User login
Remission of Psoriasis 13 Years After Autologous Stem Cell Transplant
To the Editor:
Remission of psoriasis after bone marrow transplant has been reported1; however, follow-up typically has been limited. We describe a case of a remote recurrence of psoriasis 13 years after autologous stem cell transplant.
In September 1997, a 48-year-old woman with a 20-year history of moderate to severe plaque psoriasis, previously managed with oral methotrexate (up to 30–40 mg weekly), presented to her primary care physician with a persistent cough and newly pervasive arthralgia and myalgia. A complete blood cell count showed a white blood cell count of 6.9 mg/dL (reference range, 3.8–10.8 mg/dL), hemoglobin level of 11.6 mg/dL (reference range, 11.7–15.5 mg/dL), and hematocrit level of 34% (reference range, 35.0%–45.0%). Bone survey demonstrated a 4×2-cm lytic expansile lesion in the occipital bone, and a bone marrow aspirate and biopsy revealed 40% plasma cells. She was subsequently diagnosed with stage IIIA IgA λ and free λ light chain multiple myeloma with an IgA level of 62.1 g/L (reference range, 1.24–2.17 g/L).
Therapy was initiated with dexamethasone in combination with pamidronate and she was transitioned to cyclophosphamide chemotherapy. A peripheral autologous blood stem cell transplant followed. Throughout her initial treatment, the psoriasis continued to flare and she remained on methotrexate to help maintain control of the skin disease.
In November 1998, the patient underwent an autologous CD34+ stem cell transplant. There were no complications following the transplant and the multiple myeloma has remained in complete remission to date. Following the stem cell transplant, the patient remained free of psoriatic disease for just over 13 years without any intervention. Recurrent erythematous plaques with thick scale on the bilateral lower legs prompted her return to dermatology in August 2011. To date, she continues to note intermittent though mild flares of her psoriasis. She has responded well to methotrexate 7.5 mg weekly and clobetasol propionate ointment 0.05% as needed with satisfactory control of her disease.
The development of autoimmune disease following an allogenic bone marrow transplant such as psoriasis, thyroiditis, myasthenia gravis, insulin-dependent diabetes mellitus, and vitiligo has been well described.2-4 The causal relationship between allogenic hematopoetic stem cell transplant (HSCT) and subsequent autoimmune disease has been attributed to T cells and passive transfer of autoimmune disease from the donor to the recipient.1
In the case of autologous HSCT, the remission of autoimmune disease thereafter has been attributed to myeloablative conditioning, whereby high-dose chemotherapy eliminates self-reactive lymphocytes, resulting in ablation of peripheral reactive and alloreactive T cells with depletion of thymus and reactive B cells.1
In 1997, Cooley et al5 described 4 patients with autoimmune disease who experienced complete but temporary (up to 26 months) remission following autologous bone marrow or stem cell transplant. A comprehensive review of psoriasis cases that resolved after HSCT published by Kaffenberger et al6 in 2013 cited 6 cases of autologous and 13 cases of allogenic transplant that resulted in resolution of psoriatic disease. Recurrence of disease was witnessed in 3 of 13 allogenic HSCT patients and 5 of 6 autologous HSCT patients. Of note, all 5 of these patients developed recurrent disease within 24 months following autologous HSCT, albeit with more benign disease.6
Similar to those cases presented by Kaffenberger et al,6 our patient’s psoriatic disease was notably mild compared to her disease prior to HSCT. Although an unintended but welcome outcome of multiple myeloma treatment, the nearly quiescent nature of her skin disease following autologous HSCT has led to a dramatic improvement in her quality of life.
We highlight a unique case in which psoriatic skin disease recurred more than 1 decade after autologous HSCT.
1. Braiteh F, Hymes SR, Giralt SA, et al. Complete remission of psoriasis after autologous hematopoietic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2008;26:4511-4513.
2. Kishimoto Y, Yamamoto Y, Matsumoto N, et al. Transfer of autoimmune thyroiditis and resolution of palmoplantar pustular psoriasis following allogeneic bone marrow transplantation. Bone Marrow Transpl. 1997;19:1041-1043.
3. Wahie S, Alexandroff A, Reynolds NY, et al. Psoriasis occurring after myeloablative therapy and autologous stem cell transplantation. Br J Dermatol. 2006;154:177-204.
4. Snowden JA, Heaton DC. Development of psoriasis after syngenic bone marrow transplant from psoriatic donor: further evidence for adoptive autoimmunity. Br J Dermatol. 1997;137:130-132.
5. Cooley HM, Snowden JA, Grigg AP, et al. Outcome of rheumatoid arthritis and psoriasis following autologous stem cell transplantation for hematologic malignancy. Arthritis Rheum. 1997;40:1712-1715.
6. Kaffenberger BH, Wong HK, Jarjour W, et al. Remission of psoriasis after allogenic but not autologous, hematopoietic stem-cell transplantation. J Am Acad Dermatol. 2013;68:489-492.
To the Editor:
Remission of psoriasis after bone marrow transplant has been reported1; however, follow-up typically has been limited. We describe a case of a remote recurrence of psoriasis 13 years after autologous stem cell transplant.
In September 1997, a 48-year-old woman with a 20-year history of moderate to severe plaque psoriasis, previously managed with oral methotrexate (up to 30–40 mg weekly), presented to her primary care physician with a persistent cough and newly pervasive arthralgia and myalgia. A complete blood cell count showed a white blood cell count of 6.9 mg/dL (reference range, 3.8–10.8 mg/dL), hemoglobin level of 11.6 mg/dL (reference range, 11.7–15.5 mg/dL), and hematocrit level of 34% (reference range, 35.0%–45.0%). Bone survey demonstrated a 4×2-cm lytic expansile lesion in the occipital bone, and a bone marrow aspirate and biopsy revealed 40% plasma cells. She was subsequently diagnosed with stage IIIA IgA λ and free λ light chain multiple myeloma with an IgA level of 62.1 g/L (reference range, 1.24–2.17 g/L).
Therapy was initiated with dexamethasone in combination with pamidronate and she was transitioned to cyclophosphamide chemotherapy. A peripheral autologous blood stem cell transplant followed. Throughout her initial treatment, the psoriasis continued to flare and she remained on methotrexate to help maintain control of the skin disease.
In November 1998, the patient underwent an autologous CD34+ stem cell transplant. There were no complications following the transplant and the multiple myeloma has remained in complete remission to date. Following the stem cell transplant, the patient remained free of psoriatic disease for just over 13 years without any intervention. Recurrent erythematous plaques with thick scale on the bilateral lower legs prompted her return to dermatology in August 2011. To date, she continues to note intermittent though mild flares of her psoriasis. She has responded well to methotrexate 7.5 mg weekly and clobetasol propionate ointment 0.05% as needed with satisfactory control of her disease.
The development of autoimmune disease following an allogenic bone marrow transplant such as psoriasis, thyroiditis, myasthenia gravis, insulin-dependent diabetes mellitus, and vitiligo has been well described.2-4 The causal relationship between allogenic hematopoetic stem cell transplant (HSCT) and subsequent autoimmune disease has been attributed to T cells and passive transfer of autoimmune disease from the donor to the recipient.1
In the case of autologous HSCT, the remission of autoimmune disease thereafter has been attributed to myeloablative conditioning, whereby high-dose chemotherapy eliminates self-reactive lymphocytes, resulting in ablation of peripheral reactive and alloreactive T cells with depletion of thymus and reactive B cells.1
In 1997, Cooley et al5 described 4 patients with autoimmune disease who experienced complete but temporary (up to 26 months) remission following autologous bone marrow or stem cell transplant. A comprehensive review of psoriasis cases that resolved after HSCT published by Kaffenberger et al6 in 2013 cited 6 cases of autologous and 13 cases of allogenic transplant that resulted in resolution of psoriatic disease. Recurrence of disease was witnessed in 3 of 13 allogenic HSCT patients and 5 of 6 autologous HSCT patients. Of note, all 5 of these patients developed recurrent disease within 24 months following autologous HSCT, albeit with more benign disease.6
Similar to those cases presented by Kaffenberger et al,6 our patient’s psoriatic disease was notably mild compared to her disease prior to HSCT. Although an unintended but welcome outcome of multiple myeloma treatment, the nearly quiescent nature of her skin disease following autologous HSCT has led to a dramatic improvement in her quality of life.
We highlight a unique case in which psoriatic skin disease recurred more than 1 decade after autologous HSCT.
To the Editor:
Remission of psoriasis after bone marrow transplant has been reported1; however, follow-up typically has been limited. We describe a case of a remote recurrence of psoriasis 13 years after autologous stem cell transplant.
In September 1997, a 48-year-old woman with a 20-year history of moderate to severe plaque psoriasis, previously managed with oral methotrexate (up to 30–40 mg weekly), presented to her primary care physician with a persistent cough and newly pervasive arthralgia and myalgia. A complete blood cell count showed a white blood cell count of 6.9 mg/dL (reference range, 3.8–10.8 mg/dL), hemoglobin level of 11.6 mg/dL (reference range, 11.7–15.5 mg/dL), and hematocrit level of 34% (reference range, 35.0%–45.0%). Bone survey demonstrated a 4×2-cm lytic expansile lesion in the occipital bone, and a bone marrow aspirate and biopsy revealed 40% plasma cells. She was subsequently diagnosed with stage IIIA IgA λ and free λ light chain multiple myeloma with an IgA level of 62.1 g/L (reference range, 1.24–2.17 g/L).
Therapy was initiated with dexamethasone in combination with pamidronate and she was transitioned to cyclophosphamide chemotherapy. A peripheral autologous blood stem cell transplant followed. Throughout her initial treatment, the psoriasis continued to flare and she remained on methotrexate to help maintain control of the skin disease.
In November 1998, the patient underwent an autologous CD34+ stem cell transplant. There were no complications following the transplant and the multiple myeloma has remained in complete remission to date. Following the stem cell transplant, the patient remained free of psoriatic disease for just over 13 years without any intervention. Recurrent erythematous plaques with thick scale on the bilateral lower legs prompted her return to dermatology in August 2011. To date, she continues to note intermittent though mild flares of her psoriasis. She has responded well to methotrexate 7.5 mg weekly and clobetasol propionate ointment 0.05% as needed with satisfactory control of her disease.
The development of autoimmune disease following an allogenic bone marrow transplant such as psoriasis, thyroiditis, myasthenia gravis, insulin-dependent diabetes mellitus, and vitiligo has been well described.2-4 The causal relationship between allogenic hematopoetic stem cell transplant (HSCT) and subsequent autoimmune disease has been attributed to T cells and passive transfer of autoimmune disease from the donor to the recipient.1
In the case of autologous HSCT, the remission of autoimmune disease thereafter has been attributed to myeloablative conditioning, whereby high-dose chemotherapy eliminates self-reactive lymphocytes, resulting in ablation of peripheral reactive and alloreactive T cells with depletion of thymus and reactive B cells.1
In 1997, Cooley et al5 described 4 patients with autoimmune disease who experienced complete but temporary (up to 26 months) remission following autologous bone marrow or stem cell transplant. A comprehensive review of psoriasis cases that resolved after HSCT published by Kaffenberger et al6 in 2013 cited 6 cases of autologous and 13 cases of allogenic transplant that resulted in resolution of psoriatic disease. Recurrence of disease was witnessed in 3 of 13 allogenic HSCT patients and 5 of 6 autologous HSCT patients. Of note, all 5 of these patients developed recurrent disease within 24 months following autologous HSCT, albeit with more benign disease.6
Similar to those cases presented by Kaffenberger et al,6 our patient’s psoriatic disease was notably mild compared to her disease prior to HSCT. Although an unintended but welcome outcome of multiple myeloma treatment, the nearly quiescent nature of her skin disease following autologous HSCT has led to a dramatic improvement in her quality of life.
We highlight a unique case in which psoriatic skin disease recurred more than 1 decade after autologous HSCT.
1. Braiteh F, Hymes SR, Giralt SA, et al. Complete remission of psoriasis after autologous hematopoietic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2008;26:4511-4513.
2. Kishimoto Y, Yamamoto Y, Matsumoto N, et al. Transfer of autoimmune thyroiditis and resolution of palmoplantar pustular psoriasis following allogeneic bone marrow transplantation. Bone Marrow Transpl. 1997;19:1041-1043.
3. Wahie S, Alexandroff A, Reynolds NY, et al. Psoriasis occurring after myeloablative therapy and autologous stem cell transplantation. Br J Dermatol. 2006;154:177-204.
4. Snowden JA, Heaton DC. Development of psoriasis after syngenic bone marrow transplant from psoriatic donor: further evidence for adoptive autoimmunity. Br J Dermatol. 1997;137:130-132.
5. Cooley HM, Snowden JA, Grigg AP, et al. Outcome of rheumatoid arthritis and psoriasis following autologous stem cell transplantation for hematologic malignancy. Arthritis Rheum. 1997;40:1712-1715.
6. Kaffenberger BH, Wong HK, Jarjour W, et al. Remission of psoriasis after allogenic but not autologous, hematopoietic stem-cell transplantation. J Am Acad Dermatol. 2013;68:489-492.
1. Braiteh F, Hymes SR, Giralt SA, et al. Complete remission of psoriasis after autologous hematopoietic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2008;26:4511-4513.
2. Kishimoto Y, Yamamoto Y, Matsumoto N, et al. Transfer of autoimmune thyroiditis and resolution of palmoplantar pustular psoriasis following allogeneic bone marrow transplantation. Bone Marrow Transpl. 1997;19:1041-1043.
3. Wahie S, Alexandroff A, Reynolds NY, et al. Psoriasis occurring after myeloablative therapy and autologous stem cell transplantation. Br J Dermatol. 2006;154:177-204.
4. Snowden JA, Heaton DC. Development of psoriasis after syngenic bone marrow transplant from psoriatic donor: further evidence for adoptive autoimmunity. Br J Dermatol. 1997;137:130-132.
5. Cooley HM, Snowden JA, Grigg AP, et al. Outcome of rheumatoid arthritis and psoriasis following autologous stem cell transplantation for hematologic malignancy. Arthritis Rheum. 1997;40:1712-1715.
6. Kaffenberger BH, Wong HK, Jarjour W, et al. Remission of psoriasis after allogenic but not autologous, hematopoietic stem-cell transplantation. J Am Acad Dermatol. 2013;68:489-492.
Diagnosis and Treatment of Leprosy Type 1 (Reversal) Reaction
Leprosy is a chronic granulomatous infection caused by the organism Mycobacterium leprae that primarily affects the skin and peripheral nerves.1 The organism is thought to be transmitted from person to person via the nasal secretions of infected individuals and is known to have a long incubation period, lasting 2 to 6 years.2 Leprosy has several distinct clinical presentations depending on the host immune response to the infection.3 Treatment typically involves antimicrobials (eg, clofazimine, dapsone, rifampin). Once treatment has begun, an important aspect of patient care is the recognition and treatment of leprosy reactions. Leprosy reactions are acute inflammatory complications that typically occur during the treatment course but also may occur in untreated disease. Type 1 (reversal) and type 2 (erythema nodosum leprosum) reactions are the 2 main types of leprosy reactions, which may affect 30% to 50% of all leprosy patients combined.4 Vasculonecrotic reactions (Lucio leprosy phenomenon) in leprosy are much less common.
We report a case of a 44-year-old man who repeatedly developed physical findings consistent with type 1 reactions after undergoing multiple treatments for leprosy. A discussion of leprosy, as well as its clinical manifestations, treatment options, and management of reversal reactions, also will be provided.
Case Report
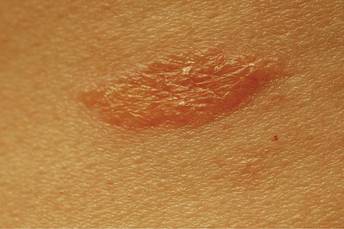
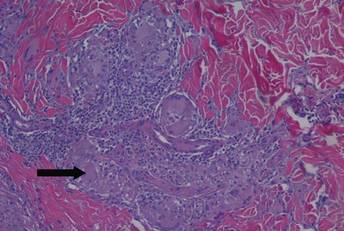
A healthy 44-year-old man presented with a several month history of elevated, erythematous to yellow, anesthetic papules and plaques on the trunk (Figure 1). No other systemic symptoms were noted. Biopsies of multiple skin lesions showed noncaseating granulomas with preferential extension in a perineural pattern and tracking along the arrector pili muscle (Figure 2). The cutaneous nerves appeared to be slightly enlarged. The patient reported a history of living in Louisiana and growing up with armadillos in the backyard, often filling the holes that they dug, but he denied having direct contact with or eating armadillos. In childhood, the patient traveled across the border to Mexico a few times but only for the day. He spent several months in the Middle East (ie, Diego Garcia, Saudi Arabia) more than 10 years prior to presentation, and he spent 2 weeks in Korea approximately 2 years prior to presentation but did not travel off the US air base. He had never traveled to South America or Africa. The clinical and histopathologic findings were consistent with Hansen disease (leprosy) and were determined to be tuberculoid in type given the limited clinical presentation, tuberculoid granulomas on biopsy, and no visible organisms on histopathologic analysis.
|
The patient initially was started on rifampin but was unable to tolerate treatment due to subsequent hepatotoxicity. He then was transitioned to a dual regimen of clofazimine and dapsone, which he tolerated well for the full 12-month treatment course. The cutaneous lesions quickly resolved after starting treatment, leaving a fine “cigarette paper–like” atrophy of the skin. After 12 months, it was subsequently presumed that the patient’s disease had been cured and treatment was stopped.
Nine months later, the patient noted new papules and plaques beginning to reappear in the truncal region. He was seen in clinic and a repeat biopsy was conducted, revealing perineural inflammation and noncaseating granulomas that were similar to the initial biopsies. Fite staining showed no acid-fast bacilli. Polymerase chain reaction was negative for M leprae. Nevertheless, a diagnosis of recurrent leprosy was made based on the patient’s clinical manifestations. He initially was started on dapsone, minocycline, and levofloxacin but was unable to tolerate the minocycline due to subsequent vertigo. After 1 month of treatment with dapsone and levofloxacin, the patient was clinically clear of all skin lesions and a repeat 12-month course of treatment was completed.
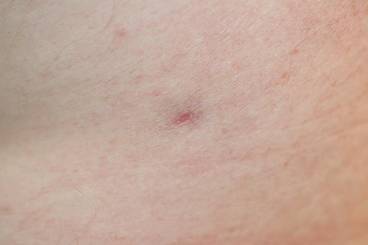
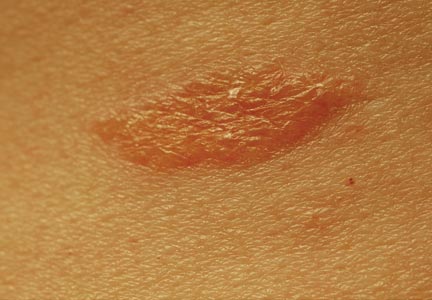
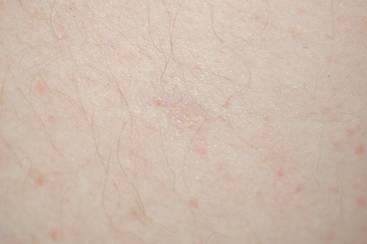
One year after completing the second 12-month treatment course, the patient again developed recurrent, indurated, erythematous papules and plaques on the trunk. Expert consultation from the National Hansen’s Disease (Leprosy) Program determined that the patient was experiencing a type 1 (reversal) reaction, not recurrent disease. Intralesional triamcinolone acetonide (10 mg/cc) was subsequently administered within the individual lesions. After a few treatments, the patient experienced notable regression of the lesions and has since been free of recurrent reactions (Figures 3 and 4).
|
Comment
Mycobacterium leprae
Mycobacterium leprae is an obligate intracellular bacillus that is confined to humans, armadillos of specific locales, and sphagnum moss. It is an acid-fast bacillus that is microscopically indistinguishable from other mycobacteria and is best detected on Fite staining of tissue sections.5
Mycobacterium leprae has 2 unique properties. It is thermolabile, growing best at 27°C to 30°C. Given its thermal sensitivity, M leprae has a preference for peripheral tissues including the skin, peripheral nerves, and the mucosa of the upper airways. It also may affect other tissues such as the bones and some viscera.2 The other unique quality of M leprae is its slow replication, with a generation time of 12 to 14 days. Because of the slow growth of M leprae, the incubation period in humans typically ranges from 2 to 6 years, with the minimal incubation period being 2 to 3 years and the maximum incubation period being as long as 40 years.6
Perhaps the greatest challenge to investigators is the fact that M leprae cannot be grown via normal laboratory culture methods. A possible explanation is reductive evolution, which may have led to a number of inactivated (pseudogenes) in the genome of this organism. In fact, close genetic examination of this organism has led to the conclusion that only half of the genome of M leprae is actually functional. This gene decay may explain the specific host tropism of M leprae as well as the inability to culture this organism in a laboratory setting.5,7
Incidence
Leprosy is primarily a disease of developing countries. More than 80% of the world’s cases of leprosy occur in India, China, Myanmar, Indonesia, Brazil, Nigeria, Madagascar, and Nepal. Although Africa has the highest prevalence, Asia is known to have had the most cases.5 In contrast, leprosy is largely absent from Europe, Canada, and the United States, except as imported cases or scattered cases along the southern border of the United States. In the United States, for example, fewer than 100 cases of leprosy are diagnosed each year, with almost all cases identified in immigrants from endemic areas.6
The global burden of leprosy, defined as the number of new cases detected annually, is stabilizing, which can be attributed in large part to the World Health Organization’s commitment in 1991 to eliminate leprosy as a public health concern by the year 2000 by implementing worldwide treatment regimes. Elimination was defined as a prevalence of less than 1 case per 10,000 persons.8 By 2012, only 3 of 122 countries had not achieved this standard, which is evidence of the program’s success.9
Disease Transmission
There is still some uncertainty involving the mode by which leprosy is transmitted. The most widely held view is that M leprae infection occurs primarily via nasal secretions.10 Transmission is thought to be respiratory, as large numbers of bacilli typically are found in the nasal secretions of untreated patients with multibacillary disease.6 Although nasal secretions often are regarded as the most common mode of M leprae transmission, other possible modes of transmission also may be important, including direct dermal inoculation and vector transmission, though neither has been proven.10 Finally, studies involving patients with confirmed exposure to armadillos have demonstrated a 2-fold increase in the incidence of leprosy versus the general population.11 Because this topic remains controversial, additional studies are needed to ascertain the mechanism of transmission of leprosy between humans and armadillos to confirm the evidence of this study.
Classification
Clinical manifestations of leprosy vary in accordance with the immune response of the host, with the more severe forms of the disease presenting in patients with the least immunity to M leprae.12 Traditionally, patient disease is classified using the Ridley-Jopling scale, which includes tuberculoid, borderline tuberculoid, borderline, borderline lepromatous, and lepromatous types of leprosy.
Tuberculoid leprosy, as noted in our patient, is characterized by a high degree of cellular immunity, a low antigen load, a small number or absence of acid-fast bacilli in skin lesions, and a predominance of helper T cells. Skin lesions in tuberculoid leprosy usually consist of 1 to 2 large hypopigmented or erythematous anesthetic lesions with raised margins and possible overlying scale.13 In tuberculoid leprosy, neural involvement often is asymmetrical and localized and may be the sole clinical finding.10
In stark contrast, lepromatous leprosy is characterized by low cellular immunity, a large antigen load, numerous acid-fast bacilli in tissues, and a predominance of suppressor T cells. Patients with lepromatous leprosy develop widespread disease that includes cutaneous findings of diffuse erythematous macules, nodules, and papules. Disease also can be demonstrated in the upper respiratory tract, anterior chambers of the eyes, testes, lymph nodes, periosteum, and superficial sensory and motor nerves of patients with lepromatous leprosy.12 Neural involvement typically is more symmetrical and diffuse than in patients with tuberculoid leprosy.10
The spectrum of disease between tuberculoid leprosy and lepromatous leprosy includes borderline tuberculoid leprosy, borderline leprosy, and borderline lepromatous leprosy.14 The clinical presentation of borderline leprosy also varies according to the patient’s immune response. Skin lesions vary in number and usually are associated with loss of sensation. Bacilli spreading throughout the bloodstream can lead to more diffuse systemic involvement. Clinical improvement of borderline leprosy to the tuberculoid type often is seen with treatment. Disease progression or deterioration to the lepromatous type can occur with immune system compromise.14
Treatment Options
Treatment of leprosy typically involves multidrug therapy. There are several effective chemotherapeutic agents against M leprae, including dapsone, clofazimine, ofloxacin, and minocycline.15 The World Health Organization recommendations for treatment are based on the classification of patient disease as either multibacillary or paucibacillary.16 Currently, patients are classified as multibacillary if they have 6 or more skin lesions and paucibacillary if they have fewer than 6 lesions.5 World Health Organization recommendations for paucibacillary leprosy include monthly doses of rifampin along with daily doses of dapsone for 6 months. Multibacillary patients usually are treated with a combination of rifampin, dapsone, and clofazimine for 12 months.1
Management of Reversal Reactions
Leprosy reactions can occur in all leprosy patients most commonly during multidrug therapy and represent a delayed hypersensitivity response to M leprae antigens.17 Type 1 and 2 reactions together affect 40% to 50% of all patients at least once during their disease course. Type 1 reactions occur in patients in the tuberculoid and borderline portion of the spectrum. These reactions manifest as erythema and induration of existing plaques. Frequently, progressive neuritis leads to sensory and motor neuropathy. These type 1 reactions typically develop gradually and may last for several weeks.4 Type 2 reactions occur in patients with borderline lepromatous leprosy and lepromatous leprosy and are characterized by the appearance of tender, erythematous, subcutaneous nodules. They are often accompanied by systemic symptoms such as malaise, fever, edema, arthralgia, and weight loss. Organ systems including the joints, eyes, testes, and nervous system also may be affected.18 The natural course of a type 2 reaction is 1 to 2 weeks, but many patients experience multiple recurrences over several months.
All leprosy reactions are believed to be immunologically mediated; however, the mechanism responsible for each reaction type remains poorly defined. The histology of type 1 reactions is that of a delayed-type hypersensitivity response with CD4+ T cells, macrophages, and expression of IL-2 in lesions. In type 1 reactions, increases in cytokines including IL-1, IL-2, IL-12, IFN-γ, and tumor necrosis factor a have been documented both locally within the skin and systemically in the serum. However, studies have not been able to differentiate if this enhanced TH1 response is related to an immunological versus an inflammatory process.19
Type 2 leprosy reactions occur in patients with poor cellular immunity to M leprae. The acute lesions typically are characterized by a neutrophilic infiltrate superimposed on a chronic lepromatous pattern, and there is a systemic inflammatory response to immune complex deposition. It has been proposed that type 2 leprosy reactions are a type of Arthus reaction characterized by deposition of an immunoglobulin-antigen complex in vascular endothelium with subsequent complement activation. Both immunoglobulin and complement have been demonstrated in the reactive nodules of type 2 reactions, and serum complement is decreased in these patients, supporting this pathogenic process.4 Other studies have identified possible immune cell activation in type 2 reactions, including increases in TH2-related cytokines.19
These immunologic reactions can ultimately lead to impaired motor, sensory, and autonomic nerve function if allowed to progress.20 As a result, anesthetic limbs are subjected to repeated trauma, infection, and pressure necrosis that may lead to limb deformity. Autonomic nerve dysfunction may lead to loss of the corneal reflex, which can result in blindness. Common motor findings include wrist and foot drop as well as clawing of the hand from damage to the nerves of the upper extremity.20
Treatment of both type 1 and 2 leprosy reactions is imperative, as these inflammatory reactions are responsible for a great deal of the permanent nerve damage, deformity, and disability that is associated with leprosy.21 Oral and intralesional corticosteroids typically are highly effective for the clinical treatment of type 1 and 2 leprosy reactions given their anti-inflammatory properties. Our patient’s type 1 leprosy reaction responded well to intralesional corticosteroid injections. Thalidomide also has proven to be highly effective in treating type 2 reactions and was used frequently prior to realization of its teratogenic effects. It is now prohibited for use in women of childbearing age but is still routinely used in many countries for the treatment of type 2 reactions in men and postmenopausal women. Other therapies for type 2 reactions that have been used with some success include cyclosporine, azathioprine, and pentoxifylline.4
Conclusion
In summary, we present a unique case of multiple cutaneous reversal reactions in a patient with leprosy years after successful antimicrobial therapy. Proper recognition of this phenomenon is important to avoid overtreatment for mistaken recurrent disease. Although rare in the United States, leprosy should be considered in the differential diagnosis of patients presenting with hypoesthetic or anesthetic skin lesions, chronic annular dermatitis, papular or nodular granulomatous skin lesions, diffuse cutaneous infiltrative disease, peripheral neuropathy, and a history of travel to regions where the disease is known to be endemic. Additionally, if left untreated, M leprae infection and subsequent type 1 or type 2 reactions can lead to devastating neurologic and cutaneous sequelae. Prompt recognition and treatment of these reactions is imperative to prevent these long-term complications.
1. Kalisiak M, Yeung R, Dytoc M. Dermacase: leprosy. Can Fam Physician. 2009;55:55-56.
2. Kustner EC, Cruz MP, Dansis CP, et al. Lepromatous leprosy: a review and case report. Med Oral Patol Cir Bucal. 2006;11:474-479.
3. Nunez-Gussman J, Hwang L, Hsu S. Targetoid erythematous plaques: an unusual morphological presentation of multibacillary Hansen’s disease. Eur J Dermatol. 2001;11:65-67.
4. Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
5. Gelber RH. Leprosy (Hansen’s disease). In: Fauci AS, Kasper DL, Longo DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill; 2008:1021-1027.
6. Simon HB. Infections due to mycobacterium. In: Dale DC, Federman DD, Antman K, et al, eds. ACP Medicine. New York, NY: WebMD Professional Publishing; 2004:1703-1720.
7. Baker LP. Mycobacterium leprae interactions with the host cell: recent advances. Indian J Med Res. 2006;123:748-759.
8. Ishii N. Recent advances in the treatment of leprosy. Dermatol Online J. 2003;9:5. http://dermatology.cdlib.org/92/reviews/leprosy/ishii.html. Accessed March 16, 2015.
9. World Health Organization. Global leprosy situation, 2012. Weekly Epidemiological Rec. 2013;88:365-380. http://www.who.int/wer/2007/wer8835.pdf. Accessed March 16, 2015.
10. Gelber RH. Hansen disease. West J Med. 1993;158:583-590.
11. Deps PD, Alves L, Gripp CG, et al. Contact with armadillos increases the risk of leprosy in Brazil: a case control study. Indian J Dermatol Venereol Leprol. 2008;74:338-342.
12. Booth AV, Kovich OI. Lepromatous leprosy. Dermatol Online J. 2007;13:9.
13. Yens DA, Asters DJ, Teitel A. Subcutaneous nodules and joint deformity in leprosy. Clin Rheumatol. 2003;9:181-186.
14. Panezai S, Saleh FG. Leprosy and peripheral neuropathy. J Clin Neuromuscul Dis. 2004;5:138-145.
15. Sasaki S, Takeshita F, Okuda K, et al. Mycobacterium leprae and leprosy. Microbiol Immunol. 2001;45:729-736.
16. Kumar A, Girdhar A, Chakma J, et al. WHO Multidrug Therapy for Leprosy: Epidemiology of default in treatment in Agra District, Uttar Pradesh, India. BioMed Research International. doi:10.1155/2015/705804.
17. Fiallo P, Clapasson A, Favre A, et al. Overexpression of vascular endothelial growth factor and its endothelial cell receptor KDR in type I leprosy reaction. Am J Med Hyg. 2002;66:180-185.
18. Sales AM, de Matos HJ, Nery JAC, et al. Double-blind trial of the efficacy of pentoxifylline vs thalidomide for the treatment of type II reaction in leprosy. Braz J Med Biol Res. 2007;40:243-248.
19. Lockwood DN, Colston MJ, Khanolkar-Young SR. The detection of Mycobacterium leprae protein and carbohydrate antigens in skin and nerve from leprosy patients with type I (reversal) reactions. Am J Trop Med Hyg. 2002;66:409-415.
20. Boggild AK, Keystone JS, Kain KC. Leprosy: a primer for Canadian physicians. CMAJ. 2004;170:71-78.
21. Rook GA, Baker R. Cortisol metabolism, cortisol sensitivity and the pathogenesis of leprosy reactions. Trop Med Int Health. 1999;4:493-498.
Leprosy is a chronic granulomatous infection caused by the organism Mycobacterium leprae that primarily affects the skin and peripheral nerves.1 The organism is thought to be transmitted from person to person via the nasal secretions of infected individuals and is known to have a long incubation period, lasting 2 to 6 years.2 Leprosy has several distinct clinical presentations depending on the host immune response to the infection.3 Treatment typically involves antimicrobials (eg, clofazimine, dapsone, rifampin). Once treatment has begun, an important aspect of patient care is the recognition and treatment of leprosy reactions. Leprosy reactions are acute inflammatory complications that typically occur during the treatment course but also may occur in untreated disease. Type 1 (reversal) and type 2 (erythema nodosum leprosum) reactions are the 2 main types of leprosy reactions, which may affect 30% to 50% of all leprosy patients combined.4 Vasculonecrotic reactions (Lucio leprosy phenomenon) in leprosy are much less common.
We report a case of a 44-year-old man who repeatedly developed physical findings consistent with type 1 reactions after undergoing multiple treatments for leprosy. A discussion of leprosy, as well as its clinical manifestations, treatment options, and management of reversal reactions, also will be provided.
Case Report
A healthy 44-year-old man presented with a several month history of elevated, erythematous to yellow, anesthetic papules and plaques on the trunk (Figure 1). No other systemic symptoms were noted. Biopsies of multiple skin lesions showed noncaseating granulomas with preferential extension in a perineural pattern and tracking along the arrector pili muscle (Figure 2). The cutaneous nerves appeared to be slightly enlarged. The patient reported a history of living in Louisiana and growing up with armadillos in the backyard, often filling the holes that they dug, but he denied having direct contact with or eating armadillos. In childhood, the patient traveled across the border to Mexico a few times but only for the day. He spent several months in the Middle East (ie, Diego Garcia, Saudi Arabia) more than 10 years prior to presentation, and he spent 2 weeks in Korea approximately 2 years prior to presentation but did not travel off the US air base. He had never traveled to South America or Africa. The clinical and histopathologic findings were consistent with Hansen disease (leprosy) and were determined to be tuberculoid in type given the limited clinical presentation, tuberculoid granulomas on biopsy, and no visible organisms on histopathologic analysis.
|
The patient initially was started on rifampin but was unable to tolerate treatment due to subsequent hepatotoxicity. He then was transitioned to a dual regimen of clofazimine and dapsone, which he tolerated well for the full 12-month treatment course. The cutaneous lesions quickly resolved after starting treatment, leaving a fine “cigarette paper–like” atrophy of the skin. After 12 months, it was subsequently presumed that the patient’s disease had been cured and treatment was stopped.
Nine months later, the patient noted new papules and plaques beginning to reappear in the truncal region. He was seen in clinic and a repeat biopsy was conducted, revealing perineural inflammation and noncaseating granulomas that were similar to the initial biopsies. Fite staining showed no acid-fast bacilli. Polymerase chain reaction was negative for M leprae. Nevertheless, a diagnosis of recurrent leprosy was made based on the patient’s clinical manifestations. He initially was started on dapsone, minocycline, and levofloxacin but was unable to tolerate the minocycline due to subsequent vertigo. After 1 month of treatment with dapsone and levofloxacin, the patient was clinically clear of all skin lesions and a repeat 12-month course of treatment was completed.
One year after completing the second 12-month treatment course, the patient again developed recurrent, indurated, erythematous papules and plaques on the trunk. Expert consultation from the National Hansen’s Disease (Leprosy) Program determined that the patient was experiencing a type 1 (reversal) reaction, not recurrent disease. Intralesional triamcinolone acetonide (10 mg/cc) was subsequently administered within the individual lesions. After a few treatments, the patient experienced notable regression of the lesions and has since been free of recurrent reactions (Figures 3 and 4).

|
Comment
Mycobacterium leprae
Mycobacterium leprae is an obligate intracellular bacillus that is confined to humans, armadillos of specific locales, and sphagnum moss. It is an acid-fast bacillus that is microscopically indistinguishable from other mycobacteria and is best detected on Fite staining of tissue sections.5
Mycobacterium leprae has 2 unique properties. It is thermolabile, growing best at 27°C to 30°C. Given its thermal sensitivity, M leprae has a preference for peripheral tissues including the skin, peripheral nerves, and the mucosa of the upper airways. It also may affect other tissues such as the bones and some viscera.2 The other unique quality of M leprae is its slow replication, with a generation time of 12 to 14 days. Because of the slow growth of M leprae, the incubation period in humans typically ranges from 2 to 6 years, with the minimal incubation period being 2 to 3 years and the maximum incubation period being as long as 40 years.6
Perhaps the greatest challenge to investigators is the fact that M leprae cannot be grown via normal laboratory culture methods. A possible explanation is reductive evolution, which may have led to a number of inactivated (pseudogenes) in the genome of this organism. In fact, close genetic examination of this organism has led to the conclusion that only half of the genome of M leprae is actually functional. This gene decay may explain the specific host tropism of M leprae as well as the inability to culture this organism in a laboratory setting.5,7
Incidence
Leprosy is primarily a disease of developing countries. More than 80% of the world’s cases of leprosy occur in India, China, Myanmar, Indonesia, Brazil, Nigeria, Madagascar, and Nepal. Although Africa has the highest prevalence, Asia is known to have had the most cases.5 In contrast, leprosy is largely absent from Europe, Canada, and the United States, except as imported cases or scattered cases along the southern border of the United States. In the United States, for example, fewer than 100 cases of leprosy are diagnosed each year, with almost all cases identified in immigrants from endemic areas.6
The global burden of leprosy, defined as the number of new cases detected annually, is stabilizing, which can be attributed in large part to the World Health Organization’s commitment in 1991 to eliminate leprosy as a public health concern by the year 2000 by implementing worldwide treatment regimes. Elimination was defined as a prevalence of less than 1 case per 10,000 persons.8 By 2012, only 3 of 122 countries had not achieved this standard, which is evidence of the program’s success.9
Disease Transmission
There is still some uncertainty involving the mode by which leprosy is transmitted. The most widely held view is that M leprae infection occurs primarily via nasal secretions.10 Transmission is thought to be respiratory, as large numbers of bacilli typically are found in the nasal secretions of untreated patients with multibacillary disease.6 Although nasal secretions often are regarded as the most common mode of M leprae transmission, other possible modes of transmission also may be important, including direct dermal inoculation and vector transmission, though neither has been proven.10 Finally, studies involving patients with confirmed exposure to armadillos have demonstrated a 2-fold increase in the incidence of leprosy versus the general population.11 Because this topic remains controversial, additional studies are needed to ascertain the mechanism of transmission of leprosy between humans and armadillos to confirm the evidence of this study.
Classification
Clinical manifestations of leprosy vary in accordance with the immune response of the host, with the more severe forms of the disease presenting in patients with the least immunity to M leprae.12 Traditionally, patient disease is classified using the Ridley-Jopling scale, which includes tuberculoid, borderline tuberculoid, borderline, borderline lepromatous, and lepromatous types of leprosy.
Tuberculoid leprosy, as noted in our patient, is characterized by a high degree of cellular immunity, a low antigen load, a small number or absence of acid-fast bacilli in skin lesions, and a predominance of helper T cells. Skin lesions in tuberculoid leprosy usually consist of 1 to 2 large hypopigmented or erythematous anesthetic lesions with raised margins and possible overlying scale.13 In tuberculoid leprosy, neural involvement often is asymmetrical and localized and may be the sole clinical finding.10
In stark contrast, lepromatous leprosy is characterized by low cellular immunity, a large antigen load, numerous acid-fast bacilli in tissues, and a predominance of suppressor T cells. Patients with lepromatous leprosy develop widespread disease that includes cutaneous findings of diffuse erythematous macules, nodules, and papules. Disease also can be demonstrated in the upper respiratory tract, anterior chambers of the eyes, testes, lymph nodes, periosteum, and superficial sensory and motor nerves of patients with lepromatous leprosy.12 Neural involvement typically is more symmetrical and diffuse than in patients with tuberculoid leprosy.10
The spectrum of disease between tuberculoid leprosy and lepromatous leprosy includes borderline tuberculoid leprosy, borderline leprosy, and borderline lepromatous leprosy.14 The clinical presentation of borderline leprosy also varies according to the patient’s immune response. Skin lesions vary in number and usually are associated with loss of sensation. Bacilli spreading throughout the bloodstream can lead to more diffuse systemic involvement. Clinical improvement of borderline leprosy to the tuberculoid type often is seen with treatment. Disease progression or deterioration to the lepromatous type can occur with immune system compromise.14
Treatment Options
Treatment of leprosy typically involves multidrug therapy. There are several effective chemotherapeutic agents against M leprae, including dapsone, clofazimine, ofloxacin, and minocycline.15 The World Health Organization recommendations for treatment are based on the classification of patient disease as either multibacillary or paucibacillary.16 Currently, patients are classified as multibacillary if they have 6 or more skin lesions and paucibacillary if they have fewer than 6 lesions.5 World Health Organization recommendations for paucibacillary leprosy include monthly doses of rifampin along with daily doses of dapsone for 6 months. Multibacillary patients usually are treated with a combination of rifampin, dapsone, and clofazimine for 12 months.1
Management of Reversal Reactions
Leprosy reactions can occur in all leprosy patients most commonly during multidrug therapy and represent a delayed hypersensitivity response to M leprae antigens.17 Type 1 and 2 reactions together affect 40% to 50% of all patients at least once during their disease course. Type 1 reactions occur in patients in the tuberculoid and borderline portion of the spectrum. These reactions manifest as erythema and induration of existing plaques. Frequently, progressive neuritis leads to sensory and motor neuropathy. These type 1 reactions typically develop gradually and may last for several weeks.4 Type 2 reactions occur in patients with borderline lepromatous leprosy and lepromatous leprosy and are characterized by the appearance of tender, erythematous, subcutaneous nodules. They are often accompanied by systemic symptoms such as malaise, fever, edema, arthralgia, and weight loss. Organ systems including the joints, eyes, testes, and nervous system also may be affected.18 The natural course of a type 2 reaction is 1 to 2 weeks, but many patients experience multiple recurrences over several months.
All leprosy reactions are believed to be immunologically mediated; however, the mechanism responsible for each reaction type remains poorly defined. The histology of type 1 reactions is that of a delayed-type hypersensitivity response with CD4+ T cells, macrophages, and expression of IL-2 in lesions. In type 1 reactions, increases in cytokines including IL-1, IL-2, IL-12, IFN-γ, and tumor necrosis factor a have been documented both locally within the skin and systemically in the serum. However, studies have not been able to differentiate if this enhanced TH1 response is related to an immunological versus an inflammatory process.19
Type 2 leprosy reactions occur in patients with poor cellular immunity to M leprae. The acute lesions typically are characterized by a neutrophilic infiltrate superimposed on a chronic lepromatous pattern, and there is a systemic inflammatory response to immune complex deposition. It has been proposed that type 2 leprosy reactions are a type of Arthus reaction characterized by deposition of an immunoglobulin-antigen complex in vascular endothelium with subsequent complement activation. Both immunoglobulin and complement have been demonstrated in the reactive nodules of type 2 reactions, and serum complement is decreased in these patients, supporting this pathogenic process.4 Other studies have identified possible immune cell activation in type 2 reactions, including increases in TH2-related cytokines.19
These immunologic reactions can ultimately lead to impaired motor, sensory, and autonomic nerve function if allowed to progress.20 As a result, anesthetic limbs are subjected to repeated trauma, infection, and pressure necrosis that may lead to limb deformity. Autonomic nerve dysfunction may lead to loss of the corneal reflex, which can result in blindness. Common motor findings include wrist and foot drop as well as clawing of the hand from damage to the nerves of the upper extremity.20
Treatment of both type 1 and 2 leprosy reactions is imperative, as these inflammatory reactions are responsible for a great deal of the permanent nerve damage, deformity, and disability that is associated with leprosy.21 Oral and intralesional corticosteroids typically are highly effective for the clinical treatment of type 1 and 2 leprosy reactions given their anti-inflammatory properties. Our patient’s type 1 leprosy reaction responded well to intralesional corticosteroid injections. Thalidomide also has proven to be highly effective in treating type 2 reactions and was used frequently prior to realization of its teratogenic effects. It is now prohibited for use in women of childbearing age but is still routinely used in many countries for the treatment of type 2 reactions in men and postmenopausal women. Other therapies for type 2 reactions that have been used with some success include cyclosporine, azathioprine, and pentoxifylline.4
Conclusion
In summary, we present a unique case of multiple cutaneous reversal reactions in a patient with leprosy years after successful antimicrobial therapy. Proper recognition of this phenomenon is important to avoid overtreatment for mistaken recurrent disease. Although rare in the United States, leprosy should be considered in the differential diagnosis of patients presenting with hypoesthetic or anesthetic skin lesions, chronic annular dermatitis, papular or nodular granulomatous skin lesions, diffuse cutaneous infiltrative disease, peripheral neuropathy, and a history of travel to regions where the disease is known to be endemic. Additionally, if left untreated, M leprae infection and subsequent type 1 or type 2 reactions can lead to devastating neurologic and cutaneous sequelae. Prompt recognition and treatment of these reactions is imperative to prevent these long-term complications.
Leprosy is a chronic granulomatous infection caused by the organism Mycobacterium leprae that primarily affects the skin and peripheral nerves.1 The organism is thought to be transmitted from person to person via the nasal secretions of infected individuals and is known to have a long incubation period, lasting 2 to 6 years.2 Leprosy has several distinct clinical presentations depending on the host immune response to the infection.3 Treatment typically involves antimicrobials (eg, clofazimine, dapsone, rifampin). Once treatment has begun, an important aspect of patient care is the recognition and treatment of leprosy reactions. Leprosy reactions are acute inflammatory complications that typically occur during the treatment course but also may occur in untreated disease. Type 1 (reversal) and type 2 (erythema nodosum leprosum) reactions are the 2 main types of leprosy reactions, which may affect 30% to 50% of all leprosy patients combined.4 Vasculonecrotic reactions (Lucio leprosy phenomenon) in leprosy are much less common.
We report a case of a 44-year-old man who repeatedly developed physical findings consistent with type 1 reactions after undergoing multiple treatments for leprosy. A discussion of leprosy, as well as its clinical manifestations, treatment options, and management of reversal reactions, also will be provided.
Case Report
A healthy 44-year-old man presented with a several month history of elevated, erythematous to yellow, anesthetic papules and plaques on the trunk (Figure 1). No other systemic symptoms were noted. Biopsies of multiple skin lesions showed noncaseating granulomas with preferential extension in a perineural pattern and tracking along the arrector pili muscle (Figure 2). The cutaneous nerves appeared to be slightly enlarged. The patient reported a history of living in Louisiana and growing up with armadillos in the backyard, often filling the holes that they dug, but he denied having direct contact with or eating armadillos. In childhood, the patient traveled across the border to Mexico a few times but only for the day. He spent several months in the Middle East (ie, Diego Garcia, Saudi Arabia) more than 10 years prior to presentation, and he spent 2 weeks in Korea approximately 2 years prior to presentation but did not travel off the US air base. He had never traveled to South America or Africa. The clinical and histopathologic findings were consistent with Hansen disease (leprosy) and were determined to be tuberculoid in type given the limited clinical presentation, tuberculoid granulomas on biopsy, and no visible organisms on histopathologic analysis.
|
The patient initially was started on rifampin but was unable to tolerate treatment due to subsequent hepatotoxicity. He then was transitioned to a dual regimen of clofazimine and dapsone, which he tolerated well for the full 12-month treatment course. The cutaneous lesions quickly resolved after starting treatment, leaving a fine “cigarette paper–like” atrophy of the skin. After 12 months, it was subsequently presumed that the patient’s disease had been cured and treatment was stopped.
Nine months later, the patient noted new papules and plaques beginning to reappear in the truncal region. He was seen in clinic and a repeat biopsy was conducted, revealing perineural inflammation and noncaseating granulomas that were similar to the initial biopsies. Fite staining showed no acid-fast bacilli. Polymerase chain reaction was negative for M leprae. Nevertheless, a diagnosis of recurrent leprosy was made based on the patient’s clinical manifestations. He initially was started on dapsone, minocycline, and levofloxacin but was unable to tolerate the minocycline due to subsequent vertigo. After 1 month of treatment with dapsone and levofloxacin, the patient was clinically clear of all skin lesions and a repeat 12-month course of treatment was completed.
One year after completing the second 12-month treatment course, the patient again developed recurrent, indurated, erythematous papules and plaques on the trunk. Expert consultation from the National Hansen’s Disease (Leprosy) Program determined that the patient was experiencing a type 1 (reversal) reaction, not recurrent disease. Intralesional triamcinolone acetonide (10 mg/cc) was subsequently administered within the individual lesions. After a few treatments, the patient experienced notable regression of the lesions and has since been free of recurrent reactions (Figures 3 and 4).

|
Comment
Mycobacterium leprae
Mycobacterium leprae is an obligate intracellular bacillus that is confined to humans, armadillos of specific locales, and sphagnum moss. It is an acid-fast bacillus that is microscopically indistinguishable from other mycobacteria and is best detected on Fite staining of tissue sections.5
Mycobacterium leprae has 2 unique properties. It is thermolabile, growing best at 27°C to 30°C. Given its thermal sensitivity, M leprae has a preference for peripheral tissues including the skin, peripheral nerves, and the mucosa of the upper airways. It also may affect other tissues such as the bones and some viscera.2 The other unique quality of M leprae is its slow replication, with a generation time of 12 to 14 days. Because of the slow growth of M leprae, the incubation period in humans typically ranges from 2 to 6 years, with the minimal incubation period being 2 to 3 years and the maximum incubation period being as long as 40 years.6
Perhaps the greatest challenge to investigators is the fact that M leprae cannot be grown via normal laboratory culture methods. A possible explanation is reductive evolution, which may have led to a number of inactivated (pseudogenes) in the genome of this organism. In fact, close genetic examination of this organism has led to the conclusion that only half of the genome of M leprae is actually functional. This gene decay may explain the specific host tropism of M leprae as well as the inability to culture this organism in a laboratory setting.5,7
Incidence
Leprosy is primarily a disease of developing countries. More than 80% of the world’s cases of leprosy occur in India, China, Myanmar, Indonesia, Brazil, Nigeria, Madagascar, and Nepal. Although Africa has the highest prevalence, Asia is known to have had the most cases.5 In contrast, leprosy is largely absent from Europe, Canada, and the United States, except as imported cases or scattered cases along the southern border of the United States. In the United States, for example, fewer than 100 cases of leprosy are diagnosed each year, with almost all cases identified in immigrants from endemic areas.6
The global burden of leprosy, defined as the number of new cases detected annually, is stabilizing, which can be attributed in large part to the World Health Organization’s commitment in 1991 to eliminate leprosy as a public health concern by the year 2000 by implementing worldwide treatment regimes. Elimination was defined as a prevalence of less than 1 case per 10,000 persons.8 By 2012, only 3 of 122 countries had not achieved this standard, which is evidence of the program’s success.9
Disease Transmission
There is still some uncertainty involving the mode by which leprosy is transmitted. The most widely held view is that M leprae infection occurs primarily via nasal secretions.10 Transmission is thought to be respiratory, as large numbers of bacilli typically are found in the nasal secretions of untreated patients with multibacillary disease.6 Although nasal secretions often are regarded as the most common mode of M leprae transmission, other possible modes of transmission also may be important, including direct dermal inoculation and vector transmission, though neither has been proven.10 Finally, studies involving patients with confirmed exposure to armadillos have demonstrated a 2-fold increase in the incidence of leprosy versus the general population.11 Because this topic remains controversial, additional studies are needed to ascertain the mechanism of transmission of leprosy between humans and armadillos to confirm the evidence of this study.
Classification
Clinical manifestations of leprosy vary in accordance with the immune response of the host, with the more severe forms of the disease presenting in patients with the least immunity to M leprae.12 Traditionally, patient disease is classified using the Ridley-Jopling scale, which includes tuberculoid, borderline tuberculoid, borderline, borderline lepromatous, and lepromatous types of leprosy.
Tuberculoid leprosy, as noted in our patient, is characterized by a high degree of cellular immunity, a low antigen load, a small number or absence of acid-fast bacilli in skin lesions, and a predominance of helper T cells. Skin lesions in tuberculoid leprosy usually consist of 1 to 2 large hypopigmented or erythematous anesthetic lesions with raised margins and possible overlying scale.13 In tuberculoid leprosy, neural involvement often is asymmetrical and localized and may be the sole clinical finding.10
In stark contrast, lepromatous leprosy is characterized by low cellular immunity, a large antigen load, numerous acid-fast bacilli in tissues, and a predominance of suppressor T cells. Patients with lepromatous leprosy develop widespread disease that includes cutaneous findings of diffuse erythematous macules, nodules, and papules. Disease also can be demonstrated in the upper respiratory tract, anterior chambers of the eyes, testes, lymph nodes, periosteum, and superficial sensory and motor nerves of patients with lepromatous leprosy.12 Neural involvement typically is more symmetrical and diffuse than in patients with tuberculoid leprosy.10
The spectrum of disease between tuberculoid leprosy and lepromatous leprosy includes borderline tuberculoid leprosy, borderline leprosy, and borderline lepromatous leprosy.14 The clinical presentation of borderline leprosy also varies according to the patient’s immune response. Skin lesions vary in number and usually are associated with loss of sensation. Bacilli spreading throughout the bloodstream can lead to more diffuse systemic involvement. Clinical improvement of borderline leprosy to the tuberculoid type often is seen with treatment. Disease progression or deterioration to the lepromatous type can occur with immune system compromise.14
Treatment Options
Treatment of leprosy typically involves multidrug therapy. There are several effective chemotherapeutic agents against M leprae, including dapsone, clofazimine, ofloxacin, and minocycline.15 The World Health Organization recommendations for treatment are based on the classification of patient disease as either multibacillary or paucibacillary.16 Currently, patients are classified as multibacillary if they have 6 or more skin lesions and paucibacillary if they have fewer than 6 lesions.5 World Health Organization recommendations for paucibacillary leprosy include monthly doses of rifampin along with daily doses of dapsone for 6 months. Multibacillary patients usually are treated with a combination of rifampin, dapsone, and clofazimine for 12 months.1
Management of Reversal Reactions
Leprosy reactions can occur in all leprosy patients most commonly during multidrug therapy and represent a delayed hypersensitivity response to M leprae antigens.17 Type 1 and 2 reactions together affect 40% to 50% of all patients at least once during their disease course. Type 1 reactions occur in patients in the tuberculoid and borderline portion of the spectrum. These reactions manifest as erythema and induration of existing plaques. Frequently, progressive neuritis leads to sensory and motor neuropathy. These type 1 reactions typically develop gradually and may last for several weeks.4 Type 2 reactions occur in patients with borderline lepromatous leprosy and lepromatous leprosy and are characterized by the appearance of tender, erythematous, subcutaneous nodules. They are often accompanied by systemic symptoms such as malaise, fever, edema, arthralgia, and weight loss. Organ systems including the joints, eyes, testes, and nervous system also may be affected.18 The natural course of a type 2 reaction is 1 to 2 weeks, but many patients experience multiple recurrences over several months.
All leprosy reactions are believed to be immunologically mediated; however, the mechanism responsible for each reaction type remains poorly defined. The histology of type 1 reactions is that of a delayed-type hypersensitivity response with CD4+ T cells, macrophages, and expression of IL-2 in lesions. In type 1 reactions, increases in cytokines including IL-1, IL-2, IL-12, IFN-γ, and tumor necrosis factor a have been documented both locally within the skin and systemically in the serum. However, studies have not been able to differentiate if this enhanced TH1 response is related to an immunological versus an inflammatory process.19
Type 2 leprosy reactions occur in patients with poor cellular immunity to M leprae. The acute lesions typically are characterized by a neutrophilic infiltrate superimposed on a chronic lepromatous pattern, and there is a systemic inflammatory response to immune complex deposition. It has been proposed that type 2 leprosy reactions are a type of Arthus reaction characterized by deposition of an immunoglobulin-antigen complex in vascular endothelium with subsequent complement activation. Both immunoglobulin and complement have been demonstrated in the reactive nodules of type 2 reactions, and serum complement is decreased in these patients, supporting this pathogenic process.4 Other studies have identified possible immune cell activation in type 2 reactions, including increases in TH2-related cytokines.19
These immunologic reactions can ultimately lead to impaired motor, sensory, and autonomic nerve function if allowed to progress.20 As a result, anesthetic limbs are subjected to repeated trauma, infection, and pressure necrosis that may lead to limb deformity. Autonomic nerve dysfunction may lead to loss of the corneal reflex, which can result in blindness. Common motor findings include wrist and foot drop as well as clawing of the hand from damage to the nerves of the upper extremity.20
Treatment of both type 1 and 2 leprosy reactions is imperative, as these inflammatory reactions are responsible for a great deal of the permanent nerve damage, deformity, and disability that is associated with leprosy.21 Oral and intralesional corticosteroids typically are highly effective for the clinical treatment of type 1 and 2 leprosy reactions given their anti-inflammatory properties. Our patient’s type 1 leprosy reaction responded well to intralesional corticosteroid injections. Thalidomide also has proven to be highly effective in treating type 2 reactions and was used frequently prior to realization of its teratogenic effects. It is now prohibited for use in women of childbearing age but is still routinely used in many countries for the treatment of type 2 reactions in men and postmenopausal women. Other therapies for type 2 reactions that have been used with some success include cyclosporine, azathioprine, and pentoxifylline.4
Conclusion
In summary, we present a unique case of multiple cutaneous reversal reactions in a patient with leprosy years after successful antimicrobial therapy. Proper recognition of this phenomenon is important to avoid overtreatment for mistaken recurrent disease. Although rare in the United States, leprosy should be considered in the differential diagnosis of patients presenting with hypoesthetic or anesthetic skin lesions, chronic annular dermatitis, papular or nodular granulomatous skin lesions, diffuse cutaneous infiltrative disease, peripheral neuropathy, and a history of travel to regions where the disease is known to be endemic. Additionally, if left untreated, M leprae infection and subsequent type 1 or type 2 reactions can lead to devastating neurologic and cutaneous sequelae. Prompt recognition and treatment of these reactions is imperative to prevent these long-term complications.
1. Kalisiak M, Yeung R, Dytoc M. Dermacase: leprosy. Can Fam Physician. 2009;55:55-56.
2. Kustner EC, Cruz MP, Dansis CP, et al. Lepromatous leprosy: a review and case report. Med Oral Patol Cir Bucal. 2006;11:474-479.
3. Nunez-Gussman J, Hwang L, Hsu S. Targetoid erythematous plaques: an unusual morphological presentation of multibacillary Hansen’s disease. Eur J Dermatol. 2001;11:65-67.
4. Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
5. Gelber RH. Leprosy (Hansen’s disease). In: Fauci AS, Kasper DL, Longo DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill; 2008:1021-1027.
6. Simon HB. Infections due to mycobacterium. In: Dale DC, Federman DD, Antman K, et al, eds. ACP Medicine. New York, NY: WebMD Professional Publishing; 2004:1703-1720.
7. Baker LP. Mycobacterium leprae interactions with the host cell: recent advances. Indian J Med Res. 2006;123:748-759.
8. Ishii N. Recent advances in the treatment of leprosy. Dermatol Online J. 2003;9:5. http://dermatology.cdlib.org/92/reviews/leprosy/ishii.html. Accessed March 16, 2015.
9. World Health Organization. Global leprosy situation, 2012. Weekly Epidemiological Rec. 2013;88:365-380. http://www.who.int/wer/2007/wer8835.pdf. Accessed March 16, 2015.
10. Gelber RH. Hansen disease. West J Med. 1993;158:583-590.
11. Deps PD, Alves L, Gripp CG, et al. Contact with armadillos increases the risk of leprosy in Brazil: a case control study. Indian J Dermatol Venereol Leprol. 2008;74:338-342.
12. Booth AV, Kovich OI. Lepromatous leprosy. Dermatol Online J. 2007;13:9.
13. Yens DA, Asters DJ, Teitel A. Subcutaneous nodules and joint deformity in leprosy. Clin Rheumatol. 2003;9:181-186.
14. Panezai S, Saleh FG. Leprosy and peripheral neuropathy. J Clin Neuromuscul Dis. 2004;5:138-145.
15. Sasaki S, Takeshita F, Okuda K, et al. Mycobacterium leprae and leprosy. Microbiol Immunol. 2001;45:729-736.
16. Kumar A, Girdhar A, Chakma J, et al. WHO Multidrug Therapy for Leprosy: Epidemiology of default in treatment in Agra District, Uttar Pradesh, India. BioMed Research International. doi:10.1155/2015/705804.
17. Fiallo P, Clapasson A, Favre A, et al. Overexpression of vascular endothelial growth factor and its endothelial cell receptor KDR in type I leprosy reaction. Am J Med Hyg. 2002;66:180-185.
18. Sales AM, de Matos HJ, Nery JAC, et al. Double-blind trial of the efficacy of pentoxifylline vs thalidomide for the treatment of type II reaction in leprosy. Braz J Med Biol Res. 2007;40:243-248.
19. Lockwood DN, Colston MJ, Khanolkar-Young SR. The detection of Mycobacterium leprae protein and carbohydrate antigens in skin and nerve from leprosy patients with type I (reversal) reactions. Am J Trop Med Hyg. 2002;66:409-415.
20. Boggild AK, Keystone JS, Kain KC. Leprosy: a primer for Canadian physicians. CMAJ. 2004;170:71-78.
21. Rook GA, Baker R. Cortisol metabolism, cortisol sensitivity and the pathogenesis of leprosy reactions. Trop Med Int Health. 1999;4:493-498.
1. Kalisiak M, Yeung R, Dytoc M. Dermacase: leprosy. Can Fam Physician. 2009;55:55-56.
2. Kustner EC, Cruz MP, Dansis CP, et al. Lepromatous leprosy: a review and case report. Med Oral Patol Cir Bucal. 2006;11:474-479.
3. Nunez-Gussman J, Hwang L, Hsu S. Targetoid erythematous plaques: an unusual morphological presentation of multibacillary Hansen’s disease. Eur J Dermatol. 2001;11:65-67.
4. Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
5. Gelber RH. Leprosy (Hansen’s disease). In: Fauci AS, Kasper DL, Longo DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill; 2008:1021-1027.
6. Simon HB. Infections due to mycobacterium. In: Dale DC, Federman DD, Antman K, et al, eds. ACP Medicine. New York, NY: WebMD Professional Publishing; 2004:1703-1720.
7. Baker LP. Mycobacterium leprae interactions with the host cell: recent advances. Indian J Med Res. 2006;123:748-759.
8. Ishii N. Recent advances in the treatment of leprosy. Dermatol Online J. 2003;9:5. http://dermatology.cdlib.org/92/reviews/leprosy/ishii.html. Accessed March 16, 2015.
9. World Health Organization. Global leprosy situation, 2012. Weekly Epidemiological Rec. 2013;88:365-380. http://www.who.int/wer/2007/wer8835.pdf. Accessed March 16, 2015.
10. Gelber RH. Hansen disease. West J Med. 1993;158:583-590.
11. Deps PD, Alves L, Gripp CG, et al. Contact with armadillos increases the risk of leprosy in Brazil: a case control study. Indian J Dermatol Venereol Leprol. 2008;74:338-342.
12. Booth AV, Kovich OI. Lepromatous leprosy. Dermatol Online J. 2007;13:9.
13. Yens DA, Asters DJ, Teitel A. Subcutaneous nodules and joint deformity in leprosy. Clin Rheumatol. 2003;9:181-186.
14. Panezai S, Saleh FG. Leprosy and peripheral neuropathy. J Clin Neuromuscul Dis. 2004;5:138-145.
15. Sasaki S, Takeshita F, Okuda K, et al. Mycobacterium leprae and leprosy. Microbiol Immunol. 2001;45:729-736.
16. Kumar A, Girdhar A, Chakma J, et al. WHO Multidrug Therapy for Leprosy: Epidemiology of default in treatment in Agra District, Uttar Pradesh, India. BioMed Research International. doi:10.1155/2015/705804.
17. Fiallo P, Clapasson A, Favre A, et al. Overexpression of vascular endothelial growth factor and its endothelial cell receptor KDR in type I leprosy reaction. Am J Med Hyg. 2002;66:180-185.
18. Sales AM, de Matos HJ, Nery JAC, et al. Double-blind trial of the efficacy of pentoxifylline vs thalidomide for the treatment of type II reaction in leprosy. Braz J Med Biol Res. 2007;40:243-248.
19. Lockwood DN, Colston MJ, Khanolkar-Young SR. The detection of Mycobacterium leprae protein and carbohydrate antigens in skin and nerve from leprosy patients with type I (reversal) reactions. Am J Trop Med Hyg. 2002;66:409-415.
20. Boggild AK, Keystone JS, Kain KC. Leprosy: a primer for Canadian physicians. CMAJ. 2004;170:71-78.
21. Rook GA, Baker R. Cortisol metabolism, cortisol sensitivity and the pathogenesis of leprosy reactions. Trop Med Int Health. 1999;4:493-498.
Practice Points
- Reversal reactions are not uncommonly witnessed in patients undergoing treatment of leprosy.
- Leprosy has several distinct clinical presentations ranging from tuberculoid leprosy to lepromatous leprosy with the extent of disease generally depending on the host’s immune response to the infection.