User login
FDA Cancels REMS for Rosiglitazone
The thiazolidinedione agent rosiglitazone now can be prescribed without complying with a Risk Evaluation and Mitigation Strategy (REMS), according to the Food and Drug Administration.
It’s been a long, strange trip for the drug that was approved in 1999 to lower blood sugar in patients with type 2 diabetes and is sold under a number of brands, including Avandia, Avandamet, and Avandaryl, as well as generics. Over the first years it was marketed, there were reports that its use was associated with an increased risk for heart failure and myocardial infarctions.
In May 2007, the FDA issued an alert that use of rosiglitazone was associated with ischemic cardiovascular events, which eventually led the agency to add a black box warning to the drug’s label in August 2007 that its use was associated with heart failure in patients with type 2 diabetes. In November 2007 the FDA extended the warning to cover an increased risk for myocardial infarction, based on “a meta-analysis of 42 clinical studies (mean duration 6 months; 14,237 total patients), most of which compared Avandia to placebo, showed Avandia to be associated with an increased risk of myocardial ischemic events such as angina or myocardial infarction. Three other studies (mean duration 41 months; 14,067 patients), comparing Avandia to some other approved oral antidiabetic agents or placebo, have not confirmed or excluded this risk. In their entirety, the available data on the risk of myocardial ischemia are inconclusive.”
In May 2011, the FDA issued a requirement that physicians who prescribed rosiglitazone participate in a REMS program. But by November 2013 the agency had withdrawn its restrictions on prescription of the drug to patients with diabetes.
Today’s action may be the final step in returning the drug to use. However, the number of new drugs that have been approved for management of type 2 diabetes in the years since rosiglitazone first appeared problematic may make this FDA action irrelevant.
The thiazolidinedione agent rosiglitazone now can be prescribed without complying with a Risk Evaluation and Mitigation Strategy (REMS), according to the Food and Drug Administration.
It’s been a long, strange trip for the drug that was approved in 1999 to lower blood sugar in patients with type 2 diabetes and is sold under a number of brands, including Avandia, Avandamet, and Avandaryl, as well as generics. Over the first years it was marketed, there were reports that its use was associated with an increased risk for heart failure and myocardial infarctions.
In May 2007, the FDA issued an alert that use of rosiglitazone was associated with ischemic cardiovascular events, which eventually led the agency to add a black box warning to the drug’s label in August 2007 that its use was associated with heart failure in patients with type 2 diabetes. In November 2007 the FDA extended the warning to cover an increased risk for myocardial infarction, based on “a meta-analysis of 42 clinical studies (mean duration 6 months; 14,237 total patients), most of which compared Avandia to placebo, showed Avandia to be associated with an increased risk of myocardial ischemic events such as angina or myocardial infarction. Three other studies (mean duration 41 months; 14,067 patients), comparing Avandia to some other approved oral antidiabetic agents or placebo, have not confirmed or excluded this risk. In their entirety, the available data on the risk of myocardial ischemia are inconclusive.”
In May 2011, the FDA issued a requirement that physicians who prescribed rosiglitazone participate in a REMS program. But by November 2013 the agency had withdrawn its restrictions on prescription of the drug to patients with diabetes.
Today’s action may be the final step in returning the drug to use. However, the number of new drugs that have been approved for management of type 2 diabetes in the years since rosiglitazone first appeared problematic may make this FDA action irrelevant.
The thiazolidinedione agent rosiglitazone now can be prescribed without complying with a Risk Evaluation and Mitigation Strategy (REMS), according to the Food and Drug Administration.
It’s been a long, strange trip for the drug that was approved in 1999 to lower blood sugar in patients with type 2 diabetes and is sold under a number of brands, including Avandia, Avandamet, and Avandaryl, as well as generics. Over the first years it was marketed, there were reports that its use was associated with an increased risk for heart failure and myocardial infarctions.
In May 2007, the FDA issued an alert that use of rosiglitazone was associated with ischemic cardiovascular events, which eventually led the agency to add a black box warning to the drug’s label in August 2007 that its use was associated with heart failure in patients with type 2 diabetes. In November 2007 the FDA extended the warning to cover an increased risk for myocardial infarction, based on “a meta-analysis of 42 clinical studies (mean duration 6 months; 14,237 total patients), most of which compared Avandia to placebo, showed Avandia to be associated with an increased risk of myocardial ischemic events such as angina or myocardial infarction. Three other studies (mean duration 41 months; 14,067 patients), comparing Avandia to some other approved oral antidiabetic agents or placebo, have not confirmed or excluded this risk. In their entirety, the available data on the risk of myocardial ischemia are inconclusive.”
In May 2011, the FDA issued a requirement that physicians who prescribed rosiglitazone participate in a REMS program. But by November 2013 the agency had withdrawn its restrictions on prescription of the drug to patients with diabetes.
Today’s action may be the final step in returning the drug to use. However, the number of new drugs that have been approved for management of type 2 diabetes in the years since rosiglitazone first appeared problematic may make this FDA action irrelevant.
FROM THE FOOD AND DRUG ADMINISTRATION
FDA cancels REMS for rosiglitazone
The thiazolidinedione agent rosiglitazone now can be prescribed without complying with a Risk Evaluation and Mitigation Strategy (REMS), according to the Food and Drug Administration.
It’s been a long, strange trip for the drug that was approved in 1999 to lower blood sugar in patients with type 2 diabetes and is sold under a number of brands, including Avandia, Avandamet, and Avandaryl, as well as generics. Over the first years it was marketed, there were reports that its use was associated with an increased risk for heart failure and myocardial infarctions.
In May 2007, the FDA issued an alert that use of rosiglitazone was associated with ischemic cardiovascular events, which eventually led the agency to add a black box warning to the drug’s label in August 2007 that its use was associated with heart failure in patients with type 2 diabetes. In November 2007 the FDA extended the warning to cover an increased risk for myocardial infarction, based on “a meta-analysis of 42 clinical studies (mean duration 6 months; 14,237 total patients), most of which compared Avandia to placebo, showed Avandia to be associated with an increased risk of myocardial ischemic events such as angina or myocardial infarction. Three other studies (mean duration 41 months; 14,067 patients), comparing Avandia to some other approved oral antidiabetic agents or placebo, have not confirmed or excluded this risk. In their entirety, the available data on the risk of myocardial ischemia are inconclusive.”
In May 2011, the FDA issued a requirement that physicians who prescribed rosiglitazone participate in a REMS program. But by November 2013 the agency had withdrawn its restrictions on prescription of the drug to patients with diabetes.
Today’s action may be the final step in returning the drug to use. However, the number of new drugs that have been approved for management of type 2 diabetes in the years since rosiglitazone first appeared problematic may make this FDA action irrelevant.
The thiazolidinedione agent rosiglitazone now can be prescribed without complying with a Risk Evaluation and Mitigation Strategy (REMS), according to the Food and Drug Administration.
It’s been a long, strange trip for the drug that was approved in 1999 to lower blood sugar in patients with type 2 diabetes and is sold under a number of brands, including Avandia, Avandamet, and Avandaryl, as well as generics. Over the first years it was marketed, there were reports that its use was associated with an increased risk for heart failure and myocardial infarctions.
In May 2007, the FDA issued an alert that use of rosiglitazone was associated with ischemic cardiovascular events, which eventually led the agency to add a black box warning to the drug’s label in August 2007 that its use was associated with heart failure in patients with type 2 diabetes. In November 2007 the FDA extended the warning to cover an increased risk for myocardial infarction, based on “a meta-analysis of 42 clinical studies (mean duration 6 months; 14,237 total patients), most of which compared Avandia to placebo, showed Avandia to be associated with an increased risk of myocardial ischemic events such as angina or myocardial infarction. Three other studies (mean duration 41 months; 14,067 patients), comparing Avandia to some other approved oral antidiabetic agents or placebo, have not confirmed or excluded this risk. In their entirety, the available data on the risk of myocardial ischemia are inconclusive.”
In May 2011, the FDA issued a requirement that physicians who prescribed rosiglitazone participate in a REMS program. But by November 2013 the agency had withdrawn its restrictions on prescription of the drug to patients with diabetes.
Today’s action may be the final step in returning the drug to use. However, the number of new drugs that have been approved for management of type 2 diabetes in the years since rosiglitazone first appeared problematic may make this FDA action irrelevant.
The thiazolidinedione agent rosiglitazone now can be prescribed without complying with a Risk Evaluation and Mitigation Strategy (REMS), according to the Food and Drug Administration.
It’s been a long, strange trip for the drug that was approved in 1999 to lower blood sugar in patients with type 2 diabetes and is sold under a number of brands, including Avandia, Avandamet, and Avandaryl, as well as generics. Over the first years it was marketed, there were reports that its use was associated with an increased risk for heart failure and myocardial infarctions.
In May 2007, the FDA issued an alert that use of rosiglitazone was associated with ischemic cardiovascular events, which eventually led the agency to add a black box warning to the drug’s label in August 2007 that its use was associated with heart failure in patients with type 2 diabetes. In November 2007 the FDA extended the warning to cover an increased risk for myocardial infarction, based on “a meta-analysis of 42 clinical studies (mean duration 6 months; 14,237 total patients), most of which compared Avandia to placebo, showed Avandia to be associated with an increased risk of myocardial ischemic events such as angina or myocardial infarction. Three other studies (mean duration 41 months; 14,067 patients), comparing Avandia to some other approved oral antidiabetic agents or placebo, have not confirmed or excluded this risk. In their entirety, the available data on the risk of myocardial ischemia are inconclusive.”
In May 2011, the FDA issued a requirement that physicians who prescribed rosiglitazone participate in a REMS program. But by November 2013 the agency had withdrawn its restrictions on prescription of the drug to patients with diabetes.
Today’s action may be the final step in returning the drug to use. However, the number of new drugs that have been approved for management of type 2 diabetes in the years since rosiglitazone first appeared problematic may make this FDA action irrelevant.
FROM THE FOOD AND DRUG ADMINISTRATION
VIDEO: Risks of long-term opioid use remain unstudied
BETHESDA, MD – When opioids first received Food and Drug Administration approval, it was for short-term use in management of acute pain. With time, these agents have become a mainstay of long-term management of chronic pain. A systematic review of the literature shows that there are no data on long-term opioid use. The longest placebo controlled randomized controlled trial being 6 months. There are ample data showing risk risks of over dose, abuse, and addiction associated with long-term opioid use for chronic pain, according to Dr. Roger Chou, director of the Pacific Northwest Evidence-based Practice Center and professor of medicine in the department of medical informatics and clinical epidemiology at the Oregon Health & Science University in Portland, who lead the review. The National Institutes of Health commissioned the review to reveal the gaps in clinical data related to long-term opioid use.
To watch Dr. Chou discuss the data gaps, watch his interview with senior reporter Elizabeth Mechcatie.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BETHESDA, MD – When opioids first received Food and Drug Administration approval, it was for short-term use in management of acute pain. With time, these agents have become a mainstay of long-term management of chronic pain. A systematic review of the literature shows that there are no data on long-term opioid use. The longest placebo controlled randomized controlled trial being 6 months. There are ample data showing risk risks of over dose, abuse, and addiction associated with long-term opioid use for chronic pain, according to Dr. Roger Chou, director of the Pacific Northwest Evidence-based Practice Center and professor of medicine in the department of medical informatics and clinical epidemiology at the Oregon Health & Science University in Portland, who lead the review. The National Institutes of Health commissioned the review to reveal the gaps in clinical data related to long-term opioid use.
To watch Dr. Chou discuss the data gaps, watch his interview with senior reporter Elizabeth Mechcatie.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BETHESDA, MD – When opioids first received Food and Drug Administration approval, it was for short-term use in management of acute pain. With time, these agents have become a mainstay of long-term management of chronic pain. A systematic review of the literature shows that there are no data on long-term opioid use. The longest placebo controlled randomized controlled trial being 6 months. There are ample data showing risk risks of over dose, abuse, and addiction associated with long-term opioid use for chronic pain, according to Dr. Roger Chou, director of the Pacific Northwest Evidence-based Practice Center and professor of medicine in the department of medical informatics and clinical epidemiology at the Oregon Health & Science University in Portland, who lead the review. The National Institutes of Health commissioned the review to reveal the gaps in clinical data related to long-term opioid use.
To watch Dr. Chou discuss the data gaps, watch his interview with senior reporter Elizabeth Mechcatie.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Primary care docs don’t use risk reduction strategies, for so many reasons
BETHESDA, MD. – Guideline-based strategies for opioid risk reduction have not been widely accepted by primary care physicians, according to Dr. Erin E. Krebs, speaking in an interview at an National Institutes of Health–sponsored pathways to prevention panel.
There are few if any data to support the effectiveness of opioid agreements, for example. Other strategies such as checking online prescription drug monitoring programs are time consuming and require use of a working computer, which may not always be present in a primary care office, said Dr. Krebs, who is medical director of the Women Veterans Comprehensive Health Center of the Minneapolis Veterans Administration. But these are crucial things to do. Patients are well aware that opioids have risks and don’t want to be harmed by them, according to Dr. Krebs. Any kind of monitoring has to be couched in terms of safety rather than mistrust, she said.
To watch her discuss the realities of trying to reduce opioid risk in a primary care world, watch her interview with senior reporter Elizabeth Mechcatie.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BETHESDA, MD. – Guideline-based strategies for opioid risk reduction have not been widely accepted by primary care physicians, according to Dr. Erin E. Krebs, speaking in an interview at an National Institutes of Health–sponsored pathways to prevention panel.
There are few if any data to support the effectiveness of opioid agreements, for example. Other strategies such as checking online prescription drug monitoring programs are time consuming and require use of a working computer, which may not always be present in a primary care office, said Dr. Krebs, who is medical director of the Women Veterans Comprehensive Health Center of the Minneapolis Veterans Administration. But these are crucial things to do. Patients are well aware that opioids have risks and don’t want to be harmed by them, according to Dr. Krebs. Any kind of monitoring has to be couched in terms of safety rather than mistrust, she said.
To watch her discuss the realities of trying to reduce opioid risk in a primary care world, watch her interview with senior reporter Elizabeth Mechcatie.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BETHESDA, MD. – Guideline-based strategies for opioid risk reduction have not been widely accepted by primary care physicians, according to Dr. Erin E. Krebs, speaking in an interview at an National Institutes of Health–sponsored pathways to prevention panel.
There are few if any data to support the effectiveness of opioid agreements, for example. Other strategies such as checking online prescription drug monitoring programs are time consuming and require use of a working computer, which may not always be present in a primary care office, said Dr. Krebs, who is medical director of the Women Veterans Comprehensive Health Center of the Minneapolis Veterans Administration. But these are crucial things to do. Patients are well aware that opioids have risks and don’t want to be harmed by them, according to Dr. Krebs. Any kind of monitoring has to be couched in terms of safety rather than mistrust, she said.
To watch her discuss the realities of trying to reduce opioid risk in a primary care world, watch her interview with senior reporter Elizabeth Mechcatie.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Opioids do not work in most forms of chronic pain
BETHESDA, MD. – The reimbursement model for managing patients with chronic pain needs to change before physicians will stop prescribing opioids, according to Dr. Daniel J. Clauw, speaking at a National Institutes of Health–sponsored pathways to prevention panel on opioids. Certain types of chronic pain that are most common in young adults are the least likely to respond to opioids. These include headache, fibromyalgia, irritable bowel syndrome, peripheral nerve damage, temporal mandibular joint pain, and interstitial cystitis. Opioids may make them worse. Unfortunately, physicians are not reimbursed to provide the types of therapy that are effective at lessening the discomfort of patients with these chronic pain conditions, such as exercise and/or cognitive-behavioral therapy, said Dr. Clauw, who is professor of anesthesiology, medicine (rheumatology), and psychiatry at the University of Michigan in Ann Arbor. He serves as director of the Chronic Pain and Fatigue Research Center there.
In interviews at the meeting, Dr. Clauw discussed the major types of chronic pain and addressed the lack of data supporting use of opioids in almost all chronic pain conditions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BETHESDA, MD. – The reimbursement model for managing patients with chronic pain needs to change before physicians will stop prescribing opioids, according to Dr. Daniel J. Clauw, speaking at a National Institutes of Health–sponsored pathways to prevention panel on opioids. Certain types of chronic pain that are most common in young adults are the least likely to respond to opioids. These include headache, fibromyalgia, irritable bowel syndrome, peripheral nerve damage, temporal mandibular joint pain, and interstitial cystitis. Opioids may make them worse. Unfortunately, physicians are not reimbursed to provide the types of therapy that are effective at lessening the discomfort of patients with these chronic pain conditions, such as exercise and/or cognitive-behavioral therapy, said Dr. Clauw, who is professor of anesthesiology, medicine (rheumatology), and psychiatry at the University of Michigan in Ann Arbor. He serves as director of the Chronic Pain and Fatigue Research Center there.
In interviews at the meeting, Dr. Clauw discussed the major types of chronic pain and addressed the lack of data supporting use of opioids in almost all chronic pain conditions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BETHESDA, MD. – The reimbursement model for managing patients with chronic pain needs to change before physicians will stop prescribing opioids, according to Dr. Daniel J. Clauw, speaking at a National Institutes of Health–sponsored pathways to prevention panel on opioids. Certain types of chronic pain that are most common in young adults are the least likely to respond to opioids. These include headache, fibromyalgia, irritable bowel syndrome, peripheral nerve damage, temporal mandibular joint pain, and interstitial cystitis. Opioids may make them worse. Unfortunately, physicians are not reimbursed to provide the types of therapy that are effective at lessening the discomfort of patients with these chronic pain conditions, such as exercise and/or cognitive-behavioral therapy, said Dr. Clauw, who is professor of anesthesiology, medicine (rheumatology), and psychiatry at the University of Michigan in Ann Arbor. He serves as director of the Chronic Pain and Fatigue Research Center there.
In interviews at the meeting, Dr. Clauw discussed the major types of chronic pain and addressed the lack of data supporting use of opioids in almost all chronic pain conditions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
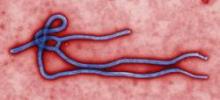
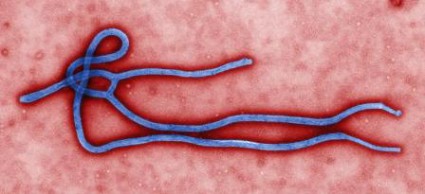
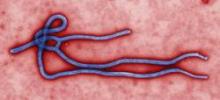
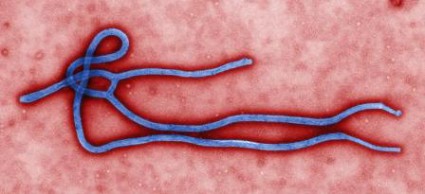
CDC: Ebola Poses Very Low Risk to US Health Care Workers
Standard barrier nursing protocols that have been in place in the United States since recognition of HIV will be adequate to protect health care workers in the unlikely event that patients who contracted the Ebola virus in Africa present to a US emergency department or clinician's office, officials from the Centers for Disease Control and Prevention said during a July 28 press briefing.
Since March, 672 people have died during the ongoing Ebola outbreak in Western Africa, according to Dr. Steve Monroe, director of the CDC Division of High-Consequence Pathogens and Pathology.
Two health care workers, both in Monvrovia, Liberia, have presented with Ebola. One is symptomatic and in isolation; the other has fever but no other symptoms.
Transmission of the virus to health care workers seems to have occurred via needle stick or contact with infected bodily fluids, according to Dr. Monroe. Some health care workers on the ground in Western Africa use only "rudimentary" safety precautions against infection, he noted.
In 2008, two cases of Marburg virus infection occurred in Western Europe. Marburg is a hemorrhagic fever virus that is a close cousin to Ebola, Dr. Monroe noted. Both patients were managed by way of standard barrier nursing precautions already in place, and those precautions were sufficient to protect physicians and nurses involved in their care.
"If a case [of Ebola] were to show up in the United States, I’m confident that we are already doing what needs to be done to prevent virus transmission to health care workers," he said.
The disease cannot be spread by asymptomatic people, according to Dr. Monroe. And if a symptomatic person travels on an airplane, it is unlikely that fellow travelers will be exposed to infected bodily secretions, he added.
One case of an Ebola patient traveling by air has been noted. The man flew from Liberia to Legos, Nigeria, where he died. The countries involved in the outbreak have stepped up efforts to screen for fever and other signs of the disease those travelers who seek to leave their countries. Only 50% of infected people present with hemorrhage; others present with the vague symptoms of fever, headache, muscle aches, and vomiting.
It is extremely unlikely that Ebola will spread outside of Africa. But, to err on the side of caution, the CDC has issued a Level 2 travel notice. Dr. Marty Centron, director of CDC Division of Global Migration and Quarantine, said the Level 2 notice does not involve any travel restrictions. It advises the traveling public to take sensible, enhanced precautions.
The CDC’s involvement in dealing with the Ebola outbreak, partnering with the World Health Organization and the health agencies of the three African countries involved, will be more of a marathon than a sprint, given how hard it can be to eradicate the virus, Dr. Monroe noted. Reseeding of regions where outbreaks seemed to be over is common, much like a wildfire that seems to have been extinguished can erupt from unexpected hot spots.
Standard barrier nursing protocols that have been in place in the United States since recognition of HIV will be adequate to protect health care workers in the unlikely event that patients who contracted the Ebola virus in Africa present to a US emergency department or clinician's office, officials from the Centers for Disease Control and Prevention said during a July 28 press briefing.
Since March, 672 people have died during the ongoing Ebola outbreak in Western Africa, according to Dr. Steve Monroe, director of the CDC Division of High-Consequence Pathogens and Pathology.
Two health care workers, both in Monvrovia, Liberia, have presented with Ebola. One is symptomatic and in isolation; the other has fever but no other symptoms.
Transmission of the virus to health care workers seems to have occurred via needle stick or contact with infected bodily fluids, according to Dr. Monroe. Some health care workers on the ground in Western Africa use only "rudimentary" safety precautions against infection, he noted.
In 2008, two cases of Marburg virus infection occurred in Western Europe. Marburg is a hemorrhagic fever virus that is a close cousin to Ebola, Dr. Monroe noted. Both patients were managed by way of standard barrier nursing precautions already in place, and those precautions were sufficient to protect physicians and nurses involved in their care.
"If a case [of Ebola] were to show up in the United States, I’m confident that we are already doing what needs to be done to prevent virus transmission to health care workers," he said.
The disease cannot be spread by asymptomatic people, according to Dr. Monroe. And if a symptomatic person travels on an airplane, it is unlikely that fellow travelers will be exposed to infected bodily secretions, he added.
One case of an Ebola patient traveling by air has been noted. The man flew from Liberia to Legos, Nigeria, where he died. The countries involved in the outbreak have stepped up efforts to screen for fever and other signs of the disease those travelers who seek to leave their countries. Only 50% of infected people present with hemorrhage; others present with the vague symptoms of fever, headache, muscle aches, and vomiting.
It is extremely unlikely that Ebola will spread outside of Africa. But, to err on the side of caution, the CDC has issued a Level 2 travel notice. Dr. Marty Centron, director of CDC Division of Global Migration and Quarantine, said the Level 2 notice does not involve any travel restrictions. It advises the traveling public to take sensible, enhanced precautions.
The CDC’s involvement in dealing with the Ebola outbreak, partnering with the World Health Organization and the health agencies of the three African countries involved, will be more of a marathon than a sprint, given how hard it can be to eradicate the virus, Dr. Monroe noted. Reseeding of regions where outbreaks seemed to be over is common, much like a wildfire that seems to have been extinguished can erupt from unexpected hot spots.
Standard barrier nursing protocols that have been in place in the United States since recognition of HIV will be adequate to protect health care workers in the unlikely event that patients who contracted the Ebola virus in Africa present to a US emergency department or clinician's office, officials from the Centers for Disease Control and Prevention said during a July 28 press briefing.
Since March, 672 people have died during the ongoing Ebola outbreak in Western Africa, according to Dr. Steve Monroe, director of the CDC Division of High-Consequence Pathogens and Pathology.
Two health care workers, both in Monvrovia, Liberia, have presented with Ebola. One is symptomatic and in isolation; the other has fever but no other symptoms.
Transmission of the virus to health care workers seems to have occurred via needle stick or contact with infected bodily fluids, according to Dr. Monroe. Some health care workers on the ground in Western Africa use only "rudimentary" safety precautions against infection, he noted.
In 2008, two cases of Marburg virus infection occurred in Western Europe. Marburg is a hemorrhagic fever virus that is a close cousin to Ebola, Dr. Monroe noted. Both patients were managed by way of standard barrier nursing precautions already in place, and those precautions were sufficient to protect physicians and nurses involved in their care.
"If a case [of Ebola] were to show up in the United States, I’m confident that we are already doing what needs to be done to prevent virus transmission to health care workers," he said.
The disease cannot be spread by asymptomatic people, according to Dr. Monroe. And if a symptomatic person travels on an airplane, it is unlikely that fellow travelers will be exposed to infected bodily secretions, he added.
One case of an Ebola patient traveling by air has been noted. The man flew from Liberia to Legos, Nigeria, where he died. The countries involved in the outbreak have stepped up efforts to screen for fever and other signs of the disease those travelers who seek to leave their countries. Only 50% of infected people present with hemorrhage; others present with the vague symptoms of fever, headache, muscle aches, and vomiting.
It is extremely unlikely that Ebola will spread outside of Africa. But, to err on the side of caution, the CDC has issued a Level 2 travel notice. Dr. Marty Centron, director of CDC Division of Global Migration and Quarantine, said the Level 2 notice does not involve any travel restrictions. It advises the traveling public to take sensible, enhanced precautions.
The CDC’s involvement in dealing with the Ebola outbreak, partnering with the World Health Organization and the health agencies of the three African countries involved, will be more of a marathon than a sprint, given how hard it can be to eradicate the virus, Dr. Monroe noted. Reseeding of regions where outbreaks seemed to be over is common, much like a wildfire that seems to have been extinguished can erupt from unexpected hot spots.
FROM A CDC BRIEFING
CDC: Ebola poses very low risk to U.S. health care workers
Standard barrier nursing protocols that have been in place in the United States since recognition of HIV will be adequate to protect health care workers in the unlikely event that patients who contracted the Ebola virus in Africa present to a U.S. emergency department or physician’s office, officials from the Centers for Disease Control and Prevention said during a July 28 press briefing.
Since March, 672 people have died during the ongoing Ebola outbreak in Western Africa, according to Dr. Steve Monroe, director of the CDC Division of High-Consequence Pathogens and Pathology.
Two health care workers, both in Monrovia, Liberia, have presented with Ebola. One is symptomatic and in isolation; the other has fever but no other symptoms.
Transmission of the virus to health care workers seems to have occurred via needle stick or contact with infected bodily fluids, according to Dr. Monroe. Some health care workers on the ground in Western Africa use only "rudimentary" safety precautions against infection, he noted.
In 2008, two cases of Marburg virus infection occurred in Western Europe. Marburg is a hemorrhagic fever virus that is a close cousin to Ebola, Dr. Monroe noted. Both patients were managed by way of standard barrier nursing precautions already in place, and those precautions were sufficient to protect physicians and nurses involved in their care.
"If a case [of Ebola] were to show up in the United States, I’m confident that we are already doing what needs to be done to prevent virus transmission to health care workers," he said.
The disease cannot be spread by asymptomatic people, according to Dr. Monroe. And if a symptomatic person travels on an airplane, it is unlikely that fellow travelers will be exposed to infected bodily secretions, he added.
One case of an Ebola patient traveling by air has been noted. The man flew from Liberia to Lagos, Nigeria, where he died. The countries involved in the outbreak have stepped up efforts to screen for fever and other signs of the disease those travelers who seek to leave their countries. Only 50% of infected people present with hemorrhage; others present with the vague symptoms of fever, headache, muscle aches, and vomiting.
It is extremely unlikely that Ebola will spread outside of Africa. But, to err on the side of caution, the CDC has issued a Level 2 travel notice. Dr. Marty Centron, director of CDC Division of Global Migration and Quarantine, said the Level 2 notice does not involve any travel restrictions. It advises the traveling public to take sensible, enhanced precautions.
The CDC’s involvement in dealing with the Ebola outbreak, partnering with the World Health Organization and the health agencies of the three African countries involved, will be more of a marathon than a sprint, given how hard it can be to eradicate the virus, Dr. Monroe noted. Reseeding of regions where outbreaks seemed to be over is common, much like a wildfire that seems to have been extinguished can erupt from unexpected hot spots.
Standard barrier nursing protocols that have been in place in the United States since recognition of HIV will be adequate to protect health care workers in the unlikely event that patients who contracted the Ebola virus in Africa present to a U.S. emergency department or physician’s office, officials from the Centers for Disease Control and Prevention said during a July 28 press briefing.
Since March, 672 people have died during the ongoing Ebola outbreak in Western Africa, according to Dr. Steve Monroe, director of the CDC Division of High-Consequence Pathogens and Pathology.
Two health care workers, both in Monrovia, Liberia, have presented with Ebola. One is symptomatic and in isolation; the other has fever but no other symptoms.
Transmission of the virus to health care workers seems to have occurred via needle stick or contact with infected bodily fluids, according to Dr. Monroe. Some health care workers on the ground in Western Africa use only "rudimentary" safety precautions against infection, he noted.
In 2008, two cases of Marburg virus infection occurred in Western Europe. Marburg is a hemorrhagic fever virus that is a close cousin to Ebola, Dr. Monroe noted. Both patients were managed by way of standard barrier nursing precautions already in place, and those precautions were sufficient to protect physicians and nurses involved in their care.
"If a case [of Ebola] were to show up in the United States, I’m confident that we are already doing what needs to be done to prevent virus transmission to health care workers," he said.
The disease cannot be spread by asymptomatic people, according to Dr. Monroe. And if a symptomatic person travels on an airplane, it is unlikely that fellow travelers will be exposed to infected bodily secretions, he added.
One case of an Ebola patient traveling by air has been noted. The man flew from Liberia to Lagos, Nigeria, where he died. The countries involved in the outbreak have stepped up efforts to screen for fever and other signs of the disease those travelers who seek to leave their countries. Only 50% of infected people present with hemorrhage; others present with the vague symptoms of fever, headache, muscle aches, and vomiting.
It is extremely unlikely that Ebola will spread outside of Africa. But, to err on the side of caution, the CDC has issued a Level 2 travel notice. Dr. Marty Centron, director of CDC Division of Global Migration and Quarantine, said the Level 2 notice does not involve any travel restrictions. It advises the traveling public to take sensible, enhanced precautions.
The CDC’s involvement in dealing with the Ebola outbreak, partnering with the World Health Organization and the health agencies of the three African countries involved, will be more of a marathon than a sprint, given how hard it can be to eradicate the virus, Dr. Monroe noted. Reseeding of regions where outbreaks seemed to be over is common, much like a wildfire that seems to have been extinguished can erupt from unexpected hot spots.
Standard barrier nursing protocols that have been in place in the United States since recognition of HIV will be adequate to protect health care workers in the unlikely event that patients who contracted the Ebola virus in Africa present to a U.S. emergency department or physician’s office, officials from the Centers for Disease Control and Prevention said during a July 28 press briefing.
Since March, 672 people have died during the ongoing Ebola outbreak in Western Africa, according to Dr. Steve Monroe, director of the CDC Division of High-Consequence Pathogens and Pathology.
Two health care workers, both in Monrovia, Liberia, have presented with Ebola. One is symptomatic and in isolation; the other has fever but no other symptoms.
Transmission of the virus to health care workers seems to have occurred via needle stick or contact with infected bodily fluids, according to Dr. Monroe. Some health care workers on the ground in Western Africa use only "rudimentary" safety precautions against infection, he noted.
In 2008, two cases of Marburg virus infection occurred in Western Europe. Marburg is a hemorrhagic fever virus that is a close cousin to Ebola, Dr. Monroe noted. Both patients were managed by way of standard barrier nursing precautions already in place, and those precautions were sufficient to protect physicians and nurses involved in their care.
"If a case [of Ebola] were to show up in the United States, I’m confident that we are already doing what needs to be done to prevent virus transmission to health care workers," he said.
The disease cannot be spread by asymptomatic people, according to Dr. Monroe. And if a symptomatic person travels on an airplane, it is unlikely that fellow travelers will be exposed to infected bodily secretions, he added.
One case of an Ebola patient traveling by air has been noted. The man flew from Liberia to Lagos, Nigeria, where he died. The countries involved in the outbreak have stepped up efforts to screen for fever and other signs of the disease those travelers who seek to leave their countries. Only 50% of infected people present with hemorrhage; others present with the vague symptoms of fever, headache, muscle aches, and vomiting.
It is extremely unlikely that Ebola will spread outside of Africa. But, to err on the side of caution, the CDC has issued a Level 2 travel notice. Dr. Marty Centron, director of CDC Division of Global Migration and Quarantine, said the Level 2 notice does not involve any travel restrictions. It advises the traveling public to take sensible, enhanced precautions.
The CDC’s involvement in dealing with the Ebola outbreak, partnering with the World Health Organization and the health agencies of the three African countries involved, will be more of a marathon than a sprint, given how hard it can be to eradicate the virus, Dr. Monroe noted. Reseeding of regions where outbreaks seemed to be over is common, much like a wildfire that seems to have been extinguished can erupt from unexpected hot spots.
FROM A CDC BRIEFING
Myth Buster: Gout Can Occur in Patients With Rheumatoid Arthritis
WASHINGTON – Gout does occur in patients with rheumatoid arthritis, though at a lower rate than in the general population, Dr. Adlene Jebakumar said at the annual meeting of the American College of Rheumatology.
This finding comes from a review of a population-based cohort of 813 people diagnosed with rheumatoid arthritis (RA) between 1980 and 2007. Diagnoses were made either clinically including typical monosodium urate crystal positivity in synovial fluid or 1977 criteria developed by an ACR precursor organization, the American Rheumatology Association criteria. All subjects were longitudinally followed through their complete community medical records until April 2012 or they died or moved away.
Of the study cohort, 537 (66%) were rheumatoid factor positive; 33% had rheumatoid nodules, and 53% had erosive joint disease. During 9,771 total person-years of follow-up (mean 12 years per RA patient), 22 patients developed gout as defined by clinical criteria. The great toe was the most common site of gout (12 of 22 patients). The 25-year cumulative incidence of gout diagnosed by clinical criteria was 5.3%. Typical intracellular monosodium urate crystals were present in 9 of 22 patients with acute gout; all had developed gout after the RA incidence date. The 25-year cumulative incidence of gout diagnosed by clinical criteria including presence of urate crystals is 1.3%. The prevalence of gout in RA on Jan 1, 2008, was 1.9% (11 of 582 patients) as opposed to expected prevalence of 5.2% (or 30 patients) based on National Health and Nutrition Examination Survey data using age and sex-specific prevalence rates.
Risk factors for gout in RA were: older age (hazard ratio, 1.5/10-year increase; P = .04), male sex (HR, 3.18; P = .03) and obesity (HR, 3.5; P = .03). The presence of erosive RA joint disease reduced the risk of gout (HR, 0.24; P = .03). Gout has become more common in patients diagnosed with RA in recent years (1995-2007) than in previous years (1980-1994; HR, 5.6; P = .007).
Dr. Eric L. Matteson noted in an interview that when an RA patient develops a hot and tender big toe, rheumatologists are likely to presume it is an RA flare. In part, this is because there is a myth in rheumatology that patients with RA cannot get gout, Dr. Eric L. Matteson noted in an interview. The literature contains reports of only 30 such cases. In fact, as the study findings show, that hot and tender toe may be gout. The best course of action is to aspirate the toe joint and look at the synovial fluid for crystals.
The treatment of gout in an RA patient can involve administration of prednisone, anakinra, allopurinol, or febuxostat. Drug-drug interactions between the agents used to treat gout and those for RA may be a problem in some cases.
Some of the treatments used in RA may explain why there are so few gout flares in RA patients. High doses of aspirin, which are an RA treatment, significantly lower uric acid levels. In what he described as being speculation, Dr. Matteson, chair of the department of rheumatology at the Mayo Clinic, Rochester, Minn., suggested that the "push away" from use of NSAIDs long term and in high doses to help manage RA may be resulting in more gout flares in these patients.
Dr. Jebakumar and Dr. Matteson reported having no relevant financial conflicts of interest.
WASHINGTON – Gout does occur in patients with rheumatoid arthritis, though at a lower rate than in the general population, Dr. Adlene Jebakumar said at the annual meeting of the American College of Rheumatology.
This finding comes from a review of a population-based cohort of 813 people diagnosed with rheumatoid arthritis (RA) between 1980 and 2007. Diagnoses were made either clinically including typical monosodium urate crystal positivity in synovial fluid or 1977 criteria developed by an ACR precursor organization, the American Rheumatology Association criteria. All subjects were longitudinally followed through their complete community medical records until April 2012 or they died or moved away.
Of the study cohort, 537 (66%) were rheumatoid factor positive; 33% had rheumatoid nodules, and 53% had erosive joint disease. During 9,771 total person-years of follow-up (mean 12 years per RA patient), 22 patients developed gout as defined by clinical criteria. The great toe was the most common site of gout (12 of 22 patients). The 25-year cumulative incidence of gout diagnosed by clinical criteria was 5.3%. Typical intracellular monosodium urate crystals were present in 9 of 22 patients with acute gout; all had developed gout after the RA incidence date. The 25-year cumulative incidence of gout diagnosed by clinical criteria including presence of urate crystals is 1.3%. The prevalence of gout in RA on Jan 1, 2008, was 1.9% (11 of 582 patients) as opposed to expected prevalence of 5.2% (or 30 patients) based on National Health and Nutrition Examination Survey data using age and sex-specific prevalence rates.
Risk factors for gout in RA were: older age (hazard ratio, 1.5/10-year increase; P = .04), male sex (HR, 3.18; P = .03) and obesity (HR, 3.5; P = .03). The presence of erosive RA joint disease reduced the risk of gout (HR, 0.24; P = .03). Gout has become more common in patients diagnosed with RA in recent years (1995-2007) than in previous years (1980-1994; HR, 5.6; P = .007).
Dr. Eric L. Matteson noted in an interview that when an RA patient develops a hot and tender big toe, rheumatologists are likely to presume it is an RA flare. In part, this is because there is a myth in rheumatology that patients with RA cannot get gout, Dr. Eric L. Matteson noted in an interview. The literature contains reports of only 30 such cases. In fact, as the study findings show, that hot and tender toe may be gout. The best course of action is to aspirate the toe joint and look at the synovial fluid for crystals.
The treatment of gout in an RA patient can involve administration of prednisone, anakinra, allopurinol, or febuxostat. Drug-drug interactions between the agents used to treat gout and those for RA may be a problem in some cases.
Some of the treatments used in RA may explain why there are so few gout flares in RA patients. High doses of aspirin, which are an RA treatment, significantly lower uric acid levels. In what he described as being speculation, Dr. Matteson, chair of the department of rheumatology at the Mayo Clinic, Rochester, Minn., suggested that the "push away" from use of NSAIDs long term and in high doses to help manage RA may be resulting in more gout flares in these patients.
Dr. Jebakumar and Dr. Matteson reported having no relevant financial conflicts of interest.
WASHINGTON – Gout does occur in patients with rheumatoid arthritis, though at a lower rate than in the general population, Dr. Adlene Jebakumar said at the annual meeting of the American College of Rheumatology.
This finding comes from a review of a population-based cohort of 813 people diagnosed with rheumatoid arthritis (RA) between 1980 and 2007. Diagnoses were made either clinically including typical monosodium urate crystal positivity in synovial fluid or 1977 criteria developed by an ACR precursor organization, the American Rheumatology Association criteria. All subjects were longitudinally followed through their complete community medical records until April 2012 or they died or moved away.
Of the study cohort, 537 (66%) were rheumatoid factor positive; 33% had rheumatoid nodules, and 53% had erosive joint disease. During 9,771 total person-years of follow-up (mean 12 years per RA patient), 22 patients developed gout as defined by clinical criteria. The great toe was the most common site of gout (12 of 22 patients). The 25-year cumulative incidence of gout diagnosed by clinical criteria was 5.3%. Typical intracellular monosodium urate crystals were present in 9 of 22 patients with acute gout; all had developed gout after the RA incidence date. The 25-year cumulative incidence of gout diagnosed by clinical criteria including presence of urate crystals is 1.3%. The prevalence of gout in RA on Jan 1, 2008, was 1.9% (11 of 582 patients) as opposed to expected prevalence of 5.2% (or 30 patients) based on National Health and Nutrition Examination Survey data using age and sex-specific prevalence rates.
Risk factors for gout in RA were: older age (hazard ratio, 1.5/10-year increase; P = .04), male sex (HR, 3.18; P = .03) and obesity (HR, 3.5; P = .03). The presence of erosive RA joint disease reduced the risk of gout (HR, 0.24; P = .03). Gout has become more common in patients diagnosed with RA in recent years (1995-2007) than in previous years (1980-1994; HR, 5.6; P = .007).
Dr. Eric L. Matteson noted in an interview that when an RA patient develops a hot and tender big toe, rheumatologists are likely to presume it is an RA flare. In part, this is because there is a myth in rheumatology that patients with RA cannot get gout, Dr. Eric L. Matteson noted in an interview. The literature contains reports of only 30 such cases. In fact, as the study findings show, that hot and tender toe may be gout. The best course of action is to aspirate the toe joint and look at the synovial fluid for crystals.
The treatment of gout in an RA patient can involve administration of prednisone, anakinra, allopurinol, or febuxostat. Drug-drug interactions between the agents used to treat gout and those for RA may be a problem in some cases.
Some of the treatments used in RA may explain why there are so few gout flares in RA patients. High doses of aspirin, which are an RA treatment, significantly lower uric acid levels. In what he described as being speculation, Dr. Matteson, chair of the department of rheumatology at the Mayo Clinic, Rochester, Minn., suggested that the "push away" from use of NSAIDs long term and in high doses to help manage RA may be resulting in more gout flares in these patients.
Dr. Jebakumar and Dr. Matteson reported having no relevant financial conflicts of interest.
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF RHEUMATOLOGY
Major Finding: The 25-year incidence of gout among 813 patients with rheumatoid arthritis was 1.9%, as diagnosed by clinical criteria.
Data Source: This finding comes from a review of an population-based incidence cohort of patients who fulfilled 1977 ARA criteria for RA in 1980-2007.
Disclosures: Dr. Jebakumar and Dr. Matteson reported having no relevant financial conflicts of interest.
Leg, Foot Ulcers on the Rise in RA
WASHINGTON – Ulcers of the lower leg and foot occurred at an incidence of 1% per year among people with rheumatoid arthritis, with the rate having doubled over the last few years, according to findings from a retrospective assessment of a population-based incidence cohort in Olmstead County, Minn.
The study cohort all met the 1987 American College of Rheumatology criteria for rheumatoid arthritis (RA) in 1980-2007. The cohort included 813 people with RA, said Dr. Adlene Jebakumar during her presentation at the annual meeting of the American College of Rheumatology. Of these, 66% were positive for rheumatoid factor, 53% had erosive joint disease, and 33% had rheumatoid nodules, all markers of severe disease.
During 9,771 total person-years of follow-up, there were 171 episodes of leg and/or foot ulcers in 125 of these people. These cases did not include ulcers resulting from animal bites, surgery, burns, biopsy, cellulitis, ingrown toenails, toenail removal, abrasion, foreign body, or herpes zoster.
Patients’ mean age at first ulcer onset was 73.5 years, and 74% were female.
The area between the ankle and knee was the most common location for ulcers (58 ulcers, 34%), followed by the tips of the toes (46 ulcers, 27%).
The major etiology was pressure (62 ulcers, 36%) or trauma (49 ulcers, 27%). Another 22 ulcers (13%) were ischemic, and 2 (1%) were vasculitic, reported Dr. Jebakumar of the division of rheumatology at the Mayo Clinic in Rochester, Minn.
The incidence of lower leg and foot ulcers was higher among patients diagnosed with RA in 1995-2007, compared with those diagnosed in 1980-1994 (hazard ratio, 2.03; P less than .001). The median time for the lower leg and foot ulcers to heal was 30 days. Ten (6%) of 171 episodes led to amputation. Lower leg and foot ulcers in RA were associated with increased mortality (HR, 2.42; P less than .001), adjusted for age, sex, and calendar year.
The risk factors for lower extremity ulcers in RA were age (HR, 1.90/10-year increase; P less than .001); current smoking (HR, 1.51; P = .048); diabetes mellitus (HR, 1.65; P =.015); coronary heart disease or heart failure (HR, 1.56; P less than .035); presence of rheumatoid nodules (HR, 1.64; P = .010); ESR of 60 mm/hr or greater on three occasions (HR, 1.78; P = .022); venous thromboembolism (HR, 2.08; P = .014); and severe extra-articular manifestations (HR, 1.67; P = .048). The patients on corticosteroid therapy accounted for 79 (46%) of 171 ulcer episodes.
Dr. Eric L. Matteson said in an interview that the incidence of leg and foot ulcers in RA patients should not come as a surprise. "Leg ulcers are a real big problem in RA because these patients have increased cardiovascular disease and peripheral artery disease. Lots of ulcers are due to poor circulation. And long-term high-dose steroids sometimes used to treat RA patients may contribute to that."
The situation is made worse in elderly patients who become sedentary in response to lower-extremity RA-related pain, he added.
"The best prevention of lower extremity ulcers is to have RA under control," said Dr. Matteson, chair of the department of rheumatology at the Mayo Clinic. And treat ulcers early in their course, when they are small, he noted, adding that patients need to be told to seek care early rather than wait until the ulcers have become large and harder to heal.
Another important aspect of prevention is harder to do than it sounds: "Keep patients active," Dr. Matteson advised.
Dr. Jebakumar and Dr. Matteson reported having no relevant financial conflicts of interest
WASHINGTON – Ulcers of the lower leg and foot occurred at an incidence of 1% per year among people with rheumatoid arthritis, with the rate having doubled over the last few years, according to findings from a retrospective assessment of a population-based incidence cohort in Olmstead County, Minn.
The study cohort all met the 1987 American College of Rheumatology criteria for rheumatoid arthritis (RA) in 1980-2007. The cohort included 813 people with RA, said Dr. Adlene Jebakumar during her presentation at the annual meeting of the American College of Rheumatology. Of these, 66% were positive for rheumatoid factor, 53% had erosive joint disease, and 33% had rheumatoid nodules, all markers of severe disease.
During 9,771 total person-years of follow-up, there were 171 episodes of leg and/or foot ulcers in 125 of these people. These cases did not include ulcers resulting from animal bites, surgery, burns, biopsy, cellulitis, ingrown toenails, toenail removal, abrasion, foreign body, or herpes zoster.
Patients’ mean age at first ulcer onset was 73.5 years, and 74% were female.
The area between the ankle and knee was the most common location for ulcers (58 ulcers, 34%), followed by the tips of the toes (46 ulcers, 27%).
The major etiology was pressure (62 ulcers, 36%) or trauma (49 ulcers, 27%). Another 22 ulcers (13%) were ischemic, and 2 (1%) were vasculitic, reported Dr. Jebakumar of the division of rheumatology at the Mayo Clinic in Rochester, Minn.
The incidence of lower leg and foot ulcers was higher among patients diagnosed with RA in 1995-2007, compared with those diagnosed in 1980-1994 (hazard ratio, 2.03; P less than .001). The median time for the lower leg and foot ulcers to heal was 30 days. Ten (6%) of 171 episodes led to amputation. Lower leg and foot ulcers in RA were associated with increased mortality (HR, 2.42; P less than .001), adjusted for age, sex, and calendar year.
The risk factors for lower extremity ulcers in RA were age (HR, 1.90/10-year increase; P less than .001); current smoking (HR, 1.51; P = .048); diabetes mellitus (HR, 1.65; P =.015); coronary heart disease or heart failure (HR, 1.56; P less than .035); presence of rheumatoid nodules (HR, 1.64; P = .010); ESR of 60 mm/hr or greater on three occasions (HR, 1.78; P = .022); venous thromboembolism (HR, 2.08; P = .014); and severe extra-articular manifestations (HR, 1.67; P = .048). The patients on corticosteroid therapy accounted for 79 (46%) of 171 ulcer episodes.
Dr. Eric L. Matteson said in an interview that the incidence of leg and foot ulcers in RA patients should not come as a surprise. "Leg ulcers are a real big problem in RA because these patients have increased cardiovascular disease and peripheral artery disease. Lots of ulcers are due to poor circulation. And long-term high-dose steroids sometimes used to treat RA patients may contribute to that."
The situation is made worse in elderly patients who become sedentary in response to lower-extremity RA-related pain, he added.
"The best prevention of lower extremity ulcers is to have RA under control," said Dr. Matteson, chair of the department of rheumatology at the Mayo Clinic. And treat ulcers early in their course, when they are small, he noted, adding that patients need to be told to seek care early rather than wait until the ulcers have become large and harder to heal.
Another important aspect of prevention is harder to do than it sounds: "Keep patients active," Dr. Matteson advised.
Dr. Jebakumar and Dr. Matteson reported having no relevant financial conflicts of interest
WASHINGTON – Ulcers of the lower leg and foot occurred at an incidence of 1% per year among people with rheumatoid arthritis, with the rate having doubled over the last few years, according to findings from a retrospective assessment of a population-based incidence cohort in Olmstead County, Minn.
The study cohort all met the 1987 American College of Rheumatology criteria for rheumatoid arthritis (RA) in 1980-2007. The cohort included 813 people with RA, said Dr. Adlene Jebakumar during her presentation at the annual meeting of the American College of Rheumatology. Of these, 66% were positive for rheumatoid factor, 53% had erosive joint disease, and 33% had rheumatoid nodules, all markers of severe disease.
During 9,771 total person-years of follow-up, there were 171 episodes of leg and/or foot ulcers in 125 of these people. These cases did not include ulcers resulting from animal bites, surgery, burns, biopsy, cellulitis, ingrown toenails, toenail removal, abrasion, foreign body, or herpes zoster.
Patients’ mean age at first ulcer onset was 73.5 years, and 74% were female.
The area between the ankle and knee was the most common location for ulcers (58 ulcers, 34%), followed by the tips of the toes (46 ulcers, 27%).
The major etiology was pressure (62 ulcers, 36%) or trauma (49 ulcers, 27%). Another 22 ulcers (13%) were ischemic, and 2 (1%) were vasculitic, reported Dr. Jebakumar of the division of rheumatology at the Mayo Clinic in Rochester, Minn.
The incidence of lower leg and foot ulcers was higher among patients diagnosed with RA in 1995-2007, compared with those diagnosed in 1980-1994 (hazard ratio, 2.03; P less than .001). The median time for the lower leg and foot ulcers to heal was 30 days. Ten (6%) of 171 episodes led to amputation. Lower leg and foot ulcers in RA were associated with increased mortality (HR, 2.42; P less than .001), adjusted for age, sex, and calendar year.
The risk factors for lower extremity ulcers in RA were age (HR, 1.90/10-year increase; P less than .001); current smoking (HR, 1.51; P = .048); diabetes mellitus (HR, 1.65; P =.015); coronary heart disease or heart failure (HR, 1.56; P less than .035); presence of rheumatoid nodules (HR, 1.64; P = .010); ESR of 60 mm/hr or greater on three occasions (HR, 1.78; P = .022); venous thromboembolism (HR, 2.08; P = .014); and severe extra-articular manifestations (HR, 1.67; P = .048). The patients on corticosteroid therapy accounted for 79 (46%) of 171 ulcer episodes.
Dr. Eric L. Matteson said in an interview that the incidence of leg and foot ulcers in RA patients should not come as a surprise. "Leg ulcers are a real big problem in RA because these patients have increased cardiovascular disease and peripheral artery disease. Lots of ulcers are due to poor circulation. And long-term high-dose steroids sometimes used to treat RA patients may contribute to that."
The situation is made worse in elderly patients who become sedentary in response to lower-extremity RA-related pain, he added.
"The best prevention of lower extremity ulcers is to have RA under control," said Dr. Matteson, chair of the department of rheumatology at the Mayo Clinic. And treat ulcers early in their course, when they are small, he noted, adding that patients need to be told to seek care early rather than wait until the ulcers have become large and harder to heal.
Another important aspect of prevention is harder to do than it sounds: "Keep patients active," Dr. Matteson advised.
Dr. Jebakumar and Dr. Matteson reported having no relevant financial conflicts of interest
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF RHEUMATOLOGY
Major Finding: Leg and foot ulcers occur among patients with rheumatoid arthritis at an incidence of 1% per year, which is double what it was just a few years ago.
Data Source: Findings come from a review of a population-based incidence cohort of 813 patients with rheumatoid arthritis.
Disclosures: Dr. Jebakumar and Dr. Matteson reported having no relevant financial conflicts of interest.
Fungal Infection Outbreak Has a New Face: Septic Arthritis
Infections have developed in one joint each in two individuals who were injected with fungus-contaminated methylprednisolone acetate in the weeks or months preceding the ongoing outbreak, according to a teleconference hosted Oct. 16 by the Food and Drug Administration and the Centers for Disease Control and Prevention.
The indications for those joint injections were not discussed at the briefing, but rheumatologists commonly use methylprednisolone injections in joints affected by rheumatoid arthritis or osteoarthritis to reduce inflammation and ease pain.
Dr. Tom Chiller, a medical epidemiologist at the Division of Foodborne, Waterborne and Environmental Diseases at the CDC, noted that both infected joints were ankles that had been injected with fungus-contaminated methylprednisolone acetate manufactured by the New England Compounding Center (NECC) in Framingham, Mass. That plant has been shut down because inspection by the FDA showed that the conditions were unsterile.
Neither culture nor polymerase chain reaction of synovial fluid aspirated from the infected ankles has grown any fungus. However, because bacteria and crystals have been ruled out as a cause of these two cases of septic arthritic and both patients received intra-articular injections with the contaminated steroid solution, investigators feel certain the patients have fungal joint infections, according to Dr. Chiller.
Three kinds of fungus have been identified in patients with fungal infections associated with the contaminated NECC products. Aspergillus was identified as the cause of the index case of fungal meningitis. Two types of black mold have been cultured from meningitis patients: Exserohilum rostratum and Cladosporium.
In total, as of Oct. 16, there have been 233 confirmed cases of fungal infection in people who received injections, usually epidural, of contaminated steroid in solution, according to Dr. Melissa Schaefer, medical officer in the Division of Healthcare Quality Promotion at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases. Among these people there have been 15 deaths, all from meningitis.
One particularly challenging aspect of this outbreak for rheumatologists is the length of incubation of septic arthritis because of a fungus.
The FDA has advised physicians who used NECC products dated May 21, 2012 or later, to contact their patients to check on their well being. Most of the patients given epidural injections with the contaminated solution are likely to present within 1-2 weeks.
The latency period of a fungus infection in a joint, however, may be months, according to Dr. Peter G. Pappas, professor of medicine in the division of infectious diseases at the University of Alabama at Birmingham, who participated in the CDC teleconference.
Patients with septic arthritis often limp into the office months after the onset of symptoms to seek medical care, he said.
The CDC has revised its treatment guidance for septic arthritis in cases of suspected fungal infection Dr. Chiller said that in cases of suspected fungal septic arthritis, the CDC now recommends empiric treatment with voriconazole (Vfend), beginning with a loading dose of 6 mg/kg every 12 hours for two doses, followed by 4 mg/kg every 12 hours for the duration of treatment. A lipid formulation of amphotericin B at a dose of 5 mg/kg IV daily should be considered in addition to voriconazole in patients with severe joint infection and/or clinical instability. Administration of 1L normal saline prior to infusion may be considered to minimize risk of nephrotoxicity. Providers and patients should be aware of and monitor for potential adverse effects of amphotericin B formulations.
The CDC plans to update the guidance on diagnosing fungal septic arthritis on Oct. 17 or 18.
The source of the outbreak is NECC, a compounding pharmacy that operated in violation of the law on a number of levels.
Infections have developed in one joint each in two individuals who were injected with fungus-contaminated methylprednisolone acetate in the weeks or months preceding the ongoing outbreak, according to a teleconference hosted Oct. 16 by the Food and Drug Administration and the Centers for Disease Control and Prevention.
The indications for those joint injections were not discussed at the briefing, but rheumatologists commonly use methylprednisolone injections in joints affected by rheumatoid arthritis or osteoarthritis to reduce inflammation and ease pain.
Dr. Tom Chiller, a medical epidemiologist at the Division of Foodborne, Waterborne and Environmental Diseases at the CDC, noted that both infected joints were ankles that had been injected with fungus-contaminated methylprednisolone acetate manufactured by the New England Compounding Center (NECC) in Framingham, Mass. That plant has been shut down because inspection by the FDA showed that the conditions were unsterile.
Neither culture nor polymerase chain reaction of synovial fluid aspirated from the infected ankles has grown any fungus. However, because bacteria and crystals have been ruled out as a cause of these two cases of septic arthritic and both patients received intra-articular injections with the contaminated steroid solution, investigators feel certain the patients have fungal joint infections, according to Dr. Chiller.
Three kinds of fungus have been identified in patients with fungal infections associated with the contaminated NECC products. Aspergillus was identified as the cause of the index case of fungal meningitis. Two types of black mold have been cultured from meningitis patients: Exserohilum rostratum and Cladosporium.
In total, as of Oct. 16, there have been 233 confirmed cases of fungal infection in people who received injections, usually epidural, of contaminated steroid in solution, according to Dr. Melissa Schaefer, medical officer in the Division of Healthcare Quality Promotion at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases. Among these people there have been 15 deaths, all from meningitis.
One particularly challenging aspect of this outbreak for rheumatologists is the length of incubation of septic arthritis because of a fungus.
The FDA has advised physicians who used NECC products dated May 21, 2012 or later, to contact their patients to check on their well being. Most of the patients given epidural injections with the contaminated solution are likely to present within 1-2 weeks.
The latency period of a fungus infection in a joint, however, may be months, according to Dr. Peter G. Pappas, professor of medicine in the division of infectious diseases at the University of Alabama at Birmingham, who participated in the CDC teleconference.
Patients with septic arthritis often limp into the office months after the onset of symptoms to seek medical care, he said.
The CDC has revised its treatment guidance for septic arthritis in cases of suspected fungal infection Dr. Chiller said that in cases of suspected fungal septic arthritis, the CDC now recommends empiric treatment with voriconazole (Vfend), beginning with a loading dose of 6 mg/kg every 12 hours for two doses, followed by 4 mg/kg every 12 hours for the duration of treatment. A lipid formulation of amphotericin B at a dose of 5 mg/kg IV daily should be considered in addition to voriconazole in patients with severe joint infection and/or clinical instability. Administration of 1L normal saline prior to infusion may be considered to minimize risk of nephrotoxicity. Providers and patients should be aware of and monitor for potential adverse effects of amphotericin B formulations.
The CDC plans to update the guidance on diagnosing fungal septic arthritis on Oct. 17 or 18.
The source of the outbreak is NECC, a compounding pharmacy that operated in violation of the law on a number of levels.
Infections have developed in one joint each in two individuals who were injected with fungus-contaminated methylprednisolone acetate in the weeks or months preceding the ongoing outbreak, according to a teleconference hosted Oct. 16 by the Food and Drug Administration and the Centers for Disease Control and Prevention.
The indications for those joint injections were not discussed at the briefing, but rheumatologists commonly use methylprednisolone injections in joints affected by rheumatoid arthritis or osteoarthritis to reduce inflammation and ease pain.
Dr. Tom Chiller, a medical epidemiologist at the Division of Foodborne, Waterborne and Environmental Diseases at the CDC, noted that both infected joints were ankles that had been injected with fungus-contaminated methylprednisolone acetate manufactured by the New England Compounding Center (NECC) in Framingham, Mass. That plant has been shut down because inspection by the FDA showed that the conditions were unsterile.
Neither culture nor polymerase chain reaction of synovial fluid aspirated from the infected ankles has grown any fungus. However, because bacteria and crystals have been ruled out as a cause of these two cases of septic arthritic and both patients received intra-articular injections with the contaminated steroid solution, investigators feel certain the patients have fungal joint infections, according to Dr. Chiller.
Three kinds of fungus have been identified in patients with fungal infections associated with the contaminated NECC products. Aspergillus was identified as the cause of the index case of fungal meningitis. Two types of black mold have been cultured from meningitis patients: Exserohilum rostratum and Cladosporium.
In total, as of Oct. 16, there have been 233 confirmed cases of fungal infection in people who received injections, usually epidural, of contaminated steroid in solution, according to Dr. Melissa Schaefer, medical officer in the Division of Healthcare Quality Promotion at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases. Among these people there have been 15 deaths, all from meningitis.
One particularly challenging aspect of this outbreak for rheumatologists is the length of incubation of septic arthritis because of a fungus.
The FDA has advised physicians who used NECC products dated May 21, 2012 or later, to contact their patients to check on their well being. Most of the patients given epidural injections with the contaminated solution are likely to present within 1-2 weeks.
The latency period of a fungus infection in a joint, however, may be months, according to Dr. Peter G. Pappas, professor of medicine in the division of infectious diseases at the University of Alabama at Birmingham, who participated in the CDC teleconference.
Patients with septic arthritis often limp into the office months after the onset of symptoms to seek medical care, he said.
The CDC has revised its treatment guidance for septic arthritis in cases of suspected fungal infection Dr. Chiller said that in cases of suspected fungal septic arthritis, the CDC now recommends empiric treatment with voriconazole (Vfend), beginning with a loading dose of 6 mg/kg every 12 hours for two doses, followed by 4 mg/kg every 12 hours for the duration of treatment. A lipid formulation of amphotericin B at a dose of 5 mg/kg IV daily should be considered in addition to voriconazole in patients with severe joint infection and/or clinical instability. Administration of 1L normal saline prior to infusion may be considered to minimize risk of nephrotoxicity. Providers and patients should be aware of and monitor for potential adverse effects of amphotericin B formulations.
The CDC plans to update the guidance on diagnosing fungal septic arthritis on Oct. 17 or 18.
The source of the outbreak is NECC, a compounding pharmacy that operated in violation of the law on a number of levels.