User login
Ptosis after motorcycle accident
A 45-year-old woman visited the clinic 6 weeks after having a stroke while on her motorcycle, which resulted in a crash. She had not been wearing a helmet and was uncertain if she had sustained a head injury. She said that during the hospital stay following the accident, she was diagnosed as hypertensive; she denied any other significant prior medical history.
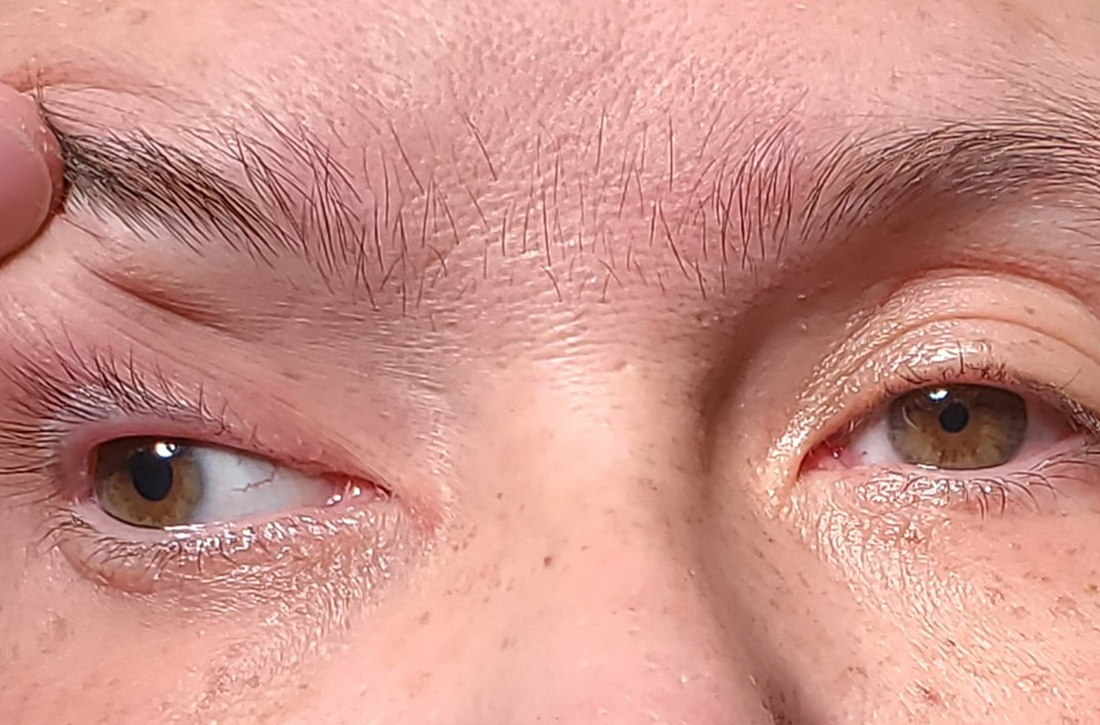
Following the crash, she said she’d been experiencing weakness in her right arm and leg and had been unable to open her right eye. When her right eye was opened manually, she said she had double vision and sensitivity to light.
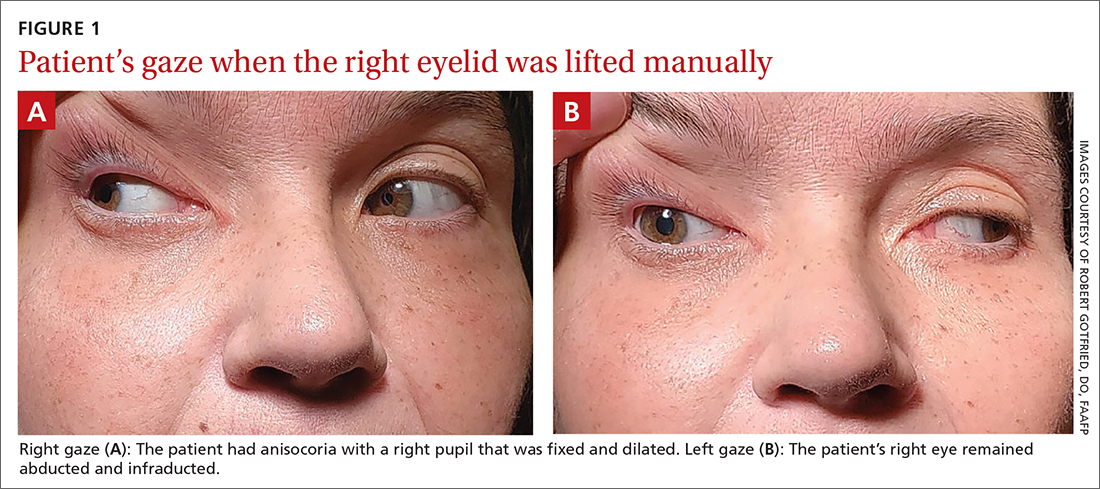
On exam, the patient had exotropia with hypotropia of her right eye. Additionally, she had anisocoria with an enlarged, nonreactive right pupil (FIGURE 1A). She was unable to adduct, supraduct, or infraduct her right eye (FIGURE 1B). Her cranial nerves were otherwise intact. On manual strength testing, she had 4/5 strength of both her right upper and lower extremities.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Third (oculomotor) nerve palsy
This patient had a complete third nerve palsy (TNP). This is defined as palsy involving all of the muscles innervated by the oculomotor nerve, with pupillary involvement.1 The oculomotor nerve supplies motor innervation to the levator palpebrae superioris, superior rectus, medial rectus, inferior rectus, and inferior oblique muscles and parasympathetic innervation to the pupillary constrictor and ciliary muscles.2 As a result, patients present with exotropia and hypotropia on exam with anisocoria. Diplopia, ptosis, and an enlarged pupil are classic symptoms of TNP.2
Computed tomography (CT) of the brain performed immediately after this patient’s accident demonstrated a 15-mm hemorrhage within the left basal ganglia with mild associated edema, and a small focus of hyperattenuation within the right aspect of the suprasellar cistern. There was no evidence of skull fracture. CT angiography (CTA) of the brain showed no evidence of aneurysm.
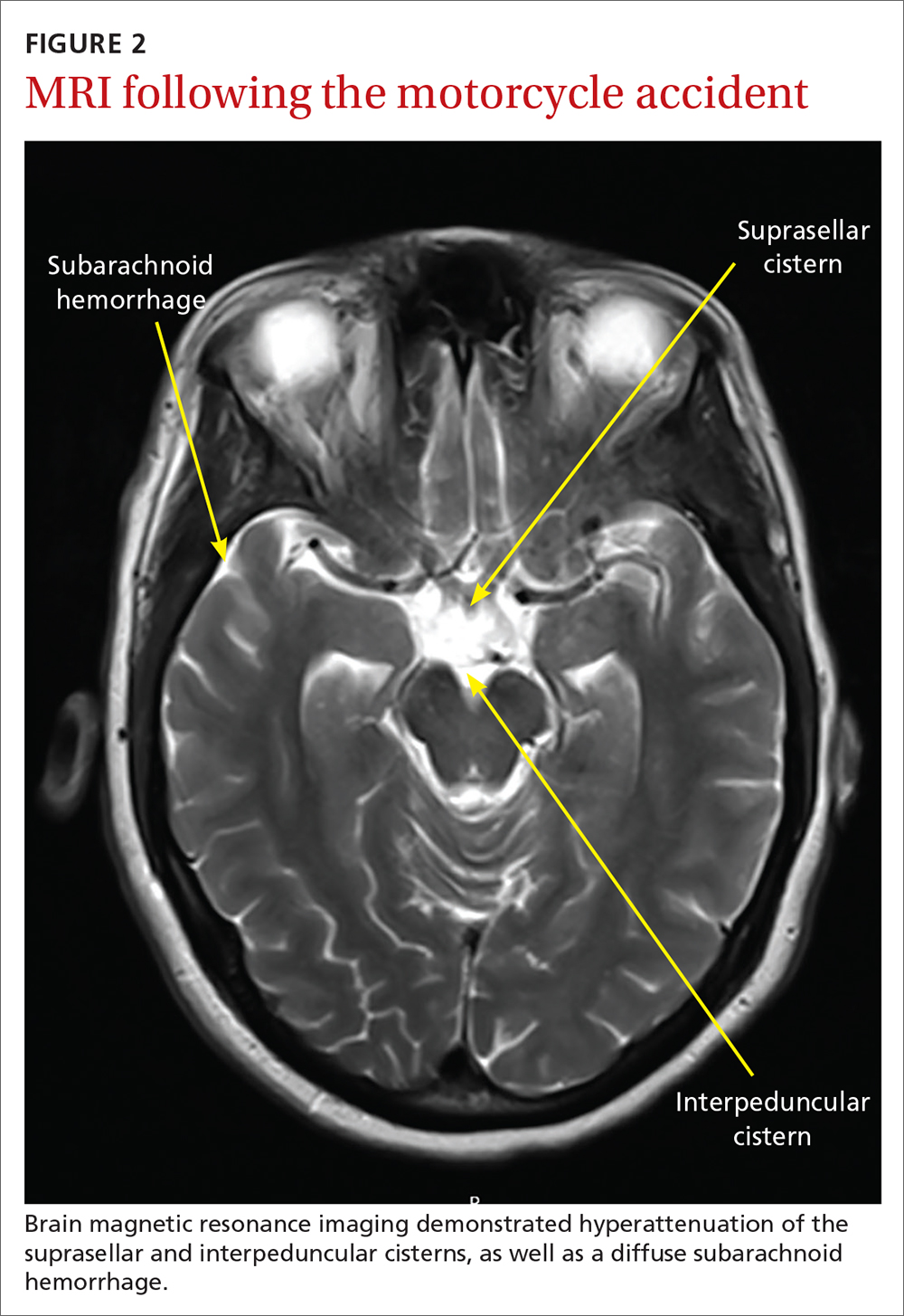
Several days later, magnetic resonance imaging (MRI) of the brain confirmed prior CT findings and revealed hemorrhagic contusions along the anterior and medial left temporal lobe. Additionally, the MRI showed subtle subdural hemorrhages along the midline falx and right parietal region, as well as diffuse subarachnoid hemorrhage around both hemispheres, the interpeduncular cistern, and the suprasellar cistern (FIGURE 2). The basal ganglia hemorrhage was believed to have been a result of uncontrolled hypertension. The hemorrhage was responsible for her right-sided weakness and was the presumed cause of the accident. The other findings were due to head trauma. Her TNP was most likely caused by both compression and irritation of the right oculomotor nerve.
An uncommon occurrence
A population-based study identified the annual incidence of TNP to be 4 per 100,000.1 The mean age of onset was 42 years. The incidence in patients older than 60 years was greater than the incidence in those younger than 60.2 Isolated TNP occurred in approximately 40% of cases.2
Complete TNP is typically indicative of compression of the ipsilateral third nerve.2 The most common region for third nerve injury is the subarachnoid space, where the oculomotor nerve is vulnerable to compression, often by an aneurysm arising from the junction of the internal carotid and posterior communicating arteries.3
Continue to: Incomplete TNP
Incomplete TNP is often microvascular in origin and requires evaluation for diabetes and hypertension. Microvascular TNP is frequently painful but usually self-resolves after 2 to 4 months.2 Giant cell arteritis may also cause an isolated, painful TNP.2
A varied differential diagnosis and a TNP link to COVID-19
The differential diagnosis for TNP includes the following:
Orbital apex injury is usually seen after high-energy craniofacial trauma.4 Orbital apex fractures present with different signs and symptoms, depending on the degree of injury to neural and vascular structures. Various syndromes come into play, the most common being superior orbital fissure syndrome, which is characterized by dysfunction of cranial nerves III, IV, V, and VI.4 Features include ophthalmoplegia, upper eyelid ptosis, a nonreactive dilated pupil, anesthesia over the ipsilateral forehead, loss of corneal reflex, orbital pain, and proptosis.4
In patients with suspected orbital apex fractures, it’s important to assess for the presence of an optic neuropathy, an evolving orbital compartment syndrome, or a ruptured globe, because these 3 things may demand acute intervention.4
Chronic progressive external ophthalmoplegia (CPEO) is a mitochondrial disorder characterized by a slow, progressive paralysis of the extraocular muscles.5 Patients usually experience bilateral, symmetrical, progressive ptosis, followed by ophthalmoparesis months to years later. Ciliary and iris muscles are not involved. CPEO often occurs with other systemic features of mitochondrial dysfunction that can cause significant morbidity and mortality.5
Continue to: Graves ophthalmopathy
Graves ophthalmopathy arises from soft-tissue enlargement in the orbit, leading to increased pressure within the bony cavity.6 Approximately 40% of patients with Graves ophthalmopathy present with restrictive extraocular myopathy; however > 90% have eyelid retraction, as opposed to ptosis.7
Guillain-Barré syndrome (GBS) is an acute, demyelinating immune-mediated polyneuropathy involving the spinal roots, peripheral nerves, and often the cranial nerves.8 The Miller Fisher variant of GBS is characterized by bilateral ophthalmoparesis, areflexia, and ataxia.8 At the early stage of illness, the presentation may be similar to TNP.8 Brain imaging is normal in patients with GBS; the diagnosis is established via characteristic electromyography and cerebrospinal fluid findings.8
Myasthenia gravis often manifests with variable ptosis associated with diplopia.9 Symptoms may be unilateral or bilateral. The ice-pack test has been identified as a simple, preliminary test for ocular myasthenia. The test involves the application of an ice-pack over the lids for 5 minutes. A 50% reduction in at least 1 component of ocular deviation is considered a positive response.10 Its specificity reportedly reaches 100%, with a sensitivity of 80%.10
COVID-19 infection may also include neurologic manifestations. There are an increasing number of case reports of central nervous system abnormalities including TNP.11,12
Trauma, tumors, or an aneurysm could be at work in TNP
TNP associated with trauma usually develops secondary to compression from an expanding hematoma, although it may also be a result of irritation of the nerve from blood in the subarachnoid space.13 Estimates of the incidence of TNP due to trauma range from 12% to 26% of cases.1,14 Vehicle-related injury is the most frequent cause of trauma-related TNP.14
Continue to: Pituitary tumors
Pituitary tumors most commonly involve the oculomotor nerve; 14% to 30% of pituitary tumors lead to TNP.13 Pituitary apoplexy secondary to infarction or hemorrhage is often associated with visual field defects and TNP.13
An underlying aneurysm manifests in a minority (10% to 15%) of patients presenting with TNP.3
Imaging is key to getting at the cause of TNP
The evaluation of patients presenting with acute TNP should be focused first on detecting an aneurysmal compressive lesion.3 CTA is the imaging modality of choice.
Once an aneurysm has been ruled out, the work-up should include a lumbar puncture and an erythrocyte sedimentation rate. Older patients should be assessed for conditions such as hypertension or diabetes that put them at risk for microvascular disease.3 If microvascular TNP is unlikely, MRI with MR angiography is recommended to exclude other potential etiologies of TNP.3 If the patient is younger than 50 years of age, consider potential infectious and inflammatory etiologies (eg, giant cell arteritis).3
Treatment options are varied
The treatment of patients with TNP is specific to the disease state. For those patients with vascular risk factors and a presumptive diagnosis of microvascular TNP, it is reasonable to observe the patient for 2 to 3 months.3 Antiplatelet therapy is usually initiated. Patching 1 eye is useful in alleviating diplopia, particularly in the short term. In most cases, deficits related to TNP resolve over weeks to months. Deficits that persist beyond 6 months may require surgical intervention.
Continue to: "The tip of the iceberg"
TNP: “The tip of the iceberg”
TNP may signal a neurologic emergency, such as an aneurysm, or other conditions such as pituitary disease or giant cell arteritis. Any patient presenting with acute onset of TNP should undergo a noninvasive neuroimaging study.3
Our patient was treated for hypertension; however, she was lost to follow-up.
1. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthalmol.2016.4456
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Margolin E, Freund P. A review of third nerve palsies. Int Ophthalmol Clin. 2019;59:99-112. doi: 10.1097/IIO.0000000000000279
4. Linnau KF, Hallam DK, Lomoschitz FM, et al. Orbital apex injury: trauma at the junction between the face and the cranium. Eur J Radiol. 2003;48:5-16. doi: 10.1016/s0720-048x(03)00203-1
5. McClelland C, Manousakis G, Lee MS. Progressive external ophthalmoplegia. Curr Neurol Neurosci Rep. 2016;16:53. doi: 10.1007/s11910-016-0652-7
6. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726-738. doi: 10.1056/NEJMra0905750
7. Subetki I, Soewond P, Soebardi S, et al. Practical guidelines management of graves ophthalmopathy. Acta Med Indones. 2019;51:364-371.
8. Wijdicks EF, Klein CJ. Guillain-Barré syndrome. Mayo Clin Proc. 2017;92:467-479. doi: 10.1016/j.mayocp.2016.12.002
9. Beloor Suresh A, Asuncion RMD. Myasthenia Gravis. In: StatPearls [Internet]. StatPearls Publishing; 2021. Accessed April 26, 2021. www.ncbi.nlm.nih.gov/books/NBK559331/
10. Chatzistefanou KI, Kouris T, Iliakis E, et al. The ice pack test in the differential diagnosis of myasthenic diplopia. Ophthalmology. 2009;116:2236-2243. doi: 10.1016/j.ophtha.2009.04.039
11. Pascual-Prieto J, Narváez-Palazón C, Porta-Etessam J, et al. COVID-19 epidemic: should ophthalmologists be aware of oculomotor paresis? Arch Soc Esp Oftalmol. 2020;95:361-362. doi: 10.1016/j.oftal.2020.05.002
12. Collantes MEV, Espiritu AI, Sy MCC, et al. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci. 2021;48:66-76. doi: 10.1017/cjn.2020.146
13. Raza HK, Chen H, Chansysouphanthong T, et al. The aetiologies of the unilateral oculomotor nerve palsy: a review of the literature. Somatosens Mot Res. 2018;35:229-239. doi :10.1080/08990220.2018.1547697
14. Keane J. Third nerve palsy: analysis of 1400 personally-examined inpatients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
A 45-year-old woman visited the clinic 6 weeks after having a stroke while on her motorcycle, which resulted in a crash. She had not been wearing a helmet and was uncertain if she had sustained a head injury. She said that during the hospital stay following the accident, she was diagnosed as hypertensive; she denied any other significant prior medical history.
Following the crash, she said she’d been experiencing weakness in her right arm and leg and had been unable to open her right eye. When her right eye was opened manually, she said she had double vision and sensitivity to light.
On exam, the patient had exotropia with hypotropia of her right eye. Additionally, she had anisocoria with an enlarged, nonreactive right pupil (FIGURE 1A). She was unable to adduct, supraduct, or infraduct her right eye (FIGURE 1B). Her cranial nerves were otherwise intact. On manual strength testing, she had 4/5 strength of both her right upper and lower extremities.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Third (oculomotor) nerve palsy
This patient had a complete third nerve palsy (TNP). This is defined as palsy involving all of the muscles innervated by the oculomotor nerve, with pupillary involvement.1 The oculomotor nerve supplies motor innervation to the levator palpebrae superioris, superior rectus, medial rectus, inferior rectus, and inferior oblique muscles and parasympathetic innervation to the pupillary constrictor and ciliary muscles.2 As a result, patients present with exotropia and hypotropia on exam with anisocoria. Diplopia, ptosis, and an enlarged pupil are classic symptoms of TNP.2
Computed tomography (CT) of the brain performed immediately after this patient’s accident demonstrated a 15-mm hemorrhage within the left basal ganglia with mild associated edema, and a small focus of hyperattenuation within the right aspect of the suprasellar cistern. There was no evidence of skull fracture. CT angiography (CTA) of the brain showed no evidence of aneurysm.
Several days later, magnetic resonance imaging (MRI) of the brain confirmed prior CT findings and revealed hemorrhagic contusions along the anterior and medial left temporal lobe. Additionally, the MRI showed subtle subdural hemorrhages along the midline falx and right parietal region, as well as diffuse subarachnoid hemorrhage around both hemispheres, the interpeduncular cistern, and the suprasellar cistern (FIGURE 2). The basal ganglia hemorrhage was believed to have been a result of uncontrolled hypertension. The hemorrhage was responsible for her right-sided weakness and was the presumed cause of the accident. The other findings were due to head trauma. Her TNP was most likely caused by both compression and irritation of the right oculomotor nerve.

An uncommon occurrence
A population-based study identified the annual incidence of TNP to be 4 per 100,000.1 The mean age of onset was 42 years. The incidence in patients older than 60 years was greater than the incidence in those younger than 60.2 Isolated TNP occurred in approximately 40% of cases.2
Complete TNP is typically indicative of compression of the ipsilateral third nerve.2 The most common region for third nerve injury is the subarachnoid space, where the oculomotor nerve is vulnerable to compression, often by an aneurysm arising from the junction of the internal carotid and posterior communicating arteries.3
Continue to: Incomplete TNP
Incomplete TNP is often microvascular in origin and requires evaluation for diabetes and hypertension. Microvascular TNP is frequently painful but usually self-resolves after 2 to 4 months.2 Giant cell arteritis may also cause an isolated, painful TNP.2
A varied differential diagnosis and a TNP link to COVID-19
The differential diagnosis for TNP includes the following:
Orbital apex injury is usually seen after high-energy craniofacial trauma.4 Orbital apex fractures present with different signs and symptoms, depending on the degree of injury to neural and vascular structures. Various syndromes come into play, the most common being superior orbital fissure syndrome, which is characterized by dysfunction of cranial nerves III, IV, V, and VI.4 Features include ophthalmoplegia, upper eyelid ptosis, a nonreactive dilated pupil, anesthesia over the ipsilateral forehead, loss of corneal reflex, orbital pain, and proptosis.4
In patients with suspected orbital apex fractures, it’s important to assess for the presence of an optic neuropathy, an evolving orbital compartment syndrome, or a ruptured globe, because these 3 things may demand acute intervention.4
Chronic progressive external ophthalmoplegia (CPEO) is a mitochondrial disorder characterized by a slow, progressive paralysis of the extraocular muscles.5 Patients usually experience bilateral, symmetrical, progressive ptosis, followed by ophthalmoparesis months to years later. Ciliary and iris muscles are not involved. CPEO often occurs with other systemic features of mitochondrial dysfunction that can cause significant morbidity and mortality.5
Continue to: Graves ophthalmopathy
Graves ophthalmopathy arises from soft-tissue enlargement in the orbit, leading to increased pressure within the bony cavity.6 Approximately 40% of patients with Graves ophthalmopathy present with restrictive extraocular myopathy; however > 90% have eyelid retraction, as opposed to ptosis.7
Guillain-Barré syndrome (GBS) is an acute, demyelinating immune-mediated polyneuropathy involving the spinal roots, peripheral nerves, and often the cranial nerves.8 The Miller Fisher variant of GBS is characterized by bilateral ophthalmoparesis, areflexia, and ataxia.8 At the early stage of illness, the presentation may be similar to TNP.8 Brain imaging is normal in patients with GBS; the diagnosis is established via characteristic electromyography and cerebrospinal fluid findings.8
Myasthenia gravis often manifests with variable ptosis associated with diplopia.9 Symptoms may be unilateral or bilateral. The ice-pack test has been identified as a simple, preliminary test for ocular myasthenia. The test involves the application of an ice-pack over the lids for 5 minutes. A 50% reduction in at least 1 component of ocular deviation is considered a positive response.10 Its specificity reportedly reaches 100%, with a sensitivity of 80%.10
COVID-19 infection may also include neurologic manifestations. There are an increasing number of case reports of central nervous system abnormalities including TNP.11,12
Trauma, tumors, or an aneurysm could be at work in TNP
TNP associated with trauma usually develops secondary to compression from an expanding hematoma, although it may also be a result of irritation of the nerve from blood in the subarachnoid space.13 Estimates of the incidence of TNP due to trauma range from 12% to 26% of cases.1,14 Vehicle-related injury is the most frequent cause of trauma-related TNP.14
Continue to: Pituitary tumors
Pituitary tumors most commonly involve the oculomotor nerve; 14% to 30% of pituitary tumors lead to TNP.13 Pituitary apoplexy secondary to infarction or hemorrhage is often associated with visual field defects and TNP.13
An underlying aneurysm manifests in a minority (10% to 15%) of patients presenting with TNP.3
Imaging is key to getting at the cause of TNP
The evaluation of patients presenting with acute TNP should be focused first on detecting an aneurysmal compressive lesion.3 CTA is the imaging modality of choice.
Once an aneurysm has been ruled out, the work-up should include a lumbar puncture and an erythrocyte sedimentation rate. Older patients should be assessed for conditions such as hypertension or diabetes that put them at risk for microvascular disease.3 If microvascular TNP is unlikely, MRI with MR angiography is recommended to exclude other potential etiologies of TNP.3 If the patient is younger than 50 years of age, consider potential infectious and inflammatory etiologies (eg, giant cell arteritis).3
Treatment options are varied
The treatment of patients with TNP is specific to the disease state. For those patients with vascular risk factors and a presumptive diagnosis of microvascular TNP, it is reasonable to observe the patient for 2 to 3 months.3 Antiplatelet therapy is usually initiated. Patching 1 eye is useful in alleviating diplopia, particularly in the short term. In most cases, deficits related to TNP resolve over weeks to months. Deficits that persist beyond 6 months may require surgical intervention.
Continue to: "The tip of the iceberg"
TNP: “The tip of the iceberg”
TNP may signal a neurologic emergency, such as an aneurysm, or other conditions such as pituitary disease or giant cell arteritis. Any patient presenting with acute onset of TNP should undergo a noninvasive neuroimaging study.3
Our patient was treated for hypertension; however, she was lost to follow-up.
A 45-year-old woman visited the clinic 6 weeks after having a stroke while on her motorcycle, which resulted in a crash. She had not been wearing a helmet and was uncertain if she had sustained a head injury. She said that during the hospital stay following the accident, she was diagnosed as hypertensive; she denied any other significant prior medical history.
Following the crash, she said she’d been experiencing weakness in her right arm and leg and had been unable to open her right eye. When her right eye was opened manually, she said she had double vision and sensitivity to light.
On exam, the patient had exotropia with hypotropia of her right eye. Additionally, she had anisocoria with an enlarged, nonreactive right pupil (FIGURE 1A). She was unable to adduct, supraduct, or infraduct her right eye (FIGURE 1B). Her cranial nerves were otherwise intact. On manual strength testing, she had 4/5 strength of both her right upper and lower extremities.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Third (oculomotor) nerve palsy
This patient had a complete third nerve palsy (TNP). This is defined as palsy involving all of the muscles innervated by the oculomotor nerve, with pupillary involvement.1 The oculomotor nerve supplies motor innervation to the levator palpebrae superioris, superior rectus, medial rectus, inferior rectus, and inferior oblique muscles and parasympathetic innervation to the pupillary constrictor and ciliary muscles.2 As a result, patients present with exotropia and hypotropia on exam with anisocoria. Diplopia, ptosis, and an enlarged pupil are classic symptoms of TNP.2
Computed tomography (CT) of the brain performed immediately after this patient’s accident demonstrated a 15-mm hemorrhage within the left basal ganglia with mild associated edema, and a small focus of hyperattenuation within the right aspect of the suprasellar cistern. There was no evidence of skull fracture. CT angiography (CTA) of the brain showed no evidence of aneurysm.
Several days later, magnetic resonance imaging (MRI) of the brain confirmed prior CT findings and revealed hemorrhagic contusions along the anterior and medial left temporal lobe. Additionally, the MRI showed subtle subdural hemorrhages along the midline falx and right parietal region, as well as diffuse subarachnoid hemorrhage around both hemispheres, the interpeduncular cistern, and the suprasellar cistern (FIGURE 2). The basal ganglia hemorrhage was believed to have been a result of uncontrolled hypertension. The hemorrhage was responsible for her right-sided weakness and was the presumed cause of the accident. The other findings were due to head trauma. Her TNP was most likely caused by both compression and irritation of the right oculomotor nerve.

An uncommon occurrence
A population-based study identified the annual incidence of TNP to be 4 per 100,000.1 The mean age of onset was 42 years. The incidence in patients older than 60 years was greater than the incidence in those younger than 60.2 Isolated TNP occurred in approximately 40% of cases.2
Complete TNP is typically indicative of compression of the ipsilateral third nerve.2 The most common region for third nerve injury is the subarachnoid space, where the oculomotor nerve is vulnerable to compression, often by an aneurysm arising from the junction of the internal carotid and posterior communicating arteries.3
Continue to: Incomplete TNP
Incomplete TNP is often microvascular in origin and requires evaluation for diabetes and hypertension. Microvascular TNP is frequently painful but usually self-resolves after 2 to 4 months.2 Giant cell arteritis may also cause an isolated, painful TNP.2
A varied differential diagnosis and a TNP link to COVID-19
The differential diagnosis for TNP includes the following:
Orbital apex injury is usually seen after high-energy craniofacial trauma.4 Orbital apex fractures present with different signs and symptoms, depending on the degree of injury to neural and vascular structures. Various syndromes come into play, the most common being superior orbital fissure syndrome, which is characterized by dysfunction of cranial nerves III, IV, V, and VI.4 Features include ophthalmoplegia, upper eyelid ptosis, a nonreactive dilated pupil, anesthesia over the ipsilateral forehead, loss of corneal reflex, orbital pain, and proptosis.4
In patients with suspected orbital apex fractures, it’s important to assess for the presence of an optic neuropathy, an evolving orbital compartment syndrome, or a ruptured globe, because these 3 things may demand acute intervention.4
Chronic progressive external ophthalmoplegia (CPEO) is a mitochondrial disorder characterized by a slow, progressive paralysis of the extraocular muscles.5 Patients usually experience bilateral, symmetrical, progressive ptosis, followed by ophthalmoparesis months to years later. Ciliary and iris muscles are not involved. CPEO often occurs with other systemic features of mitochondrial dysfunction that can cause significant morbidity and mortality.5
Continue to: Graves ophthalmopathy
Graves ophthalmopathy arises from soft-tissue enlargement in the orbit, leading to increased pressure within the bony cavity.6 Approximately 40% of patients with Graves ophthalmopathy present with restrictive extraocular myopathy; however > 90% have eyelid retraction, as opposed to ptosis.7
Guillain-Barré syndrome (GBS) is an acute, demyelinating immune-mediated polyneuropathy involving the spinal roots, peripheral nerves, and often the cranial nerves.8 The Miller Fisher variant of GBS is characterized by bilateral ophthalmoparesis, areflexia, and ataxia.8 At the early stage of illness, the presentation may be similar to TNP.8 Brain imaging is normal in patients with GBS; the diagnosis is established via characteristic electromyography and cerebrospinal fluid findings.8
Myasthenia gravis often manifests with variable ptosis associated with diplopia.9 Symptoms may be unilateral or bilateral. The ice-pack test has been identified as a simple, preliminary test for ocular myasthenia. The test involves the application of an ice-pack over the lids for 5 minutes. A 50% reduction in at least 1 component of ocular deviation is considered a positive response.10 Its specificity reportedly reaches 100%, with a sensitivity of 80%.10
COVID-19 infection may also include neurologic manifestations. There are an increasing number of case reports of central nervous system abnormalities including TNP.11,12
Trauma, tumors, or an aneurysm could be at work in TNP
TNP associated with trauma usually develops secondary to compression from an expanding hematoma, although it may also be a result of irritation of the nerve from blood in the subarachnoid space.13 Estimates of the incidence of TNP due to trauma range from 12% to 26% of cases.1,14 Vehicle-related injury is the most frequent cause of trauma-related TNP.14
Continue to: Pituitary tumors
Pituitary tumors most commonly involve the oculomotor nerve; 14% to 30% of pituitary tumors lead to TNP.13 Pituitary apoplexy secondary to infarction or hemorrhage is often associated with visual field defects and TNP.13
An underlying aneurysm manifests in a minority (10% to 15%) of patients presenting with TNP.3
Imaging is key to getting at the cause of TNP
The evaluation of patients presenting with acute TNP should be focused first on detecting an aneurysmal compressive lesion.3 CTA is the imaging modality of choice.
Once an aneurysm has been ruled out, the work-up should include a lumbar puncture and an erythrocyte sedimentation rate. Older patients should be assessed for conditions such as hypertension or diabetes that put them at risk for microvascular disease.3 If microvascular TNP is unlikely, MRI with MR angiography is recommended to exclude other potential etiologies of TNP.3 If the patient is younger than 50 years of age, consider potential infectious and inflammatory etiologies (eg, giant cell arteritis).3
Treatment options are varied
The treatment of patients with TNP is specific to the disease state. For those patients with vascular risk factors and a presumptive diagnosis of microvascular TNP, it is reasonable to observe the patient for 2 to 3 months.3 Antiplatelet therapy is usually initiated. Patching 1 eye is useful in alleviating diplopia, particularly in the short term. In most cases, deficits related to TNP resolve over weeks to months. Deficits that persist beyond 6 months may require surgical intervention.
Continue to: "The tip of the iceberg"
TNP: “The tip of the iceberg”
TNP may signal a neurologic emergency, such as an aneurysm, or other conditions such as pituitary disease or giant cell arteritis. Any patient presenting with acute onset of TNP should undergo a noninvasive neuroimaging study.3
Our patient was treated for hypertension; however, she was lost to follow-up.
1. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthalmol.2016.4456
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Margolin E, Freund P. A review of third nerve palsies. Int Ophthalmol Clin. 2019;59:99-112. doi: 10.1097/IIO.0000000000000279
4. Linnau KF, Hallam DK, Lomoschitz FM, et al. Orbital apex injury: trauma at the junction between the face and the cranium. Eur J Radiol. 2003;48:5-16. doi: 10.1016/s0720-048x(03)00203-1
5. McClelland C, Manousakis G, Lee MS. Progressive external ophthalmoplegia. Curr Neurol Neurosci Rep. 2016;16:53. doi: 10.1007/s11910-016-0652-7
6. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726-738. doi: 10.1056/NEJMra0905750
7. Subetki I, Soewond P, Soebardi S, et al. Practical guidelines management of graves ophthalmopathy. Acta Med Indones. 2019;51:364-371.
8. Wijdicks EF, Klein CJ. Guillain-Barré syndrome. Mayo Clin Proc. 2017;92:467-479. doi: 10.1016/j.mayocp.2016.12.002
9. Beloor Suresh A, Asuncion RMD. Myasthenia Gravis. In: StatPearls [Internet]. StatPearls Publishing; 2021. Accessed April 26, 2021. www.ncbi.nlm.nih.gov/books/NBK559331/
10. Chatzistefanou KI, Kouris T, Iliakis E, et al. The ice pack test in the differential diagnosis of myasthenic diplopia. Ophthalmology. 2009;116:2236-2243. doi: 10.1016/j.ophtha.2009.04.039
11. Pascual-Prieto J, Narváez-Palazón C, Porta-Etessam J, et al. COVID-19 epidemic: should ophthalmologists be aware of oculomotor paresis? Arch Soc Esp Oftalmol. 2020;95:361-362. doi: 10.1016/j.oftal.2020.05.002
12. Collantes MEV, Espiritu AI, Sy MCC, et al. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci. 2021;48:66-76. doi: 10.1017/cjn.2020.146
13. Raza HK, Chen H, Chansysouphanthong T, et al. The aetiologies of the unilateral oculomotor nerve palsy: a review of the literature. Somatosens Mot Res. 2018;35:229-239. doi :10.1080/08990220.2018.1547697
14. Keane J. Third nerve palsy: analysis of 1400 personally-examined inpatients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
1. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthalmol.2016.4456
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Margolin E, Freund P. A review of third nerve palsies. Int Ophthalmol Clin. 2019;59:99-112. doi: 10.1097/IIO.0000000000000279
4. Linnau KF, Hallam DK, Lomoschitz FM, et al. Orbital apex injury: trauma at the junction between the face and the cranium. Eur J Radiol. 2003;48:5-16. doi: 10.1016/s0720-048x(03)00203-1
5. McClelland C, Manousakis G, Lee MS. Progressive external ophthalmoplegia. Curr Neurol Neurosci Rep. 2016;16:53. doi: 10.1007/s11910-016-0652-7
6. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726-738. doi: 10.1056/NEJMra0905750
7. Subetki I, Soewond P, Soebardi S, et al. Practical guidelines management of graves ophthalmopathy. Acta Med Indones. 2019;51:364-371.
8. Wijdicks EF, Klein CJ. Guillain-Barré syndrome. Mayo Clin Proc. 2017;92:467-479. doi: 10.1016/j.mayocp.2016.12.002
9. Beloor Suresh A, Asuncion RMD. Myasthenia Gravis. In: StatPearls [Internet]. StatPearls Publishing; 2021. Accessed April 26, 2021. www.ncbi.nlm.nih.gov/books/NBK559331/
10. Chatzistefanou KI, Kouris T, Iliakis E, et al. The ice pack test in the differential diagnosis of myasthenic diplopia. Ophthalmology. 2009;116:2236-2243. doi: 10.1016/j.ophtha.2009.04.039
11. Pascual-Prieto J, Narváez-Palazón C, Porta-Etessam J, et al. COVID-19 epidemic: should ophthalmologists be aware of oculomotor paresis? Arch Soc Esp Oftalmol. 2020;95:361-362. doi: 10.1016/j.oftal.2020.05.002
12. Collantes MEV, Espiritu AI, Sy MCC, et al. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci. 2021;48:66-76. doi: 10.1017/cjn.2020.146
13. Raza HK, Chen H, Chansysouphanthong T, et al. The aetiologies of the unilateral oculomotor nerve palsy: a review of the literature. Somatosens Mot Res. 2018;35:229-239. doi :10.1080/08990220.2018.1547697
14. Keane J. Third nerve palsy: analysis of 1400 personally-examined inpatients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
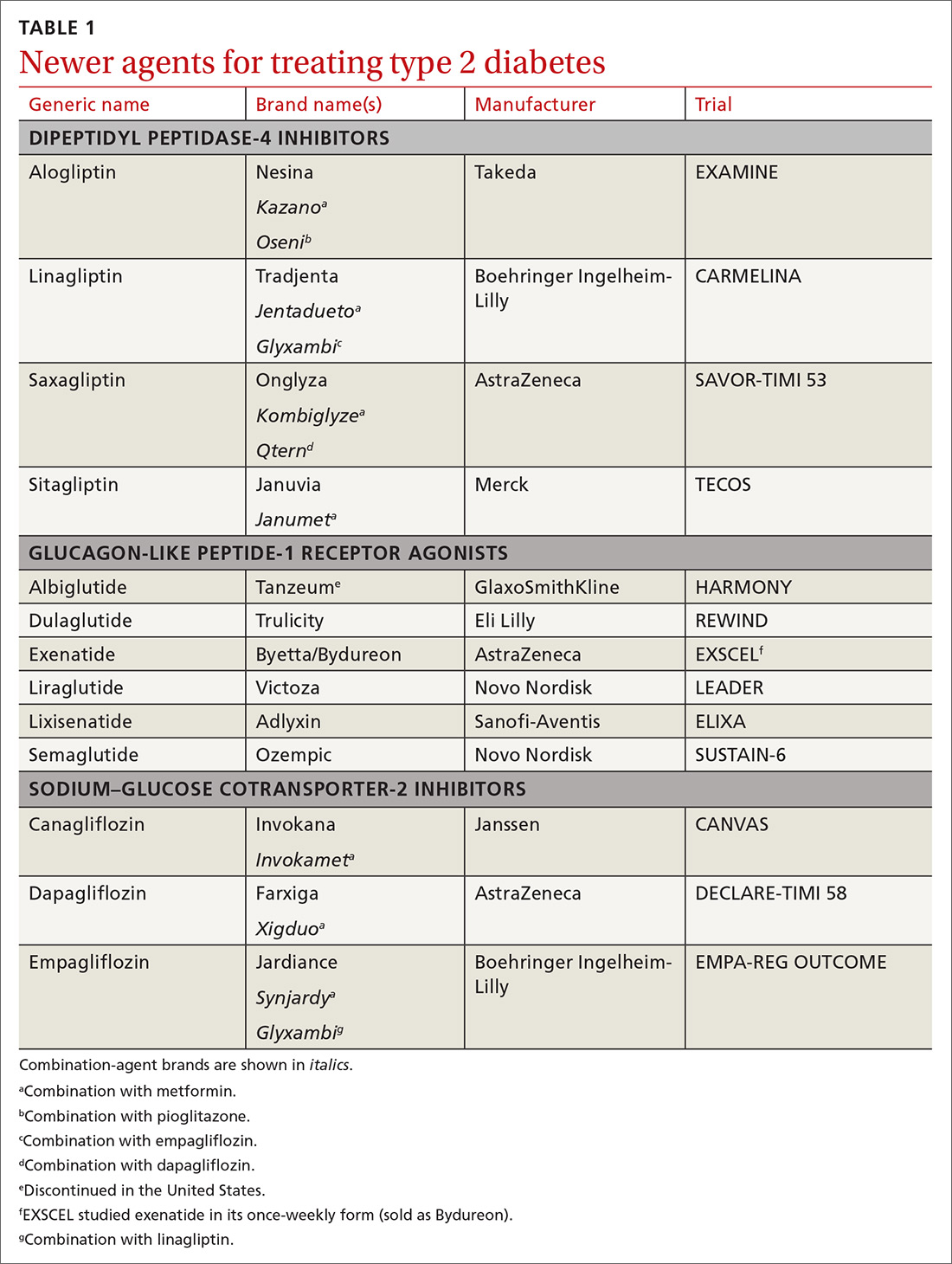
How to use type 2 diabetes meds to lower CV disease risk
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4
Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
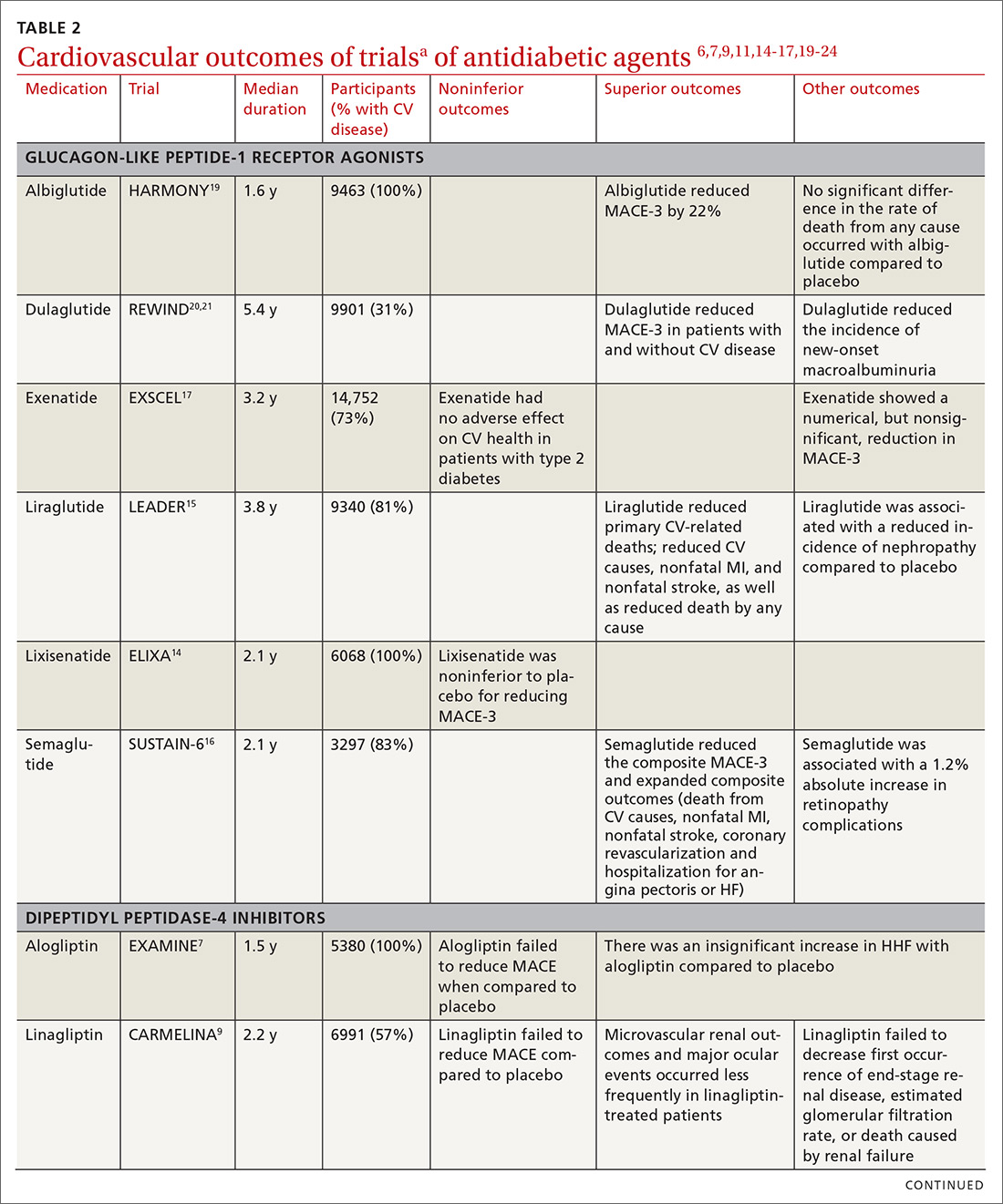
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).
Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32
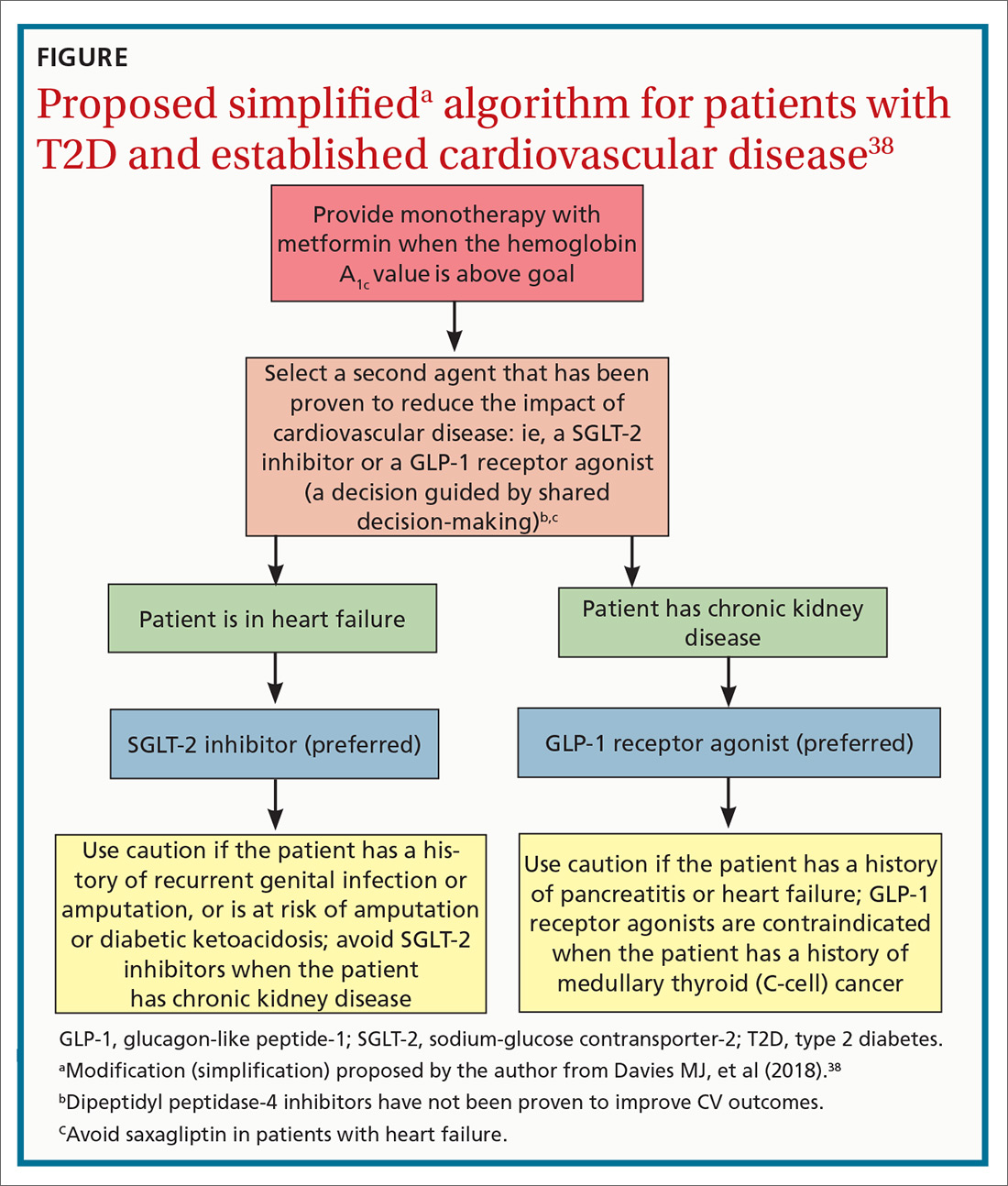
See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.
ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; [email protected].
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4

Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).

Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32

See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.

ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; [email protected].
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4

Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).

Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32

See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.

ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; [email protected].
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
PRACTICE RECOMMENDATIONS
› Consider American Diabetes Association (ADA) guidance and prescribe a sodium–glucose cotransporter-2 (SGLT-2) inhibitor or glucagon-like peptide- 1 (GLP-1) receptor agonist that has demonstrated cardiovascular (CV) disease benefit for your patients who have type 2 diabetes (T2D) and established atherosclerotic CV disease. A
› Consider ADA’s recommendation for preferred therapy and prescribe an SGLT-2 inhibitor for your patients with T2D who have atherosclerotic CV disease and are at high risk of heart failure or in whom heart failure coexists. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series