User login
Risk factors for nonsuicidal self-injury: A review of the evidence
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.
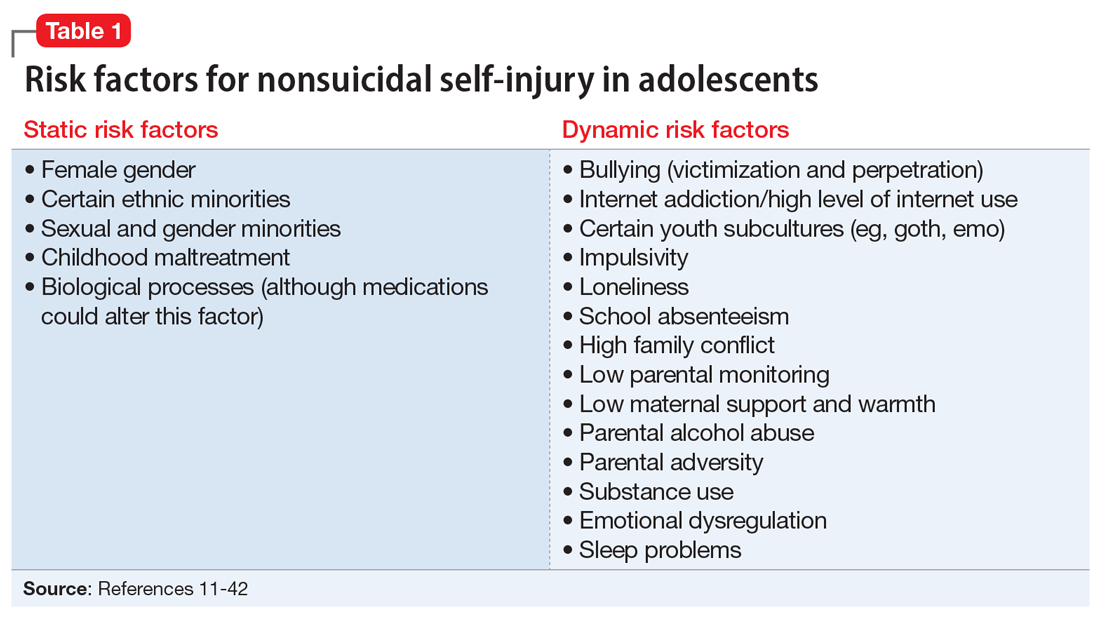
NSSI risk factors for adolescents
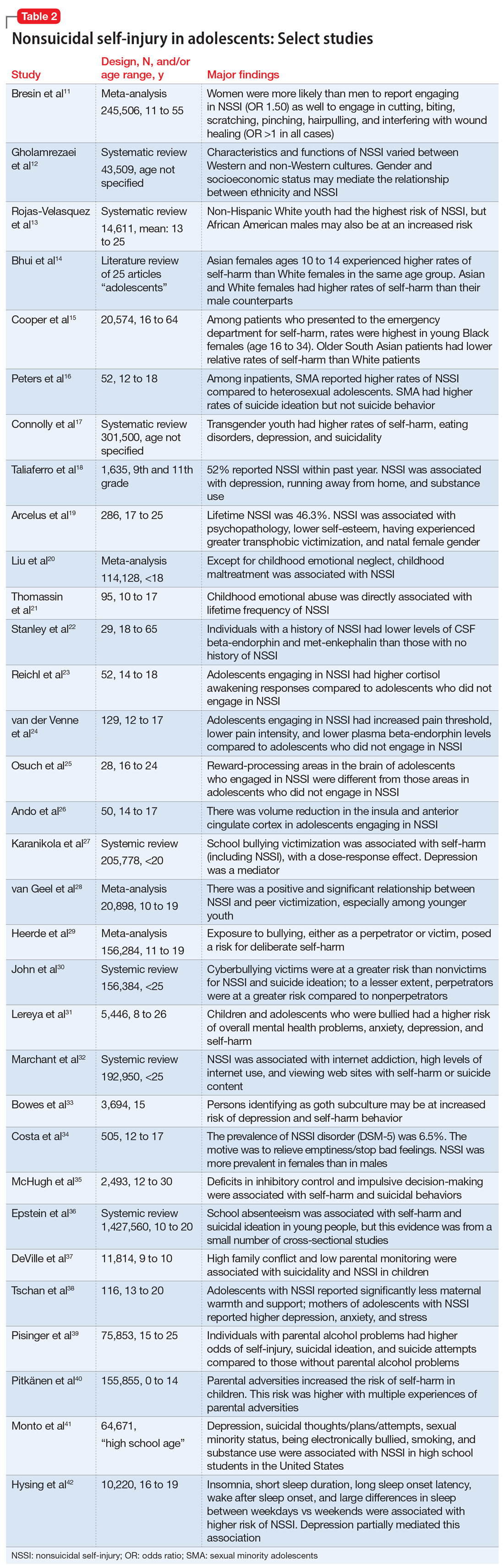
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.
Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
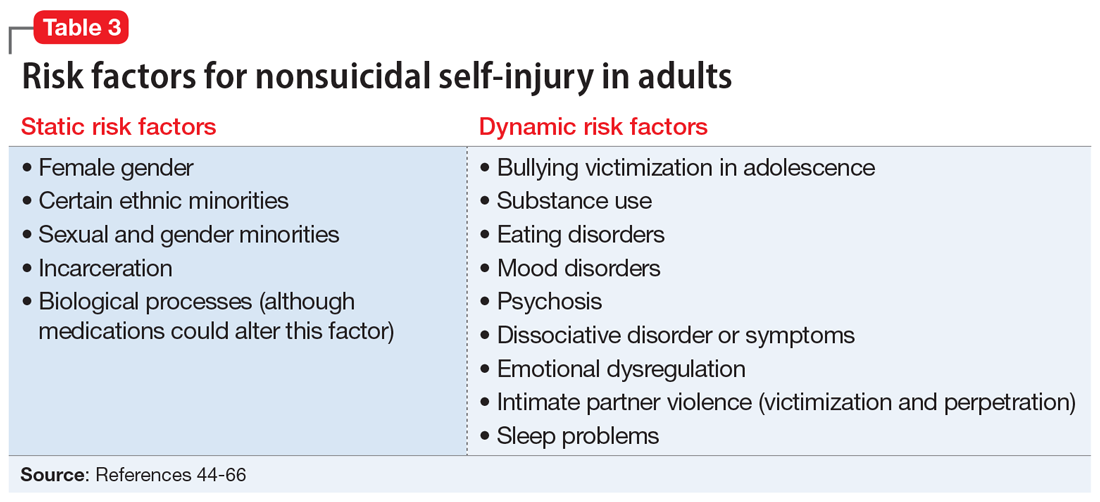
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.
Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11
As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.

NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.

Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.

Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11

As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.

NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.

Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.

Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11

As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
Off-label prescribing: How to limit your liability
The FDA defines “off-label” prescribing as prescribing an FDA-approved medication for an unapproved use, such as for an unapproved clinical indication, for a higher-than-approved dose, or for a patient who is not part of the FDA-approved population (eg, children or geriatric patients).1 Off-label prescribing is common in psychiatry; approximately 13% of psychiatry patients are prescribed off-label psychotropic medications.2 The American Psychiatric Association strongly supports “the autonomous clinical decision-making authority of a physician” and “a physician’s lawful use of an FDA-approved drug product or medical device for an off-label indication when such use is based upon sound scientific evidence in conjunction with sound medical judgment.”3 Because many psychiatric diagnoses have no FDA-approved medications, off-label prescribing often may be a psychiatrist’s only pharmacologic option.
Unfortunately, off-label prescribing can increase a psychiatrist’s risk for liability when treatment falls short of patients’ expectations, or when patients allege that they were injured by the use of an off-label medication. Off-label prescribing does not automatically lead to losing a malpractice suit because the FDA states that physicians can prescribe approved medications for any scientifically supported use, including off-label.1 Medical malpractice lawsuits alleging negligence in prescribing practices, such as off-label prescribing, typically include allegations against the psychiatrist for failure to4:
- adequately assess the patient
- consult the patient’s medical records
- obtain informed consent from the patient
- appropriately prescribe a medication for the clinical indication, dosage, patient’s age, etc.
- monitor for adverse effects and therapeutic effectiveness.
Steps to minimize your risk
When prescribing a medication off-label, the following approaches can help reduce your liability risk:
Conduct a comprehensive clinical assessment. This should include requesting and reviewing your patient’s medical records.
Explain your motivation. Explain to your patient how prescribing an off-label medication can directly benefit him/her. Make it clear that you are not conducting experimental research by prescribing off-label because some patients might perceive this as a covert form of research.
Know the medications you prescribe. Although this sounds obvious, psychiatrists should thoroughly understand how each medication they prescribe is likely to clinically affect their patient. This information is available from many sources, including the FDA’s medication information sheets and the manufacturer’s medication package inserts. If possible, make sure that your off-label prescribing is supported by reputable, peer-reviewed literature.
Obtain informed consent. Tell your patient that the medication you are recommending is being prescribed off-label. Discuss the medication’s risks, benefits, adverse effects, associated “black-box” warnings, off-label uses, and alternatives to the off-label medication.4 Allow time for the patient to ask questions about these treatments.
Continue to: Document all steps
Document all steps. There is an adage in medicine that “If it’s not written, it wasn’t done.” To help reduce your liability risk when prescribing off-label, be sure to document the following4:
- your clinical assessment
- information you gleaned from the patient’s medical records
- your review of information regarding both therapeutic and adverse effects of the medication you want to prescribe
- your discussion of informed consent, including documentation that the patient is aware that the medication is being prescribed off-label
- your clinical rationale for why the off-label medication is in the patient’s best interest.
Also, document the steps you take to monitor for adverse events and therapeutic effectiveness.4 Overall, the goal of documentation should be to support the adequate continuing care of our patients.
1. US Food and Drug Administration. Understanding unapproved use of approved drugs “off label.” https://www.fda.gov/patients/learn-about-expanded-access-and-other-treatment-options/understanding-unapproved-use-approved-drugs-label. Updated February 5, 2018. Accessed August 6, 2020.
2. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLoS One. 2018;13(7):e0198363. doi: 10.1371/journal.pone.0198363.
3. McLeer S, Mawhinney J; Council on Healthcare Systems and Financing. Position statement on off-label treatments. American Psychiatric Association. https://www.psychiatry.org/File%20Library/About-APA/Organization-Documents-Policies/Policies/Position-2016-Off-Label-Treatment.pdf. Published July 2016. Accessed August 6, 2020.
4. Funicelli A. What to consider when prescribing off-label. Psychiatric News. 2019;54(14):12.
The FDA defines “off-label” prescribing as prescribing an FDA-approved medication for an unapproved use, such as for an unapproved clinical indication, for a higher-than-approved dose, or for a patient who is not part of the FDA-approved population (eg, children or geriatric patients).1 Off-label prescribing is common in psychiatry; approximately 13% of psychiatry patients are prescribed off-label psychotropic medications.2 The American Psychiatric Association strongly supports “the autonomous clinical decision-making authority of a physician” and “a physician’s lawful use of an FDA-approved drug product or medical device for an off-label indication when such use is based upon sound scientific evidence in conjunction with sound medical judgment.”3 Because many psychiatric diagnoses have no FDA-approved medications, off-label prescribing often may be a psychiatrist’s only pharmacologic option.
Unfortunately, off-label prescribing can increase a psychiatrist’s risk for liability when treatment falls short of patients’ expectations, or when patients allege that they were injured by the use of an off-label medication. Off-label prescribing does not automatically lead to losing a malpractice suit because the FDA states that physicians can prescribe approved medications for any scientifically supported use, including off-label.1 Medical malpractice lawsuits alleging negligence in prescribing practices, such as off-label prescribing, typically include allegations against the psychiatrist for failure to4:
- adequately assess the patient
- consult the patient’s medical records
- obtain informed consent from the patient
- appropriately prescribe a medication for the clinical indication, dosage, patient’s age, etc.
- monitor for adverse effects and therapeutic effectiveness.
Steps to minimize your risk
When prescribing a medication off-label, the following approaches can help reduce your liability risk:
Conduct a comprehensive clinical assessment. This should include requesting and reviewing your patient’s medical records.
Explain your motivation. Explain to your patient how prescribing an off-label medication can directly benefit him/her. Make it clear that you are not conducting experimental research by prescribing off-label because some patients might perceive this as a covert form of research.
Know the medications you prescribe. Although this sounds obvious, psychiatrists should thoroughly understand how each medication they prescribe is likely to clinically affect their patient. This information is available from many sources, including the FDA’s medication information sheets and the manufacturer’s medication package inserts. If possible, make sure that your off-label prescribing is supported by reputable, peer-reviewed literature.
Obtain informed consent. Tell your patient that the medication you are recommending is being prescribed off-label. Discuss the medication’s risks, benefits, adverse effects, associated “black-box” warnings, off-label uses, and alternatives to the off-label medication.4 Allow time for the patient to ask questions about these treatments.
Continue to: Document all steps
Document all steps. There is an adage in medicine that “If it’s not written, it wasn’t done.” To help reduce your liability risk when prescribing off-label, be sure to document the following4:
- your clinical assessment
- information you gleaned from the patient’s medical records
- your review of information regarding both therapeutic and adverse effects of the medication you want to prescribe
- your discussion of informed consent, including documentation that the patient is aware that the medication is being prescribed off-label
- your clinical rationale for why the off-label medication is in the patient’s best interest.
Also, document the steps you take to monitor for adverse events and therapeutic effectiveness.4 Overall, the goal of documentation should be to support the adequate continuing care of our patients.
The FDA defines “off-label” prescribing as prescribing an FDA-approved medication for an unapproved use, such as for an unapproved clinical indication, for a higher-than-approved dose, or for a patient who is not part of the FDA-approved population (eg, children or geriatric patients).1 Off-label prescribing is common in psychiatry; approximately 13% of psychiatry patients are prescribed off-label psychotropic medications.2 The American Psychiatric Association strongly supports “the autonomous clinical decision-making authority of a physician” and “a physician’s lawful use of an FDA-approved drug product or medical device for an off-label indication when such use is based upon sound scientific evidence in conjunction with sound medical judgment.”3 Because many psychiatric diagnoses have no FDA-approved medications, off-label prescribing often may be a psychiatrist’s only pharmacologic option.
Unfortunately, off-label prescribing can increase a psychiatrist’s risk for liability when treatment falls short of patients’ expectations, or when patients allege that they were injured by the use of an off-label medication. Off-label prescribing does not automatically lead to losing a malpractice suit because the FDA states that physicians can prescribe approved medications for any scientifically supported use, including off-label.1 Medical malpractice lawsuits alleging negligence in prescribing practices, such as off-label prescribing, typically include allegations against the psychiatrist for failure to4:
- adequately assess the patient
- consult the patient’s medical records
- obtain informed consent from the patient
- appropriately prescribe a medication for the clinical indication, dosage, patient’s age, etc.
- monitor for adverse effects and therapeutic effectiveness.
Steps to minimize your risk
When prescribing a medication off-label, the following approaches can help reduce your liability risk:
Conduct a comprehensive clinical assessment. This should include requesting and reviewing your patient’s medical records.
Explain your motivation. Explain to your patient how prescribing an off-label medication can directly benefit him/her. Make it clear that you are not conducting experimental research by prescribing off-label because some patients might perceive this as a covert form of research.
Know the medications you prescribe. Although this sounds obvious, psychiatrists should thoroughly understand how each medication they prescribe is likely to clinically affect their patient. This information is available from many sources, including the FDA’s medication information sheets and the manufacturer’s medication package inserts. If possible, make sure that your off-label prescribing is supported by reputable, peer-reviewed literature.
Obtain informed consent. Tell your patient that the medication you are recommending is being prescribed off-label. Discuss the medication’s risks, benefits, adverse effects, associated “black-box” warnings, off-label uses, and alternatives to the off-label medication.4 Allow time for the patient to ask questions about these treatments.
Continue to: Document all steps
Document all steps. There is an adage in medicine that “If it’s not written, it wasn’t done.” To help reduce your liability risk when prescribing off-label, be sure to document the following4:
- your clinical assessment
- information you gleaned from the patient’s medical records
- your review of information regarding both therapeutic and adverse effects of the medication you want to prescribe
- your discussion of informed consent, including documentation that the patient is aware that the medication is being prescribed off-label
- your clinical rationale for why the off-label medication is in the patient’s best interest.
Also, document the steps you take to monitor for adverse events and therapeutic effectiveness.4 Overall, the goal of documentation should be to support the adequate continuing care of our patients.
1. US Food and Drug Administration. Understanding unapproved use of approved drugs “off label.” https://www.fda.gov/patients/learn-about-expanded-access-and-other-treatment-options/understanding-unapproved-use-approved-drugs-label. Updated February 5, 2018. Accessed August 6, 2020.
2. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLoS One. 2018;13(7):e0198363. doi: 10.1371/journal.pone.0198363.
3. McLeer S, Mawhinney J; Council on Healthcare Systems and Financing. Position statement on off-label treatments. American Psychiatric Association. https://www.psychiatry.org/File%20Library/About-APA/Organization-Documents-Policies/Policies/Position-2016-Off-Label-Treatment.pdf. Published July 2016. Accessed August 6, 2020.
4. Funicelli A. What to consider when prescribing off-label. Psychiatric News. 2019;54(14):12.
1. US Food and Drug Administration. Understanding unapproved use of approved drugs “off label.” https://www.fda.gov/patients/learn-about-expanded-access-and-other-treatment-options/understanding-unapproved-use-approved-drugs-label. Updated February 5, 2018. Accessed August 6, 2020.
2. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLoS One. 2018;13(7):e0198363. doi: 10.1371/journal.pone.0198363.
3. McLeer S, Mawhinney J; Council on Healthcare Systems and Financing. Position statement on off-label treatments. American Psychiatric Association. https://www.psychiatry.org/File%20Library/About-APA/Organization-Documents-Policies/Policies/Position-2016-Off-Label-Treatment.pdf. Published July 2016. Accessed August 6, 2020.
4. Funicelli A. What to consider when prescribing off-label. Psychiatric News. 2019;54(14):12.
Called to court? Tips for testifying
As a psychiatrist, you could be called to court to testify as a fact witness in a hearing or trial. Your role as a fact witness would differ from that of an expert witness in that you would likely testify about the information that you have gathered through direct observation of patients or others. Fact witnesses are generally not asked to give expert opinions regarding forensic issues, and treating psychiatrists should not do so about their patients. As a fact witness, depending on the form of litigation, you might be in one of the following 4 roles1:
- Observer. As the term implies, you have observed an event. For example, you are asked to testify about a fight that you witnessed between another clinician’s patient and a nurse while you were making your rounds on an inpatient unit.
- Non-defendant treater. You are the treating psychiatrist for a patient who is involved in litigation to recover damages for injuries sustained from a third party. For example, you are asked to testify about your patient’s premorbid functioning before a claimed injury that spurred the lawsuit.
- Plaintiff. You are suing someone else and may be claiming your own damages. For example, in your attempt to claim damages as a plaintiff, you use your clinical knowledge to testify about your own mental health symptoms and the adverse impact these have had on you.
- Defendant treater. You are being sued by one of your patients. For example, a patient brings a malpractice case against you for allegations of not meeting the standard of care. You testify about your direct observations of the patient, the diagnoses you provided, and your rationale for the implemented treatment plan.
Preparing yourself as a fact witness
For many psychiatrists, testifying can be an intimidating process. Although there are similarities between testifying in a courtroom and giving a deposition, there are also significant differences. For guidelines on providing depositions, see Knoll and Resnick’s “Deposition dos and don’ts: How to answer 8 tricky questions” (
Don’t panic. Although your first reaction may be to panic upon receiving a subpoena or court order, you should “keep your cool” and remember that the observations you made or treatment provided have already taken place.1 Your role as a fact witness is to inform the judge and jury about what you saw and did.1
Continue to: Refresh your memory and practice
Refresh your memory and practice. Gather all required information (eg, medical records, your notes, etc.) and review it before testifying. This will help you to recall the facts more accurately when you are asked a question. Consider practicing your testimony with the attorney who requested you to get feedback on how you present yourself.1 However, do not try to memorize what you are going to say because this could make your testimony sound rehearsed and unconvincing.
Plan ahead, and have a pretrial conference. Because court proceedings are unpredictable, you should clear your schedule to allow enough time to appear in court. Before your court appearance, meet with the attorney who requested you to discuss any new facts or issues as well as learn what the attorney aims to accomplish with your testimony.1
Speak clearly in your own words, and avoid jargon. Courtroom officials are unlikely to understand psychiatric jargon. Therefore, you should explain psychiatric terms in language that laypeople would comprehend. Because the court stenographer will require you to use actual words for the court transcripts, you should answer clearly and verbally or respond with a definitive “yes” or “no” (and not by nodding or shaking your head).
Testimony is also not a time for guessing. If you don’t know the answer, you should say “I don’t know.”
1. Gutheil TG. The psychiatrist in court: a survival guide. Washington, DC: American Psychiatric Press, Inc.; 1998.
2. Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
As a psychiatrist, you could be called to court to testify as a fact witness in a hearing or trial. Your role as a fact witness would differ from that of an expert witness in that you would likely testify about the information that you have gathered through direct observation of patients or others. Fact witnesses are generally not asked to give expert opinions regarding forensic issues, and treating psychiatrists should not do so about their patients. As a fact witness, depending on the form of litigation, you might be in one of the following 4 roles1:
- Observer. As the term implies, you have observed an event. For example, you are asked to testify about a fight that you witnessed between another clinician’s patient and a nurse while you were making your rounds on an inpatient unit.
- Non-defendant treater. You are the treating psychiatrist for a patient who is involved in litigation to recover damages for injuries sustained from a third party. For example, you are asked to testify about your patient’s premorbid functioning before a claimed injury that spurred the lawsuit.
- Plaintiff. You are suing someone else and may be claiming your own damages. For example, in your attempt to claim damages as a plaintiff, you use your clinical knowledge to testify about your own mental health symptoms and the adverse impact these have had on you.
- Defendant treater. You are being sued by one of your patients. For example, a patient brings a malpractice case against you for allegations of not meeting the standard of care. You testify about your direct observations of the patient, the diagnoses you provided, and your rationale for the implemented treatment plan.
Preparing yourself as a fact witness
For many psychiatrists, testifying can be an intimidating process. Although there are similarities between testifying in a courtroom and giving a deposition, there are also significant differences. For guidelines on providing depositions, see Knoll and Resnick’s “Deposition dos and don’ts: How to answer 8 tricky questions” (
Don’t panic. Although your first reaction may be to panic upon receiving a subpoena or court order, you should “keep your cool” and remember that the observations you made or treatment provided have already taken place.1 Your role as a fact witness is to inform the judge and jury about what you saw and did.1
Continue to: Refresh your memory and practice
Refresh your memory and practice. Gather all required information (eg, medical records, your notes, etc.) and review it before testifying. This will help you to recall the facts more accurately when you are asked a question. Consider practicing your testimony with the attorney who requested you to get feedback on how you present yourself.1 However, do not try to memorize what you are going to say because this could make your testimony sound rehearsed and unconvincing.
Plan ahead, and have a pretrial conference. Because court proceedings are unpredictable, you should clear your schedule to allow enough time to appear in court. Before your court appearance, meet with the attorney who requested you to discuss any new facts or issues as well as learn what the attorney aims to accomplish with your testimony.1
Speak clearly in your own words, and avoid jargon. Courtroom officials are unlikely to understand psychiatric jargon. Therefore, you should explain psychiatric terms in language that laypeople would comprehend. Because the court stenographer will require you to use actual words for the court transcripts, you should answer clearly and verbally or respond with a definitive “yes” or “no” (and not by nodding or shaking your head).
Testimony is also not a time for guessing. If you don’t know the answer, you should say “I don’t know.”
As a psychiatrist, you could be called to court to testify as a fact witness in a hearing or trial. Your role as a fact witness would differ from that of an expert witness in that you would likely testify about the information that you have gathered through direct observation of patients or others. Fact witnesses are generally not asked to give expert opinions regarding forensic issues, and treating psychiatrists should not do so about their patients. As a fact witness, depending on the form of litigation, you might be in one of the following 4 roles1:
- Observer. As the term implies, you have observed an event. For example, you are asked to testify about a fight that you witnessed between another clinician’s patient and a nurse while you were making your rounds on an inpatient unit.
- Non-defendant treater. You are the treating psychiatrist for a patient who is involved in litigation to recover damages for injuries sustained from a third party. For example, you are asked to testify about your patient’s premorbid functioning before a claimed injury that spurred the lawsuit.
- Plaintiff. You are suing someone else and may be claiming your own damages. For example, in your attempt to claim damages as a plaintiff, you use your clinical knowledge to testify about your own mental health symptoms and the adverse impact these have had on you.
- Defendant treater. You are being sued by one of your patients. For example, a patient brings a malpractice case against you for allegations of not meeting the standard of care. You testify about your direct observations of the patient, the diagnoses you provided, and your rationale for the implemented treatment plan.
Preparing yourself as a fact witness
For many psychiatrists, testifying can be an intimidating process. Although there are similarities between testifying in a courtroom and giving a deposition, there are also significant differences. For guidelines on providing depositions, see Knoll and Resnick’s “Deposition dos and don’ts: How to answer 8 tricky questions” (
Don’t panic. Although your first reaction may be to panic upon receiving a subpoena or court order, you should “keep your cool” and remember that the observations you made or treatment provided have already taken place.1 Your role as a fact witness is to inform the judge and jury about what you saw and did.1
Continue to: Refresh your memory and practice
Refresh your memory and practice. Gather all required information (eg, medical records, your notes, etc.) and review it before testifying. This will help you to recall the facts more accurately when you are asked a question. Consider practicing your testimony with the attorney who requested you to get feedback on how you present yourself.1 However, do not try to memorize what you are going to say because this could make your testimony sound rehearsed and unconvincing.
Plan ahead, and have a pretrial conference. Because court proceedings are unpredictable, you should clear your schedule to allow enough time to appear in court. Before your court appearance, meet with the attorney who requested you to discuss any new facts or issues as well as learn what the attorney aims to accomplish with your testimony.1
Speak clearly in your own words, and avoid jargon. Courtroom officials are unlikely to understand psychiatric jargon. Therefore, you should explain psychiatric terms in language that laypeople would comprehend. Because the court stenographer will require you to use actual words for the court transcripts, you should answer clearly and verbally or respond with a definitive “yes” or “no” (and not by nodding or shaking your head).
Testimony is also not a time for guessing. If you don’t know the answer, you should say “I don’t know.”
1. Gutheil TG. The psychiatrist in court: a survival guide. Washington, DC: American Psychiatric Press, Inc.; 1998.
2. Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
1. Gutheil TG. The psychiatrist in court: a survival guide. Washington, DC: American Psychiatric Press, Inc.; 1998.
2. Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
How to handle unsolicited e-mails
The ubiquitous use of e-mail has opened the proverbial “Pandora’s box” of access to psychiatrists. Our e-mail addresses are readily available online via search engines or on hospital Web sites. E-mail has become a convenient method of communicating with patients; however, it also has resulted in a proliferation of unsolicited e-mails sent to physicians from people they don’t know seeking professional advice.1 If you publish medical literature or make media appearances, you may be contacted by such individuals requesting your expertise.
Unsolicited e-mails present psychiatrists with ethical and legal quandaries that force them to consider how they can balance the human reflex to offer assistance against the potential ramifications of replying. These conundrums include:
- whether the sender is an actual person, and whether he or she is asking for advice
- the risks of replying vs not replying
- the possibility that there is a plausible crisis or danger to the sender or others
- the potential for establishing a doctor–patient relationship by replying
- the legal liability that might be incurred by replying.2
Take preemptive measures
There is guidance on how to e-mail your patients and respond to solicited e-mails, but there is a dearth of literature on how to respond to unsolicited e-mails. Anecdotal reports and limited literature suggest several possible measures you could take for managing unsolicited e-mails:
- Establish a policy of never opening unsolicited e-mails
- Create a strict junk-mail filter to prevent unsolicited e-mails from being delivered to your inbox
- Set up an automatic reply stating that unwanted or unsolicited e-mails will not be read and/or that no reply will be provided
- Read unsolicited e-mails, but immediately delete them without replying
- Acknowledge the sender in a reply, but state that you are unable to assist and decline further contact
- Send a generic reply clarifying that you are unable to provide medical assistance, and encourage the sender to seek help locally.2
Despite the urge to help, consider the consequences
In addition to taking up valuable time, unsolicited e-mails create legal and ethical predicaments that could subject you to legal liability if you choose to reply. Even though your intentions may be altruistic and you want to be helpfu
1. D’Alessandro DM, D’Alessandro MP, Colbert S. A proposed solution for addressing the challenge of patient cries for help through an analysis of unsolicited electronic email. Pediatrics. 2000;105(6):E74.
2. Friedman SH, Appel JM, Ash P, et al. Unsolicited e-mails to forensic psychiatrists. J Am Acad Psychiatry Law. 2016;44(4):470-478.
The ubiquitous use of e-mail has opened the proverbial “Pandora’s box” of access to psychiatrists. Our e-mail addresses are readily available online via search engines or on hospital Web sites. E-mail has become a convenient method of communicating with patients; however, it also has resulted in a proliferation of unsolicited e-mails sent to physicians from people they don’t know seeking professional advice.1 If you publish medical literature or make media appearances, you may be contacted by such individuals requesting your expertise.
Unsolicited e-mails present psychiatrists with ethical and legal quandaries that force them to consider how they can balance the human reflex to offer assistance against the potential ramifications of replying. These conundrums include:
- whether the sender is an actual person, and whether he or she is asking for advice
- the risks of replying vs not replying
- the possibility that there is a plausible crisis or danger to the sender or others
- the potential for establishing a doctor–patient relationship by replying
- the legal liability that might be incurred by replying.2
Take preemptive measures
There is guidance on how to e-mail your patients and respond to solicited e-mails, but there is a dearth of literature on how to respond to unsolicited e-mails. Anecdotal reports and limited literature suggest several possible measures you could take for managing unsolicited e-mails:
- Establish a policy of never opening unsolicited e-mails
- Create a strict junk-mail filter to prevent unsolicited e-mails from being delivered to your inbox
- Set up an automatic reply stating that unwanted or unsolicited e-mails will not be read and/or that no reply will be provided
- Read unsolicited e-mails, but immediately delete them without replying
- Acknowledge the sender in a reply, but state that you are unable to assist and decline further contact
- Send a generic reply clarifying that you are unable to provide medical assistance, and encourage the sender to seek help locally.2
Despite the urge to help, consider the consequences
In addition to taking up valuable time, unsolicited e-mails create legal and ethical predicaments that could subject you to legal liability if you choose to reply. Even though your intentions may be altruistic and you want to be helpfu
The ubiquitous use of e-mail has opened the proverbial “Pandora’s box” of access to psychiatrists. Our e-mail addresses are readily available online via search engines or on hospital Web sites. E-mail has become a convenient method of communicating with patients; however, it also has resulted in a proliferation of unsolicited e-mails sent to physicians from people they don’t know seeking professional advice.1 If you publish medical literature or make media appearances, you may be contacted by such individuals requesting your expertise.
Unsolicited e-mails present psychiatrists with ethical and legal quandaries that force them to consider how they can balance the human reflex to offer assistance against the potential ramifications of replying. These conundrums include:
- whether the sender is an actual person, and whether he or she is asking for advice
- the risks of replying vs not replying
- the possibility that there is a plausible crisis or danger to the sender or others
- the potential for establishing a doctor–patient relationship by replying
- the legal liability that might be incurred by replying.2
Take preemptive measures
There is guidance on how to e-mail your patients and respond to solicited e-mails, but there is a dearth of literature on how to respond to unsolicited e-mails. Anecdotal reports and limited literature suggest several possible measures you could take for managing unsolicited e-mails:
- Establish a policy of never opening unsolicited e-mails
- Create a strict junk-mail filter to prevent unsolicited e-mails from being delivered to your inbox
- Set up an automatic reply stating that unwanted or unsolicited e-mails will not be read and/or that no reply will be provided
- Read unsolicited e-mails, but immediately delete them without replying
- Acknowledge the sender in a reply, but state that you are unable to assist and decline further contact
- Send a generic reply clarifying that you are unable to provide medical assistance, and encourage the sender to seek help locally.2
Despite the urge to help, consider the consequences
In addition to taking up valuable time, unsolicited e-mails create legal and ethical predicaments that could subject you to legal liability if you choose to reply. Even though your intentions may be altruistic and you want to be helpfu
1. D’Alessandro DM, D’Alessandro MP, Colbert S. A proposed solution for addressing the challenge of patient cries for help through an analysis of unsolicited electronic email. Pediatrics. 2000;105(6):E74.
2. Friedman SH, Appel JM, Ash P, et al. Unsolicited e-mails to forensic psychiatrists. J Am Acad Psychiatry Law. 2016;44(4):470-478.
1. D’Alessandro DM, D’Alessandro MP, Colbert S. A proposed solution for addressing the challenge of patient cries for help through an analysis of unsolicited electronic email. Pediatrics. 2000;105(6):E74.
2. Friedman SH, Appel JM, Ash P, et al. Unsolicited e-mails to forensic psychiatrists. J Am Acad Psychiatry Law. 2016;44(4):470-478.
Hypnotics and driving: FDA action, clinical trials show need for precautions
“Sleep driving” blamed on the hypnotic zolpidem was used as a defense last year in Virginia in a criminal case involving impaired driving. The defendant’s attorney argued that the defendant should not be held criminally liable because he was “essentially unconscious” and the accident therefore was involuntary.
The “sleep driving” defense failed when testimony revealed the defendant had taken 5 times the recommended zolpidem dose before the accident. The judge found him guilty of a felony charge of driving under the influence of a sleep medication.1
Sedative-hypnotics are increasingly being used to treat insomnia2-4 and as a result some patients try to drive while under the drugs’ sedating effects. Also, new FDA-ordered labeling for all 13 available prescription sleep aids warns of potential risks of “complex sleep-related behaviors,” including driving, phoning, and eating while asleep (Box 1).
Hypnotics can improve quality of life and well-being by addressing insomnia’s complications—hypertension, diabetes, coronary artery disease, depression, and anxiety5-7—but they also have been associated with impaired motor coordination and somnambulism. To help you and your patients weigh sleep medications’ relative risks and benefits, we report on clinical studies and court cases in the literature. Most of the data focus on zolpidem, by far the most prescribed hypnotic (Box 2).8,9
Labeling of all sedative-hypnotic drugs now carries FDA-ordered precautions about “sleep-driving and other complex behaviors” that may occur without the patient being fully awake. FDA cited reports of patients preparing and eating food, making phone calls, and having sex after taking a sedative-hypnotic, usually without memory of the event. A warning also was added about rare, potentially fatal anaphylactic reactions in patients taking first or later doses of sleep medications.
Steven Galson, MD, MPH, director of FDA’s Center for Drug Evaluation and Research, said the labeling changes were needed to inform patients and prescribers about the risks of sleep aids that “are well-tolerated and effective for many people.”
Source: Walsh S, Rawlings K. FDA requests label change for all sleep disorder drug products. Available at www.fda.gov/bbs/topics/NEWS/2007/NEW01587.html.
Zolpidem incidents and cases
In 2005, Americans filled 43 million prescriptions for sedative-hypnotics—26.5 million for zolpidem alone—compared with 29 million prescriptions in 2001.4 In addition to the new the FDA-requested warnings about sleep-related behaviors, zolpidem’s labeling cautions patients about operating heavy machinery, driving, or engaging in hazardous occupations after taking the drug. The manufacturer tells patients:
- to ingest zolpidem only before going to bed
- that they may experience residual sedation the following day.
Impaired driving. Besides the “sleep driving” case in Virginia, a highly publicized zolpidem-related driving incident occurred May 4, 2006, when U.S. Representative Patrick Kennedy was involved in an accident after having taken zolpidem in combination with an antinausea medication. Another driving-related case has used zolpidem as a defense for impairment, but the court decided that the medication was not at fault because the defendant also had ingested alcohol.10
Other litigation. Although zolpidem-related impairment apparently has not been used successfully as a defense for a driving incident, class action suits alleging failure to disclose potentially harmful side effects have been filed against the manufacturer.
In Janet Makinen and others v. sanofi-aventis,11 at least 500 plaintiffs claim zolpidem is related to sleep-driving, sleep-eating, and other somnambulistic behaviors. Plaintiffs allege negligence, breach of implied warranties, fraud, unfair trade practices, express warranty violations, strict liability, and consumer fraud violations. Other suits claim dangerous sleep-related side effects with zolpidem use.12
What clinical evidence shows
Driving impairment. Clinical studies have shown conflicting results about driving impairment associated with zolpidem. The literature falls into 2 categories, based on treatment duration:
- Zolpidem affects performance and memory within the first 4 to 5 hours of administration (Table 1).
- Beyond 5 hours, no residual effects on performance have been identified (Table 2), and repeat nightly dosing has not caused impairment or tolerance.
- All sedative hypnotic benzodiazepines had statistically significant residual effects 10 to 11 hours after ingestion, with longer periods of impairment corresponding to medications with longer half-lives.
- Zopiclone was associated with significant residual impairment for up to 10 hours after ingestion.
- Zolpidem and zaleplon showed no significant impairment in driving 10 to 11 hours after ingestion. Impairment was found, however, when zolpidem was taken within 5 hours of driving.14-18
Table 1
Studies of zolpidem-associated driving skills impairment
(
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Wilkinson, 199514 Blinded; 29 subjects | Zolpidem, 10 mg, 15 mg, and placebo in combination with an alcoholic drink (to reach a BAC of 0.08%) or placebo drink; testing 45 min, 130 min, and 230 min after administration | Visual backward masking test (approximates driving performance) and attention tests | Zolpidem produced significant impairment in combination with alcohol and when administered alone during peak effect assessment; alcohol did not potentiate zolpidem’s effects; additive effects of alcohol seen with 10-mg dose but not 15-mg dose of zolpidem |
| Rush et al, 199815 Double-blind, crossover; 9 subjects | Zolpidem, 7.5, 15, and 22.5 mg; quazepam, 15, 30, and 45 mg; triazolam, 0.1875, 0.375, and 0.5625 mg; testing ½, 1, 1½, 2, 2½, 3, 4, 5, and 6 hours after administration | Subject- and observer-rated questionnaires; tests of recall and delayed recognition | Performance-impairing effects of zolpidem were virtually indistinguishable from those of classic benzodiazepines, such as triazolam |
| Mattila et al, 199816 Randomized, placebo-controlled, double-blind, crossover; 12 subjects | Zolpidem, 15 mg; diazepam, 15 mg; oxazepam, 30 mg; zopiclone, 7.5 mg; alcohol testing before and 1, 3½, and 5 hours after administration | Simulated driving and other measures | Zolpidem impaired coordination, reaction, and cognition at 1 and 3½ hours; tracking remained impaired at 5 hours; all agents (especially zolpidem) impaired learning and memory |
| Mintzer et al, 199917 Double-blind, placebo-controlled; 16 subjects | Zolpidem, 15 mg/70 kg (dosed by subject weight); testing ½, 1, 2, and 3 hours after administration | Memory tasks (recall, fragment completion, recognition) | Zolpidem interfered with explicit but not implicit memory after administration; zolpidem produced a specific deficit in acquisition of contextual information |
| Verster et al, 200418 2-step randomized, placebo-controlled, double-blind, crossover; 30 subjects | Zolpidem, 10 mg and 20 mg; zaleplon, 10 mg and 20 mg; middle-of-the-night dosing; testing 4 hours after dosing | On-the-road driving and other tests of attention, learning, and thinking | Zolpidem, 10 mg and 20 mg, significantly impaired driving function; zolpidem, 20 mg, produced significant impairment on all psychomotor and memory tests; zaleplon, 10 mg and 20 mg, did not differ significantly from placebo |
| BAC: Blood alcohol concentration | |||
Studies of zolpidem-associated driving skills impairment
(>5 hours after dosing)
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Fairweather et al, 199223 Randomized, placebo-controlled; 24 older volunteers taking no other medications | Zolpidem, 5 mg or 10 mg, or placebo taken before bedtime; testing 8.5 hours after administration | Numerous, including reactive time, memory, word recognition | Zolpidem consistently helped with sleep latency, with no residual performance deficits; no tolerance seen with repeated dosing |
| Bocca et al, 199924 Double-blind, crossover; 16 volunteers | Zolpidem, 10 mg; zopiclone, 7.5 mg; flunitrazepam,* 1 mg; and placebo given at 11 PM, with testing at 9 AM | Driving simulation and real time test drive; eye movements measured after driving tests | No residual effects with zolpidem; zopiclone impaired driving ability and increased saccadic latency; flunitrazepam impaired early morning driving and saccadic eye movements longer than zopiclone |
| Partinen et al, 200320 Randomized, placebo-controlled, double-blind, 3-period crossover; 18 women with insomnia | Zolpidem, 10 mg; temazepam, 20 mg; dosing at 2 AM, testing 5.5 hours after dosing | Driving simulation; delayed word recall and memory testing (FePsy test) | No statistically significant effects on driving ability with either drug; no significant differences in FePsy results compared with baseline or placebo |
| Staner et al, 200525 Randomized, placebo-controlled, double-blind, four-way crossover; 23 subjects with DSM-IV-TR diagnosis of insomnia | Zolpidem, 10 mg; zopiclone, 7.5 mg; lormetazepam,* 1 mg; 7 days of dosing; tests given 9 to 11 hours post-dosing | Driving simulation; EEG at rest and while driving | Zolpidem showed no impairment of driving ability and no EEG changes compared with placebo; driving impairment and EEG alterations were found with zopiclone and lormetazepam |
| * Hypnotics not approved in the United States but available elsewhere. | |||
Acute effects (
Combined with alcohol. Wilkinson14 conducted a randomized, 6-way crossover study in which subjects received 10- or 15-mg doses of zolpidem or placebo plus an alcoholic beverage (enough to obtain a blood alcohol concentration [BAC] of ~0.08%) or placebo beverage. Tests given shortly after patients took the study medications showed that zolpidem caused statistically significant impairment both in combination with alcohol and alone during peak drug effect—identified as 45 minutes after ingestion. Alcohol did not potentiate the impairment associated with zolpidem.
Using a similar design, Mattila et al16 compared acute performance impairment associated with zolpidem, diazepam, oxazepam, and zopiclone. In this randomized, double-blinded, crossover study, all comparison medications impaired antecedent learning and memory, but zolpidem given at 15 mg had the greatest effect. Zolpidem impaired coordination, reactive functioning, and cognitive skills at 1 and 3.5 hours after administration, and simulated driving test performance remained impaired at 5 hours (approximately two half-lives of the medication). Of note is that the 15-mg zolpidem dose used in this study was shown by Wilkinson et al14 to be more impairing than the recommended maximum 10-mg dose.
A study from the University of Toronto19 that did not include zolpidem examined potential psychomotor performance deficits and sleepiness in a comparison of time-released melatonin, 6 mg; zaleplon, 10 mg; zopiclone, 7.5 mg; temazepam, 15 mg, and placebo. Tests were given to 9 men and 14 women, ages 21 to 53, just before drug administration and 7 hours later.
Zaleplon had the greatest effect on psychomotor performance, followed by temazepam and zopiclone. Aside from prolonged perceived sleepiness, melatonin and placebo did not interfere with performance testing.
Zolpidem, a benzodiazepine receptor agonist, was the 7th most prescribed drug in the United States in 2005 (2006 data not available).8 It is FDA-approved for short-term treatment of insomnia, although “short-term” is not defined. Package labeling states:
This nonbenzodiazepine hypnotic has been shown to decrease sleep latency and increase sleep duration for up to 35 days in controlled clinical trials. Patients should be evaluated for a primary psychiatric or medical illness if insomnia does not remit after 7 to 10 days of treatment.
An imidazopyridine that acts as an agonist of GABA A1, zolpidem produces sedation while avoiding anticonvulsant, anxiolytic, and muscle relaxation effects. Available in 5- and 10-mg tablets, the drug is rapidly absorbed in the GI tract and excreted primarily through the kidneys. Its half-life is approximately 2.5 hours (approximately 3 hours in elderly patients). The most common side effects are daytime drowsiness, dizziness, and diarrhea; others include asthenia, hiccup, and diplopia.9
- driving ability 4 hours after administration
- memory and psychomotor performance 6 hours after administration.
Partinen et al20 used the recommended zolpidem dose in a similar study of after-midnight use by women with insomnia. The double-blind, randomized, controlled trial evaluated performance with a driving simulator and neuropsychological testing 5.5 hours after medication dosing. Patients taking zolpidem, 10 mg, showed no significant impairment when compared with those taking placebo. Some patients scored poorly on the driving tests alone, and the authors concluded that this group was more susceptible to zolpidem’s effect.
Memory. In a double-blind, placebo-controlled trial by Mintzner et al,17 zolpidem dosed by patient weight at 15 mg/70 kg:
- significantly impaired explicit memory (requires conscious recollection for recall)
- did not affect implicit memory (lack of conscious awareness in the act of recollection).
These findings support complaints of zolpidem-related anterograde amnestic episodes, which also occur with some benzodiazepines (such as midazolam).
Similar to benzodiazepines? Rush et al’s results21 support Mintzer’s assertion17 that zolpidem shares many side effects with benzodiazepines. Performance impairment associated with zolpidem—as rated by subjects and observers—is virtually indistinguishable from a benzodiazepine effect, except that the duration is shorter with zolpidem (5 hours), compared with up to 10 hours for benzodiazepines.
Logan and Couper22 reviewed police reports and toxicology profiles of individuals suspected of driving while impaired. Zolpidem was found in 29 subjects, 5 of whom showed no other substances. In those 5, zolpidem blood levels ranged from 0.08 to 1.40 mg/L and did not appear to correlate with the degree of impairment.
Residual effects (>5 hours)
Older patients. In a randomized, placebo-controlled trial by Fairweather et al,23 zolpidem improved sleep latency in 24 subjects ages 63 to 80. No evidence of impairment in reactive time, memory, or word recognition was found 8.5 hours after nighttime dosing, and tolerance was not seen after 1 week of repeated dosing.
Driving impairment. Bocca et al24 compared degree of driving impairment by zolpidem, zopiclone, flunitrazepam (not approved in the United States), and placebo. The 16 subjects received each medication at 11 pm, with a 2-week washout between medications. One group of 8 was tested at 9 am and the other 8 subjects at 11 am. Those taking zolpidem showed no residual performance impairment, as measured by simulated driving, a test drive, and saccadic eye movements.
Staner et al25 reported similar results when comparing zolpidem, zopiclone, lormetazepam (not approved in the United States), and placebo. Using a driving simulator and electroencephalography (EEG), they evaluated 23 subjects diagnosed with insomnia at 9 and 11 hours post-dose. Zolpidem did not significantly impair driving ability and did not differ from placebo on EEG analysis (resting or driving). The study showed driving impairment with zopiclone and lormetazepam, along with characteristic benzodiazepine EEG changes. This study further supports evidence of limited impairment on driving after appropriate use of zolpidem.
Informed consent
In the informed consent process, failing to warn a patient about medication side effects can lead to legal claims against both manufacturers and prescribers. With any medication, patients have the right to know about a drug’s risks, benefits, and alternate therapies—including no therapy.
Two standards are associated with informed consent and negligence:
- The “reasonable practitioner” standard outlined in Natanson v. Kline (1960)26 mandates that the prescribing physician has revealed all that an “average, reasonable practitioner” would disclose in similar circumstances.
- The “reasonable patient” standard set in Canterbury v. Spence (1972)27 mandates that the prescribing physician has informed the patient about the proposed treatment, its side effects, and alternatives to the proposed treatment that a reasonable patient would consider material to the decision of whether or not to undergo treatment.
The vaccine was licensed as a prescription drug but administered through county health departments. In 1970, a nurse in a Texas Department of Health clinic administered the vaccine to 8-month-old Anita Reyes without telling the girl’s parents of warnings in the package circular. Holding Wyeth Laboratories to a reasonableness standard, the court found that the company knew or should have known how the vaccine would be distributed.
The package insert was not shown to have given inadequate warning, and the vaccine was not shown to be defective (it was a trivalent live-virus Sabin oral polio vaccine, as intended).
Vioxx cases. Similarly, some plaintiffs have been awarded millions of dollars (as in Ernst v. Merck & Co., Inc.29) in rulings that Merck & Co. failed to disclose the risk of cardiotoxicity with the arthritis drug rofecoxib (Vioxx) and thus failed to provide physicians with information needed when prescribing the drug. In Humeston v. Merck & Co.,30 a Texas court in 2005 held that Vioxx’s warning labels were adequate. In a retrial, however, the New Jersey Superior Court awarded the plaintiff $47.5 million.31
As with the polio vaccine and Vioxx litigations, courts are being asked to decide if patients were adequately informed about sleep-driving and other risks associated with the use of sedative-hypnotics.
Clinical recommendations
Zolpidem—like many other medications—carries a substantial risk of side effects, even when used appropriately. However, given the medical and mental health risks of untreated insomnia, the benefits of a medication such as zolpidem will likely outweigh its risks.
Numerous studies have shown that zolpidem is effective for improving sleep latency and that there are mild, if any, residual side effects beyond what would normally be a restful night’s sleep. Impairments are evident, however, during the hours following the drug’s administration, with some effects lasting >5 hours depending on the dose.
Risk management. When prescribing nonbenzodiazepine hypnotics such as zolpidem, you may want to adopt a risk management approach as you would with other medications that can have serious side effects. An approach to benzodiazepine prescribing proposed by Bursztajn et al31 advocates:
- using the informed-consent process to build an alliance with patients
- not prescribing the medication in isolation of other beneficial therapies
- being aware of and always documenting your decision-making process.
When you make patients aware of all risks, benefits, alternate therapies, and possible outcomes with no treatment, you have informed them effectively. Patients are then left to decide whether or not to agree to the treatment. You also are responsible for monitoring the patient, addressing the patient’s questions, and relaying important safety information.
When prescribing zolpidem, discuss safety information with the patient, such as:
- Do not drive or operate heavy equipment for at least 5 to 6 hours after administration.
- Have a safety plan in place for transportation during those hours.
- Do not use this medication with alcohol or other sedative/hypnotics.
- Contact the prescriber about any suspected adverse effects.
- MedlinePlus information on sleep disorders. National Institutes of Health and National Library of Medicine. www.nlm.nih.gov/medlineplus/sleepdisorders.html.
- Zolpidem (systemic). Mayoclinic.com: Tools for healthier lives. www.mayoclinic.com/health/drug-information/DR202707.
- Diazepam • Valium
- Eszopiclone • Lunesta
- Midazolam • Versed
- Oxazepam • Serax
- Quazepam • Doral
- Rofecoxib • Vioxx
- Temazepam • Restoril
- Triazolam • Halcion
- Zolpidem • Ambien, Ambien CR
- Zaleplon • Sonata
- Zopiclone • Imovane (in Europe)
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors acknowledge the assistance and guidance of Linda T. Moore, JD, in preparing this manuscript.
1. Markon J. Sleeping Va. driver convicted in crash; man had taken too much Ambien. The Washington Post, August 2, 2006. Accessed August 26, 2006 from LexisNexis Academic Database.
2. Colten HR, Altevogt BM. Sleep disorders and sleep deprivation: an unmet public health problem. Available at: http://www.iom.edu/CMS/3740/23160/33668.aspx. Accessed February 21, 2007.
3. Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment; prevalence and correlates. Arch Gen Psychiatry 1985;42:225-32.
4. Barclay L. Driving, other erratic behaviors reported after taking zolpidem. Available at http://www.medscape.com/viewarticle/528415. Accessed February 21, 2007.
5. Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep 2006;29(8):1009-14.
6. Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005;165(8):863-7.
7. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med 2006;4(2):104-13.
8. Verispan VONA. Top 200 brand name drugs by units in 2005. Drug Topics 2006. Available at: http://www.drugtopics.com/drugtopics/data/articlestandard/drugtopics/102006/311294/article.pdf. Accessed February 22, 2007.
9. sanofi-aventis Ambien prescribing information. Available at: http://products.sanofi-aventis.us/ambien/ambien.html. Accessed February 21, 2007.
10. Pear R. Patrick Kennedy crashes car into a Capitol Hill barrier. The New York Times, May 5, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
11. Janet Makinen and others v. Sanofi-Synthelabo & Sanofi-Synthelabo, Inc. Class action suit filed March 6, 2006 in U.S. District Court for the Southern District of New York, NY.
12. Tooher NL. Ambien users are filing lawsuits. Kansas City Daily Record, April 12, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
13. Verster JC, Veldhuijzen DS, Volkerts ER. Residual effects of sleep medication on driving ability. Sleep Med Rev 2004;8(4):309-25.
14. Wilkinson CJ. The acute effects of zolpidem, administered alone and with alcohol, on cognitive and psychomotor function. J Clin Psychiatry 1995;56(7):309-18.
15. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
16. Mattila MJ, Vanakoski J, Kalska H, Seppala T. Effects of alcohol, zolpidem, and some other sedatives and hypnotics on human performance and memory. Pharmacol Biochem Behav 1998;59(4):917-23.
17. Mintzer MZ, Griffiths RR. Selective effects of zolpidem on human memory functions. J Psychopharmacol 1999;13(1):18-31.
18. Verster JC, Volkerts ER, Schreuder AH, et al. Residual effects of middle-of-the-night administration of zaleplon and zolpidem on driving ability, memory functions, and psychomotor performance. J Clin Psychopharmacol 2002;22(6):576-83.
19. Paul MA, Gray G, Kenny G, Pigeau RA. Impact of melatonin, zaleplon, zopiclone, and temazepam on psychomotor performance. Aviat Space Environ Med 2003;74(12):1263-70.
20. Partinen M, Hirvonen K, Hublin C, et al. Effects of after-midnight intake of zolpidem and temazepam on driving ability in women with non-organic insomnia. Sleep Med 2003;4(6):553-61.
21. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
22. Logan BK, Couper FJ. Zolpidem and driving impairment. J Forensic Sci 2001;46(1):105-10.
23. Fairweather DB, Kerr JS, Hindmarch I. The effects of acute and repeated doses of zolpidem on subjective sleep, psychomotor performance and cognitive function in elderly volunteers. Eur J Clin Pharmacol 1992;43(6):597-601.
24. Bocca ML, Le Doze F, Etard O, et al. Residual effect of zolpidem 10 mg and zopiclone 7.5 mg versus flunitrazepam 1 mg and placebo on driving performance and ocular saccades. Psychopharmacology (Berl) 1999;143(4):373-9.
25. Staner L, Ertle S, Boeijinga P, et al. Next-day residual effects of hypnotics in DSM-IV primary insomnia: a driving simulator study with simultaneous electroencephalogram monitoring. Psychopharmacology (Berl) 2005;181(4):790-8.
26. Natanson v. Kline 300 P.2d 1093 (1960).
27. Canterbury v. Spence 464 F.2d 772 (1972).
28. Reyes v. Wyeth Laboratories. 498 F.2d 1264 (1974).
29. Ernst v. Merck & Co. 24 PLLR 149 (2005).
30. Humeston v. Merck & Co., No. ATL-L-2272-03-MT, Super. Ct., Atlantic County, NJ, November 3, 2005.
31. Johnson LA. Jury blames Vioxx for man’s heart attack, awards $47.5 million. Available at http://news.findlaw.com/ap/o/51/03-12-2007/85f9000f67cd576a.html. Accessed March 16, 2007.
32. Bursztajn HJ, Brodsky A. Ethical and legal dimensions of benzodiazepine prescription: a commentary. Psychiatr Ann 1998;28(3):121-7.
“Sleep driving” blamed on the hypnotic zolpidem was used as a defense last year in Virginia in a criminal case involving impaired driving. The defendant’s attorney argued that the defendant should not be held criminally liable because he was “essentially unconscious” and the accident therefore was involuntary.
The “sleep driving” defense failed when testimony revealed the defendant had taken 5 times the recommended zolpidem dose before the accident. The judge found him guilty of a felony charge of driving under the influence of a sleep medication.1
Sedative-hypnotics are increasingly being used to treat insomnia2-4 and as a result some patients try to drive while under the drugs’ sedating effects. Also, new FDA-ordered labeling for all 13 available prescription sleep aids warns of potential risks of “complex sleep-related behaviors,” including driving, phoning, and eating while asleep (Box 1).
Hypnotics can improve quality of life and well-being by addressing insomnia’s complications—hypertension, diabetes, coronary artery disease, depression, and anxiety5-7—but they also have been associated with impaired motor coordination and somnambulism. To help you and your patients weigh sleep medications’ relative risks and benefits, we report on clinical studies and court cases in the literature. Most of the data focus on zolpidem, by far the most prescribed hypnotic (Box 2).8,9
Labeling of all sedative-hypnotic drugs now carries FDA-ordered precautions about “sleep-driving and other complex behaviors” that may occur without the patient being fully awake. FDA cited reports of patients preparing and eating food, making phone calls, and having sex after taking a sedative-hypnotic, usually without memory of the event. A warning also was added about rare, potentially fatal anaphylactic reactions in patients taking first or later doses of sleep medications.
Steven Galson, MD, MPH, director of FDA’s Center for Drug Evaluation and Research, said the labeling changes were needed to inform patients and prescribers about the risks of sleep aids that “are well-tolerated and effective for many people.”
Source: Walsh S, Rawlings K. FDA requests label change for all sleep disorder drug products. Available at www.fda.gov/bbs/topics/NEWS/2007/NEW01587.html.
Zolpidem incidents and cases
In 2005, Americans filled 43 million prescriptions for sedative-hypnotics—26.5 million for zolpidem alone—compared with 29 million prescriptions in 2001.4 In addition to the new the FDA-requested warnings about sleep-related behaviors, zolpidem’s labeling cautions patients about operating heavy machinery, driving, or engaging in hazardous occupations after taking the drug. The manufacturer tells patients:
- to ingest zolpidem only before going to bed
- that they may experience residual sedation the following day.
Impaired driving. Besides the “sleep driving” case in Virginia, a highly publicized zolpidem-related driving incident occurred May 4, 2006, when U.S. Representative Patrick Kennedy was involved in an accident after having taken zolpidem in combination with an antinausea medication. Another driving-related case has used zolpidem as a defense for impairment, but the court decided that the medication was not at fault because the defendant also had ingested alcohol.10
Other litigation. Although zolpidem-related impairment apparently has not been used successfully as a defense for a driving incident, class action suits alleging failure to disclose potentially harmful side effects have been filed against the manufacturer.
In Janet Makinen and others v. sanofi-aventis,11 at least 500 plaintiffs claim zolpidem is related to sleep-driving, sleep-eating, and other somnambulistic behaviors. Plaintiffs allege negligence, breach of implied warranties, fraud, unfair trade practices, express warranty violations, strict liability, and consumer fraud violations. Other suits claim dangerous sleep-related side effects with zolpidem use.12
What clinical evidence shows
Driving impairment. Clinical studies have shown conflicting results about driving impairment associated with zolpidem. The literature falls into 2 categories, based on treatment duration:
- Zolpidem affects performance and memory within the first 4 to 5 hours of administration (Table 1).
- Beyond 5 hours, no residual effects on performance have been identified (Table 2), and repeat nightly dosing has not caused impairment or tolerance.
- All sedative hypnotic benzodiazepines had statistically significant residual effects 10 to 11 hours after ingestion, with longer periods of impairment corresponding to medications with longer half-lives.
- Zopiclone was associated with significant residual impairment for up to 10 hours after ingestion.
- Zolpidem and zaleplon showed no significant impairment in driving 10 to 11 hours after ingestion. Impairment was found, however, when zolpidem was taken within 5 hours of driving.14-18
Table 1
Studies of zolpidem-associated driving skills impairment
(
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Wilkinson, 199514 Blinded; 29 subjects | Zolpidem, 10 mg, 15 mg, and placebo in combination with an alcoholic drink (to reach a BAC of 0.08%) or placebo drink; testing 45 min, 130 min, and 230 min after administration | Visual backward masking test (approximates driving performance) and attention tests | Zolpidem produced significant impairment in combination with alcohol and when administered alone during peak effect assessment; alcohol did not potentiate zolpidem’s effects; additive effects of alcohol seen with 10-mg dose but not 15-mg dose of zolpidem |
| Rush et al, 199815 Double-blind, crossover; 9 subjects | Zolpidem, 7.5, 15, and 22.5 mg; quazepam, 15, 30, and 45 mg; triazolam, 0.1875, 0.375, and 0.5625 mg; testing ½, 1, 1½, 2, 2½, 3, 4, 5, and 6 hours after administration | Subject- and observer-rated questionnaires; tests of recall and delayed recognition | Performance-impairing effects of zolpidem were virtually indistinguishable from those of classic benzodiazepines, such as triazolam |
| Mattila et al, 199816 Randomized, placebo-controlled, double-blind, crossover; 12 subjects | Zolpidem, 15 mg; diazepam, 15 mg; oxazepam, 30 mg; zopiclone, 7.5 mg; alcohol testing before and 1, 3½, and 5 hours after administration | Simulated driving and other measures | Zolpidem impaired coordination, reaction, and cognition at 1 and 3½ hours; tracking remained impaired at 5 hours; all agents (especially zolpidem) impaired learning and memory |
| Mintzer et al, 199917 Double-blind, placebo-controlled; 16 subjects | Zolpidem, 15 mg/70 kg (dosed by subject weight); testing ½, 1, 2, and 3 hours after administration | Memory tasks (recall, fragment completion, recognition) | Zolpidem interfered with explicit but not implicit memory after administration; zolpidem produced a specific deficit in acquisition of contextual information |
| Verster et al, 200418 2-step randomized, placebo-controlled, double-blind, crossover; 30 subjects | Zolpidem, 10 mg and 20 mg; zaleplon, 10 mg and 20 mg; middle-of-the-night dosing; testing 4 hours after dosing | On-the-road driving and other tests of attention, learning, and thinking | Zolpidem, 10 mg and 20 mg, significantly impaired driving function; zolpidem, 20 mg, produced significant impairment on all psychomotor and memory tests; zaleplon, 10 mg and 20 mg, did not differ significantly from placebo |
| BAC: Blood alcohol concentration | |||
Studies of zolpidem-associated driving skills impairment
(>5 hours after dosing)
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Fairweather et al, 199223 Randomized, placebo-controlled; 24 older volunteers taking no other medications | Zolpidem, 5 mg or 10 mg, or placebo taken before bedtime; testing 8.5 hours after administration | Numerous, including reactive time, memory, word recognition | Zolpidem consistently helped with sleep latency, with no residual performance deficits; no tolerance seen with repeated dosing |
| Bocca et al, 199924 Double-blind, crossover; 16 volunteers | Zolpidem, 10 mg; zopiclone, 7.5 mg; flunitrazepam,* 1 mg; and placebo given at 11 PM, with testing at 9 AM | Driving simulation and real time test drive; eye movements measured after driving tests | No residual effects with zolpidem; zopiclone impaired driving ability and increased saccadic latency; flunitrazepam impaired early morning driving and saccadic eye movements longer than zopiclone |
| Partinen et al, 200320 Randomized, placebo-controlled, double-blind, 3-period crossover; 18 women with insomnia | Zolpidem, 10 mg; temazepam, 20 mg; dosing at 2 AM, testing 5.5 hours after dosing | Driving simulation; delayed word recall and memory testing (FePsy test) | No statistically significant effects on driving ability with either drug; no significant differences in FePsy results compared with baseline or placebo |
| Staner et al, 200525 Randomized, placebo-controlled, double-blind, four-way crossover; 23 subjects with DSM-IV-TR diagnosis of insomnia | Zolpidem, 10 mg; zopiclone, 7.5 mg; lormetazepam,* 1 mg; 7 days of dosing; tests given 9 to 11 hours post-dosing | Driving simulation; EEG at rest and while driving | Zolpidem showed no impairment of driving ability and no EEG changes compared with placebo; driving impairment and EEG alterations were found with zopiclone and lormetazepam |
| * Hypnotics not approved in the United States but available elsewhere. | |||
Acute effects (
Combined with alcohol. Wilkinson14 conducted a randomized, 6-way crossover study in which subjects received 10- or 15-mg doses of zolpidem or placebo plus an alcoholic beverage (enough to obtain a blood alcohol concentration [BAC] of ~0.08%) or placebo beverage. Tests given shortly after patients took the study medications showed that zolpidem caused statistically significant impairment both in combination with alcohol and alone during peak drug effect—identified as 45 minutes after ingestion. Alcohol did not potentiate the impairment associated with zolpidem.
Using a similar design, Mattila et al16 compared acute performance impairment associated with zolpidem, diazepam, oxazepam, and zopiclone. In this randomized, double-blinded, crossover study, all comparison medications impaired antecedent learning and memory, but zolpidem given at 15 mg had the greatest effect. Zolpidem impaired coordination, reactive functioning, and cognitive skills at 1 and 3.5 hours after administration, and simulated driving test performance remained impaired at 5 hours (approximately two half-lives of the medication). Of note is that the 15-mg zolpidem dose used in this study was shown by Wilkinson et al14 to be more impairing than the recommended maximum 10-mg dose.
A study from the University of Toronto19 that did not include zolpidem examined potential psychomotor performance deficits and sleepiness in a comparison of time-released melatonin, 6 mg; zaleplon, 10 mg; zopiclone, 7.5 mg; temazepam, 15 mg, and placebo. Tests were given to 9 men and 14 women, ages 21 to 53, just before drug administration and 7 hours later.
Zaleplon had the greatest effect on psychomotor performance, followed by temazepam and zopiclone. Aside from prolonged perceived sleepiness, melatonin and placebo did not interfere with performance testing.
Zolpidem, a benzodiazepine receptor agonist, was the 7th most prescribed drug in the United States in 2005 (2006 data not available).8 It is FDA-approved for short-term treatment of insomnia, although “short-term” is not defined. Package labeling states:
This nonbenzodiazepine hypnotic has been shown to decrease sleep latency and increase sleep duration for up to 35 days in controlled clinical trials. Patients should be evaluated for a primary psychiatric or medical illness if insomnia does not remit after 7 to 10 days of treatment.
An imidazopyridine that acts as an agonist of GABA A1, zolpidem produces sedation while avoiding anticonvulsant, anxiolytic, and muscle relaxation effects. Available in 5- and 10-mg tablets, the drug is rapidly absorbed in the GI tract and excreted primarily through the kidneys. Its half-life is approximately 2.5 hours (approximately 3 hours in elderly patients). The most common side effects are daytime drowsiness, dizziness, and diarrhea; others include asthenia, hiccup, and diplopia.9
- driving ability 4 hours after administration
- memory and psychomotor performance 6 hours after administration.
Partinen et al20 used the recommended zolpidem dose in a similar study of after-midnight use by women with insomnia. The double-blind, randomized, controlled trial evaluated performance with a driving simulator and neuropsychological testing 5.5 hours after medication dosing. Patients taking zolpidem, 10 mg, showed no significant impairment when compared with those taking placebo. Some patients scored poorly on the driving tests alone, and the authors concluded that this group was more susceptible to zolpidem’s effect.
Memory. In a double-blind, placebo-controlled trial by Mintzner et al,17 zolpidem dosed by patient weight at 15 mg/70 kg:
- significantly impaired explicit memory (requires conscious recollection for recall)
- did not affect implicit memory (lack of conscious awareness in the act of recollection).
These findings support complaints of zolpidem-related anterograde amnestic episodes, which also occur with some benzodiazepines (such as midazolam).
Similar to benzodiazepines? Rush et al’s results21 support Mintzer’s assertion17 that zolpidem shares many side effects with benzodiazepines. Performance impairment associated with zolpidem—as rated by subjects and observers—is virtually indistinguishable from a benzodiazepine effect, except that the duration is shorter with zolpidem (5 hours), compared with up to 10 hours for benzodiazepines.
Logan and Couper22 reviewed police reports and toxicology profiles of individuals suspected of driving while impaired. Zolpidem was found in 29 subjects, 5 of whom showed no other substances. In those 5, zolpidem blood levels ranged from 0.08 to 1.40 mg/L and did not appear to correlate with the degree of impairment.
Residual effects (>5 hours)
Older patients. In a randomized, placebo-controlled trial by Fairweather et al,23 zolpidem improved sleep latency in 24 subjects ages 63 to 80. No evidence of impairment in reactive time, memory, or word recognition was found 8.5 hours after nighttime dosing, and tolerance was not seen after 1 week of repeated dosing.
Driving impairment. Bocca et al24 compared degree of driving impairment by zolpidem, zopiclone, flunitrazepam (not approved in the United States), and placebo. The 16 subjects received each medication at 11 pm, with a 2-week washout between medications. One group of 8 was tested at 9 am and the other 8 subjects at 11 am. Those taking zolpidem showed no residual performance impairment, as measured by simulated driving, a test drive, and saccadic eye movements.
Staner et al25 reported similar results when comparing zolpidem, zopiclone, lormetazepam (not approved in the United States), and placebo. Using a driving simulator and electroencephalography (EEG), they evaluated 23 subjects diagnosed with insomnia at 9 and 11 hours post-dose. Zolpidem did not significantly impair driving ability and did not differ from placebo on EEG analysis (resting or driving). The study showed driving impairment with zopiclone and lormetazepam, along with characteristic benzodiazepine EEG changes. This study further supports evidence of limited impairment on driving after appropriate use of zolpidem.
Informed consent
In the informed consent process, failing to warn a patient about medication side effects can lead to legal claims against both manufacturers and prescribers. With any medication, patients have the right to know about a drug’s risks, benefits, and alternate therapies—including no therapy.
Two standards are associated with informed consent and negligence:
- The “reasonable practitioner” standard outlined in Natanson v. Kline (1960)26 mandates that the prescribing physician has revealed all that an “average, reasonable practitioner” would disclose in similar circumstances.
- The “reasonable patient” standard set in Canterbury v. Spence (1972)27 mandates that the prescribing physician has informed the patient about the proposed treatment, its side effects, and alternatives to the proposed treatment that a reasonable patient would consider material to the decision of whether or not to undergo treatment.
The vaccine was licensed as a prescription drug but administered through county health departments. In 1970, a nurse in a Texas Department of Health clinic administered the vaccine to 8-month-old Anita Reyes without telling the girl’s parents of warnings in the package circular. Holding Wyeth Laboratories to a reasonableness standard, the court found that the company knew or should have known how the vaccine would be distributed.
The package insert was not shown to have given inadequate warning, and the vaccine was not shown to be defective (it was a trivalent live-virus Sabin oral polio vaccine, as intended).
Vioxx cases. Similarly, some plaintiffs have been awarded millions of dollars (as in Ernst v. Merck & Co., Inc.29) in rulings that Merck & Co. failed to disclose the risk of cardiotoxicity with the arthritis drug rofecoxib (Vioxx) and thus failed to provide physicians with information needed when prescribing the drug. In Humeston v. Merck & Co.,30 a Texas court in 2005 held that Vioxx’s warning labels were adequate. In a retrial, however, the New Jersey Superior Court awarded the plaintiff $47.5 million.31
As with the polio vaccine and Vioxx litigations, courts are being asked to decide if patients were adequately informed about sleep-driving and other risks associated with the use of sedative-hypnotics.
Clinical recommendations
Zolpidem—like many other medications—carries a substantial risk of side effects, even when used appropriately. However, given the medical and mental health risks of untreated insomnia, the benefits of a medication such as zolpidem will likely outweigh its risks.
Numerous studies have shown that zolpidem is effective for improving sleep latency and that there are mild, if any, residual side effects beyond what would normally be a restful night’s sleep. Impairments are evident, however, during the hours following the drug’s administration, with some effects lasting >5 hours depending on the dose.
Risk management. When prescribing nonbenzodiazepine hypnotics such as zolpidem, you may want to adopt a risk management approach as you would with other medications that can have serious side effects. An approach to benzodiazepine prescribing proposed by Bursztajn et al31 advocates:
- using the informed-consent process to build an alliance with patients
- not prescribing the medication in isolation of other beneficial therapies
- being aware of and always documenting your decision-making process.
When you make patients aware of all risks, benefits, alternate therapies, and possible outcomes with no treatment, you have informed them effectively. Patients are then left to decide whether or not to agree to the treatment. You also are responsible for monitoring the patient, addressing the patient’s questions, and relaying important safety information.
When prescribing zolpidem, discuss safety information with the patient, such as:
- Do not drive or operate heavy equipment for at least 5 to 6 hours after administration.
- Have a safety plan in place for transportation during those hours.
- Do not use this medication with alcohol or other sedative/hypnotics.
- Contact the prescriber about any suspected adverse effects.
- MedlinePlus information on sleep disorders. National Institutes of Health and National Library of Medicine. www.nlm.nih.gov/medlineplus/sleepdisorders.html.
- Zolpidem (systemic). Mayoclinic.com: Tools for healthier lives. www.mayoclinic.com/health/drug-information/DR202707.
- Diazepam • Valium
- Eszopiclone • Lunesta
- Midazolam • Versed
- Oxazepam • Serax
- Quazepam • Doral
- Rofecoxib • Vioxx
- Temazepam • Restoril
- Triazolam • Halcion
- Zolpidem • Ambien, Ambien CR
- Zaleplon • Sonata
- Zopiclone • Imovane (in Europe)
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors acknowledge the assistance and guidance of Linda T. Moore, JD, in preparing this manuscript.
“Sleep driving” blamed on the hypnotic zolpidem was used as a defense last year in Virginia in a criminal case involving impaired driving. The defendant’s attorney argued that the defendant should not be held criminally liable because he was “essentially unconscious” and the accident therefore was involuntary.
The “sleep driving” defense failed when testimony revealed the defendant had taken 5 times the recommended zolpidem dose before the accident. The judge found him guilty of a felony charge of driving under the influence of a sleep medication.1
Sedative-hypnotics are increasingly being used to treat insomnia2-4 and as a result some patients try to drive while under the drugs’ sedating effects. Also, new FDA-ordered labeling for all 13 available prescription sleep aids warns of potential risks of “complex sleep-related behaviors,” including driving, phoning, and eating while asleep (Box 1).
Hypnotics can improve quality of life and well-being by addressing insomnia’s complications—hypertension, diabetes, coronary artery disease, depression, and anxiety5-7—but they also have been associated with impaired motor coordination and somnambulism. To help you and your patients weigh sleep medications’ relative risks and benefits, we report on clinical studies and court cases in the literature. Most of the data focus on zolpidem, by far the most prescribed hypnotic (Box 2).8,9
Labeling of all sedative-hypnotic drugs now carries FDA-ordered precautions about “sleep-driving and other complex behaviors” that may occur without the patient being fully awake. FDA cited reports of patients preparing and eating food, making phone calls, and having sex after taking a sedative-hypnotic, usually without memory of the event. A warning also was added about rare, potentially fatal anaphylactic reactions in patients taking first or later doses of sleep medications.
Steven Galson, MD, MPH, director of FDA’s Center for Drug Evaluation and Research, said the labeling changes were needed to inform patients and prescribers about the risks of sleep aids that “are well-tolerated and effective for many people.”
Source: Walsh S, Rawlings K. FDA requests label change for all sleep disorder drug products. Available at www.fda.gov/bbs/topics/NEWS/2007/NEW01587.html.
Zolpidem incidents and cases
In 2005, Americans filled 43 million prescriptions for sedative-hypnotics—26.5 million for zolpidem alone—compared with 29 million prescriptions in 2001.4 In addition to the new the FDA-requested warnings about sleep-related behaviors, zolpidem’s labeling cautions patients about operating heavy machinery, driving, or engaging in hazardous occupations after taking the drug. The manufacturer tells patients:
- to ingest zolpidem only before going to bed
- that they may experience residual sedation the following day.
Impaired driving. Besides the “sleep driving” case in Virginia, a highly publicized zolpidem-related driving incident occurred May 4, 2006, when U.S. Representative Patrick Kennedy was involved in an accident after having taken zolpidem in combination with an antinausea medication. Another driving-related case has used zolpidem as a defense for impairment, but the court decided that the medication was not at fault because the defendant also had ingested alcohol.10
Other litigation. Although zolpidem-related impairment apparently has not been used successfully as a defense for a driving incident, class action suits alleging failure to disclose potentially harmful side effects have been filed against the manufacturer.
In Janet Makinen and others v. sanofi-aventis,11 at least 500 plaintiffs claim zolpidem is related to sleep-driving, sleep-eating, and other somnambulistic behaviors. Plaintiffs allege negligence, breach of implied warranties, fraud, unfair trade practices, express warranty violations, strict liability, and consumer fraud violations. Other suits claim dangerous sleep-related side effects with zolpidem use.12
What clinical evidence shows
Driving impairment. Clinical studies have shown conflicting results about driving impairment associated with zolpidem. The literature falls into 2 categories, based on treatment duration:
- Zolpidem affects performance and memory within the first 4 to 5 hours of administration (Table 1).
- Beyond 5 hours, no residual effects on performance have been identified (Table 2), and repeat nightly dosing has not caused impairment or tolerance.
- All sedative hypnotic benzodiazepines had statistically significant residual effects 10 to 11 hours after ingestion, with longer periods of impairment corresponding to medications with longer half-lives.
- Zopiclone was associated with significant residual impairment for up to 10 hours after ingestion.
- Zolpidem and zaleplon showed no significant impairment in driving 10 to 11 hours after ingestion. Impairment was found, however, when zolpidem was taken within 5 hours of driving.14-18
Table 1
Studies of zolpidem-associated driving skills impairment
(
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Wilkinson, 199514 Blinded; 29 subjects | Zolpidem, 10 mg, 15 mg, and placebo in combination with an alcoholic drink (to reach a BAC of 0.08%) or placebo drink; testing 45 min, 130 min, and 230 min after administration | Visual backward masking test (approximates driving performance) and attention tests | Zolpidem produced significant impairment in combination with alcohol and when administered alone during peak effect assessment; alcohol did not potentiate zolpidem’s effects; additive effects of alcohol seen with 10-mg dose but not 15-mg dose of zolpidem |
| Rush et al, 199815 Double-blind, crossover; 9 subjects | Zolpidem, 7.5, 15, and 22.5 mg; quazepam, 15, 30, and 45 mg; triazolam, 0.1875, 0.375, and 0.5625 mg; testing ½, 1, 1½, 2, 2½, 3, 4, 5, and 6 hours after administration | Subject- and observer-rated questionnaires; tests of recall and delayed recognition | Performance-impairing effects of zolpidem were virtually indistinguishable from those of classic benzodiazepines, such as triazolam |
| Mattila et al, 199816 Randomized, placebo-controlled, double-blind, crossover; 12 subjects | Zolpidem, 15 mg; diazepam, 15 mg; oxazepam, 30 mg; zopiclone, 7.5 mg; alcohol testing before and 1, 3½, and 5 hours after administration | Simulated driving and other measures | Zolpidem impaired coordination, reaction, and cognition at 1 and 3½ hours; tracking remained impaired at 5 hours; all agents (especially zolpidem) impaired learning and memory |
| Mintzer et al, 199917 Double-blind, placebo-controlled; 16 subjects | Zolpidem, 15 mg/70 kg (dosed by subject weight); testing ½, 1, 2, and 3 hours after administration | Memory tasks (recall, fragment completion, recognition) | Zolpidem interfered with explicit but not implicit memory after administration; zolpidem produced a specific deficit in acquisition of contextual information |
| Verster et al, 200418 2-step randomized, placebo-controlled, double-blind, crossover; 30 subjects | Zolpidem, 10 mg and 20 mg; zaleplon, 10 mg and 20 mg; middle-of-the-night dosing; testing 4 hours after dosing | On-the-road driving and other tests of attention, learning, and thinking | Zolpidem, 10 mg and 20 mg, significantly impaired driving function; zolpidem, 20 mg, produced significant impairment on all psychomotor and memory tests; zaleplon, 10 mg and 20 mg, did not differ significantly from placebo |
| BAC: Blood alcohol concentration | |||
Studies of zolpidem-associated driving skills impairment
(>5 hours after dosing)
| Author/design | Doses and timing | Driving skills assessments | Conclusions |
|---|---|---|---|
| Fairweather et al, 199223 Randomized, placebo-controlled; 24 older volunteers taking no other medications | Zolpidem, 5 mg or 10 mg, or placebo taken before bedtime; testing 8.5 hours after administration | Numerous, including reactive time, memory, word recognition | Zolpidem consistently helped with sleep latency, with no residual performance deficits; no tolerance seen with repeated dosing |
| Bocca et al, 199924 Double-blind, crossover; 16 volunteers | Zolpidem, 10 mg; zopiclone, 7.5 mg; flunitrazepam,* 1 mg; and placebo given at 11 PM, with testing at 9 AM | Driving simulation and real time test drive; eye movements measured after driving tests | No residual effects with zolpidem; zopiclone impaired driving ability and increased saccadic latency; flunitrazepam impaired early morning driving and saccadic eye movements longer than zopiclone |
| Partinen et al, 200320 Randomized, placebo-controlled, double-blind, 3-period crossover; 18 women with insomnia | Zolpidem, 10 mg; temazepam, 20 mg; dosing at 2 AM, testing 5.5 hours after dosing | Driving simulation; delayed word recall and memory testing (FePsy test) | No statistically significant effects on driving ability with either drug; no significant differences in FePsy results compared with baseline or placebo |
| Staner et al, 200525 Randomized, placebo-controlled, double-blind, four-way crossover; 23 subjects with DSM-IV-TR diagnosis of insomnia | Zolpidem, 10 mg; zopiclone, 7.5 mg; lormetazepam,* 1 mg; 7 days of dosing; tests given 9 to 11 hours post-dosing | Driving simulation; EEG at rest and while driving | Zolpidem showed no impairment of driving ability and no EEG changes compared with placebo; driving impairment and EEG alterations were found with zopiclone and lormetazepam |
| * Hypnotics not approved in the United States but available elsewhere. | |||
Acute effects (
Combined with alcohol. Wilkinson14 conducted a randomized, 6-way crossover study in which subjects received 10- or 15-mg doses of zolpidem or placebo plus an alcoholic beverage (enough to obtain a blood alcohol concentration [BAC] of ~0.08%) or placebo beverage. Tests given shortly after patients took the study medications showed that zolpidem caused statistically significant impairment both in combination with alcohol and alone during peak drug effect—identified as 45 minutes after ingestion. Alcohol did not potentiate the impairment associated with zolpidem.
Using a similar design, Mattila et al16 compared acute performance impairment associated with zolpidem, diazepam, oxazepam, and zopiclone. In this randomized, double-blinded, crossover study, all comparison medications impaired antecedent learning and memory, but zolpidem given at 15 mg had the greatest effect. Zolpidem impaired coordination, reactive functioning, and cognitive skills at 1 and 3.5 hours after administration, and simulated driving test performance remained impaired at 5 hours (approximately two half-lives of the medication). Of note is that the 15-mg zolpidem dose used in this study was shown by Wilkinson et al14 to be more impairing than the recommended maximum 10-mg dose.
A study from the University of Toronto19 that did not include zolpidem examined potential psychomotor performance deficits and sleepiness in a comparison of time-released melatonin, 6 mg; zaleplon, 10 mg; zopiclone, 7.5 mg; temazepam, 15 mg, and placebo. Tests were given to 9 men and 14 women, ages 21 to 53, just before drug administration and 7 hours later.
Zaleplon had the greatest effect on psychomotor performance, followed by temazepam and zopiclone. Aside from prolonged perceived sleepiness, melatonin and placebo did not interfere with performance testing.
Zolpidem, a benzodiazepine receptor agonist, was the 7th most prescribed drug in the United States in 2005 (2006 data not available).8 It is FDA-approved for short-term treatment of insomnia, although “short-term” is not defined. Package labeling states:
This nonbenzodiazepine hypnotic has been shown to decrease sleep latency and increase sleep duration for up to 35 days in controlled clinical trials. Patients should be evaluated for a primary psychiatric or medical illness if insomnia does not remit after 7 to 10 days of treatment.
An imidazopyridine that acts as an agonist of GABA A1, zolpidem produces sedation while avoiding anticonvulsant, anxiolytic, and muscle relaxation effects. Available in 5- and 10-mg tablets, the drug is rapidly absorbed in the GI tract and excreted primarily through the kidneys. Its half-life is approximately 2.5 hours (approximately 3 hours in elderly patients). The most common side effects are daytime drowsiness, dizziness, and diarrhea; others include asthenia, hiccup, and diplopia.9
- driving ability 4 hours after administration
- memory and psychomotor performance 6 hours after administration.
Partinen et al20 used the recommended zolpidem dose in a similar study of after-midnight use by women with insomnia. The double-blind, randomized, controlled trial evaluated performance with a driving simulator and neuropsychological testing 5.5 hours after medication dosing. Patients taking zolpidem, 10 mg, showed no significant impairment when compared with those taking placebo. Some patients scored poorly on the driving tests alone, and the authors concluded that this group was more susceptible to zolpidem’s effect.
Memory. In a double-blind, placebo-controlled trial by Mintzner et al,17 zolpidem dosed by patient weight at 15 mg/70 kg:
- significantly impaired explicit memory (requires conscious recollection for recall)
- did not affect implicit memory (lack of conscious awareness in the act of recollection).
These findings support complaints of zolpidem-related anterograde amnestic episodes, which also occur with some benzodiazepines (such as midazolam).
Similar to benzodiazepines? Rush et al’s results21 support Mintzer’s assertion17 that zolpidem shares many side effects with benzodiazepines. Performance impairment associated with zolpidem—as rated by subjects and observers—is virtually indistinguishable from a benzodiazepine effect, except that the duration is shorter with zolpidem (5 hours), compared with up to 10 hours for benzodiazepines.
Logan and Couper22 reviewed police reports and toxicology profiles of individuals suspected of driving while impaired. Zolpidem was found in 29 subjects, 5 of whom showed no other substances. In those 5, zolpidem blood levels ranged from 0.08 to 1.40 mg/L and did not appear to correlate with the degree of impairment.
Residual effects (>5 hours)
Older patients. In a randomized, placebo-controlled trial by Fairweather et al,23 zolpidem improved sleep latency in 24 subjects ages 63 to 80. No evidence of impairment in reactive time, memory, or word recognition was found 8.5 hours after nighttime dosing, and tolerance was not seen after 1 week of repeated dosing.
Driving impairment. Bocca et al24 compared degree of driving impairment by zolpidem, zopiclone, flunitrazepam (not approved in the United States), and placebo. The 16 subjects received each medication at 11 pm, with a 2-week washout between medications. One group of 8 was tested at 9 am and the other 8 subjects at 11 am. Those taking zolpidem showed no residual performance impairment, as measured by simulated driving, a test drive, and saccadic eye movements.
Staner et al25 reported similar results when comparing zolpidem, zopiclone, lormetazepam (not approved in the United States), and placebo. Using a driving simulator and electroencephalography (EEG), they evaluated 23 subjects diagnosed with insomnia at 9 and 11 hours post-dose. Zolpidem did not significantly impair driving ability and did not differ from placebo on EEG analysis (resting or driving). The study showed driving impairment with zopiclone and lormetazepam, along with characteristic benzodiazepine EEG changes. This study further supports evidence of limited impairment on driving after appropriate use of zolpidem.
Informed consent
In the informed consent process, failing to warn a patient about medication side effects can lead to legal claims against both manufacturers and prescribers. With any medication, patients have the right to know about a drug’s risks, benefits, and alternate therapies—including no therapy.
Two standards are associated with informed consent and negligence:
- The “reasonable practitioner” standard outlined in Natanson v. Kline (1960)26 mandates that the prescribing physician has revealed all that an “average, reasonable practitioner” would disclose in similar circumstances.
- The “reasonable patient” standard set in Canterbury v. Spence (1972)27 mandates that the prescribing physician has informed the patient about the proposed treatment, its side effects, and alternatives to the proposed treatment that a reasonable patient would consider material to the decision of whether or not to undergo treatment.
The vaccine was licensed as a prescription drug but administered through county health departments. In 1970, a nurse in a Texas Department of Health clinic administered the vaccine to 8-month-old Anita Reyes without telling the girl’s parents of warnings in the package circular. Holding Wyeth Laboratories to a reasonableness standard, the court found that the company knew or should have known how the vaccine would be distributed.
The package insert was not shown to have given inadequate warning, and the vaccine was not shown to be defective (it was a trivalent live-virus Sabin oral polio vaccine, as intended).
Vioxx cases. Similarly, some plaintiffs have been awarded millions of dollars (as in Ernst v. Merck & Co., Inc.29) in rulings that Merck & Co. failed to disclose the risk of cardiotoxicity with the arthritis drug rofecoxib (Vioxx) and thus failed to provide physicians with information needed when prescribing the drug. In Humeston v. Merck & Co.,30 a Texas court in 2005 held that Vioxx’s warning labels were adequate. In a retrial, however, the New Jersey Superior Court awarded the plaintiff $47.5 million.31
As with the polio vaccine and Vioxx litigations, courts are being asked to decide if patients were adequately informed about sleep-driving and other risks associated with the use of sedative-hypnotics.
Clinical recommendations
Zolpidem—like many other medications—carries a substantial risk of side effects, even when used appropriately. However, given the medical and mental health risks of untreated insomnia, the benefits of a medication such as zolpidem will likely outweigh its risks.
Numerous studies have shown that zolpidem is effective for improving sleep latency and that there are mild, if any, residual side effects beyond what would normally be a restful night’s sleep. Impairments are evident, however, during the hours following the drug’s administration, with some effects lasting >5 hours depending on the dose.
Risk management. When prescribing nonbenzodiazepine hypnotics such as zolpidem, you may want to adopt a risk management approach as you would with other medications that can have serious side effects. An approach to benzodiazepine prescribing proposed by Bursztajn et al31 advocates:
- using the informed-consent process to build an alliance with patients
- not prescribing the medication in isolation of other beneficial therapies
- being aware of and always documenting your decision-making process.
When you make patients aware of all risks, benefits, alternate therapies, and possible outcomes with no treatment, you have informed them effectively. Patients are then left to decide whether or not to agree to the treatment. You also are responsible for monitoring the patient, addressing the patient’s questions, and relaying important safety information.
When prescribing zolpidem, discuss safety information with the patient, such as:
- Do not drive or operate heavy equipment for at least 5 to 6 hours after administration.
- Have a safety plan in place for transportation during those hours.
- Do not use this medication with alcohol or other sedative/hypnotics.
- Contact the prescriber about any suspected adverse effects.
- MedlinePlus information on sleep disorders. National Institutes of Health and National Library of Medicine. www.nlm.nih.gov/medlineplus/sleepdisorders.html.
- Zolpidem (systemic). Mayoclinic.com: Tools for healthier lives. www.mayoclinic.com/health/drug-information/DR202707.
- Diazepam • Valium
- Eszopiclone • Lunesta
- Midazolam • Versed
- Oxazepam • Serax
- Quazepam • Doral
- Rofecoxib • Vioxx
- Temazepam • Restoril
- Triazolam • Halcion
- Zolpidem • Ambien, Ambien CR
- Zaleplon • Sonata
- Zopiclone • Imovane (in Europe)
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
The authors acknowledge the assistance and guidance of Linda T. Moore, JD, in preparing this manuscript.
1. Markon J. Sleeping Va. driver convicted in crash; man had taken too much Ambien. The Washington Post, August 2, 2006. Accessed August 26, 2006 from LexisNexis Academic Database.
2. Colten HR, Altevogt BM. Sleep disorders and sleep deprivation: an unmet public health problem. Available at: http://www.iom.edu/CMS/3740/23160/33668.aspx. Accessed February 21, 2007.
3. Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment; prevalence and correlates. Arch Gen Psychiatry 1985;42:225-32.
4. Barclay L. Driving, other erratic behaviors reported after taking zolpidem. Available at http://www.medscape.com/viewarticle/528415. Accessed February 21, 2007.
5. Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep 2006;29(8):1009-14.
6. Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005;165(8):863-7.
7. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med 2006;4(2):104-13.
8. Verispan VONA. Top 200 brand name drugs by units in 2005. Drug Topics 2006. Available at: http://www.drugtopics.com/drugtopics/data/articlestandard/drugtopics/102006/311294/article.pdf. Accessed February 22, 2007.
9. sanofi-aventis Ambien prescribing information. Available at: http://products.sanofi-aventis.us/ambien/ambien.html. Accessed February 21, 2007.
10. Pear R. Patrick Kennedy crashes car into a Capitol Hill barrier. The New York Times, May 5, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
11. Janet Makinen and others v. Sanofi-Synthelabo & Sanofi-Synthelabo, Inc. Class action suit filed March 6, 2006 in U.S. District Court for the Southern District of New York, NY.
12. Tooher NL. Ambien users are filing lawsuits. Kansas City Daily Record, April 12, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
13. Verster JC, Veldhuijzen DS, Volkerts ER. Residual effects of sleep medication on driving ability. Sleep Med Rev 2004;8(4):309-25.
14. Wilkinson CJ. The acute effects of zolpidem, administered alone and with alcohol, on cognitive and psychomotor function. J Clin Psychiatry 1995;56(7):309-18.
15. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
16. Mattila MJ, Vanakoski J, Kalska H, Seppala T. Effects of alcohol, zolpidem, and some other sedatives and hypnotics on human performance and memory. Pharmacol Biochem Behav 1998;59(4):917-23.
17. Mintzer MZ, Griffiths RR. Selective effects of zolpidem on human memory functions. J Psychopharmacol 1999;13(1):18-31.
18. Verster JC, Volkerts ER, Schreuder AH, et al. Residual effects of middle-of-the-night administration of zaleplon and zolpidem on driving ability, memory functions, and psychomotor performance. J Clin Psychopharmacol 2002;22(6):576-83.
19. Paul MA, Gray G, Kenny G, Pigeau RA. Impact of melatonin, zaleplon, zopiclone, and temazepam on psychomotor performance. Aviat Space Environ Med 2003;74(12):1263-70.
20. Partinen M, Hirvonen K, Hublin C, et al. Effects of after-midnight intake of zolpidem and temazepam on driving ability in women with non-organic insomnia. Sleep Med 2003;4(6):553-61.
21. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
22. Logan BK, Couper FJ. Zolpidem and driving impairment. J Forensic Sci 2001;46(1):105-10.
23. Fairweather DB, Kerr JS, Hindmarch I. The effects of acute and repeated doses of zolpidem on subjective sleep, psychomotor performance and cognitive function in elderly volunteers. Eur J Clin Pharmacol 1992;43(6):597-601.
24. Bocca ML, Le Doze F, Etard O, et al. Residual effect of zolpidem 10 mg and zopiclone 7.5 mg versus flunitrazepam 1 mg and placebo on driving performance and ocular saccades. Psychopharmacology (Berl) 1999;143(4):373-9.
25. Staner L, Ertle S, Boeijinga P, et al. Next-day residual effects of hypnotics in DSM-IV primary insomnia: a driving simulator study with simultaneous electroencephalogram monitoring. Psychopharmacology (Berl) 2005;181(4):790-8.
26. Natanson v. Kline 300 P.2d 1093 (1960).
27. Canterbury v. Spence 464 F.2d 772 (1972).
28. Reyes v. Wyeth Laboratories. 498 F.2d 1264 (1974).
29. Ernst v. Merck & Co. 24 PLLR 149 (2005).
30. Humeston v. Merck & Co., No. ATL-L-2272-03-MT, Super. Ct., Atlantic County, NJ, November 3, 2005.
31. Johnson LA. Jury blames Vioxx for man’s heart attack, awards $47.5 million. Available at http://news.findlaw.com/ap/o/51/03-12-2007/85f9000f67cd576a.html. Accessed March 16, 2007.
32. Bursztajn HJ, Brodsky A. Ethical and legal dimensions of benzodiazepine prescription: a commentary. Psychiatr Ann 1998;28(3):121-7.
1. Markon J. Sleeping Va. driver convicted in crash; man had taken too much Ambien. The Washington Post, August 2, 2006. Accessed August 26, 2006 from LexisNexis Academic Database.
2. Colten HR, Altevogt BM. Sleep disorders and sleep deprivation: an unmet public health problem. Available at: http://www.iom.edu/CMS/3740/23160/33668.aspx. Accessed February 21, 2007.
3. Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment; prevalence and correlates. Arch Gen Psychiatry 1985;42:225-32.
4. Barclay L. Driving, other erratic behaviors reported after taking zolpidem. Available at http://www.medscape.com/viewarticle/528415. Accessed February 21, 2007.
5. Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep 2006;29(8):1009-14.
6. Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005;165(8):863-7.
7. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med 2006;4(2):104-13.
8. Verispan VONA. Top 200 brand name drugs by units in 2005. Drug Topics 2006. Available at: http://www.drugtopics.com/drugtopics/data/articlestandard/drugtopics/102006/311294/article.pdf. Accessed February 22, 2007.
9. sanofi-aventis Ambien prescribing information. Available at: http://products.sanofi-aventis.us/ambien/ambien.html. Accessed February 21, 2007.
10. Pear R. Patrick Kennedy crashes car into a Capitol Hill barrier. The New York Times, May 5, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
11. Janet Makinen and others v. Sanofi-Synthelabo & Sanofi-Synthelabo, Inc. Class action suit filed March 6, 2006 in U.S. District Court for the Southern District of New York, NY.
12. Tooher NL. Ambien users are filing lawsuits. Kansas City Daily Record, April 12, 2006. Accessed September 25, 2006 from LexisNexis Academic Database.
13. Verster JC, Veldhuijzen DS, Volkerts ER. Residual effects of sleep medication on driving ability. Sleep Med Rev 2004;8(4):309-25.
14. Wilkinson CJ. The acute effects of zolpidem, administered alone and with alcohol, on cognitive and psychomotor function. J Clin Psychiatry 1995;56(7):309-18.
15. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
16. Mattila MJ, Vanakoski J, Kalska H, Seppala T. Effects of alcohol, zolpidem, and some other sedatives and hypnotics on human performance and memory. Pharmacol Biochem Behav 1998;59(4):917-23.
17. Mintzer MZ, Griffiths RR. Selective effects of zolpidem on human memory functions. J Psychopharmacol 1999;13(1):18-31.
18. Verster JC, Volkerts ER, Schreuder AH, et al. Residual effects of middle-of-the-night administration of zaleplon and zolpidem on driving ability, memory functions, and psychomotor performance. J Clin Psychopharmacol 2002;22(6):576-83.
19. Paul MA, Gray G, Kenny G, Pigeau RA. Impact of melatonin, zaleplon, zopiclone, and temazepam on psychomotor performance. Aviat Space Environ Med 2003;74(12):1263-70.
20. Partinen M, Hirvonen K, Hublin C, et al. Effects of after-midnight intake of zolpidem and temazepam on driving ability in women with non-organic insomnia. Sleep Med 2003;4(6):553-61.
21. Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 1998;18(2):154-65.
22. Logan BK, Couper FJ. Zolpidem and driving impairment. J Forensic Sci 2001;46(1):105-10.
23. Fairweather DB, Kerr JS, Hindmarch I. The effects of acute and repeated doses of zolpidem on subjective sleep, psychomotor performance and cognitive function in elderly volunteers. Eur J Clin Pharmacol 1992;43(6):597-601.
24. Bocca ML, Le Doze F, Etard O, et al. Residual effect of zolpidem 10 mg and zopiclone 7.5 mg versus flunitrazepam 1 mg and placebo on driving performance and ocular saccades. Psychopharmacology (Berl) 1999;143(4):373-9.
25. Staner L, Ertle S, Boeijinga P, et al. Next-day residual effects of hypnotics in DSM-IV primary insomnia: a driving simulator study with simultaneous electroencephalogram monitoring. Psychopharmacology (Berl) 2005;181(4):790-8.
26. Natanson v. Kline 300 P.2d 1093 (1960).
27. Canterbury v. Spence 464 F.2d 772 (1972).
28. Reyes v. Wyeth Laboratories. 498 F.2d 1264 (1974).
29. Ernst v. Merck & Co. 24 PLLR 149 (2005).
30. Humeston v. Merck & Co., No. ATL-L-2272-03-MT, Super. Ct., Atlantic County, NJ, November 3, 2005.
31. Johnson LA. Jury blames Vioxx for man’s heart attack, awards $47.5 million. Available at http://news.findlaw.com/ap/o/51/03-12-2007/85f9000f67cd576a.html. Accessed March 16, 2007.
32. Bursztajn HJ, Brodsky A. Ethical and legal dimensions of benzodiazepine prescription: a commentary. Psychiatr Ann 1998;28(3):121-7.


