User login
An irritable, inattentive, and disruptive child: Is it ADHD or bipolar disorder?
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
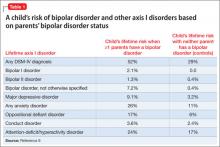
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
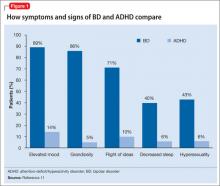
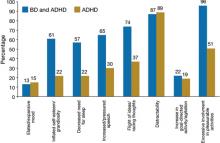
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
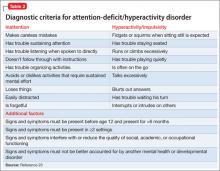
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
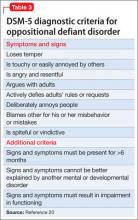
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
What to look for when evaluating mood swings in children and adolescents
Not all mood swings are bipolar disorder
M, age 13, is referred by her pediatrician with the chief complaint of “severe mood swings, rule out bipolar disorder (BD).” In the past she was treated for attention-deficit/hyperactivity disorder (ADHD) with stimulants with mixed results. M’s parents are concerned about her “flipping out” whenever she is asked to do something she does not want to do. Her mother has a history of depression and anxiety; her father had a “drinking problem.” There is no history of BD in her first- or second-degree relatives. Are M’s rapid mood swings a sign of BD or another disorder?
The differential diagnosis of “mood swings” is important because they are a common presenting symptom of many children and adolescents with mood and behavioral disorders. Mood swings often occur in children and adolescents with ADHD, oppositional defiant disorder (ODD), developmental disorders, depressive disorders, BD, anxiety disorders, and conduct disorders. Mood swings are analogous to a fever in pediatrics—they indicate something potentially is wrong with the patient, but are not diagnostic as an isolated symptom.
Mood swings in children are common, nonspecific symptoms that more often are a sign of anxiety or behavioral disorders than BD. This article discusses the differential diagnosis of mood swings in children and adolescents and how to best screen and diagnose these patients.
What are ‘mood swings’?
Mood swings is a popular term that is nonspecific and not part of DSM-IV-TR diagnostic criteria for BD. The complaint of “mood swings” may reflect severe mood lability of pediatric patients with BD. This mood lability is best described by the Kiddie-Mania Rating Scale (K-MRS) developed by Axelson and colleagues as “rapid mood variation with several mood states within a brief period of time which appears internally driven without regard to the circumstance.”1 On K-MRS mood lability items, children with mania typically score:
- Moderate—many mood changes throughout the day, can vary from elevated mood to anger to sadness within a few hours; changes in mood are clearly out of proportion to circumstances and cause impairment in functioning
- Severe—rapid mood swings nearly all of the time, with mood intensity greatly out of proportion to circumstances
- Extreme—constant, explosive variability in mood, several mood changes occurring within minutes, difficult to identify a particular mood, changes in mood radically out of proportion to circumstances.
Patients with BD typically exhibit what is best described as a “mood cycle”—a pronounced shift in mood and energy from 1 extreme to another.2 An example of this would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours and then later in the day becomes sad, depressed, and suicidal with no precipitant for either mood cycle. BD patients also will exhibit other symptoms of mania during these mood cycling periods.
Rapid cycling is a DSM-IV course specifier that indicates ≥4 mood episodes per year in patients with BD with a typical course of mania or hypomania followed by depression, or vice versa.3 The episodes must be demarcated by full or partial remission that lasts ≥2 months or by a switch to a mood state of opposite polarity. In the past, children with frequent mood swings were described incorrectly as “rapid cycling,” but this term has been dropped because it engenders confusion between adult and pediatric BD phenomenology.2
A more precise method of describing mood symptoms in a child or adolescent is to use the FIND criteria, which include:4
- Frequency of symptoms per week
- Intensity of mood symptoms
- Number of mood cycles per day
- Duration of symptoms per day.
Visit this article at CurrentPsychiatry.com to view a table that outlines what to look for when using the FIND criteria to evaluate common pediatric psychiatric disorders that include mood swings. Table 1
describes clinical characteristics and tools and resources used to differentiate these and other disorders.4
Table 1
Clinical characteristics of psychiatric disorders that often feature mood swings
| Disorder | Clinical description | Useful tools/resources |
|---|---|---|
| ADHD | Chronic symptoms of hyperactivity, distractibility, impulsivity, poor attentional skills, disorganization | Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) |
| ODD | Chronic symptoms of oppositionality, negativity; short, frequent mood swings in response to being asked to do something they do not want to do | CPRS-R:L |
| Anxiety disorders | Excessive ‘worry,’ difficulty with transitions, increased mood swings during stressful periods, psychosomatic symptoms | Self-Report for Childhood Anxiety Related Disorders |
| ARND | History of exposure to alcohol in-utero; mild dysmorphia, attentional, mood, and executive functioning problems | National Organization on Fetal Alcohol Syndrome |
| Bipolar disorder | In children: clustering together of episodes or ‘mini-episodes’ (several days) of increased energy, decreased need for sleep, increased mood cycling, pressured speech, etc. In adolescents: depressive episodes with episodes of hypomania or mania | Mood Disorders Questionnaire Kiddie Schedule for Affective Disorders and Schizophrenia Mania Rating Scale |
| ADHD: attention-deficit/hyperactivity disorder; ARND: alcohol-related neurodevelopmental disorder; ODD: oppositional defiant disorder | ||
| Source: Reference 4 | ||
Mood swings: A chart review
We recently completed a retrospective chart review of 100 patients consecutively referred to our pediatric mood disorders clinic for evaluation of “mood swings, rule out BD.” These patients were self-referred, referred by a psychiatrist for a second opinion, or referred by their primary care physician. The mean age of these patients was 8±2.8 years and 68% were male.
Two experienced clinicians (RAK and EM) interviewed each patient and their caregivers and reviewed results of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L)5 and other outside information.
Figure 1 illustrates these patients’ diagnoses. Diagnoses for each of these disorders were made using DSM-IV-TR criteria.3
The most common diagnoses among patients with the chief complaint of mood swings were ADHD (39%); ODD with ADHD (15%); an anxiety disorder, usually generalized anxiety disorder (GAD) (15%); BD (12%); and a secondary mood disorder, usually fetal alcohol spectrum disorder (10%). We were surprised at how often ADHD, ODD, and anxiety disorders were found to be responsible for these patients’ mood swings and how frequently the referring clinician did not recognize these disorders. In the following sections, we discuss each of these disorders and how they differ from BD.
Figure 1 Underlying diagnoses of 100 children/adolescents referred for ‘mood swings’
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder; MDD: major depressive disorder; ODD: oppositional defiant disorder; PDD: pervasive developmental disorder
ADHD and ODD
In our sample, patients with undiagnosed ADHD made up the largest group of those with frequent mood swings. ADHD inattentive type was missed frequently in adolescent girls who still had behavioral aspects of ADHD, including impulsivity and aggression.6
The CPRS-R:L is useful for screening and diagnosing children and adolescents with ADHD and ODD. It contains 80 items, can be used in males and females and patients age 3 to 17, and has validated norms by age and sex.5 It takes parents approximately 10 minutes to fill out this questionnaire and the results can be scored by hand. The CPRS-R:L includes the following scales: oppositional; cognitive problems/inattention; hyperactivity; anxious-shy; perfectionism; social problems; psychosomatic; Connors’ global index; DSM-IV symptom subscales; and an ADHD index. Patients with mood swings and ADHD combined typically score >2 standard deviations above their age/sex mean on the CPRS-R:L hyperactivity scale, Connors’ Global Index, and ADHD index.5
A common childhood disorder, ODD has multiple etiologies.7 The first DSM-IV criteria for ODD is “often loses temper”3—essentially mood swings that often are expressed behaviorally as anger and at times as aggressive outbursts.
Dodge and Cole8 categorized aggression as reactive (impulsivity with a high affective valence) or proactive (characterized by low arousal and premeditation, ie, predatory conduct disorder). Reactive aggression typically is an angry defensive response to frustration, threat, or provocation, whereas proactive aggression is deliberate, coercive behavior often used to obtain a goal.9 Reactive aggression is common among children with ADHD and ODD and typically begins as a mood swing that escalates into reactive aggressive behavior. In a study of 268 consecutively referred children and adolescents with ADHD and 100 community controls, Connor et al10 found significantly more reactive than proactive forms of aggression in ADHD patients.
It can be difficult to differentiate the moods swings and symptoms of ODD from those of pediatric BD. Mick et al11 found that severe irritability may be a diagnostic indicator of BD in children with ADHD. Using the Kiddie Schedule for Affective Disorders and Schizophrenia (epidemiologic version) structured diagnostic interview,12 they evaluated 274 children (mean age 10.8±3.2) with ADHD; 37% had no comorbid mood disorder, 36% had ADHD with depression, and 11% had ADHD with BD. Researchers characterized 3 types of irritability in these patients:
- ODD-type irritability characterized by a low frustration tolerance that is seen in ODD
- Mad/cranky irritability found in depressive disorders
- Super-angry/grouchy/cranky irritability with frequent, prolonged, and largely unprovoked anger episodes and characteristics of mania.
ODD-type irritability was common among all ADHD patients, was the least impairing type of irritability, and did not increase the risk of a mood disorder. Mad/cranky irritability was common only in children with ADHD and a mood disorder (depression or BD), was more impairing than ODD-type irritability, and was most predictive of unipolar depression. Super-angry/grouchy/cranky irritability was common only among children with ADHD and BD (77%), was the most impairing, and was predictive of both unipolar depression and BD. The type of irritability and clustering of DSM-IV manic symptoms best differentiated ADHD subjects from those with ADHD and BD. Figure 2 illustrates symptoms that differentiated patients with ADHD from those with ADHD and comorbid BD.11
A review of pharmacotherapy for aggression in children found the largest effects for methylphenidate for aggression in ADHD (mean effect size=0.9, combined N=844).13 Our clinical experience has been that pediatric patients with ADHD or ODD with ADHD often have high levels of reactive aggression that presents as mood swings, and aggressively treating ADHD often results in improved mood and other ADHD symptoms.
Figure 2 Symptoms that differentiate BD from BD with comorbid ADHD
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
Source: Reference 11
Anxiety disorders
The estimated prevalence of child and adolescent anxiety disorders is 10% to 20%14; in our sample the prevalence was 15%. These disorders include GAD, separation anxiety disorder, social phobias, posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. Often, children with GAD worry excessively and become upset during transitions when things don’t proceed as they expect, with resultant angry outbursts and mood swings. Mood swings and difficulty sleeping are common in children with anxiety disorders or BD. Anxiety disorders often will be missed unless specific triggers of the mood swings or angry outbursts—as well as differentiating symptoms such as excessive fear, worry, and psychosomatic symptoms—are assessed.
In our clinical experience, simply asking a child if he or she is anxious is not sufficient to uncover an anxiety disorder. Although the CPRS-L:R will screen for anxiety disorders, we have found that the Self-Report for Childhood Anxiety Related Disorders (SCARED) developed by Birmaher et al15 is more specific. This tool can be used in patients age ≥8. The parent and child versions of the SCARED contain 41 items that measure 5 factors:
- general anxiety
- separation anxiety
- social phobia
- school phobia
- physical symptoms of anxiety.
The SCARED takes 5 minutes to fill out and is available in parent and child versions.
Secondary mood disorders
Many patients in our sample had a mood disorder secondary to the neurologic effects of alcohol on the developing brain. For more about maternal alcohol use, fetal alcohol spectrum disorders, and mood swings, visit this article at CurrentPsychiatry.com.
What BD looks like in children
In our sample, 12% of patients referred for mood swings were diagnosed with bipolar I disorder (BDI), bipolar II disorder (BDII), or bipolar disorder, not otherwise specified (BD-NOS). In the United States, lifetime prevalence of BDI and BDII in adolescents age 13 to 17 is 2.9%.16 No large epidemiologic studies have looked at the lifetime prevalence of BD in children age <13.
How often a clinician sees BD in children and adolescents largely depends on the type of setting in which he or she practices. Although in the general population BD is relatively rare compared with other childhood psychiatric disorders, on child/adolescent inpatient units it is common to find that 30% to 40% of patients have BD.17
The best longitudinal study to date of the phenomenology, comorbidity, and outcome of BD in children and adolescents is the National Institute of Mental Health-funded Course and Outcome of Bipolar Youth study (COBY).18 In this ongoing, longitudinal study, 413 youths (age 7 to 17) with BDI (N=244), BDII (N=28), or BD-NOS (N=141) were rigorously diagnosed using state-of-the-art measures, including the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present version19 and re-evaluated every 9.4 months for 4 years. When organizing this study, investigators found that DSM-IV criteria for BD-NOS were too vague to be useful and developed their own criteria (Table 2).18
For BDI patients in the COBY study, the mean age of onset for bipolar symptoms was 9.0±4.1 years and the mean duration of illness was 4.4±3.1 years. Researchers reported that at the 4-year assessment approximately 70% of patients with BD recovered from their index episode, and 50% had at least 1 syndromal recurrence, particularly depressive episodes.20 Analyses of these patients’ weekly mood symptoms showed that they had syndromal or subsyndromal symptoms with numerous changes in symptoms and shifts of mood polarity 60% of the time, and psychosis 3% of the time. During this study, 20% of BDII patients progressed to BDI, and 25% of BD-NOS patients converted to BDI or BDII.
Further analysis of the COBY data revealed that onset of mood symptoms preceded onset of clear bipolar episodes by an average of 1.0±1.7 years. Depression was the most common initial and most frequent episode for adolescents; mood lability was seen more often in childhood-onset and adolescents with early-onset BD. Depressed children had more severe irritability than depressed adolescents, and older age was associated with more severe and typical mood symptomatology.21
The clinical picture of a child with BD that emerges from the COBY study is:
- a fairly young child with the onset of mood symptoms between age 5 to 12
- subsyndromal and less frequently clear syndromal episodes
- primarily mixed and depressed symptoms with rapid mood cycles during these episodes.22
It is clear that there is a spectrum of bipolar disorders in children and adolescents with varying degrees of symptom expression and children differ from adolescents and adults in their initial presentation of BD.
Table 2
COBY criteria for bipolar disorder, not otherwise specified
| Presence of clinically relevant bipolar symptoms that do not fulfill DSM-IV criteria for BDI or BDII |
| In addition, patients are required to have elevated mood plus 2 associated DSM-IV symptoms or irritable mood plus 3 DSM-IV associated symptoms, along with a change in level of functioning |
| Duration of a minimum of 4 hours within a 24-hour period |
| At least 4 cumulative lifetime days meeting the criteria |
| BDI: bipolar I disorder; BDII: bipolar II disorder; COBY: Course and Outcome of Bipolar Youth study |
| Source: Reference 18 |
Related Resources
- Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Goodwin FK, Jamison KR. Manic-depressive illness. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2007.
- Miklowitz DJ, Cicchetti D, eds. Understanding bipolar disorder: a developmental perspective. New York, NY: Guilford Press; 2010.
Drug Brand Name
- Methylphenidate • Ritalin, Concerta, others
Disclosures
Dr. Kowatch receives grant/research support from the National Institute of Child Health and Human Development and the National Institute of Mental Health and is a consultant to AstraZeneca, Forest Pharmaceuticals, Merck, and the REACH Foundation.
Dr. Delgado and Ms. Monroe report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
FIND criteria of disorders found to cause mood swings
| Criteria | BDI | BP-NOS | ODD | GAD | ARND |
|---|---|---|---|---|---|
| Frequency of symptoms/week | 7 days (more days than not in an average week) | 2 to 3 days/week | Daily (chronic) irritability and mood swings precipitated by ‘not getting their way’ | Greatest during times of change/stress | Daily |
| Intensity of symptoms | Severe—parents often are afraid to take the child out in public because of mood symptoms | Moderate | Mild/moderate | Mild/moderate when stressed | Mild/moderate |
| Number of mood cycles/day | Daily cycles of euphoria and depression | 3 to 4 | 5 to 10 | 2 to 3 | 8 to 10 |
| Duration of symptoms/day | Euphoria: 30 to 60 minutes Depression: 30 minutes to 6 hours | 4 hours total/day of mood symptoms | Short; 5 to 10 minutes | Short; 5 to 10 minutes | Short; 5 to 10 minutes |
| ARND: alcohol-related neurodevelopmental disorder; BDI: bipolar I disorder; BD-NOS: bipolar disorder, not otherwise specified; GAD: generalized anxiety disorder; ODD: oppositional defiant disorder | |||||
| Source: Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008 | |||||
Even small amounts of alcohol use by a pregnant woman can impact her child’s development. In a controlled study examining drinking behavior of 12,678 pregnant women and the effect this had on their children, Sayel et ala found that <1 drink per week during the first trimester was clinically significant for mental health problems in girls, measured at age 4 and 8, when using parent or teacher report.
Fetal alcohol spectrum disorder describes the range of effects that can occur in an individual whose mother drank alcohol during pregnancy. These disorders include fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD).
FAS. Individuals with FAS have a distinct pattern of facial abnormalities, growth deficiency, and evidence of CNS dysfunction. Characteristic facial abnormalities may include a smooth philtrum, thin upper lip, upturned nose, flat nasal bridge and midface, epicanthal folds, small palpebral fissures, and small head circumference. Growth deficiency begins in-utero and continues throughout childhood and into adulthood. CNS abnormalities can include impaired brain growth or abnormal structure, manifested differently depending on age.
ARND. Many individuals affected by alcohol exposure before birth do not have the characteristic facial abnormalities and growth retardation identified with full FAS, yet have significant brain and behavioral impairments. Individuals with ARND have either the facial anomalies, growth retardation, and other physical abnormalities, or a complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level and unexplained by genetic background or environmental conditions (ie, poor impulse control, language deficits, problems with abstraction, mathematical and social perception deficits, learning problems, and impairment in attention, memory, or judgment).b
ARBD. Persons with ARBD have malformations of the skeletal and major organ systems, such as cardiac or renal abnormalities.
Comorbid psychiatric conditions in children with prenatal alcohol exposure are 5 to 16 times more prevalent than in the general population; these children are 38% more likely to have an anger disorder.c O’Connor and Paleyd found that “…mood disorder symptoms were significantly higher for children with parental alcohol exposure compared to children without exposure.” Children with ARND are treated symptomatically depending upon which deficits and behaviors they exhibit.e
References
a. Sayal K, Heron J, Golding J, et al. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009;123(2):e289-296.
b. Warren KR, Foudin LL. Alcohol-related birth defects—the past, present, and future. Alcohol Res Health. 2001;25(3):153-158.
c. Burd L, Klug MG, Martsolf JT, et al. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol. 2003;25(6):697-705.
d. O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15(3):225-234.
e. Paley B, O’Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15(3):258-267.
1. Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463-470.
2. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10(1 Pt 2):194-214.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Conners CK. Conners’ Parent Rating Scale Long Form (CPRS-R:L) North Tonawanda, NY: Multi-Health Systems, Inc.; 1997.
6. Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50(9):1042-1051.
7. Steiner H, Remsing L. and the Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
8. Dodge KA, Cole JD. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Pers Soc Psychol. 1987;53(6):1146-1158.
9. Connor DF, Steingard RJ, Cunningham JA, et al. Proactive and reactive aggression in referred children and adolescents. Am J Orthopsychiatry. 2004;74(2):129-136.
10. Connor DF, Chartier KG, Preen EC, et al. Impulsive aggression in attention-deficit/hyperactivity disorder: symptom severity, co-morbidity, and attention-deficit/hyperactivity disorder subtype. J Child Adolesc Psychopharmacol. 2010;20(2):119-126.
11. Mick E, Spencer T, Wozniak J, et al. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58(7):576-582.
12. Orvaschel H. Schizophrenia and Affective Disorders Schedule for children—Epidemiological Version (KSADS-E). Fort Lauderdale, FL: Nova Southeastern University; 1995.
13. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
14. Achenbach TM, Howell CT, McConaughy SH, et al. Six-year predictors of problems in a national sample: IV. Young adult signs of disturbance. J Am Acad Child Adolesc Psychiatry. 1998;37(7):718-727.
15. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545-553.
16. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
17. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7):712-717.
18. Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175-183.
19. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988.
20. Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18(4):1023-1035.
21. Birmaher B, Axelson D, Strober M, et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar Disord. 2009;11(1):52-62.
22. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
M, age 13, is referred by her pediatrician with the chief complaint of “severe mood swings, rule out bipolar disorder (BD).” In the past she was treated for attention-deficit/hyperactivity disorder (ADHD) with stimulants with mixed results. M’s parents are concerned about her “flipping out” whenever she is asked to do something she does not want to do. Her mother has a history of depression and anxiety; her father had a “drinking problem.” There is no history of BD in her first- or second-degree relatives. Are M’s rapid mood swings a sign of BD or another disorder?
The differential diagnosis of “mood swings” is important because they are a common presenting symptom of many children and adolescents with mood and behavioral disorders. Mood swings often occur in children and adolescents with ADHD, oppositional defiant disorder (ODD), developmental disorders, depressive disorders, BD, anxiety disorders, and conduct disorders. Mood swings are analogous to a fever in pediatrics—they indicate something potentially is wrong with the patient, but are not diagnostic as an isolated symptom.
Mood swings in children are common, nonspecific symptoms that more often are a sign of anxiety or behavioral disorders than BD. This article discusses the differential diagnosis of mood swings in children and adolescents and how to best screen and diagnose these patients.
What are ‘mood swings’?
Mood swings is a popular term that is nonspecific and not part of DSM-IV-TR diagnostic criteria for BD. The complaint of “mood swings” may reflect severe mood lability of pediatric patients with BD. This mood lability is best described by the Kiddie-Mania Rating Scale (K-MRS) developed by Axelson and colleagues as “rapid mood variation with several mood states within a brief period of time which appears internally driven without regard to the circumstance.”1 On K-MRS mood lability items, children with mania typically score:
- Moderate—many mood changes throughout the day, can vary from elevated mood to anger to sadness within a few hours; changes in mood are clearly out of proportion to circumstances and cause impairment in functioning
- Severe—rapid mood swings nearly all of the time, with mood intensity greatly out of proportion to circumstances
- Extreme—constant, explosive variability in mood, several mood changes occurring within minutes, difficult to identify a particular mood, changes in mood radically out of proportion to circumstances.
Patients with BD typically exhibit what is best described as a “mood cycle”—a pronounced shift in mood and energy from 1 extreme to another.2 An example of this would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours and then later in the day becomes sad, depressed, and suicidal with no precipitant for either mood cycle. BD patients also will exhibit other symptoms of mania during these mood cycling periods.
Rapid cycling is a DSM-IV course specifier that indicates ≥4 mood episodes per year in patients with BD with a typical course of mania or hypomania followed by depression, or vice versa.3 The episodes must be demarcated by full or partial remission that lasts ≥2 months or by a switch to a mood state of opposite polarity. In the past, children with frequent mood swings were described incorrectly as “rapid cycling,” but this term has been dropped because it engenders confusion between adult and pediatric BD phenomenology.2
A more precise method of describing mood symptoms in a child or adolescent is to use the FIND criteria, which include:4
- Frequency of symptoms per week
- Intensity of mood symptoms
- Number of mood cycles per day
- Duration of symptoms per day.
Visit this article at CurrentPsychiatry.com to view a table that outlines what to look for when using the FIND criteria to evaluate common pediatric psychiatric disorders that include mood swings. Table 1
describes clinical characteristics and tools and resources used to differentiate these and other disorders.4
Table 1
Clinical characteristics of psychiatric disorders that often feature mood swings
| Disorder | Clinical description | Useful tools/resources |
|---|---|---|
| ADHD | Chronic symptoms of hyperactivity, distractibility, impulsivity, poor attentional skills, disorganization | Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) |
| ODD | Chronic symptoms of oppositionality, negativity; short, frequent mood swings in response to being asked to do something they do not want to do | CPRS-R:L |
| Anxiety disorders | Excessive ‘worry,’ difficulty with transitions, increased mood swings during stressful periods, psychosomatic symptoms | Self-Report for Childhood Anxiety Related Disorders |
| ARND | History of exposure to alcohol in-utero; mild dysmorphia, attentional, mood, and executive functioning problems | National Organization on Fetal Alcohol Syndrome |
| Bipolar disorder | In children: clustering together of episodes or ‘mini-episodes’ (several days) of increased energy, decreased need for sleep, increased mood cycling, pressured speech, etc. In adolescents: depressive episodes with episodes of hypomania or mania | Mood Disorders Questionnaire Kiddie Schedule for Affective Disorders and Schizophrenia Mania Rating Scale |
| ADHD: attention-deficit/hyperactivity disorder; ARND: alcohol-related neurodevelopmental disorder; ODD: oppositional defiant disorder | ||
| Source: Reference 4 | ||
Mood swings: A chart review
We recently completed a retrospective chart review of 100 patients consecutively referred to our pediatric mood disorders clinic for evaluation of “mood swings, rule out BD.” These patients were self-referred, referred by a psychiatrist for a second opinion, or referred by their primary care physician. The mean age of these patients was 8±2.8 years and 68% were male.
Two experienced clinicians (RAK and EM) interviewed each patient and their caregivers and reviewed results of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L)5 and other outside information.
Figure 1 illustrates these patients’ diagnoses. Diagnoses for each of these disorders were made using DSM-IV-TR criteria.3
The most common diagnoses among patients with the chief complaint of mood swings were ADHD (39%); ODD with ADHD (15%); an anxiety disorder, usually generalized anxiety disorder (GAD) (15%); BD (12%); and a secondary mood disorder, usually fetal alcohol spectrum disorder (10%). We were surprised at how often ADHD, ODD, and anxiety disorders were found to be responsible for these patients’ mood swings and how frequently the referring clinician did not recognize these disorders. In the following sections, we discuss each of these disorders and how they differ from BD.
Figure 1 Underlying diagnoses of 100 children/adolescents referred for ‘mood swings’
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder; MDD: major depressive disorder; ODD: oppositional defiant disorder; PDD: pervasive developmental disorder
ADHD and ODD
In our sample, patients with undiagnosed ADHD made up the largest group of those with frequent mood swings. ADHD inattentive type was missed frequently in adolescent girls who still had behavioral aspects of ADHD, including impulsivity and aggression.6
The CPRS-R:L is useful for screening and diagnosing children and adolescents with ADHD and ODD. It contains 80 items, can be used in males and females and patients age 3 to 17, and has validated norms by age and sex.5 It takes parents approximately 10 minutes to fill out this questionnaire and the results can be scored by hand. The CPRS-R:L includes the following scales: oppositional; cognitive problems/inattention; hyperactivity; anxious-shy; perfectionism; social problems; psychosomatic; Connors’ global index; DSM-IV symptom subscales; and an ADHD index. Patients with mood swings and ADHD combined typically score >2 standard deviations above their age/sex mean on the CPRS-R:L hyperactivity scale, Connors’ Global Index, and ADHD index.5
A common childhood disorder, ODD has multiple etiologies.7 The first DSM-IV criteria for ODD is “often loses temper”3—essentially mood swings that often are expressed behaviorally as anger and at times as aggressive outbursts.
Dodge and Cole8 categorized aggression as reactive (impulsivity with a high affective valence) or proactive (characterized by low arousal and premeditation, ie, predatory conduct disorder). Reactive aggression typically is an angry defensive response to frustration, threat, or provocation, whereas proactive aggression is deliberate, coercive behavior often used to obtain a goal.9 Reactive aggression is common among children with ADHD and ODD and typically begins as a mood swing that escalates into reactive aggressive behavior. In a study of 268 consecutively referred children and adolescents with ADHD and 100 community controls, Connor et al10 found significantly more reactive than proactive forms of aggression in ADHD patients.
It can be difficult to differentiate the moods swings and symptoms of ODD from those of pediatric BD. Mick et al11 found that severe irritability may be a diagnostic indicator of BD in children with ADHD. Using the Kiddie Schedule for Affective Disorders and Schizophrenia (epidemiologic version) structured diagnostic interview,12 they evaluated 274 children (mean age 10.8±3.2) with ADHD; 37% had no comorbid mood disorder, 36% had ADHD with depression, and 11% had ADHD with BD. Researchers characterized 3 types of irritability in these patients:
- ODD-type irritability characterized by a low frustration tolerance that is seen in ODD
- Mad/cranky irritability found in depressive disorders
- Super-angry/grouchy/cranky irritability with frequent, prolonged, and largely unprovoked anger episodes and characteristics of mania.
ODD-type irritability was common among all ADHD patients, was the least impairing type of irritability, and did not increase the risk of a mood disorder. Mad/cranky irritability was common only in children with ADHD and a mood disorder (depression or BD), was more impairing than ODD-type irritability, and was most predictive of unipolar depression. Super-angry/grouchy/cranky irritability was common only among children with ADHD and BD (77%), was the most impairing, and was predictive of both unipolar depression and BD. The type of irritability and clustering of DSM-IV manic symptoms best differentiated ADHD subjects from those with ADHD and BD. Figure 2 illustrates symptoms that differentiated patients with ADHD from those with ADHD and comorbid BD.11
A review of pharmacotherapy for aggression in children found the largest effects for methylphenidate for aggression in ADHD (mean effect size=0.9, combined N=844).13 Our clinical experience has been that pediatric patients with ADHD or ODD with ADHD often have high levels of reactive aggression that presents as mood swings, and aggressively treating ADHD often results in improved mood and other ADHD symptoms.
Figure 2 Symptoms that differentiate BD from BD with comorbid ADHD
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
Source: Reference 11
Anxiety disorders
The estimated prevalence of child and adolescent anxiety disorders is 10% to 20%14; in our sample the prevalence was 15%. These disorders include GAD, separation anxiety disorder, social phobias, posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. Often, children with GAD worry excessively and become upset during transitions when things don’t proceed as they expect, with resultant angry outbursts and mood swings. Mood swings and difficulty sleeping are common in children with anxiety disorders or BD. Anxiety disorders often will be missed unless specific triggers of the mood swings or angry outbursts—as well as differentiating symptoms such as excessive fear, worry, and psychosomatic symptoms—are assessed.
In our clinical experience, simply asking a child if he or she is anxious is not sufficient to uncover an anxiety disorder. Although the CPRS-L:R will screen for anxiety disorders, we have found that the Self-Report for Childhood Anxiety Related Disorders (SCARED) developed by Birmaher et al15 is more specific. This tool can be used in patients age ≥8. The parent and child versions of the SCARED contain 41 items that measure 5 factors:
- general anxiety
- separation anxiety
- social phobia
- school phobia
- physical symptoms of anxiety.
The SCARED takes 5 minutes to fill out and is available in parent and child versions.
Secondary mood disorders
Many patients in our sample had a mood disorder secondary to the neurologic effects of alcohol on the developing brain. For more about maternal alcohol use, fetal alcohol spectrum disorders, and mood swings, visit this article at CurrentPsychiatry.com.
What BD looks like in children
In our sample, 12% of patients referred for mood swings were diagnosed with bipolar I disorder (BDI), bipolar II disorder (BDII), or bipolar disorder, not otherwise specified (BD-NOS). In the United States, lifetime prevalence of BDI and BDII in adolescents age 13 to 17 is 2.9%.16 No large epidemiologic studies have looked at the lifetime prevalence of BD in children age <13.
How often a clinician sees BD in children and adolescents largely depends on the type of setting in which he or she practices. Although in the general population BD is relatively rare compared with other childhood psychiatric disorders, on child/adolescent inpatient units it is common to find that 30% to 40% of patients have BD.17
The best longitudinal study to date of the phenomenology, comorbidity, and outcome of BD in children and adolescents is the National Institute of Mental Health-funded Course and Outcome of Bipolar Youth study (COBY).18 In this ongoing, longitudinal study, 413 youths (age 7 to 17) with BDI (N=244), BDII (N=28), or BD-NOS (N=141) were rigorously diagnosed using state-of-the-art measures, including the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present version19 and re-evaluated every 9.4 months for 4 years. When organizing this study, investigators found that DSM-IV criteria for BD-NOS were too vague to be useful and developed their own criteria (Table 2).18
For BDI patients in the COBY study, the mean age of onset for bipolar symptoms was 9.0±4.1 years and the mean duration of illness was 4.4±3.1 years. Researchers reported that at the 4-year assessment approximately 70% of patients with BD recovered from their index episode, and 50% had at least 1 syndromal recurrence, particularly depressive episodes.20 Analyses of these patients’ weekly mood symptoms showed that they had syndromal or subsyndromal symptoms with numerous changes in symptoms and shifts of mood polarity 60% of the time, and psychosis 3% of the time. During this study, 20% of BDII patients progressed to BDI, and 25% of BD-NOS patients converted to BDI or BDII.
Further analysis of the COBY data revealed that onset of mood symptoms preceded onset of clear bipolar episodes by an average of 1.0±1.7 years. Depression was the most common initial and most frequent episode for adolescents; mood lability was seen more often in childhood-onset and adolescents with early-onset BD. Depressed children had more severe irritability than depressed adolescents, and older age was associated with more severe and typical mood symptomatology.21
The clinical picture of a child with BD that emerges from the COBY study is:
- a fairly young child with the onset of mood symptoms between age 5 to 12
- subsyndromal and less frequently clear syndromal episodes
- primarily mixed and depressed symptoms with rapid mood cycles during these episodes.22
It is clear that there is a spectrum of bipolar disorders in children and adolescents with varying degrees of symptom expression and children differ from adolescents and adults in their initial presentation of BD.
Table 2
COBY criteria for bipolar disorder, not otherwise specified
| Presence of clinically relevant bipolar symptoms that do not fulfill DSM-IV criteria for BDI or BDII |
| In addition, patients are required to have elevated mood plus 2 associated DSM-IV symptoms or irritable mood plus 3 DSM-IV associated symptoms, along with a change in level of functioning |
| Duration of a minimum of 4 hours within a 24-hour period |
| At least 4 cumulative lifetime days meeting the criteria |
| BDI: bipolar I disorder; BDII: bipolar II disorder; COBY: Course and Outcome of Bipolar Youth study |
| Source: Reference 18 |
Related Resources
- Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Goodwin FK, Jamison KR. Manic-depressive illness. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2007.
- Miklowitz DJ, Cicchetti D, eds. Understanding bipolar disorder: a developmental perspective. New York, NY: Guilford Press; 2010.
Drug Brand Name
- Methylphenidate • Ritalin, Concerta, others
Disclosures
Dr. Kowatch receives grant/research support from the National Institute of Child Health and Human Development and the National Institute of Mental Health and is a consultant to AstraZeneca, Forest Pharmaceuticals, Merck, and the REACH Foundation.
Dr. Delgado and Ms. Monroe report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
FIND criteria of disorders found to cause mood swings
| Criteria | BDI | BP-NOS | ODD | GAD | ARND |
|---|---|---|---|---|---|
| Frequency of symptoms/week | 7 days (more days than not in an average week) | 2 to 3 days/week | Daily (chronic) irritability and mood swings precipitated by ‘not getting their way’ | Greatest during times of change/stress | Daily |
| Intensity of symptoms | Severe—parents often are afraid to take the child out in public because of mood symptoms | Moderate | Mild/moderate | Mild/moderate when stressed | Mild/moderate |
| Number of mood cycles/day | Daily cycles of euphoria and depression | 3 to 4 | 5 to 10 | 2 to 3 | 8 to 10 |
| Duration of symptoms/day | Euphoria: 30 to 60 minutes Depression: 30 minutes to 6 hours | 4 hours total/day of mood symptoms | Short; 5 to 10 minutes | Short; 5 to 10 minutes | Short; 5 to 10 minutes |
| ARND: alcohol-related neurodevelopmental disorder; BDI: bipolar I disorder; BD-NOS: bipolar disorder, not otherwise specified; GAD: generalized anxiety disorder; ODD: oppositional defiant disorder | |||||
| Source: Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008 | |||||
Even small amounts of alcohol use by a pregnant woman can impact her child’s development. In a controlled study examining drinking behavior of 12,678 pregnant women and the effect this had on their children, Sayel et ala found that <1 drink per week during the first trimester was clinically significant for mental health problems in girls, measured at age 4 and 8, when using parent or teacher report.
Fetal alcohol spectrum disorder describes the range of effects that can occur in an individual whose mother drank alcohol during pregnancy. These disorders include fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD).
FAS. Individuals with FAS have a distinct pattern of facial abnormalities, growth deficiency, and evidence of CNS dysfunction. Characteristic facial abnormalities may include a smooth philtrum, thin upper lip, upturned nose, flat nasal bridge and midface, epicanthal folds, small palpebral fissures, and small head circumference. Growth deficiency begins in-utero and continues throughout childhood and into adulthood. CNS abnormalities can include impaired brain growth or abnormal structure, manifested differently depending on age.
ARND. Many individuals affected by alcohol exposure before birth do not have the characteristic facial abnormalities and growth retardation identified with full FAS, yet have significant brain and behavioral impairments. Individuals with ARND have either the facial anomalies, growth retardation, and other physical abnormalities, or a complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level and unexplained by genetic background or environmental conditions (ie, poor impulse control, language deficits, problems with abstraction, mathematical and social perception deficits, learning problems, and impairment in attention, memory, or judgment).b
ARBD. Persons with ARBD have malformations of the skeletal and major organ systems, such as cardiac or renal abnormalities.
Comorbid psychiatric conditions in children with prenatal alcohol exposure are 5 to 16 times more prevalent than in the general population; these children are 38% more likely to have an anger disorder.c O’Connor and Paleyd found that “…mood disorder symptoms were significantly higher for children with parental alcohol exposure compared to children without exposure.” Children with ARND are treated symptomatically depending upon which deficits and behaviors they exhibit.e
References
a. Sayal K, Heron J, Golding J, et al. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009;123(2):e289-296.
b. Warren KR, Foudin LL. Alcohol-related birth defects—the past, present, and future. Alcohol Res Health. 2001;25(3):153-158.
c. Burd L, Klug MG, Martsolf JT, et al. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol. 2003;25(6):697-705.
d. O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15(3):225-234.
e. Paley B, O’Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15(3):258-267.
M, age 13, is referred by her pediatrician with the chief complaint of “severe mood swings, rule out bipolar disorder (BD).” In the past she was treated for attention-deficit/hyperactivity disorder (ADHD) with stimulants with mixed results. M’s parents are concerned about her “flipping out” whenever she is asked to do something she does not want to do. Her mother has a history of depression and anxiety; her father had a “drinking problem.” There is no history of BD in her first- or second-degree relatives. Are M’s rapid mood swings a sign of BD or another disorder?
The differential diagnosis of “mood swings” is important because they are a common presenting symptom of many children and adolescents with mood and behavioral disorders. Mood swings often occur in children and adolescents with ADHD, oppositional defiant disorder (ODD), developmental disorders, depressive disorders, BD, anxiety disorders, and conduct disorders. Mood swings are analogous to a fever in pediatrics—they indicate something potentially is wrong with the patient, but are not diagnostic as an isolated symptom.
Mood swings in children are common, nonspecific symptoms that more often are a sign of anxiety or behavioral disorders than BD. This article discusses the differential diagnosis of mood swings in children and adolescents and how to best screen and diagnose these patients.
What are ‘mood swings’?
Mood swings is a popular term that is nonspecific and not part of DSM-IV-TR diagnostic criteria for BD. The complaint of “mood swings” may reflect severe mood lability of pediatric patients with BD. This mood lability is best described by the Kiddie-Mania Rating Scale (K-MRS) developed by Axelson and colleagues as “rapid mood variation with several mood states within a brief period of time which appears internally driven without regard to the circumstance.”1 On K-MRS mood lability items, children with mania typically score:
- Moderate—many mood changes throughout the day, can vary from elevated mood to anger to sadness within a few hours; changes in mood are clearly out of proportion to circumstances and cause impairment in functioning
- Severe—rapid mood swings nearly all of the time, with mood intensity greatly out of proportion to circumstances
- Extreme—constant, explosive variability in mood, several mood changes occurring within minutes, difficult to identify a particular mood, changes in mood radically out of proportion to circumstances.
Patients with BD typically exhibit what is best described as a “mood cycle”—a pronounced shift in mood and energy from 1 extreme to another.2 An example of this would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours and then later in the day becomes sad, depressed, and suicidal with no precipitant for either mood cycle. BD patients also will exhibit other symptoms of mania during these mood cycling periods.
Rapid cycling is a DSM-IV course specifier that indicates ≥4 mood episodes per year in patients with BD with a typical course of mania or hypomania followed by depression, or vice versa.3 The episodes must be demarcated by full or partial remission that lasts ≥2 months or by a switch to a mood state of opposite polarity. In the past, children with frequent mood swings were described incorrectly as “rapid cycling,” but this term has been dropped because it engenders confusion between adult and pediatric BD phenomenology.2
A more precise method of describing mood symptoms in a child or adolescent is to use the FIND criteria, which include:4
- Frequency of symptoms per week
- Intensity of mood symptoms
- Number of mood cycles per day
- Duration of symptoms per day.
Visit this article at CurrentPsychiatry.com to view a table that outlines what to look for when using the FIND criteria to evaluate common pediatric psychiatric disorders that include mood swings. Table 1
describes clinical characteristics and tools and resources used to differentiate these and other disorders.4
Table 1
Clinical characteristics of psychiatric disorders that often feature mood swings
| Disorder | Clinical description | Useful tools/resources |
|---|---|---|
| ADHD | Chronic symptoms of hyperactivity, distractibility, impulsivity, poor attentional skills, disorganization | Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) |
| ODD | Chronic symptoms of oppositionality, negativity; short, frequent mood swings in response to being asked to do something they do not want to do | CPRS-R:L |
| Anxiety disorders | Excessive ‘worry,’ difficulty with transitions, increased mood swings during stressful periods, psychosomatic symptoms | Self-Report for Childhood Anxiety Related Disorders |
| ARND | History of exposure to alcohol in-utero; mild dysmorphia, attentional, mood, and executive functioning problems | National Organization on Fetal Alcohol Syndrome |
| Bipolar disorder | In children: clustering together of episodes or ‘mini-episodes’ (several days) of increased energy, decreased need for sleep, increased mood cycling, pressured speech, etc. In adolescents: depressive episodes with episodes of hypomania or mania | Mood Disorders Questionnaire Kiddie Schedule for Affective Disorders and Schizophrenia Mania Rating Scale |
| ADHD: attention-deficit/hyperactivity disorder; ARND: alcohol-related neurodevelopmental disorder; ODD: oppositional defiant disorder | ||
| Source: Reference 4 | ||
Mood swings: A chart review
We recently completed a retrospective chart review of 100 patients consecutively referred to our pediatric mood disorders clinic for evaluation of “mood swings, rule out BD.” These patients were self-referred, referred by a psychiatrist for a second opinion, or referred by their primary care physician. The mean age of these patients was 8±2.8 years and 68% were male.
Two experienced clinicians (RAK and EM) interviewed each patient and their caregivers and reviewed results of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L)5 and other outside information.
Figure 1 illustrates these patients’ diagnoses. Diagnoses for each of these disorders were made using DSM-IV-TR criteria.3
The most common diagnoses among patients with the chief complaint of mood swings were ADHD (39%); ODD with ADHD (15%); an anxiety disorder, usually generalized anxiety disorder (GAD) (15%); BD (12%); and a secondary mood disorder, usually fetal alcohol spectrum disorder (10%). We were surprised at how often ADHD, ODD, and anxiety disorders were found to be responsible for these patients’ mood swings and how frequently the referring clinician did not recognize these disorders. In the following sections, we discuss each of these disorders and how they differ from BD.
Figure 1 Underlying diagnoses of 100 children/adolescents referred for ‘mood swings’
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder; MDD: major depressive disorder; ODD: oppositional defiant disorder; PDD: pervasive developmental disorder
ADHD and ODD
In our sample, patients with undiagnosed ADHD made up the largest group of those with frequent mood swings. ADHD inattentive type was missed frequently in adolescent girls who still had behavioral aspects of ADHD, including impulsivity and aggression.6
The CPRS-R:L is useful for screening and diagnosing children and adolescents with ADHD and ODD. It contains 80 items, can be used in males and females and patients age 3 to 17, and has validated norms by age and sex.5 It takes parents approximately 10 minutes to fill out this questionnaire and the results can be scored by hand. The CPRS-R:L includes the following scales: oppositional; cognitive problems/inattention; hyperactivity; anxious-shy; perfectionism; social problems; psychosomatic; Connors’ global index; DSM-IV symptom subscales; and an ADHD index. Patients with mood swings and ADHD combined typically score >2 standard deviations above their age/sex mean on the CPRS-R:L hyperactivity scale, Connors’ Global Index, and ADHD index.5
A common childhood disorder, ODD has multiple etiologies.7 The first DSM-IV criteria for ODD is “often loses temper”3—essentially mood swings that often are expressed behaviorally as anger and at times as aggressive outbursts.
Dodge and Cole8 categorized aggression as reactive (impulsivity with a high affective valence) or proactive (characterized by low arousal and premeditation, ie, predatory conduct disorder). Reactive aggression typically is an angry defensive response to frustration, threat, or provocation, whereas proactive aggression is deliberate, coercive behavior often used to obtain a goal.9 Reactive aggression is common among children with ADHD and ODD and typically begins as a mood swing that escalates into reactive aggressive behavior. In a study of 268 consecutively referred children and adolescents with ADHD and 100 community controls, Connor et al10 found significantly more reactive than proactive forms of aggression in ADHD patients.
It can be difficult to differentiate the moods swings and symptoms of ODD from those of pediatric BD. Mick et al11 found that severe irritability may be a diagnostic indicator of BD in children with ADHD. Using the Kiddie Schedule for Affective Disorders and Schizophrenia (epidemiologic version) structured diagnostic interview,12 they evaluated 274 children (mean age 10.8±3.2) with ADHD; 37% had no comorbid mood disorder, 36% had ADHD with depression, and 11% had ADHD with BD. Researchers characterized 3 types of irritability in these patients:
- ODD-type irritability characterized by a low frustration tolerance that is seen in ODD
- Mad/cranky irritability found in depressive disorders
- Super-angry/grouchy/cranky irritability with frequent, prolonged, and largely unprovoked anger episodes and characteristics of mania.
ODD-type irritability was common among all ADHD patients, was the least impairing type of irritability, and did not increase the risk of a mood disorder. Mad/cranky irritability was common only in children with ADHD and a mood disorder (depression or BD), was more impairing than ODD-type irritability, and was most predictive of unipolar depression. Super-angry/grouchy/cranky irritability was common only among children with ADHD and BD (77%), was the most impairing, and was predictive of both unipolar depression and BD. The type of irritability and clustering of DSM-IV manic symptoms best differentiated ADHD subjects from those with ADHD and BD. Figure 2 illustrates symptoms that differentiated patients with ADHD from those with ADHD and comorbid BD.11
A review of pharmacotherapy for aggression in children found the largest effects for methylphenidate for aggression in ADHD (mean effect size=0.9, combined N=844).13 Our clinical experience has been that pediatric patients with ADHD or ODD with ADHD often have high levels of reactive aggression that presents as mood swings, and aggressively treating ADHD often results in improved mood and other ADHD symptoms.
Figure 2 Symptoms that differentiate BD from BD with comorbid ADHD
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
Source: Reference 11
Anxiety disorders
The estimated prevalence of child and adolescent anxiety disorders is 10% to 20%14; in our sample the prevalence was 15%. These disorders include GAD, separation anxiety disorder, social phobias, posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. Often, children with GAD worry excessively and become upset during transitions when things don’t proceed as they expect, with resultant angry outbursts and mood swings. Mood swings and difficulty sleeping are common in children with anxiety disorders or BD. Anxiety disorders often will be missed unless specific triggers of the mood swings or angry outbursts—as well as differentiating symptoms such as excessive fear, worry, and psychosomatic symptoms—are assessed.
In our clinical experience, simply asking a child if he or she is anxious is not sufficient to uncover an anxiety disorder. Although the CPRS-L:R will screen for anxiety disorders, we have found that the Self-Report for Childhood Anxiety Related Disorders (SCARED) developed by Birmaher et al15 is more specific. This tool can be used in patients age ≥8. The parent and child versions of the SCARED contain 41 items that measure 5 factors:
- general anxiety
- separation anxiety
- social phobia
- school phobia
- physical symptoms of anxiety.
The SCARED takes 5 minutes to fill out and is available in parent and child versions.
Secondary mood disorders
Many patients in our sample had a mood disorder secondary to the neurologic effects of alcohol on the developing brain. For more about maternal alcohol use, fetal alcohol spectrum disorders, and mood swings, visit this article at CurrentPsychiatry.com.
What BD looks like in children
In our sample, 12% of patients referred for mood swings were diagnosed with bipolar I disorder (BDI), bipolar II disorder (BDII), or bipolar disorder, not otherwise specified (BD-NOS). In the United States, lifetime prevalence of BDI and BDII in adolescents age 13 to 17 is 2.9%.16 No large epidemiologic studies have looked at the lifetime prevalence of BD in children age <13.
How often a clinician sees BD in children and adolescents largely depends on the type of setting in which he or she practices. Although in the general population BD is relatively rare compared with other childhood psychiatric disorders, on child/adolescent inpatient units it is common to find that 30% to 40% of patients have BD.17
The best longitudinal study to date of the phenomenology, comorbidity, and outcome of BD in children and adolescents is the National Institute of Mental Health-funded Course and Outcome of Bipolar Youth study (COBY).18 In this ongoing, longitudinal study, 413 youths (age 7 to 17) with BDI (N=244), BDII (N=28), or BD-NOS (N=141) were rigorously diagnosed using state-of-the-art measures, including the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present version19 and re-evaluated every 9.4 months for 4 years. When organizing this study, investigators found that DSM-IV criteria for BD-NOS were too vague to be useful and developed their own criteria (Table 2).18
For BDI patients in the COBY study, the mean age of onset for bipolar symptoms was 9.0±4.1 years and the mean duration of illness was 4.4±3.1 years. Researchers reported that at the 4-year assessment approximately 70% of patients with BD recovered from their index episode, and 50% had at least 1 syndromal recurrence, particularly depressive episodes.20 Analyses of these patients’ weekly mood symptoms showed that they had syndromal or subsyndromal symptoms with numerous changes in symptoms and shifts of mood polarity 60% of the time, and psychosis 3% of the time. During this study, 20% of BDII patients progressed to BDI, and 25% of BD-NOS patients converted to BDI or BDII.
Further analysis of the COBY data revealed that onset of mood symptoms preceded onset of clear bipolar episodes by an average of 1.0±1.7 years. Depression was the most common initial and most frequent episode for adolescents; mood lability was seen more often in childhood-onset and adolescents with early-onset BD. Depressed children had more severe irritability than depressed adolescents, and older age was associated with more severe and typical mood symptomatology.21
The clinical picture of a child with BD that emerges from the COBY study is:
- a fairly young child with the onset of mood symptoms between age 5 to 12
- subsyndromal and less frequently clear syndromal episodes
- primarily mixed and depressed symptoms with rapid mood cycles during these episodes.22
It is clear that there is a spectrum of bipolar disorders in children and adolescents with varying degrees of symptom expression and children differ from adolescents and adults in their initial presentation of BD.
Table 2
COBY criteria for bipolar disorder, not otherwise specified
| Presence of clinically relevant bipolar symptoms that do not fulfill DSM-IV criteria for BDI or BDII |
| In addition, patients are required to have elevated mood plus 2 associated DSM-IV symptoms or irritable mood plus 3 DSM-IV associated symptoms, along with a change in level of functioning |
| Duration of a minimum of 4 hours within a 24-hour period |
| At least 4 cumulative lifetime days meeting the criteria |
| BDI: bipolar I disorder; BDII: bipolar II disorder; COBY: Course and Outcome of Bipolar Youth study |
| Source: Reference 18 |
Related Resources
- Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Goodwin FK, Jamison KR. Manic-depressive illness. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2007.
- Miklowitz DJ, Cicchetti D, eds. Understanding bipolar disorder: a developmental perspective. New York, NY: Guilford Press; 2010.
Drug Brand Name
- Methylphenidate • Ritalin, Concerta, others
Disclosures
Dr. Kowatch receives grant/research support from the National Institute of Child Health and Human Development and the National Institute of Mental Health and is a consultant to AstraZeneca, Forest Pharmaceuticals, Merck, and the REACH Foundation.
Dr. Delgado and Ms. Monroe report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
FIND criteria of disorders found to cause mood swings
| Criteria | BDI | BP-NOS | ODD | GAD | ARND |
|---|---|---|---|---|---|
| Frequency of symptoms/week | 7 days (more days than not in an average week) | 2 to 3 days/week | Daily (chronic) irritability and mood swings precipitated by ‘not getting their way’ | Greatest during times of change/stress | Daily |
| Intensity of symptoms | Severe—parents often are afraid to take the child out in public because of mood symptoms | Moderate | Mild/moderate | Mild/moderate when stressed | Mild/moderate |
| Number of mood cycles/day | Daily cycles of euphoria and depression | 3 to 4 | 5 to 10 | 2 to 3 | 8 to 10 |
| Duration of symptoms/day | Euphoria: 30 to 60 minutes Depression: 30 minutes to 6 hours | 4 hours total/day of mood symptoms | Short; 5 to 10 minutes | Short; 5 to 10 minutes | Short; 5 to 10 minutes |
| ARND: alcohol-related neurodevelopmental disorder; BDI: bipolar I disorder; BD-NOS: bipolar disorder, not otherwise specified; GAD: generalized anxiety disorder; ODD: oppositional defiant disorder | |||||
| Source: Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008 | |||||
Even small amounts of alcohol use by a pregnant woman can impact her child’s development. In a controlled study examining drinking behavior of 12,678 pregnant women and the effect this had on their children, Sayel et ala found that <1 drink per week during the first trimester was clinically significant for mental health problems in girls, measured at age 4 and 8, when using parent or teacher report.
Fetal alcohol spectrum disorder describes the range of effects that can occur in an individual whose mother drank alcohol during pregnancy. These disorders include fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD).
FAS. Individuals with FAS have a distinct pattern of facial abnormalities, growth deficiency, and evidence of CNS dysfunction. Characteristic facial abnormalities may include a smooth philtrum, thin upper lip, upturned nose, flat nasal bridge and midface, epicanthal folds, small palpebral fissures, and small head circumference. Growth deficiency begins in-utero and continues throughout childhood and into adulthood. CNS abnormalities can include impaired brain growth or abnormal structure, manifested differently depending on age.
ARND. Many individuals affected by alcohol exposure before birth do not have the characteristic facial abnormalities and growth retardation identified with full FAS, yet have significant brain and behavioral impairments. Individuals with ARND have either the facial anomalies, growth retardation, and other physical abnormalities, or a complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level and unexplained by genetic background or environmental conditions (ie, poor impulse control, language deficits, problems with abstraction, mathematical and social perception deficits, learning problems, and impairment in attention, memory, or judgment).b
ARBD. Persons with ARBD have malformations of the skeletal and major organ systems, such as cardiac or renal abnormalities.
Comorbid psychiatric conditions in children with prenatal alcohol exposure are 5 to 16 times more prevalent than in the general population; these children are 38% more likely to have an anger disorder.c O’Connor and Paleyd found that “…mood disorder symptoms were significantly higher for children with parental alcohol exposure compared to children without exposure.” Children with ARND are treated symptomatically depending upon which deficits and behaviors they exhibit.e
References
a. Sayal K, Heron J, Golding J, et al. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009;123(2):e289-296.
b. Warren KR, Foudin LL. Alcohol-related birth defects—the past, present, and future. Alcohol Res Health. 2001;25(3):153-158.
c. Burd L, Klug MG, Martsolf JT, et al. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol. 2003;25(6):697-705.
d. O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15(3):225-234.
e. Paley B, O’Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15(3):258-267.
1. Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463-470.
2. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10(1 Pt 2):194-214.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Conners CK. Conners’ Parent Rating Scale Long Form (CPRS-R:L) North Tonawanda, NY: Multi-Health Systems, Inc.; 1997.
6. Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50(9):1042-1051.
7. Steiner H, Remsing L. and the Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
8. Dodge KA, Cole JD. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Pers Soc Psychol. 1987;53(6):1146-1158.
9. Connor DF, Steingard RJ, Cunningham JA, et al. Proactive and reactive aggression in referred children and adolescents. Am J Orthopsychiatry. 2004;74(2):129-136.
10. Connor DF, Chartier KG, Preen EC, et al. Impulsive aggression in attention-deficit/hyperactivity disorder: symptom severity, co-morbidity, and attention-deficit/hyperactivity disorder subtype. J Child Adolesc Psychopharmacol. 2010;20(2):119-126.
11. Mick E, Spencer T, Wozniak J, et al. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58(7):576-582.
12. Orvaschel H. Schizophrenia and Affective Disorders Schedule for children—Epidemiological Version (KSADS-E). Fort Lauderdale, FL: Nova Southeastern University; 1995.
13. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
14. Achenbach TM, Howell CT, McConaughy SH, et al. Six-year predictors of problems in a national sample: IV. Young adult signs of disturbance. J Am Acad Child Adolesc Psychiatry. 1998;37(7):718-727.
15. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545-553.
16. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
17. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7):712-717.
18. Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175-183.
19. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988.
20. Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18(4):1023-1035.
21. Birmaher B, Axelson D, Strober M, et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar Disord. 2009;11(1):52-62.
22. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
1. Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463-470.
2. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10(1 Pt 2):194-214.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Conners CK. Conners’ Parent Rating Scale Long Form (CPRS-R:L) North Tonawanda, NY: Multi-Health Systems, Inc.; 1997.
6. Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50(9):1042-1051.
7. Steiner H, Remsing L. and the Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
8. Dodge KA, Cole JD. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Pers Soc Psychol. 1987;53(6):1146-1158.
9. Connor DF, Steingard RJ, Cunningham JA, et al. Proactive and reactive aggression in referred children and adolescents. Am J Orthopsychiatry. 2004;74(2):129-136.
10. Connor DF, Chartier KG, Preen EC, et al. Impulsive aggression in attention-deficit/hyperactivity disorder: symptom severity, co-morbidity, and attention-deficit/hyperactivity disorder subtype. J Child Adolesc Psychopharmacol. 2010;20(2):119-126.
11. Mick E, Spencer T, Wozniak J, et al. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58(7):576-582.
12. Orvaschel H. Schizophrenia and Affective Disorders Schedule for children—Epidemiological Version (KSADS-E). Fort Lauderdale, FL: Nova Southeastern University; 1995.
13. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
14. Achenbach TM, Howell CT, McConaughy SH, et al. Six-year predictors of problems in a national sample: IV. Young adult signs of disturbance. J Am Acad Child Adolesc Psychiatry. 1998;37(7):718-727.
15. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545-553.
16. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
17. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7):712-717.
18. Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175-183.
19. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988.
20. Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18(4):1023-1035.
21. Birmaher B, Axelson D, Strober M, et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar Disord. 2009;11(1):52-62.
22. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
Clinical trials support new algorithm for treating pediatric bipolar mania
Five recent randomized controlled trials (RCTs) have demonstrated the efficacy of atypical antipsychotics for treating bipolar disorder in children and adolescents, but 4 of these 5 trials remain unpublished. The lag time between the completion of these trials and publication of their results—typically 4 to 5 years1—leaves psychiatrists without important evidence to explain to families and critics2 why they might recommend using these powerful medications in children with mental illness.
This article previews the preliminary results of these 5 RCTs of atypical antipsychotics, offers a treatment algorithm supported by this evidence, and discusses how to manage potentially serious risks when using antipsychotics to treat children and adolescents with bipolar disorder (BPD).
Where do atypical antipsychotics fit in?
Details of the 5 industry-sponsored RCTs of atypical antipsychotics in children and adolescents with bipolar I manic or mixed episodes are summarized in Table 1.3-7 Only the olanzapine study4 has been published; data from the other 4 trials were presented at medical meetings in 2007 and 2008.
Change in Young Mania Rating Scale (YMRS) score was the primary outcome measure in these 5 trials, and each compound was more effective than placebo. The trials demonstrated statistically significant and clinically relevant differences between each antipsychotic and placebo. The number needed to treat (NNT)—how many patients need to be treated for 1 to benefit in a controlled clinical trial—ranged from 2 to 4. For comparison, the NNT for statins in the prevention of coronary events is 12 to 22,8 and the NNT in an analysis of trials of selective serotonin reuptake inhibitors for pediatric major depressive disorder was 9.9 Thus, an NNT of ≤4 represents a clinically significant effect.
Risperidone is FDA-approved for short-term treatment of acute bipolar I manic or mixed episodes in patients age 10 to 17. Aripiprazole is approved for acute and maintenance treatment of bipolar I manic or mixed episodes (with or without psychosis) as monotherapy or with lithium or valproate in patients age 10 to 17. In June, an FDA advisory committee recommended pediatric bipolar indications for olanzapine, quetiapine, and ziprasidone.
‘Mood stabilizers’ such as lithium, valproate, and carbamazepine have been used for years to treat bipolar mania in adults, adolescents, and children, despite limited supporting evidence. Preliminary results of a National Institute of Mental Health-funded double-blind RCT provide insights on their efficacy.10
The 153 outpatients age 7 to 17 in a bipolar I manic or mixed episode were randomly assigned to lithium, divalproex, or placebo for 8 weeks. Response rates—based on a Clinical Global Impressions-Improvement score of 1 or 2 (very much or much improved)—were divalproex, 54%; lithium, 42%; and placebo, 29%. Lithium showed a trend toward efficacy but did not clearly separate from placebo on the primary outcome measures. Effect sizes for lithium and divalproex were moderate.10
Only 1 study has compared a mood stabilizer with an atypical antipsychotic for treating mania in adolescents. In a double-blind trial, DelBello et al11 randomly assigned 50 patients age 12 to 18 with a bipolar I manic or mixed episode to quetiapine, 400 to 600 mg/d, or divalproex, serum level 80 to 120 μg/mL, for 28 days. Manic symptoms resolved more rapidly, and remission rates measured by the YMRS were higher with quetiapine than with divalproex. Both medications were well tolerated.
Combination therapy. BPD as it presents in children and adolescents is often difficult to treat because of the disorder’s various phases (manic, depressed, mixed), frequent psychotic symptoms, and high rate of comorbidity. Pediatric BPD patients frequently require several psychotropics, including mood stabilizers and atypical antipsychotics.
In a double-blind, placebo-controlled study, 30 adolescents in a bipolar I manic or mixed episode initially received divalproex, 20 mg/kg/d, then were randomly assigned to 6 weeks of adjunctive quetiapine, titrated to 450 mg/d in 7 days (n=15), or placebo (n=15). Those receiving divalproex plus quetiapine showed a statistically significant greater reduction in manic symptoms (P=.03) and a higher response rate (87% vs 53%, P=.05), compared with those receiving divalproex and placebo. This suggests that a mood stabilizer plus an atypical antipsychotic is more effective than a mood stabilizer alone for adolescent mania. Quetiapine was well tolerated.12
Treatment. The American Psychiatric Association’s outdated 2002 practice guideline for acute bipolar I manic or mixed episodes in adults recommends lithium, valproate, and/or an antipsychotic.13 The more recent Texas Medication Algorithm Project (TMAP) guidelines recommend monotherapy with lithium, valproate, aripiprazole, quetiapine, risperidone, or ziprasidone for adults with euphoric or irritable manic or hypomanic symptoms.14
Based on the TMAP algorithm, recent clinical trial evidence, and our experience in treating pediatric BPD, we offer an approach for treating mania/hypomania in patients age 10 to 17 (see Proposed Algorithm). For dosing and precautions when using atypical antipsychotics in children and adolescents with BPD, see Table 2.15-17
Comorbid psychiatric illnesses (such as anxiety disorders) are prevalent in adolescents with BPD. Evidence in adults and adolescents suggests that some atypical antipsychotics may provide additional benefit for these conditions as well. Thus, consider comorbid conditions and symptoms when choosing antimanic agents.
Attention-deficit/hyperactivity disorder (ADHD) is a common comorbidity in children with BPD, and stimulant medications are most often prescribed to treat inattentiveness and hyperactivity. Caution is imperative when treating bipolar youth with stimulants, which can exacerbate manic symptoms. Treat the patient’s mania before adding or reintroducing stimulant medication. Research and clinical experience suggest that if you first stabilize these patients on a mood stabilizer or atypical antipsychotic, adding a stimulant can be very helpful in treating comorbid ADHD symptoms. Start with low stimulant doses, and increase slowly.
Table 1
RCTs of atypical antipsychotics in patients age 10 to 17
with bipolar I disorder*
| Antipsychotic and source | Bipolar I episode (# of subjects) | Trial duration (days) | Dosage (mg/d) | Response rate or YMRS score change | NNT | Mean weight gain (kg) |
|---|---|---|---|---|---|---|
| Risperidone Pandina et al3 AACAP 2007 | Manic, mixed (169) | 21 | 0.5 to 2.5 3 to 6 | 59% 63% | 3.3 3.5 | 1.9 1.4 |
| Olanzapine Tohen et al4 | Manic, mixed (161) | 21 | 10.4 ± 4.5 | 49% | 4.1 | 3.7 ± 2.2 |
| Quetiapine DelBello et al5 AACAP 2007 | Manic (284) | 21 | 400 600 | 64% 58% | 4.4 4.2 | 1.7 1.7 |
| Aripiprazole Wagner et al6 ACNP 2007 | Manic, mixed (296) | 28 | 10 30 | 45% 64% | 4.1 2.4 | 0.9 0.54 |
| Ziprasidone DelBello et al7 APA 2008 | Manic, mixed (238) | 28 | 80 to 160 | –13.83 with ziprasidone, –8.61 with placebo | 3.7 | None |
| *Each trial included a 6-month open extension phase; results are pending | ||||||
| AACAP: American Academy of Child and Adolescent Psychiatry; ACNP: American College of Neuropsychopharmacology; APA: American Psychiatric Association; NNT: number needed to treat; RCT: randomized controlled trial; YMRS: Young Mania Rating Scale | ||||||
Table 2
Recommended antipsychotic use in pediatric bipolar disorder
| Drug | Starting dosage (mg) | Target dosage (mg/d) | Precautions |
|---|---|---|---|
| Aripiprazole | 2.5 to 5 at bedtime | 10 to 30 | Monitor for CYP 3A4 and 2D6 interactions, weight, BMI, cholesterol, lipids, and glucose |
| Olanzapine | 2.5 bid | 10 to 20 | Monitor for CYP 2D6 interactions, weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Quetiapine | 50 bid | 400 to 1,200 | Monitor for weight, BMI, cholesterol, lipids, and glucose |
| Risperidone | 0.25 bid | 1 to 2.5 | Monitor for EPS, hyperprolactinemia (and associated sexual side effects, including galactorrhea), weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Ziprasidone | 20 bid | 80 to 160 | Check baseline ECG and as dose increases or with reason for high level of concern; monitor prolactin levels |
| BMI: body mass index; CYP: cytochrome P450; ECG: electrocardiography; EPS: extrapyramidal symptoms | |||
| Source: References 15-17 | |||
Proposed Algorithm: Treating a bipolar mixed/manic episode in patients age 10 to 17
Stage 1. Consider patient’s experience with antipsychotics, body weight, and family history when choosing first-line monotherapy (1A). Quetiapine poses low risk for extrapyramidal symptoms and tardive dyskinesia. Aripiprazole and ziprasidone pose relatively low risk of weight gain. Risperidone is potent at low doses but increases prolactin levels (long-term effect unknown).
Second-line choices (1B) are mood stabilizers lithium and valproate (because of lower potency than atypical antipsychotics), and olanzapine (which—although potent—causes substantial weight gain). In case of lack of response or intolerable side effects with initial agent, select an alternate from 1A or 1B. If this is not effective, move to Stage 2.
Stage 2. Consider augmentation for patients who show partial response to monotherapy (in your clinical judgment “mild to moderately improved” but not “much or very much improved”).
Stage 3. Combination therapy could include 2 mood stabilizers (such as lithium and valproate) plus an atypical antipsychotic; 2 atypical antipsychotics; or other combinations based on patient’s past responses. No research has shown these combinations to be efficacious in bipolar children and adolescents, but we find they sometimes help those with treatment-resistant symptoms.
Duration. Maintain psychotropics 12 to 18 months. When patient is euthymic, slowly taper 1 medication across several months. If symptoms recur, reintroduce the mood-stabilizing agent(s).
Source: Adapted and reprinted with permission from Kowatch RA, Fristad MA, Findling R, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008
Managing adverse effects
Although clinically effective, atypical antipsychotics may cause serious side effects that must be recognized and managed. These include extrapyramidal symptoms (EPS), tardive dyskinesia (TD), weight gain and obesity, hyperlipidemia, increased prolactin levels, and QTc changes. Counsel patients and families about the risks and benefits of antipsychotics when you consider them for children and adolescents with BPD (Table 3).
EPS. Drug-induced parkinsonism and akathisia are the most common EPS in children and adolescents with BPD treated with atypical antipsychotics.18
Correll et al19 reported a 10% rate of EPS in patients treated with aripiprazole. Treatment-emergent EPS also was observed in the RCT of risperidone.20 EPS-related adverse events were associated with higher doses of risperidone, although none of the akathisia/EPS measures were thought to be “clinically significant.”
EPS frequency was relatively low and similar to placebo in the 3-week quetiapine trial,21 and no changes in movement disorder scale scores were observed during the olanzapine or ziprasidone RCTs.4,7
Recommendations. If your pediatric patient develops EPS, first try an antipsychotic dose reduction. Because anticholinergics can contribute to antipsychotic-induced weight gain, reserve them until after a dosage reduction has been unsuccessful.
Benztropine (0.25 to 0.5 mg given 2 to 3 times daily, not to exceed 3 mg/d) or diphenhydramine (25 to 50 mg given 3 to 4 times daily; maximum dosage 5 mg/kg/d) can be effective in treating EPS. Avoid anticholinergics in children with narrow-angle glaucoma or age <3.
Akathisia may be managed with propranolol (20 to 120 mg/d in divided doses). Multiple doses (typically 3 times daily) are needed to prevent interdose withdrawal symptoms. Use this beta blocker with caution in children with asthma because of the possibility of bronchospasm.
TD. Short-term trials and a meta-analysis of atypical antipsychotic trials (>11 months’ duration, subject age <18) suggest a low annual risk for TD (0.4%).22 Large, prospective, long-term trials of atypical antipsychotics are necessary to more accurately define the risk of TD in the pediatric population, however. Retrospective analyses of adolescents treated with antipsychotics suggest 3 TD risk factors:
- early age of antipsychotic use
- medication nonadherence
- concomitant use of antiparkinsonian agents.23
Kumra et al24 identified lower premorbid functioning and greater positive symptoms at baseline as factors associated with “withdrawal dyskinesia/tardive dyskinesia” in children and adolescents with early-onset psychotic-spectrum disorders treated with typical or atypical antipsychotics.
Recommendations. To minimize TD risk, use the lowest effective antipsychotic dose, monitor for abnormal involuntary movements with standardized assessments (such as the Abnormal Involuntary Movement Scale), review risks and benefits with parents and patients, and regularly evaluate the indication and need for antipsychotic therapy. It is reasonable to attempt to lower the antipsychotic dose after the patient has attained remission and been stable for 1 year.
Neuroleptic malignant syndrome (NMS). This complication of dopamine-blocking medications:
- is among the most serious adverse effects of antipsychotic treatment
- continues to be associated with a mortality rate of 10%.25
Recommendation. At least 1 recent review of pediatric NMS cases suggests that essential features (hyperthermia and severe muscular rigidity) are retained in children.26 Nonetheless, monitor for variant presentations; hyper thermia or muscle rigidity may be absent or develop slowly over several days in patients treated with atypical antipsychotics.27
Weight gain and glucose metabolism. A major adverse effect of most atypical antipsychotics is increased appetite, weight gain, and possible obesity.28 In children, “obesity” refers to a body mass index (BMI) >95th percentile for age and sex; “over-weight” refers to BMI between the 85th and 95th percentile. Mean weight gain in the 5 atypical antipsychotic pediatric bipolar trials ranged from 0 to 8 lbs across 3 to 4 weeks of treatment (Figure).3-7
Recommendations. Emphasize diet and exercise, with restriction of high-carbohydrate food, “fast foods,” and soft drinks. Another option is a trial of metformin, which decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization.
Klein et al29 studied 39 patients age 10 to 17 with mood and psychotic disorders whose weight increased by >10% during <1 year of olanzapine, risperidone, or quetiapine therapy. In this 16-week, double-blind, placebo-controlled trial, weight was stabilized in subjects receiving metformin, whereas those receiving placebo continued to gain weight (0.31 kg [0.68 lb]/week).
The usual starting metformin dose is 500 mg bid with meals. Increase in increments of 500 mg weekly, up to a maximum of 2,000 mg/d in divided doses. Potential side effects include diarrhea, nausea/vomiting, flatulence, and headache.
Hyperlipidemia. Patients who gain weight with atypical antipsychotics also may develop hyperlipidemia. Fasting serum triglycerides >150 mg/dL (1.70 mmol/L) in obese children are considered to be elevated and an early sign of metabolic syndrome.30 Fasting total cholesterol >200 mg/dL (5.18 mmol/L) or low-density lipoprotein cholesterol >130 mg/dL (3.38 mmol/L) is consistent with hyperlipidemia.
Recommendation. Monitor and treat hyperlipidemia, which increases the risk of atherosclerosis as obese children grow older.31
Prolactin. Elevated prolactin concentrations may have deleterious effects in the developing child or adolescent, including gynecomastia, oligomenorrhea, and amenorrhea.17 Long-term effects on growth and sexual maturation have not been fully evaluated.
The relative tendency of atypical antipsychotics to cause hyperprolactinemia is roughly: risperidone/paliperidone > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.18 In the risperidone RCT, mean changes in baseline prolactin levels were 41 ng/mL for boys and 59 ng/mL in girls.3 Results of the olanzapine RCT suggest a high incidence of hyperprolactinemia (26% of girls, 63% of boys).4 Decreases in serum prolactin were observed in bipolar children and adolescents treated with aripiprazole for 30 weeks.19
Recommendations. For any pediatric patient treated with an atypical antipsychotic that increases prolactin levels:
- Obtain a baseline prolactin level.
- Repeat after 6 months of treatment or with the emergence of elevated prolactin symptoms, such as gynecomastia in boys. Ask about increases in breast size, galactorrhea, changes in menstruation, sexual functioning, and pubertal development.
Switch patients who develop any of these side effects to another atypical agent that does not increase serum prolactin.32
QTc interval prolongation. All atypical antipsychotics can cause QTc prolongation. Several cases of significant QTc prolongation have been reported in children and adolescents treated with ziprasidone.33,34 In the RCT of ziprasidone, QTc prolongation was not clinically significant in most of the patients in which it was reported, and it did not lead to adverse events.34 Mean QTc change was 8.1 msec at study termination.7
Patients enrolled in clinical trails are screened very carefully, however, and those with preexisting medical abnormalities typically are excluded. Thus, these findings may have limited usefulness for “real-world” patients.
Recommendations. Until additional information is known about the cardiac effects of atypical antipsychotics in children and adolescents:
- Perform a careful history, review of symptoms, and physical exam looking for any history of palpitations, shortness of breath, or syncope.
- Query specifically about any family history of sudden cardiac death.
- Perform a baseline resting ECG for patients starting ziprasidone or clozapine, or for other atypicals if indicated by history, review of systems, physical exam, etc.
- For patients treated with ziprasidone or clozapine, repeat ECG as the dose increases or if the patient has cardiac symptoms (unexplained shortness of breath, palpitations, skipped beats, etc.).
Table 3
Talking to families about using antipsychotics
in children with bipolar disorder
| Effectiveness. Large, placebo-controlled studies have shown that atypical antipsychotics can significantly reduce manic symptoms in children and adolescents with bipolar disorder |
| Safety data. Additional 6-month safety data indicate that atypical antipsychotics continue to be effective in children and adolescents, without dramatic changes in side effects |
| Precautions. Antipsychotics are powerful medications and must be used carefully in pediatric patients |
| Potential side effects. All antipsychotics have serious potential side effects that must be recognized, monitored, and managed |
| Potential benefits from using atypical antipsychotics include mood stabilization, treatment of psychotic symptoms, and lower risk of extrapyramidal symptoms compared with typical antipsychotics |
| Risk vs benefit. On balance, the potential benefit of these agents outweighs the potential risk for children and adolescents with bipolar disorder |
Figure: Mean weight gain with atypical antipsychotics in pediatric bipolar trials
Weight gain in children and adolescents with bipolar disorder varied among atypical antipsychotics used in 5 recent randomized controlled trials. Treatment duration was 3 weeks with olanzapine, risperidone, and quetiapine and 4 weeks with aripiprazole and ziprasidone. Dosages were olanzapine, 10.4 ± 4.5 mg/d; risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d; aripiprazole, 10 or 30 mg/d; quetiapine, 400 or 600 mg/d; and ziprasidone, 80 to 160 mg/d.
Source: References 3-7Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org.
- University of Illinois at Chicago Pediatric Mood Disorders Clinic. www.psych.uic.edu/pmdc.
- Ryan Licht Sang Bipolar Foundation. www.ryanlichtsangbipolarfoundation.org.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Carbamazepine • Carbatrol
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex sodium • Depakote
- Lithium • Lithobid, others
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosures
Dr. Kowatch is a consultant to and speaker for AstraZeneca and a consultant to Forest Pharmaceuticals. He receives research support from the National Alliance for Research on Schizophrenia and Depression, the National Institute of Child Health and Human Development, the National Institute of Mental Health, and the Stanley Foundation.
Dr. Strawn has received research support from the American Academy of Child and Adolescent Psychiatry (Lilly Pilot Research Award).
Dr. Sorter receives research support from the National Institute of Mental Health and the Health Foundation of Greater Cincinnati.
1. Hopewell S, Clarke M, Stewart L, et al. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2007;(2):MR000011.-
2. Carey B. Risks found for youths in new antipsychotics. The New York Times. September 15, 2008:A17.
3. Pandina G, DelBello M, Kushner S, et al. Risperidone for the treatment of acute mania in bipolar youth. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
4. Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164(10):1547-1556.
5. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescents with bipolar mania: a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
6. Wagner K, Nyilas M, Forbes R, et al. Acute efficacy of aripiprazole for the treatment of bipolar I disorder, mixed or manic, in pediatric patients. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology, December 9-13, 2007; Boca Raton, FL.
7. DelBello M, Findling RL, Wang P, et al. Safety and efficacy of ziprasidone in pediatric bipolar disorder. Paper presented at: Annual Meeting of the American Psychiatric Association, May 3-8, 2008; Washington, DC.
8. McElduff P, Jaefarnezhad M, Durrington PN. American, British and European recommendations for statins in the primary prevention of cardiovascular disease applied to British men studied prospectively. Heart. 2006;92(9):1213-1218.
9. Tsapakis EM, Soldani F, Tondo L, et al. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry. 2008;193(1):10-17.
10. Kowatch R, Findling R, Scheffer R, et al. Placebo controlled trial of divalproex versus lithium for bipolar disorder. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 23-28, 2007; Boston, MA.
11. DelBello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45(3):305-313.
12. DelBello MP, Schwiers ML, Rosenberg HL, et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1216-1223.
13. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
14. Suppes T, Dennehy EB, Hirschfeld RM, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66(7):870-886.
15. Becker AL, Epperson CN. Female puberty: clinical implications for the use of prolactin-modulating psychotropics. Child Adolesc Psychiatr Clin N Am. 2006;15(1):207-220.
16. Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177-206.
17. Correll CU. Effect of hyperprolactinemia during development in children and adolescents. J Clin Psychiatry. 2008;69(8):e24.-
18. Correll CU. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(1):9-20.
19. Correll CU, Nyilas M, Ashfaque S, et al. Long-term safety and tolerability of aripiprazole in children (10-17 years) with bipolar disorder. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
20. Pandina GJ, Bossie CA, Youssef E, et al. Risperidone improves behavioral symptoms in children with autism in a improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007;37(2):367-373.
21. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescent with bipolar mania; a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
22. Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol. 2007;17(5):647-656.
23. McDermid SA, Hood J, Bockus S, et al. Adolescents on neuroleptic medication: is this population at risk for tardive dyskinesia? Can J Psychiatry. 1998;43(6):629-631.
24. Kumra S, Jacobsen LK, Lenane M, et al. Case series: spectrum of neuroleptic-induced movement disorders and extrapyramidal side effects in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1998;37(2):221-227.
25. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
26. Croarkin PE, Emslie GJ, Mayes TL. Neuroleptic malignant syndrome associated with atypical antipsychotics in pediatric patients: a review of published cases. J Clin Psychiatry. 2008;69(7):1157-1165.
27. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28(4):530-535.
28. Correll CU. Metabolic side effects of second-generation antipsychotics in children and adolescents: a different story? J Clin Psychiatry. 2005;66(10):1331-1332.
29. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072-2079.
30. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs. 2007;22(3):218-253.
31. O’Grady MJ, Brown AM, O’Neill MB. Cholesterol screening in an at-risk pediatric population. Pediatr Cardiol. 2008;29(3):609-613.
32. Ali J, Khemka M. Hyperprolactinemia: monitoring children on long-term risperidone. Current Psychiatry. 2008;7(11):64-72.
33. Blair J, Scahill L, State M, et al. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44(1):73-79.
34. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
Five recent randomized controlled trials (RCTs) have demonstrated the efficacy of atypical antipsychotics for treating bipolar disorder in children and adolescents, but 4 of these 5 trials remain unpublished. The lag time between the completion of these trials and publication of their results—typically 4 to 5 years1—leaves psychiatrists without important evidence to explain to families and critics2 why they might recommend using these powerful medications in children with mental illness.
This article previews the preliminary results of these 5 RCTs of atypical antipsychotics, offers a treatment algorithm supported by this evidence, and discusses how to manage potentially serious risks when using antipsychotics to treat children and adolescents with bipolar disorder (BPD).
Where do atypical antipsychotics fit in?
Details of the 5 industry-sponsored RCTs of atypical antipsychotics in children and adolescents with bipolar I manic or mixed episodes are summarized in Table 1.3-7 Only the olanzapine study4 has been published; data from the other 4 trials were presented at medical meetings in 2007 and 2008.
Change in Young Mania Rating Scale (YMRS) score was the primary outcome measure in these 5 trials, and each compound was more effective than placebo. The trials demonstrated statistically significant and clinically relevant differences between each antipsychotic and placebo. The number needed to treat (NNT)—how many patients need to be treated for 1 to benefit in a controlled clinical trial—ranged from 2 to 4. For comparison, the NNT for statins in the prevention of coronary events is 12 to 22,8 and the NNT in an analysis of trials of selective serotonin reuptake inhibitors for pediatric major depressive disorder was 9.9 Thus, an NNT of ≤4 represents a clinically significant effect.
Risperidone is FDA-approved for short-term treatment of acute bipolar I manic or mixed episodes in patients age 10 to 17. Aripiprazole is approved for acute and maintenance treatment of bipolar I manic or mixed episodes (with or without psychosis) as monotherapy or with lithium or valproate in patients age 10 to 17. In June, an FDA advisory committee recommended pediatric bipolar indications for olanzapine, quetiapine, and ziprasidone.
‘Mood stabilizers’ such as lithium, valproate, and carbamazepine have been used for years to treat bipolar mania in adults, adolescents, and children, despite limited supporting evidence. Preliminary results of a National Institute of Mental Health-funded double-blind RCT provide insights on their efficacy.10
The 153 outpatients age 7 to 17 in a bipolar I manic or mixed episode were randomly assigned to lithium, divalproex, or placebo for 8 weeks. Response rates—based on a Clinical Global Impressions-Improvement score of 1 or 2 (very much or much improved)—were divalproex, 54%; lithium, 42%; and placebo, 29%. Lithium showed a trend toward efficacy but did not clearly separate from placebo on the primary outcome measures. Effect sizes for lithium and divalproex were moderate.10
Only 1 study has compared a mood stabilizer with an atypical antipsychotic for treating mania in adolescents. In a double-blind trial, DelBello et al11 randomly assigned 50 patients age 12 to 18 with a bipolar I manic or mixed episode to quetiapine, 400 to 600 mg/d, or divalproex, serum level 80 to 120 μg/mL, for 28 days. Manic symptoms resolved more rapidly, and remission rates measured by the YMRS were higher with quetiapine than with divalproex. Both medications were well tolerated.
Combination therapy. BPD as it presents in children and adolescents is often difficult to treat because of the disorder’s various phases (manic, depressed, mixed), frequent psychotic symptoms, and high rate of comorbidity. Pediatric BPD patients frequently require several psychotropics, including mood stabilizers and atypical antipsychotics.
In a double-blind, placebo-controlled study, 30 adolescents in a bipolar I manic or mixed episode initially received divalproex, 20 mg/kg/d, then were randomly assigned to 6 weeks of adjunctive quetiapine, titrated to 450 mg/d in 7 days (n=15), or placebo (n=15). Those receiving divalproex plus quetiapine showed a statistically significant greater reduction in manic symptoms (P=.03) and a higher response rate (87% vs 53%, P=.05), compared with those receiving divalproex and placebo. This suggests that a mood stabilizer plus an atypical antipsychotic is more effective than a mood stabilizer alone for adolescent mania. Quetiapine was well tolerated.12
Treatment. The American Psychiatric Association’s outdated 2002 practice guideline for acute bipolar I manic or mixed episodes in adults recommends lithium, valproate, and/or an antipsychotic.13 The more recent Texas Medication Algorithm Project (TMAP) guidelines recommend monotherapy with lithium, valproate, aripiprazole, quetiapine, risperidone, or ziprasidone for adults with euphoric or irritable manic or hypomanic symptoms.14
Based on the TMAP algorithm, recent clinical trial evidence, and our experience in treating pediatric BPD, we offer an approach for treating mania/hypomania in patients age 10 to 17 (see Proposed Algorithm). For dosing and precautions when using atypical antipsychotics in children and adolescents with BPD, see Table 2.15-17
Comorbid psychiatric illnesses (such as anxiety disorders) are prevalent in adolescents with BPD. Evidence in adults and adolescents suggests that some atypical antipsychotics may provide additional benefit for these conditions as well. Thus, consider comorbid conditions and symptoms when choosing antimanic agents.
Attention-deficit/hyperactivity disorder (ADHD) is a common comorbidity in children with BPD, and stimulant medications are most often prescribed to treat inattentiveness and hyperactivity. Caution is imperative when treating bipolar youth with stimulants, which can exacerbate manic symptoms. Treat the patient’s mania before adding or reintroducing stimulant medication. Research and clinical experience suggest that if you first stabilize these patients on a mood stabilizer or atypical antipsychotic, adding a stimulant can be very helpful in treating comorbid ADHD symptoms. Start with low stimulant doses, and increase slowly.
Table 1
RCTs of atypical antipsychotics in patients age 10 to 17
with bipolar I disorder*
| Antipsychotic and source | Bipolar I episode (# of subjects) | Trial duration (days) | Dosage (mg/d) | Response rate or YMRS score change | NNT | Mean weight gain (kg) |
|---|---|---|---|---|---|---|
| Risperidone Pandina et al3 AACAP 2007 | Manic, mixed (169) | 21 | 0.5 to 2.5 3 to 6 | 59% 63% | 3.3 3.5 | 1.9 1.4 |
| Olanzapine Tohen et al4 | Manic, mixed (161) | 21 | 10.4 ± 4.5 | 49% | 4.1 | 3.7 ± 2.2 |
| Quetiapine DelBello et al5 AACAP 2007 | Manic (284) | 21 | 400 600 | 64% 58% | 4.4 4.2 | 1.7 1.7 |
| Aripiprazole Wagner et al6 ACNP 2007 | Manic, mixed (296) | 28 | 10 30 | 45% 64% | 4.1 2.4 | 0.9 0.54 |
| Ziprasidone DelBello et al7 APA 2008 | Manic, mixed (238) | 28 | 80 to 160 | –13.83 with ziprasidone, –8.61 with placebo | 3.7 | None |
| *Each trial included a 6-month open extension phase; results are pending | ||||||
| AACAP: American Academy of Child and Adolescent Psychiatry; ACNP: American College of Neuropsychopharmacology; APA: American Psychiatric Association; NNT: number needed to treat; RCT: randomized controlled trial; YMRS: Young Mania Rating Scale | ||||||
Table 2
Recommended antipsychotic use in pediatric bipolar disorder
| Drug | Starting dosage (mg) | Target dosage (mg/d) | Precautions |
|---|---|---|---|
| Aripiprazole | 2.5 to 5 at bedtime | 10 to 30 | Monitor for CYP 3A4 and 2D6 interactions, weight, BMI, cholesterol, lipids, and glucose |
| Olanzapine | 2.5 bid | 10 to 20 | Monitor for CYP 2D6 interactions, weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Quetiapine | 50 bid | 400 to 1,200 | Monitor for weight, BMI, cholesterol, lipids, and glucose |
| Risperidone | 0.25 bid | 1 to 2.5 | Monitor for EPS, hyperprolactinemia (and associated sexual side effects, including galactorrhea), weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Ziprasidone | 20 bid | 80 to 160 | Check baseline ECG and as dose increases or with reason for high level of concern; monitor prolactin levels |
| BMI: body mass index; CYP: cytochrome P450; ECG: electrocardiography; EPS: extrapyramidal symptoms | |||
| Source: References 15-17 | |||
Proposed Algorithm: Treating a bipolar mixed/manic episode in patients age 10 to 17
Stage 1. Consider patient’s experience with antipsychotics, body weight, and family history when choosing first-line monotherapy (1A). Quetiapine poses low risk for extrapyramidal symptoms and tardive dyskinesia. Aripiprazole and ziprasidone pose relatively low risk of weight gain. Risperidone is potent at low doses but increases prolactin levels (long-term effect unknown).
Second-line choices (1B) are mood stabilizers lithium and valproate (because of lower potency than atypical antipsychotics), and olanzapine (which—although potent—causes substantial weight gain). In case of lack of response or intolerable side effects with initial agent, select an alternate from 1A or 1B. If this is not effective, move to Stage 2.
Stage 2. Consider augmentation for patients who show partial response to monotherapy (in your clinical judgment “mild to moderately improved” but not “much or very much improved”).
Stage 3. Combination therapy could include 2 mood stabilizers (such as lithium and valproate) plus an atypical antipsychotic; 2 atypical antipsychotics; or other combinations based on patient’s past responses. No research has shown these combinations to be efficacious in bipolar children and adolescents, but we find they sometimes help those with treatment-resistant symptoms.
Duration. Maintain psychotropics 12 to 18 months. When patient is euthymic, slowly taper 1 medication across several months. If symptoms recur, reintroduce the mood-stabilizing agent(s).
Source: Adapted and reprinted with permission from Kowatch RA, Fristad MA, Findling R, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008
Managing adverse effects
Although clinically effective, atypical antipsychotics may cause serious side effects that must be recognized and managed. These include extrapyramidal symptoms (EPS), tardive dyskinesia (TD), weight gain and obesity, hyperlipidemia, increased prolactin levels, and QTc changes. Counsel patients and families about the risks and benefits of antipsychotics when you consider them for children and adolescents with BPD (Table 3).
EPS. Drug-induced parkinsonism and akathisia are the most common EPS in children and adolescents with BPD treated with atypical antipsychotics.18
Correll et al19 reported a 10% rate of EPS in patients treated with aripiprazole. Treatment-emergent EPS also was observed in the RCT of risperidone.20 EPS-related adverse events were associated with higher doses of risperidone, although none of the akathisia/EPS measures were thought to be “clinically significant.”
EPS frequency was relatively low and similar to placebo in the 3-week quetiapine trial,21 and no changes in movement disorder scale scores were observed during the olanzapine or ziprasidone RCTs.4,7
Recommendations. If your pediatric patient develops EPS, first try an antipsychotic dose reduction. Because anticholinergics can contribute to antipsychotic-induced weight gain, reserve them until after a dosage reduction has been unsuccessful.
Benztropine (0.25 to 0.5 mg given 2 to 3 times daily, not to exceed 3 mg/d) or diphenhydramine (25 to 50 mg given 3 to 4 times daily; maximum dosage 5 mg/kg/d) can be effective in treating EPS. Avoid anticholinergics in children with narrow-angle glaucoma or age <3.
Akathisia may be managed with propranolol (20 to 120 mg/d in divided doses). Multiple doses (typically 3 times daily) are needed to prevent interdose withdrawal symptoms. Use this beta blocker with caution in children with asthma because of the possibility of bronchospasm.
TD. Short-term trials and a meta-analysis of atypical antipsychotic trials (>11 months’ duration, subject age <18) suggest a low annual risk for TD (0.4%).22 Large, prospective, long-term trials of atypical antipsychotics are necessary to more accurately define the risk of TD in the pediatric population, however. Retrospective analyses of adolescents treated with antipsychotics suggest 3 TD risk factors:
- early age of antipsychotic use
- medication nonadherence
- concomitant use of antiparkinsonian agents.23
Kumra et al24 identified lower premorbid functioning and greater positive symptoms at baseline as factors associated with “withdrawal dyskinesia/tardive dyskinesia” in children and adolescents with early-onset psychotic-spectrum disorders treated with typical or atypical antipsychotics.
Recommendations. To minimize TD risk, use the lowest effective antipsychotic dose, monitor for abnormal involuntary movements with standardized assessments (such as the Abnormal Involuntary Movement Scale), review risks and benefits with parents and patients, and regularly evaluate the indication and need for antipsychotic therapy. It is reasonable to attempt to lower the antipsychotic dose after the patient has attained remission and been stable for 1 year.
Neuroleptic malignant syndrome (NMS). This complication of dopamine-blocking medications:
- is among the most serious adverse effects of antipsychotic treatment
- continues to be associated with a mortality rate of 10%.25
Recommendation. At least 1 recent review of pediatric NMS cases suggests that essential features (hyperthermia and severe muscular rigidity) are retained in children.26 Nonetheless, monitor for variant presentations; hyper thermia or muscle rigidity may be absent or develop slowly over several days in patients treated with atypical antipsychotics.27
Weight gain and glucose metabolism. A major adverse effect of most atypical antipsychotics is increased appetite, weight gain, and possible obesity.28 In children, “obesity” refers to a body mass index (BMI) >95th percentile for age and sex; “over-weight” refers to BMI between the 85th and 95th percentile. Mean weight gain in the 5 atypical antipsychotic pediatric bipolar trials ranged from 0 to 8 lbs across 3 to 4 weeks of treatment (Figure).3-7
Recommendations. Emphasize diet and exercise, with restriction of high-carbohydrate food, “fast foods,” and soft drinks. Another option is a trial of metformin, which decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization.
Klein et al29 studied 39 patients age 10 to 17 with mood and psychotic disorders whose weight increased by >10% during <1 year of olanzapine, risperidone, or quetiapine therapy. In this 16-week, double-blind, placebo-controlled trial, weight was stabilized in subjects receiving metformin, whereas those receiving placebo continued to gain weight (0.31 kg [0.68 lb]/week).
The usual starting metformin dose is 500 mg bid with meals. Increase in increments of 500 mg weekly, up to a maximum of 2,000 mg/d in divided doses. Potential side effects include diarrhea, nausea/vomiting, flatulence, and headache.
Hyperlipidemia. Patients who gain weight with atypical antipsychotics also may develop hyperlipidemia. Fasting serum triglycerides >150 mg/dL (1.70 mmol/L) in obese children are considered to be elevated and an early sign of metabolic syndrome.30 Fasting total cholesterol >200 mg/dL (5.18 mmol/L) or low-density lipoprotein cholesterol >130 mg/dL (3.38 mmol/L) is consistent with hyperlipidemia.
Recommendation. Monitor and treat hyperlipidemia, which increases the risk of atherosclerosis as obese children grow older.31
Prolactin. Elevated prolactin concentrations may have deleterious effects in the developing child or adolescent, including gynecomastia, oligomenorrhea, and amenorrhea.17 Long-term effects on growth and sexual maturation have not been fully evaluated.
The relative tendency of atypical antipsychotics to cause hyperprolactinemia is roughly: risperidone/paliperidone > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.18 In the risperidone RCT, mean changes in baseline prolactin levels were 41 ng/mL for boys and 59 ng/mL in girls.3 Results of the olanzapine RCT suggest a high incidence of hyperprolactinemia (26% of girls, 63% of boys).4 Decreases in serum prolactin were observed in bipolar children and adolescents treated with aripiprazole for 30 weeks.19
Recommendations. For any pediatric patient treated with an atypical antipsychotic that increases prolactin levels:
- Obtain a baseline prolactin level.
- Repeat after 6 months of treatment or with the emergence of elevated prolactin symptoms, such as gynecomastia in boys. Ask about increases in breast size, galactorrhea, changes in menstruation, sexual functioning, and pubertal development.
Switch patients who develop any of these side effects to another atypical agent that does not increase serum prolactin.32
QTc interval prolongation. All atypical antipsychotics can cause QTc prolongation. Several cases of significant QTc prolongation have been reported in children and adolescents treated with ziprasidone.33,34 In the RCT of ziprasidone, QTc prolongation was not clinically significant in most of the patients in which it was reported, and it did not lead to adverse events.34 Mean QTc change was 8.1 msec at study termination.7
Patients enrolled in clinical trails are screened very carefully, however, and those with preexisting medical abnormalities typically are excluded. Thus, these findings may have limited usefulness for “real-world” patients.
Recommendations. Until additional information is known about the cardiac effects of atypical antipsychotics in children and adolescents:
- Perform a careful history, review of symptoms, and physical exam looking for any history of palpitations, shortness of breath, or syncope.
- Query specifically about any family history of sudden cardiac death.
- Perform a baseline resting ECG for patients starting ziprasidone or clozapine, or for other atypicals if indicated by history, review of systems, physical exam, etc.
- For patients treated with ziprasidone or clozapine, repeat ECG as the dose increases or if the patient has cardiac symptoms (unexplained shortness of breath, palpitations, skipped beats, etc.).
Table 3
Talking to families about using antipsychotics
in children with bipolar disorder
| Effectiveness. Large, placebo-controlled studies have shown that atypical antipsychotics can significantly reduce manic symptoms in children and adolescents with bipolar disorder |
| Safety data. Additional 6-month safety data indicate that atypical antipsychotics continue to be effective in children and adolescents, without dramatic changes in side effects |
| Precautions. Antipsychotics are powerful medications and must be used carefully in pediatric patients |
| Potential side effects. All antipsychotics have serious potential side effects that must be recognized, monitored, and managed |
| Potential benefits from using atypical antipsychotics include mood stabilization, treatment of psychotic symptoms, and lower risk of extrapyramidal symptoms compared with typical antipsychotics |
| Risk vs benefit. On balance, the potential benefit of these agents outweighs the potential risk for children and adolescents with bipolar disorder |
Figure: Mean weight gain with atypical antipsychotics in pediatric bipolar trials
Weight gain in children and adolescents with bipolar disorder varied among atypical antipsychotics used in 5 recent randomized controlled trials. Treatment duration was 3 weeks with olanzapine, risperidone, and quetiapine and 4 weeks with aripiprazole and ziprasidone. Dosages were olanzapine, 10.4 ± 4.5 mg/d; risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d; aripiprazole, 10 or 30 mg/d; quetiapine, 400 or 600 mg/d; and ziprasidone, 80 to 160 mg/d.
Source: References 3-7Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org.
- University of Illinois at Chicago Pediatric Mood Disorders Clinic. www.psych.uic.edu/pmdc.
- Ryan Licht Sang Bipolar Foundation. www.ryanlichtsangbipolarfoundation.org.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Carbamazepine • Carbatrol
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex sodium • Depakote
- Lithium • Lithobid, others
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosures
Dr. Kowatch is a consultant to and speaker for AstraZeneca and a consultant to Forest Pharmaceuticals. He receives research support from the National Alliance for Research on Schizophrenia and Depression, the National Institute of Child Health and Human Development, the National Institute of Mental Health, and the Stanley Foundation.
Dr. Strawn has received research support from the American Academy of Child and Adolescent Psychiatry (Lilly Pilot Research Award).
Dr. Sorter receives research support from the National Institute of Mental Health and the Health Foundation of Greater Cincinnati.
Five recent randomized controlled trials (RCTs) have demonstrated the efficacy of atypical antipsychotics for treating bipolar disorder in children and adolescents, but 4 of these 5 trials remain unpublished. The lag time between the completion of these trials and publication of their results—typically 4 to 5 years1—leaves psychiatrists without important evidence to explain to families and critics2 why they might recommend using these powerful medications in children with mental illness.
This article previews the preliminary results of these 5 RCTs of atypical antipsychotics, offers a treatment algorithm supported by this evidence, and discusses how to manage potentially serious risks when using antipsychotics to treat children and adolescents with bipolar disorder (BPD).
Where do atypical antipsychotics fit in?
Details of the 5 industry-sponsored RCTs of atypical antipsychotics in children and adolescents with bipolar I manic or mixed episodes are summarized in Table 1.3-7 Only the olanzapine study4 has been published; data from the other 4 trials were presented at medical meetings in 2007 and 2008.
Change in Young Mania Rating Scale (YMRS) score was the primary outcome measure in these 5 trials, and each compound was more effective than placebo. The trials demonstrated statistically significant and clinically relevant differences between each antipsychotic and placebo. The number needed to treat (NNT)—how many patients need to be treated for 1 to benefit in a controlled clinical trial—ranged from 2 to 4. For comparison, the NNT for statins in the prevention of coronary events is 12 to 22,8 and the NNT in an analysis of trials of selective serotonin reuptake inhibitors for pediatric major depressive disorder was 9.9 Thus, an NNT of ≤4 represents a clinically significant effect.
Risperidone is FDA-approved for short-term treatment of acute bipolar I manic or mixed episodes in patients age 10 to 17. Aripiprazole is approved for acute and maintenance treatment of bipolar I manic or mixed episodes (with or without psychosis) as monotherapy or with lithium or valproate in patients age 10 to 17. In June, an FDA advisory committee recommended pediatric bipolar indications for olanzapine, quetiapine, and ziprasidone.
‘Mood stabilizers’ such as lithium, valproate, and carbamazepine have been used for years to treat bipolar mania in adults, adolescents, and children, despite limited supporting evidence. Preliminary results of a National Institute of Mental Health-funded double-blind RCT provide insights on their efficacy.10
The 153 outpatients age 7 to 17 in a bipolar I manic or mixed episode were randomly assigned to lithium, divalproex, or placebo for 8 weeks. Response rates—based on a Clinical Global Impressions-Improvement score of 1 or 2 (very much or much improved)—were divalproex, 54%; lithium, 42%; and placebo, 29%. Lithium showed a trend toward efficacy but did not clearly separate from placebo on the primary outcome measures. Effect sizes for lithium and divalproex were moderate.10
Only 1 study has compared a mood stabilizer with an atypical antipsychotic for treating mania in adolescents. In a double-blind trial, DelBello et al11 randomly assigned 50 patients age 12 to 18 with a bipolar I manic or mixed episode to quetiapine, 400 to 600 mg/d, or divalproex, serum level 80 to 120 μg/mL, for 28 days. Manic symptoms resolved more rapidly, and remission rates measured by the YMRS were higher with quetiapine than with divalproex. Both medications were well tolerated.
Combination therapy. BPD as it presents in children and adolescents is often difficult to treat because of the disorder’s various phases (manic, depressed, mixed), frequent psychotic symptoms, and high rate of comorbidity. Pediatric BPD patients frequently require several psychotropics, including mood stabilizers and atypical antipsychotics.
In a double-blind, placebo-controlled study, 30 adolescents in a bipolar I manic or mixed episode initially received divalproex, 20 mg/kg/d, then were randomly assigned to 6 weeks of adjunctive quetiapine, titrated to 450 mg/d in 7 days (n=15), or placebo (n=15). Those receiving divalproex plus quetiapine showed a statistically significant greater reduction in manic symptoms (P=.03) and a higher response rate (87% vs 53%, P=.05), compared with those receiving divalproex and placebo. This suggests that a mood stabilizer plus an atypical antipsychotic is more effective than a mood stabilizer alone for adolescent mania. Quetiapine was well tolerated.12
Treatment. The American Psychiatric Association’s outdated 2002 practice guideline for acute bipolar I manic or mixed episodes in adults recommends lithium, valproate, and/or an antipsychotic.13 The more recent Texas Medication Algorithm Project (TMAP) guidelines recommend monotherapy with lithium, valproate, aripiprazole, quetiapine, risperidone, or ziprasidone for adults with euphoric or irritable manic or hypomanic symptoms.14
Based on the TMAP algorithm, recent clinical trial evidence, and our experience in treating pediatric BPD, we offer an approach for treating mania/hypomania in patients age 10 to 17 (see Proposed Algorithm). For dosing and precautions when using atypical antipsychotics in children and adolescents with BPD, see Table 2.15-17
Comorbid psychiatric illnesses (such as anxiety disorders) are prevalent in adolescents with BPD. Evidence in adults and adolescents suggests that some atypical antipsychotics may provide additional benefit for these conditions as well. Thus, consider comorbid conditions and symptoms when choosing antimanic agents.
Attention-deficit/hyperactivity disorder (ADHD) is a common comorbidity in children with BPD, and stimulant medications are most often prescribed to treat inattentiveness and hyperactivity. Caution is imperative when treating bipolar youth with stimulants, which can exacerbate manic symptoms. Treat the patient’s mania before adding or reintroducing stimulant medication. Research and clinical experience suggest that if you first stabilize these patients on a mood stabilizer or atypical antipsychotic, adding a stimulant can be very helpful in treating comorbid ADHD symptoms. Start with low stimulant doses, and increase slowly.
Table 1
RCTs of atypical antipsychotics in patients age 10 to 17
with bipolar I disorder*
| Antipsychotic and source | Bipolar I episode (# of subjects) | Trial duration (days) | Dosage (mg/d) | Response rate or YMRS score change | NNT | Mean weight gain (kg) |
|---|---|---|---|---|---|---|
| Risperidone Pandina et al3 AACAP 2007 | Manic, mixed (169) | 21 | 0.5 to 2.5 3 to 6 | 59% 63% | 3.3 3.5 | 1.9 1.4 |
| Olanzapine Tohen et al4 | Manic, mixed (161) | 21 | 10.4 ± 4.5 | 49% | 4.1 | 3.7 ± 2.2 |
| Quetiapine DelBello et al5 AACAP 2007 | Manic (284) | 21 | 400 600 | 64% 58% | 4.4 4.2 | 1.7 1.7 |
| Aripiprazole Wagner et al6 ACNP 2007 | Manic, mixed (296) | 28 | 10 30 | 45% 64% | 4.1 2.4 | 0.9 0.54 |
| Ziprasidone DelBello et al7 APA 2008 | Manic, mixed (238) | 28 | 80 to 160 | –13.83 with ziprasidone, –8.61 with placebo | 3.7 | None |
| *Each trial included a 6-month open extension phase; results are pending | ||||||
| AACAP: American Academy of Child and Adolescent Psychiatry; ACNP: American College of Neuropsychopharmacology; APA: American Psychiatric Association; NNT: number needed to treat; RCT: randomized controlled trial; YMRS: Young Mania Rating Scale | ||||||
Table 2
Recommended antipsychotic use in pediatric bipolar disorder
| Drug | Starting dosage (mg) | Target dosage (mg/d) | Precautions |
|---|---|---|---|
| Aripiprazole | 2.5 to 5 at bedtime | 10 to 30 | Monitor for CYP 3A4 and 2D6 interactions, weight, BMI, cholesterol, lipids, and glucose |
| Olanzapine | 2.5 bid | 10 to 20 | Monitor for CYP 2D6 interactions, weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Quetiapine | 50 bid | 400 to 1,200 | Monitor for weight, BMI, cholesterol, lipids, and glucose |
| Risperidone | 0.25 bid | 1 to 2.5 | Monitor for EPS, hyperprolactinemia (and associated sexual side effects, including galactorrhea), weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Ziprasidone | 20 bid | 80 to 160 | Check baseline ECG and as dose increases or with reason for high level of concern; monitor prolactin levels |
| BMI: body mass index; CYP: cytochrome P450; ECG: electrocardiography; EPS: extrapyramidal symptoms | |||
| Source: References 15-17 | |||
Proposed Algorithm: Treating a bipolar mixed/manic episode in patients age 10 to 17
Stage 1. Consider patient’s experience with antipsychotics, body weight, and family history when choosing first-line monotherapy (1A). Quetiapine poses low risk for extrapyramidal symptoms and tardive dyskinesia. Aripiprazole and ziprasidone pose relatively low risk of weight gain. Risperidone is potent at low doses but increases prolactin levels (long-term effect unknown).
Second-line choices (1B) are mood stabilizers lithium and valproate (because of lower potency than atypical antipsychotics), and olanzapine (which—although potent—causes substantial weight gain). In case of lack of response or intolerable side effects with initial agent, select an alternate from 1A or 1B. If this is not effective, move to Stage 2.
Stage 2. Consider augmentation for patients who show partial response to monotherapy (in your clinical judgment “mild to moderately improved” but not “much or very much improved”).
Stage 3. Combination therapy could include 2 mood stabilizers (such as lithium and valproate) plus an atypical antipsychotic; 2 atypical antipsychotics; or other combinations based on patient’s past responses. No research has shown these combinations to be efficacious in bipolar children and adolescents, but we find they sometimes help those with treatment-resistant symptoms.
Duration. Maintain psychotropics 12 to 18 months. When patient is euthymic, slowly taper 1 medication across several months. If symptoms recur, reintroduce the mood-stabilizing agent(s).
Source: Adapted and reprinted with permission from Kowatch RA, Fristad MA, Findling R, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008
Managing adverse effects
Although clinically effective, atypical antipsychotics may cause serious side effects that must be recognized and managed. These include extrapyramidal symptoms (EPS), tardive dyskinesia (TD), weight gain and obesity, hyperlipidemia, increased prolactin levels, and QTc changes. Counsel patients and families about the risks and benefits of antipsychotics when you consider them for children and adolescents with BPD (Table 3).
EPS. Drug-induced parkinsonism and akathisia are the most common EPS in children and adolescents with BPD treated with atypical antipsychotics.18
Correll et al19 reported a 10% rate of EPS in patients treated with aripiprazole. Treatment-emergent EPS also was observed in the RCT of risperidone.20 EPS-related adverse events were associated with higher doses of risperidone, although none of the akathisia/EPS measures were thought to be “clinically significant.”
EPS frequency was relatively low and similar to placebo in the 3-week quetiapine trial,21 and no changes in movement disorder scale scores were observed during the olanzapine or ziprasidone RCTs.4,7
Recommendations. If your pediatric patient develops EPS, first try an antipsychotic dose reduction. Because anticholinergics can contribute to antipsychotic-induced weight gain, reserve them until after a dosage reduction has been unsuccessful.
Benztropine (0.25 to 0.5 mg given 2 to 3 times daily, not to exceed 3 mg/d) or diphenhydramine (25 to 50 mg given 3 to 4 times daily; maximum dosage 5 mg/kg/d) can be effective in treating EPS. Avoid anticholinergics in children with narrow-angle glaucoma or age <3.
Akathisia may be managed with propranolol (20 to 120 mg/d in divided doses). Multiple doses (typically 3 times daily) are needed to prevent interdose withdrawal symptoms. Use this beta blocker with caution in children with asthma because of the possibility of bronchospasm.
TD. Short-term trials and a meta-analysis of atypical antipsychotic trials (>11 months’ duration, subject age <18) suggest a low annual risk for TD (0.4%).22 Large, prospective, long-term trials of atypical antipsychotics are necessary to more accurately define the risk of TD in the pediatric population, however. Retrospective analyses of adolescents treated with antipsychotics suggest 3 TD risk factors:
- early age of antipsychotic use
- medication nonadherence
- concomitant use of antiparkinsonian agents.23
Kumra et al24 identified lower premorbid functioning and greater positive symptoms at baseline as factors associated with “withdrawal dyskinesia/tardive dyskinesia” in children and adolescents with early-onset psychotic-spectrum disorders treated with typical or atypical antipsychotics.
Recommendations. To minimize TD risk, use the lowest effective antipsychotic dose, monitor for abnormal involuntary movements with standardized assessments (such as the Abnormal Involuntary Movement Scale), review risks and benefits with parents and patients, and regularly evaluate the indication and need for antipsychotic therapy. It is reasonable to attempt to lower the antipsychotic dose after the patient has attained remission and been stable for 1 year.
Neuroleptic malignant syndrome (NMS). This complication of dopamine-blocking medications:
- is among the most serious adverse effects of antipsychotic treatment
- continues to be associated with a mortality rate of 10%.25
Recommendation. At least 1 recent review of pediatric NMS cases suggests that essential features (hyperthermia and severe muscular rigidity) are retained in children.26 Nonetheless, monitor for variant presentations; hyper thermia or muscle rigidity may be absent or develop slowly over several days in patients treated with atypical antipsychotics.27
Weight gain and glucose metabolism. A major adverse effect of most atypical antipsychotics is increased appetite, weight gain, and possible obesity.28 In children, “obesity” refers to a body mass index (BMI) >95th percentile for age and sex; “over-weight” refers to BMI between the 85th and 95th percentile. Mean weight gain in the 5 atypical antipsychotic pediatric bipolar trials ranged from 0 to 8 lbs across 3 to 4 weeks of treatment (Figure).3-7
Recommendations. Emphasize diet and exercise, with restriction of high-carbohydrate food, “fast foods,” and soft drinks. Another option is a trial of metformin, which decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization.
Klein et al29 studied 39 patients age 10 to 17 with mood and psychotic disorders whose weight increased by >10% during <1 year of olanzapine, risperidone, or quetiapine therapy. In this 16-week, double-blind, placebo-controlled trial, weight was stabilized in subjects receiving metformin, whereas those receiving placebo continued to gain weight (0.31 kg [0.68 lb]/week).
The usual starting metformin dose is 500 mg bid with meals. Increase in increments of 500 mg weekly, up to a maximum of 2,000 mg/d in divided doses. Potential side effects include diarrhea, nausea/vomiting, flatulence, and headache.
Hyperlipidemia. Patients who gain weight with atypical antipsychotics also may develop hyperlipidemia. Fasting serum triglycerides >150 mg/dL (1.70 mmol/L) in obese children are considered to be elevated and an early sign of metabolic syndrome.30 Fasting total cholesterol >200 mg/dL (5.18 mmol/L) or low-density lipoprotein cholesterol >130 mg/dL (3.38 mmol/L) is consistent with hyperlipidemia.
Recommendation. Monitor and treat hyperlipidemia, which increases the risk of atherosclerosis as obese children grow older.31
Prolactin. Elevated prolactin concentrations may have deleterious effects in the developing child or adolescent, including gynecomastia, oligomenorrhea, and amenorrhea.17 Long-term effects on growth and sexual maturation have not been fully evaluated.
The relative tendency of atypical antipsychotics to cause hyperprolactinemia is roughly: risperidone/paliperidone > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.18 In the risperidone RCT, mean changes in baseline prolactin levels were 41 ng/mL for boys and 59 ng/mL in girls.3 Results of the olanzapine RCT suggest a high incidence of hyperprolactinemia (26% of girls, 63% of boys).4 Decreases in serum prolactin were observed in bipolar children and adolescents treated with aripiprazole for 30 weeks.19
Recommendations. For any pediatric patient treated with an atypical antipsychotic that increases prolactin levels:
- Obtain a baseline prolactin level.
- Repeat after 6 months of treatment or with the emergence of elevated prolactin symptoms, such as gynecomastia in boys. Ask about increases in breast size, galactorrhea, changes in menstruation, sexual functioning, and pubertal development.
Switch patients who develop any of these side effects to another atypical agent that does not increase serum prolactin.32
QTc interval prolongation. All atypical antipsychotics can cause QTc prolongation. Several cases of significant QTc prolongation have been reported in children and adolescents treated with ziprasidone.33,34 In the RCT of ziprasidone, QTc prolongation was not clinically significant in most of the patients in which it was reported, and it did not lead to adverse events.34 Mean QTc change was 8.1 msec at study termination.7
Patients enrolled in clinical trails are screened very carefully, however, and those with preexisting medical abnormalities typically are excluded. Thus, these findings may have limited usefulness for “real-world” patients.
Recommendations. Until additional information is known about the cardiac effects of atypical antipsychotics in children and adolescents:
- Perform a careful history, review of symptoms, and physical exam looking for any history of palpitations, shortness of breath, or syncope.
- Query specifically about any family history of sudden cardiac death.
- Perform a baseline resting ECG for patients starting ziprasidone or clozapine, or for other atypicals if indicated by history, review of systems, physical exam, etc.
- For patients treated with ziprasidone or clozapine, repeat ECG as the dose increases or if the patient has cardiac symptoms (unexplained shortness of breath, palpitations, skipped beats, etc.).
Table 3
Talking to families about using antipsychotics
in children with bipolar disorder
| Effectiveness. Large, placebo-controlled studies have shown that atypical antipsychotics can significantly reduce manic symptoms in children and adolescents with bipolar disorder |
| Safety data. Additional 6-month safety data indicate that atypical antipsychotics continue to be effective in children and adolescents, without dramatic changes in side effects |
| Precautions. Antipsychotics are powerful medications and must be used carefully in pediatric patients |
| Potential side effects. All antipsychotics have serious potential side effects that must be recognized, monitored, and managed |
| Potential benefits from using atypical antipsychotics include mood stabilization, treatment of psychotic symptoms, and lower risk of extrapyramidal symptoms compared with typical antipsychotics |
| Risk vs benefit. On balance, the potential benefit of these agents outweighs the potential risk for children and adolescents with bipolar disorder |
Figure: Mean weight gain with atypical antipsychotics in pediatric bipolar trials
Weight gain in children and adolescents with bipolar disorder varied among atypical antipsychotics used in 5 recent randomized controlled trials. Treatment duration was 3 weeks with olanzapine, risperidone, and quetiapine and 4 weeks with aripiprazole and ziprasidone. Dosages were olanzapine, 10.4 ± 4.5 mg/d; risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d; aripiprazole, 10 or 30 mg/d; quetiapine, 400 or 600 mg/d; and ziprasidone, 80 to 160 mg/d.
Source: References 3-7Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org.
- University of Illinois at Chicago Pediatric Mood Disorders Clinic. www.psych.uic.edu/pmdc.
- Ryan Licht Sang Bipolar Foundation. www.ryanlichtsangbipolarfoundation.org.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Carbamazepine • Carbatrol
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex sodium • Depakote
- Lithium • Lithobid, others
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosures
Dr. Kowatch is a consultant to and speaker for AstraZeneca and a consultant to Forest Pharmaceuticals. He receives research support from the National Alliance for Research on Schizophrenia and Depression, the National Institute of Child Health and Human Development, the National Institute of Mental Health, and the Stanley Foundation.
Dr. Strawn has received research support from the American Academy of Child and Adolescent Psychiatry (Lilly Pilot Research Award).
Dr. Sorter receives research support from the National Institute of Mental Health and the Health Foundation of Greater Cincinnati.
1. Hopewell S, Clarke M, Stewart L, et al. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2007;(2):MR000011.-
2. Carey B. Risks found for youths in new antipsychotics. The New York Times. September 15, 2008:A17.
3. Pandina G, DelBello M, Kushner S, et al. Risperidone for the treatment of acute mania in bipolar youth. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
4. Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164(10):1547-1556.
5. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescents with bipolar mania: a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
6. Wagner K, Nyilas M, Forbes R, et al. Acute efficacy of aripiprazole for the treatment of bipolar I disorder, mixed or manic, in pediatric patients. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology, December 9-13, 2007; Boca Raton, FL.
7. DelBello M, Findling RL, Wang P, et al. Safety and efficacy of ziprasidone in pediatric bipolar disorder. Paper presented at: Annual Meeting of the American Psychiatric Association, May 3-8, 2008; Washington, DC.
8. McElduff P, Jaefarnezhad M, Durrington PN. American, British and European recommendations for statins in the primary prevention of cardiovascular disease applied to British men studied prospectively. Heart. 2006;92(9):1213-1218.
9. Tsapakis EM, Soldani F, Tondo L, et al. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry. 2008;193(1):10-17.
10. Kowatch R, Findling R, Scheffer R, et al. Placebo controlled trial of divalproex versus lithium for bipolar disorder. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 23-28, 2007; Boston, MA.
11. DelBello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45(3):305-313.
12. DelBello MP, Schwiers ML, Rosenberg HL, et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1216-1223.
13. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
14. Suppes T, Dennehy EB, Hirschfeld RM, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66(7):870-886.
15. Becker AL, Epperson CN. Female puberty: clinical implications for the use of prolactin-modulating psychotropics. Child Adolesc Psychiatr Clin N Am. 2006;15(1):207-220.
16. Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177-206.
17. Correll CU. Effect of hyperprolactinemia during development in children and adolescents. J Clin Psychiatry. 2008;69(8):e24.-
18. Correll CU. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(1):9-20.
19. Correll CU, Nyilas M, Ashfaque S, et al. Long-term safety and tolerability of aripiprazole in children (10-17 years) with bipolar disorder. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
20. Pandina GJ, Bossie CA, Youssef E, et al. Risperidone improves behavioral symptoms in children with autism in a improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007;37(2):367-373.
21. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescent with bipolar mania; a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
22. Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol. 2007;17(5):647-656.
23. McDermid SA, Hood J, Bockus S, et al. Adolescents on neuroleptic medication: is this population at risk for tardive dyskinesia? Can J Psychiatry. 1998;43(6):629-631.
24. Kumra S, Jacobsen LK, Lenane M, et al. Case series: spectrum of neuroleptic-induced movement disorders and extrapyramidal side effects in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1998;37(2):221-227.
25. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
26. Croarkin PE, Emslie GJ, Mayes TL. Neuroleptic malignant syndrome associated with atypical antipsychotics in pediatric patients: a review of published cases. J Clin Psychiatry. 2008;69(7):1157-1165.
27. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28(4):530-535.
28. Correll CU. Metabolic side effects of second-generation antipsychotics in children and adolescents: a different story? J Clin Psychiatry. 2005;66(10):1331-1332.
29. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072-2079.
30. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs. 2007;22(3):218-253.
31. O’Grady MJ, Brown AM, O’Neill MB. Cholesterol screening in an at-risk pediatric population. Pediatr Cardiol. 2008;29(3):609-613.
32. Ali J, Khemka M. Hyperprolactinemia: monitoring children on long-term risperidone. Current Psychiatry. 2008;7(11):64-72.
33. Blair J, Scahill L, State M, et al. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44(1):73-79.
34. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
1. Hopewell S, Clarke M, Stewart L, et al. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2007;(2):MR000011.-
2. Carey B. Risks found for youths in new antipsychotics. The New York Times. September 15, 2008:A17.
3. Pandina G, DelBello M, Kushner S, et al. Risperidone for the treatment of acute mania in bipolar youth. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
4. Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164(10):1547-1556.
5. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescents with bipolar mania: a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
6. Wagner K, Nyilas M, Forbes R, et al. Acute efficacy of aripiprazole for the treatment of bipolar I disorder, mixed or manic, in pediatric patients. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology, December 9-13, 2007; Boca Raton, FL.
7. DelBello M, Findling RL, Wang P, et al. Safety and efficacy of ziprasidone in pediatric bipolar disorder. Paper presented at: Annual Meeting of the American Psychiatric Association, May 3-8, 2008; Washington, DC.
8. McElduff P, Jaefarnezhad M, Durrington PN. American, British and European recommendations for statins in the primary prevention of cardiovascular disease applied to British men studied prospectively. Heart. 2006;92(9):1213-1218.
9. Tsapakis EM, Soldani F, Tondo L, et al. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry. 2008;193(1):10-17.
10. Kowatch R, Findling R, Scheffer R, et al. Placebo controlled trial of divalproex versus lithium for bipolar disorder. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 23-28, 2007; Boston, MA.
11. DelBello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45(3):305-313.
12. DelBello MP, Schwiers ML, Rosenberg HL, et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1216-1223.
13. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
14. Suppes T, Dennehy EB, Hirschfeld RM, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66(7):870-886.
15. Becker AL, Epperson CN. Female puberty: clinical implications for the use of prolactin-modulating psychotropics. Child Adolesc Psychiatr Clin N Am. 2006;15(1):207-220.
16. Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177-206.
17. Correll CU. Effect of hyperprolactinemia during development in children and adolescents. J Clin Psychiatry. 2008;69(8):e24.-
18. Correll CU. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(1):9-20.
19. Correll CU, Nyilas M, Ashfaque S, et al. Long-term safety and tolerability of aripiprazole in children (10-17 years) with bipolar disorder. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
20. Pandina GJ, Bossie CA, Youssef E, et al. Risperidone improves behavioral symptoms in children with autism in a improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007;37(2):367-373.
21. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescent with bipolar mania; a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
22. Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol. 2007;17(5):647-656.
23. McDermid SA, Hood J, Bockus S, et al. Adolescents on neuroleptic medication: is this population at risk for tardive dyskinesia? Can J Psychiatry. 1998;43(6):629-631.
24. Kumra S, Jacobsen LK, Lenane M, et al. Case series: spectrum of neuroleptic-induced movement disorders and extrapyramidal side effects in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1998;37(2):221-227.
25. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
26. Croarkin PE, Emslie GJ, Mayes TL. Neuroleptic malignant syndrome associated with atypical antipsychotics in pediatric patients: a review of published cases. J Clin Psychiatry. 2008;69(7):1157-1165.
27. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28(4):530-535.
28. Correll CU. Metabolic side effects of second-generation antipsychotics in children and adolescents: a different story? J Clin Psychiatry. 2005;66(10):1331-1332.
29. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072-2079.
30. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs. 2007;22(3):218-253.
31. O’Grady MJ, Brown AM, O’Neill MB. Cholesterol screening in an at-risk pediatric population. Pediatr Cardiol. 2008;29(3):609-613.
32. Ali J, Khemka M. Hyperprolactinemia: monitoring children on long-term risperidone. Current Psychiatry. 2008;7(11):64-72.
33. Blair J, Scahill L, State M, et al. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44(1):73-79.
34. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
Choosing antipsychotics for children with schizophrenia: Evidence plus experience
“A patient I’ve seen for a number of years had been diagnosed in the pervasive developmental disorder spectrum, but she was quite atypical. Her perseverative thinking focused on a fantasy world, and she was so preoccupied that it was very difficult to pull her out of it. Now at age 12, she has a full-blown psychotic disorder, and the fantasy world is enveloping her. She hears people talking to her all day long.”
Jean A. Frazier, MD, who treats this patient and other children with psychotic disorders, was 1 of 4 principal investigators in the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study, a randomized, double-blind, multisite trial funded by the National Institute of Mental Health. The study, published in November 2008,1 compared the efficacy and tolerability of 3 antipsychotics—olanzapine, risperidone, and molindone—in pediatric patients with schizophrenia or schizoaffective disorder (Box 1).
Dr. Frazier discusses the unexpected findings of the TEOSS trial with Current Psychiatry Section Editor Robert A. Kowatch, MD, PhD. Based on the trial findings and her experience, she tells how she makes decisions when prescribing antipsychotics for children and adolescents with schizophrenia and related disorders.
DR. KOWATCH: The TEOSS trial found no significant differences in efficacy between molindone and the atypical antipsychotics (olanzapine and risperidone) included in the study. You’ve prescribed both typical and atypical antipsychotics in research and in your clinical practice. Do you believe there’s any difference between the 2 classes?
DR. FRAZIER: There are some differences. For example, treatment-refractory patients, especially young children, sometimes need more D2 blockade than some atypical antipsychotics provide. I’ve seen more extra pyramidal side effects with the typical antipsychotics than the atypicals, although it’s not uncommon to see some akathisia with aripiprazole or some dystonia and dyskinesia with risperidone.
DR. KOWATCH: What are the benefits and risks of using antipsychotics in young children?
DR. FRAZIER: The benefit is that antipsychotics can decrease children’s suffering and get them more centered in reality so they can enjoy their friends and progress in school. And when that happens, it’s wonderful. What are the risks? With the atypicals my greatest concern is weight gain, and with the typical agents it’s tardive dyskinesia.
DR. KOWATCH: Have you changed the way you prescribe antipsychotics as a result of the TEOSS study?
DR. FRAZIER: Actually, I have. Clinicians have to be very careful about selecting psychotropic agents that can worsen pediatric-onset obesity. Olanzapine is an effective agent for targeting psychosis and mood symptoms, but the weight gain associated with it is a concern. I do not prescribe olanzapine as much as I have in the past, although I keep it in my armamentarium and tend to reserve it for third- or fourth-line therapy.
I have found molindone to be quite effective in children with schizophrenia or schizoaffective disorder, especially in those who have gained a lot of weight on atypical antipsychotics. They usually lose weight on molindone.
DR. KOWATCH: Do you think the TEOSS study had adequate power to demonstrate differences among molindone, olanzapine, and risperidone?
DR. FRAZIER: We enrolled 119 patients—which is large for a study such as this—but we did not reach our target of 168 patients, which might have increased our power to detect differences. Among the children we did enroll, the 3 antipsychotics showed no difference in efficacy, but the meaningful finding of this study to me was the side effect profile of these agents.
DR. KOWATCH: You mean weight gain with olanzapine and extrapyramidal symptoms with molindone?
DR. FRAZIER: Yes.
Managing side effects
DR. KOWATCH: How do you manage antipsychotic side effects?
DR. FRAZIER: For any of the antipsychotics’ side effects, you have to decide whether to continue the agent or switch to another anti psychotic. For example, I’ve had a number of children—many with significant weight problems—whose psychotic symptoms have responded only to risperidone. So we put them back on risperidone, and the decision then becomes what can we do to help with the weight gain while continuing that agent.
For weight gain, I think the best intervention is diet, exercise, and drinking a lot of water, but that can be effective only if you engage the patient’s entire family in the intervention as well. Short of that, a number of pharmacologic interventions have been studied, although not specifically in children.
In an open-label trial our group conducted with 11 children age 10 to 18 years who had gained weight while taking atypical antipsychotics, metformin decreased lipid levels and body mass index but not significantly. I’ve followed these children in my practice, however, and all those who continued taking metformin over a period of months lost weight.
TEOSS study adds to debate about efficacy and tolerability
The 5-year National Institute of Mental Health-funded Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) trial began with an ambitious goal: to compare the efficacy and safety of 1 typical and 2 atypical antipsychotics in children age 8 to 19 with schizophrenia. The primary hypothesis was that atypical agents would show greater efficacy and tolerability when given for 8 weeks. Instead, the atypical agents showed no greater efficacy, and adverse effects occurred with all 3 antipsychotics. Because the trial was designed for 168 subjects but enrolled 119, it may not have been adequately powered to detect differences among the 3 agents.
Medications: Most of the 116 children who received medications were severely ill with psychotic symptoms when randomly assigned to 1 of the 3 antipsychotics for 8 weeks of double-blind treatment. Administration began at the lowest dose in a set range and usually was increased to midrange within 10 to 14 days. Dosing remained flexible within these ranges:
- molindone, 10 to 140 mg/d (mean endpoint dose 59.9 mg/d)
- olanzapine, 2.5 to 20 mg/d (mean endpoint dose 11.4 mg/d)
- risperidone, 0.5 to 6 mg/d (mean endpoint dose 2.8 mg/d).
Benztropine, ≥1 mg/d, was given to all patients treated with molindone, 14% of those treated with olanzapine, and 34% of those treated with risperidone to prevent or manage akathisia.
Efficacy: Two criteria defined treatment response: a Clinical Global Impression improvement score of 1 or 2 and a ≥20% reduction in baseline Positive and Negative Syndrome Scale (PANSS) score. Tolerability outcomes included neurologic side effects, weight changes, laboratory analyses, vital signs, ECG, serious adverse events, and treatment discontinuation. Extrapyramidal symptoms were monitored with involuntary movement and akathisia scales.
Observed PANSS total score by week of treatment
Mean Positive and Negative Symptom Scale (PANSS) total scores of observed cases during each week of the TEOSS trial. Minimum possible PANSS score is 30; scores >60 typically are viewed as problematic.
Among the 70 patients who completed treatment (25 of 40 with molindone, 17 of 35 olanzapine, and 28 of 41 with risperidone), more than one-half failed to achieve an adequate response. Response rates were 50% with molindone, 34% with olanzapine, and 46% with risperidone. The atypical antipsychotics did not show greater efficacy than molindone, and mean reductions in psychotic symptoms were modest (20% to 34% on the PANSS). Mean medication doses were midrange and considered moderate.
Tolerability: Sedation, irritability, and anxiety were frequent adverse events. Patients receiving molindone reported significantly higher rates of akathisia (P < .0008). Those receiving olanzapine reported significantly higher rates of weight gain (P < .0001) and were the only group with increased lipid and insulin serum levels and liver function tests. Patients in the risperidone group reported significantly higher rates of constipation (P < .021) and were the only group that experienced elevated serum prolactin.
Source: Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431
Choosing antipsychotics
DR. KOWATCH: Let’s say you’re seeing psychosis in a 12-year-old whom you think is schizophrenic, and he or she has not yet received an antipsychotic. What are your top 3 treatment choices?
DR. FRAZIER: The first agent I usually select is risperidone. We have the most data on the use of this atypical antipsychotic in children and adolescents, and most psychotic children I see do better with a bit more D2 blockade than some of the other atypicals provide. That said, I remain concerned about risperidone’s side effects—such as weight gain and increased serum prolactin—so my usual second-line agent is aripiprazole.
I became interested in the complicated overlap between pervasive developmental disorders spectrum and psychotic disorders early in my training. More is known about prodromal symptoms in adolescents and adults than in children.
A group in the Netherlands5 compared 32 adolescents with severe early deficits in affect regulation, anxiety, disturbed social relationships, and thought disorder (characterized as “multiple complex developmental disorder” [MCDD]) with 80 adolescents with prodromal psychotic symptoms who met criteria for “at-risk mental state” (ARMS). Three-quarters of the children with MCDD (78%) were found to meet criteria for ARMS, and the 2 groups showed similar schizotypal traits, disorganization, and prodromal symptoms.
Signs of progression to psychosis and schizophrenia in children typically include:
- change in personality
- decrease in functioning or decline in ability to perform at school
- unusual thoughts or behaviors
- crippling anxiety
- supersensitivity to stress.
With experience, the clinician can more clearly differentiate the prodromal signs of psychosis from normal childhood behaviors. Children who are psychotic often don’t make good eye contact. When you try to engage them in discussion about hearing voices, they’re inattentive and internally preoccupied.
Normal vs psychotic children. You want a child in the latency age to have a rich fantasy life. If they do not, that raises concerns. Both normal and psychotic children sometimes say an imaginary friend told them something. Normal children eventually will admit this friend is imaginary. When children are psychotic, especially at an early age, you can’t pull them out of thinking about the imaginary friend, and they can’t distinguish fantasy from reality. Psychotic children also hear imaginary friends talking to them much more often.
Normal children usually are not afraid of their imaginary friends, whereas psychotic children—particularly adolescents—often are afraid of the voices they hear. However, if a psychotic child has heard voices from a young age, the voices aren’t always ego-dystonic. The girl I mentioned at the beginning of this article likes having the voices around. In fact, she gets uncomfortable when the voices are quiet.—Jean A. Frazier, MD
DR. KOWATCH: Why do you like aripiprazole for this patient population?
DR. FRAZIER: Aripiprazole doesn’t tend to be associated with as much weight gain as olanzapine or risperidone, although I’ve had children—especially in the autism spectrum—who have gained quite a bit of weight on aripiprazole. Clinically, I’ve noticed that aripiprazole seems to brighten up children’s affect. It also seems to help many children in my practice with attentional symptoms, although that’s anecdotal.
Although we don’t have a lot of data to inform this discussion about aripiprazole, a placebo-controlled study of 302 adolescents diagnosed with schizophrenia showed that aripiprazole, 10 mg/d, targeted negative symptoms fairly well, based on changes from baseline in PANSS (Positive and Negative Syndrome Scale) total scores. This was a 6-week multicenter, double-blind, randomized, trial.2
Ultimately, cognition in patients with schizophrenia is the strongest predictor of success in the workplace and in school. We need data on what happens to neurocognitive functioning with aripiprazole—and all the other atypical agents.
DR. KOWATCH: What would be your third-line agent?
DR. FRAZIER: Well, that varies for me. I’m trying to match the medication I use with the individual patient, and at this point I prescribe based on the side-effect profile more than anything else. I also consider if the child has a family member who has suffered from a similar condition and what agents the family member responded to.
Let’s say I have a child who has tried 1 or 2 atypical antipsychotics and has not had a good response. Many times I decide to try yet another atypical, and often I will try quetiapine. But after a patient has not responded to 2 atypicals, I might start thinking about a typical agent or clozapine. I use clozapine quite a bit. I find it is the most efficacious agent available, and the data speak to this as well.3,4 It has been truly remarkable for some children in my practice.
Less than 50% chance of efficacy?
DR. KOWATCH: The TEOSS study found 50% or lower response rates across 8 weeks of antipsychotic treatment. Clinically, what kind of response rates do you see with antipsychotics in children and adolescents?
DR. FRAZIER: I probably see about a 50% response rate in my practice as well. It’s variable, and the earlier the onset of the illness, the harder it is to treat.
DR. KOWATCH: Do you ever combine a typical antipsychotic with an atypical?
DR. FRAZIER: I try not to, but a number of children in the schizophrenia spectrum have enduring positive symptoms after 2 or 3 trials of atypical antipsychotics. Sometimes adding a touch of a typical agent can improve the situation. The typicals I usually try are perphenazine (around 8 to 16 mg/d) or molin done (around 20 to 60 mg/d). Sometimes I use a very low dose of haloperidol (such as 0.5 to 2 mg/d) with an atypical agent, and it can be quite effective.
DR. KOWATCH: That has been our experience as well; sometimes combining typical and atypical agents improves response. Besides medications, what do you consider an optimal treatment plan for a child or adolescent with psychosis?
DR. FRAZIER: These children need a multi-modal approach. Pharmacotherapy is the cornerstone because you want to decrease positive symptoms of psychosis, but often these children require therapeutic school placements or residential programs. If they’re old enough, cognitive-behavioral therapy can help by teaching them skills to manage ongoing psychotic symptoms. Older teens often have comorbid substance abuse and may require substance abuse intervention.
Are antipsychotics overused?
DR. KOWATCH: Do you think antipsychotics are overused in pediatric patients with psychosis?
DR. FRAZIER: In pediatric patients with psychosis? No.
DR. KOWATCH: What about in pediatric patients with behavioral disorders?
DR. FRAZIER: We need more studies to inform our practice and to be mindful of the evidence. Most children with schizophrenia have substantial developmental challenges (Box 2).5 In the autism spectrum, often an atypical antipsychotic is the only agent that can help a patient who is aggressive, self-injurious, or agitated.
In terms of bipolar disorder in children and adolescents, it would be ideal if we had more head-to-head comparator studies to inform our prescriptive practice. For example, we need more studies comparing traditional mood stabilizers such as lithium with the atypical agents.
Of course it would be ideal if we could use monotherapy in children who suffer from bipolar disorder and schizophrenia. But early-onset bipolar disorder—like early-onset schizophrenia—can be very difficult to treat and often requires more than 1 agent.
In a recent study of a pharmacotherapy algorithm for treating pediatric bipolar disorder,3 the children who did the best were on a combination of a mood stabilizer and an atypical antipsychotic. That has been my experience, too. I do my best to manage children on a mood stabilizer alone, but I rarely have been able to do that.
In terms of attention-deficit/hyperactivity disorder (ADHD), it depends on what’s going on with the child. Certain children with an ADHD diagnosis have complicated behavioral issues. First I would wonder if they have a different diagnosis, particularly if it gets to the point that an atypical agent is being considered. But sometimes it becomes a question of treating pronounced aggression. We need more studies to inform what we do. Some studies indicate that stimulants can be quite helpful for the aggressive child with ADHD.7
DR. KOWATCH: I don’t see any child and adolescent psychiatrist in the United States using antipsychotics to treat uncomplicated ADHD. The kids we see [at specialty clinics] have comorbid problems such as conduct disorder, oppositional-defiant disorder, mood instability—whatever you want to call it. And we’re seeing these patients because they haven’t done well on other medications, such as stimulants. Usually the parents are desperate because these children are moody and aggressive. I don’t think anybody wants to treat children with antipsychotics or mood stabilizers, but it’s what keeps these children well.
DR. FRAZIER: Yes, I agree.
Related resources
- Longitudinal assessment and monitoring of clinical status and brain function in adolescents and adults. Boston Center for Intervention Development and Applied Research (CIDAR) study. www.bostoncidar.org.
- Frazier JA, Hodge S, Breeze JL, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008;34(1):37-46.
- Frazier JA, McClellan J, Findling RL, et al. Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS): demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2007;46:979-988.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Metformin • Glucophage
- Molindone • Moban
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosures
Dr. Kowatch receives grant/research support from the Stanley Foundation, National Institute of Mental Health, National Institute of Child Health and Human Development, and the National Alliance for Research on Schizophrenia and Depression. He is a consultant to AstraZeneca and Forest Pharmaceuticals and a speaker for AstraZeneca.
Dr. Frazier receives grant/research support from Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Johnson & Johnson, Neuropharm, Otsuka America Pharmaceuticals, and Pfizer Inc.
1. Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431.
2. Findling RL, Robb A, Nyilas M, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165(11):1432-1441.
3. Findling RL, Frazier JA, Gerbino-Rosen G, et al. Is there a role for clozapine in the treatment of children and adolescents? J Am Acad Child Adolesc Psychiatry. 2007;46(3):423-428.
4. Kim Y, Kim BN, Cho SC, et al. Long-term sustained benefits of clozapine treatment in refractory early onset schizophrenia: a retrospective study in Korean children and adolescents. Hum Psychopharmacol. 2008;23(8):715-722.
5. Sprong M, Becker HE, Schothorst PF, et al. Pathways to psychosis: a comparison of the pervasive developmental disorder subtype multiple complex developmental disorder and the “at risk mental state.” Schizophr Res. 2008;99:38-47.
6. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004;43(7):859-867.
7. Sinzig J, Döpfner M, Lehmkuhl G, et al. Long-acting methylphenidate has an effect on aggressive behavior in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17(4):421-432.
“A patient I’ve seen for a number of years had been diagnosed in the pervasive developmental disorder spectrum, but she was quite atypical. Her perseverative thinking focused on a fantasy world, and she was so preoccupied that it was very difficult to pull her out of it. Now at age 12, she has a full-blown psychotic disorder, and the fantasy world is enveloping her. She hears people talking to her all day long.”
Jean A. Frazier, MD, who treats this patient and other children with psychotic disorders, was 1 of 4 principal investigators in the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study, a randomized, double-blind, multisite trial funded by the National Institute of Mental Health. The study, published in November 2008,1 compared the efficacy and tolerability of 3 antipsychotics—olanzapine, risperidone, and molindone—in pediatric patients with schizophrenia or schizoaffective disorder (Box 1).
Dr. Frazier discusses the unexpected findings of the TEOSS trial with Current Psychiatry Section Editor Robert A. Kowatch, MD, PhD. Based on the trial findings and her experience, she tells how she makes decisions when prescribing antipsychotics for children and adolescents with schizophrenia and related disorders.
DR. KOWATCH: The TEOSS trial found no significant differences in efficacy between molindone and the atypical antipsychotics (olanzapine and risperidone) included in the study. You’ve prescribed both typical and atypical antipsychotics in research and in your clinical practice. Do you believe there’s any difference between the 2 classes?
DR. FRAZIER: There are some differences. For example, treatment-refractory patients, especially young children, sometimes need more D2 blockade than some atypical antipsychotics provide. I’ve seen more extra pyramidal side effects with the typical antipsychotics than the atypicals, although it’s not uncommon to see some akathisia with aripiprazole or some dystonia and dyskinesia with risperidone.
DR. KOWATCH: What are the benefits and risks of using antipsychotics in young children?
DR. FRAZIER: The benefit is that antipsychotics can decrease children’s suffering and get them more centered in reality so they can enjoy their friends and progress in school. And when that happens, it’s wonderful. What are the risks? With the atypicals my greatest concern is weight gain, and with the typical agents it’s tardive dyskinesia.
DR. KOWATCH: Have you changed the way you prescribe antipsychotics as a result of the TEOSS study?
DR. FRAZIER: Actually, I have. Clinicians have to be very careful about selecting psychotropic agents that can worsen pediatric-onset obesity. Olanzapine is an effective agent for targeting psychosis and mood symptoms, but the weight gain associated with it is a concern. I do not prescribe olanzapine as much as I have in the past, although I keep it in my armamentarium and tend to reserve it for third- or fourth-line therapy.
I have found molindone to be quite effective in children with schizophrenia or schizoaffective disorder, especially in those who have gained a lot of weight on atypical antipsychotics. They usually lose weight on molindone.
DR. KOWATCH: Do you think the TEOSS study had adequate power to demonstrate differences among molindone, olanzapine, and risperidone?
DR. FRAZIER: We enrolled 119 patients—which is large for a study such as this—but we did not reach our target of 168 patients, which might have increased our power to detect differences. Among the children we did enroll, the 3 antipsychotics showed no difference in efficacy, but the meaningful finding of this study to me was the side effect profile of these agents.
DR. KOWATCH: You mean weight gain with olanzapine and extrapyramidal symptoms with molindone?
DR. FRAZIER: Yes.
Managing side effects
DR. KOWATCH: How do you manage antipsychotic side effects?
DR. FRAZIER: For any of the antipsychotics’ side effects, you have to decide whether to continue the agent or switch to another anti psychotic. For example, I’ve had a number of children—many with significant weight problems—whose psychotic symptoms have responded only to risperidone. So we put them back on risperidone, and the decision then becomes what can we do to help with the weight gain while continuing that agent.
For weight gain, I think the best intervention is diet, exercise, and drinking a lot of water, but that can be effective only if you engage the patient’s entire family in the intervention as well. Short of that, a number of pharmacologic interventions have been studied, although not specifically in children.
In an open-label trial our group conducted with 11 children age 10 to 18 years who had gained weight while taking atypical antipsychotics, metformin decreased lipid levels and body mass index but not significantly. I’ve followed these children in my practice, however, and all those who continued taking metformin over a period of months lost weight.
TEOSS study adds to debate about efficacy and tolerability
The 5-year National Institute of Mental Health-funded Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) trial began with an ambitious goal: to compare the efficacy and safety of 1 typical and 2 atypical antipsychotics in children age 8 to 19 with schizophrenia. The primary hypothesis was that atypical agents would show greater efficacy and tolerability when given for 8 weeks. Instead, the atypical agents showed no greater efficacy, and adverse effects occurred with all 3 antipsychotics. Because the trial was designed for 168 subjects but enrolled 119, it may not have been adequately powered to detect differences among the 3 agents.
Medications: Most of the 116 children who received medications were severely ill with psychotic symptoms when randomly assigned to 1 of the 3 antipsychotics for 8 weeks of double-blind treatment. Administration began at the lowest dose in a set range and usually was increased to midrange within 10 to 14 days. Dosing remained flexible within these ranges:
- molindone, 10 to 140 mg/d (mean endpoint dose 59.9 mg/d)
- olanzapine, 2.5 to 20 mg/d (mean endpoint dose 11.4 mg/d)
- risperidone, 0.5 to 6 mg/d (mean endpoint dose 2.8 mg/d).
Benztropine, ≥1 mg/d, was given to all patients treated with molindone, 14% of those treated with olanzapine, and 34% of those treated with risperidone to prevent or manage akathisia.
Efficacy: Two criteria defined treatment response: a Clinical Global Impression improvement score of 1 or 2 and a ≥20% reduction in baseline Positive and Negative Syndrome Scale (PANSS) score. Tolerability outcomes included neurologic side effects, weight changes, laboratory analyses, vital signs, ECG, serious adverse events, and treatment discontinuation. Extrapyramidal symptoms were monitored with involuntary movement and akathisia scales.
Observed PANSS total score by week of treatment
Mean Positive and Negative Symptom Scale (PANSS) total scores of observed cases during each week of the TEOSS trial. Minimum possible PANSS score is 30; scores >60 typically are viewed as problematic.
Among the 70 patients who completed treatment (25 of 40 with molindone, 17 of 35 olanzapine, and 28 of 41 with risperidone), more than one-half failed to achieve an adequate response. Response rates were 50% with molindone, 34% with olanzapine, and 46% with risperidone. The atypical antipsychotics did not show greater efficacy than molindone, and mean reductions in psychotic symptoms were modest (20% to 34% on the PANSS). Mean medication doses were midrange and considered moderate.
Tolerability: Sedation, irritability, and anxiety were frequent adverse events. Patients receiving molindone reported significantly higher rates of akathisia (P < .0008). Those receiving olanzapine reported significantly higher rates of weight gain (P < .0001) and were the only group with increased lipid and insulin serum levels and liver function tests. Patients in the risperidone group reported significantly higher rates of constipation (P < .021) and were the only group that experienced elevated serum prolactin.
Source: Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431
Choosing antipsychotics
DR. KOWATCH: Let’s say you’re seeing psychosis in a 12-year-old whom you think is schizophrenic, and he or she has not yet received an antipsychotic. What are your top 3 treatment choices?
DR. FRAZIER: The first agent I usually select is risperidone. We have the most data on the use of this atypical antipsychotic in children and adolescents, and most psychotic children I see do better with a bit more D2 blockade than some of the other atypicals provide. That said, I remain concerned about risperidone’s side effects—such as weight gain and increased serum prolactin—so my usual second-line agent is aripiprazole.
I became interested in the complicated overlap between pervasive developmental disorders spectrum and psychotic disorders early in my training. More is known about prodromal symptoms in adolescents and adults than in children.
A group in the Netherlands5 compared 32 adolescents with severe early deficits in affect regulation, anxiety, disturbed social relationships, and thought disorder (characterized as “multiple complex developmental disorder” [MCDD]) with 80 adolescents with prodromal psychotic symptoms who met criteria for “at-risk mental state” (ARMS). Three-quarters of the children with MCDD (78%) were found to meet criteria for ARMS, and the 2 groups showed similar schizotypal traits, disorganization, and prodromal symptoms.
Signs of progression to psychosis and schizophrenia in children typically include:
- change in personality
- decrease in functioning or decline in ability to perform at school
- unusual thoughts or behaviors
- crippling anxiety
- supersensitivity to stress.
With experience, the clinician can more clearly differentiate the prodromal signs of psychosis from normal childhood behaviors. Children who are psychotic often don’t make good eye contact. When you try to engage them in discussion about hearing voices, they’re inattentive and internally preoccupied.
Normal vs psychotic children. You want a child in the latency age to have a rich fantasy life. If they do not, that raises concerns. Both normal and psychotic children sometimes say an imaginary friend told them something. Normal children eventually will admit this friend is imaginary. When children are psychotic, especially at an early age, you can’t pull them out of thinking about the imaginary friend, and they can’t distinguish fantasy from reality. Psychotic children also hear imaginary friends talking to them much more often.
Normal children usually are not afraid of their imaginary friends, whereas psychotic children—particularly adolescents—often are afraid of the voices they hear. However, if a psychotic child has heard voices from a young age, the voices aren’t always ego-dystonic. The girl I mentioned at the beginning of this article likes having the voices around. In fact, she gets uncomfortable when the voices are quiet.—Jean A. Frazier, MD
DR. KOWATCH: Why do you like aripiprazole for this patient population?
DR. FRAZIER: Aripiprazole doesn’t tend to be associated with as much weight gain as olanzapine or risperidone, although I’ve had children—especially in the autism spectrum—who have gained quite a bit of weight on aripiprazole. Clinically, I’ve noticed that aripiprazole seems to brighten up children’s affect. It also seems to help many children in my practice with attentional symptoms, although that’s anecdotal.
Although we don’t have a lot of data to inform this discussion about aripiprazole, a placebo-controlled study of 302 adolescents diagnosed with schizophrenia showed that aripiprazole, 10 mg/d, targeted negative symptoms fairly well, based on changes from baseline in PANSS (Positive and Negative Syndrome Scale) total scores. This was a 6-week multicenter, double-blind, randomized, trial.2
Ultimately, cognition in patients with schizophrenia is the strongest predictor of success in the workplace and in school. We need data on what happens to neurocognitive functioning with aripiprazole—and all the other atypical agents.
DR. KOWATCH: What would be your third-line agent?
DR. FRAZIER: Well, that varies for me. I’m trying to match the medication I use with the individual patient, and at this point I prescribe based on the side-effect profile more than anything else. I also consider if the child has a family member who has suffered from a similar condition and what agents the family member responded to.
Let’s say I have a child who has tried 1 or 2 atypical antipsychotics and has not had a good response. Many times I decide to try yet another atypical, and often I will try quetiapine. But after a patient has not responded to 2 atypicals, I might start thinking about a typical agent or clozapine. I use clozapine quite a bit. I find it is the most efficacious agent available, and the data speak to this as well.3,4 It has been truly remarkable for some children in my practice.
Less than 50% chance of efficacy?
DR. KOWATCH: The TEOSS study found 50% or lower response rates across 8 weeks of antipsychotic treatment. Clinically, what kind of response rates do you see with antipsychotics in children and adolescents?
DR. FRAZIER: I probably see about a 50% response rate in my practice as well. It’s variable, and the earlier the onset of the illness, the harder it is to treat.
DR. KOWATCH: Do you ever combine a typical antipsychotic with an atypical?
DR. FRAZIER: I try not to, but a number of children in the schizophrenia spectrum have enduring positive symptoms after 2 or 3 trials of atypical antipsychotics. Sometimes adding a touch of a typical agent can improve the situation. The typicals I usually try are perphenazine (around 8 to 16 mg/d) or molin done (around 20 to 60 mg/d). Sometimes I use a very low dose of haloperidol (such as 0.5 to 2 mg/d) with an atypical agent, and it can be quite effective.
DR. KOWATCH: That has been our experience as well; sometimes combining typical and atypical agents improves response. Besides medications, what do you consider an optimal treatment plan for a child or adolescent with psychosis?
DR. FRAZIER: These children need a multi-modal approach. Pharmacotherapy is the cornerstone because you want to decrease positive symptoms of psychosis, but often these children require therapeutic school placements or residential programs. If they’re old enough, cognitive-behavioral therapy can help by teaching them skills to manage ongoing psychotic symptoms. Older teens often have comorbid substance abuse and may require substance abuse intervention.
Are antipsychotics overused?
DR. KOWATCH: Do you think antipsychotics are overused in pediatric patients with psychosis?
DR. FRAZIER: In pediatric patients with psychosis? No.
DR. KOWATCH: What about in pediatric patients with behavioral disorders?
DR. FRAZIER: We need more studies to inform our practice and to be mindful of the evidence. Most children with schizophrenia have substantial developmental challenges (Box 2).5 In the autism spectrum, often an atypical antipsychotic is the only agent that can help a patient who is aggressive, self-injurious, or agitated.
In terms of bipolar disorder in children and adolescents, it would be ideal if we had more head-to-head comparator studies to inform our prescriptive practice. For example, we need more studies comparing traditional mood stabilizers such as lithium with the atypical agents.
Of course it would be ideal if we could use monotherapy in children who suffer from bipolar disorder and schizophrenia. But early-onset bipolar disorder—like early-onset schizophrenia—can be very difficult to treat and often requires more than 1 agent.
In a recent study of a pharmacotherapy algorithm for treating pediatric bipolar disorder,3 the children who did the best were on a combination of a mood stabilizer and an atypical antipsychotic. That has been my experience, too. I do my best to manage children on a mood stabilizer alone, but I rarely have been able to do that.
In terms of attention-deficit/hyperactivity disorder (ADHD), it depends on what’s going on with the child. Certain children with an ADHD diagnosis have complicated behavioral issues. First I would wonder if they have a different diagnosis, particularly if it gets to the point that an atypical agent is being considered. But sometimes it becomes a question of treating pronounced aggression. We need more studies to inform what we do. Some studies indicate that stimulants can be quite helpful for the aggressive child with ADHD.7
DR. KOWATCH: I don’t see any child and adolescent psychiatrist in the United States using antipsychotics to treat uncomplicated ADHD. The kids we see [at specialty clinics] have comorbid problems such as conduct disorder, oppositional-defiant disorder, mood instability—whatever you want to call it. And we’re seeing these patients because they haven’t done well on other medications, such as stimulants. Usually the parents are desperate because these children are moody and aggressive. I don’t think anybody wants to treat children with antipsychotics or mood stabilizers, but it’s what keeps these children well.
DR. FRAZIER: Yes, I agree.
Related resources
- Longitudinal assessment and monitoring of clinical status and brain function in adolescents and adults. Boston Center for Intervention Development and Applied Research (CIDAR) study. www.bostoncidar.org.
- Frazier JA, Hodge S, Breeze JL, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008;34(1):37-46.
- Frazier JA, McClellan J, Findling RL, et al. Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS): demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2007;46:979-988.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Metformin • Glucophage
- Molindone • Moban
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosures
Dr. Kowatch receives grant/research support from the Stanley Foundation, National Institute of Mental Health, National Institute of Child Health and Human Development, and the National Alliance for Research on Schizophrenia and Depression. He is a consultant to AstraZeneca and Forest Pharmaceuticals and a speaker for AstraZeneca.
Dr. Frazier receives grant/research support from Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Johnson & Johnson, Neuropharm, Otsuka America Pharmaceuticals, and Pfizer Inc.
“A patient I’ve seen for a number of years had been diagnosed in the pervasive developmental disorder spectrum, but she was quite atypical. Her perseverative thinking focused on a fantasy world, and she was so preoccupied that it was very difficult to pull her out of it. Now at age 12, she has a full-blown psychotic disorder, and the fantasy world is enveloping her. She hears people talking to her all day long.”
Jean A. Frazier, MD, who treats this patient and other children with psychotic disorders, was 1 of 4 principal investigators in the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study, a randomized, double-blind, multisite trial funded by the National Institute of Mental Health. The study, published in November 2008,1 compared the efficacy and tolerability of 3 antipsychotics—olanzapine, risperidone, and molindone—in pediatric patients with schizophrenia or schizoaffective disorder (Box 1).
Dr. Frazier discusses the unexpected findings of the TEOSS trial with Current Psychiatry Section Editor Robert A. Kowatch, MD, PhD. Based on the trial findings and her experience, she tells how she makes decisions when prescribing antipsychotics for children and adolescents with schizophrenia and related disorders.
DR. KOWATCH: The TEOSS trial found no significant differences in efficacy between molindone and the atypical antipsychotics (olanzapine and risperidone) included in the study. You’ve prescribed both typical and atypical antipsychotics in research and in your clinical practice. Do you believe there’s any difference between the 2 classes?
DR. FRAZIER: There are some differences. For example, treatment-refractory patients, especially young children, sometimes need more D2 blockade than some atypical antipsychotics provide. I’ve seen more extra pyramidal side effects with the typical antipsychotics than the atypicals, although it’s not uncommon to see some akathisia with aripiprazole or some dystonia and dyskinesia with risperidone.
DR. KOWATCH: What are the benefits and risks of using antipsychotics in young children?
DR. FRAZIER: The benefit is that antipsychotics can decrease children’s suffering and get them more centered in reality so they can enjoy their friends and progress in school. And when that happens, it’s wonderful. What are the risks? With the atypicals my greatest concern is weight gain, and with the typical agents it’s tardive dyskinesia.
DR. KOWATCH: Have you changed the way you prescribe antipsychotics as a result of the TEOSS study?
DR. FRAZIER: Actually, I have. Clinicians have to be very careful about selecting psychotropic agents that can worsen pediatric-onset obesity. Olanzapine is an effective agent for targeting psychosis and mood symptoms, but the weight gain associated with it is a concern. I do not prescribe olanzapine as much as I have in the past, although I keep it in my armamentarium and tend to reserve it for third- or fourth-line therapy.
I have found molindone to be quite effective in children with schizophrenia or schizoaffective disorder, especially in those who have gained a lot of weight on atypical antipsychotics. They usually lose weight on molindone.
DR. KOWATCH: Do you think the TEOSS study had adequate power to demonstrate differences among molindone, olanzapine, and risperidone?
DR. FRAZIER: We enrolled 119 patients—which is large for a study such as this—but we did not reach our target of 168 patients, which might have increased our power to detect differences. Among the children we did enroll, the 3 antipsychotics showed no difference in efficacy, but the meaningful finding of this study to me was the side effect profile of these agents.
DR. KOWATCH: You mean weight gain with olanzapine and extrapyramidal symptoms with molindone?
DR. FRAZIER: Yes.
Managing side effects
DR. KOWATCH: How do you manage antipsychotic side effects?
DR. FRAZIER: For any of the antipsychotics’ side effects, you have to decide whether to continue the agent or switch to another anti psychotic. For example, I’ve had a number of children—many with significant weight problems—whose psychotic symptoms have responded only to risperidone. So we put them back on risperidone, and the decision then becomes what can we do to help with the weight gain while continuing that agent.
For weight gain, I think the best intervention is diet, exercise, and drinking a lot of water, but that can be effective only if you engage the patient’s entire family in the intervention as well. Short of that, a number of pharmacologic interventions have been studied, although not specifically in children.
In an open-label trial our group conducted with 11 children age 10 to 18 years who had gained weight while taking atypical antipsychotics, metformin decreased lipid levels and body mass index but not significantly. I’ve followed these children in my practice, however, and all those who continued taking metformin over a period of months lost weight.
TEOSS study adds to debate about efficacy and tolerability
The 5-year National Institute of Mental Health-funded Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) trial began with an ambitious goal: to compare the efficacy and safety of 1 typical and 2 atypical antipsychotics in children age 8 to 19 with schizophrenia. The primary hypothesis was that atypical agents would show greater efficacy and tolerability when given for 8 weeks. Instead, the atypical agents showed no greater efficacy, and adverse effects occurred with all 3 antipsychotics. Because the trial was designed for 168 subjects but enrolled 119, it may not have been adequately powered to detect differences among the 3 agents.
Medications: Most of the 116 children who received medications were severely ill with psychotic symptoms when randomly assigned to 1 of the 3 antipsychotics for 8 weeks of double-blind treatment. Administration began at the lowest dose in a set range and usually was increased to midrange within 10 to 14 days. Dosing remained flexible within these ranges:
- molindone, 10 to 140 mg/d (mean endpoint dose 59.9 mg/d)
- olanzapine, 2.5 to 20 mg/d (mean endpoint dose 11.4 mg/d)
- risperidone, 0.5 to 6 mg/d (mean endpoint dose 2.8 mg/d).
Benztropine, ≥1 mg/d, was given to all patients treated with molindone, 14% of those treated with olanzapine, and 34% of those treated with risperidone to prevent or manage akathisia.
Efficacy: Two criteria defined treatment response: a Clinical Global Impression improvement score of 1 or 2 and a ≥20% reduction in baseline Positive and Negative Syndrome Scale (PANSS) score. Tolerability outcomes included neurologic side effects, weight changes, laboratory analyses, vital signs, ECG, serious adverse events, and treatment discontinuation. Extrapyramidal symptoms were monitored with involuntary movement and akathisia scales.
Observed PANSS total score by week of treatment
Mean Positive and Negative Symptom Scale (PANSS) total scores of observed cases during each week of the TEOSS trial. Minimum possible PANSS score is 30; scores >60 typically are viewed as problematic.
Among the 70 patients who completed treatment (25 of 40 with molindone, 17 of 35 olanzapine, and 28 of 41 with risperidone), more than one-half failed to achieve an adequate response. Response rates were 50% with molindone, 34% with olanzapine, and 46% with risperidone. The atypical antipsychotics did not show greater efficacy than molindone, and mean reductions in psychotic symptoms were modest (20% to 34% on the PANSS). Mean medication doses were midrange and considered moderate.
Tolerability: Sedation, irritability, and anxiety were frequent adverse events. Patients receiving molindone reported significantly higher rates of akathisia (P < .0008). Those receiving olanzapine reported significantly higher rates of weight gain (P < .0001) and were the only group with increased lipid and insulin serum levels and liver function tests. Patients in the risperidone group reported significantly higher rates of constipation (P < .021) and were the only group that experienced elevated serum prolactin.
Source: Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431
Choosing antipsychotics
DR. KOWATCH: Let’s say you’re seeing psychosis in a 12-year-old whom you think is schizophrenic, and he or she has not yet received an antipsychotic. What are your top 3 treatment choices?
DR. FRAZIER: The first agent I usually select is risperidone. We have the most data on the use of this atypical antipsychotic in children and adolescents, and most psychotic children I see do better with a bit more D2 blockade than some of the other atypicals provide. That said, I remain concerned about risperidone’s side effects—such as weight gain and increased serum prolactin—so my usual second-line agent is aripiprazole.
I became interested in the complicated overlap between pervasive developmental disorders spectrum and psychotic disorders early in my training. More is known about prodromal symptoms in adolescents and adults than in children.
A group in the Netherlands5 compared 32 adolescents with severe early deficits in affect regulation, anxiety, disturbed social relationships, and thought disorder (characterized as “multiple complex developmental disorder” [MCDD]) with 80 adolescents with prodromal psychotic symptoms who met criteria for “at-risk mental state” (ARMS). Three-quarters of the children with MCDD (78%) were found to meet criteria for ARMS, and the 2 groups showed similar schizotypal traits, disorganization, and prodromal symptoms.
Signs of progression to psychosis and schizophrenia in children typically include:
- change in personality
- decrease in functioning or decline in ability to perform at school
- unusual thoughts or behaviors
- crippling anxiety
- supersensitivity to stress.
With experience, the clinician can more clearly differentiate the prodromal signs of psychosis from normal childhood behaviors. Children who are psychotic often don’t make good eye contact. When you try to engage them in discussion about hearing voices, they’re inattentive and internally preoccupied.
Normal vs psychotic children. You want a child in the latency age to have a rich fantasy life. If they do not, that raises concerns. Both normal and psychotic children sometimes say an imaginary friend told them something. Normal children eventually will admit this friend is imaginary. When children are psychotic, especially at an early age, you can’t pull them out of thinking about the imaginary friend, and they can’t distinguish fantasy from reality. Psychotic children also hear imaginary friends talking to them much more often.
Normal children usually are not afraid of their imaginary friends, whereas psychotic children—particularly adolescents—often are afraid of the voices they hear. However, if a psychotic child has heard voices from a young age, the voices aren’t always ego-dystonic. The girl I mentioned at the beginning of this article likes having the voices around. In fact, she gets uncomfortable when the voices are quiet.—Jean A. Frazier, MD
DR. KOWATCH: Why do you like aripiprazole for this patient population?
DR. FRAZIER: Aripiprazole doesn’t tend to be associated with as much weight gain as olanzapine or risperidone, although I’ve had children—especially in the autism spectrum—who have gained quite a bit of weight on aripiprazole. Clinically, I’ve noticed that aripiprazole seems to brighten up children’s affect. It also seems to help many children in my practice with attentional symptoms, although that’s anecdotal.
Although we don’t have a lot of data to inform this discussion about aripiprazole, a placebo-controlled study of 302 adolescents diagnosed with schizophrenia showed that aripiprazole, 10 mg/d, targeted negative symptoms fairly well, based on changes from baseline in PANSS (Positive and Negative Syndrome Scale) total scores. This was a 6-week multicenter, double-blind, randomized, trial.2
Ultimately, cognition in patients with schizophrenia is the strongest predictor of success in the workplace and in school. We need data on what happens to neurocognitive functioning with aripiprazole—and all the other atypical agents.
DR. KOWATCH: What would be your third-line agent?
DR. FRAZIER: Well, that varies for me. I’m trying to match the medication I use with the individual patient, and at this point I prescribe based on the side-effect profile more than anything else. I also consider if the child has a family member who has suffered from a similar condition and what agents the family member responded to.
Let’s say I have a child who has tried 1 or 2 atypical antipsychotics and has not had a good response. Many times I decide to try yet another atypical, and often I will try quetiapine. But after a patient has not responded to 2 atypicals, I might start thinking about a typical agent or clozapine. I use clozapine quite a bit. I find it is the most efficacious agent available, and the data speak to this as well.3,4 It has been truly remarkable for some children in my practice.
Less than 50% chance of efficacy?
DR. KOWATCH: The TEOSS study found 50% or lower response rates across 8 weeks of antipsychotic treatment. Clinically, what kind of response rates do you see with antipsychotics in children and adolescents?
DR. FRAZIER: I probably see about a 50% response rate in my practice as well. It’s variable, and the earlier the onset of the illness, the harder it is to treat.
DR. KOWATCH: Do you ever combine a typical antipsychotic with an atypical?
DR. FRAZIER: I try not to, but a number of children in the schizophrenia spectrum have enduring positive symptoms after 2 or 3 trials of atypical antipsychotics. Sometimes adding a touch of a typical agent can improve the situation. The typicals I usually try are perphenazine (around 8 to 16 mg/d) or molin done (around 20 to 60 mg/d). Sometimes I use a very low dose of haloperidol (such as 0.5 to 2 mg/d) with an atypical agent, and it can be quite effective.
DR. KOWATCH: That has been our experience as well; sometimes combining typical and atypical agents improves response. Besides medications, what do you consider an optimal treatment plan for a child or adolescent with psychosis?
DR. FRAZIER: These children need a multi-modal approach. Pharmacotherapy is the cornerstone because you want to decrease positive symptoms of psychosis, but often these children require therapeutic school placements or residential programs. If they’re old enough, cognitive-behavioral therapy can help by teaching them skills to manage ongoing psychotic symptoms. Older teens often have comorbid substance abuse and may require substance abuse intervention.
Are antipsychotics overused?
DR. KOWATCH: Do you think antipsychotics are overused in pediatric patients with psychosis?
DR. FRAZIER: In pediatric patients with psychosis? No.
DR. KOWATCH: What about in pediatric patients with behavioral disorders?
DR. FRAZIER: We need more studies to inform our practice and to be mindful of the evidence. Most children with schizophrenia have substantial developmental challenges (Box 2).5 In the autism spectrum, often an atypical antipsychotic is the only agent that can help a patient who is aggressive, self-injurious, or agitated.
In terms of bipolar disorder in children and adolescents, it would be ideal if we had more head-to-head comparator studies to inform our prescriptive practice. For example, we need more studies comparing traditional mood stabilizers such as lithium with the atypical agents.
Of course it would be ideal if we could use monotherapy in children who suffer from bipolar disorder and schizophrenia. But early-onset bipolar disorder—like early-onset schizophrenia—can be very difficult to treat and often requires more than 1 agent.
In a recent study of a pharmacotherapy algorithm for treating pediatric bipolar disorder,3 the children who did the best were on a combination of a mood stabilizer and an atypical antipsychotic. That has been my experience, too. I do my best to manage children on a mood stabilizer alone, but I rarely have been able to do that.
In terms of attention-deficit/hyperactivity disorder (ADHD), it depends on what’s going on with the child. Certain children with an ADHD diagnosis have complicated behavioral issues. First I would wonder if they have a different diagnosis, particularly if it gets to the point that an atypical agent is being considered. But sometimes it becomes a question of treating pronounced aggression. We need more studies to inform what we do. Some studies indicate that stimulants can be quite helpful for the aggressive child with ADHD.7
DR. KOWATCH: I don’t see any child and adolescent psychiatrist in the United States using antipsychotics to treat uncomplicated ADHD. The kids we see [at specialty clinics] have comorbid problems such as conduct disorder, oppositional-defiant disorder, mood instability—whatever you want to call it. And we’re seeing these patients because they haven’t done well on other medications, such as stimulants. Usually the parents are desperate because these children are moody and aggressive. I don’t think anybody wants to treat children with antipsychotics or mood stabilizers, but it’s what keeps these children well.
DR. FRAZIER: Yes, I agree.
Related resources
- Longitudinal assessment and monitoring of clinical status and brain function in adolescents and adults. Boston Center for Intervention Development and Applied Research (CIDAR) study. www.bostoncidar.org.
- Frazier JA, Hodge S, Breeze JL, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008;34(1):37-46.
- Frazier JA, McClellan J, Findling RL, et al. Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS): demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2007;46:979-988.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Clozapine • Clozaril
- Haloperidol • Haldol
- Metformin • Glucophage
- Molindone • Moban
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosures
Dr. Kowatch receives grant/research support from the Stanley Foundation, National Institute of Mental Health, National Institute of Child Health and Human Development, and the National Alliance for Research on Schizophrenia and Depression. He is a consultant to AstraZeneca and Forest Pharmaceuticals and a speaker for AstraZeneca.
Dr. Frazier receives grant/research support from Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Johnson & Johnson, Neuropharm, Otsuka America Pharmaceuticals, and Pfizer Inc.
1. Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431.
2. Findling RL, Robb A, Nyilas M, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165(11):1432-1441.
3. Findling RL, Frazier JA, Gerbino-Rosen G, et al. Is there a role for clozapine in the treatment of children and adolescents? J Am Acad Child Adolesc Psychiatry. 2007;46(3):423-428.
4. Kim Y, Kim BN, Cho SC, et al. Long-term sustained benefits of clozapine treatment in refractory early onset schizophrenia: a retrospective study in Korean children and adolescents. Hum Psychopharmacol. 2008;23(8):715-722.
5. Sprong M, Becker HE, Schothorst PF, et al. Pathways to psychosis: a comparison of the pervasive developmental disorder subtype multiple complex developmental disorder and the “at risk mental state.” Schizophr Res. 2008;99:38-47.
6. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004;43(7):859-867.
7. Sinzig J, Döpfner M, Lehmkuhl G, et al. Long-acting methylphenidate has an effect on aggressive behavior in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17(4):421-432.
1. Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431.
2. Findling RL, Robb A, Nyilas M, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165(11):1432-1441.
3. Findling RL, Frazier JA, Gerbino-Rosen G, et al. Is there a role for clozapine in the treatment of children and adolescents? J Am Acad Child Adolesc Psychiatry. 2007;46(3):423-428.
4. Kim Y, Kim BN, Cho SC, et al. Long-term sustained benefits of clozapine treatment in refractory early onset schizophrenia: a retrospective study in Korean children and adolescents. Hum Psychopharmacol. 2008;23(8):715-722.
5. Sprong M, Becker HE, Schothorst PF, et al. Pathways to psychosis: a comparison of the pervasive developmental disorder subtype multiple complex developmental disorder and the “at risk mental state.” Schizophr Res. 2008;99:38-47.
6. Pavuluri MN, Henry DB, Devineni B, et al. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004;43(7):859-867.
7. Sinzig J, Döpfner M, Lehmkuhl G, et al. Long-acting methylphenidate has an effect on aggressive behavior in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17(4):421-432.
Risperidone’s 2 new pediatric indications
Risperidone is the first second-generation antipsychotic (SGA) to receive FDA approval for treating children and adolescents with bipolar mania or schizophrenia. Specifically, the SGA is indicated for treating schizophrenia in patients age 13 to 17 and as monotherapy in short-term treatment of manic or mixed episodes of bipolar I disorder in patients age 10 to 17 (Table 1).
Risperidone also is approved for:
- schizophrenia in adults
- acute mania or mixed episodes associated with bipolar I disorder in adults, alone or in combination with lithium or valproate
- irritability associated with autistic disorder in patients age 5 to 16.
Table 1
Risperidone: Fast facts
| Brand name: Risperdal |
| Class: Second-generation antipsychotic |
| New indications: Schizophrenia in adolescents age 13 to 17 and monotherapy in short-term treatment of manic or mixed episodes of bipolar I disorder in children and adolescents age 10 to 17. (Risperidone had been approved for schizophrenia and short-term treatment of acute manic or mixed episodes associated with bipolar I disorder in adults and treatment of irritability associated with autistic disorder in children and adolescents.) |
| Approval date: August 22, 2007 for pediatric schizophrenia and mania indications |
| Manufacturer: Janssen, L.P. |
| Dosing forms: 0.25-, 0.5-, 1-, 2-, 3-, and 4-mg tablets; 0.5-, 1-, 2-, 3-, and 4-mg orally disintegrating tablets; 1 mg/mL oral solution |
| Recommended target dosage: 3 mg/d (pediatric schizophrenia) or 2.5 mg/d (pediatric bipolar mania). See Table 2 for initial dosages and titration |
Clinical implications
Risperidone is widely used off-label to treat irritability in children with pervasive developmental disorders,1,2 aggressive behaviors associated with conduct disorder,3 psychotic disorders,4 and bipolar disorder.5 It also has been used off-label to treat pediatric schizophrenia and bipolar disorder for many years.
These 2 new indications give clinicians additional support for using SGAs in children and adolescents with these serious psychiatric disorders.
How it works
Risperidone’s therapeutic activity in schizophrenia seems to be mediated through a combination of dopamine type 2 (D2) and serotonin type 2 (5HT2) receptor antagonism. Antagonism at receptors other than D2 and 5HT2 may explain some of risperidone’s other therapeutic effects.
Pharmacokinetics
In children, the half-lives of risperidone and its major active metabolite 9-hydroxyrisperidone are 3±2.3 hours and 22±46 hours, respectively.6 The pharmacologic activity of 9-hydroxyrisperidone is similar to that of risperidone.
Risperidone is extensively metabolized in the liver by the cytochrome P-450 (CYP) 2D6 enzyme system. The main metabolic pathway is through hydroxylation of risperidone to 9-hydroxyrisperidone by CYP 2D6. Food does not affect the rate or extent of the drug’s absorption.6
Efficacy studies
In schizophrenia. Approval of the indication for pediatric schizophrenia was based on data from 2 short-term (6 and 8 weeks) randomized, double-blind, controlled trials involving a total of 416 patients age 13 to 17 who met DSM-IV-TR criteria for schizophrenia and were experiencing an acute episode at enrollment.7 In one study, patients received risperidone, 1 to 3 mg/d, 4 to 6 mg/d, or placebo. In the other study, dosages were 0.15 to 0.6 mg/d or 1.5 to 6 mg/d. Except for patients in the 0.15 to 0.6 mg group (who initially received 0.05 mg/d), most patients started risperidone at 0.5 mg/d. In both trials, starting dosages were titrated to the target range in approximately 7 days.
Outcomes were measured as changes in total Positive and Negative Syndrome Scale (PANSS) and Personal and Social Performance (PSP) scale scores. The multi-item PANSS inventory measures positive and negative schizophrenia symptoms, disorganized thoughts, uncontrolled hostility/excitement, and anxiety/depression. The PSP gauges personal and social functioning in socially useful activities (work and study), personal and social relationships, self-care, and disturbing/ aggressive behaviors.
Risperidone, 1 to 6 mg/d, improved schizophrenia symptoms significantly more than placebo, as measured by PANSS scores. Doses >3 mg/d did not show greater efficacy than lower doses, as evaluated by PANSS and PSP scores.
Adverse reactions experienced by >5% of patients treated with risperidone included somnolence, parkinsonism, tremor, dystonia, dizziness, akathisia, increased salivation, and anxiety.7
In bipolar I disorder. Risperidone’s efficacy for short-term treatment of mania in children and adolescents was demonstrated in a 3-week, randomized, double-blind, placebo-controlled, multi-center study of 169 patients age 10 to 17 who were experiencing a manic or mixed episode of bipolar I disorder.7 Patients were randomly assigned to risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d, or placebo. All patients were started at 0.5 mg/d and this dose was titrated to the target dosage range in 7 days.
Risperidone, 0.5 to 6 mg/d, significantly decreased the total Young Mania Rating Scale score—a measure of the severity of elevated mood, increased motor activity energy, sexual interest, sleep, irritability, speech (rate/amount), language (thought disorder, content, disruptive), aggressive behavior, appearance, and insight. No evidence of increased efficacy was observed at doses >2.5 mg/d. In this trial, symptoms reported by >5% of patients included fatigue, dizziness, dystonia, parkinsonism, akathisia, abdominal pain, dyspepsia, nausea, vomiting, and diarrhea.7
Pediatric dosing. Based on these studies, the recommended starting dose for children and adolescents is 0.5 mg/d, with titration in 0.5-to 1-mg increments to targets of:
Table 2
Recommended dosing of risperidone
for pediatric schizophrenia and bipolar mania
| Indication | Initial dose | Titration | Target dose | Effective dose range |
|---|---|---|---|---|
| Schizophrenia, adolescents age 13 to 17 | 0.5 mg/d | 0.5 to 1 mg/d | 3 mg/d | 1 to 6 mg/d |
| Bipolar mania, children and adolescents age 10 to 17 | 0.5 mg/d | 0.5 to 1 mg/d | 2.5 mg/d | 0.5 to 6 mg/d |
| Source:Reference 7 | ||||
Tolerability studies
In long-term studies, the most commonly reported adverse events associated with risperidone in children and adolescents have been rhinitis, abdominal pain, increased saliva, body pain, gynecomastia, and weight increase.8 Specific adverse effects that pose long-term concerns are:
- tardive dyskinesia (TD)
- weight gain
- increased prolactin levels
Tardive dyskinesia. In clinical trials that included 1,885 children and adolescents with autistic disorder or other psychiatric disorders treated with risperidone, 2 patients (0.1%) were reported to have TD, which resolved when risperidone was discontinued.7 To monitor for TD, administer the Abnormal Involuntary Movement Scale at baseline and every 6 months while using risperidone in pediatric patients.
Weight gain. In long-term, open-label trials, patients with autistic or other psychiatric disorders gained an average 7.5
kg after 12 months of risperidone treatment. Most of the weight gain occurred in the first 6 months.9 Expected normal weight gain in children is 3 to 3.5 kg/year adjusted for age, based on Centers for Disease Control and Prevention normative data.
Follow the American Diabetes Association guidelines10 for monitoring metabolic parameters during antipsychotic
treatment, and intervene if clinically significant weight gain occurs.
In a 16-week, placebo-controlled study,11 metformin reversed weight gain associated with SGAs in children and adolescents. Metformin’s potential side effects include hypoglycemia, diarrhea, nausea/vomiting, and (rarely) lactic acidosis, but no adverse events were attributed to metformin.
Increased prolactin. As in adults, risperidone elevates serum prolactin in children and adolescents. All pediatric risperidone trials—of autism,2 disruptive behavior disorders in children with subaverage intelligence,9 schizophrenia,7 and bipolar mania—have shown increased serum prolactin. Risperidone’s long-term effects on growth and sexual maturation have not been fully evaluated, but hyperprolactinemia may inhibit reproductive function.
Findling et al12 analyzed data from 5 clinical trials (total 700 patients) in which children and adolescents age 5 to 15 years with subaverage IQs and conduct or other disruptive behavior disorders received risperidone for up to 55 weeks. Mean prolactin levels rose from 7.8 ng/mL
at baseline to 29.4 ng/mL at weeks 4 to 7, then progressively decreased to 16.1 ng/mL at weeks 40 to 48 (n=358) and 13.0 ng/mL at weeks 52 to 55 (n=42). Girls returned to a mean value within the normal range (≤30 ng/mL) by weeks 8 to 12, and boys were close to normal values (≤18 ng/mL) by weeks 16 to 24.
The researchers concluded that serum prolactin levels in children tend to rise and peak within the first 1 to 2 months of risperidone treatment and then steadily decline to values within or very close to normal range by 3 to 5 months.
The biological significance of chronic, mild prolactin elevations is unknown.13 Children entering puberty appear to be at highest risk for elevated prolactin and clinical symptoms while treated with risperidone.14 Therefore, ask all adolescents treated with risperidone about increases in breast size and galactorrhea. Switch those who develop these symptoms to an SGA that does not increase serum prolactin.
Contraindications. Risperidone is contraindicated in patients with a known hypersensitivity to the drug.
- Risperdal prescribing information. www.risperdal.com/risperdal/shared/pi/risperdal.pdf.
Drug brand names
- Lithium • Eskalith, Lithobid
- Risperidone • Risperdal
- Metformin • Glucophage, Fortamet
- Valproate • Depakote
Disclosures
Dr. Kowatch receives research support from Bristol-Meyers Squibb, Stanley Research Foundation, National Institute of Mental Health, and National Institute of Child Health and Human Development. He is a consultant for Creative Educational Concepts, Child and Adolescent Bipolar Foundation, Abbott Laboratories, and sanofi-aventis, and a speaker for Abbott Laboratories and AstraZeneca.
1. Aman MG, De Smedt G, Derivan A, et al. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry 2002;159:1337-46.
2. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
3. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry 2000;39(4):509-16.
4. Sikich L, Hamer R, Malekpour AH, et al. Double-blind trial comparing risperidone, olanzapine, and haloperidol in the treatment of psychotic children and adolescents. Paper presented at: Society of Biological Psychiatry Annual Meeting; May 16-18, 2002; Philadelphia, PA.
5. Frazier JA, Meyer MC, Biederman J, et al. Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry 1999;38(8):960-5.
6. Aman MG, Vinks AA, Remmerie B, et al. Plasma pharmacokinetic characteristics of risperidone and their relationship to saliva concentrations in children with psychiatric or neurodevelopmental disorders. Clin Ther 2007;29(7):1476-86.
7. Risperdal [package insert]. Titusville, NJ: Janssen, L.P; 2007.
8. Reyes MR, Olah R, Csaba K, et al. Long-term safety and efficacy of risperidone in children with disruptive behaviour disorders. Results of a 2-year extension study. Eur Child Adolesc Psychiatry 2006;15(2):97-104.
9. Croonenberghs J, Fegert JM, Findling RL, et al. Risperidone in children with disruptive behavior disorders and subaverage intelligence: a 1-year, open-label study of 504 patients. J Am Acad Child Adolesc Psychiatry 2005;44(1):64-72.
10. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry 2004;65(2):267-72.
11. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry 2006;163(12):2072-9.
12. Findling RL, Kusumakar V, Daneman D, et al. Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry 2003;64(11):1362-9.
13. Staller J. The effect of long-term antipsychotic treatment on prolactin. J Child Adolesc Psychopharmacol 2006;16(3):317-26.
14. Holzer L, Eap CB. Risperidone-induced symptomatic hyperprolactinaemia in adolescents. J Clin Psychopharmacol 2006;26(2):167-71.
Dr. Kowatch is professor of psychiatry and pediatrics at Cincinnati Children’s Hospital Medical Center and a Section Editor for Current Psychiatry.
Risperidone is the first second-generation antipsychotic (SGA) to receive FDA approval for treating children and adolescents with bipolar mania or schizophrenia. Specifically, the SGA is indicated for treating schizophrenia in patients age 13 to 17 and as monotherapy in short-term treatment of manic or mixed episodes of bipolar I disorder in patients age 10 to 17 (Table 1).
Risperidone also is approved for:
- schizophrenia in adults
- acute mania or mixed episodes associated with bipolar I disorder in adults, alone or in combination with lithium or valproate
- irritability associated with autistic disorder in patients age 5 to 16.
Table 1
Risperidone: Fast facts
| Brand name: Risperdal |
| Class: Second-generation antipsychotic |
| New indications: Schizophrenia in adolescents age 13 to 17 and monotherapy in short-term treatment of manic or mixed episodes of bipolar I disorder in children and adolescents age 10 to 17. (Risperidone had been approved for schizophrenia and short-term treatment of acute manic or mixed episodes associated with bipolar I disorder in adults and treatment of irritability associated with autistic disorder in children and adolescents.) |
| Approval date: August 22, 2007 for pediatric schizophrenia and mania indications |
| Manufacturer: Janssen, L.P. |
| Dosing forms: 0.25-, 0.5-, 1-, 2-, 3-, and 4-mg tablets; 0.5-, 1-, 2-, 3-, and 4-mg orally disintegrating tablets; 1 mg/mL oral solution |
| Recommended target dosage: 3 mg/d (pediatric schizophrenia) or 2.5 mg/d (pediatric bipolar mania). See Table 2 for initial dosages and titration |
Clinical implications
Risperidone is widely used off-label to treat irritability in children with pervasive developmental disorders,1,2 aggressive behaviors associated with conduct disorder,3 psychotic disorders,4 and bipolar disorder.5 It also has been used off-label to treat pediatric schizophrenia and bipolar disorder for many years.
These 2 new indications give clinicians additional support for using SGAs in children and adolescents with these serious psychiatric disorders.
How it works
Risperidone’s therapeutic activity in schizophrenia seems to be mediated through a combination of dopamine type 2 (D2) and serotonin type 2 (5HT2) receptor antagonism. Antagonism at receptors other than D2 and 5HT2 may explain some of risperidone’s other therapeutic effects.
Pharmacokinetics
In children, the half-lives of risperidone and its major active metabolite 9-hydroxyrisperidone are 3±2.3 hours and 22±46 hours, respectively.6 The pharmacologic activity of 9-hydroxyrisperidone is similar to that of risperidone.
Risperidone is extensively metabolized in the liver by the cytochrome P-450 (CYP) 2D6 enzyme system. The main metabolic pathway is through hydroxylation of risperidone to 9-hydroxyrisperidone by CYP 2D6. Food does not affect the rate or extent of the drug’s absorption.6
Efficacy studies
In schizophrenia. Approval of the indication for pediatric schizophrenia was based on data from 2 short-term (6 and 8 weeks) randomized, double-blind, controlled trials involving a total of 416 patients age 13 to 17 who met DSM-IV-TR criteria for schizophrenia and were experiencing an acute episode at enrollment.7 In one study, patients received risperidone, 1 to 3 mg/d, 4 to 6 mg/d, or placebo. In the other study, dosages were 0.15 to 0.6 mg/d or 1.5 to 6 mg/d. Except for patients in the 0.15 to 0.6 mg group (who initially received 0.05 mg/d), most patients started risperidone at 0.5 mg/d. In both trials, starting dosages were titrated to the target range in approximately 7 days.
Outcomes were measured as changes in total Positive and Negative Syndrome Scale (PANSS) and Personal and Social Performance (PSP) scale scores. The multi-item PANSS inventory measures positive and negative schizophrenia symptoms, disorganized thoughts, uncontrolled hostility/excitement, and anxiety/depression. The PSP gauges personal and social functioning in socially useful activities (work and study), personal and social relationships, self-care, and disturbing/ aggressive behaviors.
Risperidone, 1 to 6 mg/d, improved schizophrenia symptoms significantly more than placebo, as measured by PANSS scores. Doses >3 mg/d did not show greater efficacy than lower doses, as evaluated by PANSS and PSP scores.
Adverse reactions experienced by >5% of patients treated with risperidone included somnolence, parkinsonism, tremor, dystonia, dizziness, akathisia, increased salivation, and anxiety.7
In bipolar I disorder. Risperidone’s efficacy for short-term treatment of mania in children and adolescents was demonstrated in a 3-week, randomized, double-blind, placebo-controlled, multi-center study of 169 patients age 10 to 17 who were experiencing a manic or mixed episode of bipolar I disorder.7 Patients were randomly assigned to risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d, or placebo. All patients were started at 0.5 mg/d and this dose was titrated to the target dosage range in 7 days.
Risperidone, 0.5 to 6 mg/d, significantly decreased the total Young Mania Rating Scale score—a measure of the severity of elevated mood, increased motor activity energy, sexual interest, sleep, irritability, speech (rate/amount), language (thought disorder, content, disruptive), aggressive behavior, appearance, and insight. No evidence of increased efficacy was observed at doses >2.5 mg/d. In this trial, symptoms reported by >5% of patients included fatigue, dizziness, dystonia, parkinsonism, akathisia, abdominal pain, dyspepsia, nausea, vomiting, and diarrhea.7
Pediatric dosing. Based on these studies, the recommended starting dose for children and adolescents is 0.5 mg/d, with titration in 0.5-to 1-mg increments to targets of:
Table 2
Recommended dosing of risperidone
for pediatric schizophrenia and bipolar mania
| Indication | Initial dose | Titration | Target dose | Effective dose range |
|---|---|---|---|---|
| Schizophrenia, adolescents age 13 to 17 | 0.5 mg/d | 0.5 to 1 mg/d | 3 mg/d | 1 to 6 mg/d |
| Bipolar mania, children and adolescents age 10 to 17 | 0.5 mg/d | 0.5 to 1 mg/d | 2.5 mg/d | 0.5 to 6 mg/d |
| Source:Reference 7 | ||||
Tolerability studies
In long-term studies, the most commonly reported adverse events associated with risperidone in children and adolescents have been rhinitis, abdominal pain, increased saliva, body pain, gynecomastia, and weight increase.8 Specific adverse effects that pose long-term concerns are:
- tardive dyskinesia (TD)
- weight gain
- increased prolactin levels
Tardive dyskinesia. In clinical trials that included 1,885 children and adolescents with autistic disorder or other psychiatric disorders treated with risperidone, 2 patients (0.1%) were reported to have TD, which resolved when risperidone was discontinued.7 To monitor for TD, administer the Abnormal Involuntary Movement Scale at baseline and every 6 months while using risperidone in pediatric patients.
Weight gain. In long-term, open-label trials, patients with autistic or other psychiatric disorders gained an average 7.5
kg after 12 months of risperidone treatment. Most of the weight gain occurred in the first 6 months.9 Expected normal weight gain in children is 3 to 3.5 kg/year adjusted for age, based on Centers for Disease Control and Prevention normative data.
Follow the American Diabetes Association guidelines10 for monitoring metabolic parameters during antipsychotic
treatment, and intervene if clinically significant weight gain occurs.
In a 16-week, placebo-controlled study,11 metformin reversed weight gain associated with SGAs in children and adolescents. Metformin’s potential side effects include hypoglycemia, diarrhea, nausea/vomiting, and (rarely) lactic acidosis, but no adverse events were attributed to metformin.
Increased prolactin. As in adults, risperidone elevates serum prolactin in children and adolescents. All pediatric risperidone trials—of autism,2 disruptive behavior disorders in children with subaverage intelligence,9 schizophrenia,7 and bipolar mania—have shown increased serum prolactin. Risperidone’s long-term effects on growth and sexual maturation have not been fully evaluated, but hyperprolactinemia may inhibit reproductive function.
Findling et al12 analyzed data from 5 clinical trials (total 700 patients) in which children and adolescents age 5 to 15 years with subaverage IQs and conduct or other disruptive behavior disorders received risperidone for up to 55 weeks. Mean prolactin levels rose from 7.8 ng/mL
at baseline to 29.4 ng/mL at weeks 4 to 7, then progressively decreased to 16.1 ng/mL at weeks 40 to 48 (n=358) and 13.0 ng/mL at weeks 52 to 55 (n=42). Girls returned to a mean value within the normal range (≤30 ng/mL) by weeks 8 to 12, and boys were close to normal values (≤18 ng/mL) by weeks 16 to 24.
The researchers concluded that serum prolactin levels in children tend to rise and peak within the first 1 to 2 months of risperidone treatment and then steadily decline to values within or very close to normal range by 3 to 5 months.
The biological significance of chronic, mild prolactin elevations is unknown.13 Children entering puberty appear to be at highest risk for elevated prolactin and clinical symptoms while treated with risperidone.14 Therefore, ask all adolescents treated with risperidone about increases in breast size and galactorrhea. Switch those who develop these symptoms to an SGA that does not increase serum prolactin.
Contraindications. Risperidone is contraindicated in patients with a known hypersensitivity to the drug.
- Risperdal prescribing information. www.risperdal.com/risperdal/shared/pi/risperdal.pdf.
Drug brand names
- Lithium • Eskalith, Lithobid
- Risperidone • Risperdal
- Metformin • Glucophage, Fortamet
- Valproate • Depakote
Disclosures
Dr. Kowatch receives research support from Bristol-Meyers Squibb, Stanley Research Foundation, National Institute of Mental Health, and National Institute of Child Health and Human Development. He is a consultant for Creative Educational Concepts, Child and Adolescent Bipolar Foundation, Abbott Laboratories, and sanofi-aventis, and a speaker for Abbott Laboratories and AstraZeneca.
Risperidone is the first second-generation antipsychotic (SGA) to receive FDA approval for treating children and adolescents with bipolar mania or schizophrenia. Specifically, the SGA is indicated for treating schizophrenia in patients age 13 to 17 and as monotherapy in short-term treatment of manic or mixed episodes of bipolar I disorder in patients age 10 to 17 (Table 1).
Risperidone also is approved for:
- schizophrenia in adults
- acute mania or mixed episodes associated with bipolar I disorder in adults, alone or in combination with lithium or valproate
- irritability associated with autistic disorder in patients age 5 to 16.
Table 1
Risperidone: Fast facts
| Brand name: Risperdal |
| Class: Second-generation antipsychotic |
| New indications: Schizophrenia in adolescents age 13 to 17 and monotherapy in short-term treatment of manic or mixed episodes of bipolar I disorder in children and adolescents age 10 to 17. (Risperidone had been approved for schizophrenia and short-term treatment of acute manic or mixed episodes associated with bipolar I disorder in adults and treatment of irritability associated with autistic disorder in children and adolescents.) |
| Approval date: August 22, 2007 for pediatric schizophrenia and mania indications |
| Manufacturer: Janssen, L.P. |
| Dosing forms: 0.25-, 0.5-, 1-, 2-, 3-, and 4-mg tablets; 0.5-, 1-, 2-, 3-, and 4-mg orally disintegrating tablets; 1 mg/mL oral solution |
| Recommended target dosage: 3 mg/d (pediatric schizophrenia) or 2.5 mg/d (pediatric bipolar mania). See Table 2 for initial dosages and titration |
Clinical implications
Risperidone is widely used off-label to treat irritability in children with pervasive developmental disorders,1,2 aggressive behaviors associated with conduct disorder,3 psychotic disorders,4 and bipolar disorder.5 It also has been used off-label to treat pediatric schizophrenia and bipolar disorder for many years.
These 2 new indications give clinicians additional support for using SGAs in children and adolescents with these serious psychiatric disorders.
How it works
Risperidone’s therapeutic activity in schizophrenia seems to be mediated through a combination of dopamine type 2 (D2) and serotonin type 2 (5HT2) receptor antagonism. Antagonism at receptors other than D2 and 5HT2 may explain some of risperidone’s other therapeutic effects.
Pharmacokinetics
In children, the half-lives of risperidone and its major active metabolite 9-hydroxyrisperidone are 3±2.3 hours and 22±46 hours, respectively.6 The pharmacologic activity of 9-hydroxyrisperidone is similar to that of risperidone.
Risperidone is extensively metabolized in the liver by the cytochrome P-450 (CYP) 2D6 enzyme system. The main metabolic pathway is through hydroxylation of risperidone to 9-hydroxyrisperidone by CYP 2D6. Food does not affect the rate or extent of the drug’s absorption.6
Efficacy studies
In schizophrenia. Approval of the indication for pediatric schizophrenia was based on data from 2 short-term (6 and 8 weeks) randomized, double-blind, controlled trials involving a total of 416 patients age 13 to 17 who met DSM-IV-TR criteria for schizophrenia and were experiencing an acute episode at enrollment.7 In one study, patients received risperidone, 1 to 3 mg/d, 4 to 6 mg/d, or placebo. In the other study, dosages were 0.15 to 0.6 mg/d or 1.5 to 6 mg/d. Except for patients in the 0.15 to 0.6 mg group (who initially received 0.05 mg/d), most patients started risperidone at 0.5 mg/d. In both trials, starting dosages were titrated to the target range in approximately 7 days.
Outcomes were measured as changes in total Positive and Negative Syndrome Scale (PANSS) and Personal and Social Performance (PSP) scale scores. The multi-item PANSS inventory measures positive and negative schizophrenia symptoms, disorganized thoughts, uncontrolled hostility/excitement, and anxiety/depression. The PSP gauges personal and social functioning in socially useful activities (work and study), personal and social relationships, self-care, and disturbing/ aggressive behaviors.
Risperidone, 1 to 6 mg/d, improved schizophrenia symptoms significantly more than placebo, as measured by PANSS scores. Doses >3 mg/d did not show greater efficacy than lower doses, as evaluated by PANSS and PSP scores.
Adverse reactions experienced by >5% of patients treated with risperidone included somnolence, parkinsonism, tremor, dystonia, dizziness, akathisia, increased salivation, and anxiety.7
In bipolar I disorder. Risperidone’s efficacy for short-term treatment of mania in children and adolescents was demonstrated in a 3-week, randomized, double-blind, placebo-controlled, multi-center study of 169 patients age 10 to 17 who were experiencing a manic or mixed episode of bipolar I disorder.7 Patients were randomly assigned to risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d, or placebo. All patients were started at 0.5 mg/d and this dose was titrated to the target dosage range in 7 days.
Risperidone, 0.5 to 6 mg/d, significantly decreased the total Young Mania Rating Scale score—a measure of the severity of elevated mood, increased motor activity energy, sexual interest, sleep, irritability, speech (rate/amount), language (thought disorder, content, disruptive), aggressive behavior, appearance, and insight. No evidence of increased efficacy was observed at doses >2.5 mg/d. In this trial, symptoms reported by >5% of patients included fatigue, dizziness, dystonia, parkinsonism, akathisia, abdominal pain, dyspepsia, nausea, vomiting, and diarrhea.7
Pediatric dosing. Based on these studies, the recommended starting dose for children and adolescents is 0.5 mg/d, with titration in 0.5-to 1-mg increments to targets of:
Table 2
Recommended dosing of risperidone
for pediatric schizophrenia and bipolar mania
| Indication | Initial dose | Titration | Target dose | Effective dose range |
|---|---|---|---|---|
| Schizophrenia, adolescents age 13 to 17 | 0.5 mg/d | 0.5 to 1 mg/d | 3 mg/d | 1 to 6 mg/d |
| Bipolar mania, children and adolescents age 10 to 17 | 0.5 mg/d | 0.5 to 1 mg/d | 2.5 mg/d | 0.5 to 6 mg/d |
| Source:Reference 7 | ||||
Tolerability studies
In long-term studies, the most commonly reported adverse events associated with risperidone in children and adolescents have been rhinitis, abdominal pain, increased saliva, body pain, gynecomastia, and weight increase.8 Specific adverse effects that pose long-term concerns are:
- tardive dyskinesia (TD)
- weight gain
- increased prolactin levels
Tardive dyskinesia. In clinical trials that included 1,885 children and adolescents with autistic disorder or other psychiatric disorders treated with risperidone, 2 patients (0.1%) were reported to have TD, which resolved when risperidone was discontinued.7 To monitor for TD, administer the Abnormal Involuntary Movement Scale at baseline and every 6 months while using risperidone in pediatric patients.
Weight gain. In long-term, open-label trials, patients with autistic or other psychiatric disorders gained an average 7.5
kg after 12 months of risperidone treatment. Most of the weight gain occurred in the first 6 months.9 Expected normal weight gain in children is 3 to 3.5 kg/year adjusted for age, based on Centers for Disease Control and Prevention normative data.
Follow the American Diabetes Association guidelines10 for monitoring metabolic parameters during antipsychotic
treatment, and intervene if clinically significant weight gain occurs.
In a 16-week, placebo-controlled study,11 metformin reversed weight gain associated with SGAs in children and adolescents. Metformin’s potential side effects include hypoglycemia, diarrhea, nausea/vomiting, and (rarely) lactic acidosis, but no adverse events were attributed to metformin.
Increased prolactin. As in adults, risperidone elevates serum prolactin in children and adolescents. All pediatric risperidone trials—of autism,2 disruptive behavior disorders in children with subaverage intelligence,9 schizophrenia,7 and bipolar mania—have shown increased serum prolactin. Risperidone’s long-term effects on growth and sexual maturation have not been fully evaluated, but hyperprolactinemia may inhibit reproductive function.
Findling et al12 analyzed data from 5 clinical trials (total 700 patients) in which children and adolescents age 5 to 15 years with subaverage IQs and conduct or other disruptive behavior disorders received risperidone for up to 55 weeks. Mean prolactin levels rose from 7.8 ng/mL
at baseline to 29.4 ng/mL at weeks 4 to 7, then progressively decreased to 16.1 ng/mL at weeks 40 to 48 (n=358) and 13.0 ng/mL at weeks 52 to 55 (n=42). Girls returned to a mean value within the normal range (≤30 ng/mL) by weeks 8 to 12, and boys were close to normal values (≤18 ng/mL) by weeks 16 to 24.
The researchers concluded that serum prolactin levels in children tend to rise and peak within the first 1 to 2 months of risperidone treatment and then steadily decline to values within or very close to normal range by 3 to 5 months.
The biological significance of chronic, mild prolactin elevations is unknown.13 Children entering puberty appear to be at highest risk for elevated prolactin and clinical symptoms while treated with risperidone.14 Therefore, ask all adolescents treated with risperidone about increases in breast size and galactorrhea. Switch those who develop these symptoms to an SGA that does not increase serum prolactin.
Contraindications. Risperidone is contraindicated in patients with a known hypersensitivity to the drug.
- Risperdal prescribing information. www.risperdal.com/risperdal/shared/pi/risperdal.pdf.
Drug brand names
- Lithium • Eskalith, Lithobid
- Risperidone • Risperdal
- Metformin • Glucophage, Fortamet
- Valproate • Depakote
Disclosures
Dr. Kowatch receives research support from Bristol-Meyers Squibb, Stanley Research Foundation, National Institute of Mental Health, and National Institute of Child Health and Human Development. He is a consultant for Creative Educational Concepts, Child and Adolescent Bipolar Foundation, Abbott Laboratories, and sanofi-aventis, and a speaker for Abbott Laboratories and AstraZeneca.
1. Aman MG, De Smedt G, Derivan A, et al. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry 2002;159:1337-46.
2. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
3. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry 2000;39(4):509-16.
4. Sikich L, Hamer R, Malekpour AH, et al. Double-blind trial comparing risperidone, olanzapine, and haloperidol in the treatment of psychotic children and adolescents. Paper presented at: Society of Biological Psychiatry Annual Meeting; May 16-18, 2002; Philadelphia, PA.
5. Frazier JA, Meyer MC, Biederman J, et al. Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry 1999;38(8):960-5.
6. Aman MG, Vinks AA, Remmerie B, et al. Plasma pharmacokinetic characteristics of risperidone and their relationship to saliva concentrations in children with psychiatric or neurodevelopmental disorders. Clin Ther 2007;29(7):1476-86.
7. Risperdal [package insert]. Titusville, NJ: Janssen, L.P; 2007.
8. Reyes MR, Olah R, Csaba K, et al. Long-term safety and efficacy of risperidone in children with disruptive behaviour disorders. Results of a 2-year extension study. Eur Child Adolesc Psychiatry 2006;15(2):97-104.
9. Croonenberghs J, Fegert JM, Findling RL, et al. Risperidone in children with disruptive behavior disorders and subaverage intelligence: a 1-year, open-label study of 504 patients. J Am Acad Child Adolesc Psychiatry 2005;44(1):64-72.
10. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry 2004;65(2):267-72.
11. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry 2006;163(12):2072-9.
12. Findling RL, Kusumakar V, Daneman D, et al. Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry 2003;64(11):1362-9.
13. Staller J. The effect of long-term antipsychotic treatment on prolactin. J Child Adolesc Psychopharmacol 2006;16(3):317-26.
14. Holzer L, Eap CB. Risperidone-induced symptomatic hyperprolactinaemia in adolescents. J Clin Psychopharmacol 2006;26(2):167-71.
Dr. Kowatch is professor of psychiatry and pediatrics at Cincinnati Children’s Hospital Medical Center and a Section Editor for Current Psychiatry.
1. Aman MG, De Smedt G, Derivan A, et al. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry 2002;159:1337-46.
2. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347(5):314-21.
3. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry 2000;39(4):509-16.
4. Sikich L, Hamer R, Malekpour AH, et al. Double-blind trial comparing risperidone, olanzapine, and haloperidol in the treatment of psychotic children and adolescents. Paper presented at: Society of Biological Psychiatry Annual Meeting; May 16-18, 2002; Philadelphia, PA.
5. Frazier JA, Meyer MC, Biederman J, et al. Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry 1999;38(8):960-5.
6. Aman MG, Vinks AA, Remmerie B, et al. Plasma pharmacokinetic characteristics of risperidone and their relationship to saliva concentrations in children with psychiatric or neurodevelopmental disorders. Clin Ther 2007;29(7):1476-86.
7. Risperdal [package insert]. Titusville, NJ: Janssen, L.P; 2007.
8. Reyes MR, Olah R, Csaba K, et al. Long-term safety and efficacy of risperidone in children with disruptive behaviour disorders. Results of a 2-year extension study. Eur Child Adolesc Psychiatry 2006;15(2):97-104.
9. Croonenberghs J, Fegert JM, Findling RL, et al. Risperidone in children with disruptive behavior disorders and subaverage intelligence: a 1-year, open-label study of 504 patients. J Am Acad Child Adolesc Psychiatry 2005;44(1):64-72.
10. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry 2004;65(2):267-72.
11. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry 2006;163(12):2072-9.
12. Findling RL, Kusumakar V, Daneman D, et al. Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry 2003;64(11):1362-9.
13. Staller J. The effect of long-term antipsychotic treatment on prolactin. J Child Adolesc Psychopharmacol 2006;16(3):317-26.
14. Holzer L, Eap CB. Risperidone-induced symptomatic hyperprolactinaemia in adolescents. J Clin Psychopharmacol 2006;26(2):167-71.
Dr. Kowatch is professor of psychiatry and pediatrics at Cincinnati Children’s Hospital Medical Center and a Section Editor for Current Psychiatry.
Is this child bipolar? What’s needed to improve diagnosis
When does bipolar disorder begin? That question confounds clinicians, worries parents, and is leading researchers such as Kiki D. Chang, MD, to look for answers in families with this highly heritable disorder.
“Parents with bipolar disorder know what’s happening if their children have early symptoms,” Dr. Chang says. “They tell me, ‘I don’t want my child to go through what I went through, and he’s having the same symptoms I did.’”
Dr. Chang believes early psychotherapy and medication might prevent prodromal bipolar disorder from fully developing. His team at Stanford University is among those seeking genetic and brain imaging biomarkers to make a pediatric bipolar diagnosis more reliable. Lack of age-specific criteria may be causing overdiagnosis, as suggested by a 40-fold increase in 10 years in the number of children and adolescents being treated for bipolar disorder.1
In this interview by Robert A. Kowatch, MD, PhD, Dr. Chang describes a child with probable early signs of bipolar disorder and discusses why early intervention is both complicated and promising.
Children at risk for bipolarity
DR. KOWATCH: You’re studying children considered at high risk for developing bipolar disorder; why are these studies important?
DR. CHANG: High-risk children represent a chance to understand risk factors for developing bipolar disorder and what the early symptoms are. By “high risk,” we mean children and adolescents who possess a genetic predisposition toward bipolar disorder.
Bipolar disorder develops over time; a boy such as “Brian” (Box 1) likely would have gone 3 to 5 years on the stimulant—not doing well—until he had a manic episode at age 14 or 15. The full mood episode usually does not develop until later, with the right—or you could say wrong— combination of environment and stressors acting on a genetic predisposition.
DR. KOWATCH: Do the parents of the children you’re studying have bipolar disorder?
DR. CHANG: Yes; we’re studying what we call “bipolar offspring”—children with biological parents with bipolar disorder (Box 2).2-4 One also could look at siblings; having a brother or sister with bipolar disorder increases risk as well. If you search back in these families, usually you’ll find many relatives with bipolar disorder who reflect the child’s genetic predisposition.
Mrs. M, age 35, had early-onset depression but was not diagnosed with bipolar disorder until age 22. She requests a consultation for her 10-year-old son, Brian, whom she suspects also may have bipolar disorder. “I know there’s something going on; he’s just like I was, but no one would listen to me,” she says.
The boy’s pediatrician prescribed methylphenidate for “a little inattention” but felt that Brian was doing okay in school and had some friends. The stimulant might be helping, says Mrs. M, but she is not sure.
You talk to Brian and learn he has some anxiety. He sometimes gets very excited and runs around, and sometimes he does not sleep well. If you consider all the symptoms, this child has anxiety, attention-deficit/hyperactivity disorder, short depressive periods that affect his functioning, and a parent with bipolar disorder.
You ask further, and Brian tells you about hearing conversations and voices of old friends, his parents, and unknown people in his head, usually neutral, and not commanding or commentating. No one has asked him about parapsychotic phenomena, and he’s never reported this to anyone.
In adults, the incidence of bipolar types I and II is approximately 4%.1 Because two-thirds of adults with bipolar disorder have onset during childhood or adolescence, the incidence of pediatric bipolar disorder may be 1% to 2%. It could be as high as 3% if you include children with prodromes or early forms of the disorder.
The risk of a child developing a bipolar disorder is probably 15% to 20% when 1 biological parent—or sibling—has a bipolar disorder.2 If both parents have bipolar disorder, some older studies suggest that the child’s risk of developing at least a mood disorder would be up to 75%,3 and depression in a child might develop into a bipolar disorder.
Therefore, the risk of bipolar disorder developing in a child whose parents both have bipolar disorder may be >50% and could approach 75%.
‘Kindling’ in bipolar disorder
DR. KOWATCH: What have you seen in children whose parents have bipolar disorder?
DR. CHANG: We’ve tracked more than 200 bipolar offspring for up to 10 years. In some families we’ve seen the natural progression toward full mania and bipolar disorder.
We’ve also seen children who start to show symptoms but don’t develop full bipolar disorder. These children have had clinical treatment, so we’re not sure if the intervention prevented full bipolar disorder or if they would not have developed it anyway. Some children have developed mood symptoms and other psychiatric problems that have resolved with early intervention.
DR. CHANG: Kindling, which originally referred to seizure disorders, also has been applied to affective disorders.5 Early stressors and triggers appear to add up over time and combine with genetic predisposition to create a full mood episode. After that break, it becomes easier and easier to have the next episode, and the disorder becomes chronic and more difficult to treat.
The goal of our work is to stop kindling in bipolar disorder—to prevent environmental or developmental “sparks” from interacting with genetic predisposition and igniting a chronic, spontaneous course of mood episodes.
Brain imaging biomarkers
DR. KOWATCH: Are researchers finding biomarkers for bipolar disorder?
DR. CHANG: The field is young but light-years ahead of where we were 10 years ago. Brain imaging has revealed consistently abnormal areas in children with bipolar disorder. These abnormalities are seen in adults with bipolar disorder as well, but chronic illness, substance abuse, and medication exposure affect the findings in adults. Children have had less exposure to these confounding variables.
We and other groups have identified areas of the prefrontal cortex, amygdala, cerebellum, and striatum that could represent biomarkers, although I wouldn’t say yet that there are any markers per se. A decrease in amygdala volume has been found consistently in children with bipolar disorder, for example, but it’s not specific to bipolar disorder. So we have a way to go before we find specific biomarkers.
In the future, clinicians will probably use a set of 10 to 20 biomarkers, and the more biomarkers a patient has, the greater the risk for bipolar disorder. Once a battery of biomarkers has been put together, the more certain a bipolar disorder diagnosis will become.
Genetic biomarkers
DR. KOWATCH: We’ve talked about high-risk families; are there genetic markers for bipolar disorder?
If you look at common polymorphisms in a set of genes, eventually you’ll be able to calculate the risk that a person will develop bipolar disorder. We’re also investigating whether genes control the age of onset.
DR. KOWATCH: How are you looking for genetic markers in the high-risk children you’re studying?
DR. CHANG: We start with the proband—the child of a bipolar parent—and then study as much of the family as we can. Approximately 50% of the probands’ first- or second-degree relatives have a mood disorder—so our samples are highly loaded.
We’re interested in the interaction between genes and brain function and structure: How do genetic predispositions lead to brain differences that create vulnerability for mood disorders—in this case, bipolar disorder?
To explore that question, we’re starting a 5-year study funded by the National Institutes of Health (NIH). We’re recruiting 50 sibling pairs in which 1 child has early bipolar symptoms and the other is healthy. We will compare these pairs’ genetic and brain imaging profiles with those of 30 healthy children with no genetic risk for bipolar disorder, as far as we can tell.
Something makes 1 child develop bipolar disorder and another child not. By matching siblings with shared environments, we’re trying to eliminate environmental factors and look at their genetic and brain function differences. We’ll use functional brain imaging to look at how children respond to mood-related tasks and standard tasks involving facial emotion exposure to activate brain areas bipolar disorder is thought to affect.
Preventing bipolar ‘kindling’
DR. KOWATCH: What interventions might interrupt kindling and help prevent bipolar I disorder from developing in high risk children?
DR. CHANG: Families affected by bipolar disorder are characterized by stress and high expressed emotion; they tend to fight a lot, and we’re trying to improve communication and their ability to work together. We think reduced stress could halt the progression of the disorder in at-risk children.
Our group is collaborating with Dr. David Miklowitz at the University of Colorado to develop a family psychotherapy program for children who have at least 1 parent or sibling with bipolar disorder and are showing early bipolar symptoms. In a 3-year, NIH-funded treatment development study, 40 children will be randomly assigned to receive 12 sessions of weekly family-focused therapy (FFT) or treatment as usual.
We also think some medications have potential for protecting the brain against the progression of bipolar disorder. In vitro evidence exists for lithium, valproate, and carbamazepine to some extent, other anticonvulsants such as lamotrigine, and atypical antipsychotics such as quetiapine and olanzapine. A few preliminary clinical trials have been conducted (Box 3)9-11 but no longitudinal studies.
A 12-week, open-label study of valproate8 showed symptom improvement in 18 of 23 (78%) children ages 6 to 18 with mood or behavioral symptoms and at least 1 parent with bipolar disorder. On the other hand, a double-blind, controlled trial found no difference in mood symptom changes in 56 children receiving valproate or placebo for up to 5 years. Children in this study were ages 5 to 17, met DSM-IV-TR criteria for cyclothymia or bipolar disorder not otherwise specified, and had at least 1 biological parent with bipolar disorder.9
A small, open-label, 12-week prospective study suggested that quetiapine may be useful for treating mood symptoms in adolescents with at least 1 first-degree relative with bipolar disorder. The 20 adolescents (ages 12 to 18) had mood disorder diagnoses but did not meet DSM-IV-TR criteria for bipolar I disorder.10
RECOMMENDATIONS
DR. KOWATCH: What do you recommend that psychiatrists do to help children at risk for bipolar disorder?
DR. CHANG: Ask your adult patients with bipolar disorder how their children are doing. If a child is not doing well, consider referral to a child and adolescent psychiatrist or take an interest yourself and assess the child for early signs of bipolar disorder.
DR. KOWATCH: What are the prodromal symptoms in children and adolescents?
DR. CHANG: In the past, the earliest reported symptoms were thought to include extreme hyperactivity, inappropriate sexuality, and severe depression at a very young age (preschool or school age children). Now data point to 2 major pathways toward bipolar disorder:
- early-onset depression, which elevates risk for later mania
- early attention-deficit/hyperactivity disorder (ADHD).
DR. KOWATCH: So you’ve got a group with depression and a group with severe ADHD that might develop into bipolar disorder?
DR. CHANG: The ADHD need not be severe. In these children, ADHD may reflect an underlying brain development trajectory toward mood dysregulation. We’ve also seen anxiety as an initial condition. A cross-sectional study found anxiety to be prevalent in bipolar offspring and a possible risk factor for later mania.12
Anxiety is very common in children, so it’s hard to tell if it’s a precursor for bipolar disorder in an individual child. But looking back, a lot of children who develop bipolar disorder had early anxiety, which may be a marker that they were not coping well with stress. What starts leaking out as anxiety eventually may leak out as a full mood episode.
DR. CHANG: Yes, although sometimes the risk comes not from the parents but from a second-degree or more distant relative. We have seen plenty of families in which (as far as we can tell) the parents don’t have any mood disorders, but a child has full bipolar disorder that began over time—as it usually does in bipolar offspring.
Children or adolescents who have first-break episodes after very little pre-morbid dysfunction comprise yet another subset. This group tends to present with episodic manic depression.
DR. KOWATCH: Do you think children with bipolar disorder have clear mood episodes?
DR. CHANG: Our research is trying to bypass that debate. We’re trying to understand whether biomarkers in the brain or blood can be used to distinguish different types of bipolar disorders, rather than relying on symptomatology.
Related resources
- Chang KD, Howe M, Gallelli, K, Miklowitz D. Prevention of pediatric bipolar disorder: integration of neurobiological and psychosocial processes. Ann NY Acad Sci 2006;1094:235–47.
- Chang KD, Gallelli KA. Bipolar disorders and genetics: clinical implications of high heritability. Medscape Psychiatry & Mental Health 2004;9(2). Available at: http://www.medscape.com/viewarticle/489331.
- Miklowitz D, Biuckians A, Richards JA. Early-onset bipolar disorder: a family treatment perspective. Dev Psychopathol 2006;18(4):1247–65.
- Carbamazepine • various
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Valproate • Depakene, Depakote
Dr. Chang receives research support from AstraZeneca, Eli Lilly and Company, Otsuka, and the National Institute of Mental Health. He is a consultant to Abbott Laboratories, GlaxoSmithKline, and Shire, and a speaker for Abbott Laboratories and AstraZeneca.
Dr. Kowatch receives research support from Bristol-Myers Squibb, Stanley Research Foundation, National Institute of Mental Health, and National Institute of Child Health and Human Development. He is a speaker for Abbott Laboratories and AstraZeneca.
1. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry 2007;64(9):1032-9.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62(6):593-602.
3. Chang KD, Adleman N, Dienes K, et al. Bipolar offspring: a window into bipolar disorder evolution. Biol Psychiatry 2003;53:941-5.
4. Gershon ES, Hamovit J, Guroff JJ, et al. A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Arch Gen Psychiatry 1982;39(10):1157-67.
5. Post RM. Do the epilepsies, pain syndromes, and affective disorders share common kindling-like mechanisms? Epilepsy Res 2002;50:203-19.
6. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;18;301(5631):291-3.
7. Green EK, Raybould R, Macgregor S, et al. Genetic variation of brain-derived neurotrophic factor (BDNF) in bipolar disorder: case-control study of over 3000 individuals from the UK. Br J Psychiatry 2006;188:21-5.
8. Burdick KE, Funke B, Goldberg JF, et al. COMT genotype increases risk for bipolar I disorder and influences neurocognitive performance. Bipolar Disord 2007;9(4):370-6.
9. Chang KD, Dienes K, Blasey C, et al. Divalproex monotherapy in the treatment of bipolar offspring with mood and behavioral disorders and at least mild affective symptoms. J Clin Psychiatry 2003;64(8):936-42.
10. Findling RL, Frazier TW, Youngstrom EA, et al. Double-blind, placebo-controlled trial of divalproex monotherapy in the treatment of symptomatic youth at high risk for developing bipolar disorder. J Clin Psychiatry 2007;68(5):781-8.
11. DelBello MP, Adler CM, Whitsel RM, et al. A 12-week single-blind trial of quetiapine for the treatment of mood symptoms in adolescents at high risk for developing bipolar I disorder. J Clin Psychiatry 2007;68(5):789-95.
12. Henin A, Biederman J, Mick E, et al. Psychopathology in the offspring of parents with bipolar disorder: a controlled study. Biol Psychiatry 2005;58(7):554-61.
When does bipolar disorder begin? That question confounds clinicians, worries parents, and is leading researchers such as Kiki D. Chang, MD, to look for answers in families with this highly heritable disorder.
“Parents with bipolar disorder know what’s happening if their children have early symptoms,” Dr. Chang says. “They tell me, ‘I don’t want my child to go through what I went through, and he’s having the same symptoms I did.’”
Dr. Chang believes early psychotherapy and medication might prevent prodromal bipolar disorder from fully developing. His team at Stanford University is among those seeking genetic and brain imaging biomarkers to make a pediatric bipolar diagnosis more reliable. Lack of age-specific criteria may be causing overdiagnosis, as suggested by a 40-fold increase in 10 years in the number of children and adolescents being treated for bipolar disorder.1
In this interview by Robert A. Kowatch, MD, PhD, Dr. Chang describes a child with probable early signs of bipolar disorder and discusses why early intervention is both complicated and promising.
Children at risk for bipolarity
DR. KOWATCH: You’re studying children considered at high risk for developing bipolar disorder; why are these studies important?
DR. CHANG: High-risk children represent a chance to understand risk factors for developing bipolar disorder and what the early symptoms are. By “high risk,” we mean children and adolescents who possess a genetic predisposition toward bipolar disorder.
Bipolar disorder develops over time; a boy such as “Brian” (Box 1) likely would have gone 3 to 5 years on the stimulant—not doing well—until he had a manic episode at age 14 or 15. The full mood episode usually does not develop until later, with the right—or you could say wrong— combination of environment and stressors acting on a genetic predisposition.
DR. KOWATCH: Do the parents of the children you’re studying have bipolar disorder?
DR. CHANG: Yes; we’re studying what we call “bipolar offspring”—children with biological parents with bipolar disorder (Box 2).2-4 One also could look at siblings; having a brother or sister with bipolar disorder increases risk as well. If you search back in these families, usually you’ll find many relatives with bipolar disorder who reflect the child’s genetic predisposition.
Mrs. M, age 35, had early-onset depression but was not diagnosed with bipolar disorder until age 22. She requests a consultation for her 10-year-old son, Brian, whom she suspects also may have bipolar disorder. “I know there’s something going on; he’s just like I was, but no one would listen to me,” she says.
The boy’s pediatrician prescribed methylphenidate for “a little inattention” but felt that Brian was doing okay in school and had some friends. The stimulant might be helping, says Mrs. M, but she is not sure.
You talk to Brian and learn he has some anxiety. He sometimes gets very excited and runs around, and sometimes he does not sleep well. If you consider all the symptoms, this child has anxiety, attention-deficit/hyperactivity disorder, short depressive periods that affect his functioning, and a parent with bipolar disorder.
You ask further, and Brian tells you about hearing conversations and voices of old friends, his parents, and unknown people in his head, usually neutral, and not commanding or commentating. No one has asked him about parapsychotic phenomena, and he’s never reported this to anyone.
In adults, the incidence of bipolar types I and II is approximately 4%.1 Because two-thirds of adults with bipolar disorder have onset during childhood or adolescence, the incidence of pediatric bipolar disorder may be 1% to 2%. It could be as high as 3% if you include children with prodromes or early forms of the disorder.
The risk of a child developing a bipolar disorder is probably 15% to 20% when 1 biological parent—or sibling—has a bipolar disorder.2 If both parents have bipolar disorder, some older studies suggest that the child’s risk of developing at least a mood disorder would be up to 75%,3 and depression in a child might develop into a bipolar disorder.
Therefore, the risk of bipolar disorder developing in a child whose parents both have bipolar disorder may be >50% and could approach 75%.
‘Kindling’ in bipolar disorder
DR. KOWATCH: What have you seen in children whose parents have bipolar disorder?
DR. CHANG: We’ve tracked more than 200 bipolar offspring for up to 10 years. In some families we’ve seen the natural progression toward full mania and bipolar disorder.
We’ve also seen children who start to show symptoms but don’t develop full bipolar disorder. These children have had clinical treatment, so we’re not sure if the intervention prevented full bipolar disorder or if they would not have developed it anyway. Some children have developed mood symptoms and other psychiatric problems that have resolved with early intervention.
DR. CHANG: Kindling, which originally referred to seizure disorders, also has been applied to affective disorders.5 Early stressors and triggers appear to add up over time and combine with genetic predisposition to create a full mood episode. After that break, it becomes easier and easier to have the next episode, and the disorder becomes chronic and more difficult to treat.
The goal of our work is to stop kindling in bipolar disorder—to prevent environmental or developmental “sparks” from interacting with genetic predisposition and igniting a chronic, spontaneous course of mood episodes.
Brain imaging biomarkers
DR. KOWATCH: Are researchers finding biomarkers for bipolar disorder?
DR. CHANG: The field is young but light-years ahead of where we were 10 years ago. Brain imaging has revealed consistently abnormal areas in children with bipolar disorder. These abnormalities are seen in adults with bipolar disorder as well, but chronic illness, substance abuse, and medication exposure affect the findings in adults. Children have had less exposure to these confounding variables.
We and other groups have identified areas of the prefrontal cortex, amygdala, cerebellum, and striatum that could represent biomarkers, although I wouldn’t say yet that there are any markers per se. A decrease in amygdala volume has been found consistently in children with bipolar disorder, for example, but it’s not specific to bipolar disorder. So we have a way to go before we find specific biomarkers.
In the future, clinicians will probably use a set of 10 to 20 biomarkers, and the more biomarkers a patient has, the greater the risk for bipolar disorder. Once a battery of biomarkers has been put together, the more certain a bipolar disorder diagnosis will become.
Genetic biomarkers
DR. KOWATCH: We’ve talked about high-risk families; are there genetic markers for bipolar disorder?
If you look at common polymorphisms in a set of genes, eventually you’ll be able to calculate the risk that a person will develop bipolar disorder. We’re also investigating whether genes control the age of onset.
DR. KOWATCH: How are you looking for genetic markers in the high-risk children you’re studying?
DR. CHANG: We start with the proband—the child of a bipolar parent—and then study as much of the family as we can. Approximately 50% of the probands’ first- or second-degree relatives have a mood disorder—so our samples are highly loaded.
We’re interested in the interaction between genes and brain function and structure: How do genetic predispositions lead to brain differences that create vulnerability for mood disorders—in this case, bipolar disorder?
To explore that question, we’re starting a 5-year study funded by the National Institutes of Health (NIH). We’re recruiting 50 sibling pairs in which 1 child has early bipolar symptoms and the other is healthy. We will compare these pairs’ genetic and brain imaging profiles with those of 30 healthy children with no genetic risk for bipolar disorder, as far as we can tell.
Something makes 1 child develop bipolar disorder and another child not. By matching siblings with shared environments, we’re trying to eliminate environmental factors and look at their genetic and brain function differences. We’ll use functional brain imaging to look at how children respond to mood-related tasks and standard tasks involving facial emotion exposure to activate brain areas bipolar disorder is thought to affect.
Preventing bipolar ‘kindling’
DR. KOWATCH: What interventions might interrupt kindling and help prevent bipolar I disorder from developing in high risk children?
DR. CHANG: Families affected by bipolar disorder are characterized by stress and high expressed emotion; they tend to fight a lot, and we’re trying to improve communication and their ability to work together. We think reduced stress could halt the progression of the disorder in at-risk children.
Our group is collaborating with Dr. David Miklowitz at the University of Colorado to develop a family psychotherapy program for children who have at least 1 parent or sibling with bipolar disorder and are showing early bipolar symptoms. In a 3-year, NIH-funded treatment development study, 40 children will be randomly assigned to receive 12 sessions of weekly family-focused therapy (FFT) or treatment as usual.
We also think some medications have potential for protecting the brain against the progression of bipolar disorder. In vitro evidence exists for lithium, valproate, and carbamazepine to some extent, other anticonvulsants such as lamotrigine, and atypical antipsychotics such as quetiapine and olanzapine. A few preliminary clinical trials have been conducted (Box 3)9-11 but no longitudinal studies.
A 12-week, open-label study of valproate8 showed symptom improvement in 18 of 23 (78%) children ages 6 to 18 with mood or behavioral symptoms and at least 1 parent with bipolar disorder. On the other hand, a double-blind, controlled trial found no difference in mood symptom changes in 56 children receiving valproate or placebo for up to 5 years. Children in this study were ages 5 to 17, met DSM-IV-TR criteria for cyclothymia or bipolar disorder not otherwise specified, and had at least 1 biological parent with bipolar disorder.9
A small, open-label, 12-week prospective study suggested that quetiapine may be useful for treating mood symptoms in adolescents with at least 1 first-degree relative with bipolar disorder. The 20 adolescents (ages 12 to 18) had mood disorder diagnoses but did not meet DSM-IV-TR criteria for bipolar I disorder.10
RECOMMENDATIONS
DR. KOWATCH: What do you recommend that psychiatrists do to help children at risk for bipolar disorder?
DR. CHANG: Ask your adult patients with bipolar disorder how their children are doing. If a child is not doing well, consider referral to a child and adolescent psychiatrist or take an interest yourself and assess the child for early signs of bipolar disorder.
DR. KOWATCH: What are the prodromal symptoms in children and adolescents?
DR. CHANG: In the past, the earliest reported symptoms were thought to include extreme hyperactivity, inappropriate sexuality, and severe depression at a very young age (preschool or school age children). Now data point to 2 major pathways toward bipolar disorder:
- early-onset depression, which elevates risk for later mania
- early attention-deficit/hyperactivity disorder (ADHD).
DR. KOWATCH: So you’ve got a group with depression and a group with severe ADHD that might develop into bipolar disorder?
DR. CHANG: The ADHD need not be severe. In these children, ADHD may reflect an underlying brain development trajectory toward mood dysregulation. We’ve also seen anxiety as an initial condition. A cross-sectional study found anxiety to be prevalent in bipolar offspring and a possible risk factor for later mania.12
Anxiety is very common in children, so it’s hard to tell if it’s a precursor for bipolar disorder in an individual child. But looking back, a lot of children who develop bipolar disorder had early anxiety, which may be a marker that they were not coping well with stress. What starts leaking out as anxiety eventually may leak out as a full mood episode.
DR. CHANG: Yes, although sometimes the risk comes not from the parents but from a second-degree or more distant relative. We have seen plenty of families in which (as far as we can tell) the parents don’t have any mood disorders, but a child has full bipolar disorder that began over time—as it usually does in bipolar offspring.
Children or adolescents who have first-break episodes after very little pre-morbid dysfunction comprise yet another subset. This group tends to present with episodic manic depression.
DR. KOWATCH: Do you think children with bipolar disorder have clear mood episodes?
DR. CHANG: Our research is trying to bypass that debate. We’re trying to understand whether biomarkers in the brain or blood can be used to distinguish different types of bipolar disorders, rather than relying on symptomatology.
Related resources
- Chang KD, Howe M, Gallelli, K, Miklowitz D. Prevention of pediatric bipolar disorder: integration of neurobiological and psychosocial processes. Ann NY Acad Sci 2006;1094:235–47.
- Chang KD, Gallelli KA. Bipolar disorders and genetics: clinical implications of high heritability. Medscape Psychiatry & Mental Health 2004;9(2). Available at: http://www.medscape.com/viewarticle/489331.
- Miklowitz D, Biuckians A, Richards JA. Early-onset bipolar disorder: a family treatment perspective. Dev Psychopathol 2006;18(4):1247–65.
- Carbamazepine • various
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Valproate • Depakene, Depakote
Dr. Chang receives research support from AstraZeneca, Eli Lilly and Company, Otsuka, and the National Institute of Mental Health. He is a consultant to Abbott Laboratories, GlaxoSmithKline, and Shire, and a speaker for Abbott Laboratories and AstraZeneca.
Dr. Kowatch receives research support from Bristol-Myers Squibb, Stanley Research Foundation, National Institute of Mental Health, and National Institute of Child Health and Human Development. He is a speaker for Abbott Laboratories and AstraZeneca.
When does bipolar disorder begin? That question confounds clinicians, worries parents, and is leading researchers such as Kiki D. Chang, MD, to look for answers in families with this highly heritable disorder.
“Parents with bipolar disorder know what’s happening if their children have early symptoms,” Dr. Chang says. “They tell me, ‘I don’t want my child to go through what I went through, and he’s having the same symptoms I did.’”
Dr. Chang believes early psychotherapy and medication might prevent prodromal bipolar disorder from fully developing. His team at Stanford University is among those seeking genetic and brain imaging biomarkers to make a pediatric bipolar diagnosis more reliable. Lack of age-specific criteria may be causing overdiagnosis, as suggested by a 40-fold increase in 10 years in the number of children and adolescents being treated for bipolar disorder.1
In this interview by Robert A. Kowatch, MD, PhD, Dr. Chang describes a child with probable early signs of bipolar disorder and discusses why early intervention is both complicated and promising.
Children at risk for bipolarity
DR. KOWATCH: You’re studying children considered at high risk for developing bipolar disorder; why are these studies important?
DR. CHANG: High-risk children represent a chance to understand risk factors for developing bipolar disorder and what the early symptoms are. By “high risk,” we mean children and adolescents who possess a genetic predisposition toward bipolar disorder.
Bipolar disorder develops over time; a boy such as “Brian” (Box 1) likely would have gone 3 to 5 years on the stimulant—not doing well—until he had a manic episode at age 14 or 15. The full mood episode usually does not develop until later, with the right—or you could say wrong— combination of environment and stressors acting on a genetic predisposition.
DR. KOWATCH: Do the parents of the children you’re studying have bipolar disorder?
DR. CHANG: Yes; we’re studying what we call “bipolar offspring”—children with biological parents with bipolar disorder (Box 2).2-4 One also could look at siblings; having a brother or sister with bipolar disorder increases risk as well. If you search back in these families, usually you’ll find many relatives with bipolar disorder who reflect the child’s genetic predisposition.
Mrs. M, age 35, had early-onset depression but was not diagnosed with bipolar disorder until age 22. She requests a consultation for her 10-year-old son, Brian, whom she suspects also may have bipolar disorder. “I know there’s something going on; he’s just like I was, but no one would listen to me,” she says.
The boy’s pediatrician prescribed methylphenidate for “a little inattention” but felt that Brian was doing okay in school and had some friends. The stimulant might be helping, says Mrs. M, but she is not sure.
You talk to Brian and learn he has some anxiety. He sometimes gets very excited and runs around, and sometimes he does not sleep well. If you consider all the symptoms, this child has anxiety, attention-deficit/hyperactivity disorder, short depressive periods that affect his functioning, and a parent with bipolar disorder.
You ask further, and Brian tells you about hearing conversations and voices of old friends, his parents, and unknown people in his head, usually neutral, and not commanding or commentating. No one has asked him about parapsychotic phenomena, and he’s never reported this to anyone.
In adults, the incidence of bipolar types I and II is approximately 4%.1 Because two-thirds of adults with bipolar disorder have onset during childhood or adolescence, the incidence of pediatric bipolar disorder may be 1% to 2%. It could be as high as 3% if you include children with prodromes or early forms of the disorder.
The risk of a child developing a bipolar disorder is probably 15% to 20% when 1 biological parent—or sibling—has a bipolar disorder.2 If both parents have bipolar disorder, some older studies suggest that the child’s risk of developing at least a mood disorder would be up to 75%,3 and depression in a child might develop into a bipolar disorder.
Therefore, the risk of bipolar disorder developing in a child whose parents both have bipolar disorder may be >50% and could approach 75%.
‘Kindling’ in bipolar disorder
DR. KOWATCH: What have you seen in children whose parents have bipolar disorder?
DR. CHANG: We’ve tracked more than 200 bipolar offspring for up to 10 years. In some families we’ve seen the natural progression toward full mania and bipolar disorder.
We’ve also seen children who start to show symptoms but don’t develop full bipolar disorder. These children have had clinical treatment, so we’re not sure if the intervention prevented full bipolar disorder or if they would not have developed it anyway. Some children have developed mood symptoms and other psychiatric problems that have resolved with early intervention.
DR. CHANG: Kindling, which originally referred to seizure disorders, also has been applied to affective disorders.5 Early stressors and triggers appear to add up over time and combine with genetic predisposition to create a full mood episode. After that break, it becomes easier and easier to have the next episode, and the disorder becomes chronic and more difficult to treat.
The goal of our work is to stop kindling in bipolar disorder—to prevent environmental or developmental “sparks” from interacting with genetic predisposition and igniting a chronic, spontaneous course of mood episodes.
Brain imaging biomarkers
DR. KOWATCH: Are researchers finding biomarkers for bipolar disorder?
DR. CHANG: The field is young but light-years ahead of where we were 10 years ago. Brain imaging has revealed consistently abnormal areas in children with bipolar disorder. These abnormalities are seen in adults with bipolar disorder as well, but chronic illness, substance abuse, and medication exposure affect the findings in adults. Children have had less exposure to these confounding variables.
We and other groups have identified areas of the prefrontal cortex, amygdala, cerebellum, and striatum that could represent biomarkers, although I wouldn’t say yet that there are any markers per se. A decrease in amygdala volume has been found consistently in children with bipolar disorder, for example, but it’s not specific to bipolar disorder. So we have a way to go before we find specific biomarkers.
In the future, clinicians will probably use a set of 10 to 20 biomarkers, and the more biomarkers a patient has, the greater the risk for bipolar disorder. Once a battery of biomarkers has been put together, the more certain a bipolar disorder diagnosis will become.
Genetic biomarkers
DR. KOWATCH: We’ve talked about high-risk families; are there genetic markers for bipolar disorder?
If you look at common polymorphisms in a set of genes, eventually you’ll be able to calculate the risk that a person will develop bipolar disorder. We’re also investigating whether genes control the age of onset.
DR. KOWATCH: How are you looking for genetic markers in the high-risk children you’re studying?
DR. CHANG: We start with the proband—the child of a bipolar parent—and then study as much of the family as we can. Approximately 50% of the probands’ first- or second-degree relatives have a mood disorder—so our samples are highly loaded.
We’re interested in the interaction between genes and brain function and structure: How do genetic predispositions lead to brain differences that create vulnerability for mood disorders—in this case, bipolar disorder?
To explore that question, we’re starting a 5-year study funded by the National Institutes of Health (NIH). We’re recruiting 50 sibling pairs in which 1 child has early bipolar symptoms and the other is healthy. We will compare these pairs’ genetic and brain imaging profiles with those of 30 healthy children with no genetic risk for bipolar disorder, as far as we can tell.
Something makes 1 child develop bipolar disorder and another child not. By matching siblings with shared environments, we’re trying to eliminate environmental factors and look at their genetic and brain function differences. We’ll use functional brain imaging to look at how children respond to mood-related tasks and standard tasks involving facial emotion exposure to activate brain areas bipolar disorder is thought to affect.
Preventing bipolar ‘kindling’
DR. KOWATCH: What interventions might interrupt kindling and help prevent bipolar I disorder from developing in high risk children?
DR. CHANG: Families affected by bipolar disorder are characterized by stress and high expressed emotion; they tend to fight a lot, and we’re trying to improve communication and their ability to work together. We think reduced stress could halt the progression of the disorder in at-risk children.
Our group is collaborating with Dr. David Miklowitz at the University of Colorado to develop a family psychotherapy program for children who have at least 1 parent or sibling with bipolar disorder and are showing early bipolar symptoms. In a 3-year, NIH-funded treatment development study, 40 children will be randomly assigned to receive 12 sessions of weekly family-focused therapy (FFT) or treatment as usual.
We also think some medications have potential for protecting the brain against the progression of bipolar disorder. In vitro evidence exists for lithium, valproate, and carbamazepine to some extent, other anticonvulsants such as lamotrigine, and atypical antipsychotics such as quetiapine and olanzapine. A few preliminary clinical trials have been conducted (Box 3)9-11 but no longitudinal studies.
A 12-week, open-label study of valproate8 showed symptom improvement in 18 of 23 (78%) children ages 6 to 18 with mood or behavioral symptoms and at least 1 parent with bipolar disorder. On the other hand, a double-blind, controlled trial found no difference in mood symptom changes in 56 children receiving valproate or placebo for up to 5 years. Children in this study were ages 5 to 17, met DSM-IV-TR criteria for cyclothymia or bipolar disorder not otherwise specified, and had at least 1 biological parent with bipolar disorder.9
A small, open-label, 12-week prospective study suggested that quetiapine may be useful for treating mood symptoms in adolescents with at least 1 first-degree relative with bipolar disorder. The 20 adolescents (ages 12 to 18) had mood disorder diagnoses but did not meet DSM-IV-TR criteria for bipolar I disorder.10
RECOMMENDATIONS
DR. KOWATCH: What do you recommend that psychiatrists do to help children at risk for bipolar disorder?
DR. CHANG: Ask your adult patients with bipolar disorder how their children are doing. If a child is not doing well, consider referral to a child and adolescent psychiatrist or take an interest yourself and assess the child for early signs of bipolar disorder.
DR. KOWATCH: What are the prodromal symptoms in children and adolescents?
DR. CHANG: In the past, the earliest reported symptoms were thought to include extreme hyperactivity, inappropriate sexuality, and severe depression at a very young age (preschool or school age children). Now data point to 2 major pathways toward bipolar disorder:
- early-onset depression, which elevates risk for later mania
- early attention-deficit/hyperactivity disorder (ADHD).
DR. KOWATCH: So you’ve got a group with depression and a group with severe ADHD that might develop into bipolar disorder?
DR. CHANG: The ADHD need not be severe. In these children, ADHD may reflect an underlying brain development trajectory toward mood dysregulation. We’ve also seen anxiety as an initial condition. A cross-sectional study found anxiety to be prevalent in bipolar offspring and a possible risk factor for later mania.12
Anxiety is very common in children, so it’s hard to tell if it’s a precursor for bipolar disorder in an individual child. But looking back, a lot of children who develop bipolar disorder had early anxiety, which may be a marker that they were not coping well with stress. What starts leaking out as anxiety eventually may leak out as a full mood episode.
DR. CHANG: Yes, although sometimes the risk comes not from the parents but from a second-degree or more distant relative. We have seen plenty of families in which (as far as we can tell) the parents don’t have any mood disorders, but a child has full bipolar disorder that began over time—as it usually does in bipolar offspring.
Children or adolescents who have first-break episodes after very little pre-morbid dysfunction comprise yet another subset. This group tends to present with episodic manic depression.
DR. KOWATCH: Do you think children with bipolar disorder have clear mood episodes?
DR. CHANG: Our research is trying to bypass that debate. We’re trying to understand whether biomarkers in the brain or blood can be used to distinguish different types of bipolar disorders, rather than relying on symptomatology.
Related resources
- Chang KD, Howe M, Gallelli, K, Miklowitz D. Prevention of pediatric bipolar disorder: integration of neurobiological and psychosocial processes. Ann NY Acad Sci 2006;1094:235–47.
- Chang KD, Gallelli KA. Bipolar disorders and genetics: clinical implications of high heritability. Medscape Psychiatry & Mental Health 2004;9(2). Available at: http://www.medscape.com/viewarticle/489331.
- Miklowitz D, Biuckians A, Richards JA. Early-onset bipolar disorder: a family treatment perspective. Dev Psychopathol 2006;18(4):1247–65.
- Carbamazepine • various
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Valproate • Depakene, Depakote
Dr. Chang receives research support from AstraZeneca, Eli Lilly and Company, Otsuka, and the National Institute of Mental Health. He is a consultant to Abbott Laboratories, GlaxoSmithKline, and Shire, and a speaker for Abbott Laboratories and AstraZeneca.
Dr. Kowatch receives research support from Bristol-Myers Squibb, Stanley Research Foundation, National Institute of Mental Health, and National Institute of Child Health and Human Development. He is a speaker for Abbott Laboratories and AstraZeneca.
1. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry 2007;64(9):1032-9.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62(6):593-602.
3. Chang KD, Adleman N, Dienes K, et al. Bipolar offspring: a window into bipolar disorder evolution. Biol Psychiatry 2003;53:941-5.
4. Gershon ES, Hamovit J, Guroff JJ, et al. A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Arch Gen Psychiatry 1982;39(10):1157-67.
5. Post RM. Do the epilepsies, pain syndromes, and affective disorders share common kindling-like mechanisms? Epilepsy Res 2002;50:203-19.
6. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;18;301(5631):291-3.
7. Green EK, Raybould R, Macgregor S, et al. Genetic variation of brain-derived neurotrophic factor (BDNF) in bipolar disorder: case-control study of over 3000 individuals from the UK. Br J Psychiatry 2006;188:21-5.
8. Burdick KE, Funke B, Goldberg JF, et al. COMT genotype increases risk for bipolar I disorder and influences neurocognitive performance. Bipolar Disord 2007;9(4):370-6.
9. Chang KD, Dienes K, Blasey C, et al. Divalproex monotherapy in the treatment of bipolar offspring with mood and behavioral disorders and at least mild affective symptoms. J Clin Psychiatry 2003;64(8):936-42.
10. Findling RL, Frazier TW, Youngstrom EA, et al. Double-blind, placebo-controlled trial of divalproex monotherapy in the treatment of symptomatic youth at high risk for developing bipolar disorder. J Clin Psychiatry 2007;68(5):781-8.
11. DelBello MP, Adler CM, Whitsel RM, et al. A 12-week single-blind trial of quetiapine for the treatment of mood symptoms in adolescents at high risk for developing bipolar I disorder. J Clin Psychiatry 2007;68(5):789-95.
12. Henin A, Biederman J, Mick E, et al. Psychopathology in the offspring of parents with bipolar disorder: a controlled study. Biol Psychiatry 2005;58(7):554-61.
1. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry 2007;64(9):1032-9.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62(6):593-602.
3. Chang KD, Adleman N, Dienes K, et al. Bipolar offspring: a window into bipolar disorder evolution. Biol Psychiatry 2003;53:941-5.
4. Gershon ES, Hamovit J, Guroff JJ, et al. A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Arch Gen Psychiatry 1982;39(10):1157-67.
5. Post RM. Do the epilepsies, pain syndromes, and affective disorders share common kindling-like mechanisms? Epilepsy Res 2002;50:203-19.
6. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;18;301(5631):291-3.
7. Green EK, Raybould R, Macgregor S, et al. Genetic variation of brain-derived neurotrophic factor (BDNF) in bipolar disorder: case-control study of over 3000 individuals from the UK. Br J Psychiatry 2006;188:21-5.
8. Burdick KE, Funke B, Goldberg JF, et al. COMT genotype increases risk for bipolar I disorder and influences neurocognitive performance. Bipolar Disord 2007;9(4):370-6.
9. Chang KD, Dienes K, Blasey C, et al. Divalproex monotherapy in the treatment of bipolar offspring with mood and behavioral disorders and at least mild affective symptoms. J Clin Psychiatry 2003;64(8):936-42.
10. Findling RL, Frazier TW, Youngstrom EA, et al. Double-blind, placebo-controlled trial of divalproex monotherapy in the treatment of symptomatic youth at high risk for developing bipolar disorder. J Clin Psychiatry 2007;68(5):781-8.
11. DelBello MP, Adler CM, Whitsel RM, et al. A 12-week single-blind trial of quetiapine for the treatment of mood symptoms in adolescents at high risk for developing bipolar I disorder. J Clin Psychiatry 2007;68(5):789-95.
12. Henin A, Biederman J, Mick E, et al. Psychopathology in the offspring of parents with bipolar disorder: a controlled study. Biol Psychiatry 2005;58(7):554-61.