User login
Strategies for improving ADHD medication adherence
Attention-deficit/hyperactivity disorder (ADHD) is the most common childhood neurodevelopmental disorder, affecting 8% to 12% of school-aged children in the United States1-3 with significant impairments that often persist into adulthood.4-8 Current guidelines recommend stimulant medication and/or behavioral therapies as first-line treatments for ADHD.9,10 There is a wealth of evidence on the efficacy of stimulants in ADHD, with the most significant effects noted on core ADHD symptoms.11,12 Additional evidence links stimulants to decreased long-term negative outcomes, including reduced school absences and grade retention,13 as well as modestly but significantly improved reading and math scores.14 Other studies have reported that individuals with ADHD who receive medication have decreased criminality,15,16 motor vehicle accidents,17,18 injuries,19 substance abuse,20-22 and risk for subsequent and concurrent depression.23 Therefore, the evidence suggests that consistent medication treatment helps improve outcomes for individuals with ADHD.
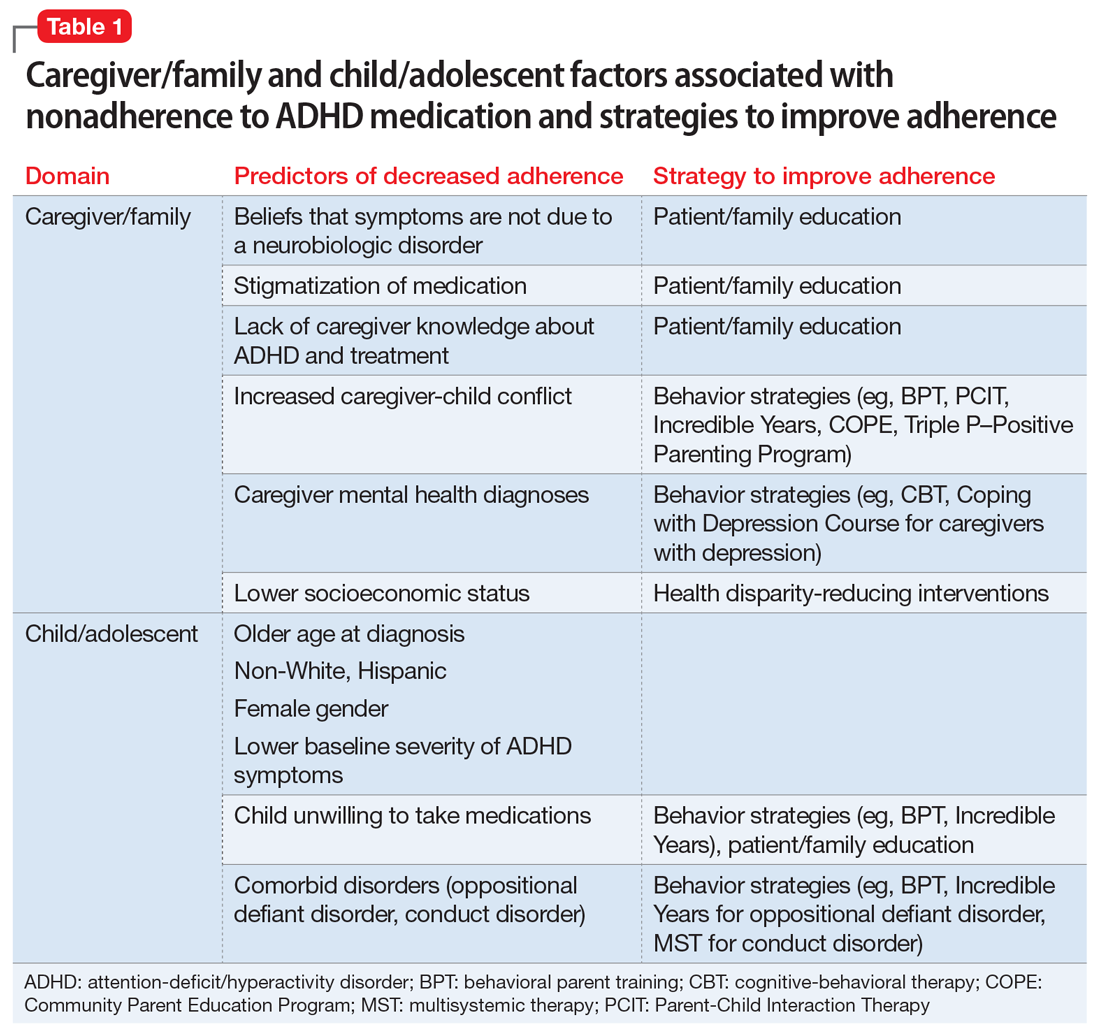
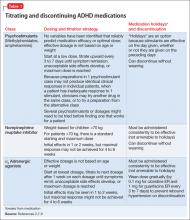
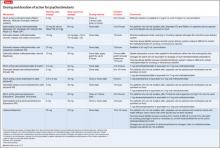
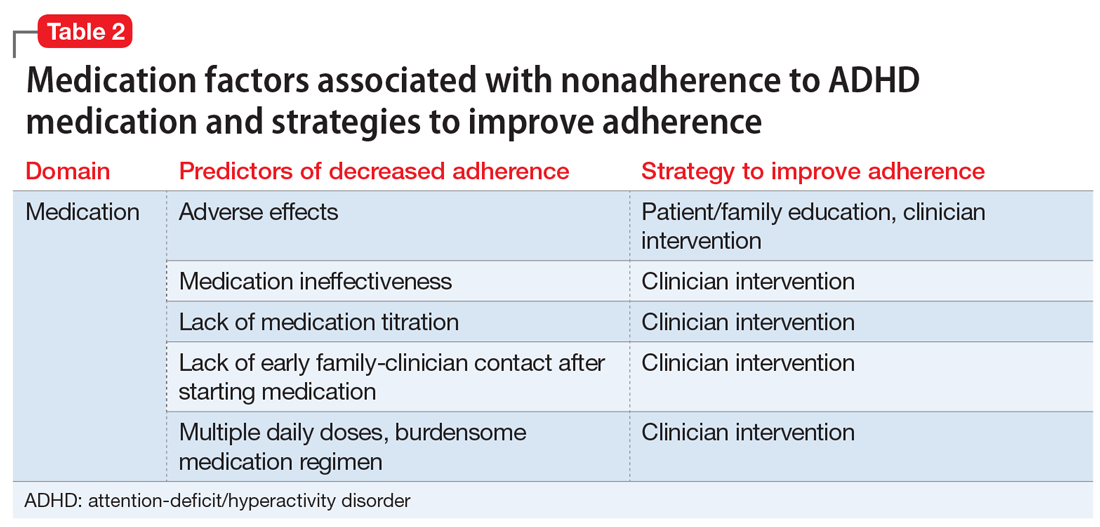
Adherence is defined as “the extent to which a person’s behavior (eg, taking medication) corresponds with agreed recommendations from a clinician.”24 Unfortunately, pediatric ADHD medication adherence has been found to be poor (approximately 64%).25-30 Nonadherence to ADHD medication has been linked to multiple factors, including caregiver/family and child/adolescent factors (Table 1), medication-related factors (Table 2), and health care/system factors (Table 3). Understanding and addressing these factors is essential to maximizing long-term outcomes. In this article, we review the factors associated with nonadherence to ADHD medication, and outline strategies to improve adherence.
Caregiver/family characteristics
Caregiver beliefs about ADHD and their attitudes toward treatment have been associated with the initiation of and adherence to ADHD medication. For example, caregivers who view a child’s difficulties as a medical disorder that requires a biologic intervention are more likely to accept and adhere to medication.31 Similarly, caregivers who perceive ADHD medication as safe, effective, and socially acceptable are more likely to be treatment-adherent.32-35
- increased caregiver knowledge about ADHD33
- receiving an ADHD diagnosis based on a thorough diagnostic process (ie, comprehensive psychological testing)36
- satisfaction with information about medicine
- comfort with the treatment plan.34
Socioeconomic status, family functioning, and caregiver mental health diagnoses (eg, ADHD, depression) have also been linked to ADHD medication adherence. Several studies, including the Multimodal Treatment Study of Children with ADHD,11 a landmark study of stimulant medication for children with ADHD, have found an association between low income and decreased likelihood of receiving ADHD medication.2,37-39 Further, Gau et al40 found that negative caregiver-child relationships and family dysfunction were associated with poor medication adherence in children with ADHD.9 Prior studies have also shown that mothers of children with ADHD are more likely to have depression and/or anxiety,41,42 and that caregivers with a history of mental health diagnoses are more accepting of initiating medication treatment for their children.43 However, additional studies have found that caregiver mental health diagnoses decreased the likelihood of ADHD medication adherence.40,44

Child characteristics
Child characteristics associated with decreased ADHD medication adherence include older age (eg, adolescents vs school-aged children),29,30,34,40,45-47 non-White race, Hispanic ethnicity,29,33,48-51 female gender,29,33,52 lower baseline ADHD symptom severity,30,37 and child unwillingness to take medications.34 However, prior studies have not been completely consistent about the relationship between child comorbid conditions (eg, oppositional defiant disorder [ODD], conduct disorder) and ADHD medication adherence. A few studies found that child comorbid conditions, especially ODD, mediate poor ADHD medication adherence, possibly secondary to an increased caregiver-child conflict.30,53,54 However, other studies have reported that the presence of comorbid ODD, depression, and anxiety predicted increased adherence to ADHD medications.37,46
Medication-related factors
Adverse effects of medications are the most commonly cited reason for ADHD medication nonadherence
On the other hand, increased ADHD medication effectiveness has been associated with improved medication adherence.5,34,54-56 Medication titration and dosing factors have also been shown to affect adherence. Specifically, adherence has been improved when ADHD medications are titrated in a systematic manner soon after starting treatment, and when families have an early first contact with a physician after starting medication (within 3 months).28 In addition, use of a simplified dose regimen has been linked to better adherence: patients who are prescribed long-acting stimulants are more likely to adhere to treatment compared with patients who take short-acting formulations.26,40,49,61-63 It is possible that long-acting stimulants increase adherence because they produce more even and sustained effects on ADHD symptoms throughout the day, compared with short-acting formulations.64 Furthermore, the inconvenience of taking multiple doses throughout the day, as well as the potential social stigma of mid-school day dosing, may negatively impact adherence to short-acting formulations.10
Continue to: Health care/system factors
Health care/system factors
Several studies have investigated the influence of health services factors on ADHD medication adherence. Specifically, limited transportation services and lack of mental health providers in the community have been linked to decreased ADHD medication adherence.47,65,66 Furthermore, limited insurance coverage and higher costs of ADHD medications, which lead to substantial out-of-pocket payments for families, have been associated with decreased likelihood of ADHD medication adherence.29,67
Clinician-related factors also can affect ADHD medication adherence. For example, a clinician’s lack knowledge of ADHD care can negatively impact ADHD medication adherence.68 Two studies have documented improved ADHD medication adherence when treatment is provided by specialists (eg, child psychiatrists) rather than by community primary care providers, possibly because specialists are more likely to provide close stimulant titration and monitoring (ie, ≥ 3 visits in the first 90 days) and use higher maximum doses.62,69 Furthermore, ADHD medication initiation and adherence are increased when patients have a strong working alliance with their clinician and trust the health care system,31,34,35 as well as when there is a match between the caregiver’s and clinician’s perception of the cause, course, and best treatment practices for a child’s ADHD.65
Strategies to improve medication adherence
A number of strategies to improve ADHD medication adherence can be derived from our knowledge of the factors that influence adherence.
Patient/family education. Unanswered questions about ADHD diagnosis, etiology, and medication adverse effects can negatively impact the ADHD treatment process. Therefore, patient/family education regarding ADHD and its management is necessary to improve medication adherence, because it helps families attain the knowledge, confidence, and motivation to manage their child’s condition.
Clinicians have an important role in educating patients about70:
- the medications they are taking
- why they are taking them
- what the medications look like
- the time of medication administration
- the potential adverse effects
- what to do if adverse effects occur
- what regular testing/monitoring is necessary.
Clinicians can provide appropriate psychoeducation by sharing written materials and trusted websites with families (see Related Resources).
Behavioral strategies. Behavioral interventions have been among the most effective strategies for improving medication adherence in other chronic conditions.71 Behavioral strategies are likely to be particularly important for families of children with ADHD and comorbid conditions such as ODD because these families experience considerable caregiver-child conflict.72 Moreover, parents of children with ADHD are at higher risk for having ADHD and depression themselves,73 both of which may interfere with a parent’s ability to obtain and administer medications consistently. Thus, for these families, using a combination of psychoeducation and behavioral strategies will be necessary to affect change in attitude and behavior. Behavioral strategies that can be used to improve medication adherence include:
- Technology-based interventions can reduce the impact of environmental barriers to adherence. For example, pharmacy automatic prescription renewal systems can reduce the likelihood of families failing to obtain ADHD medication refills. Pill reminder boxes, smartphone alerts, and setting various alarms can effectively prompt caregivers/patients to administer medication. In particular, these methods can be crucial in families for which multiple members have ADHD and its attendant difficulties with organization and task completion.
- Caregiver training may assist families in developing specific behavioral management skills that support adherence. This training can be as straightforward as instructing caregivers on the use of positive reinforcement when teaching their children to swallow pills. It may also encompass structured behavioral interventions aimed at training caregivers to utilize rewards and consequences in order to maximize medication adherence.74
Continue to: Clinician interventions
Clinician interventions. Clinicians can use decision aids to help inform families about treatment options, promote shared decision making, and decrease uncertainty about the treatment plan75 (see Related Resources). Early titration of ADHD medications and early first contact (within months of starting medication treatment) between caregivers and clinicians, whether via in-person visit, telephone, or email, have also been related to improved adherence.28 Furthermore, clinicians can improve adherence by prescribing a simplified medication regimen (ie, long-acting formulations that provide full-day coverage). To address the negative impact of high out-of-pocket ADHD medication costs on adherence, clinicians can also prescribe generic preparations and/or “preferred” medications options on an individual patient’s formulary.
Because clinician knowledge and expertise in ADHD care has been linked to improved patient medication adherence,68 clinicians are encouraged to use the American Academy of Pediatrics (AAP) guideline for diagnosis and treatment of ADHD, which includes a supplemental process of care algorithm (last published in 2011,10 with an updated guideline anticipated in 2019), as well as the AAP/National Institute for Children’s Health Quality (NICHQ) ADHD Toolkit,76 which includes items helpful for ADHD diagnosis and treatment. The Society for Developmental and Behavioral Pediatrics is also developing a clinical practice guideline for the diagnosis and treatment of complex ADHD (ie, ADHD complicated by coexisting mental health, developmental, and/or psychosocial conditions or issues), with publication anticipated in 2019. Primary care providers can also improve their expertise in ADHD care by pursuing additional mental health–related trainings (such as those conducted by the REACH Institute).77
Because receiving ADHD care from a specialist has been shown to improve medication initiation and adherence,62,69 other strategies to address the short supply of child psychiatrists include offering incentives to medical students to pursue a career in child psychiatry (eg, loan forgiveness). Telepsychiatry and co-location of mental health specialists and primary care providers are additional innovative ways in which ADHD specialty care can be delivered to more patients.64
Finally, providing culturally-sensitive care can strengthen the clinician-caregiver relationship and promote adherence to treatment. For example, clinicians can partner with local groups to increase their understanding of how different racial/ethnic groups perceive ADHD and its treatment.64
Peer support models. Peers are credible role models who have a valued role in facilitating the use of mental health services by empowering families and enhancing service satisfaction.78 In several communities in the United States, peer models using family advocates have been introduced.79 Family advocates are typically caregivers of children who have special needs or have been involved in the mental health system. Their perspective—as peers and first-hand consumers of the health care and/or mental health system—can make them powerful and effective coaches to families of children with ADHD. By helping families to navigate ADHD care systems successfully, family advocates can play an important role in enhancing ADHD medication adherence, although further investigation is needed. In addition, the stigma around ADHD medication use, which adversely impacts adherence, can be mitigated if caregivers participate in organized ADHD-related support groups (eg, Children and Adults with ADHD [CHADD]).
Continue to: Health disparity-reducing interventions
Health disparity-reducing interventions. Successful health disparity-reducing interventions—such as those developed to enhance care of other chronic disorders including asthma and diabetes—can be applied to improve ADHD care. These interventions, which include medical-legal partnerships (eg, between clinicians, social workers, legal advocates, and community partners) in primary care centers, have been shown to improve health insurance coverage and therefore health care access.80,81 Although some hardships linked to nonadherence (eg, low socioeconomic status) may not be amenable to health care–related interventions, screening for these hardships can identify children who are most at risk for poor adherence. This would alert clinicians to proactively identify barriers to adherence and implement mitigation strategies. This might include developing more streamlined, easier-to-follow management plans for these patients, such as those that can be delivered through pharmacist-physician collaborative programs82 and school-based therapy programs.83-85
Bottom Line
Suboptimal adherence to medications for attention-deficit/hyperactivity disorder (ADHD) can be addressed through patient/family education, behavioral strategies, clinician interventions, peer support models, and health disparity-reducing interventions. By improving ADHD treatment adherence, these interventions have the potential to maximize long-term outcomes.
Related Resources
- Cohen Children’s Medical Center Northwell Health. The ADHD Medication Guide. www.ADHDMedicationGuide.com. Revised December 31, 2017.
- Cincinnati Children’s Hospital Medical Center. Decision aids to facilitate shared decision making in practice. www.cincinnatichildrens.org/service/j/anderson-center/ evidence-based-care/decision-aids.
- CHADD. Children and Adults with attention-deficit/ hyperactivity disorder. www.chadd.org.
Drug Brand Name
Methylphenidate • Concerta, Ritalin
1. Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857-864.
2. Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119 (Suppl 1):S99-S106.
3. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199-212.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484-500.
5. Charach A, Dashti B, Carson P, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at-risk preschoolers; long-term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.ncbi.nlm.nih.gov/books/NBK82368/.
6. Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010;46(3):209-217.
7. Barkley RA, Fischer M, Smallish L, et al. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
8. Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631-642.
9. Pliszka S, the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
10. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
11. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
12. Abikoff H, Hechtman L, Klein RG, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43(7):802-811.
13. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. J Dev Behav Pediatr. 2007;28(4):265-273.
14. Scheffler RM, Brown TT, Fulton BD, et al. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123(5):1273-1279.
15. Dalsgaard S, Nielsen HS, Simonsen M. Five-fold increase in national prevalence rates of attention-deficit/hyperactivity disorder medications for children and adolescents with autism spectrum disorder, attention-deficit/hyperactivity disorder, and other psychiatric disorders: a Danish register-based study. J Child Adolesc Psychopharmacol. 2013;23(7):432-439.
16. Lichtenstein P, Halldner L, Zetterqvist J, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006-2014.
17. Chang Z, Lichtenstein P, D’Onofrio BM, et al. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA Psychiatry. 2014;71(3):319-325.
18. Chang Z, Quinn PD, Hur K, et al. Association between medication use for attention-deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry. 2017;74(6):597-603.
19. Dalsgaard S, Leckman JF, Mortensen PB, et al. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry. 2015;2(8):702-709.
20. Chang Z, Lichtenstein P, Halldner L, et al. Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry. 2014;55(8):878-885.
21. Fischer M, Barkley RA. Childhood stimulant treatment and risk for later substance abuse. J Clin Psychiatry. 2003;64(Suppl 11):19-23.
22. Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003;64(Suppl 11):3-8.
23. Chang Z, D’Onofrio BM, Quinn PD, et al. Medicationfor attention-deficit/hyperactivity disorder and risk for depression: a nationwide longitudinal cohort study. Biol Psychiatry. 2016;80(12):916-922.
24. World Health Organization. Adherence to long-term therapies: evidence for action. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. Published 2003. Accessed July 22, 2019.
25. Perwien A, Hall J, Swensen A, et al. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122-129.
26. Faraone SV, Biederman J, Zimmerman B. An analysis of patient adherence to treatment during a 1-year, open-label study of OROS methylphenidate in children with ADHD. J Atten Disord. 2007;11(2):157-166.
27. Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin. 2011;27(Suppl 2):13-22.
28. Brinkman WB, Baum R, Kelleher KJ, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry. 2016;55(4):289-294.
29. Bokhari FAS, Heiland F, Levine P, et al. Risk factors for discontinuing drug therapy among children with ADHD. Health Services and Outcomes Research Methodology. 2008;8(3):134-158.
30. Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40(8):922-928.
31. DosReis S, Mychailyszyn MP, Evans-Lacko SE, et al. The meaning of attention-deficit/hyperactivity disorder medication and parents’ initiation and continuity of treatment for their child. J Child Adolesc Psychopharmacol. 2009;19(4):377-383.
32. dosReis S, Myers MA. Parental attitudes and involvement in psychopharmacological treatment for ADHD: a conceptual model. Int Rev Psychiatry. 2008;20(2):135-141.
33. Bussing R, Koro-Ljungberg M, Noguchi K, et al. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med. 2012;74(1):92-100.
34. Brinkman WB, Sucharew H, Majcher JH, et al. Predictors of medication continuity in children with ADHD. Pediatrics. 2018;141(6). doi: 10.1542/peds.2017-2580.
35. Coletti DJ, Pappadopulos E, Katsiotas NJ, et al. Parent perspectives on the decision to initiate medication treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(3):226-237.
36. Bussing R, Gary FA. Practice guidelines and parental ADHD treatment evaluations: friends or foes? Harv Rev Psychiatry. 2001;9(5):223-233.
37. Charach A, Gajaria A. Improving psychostimulant adherence in children with ADHD. Expert Rev Neurother. 2008;8(10):1563-1571.
38. Rieppi R, Greenhill LL, Ford RE, et al. Socioeconomic status as a moderator of ADHD treatment outcomes. J Am Acad Child Adolesc Psychiatry. 2002;41(3):269-277.
39. Swanson JM, Hinshaw SP, Arnold LE, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1003-1014.
40. Gau SS, Shen HY, Chou MC, et al. Determinants of adherence to methylphenidate and the impact of poor adherence on maternal and family measures. J Child Adolesc Psychopharmacol. 2006;16(3):286-297.
41. Barkley RA, Fischer M, Edelbrock C, et al. The adolescent outcome of hyperactive children diagnosed by research criteria--III. Mother-child interactions, family conflicts and maternal psychopathology. J Child Psychol Psychiatry. 1991;32(2):233-255.
42. Kashdan TB, Jacob RG, Pelham WE, et al. Depression and anxiety in parents of children with ADHD and varying levels of oppositional defiant behaviors: modeling relationships with family functioning. J Clin Child Adolesc Psychol. 2004;33(1):169-181.
43. Chavira DA, Stein MB, Bailey K, et al. Parental opinions regarding treatment for social anxiety disorder in youth. J Dev Behav Pediatr. 2003;24(5):315-322.
44. Leslie LK, Aarons GA, Haine RA, et al. Caregiver depression and medication use by youths with ADHD who receive services in the public sector. Psychiatr Serv. 2007;58(1):131-134.
45. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27(1):1-10.
46. Atzori P, Usala T, Carucci S, et al. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol. 2009;19(6):673-681.
47. Chen CY, Yeh HH, Chen KH, et al. Differential effects of predictors on methylphenidate initiation and discontinuation among young people with newly diagnosed attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(3):265-273.
48. Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother. 2008;42(1):24-31.
49. Marcus SC, Wan GJ, Kemner JE, et al. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159(6):572-578.
50. Cummings JR JX, Allen L, Lally C, et al. Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatrics. 2017;139(6):e2016-e2044.
51. Hudson JL, Miller GE, Kirby JB. Explaining racial and ethnic differences in children’s use of stimulant medications. Med Care. 2007;45(11):1068-1075.
52. van den Ban E, Souverein PC, Swaab H, et al. Less discontinuation of ADHD drug use since the availability of long-acting ADHD medication in children, adolescents and adults under the age of 45 years in the Netherlands. Atten Defic Hyperact Disord. 2010;2(4):213-220.
53. Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43(5):559-567.
54. Toomey SL, Sox CM, Rusinak D, et al. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila). 2012;51(8):763-769.
55. Brinkman WB, Simon JO, Epstein JN. Reasons why children and adolescents with attention-deficit/hyperactivity disorder stop and restart taking medicine. Acad Pediatr. 2018;18(3):273-280.
56. Wehmeier PM, Dittmann RW, Banaschewski T. Treatment compliance or medication adherence in children and adolescents on ADHD medication in clinical practice: results from the COMPLY observational study. Atten Defic Hyperact Disord. 2015;7(2):165-174.
57. Frank E, Ozon C, Nair V, et al. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry. 2015;76(11):e1459-e1468.
58. Pozzi M, Carnovale C, Peeters G, et al. Adverse drug events related to mood and emotion in paediatric patients treated for ADHD: a meta-analysis. J Affect Disord. 2018;238:161-178.
59. Stuckelman ZD, Mulqueen JM, Ferracioli-Oda E, et al. Risk of irritability with psychostimulant treatment in children with ADHD: a meta-analysis. J Clin Psychiatry. 2017;78(6):e648-e655.
60. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727-738.
61. Lawson KA, Johnsrud M, Hodgkins P, et al. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clin Ther. 2012;34(4):944-956 e944.
62. Olfson M, Marcus S, Wan G. Stimulant dosing for children with ADHD: a medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48(1):51-59.
63. Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989-1002.
64. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann. 2008;37(1):19-26.
65. Leslie LK, Plemmons D, Monn AR, et al. Investigating ADHD treatment trajectories: listening to families’ stories about medication use. J Dev Behav Pediatr. 2007;28(3):179-188.
66. Fiks AG, Mayne S, Localio AR, et al. Shared decision making and behavioral impairment: a national study among children with special health care needs. BMC Pediatr. 2012;12:153.
67. Stevens J, Harman JS, Kelleher KJ. Race/ethnicity and insurance status as factors associated with ADHD treatment patterns. J Child Adolesc Psychopharmacol. 2005;15(1):88-96.
68. Charach A, Skyba A, Cook L, et al. Using stimulant medication for children with ADHD: what do parents say? A brief report. J Can Acad Child Adolesc Psychiatry. 2006;15(2):75-83.
69. Chen CY, Gerhard T, Winterstein AG. Determinants of initial pharmacological treatment for youths with attention-deficit/hyperactivity disorder. J Child Adolescent Psychopharmacol. 2009;19(2):187-195.
70. National Council on Patient Information and Education. Enhancing prescription medication adherence: a national action plan. http://www.bemedwise.org/docs/enhancingprescriptionmedicineadherence.pdf. Published August 2007. Accessed July 22, 2019.
71. Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590-611.
72. Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clin Child Fam Psychol Rev. 2001;4(3):183-207.
73. Chronis AM, Lahey BB, Pelham WE Jr., et al. Psychopathology and substance abuse in parents of young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1424-1432.
74. Chacko A, Newcorn JH, Feirsen N, et al. Improving medication adherence in chronic pediatric health conditions: a focus on ADHD in youth. Curr Pharm Des. 2010;16(22):2416-2423.
75. Brinkman WB, Hartl Majcher J, Polling LM, et al. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ Couns. 2013;93(1):95-101.
76. American Academy of Pediatrics. Caring for children with ADHD: a resource toolkit for clinicians. 2nd ed. https://www.aap.org/en-us/pubserv/adhd2/Pages/default.aspx. Published 2011. Accessed July 22, 2019.
77. The REACH Institute. Course dates and registration. http://www.thereachinstitute.org/services/for-primary-care-practitioners/training-dates-and-registration. Accessed July 22, 2019.
78. Sells D, Davidson L, Jewell C, et al. The treatment relationship in peer-based and regular case management for clients with severe mental illness. Psychiatr Serv. 2006;57(8):1179-1184.
79. Hoagwood KE, Green E, Kelleher K, et al. Family advocacy, support and education in children’s mental health: results of a national survey. Adm Policy Ment Health. 2008;35(1-2):73-83.
80. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children—outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24(3):1063-1073.
81. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(Suppl 2):157-168.
82. Bradley CL, Luder HR, Beck AF, et al. Pediatric asthma medication therapy management through community pharmacy and primary care collaboration. J Am Pharm Assoc (2003). 2016;56(4):455-460.
83. Noyes K, Bajorska A, Fisher S, et al. Cost-effectiveness of the school-based asthma therapy (SBAT) program. Pediatrics. 2013;131(3):e709-e717.
84. Halterman JS, Fagnano M, Montes G, et al. The school-based preventive asthma care trial: results of a pilot study. J Pediatr. 2012;161(6):1109-1115.
85. Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the school-based asthma therapy trial. Arch Pediatr Adolesc Med. 2011;165(3):262-268.
Attention-deficit/hyperactivity disorder (ADHD) is the most common childhood neurodevelopmental disorder, affecting 8% to 12% of school-aged children in the United States1-3 with significant impairments that often persist into adulthood.4-8 Current guidelines recommend stimulant medication and/or behavioral therapies as first-line treatments for ADHD.9,10 There is a wealth of evidence on the efficacy of stimulants in ADHD, with the most significant effects noted on core ADHD symptoms.11,12 Additional evidence links stimulants to decreased long-term negative outcomes, including reduced school absences and grade retention,13 as well as modestly but significantly improved reading and math scores.14 Other studies have reported that individuals with ADHD who receive medication have decreased criminality,15,16 motor vehicle accidents,17,18 injuries,19 substance abuse,20-22 and risk for subsequent and concurrent depression.23 Therefore, the evidence suggests that consistent medication treatment helps improve outcomes for individuals with ADHD.

Adherence is defined as “the extent to which a person’s behavior (eg, taking medication) corresponds with agreed recommendations from a clinician.”24 Unfortunately, pediatric ADHD medication adherence has been found to be poor (approximately 64%).25-30 Nonadherence to ADHD medication has been linked to multiple factors, including caregiver/family and child/adolescent factors (Table 1), medication-related factors (Table 2), and health care/system factors (Table 3). Understanding and addressing these factors is essential to maximizing long-term outcomes. In this article, we review the factors associated with nonadherence to ADHD medication, and outline strategies to improve adherence.

Caregiver/family characteristics
Caregiver beliefs about ADHD and their attitudes toward treatment have been associated with the initiation of and adherence to ADHD medication. For example, caregivers who view a child’s difficulties as a medical disorder that requires a biologic intervention are more likely to accept and adhere to medication.31 Similarly, caregivers who perceive ADHD medication as safe, effective, and socially acceptable are more likely to be treatment-adherent.32-35
- increased caregiver knowledge about ADHD33
- receiving an ADHD diagnosis based on a thorough diagnostic process (ie, comprehensive psychological testing)36
- satisfaction with information about medicine
- comfort with the treatment plan.34
Socioeconomic status, family functioning, and caregiver mental health diagnoses (eg, ADHD, depression) have also been linked to ADHD medication adherence. Several studies, including the Multimodal Treatment Study of Children with ADHD,11 a landmark study of stimulant medication for children with ADHD, have found an association between low income and decreased likelihood of receiving ADHD medication.2,37-39 Further, Gau et al40 found that negative caregiver-child relationships and family dysfunction were associated with poor medication adherence in children with ADHD.9 Prior studies have also shown that mothers of children with ADHD are more likely to have depression and/or anxiety,41,42 and that caregivers with a history of mental health diagnoses are more accepting of initiating medication treatment for their children.43 However, additional studies have found that caregiver mental health diagnoses decreased the likelihood of ADHD medication adherence.40,44

Child characteristics
Child characteristics associated with decreased ADHD medication adherence include older age (eg, adolescents vs school-aged children),29,30,34,40,45-47 non-White race, Hispanic ethnicity,29,33,48-51 female gender,29,33,52 lower baseline ADHD symptom severity,30,37 and child unwillingness to take medications.34 However, prior studies have not been completely consistent about the relationship between child comorbid conditions (eg, oppositional defiant disorder [ODD], conduct disorder) and ADHD medication adherence. A few studies found that child comorbid conditions, especially ODD, mediate poor ADHD medication adherence, possibly secondary to an increased caregiver-child conflict.30,53,54 However, other studies have reported that the presence of comorbid ODD, depression, and anxiety predicted increased adherence to ADHD medications.37,46
Medication-related factors
Adverse effects of medications are the most commonly cited reason for ADHD medication nonadherence
On the other hand, increased ADHD medication effectiveness has been associated with improved medication adherence.5,34,54-56 Medication titration and dosing factors have also been shown to affect adherence. Specifically, adherence has been improved when ADHD medications are titrated in a systematic manner soon after starting treatment, and when families have an early first contact with a physician after starting medication (within 3 months).28 In addition, use of a simplified dose regimen has been linked to better adherence: patients who are prescribed long-acting stimulants are more likely to adhere to treatment compared with patients who take short-acting formulations.26,40,49,61-63 It is possible that long-acting stimulants increase adherence because they produce more even and sustained effects on ADHD symptoms throughout the day, compared with short-acting formulations.64 Furthermore, the inconvenience of taking multiple doses throughout the day, as well as the potential social stigma of mid-school day dosing, may negatively impact adherence to short-acting formulations.10
Continue to: Health care/system factors
Health care/system factors
Several studies have investigated the influence of health services factors on ADHD medication adherence. Specifically, limited transportation services and lack of mental health providers in the community have been linked to decreased ADHD medication adherence.47,65,66 Furthermore, limited insurance coverage and higher costs of ADHD medications, which lead to substantial out-of-pocket payments for families, have been associated with decreased likelihood of ADHD medication adherence.29,67
Clinician-related factors also can affect ADHD medication adherence. For example, a clinician’s lack knowledge of ADHD care can negatively impact ADHD medication adherence.68 Two studies have documented improved ADHD medication adherence when treatment is provided by specialists (eg, child psychiatrists) rather than by community primary care providers, possibly because specialists are more likely to provide close stimulant titration and monitoring (ie, ≥ 3 visits in the first 90 days) and use higher maximum doses.62,69 Furthermore, ADHD medication initiation and adherence are increased when patients have a strong working alliance with their clinician and trust the health care system,31,34,35 as well as when there is a match between the caregiver’s and clinician’s perception of the cause, course, and best treatment practices for a child’s ADHD.65
Strategies to improve medication adherence
A number of strategies to improve ADHD medication adherence can be derived from our knowledge of the factors that influence adherence.
Patient/family education. Unanswered questions about ADHD diagnosis, etiology, and medication adverse effects can negatively impact the ADHD treatment process. Therefore, patient/family education regarding ADHD and its management is necessary to improve medication adherence, because it helps families attain the knowledge, confidence, and motivation to manage their child’s condition.
Clinicians have an important role in educating patients about70:
- the medications they are taking
- why they are taking them
- what the medications look like
- the time of medication administration
- the potential adverse effects
- what to do if adverse effects occur
- what regular testing/monitoring is necessary.
Clinicians can provide appropriate psychoeducation by sharing written materials and trusted websites with families (see Related Resources).
Behavioral strategies. Behavioral interventions have been among the most effective strategies for improving medication adherence in other chronic conditions.71 Behavioral strategies are likely to be particularly important for families of children with ADHD and comorbid conditions such as ODD because these families experience considerable caregiver-child conflict.72 Moreover, parents of children with ADHD are at higher risk for having ADHD and depression themselves,73 both of which may interfere with a parent’s ability to obtain and administer medications consistently. Thus, for these families, using a combination of psychoeducation and behavioral strategies will be necessary to affect change in attitude and behavior. Behavioral strategies that can be used to improve medication adherence include:
- Technology-based interventions can reduce the impact of environmental barriers to adherence. For example, pharmacy automatic prescription renewal systems can reduce the likelihood of families failing to obtain ADHD medication refills. Pill reminder boxes, smartphone alerts, and setting various alarms can effectively prompt caregivers/patients to administer medication. In particular, these methods can be crucial in families for which multiple members have ADHD and its attendant difficulties with organization and task completion.
- Caregiver training may assist families in developing specific behavioral management skills that support adherence. This training can be as straightforward as instructing caregivers on the use of positive reinforcement when teaching their children to swallow pills. It may also encompass structured behavioral interventions aimed at training caregivers to utilize rewards and consequences in order to maximize medication adherence.74
Continue to: Clinician interventions
Clinician interventions. Clinicians can use decision aids to help inform families about treatment options, promote shared decision making, and decrease uncertainty about the treatment plan75 (see Related Resources). Early titration of ADHD medications and early first contact (within months of starting medication treatment) between caregivers and clinicians, whether via in-person visit, telephone, or email, have also been related to improved adherence.28 Furthermore, clinicians can improve adherence by prescribing a simplified medication regimen (ie, long-acting formulations that provide full-day coverage). To address the negative impact of high out-of-pocket ADHD medication costs on adherence, clinicians can also prescribe generic preparations and/or “preferred” medications options on an individual patient’s formulary.
Because clinician knowledge and expertise in ADHD care has been linked to improved patient medication adherence,68 clinicians are encouraged to use the American Academy of Pediatrics (AAP) guideline for diagnosis and treatment of ADHD, which includes a supplemental process of care algorithm (last published in 2011,10 with an updated guideline anticipated in 2019), as well as the AAP/National Institute for Children’s Health Quality (NICHQ) ADHD Toolkit,76 which includes items helpful for ADHD diagnosis and treatment. The Society for Developmental and Behavioral Pediatrics is also developing a clinical practice guideline for the diagnosis and treatment of complex ADHD (ie, ADHD complicated by coexisting mental health, developmental, and/or psychosocial conditions or issues), with publication anticipated in 2019. Primary care providers can also improve their expertise in ADHD care by pursuing additional mental health–related trainings (such as those conducted by the REACH Institute).77
Because receiving ADHD care from a specialist has been shown to improve medication initiation and adherence,62,69 other strategies to address the short supply of child psychiatrists include offering incentives to medical students to pursue a career in child psychiatry (eg, loan forgiveness). Telepsychiatry and co-location of mental health specialists and primary care providers are additional innovative ways in which ADHD specialty care can be delivered to more patients.64
Finally, providing culturally-sensitive care can strengthen the clinician-caregiver relationship and promote adherence to treatment. For example, clinicians can partner with local groups to increase their understanding of how different racial/ethnic groups perceive ADHD and its treatment.64
Peer support models. Peers are credible role models who have a valued role in facilitating the use of mental health services by empowering families and enhancing service satisfaction.78 In several communities in the United States, peer models using family advocates have been introduced.79 Family advocates are typically caregivers of children who have special needs or have been involved in the mental health system. Their perspective—as peers and first-hand consumers of the health care and/or mental health system—can make them powerful and effective coaches to families of children with ADHD. By helping families to navigate ADHD care systems successfully, family advocates can play an important role in enhancing ADHD medication adherence, although further investigation is needed. In addition, the stigma around ADHD medication use, which adversely impacts adherence, can be mitigated if caregivers participate in organized ADHD-related support groups (eg, Children and Adults with ADHD [CHADD]).
Continue to: Health disparity-reducing interventions
Health disparity-reducing interventions. Successful health disparity-reducing interventions—such as those developed to enhance care of other chronic disorders including asthma and diabetes—can be applied to improve ADHD care. These interventions, which include medical-legal partnerships (eg, between clinicians, social workers, legal advocates, and community partners) in primary care centers, have been shown to improve health insurance coverage and therefore health care access.80,81 Although some hardships linked to nonadherence (eg, low socioeconomic status) may not be amenable to health care–related interventions, screening for these hardships can identify children who are most at risk for poor adherence. This would alert clinicians to proactively identify barriers to adherence and implement mitigation strategies. This might include developing more streamlined, easier-to-follow management plans for these patients, such as those that can be delivered through pharmacist-physician collaborative programs82 and school-based therapy programs.83-85
Bottom Line
Suboptimal adherence to medications for attention-deficit/hyperactivity disorder (ADHD) can be addressed through patient/family education, behavioral strategies, clinician interventions, peer support models, and health disparity-reducing interventions. By improving ADHD treatment adherence, these interventions have the potential to maximize long-term outcomes.
Related Resources
- Cohen Children’s Medical Center Northwell Health. The ADHD Medication Guide. www.ADHDMedicationGuide.com. Revised December 31, 2017.
- Cincinnati Children’s Hospital Medical Center. Decision aids to facilitate shared decision making in practice. www.cincinnatichildrens.org/service/j/anderson-center/ evidence-based-care/decision-aids.
- CHADD. Children and Adults with attention-deficit/ hyperactivity disorder. www.chadd.org.
Drug Brand Name
Methylphenidate • Concerta, Ritalin
Attention-deficit/hyperactivity disorder (ADHD) is the most common childhood neurodevelopmental disorder, affecting 8% to 12% of school-aged children in the United States1-3 with significant impairments that often persist into adulthood.4-8 Current guidelines recommend stimulant medication and/or behavioral therapies as first-line treatments for ADHD.9,10 There is a wealth of evidence on the efficacy of stimulants in ADHD, with the most significant effects noted on core ADHD symptoms.11,12 Additional evidence links stimulants to decreased long-term negative outcomes, including reduced school absences and grade retention,13 as well as modestly but significantly improved reading and math scores.14 Other studies have reported that individuals with ADHD who receive medication have decreased criminality,15,16 motor vehicle accidents,17,18 injuries,19 substance abuse,20-22 and risk for subsequent and concurrent depression.23 Therefore, the evidence suggests that consistent medication treatment helps improve outcomes for individuals with ADHD.

Adherence is defined as “the extent to which a person’s behavior (eg, taking medication) corresponds with agreed recommendations from a clinician.”24 Unfortunately, pediatric ADHD medication adherence has been found to be poor (approximately 64%).25-30 Nonadherence to ADHD medication has been linked to multiple factors, including caregiver/family and child/adolescent factors (Table 1), medication-related factors (Table 2), and health care/system factors (Table 3). Understanding and addressing these factors is essential to maximizing long-term outcomes. In this article, we review the factors associated with nonadherence to ADHD medication, and outline strategies to improve adherence.

Caregiver/family characteristics
Caregiver beliefs about ADHD and their attitudes toward treatment have been associated with the initiation of and adherence to ADHD medication. For example, caregivers who view a child’s difficulties as a medical disorder that requires a biologic intervention are more likely to accept and adhere to medication.31 Similarly, caregivers who perceive ADHD medication as safe, effective, and socially acceptable are more likely to be treatment-adherent.32-35
- increased caregiver knowledge about ADHD33
- receiving an ADHD diagnosis based on a thorough diagnostic process (ie, comprehensive psychological testing)36
- satisfaction with information about medicine
- comfort with the treatment plan.34
Socioeconomic status, family functioning, and caregiver mental health diagnoses (eg, ADHD, depression) have also been linked to ADHD medication adherence. Several studies, including the Multimodal Treatment Study of Children with ADHD,11 a landmark study of stimulant medication for children with ADHD, have found an association between low income and decreased likelihood of receiving ADHD medication.2,37-39 Further, Gau et al40 found that negative caregiver-child relationships and family dysfunction were associated with poor medication adherence in children with ADHD.9 Prior studies have also shown that mothers of children with ADHD are more likely to have depression and/or anxiety,41,42 and that caregivers with a history of mental health diagnoses are more accepting of initiating medication treatment for their children.43 However, additional studies have found that caregiver mental health diagnoses decreased the likelihood of ADHD medication adherence.40,44

Child characteristics
Child characteristics associated with decreased ADHD medication adherence include older age (eg, adolescents vs school-aged children),29,30,34,40,45-47 non-White race, Hispanic ethnicity,29,33,48-51 female gender,29,33,52 lower baseline ADHD symptom severity,30,37 and child unwillingness to take medications.34 However, prior studies have not been completely consistent about the relationship between child comorbid conditions (eg, oppositional defiant disorder [ODD], conduct disorder) and ADHD medication adherence. A few studies found that child comorbid conditions, especially ODD, mediate poor ADHD medication adherence, possibly secondary to an increased caregiver-child conflict.30,53,54 However, other studies have reported that the presence of comorbid ODD, depression, and anxiety predicted increased adherence to ADHD medications.37,46
Medication-related factors
Adverse effects of medications are the most commonly cited reason for ADHD medication nonadherence
On the other hand, increased ADHD medication effectiveness has been associated with improved medication adherence.5,34,54-56 Medication titration and dosing factors have also been shown to affect adherence. Specifically, adherence has been improved when ADHD medications are titrated in a systematic manner soon after starting treatment, and when families have an early first contact with a physician after starting medication (within 3 months).28 In addition, use of a simplified dose regimen has been linked to better adherence: patients who are prescribed long-acting stimulants are more likely to adhere to treatment compared with patients who take short-acting formulations.26,40,49,61-63 It is possible that long-acting stimulants increase adherence because they produce more even and sustained effects on ADHD symptoms throughout the day, compared with short-acting formulations.64 Furthermore, the inconvenience of taking multiple doses throughout the day, as well as the potential social stigma of mid-school day dosing, may negatively impact adherence to short-acting formulations.10
Continue to: Health care/system factors
Health care/system factors
Several studies have investigated the influence of health services factors on ADHD medication adherence. Specifically, limited transportation services and lack of mental health providers in the community have been linked to decreased ADHD medication adherence.47,65,66 Furthermore, limited insurance coverage and higher costs of ADHD medications, which lead to substantial out-of-pocket payments for families, have been associated with decreased likelihood of ADHD medication adherence.29,67
Clinician-related factors also can affect ADHD medication adherence. For example, a clinician’s lack knowledge of ADHD care can negatively impact ADHD medication adherence.68 Two studies have documented improved ADHD medication adherence when treatment is provided by specialists (eg, child psychiatrists) rather than by community primary care providers, possibly because specialists are more likely to provide close stimulant titration and monitoring (ie, ≥ 3 visits in the first 90 days) and use higher maximum doses.62,69 Furthermore, ADHD medication initiation and adherence are increased when patients have a strong working alliance with their clinician and trust the health care system,31,34,35 as well as when there is a match between the caregiver’s and clinician’s perception of the cause, course, and best treatment practices for a child’s ADHD.65
Strategies to improve medication adherence
A number of strategies to improve ADHD medication adherence can be derived from our knowledge of the factors that influence adherence.
Patient/family education. Unanswered questions about ADHD diagnosis, etiology, and medication adverse effects can negatively impact the ADHD treatment process. Therefore, patient/family education regarding ADHD and its management is necessary to improve medication adherence, because it helps families attain the knowledge, confidence, and motivation to manage their child’s condition.
Clinicians have an important role in educating patients about70:
- the medications they are taking
- why they are taking them
- what the medications look like
- the time of medication administration
- the potential adverse effects
- what to do if adverse effects occur
- what regular testing/monitoring is necessary.
Clinicians can provide appropriate psychoeducation by sharing written materials and trusted websites with families (see Related Resources).
Behavioral strategies. Behavioral interventions have been among the most effective strategies for improving medication adherence in other chronic conditions.71 Behavioral strategies are likely to be particularly important for families of children with ADHD and comorbid conditions such as ODD because these families experience considerable caregiver-child conflict.72 Moreover, parents of children with ADHD are at higher risk for having ADHD and depression themselves,73 both of which may interfere with a parent’s ability to obtain and administer medications consistently. Thus, for these families, using a combination of psychoeducation and behavioral strategies will be necessary to affect change in attitude and behavior. Behavioral strategies that can be used to improve medication adherence include:
- Technology-based interventions can reduce the impact of environmental barriers to adherence. For example, pharmacy automatic prescription renewal systems can reduce the likelihood of families failing to obtain ADHD medication refills. Pill reminder boxes, smartphone alerts, and setting various alarms can effectively prompt caregivers/patients to administer medication. In particular, these methods can be crucial in families for which multiple members have ADHD and its attendant difficulties with organization and task completion.
- Caregiver training may assist families in developing specific behavioral management skills that support adherence. This training can be as straightforward as instructing caregivers on the use of positive reinforcement when teaching their children to swallow pills. It may also encompass structured behavioral interventions aimed at training caregivers to utilize rewards and consequences in order to maximize medication adherence.74
Continue to: Clinician interventions
Clinician interventions. Clinicians can use decision aids to help inform families about treatment options, promote shared decision making, and decrease uncertainty about the treatment plan75 (see Related Resources). Early titration of ADHD medications and early first contact (within months of starting medication treatment) between caregivers and clinicians, whether via in-person visit, telephone, or email, have also been related to improved adherence.28 Furthermore, clinicians can improve adherence by prescribing a simplified medication regimen (ie, long-acting formulations that provide full-day coverage). To address the negative impact of high out-of-pocket ADHD medication costs on adherence, clinicians can also prescribe generic preparations and/or “preferred” medications options on an individual patient’s formulary.
Because clinician knowledge and expertise in ADHD care has been linked to improved patient medication adherence,68 clinicians are encouraged to use the American Academy of Pediatrics (AAP) guideline for diagnosis and treatment of ADHD, which includes a supplemental process of care algorithm (last published in 2011,10 with an updated guideline anticipated in 2019), as well as the AAP/National Institute for Children’s Health Quality (NICHQ) ADHD Toolkit,76 which includes items helpful for ADHD diagnosis and treatment. The Society for Developmental and Behavioral Pediatrics is also developing a clinical practice guideline for the diagnosis and treatment of complex ADHD (ie, ADHD complicated by coexisting mental health, developmental, and/or psychosocial conditions or issues), with publication anticipated in 2019. Primary care providers can also improve their expertise in ADHD care by pursuing additional mental health–related trainings (such as those conducted by the REACH Institute).77
Because receiving ADHD care from a specialist has been shown to improve medication initiation and adherence,62,69 other strategies to address the short supply of child psychiatrists include offering incentives to medical students to pursue a career in child psychiatry (eg, loan forgiveness). Telepsychiatry and co-location of mental health specialists and primary care providers are additional innovative ways in which ADHD specialty care can be delivered to more patients.64
Finally, providing culturally-sensitive care can strengthen the clinician-caregiver relationship and promote adherence to treatment. For example, clinicians can partner with local groups to increase their understanding of how different racial/ethnic groups perceive ADHD and its treatment.64
Peer support models. Peers are credible role models who have a valued role in facilitating the use of mental health services by empowering families and enhancing service satisfaction.78 In several communities in the United States, peer models using family advocates have been introduced.79 Family advocates are typically caregivers of children who have special needs or have been involved in the mental health system. Their perspective—as peers and first-hand consumers of the health care and/or mental health system—can make them powerful and effective coaches to families of children with ADHD. By helping families to navigate ADHD care systems successfully, family advocates can play an important role in enhancing ADHD medication adherence, although further investigation is needed. In addition, the stigma around ADHD medication use, which adversely impacts adherence, can be mitigated if caregivers participate in organized ADHD-related support groups (eg, Children and Adults with ADHD [CHADD]).
Continue to: Health disparity-reducing interventions
Health disparity-reducing interventions. Successful health disparity-reducing interventions—such as those developed to enhance care of other chronic disorders including asthma and diabetes—can be applied to improve ADHD care. These interventions, which include medical-legal partnerships (eg, between clinicians, social workers, legal advocates, and community partners) in primary care centers, have been shown to improve health insurance coverage and therefore health care access.80,81 Although some hardships linked to nonadherence (eg, low socioeconomic status) may not be amenable to health care–related interventions, screening for these hardships can identify children who are most at risk for poor adherence. This would alert clinicians to proactively identify barriers to adherence and implement mitigation strategies. This might include developing more streamlined, easier-to-follow management plans for these patients, such as those that can be delivered through pharmacist-physician collaborative programs82 and school-based therapy programs.83-85
Bottom Line
Suboptimal adherence to medications for attention-deficit/hyperactivity disorder (ADHD) can be addressed through patient/family education, behavioral strategies, clinician interventions, peer support models, and health disparity-reducing interventions. By improving ADHD treatment adherence, these interventions have the potential to maximize long-term outcomes.
Related Resources
- Cohen Children’s Medical Center Northwell Health. The ADHD Medication Guide. www.ADHDMedicationGuide.com. Revised December 31, 2017.
- Cincinnati Children’s Hospital Medical Center. Decision aids to facilitate shared decision making in practice. www.cincinnatichildrens.org/service/j/anderson-center/ evidence-based-care/decision-aids.
- CHADD. Children and Adults with attention-deficit/ hyperactivity disorder. www.chadd.org.
Drug Brand Name
Methylphenidate • Concerta, Ritalin
1. Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857-864.
2. Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119 (Suppl 1):S99-S106.
3. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199-212.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484-500.
5. Charach A, Dashti B, Carson P, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at-risk preschoolers; long-term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.ncbi.nlm.nih.gov/books/NBK82368/.
6. Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010;46(3):209-217.
7. Barkley RA, Fischer M, Smallish L, et al. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
8. Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631-642.
9. Pliszka S, the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
10. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
11. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
12. Abikoff H, Hechtman L, Klein RG, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43(7):802-811.
13. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. J Dev Behav Pediatr. 2007;28(4):265-273.
14. Scheffler RM, Brown TT, Fulton BD, et al. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123(5):1273-1279.
15. Dalsgaard S, Nielsen HS, Simonsen M. Five-fold increase in national prevalence rates of attention-deficit/hyperactivity disorder medications for children and adolescents with autism spectrum disorder, attention-deficit/hyperactivity disorder, and other psychiatric disorders: a Danish register-based study. J Child Adolesc Psychopharmacol. 2013;23(7):432-439.
16. Lichtenstein P, Halldner L, Zetterqvist J, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006-2014.
17. Chang Z, Lichtenstein P, D’Onofrio BM, et al. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA Psychiatry. 2014;71(3):319-325.
18. Chang Z, Quinn PD, Hur K, et al. Association between medication use for attention-deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry. 2017;74(6):597-603.
19. Dalsgaard S, Leckman JF, Mortensen PB, et al. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry. 2015;2(8):702-709.
20. Chang Z, Lichtenstein P, Halldner L, et al. Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry. 2014;55(8):878-885.
21. Fischer M, Barkley RA. Childhood stimulant treatment and risk for later substance abuse. J Clin Psychiatry. 2003;64(Suppl 11):19-23.
22. Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003;64(Suppl 11):3-8.
23. Chang Z, D’Onofrio BM, Quinn PD, et al. Medicationfor attention-deficit/hyperactivity disorder and risk for depression: a nationwide longitudinal cohort study. Biol Psychiatry. 2016;80(12):916-922.
24. World Health Organization. Adherence to long-term therapies: evidence for action. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. Published 2003. Accessed July 22, 2019.
25. Perwien A, Hall J, Swensen A, et al. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122-129.
26. Faraone SV, Biederman J, Zimmerman B. An analysis of patient adherence to treatment during a 1-year, open-label study of OROS methylphenidate in children with ADHD. J Atten Disord. 2007;11(2):157-166.
27. Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin. 2011;27(Suppl 2):13-22.
28. Brinkman WB, Baum R, Kelleher KJ, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry. 2016;55(4):289-294.
29. Bokhari FAS, Heiland F, Levine P, et al. Risk factors for discontinuing drug therapy among children with ADHD. Health Services and Outcomes Research Methodology. 2008;8(3):134-158.
30. Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40(8):922-928.
31. DosReis S, Mychailyszyn MP, Evans-Lacko SE, et al. The meaning of attention-deficit/hyperactivity disorder medication and parents’ initiation and continuity of treatment for their child. J Child Adolesc Psychopharmacol. 2009;19(4):377-383.
32. dosReis S, Myers MA. Parental attitudes and involvement in psychopharmacological treatment for ADHD: a conceptual model. Int Rev Psychiatry. 2008;20(2):135-141.
33. Bussing R, Koro-Ljungberg M, Noguchi K, et al. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med. 2012;74(1):92-100.
34. Brinkman WB, Sucharew H, Majcher JH, et al. Predictors of medication continuity in children with ADHD. Pediatrics. 2018;141(6). doi: 10.1542/peds.2017-2580.
35. Coletti DJ, Pappadopulos E, Katsiotas NJ, et al. Parent perspectives on the decision to initiate medication treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(3):226-237.
36. Bussing R, Gary FA. Practice guidelines and parental ADHD treatment evaluations: friends or foes? Harv Rev Psychiatry. 2001;9(5):223-233.
37. Charach A, Gajaria A. Improving psychostimulant adherence in children with ADHD. Expert Rev Neurother. 2008;8(10):1563-1571.
38. Rieppi R, Greenhill LL, Ford RE, et al. Socioeconomic status as a moderator of ADHD treatment outcomes. J Am Acad Child Adolesc Psychiatry. 2002;41(3):269-277.
39. Swanson JM, Hinshaw SP, Arnold LE, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1003-1014.
40. Gau SS, Shen HY, Chou MC, et al. Determinants of adherence to methylphenidate and the impact of poor adherence on maternal and family measures. J Child Adolesc Psychopharmacol. 2006;16(3):286-297.
41. Barkley RA, Fischer M, Edelbrock C, et al. The adolescent outcome of hyperactive children diagnosed by research criteria--III. Mother-child interactions, family conflicts and maternal psychopathology. J Child Psychol Psychiatry. 1991;32(2):233-255.
42. Kashdan TB, Jacob RG, Pelham WE, et al. Depression and anxiety in parents of children with ADHD and varying levels of oppositional defiant behaviors: modeling relationships with family functioning. J Clin Child Adolesc Psychol. 2004;33(1):169-181.
43. Chavira DA, Stein MB, Bailey K, et al. Parental opinions regarding treatment for social anxiety disorder in youth. J Dev Behav Pediatr. 2003;24(5):315-322.
44. Leslie LK, Aarons GA, Haine RA, et al. Caregiver depression and medication use by youths with ADHD who receive services in the public sector. Psychiatr Serv. 2007;58(1):131-134.
45. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27(1):1-10.
46. Atzori P, Usala T, Carucci S, et al. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol. 2009;19(6):673-681.
47. Chen CY, Yeh HH, Chen KH, et al. Differential effects of predictors on methylphenidate initiation and discontinuation among young people with newly diagnosed attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(3):265-273.
48. Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother. 2008;42(1):24-31.
49. Marcus SC, Wan GJ, Kemner JE, et al. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159(6):572-578.
50. Cummings JR JX, Allen L, Lally C, et al. Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatrics. 2017;139(6):e2016-e2044.
51. Hudson JL, Miller GE, Kirby JB. Explaining racial and ethnic differences in children’s use of stimulant medications. Med Care. 2007;45(11):1068-1075.
52. van den Ban E, Souverein PC, Swaab H, et al. Less discontinuation of ADHD drug use since the availability of long-acting ADHD medication in children, adolescents and adults under the age of 45 years in the Netherlands. Atten Defic Hyperact Disord. 2010;2(4):213-220.
53. Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43(5):559-567.
54. Toomey SL, Sox CM, Rusinak D, et al. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila). 2012;51(8):763-769.
55. Brinkman WB, Simon JO, Epstein JN. Reasons why children and adolescents with attention-deficit/hyperactivity disorder stop and restart taking medicine. Acad Pediatr. 2018;18(3):273-280.
56. Wehmeier PM, Dittmann RW, Banaschewski T. Treatment compliance or medication adherence in children and adolescents on ADHD medication in clinical practice: results from the COMPLY observational study. Atten Defic Hyperact Disord. 2015;7(2):165-174.
57. Frank E, Ozon C, Nair V, et al. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry. 2015;76(11):e1459-e1468.
58. Pozzi M, Carnovale C, Peeters G, et al. Adverse drug events related to mood and emotion in paediatric patients treated for ADHD: a meta-analysis. J Affect Disord. 2018;238:161-178.
59. Stuckelman ZD, Mulqueen JM, Ferracioli-Oda E, et al. Risk of irritability with psychostimulant treatment in children with ADHD: a meta-analysis. J Clin Psychiatry. 2017;78(6):e648-e655.
60. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727-738.
61. Lawson KA, Johnsrud M, Hodgkins P, et al. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clin Ther. 2012;34(4):944-956 e944.
62. Olfson M, Marcus S, Wan G. Stimulant dosing for children with ADHD: a medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48(1):51-59.
63. Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989-1002.
64. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann. 2008;37(1):19-26.
65. Leslie LK, Plemmons D, Monn AR, et al. Investigating ADHD treatment trajectories: listening to families’ stories about medication use. J Dev Behav Pediatr. 2007;28(3):179-188.
66. Fiks AG, Mayne S, Localio AR, et al. Shared decision making and behavioral impairment: a national study among children with special health care needs. BMC Pediatr. 2012;12:153.
67. Stevens J, Harman JS, Kelleher KJ. Race/ethnicity and insurance status as factors associated with ADHD treatment patterns. J Child Adolesc Psychopharmacol. 2005;15(1):88-96.
68. Charach A, Skyba A, Cook L, et al. Using stimulant medication for children with ADHD: what do parents say? A brief report. J Can Acad Child Adolesc Psychiatry. 2006;15(2):75-83.
69. Chen CY, Gerhard T, Winterstein AG. Determinants of initial pharmacological treatment for youths with attention-deficit/hyperactivity disorder. J Child Adolescent Psychopharmacol. 2009;19(2):187-195.
70. National Council on Patient Information and Education. Enhancing prescription medication adherence: a national action plan. http://www.bemedwise.org/docs/enhancingprescriptionmedicineadherence.pdf. Published August 2007. Accessed July 22, 2019.
71. Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590-611.
72. Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clin Child Fam Psychol Rev. 2001;4(3):183-207.
73. Chronis AM, Lahey BB, Pelham WE Jr., et al. Psychopathology and substance abuse in parents of young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1424-1432.
74. Chacko A, Newcorn JH, Feirsen N, et al. Improving medication adherence in chronic pediatric health conditions: a focus on ADHD in youth. Curr Pharm Des. 2010;16(22):2416-2423.
75. Brinkman WB, Hartl Majcher J, Polling LM, et al. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ Couns. 2013;93(1):95-101.
76. American Academy of Pediatrics. Caring for children with ADHD: a resource toolkit for clinicians. 2nd ed. https://www.aap.org/en-us/pubserv/adhd2/Pages/default.aspx. Published 2011. Accessed July 22, 2019.
77. The REACH Institute. Course dates and registration. http://www.thereachinstitute.org/services/for-primary-care-practitioners/training-dates-and-registration. Accessed July 22, 2019.
78. Sells D, Davidson L, Jewell C, et al. The treatment relationship in peer-based and regular case management for clients with severe mental illness. Psychiatr Serv. 2006;57(8):1179-1184.
79. Hoagwood KE, Green E, Kelleher K, et al. Family advocacy, support and education in children’s mental health: results of a national survey. Adm Policy Ment Health. 2008;35(1-2):73-83.
80. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children—outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24(3):1063-1073.
81. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(Suppl 2):157-168.
82. Bradley CL, Luder HR, Beck AF, et al. Pediatric asthma medication therapy management through community pharmacy and primary care collaboration. J Am Pharm Assoc (2003). 2016;56(4):455-460.
83. Noyes K, Bajorska A, Fisher S, et al. Cost-effectiveness of the school-based asthma therapy (SBAT) program. Pediatrics. 2013;131(3):e709-e717.
84. Halterman JS, Fagnano M, Montes G, et al. The school-based preventive asthma care trial: results of a pilot study. J Pediatr. 2012;161(6):1109-1115.
85. Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the school-based asthma therapy trial. Arch Pediatr Adolesc Med. 2011;165(3):262-268.
1. Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857-864.
2. Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119 (Suppl 1):S99-S106.
3. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199-212.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484-500.
5. Charach A, Dashti B, Carson P, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at-risk preschoolers; long-term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.ncbi.nlm.nih.gov/books/NBK82368/.
6. Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010;46(3):209-217.
7. Barkley RA, Fischer M, Smallish L, et al. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
8. Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631-642.
9. Pliszka S, the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
10. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
11. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
12. Abikoff H, Hechtman L, Klein RG, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43(7):802-811.
13. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. J Dev Behav Pediatr. 2007;28(4):265-273.
14. Scheffler RM, Brown TT, Fulton BD, et al. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123(5):1273-1279.
15. Dalsgaard S, Nielsen HS, Simonsen M. Five-fold increase in national prevalence rates of attention-deficit/hyperactivity disorder medications for children and adolescents with autism spectrum disorder, attention-deficit/hyperactivity disorder, and other psychiatric disorders: a Danish register-based study. J Child Adolesc Psychopharmacol. 2013;23(7):432-439.
16. Lichtenstein P, Halldner L, Zetterqvist J, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006-2014.
17. Chang Z, Lichtenstein P, D’Onofrio BM, et al. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA Psychiatry. 2014;71(3):319-325.
18. Chang Z, Quinn PD, Hur K, et al. Association between medication use for attention-deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry. 2017;74(6):597-603.
19. Dalsgaard S, Leckman JF, Mortensen PB, et al. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry. 2015;2(8):702-709.
20. Chang Z, Lichtenstein P, Halldner L, et al. Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry. 2014;55(8):878-885.
21. Fischer M, Barkley RA. Childhood stimulant treatment and risk for later substance abuse. J Clin Psychiatry. 2003;64(Suppl 11):19-23.
22. Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003;64(Suppl 11):3-8.
23. Chang Z, D’Onofrio BM, Quinn PD, et al. Medicationfor attention-deficit/hyperactivity disorder and risk for depression: a nationwide longitudinal cohort study. Biol Psychiatry. 2016;80(12):916-922.
24. World Health Organization. Adherence to long-term therapies: evidence for action. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. Published 2003. Accessed July 22, 2019.
25. Perwien A, Hall J, Swensen A, et al. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122-129.
26. Faraone SV, Biederman J, Zimmerman B. An analysis of patient adherence to treatment during a 1-year, open-label study of OROS methylphenidate in children with ADHD. J Atten Disord. 2007;11(2):157-166.
27. Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin. 2011;27(Suppl 2):13-22.
28. Brinkman WB, Baum R, Kelleher KJ, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry. 2016;55(4):289-294.
29. Bokhari FAS, Heiland F, Levine P, et al. Risk factors for discontinuing drug therapy among children with ADHD. Health Services and Outcomes Research Methodology. 2008;8(3):134-158.
30. Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40(8):922-928.
31. DosReis S, Mychailyszyn MP, Evans-Lacko SE, et al. The meaning of attention-deficit/hyperactivity disorder medication and parents’ initiation and continuity of treatment for their child. J Child Adolesc Psychopharmacol. 2009;19(4):377-383.
32. dosReis S, Myers MA. Parental attitudes and involvement in psychopharmacological treatment for ADHD: a conceptual model. Int Rev Psychiatry. 2008;20(2):135-141.
33. Bussing R, Koro-Ljungberg M, Noguchi K, et al. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med. 2012;74(1):92-100.
34. Brinkman WB, Sucharew H, Majcher JH, et al. Predictors of medication continuity in children with ADHD. Pediatrics. 2018;141(6). doi: 10.1542/peds.2017-2580.
35. Coletti DJ, Pappadopulos E, Katsiotas NJ, et al. Parent perspectives on the decision to initiate medication treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(3):226-237.
36. Bussing R, Gary FA. Practice guidelines and parental ADHD treatment evaluations: friends or foes? Harv Rev Psychiatry. 2001;9(5):223-233.
37. Charach A, Gajaria A. Improving psychostimulant adherence in children with ADHD. Expert Rev Neurother. 2008;8(10):1563-1571.
38. Rieppi R, Greenhill LL, Ford RE, et al. Socioeconomic status as a moderator of ADHD treatment outcomes. J Am Acad Child Adolesc Psychiatry. 2002;41(3):269-277.
39. Swanson JM, Hinshaw SP, Arnold LE, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1003-1014.
40. Gau SS, Shen HY, Chou MC, et al. Determinants of adherence to methylphenidate and the impact of poor adherence on maternal and family measures. J Child Adolesc Psychopharmacol. 2006;16(3):286-297.
41. Barkley RA, Fischer M, Edelbrock C, et al. The adolescent outcome of hyperactive children diagnosed by research criteria--III. Mother-child interactions, family conflicts and maternal psychopathology. J Child Psychol Psychiatry. 1991;32(2):233-255.
42. Kashdan TB, Jacob RG, Pelham WE, et al. Depression and anxiety in parents of children with ADHD and varying levels of oppositional defiant behaviors: modeling relationships with family functioning. J Clin Child Adolesc Psychol. 2004;33(1):169-181.
43. Chavira DA, Stein MB, Bailey K, et al. Parental opinions regarding treatment for social anxiety disorder in youth. J Dev Behav Pediatr. 2003;24(5):315-322.
44. Leslie LK, Aarons GA, Haine RA, et al. Caregiver depression and medication use by youths with ADHD who receive services in the public sector. Psychiatr Serv. 2007;58(1):131-134.
45. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27(1):1-10.
46. Atzori P, Usala T, Carucci S, et al. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol. 2009;19(6):673-681.
47. Chen CY, Yeh HH, Chen KH, et al. Differential effects of predictors on methylphenidate initiation and discontinuation among young people with newly diagnosed attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(3):265-273.
48. Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother. 2008;42(1):24-31.
49. Marcus SC, Wan GJ, Kemner JE, et al. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159(6):572-578.
50. Cummings JR JX, Allen L, Lally C, et al. Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatrics. 2017;139(6):e2016-e2044.
51. Hudson JL, Miller GE, Kirby JB. Explaining racial and ethnic differences in children’s use of stimulant medications. Med Care. 2007;45(11):1068-1075.
52. van den Ban E, Souverein PC, Swaab H, et al. Less discontinuation of ADHD drug use since the availability of long-acting ADHD medication in children, adolescents and adults under the age of 45 years in the Netherlands. Atten Defic Hyperact Disord. 2010;2(4):213-220.
53. Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43(5):559-567.
54. Toomey SL, Sox CM, Rusinak D, et al. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila). 2012;51(8):763-769.
55. Brinkman WB, Simon JO, Epstein JN. Reasons why children and adolescents with attention-deficit/hyperactivity disorder stop and restart taking medicine. Acad Pediatr. 2018;18(3):273-280.
56. Wehmeier PM, Dittmann RW, Banaschewski T. Treatment compliance or medication adherence in children and adolescents on ADHD medication in clinical practice: results from the COMPLY observational study. Atten Defic Hyperact Disord. 2015;7(2):165-174.
57. Frank E, Ozon C, Nair V, et al. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry. 2015;76(11):e1459-e1468.
58. Pozzi M, Carnovale C, Peeters G, et al. Adverse drug events related to mood and emotion in paediatric patients treated for ADHD: a meta-analysis. J Affect Disord. 2018;238:161-178.
59. Stuckelman ZD, Mulqueen JM, Ferracioli-Oda E, et al. Risk of irritability with psychostimulant treatment in children with ADHD: a meta-analysis. J Clin Psychiatry. 2017;78(6):e648-e655.
60. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727-738.
61. Lawson KA, Johnsrud M, Hodgkins P, et al. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clin Ther. 2012;34(4):944-956 e944.
62. Olfson M, Marcus S, Wan G. Stimulant dosing for children with ADHD: a medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48(1):51-59.
63. Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989-1002.
64. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann. 2008;37(1):19-26.
65. Leslie LK, Plemmons D, Monn AR, et al. Investigating ADHD treatment trajectories: listening to families’ stories about medication use. J Dev Behav Pediatr. 2007;28(3):179-188.
66. Fiks AG, Mayne S, Localio AR, et al. Shared decision making and behavioral impairment: a national study among children with special health care needs. BMC Pediatr. 2012;12:153.
67. Stevens J, Harman JS, Kelleher KJ. Race/ethnicity and insurance status as factors associated with ADHD treatment patterns. J Child Adolesc Psychopharmacol. 2005;15(1):88-96.
68. Charach A, Skyba A, Cook L, et al. Using stimulant medication for children with ADHD: what do parents say? A brief report. J Can Acad Child Adolesc Psychiatry. 2006;15(2):75-83.
69. Chen CY, Gerhard T, Winterstein AG. Determinants of initial pharmacological treatment for youths with attention-deficit/hyperactivity disorder. J Child Adolescent Psychopharmacol. 2009;19(2):187-195.
70. National Council on Patient Information and Education. Enhancing prescription medication adherence: a national action plan. http://www.bemedwise.org/docs/enhancingprescriptionmedicineadherence.pdf. Published August 2007. Accessed July 22, 2019.
71. Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590-611.
72. Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clin Child Fam Psychol Rev. 2001;4(3):183-207.
73. Chronis AM, Lahey BB, Pelham WE Jr., et al. Psychopathology and substance abuse in parents of young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1424-1432.
74. Chacko A, Newcorn JH, Feirsen N, et al. Improving medication adherence in chronic pediatric health conditions: a focus on ADHD in youth. Curr Pharm Des. 2010;16(22):2416-2423.
75. Brinkman WB, Hartl Majcher J, Polling LM, et al. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ Couns. 2013;93(1):95-101.
76. American Academy of Pediatrics. Caring for children with ADHD: a resource toolkit for clinicians. 2nd ed. https://www.aap.org/en-us/pubserv/adhd2/Pages/default.aspx. Published 2011. Accessed July 22, 2019.
77. The REACH Institute. Course dates and registration. http://www.thereachinstitute.org/services/for-primary-care-practitioners/training-dates-and-registration. Accessed July 22, 2019.
78. Sells D, Davidson L, Jewell C, et al. The treatment relationship in peer-based and regular case management for clients with severe mental illness. Psychiatr Serv. 2006;57(8):1179-1184.
79. Hoagwood KE, Green E, Kelleher K, et al. Family advocacy, support and education in children’s mental health: results of a national survey. Adm Policy Ment Health. 2008;35(1-2):73-83.
80. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children—outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24(3):1063-1073.
81. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(Suppl 2):157-168.
82. Bradley CL, Luder HR, Beck AF, et al. Pediatric asthma medication therapy management through community pharmacy and primary care collaboration. J Am Pharm Assoc (2003). 2016;56(4):455-460.
83. Noyes K, Bajorska A, Fisher S, et al. Cost-effectiveness of the school-based asthma therapy (SBAT) program. Pediatrics. 2013;131(3):e709-e717.
84. Halterman JS, Fagnano M, Montes G, et al. The school-based preventive asthma care trial: results of a pilot study. J Pediatr. 2012;161(6):1109-1115.
85. Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the school-based asthma therapy trial. Arch Pediatr Adolesc Med. 2011;165(3):262-268.
Expanding medication options for pediatric ADHD
Molly, age 9, is diagnosed with attention-deficit/hyperactivity disorder (ADHD) by her psychiatrist, who prescribes a long-acting methylphenidate formulation at 1 mg/kg. She tolerates the medication without side effects and shows significant improvement in her academic performance and on-task behavior in school. Molly takes methylphenidate before school at 7:00 am; this dose usually wears off at approximately 3:30 pm.
Molly and her parents are pleased with her response to methylphenidate, but report that she has difficulty getting ready for school because of distractibility. In the evenings Molly has trouble staying seated to do homework and often interrupts and argues with family members, but cannot tolerate afternoon dosing of immediate-release methylphenidate because of insomnia.
ADHD, the most common childhood neurobehavioral disorder, is characterized by difficulties with attention, impulse control, and modulating activity level. The pathophysiology of ADHD is thought to involve dysregulation of brain dopamine and norepinephrine systems.1 Managing ADHD includes pharmacotherapeutic and nonpharmacotherapeutic—ie, behavioral and psychoeducational—interventions.2,3
In this article, we provide an overview of the efficacy, side effects, and dosing for the 3 classes of ADHD medication—psychostimulants, atomoxetine, and α2 adrenergic agonists—including guidance on medication choice and combination treatment. We also discuss the effects of psychostimulants on tics, cardiovascular concerns, and substance abuse potential.
Psychostimulants
Methylphenidates and amphetamines are first-line agents for ADHD. Their primary mechanism of action involves blocking dopamine transporters, with additional effects including blockade of norepinephrine transporters, dampening action of monoamine oxidase (which slows dopamine and norepinephrine degradation), and enhanced release of dopamine into the synaptic space.1
Efficacy and response rates are similar for methylphenidate and amphetamine medications, although as many as 25% of patients may respond to only 1 agent.1 More than 90% of patients will have a positive response to one of the psychostimulants.1 The beneficial effects of psychostimulants on inattention, hyperactivity, and impulsivity are well documented.2Improvements in noncompliance, aggression, social interactions, and academic productivity also have been observed.4,5
Because of increased recognition of pervasive ADHD-related impairments, which can affect functioning in social, family, and extracurricular settings, practitioners have shifted to long-acting psychostimulants to reduce the need for in-school dosing, improve compliance, and obtain more after-school treatment effects. Long-acting formulations produce a slower rise and fall of psychostimulant levels in the brain, which may decrease side effects and potential for later drug abuse.6 See Table 12,7-9 and Table 22,7,9 for titration, dosing, and duration of action of psychostimulants.
The most common side effects of psychostimulants are appetite loss, abdominal pain, headaches, and sleep disturbances.2 Emotional symptoms—irritability and nervousness—may be observed with psychostimulant use, but these behaviors may improve, rather than become worse, with treatment.5 Methylphenidates and amphetamines share many of the same side effects,2 with many studies indicating no differences between their side-effect profiles.1 Other studies indicate that sleep and emotional side effects may be more prominent with amphetamines than methylphenidates,10 although response varies by individual.
There is little evidence that methylphenidate, low-dose amphetamine, or low-dose dextroamphetamine makes tics worse in most children who have them, although significant tic exacerbation has been observed with higher-dose dextroamphetamine.11,12 In patients with comorbid ADHD and tic disorders, a trial of psychostimulants with monitoring for worsening tics is appropriate.
Changes in heart rate and blood pressure generally are not clinically significant in patients taking psychostimulants (average increases: 1 or 2 beats per minute and 1 to 4 mm Hg for systolic and diastolic blood pressures).12 However, psychostimulants may be associated with more substantial increases in heart rate and blood pressure in a subset of individuals (5% to 15%).12 Large studies of children and adults in the general population have not found an association between psychostimulant use and severe cardiovascular events (sudden cardiac death, myocardial infarction, stroke).12-14 Because of reports of sudden cardiac death in children with underlying heart disease who take a psychostimulant,15 clinicians are advised to screen patients and consider an electrocardiogram or evaluation by a cardiologist before starting a psychostimulant in a patient who has a personal or family history of specific cardiovascular risk factors (see Perrin et al16 and Cortese et al12 for screening questions and conditions).
Modest reductions in height (1 or 2 cm after 3 years of psychostimulant treatment) appear to be dose-dependent, and are similar across the methylphenidate and amphetamine classes. Some studies have shown reversal of growth deficits after treatment is stopped treatment and no adverse effects on final adult height.12,17 More study is needed to clarify the effects of continuous psychostimulant treatment from childhood to adulthood on growth.
Studies have failed to show an increased risk of substance abuse in persons with ADHD who were treated with psychostimulants during childhood. Some studies document a lower rate of later substance abuse in youths who received ADHD medications, although other reports show no effect of psychostimulant treatment on subsequent substance use disorder risk.12 Be aware that psychostimulants can be misused (eg, to get “high,” for performance enhancement, to suppress appetite, etc.). Misuse of psychostimulants is most common with short-acting preparations, and generally more difficult with long-acting preparations because extracting the active ingredients for snorting is difficult.2,12 Monitor refill requests and patient behavior for signs of misuse, and be alert for signs of illegal drug use in the patient’s family.
Psychotic symptoms—including hallucinations, delusions, mania, and extreme agitation—with psychostimulant treatment are rare, occurring at a rate of 1.5%.12
Atomoxetine
Approved by the FDA in 2002 for ADHD, atomoxetine is effective and generally well tolerated, although it is not as effective as psychostimulants.2 Atomoxetine is a potent norepinephrine reuptake inhibitor18 that does not produce euphoria, does not have potential for abuse, and has not been linked to increased tic onset or severity.19 Atomoxetine treatment is associated with a lower rate of sleep initiation difficulty compared with psychostimulants.18 Some studies suggest that atomoxetine may have mild beneficial effects on anxiety disorders,18 making it a reasonable choice for patients with significant anxiety or insomnia during psychostimulant treatment. Table 12,7-9 and Table 32,7,9 include information on dosing and duration of action for atomoxetine.
Common side effects of atomoxetine include sedation and fatigue, upset stomach, nausea and vomiting, reduced appetite, headache, and irritability.18 Inform patients that atomoxetine carries an FDA black-box warning for suicide risk; a review of 14 studies showed suicidal ideation was more common with atomoxetine than placebo, although no suicides occurred in any trials.20
Hepatotoxicity is rare with atomoxetine.21 Although routine liver enzyme testing is not required, discontinue atomoxetine if jaundice develops or elevated levels of liver enzymes are noted. Other rare but potentially serious side effects include changes in heart rate (≥20 beats per min) or blood pressure that occur in 5% to 10% of patients taking atomoxetine.22 The risk of serious cardiovascular events and sudden cardiac death with atomoxetine is extremely low, but patients should be screened for a personal and family history of cardiovascular risk factors and, if any of these are present, evaluated further before starting atomoxetine. Routine heart rate and blood pressure monitoring is recommended for all patients.12-14,16
Last, atomoxetine has been linked to growth delays in the first 1 or 2 years of treatment, with a return to expected measurements after an average 2 or 3 years of treatment; persistent decreases in growth rate were observed in patients who were taller or heavier than average before treatment.23
α2 Adrenergic agonists
Guanfacine ER and clonidine ER, the extended release (ER) formulations of α2 adrenergic agonists, were FDA-approved for treating ADHD in 2009 and 2010, respectively. Short-acting guanfacine and clonidine also are used for treating ADHD.24 Their mechanism of action involves stimulation of the pre-synaptic and post-synapic α2 adrenergic receptors, which control the release of norepinephrine and the rate of cell firing.25 The α2 agonists are considered a second-line treatment for ADHD because their efficacy and response rate for core ADHD symptoms lags behind those of psychostimulants.25 In addition to treating core ADHD symptoms, guanfacine and clonidine are used to treat tics and oppositional/aggressive behavior comorbid with ADHD.24,26 Clonidine, which is more sedating than guanfacine, can be used to treat comorbid ADHD and sleep disorders.24 The α2 agonists do not produce euphoria and do not have drug abuse potential.2Table 12,7-9 and Table 32,7,9 provide guidelines for prescribing guanfacine ER and clonidine ER.
The most common adverse effect is drowsiness; other common side effects include dizziness, irritability, headache, and abdominal pain.24 Short-term studies of α2 agonist treatment of ADHD have shown small, non-clinically significant reductions in heart rate and blood pressure; α2 agonist-associated bradycardia, increased QT interval, and cardiac arrhythmias have been reported,7,24,27 as well as rebound hypertension with abrupt discontinuation.24 Screen patients for a personal and family history of cardiovascular risk factors and, if present, evaluate further before initiating α2 agonists.
Combining ADHD medication classes
Combination therapy with >1 ADHD medications is employed when 1 class does not provide adequate symptom coverage or produces problematic side effects.8,24 Psychostimulants can be combined with low-dose atomoxetine (0.5 to 1.0 mg/kg/d) when atomoxetine does not adequately cover ADHD symptoms in school, or when psychostimulants do not adequately cover evening symptoms or patients experience problems with evening psychostimulant rebound.8 To date, prospective data on the safety and efficacy of combining atomoxetine and psychostimulants are limited, but what evidence is available suggests improved symptom control for some, but not all, patients, and a lack of serious adverse events.28
Psychostimulants have been combined with α2agonists when children have an inadequate response to psychostimulants alone, or in cases of ADHD comorbid with aggression or tics.24 Although early case reports raised concern about the safety of combining psychostimulants and α2 agonists, subsequent studies suggest that clonidine and guanfacine generally are well-tolerated when co-administered with psychostimulants.24,27,29
Case continued
Molly has derived substantial benefit from long-acting methylphenidate during the school day, but continues to have significant ADHD-related impairment in the mornings and evenings. Her physician tried afternoon dosing of immediate-release methylphenidate to address evening difficulties, but Molly experienced insomnia. It would be reasonable to consider adjunctive therapy with a non-stimulant medication. A medication that can provide round-the-clock ADHD symptom coverage—such as atomoxetine, guanfacine ER, or clonidine ER—could be added to her current day-time psychostimulant treatment, potentially improving her functioning at home before school and in the evenings.
Additional considerations
Combining medication and behavior therapy offers greater improvements on academic, conduct, and family satisfaction measures than either treatment alone.2 Clinicians can choose to employ behavior therapy alone, particularly if parents feel uncomfortable with—or children have not tolerated—medication.2,3 Evidence-based behavioral parent training and classroom management strategies (implemented by teachers) have shown the strongest and most consistent effects among nonpharmacotherapeutic interventions for ADHD.2 Most studies comparing behavior therapy to psychostimulants have found a stronger effect on core ADHD symptoms from psychostimulants than from behavior therapy.
When a patient does not respond adequately to FDA-approved ADHD medications alone or in combination, consider bupropion, an antidepressant with indirect dopamine and noradrenergic effects. Off-label bupropion has been shown to be effective for ADHD in controlled trials of both children and adults.30
Clinicians often encounter children who meet criteria for ADHD and an anxiety or mood disorder. Table 48,31 summarizes treatment recommendations for these patients.
Clinical considerations
- Begin treatment with a psychostimulant at a low dosage, and titrate gradually until symptoms are controlled or side effects develop.
- Keep in mind that an effective dosage of a psychostimulant is not closely correlated with age, weight, or severity of symptoms.
- Monitor refill requests and patient behavior for signs of psychostimulant misuse. Be alert for signs of illegal drug use in patient family members.
- Lisdexamfetamine, dermal methylphenidate, and osmotic release oral system methylphenidate are the formulations least likely to be misused because their delivery systems make it difficult to extract the active ingredient for snorting or intravenous injection.
- Psychostimulants have not been shown to exacerbate tics in most children who have comorbid ADHD and a tic disorder. When a stimulant is associated with an exacerbation of tics, switching treatment to atomoxetine or α2 agonists is reasonable.
- For patients whose use of a stimulant is limited by an adverse effect on sleep, consider atomoxetine and α2 adrenergic agonists as alternative or adjunctive treatments.
- All 3 classes of FDA-approved ADHD medications (psychostimulants, atomoxetine, and adrenergic agonists) have been associated with adverse cardiac events in children who have underlying cardiovascular conditions. Before initiating treatment, screen patients for a personal or family history of cardiovascular risk factors, and undertake further evaluation as indicated.
Bottom Line
In general, the evidence supports psychostimulants as initial pharmacotherapy for ADHD, with additional options including atomoxetine and α2 agonists. When one medication class does not provide adequate coverage for ADHD symptoms, combining medication classes can be beneficial.
Related Resources
- National Institute of Mental Health. What is attention deficit hyperactivity disorder (ADHD, ADD)?” www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml.
- National Resource Center on AD/HD. Managing medication for children and adolescents with ADHD. www.help4adhd.org/en/treatment/medication/WWK3.
Drug Brand Names
Atomoxetine • Strattera
Lisdexamfetamine • Vyvanse
Bupropion • Wellbutrin, Zyban
Clonidine extended release • Kapvay
Guanfacine extended release • Intuniv
Dexmethylphenidate • Focalin, Focalin XR
Mixed amphetamine salts • Adderall, Adderall XR
Dextroamphetamine • Dexedrine, Dexedrine SR, DextroStat, ProCentra
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Disclosures
Dr. Froehlich receives support from the National Institute of Mental Health Grant K23 MH083881. Dr. Delgado has received research support from Pfizer, Inc. Dr. Anixt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998; 94(1):127-152.
2. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
3. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
4. Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340(1):40-46.
5. Goldman LS, Genel M, Bezman RJ, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(14):1100-1107.
6. Swanson J, Gupta S, Lam A, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry. 2003;60(2):204-211.
7. Vaughan B, Kratochvil CJ. Pharmacotherapy of pediatric attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2012;21(4):941-955.
8. Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642-657.
9. Antshel KM, Hargrave TM, Simonescu M, et al. Advances in understanding and treating ADHD. BMC Med. 2011;9:72.
10. Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662-666.
11. Pringsheim T, Steeves T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2011(4):CD007990.
12. Cortese S, Holtmann M, Banaschewski T, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013; 54(3):227-246.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
14. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
15. Vetter VL, Elia J, Erickson C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
16. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451-453.
17. Faraone SV, Biederman J, Morley CP, et al. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(9):994-1009.
18. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
19. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
20. Bangs ME, Tauscher-Wisniewski S, Polzer J, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47(2):209-218.
21. Bangs ME, Jin L, Zhang S, et al. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008;31(4):345-354.
22. U.S. Food and Drug Administration. Strattera (atomoxetine hydrochloride) capsule. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm223889.htm. Published August 2013. Accessed October 31, 2013.
23. Spencer TJ, Kratochvil CJ, Sangal RB, et al. Effects of atomoxetine on growth in children with attention-deficit/hyperactivity disorder following up to five years of treatment. J Child Adolesc Psychopharmacol. 2007;17(5):689-700.
24. Connor DF. Other medications. In: Barkley RA, ed. Attention-deficit/hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:658-677.
25. May DE, Kratochvil CJ. Attention-deficit hyperactivity disorder: recent advances in paediatric pharmacotherapy. Drugs. 2010;70(1):15-40.
26. Connor DF, Findling RL, Kollins SH, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010; 24(9):755-768.
27. Croxtall JD. Clonidine extended-release: in attention-deficit hyperactivity disorder. Paediatr Drugs. 2011;13(5):329-336.
28. Treuer T, Gau SS, Mendez L, et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23(3):179-193.
29. Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122(5):78-87.
30. Spencer TJ. Antidepressant and specific norepinephrine reuptake inhibitor treatments. In: Barkley RA, ed. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:648-657.
31. Singh MK, DelBello MP, Kowatch RA, et al. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8(6):710-720.
Molly, age 9, is diagnosed with attention-deficit/hyperactivity disorder (ADHD) by her psychiatrist, who prescribes a long-acting methylphenidate formulation at 1 mg/kg. She tolerates the medication without side effects and shows significant improvement in her academic performance and on-task behavior in school. Molly takes methylphenidate before school at 7:00 am; this dose usually wears off at approximately 3:30 pm.
Molly and her parents are pleased with her response to methylphenidate, but report that she has difficulty getting ready for school because of distractibility. In the evenings Molly has trouble staying seated to do homework and often interrupts and argues with family members, but cannot tolerate afternoon dosing of immediate-release methylphenidate because of insomnia.
ADHD, the most common childhood neurobehavioral disorder, is characterized by difficulties with attention, impulse control, and modulating activity level. The pathophysiology of ADHD is thought to involve dysregulation of brain dopamine and norepinephrine systems.1 Managing ADHD includes pharmacotherapeutic and nonpharmacotherapeutic—ie, behavioral and psychoeducational—interventions.2,3
In this article, we provide an overview of the efficacy, side effects, and dosing for the 3 classes of ADHD medication—psychostimulants, atomoxetine, and α2 adrenergic agonists—including guidance on medication choice and combination treatment. We also discuss the effects of psychostimulants on tics, cardiovascular concerns, and substance abuse potential.
Psychostimulants
Methylphenidates and amphetamines are first-line agents for ADHD. Their primary mechanism of action involves blocking dopamine transporters, with additional effects including blockade of norepinephrine transporters, dampening action of monoamine oxidase (which slows dopamine and norepinephrine degradation), and enhanced release of dopamine into the synaptic space.1
Efficacy and response rates are similar for methylphenidate and amphetamine medications, although as many as 25% of patients may respond to only 1 agent.1 More than 90% of patients will have a positive response to one of the psychostimulants.1 The beneficial effects of psychostimulants on inattention, hyperactivity, and impulsivity are well documented.2Improvements in noncompliance, aggression, social interactions, and academic productivity also have been observed.4,5
Because of increased recognition of pervasive ADHD-related impairments, which can affect functioning in social, family, and extracurricular settings, practitioners have shifted to long-acting psychostimulants to reduce the need for in-school dosing, improve compliance, and obtain more after-school treatment effects. Long-acting formulations produce a slower rise and fall of psychostimulant levels in the brain, which may decrease side effects and potential for later drug abuse.6 See Table 12,7-9 and Table 22,7,9 for titration, dosing, and duration of action of psychostimulants.
The most common side effects of psychostimulants are appetite loss, abdominal pain, headaches, and sleep disturbances.2 Emotional symptoms—irritability and nervousness—may be observed with psychostimulant use, but these behaviors may improve, rather than become worse, with treatment.5 Methylphenidates and amphetamines share many of the same side effects,2 with many studies indicating no differences between their side-effect profiles.1 Other studies indicate that sleep and emotional side effects may be more prominent with amphetamines than methylphenidates,10 although response varies by individual.
There is little evidence that methylphenidate, low-dose amphetamine, or low-dose dextroamphetamine makes tics worse in most children who have them, although significant tic exacerbation has been observed with higher-dose dextroamphetamine.11,12 In patients with comorbid ADHD and tic disorders, a trial of psychostimulants with monitoring for worsening tics is appropriate.
Changes in heart rate and blood pressure generally are not clinically significant in patients taking psychostimulants (average increases: 1 or 2 beats per minute and 1 to 4 mm Hg for systolic and diastolic blood pressures).12 However, psychostimulants may be associated with more substantial increases in heart rate and blood pressure in a subset of individuals (5% to 15%).12 Large studies of children and adults in the general population have not found an association between psychostimulant use and severe cardiovascular events (sudden cardiac death, myocardial infarction, stroke).12-14 Because of reports of sudden cardiac death in children with underlying heart disease who take a psychostimulant,15 clinicians are advised to screen patients and consider an electrocardiogram or evaluation by a cardiologist before starting a psychostimulant in a patient who has a personal or family history of specific cardiovascular risk factors (see Perrin et al16 and Cortese et al12 for screening questions and conditions).
Modest reductions in height (1 or 2 cm after 3 years of psychostimulant treatment) appear to be dose-dependent, and are similar across the methylphenidate and amphetamine classes. Some studies have shown reversal of growth deficits after treatment is stopped treatment and no adverse effects on final adult height.12,17 More study is needed to clarify the effects of continuous psychostimulant treatment from childhood to adulthood on growth.
Studies have failed to show an increased risk of substance abuse in persons with ADHD who were treated with psychostimulants during childhood. Some studies document a lower rate of later substance abuse in youths who received ADHD medications, although other reports show no effect of psychostimulant treatment on subsequent substance use disorder risk.12 Be aware that psychostimulants can be misused (eg, to get “high,” for performance enhancement, to suppress appetite, etc.). Misuse of psychostimulants is most common with short-acting preparations, and generally more difficult with long-acting preparations because extracting the active ingredients for snorting is difficult.2,12 Monitor refill requests and patient behavior for signs of misuse, and be alert for signs of illegal drug use in the patient’s family.
Psychotic symptoms—including hallucinations, delusions, mania, and extreme agitation—with psychostimulant treatment are rare, occurring at a rate of 1.5%.12
Atomoxetine
Approved by the FDA in 2002 for ADHD, atomoxetine is effective and generally well tolerated, although it is not as effective as psychostimulants.2 Atomoxetine is a potent norepinephrine reuptake inhibitor18 that does not produce euphoria, does not have potential for abuse, and has not been linked to increased tic onset or severity.19 Atomoxetine treatment is associated with a lower rate of sleep initiation difficulty compared with psychostimulants.18 Some studies suggest that atomoxetine may have mild beneficial effects on anxiety disorders,18 making it a reasonable choice for patients with significant anxiety or insomnia during psychostimulant treatment. Table 12,7-9 and Table 32,7,9 include information on dosing and duration of action for atomoxetine.
Common side effects of atomoxetine include sedation and fatigue, upset stomach, nausea and vomiting, reduced appetite, headache, and irritability.18 Inform patients that atomoxetine carries an FDA black-box warning for suicide risk; a review of 14 studies showed suicidal ideation was more common with atomoxetine than placebo, although no suicides occurred in any trials.20
Hepatotoxicity is rare with atomoxetine.21 Although routine liver enzyme testing is not required, discontinue atomoxetine if jaundice develops or elevated levels of liver enzymes are noted. Other rare but potentially serious side effects include changes in heart rate (≥20 beats per min) or blood pressure that occur in 5% to 10% of patients taking atomoxetine.22 The risk of serious cardiovascular events and sudden cardiac death with atomoxetine is extremely low, but patients should be screened for a personal and family history of cardiovascular risk factors and, if any of these are present, evaluated further before starting atomoxetine. Routine heart rate and blood pressure monitoring is recommended for all patients.12-14,16
Last, atomoxetine has been linked to growth delays in the first 1 or 2 years of treatment, with a return to expected measurements after an average 2 or 3 years of treatment; persistent decreases in growth rate were observed in patients who were taller or heavier than average before treatment.23
α2 Adrenergic agonists
Guanfacine ER and clonidine ER, the extended release (ER) formulations of α2 adrenergic agonists, were FDA-approved for treating ADHD in 2009 and 2010, respectively. Short-acting guanfacine and clonidine also are used for treating ADHD.24 Their mechanism of action involves stimulation of the pre-synaptic and post-synapic α2 adrenergic receptors, which control the release of norepinephrine and the rate of cell firing.25 The α2 agonists are considered a second-line treatment for ADHD because their efficacy and response rate for core ADHD symptoms lags behind those of psychostimulants.25 In addition to treating core ADHD symptoms, guanfacine and clonidine are used to treat tics and oppositional/aggressive behavior comorbid with ADHD.24,26 Clonidine, which is more sedating than guanfacine, can be used to treat comorbid ADHD and sleep disorders.24 The α2 agonists do not produce euphoria and do not have drug abuse potential.2Table 12,7-9 and Table 32,7,9 provide guidelines for prescribing guanfacine ER and clonidine ER.
The most common adverse effect is drowsiness; other common side effects include dizziness, irritability, headache, and abdominal pain.24 Short-term studies of α2 agonist treatment of ADHD have shown small, non-clinically significant reductions in heart rate and blood pressure; α2 agonist-associated bradycardia, increased QT interval, and cardiac arrhythmias have been reported,7,24,27 as well as rebound hypertension with abrupt discontinuation.24 Screen patients for a personal and family history of cardiovascular risk factors and, if present, evaluate further before initiating α2 agonists.
Combining ADHD medication classes
Combination therapy with >1 ADHD medications is employed when 1 class does not provide adequate symptom coverage or produces problematic side effects.8,24 Psychostimulants can be combined with low-dose atomoxetine (0.5 to 1.0 mg/kg/d) when atomoxetine does not adequately cover ADHD symptoms in school, or when psychostimulants do not adequately cover evening symptoms or patients experience problems with evening psychostimulant rebound.8 To date, prospective data on the safety and efficacy of combining atomoxetine and psychostimulants are limited, but what evidence is available suggests improved symptom control for some, but not all, patients, and a lack of serious adverse events.28
Psychostimulants have been combined with α2agonists when children have an inadequate response to psychostimulants alone, or in cases of ADHD comorbid with aggression or tics.24 Although early case reports raised concern about the safety of combining psychostimulants and α2 agonists, subsequent studies suggest that clonidine and guanfacine generally are well-tolerated when co-administered with psychostimulants.24,27,29
Case continued
Molly has derived substantial benefit from long-acting methylphenidate during the school day, but continues to have significant ADHD-related impairment in the mornings and evenings. Her physician tried afternoon dosing of immediate-release methylphenidate to address evening difficulties, but Molly experienced insomnia. It would be reasonable to consider adjunctive therapy with a non-stimulant medication. A medication that can provide round-the-clock ADHD symptom coverage—such as atomoxetine, guanfacine ER, or clonidine ER—could be added to her current day-time psychostimulant treatment, potentially improving her functioning at home before school and in the evenings.
Additional considerations
Combining medication and behavior therapy offers greater improvements on academic, conduct, and family satisfaction measures than either treatment alone.2 Clinicians can choose to employ behavior therapy alone, particularly if parents feel uncomfortable with—or children have not tolerated—medication.2,3 Evidence-based behavioral parent training and classroom management strategies (implemented by teachers) have shown the strongest and most consistent effects among nonpharmacotherapeutic interventions for ADHD.2 Most studies comparing behavior therapy to psychostimulants have found a stronger effect on core ADHD symptoms from psychostimulants than from behavior therapy.
When a patient does not respond adequately to FDA-approved ADHD medications alone or in combination, consider bupropion, an antidepressant with indirect dopamine and noradrenergic effects. Off-label bupropion has been shown to be effective for ADHD in controlled trials of both children and adults.30
Clinicians often encounter children who meet criteria for ADHD and an anxiety or mood disorder. Table 48,31 summarizes treatment recommendations for these patients.
Clinical considerations
- Begin treatment with a psychostimulant at a low dosage, and titrate gradually until symptoms are controlled or side effects develop.
- Keep in mind that an effective dosage of a psychostimulant is not closely correlated with age, weight, or severity of symptoms.
- Monitor refill requests and patient behavior for signs of psychostimulant misuse. Be alert for signs of illegal drug use in patient family members.
- Lisdexamfetamine, dermal methylphenidate, and osmotic release oral system methylphenidate are the formulations least likely to be misused because their delivery systems make it difficult to extract the active ingredient for snorting or intravenous injection.
- Psychostimulants have not been shown to exacerbate tics in most children who have comorbid ADHD and a tic disorder. When a stimulant is associated with an exacerbation of tics, switching treatment to atomoxetine or α2 agonists is reasonable.
- For patients whose use of a stimulant is limited by an adverse effect on sleep, consider atomoxetine and α2 adrenergic agonists as alternative or adjunctive treatments.
- All 3 classes of FDA-approved ADHD medications (psychostimulants, atomoxetine, and adrenergic agonists) have been associated with adverse cardiac events in children who have underlying cardiovascular conditions. Before initiating treatment, screen patients for a personal or family history of cardiovascular risk factors, and undertake further evaluation as indicated.
Bottom Line
In general, the evidence supports psychostimulants as initial pharmacotherapy for ADHD, with additional options including atomoxetine and α2 agonists. When one medication class does not provide adequate coverage for ADHD symptoms, combining medication classes can be beneficial.
Related Resources
- National Institute of Mental Health. What is attention deficit hyperactivity disorder (ADHD, ADD)?” www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml.
- National Resource Center on AD/HD. Managing medication for children and adolescents with ADHD. www.help4adhd.org/en/treatment/medication/WWK3.
Drug Brand Names
Atomoxetine • Strattera
Lisdexamfetamine • Vyvanse
Bupropion • Wellbutrin, Zyban
Clonidine extended release • Kapvay
Guanfacine extended release • Intuniv
Dexmethylphenidate • Focalin, Focalin XR
Mixed amphetamine salts • Adderall, Adderall XR
Dextroamphetamine • Dexedrine, Dexedrine SR, DextroStat, ProCentra
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Disclosures
Dr. Froehlich receives support from the National Institute of Mental Health Grant K23 MH083881. Dr. Delgado has received research support from Pfizer, Inc. Dr. Anixt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Molly, age 9, is diagnosed with attention-deficit/hyperactivity disorder (ADHD) by her psychiatrist, who prescribes a long-acting methylphenidate formulation at 1 mg/kg. She tolerates the medication without side effects and shows significant improvement in her academic performance and on-task behavior in school. Molly takes methylphenidate before school at 7:00 am; this dose usually wears off at approximately 3:30 pm.
Molly and her parents are pleased with her response to methylphenidate, but report that she has difficulty getting ready for school because of distractibility. In the evenings Molly has trouble staying seated to do homework and often interrupts and argues with family members, but cannot tolerate afternoon dosing of immediate-release methylphenidate because of insomnia.
ADHD, the most common childhood neurobehavioral disorder, is characterized by difficulties with attention, impulse control, and modulating activity level. The pathophysiology of ADHD is thought to involve dysregulation of brain dopamine and norepinephrine systems.1 Managing ADHD includes pharmacotherapeutic and nonpharmacotherapeutic—ie, behavioral and psychoeducational—interventions.2,3
In this article, we provide an overview of the efficacy, side effects, and dosing for the 3 classes of ADHD medication—psychostimulants, atomoxetine, and α2 adrenergic agonists—including guidance on medication choice and combination treatment. We also discuss the effects of psychostimulants on tics, cardiovascular concerns, and substance abuse potential.
Psychostimulants
Methylphenidates and amphetamines are first-line agents for ADHD. Their primary mechanism of action involves blocking dopamine transporters, with additional effects including blockade of norepinephrine transporters, dampening action of monoamine oxidase (which slows dopamine and norepinephrine degradation), and enhanced release of dopamine into the synaptic space.1
Efficacy and response rates are similar for methylphenidate and amphetamine medications, although as many as 25% of patients may respond to only 1 agent.1 More than 90% of patients will have a positive response to one of the psychostimulants.1 The beneficial effects of psychostimulants on inattention, hyperactivity, and impulsivity are well documented.2Improvements in noncompliance, aggression, social interactions, and academic productivity also have been observed.4,5
Because of increased recognition of pervasive ADHD-related impairments, which can affect functioning in social, family, and extracurricular settings, practitioners have shifted to long-acting psychostimulants to reduce the need for in-school dosing, improve compliance, and obtain more after-school treatment effects. Long-acting formulations produce a slower rise and fall of psychostimulant levels in the brain, which may decrease side effects and potential for later drug abuse.6 See Table 12,7-9 and Table 22,7,9 for titration, dosing, and duration of action of psychostimulants.
The most common side effects of psychostimulants are appetite loss, abdominal pain, headaches, and sleep disturbances.2 Emotional symptoms—irritability and nervousness—may be observed with psychostimulant use, but these behaviors may improve, rather than become worse, with treatment.5 Methylphenidates and amphetamines share many of the same side effects,2 with many studies indicating no differences between their side-effect profiles.1 Other studies indicate that sleep and emotional side effects may be more prominent with amphetamines than methylphenidates,10 although response varies by individual.
There is little evidence that methylphenidate, low-dose amphetamine, or low-dose dextroamphetamine makes tics worse in most children who have them, although significant tic exacerbation has been observed with higher-dose dextroamphetamine.11,12 In patients with comorbid ADHD and tic disorders, a trial of psychostimulants with monitoring for worsening tics is appropriate.
Changes in heart rate and blood pressure generally are not clinically significant in patients taking psychostimulants (average increases: 1 or 2 beats per minute and 1 to 4 mm Hg for systolic and diastolic blood pressures).12 However, psychostimulants may be associated with more substantial increases in heart rate and blood pressure in a subset of individuals (5% to 15%).12 Large studies of children and adults in the general population have not found an association between psychostimulant use and severe cardiovascular events (sudden cardiac death, myocardial infarction, stroke).12-14 Because of reports of sudden cardiac death in children with underlying heart disease who take a psychostimulant,15 clinicians are advised to screen patients and consider an electrocardiogram or evaluation by a cardiologist before starting a psychostimulant in a patient who has a personal or family history of specific cardiovascular risk factors (see Perrin et al16 and Cortese et al12 for screening questions and conditions).
Modest reductions in height (1 or 2 cm after 3 years of psychostimulant treatment) appear to be dose-dependent, and are similar across the methylphenidate and amphetamine classes. Some studies have shown reversal of growth deficits after treatment is stopped treatment and no adverse effects on final adult height.12,17 More study is needed to clarify the effects of continuous psychostimulant treatment from childhood to adulthood on growth.
Studies have failed to show an increased risk of substance abuse in persons with ADHD who were treated with psychostimulants during childhood. Some studies document a lower rate of later substance abuse in youths who received ADHD medications, although other reports show no effect of psychostimulant treatment on subsequent substance use disorder risk.12 Be aware that psychostimulants can be misused (eg, to get “high,” for performance enhancement, to suppress appetite, etc.). Misuse of psychostimulants is most common with short-acting preparations, and generally more difficult with long-acting preparations because extracting the active ingredients for snorting is difficult.2,12 Monitor refill requests and patient behavior for signs of misuse, and be alert for signs of illegal drug use in the patient’s family.
Psychotic symptoms—including hallucinations, delusions, mania, and extreme agitation—with psychostimulant treatment are rare, occurring at a rate of 1.5%.12
Atomoxetine
Approved by the FDA in 2002 for ADHD, atomoxetine is effective and generally well tolerated, although it is not as effective as psychostimulants.2 Atomoxetine is a potent norepinephrine reuptake inhibitor18 that does not produce euphoria, does not have potential for abuse, and has not been linked to increased tic onset or severity.19 Atomoxetine treatment is associated with a lower rate of sleep initiation difficulty compared with psychostimulants.18 Some studies suggest that atomoxetine may have mild beneficial effects on anxiety disorders,18 making it a reasonable choice for patients with significant anxiety or insomnia during psychostimulant treatment. Table 12,7-9 and Table 32,7,9 include information on dosing and duration of action for atomoxetine.
Common side effects of atomoxetine include sedation and fatigue, upset stomach, nausea and vomiting, reduced appetite, headache, and irritability.18 Inform patients that atomoxetine carries an FDA black-box warning for suicide risk; a review of 14 studies showed suicidal ideation was more common with atomoxetine than placebo, although no suicides occurred in any trials.20
Hepatotoxicity is rare with atomoxetine.21 Although routine liver enzyme testing is not required, discontinue atomoxetine if jaundice develops or elevated levels of liver enzymes are noted. Other rare but potentially serious side effects include changes in heart rate (≥20 beats per min) or blood pressure that occur in 5% to 10% of patients taking atomoxetine.22 The risk of serious cardiovascular events and sudden cardiac death with atomoxetine is extremely low, but patients should be screened for a personal and family history of cardiovascular risk factors and, if any of these are present, evaluated further before starting atomoxetine. Routine heart rate and blood pressure monitoring is recommended for all patients.12-14,16
Last, atomoxetine has been linked to growth delays in the first 1 or 2 years of treatment, with a return to expected measurements after an average 2 or 3 years of treatment; persistent decreases in growth rate were observed in patients who were taller or heavier than average before treatment.23
α2 Adrenergic agonists
Guanfacine ER and clonidine ER, the extended release (ER) formulations of α2 adrenergic agonists, were FDA-approved for treating ADHD in 2009 and 2010, respectively. Short-acting guanfacine and clonidine also are used for treating ADHD.24 Their mechanism of action involves stimulation of the pre-synaptic and post-synapic α2 adrenergic receptors, which control the release of norepinephrine and the rate of cell firing.25 The α2 agonists are considered a second-line treatment for ADHD because their efficacy and response rate for core ADHD symptoms lags behind those of psychostimulants.25 In addition to treating core ADHD symptoms, guanfacine and clonidine are used to treat tics and oppositional/aggressive behavior comorbid with ADHD.24,26 Clonidine, which is more sedating than guanfacine, can be used to treat comorbid ADHD and sleep disorders.24 The α2 agonists do not produce euphoria and do not have drug abuse potential.2Table 12,7-9 and Table 32,7,9 provide guidelines for prescribing guanfacine ER and clonidine ER.
The most common adverse effect is drowsiness; other common side effects include dizziness, irritability, headache, and abdominal pain.24 Short-term studies of α2 agonist treatment of ADHD have shown small, non-clinically significant reductions in heart rate and blood pressure; α2 agonist-associated bradycardia, increased QT interval, and cardiac arrhythmias have been reported,7,24,27 as well as rebound hypertension with abrupt discontinuation.24 Screen patients for a personal and family history of cardiovascular risk factors and, if present, evaluate further before initiating α2 agonists.
Combining ADHD medication classes
Combination therapy with >1 ADHD medications is employed when 1 class does not provide adequate symptom coverage or produces problematic side effects.8,24 Psychostimulants can be combined with low-dose atomoxetine (0.5 to 1.0 mg/kg/d) when atomoxetine does not adequately cover ADHD symptoms in school, or when psychostimulants do not adequately cover evening symptoms or patients experience problems with evening psychostimulant rebound.8 To date, prospective data on the safety and efficacy of combining atomoxetine and psychostimulants are limited, but what evidence is available suggests improved symptom control for some, but not all, patients, and a lack of serious adverse events.28
Psychostimulants have been combined with α2agonists when children have an inadequate response to psychostimulants alone, or in cases of ADHD comorbid with aggression or tics.24 Although early case reports raised concern about the safety of combining psychostimulants and α2 agonists, subsequent studies suggest that clonidine and guanfacine generally are well-tolerated when co-administered with psychostimulants.24,27,29
Case continued
Molly has derived substantial benefit from long-acting methylphenidate during the school day, but continues to have significant ADHD-related impairment in the mornings and evenings. Her physician tried afternoon dosing of immediate-release methylphenidate to address evening difficulties, but Molly experienced insomnia. It would be reasonable to consider adjunctive therapy with a non-stimulant medication. A medication that can provide round-the-clock ADHD symptom coverage—such as atomoxetine, guanfacine ER, or clonidine ER—could be added to her current day-time psychostimulant treatment, potentially improving her functioning at home before school and in the evenings.
Additional considerations
Combining medication and behavior therapy offers greater improvements on academic, conduct, and family satisfaction measures than either treatment alone.2 Clinicians can choose to employ behavior therapy alone, particularly if parents feel uncomfortable with—or children have not tolerated—medication.2,3 Evidence-based behavioral parent training and classroom management strategies (implemented by teachers) have shown the strongest and most consistent effects among nonpharmacotherapeutic interventions for ADHD.2 Most studies comparing behavior therapy to psychostimulants have found a stronger effect on core ADHD symptoms from psychostimulants than from behavior therapy.
When a patient does not respond adequately to FDA-approved ADHD medications alone or in combination, consider bupropion, an antidepressant with indirect dopamine and noradrenergic effects. Off-label bupropion has been shown to be effective for ADHD in controlled trials of both children and adults.30
Clinicians often encounter children who meet criteria for ADHD and an anxiety or mood disorder. Table 48,31 summarizes treatment recommendations for these patients.
Clinical considerations
- Begin treatment with a psychostimulant at a low dosage, and titrate gradually until symptoms are controlled or side effects develop.
- Keep in mind that an effective dosage of a psychostimulant is not closely correlated with age, weight, or severity of symptoms.
- Monitor refill requests and patient behavior for signs of psychostimulant misuse. Be alert for signs of illegal drug use in patient family members.
- Lisdexamfetamine, dermal methylphenidate, and osmotic release oral system methylphenidate are the formulations least likely to be misused because their delivery systems make it difficult to extract the active ingredient for snorting or intravenous injection.
- Psychostimulants have not been shown to exacerbate tics in most children who have comorbid ADHD and a tic disorder. When a stimulant is associated with an exacerbation of tics, switching treatment to atomoxetine or α2 agonists is reasonable.
- For patients whose use of a stimulant is limited by an adverse effect on sleep, consider atomoxetine and α2 adrenergic agonists as alternative or adjunctive treatments.
- All 3 classes of FDA-approved ADHD medications (psychostimulants, atomoxetine, and adrenergic agonists) have been associated with adverse cardiac events in children who have underlying cardiovascular conditions. Before initiating treatment, screen patients for a personal or family history of cardiovascular risk factors, and undertake further evaluation as indicated.
Bottom Line
In general, the evidence supports psychostimulants as initial pharmacotherapy for ADHD, with additional options including atomoxetine and α2 agonists. When one medication class does not provide adequate coverage for ADHD symptoms, combining medication classes can be beneficial.
Related Resources
- National Institute of Mental Health. What is attention deficit hyperactivity disorder (ADHD, ADD)?” www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml.
- National Resource Center on AD/HD. Managing medication for children and adolescents with ADHD. www.help4adhd.org/en/treatment/medication/WWK3.
Drug Brand Names
Atomoxetine • Strattera
Lisdexamfetamine • Vyvanse
Bupropion • Wellbutrin, Zyban
Clonidine extended release • Kapvay
Guanfacine extended release • Intuniv
Dexmethylphenidate • Focalin, Focalin XR
Mixed amphetamine salts • Adderall, Adderall XR
Dextroamphetamine • Dexedrine, Dexedrine SR, DextroStat, ProCentra
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Disclosures
Dr. Froehlich receives support from the National Institute of Mental Health Grant K23 MH083881. Dr. Delgado has received research support from Pfizer, Inc. Dr. Anixt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998; 94(1):127-152.
2. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
3. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
4. Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340(1):40-46.
5. Goldman LS, Genel M, Bezman RJ, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(14):1100-1107.
6. Swanson J, Gupta S, Lam A, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry. 2003;60(2):204-211.
7. Vaughan B, Kratochvil CJ. Pharmacotherapy of pediatric attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2012;21(4):941-955.
8. Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642-657.
9. Antshel KM, Hargrave TM, Simonescu M, et al. Advances in understanding and treating ADHD. BMC Med. 2011;9:72.
10. Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662-666.
11. Pringsheim T, Steeves T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2011(4):CD007990.
12. Cortese S, Holtmann M, Banaschewski T, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013; 54(3):227-246.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
14. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
15. Vetter VL, Elia J, Erickson C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
16. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451-453.
17. Faraone SV, Biederman J, Morley CP, et al. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(9):994-1009.
18. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
19. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
20. Bangs ME, Tauscher-Wisniewski S, Polzer J, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47(2):209-218.
21. Bangs ME, Jin L, Zhang S, et al. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008;31(4):345-354.
22. U.S. Food and Drug Administration. Strattera (atomoxetine hydrochloride) capsule. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm223889.htm. Published August 2013. Accessed October 31, 2013.
23. Spencer TJ, Kratochvil CJ, Sangal RB, et al. Effects of atomoxetine on growth in children with attention-deficit/hyperactivity disorder following up to five years of treatment. J Child Adolesc Psychopharmacol. 2007;17(5):689-700.
24. Connor DF. Other medications. In: Barkley RA, ed. Attention-deficit/hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:658-677.
25. May DE, Kratochvil CJ. Attention-deficit hyperactivity disorder: recent advances in paediatric pharmacotherapy. Drugs. 2010;70(1):15-40.
26. Connor DF, Findling RL, Kollins SH, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010; 24(9):755-768.
27. Croxtall JD. Clonidine extended-release: in attention-deficit hyperactivity disorder. Paediatr Drugs. 2011;13(5):329-336.
28. Treuer T, Gau SS, Mendez L, et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23(3):179-193.
29. Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122(5):78-87.
30. Spencer TJ. Antidepressant and specific norepinephrine reuptake inhibitor treatments. In: Barkley RA, ed. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:648-657.
31. Singh MK, DelBello MP, Kowatch RA, et al. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8(6):710-720.
1. Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998; 94(1):127-152.
2. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
3. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
4. Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340(1):40-46.
5. Goldman LS, Genel M, Bezman RJ, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(14):1100-1107.
6. Swanson J, Gupta S, Lam A, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry. 2003;60(2):204-211.
7. Vaughan B, Kratochvil CJ. Pharmacotherapy of pediatric attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2012;21(4):941-955.
8. Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642-657.
9. Antshel KM, Hargrave TM, Simonescu M, et al. Advances in understanding and treating ADHD. BMC Med. 2011;9:72.
10. Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662-666.
11. Pringsheim T, Steeves T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2011(4):CD007990.
12. Cortese S, Holtmann M, Banaschewski T, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013; 54(3):227-246.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
14. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
15. Vetter VL, Elia J, Erickson C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
16. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451-453.
17. Faraone SV, Biederman J, Morley CP, et al. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(9):994-1009.
18. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
19. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
20. Bangs ME, Tauscher-Wisniewski S, Polzer J, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47(2):209-218.
21. Bangs ME, Jin L, Zhang S, et al. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008;31(4):345-354.
22. U.S. Food and Drug Administration. Strattera (atomoxetine hydrochloride) capsule. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm223889.htm. Published August 2013. Accessed October 31, 2013.
23. Spencer TJ, Kratochvil CJ, Sangal RB, et al. Effects of atomoxetine on growth in children with attention-deficit/hyperactivity disorder following up to five years of treatment. J Child Adolesc Psychopharmacol. 2007;17(5):689-700.
24. Connor DF. Other medications. In: Barkley RA, ed. Attention-deficit/hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:658-677.
25. May DE, Kratochvil CJ. Attention-deficit hyperactivity disorder: recent advances in paediatric pharmacotherapy. Drugs. 2010;70(1):15-40.
26. Connor DF, Findling RL, Kollins SH, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010; 24(9):755-768.
27. Croxtall JD. Clonidine extended-release: in attention-deficit hyperactivity disorder. Paediatr Drugs. 2011;13(5):329-336.
28. Treuer T, Gau SS, Mendez L, et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23(3):179-193.
29. Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122(5):78-87.
30. Spencer TJ. Antidepressant and specific norepinephrine reuptake inhibitor treatments. In: Barkley RA, ed. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:648-657.
31. Singh MK, DelBello MP, Kowatch RA, et al. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8(6):710-720.