User login
Does taking BP medicine at night (vs morning) result in fewer cardiovascular events?
Evidence summary
Recent UK study shows no difference by timing
A 2022 UK prospective, randomized, multicenter trial assigned 21,104 predominantly White adults (58% men) with hypertension to take their usual antihypertensive medication either in the morning (6
All patient baseline characteristics were equivalent between groups. If troubled by nocturia, patients in the evening group taking diuretics were told to take only the diuretic earlier (6
The median follow-up was 5.2 years. Data were collected at regular intervals through patient completion of online questionnaires and researcher analysis of National Health Service data on hospitalization and death. The intention-to-treat analysis showed no difference in the primary outcome (a composite of vascular death, nonfatal myocardial infarction, or nonfatal stroke) between the evening and morning administration groups (0.69 events vs 0.72 events per 100 person-years; hazard ratio [HR] = 0.95; 95% CI, 0.83-1.10; P = .53).
The controversial Hygia Project favored evening
Prior to the UK study was the Hygia Chronotherapy Trial, a prospective, controlled, multicenter study conducted within the primary care setting in Spain. Caucasian Spanish adults (N = 19,168; mean age, 61 years; 56% men) with hypertension were randomly assigned to take all prescribed antihypertensive medication either at bedtime or upon waking.2
The Hygia Project initially sought to establish the value of ambulatory blood pressure monitoring (ABPM) compared to office blood pressure (BP) monitoring and to explore the prognostic value of sleeping BP.3 The study objectives evolved over time. The randomization process was not clearly described,2,3 but multiple randomizations were alluded to. The authors stated that “for any of these chronotherapy trials” randomizations were done separately for “each participating center” and “randomization of participants to treatment-time regimen is done separately for each hypertension medication or combination being tested.”
The baseline characteristics of patients in the evening and morning administration groups were similar, but statistically significant differences existed in BMI (29.6 vs 29.7; P = .030) and sleep-time systolic BP percent decline (9.3 vs 9.0; P < .001). Mean baseline 48-hour BP was 132/77 mm Hg. Hypertension was defined as an awake systolic BP ≥
Prescribers were free to prescribe medicines from 5 classes (diuretic, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or beta-blocker) as they thought appropriate, were encouraged to use fixed-dose combination pills, and were told not to use split (eg, twice per day) dosing. Annual 48-hour ABPM was completed, and patients’ electronic health records were analyzed by blinded investigators. Median follow-up was 6.3 years, and only 84 participants failed to complete the minimum 1-year participation requirement.
Continue to: The primary outcome...
The primary outcome—a composite of cardiovascular death, myocardial infarction, coronary revascularization, heart failure, or stroke—occurred in 1752 patients, favoring the bedtime group (HR = 0.55; 95% CI, 0.50-0.61; P < .001). The calculated number of events was 1130 in the morning administration group and 622 in the evening administration group; the authors did not explicitly report the event numbers in each group. Each component of the composite outcome also favored evening administration (P < .001 for all): cardiovascular death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63).
The complicated, layered study design and randomization methods limit the ability to critically appraise the study.
Smaller Spanish study also supported evening administration
A prior, smaller, prospective randomized trial conducted by the same researchers as the Hygia Project found even greater benefits to evening BP medication administration.4 The 2156 Spanish patients (52% men; average age, 55 years) from multiple primary care offices were randomized 1:1 to BP medication administration either upon awakening or at bedtime. Dozens of baseline characteristics were evenly distributed except for age (55.0 vs 56.3; P = .021) and creatinine (0.96 vs 0.98; P = .028), both of which were lower in the evening group.
After a median follow-up of 5.6 years, the bedtime group had significantly lower total events (187 events in the morning group vs 68 in the evening group; relative risk [RR] = 0.39; 95% CI, 0.29-0.51; P < .001). Individual cardiovascular outcomes also dramatically favored the evening group: total deaths (12 vs 28; P = .008), cardiovascular deaths (3 vs 14; P = .006), cardiovascular disease events (30 vs 74; P < .001), stroke (7 vs 24; P = .001), and heart failure (8 vs 33; P < .001).
Limits of both the UK trial and the Hygia Project trial included single countries of study with a lack of racial and ethnic diversity, and greater nonadherence to the evening administration of the medications.
Recommendations from others
A 2022 consensus statement from the International Society of Hypertension, published before the UK trial, recommended against bedtime dosing until more high-quality data became available. They pointed to evidence showing higher medication adherence with morning dosing, risk for asleep BP dropping, and worsening daytime BP control as reasons to continue morning administration.5 Other reviewers have questioned the Hygia Project results due to their reported implausibly large effects on cardiovascular outcomes, noting that independent attempts to verify the methods and the data have proven challenging and are not completed.6
Editor’s takeaway
I confess that I was swayed by the results of the Hygia Project; for a year or so, I advised my patients to take at least 1 BP pill at night. But after the UK study came out, I needed to reconsider. I began to worry that the great outcomes of nocturnal therapy may have been a mirage. I have returned to counseling patients to take their BP medications in whichever way fosters consistency while minimizing adverse effects for them.
1. Mackenzie IS, Rogers A, Poulter NR, et al; TIME Study Group. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial. Lancet. 2022;400:1417-1425. doi: 10.1016/S0140-6736(22)01786-X
2. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al; Hygia Project Investigators. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41:4565-4576. doi: 10.1093/eurheartj/ehz754
3. Hermida RC. Sleep-time ambulatory blood pressure as a prognostic marker of vascular and other risks and therapeutic target for prevention by hypertension chronotherapy: rationale and design of the Hygia Project. Chronobiol Int. 2016;33:906-936. doi: 10.1080/07420528.2016.1181078
4. Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651. doi: 10.3109/07420528.2010.510230
5. Stergiou G, Brunström M, MacDonald T, et al. Bedtime dosing of antihypertensive medications: systematic review and consensus statement: International Society of Hypertension position paper endorsed by World Hypertension League and European Society of Hypertension. J Hypertens. 2022;40:1847-1858. doi: 10.1097/HJH.0000000000003240
6. Brunström M, Kjeldsen SE, Kreutz R, et al. Missing verification of source data in hypertension research: The HYGIA PROJECT in Perspective. Hypertension. 2021;78:555-558. doi: 10.1161/HYPERTENSIONAHA.121.17356
Evidence summary
Recent UK study shows no difference by timing
A 2022 UK prospective, randomized, multicenter trial assigned 21,104 predominantly White adults (58% men) with hypertension to take their usual antihypertensive medication either in the morning (6
All patient baseline characteristics were equivalent between groups. If troubled by nocturia, patients in the evening group taking diuretics were told to take only the diuretic earlier (6
The median follow-up was 5.2 years. Data were collected at regular intervals through patient completion of online questionnaires and researcher analysis of National Health Service data on hospitalization and death. The intention-to-treat analysis showed no difference in the primary outcome (a composite of vascular death, nonfatal myocardial infarction, or nonfatal stroke) between the evening and morning administration groups (0.69 events vs 0.72 events per 100 person-years; hazard ratio [HR] = 0.95; 95% CI, 0.83-1.10; P = .53).
The controversial Hygia Project favored evening
Prior to the UK study was the Hygia Chronotherapy Trial, a prospective, controlled, multicenter study conducted within the primary care setting in Spain. Caucasian Spanish adults (N = 19,168; mean age, 61 years; 56% men) with hypertension were randomly assigned to take all prescribed antihypertensive medication either at bedtime or upon waking.2
The Hygia Project initially sought to establish the value of ambulatory blood pressure monitoring (ABPM) compared to office blood pressure (BP) monitoring and to explore the prognostic value of sleeping BP.3 The study objectives evolved over time. The randomization process was not clearly described,2,3 but multiple randomizations were alluded to. The authors stated that “for any of these chronotherapy trials” randomizations were done separately for “each participating center” and “randomization of participants to treatment-time regimen is done separately for each hypertension medication or combination being tested.”
The baseline characteristics of patients in the evening and morning administration groups were similar, but statistically significant differences existed in BMI (29.6 vs 29.7; P = .030) and sleep-time systolic BP percent decline (9.3 vs 9.0; P < .001). Mean baseline 48-hour BP was 132/77 mm Hg. Hypertension was defined as an awake systolic BP ≥
Prescribers were free to prescribe medicines from 5 classes (diuretic, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or beta-blocker) as they thought appropriate, were encouraged to use fixed-dose combination pills, and were told not to use split (eg, twice per day) dosing. Annual 48-hour ABPM was completed, and patients’ electronic health records were analyzed by blinded investigators. Median follow-up was 6.3 years, and only 84 participants failed to complete the minimum 1-year participation requirement.
Continue to: The primary outcome...
The primary outcome—a composite of cardiovascular death, myocardial infarction, coronary revascularization, heart failure, or stroke—occurred in 1752 patients, favoring the bedtime group (HR = 0.55; 95% CI, 0.50-0.61; P < .001). The calculated number of events was 1130 in the morning administration group and 622 in the evening administration group; the authors did not explicitly report the event numbers in each group. Each component of the composite outcome also favored evening administration (P < .001 for all): cardiovascular death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63).
The complicated, layered study design and randomization methods limit the ability to critically appraise the study.
Smaller Spanish study also supported evening administration
A prior, smaller, prospective randomized trial conducted by the same researchers as the Hygia Project found even greater benefits to evening BP medication administration.4 The 2156 Spanish patients (52% men; average age, 55 years) from multiple primary care offices were randomized 1:1 to BP medication administration either upon awakening or at bedtime. Dozens of baseline characteristics were evenly distributed except for age (55.0 vs 56.3; P = .021) and creatinine (0.96 vs 0.98; P = .028), both of which were lower in the evening group.
After a median follow-up of 5.6 years, the bedtime group had significantly lower total events (187 events in the morning group vs 68 in the evening group; relative risk [RR] = 0.39; 95% CI, 0.29-0.51; P < .001). Individual cardiovascular outcomes also dramatically favored the evening group: total deaths (12 vs 28; P = .008), cardiovascular deaths (3 vs 14; P = .006), cardiovascular disease events (30 vs 74; P < .001), stroke (7 vs 24; P = .001), and heart failure (8 vs 33; P < .001).
Limits of both the UK trial and the Hygia Project trial included single countries of study with a lack of racial and ethnic diversity, and greater nonadherence to the evening administration of the medications.
Recommendations from others
A 2022 consensus statement from the International Society of Hypertension, published before the UK trial, recommended against bedtime dosing until more high-quality data became available. They pointed to evidence showing higher medication adherence with morning dosing, risk for asleep BP dropping, and worsening daytime BP control as reasons to continue morning administration.5 Other reviewers have questioned the Hygia Project results due to their reported implausibly large effects on cardiovascular outcomes, noting that independent attempts to verify the methods and the data have proven challenging and are not completed.6
Editor’s takeaway
I confess that I was swayed by the results of the Hygia Project; for a year or so, I advised my patients to take at least 1 BP pill at night. But after the UK study came out, I needed to reconsider. I began to worry that the great outcomes of nocturnal therapy may have been a mirage. I have returned to counseling patients to take their BP medications in whichever way fosters consistency while minimizing adverse effects for them.
Evidence summary
Recent UK study shows no difference by timing
A 2022 UK prospective, randomized, multicenter trial assigned 21,104 predominantly White adults (58% men) with hypertension to take their usual antihypertensive medication either in the morning (6
All patient baseline characteristics were equivalent between groups. If troubled by nocturia, patients in the evening group taking diuretics were told to take only the diuretic earlier (6
The median follow-up was 5.2 years. Data were collected at regular intervals through patient completion of online questionnaires and researcher analysis of National Health Service data on hospitalization and death. The intention-to-treat analysis showed no difference in the primary outcome (a composite of vascular death, nonfatal myocardial infarction, or nonfatal stroke) between the evening and morning administration groups (0.69 events vs 0.72 events per 100 person-years; hazard ratio [HR] = 0.95; 95% CI, 0.83-1.10; P = .53).
The controversial Hygia Project favored evening
Prior to the UK study was the Hygia Chronotherapy Trial, a prospective, controlled, multicenter study conducted within the primary care setting in Spain. Caucasian Spanish adults (N = 19,168; mean age, 61 years; 56% men) with hypertension were randomly assigned to take all prescribed antihypertensive medication either at bedtime or upon waking.2
The Hygia Project initially sought to establish the value of ambulatory blood pressure monitoring (ABPM) compared to office blood pressure (BP) monitoring and to explore the prognostic value of sleeping BP.3 The study objectives evolved over time. The randomization process was not clearly described,2,3 but multiple randomizations were alluded to. The authors stated that “for any of these chronotherapy trials” randomizations were done separately for “each participating center” and “randomization of participants to treatment-time regimen is done separately for each hypertension medication or combination being tested.”
The baseline characteristics of patients in the evening and morning administration groups were similar, but statistically significant differences existed in BMI (29.6 vs 29.7; P = .030) and sleep-time systolic BP percent decline (9.3 vs 9.0; P < .001). Mean baseline 48-hour BP was 132/77 mm Hg. Hypertension was defined as an awake systolic BP ≥
Prescribers were free to prescribe medicines from 5 classes (diuretic, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or beta-blocker) as they thought appropriate, were encouraged to use fixed-dose combination pills, and were told not to use split (eg, twice per day) dosing. Annual 48-hour ABPM was completed, and patients’ electronic health records were analyzed by blinded investigators. Median follow-up was 6.3 years, and only 84 participants failed to complete the minimum 1-year participation requirement.
Continue to: The primary outcome...
The primary outcome—a composite of cardiovascular death, myocardial infarction, coronary revascularization, heart failure, or stroke—occurred in 1752 patients, favoring the bedtime group (HR = 0.55; 95% CI, 0.50-0.61; P < .001). The calculated number of events was 1130 in the morning administration group and 622 in the evening administration group; the authors did not explicitly report the event numbers in each group. Each component of the composite outcome also favored evening administration (P < .001 for all): cardiovascular death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63).
The complicated, layered study design and randomization methods limit the ability to critically appraise the study.
Smaller Spanish study also supported evening administration
A prior, smaller, prospective randomized trial conducted by the same researchers as the Hygia Project found even greater benefits to evening BP medication administration.4 The 2156 Spanish patients (52% men; average age, 55 years) from multiple primary care offices were randomized 1:1 to BP medication administration either upon awakening or at bedtime. Dozens of baseline characteristics were evenly distributed except for age (55.0 vs 56.3; P = .021) and creatinine (0.96 vs 0.98; P = .028), both of which were lower in the evening group.
After a median follow-up of 5.6 years, the bedtime group had significantly lower total events (187 events in the morning group vs 68 in the evening group; relative risk [RR] = 0.39; 95% CI, 0.29-0.51; P < .001). Individual cardiovascular outcomes also dramatically favored the evening group: total deaths (12 vs 28; P = .008), cardiovascular deaths (3 vs 14; P = .006), cardiovascular disease events (30 vs 74; P < .001), stroke (7 vs 24; P = .001), and heart failure (8 vs 33; P < .001).
Limits of both the UK trial and the Hygia Project trial included single countries of study with a lack of racial and ethnic diversity, and greater nonadherence to the evening administration of the medications.
Recommendations from others
A 2022 consensus statement from the International Society of Hypertension, published before the UK trial, recommended against bedtime dosing until more high-quality data became available. They pointed to evidence showing higher medication adherence with morning dosing, risk for asleep BP dropping, and worsening daytime BP control as reasons to continue morning administration.5 Other reviewers have questioned the Hygia Project results due to their reported implausibly large effects on cardiovascular outcomes, noting that independent attempts to verify the methods and the data have proven challenging and are not completed.6
Editor’s takeaway
I confess that I was swayed by the results of the Hygia Project; for a year or so, I advised my patients to take at least 1 BP pill at night. But after the UK study came out, I needed to reconsider. I began to worry that the great outcomes of nocturnal therapy may have been a mirage. I have returned to counseling patients to take their BP medications in whichever way fosters consistency while minimizing adverse effects for them.
1. Mackenzie IS, Rogers A, Poulter NR, et al; TIME Study Group. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial. Lancet. 2022;400:1417-1425. doi: 10.1016/S0140-6736(22)01786-X
2. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al; Hygia Project Investigators. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41:4565-4576. doi: 10.1093/eurheartj/ehz754
3. Hermida RC. Sleep-time ambulatory blood pressure as a prognostic marker of vascular and other risks and therapeutic target for prevention by hypertension chronotherapy: rationale and design of the Hygia Project. Chronobiol Int. 2016;33:906-936. doi: 10.1080/07420528.2016.1181078
4. Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651. doi: 10.3109/07420528.2010.510230
5. Stergiou G, Brunström M, MacDonald T, et al. Bedtime dosing of antihypertensive medications: systematic review and consensus statement: International Society of Hypertension position paper endorsed by World Hypertension League and European Society of Hypertension. J Hypertens. 2022;40:1847-1858. doi: 10.1097/HJH.0000000000003240
6. Brunström M, Kjeldsen SE, Kreutz R, et al. Missing verification of source data in hypertension research: The HYGIA PROJECT in Perspective. Hypertension. 2021;78:555-558. doi: 10.1161/HYPERTENSIONAHA.121.17356
1. Mackenzie IS, Rogers A, Poulter NR, et al; TIME Study Group. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial. Lancet. 2022;400:1417-1425. doi: 10.1016/S0140-6736(22)01786-X
2. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al; Hygia Project Investigators. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41:4565-4576. doi: 10.1093/eurheartj/ehz754
3. Hermida RC. Sleep-time ambulatory blood pressure as a prognostic marker of vascular and other risks and therapeutic target for prevention by hypertension chronotherapy: rationale and design of the Hygia Project. Chronobiol Int. 2016;33:906-936. doi: 10.1080/07420528.2016.1181078
4. Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651. doi: 10.3109/07420528.2010.510230
5. Stergiou G, Brunström M, MacDonald T, et al. Bedtime dosing of antihypertensive medications: systematic review and consensus statement: International Society of Hypertension position paper endorsed by World Hypertension League and European Society of Hypertension. J Hypertens. 2022;40:1847-1858. doi: 10.1097/HJH.0000000000003240
6. Brunström M, Kjeldsen SE, Kreutz R, et al. Missing verification of source data in hypertension research: The HYGIA PROJECT in Perspective. Hypertension. 2021;78:555-558. doi: 10.1161/HYPERTENSIONAHA.121.17356
EVIDENCE-BASED ANSWER:
Are manual therapies effective at reducing chronic tension headache frequency in adults?
Evidence summary
Small studies offer mixed evidence of benefit
Seven RCTs using manual therapies to treat chronic tension headaches have reported the change in headache frequency (TABLE1-7). Most, but not all, manual therapies significantly improved headache frequency.
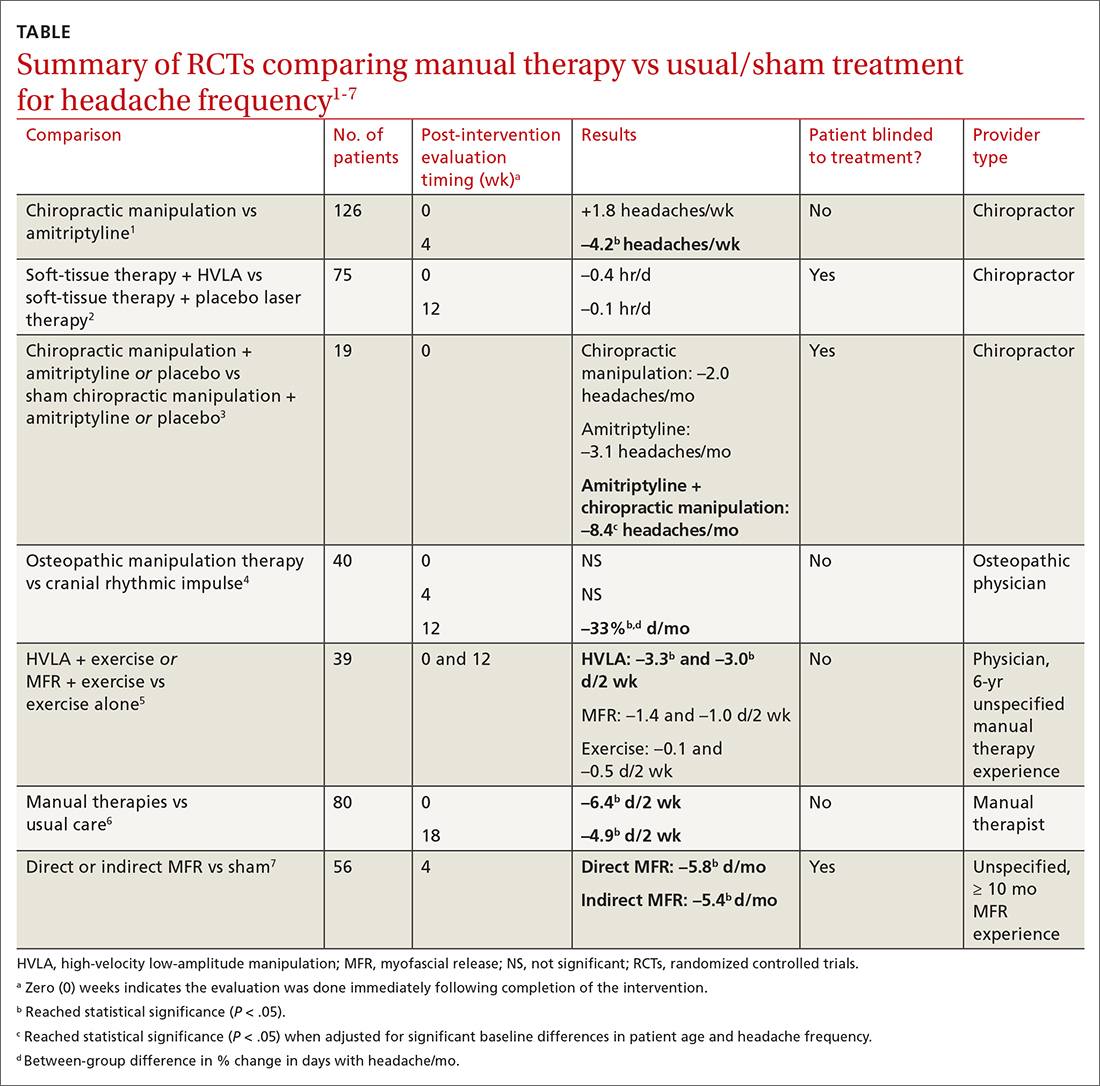
Participants ranged in age from 18 to 65 years, with mean age ranges of 33 to 42 years in each study. At baseline, patients had 10 or more tension-type headaches per month. The manual therapies varied in techniques, duration, and the training of the person performing the intervention:
- Twice-weekly chiropractic spinal manipulation for 6 weeks1
- Soft-tissue therapy plus spinal manipulation (8 treatments over 4 weeks)2
- Chiropractic spinal manipulation with or without amitriptyline for 14 weeks3
- Corrective osteopathic manipulation treatment (OMT) techniques tailored for each patient for 1 month4
- High-velocity low-amplitude manipulation (HVLA) plus exercise or myofascial release plus exercise twice weekly for 8 weeks5
- Manual therapy treatment consisting of a combination of mobilizations of the cervical and thoracic spine, exercises, and postural correction for up to 9 sessions of 30 minutes each6
- One hour of direct or indirect myofascial release treatment twice weekly for 12 weeks.7
Three studies involved chiropractic providers.1-3 One study (n = 19) found a positive effect, in which chiropractic manipulation augmented with amitriptyline performed better than chiropractic manipulation alone.3 Another chiropractic study did not find an immediate posttreatment benefit but did report significant headache reduction at the 4-week follow-up interval.1 The third chiropractic study did not show additional benefit from HVLA manipulation.2
One small study involving osteopathic physicians using OMT found reduced headache frequency after 12 weeks but not at 4 weeks.4 Another study, comparing HVLA or myofascial release with exercise to exercise alone, found benefit for the HVLA group but not for myofascial release; interventions in this study were performed by a physician with at least 6 years of unspecified manual therapy experience.5 A small study of manual therapists found improvement at the end of manual therapy but not at 18 months.6 Another small study using providers with 10 months’ experience with myofascial release found reduced headache frequency 4 weeks after a course of direct and indirect myofascial release (compared with sham release).7
Editor’s takeaway
It isn’t hard to imagine why muscle tension headaches might respond to certain forms of manual therapy. However, all available studies of these modalities have been small (< 100 patients) or lacked blinding, introducing the potential for significant bias. Nevertheless, for now it appears reasonable to refer interested patients with tension headache to an osteopathic physician for OMT or myofascial release to reduce headache frequency.
1. Boline PD, Kassak K, Bronfort G, et al. Spinal manipulation vs amitriptyline for the treatment of chronic tension-type headaches—a randomized clinical-trial. J Manipulative Physiol Ther. 1995;18:148-254.
2. Bove G. Spinal manipulation in the treatment of episodic tension-type headache: a randomized controlled trial. JAMA. 1998;280:1576-1579.
3. Vernon H, Jansz G, Goldsmith CH, et al. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manipulative Physiol Ther. 2009;32:344-351.
4. Rolle G, Tremolizzo L, Somalvico F, et al. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Am Osteopath Assoc. 2014;114:678-685. doi: 10.7556/jaoa.2014.136
5. Corum M, Aydin T, Ceylan CM, et al. The comparative effects of spinal manipulation, myofascial release and exercise in tension-type headache patients with neck pain: a randomized controlled trial. Complement Ther Clin Pract. 2021;43:101319. doi: 0.1016/j.ctcp.2021.101319
6. Castien RF, van der Windt DAWM, Grooten A, et al. Effectiveness of manual therapy compared to usual care by the general practitioner for chronic tension-type headache: a pragmatic, randomised, clinical trial. Cephalalgia. 2009;31:133-143.
7. Ajimsha MS. Effectiveness of direct vs indirect technique myofascial release in the management of tension-type headache. J Bodyw Mov Ther. 2011;15:431-435. doi: 10.1016/j.jbmt.2011.01.021
Evidence summary
Small studies offer mixed evidence of benefit
Seven RCTs using manual therapies to treat chronic tension headaches have reported the change in headache frequency (TABLE1-7). Most, but not all, manual therapies significantly improved headache frequency.

Participants ranged in age from 18 to 65 years, with mean age ranges of 33 to 42 years in each study. At baseline, patients had 10 or more tension-type headaches per month. The manual therapies varied in techniques, duration, and the training of the person performing the intervention:
- Twice-weekly chiropractic spinal manipulation for 6 weeks1
- Soft-tissue therapy plus spinal manipulation (8 treatments over 4 weeks)2
- Chiropractic spinal manipulation with or without amitriptyline for 14 weeks3
- Corrective osteopathic manipulation treatment (OMT) techniques tailored for each patient for 1 month4
- High-velocity low-amplitude manipulation (HVLA) plus exercise or myofascial release plus exercise twice weekly for 8 weeks5
- Manual therapy treatment consisting of a combination of mobilizations of the cervical and thoracic spine, exercises, and postural correction for up to 9 sessions of 30 minutes each6
- One hour of direct or indirect myofascial release treatment twice weekly for 12 weeks.7
Three studies involved chiropractic providers.1-3 One study (n = 19) found a positive effect, in which chiropractic manipulation augmented with amitriptyline performed better than chiropractic manipulation alone.3 Another chiropractic study did not find an immediate posttreatment benefit but did report significant headache reduction at the 4-week follow-up interval.1 The third chiropractic study did not show additional benefit from HVLA manipulation.2
One small study involving osteopathic physicians using OMT found reduced headache frequency after 12 weeks but not at 4 weeks.4 Another study, comparing HVLA or myofascial release with exercise to exercise alone, found benefit for the HVLA group but not for myofascial release; interventions in this study were performed by a physician with at least 6 years of unspecified manual therapy experience.5 A small study of manual therapists found improvement at the end of manual therapy but not at 18 months.6 Another small study using providers with 10 months’ experience with myofascial release found reduced headache frequency 4 weeks after a course of direct and indirect myofascial release (compared with sham release).7
Editor’s takeaway
It isn’t hard to imagine why muscle tension headaches might respond to certain forms of manual therapy. However, all available studies of these modalities have been small (< 100 patients) or lacked blinding, introducing the potential for significant bias. Nevertheless, for now it appears reasonable to refer interested patients with tension headache to an osteopathic physician for OMT or myofascial release to reduce headache frequency.
Evidence summary
Small studies offer mixed evidence of benefit
Seven RCTs using manual therapies to treat chronic tension headaches have reported the change in headache frequency (TABLE1-7). Most, but not all, manual therapies significantly improved headache frequency.

Participants ranged in age from 18 to 65 years, with mean age ranges of 33 to 42 years in each study. At baseline, patients had 10 or more tension-type headaches per month. The manual therapies varied in techniques, duration, and the training of the person performing the intervention:
- Twice-weekly chiropractic spinal manipulation for 6 weeks1
- Soft-tissue therapy plus spinal manipulation (8 treatments over 4 weeks)2
- Chiropractic spinal manipulation with or without amitriptyline for 14 weeks3
- Corrective osteopathic manipulation treatment (OMT) techniques tailored for each patient for 1 month4
- High-velocity low-amplitude manipulation (HVLA) plus exercise or myofascial release plus exercise twice weekly for 8 weeks5
- Manual therapy treatment consisting of a combination of mobilizations of the cervical and thoracic spine, exercises, and postural correction for up to 9 sessions of 30 minutes each6
- One hour of direct or indirect myofascial release treatment twice weekly for 12 weeks.7
Three studies involved chiropractic providers.1-3 One study (n = 19) found a positive effect, in which chiropractic manipulation augmented with amitriptyline performed better than chiropractic manipulation alone.3 Another chiropractic study did not find an immediate posttreatment benefit but did report significant headache reduction at the 4-week follow-up interval.1 The third chiropractic study did not show additional benefit from HVLA manipulation.2
One small study involving osteopathic physicians using OMT found reduced headache frequency after 12 weeks but not at 4 weeks.4 Another study, comparing HVLA or myofascial release with exercise to exercise alone, found benefit for the HVLA group but not for myofascial release; interventions in this study were performed by a physician with at least 6 years of unspecified manual therapy experience.5 A small study of manual therapists found improvement at the end of manual therapy but not at 18 months.6 Another small study using providers with 10 months’ experience with myofascial release found reduced headache frequency 4 weeks after a course of direct and indirect myofascial release (compared with sham release).7
Editor’s takeaway
It isn’t hard to imagine why muscle tension headaches might respond to certain forms of manual therapy. However, all available studies of these modalities have been small (< 100 patients) or lacked blinding, introducing the potential for significant bias. Nevertheless, for now it appears reasonable to refer interested patients with tension headache to an osteopathic physician for OMT or myofascial release to reduce headache frequency.
1. Boline PD, Kassak K, Bronfort G, et al. Spinal manipulation vs amitriptyline for the treatment of chronic tension-type headaches—a randomized clinical-trial. J Manipulative Physiol Ther. 1995;18:148-254.
2. Bove G. Spinal manipulation in the treatment of episodic tension-type headache: a randomized controlled trial. JAMA. 1998;280:1576-1579.
3. Vernon H, Jansz G, Goldsmith CH, et al. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manipulative Physiol Ther. 2009;32:344-351.
4. Rolle G, Tremolizzo L, Somalvico F, et al. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Am Osteopath Assoc. 2014;114:678-685. doi: 10.7556/jaoa.2014.136
5. Corum M, Aydin T, Ceylan CM, et al. The comparative effects of spinal manipulation, myofascial release and exercise in tension-type headache patients with neck pain: a randomized controlled trial. Complement Ther Clin Pract. 2021;43:101319. doi: 0.1016/j.ctcp.2021.101319
6. Castien RF, van der Windt DAWM, Grooten A, et al. Effectiveness of manual therapy compared to usual care by the general practitioner for chronic tension-type headache: a pragmatic, randomised, clinical trial. Cephalalgia. 2009;31:133-143.
7. Ajimsha MS. Effectiveness of direct vs indirect technique myofascial release in the management of tension-type headache. J Bodyw Mov Ther. 2011;15:431-435. doi: 10.1016/j.jbmt.2011.01.021
1. Boline PD, Kassak K, Bronfort G, et al. Spinal manipulation vs amitriptyline for the treatment of chronic tension-type headaches—a randomized clinical-trial. J Manipulative Physiol Ther. 1995;18:148-254.
2. Bove G. Spinal manipulation in the treatment of episodic tension-type headache: a randomized controlled trial. JAMA. 1998;280:1576-1579.
3. Vernon H, Jansz G, Goldsmith CH, et al. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manipulative Physiol Ther. 2009;32:344-351.
4. Rolle G, Tremolizzo L, Somalvico F, et al. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Am Osteopath Assoc. 2014;114:678-685. doi: 10.7556/jaoa.2014.136
5. Corum M, Aydin T, Ceylan CM, et al. The comparative effects of spinal manipulation, myofascial release and exercise in tension-type headache patients with neck pain: a randomized controlled trial. Complement Ther Clin Pract. 2021;43:101319. doi: 0.1016/j.ctcp.2021.101319
6. Castien RF, van der Windt DAWM, Grooten A, et al. Effectiveness of manual therapy compared to usual care by the general practitioner for chronic tension-type headache: a pragmatic, randomised, clinical trial. Cephalalgia. 2009;31:133-143.
7. Ajimsha MS. Effectiveness of direct vs indirect technique myofascial release in the management of tension-type headache. J Bodyw Mov Ther. 2011;15:431-435. doi: 10.1016/j.jbmt.2011.01.021
EVIDENCE-BASED ANSWER:
MAYBE. Among patients with chronic tension headaches, manual therapies may reduce headache frequency more than sham manual therapy, usual care, or exercise treatments—by 1.5 to 4.2 headaches or days with headache per week (strength of recommendation, B; preponderance of evidence from primarily small, heterogeneous randomized controlled trials [RCTs]).
Which medications work best for menorrhagia?
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
EVIDENCE-BASED ANSWER:
Four medications have been shown to reduce menstrual blood loss (MBL) significantly in placebo-controlled randomized controlled trials (RCTs): the levonorgestrel-releasing intrauterine system (LNG-IUS), tranexamic acid, nonsteroidal anti-inflammatory drugs (NSAIDs), and danazol, a synthetic steroid (strength of recommendation: A, meta-analyses of RCTs).
A single trial showed that the LNG-IUS reduced MBL by about 100 mL, compared with placebo. In a meta-analysis of 4 placebo-controlled RCTs, tranexamic acid reduced MBL by about 53 mL, roughly a 40% to 50% decrease. The 8 NSAID trials (5 mefenamic acid, 2 naproxen, 1 ibuprofen) demonstrated effectiveness, but the effect size is difficult to quantify. The single danazol RCT used a subjective scoring system without reporting MBL.
No studies compared all effective medical therapies against one another. In head-to-head comparisons, women were more likely to experience improvement with the LNG-IUS than with tranexamic acid (number needed to treat [NNT] = 2 to 6). Both treatments are superior to NSAIDs. Danazol is also more efficacious than NSAIDs, but its use is limited by its adverse effects, including teratogenicity.
No placebo-controlled trials have studied oral contraceptive pills (OCPs) or oral progesterone to treat menorrhagia. However, multiple comparative RCTs have demonstrated that these commonly prescribed medications significantly decrease MBL. Trials have shown the reduction to be inferior to LNG-IUS and danazol and equivalent to NSAIDs.