User login
Add vitamin E to NASH ‘toolkit’
VIENNA – Pooled analysis of data from three controlled trials provides further evidence to support the use of vitamin E in the treatment of nonalcoholic steatohepatitis (NASH).
Daily vitamin E intake led to resolution of NASH in a higher percentage of patients than in those not treated with vitamin E (38% vs. 20%, P < .001). It also resulted in more patients achieving histologic improvement of NASH (45% vs. 22%, P < .0001).
“The magnitude of the effect of vitamin E on NASH was comparable to pioglitazone, metformin, and obeticholic acid,” Dr. Kris Kowdley of the Swedish Medical Center, Seattle, said in an interview at the meeting sponsored by the European Association for the Study of the Liver.
The odds ratio (OR) for the resolution of NASH for vitamin E versus no vitamin E was 2.4, (P < .001) and ORs for the other anti-NASH treatments used in the trials were 3.4 for pioglitazone vs. placebo (P = .001), 1.8 for metformin vs. placebo (P = .24), and 1.8 for obeticholic acid vs. placebo (P = .13).
The respective ORs for histologic improvement for vitamin E and the other treatments were 2.9 (P < .0001), 4.1 (P = .0001), 2.7 (P = .02), and 3.1 (P = .0002).
The data combined for the analysis were derived from three controlled studies: PIVENS (Pioglitazone Versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis], TONIC (The Treatment of Nonalcoholic Fatty Liver Disease in Children), and FLINT (Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment Trial).
In the phase III, randomized, placebo-controlled, double-blind PIVENS trial, vitamin E therapy was compared to pioglitazone and placebo in 247 nondiabetic adults (N. Engl. J. Med. 2010; 362:1675-85).
In the TONIC phase III, double-blind, placebo-controlled trial, 173 children with NASH but no diabetes were randomized to treatment with vitamin E, metformin, or placebo for 96 weeks.
In FLINT, a phase II, placebo-controlled, double-blind trial of obeticholic acid, vitamin E was allowed as a concomitant therapy, and 23% of placebo-treated patients took vitamin E at baseline.
Dr. Kowdley noted that natural (d-alpha-tocopherol) not synthetic vitamin E was used in all these trials and the dosage used was 800 IU once daily in the PIVENS trial, 400 IU twice daily in the TONIC trial, and 400-800 IU in the patients who received it in the placebo arm of the FLINT trial.
In total, the pooled analysis considered 347 participants of these trials of whom 155 had been treated with vitamin E and 192 had not.
Histologic improvement was defined as at least a 2-point improvement in NASH with no worsening of fibrosis. NASH resolution was determined by pre- and posttreatment biopsies.
How vitamin E might be exerting its effect is not clear, Dr. Kowdley said. “We know that from the PIVENS trial it does not affect insulin sensitivity, and so presumably it has an antioxidant effect,” he speculated. Combination with other anti-NASH treatments, such as obeticholic acid, could produce synergistic effects perhaps, he acknowledged, but that would require further study.
“Vitamin E appears to be safe in this short-term study,” Dr. Kowdley said. Safety measures included the incidence of cardiac events and changes in lipid levels. However, there was no increased incidence in cardiovascular events with vitamin E use compared to no vitamin E use (6.9% vs. 7.6%, P =.85). There were also no significant differences in net changes in total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, or triglycerides.
Although it was not shown in the late-breaking poster, Dr. Kowdley said that vitamin E appeared to improve NASH in diabetic as well as in nondiabetic subjects with no increased risk of cardiovascular events as other studies have suggested.
“It’s a pooled analysis,” Dr. Kowdley acknowledged, “but we conclude that vitamin E was significantly associated with histologic improvement, reduction in NASH activity score and resolution of NASH, with an effect size that seems in the ballpark of the other therapies.
“So I think this suggests that vitamin E should be our toolkit for treatment of NASH,” he added.
The National Institute of Diabetes and Digestive and Kidney Diseases sponsored the studies. Dr. Kowdley had no relevant conflicts of interest to disclosure.
VIENNA – Pooled analysis of data from three controlled trials provides further evidence to support the use of vitamin E in the treatment of nonalcoholic steatohepatitis (NASH).
Daily vitamin E intake led to resolution of NASH in a higher percentage of patients than in those not treated with vitamin E (38% vs. 20%, P < .001). It also resulted in more patients achieving histologic improvement of NASH (45% vs. 22%, P < .0001).
“The magnitude of the effect of vitamin E on NASH was comparable to pioglitazone, metformin, and obeticholic acid,” Dr. Kris Kowdley of the Swedish Medical Center, Seattle, said in an interview at the meeting sponsored by the European Association for the Study of the Liver.
The odds ratio (OR) for the resolution of NASH for vitamin E versus no vitamin E was 2.4, (P < .001) and ORs for the other anti-NASH treatments used in the trials were 3.4 for pioglitazone vs. placebo (P = .001), 1.8 for metformin vs. placebo (P = .24), and 1.8 for obeticholic acid vs. placebo (P = .13).
The respective ORs for histologic improvement for vitamin E and the other treatments were 2.9 (P < .0001), 4.1 (P = .0001), 2.7 (P = .02), and 3.1 (P = .0002).
The data combined for the analysis were derived from three controlled studies: PIVENS (Pioglitazone Versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis], TONIC (The Treatment of Nonalcoholic Fatty Liver Disease in Children), and FLINT (Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment Trial).
In the phase III, randomized, placebo-controlled, double-blind PIVENS trial, vitamin E therapy was compared to pioglitazone and placebo in 247 nondiabetic adults (N. Engl. J. Med. 2010; 362:1675-85).
In the TONIC phase III, double-blind, placebo-controlled trial, 173 children with NASH but no diabetes were randomized to treatment with vitamin E, metformin, or placebo for 96 weeks.
In FLINT, a phase II, placebo-controlled, double-blind trial of obeticholic acid, vitamin E was allowed as a concomitant therapy, and 23% of placebo-treated patients took vitamin E at baseline.
Dr. Kowdley noted that natural (d-alpha-tocopherol) not synthetic vitamin E was used in all these trials and the dosage used was 800 IU once daily in the PIVENS trial, 400 IU twice daily in the TONIC trial, and 400-800 IU in the patients who received it in the placebo arm of the FLINT trial.
In total, the pooled analysis considered 347 participants of these trials of whom 155 had been treated with vitamin E and 192 had not.
Histologic improvement was defined as at least a 2-point improvement in NASH with no worsening of fibrosis. NASH resolution was determined by pre- and posttreatment biopsies.
How vitamin E might be exerting its effect is not clear, Dr. Kowdley said. “We know that from the PIVENS trial it does not affect insulin sensitivity, and so presumably it has an antioxidant effect,” he speculated. Combination with other anti-NASH treatments, such as obeticholic acid, could produce synergistic effects perhaps, he acknowledged, but that would require further study.
“Vitamin E appears to be safe in this short-term study,” Dr. Kowdley said. Safety measures included the incidence of cardiac events and changes in lipid levels. However, there was no increased incidence in cardiovascular events with vitamin E use compared to no vitamin E use (6.9% vs. 7.6%, P =.85). There were also no significant differences in net changes in total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, or triglycerides.
Although it was not shown in the late-breaking poster, Dr. Kowdley said that vitamin E appeared to improve NASH in diabetic as well as in nondiabetic subjects with no increased risk of cardiovascular events as other studies have suggested.
“It’s a pooled analysis,” Dr. Kowdley acknowledged, “but we conclude that vitamin E was significantly associated with histologic improvement, reduction in NASH activity score and resolution of NASH, with an effect size that seems in the ballpark of the other therapies.
“So I think this suggests that vitamin E should be our toolkit for treatment of NASH,” he added.
The National Institute of Diabetes and Digestive and Kidney Diseases sponsored the studies. Dr. Kowdley had no relevant conflicts of interest to disclosure.
VIENNA – Pooled analysis of data from three controlled trials provides further evidence to support the use of vitamin E in the treatment of nonalcoholic steatohepatitis (NASH).
Daily vitamin E intake led to resolution of NASH in a higher percentage of patients than in those not treated with vitamin E (38% vs. 20%, P < .001). It also resulted in more patients achieving histologic improvement of NASH (45% vs. 22%, P < .0001).
“The magnitude of the effect of vitamin E on NASH was comparable to pioglitazone, metformin, and obeticholic acid,” Dr. Kris Kowdley of the Swedish Medical Center, Seattle, said in an interview at the meeting sponsored by the European Association for the Study of the Liver.
The odds ratio (OR) for the resolution of NASH for vitamin E versus no vitamin E was 2.4, (P < .001) and ORs for the other anti-NASH treatments used in the trials were 3.4 for pioglitazone vs. placebo (P = .001), 1.8 for metformin vs. placebo (P = .24), and 1.8 for obeticholic acid vs. placebo (P = .13).
The respective ORs for histologic improvement for vitamin E and the other treatments were 2.9 (P < .0001), 4.1 (P = .0001), 2.7 (P = .02), and 3.1 (P = .0002).
The data combined for the analysis were derived from three controlled studies: PIVENS (Pioglitazone Versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis], TONIC (The Treatment of Nonalcoholic Fatty Liver Disease in Children), and FLINT (Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment Trial).
In the phase III, randomized, placebo-controlled, double-blind PIVENS trial, vitamin E therapy was compared to pioglitazone and placebo in 247 nondiabetic adults (N. Engl. J. Med. 2010; 362:1675-85).
In the TONIC phase III, double-blind, placebo-controlled trial, 173 children with NASH but no diabetes were randomized to treatment with vitamin E, metformin, or placebo for 96 weeks.
In FLINT, a phase II, placebo-controlled, double-blind trial of obeticholic acid, vitamin E was allowed as a concomitant therapy, and 23% of placebo-treated patients took vitamin E at baseline.
Dr. Kowdley noted that natural (d-alpha-tocopherol) not synthetic vitamin E was used in all these trials and the dosage used was 800 IU once daily in the PIVENS trial, 400 IU twice daily in the TONIC trial, and 400-800 IU in the patients who received it in the placebo arm of the FLINT trial.
In total, the pooled analysis considered 347 participants of these trials of whom 155 had been treated with vitamin E and 192 had not.
Histologic improvement was defined as at least a 2-point improvement in NASH with no worsening of fibrosis. NASH resolution was determined by pre- and posttreatment biopsies.
How vitamin E might be exerting its effect is not clear, Dr. Kowdley said. “We know that from the PIVENS trial it does not affect insulin sensitivity, and so presumably it has an antioxidant effect,” he speculated. Combination with other anti-NASH treatments, such as obeticholic acid, could produce synergistic effects perhaps, he acknowledged, but that would require further study.
“Vitamin E appears to be safe in this short-term study,” Dr. Kowdley said. Safety measures included the incidence of cardiac events and changes in lipid levels. However, there was no increased incidence in cardiovascular events with vitamin E use compared to no vitamin E use (6.9% vs. 7.6%, P =.85). There were also no significant differences in net changes in total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, or triglycerides.
Although it was not shown in the late-breaking poster, Dr. Kowdley said that vitamin E appeared to improve NASH in diabetic as well as in nondiabetic subjects with no increased risk of cardiovascular events as other studies have suggested.
“It’s a pooled analysis,” Dr. Kowdley acknowledged, “but we conclude that vitamin E was significantly associated with histologic improvement, reduction in NASH activity score and resolution of NASH, with an effect size that seems in the ballpark of the other therapies.
“So I think this suggests that vitamin E should be our toolkit for treatment of NASH,” he added.
The National Institute of Diabetes and Digestive and Kidney Diseases sponsored the studies. Dr. Kowdley had no relevant conflicts of interest to disclosure.
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: Vitamin E was shown to improve nonalcoholic steatohepatitis (NASH) to an extent similar to those of pioglitazone, metformin, and obeticholic acid.
Major finding: The odds ratio (OR) for the resolution of NASH was 2.4 (P < .001) and the OR for histologic improvement was 2.9 (P < .0001), comparing vitamin E to no vitamin E therapy.
Data source: Pooled analysis of data on vitamin E use in 347 adults and children with NASH from three controlled trials: PIVENS, TONIC, and FLINT.
Disclosures: The National Institute of Diabetes and Digestive and Kidney Diseases sponsored the studies. Dr. Kowdley had no conflicts of interest to disclosure.
Synovial pathotype could help guide anti-TNF therapy
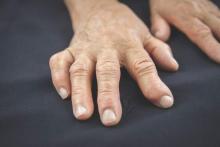
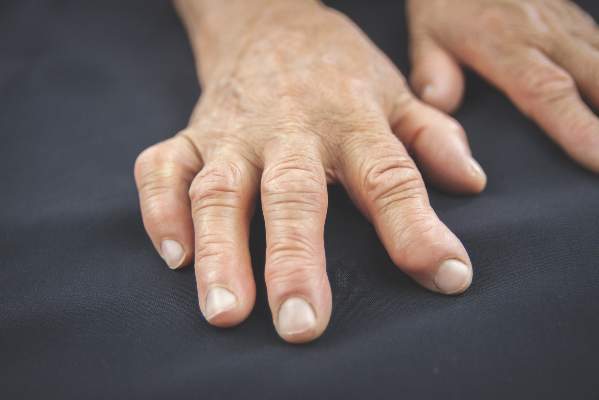
MANCHESTER, U.K. – Analysis of synovial tissue taken by ultrasound-guided biopsy could help stratify patients with rheumatoid arthritis and decide if treatment with a tumor necrosis factor inhibitor is appropriate, according to research presented at the British Society for Rheumatology annual conference.
In a small yet intriguing study, Dr. Maria Di Cicco of Queen Mary University of London and her colleagues found that patients who had a lymphoid synovial pathotype were more likely to respond to treatment with the anti-TNF inhibitor certolizumab pegol than if they had other synovial pathotypes.
“Rheumatoid arthritis is a clinically heterogeneous disease to the extent that we should think of it as a syndrome rather than a disease,” Dr. Di Cicco said. “This heterogeneity is expressed as a variable outcome and response to treatment,” she added.
Although biologic drugs have dramatically changed how patients are treated, 30%-40% of patients do not respond to treatment and the way biologic treatment is selected for RA is more down to trial and error than personalized medicine, Dr. Di Cicco observed. This is concerning, as the number of biologic medicines available is exponentially increasing and ways of matching the right drug to the right patient need to be found.
The aim of the current study was to see if different synovial subtypes could influence patients’ first response to treatment with anti-TNF therapy. Recent research looking at synovial tissue suggests that there are three clear synovial subtypes: myeloid, Pauci-immune, and lymphoid.
The myeloid and Pauci-immune pathotypes are characterized by diffuse cellular infiltration distributed evenly through the synovium. The lymphoid synovial pathotype, on the other hand, is characterized by the presence of large lymphocytic aggregates that are known as ectopic lymphoid structures (ELS). Dr. Di Cicco noted that ELS had been linked to B-cell proliferation within the synovium and to promoting in situ autoantibody production and class switching.
For the study, 28 patients with RA on stable doses of methotrexate who qualified for anti-TNF treatment according to U.K. National Institute for Health and Care Excellence guidance were recruited at a single center. All patients underwent ultrasound-guided biopsy of an active joint, which tended to be a small joint such as the wrist or metacarpophalangeal, before they were treated with certolizumab pegol. Twenty patients had a repeat synovial biopsy when the clinical efficacy of treatment was assessed at 12 weeks.
A minimum of six samples were taken from each biopsied joint, and this material underwent immunohistochemical analysis for the presence of T cells expressing CD3, B cells expressing CD20, macrophages expressing CD68, and plasma cells expressing CD138.
Samples were then classified as being the lymphoid pathotype if they contained mainly B and T cells and formed large lymphocytic aggregates or ELS. Myeloid subtype was defined as the presence of sublining macrophages and Pauci-immune if none of these features was present.
Thirteen (46.4%) patients were found to the have the Pauci-immune pathotype, with the lymphoid pathotype being the next most common (39.3% of patients), and four (14.3%) had the myeloid pathotype.
Apart from no prior oral steroid use in patients with the lymphoid pathotype versus 75% of patients with the myeloid pathotype and 38.5% of patients with the Pauci-immune pathotype, there were no significant differences among the groups in terms of demographics, clinical features, inflammatory markers, antibody or erosive status, or other treatments.
“After 12 weeks of therapy, the myeloid and the lymphoid groups showed a significantly higher fall in [Disease Activity Score] 28 compared to the Pauci-immune group,” Dr. Di Cicco reported.
“We also assessed achievement of EULAR response, and 71% of patients did respond. Half of them were good responders and half of them were moderate responders,” she added.
EULAR response by synovial pathotype was 100% for patients with the lymphoid pathotype, 75% for those with the myeloid pathotype, and 46% for those with the Pauci-immune pathotype.
Greater decreases in synovial thickening and power Doppler ultrasound also were seen in the myeloid and lymphoid pathotypes, compared with the Pauci-immune pathotype, which appeared more resistant to change in response to the anti-TNF therapy.
In the 20 patients who had a repeat biopsy, 9 had ELS at baseline. After treatment, three patients were ELS negative, four remained ELS positive, and two were ungraded.
“Our work shows that the presence of synovial ectopic lymphoid structures defining a lymphoid pathotype is a predictor of clinical response to anti-TNF-alpha,” Dr. Di Cicco said. Although further research and confirmation is needed, “we think that synovial tissue analysis could be considered as a promising tool to stratify RA patients and guide therapeutic decision,” she said.
Dr. Di Cicco had no disclosures.
MANCHESTER, U.K. – Analysis of synovial tissue taken by ultrasound-guided biopsy could help stratify patients with rheumatoid arthritis and decide if treatment with a tumor necrosis factor inhibitor is appropriate, according to research presented at the British Society for Rheumatology annual conference.
In a small yet intriguing study, Dr. Maria Di Cicco of Queen Mary University of London and her colleagues found that patients who had a lymphoid synovial pathotype were more likely to respond to treatment with the anti-TNF inhibitor certolizumab pegol than if they had other synovial pathotypes.
“Rheumatoid arthritis is a clinically heterogeneous disease to the extent that we should think of it as a syndrome rather than a disease,” Dr. Di Cicco said. “This heterogeneity is expressed as a variable outcome and response to treatment,” she added.
Although biologic drugs have dramatically changed how patients are treated, 30%-40% of patients do not respond to treatment and the way biologic treatment is selected for RA is more down to trial and error than personalized medicine, Dr. Di Cicco observed. This is concerning, as the number of biologic medicines available is exponentially increasing and ways of matching the right drug to the right patient need to be found.
The aim of the current study was to see if different synovial subtypes could influence patients’ first response to treatment with anti-TNF therapy. Recent research looking at synovial tissue suggests that there are three clear synovial subtypes: myeloid, Pauci-immune, and lymphoid.
The myeloid and Pauci-immune pathotypes are characterized by diffuse cellular infiltration distributed evenly through the synovium. The lymphoid synovial pathotype, on the other hand, is characterized by the presence of large lymphocytic aggregates that are known as ectopic lymphoid structures (ELS). Dr. Di Cicco noted that ELS had been linked to B-cell proliferation within the synovium and to promoting in situ autoantibody production and class switching.
For the study, 28 patients with RA on stable doses of methotrexate who qualified for anti-TNF treatment according to U.K. National Institute for Health and Care Excellence guidance were recruited at a single center. All patients underwent ultrasound-guided biopsy of an active joint, which tended to be a small joint such as the wrist or metacarpophalangeal, before they were treated with certolizumab pegol. Twenty patients had a repeat synovial biopsy when the clinical efficacy of treatment was assessed at 12 weeks.
A minimum of six samples were taken from each biopsied joint, and this material underwent immunohistochemical analysis for the presence of T cells expressing CD3, B cells expressing CD20, macrophages expressing CD68, and plasma cells expressing CD138.
Samples were then classified as being the lymphoid pathotype if they contained mainly B and T cells and formed large lymphocytic aggregates or ELS. Myeloid subtype was defined as the presence of sublining macrophages and Pauci-immune if none of these features was present.
Thirteen (46.4%) patients were found to the have the Pauci-immune pathotype, with the lymphoid pathotype being the next most common (39.3% of patients), and four (14.3%) had the myeloid pathotype.
Apart from no prior oral steroid use in patients with the lymphoid pathotype versus 75% of patients with the myeloid pathotype and 38.5% of patients with the Pauci-immune pathotype, there were no significant differences among the groups in terms of demographics, clinical features, inflammatory markers, antibody or erosive status, or other treatments.
“After 12 weeks of therapy, the myeloid and the lymphoid groups showed a significantly higher fall in [Disease Activity Score] 28 compared to the Pauci-immune group,” Dr. Di Cicco reported.
“We also assessed achievement of EULAR response, and 71% of patients did respond. Half of them were good responders and half of them were moderate responders,” she added.
EULAR response by synovial pathotype was 100% for patients with the lymphoid pathotype, 75% for those with the myeloid pathotype, and 46% for those with the Pauci-immune pathotype.
Greater decreases in synovial thickening and power Doppler ultrasound also were seen in the myeloid and lymphoid pathotypes, compared with the Pauci-immune pathotype, which appeared more resistant to change in response to the anti-TNF therapy.
In the 20 patients who had a repeat biopsy, 9 had ELS at baseline. After treatment, three patients were ELS negative, four remained ELS positive, and two were ungraded.
“Our work shows that the presence of synovial ectopic lymphoid structures defining a lymphoid pathotype is a predictor of clinical response to anti-TNF-alpha,” Dr. Di Cicco said. Although further research and confirmation is needed, “we think that synovial tissue analysis could be considered as a promising tool to stratify RA patients and guide therapeutic decision,” she said.
Dr. Di Cicco had no disclosures.
MANCHESTER, U.K. – Analysis of synovial tissue taken by ultrasound-guided biopsy could help stratify patients with rheumatoid arthritis and decide if treatment with a tumor necrosis factor inhibitor is appropriate, according to research presented at the British Society for Rheumatology annual conference.
In a small yet intriguing study, Dr. Maria Di Cicco of Queen Mary University of London and her colleagues found that patients who had a lymphoid synovial pathotype were more likely to respond to treatment with the anti-TNF inhibitor certolizumab pegol than if they had other synovial pathotypes.
“Rheumatoid arthritis is a clinically heterogeneous disease to the extent that we should think of it as a syndrome rather than a disease,” Dr. Di Cicco said. “This heterogeneity is expressed as a variable outcome and response to treatment,” she added.
Although biologic drugs have dramatically changed how patients are treated, 30%-40% of patients do not respond to treatment and the way biologic treatment is selected for RA is more down to trial and error than personalized medicine, Dr. Di Cicco observed. This is concerning, as the number of biologic medicines available is exponentially increasing and ways of matching the right drug to the right patient need to be found.
The aim of the current study was to see if different synovial subtypes could influence patients’ first response to treatment with anti-TNF therapy. Recent research looking at synovial tissue suggests that there are three clear synovial subtypes: myeloid, Pauci-immune, and lymphoid.
The myeloid and Pauci-immune pathotypes are characterized by diffuse cellular infiltration distributed evenly through the synovium. The lymphoid synovial pathotype, on the other hand, is characterized by the presence of large lymphocytic aggregates that are known as ectopic lymphoid structures (ELS). Dr. Di Cicco noted that ELS had been linked to B-cell proliferation within the synovium and to promoting in situ autoantibody production and class switching.
For the study, 28 patients with RA on stable doses of methotrexate who qualified for anti-TNF treatment according to U.K. National Institute for Health and Care Excellence guidance were recruited at a single center. All patients underwent ultrasound-guided biopsy of an active joint, which tended to be a small joint such as the wrist or metacarpophalangeal, before they were treated with certolizumab pegol. Twenty patients had a repeat synovial biopsy when the clinical efficacy of treatment was assessed at 12 weeks.
A minimum of six samples were taken from each biopsied joint, and this material underwent immunohistochemical analysis for the presence of T cells expressing CD3, B cells expressing CD20, macrophages expressing CD68, and plasma cells expressing CD138.
Samples were then classified as being the lymphoid pathotype if they contained mainly B and T cells and formed large lymphocytic aggregates or ELS. Myeloid subtype was defined as the presence of sublining macrophages and Pauci-immune if none of these features was present.
Thirteen (46.4%) patients were found to the have the Pauci-immune pathotype, with the lymphoid pathotype being the next most common (39.3% of patients), and four (14.3%) had the myeloid pathotype.
Apart from no prior oral steroid use in patients with the lymphoid pathotype versus 75% of patients with the myeloid pathotype and 38.5% of patients with the Pauci-immune pathotype, there were no significant differences among the groups in terms of demographics, clinical features, inflammatory markers, antibody or erosive status, or other treatments.
“After 12 weeks of therapy, the myeloid and the lymphoid groups showed a significantly higher fall in [Disease Activity Score] 28 compared to the Pauci-immune group,” Dr. Di Cicco reported.
“We also assessed achievement of EULAR response, and 71% of patients did respond. Half of them were good responders and half of them were moderate responders,” she added.
EULAR response by synovial pathotype was 100% for patients with the lymphoid pathotype, 75% for those with the myeloid pathotype, and 46% for those with the Pauci-immune pathotype.
Greater decreases in synovial thickening and power Doppler ultrasound also were seen in the myeloid and lymphoid pathotypes, compared with the Pauci-immune pathotype, which appeared more resistant to change in response to the anti-TNF therapy.
In the 20 patients who had a repeat biopsy, 9 had ELS at baseline. After treatment, three patients were ELS negative, four remained ELS positive, and two were ungraded.
“Our work shows that the presence of synovial ectopic lymphoid structures defining a lymphoid pathotype is a predictor of clinical response to anti-TNF-alpha,” Dr. Di Cicco said. Although further research and confirmation is needed, “we think that synovial tissue analysis could be considered as a promising tool to stratify RA patients and guide therapeutic decision,” she said.
Dr. Di Cicco had no disclosures.
AT RHEUMATOLOGY 2015
Key clinical point: Early data suggest that synovial tissue analysis may be a potential tool to guide anti-TNF therapy, although more data are needed.
Major finding: EULAR response by synovial pathotype was 100% for patients with the lymphoid pathotype, 75% for those with the myeloid pathotype, and 46% for those with the Pauci-immune pathotype.
Data source: 28 patients with rheumatoid arthritis needing first anti-TNF treatment.
Disclosures: Dr. Di Cicco had no disclosures.
BSR: Patient selection key to using rituximab in lupus
MANCHESTER, U.K. – Although around half of patients with refractory systemic lupus erythematous (SLE) benefit from rituximab treatment at 6 months, there is a subgroup of patients that appears to do worse, according to research presented at the British Society for Rheumatology annual conference.
Data from the British Isles Lupus Assessment Group Biologic Registry (BILAG BR) showed that 58.75% of patients showed an improvement in disease activity measured using the BILAG 2004 Index System at 3 months and 48.75% showed improvement at 6 months.
Persistent disease, however, remained in 20% of patients at 3 and 6 months, and deteriorating disease occurred in 15% at 3 months and 22.5% at 6 months.
“A similar pattern was seen with the SLEDAI-2K response, although a higher percentage of patients seemed to improve with this measure,” said BILAG BR study coordinator Emily Sutton of the Centre for Musculoskeletal Research at the University of Manchester, England, where the registry is based.
Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) responses at 3 and 6 months showed around 70% of patients had improved at both time points, 16%-18% had persistent disease, and 11%-13% had worsening disease.
BILAG BR is a UK-based, multicenter, prospective, observational study of patients with SLE who are refractory to standard immunosuppressive therapy and are starting treatment with a biologic drug or a conventional, nonbiologic therapy.
The registry was set up in 2010 with the aim of recruiting 220 patients into the biologic cohort and 220 patients into the conventional, nonbiologic therapy cohort to enable monitoring of the real-world safety and effectiveness of biologics, compared with standard immunosuppressive therapies in the treatment of SLE.
Prior data on rituximab therapy in SLE were inconclusive, Dr. Sutton noted, with two clinical trials – EXPLORER (Arthritis Rheum. 2010;62:222-33) and LUNAR (Arthritis Rheum. 2012;64:1215-26) failing to hit their primary endpoints and show a clear benefit for rituximab.
Nevertheless, data from an open-label, single-center study of 50 patients treated with rituximab show that around 42% of patients achieved remission at 6 months (Arthritis Rheum. 2009;61:482-7).
The current analysis of BILAG BR data looked at the response to rituximab at 3 and 6 months in a total of 80 patients. Most (92.5%) of the study subjects were female, with a median age at diagnosis of 29.4 years and a median age at enrollment into the registry of 39.5 years. Approximately 60% were white, 13.7% were of South Asian descent, 13.7% of African ancestry, and the remainder was of mixed ethnicity.
The BILAG 2004 Index System scores disease activity across nine organ systems, and Dr. Sutton noted that all organ systems were involved at baseline, but the most common systems involved were the mucocutaneous (41% of patients), renal (35%), and musculoskeletal (31%) systems.
“When we looked at the disease activity at 6 months by BILAG organ system, there were a number of new flares in systems not previously involved,” Dr. Sutton reported.
“SLE patients demonstrated a variable response to rituximab, not just in the organ system involved, but also in the magnitude of response and the duration of remission,” she said.
“It is, therefore, essential that we work towards a position where we are able to select those patients who are most likely to respond well to treatment,” Dr. Sutton observed.
Patients continue to be enrolled into the registry, and future work will look at whether there are any factors predictive of a response to rituximab and try to stratify patients for B-cell depletion therapy, she added.
BILAG BR is supported by unconditional educational grants from Roche and GlaxoSmithKline. Dr. Sutton has received research funding from Roche and GlaxoSmithKline.
MANCHESTER, U.K. – Although around half of patients with refractory systemic lupus erythematous (SLE) benefit from rituximab treatment at 6 months, there is a subgroup of patients that appears to do worse, according to research presented at the British Society for Rheumatology annual conference.
Data from the British Isles Lupus Assessment Group Biologic Registry (BILAG BR) showed that 58.75% of patients showed an improvement in disease activity measured using the BILAG 2004 Index System at 3 months and 48.75% showed improvement at 6 months.
Persistent disease, however, remained in 20% of patients at 3 and 6 months, and deteriorating disease occurred in 15% at 3 months and 22.5% at 6 months.
“A similar pattern was seen with the SLEDAI-2K response, although a higher percentage of patients seemed to improve with this measure,” said BILAG BR study coordinator Emily Sutton of the Centre for Musculoskeletal Research at the University of Manchester, England, where the registry is based.
Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) responses at 3 and 6 months showed around 70% of patients had improved at both time points, 16%-18% had persistent disease, and 11%-13% had worsening disease.
BILAG BR is a UK-based, multicenter, prospective, observational study of patients with SLE who are refractory to standard immunosuppressive therapy and are starting treatment with a biologic drug or a conventional, nonbiologic therapy.
The registry was set up in 2010 with the aim of recruiting 220 patients into the biologic cohort and 220 patients into the conventional, nonbiologic therapy cohort to enable monitoring of the real-world safety and effectiveness of biologics, compared with standard immunosuppressive therapies in the treatment of SLE.
Prior data on rituximab therapy in SLE were inconclusive, Dr. Sutton noted, with two clinical trials – EXPLORER (Arthritis Rheum. 2010;62:222-33) and LUNAR (Arthritis Rheum. 2012;64:1215-26) failing to hit their primary endpoints and show a clear benefit for rituximab.
Nevertheless, data from an open-label, single-center study of 50 patients treated with rituximab show that around 42% of patients achieved remission at 6 months (Arthritis Rheum. 2009;61:482-7).
The current analysis of BILAG BR data looked at the response to rituximab at 3 and 6 months in a total of 80 patients. Most (92.5%) of the study subjects were female, with a median age at diagnosis of 29.4 years and a median age at enrollment into the registry of 39.5 years. Approximately 60% were white, 13.7% were of South Asian descent, 13.7% of African ancestry, and the remainder was of mixed ethnicity.
The BILAG 2004 Index System scores disease activity across nine organ systems, and Dr. Sutton noted that all organ systems were involved at baseline, but the most common systems involved were the mucocutaneous (41% of patients), renal (35%), and musculoskeletal (31%) systems.
“When we looked at the disease activity at 6 months by BILAG organ system, there were a number of new flares in systems not previously involved,” Dr. Sutton reported.
“SLE patients demonstrated a variable response to rituximab, not just in the organ system involved, but also in the magnitude of response and the duration of remission,” she said.
“It is, therefore, essential that we work towards a position where we are able to select those patients who are most likely to respond well to treatment,” Dr. Sutton observed.
Patients continue to be enrolled into the registry, and future work will look at whether there are any factors predictive of a response to rituximab and try to stratify patients for B-cell depletion therapy, she added.
BILAG BR is supported by unconditional educational grants from Roche and GlaxoSmithKline. Dr. Sutton has received research funding from Roche and GlaxoSmithKline.
MANCHESTER, U.K. – Although around half of patients with refractory systemic lupus erythematous (SLE) benefit from rituximab treatment at 6 months, there is a subgroup of patients that appears to do worse, according to research presented at the British Society for Rheumatology annual conference.
Data from the British Isles Lupus Assessment Group Biologic Registry (BILAG BR) showed that 58.75% of patients showed an improvement in disease activity measured using the BILAG 2004 Index System at 3 months and 48.75% showed improvement at 6 months.
Persistent disease, however, remained in 20% of patients at 3 and 6 months, and deteriorating disease occurred in 15% at 3 months and 22.5% at 6 months.
“A similar pattern was seen with the SLEDAI-2K response, although a higher percentage of patients seemed to improve with this measure,” said BILAG BR study coordinator Emily Sutton of the Centre for Musculoskeletal Research at the University of Manchester, England, where the registry is based.
Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) responses at 3 and 6 months showed around 70% of patients had improved at both time points, 16%-18% had persistent disease, and 11%-13% had worsening disease.
BILAG BR is a UK-based, multicenter, prospective, observational study of patients with SLE who are refractory to standard immunosuppressive therapy and are starting treatment with a biologic drug or a conventional, nonbiologic therapy.
The registry was set up in 2010 with the aim of recruiting 220 patients into the biologic cohort and 220 patients into the conventional, nonbiologic therapy cohort to enable monitoring of the real-world safety and effectiveness of biologics, compared with standard immunosuppressive therapies in the treatment of SLE.
Prior data on rituximab therapy in SLE were inconclusive, Dr. Sutton noted, with two clinical trials – EXPLORER (Arthritis Rheum. 2010;62:222-33) and LUNAR (Arthritis Rheum. 2012;64:1215-26) failing to hit their primary endpoints and show a clear benefit for rituximab.
Nevertheless, data from an open-label, single-center study of 50 patients treated with rituximab show that around 42% of patients achieved remission at 6 months (Arthritis Rheum. 2009;61:482-7).
The current analysis of BILAG BR data looked at the response to rituximab at 3 and 6 months in a total of 80 patients. Most (92.5%) of the study subjects were female, with a median age at diagnosis of 29.4 years and a median age at enrollment into the registry of 39.5 years. Approximately 60% were white, 13.7% were of South Asian descent, 13.7% of African ancestry, and the remainder was of mixed ethnicity.
The BILAG 2004 Index System scores disease activity across nine organ systems, and Dr. Sutton noted that all organ systems were involved at baseline, but the most common systems involved were the mucocutaneous (41% of patients), renal (35%), and musculoskeletal (31%) systems.
“When we looked at the disease activity at 6 months by BILAG organ system, there were a number of new flares in systems not previously involved,” Dr. Sutton reported.
“SLE patients demonstrated a variable response to rituximab, not just in the organ system involved, but also in the magnitude of response and the duration of remission,” she said.
“It is, therefore, essential that we work towards a position where we are able to select those patients who are most likely to respond well to treatment,” Dr. Sutton observed.
Patients continue to be enrolled into the registry, and future work will look at whether there are any factors predictive of a response to rituximab and try to stratify patients for B-cell depletion therapy, she added.
BILAG BR is supported by unconditional educational grants from Roche and GlaxoSmithKline. Dr. Sutton has received research funding from Roche and GlaxoSmithKline.
AT RHEUMATOLOGY 2015
Key clinical point: Although the majority of patients benefited from rituximab, a significant proportion did not, highlighting the need to identify predictive factors to help stratify patients suitable for this therapy.
Major finding: At 6 months, around 50% of patients had improved, 20% had persistent disease, and 22.5% had deteriorating disease using the BILAG 2004 System Index.
Data source: The BILAG BR is a prospective, observational cohort study of patients with systemic lupus erythematosus who are starting treatment with a biologic drug or a conventional, nonbiologic therapy.
Disclosures: The BILAG BR is supported by unconditional educational grants from Roche and GlaxoSmithKline. Dr. Sutton has received research funding from Roche and GlaxoSmithKline.
BSR: Lengthy SLE remission is possible, late relapses occur
MANCHESTER – Complete remissions of at least 3 years were achieved by nearly 15% of patients with systemic lupus erythematous, based on a retrospective analysis of an observational, single-center cohort of 624 patients treated over a 32-year span.
Nearly 5% of the patients achieved complete remissions of at least 10 years.
“Systemic lupus erythematosus is an unpredictable disease with many periods of exacerbations and remissions, but patients can achieve complete remission,” said Dr. Carmen Medina-Quiñones, of Ramón y Cajal Hospital in Madrid, at the British Society for Rheumatology annual conference.
Nevertheless, a patient with SLE can relapse after many years of remission, she noted during a poster tour.
Indeed, SLE flared in 22% of patients who had achieved complete remission, and 3 of these 15 patients relapsed more than a decade later. One patient relapsed after 12 years, another after 16 years, and the remaining patient after 18 years.
The findings indicate that all SLE patients need to be monitored and that long-term strategies are needed to control lupus activity, Dr. Medina-Quiñones said.
The analysis consisted of patients enrolled for a minimum period of 3 years in the SLE cohort at University College Hospital, London, from January 1978 to December 2010. The main aim was to determine how many patients in the cohort were able to achieve a complete remission over the follow-up period of 32 years.
Complete remission was defined as achieving BILAG (British Isles Lupus Assessment Group) scores of C, D, or E signifying the absence of clinical features and having normal laboratory findings, including normal complement levels. The definition also required that patients were off treatment for at least 3 years, meaning no corticosteroid or immunosuppressive therapies, although antimalarial and nonsteroidal anti-inflammatory drugs were allowed.
It was found that 45 (8.5%) achieved clinical but not serologic remission, and 66 (12.4%) patients achieved only serologic remission. “Interestingly, 29 patients achieved complete remission even without any antimalarial or nonsteroidal treatment,” Dr. Medina-Quiñones said.
The researchers also found that remissions were less likely in patients who had renal (P < .001), neurologic (P = .002), or cardiopulmonary involvement (P < .001).
Dr. Anisur Rahman of University College London who moderated the poster tour commented: “Many patients ask, ‘Will I ever be free of this disease?’ and ‘Will I ever not have to take any medication?’ The message here seems to be that it is quite unlikely for people with lupus.”
Dr. Medina-Quiñones commented: “Nowadays, we have a lot of new technologies and we have had a lot of success with different kinds of treatment, so it is likely that patients’ quality of life will improve and that they will have more chances to achieve remission.”
Dr. Medina-Quiñones has no relevant financial disclosures.
MANCHESTER – Complete remissions of at least 3 years were achieved by nearly 15% of patients with systemic lupus erythematous, based on a retrospective analysis of an observational, single-center cohort of 624 patients treated over a 32-year span.
Nearly 5% of the patients achieved complete remissions of at least 10 years.
“Systemic lupus erythematosus is an unpredictable disease with many periods of exacerbations and remissions, but patients can achieve complete remission,” said Dr. Carmen Medina-Quiñones, of Ramón y Cajal Hospital in Madrid, at the British Society for Rheumatology annual conference.
Nevertheless, a patient with SLE can relapse after many years of remission, she noted during a poster tour.
Indeed, SLE flared in 22% of patients who had achieved complete remission, and 3 of these 15 patients relapsed more than a decade later. One patient relapsed after 12 years, another after 16 years, and the remaining patient after 18 years.
The findings indicate that all SLE patients need to be monitored and that long-term strategies are needed to control lupus activity, Dr. Medina-Quiñones said.
The analysis consisted of patients enrolled for a minimum period of 3 years in the SLE cohort at University College Hospital, London, from January 1978 to December 2010. The main aim was to determine how many patients in the cohort were able to achieve a complete remission over the follow-up period of 32 years.
Complete remission was defined as achieving BILAG (British Isles Lupus Assessment Group) scores of C, D, or E signifying the absence of clinical features and having normal laboratory findings, including normal complement levels. The definition also required that patients were off treatment for at least 3 years, meaning no corticosteroid or immunosuppressive therapies, although antimalarial and nonsteroidal anti-inflammatory drugs were allowed.
It was found that 45 (8.5%) achieved clinical but not serologic remission, and 66 (12.4%) patients achieved only serologic remission. “Interestingly, 29 patients achieved complete remission even without any antimalarial or nonsteroidal treatment,” Dr. Medina-Quiñones said.
The researchers also found that remissions were less likely in patients who had renal (P < .001), neurologic (P = .002), or cardiopulmonary involvement (P < .001).
Dr. Anisur Rahman of University College London who moderated the poster tour commented: “Many patients ask, ‘Will I ever be free of this disease?’ and ‘Will I ever not have to take any medication?’ The message here seems to be that it is quite unlikely for people with lupus.”
Dr. Medina-Quiñones commented: “Nowadays, we have a lot of new technologies and we have had a lot of success with different kinds of treatment, so it is likely that patients’ quality of life will improve and that they will have more chances to achieve remission.”
Dr. Medina-Quiñones has no relevant financial disclosures.
MANCHESTER – Complete remissions of at least 3 years were achieved by nearly 15% of patients with systemic lupus erythematous, based on a retrospective analysis of an observational, single-center cohort of 624 patients treated over a 32-year span.
Nearly 5% of the patients achieved complete remissions of at least 10 years.
“Systemic lupus erythematosus is an unpredictable disease with many periods of exacerbations and remissions, but patients can achieve complete remission,” said Dr. Carmen Medina-Quiñones, of Ramón y Cajal Hospital in Madrid, at the British Society for Rheumatology annual conference.
Nevertheless, a patient with SLE can relapse after many years of remission, she noted during a poster tour.
Indeed, SLE flared in 22% of patients who had achieved complete remission, and 3 of these 15 patients relapsed more than a decade later. One patient relapsed after 12 years, another after 16 years, and the remaining patient after 18 years.
The findings indicate that all SLE patients need to be monitored and that long-term strategies are needed to control lupus activity, Dr. Medina-Quiñones said.
The analysis consisted of patients enrolled for a minimum period of 3 years in the SLE cohort at University College Hospital, London, from January 1978 to December 2010. The main aim was to determine how many patients in the cohort were able to achieve a complete remission over the follow-up period of 32 years.
Complete remission was defined as achieving BILAG (British Isles Lupus Assessment Group) scores of C, D, or E signifying the absence of clinical features and having normal laboratory findings, including normal complement levels. The definition also required that patients were off treatment for at least 3 years, meaning no corticosteroid or immunosuppressive therapies, although antimalarial and nonsteroidal anti-inflammatory drugs were allowed.
It was found that 45 (8.5%) achieved clinical but not serologic remission, and 66 (12.4%) patients achieved only serologic remission. “Interestingly, 29 patients achieved complete remission even without any antimalarial or nonsteroidal treatment,” Dr. Medina-Quiñones said.
The researchers also found that remissions were less likely in patients who had renal (P < .001), neurologic (P = .002), or cardiopulmonary involvement (P < .001).
Dr. Anisur Rahman of University College London who moderated the poster tour commented: “Many patients ask, ‘Will I ever be free of this disease?’ and ‘Will I ever not have to take any medication?’ The message here seems to be that it is quite unlikely for people with lupus.”
Dr. Medina-Quiñones commented: “Nowadays, we have a lot of new technologies and we have had a lot of success with different kinds of treatment, so it is likely that patients’ quality of life will improve and that they will have more chances to achieve remission.”
Dr. Medina-Quiñones has no relevant financial disclosures.
AT RHEUMATOLOGY 2015
Key clinical point: Patients with systemic lupus erythematosus can achieve complete remission for many years, but relapse can occur decades later, so long-term follow-up and monitoring are required.
Major finding: Complete remission was achieved by 15% of patients for at least 3 years and by 5% of patients for at least 10 years.
Data source: A 32-year retrospective analysis of an observational, single-center cohort of 624 patients with SLE treated for a minimum period of 3 years.
Disclosures: Dr. Medina-Quiñones has no relevant financial disclosures.
HCV Treatment Delays Dented Efficacy in Era Before All-oral Therapy
VIENNA – Delaying hepatitis C virus (HVC) treatment can have a detrimental effect on the outcomes of some patients, according to the results of a large 10-year retrospective analysis involving more than 187,000 U.S. veterans.
Indeed, waiting until a patient with HCV infection develops advanced cirrhosis, as measured by the Fibrosis-4 (FIB4) index, can reduce the chances that treatments will be effective and significantly increases morbidity and mortality. Health economist Jeffrey McCombs, Ph.D., of the University of Southern California, Los Angeles, presented data from a 10-year retrospective analysis of the Department of Veteran’s Affairs Veterans Health Administration HCV clinical registry involving more than 187,000 U.S. veterans with detectable HCV levels.
The caveat is that older treatments rather than newer all-oral direct-acting agents were used in the study population at the time of data analyses.
The analysis built on three previous analyses of data from the VA System, the first of which found that viral load suppression reduced the risk of liver events by around 27% and death by 45% (JAMA Intern. Med. 2014;174: 204-12). This analysis also found that relative to patients with genotype 1 (GT1), patients with GT2 were at lower risk and patients with GT3 were at higher risk of liver events and death.
The second analysis looked at treatment timing and found that treatment effectiveness was significantly reduced if treatment was started after common laboratory tests to detect liver changes became abnormal.
The third analysis looked at using the FIB4 index to monitor the risk of long-term morbidity and mortality and found that a FIB4 in excess of 3.4 was associated with a significantly increased risk for liver events and death, whereas a FIB4 of 1.45 was associated with moderate risk.
“This brought us to the last research question, looking at the relationship between treatment efficacy and exceeding certain FIB4 levels,” Dr. McCombs said at the meeting sponsored by the European Association for the Study of the Liver. The analysis compared treating early versus treating late, based on FIB4 levels – FIB4 of 1.0, FIB4 of 1.45, and FIB4 of 3.25.
Initiating treatment early before patients’ FIB4 exceeded 1.0 was associated with a 41% reduction in morbidity and a 36% reduction in mortality. Waiting until the FIB4 exceeded 1.0 resulted in a 30% reduction in liver events and 40% in mortality.
Treating patients early before exceeding a FIB4 of 1.45 was associated with morbidity and mortality reductions of 39% and 40% and waiting until the FIB4 exceeded this threshold was associated with reductions of 27% and 39%, respectively.
Using the FIB4 threshold of 3.25 showed even smaller reductions in morbidity and mortality. When treating early, the reductions were 36% and 45%; when treating late, the reductions were 11% and 25%.
“This is the kind of information you need when you talk with your payers,” Dr. McCombs said. “If you restrict access to treatment, you are not going to be as effective.”
Treating early or late appeared to have a similar effect on viral load and the number needed to treat (NNT) increased using the FIB4 3.5 threshold. Indeed, if treatment is initiated before the FIB4 rises above 3.25, the NNT is 180 patients to prevent one death, Dr. McCombs said, but if treatment is delayed, then the NNT jumps to 325 patients.
In patients with low FIB4 a ‘watch and wait strategy’ could potentially be feasible, he acknowledged in an interview. “The bottom line is that we can’t afford to treat everybody now,” he said.
“The FIB4 is a parameter we can probably watch, but it is unknown exactly how long we can wait,” Dr. McCombs said.
The data were derived from a study that was supported by a grant from Bristol-Myers Squibb. Dr. McCombs had no disclosures.
VIENNA – Delaying hepatitis C virus (HVC) treatment can have a detrimental effect on the outcomes of some patients, according to the results of a large 10-year retrospective analysis involving more than 187,000 U.S. veterans.
Indeed, waiting until a patient with HCV infection develops advanced cirrhosis, as measured by the Fibrosis-4 (FIB4) index, can reduce the chances that treatments will be effective and significantly increases morbidity and mortality. Health economist Jeffrey McCombs, Ph.D., of the University of Southern California, Los Angeles, presented data from a 10-year retrospective analysis of the Department of Veteran’s Affairs Veterans Health Administration HCV clinical registry involving more than 187,000 U.S. veterans with detectable HCV levels.
The caveat is that older treatments rather than newer all-oral direct-acting agents were used in the study population at the time of data analyses.
The analysis built on three previous analyses of data from the VA System, the first of which found that viral load suppression reduced the risk of liver events by around 27% and death by 45% (JAMA Intern. Med. 2014;174: 204-12). This analysis also found that relative to patients with genotype 1 (GT1), patients with GT2 were at lower risk and patients with GT3 were at higher risk of liver events and death.
The second analysis looked at treatment timing and found that treatment effectiveness was significantly reduced if treatment was started after common laboratory tests to detect liver changes became abnormal.
The third analysis looked at using the FIB4 index to monitor the risk of long-term morbidity and mortality and found that a FIB4 in excess of 3.4 was associated with a significantly increased risk for liver events and death, whereas a FIB4 of 1.45 was associated with moderate risk.
“This brought us to the last research question, looking at the relationship between treatment efficacy and exceeding certain FIB4 levels,” Dr. McCombs said at the meeting sponsored by the European Association for the Study of the Liver. The analysis compared treating early versus treating late, based on FIB4 levels – FIB4 of 1.0, FIB4 of 1.45, and FIB4 of 3.25.
Initiating treatment early before patients’ FIB4 exceeded 1.0 was associated with a 41% reduction in morbidity and a 36% reduction in mortality. Waiting until the FIB4 exceeded 1.0 resulted in a 30% reduction in liver events and 40% in mortality.
Treating patients early before exceeding a FIB4 of 1.45 was associated with morbidity and mortality reductions of 39% and 40% and waiting until the FIB4 exceeded this threshold was associated with reductions of 27% and 39%, respectively.
Using the FIB4 threshold of 3.25 showed even smaller reductions in morbidity and mortality. When treating early, the reductions were 36% and 45%; when treating late, the reductions were 11% and 25%.
“This is the kind of information you need when you talk with your payers,” Dr. McCombs said. “If you restrict access to treatment, you are not going to be as effective.”
Treating early or late appeared to have a similar effect on viral load and the number needed to treat (NNT) increased using the FIB4 3.5 threshold. Indeed, if treatment is initiated before the FIB4 rises above 3.25, the NNT is 180 patients to prevent one death, Dr. McCombs said, but if treatment is delayed, then the NNT jumps to 325 patients.
In patients with low FIB4 a ‘watch and wait strategy’ could potentially be feasible, he acknowledged in an interview. “The bottom line is that we can’t afford to treat everybody now,” he said.
“The FIB4 is a parameter we can probably watch, but it is unknown exactly how long we can wait,” Dr. McCombs said.
The data were derived from a study that was supported by a grant from Bristol-Myers Squibb. Dr. McCombs had no disclosures.
VIENNA – Delaying hepatitis C virus (HVC) treatment can have a detrimental effect on the outcomes of some patients, according to the results of a large 10-year retrospective analysis involving more than 187,000 U.S. veterans.
Indeed, waiting until a patient with HCV infection develops advanced cirrhosis, as measured by the Fibrosis-4 (FIB4) index, can reduce the chances that treatments will be effective and significantly increases morbidity and mortality. Health economist Jeffrey McCombs, Ph.D., of the University of Southern California, Los Angeles, presented data from a 10-year retrospective analysis of the Department of Veteran’s Affairs Veterans Health Administration HCV clinical registry involving more than 187,000 U.S. veterans with detectable HCV levels.
The caveat is that older treatments rather than newer all-oral direct-acting agents were used in the study population at the time of data analyses.
The analysis built on three previous analyses of data from the VA System, the first of which found that viral load suppression reduced the risk of liver events by around 27% and death by 45% (JAMA Intern. Med. 2014;174: 204-12). This analysis also found that relative to patients with genotype 1 (GT1), patients with GT2 were at lower risk and patients with GT3 were at higher risk of liver events and death.
The second analysis looked at treatment timing and found that treatment effectiveness was significantly reduced if treatment was started after common laboratory tests to detect liver changes became abnormal.
The third analysis looked at using the FIB4 index to monitor the risk of long-term morbidity and mortality and found that a FIB4 in excess of 3.4 was associated with a significantly increased risk for liver events and death, whereas a FIB4 of 1.45 was associated with moderate risk.
“This brought us to the last research question, looking at the relationship between treatment efficacy and exceeding certain FIB4 levels,” Dr. McCombs said at the meeting sponsored by the European Association for the Study of the Liver. The analysis compared treating early versus treating late, based on FIB4 levels – FIB4 of 1.0, FIB4 of 1.45, and FIB4 of 3.25.
Initiating treatment early before patients’ FIB4 exceeded 1.0 was associated with a 41% reduction in morbidity and a 36% reduction in mortality. Waiting until the FIB4 exceeded 1.0 resulted in a 30% reduction in liver events and 40% in mortality.
Treating patients early before exceeding a FIB4 of 1.45 was associated with morbidity and mortality reductions of 39% and 40% and waiting until the FIB4 exceeded this threshold was associated with reductions of 27% and 39%, respectively.
Using the FIB4 threshold of 3.25 showed even smaller reductions in morbidity and mortality. When treating early, the reductions were 36% and 45%; when treating late, the reductions were 11% and 25%.
“This is the kind of information you need when you talk with your payers,” Dr. McCombs said. “If you restrict access to treatment, you are not going to be as effective.”
Treating early or late appeared to have a similar effect on viral load and the number needed to treat (NNT) increased using the FIB4 3.5 threshold. Indeed, if treatment is initiated before the FIB4 rises above 3.25, the NNT is 180 patients to prevent one death, Dr. McCombs said, but if treatment is delayed, then the NNT jumps to 325 patients.
In patients with low FIB4 a ‘watch and wait strategy’ could potentially be feasible, he acknowledged in an interview. “The bottom line is that we can’t afford to treat everybody now,” he said.
“The FIB4 is a parameter we can probably watch, but it is unknown exactly how long we can wait,” Dr. McCombs said.
The data were derived from a study that was supported by a grant from Bristol-Myers Squibb. Dr. McCombs had no disclosures.
AT THE INTERNATIONAL LIVER CONGRESS 2015
ILC: HCV treatment delays dented efficacy in era before all-oral therapy
VIENNA – Delaying hepatitis C virus (HVC) treatment can have a detrimental effect on the outcomes of some patients, according to the results of a large 10-year retrospective analysis involving more than 187,000 U.S. veterans.
Indeed, waiting until a patient with HCV infection develops advanced cirrhosis, as measured by the Fibrosis-4 (FIB4) index, can reduce the chances that treatments will be effective and significantly increases morbidity and mortality. Health economist Jeffrey McCombs, Ph.D., of the University of Southern California, Los Angeles, presented data from a 10-year retrospective analysis of the Department of Veteran’s Affairs Veterans Health Administration HCV clinical registry involving more than 187,000 U.S. veterans with detectable HCV levels.
The caveat is that older treatments rather than newer all-oral direct-acting agents were used in the study population at the time of data analyses.
The analysis built on three previous analyses of data from the VA System, the first of which found that viral load suppression reduced the risk of liver events by around 27% and death by 45% (JAMA Intern. Med. 2014;174: 204-12). This analysis also found that relative to patients with genotype 1 (GT1), patients with GT2 were at lower risk and patients with GT3 were at higher risk of liver events and death.
The second analysis looked at treatment timing and found that treatment effectiveness was significantly reduced if treatment was started after common laboratory tests to detect liver changes became abnormal.
The third analysis looked at using the FIB4 index to monitor the risk of long-term morbidity and mortality and found that a FIB4 in excess of 3.4 was associated with a significantly increased risk for liver events and death, whereas a FIB4 of 1.45 was associated with moderate risk.
“This brought us to the last research question, looking at the relationship between treatment efficacy and exceeding certain FIB4 levels,” Dr. McCombs said at the meeting sponsored by the European Association for the Study of the Liver. The analysis compared treating early versus treating late, based on FIB4 levels – FIB4 of 1.0, FIB4 of 1.45, and FIB4 of 3.25.
Initiating treatment early before patients’ FIB4 exceeded 1.0 was associated with a 41% reduction in morbidity and a 36% reduction in mortality. Waiting until the FIB4 exceeded 1.0 resulted in a 30% reduction in liver events and 40% in mortality.
Treating patients early before exceeding a FIB4 of 1.45 was associated with morbidity and mortality reductions of 39% and 40% and waiting until the FIB4 exceeded this threshold was associated with reductions of 27% and 39%, respectively.
Using the FIB4 threshold of 3.25 showed even smaller reductions in morbidity and mortality. When treating early, the reductions were 36% and 45%; when treating late, the reductions were 11% and 25%.
“This is the kind of information you need when you talk with your payers,” Dr. McCombs said. “If you restrict access to treatment, you are not going to be as effective.”
Treating early or late appeared to have a similar effect on viral load and the number needed to treat (NNT) increased using the FIB4 3.5 threshold. Indeed, if treatment is initiated before the FIB4 rises above 3.25, the NNT is 180 patients to prevent one death, Dr. McCombs said, but if treatment is delayed, then the NNT jumps to 325 patients.
In patients with low FIB4 a ‘watch and wait strategy’ could potentially be feasible, he acknowledged in an interview. “The bottom line is that we can’t afford to treat everybody now,” he said.
“The FIB4 is a parameter we can probably watch, but it is unknown exactly how long we can wait,” Dr. McCombs said.
The data were derived from a study that was supported by a grant from Bristol-Myers Squibb. Dr. McCombs had no disclosures.
VIENNA – Delaying hepatitis C virus (HVC) treatment can have a detrimental effect on the outcomes of some patients, according to the results of a large 10-year retrospective analysis involving more than 187,000 U.S. veterans.
Indeed, waiting until a patient with HCV infection develops advanced cirrhosis, as measured by the Fibrosis-4 (FIB4) index, can reduce the chances that treatments will be effective and significantly increases morbidity and mortality. Health economist Jeffrey McCombs, Ph.D., of the University of Southern California, Los Angeles, presented data from a 10-year retrospective analysis of the Department of Veteran’s Affairs Veterans Health Administration HCV clinical registry involving more than 187,000 U.S. veterans with detectable HCV levels.
The caveat is that older treatments rather than newer all-oral direct-acting agents were used in the study population at the time of data analyses.
The analysis built on three previous analyses of data from the VA System, the first of which found that viral load suppression reduced the risk of liver events by around 27% and death by 45% (JAMA Intern. Med. 2014;174: 204-12). This analysis also found that relative to patients with genotype 1 (GT1), patients with GT2 were at lower risk and patients with GT3 were at higher risk of liver events and death.
The second analysis looked at treatment timing and found that treatment effectiveness was significantly reduced if treatment was started after common laboratory tests to detect liver changes became abnormal.
The third analysis looked at using the FIB4 index to monitor the risk of long-term morbidity and mortality and found that a FIB4 in excess of 3.4 was associated with a significantly increased risk for liver events and death, whereas a FIB4 of 1.45 was associated with moderate risk.
“This brought us to the last research question, looking at the relationship between treatment efficacy and exceeding certain FIB4 levels,” Dr. McCombs said at the meeting sponsored by the European Association for the Study of the Liver. The analysis compared treating early versus treating late, based on FIB4 levels – FIB4 of 1.0, FIB4 of 1.45, and FIB4 of 3.25.
Initiating treatment early before patients’ FIB4 exceeded 1.0 was associated with a 41% reduction in morbidity and a 36% reduction in mortality. Waiting until the FIB4 exceeded 1.0 resulted in a 30% reduction in liver events and 40% in mortality.
Treating patients early before exceeding a FIB4 of 1.45 was associated with morbidity and mortality reductions of 39% and 40% and waiting until the FIB4 exceeded this threshold was associated with reductions of 27% and 39%, respectively.
Using the FIB4 threshold of 3.25 showed even smaller reductions in morbidity and mortality. When treating early, the reductions were 36% and 45%; when treating late, the reductions were 11% and 25%.
“This is the kind of information you need when you talk with your payers,” Dr. McCombs said. “If you restrict access to treatment, you are not going to be as effective.”
Treating early or late appeared to have a similar effect on viral load and the number needed to treat (NNT) increased using the FIB4 3.5 threshold. Indeed, if treatment is initiated before the FIB4 rises above 3.25, the NNT is 180 patients to prevent one death, Dr. McCombs said, but if treatment is delayed, then the NNT jumps to 325 patients.
In patients with low FIB4 a ‘watch and wait strategy’ could potentially be feasible, he acknowledged in an interview. “The bottom line is that we can’t afford to treat everybody now,” he said.
“The FIB4 is a parameter we can probably watch, but it is unknown exactly how long we can wait,” Dr. McCombs said.
The data were derived from a study that was supported by a grant from Bristol-Myers Squibb. Dr. McCombs had no disclosures.
VIENNA – Delaying hepatitis C virus (HVC) treatment can have a detrimental effect on the outcomes of some patients, according to the results of a large 10-year retrospective analysis involving more than 187,000 U.S. veterans.
Indeed, waiting until a patient with HCV infection develops advanced cirrhosis, as measured by the Fibrosis-4 (FIB4) index, can reduce the chances that treatments will be effective and significantly increases morbidity and mortality. Health economist Jeffrey McCombs, Ph.D., of the University of Southern California, Los Angeles, presented data from a 10-year retrospective analysis of the Department of Veteran’s Affairs Veterans Health Administration HCV clinical registry involving more than 187,000 U.S. veterans with detectable HCV levels.
The caveat is that older treatments rather than newer all-oral direct-acting agents were used in the study population at the time of data analyses.
The analysis built on three previous analyses of data from the VA System, the first of which found that viral load suppression reduced the risk of liver events by around 27% and death by 45% (JAMA Intern. Med. 2014;174: 204-12). This analysis also found that relative to patients with genotype 1 (GT1), patients with GT2 were at lower risk and patients with GT3 were at higher risk of liver events and death.
The second analysis looked at treatment timing and found that treatment effectiveness was significantly reduced if treatment was started after common laboratory tests to detect liver changes became abnormal.
The third analysis looked at using the FIB4 index to monitor the risk of long-term morbidity and mortality and found that a FIB4 in excess of 3.4 was associated with a significantly increased risk for liver events and death, whereas a FIB4 of 1.45 was associated with moderate risk.
“This brought us to the last research question, looking at the relationship between treatment efficacy and exceeding certain FIB4 levels,” Dr. McCombs said at the meeting sponsored by the European Association for the Study of the Liver. The analysis compared treating early versus treating late, based on FIB4 levels – FIB4 of 1.0, FIB4 of 1.45, and FIB4 of 3.25.
Initiating treatment early before patients’ FIB4 exceeded 1.0 was associated with a 41% reduction in morbidity and a 36% reduction in mortality. Waiting until the FIB4 exceeded 1.0 resulted in a 30% reduction in liver events and 40% in mortality.
Treating patients early before exceeding a FIB4 of 1.45 was associated with morbidity and mortality reductions of 39% and 40% and waiting until the FIB4 exceeded this threshold was associated with reductions of 27% and 39%, respectively.
Using the FIB4 threshold of 3.25 showed even smaller reductions in morbidity and mortality. When treating early, the reductions were 36% and 45%; when treating late, the reductions were 11% and 25%.
“This is the kind of information you need when you talk with your payers,” Dr. McCombs said. “If you restrict access to treatment, you are not going to be as effective.”
Treating early or late appeared to have a similar effect on viral load and the number needed to treat (NNT) increased using the FIB4 3.5 threshold. Indeed, if treatment is initiated before the FIB4 rises above 3.25, the NNT is 180 patients to prevent one death, Dr. McCombs said, but if treatment is delayed, then the NNT jumps to 325 patients.
In patients with low FIB4 a ‘watch and wait strategy’ could potentially be feasible, he acknowledged in an interview. “The bottom line is that we can’t afford to treat everybody now,” he said.
“The FIB4 is a parameter we can probably watch, but it is unknown exactly how long we can wait,” Dr. McCombs said.
The data were derived from a study that was supported by a grant from Bristol-Myers Squibb. Dr. McCombs had no disclosures.
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: Efficacy diminishes when HCV treatment is delayed, particularly in patients who progress to advanced cirrhosis.
Major finding: Delaying treatment until the FIB4 index threshold exceeded 3.25 was associated with an 11% reduction in morbidity and 25% reduction in mortality; when treatment was started earlier, the reductions were 36% and 45%, respectively.
Data source: A 10-year retrospective analysis of the Veterans Health Administration HCV clinical registry involving more than 187,000 U.S. veterans.
Disclosures: The data were derived from a study that was supported by a grant from Bristol-Myers Squibb. Dr. McCombs had no disclosures.
ILC: Real-world success for all-oral antiviral therapy in HCV-related cirrhosis
VIENNA – More than 75% of patients with decompensated cirrhosis due to chronic hepatitis C virus (HCV) infection achieved a sustained virologic response at 12 weeks (SVR12) with all-oral HCV therapy in a routine clinical practice setting.
SVR12 was achieved in 74%-81% of patients treated with various regimens containing the direct antiviral agents sofosbuvir (Sovaldi) and simeprevir (Olysio), with or without ribavirin, based on interim data from the ongoing HCV-Target study. “All-oral DAA therapy has been readily used to treat HCV cirrhosis with MELD (Model for End-Stage Liver Disease) score greater than or equal to 10,” study investigator Dr. Rajender Reddy reported at the meeting sponsored by the European Association for the Study of the Liver.
“There is limited experience with all-oral DAA [direct antiviral agent] therapy in patients who have advanced HCV liver disease,” said Dr. Reddy, who is director of hepatology and medical director of liver transplantation at the Hospital of the University of Pennsylvania in Philadelphia. “HCV therapy in decompensated cirrhosis in the era of interferon-based therapy has been associated with suboptimal response and poor tolerability.”
Alternatively, these early findings from HCV-Target suggest that all-oral DAA therapy is not only effective in patients with advanced liver disease, but also that such treatment is well tolerated, he said.
HCV-Target is a longitudinal, observational study involving an international consortium of academic and community medical centers in the U.S., Canada, and Europe. HCV treatment is administered according to the local standard of care and the regimen selected by the patient’s health care provider.
As of October 2014, 2,204 patients were enrolled and 253 had a baseline MELD score of 10 or higher; 216 had completed treatment and had 12 weeks’ follow-up data available for analysis. Of these, 76 received a combination of sofosbuvir and ribavirin, 108 had been treated with sofosbuvir and simeprevir, and 32 had received all three drugs.
The mean age of patients was 59 years, but ranged from 38 to 80 years. The majority (59%) of patients had failed prior treatment, and around 73% had a history of prior hepatic decompensation, suggesting advancement of their liver disease.
“Sustained virologic response varied by genotype and regimen,” Dr. Reddy observed. SVR12 rates ranged from 52% to 74% in patients with genotype 1 (GT1). Most (63%) of these patients had received a combination of sofosbuvir and simeprevir, which yielded the highest SVR12 rates (72% in treatment-experienced and 78% in previously untreated GT1 patients).
SVR12 was 81% in patients with GT2, the majority (97%) had been treated with sofosbuvir plus ribavirin, with just 3% receiving all three drugs in combination.
SVR12 was the lowest, at 39%, in patients with GT3, all of whom had received sofosbuvir plus ribavirin.
Relapse rates were highest in GT3 patients, at 46% versus 26% in GT1 and 7% in GT2 patients.
SVR12 rates were also evaluated in relation to the MELD score. Of interest, said Dr. Reddy, was that patients with a MELD score of 10-15 (n=187) and those with a MELD score of 16-21 (n=21) had comparable SVR12 rates that ranged from 55% to 73%, with the lowest response rates being seen in patients treated with sofosbuvir plus ribavirin. Although there were only eight patients with a MELD score greater than 21, SVR12 was 100%.
Multivariate analysis to look at predictors of response was performed exclusively in GT1 patients and showed that being GT1a and having elevated bilirubin levels were negative predictors of response, with respective odds ratios of 0.39 (P = .069) and 0.46 (P = .002). Higher albumin levels, however, were associated with better outcomes (OR = 2.52, P = .026).
In terms of safety, 86% of 234 patients experienced any adverse event. Dr. Reddy highlighted anemia, which was more common (seen in about 30% of patients) with the ribavirin regimens, and photosensitivity, which was only seen in patients treated with simeprevir.
There were 44 serious adverse events; 16 were due to hepatic decompensation, 10 resulted from infections, 3 were deaths (one of which was due to liver failure), and 12 were liver transplants.
“Long-term follow-up is needed to assess the impact of SVR on the reversibility of liver disease,” he said.
The study is sponsored by the University of Florida and the University of Carolina at Chapel Hill and funded in part by AbbVie, Gilead, Janssen, Kadmon, Merck, Bristol-Myers Squibb, and GlaxoSmithKline. Dr. Reddy has acted as an ad-hoc advisory board member for and received research support from Gilead, Bristol-Myers Squibb, Merck, AbbVie, and Janssen.
VIENNA – More than 75% of patients with decompensated cirrhosis due to chronic hepatitis C virus (HCV) infection achieved a sustained virologic response at 12 weeks (SVR12) with all-oral HCV therapy in a routine clinical practice setting.
SVR12 was achieved in 74%-81% of patients treated with various regimens containing the direct antiviral agents sofosbuvir (Sovaldi) and simeprevir (Olysio), with or without ribavirin, based on interim data from the ongoing HCV-Target study. “All-oral DAA therapy has been readily used to treat HCV cirrhosis with MELD (Model for End-Stage Liver Disease) score greater than or equal to 10,” study investigator Dr. Rajender Reddy reported at the meeting sponsored by the European Association for the Study of the Liver.
“There is limited experience with all-oral DAA [direct antiviral agent] therapy in patients who have advanced HCV liver disease,” said Dr. Reddy, who is director of hepatology and medical director of liver transplantation at the Hospital of the University of Pennsylvania in Philadelphia. “HCV therapy in decompensated cirrhosis in the era of interferon-based therapy has been associated with suboptimal response and poor tolerability.”
Alternatively, these early findings from HCV-Target suggest that all-oral DAA therapy is not only effective in patients with advanced liver disease, but also that such treatment is well tolerated, he said.
HCV-Target is a longitudinal, observational study involving an international consortium of academic and community medical centers in the U.S., Canada, and Europe. HCV treatment is administered according to the local standard of care and the regimen selected by the patient’s health care provider.
As of October 2014, 2,204 patients were enrolled and 253 had a baseline MELD score of 10 or higher; 216 had completed treatment and had 12 weeks’ follow-up data available for analysis. Of these, 76 received a combination of sofosbuvir and ribavirin, 108 had been treated with sofosbuvir and simeprevir, and 32 had received all three drugs.
The mean age of patients was 59 years, but ranged from 38 to 80 years. The majority (59%) of patients had failed prior treatment, and around 73% had a history of prior hepatic decompensation, suggesting advancement of their liver disease.
“Sustained virologic response varied by genotype and regimen,” Dr. Reddy observed. SVR12 rates ranged from 52% to 74% in patients with genotype 1 (GT1). Most (63%) of these patients had received a combination of sofosbuvir and simeprevir, which yielded the highest SVR12 rates (72% in treatment-experienced and 78% in previously untreated GT1 patients).
SVR12 was 81% in patients with GT2, the majority (97%) had been treated with sofosbuvir plus ribavirin, with just 3% receiving all three drugs in combination.
SVR12 was the lowest, at 39%, in patients with GT3, all of whom had received sofosbuvir plus ribavirin.
Relapse rates were highest in GT3 patients, at 46% versus 26% in GT1 and 7% in GT2 patients.
SVR12 rates were also evaluated in relation to the MELD score. Of interest, said Dr. Reddy, was that patients with a MELD score of 10-15 (n=187) and those with a MELD score of 16-21 (n=21) had comparable SVR12 rates that ranged from 55% to 73%, with the lowest response rates being seen in patients treated with sofosbuvir plus ribavirin. Although there were only eight patients with a MELD score greater than 21, SVR12 was 100%.
Multivariate analysis to look at predictors of response was performed exclusively in GT1 patients and showed that being GT1a and having elevated bilirubin levels were negative predictors of response, with respective odds ratios of 0.39 (P = .069) and 0.46 (P = .002). Higher albumin levels, however, were associated with better outcomes (OR = 2.52, P = .026).
In terms of safety, 86% of 234 patients experienced any adverse event. Dr. Reddy highlighted anemia, which was more common (seen in about 30% of patients) with the ribavirin regimens, and photosensitivity, which was only seen in patients treated with simeprevir.
There were 44 serious adverse events; 16 were due to hepatic decompensation, 10 resulted from infections, 3 were deaths (one of which was due to liver failure), and 12 were liver transplants.
“Long-term follow-up is needed to assess the impact of SVR on the reversibility of liver disease,” he said.
The study is sponsored by the University of Florida and the University of Carolina at Chapel Hill and funded in part by AbbVie, Gilead, Janssen, Kadmon, Merck, Bristol-Myers Squibb, and GlaxoSmithKline. Dr. Reddy has acted as an ad-hoc advisory board member for and received research support from Gilead, Bristol-Myers Squibb, Merck, AbbVie, and Janssen.
VIENNA – More than 75% of patients with decompensated cirrhosis due to chronic hepatitis C virus (HCV) infection achieved a sustained virologic response at 12 weeks (SVR12) with all-oral HCV therapy in a routine clinical practice setting.
SVR12 was achieved in 74%-81% of patients treated with various regimens containing the direct antiviral agents sofosbuvir (Sovaldi) and simeprevir (Olysio), with or without ribavirin, based on interim data from the ongoing HCV-Target study. “All-oral DAA therapy has been readily used to treat HCV cirrhosis with MELD (Model for End-Stage Liver Disease) score greater than or equal to 10,” study investigator Dr. Rajender Reddy reported at the meeting sponsored by the European Association for the Study of the Liver.
“There is limited experience with all-oral DAA [direct antiviral agent] therapy in patients who have advanced HCV liver disease,” said Dr. Reddy, who is director of hepatology and medical director of liver transplantation at the Hospital of the University of Pennsylvania in Philadelphia. “HCV therapy in decompensated cirrhosis in the era of interferon-based therapy has been associated with suboptimal response and poor tolerability.”
Alternatively, these early findings from HCV-Target suggest that all-oral DAA therapy is not only effective in patients with advanced liver disease, but also that such treatment is well tolerated, he said.
HCV-Target is a longitudinal, observational study involving an international consortium of academic and community medical centers in the U.S., Canada, and Europe. HCV treatment is administered according to the local standard of care and the regimen selected by the patient’s health care provider.
As of October 2014, 2,204 patients were enrolled and 253 had a baseline MELD score of 10 or higher; 216 had completed treatment and had 12 weeks’ follow-up data available for analysis. Of these, 76 received a combination of sofosbuvir and ribavirin, 108 had been treated with sofosbuvir and simeprevir, and 32 had received all three drugs.
The mean age of patients was 59 years, but ranged from 38 to 80 years. The majority (59%) of patients had failed prior treatment, and around 73% had a history of prior hepatic decompensation, suggesting advancement of their liver disease.
“Sustained virologic response varied by genotype and regimen,” Dr. Reddy observed. SVR12 rates ranged from 52% to 74% in patients with genotype 1 (GT1). Most (63%) of these patients had received a combination of sofosbuvir and simeprevir, which yielded the highest SVR12 rates (72% in treatment-experienced and 78% in previously untreated GT1 patients).
SVR12 was 81% in patients with GT2, the majority (97%) had been treated with sofosbuvir plus ribavirin, with just 3% receiving all three drugs in combination.
SVR12 was the lowest, at 39%, in patients with GT3, all of whom had received sofosbuvir plus ribavirin.
Relapse rates were highest in GT3 patients, at 46% versus 26% in GT1 and 7% in GT2 patients.
SVR12 rates were also evaluated in relation to the MELD score. Of interest, said Dr. Reddy, was that patients with a MELD score of 10-15 (n=187) and those with a MELD score of 16-21 (n=21) had comparable SVR12 rates that ranged from 55% to 73%, with the lowest response rates being seen in patients treated with sofosbuvir plus ribavirin. Although there were only eight patients with a MELD score greater than 21, SVR12 was 100%.
Multivariate analysis to look at predictors of response was performed exclusively in GT1 patients and showed that being GT1a and having elevated bilirubin levels were negative predictors of response, with respective odds ratios of 0.39 (P = .069) and 0.46 (P = .002). Higher albumin levels, however, were associated with better outcomes (OR = 2.52, P = .026).
In terms of safety, 86% of 234 patients experienced any adverse event. Dr. Reddy highlighted anemia, which was more common (seen in about 30% of patients) with the ribavirin regimens, and photosensitivity, which was only seen in patients treated with simeprevir.
There were 44 serious adverse events; 16 were due to hepatic decompensation, 10 resulted from infections, 3 were deaths (one of which was due to liver failure), and 12 were liver transplants.
“Long-term follow-up is needed to assess the impact of SVR on the reversibility of liver disease,” he said.
The study is sponsored by the University of Florida and the University of Carolina at Chapel Hill and funded in part by AbbVie, Gilead, Janssen, Kadmon, Merck, Bristol-Myers Squibb, and GlaxoSmithKline. Dr. Reddy has acted as an ad-hoc advisory board member for and received research support from Gilead, Bristol-Myers Squibb, Merck, AbbVie, and Janssen.
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: An all-oral antiviral regimen appears effective and safe for most patients with decompensated cirrhosis due to chronic HCV infection.
Major finding: SVR12 was achieved in 74%-81% of patients treated with the direct antiviral agents sofosbuvir and simeprevir used together or in combination with ribavirin.
Data source: Ongoing, longitudinal, observational study of patients with HCV cirrhosis being treated with all-oral DAA.
Disclosures: The study is sponsored by the University of Florida and the University of Carolina at Chapel Hill and funded in part by AbbVie, Gilead, Janssen, Kadmon, Merck, Bristol-Myers Squibb, and GlaxoSmithKline. Dr. Reddy has acted as an ad-hoc advisory board member for and received research support from Gilead, Bristol-Myers Squibb, Merck, AbbVie, and Janssen.
EASL: Study supports HCC screening in alcoholic liver disease
VIENNA – The early results of a prospective, multicenter study show a high rate of hepatic focal lesions, further supporting liver cancer screening in patients with alcoholic liver disease, according to researchers.
The first results from the National Cohort of Uncomplicated Alcoholic Cirrhosis (CIRRAL) show that the cumulative incidence of hepatic focal lesions was 10.7% at 1 year and 18.1% at 2 years. The incidence of hepatocellular carcinoma (HCC) was about 1.6% at 1 year and 4.5% at 2 years.
“Primary liver cancer has a high incidence in Europe and in France,” said Dr. Nathalie Ganne of Assistance Publique des Hôpitaux de Paris–Hôpital Jean-Verdier, Bondy, France. “However, the natural history of HCC in alcoholic cirrhosis is not well known,” she said at the meeting sponsored by the European Association for the Study of the Liver.
EASL clinical guidelines recommend screening for hepatocellular carcinoma (HCC) in cirrhotic patients and the association’s general recommendations for screening for complications should be applied to those with alcoholic cirrhosis. However, Dr. Ganne pointed out, there were conflicting data on the value of HCC surveillance in patients with alcoholic liver disease.
On the one hand, data from a large retrospective Danish Registry study involving more than 8,400 patients suggested the risk of HCC was low, at around 0.25%-0.5% per year, so screening was unlikely to save many lives or to be cost-effective (Ann. Intern. Med. 2012;156:841-7). On the other hand, prospective data from a Spanish cohort of 450 patients suggested that there was a relatively high HCC risk, at 2.5% per year, and screening could have a greater impact on mortality (Clin. Gastroenterol. Hepatol. 2013;11:95-101).
To address the controversy and better understand alcoholic liver cirrhosis, the CIRRAL cohort was established with the aim to prospectively describe the natural history of the disease, including documenting the incidence of HCC and other complications, and identifying risk factors or predictors for HCC.
The study population comprised 599 patients with histologically proven, alcohol-related cirrhosis recruited over a 4-year period at 24 centers in France and 2 centers in Belgium. For inclusion, patients needed to have a history of chronic alcohol abuse and compensated liver disease (Child-Pugh A), to be hepatitis B and hepatitis C negative, and to have no prior history of liver cancer. Patients were screened for HCC, liver biopsy samples were obtained, and ethanol breath tests were undertaken at enrollment and annually thereafter.
Most patients were male; the median age was 58.6 years. Almost 70% of patients were abstinent from alcohol, with a median time of 28.4 months since alcohol was consumed. About 17% still had mild alcohol consumption, defined as having one to six alcoholic drinks per week.
Most patients had a history of smoking, with 35% being former and 36% being current smokers. Most patients (78%) were regular coffee drinkers, at an average of two cups per day; almost 40% drank three or more cups per day.
At baseline, 24 (4%) of 548 evaluable patients had a liver nodule, of which 13 were benign hemangiomas or biliary cysts and 11 were undetermined or regenerative nodules. After a median follow-up of 17 months, 13 of these patients still had a liver nodule present, of which two were confirmed as HCC. In the group of 524 patients without a liver nodule at baseline, 60 patients had a liver nodule at follow-up, of which 16 were confirmed as HCC.
“As expected, patients with baseline nodules were more at risk of focal lesions or proven HCC at follow-up,” Dr. Ganne said. The cumulative incidences of hepatic focal lesions and HCC at 2 years’ follow-up were 13.4% and 3.7% in those without and 76.8% and 13.3% in those with liver nodules at baseline.
Because of the short duration of current follow-up, no predictive factors were found, but there was a nonsignificant trend that linked HCC to a long duration of cirrhosis and the persistence of alcohol consumption.
Looking at the characteristics and treatment of the 18 cases of HCC, the researchers noted that 17 met noninvasive criteria. Eight were uninodular, nine were multinodular, and one was infiltrative. Fifteen (55%) met Milan criteria for liver transplantation. Seven cases (38%) received curative treatment involving percutaneous ablation.
Dr. Ganne reported that the overall and event-free survival rates at 2 years were 92.1% and 80.2%. The high rate of hepatic focal lesions found suggests that about 10% of patients per year will need a recall diagnosis procedure, she said.
Also, as the incidence of HCC was greater than 1.5% during the first year and probably an additional 3% per year thereafter, judging by the second year cumulative incidence rate, surveillance for HCC in patients with alcoholic liver cirrhosis “might be cost effective,” she concluded.
The CIRRAL cohort is an ongoing study that will form the backbone for additional studies into HCC in alcoholic liver disease, Dr. Ganne observed. Biological samples are being stored and a prospective database has been set up to include details of all assessments performed. Three nested studies are underway: one is looking at genetics using biobanked material (PANGEN), another is using the database to understand the role of the intestinal microbiota (MACHA), and another is looking at the potential role of nutrition (ALICIR).
The French National Cancer Institute, the French Association for Cancer Research, and the French National Agency for Research on AIDS and Viral Hepatitis funded the study. Dr. Ganne has no conflicts of interest.
VIENNA – The early results of a prospective, multicenter study show a high rate of hepatic focal lesions, further supporting liver cancer screening in patients with alcoholic liver disease, according to researchers.
The first results from the National Cohort of Uncomplicated Alcoholic Cirrhosis (CIRRAL) show that the cumulative incidence of hepatic focal lesions was 10.7% at 1 year and 18.1% at 2 years. The incidence of hepatocellular carcinoma (HCC) was about 1.6% at 1 year and 4.5% at 2 years.
“Primary liver cancer has a high incidence in Europe and in France,” said Dr. Nathalie Ganne of Assistance Publique des Hôpitaux de Paris–Hôpital Jean-Verdier, Bondy, France. “However, the natural history of HCC in alcoholic cirrhosis is not well known,” she said at the meeting sponsored by the European Association for the Study of the Liver.
EASL clinical guidelines recommend screening for hepatocellular carcinoma (HCC) in cirrhotic patients and the association’s general recommendations for screening for complications should be applied to those with alcoholic cirrhosis. However, Dr. Ganne pointed out, there were conflicting data on the value of HCC surveillance in patients with alcoholic liver disease.
On the one hand, data from a large retrospective Danish Registry study involving more than 8,400 patients suggested the risk of HCC was low, at around 0.25%-0.5% per year, so screening was unlikely to save many lives or to be cost-effective (Ann. Intern. Med. 2012;156:841-7). On the other hand, prospective data from a Spanish cohort of 450 patients suggested that there was a relatively high HCC risk, at 2.5% per year, and screening could have a greater impact on mortality (Clin. Gastroenterol. Hepatol. 2013;11:95-101).
To address the controversy and better understand alcoholic liver cirrhosis, the CIRRAL cohort was established with the aim to prospectively describe the natural history of the disease, including documenting the incidence of HCC and other complications, and identifying risk factors or predictors for HCC.
The study population comprised 599 patients with histologically proven, alcohol-related cirrhosis recruited over a 4-year period at 24 centers in France and 2 centers in Belgium. For inclusion, patients needed to have a history of chronic alcohol abuse and compensated liver disease (Child-Pugh A), to be hepatitis B and hepatitis C negative, and to have no prior history of liver cancer. Patients were screened for HCC, liver biopsy samples were obtained, and ethanol breath tests were undertaken at enrollment and annually thereafter.
Most patients were male; the median age was 58.6 years. Almost 70% of patients were abstinent from alcohol, with a median time of 28.4 months since alcohol was consumed. About 17% still had mild alcohol consumption, defined as having one to six alcoholic drinks per week.
Most patients had a history of smoking, with 35% being former and 36% being current smokers. Most patients (78%) were regular coffee drinkers, at an average of two cups per day; almost 40% drank three or more cups per day.
At baseline, 24 (4%) of 548 evaluable patients had a liver nodule, of which 13 were benign hemangiomas or biliary cysts and 11 were undetermined or regenerative nodules. After a median follow-up of 17 months, 13 of these patients still had a liver nodule present, of which two were confirmed as HCC. In the group of 524 patients without a liver nodule at baseline, 60 patients had a liver nodule at follow-up, of which 16 were confirmed as HCC.
“As expected, patients with baseline nodules were more at risk of focal lesions or proven HCC at follow-up,” Dr. Ganne said. The cumulative incidences of hepatic focal lesions and HCC at 2 years’ follow-up were 13.4% and 3.7% in those without and 76.8% and 13.3% in those with liver nodules at baseline.
Because of the short duration of current follow-up, no predictive factors were found, but there was a nonsignificant trend that linked HCC to a long duration of cirrhosis and the persistence of alcohol consumption.
Looking at the characteristics and treatment of the 18 cases of HCC, the researchers noted that 17 met noninvasive criteria. Eight were uninodular, nine were multinodular, and one was infiltrative. Fifteen (55%) met Milan criteria for liver transplantation. Seven cases (38%) received curative treatment involving percutaneous ablation.
Dr. Ganne reported that the overall and event-free survival rates at 2 years were 92.1% and 80.2%. The high rate of hepatic focal lesions found suggests that about 10% of patients per year will need a recall diagnosis procedure, she said.
Also, as the incidence of HCC was greater than 1.5% during the first year and probably an additional 3% per year thereafter, judging by the second year cumulative incidence rate, surveillance for HCC in patients with alcoholic liver cirrhosis “might be cost effective,” she concluded.
The CIRRAL cohort is an ongoing study that will form the backbone for additional studies into HCC in alcoholic liver disease, Dr. Ganne observed. Biological samples are being stored and a prospective database has been set up to include details of all assessments performed. Three nested studies are underway: one is looking at genetics using biobanked material (PANGEN), another is using the database to understand the role of the intestinal microbiota (MACHA), and another is looking at the potential role of nutrition (ALICIR).
The French National Cancer Institute, the French Association for Cancer Research, and the French National Agency for Research on AIDS and Viral Hepatitis funded the study. Dr. Ganne has no conflicts of interest.
VIENNA – The early results of a prospective, multicenter study show a high rate of hepatic focal lesions, further supporting liver cancer screening in patients with alcoholic liver disease, according to researchers.
The first results from the National Cohort of Uncomplicated Alcoholic Cirrhosis (CIRRAL) show that the cumulative incidence of hepatic focal lesions was 10.7% at 1 year and 18.1% at 2 years. The incidence of hepatocellular carcinoma (HCC) was about 1.6% at 1 year and 4.5% at 2 years.
“Primary liver cancer has a high incidence in Europe and in France,” said Dr. Nathalie Ganne of Assistance Publique des Hôpitaux de Paris–Hôpital Jean-Verdier, Bondy, France. “However, the natural history of HCC in alcoholic cirrhosis is not well known,” she said at the meeting sponsored by the European Association for the Study of the Liver.
EASL clinical guidelines recommend screening for hepatocellular carcinoma (HCC) in cirrhotic patients and the association’s general recommendations for screening for complications should be applied to those with alcoholic cirrhosis. However, Dr. Ganne pointed out, there were conflicting data on the value of HCC surveillance in patients with alcoholic liver disease.
On the one hand, data from a large retrospective Danish Registry study involving more than 8,400 patients suggested the risk of HCC was low, at around 0.25%-0.5% per year, so screening was unlikely to save many lives or to be cost-effective (Ann. Intern. Med. 2012;156:841-7). On the other hand, prospective data from a Spanish cohort of 450 patients suggested that there was a relatively high HCC risk, at 2.5% per year, and screening could have a greater impact on mortality (Clin. Gastroenterol. Hepatol. 2013;11:95-101).
To address the controversy and better understand alcoholic liver cirrhosis, the CIRRAL cohort was established with the aim to prospectively describe the natural history of the disease, including documenting the incidence of HCC and other complications, and identifying risk factors or predictors for HCC.
The study population comprised 599 patients with histologically proven, alcohol-related cirrhosis recruited over a 4-year period at 24 centers in France and 2 centers in Belgium. For inclusion, patients needed to have a history of chronic alcohol abuse and compensated liver disease (Child-Pugh A), to be hepatitis B and hepatitis C negative, and to have no prior history of liver cancer. Patients were screened for HCC, liver biopsy samples were obtained, and ethanol breath tests were undertaken at enrollment and annually thereafter.
Most patients were male; the median age was 58.6 years. Almost 70% of patients were abstinent from alcohol, with a median time of 28.4 months since alcohol was consumed. About 17% still had mild alcohol consumption, defined as having one to six alcoholic drinks per week.
Most patients had a history of smoking, with 35% being former and 36% being current smokers. Most patients (78%) were regular coffee drinkers, at an average of two cups per day; almost 40% drank three or more cups per day.
At baseline, 24 (4%) of 548 evaluable patients had a liver nodule, of which 13 were benign hemangiomas or biliary cysts and 11 were undetermined or regenerative nodules. After a median follow-up of 17 months, 13 of these patients still had a liver nodule present, of which two were confirmed as HCC. In the group of 524 patients without a liver nodule at baseline, 60 patients had a liver nodule at follow-up, of which 16 were confirmed as HCC.
“As expected, patients with baseline nodules were more at risk of focal lesions or proven HCC at follow-up,” Dr. Ganne said. The cumulative incidences of hepatic focal lesions and HCC at 2 years’ follow-up were 13.4% and 3.7% in those without and 76.8% and 13.3% in those with liver nodules at baseline.
Because of the short duration of current follow-up, no predictive factors were found, but there was a nonsignificant trend that linked HCC to a long duration of cirrhosis and the persistence of alcohol consumption.
Looking at the characteristics and treatment of the 18 cases of HCC, the researchers noted that 17 met noninvasive criteria. Eight were uninodular, nine were multinodular, and one was infiltrative. Fifteen (55%) met Milan criteria for liver transplantation. Seven cases (38%) received curative treatment involving percutaneous ablation.
Dr. Ganne reported that the overall and event-free survival rates at 2 years were 92.1% and 80.2%. The high rate of hepatic focal lesions found suggests that about 10% of patients per year will need a recall diagnosis procedure, she said.
Also, as the incidence of HCC was greater than 1.5% during the first year and probably an additional 3% per year thereafter, judging by the second year cumulative incidence rate, surveillance for HCC in patients with alcoholic liver cirrhosis “might be cost effective,” she concluded.
The CIRRAL cohort is an ongoing study that will form the backbone for additional studies into HCC in alcoholic liver disease, Dr. Ganne observed. Biological samples are being stored and a prospective database has been set up to include details of all assessments performed. Three nested studies are underway: one is looking at genetics using biobanked material (PANGEN), another is using the database to understand the role of the intestinal microbiota (MACHA), and another is looking at the potential role of nutrition (ALICIR).
The French National Cancer Institute, the French Association for Cancer Research, and the French National Agency for Research on AIDS and Viral Hepatitis funded the study. Dr. Ganne has no conflicts of interest.
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: A high rate of hepatic focal lesions suggests that about 10% of patients per year may need a recall diagnosis procedure.
Major finding: The cumulative incidences of hepatic lesions and of liver cancer at 1 and 2 years were 10.7% and 18.1%, and 1.6% and 4.5%, respectively.
Data source: A prospective cohort of 599 patients with histologically proven, alcohol-related compensated liver cirrhosis recruited over a 4-year period at 26 centers in France and Belgium.
Disclosures: The French National Cancer Institute, the French Association for Cancer Research, and the French National Agency for Research on AIDS and Viral Hepatitis funded the study. Dr. Ganne has no conflicts of interest.
Consider HCV a "Cardiovascular Risk Factor"
VIENNA – Major cardiovascular (CV) events are significantly increased in hospital patients who are infected with the hepatitis C virus (HCV) versus those who are not, according to data presented at the meeting sponsored by the European Association for the Study of the Liver.
A retrospective analysis of more than 200,000 inpatients with and without HCV infection at discharge showed that those with HCV were 29% more likely to have had an acute MI, 98% more likely to have had a cerebral vascular accident (CVA), and 88% times more likely to have coronary artery disease (CAD). There was also an 8% increased risk for heart failure (HF).
“HCV infection is strongly linked to an increase in cardiovascular outcome, length of hospital stay, and cost of care,” Dr. Firew Wubiee and Dr. Charles Howell of Howard University, Washington, D.C., reported in an ePoster at the meeting.
For their retrospective analysis, data were obtained from the 2011 Nationwide Inpatient Sample, which is a large all-payer inpatient care database in the United States that contains information on more than 7 million hospital stays.
ICD-9 codes were used to identify all inpatient cases with and without HCV infection at discharge and those with major CV events, excluding those with liver cirrhosis, hepatocellular carcinoma, and cases of liver transplantation. Dr. Wubiee and Dr. Howell found that inpatients with HCV infection were significantly (P < .001) younger than were those without HCV (mean age 52.8 vs. 57.9 years). They were significantly more likely (P < .001) to be male (62.2% vs. 40%), have a smoking history (45.5% vs. 22.7%), be from ethnic or racial minorities (26.4% vs. 5.1%), and have a household income of less than $39,000 (41% vs. 29.1%).
While inpatients with HCV were also significantly (P < .001) more likely than were those without HCV to have diabetes mellitus (2.1% vs. 1.8%), they were significantly less likely (P < .001) to have other known CV risk factors such as obesity (8.7% vs. 11.8%), dyslipidemia (13.2% vs. 28.1%), and hypertension (49.7% vs. 51%).
Odds ratios for CV outcomes were adjusted for multiple confounding factors, including age, race, obesity, diabetes, dyslipidemia, smoking, alcohol use, hypertension, and hepatitis B infection. The adjusted odds ratios for major CV outcomes were 2.29 for AMI, 1.98 for CVA, 1.88 for CAD, and 1.08 for HF (all P < .001).
The duration of in-hospital treatment was longer for patients with HCV than for those without, with adjusted differences of 1.3 days for AMI (P < .001), 2.02 days for CVA (P < .003), 0.52 days for CAD (P < .001), and 0.37 days for HF (P < .001).
The adjusted cost of inpatient care was also higher for HCV-infected versus noninfected individuals with a mean cost difference of $10,126 for AMI, (P < .001), $10,105 for CVA (P < .002), $2,703 for CAD (P < .001), and $1,895 for HF (P < .001).
“We hypothesized that the inflammation and insulin resistance related to HCV increases the prevalence of cardiovascular diseases compared to patients without HCV,” Dr. Wubiee and Dr. Howell observed.
“The results are consistent with mounting evidence for [a] biological and epidemiological association between HCV infection and cardiovascular morbidity,” they said and suggested “HCV needs to be considered a cardiovascular risk factor.”
VIENNA – Major cardiovascular (CV) events are significantly increased in hospital patients who are infected with the hepatitis C virus (HCV) versus those who are not, according to data presented at the meeting sponsored by the European Association for the Study of the Liver.
A retrospective analysis of more than 200,000 inpatients with and without HCV infection at discharge showed that those with HCV were 29% more likely to have had an acute MI, 98% more likely to have had a cerebral vascular accident (CVA), and 88% times more likely to have coronary artery disease (CAD). There was also an 8% increased risk for heart failure (HF).
“HCV infection is strongly linked to an increase in cardiovascular outcome, length of hospital stay, and cost of care,” Dr. Firew Wubiee and Dr. Charles Howell of Howard University, Washington, D.C., reported in an ePoster at the meeting.
For their retrospective analysis, data were obtained from the 2011 Nationwide Inpatient Sample, which is a large all-payer inpatient care database in the United States that contains information on more than 7 million hospital stays.
ICD-9 codes were used to identify all inpatient cases with and without HCV infection at discharge and those with major CV events, excluding those with liver cirrhosis, hepatocellular carcinoma, and cases of liver transplantation. Dr. Wubiee and Dr. Howell found that inpatients with HCV infection were significantly (P < .001) younger than were those without HCV (mean age 52.8 vs. 57.9 years). They were significantly more likely (P < .001) to be male (62.2% vs. 40%), have a smoking history (45.5% vs. 22.7%), be from ethnic or racial minorities (26.4% vs. 5.1%), and have a household income of less than $39,000 (41% vs. 29.1%).
While inpatients with HCV were also significantly (P < .001) more likely than were those without HCV to have diabetes mellitus (2.1% vs. 1.8%), they were significantly less likely (P < .001) to have other known CV risk factors such as obesity (8.7% vs. 11.8%), dyslipidemia (13.2% vs. 28.1%), and hypertension (49.7% vs. 51%).
Odds ratios for CV outcomes were adjusted for multiple confounding factors, including age, race, obesity, diabetes, dyslipidemia, smoking, alcohol use, hypertension, and hepatitis B infection. The adjusted odds ratios for major CV outcomes were 2.29 for AMI, 1.98 for CVA, 1.88 for CAD, and 1.08 for HF (all P < .001).
The duration of in-hospital treatment was longer for patients with HCV than for those without, with adjusted differences of 1.3 days for AMI (P < .001), 2.02 days for CVA (P < .003), 0.52 days for CAD (P < .001), and 0.37 days for HF (P < .001).
The adjusted cost of inpatient care was also higher for HCV-infected versus noninfected individuals with a mean cost difference of $10,126 for AMI, (P < .001), $10,105 for CVA (P < .002), $2,703 for CAD (P < .001), and $1,895 for HF (P < .001).
“We hypothesized that the inflammation and insulin resistance related to HCV increases the prevalence of cardiovascular diseases compared to patients without HCV,” Dr. Wubiee and Dr. Howell observed.
“The results are consistent with mounting evidence for [a] biological and epidemiological association between HCV infection and cardiovascular morbidity,” they said and suggested “HCV needs to be considered a cardiovascular risk factor.”
VIENNA – Major cardiovascular (CV) events are significantly increased in hospital patients who are infected with the hepatitis C virus (HCV) versus those who are not, according to data presented at the meeting sponsored by the European Association for the Study of the Liver.
A retrospective analysis of more than 200,000 inpatients with and without HCV infection at discharge showed that those with HCV were 29% more likely to have had an acute MI, 98% more likely to have had a cerebral vascular accident (CVA), and 88% times more likely to have coronary artery disease (CAD). There was also an 8% increased risk for heart failure (HF).
“HCV infection is strongly linked to an increase in cardiovascular outcome, length of hospital stay, and cost of care,” Dr. Firew Wubiee and Dr. Charles Howell of Howard University, Washington, D.C., reported in an ePoster at the meeting.
For their retrospective analysis, data were obtained from the 2011 Nationwide Inpatient Sample, which is a large all-payer inpatient care database in the United States that contains information on more than 7 million hospital stays.
ICD-9 codes were used to identify all inpatient cases with and without HCV infection at discharge and those with major CV events, excluding those with liver cirrhosis, hepatocellular carcinoma, and cases of liver transplantation. Dr. Wubiee and Dr. Howell found that inpatients with HCV infection were significantly (P < .001) younger than were those without HCV (mean age 52.8 vs. 57.9 years). They were significantly more likely (P < .001) to be male (62.2% vs. 40%), have a smoking history (45.5% vs. 22.7%), be from ethnic or racial minorities (26.4% vs. 5.1%), and have a household income of less than $39,000 (41% vs. 29.1%).
While inpatients with HCV were also significantly (P < .001) more likely than were those without HCV to have diabetes mellitus (2.1% vs. 1.8%), they were significantly less likely (P < .001) to have other known CV risk factors such as obesity (8.7% vs. 11.8%), dyslipidemia (13.2% vs. 28.1%), and hypertension (49.7% vs. 51%).
Odds ratios for CV outcomes were adjusted for multiple confounding factors, including age, race, obesity, diabetes, dyslipidemia, smoking, alcohol use, hypertension, and hepatitis B infection. The adjusted odds ratios for major CV outcomes were 2.29 for AMI, 1.98 for CVA, 1.88 for CAD, and 1.08 for HF (all P < .001).
The duration of in-hospital treatment was longer for patients with HCV than for those without, with adjusted differences of 1.3 days for AMI (P < .001), 2.02 days for CVA (P < .003), 0.52 days for CAD (P < .001), and 0.37 days for HF (P < .001).
The adjusted cost of inpatient care was also higher for HCV-infected versus noninfected individuals with a mean cost difference of $10,126 for AMI, (P < .001), $10,105 for CVA (P < .002), $2,703 for CAD (P < .001), and $1,895 for HF (P < .001).
“We hypothesized that the inflammation and insulin resistance related to HCV increases the prevalence of cardiovascular diseases compared to patients without HCV,” Dr. Wubiee and Dr. Howell observed.
“The results are consistent with mounting evidence for [a] biological and epidemiological association between HCV infection and cardiovascular morbidity,” they said and suggested “HCV needs to be considered a cardiovascular risk factor.”
AT THE INTERNATIONAL LIVER CONGRESS 2015
Consider HCV ‘a cardiovascular risk factor’
VIENNA – Major cardiovascular (CV) events are significantly increased in hospital patients who are infected with the hepatitis C virus (HCV) versus those who are not, according to data presented at the meeting sponsored by the European Association for the Study of the Liver.
A retrospective analysis of more than 200,000 inpatients with and without HCV infection at discharge showed that those with HCV were 29% more likely to have had an acute MI, 98% more likely to have had a cerebral vascular accident (CVA), and 88% times more likely to have coronary artery disease (CAD). There was also an 8% increased risk for heart failure (HF).
“HCV infection is strongly linked to an increase in cardiovascular outcome, length of hospital stay, and cost of care,” Dr. Firew Wubiee and Dr. Charles Howell of Howard University, Washington, D.C., reported in an ePoster at the meeting.
For their retrospective analysis, data were obtained from the 2011 Nationwide Inpatient Sample, which is a large all-payer inpatient care database in the United States that contains information on more than 7 million hospital stays.
ICD-9 codes were used to identify all inpatient cases with and without HCV infection at discharge and those with major CV events, excluding those with liver cirrhosis, hepatocellular carcinoma, and cases of liver transplantation. Dr. Wubiee and Dr. Howell found that inpatients with HCV infection were significantly (P < .001) younger than were those without HCV (mean age 52.8 vs. 57.9 years). They were significantly more likely (P < .001) to be male (62.2% vs. 40%), have a smoking history (45.5% vs. 22.7%), be from ethnic or racial minorities (26.4% vs. 5.1%), and have a household income of less than $39,000 (41% vs. 29.1%).
While inpatients with HCV were also significantly (P < .001) more likely than were those without HCV to have diabetes mellitus (2.1% vs. 1.8%), they were significantly less likely (P < .001) to have other known CV risk factors such as obesity (8.7% vs. 11.8%), dyslipidemia (13.2% vs. 28.1%), and hypertension (49.7% vs. 51%).
Odds ratios for CV outcomes were adjusted for multiple confounding factors, including age, race, obesity, diabetes, dyslipidemia, smoking, alcohol use, hypertension, and hepatitis B infection. The adjusted odds ratios for major CV outcomes were 2.29 for AMI, 1.98 for CVA, 1.88 for CAD, and 1.08 for HF (all P < .001).
The duration of in-hospital treatment was longer for patients with HCV than for those without, with adjusted differences of 1.3 days for AMI (P < .001), 2.02 days for CVA (P < .003), 0.52 days for CAD (P < .001), and 0.37 days for HF (P < .001).
The adjusted cost of inpatient care was also higher for HCV-infected versus noninfected individuals with a mean cost difference of $10,126 for AMI, (P < .001), $10,105 for CVA (P < .002), $2,703 for CAD (P < .001), and $1,895 for HF (P < .001).
“We hypothesized that the inflammation and insulin resistance related to HCV increases the prevalence of cardiovascular diseases compared to patients without HCV,” Dr. Wubiee and Dr. Howell observed.
“The results are consistent with mounting evidence for [a] biological and epidemiological association between HCV infection and cardiovascular morbidity,” they said and suggested “HCV needs to be considered a cardiovascular risk factor.”
VIENNA – Major cardiovascular (CV) events are significantly increased in hospital patients who are infected with the hepatitis C virus (HCV) versus those who are not, according to data presented at the meeting sponsored by the European Association for the Study of the Liver.
A retrospective analysis of more than 200,000 inpatients with and without HCV infection at discharge showed that those with HCV were 29% more likely to have had an acute MI, 98% more likely to have had a cerebral vascular accident (CVA), and 88% times more likely to have coronary artery disease (CAD). There was also an 8% increased risk for heart failure (HF).
“HCV infection is strongly linked to an increase in cardiovascular outcome, length of hospital stay, and cost of care,” Dr. Firew Wubiee and Dr. Charles Howell of Howard University, Washington, D.C., reported in an ePoster at the meeting.
For their retrospective analysis, data were obtained from the 2011 Nationwide Inpatient Sample, which is a large all-payer inpatient care database in the United States that contains information on more than 7 million hospital stays.
ICD-9 codes were used to identify all inpatient cases with and without HCV infection at discharge and those with major CV events, excluding those with liver cirrhosis, hepatocellular carcinoma, and cases of liver transplantation. Dr. Wubiee and Dr. Howell found that inpatients with HCV infection were significantly (P < .001) younger than were those without HCV (mean age 52.8 vs. 57.9 years). They were significantly more likely (P < .001) to be male (62.2% vs. 40%), have a smoking history (45.5% vs. 22.7%), be from ethnic or racial minorities (26.4% vs. 5.1%), and have a household income of less than $39,000 (41% vs. 29.1%).
While inpatients with HCV were also significantly (P < .001) more likely than were those without HCV to have diabetes mellitus (2.1% vs. 1.8%), they were significantly less likely (P < .001) to have other known CV risk factors such as obesity (8.7% vs. 11.8%), dyslipidemia (13.2% vs. 28.1%), and hypertension (49.7% vs. 51%).
Odds ratios for CV outcomes were adjusted for multiple confounding factors, including age, race, obesity, diabetes, dyslipidemia, smoking, alcohol use, hypertension, and hepatitis B infection. The adjusted odds ratios for major CV outcomes were 2.29 for AMI, 1.98 for CVA, 1.88 for CAD, and 1.08 for HF (all P < .001).
The duration of in-hospital treatment was longer for patients with HCV than for those without, with adjusted differences of 1.3 days for AMI (P < .001), 2.02 days for CVA (P < .003), 0.52 days for CAD (P < .001), and 0.37 days for HF (P < .001).
The adjusted cost of inpatient care was also higher for HCV-infected versus noninfected individuals with a mean cost difference of $10,126 for AMI, (P < .001), $10,105 for CVA (P < .002), $2,703 for CAD (P < .001), and $1,895 for HF (P < .001).
“We hypothesized that the inflammation and insulin resistance related to HCV increases the prevalence of cardiovascular diseases compared to patients without HCV,” Dr. Wubiee and Dr. Howell observed.
“The results are consistent with mounting evidence for [a] biological and epidemiological association between HCV infection and cardiovascular morbidity,” they said and suggested “HCV needs to be considered a cardiovascular risk factor.”
VIENNA – Major cardiovascular (CV) events are significantly increased in hospital patients who are infected with the hepatitis C virus (HCV) versus those who are not, according to data presented at the meeting sponsored by the European Association for the Study of the Liver.
A retrospective analysis of more than 200,000 inpatients with and without HCV infection at discharge showed that those with HCV were 29% more likely to have had an acute MI, 98% more likely to have had a cerebral vascular accident (CVA), and 88% times more likely to have coronary artery disease (CAD). There was also an 8% increased risk for heart failure (HF).
“HCV infection is strongly linked to an increase in cardiovascular outcome, length of hospital stay, and cost of care,” Dr. Firew Wubiee and Dr. Charles Howell of Howard University, Washington, D.C., reported in an ePoster at the meeting.
For their retrospective analysis, data were obtained from the 2011 Nationwide Inpatient Sample, which is a large all-payer inpatient care database in the United States that contains information on more than 7 million hospital stays.
ICD-9 codes were used to identify all inpatient cases with and without HCV infection at discharge and those with major CV events, excluding those with liver cirrhosis, hepatocellular carcinoma, and cases of liver transplantation. Dr. Wubiee and Dr. Howell found that inpatients with HCV infection were significantly (P < .001) younger than were those without HCV (mean age 52.8 vs. 57.9 years). They were significantly more likely (P < .001) to be male (62.2% vs. 40%), have a smoking history (45.5% vs. 22.7%), be from ethnic or racial minorities (26.4% vs. 5.1%), and have a household income of less than $39,000 (41% vs. 29.1%).
While inpatients with HCV were also significantly (P < .001) more likely than were those without HCV to have diabetes mellitus (2.1% vs. 1.8%), they were significantly less likely (P < .001) to have other known CV risk factors such as obesity (8.7% vs. 11.8%), dyslipidemia (13.2% vs. 28.1%), and hypertension (49.7% vs. 51%).
Odds ratios for CV outcomes were adjusted for multiple confounding factors, including age, race, obesity, diabetes, dyslipidemia, smoking, alcohol use, hypertension, and hepatitis B infection. The adjusted odds ratios for major CV outcomes were 2.29 for AMI, 1.98 for CVA, 1.88 for CAD, and 1.08 for HF (all P < .001).
The duration of in-hospital treatment was longer for patients with HCV than for those without, with adjusted differences of 1.3 days for AMI (P < .001), 2.02 days for CVA (P < .003), 0.52 days for CAD (P < .001), and 0.37 days for HF (P < .001).
The adjusted cost of inpatient care was also higher for HCV-infected versus noninfected individuals with a mean cost difference of $10,126 for AMI, (P < .001), $10,105 for CVA (P < .002), $2,703 for CAD (P < .001), and $1,895 for HF (P < .001).
“We hypothesized that the inflammation and insulin resistance related to HCV increases the prevalence of cardiovascular diseases compared to patients without HCV,” Dr. Wubiee and Dr. Howell observed.
“The results are consistent with mounting evidence for [a] biological and epidemiological association between HCV infection and cardiovascular morbidity,” they said and suggested “HCV needs to be considered a cardiovascular risk factor.”
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: Chronic HCV infection was strongly linked to CV outcome and needs to be considered a CV risk factor.
Major finding: Adjusted odds ratios for major cardiovascular events were higher in patients with HCV than without HCV, at 2.29 for acute MI, 1.98 for cerebral vascular accident, 1.88 for coronary artery disease, and 1.08 for heart failure.
Data source: Retrospective analysis of more than 200,000 hospital inpatients with and without HCV infection at discharge from the 2011 Nationwide Inpatient Sample (NIS).
Disclosures: The authors had no disclosures.