User login
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
To the Editor:
The incidence of nonmelanoma skin cancer (NMSC) is rapidly increasing worldwide. Due to its highly curable nature when treated early, accurate diagnosis is the cornerstone to good patient outcomes.1 Accurate diagnosis of skin cancer and subsequent treatment decisions rely heavily on the congruence between clinical observations and histopathologic assessments. Clinical misdiagnosis of a malignant lesion can lead to delayed and suboptimal treatment, which may contribute to serious complications such as metastasis or even mortality. In this study, data from clinically diagnosed basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) were compared to their identified histopathologic subtype classifications. The accuracy of the clinical diagnosis of these NMSCs was assessed by determining the rate of misdiagnosis and the respective positive predictive value (PPV).
A retrospective review of medical records from a private dermatology practice in Lubbock, Texas, was conducted to identify patients diagnosed with NMSC from January 1, 2017, through December 31, 2021. A total of 11,229 NMSCs were diagnosed and treated in 5877 patients. Of the NMSCs diagnosed, 11,145 were identified as keratinocyte carcinomas and were classified as BCCs or SCCs. The accuracy of the clinical diagnoses was determined by comparison to the histologic subtype identified via biopsy of the lesion. Although the use of a dermatoscope during the clinical encounter was not formally recorded, reports from the examining dermatologists indicated it was not used in the majority of cases.
If a lesion was clinically diagnosed as a BCC but was identified as a subtype of SCC on histology (or vice versa), the lesion was considered to be mismatched. The number of mismatched lesions and the mismatch rate for each lesion type/subtype is recorded in the Table. Of the total 11,145 keratinocyte carcinomas included in our study, there was an overall 10.63% mismatch rate, with 1185 of the malignancies having a differing clinical diagnosis (eg, BCC vs SCC) from the histologic findings. The clinical mismatch rate was notably higher for SCC compared to BCC (15.83% vs 7.03%, respectively).
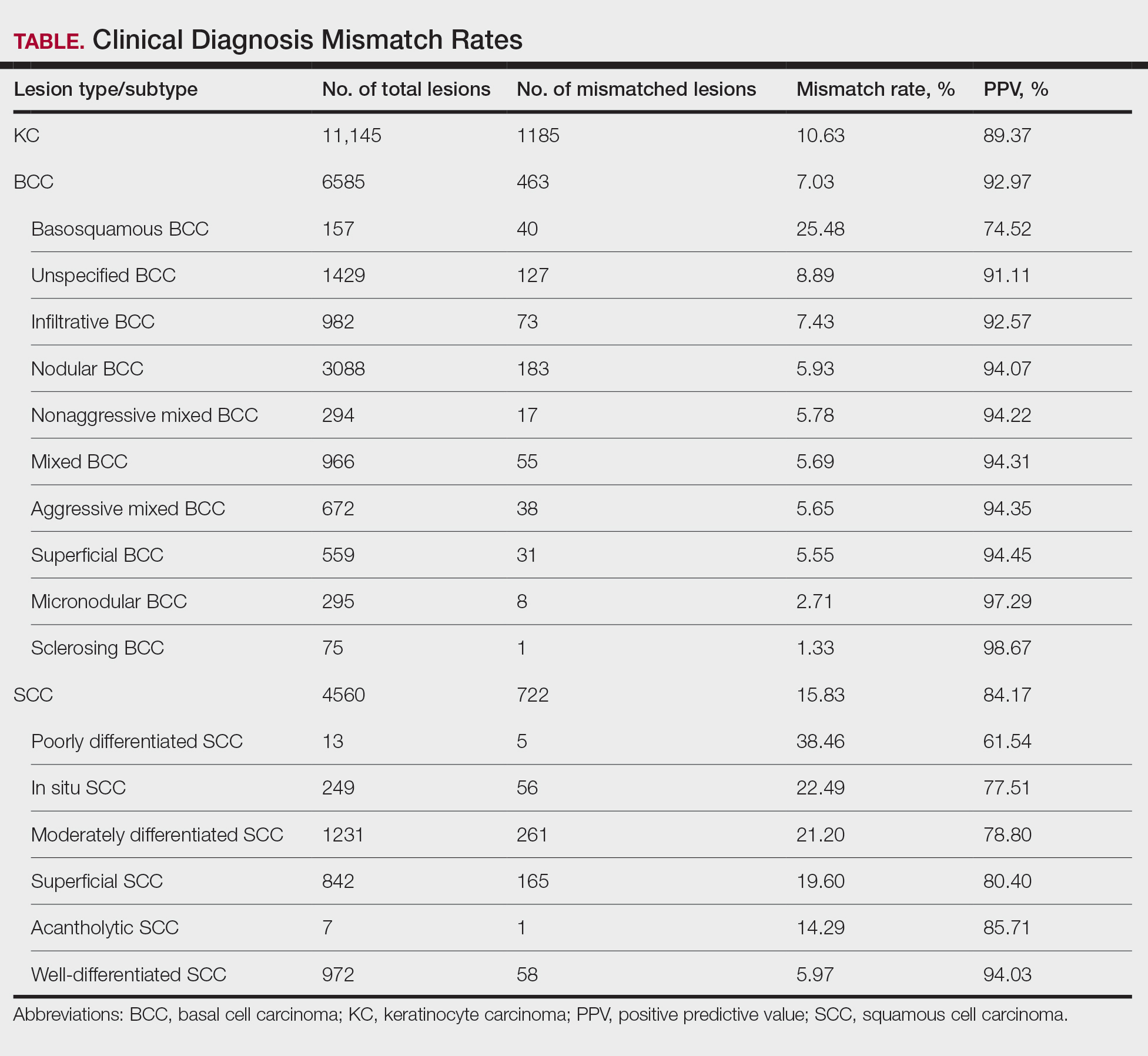
The Table provides a breakdown of the BCC subtypes identified by histology with their computed mismatch rate and PPV. It is worth clarifying that lesions classified as more than one BCC subtype per the histologic findings were diagnosed as mixed BCC; these were further classified as mixed-aggressive BCC (if at least one aggressive BCC subtype was present) and mixed nonaggressive BCC (if no aggressive BCC subtype was present). Overall, BCCs were less likely to be misdiagnosed, with an average PPV of 92.97% compared to 84.17% for SCCs. Basosquamous BCC was the BCC subtype with the highest mismatch rate (25.48%), while sclerosing BCC has the lowest overall mismatch rate (1.33%). The most common malignancy was BCC, with nodular BCC being the most common subtype.
The Table also breaks down the SCC subtypes, reporting the most commonly misdiagnosed of any BCC or SCC subtype to be poorly differentiated SCC (mismatch rate, 38.46%). The lowest mismatch rate of the SCC subtypes was 5.97% for well-differentiated SCC.
There was an overall PPV of 89.37% in clinically evaluated malignancies and their respective histologic subtypes. Basal cell carcinoma had a lower overall mismatch rate of 7.03% compared to 15.83% in SCC. The most common misdiagnosis was attributed to poorly differentiated SCC (mismatch rate, 38.46%), while the least common misdiagnosed malignancy was sclerosing BCC (1.33%). The high mismatch rate of poorly differentiated SCC may be due to its diverging presentation from a typical SCC as a flat lesion with the absence of scaling, keratin, or bleeding, leading to the misdiagnosis of BCC.2
Accurate clinical diagnosis of NMSCs is the basis for further evaluation and treatment that should ensue in a timely manner; however, accurately identifying BCCs vs SCCs solely based on clinical examination can be challenging due to variable manifestations and overlapping features. Basal cell carcinoma commonly presents as a shiny pink/flesh-colored nodule, macule, or patch with surface telangiectasia, sometimes appearing with ulceration or crusting.3 Alternatively, SCC typically appears as a firm, sharply demarcated, red nodule with a thick overlying scale.4 Definitive diagnoses can be difficult upon clinical examination since these features can be shared between the 2 subtypes. To aid in these uncertainties, a growing number of clinicians are implementing the use of dermoscopy in their everyday practice.
Dermoscopy is an extremely useful tool in improving the diagnostic accuracy of skin cancers compared to examination with the naked eye, as it provides detailed visualization of specific structures and patterns in skin cancer lesions.5 The dermoscopic appearance of BCC is characterized by pearly blue-gray or translucent globules with arborizing vessels, spoke-wheel structures, and leaflike areas.5,6 Conversely, dermoscopic features of SCC may include a milky-red globule with a scaly, sharply demarcated, crusted lesion with polymorphous vasculature, sometimes resembling a persistent sore or nonhealing wound.4,5 Though the use of dermoscopy can aid in diagnosis upon initial examination, certain factors such as trauma, ulceration, and previous treatments that distorted the lesion’s architecture may lead to misdiagnosis. Furthermore, the distinct vascular patterns found in BCC and SCC may be mistaken for each other and therefore lead to misdiagnosis upon examination.7 Other variables that may complicate diagnosis include the location of the lesion, its size, and the presence of other skin conditions or nearby lesions.
The primary limitation of the current study was the limited scope of the data, as they were derived from patients seen at one private dermatology practice, preventing the generalizability of our findings. However, our results show trends similar to those observed in other studies analyzing the clinical accuracy of skin cancer diagnoses, with higher PPVs for BCC compared to SCC. A study by Ahnlide and Bjellerup8 was based in a hospital dermatology department and demonstrated a PPV of 85.5% for BCC compared to 92.97% in our study; for SCC, the PPV was 67.3% compared to 84.17% in our study. In another study by Heal et al,9 data were collected from an Australian registry that included records of all histologically confirmed skin cancers from December 1996 to October 1999 from 202 general practitioners and 42 specialists, including 1 dermatologist. The PPVs for BCC and SCC were 72.7% and 49.4%, respectively. Although our results indicated higher PPVs compared to these 2 studies, some of the discrepancies can be accounted for by the differences in clinical setting as well as the lack of expertise of nondermatologist physicians in identifying skin malignancies in the study by Heal et al.9
The current study was further limited by the lack of data quantifying the number of lesions clinically suspected to be malignant but found to be histologically benign. It is typical for clinicians to have a low threshold to biopsy a suspicious lesion with atypical features (eg, rapid evolution and growth, bleeding, crusting). Furthermore, the identification of risk factors in the patient’s medical and family history (eg, exposure to radiation, personal or family history of skin cancers) can heavily influence a clinician’s decision to biopsy a lesion with an atypical appearance.10 Many benign lesions are biopsied to avoid missing a diagnosis of malignancy. Consequently, our results suggest a high degree of clinical misdiagnosis of BCCs and SCCs. Obtaining data on the number of lesions suspected to be BCC or SCC that were found to be histologically benign would be a valuable addition to our study, as it would provide a measurable insight into the sensitivity of clinicians’ decision-making to identify a lesion as suspicious and warranting biopsy.
While clinical diagnosis plays a vital role in identifying suspected NMSCs such as BCC and SCC, its accuracy can be limited even with the use of dermoscopy. Overall, our data have shown a high rate of diagnostic accuracy upon suspicion of malignancy, but the different variables that affect clinical presentation promote histologic diagnosis to prevail as the gold standard.
- Seyed Ahadi M, Firooz A, Rahimi H, et al. Clinical diagnosis has a high negative predictive value in evaluation of malignant skin lesions. Dermatol Res Pract. 2021;2021:6618990. doi:10.1155/2021/6618990
- Lallas A, Pyne J, Kyrgidis A, et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br J Dermatol. 2015;172:1308- 1315. doi:10.1111/bjd.13510
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. September 19, 2022. In: StatPearls. StatPearls Publishing; 2023.
- Suárez AL, Louis P, Kitts J, et al. Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC). J Am Acad Dermatol. 2015;73:968-975. doi:10.1016/j.jaad.2015.08.041
- Wolner ZJ, Yélamos O, Liopyris K, et al. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35:417-437. doi:10.1016/j.det.2017.06.003
- Reiter O, Mimouni I, Dusza S, et al. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol. 2021;85:653-664. doi:10.1016/j.jaad.2019.11.008
- Pruneda C, Ramesh M, Hope L, et al. Nonmelanoma skin cancers: diagnostic accuracy of midlevel providers versus dermatologists. The Dermatologist. March 2023. Accessed March 18, 2025. https://www.hmpgloballearningnetwork.com/site/thederm/feature-story/nonmelanoma-skin-cancers-diagnostic-accuracy-midlevel-providers-vs
- Ahnlide I, Bjellerup M. Accuracy of clinical skin tumour diagnosis in a dermatological setting. Acta Derm Venereol. 2013;93:305-308. doi:10.2340/00015555-1560
- Heal CF, Raasch BA, Buettner PG, et al. Accuracy of clinical diagnosis of skin lesions. Br J Dermatol. 2008;159:661-668.
- Fu S, Kim S, Wasko C. Dermatological guide for primary care physicians: full body skin checks, skin cancer detection, and patient education on self-skin checks and sun protection. Proc (Bayl Univ Med Cent). 2024;37:647-654. doi:10.1080/08998280.2024.2351751
To the Editor:
The incidence of nonmelanoma skin cancer (NMSC) is rapidly increasing worldwide. Due to its highly curable nature when treated early, accurate diagnosis is the cornerstone to good patient outcomes.1 Accurate diagnosis of skin cancer and subsequent treatment decisions rely heavily on the congruence between clinical observations and histopathologic assessments. Clinical misdiagnosis of a malignant lesion can lead to delayed and suboptimal treatment, which may contribute to serious complications such as metastasis or even mortality. In this study, data from clinically diagnosed basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) were compared to their identified histopathologic subtype classifications. The accuracy of the clinical diagnosis of these NMSCs was assessed by determining the rate of misdiagnosis and the respective positive predictive value (PPV).
A retrospective review of medical records from a private dermatology practice in Lubbock, Texas, was conducted to identify patients diagnosed with NMSC from January 1, 2017, through December 31, 2021. A total of 11,229 NMSCs were diagnosed and treated in 5877 patients. Of the NMSCs diagnosed, 11,145 were identified as keratinocyte carcinomas and were classified as BCCs or SCCs. The accuracy of the clinical diagnoses was determined by comparison to the histologic subtype identified via biopsy of the lesion. Although the use of a dermatoscope during the clinical encounter was not formally recorded, reports from the examining dermatologists indicated it was not used in the majority of cases.
If a lesion was clinically diagnosed as a BCC but was identified as a subtype of SCC on histology (or vice versa), the lesion was considered to be mismatched. The number of mismatched lesions and the mismatch rate for each lesion type/subtype is recorded in the Table. Of the total 11,145 keratinocyte carcinomas included in our study, there was an overall 10.63% mismatch rate, with 1185 of the malignancies having a differing clinical diagnosis (eg, BCC vs SCC) from the histologic findings. The clinical mismatch rate was notably higher for SCC compared to BCC (15.83% vs 7.03%, respectively).

The Table provides a breakdown of the BCC subtypes identified by histology with their computed mismatch rate and PPV. It is worth clarifying that lesions classified as more than one BCC subtype per the histologic findings were diagnosed as mixed BCC; these were further classified as mixed-aggressive BCC (if at least one aggressive BCC subtype was present) and mixed nonaggressive BCC (if no aggressive BCC subtype was present). Overall, BCCs were less likely to be misdiagnosed, with an average PPV of 92.97% compared to 84.17% for SCCs. Basosquamous BCC was the BCC subtype with the highest mismatch rate (25.48%), while sclerosing BCC has the lowest overall mismatch rate (1.33%). The most common malignancy was BCC, with nodular BCC being the most common subtype.
The Table also breaks down the SCC subtypes, reporting the most commonly misdiagnosed of any BCC or SCC subtype to be poorly differentiated SCC (mismatch rate, 38.46%). The lowest mismatch rate of the SCC subtypes was 5.97% for well-differentiated SCC.
There was an overall PPV of 89.37% in clinically evaluated malignancies and their respective histologic subtypes. Basal cell carcinoma had a lower overall mismatch rate of 7.03% compared to 15.83% in SCC. The most common misdiagnosis was attributed to poorly differentiated SCC (mismatch rate, 38.46%), while the least common misdiagnosed malignancy was sclerosing BCC (1.33%). The high mismatch rate of poorly differentiated SCC may be due to its diverging presentation from a typical SCC as a flat lesion with the absence of scaling, keratin, or bleeding, leading to the misdiagnosis of BCC.2
Accurate clinical diagnosis of NMSCs is the basis for further evaluation and treatment that should ensue in a timely manner; however, accurately identifying BCCs vs SCCs solely based on clinical examination can be challenging due to variable manifestations and overlapping features. Basal cell carcinoma commonly presents as a shiny pink/flesh-colored nodule, macule, or patch with surface telangiectasia, sometimes appearing with ulceration or crusting.3 Alternatively, SCC typically appears as a firm, sharply demarcated, red nodule with a thick overlying scale.4 Definitive diagnoses can be difficult upon clinical examination since these features can be shared between the 2 subtypes. To aid in these uncertainties, a growing number of clinicians are implementing the use of dermoscopy in their everyday practice.
Dermoscopy is an extremely useful tool in improving the diagnostic accuracy of skin cancers compared to examination with the naked eye, as it provides detailed visualization of specific structures and patterns in skin cancer lesions.5 The dermoscopic appearance of BCC is characterized by pearly blue-gray or translucent globules with arborizing vessels, spoke-wheel structures, and leaflike areas.5,6 Conversely, dermoscopic features of SCC may include a milky-red globule with a scaly, sharply demarcated, crusted lesion with polymorphous vasculature, sometimes resembling a persistent sore or nonhealing wound.4,5 Though the use of dermoscopy can aid in diagnosis upon initial examination, certain factors such as trauma, ulceration, and previous treatments that distorted the lesion’s architecture may lead to misdiagnosis. Furthermore, the distinct vascular patterns found in BCC and SCC may be mistaken for each other and therefore lead to misdiagnosis upon examination.7 Other variables that may complicate diagnosis include the location of the lesion, its size, and the presence of other skin conditions or nearby lesions.
The primary limitation of the current study was the limited scope of the data, as they were derived from patients seen at one private dermatology practice, preventing the generalizability of our findings. However, our results show trends similar to those observed in other studies analyzing the clinical accuracy of skin cancer diagnoses, with higher PPVs for BCC compared to SCC. A study by Ahnlide and Bjellerup8 was based in a hospital dermatology department and demonstrated a PPV of 85.5% for BCC compared to 92.97% in our study; for SCC, the PPV was 67.3% compared to 84.17% in our study. In another study by Heal et al,9 data were collected from an Australian registry that included records of all histologically confirmed skin cancers from December 1996 to October 1999 from 202 general practitioners and 42 specialists, including 1 dermatologist. The PPVs for BCC and SCC were 72.7% and 49.4%, respectively. Although our results indicated higher PPVs compared to these 2 studies, some of the discrepancies can be accounted for by the differences in clinical setting as well as the lack of expertise of nondermatologist physicians in identifying skin malignancies in the study by Heal et al.9
The current study was further limited by the lack of data quantifying the number of lesions clinically suspected to be malignant but found to be histologically benign. It is typical for clinicians to have a low threshold to biopsy a suspicious lesion with atypical features (eg, rapid evolution and growth, bleeding, crusting). Furthermore, the identification of risk factors in the patient’s medical and family history (eg, exposure to radiation, personal or family history of skin cancers) can heavily influence a clinician’s decision to biopsy a lesion with an atypical appearance.10 Many benign lesions are biopsied to avoid missing a diagnosis of malignancy. Consequently, our results suggest a high degree of clinical misdiagnosis of BCCs and SCCs. Obtaining data on the number of lesions suspected to be BCC or SCC that were found to be histologically benign would be a valuable addition to our study, as it would provide a measurable insight into the sensitivity of clinicians’ decision-making to identify a lesion as suspicious and warranting biopsy.
While clinical diagnosis plays a vital role in identifying suspected NMSCs such as BCC and SCC, its accuracy can be limited even with the use of dermoscopy. Overall, our data have shown a high rate of diagnostic accuracy upon suspicion of malignancy, but the different variables that affect clinical presentation promote histologic diagnosis to prevail as the gold standard.
To the Editor:
The incidence of nonmelanoma skin cancer (NMSC) is rapidly increasing worldwide. Due to its highly curable nature when treated early, accurate diagnosis is the cornerstone to good patient outcomes.1 Accurate diagnosis of skin cancer and subsequent treatment decisions rely heavily on the congruence between clinical observations and histopathologic assessments. Clinical misdiagnosis of a malignant lesion can lead to delayed and suboptimal treatment, which may contribute to serious complications such as metastasis or even mortality. In this study, data from clinically diagnosed basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) were compared to their identified histopathologic subtype classifications. The accuracy of the clinical diagnosis of these NMSCs was assessed by determining the rate of misdiagnosis and the respective positive predictive value (PPV).
A retrospective review of medical records from a private dermatology practice in Lubbock, Texas, was conducted to identify patients diagnosed with NMSC from January 1, 2017, through December 31, 2021. A total of 11,229 NMSCs were diagnosed and treated in 5877 patients. Of the NMSCs diagnosed, 11,145 were identified as keratinocyte carcinomas and were classified as BCCs or SCCs. The accuracy of the clinical diagnoses was determined by comparison to the histologic subtype identified via biopsy of the lesion. Although the use of a dermatoscope during the clinical encounter was not formally recorded, reports from the examining dermatologists indicated it was not used in the majority of cases.
If a lesion was clinically diagnosed as a BCC but was identified as a subtype of SCC on histology (or vice versa), the lesion was considered to be mismatched. The number of mismatched lesions and the mismatch rate for each lesion type/subtype is recorded in the Table. Of the total 11,145 keratinocyte carcinomas included in our study, there was an overall 10.63% mismatch rate, with 1185 of the malignancies having a differing clinical diagnosis (eg, BCC vs SCC) from the histologic findings. The clinical mismatch rate was notably higher for SCC compared to BCC (15.83% vs 7.03%, respectively).

The Table provides a breakdown of the BCC subtypes identified by histology with their computed mismatch rate and PPV. It is worth clarifying that lesions classified as more than one BCC subtype per the histologic findings were diagnosed as mixed BCC; these were further classified as mixed-aggressive BCC (if at least one aggressive BCC subtype was present) and mixed nonaggressive BCC (if no aggressive BCC subtype was present). Overall, BCCs were less likely to be misdiagnosed, with an average PPV of 92.97% compared to 84.17% for SCCs. Basosquamous BCC was the BCC subtype with the highest mismatch rate (25.48%), while sclerosing BCC has the lowest overall mismatch rate (1.33%). The most common malignancy was BCC, with nodular BCC being the most common subtype.
The Table also breaks down the SCC subtypes, reporting the most commonly misdiagnosed of any BCC or SCC subtype to be poorly differentiated SCC (mismatch rate, 38.46%). The lowest mismatch rate of the SCC subtypes was 5.97% for well-differentiated SCC.
There was an overall PPV of 89.37% in clinically evaluated malignancies and their respective histologic subtypes. Basal cell carcinoma had a lower overall mismatch rate of 7.03% compared to 15.83% in SCC. The most common misdiagnosis was attributed to poorly differentiated SCC (mismatch rate, 38.46%), while the least common misdiagnosed malignancy was sclerosing BCC (1.33%). The high mismatch rate of poorly differentiated SCC may be due to its diverging presentation from a typical SCC as a flat lesion with the absence of scaling, keratin, or bleeding, leading to the misdiagnosis of BCC.2
Accurate clinical diagnosis of NMSCs is the basis for further evaluation and treatment that should ensue in a timely manner; however, accurately identifying BCCs vs SCCs solely based on clinical examination can be challenging due to variable manifestations and overlapping features. Basal cell carcinoma commonly presents as a shiny pink/flesh-colored nodule, macule, or patch with surface telangiectasia, sometimes appearing with ulceration or crusting.3 Alternatively, SCC typically appears as a firm, sharply demarcated, red nodule with a thick overlying scale.4 Definitive diagnoses can be difficult upon clinical examination since these features can be shared between the 2 subtypes. To aid in these uncertainties, a growing number of clinicians are implementing the use of dermoscopy in their everyday practice.
Dermoscopy is an extremely useful tool in improving the diagnostic accuracy of skin cancers compared to examination with the naked eye, as it provides detailed visualization of specific structures and patterns in skin cancer lesions.5 The dermoscopic appearance of BCC is characterized by pearly blue-gray or translucent globules with arborizing vessels, spoke-wheel structures, and leaflike areas.5,6 Conversely, dermoscopic features of SCC may include a milky-red globule with a scaly, sharply demarcated, crusted lesion with polymorphous vasculature, sometimes resembling a persistent sore or nonhealing wound.4,5 Though the use of dermoscopy can aid in diagnosis upon initial examination, certain factors such as trauma, ulceration, and previous treatments that distorted the lesion’s architecture may lead to misdiagnosis. Furthermore, the distinct vascular patterns found in BCC and SCC may be mistaken for each other and therefore lead to misdiagnosis upon examination.7 Other variables that may complicate diagnosis include the location of the lesion, its size, and the presence of other skin conditions or nearby lesions.
The primary limitation of the current study was the limited scope of the data, as they were derived from patients seen at one private dermatology practice, preventing the generalizability of our findings. However, our results show trends similar to those observed in other studies analyzing the clinical accuracy of skin cancer diagnoses, with higher PPVs for BCC compared to SCC. A study by Ahnlide and Bjellerup8 was based in a hospital dermatology department and demonstrated a PPV of 85.5% for BCC compared to 92.97% in our study; for SCC, the PPV was 67.3% compared to 84.17% in our study. In another study by Heal et al,9 data were collected from an Australian registry that included records of all histologically confirmed skin cancers from December 1996 to October 1999 from 202 general practitioners and 42 specialists, including 1 dermatologist. The PPVs for BCC and SCC were 72.7% and 49.4%, respectively. Although our results indicated higher PPVs compared to these 2 studies, some of the discrepancies can be accounted for by the differences in clinical setting as well as the lack of expertise of nondermatologist physicians in identifying skin malignancies in the study by Heal et al.9
The current study was further limited by the lack of data quantifying the number of lesions clinically suspected to be malignant but found to be histologically benign. It is typical for clinicians to have a low threshold to biopsy a suspicious lesion with atypical features (eg, rapid evolution and growth, bleeding, crusting). Furthermore, the identification of risk factors in the patient’s medical and family history (eg, exposure to radiation, personal or family history of skin cancers) can heavily influence a clinician’s decision to biopsy a lesion with an atypical appearance.10 Many benign lesions are biopsied to avoid missing a diagnosis of malignancy. Consequently, our results suggest a high degree of clinical misdiagnosis of BCCs and SCCs. Obtaining data on the number of lesions suspected to be BCC or SCC that were found to be histologically benign would be a valuable addition to our study, as it would provide a measurable insight into the sensitivity of clinicians’ decision-making to identify a lesion as suspicious and warranting biopsy.
While clinical diagnosis plays a vital role in identifying suspected NMSCs such as BCC and SCC, its accuracy can be limited even with the use of dermoscopy. Overall, our data have shown a high rate of diagnostic accuracy upon suspicion of malignancy, but the different variables that affect clinical presentation promote histologic diagnosis to prevail as the gold standard.
- Seyed Ahadi M, Firooz A, Rahimi H, et al. Clinical diagnosis has a high negative predictive value in evaluation of malignant skin lesions. Dermatol Res Pract. 2021;2021:6618990. doi:10.1155/2021/6618990
- Lallas A, Pyne J, Kyrgidis A, et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br J Dermatol. 2015;172:1308- 1315. doi:10.1111/bjd.13510
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. September 19, 2022. In: StatPearls. StatPearls Publishing; 2023.
- Suárez AL, Louis P, Kitts J, et al. Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC). J Am Acad Dermatol. 2015;73:968-975. doi:10.1016/j.jaad.2015.08.041
- Wolner ZJ, Yélamos O, Liopyris K, et al. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35:417-437. doi:10.1016/j.det.2017.06.003
- Reiter O, Mimouni I, Dusza S, et al. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol. 2021;85:653-664. doi:10.1016/j.jaad.2019.11.008
- Pruneda C, Ramesh M, Hope L, et al. Nonmelanoma skin cancers: diagnostic accuracy of midlevel providers versus dermatologists. The Dermatologist. March 2023. Accessed March 18, 2025. https://www.hmpgloballearningnetwork.com/site/thederm/feature-story/nonmelanoma-skin-cancers-diagnostic-accuracy-midlevel-providers-vs
- Ahnlide I, Bjellerup M. Accuracy of clinical skin tumour diagnosis in a dermatological setting. Acta Derm Venereol. 2013;93:305-308. doi:10.2340/00015555-1560
- Heal CF, Raasch BA, Buettner PG, et al. Accuracy of clinical diagnosis of skin lesions. Br J Dermatol. 2008;159:661-668.
- Fu S, Kim S, Wasko C. Dermatological guide for primary care physicians: full body skin checks, skin cancer detection, and patient education on self-skin checks and sun protection. Proc (Bayl Univ Med Cent). 2024;37:647-654. doi:10.1080/08998280.2024.2351751
- Seyed Ahadi M, Firooz A, Rahimi H, et al. Clinical diagnosis has a high negative predictive value in evaluation of malignant skin lesions. Dermatol Res Pract. 2021;2021:6618990. doi:10.1155/2021/6618990
- Lallas A, Pyne J, Kyrgidis A, et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br J Dermatol. 2015;172:1308- 1315. doi:10.1111/bjd.13510
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. September 19, 2022. In: StatPearls. StatPearls Publishing; 2023.
- Suárez AL, Louis P, Kitts J, et al. Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC). J Am Acad Dermatol. 2015;73:968-975. doi:10.1016/j.jaad.2015.08.041
- Wolner ZJ, Yélamos O, Liopyris K, et al. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35:417-437. doi:10.1016/j.det.2017.06.003
- Reiter O, Mimouni I, Dusza S, et al. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol. 2021;85:653-664. doi:10.1016/j.jaad.2019.11.008
- Pruneda C, Ramesh M, Hope L, et al. Nonmelanoma skin cancers: diagnostic accuracy of midlevel providers versus dermatologists. The Dermatologist. March 2023. Accessed March 18, 2025. https://www.hmpgloballearningnetwork.com/site/thederm/feature-story/nonmelanoma-skin-cancers-diagnostic-accuracy-midlevel-providers-vs
- Ahnlide I, Bjellerup M. Accuracy of clinical skin tumour diagnosis in a dermatological setting. Acta Derm Venereol. 2013;93:305-308. doi:10.2340/00015555-1560
- Heal CF, Raasch BA, Buettner PG, et al. Accuracy of clinical diagnosis of skin lesions. Br J Dermatol. 2008;159:661-668.
- Fu S, Kim S, Wasko C. Dermatological guide for primary care physicians: full body skin checks, skin cancer detection, and patient education on self-skin checks and sun protection. Proc (Bayl Univ Med Cent). 2024;37:647-654. doi:10.1080/08998280.2024.2351751
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
PRACTICE POINTS
- Malignant lesions may be misdiagnosed when assessments are guided by clinical features that align with typical presentations of other lesion types, potentially leading to diagnostic errors among experienced clinicians.
- Although dermoscopy is a beneficial tool in examining potential skin cancers, clinical observations should not bypass the gold standard of histopathologic examination.
Merkel Cell Carcinoma in a Vein Graft Donor Site
Case Report
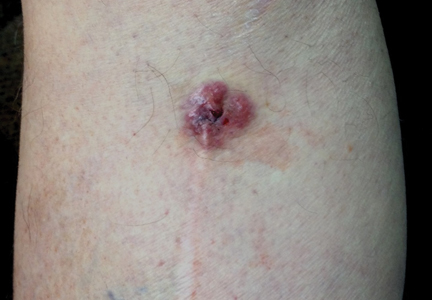
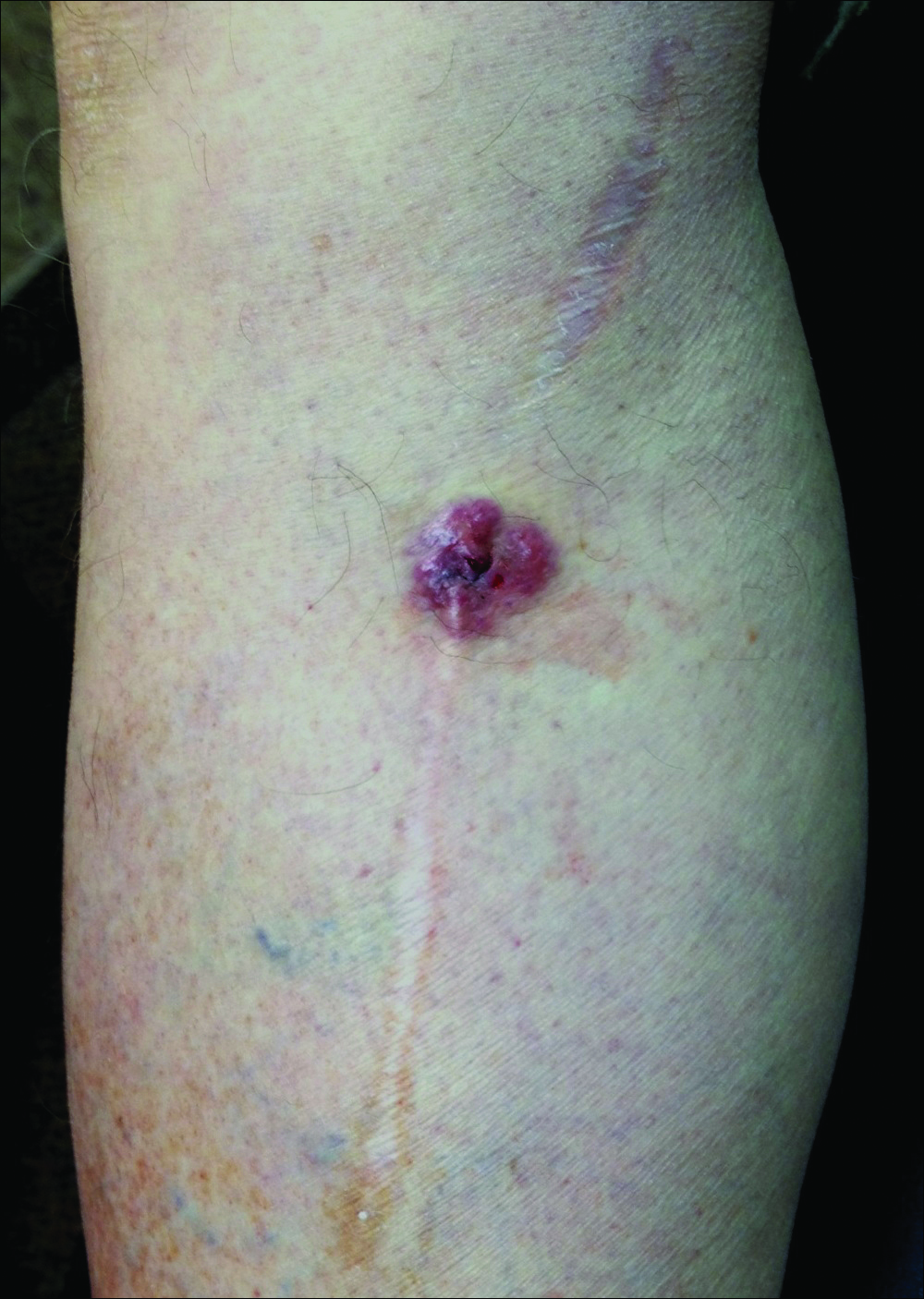
A 70-year-old man with history of coronary artery disease presented with a growing lesion on the right leg of 1 year’s duration. The lesion developed at a vein graft donor site for a coronary artery bypass that had been performed 18 years prior to presentation. The patient reported that the lesion was sensitive to touch. Physical examination revealed a 27-mm, firm, violaceous plaque on the medial aspect of the right upper shin (Figure 1). Mild pitting edema also was noted on both lower legs but was more prominent on the right leg. A 6-mm punch biopsy was performed.
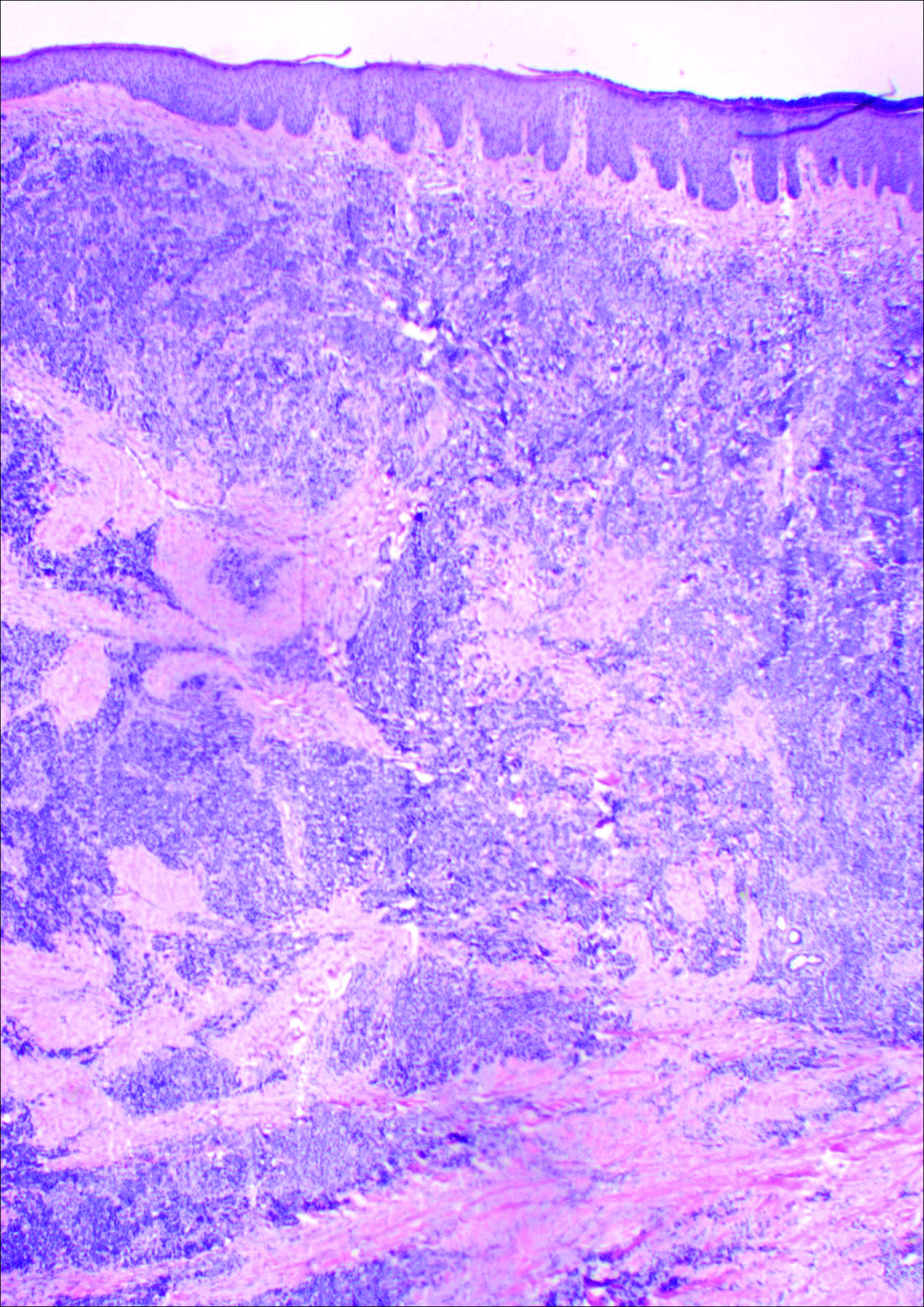
Histology showed diffuse infiltration of the dermis and subcutaneous fat by intermediate-sized atypical blue cells with scant cytoplasm (Figure 2). The tumor exhibited moderate cytologic atypia with occasional mitotic figures, and lymphovascular invasion was present. Staining for CD3 was negative within the tumor, but a few reactive lymphocytes were highlighted at the periphery. Staining for CD20 and CD30 was negative. Strong and diffuse staining for cyto-keratin 20 and pan-cytokeratin was noted within the tumor with the distinctive perinuclear pattern characteristic of Merkel cell carcinoma (MCC). Staining for cytokeratin 7 was negative. Synaptophysin and chromogranin were strongly and diffusely positive within the tumor, consistent with a diagnosis of MCC.
The patient was found to have stage IIA (T2N0M0) MCC. Computed tomography completed for staging showed no evidence of metastasis. Wide local excision of the lesion was performed. Margins were negative, as was a right inguinal sentinel lymph node dissection. Because of the size of the tumor and the presence of lymphovascular invasion, radiation therapy at the primary tumor site was recommended. Local radiation treatment (200 cGy daily) was administered for a total dose of 5000 cGy over 5 weeks. The patient currently is free of recurrence or metastases and is being followed by the oncology, surgery, and dermatology departments.
Comment
Merkel cell carcinoma is a rare aggressive cutaneous malignancy. The exact pathogenesis is unknown, but immunosuppression and UV radiation, possibly through its immunosuppressive effects, appear to be contributing factors. More recently, the Merkel cell polyomavirus has been linked to MCC in approximately 80% of cases.1,2
Merkel cell carcinoma is more common in individuals with fair skin, and the average age at diagnosis is 69 years.1 Patients typically present with an asymptomatic, firm, erythematous or violaceous, dome-shaped nodule or a small indurated plaque, most commonly on sun-exposed areas of the head and neck followed by the upper and lower extremities including the hands, feet, ankles, and wrists. Fifteen percent to 20% of MCCs develop on the legs and feet.1 Our patient presented with an MCC that developed on the right shin at a vein graft donor site.
The development of a cutaneous malignancy in a chronic wound (also known as a Marjolin ulcer) is a rare but well-recognized process. These malignancies occur in previously traumatized or chronically inflamed wounds and have been found to occur most commonly in chronic burn wounds, especially in ungrafted full-thickness burns. Squamous cell carcinomas (SCCs) are the most common malignancies to arise in chronic wounds, but basal cell carcinomas, adenocarcinomas, melanomas, malignant fibrous histiocytomas, adenoacanthomas, liposarcomas, and osteosarcomas also have been reported.3 There also have been a few reports of MCC associated with Bowen disease that developed in burn wounds.4 These malignancies generally occur years after injury (average, 35.5 years), but there have been reports of keratoacanthomas developing as early as 3 weeks after injury.5,6
In some reports, malignancies in skin graft donor sites are differentiated from Marjolin ulcers, as the former appear in healed surgical wounds rather than in chronic unstable wounds and tend to occur sooner (ie, in weeks to months after graft harvesting).7,8 The development of these malignancies in graft donor sites is not as well recognized and has been reported in donor sites for split-thickness skin grafts (STSGs), full-thickness skin grafts, tendon grafts, and bone grafts. In addition to malignancies that arise de novo, some develop due to metastatic and iatrogenic spread. The majority of reported malignancies in tendon and bone graft donor sites have been due to metastasis or iatrogenic spread.9-14
Iatrogenic implantation of tumor cells is a well-recognized phenomenon. Hussain et al10 reported a case of implantation of SCC in an STSG donor site, most likely due to direct seeding from a hollow needle used to infiltrate local anesthetic in the tumor area and the STSG. In this case, metastasis could not be completely ruled out.10 There also have been reports of osteosarcoma, ameloblastoma, scirrhous carcinoma of the breast, and malignant fibrous histiocytoma thought to be implanted at bone graft donor sites.14-17 Iatrogenic spread of malignancies can occur through seeding from contaminated gloves or instruments such as hollow bore needles or trocar placement in laparoscopic surgery.11 Airborne spread also may be possible, as viable melanoma cells have been detected in electrocautery plume in mice.13
Metastatic malignancies including metastases from SCC, adenocarcinoma, melanoma, malignant fibrous histiocytoma, angiosarcoma, and osteosarcoma also have been reported to develop in graft donor sites.11,13,18,19 Many malignancies thought to have developed from iatrogenic seeding may actually be from metastasis either by hematogenous or lymphatic spread. A possible contributing factor may be surgery-induced immunosuppression, which has been linked to increased tumor metastasis formation.20 Surgery or trauma have been shown to have an effect on cellular components of the immune system, causing changes such as a shift in T lymphocytes toward immune-suppressive T lymphocytes and impaired function of natural killer cells, neutrophils, and macrophages.20 The suppression of cell-mediated immunity has been shown to decrease over days to weeks in the postoperative period.21 In addition to surgery- or trauma-induced immunosuppression, the risk for metastasis may increase due to increased vascular, including lymphatic, flow toward a skin graft donor site.13,16 Furthermore, trauma predisposes areas to a hypercoagulable state with increased sludging as well as increased platelet counts and fibrinogen levels, which may lead to localization of metastatic lesions.22 All of these factors could potentially work simultaneously to induce the development of metastasis in graft donor sites.
We found that SCCs and keratoacanthomas, which may be a variant of SCC, are among the only primary malignancies that have been reported to develop in skin graft donor sites.6-8 Malignancies in these donor sites appear to develop sooner than those found in chronic wounds and are reported to develop within weeks to several months postoperatively, even in as few as 2 weeks.6,8 Tamir et al6 reported 2 keratoacanthomas that developed simultaneously in a burn scar and STSG donor site. The investigators believed it could be a sign of reduced immune surveillance in the 2 affected areas.6 It has been hypothesized that one cause of local immune suppression in Marjolin ulcers could be due to poor lymphatic regeneration in scar tissue, which would prevent delivery of antigens and stimulated lymphocytes.23 Haik et al7 considered this possibility when discussing a case of SCC that developed at the site of an STSG. The authors did not feel it applied, however, as the donor site had only undergone a single skin harvesting procedure.7 Ponnuvelu et al8 felt that inflammation was the underlying etiology behind the 2 cases they reported of SCCs that developed in STSG donor sites. The inflammation associated with tumors has many of the same processes involved in wound healing (eg, cellular proliferation, angiogenesis). Ponnuvelu et al8 hypothesized that the local inflammation caused by graft harvesting produced an ideal environment for early carcinogenesis. Although in chronic wounds it is believed that continual repair and regeneration in recurrent ulceration contributes to neoplastic initiation, it is thought that even a single injury may lead to malignant change, which may be because prior actinic damage or another cause has made the area more susceptible to these changes.24,25 Surgery-induced immunosuppression also may play a role in development of primary malignancies in graft donor sites.
There have been a few reports of SCCs and basal cell carcinomas occurring in other surgical scars that healed without complications.24,26-28 Similar to the malignancies in graft donor sites, some authors differentiate malignancies that occur in surgical scars that heal without complications from Marjolin ulcers, as they do not occur in chronically irritated wounds. These malignancies have been reported in scars from sternotomies, an infertility procedure, hair transplantation, thyroidectomy, colostomy, cleft lip repair, inguinal hernia repair, and paraumbilical laparoscopic port site. The time between surgery and diagnosis of malignancy ranged from 9 months to 67 years.24,26-28 The development of malignancies in these surgical scars may be due to local immunosuppression, possibly from decreased lymphatic flow; additionally, the inflammation in wound healing may provide the ideal environment for carcinogenesis. Trauma in areas already susceptible to malignant change could be a contributing factor.
Conclusion
Our patient developed an MCC in a vein graft donor site 18 years after vein harvesting. It was likely a primary tumor, as vein harvesting was done for coronary artery bypass graft. There was no evidence of any other lesions on physical examination or computed tomography, making it doubtful that an MCC serving as a primary lesion for seeding or metastasis was present. If such a lesion had been present at that time, it would likely have spread well before the time of presentation to our clinic due to the fast doubling time and high rate of metastasis characteristic of MCCs, further lessening the possibility of metastasis or implantation.
The extended length of time from procedure to lesion development in our patient is much longer than for other reported malignancies in graft donor sites, but the reported time for malignancies in other postsurgical scars is more varied. Regardless of whether the MCC in our patient is classified as a Marjolin ulcer, the pathogenesis is unclear. It is thought that a single injury could lead to malignant change in predisposed skin. Our patient’s legs did not have any evidence of prior actinic damage; however, it is likely that he had local immune suppression, which may have made him more susceptible to these changes. It is unlikely that surgery-induced immunosuppression played a role in our patient, as specific cellular components of the immune system only appear to be affected over days to weeks in the postoperative period. Although poor lymphatic regeneration in scar tissue leading to decreased immune surveillance is not generally thought to contribute to malignancies in most surgical scars, our patient underwent vein harvesting. Chronic edema commonly occurs after vein harvesting and is believed to be due to trauma to the lymphatics. Local immune suppression also may have led to increased susceptibility to infection by the MCC polyomavirus, which has been found to be associated with many MCCs. In addition, the area may have been more susceptible to carcinogenesis due to changes from inflammation from wound healing. We suspect together these factors contributed to the development of our patient’s MCC. Although rare, graft donor sites should be examined periodically for the development of malignancy.
- Swann MH, Yoon J. Merkel cell carcinoma. Semin Oncol. 2007;34:51-56.
- Schrama D, Ugurel S, Becker JC. Merkel cell carcinoma: recent insights and new treatment options. Curr Opin Oncol. 2012;24:141-149.
- Kadir AR. Burn scar neoplasm. Ann Burns Fire Disasters. 2007;20:185-188.
- Walsh NM. Primary neuroendocrine (Merkel cell) carcinoma of the skin: morphologic diversity and implications thereof. Hum Pathol. 2001;32:680-689.
- Guenther N, Menenakos C, Braumann C, et al. Squamous cell carcinoma arising on a skin graft 64 years after primary injury. Dermatol Online J. 2007;13:27.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40:870-871.
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893.
- Ponnuvelu G, Ng MF, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169.
- Bekar A, Kahveci R, Tolunay S, et al. Metastatic gliosarcoma mass extension to a donor fascia lata graft harvest site by tumor cell contamination. World Neurosurg. 2010;73:719-721.
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692.
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Serrano-Ortega S, Buendia-Eisman A, Ortega del Olmo RM, et al. Melanoma metastasis in donor site of full-thickness skin graft. Dermatology. 2000;201:377-378.
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266.
- Yip KM, Lin J, Kumta SM. A pelvic osteosarcoma with metastasis to the donor site of the bone graft. a case report. Int Orthop. 1996;20:389-391.
- Dias RG, Abudu A, Carter SR, et al. Tumour transfer to bone graft donor site: a case report and review of the literature of the mechanism of seeding. Sarcoma. 2000;4:57-59.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419.
- Singh C, Ibrahim S, Pang KS, et al. Implantation metastasis in a 13-year-old girl: a case report. J Orthop Surg (Hong Kong). 2003;11:94-96.
- Enion DS, Scott MJ, Gouldesbrough D. Cutaneous metastasis from a malignant fibrous histiocytoma to a limb skin graft donor site. Br J Surg. 1993;80:366.
- Yamasaki O, Terao K, Asagoe K, et al. Koebner phenomenon on skin graft donor site in cutaneous angiosarcoma. Eur J Dermatol. 2001;11:584-586.
- Hogan BV, Peter MB, Shenoy HG, et al. Surgery induced immunosuppression. Surgeon. 2011;9:38-43.
- Neeman E, Ben-Eliyahu S. The perioperative period and promotion of cancer metastasis: new outlooks on mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30(suppl):32-40.
- Agostino D, Cliffton EE. Trauma as a cause of localization of blood-borne metastases: preventive effect of heparin and fibrinolysin. Ann Surg. 1965;161:97-102.
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutely in a skin graft donor site. J Trauma. 1987;27:681-683.
- Korula R, Hughes CF. Squamous cell carcinoma arising in a sternotomy scar. Ann Thorac Surg. 1991;51:667-669.
- Kennedy CTC, Burd DAR, Creamer D. Mechanical and thermal injury. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 2. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2010:28.1-28.94.
- Durrani AJ, Miller RJ, Davies M. Basal cell carcinoma arising in a laparoscopic port site scar at the umbilicus. Plast Reconstr Surg. 2005;116:348-350.
- Kotwal S, Madaan S, Prescott S, et al. Unusual squamous cell carcinoma of the scrotum arising from a well healed, innocuous scar of an infertility procedure: a case report. Ann R Coll Surg Engl. 2007;89:17-19.
- Ozyazgan I, Kontas O. Previous injuries or scars as risk factors for the development of basal cell carcinoma. Scand J Plast Reconstr Surg Hand Surg. 2004;38:11-15.
Case Report
A 70-year-old man with history of coronary artery disease presented with a growing lesion on the right leg of 1 year’s duration. The lesion developed at a vein graft donor site for a coronary artery bypass that had been performed 18 years prior to presentation. The patient reported that the lesion was sensitive to touch. Physical examination revealed a 27-mm, firm, violaceous plaque on the medial aspect of the right upper shin (Figure 1). Mild pitting edema also was noted on both lower legs but was more prominent on the right leg. A 6-mm punch biopsy was performed.

Histology showed diffuse infiltration of the dermis and subcutaneous fat by intermediate-sized atypical blue cells with scant cytoplasm (Figure 2). The tumor exhibited moderate cytologic atypia with occasional mitotic figures, and lymphovascular invasion was present. Staining for CD3 was negative within the tumor, but a few reactive lymphocytes were highlighted at the periphery. Staining for CD20 and CD30 was negative. Strong and diffuse staining for cyto-keratin 20 and pan-cytokeratin was noted within the tumor with the distinctive perinuclear pattern characteristic of Merkel cell carcinoma (MCC). Staining for cytokeratin 7 was negative. Synaptophysin and chromogranin were strongly and diffusely positive within the tumor, consistent with a diagnosis of MCC.

The patient was found to have stage IIA (T2N0M0) MCC. Computed tomography completed for staging showed no evidence of metastasis. Wide local excision of the lesion was performed. Margins were negative, as was a right inguinal sentinel lymph node dissection. Because of the size of the tumor and the presence of lymphovascular invasion, radiation therapy at the primary tumor site was recommended. Local radiation treatment (200 cGy daily) was administered for a total dose of 5000 cGy over 5 weeks. The patient currently is free of recurrence or metastases and is being followed by the oncology, surgery, and dermatology departments.
Comment
Merkel cell carcinoma is a rare aggressive cutaneous malignancy. The exact pathogenesis is unknown, but immunosuppression and UV radiation, possibly through its immunosuppressive effects, appear to be contributing factors. More recently, the Merkel cell polyomavirus has been linked to MCC in approximately 80% of cases.1,2
Merkel cell carcinoma is more common in individuals with fair skin, and the average age at diagnosis is 69 years.1 Patients typically present with an asymptomatic, firm, erythematous or violaceous, dome-shaped nodule or a small indurated plaque, most commonly on sun-exposed areas of the head and neck followed by the upper and lower extremities including the hands, feet, ankles, and wrists. Fifteen percent to 20% of MCCs develop on the legs and feet.1 Our patient presented with an MCC that developed on the right shin at a vein graft donor site.
The development of a cutaneous malignancy in a chronic wound (also known as a Marjolin ulcer) is a rare but well-recognized process. These malignancies occur in previously traumatized or chronically inflamed wounds and have been found to occur most commonly in chronic burn wounds, especially in ungrafted full-thickness burns. Squamous cell carcinomas (SCCs) are the most common malignancies to arise in chronic wounds, but basal cell carcinomas, adenocarcinomas, melanomas, malignant fibrous histiocytomas, adenoacanthomas, liposarcomas, and osteosarcomas also have been reported.3 There also have been a few reports of MCC associated with Bowen disease that developed in burn wounds.4 These malignancies generally occur years after injury (average, 35.5 years), but there have been reports of keratoacanthomas developing as early as 3 weeks after injury.5,6
In some reports, malignancies in skin graft donor sites are differentiated from Marjolin ulcers, as the former appear in healed surgical wounds rather than in chronic unstable wounds and tend to occur sooner (ie, in weeks to months after graft harvesting).7,8 The development of these malignancies in graft donor sites is not as well recognized and has been reported in donor sites for split-thickness skin grafts (STSGs), full-thickness skin grafts, tendon grafts, and bone grafts. In addition to malignancies that arise de novo, some develop due to metastatic and iatrogenic spread. The majority of reported malignancies in tendon and bone graft donor sites have been due to metastasis or iatrogenic spread.9-14
Iatrogenic implantation of tumor cells is a well-recognized phenomenon. Hussain et al10 reported a case of implantation of SCC in an STSG donor site, most likely due to direct seeding from a hollow needle used to infiltrate local anesthetic in the tumor area and the STSG. In this case, metastasis could not be completely ruled out.10 There also have been reports of osteosarcoma, ameloblastoma, scirrhous carcinoma of the breast, and malignant fibrous histiocytoma thought to be implanted at bone graft donor sites.14-17 Iatrogenic spread of malignancies can occur through seeding from contaminated gloves or instruments such as hollow bore needles or trocar placement in laparoscopic surgery.11 Airborne spread also may be possible, as viable melanoma cells have been detected in electrocautery plume in mice.13
Metastatic malignancies including metastases from SCC, adenocarcinoma, melanoma, malignant fibrous histiocytoma, angiosarcoma, and osteosarcoma also have been reported to develop in graft donor sites.11,13,18,19 Many malignancies thought to have developed from iatrogenic seeding may actually be from metastasis either by hematogenous or lymphatic spread. A possible contributing factor may be surgery-induced immunosuppression, which has been linked to increased tumor metastasis formation.20 Surgery or trauma have been shown to have an effect on cellular components of the immune system, causing changes such as a shift in T lymphocytes toward immune-suppressive T lymphocytes and impaired function of natural killer cells, neutrophils, and macrophages.20 The suppression of cell-mediated immunity has been shown to decrease over days to weeks in the postoperative period.21 In addition to surgery- or trauma-induced immunosuppression, the risk for metastasis may increase due to increased vascular, including lymphatic, flow toward a skin graft donor site.13,16 Furthermore, trauma predisposes areas to a hypercoagulable state with increased sludging as well as increased platelet counts and fibrinogen levels, which may lead to localization of metastatic lesions.22 All of these factors could potentially work simultaneously to induce the development of metastasis in graft donor sites.
We found that SCCs and keratoacanthomas, which may be a variant of SCC, are among the only primary malignancies that have been reported to develop in skin graft donor sites.6-8 Malignancies in these donor sites appear to develop sooner than those found in chronic wounds and are reported to develop within weeks to several months postoperatively, even in as few as 2 weeks.6,8 Tamir et al6 reported 2 keratoacanthomas that developed simultaneously in a burn scar and STSG donor site. The investigators believed it could be a sign of reduced immune surveillance in the 2 affected areas.6 It has been hypothesized that one cause of local immune suppression in Marjolin ulcers could be due to poor lymphatic regeneration in scar tissue, which would prevent delivery of antigens and stimulated lymphocytes.23 Haik et al7 considered this possibility when discussing a case of SCC that developed at the site of an STSG. The authors did not feel it applied, however, as the donor site had only undergone a single skin harvesting procedure.7 Ponnuvelu et al8 felt that inflammation was the underlying etiology behind the 2 cases they reported of SCCs that developed in STSG donor sites. The inflammation associated with tumors has many of the same processes involved in wound healing (eg, cellular proliferation, angiogenesis). Ponnuvelu et al8 hypothesized that the local inflammation caused by graft harvesting produced an ideal environment for early carcinogenesis. Although in chronic wounds it is believed that continual repair and regeneration in recurrent ulceration contributes to neoplastic initiation, it is thought that even a single injury may lead to malignant change, which may be because prior actinic damage or another cause has made the area more susceptible to these changes.24,25 Surgery-induced immunosuppression also may play a role in development of primary malignancies in graft donor sites.
There have been a few reports of SCCs and basal cell carcinomas occurring in other surgical scars that healed without complications.24,26-28 Similar to the malignancies in graft donor sites, some authors differentiate malignancies that occur in surgical scars that heal without complications from Marjolin ulcers, as they do not occur in chronically irritated wounds. These malignancies have been reported in scars from sternotomies, an infertility procedure, hair transplantation, thyroidectomy, colostomy, cleft lip repair, inguinal hernia repair, and paraumbilical laparoscopic port site. The time between surgery and diagnosis of malignancy ranged from 9 months to 67 years.24,26-28 The development of malignancies in these surgical scars may be due to local immunosuppression, possibly from decreased lymphatic flow; additionally, the inflammation in wound healing may provide the ideal environment for carcinogenesis. Trauma in areas already susceptible to malignant change could be a contributing factor.
Conclusion
Our patient developed an MCC in a vein graft donor site 18 years after vein harvesting. It was likely a primary tumor, as vein harvesting was done for coronary artery bypass graft. There was no evidence of any other lesions on physical examination or computed tomography, making it doubtful that an MCC serving as a primary lesion for seeding or metastasis was present. If such a lesion had been present at that time, it would likely have spread well before the time of presentation to our clinic due to the fast doubling time and high rate of metastasis characteristic of MCCs, further lessening the possibility of metastasis or implantation.
The extended length of time from procedure to lesion development in our patient is much longer than for other reported malignancies in graft donor sites, but the reported time for malignancies in other postsurgical scars is more varied. Regardless of whether the MCC in our patient is classified as a Marjolin ulcer, the pathogenesis is unclear. It is thought that a single injury could lead to malignant change in predisposed skin. Our patient’s legs did not have any evidence of prior actinic damage; however, it is likely that he had local immune suppression, which may have made him more susceptible to these changes. It is unlikely that surgery-induced immunosuppression played a role in our patient, as specific cellular components of the immune system only appear to be affected over days to weeks in the postoperative period. Although poor lymphatic regeneration in scar tissue leading to decreased immune surveillance is not generally thought to contribute to malignancies in most surgical scars, our patient underwent vein harvesting. Chronic edema commonly occurs after vein harvesting and is believed to be due to trauma to the lymphatics. Local immune suppression also may have led to increased susceptibility to infection by the MCC polyomavirus, which has been found to be associated with many MCCs. In addition, the area may have been more susceptible to carcinogenesis due to changes from inflammation from wound healing. We suspect together these factors contributed to the development of our patient’s MCC. Although rare, graft donor sites should be examined periodically for the development of malignancy.
Case Report
A 70-year-old man with history of coronary artery disease presented with a growing lesion on the right leg of 1 year’s duration. The lesion developed at a vein graft donor site for a coronary artery bypass that had been performed 18 years prior to presentation. The patient reported that the lesion was sensitive to touch. Physical examination revealed a 27-mm, firm, violaceous plaque on the medial aspect of the right upper shin (Figure 1). Mild pitting edema also was noted on both lower legs but was more prominent on the right leg. A 6-mm punch biopsy was performed.

Histology showed diffuse infiltration of the dermis and subcutaneous fat by intermediate-sized atypical blue cells with scant cytoplasm (Figure 2). The tumor exhibited moderate cytologic atypia with occasional mitotic figures, and lymphovascular invasion was present. Staining for CD3 was negative within the tumor, but a few reactive lymphocytes were highlighted at the periphery. Staining for CD20 and CD30 was negative. Strong and diffuse staining for cyto-keratin 20 and pan-cytokeratin was noted within the tumor with the distinctive perinuclear pattern characteristic of Merkel cell carcinoma (MCC). Staining for cytokeratin 7 was negative. Synaptophysin and chromogranin were strongly and diffusely positive within the tumor, consistent with a diagnosis of MCC.

The patient was found to have stage IIA (T2N0M0) MCC. Computed tomography completed for staging showed no evidence of metastasis. Wide local excision of the lesion was performed. Margins were negative, as was a right inguinal sentinel lymph node dissection. Because of the size of the tumor and the presence of lymphovascular invasion, radiation therapy at the primary tumor site was recommended. Local radiation treatment (200 cGy daily) was administered for a total dose of 5000 cGy over 5 weeks. The patient currently is free of recurrence or metastases and is being followed by the oncology, surgery, and dermatology departments.
Comment
Merkel cell carcinoma is a rare aggressive cutaneous malignancy. The exact pathogenesis is unknown, but immunosuppression and UV radiation, possibly through its immunosuppressive effects, appear to be contributing factors. More recently, the Merkel cell polyomavirus has been linked to MCC in approximately 80% of cases.1,2
Merkel cell carcinoma is more common in individuals with fair skin, and the average age at diagnosis is 69 years.1 Patients typically present with an asymptomatic, firm, erythematous or violaceous, dome-shaped nodule or a small indurated plaque, most commonly on sun-exposed areas of the head and neck followed by the upper and lower extremities including the hands, feet, ankles, and wrists. Fifteen percent to 20% of MCCs develop on the legs and feet.1 Our patient presented with an MCC that developed on the right shin at a vein graft donor site.
The development of a cutaneous malignancy in a chronic wound (also known as a Marjolin ulcer) is a rare but well-recognized process. These malignancies occur in previously traumatized or chronically inflamed wounds and have been found to occur most commonly in chronic burn wounds, especially in ungrafted full-thickness burns. Squamous cell carcinomas (SCCs) are the most common malignancies to arise in chronic wounds, but basal cell carcinomas, adenocarcinomas, melanomas, malignant fibrous histiocytomas, adenoacanthomas, liposarcomas, and osteosarcomas also have been reported.3 There also have been a few reports of MCC associated with Bowen disease that developed in burn wounds.4 These malignancies generally occur years after injury (average, 35.5 years), but there have been reports of keratoacanthomas developing as early as 3 weeks after injury.5,6
In some reports, malignancies in skin graft donor sites are differentiated from Marjolin ulcers, as the former appear in healed surgical wounds rather than in chronic unstable wounds and tend to occur sooner (ie, in weeks to months after graft harvesting).7,8 The development of these malignancies in graft donor sites is not as well recognized and has been reported in donor sites for split-thickness skin grafts (STSGs), full-thickness skin grafts, tendon grafts, and bone grafts. In addition to malignancies that arise de novo, some develop due to metastatic and iatrogenic spread. The majority of reported malignancies in tendon and bone graft donor sites have been due to metastasis or iatrogenic spread.9-14
Iatrogenic implantation of tumor cells is a well-recognized phenomenon. Hussain et al10 reported a case of implantation of SCC in an STSG donor site, most likely due to direct seeding from a hollow needle used to infiltrate local anesthetic in the tumor area and the STSG. In this case, metastasis could not be completely ruled out.10 There also have been reports of osteosarcoma, ameloblastoma, scirrhous carcinoma of the breast, and malignant fibrous histiocytoma thought to be implanted at bone graft donor sites.14-17 Iatrogenic spread of malignancies can occur through seeding from contaminated gloves or instruments such as hollow bore needles or trocar placement in laparoscopic surgery.11 Airborne spread also may be possible, as viable melanoma cells have been detected in electrocautery plume in mice.13
Metastatic malignancies including metastases from SCC, adenocarcinoma, melanoma, malignant fibrous histiocytoma, angiosarcoma, and osteosarcoma also have been reported to develop in graft donor sites.11,13,18,19 Many malignancies thought to have developed from iatrogenic seeding may actually be from metastasis either by hematogenous or lymphatic spread. A possible contributing factor may be surgery-induced immunosuppression, which has been linked to increased tumor metastasis formation.20 Surgery or trauma have been shown to have an effect on cellular components of the immune system, causing changes such as a shift in T lymphocytes toward immune-suppressive T lymphocytes and impaired function of natural killer cells, neutrophils, and macrophages.20 The suppression of cell-mediated immunity has been shown to decrease over days to weeks in the postoperative period.21 In addition to surgery- or trauma-induced immunosuppression, the risk for metastasis may increase due to increased vascular, including lymphatic, flow toward a skin graft donor site.13,16 Furthermore, trauma predisposes areas to a hypercoagulable state with increased sludging as well as increased platelet counts and fibrinogen levels, which may lead to localization of metastatic lesions.22 All of these factors could potentially work simultaneously to induce the development of metastasis in graft donor sites.
We found that SCCs and keratoacanthomas, which may be a variant of SCC, are among the only primary malignancies that have been reported to develop in skin graft donor sites.6-8 Malignancies in these donor sites appear to develop sooner than those found in chronic wounds and are reported to develop within weeks to several months postoperatively, even in as few as 2 weeks.6,8 Tamir et al6 reported 2 keratoacanthomas that developed simultaneously in a burn scar and STSG donor site. The investigators believed it could be a sign of reduced immune surveillance in the 2 affected areas.6 It has been hypothesized that one cause of local immune suppression in Marjolin ulcers could be due to poor lymphatic regeneration in scar tissue, which would prevent delivery of antigens and stimulated lymphocytes.23 Haik et al7 considered this possibility when discussing a case of SCC that developed at the site of an STSG. The authors did not feel it applied, however, as the donor site had only undergone a single skin harvesting procedure.7 Ponnuvelu et al8 felt that inflammation was the underlying etiology behind the 2 cases they reported of SCCs that developed in STSG donor sites. The inflammation associated with tumors has many of the same processes involved in wound healing (eg, cellular proliferation, angiogenesis). Ponnuvelu et al8 hypothesized that the local inflammation caused by graft harvesting produced an ideal environment for early carcinogenesis. Although in chronic wounds it is believed that continual repair and regeneration in recurrent ulceration contributes to neoplastic initiation, it is thought that even a single injury may lead to malignant change, which may be because prior actinic damage or another cause has made the area more susceptible to these changes.24,25 Surgery-induced immunosuppression also may play a role in development of primary malignancies in graft donor sites.
There have been a few reports of SCCs and basal cell carcinomas occurring in other surgical scars that healed without complications.24,26-28 Similar to the malignancies in graft donor sites, some authors differentiate malignancies that occur in surgical scars that heal without complications from Marjolin ulcers, as they do not occur in chronically irritated wounds. These malignancies have been reported in scars from sternotomies, an infertility procedure, hair transplantation, thyroidectomy, colostomy, cleft lip repair, inguinal hernia repair, and paraumbilical laparoscopic port site. The time between surgery and diagnosis of malignancy ranged from 9 months to 67 years.24,26-28 The development of malignancies in these surgical scars may be due to local immunosuppression, possibly from decreased lymphatic flow; additionally, the inflammation in wound healing may provide the ideal environment for carcinogenesis. Trauma in areas already susceptible to malignant change could be a contributing factor.
Conclusion
Our patient developed an MCC in a vein graft donor site 18 years after vein harvesting. It was likely a primary tumor, as vein harvesting was done for coronary artery bypass graft. There was no evidence of any other lesions on physical examination or computed tomography, making it doubtful that an MCC serving as a primary lesion for seeding or metastasis was present. If such a lesion had been present at that time, it would likely have spread well before the time of presentation to our clinic due to the fast doubling time and high rate of metastasis characteristic of MCCs, further lessening the possibility of metastasis or implantation.
The extended length of time from procedure to lesion development in our patient is much longer than for other reported malignancies in graft donor sites, but the reported time for malignancies in other postsurgical scars is more varied. Regardless of whether the MCC in our patient is classified as a Marjolin ulcer, the pathogenesis is unclear. It is thought that a single injury could lead to malignant change in predisposed skin. Our patient’s legs did not have any evidence of prior actinic damage; however, it is likely that he had local immune suppression, which may have made him more susceptible to these changes. It is unlikely that surgery-induced immunosuppression played a role in our patient, as specific cellular components of the immune system only appear to be affected over days to weeks in the postoperative period. Although poor lymphatic regeneration in scar tissue leading to decreased immune surveillance is not generally thought to contribute to malignancies in most surgical scars, our patient underwent vein harvesting. Chronic edema commonly occurs after vein harvesting and is believed to be due to trauma to the lymphatics. Local immune suppression also may have led to increased susceptibility to infection by the MCC polyomavirus, which has been found to be associated with many MCCs. In addition, the area may have been more susceptible to carcinogenesis due to changes from inflammation from wound healing. We suspect together these factors contributed to the development of our patient’s MCC. Although rare, graft donor sites should be examined periodically for the development of malignancy.
- Swann MH, Yoon J. Merkel cell carcinoma. Semin Oncol. 2007;34:51-56.
- Schrama D, Ugurel S, Becker JC. Merkel cell carcinoma: recent insights and new treatment options. Curr Opin Oncol. 2012;24:141-149.
- Kadir AR. Burn scar neoplasm. Ann Burns Fire Disasters. 2007;20:185-188.
- Walsh NM. Primary neuroendocrine (Merkel cell) carcinoma of the skin: morphologic diversity and implications thereof. Hum Pathol. 2001;32:680-689.
- Guenther N, Menenakos C, Braumann C, et al. Squamous cell carcinoma arising on a skin graft 64 years after primary injury. Dermatol Online J. 2007;13:27.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40:870-871.
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893.
- Ponnuvelu G, Ng MF, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169.
- Bekar A, Kahveci R, Tolunay S, et al. Metastatic gliosarcoma mass extension to a donor fascia lata graft harvest site by tumor cell contamination. World Neurosurg. 2010;73:719-721.
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692.
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Serrano-Ortega S, Buendia-Eisman A, Ortega del Olmo RM, et al. Melanoma metastasis in donor site of full-thickness skin graft. Dermatology. 2000;201:377-378.
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266.
- Yip KM, Lin J, Kumta SM. A pelvic osteosarcoma with metastasis to the donor site of the bone graft. a case report. Int Orthop. 1996;20:389-391.
- Dias RG, Abudu A, Carter SR, et al. Tumour transfer to bone graft donor site: a case report and review of the literature of the mechanism of seeding. Sarcoma. 2000;4:57-59.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419.
- Singh C, Ibrahim S, Pang KS, et al. Implantation metastasis in a 13-year-old girl: a case report. J Orthop Surg (Hong Kong). 2003;11:94-96.
- Enion DS, Scott MJ, Gouldesbrough D. Cutaneous metastasis from a malignant fibrous histiocytoma to a limb skin graft donor site. Br J Surg. 1993;80:366.
- Yamasaki O, Terao K, Asagoe K, et al. Koebner phenomenon on skin graft donor site in cutaneous angiosarcoma. Eur J Dermatol. 2001;11:584-586.
- Hogan BV, Peter MB, Shenoy HG, et al. Surgery induced immunosuppression. Surgeon. 2011;9:38-43.
- Neeman E, Ben-Eliyahu S. The perioperative period and promotion of cancer metastasis: new outlooks on mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30(suppl):32-40.
- Agostino D, Cliffton EE. Trauma as a cause of localization of blood-borne metastases: preventive effect of heparin and fibrinolysin. Ann Surg. 1965;161:97-102.
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutely in a skin graft donor site. J Trauma. 1987;27:681-683.
- Korula R, Hughes CF. Squamous cell carcinoma arising in a sternotomy scar. Ann Thorac Surg. 1991;51:667-669.
- Kennedy CTC, Burd DAR, Creamer D. Mechanical and thermal injury. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 2. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2010:28.1-28.94.
- Durrani AJ, Miller RJ, Davies M. Basal cell carcinoma arising in a laparoscopic port site scar at the umbilicus. Plast Reconstr Surg. 2005;116:348-350.
- Kotwal S, Madaan S, Prescott S, et al. Unusual squamous cell carcinoma of the scrotum arising from a well healed, innocuous scar of an infertility procedure: a case report. Ann R Coll Surg Engl. 2007;89:17-19.
- Ozyazgan I, Kontas O. Previous injuries or scars as risk factors for the development of basal cell carcinoma. Scand J Plast Reconstr Surg Hand Surg. 2004;38:11-15.
- Swann MH, Yoon J. Merkel cell carcinoma. Semin Oncol. 2007;34:51-56.
- Schrama D, Ugurel S, Becker JC. Merkel cell carcinoma: recent insights and new treatment options. Curr Opin Oncol. 2012;24:141-149.
- Kadir AR. Burn scar neoplasm. Ann Burns Fire Disasters. 2007;20:185-188.
- Walsh NM. Primary neuroendocrine (Merkel cell) carcinoma of the skin: morphologic diversity and implications thereof. Hum Pathol. 2001;32:680-689.
- Guenther N, Menenakos C, Braumann C, et al. Squamous cell carcinoma arising on a skin graft 64 years after primary injury. Dermatol Online J. 2007;13:27.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40:870-871.
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893.
- Ponnuvelu G, Ng MF, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169.
- Bekar A, Kahveci R, Tolunay S, et al. Metastatic gliosarcoma mass extension to a donor fascia lata graft harvest site by tumor cell contamination. World Neurosurg. 2010;73:719-721.
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692.
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Serrano-Ortega S, Buendia-Eisman A, Ortega del Olmo RM, et al. Melanoma metastasis in donor site of full-thickness skin graft. Dermatology. 2000;201:377-378.
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266.
- Yip KM, Lin J, Kumta SM. A pelvic osteosarcoma with metastasis to the donor site of the bone graft. a case report. Int Orthop. 1996;20:389-391.
- Dias RG, Abudu A, Carter SR, et al. Tumour transfer to bone graft donor site: a case report and review of the literature of the mechanism of seeding. Sarcoma. 2000;4:57-59.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419.
- Singh C, Ibrahim S, Pang KS, et al. Implantation metastasis in a 13-year-old girl: a case report. J Orthop Surg (Hong Kong). 2003;11:94-96.
- Enion DS, Scott MJ, Gouldesbrough D. Cutaneous metastasis from a malignant fibrous histiocytoma to a limb skin graft donor site. Br J Surg. 1993;80:366.
- Yamasaki O, Terao K, Asagoe K, et al. Koebner phenomenon on skin graft donor site in cutaneous angiosarcoma. Eur J Dermatol. 2001;11:584-586.
- Hogan BV, Peter MB, Shenoy HG, et al. Surgery induced immunosuppression. Surgeon. 2011;9:38-43.
- Neeman E, Ben-Eliyahu S. The perioperative period and promotion of cancer metastasis: new outlooks on mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30(suppl):32-40.
- Agostino D, Cliffton EE. Trauma as a cause of localization of blood-borne metastases: preventive effect of heparin and fibrinolysin. Ann Surg. 1965;161:97-102.
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutely in a skin graft donor site. J Trauma. 1987;27:681-683.
- Korula R, Hughes CF. Squamous cell carcinoma arising in a sternotomy scar. Ann Thorac Surg. 1991;51:667-669.
- Kennedy CTC, Burd DAR, Creamer D. Mechanical and thermal injury. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 2. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2010:28.1-28.94.
- Durrani AJ, Miller RJ, Davies M. Basal cell carcinoma arising in a laparoscopic port site scar at the umbilicus. Plast Reconstr Surg. 2005;116:348-350.
- Kotwal S, Madaan S, Prescott S, et al. Unusual squamous cell carcinoma of the scrotum arising from a well healed, innocuous scar of an infertility procedure: a case report. Ann R Coll Surg Engl. 2007;89:17-19.
- Ozyazgan I, Kontas O. Previous injuries or scars as risk factors for the development of basal cell carcinoma. Scand J Plast Reconstr Surg Hand Surg. 2004;38:11-15.
Practice Points
- Malignancies (both primary and metastatic) can develop in graft donor sites including donor sites for split-thickness skin, full-thickness skin, tendon, bone, and vein grafts.
- Primary malignancies that develop in graft donor sites may be distinct from malignancies that develop in chronic wounds, as the former occur in healed surgical wounds and tend to occur sooner after injury (ie, weeks to months after graft harvesting versus years).
- Although the occurrence is rare, graft donor sites should be examined periodically for development of malignancies.