User login
Statin myopathy: A common dilemma not reflected in clinical trials
When a patient taking a statin complains of muscle aches, is he or she experiencing statin-induced myopathy or some other problem? Should statin therapy be discontinued?
Statins have proven efficacy in preventing heart attacks and death,1 and they are the most widely prescribed drugs worldwide. Nevertheless, they remain underused, with only 50% of those who would benefit from being on a statin receiving one.2,3 In addition, at least 25% of adults who start taking statins stop taking them by 6 months, and up to 60% stop by 2 years.4
Patient and physician fears about myopathy remain a key reason for stopping. Myopathy, a known side effect of statins, is rare in randomized controlled trials, but less so in observational studies and clinical experience. This discrepancy between clinical trials and clinical experience reduces confidence in lipid-lowering therapy and contributes to its underuse.
This review emphasizes clinical aspects of statin myopathy that are important to the practicing physician. We will define myopathy, review its purported mechanisms, and describe a clinical approach to patients with possible toxicity, including risk factors, physical findings, and consideration of alternate diagnoses. Since there is no single test to diagnose statin-induced myopathy, we offer a framework to aid clinicians in stratifying patients based on the likelihood that their symptoms are due to statin toxicity weighed against the likelihood that they will benefit from statin therapy.
DEFINITIONS DIFFER
Little consensus exists on how to define the adverse muscle effects of statins,5 which may contribute to the underdiagnosis of this complication. The magnitude of creatine kinase (CK) elevation required to define rhabdomyolysis has increased from 500 IU/L in 1982,6 to 1,000 IU/L in 1988,7 to 50 times the upper limit of normal in one current definition.8 The American Heart Association, the American College of Cardiology, the National Heart Lung and Blood Institute,9 the National Lipid Association,8 and the US Food and Drug Administration10 all differ in their definitions.
Our definitions
For the purpose of this article, we offer the following definitions:
Myalgia—muscle weakness, soreness, tenderness, stiffness, cramping, or aching, either at rest or with exertion, without any elevation in CK.
Myositis—elevated CK with or without muscle symptoms. The “-itis” suffix is unfortunate since myositis does not correspond to inflammation on biopsy.
Rhabdomyolysis—muscle symptoms with a CK level 10 times the upper limit of normal or higher. Evidence of renal dysfunction is not required for the diagnosis, as preexisting renal disease and hydration status are more closely related to kidney damage than the degree of muscle injury.11
STATIN MYOPATHY IS MORE COMMON IN THE REAL WORLD THAN IN TRIALS
The incidence of statin-induced myopathy is significantly lower in randomized controlled trials of statin efficacy than in observational studies of real-world patients. In randomized clinical trials, myalgia was reported in 1% to 5% of patients in the statin groups and placebo groups alike,9,12 whereas clinical practice would suggest it is more common.
Why is statin-induced myopathy so uncommon in clinical trials?
A reason may be that patients in clinical trials are carefully screened. To minimize toxicity, the clinical trials of statins excluded patients with renal insufficiency, hepatic insufficiency, a history of muscular complaints, and poorly controlled diabetes, as well as patients taking drugs with possible interactions. Large efficacy trials have excluded up to 30% of the participants in active prerandomization phases.13,14
Another reason is that these trials were designed to assess the efficacy of statins and were not sensitive to adverse effects like muscle pain. When they looked at myopathy, they focused on rhabdomyolysis—the most severe form—rather than on myalgia, fatigue, or other minor muscle complaints.15 Additionally, most trials enrolled too few patients and did not have long enough follow-up to reveal infrequent toxicities.
Despite the strict criteria, a significant number of trial patients discontinued statin therapy during the study period. In the Treat to New Targets (TNT) trial, 5% of patients in both the high- and low-dose atorvastatin (Lipitor) groups experienced muscle toxicity, even though 35% of eligible patients had been excluded during the open-label run-in phase.14
Also, physicians may overlook and patients may fail to report symptoms such as fatigue, malaise, or dyspnea that are not commonly accepted as signs of statin toxicity.16
Findings from observational studies
Observational studies in nonselected outpatients show a higher frequency of muscle complaints in the statin groups than in the control groups. These studies suggest the frequency of statin myopathy is 9% to 20%.17–19
The Prediction of Muscular Risk in Observational Conditions (PRIMO) study20 was one of the largest and best-defined observational studies of muscular symptoms in an unselected population. It included 7,924 French outpatients with hypercholesterolemia, ages 18 to 75 years, on high-dose statins for 3 or more months before the study. Daily statin regimens included atorvastatin 40 to 80 mg, fluvastatin (Lescol) 80 mg, pravastatin (Pravachol) 40 mg, and simvastatin (Zocor) 40 to 80 mg. In this study, 10.5% of patients reported muscle-related symptoms.
Buettner et al,21 in another cross-sectional study, interviewed and examined 3,580 adults over age 40. Of those taking statins, 22% reported having had musculoskeletal pain in at least one anatomic region in the last 30 days, compared with 16.7% of those not taking a statin.
In the United States, where an estimated 33 million adults use statins, musculoskeletal pain can be expected to occur in 7 million people, likely induced by statin therapy in 25% of cases.22
WHAT CAUSES STATIN MYOPATHY?
The causes of statin-induced myopathy are poorly understood.
Historically, statin-induced toxicity was thought to be caused by inhibition of the synthesis of mevalonate, leading to depletion of its metabolites, such as cholesterol, isoprenoids, and ubiquinone (coenzyme Q10). Depletion of intracellular cholesterol may lead to abnormal membrane behaviors; depletion of isoprenoids may affect intracellular signaling; depletion of coenzyme Q10 may in turn reduce mitochondrial respiratory function. Genetic factors may also play a role, contributing to pharmacokinetics and predisposing metabolic muscle disorders.23,24
Statin-induced myopathy seems to be different in randomized efficacy trials than in the clinical setting. In randomized trials, the mechanism appears to involve abnormal pharmacokinetics. The participants are carefully selected to have a low risk of muscle toxicity, but some develop toxicity when statin levels are elevated because of reduced drug breakdown and metabolism. However, in clinical practice, where a much larger group of unselected patients are exposed to statins, toxicity appears to be more related to a metabolic predisposition.25–27
Multiple minor metabolic abnormalities have been described in the muscles of patients with statin-induced muscle toxicity, suggesting that some patients have a predisposition for muscle complaints.23 About 25% of patients with recurrent rhabdomyolysis irrespective of lipid-lowering therapy have an underlying metabolic muscle disorder.28 In these vulnerable patients, minor metabolic defects are exacerbated by any agent that reduces the delivery of fat substrate to muscle, leading to muscle starvation.
This concept explains why patients may develop the same muscle complaint on different lipid-lowering agents.29,30 It also may explain why rhabdomyolysis can occur after apheresis of lipids, when no drugs have been given.31 In vulnerable patients, muscle toxicity may result from any agent that lessens the lipid substrate available to muscle rather than from the reduction of products downstream from mevalonate by statins.
SOME STATINS MAY BE LESS TOXIC
In the PRIMO study, muscle-related symptoms occurred with the various regimens as follows:
- Fluvastatin XL 40 mg—5.1%
- Pravastatin 40 mg—10.9%
- Atorvastatin 40 to 80 mg—14.9%
- Simvastatin 40 to 80 mg—18.2%.
Others have also shown that fluvastatin contributed to the smallest number of reported cases of rhabdomyolysis among statins: 55 (1.6%) of 3,339 cases.34
More recent studies indicate that rosuvastatin (Crestor), the most hydrophilic statin, may be well tolerated in those who do not tolerate other statins,35–37 though no head-to-head trial has been done.
RISK FACTORS FOR STATIN-INDUCED MYOPATHY
APPROACH TO SUSPECTED STATIN-INDUCED MYOPATHY
The recommendations that follow are based on observations from our statin myopathy clinic in more than 650 patients, of whom more than 60 have suffered rhabdomyolysis. This experience is largely anecdotal, since such patients have not been well studied in controlled trials.
Are the symptoms due to the statin?
In our experience, most patients who develop significant weakness and pain on statin therapy have normal CK levels.40
Since there is as yet no test to confirm or reject the diagnosis of statin toxicity, the first objective is to determine the likelihood that the muscle complaint is being caused by statin therapy. Factors that make statin toxicity more likely must be weighed against any features that are atypical or that favor an alternate diagnosis.
The final decision about future statin treatment depends on the balance between the expected benefit of statin therapy for each individual and the likelihood that the symptoms are due to statin therapy. Below, we provide an algorithmic approach based on history, physical examination, and laboratory findings.
Findings from the history that implicate statins
Many muscle symptoms resolve within 2 weeks of starting statin therapy. Therefore, if patients have a normal CK level and can tolerate the symptoms, we ask them to continue therapy and see if their symptoms resolve with continued use.
Symptoms that persist beyond the first 2 weeks of therapy are likely due to the statin. These include symmetric burning or pain in the large muscles during exercise that was not present before lipid-lowering therapy. Any symptom that reproducibly recurs with statin rechallenge and disappears within 2 weeks of discontinuing therapy is more likely to be caused by the statin.
Findings of the PRIMO study are representative of typical statin-induced symptoms that we see in our clinic.20
- Most patients did not identify a trigger, but the 40% who did had engaged in unusual physical exertion or had received a new drug in addition to the statin.
- Heaviness, stiffness, or cramps predominated in 70%, with only a quarter noting weakness and another quarter suffering myalgias during exercise. Pain was diffuse in 60% and more common in the lower extremities than the upper extremities.
- Physically active patients were more likely to suffer muscle symptoms than sedentary patients, echoing the observation by Sinzinger and O’Grady that athletes are especially intolerant of lipid-lowering therapy.41
- Patients who have had muscle complaints with other drug therapies such as bisphosphonates, 42 raloxifene (Evista),43 or diuretics may be having the same provocation of an underlying muscle predisposition by the statin.
- A personal or family history of muscle complaints predisposed patients to statin-induced myopathy.20
Finally, we have repeatedly found that when dyspnea and fatigue are associated with the muscle complaint, they are more likely to be caused by statins.44
Statins can unveil underlying musculoskeletal disorders
Atypical complaints require us to search for an alternate diagnosis before dismissing them as not statin-induced.
Previous episodes of myoglobinuria (dark urine after exertion) would raise the possibility of a metabolic myopathy. Frequent muscle cramps raise the possibility of a metabolic myopathy or motor neuron disease.
Asymmetric pain or pain involving joints and ligaments is less likely to be statin-related but in our experience occasionally occurs.
Some patients with underlying degenerative arthritis or tendinitis repeatedly develop worsening symptoms each time they take statins, perhaps because muscle weakness exacerbates the arthropathy or tendinopathy.45
Although statin-related muscle complaints are almost always symmetric, many patients with underlying peripheral vascular disease have asymmetric pain in the limb with poor vascular supply; the pain is reproducible by statin rechallenge.
In our experience, many patients whose chronic low back pain is due to a lumbar radiculopathy experience exacerbations of that pain whenever they start statins.
Weakness preceding the use of the statins or a family history of neuromuscular disorders may indicate a neurodegenerative disorder and warrants consideration of early neurological consultation.
Although these rules are not absolute, they are helpful in the initial evaluation, which must exclude alternative diagnoses.
Is the patient a vegetarian? A drinker? Taking supplements?
Taking a careful history of diet and supplement use is important to find exposures that may increase the risk of statin-related muscle complaints. Vegetarians may develop carnitine or vitamin B12 deficiencies. Alcohol and vitamin E and other supplements are occasional causes of muscle symptoms falsely attributed to statin therapy. It is also important to remember that red yeast rice contains lovastatin, which can exacerbate myopathy, especially when taken in conjunction with another statin.
Physical examination
The examination of patients with possible statin-induced myopathy begins with a general assessment for signs of hypothyroidism or excess alcohol consumption.
Ankle-brachial indices are used to exclude significant peripheral vascular disease.
The musculoskeletal examination focuses on muscle atrophy, tone, and strength but also excludes tendinopathies, arthropathies, and myofascial pain syndromes, which are often confused with muscle pain.
We conduct quantitative dynamometry, measuring handgrip with a Jamar dynamometer and hip abduction with a Nicholas Manual Muscle Tester. Precise dynamometric measurements are tracked at subsequent visits and are helpful in following recovery from myopathy as well as in tracking strength during subsequent statin rechallenges.
We routinely look for hyperreflexia, fasciculations, extensor-plantar responses, and decreased heel-to-shin movement, which would suggest myelopathy. Reflexes and a sensory examination including vibration and temperature sensation help exclude radiculopathy and peripheral neuropathy.
Laboratory evaluation
In every patient with possibly statin myopathy, the primary care physician should measure:
- The serum CK level (preferably more than 72 hours after exercise)
- The 25-hydroxy vitamin level
- The thyroid-stimulating hormone level.
Further laboratory evaluation depends on the findings and will often be directed by subspecialists. For example, we assess the sedimentation rate, anti-Ro and anti-La antibodies, and the myositis panel in patients with elevated CK whose other findings suggest an autoimmune or inflammatory process. We test serum carnitine levels (free, total, and esterified), fasting serum lactate levels, and serum cortisol in those with findings suggestive of metabolic myopathy. We order electromyography and nerve conduction studies in patients with possible myelopathy, peripheral neuropathy, or inflammatory myopathy.
Ultimately, a muscle biopsy may be necessary to exclude inflammatory or necrotizing myopathies in patients whose CK remains elevated despite withdrawal of statins. It may also be helpful when other findings suggest a metabolic myopathy. When a biopsy is needed, magnetic resonance imaging of the affected limb may identify an affected muscle for biopsy.
MANAGEMENT
Reassess the lipid goal
If the source of a complaint remains unclear after challenge and rechallenge with alternate statins, we generally recommend restarting therapy and trying to achieve the LDL-C goal. If the workup suggests a neurologic or rheumatolic etiology, a referral to a specialist is indicated. However, if the evaluation leads to a diagnosis of statin-induced myopathy, the next task is to reassess the lipid treatment goals.
The Adult Treatment Panel (ATP) III guidelines should be used to assess the patient’s risk of having major coronary heart disease in the next 10 years, and to determine appropriate LDL-C goals.46 Some patients with suspected toxicity may have been treated to more aggressive LDL-C levels than recommended by these guidelines. The first step in these patients is to reduce or discontinue the unnecessary statin, even if there is no clear evidence of toxicity.
For the rest of patients with suspected toxicity, the decision to discontinue statin therapy must be weighed against the estimated reduction in risk associated with taking a statin medication.
Prescribe a 6-week ‘statin holiday’ and see if symptoms resolve
In patients whose evaluation suggests statin myopathy, we stop all lipid-lowering therapy for 6 weeks and see if symptoms resolve and if grip and hip strength increase by dynamometry.
We often give these patient supplements of 600 mg daily of a bioavailable source of coenzyme Q10 and fish oil during this statin holiday. The data supporting the use of these supplements are mixed but the risks are minimal.5 In patients whose evaluation suggests a primary disorder in fatty acid oxidation, we add a trial of l-carnitine supplementation if symptoms do not resolve after a 6-week course of the coenzyme Q10 and fish oil.
If symptoms persist or if resolution is unclear at 6 weeks, we extend the holiday for an additional 6 weeks, except in patients with recent unstable coronary disease: for these patients, unless there is evidence of rhabdomyolysis, we believe that the benefits of continued statin therapy exceed the risks.
If the initial evaluation is consistent with statin myopathy and the neuromuscular symptoms (myalgias and weakness) do not respond within a few months of statin withdrawal, neurologic consultation is indicated to evaluate for an underlying neurologic disorder that has become symptomatic during statin therapy but whose existence is independent of the statin therapy. In some cases, the preexisting neurologic disorder may become symptomatic because of the statin therapy and remain symptomatic despite discontinuation of the statin therapy.
Restarting lipid-lowering therapy
Once the myopathy symptoms have abated or are controlled, a rechallenge of statin therapy is in order for those whose risk profile suggests greater benefit from statin therapy.
We consider the complete statin exposure history and any concomitant therapy that may have been competing with cytochrome P450 (CYP) metabolism of statins in designing an alternate lipid-lowering plan.
For patients with known coronary or vascular disease, in whom the survival benefit of statins is greatest, we generally try to find a statin regimen that is tolerable. Long-acting fluvastatin or a statin with less CYP dependence, such as pravastatin, is often successful.59 For patients whose myopathy has recurred with multiple statin rechallenges or whose lipid-lowering goal requires a more potent therapy, rosuvastatin in alternate-day or once- or twice-a-week schedules is efficacious and well tolerated in many patients.36,37,60 Of note, however, although such alternate-day therapies may produce excellent reductions in cholesterol levels, these regimens have not been proven to reduce cardiovascular end points.
Alternative lipid-lowering therapy. Occasionally, a patient cannot tolerate even intermittent rosuvastatin. In these cases, we prescribe resin therapy, which is well tolerated in those with recurrent statin myopathy.61
Although some believe that ezetimibe (Zetia) is an option for these patients, we do not agree, since it often causes similar muscle complaints in the most sensitive statin myopathy patients.30 Furthermore, ezetimibe has not been shown to improve cardiac end points.
Red yeast rice is also not a safe alternative in these patients, in whom muscle complaints and CK elevations frequently develop anew on this unregulated supplement despite its low lovastatin equivalence, 6 mg a day.62 A recent study showed that there is wide variability in the amount of lovastatin in over-the-counter red yeast rice; the median dose was 6 mg, and the maximum dose was 14.5 mg.63
The ultimate lipid-lowering plan for most of these patients will require a compromise between the ideal LDL-C goal and the LDL-C level that is achievable with these alternate attempts at lipid lowering.
While combination therapy may be attractive in patients with combined lipid disorders and no muscle complaints, fibrates are more likely to cause muscle toxicity per dose prescribed than statins, and the addition of fibrates to statin therapy increases the risks of muscle reactions.64,65 The evidence that fibrates reduce cardiovascular end points is much less robust than that for statins, which further reduces enthusiasm for combination therapy in patients with statin muscle toxicity.66,67
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Foley KA, Simpson RJ, Crouse JR, et al. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- O’Meara JG, Kardia SL, Armon JJ, et al. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med 2004; 164:1313–1318.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore) 1982; 61:141–152.
- Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med 1988; 148:1553–1557.
- McKenney JM, Davidson MH, Jacobson TA, et al. National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–84C.
- Pasternak RC, Smith SC, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002; 40:567–572.
- Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep 2007; 9:389–396.
- Linares LA, Golomb BA, Jaojoco JA, et al. The modern spectrum of rhabdomyolysis: drug toxicity revealed by creatine kinase screening. Curr Drug Saf 2009; 4:181–187.
- Armitage J. The safety of statins in clinical practice. Lancet 2007; 370:1781–1790.
- Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361:2005–2016.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol 2002; 40:163–171.
- de Sauvage Nolting PR, Buirma RJ, et al. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90:181–184.
- Franc S, Dejager S, Bruckert E, et al. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc Drugs Ther 2003; 17:459–465.
- Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation 2006; 114:2788–2797.
- Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Spatz ES, Canavan ME, Desai MM. From here to JUPITER: identifying new patients for statin therapy using data from the 1999–2004 National Health and Nutrition Examination Survey. Circ Cardiovasc Qual Outcomes 2009; 2:41–48.
- Vladutiu GD, Simmons Z, Isackson PJ, et al. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve 2006; 34:153–162.
- Vladutiu GD. Genetic predisposition to statin myopathy. Curr Opin Rheumatol 2008; 20:648–655.
- Phillips PS, Phillips CT, Sullivan MJ, et al. Statin myotoxicity is associated with changes in the cardiopulmonary function. Atherosclerosis 2004; 177:183–188.
- Phillips PS, Ciaraldi TP, Kim DL, et al. Myotoxic reactions to lipidlowering therapy are associated with altered oxidation of fatty acids. Endocrine 2009; 35:38–46.
- Phillips PS, Haas RH. Statin myopathy as a metabolic muscle disease. Expert Rev Cardiovasc Ther 2008; 6:971–978.
- Löfberg M, Jänkälä H, Paetau A, et al. Metabolic causes of recurrent rhabdomyolysis. Acta Neurol Scand 1998; 98:268–275.
- Havranek JM, Wolfsen A, Warnke GA, et al. Monotherapy with Ezetimibe Causing Myopathy. Am J Med 2006; 119:285–286.
- Phillips PS. Ezetimibe and statin-associated myopathy. Ann Intern Med 2004; 141:649.
- Durst R, Rund D, Schurr D, et al. One year experience with a low density lipoprotein apheresis system. Isr Med Assoc J 2002; 4:677–680.
- Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol 1997; 80:106–107.
- Brewer HB. Benefit-risk assessment of rosuvastatin 10 to 40 milligrams. Am J Cardiol 2003; 92:23K–29K.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Backes JM, Venero CV, Gibson CA, et al. Effectiveness and tolerability of every-other-day rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother 2008; 42:341–346.
- Backes JM, Moriarty PM, Ruisinger JF, et al. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med 2006; 119:400–409.
- Jacobson TA. Toward “pain-free” statin prescribing: clinical algorithm for diagnosis and management of myalgia. Mayo Clin Proc 2008; 83:687–700.
- Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002; 137:581–585.
- Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol 2004; 57:525–528.
- Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med 2005; 165:346–347.
- Martino S, Disch D, Dowsett SA, et al. Safety assessment of raloxifene over eight years in a clinical trial setting. Curr Med Res Opin 2005; 21:1441–1452.
- Phillips PS, Kimura BJ, Kelley R, et al. Feasibility of using the internet to perform medical research: observations from a statin-myopathy public information web site. Circulation 2003; 107:e7001–e7039.
- Chazerain P, Hayem G, Hamza S, et al. Four cases of tendinopathy in patients on statin therapy. Joint Bone Spine 2001; 68:430–433.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004; 44:720–732.
- Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation 1998; 97:1453–1460.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995; 333:1301–1307.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006; 368:1155–1163.
- Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHATLLT). JAMA 2002; 288:2998–3007.
- Stein EA, Ballantyne CM, Windler E, et al. Efficacy and tolerability of fluvastatin XL 80 mg alone, ezetimibe alone, and the combination of fluvastatin XL 80 mg with ezetimibe in patients with a history of muscle-related side effects with other statins. Am J Cardiol 2008; 101:490–496.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Phillips PS, Gray NL, McDonald FG, et al. Colesevelam is safe and effective in patients with statin myotoxicity. Arterioscler Thromb Vasc Biol 2005 25:E-97.
- Phillips PS. Balancing randomized trials with anecdote. Ann Intern Med 2009; 150:885–886.
- Gordon RY, Cooperman T, Obermeyer W, et al. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware! Arch Intern Med 2010; 170:1722–1727.
- Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004; 292:2585–2590.
- Gaist D, Rodríguez LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001; 12:565–569.
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366:1849–1861.
- ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
When a patient taking a statin complains of muscle aches, is he or she experiencing statin-induced myopathy or some other problem? Should statin therapy be discontinued?
Statins have proven efficacy in preventing heart attacks and death,1 and they are the most widely prescribed drugs worldwide. Nevertheless, they remain underused, with only 50% of those who would benefit from being on a statin receiving one.2,3 In addition, at least 25% of adults who start taking statins stop taking them by 6 months, and up to 60% stop by 2 years.4
Patient and physician fears about myopathy remain a key reason for stopping. Myopathy, a known side effect of statins, is rare in randomized controlled trials, but less so in observational studies and clinical experience. This discrepancy between clinical trials and clinical experience reduces confidence in lipid-lowering therapy and contributes to its underuse.
This review emphasizes clinical aspects of statin myopathy that are important to the practicing physician. We will define myopathy, review its purported mechanisms, and describe a clinical approach to patients with possible toxicity, including risk factors, physical findings, and consideration of alternate diagnoses. Since there is no single test to diagnose statin-induced myopathy, we offer a framework to aid clinicians in stratifying patients based on the likelihood that their symptoms are due to statin toxicity weighed against the likelihood that they will benefit from statin therapy.
DEFINITIONS DIFFER
Little consensus exists on how to define the adverse muscle effects of statins,5 which may contribute to the underdiagnosis of this complication. The magnitude of creatine kinase (CK) elevation required to define rhabdomyolysis has increased from 500 IU/L in 1982,6 to 1,000 IU/L in 1988,7 to 50 times the upper limit of normal in one current definition.8 The American Heart Association, the American College of Cardiology, the National Heart Lung and Blood Institute,9 the National Lipid Association,8 and the US Food and Drug Administration10 all differ in their definitions.
Our definitions
For the purpose of this article, we offer the following definitions:
Myalgia—muscle weakness, soreness, tenderness, stiffness, cramping, or aching, either at rest or with exertion, without any elevation in CK.
Myositis—elevated CK with or without muscle symptoms. The “-itis” suffix is unfortunate since myositis does not correspond to inflammation on biopsy.
Rhabdomyolysis—muscle symptoms with a CK level 10 times the upper limit of normal or higher. Evidence of renal dysfunction is not required for the diagnosis, as preexisting renal disease and hydration status are more closely related to kidney damage than the degree of muscle injury.11
STATIN MYOPATHY IS MORE COMMON IN THE REAL WORLD THAN IN TRIALS
The incidence of statin-induced myopathy is significantly lower in randomized controlled trials of statin efficacy than in observational studies of real-world patients. In randomized clinical trials, myalgia was reported in 1% to 5% of patients in the statin groups and placebo groups alike,9,12 whereas clinical practice would suggest it is more common.
Why is statin-induced myopathy so uncommon in clinical trials?
A reason may be that patients in clinical trials are carefully screened. To minimize toxicity, the clinical trials of statins excluded patients with renal insufficiency, hepatic insufficiency, a history of muscular complaints, and poorly controlled diabetes, as well as patients taking drugs with possible interactions. Large efficacy trials have excluded up to 30% of the participants in active prerandomization phases.13,14
Another reason is that these trials were designed to assess the efficacy of statins and were not sensitive to adverse effects like muscle pain. When they looked at myopathy, they focused on rhabdomyolysis—the most severe form—rather than on myalgia, fatigue, or other minor muscle complaints.15 Additionally, most trials enrolled too few patients and did not have long enough follow-up to reveal infrequent toxicities.
Despite the strict criteria, a significant number of trial patients discontinued statin therapy during the study period. In the Treat to New Targets (TNT) trial, 5% of patients in both the high- and low-dose atorvastatin (Lipitor) groups experienced muscle toxicity, even though 35% of eligible patients had been excluded during the open-label run-in phase.14
Also, physicians may overlook and patients may fail to report symptoms such as fatigue, malaise, or dyspnea that are not commonly accepted as signs of statin toxicity.16
Findings from observational studies
Observational studies in nonselected outpatients show a higher frequency of muscle complaints in the statin groups than in the control groups. These studies suggest the frequency of statin myopathy is 9% to 20%.17–19
The Prediction of Muscular Risk in Observational Conditions (PRIMO) study20 was one of the largest and best-defined observational studies of muscular symptoms in an unselected population. It included 7,924 French outpatients with hypercholesterolemia, ages 18 to 75 years, on high-dose statins for 3 or more months before the study. Daily statin regimens included atorvastatin 40 to 80 mg, fluvastatin (Lescol) 80 mg, pravastatin (Pravachol) 40 mg, and simvastatin (Zocor) 40 to 80 mg. In this study, 10.5% of patients reported muscle-related symptoms.
Buettner et al,21 in another cross-sectional study, interviewed and examined 3,580 adults over age 40. Of those taking statins, 22% reported having had musculoskeletal pain in at least one anatomic region in the last 30 days, compared with 16.7% of those not taking a statin.
In the United States, where an estimated 33 million adults use statins, musculoskeletal pain can be expected to occur in 7 million people, likely induced by statin therapy in 25% of cases.22
WHAT CAUSES STATIN MYOPATHY?
The causes of statin-induced myopathy are poorly understood.
Historically, statin-induced toxicity was thought to be caused by inhibition of the synthesis of mevalonate, leading to depletion of its metabolites, such as cholesterol, isoprenoids, and ubiquinone (coenzyme Q10). Depletion of intracellular cholesterol may lead to abnormal membrane behaviors; depletion of isoprenoids may affect intracellular signaling; depletion of coenzyme Q10 may in turn reduce mitochondrial respiratory function. Genetic factors may also play a role, contributing to pharmacokinetics and predisposing metabolic muscle disorders.23,24
Statin-induced myopathy seems to be different in randomized efficacy trials than in the clinical setting. In randomized trials, the mechanism appears to involve abnormal pharmacokinetics. The participants are carefully selected to have a low risk of muscle toxicity, but some develop toxicity when statin levels are elevated because of reduced drug breakdown and metabolism. However, in clinical practice, where a much larger group of unselected patients are exposed to statins, toxicity appears to be more related to a metabolic predisposition.25–27
Multiple minor metabolic abnormalities have been described in the muscles of patients with statin-induced muscle toxicity, suggesting that some patients have a predisposition for muscle complaints.23 About 25% of patients with recurrent rhabdomyolysis irrespective of lipid-lowering therapy have an underlying metabolic muscle disorder.28 In these vulnerable patients, minor metabolic defects are exacerbated by any agent that reduces the delivery of fat substrate to muscle, leading to muscle starvation.
This concept explains why patients may develop the same muscle complaint on different lipid-lowering agents.29,30 It also may explain why rhabdomyolysis can occur after apheresis of lipids, when no drugs have been given.31 In vulnerable patients, muscle toxicity may result from any agent that lessens the lipid substrate available to muscle rather than from the reduction of products downstream from mevalonate by statins.
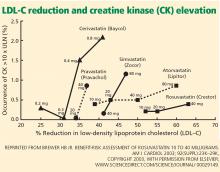
SOME STATINS MAY BE LESS TOXIC
In the PRIMO study, muscle-related symptoms occurred with the various regimens as follows:
- Fluvastatin XL 40 mg—5.1%
- Pravastatin 40 mg—10.9%
- Atorvastatin 40 to 80 mg—14.9%
- Simvastatin 40 to 80 mg—18.2%.
Others have also shown that fluvastatin contributed to the smallest number of reported cases of rhabdomyolysis among statins: 55 (1.6%) of 3,339 cases.34
More recent studies indicate that rosuvastatin (Crestor), the most hydrophilic statin, may be well tolerated in those who do not tolerate other statins,35–37 though no head-to-head trial has been done.
RISK FACTORS FOR STATIN-INDUCED MYOPATHY
APPROACH TO SUSPECTED STATIN-INDUCED MYOPATHY
The recommendations that follow are based on observations from our statin myopathy clinic in more than 650 patients, of whom more than 60 have suffered rhabdomyolysis. This experience is largely anecdotal, since such patients have not been well studied in controlled trials.
Are the symptoms due to the statin?
In our experience, most patients who develop significant weakness and pain on statin therapy have normal CK levels.40
Since there is as yet no test to confirm or reject the diagnosis of statin toxicity, the first objective is to determine the likelihood that the muscle complaint is being caused by statin therapy. Factors that make statin toxicity more likely must be weighed against any features that are atypical or that favor an alternate diagnosis.
The final decision about future statin treatment depends on the balance between the expected benefit of statin therapy for each individual and the likelihood that the symptoms are due to statin therapy. Below, we provide an algorithmic approach based on history, physical examination, and laboratory findings.
Findings from the history that implicate statins
Many muscle symptoms resolve within 2 weeks of starting statin therapy. Therefore, if patients have a normal CK level and can tolerate the symptoms, we ask them to continue therapy and see if their symptoms resolve with continued use.
Symptoms that persist beyond the first 2 weeks of therapy are likely due to the statin. These include symmetric burning or pain in the large muscles during exercise that was not present before lipid-lowering therapy. Any symptom that reproducibly recurs with statin rechallenge and disappears within 2 weeks of discontinuing therapy is more likely to be caused by the statin.
Findings of the PRIMO study are representative of typical statin-induced symptoms that we see in our clinic.20
- Most patients did not identify a trigger, but the 40% who did had engaged in unusual physical exertion or had received a new drug in addition to the statin.
- Heaviness, stiffness, or cramps predominated in 70%, with only a quarter noting weakness and another quarter suffering myalgias during exercise. Pain was diffuse in 60% and more common in the lower extremities than the upper extremities.
- Physically active patients were more likely to suffer muscle symptoms than sedentary patients, echoing the observation by Sinzinger and O’Grady that athletes are especially intolerant of lipid-lowering therapy.41
- Patients who have had muscle complaints with other drug therapies such as bisphosphonates, 42 raloxifene (Evista),43 or diuretics may be having the same provocation of an underlying muscle predisposition by the statin.
- A personal or family history of muscle complaints predisposed patients to statin-induced myopathy.20
Finally, we have repeatedly found that when dyspnea and fatigue are associated with the muscle complaint, they are more likely to be caused by statins.44
Statins can unveil underlying musculoskeletal disorders
Atypical complaints require us to search for an alternate diagnosis before dismissing them as not statin-induced.
Previous episodes of myoglobinuria (dark urine after exertion) would raise the possibility of a metabolic myopathy. Frequent muscle cramps raise the possibility of a metabolic myopathy or motor neuron disease.
Asymmetric pain or pain involving joints and ligaments is less likely to be statin-related but in our experience occasionally occurs.
Some patients with underlying degenerative arthritis or tendinitis repeatedly develop worsening symptoms each time they take statins, perhaps because muscle weakness exacerbates the arthropathy or tendinopathy.45
Although statin-related muscle complaints are almost always symmetric, many patients with underlying peripheral vascular disease have asymmetric pain in the limb with poor vascular supply; the pain is reproducible by statin rechallenge.
In our experience, many patients whose chronic low back pain is due to a lumbar radiculopathy experience exacerbations of that pain whenever they start statins.
Weakness preceding the use of the statins or a family history of neuromuscular disorders may indicate a neurodegenerative disorder and warrants consideration of early neurological consultation.
Although these rules are not absolute, they are helpful in the initial evaluation, which must exclude alternative diagnoses.
Is the patient a vegetarian? A drinker? Taking supplements?
Taking a careful history of diet and supplement use is important to find exposures that may increase the risk of statin-related muscle complaints. Vegetarians may develop carnitine or vitamin B12 deficiencies. Alcohol and vitamin E and other supplements are occasional causes of muscle symptoms falsely attributed to statin therapy. It is also important to remember that red yeast rice contains lovastatin, which can exacerbate myopathy, especially when taken in conjunction with another statin.
Physical examination
The examination of patients with possible statin-induced myopathy begins with a general assessment for signs of hypothyroidism or excess alcohol consumption.
Ankle-brachial indices are used to exclude significant peripheral vascular disease.
The musculoskeletal examination focuses on muscle atrophy, tone, and strength but also excludes tendinopathies, arthropathies, and myofascial pain syndromes, which are often confused with muscle pain.
We conduct quantitative dynamometry, measuring handgrip with a Jamar dynamometer and hip abduction with a Nicholas Manual Muscle Tester. Precise dynamometric measurements are tracked at subsequent visits and are helpful in following recovery from myopathy as well as in tracking strength during subsequent statin rechallenges.
We routinely look for hyperreflexia, fasciculations, extensor-plantar responses, and decreased heel-to-shin movement, which would suggest myelopathy. Reflexes and a sensory examination including vibration and temperature sensation help exclude radiculopathy and peripheral neuropathy.
Laboratory evaluation
In every patient with possibly statin myopathy, the primary care physician should measure:
- The serum CK level (preferably more than 72 hours after exercise)
- The 25-hydroxy vitamin level
- The thyroid-stimulating hormone level.
Further laboratory evaluation depends on the findings and will often be directed by subspecialists. For example, we assess the sedimentation rate, anti-Ro and anti-La antibodies, and the myositis panel in patients with elevated CK whose other findings suggest an autoimmune or inflammatory process. We test serum carnitine levels (free, total, and esterified), fasting serum lactate levels, and serum cortisol in those with findings suggestive of metabolic myopathy. We order electromyography and nerve conduction studies in patients with possible myelopathy, peripheral neuropathy, or inflammatory myopathy.
Ultimately, a muscle biopsy may be necessary to exclude inflammatory or necrotizing myopathies in patients whose CK remains elevated despite withdrawal of statins. It may also be helpful when other findings suggest a metabolic myopathy. When a biopsy is needed, magnetic resonance imaging of the affected limb may identify an affected muscle for biopsy.
MANAGEMENT
Reassess the lipid goal
If the source of a complaint remains unclear after challenge and rechallenge with alternate statins, we generally recommend restarting therapy and trying to achieve the LDL-C goal. If the workup suggests a neurologic or rheumatolic etiology, a referral to a specialist is indicated. However, if the evaluation leads to a diagnosis of statin-induced myopathy, the next task is to reassess the lipid treatment goals.
The Adult Treatment Panel (ATP) III guidelines should be used to assess the patient’s risk of having major coronary heart disease in the next 10 years, and to determine appropriate LDL-C goals.46 Some patients with suspected toxicity may have been treated to more aggressive LDL-C levels than recommended by these guidelines. The first step in these patients is to reduce or discontinue the unnecessary statin, even if there is no clear evidence of toxicity.
For the rest of patients with suspected toxicity, the decision to discontinue statin therapy must be weighed against the estimated reduction in risk associated with taking a statin medication.
Prescribe a 6-week ‘statin holiday’ and see if symptoms resolve
In patients whose evaluation suggests statin myopathy, we stop all lipid-lowering therapy for 6 weeks and see if symptoms resolve and if grip and hip strength increase by dynamometry.
We often give these patient supplements of 600 mg daily of a bioavailable source of coenzyme Q10 and fish oil during this statin holiday. The data supporting the use of these supplements are mixed but the risks are minimal.5 In patients whose evaluation suggests a primary disorder in fatty acid oxidation, we add a trial of l-carnitine supplementation if symptoms do not resolve after a 6-week course of the coenzyme Q10 and fish oil.
If symptoms persist or if resolution is unclear at 6 weeks, we extend the holiday for an additional 6 weeks, except in patients with recent unstable coronary disease: for these patients, unless there is evidence of rhabdomyolysis, we believe that the benefits of continued statin therapy exceed the risks.
If the initial evaluation is consistent with statin myopathy and the neuromuscular symptoms (myalgias and weakness) do not respond within a few months of statin withdrawal, neurologic consultation is indicated to evaluate for an underlying neurologic disorder that has become symptomatic during statin therapy but whose existence is independent of the statin therapy. In some cases, the preexisting neurologic disorder may become symptomatic because of the statin therapy and remain symptomatic despite discontinuation of the statin therapy.
Restarting lipid-lowering therapy
Once the myopathy symptoms have abated or are controlled, a rechallenge of statin therapy is in order for those whose risk profile suggests greater benefit from statin therapy.
We consider the complete statin exposure history and any concomitant therapy that may have been competing with cytochrome P450 (CYP) metabolism of statins in designing an alternate lipid-lowering plan.
For patients with known coronary or vascular disease, in whom the survival benefit of statins is greatest, we generally try to find a statin regimen that is tolerable. Long-acting fluvastatin or a statin with less CYP dependence, such as pravastatin, is often successful.59 For patients whose myopathy has recurred with multiple statin rechallenges or whose lipid-lowering goal requires a more potent therapy, rosuvastatin in alternate-day or once- or twice-a-week schedules is efficacious and well tolerated in many patients.36,37,60 Of note, however, although such alternate-day therapies may produce excellent reductions in cholesterol levels, these regimens have not been proven to reduce cardiovascular end points.
Alternative lipid-lowering therapy. Occasionally, a patient cannot tolerate even intermittent rosuvastatin. In these cases, we prescribe resin therapy, which is well tolerated in those with recurrent statin myopathy.61
Although some believe that ezetimibe (Zetia) is an option for these patients, we do not agree, since it often causes similar muscle complaints in the most sensitive statin myopathy patients.30 Furthermore, ezetimibe has not been shown to improve cardiac end points.
Red yeast rice is also not a safe alternative in these patients, in whom muscle complaints and CK elevations frequently develop anew on this unregulated supplement despite its low lovastatin equivalence, 6 mg a day.62 A recent study showed that there is wide variability in the amount of lovastatin in over-the-counter red yeast rice; the median dose was 6 mg, and the maximum dose was 14.5 mg.63
The ultimate lipid-lowering plan for most of these patients will require a compromise between the ideal LDL-C goal and the LDL-C level that is achievable with these alternate attempts at lipid lowering.
While combination therapy may be attractive in patients with combined lipid disorders and no muscle complaints, fibrates are more likely to cause muscle toxicity per dose prescribed than statins, and the addition of fibrates to statin therapy increases the risks of muscle reactions.64,65 The evidence that fibrates reduce cardiovascular end points is much less robust than that for statins, which further reduces enthusiasm for combination therapy in patients with statin muscle toxicity.66,67
When a patient taking a statin complains of muscle aches, is he or she experiencing statin-induced myopathy or some other problem? Should statin therapy be discontinued?
Statins have proven efficacy in preventing heart attacks and death,1 and they are the most widely prescribed drugs worldwide. Nevertheless, they remain underused, with only 50% of those who would benefit from being on a statin receiving one.2,3 In addition, at least 25% of adults who start taking statins stop taking them by 6 months, and up to 60% stop by 2 years.4
Patient and physician fears about myopathy remain a key reason for stopping. Myopathy, a known side effect of statins, is rare in randomized controlled trials, but less so in observational studies and clinical experience. This discrepancy between clinical trials and clinical experience reduces confidence in lipid-lowering therapy and contributes to its underuse.
This review emphasizes clinical aspects of statin myopathy that are important to the practicing physician. We will define myopathy, review its purported mechanisms, and describe a clinical approach to patients with possible toxicity, including risk factors, physical findings, and consideration of alternate diagnoses. Since there is no single test to diagnose statin-induced myopathy, we offer a framework to aid clinicians in stratifying patients based on the likelihood that their symptoms are due to statin toxicity weighed against the likelihood that they will benefit from statin therapy.
DEFINITIONS DIFFER
Little consensus exists on how to define the adverse muscle effects of statins,5 which may contribute to the underdiagnosis of this complication. The magnitude of creatine kinase (CK) elevation required to define rhabdomyolysis has increased from 500 IU/L in 1982,6 to 1,000 IU/L in 1988,7 to 50 times the upper limit of normal in one current definition.8 The American Heart Association, the American College of Cardiology, the National Heart Lung and Blood Institute,9 the National Lipid Association,8 and the US Food and Drug Administration10 all differ in their definitions.
Our definitions
For the purpose of this article, we offer the following definitions:
Myalgia—muscle weakness, soreness, tenderness, stiffness, cramping, or aching, either at rest or with exertion, without any elevation in CK.
Myositis—elevated CK with or without muscle symptoms. The “-itis” suffix is unfortunate since myositis does not correspond to inflammation on biopsy.
Rhabdomyolysis—muscle symptoms with a CK level 10 times the upper limit of normal or higher. Evidence of renal dysfunction is not required for the diagnosis, as preexisting renal disease and hydration status are more closely related to kidney damage than the degree of muscle injury.11
STATIN MYOPATHY IS MORE COMMON IN THE REAL WORLD THAN IN TRIALS
The incidence of statin-induced myopathy is significantly lower in randomized controlled trials of statin efficacy than in observational studies of real-world patients. In randomized clinical trials, myalgia was reported in 1% to 5% of patients in the statin groups and placebo groups alike,9,12 whereas clinical practice would suggest it is more common.
Why is statin-induced myopathy so uncommon in clinical trials?
A reason may be that patients in clinical trials are carefully screened. To minimize toxicity, the clinical trials of statins excluded patients with renal insufficiency, hepatic insufficiency, a history of muscular complaints, and poorly controlled diabetes, as well as patients taking drugs with possible interactions. Large efficacy trials have excluded up to 30% of the participants in active prerandomization phases.13,14
Another reason is that these trials were designed to assess the efficacy of statins and were not sensitive to adverse effects like muscle pain. When they looked at myopathy, they focused on rhabdomyolysis—the most severe form—rather than on myalgia, fatigue, or other minor muscle complaints.15 Additionally, most trials enrolled too few patients and did not have long enough follow-up to reveal infrequent toxicities.
Despite the strict criteria, a significant number of trial patients discontinued statin therapy during the study period. In the Treat to New Targets (TNT) trial, 5% of patients in both the high- and low-dose atorvastatin (Lipitor) groups experienced muscle toxicity, even though 35% of eligible patients had been excluded during the open-label run-in phase.14
Also, physicians may overlook and patients may fail to report symptoms such as fatigue, malaise, or dyspnea that are not commonly accepted as signs of statin toxicity.16
Findings from observational studies
Observational studies in nonselected outpatients show a higher frequency of muscle complaints in the statin groups than in the control groups. These studies suggest the frequency of statin myopathy is 9% to 20%.17–19
The Prediction of Muscular Risk in Observational Conditions (PRIMO) study20 was one of the largest and best-defined observational studies of muscular symptoms in an unselected population. It included 7,924 French outpatients with hypercholesterolemia, ages 18 to 75 years, on high-dose statins for 3 or more months before the study. Daily statin regimens included atorvastatin 40 to 80 mg, fluvastatin (Lescol) 80 mg, pravastatin (Pravachol) 40 mg, and simvastatin (Zocor) 40 to 80 mg. In this study, 10.5% of patients reported muscle-related symptoms.
Buettner et al,21 in another cross-sectional study, interviewed and examined 3,580 adults over age 40. Of those taking statins, 22% reported having had musculoskeletal pain in at least one anatomic region in the last 30 days, compared with 16.7% of those not taking a statin.
In the United States, where an estimated 33 million adults use statins, musculoskeletal pain can be expected to occur in 7 million people, likely induced by statin therapy in 25% of cases.22
WHAT CAUSES STATIN MYOPATHY?
The causes of statin-induced myopathy are poorly understood.
Historically, statin-induced toxicity was thought to be caused by inhibition of the synthesis of mevalonate, leading to depletion of its metabolites, such as cholesterol, isoprenoids, and ubiquinone (coenzyme Q10). Depletion of intracellular cholesterol may lead to abnormal membrane behaviors; depletion of isoprenoids may affect intracellular signaling; depletion of coenzyme Q10 may in turn reduce mitochondrial respiratory function. Genetic factors may also play a role, contributing to pharmacokinetics and predisposing metabolic muscle disorders.23,24
Statin-induced myopathy seems to be different in randomized efficacy trials than in the clinical setting. In randomized trials, the mechanism appears to involve abnormal pharmacokinetics. The participants are carefully selected to have a low risk of muscle toxicity, but some develop toxicity when statin levels are elevated because of reduced drug breakdown and metabolism. However, in clinical practice, where a much larger group of unselected patients are exposed to statins, toxicity appears to be more related to a metabolic predisposition.25–27
Multiple minor metabolic abnormalities have been described in the muscles of patients with statin-induced muscle toxicity, suggesting that some patients have a predisposition for muscle complaints.23 About 25% of patients with recurrent rhabdomyolysis irrespective of lipid-lowering therapy have an underlying metabolic muscle disorder.28 In these vulnerable patients, minor metabolic defects are exacerbated by any agent that reduces the delivery of fat substrate to muscle, leading to muscle starvation.
This concept explains why patients may develop the same muscle complaint on different lipid-lowering agents.29,30 It also may explain why rhabdomyolysis can occur after apheresis of lipids, when no drugs have been given.31 In vulnerable patients, muscle toxicity may result from any agent that lessens the lipid substrate available to muscle rather than from the reduction of products downstream from mevalonate by statins.
SOME STATINS MAY BE LESS TOXIC
In the PRIMO study, muscle-related symptoms occurred with the various regimens as follows:
- Fluvastatin XL 40 mg—5.1%
- Pravastatin 40 mg—10.9%
- Atorvastatin 40 to 80 mg—14.9%
- Simvastatin 40 to 80 mg—18.2%.
Others have also shown that fluvastatin contributed to the smallest number of reported cases of rhabdomyolysis among statins: 55 (1.6%) of 3,339 cases.34
More recent studies indicate that rosuvastatin (Crestor), the most hydrophilic statin, may be well tolerated in those who do not tolerate other statins,35–37 though no head-to-head trial has been done.
RISK FACTORS FOR STATIN-INDUCED MYOPATHY
APPROACH TO SUSPECTED STATIN-INDUCED MYOPATHY
The recommendations that follow are based on observations from our statin myopathy clinic in more than 650 patients, of whom more than 60 have suffered rhabdomyolysis. This experience is largely anecdotal, since such patients have not been well studied in controlled trials.
Are the symptoms due to the statin?
In our experience, most patients who develop significant weakness and pain on statin therapy have normal CK levels.40
Since there is as yet no test to confirm or reject the diagnosis of statin toxicity, the first objective is to determine the likelihood that the muscle complaint is being caused by statin therapy. Factors that make statin toxicity more likely must be weighed against any features that are atypical or that favor an alternate diagnosis.
The final decision about future statin treatment depends on the balance between the expected benefit of statin therapy for each individual and the likelihood that the symptoms are due to statin therapy. Below, we provide an algorithmic approach based on history, physical examination, and laboratory findings.
Findings from the history that implicate statins
Many muscle symptoms resolve within 2 weeks of starting statin therapy. Therefore, if patients have a normal CK level and can tolerate the symptoms, we ask them to continue therapy and see if their symptoms resolve with continued use.
Symptoms that persist beyond the first 2 weeks of therapy are likely due to the statin. These include symmetric burning or pain in the large muscles during exercise that was not present before lipid-lowering therapy. Any symptom that reproducibly recurs with statin rechallenge and disappears within 2 weeks of discontinuing therapy is more likely to be caused by the statin.
Findings of the PRIMO study are representative of typical statin-induced symptoms that we see in our clinic.20
- Most patients did not identify a trigger, but the 40% who did had engaged in unusual physical exertion or had received a new drug in addition to the statin.
- Heaviness, stiffness, or cramps predominated in 70%, with only a quarter noting weakness and another quarter suffering myalgias during exercise. Pain was diffuse in 60% and more common in the lower extremities than the upper extremities.
- Physically active patients were more likely to suffer muscle symptoms than sedentary patients, echoing the observation by Sinzinger and O’Grady that athletes are especially intolerant of lipid-lowering therapy.41
- Patients who have had muscle complaints with other drug therapies such as bisphosphonates, 42 raloxifene (Evista),43 or diuretics may be having the same provocation of an underlying muscle predisposition by the statin.
- A personal or family history of muscle complaints predisposed patients to statin-induced myopathy.20
Finally, we have repeatedly found that when dyspnea and fatigue are associated with the muscle complaint, they are more likely to be caused by statins.44
Statins can unveil underlying musculoskeletal disorders
Atypical complaints require us to search for an alternate diagnosis before dismissing them as not statin-induced.
Previous episodes of myoglobinuria (dark urine after exertion) would raise the possibility of a metabolic myopathy. Frequent muscle cramps raise the possibility of a metabolic myopathy or motor neuron disease.
Asymmetric pain or pain involving joints and ligaments is less likely to be statin-related but in our experience occasionally occurs.
Some patients with underlying degenerative arthritis or tendinitis repeatedly develop worsening symptoms each time they take statins, perhaps because muscle weakness exacerbates the arthropathy or tendinopathy.45
Although statin-related muscle complaints are almost always symmetric, many patients with underlying peripheral vascular disease have asymmetric pain in the limb with poor vascular supply; the pain is reproducible by statin rechallenge.
In our experience, many patients whose chronic low back pain is due to a lumbar radiculopathy experience exacerbations of that pain whenever they start statins.
Weakness preceding the use of the statins or a family history of neuromuscular disorders may indicate a neurodegenerative disorder and warrants consideration of early neurological consultation.
Although these rules are not absolute, they are helpful in the initial evaluation, which must exclude alternative diagnoses.
Is the patient a vegetarian? A drinker? Taking supplements?
Taking a careful history of diet and supplement use is important to find exposures that may increase the risk of statin-related muscle complaints. Vegetarians may develop carnitine or vitamin B12 deficiencies. Alcohol and vitamin E and other supplements are occasional causes of muscle symptoms falsely attributed to statin therapy. It is also important to remember that red yeast rice contains lovastatin, which can exacerbate myopathy, especially when taken in conjunction with another statin.
Physical examination
The examination of patients with possible statin-induced myopathy begins with a general assessment for signs of hypothyroidism or excess alcohol consumption.
Ankle-brachial indices are used to exclude significant peripheral vascular disease.
The musculoskeletal examination focuses on muscle atrophy, tone, and strength but also excludes tendinopathies, arthropathies, and myofascial pain syndromes, which are often confused with muscle pain.
We conduct quantitative dynamometry, measuring handgrip with a Jamar dynamometer and hip abduction with a Nicholas Manual Muscle Tester. Precise dynamometric measurements are tracked at subsequent visits and are helpful in following recovery from myopathy as well as in tracking strength during subsequent statin rechallenges.
We routinely look for hyperreflexia, fasciculations, extensor-plantar responses, and decreased heel-to-shin movement, which would suggest myelopathy. Reflexes and a sensory examination including vibration and temperature sensation help exclude radiculopathy and peripheral neuropathy.
Laboratory evaluation
In every patient with possibly statin myopathy, the primary care physician should measure:
- The serum CK level (preferably more than 72 hours after exercise)
- The 25-hydroxy vitamin level
- The thyroid-stimulating hormone level.
Further laboratory evaluation depends on the findings and will often be directed by subspecialists. For example, we assess the sedimentation rate, anti-Ro and anti-La antibodies, and the myositis panel in patients with elevated CK whose other findings suggest an autoimmune or inflammatory process. We test serum carnitine levels (free, total, and esterified), fasting serum lactate levels, and serum cortisol in those with findings suggestive of metabolic myopathy. We order electromyography and nerve conduction studies in patients with possible myelopathy, peripheral neuropathy, or inflammatory myopathy.
Ultimately, a muscle biopsy may be necessary to exclude inflammatory or necrotizing myopathies in patients whose CK remains elevated despite withdrawal of statins. It may also be helpful when other findings suggest a metabolic myopathy. When a biopsy is needed, magnetic resonance imaging of the affected limb may identify an affected muscle for biopsy.
MANAGEMENT
Reassess the lipid goal
If the source of a complaint remains unclear after challenge and rechallenge with alternate statins, we generally recommend restarting therapy and trying to achieve the LDL-C goal. If the workup suggests a neurologic or rheumatolic etiology, a referral to a specialist is indicated. However, if the evaluation leads to a diagnosis of statin-induced myopathy, the next task is to reassess the lipid treatment goals.
The Adult Treatment Panel (ATP) III guidelines should be used to assess the patient’s risk of having major coronary heart disease in the next 10 years, and to determine appropriate LDL-C goals.46 Some patients with suspected toxicity may have been treated to more aggressive LDL-C levels than recommended by these guidelines. The first step in these patients is to reduce or discontinue the unnecessary statin, even if there is no clear evidence of toxicity.
For the rest of patients with suspected toxicity, the decision to discontinue statin therapy must be weighed against the estimated reduction in risk associated with taking a statin medication.
Prescribe a 6-week ‘statin holiday’ and see if symptoms resolve
In patients whose evaluation suggests statin myopathy, we stop all lipid-lowering therapy for 6 weeks and see if symptoms resolve and if grip and hip strength increase by dynamometry.
We often give these patient supplements of 600 mg daily of a bioavailable source of coenzyme Q10 and fish oil during this statin holiday. The data supporting the use of these supplements are mixed but the risks are minimal.5 In patients whose evaluation suggests a primary disorder in fatty acid oxidation, we add a trial of l-carnitine supplementation if symptoms do not resolve after a 6-week course of the coenzyme Q10 and fish oil.
If symptoms persist or if resolution is unclear at 6 weeks, we extend the holiday for an additional 6 weeks, except in patients with recent unstable coronary disease: for these patients, unless there is evidence of rhabdomyolysis, we believe that the benefits of continued statin therapy exceed the risks.
If the initial evaluation is consistent with statin myopathy and the neuromuscular symptoms (myalgias and weakness) do not respond within a few months of statin withdrawal, neurologic consultation is indicated to evaluate for an underlying neurologic disorder that has become symptomatic during statin therapy but whose existence is independent of the statin therapy. In some cases, the preexisting neurologic disorder may become symptomatic because of the statin therapy and remain symptomatic despite discontinuation of the statin therapy.
Restarting lipid-lowering therapy
Once the myopathy symptoms have abated or are controlled, a rechallenge of statin therapy is in order for those whose risk profile suggests greater benefit from statin therapy.
We consider the complete statin exposure history and any concomitant therapy that may have been competing with cytochrome P450 (CYP) metabolism of statins in designing an alternate lipid-lowering plan.
For patients with known coronary or vascular disease, in whom the survival benefit of statins is greatest, we generally try to find a statin regimen that is tolerable. Long-acting fluvastatin or a statin with less CYP dependence, such as pravastatin, is often successful.59 For patients whose myopathy has recurred with multiple statin rechallenges or whose lipid-lowering goal requires a more potent therapy, rosuvastatin in alternate-day or once- or twice-a-week schedules is efficacious and well tolerated in many patients.36,37,60 Of note, however, although such alternate-day therapies may produce excellent reductions in cholesterol levels, these regimens have not been proven to reduce cardiovascular end points.
Alternative lipid-lowering therapy. Occasionally, a patient cannot tolerate even intermittent rosuvastatin. In these cases, we prescribe resin therapy, which is well tolerated in those with recurrent statin myopathy.61
Although some believe that ezetimibe (Zetia) is an option for these patients, we do not agree, since it often causes similar muscle complaints in the most sensitive statin myopathy patients.30 Furthermore, ezetimibe has not been shown to improve cardiac end points.
Red yeast rice is also not a safe alternative in these patients, in whom muscle complaints and CK elevations frequently develop anew on this unregulated supplement despite its low lovastatin equivalence, 6 mg a day.62 A recent study showed that there is wide variability in the amount of lovastatin in over-the-counter red yeast rice; the median dose was 6 mg, and the maximum dose was 14.5 mg.63
The ultimate lipid-lowering plan for most of these patients will require a compromise between the ideal LDL-C goal and the LDL-C level that is achievable with these alternate attempts at lipid lowering.
While combination therapy may be attractive in patients with combined lipid disorders and no muscle complaints, fibrates are more likely to cause muscle toxicity per dose prescribed than statins, and the addition of fibrates to statin therapy increases the risks of muscle reactions.64,65 The evidence that fibrates reduce cardiovascular end points is much less robust than that for statins, which further reduces enthusiasm for combination therapy in patients with statin muscle toxicity.66,67
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Foley KA, Simpson RJ, Crouse JR, et al. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- O’Meara JG, Kardia SL, Armon JJ, et al. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med 2004; 164:1313–1318.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore) 1982; 61:141–152.
- Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med 1988; 148:1553–1557.
- McKenney JM, Davidson MH, Jacobson TA, et al. National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–84C.
- Pasternak RC, Smith SC, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002; 40:567–572.
- Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep 2007; 9:389–396.
- Linares LA, Golomb BA, Jaojoco JA, et al. The modern spectrum of rhabdomyolysis: drug toxicity revealed by creatine kinase screening. Curr Drug Saf 2009; 4:181–187.
- Armitage J. The safety of statins in clinical practice. Lancet 2007; 370:1781–1790.
- Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361:2005–2016.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol 2002; 40:163–171.
- de Sauvage Nolting PR, Buirma RJ, et al. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90:181–184.
- Franc S, Dejager S, Bruckert E, et al. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc Drugs Ther 2003; 17:459–465.
- Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation 2006; 114:2788–2797.
- Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Spatz ES, Canavan ME, Desai MM. From here to JUPITER: identifying new patients for statin therapy using data from the 1999–2004 National Health and Nutrition Examination Survey. Circ Cardiovasc Qual Outcomes 2009; 2:41–48.
- Vladutiu GD, Simmons Z, Isackson PJ, et al. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve 2006; 34:153–162.
- Vladutiu GD. Genetic predisposition to statin myopathy. Curr Opin Rheumatol 2008; 20:648–655.
- Phillips PS, Phillips CT, Sullivan MJ, et al. Statin myotoxicity is associated with changes in the cardiopulmonary function. Atherosclerosis 2004; 177:183–188.
- Phillips PS, Ciaraldi TP, Kim DL, et al. Myotoxic reactions to lipidlowering therapy are associated with altered oxidation of fatty acids. Endocrine 2009; 35:38–46.
- Phillips PS, Haas RH. Statin myopathy as a metabolic muscle disease. Expert Rev Cardiovasc Ther 2008; 6:971–978.
- Löfberg M, Jänkälä H, Paetau A, et al. Metabolic causes of recurrent rhabdomyolysis. Acta Neurol Scand 1998; 98:268–275.
- Havranek JM, Wolfsen A, Warnke GA, et al. Monotherapy with Ezetimibe Causing Myopathy. Am J Med 2006; 119:285–286.
- Phillips PS. Ezetimibe and statin-associated myopathy. Ann Intern Med 2004; 141:649.
- Durst R, Rund D, Schurr D, et al. One year experience with a low density lipoprotein apheresis system. Isr Med Assoc J 2002; 4:677–680.
- Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol 1997; 80:106–107.
- Brewer HB. Benefit-risk assessment of rosuvastatin 10 to 40 milligrams. Am J Cardiol 2003; 92:23K–29K.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Backes JM, Venero CV, Gibson CA, et al. Effectiveness and tolerability of every-other-day rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother 2008; 42:341–346.
- Backes JM, Moriarty PM, Ruisinger JF, et al. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med 2006; 119:400–409.
- Jacobson TA. Toward “pain-free” statin prescribing: clinical algorithm for diagnosis and management of myalgia. Mayo Clin Proc 2008; 83:687–700.
- Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002; 137:581–585.
- Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol 2004; 57:525–528.
- Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med 2005; 165:346–347.
- Martino S, Disch D, Dowsett SA, et al. Safety assessment of raloxifene over eight years in a clinical trial setting. Curr Med Res Opin 2005; 21:1441–1452.
- Phillips PS, Kimura BJ, Kelley R, et al. Feasibility of using the internet to perform medical research: observations from a statin-myopathy public information web site. Circulation 2003; 107:e7001–e7039.
- Chazerain P, Hayem G, Hamza S, et al. Four cases of tendinopathy in patients on statin therapy. Joint Bone Spine 2001; 68:430–433.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004; 44:720–732.
- Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation 1998; 97:1453–1460.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995; 333:1301–1307.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006; 368:1155–1163.
- Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHATLLT). JAMA 2002; 288:2998–3007.
- Stein EA, Ballantyne CM, Windler E, et al. Efficacy and tolerability of fluvastatin XL 80 mg alone, ezetimibe alone, and the combination of fluvastatin XL 80 mg with ezetimibe in patients with a history of muscle-related side effects with other statins. Am J Cardiol 2008; 101:490–496.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Phillips PS, Gray NL, McDonald FG, et al. Colesevelam is safe and effective in patients with statin myotoxicity. Arterioscler Thromb Vasc Biol 2005 25:E-97.
- Phillips PS. Balancing randomized trials with anecdote. Ann Intern Med 2009; 150:885–886.
- Gordon RY, Cooperman T, Obermeyer W, et al. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware! Arch Intern Med 2010; 170:1722–1727.
- Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004; 292:2585–2590.
- Gaist D, Rodríguez LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001; 12:565–569.
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366:1849–1861.
- ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Foley KA, Simpson RJ, Crouse JR, et al. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- O’Meara JG, Kardia SL, Armon JJ, et al. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med 2004; 164:1313–1318.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore) 1982; 61:141–152.
- Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med 1988; 148:1553–1557.
- McKenney JM, Davidson MH, Jacobson TA, et al. National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–84C.
- Pasternak RC, Smith SC, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002; 40:567–572.
- Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep 2007; 9:389–396.
- Linares LA, Golomb BA, Jaojoco JA, et al. The modern spectrum of rhabdomyolysis: drug toxicity revealed by creatine kinase screening. Curr Drug Saf 2009; 4:181–187.
- Armitage J. The safety of statins in clinical practice. Lancet 2007; 370:1781–1790.
- Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361:2005–2016.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol 2002; 40:163–171.
- de Sauvage Nolting PR, Buirma RJ, et al. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90:181–184.
- Franc S, Dejager S, Bruckert E, et al. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc Drugs Ther 2003; 17:459–465.
- Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation 2006; 114:2788–2797.
- Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Spatz ES, Canavan ME, Desai MM. From here to JUPITER: identifying new patients for statin therapy using data from the 1999–2004 National Health and Nutrition Examination Survey. Circ Cardiovasc Qual Outcomes 2009; 2:41–48.
- Vladutiu GD, Simmons Z, Isackson PJ, et al. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve 2006; 34:153–162.
- Vladutiu GD. Genetic predisposition to statin myopathy. Curr Opin Rheumatol 2008; 20:648–655.
- Phillips PS, Phillips CT, Sullivan MJ, et al. Statin myotoxicity is associated with changes in the cardiopulmonary function. Atherosclerosis 2004; 177:183–188.
- Phillips PS, Ciaraldi TP, Kim DL, et al. Myotoxic reactions to lipidlowering therapy are associated with altered oxidation of fatty acids. Endocrine 2009; 35:38–46.
- Phillips PS, Haas RH. Statin myopathy as a metabolic muscle disease. Expert Rev Cardiovasc Ther 2008; 6:971–978.
- Löfberg M, Jänkälä H, Paetau A, et al. Metabolic causes of recurrent rhabdomyolysis. Acta Neurol Scand 1998; 98:268–275.
- Havranek JM, Wolfsen A, Warnke GA, et al. Monotherapy with Ezetimibe Causing Myopathy. Am J Med 2006; 119:285–286.
- Phillips PS. Ezetimibe and statin-associated myopathy. Ann Intern Med 2004; 141:649.
- Durst R, Rund D, Schurr D, et al. One year experience with a low density lipoprotein apheresis system. Isr Med Assoc J 2002; 4:677–680.
- Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol 1997; 80:106–107.
- Brewer HB. Benefit-risk assessment of rosuvastatin 10 to 40 milligrams. Am J Cardiol 2003; 92:23K–29K.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Backes JM, Venero CV, Gibson CA, et al. Effectiveness and tolerability of every-other-day rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother 2008; 42:341–346.
- Backes JM, Moriarty PM, Ruisinger JF, et al. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med 2006; 119:400–409.
- Jacobson TA. Toward “pain-free” statin prescribing: clinical algorithm for diagnosis and management of myalgia. Mayo Clin Proc 2008; 83:687–700.
- Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002; 137:581–585.
- Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol 2004; 57:525–528.
- Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med 2005; 165:346–347.
- Martino S, Disch D, Dowsett SA, et al. Safety assessment of raloxifene over eight years in a clinical trial setting. Curr Med Res Opin 2005; 21:1441–1452.
- Phillips PS, Kimura BJ, Kelley R, et al. Feasibility of using the internet to perform medical research: observations from a statin-myopathy public information web site. Circulation 2003; 107:e7001–e7039.
- Chazerain P, Hayem G, Hamza S, et al. Four cases of tendinopathy in patients on statin therapy. Joint Bone Spine 2001; 68:430–433.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004; 44:720–732.
- Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation 1998; 97:1453–1460.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995; 333:1301–1307.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006; 368:1155–1163.
- Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHATLLT). JAMA 2002; 288:2998–3007.
- Stein EA, Ballantyne CM, Windler E, et al. Efficacy and tolerability of fluvastatin XL 80 mg alone, ezetimibe alone, and the combination of fluvastatin XL 80 mg with ezetimibe in patients with a history of muscle-related side effects with other statins. Am J Cardiol 2008; 101:490–496.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Phillips PS, Gray NL, McDonald FG, et al. Colesevelam is safe and effective in patients with statin myotoxicity. Arterioscler Thromb Vasc Biol 2005 25:E-97.
- Phillips PS. Balancing randomized trials with anecdote. Ann Intern Med 2009; 150:885–886.
- Gordon RY, Cooperman T, Obermeyer W, et al. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware! Arch Intern Med 2010; 170:1722–1727.
- Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004; 292:2585–2590.
- Gaist D, Rodríguez LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001; 12:565–569.
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366:1849–1861.
- ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
KEY POINTS
- There is little consensus on the definition of statin-induced myopathy, and it is underdiagnosed. The incidence of statin-induced muscle toxicity in randomized controlled trials is lower than in clinical practice.
- Abnormal pharmacokinetic activity contributes to toxicity, but some patients may be predisposed by underlying metabolic muscle disorders.
- A focused history and neuromusculoskeletal examination are important in the evaluation of muscle complaints that may be induced by statins.
- In patients with possible statin-induced myopathy, assessing the risks and benefits of statin therapy is essential.
- For patients who cannot tolerate statin therapy, alternatives include a “statin holiday” followed by a rechallenge with a different statin, intermittent rosuvastatin (Crestor), or resin therapy. Sometimes the best alternative is a compromise between the goal level for low-density-lipoprotein cholesterol and the level achievable with alternative therapy.