User login
Hospital medicine and perioperative care: A framework for high-quality, high-value collaborative care
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
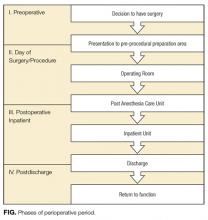
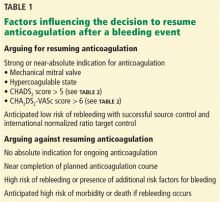
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
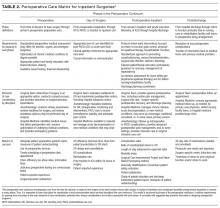
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
© 2017 Society of Hospital Medicine
In reply: Resuming anticoagulation after hemorrhage
In Reply: We thank Dr. Jandali for his thoughtful comments on our article. We acknowledge that there may be a small subset of patients in whom low-intensity warfarin may be worth trying—such as patients with a history of idiopathic or recurrent venous thromboembolism in whom problematic (but not life-threatening) bleeding recurs—but only when the international normalized ratio (INR) is at the high end of the therapeutic range or slightly above it. However, when attempting to apply the results from PREVENT1 and ELATE2 to clinical practice and the management of anticoagulation after hemorrhage, it is important to note that in ELATE there was a higher incidence of recurrent thromboembolism in patients on lower-intensity anticoagulation than in those on conventional treatment, and no significant difference in major bleeding was noted between the high- and low-intensity groups.
We acknowledge, though, that the rates of major bleeding were surprisingly low in the high-intensity group in this study relative to historical controls and so may not apply to all patients.
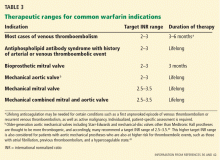
It is also important to recognize that several studies have evaluated low-intensity dosing for stroke prophylaxis in atrial fibrillation with generally disappointing results, and at present, expert opinion continues to support a therapeutic INR goal of 2.0 to 3.0.3
Therefore, we believe that low-intensity warfarin treatment is only appropriate to try in a very small subset of carefully selected patients with a history of venous thromboembolism who have proven that they cannot tolerate full-dose warfarin and in whom a trial of low-dose warfarin treatment carries acceptable risk.
- Ridker PM, Goldhaber SZ, Danielson E, et al; PREVENT Investigators. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003; 348:1425–1434.
- Kearon C, Ginsberg JS, Kovacs MJ, et al; Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:631–639.
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
In Reply: We thank Dr. Jandali for his thoughtful comments on our article. We acknowledge that there may be a small subset of patients in whom low-intensity warfarin may be worth trying—such as patients with a history of idiopathic or recurrent venous thromboembolism in whom problematic (but not life-threatening) bleeding recurs—but only when the international normalized ratio (INR) is at the high end of the therapeutic range or slightly above it. However, when attempting to apply the results from PREVENT1 and ELATE2 to clinical practice and the management of anticoagulation after hemorrhage, it is important to note that in ELATE there was a higher incidence of recurrent thromboembolism in patients on lower-intensity anticoagulation than in those on conventional treatment, and no significant difference in major bleeding was noted between the high- and low-intensity groups.
We acknowledge, though, that the rates of major bleeding were surprisingly low in the high-intensity group in this study relative to historical controls and so may not apply to all patients.
It is also important to recognize that several studies have evaluated low-intensity dosing for stroke prophylaxis in atrial fibrillation with generally disappointing results, and at present, expert opinion continues to support a therapeutic INR goal of 2.0 to 3.0.3
Therefore, we believe that low-intensity warfarin treatment is only appropriate to try in a very small subset of carefully selected patients with a history of venous thromboembolism who have proven that they cannot tolerate full-dose warfarin and in whom a trial of low-dose warfarin treatment carries acceptable risk.
In Reply: We thank Dr. Jandali for his thoughtful comments on our article. We acknowledge that there may be a small subset of patients in whom low-intensity warfarin may be worth trying—such as patients with a history of idiopathic or recurrent venous thromboembolism in whom problematic (but not life-threatening) bleeding recurs—but only when the international normalized ratio (INR) is at the high end of the therapeutic range or slightly above it. However, when attempting to apply the results from PREVENT1 and ELATE2 to clinical practice and the management of anticoagulation after hemorrhage, it is important to note that in ELATE there was a higher incidence of recurrent thromboembolism in patients on lower-intensity anticoagulation than in those on conventional treatment, and no significant difference in major bleeding was noted between the high- and low-intensity groups.
We acknowledge, though, that the rates of major bleeding were surprisingly low in the high-intensity group in this study relative to historical controls and so may not apply to all patients.
It is also important to recognize that several studies have evaluated low-intensity dosing for stroke prophylaxis in atrial fibrillation with generally disappointing results, and at present, expert opinion continues to support a therapeutic INR goal of 2.0 to 3.0.3
Therefore, we believe that low-intensity warfarin treatment is only appropriate to try in a very small subset of carefully selected patients with a history of venous thromboembolism who have proven that they cannot tolerate full-dose warfarin and in whom a trial of low-dose warfarin treatment carries acceptable risk.
- Ridker PM, Goldhaber SZ, Danielson E, et al; PREVENT Investigators. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003; 348:1425–1434.
- Kearon C, Ginsberg JS, Kovacs MJ, et al; Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:631–639.
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Ridker PM, Goldhaber SZ, Danielson E, et al; PREVENT Investigators. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003; 348:1425–1434.
- Kearon C, Ginsberg JS, Kovacs MJ, et al; Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:631–639.
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
Updates in Perioperative Medicine
Given the rapid expansion of the field of perioperative medicine, clinicians need to remain apprised of the current evidence to ensure optimization of patient care. In this update, we review 10 key articles from the perioperative literature, with the goal of summarizing the most clinically important evidence over the past year. This summary of recent literature in perioperative medicine is derived from the Update in Perioperative Medicine sessions presented at the 10th Annual Perioperative Medicine Summit and the Society of General Internal Medicine 38th Annual Meeting. A systematic search strategy was used to identify pertinent articles, and the following were selected by the authors based on their relevance to the clinical practice of perioperative medicine.
PERIOPERATIVE CARDIOVASCULAR CARE
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:e278e333.
Background
The American College of Cardiology/American Heart Association (ACC/AHA) perioperative guideline provides recommendations for the evaluation and management of cardiovascular disease in patients undergoing noncardiac surgery.
Findings
The new guideline combines the evaluation of surgery‐ and patient‐specific risk in the algorithm for preoperative cardiovascular evaluation into a single step and recommends the use of 1 of 3 tools: the Revised Cardiac Risk Index (RCRI),[1] National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator,[2] or the NSQIP‐derived myocardial infarction and cardiac arrest calculator.[3] Estimation of risk is also simplified by stratification into only 2 groups: low risk (risk of major adverse cardiac event 1%) and elevated risk (1% risk). Coronary evaluation can be considered for patients with elevated cardiac risk and poor functional capacity, but is advised only if the results would alter perioperative management. For example, a patient with very high risk who has evidence of ischemia on stress testing may choose to forego surgery. Preoperative coronary revascularization is only indicated for patients meeting criteria in the nonsurgical setting.
For patients with previous percutaneous coronary intervention, the ACC/AHA has not changed its recommendations to optimally delay surgery for at least 30 days after bare‐metal stenting and at least 1 year after drug‐eluting stent (DES) placement. However, in patients with a DES placed 6 to 12 months previously, surgery can be performed if the risks of surgical delay outweigh the risks of DES thrombosis. After any type of coronary stenting, dual antiplatelet therapy should be continued uninterrupted through the first 4 to 6 weeks and even later whenever feasible. If not possible, aspirin therapy should be maintained through surgery unless bleeding risk is too high.
The guideline recommends perioperative continuation of ‐blockers in patients taking them chronically. Preoperative initiation of ‐blocker therapy may be considered for patients with myocardial ischemia on stress testing or 3 RCRI factors and should be started far enough in advance to allow determination of patient's tolerance prior to surgery.
Cautions
Many recommendations are based on data from nonrandomized trials or expert opinion, and the data in areas such as perioperative ‐blockade continue to evolve.
Implications
The ACC/AHA guideline continues to be a critically valuable resource for hospitalists providing perioperative care to noncardiac surgery patients.
Wijeysundera DN, Duncan D, Nkonde‐Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines.
J Am Coll Cardiol. 2014;64(22):24062425.
Background
Various clinical trials have reported conflicting results regarding the efficacy and safety of perioperative ‐blockers resulting in guideline committees changing their recommendations. Because of questions raised regarding the scientific integrity of the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography)‐I[4] and DECREASE‐IV[5] trials as well as the dosing of ‐blockers in POISE (PeriOperative Ischemic Evaluation) study,[6] this systematic review was performed in conjunction with the ACC/AHA guideline update[7] to evaluate the data with and without these trials.
Findings
Sixteen randomized control trials (RCTs) (n=12,043) and 1 cohort study (n=348) were included in the analysis. Perioperative ‐blockers were associated with a reduction in nonfatal myocardial infarction (MI) (relative risk [RR]: 0.69; 95% confidence interval [CI]: 0.58‐0.82; P0.001) but an increase in bradycardia (RR: 2.61; 95% CI: 2.18‐3.12), hypotension (RR: 1.47; 95% CI: 1.34‐1.6), and nonfatal strokes (RR: 1.76; 95% CI: 1.07‐2.91; P=0.02). The POISE trial was the only one demonstrating a statistically significant increase in stroke.
The major discrepancy between the DECREASE trials and the other RCTs was related to mortalitya reduction in both cardiovascular and all‐cause death in DECREASE but an increased risk of all‐cause death in the other trials.
Cautions
Because of its size, the POISE trial heavily influences the results, particularly for mortality and stroke. Including the DECREASE trials reduces the otherwise increased risk for death to a null effect. Exclusion of the POISE and DECREASE trials leaves few data to make conclusions about safety and efficacy of perioperative ‐blockade. Several cohort studies have found metoprolol to be associated with worse outcomes than with atenolol or bisoprolol (which were preferred by the European Society of Cardiology guidelines).[8]
Implications
Perioperative ‐blockade started within 1 day of noncardiac surgery was associated with fewer nonfatal MIs but at the cost of an increase in hypotension, bradycardia, and a possible increase in stroke and death. Long‐term ‐blockade should be continued perioperatively, whereas the decision to initiate a ‐blocker should be individualized. If starting a ‐blocker perioperatively, it should be done 2 days before surgery.
Botto F, Alonso‐Coello P, Chan MT, et al.; on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology. 2014;120(3):564578.
Background
Many patients sustain myocardial injury in the perioperative period as evidenced by troponin elevations, but most do not meet diagnostic criteria for MI. Myocardial injury after noncardiac surgery (MINS) is defined as prognostically relevant myocardial injury due to ischemia that occurs within 30 days after noncardiac surgery. This international, prospective cohort study of 15,065 patients 45 years old who underwent in‐patient noncardiac surgery determined diagnostic criteria, characteristics, predictors, and 30‐day outcomes of MINS.
Findings
The diagnostic criterion for MINS was a peak troponin T level 0.03 ng/mL judged to be due to an ischemic etiology. Twelve independent predictors of MINS were identified including age 75 years, known cardiovascular disease or risk factors, and surgical factors. MINS was an independent predictor of 30‐day mortality (adjusted hazard ratio [HR]: 3.87; 95% CI: 2.96‐5.08). Age >75 years, ST elevation, or new left bundle branch block, and anterior ischemic findings were independent predictors of 30‐day mortality among patients with MINS.
Cautions
Although screening high‐risk surgical patients without signs or symptoms of ischemia with postoperative troponins will increase the frequency of diagnosing MINS, evidence for an effective treatment has not yet been established. The ACC/AHA guidelines state that routine screening is of uncertain benefit for this reason.
Implications
Because MINS is common and carries a poor 30‐day prognosis, clinical trials are needed to determine when to obtain postoperative troponins and how to prevent and treat this complication.[9] Some observational data from POISE suggest that aspirin and statins can reduce the risk of 30‐day mortality in patients with postoperative MIs.
Devereaux PJ, Mrkobrada M, Sessler DI, et al. for the POISE‐2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):14941503.
Devereaux PJ, Sessler DI, Leslie K, et al. for the POISE‐2 Investigators. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):15041513.
Background
Medical risk reduction with aspirin and other agents in perioperative patients remains controversial. The POISE‐2 trial is a blinded RCT examining the effects of aspirin and clonidine on outcomes in >10,000 noncardiac surgery patients at risk of cardiovascular complications. The aspirin arm of the study included the initiation group and the continuation stratum, as well as placebo. Patients in the clonidine portion of the trial received 0.2 mg of clonidine or placebo daily for the same time periods.
Findings
The primary outcome was a composite of death or nonfatal MI within 30 days of surgery. Outcomes were similar in patients initiated or continued on aspirin. No difference was seen between aspirin or placebo in the primary outcome (7.0% vs 7.1%; HR: 0.86; 95% CI: 0.86‐1.15; P=0.92). There were no differences in rates of MI, venous thromboembolism, or stroke. Major bleeding rates were higher in aspirin versus placebo‐treated patients (4.6% vs 3.8%; HR: 1.23; 95% CI: 1.01‐1.49; P=0.04).
Clonidine did not alter the composite outcome of death or nonfatal MI (7.3% vs 6.8%; HR: 1.08; 95% CI: 0.93‐1.26; P=0.29). Clinically significant hypotension, bradycardia, and nonfatal cardiac arrest were more common in clonidine‐treated patients, although no difference was detected in stroke rates.
Cautions
Although patients in the trial had cardiovascular risk factors, 24% of patients had known coronary artery disease, and 5% had coronary stents. Conclusions based on this trial regarding perioperative management of antiplatelet therapy should not include patients with coronary artery stents.
Implications
Aspirin started before surgery and continued perioperatively did not decrease the rate of death or nonfatal MI but increased the risk of major bleeding. Perioperative management of aspirin needs to be undertaken in the context of cardiac and bleeding risks. Clonidine also did not improve outcomes and increased the risk of bradycardia and hypotension. Current guidelines recommend against using alpha‐2 agonists for prevention of perioperative cardiac events7; however, patients already on alpha‐2 agonists should not stop them abruptly.
PERIOPERATIVE PULMONARY CARE
Mutter TC, Chateau D, Moffatt M, et al. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology. 2014;121(4):707718.
Background
An increasing body of literature associates obstructive sleep apnea (OSA) with an increased risk of postoperative complications. Despite evidence of risk, potential benefits of preoperative diagnosis and treatment of OSA remain unclear.
Findings
Using databases to identify patients prescribed continuous positive airway pressure (CPAP) therapy, the study compared postoperative outcomes of patients who underwent surgery any time after polysomnography (PSG) and CPAP prescription (diagnosed OSA [DOSA]) and those who had surgery during the 5 years preceding their PSG (undiagnosed OSA [UOSA]). These patients were matched with patients who underwent the same procedure for the same indication and had no insurance claims for PSG or diagnosis of sleep‐disordered breathing.
After multivariate analysis, OSA of any type was associated with increased pulmonary complications (odds ratio [OR]: 2.08; 95% CI: 1.35‐2.19). However, no significant differences in respiratory outcomes were noted between DOSA patients (N=2640) and those with UOSA (N=1571). DOSA patients did have fewer cardiovascular complications than UOSA patients (OR: 0.34; 95% CI: 0.15‐0.77). Only severe OSA (apnea‐hypopnea index >30) was associated with increased pulmonary and cardiovascular complications.
Cautions
Although this study suggests an association between preoperative diagnosis and treatment of OSA and reduced cardiovascular complications, the results are not definitive due to the inability to control for all confounding variables in a retrospective study utilizing an administrative database.
Implications
OSA is an important risk factor for postoperative complications, and this study suggests that preoperative treatment with CPAP is associated with reduced risk of cardiovascular complications, particularly in patients with severe OSA. Future controlled trials should focus on the risk‐reduction potential of preoperative diagnosis and treatment of OSA.
Mazo V, Sabat S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219231.
Background
In 2010, Canet et al. published a novel risk index, the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) index, to provide a quantitative estimate of the risk of postoperative pulmonary complications (PPCs).[10]
In the current report, Mazo and colleagues studied the ARISCAT index in a broader sample to characterize its accuracy in predicting PPC risk. The ARISCAT index is derived from clinical risk factors: (1) age, (2) preoperative oxygen saturation, (3) respiratory infection in the prior month, (4) anemia, (5) surgical site, (6) duration of surgery, and (7) emergency surgery, with varying weights based on the strength of the association in a multivariable analysis. This score can be calculated via addition of these weighted risk factors, with a score>45 equal to high risk for PPC.
Findings
Examining 5099 patients from 63 European hospitals, the authors definition of PPC included respiratory failure, pulmonary infection, pleural effusion, atelectasis, pneumothorax, bronchospasm, and aspiration pneumonitis. PPC rates were as follows: low risk (3.39%), intermediate risk (12.98%), and high risk (38.01%). The positive likelihood ratio for PPC among the highest risk group was 7.12. The C statistic for fit was 0.80. Observed PPC rates were higher than predicted for the low (3.39% vs 0.87%) and intermediate (12.98% vs 7.82%) risk groups.
Cautions
The calibration slopes were less than ideal in all subsamples, with the Western European sample performing better than the other geographic areas; suggesting that the coefficients on the ARISCAT index may benefit from recalibration to match specific populations.
Implications
This is the first major pulmonary risk index that has been externally validated. Its use of readily available clinical information, simplicity, and accuracy in estimating PPC risk make it an important addition to the toolkit during a preoperative evaluation.
PERIOPERATIVE ATRIAL FIBRILLATION/ANTICOAGULATION
Gialdini G, Nearing K, Bhave P, et al. Perioperative atrial fibrillation and the long term risk of ischemic stroke. JAMA. 2014;312(6):616622.
Background
New‐onset atrial fibrillation (AF) is the most common perioperative arrhythmia.[11] However, little is known regarding the long‐term risks of ischemic stroke in patients who develop perioperative AF. This retrospective cohort study examined adults with no preexisting history of AF, hospitalized for surgery, and discharged free of cerebrovascular disease between 2007 and 2011 (n=1,729,360).
Findings
Of the eligible patients, 1.43% (95% CI: 1.41%‐1.45%) developed perioperative AF, and 0.81% (95% CI: 0.79%‐0.82%) had a stroke up to 1 year after discharge. Perioperative AF was associated with subsequent stroke after both cardiac (HR: 1.3; 95% CI: 1.1‐1.6) and noncardiac surgery (HR: 2; 95% CI: 1.7‐2.3). The association with stroke was stronger for perioperative AF after noncardiac versus cardiac surgery (P0.001 for interaction).
Cautions
This is a retrospective cohort study, using claims data to identify AF and stroke. Data on duration of the perioperative AF episodes or use of antithrombotic therapies were not available.
Implications
The association found between perioperative AF and long‐term risk of ischemic stroke may suggest that perioperative AF, especially after noncardiac surgery, should be treated aggressively in terms of thromboembolic risk; however, further data will be required to validate this association.
Van Diepen S, Youngson E, Ezekowitz J, McAlister F. Which risk score best predicts perioperative outcomes in nonvalvular atrial fibrillation patients undergoing noncardiac surgery? Am Heart J. 2014;168(1):6067.
Background
Patients with nonvalvular AF (NVAF) are at increased risk for adverse perioperative outcomes after noncardiac surgery.[12] The RCRI is commonly used to predict perioperative cardiovascular events for all patients, including those with NVAF, though AF is not part of this risk assessment. The goal of this retrospective cohort study was to examine the prognostic utility of already existing NVAF risk indices, including the CHADS2 (Congestive heart failure, Hypertension, Age 75 years, Diabetes mellitus, prior stroke or transient ischemic attack), CHA2DS2‐VASc (Congestive heart failure; Hypertension; Age 75 years; Diabetes mellitus; Stroke, TIA, or thromboembolism [TE]; Vascular disease; Age 65 to 74 years; Sex category [female]), and R2CHADS2 (Renal dysfunction, Congestive heart failure, Hypertension, Age, Diabetes, Stroke/TIA) for perioperative outcomes in patients undergoing noncardiac surgery.
Findings
A population dataset of NVAF patients (n=32,160) who underwent noncardiac surgery was examined, with outcome measures including 30‐day mortality, stroke, TIA, or systemic embolism. The incidence of the 30‐day composite outcome was 4.2% and the C indices were 0.65 for the RCRI, 0.67 for CHADS2, 0.67 for CHA2DS2‐VASc, and 0.68 for R2CHADS2. The Net Reclassification Index (NRI), a measure evaluating the improvement in prediction performance gained by adding a marker to a set of baseline predictors, was calculated. All NVAF scores performed better than the RCRI for predicting mortality risk (NRI: 12.3%, 8.4%, and 13.3% respectively, all P0.01).
Cautions
Patients in the highest risk category by RCRI appear to have an unadjusted higher 30‐day mortality risk (8%) than that predicted by the other 3 scores (5%, 5.6%, and 5%), indicating that these risk scores should not completely supplant the RCRI for risk stratification in this population. In addition, the overall improvement in predictive capacity of the CHADS2, CHA2DS2‐VASc, and R2CHADS2, although superior to the RCRI, is modest.
Implications
These findings indicate that the preoperative risk stratification for patients with NVAF can be improved by utilizing the CHADS2, CHA2DS2‐VASc, or R2CHADS2 scores when undergoing noncardiac surgery. For patients with NVAF identified as high risk for adverse outcomes, this assessment can be integrated into the preoperative discussion on the risks/benefits of surgery.
Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Circulation. 2015;131:488494
Background
Oral anticoagulation (OAC) significantly reduces the risk of stroke in patients with AF. Many AF patients on long‐term anticoagulation undergo procedures requiring temporary interruption of OAC. Although guidelines have been published on when and how to initiate bridging therapy, they are based on observational data. Thus, it remains unclear which patients should receive bridging anticoagulation.
Findings
This is a US registry of outpatients with AF with temporary interruptions of OAC for a procedure. Of 7372 patients treated with OAC, 2803 overall interruption events occurred in 2200 patients (30%). Bridging anticoagulants were used in 24% (n=665). Bleeding events were more common in bridged than nonbridged patients (5.0% vs 1.3%; adjusted OR: 3.84; P0.0001). The overall composite end point of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days was significantly higher in patients receiving bridging (13% vs 6.3%; adjusted OR: 1.94; P=0.0001). This statistically significant increase in the composite outcome, which includes cardiovascular events, is most likely in part secondary to inclusion of bleeding events. The recently published BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial did not find a statistically significant difference in cardiovascular events between bridged and nonbridged patients.[13]
Cautions
Although patients who were bridged appear to have had more comorbidities and a higher mean CHADS2 score than patients who were not bridged, it is difficult to determine which population of patients may be high risk enough to warrant bridging, as indicated by current American College of Chest Physicians guidelines, as this was not evaluated in this study
Implications
The use of bridging anticoagulation was significantly associated with higher overall bleeding and adverse event rates. The BRIDGE trial also found that forgoing bridging anticoagulation decreased the risk of major bleeding in patients with AF and was noninferior to bridging for the prevention of arterial TE.[13]
- , , , et al. Derivation and prospective evaluation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842.
- , , , et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387.
- , , , et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789–1794.
- , , , et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- POISE Study Group, , , , et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
- , , , et al. American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137.
- , , , et al. 2014 ESC/ESA Guidelines on non‐cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non‐cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431.
- , , , et al. The long‐term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053–1063.
- , , , et al. ARISCAT Group: Prediction of postoperative pulmonary complications in a population‐based surgical cohort. Anesthesiology. 2010;113:1338–1350.
- , . Noncardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28(10 suppl):N145–N150.
- , , , et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major cardiac surgery. Am Heart J. 2012;164(6):918–924.
- , , , et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–833.
Given the rapid expansion of the field of perioperative medicine, clinicians need to remain apprised of the current evidence to ensure optimization of patient care. In this update, we review 10 key articles from the perioperative literature, with the goal of summarizing the most clinically important evidence over the past year. This summary of recent literature in perioperative medicine is derived from the Update in Perioperative Medicine sessions presented at the 10th Annual Perioperative Medicine Summit and the Society of General Internal Medicine 38th Annual Meeting. A systematic search strategy was used to identify pertinent articles, and the following were selected by the authors based on their relevance to the clinical practice of perioperative medicine.
PERIOPERATIVE CARDIOVASCULAR CARE
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:e278e333.
Background
The American College of Cardiology/American Heart Association (ACC/AHA) perioperative guideline provides recommendations for the evaluation and management of cardiovascular disease in patients undergoing noncardiac surgery.
Findings
The new guideline combines the evaluation of surgery‐ and patient‐specific risk in the algorithm for preoperative cardiovascular evaluation into a single step and recommends the use of 1 of 3 tools: the Revised Cardiac Risk Index (RCRI),[1] National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator,[2] or the NSQIP‐derived myocardial infarction and cardiac arrest calculator.[3] Estimation of risk is also simplified by stratification into only 2 groups: low risk (risk of major adverse cardiac event 1%) and elevated risk (1% risk). Coronary evaluation can be considered for patients with elevated cardiac risk and poor functional capacity, but is advised only if the results would alter perioperative management. For example, a patient with very high risk who has evidence of ischemia on stress testing may choose to forego surgery. Preoperative coronary revascularization is only indicated for patients meeting criteria in the nonsurgical setting.
For patients with previous percutaneous coronary intervention, the ACC/AHA has not changed its recommendations to optimally delay surgery for at least 30 days after bare‐metal stenting and at least 1 year after drug‐eluting stent (DES) placement. However, in patients with a DES placed 6 to 12 months previously, surgery can be performed if the risks of surgical delay outweigh the risks of DES thrombosis. After any type of coronary stenting, dual antiplatelet therapy should be continued uninterrupted through the first 4 to 6 weeks and even later whenever feasible. If not possible, aspirin therapy should be maintained through surgery unless bleeding risk is too high.
The guideline recommends perioperative continuation of ‐blockers in patients taking them chronically. Preoperative initiation of ‐blocker therapy may be considered for patients with myocardial ischemia on stress testing or 3 RCRI factors and should be started far enough in advance to allow determination of patient's tolerance prior to surgery.
Cautions
Many recommendations are based on data from nonrandomized trials or expert opinion, and the data in areas such as perioperative ‐blockade continue to evolve.
Implications
The ACC/AHA guideline continues to be a critically valuable resource for hospitalists providing perioperative care to noncardiac surgery patients.
Wijeysundera DN, Duncan D, Nkonde‐Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines.
J Am Coll Cardiol. 2014;64(22):24062425.
Background
Various clinical trials have reported conflicting results regarding the efficacy and safety of perioperative ‐blockers resulting in guideline committees changing their recommendations. Because of questions raised regarding the scientific integrity of the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography)‐I[4] and DECREASE‐IV[5] trials as well as the dosing of ‐blockers in POISE (PeriOperative Ischemic Evaluation) study,[6] this systematic review was performed in conjunction with the ACC/AHA guideline update[7] to evaluate the data with and without these trials.
Findings
Sixteen randomized control trials (RCTs) (n=12,043) and 1 cohort study (n=348) were included in the analysis. Perioperative ‐blockers were associated with a reduction in nonfatal myocardial infarction (MI) (relative risk [RR]: 0.69; 95% confidence interval [CI]: 0.58‐0.82; P0.001) but an increase in bradycardia (RR: 2.61; 95% CI: 2.18‐3.12), hypotension (RR: 1.47; 95% CI: 1.34‐1.6), and nonfatal strokes (RR: 1.76; 95% CI: 1.07‐2.91; P=0.02). The POISE trial was the only one demonstrating a statistically significant increase in stroke.
The major discrepancy between the DECREASE trials and the other RCTs was related to mortalitya reduction in both cardiovascular and all‐cause death in DECREASE but an increased risk of all‐cause death in the other trials.
Cautions
Because of its size, the POISE trial heavily influences the results, particularly for mortality and stroke. Including the DECREASE trials reduces the otherwise increased risk for death to a null effect. Exclusion of the POISE and DECREASE trials leaves few data to make conclusions about safety and efficacy of perioperative ‐blockade. Several cohort studies have found metoprolol to be associated with worse outcomes than with atenolol or bisoprolol (which were preferred by the European Society of Cardiology guidelines).[8]
Implications
Perioperative ‐blockade started within 1 day of noncardiac surgery was associated with fewer nonfatal MIs but at the cost of an increase in hypotension, bradycardia, and a possible increase in stroke and death. Long‐term ‐blockade should be continued perioperatively, whereas the decision to initiate a ‐blocker should be individualized. If starting a ‐blocker perioperatively, it should be done 2 days before surgery.
Botto F, Alonso‐Coello P, Chan MT, et al.; on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology. 2014;120(3):564578.
Background
Many patients sustain myocardial injury in the perioperative period as evidenced by troponin elevations, but most do not meet diagnostic criteria for MI. Myocardial injury after noncardiac surgery (MINS) is defined as prognostically relevant myocardial injury due to ischemia that occurs within 30 days after noncardiac surgery. This international, prospective cohort study of 15,065 patients 45 years old who underwent in‐patient noncardiac surgery determined diagnostic criteria, characteristics, predictors, and 30‐day outcomes of MINS.
Findings
The diagnostic criterion for MINS was a peak troponin T level 0.03 ng/mL judged to be due to an ischemic etiology. Twelve independent predictors of MINS were identified including age 75 years, known cardiovascular disease or risk factors, and surgical factors. MINS was an independent predictor of 30‐day mortality (adjusted hazard ratio [HR]: 3.87; 95% CI: 2.96‐5.08). Age >75 years, ST elevation, or new left bundle branch block, and anterior ischemic findings were independent predictors of 30‐day mortality among patients with MINS.
Cautions
Although screening high‐risk surgical patients without signs or symptoms of ischemia with postoperative troponins will increase the frequency of diagnosing MINS, evidence for an effective treatment has not yet been established. The ACC/AHA guidelines state that routine screening is of uncertain benefit for this reason.
Implications
Because MINS is common and carries a poor 30‐day prognosis, clinical trials are needed to determine when to obtain postoperative troponins and how to prevent and treat this complication.[9] Some observational data from POISE suggest that aspirin and statins can reduce the risk of 30‐day mortality in patients with postoperative MIs.
Devereaux PJ, Mrkobrada M, Sessler DI, et al. for the POISE‐2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):14941503.
Devereaux PJ, Sessler DI, Leslie K, et al. for the POISE‐2 Investigators. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):15041513.
Background
Medical risk reduction with aspirin and other agents in perioperative patients remains controversial. The POISE‐2 trial is a blinded RCT examining the effects of aspirin and clonidine on outcomes in >10,000 noncardiac surgery patients at risk of cardiovascular complications. The aspirin arm of the study included the initiation group and the continuation stratum, as well as placebo. Patients in the clonidine portion of the trial received 0.2 mg of clonidine or placebo daily for the same time periods.
Findings
The primary outcome was a composite of death or nonfatal MI within 30 days of surgery. Outcomes were similar in patients initiated or continued on aspirin. No difference was seen between aspirin or placebo in the primary outcome (7.0% vs 7.1%; HR: 0.86; 95% CI: 0.86‐1.15; P=0.92). There were no differences in rates of MI, venous thromboembolism, or stroke. Major bleeding rates were higher in aspirin versus placebo‐treated patients (4.6% vs 3.8%; HR: 1.23; 95% CI: 1.01‐1.49; P=0.04).
Clonidine did not alter the composite outcome of death or nonfatal MI (7.3% vs 6.8%; HR: 1.08; 95% CI: 0.93‐1.26; P=0.29). Clinically significant hypotension, bradycardia, and nonfatal cardiac arrest were more common in clonidine‐treated patients, although no difference was detected in stroke rates.
Cautions
Although patients in the trial had cardiovascular risk factors, 24% of patients had known coronary artery disease, and 5% had coronary stents. Conclusions based on this trial regarding perioperative management of antiplatelet therapy should not include patients with coronary artery stents.
Implications
Aspirin started before surgery and continued perioperatively did not decrease the rate of death or nonfatal MI but increased the risk of major bleeding. Perioperative management of aspirin needs to be undertaken in the context of cardiac and bleeding risks. Clonidine also did not improve outcomes and increased the risk of bradycardia and hypotension. Current guidelines recommend against using alpha‐2 agonists for prevention of perioperative cardiac events7; however, patients already on alpha‐2 agonists should not stop them abruptly.
PERIOPERATIVE PULMONARY CARE
Mutter TC, Chateau D, Moffatt M, et al. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology. 2014;121(4):707718.
Background
An increasing body of literature associates obstructive sleep apnea (OSA) with an increased risk of postoperative complications. Despite evidence of risk, potential benefits of preoperative diagnosis and treatment of OSA remain unclear.
Findings
Using databases to identify patients prescribed continuous positive airway pressure (CPAP) therapy, the study compared postoperative outcomes of patients who underwent surgery any time after polysomnography (PSG) and CPAP prescription (diagnosed OSA [DOSA]) and those who had surgery during the 5 years preceding their PSG (undiagnosed OSA [UOSA]). These patients were matched with patients who underwent the same procedure for the same indication and had no insurance claims for PSG or diagnosis of sleep‐disordered breathing.
After multivariate analysis, OSA of any type was associated with increased pulmonary complications (odds ratio [OR]: 2.08; 95% CI: 1.35‐2.19). However, no significant differences in respiratory outcomes were noted between DOSA patients (N=2640) and those with UOSA (N=1571). DOSA patients did have fewer cardiovascular complications than UOSA patients (OR: 0.34; 95% CI: 0.15‐0.77). Only severe OSA (apnea‐hypopnea index >30) was associated with increased pulmonary and cardiovascular complications.
Cautions
Although this study suggests an association between preoperative diagnosis and treatment of OSA and reduced cardiovascular complications, the results are not definitive due to the inability to control for all confounding variables in a retrospective study utilizing an administrative database.
Implications
OSA is an important risk factor for postoperative complications, and this study suggests that preoperative treatment with CPAP is associated with reduced risk of cardiovascular complications, particularly in patients with severe OSA. Future controlled trials should focus on the risk‐reduction potential of preoperative diagnosis and treatment of OSA.
Mazo V, Sabat S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219231.
Background
In 2010, Canet et al. published a novel risk index, the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) index, to provide a quantitative estimate of the risk of postoperative pulmonary complications (PPCs).[10]
In the current report, Mazo and colleagues studied the ARISCAT index in a broader sample to characterize its accuracy in predicting PPC risk. The ARISCAT index is derived from clinical risk factors: (1) age, (2) preoperative oxygen saturation, (3) respiratory infection in the prior month, (4) anemia, (5) surgical site, (6) duration of surgery, and (7) emergency surgery, with varying weights based on the strength of the association in a multivariable analysis. This score can be calculated via addition of these weighted risk factors, with a score>45 equal to high risk for PPC.
Findings
Examining 5099 patients from 63 European hospitals, the authors definition of PPC included respiratory failure, pulmonary infection, pleural effusion, atelectasis, pneumothorax, bronchospasm, and aspiration pneumonitis. PPC rates were as follows: low risk (3.39%), intermediate risk (12.98%), and high risk (38.01%). The positive likelihood ratio for PPC among the highest risk group was 7.12. The C statistic for fit was 0.80. Observed PPC rates were higher than predicted for the low (3.39% vs 0.87%) and intermediate (12.98% vs 7.82%) risk groups.
Cautions
The calibration slopes were less than ideal in all subsamples, with the Western European sample performing better than the other geographic areas; suggesting that the coefficients on the ARISCAT index may benefit from recalibration to match specific populations.
Implications
This is the first major pulmonary risk index that has been externally validated. Its use of readily available clinical information, simplicity, and accuracy in estimating PPC risk make it an important addition to the toolkit during a preoperative evaluation.
PERIOPERATIVE ATRIAL FIBRILLATION/ANTICOAGULATION
Gialdini G, Nearing K, Bhave P, et al. Perioperative atrial fibrillation and the long term risk of ischemic stroke. JAMA. 2014;312(6):616622.
Background
New‐onset atrial fibrillation (AF) is the most common perioperative arrhythmia.[11] However, little is known regarding the long‐term risks of ischemic stroke in patients who develop perioperative AF. This retrospective cohort study examined adults with no preexisting history of AF, hospitalized for surgery, and discharged free of cerebrovascular disease between 2007 and 2011 (n=1,729,360).
Findings
Of the eligible patients, 1.43% (95% CI: 1.41%‐1.45%) developed perioperative AF, and 0.81% (95% CI: 0.79%‐0.82%) had a stroke up to 1 year after discharge. Perioperative AF was associated with subsequent stroke after both cardiac (HR: 1.3; 95% CI: 1.1‐1.6) and noncardiac surgery (HR: 2; 95% CI: 1.7‐2.3). The association with stroke was stronger for perioperative AF after noncardiac versus cardiac surgery (P0.001 for interaction).
Cautions
This is a retrospective cohort study, using claims data to identify AF and stroke. Data on duration of the perioperative AF episodes or use of antithrombotic therapies were not available.
Implications
The association found between perioperative AF and long‐term risk of ischemic stroke may suggest that perioperative AF, especially after noncardiac surgery, should be treated aggressively in terms of thromboembolic risk; however, further data will be required to validate this association.
Van Diepen S, Youngson E, Ezekowitz J, McAlister F. Which risk score best predicts perioperative outcomes in nonvalvular atrial fibrillation patients undergoing noncardiac surgery? Am Heart J. 2014;168(1):6067.
Background
Patients with nonvalvular AF (NVAF) are at increased risk for adverse perioperative outcomes after noncardiac surgery.[12] The RCRI is commonly used to predict perioperative cardiovascular events for all patients, including those with NVAF, though AF is not part of this risk assessment. The goal of this retrospective cohort study was to examine the prognostic utility of already existing NVAF risk indices, including the CHADS2 (Congestive heart failure, Hypertension, Age 75 years, Diabetes mellitus, prior stroke or transient ischemic attack), CHA2DS2‐VASc (Congestive heart failure; Hypertension; Age 75 years; Diabetes mellitus; Stroke, TIA, or thromboembolism [TE]; Vascular disease; Age 65 to 74 years; Sex category [female]), and R2CHADS2 (Renal dysfunction, Congestive heart failure, Hypertension, Age, Diabetes, Stroke/TIA) for perioperative outcomes in patients undergoing noncardiac surgery.
Findings
A population dataset of NVAF patients (n=32,160) who underwent noncardiac surgery was examined, with outcome measures including 30‐day mortality, stroke, TIA, or systemic embolism. The incidence of the 30‐day composite outcome was 4.2% and the C indices were 0.65 for the RCRI, 0.67 for CHADS2, 0.67 for CHA2DS2‐VASc, and 0.68 for R2CHADS2. The Net Reclassification Index (NRI), a measure evaluating the improvement in prediction performance gained by adding a marker to a set of baseline predictors, was calculated. All NVAF scores performed better than the RCRI for predicting mortality risk (NRI: 12.3%, 8.4%, and 13.3% respectively, all P0.01).
Cautions
Patients in the highest risk category by RCRI appear to have an unadjusted higher 30‐day mortality risk (8%) than that predicted by the other 3 scores (5%, 5.6%, and 5%), indicating that these risk scores should not completely supplant the RCRI for risk stratification in this population. In addition, the overall improvement in predictive capacity of the CHADS2, CHA2DS2‐VASc, and R2CHADS2, although superior to the RCRI, is modest.
Implications
These findings indicate that the preoperative risk stratification for patients with NVAF can be improved by utilizing the CHADS2, CHA2DS2‐VASc, or R2CHADS2 scores when undergoing noncardiac surgery. For patients with NVAF identified as high risk for adverse outcomes, this assessment can be integrated into the preoperative discussion on the risks/benefits of surgery.
Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Circulation. 2015;131:488494
Background
Oral anticoagulation (OAC) significantly reduces the risk of stroke in patients with AF. Many AF patients on long‐term anticoagulation undergo procedures requiring temporary interruption of OAC. Although guidelines have been published on when and how to initiate bridging therapy, they are based on observational data. Thus, it remains unclear which patients should receive bridging anticoagulation.
Findings
This is a US registry of outpatients with AF with temporary interruptions of OAC for a procedure. Of 7372 patients treated with OAC, 2803 overall interruption events occurred in 2200 patients (30%). Bridging anticoagulants were used in 24% (n=665). Bleeding events were more common in bridged than nonbridged patients (5.0% vs 1.3%; adjusted OR: 3.84; P0.0001). The overall composite end point of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days was significantly higher in patients receiving bridging (13% vs 6.3%; adjusted OR: 1.94; P=0.0001). This statistically significant increase in the composite outcome, which includes cardiovascular events, is most likely in part secondary to inclusion of bleeding events. The recently published BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial did not find a statistically significant difference in cardiovascular events between bridged and nonbridged patients.[13]
Cautions
Although patients who were bridged appear to have had more comorbidities and a higher mean CHADS2 score than patients who were not bridged, it is difficult to determine which population of patients may be high risk enough to warrant bridging, as indicated by current American College of Chest Physicians guidelines, as this was not evaluated in this study
Implications
The use of bridging anticoagulation was significantly associated with higher overall bleeding and adverse event rates. The BRIDGE trial also found that forgoing bridging anticoagulation decreased the risk of major bleeding in patients with AF and was noninferior to bridging for the prevention of arterial TE.[13]
Given the rapid expansion of the field of perioperative medicine, clinicians need to remain apprised of the current evidence to ensure optimization of patient care. In this update, we review 10 key articles from the perioperative literature, with the goal of summarizing the most clinically important evidence over the past year. This summary of recent literature in perioperative medicine is derived from the Update in Perioperative Medicine sessions presented at the 10th Annual Perioperative Medicine Summit and the Society of General Internal Medicine 38th Annual Meeting. A systematic search strategy was used to identify pertinent articles, and the following were selected by the authors based on their relevance to the clinical practice of perioperative medicine.
PERIOPERATIVE CARDIOVASCULAR CARE
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:e278e333.
Background
The American College of Cardiology/American Heart Association (ACC/AHA) perioperative guideline provides recommendations for the evaluation and management of cardiovascular disease in patients undergoing noncardiac surgery.
Findings
The new guideline combines the evaluation of surgery‐ and patient‐specific risk in the algorithm for preoperative cardiovascular evaluation into a single step and recommends the use of 1 of 3 tools: the Revised Cardiac Risk Index (RCRI),[1] National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator,[2] or the NSQIP‐derived myocardial infarction and cardiac arrest calculator.[3] Estimation of risk is also simplified by stratification into only 2 groups: low risk (risk of major adverse cardiac event 1%) and elevated risk (1% risk). Coronary evaluation can be considered for patients with elevated cardiac risk and poor functional capacity, but is advised only if the results would alter perioperative management. For example, a patient with very high risk who has evidence of ischemia on stress testing may choose to forego surgery. Preoperative coronary revascularization is only indicated for patients meeting criteria in the nonsurgical setting.
For patients with previous percutaneous coronary intervention, the ACC/AHA has not changed its recommendations to optimally delay surgery for at least 30 days after bare‐metal stenting and at least 1 year after drug‐eluting stent (DES) placement. However, in patients with a DES placed 6 to 12 months previously, surgery can be performed if the risks of surgical delay outweigh the risks of DES thrombosis. After any type of coronary stenting, dual antiplatelet therapy should be continued uninterrupted through the first 4 to 6 weeks and even later whenever feasible. If not possible, aspirin therapy should be maintained through surgery unless bleeding risk is too high.
The guideline recommends perioperative continuation of ‐blockers in patients taking them chronically. Preoperative initiation of ‐blocker therapy may be considered for patients with myocardial ischemia on stress testing or 3 RCRI factors and should be started far enough in advance to allow determination of patient's tolerance prior to surgery.
Cautions
Many recommendations are based on data from nonrandomized trials or expert opinion, and the data in areas such as perioperative ‐blockade continue to evolve.
Implications
The ACC/AHA guideline continues to be a critically valuable resource for hospitalists providing perioperative care to noncardiac surgery patients.
Wijeysundera DN, Duncan D, Nkonde‐Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines.
J Am Coll Cardiol. 2014;64(22):24062425.
Background
Various clinical trials have reported conflicting results regarding the efficacy and safety of perioperative ‐blockers resulting in guideline committees changing their recommendations. Because of questions raised regarding the scientific integrity of the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography)‐I[4] and DECREASE‐IV[5] trials as well as the dosing of ‐blockers in POISE (PeriOperative Ischemic Evaluation) study,[6] this systematic review was performed in conjunction with the ACC/AHA guideline update[7] to evaluate the data with and without these trials.
Findings
Sixteen randomized control trials (RCTs) (n=12,043) and 1 cohort study (n=348) were included in the analysis. Perioperative ‐blockers were associated with a reduction in nonfatal myocardial infarction (MI) (relative risk [RR]: 0.69; 95% confidence interval [CI]: 0.58‐0.82; P0.001) but an increase in bradycardia (RR: 2.61; 95% CI: 2.18‐3.12), hypotension (RR: 1.47; 95% CI: 1.34‐1.6), and nonfatal strokes (RR: 1.76; 95% CI: 1.07‐2.91; P=0.02). The POISE trial was the only one demonstrating a statistically significant increase in stroke.
The major discrepancy between the DECREASE trials and the other RCTs was related to mortalitya reduction in both cardiovascular and all‐cause death in DECREASE but an increased risk of all‐cause death in the other trials.
Cautions
Because of its size, the POISE trial heavily influences the results, particularly for mortality and stroke. Including the DECREASE trials reduces the otherwise increased risk for death to a null effect. Exclusion of the POISE and DECREASE trials leaves few data to make conclusions about safety and efficacy of perioperative ‐blockade. Several cohort studies have found metoprolol to be associated with worse outcomes than with atenolol or bisoprolol (which were preferred by the European Society of Cardiology guidelines).[8]
Implications
Perioperative ‐blockade started within 1 day of noncardiac surgery was associated with fewer nonfatal MIs but at the cost of an increase in hypotension, bradycardia, and a possible increase in stroke and death. Long‐term ‐blockade should be continued perioperatively, whereas the decision to initiate a ‐blocker should be individualized. If starting a ‐blocker perioperatively, it should be done 2 days before surgery.
Botto F, Alonso‐Coello P, Chan MT, et al.; on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology. 2014;120(3):564578.
Background
Many patients sustain myocardial injury in the perioperative period as evidenced by troponin elevations, but most do not meet diagnostic criteria for MI. Myocardial injury after noncardiac surgery (MINS) is defined as prognostically relevant myocardial injury due to ischemia that occurs within 30 days after noncardiac surgery. This international, prospective cohort study of 15,065 patients 45 years old who underwent in‐patient noncardiac surgery determined diagnostic criteria, characteristics, predictors, and 30‐day outcomes of MINS.
Findings
The diagnostic criterion for MINS was a peak troponin T level 0.03 ng/mL judged to be due to an ischemic etiology. Twelve independent predictors of MINS were identified including age 75 years, known cardiovascular disease or risk factors, and surgical factors. MINS was an independent predictor of 30‐day mortality (adjusted hazard ratio [HR]: 3.87; 95% CI: 2.96‐5.08). Age >75 years, ST elevation, or new left bundle branch block, and anterior ischemic findings were independent predictors of 30‐day mortality among patients with MINS.
Cautions
Although screening high‐risk surgical patients without signs or symptoms of ischemia with postoperative troponins will increase the frequency of diagnosing MINS, evidence for an effective treatment has not yet been established. The ACC/AHA guidelines state that routine screening is of uncertain benefit for this reason.
Implications
Because MINS is common and carries a poor 30‐day prognosis, clinical trials are needed to determine when to obtain postoperative troponins and how to prevent and treat this complication.[9] Some observational data from POISE suggest that aspirin and statins can reduce the risk of 30‐day mortality in patients with postoperative MIs.
Devereaux PJ, Mrkobrada M, Sessler DI, et al. for the POISE‐2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):14941503.
Devereaux PJ, Sessler DI, Leslie K, et al. for the POISE‐2 Investigators. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):15041513.
Background
Medical risk reduction with aspirin and other agents in perioperative patients remains controversial. The POISE‐2 trial is a blinded RCT examining the effects of aspirin and clonidine on outcomes in >10,000 noncardiac surgery patients at risk of cardiovascular complications. The aspirin arm of the study included the initiation group and the continuation stratum, as well as placebo. Patients in the clonidine portion of the trial received 0.2 mg of clonidine or placebo daily for the same time periods.
Findings
The primary outcome was a composite of death or nonfatal MI within 30 days of surgery. Outcomes were similar in patients initiated or continued on aspirin. No difference was seen between aspirin or placebo in the primary outcome (7.0% vs 7.1%; HR: 0.86; 95% CI: 0.86‐1.15; P=0.92). There were no differences in rates of MI, venous thromboembolism, or stroke. Major bleeding rates were higher in aspirin versus placebo‐treated patients (4.6% vs 3.8%; HR: 1.23; 95% CI: 1.01‐1.49; P=0.04).
Clonidine did not alter the composite outcome of death or nonfatal MI (7.3% vs 6.8%; HR: 1.08; 95% CI: 0.93‐1.26; P=0.29). Clinically significant hypotension, bradycardia, and nonfatal cardiac arrest were more common in clonidine‐treated patients, although no difference was detected in stroke rates.
Cautions
Although patients in the trial had cardiovascular risk factors, 24% of patients had known coronary artery disease, and 5% had coronary stents. Conclusions based on this trial regarding perioperative management of antiplatelet therapy should not include patients with coronary artery stents.
Implications
Aspirin started before surgery and continued perioperatively did not decrease the rate of death or nonfatal MI but increased the risk of major bleeding. Perioperative management of aspirin needs to be undertaken in the context of cardiac and bleeding risks. Clonidine also did not improve outcomes and increased the risk of bradycardia and hypotension. Current guidelines recommend against using alpha‐2 agonists for prevention of perioperative cardiac events7; however, patients already on alpha‐2 agonists should not stop them abruptly.
PERIOPERATIVE PULMONARY CARE
Mutter TC, Chateau D, Moffatt M, et al. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology. 2014;121(4):707718.
Background
An increasing body of literature associates obstructive sleep apnea (OSA) with an increased risk of postoperative complications. Despite evidence of risk, potential benefits of preoperative diagnosis and treatment of OSA remain unclear.
Findings
Using databases to identify patients prescribed continuous positive airway pressure (CPAP) therapy, the study compared postoperative outcomes of patients who underwent surgery any time after polysomnography (PSG) and CPAP prescription (diagnosed OSA [DOSA]) and those who had surgery during the 5 years preceding their PSG (undiagnosed OSA [UOSA]). These patients were matched with patients who underwent the same procedure for the same indication and had no insurance claims for PSG or diagnosis of sleep‐disordered breathing.
After multivariate analysis, OSA of any type was associated with increased pulmonary complications (odds ratio [OR]: 2.08; 95% CI: 1.35‐2.19). However, no significant differences in respiratory outcomes were noted between DOSA patients (N=2640) and those with UOSA (N=1571). DOSA patients did have fewer cardiovascular complications than UOSA patients (OR: 0.34; 95% CI: 0.15‐0.77). Only severe OSA (apnea‐hypopnea index >30) was associated with increased pulmonary and cardiovascular complications.
Cautions
Although this study suggests an association between preoperative diagnosis and treatment of OSA and reduced cardiovascular complications, the results are not definitive due to the inability to control for all confounding variables in a retrospective study utilizing an administrative database.
Implications
OSA is an important risk factor for postoperative complications, and this study suggests that preoperative treatment with CPAP is associated with reduced risk of cardiovascular complications, particularly in patients with severe OSA. Future controlled trials should focus on the risk‐reduction potential of preoperative diagnosis and treatment of OSA.
Mazo V, Sabat S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219231.
Background
In 2010, Canet et al. published a novel risk index, the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) index, to provide a quantitative estimate of the risk of postoperative pulmonary complications (PPCs).[10]
In the current report, Mazo and colleagues studied the ARISCAT index in a broader sample to characterize its accuracy in predicting PPC risk. The ARISCAT index is derived from clinical risk factors: (1) age, (2) preoperative oxygen saturation, (3) respiratory infection in the prior month, (4) anemia, (5) surgical site, (6) duration of surgery, and (7) emergency surgery, with varying weights based on the strength of the association in a multivariable analysis. This score can be calculated via addition of these weighted risk factors, with a score>45 equal to high risk for PPC.
Findings
Examining 5099 patients from 63 European hospitals, the authors definition of PPC included respiratory failure, pulmonary infection, pleural effusion, atelectasis, pneumothorax, bronchospasm, and aspiration pneumonitis. PPC rates were as follows: low risk (3.39%), intermediate risk (12.98%), and high risk (38.01%). The positive likelihood ratio for PPC among the highest risk group was 7.12. The C statistic for fit was 0.80. Observed PPC rates were higher than predicted for the low (3.39% vs 0.87%) and intermediate (12.98% vs 7.82%) risk groups.
Cautions
The calibration slopes were less than ideal in all subsamples, with the Western European sample performing better than the other geographic areas; suggesting that the coefficients on the ARISCAT index may benefit from recalibration to match specific populations.
Implications
This is the first major pulmonary risk index that has been externally validated. Its use of readily available clinical information, simplicity, and accuracy in estimating PPC risk make it an important addition to the toolkit during a preoperative evaluation.
PERIOPERATIVE ATRIAL FIBRILLATION/ANTICOAGULATION
Gialdini G, Nearing K, Bhave P, et al. Perioperative atrial fibrillation and the long term risk of ischemic stroke. JAMA. 2014;312(6):616622.
Background
New‐onset atrial fibrillation (AF) is the most common perioperative arrhythmia.[11] However, little is known regarding the long‐term risks of ischemic stroke in patients who develop perioperative AF. This retrospective cohort study examined adults with no preexisting history of AF, hospitalized for surgery, and discharged free of cerebrovascular disease between 2007 and 2011 (n=1,729,360).
Findings
Of the eligible patients, 1.43% (95% CI: 1.41%‐1.45%) developed perioperative AF, and 0.81% (95% CI: 0.79%‐0.82%) had a stroke up to 1 year after discharge. Perioperative AF was associated with subsequent stroke after both cardiac (HR: 1.3; 95% CI: 1.1‐1.6) and noncardiac surgery (HR: 2; 95% CI: 1.7‐2.3). The association with stroke was stronger for perioperative AF after noncardiac versus cardiac surgery (P0.001 for interaction).
Cautions
This is a retrospective cohort study, using claims data to identify AF and stroke. Data on duration of the perioperative AF episodes or use of antithrombotic therapies were not available.
Implications
The association found between perioperative AF and long‐term risk of ischemic stroke may suggest that perioperative AF, especially after noncardiac surgery, should be treated aggressively in terms of thromboembolic risk; however, further data will be required to validate this association.
Van Diepen S, Youngson E, Ezekowitz J, McAlister F. Which risk score best predicts perioperative outcomes in nonvalvular atrial fibrillation patients undergoing noncardiac surgery? Am Heart J. 2014;168(1):6067.
Background
Patients with nonvalvular AF (NVAF) are at increased risk for adverse perioperative outcomes after noncardiac surgery.[12] The RCRI is commonly used to predict perioperative cardiovascular events for all patients, including those with NVAF, though AF is not part of this risk assessment. The goal of this retrospective cohort study was to examine the prognostic utility of already existing NVAF risk indices, including the CHADS2 (Congestive heart failure, Hypertension, Age 75 years, Diabetes mellitus, prior stroke or transient ischemic attack), CHA2DS2‐VASc (Congestive heart failure; Hypertension; Age 75 years; Diabetes mellitus; Stroke, TIA, or thromboembolism [TE]; Vascular disease; Age 65 to 74 years; Sex category [female]), and R2CHADS2 (Renal dysfunction, Congestive heart failure, Hypertension, Age, Diabetes, Stroke/TIA) for perioperative outcomes in patients undergoing noncardiac surgery.
Findings
A population dataset of NVAF patients (n=32,160) who underwent noncardiac surgery was examined, with outcome measures including 30‐day mortality, stroke, TIA, or systemic embolism. The incidence of the 30‐day composite outcome was 4.2% and the C indices were 0.65 for the RCRI, 0.67 for CHADS2, 0.67 for CHA2DS2‐VASc, and 0.68 for R2CHADS2. The Net Reclassification Index (NRI), a measure evaluating the improvement in prediction performance gained by adding a marker to a set of baseline predictors, was calculated. All NVAF scores performed better than the RCRI for predicting mortality risk (NRI: 12.3%, 8.4%, and 13.3% respectively, all P0.01).
Cautions
Patients in the highest risk category by RCRI appear to have an unadjusted higher 30‐day mortality risk (8%) than that predicted by the other 3 scores (5%, 5.6%, and 5%), indicating that these risk scores should not completely supplant the RCRI for risk stratification in this population. In addition, the overall improvement in predictive capacity of the CHADS2, CHA2DS2‐VASc, and R2CHADS2, although superior to the RCRI, is modest.
Implications
These findings indicate that the preoperative risk stratification for patients with NVAF can be improved by utilizing the CHADS2, CHA2DS2‐VASc, or R2CHADS2 scores when undergoing noncardiac surgery. For patients with NVAF identified as high risk for adverse outcomes, this assessment can be integrated into the preoperative discussion on the risks/benefits of surgery.
Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Circulation. 2015;131:488494
Background
Oral anticoagulation (OAC) significantly reduces the risk of stroke in patients with AF. Many AF patients on long‐term anticoagulation undergo procedures requiring temporary interruption of OAC. Although guidelines have been published on when and how to initiate bridging therapy, they are based on observational data. Thus, it remains unclear which patients should receive bridging anticoagulation.
Findings
This is a US registry of outpatients with AF with temporary interruptions of OAC for a procedure. Of 7372 patients treated with OAC, 2803 overall interruption events occurred in 2200 patients (30%). Bridging anticoagulants were used in 24% (n=665). Bleeding events were more common in bridged than nonbridged patients (5.0% vs 1.3%; adjusted OR: 3.84; P0.0001). The overall composite end point of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days was significantly higher in patients receiving bridging (13% vs 6.3%; adjusted OR: 1.94; P=0.0001). This statistically significant increase in the composite outcome, which includes cardiovascular events, is most likely in part secondary to inclusion of bleeding events. The recently published BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial did not find a statistically significant difference in cardiovascular events between bridged and nonbridged patients.[13]
Cautions
Although patients who were bridged appear to have had more comorbidities and a higher mean CHADS2 score than patients who were not bridged, it is difficult to determine which population of patients may be high risk enough to warrant bridging, as indicated by current American College of Chest Physicians guidelines, as this was not evaluated in this study
Implications
The use of bridging anticoagulation was significantly associated with higher overall bleeding and adverse event rates. The BRIDGE trial also found that forgoing bridging anticoagulation decreased the risk of major bleeding in patients with AF and was noninferior to bridging for the prevention of arterial TE.[13]
- , , , et al. Derivation and prospective evaluation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842.
- , , , et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387.
- , , , et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789–1794.
- , , , et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- POISE Study Group, , , , et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
- , , , et al. American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137.
- , , , et al. 2014 ESC/ESA Guidelines on non‐cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non‐cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431.
- , , , et al. The long‐term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053–1063.
- , , , et al. ARISCAT Group: Prediction of postoperative pulmonary complications in a population‐based surgical cohort. Anesthesiology. 2010;113:1338–1350.
- , . Noncardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28(10 suppl):N145–N150.
- , , , et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major cardiac surgery. Am Heart J. 2012;164(6):918–924.
- , , , et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–833.
- , , , et al. Derivation and prospective evaluation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842.
- , , , et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387.
- , , , et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789–1794.
- , , , et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- POISE Study Group, , , , et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
- , , , et al. American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137.
- , , , et al. 2014 ESC/ESA Guidelines on non‐cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non‐cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431.
- , , , et al. The long‐term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053–1063.
- , , , et al. ARISCAT Group: Prediction of postoperative pulmonary complications in a population‐based surgical cohort. Anesthesiology. 2010;113:1338–1350.
- , . Noncardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28(10 suppl):N145–N150.
- , , , et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major cardiac surgery. Am Heart J. 2012;164(6):918–924.
- , , , et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–833.
Resuming anticoagulation after hemorrhage: A practical approach
If a patient receiving anticoagulant therapy suffers a bleeding event, the patient and physician must decide whether and how soon to restart the therapy, and with what agent.
Foremost on our minds tends to be the risk of another hemorrhage. Subtler to appreciate immediately after an event is the continued risk of thrombosis, often from the same medical condition that prompted anticoagulation therapy in the first place (Table 1).
Complicating the decision, there may be a rebound effect: some thrombotic events such as pulmonary embolism and atrial fibrillation-related stroke may be more likely to occur in the first weeks after stopping warfarin than during similar intervals in patients who have not been taking it.1–3 The same thing may happen with the newer, target-specific oral anticoagulants.4–6
Although we have evidence-based guidelines for initiating and managing anticoagulant therapy, ample data on adverse events, and protocols for reversing anticoagulation if bleeding occurs, we do not have clear guidelines on restarting anticoagulation after a hemorrhagic event.
In this article, we outline a practical framework for approaching this clinical dilemma. Used in conjunction with consideration of a patient’s values and preferences as well as input from experts, this framework can help clinicians guide their patients through this challenging clinical decision. It consists of five questions:
- Why is the patient on anticoagulation, and what is the risk of thromboembolism without it?
- What was the clinical impact of the hemorrhage, and what is the risk of rebleeding if anticoagulation is resumed?
- What additional patient factors should be taken into consideration?
- How long should we wait before restarting anticoagulation?
- Would a newer drug be a better choice?
BLEEDING OCCURS IN 2% TO 3% OF PATIENTS PER YEAR
Most of our information on anticoagulation is about vitamin K antagonists—principally warfarin, in use since the 1950s. Among patients taking warfarin outside of clinical trials, the risk of major bleeding is estimated at 2% to 3% per year.7
However, the target-specific oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa) and edoxaban (Savaysa) are being used more and more, and we include them in our discussion insofar as we have information on them. The rates of bleeding with these new drugs in clinical trials have been comparable to or lower than those with warfarin.8 Postmarketing surveillance is under way.
WHY IS THE PATIENT ON ANTICOAGULATION? WHAT IS THE RISK WITHOUT IT?
Common, evidence-based indications for anticoagulation are to prevent complications in patients with venous thromboembolism and to prevent stroke in patients with atrial fibrillation or a mechanical heart valve. Other uses, such as in heart failure and its sequelae, pulmonary hypertension, and splanchnic or hepatic vein thrombosis, have less robust evidence to support them.
When anticoagulation-related bleeding occurs, it is essential to review why the patient is taking the drug and the risk of thromboembolism without it. Some indications pose a higher risk of thromboembolism than others and so argue more strongly for continuing the treatment.
Douketis et al9 developed a risk-stratification scheme for perioperative thromboembolism. We have modified it by adding the CHA2DS2-VASc score (Table 2),9–11 and believe it can be used more widely.
High-risk indications
Conditions that pose a high risk of thrombosis almost always require restarting anticoagulation. Here, the most appropriate question nearly always is not if anticoagulation should be restarted, but when. Examples:
- A mechanical mitral valve
- Antiphospholipid antibody syndrome with recurrent thromboembolic events.
Lower-risk indications
Lower-risk indications allow more leeway in determining if anticoagulation should be resumed. The most straightforward cases fall well within established guidelines. Examples:
- Atrial fibrillation and a CHA2DS2-VASc score of 1. The 2014 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society10 suggest that patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of 1 have three options: an oral anticoagulant, aspirin, and no antithrombotic therapy. If such a patient on anticoagulant therapy subsequently experiences a major gastrointestinal hemorrhage requiring transfusion and intensive care and no definitively treatable source of bleeding is found on endoscopy, one can argue that the risks of continued anticoagulation (recurrent bleeding) now exceed the benefits and that the patient would be better served by aspirin or even no antithrombotic therapy.
- After 6 months of anticoagulation for unprovoked deep vein thrombosis. Several studies showed that aspirin reduced the risk of recurrent venous thromboembolism in patients who completed an initial 6-month course of anticoagulation.12–15 Though these studies did not specifically compare aspirin with warfarin or target-specific oral anticoagulants in preventing recurrent venous thromboembolism after a hemorrhage, it is reasonable to extrapolate their results to this situation.
If the risk of recurrent hemorrhage on anticoagulation is considered to be too great, then aspirin is an alternative to no anticoagulation, as it reduces the risk of recurrent venous thromboembolism.16 However, we advise caution if the bleeding lesion may be specifically exacerbated by aspirin, particularly upper gastrointestinal ulcers.
Moderate-risk indications
- After a partial course of anticoagulation for provoked venous thromboembolism. Suppose a patient in the 10th week of a planned 12-week course of anticoagulation for a surgically provoked, first deep vein thrombosis presents with abdominal pain and is found to have a retroperitoneal hematoma. In light of the risk of recurrent bleeding vs the benefit of resuming anticoagulation for the limited remaining period, her 12-week treatment course can reasonably be shortened to 10 weeks.
The risk of recurrent venous thromboembolism when a patient is off anticoagulation decreases with time from the initial event. The highest risk, estimated at 0.3% to 1.3% per day, is in the first 4 weeks, falling to 0.03% to 0.2% per day in weeks 5 through 12, and 0.05% per day thereafter.17–20
Additionally, a pooled analysis of seven randomized trials suggests that patients with isolated, distal deep vein thrombosis provoked by a temporary risk factor did not have a high risk of recurrence after being treated for 4 to 6 weeks.21 These analyses are based on vitamin K antagonists, though it seems reasonable to extrapolate this information to the target-specific oral anticoagulants.
More challenging are situations in which the evidence supporting the initial or continued need for anticoagulation is less robust, such as in heart failure, pulmonary hypertension, or splanchnic and hepatic vein thrombosis. In these cases, the lack of strong evidence supporting the use of anticoagulation should make us hesitate to resume it after bleeding.
WHAT WAS THE CLINICAL IMPACT? WHAT IS THE RISK OF REBLEEDING?
Different groups have defined major and minor bleeding in different ways.22,23 Several have proposed criteria to standardize how bleeding events (on warfarin and otherwise) are classified,23–25 but the definitions differ.
Specifically, all agree that a “major” bleeding event is one that is fatal, involves bleeding into a major organ, or leads to a substantial decline in hemoglobin level. However, the Thrombolysis in Myocardial Infarction trials use a decline of more than 5 g/dL in their definition,23,25 while the International Society on Thrombosis and Haemostasis uses 2 g/dL.24
Here, we review the clinical impact of the most common sources of anticoagulation-related hemorrhage—gastrointestinal, soft tissue, and urinary tract26—as well as intracerebral hemorrhage, a less common but more uniformly devastating event.27
Clinical impact of gastrointestinal hemorrhage
Each year, about 4.5% of patients taking warfarin have a gastrointestinal hemorrhage, though not all of these events are major.28 Evolving data suggest that the newer agents (particularly dabigatran, rivaroxaban, and edoxaban) pose a higher risk of gastrointestinal bleeding than warfarin.29 Patients may need plasma and blood transfusions and intravenous phytonadione, all of which carry risks, albeit small.
Frequently, endoscopy is needed to find the source of bleeding and to control it. If this does not work, angiographic intervention to infuse vasoconstrictors or embolic coils into the culprit artery may be required, and some patients need surgery. Each intervention carries its own risk.
Clinical impact of soft-tissue hemorrhage
Soft-tissue hemorrhage accounts for more than 20% of warfarin-related bleeding events26; as yet, we know of no data on the rate with the new drugs. Soft-tissue hemorrhage is often localized to the large muscles of the retroperitoneum and legs. Though retroperitoneal hemorrhage accounts for a relatively small portion of soft-tissue hemorrhages, it is associated with high rates of morbidity and death and will therefore be our focus.26
Much of the clinical impact of retroperitoneal hemorrhage is from a mass effect that causes abdominal compartment syndrome, hydroureter, ileus, abscess formation, and acute and chronic pain. At least 20% of cases are associated with femoral neuropathy. It can also lead to deep vein thrombosis from venous compression, coupled with hypercoagulability in response to bleeding. Brisk bleeding can lead to shock and death, and the mortality rate in retroperitoneal hemorrhage is estimated at 20% or higher.30
In many cases, the retroperitoneal hemorrhage will self-tamponade and the blood will be reabsorbed once the bleeding has stopped, but uncontrolled bleeding may require surgical or angiographic intervention.30
Clinical impact of urinary tract hemorrhage
Gross or microscopic hematuria can be found in an estimated 2% to 24% of patients taking warfarin31–33; data are lacking for the target-specific oral anticoagulants. Interventions required to manage urinary tract bleeding include bladder irrigation and, less often, transfusion.31 Since a significant number of cases of hematuria are due to neoplastic disease,32 a diagnostic workup with radiographic imaging of the upper tract and cystoscopy of the lower tract is usually required.31 While life-threatening hemorrhage is uncommon, complications such as transient urinary obstruction from clots may occur.
Clinical impact of intracranial hemorrhage
Intracranial hemorrhage is the most feared and deadly of the bleeding complications of anticoagulation. The incidence in patients on warfarin is estimated at 2% to 3% per year, which is markedly higher than the estimated incidence of 25 per 100,000 person-years in the general population.34 Emerging data indicate that the newer drugs are also associated with a risk of intracranial hemorrhage, though the risk is about half that with vitamin K antagonists.35 Intracranial hemorrhage leads to death or disability in 76% of cases, compared with 3% of cases of bleeding from the gastrointestinal or urinary tract.27
Regardless of the source of bleeding, hospitalization is likely to be required and may be prolonged, with attendant risks of nosocomial harms such as infection.
Risk of rebleeding
Given the scope and severity of anticoagulation-related bleeding, there is strong interest in predicting and preventing it. By some estimates, the incidence of recurrent bleeding after resuming vitamin K antagonists is 8% to 13%.22 Although there are several indices for predicting the risk of major bleeding when starting anticoagulation, there are currently no validated tools to estimate a patient’s risk of rebleeding.36
The patient factor that most consistently predicts major bleeding is a history of bleeding, particularly from the gastrointestinal tract. Finding and controlling the source of bleeding is important.26,37 For example, a patient with gross hematuria who is found on cystoscopy to have a urothelial papilloma is unlikely to have rebleeding if the tumor is successfully resected and serial follow-up shows no regrowth. In contrast, consider a patient with a major gastrointestinal hemorrhage, the source of which remains elusive after upper, lower, and capsule endoscopy or, alternatively, is suspected to be from one of multiple angiodysplastic lesions. Without definitive source management, this patient faces a high risk of rebleeding.
With or without anticoagulation, after a first intracranial hemorrhage the risk of another one is estimated at 2% to 4% per year.34 An observational study found a recurrence rate of 7.5% when vitamin K antagonist therapy was started after an intracranial hemorrhage (though not all patients were on a vitamin K antagonist at the time of the first hemorrhage).38
Patients with lobar hemorrhage and those with suspected cerebral amyloid angiopathy may be at particularly high risk if anticoagulation is resumed. Conversely, initial events attributed to uncontrolled hypertension that subsequently can be well controlled may portend a lower risk of rebleeding.34 For other types of intracranial hemorrhage, recurrence rates can be even higher. Irrespective of anticoagulation, one prospective study estimated the crude annual rebleeding rate with untreated arteriovenous malformations to be 7%.39 In chronic subdural hematoma, the recurrence rate after initial drainage has been estimated at 9.2% to 26.5%, with use of anticoagulants (in this case, vitamin K antagonists) being an independent predictor of recurrence.40
WHAT OTHER PATIENT FACTORS NEED CONSIDERATION?
Target INR on warfarin
An important factor influencing the risk of bleeding with warfarin is the intensity of this therapy.37 A meta-analysis41 found that the risks of major hemorrhage and thromboembolism are minimized if the goal international normalized ratio (INR) is 2.0 to 3.0. When considering resuming anticoagulation after bleeding, make sure the therapeutic target is appropriate.37
Table 3 summarizes recommended therapeutic ranges for frequently encountered indications for warfarin.36,42,43
INR at time of the event and challenges in controlling it
The decision to resume anticoagulation in patients who bled while using warfarin must take into account the actual INR at the time of the event.
For example, consider a patient whose INR values are consistently in the therapeutic range. While on vacation, he receives ciprofloxacin for acute prostatitis from an urgent care team, and no adjustment to INR monitoring or warfarin dose is made. Several days later, he presents with lower gastrointestinal bleeding. His INR is 8, and colonoscopy reveals diverticulosis with a bleeding vessel, responsive to endoscopic therapy. After controlling the source of bleeding and reinforcing the need to always review new medications for potential interactions with anticoagulation, it is reasonable to expect that he once again will be able to keep his INR in the therapeutic range.
A patient on anticoagulation for the same indication but who has a history of repeated supratherapeutic levels, poor adherence, or poor access to INR monitoring poses very different concerns about resuming anticoagulation (as well as which agent to use, as we discuss below).
Of note, a high INR alone does not explain bleeding. It is estimated that a workup for gastrointestinal bleeding and gross hematuria uncovers previously undetected lesions in approximately one-third of cases involving warfarin.26 A similar malignancy-unmasking effect is now recognized in patients using the target-specific oral agents who experience gastrointestinal bleeding.44 Accordingly, we recommend a comprehensive source evaluation for any anticoagulation-related hemorrhage.
Comorbid conditions
Comorbid conditions associated with bleeding include cancer, end-stage renal disease, liver disease, arterial hypertension, prior stroke, and alcohol abuse.37,45 Gait instability, regardless of cause, may also increase the risk of trauma-related hemorrhage, but some have estimated that a patient would need to fall multiple times per week to contraindicate anticoagulation on the basis of falls alone.46
Concurrent medications
Concomitant therapies, including antiplatelet drugs and nonsteroidal anti-inflammatory drugs, increase bleeding risk.47,48 Aspirin and the nonsteroidals, in addition to having antiplatelet effects, also can cause gastric erosion.37 In evaluating whether and when to restart anticoagulation, it is advisable to review the role that concomitant therapies may have had in the index bleeding event and to evaluate the risks and benefits of these other agents.
Additionally, warfarin has many interactions. Although the newer drugs are lauded for having fewer interactions, they are not completely free of them, and the potential for interactions must always be reviewed.49 Further, unlike warfarin therapy, therapy with the newer agents is not routinely monitored with laboratory tests, so toxicity (or underdosing) may not be recognized until an adverse clinical event occurs. Ultimately, it may be safer to resume anticoagulation after a contributing drug can be safely discontinued.
Advanced age
The influence that the patient’s age should have on the decision to restart anticoagulation is unclear. Although the risk of intracranial hemorrhage increases with age, particularly after age 80, limited data exist in this population, particularly with regard to rebleeding. Further, age is a major risk factor for most thrombotic events, including venous thromboembolism and stroke from atrial fibrillation, so although the risks of anticoagulation may be higher, the benefits may also be higher than in younger patients.37,46 We discourage using age alone as a reason to withhold anticoagulation after a hemorrhage.
HOW LONG SHOULD WE WAIT TO RESTART ANTICOAGULATION?
We lack conclusive data on how long to wait to restart anticoagulation after an anticoagulation-associated hemorrhage.
The decision is complicated by evidence suggesting a rebound effect, with an increased risk of pulmonary embolism and atrial fibrillation-related stroke during the first 90 days of interruption of therapy with warfarin as well as with target-specific oral anticoagulants.3–8 In anticoagulation-associated retroperitoneal bleeding, there is increased risk of deep vein thrombosis from compression, even if venous thromboembolism was not the initial indication for anticoagulation.30
In patients with intracranial hemorrhage, evidence suggests that the intracranial hemorrhage itself increases the risk of arterial and venous thromboembolic events. Irrespective of whether a patient was previously on anticoagulation, the risk of arterial and venous thromboembolic events approaches 7% during the initial intracranial hemorrhage-related hospitalization and 9% during the first 90 days.34,50,51
To date, the only information we have about when to resume anticoagulation comes from patients taking vitamin K antagonists.
Timing after gastrointestinal bleeding
Small case series suggest that in the first 2 months after warfarin-associated gastrointestinal bleeding, there is substantial risk of rebleeding when anticoagulation is resumed—and of thrombosis when it is not.52,53 Two retrospective cohort studies may provide some guidance in this dilemma.28,54
Witt et al28 followed 442 patients who presented with gastrointestinal bleeding from any site during warfarin therapy for varied indications for up to 90 days after the index bleeding event. The risk of death was three times lower in patients who restarted warfarin than in those who did not, and their rate of thrombotic events was 10 times lower. The risk of recurrent gastrointestinal bleeding was statistically insignificant, and there were no fatal bleeding events. Anticoagulant therapy was generally resumed within 1 week of the bleeding event, at a median of 4 days.28,55
Qureshi et al54 performed a retrospective cohort study of 1,329 patients with nonvalvular atrial fibrillation who had experienced a gastrointestinal hemorrhage while taking warfarin. They found that resuming warfarin after 7 days was not associated with a higher risk of recurrent gastrointestinal bleeding and that the rates of death and thromboembolism were lower than in patients who resumed warfarin after 30 days. On the other hand, the risk of recurrent gastrointestinal bleeding was significantly greater if therapy was resumed within the first week.
In view of these studies, we believe that most patients should resume anticoagulation after 4 to 7 days of interruption after gastrointestinal bleeding.55
Timing after soft-tissue hemorrhage
The literature on resuming anticoagulation after soft-tissue hemorrhage is sparse. A retrospective study52 looked at this question in patients with spontaneous rectal sheath hematoma who had been receiving antiplatelet drugs, intravenous heparin, vitamin K antagonists, or a combination of these, but not target-specific agents. More than half of the patients were on vitamin K antagonists at the time of hemorrhage. Analysis suggested that when benefits of resuming anticoagulation are believed to outweigh risks, it is reasonable to resume anticoagulation 4 days after the index event.56
Timing after intracranial hemorrhage
Anticoagulation should not be considered within the first 24 hours after intracranial hemorrhage, as over 70% of patients develop some amount of hematoma expansion during this time.34,57 The period thereafter poses a challenge, as the risk of hematoma expansion decreases while the risk of arterial and venous thromboembolism is ongoing and cumulative.50
Perhaps surprisingly, national guidelines suggest starting prophylactic-dosed anticoagulation early in all intracranial hemorrhage patients, including those not previously on warfarin.58,59 In a randomized trial, Boeer et al60 concluded that starting low-dose subcutaneous heparin the day after an intracranial hemorrhage decreased the risk of thromboembolism without increasing the risk of rebleeding.60 Dickmann et al61 similarly concluded that there was no increased risk of rebleeding with early prophylactic-dosed subcutaneous heparin.61 Optimal mechanical thromboprophylaxis, including graduated compression stockings and intermittent pneumatic compression stockings, is also encouraged.34
Expert opinion remains divided on when and if anticoagulants should be resumed.34,62 The American Heart Association suggests that in nonvalvular atrial fibrillation, long-term anticoagulation should be avoided after spontaneous lobar hemorrhage; antiplatelet agents can be considered instead.58 In nonlobar hemorrhage, the American Heart Association suggests that anticoagulation be considered, depending on strength of indication, 7 to 10 days after the onset.58 The European Stroke Initiative suggests patients with strong indications for anticoagulation be restarted on warfarin 10 to 14 days after the event, depending on the risk of thromboembolism and recurrent intracranial hemorrhage.59 Others suggest delaying resumption to 10 to 30 weeks after an index intracranial hemorrhage.63
Overall, in the immediate acute period of intracranial hemorrhage, most patients will likely benefit from acute reversal of anticoagulation, followed by institution of prophylactic-dose anticoagulation after the first 24 hours. Going forward, patients who remain at higher risk of a recurrence of anticoagulant-related intracranial hemorrhage (such as those with lobar hemorrhage, suspected cerebral amyloid angiopathy, and other high-risk factors) than of thromboembolic events may be best managed without anticoagulants. Alternatively, patients with deep hemispheric intracranial hemorrhage, hypertension that can be well controlled, and a high risk of serious thromboembolism may experience net benefit from restarting anticoagulation.34
We recommend considering restarting anticoagulation 7 days after the onset of intracranial hemorrhage in patients at high risk of thromboembolism and after at least 14 days for patients at lower risk (Table 2). Discussions with neurologic and neurosurgical consultants should also inform this timing decision.
WOULD A NEWER DRUG BE A BETTER CHOICE?
The emergence of target-specific oral anticoagulants, including factor Xa inhibitors such as rivaroxaban, apixaban, and edoxaban and the direct thrombin inhibitor dabigatran etexilate, presents further challenges in managing anticoagulation after hemorrhage. Table 4 summarizes the current FDA-approved indications.64–67
These newer agents are attractive because, compared with warfarin, they have wider therapeutic windows, faster onset and offset of action, and fewer drug and food interactions.68 A meta-analysis of data available to date suggests that the new drugs, compared with warfarin, show a favorable risk-benefit profile with reductions in stroke, intracranial hemorrhage, and mortality with similar overall major bleeding rates, except for a possible increase in gastrointestinal bleeding.68
However, when managing anticoagulation after a bleeding event, the newer agents are challenging for two reasons: they may be associated with a higher incidence of gastrointestinal bleeding than warfarin, and they lack the typical reversal agents that can be used to manage an acute bleeding event.68,69
In individual studies comparing warfarin with dabigatran,70 rivaroxaban,71 apixaban,72 or edoxaban73 for stroke prevention in patients with atrial fibrillation, there was no significant difference in the rate of major bleeding between dabigatran in its higher dose (150 mg twice a day) or rivaroxaban compared with warfarin.70,71 The risk of major bleeding was actually lower with apixaban72 and edoxaban.73
In regard to specific types of major bleeding, the rate of intracranial hemorrhage was significantly lower with dabigatran, rivaroxaban, apixaban, and edoxaban than with warfarin.35,68–73 Some have proposed that since the brain is high in tissue factor, inhibition of tissue factor-factor VIIa complexes by vitamin K antagonists leaves the brain vulnerable to hemorrhage. Others suggest that the targeted mechanism of target-specific agents, as opposed to the multiple pathways in both the intrinsic and extrinsic coagulation cascade that vitamin K antagonists affect, may explain this difference.35,74,75
However, some studies suggest that rivaroxaban and the higher doses of dabigatran and edoxaban are associated with higher rates of major gastrointestinal bleeding compared with warfarin.69–71,76 But apixaban demonstrated no significant difference in gastrointestinal bleeding, and instead demonstrated rates of gastrointestinal bleeding comparable to that with aspirin for stroke prevention in atrial fibrillation.72
The new oral anticoagulants lack antidotes or reversal agents such as phytonadione and fresh-frozen plasma that are available to manage warfarin-associated bleeding events. Other proposed reversal options for the new agents include activated charcoal (if the drugs were taken recently enough to remain in the gastrointestinal tract) and concentrated clotting factor product, though research is ongoing in regards to the most appropriate use in clinical practice.37,69 Unlike rivaroxaban and apixaban, dabigatran has low plasma protein binding and is dialyzable, which provides another strategy in managing dabigatran-related bleeding.69
Of note, the above bleeding risk calculations relate to the first anticoagulant-related bleeding event, though presumably the same risk comparison across agents may be applicable to rebleeding events. Given the data above, when anticoagulation is to be resumed after an intracranial hemorrhage, the risk of rebleeding, particularly in the form of recurrent intracranial hemorrhage, may be lower if a target-specific oral anticoagulant is used.75 Similarly, when anticoagulation is to be resumed after a gastrointestinal bleeding event, reinitiation with warfarin or apixaban therapy may present the lowest risk of recurrent gastrointestinal rebleeding. In other sources of bleeding, such as retroperitoneal bleeding, we suggest consideration of transitioning to warfarin, given the availability of reversal agents in the event of recurrent bleeding.
Other important drug-specific factors that must be noted when selecting an agent with which to resume anticoagulation after a hemorrhage include the following:
- In patients with significant renal impairment, the choice of agent will be limited to a vitamin K antagonist.77
- A meta-analysis of randomized clinical trials suggests that in the elderly (age 75 and older) target-specific oral anticoagulants did not cause excess bleeding and were associated with at least equal efficacy compared with vitamin K antagonists.78
- Target-specific oral anticoagulants may be beneficial in patients who have challenges in achieving INR targets, as evidence suggests that switching to them is associated with a reduction in bleeding for patients who struggle to maintain an appropriately therapeutic INR.68 On the other hand, if there is concern that a patient may occasionally miss doses of an anticoagulant, given the rapid onset and offset of action of target-specific agents compared with warfarin, a missed dose of a target-specific agent may result in faster dissolution of anticoagulant effect and increased risk of thrombotic events, and lapses in anticoagulation will not be identified by routine drug monitoring.6–8,75 As such, it is vital to have a frank discussion with any patient who has difficulty maintaining therapeutic INRs on warfarin treatment to make sure that he or she is not missing doses.
- If there is no clear and compelling reason to select a particular agent, cost considerations should be taken into account. We have included estimated 30-day pricing for the various agents in Table 4.
- Jaffer AK, Brotman DJ, Bash LD, Mahmood SK, Lott B, White RH. Variations in perioperative warfarin management: outcomes and practice patterns at nine hospitals. Am J Med 2010; 123:141–150.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- Raunsø J, Selmer C, Olesen JB, et al. Increased short-term risk of thrombo-embolism or death after interruption of warfarin treatment in patients with atrial fibrillation. Eur Heart J 2012; 33:1886–1892.
- Xarelto (rivaroxaban). Highlights of prescribing information. Jansen Pharmaceuticals, Inc. www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100. Accessed March 9, 2015.
- Pradaxa (dabigatran etexilate mesylate). Highlights of prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed March 9, 2015.
- Eliquis (apixaban). Highlights of prescribing information. Bristol-Myers Squibb Company. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed March 9, 2015.
- Schulman S, Beyth RJ, Kearon C, Levine MN; American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest 2008; 133(suppl 6):257S–298S.
- Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014; 123:1152–1158.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994; 89:635–641.
- Warkentin TE. Aspirin for dual prevention of venous and arterial thrombosis. N Engl J Med 2012; 367:2039–2041.
- Simes J, Becattini C, Agnelli G, et al; INSPIRE Study Investigators* (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the Prevention of Recurrent Venous Thromboembolism: The INSPIRE Collaboration. Circulation 2014; 130:1062–1071.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Wakefield TW, Obi AT, Henke PK. An aspirin a day to keep the clots away: can aspirin prevent recurrent thrombosis in extended treatment for venous thromboembolism? Circulation 2014; 130:1031–1033.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Coon WW, Willis PW 3rd. Recurrence of venous thromboembolism. Surgery 1973; 73:823–827.
- Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979; 301:855–858.
- Jaffer AK, Brotman DJ, Chukwumerije N. When patients on warfarin need surgery. Cleve Clin J Med 2003; 70:973–984.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis 2011; 31:419–423.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123:2736–2747.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Wiviott SD, Antman EM, Gibson CM, et al; TRITON-TIMI 38 Investigators. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006; 152:627–635.
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993; 95:315–328.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med 2012; 172:1484–1491.
- Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013; 145:105-112.e15.
- Loor G, Bassiouny H, Valentin C, Shao MY, Funaki B, Desai T. Local and systemic consequences of large retroperitoneal clot burdens. World J Surg 2009; 33:1618–1625.
- Satasivam P, Reeves F, Lin M, et al. The effect of oral anticoagulation on the prevalence and management of haematuria in a contemporary Australian patient cohort. BJU Int 2012; 110(suppl 4):80–84.
- Van Savage JG, Fried FA. Anticoagulant associated hematuria: a prospective study. J Urol 1995; 153:1594–1596.
- Mosley DH, Schatz IJ, Breneman GM, Keyes JW. Long-term anticoagulant therapy. Complications and control in a review of 978 cases. JAMA 1963; 186:914–916.
- Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med 2010; 77:791–799.
- Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 2014 Aug 14. [Epub ahead of print]
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Poli D, Antonucci E, Dentali F, et al; Italian Federation of Anticoagulation Clinics (FCSA). Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014; 82:1020–1026.
- Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke 2006; 37:1243–1247.
- Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012; 154:1541–1548.
- Oake N, Jennings A, Forster AJ, et al. Anticoagulation intensity and outcomes among patients prescribed oral anticoagulant therapy: a systematic review and meta-analysis. CMAJ 2008; 179:235–244.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation 2008; 118:e523–e661.
- Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014; 46:672–678.
- Khalid F, Qureshi W, Qureshi S, Alirhayim Z, Garikapati K, Patsias I. Impact of restarting warfarin therapy in renal disease anticoagulated patients with gastrointestinal hemorrhage. Ren Fail 2013; 35:1228–1235.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Davidson BL, Verheijen S, Lensing AW, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 2014; 174:947–953.
- Knijff-Dutmer EA, Schut GA, van de Laar MA. Concomitant coumarin-NSAID therapy and risk for bleeding. Ann Pharmacother 2003; 37:12–16.
- Heidbuchel H, Verhamme P, Alings M, et al; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013; 15:625–651.
- Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care 2009; 10:28–34.
- Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther 2008; 25:831–841.
- Ananthasubramaniam K, Beattie JN, Rosman HS, Jayam V, Borzak S. How safely and for how long can warfarin therapy be withheld in prosthetic heart valve patients hospitalized with a major hemorrhage? Chest 2001; 119:478–484.
- Lee JK, Kang HW, Kim SG, Kim JS, Jung HC. Risks related with withholding and resuming anticoagulation in patients with non-variceal upper gastrointestinal bleeding while on warfarin therapy. Int J Clin Pract 2012; 66:64–68.
- Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol 2014; 113:662–668.
- Brotman DJ, Jaffer AK. Resuming anticoagulation in the first week following gastrointestinal tract hemorrhage: should we adopt a 4-day rule? Arch Intern Med 2012; 172:1492–1493.
- Kunkala MR1, Kehl J, Zielinski MD. Spontaneous rectus sheath hematomas: when to restart anticoagulation? World J Surg 2013; 37:2555–2559.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke 2007; 38:2001–2023.
- Steiner T, Kaste M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 2006; 22:294–316. Erratum in: Cerebrovasc Dis 2006; 22:461.
- Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 1991; 54:466–467.
- Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral hemorrhage. Klin Wochenschr 1988; 66:1182–1183.
- Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc 2007; 82:82–92. Erratum in: Mayo Clin Proc 2007; 82:387.
- Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010; 41:2860–2866.
- US Food and Drug Administration. Drug Information. XARELTO (rivaroxaban) tablets, for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. ELIQUIS® (apixaban) tablets for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s009lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. PRADAXA® (dabigatran etexilate mesylate) capsules for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/022512s023lbl.pdf. Accessed March 9, 2015.
- New oral anticoagulants for acute venous thromboembolism. Med Lett Drugs Ther 2014; 56:3–4.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 2009; 108:1447–1452.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Loffredo L, Perri L, Violi F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: a meta-analysis of interventional trials. Dig Liver Dis 2015 Feb 7. pii: S1590-8658(15)00189-9. doi: 10.1016/j.dld.2015.01.159. [Epub ahead of print]
- Thachil J. The newer direct oral anticoagulants: a practical guide. Clin Med 2014; 14:165–175.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
If a patient receiving anticoagulant therapy suffers a bleeding event, the patient and physician must decide whether and how soon to restart the therapy, and with what agent.
Foremost on our minds tends to be the risk of another hemorrhage. Subtler to appreciate immediately after an event is the continued risk of thrombosis, often from the same medical condition that prompted anticoagulation therapy in the first place (Table 1).
Complicating the decision, there may be a rebound effect: some thrombotic events such as pulmonary embolism and atrial fibrillation-related stroke may be more likely to occur in the first weeks after stopping warfarin than during similar intervals in patients who have not been taking it.1–3 The same thing may happen with the newer, target-specific oral anticoagulants.4–6
Although we have evidence-based guidelines for initiating and managing anticoagulant therapy, ample data on adverse events, and protocols for reversing anticoagulation if bleeding occurs, we do not have clear guidelines on restarting anticoagulation after a hemorrhagic event.
In this article, we outline a practical framework for approaching this clinical dilemma. Used in conjunction with consideration of a patient’s values and preferences as well as input from experts, this framework can help clinicians guide their patients through this challenging clinical decision. It consists of five questions:
- Why is the patient on anticoagulation, and what is the risk of thromboembolism without it?
- What was the clinical impact of the hemorrhage, and what is the risk of rebleeding if anticoagulation is resumed?
- What additional patient factors should be taken into consideration?
- How long should we wait before restarting anticoagulation?
- Would a newer drug be a better choice?
BLEEDING OCCURS IN 2% TO 3% OF PATIENTS PER YEAR
Most of our information on anticoagulation is about vitamin K antagonists—principally warfarin, in use since the 1950s. Among patients taking warfarin outside of clinical trials, the risk of major bleeding is estimated at 2% to 3% per year.7
However, the target-specific oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa) and edoxaban (Savaysa) are being used more and more, and we include them in our discussion insofar as we have information on them. The rates of bleeding with these new drugs in clinical trials have been comparable to or lower than those with warfarin.8 Postmarketing surveillance is under way.
WHY IS THE PATIENT ON ANTICOAGULATION? WHAT IS THE RISK WITHOUT IT?
Common, evidence-based indications for anticoagulation are to prevent complications in patients with venous thromboembolism and to prevent stroke in patients with atrial fibrillation or a mechanical heart valve. Other uses, such as in heart failure and its sequelae, pulmonary hypertension, and splanchnic or hepatic vein thrombosis, have less robust evidence to support them.
When anticoagulation-related bleeding occurs, it is essential to review why the patient is taking the drug and the risk of thromboembolism without it. Some indications pose a higher risk of thromboembolism than others and so argue more strongly for continuing the treatment.
Douketis et al9 developed a risk-stratification scheme for perioperative thromboembolism. We have modified it by adding the CHA2DS2-VASc score (Table 2),9–11 and believe it can be used more widely.
High-risk indications
Conditions that pose a high risk of thrombosis almost always require restarting anticoagulation. Here, the most appropriate question nearly always is not if anticoagulation should be restarted, but when. Examples:
- A mechanical mitral valve
- Antiphospholipid antibody syndrome with recurrent thromboembolic events.
Lower-risk indications
Lower-risk indications allow more leeway in determining if anticoagulation should be resumed. The most straightforward cases fall well within established guidelines. Examples:
- Atrial fibrillation and a CHA2DS2-VASc score of 1. The 2014 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society10 suggest that patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of 1 have three options: an oral anticoagulant, aspirin, and no antithrombotic therapy. If such a patient on anticoagulant therapy subsequently experiences a major gastrointestinal hemorrhage requiring transfusion and intensive care and no definitively treatable source of bleeding is found on endoscopy, one can argue that the risks of continued anticoagulation (recurrent bleeding) now exceed the benefits and that the patient would be better served by aspirin or even no antithrombotic therapy.
- After 6 months of anticoagulation for unprovoked deep vein thrombosis. Several studies showed that aspirin reduced the risk of recurrent venous thromboembolism in patients who completed an initial 6-month course of anticoagulation.12–15 Though these studies did not specifically compare aspirin with warfarin or target-specific oral anticoagulants in preventing recurrent venous thromboembolism after a hemorrhage, it is reasonable to extrapolate their results to this situation.
If the risk of recurrent hemorrhage on anticoagulation is considered to be too great, then aspirin is an alternative to no anticoagulation, as it reduces the risk of recurrent venous thromboembolism.16 However, we advise caution if the bleeding lesion may be specifically exacerbated by aspirin, particularly upper gastrointestinal ulcers.
Moderate-risk indications
- After a partial course of anticoagulation for provoked venous thromboembolism. Suppose a patient in the 10th week of a planned 12-week course of anticoagulation for a surgically provoked, first deep vein thrombosis presents with abdominal pain and is found to have a retroperitoneal hematoma. In light of the risk of recurrent bleeding vs the benefit of resuming anticoagulation for the limited remaining period, her 12-week treatment course can reasonably be shortened to 10 weeks.
The risk of recurrent venous thromboembolism when a patient is off anticoagulation decreases with time from the initial event. The highest risk, estimated at 0.3% to 1.3% per day, is in the first 4 weeks, falling to 0.03% to 0.2% per day in weeks 5 through 12, and 0.05% per day thereafter.17–20
Additionally, a pooled analysis of seven randomized trials suggests that patients with isolated, distal deep vein thrombosis provoked by a temporary risk factor did not have a high risk of recurrence after being treated for 4 to 6 weeks.21 These analyses are based on vitamin K antagonists, though it seems reasonable to extrapolate this information to the target-specific oral anticoagulants.
More challenging are situations in which the evidence supporting the initial or continued need for anticoagulation is less robust, such as in heart failure, pulmonary hypertension, or splanchnic and hepatic vein thrombosis. In these cases, the lack of strong evidence supporting the use of anticoagulation should make us hesitate to resume it after bleeding.
WHAT WAS THE CLINICAL IMPACT? WHAT IS THE RISK OF REBLEEDING?
Different groups have defined major and minor bleeding in different ways.22,23 Several have proposed criteria to standardize how bleeding events (on warfarin and otherwise) are classified,23–25 but the definitions differ.
Specifically, all agree that a “major” bleeding event is one that is fatal, involves bleeding into a major organ, or leads to a substantial decline in hemoglobin level. However, the Thrombolysis in Myocardial Infarction trials use a decline of more than 5 g/dL in their definition,23,25 while the International Society on Thrombosis and Haemostasis uses 2 g/dL.24
Here, we review the clinical impact of the most common sources of anticoagulation-related hemorrhage—gastrointestinal, soft tissue, and urinary tract26—as well as intracerebral hemorrhage, a less common but more uniformly devastating event.27
Clinical impact of gastrointestinal hemorrhage
Each year, about 4.5% of patients taking warfarin have a gastrointestinal hemorrhage, though not all of these events are major.28 Evolving data suggest that the newer agents (particularly dabigatran, rivaroxaban, and edoxaban) pose a higher risk of gastrointestinal bleeding than warfarin.29 Patients may need plasma and blood transfusions and intravenous phytonadione, all of which carry risks, albeit small.
Frequently, endoscopy is needed to find the source of bleeding and to control it. If this does not work, angiographic intervention to infuse vasoconstrictors or embolic coils into the culprit artery may be required, and some patients need surgery. Each intervention carries its own risk.
Clinical impact of soft-tissue hemorrhage
Soft-tissue hemorrhage accounts for more than 20% of warfarin-related bleeding events26; as yet, we know of no data on the rate with the new drugs. Soft-tissue hemorrhage is often localized to the large muscles of the retroperitoneum and legs. Though retroperitoneal hemorrhage accounts for a relatively small portion of soft-tissue hemorrhages, it is associated with high rates of morbidity and death and will therefore be our focus.26
Much of the clinical impact of retroperitoneal hemorrhage is from a mass effect that causes abdominal compartment syndrome, hydroureter, ileus, abscess formation, and acute and chronic pain. At least 20% of cases are associated with femoral neuropathy. It can also lead to deep vein thrombosis from venous compression, coupled with hypercoagulability in response to bleeding. Brisk bleeding can lead to shock and death, and the mortality rate in retroperitoneal hemorrhage is estimated at 20% or higher.30
In many cases, the retroperitoneal hemorrhage will self-tamponade and the blood will be reabsorbed once the bleeding has stopped, but uncontrolled bleeding may require surgical or angiographic intervention.30
Clinical impact of urinary tract hemorrhage
Gross or microscopic hematuria can be found in an estimated 2% to 24% of patients taking warfarin31–33; data are lacking for the target-specific oral anticoagulants. Interventions required to manage urinary tract bleeding include bladder irrigation and, less often, transfusion.31 Since a significant number of cases of hematuria are due to neoplastic disease,32 a diagnostic workup with radiographic imaging of the upper tract and cystoscopy of the lower tract is usually required.31 While life-threatening hemorrhage is uncommon, complications such as transient urinary obstruction from clots may occur.
Clinical impact of intracranial hemorrhage
Intracranial hemorrhage is the most feared and deadly of the bleeding complications of anticoagulation. The incidence in patients on warfarin is estimated at 2% to 3% per year, which is markedly higher than the estimated incidence of 25 per 100,000 person-years in the general population.34 Emerging data indicate that the newer drugs are also associated with a risk of intracranial hemorrhage, though the risk is about half that with vitamin K antagonists.35 Intracranial hemorrhage leads to death or disability in 76% of cases, compared with 3% of cases of bleeding from the gastrointestinal or urinary tract.27
Regardless of the source of bleeding, hospitalization is likely to be required and may be prolonged, with attendant risks of nosocomial harms such as infection.
Risk of rebleeding
Given the scope and severity of anticoagulation-related bleeding, there is strong interest in predicting and preventing it. By some estimates, the incidence of recurrent bleeding after resuming vitamin K antagonists is 8% to 13%.22 Although there are several indices for predicting the risk of major bleeding when starting anticoagulation, there are currently no validated tools to estimate a patient’s risk of rebleeding.36
The patient factor that most consistently predicts major bleeding is a history of bleeding, particularly from the gastrointestinal tract. Finding and controlling the source of bleeding is important.26,37 For example, a patient with gross hematuria who is found on cystoscopy to have a urothelial papilloma is unlikely to have rebleeding if the tumor is successfully resected and serial follow-up shows no regrowth. In contrast, consider a patient with a major gastrointestinal hemorrhage, the source of which remains elusive after upper, lower, and capsule endoscopy or, alternatively, is suspected to be from one of multiple angiodysplastic lesions. Without definitive source management, this patient faces a high risk of rebleeding.
With or without anticoagulation, after a first intracranial hemorrhage the risk of another one is estimated at 2% to 4% per year.34 An observational study found a recurrence rate of 7.5% when vitamin K antagonist therapy was started after an intracranial hemorrhage (though not all patients were on a vitamin K antagonist at the time of the first hemorrhage).38
Patients with lobar hemorrhage and those with suspected cerebral amyloid angiopathy may be at particularly high risk if anticoagulation is resumed. Conversely, initial events attributed to uncontrolled hypertension that subsequently can be well controlled may portend a lower risk of rebleeding.34 For other types of intracranial hemorrhage, recurrence rates can be even higher. Irrespective of anticoagulation, one prospective study estimated the crude annual rebleeding rate with untreated arteriovenous malformations to be 7%.39 In chronic subdural hematoma, the recurrence rate after initial drainage has been estimated at 9.2% to 26.5%, with use of anticoagulants (in this case, vitamin K antagonists) being an independent predictor of recurrence.40
WHAT OTHER PATIENT FACTORS NEED CONSIDERATION?
Target INR on warfarin
An important factor influencing the risk of bleeding with warfarin is the intensity of this therapy.37 A meta-analysis41 found that the risks of major hemorrhage and thromboembolism are minimized if the goal international normalized ratio (INR) is 2.0 to 3.0. When considering resuming anticoagulation after bleeding, make sure the therapeutic target is appropriate.37
Table 3 summarizes recommended therapeutic ranges for frequently encountered indications for warfarin.36,42,43
INR at time of the event and challenges in controlling it
The decision to resume anticoagulation in patients who bled while using warfarin must take into account the actual INR at the time of the event.
For example, consider a patient whose INR values are consistently in the therapeutic range. While on vacation, he receives ciprofloxacin for acute prostatitis from an urgent care team, and no adjustment to INR monitoring or warfarin dose is made. Several days later, he presents with lower gastrointestinal bleeding. His INR is 8, and colonoscopy reveals diverticulosis with a bleeding vessel, responsive to endoscopic therapy. After controlling the source of bleeding and reinforcing the need to always review new medications for potential interactions with anticoagulation, it is reasonable to expect that he once again will be able to keep his INR in the therapeutic range.
A patient on anticoagulation for the same indication but who has a history of repeated supratherapeutic levels, poor adherence, or poor access to INR monitoring poses very different concerns about resuming anticoagulation (as well as which agent to use, as we discuss below).
Of note, a high INR alone does not explain bleeding. It is estimated that a workup for gastrointestinal bleeding and gross hematuria uncovers previously undetected lesions in approximately one-third of cases involving warfarin.26 A similar malignancy-unmasking effect is now recognized in patients using the target-specific oral agents who experience gastrointestinal bleeding.44 Accordingly, we recommend a comprehensive source evaluation for any anticoagulation-related hemorrhage.
Comorbid conditions
Comorbid conditions associated with bleeding include cancer, end-stage renal disease, liver disease, arterial hypertension, prior stroke, and alcohol abuse.37,45 Gait instability, regardless of cause, may also increase the risk of trauma-related hemorrhage, but some have estimated that a patient would need to fall multiple times per week to contraindicate anticoagulation on the basis of falls alone.46
Concurrent medications
Concomitant therapies, including antiplatelet drugs and nonsteroidal anti-inflammatory drugs, increase bleeding risk.47,48 Aspirin and the nonsteroidals, in addition to having antiplatelet effects, also can cause gastric erosion.37 In evaluating whether and when to restart anticoagulation, it is advisable to review the role that concomitant therapies may have had in the index bleeding event and to evaluate the risks and benefits of these other agents.
Additionally, warfarin has many interactions. Although the newer drugs are lauded for having fewer interactions, they are not completely free of them, and the potential for interactions must always be reviewed.49 Further, unlike warfarin therapy, therapy with the newer agents is not routinely monitored with laboratory tests, so toxicity (or underdosing) may not be recognized until an adverse clinical event occurs. Ultimately, it may be safer to resume anticoagulation after a contributing drug can be safely discontinued.
Advanced age
The influence that the patient’s age should have on the decision to restart anticoagulation is unclear. Although the risk of intracranial hemorrhage increases with age, particularly after age 80, limited data exist in this population, particularly with regard to rebleeding. Further, age is a major risk factor for most thrombotic events, including venous thromboembolism and stroke from atrial fibrillation, so although the risks of anticoagulation may be higher, the benefits may also be higher than in younger patients.37,46 We discourage using age alone as a reason to withhold anticoagulation after a hemorrhage.
HOW LONG SHOULD WE WAIT TO RESTART ANTICOAGULATION?
We lack conclusive data on how long to wait to restart anticoagulation after an anticoagulation-associated hemorrhage.
The decision is complicated by evidence suggesting a rebound effect, with an increased risk of pulmonary embolism and atrial fibrillation-related stroke during the first 90 days of interruption of therapy with warfarin as well as with target-specific oral anticoagulants.3–8 In anticoagulation-associated retroperitoneal bleeding, there is increased risk of deep vein thrombosis from compression, even if venous thromboembolism was not the initial indication for anticoagulation.30
In patients with intracranial hemorrhage, evidence suggests that the intracranial hemorrhage itself increases the risk of arterial and venous thromboembolic events. Irrespective of whether a patient was previously on anticoagulation, the risk of arterial and venous thromboembolic events approaches 7% during the initial intracranial hemorrhage-related hospitalization and 9% during the first 90 days.34,50,51
To date, the only information we have about when to resume anticoagulation comes from patients taking vitamin K antagonists.
Timing after gastrointestinal bleeding
Small case series suggest that in the first 2 months after warfarin-associated gastrointestinal bleeding, there is substantial risk of rebleeding when anticoagulation is resumed—and of thrombosis when it is not.52,53 Two retrospective cohort studies may provide some guidance in this dilemma.28,54
Witt et al28 followed 442 patients who presented with gastrointestinal bleeding from any site during warfarin therapy for varied indications for up to 90 days after the index bleeding event. The risk of death was three times lower in patients who restarted warfarin than in those who did not, and their rate of thrombotic events was 10 times lower. The risk of recurrent gastrointestinal bleeding was statistically insignificant, and there were no fatal bleeding events. Anticoagulant therapy was generally resumed within 1 week of the bleeding event, at a median of 4 days.28,55
Qureshi et al54 performed a retrospective cohort study of 1,329 patients with nonvalvular atrial fibrillation who had experienced a gastrointestinal hemorrhage while taking warfarin. They found that resuming warfarin after 7 days was not associated with a higher risk of recurrent gastrointestinal bleeding and that the rates of death and thromboembolism were lower than in patients who resumed warfarin after 30 days. On the other hand, the risk of recurrent gastrointestinal bleeding was significantly greater if therapy was resumed within the first week.
In view of these studies, we believe that most patients should resume anticoagulation after 4 to 7 days of interruption after gastrointestinal bleeding.55
Timing after soft-tissue hemorrhage
The literature on resuming anticoagulation after soft-tissue hemorrhage is sparse. A retrospective study52 looked at this question in patients with spontaneous rectal sheath hematoma who had been receiving antiplatelet drugs, intravenous heparin, vitamin K antagonists, or a combination of these, but not target-specific agents. More than half of the patients were on vitamin K antagonists at the time of hemorrhage. Analysis suggested that when benefits of resuming anticoagulation are believed to outweigh risks, it is reasonable to resume anticoagulation 4 days after the index event.56
Timing after intracranial hemorrhage
Anticoagulation should not be considered within the first 24 hours after intracranial hemorrhage, as over 70% of patients develop some amount of hematoma expansion during this time.34,57 The period thereafter poses a challenge, as the risk of hematoma expansion decreases while the risk of arterial and venous thromboembolism is ongoing and cumulative.50
Perhaps surprisingly, national guidelines suggest starting prophylactic-dosed anticoagulation early in all intracranial hemorrhage patients, including those not previously on warfarin.58,59 In a randomized trial, Boeer et al60 concluded that starting low-dose subcutaneous heparin the day after an intracranial hemorrhage decreased the risk of thromboembolism without increasing the risk of rebleeding.60 Dickmann et al61 similarly concluded that there was no increased risk of rebleeding with early prophylactic-dosed subcutaneous heparin.61 Optimal mechanical thromboprophylaxis, including graduated compression stockings and intermittent pneumatic compression stockings, is also encouraged.34
Expert opinion remains divided on when and if anticoagulants should be resumed.34,62 The American Heart Association suggests that in nonvalvular atrial fibrillation, long-term anticoagulation should be avoided after spontaneous lobar hemorrhage; antiplatelet agents can be considered instead.58 In nonlobar hemorrhage, the American Heart Association suggests that anticoagulation be considered, depending on strength of indication, 7 to 10 days after the onset.58 The European Stroke Initiative suggests patients with strong indications for anticoagulation be restarted on warfarin 10 to 14 days after the event, depending on the risk of thromboembolism and recurrent intracranial hemorrhage.59 Others suggest delaying resumption to 10 to 30 weeks after an index intracranial hemorrhage.63
Overall, in the immediate acute period of intracranial hemorrhage, most patients will likely benefit from acute reversal of anticoagulation, followed by institution of prophylactic-dose anticoagulation after the first 24 hours. Going forward, patients who remain at higher risk of a recurrence of anticoagulant-related intracranial hemorrhage (such as those with lobar hemorrhage, suspected cerebral amyloid angiopathy, and other high-risk factors) than of thromboembolic events may be best managed without anticoagulants. Alternatively, patients with deep hemispheric intracranial hemorrhage, hypertension that can be well controlled, and a high risk of serious thromboembolism may experience net benefit from restarting anticoagulation.34
We recommend considering restarting anticoagulation 7 days after the onset of intracranial hemorrhage in patients at high risk of thromboembolism and after at least 14 days for patients at lower risk (Table 2). Discussions with neurologic and neurosurgical consultants should also inform this timing decision.
WOULD A NEWER DRUG BE A BETTER CHOICE?
The emergence of target-specific oral anticoagulants, including factor Xa inhibitors such as rivaroxaban, apixaban, and edoxaban and the direct thrombin inhibitor dabigatran etexilate, presents further challenges in managing anticoagulation after hemorrhage. Table 4 summarizes the current FDA-approved indications.64–67
These newer agents are attractive because, compared with warfarin, they have wider therapeutic windows, faster onset and offset of action, and fewer drug and food interactions.68 A meta-analysis of data available to date suggests that the new drugs, compared with warfarin, show a favorable risk-benefit profile with reductions in stroke, intracranial hemorrhage, and mortality with similar overall major bleeding rates, except for a possible increase in gastrointestinal bleeding.68
However, when managing anticoagulation after a bleeding event, the newer agents are challenging for two reasons: they may be associated with a higher incidence of gastrointestinal bleeding than warfarin, and they lack the typical reversal agents that can be used to manage an acute bleeding event.68,69
In individual studies comparing warfarin with dabigatran,70 rivaroxaban,71 apixaban,72 or edoxaban73 for stroke prevention in patients with atrial fibrillation, there was no significant difference in the rate of major bleeding between dabigatran in its higher dose (150 mg twice a day) or rivaroxaban compared with warfarin.70,71 The risk of major bleeding was actually lower with apixaban72 and edoxaban.73
In regard to specific types of major bleeding, the rate of intracranial hemorrhage was significantly lower with dabigatran, rivaroxaban, apixaban, and edoxaban than with warfarin.35,68–73 Some have proposed that since the brain is high in tissue factor, inhibition of tissue factor-factor VIIa complexes by vitamin K antagonists leaves the brain vulnerable to hemorrhage. Others suggest that the targeted mechanism of target-specific agents, as opposed to the multiple pathways in both the intrinsic and extrinsic coagulation cascade that vitamin K antagonists affect, may explain this difference.35,74,75
However, some studies suggest that rivaroxaban and the higher doses of dabigatran and edoxaban are associated with higher rates of major gastrointestinal bleeding compared with warfarin.69–71,76 But apixaban demonstrated no significant difference in gastrointestinal bleeding, and instead demonstrated rates of gastrointestinal bleeding comparable to that with aspirin for stroke prevention in atrial fibrillation.72
The new oral anticoagulants lack antidotes or reversal agents such as phytonadione and fresh-frozen plasma that are available to manage warfarin-associated bleeding events. Other proposed reversal options for the new agents include activated charcoal (if the drugs were taken recently enough to remain in the gastrointestinal tract) and concentrated clotting factor product, though research is ongoing in regards to the most appropriate use in clinical practice.37,69 Unlike rivaroxaban and apixaban, dabigatran has low plasma protein binding and is dialyzable, which provides another strategy in managing dabigatran-related bleeding.69
Of note, the above bleeding risk calculations relate to the first anticoagulant-related bleeding event, though presumably the same risk comparison across agents may be applicable to rebleeding events. Given the data above, when anticoagulation is to be resumed after an intracranial hemorrhage, the risk of rebleeding, particularly in the form of recurrent intracranial hemorrhage, may be lower if a target-specific oral anticoagulant is used.75 Similarly, when anticoagulation is to be resumed after a gastrointestinal bleeding event, reinitiation with warfarin or apixaban therapy may present the lowest risk of recurrent gastrointestinal rebleeding. In other sources of bleeding, such as retroperitoneal bleeding, we suggest consideration of transitioning to warfarin, given the availability of reversal agents in the event of recurrent bleeding.
Other important drug-specific factors that must be noted when selecting an agent with which to resume anticoagulation after a hemorrhage include the following:
- In patients with significant renal impairment, the choice of agent will be limited to a vitamin K antagonist.77
- A meta-analysis of randomized clinical trials suggests that in the elderly (age 75 and older) target-specific oral anticoagulants did not cause excess bleeding and were associated with at least equal efficacy compared with vitamin K antagonists.78
- Target-specific oral anticoagulants may be beneficial in patients who have challenges in achieving INR targets, as evidence suggests that switching to them is associated with a reduction in bleeding for patients who struggle to maintain an appropriately therapeutic INR.68 On the other hand, if there is concern that a patient may occasionally miss doses of an anticoagulant, given the rapid onset and offset of action of target-specific agents compared with warfarin, a missed dose of a target-specific agent may result in faster dissolution of anticoagulant effect and increased risk of thrombotic events, and lapses in anticoagulation will not be identified by routine drug monitoring.6–8,75 As such, it is vital to have a frank discussion with any patient who has difficulty maintaining therapeutic INRs on warfarin treatment to make sure that he or she is not missing doses.
- If there is no clear and compelling reason to select a particular agent, cost considerations should be taken into account. We have included estimated 30-day pricing for the various agents in Table 4.
If a patient receiving anticoagulant therapy suffers a bleeding event, the patient and physician must decide whether and how soon to restart the therapy, and with what agent.
Foremost on our minds tends to be the risk of another hemorrhage. Subtler to appreciate immediately after an event is the continued risk of thrombosis, often from the same medical condition that prompted anticoagulation therapy in the first place (Table 1).
Complicating the decision, there may be a rebound effect: some thrombotic events such as pulmonary embolism and atrial fibrillation-related stroke may be more likely to occur in the first weeks after stopping warfarin than during similar intervals in patients who have not been taking it.1–3 The same thing may happen with the newer, target-specific oral anticoagulants.4–6
Although we have evidence-based guidelines for initiating and managing anticoagulant therapy, ample data on adverse events, and protocols for reversing anticoagulation if bleeding occurs, we do not have clear guidelines on restarting anticoagulation after a hemorrhagic event.
In this article, we outline a practical framework for approaching this clinical dilemma. Used in conjunction with consideration of a patient’s values and preferences as well as input from experts, this framework can help clinicians guide their patients through this challenging clinical decision. It consists of five questions:
- Why is the patient on anticoagulation, and what is the risk of thromboembolism without it?
- What was the clinical impact of the hemorrhage, and what is the risk of rebleeding if anticoagulation is resumed?
- What additional patient factors should be taken into consideration?
- How long should we wait before restarting anticoagulation?
- Would a newer drug be a better choice?
BLEEDING OCCURS IN 2% TO 3% OF PATIENTS PER YEAR
Most of our information on anticoagulation is about vitamin K antagonists—principally warfarin, in use since the 1950s. Among patients taking warfarin outside of clinical trials, the risk of major bleeding is estimated at 2% to 3% per year.7
However, the target-specific oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa) and edoxaban (Savaysa) are being used more and more, and we include them in our discussion insofar as we have information on them. The rates of bleeding with these new drugs in clinical trials have been comparable to or lower than those with warfarin.8 Postmarketing surveillance is under way.
WHY IS THE PATIENT ON ANTICOAGULATION? WHAT IS THE RISK WITHOUT IT?
Common, evidence-based indications for anticoagulation are to prevent complications in patients with venous thromboembolism and to prevent stroke in patients with atrial fibrillation or a mechanical heart valve. Other uses, such as in heart failure and its sequelae, pulmonary hypertension, and splanchnic or hepatic vein thrombosis, have less robust evidence to support them.
When anticoagulation-related bleeding occurs, it is essential to review why the patient is taking the drug and the risk of thromboembolism without it. Some indications pose a higher risk of thromboembolism than others and so argue more strongly for continuing the treatment.
Douketis et al9 developed a risk-stratification scheme for perioperative thromboembolism. We have modified it by adding the CHA2DS2-VASc score (Table 2),9–11 and believe it can be used more widely.
High-risk indications
Conditions that pose a high risk of thrombosis almost always require restarting anticoagulation. Here, the most appropriate question nearly always is not if anticoagulation should be restarted, but when. Examples:
- A mechanical mitral valve
- Antiphospholipid antibody syndrome with recurrent thromboembolic events.
Lower-risk indications
Lower-risk indications allow more leeway in determining if anticoagulation should be resumed. The most straightforward cases fall well within established guidelines. Examples:
- Atrial fibrillation and a CHA2DS2-VASc score of 1. The 2014 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society10 suggest that patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of 1 have three options: an oral anticoagulant, aspirin, and no antithrombotic therapy. If such a patient on anticoagulant therapy subsequently experiences a major gastrointestinal hemorrhage requiring transfusion and intensive care and no definitively treatable source of bleeding is found on endoscopy, one can argue that the risks of continued anticoagulation (recurrent bleeding) now exceed the benefits and that the patient would be better served by aspirin or even no antithrombotic therapy.
- After 6 months of anticoagulation for unprovoked deep vein thrombosis. Several studies showed that aspirin reduced the risk of recurrent venous thromboembolism in patients who completed an initial 6-month course of anticoagulation.12–15 Though these studies did not specifically compare aspirin with warfarin or target-specific oral anticoagulants in preventing recurrent venous thromboembolism after a hemorrhage, it is reasonable to extrapolate their results to this situation.
If the risk of recurrent hemorrhage on anticoagulation is considered to be too great, then aspirin is an alternative to no anticoagulation, as it reduces the risk of recurrent venous thromboembolism.16 However, we advise caution if the bleeding lesion may be specifically exacerbated by aspirin, particularly upper gastrointestinal ulcers.
Moderate-risk indications
- After a partial course of anticoagulation for provoked venous thromboembolism. Suppose a patient in the 10th week of a planned 12-week course of anticoagulation for a surgically provoked, first deep vein thrombosis presents with abdominal pain and is found to have a retroperitoneal hematoma. In light of the risk of recurrent bleeding vs the benefit of resuming anticoagulation for the limited remaining period, her 12-week treatment course can reasonably be shortened to 10 weeks.
The risk of recurrent venous thromboembolism when a patient is off anticoagulation decreases with time from the initial event. The highest risk, estimated at 0.3% to 1.3% per day, is in the first 4 weeks, falling to 0.03% to 0.2% per day in weeks 5 through 12, and 0.05% per day thereafter.17–20
Additionally, a pooled analysis of seven randomized trials suggests that patients with isolated, distal deep vein thrombosis provoked by a temporary risk factor did not have a high risk of recurrence after being treated for 4 to 6 weeks.21 These analyses are based on vitamin K antagonists, though it seems reasonable to extrapolate this information to the target-specific oral anticoagulants.
More challenging are situations in which the evidence supporting the initial or continued need for anticoagulation is less robust, such as in heart failure, pulmonary hypertension, or splanchnic and hepatic vein thrombosis. In these cases, the lack of strong evidence supporting the use of anticoagulation should make us hesitate to resume it after bleeding.
WHAT WAS THE CLINICAL IMPACT? WHAT IS THE RISK OF REBLEEDING?
Different groups have defined major and minor bleeding in different ways.22,23 Several have proposed criteria to standardize how bleeding events (on warfarin and otherwise) are classified,23–25 but the definitions differ.
Specifically, all agree that a “major” bleeding event is one that is fatal, involves bleeding into a major organ, or leads to a substantial decline in hemoglobin level. However, the Thrombolysis in Myocardial Infarction trials use a decline of more than 5 g/dL in their definition,23,25 while the International Society on Thrombosis and Haemostasis uses 2 g/dL.24
Here, we review the clinical impact of the most common sources of anticoagulation-related hemorrhage—gastrointestinal, soft tissue, and urinary tract26—as well as intracerebral hemorrhage, a less common but more uniformly devastating event.27
Clinical impact of gastrointestinal hemorrhage
Each year, about 4.5% of patients taking warfarin have a gastrointestinal hemorrhage, though not all of these events are major.28 Evolving data suggest that the newer agents (particularly dabigatran, rivaroxaban, and edoxaban) pose a higher risk of gastrointestinal bleeding than warfarin.29 Patients may need plasma and blood transfusions and intravenous phytonadione, all of which carry risks, albeit small.
Frequently, endoscopy is needed to find the source of bleeding and to control it. If this does not work, angiographic intervention to infuse vasoconstrictors or embolic coils into the culprit artery may be required, and some patients need surgery. Each intervention carries its own risk.
Clinical impact of soft-tissue hemorrhage
Soft-tissue hemorrhage accounts for more than 20% of warfarin-related bleeding events26; as yet, we know of no data on the rate with the new drugs. Soft-tissue hemorrhage is often localized to the large muscles of the retroperitoneum and legs. Though retroperitoneal hemorrhage accounts for a relatively small portion of soft-tissue hemorrhages, it is associated with high rates of morbidity and death and will therefore be our focus.26
Much of the clinical impact of retroperitoneal hemorrhage is from a mass effect that causes abdominal compartment syndrome, hydroureter, ileus, abscess formation, and acute and chronic pain. At least 20% of cases are associated with femoral neuropathy. It can also lead to deep vein thrombosis from venous compression, coupled with hypercoagulability in response to bleeding. Brisk bleeding can lead to shock and death, and the mortality rate in retroperitoneal hemorrhage is estimated at 20% or higher.30
In many cases, the retroperitoneal hemorrhage will self-tamponade and the blood will be reabsorbed once the bleeding has stopped, but uncontrolled bleeding may require surgical or angiographic intervention.30
Clinical impact of urinary tract hemorrhage
Gross or microscopic hematuria can be found in an estimated 2% to 24% of patients taking warfarin31–33; data are lacking for the target-specific oral anticoagulants. Interventions required to manage urinary tract bleeding include bladder irrigation and, less often, transfusion.31 Since a significant number of cases of hematuria are due to neoplastic disease,32 a diagnostic workup with radiographic imaging of the upper tract and cystoscopy of the lower tract is usually required.31 While life-threatening hemorrhage is uncommon, complications such as transient urinary obstruction from clots may occur.
Clinical impact of intracranial hemorrhage
Intracranial hemorrhage is the most feared and deadly of the bleeding complications of anticoagulation. The incidence in patients on warfarin is estimated at 2% to 3% per year, which is markedly higher than the estimated incidence of 25 per 100,000 person-years in the general population.34 Emerging data indicate that the newer drugs are also associated with a risk of intracranial hemorrhage, though the risk is about half that with vitamin K antagonists.35 Intracranial hemorrhage leads to death or disability in 76% of cases, compared with 3% of cases of bleeding from the gastrointestinal or urinary tract.27
Regardless of the source of bleeding, hospitalization is likely to be required and may be prolonged, with attendant risks of nosocomial harms such as infection.
Risk of rebleeding
Given the scope and severity of anticoagulation-related bleeding, there is strong interest in predicting and preventing it. By some estimates, the incidence of recurrent bleeding after resuming vitamin K antagonists is 8% to 13%.22 Although there are several indices for predicting the risk of major bleeding when starting anticoagulation, there are currently no validated tools to estimate a patient’s risk of rebleeding.36
The patient factor that most consistently predicts major bleeding is a history of bleeding, particularly from the gastrointestinal tract. Finding and controlling the source of bleeding is important.26,37 For example, a patient with gross hematuria who is found on cystoscopy to have a urothelial papilloma is unlikely to have rebleeding if the tumor is successfully resected and serial follow-up shows no regrowth. In contrast, consider a patient with a major gastrointestinal hemorrhage, the source of which remains elusive after upper, lower, and capsule endoscopy or, alternatively, is suspected to be from one of multiple angiodysplastic lesions. Without definitive source management, this patient faces a high risk of rebleeding.
With or without anticoagulation, after a first intracranial hemorrhage the risk of another one is estimated at 2% to 4% per year.34 An observational study found a recurrence rate of 7.5% when vitamin K antagonist therapy was started after an intracranial hemorrhage (though not all patients were on a vitamin K antagonist at the time of the first hemorrhage).38
Patients with lobar hemorrhage and those with suspected cerebral amyloid angiopathy may be at particularly high risk if anticoagulation is resumed. Conversely, initial events attributed to uncontrolled hypertension that subsequently can be well controlled may portend a lower risk of rebleeding.34 For other types of intracranial hemorrhage, recurrence rates can be even higher. Irrespective of anticoagulation, one prospective study estimated the crude annual rebleeding rate with untreated arteriovenous malformations to be 7%.39 In chronic subdural hematoma, the recurrence rate after initial drainage has been estimated at 9.2% to 26.5%, with use of anticoagulants (in this case, vitamin K antagonists) being an independent predictor of recurrence.40
WHAT OTHER PATIENT FACTORS NEED CONSIDERATION?
Target INR on warfarin
An important factor influencing the risk of bleeding with warfarin is the intensity of this therapy.37 A meta-analysis41 found that the risks of major hemorrhage and thromboembolism are minimized if the goal international normalized ratio (INR) is 2.0 to 3.0. When considering resuming anticoagulation after bleeding, make sure the therapeutic target is appropriate.37
Table 3 summarizes recommended therapeutic ranges for frequently encountered indications for warfarin.36,42,43
INR at time of the event and challenges in controlling it
The decision to resume anticoagulation in patients who bled while using warfarin must take into account the actual INR at the time of the event.
For example, consider a patient whose INR values are consistently in the therapeutic range. While on vacation, he receives ciprofloxacin for acute prostatitis from an urgent care team, and no adjustment to INR monitoring or warfarin dose is made. Several days later, he presents with lower gastrointestinal bleeding. His INR is 8, and colonoscopy reveals diverticulosis with a bleeding vessel, responsive to endoscopic therapy. After controlling the source of bleeding and reinforcing the need to always review new medications for potential interactions with anticoagulation, it is reasonable to expect that he once again will be able to keep his INR in the therapeutic range.
A patient on anticoagulation for the same indication but who has a history of repeated supratherapeutic levels, poor adherence, or poor access to INR monitoring poses very different concerns about resuming anticoagulation (as well as which agent to use, as we discuss below).
Of note, a high INR alone does not explain bleeding. It is estimated that a workup for gastrointestinal bleeding and gross hematuria uncovers previously undetected lesions in approximately one-third of cases involving warfarin.26 A similar malignancy-unmasking effect is now recognized in patients using the target-specific oral agents who experience gastrointestinal bleeding.44 Accordingly, we recommend a comprehensive source evaluation for any anticoagulation-related hemorrhage.
Comorbid conditions
Comorbid conditions associated with bleeding include cancer, end-stage renal disease, liver disease, arterial hypertension, prior stroke, and alcohol abuse.37,45 Gait instability, regardless of cause, may also increase the risk of trauma-related hemorrhage, but some have estimated that a patient would need to fall multiple times per week to contraindicate anticoagulation on the basis of falls alone.46
Concurrent medications
Concomitant therapies, including antiplatelet drugs and nonsteroidal anti-inflammatory drugs, increase bleeding risk.47,48 Aspirin and the nonsteroidals, in addition to having antiplatelet effects, also can cause gastric erosion.37 In evaluating whether and when to restart anticoagulation, it is advisable to review the role that concomitant therapies may have had in the index bleeding event and to evaluate the risks and benefits of these other agents.
Additionally, warfarin has many interactions. Although the newer drugs are lauded for having fewer interactions, they are not completely free of them, and the potential for interactions must always be reviewed.49 Further, unlike warfarin therapy, therapy with the newer agents is not routinely monitored with laboratory tests, so toxicity (or underdosing) may not be recognized until an adverse clinical event occurs. Ultimately, it may be safer to resume anticoagulation after a contributing drug can be safely discontinued.
Advanced age
The influence that the patient’s age should have on the decision to restart anticoagulation is unclear. Although the risk of intracranial hemorrhage increases with age, particularly after age 80, limited data exist in this population, particularly with regard to rebleeding. Further, age is a major risk factor for most thrombotic events, including venous thromboembolism and stroke from atrial fibrillation, so although the risks of anticoagulation may be higher, the benefits may also be higher than in younger patients.37,46 We discourage using age alone as a reason to withhold anticoagulation after a hemorrhage.
HOW LONG SHOULD WE WAIT TO RESTART ANTICOAGULATION?
We lack conclusive data on how long to wait to restart anticoagulation after an anticoagulation-associated hemorrhage.
The decision is complicated by evidence suggesting a rebound effect, with an increased risk of pulmonary embolism and atrial fibrillation-related stroke during the first 90 days of interruption of therapy with warfarin as well as with target-specific oral anticoagulants.3–8 In anticoagulation-associated retroperitoneal bleeding, there is increased risk of deep vein thrombosis from compression, even if venous thromboembolism was not the initial indication for anticoagulation.30
In patients with intracranial hemorrhage, evidence suggests that the intracranial hemorrhage itself increases the risk of arterial and venous thromboembolic events. Irrespective of whether a patient was previously on anticoagulation, the risk of arterial and venous thromboembolic events approaches 7% during the initial intracranial hemorrhage-related hospitalization and 9% during the first 90 days.34,50,51
To date, the only information we have about when to resume anticoagulation comes from patients taking vitamin K antagonists.
Timing after gastrointestinal bleeding
Small case series suggest that in the first 2 months after warfarin-associated gastrointestinal bleeding, there is substantial risk of rebleeding when anticoagulation is resumed—and of thrombosis when it is not.52,53 Two retrospective cohort studies may provide some guidance in this dilemma.28,54
Witt et al28 followed 442 patients who presented with gastrointestinal bleeding from any site during warfarin therapy for varied indications for up to 90 days after the index bleeding event. The risk of death was three times lower in patients who restarted warfarin than in those who did not, and their rate of thrombotic events was 10 times lower. The risk of recurrent gastrointestinal bleeding was statistically insignificant, and there were no fatal bleeding events. Anticoagulant therapy was generally resumed within 1 week of the bleeding event, at a median of 4 days.28,55
Qureshi et al54 performed a retrospective cohort study of 1,329 patients with nonvalvular atrial fibrillation who had experienced a gastrointestinal hemorrhage while taking warfarin. They found that resuming warfarin after 7 days was not associated with a higher risk of recurrent gastrointestinal bleeding and that the rates of death and thromboembolism were lower than in patients who resumed warfarin after 30 days. On the other hand, the risk of recurrent gastrointestinal bleeding was significantly greater if therapy was resumed within the first week.
In view of these studies, we believe that most patients should resume anticoagulation after 4 to 7 days of interruption after gastrointestinal bleeding.55
Timing after soft-tissue hemorrhage
The literature on resuming anticoagulation after soft-tissue hemorrhage is sparse. A retrospective study52 looked at this question in patients with spontaneous rectal sheath hematoma who had been receiving antiplatelet drugs, intravenous heparin, vitamin K antagonists, or a combination of these, but not target-specific agents. More than half of the patients were on vitamin K antagonists at the time of hemorrhage. Analysis suggested that when benefits of resuming anticoagulation are believed to outweigh risks, it is reasonable to resume anticoagulation 4 days after the index event.56
Timing after intracranial hemorrhage
Anticoagulation should not be considered within the first 24 hours after intracranial hemorrhage, as over 70% of patients develop some amount of hematoma expansion during this time.34,57 The period thereafter poses a challenge, as the risk of hematoma expansion decreases while the risk of arterial and venous thromboembolism is ongoing and cumulative.50
Perhaps surprisingly, national guidelines suggest starting prophylactic-dosed anticoagulation early in all intracranial hemorrhage patients, including those not previously on warfarin.58,59 In a randomized trial, Boeer et al60 concluded that starting low-dose subcutaneous heparin the day after an intracranial hemorrhage decreased the risk of thromboembolism without increasing the risk of rebleeding.60 Dickmann et al61 similarly concluded that there was no increased risk of rebleeding with early prophylactic-dosed subcutaneous heparin.61 Optimal mechanical thromboprophylaxis, including graduated compression stockings and intermittent pneumatic compression stockings, is also encouraged.34
Expert opinion remains divided on when and if anticoagulants should be resumed.34,62 The American Heart Association suggests that in nonvalvular atrial fibrillation, long-term anticoagulation should be avoided after spontaneous lobar hemorrhage; antiplatelet agents can be considered instead.58 In nonlobar hemorrhage, the American Heart Association suggests that anticoagulation be considered, depending on strength of indication, 7 to 10 days after the onset.58 The European Stroke Initiative suggests patients with strong indications for anticoagulation be restarted on warfarin 10 to 14 days after the event, depending on the risk of thromboembolism and recurrent intracranial hemorrhage.59 Others suggest delaying resumption to 10 to 30 weeks after an index intracranial hemorrhage.63
Overall, in the immediate acute period of intracranial hemorrhage, most patients will likely benefit from acute reversal of anticoagulation, followed by institution of prophylactic-dose anticoagulation after the first 24 hours. Going forward, patients who remain at higher risk of a recurrence of anticoagulant-related intracranial hemorrhage (such as those with lobar hemorrhage, suspected cerebral amyloid angiopathy, and other high-risk factors) than of thromboembolic events may be best managed without anticoagulants. Alternatively, patients with deep hemispheric intracranial hemorrhage, hypertension that can be well controlled, and a high risk of serious thromboembolism may experience net benefit from restarting anticoagulation.34
We recommend considering restarting anticoagulation 7 days after the onset of intracranial hemorrhage in patients at high risk of thromboembolism and after at least 14 days for patients at lower risk (Table 2). Discussions with neurologic and neurosurgical consultants should also inform this timing decision.
WOULD A NEWER DRUG BE A BETTER CHOICE?
The emergence of target-specific oral anticoagulants, including factor Xa inhibitors such as rivaroxaban, apixaban, and edoxaban and the direct thrombin inhibitor dabigatran etexilate, presents further challenges in managing anticoagulation after hemorrhage. Table 4 summarizes the current FDA-approved indications.64–67
These newer agents are attractive because, compared with warfarin, they have wider therapeutic windows, faster onset and offset of action, and fewer drug and food interactions.68 A meta-analysis of data available to date suggests that the new drugs, compared with warfarin, show a favorable risk-benefit profile with reductions in stroke, intracranial hemorrhage, and mortality with similar overall major bleeding rates, except for a possible increase in gastrointestinal bleeding.68
However, when managing anticoagulation after a bleeding event, the newer agents are challenging for two reasons: they may be associated with a higher incidence of gastrointestinal bleeding than warfarin, and they lack the typical reversal agents that can be used to manage an acute bleeding event.68,69
In individual studies comparing warfarin with dabigatran,70 rivaroxaban,71 apixaban,72 or edoxaban73 for stroke prevention in patients with atrial fibrillation, there was no significant difference in the rate of major bleeding between dabigatran in its higher dose (150 mg twice a day) or rivaroxaban compared with warfarin.70,71 The risk of major bleeding was actually lower with apixaban72 and edoxaban.73
In regard to specific types of major bleeding, the rate of intracranial hemorrhage was significantly lower with dabigatran, rivaroxaban, apixaban, and edoxaban than with warfarin.35,68–73 Some have proposed that since the brain is high in tissue factor, inhibition of tissue factor-factor VIIa complexes by vitamin K antagonists leaves the brain vulnerable to hemorrhage. Others suggest that the targeted mechanism of target-specific agents, as opposed to the multiple pathways in both the intrinsic and extrinsic coagulation cascade that vitamin K antagonists affect, may explain this difference.35,74,75
However, some studies suggest that rivaroxaban and the higher doses of dabigatran and edoxaban are associated with higher rates of major gastrointestinal bleeding compared with warfarin.69–71,76 But apixaban demonstrated no significant difference in gastrointestinal bleeding, and instead demonstrated rates of gastrointestinal bleeding comparable to that with aspirin for stroke prevention in atrial fibrillation.72
The new oral anticoagulants lack antidotes or reversal agents such as phytonadione and fresh-frozen plasma that are available to manage warfarin-associated bleeding events. Other proposed reversal options for the new agents include activated charcoal (if the drugs were taken recently enough to remain in the gastrointestinal tract) and concentrated clotting factor product, though research is ongoing in regards to the most appropriate use in clinical practice.37,69 Unlike rivaroxaban and apixaban, dabigatran has low plasma protein binding and is dialyzable, which provides another strategy in managing dabigatran-related bleeding.69
Of note, the above bleeding risk calculations relate to the first anticoagulant-related bleeding event, though presumably the same risk comparison across agents may be applicable to rebleeding events. Given the data above, when anticoagulation is to be resumed after an intracranial hemorrhage, the risk of rebleeding, particularly in the form of recurrent intracranial hemorrhage, may be lower if a target-specific oral anticoagulant is used.75 Similarly, when anticoagulation is to be resumed after a gastrointestinal bleeding event, reinitiation with warfarin or apixaban therapy may present the lowest risk of recurrent gastrointestinal rebleeding. In other sources of bleeding, such as retroperitoneal bleeding, we suggest consideration of transitioning to warfarin, given the availability of reversal agents in the event of recurrent bleeding.
Other important drug-specific factors that must be noted when selecting an agent with which to resume anticoagulation after a hemorrhage include the following:
- In patients with significant renal impairment, the choice of agent will be limited to a vitamin K antagonist.77
- A meta-analysis of randomized clinical trials suggests that in the elderly (age 75 and older) target-specific oral anticoagulants did not cause excess bleeding and were associated with at least equal efficacy compared with vitamin K antagonists.78
- Target-specific oral anticoagulants may be beneficial in patients who have challenges in achieving INR targets, as evidence suggests that switching to them is associated with a reduction in bleeding for patients who struggle to maintain an appropriately therapeutic INR.68 On the other hand, if there is concern that a patient may occasionally miss doses of an anticoagulant, given the rapid onset and offset of action of target-specific agents compared with warfarin, a missed dose of a target-specific agent may result in faster dissolution of anticoagulant effect and increased risk of thrombotic events, and lapses in anticoagulation will not be identified by routine drug monitoring.6–8,75 As such, it is vital to have a frank discussion with any patient who has difficulty maintaining therapeutic INRs on warfarin treatment to make sure that he or she is not missing doses.
- If there is no clear and compelling reason to select a particular agent, cost considerations should be taken into account. We have included estimated 30-day pricing for the various agents in Table 4.
- Jaffer AK, Brotman DJ, Bash LD, Mahmood SK, Lott B, White RH. Variations in perioperative warfarin management: outcomes and practice patterns at nine hospitals. Am J Med 2010; 123:141–150.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- Raunsø J, Selmer C, Olesen JB, et al. Increased short-term risk of thrombo-embolism or death after interruption of warfarin treatment in patients with atrial fibrillation. Eur Heart J 2012; 33:1886–1892.
- Xarelto (rivaroxaban). Highlights of prescribing information. Jansen Pharmaceuticals, Inc. www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100. Accessed March 9, 2015.
- Pradaxa (dabigatran etexilate mesylate). Highlights of prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed March 9, 2015.
- Eliquis (apixaban). Highlights of prescribing information. Bristol-Myers Squibb Company. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed March 9, 2015.
- Schulman S, Beyth RJ, Kearon C, Levine MN; American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest 2008; 133(suppl 6):257S–298S.
- Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014; 123:1152–1158.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994; 89:635–641.
- Warkentin TE. Aspirin for dual prevention of venous and arterial thrombosis. N Engl J Med 2012; 367:2039–2041.
- Simes J, Becattini C, Agnelli G, et al; INSPIRE Study Investigators* (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the Prevention of Recurrent Venous Thromboembolism: The INSPIRE Collaboration. Circulation 2014; 130:1062–1071.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Wakefield TW, Obi AT, Henke PK. An aspirin a day to keep the clots away: can aspirin prevent recurrent thrombosis in extended treatment for venous thromboembolism? Circulation 2014; 130:1031–1033.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Coon WW, Willis PW 3rd. Recurrence of venous thromboembolism. Surgery 1973; 73:823–827.
- Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979; 301:855–858.
- Jaffer AK, Brotman DJ, Chukwumerije N. When patients on warfarin need surgery. Cleve Clin J Med 2003; 70:973–984.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis 2011; 31:419–423.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123:2736–2747.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Wiviott SD, Antman EM, Gibson CM, et al; TRITON-TIMI 38 Investigators. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006; 152:627–635.
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993; 95:315–328.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med 2012; 172:1484–1491.
- Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013; 145:105-112.e15.
- Loor G, Bassiouny H, Valentin C, Shao MY, Funaki B, Desai T. Local and systemic consequences of large retroperitoneal clot burdens. World J Surg 2009; 33:1618–1625.
- Satasivam P, Reeves F, Lin M, et al. The effect of oral anticoagulation on the prevalence and management of haematuria in a contemporary Australian patient cohort. BJU Int 2012; 110(suppl 4):80–84.
- Van Savage JG, Fried FA. Anticoagulant associated hematuria: a prospective study. J Urol 1995; 153:1594–1596.
- Mosley DH, Schatz IJ, Breneman GM, Keyes JW. Long-term anticoagulant therapy. Complications and control in a review of 978 cases. JAMA 1963; 186:914–916.
- Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med 2010; 77:791–799.
- Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 2014 Aug 14. [Epub ahead of print]
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Poli D, Antonucci E, Dentali F, et al; Italian Federation of Anticoagulation Clinics (FCSA). Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014; 82:1020–1026.
- Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke 2006; 37:1243–1247.
- Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012; 154:1541–1548.
- Oake N, Jennings A, Forster AJ, et al. Anticoagulation intensity and outcomes among patients prescribed oral anticoagulant therapy: a systematic review and meta-analysis. CMAJ 2008; 179:235–244.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation 2008; 118:e523–e661.
- Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014; 46:672–678.
- Khalid F, Qureshi W, Qureshi S, Alirhayim Z, Garikapati K, Patsias I. Impact of restarting warfarin therapy in renal disease anticoagulated patients with gastrointestinal hemorrhage. Ren Fail 2013; 35:1228–1235.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Davidson BL, Verheijen S, Lensing AW, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 2014; 174:947–953.
- Knijff-Dutmer EA, Schut GA, van de Laar MA. Concomitant coumarin-NSAID therapy and risk for bleeding. Ann Pharmacother 2003; 37:12–16.
- Heidbuchel H, Verhamme P, Alings M, et al; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013; 15:625–651.
- Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care 2009; 10:28–34.
- Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther 2008; 25:831–841.
- Ananthasubramaniam K, Beattie JN, Rosman HS, Jayam V, Borzak S. How safely and for how long can warfarin therapy be withheld in prosthetic heart valve patients hospitalized with a major hemorrhage? Chest 2001; 119:478–484.
- Lee JK, Kang HW, Kim SG, Kim JS, Jung HC. Risks related with withholding and resuming anticoagulation in patients with non-variceal upper gastrointestinal bleeding while on warfarin therapy. Int J Clin Pract 2012; 66:64–68.
- Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol 2014; 113:662–668.
- Brotman DJ, Jaffer AK. Resuming anticoagulation in the first week following gastrointestinal tract hemorrhage: should we adopt a 4-day rule? Arch Intern Med 2012; 172:1492–1493.
- Kunkala MR1, Kehl J, Zielinski MD. Spontaneous rectus sheath hematomas: when to restart anticoagulation? World J Surg 2013; 37:2555–2559.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke 2007; 38:2001–2023.
- Steiner T, Kaste M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 2006; 22:294–316. Erratum in: Cerebrovasc Dis 2006; 22:461.
- Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 1991; 54:466–467.
- Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral hemorrhage. Klin Wochenschr 1988; 66:1182–1183.
- Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc 2007; 82:82–92. Erratum in: Mayo Clin Proc 2007; 82:387.
- Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010; 41:2860–2866.
- US Food and Drug Administration. Drug Information. XARELTO (rivaroxaban) tablets, for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. ELIQUIS® (apixaban) tablets for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s009lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. PRADAXA® (dabigatran etexilate mesylate) capsules for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/022512s023lbl.pdf. Accessed March 9, 2015.
- New oral anticoagulants for acute venous thromboembolism. Med Lett Drugs Ther 2014; 56:3–4.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 2009; 108:1447–1452.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Loffredo L, Perri L, Violi F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: a meta-analysis of interventional trials. Dig Liver Dis 2015 Feb 7. pii: S1590-8658(15)00189-9. doi: 10.1016/j.dld.2015.01.159. [Epub ahead of print]
- Thachil J. The newer direct oral anticoagulants: a practical guide. Clin Med 2014; 14:165–175.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Jaffer AK, Brotman DJ, Bash LD, Mahmood SK, Lott B, White RH. Variations in perioperative warfarin management: outcomes and practice patterns at nine hospitals. Am J Med 2010; 123:141–150.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- Raunsø J, Selmer C, Olesen JB, et al. Increased short-term risk of thrombo-embolism or death after interruption of warfarin treatment in patients with atrial fibrillation. Eur Heart J 2012; 33:1886–1892.
- Xarelto (rivaroxaban). Highlights of prescribing information. Jansen Pharmaceuticals, Inc. www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100. Accessed March 9, 2015.
- Pradaxa (dabigatran etexilate mesylate). Highlights of prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed March 9, 2015.
- Eliquis (apixaban). Highlights of prescribing information. Bristol-Myers Squibb Company. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed March 9, 2015.
- Schulman S, Beyth RJ, Kearon C, Levine MN; American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest 2008; 133(suppl 6):257S–298S.
- Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014; 123:1152–1158.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994; 89:635–641.
- Warkentin TE. Aspirin for dual prevention of venous and arterial thrombosis. N Engl J Med 2012; 367:2039–2041.
- Simes J, Becattini C, Agnelli G, et al; INSPIRE Study Investigators* (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the Prevention of Recurrent Venous Thromboembolism: The INSPIRE Collaboration. Circulation 2014; 130:1062–1071.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Wakefield TW, Obi AT, Henke PK. An aspirin a day to keep the clots away: can aspirin prevent recurrent thrombosis in extended treatment for venous thromboembolism? Circulation 2014; 130:1031–1033.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Coon WW, Willis PW 3rd. Recurrence of venous thromboembolism. Surgery 1973; 73:823–827.
- Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979; 301:855–858.
- Jaffer AK, Brotman DJ, Chukwumerije N. When patients on warfarin need surgery. Cleve Clin J Med 2003; 70:973–984.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis 2011; 31:419–423.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123:2736–2747.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Wiviott SD, Antman EM, Gibson CM, et al; TRITON-TIMI 38 Investigators. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006; 152:627–635.
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993; 95:315–328.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med 2012; 172:1484–1491.
- Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013; 145:105-112.e15.
- Loor G, Bassiouny H, Valentin C, Shao MY, Funaki B, Desai T. Local and systemic consequences of large retroperitoneal clot burdens. World J Surg 2009; 33:1618–1625.
- Satasivam P, Reeves F, Lin M, et al. The effect of oral anticoagulation on the prevalence and management of haematuria in a contemporary Australian patient cohort. BJU Int 2012; 110(suppl 4):80–84.
- Van Savage JG, Fried FA. Anticoagulant associated hematuria: a prospective study. J Urol 1995; 153:1594–1596.
- Mosley DH, Schatz IJ, Breneman GM, Keyes JW. Long-term anticoagulant therapy. Complications and control in a review of 978 cases. JAMA 1963; 186:914–916.
- Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med 2010; 77:791–799.
- Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 2014 Aug 14. [Epub ahead of print]
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Poli D, Antonucci E, Dentali F, et al; Italian Federation of Anticoagulation Clinics (FCSA). Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014; 82:1020–1026.
- Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke 2006; 37:1243–1247.
- Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012; 154:1541–1548.
- Oake N, Jennings A, Forster AJ, et al. Anticoagulation intensity and outcomes among patients prescribed oral anticoagulant therapy: a systematic review and meta-analysis. CMAJ 2008; 179:235–244.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation 2008; 118:e523–e661.
- Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014; 46:672–678.
- Khalid F, Qureshi W, Qureshi S, Alirhayim Z, Garikapati K, Patsias I. Impact of restarting warfarin therapy in renal disease anticoagulated patients with gastrointestinal hemorrhage. Ren Fail 2013; 35:1228–1235.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Davidson BL, Verheijen S, Lensing AW, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 2014; 174:947–953.
- Knijff-Dutmer EA, Schut GA, van de Laar MA. Concomitant coumarin-NSAID therapy and risk for bleeding. Ann Pharmacother 2003; 37:12–16.
- Heidbuchel H, Verhamme P, Alings M, et al; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013; 15:625–651.
- Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care 2009; 10:28–34.
- Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther 2008; 25:831–841.
- Ananthasubramaniam K, Beattie JN, Rosman HS, Jayam V, Borzak S. How safely and for how long can warfarin therapy be withheld in prosthetic heart valve patients hospitalized with a major hemorrhage? Chest 2001; 119:478–484.
- Lee JK, Kang HW, Kim SG, Kim JS, Jung HC. Risks related with withholding and resuming anticoagulation in patients with non-variceal upper gastrointestinal bleeding while on warfarin therapy. Int J Clin Pract 2012; 66:64–68.
- Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol 2014; 113:662–668.
- Brotman DJ, Jaffer AK. Resuming anticoagulation in the first week following gastrointestinal tract hemorrhage: should we adopt a 4-day rule? Arch Intern Med 2012; 172:1492–1493.
- Kunkala MR1, Kehl J, Zielinski MD. Spontaneous rectus sheath hematomas: when to restart anticoagulation? World J Surg 2013; 37:2555–2559.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke 2007; 38:2001–2023.
- Steiner T, Kaste M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 2006; 22:294–316. Erratum in: Cerebrovasc Dis 2006; 22:461.
- Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 1991; 54:466–467.
- Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral hemorrhage. Klin Wochenschr 1988; 66:1182–1183.
- Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc 2007; 82:82–92. Erratum in: Mayo Clin Proc 2007; 82:387.
- Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010; 41:2860–2866.
- US Food and Drug Administration. Drug Information. XARELTO (rivaroxaban) tablets, for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. ELIQUIS® (apixaban) tablets for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s009lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. PRADAXA® (dabigatran etexilate mesylate) capsules for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/022512s023lbl.pdf. Accessed March 9, 2015.
- New oral anticoagulants for acute venous thromboembolism. Med Lett Drugs Ther 2014; 56:3–4.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 2009; 108:1447–1452.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Loffredo L, Perri L, Violi F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: a meta-analysis of interventional trials. Dig Liver Dis 2015 Feb 7. pii: S1590-8658(15)00189-9. doi: 10.1016/j.dld.2015.01.159. [Epub ahead of print]
- Thachil J. The newer direct oral anticoagulants: a practical guide. Clin Med 2014; 14:165–175.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
KEY POINTS
- Not all patients on anticoagulation at the time of a bleeding event have a strong indication to continue anticoagulation afterward.
- Important considerations when deciding whether to resume anticoagulation after hemorrhage are whether the source of bleeding has been found and controlled and, if the patient is receiving warfarin, whether he or she can be expected to maintain the target international normalized ratio.
- The newer oral anticoagulants, including factor Xa inhibitors and direct thrombin inhibitors, lack antidotes or reversal agents, and their risk of causing bleeding compared with warfarin varies by site of bleeding.
Update in hospital medicine: Studies likely to affect inpatient practice in 2011
A number of studies published in the last few years will likely affect the way we practice medicine in the hospital. Here, we will use a hypothetical case scenario to focus on the issues of anticoagulants, patient safety, quality improvement, critical care, transitions of care, and perioperative medicine.
AN ELDERLY MAN WITH NEW-ONSET ATRIAL FIBRILLATION
P.G. is an 80-year-old man with a history of hypertension and type 2 diabetes mellitus who is admitted with new-onset atrial fibrillation. In the hospital, his heart rate is brought under control with intravenous metoprolol (Lopressor). On discharge, he will be followed by his primary care physician (PCP). He does not have access to an anticoagulation clinic.
1. What are this patient’s options for stroke prevention?
- Aspirin 81 mg daily and clopidogrel (Plavix) 75 mg daily
- Warfarin (Coumadin) with a target international normalized ratio (INR) of 2.0 to 3.0
- Aspirin mg daily by itself
- Dabigatran (Pradaxa) 150 mg daily
A new oral anticoagulant agent
In deciding what type of anticoagulation to give to a patient with atrial fibrillation, it is useful to look at the CHADS2 score (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack. This patient has a CHADS2 score of 3, indicating that he should receive warfarin. An alternative is dabigatran, the first new anticoagulant agent in more than 50 years.
In a multicenter, international trial, Connolly et al1 randomized 18,113 patients (mean age 71, 64% men) to receive dabigatran 110 mg twice daily, dabigatran 150 mg twice daily, or warfarin with a target INR of 2.0 to 3.0. In this noninferiority trial, dabigatran was given in a blinded manner, but the use of warfarin was open-label. Patients were eligible if they had atrial fibrillation at screening or within the previous 6 months and were at risk of stroke—ie, if they had at least one of the following: a history of stroke or transient ischemic attack, a left ventricular ejection fraction of less than 40%, symptoms of congestive heart failure (New York Heart Association class II or higher), and an age of 75 or older or an age of 65 to 74 with diabetes mellitus, hypertension, or coronary artery disease.
At a mean follow-up of 2 years, the rate of stroke or systolic embolism was 1.69% per year in the warfarin group compared with 1.1% in the higher-dose dabigatran group (relative risk 0.66, 95% confidence interval [CI] 0.53–0.82, P < .001). The rates of major hemorrhage were similar between these two groups. Comparing lower-dose dabigatran and warfarin, the rates of stroke or systolic embolism were not significantly different, but the rate of major bleeding was significantly lower with lower-dose dabigatran.
In a trial in patients with acute venous thromboembolism, Schulman et al2 found that dabigatran was not inferior to warfarin in preventing venous thromboembolism.
Guidelines from the American College of Cardiology Foundation and the American Heart Association now endorse dabigatran as an alternative to warfarin for patients with atrial fibrillation.3 However, the guidelines state that it should be reserved for those patients who:
- Do not have a prosthetic heart valve or hemodynamically significant valve disease
- Have good kidney function (dabigatran is cleared by the kidney; the creatinine clearance rate should be greater than 30 mL/min for patients to receive dabigatran 150 mg twice a day, and at least 15 mL/min to receive 75 mg twice a day)
- Do not have severe hepatic dysfunction (which would impair baseline clotting function).
They note that other factors to consider are whether the patient:
- Can comply with the twice-daily dosing required
- Can afford the drug
- Has access to an anticoagulation management program (which would argue in favor of using warfarin).
Dabigatran is not yet approved to prevent venous thromboembolism.
CASE CONTINUED: HE GETS AN INFECTION
P.G. is started on dabigatran 150 mg by mouth twice a day.
While in the hospital he develops shortness of breath and needs intravenous furosemide (Lasix). Because he has bad veins, a percutaneous intravenous central catheter (PICC) line is placed. However, 2 days later, his temperature is 101.5°F, and his systolic blood pressure is 70 mm Hg. He is transferred to the medical intensive care unit (ICU) for treatment of sepsis. The anticoagulant is held, the PICC line is removed, and a new central catheter is inserted.
2. Which of the following directions is incorrect?
- Wash your hands before inserting the catheter. The accompanying nurse is required to directly observe this procedure or, if this step is not observed, to confirm that the physician did it.
- Before inserting the catheter, clean the patient’s skin with chlorhexidine antiseptic.
- Place sterile drapes over the entire patient.
- Wear any mask, hat, gown, and gloves available.
- Put a sterile dressing over the catheter.
A checklist can prevent infections when inserting central catheters
A checklist developed at Johns Hopkins Hospital consists of the five statements above, except for the second to last one—you should wear a sterile mask, hat, gown and gloves. This is important to ensure that sterility is not broken at any point during the procedure.
Pronovost et al4 launched a multicenter initiative at 90 ICUs, predominantly in the state of Michigan, to implement interventions to improve staff culture and teamwork and to translate research into practice by increasing the extent to which these five evidence-based recommendations were applied. The mean rate of catheter-related blood stream infections at baseline was 7.7%; this dropped to 2.8% during the implementation period, 2.3% in the first 3 months after implementation, 1.3% in months 16 through 18, and 1.1% in months 34 through 36, demonstrating that the gains from this quality-improvement project were sustainable.
If this intervention and collaborative model were implemented in all ICUs across the United States and if similar success rates were achieved, substantial and sustained reductions could be made in the 82,000 infections, 28,000 deaths, and $2.3 billion in costs attributed to these infections annually.
CASE CONTINUED: HE IS RESUSCITATED
P.G. is started on a 1-L fluid bolus but he remains hypotensive, necessitating a norepinephrine drip. He does well for about 6 hours, but in the middle of the night he develops ventricular tachycardia and ventricular fibrillation, and a code is called. He is successfully resuscitated, but the family is looking for prognostic information.
3. What are P.G.’s chances of surviving and leaving the hospital?
- 5%
- 8%
- 15%
- 23%
A registry of cardiopulmonary resuscitation
Tian et al5 evaluated outcomes in the largest registry of cardiopulmonary resuscitation to date. In this analysis, 49,656 adult patients with a first cardiopulmonary arrest occurring in an ICU between January 1, 2000, and August 26, 2008, were evaluated for their outcomes on pressors vs those not on pressors.
Other independent predictors of a lower survival rate were nonwhite race, mechanical ventilation, having three or more immediate causes of cardiopulmonary arrest, age 65 years or older, and cardiopulmonary arrest occurring at night or over the weekend.
Fortunately, for our patient, survival rates were higher for patients with ventricular tachycardia or fibrillation than with other causes of cardiopulmonary arrest: 22.6% for those on pressors (like our patient) and 40.7% for those on no pressors.
CASE CONTINUED: HE RECOVERS AND GOES HOME
P.G. makes a remarkable recovery and is now ready to go home. It is the weekend, and you are unable to schedule a follow-up appointment before his discharge, so you ask him to make an appointment with his PCP.
4. What is the likelihood that P.G. will be readmitted within 1 month?
- 5%
- 12%
- 20%
- 25%
- 30%
The importance of follow-up with a primary care physician
Misky et al,6 in a small study, attempted to identify the characteristics and outcomes of discharged patients who lack timely follow-up with a PCP. They prospectively enrolled 65 patients admitted to University of Colorado Hospital, an urban 425-bed tertiary care center, collecting information about patient demographics, diagnosis, payer source, and PCPs. After discharge, they called the patients to determine their PCP follow-up and readmission status. Thirty-day readmission rates and hospital length of stay were compared in patients with and without timely PCP follow-up (ie, within 4 weeks).
Patients lacking timely PCP follow-up were 10 times more likely to be readmitted (odds ratio [OR] = 9.9, P = .04): the rate was 21% in patients lacking timely PCP follow-up vs 3% in patients with timely PCP follow-up, P = .03. Lack of insurance was associated with lower rates of timely PCP follow-up: 29% vs 56% (P = .06), but did not independently increase the readmission rate or length of stay (OR = 1.0, P = .96). Index hospital length of stay was longer in patients lacking timely PCP follow-up: 4.4 days vs 6.3 days, P = 0.11.
Comment. Nearly half of the patients in this study, who were discharged from a large urban academic center, lacked timely follow-up with a PCP, resulting in higher rates of readmission and a nonsignificant trend toward longer length of stay. Timely follow-up is necessary for vulnerable patients.
Since the lack of timely PCP follow-up results in higher readmission rates and possibly a longer length of stay, a PCP appointment at discharge should perhaps be considered a core quality measure. This would be problematic in our American health care system, in which many patients lack health insurance and do not have a PCP.
A MAN UNDERGOING GASTRIC BYPASS SURGERY
A 55-year-old morbidly obese man (body mass index 45 kg/m2) with a history of type 2 diabetes mellitus, chronic renal insufficiency (serum creatinine level 2.1 mg/dL), hypercholesterolemia, and previous stroke is scheduled for gastric bypass surgery. His functional capacity is low, but he is able to do his activities of daily living. He reports having dyspnea on exertion and intermittently at rest, but no chest pain. His medications include insulin, atorvastatin (Lipitor), aspirin, and atenolol (Tenormin). He is afebrile; his blood pressure is 130/80 mm Hg, pulse 75, and oxygen saturation 97% on room air. His baseline electrocardiogram shows no Q waves.
5. Which of the following is an appropriate next step before proceeding to surgery?
- Echocardiography
- Cardiac catheterization
- Dobutamine stress echocardiography or adenosine thallium scanning
- No cardiac testing is necessary before surgery
Is cardiac testing necessary before noncardiac surgery?
Wijeysundera et al7 performed a retrospective cohort study of patients who underwent elective surgery at acute care hospitals in Ontario, Canada, in the years 1994 through 2004. The aim was to determine the association of noninvasive cardiac stress testing before surgery with survival rates and length of hospital stay. Included were 271,082 patients, of whom 23,991 (8.9%) underwent stress testing less than 6 months before surgery. These patients were matched with 46,120 who did not undergo testing.
One year after surgery, fewer patients who underwent stress testing had died: 1,622 (7.0%) vs 1,738 (7.5%); hazard ratio 0.92, 95% CI 0.86–0.99, P = .03. The number needed to treat (ie, to be tested) to prevent one death was 221. The tested patients also had a shorter mean hospital stay: 8.72 vs 8.96 days, a difference of 0.24 days (95% CI −0.07 to −0.43; P < .001).
However, the elderly patients (ie, older than 66 years) who underwent testing were more likely to be on beta-blockers and statins than those who did not undergo testing, which may be a confounding factor.
Furthermore, the benefit was all in the patients at intermediate or high risk. The authors performed a subgroup analysis, dividing the patients on the basis of their Revised Cardiac Risk Index (RCRI; 1 point each for ischemic heart disease, congestive heart failure, cerebrovascular disease, diabetes, renal insufficiency, and high-risk surgery).8 Patients with an RCRI of 0 points (indicating low risk) actually had a higher risk of death with testing than without testing: hazard ratio 1.35 (95% CI 1.03–1.74), number needed to harm 179—ie, for every 179 low-risk patients tested, one excess death occurred. Those with an RCRI of 1 or 2 points (indicating intermediate risk) had a hazard ratio of 0.92 with testing (95% CI 085–0.99), and those with an RCRI of 3 to 6 points (indicating high risk) had a hazard ratio of 0.80 with testing (95% CI 0.67- 0.97; number needed to treat = 38).
Comment. These findings indicate that cardiac stress testing should be done selectively before noncardiac surgery, and primarily for patients at high risk (with an RCRI of 3 or higher) and in some patients at intermediate risk, but not in patients at low risk, in whom it may be harmful. Stress testing may change patient management because a positive stress test allows one to start a beta-blocker or a statin, use more aggressive intraoperative and postoperative care, and identify patients who have indications for revascularization.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:1144–1150.
- Pronovost PJ, Goeschel CA, Colantuoni E, et al. Sustaining reductions in catheter-related bloodstream infections in Michigan intensive care units: observational study. BMJ 2010; 340:c309.
- Tian J, Kaufman DA, Zarich S, et al; American Heart Association National Registry for Cardiopulmonary Resuscitation Investigators. Outcomes of critically ill patients who received cardiopulmonary resuscitation. Am J Respir Crit Care Med 2010; 182:501–506.
- Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med 2010; 5:392–397.
- Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Non-invasive cardiac stress testing before elective major non-cardiac surgery: population based cohort study. BMJ 2010; 340:b5526.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
A number of studies published in the last few years will likely affect the way we practice medicine in the hospital. Here, we will use a hypothetical case scenario to focus on the issues of anticoagulants, patient safety, quality improvement, critical care, transitions of care, and perioperative medicine.
AN ELDERLY MAN WITH NEW-ONSET ATRIAL FIBRILLATION
P.G. is an 80-year-old man with a history of hypertension and type 2 diabetes mellitus who is admitted with new-onset atrial fibrillation. In the hospital, his heart rate is brought under control with intravenous metoprolol (Lopressor). On discharge, he will be followed by his primary care physician (PCP). He does not have access to an anticoagulation clinic.
1. What are this patient’s options for stroke prevention?
- Aspirin 81 mg daily and clopidogrel (Plavix) 75 mg daily
- Warfarin (Coumadin) with a target international normalized ratio (INR) of 2.0 to 3.0
- Aspirin mg daily by itself
- Dabigatran (Pradaxa) 150 mg daily
A new oral anticoagulant agent
In deciding what type of anticoagulation to give to a patient with atrial fibrillation, it is useful to look at the CHADS2 score (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack. This patient has a CHADS2 score of 3, indicating that he should receive warfarin. An alternative is dabigatran, the first new anticoagulant agent in more than 50 years.
In a multicenter, international trial, Connolly et al1 randomized 18,113 patients (mean age 71, 64% men) to receive dabigatran 110 mg twice daily, dabigatran 150 mg twice daily, or warfarin with a target INR of 2.0 to 3.0. In this noninferiority trial, dabigatran was given in a blinded manner, but the use of warfarin was open-label. Patients were eligible if they had atrial fibrillation at screening or within the previous 6 months and were at risk of stroke—ie, if they had at least one of the following: a history of stroke or transient ischemic attack, a left ventricular ejection fraction of less than 40%, symptoms of congestive heart failure (New York Heart Association class II or higher), and an age of 75 or older or an age of 65 to 74 with diabetes mellitus, hypertension, or coronary artery disease.
At a mean follow-up of 2 years, the rate of stroke or systolic embolism was 1.69% per year in the warfarin group compared with 1.1% in the higher-dose dabigatran group (relative risk 0.66, 95% confidence interval [CI] 0.53–0.82, P < .001). The rates of major hemorrhage were similar between these two groups. Comparing lower-dose dabigatran and warfarin, the rates of stroke or systolic embolism were not significantly different, but the rate of major bleeding was significantly lower with lower-dose dabigatran.
In a trial in patients with acute venous thromboembolism, Schulman et al2 found that dabigatran was not inferior to warfarin in preventing venous thromboembolism.
Guidelines from the American College of Cardiology Foundation and the American Heart Association now endorse dabigatran as an alternative to warfarin for patients with atrial fibrillation.3 However, the guidelines state that it should be reserved for those patients who:
- Do not have a prosthetic heart valve or hemodynamically significant valve disease
- Have good kidney function (dabigatran is cleared by the kidney; the creatinine clearance rate should be greater than 30 mL/min for patients to receive dabigatran 150 mg twice a day, and at least 15 mL/min to receive 75 mg twice a day)
- Do not have severe hepatic dysfunction (which would impair baseline clotting function).
They note that other factors to consider are whether the patient:
- Can comply with the twice-daily dosing required
- Can afford the drug
- Has access to an anticoagulation management program (which would argue in favor of using warfarin).
Dabigatran is not yet approved to prevent venous thromboembolism.
CASE CONTINUED: HE GETS AN INFECTION
P.G. is started on dabigatran 150 mg by mouth twice a day.
While in the hospital he develops shortness of breath and needs intravenous furosemide (Lasix). Because he has bad veins, a percutaneous intravenous central catheter (PICC) line is placed. However, 2 days later, his temperature is 101.5°F, and his systolic blood pressure is 70 mm Hg. He is transferred to the medical intensive care unit (ICU) for treatment of sepsis. The anticoagulant is held, the PICC line is removed, and a new central catheter is inserted.
2. Which of the following directions is incorrect?
- Wash your hands before inserting the catheter. The accompanying nurse is required to directly observe this procedure or, if this step is not observed, to confirm that the physician did it.
- Before inserting the catheter, clean the patient’s skin with chlorhexidine antiseptic.
- Place sterile drapes over the entire patient.
- Wear any mask, hat, gown, and gloves available.
- Put a sterile dressing over the catheter.
A checklist can prevent infections when inserting central catheters
A checklist developed at Johns Hopkins Hospital consists of the five statements above, except for the second to last one—you should wear a sterile mask, hat, gown and gloves. This is important to ensure that sterility is not broken at any point during the procedure.
Pronovost et al4 launched a multicenter initiative at 90 ICUs, predominantly in the state of Michigan, to implement interventions to improve staff culture and teamwork and to translate research into practice by increasing the extent to which these five evidence-based recommendations were applied. The mean rate of catheter-related blood stream infections at baseline was 7.7%; this dropped to 2.8% during the implementation period, 2.3% in the first 3 months after implementation, 1.3% in months 16 through 18, and 1.1% in months 34 through 36, demonstrating that the gains from this quality-improvement project were sustainable.
If this intervention and collaborative model were implemented in all ICUs across the United States and if similar success rates were achieved, substantial and sustained reductions could be made in the 82,000 infections, 28,000 deaths, and $2.3 billion in costs attributed to these infections annually.
CASE CONTINUED: HE IS RESUSCITATED
P.G. is started on a 1-L fluid bolus but he remains hypotensive, necessitating a norepinephrine drip. He does well for about 6 hours, but in the middle of the night he develops ventricular tachycardia and ventricular fibrillation, and a code is called. He is successfully resuscitated, but the family is looking for prognostic information.
3. What are P.G.’s chances of surviving and leaving the hospital?
- 5%
- 8%
- 15%
- 23%
A registry of cardiopulmonary resuscitation
Tian et al5 evaluated outcomes in the largest registry of cardiopulmonary resuscitation to date. In this analysis, 49,656 adult patients with a first cardiopulmonary arrest occurring in an ICU between January 1, 2000, and August 26, 2008, were evaluated for their outcomes on pressors vs those not on pressors.
Other independent predictors of a lower survival rate were nonwhite race, mechanical ventilation, having three or more immediate causes of cardiopulmonary arrest, age 65 years or older, and cardiopulmonary arrest occurring at night or over the weekend.
Fortunately, for our patient, survival rates were higher for patients with ventricular tachycardia or fibrillation than with other causes of cardiopulmonary arrest: 22.6% for those on pressors (like our patient) and 40.7% for those on no pressors.
CASE CONTINUED: HE RECOVERS AND GOES HOME
P.G. makes a remarkable recovery and is now ready to go home. It is the weekend, and you are unable to schedule a follow-up appointment before his discharge, so you ask him to make an appointment with his PCP.
4. What is the likelihood that P.G. will be readmitted within 1 month?
- 5%
- 12%
- 20%
- 25%
- 30%
The importance of follow-up with a primary care physician
Misky et al,6 in a small study, attempted to identify the characteristics and outcomes of discharged patients who lack timely follow-up with a PCP. They prospectively enrolled 65 patients admitted to University of Colorado Hospital, an urban 425-bed tertiary care center, collecting information about patient demographics, diagnosis, payer source, and PCPs. After discharge, they called the patients to determine their PCP follow-up and readmission status. Thirty-day readmission rates and hospital length of stay were compared in patients with and without timely PCP follow-up (ie, within 4 weeks).
Patients lacking timely PCP follow-up were 10 times more likely to be readmitted (odds ratio [OR] = 9.9, P = .04): the rate was 21% in patients lacking timely PCP follow-up vs 3% in patients with timely PCP follow-up, P = .03. Lack of insurance was associated with lower rates of timely PCP follow-up: 29% vs 56% (P = .06), but did not independently increase the readmission rate or length of stay (OR = 1.0, P = .96). Index hospital length of stay was longer in patients lacking timely PCP follow-up: 4.4 days vs 6.3 days, P = 0.11.
Comment. Nearly half of the patients in this study, who were discharged from a large urban academic center, lacked timely follow-up with a PCP, resulting in higher rates of readmission and a nonsignificant trend toward longer length of stay. Timely follow-up is necessary for vulnerable patients.
Since the lack of timely PCP follow-up results in higher readmission rates and possibly a longer length of stay, a PCP appointment at discharge should perhaps be considered a core quality measure. This would be problematic in our American health care system, in which many patients lack health insurance and do not have a PCP.
A MAN UNDERGOING GASTRIC BYPASS SURGERY
A 55-year-old morbidly obese man (body mass index 45 kg/m2) with a history of type 2 diabetes mellitus, chronic renal insufficiency (serum creatinine level 2.1 mg/dL), hypercholesterolemia, and previous stroke is scheduled for gastric bypass surgery. His functional capacity is low, but he is able to do his activities of daily living. He reports having dyspnea on exertion and intermittently at rest, but no chest pain. His medications include insulin, atorvastatin (Lipitor), aspirin, and atenolol (Tenormin). He is afebrile; his blood pressure is 130/80 mm Hg, pulse 75, and oxygen saturation 97% on room air. His baseline electrocardiogram shows no Q waves.
5. Which of the following is an appropriate next step before proceeding to surgery?
- Echocardiography
- Cardiac catheterization
- Dobutamine stress echocardiography or adenosine thallium scanning
- No cardiac testing is necessary before surgery
Is cardiac testing necessary before noncardiac surgery?
Wijeysundera et al7 performed a retrospective cohort study of patients who underwent elective surgery at acute care hospitals in Ontario, Canada, in the years 1994 through 2004. The aim was to determine the association of noninvasive cardiac stress testing before surgery with survival rates and length of hospital stay. Included were 271,082 patients, of whom 23,991 (8.9%) underwent stress testing less than 6 months before surgery. These patients were matched with 46,120 who did not undergo testing.
One year after surgery, fewer patients who underwent stress testing had died: 1,622 (7.0%) vs 1,738 (7.5%); hazard ratio 0.92, 95% CI 0.86–0.99, P = .03. The number needed to treat (ie, to be tested) to prevent one death was 221. The tested patients also had a shorter mean hospital stay: 8.72 vs 8.96 days, a difference of 0.24 days (95% CI −0.07 to −0.43; P < .001).
However, the elderly patients (ie, older than 66 years) who underwent testing were more likely to be on beta-blockers and statins than those who did not undergo testing, which may be a confounding factor.
Furthermore, the benefit was all in the patients at intermediate or high risk. The authors performed a subgroup analysis, dividing the patients on the basis of their Revised Cardiac Risk Index (RCRI; 1 point each for ischemic heart disease, congestive heart failure, cerebrovascular disease, diabetes, renal insufficiency, and high-risk surgery).8 Patients with an RCRI of 0 points (indicating low risk) actually had a higher risk of death with testing than without testing: hazard ratio 1.35 (95% CI 1.03–1.74), number needed to harm 179—ie, for every 179 low-risk patients tested, one excess death occurred. Those with an RCRI of 1 or 2 points (indicating intermediate risk) had a hazard ratio of 0.92 with testing (95% CI 085–0.99), and those with an RCRI of 3 to 6 points (indicating high risk) had a hazard ratio of 0.80 with testing (95% CI 0.67- 0.97; number needed to treat = 38).
Comment. These findings indicate that cardiac stress testing should be done selectively before noncardiac surgery, and primarily for patients at high risk (with an RCRI of 3 or higher) and in some patients at intermediate risk, but not in patients at low risk, in whom it may be harmful. Stress testing may change patient management because a positive stress test allows one to start a beta-blocker or a statin, use more aggressive intraoperative and postoperative care, and identify patients who have indications for revascularization.
A number of studies published in the last few years will likely affect the way we practice medicine in the hospital. Here, we will use a hypothetical case scenario to focus on the issues of anticoagulants, patient safety, quality improvement, critical care, transitions of care, and perioperative medicine.
AN ELDERLY MAN WITH NEW-ONSET ATRIAL FIBRILLATION
P.G. is an 80-year-old man with a history of hypertension and type 2 diabetes mellitus who is admitted with new-onset atrial fibrillation. In the hospital, his heart rate is brought under control with intravenous metoprolol (Lopressor). On discharge, he will be followed by his primary care physician (PCP). He does not have access to an anticoagulation clinic.
1. What are this patient’s options for stroke prevention?
- Aspirin 81 mg daily and clopidogrel (Plavix) 75 mg daily
- Warfarin (Coumadin) with a target international normalized ratio (INR) of 2.0 to 3.0
- Aspirin mg daily by itself
- Dabigatran (Pradaxa) 150 mg daily
A new oral anticoagulant agent
In deciding what type of anticoagulation to give to a patient with atrial fibrillation, it is useful to look at the CHADS2 score (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack. This patient has a CHADS2 score of 3, indicating that he should receive warfarin. An alternative is dabigatran, the first new anticoagulant agent in more than 50 years.
In a multicenter, international trial, Connolly et al1 randomized 18,113 patients (mean age 71, 64% men) to receive dabigatran 110 mg twice daily, dabigatran 150 mg twice daily, or warfarin with a target INR of 2.0 to 3.0. In this noninferiority trial, dabigatran was given in a blinded manner, but the use of warfarin was open-label. Patients were eligible if they had atrial fibrillation at screening or within the previous 6 months and were at risk of stroke—ie, if they had at least one of the following: a history of stroke or transient ischemic attack, a left ventricular ejection fraction of less than 40%, symptoms of congestive heart failure (New York Heart Association class II or higher), and an age of 75 or older or an age of 65 to 74 with diabetes mellitus, hypertension, or coronary artery disease.
At a mean follow-up of 2 years, the rate of stroke or systolic embolism was 1.69% per year in the warfarin group compared with 1.1% in the higher-dose dabigatran group (relative risk 0.66, 95% confidence interval [CI] 0.53–0.82, P < .001). The rates of major hemorrhage were similar between these two groups. Comparing lower-dose dabigatran and warfarin, the rates of stroke or systolic embolism were not significantly different, but the rate of major bleeding was significantly lower with lower-dose dabigatran.
In a trial in patients with acute venous thromboembolism, Schulman et al2 found that dabigatran was not inferior to warfarin in preventing venous thromboembolism.
Guidelines from the American College of Cardiology Foundation and the American Heart Association now endorse dabigatran as an alternative to warfarin for patients with atrial fibrillation.3 However, the guidelines state that it should be reserved for those patients who:
- Do not have a prosthetic heart valve or hemodynamically significant valve disease
- Have good kidney function (dabigatran is cleared by the kidney; the creatinine clearance rate should be greater than 30 mL/min for patients to receive dabigatran 150 mg twice a day, and at least 15 mL/min to receive 75 mg twice a day)
- Do not have severe hepatic dysfunction (which would impair baseline clotting function).
They note that other factors to consider are whether the patient:
- Can comply with the twice-daily dosing required
- Can afford the drug
- Has access to an anticoagulation management program (which would argue in favor of using warfarin).
Dabigatran is not yet approved to prevent venous thromboembolism.
CASE CONTINUED: HE GETS AN INFECTION
P.G. is started on dabigatran 150 mg by mouth twice a day.
While in the hospital he develops shortness of breath and needs intravenous furosemide (Lasix). Because he has bad veins, a percutaneous intravenous central catheter (PICC) line is placed. However, 2 days later, his temperature is 101.5°F, and his systolic blood pressure is 70 mm Hg. He is transferred to the medical intensive care unit (ICU) for treatment of sepsis. The anticoagulant is held, the PICC line is removed, and a new central catheter is inserted.
2. Which of the following directions is incorrect?
- Wash your hands before inserting the catheter. The accompanying nurse is required to directly observe this procedure or, if this step is not observed, to confirm that the physician did it.
- Before inserting the catheter, clean the patient’s skin with chlorhexidine antiseptic.
- Place sterile drapes over the entire patient.
- Wear any mask, hat, gown, and gloves available.
- Put a sterile dressing over the catheter.
A checklist can prevent infections when inserting central catheters
A checklist developed at Johns Hopkins Hospital consists of the five statements above, except for the second to last one—you should wear a sterile mask, hat, gown and gloves. This is important to ensure that sterility is not broken at any point during the procedure.
Pronovost et al4 launched a multicenter initiative at 90 ICUs, predominantly in the state of Michigan, to implement interventions to improve staff culture and teamwork and to translate research into practice by increasing the extent to which these five evidence-based recommendations were applied. The mean rate of catheter-related blood stream infections at baseline was 7.7%; this dropped to 2.8% during the implementation period, 2.3% in the first 3 months after implementation, 1.3% in months 16 through 18, and 1.1% in months 34 through 36, demonstrating that the gains from this quality-improvement project were sustainable.
If this intervention and collaborative model were implemented in all ICUs across the United States and if similar success rates were achieved, substantial and sustained reductions could be made in the 82,000 infections, 28,000 deaths, and $2.3 billion in costs attributed to these infections annually.
CASE CONTINUED: HE IS RESUSCITATED
P.G. is started on a 1-L fluid bolus but he remains hypotensive, necessitating a norepinephrine drip. He does well for about 6 hours, but in the middle of the night he develops ventricular tachycardia and ventricular fibrillation, and a code is called. He is successfully resuscitated, but the family is looking for prognostic information.
3. What are P.G.’s chances of surviving and leaving the hospital?
- 5%
- 8%
- 15%
- 23%
A registry of cardiopulmonary resuscitation
Tian et al5 evaluated outcomes in the largest registry of cardiopulmonary resuscitation to date. In this analysis, 49,656 adult patients with a first cardiopulmonary arrest occurring in an ICU between January 1, 2000, and August 26, 2008, were evaluated for their outcomes on pressors vs those not on pressors.
Other independent predictors of a lower survival rate were nonwhite race, mechanical ventilation, having three or more immediate causes of cardiopulmonary arrest, age 65 years or older, and cardiopulmonary arrest occurring at night or over the weekend.
Fortunately, for our patient, survival rates were higher for patients with ventricular tachycardia or fibrillation than with other causes of cardiopulmonary arrest: 22.6% for those on pressors (like our patient) and 40.7% for those on no pressors.
CASE CONTINUED: HE RECOVERS AND GOES HOME
P.G. makes a remarkable recovery and is now ready to go home. It is the weekend, and you are unable to schedule a follow-up appointment before his discharge, so you ask him to make an appointment with his PCP.
4. What is the likelihood that P.G. will be readmitted within 1 month?
- 5%
- 12%
- 20%
- 25%
- 30%
The importance of follow-up with a primary care physician
Misky et al,6 in a small study, attempted to identify the characteristics and outcomes of discharged patients who lack timely follow-up with a PCP. They prospectively enrolled 65 patients admitted to University of Colorado Hospital, an urban 425-bed tertiary care center, collecting information about patient demographics, diagnosis, payer source, and PCPs. After discharge, they called the patients to determine their PCP follow-up and readmission status. Thirty-day readmission rates and hospital length of stay were compared in patients with and without timely PCP follow-up (ie, within 4 weeks).
Patients lacking timely PCP follow-up were 10 times more likely to be readmitted (odds ratio [OR] = 9.9, P = .04): the rate was 21% in patients lacking timely PCP follow-up vs 3% in patients with timely PCP follow-up, P = .03. Lack of insurance was associated with lower rates of timely PCP follow-up: 29% vs 56% (P = .06), but did not independently increase the readmission rate or length of stay (OR = 1.0, P = .96). Index hospital length of stay was longer in patients lacking timely PCP follow-up: 4.4 days vs 6.3 days, P = 0.11.
Comment. Nearly half of the patients in this study, who were discharged from a large urban academic center, lacked timely follow-up with a PCP, resulting in higher rates of readmission and a nonsignificant trend toward longer length of stay. Timely follow-up is necessary for vulnerable patients.
Since the lack of timely PCP follow-up results in higher readmission rates and possibly a longer length of stay, a PCP appointment at discharge should perhaps be considered a core quality measure. This would be problematic in our American health care system, in which many patients lack health insurance and do not have a PCP.
A MAN UNDERGOING GASTRIC BYPASS SURGERY
A 55-year-old morbidly obese man (body mass index 45 kg/m2) with a history of type 2 diabetes mellitus, chronic renal insufficiency (serum creatinine level 2.1 mg/dL), hypercholesterolemia, and previous stroke is scheduled for gastric bypass surgery. His functional capacity is low, but he is able to do his activities of daily living. He reports having dyspnea on exertion and intermittently at rest, but no chest pain. His medications include insulin, atorvastatin (Lipitor), aspirin, and atenolol (Tenormin). He is afebrile; his blood pressure is 130/80 mm Hg, pulse 75, and oxygen saturation 97% on room air. His baseline electrocardiogram shows no Q waves.
5. Which of the following is an appropriate next step before proceeding to surgery?
- Echocardiography
- Cardiac catheterization
- Dobutamine stress echocardiography or adenosine thallium scanning
- No cardiac testing is necessary before surgery
Is cardiac testing necessary before noncardiac surgery?
Wijeysundera et al7 performed a retrospective cohort study of patients who underwent elective surgery at acute care hospitals in Ontario, Canada, in the years 1994 through 2004. The aim was to determine the association of noninvasive cardiac stress testing before surgery with survival rates and length of hospital stay. Included were 271,082 patients, of whom 23,991 (8.9%) underwent stress testing less than 6 months before surgery. These patients were matched with 46,120 who did not undergo testing.
One year after surgery, fewer patients who underwent stress testing had died: 1,622 (7.0%) vs 1,738 (7.5%); hazard ratio 0.92, 95% CI 0.86–0.99, P = .03. The number needed to treat (ie, to be tested) to prevent one death was 221. The tested patients also had a shorter mean hospital stay: 8.72 vs 8.96 days, a difference of 0.24 days (95% CI −0.07 to −0.43; P < .001).
However, the elderly patients (ie, older than 66 years) who underwent testing were more likely to be on beta-blockers and statins than those who did not undergo testing, which may be a confounding factor.
Furthermore, the benefit was all in the patients at intermediate or high risk. The authors performed a subgroup analysis, dividing the patients on the basis of their Revised Cardiac Risk Index (RCRI; 1 point each for ischemic heart disease, congestive heart failure, cerebrovascular disease, diabetes, renal insufficiency, and high-risk surgery).8 Patients with an RCRI of 0 points (indicating low risk) actually had a higher risk of death with testing than without testing: hazard ratio 1.35 (95% CI 1.03–1.74), number needed to harm 179—ie, for every 179 low-risk patients tested, one excess death occurred. Those with an RCRI of 1 or 2 points (indicating intermediate risk) had a hazard ratio of 0.92 with testing (95% CI 085–0.99), and those with an RCRI of 3 to 6 points (indicating high risk) had a hazard ratio of 0.80 with testing (95% CI 0.67- 0.97; number needed to treat = 38).
Comment. These findings indicate that cardiac stress testing should be done selectively before noncardiac surgery, and primarily for patients at high risk (with an RCRI of 3 or higher) and in some patients at intermediate risk, but not in patients at low risk, in whom it may be harmful. Stress testing may change patient management because a positive stress test allows one to start a beta-blocker or a statin, use more aggressive intraoperative and postoperative care, and identify patients who have indications for revascularization.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:1144–1150.
- Pronovost PJ, Goeschel CA, Colantuoni E, et al. Sustaining reductions in catheter-related bloodstream infections in Michigan intensive care units: observational study. BMJ 2010; 340:c309.
- Tian J, Kaufman DA, Zarich S, et al; American Heart Association National Registry for Cardiopulmonary Resuscitation Investigators. Outcomes of critically ill patients who received cardiopulmonary resuscitation. Am J Respir Crit Care Med 2010; 182:501–506.
- Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med 2010; 5:392–397.
- Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Non-invasive cardiac stress testing before elective major non-cardiac surgery: population based cohort study. BMJ 2010; 340:b5526.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:1144–1150.
- Pronovost PJ, Goeschel CA, Colantuoni E, et al. Sustaining reductions in catheter-related bloodstream infections in Michigan intensive care units: observational study. BMJ 2010; 340:c309.
- Tian J, Kaufman DA, Zarich S, et al; American Heart Association National Registry for Cardiopulmonary Resuscitation Investigators. Outcomes of critically ill patients who received cardiopulmonary resuscitation. Am J Respir Crit Care Med 2010; 182:501–506.
- Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med 2010; 5:392–397.
- Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Non-invasive cardiac stress testing before elective major non-cardiac surgery: population based cohort study. BMJ 2010; 340:b5526.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
KEY POINTS
- Dabigatran (Pradaxa) will likely start to replace warfarin (Coumadin) both to prevent stroke in patients with atrial fibrillation and to prevent recurrent venous thromboembolism.
- Using a checklist during insertion of central venous catheters can decrease the rate of catheter-related bloodstream infections in the intensive care unit.
- The overall survival rate of patients who undergo cardiopulmonary resuscitation in the intensive care unit is approximately 16%; the rate is lower in patients who are receiving pressor drugs and higher in those with ventricular tachycardia or ventricular fibrillation.
- Patients lacking follow-up with a primary care physician within 30 days of discharge are at high risk of readmission and have a trend for longer length of hospital stay.
- Preoperative stress testing for patients undergoing noncardiac surgery should be done selectively, ie, in patients at high risk.