User login
Palmoplantar Pustular Eruption Due to Dabigatran
To the Editor:
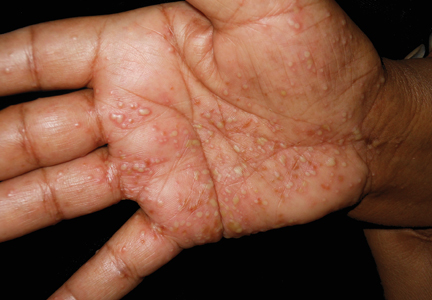
A 71-year-old woman with hypertension and atrial fibrillation due to thyrotoxicosis was prescribed dabigatran for stroke prevention by her cardiologist. She also was taking pantoprazole, methimazole, and amiodarone at the time of presentation, all managed by her endocrinologist. She had no known drug allergies but reported a remote history of a palmar rash after eating shellfish. She otherwise had never had any problems with her skin and had no family history of psoriasis. She had a history of smoking 50 packs per year but had quit 6 months prior to presentation. After two 150-mg doses of dabigatran, she noticed numerous mildly tender and itchy eruptions on the palmar and plantar surfaces with no associated respiratory, oropharyngeal, or constitutional symptoms. She denied any recent shellfish ingestion. On dermatologic examination, numerous discreet pustules were present on the bilateral palmar and plantar surfaces with minimal erythema of the underlying skin (Figure).
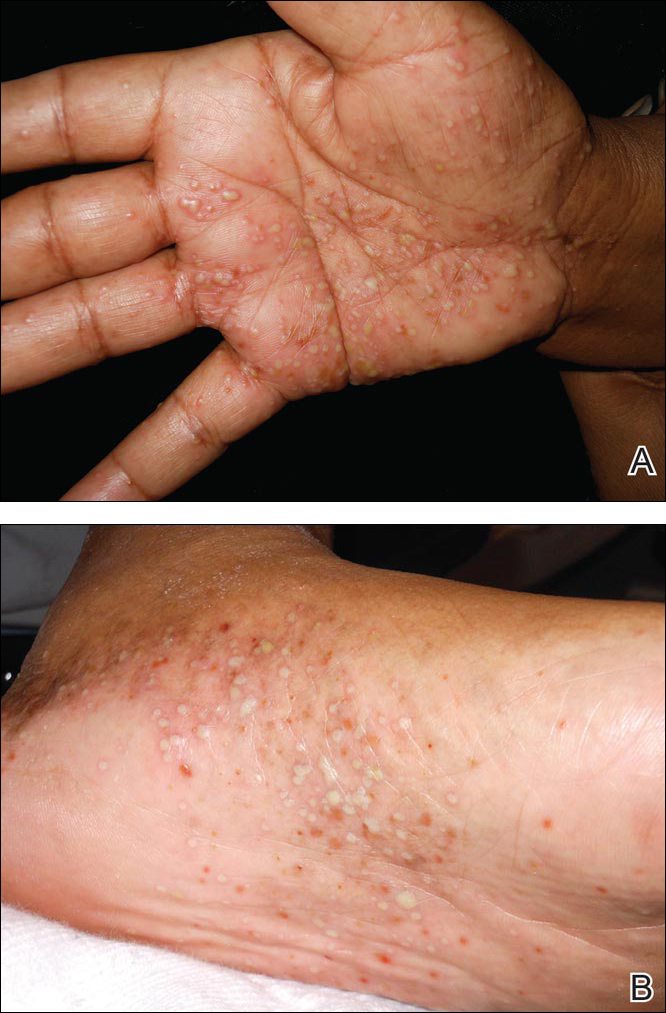
A punch biopsy was taken from a newly forming lesion on the right palm. Histopathology revealed mild hyperkeratosis, spongiosis with lymphocyte exocytosis, intraepidermal vesiculation, and a sparse upper dermal and perivascular lymphohistiocytic infiltration. No neutrophils or microabscesses were seen. Staining with periodic acid–Schiff revealed no fungi, and S-100 staining revealed numerous Langerhans cells in the epidermis. Although the skin lesions clinically appeared pustular, the results were consistent with an eczematous drug reaction. Laboratory values, including a complete blood cell count, iron studies, chemistry panels, liver function, thyroid function, and coagulation studies, were remarkable only for mild anemia. The patient declined any topical or systemic skin treatment. Dabigatran was discontinued, and the lesions began to clear immediately thereafter. Dabigatran was not reintroduced. Enoxaparin subsequently was prescribed for anticoagulation. The diagnosis of a drug reaction due to dabigatran was made, which was supported with a score of 7 on the Naranjo scale (0=doubtful; 1–4=possible; 5–8=probable; ≥9=definite) for determining probability of drug-induced adverse reactions.1 The differential diagnosis for the skin eruption included palmoplantar pustular psoriasis, dyshidrotic eczema, and allergic contact dermatitis, but the clinical history did not support these diagnoses.
Dabigatran is a direct thrombin inhibitor used to reduce the risk for stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Based on results of the RE-LY (Randomization Evaluation of Long-term Anticoagulation Therapy) trial published in 2009, dabigatran 150 mg twice daily significantly reduced the risk for stroke and systemic emboli in patients with atrial fibrillation compared to warfarin (annual risk, 1.11% vs 1.69%; relative risk, 0.66; 95% CI, 0.53-0.82; P<.001) with the advantage of not requiring frequent monitoring of the international normalized ratio.2 The most common adverse effect of dabigatran in this trial was dyspepsia (11.3% vs 5.8%). Drug hypersensitivity, allergic edema, and anaphylaxis were reported in less than 0.1% of patients taking dabigatran.2
According to a PubMed search of articles indexed for MEDLINE using the search terms dabigatran cutaneous reaction and dabigatran rash, 4 case reports of cutaneous eruption due to dabigatran were identified. In one report, a 20-year-old man with atrial fibrillation developed an eruption similar to our patient on the thigh and forearm after 2 weeks of taking oral dabigatran 150 mg twice daily. It resolved without complication after topical corticosteroid use and discontinuation of dabigatran.3 In another report, a 78-year-old man presented to the emergency department after taking two 150-mg doses of dabigatran with a diffuse, full-body, pruritic rash that resolved with oral diphenhydramine and discontinuation of dabigatran.4 A third case described a 59-year-old man who was taking 150 mg dabigatran twice daily for 5 days before developing a rash.5 The fourth case involved a 74-year-old woman who developed leukocytoclastic vasculitis 1 week after taking dabigatran 150 mg twice daily.6
It is important to monitor for and report hypersensitivity reactions in patients taking dabigatran. Drug exanthems may cause discomfort or even herald more serious hypersensitivity reactions. Patients experiencing these reactions may discontinue therapy without notifying a physician and consequently place themselves at risk for embolism or stroke.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation [published online August 30, 2009]. N Engl J Med. 2009;361:1139-1151.
- Whitehead H, Boyd J, Blais D, et al. Drug induced exanthem following dabigatran. Ann Pharmacother. 2011;45:e53.
- Eid TJ, Shah SA. Dabigatran-induced rash. Am J Health Syst Pharm. 2011;68:1489-1490.
- To K, Reynolds C, Spinler SA. Rash associated with dabigatran etrexilate. Pharmacotherapy. 2013;33:e23-e27.
- Cakmak MA, Sahin S, Cinar N, et al. Adverse skin reaction caused by dabigatran. Eur Rev Med Pharmacol Sci. 2014;18:2595.
To the Editor:
A 71-year-old woman with hypertension and atrial fibrillation due to thyrotoxicosis was prescribed dabigatran for stroke prevention by her cardiologist. She also was taking pantoprazole, methimazole, and amiodarone at the time of presentation, all managed by her endocrinologist. She had no known drug allergies but reported a remote history of a palmar rash after eating shellfish. She otherwise had never had any problems with her skin and had no family history of psoriasis. She had a history of smoking 50 packs per year but had quit 6 months prior to presentation. After two 150-mg doses of dabigatran, she noticed numerous mildly tender and itchy eruptions on the palmar and plantar surfaces with no associated respiratory, oropharyngeal, or constitutional symptoms. She denied any recent shellfish ingestion. On dermatologic examination, numerous discreet pustules were present on the bilateral palmar and plantar surfaces with minimal erythema of the underlying skin (Figure).

A punch biopsy was taken from a newly forming lesion on the right palm. Histopathology revealed mild hyperkeratosis, spongiosis with lymphocyte exocytosis, intraepidermal vesiculation, and a sparse upper dermal and perivascular lymphohistiocytic infiltration. No neutrophils or microabscesses were seen. Staining with periodic acid–Schiff revealed no fungi, and S-100 staining revealed numerous Langerhans cells in the epidermis. Although the skin lesions clinically appeared pustular, the results were consistent with an eczematous drug reaction. Laboratory values, including a complete blood cell count, iron studies, chemistry panels, liver function, thyroid function, and coagulation studies, were remarkable only for mild anemia. The patient declined any topical or systemic skin treatment. Dabigatran was discontinued, and the lesions began to clear immediately thereafter. Dabigatran was not reintroduced. Enoxaparin subsequently was prescribed for anticoagulation. The diagnosis of a drug reaction due to dabigatran was made, which was supported with a score of 7 on the Naranjo scale (0=doubtful; 1–4=possible; 5–8=probable; ≥9=definite) for determining probability of drug-induced adverse reactions.1 The differential diagnosis for the skin eruption included palmoplantar pustular psoriasis, dyshidrotic eczema, and allergic contact dermatitis, but the clinical history did not support these diagnoses.
Dabigatran is a direct thrombin inhibitor used to reduce the risk for stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Based on results of the RE-LY (Randomization Evaluation of Long-term Anticoagulation Therapy) trial published in 2009, dabigatran 150 mg twice daily significantly reduced the risk for stroke and systemic emboli in patients with atrial fibrillation compared to warfarin (annual risk, 1.11% vs 1.69%; relative risk, 0.66; 95% CI, 0.53-0.82; P<.001) with the advantage of not requiring frequent monitoring of the international normalized ratio.2 The most common adverse effect of dabigatran in this trial was dyspepsia (11.3% vs 5.8%). Drug hypersensitivity, allergic edema, and anaphylaxis were reported in less than 0.1% of patients taking dabigatran.2
According to a PubMed search of articles indexed for MEDLINE using the search terms dabigatran cutaneous reaction and dabigatran rash, 4 case reports of cutaneous eruption due to dabigatran were identified. In one report, a 20-year-old man with atrial fibrillation developed an eruption similar to our patient on the thigh and forearm after 2 weeks of taking oral dabigatran 150 mg twice daily. It resolved without complication after topical corticosteroid use and discontinuation of dabigatran.3 In another report, a 78-year-old man presented to the emergency department after taking two 150-mg doses of dabigatran with a diffuse, full-body, pruritic rash that resolved with oral diphenhydramine and discontinuation of dabigatran.4 A third case described a 59-year-old man who was taking 150 mg dabigatran twice daily for 5 days before developing a rash.5 The fourth case involved a 74-year-old woman who developed leukocytoclastic vasculitis 1 week after taking dabigatran 150 mg twice daily.6
It is important to monitor for and report hypersensitivity reactions in patients taking dabigatran. Drug exanthems may cause discomfort or even herald more serious hypersensitivity reactions. Patients experiencing these reactions may discontinue therapy without notifying a physician and consequently place themselves at risk for embolism or stroke.
To the Editor:
A 71-year-old woman with hypertension and atrial fibrillation due to thyrotoxicosis was prescribed dabigatran for stroke prevention by her cardiologist. She also was taking pantoprazole, methimazole, and amiodarone at the time of presentation, all managed by her endocrinologist. She had no known drug allergies but reported a remote history of a palmar rash after eating shellfish. She otherwise had never had any problems with her skin and had no family history of psoriasis. She had a history of smoking 50 packs per year but had quit 6 months prior to presentation. After two 150-mg doses of dabigatran, she noticed numerous mildly tender and itchy eruptions on the palmar and plantar surfaces with no associated respiratory, oropharyngeal, or constitutional symptoms. She denied any recent shellfish ingestion. On dermatologic examination, numerous discreet pustules were present on the bilateral palmar and plantar surfaces with minimal erythema of the underlying skin (Figure).

A punch biopsy was taken from a newly forming lesion on the right palm. Histopathology revealed mild hyperkeratosis, spongiosis with lymphocyte exocytosis, intraepidermal vesiculation, and a sparse upper dermal and perivascular lymphohistiocytic infiltration. No neutrophils or microabscesses were seen. Staining with periodic acid–Schiff revealed no fungi, and S-100 staining revealed numerous Langerhans cells in the epidermis. Although the skin lesions clinically appeared pustular, the results were consistent with an eczematous drug reaction. Laboratory values, including a complete blood cell count, iron studies, chemistry panels, liver function, thyroid function, and coagulation studies, were remarkable only for mild anemia. The patient declined any topical or systemic skin treatment. Dabigatran was discontinued, and the lesions began to clear immediately thereafter. Dabigatran was not reintroduced. Enoxaparin subsequently was prescribed for anticoagulation. The diagnosis of a drug reaction due to dabigatran was made, which was supported with a score of 7 on the Naranjo scale (0=doubtful; 1–4=possible; 5–8=probable; ≥9=definite) for determining probability of drug-induced adverse reactions.1 The differential diagnosis for the skin eruption included palmoplantar pustular psoriasis, dyshidrotic eczema, and allergic contact dermatitis, but the clinical history did not support these diagnoses.
Dabigatran is a direct thrombin inhibitor used to reduce the risk for stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Based on results of the RE-LY (Randomization Evaluation of Long-term Anticoagulation Therapy) trial published in 2009, dabigatran 150 mg twice daily significantly reduced the risk for stroke and systemic emboli in patients with atrial fibrillation compared to warfarin (annual risk, 1.11% vs 1.69%; relative risk, 0.66; 95% CI, 0.53-0.82; P<.001) with the advantage of not requiring frequent monitoring of the international normalized ratio.2 The most common adverse effect of dabigatran in this trial was dyspepsia (11.3% vs 5.8%). Drug hypersensitivity, allergic edema, and anaphylaxis were reported in less than 0.1% of patients taking dabigatran.2
According to a PubMed search of articles indexed for MEDLINE using the search terms dabigatran cutaneous reaction and dabigatran rash, 4 case reports of cutaneous eruption due to dabigatran were identified. In one report, a 20-year-old man with atrial fibrillation developed an eruption similar to our patient on the thigh and forearm after 2 weeks of taking oral dabigatran 150 mg twice daily. It resolved without complication after topical corticosteroid use and discontinuation of dabigatran.3 In another report, a 78-year-old man presented to the emergency department after taking two 150-mg doses of dabigatran with a diffuse, full-body, pruritic rash that resolved with oral diphenhydramine and discontinuation of dabigatran.4 A third case described a 59-year-old man who was taking 150 mg dabigatran twice daily for 5 days before developing a rash.5 The fourth case involved a 74-year-old woman who developed leukocytoclastic vasculitis 1 week after taking dabigatran 150 mg twice daily.6
It is important to monitor for and report hypersensitivity reactions in patients taking dabigatran. Drug exanthems may cause discomfort or even herald more serious hypersensitivity reactions. Patients experiencing these reactions may discontinue therapy without notifying a physician and consequently place themselves at risk for embolism or stroke.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation [published online August 30, 2009]. N Engl J Med. 2009;361:1139-1151.
- Whitehead H, Boyd J, Blais D, et al. Drug induced exanthem following dabigatran. Ann Pharmacother. 2011;45:e53.
- Eid TJ, Shah SA. Dabigatran-induced rash. Am J Health Syst Pharm. 2011;68:1489-1490.
- To K, Reynolds C, Spinler SA. Rash associated with dabigatran etrexilate. Pharmacotherapy. 2013;33:e23-e27.
- Cakmak MA, Sahin S, Cinar N, et al. Adverse skin reaction caused by dabigatran. Eur Rev Med Pharmacol Sci. 2014;18:2595.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation [published online August 30, 2009]. N Engl J Med. 2009;361:1139-1151.
- Whitehead H, Boyd J, Blais D, et al. Drug induced exanthem following dabigatran. Ann Pharmacother. 2011;45:e53.
- Eid TJ, Shah SA. Dabigatran-induced rash. Am J Health Syst Pharm. 2011;68:1489-1490.
- To K, Reynolds C, Spinler SA. Rash associated with dabigatran etrexilate. Pharmacotherapy. 2013;33:e23-e27.
- Cakmak MA, Sahin S, Cinar N, et al. Adverse skin reaction caused by dabigatran. Eur Rev Med Pharmacol Sci. 2014;18:2595.
Practice Points
- Dabigatran is a direct thrombin inhibitor used in patients with atrial fibrillation to prevent thromboembolic events.
- Although the most common adverse effects of dabigatran are bleeding and dyspepsia, clinicians also should be aware of the potential for cutaneous hypersensitivity reactions to this drug.