User login
Alzheimer disease: Time to improve its diagnosis and treatment
The number of patients with Alzheimer disease, the most common cause of disability in the elderly, is about to rise dramatically. More than 5 million people in the United States are affected, and by 2050 this figure may rise to between 11 and 16 million.1 The prevalence doubles every 5 years from ages 65 to 85, so that Alzheimer disease affects 30% to 50% of all people at age 85.1,2
Primary care physicians bear the brunt of diagnosing and treating all these patients,3 requiring that they have the training to meet this critical public health problem.
But diagnosing this disease is not easy. In the early stages, it can be difficult to distinguish between the decline in certain cognitive functions due to normal aging (eg, name recall) and the mild cognitive impairment that often precedes Alzheimer disease.
Once a patient is diagnosed with Alzheimer disease, there needs to be a realistic discussion with the patient and family about what treatment with different drugs can—and cannot—accomplish.
ALZHEIMER DISEASE DIAGNOSIS: THE EARLIER, THE BETTER
While much has been accomplished in Alzheimer disease research in the last 20 years, a great deal remains to be done to improve its diagnosis and treatment. There is increasing evidence that early diagnosis of Alzheimer disease will be key to maximizing treatment benefits. But too often, patients are diagnosed in later stages of the disease, when disabling symptoms and neuropathologic changes have become well established.
Mild cognitive impairment: A predementia phase
The pathologic changes of Alzheimer disease typically begin many years before its clinical signs are apparent. Most patients pass through a predementia phase called mild cognitive impairment, with early memory loss but with relatively well-preserved activities of daily living.
From 6% to 25% of patients with mild cognitive impairment progress to dementia annually, a rate far higher than the incidence rate in the general population of 0.3% to 3.9% per year, depending on age.4,5 Therefore, patients with mild cognitive impairment are a good population in which to test interventions to prevent dementia.6,7
The concept of mild cognitive impairment is controversial because it is a transitional stage between normal aging and dementia rather than a distinct pathologic entity.8 Moreover, in some large community-based studies,9,10 a sizeable number of people with mild cognitive impairment reverted to normal cognitive function over 5 years, suggesting that mild cognitive impairment may be unstable over time.
Are other factors causing the dementia?
The Diagnostic and Statistical Manual IV-Text Revision11 defines dementia as memory loss and at least one other area of cognitive impairment, not due to delirium, that interferes with social and occupational functioning. Alzheimer disease is the most common cause of dementia in the United States.1
Still, Alzheimer disease does not typically exist in isolation. For example, while Alzheimer disease was the predominant cause of dementia in a recent postmortem series, 38% of dementia cases featured Alzheimer disease with lacunar infarction.12 Accordingly, clinicians must consider factors other than Alzheimer disease that could contribute to (or even fully account for) the complaints or observed deficits.
Is it Alzheimer disease or normal aging?
Although cognitive impairment and changes in behavior are common in the elderly, they are not a normal part of aging. Like other chronic disorders associated with aging, Alzheimer disease can be diagnosed and treated. Cognitive impairment may come to light when the patient or a family member reports a problem or the clinician asks about problems or observes signs of impairment in the office. The cognitive difficulties should be taken seriously, and their impact on daily functioning should be evaluated.
Certain cognitive functions such as mental flexibility and speed of processing decline in normal aging,13 and many older people report cognitive symptoms. Therefore, it is important to differentiate mild age-associated cognitive changes from the beginning of a cognitive disorder such as Alzheimer disease. This can be difficult because the cognitive complaints of normal aging overlap with the symptoms of early Alzheimer disease, and there are no clear rules for distinguishing the two.13
Clues that a more serious problem exists may come from details such as a history of decline in cognitive abilities, involvement of more than one domain, and the extent to which the problem disrupts daily functioning. 13 When evaluating the nature and severity of a cognitive disorder, one must take into account the patient’s level of education, premorbid intellectual and occupational functioning, and concurrent medical and psychiatric conditions. Clinicians must also consider the impact that medications, possible substance abuse, and language and culture of origin have on cognition.
The most common cognitive complaints in the elderly tend to be related to working memory (eg, recalling the name of a recent acquaintance or a telephone number that was just looked up).14 Other common complaints center on the speed of mental processing (eg, thinking quickly), memory (eg, recalling the name of an old acquaintance, or remembering where objects were placed or why one entered a room), executive function (eg, multitasking or planning a series of events), attention, and concentration.14
People with age-related cognitive changes can learn new information and recall previously learned information, although they may do so less rapidly and less efficiently. Furthermore, age-related cognitive change is not substantially progressive and does not significantly impair daily functioning.14 Nevertheless, complaints of cognitive changes should be monitored, since several studies showed that elderly people with cognitive complaints have an increased risk of developing dementia.15,16
Key warning signs
The Alzheimer’s Association17 lists 10 key warning signs of Alzheimer disease:
- Memory loss
- Difficulty performing familiar tasks
- Problems with language
- Disorientation to time and place
- Poor or decreased judgment
- Problems with abstract thought
- Misplacing things
- Changes in mood or behavior
- Changes in personality
- Loss of initiative.
As annotated on the Alzheimer’s Association Web site,17 the list also highlights key differences between normal aging and more serious symptoms of possible Alzheimer disease. For example, patients with Alzheimer disease are more likely to forget entire experiences and not remember them later, whereas normal elderly may forget parts of events and then recall the missing details later. Also, patients with Alzheimer disease are more likely to lose the ability to complete familiar tasks or to follow written or spoken directions. Additional signs that a more serious cognitive problem exists include misplacing items so often that it interferes with daily activities, frequently losing the thread of conversations, and repeating the same questions, stories, or comments within a short time without being aware of it.18
Talk to a family member
Interviewing a reliable informant who knows the patient well is extremely helpful for determining the presence and extent of a cognitive problem. This is particularly important because even patients with mild cognitive impairment may have impaired awareness of their memory problems and may underestimate20 or overestimate21 the problem.
Ask the informant about symptoms such as being repetitive, misplacing items, or having trouble with finding words or names, remembering to take medication, managing finances, navigating while driving, or performing multiple tasks or all steps of a task. A change in behavior may be the first sign of a cognitive disorder, so the patient and informant should also be asked about signs of irritability, anxiety, increased social isolation, and decline in motivation.
Screening tests
Memory can be tested with a three- or four-word recall test during the physical examination, with the addition of a clock-drawing task,22 or can be assessed with a composite cognitive measure. For example:
The Mini-Mental State Exam (MMSE)23 can be given by the clinician or a trained member of the office staff. People with Alzheimer disease show progressive disability and a predictable rate of decline of approximately 2.8 points on the MMSE per year, with slower decline in the milder stages and faster decline in the moderate and severe stages of the illness.
The Montreal Cognitive Assessment Battery (MOCA, www.mocatest.org),24 is a new cognitive screening test with a 30-point format similar to that of the MMSE. It includes a five-word recall, clock-drawing, and executive and visuospatial items that make it more sensitive for mild cognitive impairment and vascular dementia.
The Alzheimer’s Disease 8 (AD8),25 a sensitive eight-question scale developed at Washington University, St. Louis, MO, can be completed by an informant in the waiting room.
New computerized screening measures are being developed that can be completed online or in the waiting room, and some simulate practical tasks such as using an automated teller machine and driving.
Care should be taken when interpreting performance on screening tests in patients who have very low or very high education levels, who are not tested in their native language, or who have physical or sensory deficits that might limit their performance.
Brain imaging and other tests
Patients with evidence of cognitive impairment should undergo a structural brain imaging test such as noncontrast computed tomography (CT) or magnetic resonance imaging (MRI) to evaluate for changes consistent with Alzheimer disease and to help rule out alternative causes of the cognitive impairment.26,27
Neuropsychologic testing can be done by a dementia specialist, who can also help with diagnosis and treatment.
Positron emission tomography (PET) using fluorodeoxyglucose shows patterns of brain metabolism and can help differentiate Alzheimer disease from non-Alzheimer dementia, as patients with the former typically show hypometabolism in the temporal and parietal cortices.28
Quantitative MRI and PET amyloid imaging are exciting new techniques currently being developed to diagnose Alzheimer disease earlier in clinical practice.29,30
Cerebrospinal fluid markers. A decrease in the amyloid beta 1–42 peptide and an increase in the tau and phosphotau proteins may be the earliest signs of Alzheimer disease.31,32 However, before these tests can be widely used in clinical practice, their sensitivity and specificity need to be established, people’s reluctance to undergo lumbar puncture will have to be overcome, and third-party reimbursement will have to be obtained.
Genetic factors also play an important role in the development of Alzheimer disease. The apolipoprotein E4 (ApoE4) allele is a marker for Alzheimer disease. People of European descent who possess one copy of the allele have three times the risk (with onset typically in their 70s), and individuals who are homozygous have 15 times the risk (with typical onset in their 60s), compared with people lacking ApoE4.33,34 This test is commercially available but is still considered a research tool.
CURRENT AND FUTURE TREATMENTS
Five drugs have been approved for treating Alzheimer disease: four cholinesterase inhibitors approved for mild to moderate disease and a glutamate N-methyl D-aspartate (NMDA) antagonist approved for moderate to severe disease.
Cholinesterase inhibitors for mild to moderate disease
The cholinesterase inhibitors tend to stabilize memory during the first year of treatment, and they may make the subsequent decline more gradual. The four current drugs have similar efficacy, so the choice is usually based on tolerability and ease of use.
Tacrine (Cognex) is rarely used because it must be taken four times a day, it can cause gastrointestinal adverse effects, and it can raise hepatic enzyme levels.
Donepezil (Aricept) is the drug most often prescribed because it can be taken once daily, a major benefit in older patients with memory loss. Also, its starting dose (5 mg) is a therapeutic dose. Donepezil was also recently approved for the treatment of severe Alzheimer disease on the basis of positive results in trials in patients with moderate to severe disease.35,36
Galantamine (Razadyne) comes in an extended-release formulation that can be taken once daily.
Rivastigmine (Exelon) is taken twice a day with food to reduce the risk of gastrointestinal adverse effects. It is also now available as a daily patch, which has a more favorable adverse-effect profile than oral rivastigmine.37
Memantine for moderate to severe disease
Memantine (Namenda), an NMDA antagonist, is approved for moderate to severe Alzheimer disease. The approval was based on a trial in which patients with advanced Alzheimer disease who received memantine monotherapy showed less decline in cognition and function after 6 months than those who received placebo,38 and another trial in which patients who received the combination of donepezil plus memantine showed more benefit than with donepezil alone.39
A treatment strategy
Recent guidelines recommend starting treatment with a cholinesterase inhibitor soon after Alzheimer disease is diagnosed and titrating the dose, as tolerated, to the high end of the therapeutic range.40 Once patients decline to the moderate stage of the illness, usually with an MMSE score of 10 to 20, memantine should be added and titrated upward to 10 mg twice a day. The medications should be continued as long as they are tolerated and the clinician feels there is some evidence they are helping.
The main benefit of the cholinesterase inhibitors in clinical trials is an attenuation of decline over time rather than an improvement in cognitive or behavioral symptoms. This should be considered when judging whether there has been a positive effect. It is also important to discuss this point with patients and their families, who may expect improvement rather than relative stability. Benefits of these drugs in later stages of the illness usually involve better recognition and engagement with family members and people around them and less severe behavioral disturbances, making care easier.36,41–43
Will drug therapy help in mild cognitive impairment?
Currently, no drugs are approved by the US Food and Drug Administration for patients with mild cognitive impairment, and the use of cholinesterase inhibitors in this population may not be reimbursed.
Six trials of cholinesterase inhibitors for mild cognitive impairment have been completed.44–47 On the whole, donepezil, rivastigmine, and galantamine had no effect on the primary end points in these trials, but they had some effects on some secondary ones.
In the Alzheimer Disease Cooperative Study,44 donepezil had no effect on the rate of progression from mild cognitive impairment to Alzheimer disease over the entire 3 years of the study, but it did reduce the rate in the first year of treatment. Moreover, the subgroup with one or two ApoE4 alleles benefitted over the entire 3 years.
A 24-week trial of donepezil in patients with mild cognitive impairment45 had negative results with regard to the selected study end points (two standardized tests), but there was evidence of cognitive benefit with donepezil on secondary measures such as the Alzheimer’s Disease Cognitive Assessment Scale-Cognitive Subscale,48 a widely used cognitive measure in Alzheimer disease trials, and in patients’ self-assessment of their memory.
One should discuss the risks and benefits of cholinesterase treatment with patients with mild cognitive impairment in whom underlying Alzheimer disease is strongly suspected.
Addressing behavioral problems
Behavioral problems are often the most disturbing symptoms in dementia, often leading to higher levels of care.
Apathy is the most common behavioral symptom in Alzheimer disease, increasing with disease severity.49 There is no approved treatment for these apathetic symptoms, though methylphenidate (Ritalin) and modafanil (Provigil) are being tested in small clinical trials.
Depression and irritability are common and may respond well to low doses of serotonin reuptake inhibitors.
Agitation and psychosis are distressing and are likely to overwhelm the caregiver’s ability to cope. Recent studies have raised concern about the safety and efficacy of atypical neuroleptics in patients with dementia and suggest that these drugs be used with careful monitoring.50
A safe, calm, predictable environment
Patients with Alzheimer disease function best in an environment that is safe, calm, and predictable, and their caregivers require ongoing support and education to develop realistic expectations throughout the course of the illness.
Behavioral treatments for problematic behaviors for which supportive evidence exists include reduction of environmental stressors and behavioral management of problematic behaviors.51–53 Such interventions typically include carefully observing and recording the problematic behavior, including its antecedents and situations under which it is most likely to occur, and then modifying the physical and interpersonal environment and schedule to reduce its occurrence.51
A challenge to the use of behavioral interventions in dementia is that the patient’s cognitive functioning is gradually declining, and this may require adjustments of interventions with time and in response to new behaviors that emerge.51 Referral to a behavioral specialist such as a geriatric psychiatrist may be helpful in managing disruptive and hard-to-treat behavioral problems.
Amyloid-lowering drugs are being tested
New disease-modifying agents are being tested to see if they delay disability, promote independence, and improve quality of life. Chief among these are compounds that reduce brain amyloid.
The amyloid cascade hypothesis is the current prevailing view of the pathogenesis of Alzheimer disease.56 Small molecules of extracellular amyloid are deposited in the brain early in the course of the disease. These oligomers of beta-amyloid gradually coalesce into fibrillar sheets that form the core of amyloid plaques. Amyloid invokes an immune response and stimulates the hyperphosphorylation of tau into intraneuronal neurofibrillary tangles. The accumulation of these tangles contributes to neuronal and synaptic loss, which correlates with dementia and disability.
The current disease-modifying strategies are designed to decrease the production of amyloid, inhibit fibrillogenesis, and promote clearance of the toxic amyloid beta 1–42 fragment. We should note, however, that the correlation between amyloid burden and clinical decline is not strong, and that lowering brain amyloid may not produce a measurable clinical benefit.57
Large-scale trials are being conducted with agents that modulate and inhibit gamma secretase, an enzyme involved in cleaving the amyloid precursor protein into the toxic fragment of beta amyloid.58 Early results show improvement in cognition and decreased amyloid levels in transgenic animals treated with these agents.
A recently completed phase III trial of tramiprosate, a glycosaminoglycan receptor inhibitor that interferes with fibrillization of amyloid, did not show positive results, but other antifibrillization agents are currently being tested.59,60
Exciting immunotherapeutic approaches that target the toxic fragment of beta amyloid have been developed. In the active vaccine approach, a small fragment of beta amyloid is injected to stimulate the production of beta amyloid antibodies to lower brain amyloid levels. However, although active vaccines are designed primarily to stimulate a B-cell response, they can cause adverse effects through unplanned stimulation of T cells.
Passive immunization with a monoclonal antibody against beta amyloid may be a safer strategy, and a number of compounds are undergoing clinical trials.61–63 Intravenous immune globulin contains antiamyloid antibodies and other immunomodulatory factors that may be useful in treating Alzheimer disease, and a phase III trial is being planned in view of positive results from earlier-phase studies.64
Future disease-modifying treatments
Treatments designed to prevent hyperphosphorylation of tau are also being pursued. Currently approved compounds such as lithium (Eskalith) and valproic acid (Depakene) have been shown to decrease the formation of neurofibrillary tangles in laboratory models. There is some retrospective epidemiologic evidence that statin treatment is associated with a lower incidence of Alzheimer disease and decreased amyloid deposition in in vitro preparations, and two large phase III trials of statins in addition to cholinesterase inhibitors are nearing completion.65,66
Future mechanism-based treatments will be directed at reducing oxidative stress, promoting neurorestoration, and genetic modification. A summary of the disease-modifying strategies now being tested has recently been published (Table 3).
SOME RISK FACTORS ARE MODIFIABLE
Evidence is growing that nutritional factors (eg, dietary restriction, antioxidant intake, and the Mediterranean diet) and lifestyle factors (eg, social and mental activity and exercise) can promote healthy brain aging and delay the onset of Alzheimer disease.69,70
Animals on low-calorie diets live longer, and some epidemiologic studies have shown that people who consume fewer calories have a lower incidence of Alzheimer disease.69
Consuming fish two to three times per week appears to lower the incidence of Alzheimer disease, and a recent report in 2,254 elderly people followed for 4 years found that a Mediterranean diet of fish, olive oil, vegetables, and fruit had a protective effect.71
Resveratrol, a chemical found in red wine, is associated with longevity. A clinical trial of resveratrol to prevent Alzheimer disease is being planned.72–74 Compounds such as docosahexaenoic acid (an omega-3 fatty acid), a flavanoid found in green tea, and curcumin (a component of many curry dishes) are also associated with lowering levels of amyloid.75–77
Foods high in antioxidants may reduce the risk of Alzheimer disease.69,78 Daily folate supplements may have a protective effect, but antioxidants such as vitamin E and anti-inflammatory medications have shown disappointing results in preventing or treating Alzheimer disease and may have significant adverse effects.44,59,79,80
Evidence is also increasing that education, learning new skills, frequent socializing, and regularly engaging in physical exercise and mentally stimulating activities delay the onset of Alzheimer disease, and these pursuits should be encouraged.81–83 Also, treatment of cardiovascular risk factors such as hypertension and hyperlipidemia in mid-life and in older age may lower the rate of cognitive impairment in the elderly.84–86
ACKNOWLEDGMENT
The authors would like to thank Caroline O’Connor and Martha Dunlap for their assistance in preparing this manuscript.
- Alzheimer’s Association. 2008 Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia 2008; 4:110–133.
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003; 60:1119–1122.
- Knopman DS. Management of cognition and function: new results from the clinical trials programme of Aricept(R) (donepezil HCl). Int J Neuropsychopharmacol 2000; 3:13–20.
- Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001; 58:1985–1992.
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1133–1142.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr 1997; 9(suppl 1):65–69.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J Alzheimers Dis 2005; 7:235–239.
- Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59:1594–1599.
- Hsiung GY, Donald A, Grand J, et al. Outcomes of cognitively impaired not demented at 2 years in the Canadian Cohort Study of Cognitive Impairment and Related Dementias. Dement Geriatr Cogn Disord 2006; 22:413–420.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition (Text Revision). 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69:2197–2204.
- Anstey KJ, Low LF. Normal cognitive changes in aging. Aust Fam Physician 2004; 33:783–787.
- Newson RS, Kemps EB. The nature of subjective cognitive complaints of older adults. Int J Aging Hum Dev 2006; 63:139–151.
- Jorm AF, Masaki KH, Davis DG, et al. Memory complaints in non-demented men predict future pathologic diagnosis of Alzheimer disease. Neurology 2004; 63:1960–1961.
- Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000; 15:983–991.
- Alzheimer’s Association. Warning Signs of Alzheimer’s. www.alz.org/alzheimers_disease_symptoms_of_alzheimers.asp. Accessed 10/23/2008.
- Rockwood K, Black S, Bedard MA, Tran T, Lussier I. Specific symptomatic changes following donepezil treatment of Alzheimer’s disease: a multi-centre, primary care, open-label study. Int J Geriatr Psychiatry 2007; 22:312–319.
- Perneczky R, Pohl C, Sorg C, et al. Complex activities of daily living in mild cognitive impairment: conceptual and diagnostic issues. Age Ageing 2006; 35:240–245.
- Vogel A, Stokholm J, Gade A, Andersen BB, Hejl AM, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer’s disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord 2004; 17:181–187.
- Kalbe E, Salmon E, Perani D, et al. Anosognosia in very mild Alzheimer’s disease but not in mild cognitive impairment. Dement Geriatr Cogn Disord 2005; 19:349–356.
- Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc 2003; 51:1451–1454.
- Folstein MF, Folstein ES, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive status of patients for the clinician”. J Psychiatr Res 1975; 12:189–198.
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699.
- Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology 2005; 65:559–564.
- Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1154–1166.
- Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1143–1153.
- Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology 2007; 69:871–877.
- Jack CR, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 2002; 58:750–757.
- Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004; 55:306–319.
- Sunderland T, Linker G, Mirza N, et al. Decreased beta-amyloid 1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 2003; 289:2094–2103.
- Burger PC, Vogel FS. The development of the pathologic changes of Alzheimer’s disease and senile dementia in patients with Down’s syndrome. Am J Pathol 1973; 73:457–476.
- Mayeux R, Saunders AM, Shea S, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s Disease Centers Consortium on Apolipoprotein E and Alzheimer’s Disease. N Engl J Med 1998; 338:506–511.
- Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997; 278:1349–1356.
- Winblad B, Kilander L, Eriksson S, et al. Donepezil in patients with severe Alzheimer’s disease: double-blind, parallel-group, placebo-controlled study. Lancet 2006; 367:1057–1065.
- Black SE, Doody R, Li H, et al. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology 2007; 69:459–469.
- Winblad B, Cummings J, Andreasen N, et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007; 22:456–467.
- Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Fillit HM, Doody RS, Binaso K, et al. Recommendations for best practices in the treatment of Alzheimer’s disease in managed care. Am J Geriatr Pharmacother 2006; 4( suppl A):S9–S24.
- Feldman H, Gauthier S, Hecker J, et al. Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer’s disease and the effect on caregiver burden. J Am Geriatr Soc 2003; 51:737–744.
- Cummings JL, Mackell J, Kaufer D. Behavioral effects of current Alzheimer’s disease treatment: a descriptive review. Alzheimers Dement 2008; 4:49–60.
- Wilcock GK, Ballard CG, Cooper JA, Henrik L. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005; 352:2379–2388.
- Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 2004; 63:651–657.
- Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol 2007; 6:501–512.
- Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 2008; 70:2024–2035.
- Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11( suppl 2):S13–S21.
- Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 1994; 6:358–370.
- Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging 2007; 22:28–36.
- Overshott R, Byrne J, Burns A. Nonpharmacological and pharmacological interventions for symptoms in Alzheimer’s disease. Expert Rev Neurother 2004; 4:809–821.
- Spira AP, Edelstein BA. Behavioral interventions for agitation in older adults with dementia: an evaluative review. Int Psychogeriatr 2006; 18:195–225.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Qaseem A, Snow V, Cross J, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002; 297:353–356.
- Giannakopoulos P, Herrmann FR, Bussiere T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 2003; 60:1495–1500.
- Geerts H. Drug evaluation: (R)-flurbiprofen—an enantiomer of flurbiprofen for the treatment of Alzheimer’s disease. IDrugs 2007; 10:121–133.
- Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide treatment in Alzheimer’s disease. Neurology 2002; 58:1050–1054.
- Fenili D, Brown M, Rappaport R, McLaurin J. Properties of scylloinositol as a therapeutic treatment of AD-like pathology. J Mol Med 2007; 85:603–611.
- Lee M, Bard F, Johnson-Wood K, Lee C, et al. Abeta42 immunization in Alzheimer’s disease generates Abeta N-terminal antibodies. Ann Neurol 2005; 58:430–435.
- Masliah E, Hansen L, Adame A, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology 2005; 64:129–131.
- Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005; 64:1553–1562.
- Dodel RC, Du Y, Depboylu C, et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2004; 75:1472–1474.
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet 2000; 356:1627–1631.
- Sparks DL, Sabbagh MN, Connor DJ, et al. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol 2005; 62:753–757.
- Labie C, Lafon C, Marmouget C, et al. Effect of the neuroprotective compound SR57746A on nerve growth factor synthesis in cultured astrocytes from neonatal rat cortex. Br J Pharmacol 1999; 127:139–144.
- Salloway S, Mintzer J, Weiner MF, Cummings JL. Disease-modifying therapies in Alzheimer’s disease. Alzheimer Dement 2008; 4:65–79.
- Burgener SC, Buettner L, Coen Buckwalter K, et al. Evidence supporting nutritional interventions for persons in early stage Alzheimer’s disease (AD). J Nutr Health Aging 2008; 12:18–21.
- Kivipelto M, Solomon A. Alzheimer’s disease - the ways of prevention. J Nutr Health Aging 2008; 12:89S–94S.
- Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol 2006; 59:912–921.
- Anekonda TS. Resveratrol—a boon for treating Alzheimer’s disease? Brain Res Rev 2006; 52:316–326.
- Wang J, Ho L, Zhao Z, et al. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J 2006; 20:2313–2320.
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem 2005; 280:37377–37382.
- Cole GM, Frautschy SA. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer’s disease mouse model. Nutr Health 2006; 18:249–259.
- Rezai-Zadeh K, Shytle D, Sun N, et al. Green tea epigallocatechin-3 gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci 2005; 25:8807–8814.
- Zhang L, Fiala M, Cashman J, et al. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J Alzheimers Dis 2006; 10:1–7.
- Frank B, Gupta S. A review of antioxidants and Alzheimer’s disease. Ann Clin Psychiatry 2005; 17:269–286.
- Thal LJ, Ferris SH, Kirby L, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology 2005; 30:1204–1215.
- Hayden KM, Welsh-Bohmer KA, Wengreen HJ, Zandi PP, Lyketsos CG, Breitner JC. Risk of mortality with vitamin E supplements: the Cache County study. Am J Med 2007; 120:180–184.
- Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003; 348:2508–2516.
- Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006; 144:73–81.
- Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord 2004; 18:57–64.
- Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med 2002; 162:2046–2052.
- Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology 2001; 56:1683–1689.
- Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000; 21:49–55.
The number of patients with Alzheimer disease, the most common cause of disability in the elderly, is about to rise dramatically. More than 5 million people in the United States are affected, and by 2050 this figure may rise to between 11 and 16 million.1 The prevalence doubles every 5 years from ages 65 to 85, so that Alzheimer disease affects 30% to 50% of all people at age 85.1,2
Primary care physicians bear the brunt of diagnosing and treating all these patients,3 requiring that they have the training to meet this critical public health problem.
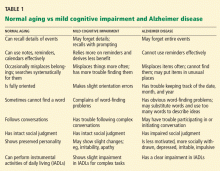
But diagnosing this disease is not easy. In the early stages, it can be difficult to distinguish between the decline in certain cognitive functions due to normal aging (eg, name recall) and the mild cognitive impairment that often precedes Alzheimer disease.
Once a patient is diagnosed with Alzheimer disease, there needs to be a realistic discussion with the patient and family about what treatment with different drugs can—and cannot—accomplish.
ALZHEIMER DISEASE DIAGNOSIS: THE EARLIER, THE BETTER
While much has been accomplished in Alzheimer disease research in the last 20 years, a great deal remains to be done to improve its diagnosis and treatment. There is increasing evidence that early diagnosis of Alzheimer disease will be key to maximizing treatment benefits. But too often, patients are diagnosed in later stages of the disease, when disabling symptoms and neuropathologic changes have become well established.
Mild cognitive impairment: A predementia phase
The pathologic changes of Alzheimer disease typically begin many years before its clinical signs are apparent. Most patients pass through a predementia phase called mild cognitive impairment, with early memory loss but with relatively well-preserved activities of daily living.
From 6% to 25% of patients with mild cognitive impairment progress to dementia annually, a rate far higher than the incidence rate in the general population of 0.3% to 3.9% per year, depending on age.4,5 Therefore, patients with mild cognitive impairment are a good population in which to test interventions to prevent dementia.6,7
The concept of mild cognitive impairment is controversial because it is a transitional stage between normal aging and dementia rather than a distinct pathologic entity.8 Moreover, in some large community-based studies,9,10 a sizeable number of people with mild cognitive impairment reverted to normal cognitive function over 5 years, suggesting that mild cognitive impairment may be unstable over time.
Are other factors causing the dementia?
The Diagnostic and Statistical Manual IV-Text Revision11 defines dementia as memory loss and at least one other area of cognitive impairment, not due to delirium, that interferes with social and occupational functioning. Alzheimer disease is the most common cause of dementia in the United States.1
Still, Alzheimer disease does not typically exist in isolation. For example, while Alzheimer disease was the predominant cause of dementia in a recent postmortem series, 38% of dementia cases featured Alzheimer disease with lacunar infarction.12 Accordingly, clinicians must consider factors other than Alzheimer disease that could contribute to (or even fully account for) the complaints or observed deficits.
Is it Alzheimer disease or normal aging?
Although cognitive impairment and changes in behavior are common in the elderly, they are not a normal part of aging. Like other chronic disorders associated with aging, Alzheimer disease can be diagnosed and treated. Cognitive impairment may come to light when the patient or a family member reports a problem or the clinician asks about problems or observes signs of impairment in the office. The cognitive difficulties should be taken seriously, and their impact on daily functioning should be evaluated.
Certain cognitive functions such as mental flexibility and speed of processing decline in normal aging,13 and many older people report cognitive symptoms. Therefore, it is important to differentiate mild age-associated cognitive changes from the beginning of a cognitive disorder such as Alzheimer disease. This can be difficult because the cognitive complaints of normal aging overlap with the symptoms of early Alzheimer disease, and there are no clear rules for distinguishing the two.13
Clues that a more serious problem exists may come from details such as a history of decline in cognitive abilities, involvement of more than one domain, and the extent to which the problem disrupts daily functioning. 13 When evaluating the nature and severity of a cognitive disorder, one must take into account the patient’s level of education, premorbid intellectual and occupational functioning, and concurrent medical and psychiatric conditions. Clinicians must also consider the impact that medications, possible substance abuse, and language and culture of origin have on cognition.
The most common cognitive complaints in the elderly tend to be related to working memory (eg, recalling the name of a recent acquaintance or a telephone number that was just looked up).14 Other common complaints center on the speed of mental processing (eg, thinking quickly), memory (eg, recalling the name of an old acquaintance, or remembering where objects were placed or why one entered a room), executive function (eg, multitasking or planning a series of events), attention, and concentration.14
People with age-related cognitive changes can learn new information and recall previously learned information, although they may do so less rapidly and less efficiently. Furthermore, age-related cognitive change is not substantially progressive and does not significantly impair daily functioning.14 Nevertheless, complaints of cognitive changes should be monitored, since several studies showed that elderly people with cognitive complaints have an increased risk of developing dementia.15,16
Key warning signs
The Alzheimer’s Association17 lists 10 key warning signs of Alzheimer disease:
- Memory loss
- Difficulty performing familiar tasks
- Problems with language
- Disorientation to time and place
- Poor or decreased judgment
- Problems with abstract thought
- Misplacing things
- Changes in mood or behavior
- Changes in personality
- Loss of initiative.
As annotated on the Alzheimer’s Association Web site,17 the list also highlights key differences between normal aging and more serious symptoms of possible Alzheimer disease. For example, patients with Alzheimer disease are more likely to forget entire experiences and not remember them later, whereas normal elderly may forget parts of events and then recall the missing details later. Also, patients with Alzheimer disease are more likely to lose the ability to complete familiar tasks or to follow written or spoken directions. Additional signs that a more serious cognitive problem exists include misplacing items so often that it interferes with daily activities, frequently losing the thread of conversations, and repeating the same questions, stories, or comments within a short time without being aware of it.18
Talk to a family member
Interviewing a reliable informant who knows the patient well is extremely helpful for determining the presence and extent of a cognitive problem. This is particularly important because even patients with mild cognitive impairment may have impaired awareness of their memory problems and may underestimate20 or overestimate21 the problem.
Ask the informant about symptoms such as being repetitive, misplacing items, or having trouble with finding words or names, remembering to take medication, managing finances, navigating while driving, or performing multiple tasks or all steps of a task. A change in behavior may be the first sign of a cognitive disorder, so the patient and informant should also be asked about signs of irritability, anxiety, increased social isolation, and decline in motivation.
Screening tests
Memory can be tested with a three- or four-word recall test during the physical examination, with the addition of a clock-drawing task,22 or can be assessed with a composite cognitive measure. For example:
The Mini-Mental State Exam (MMSE)23 can be given by the clinician or a trained member of the office staff. People with Alzheimer disease show progressive disability and a predictable rate of decline of approximately 2.8 points on the MMSE per year, with slower decline in the milder stages and faster decline in the moderate and severe stages of the illness.
The Montreal Cognitive Assessment Battery (MOCA, www.mocatest.org),24 is a new cognitive screening test with a 30-point format similar to that of the MMSE. It includes a five-word recall, clock-drawing, and executive and visuospatial items that make it more sensitive for mild cognitive impairment and vascular dementia.
The Alzheimer’s Disease 8 (AD8),25 a sensitive eight-question scale developed at Washington University, St. Louis, MO, can be completed by an informant in the waiting room.
New computerized screening measures are being developed that can be completed online or in the waiting room, and some simulate practical tasks such as using an automated teller machine and driving.
Care should be taken when interpreting performance on screening tests in patients who have very low or very high education levels, who are not tested in their native language, or who have physical or sensory deficits that might limit their performance.
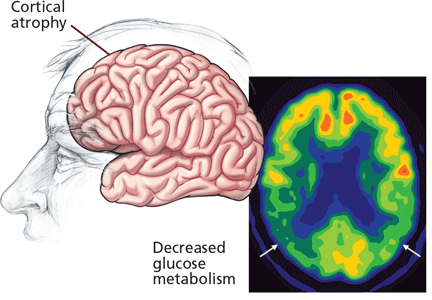
Brain imaging and other tests
Patients with evidence of cognitive impairment should undergo a structural brain imaging test such as noncontrast computed tomography (CT) or magnetic resonance imaging (MRI) to evaluate for changes consistent with Alzheimer disease and to help rule out alternative causes of the cognitive impairment.26,27
Neuropsychologic testing can be done by a dementia specialist, who can also help with diagnosis and treatment.
Positron emission tomography (PET) using fluorodeoxyglucose shows patterns of brain metabolism and can help differentiate Alzheimer disease from non-Alzheimer dementia, as patients with the former typically show hypometabolism in the temporal and parietal cortices.28
Quantitative MRI and PET amyloid imaging are exciting new techniques currently being developed to diagnose Alzheimer disease earlier in clinical practice.29,30
Cerebrospinal fluid markers. A decrease in the amyloid beta 1–42 peptide and an increase in the tau and phosphotau proteins may be the earliest signs of Alzheimer disease.31,32 However, before these tests can be widely used in clinical practice, their sensitivity and specificity need to be established, people’s reluctance to undergo lumbar puncture will have to be overcome, and third-party reimbursement will have to be obtained.
Genetic factors also play an important role in the development of Alzheimer disease. The apolipoprotein E4 (ApoE4) allele is a marker for Alzheimer disease. People of European descent who possess one copy of the allele have three times the risk (with onset typically in their 70s), and individuals who are homozygous have 15 times the risk (with typical onset in their 60s), compared with people lacking ApoE4.33,34 This test is commercially available but is still considered a research tool.
CURRENT AND FUTURE TREATMENTS
Five drugs have been approved for treating Alzheimer disease: four cholinesterase inhibitors approved for mild to moderate disease and a glutamate N-methyl D-aspartate (NMDA) antagonist approved for moderate to severe disease.
Cholinesterase inhibitors for mild to moderate disease
The cholinesterase inhibitors tend to stabilize memory during the first year of treatment, and they may make the subsequent decline more gradual. The four current drugs have similar efficacy, so the choice is usually based on tolerability and ease of use.
Tacrine (Cognex) is rarely used because it must be taken four times a day, it can cause gastrointestinal adverse effects, and it can raise hepatic enzyme levels.
Donepezil (Aricept) is the drug most often prescribed because it can be taken once daily, a major benefit in older patients with memory loss. Also, its starting dose (5 mg) is a therapeutic dose. Donepezil was also recently approved for the treatment of severe Alzheimer disease on the basis of positive results in trials in patients with moderate to severe disease.35,36
Galantamine (Razadyne) comes in an extended-release formulation that can be taken once daily.
Rivastigmine (Exelon) is taken twice a day with food to reduce the risk of gastrointestinal adverse effects. It is also now available as a daily patch, which has a more favorable adverse-effect profile than oral rivastigmine.37
Memantine for moderate to severe disease
Memantine (Namenda), an NMDA antagonist, is approved for moderate to severe Alzheimer disease. The approval was based on a trial in which patients with advanced Alzheimer disease who received memantine monotherapy showed less decline in cognition and function after 6 months than those who received placebo,38 and another trial in which patients who received the combination of donepezil plus memantine showed more benefit than with donepezil alone.39
A treatment strategy
Recent guidelines recommend starting treatment with a cholinesterase inhibitor soon after Alzheimer disease is diagnosed and titrating the dose, as tolerated, to the high end of the therapeutic range.40 Once patients decline to the moderate stage of the illness, usually with an MMSE score of 10 to 20, memantine should be added and titrated upward to 10 mg twice a day. The medications should be continued as long as they are tolerated and the clinician feels there is some evidence they are helping.
The main benefit of the cholinesterase inhibitors in clinical trials is an attenuation of decline over time rather than an improvement in cognitive or behavioral symptoms. This should be considered when judging whether there has been a positive effect. It is also important to discuss this point with patients and their families, who may expect improvement rather than relative stability. Benefits of these drugs in later stages of the illness usually involve better recognition and engagement with family members and people around them and less severe behavioral disturbances, making care easier.36,41–43
Will drug therapy help in mild cognitive impairment?
Currently, no drugs are approved by the US Food and Drug Administration for patients with mild cognitive impairment, and the use of cholinesterase inhibitors in this population may not be reimbursed.
Six trials of cholinesterase inhibitors for mild cognitive impairment have been completed.44–47 On the whole, donepezil, rivastigmine, and galantamine had no effect on the primary end points in these trials, but they had some effects on some secondary ones.
In the Alzheimer Disease Cooperative Study,44 donepezil had no effect on the rate of progression from mild cognitive impairment to Alzheimer disease over the entire 3 years of the study, but it did reduce the rate in the first year of treatment. Moreover, the subgroup with one or two ApoE4 alleles benefitted over the entire 3 years.
A 24-week trial of donepezil in patients with mild cognitive impairment45 had negative results with regard to the selected study end points (two standardized tests), but there was evidence of cognitive benefit with donepezil on secondary measures such as the Alzheimer’s Disease Cognitive Assessment Scale-Cognitive Subscale,48 a widely used cognitive measure in Alzheimer disease trials, and in patients’ self-assessment of their memory.
One should discuss the risks and benefits of cholinesterase treatment with patients with mild cognitive impairment in whom underlying Alzheimer disease is strongly suspected.
Addressing behavioral problems
Behavioral problems are often the most disturbing symptoms in dementia, often leading to higher levels of care.
Apathy is the most common behavioral symptom in Alzheimer disease, increasing with disease severity.49 There is no approved treatment for these apathetic symptoms, though methylphenidate (Ritalin) and modafanil (Provigil) are being tested in small clinical trials.
Depression and irritability are common and may respond well to low doses of serotonin reuptake inhibitors.
Agitation and psychosis are distressing and are likely to overwhelm the caregiver’s ability to cope. Recent studies have raised concern about the safety and efficacy of atypical neuroleptics in patients with dementia and suggest that these drugs be used with careful monitoring.50
A safe, calm, predictable environment
Patients with Alzheimer disease function best in an environment that is safe, calm, and predictable, and their caregivers require ongoing support and education to develop realistic expectations throughout the course of the illness.
Behavioral treatments for problematic behaviors for which supportive evidence exists include reduction of environmental stressors and behavioral management of problematic behaviors.51–53 Such interventions typically include carefully observing and recording the problematic behavior, including its antecedents and situations under which it is most likely to occur, and then modifying the physical and interpersonal environment and schedule to reduce its occurrence.51
A challenge to the use of behavioral interventions in dementia is that the patient’s cognitive functioning is gradually declining, and this may require adjustments of interventions with time and in response to new behaviors that emerge.51 Referral to a behavioral specialist such as a geriatric psychiatrist may be helpful in managing disruptive and hard-to-treat behavioral problems.
Amyloid-lowering drugs are being tested
New disease-modifying agents are being tested to see if they delay disability, promote independence, and improve quality of life. Chief among these are compounds that reduce brain amyloid.
The amyloid cascade hypothesis is the current prevailing view of the pathogenesis of Alzheimer disease.56 Small molecules of extracellular amyloid are deposited in the brain early in the course of the disease. These oligomers of beta-amyloid gradually coalesce into fibrillar sheets that form the core of amyloid plaques. Amyloid invokes an immune response and stimulates the hyperphosphorylation of tau into intraneuronal neurofibrillary tangles. The accumulation of these tangles contributes to neuronal and synaptic loss, which correlates with dementia and disability.
The current disease-modifying strategies are designed to decrease the production of amyloid, inhibit fibrillogenesis, and promote clearance of the toxic amyloid beta 1–42 fragment. We should note, however, that the correlation between amyloid burden and clinical decline is not strong, and that lowering brain amyloid may not produce a measurable clinical benefit.57
Large-scale trials are being conducted with agents that modulate and inhibit gamma secretase, an enzyme involved in cleaving the amyloid precursor protein into the toxic fragment of beta amyloid.58 Early results show improvement in cognition and decreased amyloid levels in transgenic animals treated with these agents.
A recently completed phase III trial of tramiprosate, a glycosaminoglycan receptor inhibitor that interferes with fibrillization of amyloid, did not show positive results, but other antifibrillization agents are currently being tested.59,60
Exciting immunotherapeutic approaches that target the toxic fragment of beta amyloid have been developed. In the active vaccine approach, a small fragment of beta amyloid is injected to stimulate the production of beta amyloid antibodies to lower brain amyloid levels. However, although active vaccines are designed primarily to stimulate a B-cell response, they can cause adverse effects through unplanned stimulation of T cells.
Passive immunization with a monoclonal antibody against beta amyloid may be a safer strategy, and a number of compounds are undergoing clinical trials.61–63 Intravenous immune globulin contains antiamyloid antibodies and other immunomodulatory factors that may be useful in treating Alzheimer disease, and a phase III trial is being planned in view of positive results from earlier-phase studies.64
Future disease-modifying treatments
Treatments designed to prevent hyperphosphorylation of tau are also being pursued. Currently approved compounds such as lithium (Eskalith) and valproic acid (Depakene) have been shown to decrease the formation of neurofibrillary tangles in laboratory models. There is some retrospective epidemiologic evidence that statin treatment is associated with a lower incidence of Alzheimer disease and decreased amyloid deposition in in vitro preparations, and two large phase III trials of statins in addition to cholinesterase inhibitors are nearing completion.65,66
Future mechanism-based treatments will be directed at reducing oxidative stress, promoting neurorestoration, and genetic modification. A summary of the disease-modifying strategies now being tested has recently been published (Table 3).
SOME RISK FACTORS ARE MODIFIABLE
Evidence is growing that nutritional factors (eg, dietary restriction, antioxidant intake, and the Mediterranean diet) and lifestyle factors (eg, social and mental activity and exercise) can promote healthy brain aging and delay the onset of Alzheimer disease.69,70
Animals on low-calorie diets live longer, and some epidemiologic studies have shown that people who consume fewer calories have a lower incidence of Alzheimer disease.69
Consuming fish two to three times per week appears to lower the incidence of Alzheimer disease, and a recent report in 2,254 elderly people followed for 4 years found that a Mediterranean diet of fish, olive oil, vegetables, and fruit had a protective effect.71
Resveratrol, a chemical found in red wine, is associated with longevity. A clinical trial of resveratrol to prevent Alzheimer disease is being planned.72–74 Compounds such as docosahexaenoic acid (an omega-3 fatty acid), a flavanoid found in green tea, and curcumin (a component of many curry dishes) are also associated with lowering levels of amyloid.75–77
Foods high in antioxidants may reduce the risk of Alzheimer disease.69,78 Daily folate supplements may have a protective effect, but antioxidants such as vitamin E and anti-inflammatory medications have shown disappointing results in preventing or treating Alzheimer disease and may have significant adverse effects.44,59,79,80
Evidence is also increasing that education, learning new skills, frequent socializing, and regularly engaging in physical exercise and mentally stimulating activities delay the onset of Alzheimer disease, and these pursuits should be encouraged.81–83 Also, treatment of cardiovascular risk factors such as hypertension and hyperlipidemia in mid-life and in older age may lower the rate of cognitive impairment in the elderly.84–86
ACKNOWLEDGMENT
The authors would like to thank Caroline O’Connor and Martha Dunlap for their assistance in preparing this manuscript.
The number of patients with Alzheimer disease, the most common cause of disability in the elderly, is about to rise dramatically. More than 5 million people in the United States are affected, and by 2050 this figure may rise to between 11 and 16 million.1 The prevalence doubles every 5 years from ages 65 to 85, so that Alzheimer disease affects 30% to 50% of all people at age 85.1,2
Primary care physicians bear the brunt of diagnosing and treating all these patients,3 requiring that they have the training to meet this critical public health problem.
But diagnosing this disease is not easy. In the early stages, it can be difficult to distinguish between the decline in certain cognitive functions due to normal aging (eg, name recall) and the mild cognitive impairment that often precedes Alzheimer disease.
Once a patient is diagnosed with Alzheimer disease, there needs to be a realistic discussion with the patient and family about what treatment with different drugs can—and cannot—accomplish.
ALZHEIMER DISEASE DIAGNOSIS: THE EARLIER, THE BETTER
While much has been accomplished in Alzheimer disease research in the last 20 years, a great deal remains to be done to improve its diagnosis and treatment. There is increasing evidence that early diagnosis of Alzheimer disease will be key to maximizing treatment benefits. But too often, patients are diagnosed in later stages of the disease, when disabling symptoms and neuropathologic changes have become well established.
Mild cognitive impairment: A predementia phase
The pathologic changes of Alzheimer disease typically begin many years before its clinical signs are apparent. Most patients pass through a predementia phase called mild cognitive impairment, with early memory loss but with relatively well-preserved activities of daily living.
From 6% to 25% of patients with mild cognitive impairment progress to dementia annually, a rate far higher than the incidence rate in the general population of 0.3% to 3.9% per year, depending on age.4,5 Therefore, patients with mild cognitive impairment are a good population in which to test interventions to prevent dementia.6,7
The concept of mild cognitive impairment is controversial because it is a transitional stage between normal aging and dementia rather than a distinct pathologic entity.8 Moreover, in some large community-based studies,9,10 a sizeable number of people with mild cognitive impairment reverted to normal cognitive function over 5 years, suggesting that mild cognitive impairment may be unstable over time.
Are other factors causing the dementia?
The Diagnostic and Statistical Manual IV-Text Revision11 defines dementia as memory loss and at least one other area of cognitive impairment, not due to delirium, that interferes with social and occupational functioning. Alzheimer disease is the most common cause of dementia in the United States.1
Still, Alzheimer disease does not typically exist in isolation. For example, while Alzheimer disease was the predominant cause of dementia in a recent postmortem series, 38% of dementia cases featured Alzheimer disease with lacunar infarction.12 Accordingly, clinicians must consider factors other than Alzheimer disease that could contribute to (or even fully account for) the complaints or observed deficits.
Is it Alzheimer disease or normal aging?
Although cognitive impairment and changes in behavior are common in the elderly, they are not a normal part of aging. Like other chronic disorders associated with aging, Alzheimer disease can be diagnosed and treated. Cognitive impairment may come to light when the patient or a family member reports a problem or the clinician asks about problems or observes signs of impairment in the office. The cognitive difficulties should be taken seriously, and their impact on daily functioning should be evaluated.
Certain cognitive functions such as mental flexibility and speed of processing decline in normal aging,13 and many older people report cognitive symptoms. Therefore, it is important to differentiate mild age-associated cognitive changes from the beginning of a cognitive disorder such as Alzheimer disease. This can be difficult because the cognitive complaints of normal aging overlap with the symptoms of early Alzheimer disease, and there are no clear rules for distinguishing the two.13
Clues that a more serious problem exists may come from details such as a history of decline in cognitive abilities, involvement of more than one domain, and the extent to which the problem disrupts daily functioning. 13 When evaluating the nature and severity of a cognitive disorder, one must take into account the patient’s level of education, premorbid intellectual and occupational functioning, and concurrent medical and psychiatric conditions. Clinicians must also consider the impact that medications, possible substance abuse, and language and culture of origin have on cognition.
The most common cognitive complaints in the elderly tend to be related to working memory (eg, recalling the name of a recent acquaintance or a telephone number that was just looked up).14 Other common complaints center on the speed of mental processing (eg, thinking quickly), memory (eg, recalling the name of an old acquaintance, or remembering where objects were placed or why one entered a room), executive function (eg, multitasking or planning a series of events), attention, and concentration.14
People with age-related cognitive changes can learn new information and recall previously learned information, although they may do so less rapidly and less efficiently. Furthermore, age-related cognitive change is not substantially progressive and does not significantly impair daily functioning.14 Nevertheless, complaints of cognitive changes should be monitored, since several studies showed that elderly people with cognitive complaints have an increased risk of developing dementia.15,16
Key warning signs
The Alzheimer’s Association17 lists 10 key warning signs of Alzheimer disease:
- Memory loss
- Difficulty performing familiar tasks
- Problems with language
- Disorientation to time and place
- Poor or decreased judgment
- Problems with abstract thought
- Misplacing things
- Changes in mood or behavior
- Changes in personality
- Loss of initiative.
As annotated on the Alzheimer’s Association Web site,17 the list also highlights key differences between normal aging and more serious symptoms of possible Alzheimer disease. For example, patients with Alzheimer disease are more likely to forget entire experiences and not remember them later, whereas normal elderly may forget parts of events and then recall the missing details later. Also, patients with Alzheimer disease are more likely to lose the ability to complete familiar tasks or to follow written or spoken directions. Additional signs that a more serious cognitive problem exists include misplacing items so often that it interferes with daily activities, frequently losing the thread of conversations, and repeating the same questions, stories, or comments within a short time without being aware of it.18
Talk to a family member
Interviewing a reliable informant who knows the patient well is extremely helpful for determining the presence and extent of a cognitive problem. This is particularly important because even patients with mild cognitive impairment may have impaired awareness of their memory problems and may underestimate20 or overestimate21 the problem.
Ask the informant about symptoms such as being repetitive, misplacing items, or having trouble with finding words or names, remembering to take medication, managing finances, navigating while driving, or performing multiple tasks or all steps of a task. A change in behavior may be the first sign of a cognitive disorder, so the patient and informant should also be asked about signs of irritability, anxiety, increased social isolation, and decline in motivation.
Screening tests
Memory can be tested with a three- or four-word recall test during the physical examination, with the addition of a clock-drawing task,22 or can be assessed with a composite cognitive measure. For example:
The Mini-Mental State Exam (MMSE)23 can be given by the clinician or a trained member of the office staff. People with Alzheimer disease show progressive disability and a predictable rate of decline of approximately 2.8 points on the MMSE per year, with slower decline in the milder stages and faster decline in the moderate and severe stages of the illness.
The Montreal Cognitive Assessment Battery (MOCA, www.mocatest.org),24 is a new cognitive screening test with a 30-point format similar to that of the MMSE. It includes a five-word recall, clock-drawing, and executive and visuospatial items that make it more sensitive for mild cognitive impairment and vascular dementia.
The Alzheimer’s Disease 8 (AD8),25 a sensitive eight-question scale developed at Washington University, St. Louis, MO, can be completed by an informant in the waiting room.
New computerized screening measures are being developed that can be completed online or in the waiting room, and some simulate practical tasks such as using an automated teller machine and driving.
Care should be taken when interpreting performance on screening tests in patients who have very low or very high education levels, who are not tested in their native language, or who have physical or sensory deficits that might limit their performance.
Brain imaging and other tests
Patients with evidence of cognitive impairment should undergo a structural brain imaging test such as noncontrast computed tomography (CT) or magnetic resonance imaging (MRI) to evaluate for changes consistent with Alzheimer disease and to help rule out alternative causes of the cognitive impairment.26,27
Neuropsychologic testing can be done by a dementia specialist, who can also help with diagnosis and treatment.
Positron emission tomography (PET) using fluorodeoxyglucose shows patterns of brain metabolism and can help differentiate Alzheimer disease from non-Alzheimer dementia, as patients with the former typically show hypometabolism in the temporal and parietal cortices.28
Quantitative MRI and PET amyloid imaging are exciting new techniques currently being developed to diagnose Alzheimer disease earlier in clinical practice.29,30
Cerebrospinal fluid markers. A decrease in the amyloid beta 1–42 peptide and an increase in the tau and phosphotau proteins may be the earliest signs of Alzheimer disease.31,32 However, before these tests can be widely used in clinical practice, their sensitivity and specificity need to be established, people’s reluctance to undergo lumbar puncture will have to be overcome, and third-party reimbursement will have to be obtained.
Genetic factors also play an important role in the development of Alzheimer disease. The apolipoprotein E4 (ApoE4) allele is a marker for Alzheimer disease. People of European descent who possess one copy of the allele have three times the risk (with onset typically in their 70s), and individuals who are homozygous have 15 times the risk (with typical onset in their 60s), compared with people lacking ApoE4.33,34 This test is commercially available but is still considered a research tool.
CURRENT AND FUTURE TREATMENTS
Five drugs have been approved for treating Alzheimer disease: four cholinesterase inhibitors approved for mild to moderate disease and a glutamate N-methyl D-aspartate (NMDA) antagonist approved for moderate to severe disease.
Cholinesterase inhibitors for mild to moderate disease
The cholinesterase inhibitors tend to stabilize memory during the first year of treatment, and they may make the subsequent decline more gradual. The four current drugs have similar efficacy, so the choice is usually based on tolerability and ease of use.
Tacrine (Cognex) is rarely used because it must be taken four times a day, it can cause gastrointestinal adverse effects, and it can raise hepatic enzyme levels.
Donepezil (Aricept) is the drug most often prescribed because it can be taken once daily, a major benefit in older patients with memory loss. Also, its starting dose (5 mg) is a therapeutic dose. Donepezil was also recently approved for the treatment of severe Alzheimer disease on the basis of positive results in trials in patients with moderate to severe disease.35,36
Galantamine (Razadyne) comes in an extended-release formulation that can be taken once daily.
Rivastigmine (Exelon) is taken twice a day with food to reduce the risk of gastrointestinal adverse effects. It is also now available as a daily patch, which has a more favorable adverse-effect profile than oral rivastigmine.37
Memantine for moderate to severe disease
Memantine (Namenda), an NMDA antagonist, is approved for moderate to severe Alzheimer disease. The approval was based on a trial in which patients with advanced Alzheimer disease who received memantine monotherapy showed less decline in cognition and function after 6 months than those who received placebo,38 and another trial in which patients who received the combination of donepezil plus memantine showed more benefit than with donepezil alone.39
A treatment strategy
Recent guidelines recommend starting treatment with a cholinesterase inhibitor soon after Alzheimer disease is diagnosed and titrating the dose, as tolerated, to the high end of the therapeutic range.40 Once patients decline to the moderate stage of the illness, usually with an MMSE score of 10 to 20, memantine should be added and titrated upward to 10 mg twice a day. The medications should be continued as long as they are tolerated and the clinician feels there is some evidence they are helping.
The main benefit of the cholinesterase inhibitors in clinical trials is an attenuation of decline over time rather than an improvement in cognitive or behavioral symptoms. This should be considered when judging whether there has been a positive effect. It is also important to discuss this point with patients and their families, who may expect improvement rather than relative stability. Benefits of these drugs in later stages of the illness usually involve better recognition and engagement with family members and people around them and less severe behavioral disturbances, making care easier.36,41–43
Will drug therapy help in mild cognitive impairment?
Currently, no drugs are approved by the US Food and Drug Administration for patients with mild cognitive impairment, and the use of cholinesterase inhibitors in this population may not be reimbursed.
Six trials of cholinesterase inhibitors for mild cognitive impairment have been completed.44–47 On the whole, donepezil, rivastigmine, and galantamine had no effect on the primary end points in these trials, but they had some effects on some secondary ones.
In the Alzheimer Disease Cooperative Study,44 donepezil had no effect on the rate of progression from mild cognitive impairment to Alzheimer disease over the entire 3 years of the study, but it did reduce the rate in the first year of treatment. Moreover, the subgroup with one or two ApoE4 alleles benefitted over the entire 3 years.
A 24-week trial of donepezil in patients with mild cognitive impairment45 had negative results with regard to the selected study end points (two standardized tests), but there was evidence of cognitive benefit with donepezil on secondary measures such as the Alzheimer’s Disease Cognitive Assessment Scale-Cognitive Subscale,48 a widely used cognitive measure in Alzheimer disease trials, and in patients’ self-assessment of their memory.
One should discuss the risks and benefits of cholinesterase treatment with patients with mild cognitive impairment in whom underlying Alzheimer disease is strongly suspected.
Addressing behavioral problems
Behavioral problems are often the most disturbing symptoms in dementia, often leading to higher levels of care.
Apathy is the most common behavioral symptom in Alzheimer disease, increasing with disease severity.49 There is no approved treatment for these apathetic symptoms, though methylphenidate (Ritalin) and modafanil (Provigil) are being tested in small clinical trials.
Depression and irritability are common and may respond well to low doses of serotonin reuptake inhibitors.
Agitation and psychosis are distressing and are likely to overwhelm the caregiver’s ability to cope. Recent studies have raised concern about the safety and efficacy of atypical neuroleptics in patients with dementia and suggest that these drugs be used with careful monitoring.50
A safe, calm, predictable environment
Patients with Alzheimer disease function best in an environment that is safe, calm, and predictable, and their caregivers require ongoing support and education to develop realistic expectations throughout the course of the illness.
Behavioral treatments for problematic behaviors for which supportive evidence exists include reduction of environmental stressors and behavioral management of problematic behaviors.51–53 Such interventions typically include carefully observing and recording the problematic behavior, including its antecedents and situations under which it is most likely to occur, and then modifying the physical and interpersonal environment and schedule to reduce its occurrence.51
A challenge to the use of behavioral interventions in dementia is that the patient’s cognitive functioning is gradually declining, and this may require adjustments of interventions with time and in response to new behaviors that emerge.51 Referral to a behavioral specialist such as a geriatric psychiatrist may be helpful in managing disruptive and hard-to-treat behavioral problems.
Amyloid-lowering drugs are being tested
New disease-modifying agents are being tested to see if they delay disability, promote independence, and improve quality of life. Chief among these are compounds that reduce brain amyloid.
The amyloid cascade hypothesis is the current prevailing view of the pathogenesis of Alzheimer disease.56 Small molecules of extracellular amyloid are deposited in the brain early in the course of the disease. These oligomers of beta-amyloid gradually coalesce into fibrillar sheets that form the core of amyloid plaques. Amyloid invokes an immune response and stimulates the hyperphosphorylation of tau into intraneuronal neurofibrillary tangles. The accumulation of these tangles contributes to neuronal and synaptic loss, which correlates with dementia and disability.
The current disease-modifying strategies are designed to decrease the production of amyloid, inhibit fibrillogenesis, and promote clearance of the toxic amyloid beta 1–42 fragment. We should note, however, that the correlation between amyloid burden and clinical decline is not strong, and that lowering brain amyloid may not produce a measurable clinical benefit.57
Large-scale trials are being conducted with agents that modulate and inhibit gamma secretase, an enzyme involved in cleaving the amyloid precursor protein into the toxic fragment of beta amyloid.58 Early results show improvement in cognition and decreased amyloid levels in transgenic animals treated with these agents.
A recently completed phase III trial of tramiprosate, a glycosaminoglycan receptor inhibitor that interferes with fibrillization of amyloid, did not show positive results, but other antifibrillization agents are currently being tested.59,60
Exciting immunotherapeutic approaches that target the toxic fragment of beta amyloid have been developed. In the active vaccine approach, a small fragment of beta amyloid is injected to stimulate the production of beta amyloid antibodies to lower brain amyloid levels. However, although active vaccines are designed primarily to stimulate a B-cell response, they can cause adverse effects through unplanned stimulation of T cells.
Passive immunization with a monoclonal antibody against beta amyloid may be a safer strategy, and a number of compounds are undergoing clinical trials.61–63 Intravenous immune globulin contains antiamyloid antibodies and other immunomodulatory factors that may be useful in treating Alzheimer disease, and a phase III trial is being planned in view of positive results from earlier-phase studies.64
Future disease-modifying treatments
Treatments designed to prevent hyperphosphorylation of tau are also being pursued. Currently approved compounds such as lithium (Eskalith) and valproic acid (Depakene) have been shown to decrease the formation of neurofibrillary tangles in laboratory models. There is some retrospective epidemiologic evidence that statin treatment is associated with a lower incidence of Alzheimer disease and decreased amyloid deposition in in vitro preparations, and two large phase III trials of statins in addition to cholinesterase inhibitors are nearing completion.65,66
Future mechanism-based treatments will be directed at reducing oxidative stress, promoting neurorestoration, and genetic modification. A summary of the disease-modifying strategies now being tested has recently been published (Table 3).
SOME RISK FACTORS ARE MODIFIABLE
Evidence is growing that nutritional factors (eg, dietary restriction, antioxidant intake, and the Mediterranean diet) and lifestyle factors (eg, social and mental activity and exercise) can promote healthy brain aging and delay the onset of Alzheimer disease.69,70
Animals on low-calorie diets live longer, and some epidemiologic studies have shown that people who consume fewer calories have a lower incidence of Alzheimer disease.69
Consuming fish two to three times per week appears to lower the incidence of Alzheimer disease, and a recent report in 2,254 elderly people followed for 4 years found that a Mediterranean diet of fish, olive oil, vegetables, and fruit had a protective effect.71
Resveratrol, a chemical found in red wine, is associated with longevity. A clinical trial of resveratrol to prevent Alzheimer disease is being planned.72–74 Compounds such as docosahexaenoic acid (an omega-3 fatty acid), a flavanoid found in green tea, and curcumin (a component of many curry dishes) are also associated with lowering levels of amyloid.75–77
Foods high in antioxidants may reduce the risk of Alzheimer disease.69,78 Daily folate supplements may have a protective effect, but antioxidants such as vitamin E and anti-inflammatory medications have shown disappointing results in preventing or treating Alzheimer disease and may have significant adverse effects.44,59,79,80
Evidence is also increasing that education, learning new skills, frequent socializing, and regularly engaging in physical exercise and mentally stimulating activities delay the onset of Alzheimer disease, and these pursuits should be encouraged.81–83 Also, treatment of cardiovascular risk factors such as hypertension and hyperlipidemia in mid-life and in older age may lower the rate of cognitive impairment in the elderly.84–86
ACKNOWLEDGMENT
The authors would like to thank Caroline O’Connor and Martha Dunlap for their assistance in preparing this manuscript.
- Alzheimer’s Association. 2008 Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia 2008; 4:110–133.
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003; 60:1119–1122.
- Knopman DS. Management of cognition and function: new results from the clinical trials programme of Aricept(R) (donepezil HCl). Int J Neuropsychopharmacol 2000; 3:13–20.
- Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001; 58:1985–1992.
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1133–1142.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr 1997; 9(suppl 1):65–69.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J Alzheimers Dis 2005; 7:235–239.
- Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59:1594–1599.
- Hsiung GY, Donald A, Grand J, et al. Outcomes of cognitively impaired not demented at 2 years in the Canadian Cohort Study of Cognitive Impairment and Related Dementias. Dement Geriatr Cogn Disord 2006; 22:413–420.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition (Text Revision). 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69:2197–2204.
- Anstey KJ, Low LF. Normal cognitive changes in aging. Aust Fam Physician 2004; 33:783–787.
- Newson RS, Kemps EB. The nature of subjective cognitive complaints of older adults. Int J Aging Hum Dev 2006; 63:139–151.
- Jorm AF, Masaki KH, Davis DG, et al. Memory complaints in non-demented men predict future pathologic diagnosis of Alzheimer disease. Neurology 2004; 63:1960–1961.
- Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000; 15:983–991.
- Alzheimer’s Association. Warning Signs of Alzheimer’s. www.alz.org/alzheimers_disease_symptoms_of_alzheimers.asp. Accessed 10/23/2008.
- Rockwood K, Black S, Bedard MA, Tran T, Lussier I. Specific symptomatic changes following donepezil treatment of Alzheimer’s disease: a multi-centre, primary care, open-label study. Int J Geriatr Psychiatry 2007; 22:312–319.
- Perneczky R, Pohl C, Sorg C, et al. Complex activities of daily living in mild cognitive impairment: conceptual and diagnostic issues. Age Ageing 2006; 35:240–245.
- Vogel A, Stokholm J, Gade A, Andersen BB, Hejl AM, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer’s disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord 2004; 17:181–187.
- Kalbe E, Salmon E, Perani D, et al. Anosognosia in very mild Alzheimer’s disease but not in mild cognitive impairment. Dement Geriatr Cogn Disord 2005; 19:349–356.
- Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc 2003; 51:1451–1454.
- Folstein MF, Folstein ES, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive status of patients for the clinician”. J Psychiatr Res 1975; 12:189–198.
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699.
- Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology 2005; 65:559–564.
- Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1154–1166.
- Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1143–1153.
- Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology 2007; 69:871–877.
- Jack CR, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 2002; 58:750–757.
- Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004; 55:306–319.
- Sunderland T, Linker G, Mirza N, et al. Decreased beta-amyloid 1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 2003; 289:2094–2103.
- Burger PC, Vogel FS. The development of the pathologic changes of Alzheimer’s disease and senile dementia in patients with Down’s syndrome. Am J Pathol 1973; 73:457–476.
- Mayeux R, Saunders AM, Shea S, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s Disease Centers Consortium on Apolipoprotein E and Alzheimer’s Disease. N Engl J Med 1998; 338:506–511.
- Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997; 278:1349–1356.
- Winblad B, Kilander L, Eriksson S, et al. Donepezil in patients with severe Alzheimer’s disease: double-blind, parallel-group, placebo-controlled study. Lancet 2006; 367:1057–1065.
- Black SE, Doody R, Li H, et al. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology 2007; 69:459–469.
- Winblad B, Cummings J, Andreasen N, et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007; 22:456–467.
- Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Fillit HM, Doody RS, Binaso K, et al. Recommendations for best practices in the treatment of Alzheimer’s disease in managed care. Am J Geriatr Pharmacother 2006; 4( suppl A):S9–S24.
- Feldman H, Gauthier S, Hecker J, et al. Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer’s disease and the effect on caregiver burden. J Am Geriatr Soc 2003; 51:737–744.
- Cummings JL, Mackell J, Kaufer D. Behavioral effects of current Alzheimer’s disease treatment: a descriptive review. Alzheimers Dement 2008; 4:49–60.
- Wilcock GK, Ballard CG, Cooper JA, Henrik L. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005; 352:2379–2388.
- Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 2004; 63:651–657.
- Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol 2007; 6:501–512.
- Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 2008; 70:2024–2035.
- Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11( suppl 2):S13–S21.
- Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 1994; 6:358–370.
- Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging 2007; 22:28–36.
- Overshott R, Byrne J, Burns A. Nonpharmacological and pharmacological interventions for symptoms in Alzheimer’s disease. Expert Rev Neurother 2004; 4:809–821.
- Spira AP, Edelstein BA. Behavioral interventions for agitation in older adults with dementia: an evaluative review. Int Psychogeriatr 2006; 18:195–225.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Qaseem A, Snow V, Cross J, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002; 297:353–356.
- Giannakopoulos P, Herrmann FR, Bussiere T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 2003; 60:1495–1500.
- Geerts H. Drug evaluation: (R)-flurbiprofen—an enantiomer of flurbiprofen for the treatment of Alzheimer’s disease. IDrugs 2007; 10:121–133.
- Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide treatment in Alzheimer’s disease. Neurology 2002; 58:1050–1054.
- Fenili D, Brown M, Rappaport R, McLaurin J. Properties of scylloinositol as a therapeutic treatment of AD-like pathology. J Mol Med 2007; 85:603–611.
- Lee M, Bard F, Johnson-Wood K, Lee C, et al. Abeta42 immunization in Alzheimer’s disease generates Abeta N-terminal antibodies. Ann Neurol 2005; 58:430–435.
- Masliah E, Hansen L, Adame A, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology 2005; 64:129–131.
- Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005; 64:1553–1562.
- Dodel RC, Du Y, Depboylu C, et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2004; 75:1472–1474.
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet 2000; 356:1627–1631.
- Sparks DL, Sabbagh MN, Connor DJ, et al. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol 2005; 62:753–757.
- Labie C, Lafon C, Marmouget C, et al. Effect of the neuroprotective compound SR57746A on nerve growth factor synthesis in cultured astrocytes from neonatal rat cortex. Br J Pharmacol 1999; 127:139–144.
- Salloway S, Mintzer J, Weiner MF, Cummings JL. Disease-modifying therapies in Alzheimer’s disease. Alzheimer Dement 2008; 4:65–79.
- Burgener SC, Buettner L, Coen Buckwalter K, et al. Evidence supporting nutritional interventions for persons in early stage Alzheimer’s disease (AD). J Nutr Health Aging 2008; 12:18–21.
- Kivipelto M, Solomon A. Alzheimer’s disease - the ways of prevention. J Nutr Health Aging 2008; 12:89S–94S.
- Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol 2006; 59:912–921.
- Anekonda TS. Resveratrol—a boon for treating Alzheimer’s disease? Brain Res Rev 2006; 52:316–326.
- Wang J, Ho L, Zhao Z, et al. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J 2006; 20:2313–2320.
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem 2005; 280:37377–37382.
- Cole GM, Frautschy SA. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer’s disease mouse model. Nutr Health 2006; 18:249–259.
- Rezai-Zadeh K, Shytle D, Sun N, et al. Green tea epigallocatechin-3 gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci 2005; 25:8807–8814.
- Zhang L, Fiala M, Cashman J, et al. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J Alzheimers Dis 2006; 10:1–7.
- Frank B, Gupta S. A review of antioxidants and Alzheimer’s disease. Ann Clin Psychiatry 2005; 17:269–286.
- Thal LJ, Ferris SH, Kirby L, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology 2005; 30:1204–1215.
- Hayden KM, Welsh-Bohmer KA, Wengreen HJ, Zandi PP, Lyketsos CG, Breitner JC. Risk of mortality with vitamin E supplements: the Cache County study. Am J Med 2007; 120:180–184.
- Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003; 348:2508–2516.
- Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006; 144:73–81.
- Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord 2004; 18:57–64.
- Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med 2002; 162:2046–2052.
- Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology 2001; 56:1683–1689.
- Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000; 21:49–55.
- Alzheimer’s Association. 2008 Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia 2008; 4:110–133.
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003; 60:1119–1122.
- Knopman DS. Management of cognition and function: new results from the clinical trials programme of Aricept(R) (donepezil HCl). Int J Neuropsychopharmacol 2000; 3:13–20.
- Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001; 58:1985–1992.
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1133–1142.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr 1997; 9(suppl 1):65–69.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J Alzheimers Dis 2005; 7:235–239.
- Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59:1594–1599.
- Hsiung GY, Donald A, Grand J, et al. Outcomes of cognitively impaired not demented at 2 years in the Canadian Cohort Study of Cognitive Impairment and Related Dementias. Dement Geriatr Cogn Disord 2006; 22:413–420.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition (Text Revision). 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69:2197–2204.
- Anstey KJ, Low LF. Normal cognitive changes in aging. Aust Fam Physician 2004; 33:783–787.
- Newson RS, Kemps EB. The nature of subjective cognitive complaints of older adults. Int J Aging Hum Dev 2006; 63:139–151.
- Jorm AF, Masaki KH, Davis DG, et al. Memory complaints in non-demented men predict future pathologic diagnosis of Alzheimer disease. Neurology 2004; 63:1960–1961.
- Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000; 15:983–991.
- Alzheimer’s Association. Warning Signs of Alzheimer’s. www.alz.org/alzheimers_disease_symptoms_of_alzheimers.asp. Accessed 10/23/2008.
- Rockwood K, Black S, Bedard MA, Tran T, Lussier I. Specific symptomatic changes following donepezil treatment of Alzheimer’s disease: a multi-centre, primary care, open-label study. Int J Geriatr Psychiatry 2007; 22:312–319.
- Perneczky R, Pohl C, Sorg C, et al. Complex activities of daily living in mild cognitive impairment: conceptual and diagnostic issues. Age Ageing 2006; 35:240–245.
- Vogel A, Stokholm J, Gade A, Andersen BB, Hejl AM, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer’s disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord 2004; 17:181–187.
- Kalbe E, Salmon E, Perani D, et al. Anosognosia in very mild Alzheimer’s disease but not in mild cognitive impairment. Dement Geriatr Cogn Disord 2005; 19:349–356.
- Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc 2003; 51:1451–1454.
- Folstein MF, Folstein ES, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive status of patients for the clinician”. J Psychiatr Res 1975; 12:189–198.
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699.
- Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology 2005; 65:559–564.
- Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1154–1166.
- Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1143–1153.
- Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology 2007; 69:871–877.
- Jack CR, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 2002; 58:750–757.
- Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004; 55:306–319.
- Sunderland T, Linker G, Mirza N, et al. Decreased beta-amyloid 1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 2003; 289:2094–2103.
- Burger PC, Vogel FS. The development of the pathologic changes of Alzheimer’s disease and senile dementia in patients with Down’s syndrome. Am J Pathol 1973; 73:457–476.
- Mayeux R, Saunders AM, Shea S, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s Disease Centers Consortium on Apolipoprotein E and Alzheimer’s Disease. N Engl J Med 1998; 338:506–511.
- Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997; 278:1349–1356.
- Winblad B, Kilander L, Eriksson S, et al. Donepezil in patients with severe Alzheimer’s disease: double-blind, parallel-group, placebo-controlled study. Lancet 2006; 367:1057–1065.
- Black SE, Doody R, Li H, et al. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology 2007; 69:459–469.
- Winblad B, Cummings J, Andreasen N, et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007; 22:456–467.
- Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Fillit HM, Doody RS, Binaso K, et al. Recommendations for best practices in the treatment of Alzheimer’s disease in managed care. Am J Geriatr Pharmacother 2006; 4( suppl A):S9–S24.
- Feldman H, Gauthier S, Hecker J, et al. Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer’s disease and the effect on caregiver burden. J Am Geriatr Soc 2003; 51:737–744.
- Cummings JL, Mackell J, Kaufer D. Behavioral effects of current Alzheimer’s disease treatment: a descriptive review. Alzheimers Dement 2008; 4:49–60.
- Wilcock GK, Ballard CG, Cooper JA, Henrik L. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005; 352:2379–2388.
- Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 2004; 63:651–657.
- Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol 2007; 6:501–512.
- Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 2008; 70:2024–2035.
- Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11( suppl 2):S13–S21.
- Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 1994; 6:358–370.
- Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging 2007; 22:28–36.
- Overshott R, Byrne J, Burns A. Nonpharmacological and pharmacological interventions for symptoms in Alzheimer’s disease. Expert Rev Neurother 2004; 4:809–821.
- Spira AP, Edelstein BA. Behavioral interventions for agitation in older adults with dementia: an evaluative review. Int Psychogeriatr 2006; 18:195–225.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Qaseem A, Snow V, Cross J, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002; 297:353–356.
- Giannakopoulos P, Herrmann FR, Bussiere T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 2003; 60:1495–1500.
- Geerts H. Drug evaluation: (R)-flurbiprofen—an enantiomer of flurbiprofen for the treatment of Alzheimer’s disease. IDrugs 2007; 10:121–133.
- Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide treatment in Alzheimer’s disease. Neurology 2002; 58:1050–1054.
- Fenili D, Brown M, Rappaport R, McLaurin J. Properties of scylloinositol as a therapeutic treatment of AD-like pathology. J Mol Med 2007; 85:603–611.
- Lee M, Bard F, Johnson-Wood K, Lee C, et al. Abeta42 immunization in Alzheimer’s disease generates Abeta N-terminal antibodies. Ann Neurol 2005; 58:430–435.
- Masliah E, Hansen L, Adame A, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology 2005; 64:129–131.
- Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005; 64:1553–1562.
- Dodel RC, Du Y, Depboylu C, et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2004; 75:1472–1474.
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet 2000; 356:1627–1631.
- Sparks DL, Sabbagh MN, Connor DJ, et al. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol 2005; 62:753–757.
- Labie C, Lafon C, Marmouget C, et al. Effect of the neuroprotective compound SR57746A on nerve growth factor synthesis in cultured astrocytes from neonatal rat cortex. Br J Pharmacol 1999; 127:139–144.
- Salloway S, Mintzer J, Weiner MF, Cummings JL. Disease-modifying therapies in Alzheimer’s disease. Alzheimer Dement 2008; 4:65–79.
- Burgener SC, Buettner L, Coen Buckwalter K, et al. Evidence supporting nutritional interventions for persons in early stage Alzheimer’s disease (AD). J Nutr Health Aging 2008; 12:18–21.
- Kivipelto M, Solomon A. Alzheimer’s disease - the ways of prevention. J Nutr Health Aging 2008; 12:89S–94S.
- Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol 2006; 59:912–921.
- Anekonda TS. Resveratrol—a boon for treating Alzheimer’s disease? Brain Res Rev 2006; 52:316–326.
- Wang J, Ho L, Zhao Z, et al. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J 2006; 20:2313–2320.
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem 2005; 280:37377–37382.
- Cole GM, Frautschy SA. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer’s disease mouse model. Nutr Health 2006; 18:249–259.
- Rezai-Zadeh K, Shytle D, Sun N, et al. Green tea epigallocatechin-3 gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci 2005; 25:8807–8814.
- Zhang L, Fiala M, Cashman J, et al. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J Alzheimers Dis 2006; 10:1–7.
- Frank B, Gupta S. A review of antioxidants and Alzheimer’s disease. Ann Clin Psychiatry 2005; 17:269–286.
- Thal LJ, Ferris SH, Kirby L, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology 2005; 30:1204–1215.
- Hayden KM, Welsh-Bohmer KA, Wengreen HJ, Zandi PP, Lyketsos CG, Breitner JC. Risk of mortality with vitamin E supplements: the Cache County study. Am J Med 2007; 120:180–184.
- Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003; 348:2508–2516.
- Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006; 144:73–81.
- Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord 2004; 18:57–64.
- Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med 2002; 162:2046–2052.
- Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology 2001; 56:1683–1689.
- Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000; 21:49–55.
KEY POINTS
- Interpret screening tests carefully in patients who have very low or very high education levels, who are not tested in their native language, or who have deficits that might limit their performance.
- Patients with Alzheimer disease function best in a safe, calm, and predictable environment. Their caregivers require ongoing support and education to develop realistic expectations throughout the course of the illness.
- It is important that families be made aware that treatments for Alzheimer disease, such as cholinesterase inhibitors, attenuate decline over time rather than improve cognitive and behavioral symptoms.
- Evidence is growing that nutritional factors (eg, dietary restriction, antioxidant intake, and the Mediterranean diet) and lifestyle factors (eg, social and mental activity and exercise) can delay the onset of Alzheimer disease.