User login
Effects of Computer-Based Documentation Procedures on Health Care Workload Assessment and Resource Allocation: An Example From VA Sleep Medicine Programs
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
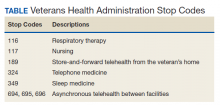
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
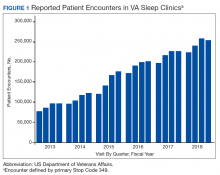
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
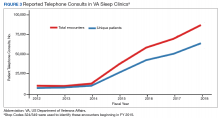
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
Health care systems are faced with the challenge of meeting increasing patient care demands with finite resources.1 Advocating for additional capital—specifically, human resources—requires compelling data that accurately capture workload credit. When workload is not captured accurately, clinicians may be tasked with providing care to a high volume of patients without appropriate resource allocation. This understaffing can delay care delivery and increase the risk of diagnostic and treatment errors.2 Furthermore, workers in understaffed medical facilities are more likely to experience burnout, which leads to high workforce turnover.
Computer based documentation (CBD) is used often in medical practices to track patient care and clinical workload. However, improperly designed and implemented CBD systems can contribute to cumbersome documentation tasks and inaccurate or incomplete data capture.3 Conversely, CBD can be a useful tool to capture workload credit and can subsequently facilitate justification for medical staff allocation to meet patient care demands. This article uses our experience with US Department of Veterans Affairs (VA) national sleep medicine programs to illustrate the impact of CBD procedures on health care workload assessment and allocation. Specifically, we examine how appropriate workload capture facilitates growth and improves the efficiency of health care programs.
The VA is the largest integrated health care system in the US, serving 9 million veterans at 1,255 facilities, including 170 VA Medical Centers (VAMCs).4 As veterans’ demands for VA medical services have outpaced available resources, there have been several media reports of lapses in timely care delivery.5-7 These lapses have been due, in part, to insufficient workforce resource allocation within the Veterans Health Administration (VHA) facilities. A 2012 audit of physician staffing levels conducted by the VA Inspector General concluded that the VA did not have an effective staffing methodology to ensure appropriate staffing levels for specialty care services.8 The lack of staffing plans and productivity standards limits the ability of medical facility officials to make informed business decisions regarding the appropriate number of specialty physicians required to meet patient care needs.8 In 2017, the Government Accountability Office (GAO) issued a report to Congress that stated the “VA’s productivity metrics and efficiency models do not provide complete and accurate information, they may misrepresent the true level of productivity and efficiency across VAMCs and limit the VA’s ability to determine the extent to which its resources are being used effectively.”9 To understand how and why many VA medical facilities remain understaffed, and therefore struggle to provide health care to veterans in a timely fashion, a description of VA CBD procedures is provided.
Background
VA Directive 1082 on Patient Care Data requires the capture of all outpatient and inpatient billable encounter data.10 Accurate capture of workload informs budget allocation models and is necessary for health care provider (HCP) productivity metrics. These data points help identify staff shortages relative to the generated workload. The Veterans Equitable Resource Allocation (VERA) model is used to allocate general purpose funds to the Veterans Integrated Service Networks (VISNs) regional network of VHA facilities. The underlying data components of the VERA model rely on comprehensive data systems that track and analyze the many management information systems used in VHA. Historically, at least 90% of the funds allocated by the VERA model have been attributed directly to patient care. All workload that is appropriately documented is accounted for in the VERA patient classification process, which is the official data source for funding patient care in VHA.
VA medical facilities use Stop Codes (formerly known as Decision Support System Identifiers) to identify workload for all outpatient encounters and inpatient professional services. Each code is composed of a 6-character descriptor that includes a primary Stop Code and a credit (secondary) Stop Code. Primary Stop Codes—the first 3 numbers in the sequence—designate the main clinical group responsible for patient care, such as sleep medicine or neurology. Secondary Stop Codes—the last 3 numbers in the sequence—further define the primary workgroup, such as the type of services provided (eg, telehealth) or the type of HCP (eg, nurse practitioner). These codes help ensure that workload and generated revenue are allocated or credited to the proper specialty care service.11 An example of how changes or inaccuracies in Stop Code reporting can affect VHA clinical workload assessment and resource allocation is provided by the VHA sleep medicine program.
The prevalence of sleep disorders—particularly apnea and insomnia—among US military service members and veterans has increased dramatically over the past 2 decades and continues to rise.12-14 Consequently, demand for sleep care services at VHA facilities also has increased substantially (Figure 1). Unfortunately, this demand has outpaced the VHA’s staffing models, sometimes resulting in long wait times for appointments.15 In fact, sleep medicine remains one of the most backlogged services in the VHA, despite significant improvements in program efficiency achieved by incorporating telehealth modalities.16 Untreated sleep disorders are associated with increased risk of depression, anxiety, impaired neurocognitive functions, cardiovascular disease, motor vehicle accidents, and premature death.17-23
A major contributor to understaffing of VHA sleep medicine programs is the CBD system’s historical inability to accurately track sleep resources and demand for sleep care services. For many years, Stop Codes attributed sleep workload credit primarily to pulmonary medicine, neurology, and internal medicine workgroups. Within these workgroups, few individuals contributed to sleep care, but the entire workgroup received credit for these services, masking the workload of sleep care providers. Additional barriers to accurate sleep medicine workload capture within the VHA included (1) inability to centrally identify personnel, including physicians, as providers of sleep care; (2) limited and variable understanding among VA sleep physicians of the importance of proper encounter form completion (the mechanism by which the cost of a service is calculated); and (3) a lack of awareness that encounter closure is directly linked to productivity measures such as relative value units (RVUs) that support sleep medicine programs and the salaries of those who provide care.
Methods
The critical role of accurate CBD in health care administration is illustrated by the proper use of Stop Codes as a foundational step in tracking services provided to justify adequate resource allocation within VA. A complete redesign of tracking sleep service documentation was initiated in 2014 and resulted in national changes to sleep medicine Stop Codes. The Stop Code initiative was the first step of several to improve CBD for VA sleep services.
Primary Stop Code 349 designates sleep medicine encounters in VA facilities (Table). However, before changes were implemented in fiscal year (FY) 2015, Stop Codes for VHA sleep care did not differentiate between specific services provided, such as laboratory-based sleep testing, at-home sleep testing, education/training sessions, follow-up appointments, equipment consults, telephone or video consults, or administrative tasks. In early FY 2015, several changes were made to Stop Codes used for VHA sleep medicine services nationwide to capture the breadth of services that were being provided; services that had previously been performed but were not documented. A new standardized coding methodology was established for continuous positive airway pressure (CPAP) clinics (349/116 or 349/117); telephone consults for sleep care (324/349); and store and forward sleep telehealth encounters (349/694, 349/695, or 349/696).
In the VA, store-and-forward telehealth refers to asynchronous telemedicine involving the acquisition and storing of clinical information (eg, data, image, sound, or video) that another site or clinician reviews later for evaluation and interpretation. In sleep medicine, data uploaded from home sleep apnea test units or CPAP devices are examples of this asynchronous telehealth model. The goal of these changes in VA Stop Codes was to accurately assess the volume of sleep care delivered and the demand for sleep care (consult volumes); enable planning for resource allocation and utilization appropriately; provide veterans with consistent access to sleep services across the country; and facilitate reductions in wait times for sleep care appointments. Results of these changes were immediate and dramatic in terms of data capture and reporting.
Results
Figure 1 illustrates an increase in patient encounters in VA sleep clinics by 24,197 (19.6%) in the first quarter of Stop Code change implementation (FY 2015, quarter 2) compared with those of the previous quarter. VHA sleep clinic patient encounters increased in subsequent quarters of FY 2015 by 29,910 (20.2%) and 11,206 (6.3%) respectively. By the end of FY 2015, reported sleep clinic encounters increased by 190,803 compared with the those at the end of FY 2014, an increase of 42.7%.
Figures 2, 3, and 4 show the additional effects of sleep Stop Code changes that were implemented in FY 2015 for CPAP clinics, telephone encounters, and store-and-forward telehealth encounters, respectively. The large increases in reported sleep patient encounters between FY 2014 and FY 2016 reflect changes in CBD and are not entirely due to actual changes in clinical workloads. These results indicate that workloads in many VHA sleep medicine clinics were grossly underreported or misallocated to other specialty services prior to the changes implemented in FY 2015. This discrepancy in care delivery vs workload capture is a contributing factor to the understaffing that continues to challenge VHA sleep programs. However, the improved accuracy of workload reporting that resulted from Stop Code modifications has resulted in only a small proportional increase in VHA clinical resources allocated to provide adequate services and care for veterans with sleep disorders.
In response to the substantial and increasing demand for sleep services by veterans, the VA Office of Rural Health (ORH) funded an enterprise-wide initiative (EWI) to develop and implement a national TeleSleep Program.16 The goal of this program is to improve the health and well-being of rural veterans by increasing their access to sleep care and services.
Discussion
Inaccuracies in CBD procedures can adversely affect health care workload assessment and allocation, contributing to ongoing challenges faced by sleep medicine clinics and other VHA programs that have limited staff yet strive to provide timely and high-quality care to veterans. “Not only does inaccurate coding contribute to miscalculations in staffing and resource allocation, it can also contribute to inaccuracies in overall measures of VA healthcare efficiency,” the GAO reported to Congress.9 The GAO went on to recommend that the VA should ensure the accuracy of underlying staffing and workload data. VHA sleep medicine programs have made efforts to educate HCPs and administrators on the importance of accurate CBD as a tool for accurate data capture that is necessary to facilitate improvements in health care availability and delivery.
In 2018, the VA Sleep Program Office released an updated set of Stop Code changes, including expansion of telehealth codes and improved designation of laboratory and home sleep testing services. These changes are anticipated to result in accurate documentation of VA sleep clinic workload and services, especially as the VA TeleSleep EWI to reach rural veterans expands.16 In light of the improved accuracy of reporting of delivered sleep services due to changes in Stop Codes over the past 4 years, VHA sleep medicine providers continue to advocate for allocation of resources commensurate with their clinical workload. An appropriate administrative response to the significant clinical workload performed by disproportionately few providers should include the authorization of increased resources and personnel for sleep medicine as well as providing the tools needed to further streamline workflow efficiency (eg, artificial intelligence, machine learning, and population health management).
Conclusions
Despite the barriers faced by many large integrated health care systems, VHA sleep medicine leadership continues to implement changes in CBD protocols that improve the accuracy of clinical workload tracking and reporting. Ultimately, these changes will support proposals for increased resources necessary to improve the quality and availability of sleep care for veterans. This example from VA illustrates the importance of accurate workload capture and its role in informing administrators of health care systems as they strive to meet the needs of patients. Although some VA sleep medicine programs continue to face challenges imposed by systemwide limitations, the ORH TeleSleep Program is a major initiative that improves veterans’ access to care by disseminating and implementing effective telehealth technologies and strategies.16
Acknowledgments
This work was supported by a VA Office of Rural Health Enterprise-Wide Initiative.
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004
1. World Health Organization. Workload indicators of staffing need (WISN). https://www.who.int/hrh/resources/WISN_Eng_UsersManual.pdf?ua=1. Published December 2015. Accessed June 24, 2020.
2. American Association for Respiratory Care. Position statement: best practices in respiratory care productivity and staffing. https://www.aarc.org/wp-content/uploads/2017/03/statement-of-best-practices_productivity-and-staffing.pdf. Revised July 2015. Accessed June 24, 2020.
3. Wu DTY, Smart N, Ciemins EL, Lanham HJ, Lindberg C, Zheng K. Using EHR audit trail logs to analyze clinical workflow: a case study from community-based ambulatory clinics. AMIA Annu Symp Proc. 2018;2017:1820-1827. Published 2018 Apr 16.
4. US Department of Veterans Affairs, Veterans Health Administration. https://www.va.gov/health.
5. Cohen T. VA crisis: solutions exist, but haven’t happened, panel hears. https://www.cnn.com/2014/06/12/politics/va-reforms/index.html. Published June 12, 2014. Accessed June 24, 2020.
6. Richardson B. IG probes uncover more problems at VA hospitals. https://thehill.com/policy/defense/258652-ig-probes-uncover-more-problems-at-va-hospitals. Published October 30, 2015. Accessed June 24, 2020.
7. Slack D. Inaccurate VA wait times prelude thousands of vets from getting outside care, probe finds. USA Today. March 3, 2017. https://www.usatoday.com/story/news/politics/2017/03/03/veterans-affairs-inspector-general-widespread-inaccuracies-wait-times/98693856. Accessed June 24, 2020.
8. US Department of Veterans Affairs, Office of the Inspector General. Veterans Health Administration: audit of physician staffing levels for specialty care services. https://www.va.gov/oig/pubs/VAOIG-11-01827-36.pdf. Published December 27, 2012. Accessed June 24, 2020.
9. Government Accountability Office. VA health care: improvements needed in data and monitoring of clinical productivity and efficiency. https://www.gao.gov/assets/690/684869.pdf. Published May 2017. Accessed June 24, 2020.
10. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1082. Patient care data capture. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3091. Published March 24, 2015. Accessed June 24, 2020.
11. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1006.02. VHA site classifications and definitions. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2970. Published December 30, 2013. Accessed June 24, 2020.
12. Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000-2010. Sleep. 2016;39(7):1399-1410. Published 2016 Jul 1. doi:10.5665/sleep.5972.
13. A Caldwell J, Knapik JJ, Lieberman HR. Trends and factors associated with insomnia and sleep apnea in all United States military service members from 2005 to 2014. J Sleep Res. 2017;26(5):665-670. doi:10.1111/jsr.12543
14. Klingaman EA, Brownlow JA, Boland EM, Mosti C, Gehrman PR. Prevalence, predictors and correlates of insomnia in US army soldiers. J Sleep Res. 2018;27(3):e12612. doi:10.1111/jsr.12612
15. Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345-350. doi:10.1016/j.sleep.2004.01.019.
16. Sarmiento KF, Folmer RL, Stepnowsky CJ, et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J Clin Sleep Med. 2019;15(9):1355-1364. doi:10.5664/jcsm.7934
17. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation [published correction appears in Sleep. 2004 Jun 15;27(4):600]. Sleep. 2003;26(2):117-126. doi:10.1093/sleep/26.2.117
18. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700-708. doi:10.1016/j.jpsychires.2006.07.008
19. Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi:10.1111/jsr.12104
20. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311-1322. doi:10.3978/j.issn.2072-1439.2015.06.11
21. Javaheri S, Redline S. Insomnia and Risk of Cardiovascular Disease. Chest. 2017;152(2):435-444. doi:10.1016/j.chest.2017.01.026
22. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructivesSleepaApnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532-540. doi:10.1001/jamacardio.2018.0095
23. Ogilvie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. 2018;44:32-37. doi:10.1016/j.sleep.2018.01.004
Tinnitus: Questions to reveal the cause, answers to provide relief
- If no treatable cause of tinnitus is found, assess the severity of tinnitus, secondary problems (such as depression, anxiety, and insomnia), and implement tinnitus management strategies (SOR:B).
- Acoustic therapy is effective for tinnitus management (SOR:B).
- All patients should wear hearing protection when they are exposed to loud sounds such as a gas lawnmower, leaf blower, power tools, or gunfire (SOR:A).
- Successful management of insomnia, anxiety or depression will decrease the severity of tinnitus for most patients (SOR:B).
Tinnitus—the perception of sound that does not have an external source—can be constant or intermittent and perceived as ringing, buzzing, hissing, sizzling, roaring, chirping, or other sounds.
Acute tinnitus, which can last days or weeks, may be caused by ear infection, medications, head or neck injury, excessive sound exposure, earwax, and changes in blood pressure or metabolism. With appropriate evaluation, such underlying conditions usually can be identified and treated, often with resultant resolution of tinnitus.
Chronic tinnitus (persistence for 6 months or more) can also result from these conditions and is more likely to occur in people who have hearing loss.1 (See Prevalence of tinnitus.) Even though a true “cure” for most cases of chronic tinnitus is not available, patients can obtain relief from the symptom with assistance from clinicians who are familiar with tinnitus management strategies.
Seidman and Jacobson2 estimated that 40 million people in the United States experience chronic tinnitus. The prevalence of tinnitus increases with age: 27% of males and 15% of females aged 45 years or older experience the symptom.3
Tinnitus is rare in children who have normal hearing.4 However, the prevalence of tinnitus in children with severe or profound hearing loss has been reported as 33%5 or 64%.6 More males than females experience tinnitus because men traditionally have had a greater amount of noise exposure in military service, in the workplace, and during recreational activities. Consequently, hearing loss and tinnitus are both more prevalent among men aged 45 years or older compared with women in the same age group.3
Damage depends on intensity, length of exposure
Tinnitus is most commonly caused by exposure to excessively loud sounds such as gunfire, power tools, machinery, or music. Ringing in the ears occurs because of damage to stereocilia, microscopic appendages attached to the apical ends of hair cells in the cochlea.
Moderate sounds (80 decibels sound pressure level [dB SPL] or lower) normally cause stereocilia to make tiny movements, triggering the releaseof neurotransmitter molecules from the basal ends of hair cells that activate auditory neurons in the eighth cranial nerve.
Excessive sound exposure (85 dB SPL or louder) causes stereocilia to bend more than they should. People then perceive high-pitched ringing tinnitus because hair cells that respond to higher-frequency sounds are located at the base of the cochlea and are the first to be damaged by loud noise.
If the damage is modest and infrequent, stereocilia can recover, returning to their normal function in a few minutes or hours. The patient’s hearing will be restored and the tinnitus will stop. However, repeated exposure to hazardous sounds eventually causes irreparable damage to stereocilia and hair cells, resulting in permanent sensorineural hearing loss and possibly chronic tinnitus.
In addition to noise exposure, any condition that causes hearing loss or damages the auditory system can contribute to the generation of tinnitus (Table). Imaging studies using functional magnetic resonance imaging7 or positron-emission tomography8-9 demonstrated that the perception of chronic tinnitus usually occurs as a result of hyperactivity within central auditory areas of the human brain, especially the auditory cortex. As portions of the auditory system degenerate during the aging process or acquire damage from noise exposure, disease, and accidents, the natural balance of central auditory excitation vs inhibition is disrupted. In patients who hear tinnitus, excitatory pathways within the auditory system are active when they shouldn’t be: in quiet environments. This gives patients the perception of tinnitus sounds.
TABLE
Causes of subjective tinnitus
| Presbycusis: hearing loss due to aging |
| Prolonged noise exposure: Noise-induced hearing loss |
| Acoustic trauma: one-time exposure to high intensity sound |
| Otosclerosis: abnormal accumulation of calcium on middle ear ossicles or cochlea |
| Infections: bacterial, viral, fungal |
| Autoimmune hearing loss |
| Meniere’s disease or endolymphatic hydrops: abnormally high inner ear pressure |
| Neoplasms: for example, acoustic neuroma or cholesteatoma |
| Genetic predisposition |
Ototoxicity
|
Vascular
|
Metabolic
|
| Head or neck injury |
Objective tinnitus
Objective tinnitus—which can be heard also by people in proximity to the patient’s ear—can be caused by vascular abnormalities (congenital arteriovenous fistula, acquired arteriovenous shunt, glomus jugulare, high-riding carotid artery, carotid stenosis, persistent stapedial artery, dehiscent jugular bulb or a vascular loop such as anterior inferior communicating artery [AICA] or posterior inferior communicating artery [PICA] compressing the auditory nerve) or mechanical disorders (abnormally patent Eustachian tube, palatal myoclonus, temporo-mandibular joint disorder, or stapedial muscle spasticity).10 However, objective tinnitus is rare, accounting for <1% of all cases. The vast majority of tinnitus cases are subjective—sounds are perceived only by the patient.
Patient evaluation
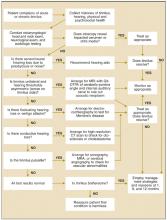
Figure 1 is an algorithm that outlines steps in the evaluation and management of patients who experience tinnitus. The first step is to collect as much information as possible about the patient and his condition.
FIGURE 1
Evaluation and management of tinnitus
Tinnitus history
Determine the duration of tinnitus and whether circumstances such as upper respiratory infection, otalgia, noise exposure, head trauma, sudden hearing loss, or vertigo occurred at the time of tinnitus onset. Ask the patient to describe the tinnitus: Is it intermittent or constant? High- or low-pitched? Unilateral or bilateral? Pulsatile or steady?
Unilateral tinnitus and hearing loss provide preliminary evidence for acoustic neuroma or cerebrovascular accident. High-pitched tinnitus is usually associated with high-frequency hearing loss caused by presbycusis (hearing impairment in the aged) or excessive noise exposure. Low-pitched roaring tinnitus is sometimes associated with low-frequency hearing loss exhibited by patients with Meniere’s disease. Pulsatile tinnitus, especially if synchronous with the patient’s pulse, can indicate vascular abnormalities.
Ask the patient if fatigue, stress, noise exposure, or any medications exacerbate the tinnitus. Also ask if masking sounds (such as water running in the shower), medications, or any other factors provide relief from tinnitus. This information can be used to formulate a tinnitus management program.
Assess the severity of the patient’s tinnitus using an instrument such as the Tinnitus Severity Index (Figure 2).11 A score of 36 or higher indicates bothersome tinnitus (level of evidence [LOE]: 2).12 Higher scores indicate that patients perceive their tinnitus to be a significant, even debilitating problem.
FIGURE 2
Tinnitus Severity Index
Hearing history
If possible, determine the presence and type of hearing loss (congenital, sudden, sensorineural, conductive, or mixed). Note the patient’s history of ear infections, surgeries, noise exposure (occupational or recreational), otalgia, otorrhea, and vertigo or other balance problems. Ask whether immediate family members have experienced hearing loss or tinnitus.
Health history
Look particularly for conditions that can contribute to hearing loss and tinnitus, such as hypertension, hypothyroidism, diabetes mellitus, arteriosclerosis, and autoimmune disorders (eg, lupus or rheumatoid arthritis). Also consider ototoxic medications, such as aminoglycoside antibiotics, cisplatin, furosemide, valproic acid, and high doses of quinine-containing compounds. When possible, patients with hearing loss or tinnitus should be given alternative medications free from ototoxicity.
Excessive use of alcohol, caffeine, and aspirin or other nonsteroidal anti-inflammatory drugs can exacerbate tinnitus for some patients. However, moderate use of these products is often possible.
Psychosocial history
Inquire about the patient’s marital and occupational status. Unemployed patients living alone often perceive tinnitus to be more severe than do employed patients who have supportive social networks. Also ask about any history of insomnia, anxiety, depression, obsessive-compulsive disorder, or psychosis. A questionnaire such as the abbreviated Beck Depression Inventory13 can be used to assess the presence and severity of depression.
Physical exam and testing
Patient evaluation should include the following physical examinations and tests.
Otolaryngologic/head and neck exam. Otoscopic examination can detect infections such as otitis media, which will usually be accompanied by complaints of ear pain or fullness, and possibly hearing loss in combination with tinnitus. Otoscopy can also detect impacted earwax (cerumen), which can occlude the ear canal or cause immobilization of the tympanic membrane, resulting in conductive hearing loss, tinnitus, and a feeling of fullness in the ear. Symptoms usually resolve when the earwax is removed.
If the tinnitus is synchronous with the patient’s pulse, it suggests a vascular contribution for the symptom. Auscultation of blood vessels in the neck can reveal venous hums or other types of bruits audible to the patient. Venous hum can be diagnosed by temporarily blocking blood flow through the jugular vein on the side where tinnitus is perceived.
Neurologic exam. A complete neurologic exam should include the Romberg test, Dix-Hallpike maneuver (if the patient experiences vertigo), gait testing, and cranial nerve function tests.
Audiologic testing. Audiologic tests should include pure tone air and bone conduction thresholds, speech discrimination testing, tympanometry, and most comfortable loudness (MCL) and uncomfortable loudness level (UCL) tests. Tympanometry is used to assess middle-ear function. Abnormal tympanograms and significant differences between air and bone conduction thresholds can indicate otitis media, otosclerosis, or cholesteatoma.
MCL and UCL tests are used to assess the dynamic range of patients’ hearing. Patients with UCLs that are only 5 to 20 dBs above their MCLs have a reduced dynamic range of hearing that can be caused by recruitment or hyperacusis. The audiometer can also be used to match the tinnitus for pitch and loudness and to test the effects of masking sounds on the patient’s tinnitus.
Additional evaluations. Results of patient examinations and history collection might warrant additional evaluations. For example, asymmetrical hearing loss (15 dB or greater asymmetry at 2 or more consecutive test frequencies) and unilateral tinnitus can indicate a retrocochlear lesion such as acoustic neuroma (also known as vestibular schwannoma).
One test for retrocochlear pathology is the auditory brainstem response (ABR). In this test, clicks are presented through earphones while scalp electrodes record brain responses to the sounds. Abnormal ABR waveforms can indicate retrocochlear lesion (such as acoustic neuroma) as a possible cause of ipsilateral hearing loss and tinnitus. If positive ABR results are obtained, MRI evaluation of the cerebellopontine angle with contrast material (such as gadolinium) should be performed.
Low-pitched roaring, ringing, or hissing tinnitus; hearing loss, which may be temporary or permanent; vertigo; and a feeling of pressure or fullness in the ear can indicate endolymphatic hydrops or Meniere’s disease. Symptoms usually occur in the form of “attacks” that increase in frequency during the first few years of the disease, then decrease in frequency as hearing thresholds stabilize. Electrocochleography testing is one way to diagnose endolymphatic hydrops. Patients who exhibit vestibular disorders should undergo electronystagmography testing to assess the severity and characteristics of their symptoms.
Pulsatile tinnitus associated with abnormalities of blood vessels in the neck can be evaluated with sonography, conventional angiography, or magnetic resonance angiography. Conditions such as a dehiscent jugular bulb or stenosis of carotid arteries can sometimes be treated surgically. However, many forms of pulsatile tinnitus are not caused by these conditions. Pulsatile tinnitus is often a consequence of hearing loss, arteriosclerosis, or weight loss or weight gain. These physiologic changes can cause patients to hear blood pulsing or “swishing” in vessels—sounds they did not perceive previously. Surgery is not recommended for most cases of pulsatile tinnitus.
Sudden hearing loss, especially if bilateral, might indicate autoimmune inner ear disease. Diagnostic tests include the Western blot immunoassay.
Treatment of active disease processes
Many contributors to tinnitus can be treated surgically or with medication.
Otitis media. Successful treatment of the infection with oral antibiotics usually resolves all auditory symptoms.
Allergies, sinus congestion, or infection. When inflammation subsides, tinnitus associated with these conditions usually resolves.
Otosclerosis. Abnormal accumulations of calcium on middle-ear ossicles (especially the stapes) or the cochlea can result in slowly progressing conductive or sensorineural hearing loss, tinnitus, and vestibular disturbances. Stapedectomy surgery—including implantation of ossicular prostheses—is often successful for advanced cases associated with significant hearing loss. Hearing aids also benefit some patients.
Meniere’s disease or other forms of endolymphatic hydrops. Meniere’s disease, characterized by abnormally high fluid pressure within the cochlea, has an estimated prevalence of 1% in the US.14 Management includes meclizine, antiemetics and diuretics, and a low-sodium diet.15 If patients do not respond to meclizine, diazepam can be prescribed to reduce the severity of vertigo attacks. Surgical intervention—including installation of an endolymphatic shunt, labyrinthectomy, or vestibular neurectomy16—or transtympanic injections of gentamicin17 are options in severe cases.
Autoimmune inner ear disease. This disease has an estimated prevalence of 0.1% in the US.18 Symptoms include sudden hearing loss in one ear that usually progresses to the second ear. Patients may also feel fullness in the ear and experience vertigo as well as ringing, hissing, or roaring tinnitus. Most patients with autoimmune inner ear disease respond to initial treatment with oral prednisone.
Auditory neoplasms. Growths such as acoustic neuroma or cholesteatoma can cause tinnitus. Acoustic neuroma (or vestibular schwannoma) is a benign neoplasm that arises from the vestibular division of the eighth cranial nerve. Symptoms include unilateral hearing loss, tinnitus, and vestibular disturbances. Surgical resection or radiation treatment of the tumor can resolve these symptoms, especially if the neoplasm is detected while it is small.
Cholesteatoma is a benign epithelial cell mass that grows in the middle-ear cavity. Over time, cholesteatomas can enlarge and destroy middleear ossicles. Hearing loss, tinnitus, dizziness, and facial muscle paralysis can result from continued cholesteatoma growth. Early detection and surgical resection of auditory neoplasms can reduce the likelihood of residual symptoms.
Hyper- or hypotension. Of these two disorders, hypertension is more likely to contribute to tinnitus. Maintenance of blood pressure within the optimum range can decrease or resolve tinnitus for some patients.
Metabolic disorders. Disorders such as diabetes mellitus, hyperthyroidism, or hypothyroidism can contribute to tinnitus. Successful management of these conditions can reduce or resolve the patient’s tinnitus.
Managing persistent tinnitus
Successful treatment of the disorders discussed can resolve or reduce tinnitus. However, if tinnitus continues to bother the patient after other diseases have been treated, shift the clinical focus from treatment to management of the symptom. At this point, the clinician should do 1 of 2 things: 1) spend the time necessary to help the patient manage tinnitus using strategies described in the following sections of this article; or 2) refer the patient to a comprehensive tinnitus management program with experienced personnel who are willing and able to spend a substantial amount of time with each patient.
Like other neurologic symptoms, tinnitus can be considered chronic if it persists for 6 months or more. Approximately 90% of cases of chronic tinnitus are associated with some degree of sensorineural hearing loss.19 Because sensorineural hearing loss is irreversible, most cases of chronic tinnitus cannot be “cured.” Duckro et al20 wrote: “As with chronic pain, the treatment of chronic tinnitus is more accurately described in terms of management rather than cure.”
The goal of management is not necessarily to mask or remove the patient’s perception of tinnitus. In many cases, this is not possible. Successful management enables patients to pay less attention to their tinnitus. An effective management program helps patients to understand and gain control over their tinnitus, rather than allowing it to control them. The ultimate goal is to reduce the severity of tinnitus. Clinicians should strive to help patients progress to where tinnitus is no longer a negative factor in their lives.
Establishing tinnitus severity
Only 25% of people who experience chronic tinnitus consider the symptom to be a significant problem.2 These are the patients most likely to seek treatment. If a patient is not bothered by tinnitus and no active disease processes are detected, no treatments are necessary. The clinician should reassure such patients that tinnitus is a harmless perception of sound and does not usually portend more serious medical conditions.
What differentiates the majority of people not bothered by tinnitus from the minority who perceive it as a significant, even debilitating problem? Is it the matched loudness, pitch, or other qualities of the sound(s) they hear? Several studies have concluded that tinnitus severity is not correlated with any of these psychoacoustic parameters.21-23
Tinnitus severity can be defined and quantified several ways: by how much or how often a patient is bothered by tinnitus; by how much or how often tinnitus detracts from the patient’s enjoyment of life; or by how disabling patients perceive their tinnitus to be. Instruments such as the Tinnitus Severity Index11 can be used to assess tinnitus severity (Figure 2).
Tinnitus management strategies
Once underlying conditions have been treated or ruled out, reassure and counsel patients regarding factors that could exacerbate or improve their condition. If patients understand their tinnitus is nothing more than a perception of sound, they will be better able to pay less attention to it. This process of patient education and counseling helps to “demystify” the symptom of tinnitus and encourages patients to view their tinnitus with a more realistic perspective.
The severity of tinnitus is often associated with problems such as insomnia,24 anxiety,25 and depression.26 Such issues can form a vicious circle, with each one exacerbating the others.23 Tinnitus is not always the starting point of this cycle—many patients experience depression, insomnia, or anxiety before tinnitus. Medication or psychotherapy will often reduce the severity of these symptoms and associated tinnitus (LOE: 2).27-28
Because each patient has a unique medical, psychological, and social history, management programs should be individualized. In fact, the most successful tinnitus management programs employ multimodal strategies designed to address the specific needs of each patient (LOE: 2).27,29
Recommendations should be formulated and explained to the patient: appropriate acoustic therapy; use of hearing protection (all patients should wear earplugs or ear muffs when they are exposed to excessively loud sounds [LOE: 1]);30 and strategies for management of insomnia, anxiety, or depression. As appropriate, provide patients with referral and contact information for physical or psychiatric evaluations, psychological counseling, and other recommended services or products.
Acoustic therapy
Patients should add pleasant sounds (music, relaxation CDs, or a tabletop sound machine) to any environment that is too quiet, and listen to them through speakers or headphones. Patients who experience chronic insomnia because of tinnitus may find relief in using a tabletop sound machine in combination with a pillow embedded with speakers (such as the Sound Pillow, distributed by Phoenix Productions, San Antonio, TX).
Patients with normal or nearly normal hearing might benefit from in-the-ear sound generators (such as those manufactured by General Hearing Instruments, Harahan, LA) that produce a broad-band sound to muffle or mask the tinnitus.31 Significant, aidable hearing loss can often be lessened with hearing aids or combination instruments (hearing aid and sound generator in one unit). Hearing aids not only improve communication ability, the devices can also reduce the perception of tinnitus.32
Follow-up
Encourage patients to ask questions about recommended tinnitus management procedures and to report their progress. Reassess patients at 1 month. If necessary, recommendations can be modified to facilitate patient improvement. Follow-up questionnaires can be mailed to patients 6 and 12 months after their initial appointment to assess the effectiveness of the tinnitus management program.
A customized combination of recommendations is effective for many patients with chronic and bothersome tinnitus (LOE: 2),27 but the process can be very time consuming. For a certain number of patients with severe tinnitus, only a comprehensive management program can help them to improve their condition.
Corresponding author
Robert L. Folmer, PhD, OHSU Tinnitus Clinic, Mail Code NRC04, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239. E-mail: [email protected].
1. Schleuning A. Medical aspects of tinnitus. In: Vernon JA, ed. Tinnitus Treatment and Relief. Boston, Mass: Allyn and Bacon; 1998;20-27.
2. Seidman MD, Jacobson GP. Update on tinnitus. Otolaryngol Clin N Amer 1996;29:455-465.
3. Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey, 1996. Hyattsville, Md: National Center for Health Statistics, 1999.
4. Stouffer JL, Tyler RS, Booth JC, Buckrell B. Tinnitus in normal-hearing and hearing-impaired children. In: Aran J-M, Dauman R, eds. Tinnitus 91: Proceedings of the Fourth International Tinnitus Seminar. Amsterdam: Kugler Publications 1992;255-258.
5. Drukier GS. The prevalence and characteristics of tinnitus with profound sensori-neural hearing impairment. Amer Ann Deaf 1989;134:260-264.
6. Graham J. Paediatric tinnitus. J Laryngol Otol 1981;95(Suppl):117-120.
7. Levine RA, Benson RR, Talavage TM, Melcher JR, Rosen BR. Functional magnetic resonance imaging and tinnitus: preliminary results. Abstr Assoc Res Otolaryngol 1997;20:65.-
8. Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec 1996;58:195-199.
9. Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wach DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neuroplasticity. Neurology 1998;50:114-120.
10. Ciocon JO, Amede F, Lechtenberg C, Astor F. Tinnitus: a stepwise workup to quiet the noise within. Geriatrics 1995;50:18-25.
11. Meikle MB, Griest SE, Stewart BJ, Press LS. Measuring the negative impact of tinnitus: a brief severity index. Abstr Assoc Res Otolaryngol 1995;167.-
12. Folmer RL, Stevenson EA, Tran A. Factors associated with long-term improvements in tinnitus severity. In Patuzzi R, ed: Proceedings of the Seventh International Tinnitus Seminar. Crawley: University of Western Australia; 2002;115-123.
13. Beck AT, Beck RW. Screening depressed patients in family practice: a rapid technic. Postgrad Medicine 1972;52:81-85.
14. daCosta SS, deSousa LC, Piza MR. Meniere’s Disease: overview, epidemiology, and natural history. Otolaryngol Clin N Amer 2002;35:455-495.
15. Thai-Van H, Bounaix MJ, Fraysse B. Meniere’s disease: pathophysiology and treatment. Drugs 2001;61:1089-1102.
16. Hillman TA, Chen DA, Arriaga MA. Vestibular nerve section versus intratympanic gentamicin for Meniere’s disease. Laryngoscope 2004;114:216-222.
17. Diamond C, Hornig JD, Liu R, O’Connell DA. Systematic review of intratympanic gentamicin in Meniere’s disease. J Otolaryngol 2003;32:351-361.
18. Hain TC. Autoimmune inner ear disease. American Hearing Research Foundation Web site. March 23, 2002. Available at www.american-hearing.org/name/autoimmune.html. Accessed on May 15, 2004.
19. Meikle MB. Electronic access to tinnitus data: The Oregon Tinnitus Data Archive. Otolaryngol Head Neck Surg 1997;117:698-700.
20. Duckro PN, Pollard CA, Bray HD, Scheiter L. Comprehensive behavioral management of complex tinnitus: a case illustration. Biofeedback and Self-Regulation 1984;9:459-469.
21. Meikle MB, Vernon J, Johnson RM. The perceived severity of tinnitus. Otolaryngol Head Neck Surg 1984;92:689-696.
22. van Veen ED, Jacobs JB, Bensing JM. Assessment of distress associated with tinnitus. J Laryngol Otol 1998;112:258-263.
23. Folmer RL, Griest SE, Martin WH. Chronic tinnitus as phantom auditory pain. Otolaryngol Head Neck Surg 2001;124:394-400.
24. Folmer RL, Griest SE. Tinnitus and insomnia. Am J Otolaryngol 2000;21:287-293.
25. Attias J, Shemesh Z, Bleich A, Solomon Z, Bar-Or G, Alster J, Sohmer H. Psychological profile of help-seeking and non-help-seeking tinnitus patients. Scand Audiol 1995;24:13-18.
26. Folmer RL, Griest SE, Meikle MB, Martin WH. Tinnitus severity, loudness and depression. Otolaryngol Head Neck Surg 1999;121:48-51.
27. Folmer R. Long-term reductions in tinnitus severity. BMC Ear Nose and Throat Disorders 2002;2:3.-Available at: www.biomedcentral.com/1472-6815/2/3.
28. Folmer RL, Shi YB. SSRI use by tinnitus patients: interactions between depression and tinnitus severity ENT J 2004;83:107-117.
29. Sullivan M, Katon W, Russo J, Dobie R, Sakai C. Coping and marital support as correlates of tinnitus disability. Gen Hosp Psychiatry 1994;16:259-266.
30. Lusk SL. Preventing noise-induced hearing loss. Nurs Clin North Amer 2002;37:257-262.
31. Henry JA, Schechter MA, Nagler SM, Fausti SA. Comparison of tinnitus masking and tinnitus retraining therapy. J Amer Acad Audiol 2002;13:559-581.
32. Surr RK, Montgomery AA, Mueller HG. Effect of amplification on tinnitus among new hearing aid users. Ear and Hearing 1985;6:71-75.
- If no treatable cause of tinnitus is found, assess the severity of tinnitus, secondary problems (such as depression, anxiety, and insomnia), and implement tinnitus management strategies (SOR:B).
- Acoustic therapy is effective for tinnitus management (SOR:B).
- All patients should wear hearing protection when they are exposed to loud sounds such as a gas lawnmower, leaf blower, power tools, or gunfire (SOR:A).
- Successful management of insomnia, anxiety or depression will decrease the severity of tinnitus for most patients (SOR:B).
Tinnitus—the perception of sound that does not have an external source—can be constant or intermittent and perceived as ringing, buzzing, hissing, sizzling, roaring, chirping, or other sounds.
Acute tinnitus, which can last days or weeks, may be caused by ear infection, medications, head or neck injury, excessive sound exposure, earwax, and changes in blood pressure or metabolism. With appropriate evaluation, such underlying conditions usually can be identified and treated, often with resultant resolution of tinnitus.
Chronic tinnitus (persistence for 6 months or more) can also result from these conditions and is more likely to occur in people who have hearing loss.1 (See Prevalence of tinnitus.) Even though a true “cure” for most cases of chronic tinnitus is not available, patients can obtain relief from the symptom with assistance from clinicians who are familiar with tinnitus management strategies.
Seidman and Jacobson2 estimated that 40 million people in the United States experience chronic tinnitus. The prevalence of tinnitus increases with age: 27% of males and 15% of females aged 45 years or older experience the symptom.3
Tinnitus is rare in children who have normal hearing.4 However, the prevalence of tinnitus in children with severe or profound hearing loss has been reported as 33%5 or 64%.6 More males than females experience tinnitus because men traditionally have had a greater amount of noise exposure in military service, in the workplace, and during recreational activities. Consequently, hearing loss and tinnitus are both more prevalent among men aged 45 years or older compared with women in the same age group.3
Damage depends on intensity, length of exposure
Tinnitus is most commonly caused by exposure to excessively loud sounds such as gunfire, power tools, machinery, or music. Ringing in the ears occurs because of damage to stereocilia, microscopic appendages attached to the apical ends of hair cells in the cochlea.
Moderate sounds (80 decibels sound pressure level [dB SPL] or lower) normally cause stereocilia to make tiny movements, triggering the releaseof neurotransmitter molecules from the basal ends of hair cells that activate auditory neurons in the eighth cranial nerve.
Excessive sound exposure (85 dB SPL or louder) causes stereocilia to bend more than they should. People then perceive high-pitched ringing tinnitus because hair cells that respond to higher-frequency sounds are located at the base of the cochlea and are the first to be damaged by loud noise.
If the damage is modest and infrequent, stereocilia can recover, returning to their normal function in a few minutes or hours. The patient’s hearing will be restored and the tinnitus will stop. However, repeated exposure to hazardous sounds eventually causes irreparable damage to stereocilia and hair cells, resulting in permanent sensorineural hearing loss and possibly chronic tinnitus.
In addition to noise exposure, any condition that causes hearing loss or damages the auditory system can contribute to the generation of tinnitus (Table). Imaging studies using functional magnetic resonance imaging7 or positron-emission tomography8-9 demonstrated that the perception of chronic tinnitus usually occurs as a result of hyperactivity within central auditory areas of the human brain, especially the auditory cortex. As portions of the auditory system degenerate during the aging process or acquire damage from noise exposure, disease, and accidents, the natural balance of central auditory excitation vs inhibition is disrupted. In patients who hear tinnitus, excitatory pathways within the auditory system are active when they shouldn’t be: in quiet environments. This gives patients the perception of tinnitus sounds.
TABLE
Causes of subjective tinnitus
| Presbycusis: hearing loss due to aging |
| Prolonged noise exposure: Noise-induced hearing loss |
| Acoustic trauma: one-time exposure to high intensity sound |
| Otosclerosis: abnormal accumulation of calcium on middle ear ossicles or cochlea |
| Infections: bacterial, viral, fungal |
| Autoimmune hearing loss |
| Meniere’s disease or endolymphatic hydrops: abnormally high inner ear pressure |
| Neoplasms: for example, acoustic neuroma or cholesteatoma |
| Genetic predisposition |
Ototoxicity
|
Vascular
|
Metabolic
|
| Head or neck injury |
Objective tinnitus
Objective tinnitus—which can be heard also by people in proximity to the patient’s ear—can be caused by vascular abnormalities (congenital arteriovenous fistula, acquired arteriovenous shunt, glomus jugulare, high-riding carotid artery, carotid stenosis, persistent stapedial artery, dehiscent jugular bulb or a vascular loop such as anterior inferior communicating artery [AICA] or posterior inferior communicating artery [PICA] compressing the auditory nerve) or mechanical disorders (abnormally patent Eustachian tube, palatal myoclonus, temporo-mandibular joint disorder, or stapedial muscle spasticity).10 However, objective tinnitus is rare, accounting for <1% of all cases. The vast majority of tinnitus cases are subjective—sounds are perceived only by the patient.
Patient evaluation
Figure 1 is an algorithm that outlines steps in the evaluation and management of patients who experience tinnitus. The first step is to collect as much information as possible about the patient and his condition.
FIGURE 1
Evaluation and management of tinnitus
Tinnitus history
Determine the duration of tinnitus and whether circumstances such as upper respiratory infection, otalgia, noise exposure, head trauma, sudden hearing loss, or vertigo occurred at the time of tinnitus onset. Ask the patient to describe the tinnitus: Is it intermittent or constant? High- or low-pitched? Unilateral or bilateral? Pulsatile or steady?
Unilateral tinnitus and hearing loss provide preliminary evidence for acoustic neuroma or cerebrovascular accident. High-pitched tinnitus is usually associated with high-frequency hearing loss caused by presbycusis (hearing impairment in the aged) or excessive noise exposure. Low-pitched roaring tinnitus is sometimes associated with low-frequency hearing loss exhibited by patients with Meniere’s disease. Pulsatile tinnitus, especially if synchronous with the patient’s pulse, can indicate vascular abnormalities.
Ask the patient if fatigue, stress, noise exposure, or any medications exacerbate the tinnitus. Also ask if masking sounds (such as water running in the shower), medications, or any other factors provide relief from tinnitus. This information can be used to formulate a tinnitus management program.
Assess the severity of the patient’s tinnitus using an instrument such as the Tinnitus Severity Index (Figure 2).11 A score of 36 or higher indicates bothersome tinnitus (level of evidence [LOE]: 2).12 Higher scores indicate that patients perceive their tinnitus to be a significant, even debilitating problem.
FIGURE 2
Tinnitus Severity Index
Hearing history
If possible, determine the presence and type of hearing loss (congenital, sudden, sensorineural, conductive, or mixed). Note the patient’s history of ear infections, surgeries, noise exposure (occupational or recreational), otalgia, otorrhea, and vertigo or other balance problems. Ask whether immediate family members have experienced hearing loss or tinnitus.
Health history
Look particularly for conditions that can contribute to hearing loss and tinnitus, such as hypertension, hypothyroidism, diabetes mellitus, arteriosclerosis, and autoimmune disorders (eg, lupus or rheumatoid arthritis). Also consider ototoxic medications, such as aminoglycoside antibiotics, cisplatin, furosemide, valproic acid, and high doses of quinine-containing compounds. When possible, patients with hearing loss or tinnitus should be given alternative medications free from ototoxicity.
Excessive use of alcohol, caffeine, and aspirin or other nonsteroidal anti-inflammatory drugs can exacerbate tinnitus for some patients. However, moderate use of these products is often possible.
Psychosocial history
Inquire about the patient’s marital and occupational status. Unemployed patients living alone often perceive tinnitus to be more severe than do employed patients who have supportive social networks. Also ask about any history of insomnia, anxiety, depression, obsessive-compulsive disorder, or psychosis. A questionnaire such as the abbreviated Beck Depression Inventory13 can be used to assess the presence and severity of depression.
Physical exam and testing
Patient evaluation should include the following physical examinations and tests.
Otolaryngologic/head and neck exam. Otoscopic examination can detect infections such as otitis media, which will usually be accompanied by complaints of ear pain or fullness, and possibly hearing loss in combination with tinnitus. Otoscopy can also detect impacted earwax (cerumen), which can occlude the ear canal or cause immobilization of the tympanic membrane, resulting in conductive hearing loss, tinnitus, and a feeling of fullness in the ear. Symptoms usually resolve when the earwax is removed.
If the tinnitus is synchronous with the patient’s pulse, it suggests a vascular contribution for the symptom. Auscultation of blood vessels in the neck can reveal venous hums or other types of bruits audible to the patient. Venous hum can be diagnosed by temporarily blocking blood flow through the jugular vein on the side where tinnitus is perceived.
Neurologic exam. A complete neurologic exam should include the Romberg test, Dix-Hallpike maneuver (if the patient experiences vertigo), gait testing, and cranial nerve function tests.
Audiologic testing. Audiologic tests should include pure tone air and bone conduction thresholds, speech discrimination testing, tympanometry, and most comfortable loudness (MCL) and uncomfortable loudness level (UCL) tests. Tympanometry is used to assess middle-ear function. Abnormal tympanograms and significant differences between air and bone conduction thresholds can indicate otitis media, otosclerosis, or cholesteatoma.
MCL and UCL tests are used to assess the dynamic range of patients’ hearing. Patients with UCLs that are only 5 to 20 dBs above their MCLs have a reduced dynamic range of hearing that can be caused by recruitment or hyperacusis. The audiometer can also be used to match the tinnitus for pitch and loudness and to test the effects of masking sounds on the patient’s tinnitus.
Additional evaluations. Results of patient examinations and history collection might warrant additional evaluations. For example, asymmetrical hearing loss (15 dB or greater asymmetry at 2 or more consecutive test frequencies) and unilateral tinnitus can indicate a retrocochlear lesion such as acoustic neuroma (also known as vestibular schwannoma).
One test for retrocochlear pathology is the auditory brainstem response (ABR). In this test, clicks are presented through earphones while scalp electrodes record brain responses to the sounds. Abnormal ABR waveforms can indicate retrocochlear lesion (such as acoustic neuroma) as a possible cause of ipsilateral hearing loss and tinnitus. If positive ABR results are obtained, MRI evaluation of the cerebellopontine angle with contrast material (such as gadolinium) should be performed.
Low-pitched roaring, ringing, or hissing tinnitus; hearing loss, which may be temporary or permanent; vertigo; and a feeling of pressure or fullness in the ear can indicate endolymphatic hydrops or Meniere’s disease. Symptoms usually occur in the form of “attacks” that increase in frequency during the first few years of the disease, then decrease in frequency as hearing thresholds stabilize. Electrocochleography testing is one way to diagnose endolymphatic hydrops. Patients who exhibit vestibular disorders should undergo electronystagmography testing to assess the severity and characteristics of their symptoms.
Pulsatile tinnitus associated with abnormalities of blood vessels in the neck can be evaluated with sonography, conventional angiography, or magnetic resonance angiography. Conditions such as a dehiscent jugular bulb or stenosis of carotid arteries can sometimes be treated surgically. However, many forms of pulsatile tinnitus are not caused by these conditions. Pulsatile tinnitus is often a consequence of hearing loss, arteriosclerosis, or weight loss or weight gain. These physiologic changes can cause patients to hear blood pulsing or “swishing” in vessels—sounds they did not perceive previously. Surgery is not recommended for most cases of pulsatile tinnitus.
Sudden hearing loss, especially if bilateral, might indicate autoimmune inner ear disease. Diagnostic tests include the Western blot immunoassay.
Treatment of active disease processes
Many contributors to tinnitus can be treated surgically or with medication.
Otitis media. Successful treatment of the infection with oral antibiotics usually resolves all auditory symptoms.
Allergies, sinus congestion, or infection. When inflammation subsides, tinnitus associated with these conditions usually resolves.
Otosclerosis. Abnormal accumulations of calcium on middle-ear ossicles (especially the stapes) or the cochlea can result in slowly progressing conductive or sensorineural hearing loss, tinnitus, and vestibular disturbances. Stapedectomy surgery—including implantation of ossicular prostheses—is often successful for advanced cases associated with significant hearing loss. Hearing aids also benefit some patients.
Meniere’s disease or other forms of endolymphatic hydrops. Meniere’s disease, characterized by abnormally high fluid pressure within the cochlea, has an estimated prevalence of 1% in the US.14 Management includes meclizine, antiemetics and diuretics, and a low-sodium diet.15 If patients do not respond to meclizine, diazepam can be prescribed to reduce the severity of vertigo attacks. Surgical intervention—including installation of an endolymphatic shunt, labyrinthectomy, or vestibular neurectomy16—or transtympanic injections of gentamicin17 are options in severe cases.
Autoimmune inner ear disease. This disease has an estimated prevalence of 0.1% in the US.18 Symptoms include sudden hearing loss in one ear that usually progresses to the second ear. Patients may also feel fullness in the ear and experience vertigo as well as ringing, hissing, or roaring tinnitus. Most patients with autoimmune inner ear disease respond to initial treatment with oral prednisone.
Auditory neoplasms. Growths such as acoustic neuroma or cholesteatoma can cause tinnitus. Acoustic neuroma (or vestibular schwannoma) is a benign neoplasm that arises from the vestibular division of the eighth cranial nerve. Symptoms include unilateral hearing loss, tinnitus, and vestibular disturbances. Surgical resection or radiation treatment of the tumor can resolve these symptoms, especially if the neoplasm is detected while it is small.
Cholesteatoma is a benign epithelial cell mass that grows in the middle-ear cavity. Over time, cholesteatomas can enlarge and destroy middleear ossicles. Hearing loss, tinnitus, dizziness, and facial muscle paralysis can result from continued cholesteatoma growth. Early detection and surgical resection of auditory neoplasms can reduce the likelihood of residual symptoms.
Hyper- or hypotension. Of these two disorders, hypertension is more likely to contribute to tinnitus. Maintenance of blood pressure within the optimum range can decrease or resolve tinnitus for some patients.
Metabolic disorders. Disorders such as diabetes mellitus, hyperthyroidism, or hypothyroidism can contribute to tinnitus. Successful management of these conditions can reduce or resolve the patient’s tinnitus.
Managing persistent tinnitus
Successful treatment of the disorders discussed can resolve or reduce tinnitus. However, if tinnitus continues to bother the patient after other diseases have been treated, shift the clinical focus from treatment to management of the symptom. At this point, the clinician should do 1 of 2 things: 1) spend the time necessary to help the patient manage tinnitus using strategies described in the following sections of this article; or 2) refer the patient to a comprehensive tinnitus management program with experienced personnel who are willing and able to spend a substantial amount of time with each patient.
Like other neurologic symptoms, tinnitus can be considered chronic if it persists for 6 months or more. Approximately 90% of cases of chronic tinnitus are associated with some degree of sensorineural hearing loss.19 Because sensorineural hearing loss is irreversible, most cases of chronic tinnitus cannot be “cured.” Duckro et al20 wrote: “As with chronic pain, the treatment of chronic tinnitus is more accurately described in terms of management rather than cure.”
The goal of management is not necessarily to mask or remove the patient’s perception of tinnitus. In many cases, this is not possible. Successful management enables patients to pay less attention to their tinnitus. An effective management program helps patients to understand and gain control over their tinnitus, rather than allowing it to control them. The ultimate goal is to reduce the severity of tinnitus. Clinicians should strive to help patients progress to where tinnitus is no longer a negative factor in their lives.
Establishing tinnitus severity
Only 25% of people who experience chronic tinnitus consider the symptom to be a significant problem.2 These are the patients most likely to seek treatment. If a patient is not bothered by tinnitus and no active disease processes are detected, no treatments are necessary. The clinician should reassure such patients that tinnitus is a harmless perception of sound and does not usually portend more serious medical conditions.
What differentiates the majority of people not bothered by tinnitus from the minority who perceive it as a significant, even debilitating problem? Is it the matched loudness, pitch, or other qualities of the sound(s) they hear? Several studies have concluded that tinnitus severity is not correlated with any of these psychoacoustic parameters.21-23
Tinnitus severity can be defined and quantified several ways: by how much or how often a patient is bothered by tinnitus; by how much or how often tinnitus detracts from the patient’s enjoyment of life; or by how disabling patients perceive their tinnitus to be. Instruments such as the Tinnitus Severity Index11 can be used to assess tinnitus severity (Figure 2).
Tinnitus management strategies
Once underlying conditions have been treated or ruled out, reassure and counsel patients regarding factors that could exacerbate or improve their condition. If patients understand their tinnitus is nothing more than a perception of sound, they will be better able to pay less attention to it. This process of patient education and counseling helps to “demystify” the symptom of tinnitus and encourages patients to view their tinnitus with a more realistic perspective.
The severity of tinnitus is often associated with problems such as insomnia,24 anxiety,25 and depression.26 Such issues can form a vicious circle, with each one exacerbating the others.23 Tinnitus is not always the starting point of this cycle—many patients experience depression, insomnia, or anxiety before tinnitus. Medication or psychotherapy will often reduce the severity of these symptoms and associated tinnitus (LOE: 2).27-28
Because each patient has a unique medical, psychological, and social history, management programs should be individualized. In fact, the most successful tinnitus management programs employ multimodal strategies designed to address the specific needs of each patient (LOE: 2).27,29
Recommendations should be formulated and explained to the patient: appropriate acoustic therapy; use of hearing protection (all patients should wear earplugs or ear muffs when they are exposed to excessively loud sounds [LOE: 1]);30 and strategies for management of insomnia, anxiety, or depression. As appropriate, provide patients with referral and contact information for physical or psychiatric evaluations, psychological counseling, and other recommended services or products.
Acoustic therapy
Patients should add pleasant sounds (music, relaxation CDs, or a tabletop sound machine) to any environment that is too quiet, and listen to them through speakers or headphones. Patients who experience chronic insomnia because of tinnitus may find relief in using a tabletop sound machine in combination with a pillow embedded with speakers (such as the Sound Pillow, distributed by Phoenix Productions, San Antonio, TX).
Patients with normal or nearly normal hearing might benefit from in-the-ear sound generators (such as those manufactured by General Hearing Instruments, Harahan, LA) that produce a broad-band sound to muffle or mask the tinnitus.31 Significant, aidable hearing loss can often be lessened with hearing aids or combination instruments (hearing aid and sound generator in one unit). Hearing aids not only improve communication ability, the devices can also reduce the perception of tinnitus.32
Follow-up
Encourage patients to ask questions about recommended tinnitus management procedures and to report their progress. Reassess patients at 1 month. If necessary, recommendations can be modified to facilitate patient improvement. Follow-up questionnaires can be mailed to patients 6 and 12 months after their initial appointment to assess the effectiveness of the tinnitus management program.
A customized combination of recommendations is effective for many patients with chronic and bothersome tinnitus (LOE: 2),27 but the process can be very time consuming. For a certain number of patients with severe tinnitus, only a comprehensive management program can help them to improve their condition.
Corresponding author
Robert L. Folmer, PhD, OHSU Tinnitus Clinic, Mail Code NRC04, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239. E-mail: [email protected].
- If no treatable cause of tinnitus is found, assess the severity of tinnitus, secondary problems (such as depression, anxiety, and insomnia), and implement tinnitus management strategies (SOR:B).
- Acoustic therapy is effective for tinnitus management (SOR:B).
- All patients should wear hearing protection when they are exposed to loud sounds such as a gas lawnmower, leaf blower, power tools, or gunfire (SOR:A).
- Successful management of insomnia, anxiety or depression will decrease the severity of tinnitus for most patients (SOR:B).
Tinnitus—the perception of sound that does not have an external source—can be constant or intermittent and perceived as ringing, buzzing, hissing, sizzling, roaring, chirping, or other sounds.
Acute tinnitus, which can last days or weeks, may be caused by ear infection, medications, head or neck injury, excessive sound exposure, earwax, and changes in blood pressure or metabolism. With appropriate evaluation, such underlying conditions usually can be identified and treated, often with resultant resolution of tinnitus.
Chronic tinnitus (persistence for 6 months or more) can also result from these conditions and is more likely to occur in people who have hearing loss.1 (See Prevalence of tinnitus.) Even though a true “cure” for most cases of chronic tinnitus is not available, patients can obtain relief from the symptom with assistance from clinicians who are familiar with tinnitus management strategies.
Seidman and Jacobson2 estimated that 40 million people in the United States experience chronic tinnitus. The prevalence of tinnitus increases with age: 27% of males and 15% of females aged 45 years or older experience the symptom.3
Tinnitus is rare in children who have normal hearing.4 However, the prevalence of tinnitus in children with severe or profound hearing loss has been reported as 33%5 or 64%.6 More males than females experience tinnitus because men traditionally have had a greater amount of noise exposure in military service, in the workplace, and during recreational activities. Consequently, hearing loss and tinnitus are both more prevalent among men aged 45 years or older compared with women in the same age group.3
Damage depends on intensity, length of exposure
Tinnitus is most commonly caused by exposure to excessively loud sounds such as gunfire, power tools, machinery, or music. Ringing in the ears occurs because of damage to stereocilia, microscopic appendages attached to the apical ends of hair cells in the cochlea.
Moderate sounds (80 decibels sound pressure level [dB SPL] or lower) normally cause stereocilia to make tiny movements, triggering the releaseof neurotransmitter molecules from the basal ends of hair cells that activate auditory neurons in the eighth cranial nerve.
Excessive sound exposure (85 dB SPL or louder) causes stereocilia to bend more than they should. People then perceive high-pitched ringing tinnitus because hair cells that respond to higher-frequency sounds are located at the base of the cochlea and are the first to be damaged by loud noise.
If the damage is modest and infrequent, stereocilia can recover, returning to their normal function in a few minutes or hours. The patient’s hearing will be restored and the tinnitus will stop. However, repeated exposure to hazardous sounds eventually causes irreparable damage to stereocilia and hair cells, resulting in permanent sensorineural hearing loss and possibly chronic tinnitus.
In addition to noise exposure, any condition that causes hearing loss or damages the auditory system can contribute to the generation of tinnitus (Table). Imaging studies using functional magnetic resonance imaging7 or positron-emission tomography8-9 demonstrated that the perception of chronic tinnitus usually occurs as a result of hyperactivity within central auditory areas of the human brain, especially the auditory cortex. As portions of the auditory system degenerate during the aging process or acquire damage from noise exposure, disease, and accidents, the natural balance of central auditory excitation vs inhibition is disrupted. In patients who hear tinnitus, excitatory pathways within the auditory system are active when they shouldn’t be: in quiet environments. This gives patients the perception of tinnitus sounds.
TABLE
Causes of subjective tinnitus
| Presbycusis: hearing loss due to aging |
| Prolonged noise exposure: Noise-induced hearing loss |
| Acoustic trauma: one-time exposure to high intensity sound |
| Otosclerosis: abnormal accumulation of calcium on middle ear ossicles or cochlea |
| Infections: bacterial, viral, fungal |
| Autoimmune hearing loss |
| Meniere’s disease or endolymphatic hydrops: abnormally high inner ear pressure |
| Neoplasms: for example, acoustic neuroma or cholesteatoma |
| Genetic predisposition |
Ototoxicity
|
Vascular
|
Metabolic
|
| Head or neck injury |
Objective tinnitus
Objective tinnitus—which can be heard also by people in proximity to the patient’s ear—can be caused by vascular abnormalities (congenital arteriovenous fistula, acquired arteriovenous shunt, glomus jugulare, high-riding carotid artery, carotid stenosis, persistent stapedial artery, dehiscent jugular bulb or a vascular loop such as anterior inferior communicating artery [AICA] or posterior inferior communicating artery [PICA] compressing the auditory nerve) or mechanical disorders (abnormally patent Eustachian tube, palatal myoclonus, temporo-mandibular joint disorder, or stapedial muscle spasticity).10 However, objective tinnitus is rare, accounting for <1% of all cases. The vast majority of tinnitus cases are subjective—sounds are perceived only by the patient.
Patient evaluation
Figure 1 is an algorithm that outlines steps in the evaluation and management of patients who experience tinnitus. The first step is to collect as much information as possible about the patient and his condition.
FIGURE 1
Evaluation and management of tinnitus
Tinnitus history
Determine the duration of tinnitus and whether circumstances such as upper respiratory infection, otalgia, noise exposure, head trauma, sudden hearing loss, or vertigo occurred at the time of tinnitus onset. Ask the patient to describe the tinnitus: Is it intermittent or constant? High- or low-pitched? Unilateral or bilateral? Pulsatile or steady?
Unilateral tinnitus and hearing loss provide preliminary evidence for acoustic neuroma or cerebrovascular accident. High-pitched tinnitus is usually associated with high-frequency hearing loss caused by presbycusis (hearing impairment in the aged) or excessive noise exposure. Low-pitched roaring tinnitus is sometimes associated with low-frequency hearing loss exhibited by patients with Meniere’s disease. Pulsatile tinnitus, especially if synchronous with the patient’s pulse, can indicate vascular abnormalities.
Ask the patient if fatigue, stress, noise exposure, or any medications exacerbate the tinnitus. Also ask if masking sounds (such as water running in the shower), medications, or any other factors provide relief from tinnitus. This information can be used to formulate a tinnitus management program.
Assess the severity of the patient’s tinnitus using an instrument such as the Tinnitus Severity Index (Figure 2).11 A score of 36 or higher indicates bothersome tinnitus (level of evidence [LOE]: 2).12 Higher scores indicate that patients perceive their tinnitus to be a significant, even debilitating problem.
FIGURE 2
Tinnitus Severity Index
Hearing history
If possible, determine the presence and type of hearing loss (congenital, sudden, sensorineural, conductive, or mixed). Note the patient’s history of ear infections, surgeries, noise exposure (occupational or recreational), otalgia, otorrhea, and vertigo or other balance problems. Ask whether immediate family members have experienced hearing loss or tinnitus.
Health history
Look particularly for conditions that can contribute to hearing loss and tinnitus, such as hypertension, hypothyroidism, diabetes mellitus, arteriosclerosis, and autoimmune disorders (eg, lupus or rheumatoid arthritis). Also consider ototoxic medications, such as aminoglycoside antibiotics, cisplatin, furosemide, valproic acid, and high doses of quinine-containing compounds. When possible, patients with hearing loss or tinnitus should be given alternative medications free from ototoxicity.
Excessive use of alcohol, caffeine, and aspirin or other nonsteroidal anti-inflammatory drugs can exacerbate tinnitus for some patients. However, moderate use of these products is often possible.
Psychosocial history
Inquire about the patient’s marital and occupational status. Unemployed patients living alone often perceive tinnitus to be more severe than do employed patients who have supportive social networks. Also ask about any history of insomnia, anxiety, depression, obsessive-compulsive disorder, or psychosis. A questionnaire such as the abbreviated Beck Depression Inventory13 can be used to assess the presence and severity of depression.
Physical exam and testing
Patient evaluation should include the following physical examinations and tests.
Otolaryngologic/head and neck exam. Otoscopic examination can detect infections such as otitis media, which will usually be accompanied by complaints of ear pain or fullness, and possibly hearing loss in combination with tinnitus. Otoscopy can also detect impacted earwax (cerumen), which can occlude the ear canal or cause immobilization of the tympanic membrane, resulting in conductive hearing loss, tinnitus, and a feeling of fullness in the ear. Symptoms usually resolve when the earwax is removed.
If the tinnitus is synchronous with the patient’s pulse, it suggests a vascular contribution for the symptom. Auscultation of blood vessels in the neck can reveal venous hums or other types of bruits audible to the patient. Venous hum can be diagnosed by temporarily blocking blood flow through the jugular vein on the side where tinnitus is perceived.
Neurologic exam. A complete neurologic exam should include the Romberg test, Dix-Hallpike maneuver (if the patient experiences vertigo), gait testing, and cranial nerve function tests.
Audiologic testing. Audiologic tests should include pure tone air and bone conduction thresholds, speech discrimination testing, tympanometry, and most comfortable loudness (MCL) and uncomfortable loudness level (UCL) tests. Tympanometry is used to assess middle-ear function. Abnormal tympanograms and significant differences between air and bone conduction thresholds can indicate otitis media, otosclerosis, or cholesteatoma.
MCL and UCL tests are used to assess the dynamic range of patients’ hearing. Patients with UCLs that are only 5 to 20 dBs above their MCLs have a reduced dynamic range of hearing that can be caused by recruitment or hyperacusis. The audiometer can also be used to match the tinnitus for pitch and loudness and to test the effects of masking sounds on the patient’s tinnitus.
Additional evaluations. Results of patient examinations and history collection might warrant additional evaluations. For example, asymmetrical hearing loss (15 dB or greater asymmetry at 2 or more consecutive test frequencies) and unilateral tinnitus can indicate a retrocochlear lesion such as acoustic neuroma (also known as vestibular schwannoma).
One test for retrocochlear pathology is the auditory brainstem response (ABR). In this test, clicks are presented through earphones while scalp electrodes record brain responses to the sounds. Abnormal ABR waveforms can indicate retrocochlear lesion (such as acoustic neuroma) as a possible cause of ipsilateral hearing loss and tinnitus. If positive ABR results are obtained, MRI evaluation of the cerebellopontine angle with contrast material (such as gadolinium) should be performed.
Low-pitched roaring, ringing, or hissing tinnitus; hearing loss, which may be temporary or permanent; vertigo; and a feeling of pressure or fullness in the ear can indicate endolymphatic hydrops or Meniere’s disease. Symptoms usually occur in the form of “attacks” that increase in frequency during the first few years of the disease, then decrease in frequency as hearing thresholds stabilize. Electrocochleography testing is one way to diagnose endolymphatic hydrops. Patients who exhibit vestibular disorders should undergo electronystagmography testing to assess the severity and characteristics of their symptoms.
Pulsatile tinnitus associated with abnormalities of blood vessels in the neck can be evaluated with sonography, conventional angiography, or magnetic resonance angiography. Conditions such as a dehiscent jugular bulb or stenosis of carotid arteries can sometimes be treated surgically. However, many forms of pulsatile tinnitus are not caused by these conditions. Pulsatile tinnitus is often a consequence of hearing loss, arteriosclerosis, or weight loss or weight gain. These physiologic changes can cause patients to hear blood pulsing or “swishing” in vessels—sounds they did not perceive previously. Surgery is not recommended for most cases of pulsatile tinnitus.
Sudden hearing loss, especially if bilateral, might indicate autoimmune inner ear disease. Diagnostic tests include the Western blot immunoassay.
Treatment of active disease processes
Many contributors to tinnitus can be treated surgically or with medication.
Otitis media. Successful treatment of the infection with oral antibiotics usually resolves all auditory symptoms.
Allergies, sinus congestion, or infection. When inflammation subsides, tinnitus associated with these conditions usually resolves.
Otosclerosis. Abnormal accumulations of calcium on middle-ear ossicles (especially the stapes) or the cochlea can result in slowly progressing conductive or sensorineural hearing loss, tinnitus, and vestibular disturbances. Stapedectomy surgery—including implantation of ossicular prostheses—is often successful for advanced cases associated with significant hearing loss. Hearing aids also benefit some patients.
Meniere’s disease or other forms of endolymphatic hydrops. Meniere’s disease, characterized by abnormally high fluid pressure within the cochlea, has an estimated prevalence of 1% in the US.14 Management includes meclizine, antiemetics and diuretics, and a low-sodium diet.15 If patients do not respond to meclizine, diazepam can be prescribed to reduce the severity of vertigo attacks. Surgical intervention—including installation of an endolymphatic shunt, labyrinthectomy, or vestibular neurectomy16—or transtympanic injections of gentamicin17 are options in severe cases.
Autoimmune inner ear disease. This disease has an estimated prevalence of 0.1% in the US.18 Symptoms include sudden hearing loss in one ear that usually progresses to the second ear. Patients may also feel fullness in the ear and experience vertigo as well as ringing, hissing, or roaring tinnitus. Most patients with autoimmune inner ear disease respond to initial treatment with oral prednisone.
Auditory neoplasms. Growths such as acoustic neuroma or cholesteatoma can cause tinnitus. Acoustic neuroma (or vestibular schwannoma) is a benign neoplasm that arises from the vestibular division of the eighth cranial nerve. Symptoms include unilateral hearing loss, tinnitus, and vestibular disturbances. Surgical resection or radiation treatment of the tumor can resolve these symptoms, especially if the neoplasm is detected while it is small.
Cholesteatoma is a benign epithelial cell mass that grows in the middle-ear cavity. Over time, cholesteatomas can enlarge and destroy middleear ossicles. Hearing loss, tinnitus, dizziness, and facial muscle paralysis can result from continued cholesteatoma growth. Early detection and surgical resection of auditory neoplasms can reduce the likelihood of residual symptoms.
Hyper- or hypotension. Of these two disorders, hypertension is more likely to contribute to tinnitus. Maintenance of blood pressure within the optimum range can decrease or resolve tinnitus for some patients.
Metabolic disorders. Disorders such as diabetes mellitus, hyperthyroidism, or hypothyroidism can contribute to tinnitus. Successful management of these conditions can reduce or resolve the patient’s tinnitus.
Managing persistent tinnitus
Successful treatment of the disorders discussed can resolve or reduce tinnitus. However, if tinnitus continues to bother the patient after other diseases have been treated, shift the clinical focus from treatment to management of the symptom. At this point, the clinician should do 1 of 2 things: 1) spend the time necessary to help the patient manage tinnitus using strategies described in the following sections of this article; or 2) refer the patient to a comprehensive tinnitus management program with experienced personnel who are willing and able to spend a substantial amount of time with each patient.
Like other neurologic symptoms, tinnitus can be considered chronic if it persists for 6 months or more. Approximately 90% of cases of chronic tinnitus are associated with some degree of sensorineural hearing loss.19 Because sensorineural hearing loss is irreversible, most cases of chronic tinnitus cannot be “cured.” Duckro et al20 wrote: “As with chronic pain, the treatment of chronic tinnitus is more accurately described in terms of management rather than cure.”
The goal of management is not necessarily to mask or remove the patient’s perception of tinnitus. In many cases, this is not possible. Successful management enables patients to pay less attention to their tinnitus. An effective management program helps patients to understand and gain control over their tinnitus, rather than allowing it to control them. The ultimate goal is to reduce the severity of tinnitus. Clinicians should strive to help patients progress to where tinnitus is no longer a negative factor in their lives.
Establishing tinnitus severity
Only 25% of people who experience chronic tinnitus consider the symptom to be a significant problem.2 These are the patients most likely to seek treatment. If a patient is not bothered by tinnitus and no active disease processes are detected, no treatments are necessary. The clinician should reassure such patients that tinnitus is a harmless perception of sound and does not usually portend more serious medical conditions.
What differentiates the majority of people not bothered by tinnitus from the minority who perceive it as a significant, even debilitating problem? Is it the matched loudness, pitch, or other qualities of the sound(s) they hear? Several studies have concluded that tinnitus severity is not correlated with any of these psychoacoustic parameters.21-23
Tinnitus severity can be defined and quantified several ways: by how much or how often a patient is bothered by tinnitus; by how much or how often tinnitus detracts from the patient’s enjoyment of life; or by how disabling patients perceive their tinnitus to be. Instruments such as the Tinnitus Severity Index11 can be used to assess tinnitus severity (Figure 2).
Tinnitus management strategies
Once underlying conditions have been treated or ruled out, reassure and counsel patients regarding factors that could exacerbate or improve their condition. If patients understand their tinnitus is nothing more than a perception of sound, they will be better able to pay less attention to it. This process of patient education and counseling helps to “demystify” the symptom of tinnitus and encourages patients to view their tinnitus with a more realistic perspective.
The severity of tinnitus is often associated with problems such as insomnia,24 anxiety,25 and depression.26 Such issues can form a vicious circle, with each one exacerbating the others.23 Tinnitus is not always the starting point of this cycle—many patients experience depression, insomnia, or anxiety before tinnitus. Medication or psychotherapy will often reduce the severity of these symptoms and associated tinnitus (LOE: 2).27-28
Because each patient has a unique medical, psychological, and social history, management programs should be individualized. In fact, the most successful tinnitus management programs employ multimodal strategies designed to address the specific needs of each patient (LOE: 2).27,29
Recommendations should be formulated and explained to the patient: appropriate acoustic therapy; use of hearing protection (all patients should wear earplugs or ear muffs when they are exposed to excessively loud sounds [LOE: 1]);30 and strategies for management of insomnia, anxiety, or depression. As appropriate, provide patients with referral and contact information for physical or psychiatric evaluations, psychological counseling, and other recommended services or products.
Acoustic therapy
Patients should add pleasant sounds (music, relaxation CDs, or a tabletop sound machine) to any environment that is too quiet, and listen to them through speakers or headphones. Patients who experience chronic insomnia because of tinnitus may find relief in using a tabletop sound machine in combination with a pillow embedded with speakers (such as the Sound Pillow, distributed by Phoenix Productions, San Antonio, TX).
Patients with normal or nearly normal hearing might benefit from in-the-ear sound generators (such as those manufactured by General Hearing Instruments, Harahan, LA) that produce a broad-band sound to muffle or mask the tinnitus.31 Significant, aidable hearing loss can often be lessened with hearing aids or combination instruments (hearing aid and sound generator in one unit). Hearing aids not only improve communication ability, the devices can also reduce the perception of tinnitus.32
Follow-up
Encourage patients to ask questions about recommended tinnitus management procedures and to report their progress. Reassess patients at 1 month. If necessary, recommendations can be modified to facilitate patient improvement. Follow-up questionnaires can be mailed to patients 6 and 12 months after their initial appointment to assess the effectiveness of the tinnitus management program.
A customized combination of recommendations is effective for many patients with chronic and bothersome tinnitus (LOE: 2),27 but the process can be very time consuming. For a certain number of patients with severe tinnitus, only a comprehensive management program can help them to improve their condition.
Corresponding author
Robert L. Folmer, PhD, OHSU Tinnitus Clinic, Mail Code NRC04, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239. E-mail: [email protected].
1. Schleuning A. Medical aspects of tinnitus. In: Vernon JA, ed. Tinnitus Treatment and Relief. Boston, Mass: Allyn and Bacon; 1998;20-27.
2. Seidman MD, Jacobson GP. Update on tinnitus. Otolaryngol Clin N Amer 1996;29:455-465.
3. Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey, 1996. Hyattsville, Md: National Center for Health Statistics, 1999.
4. Stouffer JL, Tyler RS, Booth JC, Buckrell B. Tinnitus in normal-hearing and hearing-impaired children. In: Aran J-M, Dauman R, eds. Tinnitus 91: Proceedings of the Fourth International Tinnitus Seminar. Amsterdam: Kugler Publications 1992;255-258.
5. Drukier GS. The prevalence and characteristics of tinnitus with profound sensori-neural hearing impairment. Amer Ann Deaf 1989;134:260-264.
6. Graham J. Paediatric tinnitus. J Laryngol Otol 1981;95(Suppl):117-120.
7. Levine RA, Benson RR, Talavage TM, Melcher JR, Rosen BR. Functional magnetic resonance imaging and tinnitus: preliminary results. Abstr Assoc Res Otolaryngol 1997;20:65.-
8. Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec 1996;58:195-199.
9. Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wach DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neuroplasticity. Neurology 1998;50:114-120.
10. Ciocon JO, Amede F, Lechtenberg C, Astor F. Tinnitus: a stepwise workup to quiet the noise within. Geriatrics 1995;50:18-25.
11. Meikle MB, Griest SE, Stewart BJ, Press LS. Measuring the negative impact of tinnitus: a brief severity index. Abstr Assoc Res Otolaryngol 1995;167.-
12. Folmer RL, Stevenson EA, Tran A. Factors associated with long-term improvements in tinnitus severity. In Patuzzi R, ed: Proceedings of the Seventh International Tinnitus Seminar. Crawley: University of Western Australia; 2002;115-123.
13. Beck AT, Beck RW. Screening depressed patients in family practice: a rapid technic. Postgrad Medicine 1972;52:81-85.
14. daCosta SS, deSousa LC, Piza MR. Meniere’s Disease: overview, epidemiology, and natural history. Otolaryngol Clin N Amer 2002;35:455-495.
15. Thai-Van H, Bounaix MJ, Fraysse B. Meniere’s disease: pathophysiology and treatment. Drugs 2001;61:1089-1102.
16. Hillman TA, Chen DA, Arriaga MA. Vestibular nerve section versus intratympanic gentamicin for Meniere’s disease. Laryngoscope 2004;114:216-222.
17. Diamond C, Hornig JD, Liu R, O’Connell DA. Systematic review of intratympanic gentamicin in Meniere’s disease. J Otolaryngol 2003;32:351-361.
18. Hain TC. Autoimmune inner ear disease. American Hearing Research Foundation Web site. March 23, 2002. Available at www.american-hearing.org/name/autoimmune.html. Accessed on May 15, 2004.
19. Meikle MB. Electronic access to tinnitus data: The Oregon Tinnitus Data Archive. Otolaryngol Head Neck Surg 1997;117:698-700.
20. Duckro PN, Pollard CA, Bray HD, Scheiter L. Comprehensive behavioral management of complex tinnitus: a case illustration. Biofeedback and Self-Regulation 1984;9:459-469.
21. Meikle MB, Vernon J, Johnson RM. The perceived severity of tinnitus. Otolaryngol Head Neck Surg 1984;92:689-696.
22. van Veen ED, Jacobs JB, Bensing JM. Assessment of distress associated with tinnitus. J Laryngol Otol 1998;112:258-263.
23. Folmer RL, Griest SE, Martin WH. Chronic tinnitus as phantom auditory pain. Otolaryngol Head Neck Surg 2001;124:394-400.
24. Folmer RL, Griest SE. Tinnitus and insomnia. Am J Otolaryngol 2000;21:287-293.
25. Attias J, Shemesh Z, Bleich A, Solomon Z, Bar-Or G, Alster J, Sohmer H. Psychological profile of help-seeking and non-help-seeking tinnitus patients. Scand Audiol 1995;24:13-18.
26. Folmer RL, Griest SE, Meikle MB, Martin WH. Tinnitus severity, loudness and depression. Otolaryngol Head Neck Surg 1999;121:48-51.
27. Folmer R. Long-term reductions in tinnitus severity. BMC Ear Nose and Throat Disorders 2002;2:3.-Available at: www.biomedcentral.com/1472-6815/2/3.
28. Folmer RL, Shi YB. SSRI use by tinnitus patients: interactions between depression and tinnitus severity ENT J 2004;83:107-117.
29. Sullivan M, Katon W, Russo J, Dobie R, Sakai C. Coping and marital support as correlates of tinnitus disability. Gen Hosp Psychiatry 1994;16:259-266.
30. Lusk SL. Preventing noise-induced hearing loss. Nurs Clin North Amer 2002;37:257-262.
31. Henry JA, Schechter MA, Nagler SM, Fausti SA. Comparison of tinnitus masking and tinnitus retraining therapy. J Amer Acad Audiol 2002;13:559-581.
32. Surr RK, Montgomery AA, Mueller HG. Effect of amplification on tinnitus among new hearing aid users. Ear and Hearing 1985;6:71-75.
1. Schleuning A. Medical aspects of tinnitus. In: Vernon JA, ed. Tinnitus Treatment and Relief. Boston, Mass: Allyn and Bacon; 1998;20-27.
2. Seidman MD, Jacobson GP. Update on tinnitus. Otolaryngol Clin N Amer 1996;29:455-465.
3. Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey, 1996. Hyattsville, Md: National Center for Health Statistics, 1999.
4. Stouffer JL, Tyler RS, Booth JC, Buckrell B. Tinnitus in normal-hearing and hearing-impaired children. In: Aran J-M, Dauman R, eds. Tinnitus 91: Proceedings of the Fourth International Tinnitus Seminar. Amsterdam: Kugler Publications 1992;255-258.
5. Drukier GS. The prevalence and characteristics of tinnitus with profound sensori-neural hearing impairment. Amer Ann Deaf 1989;134:260-264.
6. Graham J. Paediatric tinnitus. J Laryngol Otol 1981;95(Suppl):117-120.
7. Levine RA, Benson RR, Talavage TM, Melcher JR, Rosen BR. Functional magnetic resonance imaging and tinnitus: preliminary results. Abstr Assoc Res Otolaryngol 1997;20:65.-
8. Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec 1996;58:195-199.
9. Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wach DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neuroplasticity. Neurology 1998;50:114-120.
10. Ciocon JO, Amede F, Lechtenberg C, Astor F. Tinnitus: a stepwise workup to quiet the noise within. Geriatrics 1995;50:18-25.
11. Meikle MB, Griest SE, Stewart BJ, Press LS. Measuring the negative impact of tinnitus: a brief severity index. Abstr Assoc Res Otolaryngol 1995;167.-
12. Folmer RL, Stevenson EA, Tran A. Factors associated with long-term improvements in tinnitus severity. In Patuzzi R, ed: Proceedings of the Seventh International Tinnitus Seminar. Crawley: University of Western Australia; 2002;115-123.
13. Beck AT, Beck RW. Screening depressed patients in family practice: a rapid technic. Postgrad Medicine 1972;52:81-85.
14. daCosta SS, deSousa LC, Piza MR. Meniere’s Disease: overview, epidemiology, and natural history. Otolaryngol Clin N Amer 2002;35:455-495.
15. Thai-Van H, Bounaix MJ, Fraysse B. Meniere’s disease: pathophysiology and treatment. Drugs 2001;61:1089-1102.
16. Hillman TA, Chen DA, Arriaga MA. Vestibular nerve section versus intratympanic gentamicin for Meniere’s disease. Laryngoscope 2004;114:216-222.
17. Diamond C, Hornig JD, Liu R, O’Connell DA. Systematic review of intratympanic gentamicin in Meniere’s disease. J Otolaryngol 2003;32:351-361.
18. Hain TC. Autoimmune inner ear disease. American Hearing Research Foundation Web site. March 23, 2002. Available at www.american-hearing.org/name/autoimmune.html. Accessed on May 15, 2004.
19. Meikle MB. Electronic access to tinnitus data: The Oregon Tinnitus Data Archive. Otolaryngol Head Neck Surg 1997;117:698-700.
20. Duckro PN, Pollard CA, Bray HD, Scheiter L. Comprehensive behavioral management of complex tinnitus: a case illustration. Biofeedback and Self-Regulation 1984;9:459-469.
21. Meikle MB, Vernon J, Johnson RM. The perceived severity of tinnitus. Otolaryngol Head Neck Surg 1984;92:689-696.
22. van Veen ED, Jacobs JB, Bensing JM. Assessment of distress associated with tinnitus. J Laryngol Otol 1998;112:258-263.
23. Folmer RL, Griest SE, Martin WH. Chronic tinnitus as phantom auditory pain. Otolaryngol Head Neck Surg 2001;124:394-400.
24. Folmer RL, Griest SE. Tinnitus and insomnia. Am J Otolaryngol 2000;21:287-293.
25. Attias J, Shemesh Z, Bleich A, Solomon Z, Bar-Or G, Alster J, Sohmer H. Psychological profile of help-seeking and non-help-seeking tinnitus patients. Scand Audiol 1995;24:13-18.
26. Folmer RL, Griest SE, Meikle MB, Martin WH. Tinnitus severity, loudness and depression. Otolaryngol Head Neck Surg 1999;121:48-51.
27. Folmer R. Long-term reductions in tinnitus severity. BMC Ear Nose and Throat Disorders 2002;2:3.-Available at: www.biomedcentral.com/1472-6815/2/3.
28. Folmer RL, Shi YB. SSRI use by tinnitus patients: interactions between depression and tinnitus severity ENT J 2004;83:107-117.
29. Sullivan M, Katon W, Russo J, Dobie R, Sakai C. Coping and marital support as correlates of tinnitus disability. Gen Hosp Psychiatry 1994;16:259-266.
30. Lusk SL. Preventing noise-induced hearing loss. Nurs Clin North Amer 2002;37:257-262.
31. Henry JA, Schechter MA, Nagler SM, Fausti SA. Comparison of tinnitus masking and tinnitus retraining therapy. J Amer Acad Audiol 2002;13:559-581.
32. Surr RK, Montgomery AA, Mueller HG. Effect of amplification on tinnitus among new hearing aid users. Ear and Hearing 1985;6:71-75.