User login
Hospital medicine and perioperative care: A framework for high-quality, high-value collaborative care
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
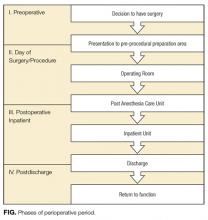
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
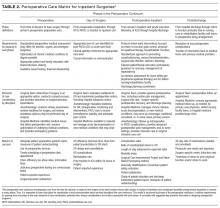
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
© 2017 Society of Hospital Medicine
Are You Ready to Work?
Physicians generally have little experience hunting for jobs. After more than a decade of education and training, graduating residents are in their late 20s—or older—when they begin searching for full-time work, and many struggle with the transition. The following seasonal tips will help you find your first hospitalist job. For more details, check out The Hospitalist's “Resident’s Corner.”
July-September
- Choose a mentor. Find an experienced hospitalist who can provide valuable feedback during your job search.
- Choose your senior-year electives carefully. Focus on areas of weakness, or areas that are pertinent to HM (e.g., infectious disease, cardiology, neurology, critical-care medicine).
- Create or update your curriculum vitae and cover letter. Edit your words carefully; spelling errors or typos in documents are costly.
- • Request letters of recommendation. Think hard about who you want before asking for a letter, as these typically carry a lot of weight in the interview selection process. Although program directors, chiefs of medicine, and hospitalists can be good choices, it is important to choose people who know you well, as they tend to generate a more personal and powerful letter.
October-December
- Start your job search by applying for desired positions. Hospitalists are in high demand; check out these sites for openings: SHM’s Career Center; classified ad sections in the Journal of Hospital Medicine; general medicine journals and The Hospitalist; and hospitals and HM groups of interest. Even if they are not advertising, contact them personally.
- Research potential employers. Prepare appropriate interview questions.
- Bring extra copies of your updated CV and look sharp. Shine your shoes. Is it time to replace the suit you used to apply for residency?
- Send a thank-you note or e-mail to the person(s) you interviewed with.
January-March
- When you receive an offer, it’s time to review the contract and negotiate terms. Don’t hesitate to ask for clarification of unclear points. You might want to have a lawyer review the contract.
- Register for your board examination.
- Apply for state medical licensure. This process varies by state, but it can take several months to complete, especially if you are applying in a state other than where you trained.
- Apply for hospital credentials.
April-June
- Moving to a different city or state can be exciting—and stressful. Talk to your new co-workers to get a feel for the city and recommendations for places to live. Some employers are very helpful with a move; some provide new hires with a real estate agent. Moving expenses often are covered as a condition of employment, but it depends on your contract.
- Consider taking a vacation to either further explore relocation options or to simply relax. You might need time to unwind as your residency concludes. Some future hospitalists like to use this time to intensify their board review; others cringe at the thought.
Dr. Grant is a hospitalist at the University of Michigan Health System in Ann Arbor. Dr. Warren-Marzola is a hospitalist at St. Luke’s Hospital in Toledo, Ohio. Both are members of SHM’s Young Physicians Committee.
Physicians generally have little experience hunting for jobs. After more than a decade of education and training, graduating residents are in their late 20s—or older—when they begin searching for full-time work, and many struggle with the transition. The following seasonal tips will help you find your first hospitalist job. For more details, check out The Hospitalist's “Resident’s Corner.”
July-September
- Choose a mentor. Find an experienced hospitalist who can provide valuable feedback during your job search.
- Choose your senior-year electives carefully. Focus on areas of weakness, or areas that are pertinent to HM (e.g., infectious disease, cardiology, neurology, critical-care medicine).
- Create or update your curriculum vitae and cover letter. Edit your words carefully; spelling errors or typos in documents are costly.
- • Request letters of recommendation. Think hard about who you want before asking for a letter, as these typically carry a lot of weight in the interview selection process. Although program directors, chiefs of medicine, and hospitalists can be good choices, it is important to choose people who know you well, as they tend to generate a more personal and powerful letter.
October-December
- Start your job search by applying for desired positions. Hospitalists are in high demand; check out these sites for openings: SHM’s Career Center; classified ad sections in the Journal of Hospital Medicine; general medicine journals and The Hospitalist; and hospitals and HM groups of interest. Even if they are not advertising, contact them personally.
- Research potential employers. Prepare appropriate interview questions.
- Bring extra copies of your updated CV and look sharp. Shine your shoes. Is it time to replace the suit you used to apply for residency?
- Send a thank-you note or e-mail to the person(s) you interviewed with.
January-March
- When you receive an offer, it’s time to review the contract and negotiate terms. Don’t hesitate to ask for clarification of unclear points. You might want to have a lawyer review the contract.
- Register for your board examination.
- Apply for state medical licensure. This process varies by state, but it can take several months to complete, especially if you are applying in a state other than where you trained.
- Apply for hospital credentials.
April-June
- Moving to a different city or state can be exciting—and stressful. Talk to your new co-workers to get a feel for the city and recommendations for places to live. Some employers are very helpful with a move; some provide new hires with a real estate agent. Moving expenses often are covered as a condition of employment, but it depends on your contract.
- Consider taking a vacation to either further explore relocation options or to simply relax. You might need time to unwind as your residency concludes. Some future hospitalists like to use this time to intensify their board review; others cringe at the thought.
Dr. Grant is a hospitalist at the University of Michigan Health System in Ann Arbor. Dr. Warren-Marzola is a hospitalist at St. Luke’s Hospital in Toledo, Ohio. Both are members of SHM’s Young Physicians Committee.
Physicians generally have little experience hunting for jobs. After more than a decade of education and training, graduating residents are in their late 20s—or older—when they begin searching for full-time work, and many struggle with the transition. The following seasonal tips will help you find your first hospitalist job. For more details, check out The Hospitalist's “Resident’s Corner.”
July-September
- Choose a mentor. Find an experienced hospitalist who can provide valuable feedback during your job search.
- Choose your senior-year electives carefully. Focus on areas of weakness, or areas that are pertinent to HM (e.g., infectious disease, cardiology, neurology, critical-care medicine).
- Create or update your curriculum vitae and cover letter. Edit your words carefully; spelling errors or typos in documents are costly.
- • Request letters of recommendation. Think hard about who you want before asking for a letter, as these typically carry a lot of weight in the interview selection process. Although program directors, chiefs of medicine, and hospitalists can be good choices, it is important to choose people who know you well, as they tend to generate a more personal and powerful letter.
October-December
- Start your job search by applying for desired positions. Hospitalists are in high demand; check out these sites for openings: SHM’s Career Center; classified ad sections in the Journal of Hospital Medicine; general medicine journals and The Hospitalist; and hospitals and HM groups of interest. Even if they are not advertising, contact them personally.
- Research potential employers. Prepare appropriate interview questions.
- Bring extra copies of your updated CV and look sharp. Shine your shoes. Is it time to replace the suit you used to apply for residency?
- Send a thank-you note or e-mail to the person(s) you interviewed with.
January-March
- When you receive an offer, it’s time to review the contract and negotiate terms. Don’t hesitate to ask for clarification of unclear points. You might want to have a lawyer review the contract.
- Register for your board examination.
- Apply for state medical licensure. This process varies by state, but it can take several months to complete, especially if you are applying in a state other than where you trained.
- Apply for hospital credentials.
April-June
- Moving to a different city or state can be exciting—and stressful. Talk to your new co-workers to get a feel for the city and recommendations for places to live. Some employers are very helpful with a move; some provide new hires with a real estate agent. Moving expenses often are covered as a condition of employment, but it depends on your contract.
- Consider taking a vacation to either further explore relocation options or to simply relax. You might need time to unwind as your residency concludes. Some future hospitalists like to use this time to intensify their board review; others cringe at the thought.
Dr. Grant is a hospitalist at the University of Michigan Health System in Ann Arbor. Dr. Warren-Marzola is a hospitalist at St. Luke’s Hospital in Toledo, Ohio. Both are members of SHM’s Young Physicians Committee.
Job Hunter’s Checklist
Apart from the part-time job that provided pocket money while you were in high school or during your undergraduate years, physicians generally have little experience in the job-hunting arena. A physician’s career path requires much skill at applying for such educational endeavors as medical school and residency training, but applying for a “real” job can be a strange concept for most.
Not lost in the equation is the fact that the application process doesn’t begin until most physicians are in their late 20s. While many of our non-physician friends are on their second or third jobs, graduating residents looking to launch their careers often struggle with the transition to the world of HM. In order to help navigate these waters, we have put together a yearlong guide to help make the transition from third-year resident to hospitalist a little smoother.
July-September
The first step in landing a job is to find a mentor who can assist you through the entire process. Choose your mentor wisely; an experienced hospitalist can provide valuable feedback during your job search. If your goal is employment with a private hospitalist group, find a hospitalist with private-practice experience.
Choose your senior-year electives carefully. Consider focusing on areas of weakness or areas that are pertinent to HM (e.g., infectious disease, cardiology, neurology, critical-care medicine). Think about an outside elective in HM.
If you haven’t done so already, now is the time to create a curriculum vitae, also known as a CV, and a cover letter. The CV is a vital document. It might be the key element in determining whether you are worthy of an interview. Work on this document early, as you will need time for edits, updates, and mentor review. The cover letter should clearly describe the type of position you want and confidently state why you would be an asset to a particular hospitalist program. Edit your words carefully; spelling errors or typos in documents can be costly.
Once the Labor Day holiday has passed, you should start requesting letters of recommendation. Think hard about who you want before asking for a letter of recommendation, as these typically carry a lot of weight in the interview selection process. Although program directors, chiefs of medicine, and hospitalists can be good choices, it is important to choose people who know you well, as they tend to generate a more personal and powerful letter. Because letter-writers often are busy people, it is appropriate to give a deadline for when you need the letter.
October-December
Actively start the job search and apply for desired positions. This is the time of year when HM jobs are heavily advertised and programs are looking to fill positions. Hospitalists are in high demand throughout the country. Some great places to find job openings are:
- SHM’s Career Center (www.hospitalmedicine.org/careers);
- Classified ad sections in the Journal of Hospital Medicine, general medicine journals, and HM news magazines like The Hospitalist (see “SHM Career Center,” p. 35); and
- Hospitals and HM groups of interest, even if they are not advertising; contact them personally.
Begin the interview process by researching the hospital and HM group in advance. Prepare appropriate interview questions. When you interview, try to meet with as many people as possible to get a feel for what the job entails. Talk to the everyday hospitalists and try to gauge how satisfied they are in their jobs.
Bring extra copies of your updated CV and look sharp. Shine your shoes. Is it time to replace the suit you used to apply for residency?
Send a thank-you note or e-mail to the person(s) you interviewed with. If possible, do this within three days of your visit. It’s an important step in the process, yet this simple task often is overlooked. Remain in contact with the HM programs you are most interested in. Think about a follow-up visit or phone call to address any unanswered questions.
January-March
Hopefully you will have one or more offers by now. This is the time to negotiate a contract and accept an offer. Review the contract carefully and don’t hesitate to ask for clarification of unclear points. Some applicants prefer to have a lawyer review the contract prior to signing (see “The Art of Negotiation,” December 2008, p. 20).
Register for your board examination. Most specialties, including internal medicine, family medicine, and pediatrics, as well as board exams for osteopathic medicine, have registration deadlines in February. Given the significant cost of applying for these exams, it pays not to be tardy, as late fees can set you back hundreds of dollars.
Apply for state medical licensure. This process varies by state, but it can take several months to complete, especially if you are applying in a state other than where you trained. For example, California recommends starting the application process six to nine months in advance. International medical graduates who require a work visa need to ensure their paperwork is processed in a timely manner.
Each hospital is different, but applications for hospital credentialing generally means filling out a mountain of paperwork. Most hospitals will perform a thorough background check, so don’t be surprised if fingerprinting is required. The hospital or hospitalist group usually helps new hires navigate through this process, which can take several weeks or even months.
April-June
Moving to a different city or state can be exciting—and stressful. Start talking to hospitalists at the facility where you will be working to get a feel for the city and recommendations for places to live. Revisit the location to become more familiar with the surroundings. Some hospitals are very helpful; some provide new hires with a real estate agent. Moving expenses often are covered as a condition of employment, but it depends on your contract.
Consider taking a vacation to either further explore relocation options or to simply relax. If you have followed the recommendations outlined in the previous months, you should have time to unwind as your residency comes to an end. Some future hospitalists like to use this time to intensify board review; others cringe at the thought.
Transitioning from resident to hospitalist is no easy task, and it shouldn’t be taken lightly. It’s not a one-month process, either, so planning is essential. Although it might seem to be a daunting journey, it’s very rewarding in the long run. TH
Dr. Grant is a hospitalist at the University of Michigan Health System in Ann Arbor. Dr. Warren-Marzola is a hospitalist at St. Luke’s Hospital in Toledo, Ohio. Both are members of SHM’s Young Physicians Committee.
Apart from the part-time job that provided pocket money while you were in high school or during your undergraduate years, physicians generally have little experience in the job-hunting arena. A physician’s career path requires much skill at applying for such educational endeavors as medical school and residency training, but applying for a “real” job can be a strange concept for most.
Not lost in the equation is the fact that the application process doesn’t begin until most physicians are in their late 20s. While many of our non-physician friends are on their second or third jobs, graduating residents looking to launch their careers often struggle with the transition to the world of HM. In order to help navigate these waters, we have put together a yearlong guide to help make the transition from third-year resident to hospitalist a little smoother.
July-September
The first step in landing a job is to find a mentor who can assist you through the entire process. Choose your mentor wisely; an experienced hospitalist can provide valuable feedback during your job search. If your goal is employment with a private hospitalist group, find a hospitalist with private-practice experience.
Choose your senior-year electives carefully. Consider focusing on areas of weakness or areas that are pertinent to HM (e.g., infectious disease, cardiology, neurology, critical-care medicine). Think about an outside elective in HM.
If you haven’t done so already, now is the time to create a curriculum vitae, also known as a CV, and a cover letter. The CV is a vital document. It might be the key element in determining whether you are worthy of an interview. Work on this document early, as you will need time for edits, updates, and mentor review. The cover letter should clearly describe the type of position you want and confidently state why you would be an asset to a particular hospitalist program. Edit your words carefully; spelling errors or typos in documents can be costly.
Once the Labor Day holiday has passed, you should start requesting letters of recommendation. Think hard about who you want before asking for a letter of recommendation, as these typically carry a lot of weight in the interview selection process. Although program directors, chiefs of medicine, and hospitalists can be good choices, it is important to choose people who know you well, as they tend to generate a more personal and powerful letter. Because letter-writers often are busy people, it is appropriate to give a deadline for when you need the letter.
October-December
Actively start the job search and apply for desired positions. This is the time of year when HM jobs are heavily advertised and programs are looking to fill positions. Hospitalists are in high demand throughout the country. Some great places to find job openings are:
- SHM’s Career Center (www.hospitalmedicine.org/careers);
- Classified ad sections in the Journal of Hospital Medicine, general medicine journals, and HM news magazines like The Hospitalist (see “SHM Career Center,” p. 35); and
- Hospitals and HM groups of interest, even if they are not advertising; contact them personally.
Begin the interview process by researching the hospital and HM group in advance. Prepare appropriate interview questions. When you interview, try to meet with as many people as possible to get a feel for what the job entails. Talk to the everyday hospitalists and try to gauge how satisfied they are in their jobs.
Bring extra copies of your updated CV and look sharp. Shine your shoes. Is it time to replace the suit you used to apply for residency?
Send a thank-you note or e-mail to the person(s) you interviewed with. If possible, do this within three days of your visit. It’s an important step in the process, yet this simple task often is overlooked. Remain in contact with the HM programs you are most interested in. Think about a follow-up visit or phone call to address any unanswered questions.
January-March
Hopefully you will have one or more offers by now. This is the time to negotiate a contract and accept an offer. Review the contract carefully and don’t hesitate to ask for clarification of unclear points. Some applicants prefer to have a lawyer review the contract prior to signing (see “The Art of Negotiation,” December 2008, p. 20).
Register for your board examination. Most specialties, including internal medicine, family medicine, and pediatrics, as well as board exams for osteopathic medicine, have registration deadlines in February. Given the significant cost of applying for these exams, it pays not to be tardy, as late fees can set you back hundreds of dollars.
Apply for state medical licensure. This process varies by state, but it can take several months to complete, especially if you are applying in a state other than where you trained. For example, California recommends starting the application process six to nine months in advance. International medical graduates who require a work visa need to ensure their paperwork is processed in a timely manner.
Each hospital is different, but applications for hospital credentialing generally means filling out a mountain of paperwork. Most hospitals will perform a thorough background check, so don’t be surprised if fingerprinting is required. The hospital or hospitalist group usually helps new hires navigate through this process, which can take several weeks or even months.
April-June
Moving to a different city or state can be exciting—and stressful. Start talking to hospitalists at the facility where you will be working to get a feel for the city and recommendations for places to live. Revisit the location to become more familiar with the surroundings. Some hospitals are very helpful; some provide new hires with a real estate agent. Moving expenses often are covered as a condition of employment, but it depends on your contract.
Consider taking a vacation to either further explore relocation options or to simply relax. If you have followed the recommendations outlined in the previous months, you should have time to unwind as your residency comes to an end. Some future hospitalists like to use this time to intensify board review; others cringe at the thought.
Transitioning from resident to hospitalist is no easy task, and it shouldn’t be taken lightly. It’s not a one-month process, either, so planning is essential. Although it might seem to be a daunting journey, it’s very rewarding in the long run. TH
Dr. Grant is a hospitalist at the University of Michigan Health System in Ann Arbor. Dr. Warren-Marzola is a hospitalist at St. Luke’s Hospital in Toledo, Ohio. Both are members of SHM’s Young Physicians Committee.
Apart from the part-time job that provided pocket money while you were in high school or during your undergraduate years, physicians generally have little experience in the job-hunting arena. A physician’s career path requires much skill at applying for such educational endeavors as medical school and residency training, but applying for a “real” job can be a strange concept for most.
Not lost in the equation is the fact that the application process doesn’t begin until most physicians are in their late 20s. While many of our non-physician friends are on their second or third jobs, graduating residents looking to launch their careers often struggle with the transition to the world of HM. In order to help navigate these waters, we have put together a yearlong guide to help make the transition from third-year resident to hospitalist a little smoother.
July-September
The first step in landing a job is to find a mentor who can assist you through the entire process. Choose your mentor wisely; an experienced hospitalist can provide valuable feedback during your job search. If your goal is employment with a private hospitalist group, find a hospitalist with private-practice experience.
Choose your senior-year electives carefully. Consider focusing on areas of weakness or areas that are pertinent to HM (e.g., infectious disease, cardiology, neurology, critical-care medicine). Think about an outside elective in HM.
If you haven’t done so already, now is the time to create a curriculum vitae, also known as a CV, and a cover letter. The CV is a vital document. It might be the key element in determining whether you are worthy of an interview. Work on this document early, as you will need time for edits, updates, and mentor review. The cover letter should clearly describe the type of position you want and confidently state why you would be an asset to a particular hospitalist program. Edit your words carefully; spelling errors or typos in documents can be costly.
Once the Labor Day holiday has passed, you should start requesting letters of recommendation. Think hard about who you want before asking for a letter of recommendation, as these typically carry a lot of weight in the interview selection process. Although program directors, chiefs of medicine, and hospitalists can be good choices, it is important to choose people who know you well, as they tend to generate a more personal and powerful letter. Because letter-writers often are busy people, it is appropriate to give a deadline for when you need the letter.
October-December
Actively start the job search and apply for desired positions. This is the time of year when HM jobs are heavily advertised and programs are looking to fill positions. Hospitalists are in high demand throughout the country. Some great places to find job openings are:
- SHM’s Career Center (www.hospitalmedicine.org/careers);
- Classified ad sections in the Journal of Hospital Medicine, general medicine journals, and HM news magazines like The Hospitalist (see “SHM Career Center,” p. 35); and
- Hospitals and HM groups of interest, even if they are not advertising; contact them personally.
Begin the interview process by researching the hospital and HM group in advance. Prepare appropriate interview questions. When you interview, try to meet with as many people as possible to get a feel for what the job entails. Talk to the everyday hospitalists and try to gauge how satisfied they are in their jobs.
Bring extra copies of your updated CV and look sharp. Shine your shoes. Is it time to replace the suit you used to apply for residency?
Send a thank-you note or e-mail to the person(s) you interviewed with. If possible, do this within three days of your visit. It’s an important step in the process, yet this simple task often is overlooked. Remain in contact with the HM programs you are most interested in. Think about a follow-up visit or phone call to address any unanswered questions.
January-March
Hopefully you will have one or more offers by now. This is the time to negotiate a contract and accept an offer. Review the contract carefully and don’t hesitate to ask for clarification of unclear points. Some applicants prefer to have a lawyer review the contract prior to signing (see “The Art of Negotiation,” December 2008, p. 20).
Register for your board examination. Most specialties, including internal medicine, family medicine, and pediatrics, as well as board exams for osteopathic medicine, have registration deadlines in February. Given the significant cost of applying for these exams, it pays not to be tardy, as late fees can set you back hundreds of dollars.
Apply for state medical licensure. This process varies by state, but it can take several months to complete, especially if you are applying in a state other than where you trained. For example, California recommends starting the application process six to nine months in advance. International medical graduates who require a work visa need to ensure their paperwork is processed in a timely manner.
Each hospital is different, but applications for hospital credentialing generally means filling out a mountain of paperwork. Most hospitals will perform a thorough background check, so don’t be surprised if fingerprinting is required. The hospital or hospitalist group usually helps new hires navigate through this process, which can take several weeks or even months.
April-June
Moving to a different city or state can be exciting—and stressful. Start talking to hospitalists at the facility where you will be working to get a feel for the city and recommendations for places to live. Revisit the location to become more familiar with the surroundings. Some hospitals are very helpful; some provide new hires with a real estate agent. Moving expenses often are covered as a condition of employment, but it depends on your contract.
Consider taking a vacation to either further explore relocation options or to simply relax. If you have followed the recommendations outlined in the previous months, you should have time to unwind as your residency comes to an end. Some future hospitalists like to use this time to intensify board review; others cringe at the thought.
Transitioning from resident to hospitalist is no easy task, and it shouldn’t be taken lightly. It’s not a one-month process, either, so planning is essential. Although it might seem to be a daunting journey, it’s very rewarding in the long run. TH
Dr. Grant is a hospitalist at the University of Michigan Health System in Ann Arbor. Dr. Warren-Marzola is a hospitalist at St. Luke’s Hospital in Toledo, Ohio. Both are members of SHM’s Young Physicians Committee.
In the Literature
Statins for Stroke Prevention
By Paul J. Grant, MD
Amarenco P, Bogousslavsky J, Callahan A III, et al. Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006 Aug 22;355:549-559.
Despite recent advances, the physician’s armamentarium for secondary stroke prevention is limited. The literature regarding optimal blood pressure management for stroke prevention is sparse, and the data addressing the best antiplatelet regimen remain controversial. This is troubling, given the fact that cerebrovascular disease remains the third leading cause of death in the United States.
Although extensive data exists for the benefits of using 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) for the prevention and treatment of cardiovascular disease, little is known about their role in decreasing the risk of stroke. The highly anticipated Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial sought to determine if statin therapy would decrease the risk of recurrent stroke in patients with no known coronary heart disease.
This prospective, randomized, double-blind, placebo-controlled trial included 4,731 men and women with no history of coronary heart disease. Eligible patients had a history of stroke (ischemic or hemorrhagic) or a transient ischemic attack (TIA) within a one- to six-month period before randomization as diagnosed by a neurologist. All patients required a low-density lipoprotein (LDL) cholesterol level between 100 and 190 mg/dL, while exclusion criteria included atrial fibrillation. Patients were randomized either to a dosage of 80 mg of atorvastatin daily or to a placebo and were followed for a median duration of 4.9 years. The primary endpoint was fatal or nonfatal stroke.
The average patient age in this trial was 63; approximately 60% of the patients were male. A total of 265 patients reached the primary endpoint in the atorvastatin group, versus 311 patients in the placebo group. This translates to an adjusted relative risk reduction of 16% in the primary endpoint for patients receiving atorvastatin (hazard ratio 0.84; 95% confidence interval 0.71 to 0.99; p=0.03). Although there was no difference in overall mortality between the two groups, the incidence of cardiovascular events was significantly lower in those receiving atorvastatin. Interestingly, more hemorrhagic strokes were noted in the atorvastatin group. With respect to safety, no significant differences in serious adverse events were noted. The atorvastatin group did, however, encounter significantly more cases of persistently elevated aspartate aminotransferase (AST) or alanine aminotransferase (ALT), at 2.2% versus 0.5% in the placebo group.
The findings by the SPARCL investigators provide strong evidence that atorvastatin reduces the incidence of stroke recurrence. The mechanism for risk reduction with statin exposure is most likely due to the dramatic lowering of LDL cholesterol. This effect has been shown in numerous trials resulting in the reduction of cardiovascular events. The present trial observed a 53% decrease in LDL cholesterol in the atorvastatin group compared with no change in the placebo arm. In addition to their powerful lipid-lowering role, statins also appear to prevent plaque rupture, optimize endothelial function, and provide anti-inflammatory effects. These are the so-called “pleiotropic effects” of statins and may be another factor contributing to the benefits observed.
Although some physicians are already prescribing statins for stroke patients, the literature supporting this practice has been sparse. The latest guidelines for prevention of stroke in patients with ischemic stroke or TIA were published in February 2006 by the American Heart Association/American Stroke Association Council on Stroke. These guidelines state that patients with a history of ischemic stroke or TIA are “reasonable candidates” for statin therapy. One could argue that these guidelines should now be revised to include a strong recommendation for statin therapy in secondary stroke prevention.
MRSA in the Community
By Matthew T. Harbison, MD
Moran GJ, Krishnadasan A, Gorwitz RJ, et al. EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006 Aug 17;355(7):666-674.
Methicillin-resistant Staphylococcus aureus (MRSA) emerged as an issue in the healthcare community not long after the introduction of methicillin in 1959. MRSA has traditionally been thought of as an issue for those individuals who have contact with the healthcare system; however, there is growing evidence that MRSA has become an entity in the greater community at large, affecting individuals who have not spent significant time in healthcare facilities. Descriptions of several community-based outbreaks have led to the understanding that community-associated MRSA has different characteristics than MRSA infections contracted in the hospital setting. The community-associated isolates are resistant to fewer antibiotics, produce different toxins, and have differing genetic complexes responsible for antibiotic resistance. The majority of the community-acquired infections are skin and soft tissue infections, although more serious infections have been reported.
Moran and colleagues conducted a prospective prevalence study in adult patients presenting to emergency departments with skin and soft tissue infections in 11 metropolitan areas in geographically diverse regions of the United States. Eligible patients 18 and older with purulent skin or soft tissue infections of less than one week’s duration had demographic and historical data collected; a wound culture was also taken. If Staphylococcus aureus was isolated, it was further evaluated by the Centers for Disease Control and Prevention (CDC) to characterize antibiotic resistance patterns, toxin production, and the type of staphylococcal cassette chromosome present.
A total of 422 patients were enrolled, with S. aureus isolated in 320 patients (76%). Of those with isolated S. aureus, 78% had MRSA (59% of the total patients enrolled). The individual site prevalence of MRSA ranged from 15 to 74% and was the predominant etiology of skin and soft tissue infections in 10 of 11 emergency departments. MRSA susceptibilities in this study were 100% to trimethoprim-sulfamethoxazole and rifampin, 95% to clindamycin, 92% to tetracycline, 60% to fluoroquinolones, and 6% to erythromycin. The authors point out that clindamycin resistance in one center was 60%; thus, individual site resistance patterns may differ significantly. Treatment data was available for 406 of the 422 patients, with the majority of those treated with incision, drainage, and antibiotics. In 100 of the 175 MRSA patients treated with antibiotics, the choice of agent was discordant with susceptibility patterns. The authors were able to contact 248 patients between two and three weeks after their visits and, of those contacted, 96% reported resolution or improvement of the wound.
Using multivariate logistic-regression analyses, the authors identified several potential risk factors for MRSA infection. These included use of any antibiotic in the past month, underlying illness, history of MRSA infection, close contact with someone with similar infection, and reported spider bite. Interestingly, being a healthcare worker, living in a long-term care facility, and being hospitalized in the past year were not shown to be significant risk factors in this study.
The results of this study highlight the emerging difficulty, which continues to evolve, with antibiotic resistance patterns. The healthcare community must be vigilant to new entities that challenge the traditional views of antibiotic resistance patterns. The high rate of community-acquired MRSA skin and soft tissue infection demonstrated in this study, in addition to the large percentage of patients prescribed antibiotics that were resistant for the strain involved, emphasizes the need to reconsider the empiric antibiotic choices for this patient population. The variability in regional resistance patterns further complicates the issue. Given the high prevalence of MRSA skin and soft tissue infections reported in this study, use of routine wound cultures appears prudent, as does the need for effective follow-up strategies for alteration of antibiotic choice if necessary. At an institutional level, development of surveillance and isolation strategies for community-acquired MRSA should be considered.
More Options for Venous Thromboembolism Treatment
By Kirsten N. Kangelaris, MD
Kearon C, Ginsberg JS, Julian JA, et al. Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA. 2006 Aug 23;296(8):935-942.
The standard approach to using unfractionated heparin (UFH) in the treatment of acute venous thromboembolism as a bridge to warfarin therapy requires continuous intravenous infusion with frequent dose adjustments in response to measurements of activated partial thromboplastin time (aPTT). This therapy inevitably requires inpatient management. Subcutaneous administration of weight-based low molecular weight heparin (LMWH) has been the modality of choice for outpatient treatment of venous thromboembolism because it does not require laboratory monitoring. Its use has been limited by the high cost of treatment, however. A preliminary study, released in 2000 by the FIDO group (Fixed-Dose Heparin Investigators, Kearon and colleagues), suggested that subcutaneously administered UFH could be optimally dosed based on weight rather than monitoring aPTT levels.
This follow-up, randomized, open-label, adjudicator-blinded, multi-centered, non-inferiority trial enrolled 708 patients and compared fixed-dose, subcutaneously administered UFH to LMWH in acute deep venous thrombosis and pulmonary embolism. Administration in both groups was twice daily, subcutaneous, and weight-based. UFH was given as a first dose of 333 U/kg, followed by 250 U/kg every 12 hours. LMWH was administered at a dose of 100 IU/kg every 12 hours. Both treatments overlapped with three months of warfarin therapy, and both could be administered out of hospital.
Exclusion criteria were age <18, contraindication to subcutaneous therapy, active bleeding, life expectancy under three months, long-term anticoagulation therapy, pregnancy, and creatinine level >2.3 mg/dL.
The primary endpoints were efficacy as determined by recurrent venous thromboembolism within three months and safety as determined by major bleeding within 10 days of randomization. A secondary endpoint was relationship of efficacy and safety outcomes to aPTT levels measured on day two to three of therapy for the UFH group.
Results revealed that UFH was statistically non-inferior to LMWH by all endpoints, including treatment duration, efficacy, and safety. At three months, there was no significant difference between the groups in frequency of recurrent venous thromboembolism (3.8% UFH versus 3.4% LMWH), bleeding (1.1% UFH versus 1.4% LMWH), or death. There was no association between aPTT levels and recurrent venous thromboembolism or bleeding.
Limitations of the study included reduced enrollment from the initial study design, though power was adequate due to a lower than expected incidence of recurrent venous thromboembolism in both arms (~3.6% versus the expected 6%); possible biases related to open-label design; and more post-randomization exclusions in the UFH group versus the LMWH group.
In summary, fixed-dose, unmonitored, subcutaneous UFH appears to be an effective, safe alternative to LMWH as a bridge to warfarin therapy for venous thromboembolism. Clinically, this is relevant, because UFH is approximately 15 to 20 times less expensive than LMWH. The authors appropriately call attention to two developments in clinical practice that occurred during the course of the present study and that could potentially limit the use of UFH. These are 1) the dosing option for once-daily LMWH, which improves convenience, and 2) the preference for long-term LMWH therapy over warfarin for treating cancer patients with venous thromboembolism. Despite these exceptions, UFH may prove to be a viable and economic option for venous thromboembolism treatment.
In-Hospital MI Versus MI at Presentation
By Erin M. Galbraith, MD
Maynard C, Lowy E, Rumsfeld J, et al. The prevalence and outcomes of in-hospital acute myocardial infarction in the Department of Veterans Affairs Health System. Arch Intern Med. 2006 Jul 10;166(13):1410-1416.
Much is known about the prevalence, treatment, and prognosis of acute myocardial infarction (AMI) when it occurs in the community and is the presenting diagnosis. Few studies, however, have addressed the epidemiology of in-hospital AMIs. This study by Maynard and colleagues attempts to elucidate the basic epidemiologic characteristics, treatments, and outcomes of patients who suffer in-hospital AMIs.
This retrospective cohort consisted of 7,054 patients who had been discharged with a diagnosis of AMI from 127 Veterans Health Administration (VHA) medical centers between July and August 2003. Patients who had suffered a postoperative MI or were transferred in from another hospital were excluded. Data was obtained from both the electronic and paper medical records. Of the 7,054 patients in the study, 792 (11.2%) had experienced an AMI while hospitalized for other medical problems. These 792 patients were older by approximately 4.5 years and more frequently suffered from heart failure, diabetes, chronic renal insufficiency, COPD, cerebrovascular disease, dementia, and cancer. These patients were less likely, however, to have had a previous MI, to be current smokers, or to have undergone previous angioplasty. They were also less likely to have known lipid disorders or to be taking aspirin or lipid-lowering agents.
Regarding their presentations and management, the patients who suffered in-hospital AMIs had faster heart rates and lower blood pressures. They were also up to 75% less likely to report typical symptoms of cardiac ischemia, including chest pain/pressure, shoulder pain, nausea, and diaphoresis. They were less often seen by an attending cardiologist and had more contraindications to AMI therapy; thus, these patients underwent reperfusion therapy at much lower rates, both initially and at 30 days. Their troponin levels were more frequently elevated, but they were only half as likely to have ST segment elevations at the time of diagnosis. Hospitalizations were longer for the in-hospital group, and there were higher rates of in-hospital cardiogenic shock, cardiac arrest, and death (27.3% versus 8.6%). The 30-day mortality rate was also higher (33% versus 11.9%). Multivariate logistic regression revealed an adjusted odds ratio of 2.0 (95% confidence interval 1.7 to 2.4; p<0.001) for 30-day mortality in those who experienced an in-hospital AMI versus those who presented with an AMI.
Potential reasons for the increased severity of outcomes include, but are not limited to, their many chronic comorbidities, their other acute diagnoses, the failure of the medical team to recognize cardiac ischemia in a timely manner (i.e., higher initial troponins), the inability to treat MIs appropriately secondary to contraindications to acute intervention, and the lack of an attending cardiologist presiding over their medical care. Clearly, further studies are needed to elucidate the causes of death in the 33% of patients who died, because it is unclear whether the patients died of complications from their MIs or as a result of their multiple other medical problems. Knowledge of the extent to which these patients could be managed, both medically and via interventional procedures (and why these therapies were not pursued), would also be of value.
This study emphasizes the importance of recognizing atypical presentations of AMIs and exercising vigilance in pursuing the most aggressive therapy possible, as dictated by a patient’s ability to tolerate medical and procedural interventions.
Hyperglycemia in Heart Failure
By David H. Wesorick, MD
Barsheshet A, Garty M, Grossman E, et al. Admission blood glucose level and mortality among hospitalized nondiabetic patients with heart failure. Arch Intern Med. 2006 Aug 14-28;166(15):1613-1619.
The medical literature strongly suggests that inpatient hyperglycemia is associated with a variety of poor outcomes. Little is known, however, about the relationship between hyperglycemia and heart failure. These investigators examined the association of admission blood glucose and mortality in patients who were admitted to the hospital with acute heart failure.
In this study, 1,122 patients admitted to the hospital with acute heart failure and without diabetes were divided into tertiles depending on their admission blood glucose levels. Diabetes was defined as an admission blood glucose greater than or equal to 200 mg/dl, a known diagnosis of diabetes recorded in the chart, or the presence of anti-diabetic medications on the patient’s medication list. Tertile #1 had an average admission blood glucose of 92 mg/dl (with a range of 54-102); tertile #2 had an average admission blood glucose of 113 mg/dl (with a range of 103-127); and tertile #3 had an average admission blood glucose of 147 mg/dl (with a range of 128-199). Mortality was evaluated according to tertile.
In this study, patients in tertile #3 had significantly higher inpatient mortality (7.2%) than patients in tertile #1 or #2 (3% and 4%, respectively). There was a significant association between hyperglycemia and mortality, even at 60 days follow-up, although not at six and 12 months follow-up. The association remained significant, even when patients with acute MI were excluded. Besides hyperglycemia, the authors noted that increasing age, increasing creatinine, a New York Heart Association (NYHA) functional class of III or IV, and a systolic blood pressure of lower than 115 were also significant, independent predictors of in-hospital mortality in this patient population.
For a hospitalist, the intriguing question is this: Is hyperglycemia just a marker of worse disease, or might it contribute to poorer outcomes? Clearly, hyperglycemia is associated with poorer outcomes in other types of patients, including post-surgical patients, critically ill patients, MI patients, and general medical patients.1 But is hyperglycemia just a marker of more severe illness? In heart failure, perhaps more severe decompensation results in a more profound activation of the sympathetic nervous system and a more vigorous release of stress hormones, such as cortisol and catecholamines. In that case, one might expect a sicker patient to have a higher blood glucose.
More recent studies, however, show that better control of hyperglycemia in some acutely ill patients actually results in improved outcomes, suggesting that the hyperglycemia itself might be contributing to the poorer outcomes in some cases.2-5 Hyperglycemia is known to alter human physiology in a variety of adverse ways.1 For example, hyperglycemia is known to inhibit nitric oxide production and to alter endothelial dysfunction. In a patient with acute heart failure, these alterations might be expected to have a significant effect on outcomes.
This study does not intend to answer these questions, but it does add to our understanding of the association of hyperglycemia and poor outcomes in acutely ill patients. More research is needed to examine whether or not heart failure patients, specifically, will benefit from better glycemic control in the acute setting. TH
References
- Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004 Feb;27(2):553-591.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001 Nov 8;345(19):1359-1367.
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006 Feb 2;354(5):449-461.
- Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999 Feb;67(2):352-362.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003 May;125(5):1007-1021.
Statins for Stroke Prevention
By Paul J. Grant, MD
Amarenco P, Bogousslavsky J, Callahan A III, et al. Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006 Aug 22;355:549-559.
Despite recent advances, the physician’s armamentarium for secondary stroke prevention is limited. The literature regarding optimal blood pressure management for stroke prevention is sparse, and the data addressing the best antiplatelet regimen remain controversial. This is troubling, given the fact that cerebrovascular disease remains the third leading cause of death in the United States.
Although extensive data exists for the benefits of using 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) for the prevention and treatment of cardiovascular disease, little is known about their role in decreasing the risk of stroke. The highly anticipated Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial sought to determine if statin therapy would decrease the risk of recurrent stroke in patients with no known coronary heart disease.
This prospective, randomized, double-blind, placebo-controlled trial included 4,731 men and women with no history of coronary heart disease. Eligible patients had a history of stroke (ischemic or hemorrhagic) or a transient ischemic attack (TIA) within a one- to six-month period before randomization as diagnosed by a neurologist. All patients required a low-density lipoprotein (LDL) cholesterol level between 100 and 190 mg/dL, while exclusion criteria included atrial fibrillation. Patients were randomized either to a dosage of 80 mg of atorvastatin daily or to a placebo and were followed for a median duration of 4.9 years. The primary endpoint was fatal or nonfatal stroke.
The average patient age in this trial was 63; approximately 60% of the patients were male. A total of 265 patients reached the primary endpoint in the atorvastatin group, versus 311 patients in the placebo group. This translates to an adjusted relative risk reduction of 16% in the primary endpoint for patients receiving atorvastatin (hazard ratio 0.84; 95% confidence interval 0.71 to 0.99; p=0.03). Although there was no difference in overall mortality between the two groups, the incidence of cardiovascular events was significantly lower in those receiving atorvastatin. Interestingly, more hemorrhagic strokes were noted in the atorvastatin group. With respect to safety, no significant differences in serious adverse events were noted. The atorvastatin group did, however, encounter significantly more cases of persistently elevated aspartate aminotransferase (AST) or alanine aminotransferase (ALT), at 2.2% versus 0.5% in the placebo group.
The findings by the SPARCL investigators provide strong evidence that atorvastatin reduces the incidence of stroke recurrence. The mechanism for risk reduction with statin exposure is most likely due to the dramatic lowering of LDL cholesterol. This effect has been shown in numerous trials resulting in the reduction of cardiovascular events. The present trial observed a 53% decrease in LDL cholesterol in the atorvastatin group compared with no change in the placebo arm. In addition to their powerful lipid-lowering role, statins also appear to prevent plaque rupture, optimize endothelial function, and provide anti-inflammatory effects. These are the so-called “pleiotropic effects” of statins and may be another factor contributing to the benefits observed.
Although some physicians are already prescribing statins for stroke patients, the literature supporting this practice has been sparse. The latest guidelines for prevention of stroke in patients with ischemic stroke or TIA were published in February 2006 by the American Heart Association/American Stroke Association Council on Stroke. These guidelines state that patients with a history of ischemic stroke or TIA are “reasonable candidates” for statin therapy. One could argue that these guidelines should now be revised to include a strong recommendation for statin therapy in secondary stroke prevention.
MRSA in the Community
By Matthew T. Harbison, MD
Moran GJ, Krishnadasan A, Gorwitz RJ, et al. EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006 Aug 17;355(7):666-674.
Methicillin-resistant Staphylococcus aureus (MRSA) emerged as an issue in the healthcare community not long after the introduction of methicillin in 1959. MRSA has traditionally been thought of as an issue for those individuals who have contact with the healthcare system; however, there is growing evidence that MRSA has become an entity in the greater community at large, affecting individuals who have not spent significant time in healthcare facilities. Descriptions of several community-based outbreaks have led to the understanding that community-associated MRSA has different characteristics than MRSA infections contracted in the hospital setting. The community-associated isolates are resistant to fewer antibiotics, produce different toxins, and have differing genetic complexes responsible for antibiotic resistance. The majority of the community-acquired infections are skin and soft tissue infections, although more serious infections have been reported.
Moran and colleagues conducted a prospective prevalence study in adult patients presenting to emergency departments with skin and soft tissue infections in 11 metropolitan areas in geographically diverse regions of the United States. Eligible patients 18 and older with purulent skin or soft tissue infections of less than one week’s duration had demographic and historical data collected; a wound culture was also taken. If Staphylococcus aureus was isolated, it was further evaluated by the Centers for Disease Control and Prevention (CDC) to characterize antibiotic resistance patterns, toxin production, and the type of staphylococcal cassette chromosome present.
A total of 422 patients were enrolled, with S. aureus isolated in 320 patients (76%). Of those with isolated S. aureus, 78% had MRSA (59% of the total patients enrolled). The individual site prevalence of MRSA ranged from 15 to 74% and was the predominant etiology of skin and soft tissue infections in 10 of 11 emergency departments. MRSA susceptibilities in this study were 100% to trimethoprim-sulfamethoxazole and rifampin, 95% to clindamycin, 92% to tetracycline, 60% to fluoroquinolones, and 6% to erythromycin. The authors point out that clindamycin resistance in one center was 60%; thus, individual site resistance patterns may differ significantly. Treatment data was available for 406 of the 422 patients, with the majority of those treated with incision, drainage, and antibiotics. In 100 of the 175 MRSA patients treated with antibiotics, the choice of agent was discordant with susceptibility patterns. The authors were able to contact 248 patients between two and three weeks after their visits and, of those contacted, 96% reported resolution or improvement of the wound.
Using multivariate logistic-regression analyses, the authors identified several potential risk factors for MRSA infection. These included use of any antibiotic in the past month, underlying illness, history of MRSA infection, close contact with someone with similar infection, and reported spider bite. Interestingly, being a healthcare worker, living in a long-term care facility, and being hospitalized in the past year were not shown to be significant risk factors in this study.
The results of this study highlight the emerging difficulty, which continues to evolve, with antibiotic resistance patterns. The healthcare community must be vigilant to new entities that challenge the traditional views of antibiotic resistance patterns. The high rate of community-acquired MRSA skin and soft tissue infection demonstrated in this study, in addition to the large percentage of patients prescribed antibiotics that were resistant for the strain involved, emphasizes the need to reconsider the empiric antibiotic choices for this patient population. The variability in regional resistance patterns further complicates the issue. Given the high prevalence of MRSA skin and soft tissue infections reported in this study, use of routine wound cultures appears prudent, as does the need for effective follow-up strategies for alteration of antibiotic choice if necessary. At an institutional level, development of surveillance and isolation strategies for community-acquired MRSA should be considered.
More Options for Venous Thromboembolism Treatment
By Kirsten N. Kangelaris, MD
Kearon C, Ginsberg JS, Julian JA, et al. Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA. 2006 Aug 23;296(8):935-942.
The standard approach to using unfractionated heparin (UFH) in the treatment of acute venous thromboembolism as a bridge to warfarin therapy requires continuous intravenous infusion with frequent dose adjustments in response to measurements of activated partial thromboplastin time (aPTT). This therapy inevitably requires inpatient management. Subcutaneous administration of weight-based low molecular weight heparin (LMWH) has been the modality of choice for outpatient treatment of venous thromboembolism because it does not require laboratory monitoring. Its use has been limited by the high cost of treatment, however. A preliminary study, released in 2000 by the FIDO group (Fixed-Dose Heparin Investigators, Kearon and colleagues), suggested that subcutaneously administered UFH could be optimally dosed based on weight rather than monitoring aPTT levels.
This follow-up, randomized, open-label, adjudicator-blinded, multi-centered, non-inferiority trial enrolled 708 patients and compared fixed-dose, subcutaneously administered UFH to LMWH in acute deep venous thrombosis and pulmonary embolism. Administration in both groups was twice daily, subcutaneous, and weight-based. UFH was given as a first dose of 333 U/kg, followed by 250 U/kg every 12 hours. LMWH was administered at a dose of 100 IU/kg every 12 hours. Both treatments overlapped with three months of warfarin therapy, and both could be administered out of hospital.
Exclusion criteria were age <18, contraindication to subcutaneous therapy, active bleeding, life expectancy under three months, long-term anticoagulation therapy, pregnancy, and creatinine level >2.3 mg/dL.
The primary endpoints were efficacy as determined by recurrent venous thromboembolism within three months and safety as determined by major bleeding within 10 days of randomization. A secondary endpoint was relationship of efficacy and safety outcomes to aPTT levels measured on day two to three of therapy for the UFH group.
Results revealed that UFH was statistically non-inferior to LMWH by all endpoints, including treatment duration, efficacy, and safety. At three months, there was no significant difference between the groups in frequency of recurrent venous thromboembolism (3.8% UFH versus 3.4% LMWH), bleeding (1.1% UFH versus 1.4% LMWH), or death. There was no association between aPTT levels and recurrent venous thromboembolism or bleeding.
Limitations of the study included reduced enrollment from the initial study design, though power was adequate due to a lower than expected incidence of recurrent venous thromboembolism in both arms (~3.6% versus the expected 6%); possible biases related to open-label design; and more post-randomization exclusions in the UFH group versus the LMWH group.
In summary, fixed-dose, unmonitored, subcutaneous UFH appears to be an effective, safe alternative to LMWH as a bridge to warfarin therapy for venous thromboembolism. Clinically, this is relevant, because UFH is approximately 15 to 20 times less expensive than LMWH. The authors appropriately call attention to two developments in clinical practice that occurred during the course of the present study and that could potentially limit the use of UFH. These are 1) the dosing option for once-daily LMWH, which improves convenience, and 2) the preference for long-term LMWH therapy over warfarin for treating cancer patients with venous thromboembolism. Despite these exceptions, UFH may prove to be a viable and economic option for venous thromboembolism treatment.
In-Hospital MI Versus MI at Presentation
By Erin M. Galbraith, MD
Maynard C, Lowy E, Rumsfeld J, et al. The prevalence and outcomes of in-hospital acute myocardial infarction in the Department of Veterans Affairs Health System. Arch Intern Med. 2006 Jul 10;166(13):1410-1416.
Much is known about the prevalence, treatment, and prognosis of acute myocardial infarction (AMI) when it occurs in the community and is the presenting diagnosis. Few studies, however, have addressed the epidemiology of in-hospital AMIs. This study by Maynard and colleagues attempts to elucidate the basic epidemiologic characteristics, treatments, and outcomes of patients who suffer in-hospital AMIs.
This retrospective cohort consisted of 7,054 patients who had been discharged with a diagnosis of AMI from 127 Veterans Health Administration (VHA) medical centers between July and August 2003. Patients who had suffered a postoperative MI or were transferred in from another hospital were excluded. Data was obtained from both the electronic and paper medical records. Of the 7,054 patients in the study, 792 (11.2%) had experienced an AMI while hospitalized for other medical problems. These 792 patients were older by approximately 4.5 years and more frequently suffered from heart failure, diabetes, chronic renal insufficiency, COPD, cerebrovascular disease, dementia, and cancer. These patients were less likely, however, to have had a previous MI, to be current smokers, or to have undergone previous angioplasty. They were also less likely to have known lipid disorders or to be taking aspirin or lipid-lowering agents.
Regarding their presentations and management, the patients who suffered in-hospital AMIs had faster heart rates and lower blood pressures. They were also up to 75% less likely to report typical symptoms of cardiac ischemia, including chest pain/pressure, shoulder pain, nausea, and diaphoresis. They were less often seen by an attending cardiologist and had more contraindications to AMI therapy; thus, these patients underwent reperfusion therapy at much lower rates, both initially and at 30 days. Their troponin levels were more frequently elevated, but they were only half as likely to have ST segment elevations at the time of diagnosis. Hospitalizations were longer for the in-hospital group, and there were higher rates of in-hospital cardiogenic shock, cardiac arrest, and death (27.3% versus 8.6%). The 30-day mortality rate was also higher (33% versus 11.9%). Multivariate logistic regression revealed an adjusted odds ratio of 2.0 (95% confidence interval 1.7 to 2.4; p<0.001) for 30-day mortality in those who experienced an in-hospital AMI versus those who presented with an AMI.
Potential reasons for the increased severity of outcomes include, but are not limited to, their many chronic comorbidities, their other acute diagnoses, the failure of the medical team to recognize cardiac ischemia in a timely manner (i.e., higher initial troponins), the inability to treat MIs appropriately secondary to contraindications to acute intervention, and the lack of an attending cardiologist presiding over their medical care. Clearly, further studies are needed to elucidate the causes of death in the 33% of patients who died, because it is unclear whether the patients died of complications from their MIs or as a result of their multiple other medical problems. Knowledge of the extent to which these patients could be managed, both medically and via interventional procedures (and why these therapies were not pursued), would also be of value.
This study emphasizes the importance of recognizing atypical presentations of AMIs and exercising vigilance in pursuing the most aggressive therapy possible, as dictated by a patient’s ability to tolerate medical and procedural interventions.
Hyperglycemia in Heart Failure
By David H. Wesorick, MD
Barsheshet A, Garty M, Grossman E, et al. Admission blood glucose level and mortality among hospitalized nondiabetic patients with heart failure. Arch Intern Med. 2006 Aug 14-28;166(15):1613-1619.
The medical literature strongly suggests that inpatient hyperglycemia is associated with a variety of poor outcomes. Little is known, however, about the relationship between hyperglycemia and heart failure. These investigators examined the association of admission blood glucose and mortality in patients who were admitted to the hospital with acute heart failure.
In this study, 1,122 patients admitted to the hospital with acute heart failure and without diabetes were divided into tertiles depending on their admission blood glucose levels. Diabetes was defined as an admission blood glucose greater than or equal to 200 mg/dl, a known diagnosis of diabetes recorded in the chart, or the presence of anti-diabetic medications on the patient’s medication list. Tertile #1 had an average admission blood glucose of 92 mg/dl (with a range of 54-102); tertile #2 had an average admission blood glucose of 113 mg/dl (with a range of 103-127); and tertile #3 had an average admission blood glucose of 147 mg/dl (with a range of 128-199). Mortality was evaluated according to tertile.
In this study, patients in tertile #3 had significantly higher inpatient mortality (7.2%) than patients in tertile #1 or #2 (3% and 4%, respectively). There was a significant association between hyperglycemia and mortality, even at 60 days follow-up, although not at six and 12 months follow-up. The association remained significant, even when patients with acute MI were excluded. Besides hyperglycemia, the authors noted that increasing age, increasing creatinine, a New York Heart Association (NYHA) functional class of III or IV, and a systolic blood pressure of lower than 115 were also significant, independent predictors of in-hospital mortality in this patient population.
For a hospitalist, the intriguing question is this: Is hyperglycemia just a marker of worse disease, or might it contribute to poorer outcomes? Clearly, hyperglycemia is associated with poorer outcomes in other types of patients, including post-surgical patients, critically ill patients, MI patients, and general medical patients.1 But is hyperglycemia just a marker of more severe illness? In heart failure, perhaps more severe decompensation results in a more profound activation of the sympathetic nervous system and a more vigorous release of stress hormones, such as cortisol and catecholamines. In that case, one might expect a sicker patient to have a higher blood glucose.
More recent studies, however, show that better control of hyperglycemia in some acutely ill patients actually results in improved outcomes, suggesting that the hyperglycemia itself might be contributing to the poorer outcomes in some cases.2-5 Hyperglycemia is known to alter human physiology in a variety of adverse ways.1 For example, hyperglycemia is known to inhibit nitric oxide production and to alter endothelial dysfunction. In a patient with acute heart failure, these alterations might be expected to have a significant effect on outcomes.
This study does not intend to answer these questions, but it does add to our understanding of the association of hyperglycemia and poor outcomes in acutely ill patients. More research is needed to examine whether or not heart failure patients, specifically, will benefit from better glycemic control in the acute setting. TH
References
- Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004 Feb;27(2):553-591.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001 Nov 8;345(19):1359-1367.
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006 Feb 2;354(5):449-461.
- Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999 Feb;67(2):352-362.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003 May;125(5):1007-1021.
Statins for Stroke Prevention
By Paul J. Grant, MD
Amarenco P, Bogousslavsky J, Callahan A III, et al. Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006 Aug 22;355:549-559.
Despite recent advances, the physician’s armamentarium for secondary stroke prevention is limited. The literature regarding optimal blood pressure management for stroke prevention is sparse, and the data addressing the best antiplatelet regimen remain controversial. This is troubling, given the fact that cerebrovascular disease remains the third leading cause of death in the United States.
Although extensive data exists for the benefits of using 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) for the prevention and treatment of cardiovascular disease, little is known about their role in decreasing the risk of stroke. The highly anticipated Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial sought to determine if statin therapy would decrease the risk of recurrent stroke in patients with no known coronary heart disease.
This prospective, randomized, double-blind, placebo-controlled trial included 4,731 men and women with no history of coronary heart disease. Eligible patients had a history of stroke (ischemic or hemorrhagic) or a transient ischemic attack (TIA) within a one- to six-month period before randomization as diagnosed by a neurologist. All patients required a low-density lipoprotein (LDL) cholesterol level between 100 and 190 mg/dL, while exclusion criteria included atrial fibrillation. Patients were randomized either to a dosage of 80 mg of atorvastatin daily or to a placebo and were followed for a median duration of 4.9 years. The primary endpoint was fatal or nonfatal stroke.
The average patient age in this trial was 63; approximately 60% of the patients were male. A total of 265 patients reached the primary endpoint in the atorvastatin group, versus 311 patients in the placebo group. This translates to an adjusted relative risk reduction of 16% in the primary endpoint for patients receiving atorvastatin (hazard ratio 0.84; 95% confidence interval 0.71 to 0.99; p=0.03). Although there was no difference in overall mortality between the two groups, the incidence of cardiovascular events was significantly lower in those receiving atorvastatin. Interestingly, more hemorrhagic strokes were noted in the atorvastatin group. With respect to safety, no significant differences in serious adverse events were noted. The atorvastatin group did, however, encounter significantly more cases of persistently elevated aspartate aminotransferase (AST) or alanine aminotransferase (ALT), at 2.2% versus 0.5% in the placebo group.
The findings by the SPARCL investigators provide strong evidence that atorvastatin reduces the incidence of stroke recurrence. The mechanism for risk reduction with statin exposure is most likely due to the dramatic lowering of LDL cholesterol. This effect has been shown in numerous trials resulting in the reduction of cardiovascular events. The present trial observed a 53% decrease in LDL cholesterol in the atorvastatin group compared with no change in the placebo arm. In addition to their powerful lipid-lowering role, statins also appear to prevent plaque rupture, optimize endothelial function, and provide anti-inflammatory effects. These are the so-called “pleiotropic effects” of statins and may be another factor contributing to the benefits observed.
Although some physicians are already prescribing statins for stroke patients, the literature supporting this practice has been sparse. The latest guidelines for prevention of stroke in patients with ischemic stroke or TIA were published in February 2006 by the American Heart Association/American Stroke Association Council on Stroke. These guidelines state that patients with a history of ischemic stroke or TIA are “reasonable candidates” for statin therapy. One could argue that these guidelines should now be revised to include a strong recommendation for statin therapy in secondary stroke prevention.
MRSA in the Community
By Matthew T. Harbison, MD
Moran GJ, Krishnadasan A, Gorwitz RJ, et al. EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006 Aug 17;355(7):666-674.
Methicillin-resistant Staphylococcus aureus (MRSA) emerged as an issue in the healthcare community not long after the introduction of methicillin in 1959. MRSA has traditionally been thought of as an issue for those individuals who have contact with the healthcare system; however, there is growing evidence that MRSA has become an entity in the greater community at large, affecting individuals who have not spent significant time in healthcare facilities. Descriptions of several community-based outbreaks have led to the understanding that community-associated MRSA has different characteristics than MRSA infections contracted in the hospital setting. The community-associated isolates are resistant to fewer antibiotics, produce different toxins, and have differing genetic complexes responsible for antibiotic resistance. The majority of the community-acquired infections are skin and soft tissue infections, although more serious infections have been reported.
Moran and colleagues conducted a prospective prevalence study in adult patients presenting to emergency departments with skin and soft tissue infections in 11 metropolitan areas in geographically diverse regions of the United States. Eligible patients 18 and older with purulent skin or soft tissue infections of less than one week’s duration had demographic and historical data collected; a wound culture was also taken. If Staphylococcus aureus was isolated, it was further evaluated by the Centers for Disease Control and Prevention (CDC) to characterize antibiotic resistance patterns, toxin production, and the type of staphylococcal cassette chromosome present.
A total of 422 patients were enrolled, with S. aureus isolated in 320 patients (76%). Of those with isolated S. aureus, 78% had MRSA (59% of the total patients enrolled). The individual site prevalence of MRSA ranged from 15 to 74% and was the predominant etiology of skin and soft tissue infections in 10 of 11 emergency departments. MRSA susceptibilities in this study were 100% to trimethoprim-sulfamethoxazole and rifampin, 95% to clindamycin, 92% to tetracycline, 60% to fluoroquinolones, and 6% to erythromycin. The authors point out that clindamycin resistance in one center was 60%; thus, individual site resistance patterns may differ significantly. Treatment data was available for 406 of the 422 patients, with the majority of those treated with incision, drainage, and antibiotics. In 100 of the 175 MRSA patients treated with antibiotics, the choice of agent was discordant with susceptibility patterns. The authors were able to contact 248 patients between two and three weeks after their visits and, of those contacted, 96% reported resolution or improvement of the wound.
Using multivariate logistic-regression analyses, the authors identified several potential risk factors for MRSA infection. These included use of any antibiotic in the past month, underlying illness, history of MRSA infection, close contact with someone with similar infection, and reported spider bite. Interestingly, being a healthcare worker, living in a long-term care facility, and being hospitalized in the past year were not shown to be significant risk factors in this study.
The results of this study highlight the emerging difficulty, which continues to evolve, with antibiotic resistance patterns. The healthcare community must be vigilant to new entities that challenge the traditional views of antibiotic resistance patterns. The high rate of community-acquired MRSA skin and soft tissue infection demonstrated in this study, in addition to the large percentage of patients prescribed antibiotics that were resistant for the strain involved, emphasizes the need to reconsider the empiric antibiotic choices for this patient population. The variability in regional resistance patterns further complicates the issue. Given the high prevalence of MRSA skin and soft tissue infections reported in this study, use of routine wound cultures appears prudent, as does the need for effective follow-up strategies for alteration of antibiotic choice if necessary. At an institutional level, development of surveillance and isolation strategies for community-acquired MRSA should be considered.
More Options for Venous Thromboembolism Treatment
By Kirsten N. Kangelaris, MD
Kearon C, Ginsberg JS, Julian JA, et al. Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA. 2006 Aug 23;296(8):935-942.
The standard approach to using unfractionated heparin (UFH) in the treatment of acute venous thromboembolism as a bridge to warfarin therapy requires continuous intravenous infusion with frequent dose adjustments in response to measurements of activated partial thromboplastin time (aPTT). This therapy inevitably requires inpatient management. Subcutaneous administration of weight-based low molecular weight heparin (LMWH) has been the modality of choice for outpatient treatment of venous thromboembolism because it does not require laboratory monitoring. Its use has been limited by the high cost of treatment, however. A preliminary study, released in 2000 by the FIDO group (Fixed-Dose Heparin Investigators, Kearon and colleagues), suggested that subcutaneously administered UFH could be optimally dosed based on weight rather than monitoring aPTT levels.
This follow-up, randomized, open-label, adjudicator-blinded, multi-centered, non-inferiority trial enrolled 708 patients and compared fixed-dose, subcutaneously administered UFH to LMWH in acute deep venous thrombosis and pulmonary embolism. Administration in both groups was twice daily, subcutaneous, and weight-based. UFH was given as a first dose of 333 U/kg, followed by 250 U/kg every 12 hours. LMWH was administered at a dose of 100 IU/kg every 12 hours. Both treatments overlapped with three months of warfarin therapy, and both could be administered out of hospital.
Exclusion criteria were age <18, contraindication to subcutaneous therapy, active bleeding, life expectancy under three months, long-term anticoagulation therapy, pregnancy, and creatinine level >2.3 mg/dL.
The primary endpoints were efficacy as determined by recurrent venous thromboembolism within three months and safety as determined by major bleeding within 10 days of randomization. A secondary endpoint was relationship of efficacy and safety outcomes to aPTT levels measured on day two to three of therapy for the UFH group.
Results revealed that UFH was statistically non-inferior to LMWH by all endpoints, including treatment duration, efficacy, and safety. At three months, there was no significant difference between the groups in frequency of recurrent venous thromboembolism (3.8% UFH versus 3.4% LMWH), bleeding (1.1% UFH versus 1.4% LMWH), or death. There was no association between aPTT levels and recurrent venous thromboembolism or bleeding.
Limitations of the study included reduced enrollment from the initial study design, though power was adequate due to a lower than expected incidence of recurrent venous thromboembolism in both arms (~3.6% versus the expected 6%); possible biases related to open-label design; and more post-randomization exclusions in the UFH group versus the LMWH group.
In summary, fixed-dose, unmonitored, subcutaneous UFH appears to be an effective, safe alternative to LMWH as a bridge to warfarin therapy for venous thromboembolism. Clinically, this is relevant, because UFH is approximately 15 to 20 times less expensive than LMWH. The authors appropriately call attention to two developments in clinical practice that occurred during the course of the present study and that could potentially limit the use of UFH. These are 1) the dosing option for once-daily LMWH, which improves convenience, and 2) the preference for long-term LMWH therapy over warfarin for treating cancer patients with venous thromboembolism. Despite these exceptions, UFH may prove to be a viable and economic option for venous thromboembolism treatment.
In-Hospital MI Versus MI at Presentation
By Erin M. Galbraith, MD
Maynard C, Lowy E, Rumsfeld J, et al. The prevalence and outcomes of in-hospital acute myocardial infarction in the Department of Veterans Affairs Health System. Arch Intern Med. 2006 Jul 10;166(13):1410-1416.
Much is known about the prevalence, treatment, and prognosis of acute myocardial infarction (AMI) when it occurs in the community and is the presenting diagnosis. Few studies, however, have addressed the epidemiology of in-hospital AMIs. This study by Maynard and colleagues attempts to elucidate the basic epidemiologic characteristics, treatments, and outcomes of patients who suffer in-hospital AMIs.
This retrospective cohort consisted of 7,054 patients who had been discharged with a diagnosis of AMI from 127 Veterans Health Administration (VHA) medical centers between July and August 2003. Patients who had suffered a postoperative MI or were transferred in from another hospital were excluded. Data was obtained from both the electronic and paper medical records. Of the 7,054 patients in the study, 792 (11.2%) had experienced an AMI while hospitalized for other medical problems. These 792 patients were older by approximately 4.5 years and more frequently suffered from heart failure, diabetes, chronic renal insufficiency, COPD, cerebrovascular disease, dementia, and cancer. These patients were less likely, however, to have had a previous MI, to be current smokers, or to have undergone previous angioplasty. They were also less likely to have known lipid disorders or to be taking aspirin or lipid-lowering agents.
Regarding their presentations and management, the patients who suffered in-hospital AMIs had faster heart rates and lower blood pressures. They were also up to 75% less likely to report typical symptoms of cardiac ischemia, including chest pain/pressure, shoulder pain, nausea, and diaphoresis. They were less often seen by an attending cardiologist and had more contraindications to AMI therapy; thus, these patients underwent reperfusion therapy at much lower rates, both initially and at 30 days. Their troponin levels were more frequently elevated, but they were only half as likely to have ST segment elevations at the time of diagnosis. Hospitalizations were longer for the in-hospital group, and there were higher rates of in-hospital cardiogenic shock, cardiac arrest, and death (27.3% versus 8.6%). The 30-day mortality rate was also higher (33% versus 11.9%). Multivariate logistic regression revealed an adjusted odds ratio of 2.0 (95% confidence interval 1.7 to 2.4; p<0.001) for 30-day mortality in those who experienced an in-hospital AMI versus those who presented with an AMI.
Potential reasons for the increased severity of outcomes include, but are not limited to, their many chronic comorbidities, their other acute diagnoses, the failure of the medical team to recognize cardiac ischemia in a timely manner (i.e., higher initial troponins), the inability to treat MIs appropriately secondary to contraindications to acute intervention, and the lack of an attending cardiologist presiding over their medical care. Clearly, further studies are needed to elucidate the causes of death in the 33% of patients who died, because it is unclear whether the patients died of complications from their MIs or as a result of their multiple other medical problems. Knowledge of the extent to which these patients could be managed, both medically and via interventional procedures (and why these therapies were not pursued), would also be of value.
This study emphasizes the importance of recognizing atypical presentations of AMIs and exercising vigilance in pursuing the most aggressive therapy possible, as dictated by a patient’s ability to tolerate medical and procedural interventions.
Hyperglycemia in Heart Failure
By David H. Wesorick, MD
Barsheshet A, Garty M, Grossman E, et al. Admission blood glucose level and mortality among hospitalized nondiabetic patients with heart failure. Arch Intern Med. 2006 Aug 14-28;166(15):1613-1619.
The medical literature strongly suggests that inpatient hyperglycemia is associated with a variety of poor outcomes. Little is known, however, about the relationship between hyperglycemia and heart failure. These investigators examined the association of admission blood glucose and mortality in patients who were admitted to the hospital with acute heart failure.
In this study, 1,122 patients admitted to the hospital with acute heart failure and without diabetes were divided into tertiles depending on their admission blood glucose levels. Diabetes was defined as an admission blood glucose greater than or equal to 200 mg/dl, a known diagnosis of diabetes recorded in the chart, or the presence of anti-diabetic medications on the patient’s medication list. Tertile #1 had an average admission blood glucose of 92 mg/dl (with a range of 54-102); tertile #2 had an average admission blood glucose of 113 mg/dl (with a range of 103-127); and tertile #3 had an average admission blood glucose of 147 mg/dl (with a range of 128-199). Mortality was evaluated according to tertile.
In this study, patients in tertile #3 had significantly higher inpatient mortality (7.2%) than patients in tertile #1 or #2 (3% and 4%, respectively). There was a significant association between hyperglycemia and mortality, even at 60 days follow-up, although not at six and 12 months follow-up. The association remained significant, even when patients with acute MI were excluded. Besides hyperglycemia, the authors noted that increasing age, increasing creatinine, a New York Heart Association (NYHA) functional class of III or IV, and a systolic blood pressure of lower than 115 were also significant, independent predictors of in-hospital mortality in this patient population.
For a hospitalist, the intriguing question is this: Is hyperglycemia just a marker of worse disease, or might it contribute to poorer outcomes? Clearly, hyperglycemia is associated with poorer outcomes in other types of patients, including post-surgical patients, critically ill patients, MI patients, and general medical patients.1 But is hyperglycemia just a marker of more severe illness? In heart failure, perhaps more severe decompensation results in a more profound activation of the sympathetic nervous system and a more vigorous release of stress hormones, such as cortisol and catecholamines. In that case, one might expect a sicker patient to have a higher blood glucose.
More recent studies, however, show that better control of hyperglycemia in some acutely ill patients actually results in improved outcomes, suggesting that the hyperglycemia itself might be contributing to the poorer outcomes in some cases.2-5 Hyperglycemia is known to alter human physiology in a variety of adverse ways.1 For example, hyperglycemia is known to inhibit nitric oxide production and to alter endothelial dysfunction. In a patient with acute heart failure, these alterations might be expected to have a significant effect on outcomes.
This study does not intend to answer these questions, but it does add to our understanding of the association of hyperglycemia and poor outcomes in acutely ill patients. More research is needed to examine whether or not heart failure patients, specifically, will benefit from better glycemic control in the acute setting. TH
References
- Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004 Feb;27(2):553-591.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001 Nov 8;345(19):1359-1367.
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006 Feb 2;354(5):449-461.
- Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999 Feb;67(2):352-362.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003 May;125(5):1007-1021.