User login
Think ESUS, not cryptogenic stroke
VIENNA – The concept of cryptogenic stroke is outdated and should be replaced by the relatively new clinical construct of embolic stroke of undetermined source, or ESUS, Dr. Hans Christoph Diener said at the annual European Stroke Conference.
The underlying premise is that these strokes of so-called unknown cause are actually due to thromboembolic events from more minor-risk or covert cardiac sources and thus could potentially be prevented by anticoagulation with the newer non-vitamin K oral anticoagulants (NOACs). In fact, Dr. Diener noted that the concept of ESUS came about because researchers wanted to do secondary prevention trials in cryptogenic stoke but the U.S. Food and Drug Administration would not allow it as there was no way to truly diagnose it.
The paradigm of cryptogenic stroke was developed at a time when sophisticated imaging was not available, Dr. Diener of the University of Duisburg-Essen, Germany, observed, noting that it was a diagnosis of exclusion. The term could also encompass cases where incomplete diagnostic evaluation had been performed.
ESUS on the other hand was “positively defined” and required a clear set of criteria to be met as set out recently by the Cryptogenic Stroke/ESUS International Working Group (Lancet Neurol. 2014;13:429–438). These criteria relied on a minimum diagnostic work-up including brain computed tomography (CT) or magnetic resonance imagine (MRI), 12-lead ECG, precordial echocardiography, cardiac monitoring for 24 hours or longer, and imaging of both the extra- and intracranial arteries supplying the area of brain ischemia.
The criteria are:
* Stroke detected by CT or MRI that is not lacunar
* Absence of extracranial or intracranial atherosclerosis causing ≥50% luminal stenosis in the arteries supplying the area of ischemia
* No major-risk cardioembolic source of embolism
* No other specific cause of stroke identified (e.g. arteritis, dissection, migraine/vasospasm, drug misuse)
According to the established TOAST (Trial of Org 10172) Criteria (Stroke. 1993;23:35–41) there are five main categories of ischemic stroke: large vessel disease, cardio-embolic disease, small vessel disease unknown (cryptogenic), or other causes or those with conflicting etiology. “These criteria were developed to include people with a particular pathophysiology into randomized trials,” Dr. Diener noted and using this classification a quarter of all ischemic strokes are generally classified as cryptogenic. Another quarter can be due to large artery atherosclerotic stenosis, another quarter to small artery disease (lacunar stroke), a fifth due to major-risk cardio-embolic disease, and the remainder to unusual events, such as dissection or arteritis.
“These different entities have different consequences for treatment,” Dr. Diener noted. “In someone who had a significant large artery atherosclerotic stenosis most probably it would be surgery or stenting,” he said while “in someone who has an identified cardiogenic source of embolism it is clearly anticoagulation.” Patients with small vessel disease would probably be treated via risk factor management.
“And then we have this entity called cryptogenic stroke,” he said. Because this category of stroke was so heterogeneous and defined by what it is not rather than what it is, treatment options were not clearly defined and performing secondary prevention trials just not possible, until now.
ESUS could include a range of pathophysiologies, he acknowledged, although the majority were likely to be unrecognized paroxysmal atrial fibrillation (AF). Others could be atrial high rate episodes, heart failure, silent myocardial infarction, patent foramen ovale, atherosclerotic plaques in the aortic arch, or nonstenotic atherosclerotic plaques in the cervical or intracranial arteries.
There are two possible strategies for stroke prevention in patients with ESUS, Dr. Diener suggested. The first was to use more sophisticated tools to detect clinically silent AF. Methods of detecting clinically silent AF via cardiac monitoring has evolved greatly from the original Holter monitoring equipment to the ambulatory devices and implantable recorders available today. Trials such as EMBRACE (N Engl J Med. 2014;370:2467-77) and CRYSTAL-AF (N Engl J Med. 2014; 370:2478-2486) had shown the respective benefits of prolonged ambulatory and implantable device monitoring, he said. The next generation of technology could include ECG- and perhaps even biomarker monitoring via the iPhone and Apple Watch, Dr. Diener said.
The second strategy for secondary stroke prevention in ESUS was to treat irrespective of the possible etiology or to develop new and more effective anticoagulatnt therapies. Dr. Diener is co-chair of the RE-SPECT ESUS-trial, which is currently recruiting patients using the new ESUS definition and will randomize a target of 6,000 patients to treatment with dabigatran, aspirin, or matching placebos. There is also another trial, NAVIGATE ESUS, comparing the NOAC rivaroxaban to aspirin with a similar design and 7,000-patient target accrual, he said. Both trials are event driven and are expected to run for 2-3 years. Results should be available in 2018.
“The old concept of cryptogenic stroke is not very useful if you want to do randomized trials in a defined population and this is why we created the definition of ESUS,” Dr. Diener said.
“In the future, we have two ways to address this problem. One way is much more sophisticated diagnostic testing to detect silent AF and the other way is to treat and to improve the medical treatment of these patients. Hopefully, three years from now, we have evidence that we have something to offer that is superior to aspirin and has a superior side effect profile.”
Introducing the concept of ESUS has not been not without its critics, however, and in a comment published in the Lancet Neurology, Dr. Martin Dennis of the Western General Hospital in Edinburgh, Scotland, pointed out that the name selected might mislead clinicians.
“An unknown proportion of patients with ESUS will not have an embolic stroke, but a stroke due to in-situ thrombosis,” he argued. “Although one can see the advantages of the name for gaining support from funders and clinicians for a trial, and for marketing new oral anticoagulants if they prove effective, the name will mislead clinicians.”
Dr. Dennis suggested that a better name might be to refer to these strokes as “non-lacunar ischaemic stroke without a defined cause”.
Dr. Diener and his institution have received financial support from a number of German and European funding bodies, and wide range of pharmaceutical companies.
VIENNA – The concept of cryptogenic stroke is outdated and should be replaced by the relatively new clinical construct of embolic stroke of undetermined source, or ESUS, Dr. Hans Christoph Diener said at the annual European Stroke Conference.
The underlying premise is that these strokes of so-called unknown cause are actually due to thromboembolic events from more minor-risk or covert cardiac sources and thus could potentially be prevented by anticoagulation with the newer non-vitamin K oral anticoagulants (NOACs). In fact, Dr. Diener noted that the concept of ESUS came about because researchers wanted to do secondary prevention trials in cryptogenic stoke but the U.S. Food and Drug Administration would not allow it as there was no way to truly diagnose it.
The paradigm of cryptogenic stroke was developed at a time when sophisticated imaging was not available, Dr. Diener of the University of Duisburg-Essen, Germany, observed, noting that it was a diagnosis of exclusion. The term could also encompass cases where incomplete diagnostic evaluation had been performed.
ESUS on the other hand was “positively defined” and required a clear set of criteria to be met as set out recently by the Cryptogenic Stroke/ESUS International Working Group (Lancet Neurol. 2014;13:429–438). These criteria relied on a minimum diagnostic work-up including brain computed tomography (CT) or magnetic resonance imagine (MRI), 12-lead ECG, precordial echocardiography, cardiac monitoring for 24 hours or longer, and imaging of both the extra- and intracranial arteries supplying the area of brain ischemia.
The criteria are:
* Stroke detected by CT or MRI that is not lacunar
* Absence of extracranial or intracranial atherosclerosis causing ≥50% luminal stenosis in the arteries supplying the area of ischemia
* No major-risk cardioembolic source of embolism
* No other specific cause of stroke identified (e.g. arteritis, dissection, migraine/vasospasm, drug misuse)
According to the established TOAST (Trial of Org 10172) Criteria (Stroke. 1993;23:35–41) there are five main categories of ischemic stroke: large vessel disease, cardio-embolic disease, small vessel disease unknown (cryptogenic), or other causes or those with conflicting etiology. “These criteria were developed to include people with a particular pathophysiology into randomized trials,” Dr. Diener noted and using this classification a quarter of all ischemic strokes are generally classified as cryptogenic. Another quarter can be due to large artery atherosclerotic stenosis, another quarter to small artery disease (lacunar stroke), a fifth due to major-risk cardio-embolic disease, and the remainder to unusual events, such as dissection or arteritis.
“These different entities have different consequences for treatment,” Dr. Diener noted. “In someone who had a significant large artery atherosclerotic stenosis most probably it would be surgery or stenting,” he said while “in someone who has an identified cardiogenic source of embolism it is clearly anticoagulation.” Patients with small vessel disease would probably be treated via risk factor management.
“And then we have this entity called cryptogenic stroke,” he said. Because this category of stroke was so heterogeneous and defined by what it is not rather than what it is, treatment options were not clearly defined and performing secondary prevention trials just not possible, until now.
ESUS could include a range of pathophysiologies, he acknowledged, although the majority were likely to be unrecognized paroxysmal atrial fibrillation (AF). Others could be atrial high rate episodes, heart failure, silent myocardial infarction, patent foramen ovale, atherosclerotic plaques in the aortic arch, or nonstenotic atherosclerotic plaques in the cervical or intracranial arteries.
There are two possible strategies for stroke prevention in patients with ESUS, Dr. Diener suggested. The first was to use more sophisticated tools to detect clinically silent AF. Methods of detecting clinically silent AF via cardiac monitoring has evolved greatly from the original Holter monitoring equipment to the ambulatory devices and implantable recorders available today. Trials such as EMBRACE (N Engl J Med. 2014;370:2467-77) and CRYSTAL-AF (N Engl J Med. 2014; 370:2478-2486) had shown the respective benefits of prolonged ambulatory and implantable device monitoring, he said. The next generation of technology could include ECG- and perhaps even biomarker monitoring via the iPhone and Apple Watch, Dr. Diener said.
The second strategy for secondary stroke prevention in ESUS was to treat irrespective of the possible etiology or to develop new and more effective anticoagulatnt therapies. Dr. Diener is co-chair of the RE-SPECT ESUS-trial, which is currently recruiting patients using the new ESUS definition and will randomize a target of 6,000 patients to treatment with dabigatran, aspirin, or matching placebos. There is also another trial, NAVIGATE ESUS, comparing the NOAC rivaroxaban to aspirin with a similar design and 7,000-patient target accrual, he said. Both trials are event driven and are expected to run for 2-3 years. Results should be available in 2018.
“The old concept of cryptogenic stroke is not very useful if you want to do randomized trials in a defined population and this is why we created the definition of ESUS,” Dr. Diener said.
“In the future, we have two ways to address this problem. One way is much more sophisticated diagnostic testing to detect silent AF and the other way is to treat and to improve the medical treatment of these patients. Hopefully, three years from now, we have evidence that we have something to offer that is superior to aspirin and has a superior side effect profile.”
Introducing the concept of ESUS has not been not without its critics, however, and in a comment published in the Lancet Neurology, Dr. Martin Dennis of the Western General Hospital in Edinburgh, Scotland, pointed out that the name selected might mislead clinicians.
“An unknown proportion of patients with ESUS will not have an embolic stroke, but a stroke due to in-situ thrombosis,” he argued. “Although one can see the advantages of the name for gaining support from funders and clinicians for a trial, and for marketing new oral anticoagulants if they prove effective, the name will mislead clinicians.”
Dr. Dennis suggested that a better name might be to refer to these strokes as “non-lacunar ischaemic stroke without a defined cause”.
Dr. Diener and his institution have received financial support from a number of German and European funding bodies, and wide range of pharmaceutical companies.
VIENNA – The concept of cryptogenic stroke is outdated and should be replaced by the relatively new clinical construct of embolic stroke of undetermined source, or ESUS, Dr. Hans Christoph Diener said at the annual European Stroke Conference.
The underlying premise is that these strokes of so-called unknown cause are actually due to thromboembolic events from more minor-risk or covert cardiac sources and thus could potentially be prevented by anticoagulation with the newer non-vitamin K oral anticoagulants (NOACs). In fact, Dr. Diener noted that the concept of ESUS came about because researchers wanted to do secondary prevention trials in cryptogenic stoke but the U.S. Food and Drug Administration would not allow it as there was no way to truly diagnose it.
The paradigm of cryptogenic stroke was developed at a time when sophisticated imaging was not available, Dr. Diener of the University of Duisburg-Essen, Germany, observed, noting that it was a diagnosis of exclusion. The term could also encompass cases where incomplete diagnostic evaluation had been performed.
ESUS on the other hand was “positively defined” and required a clear set of criteria to be met as set out recently by the Cryptogenic Stroke/ESUS International Working Group (Lancet Neurol. 2014;13:429–438). These criteria relied on a minimum diagnostic work-up including brain computed tomography (CT) or magnetic resonance imagine (MRI), 12-lead ECG, precordial echocardiography, cardiac monitoring for 24 hours or longer, and imaging of both the extra- and intracranial arteries supplying the area of brain ischemia.
The criteria are:
* Stroke detected by CT or MRI that is not lacunar
* Absence of extracranial or intracranial atherosclerosis causing ≥50% luminal stenosis in the arteries supplying the area of ischemia
* No major-risk cardioembolic source of embolism
* No other specific cause of stroke identified (e.g. arteritis, dissection, migraine/vasospasm, drug misuse)
According to the established TOAST (Trial of Org 10172) Criteria (Stroke. 1993;23:35–41) there are five main categories of ischemic stroke: large vessel disease, cardio-embolic disease, small vessel disease unknown (cryptogenic), or other causes or those with conflicting etiology. “These criteria were developed to include people with a particular pathophysiology into randomized trials,” Dr. Diener noted and using this classification a quarter of all ischemic strokes are generally classified as cryptogenic. Another quarter can be due to large artery atherosclerotic stenosis, another quarter to small artery disease (lacunar stroke), a fifth due to major-risk cardio-embolic disease, and the remainder to unusual events, such as dissection or arteritis.
“These different entities have different consequences for treatment,” Dr. Diener noted. “In someone who had a significant large artery atherosclerotic stenosis most probably it would be surgery or stenting,” he said while “in someone who has an identified cardiogenic source of embolism it is clearly anticoagulation.” Patients with small vessel disease would probably be treated via risk factor management.
“And then we have this entity called cryptogenic stroke,” he said. Because this category of stroke was so heterogeneous and defined by what it is not rather than what it is, treatment options were not clearly defined and performing secondary prevention trials just not possible, until now.
ESUS could include a range of pathophysiologies, he acknowledged, although the majority were likely to be unrecognized paroxysmal atrial fibrillation (AF). Others could be atrial high rate episodes, heart failure, silent myocardial infarction, patent foramen ovale, atherosclerotic plaques in the aortic arch, or nonstenotic atherosclerotic plaques in the cervical or intracranial arteries.
There are two possible strategies for stroke prevention in patients with ESUS, Dr. Diener suggested. The first was to use more sophisticated tools to detect clinically silent AF. Methods of detecting clinically silent AF via cardiac monitoring has evolved greatly from the original Holter monitoring equipment to the ambulatory devices and implantable recorders available today. Trials such as EMBRACE (N Engl J Med. 2014;370:2467-77) and CRYSTAL-AF (N Engl J Med. 2014; 370:2478-2486) had shown the respective benefits of prolonged ambulatory and implantable device monitoring, he said. The next generation of technology could include ECG- and perhaps even biomarker monitoring via the iPhone and Apple Watch, Dr. Diener said.
The second strategy for secondary stroke prevention in ESUS was to treat irrespective of the possible etiology or to develop new and more effective anticoagulatnt therapies. Dr. Diener is co-chair of the RE-SPECT ESUS-trial, which is currently recruiting patients using the new ESUS definition and will randomize a target of 6,000 patients to treatment with dabigatran, aspirin, or matching placebos. There is also another trial, NAVIGATE ESUS, comparing the NOAC rivaroxaban to aspirin with a similar design and 7,000-patient target accrual, he said. Both trials are event driven and are expected to run for 2-3 years. Results should be available in 2018.
“The old concept of cryptogenic stroke is not very useful if you want to do randomized trials in a defined population and this is why we created the definition of ESUS,” Dr. Diener said.
“In the future, we have two ways to address this problem. One way is much more sophisticated diagnostic testing to detect silent AF and the other way is to treat and to improve the medical treatment of these patients. Hopefully, three years from now, we have evidence that we have something to offer that is superior to aspirin and has a superior side effect profile.”
Introducing the concept of ESUS has not been not without its critics, however, and in a comment published in the Lancet Neurology, Dr. Martin Dennis of the Western General Hospital in Edinburgh, Scotland, pointed out that the name selected might mislead clinicians.
“An unknown proportion of patients with ESUS will not have an embolic stroke, but a stroke due to in-situ thrombosis,” he argued. “Although one can see the advantages of the name for gaining support from funders and clinicians for a trial, and for marketing new oral anticoagulants if they prove effective, the name will mislead clinicians.”
Dr. Dennis suggested that a better name might be to refer to these strokes as “non-lacunar ischaemic stroke without a defined cause”.
Dr. Diener and his institution have received financial support from a number of German and European funding bodies, and wide range of pharmaceutical companies.
EXPERT ANALYSIS FROM THE EUROPEAN STROKE CONFERENCE
Carotid artery stenosis guidelines need modernizing
VIENNA – Guidelines used around the world for the management of carotid stenosis, both in asymptomatic and symptomatic cases, are often outdated and do not match current evidence, according to the results of a systematic review undertaken by an international group of experts.*
Furthermore, the qualifying statements used to back up the recommendations are often confused and too simplistic, being based only on the degree of randomized data used.
“Other problems were that the guidelines often didn’t even define asymptomatic carotid artery stenosis or symptomatic carotid artery stenosis or they left out procedural standards,” said Dr. Anne Abbott, who presented the findings at the annual European Stroke Conference.
“A number of important discoveries have been made in recent years to better inform treatment decisions for patients with carotid stenosis,” Dr. Abbott, a neurologist at Monash University in Melbourne, Australia, observed.
For instance, based on evidence available today, it is clear that medical therapy alone is best for patients with moderate or severe (50%-99%) asymptomatic disease. Surgery in these patients might actually be harmful, she noted, and it is unknown if or by how much carotid endarterectomy improves stroke prevention versus medical therapy alone.
“We just haven’t done the studies, and this is where we should be concentrating our efforts with respect to randomized, placebo-controlled trials,” Dr. Abbott proposed. “But we do know that the 6% 30-day stroke/death rate [with endarterectomy] is really now too high.”
It’s also now apparent that carotid angioplasty or stenting is more harmful than carotid endarterectomy in asymptomatic patients and “shouldn’t be recommended for routine practice.” Many of the guidelines were still supporting this as an option, she observed, based on the supposed counterbalancing argument that surgical intervention was more likely to increase the risk for heart attacks than stenting. However, the evidence shows that the greatest risk to patients in the periprocedural period is the stroke risk, which is increased by stenting.
Dr. Abbott explained how the team of 16 experts had reviewed all the latest available guidelines for asymptomatic and symptomatic carotid stenosis that they could find from published from January 2008 until January 2015. She noted that guidelines were often difficult to access were often only found because the team knew of their existence through their professional networks.
A total of 34 guidelines from 23 regions or countries in six languages were identified and included in the review. Each of these was independently assessed by two to six of the team, looking at the clinical scenarios covered, the nature of the recommendations made and what evidence was being used to support the recommendations.
Of 28 guidelines that gave recommendations for asymptomatic carotid stenosis, surgery was endorsed for patients at average surgical risk and only one (4%) endorsed medical treatment alone. Eighteen (64%) recommended that stenting be performed or considered, and 24 (86%) supported the use of endarterectomy. “This is despite current evidence that these procedures are now more likely to harm than help patients,” Dr. Abbott said.
“Of major concern I think is that a high proportion, about half, of these guidelines are recommending stenting for high surgical risk asymptomatic patients, she cautioned. “This includes patients with major medical comorbidities – heart failure, respiratory failure – who have a very short life expectancy and are least likely to benefit and are more likely to be at risk from the procedures.”
Somewhat similar findings were seen regarding the use of stenting and surgery in the 33 guidelines that gave recommendations for the management of symptomatic carotid stenosis. Endarterectomy was recommended for average-surgical-risk symptomatic patients by 31 (94%) guidelines and, worryingly, stenting was still being advocated in 19 (58%) guidelines with only nine (27%) saying that stenting should not be used. Stenting was also being endorsed in symptomatic patients at high surgical risk.
Dr. Abbott said that the guidelines were hard to compare because they used a variety of qualifying statements to try to advise on the degree to which a procedure was recommended. There was no consistency or standardization: six guidelines did not use any qualifying statements or were not defined in two guidelines, 10 guidelines used class or grade to denote the strength of the recommendation being made, and 27 guidelines used class, grade, or other means to denote the strength of the evidence the recommendations were being based on.
All this means, however, that there are many opportunities to modernize the guidelines and bring them up-to-date with current knowledge. They shouldn’t be recommending stenting over surgery, for example, and they need to standardize what the recommendations are based on.
“The guidelines should always define their target population properly and that comes straight from randomized trials usually,” Dr. Abbott noted. Procedural standards also need to be given. “Guidelines also need to be consistent throughout, self-contained, and be more accessible.”
Dr. Abbott had no relevant disclosures.
*CORRECTION: 6/21/2015 An error in identification of the stent was corrected.
VIENNA – Guidelines used around the world for the management of carotid stenosis, both in asymptomatic and symptomatic cases, are often outdated and do not match current evidence, according to the results of a systematic review undertaken by an international group of experts.*
Furthermore, the qualifying statements used to back up the recommendations are often confused and too simplistic, being based only on the degree of randomized data used.
“Other problems were that the guidelines often didn’t even define asymptomatic carotid artery stenosis or symptomatic carotid artery stenosis or they left out procedural standards,” said Dr. Anne Abbott, who presented the findings at the annual European Stroke Conference.
“A number of important discoveries have been made in recent years to better inform treatment decisions for patients with carotid stenosis,” Dr. Abbott, a neurologist at Monash University in Melbourne, Australia, observed.
For instance, based on evidence available today, it is clear that medical therapy alone is best for patients with moderate or severe (50%-99%) asymptomatic disease. Surgery in these patients might actually be harmful, she noted, and it is unknown if or by how much carotid endarterectomy improves stroke prevention versus medical therapy alone.
“We just haven’t done the studies, and this is where we should be concentrating our efforts with respect to randomized, placebo-controlled trials,” Dr. Abbott proposed. “But we do know that the 6% 30-day stroke/death rate [with endarterectomy] is really now too high.”
It’s also now apparent that carotid angioplasty or stenting is more harmful than carotid endarterectomy in asymptomatic patients and “shouldn’t be recommended for routine practice.” Many of the guidelines were still supporting this as an option, she observed, based on the supposed counterbalancing argument that surgical intervention was more likely to increase the risk for heart attacks than stenting. However, the evidence shows that the greatest risk to patients in the periprocedural period is the stroke risk, which is increased by stenting.
Dr. Abbott explained how the team of 16 experts had reviewed all the latest available guidelines for asymptomatic and symptomatic carotid stenosis that they could find from published from January 2008 until January 2015. She noted that guidelines were often difficult to access were often only found because the team knew of their existence through their professional networks.
A total of 34 guidelines from 23 regions or countries in six languages were identified and included in the review. Each of these was independently assessed by two to six of the team, looking at the clinical scenarios covered, the nature of the recommendations made and what evidence was being used to support the recommendations.
Of 28 guidelines that gave recommendations for asymptomatic carotid stenosis, surgery was endorsed for patients at average surgical risk and only one (4%) endorsed medical treatment alone. Eighteen (64%) recommended that stenting be performed or considered, and 24 (86%) supported the use of endarterectomy. “This is despite current evidence that these procedures are now more likely to harm than help patients,” Dr. Abbott said.
“Of major concern I think is that a high proportion, about half, of these guidelines are recommending stenting for high surgical risk asymptomatic patients, she cautioned. “This includes patients with major medical comorbidities – heart failure, respiratory failure – who have a very short life expectancy and are least likely to benefit and are more likely to be at risk from the procedures.”
Somewhat similar findings were seen regarding the use of stenting and surgery in the 33 guidelines that gave recommendations for the management of symptomatic carotid stenosis. Endarterectomy was recommended for average-surgical-risk symptomatic patients by 31 (94%) guidelines and, worryingly, stenting was still being advocated in 19 (58%) guidelines with only nine (27%) saying that stenting should not be used. Stenting was also being endorsed in symptomatic patients at high surgical risk.
Dr. Abbott said that the guidelines were hard to compare because they used a variety of qualifying statements to try to advise on the degree to which a procedure was recommended. There was no consistency or standardization: six guidelines did not use any qualifying statements or were not defined in two guidelines, 10 guidelines used class or grade to denote the strength of the recommendation being made, and 27 guidelines used class, grade, or other means to denote the strength of the evidence the recommendations were being based on.
All this means, however, that there are many opportunities to modernize the guidelines and bring them up-to-date with current knowledge. They shouldn’t be recommending stenting over surgery, for example, and they need to standardize what the recommendations are based on.
“The guidelines should always define their target population properly and that comes straight from randomized trials usually,” Dr. Abbott noted. Procedural standards also need to be given. “Guidelines also need to be consistent throughout, self-contained, and be more accessible.”
Dr. Abbott had no relevant disclosures.
*CORRECTION: 6/21/2015 An error in identification of the stent was corrected.
VIENNA – Guidelines used around the world for the management of carotid stenosis, both in asymptomatic and symptomatic cases, are often outdated and do not match current evidence, according to the results of a systematic review undertaken by an international group of experts.*
Furthermore, the qualifying statements used to back up the recommendations are often confused and too simplistic, being based only on the degree of randomized data used.
“Other problems were that the guidelines often didn’t even define asymptomatic carotid artery stenosis or symptomatic carotid artery stenosis or they left out procedural standards,” said Dr. Anne Abbott, who presented the findings at the annual European Stroke Conference.
“A number of important discoveries have been made in recent years to better inform treatment decisions for patients with carotid stenosis,” Dr. Abbott, a neurologist at Monash University in Melbourne, Australia, observed.
For instance, based on evidence available today, it is clear that medical therapy alone is best for patients with moderate or severe (50%-99%) asymptomatic disease. Surgery in these patients might actually be harmful, she noted, and it is unknown if or by how much carotid endarterectomy improves stroke prevention versus medical therapy alone.
“We just haven’t done the studies, and this is where we should be concentrating our efforts with respect to randomized, placebo-controlled trials,” Dr. Abbott proposed. “But we do know that the 6% 30-day stroke/death rate [with endarterectomy] is really now too high.”
It’s also now apparent that carotid angioplasty or stenting is more harmful than carotid endarterectomy in asymptomatic patients and “shouldn’t be recommended for routine practice.” Many of the guidelines were still supporting this as an option, she observed, based on the supposed counterbalancing argument that surgical intervention was more likely to increase the risk for heart attacks than stenting. However, the evidence shows that the greatest risk to patients in the periprocedural period is the stroke risk, which is increased by stenting.
Dr. Abbott explained how the team of 16 experts had reviewed all the latest available guidelines for asymptomatic and symptomatic carotid stenosis that they could find from published from January 2008 until January 2015. She noted that guidelines were often difficult to access were often only found because the team knew of their existence through their professional networks.
A total of 34 guidelines from 23 regions or countries in six languages were identified and included in the review. Each of these was independently assessed by two to six of the team, looking at the clinical scenarios covered, the nature of the recommendations made and what evidence was being used to support the recommendations.
Of 28 guidelines that gave recommendations for asymptomatic carotid stenosis, surgery was endorsed for patients at average surgical risk and only one (4%) endorsed medical treatment alone. Eighteen (64%) recommended that stenting be performed or considered, and 24 (86%) supported the use of endarterectomy. “This is despite current evidence that these procedures are now more likely to harm than help patients,” Dr. Abbott said.
“Of major concern I think is that a high proportion, about half, of these guidelines are recommending stenting for high surgical risk asymptomatic patients, she cautioned. “This includes patients with major medical comorbidities – heart failure, respiratory failure – who have a very short life expectancy and are least likely to benefit and are more likely to be at risk from the procedures.”
Somewhat similar findings were seen regarding the use of stenting and surgery in the 33 guidelines that gave recommendations for the management of symptomatic carotid stenosis. Endarterectomy was recommended for average-surgical-risk symptomatic patients by 31 (94%) guidelines and, worryingly, stenting was still being advocated in 19 (58%) guidelines with only nine (27%) saying that stenting should not be used. Stenting was also being endorsed in symptomatic patients at high surgical risk.
Dr. Abbott said that the guidelines were hard to compare because they used a variety of qualifying statements to try to advise on the degree to which a procedure was recommended. There was no consistency or standardization: six guidelines did not use any qualifying statements or were not defined in two guidelines, 10 guidelines used class or grade to denote the strength of the recommendation being made, and 27 guidelines used class, grade, or other means to denote the strength of the evidence the recommendations were being based on.
All this means, however, that there are many opportunities to modernize the guidelines and bring them up-to-date with current knowledge. They shouldn’t be recommending stenting over surgery, for example, and they need to standardize what the recommendations are based on.
“The guidelines should always define their target population properly and that comes straight from randomized trials usually,” Dr. Abbott noted. Procedural standards also need to be given. “Guidelines also need to be consistent throughout, self-contained, and be more accessible.”
Dr. Abbott had no relevant disclosures.
*CORRECTION: 6/21/2015 An error in identification of the stent was corrected.
AT THE EUROPEAN STROKE CONFERENCE
Key clinical point: Guidelines for carotid stenosis need reviewing and updating in line with modern evidence and practice.
Major finding: Carotid angiography/stenting was recommended for both asymptomatic and symptomatic patients despite current evidence showing that it is more likely to cause harm than provide benefit.
Data source: Systematic review of 33 guidelines for asymptomatic and symptomatic carotid stenosis.
Disclosures: Dr. Abbott had no relevant disclosures.
Digital handgrip helps self-recovery after stroke
VIENNA – A digital handgrip developed by U.K. researchers allowed more patients with arm weakness caused by a stroke to self-rehabilitate than did the use of standard mobile device touch screen controls in a 6-month, single-center survey.
Results showed that almost all patients (94%) could use the novel wireless controller, compared with 56%-62% of patients who could successfully use the gestures that commonly control mobile devices, such as swiping, tilting, or touching a screen.
“Standard mobile gaming technology can be used by the majority of stroke patients with moderate and mild arm weakness” Paul Rinne said at the annual European Stroke Conference. “It could increase the amount of complementary therapy given and hopefully be more economical” than existing technology used in motor rehabilitation programs, he suggested.
The device could bring stroke rehabilitation to the patients’ bedside and allow additional self-therapy, said Mr. Rinne, who is completing his PhD in brain sciences and is a researcher at the Human Robotics Group, Imperial College London, where the handgrip was developed.
Physiotherapist-led physical therapy is one of the main components of poststroke rehabilitation programs and evidence shows that the success of such therapy is greatly influenced by how intensively, long, and often patients perform task-specific exercises (PLoS One 2014;9:e87987). The problem, of course, is having sufficient human and financial resources to maximize potential benefits, Mr. Rinne observed. According to a National Institute for Health and Care Excellence estimate, 55% of stroke patients receive less than 45 minutes of motor rehabilitation exercise per day. Other data suggest that patients do not receive therapy for very long or perform too few repetitions during a session.
Although gaming technology is already being used as an adjunct to traditional physical therapy in some centers, the cost of equipment currently used is often high and it is often geared toward patients with high motor function. It cannot be used by patients at the bedside or at home at the moment, which would help increase the “dose” of treatment. The use of mobile devices is thus gaining interest as a possible alternative means of supplementing current rehabilitation programs, making it both more accessible – around 75% of the general public have access to a mobile device, Mr. Rinne observed – and more engaging or motivating for patients.
Mr. Rinne and coinvestigators surveyed all patients presenting with arm weakness on admission to a large hyper-acute stroke unit over a 6-month period. Of 342 patients who were screened, 89 were included. Reasons for exclusion were cognitive impairment or comorbidities (130 patients), lack of communication or language barrier (36 patients), resolution of arm weakness (34 patients), preexisting arm weakness (24 patients), arm weakness not due to stroke (5 patients), or patient refusal (24 patients).
The mean age of patients who participated was 65 years and 57% were male. The baseline National Institutes of Health Stroke Scale score was 5.8 and the NIHSS Motor subscale score was 1 on a scale of 0 to 4, signifying mild impairment. Other functional measures used were the 66-item Fugl-Meyer Assessment-Upper Extremity (FMA-UE), the 12-item Short-Form Fugl-Meyer (S-FM) scale, and the 14-item Fugl-Meyer Assessment Hand Subscale (FMA-Hand).
During the assessment period, study participants were given a mobile tablet and asked to perform a variety of touch-screen hand movements with their paretic and unaffected arms to move an object on screen. The results were given a movement score, ranging from 0 (no movement) to 3 (full range of movement) and were compared against those obtained by use of a gaming joystick and the digital handgrip developed by the Imperial College London team.
The S-FM was used to divide patients into groups depending on their baseline arm weakness. Patients with severe impairment had lower movement scores in both their paretic and unaffected hands than did those with more moderate or mild impairment. Mr. Rinne noted that there was no difference in the results seen with the conventional controllers, and a similar proportion of patients could swipe, press a button, tilt, or use the joystick successfully.
However, comparing the results obtained with the digital handgrip and mobile tablet, he noted that patients who used the digital handgrip could follow the object on screen much more accurately than when they used a swiping motion on a tablet. Even patients with severe impairment could use the handgrip.
“We found that 89% of our severely weak patients could still interact with these games and perform self-rehabilitation when using our controller,” Mr. Rinne reported. In contrast, no severely impaired patient could perform the screen swipe on a tablet.
“Performance using the handgrip was very similar across patients with different severities,” he added, noting that patients found the novel controller more comfortable and easier to use for long periods of time.
Novel control devices adapted for patients can broaden the accessibility to stroke rehabilitation, he concluded, even in those with more severe impairments.
Mr. Rinne had no conflicts of interest.
VIENNA – A digital handgrip developed by U.K. researchers allowed more patients with arm weakness caused by a stroke to self-rehabilitate than did the use of standard mobile device touch screen controls in a 6-month, single-center survey.
Results showed that almost all patients (94%) could use the novel wireless controller, compared with 56%-62% of patients who could successfully use the gestures that commonly control mobile devices, such as swiping, tilting, or touching a screen.
“Standard mobile gaming technology can be used by the majority of stroke patients with moderate and mild arm weakness” Paul Rinne said at the annual European Stroke Conference. “It could increase the amount of complementary therapy given and hopefully be more economical” than existing technology used in motor rehabilitation programs, he suggested.
The device could bring stroke rehabilitation to the patients’ bedside and allow additional self-therapy, said Mr. Rinne, who is completing his PhD in brain sciences and is a researcher at the Human Robotics Group, Imperial College London, where the handgrip was developed.
Physiotherapist-led physical therapy is one of the main components of poststroke rehabilitation programs and evidence shows that the success of such therapy is greatly influenced by how intensively, long, and often patients perform task-specific exercises (PLoS One 2014;9:e87987). The problem, of course, is having sufficient human and financial resources to maximize potential benefits, Mr. Rinne observed. According to a National Institute for Health and Care Excellence estimate, 55% of stroke patients receive less than 45 minutes of motor rehabilitation exercise per day. Other data suggest that patients do not receive therapy for very long or perform too few repetitions during a session.
Although gaming technology is already being used as an adjunct to traditional physical therapy in some centers, the cost of equipment currently used is often high and it is often geared toward patients with high motor function. It cannot be used by patients at the bedside or at home at the moment, which would help increase the “dose” of treatment. The use of mobile devices is thus gaining interest as a possible alternative means of supplementing current rehabilitation programs, making it both more accessible – around 75% of the general public have access to a mobile device, Mr. Rinne observed – and more engaging or motivating for patients.
Mr. Rinne and coinvestigators surveyed all patients presenting with arm weakness on admission to a large hyper-acute stroke unit over a 6-month period. Of 342 patients who were screened, 89 were included. Reasons for exclusion were cognitive impairment or comorbidities (130 patients), lack of communication or language barrier (36 patients), resolution of arm weakness (34 patients), preexisting arm weakness (24 patients), arm weakness not due to stroke (5 patients), or patient refusal (24 patients).
The mean age of patients who participated was 65 years and 57% were male. The baseline National Institutes of Health Stroke Scale score was 5.8 and the NIHSS Motor subscale score was 1 on a scale of 0 to 4, signifying mild impairment. Other functional measures used were the 66-item Fugl-Meyer Assessment-Upper Extremity (FMA-UE), the 12-item Short-Form Fugl-Meyer (S-FM) scale, and the 14-item Fugl-Meyer Assessment Hand Subscale (FMA-Hand).
During the assessment period, study participants were given a mobile tablet and asked to perform a variety of touch-screen hand movements with their paretic and unaffected arms to move an object on screen. The results were given a movement score, ranging from 0 (no movement) to 3 (full range of movement) and were compared against those obtained by use of a gaming joystick and the digital handgrip developed by the Imperial College London team.
The S-FM was used to divide patients into groups depending on their baseline arm weakness. Patients with severe impairment had lower movement scores in both their paretic and unaffected hands than did those with more moderate or mild impairment. Mr. Rinne noted that there was no difference in the results seen with the conventional controllers, and a similar proportion of patients could swipe, press a button, tilt, or use the joystick successfully.
However, comparing the results obtained with the digital handgrip and mobile tablet, he noted that patients who used the digital handgrip could follow the object on screen much more accurately than when they used a swiping motion on a tablet. Even patients with severe impairment could use the handgrip.
“We found that 89% of our severely weak patients could still interact with these games and perform self-rehabilitation when using our controller,” Mr. Rinne reported. In contrast, no severely impaired patient could perform the screen swipe on a tablet.
“Performance using the handgrip was very similar across patients with different severities,” he added, noting that patients found the novel controller more comfortable and easier to use for long periods of time.
Novel control devices adapted for patients can broaden the accessibility to stroke rehabilitation, he concluded, even in those with more severe impairments.
Mr. Rinne had no conflicts of interest.
VIENNA – A digital handgrip developed by U.K. researchers allowed more patients with arm weakness caused by a stroke to self-rehabilitate than did the use of standard mobile device touch screen controls in a 6-month, single-center survey.
Results showed that almost all patients (94%) could use the novel wireless controller, compared with 56%-62% of patients who could successfully use the gestures that commonly control mobile devices, such as swiping, tilting, or touching a screen.
“Standard mobile gaming technology can be used by the majority of stroke patients with moderate and mild arm weakness” Paul Rinne said at the annual European Stroke Conference. “It could increase the amount of complementary therapy given and hopefully be more economical” than existing technology used in motor rehabilitation programs, he suggested.
The device could bring stroke rehabilitation to the patients’ bedside and allow additional self-therapy, said Mr. Rinne, who is completing his PhD in brain sciences and is a researcher at the Human Robotics Group, Imperial College London, where the handgrip was developed.
Physiotherapist-led physical therapy is one of the main components of poststroke rehabilitation programs and evidence shows that the success of such therapy is greatly influenced by how intensively, long, and often patients perform task-specific exercises (PLoS One 2014;9:e87987). The problem, of course, is having sufficient human and financial resources to maximize potential benefits, Mr. Rinne observed. According to a National Institute for Health and Care Excellence estimate, 55% of stroke patients receive less than 45 minutes of motor rehabilitation exercise per day. Other data suggest that patients do not receive therapy for very long or perform too few repetitions during a session.
Although gaming technology is already being used as an adjunct to traditional physical therapy in some centers, the cost of equipment currently used is often high and it is often geared toward patients with high motor function. It cannot be used by patients at the bedside or at home at the moment, which would help increase the “dose” of treatment. The use of mobile devices is thus gaining interest as a possible alternative means of supplementing current rehabilitation programs, making it both more accessible – around 75% of the general public have access to a mobile device, Mr. Rinne observed – and more engaging or motivating for patients.
Mr. Rinne and coinvestigators surveyed all patients presenting with arm weakness on admission to a large hyper-acute stroke unit over a 6-month period. Of 342 patients who were screened, 89 were included. Reasons for exclusion were cognitive impairment or comorbidities (130 patients), lack of communication or language barrier (36 patients), resolution of arm weakness (34 patients), preexisting arm weakness (24 patients), arm weakness not due to stroke (5 patients), or patient refusal (24 patients).
The mean age of patients who participated was 65 years and 57% were male. The baseline National Institutes of Health Stroke Scale score was 5.8 and the NIHSS Motor subscale score was 1 on a scale of 0 to 4, signifying mild impairment. Other functional measures used were the 66-item Fugl-Meyer Assessment-Upper Extremity (FMA-UE), the 12-item Short-Form Fugl-Meyer (S-FM) scale, and the 14-item Fugl-Meyer Assessment Hand Subscale (FMA-Hand).
During the assessment period, study participants were given a mobile tablet and asked to perform a variety of touch-screen hand movements with their paretic and unaffected arms to move an object on screen. The results were given a movement score, ranging from 0 (no movement) to 3 (full range of movement) and were compared against those obtained by use of a gaming joystick and the digital handgrip developed by the Imperial College London team.
The S-FM was used to divide patients into groups depending on their baseline arm weakness. Patients with severe impairment had lower movement scores in both their paretic and unaffected hands than did those with more moderate or mild impairment. Mr. Rinne noted that there was no difference in the results seen with the conventional controllers, and a similar proportion of patients could swipe, press a button, tilt, or use the joystick successfully.
However, comparing the results obtained with the digital handgrip and mobile tablet, he noted that patients who used the digital handgrip could follow the object on screen much more accurately than when they used a swiping motion on a tablet. Even patients with severe impairment could use the handgrip.
“We found that 89% of our severely weak patients could still interact with these games and perform self-rehabilitation when using our controller,” Mr. Rinne reported. In contrast, no severely impaired patient could perform the screen swipe on a tablet.
“Performance using the handgrip was very similar across patients with different severities,” he added, noting that patients found the novel controller more comfortable and easier to use for long periods of time.
Novel control devices adapted for patients can broaden the accessibility to stroke rehabilitation, he concluded, even in those with more severe impairments.
Mr. Rinne had no conflicts of interest.
AT THE EUROPEAN STROKE CONFERENCE
Key clinical point: A novel digital handgrip device could broaden the accessibility of rehabilitation to stroke patients with arm weakness.
Major finding: A total of 94% of patients could use a digital handgrip versus 56%-62% of patients who could use conventional tablet controls.
Data source: A 6-month, single-center survey of 89 patients with poststroke arm weakness.
Disclosures: Paul Rinne had no disclosures.
BSR: Multiple benefits seen with intensive psoriatic arthritis therapy
MANCHESTER, U.K. – Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis until they achieve a set of minimal disease activity criteria, an expert said at the British Society for Rheumatology annual conference.
While data are mounting on the value of treating to target (otherwise known as tight control) in psoriatic arthritis (PsA), these data lag significantly behind that for inducing and maintaining remission in rheumatoid arthritis (RA), noted Dr. Philip Helliwell of the University of Leeds (England).
“There is half as much evidence in PsA as there is in rheumatoid arthritis,” he observed. “But we’ve also got a heterogeneous disease and what we are going to have to do moving forward is to find out how to treat different phenotypic expressions of psoriatic disease, and we’re beginning to work towards that now,” he said during a Special Interest Group on Spondyloarthropathies.
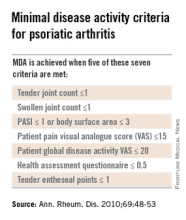
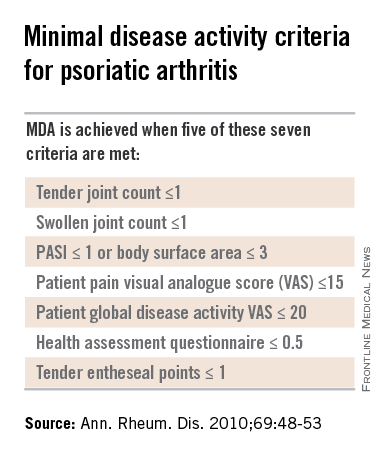
Dr. Helliwell is the principal investigator of the Tight Control of Psoriatic Arthritis (TICOPA) study, which he highlighted as an example of how targeting treatment to achieve minimal disease activity (MDA) criteria (Ann. Rheum. Dis. 2010;69:48-53) could be beneficial versus standard care.
A total of 206 patients with newly-diagnosed PsA were enrolled into the study and were randomized to receive either ‘tight control’ – meaning an intensive management strategy – or to standard care for 48 weeks.
Intensive treatment involved starting with methotrexate at a dose of 15 mg/kg per week and rapidly escalating to 25 mg/kg per week at 6 weeks if needed. If patients did not achieve five out of a set of seven MDA criteria for PsA, then methotrexate was continued and sulfasalazine was added and dose escalated after 4-8 weeks.
If MDA was still not achieved and criteria for biologics were not met according to NICE guidance, then alternatives were swapping out sulfasalazine for cyclosporine or leflunomide, again increasing the doses. Patients who did get put onto a biologic could switch to another anti-TNF if they did not respond after 12 weeks.
The primary endpoint data from the trial have been reported previously and showed that a higher proportion of patients in the intensive management group achieved ACR20 at 48 weeks, compared with those in the standard care group (62% vs. 45%, respectively; P = .02). A higher percentage of intensively managed patients also achieved ACR50 (51% vs. 25%; P = .0004) and ACR70 (38% vs. 17%, P = .002).
Skin symptoms, measured via the Psoriasis Area and Severity Index (PASI) showed significant improvement favoring intensive therapy over standard care. PASI20 was achieved by 72% of intensively managed patients versus 52% of those who received standard care. PASI75 was achieved by 59% versus 33%, respectively, and PASI90 by 40% versus 20%, respectively.
The full results of the study will be published soon in The Lancet and will include details of a variety of secondary outcomes and the cost-effectiveness of the intensive management strategy versus the standard care approach.
One of the key secondary outcomes of the trial was to look at the effects of the two treatment strategies on other PsA symptoms, such as enthesitis, dactylitis, and nail symptoms. No differences between the groups were observed, however, with similar decreases seen in the tight control and standard care groups.
There were no statistically significant differences in radiologic outcomes, which included baseline and end-of-treatment changes in the total modified Sharp van der Heijde (SVdH) score, the erosion score, and joint-space narrowing (JSN) score.
While this might seem somewhat disappointing, “there wasn’t a lot of radiologic progression going on anyway,” Dr. Helliwell pointed out. Overall, there was a difference of about 5% in the percentage of patients with at least one joint erosion at baseline and after 48 weeks of treatment.
The majority of radiographic change that did occur was JSN of the hands, Dr. Helliwell said, adding that patients with JSN tended to be slightly older than those without, although this observation did not reach statistical significance.
“We’ve since looked for associations with ACPA [anticitrullinated protein antibodies] – about 7% of our patients were ACPA positive – but there is no relationship,” he added. There was also no significant association between levels of C-reactive protein levels and erosive disease.
There was a trend suggesting that patients with erosions may be more likely to have polyarticular disease than oligoarticular disease, so a next step would be to look at radiologic progression in these two groups of patients in more detail.
More adverse events were seen in the tight control arm, compared with the standard care arm, including more serious adverse events, but not all of these were related to study treatment and many adverse events were those to be expected with methotrexate treatment, Dr. Helliwell observed.
But is the intensive approach cost-effective when compared to standard care? Data suggest that it is. Although the incremental cost-effectiveness ratio (ICER) initially exceeded the £20,000–£30,000 (about $31,000-$47,000) threshold used by the U.K. National Institute for Health and Care Excellence to judge if a new treatment is cost effective, allowing for certain factors enabled the ICER to be brought down to about £28,000 ($44,000), making it a cost-effective strategy.
PsA consists of five classical subtypes. The most common of these subtypes is polyarthritis (60% of patients), followed by oligoarthritis (30%). The remaining 10% of patients comprise those with arthritis mutilans, distal interphalangeal predominant disease, or spinal predominant disease. The clinical features of dactylitis and enthesitis are prevalent in about 40% and 50% of patients, respectively, and can occur in any subgroup.
Considering such heterogeneity in its presentation, the challenge now will be to determine if all clinical subgroups of PsA could benefit from treating to an MDA target with intensive management, or if one or other subgroups benefit more than another.
The TICOPA study was funded by Arthritis Research UK with support from Pfizer. Dr. Helliwell has received consulting fees from Pfizer.
Results of this study were published in the Lancet Sept. 30, 2015.
This article was updated October 6, 2015.
MANCHESTER, U.K. – Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis until they achieve a set of minimal disease activity criteria, an expert said at the British Society for Rheumatology annual conference.
While data are mounting on the value of treating to target (otherwise known as tight control) in psoriatic arthritis (PsA), these data lag significantly behind that for inducing and maintaining remission in rheumatoid arthritis (RA), noted Dr. Philip Helliwell of the University of Leeds (England).
“There is half as much evidence in PsA as there is in rheumatoid arthritis,” he observed. “But we’ve also got a heterogeneous disease and what we are going to have to do moving forward is to find out how to treat different phenotypic expressions of psoriatic disease, and we’re beginning to work towards that now,” he said during a Special Interest Group on Spondyloarthropathies.
Dr. Helliwell is the principal investigator of the Tight Control of Psoriatic Arthritis (TICOPA) study, which he highlighted as an example of how targeting treatment to achieve minimal disease activity (MDA) criteria (Ann. Rheum. Dis. 2010;69:48-53) could be beneficial versus standard care.
A total of 206 patients with newly-diagnosed PsA were enrolled into the study and were randomized to receive either ‘tight control’ – meaning an intensive management strategy – or to standard care for 48 weeks.
Intensive treatment involved starting with methotrexate at a dose of 15 mg/kg per week and rapidly escalating to 25 mg/kg per week at 6 weeks if needed. If patients did not achieve five out of a set of seven MDA criteria for PsA, then methotrexate was continued and sulfasalazine was added and dose escalated after 4-8 weeks.
If MDA was still not achieved and criteria for biologics were not met according to NICE guidance, then alternatives were swapping out sulfasalazine for cyclosporine or leflunomide, again increasing the doses. Patients who did get put onto a biologic could switch to another anti-TNF if they did not respond after 12 weeks.
The primary endpoint data from the trial have been reported previously and showed that a higher proportion of patients in the intensive management group achieved ACR20 at 48 weeks, compared with those in the standard care group (62% vs. 45%, respectively; P = .02). A higher percentage of intensively managed patients also achieved ACR50 (51% vs. 25%; P = .0004) and ACR70 (38% vs. 17%, P = .002).
Skin symptoms, measured via the Psoriasis Area and Severity Index (PASI) showed significant improvement favoring intensive therapy over standard care. PASI20 was achieved by 72% of intensively managed patients versus 52% of those who received standard care. PASI75 was achieved by 59% versus 33%, respectively, and PASI90 by 40% versus 20%, respectively.
The full results of the study will be published soon in The Lancet and will include details of a variety of secondary outcomes and the cost-effectiveness of the intensive management strategy versus the standard care approach.
One of the key secondary outcomes of the trial was to look at the effects of the two treatment strategies on other PsA symptoms, such as enthesitis, dactylitis, and nail symptoms. No differences between the groups were observed, however, with similar decreases seen in the tight control and standard care groups.
There were no statistically significant differences in radiologic outcomes, which included baseline and end-of-treatment changes in the total modified Sharp van der Heijde (SVdH) score, the erosion score, and joint-space narrowing (JSN) score.
While this might seem somewhat disappointing, “there wasn’t a lot of radiologic progression going on anyway,” Dr. Helliwell pointed out. Overall, there was a difference of about 5% in the percentage of patients with at least one joint erosion at baseline and after 48 weeks of treatment.
The majority of radiographic change that did occur was JSN of the hands, Dr. Helliwell said, adding that patients with JSN tended to be slightly older than those without, although this observation did not reach statistical significance.
“We’ve since looked for associations with ACPA [anticitrullinated protein antibodies] – about 7% of our patients were ACPA positive – but there is no relationship,” he added. There was also no significant association between levels of C-reactive protein levels and erosive disease.
There was a trend suggesting that patients with erosions may be more likely to have polyarticular disease than oligoarticular disease, so a next step would be to look at radiologic progression in these two groups of patients in more detail.
More adverse events were seen in the tight control arm, compared with the standard care arm, including more serious adverse events, but not all of these were related to study treatment and many adverse events were those to be expected with methotrexate treatment, Dr. Helliwell observed.
But is the intensive approach cost-effective when compared to standard care? Data suggest that it is. Although the incremental cost-effectiveness ratio (ICER) initially exceeded the £20,000–£30,000 (about $31,000-$47,000) threshold used by the U.K. National Institute for Health and Care Excellence to judge if a new treatment is cost effective, allowing for certain factors enabled the ICER to be brought down to about £28,000 ($44,000), making it a cost-effective strategy.
PsA consists of five classical subtypes. The most common of these subtypes is polyarthritis (60% of patients), followed by oligoarthritis (30%). The remaining 10% of patients comprise those with arthritis mutilans, distal interphalangeal predominant disease, or spinal predominant disease. The clinical features of dactylitis and enthesitis are prevalent in about 40% and 50% of patients, respectively, and can occur in any subgroup.
Considering such heterogeneity in its presentation, the challenge now will be to determine if all clinical subgroups of PsA could benefit from treating to an MDA target with intensive management, or if one or other subgroups benefit more than another.
The TICOPA study was funded by Arthritis Research UK with support from Pfizer. Dr. Helliwell has received consulting fees from Pfizer.
Results of this study were published in the Lancet Sept. 30, 2015.
This article was updated October 6, 2015.
MANCHESTER, U.K. – Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis until they achieve a set of minimal disease activity criteria, an expert said at the British Society for Rheumatology annual conference.
While data are mounting on the value of treating to target (otherwise known as tight control) in psoriatic arthritis (PsA), these data lag significantly behind that for inducing and maintaining remission in rheumatoid arthritis (RA), noted Dr. Philip Helliwell of the University of Leeds (England).
“There is half as much evidence in PsA as there is in rheumatoid arthritis,” he observed. “But we’ve also got a heterogeneous disease and what we are going to have to do moving forward is to find out how to treat different phenotypic expressions of psoriatic disease, and we’re beginning to work towards that now,” he said during a Special Interest Group on Spondyloarthropathies.
Dr. Helliwell is the principal investigator of the Tight Control of Psoriatic Arthritis (TICOPA) study, which he highlighted as an example of how targeting treatment to achieve minimal disease activity (MDA) criteria (Ann. Rheum. Dis. 2010;69:48-53) could be beneficial versus standard care.
A total of 206 patients with newly-diagnosed PsA were enrolled into the study and were randomized to receive either ‘tight control’ – meaning an intensive management strategy – or to standard care for 48 weeks.
Intensive treatment involved starting with methotrexate at a dose of 15 mg/kg per week and rapidly escalating to 25 mg/kg per week at 6 weeks if needed. If patients did not achieve five out of a set of seven MDA criteria for PsA, then methotrexate was continued and sulfasalazine was added and dose escalated after 4-8 weeks.
If MDA was still not achieved and criteria for biologics were not met according to NICE guidance, then alternatives were swapping out sulfasalazine for cyclosporine or leflunomide, again increasing the doses. Patients who did get put onto a biologic could switch to another anti-TNF if they did not respond after 12 weeks.
The primary endpoint data from the trial have been reported previously and showed that a higher proportion of patients in the intensive management group achieved ACR20 at 48 weeks, compared with those in the standard care group (62% vs. 45%, respectively; P = .02). A higher percentage of intensively managed patients also achieved ACR50 (51% vs. 25%; P = .0004) and ACR70 (38% vs. 17%, P = .002).
Skin symptoms, measured via the Psoriasis Area and Severity Index (PASI) showed significant improvement favoring intensive therapy over standard care. PASI20 was achieved by 72% of intensively managed patients versus 52% of those who received standard care. PASI75 was achieved by 59% versus 33%, respectively, and PASI90 by 40% versus 20%, respectively.
The full results of the study will be published soon in The Lancet and will include details of a variety of secondary outcomes and the cost-effectiveness of the intensive management strategy versus the standard care approach.
One of the key secondary outcomes of the trial was to look at the effects of the two treatment strategies on other PsA symptoms, such as enthesitis, dactylitis, and nail symptoms. No differences between the groups were observed, however, with similar decreases seen in the tight control and standard care groups.
There were no statistically significant differences in radiologic outcomes, which included baseline and end-of-treatment changes in the total modified Sharp van der Heijde (SVdH) score, the erosion score, and joint-space narrowing (JSN) score.
While this might seem somewhat disappointing, “there wasn’t a lot of radiologic progression going on anyway,” Dr. Helliwell pointed out. Overall, there was a difference of about 5% in the percentage of patients with at least one joint erosion at baseline and after 48 weeks of treatment.
The majority of radiographic change that did occur was JSN of the hands, Dr. Helliwell said, adding that patients with JSN tended to be slightly older than those without, although this observation did not reach statistical significance.
“We’ve since looked for associations with ACPA [anticitrullinated protein antibodies] – about 7% of our patients were ACPA positive – but there is no relationship,” he added. There was also no significant association between levels of C-reactive protein levels and erosive disease.
There was a trend suggesting that patients with erosions may be more likely to have polyarticular disease than oligoarticular disease, so a next step would be to look at radiologic progression in these two groups of patients in more detail.
More adverse events were seen in the tight control arm, compared with the standard care arm, including more serious adverse events, but not all of these were related to study treatment and many adverse events were those to be expected with methotrexate treatment, Dr. Helliwell observed.
But is the intensive approach cost-effective when compared to standard care? Data suggest that it is. Although the incremental cost-effectiveness ratio (ICER) initially exceeded the £20,000–£30,000 (about $31,000-$47,000) threshold used by the U.K. National Institute for Health and Care Excellence to judge if a new treatment is cost effective, allowing for certain factors enabled the ICER to be brought down to about £28,000 ($44,000), making it a cost-effective strategy.
PsA consists of five classical subtypes. The most common of these subtypes is polyarthritis (60% of patients), followed by oligoarthritis (30%). The remaining 10% of patients comprise those with arthritis mutilans, distal interphalangeal predominant disease, or spinal predominant disease. The clinical features of dactylitis and enthesitis are prevalent in about 40% and 50% of patients, respectively, and can occur in any subgroup.
Considering such heterogeneity in its presentation, the challenge now will be to determine if all clinical subgroups of PsA could benefit from treating to an MDA target with intensive management, or if one or other subgroups benefit more than another.
The TICOPA study was funded by Arthritis Research UK with support from Pfizer. Dr. Helliwell has received consulting fees from Pfizer.
Results of this study were published in the Lancet Sept. 30, 2015.
This article was updated October 6, 2015.
EXPERT ANALYSIS AT RHEUMATOLOGY 2015
Rate ratio of comorbidity high in SLE patients under 40
MANCHESTER, U.K. – When systemic lupus erythematosus (SLE) occurs before age 40, patients run a high relative risk of end-stage renal disease, data from a retrospective U.K.-based cohort study have shown.
While the risk for cardiovascular disease and stroke has been reported previously, particularly in younger SLE patients, the risks for comorbidities such as end-stage renal failure (ESRF), osteoporosis, and infection were not as clear. “We know that comorbidities are increased in patients with lupus, but we didn’t know by how much,” Dr. Frances Rees of Nottingham University Hospitals NHS Trust, Nottingham, England, explained at the British Society for Rheumatology annual conference.The adjusted incidence rate ratio (IRR) for ESRF was greater than 60 for lupus patients under age 40 and about 10 for those aged 40-69 years.“Although the absolute risk increased with age, the relative risk difference between cases and controls was highest in those at younger ages, so don’t forget primary prevention and screening in younger patients,” Dr. Rees said.
The risk was based on data obtained from the Clinical Practice Research Datalink, an anonymized database of primary care records for approximately 12 million people, on all prevalent cases of SLE occurring between 1999 and 2012 in the United Kingdom. Each of the 7,732 cases was matched to up to four patients who did not have lupus and were seen at the same practice. The control population exceeded 28,000 individuals.
Around 55% of lupus patients had a Charlson Comorbidity Index (CCI) of zero while around 75% of patients without lupus had no comorbidities. About 33% of lupus patients had a CCI of 1-2 as did about 20% of controls; less than 10% of patients had a CCI of 3-5 or more than 5.
“The highest difference between the two groups was for end-stage renal failure even after adjusting for confounders,” she added. IRRs for the other comorbidities were around or just under 2.“When we compared men and women, men had higher risks of cardiovascular disease, stroke, and cancer, but women had higher rates of infection and osteoporosis, which would fit with the underlying population,” Dr. Rees observed.
“What was interesting, however, was the difference in the incidence rates for osteoporosis between cases and controls in men, which was of a bigger relative risk than it was in women,” Dr. Rees noted. “So don’t forget to consider osteoporosis in men,” she advised. For cardiovascular disease, the IRR was much higher in patients under age 40 years than for the older patients (IRR <5).In an interview, Dr. Rees explained that while these data partly confirm what was already known, the research is the first to look at comorbidity in SLE from a community perspective. “Also, some of the previous studies done in hospitals have only really shown that the risk of cardiovascular disease and stroke was in younger people, but we have found that the risk was increased across all age groups.”
The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
MANCHESTER, U.K. – When systemic lupus erythematosus (SLE) occurs before age 40, patients run a high relative risk of end-stage renal disease, data from a retrospective U.K.-based cohort study have shown.
While the risk for cardiovascular disease and stroke has been reported previously, particularly in younger SLE patients, the risks for comorbidities such as end-stage renal failure (ESRF), osteoporosis, and infection were not as clear. “We know that comorbidities are increased in patients with lupus, but we didn’t know by how much,” Dr. Frances Rees of Nottingham University Hospitals NHS Trust, Nottingham, England, explained at the British Society for Rheumatology annual conference.The adjusted incidence rate ratio (IRR) for ESRF was greater than 60 for lupus patients under age 40 and about 10 for those aged 40-69 years.“Although the absolute risk increased with age, the relative risk difference between cases and controls was highest in those at younger ages, so don’t forget primary prevention and screening in younger patients,” Dr. Rees said.
The risk was based on data obtained from the Clinical Practice Research Datalink, an anonymized database of primary care records for approximately 12 million people, on all prevalent cases of SLE occurring between 1999 and 2012 in the United Kingdom. Each of the 7,732 cases was matched to up to four patients who did not have lupus and were seen at the same practice. The control population exceeded 28,000 individuals.
Around 55% of lupus patients had a Charlson Comorbidity Index (CCI) of zero while around 75% of patients without lupus had no comorbidities. About 33% of lupus patients had a CCI of 1-2 as did about 20% of controls; less than 10% of patients had a CCI of 3-5 or more than 5.
“The highest difference between the two groups was for end-stage renal failure even after adjusting for confounders,” she added. IRRs for the other comorbidities were around or just under 2.“When we compared men and women, men had higher risks of cardiovascular disease, stroke, and cancer, but women had higher rates of infection and osteoporosis, which would fit with the underlying population,” Dr. Rees observed.
“What was interesting, however, was the difference in the incidence rates for osteoporosis between cases and controls in men, which was of a bigger relative risk than it was in women,” Dr. Rees noted. “So don’t forget to consider osteoporosis in men,” she advised. For cardiovascular disease, the IRR was much higher in patients under age 40 years than for the older patients (IRR <5).In an interview, Dr. Rees explained that while these data partly confirm what was already known, the research is the first to look at comorbidity in SLE from a community perspective. “Also, some of the previous studies done in hospitals have only really shown that the risk of cardiovascular disease and stroke was in younger people, but we have found that the risk was increased across all age groups.”
The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
MANCHESTER, U.K. – When systemic lupus erythematosus (SLE) occurs before age 40, patients run a high relative risk of end-stage renal disease, data from a retrospective U.K.-based cohort study have shown.
While the risk for cardiovascular disease and stroke has been reported previously, particularly in younger SLE patients, the risks for comorbidities such as end-stage renal failure (ESRF), osteoporosis, and infection were not as clear. “We know that comorbidities are increased in patients with lupus, but we didn’t know by how much,” Dr. Frances Rees of Nottingham University Hospitals NHS Trust, Nottingham, England, explained at the British Society for Rheumatology annual conference.The adjusted incidence rate ratio (IRR) for ESRF was greater than 60 for lupus patients under age 40 and about 10 for those aged 40-69 years.“Although the absolute risk increased with age, the relative risk difference between cases and controls was highest in those at younger ages, so don’t forget primary prevention and screening in younger patients,” Dr. Rees said.
The risk was based on data obtained from the Clinical Practice Research Datalink, an anonymized database of primary care records for approximately 12 million people, on all prevalent cases of SLE occurring between 1999 and 2012 in the United Kingdom. Each of the 7,732 cases was matched to up to four patients who did not have lupus and were seen at the same practice. The control population exceeded 28,000 individuals.
Around 55% of lupus patients had a Charlson Comorbidity Index (CCI) of zero while around 75% of patients without lupus had no comorbidities. About 33% of lupus patients had a CCI of 1-2 as did about 20% of controls; less than 10% of patients had a CCI of 3-5 or more than 5.
“The highest difference between the two groups was for end-stage renal failure even after adjusting for confounders,” she added. IRRs for the other comorbidities were around or just under 2.“When we compared men and women, men had higher risks of cardiovascular disease, stroke, and cancer, but women had higher rates of infection and osteoporosis, which would fit with the underlying population,” Dr. Rees observed.
“What was interesting, however, was the difference in the incidence rates for osteoporosis between cases and controls in men, which was of a bigger relative risk than it was in women,” Dr. Rees noted. “So don’t forget to consider osteoporosis in men,” she advised. For cardiovascular disease, the IRR was much higher in patients under age 40 years than for the older patients (IRR <5).In an interview, Dr. Rees explained that while these data partly confirm what was already known, the research is the first to look at comorbidity in SLE from a community perspective. “Also, some of the previous studies done in hospitals have only really shown that the risk of cardiovascular disease and stroke was in younger people, but we have found that the risk was increased across all age groups.”
The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
Key clinical point: The relative comorbidity burden is highest in SLE patients under age 40.
Major finding: The adjusted incidence rate ratio (IRR) for end-stage renal failure was 60-fold higher in patients under age 40 years and 10-fold higher in patients aged 40-69 years, compared to controls.
Data source: Retrospective cohort study of 7,732 patients with systemic lupus erythematosus and 29,079 lupus-free individuals.
Disclosures: The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
BSR: Flagging early symptoms could diagnose lupus sooner
MANCHESTER, U.K. – In a general practice setting, people who developed systemic lupus erythematosus were more than twice as likely to consult a primary care physician in the 5-year run-up to their diagnosis, according to the results of a case-control study.
The median annual consultation rates were 9.2 times for systemic lupus erythematosus (SLE) patients versus 3.8 times for controls (P < .001).
Lupus patients also started to exhibit telltale symptoms during this time, providing an opportunity for making a diagnosis sooner through better recognition of these early symptoms.
The aim of the study was “to find out whether people with lupus came to see their GP [general practitioner] more frequently and what they were going to see their GP for,” Dr. Frances Rees of Nottingham (England) University Hospitals NHS Trust explained in a poster session on connective tissue disease at the British Society for Rheumatology annual conference.
“After that, we developed a risk-prediction model which was a multivariate analysis taking some of the variables that we decided in advance would be most significant,” she added. These variables included age, gender, arthritis or arthralgia, rash, fatigue, alopecia, serositis, sicca, Raynaud’s phenomenon, and the consultation rate.
“We found that the model performed well in both the model development data set and in the model validation data set,” Dr. Rees said.
The rationale for the study was that SLE can be difficult to diagnose early, and that delays of more than 7 years have been found between symptom onset and diagnosis. Perhaps, if SLE is diagnosed earlier, outcomes could be improved, Dr. Rees and her coauthors reasoned.
Using the UK Clinical Practice Research Datalink, an anonymized database of general practice records for approximately 12 million people, all incident cases of SLE occurring between 1999 and 2012 were identified and matched to four individuals without lupus who were seen at the same primary care practices.
A total of 1,739 patients with lupus and 6,952 controls were identified and separated into two cohorts: approximately two-thirds formed the development cohort and the remainder a validation cohort.
Individual odds ratios (OR) were calculated for age, gender, consultation rates, 27 clinical features, and five previous diagnoses of rheumatic disease in the 5 years preceding SLE diagnosis. Some of the clinical features considered were arthritis or arthralgia, rash, fatigue, alopecia, serositis, and sicca; the associated diseases were another connective tissue disease, rheumatoid arthritis (RA), fibromyalgia, chronic fatigue syndrome (CFS), and Epstein-Barr virus (EBV) infection.
Results showed that cases were more than five times more likely than controls to be female (84% vs. 51%; OR, 5.23; P < .001).
Early symptoms suggestive of SLE occurred more often in cases than in controls, with arthritis or arthralgia in 36% vs. 10% (OR, 4.77; P < .001), rash in 43% vs. 10% (OR, 6.84; P < .001), fatigue in 17% vs. 7% (OR, 2.76; P < .001), alopecia in 4% vs. 1% (OR, 4.77; P < .001), serositis in 4% vs. 1% (OR, 5.05; P < .001), and sicca in 6% vs 1% (OR, 4.97; P < .001). Raynaud’s phenomenon was seen in 4% vs. 0%, with an OR of 15.29, which was significant (P < .001), but the confidence interval (CI) was wide (CI, 9.41-24.85).
Patients with SLE also were more than 20 times more likely to have a prior diagnosis of another connective tissue disease (OR, 21.68; P < .001) and 10 times more likely to have been diagnosed with RA (OR, 10.77; P < .001). Fibromyalgia (OR, 7.89; P < .001) and CFS (OR, 6.39; P < .001) also were more frequent among those who developed lupus than in those who did not.
“This suggests that, although there probably is a delay in diagnosis, patients are getting other diagnoses first,” Dr. Rees noted. “So, we would propose that, in the future, our risk-prediction model may be able to reduce that delay in conjunction with educational programs to say if you have a combination of these symptoms think about testing for [antinuclear antibodies] and/or referring to rheumatology.”
The team’s risk-prediction model now needs further external and economic validation, but the hope is that perhaps it could be incorporated into electronic systems used in primary care to flag potential lupus cases.
“The clinical message is to keep SLE in mind if someone is presenting multiple times with these kind of symptoms,” Dr. Rees said in an interview.
“Lupus is very rare,” she acknowledged, nothing that some primary care physicians may only see one or two cases of lupus in their career. So the risk-prediction model would aim to be an aid to help them consider SLE as a possibility in patients with several symptoms that, on their own, might not be suggestive of lupus.
The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
MANCHESTER, U.K. – In a general practice setting, people who developed systemic lupus erythematosus were more than twice as likely to consult a primary care physician in the 5-year run-up to their diagnosis, according to the results of a case-control study.
The median annual consultation rates were 9.2 times for systemic lupus erythematosus (SLE) patients versus 3.8 times for controls (P < .001).
Lupus patients also started to exhibit telltale symptoms during this time, providing an opportunity for making a diagnosis sooner through better recognition of these early symptoms.
The aim of the study was “to find out whether people with lupus came to see their GP [general practitioner] more frequently and what they were going to see their GP for,” Dr. Frances Rees of Nottingham (England) University Hospitals NHS Trust explained in a poster session on connective tissue disease at the British Society for Rheumatology annual conference.
“After that, we developed a risk-prediction model which was a multivariate analysis taking some of the variables that we decided in advance would be most significant,” she added. These variables included age, gender, arthritis or arthralgia, rash, fatigue, alopecia, serositis, sicca, Raynaud’s phenomenon, and the consultation rate.
“We found that the model performed well in both the model development data set and in the model validation data set,” Dr. Rees said.
The rationale for the study was that SLE can be difficult to diagnose early, and that delays of more than 7 years have been found between symptom onset and diagnosis. Perhaps, if SLE is diagnosed earlier, outcomes could be improved, Dr. Rees and her coauthors reasoned.
Using the UK Clinical Practice Research Datalink, an anonymized database of general practice records for approximately 12 million people, all incident cases of SLE occurring between 1999 and 2012 were identified and matched to four individuals without lupus who were seen at the same primary care practices.
A total of 1,739 patients with lupus and 6,952 controls were identified and separated into two cohorts: approximately two-thirds formed the development cohort and the remainder a validation cohort.
Individual odds ratios (OR) were calculated for age, gender, consultation rates, 27 clinical features, and five previous diagnoses of rheumatic disease in the 5 years preceding SLE diagnosis. Some of the clinical features considered were arthritis or arthralgia, rash, fatigue, alopecia, serositis, and sicca; the associated diseases were another connective tissue disease, rheumatoid arthritis (RA), fibromyalgia, chronic fatigue syndrome (CFS), and Epstein-Barr virus (EBV) infection.
Results showed that cases were more than five times more likely than controls to be female (84% vs. 51%; OR, 5.23; P < .001).
Early symptoms suggestive of SLE occurred more often in cases than in controls, with arthritis or arthralgia in 36% vs. 10% (OR, 4.77; P < .001), rash in 43% vs. 10% (OR, 6.84; P < .001), fatigue in 17% vs. 7% (OR, 2.76; P < .001), alopecia in 4% vs. 1% (OR, 4.77; P < .001), serositis in 4% vs. 1% (OR, 5.05; P < .001), and sicca in 6% vs 1% (OR, 4.97; P < .001). Raynaud’s phenomenon was seen in 4% vs. 0%, with an OR of 15.29, which was significant (P < .001), but the confidence interval (CI) was wide (CI, 9.41-24.85).
Patients with SLE also were more than 20 times more likely to have a prior diagnosis of another connective tissue disease (OR, 21.68; P < .001) and 10 times more likely to have been diagnosed with RA (OR, 10.77; P < .001). Fibromyalgia (OR, 7.89; P < .001) and CFS (OR, 6.39; P < .001) also were more frequent among those who developed lupus than in those who did not.
“This suggests that, although there probably is a delay in diagnosis, patients are getting other diagnoses first,” Dr. Rees noted. “So, we would propose that, in the future, our risk-prediction model may be able to reduce that delay in conjunction with educational programs to say if you have a combination of these symptoms think about testing for [antinuclear antibodies] and/or referring to rheumatology.”
The team’s risk-prediction model now needs further external and economic validation, but the hope is that perhaps it could be incorporated into electronic systems used in primary care to flag potential lupus cases.
“The clinical message is to keep SLE in mind if someone is presenting multiple times with these kind of symptoms,” Dr. Rees said in an interview.
“Lupus is very rare,” she acknowledged, nothing that some primary care physicians may only see one or two cases of lupus in their career. So the risk-prediction model would aim to be an aid to help them consider SLE as a possibility in patients with several symptoms that, on their own, might not be suggestive of lupus.
The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
MANCHESTER, U.K. – In a general practice setting, people who developed systemic lupus erythematosus were more than twice as likely to consult a primary care physician in the 5-year run-up to their diagnosis, according to the results of a case-control study.
The median annual consultation rates were 9.2 times for systemic lupus erythematosus (SLE) patients versus 3.8 times for controls (P < .001).
Lupus patients also started to exhibit telltale symptoms during this time, providing an opportunity for making a diagnosis sooner through better recognition of these early symptoms.
The aim of the study was “to find out whether people with lupus came to see their GP [general practitioner] more frequently and what they were going to see their GP for,” Dr. Frances Rees of Nottingham (England) University Hospitals NHS Trust explained in a poster session on connective tissue disease at the British Society for Rheumatology annual conference.
“After that, we developed a risk-prediction model which was a multivariate analysis taking some of the variables that we decided in advance would be most significant,” she added. These variables included age, gender, arthritis or arthralgia, rash, fatigue, alopecia, serositis, sicca, Raynaud’s phenomenon, and the consultation rate.
“We found that the model performed well in both the model development data set and in the model validation data set,” Dr. Rees said.
The rationale for the study was that SLE can be difficult to diagnose early, and that delays of more than 7 years have been found between symptom onset and diagnosis. Perhaps, if SLE is diagnosed earlier, outcomes could be improved, Dr. Rees and her coauthors reasoned.
Using the UK Clinical Practice Research Datalink, an anonymized database of general practice records for approximately 12 million people, all incident cases of SLE occurring between 1999 and 2012 were identified and matched to four individuals without lupus who were seen at the same primary care practices.
A total of 1,739 patients with lupus and 6,952 controls were identified and separated into two cohorts: approximately two-thirds formed the development cohort and the remainder a validation cohort.
Individual odds ratios (OR) were calculated for age, gender, consultation rates, 27 clinical features, and five previous diagnoses of rheumatic disease in the 5 years preceding SLE diagnosis. Some of the clinical features considered were arthritis or arthralgia, rash, fatigue, alopecia, serositis, and sicca; the associated diseases were another connective tissue disease, rheumatoid arthritis (RA), fibromyalgia, chronic fatigue syndrome (CFS), and Epstein-Barr virus (EBV) infection.
Results showed that cases were more than five times more likely than controls to be female (84% vs. 51%; OR, 5.23; P < .001).
Early symptoms suggestive of SLE occurred more often in cases than in controls, with arthritis or arthralgia in 36% vs. 10% (OR, 4.77; P < .001), rash in 43% vs. 10% (OR, 6.84; P < .001), fatigue in 17% vs. 7% (OR, 2.76; P < .001), alopecia in 4% vs. 1% (OR, 4.77; P < .001), serositis in 4% vs. 1% (OR, 5.05; P < .001), and sicca in 6% vs 1% (OR, 4.97; P < .001). Raynaud’s phenomenon was seen in 4% vs. 0%, with an OR of 15.29, which was significant (P < .001), but the confidence interval (CI) was wide (CI, 9.41-24.85).
Patients with SLE also were more than 20 times more likely to have a prior diagnosis of another connective tissue disease (OR, 21.68; P < .001) and 10 times more likely to have been diagnosed with RA (OR, 10.77; P < .001). Fibromyalgia (OR, 7.89; P < .001) and CFS (OR, 6.39; P < .001) also were more frequent among those who developed lupus than in those who did not.
“This suggests that, although there probably is a delay in diagnosis, patients are getting other diagnoses first,” Dr. Rees noted. “So, we would propose that, in the future, our risk-prediction model may be able to reduce that delay in conjunction with educational programs to say if you have a combination of these symptoms think about testing for [antinuclear antibodies] and/or referring to rheumatology.”
The team’s risk-prediction model now needs further external and economic validation, but the hope is that perhaps it could be incorporated into electronic systems used in primary care to flag potential lupus cases.
“The clinical message is to keep SLE in mind if someone is presenting multiple times with these kind of symptoms,” Dr. Rees said in an interview.
“Lupus is very rare,” she acknowledged, nothing that some primary care physicians may only see one or two cases of lupus in their career. So the risk-prediction model would aim to be an aid to help them consider SLE as a possibility in patients with several symptoms that, on their own, might not be suggestive of lupus.
The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
AT RHEUMATOLOGY 2015
Key clinical point: A risk-prediction model may help primary care physicians diagnose lupus earlier.
Major finding: Lupus patients consulted more frequently than controls (median of 9.2 vs.<b/>3.8 times a year, P < .001).
Data source: 1,739 patients with newly diagnosed systemic lupus erythematosus and 6,952 practice-matched controls.
Disclosures: The work was supported by a research grant from Lupus UK. Dr. Rees had no conflicts of interest.
Shortening all-oral HCV therapy feasible
VIENNA – All-oral HCV therapy with grazoprevir and elbasvir plus sofosbuvir achieved high, sustained virologic response (SVR) rates at 12 weeks after only 6-8 weeks of therapy in a proof-of-concept phase II study.
“HCV therapy is evolving toward a once-daily, short duration, pan-genotypic, highly effective and safe regimen,” said Dr. Fred Poordad of the Texas Liver Institute, San Antonio, who reported the findings of the C-SWIFT study at the meeting sponsored by the European Association for the Study of the Liver.
“The aim of this study was to combine three highly potent direct-acting antivirals (DAAs) grazoprevir, elbasvir and sofosbuvir, each with a different mechanisms of action, to see if we could shorten therapy in two of the most difficult-to-cure populations, genotype 1 [GT1] and genotype 3 [GT3] with and without cirrhosis,” he said.
For inclusion, patients had to be treatment naive and older than 18 years of age. Cirrhosis had to be present, assessed either by liver biopsy or via noninvasive testing, and the minimum hemoglobin at enrollment needed to be 9.5 g/dL. Patients also had to be negative for HIV and the hepatitis B virus, and have liver enzyme below 350 IU/L.
Treatment consisted of a fixed-dose, once daily combination of grazoprevir 100 mg and elbasvir 50 mg plus additional sofosbuvir 400 mg, once daily. The duration of therapy depended on genotype and the presence or absence of cirrhosis.
A total of 102 patients with GT1 were enrolled, of whom 61 had no cirrhosis and were treated with the three-drug combination for a total of 4 (n = 31) or 6 (n = 30) weeks. Patients with GT1 and cirrhosis were treated with the regimen for 6 (n = 20) or 8 (n = 21) weeks.
There were 41 GT3 patients enrolled, of whom 29 had no cirrhosis and were treated for 8 (n = 15) or 12 weeks (n = 14). The 12 GT3 cirrhotic patients received 12 weeks of the regimen.
Dr. Poordad noted that the majority of patients studied were male, in their mid-50s, and of white ethnicity. The majority of the GT1 patients were subtype 1a.
Results showed that 33% of GT1 patients without cirrhosis achieved SVR12 with 4 weeks of treatment, and 87% with 6 weeks therapy. SVR12 was achieved in 80% and 94% of patients with GT1 and cirrhosis after 6 and 8 weeks of therapy, respectively.
No patient experienced breakthrough. Twenty of the noncirrhotic GT1 patients treated for 4 weeks relapsed, as did four who were treated for 6 weeks. Four relapses also occurred in cirrhotic GT1 patients treated for 6 weeks and one patient with cirrhosis relapsed after 8 weeks of treatment.
Dr. Poordad noted that patients relapsed most commonly if they had either wild-type virus or resistant-associated variants (RAV) already present at baseline.
In the GT3 arms, SVR12 was achieved by 93% and 100% of the noncirrhotic patients treated for 8 weeks and 12 weeks, respectively, and by 91% of those with cirrhosis who had 12 weeks of treatment. One patient discontinued treatment early for a reason other than virologic failure and two patients relapsed – one patient without cirrhosis in the 8-week treatment group and one in the cirrhotic group.
“All of the patients became ‘target not detected’ at the end of treatment,” Dr. Poordad observed. “The patient in the 8-week arm relapsed somewhere between SVR4 and SVR12, while the cirrhotic patient relapsed in the first 4 weeks of follow-up” he said.
Of the two GT3 patients with biologic failures, one did not have baseline RAVS and also had wild-type virus at relapse. The other patient had RAVs at baseline in NS3 and in NS5A at relapse.
There were “very few adverse events,” Dr. Poordad said, noting that there were no significant declines in hemoglobin or elevations in liver enzymes or bilirubin. The most common adverse events were headache, fatigue, and nausea, all of which occurred in fairly low numbers.
“This novel regimen of grazoprevir/elbasvir with sofosbuvir was able to shorten therapy duration to 8 weeks or less among patients who were cirrhotic and noncirrhotic with genotype 1,” Dr. Poordad said in summary.
Even GT3 patients were able to achieve high SVR12 rates with 8-12 weeks of therapy, including those who had cirrhosis.
“The concept of shortening therapy, even in the cirrhotic patients, has been demonstrated by this study,” he concluded.
He noted that retreatment of virologic failures was ongoing.
The study was sponsored by Merck & Co. Dr. Poordad has received grant/research support from Merck & Co. and numerous other pharmaceutical companies.
VIENNA – All-oral HCV therapy with grazoprevir and elbasvir plus sofosbuvir achieved high, sustained virologic response (SVR) rates at 12 weeks after only 6-8 weeks of therapy in a proof-of-concept phase II study.
“HCV therapy is evolving toward a once-daily, short duration, pan-genotypic, highly effective and safe regimen,” said Dr. Fred Poordad of the Texas Liver Institute, San Antonio, who reported the findings of the C-SWIFT study at the meeting sponsored by the European Association for the Study of the Liver.
“The aim of this study was to combine three highly potent direct-acting antivirals (DAAs) grazoprevir, elbasvir and sofosbuvir, each with a different mechanisms of action, to see if we could shorten therapy in two of the most difficult-to-cure populations, genotype 1 [GT1] and genotype 3 [GT3] with and without cirrhosis,” he said.
For inclusion, patients had to be treatment naive and older than 18 years of age. Cirrhosis had to be present, assessed either by liver biopsy or via noninvasive testing, and the minimum hemoglobin at enrollment needed to be 9.5 g/dL. Patients also had to be negative for HIV and the hepatitis B virus, and have liver enzyme below 350 IU/L.
Treatment consisted of a fixed-dose, once daily combination of grazoprevir 100 mg and elbasvir 50 mg plus additional sofosbuvir 400 mg, once daily. The duration of therapy depended on genotype and the presence or absence of cirrhosis.
A total of 102 patients with GT1 were enrolled, of whom 61 had no cirrhosis and were treated with the three-drug combination for a total of 4 (n = 31) or 6 (n = 30) weeks. Patients with GT1 and cirrhosis were treated with the regimen for 6 (n = 20) or 8 (n = 21) weeks.
There were 41 GT3 patients enrolled, of whom 29 had no cirrhosis and were treated for 8 (n = 15) or 12 weeks (n = 14). The 12 GT3 cirrhotic patients received 12 weeks of the regimen.
Dr. Poordad noted that the majority of patients studied were male, in their mid-50s, and of white ethnicity. The majority of the GT1 patients were subtype 1a.
Results showed that 33% of GT1 patients without cirrhosis achieved SVR12 with 4 weeks of treatment, and 87% with 6 weeks therapy. SVR12 was achieved in 80% and 94% of patients with GT1 and cirrhosis after 6 and 8 weeks of therapy, respectively.
No patient experienced breakthrough. Twenty of the noncirrhotic GT1 patients treated for 4 weeks relapsed, as did four who were treated for 6 weeks. Four relapses also occurred in cirrhotic GT1 patients treated for 6 weeks and one patient with cirrhosis relapsed after 8 weeks of treatment.
Dr. Poordad noted that patients relapsed most commonly if they had either wild-type virus or resistant-associated variants (RAV) already present at baseline.
In the GT3 arms, SVR12 was achieved by 93% and 100% of the noncirrhotic patients treated for 8 weeks and 12 weeks, respectively, and by 91% of those with cirrhosis who had 12 weeks of treatment. One patient discontinued treatment early for a reason other than virologic failure and two patients relapsed – one patient without cirrhosis in the 8-week treatment group and one in the cirrhotic group.
“All of the patients became ‘target not detected’ at the end of treatment,” Dr. Poordad observed. “The patient in the 8-week arm relapsed somewhere between SVR4 and SVR12, while the cirrhotic patient relapsed in the first 4 weeks of follow-up” he said.
Of the two GT3 patients with biologic failures, one did not have baseline RAVS and also had wild-type virus at relapse. The other patient had RAVs at baseline in NS3 and in NS5A at relapse.
There were “very few adverse events,” Dr. Poordad said, noting that there were no significant declines in hemoglobin or elevations in liver enzymes or bilirubin. The most common adverse events were headache, fatigue, and nausea, all of which occurred in fairly low numbers.
“This novel regimen of grazoprevir/elbasvir with sofosbuvir was able to shorten therapy duration to 8 weeks or less among patients who were cirrhotic and noncirrhotic with genotype 1,” Dr. Poordad said in summary.
Even GT3 patients were able to achieve high SVR12 rates with 8-12 weeks of therapy, including those who had cirrhosis.
“The concept of shortening therapy, even in the cirrhotic patients, has been demonstrated by this study,” he concluded.
He noted that retreatment of virologic failures was ongoing.
The study was sponsored by Merck & Co. Dr. Poordad has received grant/research support from Merck & Co. and numerous other pharmaceutical companies.
VIENNA – All-oral HCV therapy with grazoprevir and elbasvir plus sofosbuvir achieved high, sustained virologic response (SVR) rates at 12 weeks after only 6-8 weeks of therapy in a proof-of-concept phase II study.
“HCV therapy is evolving toward a once-daily, short duration, pan-genotypic, highly effective and safe regimen,” said Dr. Fred Poordad of the Texas Liver Institute, San Antonio, who reported the findings of the C-SWIFT study at the meeting sponsored by the European Association for the Study of the Liver.
“The aim of this study was to combine three highly potent direct-acting antivirals (DAAs) grazoprevir, elbasvir and sofosbuvir, each with a different mechanisms of action, to see if we could shorten therapy in two of the most difficult-to-cure populations, genotype 1 [GT1] and genotype 3 [GT3] with and without cirrhosis,” he said.
For inclusion, patients had to be treatment naive and older than 18 years of age. Cirrhosis had to be present, assessed either by liver biopsy or via noninvasive testing, and the minimum hemoglobin at enrollment needed to be 9.5 g/dL. Patients also had to be negative for HIV and the hepatitis B virus, and have liver enzyme below 350 IU/L.
Treatment consisted of a fixed-dose, once daily combination of grazoprevir 100 mg and elbasvir 50 mg plus additional sofosbuvir 400 mg, once daily. The duration of therapy depended on genotype and the presence or absence of cirrhosis.
A total of 102 patients with GT1 were enrolled, of whom 61 had no cirrhosis and were treated with the three-drug combination for a total of 4 (n = 31) or 6 (n = 30) weeks. Patients with GT1 and cirrhosis were treated with the regimen for 6 (n = 20) or 8 (n = 21) weeks.
There were 41 GT3 patients enrolled, of whom 29 had no cirrhosis and were treated for 8 (n = 15) or 12 weeks (n = 14). The 12 GT3 cirrhotic patients received 12 weeks of the regimen.
Dr. Poordad noted that the majority of patients studied were male, in their mid-50s, and of white ethnicity. The majority of the GT1 patients were subtype 1a.
Results showed that 33% of GT1 patients without cirrhosis achieved SVR12 with 4 weeks of treatment, and 87% with 6 weeks therapy. SVR12 was achieved in 80% and 94% of patients with GT1 and cirrhosis after 6 and 8 weeks of therapy, respectively.
No patient experienced breakthrough. Twenty of the noncirrhotic GT1 patients treated for 4 weeks relapsed, as did four who were treated for 6 weeks. Four relapses also occurred in cirrhotic GT1 patients treated for 6 weeks and one patient with cirrhosis relapsed after 8 weeks of treatment.
Dr. Poordad noted that patients relapsed most commonly if they had either wild-type virus or resistant-associated variants (RAV) already present at baseline.
In the GT3 arms, SVR12 was achieved by 93% and 100% of the noncirrhotic patients treated for 8 weeks and 12 weeks, respectively, and by 91% of those with cirrhosis who had 12 weeks of treatment. One patient discontinued treatment early for a reason other than virologic failure and two patients relapsed – one patient without cirrhosis in the 8-week treatment group and one in the cirrhotic group.
“All of the patients became ‘target not detected’ at the end of treatment,” Dr. Poordad observed. “The patient in the 8-week arm relapsed somewhere between SVR4 and SVR12, while the cirrhotic patient relapsed in the first 4 weeks of follow-up” he said.
Of the two GT3 patients with biologic failures, one did not have baseline RAVS and also had wild-type virus at relapse. The other patient had RAVs at baseline in NS3 and in NS5A at relapse.
There were “very few adverse events,” Dr. Poordad said, noting that there were no significant declines in hemoglobin or elevations in liver enzymes or bilirubin. The most common adverse events were headache, fatigue, and nausea, all of which occurred in fairly low numbers.
“This novel regimen of grazoprevir/elbasvir with sofosbuvir was able to shorten therapy duration to 8 weeks or less among patients who were cirrhotic and noncirrhotic with genotype 1,” Dr. Poordad said in summary.
Even GT3 patients were able to achieve high SVR12 rates with 8-12 weeks of therapy, including those who had cirrhosis.
“The concept of shortening therapy, even in the cirrhotic patients, has been demonstrated by this study,” he concluded.
He noted that retreatment of virologic failures was ongoing.
The study was sponsored by Merck & Co. Dr. Poordad has received grant/research support from Merck & Co. and numerous other pharmaceutical companies.
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: Shortening HCV therapy to 6-8 weeks is possible, even in cirrhotic patients.
Major finding: SVR12 was achieved by 87%-94% of GT1 patients treated for 6-8 weeks and by 91%-100% of GT3 patients treated for 8-12 weeks.
Data source: Phase II, open-label study of 143 treatment-naive patients with HCV genotype 1 or 3 with or without cirrhosis treated with a fixed-dose combination of grazoprevir (100 mg) and elbasvir (50 mg) plus sofosbuvir (400 mg) for 4, 6, 8, or 12 weeks.
Disclosures: The study was sponsored by Merck & Co. Dr. Poordad has received grant/research support from Merck & Co. and numerous other pharmaceutical companies.
Anti-CarP antibodies linked to worse outcome in early arthritis
MANCHESTER, ENGLAND – Anti–carbamylated protein antibodies were associated with both increased disability and greater disease progression over time in patients with early inflammatory arthritis, data from a 20-year retrospective, observational analysis showed.
According to the research, presented at the British Society for Rheumatology annual conference, patients who were also positive for anti–citrullinated protein antibodies (ACPAs) fared worse long term than those who were ACPA negative.
The results showed that anti–carbamylated protein (anti-CarP) antibodies may provide additional prognostic information to current antibody tests in inflammatory arthritis (IA), but their measurement is not yet ready for general use, said Dr. Jennifer Humphreys of the Arthritis Research UK Centre for Epidemiology at the University of Manchester.
“We know that inflammatory arthritis patients who are rheumatoid factor [RF] or ACPA positive have an increased risk of persistent disease and a poor prognosis,” she said.
“In particular, ACPA[s] are associated with severe radiological progression [and] increased disability and mortality. However, not all patients who lack these antibodies do well,” Dr. Humphreys said.
Research at the University of Leiden (the Netherlands) has found that ACPA-negative patients have anti-CarP antibodies in their sera and that these autoantibodies precede the onset of symptoms and predict the development of rheumatoid arthritis (RA) in patients with arthralgia. These antibodies have also been associated with radiological damage, she said.
Because there were few data on the relationship to disease activity and long-term outcomes in the presence of these antibodies, Dr. Humphreys and her associates decided to look at this using data from the Norfolk Arthritis Register (NOAR), she said. The U.K. team worked in collaboration with the Dutch researchers who performed the anti-CarP tests on serum samples taken from 1,995 patients with early IA enrolled in NOAR from 1990 onward.
At baseline, 1,222 patients met American College of Rheumatology/European League Against Rheumatism 2010 criteria for RA. The mean age of onset was 55 years for both the total population with IA and for those with RA. About two-thirds of the participants were women, and their median symptom duration at enrollment was 33 weeks.
Long-term disability was assessed using the health assessment questionnaire (HAQ), and disease activity was evaluated using the disease activity in 28 joints (DAS28) score. The median baseline DAS28 score was 3.81 for the total cohort and 4.45 for patients with RA, and the median baseline HAQ scores were a respective 0.875 and 1.125.
When the team looked at patient baseline characteristics according to baseline anti-CarP status, they found that the 460 (23%) patients who were positive for these autoantibodies were more likely to have higher baseline DAS28 scores (4.23 vs. 3.73), HAQ scores (1.125 vs. 0.875), and C-reactive protein levels (14 mg/L vs. 7 mg/L) than the 1,543 patients who tested anti-CarP negative. Patients positive for anti-Carp antibodies were also less likely to be female (58% vs 68%) and were slightly older at onset (55 vs. 53 years).
Dr. Humphreys noted that there was a lot of overlap among the presence of anti-CarP, ACPA, and RF. Of the 460 who were anti-CarP positive, 124 were positive only for anti-CarP, 148 were also RF positive, 30 were also ACPA positive, and 158 had all three antibodies.
“Patients who were anti-CarP antibody positive had higher HAQ scores at baseline, and this higher level of disability persisted throughout follow-up, and that association remained significant in the multivariate model,” Dr. Humphreys said.
“When we stratified by ACPA status, there was a statistically significant association in the ACPA-negative subgroup but not in the ACPA-positive subgroup,” she added. However, she noted that the beta coefficients for the association between anti-CarP antibodies and HAQ scores over time were similar (0.12 for the total cohort, 0.14 for the ACPA-negative patients, and 0.09 for the ACPA-positive patients) and that confidence intervals were wide.
Similar findings were seen for the association between anti-CarP antibodies and the DAS28 over time, with respective beta coefficients of 0.23, 01.18, and 0.25 for the total cohort, the ACPA-negative subgroup, and the ACPA-positive subgroup. Again, confidence intervals were wide.
The researchers looked at the additive effect of having anti-CarP and ACPA, and found that while there is no association between RF positivity and long-term disability, there were independent associations with both ACPA positivity and anti-CarP antibody positivity, with respective beta coefficients of –0.05, 0.12, and 0.14.
“And we see the exact same pattern with disease activity over time,” Dr. Humphreys reported.
Thus, measuring anti-CarP antibodies “may be a useful test to do in addition to both RF and ACPA,” she suggested, adding that measuring these antibodies “may be particularly valuable in patients who lack other antibodies.”
NOAR is funded by Arthritis Research UK. Dr. Humphreys had no relevant financial disclosures.
MANCHESTER, ENGLAND – Anti–carbamylated protein antibodies were associated with both increased disability and greater disease progression over time in patients with early inflammatory arthritis, data from a 20-year retrospective, observational analysis showed.
According to the research, presented at the British Society for Rheumatology annual conference, patients who were also positive for anti–citrullinated protein antibodies (ACPAs) fared worse long term than those who were ACPA negative.
The results showed that anti–carbamylated protein (anti-CarP) antibodies may provide additional prognostic information to current antibody tests in inflammatory arthritis (IA), but their measurement is not yet ready for general use, said Dr. Jennifer Humphreys of the Arthritis Research UK Centre for Epidemiology at the University of Manchester.
“We know that inflammatory arthritis patients who are rheumatoid factor [RF] or ACPA positive have an increased risk of persistent disease and a poor prognosis,” she said.
“In particular, ACPA[s] are associated with severe radiological progression [and] increased disability and mortality. However, not all patients who lack these antibodies do well,” Dr. Humphreys said.
Research at the University of Leiden (the Netherlands) has found that ACPA-negative patients have anti-CarP antibodies in their sera and that these autoantibodies precede the onset of symptoms and predict the development of rheumatoid arthritis (RA) in patients with arthralgia. These antibodies have also been associated with radiological damage, she said.
Because there were few data on the relationship to disease activity and long-term outcomes in the presence of these antibodies, Dr. Humphreys and her associates decided to look at this using data from the Norfolk Arthritis Register (NOAR), she said. The U.K. team worked in collaboration with the Dutch researchers who performed the anti-CarP tests on serum samples taken from 1,995 patients with early IA enrolled in NOAR from 1990 onward.
At baseline, 1,222 patients met American College of Rheumatology/European League Against Rheumatism 2010 criteria for RA. The mean age of onset was 55 years for both the total population with IA and for those with RA. About two-thirds of the participants were women, and their median symptom duration at enrollment was 33 weeks.
Long-term disability was assessed using the health assessment questionnaire (HAQ), and disease activity was evaluated using the disease activity in 28 joints (DAS28) score. The median baseline DAS28 score was 3.81 for the total cohort and 4.45 for patients with RA, and the median baseline HAQ scores were a respective 0.875 and 1.125.
When the team looked at patient baseline characteristics according to baseline anti-CarP status, they found that the 460 (23%) patients who were positive for these autoantibodies were more likely to have higher baseline DAS28 scores (4.23 vs. 3.73), HAQ scores (1.125 vs. 0.875), and C-reactive protein levels (14 mg/L vs. 7 mg/L) than the 1,543 patients who tested anti-CarP negative. Patients positive for anti-Carp antibodies were also less likely to be female (58% vs 68%) and were slightly older at onset (55 vs. 53 years).
Dr. Humphreys noted that there was a lot of overlap among the presence of anti-CarP, ACPA, and RF. Of the 460 who were anti-CarP positive, 124 were positive only for anti-CarP, 148 were also RF positive, 30 were also ACPA positive, and 158 had all three antibodies.
“Patients who were anti-CarP antibody positive had higher HAQ scores at baseline, and this higher level of disability persisted throughout follow-up, and that association remained significant in the multivariate model,” Dr. Humphreys said.
“When we stratified by ACPA status, there was a statistically significant association in the ACPA-negative subgroup but not in the ACPA-positive subgroup,” she added. However, she noted that the beta coefficients for the association between anti-CarP antibodies and HAQ scores over time were similar (0.12 for the total cohort, 0.14 for the ACPA-negative patients, and 0.09 for the ACPA-positive patients) and that confidence intervals were wide.
Similar findings were seen for the association between anti-CarP antibodies and the DAS28 over time, with respective beta coefficients of 0.23, 01.18, and 0.25 for the total cohort, the ACPA-negative subgroup, and the ACPA-positive subgroup. Again, confidence intervals were wide.
The researchers looked at the additive effect of having anti-CarP and ACPA, and found that while there is no association between RF positivity and long-term disability, there were independent associations with both ACPA positivity and anti-CarP antibody positivity, with respective beta coefficients of –0.05, 0.12, and 0.14.
“And we see the exact same pattern with disease activity over time,” Dr. Humphreys reported.
Thus, measuring anti-CarP antibodies “may be a useful test to do in addition to both RF and ACPA,” she suggested, adding that measuring these antibodies “may be particularly valuable in patients who lack other antibodies.”
NOAR is funded by Arthritis Research UK. Dr. Humphreys had no relevant financial disclosures.
MANCHESTER, ENGLAND – Anti–carbamylated protein antibodies were associated with both increased disability and greater disease progression over time in patients with early inflammatory arthritis, data from a 20-year retrospective, observational analysis showed.
According to the research, presented at the British Society for Rheumatology annual conference, patients who were also positive for anti–citrullinated protein antibodies (ACPAs) fared worse long term than those who were ACPA negative.
The results showed that anti–carbamylated protein (anti-CarP) antibodies may provide additional prognostic information to current antibody tests in inflammatory arthritis (IA), but their measurement is not yet ready for general use, said Dr. Jennifer Humphreys of the Arthritis Research UK Centre for Epidemiology at the University of Manchester.
“We know that inflammatory arthritis patients who are rheumatoid factor [RF] or ACPA positive have an increased risk of persistent disease and a poor prognosis,” she said.
“In particular, ACPA[s] are associated with severe radiological progression [and] increased disability and mortality. However, not all patients who lack these antibodies do well,” Dr. Humphreys said.
Research at the University of Leiden (the Netherlands) has found that ACPA-negative patients have anti-CarP antibodies in their sera and that these autoantibodies precede the onset of symptoms and predict the development of rheumatoid arthritis (RA) in patients with arthralgia. These antibodies have also been associated with radiological damage, she said.
Because there were few data on the relationship to disease activity and long-term outcomes in the presence of these antibodies, Dr. Humphreys and her associates decided to look at this using data from the Norfolk Arthritis Register (NOAR), she said. The U.K. team worked in collaboration with the Dutch researchers who performed the anti-CarP tests on serum samples taken from 1,995 patients with early IA enrolled in NOAR from 1990 onward.
At baseline, 1,222 patients met American College of Rheumatology/European League Against Rheumatism 2010 criteria for RA. The mean age of onset was 55 years for both the total population with IA and for those with RA. About two-thirds of the participants were women, and their median symptom duration at enrollment was 33 weeks.
Long-term disability was assessed using the health assessment questionnaire (HAQ), and disease activity was evaluated using the disease activity in 28 joints (DAS28) score. The median baseline DAS28 score was 3.81 for the total cohort and 4.45 for patients with RA, and the median baseline HAQ scores were a respective 0.875 and 1.125.
When the team looked at patient baseline characteristics according to baseline anti-CarP status, they found that the 460 (23%) patients who were positive for these autoantibodies were more likely to have higher baseline DAS28 scores (4.23 vs. 3.73), HAQ scores (1.125 vs. 0.875), and C-reactive protein levels (14 mg/L vs. 7 mg/L) than the 1,543 patients who tested anti-CarP negative. Patients positive for anti-Carp antibodies were also less likely to be female (58% vs 68%) and were slightly older at onset (55 vs. 53 years).
Dr. Humphreys noted that there was a lot of overlap among the presence of anti-CarP, ACPA, and RF. Of the 460 who were anti-CarP positive, 124 were positive only for anti-CarP, 148 were also RF positive, 30 were also ACPA positive, and 158 had all three antibodies.
“Patients who were anti-CarP antibody positive had higher HAQ scores at baseline, and this higher level of disability persisted throughout follow-up, and that association remained significant in the multivariate model,” Dr. Humphreys said.
“When we stratified by ACPA status, there was a statistically significant association in the ACPA-negative subgroup but not in the ACPA-positive subgroup,” she added. However, she noted that the beta coefficients for the association between anti-CarP antibodies and HAQ scores over time were similar (0.12 for the total cohort, 0.14 for the ACPA-negative patients, and 0.09 for the ACPA-positive patients) and that confidence intervals were wide.
Similar findings were seen for the association between anti-CarP antibodies and the DAS28 over time, with respective beta coefficients of 0.23, 01.18, and 0.25 for the total cohort, the ACPA-negative subgroup, and the ACPA-positive subgroup. Again, confidence intervals were wide.
The researchers looked at the additive effect of having anti-CarP and ACPA, and found that while there is no association between RF positivity and long-term disability, there were independent associations with both ACPA positivity and anti-CarP antibody positivity, with respective beta coefficients of –0.05, 0.12, and 0.14.
“And we see the exact same pattern with disease activity over time,” Dr. Humphreys reported.
Thus, measuring anti-CarP antibodies “may be a useful test to do in addition to both RF and ACPA,” she suggested, adding that measuring these antibodies “may be particularly valuable in patients who lack other antibodies.”
NOAR is funded by Arthritis Research UK. Dr. Humphreys had no relevant financial disclosures.
AT RHEUMATOLOGY 2015
Key clinical point: Anti-CarP antibodies may provide additional prognostic information to current antibody tests in early inflammatory arthritis.
Major finding: Anti-CarP antibodies were associated with both increased disability and higher disease progression over time.
Data source: A 20-year retrospective, observational study of 1,995 patients who were a median of 55 years old at diagnosis of early IA and who were enrolled in the Norfolk Arthritis Register.
Disclosures: NOAR is funded by Arthritis Research UK. Dr. Humphreys had no relevant financial disclosures.
BSR: Subclinical synovitis common in RA remission
MANCHESTER, U.K. – Subclinical synovitis on ultrasound was apparent in almost half of patients with early rheumatoid arthritis deemed to be in clinical remission and in two-thirds of those with low-disease activity in an observational study.
Power Doppler ultrasound changes were seen in 43% of patients in remission and in 65% of those with LDA. Grey-scale ultrasound changes also were found in 78% of the cohort studied, suggesting synovial hypertrophy. The changes correlated with disease activity. Alterations in synovial T-cell populations also were observed.
Attaining and maintaining clinical remission is a long-established primary treatment goal in rheumatoid arthritis (RA), but current criteria used to determine if this is being achieved are largely subjective, noted Dr. Hanna Gul, who presented the research at the for Rheumatology annual conference.
“Over the years there have been many attempts to define clinical remission criteria using disease activity indices,” said Dr. Gul, a clinical research fellow and specialist registrar at the Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England).
However, current criteria used in clinical practice and in clinical trials – most commonly the DAS28 – tend to be based on composite scores of disease, can vary in their interpretation, and do not include objective imaging or immunological assessments, she said.
While patients do seem to be increasingly achieving remission, this is not always associated with good outcomes as structural and functional progression still occurs, Dr. Gul said.
“This raises the question regarding the validity of clinical criteria,” she suggested. “Subsequently, the concept of ‘true remission’ has arisen, which is described as the absence of intra- and extra-articular inflammation, immunologic activity, and structural and functional progression.”
To look at the true remission state in more depth using disease activity criteria, imaging, and immunologic markers, Dr. Gul and her colleagues conducted a retrospective, cross-sectional study of 632 patients with early inflammatory arthritis who were considered to be in clinical remission.
Clinical remission was defined as a DAS28-CRP of less than 3.2. A total of 512 patients had a score of less than 2.6, considered as being in ‘strict’ remission, and the remaining 120 had a score of 2.6 to 3.2, having low disease activity (LDA).
The mean age of patients in the strict remission group was 58 years, and in the LDA group it was 57 years. Most of study subjects were female (70% vs. 67%), had anti-citrullinated antibodies (70% and 68%), and were rheumatoid factor positive (59% and 67%).
The median duration of disease was 6.7 months longer in the patients achieving clinical remission, at 12.9 months versus 6.2 months in the LDA group, and patients in the strict remission group had been in remission for an average of 7.6 months (in the LDA group no patient was in strict remission by definition).
The majority (75% in both groups) had been or was being treated with conventional disease-modifying drugs (csDMARDs) either alone (40%-45%) or in combination (30%-34%). About 12.5% of patients in the remission group, and 7.5% of those in the LDA group were being treated with biologics, mostly in combination with a csDMARD.
“In terms of clinical characteristics, the levels of CRP and swollen and tender joints were generally low,” Dr. Gul reported. “There was also overlap between remission and low disease activity states.”
Various patient-reported outcomes were assessed and showed that the levels of disability and functional impairment were generally low, but that there were differences between the remission and LDA states, with the best outcomes reported in patients with a CRP, tender and swollen joint count of zero.
Flow cytometry was used to analyze T-cell populations in 51 patients compared to 120 healthy controls. Results showed that there was a 9% decrease from the normal range in the percentage of naive CD4+ T-cells, a 26% increase in inflammation-related cells, and a 50% decrease in regulatory T-cells.
“This is the most extensive examination of T-cell abnormalities in RA and confirms T-regulatory abnormalities in patients with clinical remission,” Dr. Gul observed.
“Further research is required for understanding the implications, specifically with regard to T-cell abnormalities as predictors of flare,” she said.
The research was supported by the U.K. National Institute for Health Research. Dr. Gul had no disclosures.
MANCHESTER, U.K. – Subclinical synovitis on ultrasound was apparent in almost half of patients with early rheumatoid arthritis deemed to be in clinical remission and in two-thirds of those with low-disease activity in an observational study.
Power Doppler ultrasound changes were seen in 43% of patients in remission and in 65% of those with LDA. Grey-scale ultrasound changes also were found in 78% of the cohort studied, suggesting synovial hypertrophy. The changes correlated with disease activity. Alterations in synovial T-cell populations also were observed.
Attaining and maintaining clinical remission is a long-established primary treatment goal in rheumatoid arthritis (RA), but current criteria used to determine if this is being achieved are largely subjective, noted Dr. Hanna Gul, who presented the research at the for Rheumatology annual conference.
“Over the years there have been many attempts to define clinical remission criteria using disease activity indices,” said Dr. Gul, a clinical research fellow and specialist registrar at the Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England).
However, current criteria used in clinical practice and in clinical trials – most commonly the DAS28 – tend to be based on composite scores of disease, can vary in their interpretation, and do not include objective imaging or immunological assessments, she said.
While patients do seem to be increasingly achieving remission, this is not always associated with good outcomes as structural and functional progression still occurs, Dr. Gul said.
“This raises the question regarding the validity of clinical criteria,” she suggested. “Subsequently, the concept of ‘true remission’ has arisen, which is described as the absence of intra- and extra-articular inflammation, immunologic activity, and structural and functional progression.”
To look at the true remission state in more depth using disease activity criteria, imaging, and immunologic markers, Dr. Gul and her colleagues conducted a retrospective, cross-sectional study of 632 patients with early inflammatory arthritis who were considered to be in clinical remission.
Clinical remission was defined as a DAS28-CRP of less than 3.2. A total of 512 patients had a score of less than 2.6, considered as being in ‘strict’ remission, and the remaining 120 had a score of 2.6 to 3.2, having low disease activity (LDA).
The mean age of patients in the strict remission group was 58 years, and in the LDA group it was 57 years. Most of study subjects were female (70% vs. 67%), had anti-citrullinated antibodies (70% and 68%), and were rheumatoid factor positive (59% and 67%).
The median duration of disease was 6.7 months longer in the patients achieving clinical remission, at 12.9 months versus 6.2 months in the LDA group, and patients in the strict remission group had been in remission for an average of 7.6 months (in the LDA group no patient was in strict remission by definition).
The majority (75% in both groups) had been or was being treated with conventional disease-modifying drugs (csDMARDs) either alone (40%-45%) or in combination (30%-34%). About 12.5% of patients in the remission group, and 7.5% of those in the LDA group were being treated with biologics, mostly in combination with a csDMARD.
“In terms of clinical characteristics, the levels of CRP and swollen and tender joints were generally low,” Dr. Gul reported. “There was also overlap between remission and low disease activity states.”
Various patient-reported outcomes were assessed and showed that the levels of disability and functional impairment were generally low, but that there were differences between the remission and LDA states, with the best outcomes reported in patients with a CRP, tender and swollen joint count of zero.
Flow cytometry was used to analyze T-cell populations in 51 patients compared to 120 healthy controls. Results showed that there was a 9% decrease from the normal range in the percentage of naive CD4+ T-cells, a 26% increase in inflammation-related cells, and a 50% decrease in regulatory T-cells.
“This is the most extensive examination of T-cell abnormalities in RA and confirms T-regulatory abnormalities in patients with clinical remission,” Dr. Gul observed.
“Further research is required for understanding the implications, specifically with regard to T-cell abnormalities as predictors of flare,” she said.
The research was supported by the U.K. National Institute for Health Research. Dr. Gul had no disclosures.
MANCHESTER, U.K. – Subclinical synovitis on ultrasound was apparent in almost half of patients with early rheumatoid arthritis deemed to be in clinical remission and in two-thirds of those with low-disease activity in an observational study.
Power Doppler ultrasound changes were seen in 43% of patients in remission and in 65% of those with LDA. Grey-scale ultrasound changes also were found in 78% of the cohort studied, suggesting synovial hypertrophy. The changes correlated with disease activity. Alterations in synovial T-cell populations also were observed.
Attaining and maintaining clinical remission is a long-established primary treatment goal in rheumatoid arthritis (RA), but current criteria used to determine if this is being achieved are largely subjective, noted Dr. Hanna Gul, who presented the research at the for Rheumatology annual conference.
“Over the years there have been many attempts to define clinical remission criteria using disease activity indices,” said Dr. Gul, a clinical research fellow and specialist registrar at the Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England).
However, current criteria used in clinical practice and in clinical trials – most commonly the DAS28 – tend to be based on composite scores of disease, can vary in their interpretation, and do not include objective imaging or immunological assessments, she said.
While patients do seem to be increasingly achieving remission, this is not always associated with good outcomes as structural and functional progression still occurs, Dr. Gul said.
“This raises the question regarding the validity of clinical criteria,” she suggested. “Subsequently, the concept of ‘true remission’ has arisen, which is described as the absence of intra- and extra-articular inflammation, immunologic activity, and structural and functional progression.”
To look at the true remission state in more depth using disease activity criteria, imaging, and immunologic markers, Dr. Gul and her colleagues conducted a retrospective, cross-sectional study of 632 patients with early inflammatory arthritis who were considered to be in clinical remission.
Clinical remission was defined as a DAS28-CRP of less than 3.2. A total of 512 patients had a score of less than 2.6, considered as being in ‘strict’ remission, and the remaining 120 had a score of 2.6 to 3.2, having low disease activity (LDA).
The mean age of patients in the strict remission group was 58 years, and in the LDA group it was 57 years. Most of study subjects were female (70% vs. 67%), had anti-citrullinated antibodies (70% and 68%), and were rheumatoid factor positive (59% and 67%).
The median duration of disease was 6.7 months longer in the patients achieving clinical remission, at 12.9 months versus 6.2 months in the LDA group, and patients in the strict remission group had been in remission for an average of 7.6 months (in the LDA group no patient was in strict remission by definition).
The majority (75% in both groups) had been or was being treated with conventional disease-modifying drugs (csDMARDs) either alone (40%-45%) or in combination (30%-34%). About 12.5% of patients in the remission group, and 7.5% of those in the LDA group were being treated with biologics, mostly in combination with a csDMARD.
“In terms of clinical characteristics, the levels of CRP and swollen and tender joints were generally low,” Dr. Gul reported. “There was also overlap between remission and low disease activity states.”
Various patient-reported outcomes were assessed and showed that the levels of disability and functional impairment were generally low, but that there were differences between the remission and LDA states, with the best outcomes reported in patients with a CRP, tender and swollen joint count of zero.
Flow cytometry was used to analyze T-cell populations in 51 patients compared to 120 healthy controls. Results showed that there was a 9% decrease from the normal range in the percentage of naive CD4+ T-cells, a 26% increase in inflammation-related cells, and a 50% decrease in regulatory T-cells.
“This is the most extensive examination of T-cell abnormalities in RA and confirms T-regulatory abnormalities in patients with clinical remission,” Dr. Gul observed.
“Further research is required for understanding the implications, specifically with regard to T-cell abnormalities as predictors of flare,” she said.
The research was supported by the U.K. National Institute for Health Research. Dr. Gul had no disclosures.
AT RHEUMATOLOGY 2015
Key clinical point: Patients with RA in clinical remission defined via clinical criteria alone may still have subclinical inflammation.
Major finding: Power Doppler ultrasound changes were seen in 43% of patients in remission and in 65% of those with LDA.
Data source: Retrospective, cross-sectional, observational study of 632 patients with early inflammatory arthritis in clinical remission (DAS28-CRP < 3.2).
Disclosures: The research was supported by the U.K. National Institute for Health Research. Dr. Gul had no disclosures.
Ultrasound sign could help diagnose giant cell arteritis
MANCHESTER, U.K. – An early halo sign on ultrasound is both diagnostic and prognostic according to the findings of a substudy of the ongoing TABUL trial.
In newly diagnosed giant cell arteritis (GCA), halo on ultrasound was seen in 46% of cases, and its presence correlated significantly with both ischemic symptoms and abnormal physical examination of the temporal arteries.
The finding “supports the early use of the halo as a diagnostic and potentially prognostic marker,” Dr. Raashid Luqmani, professor of rheumatology at the University of Oxford, England, said at the British Society for Rheumatology annual conference.
The halo sign is an abnormal shadow seen around the temporal arteries on ultrasound, Dr. Luqmani explained.
“The value of halo size change over time in individual patients is being investigated as a marker of response to treatment,” he added, noting that the size of the halo decreased rapidly with longer duration of early, high-dose steroid treatment.
The main TABUL (Temporal Artery Biopsy Versus Ultrasound for the Diagnosis of Giant Cell Arteritis) trial is looking at the overall diagnostic performance, accuracy, and cost-effectiveness of ultrasound versus biopsy in patients with newly suspected GCA. A total of 430 patients have been recruited and have undergone a single ultrasound scan of the temporal and axillary arteries followed by temporal artery biopsy within 7 days of commencing steroids.
Clinicians are blinded to the results of the ultrasound scan until 2 weeks after a treatment decision has been made and they intend to start rapid steroid withdrawal. Following this, patients are seen at a 6-month follow-up visit.
The aim of the substudy reported by Dr. Luqmani was to describe the features of the early halo sign in response to steroid therapy and whether it correlated to ischemic symptoms.
A cross-sectional analysis was performed on data from 312 patients to look at the extent of arterial involvement, the maximum thickness of the halo, the duration of steroid treatment when the ultrasound was performed, and what ischemic symptoms were present.
The mean age of the 220 women studied was 72.5 years, and that of the 92 men was 71.2 years.
Most patients had one (30.6%), two (22.2%), or three (13.9%) temporal arterial segments involved, with the remaining third having four or more. Bilateral halos were seen in 30% of patients, temporal or axillary artery halos in 13.5%, and isolated axillary halos in 2.6%.
The fact that 13.5% had both temporal and axillary artery involvement and 2.6% had only isolated axillary halos suggests a role for scanning both the axillary and temporal arteries, Dr. Luqmani suggested.
The likelihood of finding a halo diminished with the duration of steroid therapy, he reported. “Patients who received no steroid therapy had a much bigger halo and it got progressively smaller and significantly less by day 4,” he observed.
“Although there are still some patients who still have a halo at days 5 and 6 of steroid treatment, the likelihood of finding that halo is much, much less,” he added.
Looking at the presence of halo in relation to ischemic symptoms, there did appear to be an association but it was only significant (P < .004) for jaw claudication.
“If you have a patient with newly suspected GCA, you are likely to see a significant halo, but you need to be quick, you need to be seeing that halo within 4 days of starting steroids or evaluating patients who have not had any steroid treatment,” Dr. Luqmani said.
The TABUL study is funded by the U.K. Health Technology Assessment, a program of the National Institute for Health Research,and sponsored by the University of Oxford. Dr. Luqmani had received consulting fees from GlaxoSmithKline, Novartis, Roche, and Pfizer.
MANCHESTER, U.K. – An early halo sign on ultrasound is both diagnostic and prognostic according to the findings of a substudy of the ongoing TABUL trial.
In newly diagnosed giant cell arteritis (GCA), halo on ultrasound was seen in 46% of cases, and its presence correlated significantly with both ischemic symptoms and abnormal physical examination of the temporal arteries.
The finding “supports the early use of the halo as a diagnostic and potentially prognostic marker,” Dr. Raashid Luqmani, professor of rheumatology at the University of Oxford, England, said at the British Society for Rheumatology annual conference.
The halo sign is an abnormal shadow seen around the temporal arteries on ultrasound, Dr. Luqmani explained.
“The value of halo size change over time in individual patients is being investigated as a marker of response to treatment,” he added, noting that the size of the halo decreased rapidly with longer duration of early, high-dose steroid treatment.
The main TABUL (Temporal Artery Biopsy Versus Ultrasound for the Diagnosis of Giant Cell Arteritis) trial is looking at the overall diagnostic performance, accuracy, and cost-effectiveness of ultrasound versus biopsy in patients with newly suspected GCA. A total of 430 patients have been recruited and have undergone a single ultrasound scan of the temporal and axillary arteries followed by temporal artery biopsy within 7 days of commencing steroids.
Clinicians are blinded to the results of the ultrasound scan until 2 weeks after a treatment decision has been made and they intend to start rapid steroid withdrawal. Following this, patients are seen at a 6-month follow-up visit.
The aim of the substudy reported by Dr. Luqmani was to describe the features of the early halo sign in response to steroid therapy and whether it correlated to ischemic symptoms.
A cross-sectional analysis was performed on data from 312 patients to look at the extent of arterial involvement, the maximum thickness of the halo, the duration of steroid treatment when the ultrasound was performed, and what ischemic symptoms were present.
The mean age of the 220 women studied was 72.5 years, and that of the 92 men was 71.2 years.
Most patients had one (30.6%), two (22.2%), or three (13.9%) temporal arterial segments involved, with the remaining third having four or more. Bilateral halos were seen in 30% of patients, temporal or axillary artery halos in 13.5%, and isolated axillary halos in 2.6%.
The fact that 13.5% had both temporal and axillary artery involvement and 2.6% had only isolated axillary halos suggests a role for scanning both the axillary and temporal arteries, Dr. Luqmani suggested.
The likelihood of finding a halo diminished with the duration of steroid therapy, he reported. “Patients who received no steroid therapy had a much bigger halo and it got progressively smaller and significantly less by day 4,” he observed.
“Although there are still some patients who still have a halo at days 5 and 6 of steroid treatment, the likelihood of finding that halo is much, much less,” he added.
Looking at the presence of halo in relation to ischemic symptoms, there did appear to be an association but it was only significant (P < .004) for jaw claudication.
“If you have a patient with newly suspected GCA, you are likely to see a significant halo, but you need to be quick, you need to be seeing that halo within 4 days of starting steroids or evaluating patients who have not had any steroid treatment,” Dr. Luqmani said.
The TABUL study is funded by the U.K. Health Technology Assessment, a program of the National Institute for Health Research,and sponsored by the University of Oxford. Dr. Luqmani had received consulting fees from GlaxoSmithKline, Novartis, Roche, and Pfizer.
MANCHESTER, U.K. – An early halo sign on ultrasound is both diagnostic and prognostic according to the findings of a substudy of the ongoing TABUL trial.
In newly diagnosed giant cell arteritis (GCA), halo on ultrasound was seen in 46% of cases, and its presence correlated significantly with both ischemic symptoms and abnormal physical examination of the temporal arteries.
The finding “supports the early use of the halo as a diagnostic and potentially prognostic marker,” Dr. Raashid Luqmani, professor of rheumatology at the University of Oxford, England, said at the British Society for Rheumatology annual conference.
The halo sign is an abnormal shadow seen around the temporal arteries on ultrasound, Dr. Luqmani explained.
“The value of halo size change over time in individual patients is being investigated as a marker of response to treatment,” he added, noting that the size of the halo decreased rapidly with longer duration of early, high-dose steroid treatment.
The main TABUL (Temporal Artery Biopsy Versus Ultrasound for the Diagnosis of Giant Cell Arteritis) trial is looking at the overall diagnostic performance, accuracy, and cost-effectiveness of ultrasound versus biopsy in patients with newly suspected GCA. A total of 430 patients have been recruited and have undergone a single ultrasound scan of the temporal and axillary arteries followed by temporal artery biopsy within 7 days of commencing steroids.
Clinicians are blinded to the results of the ultrasound scan until 2 weeks after a treatment decision has been made and they intend to start rapid steroid withdrawal. Following this, patients are seen at a 6-month follow-up visit.
The aim of the substudy reported by Dr. Luqmani was to describe the features of the early halo sign in response to steroid therapy and whether it correlated to ischemic symptoms.
A cross-sectional analysis was performed on data from 312 patients to look at the extent of arterial involvement, the maximum thickness of the halo, the duration of steroid treatment when the ultrasound was performed, and what ischemic symptoms were present.
The mean age of the 220 women studied was 72.5 years, and that of the 92 men was 71.2 years.
Most patients had one (30.6%), two (22.2%), or three (13.9%) temporal arterial segments involved, with the remaining third having four or more. Bilateral halos were seen in 30% of patients, temporal or axillary artery halos in 13.5%, and isolated axillary halos in 2.6%.
The fact that 13.5% had both temporal and axillary artery involvement and 2.6% had only isolated axillary halos suggests a role for scanning both the axillary and temporal arteries, Dr. Luqmani suggested.
The likelihood of finding a halo diminished with the duration of steroid therapy, he reported. “Patients who received no steroid therapy had a much bigger halo and it got progressively smaller and significantly less by day 4,” he observed.
“Although there are still some patients who still have a halo at days 5 and 6 of steroid treatment, the likelihood of finding that halo is much, much less,” he added.
Looking at the presence of halo in relation to ischemic symptoms, there did appear to be an association but it was only significant (P < .004) for jaw claudication.
“If you have a patient with newly suspected GCA, you are likely to see a significant halo, but you need to be quick, you need to be seeing that halo within 4 days of starting steroids or evaluating patients who have not had any steroid treatment,” Dr. Luqmani said.
The TABUL study is funded by the U.K. Health Technology Assessment, a program of the National Institute for Health Research,and sponsored by the University of Oxford. Dr. Luqmani had received consulting fees from GlaxoSmithKline, Novartis, Roche, and Pfizer.
AT RHEUMATOLOGY 2015
Key clinical point: The halo sign on ultrasound is a diagnostic and potentially prognostic marker, but its use is limited by the effect of early high-dose steroids from about day 4 onward.
Major finding: Halo on ultrasound was seen in 46% of cases; its presence correlated with both ischemic symptoms and abnormal physical examination of the temporal arteries.
Data source: The TABUL trial, involving 312 patients suspected of giant cell arteritis.
Disclosures: TABUL is funded by the U.K. Health Technology Assessment, a program of the National Institute for Health Research, and sponsored by the University of Oxford. Dr. Luqmani had received consulting fees from GlaxoSmithKline, Novartis, Roche, and Pfizer.