User login
HIV Pre-exposure Prophylaxis (PrEP): A Survey of Dermatologists’ Knowledge and Practice Patterns
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.
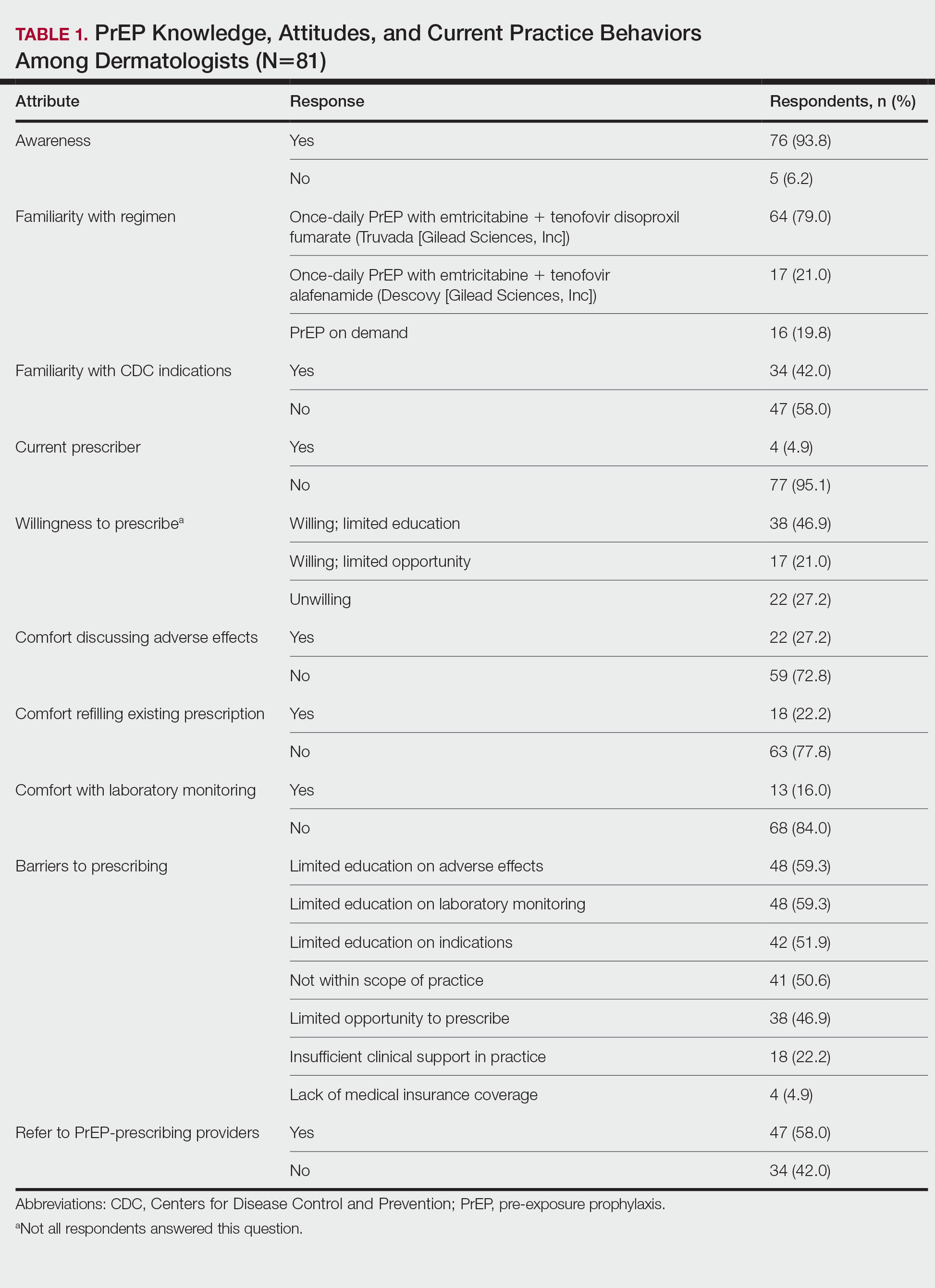
Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
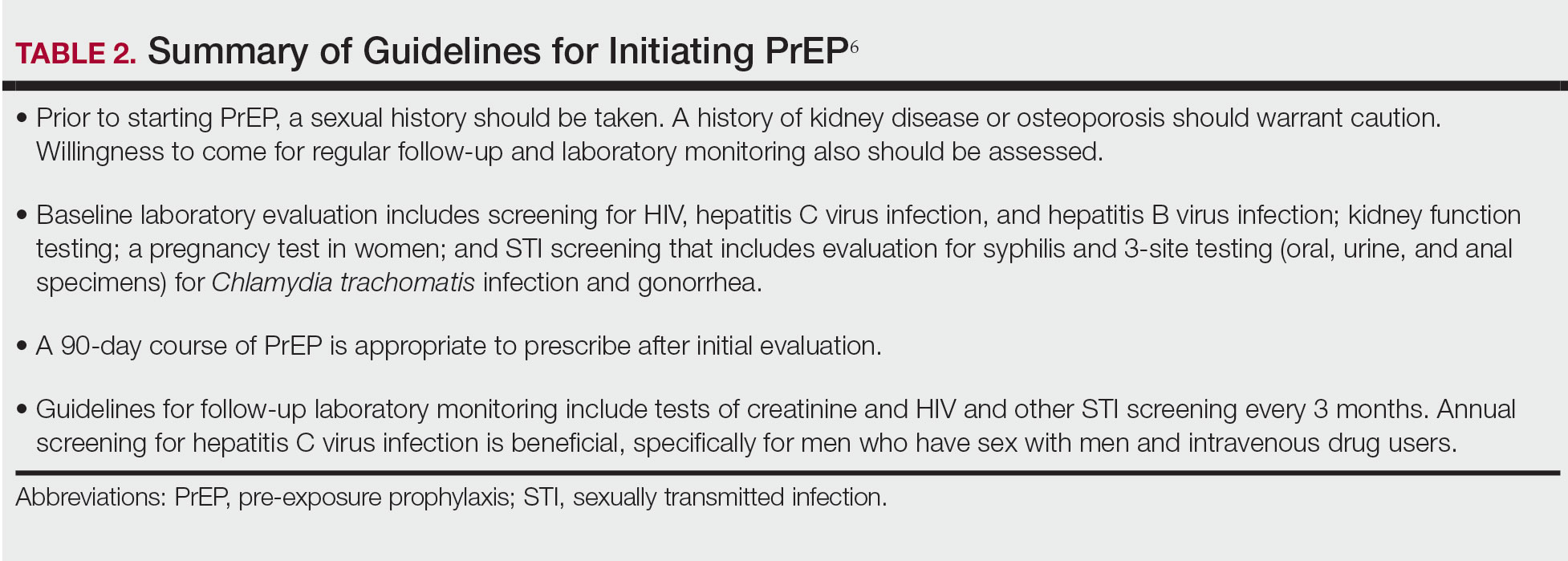
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).
In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.

Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).

In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.

Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).

In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
Practice Points
- Sexually transmitted infections (STIs) often have skin manifestations, with patients presenting to dermatologists.
- Pre-exposure prophylaxis (PrEP) uses antiretrovirals taken prophylactically to prevent transmission of and infection with HIV. Dermatologists are aware of PrEP, but several barriers prevent them from being prescribers.
- Patients with a history of an STI should be considered for PrEP.