User login
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Richard P. Usatine, MD
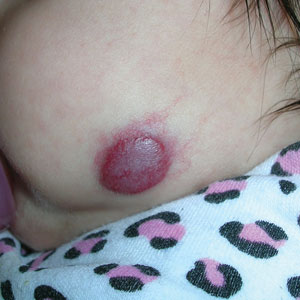
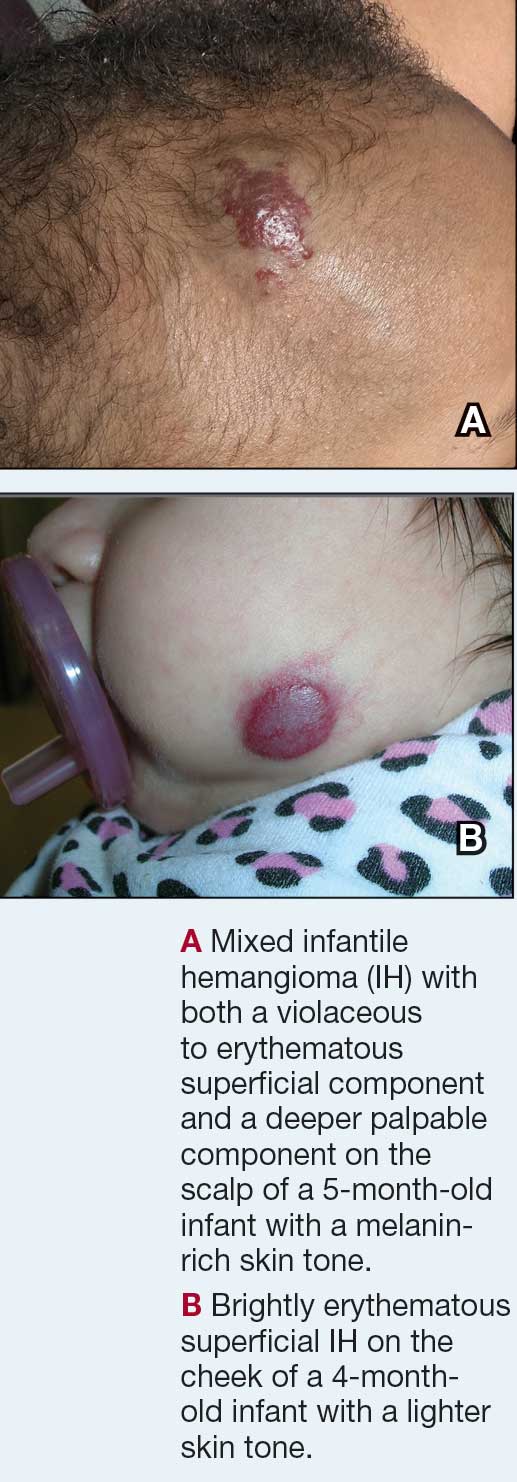
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (< 1000 g).1,3 Maternal risk factors include advanced gestational age (ie, > 35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥ 5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (> 5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. Part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. Part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.

Richard P. Usatine, MD
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (< 1000 g).1,3 Maternal risk factors include advanced gestational age (ie, > 35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥ 5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (> 5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.

Richard P. Usatine, MD
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (< 1000 g).1,3 Maternal risk factors include advanced gestational age (ie, > 35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥ 5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (> 5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. Part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. Part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. Part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. Part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (<1000 g).1,3 Maternal risk factors include advanced gestational age (ie, >35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (>5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (<1000 g).1,3 Maternal risk factors include advanced gestational age (ie, >35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (>5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (<1000 g).1,3 Maternal risk factors include advanced gestational age (ie, >35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (>5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Don’t Miss These Signs of Rosacea in Darker Skin Types
Don’t Miss These Signs of Rosacea in Darker Skin Types
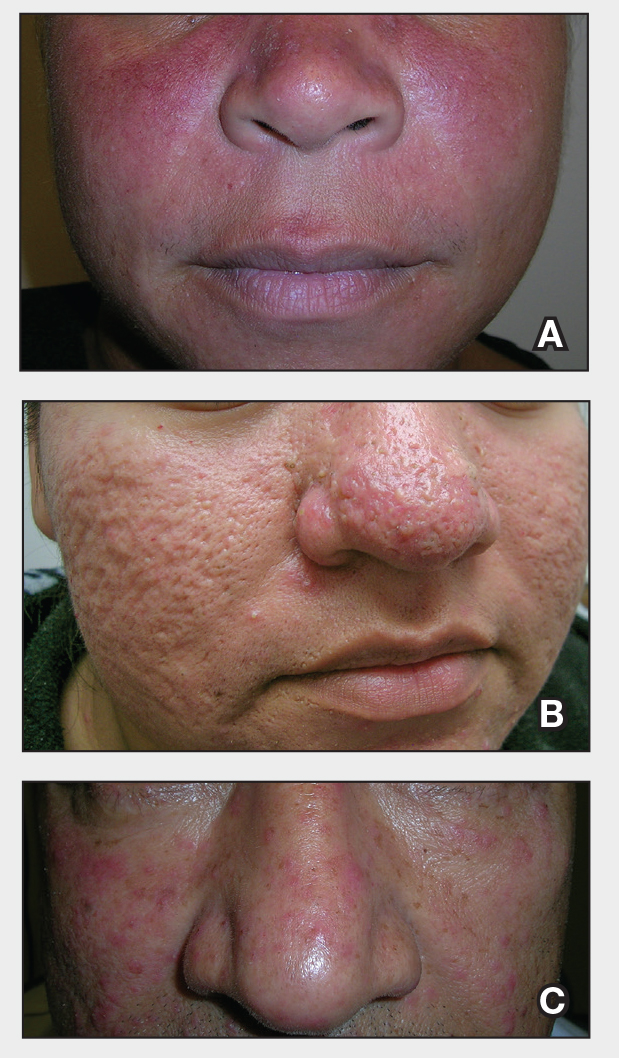
THE COMPARISON:
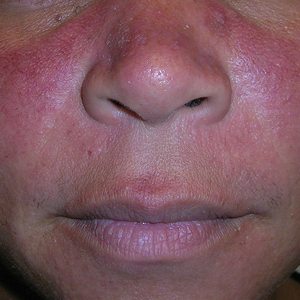
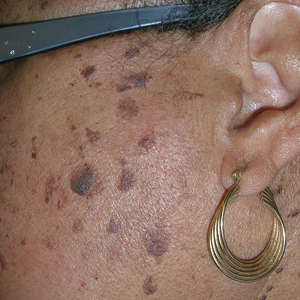
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
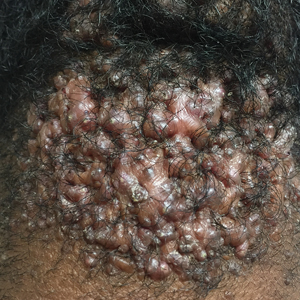
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2
Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
THE COMPARISON:
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2

Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
THE COMPARISON:
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2

Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
Don’t Miss These Signs of Rosacea in Darker Skin Types
Don’t Miss These Signs of Rosacea in Darker Skin Types
Consider Cultural Practices and Barriers to Care When Treating Alopecia Areata
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.
Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
Alopecia areata is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
Alopecia areata frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
Alopecia areata clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), alopecia areata severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, <4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
Alopecia areata is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
Alopecia areata frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
Alopecia areata clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), alopecia areata severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, <4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
The Comparison
A. Alopecia areata in a young girl with a lighter skin tone. The fine white vellus hairs are signs of regrowth.
B. Alopecia areata in a 49-year-old man with tightly coiled hair and darker skin tone. Coiled white hairs are noted in the alopecia patches.

Alopecia areata (AA) is a common autoimmune condition characterized by hair loss resulting from a T cell–mediated attack on the hair follicles. It manifests as nonscarring patches of hair loss on the scalp, eyebrows, eyelashes, and beard area as well as more extensive complete loss of scalp and body hair. While AA may affect individuals of any age, most patients develop their first patch(es) of hair loss during childhood.1 The treatment landscape for AA has evolved considerably in recent years, but barriers to access to newer treatments persist.
Epidemiology
Alopecia areata is most prevalent among pediatric and adult individuals of African, Asian, or Hispanic/Latino descent.2-4 In some studies, Black individuals had higher odds and Asian individuals had lower odds of developing AA, while other studies have reported the highest standardized prevalence among Asian individuals.5 In the United States, AA affects about 1.47% of adults and as many as 0.11% of children.6-8 In Black patients, AA often manifests early with a female predominance.5
Alopecia areata frequently is associated with autoimmune comorbidities, the most common being thyroid disease.3,5 In Black patients, AA is associated with more atopic comorbidities, including asthma, atopic dermatitis, and allergic rhinitis.5
Key Clinical Features
Alopecia areata clinically manifests similarly across different skin tones; however, in patients with more tightly coiled or curly hair, the extent of scalp hair loss may be underestimated without a full examination. Culturally sensitive approaches to hair and scalp evaluation are essential, especially for Black women, whose hair care practices and scalp conditions may be overlooked or misunderstood during visits to evaluate hair loss. A thoughtful history and gentle examination of the hair and scalp that considers hair texture, cultural practices such as head coverings (eg, headwraps, turbans, hijabs), use of hair adornments (eg, clips, beads, bows), traditional braiding, and use of natural oils or herbal treatments, as well as styling methods including tight hairstyles, use of heat styling tools (eg, flat irons, curling irons), chemical application (eg, straighteners, hair color), and washing or styling frequency can improve diagnostic accuracy and help build trust in the patient-provider relationship.
Classic signs of AA visualized with dermoscopy include yellow and/or black dots on the scalp and exclamation point hairs. The appearance of fine white vellus hairs within the alopecic patches also may indicate early regrowth. On scalp trichoscopy, black dots are more prominent, and yellow dots are less prominent, in individuals with darker skin tones vs lighter skin tones.9
Worth Noting
In addition to a full examination of the scalp, documenting the extent of hair loss using validated severity scales, including the severity of alopecia tool (SALT), alopecia areata severity index (AASI), clinician-reported outcome assessment, and patient-reported outcome measures, can standardize disease severity assessment, facilitate timely insurance or medication approvals, and support objective tracking of treatment response, which may ultimately enhance access to care.10
Prompt treatment of AA is essential. Not surprisingly, patients given a diagnosis of AA may experience considerable emotional and psychological distress—regardless of the extent of the loss.11 Treatment options include mid- to high-potency topical or intralesional corticosteroids and newer and more targeted systemic options, including 3 Janus kinase (JAK) inhibitors—baricitinib, ritlecitinib, and deuruxolitinib—for more extensive disease.12 Treatment with intralesional corticosteroids may cause transient hypopigmentation, which may be more noticeable in patients with darker skin tones. Delays in treatment with JAK inhibitors can lead to a less-than-optimal response. Of the 3 JAK inhibitors that are approved by the US Food and Drug Administration for AA, only ritlecitinib is approved for children 12 years and older, leaving a therapeutic gap for younger patients that often leads to uncomfortable scalp injections, delayed or no treatment, off-label use of JAK inhibitors as well as the pairing of off-label dupilumab with oral minoxidil.12
Based on adult data, patients with severe disease and a shorter duration of hair loss (ie, <4 years) tend to respond better to JAK inhibitors than those experiencing hair loss for longer periods. Also, those with more severe AA tend to have poorer outcomes than those with less severe disease.13 If treatment proves less than optimal, wigs and hair pieces may need to be considered. It is worth noting that some insurance companies will cover the cost of wigs for patients when prescribed as cranial prostheses.
Health Disparity Highlight
Health disparities in AA can be influenced by socioeconomic status and access to care. Patients from lower-income backgrounds often face barriers to accessing dermatologic care and treatments such as JAK inhibitors, which may remain inaccessible due to high costs and insurance limitations.14 These barriers can intersect with other factors such as age, sex, and race, potentially exacerbating disparities. Women with skin of color in underserved communities may experience delayed diagnosis, limited treatment options, and greater psychosocial distress from hair loss.14 Addressing these inequities requires advocacy, education for both patients and clinicians, and improved access to treatment to ensure comprehensive care for all patients.
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
- Kara T, Topkarcı Z. Interactions between posttraumatic stress disorder and alopecia areata in child with trauma exposure: two case reports. Int J Trichology. 2018;10:131-134. doi:10.4103/ijt.ijt_2_18
- Sy N, Mastacouris N, Strunk A, et al. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA Dermatol. 2023;159:419-423.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123.
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675-682.
- Mostaghimi A, Gao W, Ray M, et al. Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol. 2023;159:411-418.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1(suppl 1):12-23.
- Karampinis E, Toli O, Georgopoulou KE, et al. Exploring pediatric dermatology in skin of color: focus on dermoscopy. Life (Basel). 2024;14:1604.
- King BA, Senna MM, Ohyama M, et al. Defining severity in alopecia areata: current perspectives and a multidimensional framework. Dermatol Ther (Heidelb). 2022;12:825-834.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85:162-175.
- Kalil L, Welch D, Heath CR, et al. Systemic therapies for pediatric alopecia areata. Pediatr Dermatol. 2025;42 suppl 1:36-42.
- King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89:S29-S32.
- Klein EJ, Taiwò D, Kakpovbia E, et al. Disparities in Janus kinase inhibitor access for alopecia areata: a retrospective analysis. Int J Womens Dermatol. 2024;10:E155.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547-551. doi:10.1001/jamadermatol.2022.0351
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.
Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 Pseudofolliculitis barbae is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
Pseudofolliculitis barbae is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? em>Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.

Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 Pseudofolliculitis barbae is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
Pseudofolliculitis barbae is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.

Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 Pseudofolliculitis barbae is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
Pseudofolliculitis barbae is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? em>Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? em>Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
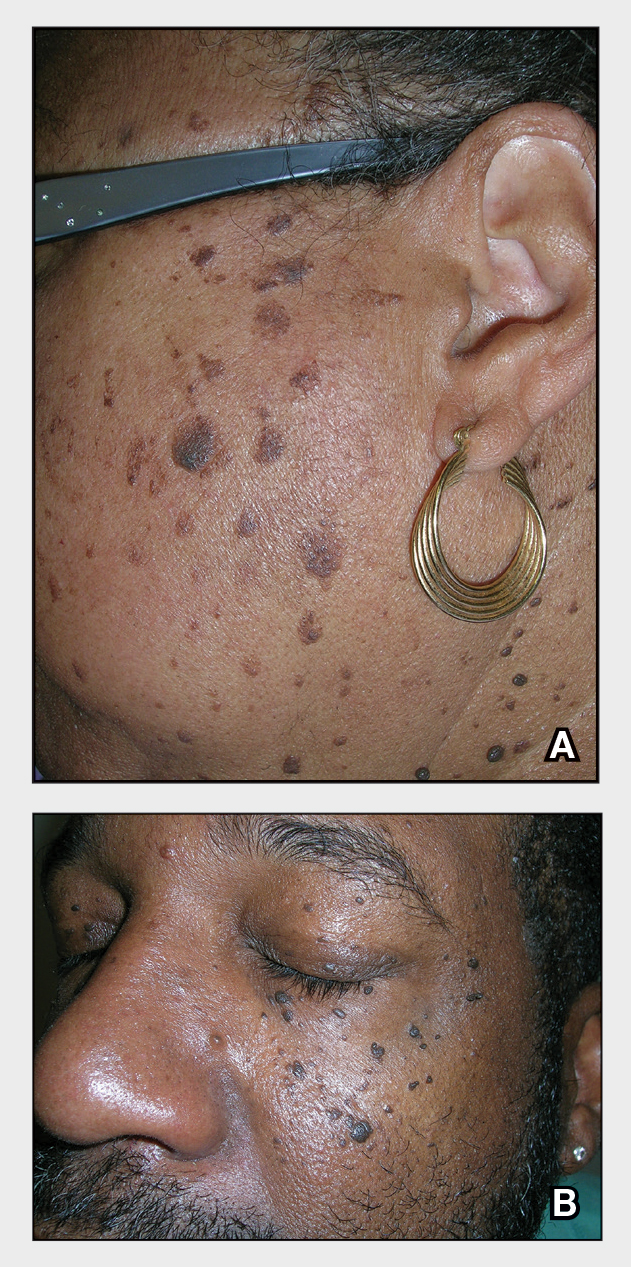
THE COMPARISON
- A A Black woman with dermatosis papulosa nigra manifesting as a cluster of light brown flat seborrheic keratoses that covered the cheeks and lateral face and extended to the neck.
- B A Black man with dermatosis papulosa nigra manifesting as small black papules on the cheeks and eyelids involving the central face.
Dermatosis papulosa nigra (DPN), a subvariant of seborrheic keratosis (SK), is characterized by benign pigmented epidermal neoplasms that typically manifest on the face, neck, and trunk in individuals with darker skin tones.1,2 While DPN meets the diagnostic criteria for SK, certain characteristics can help distinguish these lesions from other SK types. Treatment of DPN in patients with skin of color requires caution, particularly regarding the use of abrasive methods as well as cryotherapy, which generally should be avoided.
Epidemiology
The incidence of SKs increases with age.3,4 Although it can occur in patients of all skin tones, SK is more common in lighter skin tones, while DPN predominantly is diagnosed in darker skin types.1,4 The prevalence of DPN in Black patients ranges from 10% to 30%, and Black women are twice as likely to be diagnosed with DPN as men.2 One study reported a first-degree relative with DPN in 84% (42/50) of patients.5 The number and size of DPN papules increase with age.1
Key Clinical Features
Dermatosis papulosa nigra and SK have distinctive morphologies: DPN typically manifests as raised, round or filiform, sessile, brown to black, 1- to 5-mm papules.2 Seborrheic keratoses tend to be larger with a “stuck on” appearance and manifest as well-demarcated, pink to black papules or plaques that can range in size from millimeters to a few centimeters.3,4 In DPN, the lesions usually are asymptomatic but may be tender, pruritic, dry, or scaly and may become irritated.1,2 They develop symmetrically in sun-exposed areas, and the most common sites are the malar face, temporal region, neck, and trunk.1,2,6,7 Seborrheic keratoses can appear throughout the body, including in sun-exposed areas, but have varying textures (eg, greasy, waxy, verrucous).3,4
Worth Noting
Dermatosis papulosa nigra and SK can resemble each other histologically: DPN demonstrates a fibrous stroma, papillomatosis, hyperkeratosis, and acanthosis at the intraepidermal layer, which are diagnostic criteria for SK.2,4,8 However, other histologic features characteristic of SK that are not seen in DPN include pseudohorn cysts, spindle tumor cells, and basaloid cell nests.8
Dermoscopy can be useful in ruling out malignant skin cancers when evaluating pigmented lesions. The most common dermoscopic features of SK are cerebriform patterns such as fissures and ridges, comedolike openings, and pigmented fingerprintlike structures.3,4 To a lesser degree, milialike cysts, sharp demarcation, and hairpin-shaped vascular structures also may be present.4 The dermoscopic findings of DPN have not been well evaluated, but one study revealed that DPN had similar dermoscopic features to SK with some predominant features.6 Ridges and fissures were seen in 59% of patients diagnosed with DPN followed by comedolike openings seen in 27% of patients. The coexistence of a cerebriform pattern with comedolike openings was infrequent, and milialike cysts were rare.6
While DPN and SK are benign, patients often seek treatment for cosmetic reasons. Factors to consider when choosing a treatment modality include location of the lesions, the patient’s skin tone, and postprocedural outcomes (eg, depigmentation, wound healing). In general, treatments for SK include cryotherapy, electrodesiccation and curettage, and topical therapeutics such as hydrogen peroxide 40%, topical vitamin D3, and nitric-zinc 30%-50% solutions.4,8 Well-established treatment options for DPN include electrodesiccation, laser therapies, scissor excision, and cryotherapy, but topical options such as tazarotene also have been reported.1,9 Of the treatments for DPN, electrodesiccation and laser therapy routinely are used.10
The efficacy of electrodessication and potassium titanyl phosphate (KTP) laser were assessed in a randomized, investigator-blinded split-face study.11 Both modalities received high improvement ratings, with the results favoring the KTP laser. The patients (most of whom were Black) reported that KTP laser was more effective but more painful than electrodessication (P=.002).11 In another randomized study, patients received 3 treatments—electrodessication, pulsed dye laser, and curettage—for select DPN papules.10 There was no difference in the degree of clearance, cosmetic outcome, or postinflammatory hyperpigmentation between the 3 modalities, but patients found the laser to be the most painful.
It is important to exercise caution when using abrasive methods (eg, laser therapy, electrodesiccation, curettage) in patients with darker skin tones because of the increased risk for postinflammatory pigment alteration.1,2,12 Adverse effects of treatment are a top concern in the management of DPN.5,13 While cryotherapy is a preferred treatment of SK in lighter skin tones, it generally is avoided for DPN in darker skin types because melanocyte destruction can lead to cosmetically unsatisfactory and easily visible depigmentation.9
To mitigate postprocedural adverse effects, proper aftercare can promote wound healing and minimize postinflammatory pigment alteration. In one split-face study of Black patients, 2 DPN papules were removed from each side of the face using fine-curved surgical scissors.14 Next, a petrolatum-based ointment and an antibiotic ointment with polymyxin B sulfate/bacitracin zinc was applied twice daily for 21 days to opposite sides of the face. Patients did not develop infection, tolerated both treatments well, and demonstrated improved general wound appearance according to investigator- rated clinical assessment.14 Other reported postprocedural approaches include using topical agents with ingredients shown to improve hyperpigmentation (eg, niacinamide, azelaic acid) as well as photoprotection.12
Health Disparity Highlight
While DPN is benign, it can have adverse psychosocial effects on patients. A study in Senegal revealed that 60% (19/30) of patients with DPN experienced anxiety related to their condition, while others noted that DPN hindered their social relationships.13 In one US study of 50 Black patients with DPN, there was a moderate effect on quality of life, and 36% (18/50) of patients had the lesions removed. However, of the treated patients, 67% (12/18) reported few—if any—symptoms prior to removal.5 Although treatment of DPN is widely considered a cosmetic procedure, therapeutic management can address—and may improve—mental health in patients with skin of color.1,5,13 Despite the high prevalence of DPN in patients with darker skin tones, data on treatment frequency and insurance coverage are not widely available, thus limiting our understanding of treatment accessibility and economic burden.
- Frazier WT, Proddutur S, Swope K. Common dermatologic conditions in skin of color. Am Fam Physician.2023;107:26-34.
- Metin SA, Lee BW, Lambert WC, et al. Dermatosis papulosa nigra: a clinically and histopathologically distinct entity. Clin Dermatol. 2017;35:491-496.
- Braun RP, Ludwig S, Marghoob AA. Differential diagnosis of seborrheic keratosis: clinical and dermoscopic features. J Drugs Dermatol. 2017; 16: 835-842.
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217.
- Uwakwe LN, De Souza B, Subash J, et al. Dermatosis papulosa nigra: a quality of life survey study. J Clin Aesthet Dermatol. 2020;13:17-19.
- Bhat RM, Patrao N, Monteiro R, et al. A clinical, dermoscopic, and histopathological study of dermatosis papulosa nigra (DPN)—an Indian perspective. Int J Dermatol. 2017;56:957-960.
- Karampinis E, Georgopoulou KE, Kampra E, et al. Clinical and dermoscopic patterns of basal cell carcinoma and its mimickers in skin of color: a practical summary. Medicina (Kaunas). 2024;60:1386.
- Gorai S, Ahmad S, Raza SSM, et al. Update of pathophysiology and treatment options of seborrheic keratosis. Dermatol Ther. 2022;35:E15934.
- Jain S, Caire H, Haas CJ. Management of dermatosis papulosa nigra: a systematic review. Int J Dermatol. Published online October 4, 2024.
- Garcia MS, Azari R, Eisen DB. Treatment of dermatosis papulosa nigra in 10 patients: a comparison trial of electrodesiccation, pulsed dye laser, and curettage. Dermatol Surg. 2010;36:1968-1972.
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083.
- Markiewicz E, Karaman-Jurukovska N, Mammone T, et al. Postinflammatory hyperpigmentation in dark skin: molecular mechanism and skincare implications. Clin Cosmet Investig Dermatol. 2022;15: 2555-2565.
- Niang SO, Kane A, Diallo M, et al. Dermatosis papulosa nigra in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):45-47.
- Taylor SC, Averyhart AN, Heath CR. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. J Am Acad Dermatol. 2011;64(suppl 3):S30-S35.

THE COMPARISON
- A A Black woman with dermatosis papulosa nigra manifesting as a cluster of light brown flat seborrheic keratoses that covered the cheeks and lateral face and extended to the neck.
- B A Black man with dermatosis papulosa nigra manifesting as small black papules on the cheeks and eyelids involving the central face.
Dermatosis papulosa nigra (DPN), a subvariant of seborrheic keratosis (SK), is characterized by benign pigmented epidermal neoplasms that typically manifest on the face, neck, and trunk in individuals with darker skin tones.1,2 While DPN meets the diagnostic criteria for SK, certain characteristics can help distinguish these lesions from other SK types. Treatment of DPN in patients with skin of color requires caution, particularly regarding the use of abrasive methods as well as cryotherapy, which generally should be avoided.
Epidemiology
The incidence of SKs increases with age.3,4 Although it can occur in patients of all skin tones, SK is more common in lighter skin tones, while DPN predominantly is diagnosed in darker skin types.1,4 The prevalence of DPN in Black patients ranges from 10% to 30%, and Black women are twice as likely to be diagnosed with DPN as men.2 One study reported a first-degree relative with DPN in 84% (42/50) of patients.5 The number and size of DPN papules increase with age.1
Key Clinical Features
Dermatosis papulosa nigra and SK have distinctive morphologies: DPN typically manifests as raised, round or filiform, sessile, brown to black, 1- to 5-mm papules.2 Seborrheic keratoses tend to be larger with a “stuck on” appearance and manifest as well-demarcated, pink to black papules or plaques that can range in size from millimeters to a few centimeters.3,4 In DPN, the lesions usually are asymptomatic but may be tender, pruritic, dry, or scaly and may become irritated.1,2 They develop symmetrically in sun-exposed areas, and the most common sites are the malar face, temporal region, neck, and trunk.1,2,6,7 Seborrheic keratoses can appear throughout the body, including in sun-exposed areas, but have varying textures (eg, greasy, waxy, verrucous).3,4
Worth Noting
Dermatosis papulosa nigra and SK can resemble each other histologically: DPN demonstrates a fibrous stroma, papillomatosis, hyperkeratosis, and acanthosis at the intraepidermal layer, which are diagnostic criteria for SK.2,4,8 However, other histologic features characteristic of SK that are not seen in DPN include pseudohorn cysts, spindle tumor cells, and basaloid cell nests.8
Dermoscopy can be useful in ruling out malignant skin cancers when evaluating pigmented lesions. The most common dermoscopic features of SK are cerebriform patterns such as fissures and ridges, comedolike openings, and pigmented fingerprintlike structures.3,4 To a lesser degree, milialike cysts, sharp demarcation, and hairpin-shaped vascular structures also may be present.4 The dermoscopic findings of DPN have not been well evaluated, but one study revealed that DPN had similar dermoscopic features to SK with some predominant features.6 Ridges and fissures were seen in 59% of patients diagnosed with DPN followed by comedolike openings seen in 27% of patients. The coexistence of a cerebriform pattern with comedolike openings was infrequent, and milialike cysts were rare.6
While DPN and SK are benign, patients often seek treatment for cosmetic reasons. Factors to consider when choosing a treatment modality include location of the lesions, the patient’s skin tone, and postprocedural outcomes (eg, depigmentation, wound healing). In general, treatments for SK include cryotherapy, electrodesiccation and curettage, and topical therapeutics such as hydrogen peroxide 40%, topical vitamin D3, and nitric-zinc 30%-50% solutions.4,8 Well-established treatment options for DPN include electrodesiccation, laser therapies, scissor excision, and cryotherapy, but topical options such as tazarotene also have been reported.1,9 Of the treatments for DPN, electrodesiccation and laser therapy routinely are used.10
The efficacy of electrodessication and potassium titanyl phosphate (KTP) laser were assessed in a randomized, investigator-blinded split-face study.11 Both modalities received high improvement ratings, with the results favoring the KTP laser. The patients (most of whom were Black) reported that KTP laser was more effective but more painful than electrodessication (P=.002).11 In another randomized study, patients received 3 treatments—electrodessication, pulsed dye laser, and curettage—for select DPN papules.10 There was no difference in the degree of clearance, cosmetic outcome, or postinflammatory hyperpigmentation between the 3 modalities, but patients found the laser to be the most painful.
It is important to exercise caution when using abrasive methods (eg, laser therapy, electrodesiccation, curettage) in patients with darker skin tones because of the increased risk for postinflammatory pigment alteration.1,2,12 Adverse effects of treatment are a top concern in the management of DPN.5,13 While cryotherapy is a preferred treatment of SK in lighter skin tones, it generally is avoided for DPN in darker skin types because melanocyte destruction can lead to cosmetically unsatisfactory and easily visible depigmentation.9
To mitigate postprocedural adverse effects, proper aftercare can promote wound healing and minimize postinflammatory pigment alteration. In one split-face study of Black patients, 2 DPN papules were removed from each side of the face using fine-curved surgical scissors.14 Next, a petrolatum-based ointment and an antibiotic ointment with polymyxin B sulfate/bacitracin zinc was applied twice daily for 21 days to opposite sides of the face. Patients did not develop infection, tolerated both treatments well, and demonstrated improved general wound appearance according to investigator- rated clinical assessment.14 Other reported postprocedural approaches include using topical agents with ingredients shown to improve hyperpigmentation (eg, niacinamide, azelaic acid) as well as photoprotection.12
Health Disparity Highlight
While DPN is benign, it can have adverse psychosocial effects on patients. A study in Senegal revealed that 60% (19/30) of patients with DPN experienced anxiety related to their condition, while others noted that DPN hindered their social relationships.13 In one US study of 50 Black patients with DPN, there was a moderate effect on quality of life, and 36% (18/50) of patients had the lesions removed. However, of the treated patients, 67% (12/18) reported few—if any—symptoms prior to removal.5 Although treatment of DPN is widely considered a cosmetic procedure, therapeutic management can address—and may improve—mental health in patients with skin of color.1,5,13 Despite the high prevalence of DPN in patients with darker skin tones, data on treatment frequency and insurance coverage are not widely available, thus limiting our understanding of treatment accessibility and economic burden.

THE COMPARISON
- A A Black woman with dermatosis papulosa nigra manifesting as a cluster of light brown flat seborrheic keratoses that covered the cheeks and lateral face and extended to the neck.
- B A Black man with dermatosis papulosa nigra manifesting as small black papules on the cheeks and eyelids involving the central face.
Dermatosis papulosa nigra (DPN), a subvariant of seborrheic keratosis (SK), is characterized by benign pigmented epidermal neoplasms that typically manifest on the face, neck, and trunk in individuals with darker skin tones.1,2 While DPN meets the diagnostic criteria for SK, certain characteristics can help distinguish these lesions from other SK types. Treatment of DPN in patients with skin of color requires caution, particularly regarding the use of abrasive methods as well as cryotherapy, which generally should be avoided.
Epidemiology
The incidence of SKs increases with age.3,4 Although it can occur in patients of all skin tones, SK is more common in lighter skin tones, while DPN predominantly is diagnosed in darker skin types.1,4 The prevalence of DPN in Black patients ranges from 10% to 30%, and Black women are twice as likely to be diagnosed with DPN as men.2 One study reported a first-degree relative with DPN in 84% (42/50) of patients.5 The number and size of DPN papules increase with age.1
Key Clinical Features
Dermatosis papulosa nigra and SK have distinctive morphologies: DPN typically manifests as raised, round or filiform, sessile, brown to black, 1- to 5-mm papules.2 Seborrheic keratoses tend to be larger with a “stuck on” appearance and manifest as well-demarcated, pink to black papules or plaques that can range in size from millimeters to a few centimeters.3,4 In DPN, the lesions usually are asymptomatic but may be tender, pruritic, dry, or scaly and may become irritated.1,2 They develop symmetrically in sun-exposed areas, and the most common sites are the malar face, temporal region, neck, and trunk.1,2,6,7 Seborrheic keratoses can appear throughout the body, including in sun-exposed areas, but have varying textures (eg, greasy, waxy, verrucous).3,4
Worth Noting
Dermatosis papulosa nigra and SK can resemble each other histologically: DPN demonstrates a fibrous stroma, papillomatosis, hyperkeratosis, and acanthosis at the intraepidermal layer, which are diagnostic criteria for SK.2,4,8 However, other histologic features characteristic of SK that are not seen in DPN include pseudohorn cysts, spindle tumor cells, and basaloid cell nests.8
Dermoscopy can be useful in ruling out malignant skin cancers when evaluating pigmented lesions. The most common dermoscopic features of SK are cerebriform patterns such as fissures and ridges, comedolike openings, and pigmented fingerprintlike structures.3,4 To a lesser degree, milialike cysts, sharp demarcation, and hairpin-shaped vascular structures also may be present.4 The dermoscopic findings of DPN have not been well evaluated, but one study revealed that DPN had similar dermoscopic features to SK with some predominant features.6 Ridges and fissures were seen in 59% of patients diagnosed with DPN followed by comedolike openings seen in 27% of patients. The coexistence of a cerebriform pattern with comedolike openings was infrequent, and milialike cysts were rare.6
While DPN and SK are benign, patients often seek treatment for cosmetic reasons. Factors to consider when choosing a treatment modality include location of the lesions, the patient’s skin tone, and postprocedural outcomes (eg, depigmentation, wound healing). In general, treatments for SK include cryotherapy, electrodesiccation and curettage, and topical therapeutics such as hydrogen peroxide 40%, topical vitamin D3, and nitric-zinc 30%-50% solutions.4,8 Well-established treatment options for DPN include electrodesiccation, laser therapies, scissor excision, and cryotherapy, but topical options such as tazarotene also have been reported.1,9 Of the treatments for DPN, electrodesiccation and laser therapy routinely are used.10
The efficacy of electrodessication and potassium titanyl phosphate (KTP) laser were assessed in a randomized, investigator-blinded split-face study.11 Both modalities received high improvement ratings, with the results favoring the KTP laser. The patients (most of whom were Black) reported that KTP laser was more effective but more painful than electrodessication (P=.002).11 In another randomized study, patients received 3 treatments—electrodessication, pulsed dye laser, and curettage—for select DPN papules.10 There was no difference in the degree of clearance, cosmetic outcome, or postinflammatory hyperpigmentation between the 3 modalities, but patients found the laser to be the most painful.
It is important to exercise caution when using abrasive methods (eg, laser therapy, electrodesiccation, curettage) in patients with darker skin tones because of the increased risk for postinflammatory pigment alteration.1,2,12 Adverse effects of treatment are a top concern in the management of DPN.5,13 While cryotherapy is a preferred treatment of SK in lighter skin tones, it generally is avoided for DPN in darker skin types because melanocyte destruction can lead to cosmetically unsatisfactory and easily visible depigmentation.9
To mitigate postprocedural adverse effects, proper aftercare can promote wound healing and minimize postinflammatory pigment alteration. In one split-face study of Black patients, 2 DPN papules were removed from each side of the face using fine-curved surgical scissors.14 Next, a petrolatum-based ointment and an antibiotic ointment with polymyxin B sulfate/bacitracin zinc was applied twice daily for 21 days to opposite sides of the face. Patients did not develop infection, tolerated both treatments well, and demonstrated improved general wound appearance according to investigator- rated clinical assessment.14 Other reported postprocedural approaches include using topical agents with ingredients shown to improve hyperpigmentation (eg, niacinamide, azelaic acid) as well as photoprotection.12
Health Disparity Highlight
While DPN is benign, it can have adverse psychosocial effects on patients. A study in Senegal revealed that 60% (19/30) of patients with DPN experienced anxiety related to their condition, while others noted that DPN hindered their social relationships.13 In one US study of 50 Black patients with DPN, there was a moderate effect on quality of life, and 36% (18/50) of patients had the lesions removed. However, of the treated patients, 67% (12/18) reported few—if any—symptoms prior to removal.5 Although treatment of DPN is widely considered a cosmetic procedure, therapeutic management can address—and may improve—mental health in patients with skin of color.1,5,13 Despite the high prevalence of DPN in patients with darker skin tones, data on treatment frequency and insurance coverage are not widely available, thus limiting our understanding of treatment accessibility and economic burden.
- Frazier WT, Proddutur S, Swope K. Common dermatologic conditions in skin of color. Am Fam Physician.2023;107:26-34.
- Metin SA, Lee BW, Lambert WC, et al. Dermatosis papulosa nigra: a clinically and histopathologically distinct entity. Clin Dermatol. 2017;35:491-496.
- Braun RP, Ludwig S, Marghoob AA. Differential diagnosis of seborrheic keratosis: clinical and dermoscopic features. J Drugs Dermatol. 2017; 16: 835-842.
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217.
- Uwakwe LN, De Souza B, Subash J, et al. Dermatosis papulosa nigra: a quality of life survey study. J Clin Aesthet Dermatol. 2020;13:17-19.
- Bhat RM, Patrao N, Monteiro R, et al. A clinical, dermoscopic, and histopathological study of dermatosis papulosa nigra (DPN)—an Indian perspective. Int J Dermatol. 2017;56:957-960.
- Karampinis E, Georgopoulou KE, Kampra E, et al. Clinical and dermoscopic patterns of basal cell carcinoma and its mimickers in skin of color: a practical summary. Medicina (Kaunas). 2024;60:1386.
- Gorai S, Ahmad S, Raza SSM, et al. Update of pathophysiology and treatment options of seborrheic keratosis. Dermatol Ther. 2022;35:E15934.
- Jain S, Caire H, Haas CJ. Management of dermatosis papulosa nigra: a systematic review. Int J Dermatol. Published online October 4, 2024.
- Garcia MS, Azari R, Eisen DB. Treatment of dermatosis papulosa nigra in 10 patients: a comparison trial of electrodesiccation, pulsed dye laser, and curettage. Dermatol Surg. 2010;36:1968-1972.
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083.
- Markiewicz E, Karaman-Jurukovska N, Mammone T, et al. Postinflammatory hyperpigmentation in dark skin: molecular mechanism and skincare implications. Clin Cosmet Investig Dermatol. 2022;15: 2555-2565.
- Niang SO, Kane A, Diallo M, et al. Dermatosis papulosa nigra in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):45-47.
- Taylor SC, Averyhart AN, Heath CR. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. J Am Acad Dermatol. 2011;64(suppl 3):S30-S35.
- Frazier WT, Proddutur S, Swope K. Common dermatologic conditions in skin of color. Am Fam Physician.2023;107:26-34.
- Metin SA, Lee BW, Lambert WC, et al. Dermatosis papulosa nigra: a clinically and histopathologically distinct entity. Clin Dermatol. 2017;35:491-496.
- Braun RP, Ludwig S, Marghoob AA. Differential diagnosis of seborrheic keratosis: clinical and dermoscopic features. J Drugs Dermatol. 2017; 16: 835-842.
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217.
- Uwakwe LN, De Souza B, Subash J, et al. Dermatosis papulosa nigra: a quality of life survey study. J Clin Aesthet Dermatol. 2020;13:17-19.
- Bhat RM, Patrao N, Monteiro R, et al. A clinical, dermoscopic, and histopathological study of dermatosis papulosa nigra (DPN)—an Indian perspective. Int J Dermatol. 2017;56:957-960.
- Karampinis E, Georgopoulou KE, Kampra E, et al. Clinical and dermoscopic patterns of basal cell carcinoma and its mimickers in skin of color: a practical summary. Medicina (Kaunas). 2024;60:1386.
- Gorai S, Ahmad S, Raza SSM, et al. Update of pathophysiology and treatment options of seborrheic keratosis. Dermatol Ther. 2022;35:E15934.
- Jain S, Caire H, Haas CJ. Management of dermatosis papulosa nigra: a systematic review. Int J Dermatol. Published online October 4, 2024.
- Garcia MS, Azari R, Eisen DB. Treatment of dermatosis papulosa nigra in 10 patients: a comparison trial of electrodesiccation, pulsed dye laser, and curettage. Dermatol Surg. 2010;36:1968-1972.
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083.
- Markiewicz E, Karaman-Jurukovska N, Mammone T, et al. Postinflammatory hyperpigmentation in dark skin: molecular mechanism and skincare implications. Clin Cosmet Investig Dermatol. 2022;15: 2555-2565.
- Niang SO, Kane A, Diallo M, et al. Dermatosis papulosa nigra in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):45-47.
- Taylor SC, Averyhart AN, Heath CR. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. J Am Acad Dermatol. 2011;64(suppl 3):S30-S35.
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develop when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
Prurigo nodularis can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, self-consciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10
Epidemiology
Prurigo nodularis has a prevalence of 72 per 100,000 individuals in the United States,11 most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.12,13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms.10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not suprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease.11,13 Work-up for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel also should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrowband UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus.10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 Prurigo nodularis is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
Social drivers of health (eg, socioeconomic challenges, education, access to high-quality health care) likely contribute to PN. Historically, there has been a paucity of research on PN, as with most conditions that disproportionately affect patients with skin of color. Several PN clinical trials currently are underway to explore additional therapeutic options.11
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Investigative Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;82:487-490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018; 79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebocontrolled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develop when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
Prurigo nodularis can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, self-consciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10
Epidemiology
Prurigo nodularis has a prevalence of 72 per 100,000 individuals in the United States,11 most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.12,13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms.10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not suprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease.11,13 Work-up for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel also should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrowband UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus.10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 Prurigo nodularis is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
Social drivers of health (eg, socioeconomic challenges, education, access to high-quality health care) likely contribute to PN. Historically, there has been a paucity of research on PN, as with most conditions that disproportionately affect patients with skin of color. Several PN clinical trials currently are underway to explore additional therapeutic options.11
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develop when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
Prurigo nodularis can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, self-consciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10
Epidemiology
Prurigo nodularis has a prevalence of 72 per 100,000 individuals in the United States,11 most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.12,13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms.10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not suprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease.11,13 Work-up for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel also should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrowband UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus.10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 Prurigo nodularis is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
Social drivers of health (eg, socioeconomic challenges, education, access to high-quality health care) likely contribute to PN. Historically, there has been a paucity of research on PN, as with most conditions that disproportionately affect patients with skin of color. Several PN clinical trials currently are underway to explore additional therapeutic options.11
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Investigative Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;82:487-490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018; 79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebocontrolled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Investigative Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;82:487-490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018; 79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebocontrolled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Moving Beyond Traditional Methods for Treatment of Acne Keloidalis Nuchae
The Comparison
Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al16 found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace,21 which leads to hairstyling practices that may increase the risk for AKN.
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/ 3.
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16 8.
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
The Comparison

Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al16 found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace,21 which leads to hairstyling practices that may increase the risk for AKN.
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
The Comparison

Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al16 found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace,21 which leads to hairstyling practices that may increase the risk for AKN.
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/ 3.
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16 8.
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/ 3.
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16 8.
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
Act Fast With Traction Alopecia to Avoid Permanent Hair Loss
The Comparison
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296

The Comparison
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.

The Comparison
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Plantar Hyperpigmentation
The Comparison
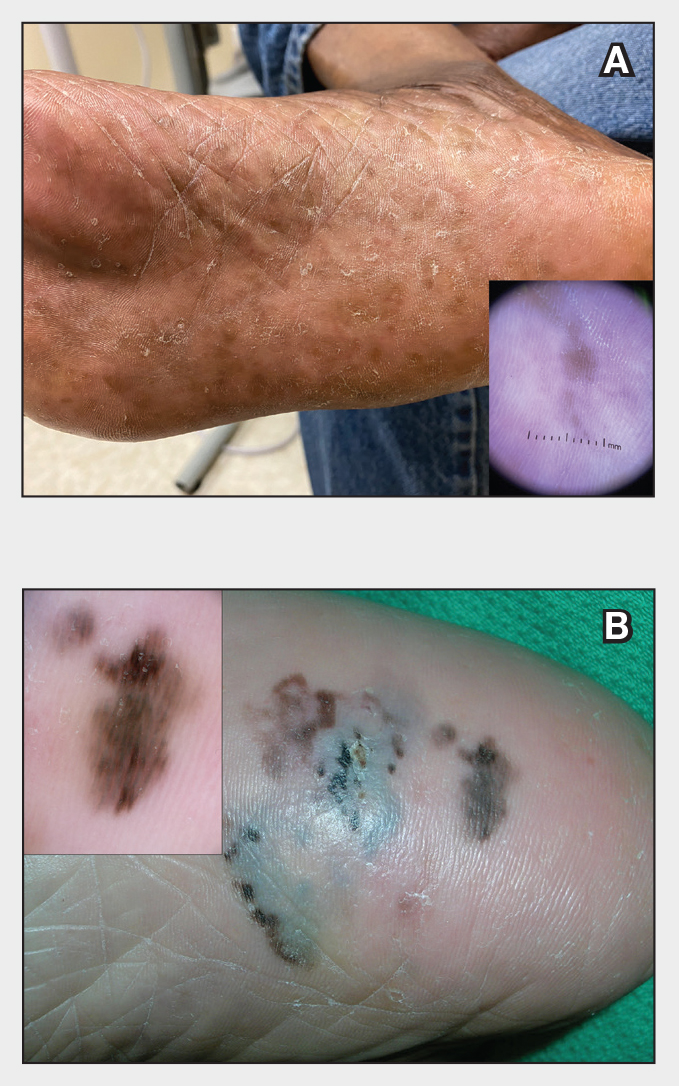
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
The Comparison

Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
The Comparison

Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyperpigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key clinical features in individuals with darker skin tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
- Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
- Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
- Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
- Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
- Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
- Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
- Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
- Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
- Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
- Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285.doi:10.12788/cutis.0513.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
- Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
- Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity