User login
Cellulitis unresponsive to antibiotics
A 36-year-old white woman sought care at our outpatient clinic for 2 days of right forearm and hand redness, swelling, and severe pain at a previous intravenous (IV) catheter site. The patient had received intramuscular antibiotics for culture-positive streptococcal pharyngitis 2 weeks earlier, and then IV fluids and steroids due to poor oral intake from odynophagia.
She indicated that since the time of the pharyngitis diagnosis, she’d had a persistent fever. At our clinic, she was given a diagnosis of cellulitis and started on a course of oral cephalexin.
Three days later, she returned. She had a temperature of 100°F and her right forearm and wrist were exquisitely sensitive to touch over a warm and indurated violaceous rash with pseudovesicles over the most edematous portions (FIGURE 1). This rash extended onto her right hand and proximal phalanges. Her fingers hurt when she moved them; sensation and pulses were intact.
Her left wrist was now mildly swollen, with associated warmth and erythema, but no vesicles. Her left ankle was also warm, erythematous, and moderately swollen with significant tenderness to palpation. She had a few scattered nodular, erythematous lesions on her upper right arm and chest.
We (RW and VK) admitted the patient and started her on IV vancomycin and clindamycin to treat presumed refractory cellulitis. On Day 2 she hadn’t improved, so we added gatifloxacin for gram-negative coverage. On Day 3, her lesions worsened, so we transferred her to a higher-level facility.
FIGURE 1
Worsening lesions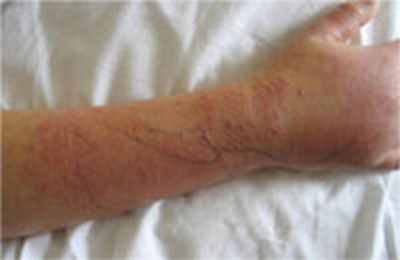
The patient’s right forearm and wrist were exquisitely sensitive to touch over the rash, with pseudovesicles. Ink markings denote original boundaries of the lesion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sweet’s syndrome
A skin biopsy revealed subepidermal bullae with marked dermal and mixed lymphocytic and neutrophilic infiltrate consistent with Sweet’s syndrome.
Sweet’s syndrome, also known as acute febrile neutrophilic dermatoses, is a condition that was originally described by Dr. Robert Sweet in 1964.1 The condition can be classified based on etiology as "classical" or idiopathic, malignancy-associated, or drug-induced.2 Classical Sweet’s syndrome typically affects women 30 to 50 years of age, but has been reported in men and women of all ages.3-5 More than 500 cases have been reported in the literature, but incidence and prevalence data are limited.6 Conditions associated with classical Sweet’s syndrome include upper respiratory infection, gastrointestinal infection, inflammatory bowel disease, and pregnancy.5
Cellulitis and lupus erythematosus are part of the differential
The differential diagnosis for erythematous, indurated, tender skin and soft tissue lesions includes cellulitis, erysipelas, thrombophlebitis, urticaria, shingles, drug-induced eruptions, and herpes simplex virus infections.4-6
Less common conditions include erythema multiforme, erythema nodusum, tuberculosis, leukemia cutis, lupus erythematosus, vasculitis, pyoderma gangrenosa, Behçet’s disease, erythema elevatum diutinum, familial Mediterranean fever, and Sweet’s syndrome.4-6
Diagnosis hinges on these criteria
Two major criteria are required to make a diagnosis of Sweet’s syndrome. They are:2
- the abrupt onset of tender, erythematous plaques or nodules (FIGURE 2)
- a predominately neutrophilic infiltration in the dermis without leukocytoclastic vasculitis.
At least 2 of 4 minor criteria must also be present:2
- a precedent respiratory or gastrointestinal (GI) infection, vaccination, inflammatory disease, malignancy, or pregnancy
- malaise and fever
- elevated erythrocyte sedimentation rate, C-reactive protein, and leukocytosis with a left shift. (Our patient’s erythrocyte sedimentation rate was 93 mm/h; her C-reactive protein was 11.47 mg/dL.)
- excellent response to corticosteroids or potassium iodide.
Although skin manifestations are a hallmark sign (which makes Sweet’s syndrome an important differential diagnosis for an unusual case of cellulitis, or one that is unresponsive to antibiotics), many possible coincident manifestations can occur. These include arthralgia, myalgia, headache, and malaise.5 Multi-system involvement has also been reported, affecting bone, the central nervous system, eyes, kidneys, heart, lungs, and GI tract.5
FIGURE 2
Another presentation of Sweet’s syndrome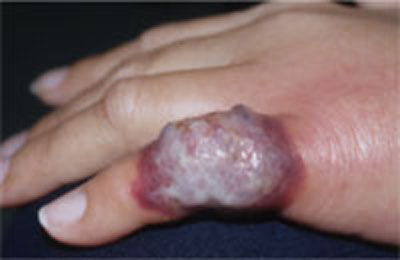
Some Sweet’s syndrome lesions appear "juicy." This lesion occurred at the site of minor trauma (pathergy) in a patient who was febrile and systemically ill.
Treatment: Corticosteroids, not antibiotics
A high clinical suspicion for Sweet’s syndrome is critical to management decisions because the primary treatment is corticosteroids, rather than antibiotics.5,6 Oral prednisone at 0.5 to 1.5 mg/kg per day with taper over 2 to 6 weeks is a standard regimen.2 IV methylprednisolone of up to 1000 mg per day for 3 to 5 days, followed by a tapered oral dose of corticosteroids over several weeks is another option.5 Other first-line treatment options include colchicine (0.5 mg 3 times a day) and oral potassium iodide (300 mg enteric-coated tablets 3 times a day).5,6 Indomethacin, cyclosporine, surgery, and dapsone are other options/adjuncts.
Recurrences of Sweet’s syndrome may occur, regardless of treatment. They are more likely to occur in patients with an underlying malignancy and may herald recurrence of malignancy in a previously treated patient.5 Treating an underlying malignancy (or discontinuing the causative medication in drug-induced Sweet’s syndrome) may resolve symptoms. IV administration of methylprednisolone, as discussed earlier, has been successful for refractory cases.5
Our patient responded well to treatment
Our patient was started on prednisone 60 mg daily and her antibiotics were discontinued. Within 24 hours, her skin lesions regressed. She was discharged on a tapering course of the prednisone.
After several weeks, her lesions completely cleared without scarring or recurrence.
CORRESPONDENCE
Ryan A. Withrow, DO, 4-2807 Reilly Road, Family Medicine Residency Clinic, Fort Bragg, NC 28314; [email protected]
1. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Edinburgh, Scotland: Mosby/Elsevier; 2010.
3. Kemmett D, Hunter JA. Sweet’s syndrome: a clinicopathologic review of twenty-nine cases. J Am Acad Dermatol. 1990;23:503-507.
4. Boatman BW, Taylor RC, Klein LE, et al. Sweet’s syndrome in children. South Med J. 1994;87:193-196.
5. Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
6. Von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
A 36-year-old white woman sought care at our outpatient clinic for 2 days of right forearm and hand redness, swelling, and severe pain at a previous intravenous (IV) catheter site. The patient had received intramuscular antibiotics for culture-positive streptococcal pharyngitis 2 weeks earlier, and then IV fluids and steroids due to poor oral intake from odynophagia.
She indicated that since the time of the pharyngitis diagnosis, she’d had a persistent fever. At our clinic, she was given a diagnosis of cellulitis and started on a course of oral cephalexin.
Three days later, she returned. She had a temperature of 100°F and her right forearm and wrist were exquisitely sensitive to touch over a warm and indurated violaceous rash with pseudovesicles over the most edematous portions (FIGURE 1). This rash extended onto her right hand and proximal phalanges. Her fingers hurt when she moved them; sensation and pulses were intact.
Her left wrist was now mildly swollen, with associated warmth and erythema, but no vesicles. Her left ankle was also warm, erythematous, and moderately swollen with significant tenderness to palpation. She had a few scattered nodular, erythematous lesions on her upper right arm and chest.
We (RW and VK) admitted the patient and started her on IV vancomycin and clindamycin to treat presumed refractory cellulitis. On Day 2 she hadn’t improved, so we added gatifloxacin for gram-negative coverage. On Day 3, her lesions worsened, so we transferred her to a higher-level facility.
FIGURE 1
Worsening lesions
The patient’s right forearm and wrist were exquisitely sensitive to touch over the rash, with pseudovesicles. Ink markings denote original boundaries of the lesion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sweet’s syndrome
A skin biopsy revealed subepidermal bullae with marked dermal and mixed lymphocytic and neutrophilic infiltrate consistent with Sweet’s syndrome.
Sweet’s syndrome, also known as acute febrile neutrophilic dermatoses, is a condition that was originally described by Dr. Robert Sweet in 1964.1 The condition can be classified based on etiology as "classical" or idiopathic, malignancy-associated, or drug-induced.2 Classical Sweet’s syndrome typically affects women 30 to 50 years of age, but has been reported in men and women of all ages.3-5 More than 500 cases have been reported in the literature, but incidence and prevalence data are limited.6 Conditions associated with classical Sweet’s syndrome include upper respiratory infection, gastrointestinal infection, inflammatory bowel disease, and pregnancy.5
Cellulitis and lupus erythematosus are part of the differential
The differential diagnosis for erythematous, indurated, tender skin and soft tissue lesions includes cellulitis, erysipelas, thrombophlebitis, urticaria, shingles, drug-induced eruptions, and herpes simplex virus infections.4-6
Less common conditions include erythema multiforme, erythema nodusum, tuberculosis, leukemia cutis, lupus erythematosus, vasculitis, pyoderma gangrenosa, Behçet’s disease, erythema elevatum diutinum, familial Mediterranean fever, and Sweet’s syndrome.4-6
Diagnosis hinges on these criteria
Two major criteria are required to make a diagnosis of Sweet’s syndrome. They are:2
- the abrupt onset of tender, erythematous plaques or nodules (FIGURE 2)
- a predominately neutrophilic infiltration in the dermis without leukocytoclastic vasculitis.
At least 2 of 4 minor criteria must also be present:2
- a precedent respiratory or gastrointestinal (GI) infection, vaccination, inflammatory disease, malignancy, or pregnancy
- malaise and fever
- elevated erythrocyte sedimentation rate, C-reactive protein, and leukocytosis with a left shift. (Our patient’s erythrocyte sedimentation rate was 93 mm/h; her C-reactive protein was 11.47 mg/dL.)
- excellent response to corticosteroids or potassium iodide.
Although skin manifestations are a hallmark sign (which makes Sweet’s syndrome an important differential diagnosis for an unusual case of cellulitis, or one that is unresponsive to antibiotics), many possible coincident manifestations can occur. These include arthralgia, myalgia, headache, and malaise.5 Multi-system involvement has also been reported, affecting bone, the central nervous system, eyes, kidneys, heart, lungs, and GI tract.5
FIGURE 2
Another presentation of Sweet’s syndrome
Some Sweet’s syndrome lesions appear "juicy." This lesion occurred at the site of minor trauma (pathergy) in a patient who was febrile and systemically ill.
Treatment: Corticosteroids, not antibiotics
A high clinical suspicion for Sweet’s syndrome is critical to management decisions because the primary treatment is corticosteroids, rather than antibiotics.5,6 Oral prednisone at 0.5 to 1.5 mg/kg per day with taper over 2 to 6 weeks is a standard regimen.2 IV methylprednisolone of up to 1000 mg per day for 3 to 5 days, followed by a tapered oral dose of corticosteroids over several weeks is another option.5 Other first-line treatment options include colchicine (0.5 mg 3 times a day) and oral potassium iodide (300 mg enteric-coated tablets 3 times a day).5,6 Indomethacin, cyclosporine, surgery, and dapsone are other options/adjuncts.
Recurrences of Sweet’s syndrome may occur, regardless of treatment. They are more likely to occur in patients with an underlying malignancy and may herald recurrence of malignancy in a previously treated patient.5 Treating an underlying malignancy (or discontinuing the causative medication in drug-induced Sweet’s syndrome) may resolve symptoms. IV administration of methylprednisolone, as discussed earlier, has been successful for refractory cases.5
Our patient responded well to treatment
Our patient was started on prednisone 60 mg daily and her antibiotics were discontinued. Within 24 hours, her skin lesions regressed. She was discharged on a tapering course of the prednisone.
After several weeks, her lesions completely cleared without scarring or recurrence.
CORRESPONDENCE
Ryan A. Withrow, DO, 4-2807 Reilly Road, Family Medicine Residency Clinic, Fort Bragg, NC 28314; [email protected]
A 36-year-old white woman sought care at our outpatient clinic for 2 days of right forearm and hand redness, swelling, and severe pain at a previous intravenous (IV) catheter site. The patient had received intramuscular antibiotics for culture-positive streptococcal pharyngitis 2 weeks earlier, and then IV fluids and steroids due to poor oral intake from odynophagia.
She indicated that since the time of the pharyngitis diagnosis, she’d had a persistent fever. At our clinic, she was given a diagnosis of cellulitis and started on a course of oral cephalexin.
Three days later, she returned. She had a temperature of 100°F and her right forearm and wrist were exquisitely sensitive to touch over a warm and indurated violaceous rash with pseudovesicles over the most edematous portions (FIGURE 1). This rash extended onto her right hand and proximal phalanges. Her fingers hurt when she moved them; sensation and pulses were intact.
Her left wrist was now mildly swollen, with associated warmth and erythema, but no vesicles. Her left ankle was also warm, erythematous, and moderately swollen with significant tenderness to palpation. She had a few scattered nodular, erythematous lesions on her upper right arm and chest.
We (RW and VK) admitted the patient and started her on IV vancomycin and clindamycin to treat presumed refractory cellulitis. On Day 2 she hadn’t improved, so we added gatifloxacin for gram-negative coverage. On Day 3, her lesions worsened, so we transferred her to a higher-level facility.
FIGURE 1
Worsening lesions
The patient’s right forearm and wrist were exquisitely sensitive to touch over the rash, with pseudovesicles. Ink markings denote original boundaries of the lesion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sweet’s syndrome
A skin biopsy revealed subepidermal bullae with marked dermal and mixed lymphocytic and neutrophilic infiltrate consistent with Sweet’s syndrome.
Sweet’s syndrome, also known as acute febrile neutrophilic dermatoses, is a condition that was originally described by Dr. Robert Sweet in 1964.1 The condition can be classified based on etiology as "classical" or idiopathic, malignancy-associated, or drug-induced.2 Classical Sweet’s syndrome typically affects women 30 to 50 years of age, but has been reported in men and women of all ages.3-5 More than 500 cases have been reported in the literature, but incidence and prevalence data are limited.6 Conditions associated with classical Sweet’s syndrome include upper respiratory infection, gastrointestinal infection, inflammatory bowel disease, and pregnancy.5
Cellulitis and lupus erythematosus are part of the differential
The differential diagnosis for erythematous, indurated, tender skin and soft tissue lesions includes cellulitis, erysipelas, thrombophlebitis, urticaria, shingles, drug-induced eruptions, and herpes simplex virus infections.4-6
Less common conditions include erythema multiforme, erythema nodusum, tuberculosis, leukemia cutis, lupus erythematosus, vasculitis, pyoderma gangrenosa, Behçet’s disease, erythema elevatum diutinum, familial Mediterranean fever, and Sweet’s syndrome.4-6
Diagnosis hinges on these criteria
Two major criteria are required to make a diagnosis of Sweet’s syndrome. They are:2
- the abrupt onset of tender, erythematous plaques or nodules (FIGURE 2)
- a predominately neutrophilic infiltration in the dermis without leukocytoclastic vasculitis.
At least 2 of 4 minor criteria must also be present:2
- a precedent respiratory or gastrointestinal (GI) infection, vaccination, inflammatory disease, malignancy, or pregnancy
- malaise and fever
- elevated erythrocyte sedimentation rate, C-reactive protein, and leukocytosis with a left shift. (Our patient’s erythrocyte sedimentation rate was 93 mm/h; her C-reactive protein was 11.47 mg/dL.)
- excellent response to corticosteroids or potassium iodide.
Although skin manifestations are a hallmark sign (which makes Sweet’s syndrome an important differential diagnosis for an unusual case of cellulitis, or one that is unresponsive to antibiotics), many possible coincident manifestations can occur. These include arthralgia, myalgia, headache, and malaise.5 Multi-system involvement has also been reported, affecting bone, the central nervous system, eyes, kidneys, heart, lungs, and GI tract.5
FIGURE 2
Another presentation of Sweet’s syndrome
Some Sweet’s syndrome lesions appear "juicy." This lesion occurred at the site of minor trauma (pathergy) in a patient who was febrile and systemically ill.
Treatment: Corticosteroids, not antibiotics
A high clinical suspicion for Sweet’s syndrome is critical to management decisions because the primary treatment is corticosteroids, rather than antibiotics.5,6 Oral prednisone at 0.5 to 1.5 mg/kg per day with taper over 2 to 6 weeks is a standard regimen.2 IV methylprednisolone of up to 1000 mg per day for 3 to 5 days, followed by a tapered oral dose of corticosteroids over several weeks is another option.5 Other first-line treatment options include colchicine (0.5 mg 3 times a day) and oral potassium iodide (300 mg enteric-coated tablets 3 times a day).5,6 Indomethacin, cyclosporine, surgery, and dapsone are other options/adjuncts.
Recurrences of Sweet’s syndrome may occur, regardless of treatment. They are more likely to occur in patients with an underlying malignancy and may herald recurrence of malignancy in a previously treated patient.5 Treating an underlying malignancy (or discontinuing the causative medication in drug-induced Sweet’s syndrome) may resolve symptoms. IV administration of methylprednisolone, as discussed earlier, has been successful for refractory cases.5
Our patient responded well to treatment
Our patient was started on prednisone 60 mg daily and her antibiotics were discontinued. Within 24 hours, her skin lesions regressed. She was discharged on a tapering course of the prednisone.
After several weeks, her lesions completely cleared without scarring or recurrence.
CORRESPONDENCE
Ryan A. Withrow, DO, 4-2807 Reilly Road, Family Medicine Residency Clinic, Fort Bragg, NC 28314; [email protected]
1. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Edinburgh, Scotland: Mosby/Elsevier; 2010.
3. Kemmett D, Hunter JA. Sweet’s syndrome: a clinicopathologic review of twenty-nine cases. J Am Acad Dermatol. 1990;23:503-507.
4. Boatman BW, Taylor RC, Klein LE, et al. Sweet’s syndrome in children. South Med J. 1994;87:193-196.
5. Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
6. Von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
1. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
2. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Edinburgh, Scotland: Mosby/Elsevier; 2010.
3. Kemmett D, Hunter JA. Sweet’s syndrome: a clinicopathologic review of twenty-nine cases. J Am Acad Dermatol. 1990;23:503-507.
4. Boatman BW, Taylor RC, Klein LE, et al. Sweet’s syndrome in children. South Med J. 1994;87:193-196.
5. Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
6. Von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
What is the best treatment for chronic constipation in the elderly?
There is no one best evidence-based treatment for chronic constipation in the elderly. While the most common first-line treatments are dietary fiber and exercise, the evidence is insufficient to support this approach in the geriatric population (strength of recommendation [SOR]: for dietary fiber: A, based on a systematic review; for exercise: SOR: B, based on 1 good- and 1 fair-quality randomized controlled trial [RCT]).
Herbal supplements (such as aloe), alternative treatments (biofeedback), lubricants (mineral oil), and combination laxatives sold in the US have not been sufficiently studied in controlled trials to make a recommendation (SOR: A, based on systematic review).
An abdominal kneading device can be used to treat chronic constipation, but the evidence is limited (SOR: B, based on 1 cohort study.)
Polyethylene glycol has not been studied in the elderly. A newer agent, lubiprostone (Amitiza), appears to be effective for the treatment of chronic constipation for elderly patients (SOR: B, based on subgroup analysis of RCTs.)
Is the patient truly constipated?
Mandi Sehgal, MD
Department of Family Medicine/Geriatrics, University of Cincinnati
Many older people feel that if they do not have a bowel movement every day they are constipated. However, constipation is defined as fewer than 3 bowel movements per week. So, the first thing we must do is to confirm that the patient is truly constipated.
Before I start my patients on any medicine, I suggest a trial of increased daily water and fiber intake along with exercise, followed by a trial of stool softeners and stimulant laxatives, if needed. If all of these methods fail, I consider trying polyethylene glycol, which can be titrated to effect. As with all medication use by the elderly, it is important to titrate cautiously (“start low and go slow”) and add other medications only when necessary.
Evidence summary
Few well-designed studies have focused on constipation treatment among the elderly. Our search located 1 systematic review of pharmacologic management, a systematic review of fiber management, 2 RCTs on the effect of exercise, and 1 before-after cohort study on abdominal massage. These studies were all conducted among geriatric patients with constipation. Two high-quality systematic reviews regarding chronic constipation management for adults of all ages included management options not studied in exclusively geriatric populations, such as herbal supplements, biofeedback, tegaserod, and polyethylene glycol.
Laxatives, fiber, and exercise: Studies are inconclusive
Two good-quality systematic reviews looked at 10 RCTs comparing laxatives with placebo, and 10 RCTs comparing 1 laxative with another.1,2 The studies generally had few participants, were of short duration, and were conducted in institutional settings. Most lacked power to make valid conclusions. These studies varied in the reported outcome measures, including stool frequency, stool consistency, straining, decrease in laxative use, and symptom scores. The reviews concluded that the best pharmacologic treatment for chronic constipation in the elderly has not been established.
Five of the higher-quality studies attained statistical significance. They showed a small but significant improvement in bowel movement frequency with a laxative when compared with placebo or another laxative (TABLE). The authors noted that multiple poor-quality studies have shown nonsignificant trends for improved constipation symptoms with laxatives compared with placebo.
Inconsistent findings on fiber. A good-quality systematic review3 of dietary fiber in the treatment of constipation for older patients located 8 moderate- to high-quality studies (6 RCTs and 2 blinded before-after studies), with 269 study participants in institutional settings. Results among studies were inconsistent, casting doubt on the efficacy of fiber treatment for constipation in the institutionalized elder.
Two RCTs4,5 investigating the effect of exercise on 246 institutionalized older patients showed no improvement in constipation. One study was of good quality, reporting adequate power and used an intention-to-treat analysis. The other was of fair quality.
Alternative TXs not well studied
A high-quality systematic review6 of constipation management among adults of all ages in North America found a lack of quality RCTs examining herbal supplement treatment. Biofeedback has been studied in adult populations, but no RCTs with placebo or sham-controls have been published.
One before-after cohort study7 investigated an external kneading mechanical device (Free-Lax) that was applied to the abdomen for 20 minutes once daily in 30 randomly selected chronically constipated nursing home residents. Researchers found significant improvements in bowel movement frequency, stool consistency and volume, and colonic transit time without side effects (TABLE).
A look beyond geriatric patients
Polyethylene glycol, tegaserod, and lubiprostone have not been studied in trials of exclusively geriatric populations. Two high-quality systematic reviews,6,8 including medium- to high-quality RCTs of pharmacologic management of chronic constipation, found good evidence to support treatment with polyethylene glycol and tegaserod in adults of all ages. Of the 8 RCTs looking at polyethylene glycol, only 1 of the studies—a high-quality crossover comparison of polyethylene glycol vs placebo with 37 out-patient subjects—included a population with a mean age >60 years (mean age 62, range 42–89 years).
TABLE
How well do these interventions work for older patients with chronic constipation?
| INTERVENTION VS COMPARISON | STOOL FREQUENCY (STOOLS PER WEEK) | NNT‡ |
|---|---|---|
| Agiolax* vs lactulose | 4.5 vs 2.2 | 43 |
| Agiolax* vs lactulose | 5.6 vs 4.2 | 71 |
| Lactitol† vs placebo | 4.9 vs 3.6 | 77 |
| Lactitol† vs lactulose | 5.5 vs 4.9 | 160 |
| Lactulose vs sorbitol† | 7.0 vs 6.7 | 330 |
| External abdominal kneading (before-after) | 3.9 vs 1.4 | 40 |
| * Agiolax is a combination bulk and stimulant laxative not readily found in the United States. | ||
| † Lactitol and sorbitol are sugar alcohols used as replacement sweeteners and approved by the FDA as food additives. | ||
| ‡ Number needed to treat (NNT) for 1 person to have 1 more stool per week. | ||
A subgroup analysis9 of 331 elderly patients enrolled in 2 RCTs of tegaserod found no difference in outcomes between treatment with tegaserod and placebo, although this analysis was limited by inadequate power.
Tegaserod linked to ischemic events. A recent analysis of clinical trials found a statistically significant increase in cardiovascular ischemic events associated with tegaserod. The manufacturer took the product off the market in compliance with an FDA request in March 2007.
Lubiprostone offers promise. Lubiprostone, a chloride channel activator approved by the FDA for the treatment of chronic idiopathic constipation, has been studied in 6 placebo-controlled, double-blind, randomized Phase II and III clinical trials. In 2 unpublished pooled analyses of 3 of the trials, lubiprostone was found to be effective in a total of 220 elderly patients 65 years of age and older.10,11
Recommendations from others
The American College of Gastroenterology Chronic Constipation Task Force evidence-based guidelines make no reference to age, but state that evidence is best for treatment with psyllium, tegaserod, polyethylene glycol, and lactulose.12 They found insufficient evidence to support use of stimulants, stool softeners, lubricants, herbal supplements, biofeedback, and alternative treatments.
The American Gastroenterological Association guidelines on constipation are primarily based on expert opinion.13 Age is not specified in their recommendations. Dietary and exercise modifications are recommended as first-line treatments, followed by laxatives. Laxatives are recommended based on cost, in order from the least to most expensive agents. Suppositories, enemas, biofeedback, and (in refractory cases) surgery are recommended for patients with pelvic floor dysfunction.
The Registered Nurses Association of Ontario guidelines for constipation prevention in the older adult population recommend fluid and dietary fiber, regular exercise, and consistent toileting.14
1. Petticrew M, Watt I, Brand M. What’s the “best buy” for treatment of constipation? Results of a systematic review of the efficacy and comparative efficacy of laxatives in the elderly. Br J Gen Pract 1999;49:387-393.
2. Petticrew M, Watt I, Sheldon T. Systematic review of the effectiveness of laxatives in the elderly. Health Technol Assess 1997;1:i–iv-1–52.
3. Kenny KA, Skelly JM. Dietary fiber for constipation in older adults: a systematic review. Clinical Effectiveness in Nursing 2001;5:120-128.
4. Chin APMJ, van Poppel MN, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr 2006;6:9.-
5. Simmons SF, Schnelle JF. Effects of an exercise and scheduled-toileting intervention on appetite and constipation in nursing home residents. J Nutr Health Aging 2004;8:116-121.
6. Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005;100 suppl 1:S5-S21.
7. Mimidis K, Galinsky D, Rimon E, Papadopoulos V, Zicherman Y, Oreopoulos D. Use of a device that applies external kneading-like force on the abdomen for treatment of constipation. World J Gastroenterol 2005;11:1971-1975.
8. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol 2005;100:936-971.
9. Baun RF, Levy HB. Tegaserod for treating chronic constipation in elderly patients. Ann Pharmacother 2007;41:309-313.
10. Ueno R, Joswick TR, Wahle A, et al. Efficacy and safety of lubiprostone for the treatment of chronic constipation in elderly vs non-elderly subjects. Gastroenterology 2006;130(suppl 2):A189.-
11. Ueno R, Panas R, Wahle A, et al. Long-term safety and efficacy of lubiprostone for the treatment of chronic constipation in elderly subjects. Gastroenterology 2006;130(suppl 2):A188.-
12. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;100 Suppl 1:S1-S4.
13. Locke GR, 3rd, Pemberton JH, Phillips SF. American Gastroenterological Association Medical Position Statement: guidelines on constipation. Gastroenterology 2000;119:1761-1766.
14. Registered Nurses Association of Ontario (RNAO). Prevention of constipation in the older adult population. Toronto, Ontario: RNAO; 2005. Available at: www.guideline.gov/summary/summary.aspx?ss=15&doc_id=7004&nbr=4213. Accessed on November 8, 2007.
There is no one best evidence-based treatment for chronic constipation in the elderly. While the most common first-line treatments are dietary fiber and exercise, the evidence is insufficient to support this approach in the geriatric population (strength of recommendation [SOR]: for dietary fiber: A, based on a systematic review; for exercise: SOR: B, based on 1 good- and 1 fair-quality randomized controlled trial [RCT]).
Herbal supplements (such as aloe), alternative treatments (biofeedback), lubricants (mineral oil), and combination laxatives sold in the US have not been sufficiently studied in controlled trials to make a recommendation (SOR: A, based on systematic review).
An abdominal kneading device can be used to treat chronic constipation, but the evidence is limited (SOR: B, based on 1 cohort study.)
Polyethylene glycol has not been studied in the elderly. A newer agent, lubiprostone (Amitiza), appears to be effective for the treatment of chronic constipation for elderly patients (SOR: B, based on subgroup analysis of RCTs.)
Is the patient truly constipated?
Mandi Sehgal, MD
Department of Family Medicine/Geriatrics, University of Cincinnati
Many older people feel that if they do not have a bowel movement every day they are constipated. However, constipation is defined as fewer than 3 bowel movements per week. So, the first thing we must do is to confirm that the patient is truly constipated.
Before I start my patients on any medicine, I suggest a trial of increased daily water and fiber intake along with exercise, followed by a trial of stool softeners and stimulant laxatives, if needed. If all of these methods fail, I consider trying polyethylene glycol, which can be titrated to effect. As with all medication use by the elderly, it is important to titrate cautiously (“start low and go slow”) and add other medications only when necessary.
Evidence summary
Few well-designed studies have focused on constipation treatment among the elderly. Our search located 1 systematic review of pharmacologic management, a systematic review of fiber management, 2 RCTs on the effect of exercise, and 1 before-after cohort study on abdominal massage. These studies were all conducted among geriatric patients with constipation. Two high-quality systematic reviews regarding chronic constipation management for adults of all ages included management options not studied in exclusively geriatric populations, such as herbal supplements, biofeedback, tegaserod, and polyethylene glycol.
Laxatives, fiber, and exercise: Studies are inconclusive
Two good-quality systematic reviews looked at 10 RCTs comparing laxatives with placebo, and 10 RCTs comparing 1 laxative with another.1,2 The studies generally had few participants, were of short duration, and were conducted in institutional settings. Most lacked power to make valid conclusions. These studies varied in the reported outcome measures, including stool frequency, stool consistency, straining, decrease in laxative use, and symptom scores. The reviews concluded that the best pharmacologic treatment for chronic constipation in the elderly has not been established.
Five of the higher-quality studies attained statistical significance. They showed a small but significant improvement in bowel movement frequency with a laxative when compared with placebo or another laxative (TABLE). The authors noted that multiple poor-quality studies have shown nonsignificant trends for improved constipation symptoms with laxatives compared with placebo.
Inconsistent findings on fiber. A good-quality systematic review3 of dietary fiber in the treatment of constipation for older patients located 8 moderate- to high-quality studies (6 RCTs and 2 blinded before-after studies), with 269 study participants in institutional settings. Results among studies were inconsistent, casting doubt on the efficacy of fiber treatment for constipation in the institutionalized elder.
Two RCTs4,5 investigating the effect of exercise on 246 institutionalized older patients showed no improvement in constipation. One study was of good quality, reporting adequate power and used an intention-to-treat analysis. The other was of fair quality.
Alternative TXs not well studied
A high-quality systematic review6 of constipation management among adults of all ages in North America found a lack of quality RCTs examining herbal supplement treatment. Biofeedback has been studied in adult populations, but no RCTs with placebo or sham-controls have been published.
One before-after cohort study7 investigated an external kneading mechanical device (Free-Lax) that was applied to the abdomen for 20 minutes once daily in 30 randomly selected chronically constipated nursing home residents. Researchers found significant improvements in bowel movement frequency, stool consistency and volume, and colonic transit time without side effects (TABLE).
A look beyond geriatric patients
Polyethylene glycol, tegaserod, and lubiprostone have not been studied in trials of exclusively geriatric populations. Two high-quality systematic reviews,6,8 including medium- to high-quality RCTs of pharmacologic management of chronic constipation, found good evidence to support treatment with polyethylene glycol and tegaserod in adults of all ages. Of the 8 RCTs looking at polyethylene glycol, only 1 of the studies—a high-quality crossover comparison of polyethylene glycol vs placebo with 37 out-patient subjects—included a population with a mean age >60 years (mean age 62, range 42–89 years).
TABLE
How well do these interventions work for older patients with chronic constipation?
| INTERVENTION VS COMPARISON | STOOL FREQUENCY (STOOLS PER WEEK) | NNT‡ |
|---|---|---|
| Agiolax* vs lactulose | 4.5 vs 2.2 | 43 |
| Agiolax* vs lactulose | 5.6 vs 4.2 | 71 |
| Lactitol† vs placebo | 4.9 vs 3.6 | 77 |
| Lactitol† vs lactulose | 5.5 vs 4.9 | 160 |
| Lactulose vs sorbitol† | 7.0 vs 6.7 | 330 |
| External abdominal kneading (before-after) | 3.9 vs 1.4 | 40 |
| * Agiolax is a combination bulk and stimulant laxative not readily found in the United States. | ||
| † Lactitol and sorbitol are sugar alcohols used as replacement sweeteners and approved by the FDA as food additives. | ||
| ‡ Number needed to treat (NNT) for 1 person to have 1 more stool per week. | ||
A subgroup analysis9 of 331 elderly patients enrolled in 2 RCTs of tegaserod found no difference in outcomes between treatment with tegaserod and placebo, although this analysis was limited by inadequate power.
Tegaserod linked to ischemic events. A recent analysis of clinical trials found a statistically significant increase in cardiovascular ischemic events associated with tegaserod. The manufacturer took the product off the market in compliance with an FDA request in March 2007.
Lubiprostone offers promise. Lubiprostone, a chloride channel activator approved by the FDA for the treatment of chronic idiopathic constipation, has been studied in 6 placebo-controlled, double-blind, randomized Phase II and III clinical trials. In 2 unpublished pooled analyses of 3 of the trials, lubiprostone was found to be effective in a total of 220 elderly patients 65 years of age and older.10,11
Recommendations from others
The American College of Gastroenterology Chronic Constipation Task Force evidence-based guidelines make no reference to age, but state that evidence is best for treatment with psyllium, tegaserod, polyethylene glycol, and lactulose.12 They found insufficient evidence to support use of stimulants, stool softeners, lubricants, herbal supplements, biofeedback, and alternative treatments.
The American Gastroenterological Association guidelines on constipation are primarily based on expert opinion.13 Age is not specified in their recommendations. Dietary and exercise modifications are recommended as first-line treatments, followed by laxatives. Laxatives are recommended based on cost, in order from the least to most expensive agents. Suppositories, enemas, biofeedback, and (in refractory cases) surgery are recommended for patients with pelvic floor dysfunction.
The Registered Nurses Association of Ontario guidelines for constipation prevention in the older adult population recommend fluid and dietary fiber, regular exercise, and consistent toileting.14
There is no one best evidence-based treatment for chronic constipation in the elderly. While the most common first-line treatments are dietary fiber and exercise, the evidence is insufficient to support this approach in the geriatric population (strength of recommendation [SOR]: for dietary fiber: A, based on a systematic review; for exercise: SOR: B, based on 1 good- and 1 fair-quality randomized controlled trial [RCT]).
Herbal supplements (such as aloe), alternative treatments (biofeedback), lubricants (mineral oil), and combination laxatives sold in the US have not been sufficiently studied in controlled trials to make a recommendation (SOR: A, based on systematic review).
An abdominal kneading device can be used to treat chronic constipation, but the evidence is limited (SOR: B, based on 1 cohort study.)
Polyethylene glycol has not been studied in the elderly. A newer agent, lubiprostone (Amitiza), appears to be effective for the treatment of chronic constipation for elderly patients (SOR: B, based on subgroup analysis of RCTs.)
Is the patient truly constipated?
Mandi Sehgal, MD
Department of Family Medicine/Geriatrics, University of Cincinnati
Many older people feel that if they do not have a bowel movement every day they are constipated. However, constipation is defined as fewer than 3 bowel movements per week. So, the first thing we must do is to confirm that the patient is truly constipated.
Before I start my patients on any medicine, I suggest a trial of increased daily water and fiber intake along with exercise, followed by a trial of stool softeners and stimulant laxatives, if needed. If all of these methods fail, I consider trying polyethylene glycol, which can be titrated to effect. As with all medication use by the elderly, it is important to titrate cautiously (“start low and go slow”) and add other medications only when necessary.
Evidence summary
Few well-designed studies have focused on constipation treatment among the elderly. Our search located 1 systematic review of pharmacologic management, a systematic review of fiber management, 2 RCTs on the effect of exercise, and 1 before-after cohort study on abdominal massage. These studies were all conducted among geriatric patients with constipation. Two high-quality systematic reviews regarding chronic constipation management for adults of all ages included management options not studied in exclusively geriatric populations, such as herbal supplements, biofeedback, tegaserod, and polyethylene glycol.
Laxatives, fiber, and exercise: Studies are inconclusive
Two good-quality systematic reviews looked at 10 RCTs comparing laxatives with placebo, and 10 RCTs comparing 1 laxative with another.1,2 The studies generally had few participants, were of short duration, and were conducted in institutional settings. Most lacked power to make valid conclusions. These studies varied in the reported outcome measures, including stool frequency, stool consistency, straining, decrease in laxative use, and symptom scores. The reviews concluded that the best pharmacologic treatment for chronic constipation in the elderly has not been established.
Five of the higher-quality studies attained statistical significance. They showed a small but significant improvement in bowel movement frequency with a laxative when compared with placebo or another laxative (TABLE). The authors noted that multiple poor-quality studies have shown nonsignificant trends for improved constipation symptoms with laxatives compared with placebo.
Inconsistent findings on fiber. A good-quality systematic review3 of dietary fiber in the treatment of constipation for older patients located 8 moderate- to high-quality studies (6 RCTs and 2 blinded before-after studies), with 269 study participants in institutional settings. Results among studies were inconsistent, casting doubt on the efficacy of fiber treatment for constipation in the institutionalized elder.
Two RCTs4,5 investigating the effect of exercise on 246 institutionalized older patients showed no improvement in constipation. One study was of good quality, reporting adequate power and used an intention-to-treat analysis. The other was of fair quality.
Alternative TXs not well studied
A high-quality systematic review6 of constipation management among adults of all ages in North America found a lack of quality RCTs examining herbal supplement treatment. Biofeedback has been studied in adult populations, but no RCTs with placebo or sham-controls have been published.
One before-after cohort study7 investigated an external kneading mechanical device (Free-Lax) that was applied to the abdomen for 20 minutes once daily in 30 randomly selected chronically constipated nursing home residents. Researchers found significant improvements in bowel movement frequency, stool consistency and volume, and colonic transit time without side effects (TABLE).
A look beyond geriatric patients
Polyethylene glycol, tegaserod, and lubiprostone have not been studied in trials of exclusively geriatric populations. Two high-quality systematic reviews,6,8 including medium- to high-quality RCTs of pharmacologic management of chronic constipation, found good evidence to support treatment with polyethylene glycol and tegaserod in adults of all ages. Of the 8 RCTs looking at polyethylene glycol, only 1 of the studies—a high-quality crossover comparison of polyethylene glycol vs placebo with 37 out-patient subjects—included a population with a mean age >60 years (mean age 62, range 42–89 years).
TABLE
How well do these interventions work for older patients with chronic constipation?
| INTERVENTION VS COMPARISON | STOOL FREQUENCY (STOOLS PER WEEK) | NNT‡ |
|---|---|---|
| Agiolax* vs lactulose | 4.5 vs 2.2 | 43 |
| Agiolax* vs lactulose | 5.6 vs 4.2 | 71 |
| Lactitol† vs placebo | 4.9 vs 3.6 | 77 |
| Lactitol† vs lactulose | 5.5 vs 4.9 | 160 |
| Lactulose vs sorbitol† | 7.0 vs 6.7 | 330 |
| External abdominal kneading (before-after) | 3.9 vs 1.4 | 40 |
| * Agiolax is a combination bulk and stimulant laxative not readily found in the United States. | ||
| † Lactitol and sorbitol are sugar alcohols used as replacement sweeteners and approved by the FDA as food additives. | ||
| ‡ Number needed to treat (NNT) for 1 person to have 1 more stool per week. | ||
A subgroup analysis9 of 331 elderly patients enrolled in 2 RCTs of tegaserod found no difference in outcomes between treatment with tegaserod and placebo, although this analysis was limited by inadequate power.
Tegaserod linked to ischemic events. A recent analysis of clinical trials found a statistically significant increase in cardiovascular ischemic events associated with tegaserod. The manufacturer took the product off the market in compliance with an FDA request in March 2007.
Lubiprostone offers promise. Lubiprostone, a chloride channel activator approved by the FDA for the treatment of chronic idiopathic constipation, has been studied in 6 placebo-controlled, double-blind, randomized Phase II and III clinical trials. In 2 unpublished pooled analyses of 3 of the trials, lubiprostone was found to be effective in a total of 220 elderly patients 65 years of age and older.10,11
Recommendations from others
The American College of Gastroenterology Chronic Constipation Task Force evidence-based guidelines make no reference to age, but state that evidence is best for treatment with psyllium, tegaserod, polyethylene glycol, and lactulose.12 They found insufficient evidence to support use of stimulants, stool softeners, lubricants, herbal supplements, biofeedback, and alternative treatments.
The American Gastroenterological Association guidelines on constipation are primarily based on expert opinion.13 Age is not specified in their recommendations. Dietary and exercise modifications are recommended as first-line treatments, followed by laxatives. Laxatives are recommended based on cost, in order from the least to most expensive agents. Suppositories, enemas, biofeedback, and (in refractory cases) surgery are recommended for patients with pelvic floor dysfunction.
The Registered Nurses Association of Ontario guidelines for constipation prevention in the older adult population recommend fluid and dietary fiber, regular exercise, and consistent toileting.14
1. Petticrew M, Watt I, Brand M. What’s the “best buy” for treatment of constipation? Results of a systematic review of the efficacy and comparative efficacy of laxatives in the elderly. Br J Gen Pract 1999;49:387-393.
2. Petticrew M, Watt I, Sheldon T. Systematic review of the effectiveness of laxatives in the elderly. Health Technol Assess 1997;1:i–iv-1–52.
3. Kenny KA, Skelly JM. Dietary fiber for constipation in older adults: a systematic review. Clinical Effectiveness in Nursing 2001;5:120-128.
4. Chin APMJ, van Poppel MN, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr 2006;6:9.-
5. Simmons SF, Schnelle JF. Effects of an exercise and scheduled-toileting intervention on appetite and constipation in nursing home residents. J Nutr Health Aging 2004;8:116-121.
6. Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005;100 suppl 1:S5-S21.
7. Mimidis K, Galinsky D, Rimon E, Papadopoulos V, Zicherman Y, Oreopoulos D. Use of a device that applies external kneading-like force on the abdomen for treatment of constipation. World J Gastroenterol 2005;11:1971-1975.
8. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol 2005;100:936-971.
9. Baun RF, Levy HB. Tegaserod for treating chronic constipation in elderly patients. Ann Pharmacother 2007;41:309-313.
10. Ueno R, Joswick TR, Wahle A, et al. Efficacy and safety of lubiprostone for the treatment of chronic constipation in elderly vs non-elderly subjects. Gastroenterology 2006;130(suppl 2):A189.-
11. Ueno R, Panas R, Wahle A, et al. Long-term safety and efficacy of lubiprostone for the treatment of chronic constipation in elderly subjects. Gastroenterology 2006;130(suppl 2):A188.-
12. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;100 Suppl 1:S1-S4.
13. Locke GR, 3rd, Pemberton JH, Phillips SF. American Gastroenterological Association Medical Position Statement: guidelines on constipation. Gastroenterology 2000;119:1761-1766.
14. Registered Nurses Association of Ontario (RNAO). Prevention of constipation in the older adult population. Toronto, Ontario: RNAO; 2005. Available at: www.guideline.gov/summary/summary.aspx?ss=15&doc_id=7004&nbr=4213. Accessed on November 8, 2007.
1. Petticrew M, Watt I, Brand M. What’s the “best buy” for treatment of constipation? Results of a systematic review of the efficacy and comparative efficacy of laxatives in the elderly. Br J Gen Pract 1999;49:387-393.
2. Petticrew M, Watt I, Sheldon T. Systematic review of the effectiveness of laxatives in the elderly. Health Technol Assess 1997;1:i–iv-1–52.
3. Kenny KA, Skelly JM. Dietary fiber for constipation in older adults: a systematic review. Clinical Effectiveness in Nursing 2001;5:120-128.
4. Chin APMJ, van Poppel MN, van Mechelen W. Effects of resistance and functional-skills training on habitual activity and constipation among older adults living in long-term care facilities: a randomized controlled trial. BMC Geriatr 2006;6:9.-
5. Simmons SF, Schnelle JF. Effects of an exercise and scheduled-toileting intervention on appetite and constipation in nursing home residents. J Nutr Health Aging 2004;8:116-121.
6. Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005;100 suppl 1:S5-S21.
7. Mimidis K, Galinsky D, Rimon E, Papadopoulos V, Zicherman Y, Oreopoulos D. Use of a device that applies external kneading-like force on the abdomen for treatment of constipation. World J Gastroenterol 2005;11:1971-1975.
8. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol 2005;100:936-971.
9. Baun RF, Levy HB. Tegaserod for treating chronic constipation in elderly patients. Ann Pharmacother 2007;41:309-313.
10. Ueno R, Joswick TR, Wahle A, et al. Efficacy and safety of lubiprostone for the treatment of chronic constipation in elderly vs non-elderly subjects. Gastroenterology 2006;130(suppl 2):A189.-
11. Ueno R, Panas R, Wahle A, et al. Long-term safety and efficacy of lubiprostone for the treatment of chronic constipation in elderly subjects. Gastroenterology 2006;130(suppl 2):A188.-
12. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;100 Suppl 1:S1-S4.
13. Locke GR, 3rd, Pemberton JH, Phillips SF. American Gastroenterological Association Medical Position Statement: guidelines on constipation. Gastroenterology 2000;119:1761-1766.
14. Registered Nurses Association of Ontario (RNAO). Prevention of constipation in the older adult population. Toronto, Ontario: RNAO; 2005. Available at: www.guideline.gov/summary/summary.aspx?ss=15&doc_id=7004&nbr=4213. Accessed on November 8, 2007.
Evidence-based answers from the Family Physicians Inquiries Network