User login
Can CBT effectively treat adult insomnia disorder?
EVIDENCE SUMMARY
Three meta-analyses that included only randomized controlled trials (RCTs) compared various CBT delivery methods with controls (wait-listed for treatment or general sleep hygiene education) to assess sleep outcomes for adults with insomnia.1-3 TABLE 11-3 summarizes the results.

CBT is comparable to pharmacotherapy
A 2002 comparative meta-analysis of 21 RCTs with a total of 470 patients examined the effectiveness of CBT (stimulus control and/or sleep restriction) compared with pharmacotherapy (benzodiazepines or benzodiazepine agonists) for treating primary insomnia of longer than one month’s duration in adults with no comorbid medical or psychiatric diagnoses.4 The CBT group received intervention over an average of 5 weeks, and the pharmacotherapy group received intervention over an average of 2 weeks.
CBT produced greater reductions in sleep onset latency than pharmacotherapy based on mean weighted effect size (1.05 vs 0.45 weighted effect size; 95% confidence interval, 0.17-1.04; P=.01). Although both CBT and pharmacotherapy improved sleep outcomes, no statistical differences were found in wake after sleep onset time, total sleep time, number of awakenings, or sleep quality ratings (TABLE 24).

Continue to: CBT has significant benefit for comorbid insomnia
CBT has significant benefit for comorbid insomnia
A 2015 meta-analysis of 23 studies enrolling a total of 1379 adults with a number of illnesses (chronic pain, alcohol dependence, breast cancer, psychiatric disorders, chronic obstructive pulmonary disease, fibromyalgia) and comorbid insomnia investigated the qualitative effectiveness of individual or group CBT therapy.5 Subjects received at least 4 face-to-face sessions and at least 2 components of CBT.
The primary outcome showed that sleep quality improved, as measured by a 6.36-point reduction in the Insomnia Severity Index (ISI; a 7-question scale on which 0=no insomnia and 28=severe insomnia) and a 3.3-point reduction in the Pittsburgh Sleep Quality Index (PSQI; a 7-category assessment tool on which 0=perfect quality and 21=poor quality). The effect size was large for both ISI and PSQI, as indicated by standard mean differences greater than 0.8 (1.22 and 0.88, respectively) and was sustained for as long as 18 months.
RECOMMENDATIONS
The American College of Physicians strongly recommends that all adult patients receive CBT as initial treatment for chronic insomnia disorder. It can be performed in multiple settings, including the primary care setting. Compared with hypnotics, CBT is unlikely to have any adverse effects.6
1. Trauer J, Qian M, Doyle J, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191-204.
2. Koffel E, Koffel J, Gehrman P. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6-16.
3. Ye Y, Chen N, Chen J, et al. Internet-based cognitive-behavioral therapy for insomnia (ICBT-i): a meta-analysis of randomized controlled trials. BMJ Open. 2016;6:e010707.
4. Smith M, Perlis M, Park S, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5-11.
5. Geiger-Brown J, Rogers V, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67.
6. Qaseem A, Kansagara D, Forciea M, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
EVIDENCE SUMMARY
Three meta-analyses that included only randomized controlled trials (RCTs) compared various CBT delivery methods with controls (wait-listed for treatment or general sleep hygiene education) to assess sleep outcomes for adults with insomnia.1-3 TABLE 11-3 summarizes the results.

CBT is comparable to pharmacotherapy
A 2002 comparative meta-analysis of 21 RCTs with a total of 470 patients examined the effectiveness of CBT (stimulus control and/or sleep restriction) compared with pharmacotherapy (benzodiazepines or benzodiazepine agonists) for treating primary insomnia of longer than one month’s duration in adults with no comorbid medical or psychiatric diagnoses.4 The CBT group received intervention over an average of 5 weeks, and the pharmacotherapy group received intervention over an average of 2 weeks.
CBT produced greater reductions in sleep onset latency than pharmacotherapy based on mean weighted effect size (1.05 vs 0.45 weighted effect size; 95% confidence interval, 0.17-1.04; P=.01). Although both CBT and pharmacotherapy improved sleep outcomes, no statistical differences were found in wake after sleep onset time, total sleep time, number of awakenings, or sleep quality ratings (TABLE 24).

Continue to: CBT has significant benefit for comorbid insomnia
CBT has significant benefit for comorbid insomnia
A 2015 meta-analysis of 23 studies enrolling a total of 1379 adults with a number of illnesses (chronic pain, alcohol dependence, breast cancer, psychiatric disorders, chronic obstructive pulmonary disease, fibromyalgia) and comorbid insomnia investigated the qualitative effectiveness of individual or group CBT therapy.5 Subjects received at least 4 face-to-face sessions and at least 2 components of CBT.
The primary outcome showed that sleep quality improved, as measured by a 6.36-point reduction in the Insomnia Severity Index (ISI; a 7-question scale on which 0=no insomnia and 28=severe insomnia) and a 3.3-point reduction in the Pittsburgh Sleep Quality Index (PSQI; a 7-category assessment tool on which 0=perfect quality and 21=poor quality). The effect size was large for both ISI and PSQI, as indicated by standard mean differences greater than 0.8 (1.22 and 0.88, respectively) and was sustained for as long as 18 months.
RECOMMENDATIONS
The American College of Physicians strongly recommends that all adult patients receive CBT as initial treatment for chronic insomnia disorder. It can be performed in multiple settings, including the primary care setting. Compared with hypnotics, CBT is unlikely to have any adverse effects.6
EVIDENCE SUMMARY
Three meta-analyses that included only randomized controlled trials (RCTs) compared various CBT delivery methods with controls (wait-listed for treatment or general sleep hygiene education) to assess sleep outcomes for adults with insomnia.1-3 TABLE 11-3 summarizes the results.

CBT is comparable to pharmacotherapy
A 2002 comparative meta-analysis of 21 RCTs with a total of 470 patients examined the effectiveness of CBT (stimulus control and/or sleep restriction) compared with pharmacotherapy (benzodiazepines or benzodiazepine agonists) for treating primary insomnia of longer than one month’s duration in adults with no comorbid medical or psychiatric diagnoses.4 The CBT group received intervention over an average of 5 weeks, and the pharmacotherapy group received intervention over an average of 2 weeks.
CBT produced greater reductions in sleep onset latency than pharmacotherapy based on mean weighted effect size (1.05 vs 0.45 weighted effect size; 95% confidence interval, 0.17-1.04; P=.01). Although both CBT and pharmacotherapy improved sleep outcomes, no statistical differences were found in wake after sleep onset time, total sleep time, number of awakenings, or sleep quality ratings (TABLE 24).

Continue to: CBT has significant benefit for comorbid insomnia
CBT has significant benefit for comorbid insomnia
A 2015 meta-analysis of 23 studies enrolling a total of 1379 adults with a number of illnesses (chronic pain, alcohol dependence, breast cancer, psychiatric disorders, chronic obstructive pulmonary disease, fibromyalgia) and comorbid insomnia investigated the qualitative effectiveness of individual or group CBT therapy.5 Subjects received at least 4 face-to-face sessions and at least 2 components of CBT.
The primary outcome showed that sleep quality improved, as measured by a 6.36-point reduction in the Insomnia Severity Index (ISI; a 7-question scale on which 0=no insomnia and 28=severe insomnia) and a 3.3-point reduction in the Pittsburgh Sleep Quality Index (PSQI; a 7-category assessment tool on which 0=perfect quality and 21=poor quality). The effect size was large for both ISI and PSQI, as indicated by standard mean differences greater than 0.8 (1.22 and 0.88, respectively) and was sustained for as long as 18 months.
RECOMMENDATIONS
The American College of Physicians strongly recommends that all adult patients receive CBT as initial treatment for chronic insomnia disorder. It can be performed in multiple settings, including the primary care setting. Compared with hypnotics, CBT is unlikely to have any adverse effects.6
1. Trauer J, Qian M, Doyle J, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191-204.
2. Koffel E, Koffel J, Gehrman P. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6-16.
3. Ye Y, Chen N, Chen J, et al. Internet-based cognitive-behavioral therapy for insomnia (ICBT-i): a meta-analysis of randomized controlled trials. BMJ Open. 2016;6:e010707.
4. Smith M, Perlis M, Park S, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5-11.
5. Geiger-Brown J, Rogers V, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67.
6. Qaseem A, Kansagara D, Forciea M, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
1. Trauer J, Qian M, Doyle J, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191-204.
2. Koffel E, Koffel J, Gehrman P. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6-16.
3. Ye Y, Chen N, Chen J, et al. Internet-based cognitive-behavioral therapy for insomnia (ICBT-i): a meta-analysis of randomized controlled trials. BMJ Open. 2016;6:e010707.
4. Smith M, Perlis M, Park S, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5-11.
5. Geiger-Brown J, Rogers V, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67.
6. Qaseem A, Kansagara D, Forciea M, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
EVIDENCE-BASED ANSWER:
Yes. Cognitive behavioral therapy (CBT) administered individually, in a group setting, or on the internet is effective for treating insomnia in adults compared with control (strength of recommendation [SOR]: A, meta-analyses).
CBT is comparable to pharmacotherapy for improving measures of sleep (SOR: A, comparative meta-analysis).
CBT produces sustainable improvements in subjective sleep quality for adults with comorbid insomnia (SOR: A, meta-analysis).
When can infants and children benefit from probiotics?
PRACTICE RECOMMENDATIONS
› Recommend a trial of Lactobacillus reuteri for breastfed infants with colic. A
› Consider Lactobacillus and Bifidobacterium species for the prevention of upper respiratory infections (URIs) and to shorten the course of URI illness. B
› Do not recommend probiotics for the prevention of respiratory or gastrointestinal allergies. A
› Consider probiotics for the reduction of abdominal pain in pediatric irritable bowel syndrome, as well as to reduce diarrhea associated with antibiotic use and acute gastroenteritis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Ms. B, a 26-year-old woman, presents to your office with her 3-year-old son for a well-child examination. During the course of the conversation, she asks you if she should be giving her child probiotics to improve his general health. Many of her friends, who also have their children in day care, have told her that probiotics, “are nature’s way of fighting infection.” Her son currently takes no medications, and has no history of asthma or recent gastrointestinal disturbances. He was treated for 2 ear infections last winter, approximately 3 months apart. His physical exam is normal and, after today, his immunizations will be up to date. How should you respond?
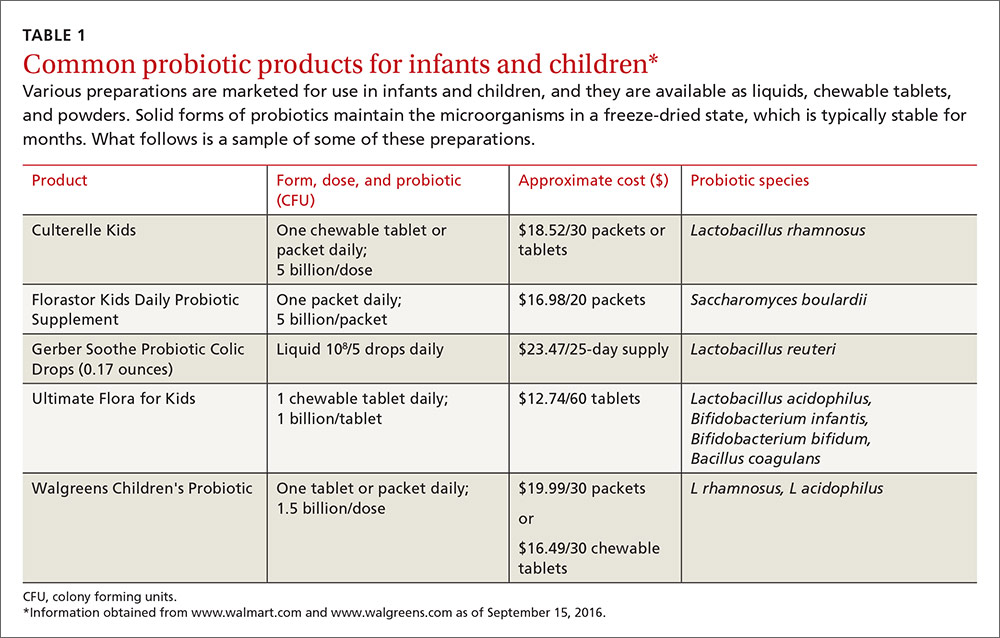
The use of probiotics as over-the-counter treatments for a variety of conditions continues to grow, with retail sales of functional probiotic foods and supplements topping $35 billion worldwide in 2014.1 In children, claims of benefit for gastrointestinal (GI) disorders, colic, and allergy prevention, as well as prevention and treatment of upper respiratory infections (URIs) have existed for over 10 years.2-4 The human gut flora develops rapidly after birth and is known to be influenced by route of delivery (vaginal vs cesarean), type of feeding (breast vs formula), and other environmental factors.5 The use of probiotics to influence the types of bacteria in a child’s intestinal tract continues to be an area of active research. (For more on probiotic formulations, see TABLE 1.)
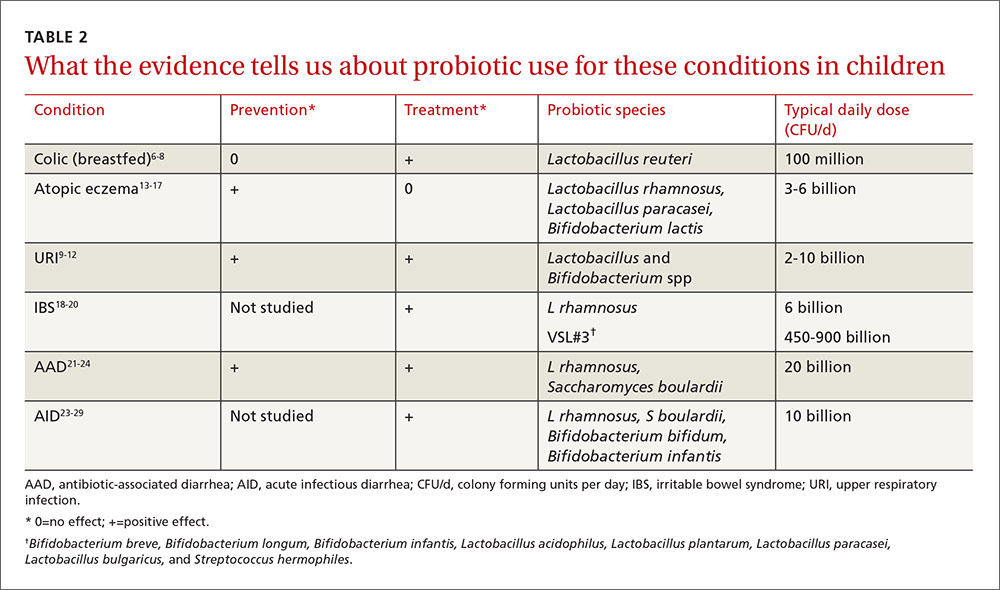
This article summarizes recent research on probiotic use in infants and children. New data support the use of probiotics for the treatment of colic and atopic eczema; however, the data on using probiotics in the management of URIs is less robust and mixed. And while probiotics improve irritable bowel syndrome (IBS) stomach pain, they do not help with related diarrhea or constipation. All of these data are summarized in TABLE 2.6-29
L reuteri improves symptoms in breastfed infants with colic
Infant colic is a relatively common condition known to negatively impact maternal mental health and the mother/child relationship.6 Numerous randomized controlled trials (RCTs) over the years have demonstrated mixed results with using probiotics to decrease crying times, with differences noted between infants who are solely breastfed and those who are not.7
In the most recent meta-analysis of 6 studies (n=427) that focused only on the probiotic Lactobacillus reuteri, breastfed infants with colic receiving a daily dose of 108 colony forming units (CFU) cried an average of 56 fewer minutes/day than those in the control group (95% confidence interval [CI], -64.4 to -47.3; P=.001) at day 21 of treatment.8 Although 2 studies in this meta-analysis included a small number of mixed-fed and formula-fed infants, the majority of trials do not show benefit for these infants. Trials assessing the use of L reuteri for prevention of colic have not shown positive results.7
Probiotics may help p
The mechanisms by which probiotics may prevent or shorten the course of URIs are not obvious. Current theories include boosting the immune function of the respiratory mucosa, acting as a competitive inhibitor for viruses, and secreting antiviral compounds.9 Multiple reviews published in the last 3 years, however, add to the evidence that the apparent benefit is real.
A 2013 meta-analysis assessed data from 4 RCTs (N=1805), which used Lactobacillus rhamnosus as the sole probiotic for prevention of URIs. In treated children, otitis media incidence was reduced by 24% (relative risk [RR] 0.76; 95% CI, 0.64-0.91) and risk of URI was reduced by 38% (RR 0.62; 95% CI, 0.50-0.78).10 The number needed to treat (NNT) was 4 for URI prevention, and the authors noted that adverse events were similar in the treatment and control groups.
A 2014 systematic review and meta-analysis of 20 RCTs examining duration of illness included 10 studies dedicated to pediatric subjects (age 12 months to 12 years).11 There were significantly fewer days of illness per person (standardized mean difference -0.31; 95% CI, -0.41 to -0.11) and each illness episode was shorter by three-quarters of a day (weighted mean difference -0.77; 95% CI, -1.5 to -0.04) in participants who received a probiotic vs those who received a placebo. Probiotics used in these studies belonged to the Lactobacillus and Bifidobacterium genera.
A 2015 systematic review of 14 RCTs assessing the benefits of probiotics, particularly Lactobacillus and Bifidobacterium strains, on URI occurrence and symptoms, showed mixed results.12 Seven of 12 studies found lowered rates of URI and otitis media incidence, 7 of 11 RCTs reported a significant reduction in severity scores for URI, and 4 of 8 RCTs reported significant reductions in school absenteeism between the probiotic and control groups. In a summary statement, the authors noted that “at least one beneficial effect of prophylactic probiotics was observed in the majority of RCTs,” and that “none of the studies reported any serious adverse events.”
Perinatal probiotics: No benefit for allergic conditions—except eczema
Allergic disease is on the rise and continues to plague children with reduced quality of life, potentially life-threatening reactions, and missed activities, including school. The gut microbiome likely influences a child’s allergic propensity through its effects on T-helper cells, transforming growth factor (TGF), and immunoglobulin A (IgA)—all known components of the allergic response. As the hygiene hypothesis suggests, the quantity and types of bacteria that inhabit the GI tract early in life play a significant role in determining a person’s later allergic responses.13
In a 2013 meta-analysis of 20 trials (N=4866), researchers looked specifically at probiotic use and the diagnosis of asthma and incident wheezing. Single and combination products of Lactobacillus and Bifidobacterium given prenatally and/or postnatally were included in the studies. The authors found no evidence to support a protective association between perinatal use of probiotics and diagnosed asthma (RR=0.99; 95% CI, 0.81-0.21) or childhood incident wheezing (RR=0.97; 95% CI, 0.87-1.09; 9 trials, 1949 infants).14
In a more recent meta-analysis (2015) conducted to inform the World Allergy Organization, 29 studies were evaluated to assess the impact of probiotics on allergic symptoms of the skin, respiratory system, and GI tract.15 No significant benefit was noted for any allergic condition except for eczema. Probiotics reduced the risk of eczema when given during the last trimester of pregnancy (RR=0.71; 95% CI, 0.60-0.84), when used by breastfeeding mothers (RR=0.57; 95% CI, 0.47-0.69), and when given to infants (RR=0.80; 95% CI, 0.68-0.94).
A 2014 systematic review and meta-analysis (N=2797) explored probiotic use specifically for the prevention of eczema.16 The pooled relative risk for all the studies was 0.74 (95% CI, 0.67-0.82). Evidence was strongest for probiotics containing the Lactobacillus species rhamnosus and paracasei, as well as for Bifidobacterium lactis. No benefit was noted with Lactobacillus acidophilus or other Bifidobacterium species. These newer reviews on eczema prevention contrast with an older Cochrane review published in 2008 (12 RCTs, N=781), which did not show significant benefit for the treatment of eczema.17
Probiotics improve IBS stomach pain, but not diarrhea or constipation
IBS is a functional disorder of the GI tract that affects up to 20% of children and teenagers and leads to a significant decrease in quality of life.18 Current theories of causation include bacterial overgrowth and neuronal hyperactivity, which may be amenable to change with supplemental probiotics.
A 2015 systematic review of non-pharmacological treatments for functional abdominal pain disorders identified 4 studies dedicated to IBS in children.19 A subgroup analysis of 3 RCTs (n=309) that looked at giving L rhamnosus to 5- to 17-year-olds with IBS showed improved abdominal pain (according to various pain scales) compared to the placebo group. Study participants received at least 3 x 109 CFU twice a day for 4 to 8 weeks. Relative risk for improvement was 1.7 (95% CI, 1.27-2.27) with an NNT of 4. None of these studies showed significant improvement in either frequency or severity of diarrhea or constipation.
A separate crossover RCT (N=59) compared placebo to VSL#3, a product containing 8 probiotics (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, L acidophilus, Lactobacillus plantarum, L paracasei, Lactobacillus bulgaricus, and Streptococcus hermophiles), given in age-dependent doses for 6 weeks to children aged 4 to 18 years.20 The frequency and intensity of abdominal pain were measured on a 5-point Likert scale. The group treated with VSL#3 dropped 1.0 ± 0.2 points vs 0.5 ± 0.2 points in the control group (P<.05) and reported an improved quality of life.
These agents reduce antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) occurs in 5% to 30% of children who receive antibiotic therapy.21 It occurs most frequently with the use of cephalosporins, penicillin, fluoroquinolones, and clindamycin, and is likely caused by an alteration of the normal gut flora. Colitis caused by Clostridium difficile remains the most serious antibiotic-associated GI complication.
A systematic review of the specific probiotic Saccharomyces boulardii conducted in 2015 analyzed data from 6 RCTs (n=1653) to determine the effect of co-administration of this probiotic with antibiotics.22 The pooled relative risk for AAD in children receiving the probiotic was 0.43 (95% CI, 0.3-0.6) compared to antibiotics alone. The absolute risk of AAD dropped from 20.9% to 8.8%, translating to a NNT of 8. Two of the RCTs specifically looked at rates of C difficile infection (n=579). C difficile infection rates dropped by 75% (RR=.25; 95% CI, 0.08-0.73) in the treatment group. This dramatic treatment effect was not seen in studies involving adults.
A similar systematic review focusing on L rhamnosus conducted in 2015 pooled data from 5 RCTs (n=445) to see if the probiotic would decrease AAD in children if it was co-administered with antibiotics.23 The relative risk for AAD in this treatment group was 0.48 (95% CI, 0.26-0.89) with an absolute risk reduction of 13.4% (23% compared to 9.6%), translating to an NNT of 7.
A Cochrane review published in 2015 included 23 studies (N=3938) and found similar results with an RR for AAD of 0.46 for treated children (95% CI, 0.35-0.61).24 Doses of probiotics ranged from 5 to 40 billion CFU/day. Although many probiotic species were used in these studies, S boulardii and L rhamnosus were cited as having the strongest data to support use in this context.
Probiotics reduce the duration, frequency of acute infectious diarrhea
Diarrhea remains the second leading cause of death among children one to 59 months of age worldwide.25 Current World Health Organization recommendations include oral rehydration salts, continued feeding to avoid dehydration, and zinc to decrease the duration and severity of illness.26 Multiple studies in adults confirm that a variety of probiotics decrease both the duration and severity of diarrhea in acute gastroenteritis.27
The authors of a 2013 systematic review of probiotics for the treatment of community-acquired acute diarrhea in children less than 5 years of age analyzed data from 8 RCTs (N=1755).28 Various probiotics were used including Lactobacillus species, Streptococcus thermophilus, Bifidobacterium species, and Saccharomyces boulardii for between 4 and 10 days. Six of these studies (n=1164) measured diarrhea duration and found a 14% reduction (95% CI, 3.8%-24.2%) in days of illness for those children treated vs those receiving placebo. Five studies (n=925) measured the difference in stool frequency on Day 2 of illness and reported a reduction of 13.1% (95% CI, 0.8%-5.3%) in the number of stools in the treated group vs the placebo group.
This review augments a Cochrane meta-analysis of 63 studies (N=8014) published in 2010.27 Fifty-six of these studies included infants and children. Pooled analysis of the varied probiotic treatments showed a mean reduction in duration of diarrhea of just over a day (24.76 hours; 95% CI, 15.9-33.6 hours; n=4555, trials=35) and decreased stool frequency on Day 2 of treatment (mean difference 0.80; 95% CI, 0.45-1.14; n=2751, trials=20). The authors concluded that probiotics “have clear beneficial effects in shortening the duration and reducing stool frequency in acute infectious diarrhea.”
Pediatric society weighs in. In 2014, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition issued guidelines regarding probiotic use for the treatment of acute gastroenteritis.29 In addition to rehydration therapy, these guidelines recommend the use of L rhamnosus and/or S boulardii as first-line treatments. Lower quality evidence is available for the use of L reuteri.
CASE › In response to Ms. B’s query about starting her young son on probiotics, you tell her that studies have shown that probiotics are safe for children when given in appropriate doses. They have been shown to help children recover from diarrheal illnesses and can help reduce the number of colds and ear infections when taken regularly. The reason you are giving them determines which strains you should use. You recommend giving her child a formulation of probiotic that contains Lactobacillus or Bifidobacterium with a dose range of 2 to 10 billion CFUs taken daily to reduce the risk of her child getting another ear infection.
CORRESPONDENCE
Paul Dassow, MD, MSPH, 1100 E. 3rd St, Chattanooga, TN 37403; [email protected].
1. Euromonitor International. Global and regional trends of the probiotics and omega fatty acids market. June 23, 2015. Available at: http://uschinahpa.org/wp-content/uploads/2015/07/EMI-US-China-HPA-Probiotic-and-Omega-2015-Final.pdf. Accessed September 9, 2016.
2. Du Toit G, Lack G. Can food allergy be prevented? The current evidence. Pediatr Clin North Am. 2011;58:481-509.
3. Gerritsen J, Smidt H, Rijkers GT, et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209-240.
4. Versalovic J. The human microbiome and probiotics: implications for pediatrics. Ann Nutr Metab. 2013;63:42-52.
5. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
6. Akman I, Kușçu K, Özdemir N, et al. Mothers’ postpartum psychological adjustment and infantile colic. Arch Dis Child. 2006;91:417-419.
7. Sung V, Collett S, de Gooyer T, et al. Probiotics to prevent or treat excessive infant crying systematic review and meta-analysis. JAMA Pediatr. 2013:167:1150-1157.
8. Harb T, Matsuyama M, David M, et al. Infant colic—what works: a systematic review of interventions for breastfed infants. J Pediatr Gastroenterol Nutr. 2016;62:668-686.
9. Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
10. Liu S, Hu P, Du X, et al. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50:377-381.
11. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
12. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. 2015;15:9-20.
13. Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15.
14. Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. Brit Med J. 2013;347:f6471.
15. Cuello-Garcia CA, Bro˙zek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952-961.
16. Mansfield JA, Bergin SW, Cooper JR, et al. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 2014;179:580-592.
17. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
18. Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4:293-304.
19. Rutten JMTM, Korterink JL, Venmans LMAJ, et al. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics. 2015;135:522-535.
20. Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24-30.
21. Turck D, Bernet JP, Marx J, et al. Incidence and risk factors of oral antibiotic associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22-26.
22. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
23. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149-1157.
24. Goldenberg JZ, Lytvyn L, Steurich J, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;12:CD004827.
25. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161.
26. WHO/UNICEF Joint Statement: Clinical Management of Acute Diarrhea. August 2004. Available at: http://www.unicef.org/publications/files/ENAcute_Diarrhoea_reprint.pdf. Accessed September 9, 2016.
27. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst Rev. 2010;(11):CD003048.
28. Applegate JA, Fischer Walker CL, Ambikapathi R, et al. Systematic review of probiotics for the treatment of community-acquired acute diarrhea in children. BMC Public Health. 2013;13:S16.
29. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132-152.
PRACTICE RECOMMENDATIONS
› Recommend a trial of Lactobacillus reuteri for breastfed infants with colic. A
› Consider Lactobacillus and Bifidobacterium species for the prevention of upper respiratory infections (URIs) and to shorten the course of URI illness. B
› Do not recommend probiotics for the prevention of respiratory or gastrointestinal allergies. A
› Consider probiotics for the reduction of abdominal pain in pediatric irritable bowel syndrome, as well as to reduce diarrhea associated with antibiotic use and acute gastroenteritis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Ms. B, a 26-year-old woman, presents to your office with her 3-year-old son for a well-child examination. During the course of the conversation, she asks you if she should be giving her child probiotics to improve his general health. Many of her friends, who also have their children in day care, have told her that probiotics, “are nature’s way of fighting infection.” Her son currently takes no medications, and has no history of asthma or recent gastrointestinal disturbances. He was treated for 2 ear infections last winter, approximately 3 months apart. His physical exam is normal and, after today, his immunizations will be up to date. How should you respond?
The use of probiotics as over-the-counter treatments for a variety of conditions continues to grow, with retail sales of functional probiotic foods and supplements topping $35 billion worldwide in 2014.1 In children, claims of benefit for gastrointestinal (GI) disorders, colic, and allergy prevention, as well as prevention and treatment of upper respiratory infections (URIs) have existed for over 10 years.2-4 The human gut flora develops rapidly after birth and is known to be influenced by route of delivery (vaginal vs cesarean), type of feeding (breast vs formula), and other environmental factors.5 The use of probiotics to influence the types of bacteria in a child’s intestinal tract continues to be an area of active research. (For more on probiotic formulations, see TABLE 1.)

This article summarizes recent research on probiotic use in infants and children. New data support the use of probiotics for the treatment of colic and atopic eczema; however, the data on using probiotics in the management of URIs is less robust and mixed. And while probiotics improve irritable bowel syndrome (IBS) stomach pain, they do not help with related diarrhea or constipation. All of these data are summarized in TABLE 2.6-29

L reuteri improves symptoms in breastfed infants with colic
Infant colic is a relatively common condition known to negatively impact maternal mental health and the mother/child relationship.6 Numerous randomized controlled trials (RCTs) over the years have demonstrated mixed results with using probiotics to decrease crying times, with differences noted between infants who are solely breastfed and those who are not.7
In the most recent meta-analysis of 6 studies (n=427) that focused only on the probiotic Lactobacillus reuteri, breastfed infants with colic receiving a daily dose of 108 colony forming units (CFU) cried an average of 56 fewer minutes/day than those in the control group (95% confidence interval [CI], -64.4 to -47.3; P=.001) at day 21 of treatment.8 Although 2 studies in this meta-analysis included a small number of mixed-fed and formula-fed infants, the majority of trials do not show benefit for these infants. Trials assessing the use of L reuteri for prevention of colic have not shown positive results.7
Probiotics may help p
The mechanisms by which probiotics may prevent or shorten the course of URIs are not obvious. Current theories include boosting the immune function of the respiratory mucosa, acting as a competitive inhibitor for viruses, and secreting antiviral compounds.9 Multiple reviews published in the last 3 years, however, add to the evidence that the apparent benefit is real.
A 2013 meta-analysis assessed data from 4 RCTs (N=1805), which used Lactobacillus rhamnosus as the sole probiotic for prevention of URIs. In treated children, otitis media incidence was reduced by 24% (relative risk [RR] 0.76; 95% CI, 0.64-0.91) and risk of URI was reduced by 38% (RR 0.62; 95% CI, 0.50-0.78).10 The number needed to treat (NNT) was 4 for URI prevention, and the authors noted that adverse events were similar in the treatment and control groups.
A 2014 systematic review and meta-analysis of 20 RCTs examining duration of illness included 10 studies dedicated to pediatric subjects (age 12 months to 12 years).11 There were significantly fewer days of illness per person (standardized mean difference -0.31; 95% CI, -0.41 to -0.11) and each illness episode was shorter by three-quarters of a day (weighted mean difference -0.77; 95% CI, -1.5 to -0.04) in participants who received a probiotic vs those who received a placebo. Probiotics used in these studies belonged to the Lactobacillus and Bifidobacterium genera.
A 2015 systematic review of 14 RCTs assessing the benefits of probiotics, particularly Lactobacillus and Bifidobacterium strains, on URI occurrence and symptoms, showed mixed results.12 Seven of 12 studies found lowered rates of URI and otitis media incidence, 7 of 11 RCTs reported a significant reduction in severity scores for URI, and 4 of 8 RCTs reported significant reductions in school absenteeism between the probiotic and control groups. In a summary statement, the authors noted that “at least one beneficial effect of prophylactic probiotics was observed in the majority of RCTs,” and that “none of the studies reported any serious adverse events.”
Perinatal probiotics: No benefit for allergic conditions—except eczema
Allergic disease is on the rise and continues to plague children with reduced quality of life, potentially life-threatening reactions, and missed activities, including school. The gut microbiome likely influences a child’s allergic propensity through its effects on T-helper cells, transforming growth factor (TGF), and immunoglobulin A (IgA)—all known components of the allergic response. As the hygiene hypothesis suggests, the quantity and types of bacteria that inhabit the GI tract early in life play a significant role in determining a person’s later allergic responses.13
In a 2013 meta-analysis of 20 trials (N=4866), researchers looked specifically at probiotic use and the diagnosis of asthma and incident wheezing. Single and combination products of Lactobacillus and Bifidobacterium given prenatally and/or postnatally were included in the studies. The authors found no evidence to support a protective association between perinatal use of probiotics and diagnosed asthma (RR=0.99; 95% CI, 0.81-0.21) or childhood incident wheezing (RR=0.97; 95% CI, 0.87-1.09; 9 trials, 1949 infants).14
In a more recent meta-analysis (2015) conducted to inform the World Allergy Organization, 29 studies were evaluated to assess the impact of probiotics on allergic symptoms of the skin, respiratory system, and GI tract.15 No significant benefit was noted for any allergic condition except for eczema. Probiotics reduced the risk of eczema when given during the last trimester of pregnancy (RR=0.71; 95% CI, 0.60-0.84), when used by breastfeeding mothers (RR=0.57; 95% CI, 0.47-0.69), and when given to infants (RR=0.80; 95% CI, 0.68-0.94).
A 2014 systematic review and meta-analysis (N=2797) explored probiotic use specifically for the prevention of eczema.16 The pooled relative risk for all the studies was 0.74 (95% CI, 0.67-0.82). Evidence was strongest for probiotics containing the Lactobacillus species rhamnosus and paracasei, as well as for Bifidobacterium lactis. No benefit was noted with Lactobacillus acidophilus or other Bifidobacterium species. These newer reviews on eczema prevention contrast with an older Cochrane review published in 2008 (12 RCTs, N=781), which did not show significant benefit for the treatment of eczema.17
Probiotics improve IBS stomach pain, but not diarrhea or constipation
IBS is a functional disorder of the GI tract that affects up to 20% of children and teenagers and leads to a significant decrease in quality of life.18 Current theories of causation include bacterial overgrowth and neuronal hyperactivity, which may be amenable to change with supplemental probiotics.
A 2015 systematic review of non-pharmacological treatments for functional abdominal pain disorders identified 4 studies dedicated to IBS in children.19 A subgroup analysis of 3 RCTs (n=309) that looked at giving L rhamnosus to 5- to 17-year-olds with IBS showed improved abdominal pain (according to various pain scales) compared to the placebo group. Study participants received at least 3 x 109 CFU twice a day for 4 to 8 weeks. Relative risk for improvement was 1.7 (95% CI, 1.27-2.27) with an NNT of 4. None of these studies showed significant improvement in either frequency or severity of diarrhea or constipation.
A separate crossover RCT (N=59) compared placebo to VSL#3, a product containing 8 probiotics (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, L acidophilus, Lactobacillus plantarum, L paracasei, Lactobacillus bulgaricus, and Streptococcus hermophiles), given in age-dependent doses for 6 weeks to children aged 4 to 18 years.20 The frequency and intensity of abdominal pain were measured on a 5-point Likert scale. The group treated with VSL#3 dropped 1.0 ± 0.2 points vs 0.5 ± 0.2 points in the control group (P<.05) and reported an improved quality of life.
These agents reduce antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) occurs in 5% to 30% of children who receive antibiotic therapy.21 It occurs most frequently with the use of cephalosporins, penicillin, fluoroquinolones, and clindamycin, and is likely caused by an alteration of the normal gut flora. Colitis caused by Clostridium difficile remains the most serious antibiotic-associated GI complication.
A systematic review of the specific probiotic Saccharomyces boulardii conducted in 2015 analyzed data from 6 RCTs (n=1653) to determine the effect of co-administration of this probiotic with antibiotics.22 The pooled relative risk for AAD in children receiving the probiotic was 0.43 (95% CI, 0.3-0.6) compared to antibiotics alone. The absolute risk of AAD dropped from 20.9% to 8.8%, translating to a NNT of 8. Two of the RCTs specifically looked at rates of C difficile infection (n=579). C difficile infection rates dropped by 75% (RR=.25; 95% CI, 0.08-0.73) in the treatment group. This dramatic treatment effect was not seen in studies involving adults.
A similar systematic review focusing on L rhamnosus conducted in 2015 pooled data from 5 RCTs (n=445) to see if the probiotic would decrease AAD in children if it was co-administered with antibiotics.23 The relative risk for AAD in this treatment group was 0.48 (95% CI, 0.26-0.89) with an absolute risk reduction of 13.4% (23% compared to 9.6%), translating to an NNT of 7.
A Cochrane review published in 2015 included 23 studies (N=3938) and found similar results with an RR for AAD of 0.46 for treated children (95% CI, 0.35-0.61).24 Doses of probiotics ranged from 5 to 40 billion CFU/day. Although many probiotic species were used in these studies, S boulardii and L rhamnosus were cited as having the strongest data to support use in this context.
Probiotics reduce the duration, frequency of acute infectious diarrhea
Diarrhea remains the second leading cause of death among children one to 59 months of age worldwide.25 Current World Health Organization recommendations include oral rehydration salts, continued feeding to avoid dehydration, and zinc to decrease the duration and severity of illness.26 Multiple studies in adults confirm that a variety of probiotics decrease both the duration and severity of diarrhea in acute gastroenteritis.27
The authors of a 2013 systematic review of probiotics for the treatment of community-acquired acute diarrhea in children less than 5 years of age analyzed data from 8 RCTs (N=1755).28 Various probiotics were used including Lactobacillus species, Streptococcus thermophilus, Bifidobacterium species, and Saccharomyces boulardii for between 4 and 10 days. Six of these studies (n=1164) measured diarrhea duration and found a 14% reduction (95% CI, 3.8%-24.2%) in days of illness for those children treated vs those receiving placebo. Five studies (n=925) measured the difference in stool frequency on Day 2 of illness and reported a reduction of 13.1% (95% CI, 0.8%-5.3%) in the number of stools in the treated group vs the placebo group.
This review augments a Cochrane meta-analysis of 63 studies (N=8014) published in 2010.27 Fifty-six of these studies included infants and children. Pooled analysis of the varied probiotic treatments showed a mean reduction in duration of diarrhea of just over a day (24.76 hours; 95% CI, 15.9-33.6 hours; n=4555, trials=35) and decreased stool frequency on Day 2 of treatment (mean difference 0.80; 95% CI, 0.45-1.14; n=2751, trials=20). The authors concluded that probiotics “have clear beneficial effects in shortening the duration and reducing stool frequency in acute infectious diarrhea.”
Pediatric society weighs in. In 2014, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition issued guidelines regarding probiotic use for the treatment of acute gastroenteritis.29 In addition to rehydration therapy, these guidelines recommend the use of L rhamnosus and/or S boulardii as first-line treatments. Lower quality evidence is available for the use of L reuteri.
CASE › In response to Ms. B’s query about starting her young son on probiotics, you tell her that studies have shown that probiotics are safe for children when given in appropriate doses. They have been shown to help children recover from diarrheal illnesses and can help reduce the number of colds and ear infections when taken regularly. The reason you are giving them determines which strains you should use. You recommend giving her child a formulation of probiotic that contains Lactobacillus or Bifidobacterium with a dose range of 2 to 10 billion CFUs taken daily to reduce the risk of her child getting another ear infection.
CORRESPONDENCE
Paul Dassow, MD, MSPH, 1100 E. 3rd St, Chattanooga, TN 37403; [email protected].
PRACTICE RECOMMENDATIONS
› Recommend a trial of Lactobacillus reuteri for breastfed infants with colic. A
› Consider Lactobacillus and Bifidobacterium species for the prevention of upper respiratory infections (URIs) and to shorten the course of URI illness. B
› Do not recommend probiotics for the prevention of respiratory or gastrointestinal allergies. A
› Consider probiotics for the reduction of abdominal pain in pediatric irritable bowel syndrome, as well as to reduce diarrhea associated with antibiotic use and acute gastroenteritis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Ms. B, a 26-year-old woman, presents to your office with her 3-year-old son for a well-child examination. During the course of the conversation, she asks you if she should be giving her child probiotics to improve his general health. Many of her friends, who also have their children in day care, have told her that probiotics, “are nature’s way of fighting infection.” Her son currently takes no medications, and has no history of asthma or recent gastrointestinal disturbances. He was treated for 2 ear infections last winter, approximately 3 months apart. His physical exam is normal and, after today, his immunizations will be up to date. How should you respond?
The use of probiotics as over-the-counter treatments for a variety of conditions continues to grow, with retail sales of functional probiotic foods and supplements topping $35 billion worldwide in 2014.1 In children, claims of benefit for gastrointestinal (GI) disorders, colic, and allergy prevention, as well as prevention and treatment of upper respiratory infections (URIs) have existed for over 10 years.2-4 The human gut flora develops rapidly after birth and is known to be influenced by route of delivery (vaginal vs cesarean), type of feeding (breast vs formula), and other environmental factors.5 The use of probiotics to influence the types of bacteria in a child’s intestinal tract continues to be an area of active research. (For more on probiotic formulations, see TABLE 1.)

This article summarizes recent research on probiotic use in infants and children. New data support the use of probiotics for the treatment of colic and atopic eczema; however, the data on using probiotics in the management of URIs is less robust and mixed. And while probiotics improve irritable bowel syndrome (IBS) stomach pain, they do not help with related diarrhea or constipation. All of these data are summarized in TABLE 2.6-29

L reuteri improves symptoms in breastfed infants with colic
Infant colic is a relatively common condition known to negatively impact maternal mental health and the mother/child relationship.6 Numerous randomized controlled trials (RCTs) over the years have demonstrated mixed results with using probiotics to decrease crying times, with differences noted between infants who are solely breastfed and those who are not.7
In the most recent meta-analysis of 6 studies (n=427) that focused only on the probiotic Lactobacillus reuteri, breastfed infants with colic receiving a daily dose of 108 colony forming units (CFU) cried an average of 56 fewer minutes/day than those in the control group (95% confidence interval [CI], -64.4 to -47.3; P=.001) at day 21 of treatment.8 Although 2 studies in this meta-analysis included a small number of mixed-fed and formula-fed infants, the majority of trials do not show benefit for these infants. Trials assessing the use of L reuteri for prevention of colic have not shown positive results.7
Probiotics may help p
The mechanisms by which probiotics may prevent or shorten the course of URIs are not obvious. Current theories include boosting the immune function of the respiratory mucosa, acting as a competitive inhibitor for viruses, and secreting antiviral compounds.9 Multiple reviews published in the last 3 years, however, add to the evidence that the apparent benefit is real.
A 2013 meta-analysis assessed data from 4 RCTs (N=1805), which used Lactobacillus rhamnosus as the sole probiotic for prevention of URIs. In treated children, otitis media incidence was reduced by 24% (relative risk [RR] 0.76; 95% CI, 0.64-0.91) and risk of URI was reduced by 38% (RR 0.62; 95% CI, 0.50-0.78).10 The number needed to treat (NNT) was 4 for URI prevention, and the authors noted that adverse events were similar in the treatment and control groups.
A 2014 systematic review and meta-analysis of 20 RCTs examining duration of illness included 10 studies dedicated to pediatric subjects (age 12 months to 12 years).11 There were significantly fewer days of illness per person (standardized mean difference -0.31; 95% CI, -0.41 to -0.11) and each illness episode was shorter by three-quarters of a day (weighted mean difference -0.77; 95% CI, -1.5 to -0.04) in participants who received a probiotic vs those who received a placebo. Probiotics used in these studies belonged to the Lactobacillus and Bifidobacterium genera.
A 2015 systematic review of 14 RCTs assessing the benefits of probiotics, particularly Lactobacillus and Bifidobacterium strains, on URI occurrence and symptoms, showed mixed results.12 Seven of 12 studies found lowered rates of URI and otitis media incidence, 7 of 11 RCTs reported a significant reduction in severity scores for URI, and 4 of 8 RCTs reported significant reductions in school absenteeism between the probiotic and control groups. In a summary statement, the authors noted that “at least one beneficial effect of prophylactic probiotics was observed in the majority of RCTs,” and that “none of the studies reported any serious adverse events.”
Perinatal probiotics: No benefit for allergic conditions—except eczema
Allergic disease is on the rise and continues to plague children with reduced quality of life, potentially life-threatening reactions, and missed activities, including school. The gut microbiome likely influences a child’s allergic propensity through its effects on T-helper cells, transforming growth factor (TGF), and immunoglobulin A (IgA)—all known components of the allergic response. As the hygiene hypothesis suggests, the quantity and types of bacteria that inhabit the GI tract early in life play a significant role in determining a person’s later allergic responses.13
In a 2013 meta-analysis of 20 trials (N=4866), researchers looked specifically at probiotic use and the diagnosis of asthma and incident wheezing. Single and combination products of Lactobacillus and Bifidobacterium given prenatally and/or postnatally were included in the studies. The authors found no evidence to support a protective association between perinatal use of probiotics and diagnosed asthma (RR=0.99; 95% CI, 0.81-0.21) or childhood incident wheezing (RR=0.97; 95% CI, 0.87-1.09; 9 trials, 1949 infants).14
In a more recent meta-analysis (2015) conducted to inform the World Allergy Organization, 29 studies were evaluated to assess the impact of probiotics on allergic symptoms of the skin, respiratory system, and GI tract.15 No significant benefit was noted for any allergic condition except for eczema. Probiotics reduced the risk of eczema when given during the last trimester of pregnancy (RR=0.71; 95% CI, 0.60-0.84), when used by breastfeeding mothers (RR=0.57; 95% CI, 0.47-0.69), and when given to infants (RR=0.80; 95% CI, 0.68-0.94).
A 2014 systematic review and meta-analysis (N=2797) explored probiotic use specifically for the prevention of eczema.16 The pooled relative risk for all the studies was 0.74 (95% CI, 0.67-0.82). Evidence was strongest for probiotics containing the Lactobacillus species rhamnosus and paracasei, as well as for Bifidobacterium lactis. No benefit was noted with Lactobacillus acidophilus or other Bifidobacterium species. These newer reviews on eczema prevention contrast with an older Cochrane review published in 2008 (12 RCTs, N=781), which did not show significant benefit for the treatment of eczema.17
Probiotics improve IBS stomach pain, but not diarrhea or constipation
IBS is a functional disorder of the GI tract that affects up to 20% of children and teenagers and leads to a significant decrease in quality of life.18 Current theories of causation include bacterial overgrowth and neuronal hyperactivity, which may be amenable to change with supplemental probiotics.
A 2015 systematic review of non-pharmacological treatments for functional abdominal pain disorders identified 4 studies dedicated to IBS in children.19 A subgroup analysis of 3 RCTs (n=309) that looked at giving L rhamnosus to 5- to 17-year-olds with IBS showed improved abdominal pain (according to various pain scales) compared to the placebo group. Study participants received at least 3 x 109 CFU twice a day for 4 to 8 weeks. Relative risk for improvement was 1.7 (95% CI, 1.27-2.27) with an NNT of 4. None of these studies showed significant improvement in either frequency or severity of diarrhea or constipation.
A separate crossover RCT (N=59) compared placebo to VSL#3, a product containing 8 probiotics (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, L acidophilus, Lactobacillus plantarum, L paracasei, Lactobacillus bulgaricus, and Streptococcus hermophiles), given in age-dependent doses for 6 weeks to children aged 4 to 18 years.20 The frequency and intensity of abdominal pain were measured on a 5-point Likert scale. The group treated with VSL#3 dropped 1.0 ± 0.2 points vs 0.5 ± 0.2 points in the control group (P<.05) and reported an improved quality of life.
These agents reduce antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) occurs in 5% to 30% of children who receive antibiotic therapy.21 It occurs most frequently with the use of cephalosporins, penicillin, fluoroquinolones, and clindamycin, and is likely caused by an alteration of the normal gut flora. Colitis caused by Clostridium difficile remains the most serious antibiotic-associated GI complication.
A systematic review of the specific probiotic Saccharomyces boulardii conducted in 2015 analyzed data from 6 RCTs (n=1653) to determine the effect of co-administration of this probiotic with antibiotics.22 The pooled relative risk for AAD in children receiving the probiotic was 0.43 (95% CI, 0.3-0.6) compared to antibiotics alone. The absolute risk of AAD dropped from 20.9% to 8.8%, translating to a NNT of 8. Two of the RCTs specifically looked at rates of C difficile infection (n=579). C difficile infection rates dropped by 75% (RR=.25; 95% CI, 0.08-0.73) in the treatment group. This dramatic treatment effect was not seen in studies involving adults.
A similar systematic review focusing on L rhamnosus conducted in 2015 pooled data from 5 RCTs (n=445) to see if the probiotic would decrease AAD in children if it was co-administered with antibiotics.23 The relative risk for AAD in this treatment group was 0.48 (95% CI, 0.26-0.89) with an absolute risk reduction of 13.4% (23% compared to 9.6%), translating to an NNT of 7.
A Cochrane review published in 2015 included 23 studies (N=3938) and found similar results with an RR for AAD of 0.46 for treated children (95% CI, 0.35-0.61).24 Doses of probiotics ranged from 5 to 40 billion CFU/day. Although many probiotic species were used in these studies, S boulardii and L rhamnosus were cited as having the strongest data to support use in this context.
Probiotics reduce the duration, frequency of acute infectious diarrhea
Diarrhea remains the second leading cause of death among children one to 59 months of age worldwide.25 Current World Health Organization recommendations include oral rehydration salts, continued feeding to avoid dehydration, and zinc to decrease the duration and severity of illness.26 Multiple studies in adults confirm that a variety of probiotics decrease both the duration and severity of diarrhea in acute gastroenteritis.27
The authors of a 2013 systematic review of probiotics for the treatment of community-acquired acute diarrhea in children less than 5 years of age analyzed data from 8 RCTs (N=1755).28 Various probiotics were used including Lactobacillus species, Streptococcus thermophilus, Bifidobacterium species, and Saccharomyces boulardii for between 4 and 10 days. Six of these studies (n=1164) measured diarrhea duration and found a 14% reduction (95% CI, 3.8%-24.2%) in days of illness for those children treated vs those receiving placebo. Five studies (n=925) measured the difference in stool frequency on Day 2 of illness and reported a reduction of 13.1% (95% CI, 0.8%-5.3%) in the number of stools in the treated group vs the placebo group.
This review augments a Cochrane meta-analysis of 63 studies (N=8014) published in 2010.27 Fifty-six of these studies included infants and children. Pooled analysis of the varied probiotic treatments showed a mean reduction in duration of diarrhea of just over a day (24.76 hours; 95% CI, 15.9-33.6 hours; n=4555, trials=35) and decreased stool frequency on Day 2 of treatment (mean difference 0.80; 95% CI, 0.45-1.14; n=2751, trials=20). The authors concluded that probiotics “have clear beneficial effects in shortening the duration and reducing stool frequency in acute infectious diarrhea.”
Pediatric society weighs in. In 2014, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition issued guidelines regarding probiotic use for the treatment of acute gastroenteritis.29 In addition to rehydration therapy, these guidelines recommend the use of L rhamnosus and/or S boulardii as first-line treatments. Lower quality evidence is available for the use of L reuteri.
CASE › In response to Ms. B’s query about starting her young son on probiotics, you tell her that studies have shown that probiotics are safe for children when given in appropriate doses. They have been shown to help children recover from diarrheal illnesses and can help reduce the number of colds and ear infections when taken regularly. The reason you are giving them determines which strains you should use. You recommend giving her child a formulation of probiotic that contains Lactobacillus or Bifidobacterium with a dose range of 2 to 10 billion CFUs taken daily to reduce the risk of her child getting another ear infection.
CORRESPONDENCE
Paul Dassow, MD, MSPH, 1100 E. 3rd St, Chattanooga, TN 37403; [email protected].
1. Euromonitor International. Global and regional trends of the probiotics and omega fatty acids market. June 23, 2015. Available at: http://uschinahpa.org/wp-content/uploads/2015/07/EMI-US-China-HPA-Probiotic-and-Omega-2015-Final.pdf. Accessed September 9, 2016.
2. Du Toit G, Lack G. Can food allergy be prevented? The current evidence. Pediatr Clin North Am. 2011;58:481-509.
3. Gerritsen J, Smidt H, Rijkers GT, et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209-240.
4. Versalovic J. The human microbiome and probiotics: implications for pediatrics. Ann Nutr Metab. 2013;63:42-52.
5. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
6. Akman I, Kușçu K, Özdemir N, et al. Mothers’ postpartum psychological adjustment and infantile colic. Arch Dis Child. 2006;91:417-419.
7. Sung V, Collett S, de Gooyer T, et al. Probiotics to prevent or treat excessive infant crying systematic review and meta-analysis. JAMA Pediatr. 2013:167:1150-1157.
8. Harb T, Matsuyama M, David M, et al. Infant colic—what works: a systematic review of interventions for breastfed infants. J Pediatr Gastroenterol Nutr. 2016;62:668-686.
9. Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
10. Liu S, Hu P, Du X, et al. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50:377-381.
11. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
12. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. 2015;15:9-20.
13. Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15.
14. Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. Brit Med J. 2013;347:f6471.
15. Cuello-Garcia CA, Bro˙zek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952-961.
16. Mansfield JA, Bergin SW, Cooper JR, et al. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 2014;179:580-592.
17. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
18. Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4:293-304.
19. Rutten JMTM, Korterink JL, Venmans LMAJ, et al. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics. 2015;135:522-535.
20. Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24-30.
21. Turck D, Bernet JP, Marx J, et al. Incidence and risk factors of oral antibiotic associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22-26.
22. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
23. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149-1157.
24. Goldenberg JZ, Lytvyn L, Steurich J, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;12:CD004827.
25. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161.
26. WHO/UNICEF Joint Statement: Clinical Management of Acute Diarrhea. August 2004. Available at: http://www.unicef.org/publications/files/ENAcute_Diarrhoea_reprint.pdf. Accessed September 9, 2016.
27. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst Rev. 2010;(11):CD003048.
28. Applegate JA, Fischer Walker CL, Ambikapathi R, et al. Systematic review of probiotics for the treatment of community-acquired acute diarrhea in children. BMC Public Health. 2013;13:S16.
29. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132-152.
1. Euromonitor International. Global and regional trends of the probiotics and omega fatty acids market. June 23, 2015. Available at: http://uschinahpa.org/wp-content/uploads/2015/07/EMI-US-China-HPA-Probiotic-and-Omega-2015-Final.pdf. Accessed September 9, 2016.
2. Du Toit G, Lack G. Can food allergy be prevented? The current evidence. Pediatr Clin North Am. 2011;58:481-509.
3. Gerritsen J, Smidt H, Rijkers GT, et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209-240.
4. Versalovic J. The human microbiome and probiotics: implications for pediatrics. Ann Nutr Metab. 2013;63:42-52.
5. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
6. Akman I, Kușçu K, Özdemir N, et al. Mothers’ postpartum psychological adjustment and infantile colic. Arch Dis Child. 2006;91:417-419.
7. Sung V, Collett S, de Gooyer T, et al. Probiotics to prevent or treat excessive infant crying systematic review and meta-analysis. JAMA Pediatr. 2013:167:1150-1157.
8. Harb T, Matsuyama M, David M, et al. Infant colic—what works: a systematic review of interventions for breastfed infants. J Pediatr Gastroenterol Nutr. 2016;62:668-686.
9. Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
10. Liu S, Hu P, Du X, et al. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50:377-381.
11. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
12. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. 2015;15:9-20.
13. Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15.
14. Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. Brit Med J. 2013;347:f6471.
15. Cuello-Garcia CA, Bro˙zek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952-961.
16. Mansfield JA, Bergin SW, Cooper JR, et al. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 2014;179:580-592.
17. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
18. Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Expert Rev Gastroenterol Hepatol. 2010;4:293-304.
19. Rutten JMTM, Korterink JL, Venmans LMAJ, et al. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics. 2015;135:522-535.
20. Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24-30.
21. Turck D, Bernet JP, Marx J, et al. Incidence and risk factors of oral antibiotic associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22-26.
22. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
23. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149-1157.
24. Goldenberg JZ, Lytvyn L, Steurich J, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;12:CD004827.
25. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161.
26. WHO/UNICEF Joint Statement: Clinical Management of Acute Diarrhea. August 2004. Available at: http://www.unicef.org/publications/files/ENAcute_Diarrhoea_reprint.pdf. Accessed September 9, 2016.
27. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst Rev. 2010;(11):CD003048.
28. Applegate JA, Fischer Walker CL, Ambikapathi R, et al. Systematic review of probiotics for the treatment of community-acquired acute diarrhea in children. BMC Public Health. 2013;13:S16.
29. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132-152.