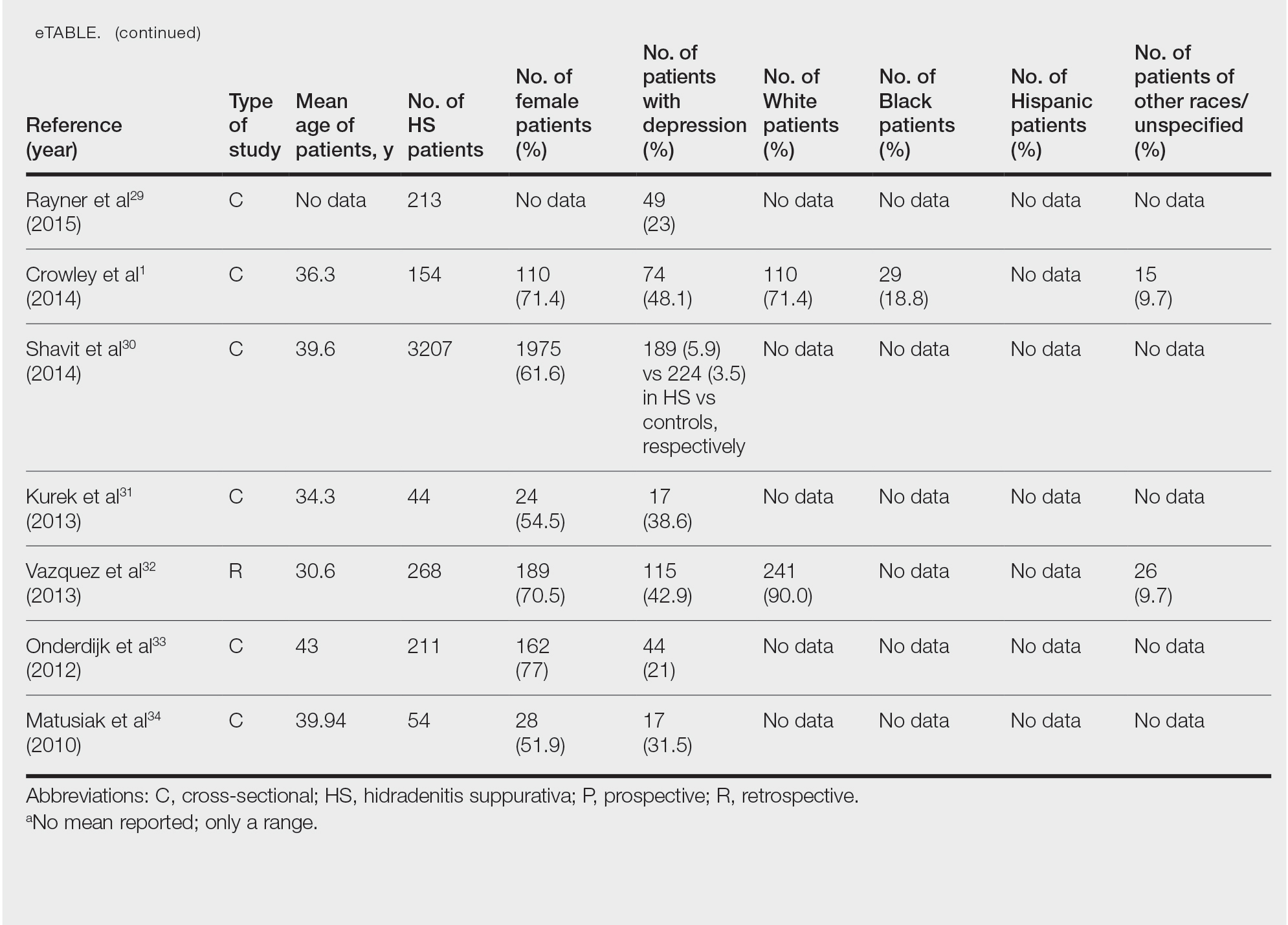
Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28


Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
Hidradenitis suppurativa (HS)—a chronic, relapsing, inflammatory disorder involving terminal hair follicles in apocrine gland–rich skin—manifests as tender inflamed nodules that transform into abscesses, sinus tracts, and scarring.1,2 The etiology of HS is multifactorial, encompassing lifestyle, microbiota, hormonal status, and genetic and environmental factors. These factors activate the immune system around the terminal hair follicles and lead to hyperkeratosis of the infundibulum of the hair follicles in intertriginous regions. This progresses to follicular occlusion, stasis, and eventual rupture. Bacterial multiplication within the plugged pilosebaceous units further boosts immune activation. Resident and migrated cells of the innate and adaptive immune system then release proinflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-17, which further enhance immune cell influx and inflammation.3,4 This aberrant immune response propagates the production of deep-seated inflammatory nodules and abscesses.3-8
The estimated prevalence of HS is 1% worldwide.9 It is more prevalent in female and Black patients (0.30%) than White patients (0.09%) and is intermediate in prevalence in the biracial population (0.22%).10 Hidradenitis suppurativa is thought to be associated with lower socioeconomic status (SES). In a retrospective analysis of HS patients (N=375), approximately one-third of patients were Black, had advanced disease, and had a notably lower SES.11 Furthermore, HS has been reported to be associated with systemic inflammation and comorbidities such as morbid obesity (38.3%) and hypertension (39.6%) as well as other metabolic syndrome–related disorders and depression (48.1%).1
Hidradenitis suppurativa may contribute to the risk for depression through its substantial impact on health-related quality of life, which culminates in social withdrawal, unemployment, and suicidal thoughts.12 The high prevalence of depression in individuals with HS1 and its association with systemic inflammation13 increases the likelihood that a common genetic predisposition also may exist between both conditions. Because depression frequently has been discovered as a concomitant diagnosis in patients with HS, we hypothesize that a shared genetic susceptibility also may exist between the 2 disorders. Our study sought to explore data on the co-occurrence of depression with HS, including its demographics and racial data.
Methods
We conducted a PubMed search of articles indexed for MEDLINE as well as Google Scholar using the terms depression and hidradenitis suppurativa to obtain all research articles published from 2000 to 2022. Articles were selected based on relevance to the topic of exploration. English-language articles that directly addressed the epidemiology, etiology, pathophysiology, and co-occurrence of both depression and HS with numerical data were included. Articles were excluded if they did not explore the information of interest on these 2 disorders or did not contain clear statistical data of patients with the 2 concurrent medical conditions.
Results
Twenty-two cross-sectional, prospective, and retrospective studies that fit the search criteria were identified and included in the analysis (eTable).1,14-34 Sixteen (72.7%) studies were cross-sectional, 5 (22.7%) were retrospective, and only 1 (4.5%) was a prospective study. Only 6 of the studies provided racial data,1,14,17,26,28,32 and of them, 4 had predominately White patients,1,14,26,32 whereas the other 2 had predominantly Black patients.17,28


Hidradenitis suppurativa was found to coexist with depression in all the studies, with a prevalence of 1.2% to 48.1%. There also was a higher prevalence of depression in HS patients than in the control patients without HS. Furthermore, a recent study by Wright and colleagues14 stratified the depression prevalence data by age and found a higher prevalence of depression in adults vs children with HS (30% vs 12%).
Comment
Major depression—a chronic and debilitating illness—is the chief cause of disability globally and in the United States alone and has a global lifetime prevalence of 17%.35 In a study of 388 patients diagnosed with depression and 404 community-matched controls who were observed for 10 years, depressed patients had a two-thirds higher likelihood of developing a serious physical illness than controls. The depression-associated elevated risk for serious physical illness persisted after controlling for confounding variables such as alcohol abuse, smoking, and level of physical activity.36 Studies also have demonstrated that HS is more prevalent in Black individuals10 and in individuals of low SES,37 who are mostly the Black and Hispanic populations that experience the highest burden of racial microaggression38 and disparities in health access and outcomes.39,40 The severity and chronicity of major depressive disorder also is higher in Black patients compared with White patients (57% vs 39%).41 Because major depression and HS are most common among Black patients who experience the highest-burden negative financial and health disparities, there may be a shared genetic disposition to both medical conditions.
Moreover, the common detrimental lifestyle choices associated with patients with depression and HS also suggest the possibility of a collective genetic susceptibility. Patients with depression also report increased consumption of alcohol, tobacco, and illicit substances; sedentary lifestyle leading to obesity; and poor compliance with prescribed medical treatment.42 Smoking and obesity are known contributors to the pathogenesis of HS, and their modification also is known to positively impact the disease course. In a retrospective single-cohort study, 50% of obese HS patients (n=35) reported a substantial decrease in disease severity after a reduction of more than 15% in body mass index over 2 years following bariatric surgery (n=35).43 Patients with HS also have reported disease remission following extensive weight loss.44 In addition, evidence has supported smoking cessation in improving the disease course of HS.43 Because these detrimental lifestyle choices are prevalent in both patients with HS and those with depression, a co-genetic susceptibility also may exist.
Furthermore, depression is characterized by a persistent inflammatory state,13,45 similar to HS.46 Elevated levels of a variety of inflammatory markers, such as C-reactive protein (CRP), IL-6, and soluble intercellular adhesion molecule 1, have been reported in patients with depression compared with healthy controls.13,45 Further analysis found a positive correlation and a strong association between depression and these inflammatory markers.47 Moreover, adipokines regulate inflammatory responses, and adipokines play a role in the pathogenesis of HS. Adipokine levels such as elevated omentin-1 (a recently identified adipokine) were found to be altered in patients with HS compared with controls.48 Results from clinical studies and meta-analyses of patients with depression also have demonstrated that adipokines are dysregulated in this population,49,50 which may be another potential genetic link between depression and HS.
In addition, genetic susceptibility to depression and HS may be shared because the inflammatory markers that have a strong association with depression also have been found to play an important role in HS treatment and disease severity prediction. In a retrospective cohort study of 404 patients, CRP or IL-6 levels were found to be reliable predictors of HS disease severity, which may explain why anti–tumor necrosis factor antibody regimens such as adalimumab and infliximab have clinically ameliorated disease activity in several cases of HS.51 In a study evaluating these drugs, high baseline levels of high-sensitivity CRP and IL-6 were predictive of patient response to infliximab.52 In a meta-analysis evaluating 20,791 participants, an association was found between concurrent depression and CRP. Furthermore, inflammation measured by high levels of CRP or IL-6 was observed to predict future depression.53 If the same inflammatory markers—CRP and IL-6—both play a major role in the disease activity of depression and HS, then a concurrent genetic predisposition may exist.
Conclusion
Understanding the comorbidities, etiologies, and risk factors for the development and progression of HS is an important step toward improved disease management. Available studies on comorbid depression in HS largely involve White patients, and more studies are needed in patients with skin of color, particularly the Black population, who have the highest prevalence of HS.10 Given the evidence for an association between depression and HS, we suggest a large-scale investigation of this patient population that includes a complete medical history, onset of HS in comparison to the onset of depression, and specific measures of disease progress and lifetime management of depression, which may help to increase knowledge about the role of depression in HS and encourage more research in this area. If shared genetic susceptibility is established, aggressive management of depression in patients at risk for HS may reduce disease incidence and severity as well as the psychological burden on patients.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
- Crowley JJ, Mekkes JR, Zouboulis CC, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561-1565.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:1-20.
- Wolk K, Warszawska K, Hoeflich C, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228-1239.
- von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537.
- Van Der Zee HH, De Ruiter L, Van Den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292-1298.
- Schlapbach C, Hänni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173:1431-1439.
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4-S7.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Soliman YS, Hoffman LK, Guzman AK, et al. African American patients with hidradenitis suppurativa have significant health care disparities: a retrospective study. J Cutan Med Surg. 2019;23:334-336.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101.
- Beatriz Currier M, Nemeroff CB. Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Antiinflamm Antiallergy Agents Med Chem. 2012;9:212-220.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol. 2022;86:55-60.
- Sampogna F, Fania L, Mastroeni S, et al. Correlation between depression, quality of life and clinical severity in patients with hidradenitis suppurativa. Acta Derm Venereol. 2020;100:1-6.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Senthilnathan A, Kolli SS, Cardwell LA, et al. Depression in hidradenitis suppurativa. Br J Dermatol. 2019;181:1087-1088.
- Pavon Blanco A, Turner MA, Petrof G, et al. To what extent do disease severity and illness perceptions explain depression, anxiety and quality of life in hidradenitis suppurativa? Br J Dermatol. 2019;180:338-345.
- Butt M, Sisic M, Silva C, et al. The associations of depression and coping methods on health-related quality of life for those with hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:1137-1139.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13:e0200683.
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924.
- Kimball AB, Sundaram M, Gauthier G, et al. The comorbidity burden of hidradenitis suppurativa in the United States: a claims data analysis. Dermatol Ther (Heidelb). 2018;8:557.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Tiri H, Jokelainen J, Timonen M, et al. Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol. 2018;79:514-519.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Kirby JS, Butt M, Esmann S, et al. Association of resilience with depression and health-related quality of life for patients with hidradenitis suppurativa. JAMA Dermatol. 2017;153:1263.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152:429-434.
- Vangipuram R, Vaidya T, Jandarov R, et al. Factors contributing to depression and chronic pain in patients with hidradenitis suppurativa: results from a single-center retrospective review. Dermatology. 2016;232:692-695.
- Rayner L, Jackson K, Turner M, et al. Integrated mental health assessment in a tertiary medical dermatology service: feasibility and the prevalence of common mental disorder. Br J Dermatol. 2015;173:201.
- Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa [published online June 9, 2014]. J Eur Acad Dermatol Venereol. 2015;29:371-376.
- Kurek A, Johanne Peters EM, Sabat R, et al. Depression is a frequent co-morbidity in patients with acne inversa. J Dtsch Dermatol Ges. 2013;11:743-749.
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97.
- Onderdijk AJ, Van Der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa [published online February 20, 2012]. J Eur Acad Dermatol Venereol. 2013;27:473-478.
- Matusiak Ł, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268.
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627.
- Holahan CJ, Pahl SA, Cronkite RC, et al. Depression and vulnerability to incident physical illness across 10 years. J Affect Disord. 2009;123:222-229.
- Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755-759.e1.
- Williams MT, Skinta MD, Kanter JW, et al. A qualitative study of microaggressions against African Americans on predominantly White campuses. BMC Psychol. 2020;8:1-13.
- Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93:1945-1952.
- Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407-411.
- Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305-315.
- Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283:506-511.
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824.
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg . 2020;24:64-72.
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519-525.
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399-409.
- Davidson KW, Schwartz JE, Kirkland SA, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study). Am J Cardiol. 2009;103:755-761.
- González-López MA, Ocejo-Viñals JG, Mata C, et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol Alergol. 2021;38:450-454.
- Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048.
- Setayesh L, Ebrahimi R, Pooyan S, et al. The possible mediatory role of adipokines in the association between low carbohydrate diet and depressive symptoms among overweight and obese women. PLoS One. 2021;16:e0257275 .
- Andriano TM, Benesh G, Babbush KM, et al. Serum inflammatory markers and leukocyte profiles accurately describe hidradenitis suppurativa disease severity. Int J Dermatol. 2022;61:1270-1275.
- Montaudié H, Seitz-Polski B, Cornille A, et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;6:156-158.
- Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940-948.
Practice Points
- Hidradenitis suppurativa (HS) is known to be associated with systemic inflammation and comorbidities, including depression.
- Depression may be a potential contributing factor to HS in affected patients, and studies on HS with comorbid depression in patients with skin of color are lacking.
Adherence to Topical Treatment Can Improve Treatment-Resistant Moderate Psoriasis
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
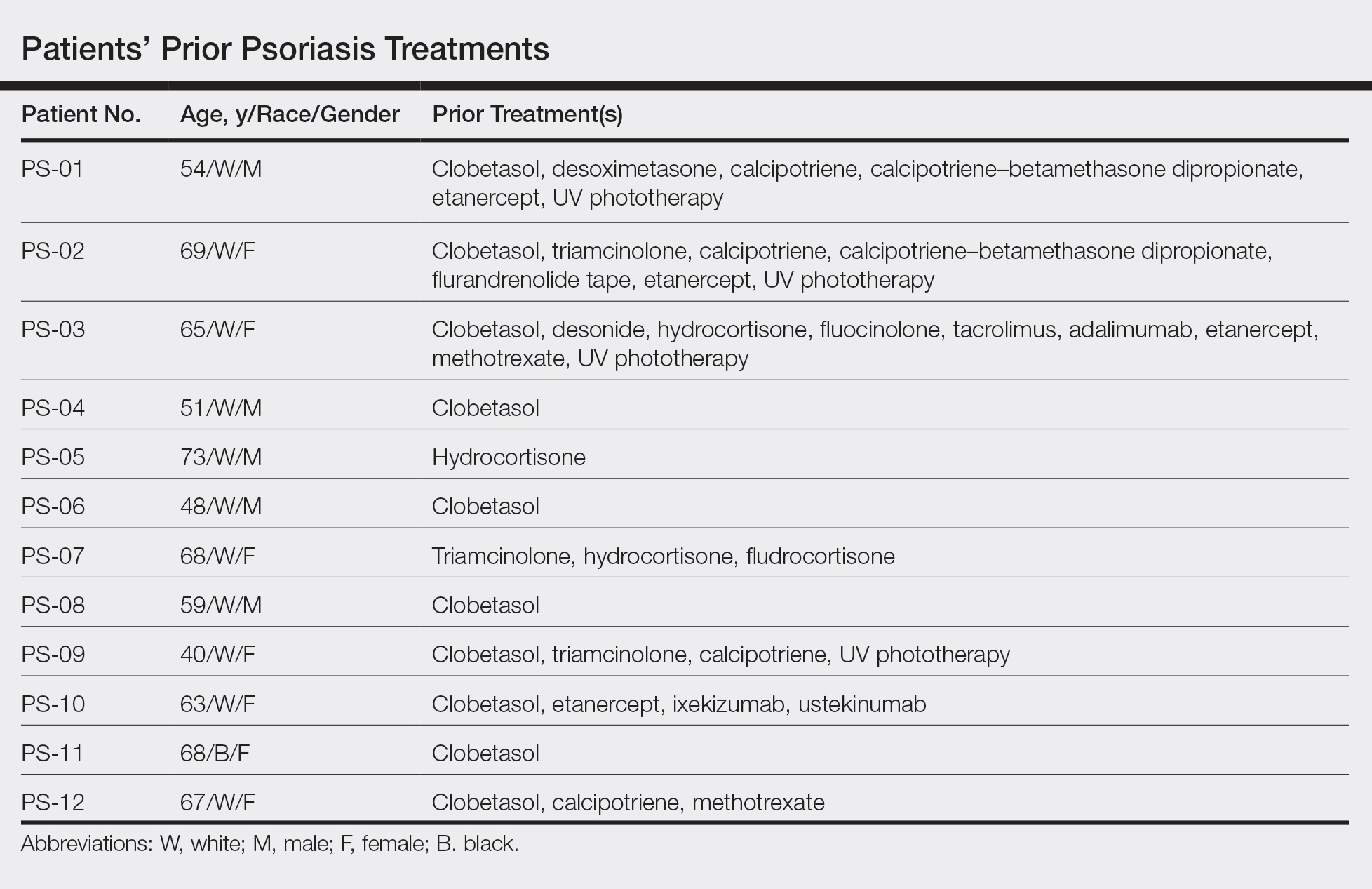
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.
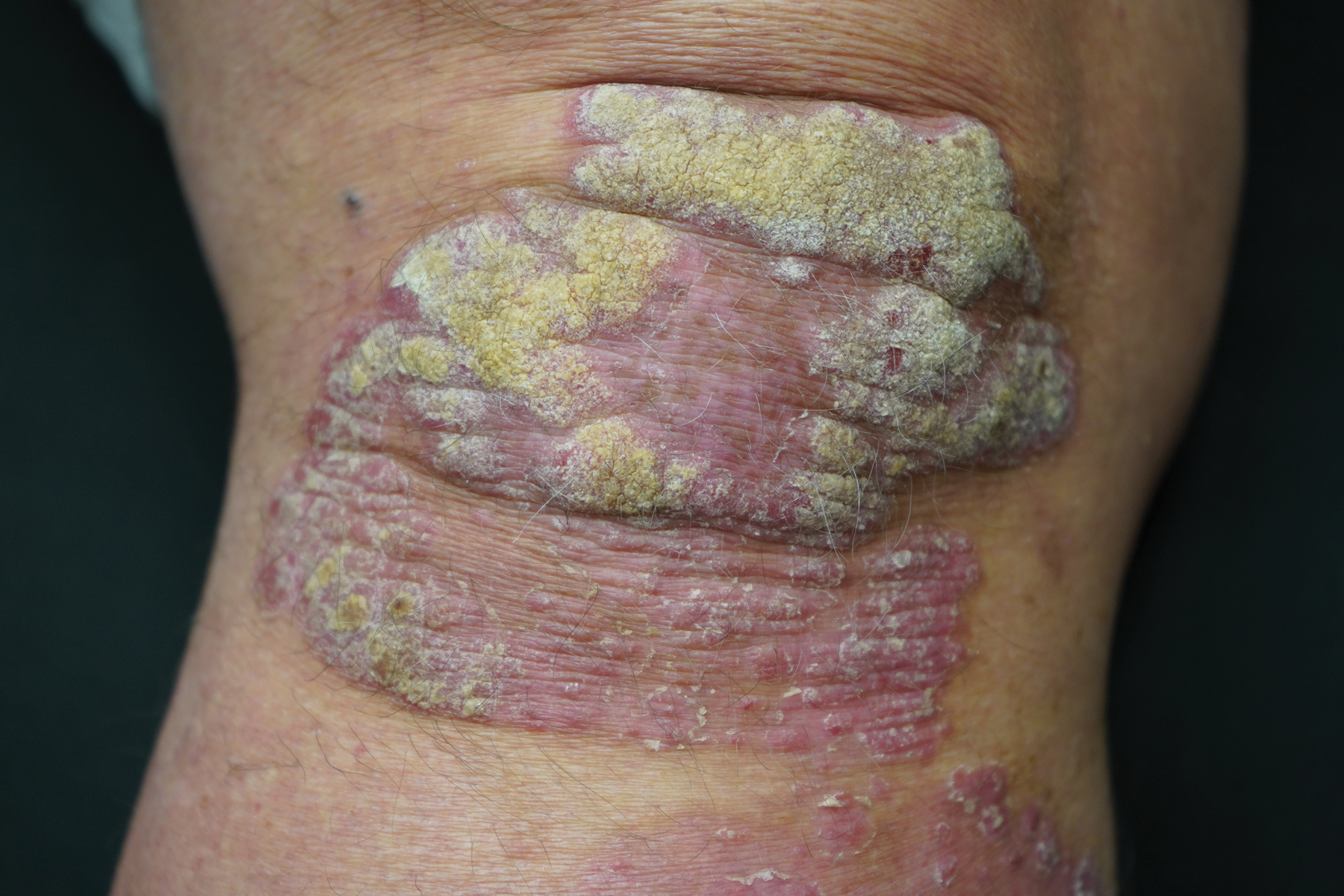
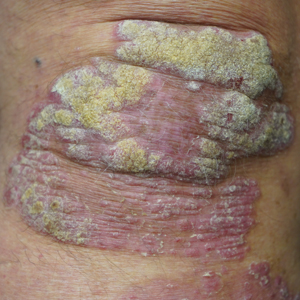
All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).
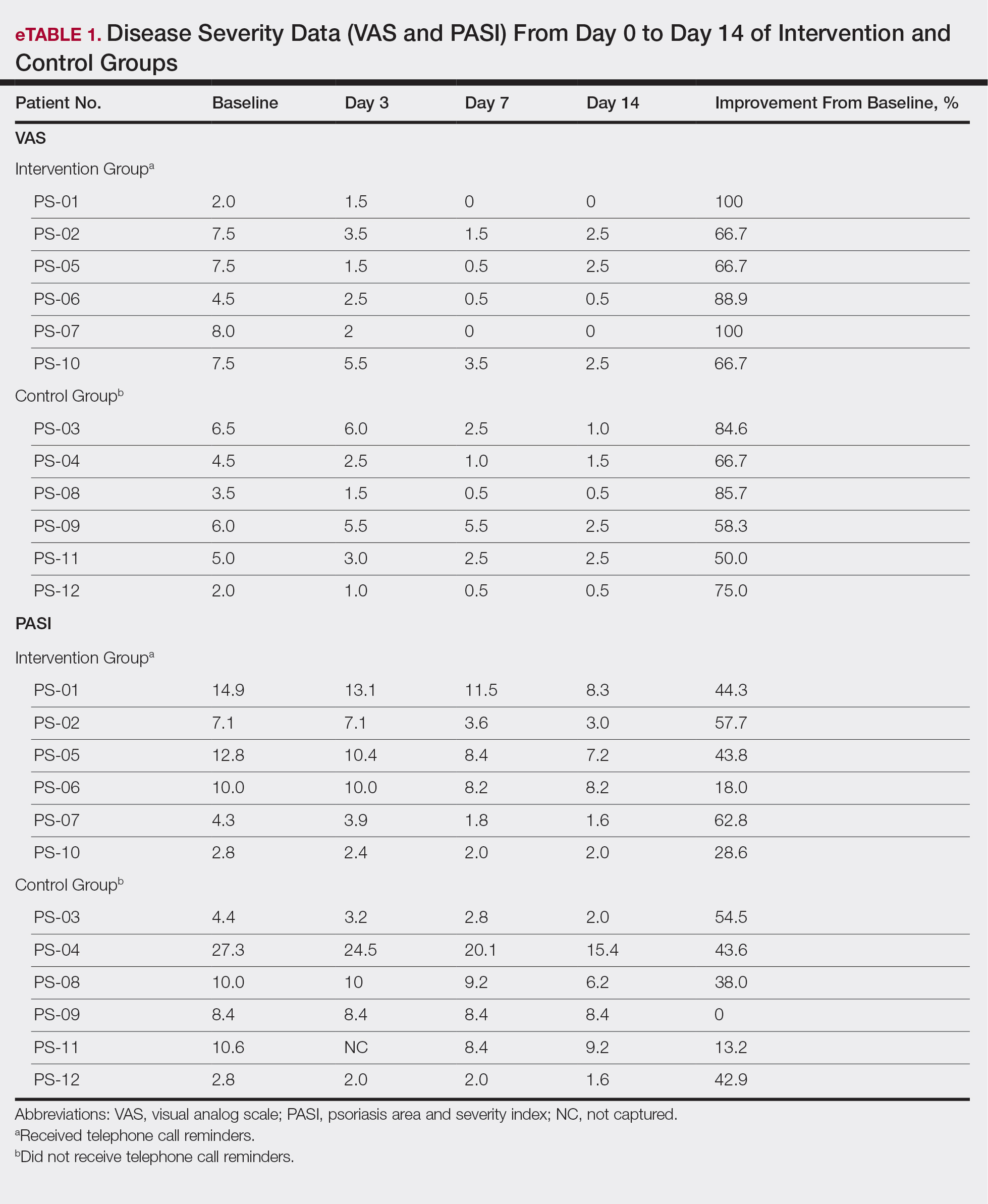
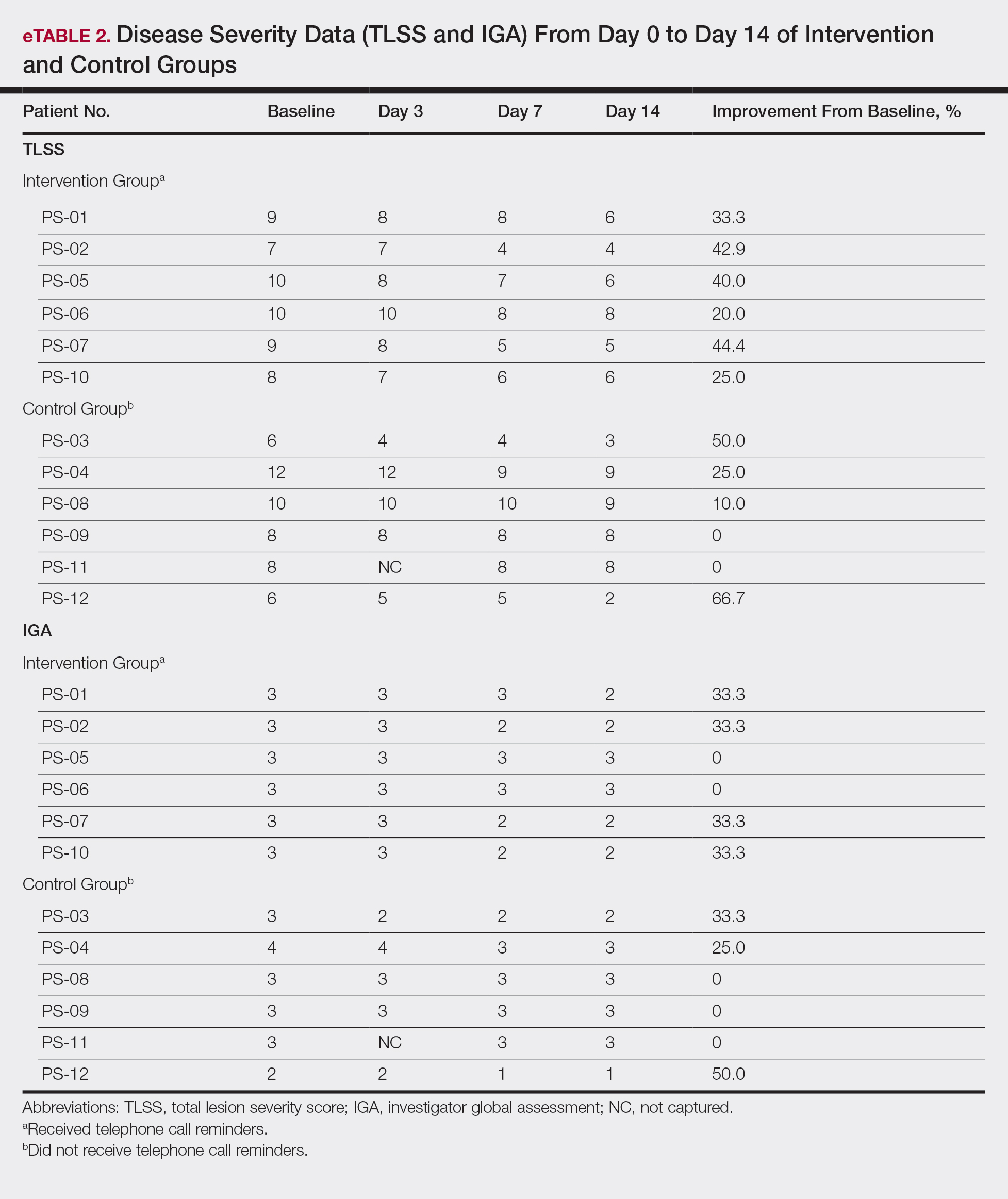
The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.


All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).


The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.


All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).


The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
Practice Points
- Most patients with psoriasis are good candidates for topical treatment.
- Topical treatment of psoriasis often is ineffective.
- Topical treatment of psoriasis can be rapidly effective, even in patients who reported disease that was resistant to topical treatment.
Topical Corticosteroids for Treatment-Resistant Atopic Dermatitis
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.
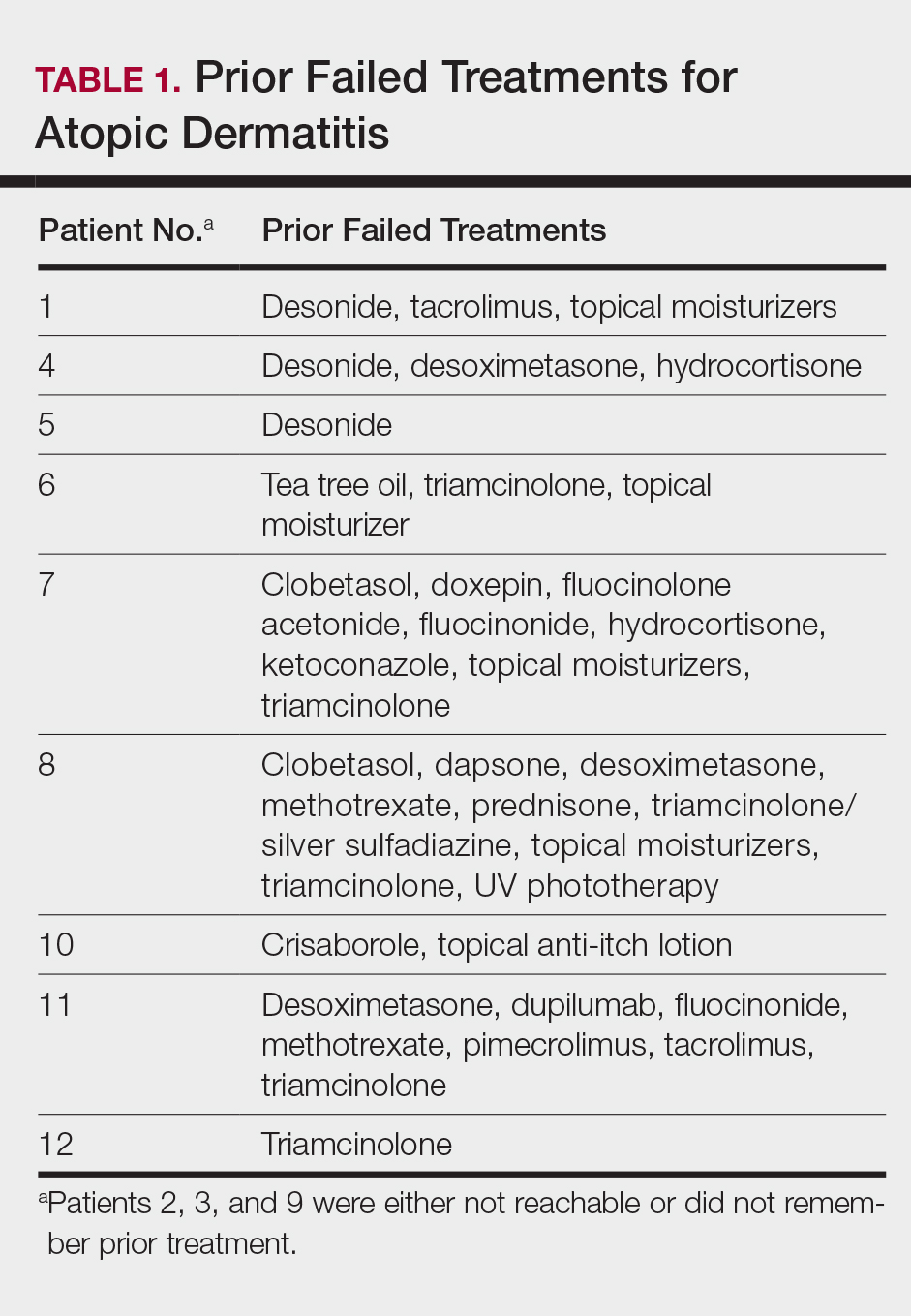
Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.
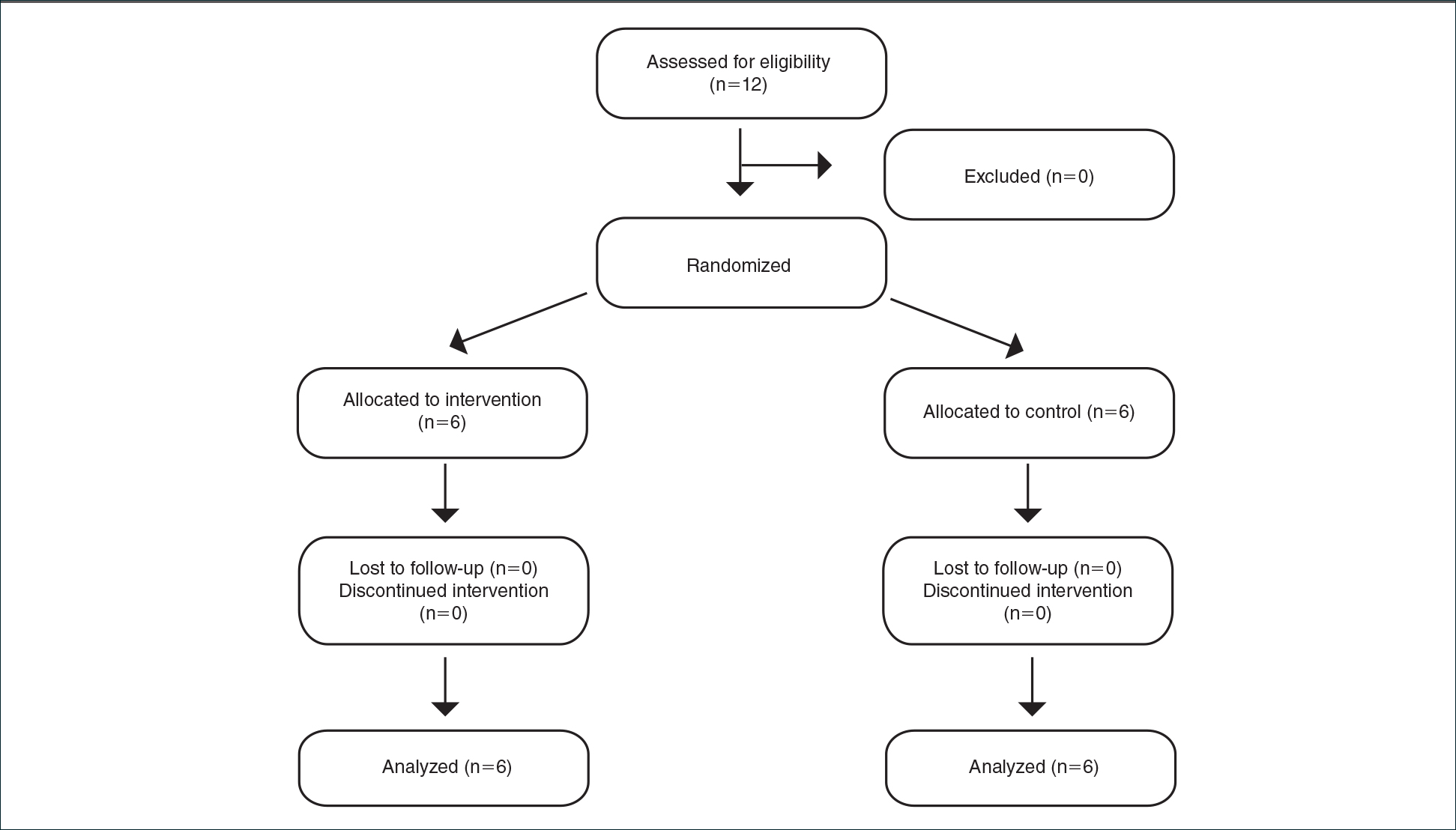
All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.
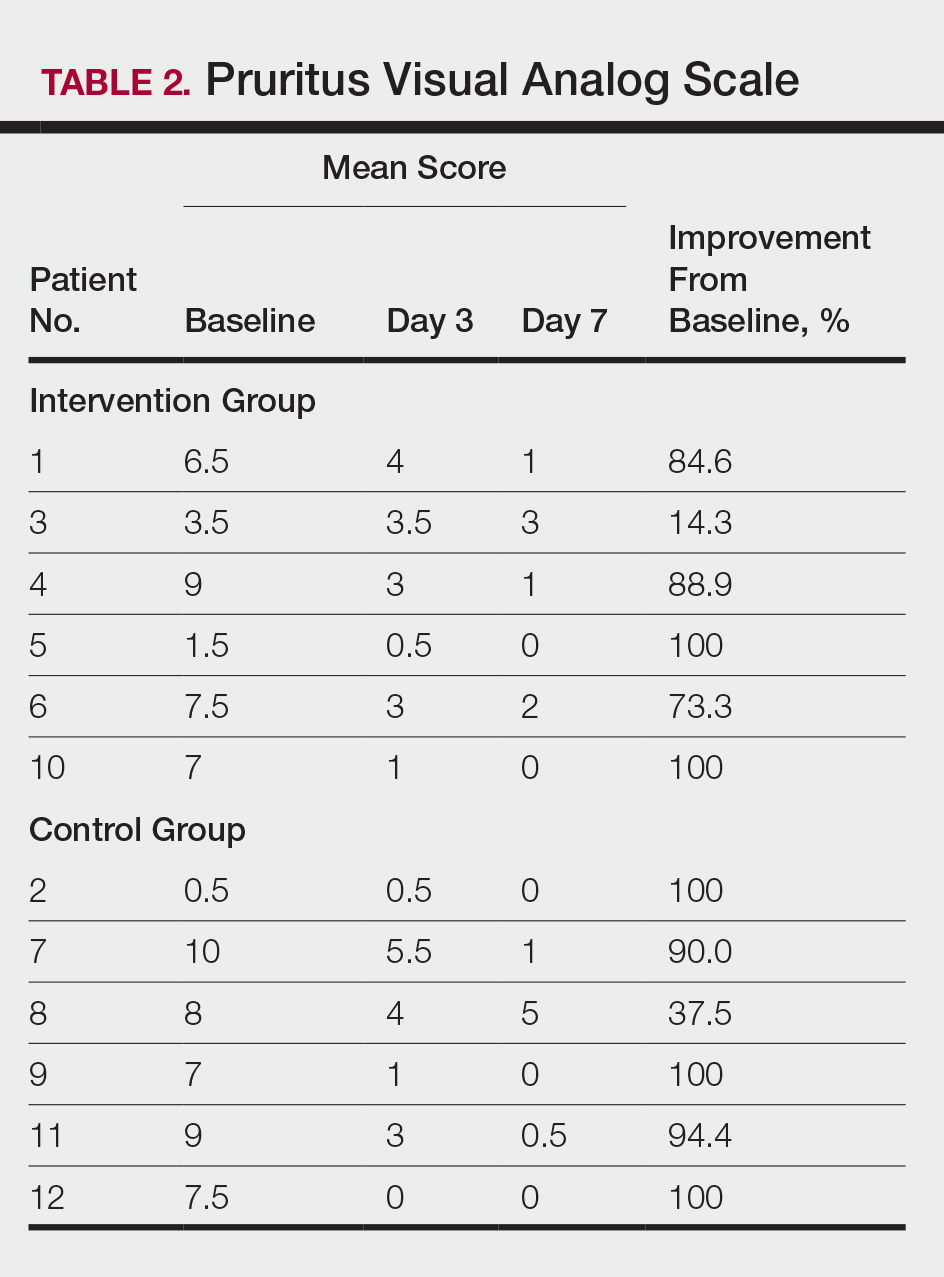
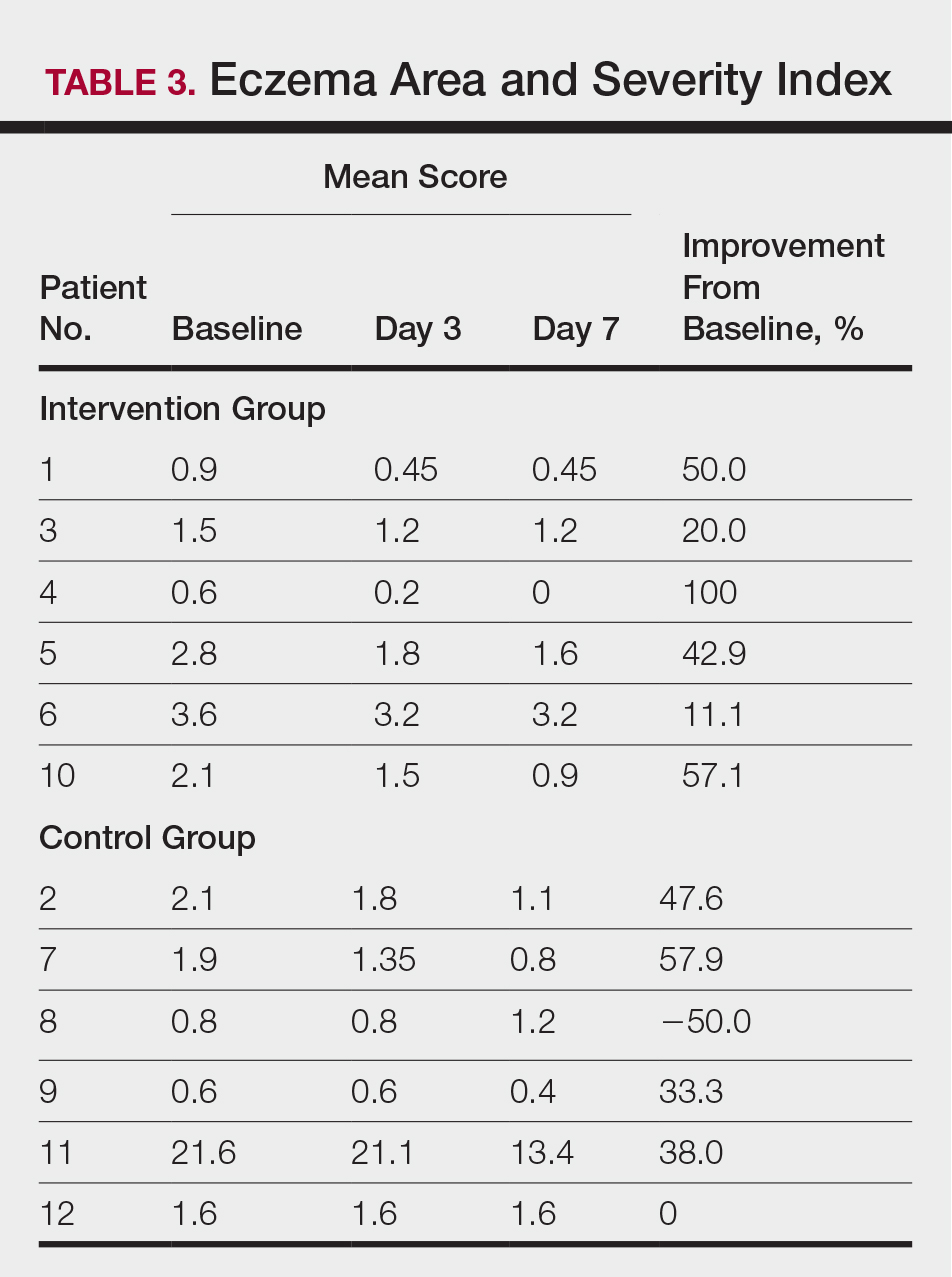
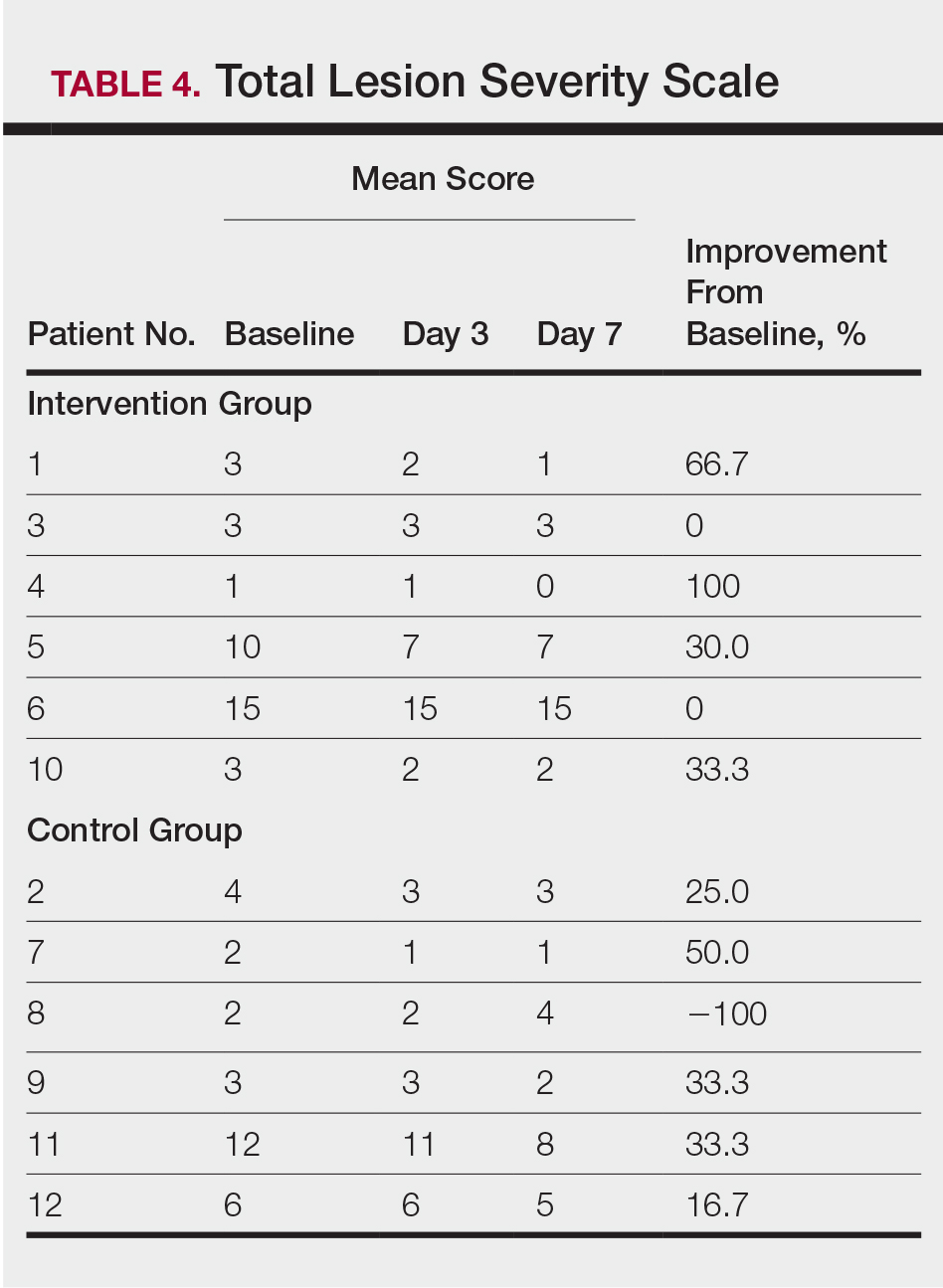
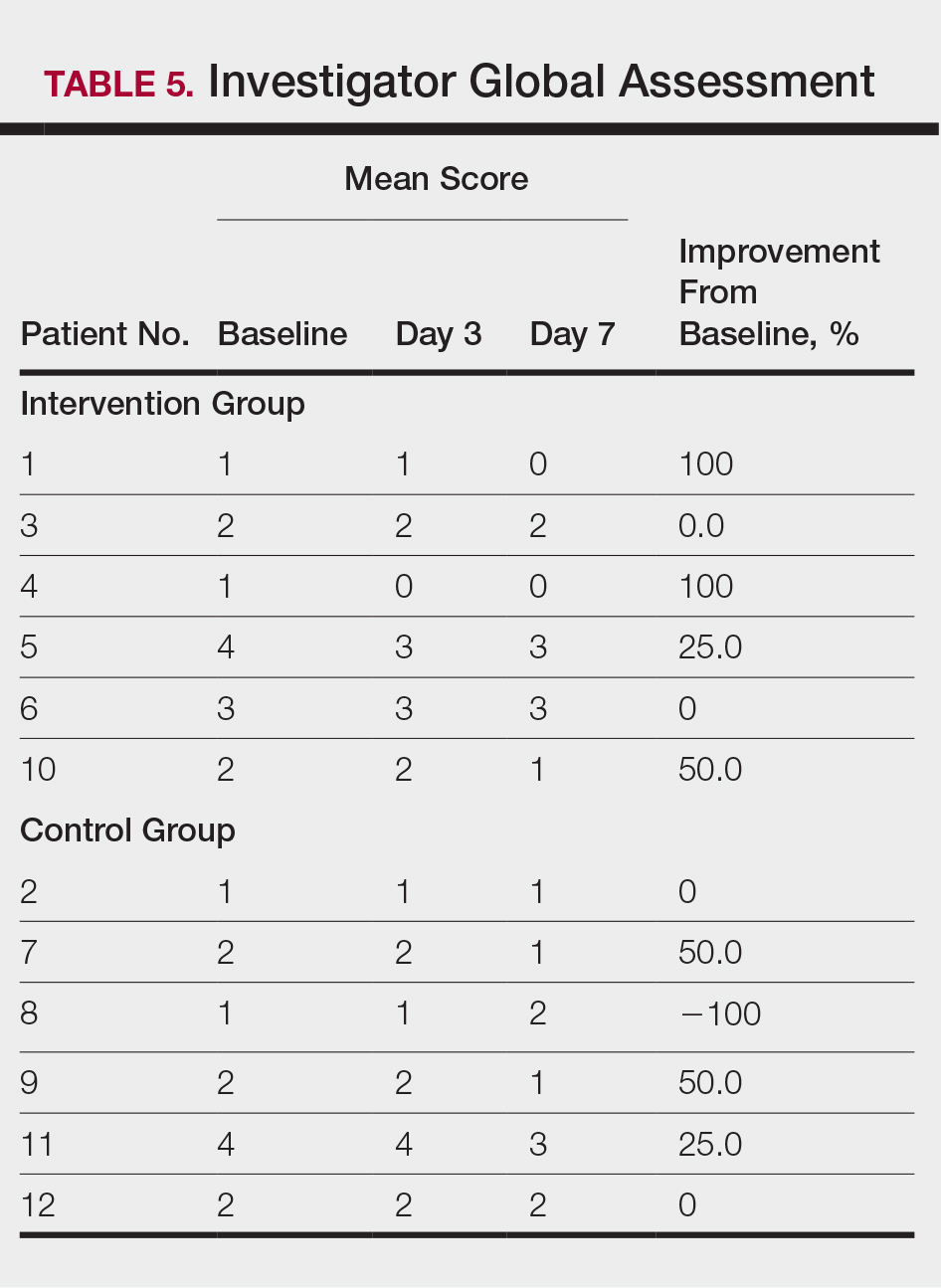
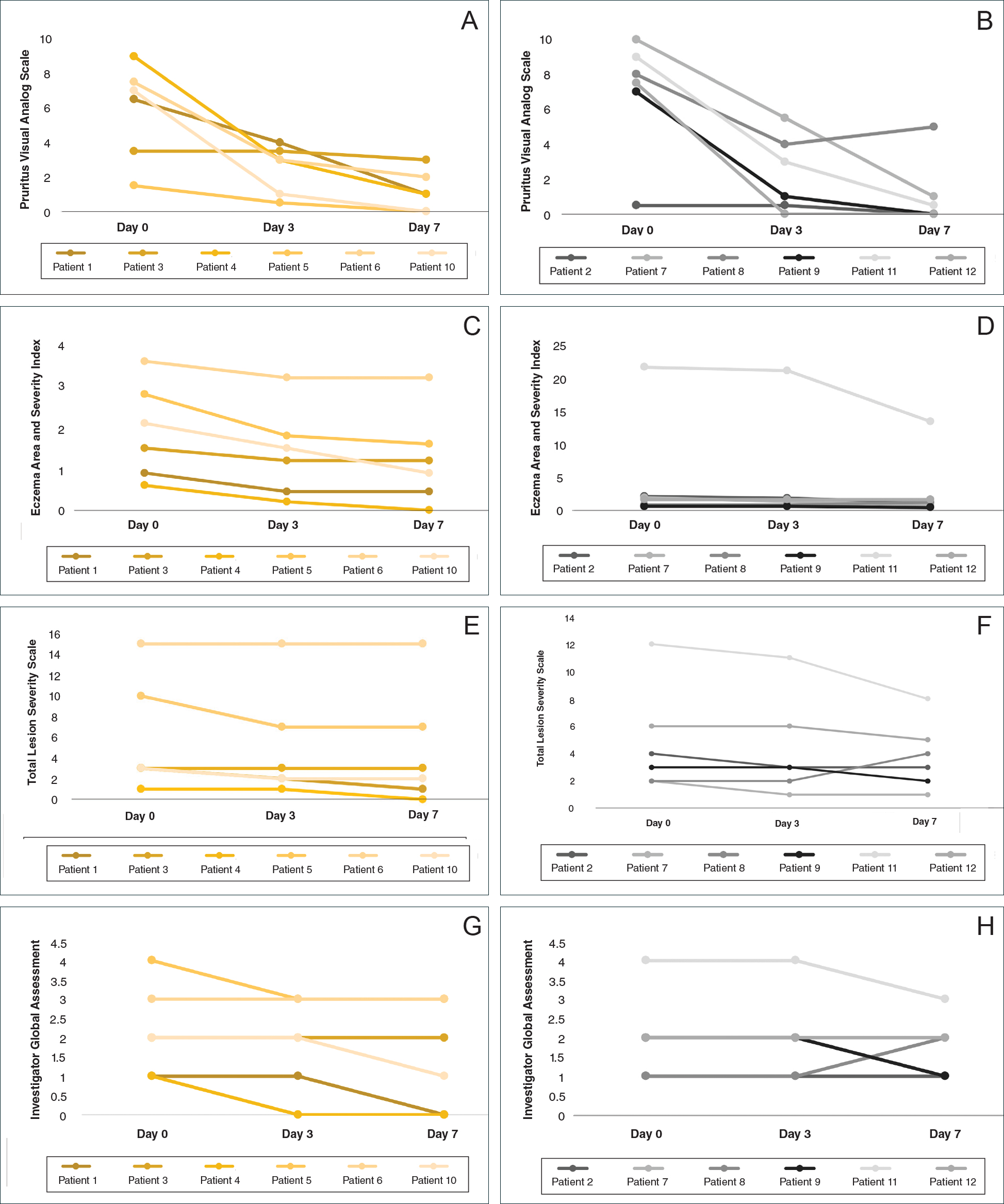
Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.

Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.

All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.





Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.

Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.

All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.





Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
Practice Points
- Mid-potency corticosteroids are the first-line treatment of atopic dermatitis (AD).
- Atopic dermatitis may fail to respond to topical corticosteroids initially or lose response over time, a phenomenon known as tachyphylaxis.
- Nonadherence to medication is the most likely cause of treatment resistance in patients with AD.