User login
Should I evaluate my patient with atrial fibrillation for sleep apnea?
Yes. The prevalence of sleep apnea is exceedingly high in patients with atrial fibrillation—50% to 80% compared with 30% to 60% in respective control groups.1–3 Conversely, atrial fibrillation is more prevalent in those with sleep-disordered breathing than in those without (4.8% vs 0.9%).4
Sleep-disordered breathing comprises obstructive sleep apnea and central sleep apnea. Obstructive sleep apnea, characterized by repetitive upper-airway obstruction during sleep, is accompanied by intermittent hypoxia, rises in carbon dioxide, autonomic nervous system fluctuations, and intrathoracic pressure alterations.5 Central sleep apnea may be neurally mediated and, in the setting of cardiac disease, is characterized by alterations in chemosensitivity and chemoresponsiveness, leading to a state of high loop gain—ie, a hypersensitive ventilatory control system leading to ventilatory drive oscillations.6
Both obstructive and central sleep apnea have been associated with atrial fibrillation. Experimental data implicate obstructive sleep apnea as a trigger of atrial arrhythmogenesis,7,8 and epidemiologic studies support an association between central sleep apnea, Cheyne-Stokes respiration, and incident atrial fibrillation.9
HOW SLEEP APNEA COULD LEAD TO ATRIAL FIBRILLATION
In experiments in animals, intermittent upper-airway obstruction led to forced inspiration, substantial negative intrathoracic pressure, subsequent left atrial distention, and increased susceptibility to atrial fibrillation.10 The autonomic nervous system may be a mediator of apnea-induced atrial fibrillation, as apnea-induced atrial fibrillation is suppressed with autonomic blockade.10
Emerging data also support the hypothesis that intermittent hypoxia7 and resolution of hypercapnia,8 as observed in obstructive sleep apnea, exert atrial electrophysiologic changes that increase vulnerability to atrial arrhythmogenesis.
In a case-crossover study,11 the odds of paroxysmal atrial fibrillation occurring after a respiratory disturbance were 17.9 times higher than after normal breathing (95% confidence interval [CI] 2.2–144.2), though the absolute rate of overall arrhythmia events (including both atrial fibrillation and nonsustained ventricular tachycardia) associated with respiratory disturbances was low (1 excess arrhythmia event per 40,000 respiratory disturbances).
EFFECT OF SLEEP APNEA ON ATRIAL FIBRILLATION MANAGEMENT
Sleep apnea also seems to affect the efficacy of a rhythm-control strategy for atrial fibrillation. For example, patients with obstructive sleep apnea have a higher risk of recurrent atrial fibrillation after cardioversion (82% vs 42% in controls)12 and up to a 25% greater risk of recurrence after catheter ablation compared with those without obstructive sleep apnea (risk ratio 1.25, 95% CI 1.08–1.45).13
Several observational studies showed a higher rate of atrial fibrillation after pulmonary vein isolation in obstructive sleep apnea patients who do not use continuous positive airway pressure (CPAP) than in those who do.14–17 CPAP therapy appears to exert beneficial effects on cardiac structural remodeling; cardiac magnetic resonance imaging shows that patients with sleep apnea who received less than 4 hours of CPAP per night had larger left atrial dimensions and increased left ventricular mass compared with those who received more than 4 hours of CPAP at night.17 However, a need remains for high-quality, large randomized controlled trials to eliminate potential unmeasured biases due to differences that may exist between CPAP users and non-users, such as general adherence to medical therapy and healthcare interventions.
An additional consideration is that the overall utility and value of obtaining a diagnosis of obstructive sleep apnea strictly as it pertains to atrial fibrillation management is affected by whether a rhythm- or rate-control strategy is pursued. In other words, if a patient is deemed to be in permanent atrial fibrillation and a rhythm-control strategy is therefore not pursued, the potential effect of untreated obstructive sleep apnea on atrial fibrillation recurrence could be less important. In this case, however, the other beneficial cardiovascular and systemic effects of diagnosing and treating underlying obstructive sleep apnea would remain.
POPULATION STUDIES
Epidemiologic and clinic-based studies have supported an association between sleep apnea (mostly central, but also obstructive) and atrial fibrillation.4,18
Community-based studies such as the Sleep Heart Health Study4 and the Outcomes of Sleep Disorders in Older Men Study (MrOS Sleep),18 involving thousands of participants, have found the strongest cross-sectional associations of both obstructive and central sleep apnea with nocturnal atrial fibrillation. The findings included a 2 to 5 times higher odds of nocturnal atrial fibrillation, particularly in those with a moderate to severe degree of sleep-disordered breathing—even after adjusting for confounding influences (eg, obesity) and self-reported cardiac disease such as heart failure.
In MrOS Sleep, in an older male cohort, both obstructive and central sleep apnea were associated with nocturnal atrial fibrillation, though central sleep apnea and Cheyne-Stokes respirations had a stronger magnitude of association.18
Further insights can be drawn specifically from patients with heart failure. Sin et al,19 in a 1999 study, found that in 450 patients with systolic heart failure (85% men), the prevalence of sleep-disordered breathing was 25% to 33% (depending on the apnea-hypopnea index cutoff used) for central sleep apnea, and similarly 27% to 38% for obstructive sleep apnea. The prevalence of atrial fibrillation in this group was 10% in women and 15% in men. Atrial fibrillation was reported as a significant risk factor for central sleep apnea, but not for obstructive sleep apnea (for which only male sex and increasing body mass index were significant risk factors). Directionality was not clearly reported in this retrospective study in terms of timing of sleep studies and other assessments: ie, the report did not clearly state which came first, the atrial fibrillation or the sleep apnea. Therefore, the possibility that central sleep apnea is a predictor of atrial fibrillation cannot be excluded.
Yumino et al,20 in a study published in 2009, evaluated 218 patients with heart failure (with a left ventricular ejection fraction of ≤ 45%) and reported a prevalence of moderate to severe sleep apnea of 21% for central sleep apnea and 26% for obstructive sleep apnea. In multivariate analysis, atrial fibrillation was independently associated with central sleep apnea but not obstructive sleep apnea.
In recent cohort studies, central sleep apnea was associated with 2 to 3 times higher odds of developing atrial fibrillation, while obstructive sleep apnea was not a predictor of incident atrial fibrillation.9,21
Although most available studies associate sleep apnea with atrial fibrillation, findings of a case-control study22 did not support a difference in the prevalence of sleep apnea syndrome (defined as apnea index ≥ 5 and apnea-hypopnea index ≥ 15, and the presence of sleep symptoms) in patients with lone atrial fibrillation (no evident cardiovascular disease) compared with controls matched for age, sex, and cardiovascular morbidity.
But observational studies are limited by the potential for residual unmeasured confounding factors and lack of objective cardiac structural data, such as left ventricular ejection fraction and atrial enlargement. Moreover, there can be significant differences in sleep apnea definitions among studies, thus limiting the ability to reach a definitive conclusion about the relationship between sleep apnea and atrial fibrillation.
SCREENING AND DIAGNOSIS
The 2014 joint guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society for the management of atrial fibrillation state that a sleep study may be useful if sleep apnea is suspected.23 The 2019 focused update of the 2014 guidelines24 state that for overweight and obese patients with atrial fibrillation, weight loss combined with risk-factor modification is recommended (class I recommendation, level of evidence B-R, ie, data derived from 1 or more randomized trials or meta-analysis of such studies). Risk-factor modification in this case includes assessment and treatment of underlying sleep apnea, hypertension, hyperlipidemia, glucose intolerance, and alcohol and tobacco use.
Laboratory polysomnography has long been considered the gold standard for sleep apnea diagnosis. In one study,13 obstructive sleep apnea was a greater predictor of atrial fibrillation when diagnosed by polysomnography (risk ratio 1.40, 95% CI 1.16–1.68) compared with identification by screening using the Berlin questionnaire (risk ratio 1.07, 95% CI 0.91–1.27). However, a laboratory sleep study is associated with increased patient burden and limited availability.
Home sleep apnea testing is being increasingly used in the diagnostic evaluation of obstructive sleep apnea and may be a less costly, more available alternative. However, since a home sleep apnea test is less sensitive than polysomnography in detecting obstructive sleep apnea, the American Academy of Sleep Medicine guidelines28 state that if a single home sleep apnea test is negative or inconclusive, polysomnography should be done if there is clinical suspicion of sleep apnea. Moreover, current guidelines from this group recommend that patients with significant cardiorespiratory disease should be tested with polysomnography rather than home sleep apnea testing.22
Further study is needed to determine the optimal screening method for sleep apnea in patients with atrial fibrillation and to clarify the role of home sleep apnea testing. While keeping in mind the limitations of a screening questionnaire in this population, as a general approach it is reasonable to use a screening questionnaire for sleep apnea. And if the screen is positive, further evaluation with a sleep study is merited, whether by laboratory polysomnography, a home sleep apnea test, or referral to a sleep specialist.
MULTIDISCIPLINARY CARE MAY BE IDEAL
Overall, given the high prevalence of sleep apnea in patients with atrial fibrillation, the deleterious effects of sleep apnea in general, the influence of sleep apnea on atrial fibrillation, and the cardiovascular and other beneficial effects of adequate treatment of sleep apnea, patients with atrial fibrillation should be assessed for sleep apnea.
While the optimal strategy in evaluating for sleep apnea in these patients needs to be further defined, a multidisciplinary approach to care involving a primary care provider, cardiologist, and sleep specialist may be ideal.
- Braga B, Poyares D, Cintra F, et al. Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med 2009; 10(2):212–216. doi:10.1016/j.sleep.2007.12.007
- Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004; 110(4):364–367. doi:10.1161/01.CIR.0000136587.68725.8E
- Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J 2008; 29(13):1662–1669. doi:10.1093/eurheartj/ehn214
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916. doi:10.1164/rccm.200509-1442OC
- Cooper VL, Bowker CM, Pearson SB, Elliott MW, Hainsworth R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor-vascular resistance reflex. J Physiol 2004; 557(pt 3):1055–1065. doi:10.1113/jphysiol.2004.062513
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607. doi:10.1378/chest.06.2287
- Lévy P, Pépin JL, Arnaud C, et al. Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives. Eur Respir J 2008; 32(4):1082–1095. doi:10.1183/09031936.00013308
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Tung P, Levitzky YS, Wang R, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc 2017; 6(7). doi:10.1161/JAHA.116.004500
- Iwasaki YK, Shi Y, Benito B, et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm 2012; 9(9):1409–1416.e1. doi:10.1016/j.hrthm.2012.03.024
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804. doi:10.1016/j.jacc.2009.06.038
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107(20):2589–2594. doi:10.1161/01.CIR.0000068337.25994.21
- Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol 2011; 108(1):47–51. doi:10.1016/j.amjcard.2011.02.343
- Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013; 10(3):331–337. doi:10.1016/j.hrthm.2012.11.015
- Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013; 62(4):300–305. doi:10.1016/j.jacc.2013.03.052
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3(5):445–451. doi:10.1161/CIRCEP.109.858381
- Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013; 2(6):e000421. doi:10.1161/JAHA.113.000421
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155. doi:10.1001/archinternmed.2009.138
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail 2009; 15(4):279–285. doi:10.1016/j.cardfail.2008.11.015
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med 2016; 193(7):783–791. doi:10.1164/rccm.201508-1523OC
- Porthan KM, Melin JH, Kupila JT, Venho KK, Partinen MM. Prevalence of sleep apnea syndrome in lone atrial fibrillation: a case-control study. Chest 2004; 125(3):879–885. doi:10.1378/chest.125.3.879
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130(23):e199–e267. doi:10.1161/CIR.0000000000000041
- Writing Group Members; January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019; 16(8):e66–e93. doi:10.1016/j.hrthm.2019.01.024
- Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999; 131(7):485–491. doi:10.7326/0003-4819-131-7-199910050-00002
- Chung F, Abdullah HR, Liao P. STOP-bang questionnaire a practical approach to screen for obstructive sleep apnea. Chest 2016; 149(3):631–638. doi:10.1378/chest.15-0903
- Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med 2016; 4(9):742–748. doi:10.1016/S2213-2600(16)30075-3
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504. doi:10.5664/jcsm.6506
Yes. The prevalence of sleep apnea is exceedingly high in patients with atrial fibrillation—50% to 80% compared with 30% to 60% in respective control groups.1–3 Conversely, atrial fibrillation is more prevalent in those with sleep-disordered breathing than in those without (4.8% vs 0.9%).4
Sleep-disordered breathing comprises obstructive sleep apnea and central sleep apnea. Obstructive sleep apnea, characterized by repetitive upper-airway obstruction during sleep, is accompanied by intermittent hypoxia, rises in carbon dioxide, autonomic nervous system fluctuations, and intrathoracic pressure alterations.5 Central sleep apnea may be neurally mediated and, in the setting of cardiac disease, is characterized by alterations in chemosensitivity and chemoresponsiveness, leading to a state of high loop gain—ie, a hypersensitive ventilatory control system leading to ventilatory drive oscillations.6
Both obstructive and central sleep apnea have been associated with atrial fibrillation. Experimental data implicate obstructive sleep apnea as a trigger of atrial arrhythmogenesis,7,8 and epidemiologic studies support an association between central sleep apnea, Cheyne-Stokes respiration, and incident atrial fibrillation.9
HOW SLEEP APNEA COULD LEAD TO ATRIAL FIBRILLATION
In experiments in animals, intermittent upper-airway obstruction led to forced inspiration, substantial negative intrathoracic pressure, subsequent left atrial distention, and increased susceptibility to atrial fibrillation.10 The autonomic nervous system may be a mediator of apnea-induced atrial fibrillation, as apnea-induced atrial fibrillation is suppressed with autonomic blockade.10
Emerging data also support the hypothesis that intermittent hypoxia7 and resolution of hypercapnia,8 as observed in obstructive sleep apnea, exert atrial electrophysiologic changes that increase vulnerability to atrial arrhythmogenesis.
In a case-crossover study,11 the odds of paroxysmal atrial fibrillation occurring after a respiratory disturbance were 17.9 times higher than after normal breathing (95% confidence interval [CI] 2.2–144.2), though the absolute rate of overall arrhythmia events (including both atrial fibrillation and nonsustained ventricular tachycardia) associated with respiratory disturbances was low (1 excess arrhythmia event per 40,000 respiratory disturbances).
EFFECT OF SLEEP APNEA ON ATRIAL FIBRILLATION MANAGEMENT
Sleep apnea also seems to affect the efficacy of a rhythm-control strategy for atrial fibrillation. For example, patients with obstructive sleep apnea have a higher risk of recurrent atrial fibrillation after cardioversion (82% vs 42% in controls)12 and up to a 25% greater risk of recurrence after catheter ablation compared with those without obstructive sleep apnea (risk ratio 1.25, 95% CI 1.08–1.45).13
Several observational studies showed a higher rate of atrial fibrillation after pulmonary vein isolation in obstructive sleep apnea patients who do not use continuous positive airway pressure (CPAP) than in those who do.14–17 CPAP therapy appears to exert beneficial effects on cardiac structural remodeling; cardiac magnetic resonance imaging shows that patients with sleep apnea who received less than 4 hours of CPAP per night had larger left atrial dimensions and increased left ventricular mass compared with those who received more than 4 hours of CPAP at night.17 However, a need remains for high-quality, large randomized controlled trials to eliminate potential unmeasured biases due to differences that may exist between CPAP users and non-users, such as general adherence to medical therapy and healthcare interventions.
An additional consideration is that the overall utility and value of obtaining a diagnosis of obstructive sleep apnea strictly as it pertains to atrial fibrillation management is affected by whether a rhythm- or rate-control strategy is pursued. In other words, if a patient is deemed to be in permanent atrial fibrillation and a rhythm-control strategy is therefore not pursued, the potential effect of untreated obstructive sleep apnea on atrial fibrillation recurrence could be less important. In this case, however, the other beneficial cardiovascular and systemic effects of diagnosing and treating underlying obstructive sleep apnea would remain.
POPULATION STUDIES
Epidemiologic and clinic-based studies have supported an association between sleep apnea (mostly central, but also obstructive) and atrial fibrillation.4,18
Community-based studies such as the Sleep Heart Health Study4 and the Outcomes of Sleep Disorders in Older Men Study (MrOS Sleep),18 involving thousands of participants, have found the strongest cross-sectional associations of both obstructive and central sleep apnea with nocturnal atrial fibrillation. The findings included a 2 to 5 times higher odds of nocturnal atrial fibrillation, particularly in those with a moderate to severe degree of sleep-disordered breathing—even after adjusting for confounding influences (eg, obesity) and self-reported cardiac disease such as heart failure.
In MrOS Sleep, in an older male cohort, both obstructive and central sleep apnea were associated with nocturnal atrial fibrillation, though central sleep apnea and Cheyne-Stokes respirations had a stronger magnitude of association.18
Further insights can be drawn specifically from patients with heart failure. Sin et al,19 in a 1999 study, found that in 450 patients with systolic heart failure (85% men), the prevalence of sleep-disordered breathing was 25% to 33% (depending on the apnea-hypopnea index cutoff used) for central sleep apnea, and similarly 27% to 38% for obstructive sleep apnea. The prevalence of atrial fibrillation in this group was 10% in women and 15% in men. Atrial fibrillation was reported as a significant risk factor for central sleep apnea, but not for obstructive sleep apnea (for which only male sex and increasing body mass index were significant risk factors). Directionality was not clearly reported in this retrospective study in terms of timing of sleep studies and other assessments: ie, the report did not clearly state which came first, the atrial fibrillation or the sleep apnea. Therefore, the possibility that central sleep apnea is a predictor of atrial fibrillation cannot be excluded.
Yumino et al,20 in a study published in 2009, evaluated 218 patients with heart failure (with a left ventricular ejection fraction of ≤ 45%) and reported a prevalence of moderate to severe sleep apnea of 21% for central sleep apnea and 26% for obstructive sleep apnea. In multivariate analysis, atrial fibrillation was independently associated with central sleep apnea but not obstructive sleep apnea.
In recent cohort studies, central sleep apnea was associated with 2 to 3 times higher odds of developing atrial fibrillation, while obstructive sleep apnea was not a predictor of incident atrial fibrillation.9,21
Although most available studies associate sleep apnea with atrial fibrillation, findings of a case-control study22 did not support a difference in the prevalence of sleep apnea syndrome (defined as apnea index ≥ 5 and apnea-hypopnea index ≥ 15, and the presence of sleep symptoms) in patients with lone atrial fibrillation (no evident cardiovascular disease) compared with controls matched for age, sex, and cardiovascular morbidity.
But observational studies are limited by the potential for residual unmeasured confounding factors and lack of objective cardiac structural data, such as left ventricular ejection fraction and atrial enlargement. Moreover, there can be significant differences in sleep apnea definitions among studies, thus limiting the ability to reach a definitive conclusion about the relationship between sleep apnea and atrial fibrillation.
SCREENING AND DIAGNOSIS
The 2014 joint guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society for the management of atrial fibrillation state that a sleep study may be useful if sleep apnea is suspected.23 The 2019 focused update of the 2014 guidelines24 state that for overweight and obese patients with atrial fibrillation, weight loss combined with risk-factor modification is recommended (class I recommendation, level of evidence B-R, ie, data derived from 1 or more randomized trials or meta-analysis of such studies). Risk-factor modification in this case includes assessment and treatment of underlying sleep apnea, hypertension, hyperlipidemia, glucose intolerance, and alcohol and tobacco use.
Laboratory polysomnography has long been considered the gold standard for sleep apnea diagnosis. In one study,13 obstructive sleep apnea was a greater predictor of atrial fibrillation when diagnosed by polysomnography (risk ratio 1.40, 95% CI 1.16–1.68) compared with identification by screening using the Berlin questionnaire (risk ratio 1.07, 95% CI 0.91–1.27). However, a laboratory sleep study is associated with increased patient burden and limited availability.
Home sleep apnea testing is being increasingly used in the diagnostic evaluation of obstructive sleep apnea and may be a less costly, more available alternative. However, since a home sleep apnea test is less sensitive than polysomnography in detecting obstructive sleep apnea, the American Academy of Sleep Medicine guidelines28 state that if a single home sleep apnea test is negative or inconclusive, polysomnography should be done if there is clinical suspicion of sleep apnea. Moreover, current guidelines from this group recommend that patients with significant cardiorespiratory disease should be tested with polysomnography rather than home sleep apnea testing.22
Further study is needed to determine the optimal screening method for sleep apnea in patients with atrial fibrillation and to clarify the role of home sleep apnea testing. While keeping in mind the limitations of a screening questionnaire in this population, as a general approach it is reasonable to use a screening questionnaire for sleep apnea. And if the screen is positive, further evaluation with a sleep study is merited, whether by laboratory polysomnography, a home sleep apnea test, or referral to a sleep specialist.
MULTIDISCIPLINARY CARE MAY BE IDEAL
Overall, given the high prevalence of sleep apnea in patients with atrial fibrillation, the deleterious effects of sleep apnea in general, the influence of sleep apnea on atrial fibrillation, and the cardiovascular and other beneficial effects of adequate treatment of sleep apnea, patients with atrial fibrillation should be assessed for sleep apnea.
While the optimal strategy in evaluating for sleep apnea in these patients needs to be further defined, a multidisciplinary approach to care involving a primary care provider, cardiologist, and sleep specialist may be ideal.
Yes. The prevalence of sleep apnea is exceedingly high in patients with atrial fibrillation—50% to 80% compared with 30% to 60% in respective control groups.1–3 Conversely, atrial fibrillation is more prevalent in those with sleep-disordered breathing than in those without (4.8% vs 0.9%).4
Sleep-disordered breathing comprises obstructive sleep apnea and central sleep apnea. Obstructive sleep apnea, characterized by repetitive upper-airway obstruction during sleep, is accompanied by intermittent hypoxia, rises in carbon dioxide, autonomic nervous system fluctuations, and intrathoracic pressure alterations.5 Central sleep apnea may be neurally mediated and, in the setting of cardiac disease, is characterized by alterations in chemosensitivity and chemoresponsiveness, leading to a state of high loop gain—ie, a hypersensitive ventilatory control system leading to ventilatory drive oscillations.6
Both obstructive and central sleep apnea have been associated with atrial fibrillation. Experimental data implicate obstructive sleep apnea as a trigger of atrial arrhythmogenesis,7,8 and epidemiologic studies support an association between central sleep apnea, Cheyne-Stokes respiration, and incident atrial fibrillation.9
HOW SLEEP APNEA COULD LEAD TO ATRIAL FIBRILLATION
In experiments in animals, intermittent upper-airway obstruction led to forced inspiration, substantial negative intrathoracic pressure, subsequent left atrial distention, and increased susceptibility to atrial fibrillation.10 The autonomic nervous system may be a mediator of apnea-induced atrial fibrillation, as apnea-induced atrial fibrillation is suppressed with autonomic blockade.10
Emerging data also support the hypothesis that intermittent hypoxia7 and resolution of hypercapnia,8 as observed in obstructive sleep apnea, exert atrial electrophysiologic changes that increase vulnerability to atrial arrhythmogenesis.
In a case-crossover study,11 the odds of paroxysmal atrial fibrillation occurring after a respiratory disturbance were 17.9 times higher than after normal breathing (95% confidence interval [CI] 2.2–144.2), though the absolute rate of overall arrhythmia events (including both atrial fibrillation and nonsustained ventricular tachycardia) associated with respiratory disturbances was low (1 excess arrhythmia event per 40,000 respiratory disturbances).
EFFECT OF SLEEP APNEA ON ATRIAL FIBRILLATION MANAGEMENT
Sleep apnea also seems to affect the efficacy of a rhythm-control strategy for atrial fibrillation. For example, patients with obstructive sleep apnea have a higher risk of recurrent atrial fibrillation after cardioversion (82% vs 42% in controls)12 and up to a 25% greater risk of recurrence after catheter ablation compared with those without obstructive sleep apnea (risk ratio 1.25, 95% CI 1.08–1.45).13
Several observational studies showed a higher rate of atrial fibrillation after pulmonary vein isolation in obstructive sleep apnea patients who do not use continuous positive airway pressure (CPAP) than in those who do.14–17 CPAP therapy appears to exert beneficial effects on cardiac structural remodeling; cardiac magnetic resonance imaging shows that patients with sleep apnea who received less than 4 hours of CPAP per night had larger left atrial dimensions and increased left ventricular mass compared with those who received more than 4 hours of CPAP at night.17 However, a need remains for high-quality, large randomized controlled trials to eliminate potential unmeasured biases due to differences that may exist between CPAP users and non-users, such as general adherence to medical therapy and healthcare interventions.
An additional consideration is that the overall utility and value of obtaining a diagnosis of obstructive sleep apnea strictly as it pertains to atrial fibrillation management is affected by whether a rhythm- or rate-control strategy is pursued. In other words, if a patient is deemed to be in permanent atrial fibrillation and a rhythm-control strategy is therefore not pursued, the potential effect of untreated obstructive sleep apnea on atrial fibrillation recurrence could be less important. In this case, however, the other beneficial cardiovascular and systemic effects of diagnosing and treating underlying obstructive sleep apnea would remain.
POPULATION STUDIES
Epidemiologic and clinic-based studies have supported an association between sleep apnea (mostly central, but also obstructive) and atrial fibrillation.4,18
Community-based studies such as the Sleep Heart Health Study4 and the Outcomes of Sleep Disorders in Older Men Study (MrOS Sleep),18 involving thousands of participants, have found the strongest cross-sectional associations of both obstructive and central sleep apnea with nocturnal atrial fibrillation. The findings included a 2 to 5 times higher odds of nocturnal atrial fibrillation, particularly in those with a moderate to severe degree of sleep-disordered breathing—even after adjusting for confounding influences (eg, obesity) and self-reported cardiac disease such as heart failure.
In MrOS Sleep, in an older male cohort, both obstructive and central sleep apnea were associated with nocturnal atrial fibrillation, though central sleep apnea and Cheyne-Stokes respirations had a stronger magnitude of association.18
Further insights can be drawn specifically from patients with heart failure. Sin et al,19 in a 1999 study, found that in 450 patients with systolic heart failure (85% men), the prevalence of sleep-disordered breathing was 25% to 33% (depending on the apnea-hypopnea index cutoff used) for central sleep apnea, and similarly 27% to 38% for obstructive sleep apnea. The prevalence of atrial fibrillation in this group was 10% in women and 15% in men. Atrial fibrillation was reported as a significant risk factor for central sleep apnea, but not for obstructive sleep apnea (for which only male sex and increasing body mass index were significant risk factors). Directionality was not clearly reported in this retrospective study in terms of timing of sleep studies and other assessments: ie, the report did not clearly state which came first, the atrial fibrillation or the sleep apnea. Therefore, the possibility that central sleep apnea is a predictor of atrial fibrillation cannot be excluded.
Yumino et al,20 in a study published in 2009, evaluated 218 patients with heart failure (with a left ventricular ejection fraction of ≤ 45%) and reported a prevalence of moderate to severe sleep apnea of 21% for central sleep apnea and 26% for obstructive sleep apnea. In multivariate analysis, atrial fibrillation was independently associated with central sleep apnea but not obstructive sleep apnea.
In recent cohort studies, central sleep apnea was associated with 2 to 3 times higher odds of developing atrial fibrillation, while obstructive sleep apnea was not a predictor of incident atrial fibrillation.9,21
Although most available studies associate sleep apnea with atrial fibrillation, findings of a case-control study22 did not support a difference in the prevalence of sleep apnea syndrome (defined as apnea index ≥ 5 and apnea-hypopnea index ≥ 15, and the presence of sleep symptoms) in patients with lone atrial fibrillation (no evident cardiovascular disease) compared with controls matched for age, sex, and cardiovascular morbidity.
But observational studies are limited by the potential for residual unmeasured confounding factors and lack of objective cardiac structural data, such as left ventricular ejection fraction and atrial enlargement. Moreover, there can be significant differences in sleep apnea definitions among studies, thus limiting the ability to reach a definitive conclusion about the relationship between sleep apnea and atrial fibrillation.
SCREENING AND DIAGNOSIS
The 2014 joint guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society for the management of atrial fibrillation state that a sleep study may be useful if sleep apnea is suspected.23 The 2019 focused update of the 2014 guidelines24 state that for overweight and obese patients with atrial fibrillation, weight loss combined with risk-factor modification is recommended (class I recommendation, level of evidence B-R, ie, data derived from 1 or more randomized trials or meta-analysis of such studies). Risk-factor modification in this case includes assessment and treatment of underlying sleep apnea, hypertension, hyperlipidemia, glucose intolerance, and alcohol and tobacco use.
Laboratory polysomnography has long been considered the gold standard for sleep apnea diagnosis. In one study,13 obstructive sleep apnea was a greater predictor of atrial fibrillation when diagnosed by polysomnography (risk ratio 1.40, 95% CI 1.16–1.68) compared with identification by screening using the Berlin questionnaire (risk ratio 1.07, 95% CI 0.91–1.27). However, a laboratory sleep study is associated with increased patient burden and limited availability.
Home sleep apnea testing is being increasingly used in the diagnostic evaluation of obstructive sleep apnea and may be a less costly, more available alternative. However, since a home sleep apnea test is less sensitive than polysomnography in detecting obstructive sleep apnea, the American Academy of Sleep Medicine guidelines28 state that if a single home sleep apnea test is negative or inconclusive, polysomnography should be done if there is clinical suspicion of sleep apnea. Moreover, current guidelines from this group recommend that patients with significant cardiorespiratory disease should be tested with polysomnography rather than home sleep apnea testing.22
Further study is needed to determine the optimal screening method for sleep apnea in patients with atrial fibrillation and to clarify the role of home sleep apnea testing. While keeping in mind the limitations of a screening questionnaire in this population, as a general approach it is reasonable to use a screening questionnaire for sleep apnea. And if the screen is positive, further evaluation with a sleep study is merited, whether by laboratory polysomnography, a home sleep apnea test, or referral to a sleep specialist.
MULTIDISCIPLINARY CARE MAY BE IDEAL
Overall, given the high prevalence of sleep apnea in patients with atrial fibrillation, the deleterious effects of sleep apnea in general, the influence of sleep apnea on atrial fibrillation, and the cardiovascular and other beneficial effects of adequate treatment of sleep apnea, patients with atrial fibrillation should be assessed for sleep apnea.
While the optimal strategy in evaluating for sleep apnea in these patients needs to be further defined, a multidisciplinary approach to care involving a primary care provider, cardiologist, and sleep specialist may be ideal.
- Braga B, Poyares D, Cintra F, et al. Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med 2009; 10(2):212–216. doi:10.1016/j.sleep.2007.12.007
- Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004; 110(4):364–367. doi:10.1161/01.CIR.0000136587.68725.8E
- Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J 2008; 29(13):1662–1669. doi:10.1093/eurheartj/ehn214
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916. doi:10.1164/rccm.200509-1442OC
- Cooper VL, Bowker CM, Pearson SB, Elliott MW, Hainsworth R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor-vascular resistance reflex. J Physiol 2004; 557(pt 3):1055–1065. doi:10.1113/jphysiol.2004.062513
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607. doi:10.1378/chest.06.2287
- Lévy P, Pépin JL, Arnaud C, et al. Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives. Eur Respir J 2008; 32(4):1082–1095. doi:10.1183/09031936.00013308
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Tung P, Levitzky YS, Wang R, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc 2017; 6(7). doi:10.1161/JAHA.116.004500
- Iwasaki YK, Shi Y, Benito B, et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm 2012; 9(9):1409–1416.e1. doi:10.1016/j.hrthm.2012.03.024
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804. doi:10.1016/j.jacc.2009.06.038
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107(20):2589–2594. doi:10.1161/01.CIR.0000068337.25994.21
- Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol 2011; 108(1):47–51. doi:10.1016/j.amjcard.2011.02.343
- Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013; 10(3):331–337. doi:10.1016/j.hrthm.2012.11.015
- Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013; 62(4):300–305. doi:10.1016/j.jacc.2013.03.052
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3(5):445–451. doi:10.1161/CIRCEP.109.858381
- Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013; 2(6):e000421. doi:10.1161/JAHA.113.000421
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155. doi:10.1001/archinternmed.2009.138
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail 2009; 15(4):279–285. doi:10.1016/j.cardfail.2008.11.015
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med 2016; 193(7):783–791. doi:10.1164/rccm.201508-1523OC
- Porthan KM, Melin JH, Kupila JT, Venho KK, Partinen MM. Prevalence of sleep apnea syndrome in lone atrial fibrillation: a case-control study. Chest 2004; 125(3):879–885. doi:10.1378/chest.125.3.879
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130(23):e199–e267. doi:10.1161/CIR.0000000000000041
- Writing Group Members; January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019; 16(8):e66–e93. doi:10.1016/j.hrthm.2019.01.024
- Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999; 131(7):485–491. doi:10.7326/0003-4819-131-7-199910050-00002
- Chung F, Abdullah HR, Liao P. STOP-bang questionnaire a practical approach to screen for obstructive sleep apnea. Chest 2016; 149(3):631–638. doi:10.1378/chest.15-0903
- Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med 2016; 4(9):742–748. doi:10.1016/S2213-2600(16)30075-3
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504. doi:10.5664/jcsm.6506
- Braga B, Poyares D, Cintra F, et al. Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med 2009; 10(2):212–216. doi:10.1016/j.sleep.2007.12.007
- Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004; 110(4):364–367. doi:10.1161/01.CIR.0000136587.68725.8E
- Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J 2008; 29(13):1662–1669. doi:10.1093/eurheartj/ehn214
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916. doi:10.1164/rccm.200509-1442OC
- Cooper VL, Bowker CM, Pearson SB, Elliott MW, Hainsworth R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor-vascular resistance reflex. J Physiol 2004; 557(pt 3):1055–1065. doi:10.1113/jphysiol.2004.062513
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607. doi:10.1378/chest.06.2287
- Lévy P, Pépin JL, Arnaud C, et al. Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives. Eur Respir J 2008; 32(4):1082–1095. doi:10.1183/09031936.00013308
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Tung P, Levitzky YS, Wang R, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc 2017; 6(7). doi:10.1161/JAHA.116.004500
- Iwasaki YK, Shi Y, Benito B, et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm 2012; 9(9):1409–1416.e1. doi:10.1016/j.hrthm.2012.03.024
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804. doi:10.1016/j.jacc.2009.06.038
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107(20):2589–2594. doi:10.1161/01.CIR.0000068337.25994.21
- Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol 2011; 108(1):47–51. doi:10.1016/j.amjcard.2011.02.343
- Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013; 10(3):331–337. doi:10.1016/j.hrthm.2012.11.015
- Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013; 62(4):300–305. doi:10.1016/j.jacc.2013.03.052
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3(5):445–451. doi:10.1161/CIRCEP.109.858381
- Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013; 2(6):e000421. doi:10.1161/JAHA.113.000421
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155. doi:10.1001/archinternmed.2009.138
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail 2009; 15(4):279–285. doi:10.1016/j.cardfail.2008.11.015
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med 2016; 193(7):783–791. doi:10.1164/rccm.201508-1523OC
- Porthan KM, Melin JH, Kupila JT, Venho KK, Partinen MM. Prevalence of sleep apnea syndrome in lone atrial fibrillation: a case-control study. Chest 2004; 125(3):879–885. doi:10.1378/chest.125.3.879
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130(23):e199–e267. doi:10.1161/CIR.0000000000000041
- Writing Group Members; January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019; 16(8):e66–e93. doi:10.1016/j.hrthm.2019.01.024
- Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999; 131(7):485–491. doi:10.7326/0003-4819-131-7-199910050-00002
- Chung F, Abdullah HR, Liao P. STOP-bang questionnaire a practical approach to screen for obstructive sleep apnea. Chest 2016; 149(3):631–638. doi:10.1378/chest.15-0903
- Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med 2016; 4(9):742–748. doi:10.1016/S2213-2600(16)30075-3
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504. doi:10.5664/jcsm.6506
Sleep apnea and the heart
SLEEP AND CARDIOVASCULAR PHYSIOLOGY
Wakefullness and sleep, the latter comprised of non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep, comprise our primary states of being. Sleep states oscillate between NREM and REM sleep. The first and shortest period of REM sleep typically occurs 90 to 120 minutes into the sleep cycle. Most REM sleep, including the longest period of REM sleep, occurs during the latter part of the sleep cycle.
With these sleep state changes, physiologic changes also occur, such as reduced heart rate and blood pressure because of enhanced parasympathetic tone. During REM sleep, there are also intermittent sympathetic nervous system surges. Other physiologic changes include a regular respiratory rate during NREM sleep and an irregular respiratory rate during REM sleep. Body temperature is normal during NREM sleep and poikilothermic (ie, tends to flucuate) during REM sleep. Blood pressure is reduced 10% to 15% during sleep1 and then rises, so that the highest blood pressure occurs in the morning. Data from 10 million users of activity-monitoring devices show that the heart rate changes during sleep.2 The heart rate is decreased in those who get less than 7 hours of sleep, then increases with longer sleep duration in a U-shaped distribution.
Cardiovascular events are more likely to occur at certain times of day. Myocardial infarction is more likely in the morning, with a threefold increased risk within the first 3 hours of awakening that peaks around 9 AM.3,4 Similar diurnal patterns have been observed with other cardiovascular conditions such as sudden cardiac death and ischemic episodes, with the highest risk during morning hours (6 to 9 AM).4
The reason for this morning predisposition for cardiovascular events is unclear, but it is thought that perhaps the autonomic fluctuations that occur during REM sleep and the predominance of REM sleep in early morning may be a factor. Diurnal changes in blood pressure and cortisol levels may also contribute, as well as levels of systemic inflammatory and thrombotic markers such as plasminogen activator inhibitor 1.
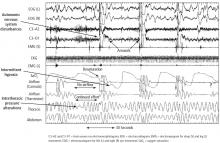
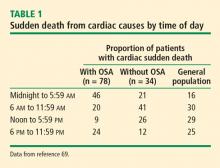
Arrhythmias are also more likely to occur in a diurnal pattern. Atrial fibrillation (AF), particularly paroxysmal AF, is believed to be vagally mediated in 10% to 25% of patients.5 Therefore, for those who are predisposed, sleep may represent a period of increased risk for AF. In a study of individuals 60 years and older, the maximum duration and peak frequency of AF occurred from midnight to 2 AM.5
Recent studies have found that REM-related obstructive sleep apnea (OSA) is associated with increased cardiovascular risk. Experimental models show that REM sleep may increase the risk for compromised coronary blood flow.6 Increased heart rate corresponds to reduced coronary blood flow and thus, to decreased coronary perfusion time and less time for relaxation of the heart, increasing the risk for coronary artery disease, thrombosis, and ischemia.
SLEEP APNEA PATHOPHYSIOLOGY
The normal physiology of the sleep-heart interaction is disrupted by sleep apnea. OSA is defined as episodes of complete or partial airway obstruction that occur during sleep with thoracoabdominal effort. Central sleep apnea (CSA) is the cessation of breathing with no thoracoabdominal effort. The pathophysiology of the sleep-heart interaction varies for OSA and CSA.
Obstructive sleep apnea
OSA is a nocturnal physiologic stressor that is highly prevalent and underrecognized. It affects approximately 17% of the adult population, and the prevalence is increasing with the obesity epidemic. Nearly 1 in 15 individuals is estimated to be affected by at least moderate OSA.7,8 OSA is underdiagnosed particularly in minority populations.9 Data from the 2015 Multi-Ethnic Study of Atherosclerosis (MESA) showed undiagnosed moderate to severe sleep apnea in 84% to 93% of individuals,9 similar to an estimated 85% of undiagnosed cases in 2002.10
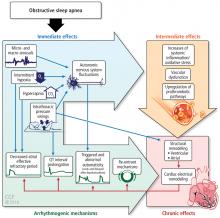
OSA is highly prevalent in individuals with underlying coronary disease11–13 and in those with cardiovascular risk factors such as diabetes, hypertension, and heart failure. The prevalence of OSA in patients with cardiovascular disease ranges from 30% (hypertension) to 60% (stroke or transient ischemic attack, arrhythmia, end-stage renal disease).14
Pathophysiology of OSA
The alterations in sympathetic activation that occur during sleep in patients with OSA persist during wakefulness. Microneurographic recording of sympathetic nerve activity in the peroneal nerve reveal that the rate of sympathetic bursts doubles and the amplitude is greater in individuals with OSA compared with a control group.15
Sympathetic nerve activity, blood pressure, and heart rate were shown to increase during REM sleep in individuals with OSA on continuous positive airway pressure (CPAP) during an induced apneic event (pressure reduction from 8 cm to 6 cm of water).15
During OSA episodes, there is an increased cardiac load. Impaired diastolic function and atrial and aortic enlargement, and in particular, the thin-walled atria are very susceptible to the intrathoracic pressure swings caused by OSA. Physiologic changes with OSA from pressure changes in the chest result in shift of the intraventricular septum, causing a reduction in cardiac output.16 With the lowering of oxygen during episodes of apnea, constriction of the pulmonary vasculature leads to elevation of pressure in the pulmonary vasculature reflected by the increase in mean pulmonary arterial pressures.17
Other studies have shown that OSA increases upregulation of markers of systemic inflammation and prothrombotic markers, the very markers that can increase cardiovascular or atherogenic risk.18–22 One example is the soluble interleukin 6 receptor, shown to be elevated in the morning relative to sleep apnea compared with the evening.20 Other biomarkers observed to be associated with sleep apnea include markers of prothrombotic potentials such as plasminogen activator inhibitor 1.19 Oxidative stress occurs because intermittent bouts of lower oxygen can lead to oxidation of serum proteins and lipids. Endothelial dysfunction has been observed as well as insulin resistance and dyslipidemia.23 Taken together, these are pathways that lead to atherogenesis and increased cardiovascular risk.
Central sleep apnea
CSA episodes are the cessation of breathing without thoracoabdominal effort, in contrast to the persistence of thoracoabdominal effort in OSA. CSA is characterized by breathing instability with highly sensitive chemoresponses and prolonged circulation time.24 This can be physiologic in some cases, as when it occurs after a very large breath or sigh and then a central apnea event occurs after the sigh. The alterations in oxygen and CO2 and the stretch of the receptors in the alveoli of the lungs initiate the Hering-Breuer inhalation reflex.
Pathophysiology of CSA
Complex pathways of medullary and aortic receptor chemosensitivity are at the root of the pathophysiology of CSA.24 With CSA there is often a relative state of hypocapnia at baseline. During sleep, there is reduction in drive, thus chemosensitivity can be activated so that central apnea episodes can ensue as a result of alterations in CO2 (ie, hypocapnia). Another factor that can contribute to the pathophysiology of CSA is arousal from sleep that can reduce CO2 levels and therefore perpetuate central events.
The concept of loop gain is used to understand the pathophysiology of CSA. Loop gain is a measure of the relative stability of a ventilation system and indicates the likelihood of an individual to have periodic breathing. It is calculated by the response to a disturbance divided by the disturbance itself.25 With a high loop gain, there is a more pronounced or exuberant response to the disturbance, indicating more instability in the system and increasing the tendency for irregular breathing and CSA episodes.
Hunter-Cheyne-Stokes respiration occurs with CSA and is characterized by cyclical crescendo-decrescendo respiratory effort that occurs during wakefulness and sleep without upper-airway obstruction.26,27 Unlike OSA, which is worse during REM sleep, Hunter-Cheyne-Stokes breathing in CSA is typically worse in NREM sleep, during N1 and N2 in particular.
SLEEP APNEA AND HEART FAILURE
Both OSA and CSA are prevalent in patients with heart failure and may be associated with the progression of heart failure. CSA often occurs in patients with heart failure. The pathophysiology is multifactorial, including pulmonary congestion that results in stretch of the J receptors in the alveoli, prolonged circulation time, and increased chemosensitivity.
Complex pathways in the neuroaxis or somnogenic biomarkers of inflammation or both may be implicated in the paradoxical lack of subjective sleepiness in the presence of increased objective measures of sleepiness in systolic heart failure. One study found a relationship with one biomarker of inflammation and oxidative stress as it relates to objective symptoms of sleepiness but not subjective symptoms of sleepiness.28
Another contributing factor in the relationship between OSA and CSA in heart failure has also been described related to rostral shifts in fluid to the neck and to the pulmonary receptors in the alveoli of the lungs.29 These rostral shifts in fluids may contribute to sleep apnea with parapharyngeal edema leading to OSA and pulmonary congestion leading to CSA.
Sleep apnea is associated with increased post-discharge mortality and hospitalization readmissions in the setting of acute heart failure.30 Mortality analysis of 1,096 patients admitted for decompensated heart failure found CSA and OSA were independently associated with mortality in patients compared with patients with no or minimal sleep-disordered breathing.30
CSA has also been shown to be a predictor of readmission in patients admitted for heart failure exacerbations.31 Targeting underlying CSA may reduce readmissions in those admitted with acute decompensated heart failure. While men were identified to be at increased risk of death relative to sleep-disordered breathing based on the initial results of the Sleep Heart Health Study, a subsequent epidemiologic substudy reflective of an older age group showed that OSA was more strongly associated with left ventricular mass index, risk of heart failure, or death in women compared with men.32
Treatment
Standard therapy for treatment of OSA is CPAP. Adaptive servo-ventilation (ASV) and transvenous phrenic nerve stimulation are also available as treatment options in certain cases of CSA.
One of the first randomized controlled trials designed to assess the impact of CSA treatment on survival in patients with heart failure initially favored the control group then later the CPAP group and was terminated early based on stopping rules.33,34 While adherence to therapy was suboptimal at an average of 3.6 hours, post hoc analysis showed that patients with CSA using CPAP with effective suppression of CSA had improved survival compared with patients who did not have effective suppression using CPAP.34
ASV is mainly used for treatment of CSA. In ASV, positive airway pressure for ventilation support is provided and adjusts as apneic episodes are detected during sleep. The support provided adapts to the physiology of the patient and can deliver breaths and utilize anticyclic modes of ventilation to address crescendo-decrescendo breathing patterns observed in Hunter-Cheyne-Stokes respiration.
In the Treatment of Sleep-Disordered Breathing With Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) trial, 1,300 patients with systolic heart failure and predominantly CSA were randomized to receive ASV vs solely standard medical management.35 The primary composite end point included all-cause mortality or unplanned admission or hospitalization for heart failure. No difference was found in the primary end point between the ASV and the control group; however, there was an unanticipated negative impact of ASV on cardiovascular outcomes in some secondary end points. Based on the secondary outcome of cardiovascular-specific mortality, clinicians were advised that ASV was contraindicated for the treatment of CSA in patients with symptomatic heart failure with a left ventricular ejection fraction less than 45%. The interpretation of this study was complicated by several methodologic limitations.36
The Cardiovascular Improvements With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure (CAT-HF) randomized controlled trial also evaluated ASV compared with standard medical management in 126 patients with heart failure.37 This trial was terminated early because of the results of the SERVE-HF trial. Compliance with therapy was suboptimal at an average of 2.7 hours per day. The composite end point did not differ between the 2 groups; however, this was likely because the study was underpowered and was terminated early. Subgroup analysis revealed that patients with heart failure with preserved ejection fraction may benefit from ASV; however, additional studies are needed to confirm these findings.
Therefore, although ASV is not indicated when there is predominantly CSA in patients with systolic heart failure, preliminary results support potential benefit in patients with OSA and preserved ejection fraction.
Another novel treatment for CSA is transvenous phrenic nerve stimulation. A device is implanted that stimulates the phrenic nerve to initiate breaths. The initial study of transvenous phrenic nerve stimulation reported a significant reduction in the number of episodes of central apnea per hour of sleep.38,39 The apnea–hypopnea index improved overall and some types of obstructive apneic events were reduced with transvenous phrenic nerve stimulation.
A multicenter randomized control trial of transvenous phrenic nerve stimulation found improvement in several sleep apnea indices, including central apnea, hypoxia, reduced arousals from sleep, and patient reported well-being.40 Transvenous phrenic nerve stimulation holds promise as a novel therapy for central predominant sleep apnea not only in terms of improving the degree of central apnea and sleep-disordered breathing, but also in improving functional outcomes. Longitudinal and intereventional trial data are needed to clarify the impact of transvenous phrenic nerve stimulation on long-term cardiac outcomes.
SLEEP APNEA AND ATRIAL FIBRILLATION AND STROKE
Atrial fibrillation
AF is the most common sustained cardiac arrhythmia. The number of Americans with AF is projected to increase from 2.3 million to more than 10 million by the year 2050.41 The increasing incidence and prevalence of AF is not fully explained by the aging population and established risk factors.42 Unrecognized sleep apnea, estimated to exist in 85% or more of the population, may partially account for the increasing incidence of AF.43
There are 3 types of AF, which are thought to follow a continuum: paroxysmal AF is characterized by episodes that occur intermittently; persistent AF is characterized by episodes that last longer than 7 days; chronic or permanent AF is typically characterized by AF that is ongoing over many years.44 As with sleep apnea, AF is often asymptomatic and is likely underdiagnosed.
Sleep apnea and AF share several risk factors. Obesity is a risk factor for both OSA and AF; however, a meta-analysis supported a stronger association of OSA and AF vs obesity and AF.45 Increasing age is a risk factor for both OSA and AF.46,47 Although white populations are at higher risk for AF, OSA is associated with a 58% increased risk of AF in African Americans.48 Nocturnal hypoxia has been associated with increased risk of AF in Asians.49
Experimental data continue to accrue providing biologic plausibility of the relationship between sleep apnea and AF. OSA contributes to structural and electrical remodeling of the heart with evidence supporting increased fibrosis and electrical remodeling in patients with OSA compared with a control group.51 Markers of structural remodeling, such as atrial size, electrical silence, and atrial voltage conduction velocity, are altered in OSA.50
Data from the Sleep Heart Health Study show very strong associations between atrial and ventricular cardiac arrhythmias and sleep apnea with two- to fivefold higher odds of arrhythmias in patients with severe OSA compared with controls even after accounting for confounding factors such as obesity.52
A multicenter, epidemiological study of older men showed that increasing severity of sleep apnea corresponds with an increased prevalence of AF and ventricular ectopy.53 This graded dose-response relationship suggests a causal relationship between sleep apnea and AF and ventricular ectopy. There also appears to be an immediate influence of apneic events and hypopneic events as it relates to arrhythmia. A case-crossover study showed an associated 18-fold increased risk of nocturnal arrhythmia within 90 seconds of an apneic or hypopneic event.54 This association was found with paroxysms of AF and with episodes of nonsustained ventricular tachycardia.
Data from a clinic-based cohort study show an association between AF and OSA.55 Specifically, increased severity of sleep apnea was associated with an increased prevalence of AF. Increasing degree of hypoxia or oxygen-lowering was also associated with increased incidence of AF or newly identified AF identified over time.
Longitudinal examination of 2 epidemiologic studies, the Sleep Heart Health Study and Outcomes of Sleep Disorders Study in Older Men, found CSA to be predictive of AF with a two- to threefold higher odds of developing incident AF as it related to baseline CSA.56 According to these data, CSA may pose a greater risk for development of AF than OSA.
With respect to AF after cardiac surgery, patients with sleep apnea and obesity appear to be at higher risk for developing AF as measured by the apnea–hypopnea index and oxygen desaturation index.57
Treatment of sleep apnea may improve arrhythmic burden. Case-based studies have shown reduced burden and resolution of baseline arrhythmia with CPAP treatment for OSA as therapeutic pressure was achieved.58 Another case-based study involved an individual with snoring and OSA and AF at baseline.59 Several retrospective studies have shown that treatment of OSA after ablation and after cardioversion results in reduced recurrence of AF; however, large definitive clinical trials are lacking.
Stroke
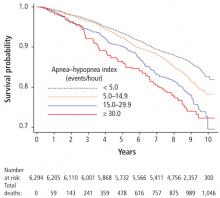
Sleep apnea is a risk factor for stroke due to intermittent hypoxia-mediated elevation of oxidative stress and systemic inflammation, hypercoaguability, and impairment of cerebral autoregulation.60 However, the relationship may be bidirectional in that stroke may be a risk factor for sleep apnea in the post-stroke period. The prevalence of sleep apnea post-stroke has been reported to be up to 70%. CSA can occur in up to 26% during the post-stroke phase.61 Data are inconsistent in terms of the location and size of stroke and the risk of sleep apnea, though cerebrovascular neuronal damage to the brainstem and cortical areas are evident.62 In one study, the incidence of stroke appeared to increase with the severity of sleep apnea.63 These findings were more pronounced in men than in women; however, this study may not have captured the increased cardiovascular risk in postmenopausal women. The Outcomes of Sleep Disorders in Older Men study found that severe hypoxia increased the incidence of stroke, and that hypoxia may be a predictor of newly diagnosed stroke in older men.64 Although definitive clinical trials are underway, post-hoc propensity-score matched analysis from the Sleep Apnea Cardiovascular Endpoints (SAVE) study showed a lower stroke risk in those adherent to CPAP compared with the control group (HR=0.56, 95% CI: 0.30-0.90).65
SLEEP APNEA, CORONARY ARTERY DISEASE, AND CARIOVASCULAR MORTALITY
The association between sleep apnea and coronary artery disease and cardiovascular mortality was considered in a Spanish study of 1,500 patients followed for 10 years, which reported that CPAP therapy reduced cardiac events in patients with OSA.66 Patients with sleep apnea had an increased risk of fatal myocardial infarction or stroke. Survival of patients treated for sleep apnea approached that of patients without OSA.
In a study of a racially diverse cohort, an association of physician diagnosed sleep apnea with cardiovascular events and survival was identified.67 Diagnosed sleep apnea was estimated to confer a two- to threefold increase in various cardiovascular outcomes and all-cause mortality.
The effect of treatment for sleep apnea on cardiovascular outcomes was the focus of a recent randomized controlled trial of nearly 3,000 participants with a mean follow-up of 4 years.65 Use of CPAP compared with usual care found no difference in cardiovascular outcomes. However, secondary analysis revealed a possible benefit of a lower risk of stroke with use of CPAP therapy. Several factors should be considered in interpreting these findings: ie, low adherence with CPAP therapy (3 hours), whether the study was sufficiently powered to detect a change in cardiovascular outcomes, and if the duration of follow-up was adequate. In terms of patient demographics and study generalizability, the study did not include patients with severe sleep apnea and hypoxia, and most participants were men, of Asian descent, with a mean body mass index of 28 kg/m2, and low levels of sleepiness at baseline.
- Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010; 56(5):765–773.
- FitBit: 150 billion data hrs shows sleep hours sweet spot, optimal health strategy. True Strange Library website. https://truestrange.com/2018/08/29/fitbit-150-billion-data-hrs-shows-sleep-hours-sweet-spot-optimal-health-strategy. Accessed August 19, 2019.
- Muller JE, Stone PH, Turi ZG, et al; MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985; 313(21):1315–1322.
- Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989; 20(4):473–476.
- Yamashita T, Murakawa Y, Hayami N, et al. Relation between aging and circadian variation of paroxysmal atrial fibrillation. Am J Cardiol 1998; 82(11):1364–1367.
- Kirby DA, Verrier RL. Differential effects of sleep stage on coronary hemodynamic function. Am J Physiol 1989; 256(5 Pt 2):H1378–H1383.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328(17):1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6(2):49–54.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109(3):659–663.
- Schäfer H, Koehler U, Ewig S, Hasper E, Tasci S, Lüderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92(2):79–84.
- Leung RST, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164(12):2147–2165.
- Cepeda-Valery B, Acharjee S, Romero-Corral A, Pressman GS, Gami AS. Obstructive sleep apnea and acute coronary syndromes: etiology, risk, and management. Curr Cardiol Rep 2014; 16(10):535.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96(4):1897–1904.
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127.
- Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51(5):363–370.
- Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013; 9(10):1003–1012.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182(6):826–833.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166(16):1725–1731.
- Paz y Mar HL, Hazen SL, Tracy RP, et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the sleep apnea stress randomized controlled trial. Chest 2016; 150(1):80–90.
- Xie X, Pan L, Ren D, Du C, Guo Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med 2013; 14(11):1139–1150.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695.
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607.
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005; 172(11):1363–1370.
- Javaheri S. Heart failure. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017:1271–1285.
- Olson LJ, Somers VK. Treating central sleep apnea in heart failure: outcomes revisited. Circulation 2007; 115(25):3140–3142.
- Mehra R, Wang L, Andrews N, et al. Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med 2017; 13(12):1411–1422.
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012; 126(12):1495–1510.
- Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J 2015; 36(23):1463–1469.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18(7):534–540.
- Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities–Sleep Heart Health Study. Circulation 2015; 132(14):1329–1337.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180.
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105.
- Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest 2015; 148(4):848–851.
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol 2017; 69(12):1577–1587.
- Abraham WT, Jagielski D, Oldenburg O, et al; remede Pilot Study Investigators. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail 2015; 3(5):360–369.
- Ponikowski P, Javaheri S, Michalkiewicz D, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 2012; 33(7):889–894.
- Costanzo MR, Ponikowski P, Javaheri S, et al; remede System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016; 388(10048):974–982.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370-2375.
- Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J 1996; 131(4):790–795.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125.
- Camm AJ, Kirchhof P, Lip GYH, et al; European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31(19):2369–2429.
- Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014; 64(7):710–721.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA 2001; 258(18):2370–2375.
- Kwon Y, Mehra R. Obstructive sleep apnea and atrial fibrillation: honing in on race-specific susceptibilities. J Clin Sleep Med 2018; 14(9):1459–1461.
- Mehra R. Sleep apnea and nocturnal cardiac arrhythmia: understanding differences across ethnicity. J Clin Sleep Med 2017; 13(11):1229–1231.
- May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhymogenesis: mechanistic insights. Chest 2017; 151(1):225–241.
- Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm 2012; 9(3):321–327.
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916.
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804.
- Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49(5):565–571.
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Am J Respir Crit Care Med 2016; 193(7):783–791.
- Kaw R, El Zarif S, Wang L, Bena J, Blackstone EH, Mehra R. Obesity as an effect modifier in sleep-disordered breathing and postcardiac surgery atrial fibrillation. Chest 2017; 151(6):1279–1287.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7(4):397–398.
- Walia HK, Chung MK, Ibrahim S, Mehra R. Positive airway pressure-induced conversion of atrial fibrillation to normal sinus rhythm in severe obstructive sleep apnea. J Clin Sleep Med 2016; 12(9):1301–1303.
- Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004; 27(2):194–201.
- Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med 2000; 161(2I):375–380.
- Song TJ, Park JH, Choi K, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med 2017; 30:36–42.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182(2):269–277.
- Stone KL, Blackwell TL, Ancoli-Israel S, et al; Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep 2016; 39(3):531–540.
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 2011; 219(2):963–968.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6(8):e1000132.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352(12):1206–1214.
SLEEP AND CARDIOVASCULAR PHYSIOLOGY
Wakefullness and sleep, the latter comprised of non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep, comprise our primary states of being. Sleep states oscillate between NREM and REM sleep. The first and shortest period of REM sleep typically occurs 90 to 120 minutes into the sleep cycle. Most REM sleep, including the longest period of REM sleep, occurs during the latter part of the sleep cycle.
With these sleep state changes, physiologic changes also occur, such as reduced heart rate and blood pressure because of enhanced parasympathetic tone. During REM sleep, there are also intermittent sympathetic nervous system surges. Other physiologic changes include a regular respiratory rate during NREM sleep and an irregular respiratory rate during REM sleep. Body temperature is normal during NREM sleep and poikilothermic (ie, tends to flucuate) during REM sleep. Blood pressure is reduced 10% to 15% during sleep1 and then rises, so that the highest blood pressure occurs in the morning. Data from 10 million users of activity-monitoring devices show that the heart rate changes during sleep.2 The heart rate is decreased in those who get less than 7 hours of sleep, then increases with longer sleep duration in a U-shaped distribution.
Cardiovascular events are more likely to occur at certain times of day. Myocardial infarction is more likely in the morning, with a threefold increased risk within the first 3 hours of awakening that peaks around 9 AM.3,4 Similar diurnal patterns have been observed with other cardiovascular conditions such as sudden cardiac death and ischemic episodes, with the highest risk during morning hours (6 to 9 AM).4
The reason for this morning predisposition for cardiovascular events is unclear, but it is thought that perhaps the autonomic fluctuations that occur during REM sleep and the predominance of REM sleep in early morning may be a factor. Diurnal changes in blood pressure and cortisol levels may also contribute, as well as levels of systemic inflammatory and thrombotic markers such as plasminogen activator inhibitor 1.
Arrhythmias are also more likely to occur in a diurnal pattern. Atrial fibrillation (AF), particularly paroxysmal AF, is believed to be vagally mediated in 10% to 25% of patients.5 Therefore, for those who are predisposed, sleep may represent a period of increased risk for AF. In a study of individuals 60 years and older, the maximum duration and peak frequency of AF occurred from midnight to 2 AM.5
Recent studies have found that REM-related obstructive sleep apnea (OSA) is associated with increased cardiovascular risk. Experimental models show that REM sleep may increase the risk for compromised coronary blood flow.6 Increased heart rate corresponds to reduced coronary blood flow and thus, to decreased coronary perfusion time and less time for relaxation of the heart, increasing the risk for coronary artery disease, thrombosis, and ischemia.
SLEEP APNEA PATHOPHYSIOLOGY
The normal physiology of the sleep-heart interaction is disrupted by sleep apnea. OSA is defined as episodes of complete or partial airway obstruction that occur during sleep with thoracoabdominal effort. Central sleep apnea (CSA) is the cessation of breathing with no thoracoabdominal effort. The pathophysiology of the sleep-heart interaction varies for OSA and CSA.
Obstructive sleep apnea
OSA is a nocturnal physiologic stressor that is highly prevalent and underrecognized. It affects approximately 17% of the adult population, and the prevalence is increasing with the obesity epidemic. Nearly 1 in 15 individuals is estimated to be affected by at least moderate OSA.7,8 OSA is underdiagnosed particularly in minority populations.9 Data from the 2015 Multi-Ethnic Study of Atherosclerosis (MESA) showed undiagnosed moderate to severe sleep apnea in 84% to 93% of individuals,9 similar to an estimated 85% of undiagnosed cases in 2002.10
OSA is highly prevalent in individuals with underlying coronary disease11–13 and in those with cardiovascular risk factors such as diabetes, hypertension, and heart failure. The prevalence of OSA in patients with cardiovascular disease ranges from 30% (hypertension) to 60% (stroke or transient ischemic attack, arrhythmia, end-stage renal disease).14
Pathophysiology of OSA
The alterations in sympathetic activation that occur during sleep in patients with OSA persist during wakefulness. Microneurographic recording of sympathetic nerve activity in the peroneal nerve reveal that the rate of sympathetic bursts doubles and the amplitude is greater in individuals with OSA compared with a control group.15
Sympathetic nerve activity, blood pressure, and heart rate were shown to increase during REM sleep in individuals with OSA on continuous positive airway pressure (CPAP) during an induced apneic event (pressure reduction from 8 cm to 6 cm of water).15
During OSA episodes, there is an increased cardiac load. Impaired diastolic function and atrial and aortic enlargement, and in particular, the thin-walled atria are very susceptible to the intrathoracic pressure swings caused by OSA. Physiologic changes with OSA from pressure changes in the chest result in shift of the intraventricular septum, causing a reduction in cardiac output.16 With the lowering of oxygen during episodes of apnea, constriction of the pulmonary vasculature leads to elevation of pressure in the pulmonary vasculature reflected by the increase in mean pulmonary arterial pressures.17
Other studies have shown that OSA increases upregulation of markers of systemic inflammation and prothrombotic markers, the very markers that can increase cardiovascular or atherogenic risk.18–22 One example is the soluble interleukin 6 receptor, shown to be elevated in the morning relative to sleep apnea compared with the evening.20 Other biomarkers observed to be associated with sleep apnea include markers of prothrombotic potentials such as plasminogen activator inhibitor 1.19 Oxidative stress occurs because intermittent bouts of lower oxygen can lead to oxidation of serum proteins and lipids. Endothelial dysfunction has been observed as well as insulin resistance and dyslipidemia.23 Taken together, these are pathways that lead to atherogenesis and increased cardiovascular risk.
Central sleep apnea
CSA episodes are the cessation of breathing without thoracoabdominal effort, in contrast to the persistence of thoracoabdominal effort in OSA. CSA is characterized by breathing instability with highly sensitive chemoresponses and prolonged circulation time.24 This can be physiologic in some cases, as when it occurs after a very large breath or sigh and then a central apnea event occurs after the sigh. The alterations in oxygen and CO2 and the stretch of the receptors in the alveoli of the lungs initiate the Hering-Breuer inhalation reflex.
Pathophysiology of CSA
Complex pathways of medullary and aortic receptor chemosensitivity are at the root of the pathophysiology of CSA.24 With CSA there is often a relative state of hypocapnia at baseline. During sleep, there is reduction in drive, thus chemosensitivity can be activated so that central apnea episodes can ensue as a result of alterations in CO2 (ie, hypocapnia). Another factor that can contribute to the pathophysiology of CSA is arousal from sleep that can reduce CO2 levels and therefore perpetuate central events.
The concept of loop gain is used to understand the pathophysiology of CSA. Loop gain is a measure of the relative stability of a ventilation system and indicates the likelihood of an individual to have periodic breathing. It is calculated by the response to a disturbance divided by the disturbance itself.25 With a high loop gain, there is a more pronounced or exuberant response to the disturbance, indicating more instability in the system and increasing the tendency for irregular breathing and CSA episodes.
Hunter-Cheyne-Stokes respiration occurs with CSA and is characterized by cyclical crescendo-decrescendo respiratory effort that occurs during wakefulness and sleep without upper-airway obstruction.26,27 Unlike OSA, which is worse during REM sleep, Hunter-Cheyne-Stokes breathing in CSA is typically worse in NREM sleep, during N1 and N2 in particular.
SLEEP APNEA AND HEART FAILURE
Both OSA and CSA are prevalent in patients with heart failure and may be associated with the progression of heart failure. CSA often occurs in patients with heart failure. The pathophysiology is multifactorial, including pulmonary congestion that results in stretch of the J receptors in the alveoli, prolonged circulation time, and increased chemosensitivity.
Complex pathways in the neuroaxis or somnogenic biomarkers of inflammation or both may be implicated in the paradoxical lack of subjective sleepiness in the presence of increased objective measures of sleepiness in systolic heart failure. One study found a relationship with one biomarker of inflammation and oxidative stress as it relates to objective symptoms of sleepiness but not subjective symptoms of sleepiness.28
Another contributing factor in the relationship between OSA and CSA in heart failure has also been described related to rostral shifts in fluid to the neck and to the pulmonary receptors in the alveoli of the lungs.29 These rostral shifts in fluids may contribute to sleep apnea with parapharyngeal edema leading to OSA and pulmonary congestion leading to CSA.
Sleep apnea is associated with increased post-discharge mortality and hospitalization readmissions in the setting of acute heart failure.30 Mortality analysis of 1,096 patients admitted for decompensated heart failure found CSA and OSA were independently associated with mortality in patients compared with patients with no or minimal sleep-disordered breathing.30
CSA has also been shown to be a predictor of readmission in patients admitted for heart failure exacerbations.31 Targeting underlying CSA may reduce readmissions in those admitted with acute decompensated heart failure. While men were identified to be at increased risk of death relative to sleep-disordered breathing based on the initial results of the Sleep Heart Health Study, a subsequent epidemiologic substudy reflective of an older age group showed that OSA was more strongly associated with left ventricular mass index, risk of heart failure, or death in women compared with men.32
Treatment
Standard therapy for treatment of OSA is CPAP. Adaptive servo-ventilation (ASV) and transvenous phrenic nerve stimulation are also available as treatment options in certain cases of CSA.
One of the first randomized controlled trials designed to assess the impact of CSA treatment on survival in patients with heart failure initially favored the control group then later the CPAP group and was terminated early based on stopping rules.33,34 While adherence to therapy was suboptimal at an average of 3.6 hours, post hoc analysis showed that patients with CSA using CPAP with effective suppression of CSA had improved survival compared with patients who did not have effective suppression using CPAP.34
ASV is mainly used for treatment of CSA. In ASV, positive airway pressure for ventilation support is provided and adjusts as apneic episodes are detected during sleep. The support provided adapts to the physiology of the patient and can deliver breaths and utilize anticyclic modes of ventilation to address crescendo-decrescendo breathing patterns observed in Hunter-Cheyne-Stokes respiration.
In the Treatment of Sleep-Disordered Breathing With Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) trial, 1,300 patients with systolic heart failure and predominantly CSA were randomized to receive ASV vs solely standard medical management.35 The primary composite end point included all-cause mortality or unplanned admission or hospitalization for heart failure. No difference was found in the primary end point between the ASV and the control group; however, there was an unanticipated negative impact of ASV on cardiovascular outcomes in some secondary end points. Based on the secondary outcome of cardiovascular-specific mortality, clinicians were advised that ASV was contraindicated for the treatment of CSA in patients with symptomatic heart failure with a left ventricular ejection fraction less than 45%. The interpretation of this study was complicated by several methodologic limitations.36
The Cardiovascular Improvements With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure (CAT-HF) randomized controlled trial also evaluated ASV compared with standard medical management in 126 patients with heart failure.37 This trial was terminated early because of the results of the SERVE-HF trial. Compliance with therapy was suboptimal at an average of 2.7 hours per day. The composite end point did not differ between the 2 groups; however, this was likely because the study was underpowered and was terminated early. Subgroup analysis revealed that patients with heart failure with preserved ejection fraction may benefit from ASV; however, additional studies are needed to confirm these findings.
Therefore, although ASV is not indicated when there is predominantly CSA in patients with systolic heart failure, preliminary results support potential benefit in patients with OSA and preserved ejection fraction.
Another novel treatment for CSA is transvenous phrenic nerve stimulation. A device is implanted that stimulates the phrenic nerve to initiate breaths. The initial study of transvenous phrenic nerve stimulation reported a significant reduction in the number of episodes of central apnea per hour of sleep.38,39 The apnea–hypopnea index improved overall and some types of obstructive apneic events were reduced with transvenous phrenic nerve stimulation.
A multicenter randomized control trial of transvenous phrenic nerve stimulation found improvement in several sleep apnea indices, including central apnea, hypoxia, reduced arousals from sleep, and patient reported well-being.40 Transvenous phrenic nerve stimulation holds promise as a novel therapy for central predominant sleep apnea not only in terms of improving the degree of central apnea and sleep-disordered breathing, but also in improving functional outcomes. Longitudinal and intereventional trial data are needed to clarify the impact of transvenous phrenic nerve stimulation on long-term cardiac outcomes.
SLEEP APNEA AND ATRIAL FIBRILLATION AND STROKE
Atrial fibrillation
AF is the most common sustained cardiac arrhythmia. The number of Americans with AF is projected to increase from 2.3 million to more than 10 million by the year 2050.41 The increasing incidence and prevalence of AF is not fully explained by the aging population and established risk factors.42 Unrecognized sleep apnea, estimated to exist in 85% or more of the population, may partially account for the increasing incidence of AF.43
There are 3 types of AF, which are thought to follow a continuum: paroxysmal AF is characterized by episodes that occur intermittently; persistent AF is characterized by episodes that last longer than 7 days; chronic or permanent AF is typically characterized by AF that is ongoing over many years.44 As with sleep apnea, AF is often asymptomatic and is likely underdiagnosed.
Sleep apnea and AF share several risk factors. Obesity is a risk factor for both OSA and AF; however, a meta-analysis supported a stronger association of OSA and AF vs obesity and AF.45 Increasing age is a risk factor for both OSA and AF.46,47 Although white populations are at higher risk for AF, OSA is associated with a 58% increased risk of AF in African Americans.48 Nocturnal hypoxia has been associated with increased risk of AF in Asians.49
Experimental data continue to accrue providing biologic plausibility of the relationship between sleep apnea and AF. OSA contributes to structural and electrical remodeling of the heart with evidence supporting increased fibrosis and electrical remodeling in patients with OSA compared with a control group.51 Markers of structural remodeling, such as atrial size, electrical silence, and atrial voltage conduction velocity, are altered in OSA.50
Data from the Sleep Heart Health Study show very strong associations between atrial and ventricular cardiac arrhythmias and sleep apnea with two- to fivefold higher odds of arrhythmias in patients with severe OSA compared with controls even after accounting for confounding factors such as obesity.52
A multicenter, epidemiological study of older men showed that increasing severity of sleep apnea corresponds with an increased prevalence of AF and ventricular ectopy.53 This graded dose-response relationship suggests a causal relationship between sleep apnea and AF and ventricular ectopy. There also appears to be an immediate influence of apneic events and hypopneic events as it relates to arrhythmia. A case-crossover study showed an associated 18-fold increased risk of nocturnal arrhythmia within 90 seconds of an apneic or hypopneic event.54 This association was found with paroxysms of AF and with episodes of nonsustained ventricular tachycardia.
Data from a clinic-based cohort study show an association between AF and OSA.55 Specifically, increased severity of sleep apnea was associated with an increased prevalence of AF. Increasing degree of hypoxia or oxygen-lowering was also associated with increased incidence of AF or newly identified AF identified over time.
Longitudinal examination of 2 epidemiologic studies, the Sleep Heart Health Study and Outcomes of Sleep Disorders Study in Older Men, found CSA to be predictive of AF with a two- to threefold higher odds of developing incident AF as it related to baseline CSA.56 According to these data, CSA may pose a greater risk for development of AF than OSA.
With respect to AF after cardiac surgery, patients with sleep apnea and obesity appear to be at higher risk for developing AF as measured by the apnea–hypopnea index and oxygen desaturation index.57
Treatment of sleep apnea may improve arrhythmic burden. Case-based studies have shown reduced burden and resolution of baseline arrhythmia with CPAP treatment for OSA as therapeutic pressure was achieved.58 Another case-based study involved an individual with snoring and OSA and AF at baseline.59 Several retrospective studies have shown that treatment of OSA after ablation and after cardioversion results in reduced recurrence of AF; however, large definitive clinical trials are lacking.
Stroke
Sleep apnea is a risk factor for stroke due to intermittent hypoxia-mediated elevation of oxidative stress and systemic inflammation, hypercoaguability, and impairment of cerebral autoregulation.60 However, the relationship may be bidirectional in that stroke may be a risk factor for sleep apnea in the post-stroke period. The prevalence of sleep apnea post-stroke has been reported to be up to 70%. CSA can occur in up to 26% during the post-stroke phase.61 Data are inconsistent in terms of the location and size of stroke and the risk of sleep apnea, though cerebrovascular neuronal damage to the brainstem and cortical areas are evident.62 In one study, the incidence of stroke appeared to increase with the severity of sleep apnea.63 These findings were more pronounced in men than in women; however, this study may not have captured the increased cardiovascular risk in postmenopausal women. The Outcomes of Sleep Disorders in Older Men study found that severe hypoxia increased the incidence of stroke, and that hypoxia may be a predictor of newly diagnosed stroke in older men.64 Although definitive clinical trials are underway, post-hoc propensity-score matched analysis from the Sleep Apnea Cardiovascular Endpoints (SAVE) study showed a lower stroke risk in those adherent to CPAP compared with the control group (HR=0.56, 95% CI: 0.30-0.90).65
SLEEP APNEA, CORONARY ARTERY DISEASE, AND CARIOVASCULAR MORTALITY
The association between sleep apnea and coronary artery disease and cardiovascular mortality was considered in a Spanish study of 1,500 patients followed for 10 years, which reported that CPAP therapy reduced cardiac events in patients with OSA.66 Patients with sleep apnea had an increased risk of fatal myocardial infarction or stroke. Survival of patients treated for sleep apnea approached that of patients without OSA.
In a study of a racially diverse cohort, an association of physician diagnosed sleep apnea with cardiovascular events and survival was identified.67 Diagnosed sleep apnea was estimated to confer a two- to threefold increase in various cardiovascular outcomes and all-cause mortality.
The effect of treatment for sleep apnea on cardiovascular outcomes was the focus of a recent randomized controlled trial of nearly 3,000 participants with a mean follow-up of 4 years.65 Use of CPAP compared with usual care found no difference in cardiovascular outcomes. However, secondary analysis revealed a possible benefit of a lower risk of stroke with use of CPAP therapy. Several factors should be considered in interpreting these findings: ie, low adherence with CPAP therapy (3 hours), whether the study was sufficiently powered to detect a change in cardiovascular outcomes, and if the duration of follow-up was adequate. In terms of patient demographics and study generalizability, the study did not include patients with severe sleep apnea and hypoxia, and most participants were men, of Asian descent, with a mean body mass index of 28 kg/m2, and low levels of sleepiness at baseline.
SLEEP AND CARDIOVASCULAR PHYSIOLOGY
Wakefullness and sleep, the latter comprised of non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep, comprise our primary states of being. Sleep states oscillate between NREM and REM sleep. The first and shortest period of REM sleep typically occurs 90 to 120 minutes into the sleep cycle. Most REM sleep, including the longest period of REM sleep, occurs during the latter part of the sleep cycle.
With these sleep state changes, physiologic changes also occur, such as reduced heart rate and blood pressure because of enhanced parasympathetic tone. During REM sleep, there are also intermittent sympathetic nervous system surges. Other physiologic changes include a regular respiratory rate during NREM sleep and an irregular respiratory rate during REM sleep. Body temperature is normal during NREM sleep and poikilothermic (ie, tends to flucuate) during REM sleep. Blood pressure is reduced 10% to 15% during sleep1 and then rises, so that the highest blood pressure occurs in the morning. Data from 10 million users of activity-monitoring devices show that the heart rate changes during sleep.2 The heart rate is decreased in those who get less than 7 hours of sleep, then increases with longer sleep duration in a U-shaped distribution.
Cardiovascular events are more likely to occur at certain times of day. Myocardial infarction is more likely in the morning, with a threefold increased risk within the first 3 hours of awakening that peaks around 9 AM.3,4 Similar diurnal patterns have been observed with other cardiovascular conditions such as sudden cardiac death and ischemic episodes, with the highest risk during morning hours (6 to 9 AM).4
The reason for this morning predisposition for cardiovascular events is unclear, but it is thought that perhaps the autonomic fluctuations that occur during REM sleep and the predominance of REM sleep in early morning may be a factor. Diurnal changes in blood pressure and cortisol levels may also contribute, as well as levels of systemic inflammatory and thrombotic markers such as plasminogen activator inhibitor 1.
Arrhythmias are also more likely to occur in a diurnal pattern. Atrial fibrillation (AF), particularly paroxysmal AF, is believed to be vagally mediated in 10% to 25% of patients.5 Therefore, for those who are predisposed, sleep may represent a period of increased risk for AF. In a study of individuals 60 years and older, the maximum duration and peak frequency of AF occurred from midnight to 2 AM.5
Recent studies have found that REM-related obstructive sleep apnea (OSA) is associated with increased cardiovascular risk. Experimental models show that REM sleep may increase the risk for compromised coronary blood flow.6 Increased heart rate corresponds to reduced coronary blood flow and thus, to decreased coronary perfusion time and less time for relaxation of the heart, increasing the risk for coronary artery disease, thrombosis, and ischemia.
SLEEP APNEA PATHOPHYSIOLOGY
The normal physiology of the sleep-heart interaction is disrupted by sleep apnea. OSA is defined as episodes of complete or partial airway obstruction that occur during sleep with thoracoabdominal effort. Central sleep apnea (CSA) is the cessation of breathing with no thoracoabdominal effort. The pathophysiology of the sleep-heart interaction varies for OSA and CSA.
Obstructive sleep apnea
OSA is a nocturnal physiologic stressor that is highly prevalent and underrecognized. It affects approximately 17% of the adult population, and the prevalence is increasing with the obesity epidemic. Nearly 1 in 15 individuals is estimated to be affected by at least moderate OSA.7,8 OSA is underdiagnosed particularly in minority populations.9 Data from the 2015 Multi-Ethnic Study of Atherosclerosis (MESA) showed undiagnosed moderate to severe sleep apnea in 84% to 93% of individuals,9 similar to an estimated 85% of undiagnosed cases in 2002.10
OSA is highly prevalent in individuals with underlying coronary disease11–13 and in those with cardiovascular risk factors such as diabetes, hypertension, and heart failure. The prevalence of OSA in patients with cardiovascular disease ranges from 30% (hypertension) to 60% (stroke or transient ischemic attack, arrhythmia, end-stage renal disease).14
Pathophysiology of OSA
The alterations in sympathetic activation that occur during sleep in patients with OSA persist during wakefulness. Microneurographic recording of sympathetic nerve activity in the peroneal nerve reveal that the rate of sympathetic bursts doubles and the amplitude is greater in individuals with OSA compared with a control group.15
Sympathetic nerve activity, blood pressure, and heart rate were shown to increase during REM sleep in individuals with OSA on continuous positive airway pressure (CPAP) during an induced apneic event (pressure reduction from 8 cm to 6 cm of water).15
During OSA episodes, there is an increased cardiac load. Impaired diastolic function and atrial and aortic enlargement, and in particular, the thin-walled atria are very susceptible to the intrathoracic pressure swings caused by OSA. Physiologic changes with OSA from pressure changes in the chest result in shift of the intraventricular septum, causing a reduction in cardiac output.16 With the lowering of oxygen during episodes of apnea, constriction of the pulmonary vasculature leads to elevation of pressure in the pulmonary vasculature reflected by the increase in mean pulmonary arterial pressures.17
Other studies have shown that OSA increases upregulation of markers of systemic inflammation and prothrombotic markers, the very markers that can increase cardiovascular or atherogenic risk.18–22 One example is the soluble interleukin 6 receptor, shown to be elevated in the morning relative to sleep apnea compared with the evening.20 Other biomarkers observed to be associated with sleep apnea include markers of prothrombotic potentials such as plasminogen activator inhibitor 1.19 Oxidative stress occurs because intermittent bouts of lower oxygen can lead to oxidation of serum proteins and lipids. Endothelial dysfunction has been observed as well as insulin resistance and dyslipidemia.23 Taken together, these are pathways that lead to atherogenesis and increased cardiovascular risk.
Central sleep apnea
CSA episodes are the cessation of breathing without thoracoabdominal effort, in contrast to the persistence of thoracoabdominal effort in OSA. CSA is characterized by breathing instability with highly sensitive chemoresponses and prolonged circulation time.24 This can be physiologic in some cases, as when it occurs after a very large breath or sigh and then a central apnea event occurs after the sigh. The alterations in oxygen and CO2 and the stretch of the receptors in the alveoli of the lungs initiate the Hering-Breuer inhalation reflex.
Pathophysiology of CSA
Complex pathways of medullary and aortic receptor chemosensitivity are at the root of the pathophysiology of CSA.24 With CSA there is often a relative state of hypocapnia at baseline. During sleep, there is reduction in drive, thus chemosensitivity can be activated so that central apnea episodes can ensue as a result of alterations in CO2 (ie, hypocapnia). Another factor that can contribute to the pathophysiology of CSA is arousal from sleep that can reduce CO2 levels and therefore perpetuate central events.
The concept of loop gain is used to understand the pathophysiology of CSA. Loop gain is a measure of the relative stability of a ventilation system and indicates the likelihood of an individual to have periodic breathing. It is calculated by the response to a disturbance divided by the disturbance itself.25 With a high loop gain, there is a more pronounced or exuberant response to the disturbance, indicating more instability in the system and increasing the tendency for irregular breathing and CSA episodes.
Hunter-Cheyne-Stokes respiration occurs with CSA and is characterized by cyclical crescendo-decrescendo respiratory effort that occurs during wakefulness and sleep without upper-airway obstruction.26,27 Unlike OSA, which is worse during REM sleep, Hunter-Cheyne-Stokes breathing in CSA is typically worse in NREM sleep, during N1 and N2 in particular.
SLEEP APNEA AND HEART FAILURE
Both OSA and CSA are prevalent in patients with heart failure and may be associated with the progression of heart failure. CSA often occurs in patients with heart failure. The pathophysiology is multifactorial, including pulmonary congestion that results in stretch of the J receptors in the alveoli, prolonged circulation time, and increased chemosensitivity.
Complex pathways in the neuroaxis or somnogenic biomarkers of inflammation or both may be implicated in the paradoxical lack of subjective sleepiness in the presence of increased objective measures of sleepiness in systolic heart failure. One study found a relationship with one biomarker of inflammation and oxidative stress as it relates to objective symptoms of sleepiness but not subjective symptoms of sleepiness.28
Another contributing factor in the relationship between OSA and CSA in heart failure has also been described related to rostral shifts in fluid to the neck and to the pulmonary receptors in the alveoli of the lungs.29 These rostral shifts in fluids may contribute to sleep apnea with parapharyngeal edema leading to OSA and pulmonary congestion leading to CSA.
Sleep apnea is associated with increased post-discharge mortality and hospitalization readmissions in the setting of acute heart failure.30 Mortality analysis of 1,096 patients admitted for decompensated heart failure found CSA and OSA were independently associated with mortality in patients compared with patients with no or minimal sleep-disordered breathing.30
CSA has also been shown to be a predictor of readmission in patients admitted for heart failure exacerbations.31 Targeting underlying CSA may reduce readmissions in those admitted with acute decompensated heart failure. While men were identified to be at increased risk of death relative to sleep-disordered breathing based on the initial results of the Sleep Heart Health Study, a subsequent epidemiologic substudy reflective of an older age group showed that OSA was more strongly associated with left ventricular mass index, risk of heart failure, or death in women compared with men.32
Treatment
Standard therapy for treatment of OSA is CPAP. Adaptive servo-ventilation (ASV) and transvenous phrenic nerve stimulation are also available as treatment options in certain cases of CSA.
One of the first randomized controlled trials designed to assess the impact of CSA treatment on survival in patients with heart failure initially favored the control group then later the CPAP group and was terminated early based on stopping rules.33,34 While adherence to therapy was suboptimal at an average of 3.6 hours, post hoc analysis showed that patients with CSA using CPAP with effective suppression of CSA had improved survival compared with patients who did not have effective suppression using CPAP.34
ASV is mainly used for treatment of CSA. In ASV, positive airway pressure for ventilation support is provided and adjusts as apneic episodes are detected during sleep. The support provided adapts to the physiology of the patient and can deliver breaths and utilize anticyclic modes of ventilation to address crescendo-decrescendo breathing patterns observed in Hunter-Cheyne-Stokes respiration.
In the Treatment of Sleep-Disordered Breathing With Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) trial, 1,300 patients with systolic heart failure and predominantly CSA were randomized to receive ASV vs solely standard medical management.35 The primary composite end point included all-cause mortality or unplanned admission or hospitalization for heart failure. No difference was found in the primary end point between the ASV and the control group; however, there was an unanticipated negative impact of ASV on cardiovascular outcomes in some secondary end points. Based on the secondary outcome of cardiovascular-specific mortality, clinicians were advised that ASV was contraindicated for the treatment of CSA in patients with symptomatic heart failure with a left ventricular ejection fraction less than 45%. The interpretation of this study was complicated by several methodologic limitations.36
The Cardiovascular Improvements With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure (CAT-HF) randomized controlled trial also evaluated ASV compared with standard medical management in 126 patients with heart failure.37 This trial was terminated early because of the results of the SERVE-HF trial. Compliance with therapy was suboptimal at an average of 2.7 hours per day. The composite end point did not differ between the 2 groups; however, this was likely because the study was underpowered and was terminated early. Subgroup analysis revealed that patients with heart failure with preserved ejection fraction may benefit from ASV; however, additional studies are needed to confirm these findings.
Therefore, although ASV is not indicated when there is predominantly CSA in patients with systolic heart failure, preliminary results support potential benefit in patients with OSA and preserved ejection fraction.
Another novel treatment for CSA is transvenous phrenic nerve stimulation. A device is implanted that stimulates the phrenic nerve to initiate breaths. The initial study of transvenous phrenic nerve stimulation reported a significant reduction in the number of episodes of central apnea per hour of sleep.38,39 The apnea–hypopnea index improved overall and some types of obstructive apneic events were reduced with transvenous phrenic nerve stimulation.
A multicenter randomized control trial of transvenous phrenic nerve stimulation found improvement in several sleep apnea indices, including central apnea, hypoxia, reduced arousals from sleep, and patient reported well-being.40 Transvenous phrenic nerve stimulation holds promise as a novel therapy for central predominant sleep apnea not only in terms of improving the degree of central apnea and sleep-disordered breathing, but also in improving functional outcomes. Longitudinal and intereventional trial data are needed to clarify the impact of transvenous phrenic nerve stimulation on long-term cardiac outcomes.
SLEEP APNEA AND ATRIAL FIBRILLATION AND STROKE
Atrial fibrillation
AF is the most common sustained cardiac arrhythmia. The number of Americans with AF is projected to increase from 2.3 million to more than 10 million by the year 2050.41 The increasing incidence and prevalence of AF is not fully explained by the aging population and established risk factors.42 Unrecognized sleep apnea, estimated to exist in 85% or more of the population, may partially account for the increasing incidence of AF.43
There are 3 types of AF, which are thought to follow a continuum: paroxysmal AF is characterized by episodes that occur intermittently; persistent AF is characterized by episodes that last longer than 7 days; chronic or permanent AF is typically characterized by AF that is ongoing over many years.44 As with sleep apnea, AF is often asymptomatic and is likely underdiagnosed.
Sleep apnea and AF share several risk factors. Obesity is a risk factor for both OSA and AF; however, a meta-analysis supported a stronger association of OSA and AF vs obesity and AF.45 Increasing age is a risk factor for both OSA and AF.46,47 Although white populations are at higher risk for AF, OSA is associated with a 58% increased risk of AF in African Americans.48 Nocturnal hypoxia has been associated with increased risk of AF in Asians.49
Experimental data continue to accrue providing biologic plausibility of the relationship between sleep apnea and AF. OSA contributes to structural and electrical remodeling of the heart with evidence supporting increased fibrosis and electrical remodeling in patients with OSA compared with a control group.51 Markers of structural remodeling, such as atrial size, electrical silence, and atrial voltage conduction velocity, are altered in OSA.50
Data from the Sleep Heart Health Study show very strong associations between atrial and ventricular cardiac arrhythmias and sleep apnea with two- to fivefold higher odds of arrhythmias in patients with severe OSA compared with controls even after accounting for confounding factors such as obesity.52
A multicenter, epidemiological study of older men showed that increasing severity of sleep apnea corresponds with an increased prevalence of AF and ventricular ectopy.53 This graded dose-response relationship suggests a causal relationship between sleep apnea and AF and ventricular ectopy. There also appears to be an immediate influence of apneic events and hypopneic events as it relates to arrhythmia. A case-crossover study showed an associated 18-fold increased risk of nocturnal arrhythmia within 90 seconds of an apneic or hypopneic event.54 This association was found with paroxysms of AF and with episodes of nonsustained ventricular tachycardia.
Data from a clinic-based cohort study show an association between AF and OSA.55 Specifically, increased severity of sleep apnea was associated with an increased prevalence of AF. Increasing degree of hypoxia or oxygen-lowering was also associated with increased incidence of AF or newly identified AF identified over time.
Longitudinal examination of 2 epidemiologic studies, the Sleep Heart Health Study and Outcomes of Sleep Disorders Study in Older Men, found CSA to be predictive of AF with a two- to threefold higher odds of developing incident AF as it related to baseline CSA.56 According to these data, CSA may pose a greater risk for development of AF than OSA.
With respect to AF after cardiac surgery, patients with sleep apnea and obesity appear to be at higher risk for developing AF as measured by the apnea–hypopnea index and oxygen desaturation index.57
Treatment of sleep apnea may improve arrhythmic burden. Case-based studies have shown reduced burden and resolution of baseline arrhythmia with CPAP treatment for OSA as therapeutic pressure was achieved.58 Another case-based study involved an individual with snoring and OSA and AF at baseline.59 Several retrospective studies have shown that treatment of OSA after ablation and after cardioversion results in reduced recurrence of AF; however, large definitive clinical trials are lacking.
Stroke
Sleep apnea is a risk factor for stroke due to intermittent hypoxia-mediated elevation of oxidative stress and systemic inflammation, hypercoaguability, and impairment of cerebral autoregulation.60 However, the relationship may be bidirectional in that stroke may be a risk factor for sleep apnea in the post-stroke period. The prevalence of sleep apnea post-stroke has been reported to be up to 70%. CSA can occur in up to 26% during the post-stroke phase.61 Data are inconsistent in terms of the location and size of stroke and the risk of sleep apnea, though cerebrovascular neuronal damage to the brainstem and cortical areas are evident.62 In one study, the incidence of stroke appeared to increase with the severity of sleep apnea.63 These findings were more pronounced in men than in women; however, this study may not have captured the increased cardiovascular risk in postmenopausal women. The Outcomes of Sleep Disorders in Older Men study found that severe hypoxia increased the incidence of stroke, and that hypoxia may be a predictor of newly diagnosed stroke in older men.64 Although definitive clinical trials are underway, post-hoc propensity-score matched analysis from the Sleep Apnea Cardiovascular Endpoints (SAVE) study showed a lower stroke risk in those adherent to CPAP compared with the control group (HR=0.56, 95% CI: 0.30-0.90).65
SLEEP APNEA, CORONARY ARTERY DISEASE, AND CARIOVASCULAR MORTALITY
The association between sleep apnea and coronary artery disease and cardiovascular mortality was considered in a Spanish study of 1,500 patients followed for 10 years, which reported that CPAP therapy reduced cardiac events in patients with OSA.66 Patients with sleep apnea had an increased risk of fatal myocardial infarction or stroke. Survival of patients treated for sleep apnea approached that of patients without OSA.
In a study of a racially diverse cohort, an association of physician diagnosed sleep apnea with cardiovascular events and survival was identified.67 Diagnosed sleep apnea was estimated to confer a two- to threefold increase in various cardiovascular outcomes and all-cause mortality.
The effect of treatment for sleep apnea on cardiovascular outcomes was the focus of a recent randomized controlled trial of nearly 3,000 participants with a mean follow-up of 4 years.65 Use of CPAP compared with usual care found no difference in cardiovascular outcomes. However, secondary analysis revealed a possible benefit of a lower risk of stroke with use of CPAP therapy. Several factors should be considered in interpreting these findings: ie, low adherence with CPAP therapy (3 hours), whether the study was sufficiently powered to detect a change in cardiovascular outcomes, and if the duration of follow-up was adequate. In terms of patient demographics and study generalizability, the study did not include patients with severe sleep apnea and hypoxia, and most participants were men, of Asian descent, with a mean body mass index of 28 kg/m2, and low levels of sleepiness at baseline.
- Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010; 56(5):765–773.
- FitBit: 150 billion data hrs shows sleep hours sweet spot, optimal health strategy. True Strange Library website. https://truestrange.com/2018/08/29/fitbit-150-billion-data-hrs-shows-sleep-hours-sweet-spot-optimal-health-strategy. Accessed August 19, 2019.
- Muller JE, Stone PH, Turi ZG, et al; MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985; 313(21):1315–1322.
- Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989; 20(4):473–476.
- Yamashita T, Murakawa Y, Hayami N, et al. Relation between aging and circadian variation of paroxysmal atrial fibrillation. Am J Cardiol 1998; 82(11):1364–1367.
- Kirby DA, Verrier RL. Differential effects of sleep stage on coronary hemodynamic function. Am J Physiol 1989; 256(5 Pt 2):H1378–H1383.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328(17):1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6(2):49–54.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109(3):659–663.
- Schäfer H, Koehler U, Ewig S, Hasper E, Tasci S, Lüderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92(2):79–84.
- Leung RST, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164(12):2147–2165.
- Cepeda-Valery B, Acharjee S, Romero-Corral A, Pressman GS, Gami AS. Obstructive sleep apnea and acute coronary syndromes: etiology, risk, and management. Curr Cardiol Rep 2014; 16(10):535.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96(4):1897–1904.
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127.
- Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51(5):363–370.
- Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013; 9(10):1003–1012.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182(6):826–833.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166(16):1725–1731.
- Paz y Mar HL, Hazen SL, Tracy RP, et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the sleep apnea stress randomized controlled trial. Chest 2016; 150(1):80–90.
- Xie X, Pan L, Ren D, Du C, Guo Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med 2013; 14(11):1139–1150.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695.
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607.
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005; 172(11):1363–1370.
- Javaheri S. Heart failure. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017:1271–1285.
- Olson LJ, Somers VK. Treating central sleep apnea in heart failure: outcomes revisited. Circulation 2007; 115(25):3140–3142.
- Mehra R, Wang L, Andrews N, et al. Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med 2017; 13(12):1411–1422.
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012; 126(12):1495–1510.
- Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J 2015; 36(23):1463–1469.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18(7):534–540.
- Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities–Sleep Heart Health Study. Circulation 2015; 132(14):1329–1337.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180.
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105.
- Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest 2015; 148(4):848–851.
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol 2017; 69(12):1577–1587.
- Abraham WT, Jagielski D, Oldenburg O, et al; remede Pilot Study Investigators. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail 2015; 3(5):360–369.
- Ponikowski P, Javaheri S, Michalkiewicz D, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 2012; 33(7):889–894.
- Costanzo MR, Ponikowski P, Javaheri S, et al; remede System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016; 388(10048):974–982.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370-2375.
- Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J 1996; 131(4):790–795.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125.
- Camm AJ, Kirchhof P, Lip GYH, et al; European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31(19):2369–2429.
- Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014; 64(7):710–721.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA 2001; 258(18):2370–2375.
- Kwon Y, Mehra R. Obstructive sleep apnea and atrial fibrillation: honing in on race-specific susceptibilities. J Clin Sleep Med 2018; 14(9):1459–1461.
- Mehra R. Sleep apnea and nocturnal cardiac arrhythmia: understanding differences across ethnicity. J Clin Sleep Med 2017; 13(11):1229–1231.
- May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhymogenesis: mechanistic insights. Chest 2017; 151(1):225–241.
- Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm 2012; 9(3):321–327.
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916.
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804.
- Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49(5):565–571.
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Am J Respir Crit Care Med 2016; 193(7):783–791.
- Kaw R, El Zarif S, Wang L, Bena J, Blackstone EH, Mehra R. Obesity as an effect modifier in sleep-disordered breathing and postcardiac surgery atrial fibrillation. Chest 2017; 151(6):1279–1287.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7(4):397–398.
- Walia HK, Chung MK, Ibrahim S, Mehra R. Positive airway pressure-induced conversion of atrial fibrillation to normal sinus rhythm in severe obstructive sleep apnea. J Clin Sleep Med 2016; 12(9):1301–1303.
- Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004; 27(2):194–201.
- Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med 2000; 161(2I):375–380.
- Song TJ, Park JH, Choi K, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med 2017; 30:36–42.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182(2):269–277.
- Stone KL, Blackwell TL, Ancoli-Israel S, et al; Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep 2016; 39(3):531–540.
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 2011; 219(2):963–968.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6(8):e1000132.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352(12):1206–1214.
- Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010; 56(5):765–773.
- FitBit: 150 billion data hrs shows sleep hours sweet spot, optimal health strategy. True Strange Library website. https://truestrange.com/2018/08/29/fitbit-150-billion-data-hrs-shows-sleep-hours-sweet-spot-optimal-health-strategy. Accessed August 19, 2019.
- Muller JE, Stone PH, Turi ZG, et al; MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985; 313(21):1315–1322.
- Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989; 20(4):473–476.
- Yamashita T, Murakawa Y, Hayami N, et al. Relation between aging and circadian variation of paroxysmal atrial fibrillation. Am J Cardiol 1998; 82(11):1364–1367.
- Kirby DA, Verrier RL. Differential effects of sleep stage on coronary hemodynamic function. Am J Physiol 1989; 256(5 Pt 2):H1378–H1383.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328(17):1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6(2):49–54.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109(3):659–663.
- Schäfer H, Koehler U, Ewig S, Hasper E, Tasci S, Lüderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92(2):79–84.
- Leung RST, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164(12):2147–2165.
- Cepeda-Valery B, Acharjee S, Romero-Corral A, Pressman GS, Gami AS. Obstructive sleep apnea and acute coronary syndromes: etiology, risk, and management. Curr Cardiol Rep 2014; 16(10):535.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96(4):1897–1904.
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127.
- Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51(5):363–370.
- Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013; 9(10):1003–1012.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182(6):826–833.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166(16):1725–1731.
- Paz y Mar HL, Hazen SL, Tracy RP, et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the sleep apnea stress randomized controlled trial. Chest 2016; 150(1):80–90.
- Xie X, Pan L, Ren D, Du C, Guo Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med 2013; 14(11):1139–1150.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695.
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607.
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005; 172(11):1363–1370.
- Javaheri S. Heart failure. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017:1271–1285.
- Olson LJ, Somers VK. Treating central sleep apnea in heart failure: outcomes revisited. Circulation 2007; 115(25):3140–3142.
- Mehra R, Wang L, Andrews N, et al. Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med 2017; 13(12):1411–1422.
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012; 126(12):1495–1510.
- Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J 2015; 36(23):1463–1469.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18(7):534–540.
- Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities–Sleep Heart Health Study. Circulation 2015; 132(14):1329–1337.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180.
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105.
- Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest 2015; 148(4):848–851.
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol 2017; 69(12):1577–1587.
- Abraham WT, Jagielski D, Oldenburg O, et al; remede Pilot Study Investigators. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail 2015; 3(5):360–369.
- Ponikowski P, Javaheri S, Michalkiewicz D, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 2012; 33(7):889–894.
- Costanzo MR, Ponikowski P, Javaheri S, et al; remede System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016; 388(10048):974–982.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370-2375.
- Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J 1996; 131(4):790–795.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125.
- Camm AJ, Kirchhof P, Lip GYH, et al; European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31(19):2369–2429.
- Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014; 64(7):710–721.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA 2001; 258(18):2370–2375.
- Kwon Y, Mehra R. Obstructive sleep apnea and atrial fibrillation: honing in on race-specific susceptibilities. J Clin Sleep Med 2018; 14(9):1459–1461.
- Mehra R. Sleep apnea and nocturnal cardiac arrhythmia: understanding differences across ethnicity. J Clin Sleep Med 2017; 13(11):1229–1231.
- May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhymogenesis: mechanistic insights. Chest 2017; 151(1):225–241.
- Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm 2012; 9(3):321–327.
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916.
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804.
- Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49(5):565–571.
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Am J Respir Crit Care Med 2016; 193(7):783–791.
- Kaw R, El Zarif S, Wang L, Bena J, Blackstone EH, Mehra R. Obesity as an effect modifier in sleep-disordered breathing and postcardiac surgery atrial fibrillation. Chest 2017; 151(6):1279–1287.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7(4):397–398.
- Walia HK, Chung MK, Ibrahim S, Mehra R. Positive airway pressure-induced conversion of atrial fibrillation to normal sinus rhythm in severe obstructive sleep apnea. J Clin Sleep Med 2016; 12(9):1301–1303.
- Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004; 27(2):194–201.
- Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med 2000; 161(2I):375–380.
- Song TJ, Park JH, Choi K, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med 2017; 30:36–42.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182(2):269–277.
- Stone KL, Blackwell TL, Ancoli-Israel S, et al; Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep 2016; 39(3):531–540.
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 2011; 219(2):963–968.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6(8):e1000132.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352(12):1206–1214.
KEY POINTS
- Diurnal variations in blood pressure, heart rate, and cardiac events occur during normal sleep.
- While normal sleep may be cardioprotective, sleep apnea disrupts the normal sleep-heart interaction.
- Untreated severe sleep apnea increases the risk for cardiovascular events.
- Treatment with continuous positive airway pressure (CPAP) may reduce the risk of cardiac events based on some data, though randomized studies suggest no improvement in cardiovascular mortality.
- Poor patient adherence to CPAP makes it difficult to evaluate the efficacy of CPAP treatment in clinical trials.
The USPSTF and screening for obstructive sleep apnea: Dispelling misconceptions
Recent guidelines from the United States Preventive Services Task Force (USPSTF) say that there is insufficient evidence to recommend screening for obstructive sleep apnea in people who have no symptoms of it.1–3
The USPSTF committee systematically reviewed the evidence, sifting through 1,315 articles,3 and found no randomized controlled trials that compared screening with no screening in adults who have no symptoms (or no recognized symptoms) of obstructive sleep apnea. Conclusion: “The current evidence is insufficient to assess the balance of benefits and harms of screening for [obstructive sleep apnea] in asymptomatic adults.”1
This is logical, rigorous, and evidence-based. However, the conclusions might be misinterpreted and need to be put into context.
SCREENING IS WARRANTED IF PATIENTS HAVE SYMPTOMS
First, note that the USPSTF is referring to people who have no symptoms. The American Academy of Sleep Medicine has issued recommendations about screening and diagnostic testing in people who do have symptoms,4 in whom it is important to pursue screening and diagnostic testing.
Symptoms of obstructive sleep apnea include excessive daytime sleepiness, fatigue, drowsy driving, disrupted or fragmented sleep, nocturia, witnessed apnea, snoring, restless sleep, neurocognitive deficits, and depressed mood. Treating it improves these symptoms, as clinical trials have shown unequivocally and consistently.5
Moreover, the third edition of the International Classification of Sleep Disorders defines obstructive sleep apnea as an obstructive apnea-hypopnea index of 15 or more events per hour even in the absence of symptoms. This threshold recognizes the risk of adverse health outcomes observed in population-based studies (ie, in participants recruited irrespective of symptoms).6
ABSENCE OF EVIDENCE, NOT EVIDENCE OF ABSENCE
Second, the absence of sufficient evidence cited by the USPSTF does not necessarily mean that screening for obstructive sleep apnea in asymptomatic people is not beneficial—it has just not been systematically studied. There was insufficient evidence available to make a recommendation to allocate resources to screen all patients irrespective of symptoms.
The Sleep Heart Health Study suggested that few people with obstructive sleep apnea were diagnosed with it and that even fewer were treated for it.7 More recent data indicate that this underdiagnosis persists and is more pervasive in underserved minority groups.8,9
SCREENING VS CASE-FINDING
Moreover, screening is not the same as case-finding. The purpose of screening, as defined 50 years ago by Wilson and Jungner in a report for the World Health Organization, is “to discover those among the apparently well who are in fact suffering from disease.”10
Case-finding, on the other hand, focuses on those suspected of being at risk of the disease. In the case of obstructive sleep apnea, this is a lot of people. The overall prevalence of obstructive sleep apnea is about 26% by one estimate,11 and many more people have risk factors for it. For example, in one study, 69% of patients presenting to a primary care clinic were overweight or obese,12 and many primary care patients have diseases that obstructive sleep apnea can exacerbate. One can therefore argue that in clinical practice, testing for obstructive sleep apnea is more like case-finding than screening—most patients that you see have unrecognized symptoms of it or risk factors for it.
CRITERIA FOR A GOOD SCREENING TEST
Principles for screening outlined by Wilson and Jungner10 were:
- The condition we are trying to detect should be important
- There should be an accepted treatment for it
- Facilities for diagnosis and treatment should be available
- Testing should be acceptable to the population
- There should be cost benefit to the expense of case-finding
- There should be an agreed-upon policy on whom to treat as patients.
Screening for obstructive sleep apnea meets many of these criteria.
Obstructive sleep apnea is important
Solid evidence exists that obstructive sleep apnea exerts a bad effect on health and quality of life. Population-based studies that enrolled participants irrespective of symptoms indicate that the risk of death is about twice as high in those with severe obstructive sleep apnea as in those without, and treatment exerts benefit especially in those with cardiovascular risk.13,14 Therefore, the criterion for screening that says the disease must be important is met.
Pathophysiologic pathways by which obstructive sleep apnea causes harm include intermittent hypoxia, hypercapnia, intrathoracic pressure swings, and autonomic nervous system fluctuations.
Treatment is beneficial
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as a cause of hypertension.15
Treating obstructive sleep apnea lowers blood pressure, which in turn improves cardiovascular outcomes. Effects are most pronounced in those with resistant hypertension. The reduction in blood pressure is only about 2 to 3 mm Hg, but this translates to a 4% to 8% reduction in future risk of stroke and coronary heart disease.16,17
The Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea to Prevent Cardiovascular Disease multicenter randomized clinical trial investigated the impact of treating obstructive sleep apnea with continuous positive airway pressure (CPAP) compared with usual care.18 Although no statistically significant difference was seen in the composite cardiovascular outcome, propensity-score analysis in the subgroup adherent to CPAP demonstrated a lower composite of cerebral events in those who used CPAP for at least 4 hours a day.
The findings from this trial are difficult to interpret for several reasons. Adherence to CPAP was suboptimal, the severity of obstructive sleep apnea might not have been bad enough to permit observation of a significant treatment effect, and the generalizability of the findings is unclear, given that many of the participants were from underresourced regions.19
In a meta-analysis of cohort studies comprising more than 3 million participants, Fu et al found that the cardiovascular mortality rate was 63% lower in those with obstructive sleep apnea using CPAP than in untreated patients.20
APPLY CLINICAL JUDGMENT
Overall, the USPSTF report is intended to guide healthcare decision-makers. However, it includes a caveat to not substitute the findings for clinical judgment and to interpret the findings in the context of collateral pertinent information.2
Although no high-quality data exist to support or refute global screening for obstructive sleep apnea in the primary care setting, the high prevalence of this disease and its detrimental effects on health and quality of life if left untreated should not be dismissed.
Arguably, most patients who present to primary care clinics are not healthy, are not free of symptoms, and are at risk of obstructive sleep apnea because they are obese. Testing for it is therefore more like case-finding than screening.
In view of the serious consequences of obstructive sleep apnea, we should view the situation as an opportunity to examine the impact of screening. Perhaps using electronic medical records, we could collect sleep-specific measures, implement case-finding strategies, and perform pragmatic clinical trials to inform and guide optimal and cost-effective screening approaches.
Patients with common disorders such as obstructive sleep apnea are often considered asymptomatic until asked about symptoms. Therefore, careful review of systems incorporating sleep health is important, particularly as patients do not typically volunteer this information. Obtaining this history does not necessarily fall under the USPSTF’s recommendation not to screen.
Future efforts should focus on leveraging the electronic medical record platform to collect sleep-specific measures, implementing case-finding strategies, and performing pragmatic clinical trials in the primary care setting to inform and guide optimal and cost-effective approaches to screening.
- US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force Recommendation Statement. JAMA 2017; 317:407–414.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017; 317:415–433.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: an evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 146. AHRQ Publication No. 14-05216-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.uspreventiveservicestaskforce.org/Page/Document/final-evidence-review152/obstructive-sleep-apnea-in-adults-screening. Accessed May 2, 2017.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13:479–504.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38:877–888.
- Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 2014; 189:335–344.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. www.who.int/ionizing_radiation/medical_radiation_exposure/munich-WHO-1968-Screening-Disease.pdf?ua=1. Accessed May 2, 2017.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Stecker T, Sparks S. Prevalence of obese patients in a primary care setting. Obesity (Silver Spring) 2006; 14:373–376.
- Zhao YY, Wang R, Gleason KJ, et al; BestAIR Investigators. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep 2017; Apr 17. doi: 10.1093/sleep/zsx040. [Epub ahead of print].
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009 Aug;6(8) e1000132. doi: 10.1371/journal.pmed.1000132. Epub 2009 Aug 18.
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schein AS, Kerkhoff AC, Coronel CC, Plentz RD, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens 2014; 32:1762–1773.
- He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J 1999; 138:211–219.
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375:919–931.
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69:841–858.
- Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath 2017; 21:181–189.
Recent guidelines from the United States Preventive Services Task Force (USPSTF) say that there is insufficient evidence to recommend screening for obstructive sleep apnea in people who have no symptoms of it.1–3
The USPSTF committee systematically reviewed the evidence, sifting through 1,315 articles,3 and found no randomized controlled trials that compared screening with no screening in adults who have no symptoms (or no recognized symptoms) of obstructive sleep apnea. Conclusion: “The current evidence is insufficient to assess the balance of benefits and harms of screening for [obstructive sleep apnea] in asymptomatic adults.”1
This is logical, rigorous, and evidence-based. However, the conclusions might be misinterpreted and need to be put into context.
SCREENING IS WARRANTED IF PATIENTS HAVE SYMPTOMS
First, note that the USPSTF is referring to people who have no symptoms. The American Academy of Sleep Medicine has issued recommendations about screening and diagnostic testing in people who do have symptoms,4 in whom it is important to pursue screening and diagnostic testing.
Symptoms of obstructive sleep apnea include excessive daytime sleepiness, fatigue, drowsy driving, disrupted or fragmented sleep, nocturia, witnessed apnea, snoring, restless sleep, neurocognitive deficits, and depressed mood. Treating it improves these symptoms, as clinical trials have shown unequivocally and consistently.5
Moreover, the third edition of the International Classification of Sleep Disorders defines obstructive sleep apnea as an obstructive apnea-hypopnea index of 15 or more events per hour even in the absence of symptoms. This threshold recognizes the risk of adverse health outcomes observed in population-based studies (ie, in participants recruited irrespective of symptoms).6
ABSENCE OF EVIDENCE, NOT EVIDENCE OF ABSENCE
Second, the absence of sufficient evidence cited by the USPSTF does not necessarily mean that screening for obstructive sleep apnea in asymptomatic people is not beneficial—it has just not been systematically studied. There was insufficient evidence available to make a recommendation to allocate resources to screen all patients irrespective of symptoms.
The Sleep Heart Health Study suggested that few people with obstructive sleep apnea were diagnosed with it and that even fewer were treated for it.7 More recent data indicate that this underdiagnosis persists and is more pervasive in underserved minority groups.8,9
SCREENING VS CASE-FINDING
Moreover, screening is not the same as case-finding. The purpose of screening, as defined 50 years ago by Wilson and Jungner in a report for the World Health Organization, is “to discover those among the apparently well who are in fact suffering from disease.”10
Case-finding, on the other hand, focuses on those suspected of being at risk of the disease. In the case of obstructive sleep apnea, this is a lot of people. The overall prevalence of obstructive sleep apnea is about 26% by one estimate,11 and many more people have risk factors for it. For example, in one study, 69% of patients presenting to a primary care clinic were overweight or obese,12 and many primary care patients have diseases that obstructive sleep apnea can exacerbate. One can therefore argue that in clinical practice, testing for obstructive sleep apnea is more like case-finding than screening—most patients that you see have unrecognized symptoms of it or risk factors for it.
CRITERIA FOR A GOOD SCREENING TEST
Principles for screening outlined by Wilson and Jungner10 were:
- The condition we are trying to detect should be important
- There should be an accepted treatment for it
- Facilities for diagnosis and treatment should be available
- Testing should be acceptable to the population
- There should be cost benefit to the expense of case-finding
- There should be an agreed-upon policy on whom to treat as patients.
Screening for obstructive sleep apnea meets many of these criteria.
Obstructive sleep apnea is important
Solid evidence exists that obstructive sleep apnea exerts a bad effect on health and quality of life. Population-based studies that enrolled participants irrespective of symptoms indicate that the risk of death is about twice as high in those with severe obstructive sleep apnea as in those without, and treatment exerts benefit especially in those with cardiovascular risk.13,14 Therefore, the criterion for screening that says the disease must be important is met.
Pathophysiologic pathways by which obstructive sleep apnea causes harm include intermittent hypoxia, hypercapnia, intrathoracic pressure swings, and autonomic nervous system fluctuations.
Treatment is beneficial
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as a cause of hypertension.15
Treating obstructive sleep apnea lowers blood pressure, which in turn improves cardiovascular outcomes. Effects are most pronounced in those with resistant hypertension. The reduction in blood pressure is only about 2 to 3 mm Hg, but this translates to a 4% to 8% reduction in future risk of stroke and coronary heart disease.16,17
The Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea to Prevent Cardiovascular Disease multicenter randomized clinical trial investigated the impact of treating obstructive sleep apnea with continuous positive airway pressure (CPAP) compared with usual care.18 Although no statistically significant difference was seen in the composite cardiovascular outcome, propensity-score analysis in the subgroup adherent to CPAP demonstrated a lower composite of cerebral events in those who used CPAP for at least 4 hours a day.
The findings from this trial are difficult to interpret for several reasons. Adherence to CPAP was suboptimal, the severity of obstructive sleep apnea might not have been bad enough to permit observation of a significant treatment effect, and the generalizability of the findings is unclear, given that many of the participants were from underresourced regions.19
In a meta-analysis of cohort studies comprising more than 3 million participants, Fu et al found that the cardiovascular mortality rate was 63% lower in those with obstructive sleep apnea using CPAP than in untreated patients.20
APPLY CLINICAL JUDGMENT
Overall, the USPSTF report is intended to guide healthcare decision-makers. However, it includes a caveat to not substitute the findings for clinical judgment and to interpret the findings in the context of collateral pertinent information.2
Although no high-quality data exist to support or refute global screening for obstructive sleep apnea in the primary care setting, the high prevalence of this disease and its detrimental effects on health and quality of life if left untreated should not be dismissed.
Arguably, most patients who present to primary care clinics are not healthy, are not free of symptoms, and are at risk of obstructive sleep apnea because they are obese. Testing for it is therefore more like case-finding than screening.
In view of the serious consequences of obstructive sleep apnea, we should view the situation as an opportunity to examine the impact of screening. Perhaps using electronic medical records, we could collect sleep-specific measures, implement case-finding strategies, and perform pragmatic clinical trials to inform and guide optimal and cost-effective screening approaches.
Patients with common disorders such as obstructive sleep apnea are often considered asymptomatic until asked about symptoms. Therefore, careful review of systems incorporating sleep health is important, particularly as patients do not typically volunteer this information. Obtaining this history does not necessarily fall under the USPSTF’s recommendation not to screen.
Future efforts should focus on leveraging the electronic medical record platform to collect sleep-specific measures, implementing case-finding strategies, and performing pragmatic clinical trials in the primary care setting to inform and guide optimal and cost-effective approaches to screening.
Recent guidelines from the United States Preventive Services Task Force (USPSTF) say that there is insufficient evidence to recommend screening for obstructive sleep apnea in people who have no symptoms of it.1–3
The USPSTF committee systematically reviewed the evidence, sifting through 1,315 articles,3 and found no randomized controlled trials that compared screening with no screening in adults who have no symptoms (or no recognized symptoms) of obstructive sleep apnea. Conclusion: “The current evidence is insufficient to assess the balance of benefits and harms of screening for [obstructive sleep apnea] in asymptomatic adults.”1
This is logical, rigorous, and evidence-based. However, the conclusions might be misinterpreted and need to be put into context.
SCREENING IS WARRANTED IF PATIENTS HAVE SYMPTOMS
First, note that the USPSTF is referring to people who have no symptoms. The American Academy of Sleep Medicine has issued recommendations about screening and diagnostic testing in people who do have symptoms,4 in whom it is important to pursue screening and diagnostic testing.
Symptoms of obstructive sleep apnea include excessive daytime sleepiness, fatigue, drowsy driving, disrupted or fragmented sleep, nocturia, witnessed apnea, snoring, restless sleep, neurocognitive deficits, and depressed mood. Treating it improves these symptoms, as clinical trials have shown unequivocally and consistently.5
Moreover, the third edition of the International Classification of Sleep Disorders defines obstructive sleep apnea as an obstructive apnea-hypopnea index of 15 or more events per hour even in the absence of symptoms. This threshold recognizes the risk of adverse health outcomes observed in population-based studies (ie, in participants recruited irrespective of symptoms).6
ABSENCE OF EVIDENCE, NOT EVIDENCE OF ABSENCE
Second, the absence of sufficient evidence cited by the USPSTF does not necessarily mean that screening for obstructive sleep apnea in asymptomatic people is not beneficial—it has just not been systematically studied. There was insufficient evidence available to make a recommendation to allocate resources to screen all patients irrespective of symptoms.
The Sleep Heart Health Study suggested that few people with obstructive sleep apnea were diagnosed with it and that even fewer were treated for it.7 More recent data indicate that this underdiagnosis persists and is more pervasive in underserved minority groups.8,9
SCREENING VS CASE-FINDING
Moreover, screening is not the same as case-finding. The purpose of screening, as defined 50 years ago by Wilson and Jungner in a report for the World Health Organization, is “to discover those among the apparently well who are in fact suffering from disease.”10
Case-finding, on the other hand, focuses on those suspected of being at risk of the disease. In the case of obstructive sleep apnea, this is a lot of people. The overall prevalence of obstructive sleep apnea is about 26% by one estimate,11 and many more people have risk factors for it. For example, in one study, 69% of patients presenting to a primary care clinic were overweight or obese,12 and many primary care patients have diseases that obstructive sleep apnea can exacerbate. One can therefore argue that in clinical practice, testing for obstructive sleep apnea is more like case-finding than screening—most patients that you see have unrecognized symptoms of it or risk factors for it.
CRITERIA FOR A GOOD SCREENING TEST
Principles for screening outlined by Wilson and Jungner10 were:
- The condition we are trying to detect should be important
- There should be an accepted treatment for it
- Facilities for diagnosis and treatment should be available
- Testing should be acceptable to the population
- There should be cost benefit to the expense of case-finding
- There should be an agreed-upon policy on whom to treat as patients.
Screening for obstructive sleep apnea meets many of these criteria.
Obstructive sleep apnea is important
Solid evidence exists that obstructive sleep apnea exerts a bad effect on health and quality of life. Population-based studies that enrolled participants irrespective of symptoms indicate that the risk of death is about twice as high in those with severe obstructive sleep apnea as in those without, and treatment exerts benefit especially in those with cardiovascular risk.13,14 Therefore, the criterion for screening that says the disease must be important is met.
Pathophysiologic pathways by which obstructive sleep apnea causes harm include intermittent hypoxia, hypercapnia, intrathoracic pressure swings, and autonomic nervous system fluctuations.
Treatment is beneficial
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as a cause of hypertension.15
Treating obstructive sleep apnea lowers blood pressure, which in turn improves cardiovascular outcomes. Effects are most pronounced in those with resistant hypertension. The reduction in blood pressure is only about 2 to 3 mm Hg, but this translates to a 4% to 8% reduction in future risk of stroke and coronary heart disease.16,17
The Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea to Prevent Cardiovascular Disease multicenter randomized clinical trial investigated the impact of treating obstructive sleep apnea with continuous positive airway pressure (CPAP) compared with usual care.18 Although no statistically significant difference was seen in the composite cardiovascular outcome, propensity-score analysis in the subgroup adherent to CPAP demonstrated a lower composite of cerebral events in those who used CPAP for at least 4 hours a day.
The findings from this trial are difficult to interpret for several reasons. Adherence to CPAP was suboptimal, the severity of obstructive sleep apnea might not have been bad enough to permit observation of a significant treatment effect, and the generalizability of the findings is unclear, given that many of the participants were from underresourced regions.19
In a meta-analysis of cohort studies comprising more than 3 million participants, Fu et al found that the cardiovascular mortality rate was 63% lower in those with obstructive sleep apnea using CPAP than in untreated patients.20
APPLY CLINICAL JUDGMENT
Overall, the USPSTF report is intended to guide healthcare decision-makers. However, it includes a caveat to not substitute the findings for clinical judgment and to interpret the findings in the context of collateral pertinent information.2
Although no high-quality data exist to support or refute global screening for obstructive sleep apnea in the primary care setting, the high prevalence of this disease and its detrimental effects on health and quality of life if left untreated should not be dismissed.
Arguably, most patients who present to primary care clinics are not healthy, are not free of symptoms, and are at risk of obstructive sleep apnea because they are obese. Testing for it is therefore more like case-finding than screening.
In view of the serious consequences of obstructive sleep apnea, we should view the situation as an opportunity to examine the impact of screening. Perhaps using electronic medical records, we could collect sleep-specific measures, implement case-finding strategies, and perform pragmatic clinical trials to inform and guide optimal and cost-effective screening approaches.
Patients with common disorders such as obstructive sleep apnea are often considered asymptomatic until asked about symptoms. Therefore, careful review of systems incorporating sleep health is important, particularly as patients do not typically volunteer this information. Obtaining this history does not necessarily fall under the USPSTF’s recommendation not to screen.
Future efforts should focus on leveraging the electronic medical record platform to collect sleep-specific measures, implementing case-finding strategies, and performing pragmatic clinical trials in the primary care setting to inform and guide optimal and cost-effective approaches to screening.
- US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force Recommendation Statement. JAMA 2017; 317:407–414.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017; 317:415–433.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: an evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 146. AHRQ Publication No. 14-05216-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.uspreventiveservicestaskforce.org/Page/Document/final-evidence-review152/obstructive-sleep-apnea-in-adults-screening. Accessed May 2, 2017.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13:479–504.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38:877–888.
- Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 2014; 189:335–344.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. www.who.int/ionizing_radiation/medical_radiation_exposure/munich-WHO-1968-Screening-Disease.pdf?ua=1. Accessed May 2, 2017.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Stecker T, Sparks S. Prevalence of obese patients in a primary care setting. Obesity (Silver Spring) 2006; 14:373–376.
- Zhao YY, Wang R, Gleason KJ, et al; BestAIR Investigators. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep 2017; Apr 17. doi: 10.1093/sleep/zsx040. [Epub ahead of print].
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009 Aug;6(8) e1000132. doi: 10.1371/journal.pmed.1000132. Epub 2009 Aug 18.
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schein AS, Kerkhoff AC, Coronel CC, Plentz RD, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens 2014; 32:1762–1773.
- He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J 1999; 138:211–219.
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375:919–931.
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69:841–858.
- Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath 2017; 21:181–189.
- US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force Recommendation Statement. JAMA 2017; 317:407–414.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017; 317:415–433.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: an evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 146. AHRQ Publication No. 14-05216-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.uspreventiveservicestaskforce.org/Page/Document/final-evidence-review152/obstructive-sleep-apnea-in-adults-screening. Accessed May 2, 2017.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13:479–504.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38:877–888.
- Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 2014; 189:335–344.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. www.who.int/ionizing_radiation/medical_radiation_exposure/munich-WHO-1968-Screening-Disease.pdf?ua=1. Accessed May 2, 2017.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Stecker T, Sparks S. Prevalence of obese patients in a primary care setting. Obesity (Silver Spring) 2006; 14:373–376.
- Zhao YY, Wang R, Gleason KJ, et al; BestAIR Investigators. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep 2017; Apr 17. doi: 10.1093/sleep/zsx040. [Epub ahead of print].
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009 Aug;6(8) e1000132. doi: 10.1371/journal.pmed.1000132. Epub 2009 Aug 18.
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schein AS, Kerkhoff AC, Coronel CC, Plentz RD, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens 2014; 32:1762–1773.
- He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J 1999; 138:211–219.
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375:919–931.
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69:841–858.
- Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath 2017; 21:181–189.
In reply: Sleep apnea ABCs
In Reply: We thank Dr. Abouda for underscoring the role of arousals in the pathophysiology of obstructive sleep apnea (OSA). Although the focus of the referenced article was to provide a general overview of the epidemiology, diagnostic testing, and cardiovascular ramifications of untreated OSA and not a detailed summary of the underlying pathophysiology, we welcome the comments from Dr. Abouda to highlight the importance of cortical or microarousals in OSA.
Whether cortical arousal during sleep is bad or good is controversial. During the development of the American Academy of Sleep Medicine respiratory event guidelines, the assignment of detriment or benefit to the arousal when considering defining and scoring of a hypopnea event was a topic of much discussion.1,2 Supporters of including arousal in the hypopnea definition cite data that sleep fragmentation without attendant hypoxia is associated with symptoms such as excessive daytime somnolence, which is recognized to be effectively addressed with OSA treatment.3,4 Moreover, experimental data indicate that arousals lead to activation of the sympathetic nervous system.5 On the other hand, those who question the inclusion of cortical arousal in the hypopnea definition cite large-scale epidemiologic studies that have failed to find a significantly increased cardiovascular risk in relation to increasing arousal index, as well as the enhanced potential to introduce measurement variability.1
The effects of cortical arousals as a purported source of sympathetic activation may operate in concert with hypoxic influences, the latter resulting in sustained increases in blood pressure in both animal models and human studies.6,7 Gottlieb et al8 examined the effect of supplemental oxygen vs continuous positive airway pressure (CPAP) on 24-hour mean arterial pressure in a multicenter randomized controlled trial. Although CPAP reduced blood pressure, as expected, the somewhat unanticipated finding that supplemental oxygen did not suggests that other factors such as hypercapnia and cortical arousals with attendant sympathetic activation may represent potential culprits. Along these lines, in patients with OSA and increased loop gain, benefit in response to sedative hypnotics has been shown to reduce ventilatory instability through an increase in arousal threshold.9 A genetic predisposition may influence the intensity of cortical arousals and accompanying cardiovascular influences that appear to be consistent within individuals but that are heterogeneous within populations.10
Few studies have identified increased cortical arousals as a cardiovascular risk factor. In the Cleveland Family Study, an elevated arousal index was associated with hypertension, but respiratory event-specific arousals was not specifically examined.11 Not only have large-scale epidemiologic studies failed to identify an association between arousal index and cardiovascular outcomes, existing data appear to support the contrary. For example, the extent of incident white matter disease identified on brain magnetic resonance imaging was inversely related to the arousal index in a subset of participants of the Sleep Heart Health Study, a large population-based study focused on sleep and cardiovascular outcomes.12 Furthermore, elevated arousal indices in women were associated with reduced incidence of stroke in the Sleep Heart Health Study.13 These data suggest that arousals may represent beneficial, protective biomarkers reflecting truncation of respiratory events translating into reduced duration of hypoxic exposure and decreased work of breathing.
Needed is further investigation dedicated to understanding the impact of cortical arousals on health outcomes in population-based studies and elucidating the mechanistic role of cortical arousals in the autonomic nervous system physiology in various subtypes of sleep-disordered breathing (eg, obstructive vs central sleep apnea) as well as periodic limb movements.
As the upper Airway is central to the pathophysiology of OSA leading to compromise in Breathing and Circulatory or Cardiovascular ramifications, we think it logical that the “A” in ABCs should stand for “airway.” Hopefully, future research will allow us to better understand the associated benefit vs detriment of cortical arousals as they pertain to subgroup susceptibilities and enhance our ability to tailor a personalized medicine approach to the treatment of sleep disorders.
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8:597–619.
- Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep 2009; 32:150-157.
- Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest 1993; 104:781–787.
- Bonnet MH, Doghramji K, Roehrs T, et al. The scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med 2007; 3:133–145.
- Loredo JS, Ziegler MG, Ancoli-Israel S, Clausen JL, Dimsdale JE. Relationship of arousals from sleep to sympathetic nervous system activity and BP in obstructive sleep apnea. Chest J 1999; 116:655–659.
- Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 1992; 20:612–619.
- Tamisier R, Pépin JL, Rémy J, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 2011; 37:119–128.
- Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med 2014; 370:2276–2285.
- Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci Lond Engl 1979. 2011; 120:505–514.
- Azarbarzin A, Ostrowski M, Hanly P, Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep 2014; 37:645–653.
- Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep 2006; 29:777–783.
- Ding J, Nieto FJ, Beauchamp NJ, et al. Sleep-disordered breathing and white matter disease in the brainstem in older adults. Sleep 2004; 27:474–479.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182:269–277.
In Reply: We thank Dr. Abouda for underscoring the role of arousals in the pathophysiology of obstructive sleep apnea (OSA). Although the focus of the referenced article was to provide a general overview of the epidemiology, diagnostic testing, and cardiovascular ramifications of untreated OSA and not a detailed summary of the underlying pathophysiology, we welcome the comments from Dr. Abouda to highlight the importance of cortical or microarousals in OSA.
Whether cortical arousal during sleep is bad or good is controversial. During the development of the American Academy of Sleep Medicine respiratory event guidelines, the assignment of detriment or benefit to the arousal when considering defining and scoring of a hypopnea event was a topic of much discussion.1,2 Supporters of including arousal in the hypopnea definition cite data that sleep fragmentation without attendant hypoxia is associated with symptoms such as excessive daytime somnolence, which is recognized to be effectively addressed with OSA treatment.3,4 Moreover, experimental data indicate that arousals lead to activation of the sympathetic nervous system.5 On the other hand, those who question the inclusion of cortical arousal in the hypopnea definition cite large-scale epidemiologic studies that have failed to find a significantly increased cardiovascular risk in relation to increasing arousal index, as well as the enhanced potential to introduce measurement variability.1
The effects of cortical arousals as a purported source of sympathetic activation may operate in concert with hypoxic influences, the latter resulting in sustained increases in blood pressure in both animal models and human studies.6,7 Gottlieb et al8 examined the effect of supplemental oxygen vs continuous positive airway pressure (CPAP) on 24-hour mean arterial pressure in a multicenter randomized controlled trial. Although CPAP reduced blood pressure, as expected, the somewhat unanticipated finding that supplemental oxygen did not suggests that other factors such as hypercapnia and cortical arousals with attendant sympathetic activation may represent potential culprits. Along these lines, in patients with OSA and increased loop gain, benefit in response to sedative hypnotics has been shown to reduce ventilatory instability through an increase in arousal threshold.9 A genetic predisposition may influence the intensity of cortical arousals and accompanying cardiovascular influences that appear to be consistent within individuals but that are heterogeneous within populations.10
Few studies have identified increased cortical arousals as a cardiovascular risk factor. In the Cleveland Family Study, an elevated arousal index was associated with hypertension, but respiratory event-specific arousals was not specifically examined.11 Not only have large-scale epidemiologic studies failed to identify an association between arousal index and cardiovascular outcomes, existing data appear to support the contrary. For example, the extent of incident white matter disease identified on brain magnetic resonance imaging was inversely related to the arousal index in a subset of participants of the Sleep Heart Health Study, a large population-based study focused on sleep and cardiovascular outcomes.12 Furthermore, elevated arousal indices in women were associated with reduced incidence of stroke in the Sleep Heart Health Study.13 These data suggest that arousals may represent beneficial, protective biomarkers reflecting truncation of respiratory events translating into reduced duration of hypoxic exposure and decreased work of breathing.
Needed is further investigation dedicated to understanding the impact of cortical arousals on health outcomes in population-based studies and elucidating the mechanistic role of cortical arousals in the autonomic nervous system physiology in various subtypes of sleep-disordered breathing (eg, obstructive vs central sleep apnea) as well as periodic limb movements.
As the upper Airway is central to the pathophysiology of OSA leading to compromise in Breathing and Circulatory or Cardiovascular ramifications, we think it logical that the “A” in ABCs should stand for “airway.” Hopefully, future research will allow us to better understand the associated benefit vs detriment of cortical arousals as they pertain to subgroup susceptibilities and enhance our ability to tailor a personalized medicine approach to the treatment of sleep disorders.
In Reply: We thank Dr. Abouda for underscoring the role of arousals in the pathophysiology of obstructive sleep apnea (OSA). Although the focus of the referenced article was to provide a general overview of the epidemiology, diagnostic testing, and cardiovascular ramifications of untreated OSA and not a detailed summary of the underlying pathophysiology, we welcome the comments from Dr. Abouda to highlight the importance of cortical or microarousals in OSA.
Whether cortical arousal during sleep is bad or good is controversial. During the development of the American Academy of Sleep Medicine respiratory event guidelines, the assignment of detriment or benefit to the arousal when considering defining and scoring of a hypopnea event was a topic of much discussion.1,2 Supporters of including arousal in the hypopnea definition cite data that sleep fragmentation without attendant hypoxia is associated with symptoms such as excessive daytime somnolence, which is recognized to be effectively addressed with OSA treatment.3,4 Moreover, experimental data indicate that arousals lead to activation of the sympathetic nervous system.5 On the other hand, those who question the inclusion of cortical arousal in the hypopnea definition cite large-scale epidemiologic studies that have failed to find a significantly increased cardiovascular risk in relation to increasing arousal index, as well as the enhanced potential to introduce measurement variability.1
The effects of cortical arousals as a purported source of sympathetic activation may operate in concert with hypoxic influences, the latter resulting in sustained increases in blood pressure in both animal models and human studies.6,7 Gottlieb et al8 examined the effect of supplemental oxygen vs continuous positive airway pressure (CPAP) on 24-hour mean arterial pressure in a multicenter randomized controlled trial. Although CPAP reduced blood pressure, as expected, the somewhat unanticipated finding that supplemental oxygen did not suggests that other factors such as hypercapnia and cortical arousals with attendant sympathetic activation may represent potential culprits. Along these lines, in patients with OSA and increased loop gain, benefit in response to sedative hypnotics has been shown to reduce ventilatory instability through an increase in arousal threshold.9 A genetic predisposition may influence the intensity of cortical arousals and accompanying cardiovascular influences that appear to be consistent within individuals but that are heterogeneous within populations.10
Few studies have identified increased cortical arousals as a cardiovascular risk factor. In the Cleveland Family Study, an elevated arousal index was associated with hypertension, but respiratory event-specific arousals was not specifically examined.11 Not only have large-scale epidemiologic studies failed to identify an association between arousal index and cardiovascular outcomes, existing data appear to support the contrary. For example, the extent of incident white matter disease identified on brain magnetic resonance imaging was inversely related to the arousal index in a subset of participants of the Sleep Heart Health Study, a large population-based study focused on sleep and cardiovascular outcomes.12 Furthermore, elevated arousal indices in women were associated with reduced incidence of stroke in the Sleep Heart Health Study.13 These data suggest that arousals may represent beneficial, protective biomarkers reflecting truncation of respiratory events translating into reduced duration of hypoxic exposure and decreased work of breathing.
Needed is further investigation dedicated to understanding the impact of cortical arousals on health outcomes in population-based studies and elucidating the mechanistic role of cortical arousals in the autonomic nervous system physiology in various subtypes of sleep-disordered breathing (eg, obstructive vs central sleep apnea) as well as periodic limb movements.
As the upper Airway is central to the pathophysiology of OSA leading to compromise in Breathing and Circulatory or Cardiovascular ramifications, we think it logical that the “A” in ABCs should stand for “airway.” Hopefully, future research will allow us to better understand the associated benefit vs detriment of cortical arousals as they pertain to subgroup susceptibilities and enhance our ability to tailor a personalized medicine approach to the treatment of sleep disorders.
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8:597–619.
- Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep 2009; 32:150-157.
- Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest 1993; 104:781–787.
- Bonnet MH, Doghramji K, Roehrs T, et al. The scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med 2007; 3:133–145.
- Loredo JS, Ziegler MG, Ancoli-Israel S, Clausen JL, Dimsdale JE. Relationship of arousals from sleep to sympathetic nervous system activity and BP in obstructive sleep apnea. Chest J 1999; 116:655–659.
- Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 1992; 20:612–619.
- Tamisier R, Pépin JL, Rémy J, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 2011; 37:119–128.
- Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med 2014; 370:2276–2285.
- Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci Lond Engl 1979. 2011; 120:505–514.
- Azarbarzin A, Ostrowski M, Hanly P, Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep 2014; 37:645–653.
- Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep 2006; 29:777–783.
- Ding J, Nieto FJ, Beauchamp NJ, et al. Sleep-disordered breathing and white matter disease in the brainstem in older adults. Sleep 2004; 27:474–479.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182:269–277.
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8:597–619.
- Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep 2009; 32:150-157.
- Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest 1993; 104:781–787.
- Bonnet MH, Doghramji K, Roehrs T, et al. The scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med 2007; 3:133–145.
- Loredo JS, Ziegler MG, Ancoli-Israel S, Clausen JL, Dimsdale JE. Relationship of arousals from sleep to sympathetic nervous system activity and BP in obstructive sleep apnea. Chest J 1999; 116:655–659.
- Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 1992; 20:612–619.
- Tamisier R, Pépin JL, Rémy J, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 2011; 37:119–128.
- Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med 2014; 370:2276–2285.
- Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci Lond Engl 1979. 2011; 120:505–514.
- Azarbarzin A, Ostrowski M, Hanly P, Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep 2014; 37:645–653.
- Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep 2006; 29:777–783.
- Ding J, Nieto FJ, Beauchamp NJ, et al. Sleep-disordered breathing and white matter disease in the brainstem in older adults. Sleep 2004; 27:474–479.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182:269–277.
Sleep apnea ABCs: Airway, breathing, circulation
Obstructive sleep apnea (OSA) is common and poorly recognized and, if untreated, leads to serious health consequences. This article discusses the epidemiology of OSA, describes common presenting signs and symptoms, and reviews diagnostic testing and treatment options. Adverse health effects related to untreated sleep apnea are also discussed.
COMMON, POORLY RECOGNIZED, AND COSTLY IF UNTREATED
OSA is very common in the general population and is associated with substantial morbidity and mortality. An estimated 17% of the general adult population has OSA, and the numbers are increasing with the obesity epidemic. Nearly 1 in 15 adults has at least moderate sleep apnea,1,2 and approximately 85% of cases are estimated to be undiagnosed.3 A 1999 study estimated that untreated OSA resulted in approximately $3.4 billion in additional medical costs per year in the United States,4 a figure that is likely to be higher now, given the rising prevalence of OSA. The prevalence of OSA in primary care and subspecialty clinics is even higher than in the community, as more than half of patients who have diabetes or hypertension and 30% to 40% of patients with coronary artery disease are estimated to have OSA.5–7
REPETITIVE UPPER-AIRWAY COLLAPSE
During sleep, parasympathetic activity is enhanced and the muscle tone of the upper airway is decreased, particularly in the pharyngeal dilator muscles. Still, even in the supine position, a healthy person maintains patency of the airway and adequate airflow during sleep.
OSA is characterized by repetitive complete or partial collapse of the upper airway during sleep, resulting in an apneic or hypopneic event, respectively, and often causing snoring from upper-airway tissue vibration.
People who are susceptible to OSA typically have a smaller, more collapsible airway that is often less distensible and has a higher critical closing pressure. Radiographic and physiologic data have shown that the airway dimensions of patients with OSA are smaller than in those without OSA. The shape of the airway of a patient with OSA is often elliptical, given the extrinsic compression of the lateral aspects of the airway by increased size of the parapharyngeal fat pads. OSA episodes are characterized by closure of the upper airway and by progressively increasing respiratory efforts driven by chemoreceptor and mechanoreceptor stimuli, culminating in an arousal from sleep and a reopening of the airway.
The disease-defining metric used for assessing OSA severity is the apnea-hypopnea index, ie, the number of apneas and hypopneas that occur per hour of sleep.8 An apneic or hypopneic event is identified during polysomnography by the complete cessation of airflow or by a reduction in airflow for 10 seconds or longer (Figure 1).
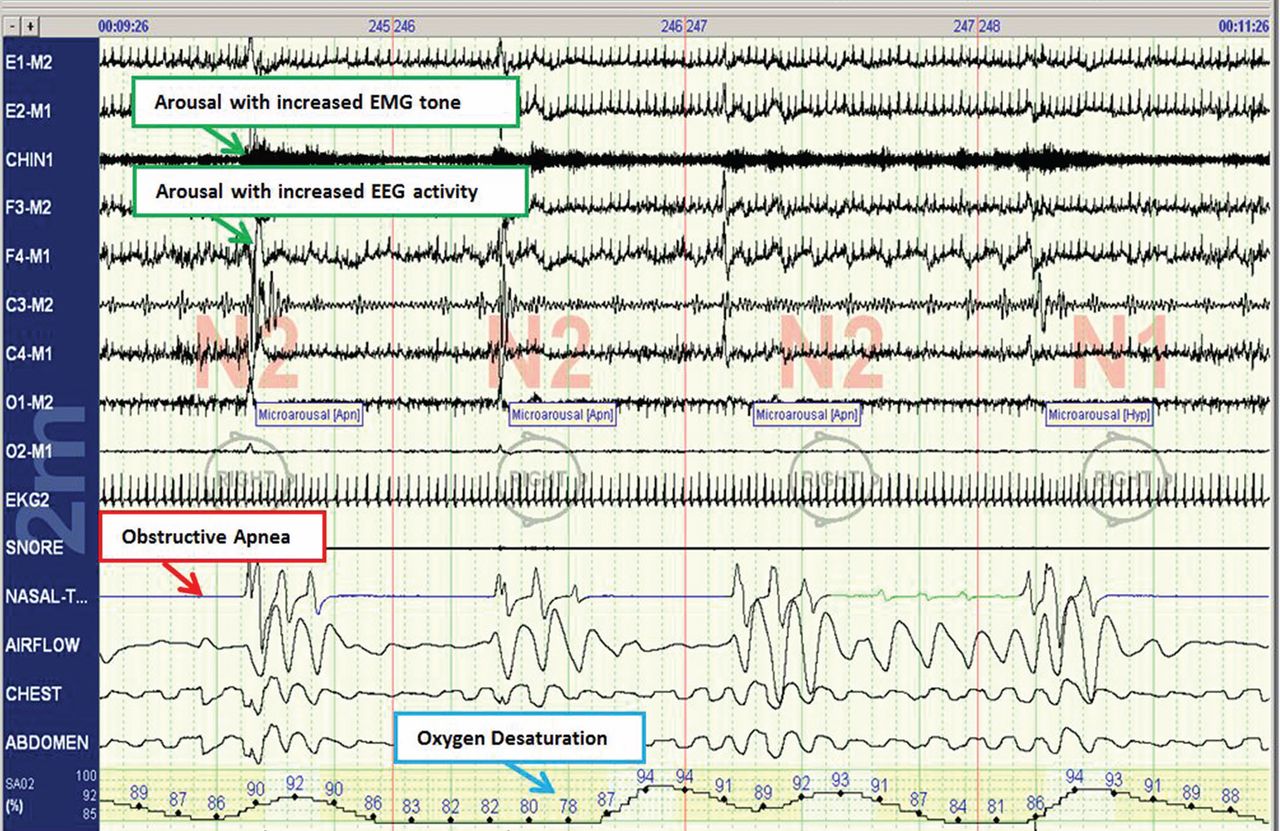
HEALTH CONSEQUENCES IF UNTREATED
Untreated sleep apnea causes numerous pathophysiologic perturbations, including chronic intermittent hypoxia, ventilatory overshoot hyperoxia, increased sympathetic nervous system activity, intrathoracic pressure swings, hypercapnea, sleep fragmentation, increased arousals, reduced sleep duration, and fragmentation of rapid-eye-movement sleep.
Intermittent hypoxia activates the sympathetic nervous system and causes pulmonary vasoconstriction, with increases in pulmonary arterial pressures and myocardial workload. Sympathetic activation, ascertained by peroneal microneurography, has been shown to be increased not only during sleep but also persisting during wakefulness in patients with untreated OSA vs those without OSA.9 Autonomic nervous system fluctuations accompany apneic episodes, resulting in enhanced parasympathetic tone and sympathetic activation associated with a rise in blood pressure and heart rate that occur after the respiratory event.
Intermediate pathways that link the negative pathophysiologic effects of OSA with adverse health outcomes include increased systemic inflammation, increased oxidative stress, metabolic dysfunction, insulin resistance, hypercoagulability, endothelial dysfunction, and autonomic dysfunction.
As a result, a variety of adverse clinical outcomes are associated with untreated OSA, including systemic hypertension, ischemic heart disease and atherosclerosis, diastolic dysfunction, congestive heart failure, cardiac arrhythmias, stroke, increased risk of death, and sudden death, as well as noncardiovascular outcomes such as gout, neurocognitive deficits, and mood disorders.10
Inflammatory and atherogenic effects
Increased levels of markers of systemic inflammation, prothrombosis, and oxidative stress have been observed in OSA and may be key pathophysiologic links between OSA and cardiovascular sequelae. OSA has been associated with up-regulation of a number of inflammatory mediators: interleukin (IL) 6, soluble IL-6 receptor, IL-8, tumor necrosis factor alpha, and C-reactive protein. Soluble IL-6 levels in particular are higher in people who have sleep-disordered breathing, as reflected by the apnea-hypopnea index independent of obesity, with relationships stronger in the morning than in the evening. This likely reflects the overnight OSA-related physiologic stress.11
Thrombotic potential is also enhanced, with higher levels of plasminogen activator inhibitor 1, fibrinogen, P-selectin, and vascular endothelial growth factor. Some of these factors normally have a diurnal cycle, with higher levels in the morning, but in OSA, increasing OSA severity is associated with increased prothrombotic potential in the morning hours. Of interest, levels of these substances showed a plateau effect, rising in people who had only mildly elevated apnea-hypopnea indices and then leveling off.12 Intermittent hypoxia followed by ventilatory overshoot hyperoxia, characteristic of sleep apnea, provides the ideal environment for augmentation of oxidative stress, with evidence of increased oxidation of serum proteins and lipids. Hypoxia and oxygen-derived free radicals may result in cardiac myocyte injury. Experimental data demonstrate that intermittent hypoxia combined with a high-fat diet results in synergistic acceleration of evidence of atherogenic lesions.
Patients with OSA also have evidence of endothelial dysfunction, insulin resistance, and dyslipidemia, all pathways that can facilitate the progression of atherosclerosis in OSA.13–15
Cardiac arrhythmias
In the Sleep Heart Health Study, a multicenter epidemiologic study designed to examine the relationships of OSA and cardiovascular outcomes, those who had moderate to severe OSA had a risk of ventricular and atrial arrhythmias two to four times higher than those without OSA, even after correction for the confounding influences of obesity and underlying cardiovascular risk.14 These findings were corroborated in subsequent work highlighting monotonic dose-response relationships with increasing OSA severity and increased odds of atrial and ventricular arrhythmia in a cohort of about 3,000 older men.11 Additional compelling evidence of a causal relationship is that the risk of discrete arrhythmic events is markedly increased after a respiratory disturbance in sleep.16
In patients who successfully underwent cardioversion for atrial fibrillation, those who had sleep apnea but were not treated with continuous positive airway pressure (CPAP) had a much higher rate of recurrence of atrial fibrillation during the subsequent year than those with CPAP-treated sleep apnea and than controls never diagnosed with sleep apnea. In the untreated patients with sleep apnea, the mean nocturnal fall in oxygen saturation was significantly greater in those who had recurrence of atrial fibrillation than in those who did not, suggesting hypoxia as an important mechanism contributing to atrial fibrillation.17
Since then, several other retrospective studies have shown similar findings after pulmonary vein antrum isolation and ablation in terms of reduction of atrial fibrillation recurrence with CPAP treatment in OSA.18
Walia et al19 described a patient with moderate sleep apnea who underwent a split-night study. During the baseline part of the study, the patient had about 18 ectopic beats per minute. During the second portion of the study while CPAP was applied, progressively fewer ectopic beats occurred as airway pressure was increased until a normal rhythm without ectopic beats was achieved at the goal treatment CPAP pressure setting.
Cardiovascular disease, stroke, and death
Marin et al20 followed about 1,500 men for 10 years, including some who had severe OSA, some with sleep apnea who were treated with CPAP, and controls. The risk of nonfatal and fatal cardiovascular disease events was nearly three times higher in those with severe disease than in healthy participants. Those treated with CPAP had a risk approximately the same as in the control group.
The Sleep Heart Study followed approximately 6,000 people with untreated sleep apnea for a median of nearly 9 years. It found a significant association between the apnea-hypopnea index and ischemic stroke, especially in men.21 Survival in patients with heart failure is also associated with the degree of OSA; patients with more severe disease (an apnea-hypopnea index ≥ 15) have a nearly three times greater risk of death than those with no disease or only mild disease (apnea-hypopnea index < 15).22
From the standpoint of health care utilization, findings that central sleep apnea predicts an increased risk of hospital readmission in heart failure are of particular interest.23
People with OSA are at increased risk of nocturnal sudden cardiac death.24 Sleep apnea is also associated with an increased overall death rate, and the higher the apnea-hypopnea index, the higher the death rate,25 even after adjusting for age, sex, body mass index, and underlying cardiovascular risk, with findings most pronounced in men under age 70.
Motor vehicle accidents
The need for caution during driving should be discussed with every patient, as motor vehicle accidents are an immediate danger to the patient and others. The association with motor vehicle accidents is independent of sleepiness, and drivers with sleep apnea often do not perceive performance impairment. Young et al26 found that men who snored were 3.4 times as likely to have an accident over a 5-year period, and that men and women with an apnea-hypopnea index greater than 15 were more than 7 times as likely to have multiple accidents over a 5-year period, highlighting the importance of discussing, documenting, and expeditiously diagnosing and treating OSA, particularly in those who report drowsiness while driving.
CLINICAL RISK FACTORS
Risk factors can be divided into nonmodifiable and modifiable ones.
Nonmodifiable factors
Age. Bimodal distributions in OSA prevalence have been observed; ie, that the pediatric population and people who are middle-aged have the highest prevalence of OSA. A linear relationship between sleep apnea prevalence and age until about age 65 was identified in data from the Sleep Heart Health Study.27 After that, the prevalence rates plateau; it is unclear if this is secondary to natural remission of the disease after a certain age or because patients with more severe disease have died by that age (ie, survivorship bias), blunting an increase in prevalence.
Sex. Men develop sleep apnea at a rate three to five times that of women. Several explanations have been proposed to account for this.28,29 Sex hormones are one factor; women with sleep apnea on hormone replacement therapy have a significantly less-severe sleep apnea burden than other postmenopausal women,30 suggesting a positive effect from estrogen. Sex-based differences in fat distribution, length and collapsibility of the upper airway, genioglossal activity, neurochemical control mechanisms, and arousal response may also contribute to prevalence differences between men and women.
As with coronary artery disease, the presentation of sleep apnea may be atypical in women, particularly around menopause. Sleep apnea should be considered in women who have snoring and daytime sleepiness.
Race. Whites, African Americans, and Asians have a similar prevalence of sleep apnea, but groups differ in obesity rates and craniofacial anatomy.31–34 Asians tend to have craniofacial skeletal restriction. African Americans are more likely to have upper-airway soft-tissue risk and to develop more severe OSA. Whites tend to have both craniofacial and soft-tissue risk. For those with craniofacial anatomy predisposing to OSA, even mild obesity can make it manifest.
Syndromes that predispose to OSA can include craniofacial structural abnormalities, connective tissue problems, or alterations in ventilatory control (eg, Marfan, Down, and Pierre Robin syndromes).
Modifiable risk factors
Obesity (body mass index ≥ 30 kg/m2) is a firmly established risk factor, but not all obese patients develop obstructive sleep apnea, and not all people with sleep apnea are obese.
Obesity increases risk by altering the geometry and function of the upper airway, increasing collapsibility. The changes are particularly pronounced in the lateral aspects of the pharynx.35
Obesity also affects respiratory drive, likely in part from leptin resistance. Load compensation is another contributing factor: the increased mass in the thorax and abdomen increases the work of breathing and reduces functional residual capacity, increasing oxygen demands and leading to atelectasis and ventilation-perfusion mismatch.
Although obesity is an important risk factor, it is important to recognize that obesity is not the only one to consider: most people with an apnea-hypopnea index of 5 or greater are not obese. The relationship between body mass index and sleep apnea is weaker in children and in the elderly, probably because other risk factors are more pronounced.36
Craniofacial structural abnormalities such as retrognathia (abnormal posterior position of the mandible) and micrognathia (undersized mandible) can increase the risk of OSA because of a resulting posteriorly displaced genioglossus muscle. Other conditions can alter chemosensitivity, affecting the pH and carbon dioxide level of the blood and therefore affecting ventilatory control mechanisms, making the person more prone to developing sleep apnea. Children and young adults may have tonsillar tissue that obstructs the airway.
The site of obstruction can be behind the palate (retropalatal), behind the tongue (retroglossal), or below the pharynx (hypopharyngeal). This helps explain why positive air way pressure—unlike surgery, which addresses a specific area—is often successful, as it serves to splint or treat all aspects of the airway.
FATIGUE, SLEEPINESS, SNORING, RESTLESS SLEEP
Sleep apnea can result in presentation of multiple signs and symptoms (Table 1).
Daytime sleepiness and fatigue are the most common symptoms. Although nonspecific, they are often quite pronounced. Two short questionnaires—the Epworth Sleepiness Scale37 and the Fatigue Severity Scale—can help distinguish between these two symptoms and assess their impact on a patient’s daily life. In the Epworth Sleepiness Scale, the patient rates his or her chance of dozing on a 4-point scale (0 = would never doze, to 3 = high chance of dozing) in eight situations:
- Sitting and reading
- Watching television
- Sitting inactive in a public place
- As a passenger in a car for an hour without a break
- Lying down to rest in the afternoon
- Sitting and talking to someone
- Sitting quietly after a lunch without alcohol
- In a car while stopped for a few minutes in traffic.
A score of 10 or more is consistent with significant subjective sleepiness.
The Fatigue Severity Scale assesses the impact of fatigue on daily living.
Snoring is a common and specific symptom of sleep apnea; however, not all patients who snore have OSA.
Restlessness during sleep is very common—patients may disturb their bed partner by moving around a lot during sleep or report that the sheets are “all over the place” by morning.
Nocturia can also be a sign of sleep apnea and can contribute to sleep fragmentation. A proposed mechanism of this symptom includes alterations of intrathoracic pressure resulting in atrial stretch, which release atrial natriuretic peptide, leading to nocturia. Treating with CPAP has been found to reduce levels of atrial natriuretic peptide, contributing to better sleep.38
Morning headache may occur and is likely related to increased CO2 levels, which appear to culminate in the morning hours. End-tidal or transcutaneous CO2 monitoring during polysomnography can help elucidate the presence of sleep-related hypoventilation.
Libido is often diminished and can actually be improved with CPAP. This is therefore an important point to discuss with patients, as improved libido can often serve as an incentive for adherence to OSA treatment.
Insomnia exists in about 15% of patients, primarily as a result of sleep apnea-related with treatment.
Sweating, particularly forehead sweating associated with sleep apnea, more commonly occurs in children.
The STOP-BANG questionnaire (Table 2)39 was primarily validated in preoperative anesthesia testing. However, because of its ease of use and favorable performance characteristics, it is increasingly used to predict the likelihood of finding OSA before polysomnography. A score of 3 or more has a sensitivity of 93%.
PHYSICAL EXAMINATION PROVIDES CLUES
Although the physical examination may be normal, certain findings indicate risk (Table 3). Obesity alone is not an accepted indication for polysomnography unless there are concomitant worrisome signs or symptoms. Of note, those who are morbidly obese (BMI > 40 kg/m2) have a prevalence of sleep apnea greater than 70%.
The classification by Friedman et al40 provides an indicator of risk. The patient is examined with the mouth opened wide and the tongue in a neutral natural position. Grades:
- I—Entire uvula and tonsils are visible
- II—Entire uvula is visible, but tonsils are not
- III—Soft palate is visible, but uvula is not
- IV—Only the hard palate is visible.
Especially in children and young adults, enlarged tonsils (or “kissing tonsils”) and a boggy edematous uvula set the stage for obstructive sleep apnea.
DIAGNOSIS REQUIRES SLEEP TESTING
A sleep study is the primary means of diagnosing OSA. Polysomnography includes electrooculography to determine when rapid-eye-movement sleep occurs; electromyography to measure muscle activity in the chin to help determine onset of sleep, with peripheral leads in the leg to measure leg movements; electroencephalography (EEG) to measure neural activity; electrocardiography; pulse oximetry to measure oxygen saturation; measurement of oronasal flow; and measurements of chest wall effort and body position using thoracic and abdominal belts that expand and contract with breathing; and audio recording to detect snoring.
Attended polysomnography requires the constant presence of a trained sleep technologist to monitor for technical issues and patient adherence.
End-tidal CO2 monitoring is a reasonable method to detect sleep-related hypoventilation but is not routinely performed in the United States. Transcutaneous CO2 monitoring is a different way to monitor CO2 used in the setting of positive airway pressure.
Polysomnography in a normal patient shows a regular pattern of increasing and decreasing airflow with inspiration and expiration while stable oxygen saturation is maintained.
In contrast, polysomnography of a patient with sleep apnea shows repetitive periods of no airflow, oxygen desaturation, and often evidence of thoracoabdominal paradox, punctuated by arousals on EEG associated with sympathetic activation (Figure 1). When the patient falls asleep, upper-airway muscle tone is reduced, causing an apneic event with hypoxia and pleural pressure swings. These prompt arousals with sympathetic activation that reestablish upper-airway muscle tone, allowing ventilation and reoxygenation to resume with a return to sleep.
Apnea-hypopnea index indicates severity
Sleep apnea severity is graded using the apnea-hypopnea index, ie, the number of apneic and hypopneic events per hour of sleep (Table 4).41 Events must last at least 10 seconds to be considered, ie, two consecutive missed breaths based on an average normal respiratory rate of about 12 breaths per minute for the typical adult.
The apnea-hypopnea index usually correlates with the severity of oxygen desaturation and with electrocardiographic abnormalities, including tachybradycardia and arrhythmias.
Although history, physical examination, and prediction tools are helpful in determining the likelihood that a patient has OSA, only polysomnography testing can establish the diagnosis. To diagnose OSA, 15 or more obstructive events per hour must be observed by polysomnography, or at least 5 events per hour with one of the following:
- Daytime sleepiness, sleep attacks, unrefreshing sleep, fatigue, or insomnia
- Waking with breath-holding, gasping, or choking
- Observer-reported loud snoring or breathing interruptions.41
Split-night study determines diagnosis and optimum treatment
The split-night study has two parts: the first is diagnostic polysomnography, followed by identification of the positive airway pressure that optimally treats the sleep apnea. The apnea-hypopnea index guides the need for the split-night study, with 40 being the established threshold according to the American Academy of Sleep Medicine.
A home sleep study is appropriate for some patients
Home sleep testing is typically more limited than standard polysomnography; it monitors airflow, effort, and oxygenation. The test is intended for adults with a high pretest probability of moderate to severe obstructive sleep apnea (STOP-BANG score ≥ 3). It is not intended for screening of asymptomatic patients or for those with coexisting sleep disorders (eg, central sleep apnea, sleep hypoventilation, periodic limb movements, insomnia, circadian rhythm disorders, parasomnias, narcolepsy) or medical disorders (eg, moderate to severe heart failure or other cardiac disease, symptomatic neurologic disease, moderate to severe pulmonary disease).42 Since March 2008, the Centers for Medicare and Medicaid Services has covered CPAP for obstructive sleep apnea based on diagnosis by home sleep study testing.43
TREATMENT OF SLEEP APNEA
Basic steps for reducing OSA are:
Weight loss. Even small weight changes can significantly affect the severity of sleep apnea, perhaps even leading to a reassessment of the degree of OSA and CPAP requirements. Longitudinal epidemiologic data demonstrate that a 10% weight loss correlates with a 26% reduction in the apnea-hypopnea index, and conversely, a 10% weight gain is associated with a 32% increase.44
Some studies have found that bariatric surgery cures OSA in 75% to 88% of cases, independent of approach.45,46 However, a trial in 60 obese patients with OSA who were randomized to either a low-calorie diet or bariatric surgery found no statistical difference in the apnea-hypopnea index between the two groups despite greater weight loss in the surgery group.47
Avoiding certain medications. Benzodiazepines, narcotics, and alcohol reduce upper airway muscle tone and should be avoided. No medications are associated with improvement of OSA, although acetazolamide may be used to treat central sleep apnea.
Positional therapy. Sleeping on the back exacerbates the problem. Supine-related OSA occurs as a result of several factors, including gravity, airway anatomy, airway critical closing pressures, and effects on upper-airway dilator muscle function.
Sleep hygiene. General recommendations to engage in behaviors to promote sleep are recommended, including keeping consistent sleep-wake times, not watching television in bed, and avoidance of caffeine intake, particularly within 4 to 6 hours of bedtime.
POSITIVE AIRWAY PRESSURE THERAPY
Nasal CPAP is the treatment of choice and is successful in 95% of patients when used consistently. It is not as costly as surgery, and results in improved long-term survival compared with uvulopalatopharyngoplasty. Another advantage is that the pressure can be retitrated as the patient’s condition changes, for example after a weight change or during pregnancy.
More than 15 randomized controlled trials have examined the effect of sleep apnea treatment with CPAP compared with either sham CPAP or another control. In a meta-analysis, CPAP was found to lead to an average systolic blood pressure reduction of about 2.5 mm Hg and a diastolic blood pressure reduction of 1.8 mm Hg. Although these reductions may seem negligible, benefits may be significant for cardiovascular outcomes.48,49
Challenges to treatment adherence
Adherence is the most commonly discussed problem with CPAP, but long-term adherence rates are comparable to medication compliance—about 60% to 70%. To optimize adherence, communication is important to ensure that problems are identified and addressed as they arise. Showing patients examples of apneic events and oxygen desaturation from their sleep study can enhance their understanding of OSA and its importance. Patients need to understand the serious nature of the disease and that CPAP therapy can significantly improve their quality of life and overall health, particularly from a cardiovascular perspective.
CPAP masks can be uncomfortable, posing a major barrier to compliance. But a number of mask designs are available, such as the nasal mask, the nasal pillow mask, and the oronasal mask. For patients with claustrophobia, the nasal pillow mask is an option, as it does not cover the face.
Some patients note symptoms of nasal congestion, although in many patients CPAP improves it. If congestion is a problem, the use of heated humidification with the machine, intranasal saline or gel, or nasal corticosteroids can help relieve it.
Pressure intolerance is a common problem. For those who feel that the pressure is too high, settings can be adjusted so that the pressure is gradually reduced between inspiration and expiration, ie, the use of expiratory pressure relief or consideration of the use of bilevel positive airway pressure.
Aerophagia (swallowing air) is a less common problem. It can also potentially be relieved with use of bilevel positive airway pressure.
Many patients develop skin irritation, which can be helped with moleskin, available at any pharmacy.
Social stigma can be a problem. Education regarding the importance of the treatment to health is essential.
Machine noise is less of a problem with the new machine models, but if it is a problem, a white-noise device or earplugs may help.
Other measures to improve compliance are keeping the regimen simple and ensuring that family support is adequate.
Medicare requires evidence of use and benefit
Medicare requires that clinical benefit be documented between the 31st and 91st day after initiating CPAP therapy. This requires face-to-face clinical reevaluation by the treating physician to document improved symptoms and objective evidence of adherence to use of the device. The devices can store usage patterns, and Medicare requires at least 4 hours per night on 70% of nights during a consecutive 30-day period in the first 3 months of use.
ALTERNATIVE THERAPIES
Alternative therapies may be options for some patients, in particular those who cannot use CPAP or who get no benefit from it. These include oral appliances for those with mild to moderate OSA50–53 and various surgical procedures, eg, uvulopalatopharyngoplasty,54,55 maxillomanibular advancement,56 tracheostomy (standard treatment before CPAP was identified as an effective treatment),57,58 and adenotonsillectomy (in children).59
Supplemental oxygen is not a first-line treatment for OSA and in general has not been found to be very effective, particularly in terms of intermediate cardiovascular outcomes,60–62 although a subset of patients with high loop gain may benefit from it.63 Loop gain is a measure of the tendency of the ventilatory control system to amplify respiration in response to a change, conferring less stable control of breathing.
Several novel alternative therapies are starting to be used. Although all of them have been shown to improve measures of OSA, none is as effective as CPAP in improving OSA severity. New therapies include the nasal expiratory positive airway pressure device,64 oral pressure therapy,65 and hypoglossal nerve stimulation.66
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA; Sleep Heart Health Research Group. The relationship between chronically disrupted sleep and healthcare use. Sleep 2002; 25:289–296.
- Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep 1999; 22:749–755.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109:659–663.
- Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92:79–84.
- Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164:2147–2165.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, Second Edition: Diagnostic and coding manual. Westchester, IL; American Academy of Sleep Medicine, 2005.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Mehra R. Sleep-disordered breathing and cardiovascular disease: exploring pathophysiology and existing data. Curr Resp Med Rev 2007; 3:258–269.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166:1725–1731.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Mehra R, Storfer-Isser A, Tracy R, Jenny N, Redline S. Association of sleep disordered breathing and oxidized LDL [abstract]. Am J Respir Crit Care Med 2010; 181:A2474.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54:1797–1804.
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107:2589–2594.
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3:445–451.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7:397–398.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010; 182:269–277.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18:534–540.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352:1206–1214.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6( 8):e1000132. doi: 10.1371/journal.pmed.1000132.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20:608–613.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165:1217–1239.
- Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 2008; 12:481–496.
- Shaher E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167:1186–1192.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167:1181–1185.
- Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med 1995; 152:1946–1949.
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162:893–900.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155:186–192. Erratum in: Am J Respir Crit Care Med 1997; 155:1820.
- Sutherland K, Lee RWW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology 2012; 17:213–222.
- Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 1995; 152:1673–1689.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14:540–545.
- Krieger J, Imbs J-L, Schmidt M, Kurtz D. Renal function in patients with obstructive sleep apnea. Effects of nasal continuous positive airway pressure. Arch Intern Med 1988; 148:1337–1340.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngal Head Neck Surg 2002; 127:13–21.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Centers for Medicare & Medicaid Services (CMS). Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). MLN Matters 2008. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM6048.pdf. Accessed June 2, 2014.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284:3015–3021.
- Guardiano SA, Scott JA, Ware JC, Schechner SA. The long-term results of gastric bypass on indexes of sleep apnea. Chest 2003; 124:1615–1619.
- Crooks PF. Surgical treatment of morbid obesity. Annu Rev Med 2006; 57:243–264.
- Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012; 308:1142–1149.
- Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 2007; 50:417–423.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Kushida CA, Morgenthaler TI, Littner MR, et al; American Academy of Sleep. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 2006; 29:240–243.
- Otsuka R, Ribeiro de Almeida F, Lowe AA, Linden W, Ryan F. The effect of oral appliance therapy on blood pressure in patients with obstructive sleep apnea. Sleep Breath 2006; 10:29–36.
- Yoshida K. Effect on blood pressure of oral appliance therapy for sleep apnea syndrome. Int J Prosthodont 2006; 19:61–66.
- Inazawa T, Ayuse T, Kurata S, et al. Effect of mandibular position on upper airway collapsibility and resistance. J Dent Res 2005; 84:554–558.
- Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981; 89:923–934.
- Schwab RJ. Imaging for the snoring and sleep apnea patient. Dent Clin North Am 2001; 45:759–796.
- Prinsell JR. Maxillomandibular advancement surgery for obstructive sleep apnea syndrome. J Am Dent Assoc 2002; 133:1489–1497.
- Thatcher GW, Maisel RH. The long-term evaluation of tracheostomy in the management of severe obstructive sleep apnea. Laryngoscope 2003; 113:201–204.
- Conway WA, Victor LD, Magilligan DJ, Fujita S, Zorick FJ, Roth T. Adverse effects of tracheostomy for sleep apnea. JAMA 1981; 246:347–350.
- Marcus CL, Moore RH, Rosen CL, et al; Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013; 368:2366–2376.
- Gottlieb DJ, Craig SE, Lorenzi-Filho G, et al. Sleep apnea cardiovascular clinical trials-current status and steps forward: The International Collaboration of Sleep Apnea Cardiovascular Trialists. Sleep 2013; 36:975–980.
- Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep 2006; 29:564–571.
- Phillips BA, McConnell JW, Smith MD. The effects of hypoxemia on cardiac output. A dose-response curve. Chest 1988; 93:471–475.
- Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respire Physiol Neurobiol 2008; 162:144–151.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34:479–485.
- Colrain IM, Black J, Siegel LC, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med 2013; 14:830–837.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370:139–149.
Obstructive sleep apnea (OSA) is common and poorly recognized and, if untreated, leads to serious health consequences. This article discusses the epidemiology of OSA, describes common presenting signs and symptoms, and reviews diagnostic testing and treatment options. Adverse health effects related to untreated sleep apnea are also discussed.
COMMON, POORLY RECOGNIZED, AND COSTLY IF UNTREATED
OSA is very common in the general population and is associated with substantial morbidity and mortality. An estimated 17% of the general adult population has OSA, and the numbers are increasing with the obesity epidemic. Nearly 1 in 15 adults has at least moderate sleep apnea,1,2 and approximately 85% of cases are estimated to be undiagnosed.3 A 1999 study estimated that untreated OSA resulted in approximately $3.4 billion in additional medical costs per year in the United States,4 a figure that is likely to be higher now, given the rising prevalence of OSA. The prevalence of OSA in primary care and subspecialty clinics is even higher than in the community, as more than half of patients who have diabetes or hypertension and 30% to 40% of patients with coronary artery disease are estimated to have OSA.5–7
REPETITIVE UPPER-AIRWAY COLLAPSE
During sleep, parasympathetic activity is enhanced and the muscle tone of the upper airway is decreased, particularly in the pharyngeal dilator muscles. Still, even in the supine position, a healthy person maintains patency of the airway and adequate airflow during sleep.
OSA is characterized by repetitive complete or partial collapse of the upper airway during sleep, resulting in an apneic or hypopneic event, respectively, and often causing snoring from upper-airway tissue vibration.
People who are susceptible to OSA typically have a smaller, more collapsible airway that is often less distensible and has a higher critical closing pressure. Radiographic and physiologic data have shown that the airway dimensions of patients with OSA are smaller than in those without OSA. The shape of the airway of a patient with OSA is often elliptical, given the extrinsic compression of the lateral aspects of the airway by increased size of the parapharyngeal fat pads. OSA episodes are characterized by closure of the upper airway and by progressively increasing respiratory efforts driven by chemoreceptor and mechanoreceptor stimuli, culminating in an arousal from sleep and a reopening of the airway.
The disease-defining metric used for assessing OSA severity is the apnea-hypopnea index, ie, the number of apneas and hypopneas that occur per hour of sleep.8 An apneic or hypopneic event is identified during polysomnography by the complete cessation of airflow or by a reduction in airflow for 10 seconds or longer (Figure 1).

HEALTH CONSEQUENCES IF UNTREATED
Untreated sleep apnea causes numerous pathophysiologic perturbations, including chronic intermittent hypoxia, ventilatory overshoot hyperoxia, increased sympathetic nervous system activity, intrathoracic pressure swings, hypercapnea, sleep fragmentation, increased arousals, reduced sleep duration, and fragmentation of rapid-eye-movement sleep.
Intermittent hypoxia activates the sympathetic nervous system and causes pulmonary vasoconstriction, with increases in pulmonary arterial pressures and myocardial workload. Sympathetic activation, ascertained by peroneal microneurography, has been shown to be increased not only during sleep but also persisting during wakefulness in patients with untreated OSA vs those without OSA.9 Autonomic nervous system fluctuations accompany apneic episodes, resulting in enhanced parasympathetic tone and sympathetic activation associated with a rise in blood pressure and heart rate that occur after the respiratory event.
Intermediate pathways that link the negative pathophysiologic effects of OSA with adverse health outcomes include increased systemic inflammation, increased oxidative stress, metabolic dysfunction, insulin resistance, hypercoagulability, endothelial dysfunction, and autonomic dysfunction.
As a result, a variety of adverse clinical outcomes are associated with untreated OSA, including systemic hypertension, ischemic heart disease and atherosclerosis, diastolic dysfunction, congestive heart failure, cardiac arrhythmias, stroke, increased risk of death, and sudden death, as well as noncardiovascular outcomes such as gout, neurocognitive deficits, and mood disorders.10
Inflammatory and atherogenic effects
Increased levels of markers of systemic inflammation, prothrombosis, and oxidative stress have been observed in OSA and may be key pathophysiologic links between OSA and cardiovascular sequelae. OSA has been associated with up-regulation of a number of inflammatory mediators: interleukin (IL) 6, soluble IL-6 receptor, IL-8, tumor necrosis factor alpha, and C-reactive protein. Soluble IL-6 levels in particular are higher in people who have sleep-disordered breathing, as reflected by the apnea-hypopnea index independent of obesity, with relationships stronger in the morning than in the evening. This likely reflects the overnight OSA-related physiologic stress.11
Thrombotic potential is also enhanced, with higher levels of plasminogen activator inhibitor 1, fibrinogen, P-selectin, and vascular endothelial growth factor. Some of these factors normally have a diurnal cycle, with higher levels in the morning, but in OSA, increasing OSA severity is associated with increased prothrombotic potential in the morning hours. Of interest, levels of these substances showed a plateau effect, rising in people who had only mildly elevated apnea-hypopnea indices and then leveling off.12 Intermittent hypoxia followed by ventilatory overshoot hyperoxia, characteristic of sleep apnea, provides the ideal environment for augmentation of oxidative stress, with evidence of increased oxidation of serum proteins and lipids. Hypoxia and oxygen-derived free radicals may result in cardiac myocyte injury. Experimental data demonstrate that intermittent hypoxia combined with a high-fat diet results in synergistic acceleration of evidence of atherogenic lesions.
Patients with OSA also have evidence of endothelial dysfunction, insulin resistance, and dyslipidemia, all pathways that can facilitate the progression of atherosclerosis in OSA.13–15
Cardiac arrhythmias
In the Sleep Heart Health Study, a multicenter epidemiologic study designed to examine the relationships of OSA and cardiovascular outcomes, those who had moderate to severe OSA had a risk of ventricular and atrial arrhythmias two to four times higher than those without OSA, even after correction for the confounding influences of obesity and underlying cardiovascular risk.14 These findings were corroborated in subsequent work highlighting monotonic dose-response relationships with increasing OSA severity and increased odds of atrial and ventricular arrhythmia in a cohort of about 3,000 older men.11 Additional compelling evidence of a causal relationship is that the risk of discrete arrhythmic events is markedly increased after a respiratory disturbance in sleep.16
In patients who successfully underwent cardioversion for atrial fibrillation, those who had sleep apnea but were not treated with continuous positive airway pressure (CPAP) had a much higher rate of recurrence of atrial fibrillation during the subsequent year than those with CPAP-treated sleep apnea and than controls never diagnosed with sleep apnea. In the untreated patients with sleep apnea, the mean nocturnal fall in oxygen saturation was significantly greater in those who had recurrence of atrial fibrillation than in those who did not, suggesting hypoxia as an important mechanism contributing to atrial fibrillation.17
Since then, several other retrospective studies have shown similar findings after pulmonary vein antrum isolation and ablation in terms of reduction of atrial fibrillation recurrence with CPAP treatment in OSA.18
Walia et al19 described a patient with moderate sleep apnea who underwent a split-night study. During the baseline part of the study, the patient had about 18 ectopic beats per minute. During the second portion of the study while CPAP was applied, progressively fewer ectopic beats occurred as airway pressure was increased until a normal rhythm without ectopic beats was achieved at the goal treatment CPAP pressure setting.
Cardiovascular disease, stroke, and death
Marin et al20 followed about 1,500 men for 10 years, including some who had severe OSA, some with sleep apnea who were treated with CPAP, and controls. The risk of nonfatal and fatal cardiovascular disease events was nearly three times higher in those with severe disease than in healthy participants. Those treated with CPAP had a risk approximately the same as in the control group.
The Sleep Heart Study followed approximately 6,000 people with untreated sleep apnea for a median of nearly 9 years. It found a significant association between the apnea-hypopnea index and ischemic stroke, especially in men.21 Survival in patients with heart failure is also associated with the degree of OSA; patients with more severe disease (an apnea-hypopnea index ≥ 15) have a nearly three times greater risk of death than those with no disease or only mild disease (apnea-hypopnea index < 15).22
From the standpoint of health care utilization, findings that central sleep apnea predicts an increased risk of hospital readmission in heart failure are of particular interest.23
People with OSA are at increased risk of nocturnal sudden cardiac death.24 Sleep apnea is also associated with an increased overall death rate, and the higher the apnea-hypopnea index, the higher the death rate,25 even after adjusting for age, sex, body mass index, and underlying cardiovascular risk, with findings most pronounced in men under age 70.
Motor vehicle accidents
The need for caution during driving should be discussed with every patient, as motor vehicle accidents are an immediate danger to the patient and others. The association with motor vehicle accidents is independent of sleepiness, and drivers with sleep apnea often do not perceive performance impairment. Young et al26 found that men who snored were 3.4 times as likely to have an accident over a 5-year period, and that men and women with an apnea-hypopnea index greater than 15 were more than 7 times as likely to have multiple accidents over a 5-year period, highlighting the importance of discussing, documenting, and expeditiously diagnosing and treating OSA, particularly in those who report drowsiness while driving.
CLINICAL RISK FACTORS
Risk factors can be divided into nonmodifiable and modifiable ones.
Nonmodifiable factors
Age. Bimodal distributions in OSA prevalence have been observed; ie, that the pediatric population and people who are middle-aged have the highest prevalence of OSA. A linear relationship between sleep apnea prevalence and age until about age 65 was identified in data from the Sleep Heart Health Study.27 After that, the prevalence rates plateau; it is unclear if this is secondary to natural remission of the disease after a certain age or because patients with more severe disease have died by that age (ie, survivorship bias), blunting an increase in prevalence.
Sex. Men develop sleep apnea at a rate three to five times that of women. Several explanations have been proposed to account for this.28,29 Sex hormones are one factor; women with sleep apnea on hormone replacement therapy have a significantly less-severe sleep apnea burden than other postmenopausal women,30 suggesting a positive effect from estrogen. Sex-based differences in fat distribution, length and collapsibility of the upper airway, genioglossal activity, neurochemical control mechanisms, and arousal response may also contribute to prevalence differences between men and women.
As with coronary artery disease, the presentation of sleep apnea may be atypical in women, particularly around menopause. Sleep apnea should be considered in women who have snoring and daytime sleepiness.
Race. Whites, African Americans, and Asians have a similar prevalence of sleep apnea, but groups differ in obesity rates and craniofacial anatomy.31–34 Asians tend to have craniofacial skeletal restriction. African Americans are more likely to have upper-airway soft-tissue risk and to develop more severe OSA. Whites tend to have both craniofacial and soft-tissue risk. For those with craniofacial anatomy predisposing to OSA, even mild obesity can make it manifest.
Syndromes that predispose to OSA can include craniofacial structural abnormalities, connective tissue problems, or alterations in ventilatory control (eg, Marfan, Down, and Pierre Robin syndromes).
Modifiable risk factors
Obesity (body mass index ≥ 30 kg/m2) is a firmly established risk factor, but not all obese patients develop obstructive sleep apnea, and not all people with sleep apnea are obese.
Obesity increases risk by altering the geometry and function of the upper airway, increasing collapsibility. The changes are particularly pronounced in the lateral aspects of the pharynx.35
Obesity also affects respiratory drive, likely in part from leptin resistance. Load compensation is another contributing factor: the increased mass in the thorax and abdomen increases the work of breathing and reduces functional residual capacity, increasing oxygen demands and leading to atelectasis and ventilation-perfusion mismatch.
Although obesity is an important risk factor, it is important to recognize that obesity is not the only one to consider: most people with an apnea-hypopnea index of 5 or greater are not obese. The relationship between body mass index and sleep apnea is weaker in children and in the elderly, probably because other risk factors are more pronounced.36
Craniofacial structural abnormalities such as retrognathia (abnormal posterior position of the mandible) and micrognathia (undersized mandible) can increase the risk of OSA because of a resulting posteriorly displaced genioglossus muscle. Other conditions can alter chemosensitivity, affecting the pH and carbon dioxide level of the blood and therefore affecting ventilatory control mechanisms, making the person more prone to developing sleep apnea. Children and young adults may have tonsillar tissue that obstructs the airway.
The site of obstruction can be behind the palate (retropalatal), behind the tongue (retroglossal), or below the pharynx (hypopharyngeal). This helps explain why positive air way pressure—unlike surgery, which addresses a specific area—is often successful, as it serves to splint or treat all aspects of the airway.
FATIGUE, SLEEPINESS, SNORING, RESTLESS SLEEP
Sleep apnea can result in presentation of multiple signs and symptoms (Table 1).
Daytime sleepiness and fatigue are the most common symptoms. Although nonspecific, they are often quite pronounced. Two short questionnaires—the Epworth Sleepiness Scale37 and the Fatigue Severity Scale—can help distinguish between these two symptoms and assess their impact on a patient’s daily life. In the Epworth Sleepiness Scale, the patient rates his or her chance of dozing on a 4-point scale (0 = would never doze, to 3 = high chance of dozing) in eight situations:
- Sitting and reading
- Watching television
- Sitting inactive in a public place
- As a passenger in a car for an hour without a break
- Lying down to rest in the afternoon
- Sitting and talking to someone
- Sitting quietly after a lunch without alcohol
- In a car while stopped for a few minutes in traffic.
A score of 10 or more is consistent with significant subjective sleepiness.
The Fatigue Severity Scale assesses the impact of fatigue on daily living.
Snoring is a common and specific symptom of sleep apnea; however, not all patients who snore have OSA.
Restlessness during sleep is very common—patients may disturb their bed partner by moving around a lot during sleep or report that the sheets are “all over the place” by morning.
Nocturia can also be a sign of sleep apnea and can contribute to sleep fragmentation. A proposed mechanism of this symptom includes alterations of intrathoracic pressure resulting in atrial stretch, which release atrial natriuretic peptide, leading to nocturia. Treating with CPAP has been found to reduce levels of atrial natriuretic peptide, contributing to better sleep.38
Morning headache may occur and is likely related to increased CO2 levels, which appear to culminate in the morning hours. End-tidal or transcutaneous CO2 monitoring during polysomnography can help elucidate the presence of sleep-related hypoventilation.
Libido is often diminished and can actually be improved with CPAP. This is therefore an important point to discuss with patients, as improved libido can often serve as an incentive for adherence to OSA treatment.
Insomnia exists in about 15% of patients, primarily as a result of sleep apnea-related with treatment.
Sweating, particularly forehead sweating associated with sleep apnea, more commonly occurs in children.
The STOP-BANG questionnaire (Table 2)39 was primarily validated in preoperative anesthesia testing. However, because of its ease of use and favorable performance characteristics, it is increasingly used to predict the likelihood of finding OSA before polysomnography. A score of 3 or more has a sensitivity of 93%.
PHYSICAL EXAMINATION PROVIDES CLUES
Although the physical examination may be normal, certain findings indicate risk (Table 3). Obesity alone is not an accepted indication for polysomnography unless there are concomitant worrisome signs or symptoms. Of note, those who are morbidly obese (BMI > 40 kg/m2) have a prevalence of sleep apnea greater than 70%.
The classification by Friedman et al40 provides an indicator of risk. The patient is examined with the mouth opened wide and the tongue in a neutral natural position. Grades:
- I—Entire uvula and tonsils are visible
- II—Entire uvula is visible, but tonsils are not
- III—Soft palate is visible, but uvula is not
- IV—Only the hard palate is visible.
Especially in children and young adults, enlarged tonsils (or “kissing tonsils”) and a boggy edematous uvula set the stage for obstructive sleep apnea.
DIAGNOSIS REQUIRES SLEEP TESTING
A sleep study is the primary means of diagnosing OSA. Polysomnography includes electrooculography to determine when rapid-eye-movement sleep occurs; electromyography to measure muscle activity in the chin to help determine onset of sleep, with peripheral leads in the leg to measure leg movements; electroencephalography (EEG) to measure neural activity; electrocardiography; pulse oximetry to measure oxygen saturation; measurement of oronasal flow; and measurements of chest wall effort and body position using thoracic and abdominal belts that expand and contract with breathing; and audio recording to detect snoring.
Attended polysomnography requires the constant presence of a trained sleep technologist to monitor for technical issues and patient adherence.
End-tidal CO2 monitoring is a reasonable method to detect sleep-related hypoventilation but is not routinely performed in the United States. Transcutaneous CO2 monitoring is a different way to monitor CO2 used in the setting of positive airway pressure.
Polysomnography in a normal patient shows a regular pattern of increasing and decreasing airflow with inspiration and expiration while stable oxygen saturation is maintained.
In contrast, polysomnography of a patient with sleep apnea shows repetitive periods of no airflow, oxygen desaturation, and often evidence of thoracoabdominal paradox, punctuated by arousals on EEG associated with sympathetic activation (Figure 1). When the patient falls asleep, upper-airway muscle tone is reduced, causing an apneic event with hypoxia and pleural pressure swings. These prompt arousals with sympathetic activation that reestablish upper-airway muscle tone, allowing ventilation and reoxygenation to resume with a return to sleep.
Apnea-hypopnea index indicates severity
Sleep apnea severity is graded using the apnea-hypopnea index, ie, the number of apneic and hypopneic events per hour of sleep (Table 4).41 Events must last at least 10 seconds to be considered, ie, two consecutive missed breaths based on an average normal respiratory rate of about 12 breaths per minute for the typical adult.
The apnea-hypopnea index usually correlates with the severity of oxygen desaturation and with electrocardiographic abnormalities, including tachybradycardia and arrhythmias.
Although history, physical examination, and prediction tools are helpful in determining the likelihood that a patient has OSA, only polysomnography testing can establish the diagnosis. To diagnose OSA, 15 or more obstructive events per hour must be observed by polysomnography, or at least 5 events per hour with one of the following:
- Daytime sleepiness, sleep attacks, unrefreshing sleep, fatigue, or insomnia
- Waking with breath-holding, gasping, or choking
- Observer-reported loud snoring or breathing interruptions.41
Split-night study determines diagnosis and optimum treatment
The split-night study has two parts: the first is diagnostic polysomnography, followed by identification of the positive airway pressure that optimally treats the sleep apnea. The apnea-hypopnea index guides the need for the split-night study, with 40 being the established threshold according to the American Academy of Sleep Medicine.
A home sleep study is appropriate for some patients
Home sleep testing is typically more limited than standard polysomnography; it monitors airflow, effort, and oxygenation. The test is intended for adults with a high pretest probability of moderate to severe obstructive sleep apnea (STOP-BANG score ≥ 3). It is not intended for screening of asymptomatic patients or for those with coexisting sleep disorders (eg, central sleep apnea, sleep hypoventilation, periodic limb movements, insomnia, circadian rhythm disorders, parasomnias, narcolepsy) or medical disorders (eg, moderate to severe heart failure or other cardiac disease, symptomatic neurologic disease, moderate to severe pulmonary disease).42 Since March 2008, the Centers for Medicare and Medicaid Services has covered CPAP for obstructive sleep apnea based on diagnosis by home sleep study testing.43
TREATMENT OF SLEEP APNEA
Basic steps for reducing OSA are:
Weight loss. Even small weight changes can significantly affect the severity of sleep apnea, perhaps even leading to a reassessment of the degree of OSA and CPAP requirements. Longitudinal epidemiologic data demonstrate that a 10% weight loss correlates with a 26% reduction in the apnea-hypopnea index, and conversely, a 10% weight gain is associated with a 32% increase.44
Some studies have found that bariatric surgery cures OSA in 75% to 88% of cases, independent of approach.45,46 However, a trial in 60 obese patients with OSA who were randomized to either a low-calorie diet or bariatric surgery found no statistical difference in the apnea-hypopnea index between the two groups despite greater weight loss in the surgery group.47
Avoiding certain medications. Benzodiazepines, narcotics, and alcohol reduce upper airway muscle tone and should be avoided. No medications are associated with improvement of OSA, although acetazolamide may be used to treat central sleep apnea.
Positional therapy. Sleeping on the back exacerbates the problem. Supine-related OSA occurs as a result of several factors, including gravity, airway anatomy, airway critical closing pressures, and effects on upper-airway dilator muscle function.
Sleep hygiene. General recommendations to engage in behaviors to promote sleep are recommended, including keeping consistent sleep-wake times, not watching television in bed, and avoidance of caffeine intake, particularly within 4 to 6 hours of bedtime.
POSITIVE AIRWAY PRESSURE THERAPY
Nasal CPAP is the treatment of choice and is successful in 95% of patients when used consistently. It is not as costly as surgery, and results in improved long-term survival compared with uvulopalatopharyngoplasty. Another advantage is that the pressure can be retitrated as the patient’s condition changes, for example after a weight change or during pregnancy.
More than 15 randomized controlled trials have examined the effect of sleep apnea treatment with CPAP compared with either sham CPAP or another control. In a meta-analysis, CPAP was found to lead to an average systolic blood pressure reduction of about 2.5 mm Hg and a diastolic blood pressure reduction of 1.8 mm Hg. Although these reductions may seem negligible, benefits may be significant for cardiovascular outcomes.48,49
Challenges to treatment adherence
Adherence is the most commonly discussed problem with CPAP, but long-term adherence rates are comparable to medication compliance—about 60% to 70%. To optimize adherence, communication is important to ensure that problems are identified and addressed as they arise. Showing patients examples of apneic events and oxygen desaturation from their sleep study can enhance their understanding of OSA and its importance. Patients need to understand the serious nature of the disease and that CPAP therapy can significantly improve their quality of life and overall health, particularly from a cardiovascular perspective.
CPAP masks can be uncomfortable, posing a major barrier to compliance. But a number of mask designs are available, such as the nasal mask, the nasal pillow mask, and the oronasal mask. For patients with claustrophobia, the nasal pillow mask is an option, as it does not cover the face.
Some patients note symptoms of nasal congestion, although in many patients CPAP improves it. If congestion is a problem, the use of heated humidification with the machine, intranasal saline or gel, or nasal corticosteroids can help relieve it.
Pressure intolerance is a common problem. For those who feel that the pressure is too high, settings can be adjusted so that the pressure is gradually reduced between inspiration and expiration, ie, the use of expiratory pressure relief or consideration of the use of bilevel positive airway pressure.
Aerophagia (swallowing air) is a less common problem. It can also potentially be relieved with use of bilevel positive airway pressure.
Many patients develop skin irritation, which can be helped with moleskin, available at any pharmacy.
Social stigma can be a problem. Education regarding the importance of the treatment to health is essential.
Machine noise is less of a problem with the new machine models, but if it is a problem, a white-noise device or earplugs may help.
Other measures to improve compliance are keeping the regimen simple and ensuring that family support is adequate.
Medicare requires evidence of use and benefit
Medicare requires that clinical benefit be documented between the 31st and 91st day after initiating CPAP therapy. This requires face-to-face clinical reevaluation by the treating physician to document improved symptoms and objective evidence of adherence to use of the device. The devices can store usage patterns, and Medicare requires at least 4 hours per night on 70% of nights during a consecutive 30-day period in the first 3 months of use.
ALTERNATIVE THERAPIES
Alternative therapies may be options for some patients, in particular those who cannot use CPAP or who get no benefit from it. These include oral appliances for those with mild to moderate OSA50–53 and various surgical procedures, eg, uvulopalatopharyngoplasty,54,55 maxillomanibular advancement,56 tracheostomy (standard treatment before CPAP was identified as an effective treatment),57,58 and adenotonsillectomy (in children).59
Supplemental oxygen is not a first-line treatment for OSA and in general has not been found to be very effective, particularly in terms of intermediate cardiovascular outcomes,60–62 although a subset of patients with high loop gain may benefit from it.63 Loop gain is a measure of the tendency of the ventilatory control system to amplify respiration in response to a change, conferring less stable control of breathing.
Several novel alternative therapies are starting to be used. Although all of them have been shown to improve measures of OSA, none is as effective as CPAP in improving OSA severity. New therapies include the nasal expiratory positive airway pressure device,64 oral pressure therapy,65 and hypoglossal nerve stimulation.66
Obstructive sleep apnea (OSA) is common and poorly recognized and, if untreated, leads to serious health consequences. This article discusses the epidemiology of OSA, describes common presenting signs and symptoms, and reviews diagnostic testing and treatment options. Adverse health effects related to untreated sleep apnea are also discussed.
COMMON, POORLY RECOGNIZED, AND COSTLY IF UNTREATED
OSA is very common in the general population and is associated with substantial morbidity and mortality. An estimated 17% of the general adult population has OSA, and the numbers are increasing with the obesity epidemic. Nearly 1 in 15 adults has at least moderate sleep apnea,1,2 and approximately 85% of cases are estimated to be undiagnosed.3 A 1999 study estimated that untreated OSA resulted in approximately $3.4 billion in additional medical costs per year in the United States,4 a figure that is likely to be higher now, given the rising prevalence of OSA. The prevalence of OSA in primary care and subspecialty clinics is even higher than in the community, as more than half of patients who have diabetes or hypertension and 30% to 40% of patients with coronary artery disease are estimated to have OSA.5–7
REPETITIVE UPPER-AIRWAY COLLAPSE
During sleep, parasympathetic activity is enhanced and the muscle tone of the upper airway is decreased, particularly in the pharyngeal dilator muscles. Still, even in the supine position, a healthy person maintains patency of the airway and adequate airflow during sleep.
OSA is characterized by repetitive complete or partial collapse of the upper airway during sleep, resulting in an apneic or hypopneic event, respectively, and often causing snoring from upper-airway tissue vibration.
People who are susceptible to OSA typically have a smaller, more collapsible airway that is often less distensible and has a higher critical closing pressure. Radiographic and physiologic data have shown that the airway dimensions of patients with OSA are smaller than in those without OSA. The shape of the airway of a patient with OSA is often elliptical, given the extrinsic compression of the lateral aspects of the airway by increased size of the parapharyngeal fat pads. OSA episodes are characterized by closure of the upper airway and by progressively increasing respiratory efforts driven by chemoreceptor and mechanoreceptor stimuli, culminating in an arousal from sleep and a reopening of the airway.
The disease-defining metric used for assessing OSA severity is the apnea-hypopnea index, ie, the number of apneas and hypopneas that occur per hour of sleep.8 An apneic or hypopneic event is identified during polysomnography by the complete cessation of airflow or by a reduction in airflow for 10 seconds or longer (Figure 1).

HEALTH CONSEQUENCES IF UNTREATED
Untreated sleep apnea causes numerous pathophysiologic perturbations, including chronic intermittent hypoxia, ventilatory overshoot hyperoxia, increased sympathetic nervous system activity, intrathoracic pressure swings, hypercapnea, sleep fragmentation, increased arousals, reduced sleep duration, and fragmentation of rapid-eye-movement sleep.
Intermittent hypoxia activates the sympathetic nervous system and causes pulmonary vasoconstriction, with increases in pulmonary arterial pressures and myocardial workload. Sympathetic activation, ascertained by peroneal microneurography, has been shown to be increased not only during sleep but also persisting during wakefulness in patients with untreated OSA vs those without OSA.9 Autonomic nervous system fluctuations accompany apneic episodes, resulting in enhanced parasympathetic tone and sympathetic activation associated with a rise in blood pressure and heart rate that occur after the respiratory event.
Intermediate pathways that link the negative pathophysiologic effects of OSA with adverse health outcomes include increased systemic inflammation, increased oxidative stress, metabolic dysfunction, insulin resistance, hypercoagulability, endothelial dysfunction, and autonomic dysfunction.
As a result, a variety of adverse clinical outcomes are associated with untreated OSA, including systemic hypertension, ischemic heart disease and atherosclerosis, diastolic dysfunction, congestive heart failure, cardiac arrhythmias, stroke, increased risk of death, and sudden death, as well as noncardiovascular outcomes such as gout, neurocognitive deficits, and mood disorders.10
Inflammatory and atherogenic effects
Increased levels of markers of systemic inflammation, prothrombosis, and oxidative stress have been observed in OSA and may be key pathophysiologic links between OSA and cardiovascular sequelae. OSA has been associated with up-regulation of a number of inflammatory mediators: interleukin (IL) 6, soluble IL-6 receptor, IL-8, tumor necrosis factor alpha, and C-reactive protein. Soluble IL-6 levels in particular are higher in people who have sleep-disordered breathing, as reflected by the apnea-hypopnea index independent of obesity, with relationships stronger in the morning than in the evening. This likely reflects the overnight OSA-related physiologic stress.11
Thrombotic potential is also enhanced, with higher levels of plasminogen activator inhibitor 1, fibrinogen, P-selectin, and vascular endothelial growth factor. Some of these factors normally have a diurnal cycle, with higher levels in the morning, but in OSA, increasing OSA severity is associated with increased prothrombotic potential in the morning hours. Of interest, levels of these substances showed a plateau effect, rising in people who had only mildly elevated apnea-hypopnea indices and then leveling off.12 Intermittent hypoxia followed by ventilatory overshoot hyperoxia, characteristic of sleep apnea, provides the ideal environment for augmentation of oxidative stress, with evidence of increased oxidation of serum proteins and lipids. Hypoxia and oxygen-derived free radicals may result in cardiac myocyte injury. Experimental data demonstrate that intermittent hypoxia combined with a high-fat diet results in synergistic acceleration of evidence of atherogenic lesions.
Patients with OSA also have evidence of endothelial dysfunction, insulin resistance, and dyslipidemia, all pathways that can facilitate the progression of atherosclerosis in OSA.13–15
Cardiac arrhythmias
In the Sleep Heart Health Study, a multicenter epidemiologic study designed to examine the relationships of OSA and cardiovascular outcomes, those who had moderate to severe OSA had a risk of ventricular and atrial arrhythmias two to four times higher than those without OSA, even after correction for the confounding influences of obesity and underlying cardiovascular risk.14 These findings were corroborated in subsequent work highlighting monotonic dose-response relationships with increasing OSA severity and increased odds of atrial and ventricular arrhythmia in a cohort of about 3,000 older men.11 Additional compelling evidence of a causal relationship is that the risk of discrete arrhythmic events is markedly increased after a respiratory disturbance in sleep.16
In patients who successfully underwent cardioversion for atrial fibrillation, those who had sleep apnea but were not treated with continuous positive airway pressure (CPAP) had a much higher rate of recurrence of atrial fibrillation during the subsequent year than those with CPAP-treated sleep apnea and than controls never diagnosed with sleep apnea. In the untreated patients with sleep apnea, the mean nocturnal fall in oxygen saturation was significantly greater in those who had recurrence of atrial fibrillation than in those who did not, suggesting hypoxia as an important mechanism contributing to atrial fibrillation.17
Since then, several other retrospective studies have shown similar findings after pulmonary vein antrum isolation and ablation in terms of reduction of atrial fibrillation recurrence with CPAP treatment in OSA.18
Walia et al19 described a patient with moderate sleep apnea who underwent a split-night study. During the baseline part of the study, the patient had about 18 ectopic beats per minute. During the second portion of the study while CPAP was applied, progressively fewer ectopic beats occurred as airway pressure was increased until a normal rhythm without ectopic beats was achieved at the goal treatment CPAP pressure setting.
Cardiovascular disease, stroke, and death
Marin et al20 followed about 1,500 men for 10 years, including some who had severe OSA, some with sleep apnea who were treated with CPAP, and controls. The risk of nonfatal and fatal cardiovascular disease events was nearly three times higher in those with severe disease than in healthy participants. Those treated with CPAP had a risk approximately the same as in the control group.
The Sleep Heart Study followed approximately 6,000 people with untreated sleep apnea for a median of nearly 9 years. It found a significant association between the apnea-hypopnea index and ischemic stroke, especially in men.21 Survival in patients with heart failure is also associated with the degree of OSA; patients with more severe disease (an apnea-hypopnea index ≥ 15) have a nearly three times greater risk of death than those with no disease or only mild disease (apnea-hypopnea index < 15).22
From the standpoint of health care utilization, findings that central sleep apnea predicts an increased risk of hospital readmission in heart failure are of particular interest.23
People with OSA are at increased risk of nocturnal sudden cardiac death.24 Sleep apnea is also associated with an increased overall death rate, and the higher the apnea-hypopnea index, the higher the death rate,25 even after adjusting for age, sex, body mass index, and underlying cardiovascular risk, with findings most pronounced in men under age 70.
Motor vehicle accidents
The need for caution during driving should be discussed with every patient, as motor vehicle accidents are an immediate danger to the patient and others. The association with motor vehicle accidents is independent of sleepiness, and drivers with sleep apnea often do not perceive performance impairment. Young et al26 found that men who snored were 3.4 times as likely to have an accident over a 5-year period, and that men and women with an apnea-hypopnea index greater than 15 were more than 7 times as likely to have multiple accidents over a 5-year period, highlighting the importance of discussing, documenting, and expeditiously diagnosing and treating OSA, particularly in those who report drowsiness while driving.
CLINICAL RISK FACTORS
Risk factors can be divided into nonmodifiable and modifiable ones.
Nonmodifiable factors
Age. Bimodal distributions in OSA prevalence have been observed; ie, that the pediatric population and people who are middle-aged have the highest prevalence of OSA. A linear relationship between sleep apnea prevalence and age until about age 65 was identified in data from the Sleep Heart Health Study.27 After that, the prevalence rates plateau; it is unclear if this is secondary to natural remission of the disease after a certain age or because patients with more severe disease have died by that age (ie, survivorship bias), blunting an increase in prevalence.
Sex. Men develop sleep apnea at a rate three to five times that of women. Several explanations have been proposed to account for this.28,29 Sex hormones are one factor; women with sleep apnea on hormone replacement therapy have a significantly less-severe sleep apnea burden than other postmenopausal women,30 suggesting a positive effect from estrogen. Sex-based differences in fat distribution, length and collapsibility of the upper airway, genioglossal activity, neurochemical control mechanisms, and arousal response may also contribute to prevalence differences between men and women.
As with coronary artery disease, the presentation of sleep apnea may be atypical in women, particularly around menopause. Sleep apnea should be considered in women who have snoring and daytime sleepiness.
Race. Whites, African Americans, and Asians have a similar prevalence of sleep apnea, but groups differ in obesity rates and craniofacial anatomy.31–34 Asians tend to have craniofacial skeletal restriction. African Americans are more likely to have upper-airway soft-tissue risk and to develop more severe OSA. Whites tend to have both craniofacial and soft-tissue risk. For those with craniofacial anatomy predisposing to OSA, even mild obesity can make it manifest.
Syndromes that predispose to OSA can include craniofacial structural abnormalities, connective tissue problems, or alterations in ventilatory control (eg, Marfan, Down, and Pierre Robin syndromes).
Modifiable risk factors
Obesity (body mass index ≥ 30 kg/m2) is a firmly established risk factor, but not all obese patients develop obstructive sleep apnea, and not all people with sleep apnea are obese.
Obesity increases risk by altering the geometry and function of the upper airway, increasing collapsibility. The changes are particularly pronounced in the lateral aspects of the pharynx.35
Obesity also affects respiratory drive, likely in part from leptin resistance. Load compensation is another contributing factor: the increased mass in the thorax and abdomen increases the work of breathing and reduces functional residual capacity, increasing oxygen demands and leading to atelectasis and ventilation-perfusion mismatch.
Although obesity is an important risk factor, it is important to recognize that obesity is not the only one to consider: most people with an apnea-hypopnea index of 5 or greater are not obese. The relationship between body mass index and sleep apnea is weaker in children and in the elderly, probably because other risk factors are more pronounced.36
Craniofacial structural abnormalities such as retrognathia (abnormal posterior position of the mandible) and micrognathia (undersized mandible) can increase the risk of OSA because of a resulting posteriorly displaced genioglossus muscle. Other conditions can alter chemosensitivity, affecting the pH and carbon dioxide level of the blood and therefore affecting ventilatory control mechanisms, making the person more prone to developing sleep apnea. Children and young adults may have tonsillar tissue that obstructs the airway.
The site of obstruction can be behind the palate (retropalatal), behind the tongue (retroglossal), or below the pharynx (hypopharyngeal). This helps explain why positive air way pressure—unlike surgery, which addresses a specific area—is often successful, as it serves to splint or treat all aspects of the airway.
FATIGUE, SLEEPINESS, SNORING, RESTLESS SLEEP
Sleep apnea can result in presentation of multiple signs and symptoms (Table 1).
Daytime sleepiness and fatigue are the most common symptoms. Although nonspecific, they are often quite pronounced. Two short questionnaires—the Epworth Sleepiness Scale37 and the Fatigue Severity Scale—can help distinguish between these two symptoms and assess their impact on a patient’s daily life. In the Epworth Sleepiness Scale, the patient rates his or her chance of dozing on a 4-point scale (0 = would never doze, to 3 = high chance of dozing) in eight situations:
- Sitting and reading
- Watching television
- Sitting inactive in a public place
- As a passenger in a car for an hour without a break
- Lying down to rest in the afternoon
- Sitting and talking to someone
- Sitting quietly after a lunch without alcohol
- In a car while stopped for a few minutes in traffic.
A score of 10 or more is consistent with significant subjective sleepiness.
The Fatigue Severity Scale assesses the impact of fatigue on daily living.
Snoring is a common and specific symptom of sleep apnea; however, not all patients who snore have OSA.
Restlessness during sleep is very common—patients may disturb their bed partner by moving around a lot during sleep or report that the sheets are “all over the place” by morning.
Nocturia can also be a sign of sleep apnea and can contribute to sleep fragmentation. A proposed mechanism of this symptom includes alterations of intrathoracic pressure resulting in atrial stretch, which release atrial natriuretic peptide, leading to nocturia. Treating with CPAP has been found to reduce levels of atrial natriuretic peptide, contributing to better sleep.38
Morning headache may occur and is likely related to increased CO2 levels, which appear to culminate in the morning hours. End-tidal or transcutaneous CO2 monitoring during polysomnography can help elucidate the presence of sleep-related hypoventilation.
Libido is often diminished and can actually be improved with CPAP. This is therefore an important point to discuss with patients, as improved libido can often serve as an incentive for adherence to OSA treatment.
Insomnia exists in about 15% of patients, primarily as a result of sleep apnea-related with treatment.
Sweating, particularly forehead sweating associated with sleep apnea, more commonly occurs in children.
The STOP-BANG questionnaire (Table 2)39 was primarily validated in preoperative anesthesia testing. However, because of its ease of use and favorable performance characteristics, it is increasingly used to predict the likelihood of finding OSA before polysomnography. A score of 3 or more has a sensitivity of 93%.
PHYSICAL EXAMINATION PROVIDES CLUES
Although the physical examination may be normal, certain findings indicate risk (Table 3). Obesity alone is not an accepted indication for polysomnography unless there are concomitant worrisome signs or symptoms. Of note, those who are morbidly obese (BMI > 40 kg/m2) have a prevalence of sleep apnea greater than 70%.
The classification by Friedman et al40 provides an indicator of risk. The patient is examined with the mouth opened wide and the tongue in a neutral natural position. Grades:
- I—Entire uvula and tonsils are visible
- II—Entire uvula is visible, but tonsils are not
- III—Soft palate is visible, but uvula is not
- IV—Only the hard palate is visible.
Especially in children and young adults, enlarged tonsils (or “kissing tonsils”) and a boggy edematous uvula set the stage for obstructive sleep apnea.
DIAGNOSIS REQUIRES SLEEP TESTING
A sleep study is the primary means of diagnosing OSA. Polysomnography includes electrooculography to determine when rapid-eye-movement sleep occurs; electromyography to measure muscle activity in the chin to help determine onset of sleep, with peripheral leads in the leg to measure leg movements; electroencephalography (EEG) to measure neural activity; electrocardiography; pulse oximetry to measure oxygen saturation; measurement of oronasal flow; and measurements of chest wall effort and body position using thoracic and abdominal belts that expand and contract with breathing; and audio recording to detect snoring.
Attended polysomnography requires the constant presence of a trained sleep technologist to monitor for technical issues and patient adherence.
End-tidal CO2 monitoring is a reasonable method to detect sleep-related hypoventilation but is not routinely performed in the United States. Transcutaneous CO2 monitoring is a different way to monitor CO2 used in the setting of positive airway pressure.
Polysomnography in a normal patient shows a regular pattern of increasing and decreasing airflow with inspiration and expiration while stable oxygen saturation is maintained.
In contrast, polysomnography of a patient with sleep apnea shows repetitive periods of no airflow, oxygen desaturation, and often evidence of thoracoabdominal paradox, punctuated by arousals on EEG associated with sympathetic activation (Figure 1). When the patient falls asleep, upper-airway muscle tone is reduced, causing an apneic event with hypoxia and pleural pressure swings. These prompt arousals with sympathetic activation that reestablish upper-airway muscle tone, allowing ventilation and reoxygenation to resume with a return to sleep.
Apnea-hypopnea index indicates severity
Sleep apnea severity is graded using the apnea-hypopnea index, ie, the number of apneic and hypopneic events per hour of sleep (Table 4).41 Events must last at least 10 seconds to be considered, ie, two consecutive missed breaths based on an average normal respiratory rate of about 12 breaths per minute for the typical adult.
The apnea-hypopnea index usually correlates with the severity of oxygen desaturation and with electrocardiographic abnormalities, including tachybradycardia and arrhythmias.
Although history, physical examination, and prediction tools are helpful in determining the likelihood that a patient has OSA, only polysomnography testing can establish the diagnosis. To diagnose OSA, 15 or more obstructive events per hour must be observed by polysomnography, or at least 5 events per hour with one of the following:
- Daytime sleepiness, sleep attacks, unrefreshing sleep, fatigue, or insomnia
- Waking with breath-holding, gasping, or choking
- Observer-reported loud snoring or breathing interruptions.41
Split-night study determines diagnosis and optimum treatment
The split-night study has two parts: the first is diagnostic polysomnography, followed by identification of the positive airway pressure that optimally treats the sleep apnea. The apnea-hypopnea index guides the need for the split-night study, with 40 being the established threshold according to the American Academy of Sleep Medicine.
A home sleep study is appropriate for some patients
Home sleep testing is typically more limited than standard polysomnography; it monitors airflow, effort, and oxygenation. The test is intended for adults with a high pretest probability of moderate to severe obstructive sleep apnea (STOP-BANG score ≥ 3). It is not intended for screening of asymptomatic patients or for those with coexisting sleep disorders (eg, central sleep apnea, sleep hypoventilation, periodic limb movements, insomnia, circadian rhythm disorders, parasomnias, narcolepsy) or medical disorders (eg, moderate to severe heart failure or other cardiac disease, symptomatic neurologic disease, moderate to severe pulmonary disease).42 Since March 2008, the Centers for Medicare and Medicaid Services has covered CPAP for obstructive sleep apnea based on diagnosis by home sleep study testing.43
TREATMENT OF SLEEP APNEA
Basic steps for reducing OSA are:
Weight loss. Even small weight changes can significantly affect the severity of sleep apnea, perhaps even leading to a reassessment of the degree of OSA and CPAP requirements. Longitudinal epidemiologic data demonstrate that a 10% weight loss correlates with a 26% reduction in the apnea-hypopnea index, and conversely, a 10% weight gain is associated with a 32% increase.44
Some studies have found that bariatric surgery cures OSA in 75% to 88% of cases, independent of approach.45,46 However, a trial in 60 obese patients with OSA who were randomized to either a low-calorie diet or bariatric surgery found no statistical difference in the apnea-hypopnea index between the two groups despite greater weight loss in the surgery group.47
Avoiding certain medications. Benzodiazepines, narcotics, and alcohol reduce upper airway muscle tone and should be avoided. No medications are associated with improvement of OSA, although acetazolamide may be used to treat central sleep apnea.
Positional therapy. Sleeping on the back exacerbates the problem. Supine-related OSA occurs as a result of several factors, including gravity, airway anatomy, airway critical closing pressures, and effects on upper-airway dilator muscle function.
Sleep hygiene. General recommendations to engage in behaviors to promote sleep are recommended, including keeping consistent sleep-wake times, not watching television in bed, and avoidance of caffeine intake, particularly within 4 to 6 hours of bedtime.
POSITIVE AIRWAY PRESSURE THERAPY
Nasal CPAP is the treatment of choice and is successful in 95% of patients when used consistently. It is not as costly as surgery, and results in improved long-term survival compared with uvulopalatopharyngoplasty. Another advantage is that the pressure can be retitrated as the patient’s condition changes, for example after a weight change or during pregnancy.
More than 15 randomized controlled trials have examined the effect of sleep apnea treatment with CPAP compared with either sham CPAP or another control. In a meta-analysis, CPAP was found to lead to an average systolic blood pressure reduction of about 2.5 mm Hg and a diastolic blood pressure reduction of 1.8 mm Hg. Although these reductions may seem negligible, benefits may be significant for cardiovascular outcomes.48,49
Challenges to treatment adherence
Adherence is the most commonly discussed problem with CPAP, but long-term adherence rates are comparable to medication compliance—about 60% to 70%. To optimize adherence, communication is important to ensure that problems are identified and addressed as they arise. Showing patients examples of apneic events and oxygen desaturation from their sleep study can enhance their understanding of OSA and its importance. Patients need to understand the serious nature of the disease and that CPAP therapy can significantly improve their quality of life and overall health, particularly from a cardiovascular perspective.
CPAP masks can be uncomfortable, posing a major barrier to compliance. But a number of mask designs are available, such as the nasal mask, the nasal pillow mask, and the oronasal mask. For patients with claustrophobia, the nasal pillow mask is an option, as it does not cover the face.
Some patients note symptoms of nasal congestion, although in many patients CPAP improves it. If congestion is a problem, the use of heated humidification with the machine, intranasal saline or gel, or nasal corticosteroids can help relieve it.
Pressure intolerance is a common problem. For those who feel that the pressure is too high, settings can be adjusted so that the pressure is gradually reduced between inspiration and expiration, ie, the use of expiratory pressure relief or consideration of the use of bilevel positive airway pressure.
Aerophagia (swallowing air) is a less common problem. It can also potentially be relieved with use of bilevel positive airway pressure.
Many patients develop skin irritation, which can be helped with moleskin, available at any pharmacy.
Social stigma can be a problem. Education regarding the importance of the treatment to health is essential.
Machine noise is less of a problem with the new machine models, but if it is a problem, a white-noise device or earplugs may help.
Other measures to improve compliance are keeping the regimen simple and ensuring that family support is adequate.
Medicare requires evidence of use and benefit
Medicare requires that clinical benefit be documented between the 31st and 91st day after initiating CPAP therapy. This requires face-to-face clinical reevaluation by the treating physician to document improved symptoms and objective evidence of adherence to use of the device. The devices can store usage patterns, and Medicare requires at least 4 hours per night on 70% of nights during a consecutive 30-day period in the first 3 months of use.
ALTERNATIVE THERAPIES
Alternative therapies may be options for some patients, in particular those who cannot use CPAP or who get no benefit from it. These include oral appliances for those with mild to moderate OSA50–53 and various surgical procedures, eg, uvulopalatopharyngoplasty,54,55 maxillomanibular advancement,56 tracheostomy (standard treatment before CPAP was identified as an effective treatment),57,58 and adenotonsillectomy (in children).59
Supplemental oxygen is not a first-line treatment for OSA and in general has not been found to be very effective, particularly in terms of intermediate cardiovascular outcomes,60–62 although a subset of patients with high loop gain may benefit from it.63 Loop gain is a measure of the tendency of the ventilatory control system to amplify respiration in response to a change, conferring less stable control of breathing.
Several novel alternative therapies are starting to be used. Although all of them have been shown to improve measures of OSA, none is as effective as CPAP in improving OSA severity. New therapies include the nasal expiratory positive airway pressure device,64 oral pressure therapy,65 and hypoglossal nerve stimulation.66
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA; Sleep Heart Health Research Group. The relationship between chronically disrupted sleep and healthcare use. Sleep 2002; 25:289–296.
- Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep 1999; 22:749–755.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109:659–663.
- Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92:79–84.
- Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164:2147–2165.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, Second Edition: Diagnostic and coding manual. Westchester, IL; American Academy of Sleep Medicine, 2005.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Mehra R. Sleep-disordered breathing and cardiovascular disease: exploring pathophysiology and existing data. Curr Resp Med Rev 2007; 3:258–269.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166:1725–1731.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Mehra R, Storfer-Isser A, Tracy R, Jenny N, Redline S. Association of sleep disordered breathing and oxidized LDL [abstract]. Am J Respir Crit Care Med 2010; 181:A2474.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54:1797–1804.
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107:2589–2594.
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3:445–451.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7:397–398.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010; 182:269–277.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18:534–540.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352:1206–1214.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6( 8):e1000132. doi: 10.1371/journal.pmed.1000132.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20:608–613.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165:1217–1239.
- Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 2008; 12:481–496.
- Shaher E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167:1186–1192.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167:1181–1185.
- Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med 1995; 152:1946–1949.
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162:893–900.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155:186–192. Erratum in: Am J Respir Crit Care Med 1997; 155:1820.
- Sutherland K, Lee RWW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology 2012; 17:213–222.
- Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 1995; 152:1673–1689.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14:540–545.
- Krieger J, Imbs J-L, Schmidt M, Kurtz D. Renal function in patients with obstructive sleep apnea. Effects of nasal continuous positive airway pressure. Arch Intern Med 1988; 148:1337–1340.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngal Head Neck Surg 2002; 127:13–21.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Centers for Medicare & Medicaid Services (CMS). Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). MLN Matters 2008. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM6048.pdf. Accessed June 2, 2014.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284:3015–3021.
- Guardiano SA, Scott JA, Ware JC, Schechner SA. The long-term results of gastric bypass on indexes of sleep apnea. Chest 2003; 124:1615–1619.
- Crooks PF. Surgical treatment of morbid obesity. Annu Rev Med 2006; 57:243–264.
- Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012; 308:1142–1149.
- Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 2007; 50:417–423.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Kushida CA, Morgenthaler TI, Littner MR, et al; American Academy of Sleep. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 2006; 29:240–243.
- Otsuka R, Ribeiro de Almeida F, Lowe AA, Linden W, Ryan F. The effect of oral appliance therapy on blood pressure in patients with obstructive sleep apnea. Sleep Breath 2006; 10:29–36.
- Yoshida K. Effect on blood pressure of oral appliance therapy for sleep apnea syndrome. Int J Prosthodont 2006; 19:61–66.
- Inazawa T, Ayuse T, Kurata S, et al. Effect of mandibular position on upper airway collapsibility and resistance. J Dent Res 2005; 84:554–558.
- Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981; 89:923–934.
- Schwab RJ. Imaging for the snoring and sleep apnea patient. Dent Clin North Am 2001; 45:759–796.
- Prinsell JR. Maxillomandibular advancement surgery for obstructive sleep apnea syndrome. J Am Dent Assoc 2002; 133:1489–1497.
- Thatcher GW, Maisel RH. The long-term evaluation of tracheostomy in the management of severe obstructive sleep apnea. Laryngoscope 2003; 113:201–204.
- Conway WA, Victor LD, Magilligan DJ, Fujita S, Zorick FJ, Roth T. Adverse effects of tracheostomy for sleep apnea. JAMA 1981; 246:347–350.
- Marcus CL, Moore RH, Rosen CL, et al; Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013; 368:2366–2376.
- Gottlieb DJ, Craig SE, Lorenzi-Filho G, et al. Sleep apnea cardiovascular clinical trials-current status and steps forward: The International Collaboration of Sleep Apnea Cardiovascular Trialists. Sleep 2013; 36:975–980.
- Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep 2006; 29:564–571.
- Phillips BA, McConnell JW, Smith MD. The effects of hypoxemia on cardiac output. A dose-response curve. Chest 1988; 93:471–475.
- Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respire Physiol Neurobiol 2008; 162:144–151.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34:479–485.
- Colrain IM, Black J, Siegel LC, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med 2013; 14:830–837.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370:139–149.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA; Sleep Heart Health Research Group. The relationship between chronically disrupted sleep and healthcare use. Sleep 2002; 25:289–296.
- Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep 1999; 22:749–755.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109:659–663.
- Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92:79–84.
- Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164:2147–2165.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, Second Edition: Diagnostic and coding manual. Westchester, IL; American Academy of Sleep Medicine, 2005.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Mehra R. Sleep-disordered breathing and cardiovascular disease: exploring pathophysiology and existing data. Curr Resp Med Rev 2007; 3:258–269.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166:1725–1731.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Mehra R, Storfer-Isser A, Tracy R, Jenny N, Redline S. Association of sleep disordered breathing and oxidized LDL [abstract]. Am J Respir Crit Care Med 2010; 181:A2474.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54:1797–1804.
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107:2589–2594.
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3:445–451.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7:397–398.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010; 182:269–277.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18:534–540.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352:1206–1214.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6( 8):e1000132. doi: 10.1371/journal.pmed.1000132.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20:608–613.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165:1217–1239.
- Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 2008; 12:481–496.
- Shaher E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167:1186–1192.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167:1181–1185.
- Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med 1995; 152:1946–1949.
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162:893–900.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155:186–192. Erratum in: Am J Respir Crit Care Med 1997; 155:1820.
- Sutherland K, Lee RWW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology 2012; 17:213–222.
- Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 1995; 152:1673–1689.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14:540–545.
- Krieger J, Imbs J-L, Schmidt M, Kurtz D. Renal function in patients with obstructive sleep apnea. Effects of nasal continuous positive airway pressure. Arch Intern Med 1988; 148:1337–1340.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngal Head Neck Surg 2002; 127:13–21.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Centers for Medicare & Medicaid Services (CMS). Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). MLN Matters 2008. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM6048.pdf. Accessed June 2, 2014.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284:3015–3021.
- Guardiano SA, Scott JA, Ware JC, Schechner SA. The long-term results of gastric bypass on indexes of sleep apnea. Chest 2003; 124:1615–1619.
- Crooks PF. Surgical treatment of morbid obesity. Annu Rev Med 2006; 57:243–264.
- Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012; 308:1142–1149.
- Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 2007; 50:417–423.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Kushida CA, Morgenthaler TI, Littner MR, et al; American Academy of Sleep. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 2006; 29:240–243.
- Otsuka R, Ribeiro de Almeida F, Lowe AA, Linden W, Ryan F. The effect of oral appliance therapy on blood pressure in patients with obstructive sleep apnea. Sleep Breath 2006; 10:29–36.
- Yoshida K. Effect on blood pressure of oral appliance therapy for sleep apnea syndrome. Int J Prosthodont 2006; 19:61–66.
- Inazawa T, Ayuse T, Kurata S, et al. Effect of mandibular position on upper airway collapsibility and resistance. J Dent Res 2005; 84:554–558.
- Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981; 89:923–934.
- Schwab RJ. Imaging for the snoring and sleep apnea patient. Dent Clin North Am 2001; 45:759–796.
- Prinsell JR. Maxillomandibular advancement surgery for obstructive sleep apnea syndrome. J Am Dent Assoc 2002; 133:1489–1497.
- Thatcher GW, Maisel RH. The long-term evaluation of tracheostomy in the management of severe obstructive sleep apnea. Laryngoscope 2003; 113:201–204.
- Conway WA, Victor LD, Magilligan DJ, Fujita S, Zorick FJ, Roth T. Adverse effects of tracheostomy for sleep apnea. JAMA 1981; 246:347–350.
- Marcus CL, Moore RH, Rosen CL, et al; Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013; 368:2366–2376.
- Gottlieb DJ, Craig SE, Lorenzi-Filho G, et al. Sleep apnea cardiovascular clinical trials-current status and steps forward: The International Collaboration of Sleep Apnea Cardiovascular Trialists. Sleep 2013; 36:975–980.
- Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep 2006; 29:564–571.
- Phillips BA, McConnell JW, Smith MD. The effects of hypoxemia on cardiac output. A dose-response curve. Chest 1988; 93:471–475.
- Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respire Physiol Neurobiol 2008; 162:144–151.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34:479–485.
- Colrain IM, Black J, Siegel LC, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med 2013; 14:830–837.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370:139–149.
KEY POINTS
- Although obesity and snoring are common features of OSA, they are not always present.
- Home sleep testing is appropriate for those highly likely to have sleep apnea and without other significant sleep or cardiovascular, respiratory, or neurologic disorders.
- Upper-airway surgery has a limited role—it is indicated primarily for those unable to tolerate CPAP.
- Risk of motor vehicle accidents is dramatically increased in untreated sleep apnea; patients should be counseled on the dangers of drowsy driving.