User login
Topical Clobetasol Propionate Treatment and Cutaneous Adverse Effects in Patients With Early-Stage Mycosis Fungoides: An Observational Study
Mycosis fungoides (MF), the most common variant of cutaneous T-cell lymphoma, is a non-Hodgkin lymphoma of T-cell origin that primarily develops in the skin and has a chronic relapsing course. Early-stage MF (stages IA–IIA) is defined as papules, patches, or plaques with limited (if any) lymph node and blood involvement and no visceral involvement.1 Early-stage MF has a favorable prognosis, and first-line treatments are skin-directed therapies including topical corticosteroids (CSs), topical chemotherapy (nitrogen mustard or carmustine), topical retinoids, topical imiquimod, local radiation, or phototherapy.2 Topical CSs are effective in treating early-stage MF and have been widely used for this indication for several decades; however, there are very little data in the literature on topical CS use in MF.3 Superpotent topical CSs have been shown to have a high overall response rate in early-stage MF3; however, cutaneous side effects associated with long-term topical use include cutaneous atrophy, striae formation, skin fragility, and irritation.
The US Food and Drug Administration (FDA) approved bexarotene gel and mechlorethamine gel for topical treatment of cutaneous lesions in patients with stage IA and IB MF in 2000 and 2013, respectively. Although each may be effective in achieving complete or partial response in MF, both agents are associated with cutaneous side effects, mainly irritation and frequent contact hypersensitivity reactions, respectively.4,5 Additionally, their high prices and limited availability are other major drawbacks of treatment.
At our institution, high-potency topical CSs, specifically once or twice daily clobetasol propionate cream 0.05% prescribed as monotherapy for at least several months, remain the mainstay of treatment in patients with limited patches, papules, and plaques covering less than 10% of the skin surface (stage IA). In this study, we aimed to assess the risk of cutaneous side effects in patients with early-stage MF who were treated with long-term, high-potency topical CSs.
Methods
This prospective observational cohort study included patients with early-stage MF who were seen at the Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, and were started on a superpotent (class I) topical CS (clobetasol propionate cream 0.05%) as monotherapy for MF from July 2016 to July 2017. The diagnosis of MF had to be supported by clinical findings and histopathologic features. All patients were Fitzpatrick skin types I, II, or III. Eligible patients were evaluated for development of CS-induced cutaneous AEs by physical examination and clinical photography of the treated lesions performed at baseline and as part of routine follow-up visits (usually scheduled every 2 to 6 months) at the MSKCC Cutaneous Lymphoma Clinic. Patients’ skin was evaluated clinically for MF activity, atrophy, telangiectasia, purpura, hypopigmentation, and stretch marks (striae). Use of the topical CS was self-reported and also was documented at follow-up visits. Treatment response was defined as follows: complete clinical response (CCR) if the treated lesions resolved completely compared to initial photography; minimal active disease (MAD) if resolution of the vast majority (≥75%) of lesions was seen; and partial response (PR) if some of the lesions resolved (<75%). We analyzed the treatment response rates and adverse effects (AEs). Results were summarized using descriptive statistics.
Results
We identified 13 patients who were started on topical clobetasol propionate as monotherapy for early-stage MF during the study period. Our cohort included 6 males and 7 females aged 36 to 76 years (median age, 61 years). All but 1 participant were diagnosed with stage IA MF (12/13 [92.3%]); of those, 9 (75.0%) had patch-stage disease and 3 (25.0%) presented with plaques. One (7.7%) participant presented with hyperpigmented patches and plaques that involved a little more than 10% of the skin surface (stage IB), and involvement of the hair follicles was noted on histology (folliculotropic MF). All prior treatments were stopped when participants started the superpotent topical CS: 6 (46.2%) participants had been treated with lower-potency topical agents and 1 (7.7%) participant was getting psoralen plus UVA therapy, while the other 6 (46.2%) participants were receiving no therapy for MF prior to starting the study. All participants were prescribed clobetasol propionate cream 0.05% once or twice daily as monotherapy and were instructed to apply it to the MF lesions only, avoiding skin folds and the face. One participant was lost to follow-up, and another stopped using the clobetasol propionate cream after 1.5 months due to local irritation associated with treatment. At their follow-up visits, the other 11 participants were advised to continue with once-daily treatment with clobetasol propionate or were tapered to once every other day, twice weekly, or once weekly depending on their response to treatment and AEs (Table). Participants were advised not to use more than 50 g of clobetasol propionate cream weekly.
All participants responded to the clobetasol propionate cream, and improvement was noted in the treated lesions; however, progression of disease (from stage IA to stage IB) occurred in 1 (8.3%) participant, and phototherapy was added with good response. The participants in our cohort were followed for 4 to 17 months (median, 11.5 months). At the last follow-up visit, all 12 participants showed treatment response: 4 (33.3%) had CCR, 5 (41.7%) had MAD; and 3 (25.0%) had PR. In one participant with a history of partial response to bexarotene gel 1%, daily clobetasol propionate cream 0.05% initially was used alone for 9 months and was later combined with bexarotene gel once weekly, resulting in MAD.
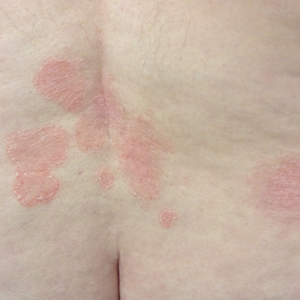
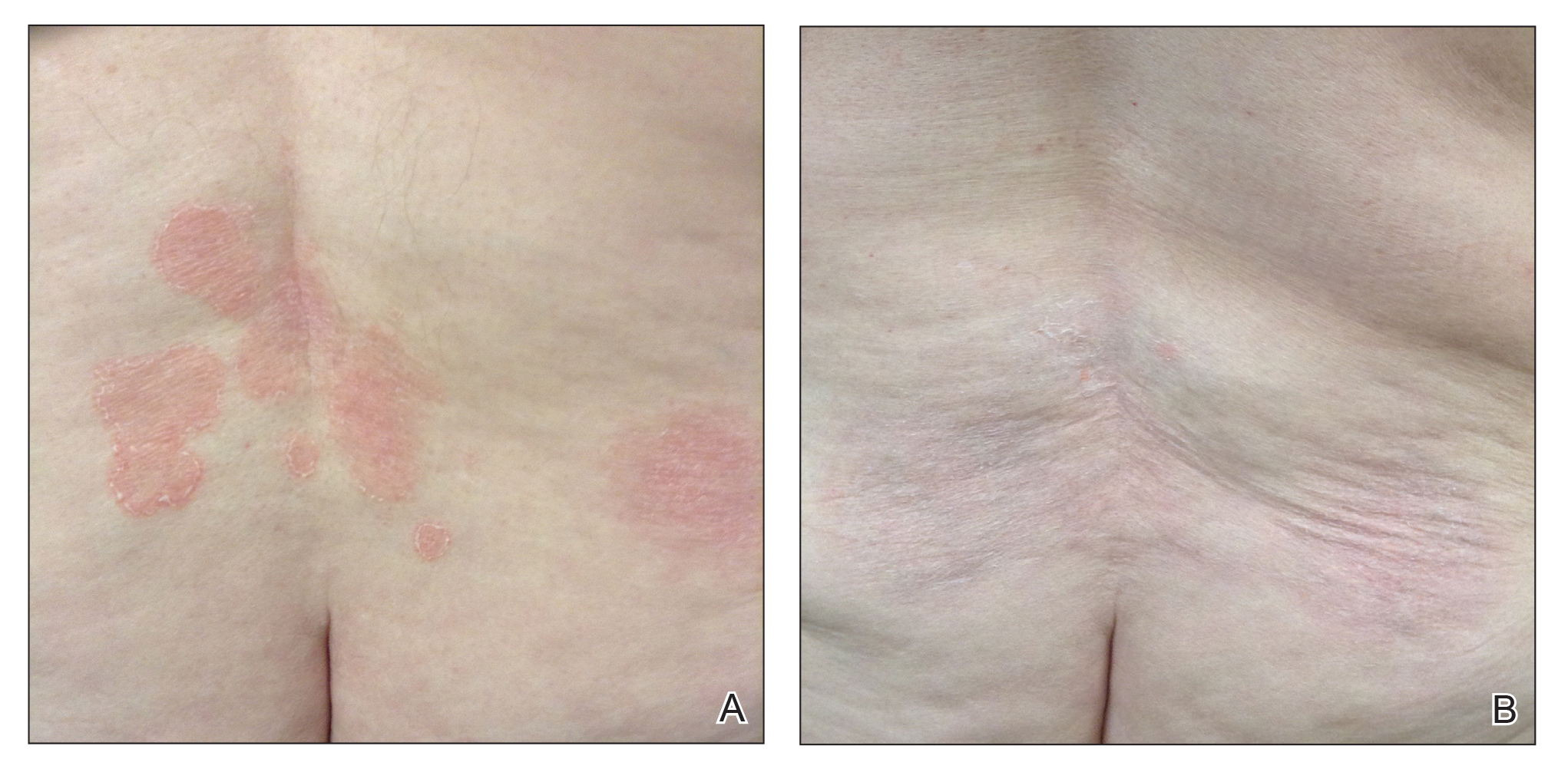
In 7 (58.3%) participants, no AEs to topical clobetasol propionate were recorded. Four (33.3%) participants developed local hypopigmentation at the application site, and 2 (16.7%) developed cutaneous atrophy with local fine wrinkling of the skin (Figure 1); none of the participants developed stretch marks (striae), telangiectases, or skin fragility. One (8.3%) participant developed a petechial rash at the clobetasol propionate application site that resolved once treatment was discontinued and did not recur after restarting clobetasol propionate twice weekly.
Comment
Topical CSs are the most commonly prescribed agents, either as monotherapy or in combination with other agents, in the treatment of numerous dermatologic conditions, including cutaneous T-cell lymphoma and MF. Cutaneous and systemic AEs have been associated with topical CS use. Local AEs are encountered more frequently and include cutaneous atrophy, striae, telangiectasia, purpura, skin fragility, hypopigmentation, hyperpigmentation, acneform eruptions, and hypertrichosis.6 Factors other than potency of the topical CS agent may affect the development of skin atrophy, including anatomic location, duration of therapy, vehicle, and method and frequency of application.7 The potential for systemic AEs due to percutaneous absorption of high-potency CSs, specifically Cushing syndrome and pathologic adrenal suppression, has been a long-standing concern and led the FDA to recommend limiting the use of superpotent CSs to 50 g weekly for 2 or 4 consecutive weeks.8 However, if using an excess of 50 g weekly is avoided, superpotent topical CSs may be safe to use consecutively for months, perhaps even years, without causing systemic effects.9
The effects of topical CSs in MF include induction of apoptosis; inhibition of lymphocyte binding to the endothelium; and downregulation of transcription factors with decreased cytokines, adhesion molecules, and production of growth factors.2 For patients with limited early-stage MF patches and thin plaques, topical CSs often control the disease for many years and frequently are the only form of therapy required. Intralesional steroids can be effective in treating thicker lesions, such as plaques or tumors.10 In an uncontrolled study, Zackheim et al11 prospectively evaluated the effectiveness and safety of twice-daily use of mainly high-potency topical CSs in 79 patients with MF stages IA to IB and observed an overall response rate of 94%. None of the patients were using systemic agents while being treated with topical CSs. Adverse effects were rare: 2 (2.5%) patients experienced temporary minor irritation from the topical CS, 1 (1.3%) patient developed localized skin atrophy under the breast that resolved several months after she stopped treatment, and 1 (1.3%) patient developed stretch marks on the thighs.11 Zackheim12 later reported treatment of approximately 200 patients with class I topical CSs, and overall response rates were over 90% in stage T1 and over 80% in stage T2 patients. Response to topical CS was reported to be evident within 3 months and often much sooner. Side effects were most likely related to the more prolonged treatment periods. Irritant dermatitis or purpura developed in approximately 10% to 20% of patients, and purpura was seen at the sites of treatment as well as at distant sites. Only a small number of patients developed cutaneous atrophy and striae, which were reversible.12 Successful use of intralesional steroids for treatment-resistant MF was reported in 4 patients who tolerated treatment well without any side effects other than local hypopigmentation in a single patient.13
At MSKCC, the first line of treatment in localized (stage IA) MF in light-skinned individuals most frequently is class I topical CSs, usually clobetasol propionate cream 0.05%. Patients are instructed to apply the cream twice daily on active MF lesions uninterruptedly until completely clear and to avoid using it on the face and in skin folds (axillary, inguinal, and abdominal). Patients are instructed to observe themselves for possible cutaneous AEs related to treatment and to stop or taper treatment if any AEs are noticed. In patients with darker skin, we may recommend other modalities such as narrowband UVB phototherapy for even limited MF disease because of the risk for uneven/hypopigmentation with superpotent CSs.
The current study offers a real-life observation of topical high-potency CSs for treatment of early-stage MF and the associated cutaneous AEs. Local hypopigmentation was identified in 4 participants (33.3%), local skin atrophy was seen in 2 participants (16.7%), and local purpura and irritation were seen in 1 participant each (8.3%). All patients responded to therapy and 75.0% (9/12) achieved CCR or showed only MAD at their last follow-up visit. The limitations of our study were the small number of patients included and the relatively short follow-up period.
In MF patients, patches can present as fine wrinkling of the skin resembling atrophy, which can make it difficult to differentiate active MF from CS-induced atrophy in patients treated with topical CSs (Figure 1) and may have caused us to overestimate the occurrence of this AE. Corticosteroid-induced skin atrophy has been studied mainly in normal skin and to a lesser extent in pathological skin in psoriasis and atopic dermatitis. Some of these studies reported that CS-induced atrophy is reversible, and skin thickness can return to normal after topical application of CS is stopped.7
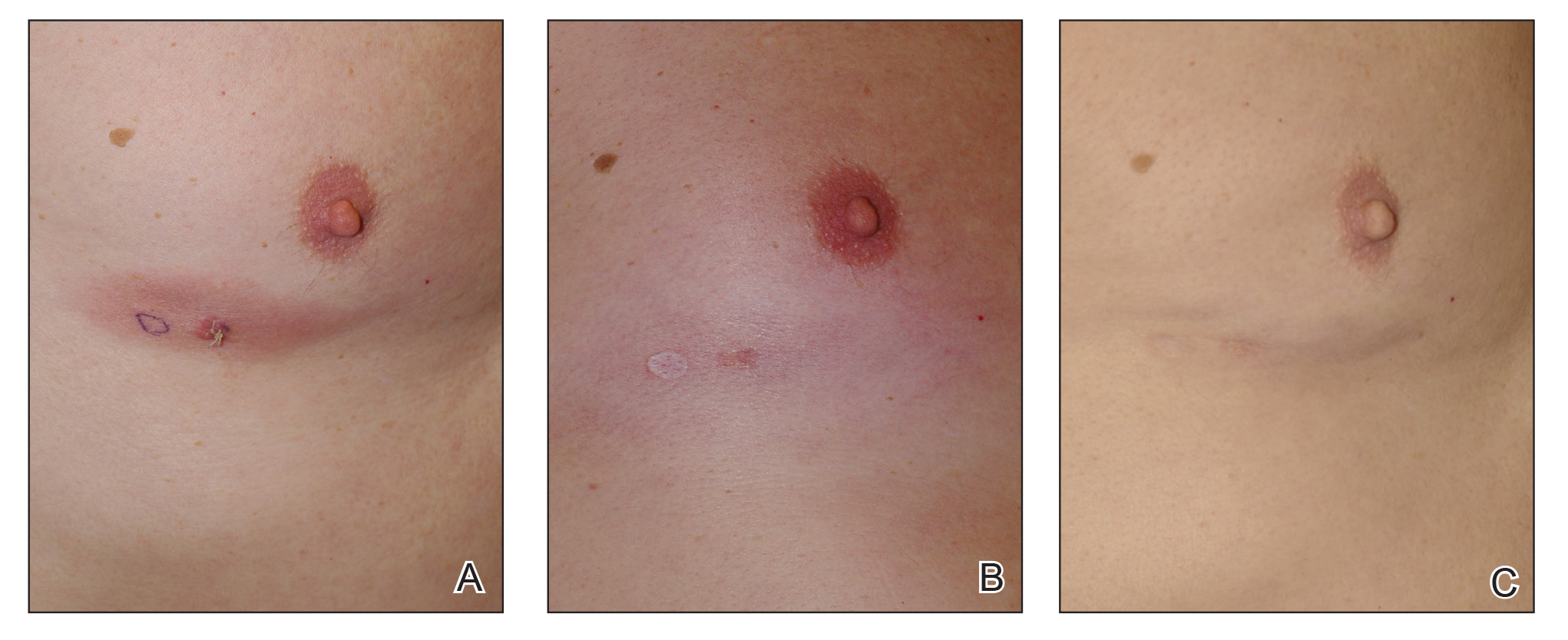
When hypopigmentation is seen around MF lesions, it is a confirmation that the patient is compliant with the therapy. From our experience, local hypopigmentation due to topical CSs is reversible (Figure 2). In some cases, MF patients have applied topical clobetasol propionate to lesional and surrounding skin, and hypopigmentation can be lessened with more careful limited application. In most cases, after discontinuation or tapering of the therapy, the skin returns to its normal color.
Based on our experience and the results of the current study, we conclude that topical superpotent CSs should remain the first-choice treatment for patients with early-stage MF (stage IA). Although bexarotene gel and mechlorethamine gel are FDA approved for early-stage MF, they are not widely available outside of the United States and are associated with AEs, mainly local skin irritation, rash, and pruritus.4,5 In contrast to bexarotene gel and mechlorethamine gel, topical clobetasol propionate can be used in young children (>12 years) and is classified as pregnancy category C.8
Conclusion
Patients with early-stage MF should be treated with skin-directed therapies, and the choice between different therapeutic options is made based on the physician’s experience with the treatment, patient characteristics, location and morphology of the MF lesions, and the AE profile of the treatment. Based on our experience, superpotent topical CSs are readily available and easily applied, have minor side effects, and remain the mainstay of therapy in patients with stage IA disease. Patients with MF on superpotent topical CS therapy should be monitored periodically and instructed how to identify cutaneous AEs related to treatment.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e221-217; quiz 240-222.
- Weberschock T, Strametz R, Lorenz M, et al. Interventions for mycosis fungoides [published online September 12, 2012]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008946.pub2.
- Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49:801-815.
- Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
- Tadicherla S, Ross K, Shenefelt PD, et al. Topical corticosteroids in dermatology. J Drugs Dermatol. 2009;8:1093-1105.
- Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: a comprehensive review. Drug Saf. 2015;38:493-509.
- Temovate E (Clobetasol Propionate) Cream, 0.05% [package insert]. Melville, NY: PharmaDerm, a division of Fougera Pharmaceuticals Inc; 2012.
- Nakamura M, Abrouk M, Zhu H, et al. Update on the systemic risks of superpotent topical steroids. J Drugs Dermatol. 2017;16:643-648.
- Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sezary syndrome. Blood. 2009;114:4337-4353.
- Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. experience in 79 patients. Arch Dermatol. 1998;134:949-954.
- Zackheim HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. 2003;16:283-287.
- Liu DY, Shaath T, Rajpara AN, et al. Safe and efficacious use of intralesional steroids for the treatment of focally resistant mycosis fungoides. J Drugs Dermatol. 2015;14:466-471.
Mycosis fungoides (MF), the most common variant of cutaneous T-cell lymphoma, is a non-Hodgkin lymphoma of T-cell origin that primarily develops in the skin and has a chronic relapsing course. Early-stage MF (stages IA–IIA) is defined as papules, patches, or plaques with limited (if any) lymph node and blood involvement and no visceral involvement.1 Early-stage MF has a favorable prognosis, and first-line treatments are skin-directed therapies including topical corticosteroids (CSs), topical chemotherapy (nitrogen mustard or carmustine), topical retinoids, topical imiquimod, local radiation, or phototherapy.2 Topical CSs are effective in treating early-stage MF and have been widely used for this indication for several decades; however, there are very little data in the literature on topical CS use in MF.3 Superpotent topical CSs have been shown to have a high overall response rate in early-stage MF3; however, cutaneous side effects associated with long-term topical use include cutaneous atrophy, striae formation, skin fragility, and irritation.
The US Food and Drug Administration (FDA) approved bexarotene gel and mechlorethamine gel for topical treatment of cutaneous lesions in patients with stage IA and IB MF in 2000 and 2013, respectively. Although each may be effective in achieving complete or partial response in MF, both agents are associated with cutaneous side effects, mainly irritation and frequent contact hypersensitivity reactions, respectively.4,5 Additionally, their high prices and limited availability are other major drawbacks of treatment.
At our institution, high-potency topical CSs, specifically once or twice daily clobetasol propionate cream 0.05% prescribed as monotherapy for at least several months, remain the mainstay of treatment in patients with limited patches, papules, and plaques covering less than 10% of the skin surface (stage IA). In this study, we aimed to assess the risk of cutaneous side effects in patients with early-stage MF who were treated with long-term, high-potency topical CSs.
Methods
This prospective observational cohort study included patients with early-stage MF who were seen at the Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, and were started on a superpotent (class I) topical CS (clobetasol propionate cream 0.05%) as monotherapy for MF from July 2016 to July 2017. The diagnosis of MF had to be supported by clinical findings and histopathologic features. All patients were Fitzpatrick skin types I, II, or III. Eligible patients were evaluated for development of CS-induced cutaneous AEs by physical examination and clinical photography of the treated lesions performed at baseline and as part of routine follow-up visits (usually scheduled every 2 to 6 months) at the MSKCC Cutaneous Lymphoma Clinic. Patients’ skin was evaluated clinically for MF activity, atrophy, telangiectasia, purpura, hypopigmentation, and stretch marks (striae). Use of the topical CS was self-reported and also was documented at follow-up visits. Treatment response was defined as follows: complete clinical response (CCR) if the treated lesions resolved completely compared to initial photography; minimal active disease (MAD) if resolution of the vast majority (≥75%) of lesions was seen; and partial response (PR) if some of the lesions resolved (<75%). We analyzed the treatment response rates and adverse effects (AEs). Results were summarized using descriptive statistics.
Results
We identified 13 patients who were started on topical clobetasol propionate as monotherapy for early-stage MF during the study period. Our cohort included 6 males and 7 females aged 36 to 76 years (median age, 61 years). All but 1 participant were diagnosed with stage IA MF (12/13 [92.3%]); of those, 9 (75.0%) had patch-stage disease and 3 (25.0%) presented with plaques. One (7.7%) participant presented with hyperpigmented patches and plaques that involved a little more than 10% of the skin surface (stage IB), and involvement of the hair follicles was noted on histology (folliculotropic MF). All prior treatments were stopped when participants started the superpotent topical CS: 6 (46.2%) participants had been treated with lower-potency topical agents and 1 (7.7%) participant was getting psoralen plus UVA therapy, while the other 6 (46.2%) participants were receiving no therapy for MF prior to starting the study. All participants were prescribed clobetasol propionate cream 0.05% once or twice daily as monotherapy and were instructed to apply it to the MF lesions only, avoiding skin folds and the face. One participant was lost to follow-up, and another stopped using the clobetasol propionate cream after 1.5 months due to local irritation associated with treatment. At their follow-up visits, the other 11 participants were advised to continue with once-daily treatment with clobetasol propionate or were tapered to once every other day, twice weekly, or once weekly depending on their response to treatment and AEs (Table). Participants were advised not to use more than 50 g of clobetasol propionate cream weekly.

All participants responded to the clobetasol propionate cream, and improvement was noted in the treated lesions; however, progression of disease (from stage IA to stage IB) occurred in 1 (8.3%) participant, and phototherapy was added with good response. The participants in our cohort were followed for 4 to 17 months (median, 11.5 months). At the last follow-up visit, all 12 participants showed treatment response: 4 (33.3%) had CCR, 5 (41.7%) had MAD; and 3 (25.0%) had PR. In one participant with a history of partial response to bexarotene gel 1%, daily clobetasol propionate cream 0.05% initially was used alone for 9 months and was later combined with bexarotene gel once weekly, resulting in MAD.
In 7 (58.3%) participants, no AEs to topical clobetasol propionate were recorded. Four (33.3%) participants developed local hypopigmentation at the application site, and 2 (16.7%) developed cutaneous atrophy with local fine wrinkling of the skin (Figure 1); none of the participants developed stretch marks (striae), telangiectases, or skin fragility. One (8.3%) participant developed a petechial rash at the clobetasol propionate application site that resolved once treatment was discontinued and did not recur after restarting clobetasol propionate twice weekly.

Comment
Topical CSs are the most commonly prescribed agents, either as monotherapy or in combination with other agents, in the treatment of numerous dermatologic conditions, including cutaneous T-cell lymphoma and MF. Cutaneous and systemic AEs have been associated with topical CS use. Local AEs are encountered more frequently and include cutaneous atrophy, striae, telangiectasia, purpura, skin fragility, hypopigmentation, hyperpigmentation, acneform eruptions, and hypertrichosis.6 Factors other than potency of the topical CS agent may affect the development of skin atrophy, including anatomic location, duration of therapy, vehicle, and method and frequency of application.7 The potential for systemic AEs due to percutaneous absorption of high-potency CSs, specifically Cushing syndrome and pathologic adrenal suppression, has been a long-standing concern and led the FDA to recommend limiting the use of superpotent CSs to 50 g weekly for 2 or 4 consecutive weeks.8 However, if using an excess of 50 g weekly is avoided, superpotent topical CSs may be safe to use consecutively for months, perhaps even years, without causing systemic effects.9
The effects of topical CSs in MF include induction of apoptosis; inhibition of lymphocyte binding to the endothelium; and downregulation of transcription factors with decreased cytokines, adhesion molecules, and production of growth factors.2 For patients with limited early-stage MF patches and thin plaques, topical CSs often control the disease for many years and frequently are the only form of therapy required. Intralesional steroids can be effective in treating thicker lesions, such as plaques or tumors.10 In an uncontrolled study, Zackheim et al11 prospectively evaluated the effectiveness and safety of twice-daily use of mainly high-potency topical CSs in 79 patients with MF stages IA to IB and observed an overall response rate of 94%. None of the patients were using systemic agents while being treated with topical CSs. Adverse effects were rare: 2 (2.5%) patients experienced temporary minor irritation from the topical CS, 1 (1.3%) patient developed localized skin atrophy under the breast that resolved several months after she stopped treatment, and 1 (1.3%) patient developed stretch marks on the thighs.11 Zackheim12 later reported treatment of approximately 200 patients with class I topical CSs, and overall response rates were over 90% in stage T1 and over 80% in stage T2 patients. Response to topical CS was reported to be evident within 3 months and often much sooner. Side effects were most likely related to the more prolonged treatment periods. Irritant dermatitis or purpura developed in approximately 10% to 20% of patients, and purpura was seen at the sites of treatment as well as at distant sites. Only a small number of patients developed cutaneous atrophy and striae, which were reversible.12 Successful use of intralesional steroids for treatment-resistant MF was reported in 4 patients who tolerated treatment well without any side effects other than local hypopigmentation in a single patient.13
At MSKCC, the first line of treatment in localized (stage IA) MF in light-skinned individuals most frequently is class I topical CSs, usually clobetasol propionate cream 0.05%. Patients are instructed to apply the cream twice daily on active MF lesions uninterruptedly until completely clear and to avoid using it on the face and in skin folds (axillary, inguinal, and abdominal). Patients are instructed to observe themselves for possible cutaneous AEs related to treatment and to stop or taper treatment if any AEs are noticed. In patients with darker skin, we may recommend other modalities such as narrowband UVB phototherapy for even limited MF disease because of the risk for uneven/hypopigmentation with superpotent CSs.
The current study offers a real-life observation of topical high-potency CSs for treatment of early-stage MF and the associated cutaneous AEs. Local hypopigmentation was identified in 4 participants (33.3%), local skin atrophy was seen in 2 participants (16.7%), and local purpura and irritation were seen in 1 participant each (8.3%). All patients responded to therapy and 75.0% (9/12) achieved CCR or showed only MAD at their last follow-up visit. The limitations of our study were the small number of patients included and the relatively short follow-up period.
In MF patients, patches can present as fine wrinkling of the skin resembling atrophy, which can make it difficult to differentiate active MF from CS-induced atrophy in patients treated with topical CSs (Figure 1) and may have caused us to overestimate the occurrence of this AE. Corticosteroid-induced skin atrophy has been studied mainly in normal skin and to a lesser extent in pathological skin in psoriasis and atopic dermatitis. Some of these studies reported that CS-induced atrophy is reversible, and skin thickness can return to normal after topical application of CS is stopped.7
When hypopigmentation is seen around MF lesions, it is a confirmation that the patient is compliant with the therapy. From our experience, local hypopigmentation due to topical CSs is reversible (Figure 2). In some cases, MF patients have applied topical clobetasol propionate to lesional and surrounding skin, and hypopigmentation can be lessened with more careful limited application. In most cases, after discontinuation or tapering of the therapy, the skin returns to its normal color.

Based on our experience and the results of the current study, we conclude that topical superpotent CSs should remain the first-choice treatment for patients with early-stage MF (stage IA). Although bexarotene gel and mechlorethamine gel are FDA approved for early-stage MF, they are not widely available outside of the United States and are associated with AEs, mainly local skin irritation, rash, and pruritus.4,5 In contrast to bexarotene gel and mechlorethamine gel, topical clobetasol propionate can be used in young children (>12 years) and is classified as pregnancy category C.8
Conclusion
Patients with early-stage MF should be treated with skin-directed therapies, and the choice between different therapeutic options is made based on the physician’s experience with the treatment, patient characteristics, location and morphology of the MF lesions, and the AE profile of the treatment. Based on our experience, superpotent topical CSs are readily available and easily applied, have minor side effects, and remain the mainstay of therapy in patients with stage IA disease. Patients with MF on superpotent topical CS therapy should be monitored periodically and instructed how to identify cutaneous AEs related to treatment.
Mycosis fungoides (MF), the most common variant of cutaneous T-cell lymphoma, is a non-Hodgkin lymphoma of T-cell origin that primarily develops in the skin and has a chronic relapsing course. Early-stage MF (stages IA–IIA) is defined as papules, patches, or plaques with limited (if any) lymph node and blood involvement and no visceral involvement.1 Early-stage MF has a favorable prognosis, and first-line treatments are skin-directed therapies including topical corticosteroids (CSs), topical chemotherapy (nitrogen mustard or carmustine), topical retinoids, topical imiquimod, local radiation, or phototherapy.2 Topical CSs are effective in treating early-stage MF and have been widely used for this indication for several decades; however, there are very little data in the literature on topical CS use in MF.3 Superpotent topical CSs have been shown to have a high overall response rate in early-stage MF3; however, cutaneous side effects associated with long-term topical use include cutaneous atrophy, striae formation, skin fragility, and irritation.
The US Food and Drug Administration (FDA) approved bexarotene gel and mechlorethamine gel for topical treatment of cutaneous lesions in patients with stage IA and IB MF in 2000 and 2013, respectively. Although each may be effective in achieving complete or partial response in MF, both agents are associated with cutaneous side effects, mainly irritation and frequent contact hypersensitivity reactions, respectively.4,5 Additionally, their high prices and limited availability are other major drawbacks of treatment.
At our institution, high-potency topical CSs, specifically once or twice daily clobetasol propionate cream 0.05% prescribed as monotherapy for at least several months, remain the mainstay of treatment in patients with limited patches, papules, and plaques covering less than 10% of the skin surface (stage IA). In this study, we aimed to assess the risk of cutaneous side effects in patients with early-stage MF who were treated with long-term, high-potency topical CSs.
Methods
This prospective observational cohort study included patients with early-stage MF who were seen at the Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, and were started on a superpotent (class I) topical CS (clobetasol propionate cream 0.05%) as monotherapy for MF from July 2016 to July 2017. The diagnosis of MF had to be supported by clinical findings and histopathologic features. All patients were Fitzpatrick skin types I, II, or III. Eligible patients were evaluated for development of CS-induced cutaneous AEs by physical examination and clinical photography of the treated lesions performed at baseline and as part of routine follow-up visits (usually scheduled every 2 to 6 months) at the MSKCC Cutaneous Lymphoma Clinic. Patients’ skin was evaluated clinically for MF activity, atrophy, telangiectasia, purpura, hypopigmentation, and stretch marks (striae). Use of the topical CS was self-reported and also was documented at follow-up visits. Treatment response was defined as follows: complete clinical response (CCR) if the treated lesions resolved completely compared to initial photography; minimal active disease (MAD) if resolution of the vast majority (≥75%) of lesions was seen; and partial response (PR) if some of the lesions resolved (<75%). We analyzed the treatment response rates and adverse effects (AEs). Results were summarized using descriptive statistics.
Results
We identified 13 patients who were started on topical clobetasol propionate as monotherapy for early-stage MF during the study period. Our cohort included 6 males and 7 females aged 36 to 76 years (median age, 61 years). All but 1 participant were diagnosed with stage IA MF (12/13 [92.3%]); of those, 9 (75.0%) had patch-stage disease and 3 (25.0%) presented with plaques. One (7.7%) participant presented with hyperpigmented patches and plaques that involved a little more than 10% of the skin surface (stage IB), and involvement of the hair follicles was noted on histology (folliculotropic MF). All prior treatments were stopped when participants started the superpotent topical CS: 6 (46.2%) participants had been treated with lower-potency topical agents and 1 (7.7%) participant was getting psoralen plus UVA therapy, while the other 6 (46.2%) participants were receiving no therapy for MF prior to starting the study. All participants were prescribed clobetasol propionate cream 0.05% once or twice daily as monotherapy and were instructed to apply it to the MF lesions only, avoiding skin folds and the face. One participant was lost to follow-up, and another stopped using the clobetasol propionate cream after 1.5 months due to local irritation associated with treatment. At their follow-up visits, the other 11 participants were advised to continue with once-daily treatment with clobetasol propionate or were tapered to once every other day, twice weekly, or once weekly depending on their response to treatment and AEs (Table). Participants were advised not to use more than 50 g of clobetasol propionate cream weekly.

All participants responded to the clobetasol propionate cream, and improvement was noted in the treated lesions; however, progression of disease (from stage IA to stage IB) occurred in 1 (8.3%) participant, and phototherapy was added with good response. The participants in our cohort were followed for 4 to 17 months (median, 11.5 months). At the last follow-up visit, all 12 participants showed treatment response: 4 (33.3%) had CCR, 5 (41.7%) had MAD; and 3 (25.0%) had PR. In one participant with a history of partial response to bexarotene gel 1%, daily clobetasol propionate cream 0.05% initially was used alone for 9 months and was later combined with bexarotene gel once weekly, resulting in MAD.
In 7 (58.3%) participants, no AEs to topical clobetasol propionate were recorded. Four (33.3%) participants developed local hypopigmentation at the application site, and 2 (16.7%) developed cutaneous atrophy with local fine wrinkling of the skin (Figure 1); none of the participants developed stretch marks (striae), telangiectases, or skin fragility. One (8.3%) participant developed a petechial rash at the clobetasol propionate application site that resolved once treatment was discontinued and did not recur after restarting clobetasol propionate twice weekly.

Comment
Topical CSs are the most commonly prescribed agents, either as monotherapy or in combination with other agents, in the treatment of numerous dermatologic conditions, including cutaneous T-cell lymphoma and MF. Cutaneous and systemic AEs have been associated with topical CS use. Local AEs are encountered more frequently and include cutaneous atrophy, striae, telangiectasia, purpura, skin fragility, hypopigmentation, hyperpigmentation, acneform eruptions, and hypertrichosis.6 Factors other than potency of the topical CS agent may affect the development of skin atrophy, including anatomic location, duration of therapy, vehicle, and method and frequency of application.7 The potential for systemic AEs due to percutaneous absorption of high-potency CSs, specifically Cushing syndrome and pathologic adrenal suppression, has been a long-standing concern and led the FDA to recommend limiting the use of superpotent CSs to 50 g weekly for 2 or 4 consecutive weeks.8 However, if using an excess of 50 g weekly is avoided, superpotent topical CSs may be safe to use consecutively for months, perhaps even years, without causing systemic effects.9
The effects of topical CSs in MF include induction of apoptosis; inhibition of lymphocyte binding to the endothelium; and downregulation of transcription factors with decreased cytokines, adhesion molecules, and production of growth factors.2 For patients with limited early-stage MF patches and thin plaques, topical CSs often control the disease for many years and frequently are the only form of therapy required. Intralesional steroids can be effective in treating thicker lesions, such as plaques or tumors.10 In an uncontrolled study, Zackheim et al11 prospectively evaluated the effectiveness and safety of twice-daily use of mainly high-potency topical CSs in 79 patients with MF stages IA to IB and observed an overall response rate of 94%. None of the patients were using systemic agents while being treated with topical CSs. Adverse effects were rare: 2 (2.5%) patients experienced temporary minor irritation from the topical CS, 1 (1.3%) patient developed localized skin atrophy under the breast that resolved several months after she stopped treatment, and 1 (1.3%) patient developed stretch marks on the thighs.11 Zackheim12 later reported treatment of approximately 200 patients with class I topical CSs, and overall response rates were over 90% in stage T1 and over 80% in stage T2 patients. Response to topical CS was reported to be evident within 3 months and often much sooner. Side effects were most likely related to the more prolonged treatment periods. Irritant dermatitis or purpura developed in approximately 10% to 20% of patients, and purpura was seen at the sites of treatment as well as at distant sites. Only a small number of patients developed cutaneous atrophy and striae, which were reversible.12 Successful use of intralesional steroids for treatment-resistant MF was reported in 4 patients who tolerated treatment well without any side effects other than local hypopigmentation in a single patient.13
At MSKCC, the first line of treatment in localized (stage IA) MF in light-skinned individuals most frequently is class I topical CSs, usually clobetasol propionate cream 0.05%. Patients are instructed to apply the cream twice daily on active MF lesions uninterruptedly until completely clear and to avoid using it on the face and in skin folds (axillary, inguinal, and abdominal). Patients are instructed to observe themselves for possible cutaneous AEs related to treatment and to stop or taper treatment if any AEs are noticed. In patients with darker skin, we may recommend other modalities such as narrowband UVB phototherapy for even limited MF disease because of the risk for uneven/hypopigmentation with superpotent CSs.
The current study offers a real-life observation of topical high-potency CSs for treatment of early-stage MF and the associated cutaneous AEs. Local hypopigmentation was identified in 4 participants (33.3%), local skin atrophy was seen in 2 participants (16.7%), and local purpura and irritation were seen in 1 participant each (8.3%). All patients responded to therapy and 75.0% (9/12) achieved CCR or showed only MAD at their last follow-up visit. The limitations of our study were the small number of patients included and the relatively short follow-up period.
In MF patients, patches can present as fine wrinkling of the skin resembling atrophy, which can make it difficult to differentiate active MF from CS-induced atrophy in patients treated with topical CSs (Figure 1) and may have caused us to overestimate the occurrence of this AE. Corticosteroid-induced skin atrophy has been studied mainly in normal skin and to a lesser extent in pathological skin in psoriasis and atopic dermatitis. Some of these studies reported that CS-induced atrophy is reversible, and skin thickness can return to normal after topical application of CS is stopped.7
When hypopigmentation is seen around MF lesions, it is a confirmation that the patient is compliant with the therapy. From our experience, local hypopigmentation due to topical CSs is reversible (Figure 2). In some cases, MF patients have applied topical clobetasol propionate to lesional and surrounding skin, and hypopigmentation can be lessened with more careful limited application. In most cases, after discontinuation or tapering of the therapy, the skin returns to its normal color.

Based on our experience and the results of the current study, we conclude that topical superpotent CSs should remain the first-choice treatment for patients with early-stage MF (stage IA). Although bexarotene gel and mechlorethamine gel are FDA approved for early-stage MF, they are not widely available outside of the United States and are associated with AEs, mainly local skin irritation, rash, and pruritus.4,5 In contrast to bexarotene gel and mechlorethamine gel, topical clobetasol propionate can be used in young children (>12 years) and is classified as pregnancy category C.8
Conclusion
Patients with early-stage MF should be treated with skin-directed therapies, and the choice between different therapeutic options is made based on the physician’s experience with the treatment, patient characteristics, location and morphology of the MF lesions, and the AE profile of the treatment. Based on our experience, superpotent topical CSs are readily available and easily applied, have minor side effects, and remain the mainstay of therapy in patients with stage IA disease. Patients with MF on superpotent topical CS therapy should be monitored periodically and instructed how to identify cutaneous AEs related to treatment.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e221-217; quiz 240-222.
- Weberschock T, Strametz R, Lorenz M, et al. Interventions for mycosis fungoides [published online September 12, 2012]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008946.pub2.
- Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49:801-815.
- Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
- Tadicherla S, Ross K, Shenefelt PD, et al. Topical corticosteroids in dermatology. J Drugs Dermatol. 2009;8:1093-1105.
- Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: a comprehensive review. Drug Saf. 2015;38:493-509.
- Temovate E (Clobetasol Propionate) Cream, 0.05% [package insert]. Melville, NY: PharmaDerm, a division of Fougera Pharmaceuticals Inc; 2012.
- Nakamura M, Abrouk M, Zhu H, et al. Update on the systemic risks of superpotent topical steroids. J Drugs Dermatol. 2017;16:643-648.
- Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sezary syndrome. Blood. 2009;114:4337-4353.
- Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. experience in 79 patients. Arch Dermatol. 1998;134:949-954.
- Zackheim HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. 2003;16:283-287.
- Liu DY, Shaath T, Rajpara AN, et al. Safe and efficacious use of intralesional steroids for the treatment of focally resistant mycosis fungoides. J Drugs Dermatol. 2015;14:466-471.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e221-217; quiz 240-222.
- Weberschock T, Strametz R, Lorenz M, et al. Interventions for mycosis fungoides [published online September 12, 2012]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008946.pub2.
- Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49:801-815.
- Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
- Tadicherla S, Ross K, Shenefelt PD, et al. Topical corticosteroids in dermatology. J Drugs Dermatol. 2009;8:1093-1105.
- Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: a comprehensive review. Drug Saf. 2015;38:493-509.
- Temovate E (Clobetasol Propionate) Cream, 0.05% [package insert]. Melville, NY: PharmaDerm, a division of Fougera Pharmaceuticals Inc; 2012.
- Nakamura M, Abrouk M, Zhu H, et al. Update on the systemic risks of superpotent topical steroids. J Drugs Dermatol. 2017;16:643-648.
- Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sezary syndrome. Blood. 2009;114:4337-4353.
- Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. experience in 79 patients. Arch Dermatol. 1998;134:949-954.
- Zackheim HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. 2003;16:283-287.
- Liu DY, Shaath T, Rajpara AN, et al. Safe and efficacious use of intralesional steroids for the treatment of focally resistant mycosis fungoides. J Drugs Dermatol. 2015;14:466-471.
Practice Points
- Topical corticosteroid (CS) treatment is a safe skin-directed therapy that can effectively obtain complete and long-term response in patients with early-stage mycosis fungoides (MF).
- Despite the availability of optional topical treatments in MF, topical superpotent class I CSs are still considered the first-line treatment in patients with limited disease (stage IA).
- Patients using prolonged topical superpotent CSs should be monitored periodically and instructed on how to identify cutaneous adverse effects related to treatment, mainly local hypopigmentation and skin atrophy.