User login
More than half of U.S. women enter pregnancy at higher CVD risk
Less than half of women in the United States enter pregnancy in favorable cardiovascular health, new research suggests.
In 2019, among women aged 20 to 44 years with live births in the United States, only 40.2% were in favorable cardiovascular health prior to pregnancy, defined as normal weight, no diabetes, and no hypertension.
Although all regions and states showed a decline in prepregnancy favorable cardiometabolic health, there were significant differences among geographic regions in the country, the authors report. “These data reveal critical deficiencies and geographic disparities in prepregnancy cardiometabolic health,” they conclude.
“One of the things that we know in the U.S. is that the maternal mortality rate has been increasing, and there are significant differences at the state level in both adverse maternal outcomes, such as maternal mortality, as well as adverse pregnancy outcomes,” corresponding author Sadiya S. Khan, MD, MS, FACC, Northwestern University Feinberg School of Medicine, Chicago, told this news organization.
“These outcomes are often related to health factors that predate pregnancy,” Dr. Khan explained, “and the processes that begin at the very, very beginning of conception are informed by health factors prior to pregnancy, in particular cardiometabolic factors like body mass index or obesity, high blood pressure, and diabetes.”
The results were published online on Feb. 14 in a special “Go Red for Women” spotlight issue of Circulation.
Cardiometabolic health factors
Using maternal birth records from live births in the Centers for Disease Control and Prevention Natality Database between 2016 and 2019, the authors analyzed data on 14,174,625 women with live births aged 20 to 44 years. The majority (81.4%) were 20 to 34 years of age, 22.7% were Hispanic or Latina, and 52.7% were non-Hispanic White.
Favorable cardiometabolic health was defined as a BMI of 18 to 24.9 kg/m2, absence of diabetes, and absence of hypertension.
Although all regions and states experienced a decline in favorable cardiometabolic health during the study period of 2016 to 2019, with a drop overall of 3.2% – from 43.5 to 40.2 per 100 live births – it was especially true of the South and Midwest regions.
In 2019, favorable prepregnancy cardiometabolic health was lowest in the South (38.1%) and Midwest (38.8%) and highest in the West (42.2%) and Northeast (43.6%).
State by state, the lowest prevalence of favorable cardiometabolic health was found in Mississippi, at 31.2%, and highest in Utah, at 47.2%.
They also found a correlation between favorable cardiometabolic health and state-level percentages of high-school education or less and enrollment in Medicaid in 2019.
Similar to what has been seen with cardiovascular disease, “we observe that the states with the lowest prevalence of favorable cardiometabolic health were in the Southeast United States,” said Dr. Khan, “and similar geographic variation was observed with some more patterns in education and Medicaid coverage for birth, and these were used as proxies for socioeconomic status in those areas.”
Although Dr. Khan notes that the relationships cannot be determined to be causal from this analysis, she said that “it does suggest that upstream social determinants of health are important determinants of cardiometabolic health.”
Socioeconomic intervention
Dr. Khan noted that policies at the federal and state level can identify ways to “ensure that individuals who are thinking about pregnancy have access to health care and have access to resources, too, from a broad range of health determinants, including housing stability, food security, as well as access to health care be optimized prior to pregnancy.”
The authors note that this analysis may actually overestimate the prevalence of favorable cardiometabolic health, and data on cholesterol, diet, a distinction between type 1 and type 2 diabetes, and physical activity were not available.
Only individuals with live births were included, which could result in the elimination of a potentially high-risk group; however, late pregnancy losses represent less than 0.3% of all pregnancies, they say.
The authors conclude that “future research is needed to equitably improve health prior to pregnancy and quantify the potential benefits in cardiovascular disease outcomes for birthing individuals and their offspring.”
This work was supported by grants from the National Heart, Lung, and Blood Institute and American Heart Association Transformational Project Award awarded to Sadiya S. Khan.
A version of this article first appeared on Medscape.com.
Less than half of women in the United States enter pregnancy in favorable cardiovascular health, new research suggests.
In 2019, among women aged 20 to 44 years with live births in the United States, only 40.2% were in favorable cardiovascular health prior to pregnancy, defined as normal weight, no diabetes, and no hypertension.
Although all regions and states showed a decline in prepregnancy favorable cardiometabolic health, there were significant differences among geographic regions in the country, the authors report. “These data reveal critical deficiencies and geographic disparities in prepregnancy cardiometabolic health,” they conclude.
“One of the things that we know in the U.S. is that the maternal mortality rate has been increasing, and there are significant differences at the state level in both adverse maternal outcomes, such as maternal mortality, as well as adverse pregnancy outcomes,” corresponding author Sadiya S. Khan, MD, MS, FACC, Northwestern University Feinberg School of Medicine, Chicago, told this news organization.
“These outcomes are often related to health factors that predate pregnancy,” Dr. Khan explained, “and the processes that begin at the very, very beginning of conception are informed by health factors prior to pregnancy, in particular cardiometabolic factors like body mass index or obesity, high blood pressure, and diabetes.”
The results were published online on Feb. 14 in a special “Go Red for Women” spotlight issue of Circulation.
Cardiometabolic health factors
Using maternal birth records from live births in the Centers for Disease Control and Prevention Natality Database between 2016 and 2019, the authors analyzed data on 14,174,625 women with live births aged 20 to 44 years. The majority (81.4%) were 20 to 34 years of age, 22.7% were Hispanic or Latina, and 52.7% were non-Hispanic White.
Favorable cardiometabolic health was defined as a BMI of 18 to 24.9 kg/m2, absence of diabetes, and absence of hypertension.
Although all regions and states experienced a decline in favorable cardiometabolic health during the study period of 2016 to 2019, with a drop overall of 3.2% – from 43.5 to 40.2 per 100 live births – it was especially true of the South and Midwest regions.
In 2019, favorable prepregnancy cardiometabolic health was lowest in the South (38.1%) and Midwest (38.8%) and highest in the West (42.2%) and Northeast (43.6%).
State by state, the lowest prevalence of favorable cardiometabolic health was found in Mississippi, at 31.2%, and highest in Utah, at 47.2%.
They also found a correlation between favorable cardiometabolic health and state-level percentages of high-school education or less and enrollment in Medicaid in 2019.
Similar to what has been seen with cardiovascular disease, “we observe that the states with the lowest prevalence of favorable cardiometabolic health were in the Southeast United States,” said Dr. Khan, “and similar geographic variation was observed with some more patterns in education and Medicaid coverage for birth, and these were used as proxies for socioeconomic status in those areas.”
Although Dr. Khan notes that the relationships cannot be determined to be causal from this analysis, she said that “it does suggest that upstream social determinants of health are important determinants of cardiometabolic health.”
Socioeconomic intervention
Dr. Khan noted that policies at the federal and state level can identify ways to “ensure that individuals who are thinking about pregnancy have access to health care and have access to resources, too, from a broad range of health determinants, including housing stability, food security, as well as access to health care be optimized prior to pregnancy.”
The authors note that this analysis may actually overestimate the prevalence of favorable cardiometabolic health, and data on cholesterol, diet, a distinction between type 1 and type 2 diabetes, and physical activity were not available.
Only individuals with live births were included, which could result in the elimination of a potentially high-risk group; however, late pregnancy losses represent less than 0.3% of all pregnancies, they say.
The authors conclude that “future research is needed to equitably improve health prior to pregnancy and quantify the potential benefits in cardiovascular disease outcomes for birthing individuals and their offspring.”
This work was supported by grants from the National Heart, Lung, and Blood Institute and American Heart Association Transformational Project Award awarded to Sadiya S. Khan.
A version of this article first appeared on Medscape.com.
Less than half of women in the United States enter pregnancy in favorable cardiovascular health, new research suggests.
In 2019, among women aged 20 to 44 years with live births in the United States, only 40.2% were in favorable cardiovascular health prior to pregnancy, defined as normal weight, no diabetes, and no hypertension.
Although all regions and states showed a decline in prepregnancy favorable cardiometabolic health, there were significant differences among geographic regions in the country, the authors report. “These data reveal critical deficiencies and geographic disparities in prepregnancy cardiometabolic health,” they conclude.
“One of the things that we know in the U.S. is that the maternal mortality rate has been increasing, and there are significant differences at the state level in both adverse maternal outcomes, such as maternal mortality, as well as adverse pregnancy outcomes,” corresponding author Sadiya S. Khan, MD, MS, FACC, Northwestern University Feinberg School of Medicine, Chicago, told this news organization.
“These outcomes are often related to health factors that predate pregnancy,” Dr. Khan explained, “and the processes that begin at the very, very beginning of conception are informed by health factors prior to pregnancy, in particular cardiometabolic factors like body mass index or obesity, high blood pressure, and diabetes.”
The results were published online on Feb. 14 in a special “Go Red for Women” spotlight issue of Circulation.
Cardiometabolic health factors
Using maternal birth records from live births in the Centers for Disease Control and Prevention Natality Database between 2016 and 2019, the authors analyzed data on 14,174,625 women with live births aged 20 to 44 years. The majority (81.4%) were 20 to 34 years of age, 22.7% were Hispanic or Latina, and 52.7% were non-Hispanic White.
Favorable cardiometabolic health was defined as a BMI of 18 to 24.9 kg/m2, absence of diabetes, and absence of hypertension.
Although all regions and states experienced a decline in favorable cardiometabolic health during the study period of 2016 to 2019, with a drop overall of 3.2% – from 43.5 to 40.2 per 100 live births – it was especially true of the South and Midwest regions.
In 2019, favorable prepregnancy cardiometabolic health was lowest in the South (38.1%) and Midwest (38.8%) and highest in the West (42.2%) and Northeast (43.6%).
State by state, the lowest prevalence of favorable cardiometabolic health was found in Mississippi, at 31.2%, and highest in Utah, at 47.2%.
They also found a correlation between favorable cardiometabolic health and state-level percentages of high-school education or less and enrollment in Medicaid in 2019.
Similar to what has been seen with cardiovascular disease, “we observe that the states with the lowest prevalence of favorable cardiometabolic health were in the Southeast United States,” said Dr. Khan, “and similar geographic variation was observed with some more patterns in education and Medicaid coverage for birth, and these were used as proxies for socioeconomic status in those areas.”
Although Dr. Khan notes that the relationships cannot be determined to be causal from this analysis, she said that “it does suggest that upstream social determinants of health are important determinants of cardiometabolic health.”
Socioeconomic intervention
Dr. Khan noted that policies at the federal and state level can identify ways to “ensure that individuals who are thinking about pregnancy have access to health care and have access to resources, too, from a broad range of health determinants, including housing stability, food security, as well as access to health care be optimized prior to pregnancy.”
The authors note that this analysis may actually overestimate the prevalence of favorable cardiometabolic health, and data on cholesterol, diet, a distinction between type 1 and type 2 diabetes, and physical activity were not available.
Only individuals with live births were included, which could result in the elimination of a potentially high-risk group; however, late pregnancy losses represent less than 0.3% of all pregnancies, they say.
The authors conclude that “future research is needed to equitably improve health prior to pregnancy and quantify the potential benefits in cardiovascular disease outcomes for birthing individuals and their offspring.”
This work was supported by grants from the National Heart, Lung, and Blood Institute and American Heart Association Transformational Project Award awarded to Sadiya S. Khan.
A version of this article first appeared on Medscape.com.
PTSD linked to ischemic heart disease
A study using data from Veterans Health Administration (VHA) electronic medical records shows a significant association between posttraumatic stress disorder (PTSD) among female veterans and an increased risk for incident ischemic heart disease (IHD).
The increased risk for IHD was highest among women younger than 40 with PTSD, and among racial and ethnic minorities.
“These women have been emerging as important targets for cardiovascular prevention, and our study suggests that PTSD may be an important psychosocial risk factor for IHD in these individuals,” wrote the researchers, led by Ramin Ebrahimi, MD, department of medicine, cardiology section, Veterans Affairs Greater Los Angeles Health Care System. “With the number of women veterans growing, it is critical to appreciate the health care needs of this relatively young and diverse patient population.”
The study results also have “important implications for earlier and more aggressive IHD risk assessment, monitoring and management in vulnerable women veterans,” they added. “Indeed, our findings support recent calls for cardiovascular risk screening in younger individuals and for the need to harness a broad range of clinicians who routinely treat younger women to maximize prevention efforts.”
The article was published online in JAMA Cardiology on March 17.
Increasing number of VHA users
“As an interventional cardiologist and the director of the cardiac catheterization laboratory, I noticed a significant number of the patients referred to the cath lab carried a diagnosis of posttraumatic stress disorder,” Dr. Ebrahimi said in an interview. “This intrigued me and started my journey into trying to understand how psychiatric disorders in general, and PTSD, may impact/interact with cardiovascular disorders,” he added.
The number of female veterans in the military has been increasing, and they now make up about 10% of the 20 million American veterans; that number is projected to exceed 2.2 million in the next 20 years, the authors wrote. Female veterans are also the fastest growing group of users of the VHA, they added.
IHD is the leading cause of death in women in the United States, despite the advancements in prevention and treatment. Although women are twice as likely to develop PTSD as are men, and it is even more likely in female veterans, much of the research has predominately been on male veterans, the authors wrote.
For this retrospective study, which used data from the VHA Corporate Data Warehouse, the authors examined a cohort of female veterans who were 18 years or older who had used the VHA health care system between Jan. 1, 2000, and Dec. 31, 2017.
Of the 828,997 female veterans, 151,030 had PTSD. Women excluded from the study were those who did not have any clinical encounters after their index visit, participants who had a diagnosis of IHD at or before the index visit, and those with incident IHD within 90 days of the index visit, allowing time between a PTSD diagnosis and IHD.
Propensity score matching on age at index visit, the number of previous visits, and the presence of traditional and female-specific cardiovascular risk factors, as well as mental and physical health conditions, was conducted to identify female veterans ever diagnosed with PTSD, who were matched in a 1:2 ratio to those never diagnosed with PTSD. In all, 132,923 women with PTSD and 265,846 women without PTSD were included, and data were analyzed for the period of Oct. 1, 2018, to Oct. 30, 2020.
IHD was defined as new-onset coronary artery disease, angina, or myocardial infarction–based ICD-9 and ICD-10 diagnostic codes. Age, race, and ethnicity were self-reported.
The analytic sample consisted of relatively young female veterans (mean [SD] age at baseline, 40.1 [12.2] years) of various races (White, 57.6%; Black, 29.8%) and ethnicities, the authors reported.
Of the 9,940 women who experienced incident IHD during follow-up, 5,559 did not have PTSD (2.1% of the overall population examined) and 4,381 had PTSD (3.3%). PTSD was significantly associated with an increased risk for IHD. Over the median follow-up of 4.9 years, female veterans with PTSD had a 44% higher rate of developing incident IHD compared with the female veterans without PTSD (hazard ratio [HR], 1.44; 95% confidence interval [CI], 1.38-1.50).
In addition, those with PTSD who developed IHD were younger at diagnosis (mean [SD] age, 55.5 [9.7]) than were patients without PTSD (mean [SD] age, 57.8 [10.7]). Effect sizes were largest in the group younger than 40 years (HR, 1.72; 95% CI, 1.55-1.90) and decreased for older participants (HR for those ≥60 years, 1.24; 95% CI, 1.12-1.38)
The authors found a 49% to 66% increase in risk for IHD associated with PTSD in Black women (HR, 1.49; 95% CI, 1.38-1.62) and those identified as non-White and non-Black (HR, 1.66; 95%, 1.33-2.08).
Women of all ethnic groups with PTSD were at higher risk of developing IHD, but this was especially true for Hispanic/Latina women (HR, 1.50; 95% CI 1.22-1.84), they noted.
The authors reported some limitations to their findings. The analytic sample could result in a lower ascertainment of certain conditions, such as psychiatric disorders, they wrote. Substance disorders were low in this study, possibly because of the younger age of female veterans in the sample. Because this study used VHA electronic medical records data, medical care outside of the VHA that was not paid for by the VHA could not be considered.
In addition, although this study used a large sample of female veterans, the findings cannot be generalized to female veterans outside of the VHA system, nonveteran women, or men, the researchers wrote.
A call to action
In an accompanying comment, Beth E. Cohen, MD, of the University of California, San Francisco, and the San Francisco Veterans Affairs Health Care System, points out that the physical implications for psychosocial conditions, including depression and PTSD, have been recognized for quite some time. For example, results of the INTERHEART case-control study of 30,000 people showed stress, depression, and stressful life events accounted for one-third the population-attributable risk for myocardial infarction.
As was also noted by Dr. Ebrahimi and colleagues, much of the current research has been on male veterans, yet types of trauma differ among genders; women experience higher rates of military sexual trauma but lower rates of combat trauma, Dr. Cohen wrote. The PTSD symptoms, trajectory, and biological effects can differ for women and men, as can the pathogenesis, presentation, and outcomes of cardiovascular disease (CVD).
These findings, she said, “are an important extension of the prior literature and represent the largest study in female veterans to date. Although methods differ across studies, the magnitude of risk associated with PTSD was consistent with that found in prior studies of male veterans and nonveteran samples.”
The assessment of age-specific risk is also a strength of the study, “and has implications for clinical practice, because PTSD-associated risk was greatest in a younger group in whom CVD may be overlooked.”
Dr. Cohen addressed the limitations outlined by the authors, including ascertainment bias, severity of PTSD symptoms, and their chronicity, but added that “even in the context of these limitations, this study illustrates the importance of PTSD to the health of women veterans and the additional work needed to reduce their CVD risk.”
Clinical questions remain, she added. Screens for PTSD are widely used in the VHA, yet no studies have examined whether screening or early detection decrease CVD risk. In addition, no evidence suggests that screening for or treatment of PTSD improves cardiovascular outcomes.
“Given the challenges of answering these questions in observational studies, it will be important to incorporate measures of CVD risk and outcomes in trials of behavioral and medical therapies for patients with PTSD,” she wrote.
She added that collaborations among multidisciplinary patient care teams will be important. “The findings of this study represent a call to action for this important work to understand the cardiovascular effects of PTSD and improve the health and well-being of women veterans,” Dr. Cohen concluded.
This research was supported by Investigator-Initiated Research Award from the Department of Defense U.S. Army Medical Research and Material Command Congressionally Directed Medical Research Programs (Dr. Ebrahimi) and in part by grants from the VA Informatics and Computing Infrastructure and the Offices of Research and Development at the Northport, Durham, and Greater Los Angeles Veterans Affairs medical centers. Dr. Ebrahimi reported receiving grants from the Department of Defense during the conduct of the study. Disclosures for other authors are available in the paper. Dr. Cohen reports no disclosures.
A version of this article first appeared on Medscape.com.
A study using data from Veterans Health Administration (VHA) electronic medical records shows a significant association between posttraumatic stress disorder (PTSD) among female veterans and an increased risk for incident ischemic heart disease (IHD).
The increased risk for IHD was highest among women younger than 40 with PTSD, and among racial and ethnic minorities.
“These women have been emerging as important targets for cardiovascular prevention, and our study suggests that PTSD may be an important psychosocial risk factor for IHD in these individuals,” wrote the researchers, led by Ramin Ebrahimi, MD, department of medicine, cardiology section, Veterans Affairs Greater Los Angeles Health Care System. “With the number of women veterans growing, it is critical to appreciate the health care needs of this relatively young and diverse patient population.”
The study results also have “important implications for earlier and more aggressive IHD risk assessment, monitoring and management in vulnerable women veterans,” they added. “Indeed, our findings support recent calls for cardiovascular risk screening in younger individuals and for the need to harness a broad range of clinicians who routinely treat younger women to maximize prevention efforts.”
The article was published online in JAMA Cardiology on March 17.
Increasing number of VHA users
“As an interventional cardiologist and the director of the cardiac catheterization laboratory, I noticed a significant number of the patients referred to the cath lab carried a diagnosis of posttraumatic stress disorder,” Dr. Ebrahimi said in an interview. “This intrigued me and started my journey into trying to understand how psychiatric disorders in general, and PTSD, may impact/interact with cardiovascular disorders,” he added.
The number of female veterans in the military has been increasing, and they now make up about 10% of the 20 million American veterans; that number is projected to exceed 2.2 million in the next 20 years, the authors wrote. Female veterans are also the fastest growing group of users of the VHA, they added.
IHD is the leading cause of death in women in the United States, despite the advancements in prevention and treatment. Although women are twice as likely to develop PTSD as are men, and it is even more likely in female veterans, much of the research has predominately been on male veterans, the authors wrote.
For this retrospective study, which used data from the VHA Corporate Data Warehouse, the authors examined a cohort of female veterans who were 18 years or older who had used the VHA health care system between Jan. 1, 2000, and Dec. 31, 2017.
Of the 828,997 female veterans, 151,030 had PTSD. Women excluded from the study were those who did not have any clinical encounters after their index visit, participants who had a diagnosis of IHD at or before the index visit, and those with incident IHD within 90 days of the index visit, allowing time between a PTSD diagnosis and IHD.
Propensity score matching on age at index visit, the number of previous visits, and the presence of traditional and female-specific cardiovascular risk factors, as well as mental and physical health conditions, was conducted to identify female veterans ever diagnosed with PTSD, who were matched in a 1:2 ratio to those never diagnosed with PTSD. In all, 132,923 women with PTSD and 265,846 women without PTSD were included, and data were analyzed for the period of Oct. 1, 2018, to Oct. 30, 2020.
IHD was defined as new-onset coronary artery disease, angina, or myocardial infarction–based ICD-9 and ICD-10 diagnostic codes. Age, race, and ethnicity were self-reported.
The analytic sample consisted of relatively young female veterans (mean [SD] age at baseline, 40.1 [12.2] years) of various races (White, 57.6%; Black, 29.8%) and ethnicities, the authors reported.
Of the 9,940 women who experienced incident IHD during follow-up, 5,559 did not have PTSD (2.1% of the overall population examined) and 4,381 had PTSD (3.3%). PTSD was significantly associated with an increased risk for IHD. Over the median follow-up of 4.9 years, female veterans with PTSD had a 44% higher rate of developing incident IHD compared with the female veterans without PTSD (hazard ratio [HR], 1.44; 95% confidence interval [CI], 1.38-1.50).
In addition, those with PTSD who developed IHD were younger at diagnosis (mean [SD] age, 55.5 [9.7]) than were patients without PTSD (mean [SD] age, 57.8 [10.7]). Effect sizes were largest in the group younger than 40 years (HR, 1.72; 95% CI, 1.55-1.90) and decreased for older participants (HR for those ≥60 years, 1.24; 95% CI, 1.12-1.38)
The authors found a 49% to 66% increase in risk for IHD associated with PTSD in Black women (HR, 1.49; 95% CI, 1.38-1.62) and those identified as non-White and non-Black (HR, 1.66; 95%, 1.33-2.08).
Women of all ethnic groups with PTSD were at higher risk of developing IHD, but this was especially true for Hispanic/Latina women (HR, 1.50; 95% CI 1.22-1.84), they noted.
The authors reported some limitations to their findings. The analytic sample could result in a lower ascertainment of certain conditions, such as psychiatric disorders, they wrote. Substance disorders were low in this study, possibly because of the younger age of female veterans in the sample. Because this study used VHA electronic medical records data, medical care outside of the VHA that was not paid for by the VHA could not be considered.
In addition, although this study used a large sample of female veterans, the findings cannot be generalized to female veterans outside of the VHA system, nonveteran women, or men, the researchers wrote.
A call to action
In an accompanying comment, Beth E. Cohen, MD, of the University of California, San Francisco, and the San Francisco Veterans Affairs Health Care System, points out that the physical implications for psychosocial conditions, including depression and PTSD, have been recognized for quite some time. For example, results of the INTERHEART case-control study of 30,000 people showed stress, depression, and stressful life events accounted for one-third the population-attributable risk for myocardial infarction.
As was also noted by Dr. Ebrahimi and colleagues, much of the current research has been on male veterans, yet types of trauma differ among genders; women experience higher rates of military sexual trauma but lower rates of combat trauma, Dr. Cohen wrote. The PTSD symptoms, trajectory, and biological effects can differ for women and men, as can the pathogenesis, presentation, and outcomes of cardiovascular disease (CVD).
These findings, she said, “are an important extension of the prior literature and represent the largest study in female veterans to date. Although methods differ across studies, the magnitude of risk associated with PTSD was consistent with that found in prior studies of male veterans and nonveteran samples.”
The assessment of age-specific risk is also a strength of the study, “and has implications for clinical practice, because PTSD-associated risk was greatest in a younger group in whom CVD may be overlooked.”
Dr. Cohen addressed the limitations outlined by the authors, including ascertainment bias, severity of PTSD symptoms, and their chronicity, but added that “even in the context of these limitations, this study illustrates the importance of PTSD to the health of women veterans and the additional work needed to reduce their CVD risk.”
Clinical questions remain, she added. Screens for PTSD are widely used in the VHA, yet no studies have examined whether screening or early detection decrease CVD risk. In addition, no evidence suggests that screening for or treatment of PTSD improves cardiovascular outcomes.
“Given the challenges of answering these questions in observational studies, it will be important to incorporate measures of CVD risk and outcomes in trials of behavioral and medical therapies for patients with PTSD,” she wrote.
She added that collaborations among multidisciplinary patient care teams will be important. “The findings of this study represent a call to action for this important work to understand the cardiovascular effects of PTSD and improve the health and well-being of women veterans,” Dr. Cohen concluded.
This research was supported by Investigator-Initiated Research Award from the Department of Defense U.S. Army Medical Research and Material Command Congressionally Directed Medical Research Programs (Dr. Ebrahimi) and in part by grants from the VA Informatics and Computing Infrastructure and the Offices of Research and Development at the Northport, Durham, and Greater Los Angeles Veterans Affairs medical centers. Dr. Ebrahimi reported receiving grants from the Department of Defense during the conduct of the study. Disclosures for other authors are available in the paper. Dr. Cohen reports no disclosures.
A version of this article first appeared on Medscape.com.
A study using data from Veterans Health Administration (VHA) electronic medical records shows a significant association between posttraumatic stress disorder (PTSD) among female veterans and an increased risk for incident ischemic heart disease (IHD).
The increased risk for IHD was highest among women younger than 40 with PTSD, and among racial and ethnic minorities.
“These women have been emerging as important targets for cardiovascular prevention, and our study suggests that PTSD may be an important psychosocial risk factor for IHD in these individuals,” wrote the researchers, led by Ramin Ebrahimi, MD, department of medicine, cardiology section, Veterans Affairs Greater Los Angeles Health Care System. “With the number of women veterans growing, it is critical to appreciate the health care needs of this relatively young and diverse patient population.”
The study results also have “important implications for earlier and more aggressive IHD risk assessment, monitoring and management in vulnerable women veterans,” they added. “Indeed, our findings support recent calls for cardiovascular risk screening in younger individuals and for the need to harness a broad range of clinicians who routinely treat younger women to maximize prevention efforts.”
The article was published online in JAMA Cardiology on March 17.
Increasing number of VHA users
“As an interventional cardiologist and the director of the cardiac catheterization laboratory, I noticed a significant number of the patients referred to the cath lab carried a diagnosis of posttraumatic stress disorder,” Dr. Ebrahimi said in an interview. “This intrigued me and started my journey into trying to understand how psychiatric disorders in general, and PTSD, may impact/interact with cardiovascular disorders,” he added.
The number of female veterans in the military has been increasing, and they now make up about 10% of the 20 million American veterans; that number is projected to exceed 2.2 million in the next 20 years, the authors wrote. Female veterans are also the fastest growing group of users of the VHA, they added.
IHD is the leading cause of death in women in the United States, despite the advancements in prevention and treatment. Although women are twice as likely to develop PTSD as are men, and it is even more likely in female veterans, much of the research has predominately been on male veterans, the authors wrote.
For this retrospective study, which used data from the VHA Corporate Data Warehouse, the authors examined a cohort of female veterans who were 18 years or older who had used the VHA health care system between Jan. 1, 2000, and Dec. 31, 2017.
Of the 828,997 female veterans, 151,030 had PTSD. Women excluded from the study were those who did not have any clinical encounters after their index visit, participants who had a diagnosis of IHD at or before the index visit, and those with incident IHD within 90 days of the index visit, allowing time between a PTSD diagnosis and IHD.
Propensity score matching on age at index visit, the number of previous visits, and the presence of traditional and female-specific cardiovascular risk factors, as well as mental and physical health conditions, was conducted to identify female veterans ever diagnosed with PTSD, who were matched in a 1:2 ratio to those never diagnosed with PTSD. In all, 132,923 women with PTSD and 265,846 women without PTSD were included, and data were analyzed for the period of Oct. 1, 2018, to Oct. 30, 2020.
IHD was defined as new-onset coronary artery disease, angina, or myocardial infarction–based ICD-9 and ICD-10 diagnostic codes. Age, race, and ethnicity were self-reported.
The analytic sample consisted of relatively young female veterans (mean [SD] age at baseline, 40.1 [12.2] years) of various races (White, 57.6%; Black, 29.8%) and ethnicities, the authors reported.
Of the 9,940 women who experienced incident IHD during follow-up, 5,559 did not have PTSD (2.1% of the overall population examined) and 4,381 had PTSD (3.3%). PTSD was significantly associated with an increased risk for IHD. Over the median follow-up of 4.9 years, female veterans with PTSD had a 44% higher rate of developing incident IHD compared with the female veterans without PTSD (hazard ratio [HR], 1.44; 95% confidence interval [CI], 1.38-1.50).
In addition, those with PTSD who developed IHD were younger at diagnosis (mean [SD] age, 55.5 [9.7]) than were patients without PTSD (mean [SD] age, 57.8 [10.7]). Effect sizes were largest in the group younger than 40 years (HR, 1.72; 95% CI, 1.55-1.90) and decreased for older participants (HR for those ≥60 years, 1.24; 95% CI, 1.12-1.38)
The authors found a 49% to 66% increase in risk for IHD associated with PTSD in Black women (HR, 1.49; 95% CI, 1.38-1.62) and those identified as non-White and non-Black (HR, 1.66; 95%, 1.33-2.08).
Women of all ethnic groups with PTSD were at higher risk of developing IHD, but this was especially true for Hispanic/Latina women (HR, 1.50; 95% CI 1.22-1.84), they noted.
The authors reported some limitations to their findings. The analytic sample could result in a lower ascertainment of certain conditions, such as psychiatric disorders, they wrote. Substance disorders were low in this study, possibly because of the younger age of female veterans in the sample. Because this study used VHA electronic medical records data, medical care outside of the VHA that was not paid for by the VHA could not be considered.
In addition, although this study used a large sample of female veterans, the findings cannot be generalized to female veterans outside of the VHA system, nonveteran women, or men, the researchers wrote.
A call to action
In an accompanying comment, Beth E. Cohen, MD, of the University of California, San Francisco, and the San Francisco Veterans Affairs Health Care System, points out that the physical implications for psychosocial conditions, including depression and PTSD, have been recognized for quite some time. For example, results of the INTERHEART case-control study of 30,000 people showed stress, depression, and stressful life events accounted for one-third the population-attributable risk for myocardial infarction.
As was also noted by Dr. Ebrahimi and colleagues, much of the current research has been on male veterans, yet types of trauma differ among genders; women experience higher rates of military sexual trauma but lower rates of combat trauma, Dr. Cohen wrote. The PTSD symptoms, trajectory, and biological effects can differ for women and men, as can the pathogenesis, presentation, and outcomes of cardiovascular disease (CVD).
These findings, she said, “are an important extension of the prior literature and represent the largest study in female veterans to date. Although methods differ across studies, the magnitude of risk associated with PTSD was consistent with that found in prior studies of male veterans and nonveteran samples.”
The assessment of age-specific risk is also a strength of the study, “and has implications for clinical practice, because PTSD-associated risk was greatest in a younger group in whom CVD may be overlooked.”
Dr. Cohen addressed the limitations outlined by the authors, including ascertainment bias, severity of PTSD symptoms, and their chronicity, but added that “even in the context of these limitations, this study illustrates the importance of PTSD to the health of women veterans and the additional work needed to reduce their CVD risk.”
Clinical questions remain, she added. Screens for PTSD are widely used in the VHA, yet no studies have examined whether screening or early detection decrease CVD risk. In addition, no evidence suggests that screening for or treatment of PTSD improves cardiovascular outcomes.
“Given the challenges of answering these questions in observational studies, it will be important to incorporate measures of CVD risk and outcomes in trials of behavioral and medical therapies for patients with PTSD,” she wrote.
She added that collaborations among multidisciplinary patient care teams will be important. “The findings of this study represent a call to action for this important work to understand the cardiovascular effects of PTSD and improve the health and well-being of women veterans,” Dr. Cohen concluded.
This research was supported by Investigator-Initiated Research Award from the Department of Defense U.S. Army Medical Research and Material Command Congressionally Directed Medical Research Programs (Dr. Ebrahimi) and in part by grants from the VA Informatics and Computing Infrastructure and the Offices of Research and Development at the Northport, Durham, and Greater Los Angeles Veterans Affairs medical centers. Dr. Ebrahimi reported receiving grants from the Department of Defense during the conduct of the study. Disclosures for other authors are available in the paper. Dr. Cohen reports no disclosures.
A version of this article first appeared on Medscape.com.
Ventricular tachycardia storm responds to magnetic stimulation
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.
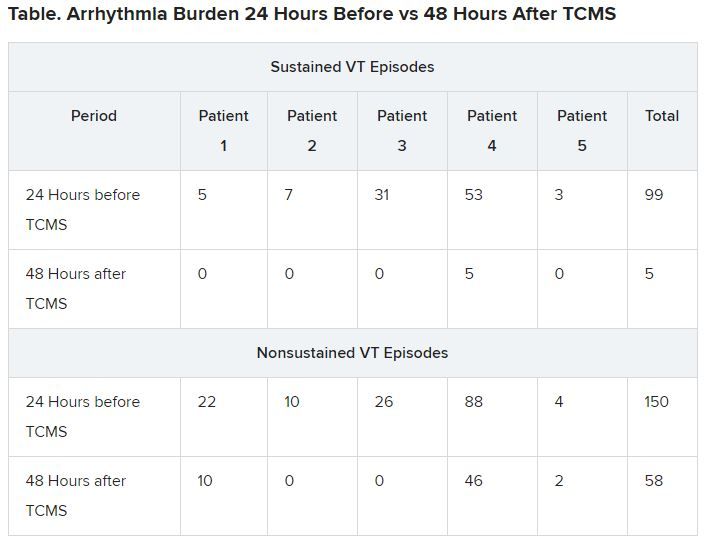
In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.

In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.

In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
