User login
Vitiligo
THE COMPARISON
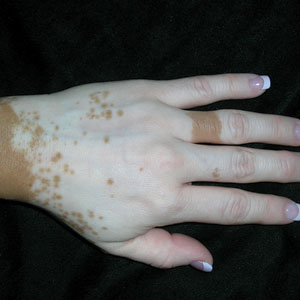
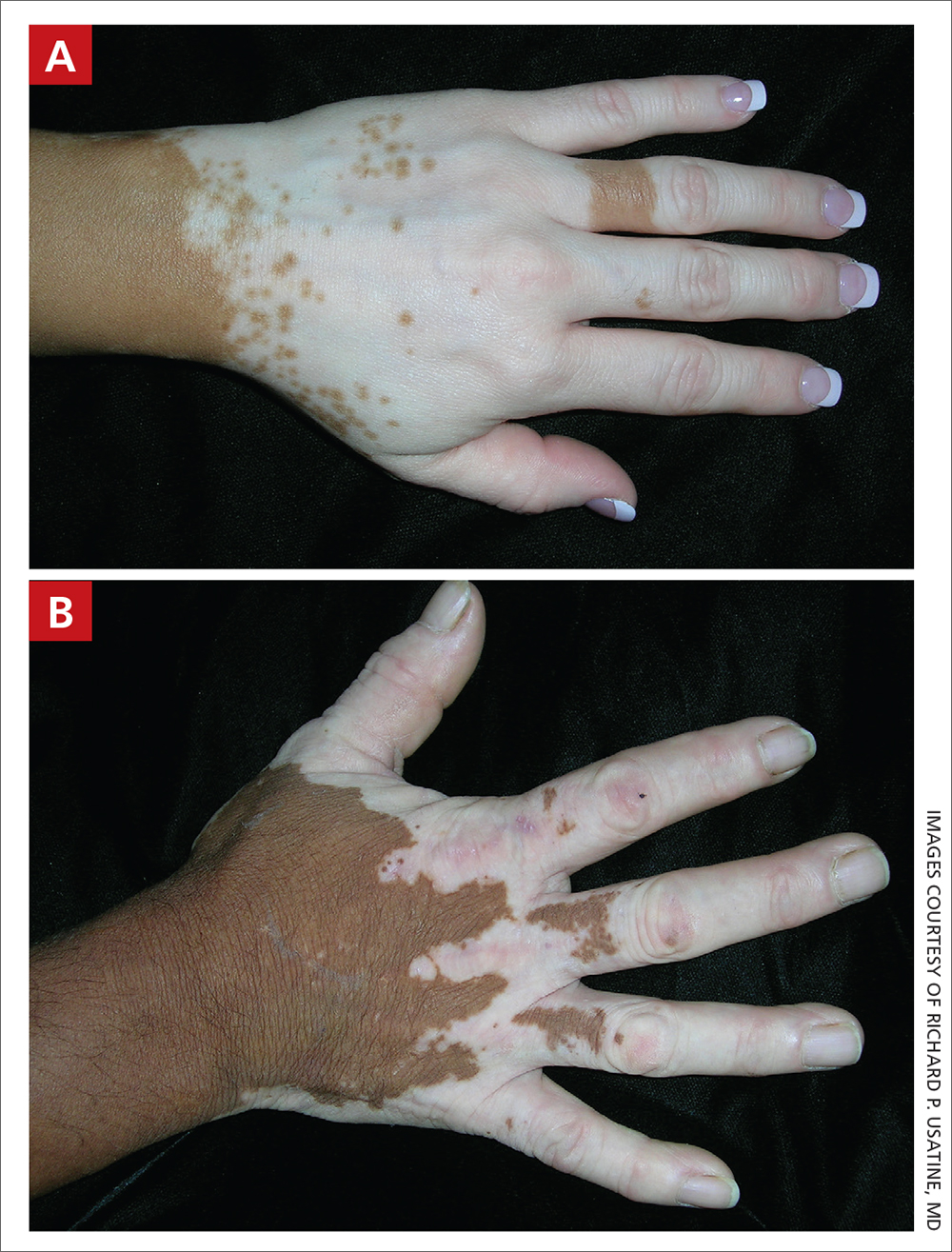
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
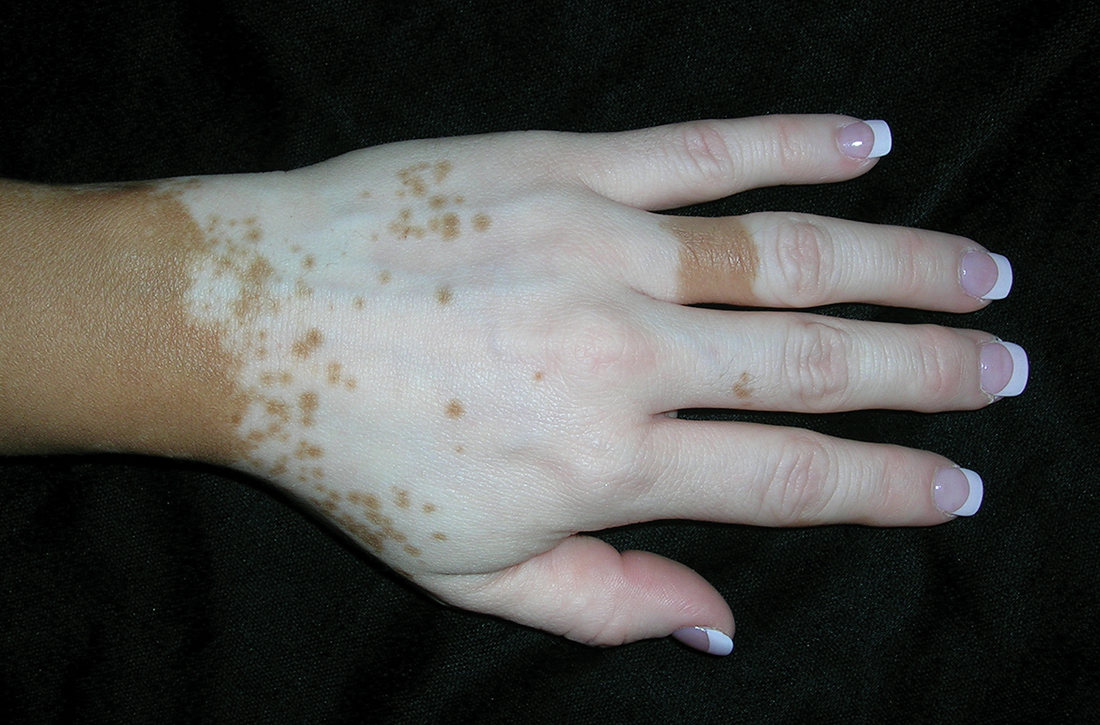
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
Infant with red eyelid lesion
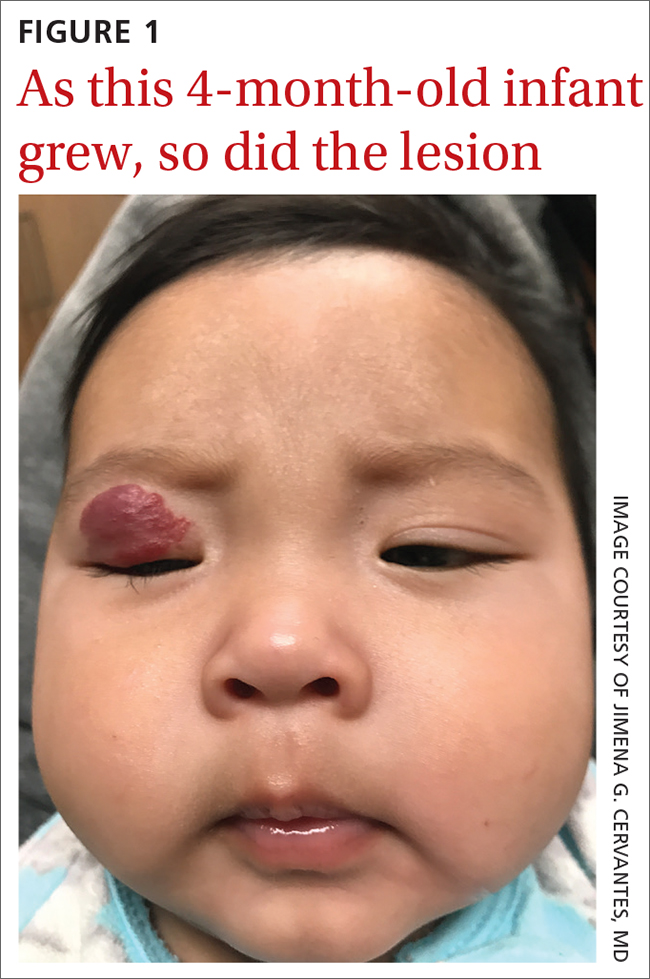
A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
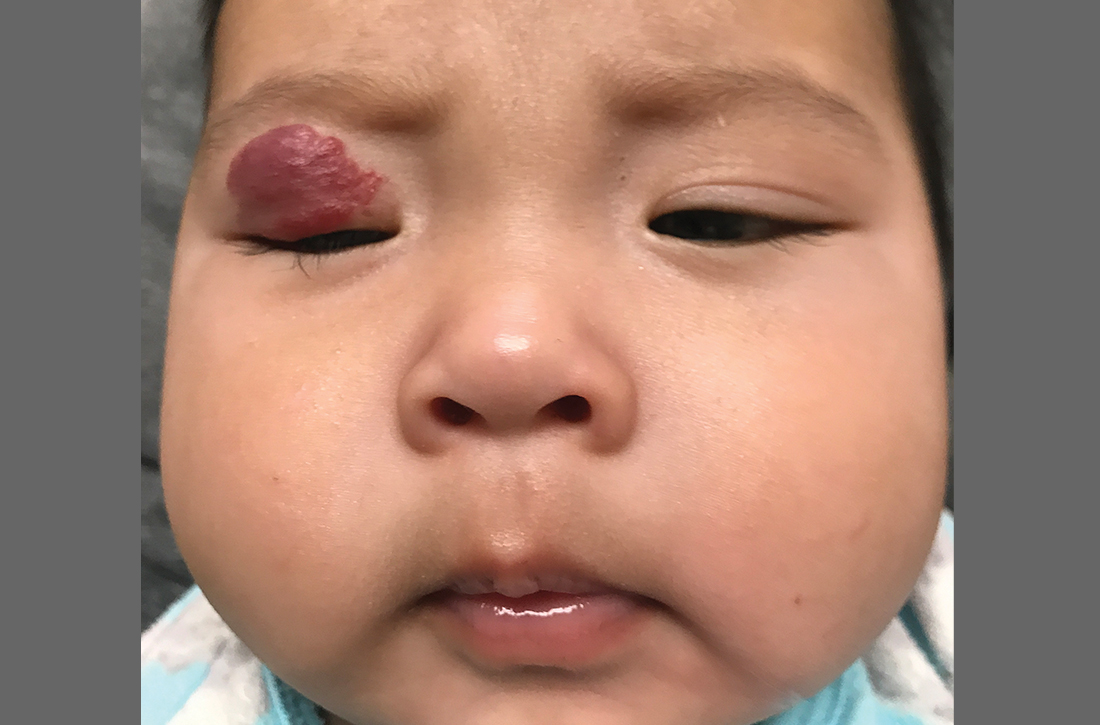
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
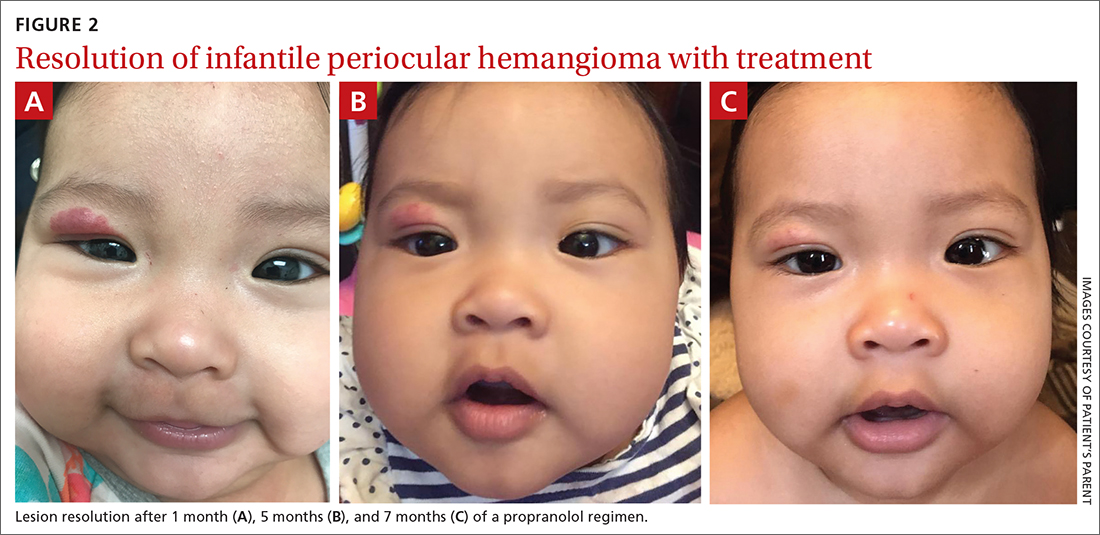
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.
1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525
A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.

A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.

1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525
1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525
Vitiligo
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband ultraviolet B (UVB) treatment.
B Vitiligo on the hand in a young Hispanic male.
Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1
Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (FIGURES A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when making the diagnosis is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Continue to: Treatment of vitiligo...
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients ages 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase–signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
FDA approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
1. Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi: 10.1016/j.det. 2016.11.014
2. Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi: 10.1016/j.jaad.2010.11.061
3. van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi: 10.1016/j.det.2016.11.005
4. Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi: 10.1016/j.ijwd.2017.11.005
5. Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review. Am J Clin Dermatol. 2021; 22:757-774. doi: 10.1007/s40257-021-00631-6
6. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical- treatment-addressing-repigmentation-vitiligo-patients-aged- 12-and-older
7. Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color. Pediatr Dermatol. 2021;38(suppl 2):79-85. doi: 10.1111/ pde.14714
8. Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. www.drugs.com/priceguide/opzelura
9. Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
10. Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. doi: 10.25251/skin.6.3.6
11. Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi: 10.2147/CCID.S185798
12. Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. www.census.gov/library/publications/2021/demo/p60-273.html
13. Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi: 10.1002/14651858.CD003263.pub4
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband ultraviolet B (UVB) treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1
Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (FIGURES A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when making the diagnosis is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Continue to: Treatment of vitiligo...
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients ages 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase–signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
FDA approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband ultraviolet B (UVB) treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1
Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (FIGURES A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when making the diagnosis is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Continue to: Treatment of vitiligo...
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients ages 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase–signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
FDA approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
1. Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi: 10.1016/j.det. 2016.11.014
2. Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi: 10.1016/j.jaad.2010.11.061
3. van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi: 10.1016/j.det.2016.11.005
4. Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi: 10.1016/j.ijwd.2017.11.005
5. Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review. Am J Clin Dermatol. 2021; 22:757-774. doi: 10.1007/s40257-021-00631-6
6. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical- treatment-addressing-repigmentation-vitiligo-patients-aged- 12-and-older
7. Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color. Pediatr Dermatol. 2021;38(suppl 2):79-85. doi: 10.1111/ pde.14714
8. Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. www.drugs.com/priceguide/opzelura
9. Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
10. Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. doi: 10.25251/skin.6.3.6
11. Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi: 10.2147/CCID.S185798
12. Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. www.census.gov/library/publications/2021/demo/p60-273.html
13. Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi: 10.1002/14651858.CD003263.pub4
1. Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi: 10.1016/j.det. 2016.11.014
2. Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi: 10.1016/j.jaad.2010.11.061
3. van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi: 10.1016/j.det.2016.11.005
4. Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi: 10.1016/j.ijwd.2017.11.005
5. Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review. Am J Clin Dermatol. 2021; 22:757-774. doi: 10.1007/s40257-021-00631-6
6. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical- treatment-addressing-repigmentation-vitiligo-patients-aged- 12-and-older
7. Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color. Pediatr Dermatol. 2021;38(suppl 2):79-85. doi: 10.1111/ pde.14714
8. Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. www.drugs.com/priceguide/opzelura
9. Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
10. Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. doi: 10.25251/skin.6.3.6
11. Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi: 10.2147/CCID.S185798
12. Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. www.census.gov/library/publications/2021/demo/p60-273.html
13. Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi: 10.1002/14651858.CD003263.pub4
Erythrasma
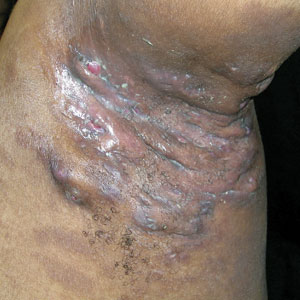
THE COMPARISON
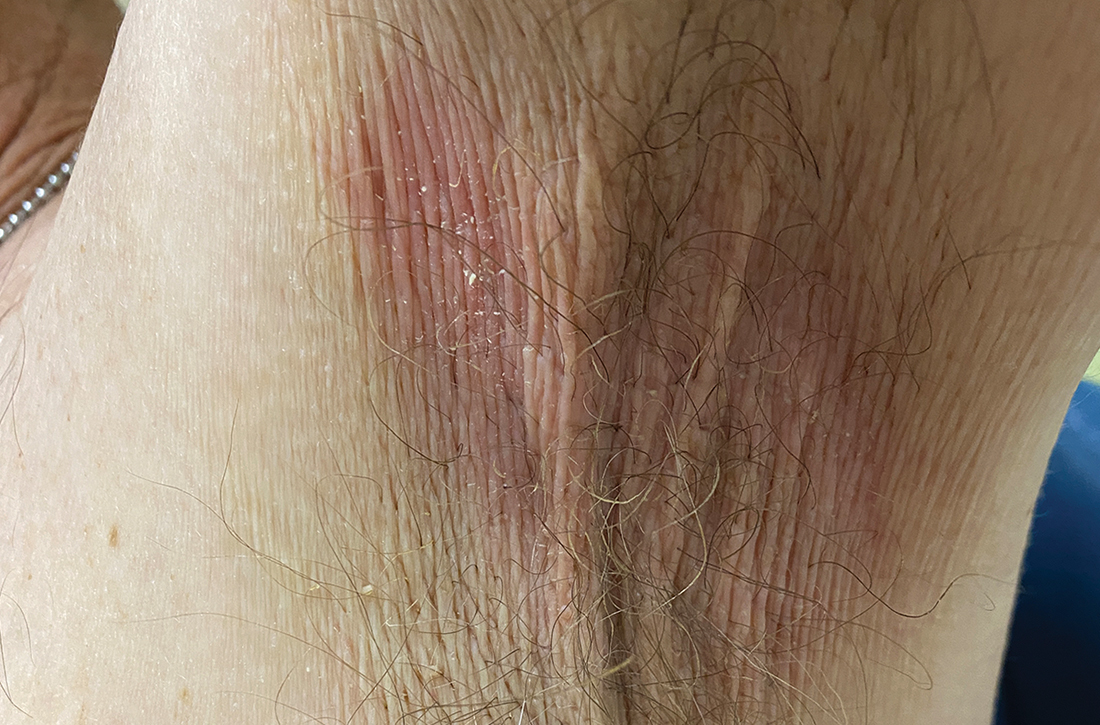
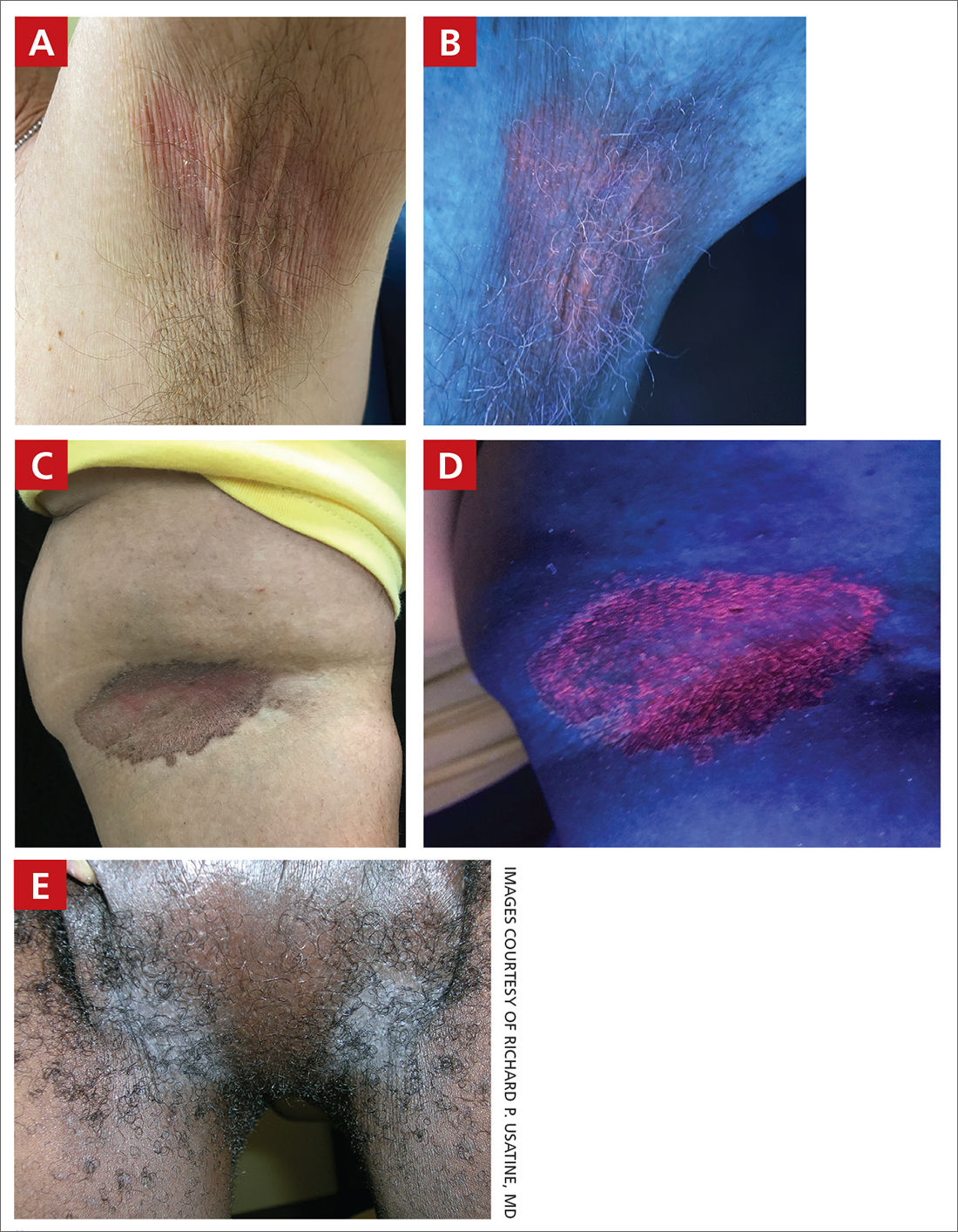
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood-lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood-lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches (with pruritus) in the groin of a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.
Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
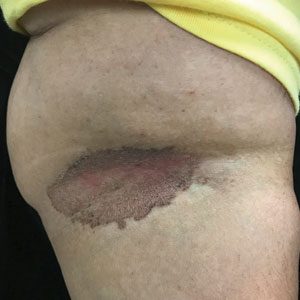
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (FIGURES A and C) or as a hypopigmented patch (FIGURE E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
- C minutissimum produces coproporphyrin III, which glows fluorescent red under Wood-lamp examination (FIGURES B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
- Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
- The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
- There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
- Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non-Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes, likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
1. Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
2. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
3. Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
4. Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
5. Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
6. Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
7. Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls. Stat- Pearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood-lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood-lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches (with pruritus) in the groin of a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (FIGURES A and C) or as a hypopigmented patch (FIGURE E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
- C minutissimum produces coproporphyrin III, which glows fluorescent red under Wood-lamp examination (FIGURES B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
- Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
- The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
- There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
- Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non-Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes, likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood-lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood-lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches (with pruritus) in the groin of a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (FIGURES A and C) or as a hypopigmented patch (FIGURE E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
- C minutissimum produces coproporphyrin III, which glows fluorescent red under Wood-lamp examination (FIGURES B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
- Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
- The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
- There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
- Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non-Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes, likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
1. Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
2. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
3. Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
4. Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
5. Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
6. Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
7. Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls. Stat- Pearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
1. Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
2. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
3. Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
4. Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
5. Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
6. Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
7. Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls. Stat- Pearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
Erythrasma
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.
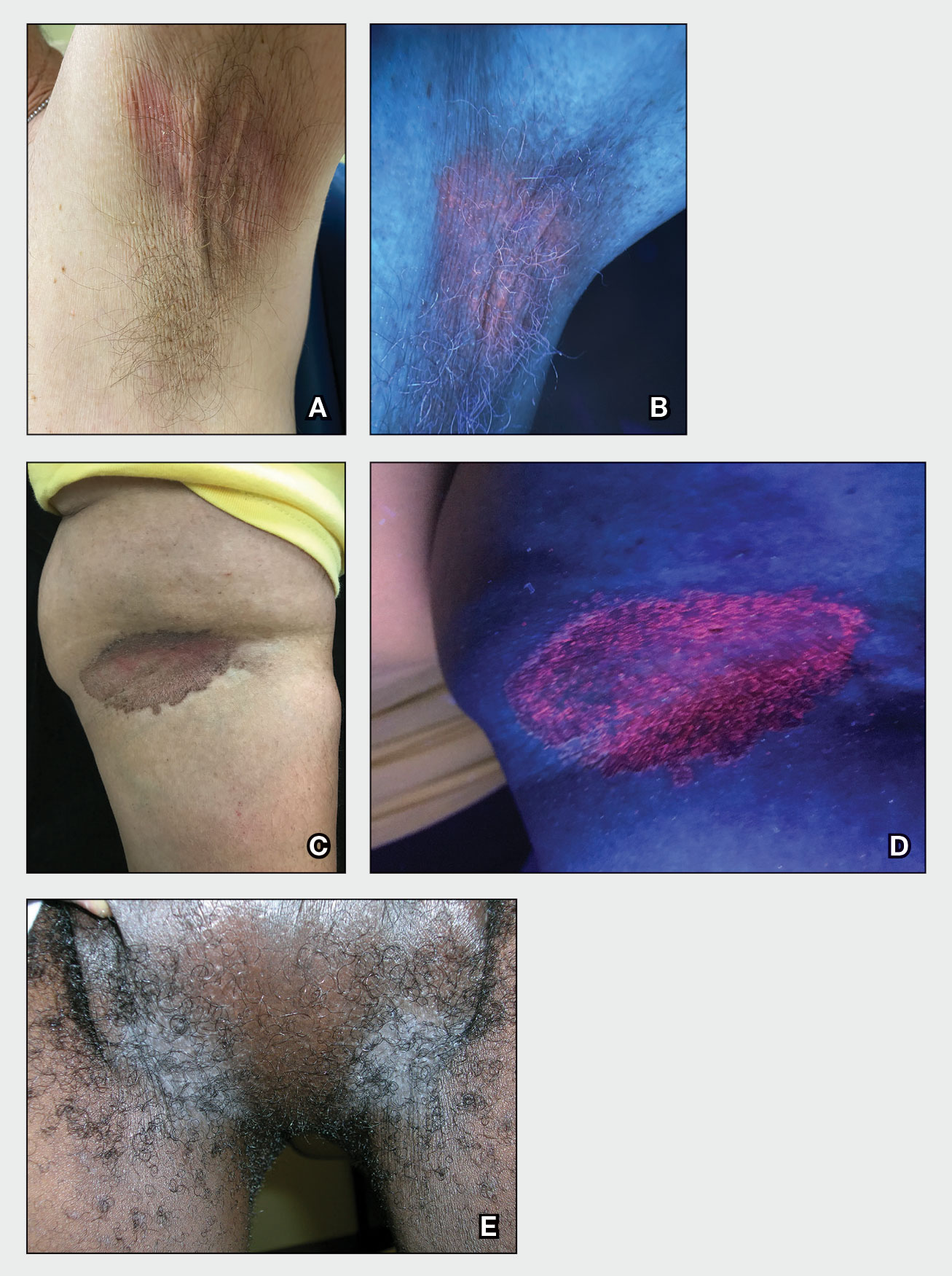
Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
Tinea capitis
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.
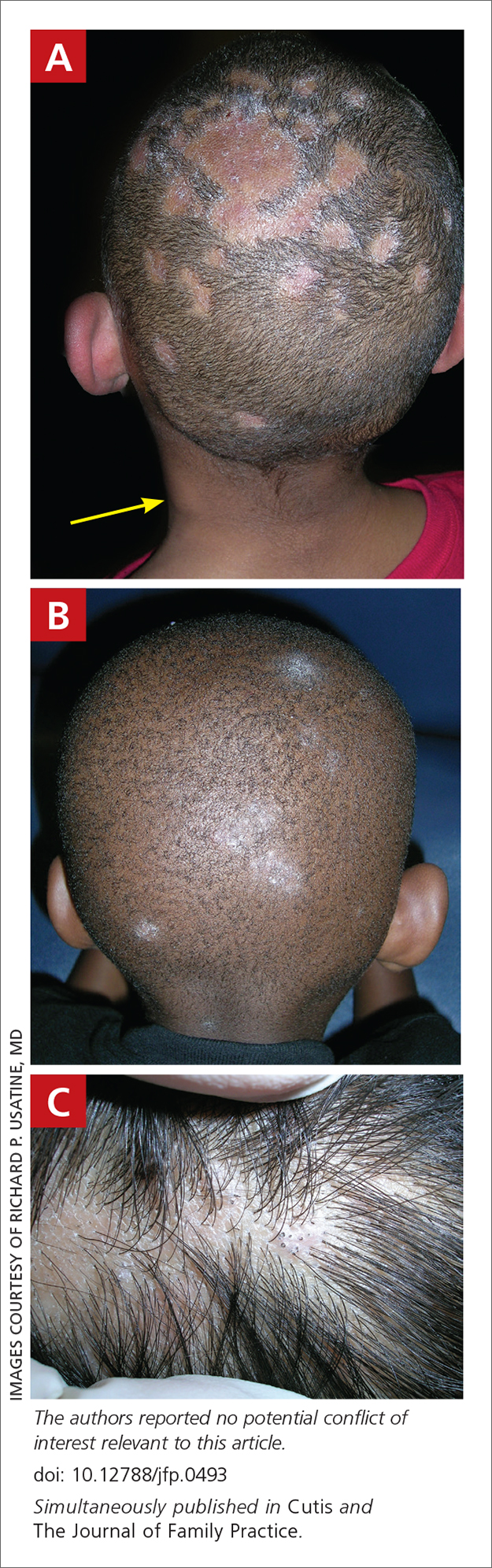
Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
Tinea Capitis
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.
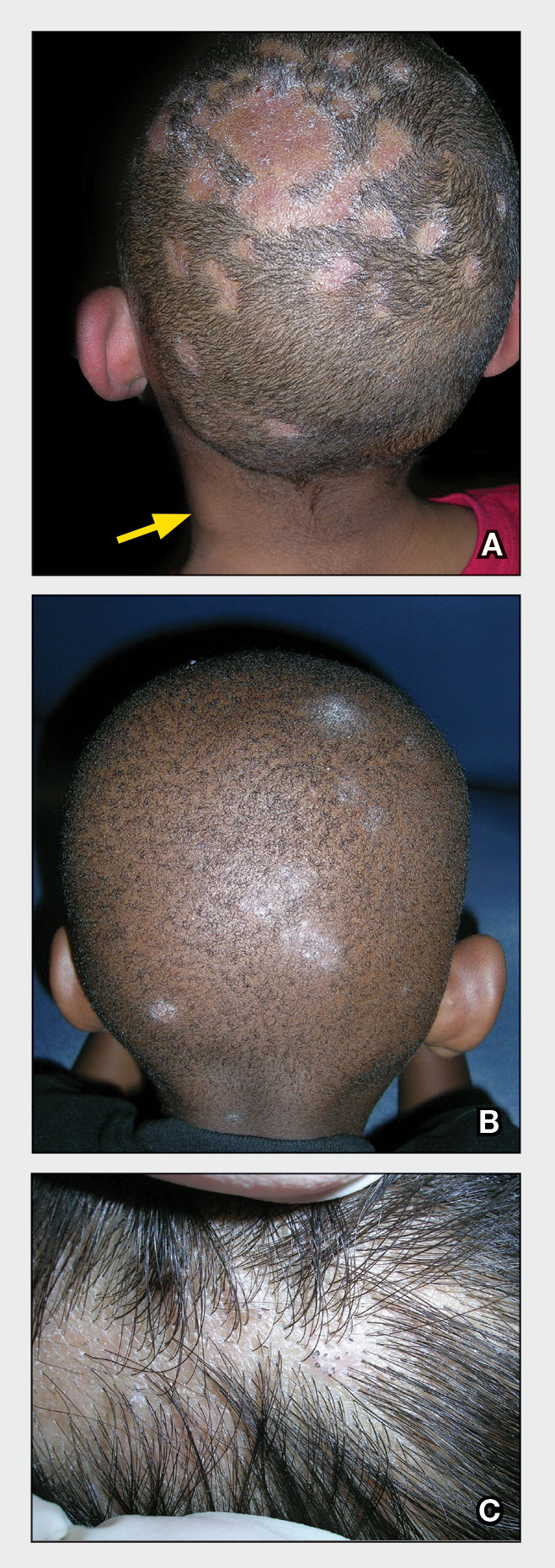
Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) such as M canis. Microsporum canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations: • broken hairs that appear as black dots on the scalp • diffuse scale mimicking seborrheic dermatitis • well-demarcated annular plaques • exudate and tenderness caused by inflammation • scalp pruritus • occipital scalp lymphadenopathy. Worth noting Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp; however, the application of heavy emollients, oils, and grease to camouflage scale contributes to falsenegative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5 Health disparity highlight A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
- Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management [published online July 12, 2018]. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi:10.1111/jdv.15088
- Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study [published online April 19, 2010]. Pediatrics. 2010;125:966-973. doi:10.1542/peds.2009-2522
- Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi:10.1067/mjd.2002.120793
- Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
- Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015 [published online January 20, 2020]. Pediatr Dermatol. 2020;37:305-310. doi:10.1111 /pde.14092
- Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria [published online April 16, 2008]. Mycoses. 2008;51:536-541. doi:10.1111/j.1439-0507.2008.01507.x
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) such as M canis. Microsporum canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations: • broken hairs that appear as black dots on the scalp • diffuse scale mimicking seborrheic dermatitis • well-demarcated annular plaques • exudate and tenderness caused by inflammation • scalp pruritus • occipital scalp lymphadenopathy. Worth noting Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp; however, the application of heavy emollients, oils, and grease to camouflage scale contributes to falsenegative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5 Health disparity highlight A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) such as M canis. Microsporum canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations: • broken hairs that appear as black dots on the scalp • diffuse scale mimicking seborrheic dermatitis • well-demarcated annular plaques • exudate and tenderness caused by inflammation • scalp pruritus • occipital scalp lymphadenopathy. Worth noting Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp; however, the application of heavy emollients, oils, and grease to camouflage scale contributes to falsenegative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5 Health disparity highlight A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
- Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management [published online July 12, 2018]. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi:10.1111/jdv.15088
- Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study [published online April 19, 2010]. Pediatrics. 2010;125:966-973. doi:10.1542/peds.2009-2522
- Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi:10.1067/mjd.2002.120793
- Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
- Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015 [published online January 20, 2020]. Pediatr Dermatol. 2020;37:305-310. doi:10.1111 /pde.14092
- Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria [published online April 16, 2008]. Mycoses. 2008;51:536-541. doi:10.1111/j.1439-0507.2008.01507.x
- Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management [published online July 12, 2018]. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi:10.1111/jdv.15088
- Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study [published online April 19, 2010]. Pediatrics. 2010;125:966-973. doi:10.1542/peds.2009-2522
- Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi:10.1067/mjd.2002.120793
- Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
- Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015 [published online January 20, 2020]. Pediatr Dermatol. 2020;37:305-310. doi:10.1111 /pde.14092
- Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria [published online April 16, 2008]. Mycoses. 2008;51:536-541. doi:10.1111/j.1439-0507.2008.01507.x
Hidradenitis suppurativa
THE COMPARISON
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.
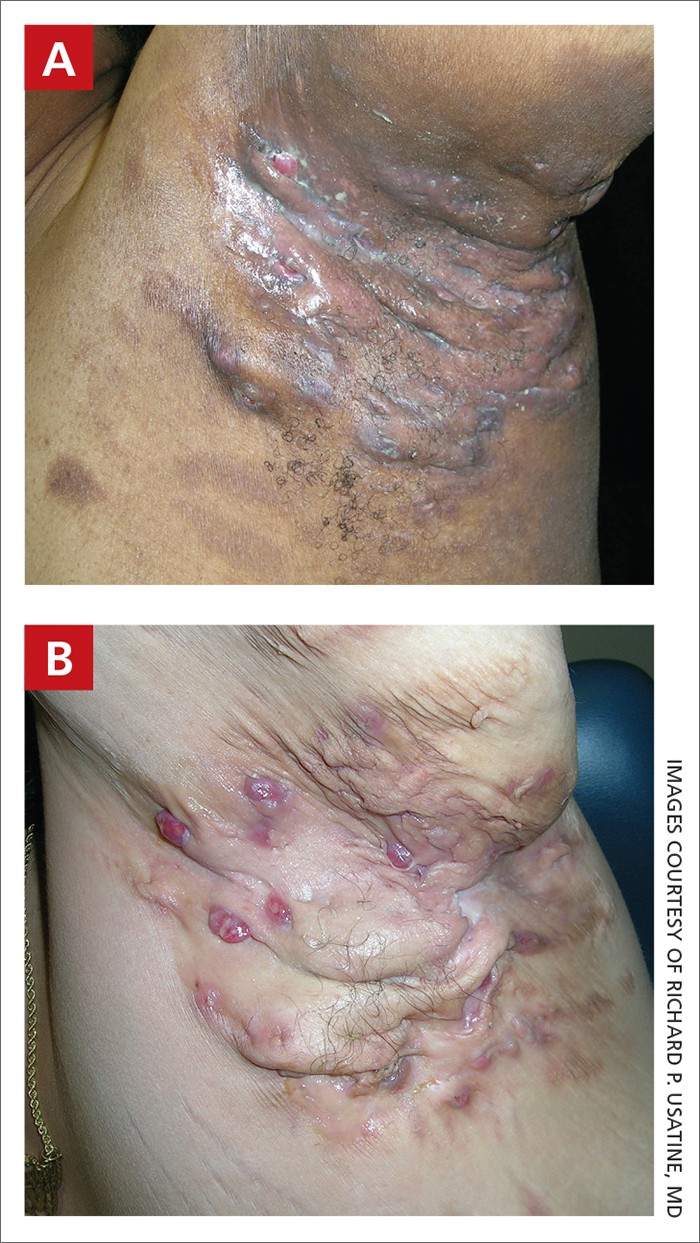
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
THE COMPARISON
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
THE COMPARISON
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
Hidradenitis Suppurativa
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).
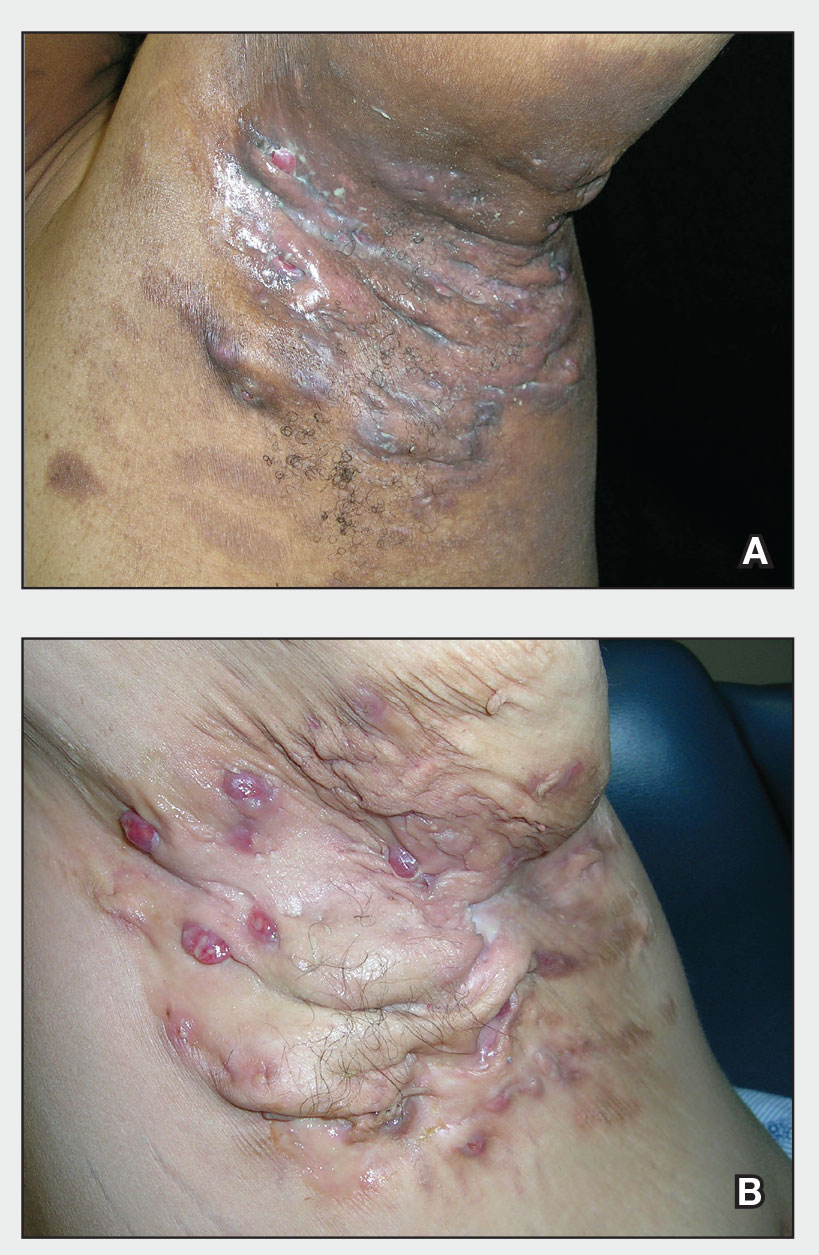
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
Basal cell carcinoma
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma (BCC) is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
BCC is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals. 5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (FIGURE, A) as in non- Hispanic White individuals.7 Any skin cancer can present with ulcerations. So, while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ethnic variability. In my practice in San Antonio (RPU), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (FIGURE, A).
There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes.1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and squamous cell carcinoma found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non-Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (FIGURE, B) should prompt consideration of skin cancer—regardless of skin color.
1. Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
2. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23: 137-151. doi:10.1007/s40257-021-00662-z
3. Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
4. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
5. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
6. Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/ S0190-9622(81)70067-7
7. Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/ S0190-9622(96)90007-9
8. Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.

Basal cell carcinoma (BCC) is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
BCC is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals. 5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (FIGURE, A) as in non- Hispanic White individuals.7 Any skin cancer can present with ulcerations. So, while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ethnic variability. In my practice in San Antonio (RPU), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (FIGURE, A).
There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes.1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and squamous cell carcinoma found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non-Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (FIGURE, B) should prompt consideration of skin cancer—regardless of skin color.
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.

Basal cell carcinoma (BCC) is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
BCC is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals. 5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (FIGURE, A) as in non- Hispanic White individuals.7 Any skin cancer can present with ulcerations. So, while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ethnic variability. In my practice in San Antonio (RPU), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (FIGURE, A).
There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes.1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and squamous cell carcinoma found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non-Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (FIGURE, B) should prompt consideration of skin cancer—regardless of skin color.
1. Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
2. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23: 137-151. doi:10.1007/s40257-021-00662-z
3. Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
4. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
5. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
6. Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/ S0190-9622(81)70067-7
7. Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/ S0190-9622(96)90007-9
8. Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
1. Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
2. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23: 137-151. doi:10.1007/s40257-021-00662-z
3. Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
4. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
5. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
6. Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/ S0190-9622(81)70067-7
7. Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/ S0190-9622(96)90007-9
8. Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005