User login
New cholesterol guidelines: Worth the wait?
On November 12, 2013, a joint task force for the American College of Cardiology and American Heart Association released new guidelines for treating high blood cholesterol to reduce the risk of atherosclerotic cardiovascular disease (ASCVD) in adults.1
This document arrives after several years of intense deliberation, 12 years after the third Adult Treatment Panel (ATP III) guidelines,2 and 8 years after an ATP III update recommending that low-density lipoprotein cholesterol (LDL-C) levels be lowered aggressively (to less than 70 mg/dL) as an option in patients at high risk.3 It represents a major shift in the approach to and management of high blood cholesterol and has sparked considerable controversy.
In the following commentary, we summarize the new guidelines and the philosophy employed by the task force in generating them. We will also examine some advantages and what we believe to be several shortcomings of the new guidelines. These latter points are illustrated through case examples.
IN RANDOMIZED CONTROLLED TRIALS WE TRUST
In collaboration with the National Heart, Lung, and Blood Institute of the National Institutes of Health, the American College of Cardiology and American Heart Association formed an expert panel task force in 2008.
The task force elected to use only evidence from randomized controlled trials, systematic reviews, and meta-analyses of randomized controlled trials (and only predefined outcomes of the trials, not post hoc analyses) in formulating its recommendations, with the goal of providing the strongest possible evidence.
The authors state that “By using [randomized controlled trial] data to identify those most likely to benefit [emphasis in original] from cholesterol-lowering statin therapy, the recommendations will be of value to primary care clinicians as well as specialists concerned with ASCVD prevention. Importantly, the recommendations were designed to be easy to use in the clinical setting, facilitating the implementation of a strategy of risk assessment and treatment focused on the prevention of ASCVD.”3 They also state the guidelines are meant to “inform clinical judgment, not replace it” and that clinician judgment in addition to discussion with patients remains vital.
During the deliberations, the National Heart, Lung, and Blood Institute removed itself from participating, stating its mission no longer included drafting new guidelines. Additionally, other initial members of the task force removed themselves because of disagreement and concerns about the direction of the new guidelines.
These guidelines, and their accompanying new cardiovascular risk calculator,4 were released without a preliminary period to allow for open discussion, comment, and critique by physicians outside the panel. No attempt was made to harmonize the guidelines with previous versions (eg, ATP III) or with current international guidelines.
WHAT’S NEW IN THE GUIDELINES?
The following are the major changes in the new guidelines for treating high blood cholesterol:
- Treatment goals for LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) are no longer recommended.
- High-intensity and moderate-intensity statin treatment is emphasized, and low-intensity statin therapy is nearly eliminated.
- “ASCVD” now includes stroke in addition to coronary heart disease and peripheral arterial disease.
- Four groups are targeted for treatment (see below).
- Nonstatin therapies have been markedly de-emphasized.
- No guidelines are provided for treating high triglyceride levels.
The new guidelines emphasize lifestyle modification as the foundation for reducing risk, regardless of cholesterol therapy. No recommendations are given for patients with New York Heart Association class II, III, or IV heart failure or for hemodialysis patients, because there were insufficient data from randomized controlled trials to support recommendations. Similarly, the guidelines apply only to people between the ages of 40 and 75 (risk calculator ages 40–79), because the authors believed there was not enough evidence from randomized controlled trials to allow development of guidelines outside of this age range.
FOUR MAJOR STATIN TREATMENT GROUPS
The new guidelines specify four groups that merit intensive or moderately intensive statin therapy (Table 1)1:
- People with clinical ASCVD
- People with LDL-C levels of 190 mg/dL or higher
- People with diabetes, age 40 to 75
- People without diabetes, age 40 to 75, with LDL-C levels 70–189 mg/dL, and a 10-year ASCVD risk of 7.5% or higher as determined by the new risk calculator4 (which also calculates the lifetime risk of ASCVD).
Below, we will address each of these four groups and provide case scenarios to consider. In general, our major disagreements with the new recommendations pertain to the first and fourth categories.
GROUP 1: PEOPLE WITH CLINICAL ASCVD
Advantages of the new guidelines
- They appropriately recommend statins in the highest tolerated doses as first-line treatment for this group at high risk.
- They designate all patients with ASCVD, including those with coronary, peripheral, and cerebrovascular disease, as a high-risk group.
- Without target LDL-C levels, treatment is simpler than before, requiring less monitoring of lipid levels. (This can also be seen as a limitation, as we discuss below.)
Limitations of the new guidelines
- They make follow-up LDL-C levels irrelevant, seeming to assume that there is no gradation in residual risk and, thus, no need to tailor therapy to the individual.
- Patients no longer have a goal to strive for or a way to monitor their progress.
- The guidelines ignore the pathophysiology of coronary artery disease and evidence of residual risk in patients on both moderate-intensity and high-intensity statin therapy.
- They also ignore the potential benefits of treating to lower LDL-C or non-HDL-C goals, thus eliminating consideration of multidrug therapy. They do not address patients with recurrent cardiovascular events already on maximal tolerated statin doses.
- They undermine the potential development and use of new therapies for dysplipidemia in patients with ASCVD.
Case 1: Is LDL-C 110 mg/dL low enough?
A 52-year-old African American man presents with newly discovered moderate coronary artery disease that is not severe enough to warrant stenting. He has no history of hypertension, diabetes mellitus, or smoking. His systolic blood pressure is 130 mm Hg, and his body mass index is 26 kg/m2. He exercises regularly and follows a low-cholesterol diet. He has the following fasting lipid values:
- Total cholesterol 290 mg/dL
- HDL-C 50 mg/dL
- Triglycerides 250 mg/dL
- Calculated LDL-C 190 mg/dL.
Two months later, after beginning atorvastatin 80 mg daily, meeting with a nutritionist, and redoubling his dietary efforts, his fasting lipid concentrations are:
- Total cholesterol 180 mg/dL
- HDL-C 55 mg/dL
- Triglycerides 75 mg/dL
- Calculated LDL-C 110 mg/dL.
Comment: Lack of LDL-C goals is a flaw
The new guidelines call for patients with known ASCVD, such as this patient, to receive intensive statin therapy—which he got.
However, once a patient is on therapy, the new guidelines do not encourage repeating the lipid panel other than to assess compliance. With intensive therapy, we expect a reduction in LDL-C of at least 50% (Table 1), but patient-to-patient differences in response to medications are common, and without repeat testing we would have no way of gauging this patient’s residual risk.
Further, the new guidelines emphasize the lack of hard outcome data supporting the addition of another lipid-lowering drug to a statin, although they do indicate that one can consider it. In a patient at high risk, such as this one, would you be comfortable with an LDL-C value of 110 mg/dL on maximum statin therapy? Would you consider adding a nonstatin drug?
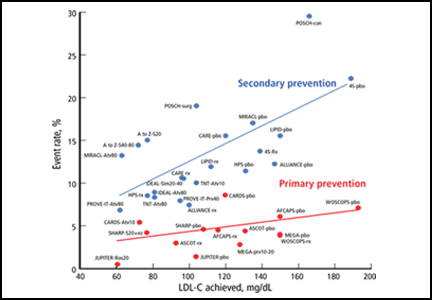
A preponderance of data shows that LDL plays a causal role in ASCVD development and adverse events. Genetic data show that the LDL particle and the LDL receptor pathway are mechanistically linked to ASCVD pathogenesis, with lifetime exposure as a critical determinant of risk.5,6 Moreover, randomized controlled trials of statins and other studies of cholesterol-lowering show a reproducible relationship between the LDL-C level achieved and absolute risk (Figure 1).7–24 We believe the totality of data constitutes a strong rationale for targeting LDL-C and establishing goals for lowering its levels. For these reasons, we believe that removing LDL-C goals is a fundamental flaw of the new guidelines.
The reason for the lack of data from randomized controlled trials demonstrating benefits of adding therapies to statins (when LDL-C is still high) or benefits of treating to specific goals is that no such trials have been performed. Even trials of nonpharmacologic means of lowering LDL-C, such as ileal bypass, which was used in the Program on the Surgical Control of the Hyperlipidemias trial,20 provide independent evidence that lowering LDL-C reduces the risk of ASCVD (Figure 1).
In addition, trials of nonstatin drugs, such as the Coronary Drug Project,25 which tested niacin, also showed outcome benefits. On the other hand, studies such as the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health26 and Treatment of HDL to Reduce the Incidence of Vascular Events27 trials did not show additional risk reduction when niacin was added to statin therapy. However, the study designs arguably had flaws, including requirement of aggressive LDL-lowering with statins, with LDL-C levels below 70 to 80 mg/dL before randomization.
Therefore, these trials do not tell us what to do for a patient on maximal intensive therapy who has recurrent ASCVD events or who, like our patient, has an LDL-C level higher than previous targets.
For this patient, we would recommend adding a second medication to further lower his LDL-C, but discussing with him the absence of proven benefit in clinical trials and the risks of side effects. At present, lacking LDL-C goals in the new guidelines, we are keeping with the ATP III goals to help guide therapeutic choices and individualize patient management.
GROUP 2: PEOPLE WITH LDL-C ≥ 190
Advantages of the new guidelines
- They state that these patients should receive statins in the highest tolerated doses, which is universally accepted.
Limitations of the new guidelines
- The new guidelines mention only that one “may consider” adding a second agent if LDL-C remains above 190 mg/dL after maximum-dose therapy. Patients with familial hypercholesterolemia or other severe forms of hypercholesterolemia typically end up on multidrug therapy to further reduce LDL-C. The absence of randomized controlled trial data in this setting to show an additive value of second and third lipid-lowering agents does not mean these agents do not provide benefit.
GROUP 3: DIABETES, AGE 40–75, LDL-C 70–189, NO CLINICAL ASCVD
Advantages of the new guidelines
- They call for aggressive treatment of people with diabetes, a group at high risk that derives significant benefit from statin therapy, as shown in randomized controlled trials.
Limitations of the new guidelines
- Although high-intensity statin therapy is indicated for this group, we believe that, using the new risk calculator, some patients may receive overly aggressive treatment, thus increasing the possibility of statin side effects.
- The guidelines do not address patients younger than 40 or older than 75.
- Diabetic patients have a high residual risk of ASCVD events, even on statin therapy. Yet the guidelines ignore the potential benefits of more aggressive LDL-lowering or non-LDL secondary targets for therapy.
Case 2: How low is too low?
A 63-year-old white woman, a nonsmoker with recently diagnosed diabetes, is seen by her primary care physician. She has hypertension, for which she takes lisinopril 5 mg daily. Her fasting lipid values are:
- Total cholesterol 160 mg/dL
- HDL-C 64 mg/dL
- Triglycerides 100 mg/dL
- Calculated LDL-C 76 mg/dL.
Her systolic blood pressure is 129 mm Hg, and based on the new risk calculator, her 10-year risk of cardiovascular disease is 10.2%. According to the new guidelines, she should be started on high-intensity statin treatment (Table 1).
Although this is an acceptable initial course of action, it necessitates close vigilance, since it may actually drive her LDL-C level too low. Randomized controlled trials have typically used an LDL-C concentration of less than or equal to 25 mg/dL as the safety cutoff. With a typical LDL-C reduction of at least 50% on high-intensity statins, our patient’s expected LDL-C level will likely be in the low 30s. We believe this would be a good outcome, provided that she tolerates the medication without adverse effects. However, responses to statins vary from patient to patient.
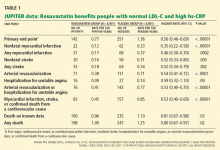
High-intensity statin therapy may not be necessary to reduce risk adequately in all patients who have diabetes without preexisting vascular disease. The Collaborative Atorvastatin Diabetes Study12 compared atorvastatin 10 mg vs placebo in people with type 2 diabetes, age 40 to 75, who had one or more cardiovascular risk factors but no signs or symptoms of preexisting ASCVD and who had only average or below-average cholesterol levels—precisely like this patient. The trial was terminated early because of a clear benefit (a 37% reduction in the composite end point of major adverse cardiovascular events) in the intervention group. For our patient, we believe an alternative and acceptable approach would be to begin moderate-intensity statin therapy (eg, with atorvastatin 10 mg) (Table 1).
Alternatively, in a patient with diabetes and previous atherosclerotic vascular disease or with a high 10-year risk and high LDL-C, limiting treatment to high-intensity statin therapy by itself may deny them the potential benefits of combination therapies and targeting to lower LDL-C levels or non-HDL-C secondary targets. Guidelines from the American Diabetes Association28 and the American Association of Clinical Endocrinologists29 continue to recommend an LDL-C goal of less than 70 mg/dL in patients at high risk, a non-HDL-C less than 100 mg/dL, an apolipoprotein B less than 80 mg/dL, and an LDL particle number less than 1,000 nmol/L.
GROUP 4: AGE 40–75, LDL-C 70–189, NO ASCVD, BUT 10-YEAR RISK ≥ 7.5%
Advantages of the new guidelines
- They may reduce ASCVD events for patients at higher risk.
- The risk calculator is easy to use and focuses on global risk, ie, all forms of ASCVD.
- The guidelines promote discussion of risks and benefits between patients and providers.
Limitations of the new guidelines
- The new risk calculator is controversial (see below).
- There is potential for overtreatment, particularly in older patients.
- There is potential for undertreatment, particularly in patients with an elevated LDL-C but whose 10-year risk is less than 7.5% because they are young.
- The guidelines do not address patients younger than 40 or older than 75.
- They do not take into account some traditional risk factors, such as family history, and nontraditional risk factors such as C-reactive protein as measured by ultrasensitive assays, lipoprotein(a), and apolipoprotein B.
Risk calculator controversy
The new risk calculator has aroused strong opinions on both sides of the aisle.
Shortly after the new guidelines were released, cardiologists Dr. Paul Ridker and Dr. Nancy Cook from Brigham and Women’s Hospital in Boston published analyses30 showing that the new risk calculator, which was based on older data from several large cohorts such as the Atherosclerosis Risk in Communities study,31 the Cardiovascular Health Study,32 the Coronary Artery Risk Development in Young Adults study,33 and the Framingham Heart Study,34,35 was inaccurate in other cohorts. Specifically, in more-recent cohorts (the Women’s Health Study,36 Physicians’ Health Study,37 and Women’s Health Initiative38), the new calculator overestimates the 10-year risk of ASCVD by 75% to 150%.30 Using the new calculator would make approximately 30 million more Americans eligible for statin treatment. The concern is that patients at lower risk would be treated and exposed to potential side effects of statin therapy.
In addition, the risk calculator relies heavily on age and sex and does not include other factors such as triglyceride level, family history, C-reactive protein, or lipoprotein(a). Importantly, and somewhat ironically given the otherwise absolute adherence to randomized controlled trial data for guideline development, the risk calculator has never been verified in prospective studies to adequately show that using it reduces ASCVD events.
Case 3: Overtreating a primary prevention patient
Based on the risk calculator, essentially any African American man in his early 60s with no other risk factors has a 10-year risk of ASCVD of 7.5% or higher and, according to the new guidelines, should receive at least moderate-intensity statin therapy.
For example, consider a 64-year-old African American man whose systolic blood pressure is 129 mm Hg, who does not smoke, does not have diabetes, and does not have hypertension, and whose total cholesterol level is 180 mg/dL, HDL-C 70 mg/dL, triglycerides 130 mg/dL, and calculated LDL-C 84 mg/dL. His calculated 10-year risk is, surprisingly, 7.5%.
Alternatively, his twin brother is a two-pack-per-day smoker with untreated hypertension and systolic blood pressure 150 mm Hg, with fasting total cholesterol 153 mg/dL, HDL-C 70 mg/dL, triglycerides 60 mg/dL, and LDL-C 71 mg/dL. His calculated 10-year risk is 10.5%, so according to the new guidelines, he too should receive high-intensity statin therapy. Yet this patient clearly needs better blood pressure control and smoking cessation as his primary risk-reduction efforts, not a statin. While assessing global risk is important, a shortcoming of the new guidelines is that they can inappropriately lead to treating the risk score, not individualizing the treatment to the patient. Because of the errors inherent in the risk calculator, some experts have called for a temporary halt on implementing the new guidelines until the risk calculator can be further validated. In November 2013, the American Heart Association and the American College of Cardiology reaffirmed their support of the new guidelines and recommended that they be implemented as planned. As of the time this manuscript goes to print, there are no plans to halt implementation of the new guidelines.
Case 4: Undertreating a primary prevention patient
A 25-year-old white man with no medical history has a total cholesterol level of 310 mg/dL, HDL-C 50 mg/dL, triglycerides 400 mg/dL, and calculated LDL-C 180 mg/dL. He does not smoke or have hypertension or diabetes but has a strong family history of premature coronary disease (his father died of myocardial infarction at age 42). His body mass index is 25 kg/m2. Because he is less than 40 years old, the risk calculator does not apply to him.
If we assume he remains untreated and returns at age 40 with the same clinical factors and laboratory values, his calculated 10-year risk of an ASCVD event according to the new risk calculator will still be only 3.1%. Assuming his medical history remains unchanged as he continues to age, his 10-year risk would not reach 7.5% until he is 58. Would you feel comfortable waiting 33 years before starting statin therapy in this patient?
Waiting for dyslipidemic patients to reach middle age before starting LDL-C-lowering therapy is a failure of prevention. For practical reasons, there are no data from randomized controlled trials with hard outcomes in younger people. Nevertheless, a tenet of preventive cardiology is that cumulative exposure accelerates the “vascular age” ahead of the chronological age. This case illustrates why individualized recommendations guided by LDL-C goals as a target for therapy are needed. For this 25-year-old patient, we would recommend starting an intermediate- or high-potency statin.
Case 5: Rheumatoid arthritis
A 60-year-old postmenopausal white woman with severe rheumatoid arthritis presents for cholesterol evaluation. Her total cholesterol level is 235 mg/dL, HDL-C 50 mg/dL, and LDL-C 165 mg/dL. She does not smoke or have hypertension or diabetes. Her systolic blood pressure is 110 mm Hg. She has elevated C-reactive protein on an ultrasensitive assay and elevated lipoprotein(a).
Her calculated 10-year risk of ASCVD is 3.0%. Assuming her medical history remains the same, she would not reach a calculated 10-year risk of at least 7.5% until age 70. We suggest starting moderate- or high-dose statin therapy in this case, based on data (not from randomized controlled trials) showing an increased risk of ASCVD events in patients with rheumatologic disease, increased lipoprotein(a), and inflammatory markers like C-reactive protein. However, the current guidelines do not address this scenario, other than to suggest that clinician consideration can be given to other risk markers such as these, and that these findings should be discussed in detail with the patient. The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin trial16 showed a dramatic ASCVD risk reduction in just such patients (Figure 1).
APPLAUSE—AND RESERVATIONS
The newest guidelines for treating high blood cholesterol represent a monumental shift away from using target levels of LDL-C and non-HDL-C and toward a focus on statin intensity for patients in the four highest-risk groups.
We applaud the expert panel for its idealistic approach of using only data from randomized controlled trials, for placing more emphasis on higher-intensity statin treatment, for including stroke in the new definition of ASCVD, and for focusing more attention on treating diabetic patients more aggressively. Simplifying the guidelines is a noble goal. Emphasizing moderate-to-high-intensity statin therapy in patients at moderate-to-high risk should have substantial long-term public health benefits.
However, as we have shown in the case examples, there are significant limitations, and some patients can end up being overtreated, while others may be undertreated.
Guidelines need to be crafted by looking at all the evidence, including the pathophysiology of the disease process, not just data from randomized controlled trials. It is difficult to implement a guideline that on one hand used randomized controlled trials exclusively for recommendations, but on the other hand used an untested risk calculator to guide therapy. Randomized controlled trials are not available for every scenario.
Further, absence of randomized controlled trial data in a given scenario should not be interpreted as evidence of lack of benefit. An example of this is a primary-prevention patient under age 40 with elevated LDL-C below the 190 mg/dL cutoff who otherwise is healthy and without risk factors (eg, Case 4). By disregarding all evidence that is not from randomized controlled trials, the expert panel fails to account for the extensive pathophysiology of ASCVD, which often begins at a young age and takes decades to develop.5,6,39 An entire generation of patients who have not reached the age of inclusion in most randomized controlled trials with hard outcomes is excluded (unless the LDL-C level is very high), potentially setting back decades of progress in the field of prevention. Prevention only works if started. With childhood and young adult obesity sharply rising, we should not fail to address the under-40-year-old patient population in our guidelines.
Guidelines are designed to be expert opinion, not to dictate practice. Focusing on the individual patient instead of the general population at risk, the expert panel appropriately emphasizes the “importance of clinician judgment, weighing potential benefits, adverse effects, drug-drug interactions and patient preferences.” However, by excluding all data that do not come from randomized controlled trials, the panel neglects a very large base of knowledge and leaves many clinicians without as much expert opinion as we had hoped for.
LDL-C goals are important: they provide a scorecard to help the patient with lifestyle and dietary changes. They provide the health care provider guidance in making treatment decisions and focusing on treatment of a single patient, not a population. Moreover, if a patient has difficulty taking standard doses of statins because of side effects, the absence of LDL-C goals makes decision-making nearly impossible. We hope physicians will rely on LDL-C goals in such situations, falling back on the ATP III recommendations, although many patients may simply go untreated until they present with ASCVD or until they “age in” to a higher risk category.
We suggest caution in strict adherence to the new guidelines and instead urge physicians to consider a hybrid of the old guidelines (using the ATP III LDL-C goals) and the new ones (emphasizing global risk assessment and high-intensity statin treatment).
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Heart Association. 2013 Prevention guidelines tools. CV risk calculator. http://my.americanheart.org/professional/StatementsGuidelines/PreventionGuidelines/Prevention-Guidelines_UCM_457698_SubHomePage.jsp. Accessed December 10, 2013.
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29:431–438.
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50(suppl):S172–S177.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- de Lemos JA, Blazing MA, Wiviott SD, et al; for the A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Downs JR, Clearfield M, Weis S, et al; for the AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/TexCAPS. JAMA 1998; 279:1615–1622.
- Koren MJ, Hunninghake DB, on behalf of the ALLIANCE investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics. J Am Coll Cardiol 2004; 44:1772–1779.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; on behalf of the CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Sacks FM, Pfeffer MA, Moye LA, et al; for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335:1001–1009.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Pedersen TR, Faegeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Ridker PM, Danielson E, Fonseca FAH, et al; for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Nakamura H, Arakawa K, Itakura H, et al; for the MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin Japan (MEGA Study): a prospective rabndomised controlled trial. Lancet 2006; 368:1155–1163.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggreessive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia: report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 1990; 323:946–955.
- Cannon CP, Braunwald E, McCabe CH, et al; for the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Shepherd J, Cobbe SM, Ford I, et al; for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995; 333:1301–1308.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1989; 8:1245–1255.
- AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-Thrive Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract 2013; 19:536–557.
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013doi: 10.1016/S0140-6736(13)62388-0. [Epub ahead of print]
- The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989; 129:687–702.
- Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276.
- Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41:1105–1116.
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham study. Ann N Y Acad Sci 1963; 107:539–556.
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979; 110:281–290.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belancer C, Buring JE, Cook N, et al; The Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Langer R, White E, Lewis C, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003; 13:S107–S121.
- Strong JP, Malcom GT, Oalmann MC, Wissler RW. The PDAY study: natural history, risk factors, and pathobiology. Ann N Y Acad Sci 1997; 811:226–235.
On November 12, 2013, a joint task force for the American College of Cardiology and American Heart Association released new guidelines for treating high blood cholesterol to reduce the risk of atherosclerotic cardiovascular disease (ASCVD) in adults.1
This document arrives after several years of intense deliberation, 12 years after the third Adult Treatment Panel (ATP III) guidelines,2 and 8 years after an ATP III update recommending that low-density lipoprotein cholesterol (LDL-C) levels be lowered aggressively (to less than 70 mg/dL) as an option in patients at high risk.3 It represents a major shift in the approach to and management of high blood cholesterol and has sparked considerable controversy.
In the following commentary, we summarize the new guidelines and the philosophy employed by the task force in generating them. We will also examine some advantages and what we believe to be several shortcomings of the new guidelines. These latter points are illustrated through case examples.
IN RANDOMIZED CONTROLLED TRIALS WE TRUST
In collaboration with the National Heart, Lung, and Blood Institute of the National Institutes of Health, the American College of Cardiology and American Heart Association formed an expert panel task force in 2008.
The task force elected to use only evidence from randomized controlled trials, systematic reviews, and meta-analyses of randomized controlled trials (and only predefined outcomes of the trials, not post hoc analyses) in formulating its recommendations, with the goal of providing the strongest possible evidence.
The authors state that “By using [randomized controlled trial] data to identify those most likely to benefit [emphasis in original] from cholesterol-lowering statin therapy, the recommendations will be of value to primary care clinicians as well as specialists concerned with ASCVD prevention. Importantly, the recommendations were designed to be easy to use in the clinical setting, facilitating the implementation of a strategy of risk assessment and treatment focused on the prevention of ASCVD.”3 They also state the guidelines are meant to “inform clinical judgment, not replace it” and that clinician judgment in addition to discussion with patients remains vital.
During the deliberations, the National Heart, Lung, and Blood Institute removed itself from participating, stating its mission no longer included drafting new guidelines. Additionally, other initial members of the task force removed themselves because of disagreement and concerns about the direction of the new guidelines.
These guidelines, and their accompanying new cardiovascular risk calculator,4 were released without a preliminary period to allow for open discussion, comment, and critique by physicians outside the panel. No attempt was made to harmonize the guidelines with previous versions (eg, ATP III) or with current international guidelines.
WHAT’S NEW IN THE GUIDELINES?
The following are the major changes in the new guidelines for treating high blood cholesterol:
- Treatment goals for LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) are no longer recommended.
- High-intensity and moderate-intensity statin treatment is emphasized, and low-intensity statin therapy is nearly eliminated.
- “ASCVD” now includes stroke in addition to coronary heart disease and peripheral arterial disease.
- Four groups are targeted for treatment (see below).
- Nonstatin therapies have been markedly de-emphasized.
- No guidelines are provided for treating high triglyceride levels.
The new guidelines emphasize lifestyle modification as the foundation for reducing risk, regardless of cholesterol therapy. No recommendations are given for patients with New York Heart Association class II, III, or IV heart failure or for hemodialysis patients, because there were insufficient data from randomized controlled trials to support recommendations. Similarly, the guidelines apply only to people between the ages of 40 and 75 (risk calculator ages 40–79), because the authors believed there was not enough evidence from randomized controlled trials to allow development of guidelines outside of this age range.
FOUR MAJOR STATIN TREATMENT GROUPS
The new guidelines specify four groups that merit intensive or moderately intensive statin therapy (Table 1)1:
- People with clinical ASCVD
- People with LDL-C levels of 190 mg/dL or higher
- People with diabetes, age 40 to 75
- People without diabetes, age 40 to 75, with LDL-C levels 70–189 mg/dL, and a 10-year ASCVD risk of 7.5% or higher as determined by the new risk calculator4 (which also calculates the lifetime risk of ASCVD).
Below, we will address each of these four groups and provide case scenarios to consider. In general, our major disagreements with the new recommendations pertain to the first and fourth categories.
GROUP 1: PEOPLE WITH CLINICAL ASCVD
Advantages of the new guidelines
- They appropriately recommend statins in the highest tolerated doses as first-line treatment for this group at high risk.
- They designate all patients with ASCVD, including those with coronary, peripheral, and cerebrovascular disease, as a high-risk group.
- Without target LDL-C levels, treatment is simpler than before, requiring less monitoring of lipid levels. (This can also be seen as a limitation, as we discuss below.)
Limitations of the new guidelines
- They make follow-up LDL-C levels irrelevant, seeming to assume that there is no gradation in residual risk and, thus, no need to tailor therapy to the individual.
- Patients no longer have a goal to strive for or a way to monitor their progress.
- The guidelines ignore the pathophysiology of coronary artery disease and evidence of residual risk in patients on both moderate-intensity and high-intensity statin therapy.
- They also ignore the potential benefits of treating to lower LDL-C or non-HDL-C goals, thus eliminating consideration of multidrug therapy. They do not address patients with recurrent cardiovascular events already on maximal tolerated statin doses.
- They undermine the potential development and use of new therapies for dysplipidemia in patients with ASCVD.
Case 1: Is LDL-C 110 mg/dL low enough?
A 52-year-old African American man presents with newly discovered moderate coronary artery disease that is not severe enough to warrant stenting. He has no history of hypertension, diabetes mellitus, or smoking. His systolic blood pressure is 130 mm Hg, and his body mass index is 26 kg/m2. He exercises regularly and follows a low-cholesterol diet. He has the following fasting lipid values:
- Total cholesterol 290 mg/dL
- HDL-C 50 mg/dL
- Triglycerides 250 mg/dL
- Calculated LDL-C 190 mg/dL.
Two months later, after beginning atorvastatin 80 mg daily, meeting with a nutritionist, and redoubling his dietary efforts, his fasting lipid concentrations are:
- Total cholesterol 180 mg/dL
- HDL-C 55 mg/dL
- Triglycerides 75 mg/dL
- Calculated LDL-C 110 mg/dL.
Comment: Lack of LDL-C goals is a flaw
The new guidelines call for patients with known ASCVD, such as this patient, to receive intensive statin therapy—which he got.
However, once a patient is on therapy, the new guidelines do not encourage repeating the lipid panel other than to assess compliance. With intensive therapy, we expect a reduction in LDL-C of at least 50% (Table 1), but patient-to-patient differences in response to medications are common, and without repeat testing we would have no way of gauging this patient’s residual risk.
Further, the new guidelines emphasize the lack of hard outcome data supporting the addition of another lipid-lowering drug to a statin, although they do indicate that one can consider it. In a patient at high risk, such as this one, would you be comfortable with an LDL-C value of 110 mg/dL on maximum statin therapy? Would you consider adding a nonstatin drug?
A preponderance of data shows that LDL plays a causal role in ASCVD development and adverse events. Genetic data show that the LDL particle and the LDL receptor pathway are mechanistically linked to ASCVD pathogenesis, with lifetime exposure as a critical determinant of risk.5,6 Moreover, randomized controlled trials of statins and other studies of cholesterol-lowering show a reproducible relationship between the LDL-C level achieved and absolute risk (Figure 1).7–24 We believe the totality of data constitutes a strong rationale for targeting LDL-C and establishing goals for lowering its levels. For these reasons, we believe that removing LDL-C goals is a fundamental flaw of the new guidelines.
The reason for the lack of data from randomized controlled trials demonstrating benefits of adding therapies to statins (when LDL-C is still high) or benefits of treating to specific goals is that no such trials have been performed. Even trials of nonpharmacologic means of lowering LDL-C, such as ileal bypass, which was used in the Program on the Surgical Control of the Hyperlipidemias trial,20 provide independent evidence that lowering LDL-C reduces the risk of ASCVD (Figure 1).
In addition, trials of nonstatin drugs, such as the Coronary Drug Project,25 which tested niacin, also showed outcome benefits. On the other hand, studies such as the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health26 and Treatment of HDL to Reduce the Incidence of Vascular Events27 trials did not show additional risk reduction when niacin was added to statin therapy. However, the study designs arguably had flaws, including requirement of aggressive LDL-lowering with statins, with LDL-C levels below 70 to 80 mg/dL before randomization.
Therefore, these trials do not tell us what to do for a patient on maximal intensive therapy who has recurrent ASCVD events or who, like our patient, has an LDL-C level higher than previous targets.
For this patient, we would recommend adding a second medication to further lower his LDL-C, but discussing with him the absence of proven benefit in clinical trials and the risks of side effects. At present, lacking LDL-C goals in the new guidelines, we are keeping with the ATP III goals to help guide therapeutic choices and individualize patient management.
GROUP 2: PEOPLE WITH LDL-C ≥ 190
Advantages of the new guidelines
- They state that these patients should receive statins in the highest tolerated doses, which is universally accepted.
Limitations of the new guidelines
- The new guidelines mention only that one “may consider” adding a second agent if LDL-C remains above 190 mg/dL after maximum-dose therapy. Patients with familial hypercholesterolemia or other severe forms of hypercholesterolemia typically end up on multidrug therapy to further reduce LDL-C. The absence of randomized controlled trial data in this setting to show an additive value of second and third lipid-lowering agents does not mean these agents do not provide benefit.
GROUP 3: DIABETES, AGE 40–75, LDL-C 70–189, NO CLINICAL ASCVD
Advantages of the new guidelines
- They call for aggressive treatment of people with diabetes, a group at high risk that derives significant benefit from statin therapy, as shown in randomized controlled trials.
Limitations of the new guidelines
- Although high-intensity statin therapy is indicated for this group, we believe that, using the new risk calculator, some patients may receive overly aggressive treatment, thus increasing the possibility of statin side effects.
- The guidelines do not address patients younger than 40 or older than 75.
- Diabetic patients have a high residual risk of ASCVD events, even on statin therapy. Yet the guidelines ignore the potential benefits of more aggressive LDL-lowering or non-LDL secondary targets for therapy.
Case 2: How low is too low?
A 63-year-old white woman, a nonsmoker with recently diagnosed diabetes, is seen by her primary care physician. She has hypertension, for which she takes lisinopril 5 mg daily. Her fasting lipid values are:
- Total cholesterol 160 mg/dL
- HDL-C 64 mg/dL
- Triglycerides 100 mg/dL
- Calculated LDL-C 76 mg/dL.
Her systolic blood pressure is 129 mm Hg, and based on the new risk calculator, her 10-year risk of cardiovascular disease is 10.2%. According to the new guidelines, she should be started on high-intensity statin treatment (Table 1).
Although this is an acceptable initial course of action, it necessitates close vigilance, since it may actually drive her LDL-C level too low. Randomized controlled trials have typically used an LDL-C concentration of less than or equal to 25 mg/dL as the safety cutoff. With a typical LDL-C reduction of at least 50% on high-intensity statins, our patient’s expected LDL-C level will likely be in the low 30s. We believe this would be a good outcome, provided that she tolerates the medication without adverse effects. However, responses to statins vary from patient to patient.
High-intensity statin therapy may not be necessary to reduce risk adequately in all patients who have diabetes without preexisting vascular disease. The Collaborative Atorvastatin Diabetes Study12 compared atorvastatin 10 mg vs placebo in people with type 2 diabetes, age 40 to 75, who had one or more cardiovascular risk factors but no signs or symptoms of preexisting ASCVD and who had only average or below-average cholesterol levels—precisely like this patient. The trial was terminated early because of a clear benefit (a 37% reduction in the composite end point of major adverse cardiovascular events) in the intervention group. For our patient, we believe an alternative and acceptable approach would be to begin moderate-intensity statin therapy (eg, with atorvastatin 10 mg) (Table 1).
Alternatively, in a patient with diabetes and previous atherosclerotic vascular disease or with a high 10-year risk and high LDL-C, limiting treatment to high-intensity statin therapy by itself may deny them the potential benefits of combination therapies and targeting to lower LDL-C levels or non-HDL-C secondary targets. Guidelines from the American Diabetes Association28 and the American Association of Clinical Endocrinologists29 continue to recommend an LDL-C goal of less than 70 mg/dL in patients at high risk, a non-HDL-C less than 100 mg/dL, an apolipoprotein B less than 80 mg/dL, and an LDL particle number less than 1,000 nmol/L.
GROUP 4: AGE 40–75, LDL-C 70–189, NO ASCVD, BUT 10-YEAR RISK ≥ 7.5%
Advantages of the new guidelines
- They may reduce ASCVD events for patients at higher risk.
- The risk calculator is easy to use and focuses on global risk, ie, all forms of ASCVD.
- The guidelines promote discussion of risks and benefits between patients and providers.
Limitations of the new guidelines
- The new risk calculator is controversial (see below).
- There is potential for overtreatment, particularly in older patients.
- There is potential for undertreatment, particularly in patients with an elevated LDL-C but whose 10-year risk is less than 7.5% because they are young.
- The guidelines do not address patients younger than 40 or older than 75.
- They do not take into account some traditional risk factors, such as family history, and nontraditional risk factors such as C-reactive protein as measured by ultrasensitive assays, lipoprotein(a), and apolipoprotein B.
Risk calculator controversy
The new risk calculator has aroused strong opinions on both sides of the aisle.
Shortly after the new guidelines were released, cardiologists Dr. Paul Ridker and Dr. Nancy Cook from Brigham and Women’s Hospital in Boston published analyses30 showing that the new risk calculator, which was based on older data from several large cohorts such as the Atherosclerosis Risk in Communities study,31 the Cardiovascular Health Study,32 the Coronary Artery Risk Development in Young Adults study,33 and the Framingham Heart Study,34,35 was inaccurate in other cohorts. Specifically, in more-recent cohorts (the Women’s Health Study,36 Physicians’ Health Study,37 and Women’s Health Initiative38), the new calculator overestimates the 10-year risk of ASCVD by 75% to 150%.30 Using the new calculator would make approximately 30 million more Americans eligible for statin treatment. The concern is that patients at lower risk would be treated and exposed to potential side effects of statin therapy.
In addition, the risk calculator relies heavily on age and sex and does not include other factors such as triglyceride level, family history, C-reactive protein, or lipoprotein(a). Importantly, and somewhat ironically given the otherwise absolute adherence to randomized controlled trial data for guideline development, the risk calculator has never been verified in prospective studies to adequately show that using it reduces ASCVD events.
Case 3: Overtreating a primary prevention patient
Based on the risk calculator, essentially any African American man in his early 60s with no other risk factors has a 10-year risk of ASCVD of 7.5% or higher and, according to the new guidelines, should receive at least moderate-intensity statin therapy.
For example, consider a 64-year-old African American man whose systolic blood pressure is 129 mm Hg, who does not smoke, does not have diabetes, and does not have hypertension, and whose total cholesterol level is 180 mg/dL, HDL-C 70 mg/dL, triglycerides 130 mg/dL, and calculated LDL-C 84 mg/dL. His calculated 10-year risk is, surprisingly, 7.5%.
Alternatively, his twin brother is a two-pack-per-day smoker with untreated hypertension and systolic blood pressure 150 mm Hg, with fasting total cholesterol 153 mg/dL, HDL-C 70 mg/dL, triglycerides 60 mg/dL, and LDL-C 71 mg/dL. His calculated 10-year risk is 10.5%, so according to the new guidelines, he too should receive high-intensity statin therapy. Yet this patient clearly needs better blood pressure control and smoking cessation as his primary risk-reduction efforts, not a statin. While assessing global risk is important, a shortcoming of the new guidelines is that they can inappropriately lead to treating the risk score, not individualizing the treatment to the patient. Because of the errors inherent in the risk calculator, some experts have called for a temporary halt on implementing the new guidelines until the risk calculator can be further validated. In November 2013, the American Heart Association and the American College of Cardiology reaffirmed their support of the new guidelines and recommended that they be implemented as planned. As of the time this manuscript goes to print, there are no plans to halt implementation of the new guidelines.
Case 4: Undertreating a primary prevention patient
A 25-year-old white man with no medical history has a total cholesterol level of 310 mg/dL, HDL-C 50 mg/dL, triglycerides 400 mg/dL, and calculated LDL-C 180 mg/dL. He does not smoke or have hypertension or diabetes but has a strong family history of premature coronary disease (his father died of myocardial infarction at age 42). His body mass index is 25 kg/m2. Because he is less than 40 years old, the risk calculator does not apply to him.
If we assume he remains untreated and returns at age 40 with the same clinical factors and laboratory values, his calculated 10-year risk of an ASCVD event according to the new risk calculator will still be only 3.1%. Assuming his medical history remains unchanged as he continues to age, his 10-year risk would not reach 7.5% until he is 58. Would you feel comfortable waiting 33 years before starting statin therapy in this patient?
Waiting for dyslipidemic patients to reach middle age before starting LDL-C-lowering therapy is a failure of prevention. For practical reasons, there are no data from randomized controlled trials with hard outcomes in younger people. Nevertheless, a tenet of preventive cardiology is that cumulative exposure accelerates the “vascular age” ahead of the chronological age. This case illustrates why individualized recommendations guided by LDL-C goals as a target for therapy are needed. For this 25-year-old patient, we would recommend starting an intermediate- or high-potency statin.
Case 5: Rheumatoid arthritis
A 60-year-old postmenopausal white woman with severe rheumatoid arthritis presents for cholesterol evaluation. Her total cholesterol level is 235 mg/dL, HDL-C 50 mg/dL, and LDL-C 165 mg/dL. She does not smoke or have hypertension or diabetes. Her systolic blood pressure is 110 mm Hg. She has elevated C-reactive protein on an ultrasensitive assay and elevated lipoprotein(a).
Her calculated 10-year risk of ASCVD is 3.0%. Assuming her medical history remains the same, she would not reach a calculated 10-year risk of at least 7.5% until age 70. We suggest starting moderate- or high-dose statin therapy in this case, based on data (not from randomized controlled trials) showing an increased risk of ASCVD events in patients with rheumatologic disease, increased lipoprotein(a), and inflammatory markers like C-reactive protein. However, the current guidelines do not address this scenario, other than to suggest that clinician consideration can be given to other risk markers such as these, and that these findings should be discussed in detail with the patient. The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin trial16 showed a dramatic ASCVD risk reduction in just such patients (Figure 1).
APPLAUSE—AND RESERVATIONS
The newest guidelines for treating high blood cholesterol represent a monumental shift away from using target levels of LDL-C and non-HDL-C and toward a focus on statin intensity for patients in the four highest-risk groups.
We applaud the expert panel for its idealistic approach of using only data from randomized controlled trials, for placing more emphasis on higher-intensity statin treatment, for including stroke in the new definition of ASCVD, and for focusing more attention on treating diabetic patients more aggressively. Simplifying the guidelines is a noble goal. Emphasizing moderate-to-high-intensity statin therapy in patients at moderate-to-high risk should have substantial long-term public health benefits.
However, as we have shown in the case examples, there are significant limitations, and some patients can end up being overtreated, while others may be undertreated.
Guidelines need to be crafted by looking at all the evidence, including the pathophysiology of the disease process, not just data from randomized controlled trials. It is difficult to implement a guideline that on one hand used randomized controlled trials exclusively for recommendations, but on the other hand used an untested risk calculator to guide therapy. Randomized controlled trials are not available for every scenario.
Further, absence of randomized controlled trial data in a given scenario should not be interpreted as evidence of lack of benefit. An example of this is a primary-prevention patient under age 40 with elevated LDL-C below the 190 mg/dL cutoff who otherwise is healthy and without risk factors (eg, Case 4). By disregarding all evidence that is not from randomized controlled trials, the expert panel fails to account for the extensive pathophysiology of ASCVD, which often begins at a young age and takes decades to develop.5,6,39 An entire generation of patients who have not reached the age of inclusion in most randomized controlled trials with hard outcomes is excluded (unless the LDL-C level is very high), potentially setting back decades of progress in the field of prevention. Prevention only works if started. With childhood and young adult obesity sharply rising, we should not fail to address the under-40-year-old patient population in our guidelines.
Guidelines are designed to be expert opinion, not to dictate practice. Focusing on the individual patient instead of the general population at risk, the expert panel appropriately emphasizes the “importance of clinician judgment, weighing potential benefits, adverse effects, drug-drug interactions and patient preferences.” However, by excluding all data that do not come from randomized controlled trials, the panel neglects a very large base of knowledge and leaves many clinicians without as much expert opinion as we had hoped for.
LDL-C goals are important: they provide a scorecard to help the patient with lifestyle and dietary changes. They provide the health care provider guidance in making treatment decisions and focusing on treatment of a single patient, not a population. Moreover, if a patient has difficulty taking standard doses of statins because of side effects, the absence of LDL-C goals makes decision-making nearly impossible. We hope physicians will rely on LDL-C goals in such situations, falling back on the ATP III recommendations, although many patients may simply go untreated until they present with ASCVD or until they “age in” to a higher risk category.
We suggest caution in strict adherence to the new guidelines and instead urge physicians to consider a hybrid of the old guidelines (using the ATP III LDL-C goals) and the new ones (emphasizing global risk assessment and high-intensity statin treatment).
On November 12, 2013, a joint task force for the American College of Cardiology and American Heart Association released new guidelines for treating high blood cholesterol to reduce the risk of atherosclerotic cardiovascular disease (ASCVD) in adults.1
This document arrives after several years of intense deliberation, 12 years after the third Adult Treatment Panel (ATP III) guidelines,2 and 8 years after an ATP III update recommending that low-density lipoprotein cholesterol (LDL-C) levels be lowered aggressively (to less than 70 mg/dL) as an option in patients at high risk.3 It represents a major shift in the approach to and management of high blood cholesterol and has sparked considerable controversy.
In the following commentary, we summarize the new guidelines and the philosophy employed by the task force in generating them. We will also examine some advantages and what we believe to be several shortcomings of the new guidelines. These latter points are illustrated through case examples.
IN RANDOMIZED CONTROLLED TRIALS WE TRUST
In collaboration with the National Heart, Lung, and Blood Institute of the National Institutes of Health, the American College of Cardiology and American Heart Association formed an expert panel task force in 2008.
The task force elected to use only evidence from randomized controlled trials, systematic reviews, and meta-analyses of randomized controlled trials (and only predefined outcomes of the trials, not post hoc analyses) in formulating its recommendations, with the goal of providing the strongest possible evidence.
The authors state that “By using [randomized controlled trial] data to identify those most likely to benefit [emphasis in original] from cholesterol-lowering statin therapy, the recommendations will be of value to primary care clinicians as well as specialists concerned with ASCVD prevention. Importantly, the recommendations were designed to be easy to use in the clinical setting, facilitating the implementation of a strategy of risk assessment and treatment focused on the prevention of ASCVD.”3 They also state the guidelines are meant to “inform clinical judgment, not replace it” and that clinician judgment in addition to discussion with patients remains vital.
During the deliberations, the National Heart, Lung, and Blood Institute removed itself from participating, stating its mission no longer included drafting new guidelines. Additionally, other initial members of the task force removed themselves because of disagreement and concerns about the direction of the new guidelines.
These guidelines, and their accompanying new cardiovascular risk calculator,4 were released without a preliminary period to allow for open discussion, comment, and critique by physicians outside the panel. No attempt was made to harmonize the guidelines with previous versions (eg, ATP III) or with current international guidelines.
WHAT’S NEW IN THE GUIDELINES?
The following are the major changes in the new guidelines for treating high blood cholesterol:
- Treatment goals for LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) are no longer recommended.
- High-intensity and moderate-intensity statin treatment is emphasized, and low-intensity statin therapy is nearly eliminated.
- “ASCVD” now includes stroke in addition to coronary heart disease and peripheral arterial disease.
- Four groups are targeted for treatment (see below).
- Nonstatin therapies have been markedly de-emphasized.
- No guidelines are provided for treating high triglyceride levels.
The new guidelines emphasize lifestyle modification as the foundation for reducing risk, regardless of cholesterol therapy. No recommendations are given for patients with New York Heart Association class II, III, or IV heart failure or for hemodialysis patients, because there were insufficient data from randomized controlled trials to support recommendations. Similarly, the guidelines apply only to people between the ages of 40 and 75 (risk calculator ages 40–79), because the authors believed there was not enough evidence from randomized controlled trials to allow development of guidelines outside of this age range.
FOUR MAJOR STATIN TREATMENT GROUPS
The new guidelines specify four groups that merit intensive or moderately intensive statin therapy (Table 1)1:
- People with clinical ASCVD
- People with LDL-C levels of 190 mg/dL or higher
- People with diabetes, age 40 to 75
- People without diabetes, age 40 to 75, with LDL-C levels 70–189 mg/dL, and a 10-year ASCVD risk of 7.5% or higher as determined by the new risk calculator4 (which also calculates the lifetime risk of ASCVD).
Below, we will address each of these four groups and provide case scenarios to consider. In general, our major disagreements with the new recommendations pertain to the first and fourth categories.
GROUP 1: PEOPLE WITH CLINICAL ASCVD
Advantages of the new guidelines
- They appropriately recommend statins in the highest tolerated doses as first-line treatment for this group at high risk.
- They designate all patients with ASCVD, including those with coronary, peripheral, and cerebrovascular disease, as a high-risk group.
- Without target LDL-C levels, treatment is simpler than before, requiring less monitoring of lipid levels. (This can also be seen as a limitation, as we discuss below.)
Limitations of the new guidelines
- They make follow-up LDL-C levels irrelevant, seeming to assume that there is no gradation in residual risk and, thus, no need to tailor therapy to the individual.
- Patients no longer have a goal to strive for or a way to monitor their progress.
- The guidelines ignore the pathophysiology of coronary artery disease and evidence of residual risk in patients on both moderate-intensity and high-intensity statin therapy.
- They also ignore the potential benefits of treating to lower LDL-C or non-HDL-C goals, thus eliminating consideration of multidrug therapy. They do not address patients with recurrent cardiovascular events already on maximal tolerated statin doses.
- They undermine the potential development and use of new therapies for dysplipidemia in patients with ASCVD.
Case 1: Is LDL-C 110 mg/dL low enough?
A 52-year-old African American man presents with newly discovered moderate coronary artery disease that is not severe enough to warrant stenting. He has no history of hypertension, diabetes mellitus, or smoking. His systolic blood pressure is 130 mm Hg, and his body mass index is 26 kg/m2. He exercises regularly and follows a low-cholesterol diet. He has the following fasting lipid values:
- Total cholesterol 290 mg/dL
- HDL-C 50 mg/dL
- Triglycerides 250 mg/dL
- Calculated LDL-C 190 mg/dL.
Two months later, after beginning atorvastatin 80 mg daily, meeting with a nutritionist, and redoubling his dietary efforts, his fasting lipid concentrations are:
- Total cholesterol 180 mg/dL
- HDL-C 55 mg/dL
- Triglycerides 75 mg/dL
- Calculated LDL-C 110 mg/dL.
Comment: Lack of LDL-C goals is a flaw
The new guidelines call for patients with known ASCVD, such as this patient, to receive intensive statin therapy—which he got.
However, once a patient is on therapy, the new guidelines do not encourage repeating the lipid panel other than to assess compliance. With intensive therapy, we expect a reduction in LDL-C of at least 50% (Table 1), but patient-to-patient differences in response to medications are common, and without repeat testing we would have no way of gauging this patient’s residual risk.
Further, the new guidelines emphasize the lack of hard outcome data supporting the addition of another lipid-lowering drug to a statin, although they do indicate that one can consider it. In a patient at high risk, such as this one, would you be comfortable with an LDL-C value of 110 mg/dL on maximum statin therapy? Would you consider adding a nonstatin drug?
A preponderance of data shows that LDL plays a causal role in ASCVD development and adverse events. Genetic data show that the LDL particle and the LDL receptor pathway are mechanistically linked to ASCVD pathogenesis, with lifetime exposure as a critical determinant of risk.5,6 Moreover, randomized controlled trials of statins and other studies of cholesterol-lowering show a reproducible relationship between the LDL-C level achieved and absolute risk (Figure 1).7–24 We believe the totality of data constitutes a strong rationale for targeting LDL-C and establishing goals for lowering its levels. For these reasons, we believe that removing LDL-C goals is a fundamental flaw of the new guidelines.
The reason for the lack of data from randomized controlled trials demonstrating benefits of adding therapies to statins (when LDL-C is still high) or benefits of treating to specific goals is that no such trials have been performed. Even trials of nonpharmacologic means of lowering LDL-C, such as ileal bypass, which was used in the Program on the Surgical Control of the Hyperlipidemias trial,20 provide independent evidence that lowering LDL-C reduces the risk of ASCVD (Figure 1).
In addition, trials of nonstatin drugs, such as the Coronary Drug Project,25 which tested niacin, also showed outcome benefits. On the other hand, studies such as the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health26 and Treatment of HDL to Reduce the Incidence of Vascular Events27 trials did not show additional risk reduction when niacin was added to statin therapy. However, the study designs arguably had flaws, including requirement of aggressive LDL-lowering with statins, with LDL-C levels below 70 to 80 mg/dL before randomization.
Therefore, these trials do not tell us what to do for a patient on maximal intensive therapy who has recurrent ASCVD events or who, like our patient, has an LDL-C level higher than previous targets.
For this patient, we would recommend adding a second medication to further lower his LDL-C, but discussing with him the absence of proven benefit in clinical trials and the risks of side effects. At present, lacking LDL-C goals in the new guidelines, we are keeping with the ATP III goals to help guide therapeutic choices and individualize patient management.
GROUP 2: PEOPLE WITH LDL-C ≥ 190
Advantages of the new guidelines
- They state that these patients should receive statins in the highest tolerated doses, which is universally accepted.
Limitations of the new guidelines
- The new guidelines mention only that one “may consider” adding a second agent if LDL-C remains above 190 mg/dL after maximum-dose therapy. Patients with familial hypercholesterolemia or other severe forms of hypercholesterolemia typically end up on multidrug therapy to further reduce LDL-C. The absence of randomized controlled trial data in this setting to show an additive value of second and third lipid-lowering agents does not mean these agents do not provide benefit.
GROUP 3: DIABETES, AGE 40–75, LDL-C 70–189, NO CLINICAL ASCVD
Advantages of the new guidelines
- They call for aggressive treatment of people with diabetes, a group at high risk that derives significant benefit from statin therapy, as shown in randomized controlled trials.
Limitations of the new guidelines
- Although high-intensity statin therapy is indicated for this group, we believe that, using the new risk calculator, some patients may receive overly aggressive treatment, thus increasing the possibility of statin side effects.
- The guidelines do not address patients younger than 40 or older than 75.
- Diabetic patients have a high residual risk of ASCVD events, even on statin therapy. Yet the guidelines ignore the potential benefits of more aggressive LDL-lowering or non-LDL secondary targets for therapy.
Case 2: How low is too low?
A 63-year-old white woman, a nonsmoker with recently diagnosed diabetes, is seen by her primary care physician. She has hypertension, for which she takes lisinopril 5 mg daily. Her fasting lipid values are:
- Total cholesterol 160 mg/dL
- HDL-C 64 mg/dL
- Triglycerides 100 mg/dL
- Calculated LDL-C 76 mg/dL.
Her systolic blood pressure is 129 mm Hg, and based on the new risk calculator, her 10-year risk of cardiovascular disease is 10.2%. According to the new guidelines, she should be started on high-intensity statin treatment (Table 1).
Although this is an acceptable initial course of action, it necessitates close vigilance, since it may actually drive her LDL-C level too low. Randomized controlled trials have typically used an LDL-C concentration of less than or equal to 25 mg/dL as the safety cutoff. With a typical LDL-C reduction of at least 50% on high-intensity statins, our patient’s expected LDL-C level will likely be in the low 30s. We believe this would be a good outcome, provided that she tolerates the medication without adverse effects. However, responses to statins vary from patient to patient.
High-intensity statin therapy may not be necessary to reduce risk adequately in all patients who have diabetes without preexisting vascular disease. The Collaborative Atorvastatin Diabetes Study12 compared atorvastatin 10 mg vs placebo in people with type 2 diabetes, age 40 to 75, who had one or more cardiovascular risk factors but no signs or symptoms of preexisting ASCVD and who had only average or below-average cholesterol levels—precisely like this patient. The trial was terminated early because of a clear benefit (a 37% reduction in the composite end point of major adverse cardiovascular events) in the intervention group. For our patient, we believe an alternative and acceptable approach would be to begin moderate-intensity statin therapy (eg, with atorvastatin 10 mg) (Table 1).
Alternatively, in a patient with diabetes and previous atherosclerotic vascular disease or with a high 10-year risk and high LDL-C, limiting treatment to high-intensity statin therapy by itself may deny them the potential benefits of combination therapies and targeting to lower LDL-C levels or non-HDL-C secondary targets. Guidelines from the American Diabetes Association28 and the American Association of Clinical Endocrinologists29 continue to recommend an LDL-C goal of less than 70 mg/dL in patients at high risk, a non-HDL-C less than 100 mg/dL, an apolipoprotein B less than 80 mg/dL, and an LDL particle number less than 1,000 nmol/L.
GROUP 4: AGE 40–75, LDL-C 70–189, NO ASCVD, BUT 10-YEAR RISK ≥ 7.5%
Advantages of the new guidelines
- They may reduce ASCVD events for patients at higher risk.
- The risk calculator is easy to use and focuses on global risk, ie, all forms of ASCVD.
- The guidelines promote discussion of risks and benefits between patients and providers.
Limitations of the new guidelines
- The new risk calculator is controversial (see below).
- There is potential for overtreatment, particularly in older patients.
- There is potential for undertreatment, particularly in patients with an elevated LDL-C but whose 10-year risk is less than 7.5% because they are young.
- The guidelines do not address patients younger than 40 or older than 75.
- They do not take into account some traditional risk factors, such as family history, and nontraditional risk factors such as C-reactive protein as measured by ultrasensitive assays, lipoprotein(a), and apolipoprotein B.
Risk calculator controversy
The new risk calculator has aroused strong opinions on both sides of the aisle.
Shortly after the new guidelines were released, cardiologists Dr. Paul Ridker and Dr. Nancy Cook from Brigham and Women’s Hospital in Boston published analyses30 showing that the new risk calculator, which was based on older data from several large cohorts such as the Atherosclerosis Risk in Communities study,31 the Cardiovascular Health Study,32 the Coronary Artery Risk Development in Young Adults study,33 and the Framingham Heart Study,34,35 was inaccurate in other cohorts. Specifically, in more-recent cohorts (the Women’s Health Study,36 Physicians’ Health Study,37 and Women’s Health Initiative38), the new calculator overestimates the 10-year risk of ASCVD by 75% to 150%.30 Using the new calculator would make approximately 30 million more Americans eligible for statin treatment. The concern is that patients at lower risk would be treated and exposed to potential side effects of statin therapy.
In addition, the risk calculator relies heavily on age and sex and does not include other factors such as triglyceride level, family history, C-reactive protein, or lipoprotein(a). Importantly, and somewhat ironically given the otherwise absolute adherence to randomized controlled trial data for guideline development, the risk calculator has never been verified in prospective studies to adequately show that using it reduces ASCVD events.
Case 3: Overtreating a primary prevention patient
Based on the risk calculator, essentially any African American man in his early 60s with no other risk factors has a 10-year risk of ASCVD of 7.5% or higher and, according to the new guidelines, should receive at least moderate-intensity statin therapy.
For example, consider a 64-year-old African American man whose systolic blood pressure is 129 mm Hg, who does not smoke, does not have diabetes, and does not have hypertension, and whose total cholesterol level is 180 mg/dL, HDL-C 70 mg/dL, triglycerides 130 mg/dL, and calculated LDL-C 84 mg/dL. His calculated 10-year risk is, surprisingly, 7.5%.
Alternatively, his twin brother is a two-pack-per-day smoker with untreated hypertension and systolic blood pressure 150 mm Hg, with fasting total cholesterol 153 mg/dL, HDL-C 70 mg/dL, triglycerides 60 mg/dL, and LDL-C 71 mg/dL. His calculated 10-year risk is 10.5%, so according to the new guidelines, he too should receive high-intensity statin therapy. Yet this patient clearly needs better blood pressure control and smoking cessation as his primary risk-reduction efforts, not a statin. While assessing global risk is important, a shortcoming of the new guidelines is that they can inappropriately lead to treating the risk score, not individualizing the treatment to the patient. Because of the errors inherent in the risk calculator, some experts have called for a temporary halt on implementing the new guidelines until the risk calculator can be further validated. In November 2013, the American Heart Association and the American College of Cardiology reaffirmed their support of the new guidelines and recommended that they be implemented as planned. As of the time this manuscript goes to print, there are no plans to halt implementation of the new guidelines.
Case 4: Undertreating a primary prevention patient
A 25-year-old white man with no medical history has a total cholesterol level of 310 mg/dL, HDL-C 50 mg/dL, triglycerides 400 mg/dL, and calculated LDL-C 180 mg/dL. He does not smoke or have hypertension or diabetes but has a strong family history of premature coronary disease (his father died of myocardial infarction at age 42). His body mass index is 25 kg/m2. Because he is less than 40 years old, the risk calculator does not apply to him.
If we assume he remains untreated and returns at age 40 with the same clinical factors and laboratory values, his calculated 10-year risk of an ASCVD event according to the new risk calculator will still be only 3.1%. Assuming his medical history remains unchanged as he continues to age, his 10-year risk would not reach 7.5% until he is 58. Would you feel comfortable waiting 33 years before starting statin therapy in this patient?
Waiting for dyslipidemic patients to reach middle age before starting LDL-C-lowering therapy is a failure of prevention. For practical reasons, there are no data from randomized controlled trials with hard outcomes in younger people. Nevertheless, a tenet of preventive cardiology is that cumulative exposure accelerates the “vascular age” ahead of the chronological age. This case illustrates why individualized recommendations guided by LDL-C goals as a target for therapy are needed. For this 25-year-old patient, we would recommend starting an intermediate- or high-potency statin.
Case 5: Rheumatoid arthritis
A 60-year-old postmenopausal white woman with severe rheumatoid arthritis presents for cholesterol evaluation. Her total cholesterol level is 235 mg/dL, HDL-C 50 mg/dL, and LDL-C 165 mg/dL. She does not smoke or have hypertension or diabetes. Her systolic blood pressure is 110 mm Hg. She has elevated C-reactive protein on an ultrasensitive assay and elevated lipoprotein(a).
Her calculated 10-year risk of ASCVD is 3.0%. Assuming her medical history remains the same, she would not reach a calculated 10-year risk of at least 7.5% until age 70. We suggest starting moderate- or high-dose statin therapy in this case, based on data (not from randomized controlled trials) showing an increased risk of ASCVD events in patients with rheumatologic disease, increased lipoprotein(a), and inflammatory markers like C-reactive protein. However, the current guidelines do not address this scenario, other than to suggest that clinician consideration can be given to other risk markers such as these, and that these findings should be discussed in detail with the patient. The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin trial16 showed a dramatic ASCVD risk reduction in just such patients (Figure 1).
APPLAUSE—AND RESERVATIONS
The newest guidelines for treating high blood cholesterol represent a monumental shift away from using target levels of LDL-C and non-HDL-C and toward a focus on statin intensity for patients in the four highest-risk groups.
We applaud the expert panel for its idealistic approach of using only data from randomized controlled trials, for placing more emphasis on higher-intensity statin treatment, for including stroke in the new definition of ASCVD, and for focusing more attention on treating diabetic patients more aggressively. Simplifying the guidelines is a noble goal. Emphasizing moderate-to-high-intensity statin therapy in patients at moderate-to-high risk should have substantial long-term public health benefits.
However, as we have shown in the case examples, there are significant limitations, and some patients can end up being overtreated, while others may be undertreated.
Guidelines need to be crafted by looking at all the evidence, including the pathophysiology of the disease process, not just data from randomized controlled trials. It is difficult to implement a guideline that on one hand used randomized controlled trials exclusively for recommendations, but on the other hand used an untested risk calculator to guide therapy. Randomized controlled trials are not available for every scenario.
Further, absence of randomized controlled trial data in a given scenario should not be interpreted as evidence of lack of benefit. An example of this is a primary-prevention patient under age 40 with elevated LDL-C below the 190 mg/dL cutoff who otherwise is healthy and without risk factors (eg, Case 4). By disregarding all evidence that is not from randomized controlled trials, the expert panel fails to account for the extensive pathophysiology of ASCVD, which often begins at a young age and takes decades to develop.5,6,39 An entire generation of patients who have not reached the age of inclusion in most randomized controlled trials with hard outcomes is excluded (unless the LDL-C level is very high), potentially setting back decades of progress in the field of prevention. Prevention only works if started. With childhood and young adult obesity sharply rising, we should not fail to address the under-40-year-old patient population in our guidelines.
Guidelines are designed to be expert opinion, not to dictate practice. Focusing on the individual patient instead of the general population at risk, the expert panel appropriately emphasizes the “importance of clinician judgment, weighing potential benefits, adverse effects, drug-drug interactions and patient preferences.” However, by excluding all data that do not come from randomized controlled trials, the panel neglects a very large base of knowledge and leaves many clinicians without as much expert opinion as we had hoped for.
LDL-C goals are important: they provide a scorecard to help the patient with lifestyle and dietary changes. They provide the health care provider guidance in making treatment decisions and focusing on treatment of a single patient, not a population. Moreover, if a patient has difficulty taking standard doses of statins because of side effects, the absence of LDL-C goals makes decision-making nearly impossible. We hope physicians will rely on LDL-C goals in such situations, falling back on the ATP III recommendations, although many patients may simply go untreated until they present with ASCVD or until they “age in” to a higher risk category.
We suggest caution in strict adherence to the new guidelines and instead urge physicians to consider a hybrid of the old guidelines (using the ATP III LDL-C goals) and the new ones (emphasizing global risk assessment and high-intensity statin treatment).
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Heart Association. 2013 Prevention guidelines tools. CV risk calculator. http://my.americanheart.org/professional/StatementsGuidelines/PreventionGuidelines/Prevention-Guidelines_UCM_457698_SubHomePage.jsp. Accessed December 10, 2013.
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29:431–438.
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50(suppl):S172–S177.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- de Lemos JA, Blazing MA, Wiviott SD, et al; for the A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Downs JR, Clearfield M, Weis S, et al; for the AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/TexCAPS. JAMA 1998; 279:1615–1622.
- Koren MJ, Hunninghake DB, on behalf of the ALLIANCE investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics. J Am Coll Cardiol 2004; 44:1772–1779.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; on behalf of the CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Sacks FM, Pfeffer MA, Moye LA, et al; for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335:1001–1009.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Pedersen TR, Faegeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Ridker PM, Danielson E, Fonseca FAH, et al; for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Nakamura H, Arakawa K, Itakura H, et al; for the MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin Japan (MEGA Study): a prospective rabndomised controlled trial. Lancet 2006; 368:1155–1163.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggreessive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia: report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 1990; 323:946–955.
- Cannon CP, Braunwald E, McCabe CH, et al; for the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Shepherd J, Cobbe SM, Ford I, et al; for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995; 333:1301–1308.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1989; 8:1245–1255.
- AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-Thrive Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract 2013; 19:536–557.
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013doi: 10.1016/S0140-6736(13)62388-0. [Epub ahead of print]
- The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989; 129:687–702.
- Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276.
- Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41:1105–1116.
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham study. Ann N Y Acad Sci 1963; 107:539–556.
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979; 110:281–290.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belancer C, Buring JE, Cook N, et al; The Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Langer R, White E, Lewis C, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003; 13:S107–S121.
- Strong JP, Malcom GT, Oalmann MC, Wissler RW. The PDAY study: natural history, risk factors, and pathobiology. Ann N Y Acad Sci 1997; 811:226–235.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Heart Association. 2013 Prevention guidelines tools. CV risk calculator. http://my.americanheart.org/professional/StatementsGuidelines/PreventionGuidelines/Prevention-Guidelines_UCM_457698_SubHomePage.jsp. Accessed December 10, 2013.
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29:431–438.
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50(suppl):S172–S177.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- de Lemos JA, Blazing MA, Wiviott SD, et al; for the A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Downs JR, Clearfield M, Weis S, et al; for the AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/TexCAPS. JAMA 1998; 279:1615–1622.
- Koren MJ, Hunninghake DB, on behalf of the ALLIANCE investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics. J Am Coll Cardiol 2004; 44:1772–1779.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; on behalf of the CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Sacks FM, Pfeffer MA, Moye LA, et al; for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335:1001–1009.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Pedersen TR, Faegeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Ridker PM, Danielson E, Fonseca FAH, et al; for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Nakamura H, Arakawa K, Itakura H, et al; for the MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin Japan (MEGA Study): a prospective rabndomised controlled trial. Lancet 2006; 368:1155–1163.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggreessive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia: report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 1990; 323:946–955.
- Cannon CP, Braunwald E, McCabe CH, et al; for the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Shepherd J, Cobbe SM, Ford I, et al; for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995; 333:1301–1308.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1989; 8:1245–1255.
- AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-Thrive Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract 2013; 19:536–557.
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013doi: 10.1016/S0140-6736(13)62388-0. [Epub ahead of print]
- The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989; 129:687–702.
- Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276.
- Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41:1105–1116.
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham study. Ann N Y Acad Sci 1963; 107:539–556.
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979; 110:281–290.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belancer C, Buring JE, Cook N, et al; The Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Langer R, White E, Lewis C, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003; 13:S107–S121.
- Strong JP, Malcom GT, Oalmann MC, Wissler RW. The PDAY study: natural history, risk factors, and pathobiology. Ann N Y Acad Sci 1997; 811:226–235.
JUPITER to Earth: A statin helps people with normal LDL-C and high hs-CRP, but what does it mean?
The medical community has struggled with two important questions for the past 10 years: When it comes to the low-density lipoprotein cholesterol (LDL-C) level, how low should one go and at what cost? And are there other markers of risk that can identify a higher-risk subpopulation in relatively healthy people? The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) provided partial answers for these questions by finding that a highly potent statin lowered the risk of cardiovascular events in patients with “normal” LDL-C but elevated levels of high-sensitivity C-reactive protein (hs-CRP).1
In this article, we will critically evaluate the methods, results, and conclusions of the JUPITER trial. Additionally, we will discuss its limitations and areas of uncertainty.
BEFORE JUPITER
The LDL-C-lowering drugs called statins have revolutionized cardiovascular medicine.2 They are beneficial in both the primary prevention setting and in acute coronary syndromes, stable angina, and unstable angina and can halt the progression of coronary artery disease—in some cases even resulting in modest regression of plaque.3–6
Many experts have credited the reduction in LDL-C as being the sole factor responsible for the decrease in major adverse events seen with statin therapy.7 However, statins have other, non-lipid-lowering properties, including anti-inflammatory and antioxidant effects, that may also contribute to their benefits.8–15
One of the anti-inflammatory actions of statins is evidenced by lower levels of the acute-phase reactant CRP.10,11,15,16 Measuring systemic CRP levels with a highly sensitive assay (yielding the so-called high-sensitivity or hs-CRP level) provides significant clinical prognostic value across a spectrum of clinical situations, ranging from risk screening in apparently healthy people to stable and unstable angina.17–22 People with higher hs-CRP levels are, on average, at higher risk of adverse cardiovascular events. However, controversy remains as to whether hs-CRP plays a mechanistic role in plaque formation and acute complications. Indeed, recent genetic studies argue strongly that hs-CRP lies outside the mechanistic path of atherosclerosis.23 Nonetheless, an overwhelming amount of data indicates that hs-CRP serves as a marker of disease.17–21
Nissen et al10 showed that the rate of progression of atherosclerosis is lower when the levels of atherogenic lipoproteins and hs-CRP are both lowered with statin therapy. Simultaneously, Ridker et al11 showed that patients who have lower hs-CRP levels after statin therapy have better clinical outcomes than those with higher hs-CRP levels, regardless of their achieved level of LDL-C.
Collectively, these studies and others have led some to believe that, in people with relatively low LDL-C but persistently elevated hs-CRP, statin therapy may reduce the rate of events.15,24 The JUPITER trial was undertaken to test this hypothesis.
JUPITER STUDY DESIGN
JUPITER was designed to see whether highly potent statin therapy is beneficial in people with elevated hs-CRP who otherwise do not meet the criteria for lipid-lowering therapy. The study was conducted at 1,315 sites in 26 countries. It was sponsored by AstraZeneca, the maker of rosuvastatin (Crestor).
Inclusion and exclusion criteria
All participants had to be free of known cardiovascular disease, have an LDL-C level lower than 130 mg/dL, and have an hs-CRP level of 2.0 mg/L or greater. Patients were excluded if they were previous or current users of lipid-lowering drugs; had severe arthritis, lupus, or inflammatory bowel disease; or were taking immune-modulating drugs such as cyclosporine (Sandimmune, others), tacrolimus (Prograf), azathioprine (Azasan, Imuran), or long-term oral corticosteroids.
Rosuvastatin therapy
Participants were randomly assigned in a 1:1 ratio to receive rosuvastatin 20 mg daily or a matching placebo in a double-blind fashion.
End points
The primary end point was the composite of nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from cardiovascular causes. Secondary end points were the individual components of the primary end point.
Statistical analysis
The study was powered to detect a 25% reduction in the primary end point among those treated with rosuvastatin. The trial was designed to run until 520 end point events had occurred. However, on March 29, 2008, after the first prespecified interim analysis, the Data and Safety Monitoring Board stopped the trial due to a significant reduction in the primary end point in the rosuvastatin group. As in most randomized clinical trials, all analyses were done on an intention-to-treat basis. Prespecified subgroup analyses were also performed.
STUDY RESULTS
Patient recruitment and eligibility
Between February 4, 2003, and December 15, 2006, a total of 89,890 people were screened. Of these, 17,802 met the inclusion and exclusion criteria and were included in the study. Of the 72,088 people who were excluded, 25,993 (36.1%) had an hs-CRP level below 2 mg/L and 37,611 (52.2%) had an LDL-C level of 130 mg/dL or higher.
A not-so-healthy population
The aim of the investigators was to include relatively healthy people. The median age was 66 years, about 16% of participants were current smokers, about 11% had a family history of heart disease, and about 41% met the criteria for metabolic syndrome, all conditions that are associated with elevated hs-CRP.25 Of note, the median hs-CRP level was 4.2 mg/L, a level indicating higher global risk according to the American College of Cardiology/American Heart Association consensus statement.26
Reduction in lipid levels and hs-CRP
By 12 months, in the rosuvastatin group, the median LDL-C level had fallen by 50% (from 108 to 55 mg/dL), and the median hs-CRP level had fallen by 37% (from 4.2 to 2.2 mg/L). Additionally, the triglyceride level had fallen by 17%. The high-density lipoprotein cholesterol levels did not change significantly.
Impact on end points
Adverse events
WHAT DOES THIS MEAN?
Is lower LDL-C better?
The JUPITER trial is the latest of several statin trials that have shown significant reductions in major adverse cardiovascular events when LDL-C was lowered below what has been recommended by the current guidelines.27,28
In 2002, the Heart Protection Study29 showed a significant reduction in major adverse cardiovascular events in patients at high risk of coronary artery disease if they received simvastatin (Zocor), even if they had LDL-C levels lower than 100 mg/dL at baseline. Similarly, the Pravastatin or Atorvastatin Evaluation and Infection-Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) trial30 showed a 16% relative risk reduction in a composite end point in patients presenting with acute coronary syndrome if they received intensive statin therapy.
These two studies led to an update by the National Cholesterol Education Program (Adult Treatment Panel III), suggesting an optimal LDL-C goal of less than 70 mg/dL in those with coronary artery disease or its risk equivalent (ie, diabetes mellitus, peripheral vascular disease). Furthermore, in support of the “lower is better” theory, a number of studies that used intravascular ultrasonography have shown regression of coronary plaque with aggressive LDL-C lowering. Notably, in a Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (the ASTEROID trial),5 rosuvastatin 40 mg daily caused significant plaque regression while lowering LDL-C to 61 mg/dL over a 24-month period.
A number of high-dose statin trials have shown that lowering LDL-C to less than 70 mg/dL significantly reduces major adverse cardiovascular events.31–39 The JUPITER trial was unique in that it extended these findings to people without known coronary disease (ie, primary prevention) or elevated cholesterol but with elevated levels of a marker of inflammation—hs-CRP. In view of the JUPITER results and of studies using intravascular ultrasonography in the primary prevention setting, it seems clear that lowering LDL-C to levels less than 70 mg/dL also reduces both atherosclerotic plaque progression and the rate of first major adverse cardiovascular events in primary prevention in patients at higher global risk.
Did the study prove that reducing hs-CRP lowers risk?
Measuring hs-CRP levels has been extensively studied in apparently healthy populations, stable angina, unstable angina, and other cardiovascular settings.18,21,40–43 It has been shown to have significant prognostic implications in a number of primary and secondary trials.44 Additionally, those with elevated LDL-C and hs-CRP levels benefit the most from statin therapy.16,45,46 Animal studies have also provided some evidence that CRP may play a role in atherogenesis.47,48 However, recent clinical and genetic studies have raised doubt about the direct causal relationship between CRP and coronary artery disease,23,49,50 and epidemiologic studies have questioned its usefulness as a marker of risk.51,52
The JUPITER study adds little to clear up the controversy about whether hs-CRP is a mechanistic participant in atherosclerotic disease. However, it also shows that this issue is somewhat irrelevant, in that selection of patients for high-potency statin therapy solely on the basis of high hs-CRP without other indications for lipid-lowering therapy clearly reduces risk and improves survival.
JUPITER did not examine whether people with higher hs-CRP levels benefited more from statin therapy than those with lower levels. The hypothesis-generating data for JUPITER came from an analysis of changes in hs-CRP and LDL-C in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS).16 Thus, JUPITER did not include people with both low LDL-C and low hs-CRP because, in the AFCAPS/TexCAPS analysis, those with low LDL-C and low hs-CRP had extremely low event rates and no clinical efficacy of statin therapy, despite good LDL-C reduction. In marked contrast, those with low LDL-C but elevated hs-CRP had high event rates and large relative risk reductions— hence the need for JUPITER to prospectively test this hypothesis. Nevertheless, the initial results of JUPITER as presented do not yet make it clear that there is a dose-response relationship between higher levels of hs-CRP and a greater reduction in events, even in a cohort with elevated hs-CRP at baseline. This analysis will no doubt be forthcoming in another manuscript from Ridker and colleagues. Specifically, it will be of interest to examine whether those with the highest hs-CRP levels benefited the most from rosuvastatin on both an absolute and relative scale, and whether those with the greatest hs-CRP reduction also benefited more. With the present data available from JUPITER, a reasonable interpretation is that an elevated hs-CRP simply widens the inclusion criterion for those for whom high-potency statin therapy improves clinical outcomes.53
Better markers are needed
Even with a nonspecific marker such as hs-CRP, patients at higher global risk and with LDL-C below the recommended levels could be identified and treated aggressively. This benefit, however, required that approximately 100 people be treated with rosuvastatin for 2 years to prevent one event. Additionally, only 20% of all patients screened were eligible for the trial. Therefore, one could argue that its generalizability is limited.
Markers of risk that are more specific and sensitive are needed to identify people at higher global risk who would otherwise be considered to be at low risk with the current risk assessment tools. A number of such inflammatory and oxidative markers are under development.54–60
Absolute vs relative risk reduction and the public health burden
The 44% reduction in the number of primary end point events in the rosuvastatin group was considerable in relative terms. However, in absolute terms, 95 people had to be treated for up to 2 years in order to prevent one event.53 In making recommendations, the United States Department of Health and Human Services has to consider the clinical benefit of a test or a drug in light of its cost. With health care costs increasing, many agencies are refusing to pay for therapies on the basis of cost or small absolute benefit.
While we do not have the answer as to whether treating 95 people for 2 years to see one benefit is cost-effective, one thing is clear: the field of medicine is in desperate need of a better way to identify individuals who may benefit from a test or therapy.61 Additionally, we think it is important to note that the “numbers-needed-to-treat” (95 at 2 years and 25 at 5 years) derived from JUPITER are actually smaller than the values observed in the AFCAPS/TexCAPS and the West of Scotland Coronary Prevention Study.62,63 This suggests that statin therapy is at least as cost-effective in those with elevated hs-CRP as in those with elevated LDL-C. Even our most robust therapies are effective in only a minority of patients treated.61
Should ‘healthy’ people be tested for hs-CRP?
In 2003, we wrote in this journal21 that measuring hs-CRP may add to the current risk-prediction models by identifying people at increased risk who would otherwise not be considered as such by current risk models. The US Centers for Disease Control and Prevention and the American Heart Association have also stated that measuring hs-CRP in those at intermediate risk may be reasonable.26
The JUPITER investigators intended to study a relatively healthy population, but, as we mentioned, a close look at the cohort’s baseline characteristics indicates a substantial proportion met the criteria for metabolic syndrome. Therefore, one could challenge whether we really need hs-CRP in such a population to identify who will benefit from statin therapy.
We agree with the recommendation from the Centers for Disease Control and Prevention and the American Heart Association that measuring hs-CRP in people at intermediate risk is a reasonable option.26 We also believe that hs-CRP should be tested as a secondary risk factor, in combination with blood pressure, lipids, diabetes, smoking, serum creatinine, and fasting blood glucose. Factors such as obesity, sedentary lifestyle, family history of heart disease, and emotional and physical stress should also be considered.
Safety of high-dose statin therapy
High-dose statin therapy has been well tolerated in clinical trials, but rates of discontinuation have been higher (7%–10%) than with moderate-dose therapy (4%–5%).64 Fortunately, the rates of serious adverse events have in general been low. For example, with simvastatin 80 mg, the rates of myopathy and rhabdomyolysis were quite low.31
Rates of elevations in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) with high-dose statin therapy have been reported to be below 1.3%. Studies have shown that reducing LDL-C to below 100 mg/dL is associated with a higher incidence of ALT and AST elevations. However, these elevations have usually been benign and often return to normal when the drug is reduced in dose or withdrawn.
In previous studies of rosuvastatin,65 the incidence of myopathy and liver function abnormalities was less than 0.1%. Rates of proteinuria were similarly low, and in many patients renal function actually improved on rosuvastatin.66,67 Furthermore, rosuvastatin may have different pharmacokinetic properties than atorvastatin (Lipitor) and simvastatin, which may result in a lower incidence of musculoskeletal toxicity.68,69
In general, the incidence of cancer has been similar in those treated with high-dose statins and those treated with placebo. The Treating to New Targets trial70 suggested that the incidence of cancer was higher with atorvastatin 80 mg daily than with 20 mg daily. However, a meta-analysis of 14 trials of moderate-dose statin therapy did not show any evidence of increased cancer rates with these agents.70 Indeed, in JUPITER, there was a reduction in cancer-related mortality rates, which could have been due to chance.
The JUPITER trial also showed an increase in the physician-reported incidence of diabetes mellitus with rosuvastatin. This is an important finding, and it may be a class effect because modest increases have similarly been reported with other statins in other major trials, eg, with pravastatin (Pravachol) in PROSPER, simvastatin in the Heart Protection Study, and atorvastatin in PROVE-IT. However, even in those with diabetes or impaired fasting glucose, the reduction in the rate of major adverse events is significant. For example, in JUPITER, almost all of the cases of “incident diabetes” were in those with impaired fasting glucose at baseline, and this group had nearly a 50% reduction in rates of myocardial infarction, stroke, and cardiovascular death. Therefore, on balance, the modest risk of earlier diagnosis of diabetes with statin therapy seems substantially offset by the marked reduction in rates of major adverse cardiovascular events in people with diabetes and impaired fasting glucose on statin therapy.
TAKE-HOME POINTS
The JUPITER trial, like previous high-dose statin trials, calls into question whether current LDL-C guidelines are appropriate for people at higher global risk with otherwise “normal” LDL-C levels.27,28 This trial heralds a new era in preventive therapy because it extends beyond LDL-C as an indication for statin therapy within the primary prevention setting. Statins have revolutionized the therapy of cardiovascular disease, and they continue to show benefit even in the “healthy.”
Clearly, hs-CRP serves as a nonlipid marker to identify those who may benefit from statin therapy. Nonetheless, more specific and sensitive markers (or panels) of cardiovascular risk are necessary. In the future, we will need markers that not only identify people at higher global risk, but that also tell us who would benefit from certain medical or surgical therapies. Elevated hs-CRP in a patient who otherwise would not be a candidate for statin therapy should trigger a reassessment of the risks vs benefits of statin therapy—JUPITER teaches us that statin therapy will benefit these patients.
Aggressive lifestyle modification that encompasses a balanced diet, routine exercise, and smoking cessation should be applied in both primary and secondary prevention. Additionally, risk factors such as elevated blood pressure and hyperlipidemia should be aggressively treated with appropriate medications.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Topol EJ. Intensive statin therapy—a sea change in cardiovascular prevention. N Engl J Med 2004; 350:1562–1564.
- Cannon CP, Murphy SA, Braunwald E. Intensive lipid lowering with atorvastatin in coronary disease. N Engl J Med 2005; 353:93–96.
- Cohen DJ, Carrozza JP, Baim DS. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. N Engl J Med 1999; 341:1853–1854.
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol 2005; 46:1855–1862.
- Aikawa M, Rabkin E, Sugiyama S, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 2001; 103:276–283.
- Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol 2005; 96:24F–33F.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Shishehbor MH, Aviles RJ, Brennan ML, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 2003; 289:1675–1680.
- Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 2003; 108:426–431.
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001; 21:1712–1719.
- Shishehbor MH, Patel T, Bhatt DL. Using statins to treat inflammation in acute coronary syndromes: Are we there yet? Cleve Clin J Med 2006; 73:760–766.
- Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001; 344:1959–1965.
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998; 98:731–733.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342:836–843.
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001; 285:2481–2485.
- Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem 2001; 47:403–411.
- Shishehbor MH, Bhatt DL, Topol EJ. Using C-reactive protein to assess cardiovascular disease risk. Cleve Clin J Med 2003; 70:634–640.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336:973–979.
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008; 359:1897–1908.
- Ridker PM. Are statins anti-inflammatory? Issues in the design and conduct of the pravastatin inflammation C-reactive protein evaluation. Curr Cardiol Rep 2000; 2:269–273.
- Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 2003; 107:391–397.
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107:499–511.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- Collins R, Peto R, Armitage J. The MRC/BHF Heart Protection Study: preliminary results. Int J Clin Pract 2002; 56:53–56.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Liem AH, van Boven AJ, Veeger NJ, et al. Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. Eur Heart J 2002; 23:1931–1937.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Pitt B, Waters D, Brown WV, et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med 1999; 341:70–76.
- Ray KK, Cannon CP, McCabe CH, et al. Early and late benefits of highdose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 2005; 46:1405–1410.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 287:3215–3222.
- Patel TN, Shishehbor MH, Bhatt DL. A review of high-dose statin therapy: targeting cholesterol and inflammation in atherosclerosis. Eur Heart J 2007; 28:664–672.
- Ridker PM. Novel risk factors and markers for coronary disease. Adv Intern Med 2000; 45:391–418.
- Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001; 103:1813–1818.
- Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998; 97:2007–2011.
- Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation 2005; 112:25–31.
- Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007; 49:2129–2138.
- Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001; 286:64–70.
- Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999; 100:230–235.
- Bisoendial RJ, Kastelein JJ, Levels JH, et al. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res 2005; 96:714–716.
- Schwedler SB, Amann K, Wernicke K, et al. Native C-reactive protein increases whereas modified C-reactive protein reduces atherosclerosis in apolipoprotein E-knockout mice. Circulation 2005; 112:1016–1023.
- Pfister R, Hellmich M. Multiple biomarkers and cardiovascular risk. N Engl J Med 2008; 359:760.
- Schunkert H, Samani NJ. Elevated C-reactive protein in atherosclerosis— chicken or egg? N Engl J Med 2008; 359:1953–1955.
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350:1387–1397.
- Kushner I, Sehgal AR. Is high-sensitivity C-reactive protein an effective screening test for cardiovascular risk? Arch Intern Med 2002; 162:867–869.
- Hlatky MA. Expanding the orbit of primary prevention—moving beyond JUPITER. N Engl J Med 2008; 359:2280–2282.
- Shishehbor MH, Hazen SL. Inflammatory and oxidative markers in atherosclerosis: relationship to outcome. Curr Atheroscler Rep 2004; 6:243–250.
- Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 2005; 25:1102–1111.
- Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008; 299:1265–1276.
- Choi SH, Chae A, Miller E, et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J Am Coll Cardiol 2008; 52:24–32.
- Hakonarson H, Thorvaldsson S, Helgadottir A, et al. Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trial. JAMA 2005; 293:2245–2256.
- Ky B, Burke A, Tsimikas S, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol 2008; 51:1653–1662.
- Levy AP, Levy JE, Kalet-Litman S, et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol 2007; 27:134–140.
- Mukherjee D, Topol EJ. Pharmacogenomics in cardiovascular diseases. Curr Probl Cardiol 2003; 28:317–347
- West of Scotland Coronary Prevention Study: identification of highrisk groups and comparison with other cardiovascular intervention trials. Lancet 1996; 348:1339–1342.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol 2007; 49:1753–1762.
- Davidson MH. Rosuvastatin safety: lessons from the FDA review and post-approval surveillance. Expert Opin Drug Saf 2004; 3:547–557.
- Kasiske BL, Wanner C, O’Neill WC. An assessment of statin safety by nephrologists. Am J Cardiol 2006; 97:82C–85C.
- McTaggart F, Buckett L, Davidson R, et al. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol 2001; 87:28B–32B.
- Jacobson TA. Statin safety: lessons from new drug applications for marketed statins. Am J Cardiol 2006; 97:44C–51C.
- Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol 2004; 94:1140–1146.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
The medical community has struggled with two important questions for the past 10 years: When it comes to the low-density lipoprotein cholesterol (LDL-C) level, how low should one go and at what cost? And are there other markers of risk that can identify a higher-risk subpopulation in relatively healthy people? The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) provided partial answers for these questions by finding that a highly potent statin lowered the risk of cardiovascular events in patients with “normal” LDL-C but elevated levels of high-sensitivity C-reactive protein (hs-CRP).1
In this article, we will critically evaluate the methods, results, and conclusions of the JUPITER trial. Additionally, we will discuss its limitations and areas of uncertainty.
BEFORE JUPITER
The LDL-C-lowering drugs called statins have revolutionized cardiovascular medicine.2 They are beneficial in both the primary prevention setting and in acute coronary syndromes, stable angina, and unstable angina and can halt the progression of coronary artery disease—in some cases even resulting in modest regression of plaque.3–6
Many experts have credited the reduction in LDL-C as being the sole factor responsible for the decrease in major adverse events seen with statin therapy.7 However, statins have other, non-lipid-lowering properties, including anti-inflammatory and antioxidant effects, that may also contribute to their benefits.8–15
One of the anti-inflammatory actions of statins is evidenced by lower levels of the acute-phase reactant CRP.10,11,15,16 Measuring systemic CRP levels with a highly sensitive assay (yielding the so-called high-sensitivity or hs-CRP level) provides significant clinical prognostic value across a spectrum of clinical situations, ranging from risk screening in apparently healthy people to stable and unstable angina.17–22 People with higher hs-CRP levels are, on average, at higher risk of adverse cardiovascular events. However, controversy remains as to whether hs-CRP plays a mechanistic role in plaque formation and acute complications. Indeed, recent genetic studies argue strongly that hs-CRP lies outside the mechanistic path of atherosclerosis.23 Nonetheless, an overwhelming amount of data indicates that hs-CRP serves as a marker of disease.17–21
Nissen et al10 showed that the rate of progression of atherosclerosis is lower when the levels of atherogenic lipoproteins and hs-CRP are both lowered with statin therapy. Simultaneously, Ridker et al11 showed that patients who have lower hs-CRP levels after statin therapy have better clinical outcomes than those with higher hs-CRP levels, regardless of their achieved level of LDL-C.
Collectively, these studies and others have led some to believe that, in people with relatively low LDL-C but persistently elevated hs-CRP, statin therapy may reduce the rate of events.15,24 The JUPITER trial was undertaken to test this hypothesis.
JUPITER STUDY DESIGN
JUPITER was designed to see whether highly potent statin therapy is beneficial in people with elevated hs-CRP who otherwise do not meet the criteria for lipid-lowering therapy. The study was conducted at 1,315 sites in 26 countries. It was sponsored by AstraZeneca, the maker of rosuvastatin (Crestor).
Inclusion and exclusion criteria
All participants had to be free of known cardiovascular disease, have an LDL-C level lower than 130 mg/dL, and have an hs-CRP level of 2.0 mg/L or greater. Patients were excluded if they were previous or current users of lipid-lowering drugs; had severe arthritis, lupus, or inflammatory bowel disease; or were taking immune-modulating drugs such as cyclosporine (Sandimmune, others), tacrolimus (Prograf), azathioprine (Azasan, Imuran), or long-term oral corticosteroids.
Rosuvastatin therapy
Participants were randomly assigned in a 1:1 ratio to receive rosuvastatin 20 mg daily or a matching placebo in a double-blind fashion.
End points
The primary end point was the composite of nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from cardiovascular causes. Secondary end points were the individual components of the primary end point.
Statistical analysis
The study was powered to detect a 25% reduction in the primary end point among those treated with rosuvastatin. The trial was designed to run until 520 end point events had occurred. However, on March 29, 2008, after the first prespecified interim analysis, the Data and Safety Monitoring Board stopped the trial due to a significant reduction in the primary end point in the rosuvastatin group. As in most randomized clinical trials, all analyses were done on an intention-to-treat basis. Prespecified subgroup analyses were also performed.
STUDY RESULTS
Patient recruitment and eligibility
Between February 4, 2003, and December 15, 2006, a total of 89,890 people were screened. Of these, 17,802 met the inclusion and exclusion criteria and were included in the study. Of the 72,088 people who were excluded, 25,993 (36.1%) had an hs-CRP level below 2 mg/L and 37,611 (52.2%) had an LDL-C level of 130 mg/dL or higher.
A not-so-healthy population
The aim of the investigators was to include relatively healthy people. The median age was 66 years, about 16% of participants were current smokers, about 11% had a family history of heart disease, and about 41% met the criteria for metabolic syndrome, all conditions that are associated with elevated hs-CRP.25 Of note, the median hs-CRP level was 4.2 mg/L, a level indicating higher global risk according to the American College of Cardiology/American Heart Association consensus statement.26
Reduction in lipid levels and hs-CRP
By 12 months, in the rosuvastatin group, the median LDL-C level had fallen by 50% (from 108 to 55 mg/dL), and the median hs-CRP level had fallen by 37% (from 4.2 to 2.2 mg/L). Additionally, the triglyceride level had fallen by 17%. The high-density lipoprotein cholesterol levels did not change significantly.
Impact on end points
Adverse events
WHAT DOES THIS MEAN?
Is lower LDL-C better?
The JUPITER trial is the latest of several statin trials that have shown significant reductions in major adverse cardiovascular events when LDL-C was lowered below what has been recommended by the current guidelines.27,28
In 2002, the Heart Protection Study29 showed a significant reduction in major adverse cardiovascular events in patients at high risk of coronary artery disease if they received simvastatin (Zocor), even if they had LDL-C levels lower than 100 mg/dL at baseline. Similarly, the Pravastatin or Atorvastatin Evaluation and Infection-Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) trial30 showed a 16% relative risk reduction in a composite end point in patients presenting with acute coronary syndrome if they received intensive statin therapy.
These two studies led to an update by the National Cholesterol Education Program (Adult Treatment Panel III), suggesting an optimal LDL-C goal of less than 70 mg/dL in those with coronary artery disease or its risk equivalent (ie, diabetes mellitus, peripheral vascular disease). Furthermore, in support of the “lower is better” theory, a number of studies that used intravascular ultrasonography have shown regression of coronary plaque with aggressive LDL-C lowering. Notably, in a Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (the ASTEROID trial),5 rosuvastatin 40 mg daily caused significant plaque regression while lowering LDL-C to 61 mg/dL over a 24-month period.
A number of high-dose statin trials have shown that lowering LDL-C to less than 70 mg/dL significantly reduces major adverse cardiovascular events.31–39 The JUPITER trial was unique in that it extended these findings to people without known coronary disease (ie, primary prevention) or elevated cholesterol but with elevated levels of a marker of inflammation—hs-CRP. In view of the JUPITER results and of studies using intravascular ultrasonography in the primary prevention setting, it seems clear that lowering LDL-C to levels less than 70 mg/dL also reduces both atherosclerotic plaque progression and the rate of first major adverse cardiovascular events in primary prevention in patients at higher global risk.
Did the study prove that reducing hs-CRP lowers risk?
Measuring hs-CRP levels has been extensively studied in apparently healthy populations, stable angina, unstable angina, and other cardiovascular settings.18,21,40–43 It has been shown to have significant prognostic implications in a number of primary and secondary trials.44 Additionally, those with elevated LDL-C and hs-CRP levels benefit the most from statin therapy.16,45,46 Animal studies have also provided some evidence that CRP may play a role in atherogenesis.47,48 However, recent clinical and genetic studies have raised doubt about the direct causal relationship between CRP and coronary artery disease,23,49,50 and epidemiologic studies have questioned its usefulness as a marker of risk.51,52
The JUPITER study adds little to clear up the controversy about whether hs-CRP is a mechanistic participant in atherosclerotic disease. However, it also shows that this issue is somewhat irrelevant, in that selection of patients for high-potency statin therapy solely on the basis of high hs-CRP without other indications for lipid-lowering therapy clearly reduces risk and improves survival.
JUPITER did not examine whether people with higher hs-CRP levels benefited more from statin therapy than those with lower levels. The hypothesis-generating data for JUPITER came from an analysis of changes in hs-CRP and LDL-C in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS).16 Thus, JUPITER did not include people with both low LDL-C and low hs-CRP because, in the AFCAPS/TexCAPS analysis, those with low LDL-C and low hs-CRP had extremely low event rates and no clinical efficacy of statin therapy, despite good LDL-C reduction. In marked contrast, those with low LDL-C but elevated hs-CRP had high event rates and large relative risk reductions— hence the need for JUPITER to prospectively test this hypothesis. Nevertheless, the initial results of JUPITER as presented do not yet make it clear that there is a dose-response relationship between higher levels of hs-CRP and a greater reduction in events, even in a cohort with elevated hs-CRP at baseline. This analysis will no doubt be forthcoming in another manuscript from Ridker and colleagues. Specifically, it will be of interest to examine whether those with the highest hs-CRP levels benefited the most from rosuvastatin on both an absolute and relative scale, and whether those with the greatest hs-CRP reduction also benefited more. With the present data available from JUPITER, a reasonable interpretation is that an elevated hs-CRP simply widens the inclusion criterion for those for whom high-potency statin therapy improves clinical outcomes.53
Better markers are needed
Even with a nonspecific marker such as hs-CRP, patients at higher global risk and with LDL-C below the recommended levels could be identified and treated aggressively. This benefit, however, required that approximately 100 people be treated with rosuvastatin for 2 years to prevent one event. Additionally, only 20% of all patients screened were eligible for the trial. Therefore, one could argue that its generalizability is limited.
Markers of risk that are more specific and sensitive are needed to identify people at higher global risk who would otherwise be considered to be at low risk with the current risk assessment tools. A number of such inflammatory and oxidative markers are under development.54–60
Absolute vs relative risk reduction and the public health burden
The 44% reduction in the number of primary end point events in the rosuvastatin group was considerable in relative terms. However, in absolute terms, 95 people had to be treated for up to 2 years in order to prevent one event.53 In making recommendations, the United States Department of Health and Human Services has to consider the clinical benefit of a test or a drug in light of its cost. With health care costs increasing, many agencies are refusing to pay for therapies on the basis of cost or small absolute benefit.
While we do not have the answer as to whether treating 95 people for 2 years to see one benefit is cost-effective, one thing is clear: the field of medicine is in desperate need of a better way to identify individuals who may benefit from a test or therapy.61 Additionally, we think it is important to note that the “numbers-needed-to-treat” (95 at 2 years and 25 at 5 years) derived from JUPITER are actually smaller than the values observed in the AFCAPS/TexCAPS and the West of Scotland Coronary Prevention Study.62,63 This suggests that statin therapy is at least as cost-effective in those with elevated hs-CRP as in those with elevated LDL-C. Even our most robust therapies are effective in only a minority of patients treated.61
Should ‘healthy’ people be tested for hs-CRP?
In 2003, we wrote in this journal21 that measuring hs-CRP may add to the current risk-prediction models by identifying people at increased risk who would otherwise not be considered as such by current risk models. The US Centers for Disease Control and Prevention and the American Heart Association have also stated that measuring hs-CRP in those at intermediate risk may be reasonable.26
The JUPITER investigators intended to study a relatively healthy population, but, as we mentioned, a close look at the cohort’s baseline characteristics indicates a substantial proportion met the criteria for metabolic syndrome. Therefore, one could challenge whether we really need hs-CRP in such a population to identify who will benefit from statin therapy.
We agree with the recommendation from the Centers for Disease Control and Prevention and the American Heart Association that measuring hs-CRP in people at intermediate risk is a reasonable option.26 We also believe that hs-CRP should be tested as a secondary risk factor, in combination with blood pressure, lipids, diabetes, smoking, serum creatinine, and fasting blood glucose. Factors such as obesity, sedentary lifestyle, family history of heart disease, and emotional and physical stress should also be considered.
Safety of high-dose statin therapy
High-dose statin therapy has been well tolerated in clinical trials, but rates of discontinuation have been higher (7%–10%) than with moderate-dose therapy (4%–5%).64 Fortunately, the rates of serious adverse events have in general been low. For example, with simvastatin 80 mg, the rates of myopathy and rhabdomyolysis were quite low.31
Rates of elevations in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) with high-dose statin therapy have been reported to be below 1.3%. Studies have shown that reducing LDL-C to below 100 mg/dL is associated with a higher incidence of ALT and AST elevations. However, these elevations have usually been benign and often return to normal when the drug is reduced in dose or withdrawn.
In previous studies of rosuvastatin,65 the incidence of myopathy and liver function abnormalities was less than 0.1%. Rates of proteinuria were similarly low, and in many patients renal function actually improved on rosuvastatin.66,67 Furthermore, rosuvastatin may have different pharmacokinetic properties than atorvastatin (Lipitor) and simvastatin, which may result in a lower incidence of musculoskeletal toxicity.68,69
In general, the incidence of cancer has been similar in those treated with high-dose statins and those treated with placebo. The Treating to New Targets trial70 suggested that the incidence of cancer was higher with atorvastatin 80 mg daily than with 20 mg daily. However, a meta-analysis of 14 trials of moderate-dose statin therapy did not show any evidence of increased cancer rates with these agents.70 Indeed, in JUPITER, there was a reduction in cancer-related mortality rates, which could have been due to chance.
The JUPITER trial also showed an increase in the physician-reported incidence of diabetes mellitus with rosuvastatin. This is an important finding, and it may be a class effect because modest increases have similarly been reported with other statins in other major trials, eg, with pravastatin (Pravachol) in PROSPER, simvastatin in the Heart Protection Study, and atorvastatin in PROVE-IT. However, even in those with diabetes or impaired fasting glucose, the reduction in the rate of major adverse events is significant. For example, in JUPITER, almost all of the cases of “incident diabetes” were in those with impaired fasting glucose at baseline, and this group had nearly a 50% reduction in rates of myocardial infarction, stroke, and cardiovascular death. Therefore, on balance, the modest risk of earlier diagnosis of diabetes with statin therapy seems substantially offset by the marked reduction in rates of major adverse cardiovascular events in people with diabetes and impaired fasting glucose on statin therapy.
TAKE-HOME POINTS
The JUPITER trial, like previous high-dose statin trials, calls into question whether current LDL-C guidelines are appropriate for people at higher global risk with otherwise “normal” LDL-C levels.27,28 This trial heralds a new era in preventive therapy because it extends beyond LDL-C as an indication for statin therapy within the primary prevention setting. Statins have revolutionized the therapy of cardiovascular disease, and they continue to show benefit even in the “healthy.”
Clearly, hs-CRP serves as a nonlipid marker to identify those who may benefit from statin therapy. Nonetheless, more specific and sensitive markers (or panels) of cardiovascular risk are necessary. In the future, we will need markers that not only identify people at higher global risk, but that also tell us who would benefit from certain medical or surgical therapies. Elevated hs-CRP in a patient who otherwise would not be a candidate for statin therapy should trigger a reassessment of the risks vs benefits of statin therapy—JUPITER teaches us that statin therapy will benefit these patients.
Aggressive lifestyle modification that encompasses a balanced diet, routine exercise, and smoking cessation should be applied in both primary and secondary prevention. Additionally, risk factors such as elevated blood pressure and hyperlipidemia should be aggressively treated with appropriate medications.
The medical community has struggled with two important questions for the past 10 years: When it comes to the low-density lipoprotein cholesterol (LDL-C) level, how low should one go and at what cost? And are there other markers of risk that can identify a higher-risk subpopulation in relatively healthy people? The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) provided partial answers for these questions by finding that a highly potent statin lowered the risk of cardiovascular events in patients with “normal” LDL-C but elevated levels of high-sensitivity C-reactive protein (hs-CRP).1
In this article, we will critically evaluate the methods, results, and conclusions of the JUPITER trial. Additionally, we will discuss its limitations and areas of uncertainty.
BEFORE JUPITER
The LDL-C-lowering drugs called statins have revolutionized cardiovascular medicine.2 They are beneficial in both the primary prevention setting and in acute coronary syndromes, stable angina, and unstable angina and can halt the progression of coronary artery disease—in some cases even resulting in modest regression of plaque.3–6
Many experts have credited the reduction in LDL-C as being the sole factor responsible for the decrease in major adverse events seen with statin therapy.7 However, statins have other, non-lipid-lowering properties, including anti-inflammatory and antioxidant effects, that may also contribute to their benefits.8–15
One of the anti-inflammatory actions of statins is evidenced by lower levels of the acute-phase reactant CRP.10,11,15,16 Measuring systemic CRP levels with a highly sensitive assay (yielding the so-called high-sensitivity or hs-CRP level) provides significant clinical prognostic value across a spectrum of clinical situations, ranging from risk screening in apparently healthy people to stable and unstable angina.17–22 People with higher hs-CRP levels are, on average, at higher risk of adverse cardiovascular events. However, controversy remains as to whether hs-CRP plays a mechanistic role in plaque formation and acute complications. Indeed, recent genetic studies argue strongly that hs-CRP lies outside the mechanistic path of atherosclerosis.23 Nonetheless, an overwhelming amount of data indicates that hs-CRP serves as a marker of disease.17–21
Nissen et al10 showed that the rate of progression of atherosclerosis is lower when the levels of atherogenic lipoproteins and hs-CRP are both lowered with statin therapy. Simultaneously, Ridker et al11 showed that patients who have lower hs-CRP levels after statin therapy have better clinical outcomes than those with higher hs-CRP levels, regardless of their achieved level of LDL-C.
Collectively, these studies and others have led some to believe that, in people with relatively low LDL-C but persistently elevated hs-CRP, statin therapy may reduce the rate of events.15,24 The JUPITER trial was undertaken to test this hypothesis.
JUPITER STUDY DESIGN
JUPITER was designed to see whether highly potent statin therapy is beneficial in people with elevated hs-CRP who otherwise do not meet the criteria for lipid-lowering therapy. The study was conducted at 1,315 sites in 26 countries. It was sponsored by AstraZeneca, the maker of rosuvastatin (Crestor).
Inclusion and exclusion criteria
All participants had to be free of known cardiovascular disease, have an LDL-C level lower than 130 mg/dL, and have an hs-CRP level of 2.0 mg/L or greater. Patients were excluded if they were previous or current users of lipid-lowering drugs; had severe arthritis, lupus, or inflammatory bowel disease; or were taking immune-modulating drugs such as cyclosporine (Sandimmune, others), tacrolimus (Prograf), azathioprine (Azasan, Imuran), or long-term oral corticosteroids.
Rosuvastatin therapy
Participants were randomly assigned in a 1:1 ratio to receive rosuvastatin 20 mg daily or a matching placebo in a double-blind fashion.
End points
The primary end point was the composite of nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from cardiovascular causes. Secondary end points were the individual components of the primary end point.
Statistical analysis
The study was powered to detect a 25% reduction in the primary end point among those treated with rosuvastatin. The trial was designed to run until 520 end point events had occurred. However, on March 29, 2008, after the first prespecified interim analysis, the Data and Safety Monitoring Board stopped the trial due to a significant reduction in the primary end point in the rosuvastatin group. As in most randomized clinical trials, all analyses were done on an intention-to-treat basis. Prespecified subgroup analyses were also performed.
STUDY RESULTS
Patient recruitment and eligibility
Between February 4, 2003, and December 15, 2006, a total of 89,890 people were screened. Of these, 17,802 met the inclusion and exclusion criteria and were included in the study. Of the 72,088 people who were excluded, 25,993 (36.1%) had an hs-CRP level below 2 mg/L and 37,611 (52.2%) had an LDL-C level of 130 mg/dL or higher.
A not-so-healthy population
The aim of the investigators was to include relatively healthy people. The median age was 66 years, about 16% of participants were current smokers, about 11% had a family history of heart disease, and about 41% met the criteria for metabolic syndrome, all conditions that are associated with elevated hs-CRP.25 Of note, the median hs-CRP level was 4.2 mg/L, a level indicating higher global risk according to the American College of Cardiology/American Heart Association consensus statement.26
Reduction in lipid levels and hs-CRP
By 12 months, in the rosuvastatin group, the median LDL-C level had fallen by 50% (from 108 to 55 mg/dL), and the median hs-CRP level had fallen by 37% (from 4.2 to 2.2 mg/L). Additionally, the triglyceride level had fallen by 17%. The high-density lipoprotein cholesterol levels did not change significantly.
Impact on end points
Adverse events
WHAT DOES THIS MEAN?
Is lower LDL-C better?
The JUPITER trial is the latest of several statin trials that have shown significant reductions in major adverse cardiovascular events when LDL-C was lowered below what has been recommended by the current guidelines.27,28
In 2002, the Heart Protection Study29 showed a significant reduction in major adverse cardiovascular events in patients at high risk of coronary artery disease if they received simvastatin (Zocor), even if they had LDL-C levels lower than 100 mg/dL at baseline. Similarly, the Pravastatin or Atorvastatin Evaluation and Infection-Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) trial30 showed a 16% relative risk reduction in a composite end point in patients presenting with acute coronary syndrome if they received intensive statin therapy.
These two studies led to an update by the National Cholesterol Education Program (Adult Treatment Panel III), suggesting an optimal LDL-C goal of less than 70 mg/dL in those with coronary artery disease or its risk equivalent (ie, diabetes mellitus, peripheral vascular disease). Furthermore, in support of the “lower is better” theory, a number of studies that used intravascular ultrasonography have shown regression of coronary plaque with aggressive LDL-C lowering. Notably, in a Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (the ASTEROID trial),5 rosuvastatin 40 mg daily caused significant plaque regression while lowering LDL-C to 61 mg/dL over a 24-month period.
A number of high-dose statin trials have shown that lowering LDL-C to less than 70 mg/dL significantly reduces major adverse cardiovascular events.31–39 The JUPITER trial was unique in that it extended these findings to people without known coronary disease (ie, primary prevention) or elevated cholesterol but with elevated levels of a marker of inflammation—hs-CRP. In view of the JUPITER results and of studies using intravascular ultrasonography in the primary prevention setting, it seems clear that lowering LDL-C to levels less than 70 mg/dL also reduces both atherosclerotic plaque progression and the rate of first major adverse cardiovascular events in primary prevention in patients at higher global risk.
Did the study prove that reducing hs-CRP lowers risk?
Measuring hs-CRP levels has been extensively studied in apparently healthy populations, stable angina, unstable angina, and other cardiovascular settings.18,21,40–43 It has been shown to have significant prognostic implications in a number of primary and secondary trials.44 Additionally, those with elevated LDL-C and hs-CRP levels benefit the most from statin therapy.16,45,46 Animal studies have also provided some evidence that CRP may play a role in atherogenesis.47,48 However, recent clinical and genetic studies have raised doubt about the direct causal relationship between CRP and coronary artery disease,23,49,50 and epidemiologic studies have questioned its usefulness as a marker of risk.51,52
The JUPITER study adds little to clear up the controversy about whether hs-CRP is a mechanistic participant in atherosclerotic disease. However, it also shows that this issue is somewhat irrelevant, in that selection of patients for high-potency statin therapy solely on the basis of high hs-CRP without other indications for lipid-lowering therapy clearly reduces risk and improves survival.
JUPITER did not examine whether people with higher hs-CRP levels benefited more from statin therapy than those with lower levels. The hypothesis-generating data for JUPITER came from an analysis of changes in hs-CRP and LDL-C in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS).16 Thus, JUPITER did not include people with both low LDL-C and low hs-CRP because, in the AFCAPS/TexCAPS analysis, those with low LDL-C and low hs-CRP had extremely low event rates and no clinical efficacy of statin therapy, despite good LDL-C reduction. In marked contrast, those with low LDL-C but elevated hs-CRP had high event rates and large relative risk reductions— hence the need for JUPITER to prospectively test this hypothesis. Nevertheless, the initial results of JUPITER as presented do not yet make it clear that there is a dose-response relationship between higher levels of hs-CRP and a greater reduction in events, even in a cohort with elevated hs-CRP at baseline. This analysis will no doubt be forthcoming in another manuscript from Ridker and colleagues. Specifically, it will be of interest to examine whether those with the highest hs-CRP levels benefited the most from rosuvastatin on both an absolute and relative scale, and whether those with the greatest hs-CRP reduction also benefited more. With the present data available from JUPITER, a reasonable interpretation is that an elevated hs-CRP simply widens the inclusion criterion for those for whom high-potency statin therapy improves clinical outcomes.53
Better markers are needed
Even with a nonspecific marker such as hs-CRP, patients at higher global risk and with LDL-C below the recommended levels could be identified and treated aggressively. This benefit, however, required that approximately 100 people be treated with rosuvastatin for 2 years to prevent one event. Additionally, only 20% of all patients screened were eligible for the trial. Therefore, one could argue that its generalizability is limited.
Markers of risk that are more specific and sensitive are needed to identify people at higher global risk who would otherwise be considered to be at low risk with the current risk assessment tools. A number of such inflammatory and oxidative markers are under development.54–60
Absolute vs relative risk reduction and the public health burden
The 44% reduction in the number of primary end point events in the rosuvastatin group was considerable in relative terms. However, in absolute terms, 95 people had to be treated for up to 2 years in order to prevent one event.53 In making recommendations, the United States Department of Health and Human Services has to consider the clinical benefit of a test or a drug in light of its cost. With health care costs increasing, many agencies are refusing to pay for therapies on the basis of cost or small absolute benefit.
While we do not have the answer as to whether treating 95 people for 2 years to see one benefit is cost-effective, one thing is clear: the field of medicine is in desperate need of a better way to identify individuals who may benefit from a test or therapy.61 Additionally, we think it is important to note that the “numbers-needed-to-treat” (95 at 2 years and 25 at 5 years) derived from JUPITER are actually smaller than the values observed in the AFCAPS/TexCAPS and the West of Scotland Coronary Prevention Study.62,63 This suggests that statin therapy is at least as cost-effective in those with elevated hs-CRP as in those with elevated LDL-C. Even our most robust therapies are effective in only a minority of patients treated.61
Should ‘healthy’ people be tested for hs-CRP?
In 2003, we wrote in this journal21 that measuring hs-CRP may add to the current risk-prediction models by identifying people at increased risk who would otherwise not be considered as such by current risk models. The US Centers for Disease Control and Prevention and the American Heart Association have also stated that measuring hs-CRP in those at intermediate risk may be reasonable.26
The JUPITER investigators intended to study a relatively healthy population, but, as we mentioned, a close look at the cohort’s baseline characteristics indicates a substantial proportion met the criteria for metabolic syndrome. Therefore, one could challenge whether we really need hs-CRP in such a population to identify who will benefit from statin therapy.
We agree with the recommendation from the Centers for Disease Control and Prevention and the American Heart Association that measuring hs-CRP in people at intermediate risk is a reasonable option.26 We also believe that hs-CRP should be tested as a secondary risk factor, in combination with blood pressure, lipids, diabetes, smoking, serum creatinine, and fasting blood glucose. Factors such as obesity, sedentary lifestyle, family history of heart disease, and emotional and physical stress should also be considered.
Safety of high-dose statin therapy
High-dose statin therapy has been well tolerated in clinical trials, but rates of discontinuation have been higher (7%–10%) than with moderate-dose therapy (4%–5%).64 Fortunately, the rates of serious adverse events have in general been low. For example, with simvastatin 80 mg, the rates of myopathy and rhabdomyolysis were quite low.31
Rates of elevations in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) with high-dose statin therapy have been reported to be below 1.3%. Studies have shown that reducing LDL-C to below 100 mg/dL is associated with a higher incidence of ALT and AST elevations. However, these elevations have usually been benign and often return to normal when the drug is reduced in dose or withdrawn.
In previous studies of rosuvastatin,65 the incidence of myopathy and liver function abnormalities was less than 0.1%. Rates of proteinuria were similarly low, and in many patients renal function actually improved on rosuvastatin.66,67 Furthermore, rosuvastatin may have different pharmacokinetic properties than atorvastatin (Lipitor) and simvastatin, which may result in a lower incidence of musculoskeletal toxicity.68,69
In general, the incidence of cancer has been similar in those treated with high-dose statins and those treated with placebo. The Treating to New Targets trial70 suggested that the incidence of cancer was higher with atorvastatin 80 mg daily than with 20 mg daily. However, a meta-analysis of 14 trials of moderate-dose statin therapy did not show any evidence of increased cancer rates with these agents.70 Indeed, in JUPITER, there was a reduction in cancer-related mortality rates, which could have been due to chance.
The JUPITER trial also showed an increase in the physician-reported incidence of diabetes mellitus with rosuvastatin. This is an important finding, and it may be a class effect because modest increases have similarly been reported with other statins in other major trials, eg, with pravastatin (Pravachol) in PROSPER, simvastatin in the Heart Protection Study, and atorvastatin in PROVE-IT. However, even in those with diabetes or impaired fasting glucose, the reduction in the rate of major adverse events is significant. For example, in JUPITER, almost all of the cases of “incident diabetes” were in those with impaired fasting glucose at baseline, and this group had nearly a 50% reduction in rates of myocardial infarction, stroke, and cardiovascular death. Therefore, on balance, the modest risk of earlier diagnosis of diabetes with statin therapy seems substantially offset by the marked reduction in rates of major adverse cardiovascular events in people with diabetes and impaired fasting glucose on statin therapy.
TAKE-HOME POINTS
The JUPITER trial, like previous high-dose statin trials, calls into question whether current LDL-C guidelines are appropriate for people at higher global risk with otherwise “normal” LDL-C levels.27,28 This trial heralds a new era in preventive therapy because it extends beyond LDL-C as an indication for statin therapy within the primary prevention setting. Statins have revolutionized the therapy of cardiovascular disease, and they continue to show benefit even in the “healthy.”
Clearly, hs-CRP serves as a nonlipid marker to identify those who may benefit from statin therapy. Nonetheless, more specific and sensitive markers (or panels) of cardiovascular risk are necessary. In the future, we will need markers that not only identify people at higher global risk, but that also tell us who would benefit from certain medical or surgical therapies. Elevated hs-CRP in a patient who otherwise would not be a candidate for statin therapy should trigger a reassessment of the risks vs benefits of statin therapy—JUPITER teaches us that statin therapy will benefit these patients.
Aggressive lifestyle modification that encompasses a balanced diet, routine exercise, and smoking cessation should be applied in both primary and secondary prevention. Additionally, risk factors such as elevated blood pressure and hyperlipidemia should be aggressively treated with appropriate medications.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Topol EJ. Intensive statin therapy—a sea change in cardiovascular prevention. N Engl J Med 2004; 350:1562–1564.
- Cannon CP, Murphy SA, Braunwald E. Intensive lipid lowering with atorvastatin in coronary disease. N Engl J Med 2005; 353:93–96.
- Cohen DJ, Carrozza JP, Baim DS. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. N Engl J Med 1999; 341:1853–1854.
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol 2005; 46:1855–1862.
- Aikawa M, Rabkin E, Sugiyama S, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 2001; 103:276–283.
- Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol 2005; 96:24F–33F.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Shishehbor MH, Aviles RJ, Brennan ML, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 2003; 289:1675–1680.
- Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 2003; 108:426–431.
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001; 21:1712–1719.
- Shishehbor MH, Patel T, Bhatt DL. Using statins to treat inflammation in acute coronary syndromes: Are we there yet? Cleve Clin J Med 2006; 73:760–766.
- Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001; 344:1959–1965.
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998; 98:731–733.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342:836–843.
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001; 285:2481–2485.
- Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem 2001; 47:403–411.
- Shishehbor MH, Bhatt DL, Topol EJ. Using C-reactive protein to assess cardiovascular disease risk. Cleve Clin J Med 2003; 70:634–640.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336:973–979.
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008; 359:1897–1908.
- Ridker PM. Are statins anti-inflammatory? Issues in the design and conduct of the pravastatin inflammation C-reactive protein evaluation. Curr Cardiol Rep 2000; 2:269–273.
- Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 2003; 107:391–397.
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107:499–511.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- Collins R, Peto R, Armitage J. The MRC/BHF Heart Protection Study: preliminary results. Int J Clin Pract 2002; 56:53–56.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Liem AH, van Boven AJ, Veeger NJ, et al. Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. Eur Heart J 2002; 23:1931–1937.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Pitt B, Waters D, Brown WV, et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med 1999; 341:70–76.
- Ray KK, Cannon CP, McCabe CH, et al. Early and late benefits of highdose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 2005; 46:1405–1410.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 287:3215–3222.
- Patel TN, Shishehbor MH, Bhatt DL. A review of high-dose statin therapy: targeting cholesterol and inflammation in atherosclerosis. Eur Heart J 2007; 28:664–672.
- Ridker PM. Novel risk factors and markers for coronary disease. Adv Intern Med 2000; 45:391–418.
- Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001; 103:1813–1818.
- Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998; 97:2007–2011.
- Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation 2005; 112:25–31.
- Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007; 49:2129–2138.
- Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001; 286:64–70.
- Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999; 100:230–235.
- Bisoendial RJ, Kastelein JJ, Levels JH, et al. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res 2005; 96:714–716.
- Schwedler SB, Amann K, Wernicke K, et al. Native C-reactive protein increases whereas modified C-reactive protein reduces atherosclerosis in apolipoprotein E-knockout mice. Circulation 2005; 112:1016–1023.
- Pfister R, Hellmich M. Multiple biomarkers and cardiovascular risk. N Engl J Med 2008; 359:760.
- Schunkert H, Samani NJ. Elevated C-reactive protein in atherosclerosis— chicken or egg? N Engl J Med 2008; 359:1953–1955.
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350:1387–1397.
- Kushner I, Sehgal AR. Is high-sensitivity C-reactive protein an effective screening test for cardiovascular risk? Arch Intern Med 2002; 162:867–869.
- Hlatky MA. Expanding the orbit of primary prevention—moving beyond JUPITER. N Engl J Med 2008; 359:2280–2282.
- Shishehbor MH, Hazen SL. Inflammatory and oxidative markers in atherosclerosis: relationship to outcome. Curr Atheroscler Rep 2004; 6:243–250.
- Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 2005; 25:1102–1111.
- Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008; 299:1265–1276.
- Choi SH, Chae A, Miller E, et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J Am Coll Cardiol 2008; 52:24–32.
- Hakonarson H, Thorvaldsson S, Helgadottir A, et al. Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trial. JAMA 2005; 293:2245–2256.
- Ky B, Burke A, Tsimikas S, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol 2008; 51:1653–1662.
- Levy AP, Levy JE, Kalet-Litman S, et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol 2007; 27:134–140.
- Mukherjee D, Topol EJ. Pharmacogenomics in cardiovascular diseases. Curr Probl Cardiol 2003; 28:317–347
- West of Scotland Coronary Prevention Study: identification of highrisk groups and comparison with other cardiovascular intervention trials. Lancet 1996; 348:1339–1342.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol 2007; 49:1753–1762.
- Davidson MH. Rosuvastatin safety: lessons from the FDA review and post-approval surveillance. Expert Opin Drug Saf 2004; 3:547–557.
- Kasiske BL, Wanner C, O’Neill WC. An assessment of statin safety by nephrologists. Am J Cardiol 2006; 97:82C–85C.
- McTaggart F, Buckett L, Davidson R, et al. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol 2001; 87:28B–32B.
- Jacobson TA. Statin safety: lessons from new drug applications for marketed statins. Am J Cardiol 2006; 97:44C–51C.
- Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol 2004; 94:1140–1146.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Topol EJ. Intensive statin therapy—a sea change in cardiovascular prevention. N Engl J Med 2004; 350:1562–1564.
- Cannon CP, Murphy SA, Braunwald E. Intensive lipid lowering with atorvastatin in coronary disease. N Engl J Med 2005; 353:93–96.
- Cohen DJ, Carrozza JP, Baim DS. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. N Engl J Med 1999; 341:1853–1854.
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol 2005; 46:1855–1862.
- Aikawa M, Rabkin E, Sugiyama S, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 2001; 103:276–283.
- Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol 2005; 96:24F–33F.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Shishehbor MH, Aviles RJ, Brennan ML, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 2003; 289:1675–1680.
- Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 2003; 108:426–431.
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001; 21:1712–1719.
- Shishehbor MH, Patel T, Bhatt DL. Using statins to treat inflammation in acute coronary syndromes: Are we there yet? Cleve Clin J Med 2006; 73:760–766.
- Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001; 344:1959–1965.
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998; 98:731–733.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342:836–843.
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001; 285:2481–2485.
- Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem 2001; 47:403–411.
- Shishehbor MH, Bhatt DL, Topol EJ. Using C-reactive protein to assess cardiovascular disease risk. Cleve Clin J Med 2003; 70:634–640.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336:973–979.
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008; 359:1897–1908.
- Ridker PM. Are statins anti-inflammatory? Issues in the design and conduct of the pravastatin inflammation C-reactive protein evaluation. Curr Cardiol Rep 2000; 2:269–273.
- Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 2003; 107:391–397.
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107:499–511.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- Collins R, Peto R, Armitage J. The MRC/BHF Heart Protection Study: preliminary results. Int J Clin Pract 2002; 56:53–56.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Liem AH, van Boven AJ, Veeger NJ, et al. Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. Eur Heart J 2002; 23:1931–1937.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Pitt B, Waters D, Brown WV, et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med 1999; 341:70–76.
- Ray KK, Cannon CP, McCabe CH, et al. Early and late benefits of highdose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 2005; 46:1405–1410.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 287:3215–3222.
- Patel TN, Shishehbor MH, Bhatt DL. A review of high-dose statin therapy: targeting cholesterol and inflammation in atherosclerosis. Eur Heart J 2007; 28:664–672.
- Ridker PM. Novel risk factors and markers for coronary disease. Adv Intern Med 2000; 45:391–418.
- Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001; 103:1813–1818.
- Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998; 97:2007–2011.
- Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation 2005; 112:25–31.
- Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007; 49:2129–2138.
- Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001; 286:64–70.
- Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999; 100:230–235.
- Bisoendial RJ, Kastelein JJ, Levels JH, et al. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res 2005; 96:714–716.
- Schwedler SB, Amann K, Wernicke K, et al. Native C-reactive protein increases whereas modified C-reactive protein reduces atherosclerosis in apolipoprotein E-knockout mice. Circulation 2005; 112:1016–1023.
- Pfister R, Hellmich M. Multiple biomarkers and cardiovascular risk. N Engl J Med 2008; 359:760.
- Schunkert H, Samani NJ. Elevated C-reactive protein in atherosclerosis— chicken or egg? N Engl J Med 2008; 359:1953–1955.
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350:1387–1397.
- Kushner I, Sehgal AR. Is high-sensitivity C-reactive protein an effective screening test for cardiovascular risk? Arch Intern Med 2002; 162:867–869.
- Hlatky MA. Expanding the orbit of primary prevention—moving beyond JUPITER. N Engl J Med 2008; 359:2280–2282.
- Shishehbor MH, Hazen SL. Inflammatory and oxidative markers in atherosclerosis: relationship to outcome. Curr Atheroscler Rep 2004; 6:243–250.
- Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 2005; 25:1102–1111.
- Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008; 299:1265–1276.
- Choi SH, Chae A, Miller E, et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J Am Coll Cardiol 2008; 52:24–32.
- Hakonarson H, Thorvaldsson S, Helgadottir A, et al. Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trial. JAMA 2005; 293:2245–2256.
- Ky B, Burke A, Tsimikas S, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol 2008; 51:1653–1662.
- Levy AP, Levy JE, Kalet-Litman S, et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol 2007; 27:134–140.
- Mukherjee D, Topol EJ. Pharmacogenomics in cardiovascular diseases. Curr Probl Cardiol 2003; 28:317–347
- West of Scotland Coronary Prevention Study: identification of highrisk groups and comparison with other cardiovascular intervention trials. Lancet 1996; 348:1339–1342.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol 2007; 49:1753–1762.
- Davidson MH. Rosuvastatin safety: lessons from the FDA review and post-approval surveillance. Expert Opin Drug Saf 2004; 3:547–557.
- Kasiske BL, Wanner C, O’Neill WC. An assessment of statin safety by nephrologists. Am J Cardiol 2006; 97:82C–85C.
- McTaggart F, Buckett L, Davidson R, et al. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol 2001; 87:28B–32B.
- Jacobson TA. Statin safety: lessons from new drug applications for marketed statins. Am J Cardiol 2006; 97:44C–51C.
- Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol 2004; 94:1140–1146.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
KEY POINTS
- LDL-C is the current gold standard diagnostic marker of risk, and elevated values should be aggressively treated in both primary and secondary prevention.
- The optional LDL-C goal of 70 mg/dL for patients at high risk may need to be extended to others at higher global risk, such as those with elevated hs-CRP.
- Although elevated hs-CRP may identify some people with low LDL-C who are nevertheless at higher global risk, more sensitive and specific markers of risk are needed.